Vitamin E bei Alzheimer‐Demenz und leichter kognitiver Beeinträchtigung
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Design: multicentre, randomised, double‐blind, placebo‐controlled trial Duration: 6 months to 4 years | |
| Participants | Diagnosis (including criteria used): possible or probable AD (NINCDS‐ADRDA) Number of participants: 613 total randomised (completers: 140 in vitamin E group, 140 in placebo group, 142 in memantine group, 139 in the vitamin E + memantine group) Age: 53 to 96 years, mean age 78.8 years Gender: 97% men Cognitive status (e.g. MMSE): 21 Ethnicity: 86% white Inclusion criteria: diagnosis of possible or probable AD (NINCDS‐ADRDA); presence of a carer (friend or relative); informed consent; MMSE 12 to 26; administration of maintenance dosage of acetylcholinesterase inhibitors for at least 4 weeks; agreement not to take vitamin E/memantine outside the study. Exclusion criteria: non‐Alzheimer's primary dementia; current major depression, delirium, alcohol or psychoactive substance abuse or dependency; schizophrenia, or delusional disorder as defined by DSM‐IV; presence of any uncontrolled systemic illness that would interfere with participation in the study or life expectancy of < 1 year; pregnant or intention to become pregnant; enrolment in another interventional clinical trial; current prescription with more than one acetylcholinesterase inhibitor; current prescription for warfarin; use of vitamin E supplements in the past 2 weeks; use of memantine in the past 4 weeks or known intolerance; estimated creatinine clearance < 5 mL/minute; use of amantadine in the past 2 weeks. | |
| Interventions | Treatment 1: vitamin E (2000 IU total daily dose divided into 2 doses) Treatment 2: memantine (20 mg/day) Treatment 3: vitamin E + memantine Treatment 4: placebo | |
| Outcomes | ADCS‐ADL (months 0, 6, 12, 18, 24, 30, 36, 42, 48) MMSE (months 0, 6, 12, 18, 24, 30, 36, 42, 48) ADAS‐Cog (months 0, 6, 12, 18, 24, 30, 36, 42, 48) DS (months 0, 6, 12, 18, 24, 30, 36, 42, 48) NPI (months 0, 6, 12, 18, 24, 30, 36, 42, 48) Caregiver Activity Survey (months 0, 6, 12, 18, 24, 30, 36, 42, 48) Adverse events | |
| Notes | On average, carers reported that vitamin E was taken on 65% of the days. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomised centrally using "...a random permuted block design of randomly varying sizes between 4 and 12." |
| Allocation concealment (selection bias) | Low risk | "…participants were randomized centrally into one of the four treatment groups…" |
| Blinding of participants and personnel (performance bias) | Low risk | "The patient, caregivers, and all site investigators were blinded to the treatment assignment." Participants received "matching placebos for vitamin E were hard‐gelatin, liquid‐filled capsules containing soybean oil." The authors did not describe whether blinding was successful. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "The patient, caregivers, and all site investigators were blinded to the treatment assignment." The authors did not describe how blinding was maintained and whether it was successful. |
| Incomplete outcome data (attrition bias) | Low risk | Incomplete outcome data was comparable between the vitamin E group and placebo group (12 participants excluded in each due to a lack of follow‐up data). |
| Selective reporting (reporting bias) | Low risk | Study protocol was published, which included the primary and secondary outcomes as included in the main paper. |
| Other bias | Low risk | No other sources of bias noted. |
| Methods | Design: randomised, placebo‐controlled, double‐blind study Duration: 6 months | |
