La vitamine E dans la démence d'Alzheimer et les troubles cognitifs légers
Résumé scientifique
Contexte
La vitamine E est naturellement présente dans le régime alimentaire. Cette substance a plusieurs fonctions biologiques, dont un effet antioxydant permettant de fixer les radicaux libres toxiques. Des preuves indiquant que les radicaux libres pourraient contribuer aux processus pathologiques menant aux troubles cognitifs ont conduit à s'intéresser à l'utilisation de suppléments de vitamine E pour traiter les troubles cognitifs légers (TCL) et la maladie d'Alzheimer (MA). Cet article est une mise à jour d'une revue Cochrane publiée pour la première fois en 2000 et précédemment mise à jour en 2006 et en 2012.
Objectifs
Évaluer l'efficacité de la vitamine E dans le traitement des TCL et de la démence en raison de la MA.
Stratégie de recherche documentaire
Nous avons effectué des recherches dans le registre spécialisé du groupe Cochrane sur la démence et les autres troubles cognitifs (ALOIS), la Bibliothèque Cochrane, MEDLINE, Embase, PsycINFO, CINAHL, LILACS, ainsi que de nombreuses bases de données d'essais et des sources de littérature grise le 22 avril 2016, en utilisant les termes : Vitamin E, vitamin‐E et alpha‐tocopherol.
Critères de sélection
Nous avons inclus tous les essais randomisés en double aveugle dans lesquels un traitement par n'importe quelle dose de vitamine E a été comparé à un placebo chez les patients atteints de MA ou de TCL.
Recueil et analyse des données
Nous avons suivi les procédures méthodologiques standard selon le Cochrane Handbook for Systematic Reviews of Interventions. Nous avons évalué la qualité des preuves en utilisant l'approche GRADE. Lorsque cela était approprié, nous avons essayé de contacter les auteurs afin d'obtenir des informations manquantes.
Résultats principaux
Quatre essais remplissaient les critères d'inclusion, mais nous n'avons pu extraire des données quant aux résultats conformément à notre protocole que pour deux essais, l'un dans une population de personnes ayant une démence de type Alzheimer (n = 304) et l'autre dans une population de personnes ayant des TCL (n = 516). Les deux essais présentaient globalement un risque de biais faible à incertain. Il n'a pas été possible de combiner les données de plusieurs études en raison d'un manque de résultats comparables.
Chez les patients atteints de MA, nous n'avons trouvé aucune preuve d'un quelconque effet cliniquement important de la vitamine E sur la cognition, mesuré par rapport aux valeurs initiales sur l'échelle Alzheimer's Disease Assessment Scale‐ Cognitive subscale (ADAS‐Cog) sur une période de six à 48 mois (différence moyenne (DM) ‐1,81, intervalle de confiance à 95 % (IC) ‐3,75 à 0,13, P = 0,07, 1 étude, n = 272 ; preuves de qualité modérée). Il n'y avait aucune preuve d'une différence entre la vitamine E et les groupes sous placebo dans le risque de subir au moins un événement indésirable grave sur six à 48 mois (risque relatif (RR) 0,86, IC à 95 % 0,71 à 1,05, P = 0,13, 1 étude, n = 304 ; preuves de qualité moyenne), ou dans le risque de décès (RR 0,84, IC à 95 % 0,52 à 1,34, P = 0,46, 1 étude, n = 304 ; preuves de qualité modérée). Les patients atteints de MA recevant de la vitamine E ont montré un plus faible déclin fonctionnel sur l'inventaire Alzheimer's Disease Cooperative Study/Activities of Daily Living que les personnes recevant un placebo au bout de six à 48 mois (différence moyenne (DM) 3,15, IC à 95 % 0,07 à 6,23, P = 0,04, 1 étude, n = 280 ; preuves de qualité modérée). Il n'y avait aucune preuve d'un quelconque effet cliniquement important sur les symptômes neuropsychiatriques mesurés avec l'Inventaire Neuropsychiatrique (DM ‐1,47, IC à 95 % ‐4,26 à 1,32, P = 0,30, 1 étude, n = 280 ; preuves de qualité modérée).
Nous n'avons trouvé aucune preuve indiquant que la vitamine E affectait la probabilité d'une progression d'un TCL vers une probable démence due à la MA sur une période de 36 mois (RR 1,03, IC à 95 % 0,79 à 1,35, P = 0,81, 1 étude, n = 516 ; preuves de qualité modérée). Cinq décès sont survenus à la fois dans le groupe de la vitamine E et dans le groupe sous placebo sur les 36 mois (RR 1,01, IC à 95 % 0,30 à 3,44, P = 0,99, 1 étude, n = 516 ; preuves de qualité modérée). Nous n'avons pas été en mesure d'extraire des données conformément au protocole de la revue pour les autres critères de jugement. Cependant, les auteurs de l'étude n'ont trouvé aucune preuve indiquant que la vitamine E différait par rapport au placebo en termes d'effet sur la fonction cognitive, la gravité globale ou les activités de la vie quotidienne. Il n'y avait aucune preuve d'une différence entre les groupes quant aux événements indésirables les plus fréquemment rapportés.
Conclusions des auteurs
Nous n'avons trouvé aucune preuve indiquant que la vitamine E sous forme d'alpha‐tocophérol administrée aux personnes atteintes de TCL prévient la progression vers la démence, ou qu'elle améliore la fonction cognitive chez les personnes atteintes de TCL ou de démence de type Alzheimer. Cependant, il existe des preuves de qualité moyenne issues d'une étude unique indiquant que la vitamine E pourrait ralentir le déclin fonctionnel dans la MA. La vitamine E n'était pas associée à un risque accru d'événements indésirables graves ou à une augmentation de la mortalité dans les essais inclus dans cette revue. Ces conclusions ont changé depuis la dernière mise à jour ; cependant, celles‐ci sont encore basées sur un petit nombre d'essais et de participants et d'autres recherches sont très susceptibles de modifier ces résultats.
PICO
Résumé simplifié
L'utilisation de la vitamine E dans le traitement des troubles cognitifs légers et de la maladie d'Alzheimer (MA)
Contexte
La vitamine E est présente dans une variété d'aliments, notamment dans les huiles végétales et les graisses, les noix et les graines. Certaines études animales non‐interventionnelles ont suggéré que la vitamine E pourrait jouer un rôle dans la prévention ou le traitement de la maladie d'Alzheimer (MA). Cependant, des preuves ont montré un lien entre la vitamine E et des effets secondaires potentiellement graves et même un risque accru de décès. Dans cette revue, nous avons examiné les preuves concernant l'effet de la vitamine E chez les personnes ayant soit une démence due à la MA ou des problèmes légers au niveau de la mémoire ou de la pensée (les troubles cognitifs légers ou TCL). Les personnes atteintes de TCL ont un risque accru de développer une démence.
Essais inclus
Nous avons recherché des essais cliniques publiés jusqu'en avril 2016 qui avaient aléatoirement assignés des personnes atteintes de démence de type Alzheimer ou atteintes de TCL à un traitement à base de suppléments de vitamine E ou à un placebo (un traitement factice). Nous avons identifié trois essais qui ont étudié les effets de la vitamine E chez les personnes souffrant de la MA, mais nous n'avons pu extraire des données que pour un seul de ces essais (304 participants). Nous n'avons trouvé qu'un seul essai avec 516 participants qui avait étudié les effets de la vitamine E chez les personnes atteintes de TCL. La qualité de ces deux essais était généralement bonne.
Résultats
La vitamine E n'a pas permis de réduire le nombre de personnes atteintes de TCL ayant développé une démence sur trois ans. Nous n'avons également pas trouvé de preuves indiquant que la vitamine E améliore la fonction cognitive (par ex. la mémoire et l'apprentissage) chez les personnes atteintes de TCL ou de démence de type Alzheimer. Un essai indiquait que les personnes atteintes de démence de type Alzheimer ayant pris de la vitamine E pourraient mieux gérer leurs activités quotidiennes (par ex. se baigner, s'habiller) comparé aux personnes ayant pris un placebo. Il n'y avait aucune preuve dans ces essais indiquant que la vitamine E avait causé des problèmes significatifs aux participants, mais ces types d'essais ne sont pas le meilleur moyen pour rechercher les effets néfastes, sauf si ceux‐ci sont très fréquents. Etant donné que les résultats provenaient tous d'essais uniques, il est probable que de futures recherches pourraient conduire à des conclusions différentes.
Conclusion
A partir de preuves limitées, nous n'avons pas trouvé d'indication qui suggère des effets bénéfiques ou délétères des suppléments de vitamine E. La qualité des preuves était modérée, d'autres essais sont nécessaires pour confirmer ces résultats. Il est possible que différents types ou doses de vitamine E puissent avoir des effets différents.
