Asesoramiento telefónico para el abandono del hábito de fumar
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [author‐defined order]
| Methods | Setting: Parents of children in a birth cohort study, Hong Kong | |
| Participants | 903 current smokers with young children (49 recent quitters not included here); 84% M, > 50% aged 36 to 45, 91% smoked ≤ 20/day | |
| Interventions | 1. Single mailing of stage‐matched S‐H (either preparation/action or contemplation/precontemplation) | |
| Outcomes | Validated abstinence at 6 m (7‐day PP). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Low risk | Numbered sealed opaque envelopes |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Independent interviewer...was unaware of subjects' group allocation... All respondents who reported they were not smoking during the preceding 7 days were invited to attend the research centre for biochemical validation." |
| Incomplete outcome data (attrition bias) | Low risk | Losses to follow‐up 11% intervention/ 4% control. Included as continuing smokers |
| Methods | Setting: 5 Veterans Administration medical centres, USA | |
| Participants | 821 smokers interested in quitting (excludes 16 deaths, 1 withdrawal); 91% M, av. age 57, av. cigs/day 26. 26% had > 7‐day abstinence in previous year, 44% ever use of bupropion, 82% ever use NRT | |
| Interventions | 1. Mailed S‐H and standard care; opportunity for intervention during routine health care and referral to individual or group cessation programmes. NRT and bupropion avail on formulary | |
| Outcomes | Abstinence at 12 m (sustained > 6 m, 7‐day PP also reported) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | Losses to follow‐up included as smokers, 16 deaths excluded |
| Methods | Setting: 65 general practices, UK | |
| Participants | 2471 smokers (2058 in relevant arms); > 80% in precontemplation or contemplation, 10% to 14% in preparation, 46% M, av. age 41, av. cigs/day 20 | |
| Interventions | 1. Standard S‐H materials, single mailing | |
| Outcomes | Abstinence at 12 m (sustained for 6 m) | |
| Notes | We included arms 3 vs 2 in the analyses. Sensitivity analysis comparing arms 3 vs 2+1. 66% received 1st phone call, 36% 2nd, 31% 3rd | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised randomisation procedure, with minimisation to balance SoC, addiction and SES |
| Allocation concealment (selection bias) | Low risk | Centralised |
| Blinding of outcome assessment (detection bias) | Low risk | 12 m PP "was confirmed with salivary cotinine, so that we had unconfirmed and confirmed prevalence of quitting." Confirmed figures used in analysis |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up 24% in group 1, 31% in 2 and 3. All included as smokers. Sensitivity analysis allowing for differential dropout did not change findings |
| Methods | Setting: North Carolina, USA; Medical Center Recruitmenet: Mailed smokers an introductory letter from the Chief of Cardiology, Chief of Oncology, or a primary care physician (the Principal Investigator (PI)) informing them of the study and encouraging smoking cessation | |
| Participants | 471 smokers enrolled in Durham Veterans Affairs Medical Center, receiving treatment for chronic illnesses (i.e. cancer, cardiovascular disease, HTN, diabetes, COPD) and wanting to quit in the next 30 days; 91.5% M, av. age 59.2, av. cigs/day not reported | |
| Interventions | 1. Standard telephone counselling, a letter from a VA physician encouraging smoking cessation, NRT, if not contra‐indicated, a S‐H cessation kit, and up to 5 TC calls (every 3 ‐ 4 weeks, av. duration 20 minutes) 2. "Family‐supported telephone counseling, included all components of the standard TC arm plus an enhanced family‐supported intervention that included a support skills booklet and additional telephone counseling content focusing on social support skills [...] The main distinction between the two arms of this comparative effectiveness study was the family‐supported intervention that aimed to help increase positive interactions between the participant and their designated support person, to facilitate smoking cessation [...] Participants randomized to family support‐based intervention also received an 8‐page disease‐specific family support booklet." | |
| Outcomes | Abstinence at 12 m (7‐day PP) Validation: available for only 50.5% of the participants | |
| Notes | New for 2018 update Funding: "This material is based upon work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, and Health Services Research and Development. IIR‐05‐202." Declarations of interest: "SCG serves as a consultant to Gilead Sciences and Watermark Research Partners. Although these relationships are not perceived to represent a conflict with the present work, it is included in the spirit of full disclosure. Presented in part at the Society of General Internal Medicine Annual Meeting, Phoenix AZ May 2011." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "blocked randomization, stratified by sex and disease type" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | High risk | The investigators mailed participants saliva‐sampling kits to measure cotinine, but the return rates for saliva samples were low at all follow‐ups. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Follow‐up rates were 86% and 81.1% at 5 months and 12 months, respectively. Loss to follow‐up was similar in both arms." |
| Methods | Setting: North Carolina, USA; Academic setting Recruitment: Investigators asked lung cancer patients’ permission to contact members of their social networks who smoked and were aged 18 and older; these received a letter describing the study and providing them a toll‐free number to call to decline participation | |
| Participants | 496 relatives of lung cancer patients, 41.5% M, av. age 46.9, av. cigs/day 19.5. More than half the participants have made more than 3 quit attempts before inclusion in the study | |
| Interventions | 1. Tailored self‐directed materials and nicotine patches 2. As above, plus proactive TC, 6 x weekly sessions scheduled over an intervention period of 12 weeks, with an av. duration of each session of 30 mins. The mean number of sessions completed was 2.4. 81 participants in this group (33%) did not complete at least 1 session | |
| Outcomes | Abstinence at 12 m (7‐day PP) Validation: saliva cotinine, not possible due to low return rates | |
| Notes | New for 2018 update. Funding: "This work was supported by National Cancer Institute grant 5U01‐CA‐92622. This research was also supported in part by the Intramural Program of the National Human Genome Research Institute, National Institutes of Health." Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was blocked by patient, with entire social network units stratified by site and size of social network enrolled (one vs. two or more) assigned to the same condition." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | High risk | Large withdrawal of participants after 12 months of follow‐up (> 50%), although similar across arms |
| Methods | Setting: Malaysia; Outpatient Quit Smoking Clinic based at 2 hospitals | |
| Participants | 231 outpatient smokers, 96.1% M, av. age 48.3, av. cigs/day 13.8 | |
| Interventions | 1. Usual care, which included a combination of nicotine gum and CBT (4 counselling sessions during the 1st month, 2 counselling sessions during the 2nd month + 2 phone calls (av. duration 20 ‐ 30 mins), and 1 counselling session during the 3rd month plus 2 phone calls (av. duration 20 ‐ 30 mins)) 2. As above, + 1 extra weekly proactive call (av. duration 10 ‐ 15 mins) during the first month of the quit attempt | |
| Outcomes | Abstinence at 6 months (4‐week PP) Validation: exhaled CO level < 7 ppm | |
| Notes | New for 2018 update Funding: not reported Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Even though "urn design was used to achieve balanced groups", participants walking in the study and being referred from outpatient clinics at the hospitals were more likely to be assigned to the intervention than those coming from other hospitals |
| Allocation concealment (selection bias) | Low risk | Quote: "the assignments of treatment within a sequence created by the urn design are not as predictable as those of other types of restricted randomisation processed" |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemical validation of self‐reported outcome. The data were collected by another research member not connected with counselling and the data analysis |
| Incomplete outcome data (attrition bias) | Low risk | Percentage lost to follow‐up was overall low (8%), although larger in the usual‐care (12%) than in the intervention group (4%). |
| Methods | Setting: Community, Australia | |
| Participants | 998 smokers interested in quitting; 48% M, 37% aged 15 ‐ 29, 26% aged 30 ‐ 39, av. cigs/day 23 | |
| Interventions | 1. Proactive call‐back TC following initial call to quitline: Multiple calls, first pre‐quit, quit, then according to need. Up to 6 m. Mailed materials | |
| Outcomes | Self‐reported abstinence at 12 m (sustained for 9 m) | |
| Notes | Average number of calls 2.8, 67% received 1 or more. 20% refused call‐back or wanted to initiate the calls, further 7% did not receive any | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up 37% intervention, 30% control. All participants included as smokers in the meta‐analysis |
| Methods | Setting: Community, Australia | |
| Participants | 1578 smokers; 46% M, modal age 30 ‐ 49, av. cigs/day 23 | |
| Interventions | 1. Standard S‐H quit pack based around SoC | |
| Outcomes | Self‐reported abstinence at 12 m (sustained for 9 m) | |
| Notes | 3 vs 2, sensitivity analysis 3 vs 2+1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation by shuffling questionnaires |
| Allocation concealment (selection bias) | Low risk | Author states "no opportunity for interviewers to influence choice"; baseline characteristics balanced, likelihood of bias judged low |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up 21% in 1, 23% in 2, 26% in 3. All participants included as smokers in the MA |
| Methods | Setting: General practice, Australia | |
| Participants | 1039 smokers, not selected for motivation but ˜80% had previously tried to quit; 45% M, av. age: 41, av. cigs/day 17 | |
| Interventions | 1. Referral: Smokers with any interest in quitting referred by fax to Victorian Quitline. Proactive contact attempted with up to 2 pre‐quit and 4 post‐quit sessions typically using relapse‐sensitive schedule. Internet support available as an alternative (4.4% reported use) | |
| Outcomes | Self‐reported abstinence at 12 m (sustained ≥ 10 m) | |
| Notes | TC as adjunct to face‐to‐face intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster‐randomised by GP (1:2 ratio). Computer allocation before GPs attended education session for their assigned intervention |
| Allocation concealment (selection bias) | Unclear risk | Initially concealed but 13 referral (30%) and 11 (42%) control GPs failed to recruit participants. Allocation not blind at time of recruitment of individual participants, so further selection bias possible. Measured characteristics at baseline were similar |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Three‐ and 12‐month questionnaires were administered...by trained interviewers who were blind to treatment condition until after the outcome data were collected." However, reliant on self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | 33% lost in referral condition, 39% in control, all included as smokers in MA. Excluding losses does not affect MA |
| Methods | Setting: Health Maintenance Organisation, USA | |
| Participants | 1329 HMO members; 42% M, av. age 47, 66% smoked > pack/day | |
| Interventions | All participants had filled a prescription. Almost 95% used; ˜51% only bupropion, 26% only NRT, remainder both | |
| Outcomes | Abstinence at 12 m (repeated 7‐day PP at 3 and 12 m) | |
| Notes | 49% of intervention group reached, 36% of those declined, 31% of total accepted counselling. Average no of calls 5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, stratified by presence of chronic disease. Method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "The follow‐up survey was conducted by the Data Collection Center within the Health Partners Research Foundation, using staff not involved in the intervention." However, reliant on self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | ˜33% lost to follow‐up, balanced across groups, included in MA as smokers |
| Methods | Setting: South Carolina, USA | |
| Participants | 121 uninsured callers to the South Carolina State Quitline who wanted to quit in the following 30 days | |
| Interventions | 1. Telephone counselling (CBT) + NRT 2. Telephone counselling (ACT) + NRT 5 weekly calls, 30‐min first session and 15‐min subsequently, were offered. All participants received standard 2‐week NRT (patch or gum) of choice | |
| Outcomes | Self‐reported abstinence at 6 m (30‐day PP) Validation: none | |
| Notes | New for 2018 update Funding: "This study was supported by the National Institutes of Health (T32MH082709 to RV, K23DA026517 to JLH, R21DA030646 to JB) and the Fred Hutchinson Cancer Research Center." Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomized study arm assignments were computer generated" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomized study arm assignments were [...] concealed" |
| Blinding of outcome assessment (detection bias) | Low risk | Abstinence not biochemically validated, but same level of personal contact in different study arms |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of individuals lost to follow‐up was greater in CBT than in ACT arm (39% and 27%, respectively) but less than 50% overall |
| Methods | Setting: Community, Australia | |
| Participants | 45 smokers attending an information evening on smoking cessation; 38% M, av. age 40, av. cigs/day 23 | |
| Interventions | 1. S‐H manual | |
| Outcomes | Abstinence at 12 m (7‐day PP) | |
| Notes | Effect of TC compared to S‐H and single information session alone | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Unclear risk | Saliva samples collected but not apparently tested |
| Incomplete outcome data (attrition bias) | Unclear risk | No details given |
| Methods | Setting: New Hampshire, USA; community mental health centres | |
| Participants | 661 medicaid beneficiaries with mental illness and low income (< USD 1317 a month) willing to initiate cessation treatment within 30 days, 36% M, av. age 45, av. cigs/day 17.3 | |
| Interventions | 1. Usual care, a prescriber visit for smoking cessation (NRT or cessation medications, i.e. bupropion/varenicline) 2. As in 1, plus referral to New Hampshire Tobacco Helpline which provides an average of 3 manualised TC sessions 3. As in 1, plus TC (av. 9 sessions) CBT initiated by a CBT therapist | |
| Outcomes | Abstinence at 12 m (7‐day PP) Validation: breath CO ≤ 4 ppm and urine cotinine < 100 ng/ml (or solely breath CO if using NRT) | |
| Notes | New for 2018 update. Funding: "This research received financial support from the Centers for Medicare and Medicaid Services (Medicaid Incentives for the Prevention of Chronic Diseases grant 1B1CMS330880) and from the New Hampshire Department of Health and Human Services (NHDHHS)." Declarations of interest: "Dr. Brunette reports receipt of research funding from Alkermes. The other authors report no financial relationships with commercial interests." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated tables for each strata within each site were used for random assignment." |
| Allocation concealment (selection bias) | High risk | Quote: "We used equipoise randomization [...] that allowed participants to opt out of one of the cessation treatment conditions or allowed randomization to any of the three options. [...] Randomization strata were defined by conditions to which the participant was willing to be randomly assigned. Within the stratum, a participant was then randomly assigned with equal probability to the selected treatment condition options." Not a true randomisation method; participants can choose what intervention they do not want to be allocated to and this can lead to selection bias. This led to different numbers between arms, and significant baseline age differences |
| Blinding of outcome assessment (detection bias) | High risk | Biochemical validation for only half of the participants in the trial (those receiving an incentive), and there are significant differences between those receiving and not receiving an incentive. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of participants lost to follow‐up was lower than 50% overall |
| Methods | Setting: Hong‐Kong, China; community‐based Recruitment: Participants were approached by investigators at shopping malls or public areas in 16 out of the 18 districts in Hong Kong. Participants who expressed an interest in joining the contest were screened for eligibility and tested on their exhaled CO to ascertain their smoking status | |
| Participants | 1003 Hong Kong residents aged 18 or older, who smoked 1 or more cig/day in the past 6 months, 82% M; 38% 18 – 39 years, 49% 40 – 59 years, 13% 60+ years, 42% 1 – 10 cigs/day, 43% 11 – 20 cigs/day, 15% > 20 cigs/day | |
| Interventions | 1. S‐H booklet and the contact information of the smoking cessation services at the enrolment 2. As 1, plus 8 mobile phone text messages corresponding to the 8 pages of the S‐H booklet (not used in review) 3. As 1, plus 4 sessions (within 1 week, after 2, 6 and 12 m) of 5‐min smoking cessation telephone counselling provided by a trained nurse, using the AWARD Protocol | |
| Outcomes | Abstinence at 12 m (7‐day PP) Validation: exhaled CO < 4 ppm and salivary cotinine level < 10 ng/ml | |
| Notes | New for 2018 update Funding: "This work was funded by Hong Kong Council on Smoking and Health." Declarations of interest: "Prof. Tai‐hing Lam is the principal investigator of the FAMILY project, which was funded by the Hong Kong Jockey Club Charities Trust. All other authors do not have connection with the tobacco, alcohol, pharmaceutical or gaming industries, and nobody was substantially funded by these organizations." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was used to ensure similar group sizes. |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization and allocation were conducted by the author who did not participate in subject recruitment to ensure allocation concealment" |
| Blinding of outcome assessment (detection bias) | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Efforts were made to minimise loss to follow‐up: Quote: "at least seven call attempts at different times were made before participants were considered as loss to follow‐up." In the end follow‐up was comparable across arms. Reasons for losses to follow‐up are provided |
| Methods | Setting: Canada | |
| Participants | 168 past‐month smokers; 27% M, av. age 56, 60 % in preparation or action SoC | |
| Interventions | 1. Counselling by research nurse (1 x 10 ‐ 60 mins, av. 40 mins, based on Transtheoretical Model, included component to enhance social support from a significant family member) | |
| Outcomes | Abstinence at 6 m (sustained at 2 and 6m) | |
| Notes | TC as adjunct to face‐to‐face counselling. 75% received 6 calls | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomised in groups of 3 ‐ 6 "to prevent contamination between groups", method not described |
| Allocation concealment (selection bias) | Low risk | Quote: "Individuals not familiar with the study were in charge of the randomization procedure which included inserting the information into envelopes that were sealed and would be opened by the investigator only at the time of recruitment." |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemical validation used |
| Incomplete outcome data (attrition bias) | Low risk | 4 deaths (3 in Grp 1, 1 in Grp 2) and 3 not meeting follow‐up criteria excluded from MA denominators. Other losses to follow‐up included |
| Methods | Setting: North and West Philadelphia, PA, US; 4 paediatric clinics | |
| Participants | 327 smoking parents from predominantly low‐income, racial‐ and ethnic‐minority families of children under the age of 11, 16.5% M, av. age 33, av. cigs/day 11.5 | |
| Interventions | 1. Individual TC health education attention control (AC) intervention that focuses on improving family nutrition on a budget 2. Individual behavioural TC intervention that focuses on reducing child SHS exposure and parent smoking cessation The TC dosage (5 sessions over 12 weeks) was similar between arms | |
| Outcomes | Abstinence at 12 m (7‐day PP) Validation: cotinine‐verified (cut‐off not reported) | |
| Notes | New for 2018 update Funding: "Supported by Temple University. Funded by the National Cancer Institute, National Institutes of Health, grant CA158361. Funded by the National Institutes of Health (NIH)." Declarations of interest: "The authors have indicated they have no potential conflicts of interest to disclose." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was seeded using values obtained from random.org." |
| Allocation concealment (selection bias) | Low risk | Quote: "The project biostatistician provided the allocations to the data collection team in opaque sealed security envelopes." |
| Blinding of outcome assessment (detection bias) | Low risk | Cotinine‐verified smoking cessation |
| Incomplete outcome data (attrition bias) | Low risk | The percentage of participants lost to follow‐up is minimal (12%), although slightly different between intervention (17%) and control (8%) arms. |
| Methods | Setting: Specialised cardiac hospital, Canada Recruitment: All smokers who were hospitalised were asked to participate by the study nurse (not selected by motivation) | |
| Participants | 40 current daily smokers with cardiovascular disease, 60% M, av. age 57. Most in preparation stage Therapists: nurse specialised in smoking cessation | |
| Interventions | All participants had 1 or more sessions with the study nurse during hospitalisation. Conditions differed after discharge 1. Intervention: 6 phone calls by study nurse at weeks 1, 2, 3, 4, 8, 12. If needed additional phone calls could be arranged between 3 and 6m post‐discharge. At week 3 appointment with the study nurse if requested by participant 2. Control: referral to a national quitline or a community centre for smoking cessation Pharmacotherapy: NRT, bupropion or varenicline were suggested during hospitalisation and follow‐up | |
| Outcomes | Self‐reported abstinence at 6 m (7‐day PP) Validation: only for 1 participant | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified, but generated by a centre for randomised controlled trials |
| Allocation concealment (selection bias) | Unclear risk | Opaque sealed envelopes |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Unclear risk | High loss to follow‐up, but missing data similar in both groups and analyses are ITT, participants lost to follow‐up considered smokers |
| Methods | Setting: California, USA; hospital‐based Recruitment: Quote: "Recruitment procedures differed between healthcare systems based on the personnel involved and the hospital’s reliance on electronic medical records (EMRs)". In 1 study site recruitment was part of the therapists' workflow, while in another academic site, research staff were involved instead | |