| Participants | Country: Spain Number of centres: 1 Diagnosis: probable AD according to NINCDS‐ADRDA Number of participants: 57 participants total randomised (completers: 19 in vitamin E group, 14 in placebo group), 18 healthy controls Age: not described Gender: not described Ethnicity: not described Cognitive status: severity of participants based on the Geriatric Dementia Scale ‐ mild (n = 25), moderate (n = 26) and severe (n = 6) Inclusion criteria: not described Exclusion criteria: people taking antioxidant supplements or any medication other than cholinesterase inhibitors | |
| Interventions | Treatment 1: vitamin E 800 IU/day Treatment 2: placebo daily | |
| Outcomes | Clock Drawing Test (months 0 and 6) BDS (months 0 and 6) MMSE (months 0 and 6) Blood total glutathione levels and oxidised glutathione (months 0 and 6) Plasma malondialdehyde (months 0 and 6) | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed using a randomized list of numbers." |
| Allocation concealment (selection bias) | Unclear risk | No reference made to how allocation concealment was ensured. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study described as 'double blind' but authors did not describe how blinding was maintained and whether it was successful. It is unclear whether the placebo was identical in appearance to the vitamin E treatment. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Study described as 'double blind' but authors did not describe how blinding was maintained and whether it was successful. |
| Incomplete outcome data (attrition bias) | High risk | 24/57 AD participants did not complete the research. "Of the patients who finished the study, 14 had been treated with placebo and 19 with vitamin E." The reasons for participant dropout were not described. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported, though the level of detail lacking. For example, means and SDs not described. |
| Other bias | Low risk | No evidence of other bias. |
| Methods | Design: randomised, placebo‐controlled, double‐blind study Duration: 36 months | |
| Participants | Country: US and Canada Number of centres: 69 Diagnosis: amnestic type of MCI Number of participants: 769 total randomised (ITT: 257 in vitamin E group, 259 in placebo group, 253 in donepezil group) Gender: 46% women Age: 55 to 90 years, mean 73 years Cognitive status: MMSE 24 to 30, mean MMSE 27.3 Inclusion criteria: amnestic MCI of a degenerative nature, impaired memory, a Logical Memory delayed‐recall score approximately 1.5 to 2 SD below an education adjusted‐norm, a CDR of 0.5, MMSE score 24 to 30, and 55 to 90 years of age. adequate vision and hearing for neuropsychological testing, normal vitamin B12 and thyroid function studies and non‐reactive RPR. ECG normal or no clinical significant abnormalities. Study informant available. Exclusion criteria: significant cerebral vascular disease, modified Hachinski > 4; Hamilton Depression Rating Scale > 12; central nervous system infarct, infection or focal lesions of clinical significance on CT or MRI scan. Medical diseases or psychiatric disorders that could interfere with study participation. Pregnant, lactating or of child‐bearing potential; taking vitamin supplements, other supplements or multivitamin. Restriction on concomitant medication usage, including those with significant cholinergic or anticholinergic effects or potential adverse effects on cognition. | |
| Interventions | Treatment 1: vitamin E group (2000 IU total daily dose divided into 2 doses, placebo donepezil and a multivitamin daily) Treatment 2: donepezil group (donepezil 10 mg, placebo vitamin E and a multivitamin daily) Treatment 3: placebo group (placebo vitamin E, placebo donepezil and a multivitamin daily) Note: the initial dose of vitamin E was 1000 IU/day, and the dose was increased to 2000 IU (1000 IU twice daily) after 6 weeks. The multivitamin contained vitamin E 15 IU | |
