Poradnictwo telefoniczne w zaprzestaniu palenia
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [author‐defined order]
| Methods | Setting: Parents of children in a birth cohort study, Hong Kong | |
| Participants | 903 current smokers with young children (49 recent quitters not included here); 84% M, > 50% aged 36 ‐ 45, 91% smoked ≤ 20/day | |
| Interventions | 1. Single mailing of stage‐matched S‐H (either preparation/action or contemplation/precontemplation) | |
| Outcomes | Abstinence at 6m, validated 7‐day PP. (Unvalidated self‐reported continuous abstinence also reported). | |
| Notes | Comparisons 4 ‐ 6. Effect on self‐reported continuous abstinence was non‐significant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Low risk | Numbered sealed opaque envelopes |
| Blinding of outcome assessment (detection bias) | Low risk | "Independent interviewer...was unaware of subjects' group allocation... All respondents who reported they were not smoking during the preceding 7 days were invited to attend the research centre for biochemical validation." |
| Incomplete outcome data (attrition bias) | Low risk | Losses to follow‐up 11% intervention/ 4% control. Included as continuing smokers |
| Methods | Setting: 5 Veterans Administration medical centres, USA | |
| Participants | 821 smokers interested in quitting (excludes 16 deaths, 1 withdrawal); 91% M, av. age 57, av. cigs/day 26. 26% had > 7d abstinence in previous year, 44% ever use of bupropion, 82% ever use NRT | |
| Interventions | 1. Mailed S‐H and standard care; opportunity for intervention during routine health care and referral to individual or group cessation programmes. NRT & bupropion avail on formulary | |
| Outcomes | Abstinence at 12m (sustained from 6m, 7‐day PP also reported) | |
| Notes | Comparisons 4 ‐ 6. TC increased use of pharmacotherapies (86% vs 30% reported use at 3m). Effect greater for sustained quitting than PP. 72% completed 3 or more calls. Mean (SD) 7.7 (4.1) including courtesy calls, relapses, repeat attempts. Mean (SD) duration of total contact 123 (71) mins. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | Losses to follow‐up included as smokers, 16 deaths excluded |
| Methods | Setting: 65 general practices, UK | |
| Participants | 2471 smokers (2058 in relevant arms); > 80% in precontemplation or contemplation, 10 ‐ 14% in preparation, 54% F, av. age 41, av. cigs/day 20 | |
| Interventions | 1. Standard S‐H materials, single mailing | |
| Outcomes | Abstinence at 12m, (reported sustained for 6m) | |
| Notes | Comparisons 4 ‐ 6. 3 vs 2. Sensitivity analysis 3 vs 2+1. 66% received 1st phone call, 36% 2nd, 31% 3rd. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised randomization procedure, with minimisation to balance SoC, addiction and SES |
| Allocation concealment (selection bias) | Low risk | Centralised |
| Blinding of outcome assessment (detection bias) | Unclear risk | 12m PP "was confirmed with salivary cotinine, so that we had unconfirmed and confirmed prevalence of quitting." Confirmed figures used in analysis. |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up 24% in group 1, 31% in 2 & 3. All included as smokers. Sensitivity analysis allowing for differential drop‐out did not change findings. |
| Methods | Setting: community, Australia | |
| Participants | 998 smokers interested in quitting; 52% F, 37% aged 15 ‐ 29, 26% aged 30 ‐ 39, av. cigs/day 23 | |
| Interventions | 1. Proactive call‐back TC following initial call to quitline: Multiple calls, first pre‐quit, quit, then according to need. Up to 6m. Mailed materials | |
| Outcomes | Abstinence at 12m (sustained for 9m) | |
| Notes | Comparison 1. Average number of calls 2.8, 67% received 1 or more. 20% refused call‐back or wanted to initiate the calls, further 7% did not receive any. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up 37% intervention, 30% control. All participants included as smokers in the meta‐analysis |
| Methods | Setting: community, Australia | |
| Participants | 1578 smokers; 54% F, modal age 30 ‐ 49, av. cigs/day 23 | |
| Interventions | 1. Standard S‐H Quit pack based around SoC | |
| Outcomes | Abstinence at 12m (sustained for 9m) | |
| Notes | Comparison 1. 3 vs 2, sensitivity analysis 3 vs 2+1. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation by shuffling questionnaires |
| Allocation concealment (selection bias) | Low risk | Author states "no opportunity for interviewers to influence choice"; baseline characteristics balanced, likelihood of bias judged low. |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up 21% in 1, 23% in 2, 26% in 3. All participants included as smokers in the MA |
| Methods | Setting: general practice, Australia | |
| Participants | 1039 smokers, not selected for motivation but ˜80% had previously tried to quit; 55% F, av.age: 41, av cigs/day 17 | |
| Interventions | 1. Referral: Smokers with any interest in quitting referred by fax to Victorian Quitline. Proactive contact attempted with up to 2 pre‐quit and 4 post‐quit sessions typically using relapse‐sensitive schedule. Internet support available as an alternative (4.4% reported use) | |
| Outcomes | Sustained abstinence at 12m (≥1m at 3m and ≥10m at 12m) | |
| Notes | New for 2009 update. Comparisons 4 ‐ 6, TC as adjunct to face‐to‐face intervention. 30.5% of referral group used call‐back service. McKay‐Brown discusses GP retention and participant recruitment problems. Reported analysis adjusts for age, gender and nicotine dependence and controls for clustering. Adjusted OR is 3.08 (1.02 to 9.28) compared to 2.81 (1.09 to 7.29) using crude data in MA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster‐randomized by GP (1:2 ratio). Computer allocation before GPs attended education session for their assigned intervention |
| Allocation concealment (selection bias) | Unclear risk | Initially concealed but 13 referral (30%) and 11 (42%) control GPs failed to recruit participants. Allocation not blind at time of recruitment of individual participants so further selection bias possible. Measured characteristics at baseline were similar |
| Blinding of outcome assessment (detection bias) | High risk | "Three‐ and 12‐month questionnaires were administered...by trained interviewers who were blind to treatment condition until after the outcome data were collected." However, reliant on self‐reported outcomes from participants not blinded to treatment condition. |
| Incomplete outcome data (attrition bias) | Low risk | 33% lost in referral condition, 39% in control, all included as smokers in MA. Excluding losses does not affect MA |
| Methods | Setting: Health Maintenance Organisation, USA | |
| Participants | 1329 HMO members; 58% F, av.age 47, 66% smoked > pack/day | |
| Interventions | All participants had filled a prescription. Almost 95% used; ˜51% only bupropion, 26% only NRT, remainder both | |
| Outcomes | Abstinence at 12m (repeated 7‐day PP at 3m & 12m) | |
| Notes | New for 2009 update. Comparisons 4 ‐ 6. 49% of intervention group reached, 36% of those declined, 31% of total accepted counselling. Average no of calls 5. There was no evidence of a greater relative effect in those reached or those accepting counselling | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, stratified by presence of chronic disease. Method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | "The follow‐up survey was conducted by the Data Collection Center within the Health Partners Research Foundation, using staff not involved in the intervention." However, reliant on self‐reported outcomes from participants not blinded to treatment condition. |
| Incomplete outcome data (attrition bias) | Low risk | ˜33% lost to follow‐up, balanced across groups, included in MA as smokers |
| Methods | Setting: community, Australia | |
| Participants | 45 smokers attending an information evening on smoking cessation; 62% F, av. age 40, av. cigs/day 23 | |
| Interventions | 1. S‐H manual | |
| Outcomes | Abstinence at 12m (7‐day PP) | |
| Notes | Comparisons 4 ‐ 6, effect of TC compared to S‐H and single information session alone | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Unclear risk | Saliva samples collected but not apparently tested. |
| Incomplete outcome data (attrition bias) | Unclear risk | No details given |
| Methods | Setting: Canada | |
| Participants | 168 past‐month smokers; 27% M, av.age 56, 60% in preparation or action SoC | |
| Interventions | 1. Counselling by research nurse (1x, 10 ‐ 60 mins, av. 40 mins, based on Transtheoretical Model, included component to enhance social support from a significant family member), 23% used pharmacotherapy. | |
| Outcomes | Abstinence at 6m (sustained at 2m & 6m) | |
| Notes | New for 2009 update. Comparisons 4 ‐ 6, TC as adjunct to face‐to‐face counselling. 75% received 6 calls | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomized in groups of 3 ‐ 6 "to prevent contamination between groups", method not described |
| Allocation concealment (selection bias) | Low risk | "Individuals not familiar with the study were in charge of the randomization procedure which included inserting the information into envelopes that were sealed and would be opened by the investigator only at the time of recruitment." |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemical validation used |
| Incomplete outcome data (attrition bias) | Low risk | 4 deaths (3 in Grp 1, 1 in Grp 2) and 3 not meeting follow‐up criteria excluded from MA denominators. Other losses to follow‐up included. |
| Methods | Setting: Specialised cardiac hospital, Canada Recruitment: all smokers who were hospitalised were asked to participate by the study nurse (not selected by motivation) | |
| Participants | 40 current daily smokers with cardiovascular disease, 40% F, av.age 57. Most in preparation stage Therapists: nurse specialised in smoking cessation | |
| Interventions | All participants had 1 or more sessions with the study nurse during hospitalisation. Conditions differed after discharge. Intervention: 6 phone calls by study nurse at wks 1, 2, 3, 4, 8, 12. If needed additional phone calls could be arranged between 3 and 6m postdischarge. At wk 3 appointment with the study nurse if asked by participant Control: referral to a national quitline or a community centre for smoking cessation Pharmacotherapy: NRT, bupropion or varenicline were suggested during hospitalisation and follow‐up | |