| Participants | 1270 hospitalised adult smokers who smoked 6 or more cigs/day, were interested in quitting, spoke English or Spanish, and were not pregnant, 56.7% M, av. age 49.9, av. cigs/day 14.6 | |
| Interventions | Factorial 2 x 2 design comparing TC vs no TC, and NRT vs no NRT 1. No TC (usual care) ± NRT patches. In general usual care consisted of providing smokers with the quitline number, but some hospitals may have also provided counselling or prescribed quitting aids 2. TCg ± NRT patches, with 10 calls scheduled, but on av. 3.6 completed. The av. number of calls in the usual care arm was 1.7 | |
| Outcomes | Abstinence at 6 m (7‐day PP) Validation: saliva cotinine < 10 ng/mL | |
| Notes | New for 2018 update. Previously listed under ongoing studies as Cummins 2012 Funding: "This research was supported by a grant from the National Cancer Institute (CA159533)." Declarations of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned by computer" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically‐confirmed abstinence |
| Incomplete outcome data (attrition bias) | Low risk | Similar loss to follow‐up across arms (˜33%) |
| Methods | Setting: California USA; pregnant women Recruitment: Callers to University of California San Diego California Smokers' Helpline | |
| Participants | 1173 pregnant (< 27 weeks) women, willing to quit within 1 month or recent quitters, av. age 26.3, av. cigs/day 11.2 | |
| Interventions | 1. Self‐help American Cancer Society's Make Yours a Fresh Start Family fact sheets, and additional tips for quitting while pregnant | |
| Outcomes | Abstinence at 6 m post‐partum (180‐day abstinence) | |
| Notes | New for 2018 update. Previously listed under studies awaiting assignment as Zhu 2004 Funding: "This research was supported by the Tobacco‐Related Disease Research Program (Grant 8RT‐0103) and First 5 California (Contract CCFC‐6810) and by funds received from the California Department of Health Services Tobacco Control Section (Contract 00–90605)." Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random allocation to condition was done by computer using blocks of 20" |
| Allocation concealment (selection bias) | Low risk | Quote: "staff were blind to group assignment until the end of the intake, when the appropriate script was presented" |
| Blinding of outcome assessment (detection bias) | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) | Low risk | Higher % lost to follow‐up in intervention arm |
| Methods | Setting: Health Maintenance Organisation, USA | |
| Participants | 1137 smokers, 479 in relevant arms, not selected by motivation to quit; 48% M, av. age 41, av. cigs/day 17 | |
| Interventions | 1. Control ‐ no materials or counselling | |
| Outcomes | Abstinence at 12 m (sustained from 3 m ‐ 12 m) | |
| Notes | 4 vs 3, effect of TC compared to S‐H and feedback alone. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Collecting saliva cotinine...was challenging because participants had neither explicitly volunteered for a study of smoking behavior nor requested treatment for smoking cessation... nearly one fourth of those contacted refused to provide a sample." Higher disconfirmation in control group but difference was not significant |
| Incomplete outcome data (attrition bias) | Low risk | 88% provided data at all 3 and 12 m. No difference in response rates across groups. Missing counted as smoking in MA |
| Methods | Setting: ENT clinics at 4 hospitals, USA | |
| Participants | 89 current smokers used in MA, out of 184 trial participants who also included 26 quit within last month and 21 within last 6 m . Demographics are for all participants; 84% M, av. age 57 | |
| Interventions | 1. Proactive counselling; 9 ‐ 11 CBT‐based calls from trained nurses, linked to use of CBT workbook. Smokers with problem drinking or depression received counselling for these too | |
| Outcomes | Abstinence at 6 m (sustained) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given. Smokers were a higher proportion of the intervention than control groups, and a higher proportion of those randomised than those who refused, raising possibility of selection bias |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms. |
| Incomplete outcome data (attrition bias) | Unclear risk | 22 in total (including non‐smokers) lost to follow‐up, evenly distributed. Losses appear to have been included as smokers |
| Methods | Setting: 8 dental practices, USA | |
| Participants | 82 smokers (60 intervention, 22 control). No baseline data for controls | |
| Interventions | 1. Control: Brief counselling (10 mins) from hygienist, reinforced by dentist | |
| Outcomes | Abstinence at 6 m (7‐day PP) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomised by practice, method not described |
| Allocation concealment (selection bias) | High risk | Hygienists who recruited participants after screening not blind, large difference in numbers recruited, not possible to establish baseline similarity |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Unclear risk | No description of number lost at follow‐up |
| Methods | Settng: Primary care patients, 50 rural practices, Kansas, USA | |
| Participants | 750 smokers of > 10 cigs/day, 41% M, av. age 47, av. cigs/day 24, 61% contemplation, 30% preparation | |
| Interventions | All participants mailed an offer of free pharmacotherapy every 6 m, 4 times in total. Nicotine patch 21 mg for 6 weeks or bupropion SR (150 mg twice daily) for 7 weeks 1. Control. No other contact. 2. Moderate‐intensity disease management: up to 2 calls from counsellor in each cycle encouraging uptake of pharmacotherapy, newsletter mailings and periodic progress reports with counselling suggestions faxed to physician 3. High‐intensity disease management, up to 6 calls at approx 1, 3, 6, 9, 12 weeks from start of each cycle | |
| Outcomes | Abstinence at 24 m (PP). Study also reported analysis based on combination of effects at all follow‐up points. Sustained abstinence not a suitable outcome since no quit date and repeated intervention Validation: attempted saliva cotinine (< 15 ng/ml) by mail at 12 and 24 m. Proxy report used at 24 m for non‐returners. Rate of validation similar across groups | |
| Notes | For analysis on counselling intensity, classified on basis of average calls; moderate in 3 ‐ 6 sessions, high in 7+ subgroups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated random‐number table" in blocks of 24 |
| Allocation concealment (selection bias) | Low risk | Quote: "To conceal allocation, we placed these cards in sequentially numbered, opaque, sealed envelopes." |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemical validation used |
| Incomplete outcome data (attrition bias) | Low risk | Differential rates of loss to follow‐up (1: 22.0%; 2: 31.3%; 3: 31.1%). Participants lost to follow‐up counted as smokers but sensitivity analysis shows no significant difference in analysis outcome if excluding those lost to follow‐up |
| Methods | Setting: Childhood Cancer Survivors Study cohort, USA | |
| Participants | 794 smokers (excludes 2 deaths in control); 53% M, av. age 31, av. cigs/day 12 | |
| Interventions | 1. S‐H control. Mailed manual (Clearing the Air) and letter from study physician | |
| Outcomes | Abstinence at 12 m (7‐day PP) | |
| Notes | No data on average number of calls. Longer‐term follow‐up, assessed at 2 ‐ 4 years, reported in Emmons 2009. Not used in MA ‐ sustained rates not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Unclear risk | Bogus pipeline procedure used, no further details provided |
| Incomplete outcome data (attrition bias) | Unclear risk | 19% lost in intervention vs 24% in control at 12 m. All included as smokers in MA. Excluding losses does not affect MA |
| Methods | Setting: English Quitline Recruitment: Callers to the NHS Smoking Helpline from any location in England | |
| Participants | 2591 smokers aged 16 or older, motivated to quit in 4 days ‐ 4 weeks. 45% M; av. age 38; 47% smoking 11 ‐ 20 cigs/day | |
| Interventions | 1. Standard telephone support (after call, further support by email, letter or text message, offer of proactive contact) 2. As 1 plus additional proactive telephone support (up to 2 calls pre‐quit date, 1 call on quit date, then calls at 3, 7, 14 and 21 days post‐quit date). Structured call content using MI template (except for 7‐ and 14‐day calls) 3. As 1, plus offer of free NRT 4. As 2, plus offer of free NRT | |
| Outcomes | Prolonged abstinence at 6 m (allowing grace period of up to 5 cigs smoked). 7‐day PP also recorded Validation: exhaled CO < 10 ppm | |
| Notes | Arms 1 and 3 combined and compared with arms 2 and 4 combined. No difference in cessation outcomes between participants offered NRT and those not offered NRT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated random number sequence" |
| Allocation concealment (selection bias) | Low risk | Subjects allocated by central computerised system |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemical validation rates used |
| Incomplete outcome data (attrition bias) | Low risk | High rates of dropout but similar across groups (standard 43%, proactive 45%). Dropouts counted as smokers Quote: "this conservative supposition could possibly mask variation...and we explored this possibility by trying alternative associations between missingness and smoking status. This analysis did not change our findings." |
| Methods | Setting: Primary care patients, 16 clinics, USA | |
| Participants | 961 smokers of ≧ 10 cigs/day. (643 in relevant arms, a further 908 were allowed to select treatment. Demographic details based on 1869); 42% M, av. age 40, av. cigs/day 22 | |
| Interventions | (Self‐selected group of factorial trial not included in MA) | |
| Outcomes | Continuous abstinence at 1 year (no relapse lasting 7 days, also 7‐day PP) | |
| Notes | Arms 2 vs 1, TC as adjunct to pharmacotherapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically‐validated cessation |
| Incomplete outcome data (attrition bias) | Low risk | 19% lost at 1 year, no difference by condition |
| Methods | Setting: Germany Recruitment: 21 prevention or rehabilitation clinics | |
| Participants | 527 hospitalised female smokers ≥ 1 cig during the 30 days preceding hospitalisation. Av. age 35.9, motivation to quit not required | |
| Interventions | 1. 3 face‐to‐face courses (60 mins each) in groups during clinic hospitalisation featuring CBT and MI 2. As 1, plus 3 proactive phone calls (10 mins duration) post‐discharge in a structured and directive style 3. As 2, but calls delivered in non‐directive style | |
| Outcomes | Self‐reported abstinence at 6 m (30‐day PP) Validation: none | |
| Notes | Intervention arms combined | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcome with participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Unclear risk | Number lost to follow‐up unclear (conflicting data available) |
| Methods | Setting: USA; population‐based | |
| Participants | 1034 smokers of ≥ 5 cigs/day, aged 17 or older, interest in quitting smoking within the next 30 days, 32% M, av. age 39.3, av. cigs/day 19.3 | |
| Interventions | Factorial design of the following 5 conditions: website (active/lite), S‐H brochure (full/lite), text messaging, NRT, and proactive TC ‐ 5 sessions of a duration of 30 mins upon enrolment, and 15 mins on quit day or day after, and weekly for 3 weeks | |
| Outcomes | Self‐reported abstinence at 7 m (7‐day PP) | |
| Notes | New for 2018 update Funding: "The project was funded through a contract to our university from Matthews Media Group, underwritten by ARRA funding to the National Cancer Institute. Additional funding was provided by the National Cancer Institute (5K05CA139871)." Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detail on exactly how the participants were randomised: Quote: “Randomization occurred immediately after the confirmation call, and participants completing this step were sent an automated email welcoming them to the study and outlining services they would receive (based on their randomization).” |
| Allocation concealment (selection bias) | Unclear risk | As above |
| Blinding of outcome assessment (detection bias) | High risk | Abstinence not biochemically validated. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | Small percentage of lost to follow‐up in each arm |
| Methods | Setting: Quitline, UK | |
| Participants | 1457 smokers planning quit attempt within 2 weeks; 34% M, av. age 39, av. cigs/day NS | |
| Interventions | 1. Standard QUIT information pack and counselling at initial contact. | |
| Outcomes | Self‐reported abstinence at 12 m (sustained for 6 m, also 7‐day PP) | |
| Notes | 26% received no additional calls, 42% had 4+ calls, 31% had 1 ‐ 3 calls | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Pseudo‐random by day of week |
| Allocation concealment (selection bias) | Low risk | Recruiters blind so concealment judged adequate |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | 37% lost to follow‐up in both groups. Missing counted as smoking in MA |
| Methods | Setting: Australia Recruitment: Arabic‐speaking GPs in 29 practices in southwest Sydney | |
| Participants | 407 Arabic smokers, aged 18 ‐ 65 48% M, av. age 29, av. cigs/day 19 | |
| Interventions | 1. Offer of free referral by GP to proactive TC provided by bilingual psychologist. If accepted offer, participants called by counsellor for 20‐min initial session. If prepared to quit, called again on quit date, 1, 3, 6 weeks and 3 m after specified quit date. If not ready to set quit date, assigned "less intensive schedule." Mailed quit kit and materials in Arabic and English 2. Usual care | |
| Outcomes | Self‐reported abstinence at 6 and 12 m (1‐day PP) Validation: none | |
| Notes | Low uptake: 101 of 213 participants agree to receive call, 46 receive at least 1 call, 8 completed all calls. Described narratively in 'Other studies' section | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | High risk | Quote: "From each participating GP, we recruited a consecutive sample of patients of Arabic background aged 18‐65 years during a specified 4‐week period, irrespective of their smoking status" using an "unobtrusive mark visible to only the GP to convey group randomization" on the baseline questionnaire. Suggests allocation not concealed |
| Blinding of outcome assessment (detection bias) | Low risk | No biochemical validation, but research assistants conducting follow‐up blind to assignment, low uptake of actual contact suggests risk of differential misreport low |
| Incomplete outcome data (attrition bias) | Low risk | Significantly more participants in intervention group lost to follow‐up at 12 m than control (45% vs 34%), all dropouts counted as smokers in ITT analysis |
| Methods | Setting: USA Recruitment: US residents searching for stop‐smoking advice on a major internet search engine who clicked on a link to www.quitnet.com, assumed to be motivated | |
| Participants | 2005 adult smokers of 5 or more cigs/day. 48.9% , av. age 35.9, av. cigs/day 20, av. FTND 5.0. 1326 contribute to this review | |
| Interventions | 1. Free 6 m access to www.quitnet.com (interactive commercial cessation website) 2. As 1, + up to 5 sessions of proactive TC for 3 m; counsellors had access to www.quitnet.com info and encouraged participants’ use of it; counsellors sent individual emails after counselling sessions to reinforce key points 3. Control: access to static, info‐only (non‐interactive) version of the content on QuitNet (not used in this review) | |
| Outcomes | Multiple 30‐day PP (at 3, 6, 12 and 18 m). Validation: none | |
| Notes | Arm 2 versus 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random numbers table…stratified by sex and baseline motivation to quit" |
| Allocation concealment (selection bias) | Unclear risk | Method not specified |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcome measure from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | High risk | Participants missing data counted as smokers. Sustained PP data not available for 46% EI, 49% EI+P 49% and 43% BI. Difference due to differential rate of follow‐up at 3 m. Quote: "The lower follow‐up assessment rate among EI+P participants at 3 months may have been owing to ‘telephone fatigue’...Telephone counselling was providing within the first 3 months of the study, which was the only assessment period for which higher loss to follow‐up was observed. If present, this bias could have attenuated the effectiveness of the combined intervention." |
| Methods | Setting: Health Maintenance Organisation, USA | |
| Participants | 388 smokers; 34% M, 67% age 40+, 84% smoked < a pack/day | |
| Interventions | 1. Coverage for TC and pharmacotherapy (bupropion or NRT, USD 15 co‐pay) | |
| Outcomes | Abstinence at 6 m (7‐day PP) | |
| Notes | Not included in MA, results discussed separately, alongside trials for TC as adjunct to pharmacotherapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | Number lost to follow‐up not described, all participants included in analyses |
| Methods | Setting: Hospital/community, Norway | |
| Participants | 133 daily smokers amongst 288 participants. Demographics not given for smoking subgroup | |
| Interventions | 1. Usual care; outpatient visit at 6 ‐ 8 weeks and primary care follow‐up | |
| Outcomes | Abstinence at 6, 12 and 18 m (assumed PP, not defined). Primary trial outcome was health‐related quality of life | |
| Notes | 18‐m follow‐up data added in 2013. Smoking was addressed as part of a multicomponent intervention. TC as adjunct to brief/minimal intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised by computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | Sequence in sealed opaque envelopes but not stated to be numbered. Fewer control group participants raises possibility of selection bias, so not classified as low risk |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | At 18 m, losses amongst baseline smokers 29% in 1, 30% in 2 . Losses reincluded as smokers in this MA |
| Methods | Setting: Quitline, Oregon, USA | |
| Participants | 4500 smokers willing to make a quit attempt; 40% M, av. age 41, av. cigs/day 22 | |
| Interventions | Factorial design; 3 levels of counselling, ± offer of nicotine patch (5‐week supply, 80% accepted, option for 3 weeks more, 25 ‐ 28% requested) | |
| Outcomes | Abstinence > 30 days at 12 m | |
| Notes | First included as Hollis 2005, based on unpublished abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | 69% reached at 12 m. Losses assumed smoking in main analysis, sensitivity analyses reported |
| Methods | Setting: 5 hospitals, Michigan, USA | |
| Participants | 525 participants, including 136 who smoked at admission and could be followed up. Smoker demographics not given | |
| Interventions | 1. In‐hospital care according to American College of Cardiology Guideline Applied to Practice quality improvement (QI) programme, including written discharge contract | |
| Outcomes | Abstinence at 8 m ("remained quit for the period") | |
| Notes | Data on smoking outcomes provided by authors from in‐press paper by Holtrop et al | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Blocked randomisation, method not described |
| Allocation concealment (selection bias) | Unclear risk | Change in methodology from randomisation at recruitment/consent to randomisation after baseline interview due to initial imbalance in numbers. Data collectors were blind to group |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Unclear risk | 15 people whose smoking status not confirmed and 15 losses to follow‐up excluded because group not stated. ITT analysis said not to alter results |
| Methods | Setting: Columbia, SC, Albuquerque, NM and Florence, SC; Recruitment: Through newspaper and radio ads | |
| Participants | 746 adult smokers of ≥ 15 cigs/day, interested in quitting gradually in the next 30 days, 46% M, av. age 46, av. cigs/day 23 | |
| Interventions | 1. Brief advice TC (2 sessions ‐ 5 mins before, and 10 mins after quit day) 2. Abrupt cessation TC (5 sessions ‐ 30 mins between 7 and 21 days before quit day, 10 mins subsequently 2 days before, 2, 7 and 14 days after quit day) 3. Gradual cessation TC (not used in this review due NRT being administered before and after quit day) In arms 1 and 2, participants were sent the US National Cancer Institute’s Clearing the Air booklet, as well as nicotine lozenges after quit day | |
| Outcomes | Abstinence at 6 m (prolonged 2 weeks post‐quit day to 6 m abstinence) | |
| Notes | New for 2018 update Funding: "The conduct of this study and preparation of the manuscript was funded by grant DA‐017825 (JH), Senior Scientist Award DA‐00490 (JH) and Institutional Training Grant DA‐07242 (EP) from the US National Institute on Drug Abuse." Declarations of interest: "Since 1/1/2007, Dr Hughes has received research grants from the National Institute on Health and Pfizer. Pfizer develops and sells smoking cessation medications. During this time, he has accepted honoraria or consulting fees from several non‐profit and for‐profit organizations and companies that develop, sell or promote smoking cessation products or services or educate/advocate about smoking cessation: Abbot Pharmaceuticals; Acrux; Aradigm; American Academy of Addiction Psychiatry; American Psychiatric Association; Begbies Traynor; Cambridge Hospital, Cline, Davis and Mann; Constella Group; Consultants in Behavior Change; Dean Foundation, DLA Piper, EPI‐Q, European Respiratory Society, Evotec; Exchange Limited; Fagerstrom Consulting; Free and Clear Glaxo‐Smith Kline; Golin Harris; Healthwise; Insyght; Informed, Invivodata; Johns Hopkins University; JL Reckner; Maine Medical Center; McNeil Pharmaceuticals; Novartis Pharmaceuticals; Oglivy Health PR, Ottawa Heart Institute, Pfizer Pharmaceuticals; Pinney Associates; Propagate Pharmaceuticals. Reuters; Scientia, Selecta; Temple University of Health Sciences; University of Arkansas; University of California‐San Francisco; University of Cantabria; University of Kentucky, US National Institutes on Health; Wolters Publishing, and Xenova." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "statistician generated a concealed allocation sequence and randomized participants to the gradual, abrupt, or minimal treatment conditions in a 2:2:1 ratio using blocked randomization (stratified by city and counselor) based on the SAS procedure PLAN" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemical verification |
| Incomplete outcome data (attrition bias) | Low risk | Similar low percentage (˜21%) lost to follow‐up between groups |
| Methods | Setting: 7 states, USA | |
| Participants | 7354 smoking Medicare beneficiaries aged 65+ (4295 contribute to review), ˜40% M, ˜69% contemplation, 30% preparation | |
| Interventions | Trial of 4 levels of Medicare benefit. All participants mailed a S‐H kit | |
| Outcomes | Abstinence at 12 m (7‐day PP) | |
| Notes | Main comparison 4 vs 3, which had similar levels of self‐reported use of any pharmacotherapy (60% vs 63.4%). Participants were not called unless they enrolled, so treated as trial of quitline availability | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomised, states divided into quarters balancing smoking prevalence and aged, restricted randomisation to different conditions |
| Allocation concealment (selection bias) | Unclear risk | Participants unaware of programme differences when enrolling and allocation determined by address. Low enrolment in 1 condition does not seem to have been due to bias |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Unclear risk | 25% lost to follow‐up at 12 m, absolute differences between groups small. Main analysis includes losses as smokers |