Authors' conclusions
Summary of findings
| Vitamin E (capsules 2000 IU/day in 2 divided doses) compared to placebo for people with Alzheimer's disease | ||||||
| Patient or population: people with Alzheimer's disease Settings: multicentre, US Intervention: vitamin E (capsules 2000 IU/day in 2 divided doses) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Vitamin E (capsules 2000 IU/day in 2 divided doses) | |||||
| Cognitive function Scale from: 0 to 70 Follow‐up: 6 to 48 months | The LS mean change from baseline in cognitive function in placebo group was 7.78 | The LS mean change from baseline in cognitive function in the intervention group was 1.81 lower | ‐ | 272 | ⊕⊕⊕⊝ | Higher scores represent worse cognitive function. A 4‐point difference in ADAS‐cog has been considered the MCID. |
| Adverse events Number of participants reporting ≥ 1 serious adverse event Follow‐up: 6 to 48 months | 625 per 1000 | 538 per 1000 | RR 0.86 | 304 | ⊕⊕⊕⊝ | ‐ |
| Deaths Number of deaths Follow‐up: 6 to 48 months | 204 per 1000 | 171 per 1000 | RR 0.84 | 304 | ⊕⊕⊕⊝ | ‐ |
| Activities of daily living Scale from: 0 to 78 Follow‐up: 6 to 48 months | The LS mean change from baseline in activities of daily living in the placebo group was ‐16.96 | The LS mean change from baseline in activities of daily living in the intervention group was 3.15 higher | ‐ | 280 | ⊕⊕⊕⊝ | Higher scores represent better activities of daily living. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADAS‐Cog: Alzheimer Disease Assessment Scale ‐ Cognitive Subscale; ADCS‐ADL: Alzheimer's Disease Cooperative Study/Activities of Daily Living Inventory;CI: confidence interval; LS: least square; MCID: minimum clinically important difference; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Quality downgraded one level due to imprecision. Evidence from a single study of modest size. This is supported by dichotomous data not reaching the optimal information size criterion (assuming α of 0.05, and β of 0.2) (Guyatt 2011). | ||||||
| Vitamin E (capsules 2000 IU/day in 2 divided doses) compared to placebo for people with mild cognitive impairment | ||||||
| Patient or population: people with mild cognitive impairment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Vitamin E (capsules 2000 IU/day in 2 divided doses) | |||||
| Progression to Alzheimer's disease Number of people progressing to AD Follow‐up: 36 months | 284 per 1000 | 293 per 1000 | RR 1.03 | 516 | ⊕⊕⊕⊝ | ‐ |
| Cognitive function Mean change from baseline of ADAS‐Cog Scale from: 0 to 70 Follow‐up: 36 months | Not possible to extract data for analysis. | ‐ | Not possible to extract data for analysis. | Unable to evaluate quality of evidence. | Uncertainty about how missing data were handled. Study reports no significant difference between intervention and control groups. | |
| Adverse events Number of participants reporting ≥ 1 serious adverse event. Follow‐up: 36 months | Not possible to extract data for analysis. | ‐ | 516 | Unable to evaluate quality of evidence. | Overall adverse event rates not reported. | |
| Death Number of deaths over 36 months Follow‐up: 36 months | 19 per 1000 | 19 per 1000 | RR 1.01 | 516 | ⊕⊕⊕⊝ | ‐ |
| Activities of daily living Mean change from baseline using the ADCS Scale from: 0 to 53 Follow‐up: 36 months | Not possible to extract data for analysis. | ‐ | Not possible to extract data for analysis. | Unable to evaluate quality of evidence. | Uncertainty about how missing data were handled. Study reports no significant difference between intervention and control groups. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADAS‐Cog: Alzheimer Disease Assessment Scale ‐ Cognitive Subscale; ADCS‐ADL: Alzheimer's Disease Cooperative Study/Activities of Daily Living Inventory;CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Quality downgraded one level due to imprecision. Evidence from a single study of modest size. This was supported by dichotomous data not reaching the optimal information size criterion (assuming α of 0.05, and β of 0.2) (Guyatt 2011). | ||||||
Background
Description of the condition
Dementia due to Alzheimer's disease (AD) is a progressive neurodegenerative condition in which people develop deficits in multiple cognitive domains, including memory, language and executive functioning, as well as a variety of emotional and behavioural symptoms. This leads to progressive functional impairment. AD causes huge emotional and financial burden to people, carers, and health and social care systems. Current treatments for AD have limited efficacy and cannot prevent progression of the condition. It is projected that 5.2% of the world's population will have dementia by 2050, equating to a worldwide prevalence of 131.5 million cases (Prince 2015).
Mild cognitive impairment (MCI) is a condition of cognitive deficits which are not accompanied by significant impairment in the performance of activities of daily living and hence do not meet the diagnostic criteria for dementia. People with MCI are at increased risk of developing dementia (Ganguli 2011), although this is not considered inevitable (Bruscoli 2004). In 2011, it was estimated that between 10% and 20% of people over the age of 65 years meet criteria for MCI (Petersen 2011).
Description of the intervention
Vitamin E is a generic term for a group of eight naturally occurring, fat‐soluble chemical derivatives of tocopherol and tocotrienol. It occurs naturally in a variety of food substances including vegetable oils and fats, and in nuts and seeds, such as almonds and sunflower seeds. Alpha‐tocopherol is the most commonly studied compound and it has been the only form of vitamin E used in clinical trials in AD. According to the Food and Nutrition Board (FNB) at the Institute of Medicine of the National Academies (US), the recommended daily allowance for men and women over 14 years of age is 22.4 IU (15 mg of alpha‐tocopherol) (National Academy of Sciences 2000).
Vitamin E has a number of biological activities which vary with the form of vitamin E (Boccardi 2016). One of its functions is to act as a scavenger of free radicals by working as an antioxidant (Uneri 2006). Oxygen free radicals contain oxygen atoms with unpaired electrons and are highly reactive, damaging proteins, DNA and cell membranes unless rapidly 'quenched' by antioxidants. They are produced as by‐products of the body's metabolism as well as by radiation. As vitamin E is lipophilic, it can protect cell membranes and plasma lipoproteins from peroxyl radicals, which react preferentially with vitamin E (Traber 2011). In particular, vitamin E is thought to inhibit the process of lipid peroxidation, which damages the polyunsaturated fatty acids essential to the integrity of cell membranes. Vitamin E's activity in protecting against the harmful effects of free radicals may be measured indirectly by assessing the state of the antioxidant‐oxidant system. Endogenous antioxidants such as glutathione provide an indication of the antioxidant status while the oxidised form of glutathione, glutathione disulphide (GSSG), and malondialdehyde (MDA) may provide an indication of the oxidative stress status and in particular lipid peroxidation.
High doses of vitamin E (over 3000 IU/day) are considered toxic, and have been implicated in a variety of symptoms including fatigue, gastrointestinal cramps and diarrhoea. Further, there is an increasing body of evidence that vitamin E supplementation can cause adverse effects, even at lower doses. For example, vitamin E increases bleeding tendency and can potentiate the effect of aspirin (Steiner 1995). One meta‐analysis found that vitamin E supplementation, while reducing risk of ischaemic stroke by 10%, increased the risk of haemorrhagic stroke by 22% (Schürks 2010). Evidence from the Selenium and Vitamin E Cancer Prevention Trial (SELECT) suggests that daily supplementation with vitamin E 400 IU in healthy men may significantly increase the risk of prostate cancer (Klein 2011), and there is evidence from the Heart Outcomes Prevention Evaluation (HOPE) trial that vitamin E supplementation increases the risk of heart failure and hospitalisation for heart failure in people over 55 years of age with diabetes mellitus and vascular disease (Lonn 2005). Miller 2005 carried out a meta‐analysis of 19 clinical trials testing vitamin E alone or in combination with other supplements. The median dose of vitamin E in these trials was 400 IU/day although the maximum dose was up to 2000 IU/day. The authors reported a statistically significant dose‐dependent relationship between vitamin E intake and all‐cause mortality. One Cochrane meta‐analysis also found that vitamin E given singly or combined with other antioxidants significantly increased mortality in 26 trials (risk ratio (RR) 1.04) (Bjelakovic 2010).
How the intervention might work
There are both theoretical arguments and empirical findings to suggest that free radical damage may be one of the mechanisms causing neuronal degeneration in a range of conditions including ageing, MCI and AD. This evidence was summarised by Grundman 2000. Many studies have found evidence of increased level of oxidative damage to neurons and mitochondrial DNA in AD and MCI (Butterfield 2002; Hensley 1995; Marcus 1998; Mecocci 1994; Mecocci 2004; Wang 2005; Wang 2006). Antioxidants, including vitamin E, improve cognitive functioning of aged rodents (Socci 1995), and protect against the effects of brain ischaemia (Hara 1990) and of some neurotoxins (Wortwein 1994). Transgenic mice overexpressing the amyloid precursor protein, implicated in AD, showed accelerated age‐associated brain degeneration (Hsiao 1995). Vitamin E can delay this deterioration (Behl 1992; Koppal 1998; Zhou 1996), and decrease oxidative DNA damage (Boothby 2005).
