Tratamiento a base de hierbas para la artritis reumatoide
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised, double blind, placebo control, 3 parallel groups. Duration 12 months. | |
| Participants | Randomised n=49, completed n=34. Age range 28‐74 yr. Inclusion: classical or definite RA (ARA criteria), requiring NSAIDs but not DMARDs. | |
| Interventions | Tradename not provided, Oenothera biennis (evening primrose), oil, 6000mg (12x500mg, approx 9% GLA, equivalent to 540mg GLA), capsules, oral. Concurrent intervention: usual NSAIDs for first 3 months only. | |
| Outcomes | Morning stiffness (minutes), grip strength mmHg, Ritchie index, pain VAS 0‐100, patient global. | |
| Notes | Results favour intervention for reduction in pain and NSAID use. No evidence of disease‐modifying effects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, method not reported. |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? | Low risk | Active intervention and placebo not distinguished by look, taste, smell or packaging. |
| Incomplete outcome data addressed? | Low risk | Reported withdrawals. Included intention‐to‐treat analyses. |
| Free of selective reporting? | Low risk | Reported adverse events. |
| Free of other bias? | Low risk | Diagnosis / assessment consistent with ACR criteria. |
| Methods | Randomised, double blind, placebo control, 2 parallel groups. Duration 6 weeks. | |
| Participants | Randomised n=26 (intervention n=13, control n=13), completed n=26 (intervention n=13, control n=13). Age (yr): intervention m=56.5 sd=8.9, control m=60.1 sd=11. Inclusion: ACR criteria RA stage I‐III. | |
| Interventions | Assalix*, Salix daphnoides cortex (willow bark), ethanolic extract, 1572.96mg (2x2x393.24mg, equivalent to 240mg salicin), tablets, oral. | |
| Outcomes | Pain VAS 0‐100, tender joint count, HAQ‐DI, stiffness VAS 0‐100, efficacy VAS 0‐100, SF‐36, ESR, CRP, ACR20. | |
| Notes | Results equivocal. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomised to one of three groups using a computer generated random number sequence. |
| Allocation concealment? | Low risk | Adequate. |
| Blinding? | Low risk | Active interventions and placebo not distinguished by look, taste, smell or packaging. |
| Incomplete outcome data addressed? | Low risk | Reported withdrawals. Included intention‐to‐treat and per protocol analyses. |
| Free of selective reporting? | Low risk | Confirmatory study, statistical power reported. |
| Free of other bias? | Low risk | Diagnosis / assessment consistent with ACR criteria. |
| Methods | Randomised, double blind, placebo control, 2 parallel groups. Duration 6 months. | |
| Participants | Randomised n=40 (intervention n=19, control n=21), completed n=30 (intervention n=13, control n=17). Age range 16‐75 yr. Inclusion: classical or definite RA, all with probable gastro‐intestinal lesions due to NSAIDs. | |
| Interventions | Tradename not provided, Oenothera biennis (evening primrose), oil, 6000mg (12x500mg, approx 9% GLA, equivalent to 540mg GLA), capsules, oral. | |
| Outcomes | Pain VAS 0‐100, well‐being score, morning stiffness (minutes), Ritchie index, HAQ, intake of NSAIDs and analgesics. | |
| Notes | Results favour intervention for morning stiffness, equivocal for all other outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, method not reported. |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? | Unclear risk | Described as double‐blind, method not reported. |
| Incomplete outcome data addressed? | Unclear risk | Reported withdrawals. |
| Free of other bias? | Unclear risk | Placebo capsules contained olive oil and may not be inert. Reported ethics committee approval. |
| Methods | Randomised, double blind, placebo control, 2 parallel groups. Duration 16 weeks. | |
| Participants | Randomised n=182 (intervention n=89, control n=93), completed n=165 (intervention n=80, control n=85). Age (yr): intervention m=45, control m=45. Inclusion: ACR criteria RA stage I‐III. | |
| Interventions | RA‐1, Ayurvedic formula, mixture of Withania somnifera, Boswellia serrata, Zingiberis officinale, Ciruma longa, 444mg, (3x2), tablets, oral. | |
| Outcomes | 20% or 50% reduction in individual core set variables, patient global assessment, physician global assessment, ARC20. | |
| Notes | Results equivocal. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomised to one of two groups using a computer generated random number sequence. |
| Allocation concealment? | Low risk | Adequate |
| Blinding? | Low risk | Active intervention and placebo not distinguished by look, taste, smell or packaging. |
| Incomplete outcome data addressed? | Low risk | Reported withdrawals. Included intention‐to‐treat and per protocol analyses. |
| Free of selective reporting? | Low risk | Reported adverse events. |
| Free of other bias? | Low risk | Diagnosis / assessment consistent with ACR criteria. Reported ethics committee approval. |
| Methods | Randomised, double blind, placebo control, 2 parallel groups. Duration 6 weeks. | |
| Participants | Randomised n=61 (intervention n=31, control n=30). Dropouts not reported. Age (yr): intervention m=42, control m=39. Inclusion: ACR criteria RA (any stage). | |
| Interventions | Tradename not provided, Tripterygium wilfordii (thunder god vine), tincture, 5‐6 applications/day, topical. | |
| Outcomes | Modified ACR20, 42 tender joint count, 40 swollen joint count, grip strength kPa, morning stiffness (hours), HAQ‐DI, ESR, CRP, patient global, physician global. | |
| Notes | Results favour intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomised to one of two groups using a computer generated random number sequence. |
| Allocation concealment? | Low risk | Adequate |
| Blinding? | Low risk | Active intervention and placebo not distinguished by look, taste, smell or packaging. |
| Incomplete outcome data addressed? | Unclear risk | Withdrawals not reported. Included intention‐to‐treat analyses. |
| Free of selective reporting? | Unclear risk | Adverse events not reported. Confirmatory study. |
| Free of other bias? | Unclear risk | Diagnosis / assessment consistent with ACR criteria. Reanalysis of previous study. |
| Methods | Randomised, double blind, placebo‐control, 2 parallel groups. Duration 4 weeks. | |
| Participants | Randomised n=31, completed n=29. Age range 20‐79 yr. Inclusion: primary RA one/both knees, moderate to very severe knee pain (scale of 0‐4), at least 3 ACR criteria for classic, definite, or probable RA. | |
| Interventions | Zostrix, capsaicin 0.025% w v cream, topical, QID. | |
| Outcomes | Pain VAS 0‐100, pain 0‐4, physician global ‐1‐3. | |
| Notes | Results favour intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, method not reported. |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? | Unclear risk | Described as double‐blind. Active intervention and placebo not distinguished by look, taste, smell or packaging, but placebo validity and blinding may be compromised by burning side effect of topical intervention. |
| Incomplete outcome data addressed? | Low risk | Reported withdrawals. |
| Free of selective reporting? | Unclear risk | Variances reported as standard error of measurement (SEM). When converted to standard deviation (SD), data are skewed, violating an assumption of the inferential analyses. Reported adverse events. |
| Free of other bias? | Unclear risk | Diagnosis / assessment criteria for OA not specified. |
| Methods | Randomised, double blind, placebo control, 2 parallel groups. Duration 12 months. | |
| Participants | Randomised n=37 (intervention n=20, control n=17), completed n=24 (intervention n=15, control n=9). Age (yr): intervention m=61 sd=12, control m=59 sd=10. M:F=1:36. Inclusion: ACR criteria RA stage II or III. | |
| Interventions | Phytodolor® N, mixture of ash bark, aspen leaf, aspen bark, golden rod herb, tincture, 3x30 drops, oral. Concurrent intervention: diclofenac 25mg/d, oral. | |
| Outcomes | Joint stiffness, grip strength mmHg, Ritchie index. | |
| Notes | Results favour intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, method not reported. |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? | Unclear risk | Described as double blind, method not reported. |
| Incomplete outcome data addressed? | Unclear risk | Reported withdrawals. |
| Free of selective reporting? | Low risk | Reported adverse events. Full data reported. |
| Free of other bias? | Low risk | Diagnosis / assessment consistent with ACR criteria. |
| Methods | Double‐blind, randomised, controlled study. Duration 24 weeks. | |
| Participants | Randomized n=121 (Tripterygium wilfordii n=60; Sulfasalazine n=61), completed n=62 (Tripterygium wilfordii n=37, Sufasalazine n=25). Age (yr): Tripterygium wilfordii m=54 sd=11, Sufasalazine m=51 sd=12. M:F = 1:1.2. Inclusion: ACR criteria RA, > 6 months. | |
| Interventions | Tripterygium wilfordii HF (TwHF) extract, 180 mg/day. Sufasalazine 2g/day. | |
| Outcomes | Primary end point: 20% improvement at 24 weeks, as defined by ACR criteria (ACR 20). Secondary end points: efficacy of TwHF in achieving ACR 50 and ACR 70 responses at 24 weeks, the improvement in the European League Against Rheumatism Disease Activity Score 28 (DAS 28) measure, and a change in the Sharp–van der Heijde score of the hand and foot radiographs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated, pseudo‐random code (with random, permuted blocks) |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? | Unclear risk | Described as double blind. Patients are likely blinded, though this is not stated. "A rheumatologist or trained staff member masked to treatment allocation assessed the patients." |