| Methods | Setting: USA, population‐based | |
| Participants | 560 adult smokers of ≥ 10 cigs/day with a desire to quit some day, but not in the next 30 days, 33% M, av. age 51, av. cigs/day 20 | |
| Interventions | 1. Usual care 5‐min TC 3. Smoking reduction TC Groups 2 and 3 were dosage‐matched with 1 x 15‐min call (week 0), followed by 2 x 10 – 15‐min calls (weeks 2 and 4) | |
| Outcomes | Abstinence at 12 m (7‐day PP) | |
| Notes | New for 2018 update Funding: "This work was supported by research grant NCI CA163176 from the National Cancer Institute (J.R.H.) and training grant T32 DA 7242–23 from the National Institute on Drug Abuse (E.M.K.)." Declarations of interest: "One of the authors received consulting and speaking fees from several companies that develop or market pharmacological and behavioral treatments for smoking cessation or harm reduction and from several non‐profit organizations that promote tobacco control. He also consults (without payment) for Swedish Match." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | One of the investigators designed a computer‐generated block randomisation schedule stratified by counsellor to assign participants to receive either intervention |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported abstinence. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | Large percentage (> 50%) of participants lost to follow‐up but according to authors: Quote: "The amount of missing data for all outcomes did not differ among conditions, nor were baseline characteristics associated with missing data". Sensitivity analyses were used to confirm robustness of their findings |
| Methods | Setting: Community, Minnesota, USA | |
| Participants | 1827 smokers, not selected by motivation to quit; 50% M, av. age 47, av. cigs/day 22 | |
| Interventions | 1. Proactive TC, 2 calls over 3 weeks. Offered S‐H materials | |
| Outcomes | Abstinence at 18 m (no puff, > 3 m and validated abstinent at 6 m) | |
| Notes | High level of cotinine disconfirmation. 70% agreed to second call | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | Minimal contact intervention, likelihood of bias small but since control group participants were not contacted at baseline and a large number of intervention group participants could not be reached, impossible to compare baseline characteristics |
| Blinding of outcome assessment (detection bias) | High risk | No biochemical validation at 18 m. At 6 m, validated abstinence rates "considerably lower" than self‐report |
| Incomplete outcome data (attrition bias) | Low risk | Only a sample of intervention and control participants were selected for follow‐up. Of this sample, 91% reached at 18 m in both groups. Numbers followed up used as denominator in MA |
| Methods | Setting: Health Maintenance Organisation, USA | |
| Participants | 509 smokers of > 20 cigs/day, motivated to quit; 44% M, av. age 42, av. cigs/day 28 | |
| Interventions | All participants received prescriptions for free nicotine patch (Prostep), 22 mg for a maximum of 6 weeks plus 2 weeks 11 mg. Proactive vs Reactive | |
| Outcomes | Abstinence at 12 m (from quit date) | |
| Notes | Arms 3 vs 1+2, effect of proactive TC compared to contact and quitline alone. (1 & 2 combined since fewer than 1% called quitline and no difference between quit rates). Participants who did not return questionnaires at 2, 5, 8, 12 weeks were called by telephone. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation by orientation session attended; participants did not know condition in advance, so risk of selection bias probably low |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically‐validated quit rates |
| Incomplete outcome data (attrition bias) | Low risk | 82% response rate at 12 m, no difference between groups, missing treated as smoking |
| Methods | Setting: Community, USA | |
| Participants | 1006 smokers in 714 households (651 in relevant arms); av. cigs/day 20 | |
| Interventions | 1. Standard Environmental Protection Agency leaflet on risks of radon (this arm not used in review) | |
| Outcomes | Self‐reported abstinence at 12 m (sustained at 3 and 12 m) | |
| Notes | Arms 3 vs 2, effect of TC versus S‐H alone | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised by household, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | 80% of households reached at 3 and 12 m, no difference across conditions. Missing treated as smoking |
| Methods | Setting: Community, USA | |
| Participants | 1364 households with 1821 smokers, ˜18 cigs/day | |
| Interventions | Factorial design crossing ± brief phone counselling with 15‐min video S‐H materials. All households given A Citizens Guide to Radon and letter tailored to results of radon level test | |
| Outcomes | Self‐reported abstinence at 12 m (sustained at 3 and 12 m) | |
| Notes | Results of analyses accounting for clustering of multiple smokers in households reported to yield results generally consistent with simple analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Responding households sequentially randomised to 4 conditions subject to stratification on radon test status |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | 83% of households completed 12 m assessment, 76% completed both 3 and 12 m |
| Methods | Setting: Sweden; clinic‐based Recruitment: Callers to Swedish National Tobacco Quitline were invited to participate in the study, if consented to participate, they were sent a postal baseline registration questionnaire | |
| Participants | 772 smokers distributed among 9 and 8 counsellors, missing baseline patients' characteristics, only characteristics of completers at 12 m are provided, 20% M, av. age 48, > 80% used NRT or other medications | |
| Interventions | 1. Standard TC 2. Motivational interviewing TC Total contact was similar between arms, with a duration ˜50', and av. number of sessions 3 | |
| Outcomes | Self‐reported abstinence at 12 m (continuous) | |
| Notes | New for 2018 update Funding: "The research was funded by the Swedish Cancer Society, Stockholm County Council, the Swedish Heart and Lung Association, the Swedish Research Council, the Swedish Council for Working Life and Social Research and the Swedish National Institute of Public Health." Declarations of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation of counsellors was semi‐randomised (with a flip of a coin) Quote: "The allocation of the counsellors resulted in an uneven distribution of total working hours between the groups. In order to achieve a more equal distribution between the two arms, the groups were readjusted (again by coin flip)" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | Low risk | Self‐reported abstinence, but same level of personal contact in different study arms |
| Incomplete outcome data (attrition bias) | High risk | Quote: "In total, 9 counsellors were allocated to ST and 8 counsellors to MI. During the study period, 2 (out of 8 ‐ 25%) of the MI counsellors left SNTQ. Consequently, the MI arm eventually came to consist of six counsellors." |
| Methods | Setting: Health centre, USA | |
| Participants | Low‐income African‐American smokers, 266 randomised, 160 followed up, 107 in relevant arms. Unselected by motivation; 48% M, 49% aged > 50 | |
| Interventions | 1. Physician prompts attached to chart (included other screening tests). Providers trained to use 4As (Ask/ Advise/ Assist/ Arrange follow‐up) model. Only received if participants visited doctor | |
| Outcomes | Self‐reported abstinence 16 m after last intervention (30‐day PP) | |
| Notes | Arms 3 vs 2, TC without face‐to‐face contact; physician advice was not an integral part of the intervention ‐ participants not required to have visited the doctor or received advice during the intervention period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | 38% loss to follow‐up primarily due to disconnected phone numbers. Reported rates based on numbers followed up. Authors report that an analysis with missing treated as smoking did not alter findings |
| Methods | Setting: Community, USA | |
| Participants | 412 teenage smokers (aged 15 ‐ 18, smoked in past 7 days); 49% M, 56% aged ≥ 17, av cigs/day 10, 21% contemplation | |
| Interventions | 1. S‐H, 2 booklets for teen smokers and video | |
| Outcomes | Abstinence at 8 m (7‐day PP) | |
| Notes | TC as adjunct to targeted S‐H. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described, stratified by SoC |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Biochemical validation done but final outcome figures based on self‐report only. High failure to confirm and low response rate. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Unclear risk | 46% Intervention and 51% Control reached at both follow‐ups. Losses included as smokers |
| Methods | Setting: Community, Australia | |
| Participants | 854 smokers interested in quitting; 49% M, av. age 42, av. cigs/day 24 | |
| Interventions | 1. Free 2‐week supply of nicotine patch by mail, instructed to purchase further supply. 14 or 21 mg depending on body weight | |
| Outcomes | Self‐reported abstinence at 6 m (90‐day continuous) | |
| Notes | TC as adjunct to NRT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "randomized" by shuffling folders each day after participants to be included were listed |
| Allocation concealment (selection bias) | High risk | Potential for bias, since allocation sequence not fixed in advance. Baseline characteristics similar across groups |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "To minimise misleading reports of abstinence, a bogus pipeline technique was used, with the possibility of carbon monoxide breath testing mentioned in the consent form and at the 3‐ and 6‐month monitoring calls." |
| Incomplete outcome data (attrition bias) | Low risk | 17% lost in NRT only, 15% in + counselling. Missing treated as smoking in MA |
| Methods | Setting: Health Maintenance Organisation, USA | |
| Participants | 580 current women smokers, not selected for motivation to quit; av. age 36, av. cigs/day 13 | |
| Interventions | 1. Usual care; no smoking cessation intervention | |
| Outcomes | Abstinence at 15 m (7‐day PPA) obtained by telephone interview | |
| Notes | Effect of TC and S‐H materials compared to no intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not stated, stratified on test result |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemical validation, quit rates not corrected but low level of misreport and Quote: "no differences between the two groups in the proportion of women who returned samples, the proportion confirmed/disconfirmed, or the confirmation rate." |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up at 15 m 20% in Intervention, 18% in Control. Losses included as smokers |
| Methods | Setting: 2 Health Maintenance Organisations, USA | |
| Participants | 897 pregnant smokers and recent quitters (44% already quit) not selected for motivation to quit; av. age 28, av. cigs/day 15 before pregnancy, 5 if still smoking | |
| Interventions | 1. S‐H booklet only | |
| Outcomes | Abstinence at 12 m postpartum (7‐day PP) | |
| Notes | Arms 3+2 vs 1, effect of TC versus S‐H only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemical validation used, not reported: Quote: "since there were no between‐group differences in the proportion of saliva samples returned or the proportion confirmed, the primary trial outcomes were based on self‐reported smoking status." |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up 13% at 12 m, not different by group, losses included as smokers |
| Methods | Setting: Army Medical Centre, USA | |
| Participants | 583 pregnant current smokers and recent quitters (390 in relevant arms); av. age 24 | |
| Interventions | 1. Usual care: provider advice and S‐H guide | |
| Outcomes | Abstinence at 12 m postpartum (7‐day PP at all 4 follow‐ups) | |
| Notes | Effect of TC as adjunct to brief advice | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described, stratified by smoking status |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Unclear risk | Biochemical validation conducted but not used in outcome data. Quote: "Saliva return rates did not differ by condition at either follow‐up" but rates of return low and level of misreport not specified |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up higher in Intervention (22%) than Control (16%). Losses included as smokers |
| Methods | Setting: Health Maintenance Organisation, USA | |
| Participants | 275 women smokers, not selected for motivation to quit; av. age 33, av. cigs/day 14 | |
| Interventions | 1. Usual care, S‐H, contact details for Free & Clear, a covered benefit | |
| Outcomes | Abstinence at 12 m (7‐day PP) | |
| Notes | Effect of TC versus S‐H only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Bogus pipeline for short follow‐up, biochemical validation at 12 m. Results from saliva strip test judged overly conservative, hence self‐report used in final outcome data, but relative effect not altered |
| Incomplete outcome data (attrition bias) | Unclear risk | No information on numbers not reached at follow‐up. All participants included in analysis |
| Methods | Setting: Pacific Northwest, USA Recruitment: Members of large regional health plan identified through automated records | |
| Participants | 52 adults with evidence of smoking in last year, depression in last 2 years, and without high levels of physical activity. 33% M; av. age 44.5; av. cigs/day 10.6; av. FTND 2.37 | |
| Interventions | 1. Intervention: usual care + phone‐based Step Up proactive counselling programme (1 motivational call, 9 weekly CBT calls and 2 follow‐up ‘booster calls’ according to participant need) 2. Control: usual care treatment for depression, smoking and physical activity (incl. S‐H material and referral information for phone‐based smoking cessation programme) | |
| Outcomes | Self‐reported abstinence at 6 m (7‐day PP) Validation: none | |
| Notes | Pilot study of an intervention also addressing physical activity and depression Number abstinent not provided and hence extrapolated from percentages given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned," stratified by baseline antidepressant use". Method of sequence generation not specified |
| Allocation concealment (selection bias) | Unclear risk | Method not specified |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcome, participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | Participants lost to follow‐up counted as smokers, similar numbers lost in each group (4/27 intervention, 2/25 control) |
| Methods | Setting: Community, USA | |
| Participants | 1745 smokers; 30% M, 23% age 18 ‐ 30, 40% age 31 ‐ 45, 30% 45 ‐ 64 | |
| Interventions | 1. TV programme and S‐H manual (ALA Freedom From Smoking in 20 Days) | |
| Outcomes | Abstinence at 24 m (7‐day PP) | |
| Notes | Effect of access to hotline combined with S‐H materials for maintenance of cessation Use of the hotline was low; only 7% called and spoke to a counsellor | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Unclear risk | Self‐reported outcomes from participants not blinded to treatment condition. Unclear if level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | 24% lost in maintenance condition, 27% in control. MA includes only responders; Including losses would give less conservative effect |
| Methods | Setting: 13 rehabilitation centres, Germany | |
| Participants | 290 smokers; 59% M, av. age 47, av cigs/day 15, control group significantly more dependent | |
| Interventions | All participants had inpatient group therapy of 7 x 60‐min sessions. ˜26% abstinent at discharge | |
| Outcomes | Abstinence at 12 m (7‐day PP) | |
| Notes | Effect of TC as adjunct to intensive support | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, 1:2 ratio, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | 17/316 randomised to intervention excluded, no contact post‐discharge. Differential dropout from remainder, 17% Intervention, 40% Control. No detected differences in characteristics of dropouts. Sensitivity analyses excluding losses to follow‐up removes significance |
| Methods | Setting: Community, Spain | |
| Participants | 200 smokers; 62% M, av. age 35, av cigs/day 28 | |
| Interventions | 1. Proactive TC, 6 x weekly 10‐min calls. 4 on motivation and cessation, 2 on maintenance, + S‐H | |
| Outcomes | Abstinence at 12 m (not even a puff since quitting) | |
| Notes | 10‐year follow‐up reported in 2008, not used in MA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemical validation used |
| Incomplete outcome data (attrition bias) | Unclear risk | No information on numbers not reached at follow‐up. All participants included in analysis |
| Methods | Setting: Community, Spain | |
| Participants | 228 smokers of ≥ 10 cigs/day; 54% M, av. age 37, av. cigs/day 27, 44% had prior year quit attempt | |
| Interventions | 1. Mailed S‐H programme; 6 weekly manuals, quit date intended to be set at end of week 4 | |
| Outcomes | Abstinence at 12 m (sustained since end of treatment) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Telephone interviews were conducted by a trainer interviewer who was blind with respect to the group to which each subject was assigned. To improve the reliability of these self‐reports of smoking status, all follow‐up questionnaires and interviews commenced with a reminder that the subject might at some point be asked to undergo a carbon monoxide test." |
| Incomplete outcome data (attrition bias) | Unclear risk | Missing data treated as failure, no statement of numbers lost to follow‐up |
| Methods | Setting: Hospitals, USA | |
| Participants | 1942 smokers (excludes deaths); 51% M, av. age 51, av cigs/day 20 | |
| Interventions | All groups received standardised physician advice | |
| Outcomes | Abstinence at 12 m (sustained at 3 m, 6 m, 12 m) | |
| Notes | Effect of additional telephone follow‐up. Not pooled. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Low risk | Quote: "Nurses opened sealed envelopes in front of patients" |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemical validation; verification by family member used when biochemical validation not possible |
| Incomplete outcome data (attrition bias) | Low risk | Number lost to follow‐up not specified, all randomised participants, excluding 82 deaths, included in analyses |
| Methods | Setting: USA | |
| Participants | 2485 adult smokers of ≥ 10 cigs/day, willing to set a quit date within 2 to 4 weeks, willing to use a nicotine patch, 31% M, av. age 44. | |
| Interventions | 1. NRT patch (free 8‐week) + Internet (iQuit Smoking website) | |
| Outcomes | Self‐reported abstinence at 9 m (6 m prolonged) | |
| Notes | New for 2018 update Funding: not reported Declarations of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided on ClinicalTrials.gov website |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided on ClinicalTrials.gov website |
| Blinding of outcome assessment (detection bias) | High risk | Abstinence not biochemically validated. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | Low percentage (19%) of participants lost to follow‐up and comparable across arms |
| Methods | Setting: Sweden; population‐based Recruitment: Tobacco user calls the quitline, the counsellor invites the user to participate in the study, and if the user consents verbally, the registration form and baseline questionnaire are sent by mail. If baseline questionnaire is returned the user is included in the study | |
| Participants | 1129 smokers, 22% M, age: ≤ 34: 20%, 35 ‐ 49 25%, 50 ‐ 64 39%, ≥ 65 17%, average cigs/day: 0: 27%; 1 ‐ 14: 34%; ≥ 15: 39%. Participants were interested in quitting | |
| Interventions | 1. Reactive quitline service: The study was performed within the normal run of the Sweedish national tobacco quitline. Participants in this arm were not offered to be called back 2. Proactive quitline service: The study was performed within the normal run of the Swedish national tobacco quitline. Participants in this arm were offered to be called back. The first call was about 25 mins and subsequent calls were 5 ‐ 10 mins, with a mean of 4.3 calls | |
| Outcomes | Self‐reported abstinence at 12 m (6 m continued) Validation: none | |
| Notes | New for 2018 update Funding: "The study was supported by grants from the Swedish Heart and Lung Association, the Swedish Heart Lung Foundation, the Swedish Cancer Society, the Swedish Research Council, the Swedish Research Council for Health, Working Life and Welfare, and the County Council of Västmanland, Sweden." Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Semi‐randomised Quote: "randomized to proactive service (even dates) and reactive service (odd dates)" |
| Allocation concealment (selection bias) | High risk | Quote: "As the randomization was performed at the time for the clients’ first call, the intervention has started and was known by the clients when they decided to return the baseline questionnaire and thus be included in the study base" |
| Blinding of outcome assessment (detection bias) | High risk | Abstinence not biochemically validated. Level of personal contact probably differed between arms |
| Incomplete outcome data (attrition bias) | High risk | Similar dropout across trial arms but > 50% |
| Methods | Setting: Primary care clinics, USA | |
| Participants | 1223 smokers (excludes deaths and 237 who did not receive intervention); 43% M, av. age 35, av. cigs/day 23 | |
| Interventions | 2 x 3 factorial design, physician intervention ± follow‐up | |
| Outcomes | Abstinence at 6 m (7‐day PP). 3 m sustained abstinence rates not given by condition | |
| Notes | Arm 1 vs 2, AO and CI effect of TC in addition to physician intervention. NCG arm in pharmacotherapy adjunct, both pooled in intensity and motivation subgroup analyses. 12‐m abstinence rates reported in Ockene 1994, but not given by follow‐up condition | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | Allocated prior to physician encounter |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | 19% lost to follow‐up, higher in telephone follow‐up group. All included as smokers in analysis |
| Methods | Setting: Health Maintenance Organisation, USA | |
| Participants | 2021 smokers of 3+ cigs/day, wanting to quit (1412 in relevant arms); 37% M, av. age 44, av. cigs/day 26 | |
| Interventions | 1. S‐H manual, Quit Kit and ALA Lifetime of Freedom from Smoking | |
| Outcomes | Abstinence at 16 m (sustained for > 6 m) obtained by blinded telephone interview | |
| Notes | Arms 3 vs 1 and 2 combined, effect of telephone counselling compared to S‐H materials alone. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described, stratified by living alone/not, advice to quit in last 12 m/not, and nicotine content of cig. brand |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemical validation in sample at 16 m Quote: "to improve the veracity of smoking self‐report, all follow‐up questionnaires and interviews began with a reminder that the subjects might be asked for a saliva specimen for nicotine assessment, creating a sort of 'bogus pipeline'" |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up 6% at 16 m, did not differ across treatment groups. Analyses based on respondents; including losses would marginally increase estimated effect |