| Outcomes | Self‐reported abstinence at 6m Validation: only for 1 participant | |
| Notes | New for 2013 update. Analysis 4.1.2, adjunct to counselling | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified, but generated by a centre for randomized controlled trials |
| Allocation concealment (selection bias) | Unclear risk | Opaque sealed envelopes |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Unclear risk | High loss to follow‐up but missing data similar in both groups and analyses are ITT, participants lost to follow‐up considered smokers |
| Methods | Setting: Health Maintenance Organisation, USA | |
| Participants | 1137 smokers, 479 in relevant arms, not selected by motivation to quit; 52% F, av. age 41, av. cigs/day 17 | |
| Interventions | 1. Control ‐ no materials or counselling | |
| Outcomes | Abstinence at 12m, from 3m ‐ 12m | |
| Notes | Comparisons 4 ‐ 6. 4 vs 3, effect of TC compared to S‐H and feedback alone. Over ⅔ completed 3 calls, rates did not differ by SoC | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Collecting saliva cotinine...was challenging because participants had neither explicitly volunteered for a study of smoking behavior nor requested treatment for smoking cessation... nearly one fourth of those contacted refused to provide a sample." Higher disconfirmation in control group but difference was not significant. |
| Incomplete outcome data (attrition bias) | Low risk | 88% provided data at all 3 &12m. No difference in response rates across groups. Missing counted as smoking in MA |
| Methods | Setting: ENT clinics at 4 hospitals, USA | |
| Participants | 89 current smokers used in MA, out of 184 trial participants who also included 26 quit within last month and 21 within last 6m . Demographics are for all participants; 16% F, av.age 57 | |
| Interventions | 1. Proactive counselling; 9 ‐ 11 CBT‐based calls from trained nurses, linked to use of CBT workbook. Smokers with problem drinking or depression received counselling for these too | |
| Outcomes | Abstinence at 6m (sustained) | |
| Notes | New for 2009 update, in comparisons 4 ‐ 6 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given. Smokers were a higher proportion of the intervention than control groups, and a higher proportion of those randomized than those who refused, raising possibility of selection bias |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Unclear risk | 22 in total (including non‐smokers) lost to follow‐up, evenly distributed. Losses appear to have been included as smokers. |
| Methods | Setting: 8 dental practices, USA | |
| Participants | 82 smokers (60 intervention, 22 control). No baseline data for controls | |
| Interventions | 1. Control: Brief counselling (10 mins) from hygienist, reinforced by dentist | |
| Outcomes | Abstinence at 6m (PP) | |
| Notes | New for 2009 update. Comparisons 4 ‐ 6, TC adjunct to face‐to‐face intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomized by practice, method not described |
| Allocation concealment (selection bias) | High risk | Hygienists who recruited patients after screening not blind, large difference in numbers recruited, not possible to establish baseline similarity |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Unclear risk | No description of number lost at follow‐up |
| Methods | Settng: Primary care patients, 50 rural practices, Kansas, USA | |
| Participants | 750 smokers of >10 cigs/day, 59% F, av. age 47, av. cigs/day 24, 61% contemplation, 30% preparation | |
| Interventions | All participants mailed an offer of free pharmacotherapy every 6m, 4 times in total. Nicotine patch 21 mg for 6 wks or bupropion SR (150 mg twice daily) for 7 wks 1. Control. No other contact. 2. Moderate intensity disease management: up to 2 calls from counsellor in each cycle encouraging uptake of pharmacotherapy, newsletter mailings & periodic progress reports with counselling suggestions faxed to physician. 3. High intensity disease management, up to 6 calls at approx 1, 3, 6, 9, 12 wks from start of each cycle. | |
| Outcomes | Abstinence at 24m (PP). Study also reported analysis based on combination of effects at all follow‐up points. Sustained abstinence not a suitable outcome since no quit date and repeated intervention. Validation: attempted saliva cotinine (< 15 ng/ml) by mail at 12 & 24m. Proxy report used at 24m for non‐returners. Rate of validation similar across groups. | |
| Notes | New for 2013 update. Participants could have multiple courses of pharmacotherapy; 23%, 33%, 23%, 12%, and 9% of participants requested 0, 1, 2, 3, or 4 courses. Disease management conditions increased use in first cycle and reduced it later. 41% of cycles used bupropion & 59% patch. Over 24 months average number of calls 3.6 in 2. and 8.2 in 3. Fewer calls in later cycles. No evidence of effect based on PP, but some evidence of benefit when all follow‐ups taken into account. Comparisons 4 ‐ 6. For analysis 5.1, classified on basis of average calls; moderate in 3 ‐ 6 sessions, high in 7+ subgroups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated random‐number table" in blocks of 24 |
| Allocation concealment (selection bias) | Low risk | "To conceal allocation, we placed these cards in sequentially numbered, opaque, sealed envelopes." |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemical validation used |
| Incomplete outcome data (attrition bias) | Low risk | Differential rates of loss to follow‐up (1: 22.0%; 2: 31.3%; 3: 31.1%). Participants lost to follow‐up counted as smokers but sensitivity analysis shows no significant difference in analysis outcome if excluding those lost to follow‐up. |
| Methods | Setting: Childhood Cancer Survivors Study cohort, USA | |
| Participants | 794 smokers (excludes 2 deaths in control); 47% F, av. age 31, av. cigs/day 12 | |
| Interventions | 1. S‐H control. Mailed manual (Clearing the Air) & letter from study physician | |
| Outcomes | Abstinence at 12m (7‐day PP) | |
| Notes | Comparisons 4 ‐ 6. No data on average number of calls. Longer‐term follow‐up, assessed at 2 ‐ 4 years, reported in Emmons 2009. Not used in MA ‐ sustained rates not reported. PP rates increased from 12m and remained higher in counselling group (20.6% vs 17.6%, P < 0.0003) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Unclear risk | Bogus pipeline procedure used, no further details provided |
| Incomplete outcome data (attrition bias) | Unclear risk | 19% lost in intervention vs 24% in control at 12m. all included as smokers in MA. Excluding losses does not affect MA |
| Methods | Setting: English Quitline Recruitment: Callers to the NHS Smoking Helpline from any location in England | |
| Participants | 2591 smokers aged 16 or older, motivated to quit in 4 days ‐ 4 wks. 45% M; av.age 38; 47% smoking 11 ‐ 20 cigs/day | |
| Interventions | 1. Standard telephone support (after call, further support by email, letter or text message, offer of proactive contact) 2. As 1 plus additional proactive telephone support (up to 2 calls pre‐quit date, 1 call on quit date, then calls at 3, 7, 14 and 21d post‐quit date). Structured call content using MI template (except for 7 and 14d calls). 3. As 1 plus offer of free NRT 4. As 2 plus offer of free NRT | |
| Outcomes | Prolonged abstinence at 6m (allowing grace period of up to 5 cigs smoked). 7d PP also recorded. Validation: exhaled CO < 10ppm | |
| Notes | New for 2013 update. Previously listed under ongoing studies as Coleman 2009. 1+3 vs 2+4 in Comparison 1. No difference in cessation outcomes between participants offered NRT and those not offered NRT. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated random number sequence" |
| Allocation concealment (selection bias) | Low risk | Subjects allocated via central computerised system |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemical validation rates used. |
| Incomplete outcome data (attrition bias) | Low risk | High rates of drop‐out but similar across groups (standard 43%, proactive 45%). Drop‐outs counted as smokers, "this conservative supposition could possibly mask variation...and we explored this possibility by trying alternative associations between missingness and smoking status. This analysis did not change our findings." |
| Methods | Setting: Primary care patients, 16 clinics, USA | |
| Participants | 961 smokers of ≧10 cigs/day. (643 in relevant arms, a further 908 were allowed to select treatment. Demographic details based on 1869); 58% F, av. age 40, av. cigs/day 22 | |
| Interventions | (Self‐selected group of factorial trial not included in MA) | |
| Outcomes | Continuous abstinence at 1 year (no relapse lasting 7 days, also PP) | |
| Notes | Comparisons 4 ‐ 6, 2 vs 1, TC as adjunct to pharmacotherapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated cessation |
| Incomplete outcome data (attrition bias) | Low risk | 19% lost at 1 year, no difference by condition. |
| Methods | Setting: Germany Recruitment: 21 prevention or rehabilitation clinics | |
| Participants | 527 hospitalised female smokers ≥1 cig during the 30 days preceding hospitalisation. Av.age 35.9, motivation to quit not required. | |
| Interventions | 1. 3 face‐to‐face courses (60 mins each) in groups during clinic hospitalisation featuring CBT and MI 2. As 1, plus 3 proactive phone calls (10 mins duration) post‐discharge in a structured and directive style 3. As 2, but calls delivered in non‐directive style | |
| Outcomes | 30 day PP at 6m Validation: none | |
| Notes | New for 2013 update. Intervention arms combined in comparisons 4 ‐ 6. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcome with participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Unclear risk | Number lost to follow‐up unclear (conflicting data available) |
| Methods | Setting: Quitline, UK | |
| Participants | 1457 smokers planning quit attempt within 2 wks; 66% F, av. age 39, av. cigs/day NS | |
| Interventions | 1. Standard QUIT information pack & counselling at initial contact. | |
| Outcomes | Abstinence at 12m (sustained for 6m, also PP) | |
