Witamina E w otępieniu typu Alzheimera i łagodnych zaburzeniach poznawczych
Appendices
Appendix 1. Source, search strategy and hits retrieved: June 2012 search
| Source | Search strategy | Hits received |
| 1. ALOIS (www.medicine.ox.ac.uk/alois) | Advanced search: [Study design: RCT OR CCT] AND [Health condition: Alzheimer OR MCI] AND [Intervention: "vitamin E"] (all dates) | Jun 2012: 39 (all dates) |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950 to present (OvidSP) | 1. *Vitamin E/ 2. "vitamin E".ti,ab. 3. "alpha‐tocopherol".ti,ab. 4. or/1‐3 5. Alzheimer*.ti,ab. 6. Alzheimer Disease/ 7. AD.ti,ab. 8. "cognit* impair*".ti,ab. 9. MCI.ti,ab. 10. (AACI or memory or CIND or ARCD or ACMI).ti,ab. 11. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 12. (nMCI or aMCI or mMCI).ti,ab. 13. (CDR adj2 "0.5").ab. 14. or/5‐13 15. 4 and 14 16. randomized controlled trial.pt. 17. controlled clinical trial.pt. 18. randomi?ed.ab. 19. placebo.ab. 20. drug therapy.fs. 21. randomly.ab. 22. trial.ab. 23. groups.ab. 24. or/16‐23 25. (animals not (humans and animals)).sh. 26. 24 not 25 27. 15 and 26 | Jun 2012: 16 |
| 3. EMBASE 1980 to 2011 week 27 (OvidSP) | 1. *Vitamin E/ 2. "vitamin E".ti,ab. 3. "alpha‐tocopherol".ti,ab. 4. or/1‐3 5. Alzheimer*.ti,ab. 6. Alzheimer Disease/ 7. AD.ti,ab. 8. "cognit* impair*".ti,ab. 9. MCI.ti,ab. 10. (AACI or memory or CIND or ARCD or ACMI).ti,ab. 11. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 12. (nMCI or aMCI or mMCI).ti,ab. 13. (CDR adj2 "0.5").ab. 14. or/5‐13 15. 4 and 14 16. randomi?ed.ab. 17. placebo.ab. 19. trial.ab. 20. groups.ab. 21. randomized controlled trial/ 22. controlled clinical trial/ 23. ("double‐blind*" or "single‐blind*").ti,ab. 24. or/16‐23 25. 15 to 24 | Jun 2012: 28 |
| 4. PsycINFO 1806 to July week 2 2011 (OvidSP) | 1. "vitamin E".ti,ab. 2. "alpha‐tocopherol".ti,ab. 3. Alzheimer*.ti,ab. 4. Alzheimer Disease/ 5. AD.ti,ab. 6. "cognit* impair*".ti,ab. 7. MCI.ti,ab. 8. (AACI or memory or CIND or ARCD or ACMI).ti,ab. 9. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 10. (nMCI or aMCI or mMCI).ti,ab. 11. (CDR adj2 "0.5").ab. 12. randomi?ed.ab. 13. placebo.ab. 14. randomly.ab. 15. trial.ab. 16. groups.ab. 17. ("double‐blind*" or "single‐blind*").ti,ab. 18. Clinical Trials/ 19. 1 or 2 20. or/3‐11 21. or/12‐18 22. 19 and 20 and 21 | Jun 2012: 6 |