| Incomplete outcome data addressed? | Unclear risk | There were a large number of drop‐outs, all are accounted for with reasons and they state that they used: "A protocol‐specified, last‐observation‐carried‐forward approach for handling missing data..." It does appear that they did an ITT analysis for several of the outcomes including the primary outcome and report them in the text. All tables appear to be the per‐protocol analyses. |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Unclear risk | Baseline differences: Less women in the TwHF group (73% vs 87%); CRP appeared to be slightly higher in the TwHF group (255.2 nmol/L vs 236.2 nmol/L); Slightly higher radiographic score in the TwHF group (40.0 vs 34).There is no discussion of differences in medications other than the interventions taking throughout the study. These differences were not tested for significance. |
| Methods | Randomised, double blind, placebo control, 2 parallel groups. Duration 13 weeks; 1 week washout, 12 weeks intervention. | |
| Participants | Randomised n=20 (intervention n=10, control n=10), completed n=18 (intervention n=9, control n=9). Age: intervention m=50, control m=38. M:F=2:18. Inclusion: definite or classical RA, prepared to abstain from NSAIDs for 13 weeks. | |
| Interventions | Tradename not provided, Oenothera biennis (evening primrose), oil, 20 mls (2x10ml, approx 9% GLA, equivalent to 1800mg of GLA), oral. | |
| Outcomes | Pain VAS 0‐100, joint score (swollen and tender joint counts), duration of morning stiffness, grip strength. | |
| Notes | Results equivocal. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, method not reported. |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? | Low risk | Active intervention and placebo not distinguished by look, taste, smell or packaging. |
| Incomplete outcome data addressed? | Low risk | Reported withdrawals. |
| Free of other bias? | Unclear risk | Placebo capsules contained olive oil and may not be inert. Diagnosis / assessment criteria for OA not specified. |
| Methods | Randomised, double blind, placebo control, 2 parallel groups. Duration 24 weeks. | |
| Participants | Randomised n=37 (intervention n=19, control n=18), completed n=27 (intervention n=14, control n=13). Age: intervention m=58, control m=50. Inclusion: 18‐80 yrs, ACR criteria RA stage I‐III, using NSAIDs, not using DMARDs. | |
| Interventions | Boracelle, Borago officinalis (borage seed), oil, 7.2ml (3x4x0.6ml, approx 23% GLA, equivalent to 1400mg GLA), capsules, oral. | |
| Outcomes | Pain VAS 0‐100, pain 0‐4, physician global 0‐4, patient global 0‐4, 68 tender joint count, 66 swollen joint count, joint tenderness score 0‐3, joint swelling score 0‐3, duration of morning stiffness, vocational activity score 0‐3, grip strength mmHg. | |
| Notes | Results favour intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, method not reported. Baseline parameters compared for significant differences. |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? | Unclear risk | Described as double‐blind, method not reported. |
| Incomplete outcome data addressed? | Low risk | Reported withdrawals. |
| Free of selective reporting? | Low risk | Reported adverse events. |
| Free of other bias? | Low risk | Diagnosis / assessment consistent with ACR criteria. |
| Methods | Randomised, double blind, placebo control, 2 parallel groups. Duration 24 weeks. | |
| Participants | Randomised n=34 (intervention n=14, control n=20), completed n=14 (intervention n=7, control n=7). Age m=55. Inclusion: 18‐80 yr, ACR criteria RA stage I‐III, using NSAIDs, DMARDs stable for past 3 months. | |
| Interventions | Tradename not provided, Ribes nigrum (blackcurrant seed), oil, 10500mg (15x700mg, approx 19% GLA, equivalent to 2000mg GLA), capsules, oral. | |
| Outcomes | Pain VAS 0‐100, pain 0‐4, physician global 0‐4 and VAS 0‐100, patient global 0‐4 and VAS 0‐100, 68 tender joint count, 66 swollen joint count, joint tenderness score 0‐3, joint swelling score 0‐3, morning stiffness (minutes), vocational activity score 0‐3, grip strength mmHg. | |
| Notes | Results favour intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, method not reported. Baseline parameters compared for significant differences. |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? | Unclear risk | Described as double‐blind, method not reported. |
| Incomplete outcome data addressed? | Low risk | Reported withdrawals. Included intention‐to‐treat analyses. |
| Free of selective reporting? | Low risk | Reported adverse events. |
| Free of other bias? | Low risk | Diagnosis / assessment consistent with ACR criteria. |
| Methods | Randomised, double blind, placebo control, 2 parallel groups. Duration 24 weeks. | |
| Participants | Randomised n=65 (intervention n=32, control n=33), completed n=58 (intervention n=28, control n=30). Age: intervention m=50, control m=50. M:F=1:1. Inclusion: ACR criteria. | |
| Interventions | Ganoderma lucidum 4g per day together with San Miao San (a combination of Rhizoma atractylodis, Cotex phellodendri, and Radix achyranthes Bidentatae) 2.4 grams per day. | |
| Outcomes | Primary outcome: ACR 20% response; Secondary outcomes: changes in ACR components including tender and swollen joint count, physician’s and patient’s global assessment, HAQ score, and ESR or CRP level, total antioxidant power of plasma, plasma ascorbic acid concentration. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated list in blocks of 5 |
| Allocation concealment? | Low risk | The list was generated at the Institute of Chinese Medicine, The Chinese University of Hong Kong. Study medications were dispensed as sealed packages in consecutive numbers. A research nurse was responsible for dispensing study medications. The investigators, research nurse, and participants were not aware of the treatment assignments throughout the study. |
| Blinding? | Low risk | The investigators, research nurse, and participants were not aware of the treatment assignments throughout the study. Treatment codes were only broken after completion of the |
| Incomplete outcome data addressed? | High risk | Three participants dropped out of the placebo group (2 due to inefficacy and 1 due to emigration); Four participants dropped out of the treatment group (all due to inefficacy; three at week 8 and one at week 12) |
| Free of selective reporting? | Low risk | All outcomes were reported |
| Free of other bias? | High risk | There are a selection of herbal medicines given in the active group. Also, participants in the active group had slightly longer standing RA (9.3 years VS 7.8 years) and a larger number of participants in the active group were taking sulphasalazine (8 VS 4). None of these differences were tested for statistical differences. |
| Methods | Randomised, double blind, placebo control, 2 parallel groups. Duration 4 weeks. | |
| Participants | Randomised n=7, completed n=5. Age: m=52, sd=4. Inclusion: ACR criteria RA. | |
| Interventions | Tradename not provided, capsaicin frutescens 0.075% wv cream, topical, QID. | |
| Outcomes | Pain VAS 0‐100, morning stiffness (Landsbury 2 question method), HAQ, grip strength mmHg, swelling (PIP, DIP circumference), tenderness (delorimeter). | |
| Notes | B:1, W:1. Placebo validity and blinding may be compromised by burning side effect of topical intervention. Small sample size, underpowered study. Results equivocal. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, method of randomisation incompletely reported. Described as randomised according to a previously established randomisation schedule. |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? | Unclear risk | Described as double‐blind. Active intervention and placebo not distinguished by look, taste, smell or packaging, but placebo validity and blinding possibly compromised by burning side effect of topical intervention. |
| Incomplete outcome data addressed? | Unclear risk | Reported no withdrawals. Included per protocol analyses. |
| Free of selective reporting? | Unclear risk | Variances reported as standard error of measurement (SEM). Reported adverse events. |
| Free of other bias? | Low risk | Diagnosis / assessment consistent with ACR criteria. |
| Methods | Randomised, double blind, placebo control, non‐intervention control, 3 parallel groups. Duration 2 weeks. | |
| Participants | Randomised n=15 (intervention n=5, placebo n=5, non‐intervention n=5), completed n=15 (intervention n=5, placebo n=5, non‐intervention control n=5). Age range 23‐76 yr; intervention m=62 sd=13, control m=63 sd=16. M:F=9:6. Inclusion: ACR crtieria RA stage II or III. | |
| Interventions | PhytodolorRN, mixture of ash bark, aspen leaf, aspen bark, golden rod herb, tincture, 3x30 drops, oral. | |
| Outcomes | Diclofenac use, pain 0‐3, joint swelling 0‐3. | |
| Notes | Results equivocal. Groups dissimilar at baseline. Change (reduction) in diclofenac use and pain was greatest in intervention group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, method of randomisation incompletely reported. Described as randomised according to a previously established randomisation schedule. |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? | Unclear risk | Described as double‐blind, method not reported. In other studies of Phytodolor® N, active intervention and placebo not distinguished by look, taste, smell or packaging. Non‐intervention control group not blinded. |
| Incomplete outcome data addressed? | Unclear risk | Reported no withdrawals. |
| Free of selective reporting? | Low risk | Full data reported. |
| Free of other bias? | Unclear risk | Diagnosis / assessment consistent with ACR criteria. Groups dissimilar at baseline. |
| Methods | Randomised, double blind, placebo control, 2 parallel groups. Duration 52 weeks; 24 weeks RCT, 28 weeks open trial. | |