| Notes | Comparison 1. 26% received no additional calls, 42% had 4+ calls, 31% had 1 ‐ 3 calls | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Pseudo‐random by day of week |
| Allocation concealment (selection bias) | Low risk | Recruiters blind so concealment judged adequate |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | 37% lost to follow‐up in both groups. Missing counted as smoking in MA |
| Methods | Setting: Australia Recruitment: Arabic‐speaking GPs in 29 practices in southwest Sydney | |
| Participants | 407 Arabic smokers, aged 18 ‐ 65. 52% F, av. age 29, av. cigs/day 19 | |
| Interventions | 1. Offer of free referral by GP to proactive telephone counselling provided by bilingual psychologist. If accepted offer, participants called by counsellor for 20 min initial session. If prepared to quit, called again on quit date, 1, 3, 6 wks and 3m after specified quit date. If not ready to set quit date, assigned "less intensive schedule." Mailed quit kit and materials in Arabic and English. 2. Usual care | |
| Outcomes | 1d PP at 6 and 12m Validation: none | |
| Notes | New for 2013 update. Low uptake: 101 of 213 participants agree to receive call, 46 receive at least one call, 8 completed all calls. Described narratively in 'other studies' section. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | High risk | "From each participating GP, we recruited a consecutive sample of patients of Arabic background aged 18‐65 years during a specified 4‐week period, irrespective of their smoking status" using an "unobtrusive mark visible to only the GP to convey group randomization" on the baseline questionnaire. Suggests allocation not concealed. |
| Blinding of outcome assessment (detection bias) | Low risk | No biochemical validation but research assistants conducting follow‐up blind to assignment, low uptake of actual contact suggests risk of differential misreport low. |
| Incomplete outcome data (attrition bias) | Low risk | Significantly more participants in intervention group lost to follow‐up at 12m than control (45% vs 34%), all drop‐outs counted as smokers in ITT analysis |
| Methods | Setting: USA Recruitment: US residents searching for stop‐smoking advice on a major internet search engine who clicked on a link to www.quitnet.com, assumed to be motivated | |
| Participants | 2005 adult smokers of 5 or more cigs/day. 51.1% F, av.age 35.9, av.cpd 20, av. FTND 5.0. 1326 contribute to this review | |
| Interventions | 1. Free 6m access to www.quitnet.com (interactive commercial cessation website) 2. As 1 + up to 5 sessions of proactive telephone counselling for 3m; counsellors had access to www.quitnet.com info and encouraged participants’ use of it; counsellors sent individual emails after counselling sessions to reinforce key points 3. Control: access to static, info only (non‐interactive) version of the content on QuitNet (not used in this review) | |
| Outcomes | Multiple 30‐day PP (at 3, 6, 12 and 18m). Validation: none | |
| Notes | New for 2013 update. 2 versus 1 in comparisons 4 ‐ 6. Use of 18m single PP outcome would not have shown any effect of intervention; quit rates were higher and similar across conditions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random numbers table…stratified by sex and baseline motivation to quit" |
| Allocation concealment (selection bias) | Unclear risk | Method not specified |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcome measure from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | High risk | Participants missing data counted as smokers. Sustained PP data not available for 46% EI, 49% EI+P 49% and 43% BI. Difference due to differential rate of follow‐up at 3m. Authors state: "The lower follow‐up assessment rate among EI+P participants at 3 months may have been owing to ‘telephone fatigue’...Telephone counselling was providing within the first 3 months of the study, which was the only assessment period for which higher loss to follow‐up was observed. If present, this bias could have attenuated the effectiveness of the combined intervention." |
| Methods | Setting: Health Maintenance Organisation, USA | |
| Participants | 388 smokers; 66% F, 67% age 40+, 84% smoked less than a pack/day | |
| Interventions | 1. Coverage for TC and pharmacotherapy (bupropion or NRT, USD15 co‐pay) | |
| Outcomes | Abstinence at 6m (PP) | |
| Notes | Not included in MA, results discussed separately, alongside trials for TC as adjunct to pharmacotherapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | Number lost to follow‐up not described, all participants included in analyses |
| Methods | Setting: Hospital/community, Norway | |
| Participants | 133 daily smokers amongst 288 participants. Demographics not given for smoking subgroup | |
| Interventions | 1. Usual care; outpatient visit at 6 ‐ 8 wks and primary care follow‐up | |
| Outcomes | Abstinence at 6, 12 and 18m (assumed PP, not defined). Primary trial outcome was health‐related quality of life | |
| Notes | 18m follow‐up data added in 2013. Comparisons 4 ‐ 6. Smoking was addressed as part of a multicomponent intervention. TC as adjunct to brief/minimal intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized by computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | Sequence in sealed opaque envelopes but not stated to be numbered. Fewer control group participants raises possibility of selection bias so not classified as low risk |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | At 18m, losses amongst baseline smokers 29% in 1, 30% in 2 . Losses reincluded as smokers in this MA |
| Methods | Setting: 24 worksites, USA | |
| Participants | 2402 smokers at baseline survey; 38 ‐ 48% in precontemplation, 50 ‐ 64% F, av age 36 ‐ 40 (large between‐company variations in prevalence and smoker characteristics). | |
| Interventions | Factorial design, 6 conditions: Incentives for participation and cessation/no incentive crossed with telephone, group or choice of programme format. | |
| Outcomes | Abstinence at 24m, sustained for 6m, & 7‐day PP | |
| Notes | Cluster‐randomized, and no other trial compared TC to group so not used in MA, reported narratively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomized by company, 4/condition, method not described |
| Allocation concealment (selection bias) | Unclear risk | Individuals recruited from baseline survey so selection bias less likely |
| Blinding of outcome assessment (detection bias) | Unclear risk | "a randomly selected sample of employees who reported on the 24‐month survey that they had not smoked or used nicotine‐containing products in the previous 7 days were contacted by telephone and asked to provide saliva samples to test for cotinine," but no correction made based on results from biochemical validation. |
| Incomplete outcome data (attrition bias) | Low risk | Results based on respondents only. Does not contribute to MA |
| Methods | Setting: Quitline, Oregon, USA | |
| Participants | 4500 smokers willing to make a quit attempt; 60% F, av. age 41, av.cigs/day 22 | |
| Interventions | Factorial design; 3 levels of counselling, +/‐ offer of nicotine patch (5 wk supply, 80% accepted, option for 3 wks more, 25 ‐ 28% requested) | |
| Outcomes | Abstinence > 30 days at 12m | |
| Notes | 2&3 +/‐ NRT combined vs 1 in comparison 1. First included as Hollis 2005 based on unpublished abstract. Offer of NRT increased mean number of calls and contact time. 1 session, 20 mins in brief no‐NRT, 2.9 sessions, 60 mins in Intensive + NRT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | 69% reached at 12m. Losses assumed smoking in main analysis, sensitivity analyses reported. |
| Methods | Setting: 5 hospitals, Michigan, USA | |
| Participants | 525 patients including 136 who smoked at admission and could be followed up. Smoker demographics not given. | |
| Interventions | 1. In‐hospital care according to American College of Cardiology Guideline Applied to Practice quality improvement (QI) programme including written discharge contract. | |
| Outcomes | Abstinence at 8m ("remained quit for the period") | |
| Notes | Comparisons 4 ‐ 6. Data on smoking outcomes provided by authors from in Press paper by Holtrop et al. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Blocked randomization, method not described |
| Allocation concealment (selection bias) | Unclear risk | Change in methodology from randomization at recruitment/consent to randomization after baseline interview due to initial imbalance in numbers. Data collectors were blind to group |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Unclear risk | 15 people whose smoking status not confirmed and 15 losses to follow‐up excluded because group not stated. ITT analysis said not to alter results |
| Methods | Setting: 7 states, USA | |
| Participants | 7354 smoking Medicare beneficiaries aged 65+ (4295 contribute to review), ˜60% F, ˜69% contemplation, 30% preparation | |
| Interventions | Trial of 4 levels of Medicare benefit. All participants mailed a S‐H kit | |
| Outcomes | Abstinence at 12m (7‐day PP) | |
| Notes | Main comparison 4 vs 3, which had similar levels of self‐reported use of any pharmacotherapy (60% vs 63.4%). Participants were not called unless they enrolled, so treated as trial of quitline availability, estimated effect displayed in Analysis 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomized, states divided into quarters balancing smoking prevalence & aged, restricted randomization to different conditions |
| Allocation concealment (selection bias) | Unclear risk | Participants unaware of programme differences when enrolling and allocation determined by address. Low enrolment in one condition does not seem to have been due to bias. |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Unclear risk | 25% lost to follow‐up at 12m, absolute differences between groups small. Main analysis includes losses as smokers |
| Methods | Setting: 8 primary care clinics, USA | |
| Participants | 1141 smokers (> 1 cig/day) 56% F, age 43/40, median cigs/day 20/15 | |
| Interventions | 1. Intervention based on AHRQ guidelines. Training in brief advice for intake clinicians, vital signs stamp. People willing to set TQD offered proactive TC (2 calls, pre‐ & post‐TQD) by trained nurse, smokers of > 10 cigs/day offered NRT | |