| 5. CINAHL (EBSCOhost) | S1 (MM "Vitamin E") S2 TX "vitamin E" S3 TX "alpha‐tocopherol" S4 S1 or S2 or S3 S5 (MH "Alzheimer's Disease") S6 TX AD OR alzheimer* S7 "mild cognitive impairment" S8 TX "cognit* impair*" S9 TX AACI OR memory OR CIND OR ARCD OR ACMI S10 TX MCI S11 TX nMCI OR aMCI OR mMCI S12 S5 or S6 or S7 or S8 or S9 or S10 or S11 S13 S4 and S12 S14 TX random* S15 TX placebo* S16 TX trial S17 TX groups S18 TX RCT OR CCT S19 (MH "Randomized Controlled Trials") S20 S14 or S15 or S16 or S17 or S18 or S19 S21 S13 and S20 S22 EM 2009 S23 EM 2010 S24 EM 2011 S25 S22 or S23 or S24 S26 S21 and S25 | Jun 2012: 6 |
| 6. ISI Web of Knowledge – all databases [includes: Web of Science (1945 to present); BIOSIS Previews (1926 to present); MEDLINE (1950 to present); Journal Citation Reports] | #1 Topic=(alzheimer* OR AD OR MCI OR memory OR cognitive OR "cognit* impair*") AND Topic=("vitamin e" OR "alpha‐tocopherol") AND Year Published=(2009‐2011) Timespan=All Years #2 Topic=(random* OR placebo* OR "double‐blind*" OR "single‐blind*") Timespan=All Years #3 #2 AND #1 Timespan=All Years | Jun 2012: 174 |
| 7. LILACS (BIREME) | vitamin‐e OR alpha‐tocopherol | Jun 2012: 0 |
| 8. CENTRAL (the Cochrane Library) (Issue 4 of 4, Oct 2010) | #1 “vitamin e” #2 “alpha‐tocopherol” #3 (#1 OR #2) #4 MeSH descriptor Vitamin E, this term only #5 (#3 OR #4) #6 alzheimer* OR AD OR “cognit* impair*” OR MCI #7 (#5 AND #6), from 2011 to 2012 | Jun 2012: 9 |
| 9. Clinicaltrials.gov (www.clinicaltrials.gov) | Interventional Studies | alzheimer OR alzheimer's OR alzheimers OR MCI OR cognitive OR cognition OR memory | vitamin E OR alpha‐tocopherol | received from 01/01/2011 to 07/15/2012 | Jun 2012: 2 |
| 10. ICTRP Search Portal (apps.who.int/trialsearch) (includes: Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register) | Interventional Studies | alzheimer OR alzheimer's OR alzheimers OR MCI OR cognitive OR cognition OR memory | vitamin E OR alpha‐tocopherol | received from 01/07/2011 to 15/07/2012 | Jun 2012: 2 |
| TOTAL before de‐duplication | 282 | |
| TOTAL after de‐duplication and first assessment | 14 | |
Appendix 2. Sources searched, search strategies and hits retrieved: October 2014, May 2015, April 2016
| Source | Search strategy | Hits received |
| 1. ALOIS (www.medicine.ox.ac.uk/alois) | Advanced search: [Study design: RCT OR CCT] AND [Health condition: Alzheimer OR MCI] AND [Intervention: "vitamin E"] (all dates) | Oct 2014: 11 May 2015: 0 Apr 2016: 0 |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950 to present (OvidSP) | 1. *Vitamin E/ 2. "vitamin E".ti,ab. 3. "alpha‐tocopherol".ti,ab. 4. or/1‐3 5. Alzheimer*.ti,ab. 6. Alzheimer Disease/ 7. AD.ti,ab. 8. "cognit* impair*".ti,ab. 9. MCI.ti,ab. 10. (AACI or memory or CIND or ARCD or ACMI).ti,ab. 11. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 12. (nMCI or aMCI or mMCI).ti,ab. 13. (CDR adj2 "0.5").ab. 14. or/5‐13 15. 4 and 14 16. randomized controlled trial.pt. 17. controlled clinical trial.pt. 18. randomi?ed.ab. 19. placebo.ab. 20. drug therapy.fs. 21. randomly.ab. 22. trial.ab. 23. groups.ab. 24. or/16‐23 25. (animals not (humans and animals)).sh. 26. 24 not 25 27. 15 and 26 | Oct 2014: 51 May 2015: 6 Apr 2016: 43 |