Central nervous tissue contains a high proportion of fatty material (lipid), and since vitamin E is fat‐soluble it can readily enter the brain (Vatassery 1988). In older‐adult population‐based studies, there have been reports of an association between low blood levels of vitamin E and impaired cognitive function (Cherubini 2005; Perrig 1997). Mean levels of vitamin E in the blood and cerebrospinal fluid of people with AD were reported to be lower than normal in several reports (Jeandel 1989; Jimenez‐Jimenez 1997; Polidori 2002; Tohgi 1994; Zaman 1992), though not in all (Ahlskog 1995). Lower levels of both forms of vitamin E ‐ tocotrienols and tocopherols ‐ have been observed in the plasma of people with AD and MCI (Mangialasche 2012). However, lower levels of vitamin E in AD may be a consequence of the disease rather than a cause of it, due in part to an overall decreased general dietary intake especially as the disease advances (Tabet 2001; Tabet 2002).
Results from clinical trials of vitamin E in non‐AD neurodegenerative disorders have not been promising. For example, neither people with Parkinson's disease (Parkinson's SG 1993; Pham 2005) nor Huntington's disease (Peyser 1995) have shown a significant overall benefit from vitamin E supplementation. In this review, we focused specifically on the role of vitamin E in the treatment of AD and MCI.
Why it is important to do this review
It is estimated that 46.8 million people worldwide have a diagnosis of dementia, and this is predicted to rise to 131.5 million cases by 2050 (Prince 2015), with AD being the most prevalent subtype. At present, there is no effective treatment for MCI or for dementia due to AD. Vitamin E continues to be used by some people as a non‐prescription supplement mainly because of its widely reported antioxidant properties and some previous positive findings. However, due to the potential health risks of taking vitamin E, it is important to determine whether vitamin E is an effective treatment of AD and MCI. This is an update of an earlier Cochrane Review, which was originally published in 2000 and was last updated in 2012. This review involved an updated search of the literature as well as changes to the conclusion, which better reflect emerging findings from vitamin E clinical trials in AD.
Objectives
To assess the efficacy of vitamin E in the treatment of MCI and dementia due to AD.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised, double‐blind trials in which vitamin E was compared with placebo in the treatment of people with MCI or dementia due to AD, or both.
Types of participants
Participants with AD were diagnosed with probable AD according to internationally accepted diagnostic criteria including NINCDS‐ADRDA (National Institute of Neurological and Communicative Disorders and Stroke‐Alzheimer's Disease and Related Disorders Association (McKhann 1984), Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV) (APA 1994), or International Statistical Classification of Diseases and Related Health Problem, Tenth Revision (ICD‐10). In the absence of consensus criteria for MCI, we accepted MCI defined according to published criteria such as those by Petersen 2005, or any other criteria with face validity. If only a subset of the study population was eligible for inclusion, then we sought and included data relating only to the eligible participants.
Types of interventions
We included studies using any dosage of vitamin E or any of its constituent tocopherols or tocotrienols. A placebo comparator was required. Co‐administration of another drug with vitamin E was permitted if the same drug was also taken by the placebo group. There was no restriction on the dosage, mode of delivery or duration of the vitamin E intervention.
Types of outcome measures
We sought data on the primary and secondary outcomes listed below. For continuous outcomes, we included only data derived from validated, published scales.
Primary outcomes
-
For MCI studies, development of, or time to development of, possible or probable AD.
-
Cognitive function.
-
Adverse events
-
Death.
Secondary outcomes
-
Global measure of dementia severity and deterioration.
-
Behavioural disturbance.
-
Mood.
-
Activities of daily living.
-
Carer burden.
-
Quality of life.
-
Permanent physical disability.
-
Institutionalisation.
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois) ‐ the Cochrane Dementia and Cognitive Improvement Group's Specialized Register on 22 April 2016. The search terms used were: "Vitamin E", vitamin‐E, alpha‐tocopherol.
ALOIS is maintained by the Trials Search Co‐ordinator of the Cochrane Dementia and Cognitive Improvement Group and contains studies in the areas of dementia prevention, dementia treatment and cognitive enhancement in healthy people. The studies are identified from:
-
monthly searches of a number of major healthcare databases: MEDLINE, Embase, CINAHL, PsycINFO and LILACS;
-
monthly searches of a number of trial registers: ISRCTN; UMIN (Japan's Trial Register); the World Health Organization (WHO) portal (which covers ClinicalTrials.gov; ISRCTN; the Chinese Clinical Trials Register; the German Clinical Trials Register; the Iranian Registry of Clinical Trials and the Netherlands National Trials Register, plus others);
-
quarterly searches of the Cochrane Library’s Central Register of Controlled Trials (CENTRAL);
-
six‐monthly searches of a number of grey literature sources: ISI Web of Knowledge Conference Proceedings; Index to Theses and Australasian Digital Theses.
To view a list of all sources searched for ALOIS see About ALOIS on the ALOIS website.
Details of the search strategies used for the retrieval of reports of trials from the healthcare databases, CENTRAL and conference proceedings can be viewed in the 'methods used in reviews' section within the editorial information about the Dementia and Cognitive Improvement Group.
Additional searches were performed in the sources listed above to cover the timeframe from the last searches performed for ALOIS to ensure that the search for the review was as up‐to‐date and as comprehensive as possible. The search strategies used can be seen in Appendix 1.
Searching other resources
We reviewed reference lists of included studies to identify any additional studies.
Data collection and analysis
Selection of studies
Two review authors (NF and DL) independently examined the title and abstract of the papers identified by the search and selected and retrieved them for their relevance to the review. We resolved any disagreements concerning inclusion by discussion among all review authors until a decision was made.
Data extraction and management
Two review authors (NF and DL for descriptive data, NF and NT for outcome data) independently extract data for all authors. We attempted to collect the following data:
-
report ‐ author, year and source of publication;
-
study ‐ study setting;
-
participants ‐ demographics, diagnostic criteria for AD or MCI, exclusion criteria, other concomitant medical conditions or medications that may affect cognition;
-
research design and features ‐ sampling mechanism, treatment assignment mechanism, blinding, dropout rates, length of follow‐up, pertinent design features (e.g. cross‐over design);
-
intervention ‐ type, duration, dose, timing, mode of delivery;
-
outcome measures;
-
results ‐ number of participants randomised, outcome data.
For each outcome measure, we sought data on every participant randomised We preferred intention‐to‐treat (ITT) data but if these were not available, we extracted data on participants who completed treatment. We did not extract data for any non‐randomised titration periods or any open‐label follow‐on phases.
For continuous data, we extracted the means, standard deviation (SD) and number of participants in each treatment group at each time point. We extracted change from baseline data if endpoint data were unavailable. For binary data, the data extracted was the number of participants with each outcome in each treatment group at each time point. For ordinal data, there were two possible approaches. If ordinal scale data appeared to be approximately normally distributed or if the analysis that the investigators performed suggested that parametric tests were appropriate, then ewe treated the outcome measures as continuous data. The second approach, which may not exclude the first, was to concatenate into two categories that best represent the contrasting states of interest, and to treat the variable as binary. For time‐to‐event data, we used a hazard ratio (HR).
Assessment of risk of bias in included studies
Two review authors (NF and DL) assessed risk of bias for each included study independently. This was assessed based on guidance from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the following criteria:
-
sequence generation;
-
allocation concealment;
-
blinding (participants);
-
blinding (investigators);
-
incomplete outcome reporting;
-
selective outcome reporting.
For each study, we gave a risk of bias rating ('Low risk', 'Unclear risk' or 'High risk') for each of the above criteria. Empirical research has shown that lack of adequate allocation concealment may be associated with bias. Trials with unclear concealment measures have been shown to yield more pronounced estimates of treatment effects than trials that have taken adequate measures to conceal allocation schedules, but less pronounced than inadequately concealed trials (Chalmers 1983; Schulz 1995). Thus, we included trials if they conformed to 'Low risk' or 'Unclear risk' allocation risk categories, while we excluded trials falling into the 'High risk' category.
Measures of treatment effect
For studies with continuous outcome measures, we calculated mean differences (MD) or standardised mean differences (SMD) with 95% confidence intervals (CI). For binary outcome measures, we calculated RRs. For time‐to‐event outcome measures, we calculated HRs.
Unit of analysis issues
The participant was the unit of analysis.
Dealing with missing data
An ITT analysis was carried out where possible. In cases where only completers' data were available, we explored the impact of the missing data on the findings, where possible. When appropriate, the review authors contacted relevant study authors to request missing information.