| Participants | Randomised n=40 (intervention n=21, control n=19), completed n=38 (intervention n=20, control n=18). Age: intervention m=53.1 sd=13.4, control m=54.9 sd=13.5. M:F intervention=20:1, control=15:4. Inclusion: ACR criteria RA stage II or III, DMARDs (sulfasalazine or hydrochloroquine) for 6 months, dose stable for past 6 weeks. | |
| Interventions | Krallendorn, Uncaria tomentosa (cat's claw), aqueous dry extract of pentacylcic alkaloid chemotype, 60mg (3x20mg), capsules, oral. | |
| Outcomes | 66 swollen joint count, 68 tender joint count, Ritchie index, pain VAS 0‐100, disease activity VAS 0‐100, morning stiffness 0‐5, HAQ (baseline and week 24 only). | |
| Notes | Results favour intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, method not reported. |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? | Low risk | Active intervention and placebo not distinguished by look, taste, smell or packaging. |
| Incomplete outcome data addressed? | Low risk | Reported withdrawals. |
| Free of selective reporting? | Low risk | Reported adverse events. |
| Free of other bias? | Low risk | Diagnosis / assessment consistent with ACR criteria. |
| Methods | Randomised, double blind, placebo control, 2 parallel groups. Duration 6 weeks. | |
| Participants | Randomised n=41 (intervention n=20, control n=21), completed n=40 (intervention n=20, control n=20). Age range 28‐65 yr. Inclusion: female, aged under 65 yr, classical or definite RA, poor symptomatic control. | |
| Interventions | Tanacetum parthenium (feverfew), (70‐86mg), oral. | |
| Outcomes | Morning stiffness (minutes), inactivity stiffness, pain VAS 0‐10, grip strength mmHg, Ritchie index, patient global, physician global. | |
| Notes | Results equivocal. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, method not reported. |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? | Low risk | Active intervention and placebo not distinguished by look, taste, smell or packaging. |
| Incomplete outcome data addressed? | Low risk | Reported withdrawals. |
| Free of selective reporting? | Low risk | Reported adverse events. |
| Free of other bias? | Low risk | Diagnosis / assessment consistent with ACR criteria. |
| Methods | Randomised, double blind, placebo control, multi‐centre trial, 2 parallel groups. Duration 12 weeks. | |
| Participants | Randomised n=78 (all centres). Data from one centre (Ratingen) available for analysis; randomised n=37 (intervention n=18, control n=19), completed n=36 (intervention n=17, control n=19). Inclusion: Active RA, at least one painful join, stable corticosteroids. | |
| Interventions | Boswellia serrata, 1200‐3600mg, (3x400mg to 3x3x400mg), tablets, oral. | |
| Outcomes | Ritchie index, pain VAS 0‐10, NSAID consumption, patient global VAS 0‐10. | |
| Notes | Results equivocal. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, method not reported. |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? | Low risk | Active intervention and placebo not distinguished by look, taste, smell or packaging. |
| Incomplete outcome data addressed? | Low risk | Reported withdrawals. |
| Free of selective reporting? | Low risk | Reported adverse events. Non‐normal data reported as median and range. |
| Free of other bias? | Low risk | Diagnosis / assessment consistent with ACR criteria. Reported ethics committee approval. |
| Methods | Randomised, double blind, active control (celecoxib), 2 parallel group, multicentre trial. Duration 6 weeks. | |
| Participants | Randomised n=183 (intervention n=91, control n=92), completed n=168 (intervention n=84, control n=84). Age (yr): intervention m=52.1 sd=12.6, control m=51.7 sd=10.9. M:F=1:8. Inclusion: ACR criteria RA stage I, II or III, disease duration >3 months, stable medications, pain (VAS 0‐100) increase of 10+mm, and 6+ tender joints, and 3+ swollen joints after NSAID washout. | |
| Interventions | SKI306X, extract mixture of Clematis mandshurica, Prunella vulgaris, Trichosanthes kirilowii, 600mg (3x200mg), tablets, oral. | |
| Outcomes | Pain (VAS 0‐100), rescue medication use (acetaminophen), ACR20. | |
| Notes | Results indicate that intervention is not inferior to active control. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomised to one of two groups using a computer generated random number sequence. Baseline parameters compared for significant differences. |
| Allocation concealment? | Low risk | Adequate |
| Blinding? | Low risk | Active intervention and placebo not distinguished by look, taste, smell or packaging. |
| Incomplete outcome data addressed? | Low risk | Reported withdrawals. Included intention‐to‐treat and per protocol analyses. |
| Free of selective reporting? | Low risk | Reported adverse events. Confirmatory study. |
| Free of other bias? | Low risk | Diagnosis / assessment consistent with ACR criteria. Reported ethics committee approval. |
| Methods | Randomised, double blind, placebo control, 2 groups crossover study. Duration 16 weeks: 12 weeks intervention 1st arm, 4 weeks intervention 2nd arm. | |
| Participants | Randomised n=70, completed first arm n=58. Age: m=47 yr. Inclusion: classic or definite RA of at least 6 months duration with poor response to NSAIDs for at least 2 months. | |
| Interventions | Tripterygium wilfordii hook F (thunder god vine), ethanolic extract, 60mg, capsules, oral. | |
| Outcomes | Joint tenderness and swelling, grip strength, 15 metre walking time, morning stiffness, physician global, patient global. | |
| Notes | Results favour intervention for short‐term use (12 weeks), with cautions regarding adverse events. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, method not reported. |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? | Unclear risk | Described as double‐blind, method not reported. |
| Incomplete outcome data addressed? | Low risk | Reported withdrawals. |
| Free of selective reporting? | Low risk | Reported adverse events. |
| Free of other bias? | Low risk | Reported ethics committee approval. |
| Methods | Randomised, double blind, placebo control, 3 parallel groups. Duration 20 weeks. | |
| Participants | Randomised n=35 (low dose n=12, high dose n=11, control n=12), completed 4 weeks n=32, completed n=21. Age: low dose m=54 sd=12, high dose m=57 sd=8, control m=51 sd=12. Inclusion: ACR criteria RA stage II‐IV, for at least 1 year, active disease, 2+ swollen joints, and 2 of 6+ tender joints, morning stiffness >30min, ESR >28mm/h. | |
| Interventions | Tripterygium wilfordii hook F (thunder god vine), ethanolic extract, low dose=180mg; high dose=360mg, capsules, oral. | |
| Outcomes | ACR20, ACR50, ACR70, ESR, CRP, RF. | |
| Notes | Results favour intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, method not reported. Baseline parameters compared for significant differences. |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? | Low risk | Active intervention and placebo not distinguished by look, taste, smell or packaging. |
| Incomplete outcome data addressed? | Low risk | Reported withdrawals. Included intention‐to‐treat analyses. |
| Free of selective reporting? | Low risk | Reported adverse events. |
| Free of other bias? | Low risk | Diagnosis / assessment consistent with ACR criteria. Reported ethics committee approval. |
| Methods | Randomised, double blind, placebo control, 2 parallel groups. Duration 6 weeks. | |
| Participants | Randomised n=50. Withdrawals not reported. Age: RA group m=40, health controls m=20 yr. Inclusion: definite RA, receiving only NSAIDs. | |
| Interventions | Tradename not provided, Ribes nigrum, (blackcurrant seed), oil, 3000mg (6x500mg, approx 19% GLA, equivalent to 525mg GLA), capsules, oral. | |
| Outcomes | Morning stiffness, grip strength, Ritchie index, pain score, patient global. | |
| Notes | Results equivocal. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, method not reported. |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? | Unclear risk | Described as double‐blind, method not reported. |
| Incomplete outcome data addressed? | High risk | Withdrawals not reported. |
| Free of selective reporting? | High risk | Adverse events not reported. |
| Free of other bias? | High risk | Data for clinical outcomes not reported. |
| Methods | Randomised, double blind, placebo control, 2 parallel groups. Duration 6 months (followed by 6 month single‐blind phase, followed by 3 month placebo phase). | |
| Participants | Randomised n=56, completed n=41. Age: m=56 yr. Inclusion: ACR criteria RA stage I‐III, 1st line treatment stable for past 1 month, 2nd line treatment stable for past 3 months. | |
| Interventions | GLA‐70, Borago officinalis (borage seed), oil, 4ml (8x0.5ml, approx 70% GLA, equivalent to 2800mg GLA), capsules, oral. | |
| Outcomes | Pain VAS 0‐100, pain 0‐4, physician global, patient global, joint swelling and tenderness, morning stiffness, grip strength, health assessment questionnaire, ACR20 (6 and 12 month follow up). | |
| Notes | Results favour intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised, method not reported. Baseline parameters compared for significant differences. |
| Allocation concealment? | Unclear risk | Unclear |
| Blinding? | Unclear risk | Described as double blind, method not reported. |
| Incomplete outcome data addressed? | Low risk | Reported withdrawals. |
| Free of selective reporting? | Unclear risk | After communication with author, unable to confirm SD for morning stiffness in GLA group (table 2) therefore these data excluded from analysis. |
| Free of other bias? | Unclear risk | Placebo capsules contained sunflower oil and may not be inert. Diagnosis / assessment consistent with ACR criteria. |