| 3. EMBASE 1980 to 2011 week 27 (OvidSP) | 1. *Vitamin E/ 2. "vitamin E".ti,ab. 3. "alpha‐tocopherol".ti,ab. 4. or/1‐3 5. Alzheimer*.ti,ab. 6. Alzheimer Disease/ 7. AD.ti,ab. 8. "cognit* impair*".ti,ab. 9. MCI.ti,ab. 10. (AACI or memory or CIND or ARCD or ACMI).ti,ab. 11. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 12. (nMCI or aMCI or mMCI).ti,ab. 13. (CDR adj2 "0.5").ab. 14. or/5‐13 15. 4 and 14 16. randomi?ed.ab. 17. placebo.ab. 19. trial.ab. 20. groups.ab. 21. randomized controlled trial/ 22. controlled clinical trial/ 23. ("double‐blind*" or "single‐blind*").ti,ab. 24. or/16‐23 25. 15 to 24 | Oct 2014: 100 May 2015: 65 Apr 2016: 40 |
| 4. PsycINFO 1806 to July week 2 2011 (OvidSP) | 1. "vitamin E".ti,ab. 2. "alpha‐tocopherol".ti,ab. 3. Alzheimer*.ti,ab. 4. Alzheimer Disease/ 5. AD.ti,ab. 6. "cognit* impair*".ti,ab. 7. MCI.ti,ab. 8. (AACI or memory or CIND or ARCD or ACMI).ti,ab. 9. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 10. (nMCI or aMCI or mMCI).ti,ab. 11. (CDR adj2 "0.5").ab. 12. randomi?ed.ab. 13. placebo.ab. 14. randomly.ab. 15. trial.ab. 16. groups.ab. 17. ("double‐blind*" or "single‐blind*").ti,ab. 18. Clinical Trials/ 19. 1 or 2 20. or/3‐11 21. or/12‐18 22. 19 and 20 and 21 | Oct 2014: 10 May 2015: 4 Apr 2016: 0 |
| 5. CINAHL (EBSCOhost) | S1 (MM "Vitamin E") S2 TX "vitamin E" S3 TX "alpha‐tocopherol" S4 S1 or S2 or S3 S5 (MH "Alzheimer's Disease") S6 TX AD OR alzheimer* S7 "mild cognitive impairment" S8 TX "cognit* impair*" S9 TX AACI OR memory OR CIND OR ARCD OR ACMI S10 TX MCI S11 TX nMCI OR aMCI OR mMCI S12 S5 or S6 or S7 or S8 or S9 or S10 or S11 S13 S4 and S12 S14 TX random* S15 TX placebo* S16 TX trial S17 TX groups S18 TX RCT OR CCT S19 (MH "Randomized Controlled Trials") S20 S14 or S15 or S16 or S17 or S18 or S19 S21 S13 and S20 S22 EM 2009 S23 EM 2010 S24 EM 2011 S25 S22 or S23 or S24 S26 S21 and S25 | Oct 2014: 7 May 2015: 5 Apr 2016: 3 |
| 6. ISI Web of Knowledge – all databases [includes: Web of Science (1945 to present); BIOSIS Previews (1926 to present); MEDLINE (1950 to present); Journal Citation Reports] | #1 Topic=(alzheimer* OR AD OR MCI OR memory OR cognitive OR "cognit* impair*") AND Topic=("vitamin e" OR "alpha‐tocopherol") AND Year Published=(2009‐2011) Timespan=All Years #2 Topic=(random* OR placebo* OR "double‐blind*" OR "single‐blind*") Timespan=All Years #3 #2 AND #1 Timespan=All Years | Oct 2014: 91 May 2015: 58 Apr 2016: 54 |