| Outcomes | Sustained abstinence at 2m & 6m | |
| Notes | TC part of a multicomponent intervention, not included in MA. Study also included a baseline assessment. Data from smokers recruited during implementation period used here. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomized by clinic, method not described |
| Allocation concealment (selection bias) | Low risk | Participants enrolled by completing an exit interview with researcher. |
| Blinding of outcome assessment (detection bias) | High risk | "Because of the poor return rate of saliva specimens for cotinine analysis and the possibility of nonresponse bias for reasons unrelated to smoking status, we used self‐reported abstinence as the primary outcome at both the 2‐ and 6‐month assessments." |
| Incomplete outcome data (attrition bias) | Low risk | 4 ‐ 8% lost to follow‐up |
| Methods | Setting: community, Minnesota, USA | |
| Participants | 1827 smokers, not selected by motivation to quit; 50% F, av. age 47, av. cigs/day 22 | |
| Interventions | 1. Proactive TC, 2 calls over 3 wks. Offered S‐H materials | |
| Outcomes | Abstinence at 18m (no puff, > 3m and validated abstinent at 6m) | |
| Notes | Comparisons 4 ‐ 6. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | Minimal contact intervention, likelihood of bias small but since control group participants were not contacted at baseline and a large number of intervention group participants could not be reached, impossible to compare baseline characteristics |
| Blinding of outcome assessment (detection bias) | High risk | No biochemical validation at 18m. At 6m, validated abstinence rates "considerably lower" than self report. |
| Incomplete outcome data (attrition bias) | Low risk | Only a sample of intervention and control participants were selected for follow‐up. Of this sample 91% reached at 18m in both groups. Numbers followed up used as denominator in MA |
| Methods | Setting: Health Maintenance Organisation, USA | |
| Participants | 509 smokers of > 20 cigs/day, motivated to quit; 56% F, av. age 42, av. cigs/day 28 | |
| Interventions | All participants received prescriptions for free nicotine patch (Prostep), 22 mg for a maximum of 6 wks plus 2 wks 11 mg. Proactive vs Reactive | |
| Outcomes | Abstinence at 12m (from quit date) | |
| Notes | Comparisons 4‐6, 3 vs 1+2, effect of proactive TC compared to contact & quitline alone. (1 & 2 combined since fewer than 1% called quitline and no difference between quit rates). Participants who did not return questionnaires at 2, 5, 8, 12 wks were called by telephone. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation by orientation session attended; participants did not know condition in advance so risk of selection bias probably low |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemically validated quit rates |
| Incomplete outcome data (attrition bias) | Low risk | 82% response rate at 12m, no difference between groups, missing treated as smoking |
| Methods | Setting: community, USA | |
| Participants | 1006 smokers in 714 households (651 in relevant arms); av. cigs/day 20 | |
| Interventions | 1. Standard Environmental Protection Agency leaflet on risks of radon (this arm not used in review) | |
| Outcomes | Abstinence at 12m (sustained at 3m, 12m) | |
| Notes | Comparisons 4 ‐ 6. 3 vs 2, effect of TC versus S‐H alone | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized by household, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | 80% of households reached at 3 & 12m, no difference across conditions. Missing treated as smoking |
| Methods | Setting: Community, USA | |
| Participants | 1364 households with 1821 smokers, ˜18 cigs/day | |
| Interventions | Factorial design crossing +/‐ brief phone counselling with 15‐min video S‐H materials. All households given A Citizens Guide to Radon and letter tailored to results of radon level test | |
| Outcomes | Abstinence at 12m, sustained at 3 & 12m | |
| Notes | Comparisons 4 ‐ 6. Results of analyses accounting for clustering of multiple smokers in households reported to yield results generally consistent with simple analyses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Responding households sequentially randomized to 4 conditions subject to stratification on radon test status |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | 83% of households completed 12m assessment, 76% completed both 3 & 12m |
| Methods | Setting: Health centre, USA | |
| Participants | Low‐income African‐American smokers, 266 randomized, 160 followed up, 107 in relevant arms. Unselected by motivation; 52% F, 49% aged > 50 | |
| Interventions | 1. Physician prompts attached to chart (included other screening tests). Providers trained to use 4As (Ask/ Advise/ Assist/ Arrange follow‐up) model. Only received if participants visited doctor | |
| Outcomes | Abstinence 16m after last intervention, 30‐day quit | |
| Notes | Comparisons 4 ‐ 6. 3 vs 2, TC without face‐to‐face contact; physician advice was not an integral part of the intervention ‐ participants not required to have visited the doctor or received advice during the intervention period. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | 38% loss to follow‐up primarily due to disconnected phone numbers. Reported rates based on numbers followed up. Authors report that an analysis with missing treated as smoking did not alter findings |
| Methods | Setting: community, USA | |
| Participants | 412 teen smokers (aged 15 ‐ 18, smoked in past 7 days); 51% F, 56% aged ≥ 17, av cigs/day 10, 21% contemplation | |
| Interventions | 1. S‐H, 2 booklets for teen smokers & video | |
| Outcomes | Abstinence at 8m (7‐day PP at 4m & 8m) | |
| Notes | Comparisons 4 ‐ 6. TC as adjunct to targeted S‐H. 72% received at least 1 call, 52% at least 5, 36% at least 3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described, stratified by SoC |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Biochemical validation done but final outcome figures based on self report only. High failure to confirm and low response rate. |
| Incomplete outcome data (attrition bias) | Unclear risk | 46% Int & 51% Cont reached at both follow‐ups. Losses included as smokers. |
| Methods | Setting: community, Australia | |
| Participants | 854 smokers interested in quitting; 51% F, av. age 42, av. cigs/day 24 | |
| Interventions | 1. Free 2‐wk supply of nicotine patch by mail, instructed to purchase further supply. 14 or 21 mg depending on body weight. | |
| Outcomes | Abstinence at 6m (90‐day continuous) | |
| Notes | Comparisons 4 ‐ 6, TC as adjunct to NRT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | 'randomized' by shuffling folders each day after participants to be included were listed |
| Allocation concealment (selection bias) | High risk | Potential for bias since allocation sequence not fixed in advance. Baseline characteristics similar across groups |
| Blinding of outcome assessment (detection bias) | Low risk | "To minimise misleading reports of abstinence, a bogus pipeline technique was used, with the possibility of carbon monoxide breath testing mentioned in the consent form and at the 3‐ and 6‐month monitoring calls." |
| Incomplete outcome data (attrition bias) | Low risk | 17% lost in NRT only, 15% in + counselling. Missing treated as smoking in MA |
| Methods | Setting: Health Maintenance Organisation, USA | |
| Participants | 580 F current smokers, not selected for motivation to quit; av. age 36, av. cigs/day 13 | |
| Interventions | 1. Usual care ‐ no smoking cessation intervention | |
| Outcomes | Abstinence at 15m (7 day at 6m & 15m), telephone interview | |
| Notes | Comparisons 4 ‐ 6. Effect of TC and S‐H materials compared to no intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not stated, stratified on test result |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemical validation, quit rates not corrected but low level of misreport and "no differences between the two groups in the proportion of women who returned samples, the proportion confirmed/disconfirmed, or the confirmation rate." |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up at 15m 20% in Int, 18% in Cont. Losses included as smokers. |
| Methods | Setting: 2 Health Maintenance Organisations, USA | |
| Participants | 897 pregnant smokers & recent quitters (44% already quit) not selected for motivation to quit; av. age 28, av. cigs/day 15 before pregnancy, 5 if still smoking | |
| Interventions | 1. S‐H booklet only | |
| Outcomes | Abstinence at 12m postpartum (7 day PP) | |
| Notes | Comparisons 4 ‐ 6. 3+2 vs 1, effect of TC versus S‐H only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemical validation used, not reported: "since there were no between‐group differences in the proportion of saliva samples returned or the proportion confirmed, the primary trial outcomes were based on self‐reported smoking status." |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up 13% at 12m, not different by group, losses included as smokers |
| Methods | Setting: Army Medical Centre, USA | |
| Participants | 583 pregnant F current smokers and recent quitters (390 in relevant arms); av. age 24 | |
| Interventions | 1. Usual care ‐ provider advice and S‐H guide | |
| Outcomes | Abstinence at 12m postpartum (PP at all 4 follow‐ups) | |
| Notes | Comparisons 4 ‐ 6, effect of TC as adjunct to brief advice | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described, stratified by smoking status |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Unclear risk | Biochemical validation conducted but not used in outcome data. "Saliva return rates did not differ by condition at either follow‐up," but rates of return low and level of misreport not specified. |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up higher in Int (22%) than Cont (16%). Losses included as smokers |
| Methods | Setting: Health Maintenance Organisation, USA | |
| Participants | 275 F smokers, not selected for motivation to quit; av. age 33, av. cigs/day 14 | |
| Interventions | 1. Usual care, S‐H, contact details for Free & Clear, a covered benefit | |
| Outcomes | Abstinence at 12m (PP) | |