| Methods | Setting: Community, USA | |
| Participants | 1422 African‐American smokers; 36% M, av. age not stated, 62% in 20 ‐ 39 age group, median cigs/day 20 | |
| Interventions | Reactive, for callers to quitline | |
| Outcomes | Abstinence at 6 m (7‐day PP) | |
| Notes | Comparison between 2 types of counselling. Also included in Cochrane Self‐help review since effects of counselling and S‐H materials cannot be separated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Pseudo‐randomised by last digit of caller's contact phone number |
| Allocation concealment (selection bias) | High risk | Potential for selection bias but unlikely given low contact |
| Blinding of outcome assessment (detection bias) | Low risk | Self‐reported outcomes from participants not blinded to treatment condition, but similar levels of personal contact in different study arms |
| Incomplete outcome data (attrition bias) | Low risk | 37% lost to follow‐up at 6 m. No differential dropout, losses included as smokers |
| Methods | Setting: Occupational health service, USA | |
| Participants | 58 smokers; 93% M, av. age 52, av. cigs/day 22 | |
| Interventions | All participants received brief physician advice at screening | |
| Outcomes | Self‐reported abstinence at 6 m (30‐day PP) Validation: none | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes, not stated if opaque and numbered |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | 32% lost to follow‐up, comparable across groups, losses included as smokers |
| Methods | Setting: 10 counties, USA | |
| Participants | 1813 smokers planning to quit within 3 m; av. age 43, av. cigs/day 28 | |
| Interventions | Reactive | |
| Outcomes | Abstinence at 18 m (sustained from 3 m) | |
| Notes | The authors report a range of analyses based on alternative measures of smoking status and using logistic regression to allow for cluster randomisation. The higher quit rate in the hotline counties was consistent in all analyses. 36% called hotline, 8.7% spoke with counsellors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Matched pairs of counties assigned to condition in a restricted procedure to minimise media spill‐over |
| Allocation concealment (selection bias) | Unclear risk | Participant recruitment not linked to county assignment so selection bias unlikely |
| Blinding of outcome assessment (detection bias) | Low risk | Self‐reported abstinence verified by significant other and/or saliva cotinine |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up over 90% at all points and did not differ by condition |
| Methods | Setting: Community, USA | |
| Participants | 177 smokers aged ≥ 60, planning to quit in next 3 m; 39% M, av. cigs/day 25 | |
| Interventions | 1. S‐H manual (Clear Horizons), access to 24‐hour hotline, 2 letters of support and hotline reminders | |
| Outcomes | Abstinence at 6 m (7‐day PP) | |
| Notes | 42% had called hotline and 17.5% spoken to counsellor by 6 m | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Unclear risk | Validation by significant other, number refused/misreported not specified |
| Incomplete outcome data (attrition bias) | Low risk | 97% reached at 12 m |
| Methods | Setting: Washington State, USA; High school‐based Recruitment: High school smokers were identified by self‐report using a study‐administered baseline classroom survey | |
| Participants | 2151 smokers, 52.7% M, Age: < 16 years old: 0.1%; 16 years old 30.5%; 17 years old: 62.0%; > 17 years old: 7.4%; smokers were of variable motivation and wish for help with quitting | |
| Interventions | 1. No intervention 2. Proactive telephone intervention ‐ 10 calls of about 15 mins each | |
| Outcomes | Self‐reported abstinence at 7 years (6‐year prolonged) | |
| Notes | New for 2018 update Funding: "This study was supported by NCI grant R01‐CA082569" Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A matched‐pair randomization was performed via a computerized coin flip for each of 25 pairs of high schools, using pair‐matching of schools based on number of smokers, smoking prevalence, fraction of students eligible for free/reduced‐priced meals, and average stage of readiness to quit, so that the experimental and control conditions were balanced on these criteria" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | High risk | Abstinence not biochemically validated. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | Even after 7 years percentage of participants lost to follow‐up is small and comparable between arms |
| Methods | Setting: Winsconsin, USA; primary care clinics Recruitment: Quote: "During clinic visits, clinical care staff (i.e. medical assistants) were prompted by electronic health record technology to invite identified smokers to participate in a research program to help them to quit smoking" | |
| Participants | 637 smokers, 45% M, mean age 45.8, average cigs/day 17.7, participants were motivated to quit | |
| Interventions | 1. Minimal phone counselling ± preparation gum ± preparation patch ± in‐person counselling ± medication duration. Quote: "Participants assigned to the minimal condition received one 10‐minute session on the TQD that provided support and addressed motivation to quit, strategies for coping with craving and medication use." 2. Intense phone counselling ± preparation gum ± preparation patch ± in‐person counselling ± medication duration Quote: "Participants in the intensive condition received three 15‐minute phone sessions (TQD, days 2 and 10). These calls emphasized intra‐treatment social support, skill execution and avoidance of danger situations" | |
| Outcomes | Self‐reported abstinence at 26 weeks (7‐day PP) Validation: none | |
| Notes | New for 2018 update Funding: "This research was supported by grants 9P50CA143188 and 1K05CA139871 from the National Cancer Institute to the University ofWisconsin Center for Tobacco Research and Intervention and by theWisconsin Partnership Program. L.M. C. is also supported by NIH grants P50DA10075 and R01DK097364. This work was carried out in part while T.R.S. was a Primary Care Research Fellow supported by a National Research Service Award (T32HP10010) from the Health Resources and Services Administration to the University of Wisconsin Department of Family Medicine. J.W.C. is also supported by Merit Review Award 101CX00056 from the US Department of Veterans Affairs. W.‐Y.L. is also supported by NSF grant DMS‐1305725." Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "stratified permuted block randomization; we stratified by gender and clinic with a fixed block size of 32 based on the 32 unique treatment conditions (in random order within each block)" |
| Allocation concealment (selection bias) | Low risk | Quote:"Staff were blinded to randomization until eligibility was confirmed; participants were blinded until consent was provided" |
| Blinding of outcome assessment (detection bias) | High risk | Abstinence not biochemically validated. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | Higher attrition in the intensive counselling arm but difference is less than 20% |
| Methods | Setting: Community, USA | |
| Participants | 756 smokers (12% precontemplation, 58% contemplation, 30% preparation) (378 in relevant arms); 38% M, av. age 43, av. cigs/day 27 | |
| Interventions | 1. ALA S‐H manuals | |
| Outcomes | Self‐reported abstinence at 18 m (sustained at 12 m and 18 m) | |
| Notes | Arms 4 vs 3, TC vs S‐H alone. Numbers randomised to groups and quit rates as shown in graphs obtained from authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described, stratified by SoC |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | 'Bogus pipeline' approach; names of significant others asked for but not contacted |
| Incomplete outcome data (attrition bias) | Low risk | Attrition at each assessment averaged 4.1% ‐ 7.1% across all treatment conditions, not significantly different. 70% provided data at every assessment. MA uses numbers randomised, sensitivity analysis does not alter conclusions |
| Methods | Setting: Managed care organisation, USA | |
| Participants | 1447 smokers (723 in comparisons used); 38% were precontemplators, 44% M, av. age 38, av. cigs/day 20 | |
| Interventions | 1. Assessment only (completed questionnaires on 4 occasions) | |
| Outcomes | Self‐reported abstinence at 18 m (sustained for 6 m). Other measures of abstinence also reported | |
| Notes | Arms 3 vs 2, TC vs S‐H alone. Other arms compared in Self‐help review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Abstinence not biochemically validated. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | Greater loss to follow‐up in TC (44%) than S‐H (38%). Denominators here include losses to follow‐up and refusals. Author analysis suggests this treatment of missing data is biased, but sensitivity analysis excluding losses and refusals does not alter our MA conclusions |
| Methods | Setting: Quitline, USA | |
| Participants | 3522 smokers willing to make a quit attempt within 2 weeks | |
| Interventions | 1. 3 American Cancer Society S‐H booklets | |
| Outcomes | Abstinence at 6 m (sustained). Only people abstinent at 3 m followed at 6 m | |
| Notes | 58% did not complete more than 1 session of counselling (McAlister 2004) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Small local sample biochemically tested, no responders disconfirmed |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up 50% in Intervention, 55% in Control (from McAlister 2004). Differed by age, with higher loss in younger participants. All losses treated as smokers |
| Methods | Setting: National Cancer Society quitline, USA | |
| Participants | 6322 smokers; 30% M, av. age 43, median cigs/day 20 | |
| Interventions | ¼ allocated to S‐H control, remainder into 3 x 2 factorial design | |
| Outcomes | Abstinence at 7 m post‐randomisation (PP) | |
| Notes | All interventions pooled vs control, results of different intensities included in post hoc analyses and discussed in more detail in text. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence without stratification |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Unclear risk | Self‐reported outcomes from participants not blinded to treatment condition. Varying levels of contact between arms in multifactorial trial |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up ˜50%, similar in all groups. Analysis includes losses as smokers |
| Methods | Setting: Spain; smoking cessation outpatient clinics Recruitment: smokers attending smoking cessation outpatient clinics | |
| Participants | 600 smokers, 51.3% M, mean age 47.4, average cigs/day 25.3. Paticipants attended clinics to "receive medical assistance" ‐ they were interested in quitting | |
| Interventions | 1. Individual counselling: "seven individual sessions at 3, 5, 7, 10, 12, 24, and 52 weeks after the pre‐quit session." 2. Telephone counselling + Individual Counseling: "individual counselling interventions at weeks 3, 5, and 12 after the pre‐quit session, telephone counselling at weeks 7, 10, and 24, and a control session at the clinic at week 52." Sessions were between 15 and 20 mins | |
| Outcomes | Abstinence at 52 weeks (sustained from week 2 to 52) Validation: CO concentrations of < 10 ppm | |
| Notes | New for 2018 update Funding: "This study was supported by a grant from the Spanish Health Institute, Carlos III PI080418." Declarations of interest: several authors "have received honoraria for conferences from manufacturers of smoking cessation products." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "used a computer‐generated randomization system based on a permuted block randomization list where each block was used by one centre. An independent researcher in the coordination centre generated a random sequence, and centres were informed about smoker allocation after consent to participation during the pre‐quit session." |
| Allocation concealment (selection bias) | Low risk | Quote: "used a computer‐generated randomization system based on a permuted block randomization list where each block was used by one centre. An independent researcher in the coordination centre generated a random sequence, and centres were informed about smoker allocation after consent to participation during the pre‐quit session." |
| Blinding of outcome assessment (detection bias) | Low risk | Used biochemical verification to validate self‐reported outcome |
| Incomplete outcome data (attrition bias) | Low risk | Attrition was around 20% and comparable across arms |
| Methods | Setting: Community, Canada | |
| Participants | 396 smokers interested in quitting within 30 days, smoking ≥ 15 cigs/day; 52% M, av. age 38, av. cigs/day 23 ‐ 24 | |
| Interventions | 1. Nicotine patch (15 mg x 8 weeks, 10 mg x 2 weeks, 5 mg x 2 weeks) free, physician advice (x 3 15‐min, 2 weeks before, 4 weeks, 12 weeks after quit date) | |
| Outcomes | Abstinence at 12 m (PP) | |
| Notes | Effect of adjunct TC compared to NRT and counselling alone | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised using table of random numbers, stratified by gender and nicotine dependence |
| Allocation concealment (selection bias) | Unclear risk | Concealment unclear but physician blind to allocation |
| Blinding of outcome assessment (detection bias) | Low risk | 'Bogus pipeline' procedures used for early follow‐ups; proportion of participants who provided breath samples did not differ between 2 groups; only 1 misreport identified; adjustment of abstinence rates for validation did not affect conclusions |
| Incomplete outcome data (attrition bias) | Low risk | 15% lost/dropped out in each groups, included as smokers |
| Methods | Setting: Tertiary care cardiac hospital, Canada | |
| Participants | 100 smokers; 68% M, av. age 54, 48% quit attempt in previous year | |
| Interventions | All participants received in‐hospital brief counselling, access to NRT, S‐H materials | |
| Outcomes | Abstinence at 1 year (PP) | |
| Notes | Mean 2.1 IVR calls completed, 46% received at least 1 counselling call, mean 1.8, so total calls categorised as 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "mediated through the Clinical Epidemiology Unit’s data centre, using a computer generated randomization list" Block size 6 |
| Allocation concealment (selection bias) | Low risk | Quote: "Research staff were unaware of the treatment allocation prior to randomization" |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | ˜15% lost to follow‐up, similar between groups. 1 Control death excluded, others included |
| Methods | Setting: Canada; hospital‐based | |
| Participants | 410 hospital‐admitted CHD smokers, 74.4% M, av. age 54.2, 16% < 11 cigs/day, 33% 11 ‐ 20 cigs/day, 40% 21 ‐ 30 cigs/day, 11% > 30 cigs/day Not selected for motivation | |
| Interventions | 1. Standard care including in‐hospital counselling by nurse, written information about smoking cessation and NRT | |
| Outcomes | Abstinence at 52 weeks (continuous abstinence) | |
| Notes | New for 2018 update Funding: "Heart and Stroke Foundation of Ontario Grant # NA5845" Declarations of interest: "RDR and ALP have received speaking and/or consulting fees and research grants from Pfizer and Johnson & Johnson. KAM has received speaking fees from Pfizer. AGL is supported by a Canadian Institutes of Health Research–Ottawa Model for Smoking Cessation Health Impact Fellowship" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated sequence" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | Low risk | Validation of self‐reports in a random subsample achieving high rates of verified abstinence |
| Incomplete outcome data (attrition bias) | Low risk | Similar percentage of participants lost to follow‐up across arms (˜30%) |
| Methods | Setting: Prenatal care services, USA | |
| Participants | 442 pregnant women smoking at least 1 cig in previous 7 days; av. age 29, av. cigs/day 21 prior to pregnancy, 10 at recruitment, 84% planned to quit | |
| Interventions | All participants received brief counselling at enrolment call and mailed a pregnancy‐tailored S‐H booklet | |
| Outcomes | Abstinence 3 m postpartum (sustained at end of pregnancy and 3 m) | |
| Notes | Mean of 5 calls received, 4 in pregnancy, av. 68 mins in total | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomization list arranged in balanced blocks of 4 and stratified by referral source" |
| Allocation concealment (selection bias) | Low risk | Quote: "... the application revealed the next assignment only after the smoker had consented to participate in the study" |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemical validation; those who failed biochemical validation or did not provide a sample counted as smokers |
| Incomplete outcome data (attrition bias) | Low risk | 21 miscarriages excluded. 33% Intervention, 28% Control lost to follow‐up, included as smokers |
| Methods | Setting: Community, USA | |
| Participants | 1867 smokers aged 50 ‐ 75 (12‐m data based on 1391, 1225 in relevant arms) interested in finding out about quitting; 37% M, av age 61, av cigs/day 27 | |
| Interventions | 1. Standard S‐H manual (not included in this review) | |
| Outcomes | Abstinence at 12 m (7‐day PP) | |
| Notes | Arms 3 vs 2. Preliminary 12 m results used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | ˜75% reached at 12 m with no treatment group differences in follow‐up rate |
| Methods | Setting: NY, NJ, MA, VT, NH, RI, USA; 6 Veterans Health Administration (VHA) facilities in the U.S. Northeast Recruitment: Quote: "All smokers who saw a VHA mental health provider at participating sites were eligible for referral to the care coordination program by their usual mental health providers via an EMR consult created for the study" | |
| Participants | 577 smokers, 92% M, av. age 54, av. cigs/day 15.9. Participants were referred from a VHA mental health provider and were interested in quitting | |
| Interventions | 1. State quit‐line counselling 2. Specialised proactive telephone counselling protocol developed by the study for mental health patients ‐ 10 or fewer calls, weekly and for 30 ‐ 60 mins Participants in both groups also received mailed S‐H materials and smoking cessation medications | |
| Outcomes | Self‐reported abstinence at 6 m (30‐day PP) Validation: none | |
| Notes | New for 2018 update Funding: "This trial was funded by the U.S. Department of Veterans Affairs Health Services Research and Development Quality Enhancement Research Initiative (#SDP 07‐034)." Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Pseudo‐randomised using social security number last digit (odd/even number). This resulted in nearly balanced groups except for the fact that participants in the specialised counselling arm smoked a significantly higher number of cigarettes than participants in the quitline arm |
| Allocation concealment (selection bias) | High risk | Unlikely to apply to this method of randomisation |
| Blinding of outcome assessment (detection bias) | High risk | Abstinence not biochemically validated. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | Similar rates of attrition in both arms |
| Methods | Setting: Winsconsin, USA; primary care clinics Recruitment: Recruited during primary care visits (11 primary care clinics in 2 healthcare systems). Existing clinical care staff (i.e. medical assistants), prompted by electronic health record technology, invited identified smokers during clinic visits to participate in a research programme to help them quit smoking | |
| Participants | 544 smokers, 41% M, av. age 46.2, av. cigs/day 18.6, motivated to quit | |
| Interventions | 1. No maintenance (phone) counselling ± extended medication ± (on site) medication adherence counselling ± automated adherence calls ± helping hand (HH) with feedback and counselling 2. No automated adherence calls ± extended medication ± (on site) medication adherence counselling ± maintenance (phone) counselling ± helping hand (HH) with feedback and counselling (not used in analysis) 3. Automated adherence calls ± extended medication ± (on site) medication adherence counselling ± maintenance (phone) counselling ± helping hand (HH) with feedback and counselling (not used in analysis) 4. Maintenance (phone) counselling ± extended medication ± (on site) medication adherence counselling ± automated adherence calls ± helping hand (HH) with feedback and counselling ‐ 8 x 15‐min calls at weeks 3, 4, 6, 8, 10, 14, 18 and 22 Quote: "All participants received a standard cessation intervention: 8 weeks of nicotine patch+nicotine gum and 50 minutes of counseling delivered over four sessions [in visits 1 week before and 1 week after the target quit day (TQD), and in calls on the TQD and at week 2]." | |
| Outcomes | Self‐reported abstinence at 52 weeks (7‐day PP) Validation: none | |
| Notes | New for 2018 update Funding: "This research was supported by grants 9P50CA143188 and 1K05CA139871 from the National Cancer Institute to the University ofWisconsin Center for Tobacco Research and Intervention and by theWisconsin Partnership Program. L.M. C. is also supported by NIH grants P50DA10075 and R01DK097364. This work was carried out in part while T.R.S. was a Primary Care Research Fellow supported by a National Research Service Award (T32HP10010) from the Health Resources and Services Administration to the University of Wisconsin Department of Family Medicine. J.W.C. is also supported by Merit Review Award 101CX00056 from the US Department of Veterans Affairs. W.‐Y.L. is also supported by NSF grant DMS‐1305725." Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Participants were randomized to one of 32 unique experimental conditions… via a database that used stratified, computer‐generated, permuted block randomization…” |
| Allocation concealment (selection bias) | Low risk | Quote: “Staff could not view the allocation sequence. The database did not reveal participants’ treatment condition to staff until participants’ eligibility was confirmed; participants were blinded to treatment condition until they provided consent.” |
| Blinding of outcome assessment (detection bias) | High risk | Abstinence not biochemically validated. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | Attrition is much larger in Maintenance Counselling arm (51%) than in No Maintenance Counselling arm (44%) |
| Methods | Setting: Netherlands; school‐based Recruitment: Quote: "Smoking parents were recruited through their children’s primary schools across the Netherlands. Primary schools were contacted by research assistants and asked to distribute study invitation letters to parents through children." [...] "Parents registered to take part by mail, e‐mail, telephone or via a website." | |
| Participants | 512 daily or weekly smokers and parents or caretakers of a child aged between 9 and 12 years. They were considering quitting smoking (currently or in the future). 47.5% M, av. age 42.2, av. cigs/day 16.2 | |
| Interventions | 1. "Standard Self‐Help Brochure: Participants received a 40‐page, colour‐printed self‐help brochure including didactic information on nicotine dependence and the health benefits of quitting smoking, tips and advice on how to initiate and maintain abstinence, instruction in the use of cognitive and behavioural skills to avoid triggers to smoke and cope with urges to smoke, and strategies for managing a lapse or relapse to smoking 2. Intensive Proactive Quitline Counselling + supplementary materials tailored to smoking parents; mean number of calls completed was 5.5 and these were scheduled for 10 days before quit day, 3 days, 1, 2, 4 weeks, 2, and 3 months after quit day." Quote: "In addition, all participants received three accompanying booklets entitled Smoke‐free parents which were designed for this study as tailored supplementary materials. Each booklet (four pages, colour‐print) contained didactic information, tips and advice, motivational messages, as well as ‘parent‐relevant information’; e.g. effects of second‐hand smoke (SHS) on children, strategies to manage parent‐specific stressors]." | |