| 7. LILACS (BIREME) | vitamin‐e OR alpha‐tocopherol | Oct 2014: 1 May 2015: 0 Apr 2016: 0 |
| 8. CENTRAL (the Cochrane Library) (Issue 4 of 4, Oct 2010) | #1 “vitamin e” #2 “alpha‐tocopherol” #3 (#1 OR #2) #4 MeSH descriptor Vitamin E, this term only #5 (#3 OR #4) #6 alzheimer* OR AD OR “cognit* impair*” OR MCI #7 (#5 AND #6), from 2011 to 2012 | Oct 2014: 33 May 2015: 15 Apr 2016: 0 |
| 9. Clinicaltrials.gov (www.clinicaltrials.gov) | Interventional Studies | alzheimer OR alzheimer's OR alzheimers OR MCI OR cognitive OR cognition OR memory | vitamin E OR alpha‐tocopherol | received from 01/01/2011 to 07/15/2012 | Oct 2014: 1 May 2015: 2 Apr 2016: 1 |
| 10. ICTRP Search Portal (apps.who.int/trialsearch) (includes: Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register) | Interventional Studies | alzheimer OR alzheimer's OR alzheimers OR MCI OR cognitive OR cognition OR memory | vitamin E OR alpha‐tocopherol | received from 01/07/2011 to 15/07/2012 | Oct 2014: 14 May 2015: 4 Apr 2016: 1 |
| TOTAL before de‐duplication | Oct 2014: 321 May 2015: 159 Apr 2016: 142 | |
| TOTAL after de‐duplication and first assessment | Oct 2014: 41 May 2015: 8 Apr 2016: 8 | |

Study flow diagram for study for the search April 2016.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
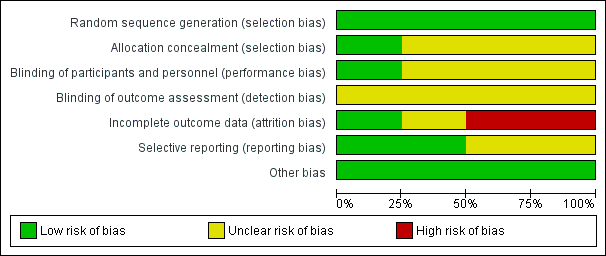
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 1 Alzheimer's Disease Assessment Scale ‐ Cognitive subscale (ADAS‐Cog) (least square (LS) mean change from baseline at 6 to 48 months): completers.
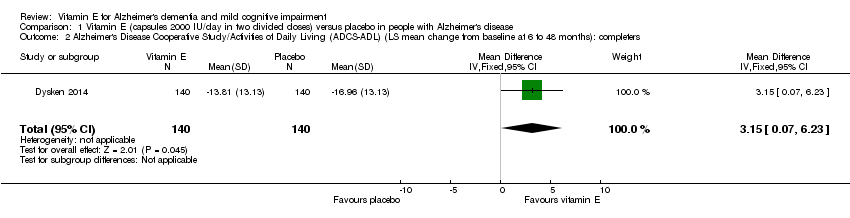
Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 2 Alzheimer's Disease Cooperative Study/Activities of Daily Living (ADCS‐ADL) (LS mean change from baseline at 6 to 48 months): completers.

Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 3 Deaths (number of deaths over 48 months).

Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 4 Serious adverse events (number of participants reporting ≥ 1 serious adverse event over 48 months).
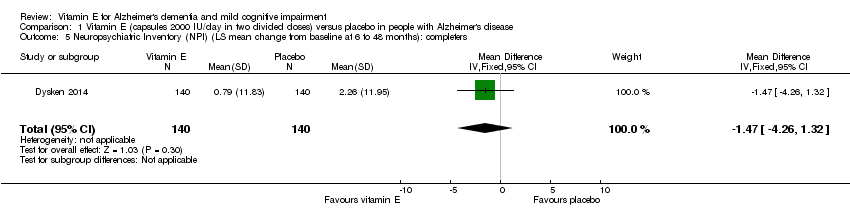
Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 5 Neuropsychiatric Inventory (NPI) (LS mean change from baseline at 6 to 48 months): completers.

Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 6 Mini‐Mental State Examination (MMSE) (LS mean change from baseline at 6 to 48 months): completers.