Unless otherwise stated, all oral medications are reported as total daily doses, which may have been administered in single or divided doses. * Indicates that the trade name was not provided in the manuscript, but has been determined through communication with the manufacturing company noted in the acknowledgements.
ARA: American Rheumatism Association
ACR: American College of Rheumatology
EULAR: European league Against Rheumatism
Allocation concealment may be listed as "unclear" if: (a) the authors reported adherence to the ICH Good Clinical Practice (GCP) guidelines did not describe the method of allocation concealment used, or (b) the reviewers were unable to agree upon the adequacy of allocation concealment as reported.
Unless subscales are named, outcome measures (eg: HAQ, SF‐36, ACR20) were used in entirety. Unless specified, all measures were used, scaled, and scored to ACR/EULAR standards.
ACR core set of disease activity measures comprises tender joint count, swollen joint count, patient's assessment of pain, patient's and physician's global assessment of disease activity, patient's assessment of physical function (global assessment or HAQ‐DI score), and laboratory investigations of one acute‐phase reactant (ESR or C‐reactive protein). ACR20 is defined as 20% improvements in tender joint count, swollen joint count, and three of the other disease activity measures. ACR50 and ACR70 are similarly defined, but at 50% and 70% thresholds.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Discussion paper. | |
| Not placebo controlled. | |
| Not randomised controlled trial. | |
| Review paper. | |
| Not placebo controlled. | |
| Review paper. | |
| Discussion paper. | |
| Discussion paper. | |
| Discussion paper. | |
| Not randomised controlled trial. | |
| Not randomised controlled trial. | |
| Not randomised controlled trial. | |
| Not randomised controlled trial. | |
| RA subgroup not distinguishable. | |
| Not a herbal intervention. | |
| No clinical outcomes reported. | |
| Case series, not a randomised controlled trial. | |
| Not truly herbal intervention. | |
| Not randomised controlled trial. | |
| Review paper. | |
| Not a randomised controlled trial. Primary measures not consistent with the topic of this review. | |
| Discussion paper. | |
| RA subgroup not distinguishable. | |
| Abstract only. Full text unavailable. | |
| Abstract only. Full text unavailable. | |
| Not randomised controlled trial. | |
| Not randomised controlled trial. | |
| Discussion paper. | |
| Not randomised controlled trial. | |
| Not randomised controlled trial. | |
| Not randomised controlled trial. | |
| Not randomised controlled trial. | |
| Not randomised controlled trial. | |
| Not randomised controlled trial. | |
| Not placebo controlled. | |
| Case series, not a randomised controlled trial. | |
| Not randomised controlled trial. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A clinical trial to study the effects of a herbal drug Qurs Mufasil in patients with joint pain (Arthritis) |
| Methods | Randomized, parallel group, placebo controlled trial |
| Participants | Patients of 20‐70 years of age fulfilling the criteria of American College of Rheumatology (ACR) for the diagnosis of Rheumatoid Arthritis, who had never received disease modifying anti/Rheumatoid Drugs (DMARDs). Presence of active disease as defined by the presence of >, 6 tender joints and >; 6 swollen joints. |
| Interventions | Qurs Mufasil:1000 mg daily for 3 months Placebo:1000 mg twice daily for 3 months |
| Outcomes | Reduction in Swollen Joint Count,Tender Joint Count, Intensity of Pain‐VAS (0‐100), Morning Stiffness, ESR and CRP Timepoint:4,8,12 weeks; Improvement in quality of life as assessed by Health Assessment Questionnaire (HAQ) Time Point: 3 months |
| Starting date | 01‐03‐2003 |
| Contact information | Yasmeen Shamsi Majeedia Hospital, Jamia Hamdard, 110062 New Delhi, India Email: [email protected] |
| Notes | Recruitment complete; http://apps.who.int/trialsearch/Trial.aspx?TrialID=CTRI/2009/091/000746 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain VAS 0‐100 Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
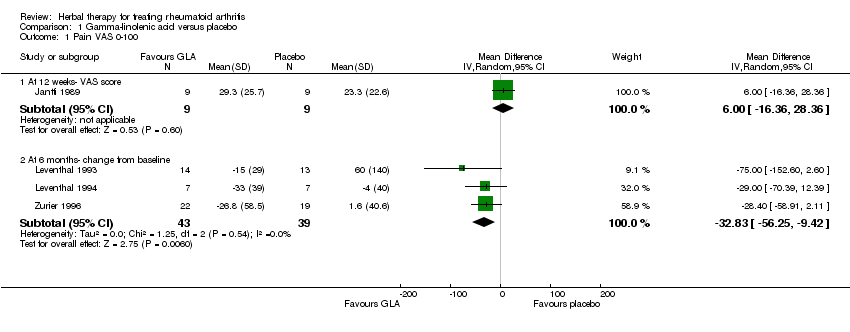
| Analysis 1.1  Comparison 1 Gamma‐linolenic acid versus placebo, Outcome 1 Pain VAS 0‐100. | ||||
| 1.1 At 12 weeks‐ VAS score | 1 | 18 | Mean Difference (IV, Random, 95% CI) | 6.00 [‐16.36, 28.36] |
| 1.2 At 6 months‐ change from baseline | 3 | 82 | Mean Difference (IV, Random, 95% CI) | ‐32.83 [‐56.25, ‐9.42] |
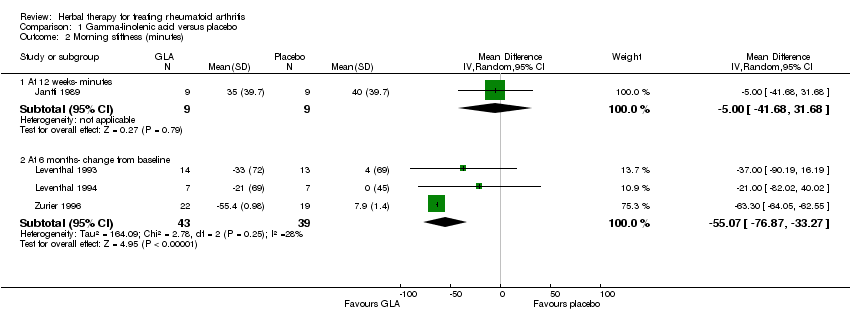
| 2 Morning stiffness (minutes) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Gamma‐linolenic acid versus placebo, Outcome 2 Morning stiffness (minutes). | ||||
| 2.1 At 12 weeks‐ minutes | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐5.0 [‐41.68, 31.68] |
| 2.2 At 6 months‐ change from baseline | 3 | 82 | Mean Difference (IV, Random, 95% CI) | ‐55.07 [‐76.87, ‐33.27] |
| 3 68 tender joint count percentage change from baseline Show forest plot | 3 | 82 | Mean Difference (IV, Random, 95% CI) | ‐53.80 [‐95.61, ‐12.00] |
| Analysis 1.3  Comparison 1 Gamma‐linolenic acid versus placebo, Outcome 3 68 tender joint count percentage change from baseline. | ||||
| 4 66 swollen joint count percentage change from baseline Show forest plot | 3 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐14.43 [‐31.43, 2.56] |
| Analysis 1.4  Comparison 1 Gamma‐linolenic acid versus placebo, Outcome 4 66 swollen joint count percentage change from baseline. | ||||
| 5 Joint tenderness (0 to 3) percentage change from baseline Show forest plot | 3 | 82 | Mean Difference (IV, Random, 95% CI) | ‐56.64 [‐98.10, ‐15.17] |
| Analysis 1.5  Comparison 1 Gamma‐linolenic acid versus placebo, Outcome 5 Joint tenderness (0 to 3) percentage change from baseline. | ||||
| 6 Joint swelling (0 to 3) percentage change from baseline Show forest plot | 3 | 82 | Mean Difference (IV, Random, 95% CI) | ‐24.02 [‐70.80, 22.76] |
| Analysis 1.6  Comparison 1 Gamma‐linolenic acid versus placebo, Outcome 6 Joint swelling (0 to 3) percentage change from baseline. | ||||
| 7 HAQ disability score percentage change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.7  Comparison 1 Gamma‐linolenic acid versus placebo, Outcome 7 HAQ disability score percentage change from baseline. | ||||
| 8 Patient global (0 to 4) percentage change from baseline Show forest plot | 3 | 82 | Mean Difference (IV, Random, 95% CI) | ‐20.87 [‐39.43, ‐2.31] |
| Analysis 1.8  Comparison 1 Gamma‐linolenic acid versus placebo, Outcome 8 Patient global (0 to 4) percentage change from baseline. | ||||
| 9 Physician global (0 to 4) percentage change from baseline Show forest plot | 3 | 82 | Mean Difference (IV, Random, 95% CI) | ‐21.28 [‐70.52, 27.95] |
| Analysis 1.9  Comparison 1 Gamma‐linolenic acid versus placebo, Outcome 9 Physician global (0 to 4) percentage change from baseline. | ||||
| 10 Participants (n) reported reduced NSAID use Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.10  Comparison 1 Gamma‐linolenic acid versus placebo, Outcome 10 Participants (n) reported reduced NSAID use. | ||||
| 10.1 At 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 At 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
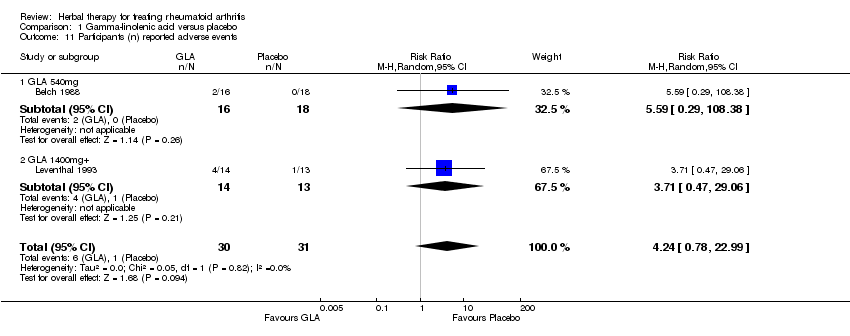
| 11 Participants (n) reported adverse events Show forest plot | 2 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 4.24 [0.78, 22.99] |
| Analysis 1.11  Comparison 1 Gamma‐linolenic acid versus placebo, Outcome 11 Participants (n) reported adverse events. | ||||
| 11.1 GLA 540mg | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 5.59 [0.29, 108.38] |
| 11.2 GLA 1400mg+ | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 3.71 [0.47, 29.06] |
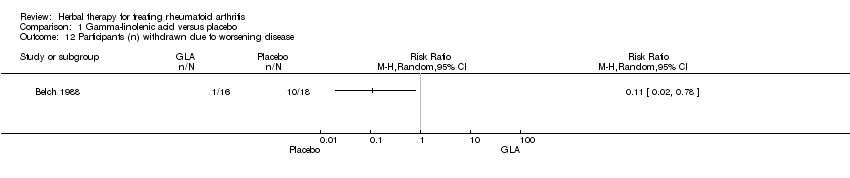
| 12 Participants (n) withdrawn due to worsening disease Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.12  Comparison 1 Gamma‐linolenic acid versus placebo, Outcome 12 Participants (n) withdrawn due to worsening disease. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Joint tenderness (0 to 3) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Tripterygium wilfordii Hook F 60 mg versus placebo, Outcome 1 Joint tenderness (0 to 3). | ||||
| 2 60 swollen joint count Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Tripterygium wilfordii Hook F 60 mg versus placebo, Outcome 2 60 swollen joint count. | ||||
| 3 Morning stiffness (hours) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Tripterygium wilfordii Hook F 60 mg versus placebo, Outcome 3 Morning stiffness (hours). | ||||
| 4 Grip strength (mmHg) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2 Tripterygium wilfordii Hook F 60 mg versus placebo, Outcome 4 Grip strength (mmHg). | ||||
| 5 15 metre walking time (seconds) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 Tripterygium wilfordii Hook F 60 mg versus placebo, Outcome 5 15 metre walking time (seconds). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ACR20 responders Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 3.1  Comparison 3 Tripterygium wilfordii Hook F 180 mg versus placebo, Outcome 1 ACR20 responders. | ||||
| 2 ACR50 responders Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 Tripterygium wilfordii Hook F 180 mg versus placebo, Outcome 2 ACR50 responders. | ||||
| 3 Participants (n) reported adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3 Tripterygium wilfordii Hook F 180 mg versus placebo, Outcome 3 Participants (n) reported adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ACR20 responders Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 4.1  Comparison 4 Triptrygium wilfordii Hook F 360 mg versus placebo, Outcome 1 ACR20 responders. | ||||