| Outcomes | Time to possible or probable development of AD MMSE (months 0, 6, 12, 18, 24, 30, 36) ADAS‐Cog (months 0, 6, 12, 18, 24, 30, 36) Global CDR (months 0, 6, 12, 18, 24, 30, 36) ADCS Mild Cognitive Impairment Activities of Daily Living Scale (months 0, 6, 12, 18, 24, 30, 36) GDS (months 0, 6, 12, 18, 24, 30, 36) Neuropsychological battery including; New York University paragraph‐recall test, the Symbol Digit Modalities Test, the category‐fluency test, a number‐cancellation test, the Boston Naming Test, the digits‐backward test, the Clock Drawing Test, and a maze‐tracing task (months 0, 6, 12, 18, 24, 30, 36) Adverse events | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly assigned to treatment groups. The authors used "…an adaptive allocation scheme for the treatment assignment, with the MMSE score, age, and APOE e4 status as balancing covariates." |
| Allocation concealment (selection bias) | Unclear risk | No reference made to the method in which allocation concealment was ensured. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study described as 'double blind' but authors did not describe how blinding was maintained and whether it was successful. Unclear whether the placebo was identical in appearance to the vitamin E treatment. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Study described as 'double blind' but authors did not describe how blinding was maintained and whether it was successful. |
| Incomplete outcome data (attrition bias) | Unclear risk | "The primary analysis was conducted according to the intention‐to‐treat principle." During the double‐blind phase, 72 participants discontinued in the vitamin E group, and 66 in placebo group. Sensitivity analyses suggested no impact of dropouts on the results regarding vitamin E and placebo groups. 76 participants in the vitamin E group and 73 in the placebo group developed AD and switched to open label donepezil. It is not clear how subsequent data from these participants was handled. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in accordance with the methods section. |
| Other bias | Low risk | No evidence of other bias. |
| Methods | Design: randomised, placebo‐controlled, double‐blind study Duration: 24 months | |
| Participants | Country: US Multicentre: 23 sites Diagnosis: probable AD according to NINCDS‐ADRDA Number of participants: 341 total randomised (completers: 85 in vitamin E group, 84 in placebo group, 87 in selegiline group, 85 in selegiline + vitamin E group) Gender: 65% women Age: mean 73 years Ethnicity: not described Cognitive status: mean MMSE 12.6 Inclusion criteria: diagnosis of probable AD, aged ≥ 45 years, fluent in English or Spanish, CDR score 2, modified Hachinski score 4, supervised by a responsible carer. Exclusion criteria: other central nervous system or psychiatric diagnosis; achieving end point on any primary outcome measure at time of entry; participation in any other investigational drug study or treatment with a psychoactive medication within 2 months of initiation of this trial; treatment with antiparkinsonian medications; recent initiated treatment for a non‐psychiatric condition with an agent to have psychoactive properties (e.g. beta‐blockers, calcium channel blockers). Entry permitted if medication and dose had been stable for 3 months prior to entry into the protocol; antilipaemic drugs specifically contradicted with selegiline (i.e. cholestyramine or colestipol) within 2 weeks of enrolment; some narcotics: demerol has been reported to interact with selegiline; ancillary vitamin E (alpha tocopherol) other than the 30 IU to 32 IU included in a standard multivitamin. | |
| Interventions | Treatment 1: placebo Treatment 2: vitamin E (2000 IU total daily dose divided into 2 doses) Treatment 3: selegiline (10 mg total daily dose divided into 2 doses) Treatment 4: vitamin E (2000 IU total daily dose divided into 2 doses) + selegiline (10 mg total daily dose divided into 2 doses) | |