Assessment of heterogeneity
Owing to the clearly significant clinical and methodological heterogeneity between studies, we performed no statistical tests for heterogeneity. As such, data did not lend itself to performing a meta‐analysis. However, we carried out critical interpretive synthesis of data for individual studies.
Assessment of reporting biases
There were an insufficient number of studies identified to make an overall quantitative assessment. We assessed biases for individual studies.
Data synthesis
It was not possible to synthesise and compare data across studies as there were no comparable outcome measures. We carried out a critical interpretive synthesis of data for individual studies separately. We included 'Summary of findings' tables.
Subgroup analysis and investigation of heterogeneity
Although not specified in the protocol, we extracted and reported data for a subgroup analysis carried out in one included study. The subgroups were participants who did or did not show a decline in markers of oxidative stress in response to vitamin E treatment. This subgroup was considered important to include as a reduction of oxidative stress is thought to be the mechanism by which vitamin E may affect cognition.
Sensitivity analysis
Sensitivity analysis was planned if a sufficient number of comparable studies were identified.
Presentation of results ‐ 'Summary of findings' tables
We used the GRADE approach to assess the quality of the supporting evidence behind the treatment effects presented in this review (Guyatt 2011). For each comparison, we presented key outcomes in a 'Summary of findings' table including, for each outcome, a summary of the amount of data, the magnitude of the effect size and the quality of the evidence.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
Only four trials met the inclusion criteria: Dysken 2014; Lloret 2009;Petersen 2005; and Sano 1996. Three were trials of alpha‐tocopherol (vitamin E) for the treatment of dementia due to AD (Dysken 2014; Lloret 2009; Sano 1996) and one was a trial of alpha‐tocopherol to delay progression from amnestic MCI to dementia (Petersen 2005). See Figure 1 for the study flow diagram of included studies.
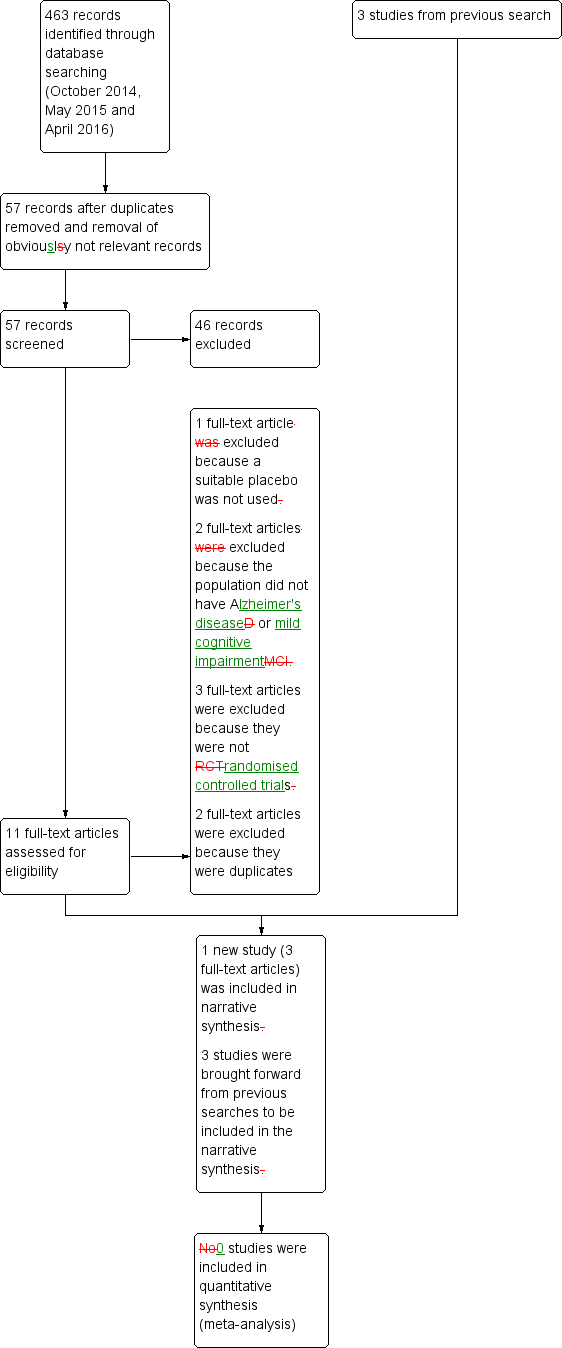
Study flow diagram for study for the search April 2016.
Included studies
Treatment of dementia due to Alzheimer's disease
The primary purpose of the first treatment study was to determine whether alpha‐tocopherol (vitamin E), selegiline (a monoamine oxidase inhibitor) or a combination of the two agents would slow the clinical deterioration associated with AD (Sano 1996). There were four groups: placebo, vitamin E (2000 IU total daily dose divided into two doses), selegiline and selegiline plus vitamin E. The study recruited 341 participants with a diagnosis of probable AD dementia of moderate severity (Clinical Dementia Rating (CDR) 2 (Berg 1988)) from 23 centres in the US. We considered only the placebo (n = 84) and vitamin E (n = 85) groups in this review. Assessments were conducted one month after enrolment and at three‐monthly intervals for two years. The primary outcome was the survival time to any one of four end points; death, institutionalisation, change in severity of dementia to a CDR of three or loss of two basic activities of daily living. After the primary end point was reached, every effort was made to continue further assessment of the secondary outcomes if possible. The secondary outcomes were the Cognitive section of the Alzheimer's Disease Assessment Scale (ADAS‐Cog) (Rosen 1984), the Mini‐Mental State Examination (MMSE) (Folstein 1975), the Blessed Dementia Scale (BDS) (Blessed 1968), the Dependence Scale (DS) (Stern 1994), the Behavior Rating Scale for Dementia (BRSD) (Tariot 1995), and adverse events. The primary analysis compared survival to end point for each of the three treatment groups in comparison with the placebo group, using Kaplan‐Meier estimation and log‐rank tests with no correction for other factors or variables, and the Cox proportional hazards model including covariates. A small number of participants were lost to follow‐up without having reached a primary end point (placebo 6/84, vitamin E 8/85).
The purpose of the second treatment study was to explore the effects of vitamin E on AD progression and markers of oxidative stress (Lloret 2009). Vitamin E 800 IU or placebo was given daily for six months. There were 57 participants diagnosed with probable AD. All AD participants were taking a cholinesterase inhibitor and were not taking any other antioxidant medication. Assessments were conducted on the first day of enrolment and after six months. Oxidative stress was assessed in two ways. The first method used blood concentrations of the oxidised glutathione molecule, GSSG, with greater levels indicating greater oxidative stress. GSSG was also assessed as a ratio with the antioxidant molecule, glutathione. The second method measured the blood marker MDA as a marker of lipid peroxidation, which is a strong indicator of oxidative damage. AD progression was measured by the Clock Drawing Test (Sunderland 1989), the BDS and MMSE. The primary analysis was to compare performance on the cognitive tasks in relation to the effects of vitamin E on oxidative stress. The effects of vitamin E on oxidative stress markers were investigated using a Mann‐Whitney test to compare GSSG and MDA values at the beginning and the end of the study in both the placebo and active treatment groups. Vitamin E‐treated participants were divided into responders and non‐responders according to whether vitamin E was effective or not in reducing markers of oxidative stress. The responder, non‐responder and placebo group means were compared using a Kruskal‐Wallis test. Comparison between groups was also undertaken following removal of participants with cerebrovascular disease. To determine the effect of modifying oxidative stress on cognitive performance, the relationship between MMSE change and GSSG change in participants treated with vitamin E was assessed using Spearman's coefficient of correlation. We contacted two authors of the Lloret 2009 study for additional information, but following an initial response from one author, we received no additional responses.
The purpose of the third treatment study was to determine if alpha tocopherol (vitamin E), memantine, or both slowed the progression of mild to moderate AD (Dysken 2014). In a double‐blind, placebo‐controlled randomised controlled trial (RCT), 613 people with mild to moderate AD were randomly assigned to one of four groups: alpha tocopherol (2000 IU total daily divided into two doses), memantine 20 mg/day, alpha tocopherol plus memantine or placebo. We considered only the placebo (n = 152) and vitamin E (n = 152) groups in this review. All participants were recruited in the US and had a diagnosis of possible or probable AD of mild to moderate severity, as defined by a MMSE score between 12 and 26. Duration of treatment ranged from six months to four years and participants were assessed every six months. The primary outcome was the Alzheimer's Disease Cooperative Study/Activities of Daily Living (ADCS‐ADL) scale (Galasko 2006). Secondary outcomes were the ADAS‐Cog, Neuropsychiatric Inventory (NPI) (Cummings 1994), the Caregiver Activity Survey (CAS) (Davis 1997), the DS and adverse events. Participants and their carers were asked specifically about participant falls, syncope and congestive heart failure as these were linked to high‐dose alpha tocopherol in previous trials (Lonn 2005; Sano 1996). Serious adverse events were coded on the basis of Medical Dictionary for Regulatory Activities. A longitudinal repeated‐measure mixed‐effects model adjusted for medical centre as a random effect and for baseline ADCS‐ADL.
