Inmunoterapia sublingual para la rinitis alérgica
Podcast
disponible enSublingual immunotherapy for allergic rhinitis
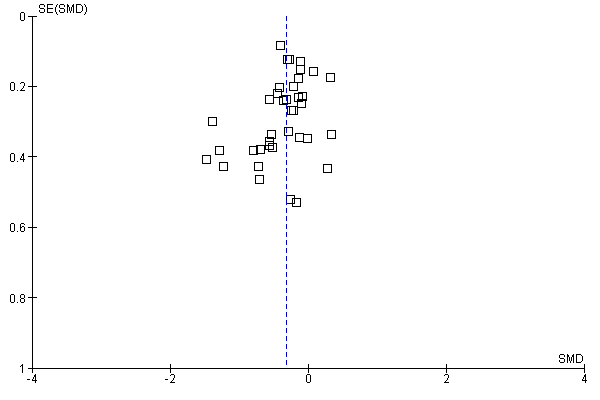
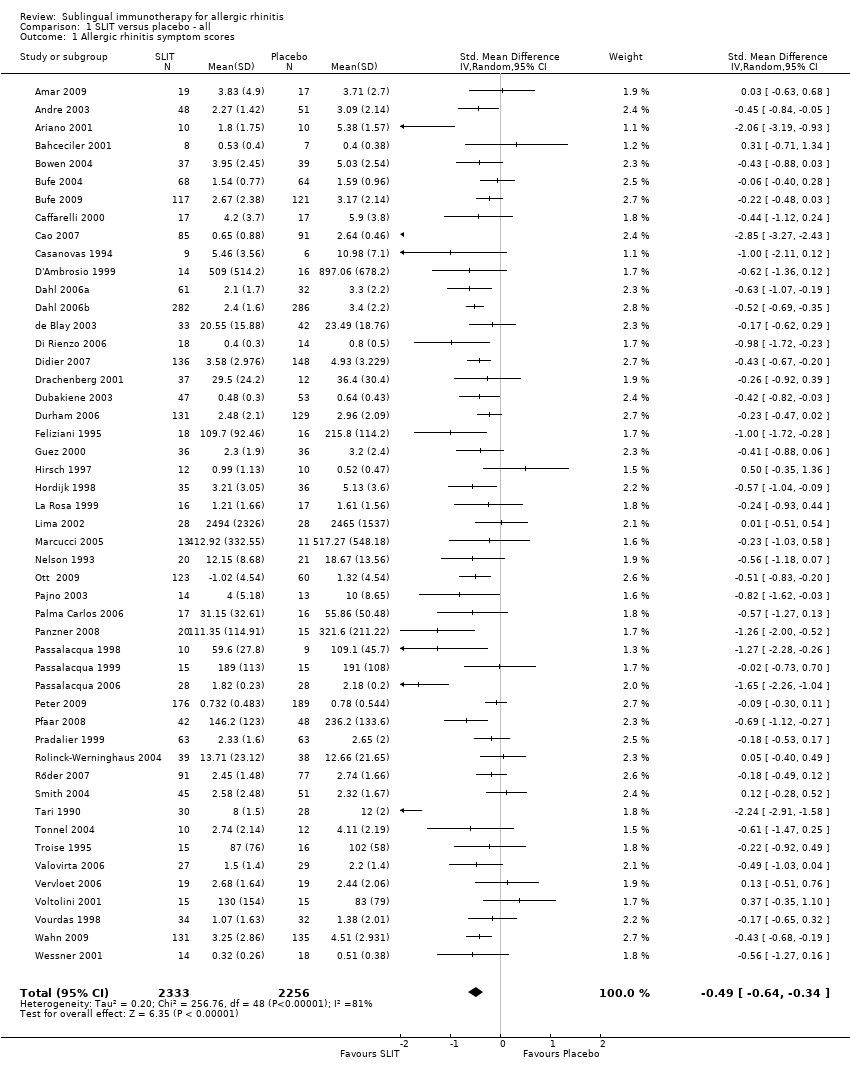
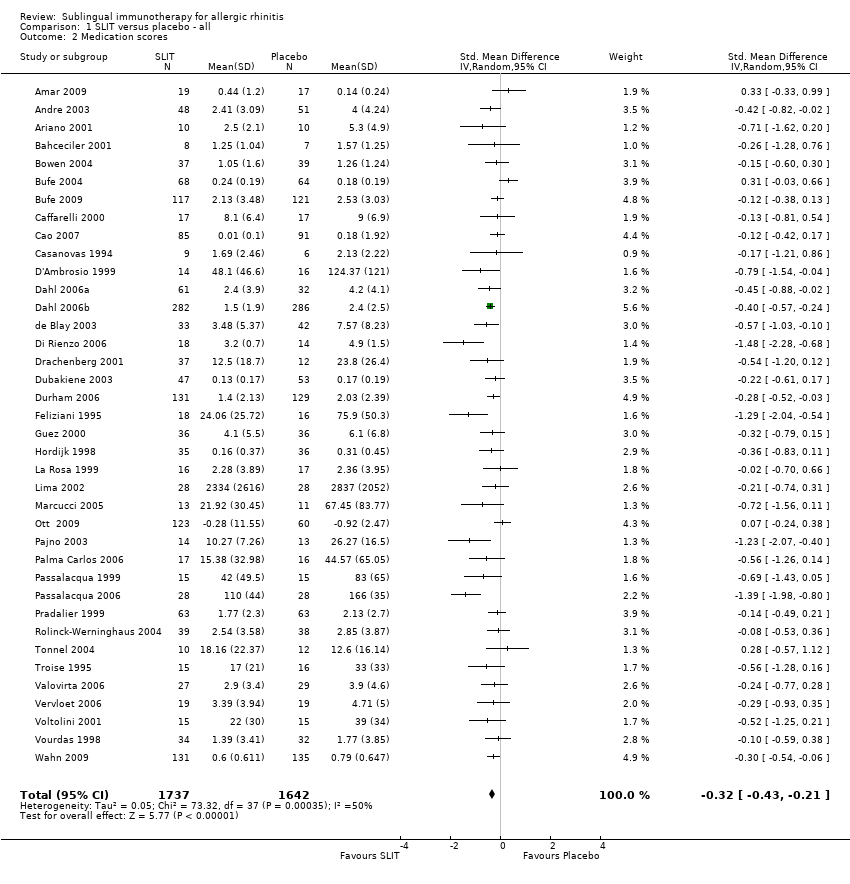