| Outcomes | Delay in any of 4 end points: death; institutionalisation; severity of dementia; loss of ADL ADAS‐Cog (1 month after enrolment and at 3‐month intervals) MMSE (1 month after enrolment and at 3‐month intervals) BDS (1 month after enrolment and at 3‐month intervals) DS (1 month after enrolment and at 3‐month intervals) BRSD (1 month after enrolment and at 3‐month intervals) Adverse events | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were "...randomly assigned (after stratification according to center with the use of a permuted‐block procedure)..." to receive vitamin E or placebo. |
| Allocation concealment (selection bias) | Unclear risk | Method in which allocation concealment was ensured was not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study described as 'double blind' but authors did not describe how blinding was maintained and whether it was successful. Unclear whether the placebo and vitamin E treatment capsules were visually identical. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Study described as 'double blind' but authors did not describe how blinding was maintained and whether it was successful. |
| Incomplete outcome data (attrition bias) | High risk | Attrition was only able to be reported up until the point a participant reached 1 of the primary end points of the study. Loss to follow‐up was defined as those who did not reach an end point and did not complete the study: 6/84 for the placebo group and 8/85 for the vitamin E group. The reasons for withdrawal were not described in each treatment group. |
| Selective reporting (reporting bias) | Unclear risk | All outcome measures were reported as per the protocol paper. No SDs reported for change scores. |
| Other bias | Low risk | No evidence of other bias. |
AD: Alzheimer's dementia; ADAS‐Cog: Alzheimer's Disease Assessment Scale ‐ Cognitive subsection; ADCS: Alzheimer's Disease Cooperative Study; ADL: activities of daily living; APoE: apolipoprotein E; BDS: Blessed Dementia Scale; BRSD: Behavior Rating Scale for Dementia; CDR: Clinical Dementia Rating; CT: computer tomography; DS: Dependence Scale; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; ECG: electrocardiography; GDS: Global Deterioration Scale; ITT: intention to treat; MCI: mild cognitive impairment; MMSE: Mini‐Mental State Examination; MRI: magnetic resonance imaging; n: number of participants; NINCDS‐ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association; NPI: Neuropsychiatric Inventory; RPR: rapid plasmin reagin; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not suitable placebo | |
| Control group did not receive a suitable placebo. | |
| Sample population did not have a diagnosis of MCI or AD. | |
| Sample population did not have a diagnosis of MCI or AD. | |
| Newsletter | |
| Not an RCT | |
| Not an RCT | |
| Review | |
| Retrospective design | |
| Review | |
| Sample population did not have a diagnosis of MCI or AD. | |
| Used a nutriceutical formulation and there was no placebo. | |
| Used a nutriceutical formulation. Placebo was just inert ingredients. | |
| Editorial | |
| Vitamin E was used in conjunction with vitamin C. | |
| Commentary | |
| Used a nutriceutical formulation, not vitamin E separately. | |
| In vitro study | |
| Review | |
| No vitamin E intervention, just a questionnaire | |
| Editorial | |
| Not suitable placebo | |
| Pioglitazone was the primary intervention. Placebo was administered alongside vitamin E. | |
| Sample population did not have a diagnosis of MCI or AD. | |
| Sample population did not have a diagnosis of MCI or AD. | |
| No relevant outcome measures | |