| Notes | Comparisons 4 ‐ 6. Effect of TC versus S‐H only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Bogus pipeline for short follow‐up, biochemical validation at 12m. Results from saliva strip test judged overly conservative, hence self report used in final outcome data, but relative effect not altered. |
| Incomplete outcome data (attrition bias) | Unclear risk | No information on numbers not reached at follow‐up. All participants included in analysis |
| Methods | Setting: Pacific Northwest, USA Recruitment: members of large regional health plan identified through automated records | |
| Participants | 52 adults with evidence of smoking in last year, depression in last 2 years, and without high levels of physical activity. 67% F; av.age 44.5; av. cigs/day 10.6; av. FTND 2.37 | |
| Interventions | 1. Intervention: usual care + phone‐based Step Up proactive counselling program (1 motivational call, 9 weekly CBT calls and 2 follow‐up ‘booster calls’ according to participant need) 2. Control: usual care treatment for depression, smoking and physical activity (incl. S‐H material and referral information for phone‐based smoking cessation programme) | |
| Outcomes | 7‐day PP at 6m, 3m also recorded Validation: none | |
| Notes | New for 2013 update. Analyses 4 ‐ 6. Pilot study of an intervention also addressing physical activity and depression Number abstinent not provided and hence extrapolated from percentages given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned," stratified by baseline antidepressant use". Method of sequence generation not specified |
| Allocation concealment (selection bias) | Unclear risk | Method not specified |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcome, participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | Participants lost to follow‐up counted as smokers, similar numbers lost in each group (4/27 intervention, 2/25 control) |
| Methods | Setting: community, USA | |
| Participants | 1745 smokers; 70% F, 23% age 18 ‐ 30, 40% age 31 ‐ 45, 30% 45 ‐ 64 | |
| Interventions | 1. TV programme and S‐H manual (ALA Freedom From Smoking in 20 Days) | |
| Outcomes | Abstinence at 24m (7‐day) | |
| Notes | Effect of access to hotline combined with S‐H materials for maintenance of cessation. Estimated effect displayed in comparison 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | 24% lost in maintenance condition, 27% in control. MA includes only responders; Including losses would give less conservative effect. |
| Methods | Setting: 13 rehabilitation centres, Germany | |
| Participants | 290 smokers; 41% F, av. age 47, av cigs/day 15, control group significantly more dependent | |
| Interventions | All participants had inpatient group therapy of 7 x 60‐min sessions. ˜26% abstinent at discharge | |
| Outcomes | Abstinence at 12m (PP) | |
| Notes | Comparisons 4 ‐ 6, effect of TC as adjunct to intensive support | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, 1:2 ratio, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | 17/316 randomized to I excluded, no contact postdischarge. Differential drop‐out from remainder, 17% Int, 40% Cont. No detected differences in characteristics of drop‐outs. Sensitivity analyses excluding losses to follow‐up removes significance |
| Methods | Setting: community, Spain | |
| Participants | 200 smokers; 38% F, av. age 35, av cigs/day 28 | |
| Interventions | 1. Proactive TC, 6 x weekly 10‐min calls. 4 on motivation & cessation, 2 on maintenance, + S‐H | |
| Outcomes | Abstinence at 12m (not even a puff since quitting) | |
| Notes | Comparisons 4 ‐ 6. 10‐year follow‐up reported in 2008, not used in MA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemical validation used |
| Incomplete outcome data (attrition bias) | Unclear risk | No information on numbers not reached at follow‐up. All participants included in analysis |
| Methods | Setting: Community, Spain | |
| Participants | 228 smokers of ≥10 cigs/day; 46% F, av. age 37, av. cigs/day 27, 44% had prior year quit attempt | |
| Interventions | 1. Mailed S‐H programme; 6 weekly manuals, quit date intended to be set at end of wk 4 | |
| Outcomes | Abstinence at 12m (sustained since end of treatment) | |
| Notes | Comparisons 4 ‐ 6. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | "Telephone interviews were conducted by a trainer interviewer who was blind with respect to the group to which each subject was assigned. To improve the reliability of these self‐reports of smoking status, all follow‐up questionnaires and interviews commenced with a reminder that the subject might at some point be asked to undergo a carbon monoxide test." |
| Incomplete outcome data (attrition bias) | Unclear risk | Missing data treated as failure, no statement of numbers lost to follow‐up |
| Methods | Setting: Hospitals, USA | |
| Participants | 1942 smokers (excludes deaths); 49% F, av. age 51, av cigs/day 20 | |
| Interventions | All groups received standardised physician advice | |
| Outcomes | Abstinence at 12m (sustained at 3m, 6m, 12m) | |
| Notes | Effect of additional telephone follow‐up. Not pooled. Intensive intervention was significantly better than usual care for confirmed PP 12m abstinence, other differences not significant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Low risk | "Nurses opened sealed envelopes in front of patients". |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemical validation; verification by family member used when biochemical validation not possible. |
| Incomplete outcome data (attrition bias) | Low risk | Number lost to follow‐up not specified, all randomized participants, excluding 82 deaths, included in analyses |
| Methods | Setting: Primary care clinics, USA | |
| Participants | 1223 smokers (excludes deaths and 237 who did not receive intervention); 57% F, av. age 35, av. cigs/day 23 | |
| Interventions | 2 x 3 factorial design, physician intervention +/‐ follow‐up | |
| Outcomes | Abstinence at 6m (7‐day); (3m sustained abstinence rates not given by condition) | |
| Notes | Comparisons 4 ‐ 6, 1 vs 2, AO and CI effect of TC in addition to physician intervention. NCG arm in pharmacotherapy adjunct, both pooled in intensity and motivation subgroup analyses. 12m abstinence rates reported in Ockene 1994 but not given by follow‐up condition | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | Allocated prior to physician encounter |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | 19% lost to follow‐up, higher in telephone follow‐up group. All included as smokers in analysis |
| Methods | Setting: Health Maintenance Organisation, USA | |
| Participants | 2021 smokers of 3+ cigs/day, wanting to quit (1412 in relevant arms); 63% F, av. age 44, av. cigs/day 26 | |
| Interventions | 1. S‐H manual, Quit Kit and ALA Lifetime of Freedom from Smoking | |
| Outcomes | Abstinence at 16m for > 6m, by blinded telephone interview. | |
| Notes | Comparisons 4 ‐ 6. 3 vs 1+2, effect of telephone counselling compared to S‐H materials alone. (No significant difference between 1 and 2) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described, stratified by living alone/not, advice to quit in last 12m/not and nicotine content of cig.brand |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemical validation in sample at 16m; "to improve the veracity of smoking self‐report, all follow‐up questionnaires and interviews began with a reminder that the subjects might be asked for a saliva specimen for nicotine assessment, creating a sort of “bogus pipeline”" |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up 6% at 16m, did not differ across treatment groups. Analyses based on respondents; including losses would marginally increase estimated effect |
| Methods | Setting: community, USA | |
| Participants | 1422 African‐American smokers; 64% F, av. age not stated, 62% in 20 ‐ 39 age group, median cigs/day 20 | |
| Interventions | Reactive, for callers to quitline | |
| Outcomes | Abstinence at 6m, 7‐day PP | |
| Notes | Comparison 2, between 2 types of counselling. Also included in Cochrane Self‐help review since effects of counselling and S‐H materials cannot be separated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Pseudo‐randomized by last digit of caller's contact phone number |
| Allocation concealment (selection bias) | High risk | Potential for selection bias but unlikely given low contact |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | 37% lost to follow‐up at 6m. No differential drop‐out, losses included as smokers. |
| Methods | Setting: occupational health service, USA | |
| Participants | 58 smokers; 93% M, av. age 52, av.cigs/day 22 | |
| Interventions | All participants received brief physician advice at screening | |
| Outcomes | Abstinence at 6m, 30 day PP, telephone | |
| Notes | Comparisons 4 ‐ 6 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes, not stated if opaque and numbered |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | 32% lost to follow‐up comparable across groups, losses included as smokers |
| Methods | Setting: 10 counties, USA | |
| Participants | 1813 smokers planning to quit within 3m; av. age 43, av. cigs/day 28 | |
| Interventions | Reactive | |
| Outcomes | Abstinence at 18m, sustained from 3m. | |
| Notes | The authors report a range of analyses based on alternative measures of smoking status and using logistic regression to allow for cluster randomization. The higher quit rate in the hotline counties was consistent in all analyses. 36% called hotline, 8.7% spoke with counsellors. Estimated effect displayed in comparison 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Matched pairs of counties assigned to condition in a restricted procedure to minimise media spill‐over. |
| Allocation concealment (selection bias) | Unclear risk | Participant recruitment not linked to county assignment so selection bias unlikely |
| Blinding of outcome assessment (detection bias) | Low risk | Self‐reported abstinence verified by significant other and/or saliva cotinine |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up over 90% at all points and did not differ by condition |
| Methods | Setting: community, USA | |
| Participants | 177 smokers aged ≥ 60, planning to quit in next 3m; 61% F, av. cigs/day 25 | |