| 2 ACR50 responders Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 4.2  Comparison 4 Triptrygium wilfordii Hook F 360 mg versus placebo, Outcome 2 ACR50 responders. | ||||
| 3 Participants (n) reported adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 4.3  Comparison 4 Triptrygium wilfordii Hook F 360 mg versus placebo, Outcome 3 Participants (n) reported adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ACR20 responders Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 Tripterygium wilfordii Hook F 180 mg versus sulfasalazine, Outcome 1 ACR20 responders. | ||||
| 2 ACR50 responders Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 5.2  Comparison 5 Tripterygium wilfordii Hook F 180 mg versus sulfasalazine, Outcome 2 ACR50 responders. | ||||
| 3 Improvement more than 0.3 units on HAQ Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 5.3  Comparison 5 Tripterygium wilfordii Hook F 180 mg versus sulfasalazine, Outcome 3 Improvement more than 0.3 units on HAQ. | ||||
| 4 Participants (n) reported adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 5.4  Comparison 5 Tripterygium wilfordii Hook F 180 mg versus sulfasalazine, Outcome 4 Participants (n) reported adverse events. | ||||
| 5 Participants (n) withdrawn due to adverse events Show forest plot | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.19, 0.94] |
| Analysis 5.5  Comparison 5 Tripterygium wilfordii Hook F 180 mg versus sulfasalazine, Outcome 5 Participants (n) withdrawn due to adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain (0 to 3) at 2 weeks Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 6.1  Comparison 6 Phytodolor N versus placebo, Outcome 1 Pain (0 to 3) at 2 weeks. | ||||
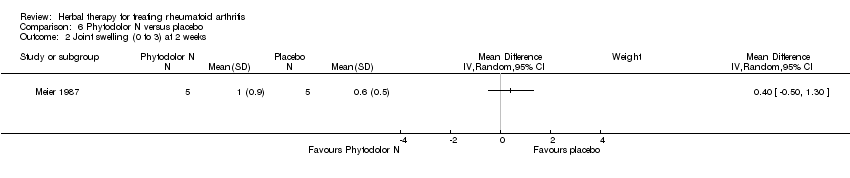
| 2 Joint swelling (0 to 3) at 2 weeks Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 6.2  Comparison 6 Phytodolor N versus placebo, Outcome 2 Joint swelling (0 to 3) at 2 weeks. | ||||
| 3 Morning stiffness (minutes) at 12 months Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 6.3  Comparison 6 Phytodolor N versus placebo, Outcome 3 Morning stiffness (minutes) at 12 months. | ||||
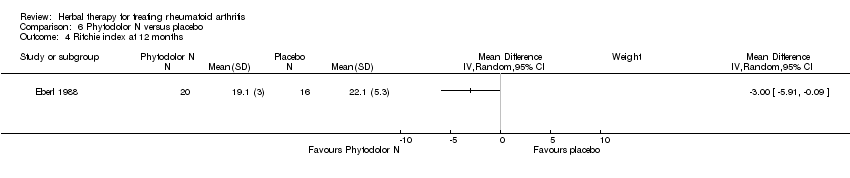
| 4 Ritchie index at 12 months Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 6.4  Comparison 6 Phytodolor N versus placebo, Outcome 4 Ritchie index at 12 months. | ||||
| 5 Cumulative NSAID use (diclofenac) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 6.5  Comparison 6 Phytodolor N versus placebo, Outcome 5 Cumulative NSAID use (diclofenac). | ||||
| 5.1 At 1 month (tablets) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 At 12 months (tablets) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain VAS 0‐100 change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.1  Comparison 7 SKI306X versus celecoxib, Outcome 1 Pain VAS 0‐100 change from baseline. | ||||
| 1.1 At 3 weeks | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 6 weeks | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 ACR20 responders Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 7.2  Comparison 7 SKI306X versus celecoxib, Outcome 2 ACR20 responders. | ||||
| 2.1 At 3 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At 6 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Participants (n) reported adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 7.3  Comparison 7 SKI306X versus celecoxib, Outcome 3 Participants (n) reported adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
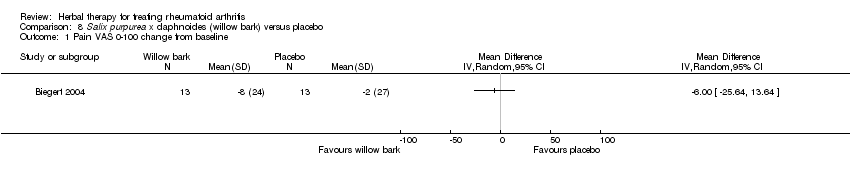
| 1 Pain VAS 0‐100 change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 8.1  Comparison 8 Salix purpurea x daphnoides (willow bark) versus placebo, Outcome 1 Pain VAS 0‐100 change from baseline. | ||||
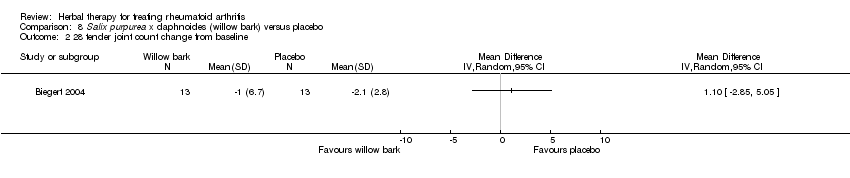
| 2 28 tender joint count change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 8.2  Comparison 8 Salix purpurea x daphnoides (willow bark) versus placebo, Outcome 2 28 tender joint count change from baseline. | ||||
| 3 28 swollen joint count change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 8.3  Comparison 8 Salix purpurea x daphnoides (willow bark) versus placebo, Outcome 3 28 swollen joint count change from baseline. | ||||
| 4 Patient assessment of efficacy VAS 0‐100 change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 8.4  Comparison 8 Salix purpurea x daphnoides (willow bark) versus placebo, Outcome 4 Patient assessment of efficacy VAS 0‐100 change from baseline. | ||||
| 5 Physician assessment of effiacy VAS 0‐100 change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 8.5  Comparison 8 Salix purpurea x daphnoides (willow bark) versus placebo, Outcome 5 Physician assessment of effiacy VAS 0‐100 change from baseline. | ||||
| 6 ACR20 responders Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 8.6  Comparison 8 Salix purpurea x daphnoides (willow bark) versus placebo, Outcome 6 ACR20 responders. | ||||
| 7 HAQ disability index change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 8.7  Comparison 8 Salix purpurea x daphnoides (willow bark) versus placebo, Outcome 7 HAQ disability index change from baseline. | ||||
| 8 SF‐36 physical component summary score change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 8.8  Comparison 8 Salix purpurea x daphnoides (willow bark) versus placebo, Outcome 8 SF‐36 physical component summary score change from baseline. | ||||
| 9 SF‐36 mental component summary score change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 8.9  Comparison 8 Salix purpurea x daphnoides (willow bark) versus placebo, Outcome 9 SF‐36 mental component summary score change from baseline. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Grip strength (mmHg) at 6 weeks Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 9.1  Comparison 9 Feverfew versus placebo, Outcome 1 Grip strength (mmHg) at 6 weeks. | ||||
| 2 Participants (n) reported adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 9.2  Comparison 9 Feverfew versus placebo, Outcome 2 Participants (n) reported adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain VAS 0‐100 change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 10.1  Comparison 10 RA‐1 versus placebo, Outcome 1 Pain VAS 0‐100 change from baseline. | ||||
| 2 68 tender joint count change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 10.2  Comparison 10 RA‐1 versus placebo, Outcome 2 68 tender joint count change from baseline. | ||||
| 3 66 swollen joint count change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 10.3  Comparison 10 RA‐1 versus placebo, Outcome 3 66 swollen joint count change from baseline. | ||||
| 4 Modified HAQ (Pune) change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 10.4  Comparison 10 RA‐1 versus placebo, Outcome 4 Modified HAQ (Pune) change from baseline. | ||||
| 5 Patient global (1 to 5) change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 10.5  Comparison 10 RA‐1 versus placebo, Outcome 5 Patient global (1 to 5) change from baseline. | ||||
| 6 Physician global (1 to 5) change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 10.6  Comparison 10 RA‐1 versus placebo, Outcome 6 Physician global (1 to 5) change from baseline. | ||||
| 7 ACR20 responders Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 10.7  Comparison 10 RA‐1 versus placebo, Outcome 7 ACR20 responders. | ||||
| 8 ACR50 responders Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 10.8  Comparison 10 RA‐1 versus placebo, Outcome 8 ACR50 responders. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants (n) reported adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 11.1  Comparison 11 Boswellia serrata versus placebo, Outcome 1 Participants (n) reported adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain VAS 0‐100 percentage change at 4 weeks Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 12.1  Comparison 12 Capsaicin versus placebo, Outcome 1 Pain VAS 0‐100 percentage change at 4 weeks. | ||||
| 2 Physician global (‐1 to 3) change from baseline at 4 weeks Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 12.2  Comparison 12 Capsaicin versus placebo, Outcome 2 Physician global (‐1 to 3) change from baseline at 4 weeks. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 42 tender joint count at 6 weeks Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 13.1  Comparison 13 Tripterygium wilfordii (topical) versus placebo, Outcome 1 42 tender joint count at 6 weeks. | ||||
| 2 40 swollen joint count at 6 weeks Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 13.2  Comparison 13 Tripterygium wilfordii (topical) versus placebo, Outcome 2 40 swollen joint count at 6 weeks. | ||||
| 3 Grip strength (kPa) at 6 weeks Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 13.3  Comparison 13 Tripterygium wilfordii (topical) versus placebo, Outcome 3 Grip strength (kPa) at 6 weeks. | ||||
| 4 Morning stiffness (hours) at 6 weeks Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 13.4  Comparison 13 Tripterygium wilfordii (topical) versus placebo, Outcome 4 Morning stiffness (hours) at 6 weeks. | ||||
| 5 ACR20 responders at 6 weeks Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 13.5  Comparison 13 Tripterygium wilfordii (topical) versus placebo, Outcome 5 ACR20 responders at 6 weeks. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ACR20 responders Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 14.1  Comparison 14 Ganoderma lucidum and SMS versus placebo, Outcome 1 ACR20 responders. | ||||
| 2 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 14.2  Comparison 14 Ganoderma lucidum and SMS versus placebo, Outcome 2 Adverse events. | ||||
Comparison 1 Gamma‐linolenic acid versus placebo, Outcome 1 Pain VAS 0‐100.
Comparison 1 Gamma‐linolenic acid versus placebo, Outcome 2 Morning stiffness (minutes).