| Sample population did not have a diagnosis of MCI or AD. | |
| Review | |
| Newsletter | |
| Vitamin E intervention given to healthy older women. | |
| Review | |
| Not an RCT | |
| Literature review about antioxidants | |
| Sample population did not have a diagnosis of MCI or AD. | |
| Sample population did not have a diagnosis of MCI or AD. | |
| Sample population did not have a diagnosis of MCI or AD. | |
| Sample population did not have a diagnosis of MCI or AD. | |
| Literature review about antioxidants | |
| No vitamin E intervention group | |
| No vitamin E intervention group | |
| Sample population did not have a diagnosis of MCI or AD. | |
| No placebo group | |
| Sample population had a diagnosis of Down's syndrome and dementia. | |
| Focus of intervention was towards depressed vs non‐depressed MCI population. | |
| Sample population did not have a diagnosis of MCI or AD. | |
| Review | |
| Survey | |
| Review | |
| Review | |
| Sample population did not have a diagnosis of MCI or AD. | |
| No co‐administration of other compounds to controls | |
| Vitamin E compared to donepezil not placebo. | |
| Retrospective in nature. No controls or placebo group | |
| Not an RCT | |
| Review | |
| Did not use vitamin E as an intervention. | |
| Not a randomised double‐blinded study; investigated the intake of fruits and vegetables. | |
| Used a nutriceutical formulation and the placebo was inert. | |
| Used a nutriceutical formulation. | |
| Systematic review | |
| Used a nutriceutical formulation. | |
| Secondary analysis (modelling) of intervention data | |
| Used a healthy population. | |
| Same data as Petersen 2005 | |
| Used a nutriceutical formulation, not vitamin E separately. | |
| Vitamin C taken by treatment arm but not controls, no relevant outcomes. | |
| Sample population did not have a diagnosis of MCI or AD. | |
| Vitamin E not administered | |
| Case study, no placebo and no controls | |
| Review | |
| Not an original paper, just a personal comment | |
| Vitamin E not administered | |
| Same data as Petersen 2005 | |
| Not an RCT |
AD: Alzheimer's dementia; MCI: mild cognitive impairment; RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by year of study]
| Trial name or title | Effects of Vitamin E on Cognition and Measures of Activities of Daily Living in Patients with Moderately Severe Alzheimer's Disease |
| Methods | Unknown |
| Participants | People with moderately severe Alzheimer's disease |
| Interventions | Vitamin E 500 mg twice daily with cholinesterase inhibitors |
| Outcomes | Changes in cognition and activities of daily living |
| Starting date | 1 November 2002 |
| Contact information | Dr Vishnu Gopal; Argyll House, 9 Williamson Road, Nether Edge, Sheffield S11 9AR; tel: (+44) 114 2718656; email: [email protected] |
| Notes | Study topic and author searched online, no evidence of trial or published data. Author no longer at contact address, emailed for an update but no response. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Alzheimer's Disease Assessment Scale ‐ Cognitive subscale (ADAS‐Cog) (least square (LS) mean change from baseline at 6 to 48 months): completers Show forest plot | 1 | 272 | Mean Difference (IV, Random, 95% CI) | ‐1.81 [‐3.75, 0.13] |
| Analysis 1.1  Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 1 Alzheimer's Disease Assessment Scale ‐ Cognitive subscale (ADAS‐Cog) (least square (LS) mean change from baseline at 6 to 48 months): completers. | ||||
| 2 Alzheimer's Disease Cooperative Study/Activities of Daily Living (ADCS‐ADL) (LS mean change from baseline at 6 to 48 months): completers Show forest plot | 1 | 280 | Mean Difference (IV, Fixed, 95% CI) | 3.15 [0.07, 6.23] |
| Analysis 1.2 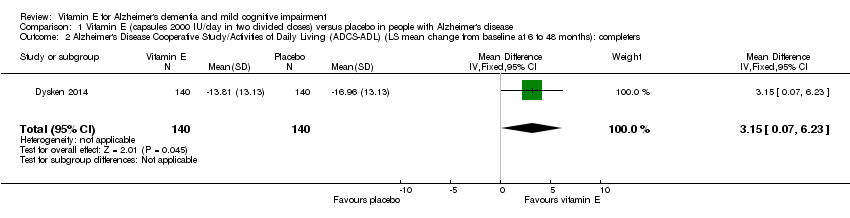 Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 2 Alzheimer's Disease Cooperative Study/Activities of Daily Living (ADCS‐ADL) (LS mean change from baseline at 6 to 48 months): completers. | ||||