| Interventions | 1. S‐H manual (Clear Horizons), access to 24‐hr hotline, 2 letters of support and hotline reminders | |
| Outcomes | Abstinence at 6m (7‐day PP) | |
| Notes | Comparisons 4 ‐ 6. 42% had called hotline and 17.5% spoken to counsellor by 6m. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Unclear risk | Validation by significant other, number refused/misreported not specified. |
| Incomplete outcome data (attrition bias) | Low risk | 97% reached at 12m |
| Methods | Setting: community, USA | |
| Participants | 756 smokers (12% precontemplation, 58% contemplation, 30% preparation) (378 in relevant arms); 62% F, av. age 43, av. cigs/day 27 | |
| Interventions | 1. ALA S‐H manuals | |
| Outcomes | Sustained abstinence at 18m (12m & 18m) | |
| Notes | Comparisons 4 ‐ 6. 4 vs 3, TC vs S‐H alone. Numbers randomized to groups and quit rates as shown in graphs obtained from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described, stratified by SoC |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | "Bogus pipeline" approach; names of significant others asked for but not contacted. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition at each assessment averaged 4.1% ‐ 7.1% across all treatment conditions, not significantly different. 70% provided data at every assessment. MA uses numbers randomized, sensitivity analysis does not alter conclusions |
| Methods | Setting: Managed care organization, USA | |
| Participants | 1447 smokers (723 in comparisons used); 38% were precontemplators, 56% F, av. age 38, av. cigs/day 20 | |
| Interventions | 1. Assessment only (completed questionnaires on 4 occasions) | |
| Outcomes | Abstinence at 18m, sustained for 6m. (Other measures of abstinence also reported) | |
| Notes | Comparisons 4 ‐ 6. 3 vs 2, TC vs S‐H alone. Other arms compared in Self‐help review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | Greater loss to follow‐up in TC (44%) than S‐H (38%). Denominators here include losses to follow‐up and refusals. Author analysis suggests this treatment of missing data is biased, but sensitivity analysis excluding losses & refusals does not alter our MA conclusions. |
| Methods | Setting: Quitline, USA | |
| Participants | 3522 smokers willing to make a quit attempt within 2 wks | |
| Interventions | 1. 3 American Cancer Society S‐H booklets | |
| Outcomes | Abstinence at 6m (sustained). Only people abstinent at 3m followed at 6m. | |
| Notes | Comparison 1. 58% did not complete more than 1 session of counselling (McAlister paper) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Small local sample biochemically tested, no responders disconfirmed. |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up 50% in Int, 55% in Cont (from McAlister paper). Differed by age ‐ higher loss in younger participants. All losses treated as smokers |
| Methods | Setting: National Cancer Society quitline, USA | |
| Participants | 6322 smokers; 70% F, av. age 43, median cigs/day 20 | |
| Interventions | ¼ allocated to S‐H control, remainder into 3 x 2 factorial design | |
| Outcomes | Abstinence at 7m postrandomization (PP) | |
| Notes | All interventions pooled vs control in comparison 1, results of different intensities discussed in more detail in text | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence without stratification |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Unclear risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up ˜50%, similar in all groups. Analysis includes losses as smokers. |
| Methods | Setting: community, Canada | |
| Participants | 396 smokers interested in quitting within 30 days, smoking ≥ 15 cigs/day; 48% F, av. age 38, av. cigs/day 23 ‐ 24 | |
| Interventions | 1. Nicotine patch (15 mg x 8 wks, 10 mg x 2 wks, 5 mg x 2 wks) free, physician advice (x 3 15‐min, 2 wks before, 4 wks, 12 wks after quit date) | |
| Outcomes | Abstinence at 12m (PP) | |
| Notes | Comparisons 4 ‐ 6, effect of adjunct TC compared to NRT and counselling alone. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized using table of random numbers, stratified by gender and nicotine dependence |
| Allocation concealment (selection bias) | Unclear risk | Concealment unclear but physician blind to allocation |
| Blinding of outcome assessment (detection bias) | Low risk | 'Bogus pipeline' procedures used for early follow‐ups; proportion of participants who provided breath samples did not differ between two groups; only one misreport identified; adjustment of abstinence rates for validation did not affect conclusions. |
| Incomplete outcome data (attrition bias) | Low risk | 15% lost/dropped out in each groups, included as smokers |
| Methods | Setting: tertiary care cardiac hospital, Canada | |
| Participants | 100 smokers; 32% F, av. age 54, 48% quit attempt in previous year | |
| Interventions | All participants received in‐hospital brief counselling, access to NRT, S‐H materials | |
| Outcomes | Abstinence at 1 year (PP) | |
| Notes | Comparisons 4 ‐ 6, mean 2.1 IVR calls completed, 46% received at least 1 counselling call, mean 1.8, so total calls categorised as 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "mediated through the Clinical Epidemiology Unit’s data centre, using a computer generated randomization list" Block size 6 |
| Allocation concealment (selection bias) | Low risk | "Research staff were unaware of the treatment allocation prior to randomization" |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | ˜15% lost to follow‐up, similar between groups. 1 Cont death excluded, others included |
| Methods | Setting: Prenatal care services, USA | |
| Participants | 442 pregnant women smoking at least 1 cig in previous 7 days; av. age 29, av. cigs/day 21 prior to pregnancy, 10 at recruitment, 84% planned to quit | |
| Interventions | All participants received brief counselling at enrolment call & mailed a pregnancy‐tailored S‐H booklet | |
| Outcomes | Abstinence 3m postpartum (sustained at end of pregnancy & 3m) | |
| Notes | Comparisons 4 ‐ 6. Mean of 5 calls received, 4 in pregnancy, av. 68 mins in total. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization list arranged in balanced blocks of 4 and stratified by referral source" |
| Allocation concealment (selection bias) | Low risk | "... the application revealed the next assignment only after the smoker had consented to participate in the study" |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemical validation; those who failed biochemical validation or did not provide a sample counted as smokers |
| Incomplete outcome data (attrition bias) | Low risk | 21 miscarriages excluded. 33% Int, 28% Cont lost to follow‐up, included as smokers. |
| Methods | Setting: community, USA | |
| Participants | 1867 smokers aged 50 ‐ 75 (12m data based on 1391, 1225 in relevant arms) interested in finding out about quitting; 63% F, av age 61, av cigs/day 27 | |
| Interventions | 1. Standard S‐H manual (not included in this review) | |
| Outcomes | Abstinence at 12m. | |
| Notes | Comparisons 4 ‐ 6. 3 vs 2. Preliminary 12m results used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | ˜75% reached at 12m with no treatment group differences in follow‐up rate |
| Methods | Setting: 40 clinics, USA | |
| Participants | 3436 smokers identified by survey, 2729 followed up, 1664 in relevant arms | |
| Interventions | Access to proactive service | |
| Outcomes | Abstinence at 6m for 7 days | |
| Notes | Does not contribute to MA. Test of providing TC to increase provider adherence to guidelines. Most of the smokers surveyed did not report use of counselling services | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomized by clinic, method not stated |
| Allocation concealment (selection bias) | Unclear risk | Smokers identified by survey, selection bias unlikely |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Unclear risk | 80.5% response to telephone survey, no difference by condition |
| Methods | Setting: Wisconsin, USA Recruitment: young adult callers to the Wisconsin Tobacco Quit Line (WTQL) | |
| Participants | 410 smokers age 18 to 24 years, smoked at least 1 cig in past 30 days and motivated to quit. 58% F; av.age 21.3 years, av. cigs/day 15 | |
| Interventions | 1. S‐H only, stage‐based booklets 2. S‐H + up to 4 proactive cessation counselling calls over 4 ‐ 6 wks via the WTQL | |
| Outcomes | 7‐day PP at 6m (1m & 3m also reported) | |
| Notes | New for 2013 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | List of random numbers |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Unclear risk | Follow‐up interviewers unaware of assignment |
| Incomplete outcome data (attrition bias) | Unclear risk | 53% not followed in Int, 50% in Cont. Missing treated as smoking. Responder analysis did not change results |
| Methods | Setting: 10 communities, Canada | |
| Participants | 632 smokers intending to quit; 61% F, av. age 42, 61% had prior use of NRT | |
| Interventions | Factorial design comparing 2 intensities of TC and 2 types of S‐H (collapsed in this review): | |
| Outcomes | Abstinence at 12m, sustained at 3m & 6m follow‐ups, also PP. | |
| Notes | All TC arms compared to S‐H only control in comparison 1. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, stratified by community, method not described |
| Allocation concealment (selection bias) | Low risk | "opening next in a series of envelopes' after enrolment" |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | 30% not available at 12m, no difference across 5 groups, missing treated as smoking |
| Methods | Seting: Quitline, USA | |
| Participants | 987 smokers, > 10 cigs/day, willing to set quit date within 30 days: av. age 42, av. cigs/day 21 | |
| Interventions | Factorial trial testing medication adherence counselling, 2 vs 6 wks NRT, and nicotine patch alone vs patch + gum. All participants received the same standard TC: 4 sessions over 4 wks. Medication adherence counselling involved additional content at each call assessing and addressing adherence | |
| Outcomes | Abstinence at 6m (30‐day PP). 7‐day PP also reported Validation: none | |