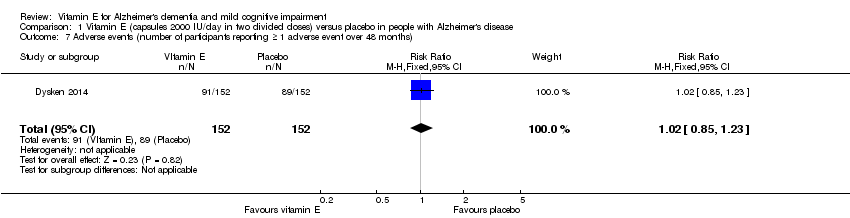
Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 7 Adverse events (number of participants reporting ≥ 1 adverse event over 48 months).
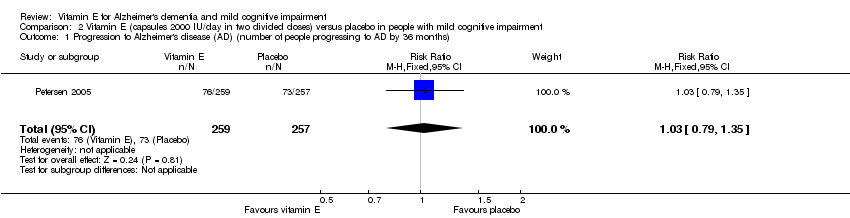
Comparison 2 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with mild cognitive impairment, Outcome 1 Progression to Alzheimer's disease (AD) (number of people progressing to AD by 36 months).
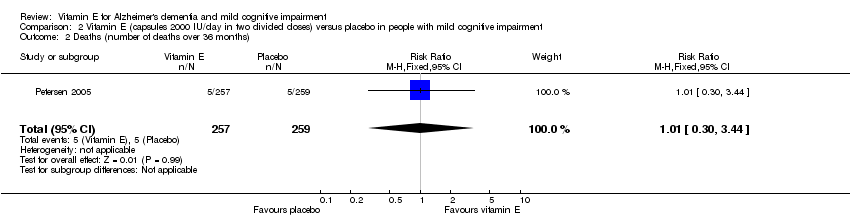
Comparison 2 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with mild cognitive impairment, Outcome 2 Deaths (number of deaths over 36 months).
| Vitamin E (capsules 2000 IU/day in 2 divided doses) compared to placebo for people with Alzheimer's disease | ||||||
| Patient or population: people with Alzheimer's disease Settings: multicentre, US Intervention: vitamin E (capsules 2000 IU/day in 2 divided doses) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Vitamin E (capsules 2000 IU/day in 2 divided doses) | |||||
| Cognitive function Scale from: 0 to 70 Follow‐up: 6 to 48 months | The LS mean change from baseline in cognitive function in placebo group was 7.78 | The LS mean change from baseline in cognitive function in the intervention group was 1.81 lower | ‐ | 272 | ⊕⊕⊕⊝ | Higher scores represent worse cognitive function. A 4‐point difference in ADAS‐cog has been considered the MCID. |
| Adverse events Number of participants reporting ≥ 1 serious adverse event Follow‐up: 6 to 48 months | 625 per 1000 | 538 per 1000 | RR 0.86 | 304 | ⊕⊕⊕⊝ | ‐ |
| Deaths Number of deaths Follow‐up: 6 to 48 months | 204 per 1000 | 171 per 1000 | RR 0.84 | 304 | ⊕⊕⊕⊝ | ‐ |
| Activities of daily living Scale from: 0 to 78 Follow‐up: 6 to 48 months | The LS mean change from baseline in activities of daily living in the placebo group was ‐16.96 | The LS mean change from baseline in activities of daily living in the intervention group was 3.15 higher | ‐ | 280 | ⊕⊕⊕⊝ | Higher scores represent better activities of daily living. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADAS‐Cog: Alzheimer Disease Assessment Scale ‐ Cognitive Subscale; ADCS‐ADL: Alzheimer's Disease Cooperative Study/Activities of Daily Living Inventory;CI: confidence interval; LS: least square; MCID: minimum clinically important difference; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Quality downgraded one level due to imprecision. Evidence from a single study of modest size. This is supported by dichotomous data not reaching the optimal information size criterion (assuming α of 0.05, and β of 0.2) (Guyatt 2011). | ||||||
| Vitamin E (capsules 2000 IU/day in 2 divided doses) compared to placebo for people with mild cognitive impairment | ||||||
| Patient or population: people with mild cognitive impairment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Vitamin E (capsules 2000 IU/day in 2 divided doses) | |||||
| Progression to Alzheimer's disease Number of people progressing to AD Follow‐up: 36 months | 284 per 1000 | 293 per 1000 | RR 1.03 | 516 | ⊕⊕⊕⊝ | ‐ |