Prevention of progression of cognitive impairment
The primary purpose of the single MCI study was to determine whether treatment with vitamin E 2000 IU/day or donepezil in people with the amnestic form of MCI decreased the conversion rate into dementia due to AD (Petersen 2005). There were three treatment groups: vitamin E, donepezil and placebo. All participants were also taking a multivitamin. This review included the vitamin E and the placebo groups only. Out of 769 participants enrolled from 69 sites in the US and Canada, 516 received either placebo or vitamin E. The secondary outcomes were scores on various measures: the MMSE (Folstein 1975), ADAS‐Cog (Rosen 1984), CDR (Sum of Boxes), the Alzheimer's Disease Assessment Scale Mild Cognitive Impairment Activities of Daily Living (ADCS‐MCI‐ADL) scale, the Global Deterioration Scale, a neuropsychological battery and adverse events. For further details about the study design refer to the Characteristics of included studies table.
The relevant primary analysis in the paper used the Cox proportional hazards model to determine whether there was a significant reduction in time to progression to AD among participants treated with vitamin E compared with participants given placebo. Participants were included on an ITT basis. Several baseline variables were included in the analysis as covariates. The secondary outcomes were examined using analysis of covariance for the change in scores without correction for multiple comparisons. Missing values were imputed using a projection method considered appropriate for assessing responses among people with neurodegenerative diseases (Aisen 2003).
Excluded studies
Following the latest search (April 2016), we initially excluded 46 articles based on the screening of the abstract and title. Of the 11 remaining articles, we excluded one because it did not use a suitable control intervention for our question (Naeini 2014), two because the study population was ineligible (Kryscio 2013a; MacPherson 2012), and three because they were not RCTs (Corbett 2014; Evans 2014; Hermann 2014). The five remaining articles all described one study which was eligible for inclusion (Dysken 2014), however, we subsequently excluded two because they were identified as duplicates. Details of the excluded studies are provided in Characteristics of excluded studies table.
Risk of bias in included studies
See Figure 2 for the summary of the risk of bias, and Figure 3 for the 'Risk of bias' graph. See Characteristics of included studies table for further details of included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
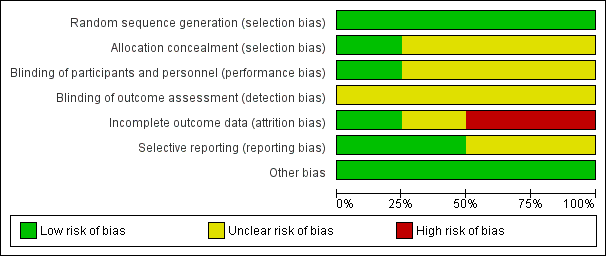
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
We judged that all four studies had a low risk of bias in terms of random sequence generation.
Only Dysken 2014 sufficiently described their method of allocation concealment. In the other three studies, the means of ensuring allocation concealment was unclear. However, we considered it sufficiently likely that allocation was concealed to include the studies.
Blinding
All studies report that they were double‐blinded, though no study described how this was maintained throughout the studies or whether blinding was successful. However, Dysken 2014 did use placebo capsules with identical appearance to the vitamin E capsules, so we considered this to have a low risk of bias. We judged the remaining studies to have an unclear risk of performance bias.
We considered all studies at unclear risk of detection bias because of a lack of information on blinding of outcome assessors.
Incomplete outcome data
Missing data was balanced across groups in Dysken 2014, thus we judged risk of bias to be low. Petersen 2005 reported using an ITT analysis with an appropriate imputation method to deal with missing data and stated that demographic and neuropsychological outcomes did not differ significantly between missing and non‐missing participants. However, it was difficult to tell how the authors had handled data from participants who had developed dementia and switched to open‐label donepezil. Only 42% of participants randomised to vitamin E and 46% randomised to placebo completed the study on their allocated treatment. We judged the study to have an unclear risk of attrition bias. Sano 1996 reported the attrition rate but did not give reasons. In addition, Sano 1996 only reported attrition up to the point a participant reached one of their primary end points, therefore we judged it to be at high risk of bias for our outcomes of interest. Lloret 2009 reported a very high attrition rate and did not give reasons; we judged it to be at high risk of bias.
Selective reporting
All studies reported outcomes stated in their protocol although often in insufficient detail for our purposes. Lloret 2009 tended to report significance values alone, without means and SDs. Similarly, Sano 1996 did not report SDs for the changes in score of their secondary outcomes. Thus, we judged the Lloret 2009 and Sano 1996 studies to have an unclear risk of bias in this domain. We judged the remaining studies to have a low risk of reporting bias.
Other potential sources of bias
We found no other sources of bias.
Effects of interventions
See: Summary of findings for the main comparison Vitamin E (capsules 2000 IU/day in two divided doses) compared to placebo for people with Alzheimer's disease; Summary of findings 2 Vitamin E (capsules 2000 IU/day in two divided doses) compared to placebo for people with mild cognitive impairment
Vitamin E for dementia due to Alzheimer's disease
Three studies assessed the efficacy of vitamin E in people with dementia due to AD (Dysken 2014; Lloret 2009; Sano 1996). For a summary of findings related to the treatment of AD, see summary of findings Table for the main comparison.
Sano 1996 had four treatment groups of which the groups receiving placebo only and vitamin E only were relevant to this review. Six of 84 participants in the placebo group and 8 of 85 participants in the vitamin E group were lost to follow‐up without reaching one of the predefined primary end points of death, institutionalisation, change in severity of dementia to a CDR score of three, or loss of two basic activities of daily living, but there was no information on the time points at which these participants were lost or on how many participants were lost to follow‐up after a primary end point.
Lloret 2009 divided participants treated with vitamin E into two groups depending on changes in oxidative stress markers with treatment. These groups had not been defined in advance of data collection. Participants who showed a decrease in GSSG values of more than 10 nmol/mL of blood after six months of vitamin E treatment were termed 'responders' and participants who did not were termed 'non‐responders'. They reported 'completers' results only (33/57 randomised participants).
Dysken 2014 had four treatment groups, but we evaluated only data for the vitamin E and placebo groups for this review (Dysken 2014). There was a wide range of durations of follow‐up within the trial (six to 48 months), with participants completing at least one follow‐up being included in the analysis. The final analysis included 140/152 (92.1%) participants in the vitamin E group and 140/152 (92.1%) participants in the placebo group. The authors calculated a least squares (LS) mean change from baseline in each treatment group and reported this with its standard error (SE). Dysken 2014 also reported results as a mean annual rate of change. For each relevant continuous outcome variable, we extracted the LS mean change from baseline, and derived SDs from reported SEs. We then calculated the MD (with 95% CI) between vitamin E and placebo groups.
Primary outcomes
Cognitive function
We were unable to extract the data we had specified for cognitive function for Sano 1996. Although the ADAS‐Cog and MMSE were secondary outcomes in the trial, only mean change scores to the time point where a participant was last assessed were reported and participants had been assessed over varying and unidentified time spans. SDs were not reported. The authors reported that changes from baseline score on the MMSE and the ADAS‐Cog did not differ significantly among the four groups.
Lloret 2009 measured cognition using the MMSE and the Clock Drawing Test, though we were unable to extract the data. The authors did not report means and SDs of their cognitive outcomes. There were no data on cognitive measures in the vitamin E‐treated group as a whole.
Dysken 2014 measured cognition using the ADAS‐Cog and MMSE. At six to 48 months, participants taking vitamin E had a smaller increase from baseline on the ADAS‐Cog (an increase in score representing worsening cognitive function) than participants taking placebo, but the result was uncertain and unlikely to be of clinical importance (completers: MD ‐1.81, 95% CI ‐3.75 to 0.13, P = 0.07, 1 study, n = 272; moderate quality evidence, downgraded due to imprecision because data were from a single small study). There was no evidence of a difference between groups in cognitive function measured with the MMSE (where a decrease represents worsening function) (completers: MD 0.19, 95% CI ‐0.72 to 1.10, P = 0.68, 1 study, n = 273; moderate quality evidence, downgraded due to imprecision).
Adverse events
Sano 1996 did not fully report adverse events. A total of 49 categories of adverse events were defined but the only results initially reported were for three categories (dental events, falls and syncopal episodes), in which there were significant differences between one or more of the treatment groups and the placebo group. However, once the authors adjusted for multiple comparisons, there were no significant differences between groups.
Lloret 2009 did not report adverse events. We requested additional information but this was not made available.