| Outcomes | Abstinence at 12 m (6‐m prolonged) Validation: breath CO and saliva cotinine analysis in a random subsample (36 out of 133) | |
| Notes | New for 2018 update Funding: "This work was supported by ZonMW, the Netherlands Organization for Health Care Research and Development (grant number: 50‐50110‐96‐639)." Declarations of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "To ensure equal group sizes, allocation was performed in blocks of 10. To ensure balance of key characteristics, stratified randomization was used based on the stratifying variables gender, educational level (low: no high school diploma/no vocational training, medium: vocational training or high school diploma, high: college degree) and cigarettes per day (fewer than 10, 10–20, 21 or more)." |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation of participants to trial conditions was conducted by an independent member of the research group using a computer‐generated allocation sequence." [...] "The independent researcher prepared a list of study participants and their allocated treatment. Based on this list, the first author prepared the mailings which informed study participants about the treatment they would receive." |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemical validation not done in the entire sample, but just in a random subset. However, verified abstinence rate of 82% (18 of 22) overall in the subsample was acceptable, with no significant different across arms |
| Incomplete outcome data (attrition bias) | Low risk | Even though "There was a significant difference in the follow‐up rate between treatment groups (85.5% in the quitline condition and 89.5% in the self‐help condition, χ2 = 4.98, P = 0.03)", overall attrition was low (11%). Furthermore "Participants lost at follow‐up did not differ on baseline characteristics compared with the remaining participants, neither across nor within conditions (all P > 0.05)." |
| Methods | Setting: California and Nevada, USA; Department of Veteran Affairs outpatient primary care clinics Recruitment: Healthcare "providers were encouraged to refer any patient who smoked and was interested in quitting" | |
| Participants | 3120 smokers, 94% M, av. age 54.2, av. cigs/day 18.3. Participants were interested in quitting | |
| Interventions | 1. Reactive Self‐Help: "Participants referred during a reactive week were mailed an invitational letter, and the co‐ordinator waited for the patient to initiate a call. If patients in the reactive condition called, they only received medication co‐ordination along with their self‐help materials." 2. Proactive Self‐Help: "Participants referred during a proactive week were sent self‐help materials and telephoned by the care co‐ordinator only to discuss medication." 3. Reactive Telephone Counselling: "Participants referred during a reactive week were mailed an invitational letter, and the co‐ordinator waited for the patient to initiate a call." 4. Proactive Telephone Counseling: "Participants referred during a proactive week were contacted by the care co‐ordinator, who tried up to five times by phone over 2 weeks to reach them." 5 or fewer calls occurred per participant "All patients referred received standard care from their primary care provider prior to referral, which typically included brief cessation advice. [...] Patients in both studies received cessation medication [...] 2 months of nicotine patches or 2 months of bupropion" | |
| Outcomes | Self‐reported abstinence at 6 m (7‐day PP) Validation: none | |
| Notes | New for 2018 update Funding: "This work was supported by VA HSR&D grant number #IMV 04–088." Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "We randomised all participating sites to either self‐help or multisession quitline counselling using a random number table." |
| Allocation concealment (selection bias) | Unclear risk | No method to conceal allocation was mentioned |
| Blinding of outcome assessment (detection bias) | High risk | Abstinence not biochemically validated. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | Attrition was low, around 17% ‐ 29% across arms |
| Methods | Setting: Wisconsin, USA Recruitment: Young adult callers to the Wisconsin Tobacco Quit Line (WTQL) | |
| Participants | 410 smokers age 18 ‐ 24 years, smoked at least 1 cig in past 30 days and motivated to quit. 42% M; av. age 21.3 years, av. cigs/day 15 | |
| Interventions | 1. S‐H only, stage‐based booklets 2. S‐H + up to 4 proactive cessation counselling calls over 4 ‐ 6 weeks through the WTQL | |
| Outcomes | Abstinence at 6 m (7‐day PP) Self‐report | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | List of random numbers |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Abstinence not biochemically validated. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Unclear risk | 53% not followed in Intervention, 50% in Control. Missing treated as smoking. Responder analysis did not change results |
| Methods | Setting: Denmark; population‐based Recruitment: Quote: "Participants were recruited from two national health surveys: the Danish Health Examination Survey (DANHES) (2007–08) and the Danish Health and Morbidity Survey (DHMS) (2010) [...] The invitation letter was sent by e‐mail or letter" | |
| Participants | 1810 smokers aged 16 and over, 42% M, av. age 51, av. cigs/day 16. Participants were willing to quit smoking within the next 12 weeks | |
| Interventions | 1. Self‐help booklet: Participants received a 36‐page booklet by letter. It was developed by the Danish National Board of Health, and included advice on how to identify difficult situations and develop coping strategies at specific stages in the smoking cessation process. Setting a quit date was encouraged. The Fagerström Test for Nicotine Dependence was also included along with information about pharmacotherapy 2. Reactive telephone counselling: A session lasted for approximately 13 – 15 mins 3. Proactive telephone counselling: 5 counsellor‐initiated sessions | |
| Outcomes | Self‐reported abstinence at 12 m (prolonged) Validation: none | |
| Notes | New for 2018 update Funding: "The study was funded by the Danish Cancer Society" Declarations of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "In order to assure equal group sizes, participants were sorted by exact date and time of enrolment and a fixed sequence of four numbers was assigned repeatedly." Not truly random method but still groups are well‐balanced |
| Allocation concealment (selection bias) | Low risk | Quote: "The procedure was conducted by a research assistant who was blinded to the participants’ names and ID numbers during the procedure." "there is little indication of bias related to the allocation procedure, as the participants were unknown to the person allocating them and a large number of participants were allocated at the same time." |
| Blinding of outcome assessment (detection bias) | High risk | Abstinence not biochemically validated. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | Attrition was low, ˜20% and similar across arms |
| Methods | Setting: 10 communities, Canada | |
| Participants | 632 smokers intending to quit; 39% M, av. age 42, 61% had prior use of NRT | |
| Interventions | Factorial design comparing 2 intensities of TC and 2 types of S‐H (collapsed in this review) | |
| Outcomes | Self‐reported abstinence at 12 m (sustained). Also at 3 m and 6 m follow‐ups, also PP | |
| Notes | All TC arms compared to S‐H‐only control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, stratified by community, method not described |
| Allocation concealment (selection bias) | Low risk | Quote: "opening next in a series of envelopes' after enrolment" |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | 30% not available at 12 m, no difference across 5 groups, missing treated as smoking |
| Methods | Seting: Quitline, USA | |
| Participants | 987 smokers, > 10 cigs/day, willing to set quit date within 30 days: av. age 42, av. cigs/day 21 | |
| Interventions | Factorial trial testing medication adherence counselling, 2 vs 6 weeks NRT, and nicotine patch alone vs patch + gum All participants received the same standard TC: 4 sessions over 4 weeks Medication adherence counselling involved additional content at each call assessing and addressing adherence | |
| Outcomes | Abstinence at 6 m (30‐day PP). 7‐day PP also reported Validation: none | |
| Notes | Not included in any MA as tested adjuncts to TC, not the efficacy of TC. Results reported narratively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | List of randomised numbers |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Abstinence not biochemically validated, but similar levels of personal contact in different study arms |
| Incomplete outcome data (attrition bias) | Unclear risk | 24% lost at 6‐m follow‐up, no difference across treatment groups |
| Methods | Setting: Community, USA | |
| Participants | 214 women smokers, > 4 cigs/day, intending to quit in next 2 weeks; av. age 33, av cigs/day 24 | |
| Interventions | 1. Free nicotine patch (dose based on smoking level) for up to 10 weeks | |
| Outcomes | Abstinence at 6 m (7‐day at 3 m and 6 m) | |
| Notes | Intervention participants received an average of 7 calls. 95% received at least 1. Participants could call Nicoderm support line, 21% of control vs 8% of intervention did so | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Biochemical validation but 7% ‐ 12% disconfirmation rate. Differential rates of return at 6 m (59% of self‐reported quitters in intervention group and 67% in control). Participants who did not provide samples classified as quitters |
| Incomplete outcome data (attrition bias) | Low risk | ˜27% lost in both groups, included as smokers |
| Methods | Setting: Community, USA | |
| Participants | 330 women smokers > 4 cigs /day, intending to quit in next 2 weeks; av. age 34, av. cigs/day 24 | |
| Interventions | 1. Free nicotine patch (dose based on smoking level) for up to 10 weeks | |
| Outcomes | Abstinence at 6 m (30‐day at 3 m and 6 m) | |
| Notes | Replication of Solomon 2000 with more extended telephone contact | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | 13% lost to follow‐up in both groups, included as smokers |
| Methods | Setting: ALA Quitline, USA | |
| Participants | 990 callers; 38% M, av. age 43, av. cigs/day 22 | |
| Interventions | 1. Reactive counselling | |
| Outcomes | Abstinence at 12 m (PP) | |
| Notes | Test of different interventions for people calling a quitline. Comparison 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number list created by independent statistician |
| Allocation concealment (selection bias) | Low risk | Enrolment and assignment by researchers independent of helpline staff. Concealment until assigned |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Interviewer assessing outcomes was blinded"; biochemical validation in a convenience sample (16/28 agreed); participants who did not agree to biochemical validation but self‐reported abstinence counted as abstinent |
| Incomplete outcome data (attrition bias) | Low risk | 47% loss to follow‐up, similar across groups, included as smokers |
| Methods | Setting: Workplaces, USA | |
| Participants | 231 smokers completed baseline survey. Demographics for all participants followed up; 94% M, av. age 40 | |
| Interventions | 1. Proactive counselling; up to 6 calls over 3 m (fruit and veg consumption also addressed), tailored feedback report and tip sheets, NRT offered to those interested in quitting | |
| Outcomes | Abstinence at 6 m (7‐day PP) | |
| Notes | Baseline denominators confirmed by author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | 18% ‐ 20% lost, assumed smokers |
| Methods | Setting: Antenatal clinics, USA | |
| Participants | 269 pregnant smokers at week 28; av. age 28, approx 50% smoked < 60 cigs/week | |
| Interventions | All participants had received brief counselling and 7 mailed S‐H booklets in early pregnancy | |
| Outcomes | Abstinence or 'a few puffs' at 6 m postpartum | |
| Notes | The common intervention in early pregnancy was not treated as face‐to‐face contact within the trial. 55% received complete intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Unclear risk | Although no biochemical validation postpartum, cotinine in subsample at week 34; no differences between experimental and control groups; Quote: "the urine samples appeared not to have been collected in a systematically biased manner." Level of misreport and refusal not specified |
| Incomplete outcome data (attrition bias) | Unclear risk | 39% lost at follow‐up in both groups, assumed to be smoking |
| Methods | Setting: Illinois and Missouri, USA; worksite employees & spouses Recruitment: Quote: "Participants called a toll free number (866‐902‐QUIT) to initiate enrolment. [...] Both organizations promoted Call‐2‐Quit through multiple channels including health fairs, employee web sites, employee news, promotional posters, fliers, and department managers. Each organization promoted Call‐2‐Quit to help smokers adapt to tobacco control policies implemented during the trial. In 2006, the hospital system implemented health insurance discounts of $10/month for employees who committed, during open enrolment in November, to pursue several health promoting activities. Smokers obtained the discount by “enrolling” in a qualifying smoking | |
| Participants | 518 employee and spouse smokers, 34% M, av. age 46.5, av. cigs/day 12.9. Participants were seeking treatment as they called the toll‐free number | |
| Interventions | 1. Directive telephone coaching ‐ Directive coaching included the following distinctive features: 2. Nondirective telephone coaching ‐ Nondirective coaching included these distinctive features: There were up to 7 weekly calls, for 15 ‐ 20 mins each | |
| Outcomes | Self‐reported abstinence at 12 m (7‐day PP) Validation: mailed saliva cotinine assays or witnessed cheek swabs attempted, but low return rate | |
| Notes | New for 2018 update Funding: "The Centers for Disease Control grant number R01 DP000098 funded this study." Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "New families were randomized to directive or nondirective coaching mode in a 1:1 ratio, based on a randomization table, when the first member enrolled. Consent to randomization was required to participate." |
| Allocation concealment (selection bias) | Low risk | Quote: "After baseline data were entered, members of a previously randomized family were assigned to the family coaching mode." |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome validation attempted but low return rate. Similar levels of personal contact in different study arms |
| Incomplete outcome data (attrition bias) | High risk | After 12 months of follow‐up the proportion lost to follow‐up was larger than half the initial sample, although similar across arms |
| Methods | Setting: Group Health Co‐operative, USA | |
| Participants | 1524 smokers ≥ 10 cigs/day; 43% M, av. age 45, av. cigs/day 23, 44% history of depression | |
| Interventions | Proactive | |
| Outcomes | Abstinence at 12 m (7‐day PP) | |
| Notes | Compares different intensities of TC. No dose/behavioural treatment interaction at 12 m so bupropion arms collapsed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation procedure built into study database |
| Allocation concealment (selection bias) | Low risk | Procedure ensured concealment |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up at 12 m 17% Intervention, 12% Control, treated as smokers |
| Methods | Setting: Community, Idaho and Washington, USA Recruitment: Community advertising, physician referral, and quitline callers | |
| Participants | 1202 adult current smokers of at least 10 cigs/day in past year and 5 cigs/day in past week, motivated to quit. 33.1% M; av. age 47.3; av. cigs/day 19.7; av.FTND 4.9 | |
| Interventions | All participants received: 12‐week course of varenicline; 5 ‐ 10‐min orientation call; S‐H materials; access to toll‐free support line for ad hoc calls 1. Telephone counselling. Proactive; from quitline counsellor using MI techniques; max 5 calls 2. Web programme with standardised content and interactive tools modelled on those used in phone intervention 3. 1+2. Phone counsellors had access to info participants entered online | |
| Outcomes | Abstinence at 6 m (30‐day PP) (abstinence at 3 m, 7‐day PP also reported) Validation: none | |
| Notes | Number abstinent not provided, estimated from percentages given in published report TC and TC+web had similar outcomes so pooled 1 + 3 vs 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Group assignment was randomly allocated using an automated algorithm built into the study database" |
| Allocation concealment (selection bias) | Low risk | Central computerised allocation, see above |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcome measure used from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | Participants lost to follow‐up counted as smokers in ITT analysis; equal losses between groups (103 Web, 107 Phone, 100 Web + phone) |
| Methods | Setting: Minnesota, Ohio, Texas, Wisconsin, USA; higher education students Recruitment: Quote: "e‐mails and promotional postcards direct‐mailed to all enrolled students" | |
| Participants | 1217 adult undergraduate smokers willing to set a quit date approximately 1 month from study eligibility assessment, 45% M, av. age 26.2, av. cigs/day 11.5 | |
| Interventions | 1. No TC ± single/multiple contests 2. TC ± single/multiple contests: 6 telephone‐administered Motivation and problem solving (MAPS) counselling sessions during the 12 week treatment, each over 20 minutes, 10 days prior to quit date, and at the discretion of the participant | |
| Outcomes | Abstinence at 6 months (continuous) Validation: Biochemical verification using urine, using NicCheck and (if NicCheck was negative) NicAlert test strips to verify self‐reported abstinence. Also, Quote: “if a participant reported that he or she had used NRT within the past 7 days, the sample was sent instead to the laboratory for analysis of concentrations of anatabine/anabasine” | |
| Notes | New for 2018 update Funding: "This study was funded by the National Heart, Lung, and Blood Institute (5R01‐HL094183‐04S1, J.L.T., Principle Investigator)." Declarations of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Low risk | Not blinded but biochemical validation so differential misreport judged unlikely |
| Incomplete outcome data (attrition bias) | Low risk | Around 19% of participants did not complete or were lost to follow‐up (similar n lost to follow‐up in each arm (1. 47/306; 2. 50/309; 3/ 67/296; 4. 71/306). Authors tested impact in sensitivity analyses and state it did not affect conclusions |
| Methods | Setting: Workplace and community, USA | |
| Participants | 382 (341 smokers, 41 recent quitters). Most in contemplation or action SoC, 24% 'blue‐collar', 41% M, av. age 41, av. cigs/day 18 ‐ 22 | |
| Interventions | 1. Callers to hotline received general information based on fact sheets, and sent S‐H material | |
| Outcomes | Abstinence at 6 m (PP) (subset followed to 12 m) | |
| Notes | Comparison between stage‐based and non‐specific brief counselling | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Saliva samples sought but not tested; surrogates asked to confirm status |
| Incomplete outcome data (attrition bias) | Low risk | 17% lost to follow‐up at 6 m, no significant difference between groups, included as smokers |
| Methods | Setting: Community, New South Wales, Australia Recruitment: Active telephone recruitment (cold‐calling) of NSW residents, motivation to quit not required | |
| Participants | 1562 adult daily smokers. 50% M, av. cigs/day 19.4, av. age 45 | |
| Interventions | 1. 6 proactive counselling calls for smokers willing to quit within 1 m, 4 for those not willing using MI techniques. Those who relapsed and set new quit date within a month offered additional 5 calls; those relapsed but did not set quit date offered a call in 1 m. Those initially not willing to quit who became motivated to quit offered additional 5 support calls. Standard S‐H materials 2. S‐H materials only | |
| Outcomes | Self‐reported abstinence at 13 m (prolonged for 12 m with 1 m grace period). Other prolonged and PP rates at 4, 7, 13 m also reported Validation: none | |
| Notes | 7.8% of control group called quitline during study period. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random number generator" |
| Allocation concealment (selection bias) | Low risk | Quote: "computer assisted telephone interview used a random number generator created by an independent programmer to allocate the smoker" |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcome measure used, participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Unclear risk | Participants lost to follow‐up counted as smokers; similar numbers in both groups (163 intervention, 154 control (21% and 19% respectively)) |
| Methods | Setting: USA; rural setting Recruitment: People meeting basic eligibility criteria were sent a letter offering them participation in the trial, to which they could respond by returning a self‐addressed postcard or contacting study staff by phone. Those expressing interest were mailed an informed consent document and baseline questionnaire, which included Vander Weg et al. BMC Public Health (2016) 16:811 Page 2 of 11 screening items to assess for eligibility for the supplemental behavioral counselling modules (described below) | |
| Participants | 63 rural Veteran daily cigarette smokers who were interested in quitting, 87.3% M, av. age 56.8, av. cigs/day 24.7 | |
| Interventions | 1. Referral to state tobacco quitline: Referred by fax to the tobacco quitline for their state of residence. Quitlines subsequently contacted participants to initiate treatment 2. Tailored telephone counselling: Combines counselling on tobacco use and related issues including depressive symptoms, risky alcohol use, and weight concerns. 6 calls, 1 per week, for 20 ‐ 30 mins The approach to pharmacotherapy was the same for both groups ‐ NRT, bupropion, varenicline | |
| Outcomes | Self‐reported abstinence at 6 m (7‐day PP) Validation: none | |
| Notes | New for 2018 update Funding: "The work reported in this manuscript was funded by the Department of Veterans Affairs Office of Rural Health (Project number 12‐CR6)." Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned to treatment conditions based on a computer‐generated algorithm on a 1:1 allocation ratio using simple randomization without blocking. The computerized random allocation sequence was generated by the study data manager." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | High risk | Abstinence not biochemically validated. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | Dropout was twice as high in the tailored intervention as in the standard tobacco quitline group |