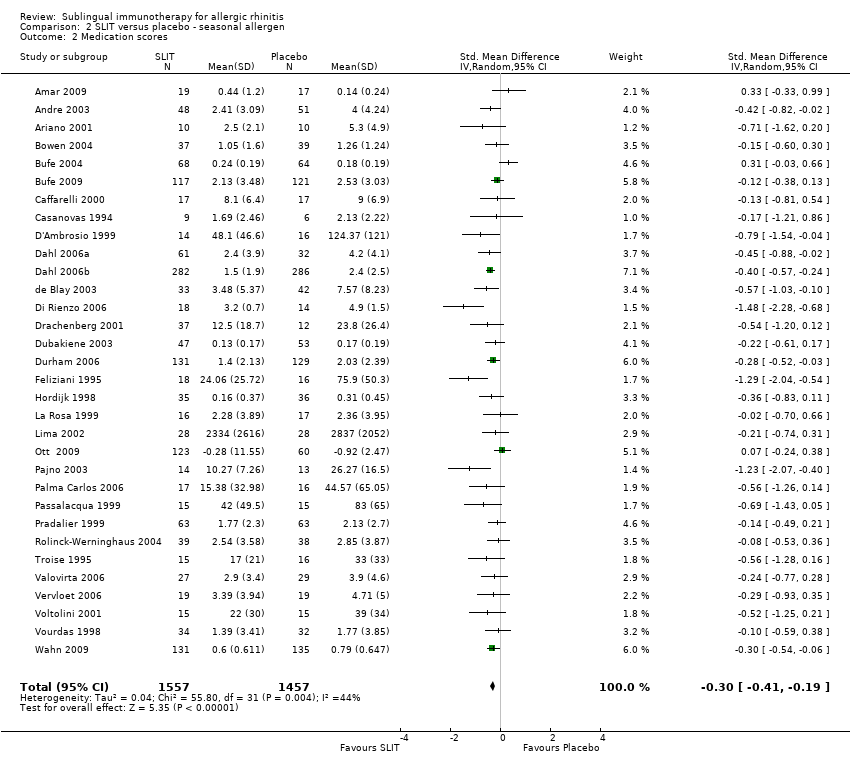
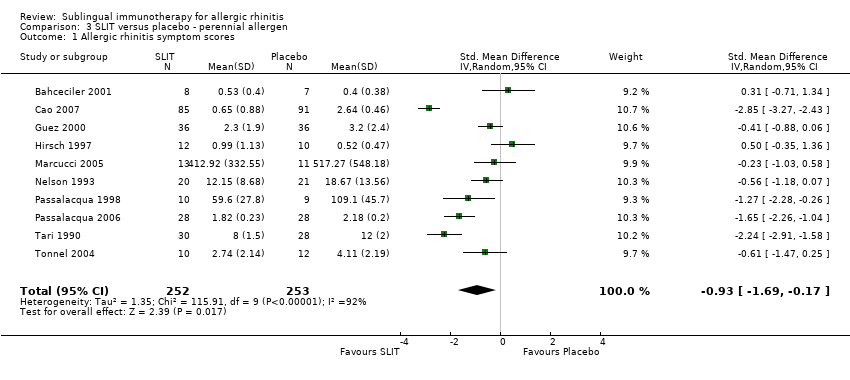
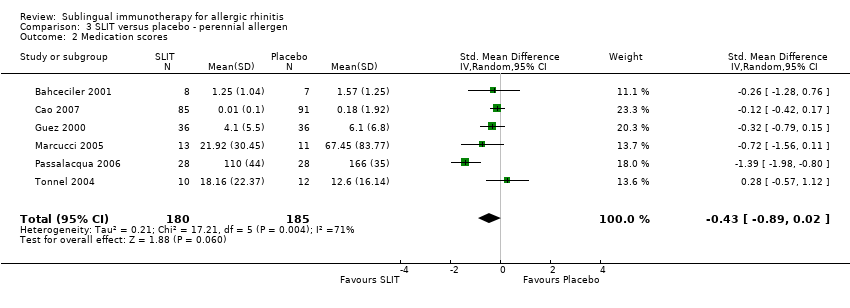
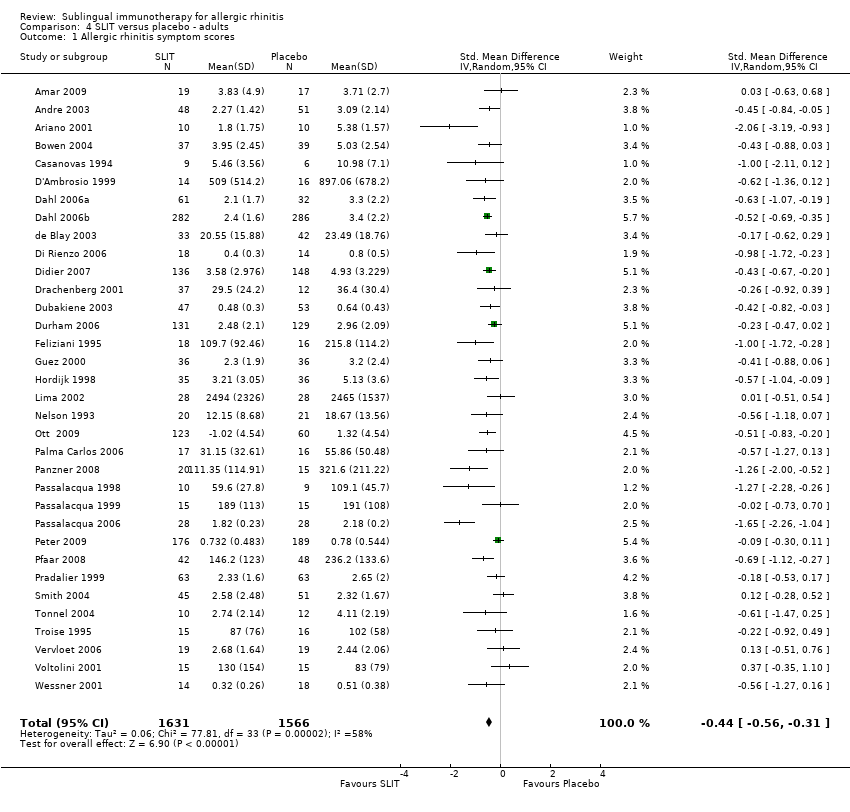
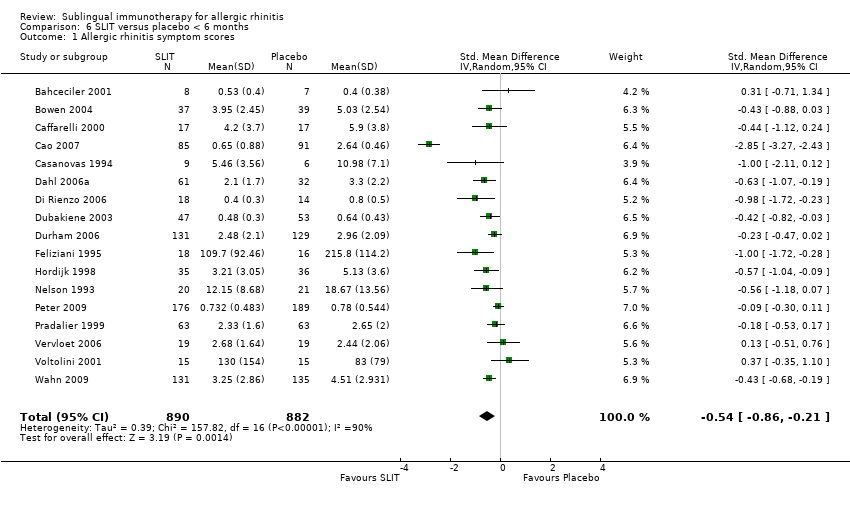
As many as 4 people in every 10 are affected by allergic rhinitis, with immunotherapy providing one of the treatment options. Duncan Wilson, from Queen Elizabeth Hospital in Birmingham England is part of a team that have updated a relevant Cochrane Review.