| Cognitive function Mean change from baseline of ADAS‐Cog Scale from: 0 to 70 Follow‐up: 36 months | Not possible to extract data for analysis. | ‐ | Not possible to extract data for analysis. | Unable to evaluate quality of evidence. | Uncertainty about how missing data were handled. Study reports no significant difference between intervention and control groups. | |
| Adverse events Number of participants reporting ≥ 1 serious adverse event. Follow‐up: 36 months | Not possible to extract data for analysis. | ‐ | 516 | Unable to evaluate quality of evidence. | Overall adverse event rates not reported. | |
| Death Number of deaths over 36 months Follow‐up: 36 months | 19 per 1000 | 19 per 1000 | RR 1.01 | 516 | ⊕⊕⊕⊝ | ‐ |
| Activities of daily living Mean change from baseline using the ADCS Scale from: 0 to 53 Follow‐up: 36 months | Not possible to extract data for analysis. | ‐ | Not possible to extract data for analysis. | Unable to evaluate quality of evidence. | Uncertainty about how missing data were handled. Study reports no significant difference between intervention and control groups. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADAS‐Cog: Alzheimer Disease Assessment Scale ‐ Cognitive Subscale; ADCS‐ADL: Alzheimer's Disease Cooperative Study/Activities of Daily Living Inventory;CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Quality downgraded one level due to imprecision. Evidence from a single study of modest size. This was supported by dichotomous data not reaching the optimal information size criterion (assuming α of 0.05, and β of 0.2) (Guyatt 2011). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Alzheimer's Disease Assessment Scale ‐ Cognitive subscale (ADAS‐Cog) (least square (LS) mean change from baseline at 6 to 48 months): completers Show forest plot | 1 | 272 | Mean Difference (IV, Random, 95% CI) | ‐1.81 [‐3.75, 0.13] |
| 2 Alzheimer's Disease Cooperative Study/Activities of Daily Living (ADCS‐ADL) (LS mean change from baseline at 6 to 48 months): completers Show forest plot | 1 | 280 | Mean Difference (IV, Fixed, 95% CI) | 3.15 [0.07, 6.23] |
| 3 Deaths (number of deaths over 48 months) Show forest plot | 1 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.52, 1.34] |
| 4 Serious adverse events (number of participants reporting ≥ 1 serious adverse event over 48 months) Show forest plot | 1 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.71, 1.05] |
| 5 Neuropsychiatric Inventory (NPI) (LS mean change from baseline at 6 to 48 months): completers Show forest plot | 1 | 280 | Mean Difference (IV, Fixed, 95% CI) | ‐1.47 [‐4.26, 1.32] |
| 6 Mini‐Mental State Examination (MMSE) (LS mean change from baseline at 6 to 48 months): completers Show forest plot | 1 | 273 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.72, 1.10] |
| 7 Adverse events (number of participants reporting ≥ 1 adverse event over 48 months) Show forest plot | 1 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.85, 1.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Progression to Alzheimer's disease (AD) (number of people progressing to AD by 36 months) Show forest plot | 1 | 516 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.79, 1.35] |
| 2 Deaths (number of deaths over 36 months) Show forest plot | 1 | 516 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.30, 3.44] |