| Methods | Setting: Community, USA | |
| Participants | 2054 smokers (1009 in relevant arms); 76% M, av. age 51, 40% precontemplators, 40% contemplators, 20% preparers | |
| Interventions | 1. Stage‐based S‐H manuals; participants sent manual for current stage and next stage. (not used in this review) | |
| Outcomes | Self‐reported abstinence at 30 m (sustained for 6 m) | |
| Notes | Comparison of arms 4 vs 3 for proactive TC. In NRT eligible groups 350 (67%) received NRT at baseline and 448 (86%) received NRT at some point, so classified as adjunct to pharmacotherapy, and in > 6 call category | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based random‐number generator |
| Allocation concealment (selection bias) | Low risk | Allocation done after completion of survey. randomised participants who did not return consent form are excluded from further analyses |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | 39% lost includes 8% refused by 30 m, no significant differences between groups. Different treatments of missing data reported not to have altered pattern of results |
| Methods | Setting: Olmsted County, MN, USA; hospital‐based Recruitment: Identified through electronic medical records. Study personnel then approached the potential participants to confirm eligibility | |
| Participants | 600 adult smokers, 51% M, av. age 46.3, av. cigs/day 14.4 | |
| Interventions | 1. Brief (˜5‐min) cessation advice: Quote: "Consisted of the first four of the 5A’s (Ask, Advise, Assess, Assist, and Arrange), including advice and brief assistance in reviewing tips to help maintain abstinence using a brochure. The brochure included the study quitline number but did not specifically encourage its use." 2. Brief (˜5‐minute) quitline facilitation intervention: Quote: "A single brief quitline facilitation intervention (also ˜5 minutes in duration, slightly modified from that piloted in the authors’ prior studies of presurgical patients) was delivered. Based on principles of Social Cognitive Therapy, it included advice to quit and quitline information. Its purpose was to facilitate quitline utilization, not to provide assistance with quitting, but to overcome cognitive barriers to quitline utilization. A written brochure that included information about the quitline and a wallet‐sized “quit‐card” were provided. If patients were amenable, study personnel then contacted the quitline provider, preferably by direct phone call (“warm handoff”) to enroll the patient for quitline services and arrange for an initial counseling call. If this direct contact could not be made, faxed referrals were sent to the quitline provider. Based on early experiences that it was difficult for the quitline to re‐contact patients while in hospital, the goal was to complete the first counseling session immediately after the in‐hospital quitline intake. Subsequent counseling sessions were scheduled by quitline counselors." NRT was offered to all participants: free 2‐week supply of nicotine patches | |
| Outcomes | Abstinence at 6 m (7‐day PP) Validation: Urine anabasine levels < 2 ng/mL | |
| Notes | New for 2018 update Funding: "This work was supported by grant RC‐2012‐0001 from ClearWay Minnesota." Declarations of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using dynamic randomization allocation based on the Mayo Clinic Study Data Management System, a proprietary web application for data entry and management. Randomization was stratified based on nursing unit to ensure the number of subjects assigned to each of the two intervention groups remained balanced within that unit, enhancing the homogeneity of admitting diagnoses between groups" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically‐confirmed smoking cessation |
| Incomplete outcome data (attrition bias) | Low risk | Low attrition ˜30% and comparable across arms |
| Methods | Setting: Beijing, China; 2 Endocrinology and Acupuncture out‐patient clinics of a general hospital | |
| Participants | 369 adult smokers who smokers 10 or more cigs/day and were not interested in quitting, 100% M, av. age 40, 43% 10 – 19 cigs/day, 57% ≥ 20 cigs/day | |
| Interventions | 1. Exercise and diet advice (EDA) control group Both groups received a single face‐to‐face brief advice (˜1 min) + 5 x TC follow‐up sessions of the same duration (˜1 min) after 1 week, and after 1, 3, 6 and 12 m | |
| Outcomes | Abstinence at 12 m (7‐day PP) | |
| Notes | New for 2018 update Funding: "This study was supported by a research grant from the National Natural Science Foundation of China (81373080), a research grant from the Beijing Municipal Science and Technology Commission (Z121107001012070) and Clinical Research Grants from the Chinese PLA General Hospital (2013FC‐TSYS‐1021 and MJ201447)." Declarations of interest: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A research assistant of the project generated the random numbers for group assignment using a computer" |
| Allocation concealment (selection bias) | Low risk | Quote: "After written consent, a trained counsellor who was not involved in preparing the randomization sequence opened a serially numbered, opaque and sealed envelope with a card inside indicating intervention or control and randomly allocated the participant accordingly, thus ensuring allocation concealment" |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically‐validated outcome. Validation rate achieved 43.2% by February 2017 (45.8% in the SRI group and 38.5% in the EDA control group) |
| Incomplete outcome data (attrition bias) | Low risk | Percentage of participants lost to follow‐up was around 30% and similar across groups. There were no differences between those who completed and those who were lost to follow‐up |
| Methods | Setting: General practices, Australia | |
| Participants | 318 smokers; 47% M, av. age ˜37, modal cigs/day 11 ‐ 20, 56% in contemplation/precontemplation | |
| Interventions | 1. GP offered referral; telephone call from a nurse trained in cessation within 3 days. 5As counselling framework. If willing to make a quit attempt mailed quit kit, encouraged to buy NRT, phoned again on TQD, 1 week, 3 weeks | |
| Outcomes | Abstinence at 12 m (PP) | |
| Notes | We classified control as minimal intervention rather than brief intervention, MA not sensitive to classification. Referral was to a research nurse not to a dedicated quitline. 5 control participants received intervention, analysed with controls as ITT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Questionnaires randomly ordered and coded prior to delivery to the practice by selecting sequential numbers from a computer‐generated random‐number list. |
| Allocation concealment (selection bias) | Unclear risk | Participants (including non‐smokers) completed the precoded questionnaire before the consultation. GP identified allocation from unobtrusive marks on questionnaire, could not influence allocation. But unclear whether selection bias by recruiters, given imbalance in numbers |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | 31% Intervention, 41% Control lost to follow‐up, included as smokers |
| Methods | Setting: Quitline, USA | |
| Participants | 3030 smokers calling smokers' helpline and were ready to quit in next week; 43% M, av. age 36, av. cigs/day 20 | |
| Interventions | 1. S‐H materials only | |
| Outcomes | Abstinence at 13 m (sustained for 12 m) | |
| Notes | Arms 2 and 3 vs 1. Arms 3 vs 2 in effect of multiple sessions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Pseudo‐random, according to last 2 digits of telephone number |
| Allocation concealment (selection bias) | High risk | Potential for selection bias but unlikely, given low contact |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemical validation in a convenience sample. Disconfirmation rate not used to correct data, but refusal and misreport rates similar in all groups |
| Incomplete outcome data (attrition bias) | Low risk | 12% ‐ 16% lost to follow‐up at 13 m, included as smokers |
| Methods | Setting: Quitline, USA | |
| Participants | 3282 smokers calling quitline, ready to quit within 1 week and wanting counselling; 44% M, av. age 38, av. cigs/day 20 | |
| Interventions | 1. S‐H pack, motivational materials, counselling provided if smoker made contact to request it | |
| Outcomes | Self‐reported abstinence at 13 m (sustained for 12 m) | |
| Notes | Authors also analysed subgroups of controls who did and did not seek counselling. 32% of Control and 72% of Intervention group received counselling | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described. 60/40 split. Only randomised when counselling demand exceeded capacity |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition. Level of personal contact differed between arms |
| Incomplete outcome data (attrition bias) | Low risk | ˜30% lost to follow‐up at 13 m in both groups, included as smokers |
| Methods | Setting: Quitline, USA Recruitment: Callers to a quitline | |
| Participants | 2278 Chinese‐, Korean‐ and Vietnamese‐speaking daily smokers, ready to quit within 1 m; 90% M; aged 18 ‐ 75 (approx. 45% 25 ‐ 44 and 45% 45 ‐ 64); av. cigs/day 15.6 | |
| Interventions | 1. S‐H pack, culturally‐tailored, translated into Chinese, Korean and Vietnamese 2. S‐H pack + proactive TC; Social Learning Theory; MI; CBT techniques. 30 ‐ 40 mins, pre‐quit, up to 5 relapse prevention calls (10 ‐ 15 min) 0, 3, 7, 14, 30 days | |
| Outcomes | Prolonged abstinence at 7 m post‐intervention, 1 m grace period immediately post‐quit Validation: none (but saliva samples collected) | |
| Notes | Number abstinent at 6 m not specified; data used in MA calculated back from percentages | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned…using blocks of 20 to keep a balance of language and sex…Random assignment tables for each strata were created using SAS 9.2." |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation was done by the computer so that staff were blinded to group assignment until the intake call" |
| Blinding of outcome assessment (detection bias) | Low risk | Self‐reported outcomes but saliva samples collected. No statistically significant differences in saliva sample return rates at 7 m between intervention and control groups and between self‐reported quitters and non‐quitters |
| Incomplete outcome data (attrition bias) | Low risk | Simlar rate of dropouts in both groups (18% in 1, 16% in 2). Participants lost to follow‐up included as smokers in outcome data |
| Methods | Setting: Australia; community‐based Recruitment: Patients were approached in the waiting room of participating practices by trained research assistants over a 2‐week period and assessed for eligibility | |
| Participants | 2390 adult smokers, 46% M, av. age 42.8, av. cigs/day 17.1 | |
| Interventions | 1. Usual care: In control group practices, the GPs were asked to assess participants’ willingness to quit and offer assistance in accordance with their usual practice. This could include advice within the practice, referral to Quitline or both, but no provision was made to facilitate either. 2. Quit with practice nurse: Individual face‐to‐face counselling with nurse. Quit kits (a printed resource used by Quitlines nationally) were also distributed to participants. Nurses were also supported by 3 proactive telephone calls from an experienced counsellor. 3. Quitline referral: GPs were asked to assess the participants’ willingness to quit and to offer brief advice. All were offered, according to clinical practice guidelines, free patches of NRT for 8 weeks | |
| Outcomes | Self‐reported abstinence at 12 m (sustained ≥ 10 m) Validation: none | |
| Notes | New for 2018 update Funding: "Australian National Health and Medical Research Council Project Grant (568617)" Declarations of interest: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Quote: "patients were given a card indicating their enrolment and the allocation group of the practice to take into the GP consultation" |
| Blinding of outcome assessment (detection bias) | High risk | Abstinence not biochemically validated, but similar levels of personal contact in different study arms |
| Incomplete outcome data (attrition bias) | Low risk | Similar % of lost to follow‐up across arms |
AHRQ: Agency for Healthcare Research and Quality; ACT: Acceptance and Commitment therapy; ALA: American Lung Association; av: average; CHD: chronic heart disease; CBT: cognitive behavioural therapy; CO: carbon monoxide; COPD: chronic obstructive pulmonary disease; F: female; FTND: Fagerström Test for Nicotine Dependence; HMO: health maintenance organisation; hrs: hours; HTN: hypertension; ITT: intention‐to‐treat (analysis); m: months; M: male; MA: meta‐analysis; MI: motivational interviewing; NCIS: National Cancer Information Service; NRT: nicotine replacement therapy; PP: point prevalence; ppm: parts per million; SES: socio‐economic status; S‐H: Self‐help materials; SHS: Second‐hand smoking; SoC: Stage of change; TC: Telephone counselling; TQD: Target quit date
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Text message intervention which encouraged participants to call a quitline | |
| Pilot study with 12 weeks follow‐up, after which the advice and control groups were offered the intervention. The intervention was 4 x weekly mailings and telephone calls from a lay facilitator. | |
| Not a fully randomised trial. Smokers assigned to behavioural condition by clinic attended | |
| Not a controlled trial. Callers to a workplace helpline set up in conjunction with a non‐smoking policy were followed up. 16% of smokers reported they had quit 3 m later, 28% of those who had tried to quit. It was estimated that between 3 and 3.3% of smokers in the company had called in the first 3 m | |
| Intervention was to increase clinic referrals to a quitline. No smoking outcomes | |
| Previously listed as ongoing. Compared proactive with reactive telephone counselling but the NRT dosage provided varied between arms | |
| Effect of TC cannot be evaluated independently of NRT | |
| Telephone compared to face‐to‐face counselling | |
| Callers to a helpline were randomised to 1 of 2 S‐H materials. No counselling was given. Follow‐up only 1 m after receipt of materials. There was no difference in cessation rates between the booklet groups. | |
| Effect of TC cannot be evaluated independently of NRT | |
| Insufficient length of follow‐up (3 months) | |
| Allocation not stated to be random. Telephone follow‐up compared to group behavioural treatment with aversive smoking only. | |
| Not a randomised trial | |
| All participants received brief TC calls. Intervention was a face‐to‐face motivational interview | |
| Not a controlled trial. Evaluation of calls to a helpline | |
| All participants called a quitline, test of different S‐H materials. Included in Cochrane Review of S‐H (Livingstone‐Banks 2019a) | |
| Intervention for smokeless tobacco use, not smoking | |
| Intervention for smokeless tobacco use, not smoking | |
| Focus on preventing relapse. See Cochrane Review on relapse prevention (Livingstone‐Banks 2019b) | |
| No data on smoking cessation | |
| Intervention condition included intensive face‐to‐face counselling as well as telephone contact | |
| Multicomponent intervention, only 12 weeks follow‐up | |
| Previously listed as ongoing. Compares an online intervention against telephone counselling with self‐help | |
| Not randomised; historical and non‐equivalent controls | |
| Evaluated a counselling component to address cessation‐related weight concerns. Will be evaluated in Cochrane Review of interventions for preventing weight gain after smoking cessation (Farley 2012) | |
| Evaluated a counselling component to address cessation‐related weight concerns. Will be evaluated in Cochrane Review of interventions for preventing weight gain after smoking cessation (Farley 2012) | |
| Only 3 months follow‐up | |
| Intervention to increase re‐enrolment in quitline services. No smoking outcomes | |
| Intervention was IVR to re‐engage relapsed smokers, no cessation outcomes | |
| Not a randomised trial. Compared intensive counselling delivered face‐to‐face or by telephone | |
| Participants in quitline arm could choose between email, telephone or sms contacts | |
| Participants in quitline arm could choose between email, telephone or sms contacts | |
| Addition of Tobacco Tactics website to nurse‐delivered TC plus NRT | |
| Focus on preventing relapse. See Cochrane Review on relapse prevention (Livingstone‐Banks 2019b) | |
| Trial identified from a paper reporting secondary outcomes. Compared 3 levels of behavioural intervention in a primary care setting. Full results have not been published and not available | |
| Callers to a helpline were randomised to one of 4 different S‐H programmes or an information control. No counselling was given. | |
| Does not measure smoking cessation. Assesses impact of a media campaign to get women smokers with young children to call a quit line. Call rates compared in media markets with and without a campaign. | |
| Not a randomised trial. Evaluated impact of free NRT as adjunct to telephone support | |
| Not a randomised trial. Evaluated impact of different amounts of free NRT as adjunct to telephone support | |
| All participants eligible for same telephone counselling intervention; test of different amounts of NRT | |
| Telephone component cannot be evaluated independently of face‐to‐face counselling | |
| Study of smokeless tobacco users only | |
| Study of smokeless tobacco smokers | |
| All participants were women with young children who called a hotline and received same stage‐based counselling. They were randomised to receive 3 different S‐H guides. See Cochrane Review of S‐H (Livingstone‐Banks 2019a) | |
| Telephone component cannot be evaluated independently of face‐to‐face counselling. The intervention included in‐hospital motivational interviewing as well as post‐discharge telephone contact, and was compared to usual care | |
| Telephone component cannot be evaluated independently of face‐to‐face counselling. The intervention included in‐hospital physician advice and counselling by a nurse as well as post‐discharge telephone contact, and was compared to usual care | |
| Not random or pseudo‐random. Interventions ran sequentially. Participants receiving mailed materials had access to a hotline | |
| Single telephone call was the brief intervention control for a 3‐session group‐based pharmacist‐conducted intervention | |
| Recent quitters were randomised to access to recorded messages, not a counsellor. Short follow‐up (4 weeks) | |
| No data on smoking cessation | |
| Multicomponent intervention including TC, individual and group counselling and an interactive web‐based programme | |
| TC as an adjunct of NRT. However, NRT is offered in a different manner in the TC arm (free) and usual care arm (discounted price) | |
| Compares 3 different lengths of telephone and face‐to‐face cognitive behavioural counselling (3, 6 and 12 months duration) | |
| Telephone component cannot be evaluated independently of text messaging | |
| Only 3‐m follow‐up. Comparison between 1 and 4 telephone follow‐ups as adjunct to face‐to‐face counselling. 19 participants per group | |
| Intervention aimed at reduction in cigarette use for people not wishing to attempt cessation | |
| Insufficient length of follow‐up (12 weeks) | |
| Telephone component cannot be evaluated independently of face‐to‐face counselling delivered by dental practitioner | |
| Intervention used cell phone. Will be evaluated in Cochrane Review of mobile phone‐based interventions for smoking cessation (Whittaker 2016) | |
| Multicomponent intervention that includes TC and free NRT versus usual care | |
| Insufficient detail in abstract to include, no full report identified | |
| Effect of TC cannot be evaluated independently of NRT | |
| Study of 2 different frequencies of telephone counselling for high blood pressure, including smoking cessation counselling. Smoking cessation not reported as an outcome, unclear if smoking cessation measured | |
| TC arm compared to a web‐based intervention | |
| Telephone component cannot be evaluated independently of face‐to‐face counselling. The intervention included in‐hospital counselling by a nurse | |
| No data on smoking | |
| Only 3 m follow‐up | |
| Included in previous updates of this review. Excluded in 2018 update due to TC being compared to group counselling | |
| Telephone component cannot be evaluated independently of face‐to‐face counselling and offer of pharmacotherapy | |
| The purpose of the telephone call was to encourage participants to enrol in quitline services | |
| Telephone component cannot be evaluated independently of face‐to‐face counselling. The intervention included in‐hospital counselling by a nurse. Quasi‐random design | |
| Complex intervention; all participants received telephone counselling | |
| Included in previous updates of this review. Excluded in 2018 update due to the fact that the effect of TC cannot be evaluated independently of NRT | |
| Insufficient length of follow‐up | |
| Main intervention component was face‐to‐face support. Telephone contact in both arms | |
| Insufficient length of follow‐up | |
| TC compared with videoconferencing among Korean‐American women. This study will be covered in a new Cochrane review about real‐time video counselling for smoking cessation (see Tzelepis 2017) | |
| TC compared with videoconferencing among women living with HIV. This study will be covered in a new Cochrane review about real‐time video counselling for smoking cessation (see Tzelepis 2017) | |
| Effect of TC cannot be evaluated independently of NRT | |
| 3 worksites allocated to different interventions. No way to distinguish variation due to worksite from effect of intervention | |
| Previously included, recruited only recent quitters so now covered in Cochrane Review of relapse prevention (Livingstone‐Banks 2019b) | |
| The intervention included 1 session of face‐to‐face counselling with telephone follow‐up. Results, which did not show any intervention effect, are given in Bobo 1998 | |
| No long‐term outcomes yet reported | |
| Effect of TC cannot be evaluated independently of NRT | |
| Not randomised. Compared participants accepting proactive calls to those choosing only 1 session | |
| Systems change intervention; trained dental staff in to assess, advise and refer to telephone counselling | |
| Quitline referral confounded with brief advice, only 3 m follow‐up | |
| The intervention included the opportunity of a motivational telephone call following provider advice and S‐H components. Follow‐up was only 5 ‐ 8 weeks | |
| Smoking status not measured | |
| Trial of a relapse prevention intervention; participants were abstinent at time of randomisation | |
| All participants had same quitline counselling | |
| The focus of the intervention was on genetic susceptibility feedback. Effect of telephone support cannot be evaluated independently | |
| Telephone component cannot be evaluated independently of website and text messaging | |
| This study includes exclusively recent quitters. This falls within the scope of a separate review on preventing relapse (Hajek 2009). | |
| Insufficient length of follow‐up (3 months) | |
| Compares 2 telephone‐based interventions for preventing relapse following group therapy. Now included in Cochrane Review of relapse prevention (Livingstone‐Banks 2019b) | |
| Trial of NRT as opposed to telephone support; same telephone support intervention offered to both groups | |