Dysken 2014 found no evidence of a difference between vitamin E and placebo groups in the number of participants reporting any adverse event or the number reporting at least one severe adverse event. The numbers of participants reporting adverse events during the study were 91/152 (59.9%) in the vitamin E group and 89/152 (58.6%) in the placebo group (RR 1.02, 95% CI 0.85 to 1.23, P = 0.82, 1 study, n = 304; moderate quality evidence, downgraded due to imprecision). The number of participants reporting at least one severe adverse event were 82/152 (54.0%) in the vitamin E group and 95/152 (62.5%) in the placebo group over the 48‐month trial (RR 0.86, 95% CI 0.71 to 1.05, P = 0.13, 1 study, n = 304; moderate quality evidence, downgraded due to imprecision).
Death
In Sano 1996, participants remained in the study until they reached one of the four predefined end points, one of which was death. In both vitamin E and placebo groups, 12% of participants died. The authors also reported that treatment had no effect on the cause of death.
Lloret 2009 did not report deaths. We requested additional information but this was not made available.
Dysken 2014 found no evidence of a difference between vitamin E and placebo groups in the number of deaths. There were 26/152 (17.1%) deaths in the vitamin E group compared to 31/152 (20.4%) deaths in the placebo group over the 48‐month trial (RR 0.84, 95% CI 0.52 to 1.34, P = 0.46, 1 study, n = 304; moderate quality evidence, downgraded due to imprecision).
Secondary outcomes
Global measure of dementia severity and deterioration
None of the studies measured dementia severity and deterioration (Dysken 2014; Lloret 2009; Sano 1996).
Behavioural disturbance
In Sano 1996, we were unable to extract the data for behavioural disturbances despite these being measured as secondary outcomes in the trial. Only the mean change scores to the time point where a participant was last assessed were reported and participants had been assessed over varying and unidentified time spans. SDs were not reported.
Lloret 2009 did not measure behavioural disturbance.
Dysken 2014 found that neuropsychiatric symptoms increased slightly more in the placebo group than in the vitamin E group over six to 48 months using the NPI but the result was uncertain and unlikely to be of clinical importance (completers: MD ‐1.47, 95% CI ‐4.26 to 1.32, P = 0.30, 1 study, n = 280; moderate quality evidence, downgraded due to imprecision).
Mood
None of the studies measured mood (Dysken 2014; Lloret 2009; Sano 1996).
Activities of daily living
In Sano 1996, we were unable to extract the data we had specified for activities of daily living, despite these being measured as secondary outcomes in the trial. Only the mean change scores to the time point where a participant was last assessed were reported and participants had been assessed over varying and unidentified time spans. SDs were not reported. The authors reported that there was significantly less decline in activities of daily living, as measured by the BDS and DS, in the vitamin E compared to the placebo group, but no significant difference between groups on the BRSD.
Lloret 2009 measured activities of daily living using the BDS. The authors did not report means and SDs, and therefore we were unable to extract the data.
Dysken 2014 measured activities of daily living using the ADCS‐ADL. Participants receiving vitamin E showed less functional decline on the ADCS‐ADL than participants receiving placebo at six to 48 months (completers: MD 3.15, 95% CI 0.07 to 6.23, P = 0.04, 1 study, n = 280; moderate quality evidence, downgraded due to imprecision).
Carer burden
None of the studies measured carer burden (Dysken 2014; Lloret 2009; Sano 1996).
Quality of life
None of the studies measured quality of life (Dysken 2014; Lloret 2009; Sano 1996).
Permanent physical disability
None of the studies measured permanent physical disability (Dysken 2014; Lloret 2009; Sano 1996).
Institutionalisation
Sano 1996 reported that participants receiving vitamin E treatment significantly delayed institutionalisation compared to participants receiving placebo (RR 0.42, no CI reported; P = 0.003).
Neither Dysken 2014 nor Lloret 2009 measured institutionalisation.
Other study outcomes
Sano 1996 reported the number in each outcome group who reached one of their primary end points (death, institutionalisation, change in severity of dementia to a CDR score of three or loss of two basic activities of daily living) within two years; this was 45/85 (53%) of participants in the vitamin E group and 58/84 (69%) in the placebo group. Because the trial was limited in time, an analysis comparing a count of events without taking into account their timing is less precise than the survival analysis reported by Sano 1996. Using Kaplan‐Meier estimation and log‐rank testing in their primary analysis, Sano 1996 found no significant difference in survival time to one of the four end points between the vitamin E and placebo groups (RR 0.70, no CI reported; P = 0.08). However, the study groups differed in baseline MMSE scores and these were correlated with clinical course. The vitamin E group began with a mean MMSE of 11.3 (SD 5.7) and the placebo group with a mean MMSE of 13.3 (SD 4.9). When the analysis was repeated using the Cox proportional hazards model and controlling for baseline MMSE, there emerged a significant difference favouring vitamin E (RR 0.47, no CI reported; P = 0.001).
The primary outcomes reported by Lloret 2009 were the percentage change in performance from baseline to six months on the BDS, Clock Drawing Test and MMSE, compared across responders (n = 9), non‐responders (n = 10) and placebo‐treated groups (n = 14), where participants who showed a decrease in GSSG values of more than 10 nmol/mL of blood after six months of vitamin E treatment were termed 'responders' and participants who did not were termed 'non‐responders'. We found no evidence that these groups had been defined before data collection. The authors reported no significant difference between groups on the Clock Drawing Test or BDS in an analysis of 'completers' only. The MMSE score increased in the responder group but decreased in the placebo group and more so in the non‐responders. There was a significant difference between the two vitamin E‐treated groups on MMSE change score (P < 0.05). The responders did not differ significantly from the placebo‐treated group. The decline in MMSE in the non‐responders was significantly greater than in the placebo group (P < 0.05). Lloret 2009 also reported a negative correlation between change in MMSE score and change in blood levels of GSSG from baseline to six months; that is, there was a greater decline in cognitive performance in people with AD whose blood GSSG levels stayed higher (reflecting higher oxidative stress).
Dysken 2014 reported that for functional decline, the mean treatment effect of 3.15 units on the ADCS‐ADL, favouring participants receiving vitamin E, translates into a clinically meaningful delay in progression of 6.2 months (95% CI 5.4 to 7.4), based on the rate of change in the control group.
Vitamin E for mild cognitive impairment
One study assessed vitamin E in people with MCI (Petersen 2005). For a summary of findings for the treatment of MCI, see summary of findings Table 2.
Primary outcomes
Development of, or time to development of, possible or probable Alzheimer's disease
Petersen 2005 found no evidence of an effect of vitamin E on the numbers of participants with MCI who progressed to possible or probable AD. As their primary outcome, Petersen 2005 reported that 33/257 participants in the vitamin E group and 38/259 participants in the placebo group progressed to possible or probable AD in the first 12 months (RR 1.02, 95% CI 0.96 to 1.10, P = 0.05, 1 study, n = 516). By 36 months, 76/257 participants in the vitamin E group and 73/259 in the placebo group had progressed to AD (RR 1.03, 95% CI 0.79 to 1.35, P = 0.81, 1 study, n = 516). We considered this moderate quality evidence, downgraded due to imprecision.
Cognitive function
Petersen 2005 reported no significant difference between those receiving vitamin E and placebo on the change from baseline of the MMSE, ADAS‐Cog and modified ADAS‐Cog. However, it was difficult to tell how missing data had been handled and no sample size was reported, so we were unable to conduct an analysis.
Adverse events
Petersen 2005 did not fully report adverse events and therefore we were unable to extract the data we required. The authors reported the rates of 10 types of adverse events that occurred in at least 5% of participants in the vitamin E and at least two times in the placebo group during the double‐blind phase of the study. Diarrhoea and cataract extraction were the most frequently reported adverse events in the vitamin E group. Neither of these adverse events differed significantly in frequency between the vitamin E and placebo groups (P > 0.05). Overall, 66/257 participants discontinued in the vitamin E group and 72/259 participants discontinued treatment in the placebo group. Reasons were not given by group, but in the study as a whole, most discontinuations resulted from withdrawal of consent or adverse events.
Death
Petersen 2005 reported that five deaths occurred in each of the vitamin E and placebo groups during the 36‐month double‐blind phase (RR 1.01, 95% CI 0.30 to 3.44, P = 0.99, 1 study, n = 516; moderate quality evidence, downgraded due to imprecision). All deaths were deemed to be unrelated to treatment.
Secondary outcomes
Global measure of dementia severity and deterioration
Petersen 2005 reported no significant difference between those receiving vitamin E and placebo on change scores of the CDR Sum of Boxes or the Global Deterioration Scale. However, it was difficult to tell how missing data had been handled and no sample size was reported, so we were unable to conduct an analysis.