| Notes | New for 2013. Not included in any meta‐analysis as tested adjuncts to TC not the efficacy of TC. Results reported narratively. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | List of randomized numbers |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors independent of quitline |
| Incomplete outcome data (attrition bias) | Unclear risk | 24% lost at 6m follow‐up, no difference across treatment groups |
| Methods | Setting: community, USA | |
| Participants | 214 F smokers, > 4 cigs/day, intending to quit in next 2 wks; av. age 33, av cigs/day 24 | |
| Interventions | 1. Free nicotine patch (dose based on smoking level) for up to 10 wks. | |
| Outcomes | Abstinence at 6m (7 days at 3m & 6m) | |
| Notes | Comparisons 4 ‐ 6. Intervention participants received an average of 7 calls. 95% received at least 1. Participants could call Nicoderm support line, 21% of control vs 8% of intervention did so. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Biochemical validation but 7% ‐ 12% disconfirmation rate. Differential rates of return at 6m (59% of self‐reported quitters in intervention group and 67% in control). Participants who did not provide samples classified as quitters. |
| Incomplete outcome data (attrition bias) | Low risk | ˜27% lost in both groups, included as smokers |
| Methods | Setting: community, USA | |
| Participants | 330 female smokers > 4 cigs /day, intending to quit in next 2 wks; av. age 34, av. cigs/day 24 | |
| Interventions | 1. Free nicotine patch (dose based on smoking level) for up to 10 wks. | |
| Outcomes | Abstinence at 6m (30 days at 3m & 6m) | |
| Notes | Comparisons 4 ‐ 6, replication of Solomon 2000 with more extended telephone contact. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | 13% lost to follow‐up in both groups, included as smokers |
| Methods | Setting: ALA Quitline, USA | |
| Participants | 990 callers; 62% F, av.age 43, av. cigs/day 22 | |
| Interventions | 1. Reactive counselling | |
| Outcomes | Abstinence at 12m (PP) | |
| Notes | Test of different interventions for people calling a quitline. Comparison 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number list created by independent statistician |
| Allocation concealment (selection bias) | Low risk | Enrolment & assignment by researchers independent of helpline staff. Concealment until assigned |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Interviewer assessing outcomes was blinded"; biochemical validation in a convenience sample (16/28 agreed); participants who did not agree to biochemical validation but self‐reported abstinence counted as abstinent |
| Incomplete outcome data (attrition bias) | Low risk | 47% loss to follow‐up, similar across groups, included as smokers. |
| Methods | Setting: Workplaces, USA | |
| Participants | 231 smokers completed baseline survey. Demographics for all participants followed up; 94% M, av. age 40 | |
| Interventions | 1. Proactive counselling; up to 6 calls over 3m (fruit & veg consumption also addressed), tailored feedback report & tip sheets, NRT offered to those interested in quitting. | |
| Outcomes | Abstinence at 6m (7‐day PP) | |
| Notes | Comparisons 4 ‐ 6. Baseline denominators confirmed by author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | 18% ‐ 20% lost, assumed smokers |
| Methods | Setting: antenatal clinics, USA | |
| Participants | 269 pregnant smokers at wk 28; av. age 28, approx 50% smoked < 60 cigs/wk | |
| Interventions | All participants had received brief counselling and 7 mailed S‐H booklets in early pregnancy. | |
| Outcomes | Abstinence or 'a few puffs' at 6m postpartum | |
| Notes | Comparisons 4 ‐ 6. The common intervention in early pregnancy was not treated as face‐to‐face contact within the trial. 55% received complete intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Unclear risk | Though no biochemical validation postpartum, cotinine in subsample at wk 34; no differences between experimental and control groups; "the urine samples appeared not to have been collected in a systematically biased manner." Level of misreport and refusal not specified. |
| Incomplete outcome data (attrition bias) | Unclear risk | 39% lost at follow‐up in both groups, assumed to be smoking |
| Methods | Setting: Group Health Co‐operative, USA | |
| Participants | 1524 smokers ≥ 10 cigs/day; 57% F, av. age 45, av. cigs/day 23, 44% history of depression | |
| Interventions | Proactive | |
| Outcomes | Abstinence at 12m (7‐day PP) | |
| Notes | Compares different intensity of TC. No dose/behavioural treatment interaction at 12m so bupropion arms collapsed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | randomization procedure built into study database |
| Allocation concealment (selection bias) | Low risk | Procedure ensured concealment |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up at 12m 17% Int, 12% Cont, treated as smokers |
| Methods | Setting: community, Idaho and Washington, USA Recruitment: community advertising, physician referral, and quitline callers | |
| Participants | 1202 adult current smokers of at least 10 cigs/day in past year and 5 cigs/day in past week, motivated to quit. 66.9% F; av.age 47.3; av.cigs/day 19.7; av.FTND 4.9 | |
| Interventions | All participants received: 12‐wk course of varenicline; 5 ‐ 10‐min orientation call; S‐H materials; access to toll‐free support line for ad hoc calls 1. Telephone counselling. Proactive; from quitline counsellor using MI techniques; max 5 calls. 2. Web programme with standardised content and interactive tools modelled on those used in phone intervention. 3. 1+2. Phone counsellors had access to info participants entered online. | |
| Outcomes | Abstinence at 6m (30‐day PP) (abstinence at 3m, 7‐day PP also reported) Validation: None | |
| Notes | New for 2013 update. Number abstinent not provided, estimated from percentages given in published report TC and TC+web had similar outcomes so pooled 1 + 3 vs 2 in comparisons 4 ‐ 6 (adjuncts to pharmacotherapy). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Group assignment was randomly allocated using an automated algorithm built into the study database" |
| Allocation concealment (selection bias) | Low risk | Central computerised allocation, see above |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcome measure used from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | Participants lost to follow‐up counted as smokers in ITT analysis; equal losses between groups (103 Web, 107 Phone, 100 Web + phone) |
| Methods | Setting: Workplace and community, USA | |
| Participants | 382 (341 smokers, 41 recent quitters). Majority in contemplation or action SoC, 24% 'blue‐collar', 59% F, av. age 41, av. cigs/day 18 ‐ 22 | |
| Interventions | 1. Callers to hotline received general information based on fact sheets, and sent S‐H material. | |
| Outcomes | Abstinence at 6m (PP) (subset followed to 12m) | |
| Notes | Comparison 2, between stage‐based and non‐specific brief counselling | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | Low risk | Saliva samples sought but not tested; surrogates asked to confirm status. |
| Incomplete outcome data (attrition bias) | Low risk | 17% lost to follow‐up at 6m, no significant difference between groups, included as smokers |
| Methods | Setting: Community, New South Wales, Australia Recruitment: Active telephone recruitment (cold‐calling) of NSW residents, motivation to quit not required | |
| Participants | 1562 adult daily smokers. 50% M, av.cigs/day 19.4, av.age 45. | |
| Interventions | 1. Six proactive counselling calls for smokers willing to quit within 1m, 4 for those not willing using MI techniques. Those who relapsed and set new quit date within a month offered additional 5 calls; those relapsed but did not set quit date offered a call in 1m. Those initially not willing to quit who became motivated to quit offered additional 5 support calls. Standard S‐H materials 2. S‐H materials only | |
| Outcomes | Prolonged abstinence at 13m (for 12m with 1m grace period). Other prolonged and PP rates at 4, 7, 13m also reported Validation: none | |
| Notes | New for 2013 update. Analyses 4 ‐ 6. 7.8% of control group called quitline during study period. No 13m outcomes showed significant effects although earlier time points did. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random number generator" |
| Allocation concealment (selection bias) | Low risk | "computer assisted telephone interview used a random number generator created by an independent programmer to allocate the smoker" |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcome measure used, participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Unclear risk | Participants lost to follow‐up counted as smokers; similar numbers in both groups (163 intervention, 154 control (21% and 19% respectively)) |
| Methods | Setting: Community, USA | |
| Participants | 2054 smokers (1009 in relevant arms); 23% F, av age 51, 40% precontemplators, 40% contemplators, 20% preparers | |
| Interventions | 1. Stage‐based S‐H manuals; participants sent manual for current stage and next stage. (not used in this review) | |
| Outcomes | Abstinence at 30m, sustained for 6m | |
| Notes | Comparisons 4 ‐ 6, 4 vs 3 for proactive TC. In NRT eligible groups 350 (67%) received NRT at baseline and 448 (86%) received NRT at some point, so classified as adjunct to pharmacotherapy, and in > 6 call category | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based random number generator |
| Allocation concealment (selection bias) | Low risk | Allocation done after completion of survey. randomized participants who did not return consent form are excluded from further analyses |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | 39% lost incl 8% refused by 30m, no significant differences between groups. Different treatments of missing data reported not to have altered pattern of results. |
| Methods | Setting: General practices, Australia | |
| Participants | 318 smokers; 53% F, av.age ˜37, modal cigs/day 11 ‐ 20, 56% in contemplation/precontemplation. | |
| Interventions | 1. GP offered referral; telephone call from a nurse trained in cessation within 3 days. 5A's counselling framework. If willing to make a quit attempt mailed quit kit, encouraged to buy NRT, phoned again on TQD, 1 wk, 3 wks. | |