Comparison 1 Gamma‐linolenic acid versus placebo, Outcome 3 68 tender joint count percentage change from baseline.

Comparison 1 Gamma‐linolenic acid versus placebo, Outcome 4 66 swollen joint count percentage change from baseline.

Comparison 1 Gamma‐linolenic acid versus placebo, Outcome 5 Joint tenderness (0 to 3) percentage change from baseline.

Comparison 1 Gamma‐linolenic acid versus placebo, Outcome 6 Joint swelling (0 to 3) percentage change from baseline.

Comparison 1 Gamma‐linolenic acid versus placebo, Outcome 7 HAQ disability score percentage change from baseline.

Comparison 1 Gamma‐linolenic acid versus placebo, Outcome 8 Patient global (0 to 4) percentage change from baseline.

Comparison 1 Gamma‐linolenic acid versus placebo, Outcome 9 Physician global (0 to 4) percentage change from baseline.

Comparison 1 Gamma‐linolenic acid versus placebo, Outcome 10 Participants (n) reported reduced NSAID use.
Comparison 1 Gamma‐linolenic acid versus placebo, Outcome 11 Participants (n) reported adverse events.
Comparison 1 Gamma‐linolenic acid versus placebo, Outcome 12 Participants (n) withdrawn due to worsening disease.

Comparison 2 Tripterygium wilfordii Hook F 60 mg versus placebo, Outcome 1 Joint tenderness (0 to 3).

Comparison 2 Tripterygium wilfordii Hook F 60 mg versus placebo, Outcome 2 60 swollen joint count.

Comparison 2 Tripterygium wilfordii Hook F 60 mg versus placebo, Outcome 3 Morning stiffness (hours).

Comparison 2 Tripterygium wilfordii Hook F 60 mg versus placebo, Outcome 4 Grip strength (mmHg).

Comparison 2 Tripterygium wilfordii Hook F 60 mg versus placebo, Outcome 5 15 metre walking time (seconds).

Comparison 3 Tripterygium wilfordii Hook F 180 mg versus placebo, Outcome 1 ACR20 responders.

Comparison 3 Tripterygium wilfordii Hook F 180 mg versus placebo, Outcome 2 ACR50 responders.

Comparison 3 Tripterygium wilfordii Hook F 180 mg versus placebo, Outcome 3 Participants (n) reported adverse events.

Comparison 4 Triptrygium wilfordii Hook F 360 mg versus placebo, Outcome 1 ACR20 responders.
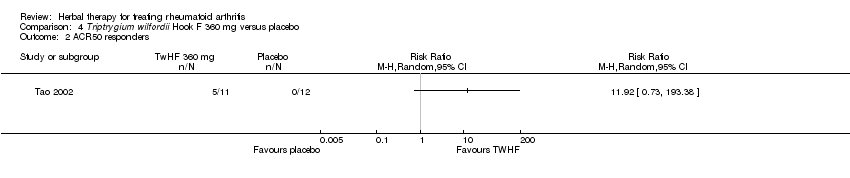
Comparison 4 Triptrygium wilfordii Hook F 360 mg versus placebo, Outcome 2 ACR50 responders.

Comparison 4 Triptrygium wilfordii Hook F 360 mg versus placebo, Outcome 3 Participants (n) reported adverse events.

Comparison 5 Tripterygium wilfordii Hook F 180 mg versus sulfasalazine, Outcome 1 ACR20 responders.
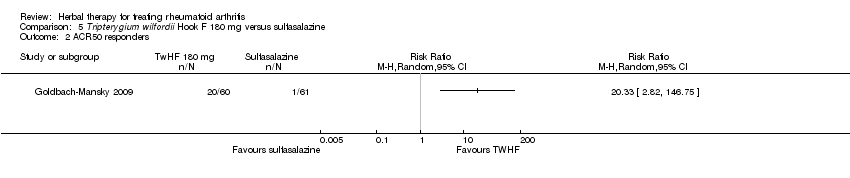
Comparison 5 Tripterygium wilfordii Hook F 180 mg versus sulfasalazine, Outcome 2 ACR50 responders.
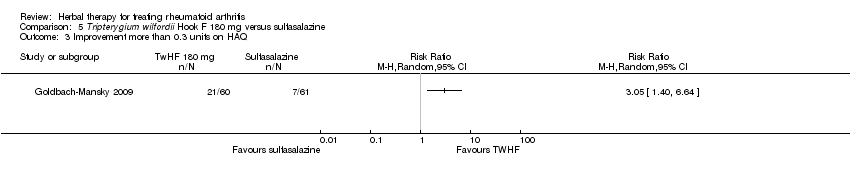
Comparison 5 Tripterygium wilfordii Hook F 180 mg versus sulfasalazine, Outcome 3 Improvement more than 0.3 units on HAQ.

Comparison 5 Tripterygium wilfordii Hook F 180 mg versus sulfasalazine, Outcome 4 Participants (n) reported adverse events.
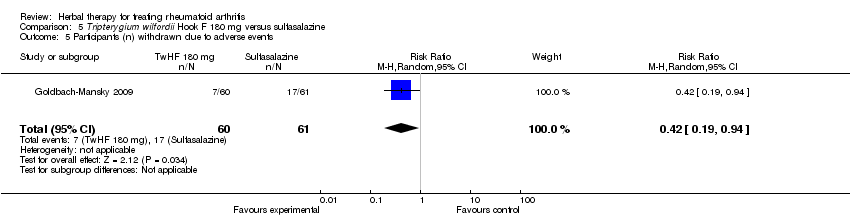
Comparison 5 Tripterygium wilfordii Hook F 180 mg versus sulfasalazine, Outcome 5 Participants (n) withdrawn due to adverse events.

Comparison 6 Phytodolor N versus placebo, Outcome 1 Pain (0 to 3) at 2 weeks.
Comparison 6 Phytodolor N versus placebo, Outcome 2 Joint swelling (0 to 3) at 2 weeks.

Comparison 6 Phytodolor N versus placebo, Outcome 3 Morning stiffness (minutes) at 12 months.
Comparison 6 Phytodolor N versus placebo, Outcome 4 Ritchie index at 12 months.

Comparison 6 Phytodolor N versus placebo, Outcome 5 Cumulative NSAID use (diclofenac).

Comparison 7 SKI306X versus celecoxib, Outcome 1 Pain VAS 0‐100 change from baseline.

Comparison 7 SKI306X versus celecoxib, Outcome 2 ACR20 responders.

Comparison 7 SKI306X versus celecoxib, Outcome 3 Participants (n) reported adverse events.
Comparison 8 Salix purpurea x daphnoides (willow bark) versus placebo, Outcome 1 Pain VAS 0‐100 change from baseline.
Comparison 8 Salix purpurea x daphnoides (willow bark) versus placebo, Outcome 2 28 tender joint count change from baseline.
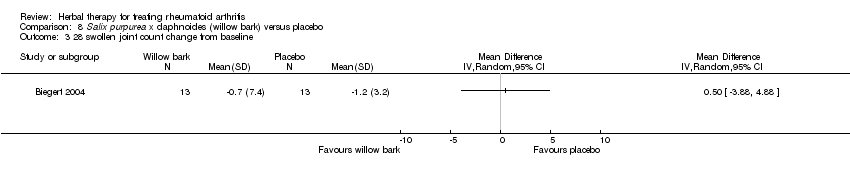
Comparison 8 Salix purpurea x daphnoides (willow bark) versus placebo, Outcome 3 28 swollen joint count change from baseline.

Comparison 8 Salix purpurea x daphnoides (willow bark) versus placebo, Outcome 4 Patient assessment of efficacy VAS 0‐100 change from baseline.