| All participants received telephone counselling and NRT, test of additional group counselling | |
| No data on smoking abstinence | |
| Insufficient length of follow‐up (1 month) | |
| Telephone support could not be evaluated independently of combined intervention | |
| The outcome of this trial is enrolment of veterans in smoking cessation services | |
| Not a controlled trial. Survey of callers to UK quitline. | |
| Insufficient length of follow‐up | |
| Trial in pregnant women | |
| Telephone intervention purpose was to assess smoking status, interest in making another quit attempt, quit challenges, and treatment preferences, not to assist cessation per se | |
| Intervention was telephone counselling for non‐smokers wanting to help a smoker. Outcome was calls by smoker to quitline, not cessation | |
| Intervention was telephone counselling for non‐smokers wanting to help a smoker. Outcome was calls by smoker to quitline, not cessation | |
| Short follow‐up | |
| School as unit of randomisation. Telephone counselling confounded by other school‐based initiatives | |
| Telephone counselling cannot be evaluated independently of telehealth counselling | |
| Not a controlled trial. A panel sample of callers to the Scottish Smokeline was followed up for 1 year. 607 (71% of original sample) were reached. | |
| The amount and timing of telephone contact is unclear. The main component was a S‐H programme, compared to a waiting list control. Total of 40 participants | |
| Intervention addressed multiple risk factors, number of smokers enrolled not specified | |
| Telephone support could not be evaluated independently of face‐to‐face counselling | |
| Not a controlled trial. Followed 258 nicotine patch purchasers who enrolled for support programme of 4 calls from a trained nurse counsellor. | |
| Traditional TC compared with telemedicine. This study will be covered in a new Cochrane Review about real‐time video counselling for smoking cessation (see Tzelepis 2017) | |
| Effect of TC cannot be evaluated independently of NRT | |
| Effect of TC cannot be evaluated independently of pharmacotherapy | |
| Effect of TC cannot be evaluated independently of pharmacotherapy | |
| Not randomised. Smokers chose intensity of support | |
| Intervention used mobile phone (including text messaging). To be covered by separate Cochrane Review (Whittaker 2016) | |
| Telephone component cannot be evaluated independently of NRT | |
| Included in previous updates of this review. Excluded in 2018 update due to TC being compared to financial incentives | |
| Systems change intervention; referral to quitline was only 1 component | |
| Small (n = 39) feasibility study in Emergency Department. Very low rate of follow‐up especially for sustained abstinence outcome (2/39 reached at both follow‐ups) | |
| Evaluated a telephone support system. All smokers recruited had access to the interactive programme. Random subsets were selected for access to messages about nicotine gum, sent a reminder to call, or sent a user's manual | |
| Study of phone counselling for relapse prevention | |
| Telephone component cannot be evaluated independently of NRT | |
| Abstinence data given only for intervention group | |
| Effect of TC cannot be evaluated independently of NRT | |
| Follow‐up 12 weeks. | |
| Multicomponent intervention which included telephone counselling, a pedometer, supporting written materials and a self‐monitoring diary | |
| Telephone component cannot be evaluated independently of face‐to‐face counselling. The intervention included brief counselling and NRT | |
| Telephone component cannot be evaluated independently of face‐to‐face counselling. The intervention included in‐hospital nurse counselling as well as post‐discharge telephone contact, and was compared to a minimal intervention | |
| Telephone component could not be evaluated independently of combined intervention | |
| Telephone intervention was a 10‐min reminder call, 2 m after face‐to‐face advice to quit prior to surgery. Outcomes combined with an arm given reminder at a face‐to‐face meeting | |
| Telephone component cannot be evaluated independently of face‐to‐face counselling. The intervention included in‐hospital physician advice and counselling by a nurse as well as post‐discharge telephone contact, and was compared to usual care | |
| Not a controlled trial. Pre‐test/post‐test study of different referral methods | |
| All participants had same basic counselling intervention. Test of a mood management component | |
| All participants had same counselling intervention. Test of tailored written materials, see Cochrane self‐help Review (Livingstone‐Banks 2019a) | |
| Not an evaluation of counselling; compared 2 strategies to encourage calls to a quitline | |
| Telephone component cannot be evaluated independently of face‐to‐face counselling. The intervention included in‐hospital physician advice and counselling by a nurse, as well as post‐discharge telephone contact, and was compared to usual care | |
| Telephone component could not be evaluated independently of combined intervention | |
| Not randomised; comparison of work‐based intervention programmes | |
| Only 3 m follow‐up | |
| Insufficient length of follow‐up (12 weeks) | |
| Only 12 weeks follow‐up | |
| All participants received telephone counselling. Test of a mood management component | |
| Intervention used mobile phone (including text messaging). To be covered by separate Cochrane Review (Whittaker 2016) | |
| Not randomised. The treated groups were recruited by different means and given different interventions, both of which included telephone counselling by nurses or counsellors | |
| Only 3 m follow‐up | |
| Trial of methods for clinic referral to quitline support. No quitting outcomes | |
| Recruitment by quitline, but test of providing samples of NRT | |
| Recruitment by quitline, but test of nicotine‐free cigarettes as an adjunct to NRT | |
| Comparison of different leaflets/booklets. One of the arms also includes referral to the quitline and other smoking cessation services | |
| Comparison of physician‐provided general help to quit smoking with intervention primarily aimed at facilitating quitline use. Both groups had same access to quitline | |
| Multicomponent intervention which includes NRT, telephone and face‐to‐face counselling | |
| Telephone component cannot be evaluated independently of face‐to‐face counselling | |
| Only 12 weeks follow‐up | |
| TC compared to face‐to‐face counselling | |
| Uncontrolled evaluation. Quitline callers followed up at 1 year | |
| Quitline component was part of a comprehensive intervention including face‐to‐face support | |
| Only 12 weeks follow‐up | |
| Not randomised; uses a concurrent matched control | |
| Not an RCT. All participants called the California Smokers' Helpline and received 1 session of counselling and planned to use NRT. Those who chose to receive further counselling were compared to those who did not |
CI: confidence interval; m: month(s); IVR: interactive voice response; NRT: nicotine replacement therapy; S‐H: self‐help; TC: telephone counselling; TQD: target quit date
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Smoking cessation: data for two years from two different interventions |
| Methods | 9‐week open‐label bupropion phase 300 mg daily and NRT for 3 weeks combined with behavioural support; smokers randomised in 2 groups, follow‐up for 3, 6, 12 and 24 months |
| Participants | No information |
| Interventions | Group A: 7 weekly one‐to‐one counselling sessions; Group B: telephone counselling |
| Outcomes | PP and continuous abstinence |
| Starting date | |
| Contact information | |
| Notes |
| Trial name or title | Reaching and treating lesbian, gay, bisexual, and transgender (LGBT) cigarette smokers |
| Methods | Randomised, open‐label, factorial (4 arm) |
| Participants | ≥ 18 years; identify as LGBT |
| Interventions | 1) self‐help manual; 2) mail‐based self‐help plus internet‐based smoking treatment; 3) self‐help manual + telephone counselling; 4) self‐help manual plus internet‐based Intervention plus telephone counselling |
| Outcomes | Primary: smoking status at 3, 6 and 12 months post‐enrolment |
| Starting date | February 2008 |
| Contact information | |
| Notes |
| Trial name or title | Acceptance and commitment therapy for smoking cessation in the primary care setting (ACT) |
| Methods | RCT, parallel assignment, double masking |
| Participants | Aged 18 years and older, currently smoking at least 1 cigarette a day in the past 30 days, Hong Kong residents, able to communicate in Cantonese, currently residing in Hong Kong and expecting to continue to do so for the next 6 months, have access to a telephone |
| Interventions | 2 telephone ACT counselling sessions, minimal face‐to‐face ACT counselling, printed self‐help leaflet on smoking cessation |
| Outcomes | Primary outcome: self‐reported 7‐day point prevalence |
| Starting date | July 2012 |
| Contact information | www.researchgate.net/profile/Yim_Mak; www.researchgate.net/profile/Alice_Loke |
| Notes | Only protocol and baseline results have been published so far |
| Trial name or title | Telephone and web‐based teen tobacco cessation in HMOs |
| Methods | RCT, parallel assignment, single (Investigator) masking |
| Participants | 600 teenagers |
| Interventions | Telephone counselling plus interactive website |
| Outcomes | Self‐reported 30 days point prevalence assessed at 12 months |
| Starting date | March 2006 |
| Contact information | |
| Notes |
| Trial name or title | Telephone counseling and the distribution of nicotine patches to smokers |
| Methods | RCT, factorial assignment, quadruple masking |
| Participants | 4200 adults residing in California including English and Spanish speakers |
| Interventions | Telephone counselling ± nicotine patch ± self‐help ± placebo |
| Outcomes | 6 months continuous abstinence assessed after 7 months of follow‐up |
| Starting date | February 2009 |
| Contact information | |
| Notes |
| Trial name or title | Dissemination of a tailored tobacco quitline for rural veteran smokers |
| Methods | RCT, Parallel Assignment, Double Masking |
| Participants | 411 rural veteran smokers, older than 18, receive primary care from the Iowa City VA Health Care System or an affiliated community‐based outpatient clinic (CBOC), live in a non‐metropolitan area (based on Rural‐Urban Commuting Area Codes (RUCA)), willing to make an attempt to quit smoking in the next 30 days, capable of providing informed consent, have access to a telephone (land line or cell phone) and have a stable residence. |
| Interventions | Tailored tobacco quitline for rural veteran smokers |
| Outcomes | 30‐day abstinence assessed after 6 months of follow‐up |
| Starting date | July 2013 |
| Contact information | mark‐[email protected] |
| Notes |
| Trial name or title | Smoke‐free randomised controlled trial |
| Methods | RCT, parallel assignment, single masking |
| Participants | 11 Singaporean current smokers among outpatients, including hospital employees, who provide informed consent for enrolment in the smoking cessation programme |
| Interventions | Proactive telephone counselling weekly for 6 months |
| Outcomes | 7‐day point prevalence assessed after 6 months of follow‐up |
| Starting date | June 2013 |
| Contact information | |
| Notes |
| Trial name or title | Smoking cessation for cervical cancer survivors |
| Methods | RCT, parallel assignment, open‐label |
| Participants | 350 women with a history of cervical cancer |
| Interventions | Self‐help + nicotine patch + referral to Oklahome quitline + 6 behavioural counselling calls |
| Outcomes | Smoking abstinence after 18 months of follow‐up |
| Starting date | January 2015 |
| Contact information | Jennifer‐[email protected] |
| Notes | Recruiting |
| Trial name or title | Evaluation of efficacy of different methods of tobacco cessation interventions among BEST employees in Mumbai |
| Methods | RCT, 4 arms, double masking |
| Participants | 4000 Mumbai male BEST employees |
| Interventions | Telephonic counselling |
| Outcomes | Tobacco cessation after 1 year of follow‐up |
| Starting date | March 2015 |
| Contact information | www.researchgate.net/profile/Sharmila_Pimple |
| Notes | Recruiting |
| Trial name or title | Telephone‐delivered interventions for smoking cessation (TALK) |
| Methods | RCT, parallel assignment, single masking |
| Participants | 1168 American adult smokers |
| Interventions | Telephone counselling (ACT) 5 weeks |
| Outcomes | 30‐day point prevalence abstinence after 12 months of follow‐up |
| Starting date | 2 November 2015 |
| Contact information | |
| Notes |
| Trial name or title | STAND Community College tobacco cessation trial |
| Methods | RCT, 3 arms, open‐label |
| Participants | 113 Californian adult smokers |
| Interventions | Usual care + referral to quitline |
| Outcomes | Biochemically‐validated smoking cessation after 6 months of follow‐up |
| Starting date | September 2014 |
| Contact information | |
| Notes | Cannot find a full‐text report |
| Trial name or title | Helping poor smokers quit |
| Methods | RCT, factorial design, double masking |
| Participants | 2000 Missouri resident adult smokers |
| Interventions | Specialised quitline services ± basic needs navigator |
| Outcomes | 7‐day point prevalence abstinence after 6 months of follow‐up |
| Starting date | 5 June 2017 |
| Contact information | |
| Notes | Recruiting |
| Trial name or title | A video‐led smoking cessation intervention in helping male smokers who is planning to have a baby to quit |
| Methods | RCT, parallel assignment, single masking |
| Participants | 888 male adult Chinese‐speaking Hong Kong‐resident smokers |
| Interventions | Telephone counselling + pamphlet |
| Outcomes | Self‐reported 7‐day point prevalence quit rate after 6 months of follow‐up |
| Starting date | 1 June 2017 |
| Contact information | www.researchgate.net/profile/Ho_Li2 |
| Notes | Recruiting |
| Trial name or title | Improving quitline support study (IQS) |
| Methods | RCT, factorial design, single masking |
| Participants | 1600 Winsconsin‐resident adult smokers |
| Interventions | 4‐call quitline counselling ± NRT ± text messaging ± financial incentives |
| Outcomes | 7‐day point prevalence abstinence after 6 months of follow‐up |
| Starting date | 7 June 2018 |
| Contact information | www.researchgate.net/profile/Danielle_Mccarthy |
| Notes | Enrolling |
| Trial name or title | Recruitment strategies for an effective smoking cessation programme for parents (De implementatie van een effectieve interventie om te stoppen met roken voor ouders) |
| Methods | RCT, parallel assignment, single masking |
| Participants | 144 smoking parents of children younger than 12 |
| Interventions | Proactive telephone counselling based on MI and cognitive‐behavioural skill building |
| Outcomes | 7‐day point prevalence abstinence after 12 months of follow‐up |
| Starting date | 15 September 2016 |
| Contact information | |
| Notes | Enroling |
| Trial name or title | Planning a change easily (PACE) |
| Methods | RCT, 4 arms |
| Participants | US adult smokers not ready to quit |
| Interventions | Motivational Interviewing Telephone Counseling |
| Outcomes | 7‐day point prevalence after 12 months of follow‐up |
| Starting date | |
| Contact information | |
| Notes | Only protocol published so far |
| Trial name or title | Antismoking interventions in stroke patients ‐ Polish perspective |
| Methods | RCT, 3 arms |
| Participants | 198 participants with first ischaemic stroke |
| Interventions | 4 telephone calls 6 weeks after discharge from hospital + brief (20‐min) counselling by physician |
| Outcomes | Smoking cessation rate after 12 months of follow‐up |
| Starting date | |
| Contact information | www.researchgate.net/profile/Halina_Sienkiewicz‐Jarosz |
| Notes | Conference abstract. First author contacted but no response received |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up Show forest plot | 14 | 32484 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.19, 1.61] |
| Analysis 1.1  Comparison 1 Interventions for callers to quitlines ‐ effect of additional proactive calls, Outcome 1 Cessation at longest follow‐up. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Interventions for callers to quitlines ‐ comparison of different intensities, Outcome 1 Cessation at longest follow‐up. | ||||
| 1.1 Seven versus three phone calls | 1 | 1908 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.09, 1.89] |
| 1.2 Five versus three phone calls | 1 | 3669 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.00, 1.64] |
| 1.3 Seven versus five phone calls | 1 | 3939 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.93, 1.36] |
| 1.4 Five versus two phone calls | 1 | 2874 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.89, 1.23] |
| 1.5 Five versus one phone call | 1 | 2189 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.01, 1.74] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up Show forest plot | 14 | 32484 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.20, 1.57] |
| Analysis 3.1  Comparison 3 Interventions for callers to quitlines ‐ subgroups by counseling intensity, Outcome 1 Cessation at longest follow‐up. | ||||
| 1.1 Two sessions or fewer | 2 | 3867 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.02, 1.46] |
| 1.2 Three to six sessions | 11 | 22612 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.17, 1.63] |
| 1.3 Seven sessions or more | 4 | 6005 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.98, 2.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 4.1  Comparison 4 Interventions for callers to quitlines ‐ comparison of different support at initial call, Outcome 1 Cessation at longest follow‐up. | ||||
| 1.1 Reactive counselling vs self‐help materials | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Tailored counselling versus standard counselling | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Stage‐based counselling versus general information | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 ACT + NRT versus CBT + NRT | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Motivational Interviewing TC versus standard TC | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.1  Comparison 5 Offer of counselling via quitlines/helplines/hotlines, Outcome 1 Cessation at longest follow‐up. | ||||
| 1.1 Hotline and self‐help materials compared to minimal intervention | 2 | 3327 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [1.16, 2.25] |
| 1.2 Hotline and self‐help materials for cessation maintenance compared to nothing | 1 | 1311 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.06] |
| 1.3 Reactive or proactive counselling vs provider counselling | 4 | 7780 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.07, 1.84] |
| 1.4 Proactive counselling vs reactive counselling | 2 | 2908 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [0.58, 7.31] |
| 1.5 Proactive counselling vs self‐help | 2 | 2498 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.76, 2.63] |
| 1.6 Reactive counselling vs self‐help | 2 | 2364 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.44, 1.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up Show forest plot | 65 | 41233 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.15, 1.35] |
| Analysis 6.1  Comparison 6 Interventions for smokers not calling quitlines ‐ subgroups by baseline support, Outcome 1 Cessation at longest follow‐up. | ||||
| 1.1 Adjunct to self‐help or minimal intervention | 35 | 22917 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [1.16, 1.57] |
| 1.2 Adjunct to brief intervention or counselling | 12 | 4234 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.12, 1.50] |
| 1.3 Adjunct to pharmacotherapy | 18 | 12865 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.03, 1.26] |
| 1.4 Adjunct to incentives for smoking cessation | 1 | 1217 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.67, 1.65] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up Show forest plot | 3 | 2602 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.12, 1.44] |
| Analysis 7.1  Comparison 7 Interventions for smokers not calling quitlines ‐ intense versus minimal telephone counselling, Outcome 1 Cessation at longest follow‐up. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up Show forest plot | 65 | 41233 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.15, 1.35] |
| Analysis 8.1  Comparison 8 Interventions for smokers not calling quitlines ‐ subgroups by counseling intensity, Outcome 1 Cessation at longest follow‐up. | ||||
| 1.1 Two sessions or fewer | 9 | 6274 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.86, 1.40] |
| 1.2 Three to six sessions | 44 | 26686 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.18, 1.42] |
| 1.3 Seven sessions or more | 13 | 8273 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.98, 1.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up Show forest plot | 65 | 41233 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.15, 1.36] |
| Analysis 9.1  Comparison 9 Interventions for smokers not calling quitlines ‐ subgroups by motivation, Outcome 1 Cessation at longest follow‐up. | ||||
| 1.1 Selected for motivation/interest in quitting | 26 | 17877 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.15, 1.49] |
| 1.2 Not selected for motivation | 39 | 23356 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [1.09, 1.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 10.1  Comparison 10 Other studies, Outcome 1 Cessation at longest follow‐up. | ||||
| 1.1 Family‐supported vs standard telephone counseling | 1 | 471 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.72, 1.45] |
| 1.2 Parental focused telephone counseling vs nutrition counseling | 1 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 2.01 [0.97, 4.17] |
| 1.3 Brief motivational vs standard telephone counseling | 1 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 2.63 [1.12, 6.14] |
| 1.4 Smoking reduction vs brief motivational telephone counseling | 1 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.47, 1.68] |
| 1.5 Smoking reduction vs standard telephone counseling | 1 | 375 | Risk Ratio (M‐H, Random, 95% CI) | 2.32 [0.98, 5.52] |
| 1.6 Nondirective vs directive telephone coaching | 1 | 518 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.82, 1.62] |
| 1.7 Tailored telephone counseling vs state tobacco quitline referral | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.47, 2.25] |
| 1.8 Brief quitline facilitation vs brief cessation advice | 1 | 600 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [0.96, 2.72] |
| 1.9 Smoking‐reduction vs exercise & diet telephone counseling | 1 | 369 | Risk Ratio (M‐H, Random, 95% CI) | 2.86 [0.93, 8.81] |
| 1.10 Medication adherence vs standard telephone counseling | 1 | 987 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.83, 1.15] |
| 1.11 Automated telephone follow‐up vs standard care | 1 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.92, 1.60] |
| 1.12 Coverage for telephone counseling and pharmacotherapy vs pharmacotherapy alone | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.38, 1.18] |

Study flow diagram for most recent update
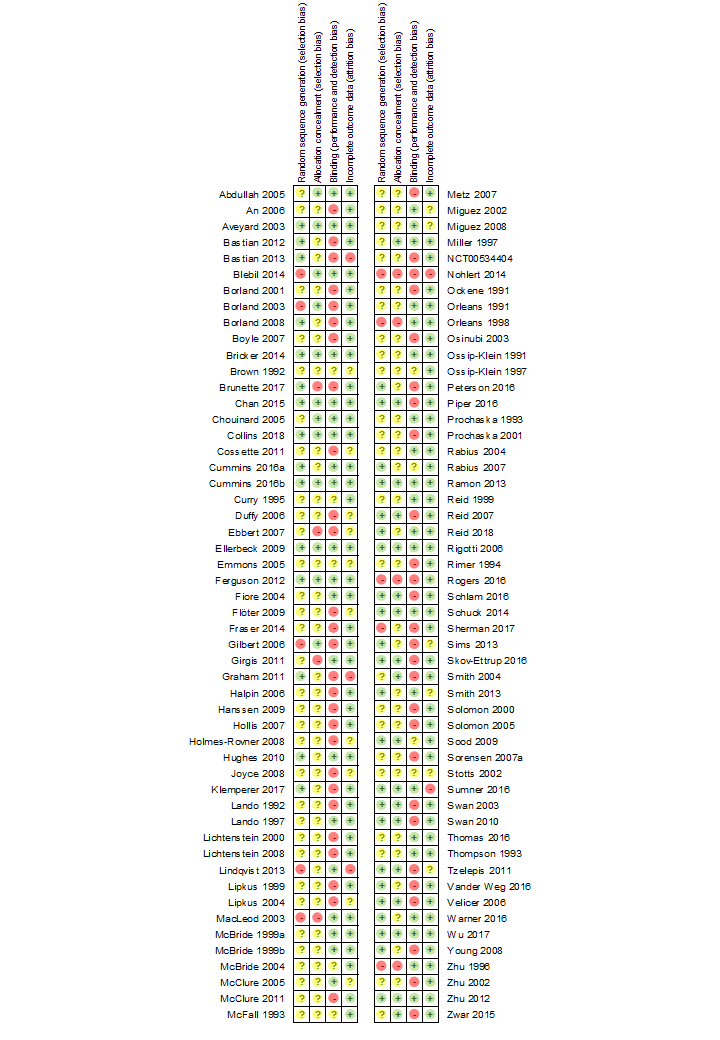
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Comparison 1. Interventions for callers to quitlines. Effect of additional proactive calls.