| 3 Deaths (number of deaths over 48 months) Show forest plot | 1 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.52, 1.34] |
| Analysis 1.3  Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 3 Deaths (number of deaths over 48 months). | ||||
| 4 Serious adverse events (number of participants reporting ≥ 1 serious adverse event over 48 months) Show forest plot | 1 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.71, 1.05] |
| Analysis 1.4 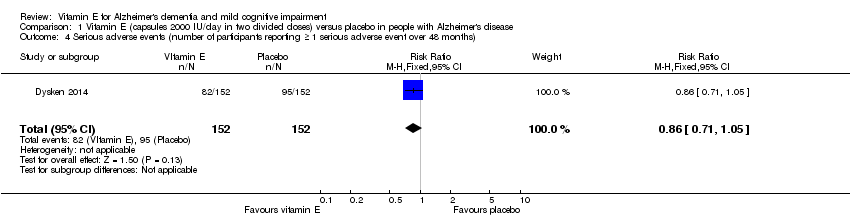 Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 4 Serious adverse events (number of participants reporting ≥ 1 serious adverse event over 48 months). | ||||
| 5 Neuropsychiatric Inventory (NPI) (LS mean change from baseline at 6 to 48 months): completers Show forest plot | 1 | 280 | Mean Difference (IV, Fixed, 95% CI) | ‐1.47 [‐4.26, 1.32] |
| Analysis 1.5  Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 5 Neuropsychiatric Inventory (NPI) (LS mean change from baseline at 6 to 48 months): completers. | ||||
| 6 Mini‐Mental State Examination (MMSE) (LS mean change from baseline at 6 to 48 months): completers Show forest plot | 1 | 273 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.72, 1.10] |
| Analysis 1.6  Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 6 Mini‐Mental State Examination (MMSE) (LS mean change from baseline at 6 to 48 months): completers. | ||||
| 7 Adverse events (number of participants reporting ≥ 1 adverse event over 48 months) Show forest plot | 1 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.85, 1.23] |
| Analysis 1.7  Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 7 Adverse events (number of participants reporting ≥ 1 adverse event over 48 months). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Progression to Alzheimer's disease (AD) (number of people progressing to AD by 36 months) Show forest plot | 1 | 516 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.79, 1.35] |
| Analysis 2.1  Comparison 2 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with mild cognitive impairment, Outcome 1 Progression to Alzheimer's disease (AD) (number of people progressing to AD by 36 months). | ||||
| 2 Deaths (number of deaths over 36 months) Show forest plot | 1 | 516 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.30, 3.44] |
| Analysis 2.2  Comparison 2 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with mild cognitive impairment, Outcome 2 Deaths (number of deaths over 36 months). | ||||

Study flow diagram for study for the search April 2016.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
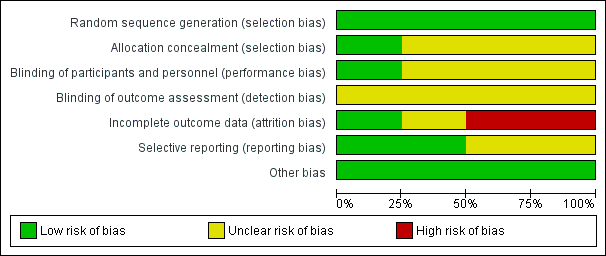
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 1 Alzheimer's Disease Assessment Scale ‐ Cognitive subscale (ADAS‐Cog) (least square (LS) mean change from baseline at 6 to 48 months): completers.
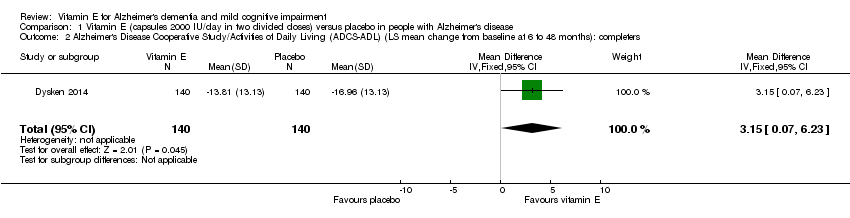
Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 2 Alzheimer's Disease Cooperative Study/Activities of Daily Living (ADCS‐ADL) (LS mean change from baseline at 6 to 48 months): completers.

Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 3 Deaths (number of deaths over 48 months).

Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 4 Serious adverse events (number of participants reporting ≥ 1 serious adverse event over 48 months).
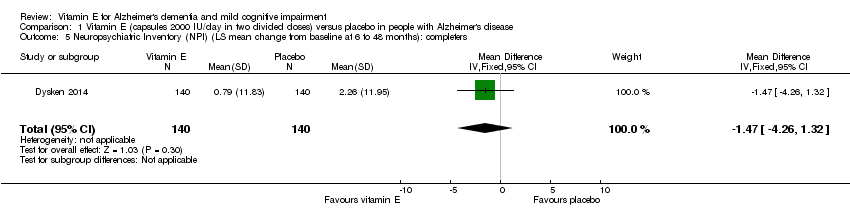
Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 5 Neuropsychiatric Inventory (NPI) (LS mean change from baseline at 6 to 48 months): completers.

Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 6 Mini‐Mental State Examination (MMSE) (LS mean change from baseline at 6 to 48 months): completers.