Comparison 8 Salix purpurea x daphnoides (willow bark) versus placebo, Outcome 5 Physician assessment of effiacy VAS 0‐100 change from baseline.
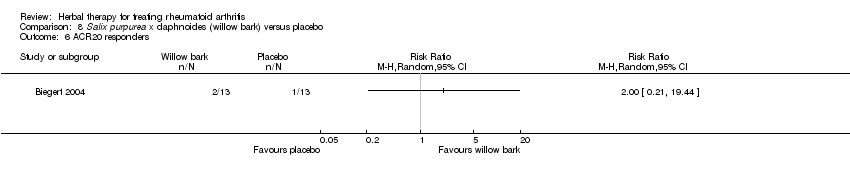
Comparison 8 Salix purpurea x daphnoides (willow bark) versus placebo, Outcome 6 ACR20 responders.
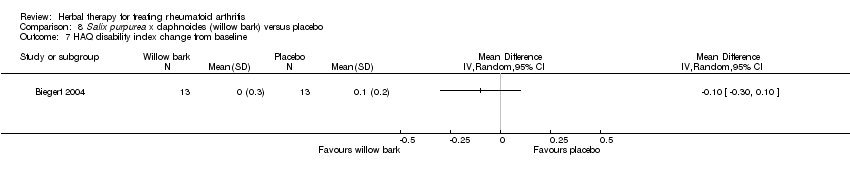
Comparison 8 Salix purpurea x daphnoides (willow bark) versus placebo, Outcome 7 HAQ disability index change from baseline.

Comparison 8 Salix purpurea x daphnoides (willow bark) versus placebo, Outcome 8 SF‐36 physical component summary score change from baseline.

Comparison 8 Salix purpurea x daphnoides (willow bark) versus placebo, Outcome 9 SF‐36 mental component summary score change from baseline.

Comparison 9 Feverfew versus placebo, Outcome 1 Grip strength (mmHg) at 6 weeks.

Comparison 9 Feverfew versus placebo, Outcome 2 Participants (n) reported adverse events.

Comparison 10 RA‐1 versus placebo, Outcome 1 Pain VAS 0‐100 change from baseline.

Comparison 10 RA‐1 versus placebo, Outcome 2 68 tender joint count change from baseline.

Comparison 10 RA‐1 versus placebo, Outcome 3 66 swollen joint count change from baseline.
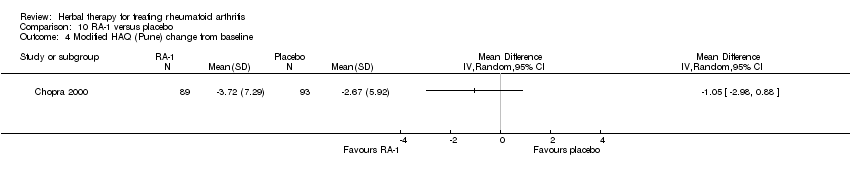
Comparison 10 RA‐1 versus placebo, Outcome 4 Modified HAQ (Pune) change from baseline.

Comparison 10 RA‐1 versus placebo, Outcome 5 Patient global (1 to 5) change from baseline.
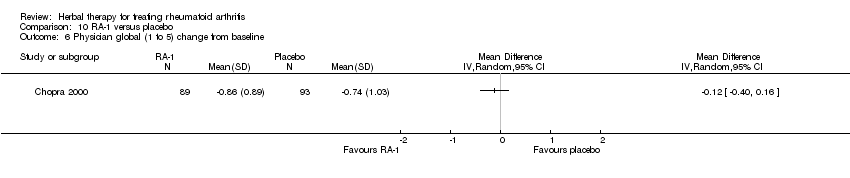
Comparison 10 RA‐1 versus placebo, Outcome 6 Physician global (1 to 5) change from baseline.

Comparison 10 RA‐1 versus placebo, Outcome 7 ACR20 responders.

Comparison 10 RA‐1 versus placebo, Outcome 8 ACR50 responders.

Comparison 11 Boswellia serrata versus placebo, Outcome 1 Participants (n) reported adverse events.
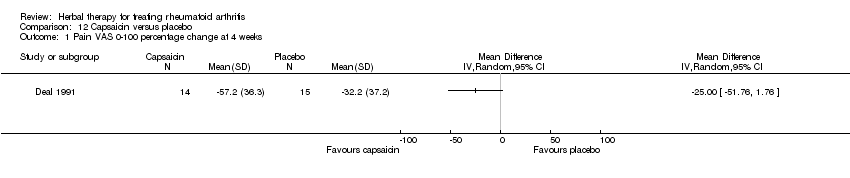
Comparison 12 Capsaicin versus placebo, Outcome 1 Pain VAS 0‐100 percentage change at 4 weeks.

Comparison 12 Capsaicin versus placebo, Outcome 2 Physician global (‐1 to 3) change from baseline at 4 weeks.
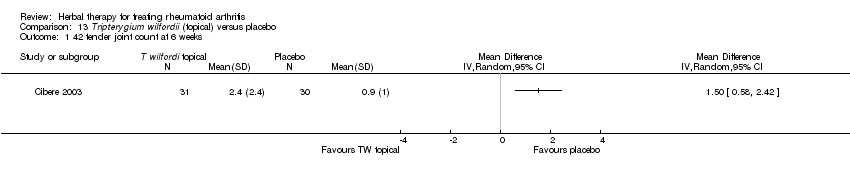
Comparison 13 Tripterygium wilfordii (topical) versus placebo, Outcome 1 42 tender joint count at 6 weeks.

Comparison 13 Tripterygium wilfordii (topical) versus placebo, Outcome 2 40 swollen joint count at 6 weeks.

Comparison 13 Tripterygium wilfordii (topical) versus placebo, Outcome 3 Grip strength (kPa) at 6 weeks.
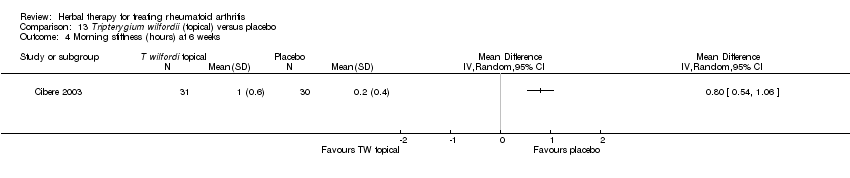
Comparison 13 Tripterygium wilfordii (topical) versus placebo, Outcome 4 Morning stiffness (hours) at 6 weeks.
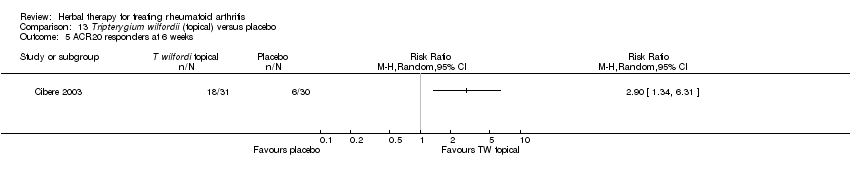
Comparison 13 Tripterygium wilfordii (topical) versus placebo, Outcome 5 ACR20 responders at 6 weeks.
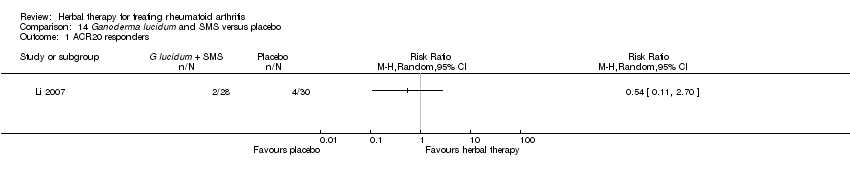
Comparison 14 Ganoderma lucidum and SMS versus placebo, Outcome 1 ACR20 responders.