Behavioural disturbance
Petersen 2005 did not measure behavioural disturbance.
Mood
Petersen 2005 did not measure mood.
Activities of daily living
Petersen 2005 reported no significant difference between those receiving vitamin E and placebo on change scores of the ADCS MCI‐ADL. However, it was difficult to tell how missing data had been handled and no sample size was reported, so we were unable to conduct an analysis.
Carer burden
Petersen 2005 did not measure carer burden.
Quality of life
Petersen 2005 did not measure quality of life.
Permanent physical disability
Petersen 2005 did not measure permanent physical disability.
Institutionalisation
Petersen 2005 did not measure institutionalisation.
Other study outcomes
Petersen 2005 also created standardised z‐scores of individual cognitive domains (memory, executive, language, visuospatial and overall cognition) and reported the change from baseline for each. There were few significant differences in cognitive function change scores between the vitamin E and placebo groups. Those that were seen (in the executive, language and overall cognitive scores) were confined to the first 18 months of the study. On average, executive function improved in both groups at six months, but the improvement was significantly greater in the vitamin E group compared to the placebo group (P < 0.05). Change in language scores significantly favoured vitamin E at six months (P < 0.05), 12 months (P < 0.05) and 18 months (P < 0.05). Overall cognitive changes scores significantly favoured vitamin E at six months (P < 0.01), with an improvement in cognition in the vitamin E group and a decline in cognition in the placebo group. There were no differences between groups in the second 18 months of the study (18 to 36 months).
Discussion
Summary of main results
Four studies met the inclusion criteria and were selected for this review. To our knowledge there are currently no relevant ongoing trials. Three studies assessed the efficacy of vitamin E in people with AD (Dysken 2014; Lloret 2009; Sano 1996), while one study assessed vitamin E in people with MCI (Petersen 2005). A significant limitation of this review is that synthesis of data from the AD studies was not possible owing to different outcome measures, heterogeneity in designs and the inability to access relevant data sets from authors' reports. The Lloret 2009 study used a dose of vitamin E of 800 IU/day in the intervention group while the studies by Dysken 2014, Sano 1996, and Petersen 2005 used a dose of vitamin E of 2000 IU/day. Participants in the Sano 1996 study withdrew from the study once they reached a series of clinical end points. We could use data only from the Dysken 2014 and Petersen 2005 studies.
Vitamin E for dementia due to Alzheimer's disease
We found no evidence from the one study from which we could extract data that vitamin E was efficacious in improving cognitive outcomes in dementia due to AD (Dysken 2014). We could not extract data from Sano 1996 or Lloret 2009.
For our other primary outcomes, adverse events and deaths, there was no evidence that participants with AD receiving vitamin E were more at risk than participants receiving placebo (Dysken 2014; Sano 1996). From this review, we were unable to comment on the safety or tolerability of higher doses of alpha‐tocopherol or of other tocopherol and tocotrienol forms. In what appeared to be a post hoc analysis, Lloret 2009 reported that the subgroup of participants with AD who responded to vitamin E with a reduction in measures of oxidative stress had similar cognitive outcomes to participants taking placebo, while participants showing no reduction in oxidative stress measures in response to vitamin E had significantly poorer cognitive outcomes. This was very low quality evidence due to the post hoc definition of response, the small sample size and other significant methodological limitations, but the apparent accelerated decline in cognitive abilities in those participants in whom vitamin E did not reduce oxidative stress measures suggests a need for caution in future trials.
Among our secondary outcomes, we could extract data from Dysken 2014 on behavioural disturbance, which did not differ between groups, and on performance of activities of daily living, which declined less over a mean follow‐up of 2.27 years in the vitamin E than in the placebo group. We were unable to extract any data on our secondary outcomes of global dementia severity, mood, carer burden, quality of life, physical disability or institutionalisation.
Vitamin E for mild cognitive impairment
Petersen 2005 was the only study identified that investigated the effects of vitamin E on the progression from MCI to AD. We found no evidence that vitamin E treatment affected the risk of progression to AD over 36 months compared to placebo. The evidence presented by Petersen 2005 also suggests that vitamin E was not efficacious in improving cognitive outcomes in MCI.
There was no evidence that vitamin E treatment increased the risk of death, and while we were unable to extract data for adverse events, the data presented by the authors indicated that the risk of adverse events also did not differ between treatment groups. As with the AD sample, we were unable to comment on the safety or tolerability of higher doses of alpha‐tocopherol or of other tocopherol and tocotrienol forms in an MCI population.
Among our secondary outcomes, we were unable to analyse data on activities of daily living and global severity because sample sizes were not clearly reported. However, the data presented by the authors showed no evidence that either outcome benefited from vitamin E treatment compared to placebo. The study did not measure mood, carer burden, quality of life, physical disability or institutionalisation, so we could not draw any conclusions about the effect of vitamin E on these outcomes in an MCI population.
Overall completeness and applicability of evidence
The review was based on a small number of RCTs that were heterogeneous in design thus preventing a meta‐analysis. Notably, no two studies included in this review had the same primary outcome, while only a single study investigated vitamin E treatment in a MCI population (Petersen 2005).
Quality of the evidence
Using the GRADE approach, we rated the overall quality of evidence for all outcomes to be moderate. We considered the studies from which we extracted data to be at low to unclear risk of bias. However, we downgraded all quality judgements one level due to imprecision because all data came from single modest‐sized studies and we therefore considered it likely that further research could have an impact on the effect estimates.
See summary of findings Table for the main comparison and summary of findings Table 2.
Potential biases in the review process
To minimise the possibility of bias, we tried to locate all RCTs by a broad and comprehensive search strategy in a number of databases. We sought additional information from the authors of one study but were unable to obtain it (Lloret 2009). The review was limited by our inability to obtain or extract the data we needed from two of the included studies.
Agreements and disagreements with other studies or reviews
Habitual intake of dietary antioxidants has not consistently been found to improve cognitive performance or reduce the risk of dementia (Crichton 2013). While vitamin E assessed in epidemiological studies in relation to cognition has consisted of the various naturally occurring forms (Engelhart 2002; Morris 2002; Morris 2005), the relationship between vitamin E and cognition is unclear. In the present systematic review, there was no evidence that vitamin E improved either cognition or risk of developing AD. Morris 2005 suggested that the combined intake of tocopherols is likely to be more important than alpha‐tocopherol alone. This is further supported by Mangialasche 2010 who studied the relationship between cognitive impairment and the eight individual forms of vitamin E and concluded that any potential neuroprotective effect for vitamin E may result from interaction of the various forms.
It has been previously reported that vitamin E, especially in the large doses used in the included studies, may be associated with potentially significant adverse effects and even an increased rate of all‐cause mortality (Miller 2005). High doses of alpha‐tocopherol also have the potential to induce enzymes involved in drug metabolism with an associated risk of serious interactions with some concomitant medication (Brigelius‐Flohé 2007). However, this review found no evidence that vitamin E treatment significantly affected the number of serious adverse events or deaths, although the relatively short study lengths and small sample sizes mean that no strong conclusions about the safety of vitamin E can be drawn from these data.

Study flow diagram for study for the search April 2016.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
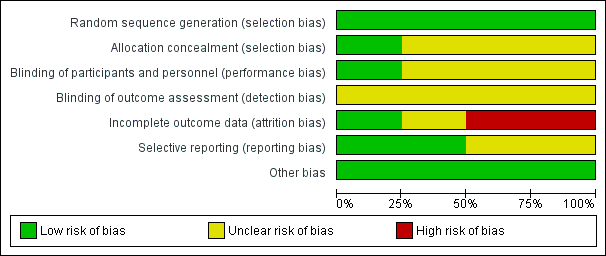
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 1 Alzheimer's Disease Assessment Scale ‐ Cognitive subscale (ADAS‐Cog) (least square (LS) mean change from baseline at 6 to 48 months): completers.
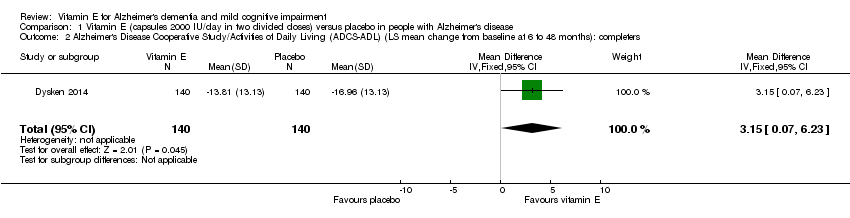
Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 2 Alzheimer's Disease Cooperative Study/Activities of Daily Living (ADCS‐ADL) (LS mean change from baseline at 6 to 48 months): completers.

Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 3 Deaths (number of deaths over 48 months).

Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 4 Serious adverse events (number of participants reporting ≥ 1 serious adverse event over 48 months).