Comparison 6. Interventions for smokers not calling quitlines ‐ subgroups by baseline support.
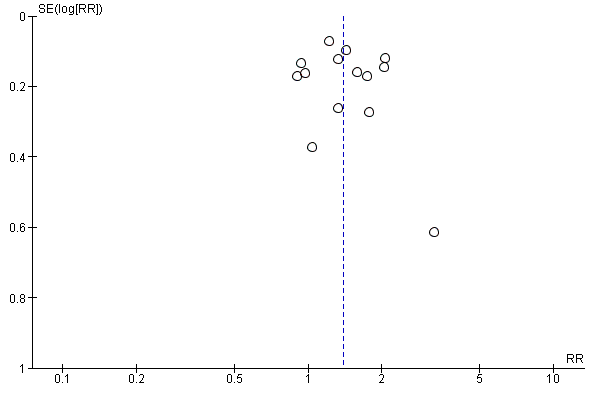
Funnel plot of studies in Comparison 1: Interventions for callers to quitlines ‐ effect of additional proactive calls.

Funnel plot of studies in Comparison 6: Interventions for smokers not calling quitlines ‐ subgroups by baseline support.
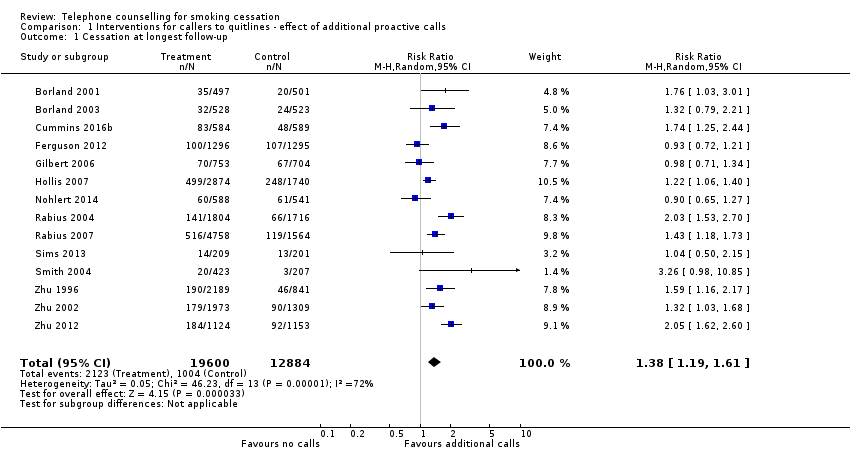
Comparison 1 Interventions for callers to quitlines ‐ effect of additional proactive calls, Outcome 1 Cessation at longest follow‐up.
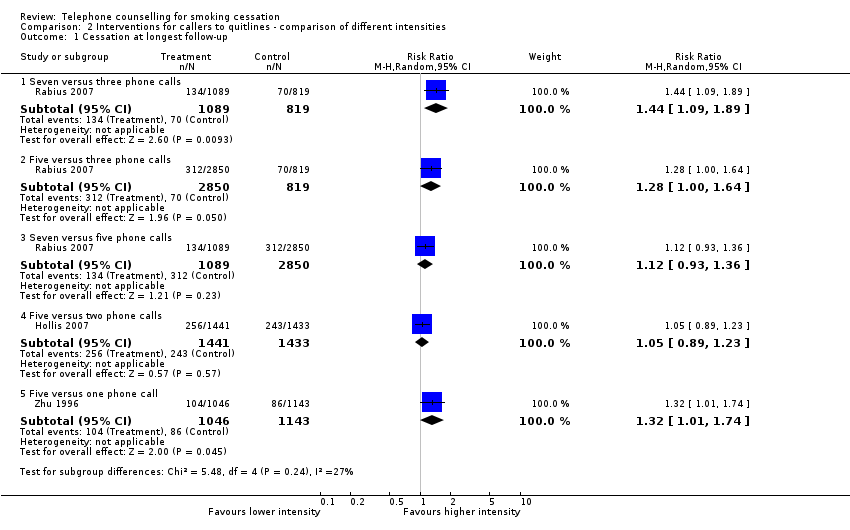
Comparison 2 Interventions for callers to quitlines ‐ comparison of different intensities, Outcome 1 Cessation at longest follow‐up.

Comparison 3 Interventions for callers to quitlines ‐ subgroups by counseling intensity, Outcome 1 Cessation at longest follow‐up.

Comparison 4 Interventions for callers to quitlines ‐ comparison of different support at initial call, Outcome 1 Cessation at longest follow‐up.
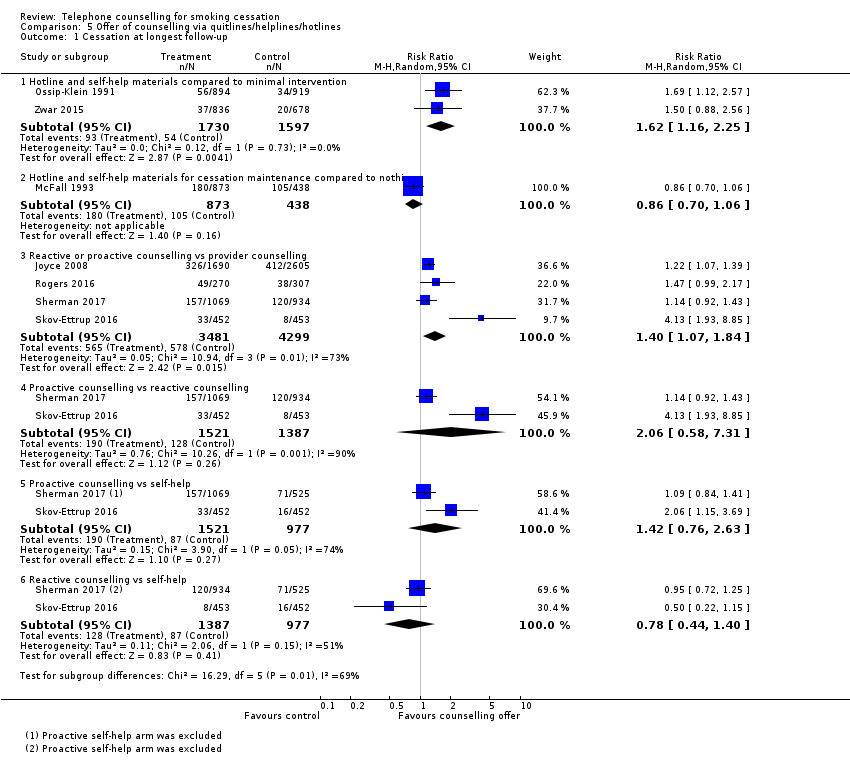
Comparison 5 Offer of counselling via quitlines/helplines/hotlines, Outcome 1 Cessation at longest follow‐up.
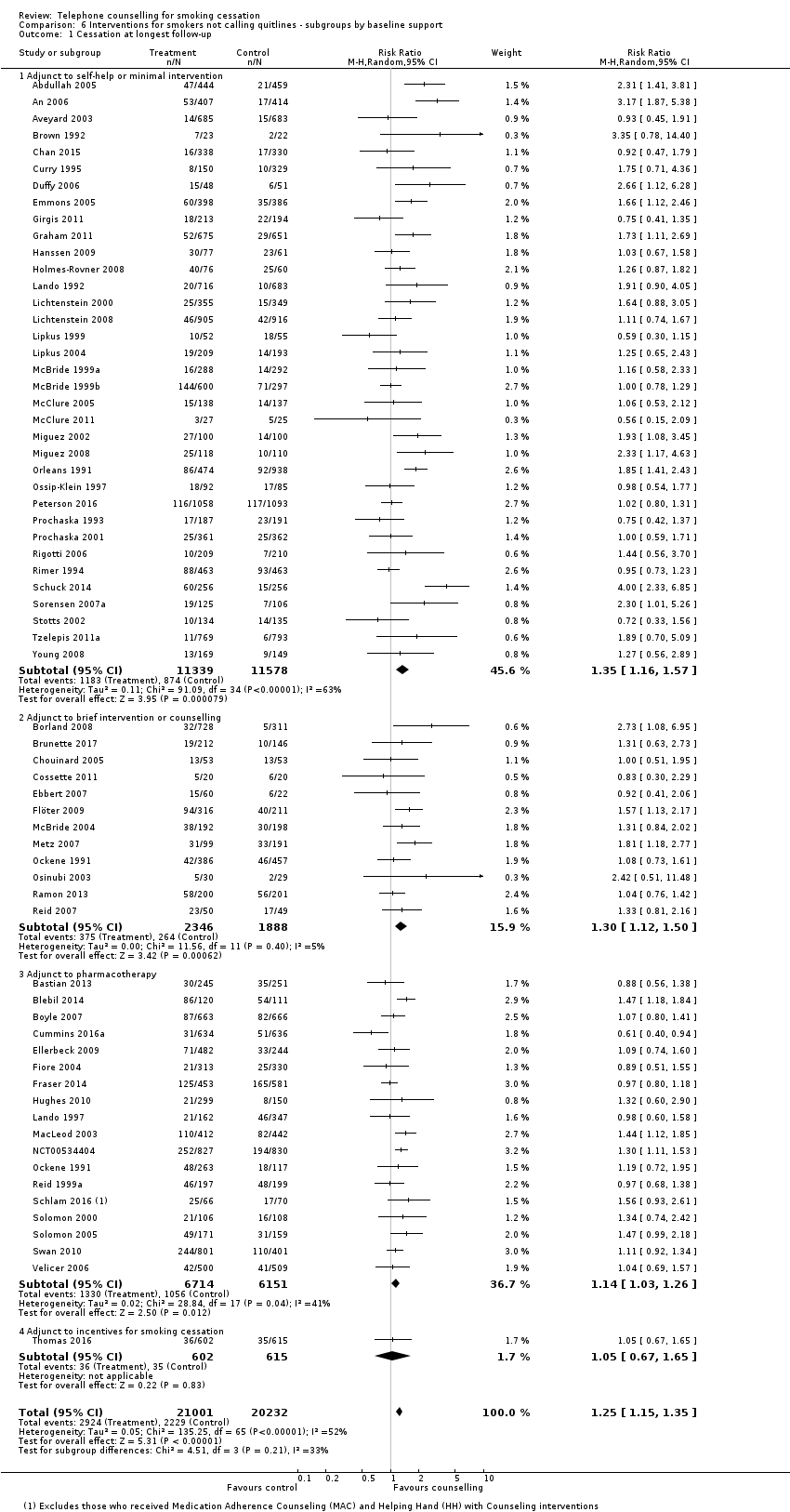
Comparison 6 Interventions for smokers not calling quitlines ‐ subgroups by baseline support, Outcome 1 Cessation at longest follow‐up.

Comparison 7 Interventions for smokers not calling quitlines ‐ intense versus minimal telephone counselling, Outcome 1 Cessation at longest follow‐up.

Comparison 8 Interventions for smokers not calling quitlines ‐ subgroups by counseling intensity, Outcome 1 Cessation at longest follow‐up.
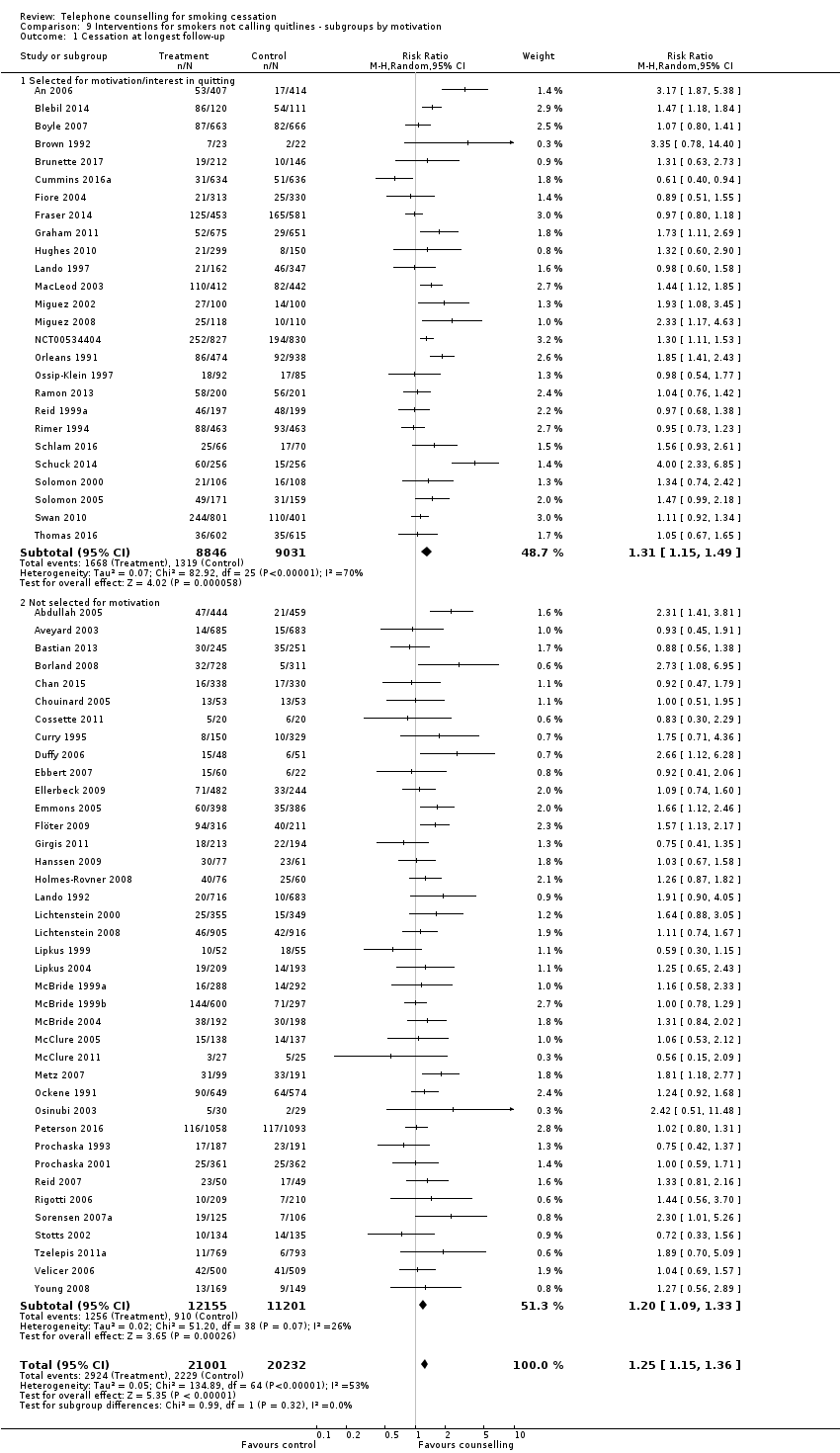
Comparison 9 Interventions for smokers not calling quitlines ‐ subgroups by motivation, Outcome 1 Cessation at longest follow‐up.

Comparison 10 Other studies, Outcome 1 Cessation at longest follow‐up.
| Interventions for callers to quitlines ‐ effect of additional proactive calls for smoking cessation | ||||||
| Patient or population: callers to quitlines | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Additional proactive calls | |||||
| Smoking cessation | Study population | RR 1.38 | 32,484 | ⊕⊕⊕⊝ | ‐ | |
| 72 per 1000 | 100 per 1000 | |||||
| Low | ||||||
| 50 per 1000a | 69 per 1000 (59 to 81) | |||||
| High | ||||||
| 150 per 1000a | 208 per 1000 (178 to 242) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aLow control rate reflects lower end of range evident in trials; 4/14 had control rates < 50 per 1000. High control rate likely to be applicable for smokers also using pharmacotherapy. | ||||||
| Proactive telephone counselling for smokers not calling quitlines | ||||||
| Patient or population: smokers not calling quitlines | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Proactive telephone counselling | |||||
| Smoking cessation | Study population | RR 1.25 | 41,233 | ⊕⊕⊕⊝ | ||
| 110 per 1000a | 137 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aBased on crude average of events/total, with participants lost to follow‐up assumed to be smoking. Interquartile range in trials 63 ‐ 200 per 1000. Higher baseline cessation rates typical amongst motivated populations receiving pharmacotherapy and some support. Relative additional benefit of telephone intervention may be smaller in this setting. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up Show forest plot | 14 | 32484 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.19, 1.61] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Seven versus three phone calls | 1 | 1908 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.09, 1.89] |
| 1.2 Five versus three phone calls | 1 | 3669 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.00, 1.64] |
| 1.3 Seven versus five phone calls | 1 | 3939 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.93, 1.36] |
| 1.4 Five versus two phone calls | 1 | 2874 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.89, 1.23] |
| 1.5 Five versus one phone call | 1 | 2189 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.01, 1.74] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up Show forest plot | 14 | 32484 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.20, 1.57] |
| 1.1 Two sessions or fewer | 2 | 3867 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.02, 1.46] |
| 1.2 Three to six sessions | 11 | 22612 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.17, 1.63] |
| 1.3 Seven sessions or more | 4 | 6005 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.98, 2.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Reactive counselling vs self‐help materials | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Tailored counselling versus standard counselling | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Stage‐based counselling versus general information | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 ACT + NRT versus CBT + NRT | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Motivational Interviewing TC versus standard TC | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Hotline and self‐help materials compared to minimal intervention | 2 | 3327 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [1.16, 2.25] |
| 1.2 Hotline and self‐help materials for cessation maintenance compared to nothing | 1 | 1311 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.06] |
| 1.3 Reactive or proactive counselling vs provider counselling | 4 | 7780 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.07, 1.84] |
| 1.4 Proactive counselling vs reactive counselling | 2 | 2908 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [0.58, 7.31] |
| 1.5 Proactive counselling vs self‐help | 2 | 2498 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.76, 2.63] |
| 1.6 Reactive counselling vs self‐help | 2 | 2364 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.44, 1.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up Show forest plot | 65 | 41233 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.15, 1.35] |
| 1.1 Adjunct to self‐help or minimal intervention | 35 | 22917 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [1.16, 1.57] |
| 1.2 Adjunct to brief intervention or counselling | 12 | 4234 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.12, 1.50] |
| 1.3 Adjunct to pharmacotherapy | 18 | 12865 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.03, 1.26] |
| 1.4 Adjunct to incentives for smoking cessation | 1 | 1217 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.67, 1.65] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up Show forest plot | 3 | 2602 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.12, 1.44] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up Show forest plot | 65 | 41233 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.15, 1.35] |
| 1.1 Two sessions or fewer | 9 | 6274 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.86, 1.40] |
| 1.2 Three to six sessions | 44 | 26686 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.18, 1.42] |
| 1.3 Seven sessions or more | 13 | 8273 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.98, 1.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up Show forest plot | 65 | 41233 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.15, 1.36] |
| 1.1 Selected for motivation/interest in quitting | 26 | 17877 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.15, 1.49] |
| 1.2 Not selected for motivation | 39 | 23356 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [1.09, 1.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Family‐supported vs standard telephone counseling | 1 | 471 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.72, 1.45] |
| 1.2 Parental focused telephone counseling vs nutrition counseling | 1 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 2.01 [0.97, 4.17] |
| 1.3 Brief motivational vs standard telephone counseling | 1 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 2.63 [1.12, 6.14] |
| 1.4 Smoking reduction vs brief motivational telephone counseling | 1 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.47, 1.68] |
| 1.5 Smoking reduction vs standard telephone counseling | 1 | 375 | Risk Ratio (M‐H, Random, 95% CI) | 2.32 [0.98, 5.52] |
| 1.6 Nondirective vs directive telephone coaching | 1 | 518 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.82, 1.62] |
| 1.7 Tailored telephone counseling vs state tobacco quitline referral | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.47, 2.25] |
| 1.8 Brief quitline facilitation vs brief cessation advice | 1 | 600 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [0.96, 2.72] |
| 1.9 Smoking‐reduction vs exercise & diet telephone counseling | 1 | 369 | Risk Ratio (M‐H, Random, 95% CI) | 2.86 [0.93, 8.81] |
| 1.10 Medication adherence vs standard telephone counseling | 1 | 987 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.83, 1.15] |
| 1.11 Automated telephone follow‐up vs standard care | 1 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.92, 1.60] |
| 1.12 Coverage for telephone counseling and pharmacotherapy vs pharmacotherapy alone | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.38, 1.18] |