Comparison 14 Ganoderma lucidum and SMS versus placebo, Outcome 2 Adverse events.
| Evening primrose oil, borage seed oil, blackcurrent seed oil (containing gamma‐linolenic acid) for rheumatoid arthritis | ||||||
| Patient or population: patients with treating rheumatoid arthritis Settings: community Intervention: Evening primrose oil, borage seed oil, blackcurrent seed oil (with gamma‐linolenic acid) versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Evening primrose oil, borage seed oil, blackcurrent seed oil (with gamma‐linolenic acid) versus placebo | |||||
| ACR 50% improvement ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Pain | The mean pain in the control groups was | The mean Pain in the intervention groups was | 82 | ⊕⊕⊕⊝ | Absolute risk difference 33 points lower (9 to 56 points lower); relative % change 54% (15 to 92%); NNTB 3 (2 to 12)3 | |
| Disability (HAQ score) | The mean disability (haq score) in the control groups was | The mean Disability (HAQ score) in the intervention groups was | 41 | ⊕⊕⊝⊝ | Absolute risk difference 16% lower (4 to 27% lower); relative % change 38% (9% to 64%); NNTB 3 (95% CI 2 to 11) | |
| Participants (n) reported adverse events | Medium risk population | RR 4.24 | 61 | ⊕⊕⊕⊝ | Absolute risk difference 15% (0 to 30%); relative percent change 324% (‐22% to 2199 %); NNT=n/a 3 | |
| 39 per 1000 | 165 per 1000 | |||||
| Change in radiographic progression ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Achievement of low disease state (DAS 28) ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidance | ||||||
| 1 From Zurier 1996, mean (SD) pain at baseline in placebo = 60.6 (21.0) 2 Unclear if randomisation was concealed or outcome assessor blinded 3 NOTE: Number needed to treat (NNT)=n/a when result is not statistically significant. NNT for dichotomous outcomes calculated using Cates NNT calculator (http://www.nntonline.net/visualrx/). NNT for continuous outcomes calculated using Wells Calculator (CMSG editorial office). 4 From Zurier 1996, mean (SD) HAQ score on 0‐100 scale at baseline in placebo = 42.5 (11.25) 5 Results based on one small trial nly | ||||||
| Triptrygium wilfordii Hook F 360 mg versus placebo for Rheumatoid arthritis | ||||||
| Patient or population: patients with Rheumatoid arthritis Settings: Community Intervention: Triptrygium wilfordii Hook F 360 mg versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Triptrygium wilfordii Hook F 360 mg versus placebo | |||||
| ACR 50% improvement | Medium risk population1 | RR 11.92 | 23 | ⊕⊕⊝⊝ | Absolute risk difference = 45% (15% to 76%) increase; relative percent change = 1090% (‐27% to 19238%); NNTB n/a4 | |
| 1 per 1000 | 12 per 1000 | |||||
| Change in pain ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| HAQ disability score ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Participants (n) reported adverse events | Medium risk population | RR 1.36 | 23 | ⊕⊕⊝⊝ | Absolute risk difference = 12% increase (‐28% to 52%); relative percent change = 36% (‐51% to 282%); NNTH = n/a | |
| 333 per 1000 | 453 per 1000 | |||||
| Change in radiographic progression ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Achievement of low disease state (DAS 28) ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidance | ||||||
| 1 Only one study available, with control event rate of 0%; thus assumed 1% control event rate for purposes of calculations; control event rates for 5 trials in review (different therapies) ranged from 0 to 35% 2 Unclear if randomisation was concealed 3 Results based on one small trial 4 NOTE: Number needed to treat (NNT)=n/a when result is not statistically significant. NNT for dichotomous outcomes calculated using Cates NNT calculator (http://www.nntonline.net/visualrx/); or for single studies as 1/RD. NNT for continuous outcomes calculated using Wells Calculator (CMSG editorial office) | ||||||
| Botanical name | Plant part | Tradename | Preparation | Drug/Extract | mg/day | Constituent marker | Marker mg/day | References |
| Populus tremula + Fraxinus excelsior + Solidago virgaurea | bark, herb, leaf | Phytodolor | fresh plant ethanolic (45,6%) extract | 3 : 1 : 1 | 5‐8 ml | total flavonoids | 0.34‐0.56 | |
| Populus tremula | bark, leaf | fresh plant ethanolic (45,6%) extract | salicin | 4.8‐8.0 | ||||
| Solidago virgaurea | herb | fresh plant ethanolic (45,6%) extract | salicyl alcohol | 0.48‐0.8 | ||||
| Fraxinus excelsior | bark | fresh plant ethanolic (45,6%) extract | isofraxidin | 0.67‐1.1 | ||||
| Salix daphnoides | bark | SM | $ ethanolic (70%) extract | 8‐14:1 | 1573 | salicin | 240 | |
| Tripterygium wilfordii Hook F | root | SM | ethanol / ethyl acetate extract | 45:1 | 180 | triptolide | 0.194, 0.056, 0.0142, 0.746 | |
| Tripterygium wilfordii Hook F | root | SM | ethanol / ethyl acetate extract | 45:1 | 360 | triptolide | 0.389, 0.112, 0.284, 1.472 | |
| Tripterygium wilfordii Hook F | root | T2 | chloroform / methanol extract | not stated | 60 | tripdiolide | 0.021, 0.041, 0.002, 0.002 | |
| Tripterygium wilfordii (local) | root | Thunder God vine | not stated | not stated | topical 5‐6 times per day | not stated | not stated | |
| Tripterygium wilfordii Hook F | root | TwHF extract | ethanol / ethyl acetate extract | not stated | 180 | triptolide and tripdiolide | not stated | |
| Withania somnifera, | RA‐1 | not stated | not stated | 444 ‐ 592 | not stated | not stated | ||
| Clematis mandshurica, | root, flower, root; 1:1:2 | SKI‐306X | ethanol 30% extracts | 7:1 | oleanolic acid 4%, rosmarinic acids 0.2%, ursolic acids 0.5%, hydroxybenzoic acid 0.03%, | |||
| Uncaria tomentosa | bark | Krallendorn | aqueous acid axtract | not stated | 60 | pentacyclic | 0.88 | |
| Tanacetum parthenium | leaf | SM | powder | 76 | parthenolide | 2‐3 micromol | ||
| Capsicum (local) | fruit | Zostrix | not stated | 0.025% | topical QID | |||
| fruit | Arlacel 165 | not stated | 0.075% | topical QID | ||||
| Oenothera biennis | semen | SM | oil | not stated | 540 | gamma‐linolenic acid (GLA) | 540 | |
| semen | SM | oil | 9% GLA | 6000 | GLA | 540 | ||
| semen | SM | oil | not stated | 20‐30 ml | GLA | not stated | ||
| Ribes nigrum | semen | SM | oil | 17% GLA | 3000 | GLA | 525 | |
| semen | SM | oil | 19% GLA | 10500 | GLA | 2000 | ||
| Borago officinalis | semen | SM | oil | 23% GLA | 7.2 ml | GLA | 1400 | |
| semen | SM | oil | 70% GLA | GLA | 2800 | |||
| Ganoderma lucida (4g) + San Miao San (Atractylodes macrocephala root, Phellodendron chinense cortex, Achyranthes bidentatae root) | not stated | not stated | aqueous extract | not stated | Rhizoma atractylodis 2.4g; Cotex phellodendri 2.4g; Radix achyranthes Bidentatae 2.4g | not stated | not stated | |
| SM = study medication | $ ethanolic extract stated in the thesis (University of Tübingen) |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain VAS 0‐100 Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 At 12 weeks‐ VAS score | 1 | 18 | Mean Difference (IV, Random, 95% CI) | 6.00 [‐16.36, 28.36] |
| 1.2 At 6 months‐ change from baseline | 3 | 82 | Mean Difference (IV, Random, 95% CI) | ‐32.83 [‐56.25, ‐9.42] |
| 2 Morning stiffness (minutes) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 At 12 weeks‐ minutes | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐5.0 [‐41.68, 31.68] |
| 2.2 At 6 months‐ change from baseline | 3 | 82 | Mean Difference (IV, Random, 95% CI) | ‐55.07 [‐76.87, ‐33.27] |
| 3 68 tender joint count percentage change from baseline Show forest plot | 3 | 82 | Mean Difference (IV, Random, 95% CI) | ‐53.80 [‐95.61, ‐12.00] |
| 4 66 swollen joint count percentage change from baseline Show forest plot | 3 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐14.43 [‐31.43, 2.56] |
| 5 Joint tenderness (0 to 3) percentage change from baseline Show forest plot | 3 | 82 | Mean Difference (IV, Random, 95% CI) | ‐56.64 [‐98.10, ‐15.17] |
| 6 Joint swelling (0 to 3) percentage change from baseline Show forest plot | 3 | 82 | Mean Difference (IV, Random, 95% CI) | ‐24.02 [‐70.80, 22.76] |
| 7 HAQ disability score percentage change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 Patient global (0 to 4) percentage change from baseline Show forest plot | 3 | 82 | Mean Difference (IV, Random, 95% CI) | ‐20.87 [‐39.43, ‐2.31] |
| 9 Physician global (0 to 4) percentage change from baseline Show forest plot | 3 | 82 | Mean Difference (IV, Random, 95% CI) | ‐21.28 [‐70.52, 27.95] |
| 10 Participants (n) reported reduced NSAID use Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.1 At 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 At 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Participants (n) reported adverse events Show forest plot | 2 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 4.24 [0.78, 22.99] |
| 11.1 GLA 540mg | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 5.59 [0.29, 108.38] |
| 11.2 GLA 1400mg+ | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 3.71 [0.47, 29.06] |
| 12 Participants (n) withdrawn due to worsening disease Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Joint tenderness (0 to 3) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 60 swollen joint count Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Morning stiffness (hours) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Grip strength (mmHg) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 15 metre walking time (seconds) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ACR20 responders Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 ACR50 responders Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Participants (n) reported adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ACR20 responders Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 ACR50 responders Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Participants (n) reported adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ACR20 responders Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 ACR50 responders Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Improvement more than 0.3 units on HAQ Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Participants (n) reported adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Participants (n) withdrawn due to adverse events Show forest plot | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.19, 0.94] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain (0 to 3) at 2 weeks Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Joint swelling (0 to 3) at 2 weeks Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 Morning stiffness (minutes) at 12 months Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Ritchie index at 12 months Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 Cumulative NSAID use (diclofenac) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 At 1 month (tablets) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 At 12 months (tablets) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain VAS 0‐100 change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 At 3 weeks | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 6 weeks | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 ACR20 responders Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 At 3 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At 6 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Participants (n) reported adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain VAS 0‐100 change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 28 tender joint count change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 28 swollen joint count change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Patient assessment of efficacy VAS 0‐100 change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Physician assessment of effiacy VAS 0‐100 change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 ACR20 responders Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 HAQ disability index change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 SF‐36 physical component summary score change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9 SF‐36 mental component summary score change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Grip strength (mmHg) at 6 weeks Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Participants (n) reported adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain VAS 0‐100 change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 68 tender joint count change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 66 swollen joint count change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Modified HAQ (Pune) change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Patient global (1 to 5) change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Physician global (1 to 5) change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 ACR20 responders Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8 ACR50 responders Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants (n) reported adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain VAS 0‐100 percentage change at 4 weeks Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Physician global (‐1 to 3) change from baseline at 4 weeks Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 42 tender joint count at 6 weeks Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 40 swollen joint count at 6 weeks Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Grip strength (kPa) at 6 weeks Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Morning stiffness (hours) at 6 weeks Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 ACR20 responders at 6 weeks Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ACR20 responders Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |