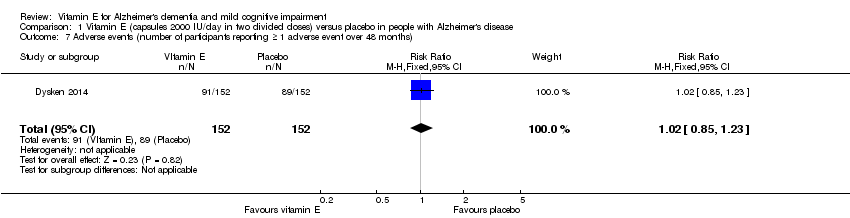
Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 7 Adverse events (number of participants reporting ≥ 1 adverse event over 48 months).
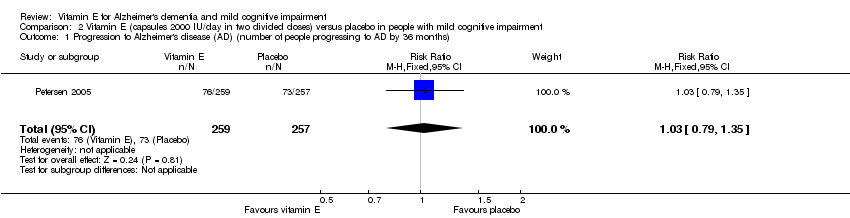
Comparison 2 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with mild cognitive impairment, Outcome 1 Progression to Alzheimer's disease (AD) (number of people progressing to AD by 36 months).
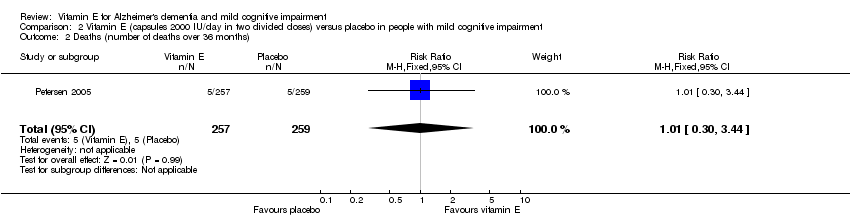
Comparison 2 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with mild cognitive impairment, Outcome 2 Deaths (number of deaths over 36 months).
| Vitamin E (capsules 2000 IU/day in 2 divided doses) compared to placebo for people with Alzheimer's disease | ||||||
| Patient or population: people with Alzheimer's disease Settings: multicentre, US Intervention: vitamin E (capsules 2000 IU/day in 2 divided doses) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Vitamin E (capsules 2000 IU/day in 2 divided doses) | |||||
| Cognitive function Scale from: 0 to 70 Follow‐up: 6 to 48 months | The LS mean change from baseline in cognitive function in placebo group was 7.78 | The LS mean change from baseline in cognitive function in the intervention group was 1.81 lower | ‐ | 272 | ⊕⊕⊕⊝ | Higher scores represent worse cognitive function. A 4‐point difference in ADAS‐cog has been considered the MCID. |
| Adverse events Number of participants reporting ≥ 1 serious adverse event Follow‐up: 6 to 48 months | 625 per 1000 | 538 per 1000 | RR 0.86 | 304 | ⊕⊕⊕⊝ | ‐ |
| Deaths Number of deaths Follow‐up: 6 to 48 months | 204 per 1000 | 171 per 1000 | RR 0.84 | 304 | ⊕⊕⊕⊝ | ‐ |
| Activities of daily living Scale from: 0 to 78 Follow‐up: 6 to 48 months | The LS mean change from baseline in activities of daily living in the placebo group was ‐16.96 | The LS mean change from baseline in activities of daily living in the intervention group was 3.15 higher | ‐ | 280 | ⊕⊕⊕⊝ | Higher scores represent better activities of daily living. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADAS‐Cog: Alzheimer Disease Assessment Scale ‐ Cognitive Subscale; ADCS‐ADL: Alzheimer's Disease Cooperative Study/Activities of Daily Living Inventory;CI: confidence interval; LS: least square; MCID: minimum clinically important difference; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Quality downgraded one level due to imprecision. Evidence from a single study of modest size. This is supported by dichotomous data not reaching the optimal information size criterion (assuming α of 0.05, and β of 0.2) (Guyatt 2011). | ||||||
| Vitamin E (capsules 2000 IU/day in 2 divided doses) compared to placebo for people with mild cognitive impairment | ||||||
| Patient or population: people with mild cognitive impairment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Vitamin E (capsules 2000 IU/day in 2 divided doses) | |||||
| Progression to Alzheimer's disease Number of people progressing to AD Follow‐up: 36 months | 284 per 1000 | 293 per 1000 | RR 1.03 | 516 | ⊕⊕⊕⊝ | ‐ |
| Cognitive function Mean change from baseline of ADAS‐Cog Scale from: 0 to 70 Follow‐up: 36 months | Not possible to extract data for analysis. | ‐ | Not possible to extract data for analysis. | Unable to evaluate quality of evidence. | Uncertainty about how missing data were handled. Study reports no significant difference between intervention and control groups. | |
| Adverse events Number of participants reporting ≥ 1 serious adverse event. Follow‐up: 36 months | Not possible to extract data for analysis. | ‐ | 516 | Unable to evaluate quality of evidence. | Overall adverse event rates not reported. | |
| Death Number of deaths over 36 months Follow‐up: 36 months | 19 per 1000 | 19 per 1000 | RR 1.01 | 516 | ⊕⊕⊕⊝ | ‐ |
| Activities of daily living Mean change from baseline using the ADCS Scale from: 0 to 53 Follow‐up: 36 months | Not possible to extract data for analysis. | ‐ | Not possible to extract data for analysis. | Unable to evaluate quality of evidence. | Uncertainty about how missing data were handled. Study reports no significant difference between intervention and control groups. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADAS‐Cog: Alzheimer Disease Assessment Scale ‐ Cognitive Subscale; ADCS‐ADL: Alzheimer's Disease Cooperative Study/Activities of Daily Living Inventory;CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Quality downgraded one level due to imprecision. Evidence from a single study of modest size. This was supported by dichotomous data not reaching the optimal information size criterion (assuming α of 0.05, and β of 0.2) (Guyatt 2011). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Alzheimer's Disease Assessment Scale ‐ Cognitive subscale (ADAS‐Cog) (least square (LS) mean change from baseline at 6 to 48 months): completers Show forest plot | 1 | 272 | Mean Difference (IV, Random, 95% CI) | ‐1.81 [‐3.75, 0.13] |
| 2 Alzheimer's Disease Cooperative Study/Activities of Daily Living (ADCS‐ADL) (LS mean change from baseline at 6 to 48 months): completers Show forest plot | 1 | 280 | Mean Difference (IV, Fixed, 95% CI) | 3.15 [0.07, 6.23] |
| 3 Deaths (number of deaths over 48 months) Show forest plot | 1 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.52, 1.34] |
| 4 Serious adverse events (number of participants reporting ≥ 1 serious adverse event over 48 months) Show forest plot | 1 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.71, 1.05] |
| 5 Neuropsychiatric Inventory (NPI) (LS mean change from baseline at 6 to 48 months): completers Show forest plot | 1 | 280 | Mean Difference (IV, Fixed, 95% CI) | ‐1.47 [‐4.26, 1.32] |
| 6 Mini‐Mental State Examination (MMSE) (LS mean change from baseline at 6 to 48 months): completers Show forest plot | 1 | 273 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.72, 1.10] |
| 7 Adverse events (number of participants reporting ≥ 1 adverse event over 48 months) Show forest plot | 1 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.85, 1.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Progression to Alzheimer's disease (AD) (number of people progressing to AD by 36 months) Show forest plot | 1 | 516 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.79, 1.35] |
| 2 Deaths (number of deaths over 36 months) Show forest plot | 1 | 516 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.30, 3.44] |