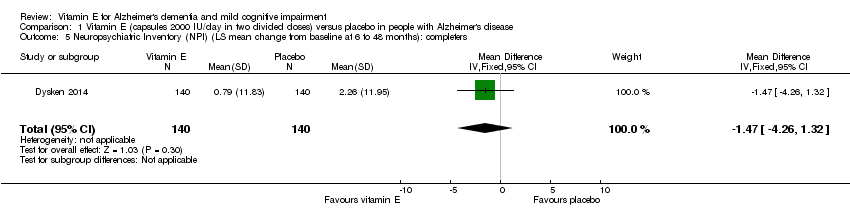
Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 5 Neuropsychiatric Inventory (NPI) (LS mean change from baseline at 6 to 48 months): completers.

Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 6 Mini‐Mental State Examination (MMSE) (LS mean change from baseline at 6 to 48 months): completers.
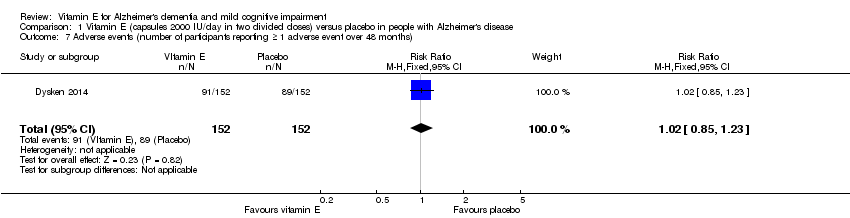
Comparison 1 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with Alzheimer's disease, Outcome 7 Adverse events (number of participants reporting ≥ 1 adverse event over 48 months).
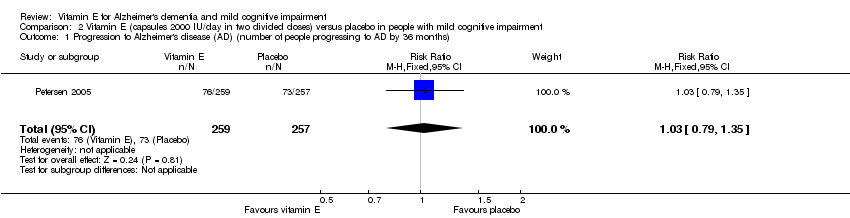
Comparison 2 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with mild cognitive impairment, Outcome 1 Progression to Alzheimer's disease (AD) (number of people progressing to AD by 36 months).
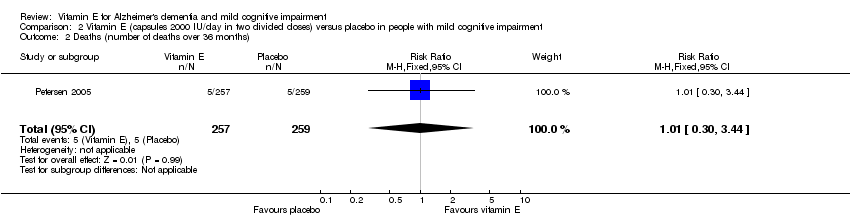
Comparison 2 Vitamin E (capsules 2000 IU/day in two divided doses) versus placebo in people with mild cognitive impairment, Outcome 2 Deaths (number of deaths over 36 months).
| Vitamin E (capsules 2000 IU/day in 2 divided doses) compared to placebo for people with Alzheimer's disease | ||||||
| Patient or population: people with Alzheimer's disease Settings: multicentre, US Intervention: vitamin E (capsules 2000 IU/day in 2 divided doses) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Vitamin E (capsules 2000 IU/day in 2 divided doses) | |||||
| Cognitive function Scale from: 0 to 70 Follow‐up: 6 to 48 months | The LS mean change from baseline in cognitive function in placebo group was 7.78 | The LS mean change from baseline in cognitive function in the intervention group was 1.81 lower | ‐ | 272 | ⊕⊕⊕⊝ | Higher scores represent worse cognitive function. A 4‐point difference in ADAS‐cog has been considered the MCID. |
| Adverse events Number of participants reporting ≥ 1 serious adverse event Follow‐up: 6 to 48 months | 625 per 1000 | 538 per 1000 | RR 0.86 | 304 | ⊕⊕⊕⊝ | ‐ |
| Deaths Number of deaths Follow‐up: 6 to 48 months | 204 per 1000 | 171 per 1000 | RR 0.84 | 304 | ⊕⊕⊕⊝ | ‐ |
| Activities of daily living Scale from: 0 to 78 Follow‐up: 6 to 48 months | The LS mean change from baseline in activities of daily living in the placebo group was ‐16.96 | The LS mean change from baseline in activities of daily living in the intervention group was 3.15 higher | ‐ | 280 | ⊕⊕⊕⊝ | Higher scores represent better activities of daily living. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADAS‐Cog: Alzheimer Disease Assessment Scale ‐ Cognitive Subscale; ADCS‐ADL: Alzheimer's Disease Cooperative Study/Activities of Daily Living Inventory;CI: confidence interval; LS: least square; MCID: minimum clinically important difference; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Quality downgraded one level due to imprecision. Evidence from a single study of modest size. This is supported by dichotomous data not reaching the optimal information size criterion (assuming α of 0.05, and β of 0.2) (Guyatt 2011). | ||||||
| Vitamin E (capsules 2000 IU/day in 2 divided doses) compared to placebo for people with mild cognitive impairment | ||||||
| Patient or population: people with mild cognitive impairment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Vitamin E (capsules 2000 IU/day in 2 divided doses) | |||||
| Progression to Alzheimer's disease Number of people progressing to AD Follow‐up: 36 months | 284 per 1000 | 293 per 1000 | RR 1.03 | 516 | ⊕⊕⊕⊝ | ‐ |
| Cognitive function Mean change from baseline of ADAS‐Cog Scale from: 0 to 70 Follow‐up: 36 months | Not possible to extract data for analysis. | ‐ | Not possible to extract data for analysis. | Unable to evaluate quality of evidence. | Uncertainty about how missing data were handled. Study reports no significant difference between intervention and control groups. | |
| Adverse events Number of participants reporting ≥ 1 serious adverse event. Follow‐up: 36 months | Not possible to extract data for analysis. | ‐ | 516 | Unable to evaluate quality of evidence. | Overall adverse event rates not reported. | |
| Death Number of deaths over 36 months Follow‐up: 36 months | 19 per 1000 | 19 per 1000 | RR 1.01 | 516 | ⊕⊕⊕⊝ | ‐ |
| Activities of daily living Mean change from baseline using the ADCS Scale from: 0 to 53 Follow‐up: 36 months | Not possible to extract data for analysis. | ‐ | Not possible to extract data for analysis. | Unable to evaluate quality of evidence. | Uncertainty about how missing data were handled. Study reports no significant difference between intervention and control groups. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADAS‐Cog: Alzheimer Disease Assessment Scale ‐ Cognitive Subscale; ADCS‐ADL: Alzheimer's Disease Cooperative Study/Activities of Daily Living Inventory;CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Quality downgraded one level due to imprecision. Evidence from a single study of modest size. This was supported by dichotomous data not reaching the optimal information size criterion (assuming α of 0.05, and β of 0.2) (Guyatt 2011). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Alzheimer's Disease Assessment Scale ‐ Cognitive subscale (ADAS‐Cog) (least square (LS) mean change from baseline at 6 to 48 months): completers Show forest plot | 1 | 272 | Mean Difference (IV, Random, 95% CI) | ‐1.81 [‐3.75, 0.13] |
| 2 Alzheimer's Disease Cooperative Study/Activities of Daily Living (ADCS‐ADL) (LS mean change from baseline at 6 to 48 months): completers Show forest plot | 1 | 280 | Mean Difference (IV, Fixed, 95% CI) | 3.15 [0.07, 6.23] |
| 3 Deaths (number of deaths over 48 months) Show forest plot | 1 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.52, 1.34] |
| 4 Serious adverse events (number of participants reporting ≥ 1 serious adverse event over 48 months) Show forest plot | 1 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.71, 1.05] |
| 5 Neuropsychiatric Inventory (NPI) (LS mean change from baseline at 6 to 48 months): completers Show forest plot | 1 | 280 | Mean Difference (IV, Fixed, 95% CI) | ‐1.47 [‐4.26, 1.32] |
| 6 Mini‐Mental State Examination (MMSE) (LS mean change from baseline at 6 to 48 months): completers Show forest plot | 1 | 273 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.72, 1.10] |
| 7 Adverse events (number of participants reporting ≥ 1 adverse event over 48 months) Show forest plot | 1 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.85, 1.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Progression to Alzheimer's disease (AD) (number of people progressing to AD by 36 months) Show forest plot | 1 | 516 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.79, 1.35] |
| 2 Deaths (number of deaths over 36 months) Show forest plot | 1 | 516 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.30, 3.44] |