| Outcomes | Abstinence at 12m (PP) | |
| Notes | Comparisons 4 ‐ 6. We classified control as minimal intervention (4.1.1) rather than brief intervention, MA not sensitive to classification. Referral was to a research nurse not to a dedicated quitline. 5 control participants received intervention, analysed with controls as ITT. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Questionnaires randomly ordered and coded prior to delivery to the practice by selecting sequential numbers from a computer generated random number list. |
| Allocation concealment (selection bias) | Unclear risk | Patients (including non‐smokers) completed the precoded questionnaire before the consultation. GP identified allocation from unobtrusive marks on questionnaire, could not influence allocation. But unclear whether selection bias by recruiters, given imbalance in numbers |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | 31% Int, 41% Cont lost to follow‐up, included as smokers |
| Methods | Setting: Quitline, USA | |
| Participants | 3030 smokers calling smokers' helpline and were ready to quit in next wk; 57% F, av. age 36, av. cigs/day 20 | |
| Interventions | 1. S‐H materials only | |
| Outcomes | Abstinence at 13m, sustained for 12m | |
| Notes | Comparison 1; 2 & 3 vs 1. 3 vs 2 in effect of multiple sessions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Pseudo‐random, according to last 2 digits of telephone number |
| Allocation concealment (selection bias) | High risk | Potential for selection bias but unlikely given low contact |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemical validation in a convenience sample. Disconfirmation rate not used to correct data, but refusal and misreport rates similar in all groups. |
| Incomplete outcome data (attrition bias) | Low risk | 12% ‐ 16% lost to follow‐up at 13m, included as smokers |
| Methods | Setting: Quitline, USA | |
| Participants | 3282 smokers calling quitline, ready to quit within 1 wk & wanting counselling; 56% F, av. age 38, av. cigs/day 20 | |
| Interventions | 1. S‐H pack, motivational materials, counselling provided if smoker made contact to request it. | |
| Outcomes | Abstinence at 13m, sustained for 12m | |
| Notes | Comparison 1. Authors also analysed subgroups of control who did and did not seek counselling. 32% of Cont and 72% of Int group received counselling | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomized, method not described. 60/40 split. Only randomized when counselling demand exceeded capacity |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) | High risk | Self‐reported outcomes from participants not blinded to treatment condition |
| Incomplete outcome data (attrition bias) | Low risk | ˜30% lost to follow‐up at 13m in both groups, included as smokers |
| Methods | Setting: Quitline, USA Recruitment: callers to a quitline | |
| Participants | 2278 Chinese‐, Korean‐ and Vietnamese‐speaking daily smokers, ready to quit within 1m; 90% M; aged 18 ‐ 75 (approx. 45% 25 ‐ 44 and 45% 45 ‐ 64); av.cigs/day 15.6 | |
| Interventions | 1. S‐H pack, culturally‐tailored translated into Chinese, Korean and Vietnamese 2. S‐H pack + proactive TC; Social Learning Theory; MI; CBT techniques. 30 ‐ 40 min, pre‐quit, up to 5 relapse prevention calls (10 ‐ 15 min) 0, 3, 7, 14, 30 days | |
| Outcomes | Prolonged abstinence at 7m post‐intervention, 1m grace period immediately postquit Validation: none (but saliva samples collected) | |
| Notes | New for 2013 update. Number abstinent at 6m not specified; data used in meta‐analysis calculated back from percentages. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly assigned…using blocks of 20 to keep a balance of language and sex…Random assignment tables for each strata were created using SAS 9.2." |
| Allocation concealment (selection bias) | Low risk | "The allocation was done by the computer so that staff were blinded to group assignment until the intake call" |
| Blinding of outcome assessment (detection bias) | Low risk | Self‐reported outcomes but saliva samples collected. No statistically significant differences in saliva sample return rates at 7m btwn intervention and control groups and btwn self‐reported quitters and non‐quitters. |
| Incomplete outcome data (attrition bias) | Low risk | Simlar rate of dropouts in both groups (18% in 1, 16% in 2). Participants lost to FU included as smokers in outcome data. |
AHRQ: Agency for Healthcare Research and Quality
ALA: American Lung Association
CBT: cognitive behavioural therapy
CO: carbon monoxide
F: female
FTND: Fagerström Test for Nicotine Dependence
HMO: health maintenance organization
hrs: hours
ITT: intention‐to‐treat (analysis)
m: months
M: male
MA: meta‐analysis
MI: motivational interviewing
NCIS: National Cancer Information Service
NRT: nicotine replacement therapy
PP: point prevalence
SES: socio‐economic status
S‐H: Self‐help materials
SoC: Stage of Change
TC: Telephone counselling
TQD: Target quit date
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Pilot study with 12 wks follow‐up, after which the advice and control groups were offered the intervention. The intervention was 4 x weekly mailings and telephone calls from a lay facilitator. No participants in any group (n = 64) quit smoking. | |
| Not a fully randomized trial. Smokers assigned to behavioural condition by clinic attended. | |
| Not a controlled trial. Callers to a workplace helpline set up in conjunction with a non‐smoking policy were followed up. 16% of smokers reported they had quit 3m later, 28% of those who had tried to quit. It was estimated that between 3 and 3.3% of smokers in the company had called in the first 3m. | |
| Intervention was to increase clinic referrals to a quitline. No smoking outcomes. | |
| Callers to a helpline were randomized to 1 of 2 S‐H materials. No counselling was given. Follow‐up only 1m after receipt of materials. There was no difference in cessation rates between the booklet groups. Overall 16% of 515 respondents reported 7‐day abstinence at 1m. | |
| Allocation not stated to be random. Telephone follow‐up compared to group behavioural treatment with aversive smoking only. Abstinence rates were lower for the telephone group. | |
| Not a randomized trial. | |
| All participants received brief TC calls. Intervention was a face‐to‐face motivational interview | |
| Not a controlled trial. Evaluation of calls to a helpline. | |
| All participants called a quitline, test of different S‐H materials. Included in Cochrane review of S‐H (Lancaster 2005b). | |
| Intervention for smokeless tobacco use, not smoking. | |
| Intervention for smokeless tobacco use, not smoking. | |
| Focus on preventing relapse. See Cochrane review on relapse prevention (Hajek 2009). | |
| Intervention condition included intensive face‐to‐face counselling as well as telephone contact. | |
| Multicomponent intervention, only 12 wks follow‐up | |
| Not randomized; historical and non‐equivalent controls. | |
| Evaluated a counselling component to address cessation related weight concerns. Will be evaluated in Cochrane review of interventions for preventing weight gain after smoking cessation (Farley 2012). | |
| Only 3 months follow‐up | |
| Intervention to increase re‐enrolment in quitline services. No smoking outcomes | |
| Intervention was IVR to re‐engage relapsed smokers, no cessation outcomes. | |
| Not a randomized trial. Compared intensive counselling delivered face‐to‐face or by telephone | |
| Focus on preventing relapse. See Cochrane review on relapse prevention (Hajek 2009). | |
| Trial identified from a paper reporting secondary outcomes. Compared 3 levels of behavioural intervention in a primary care setting. Full results have not been published and not available. | |
| Callers to a helpline were randomized to one of 4 different S‐H programmes or an information control. No counselling was given. There was no difference in outcome between any of the S‐H booklets or the control, with sustained abstinence rates of 4% ‐ 8% at 6m. | |
| Does not measure smoking cessation. Assesses impact of a media campaign to get women smokers with young children to call a quit line. Call rates compared in media markets with and without a campaign. Campaign increased call rates 10 times compared to control markets. Proportion of calls from target group also increased. Cost per caller estimated at USD 61. | |
| Not a randomized trial. Evaluated impact of free NRT as adjunct to telephone support. | |
| Not a randomized trial. Evaluated impact of different amounts of free NRT as adjunct to telephone support. | |
| All participants eligible for same telephone counselling intervention; test of different amounts of NRT. | |
| Telephone component cannot be evaluated independently of face‐to‐face counselling. | |
| Study of smokeless tobacco users only. | |
| All participants were women with young children who called a hotline and received same stage‐based counselling. They were randomized to receive 3 different S‐H guides. See Cochrane review of S‐H (Lancaster 2005b) | |
| Telephone component cannot be evaluated independently of face‐to‐face counselling. The intervention included in‐hospital motivational interviewing as well as post‐discharge telephone contact, and was compared to usual care. | |
| Telephone component cannot be evaluated independently of face‐to‐face counselling. The intervention included in‐hospital physician advice and counselling by a nurse as well as post‐discharge telephone contact, and was compared to usual care. | |
| Not random or pseudo‐random. Interventions ran sequentially. Participants receiving mailed materials had access to a hotline. | |
| Single telephone call was the brief intervention control for a 3‐session group‐based pharmacist‐conducted intervention. | |
| Recent quitters were randomized to access to recorded messages, not a counsellor. Short follow‐up (4 wks). | |
| Only 3m follow‐up. Comparison between 1 and 4 telephone follow‐ups as adjunct to face‐to‐face counselling. 19 participants per group. | |
| Intervention aimed at reduction in cigarette use for people not wishing to attempt cessation. | |
| Telephone component cannot be evaluated independently of face‐to‐face counselling delivered by dental practitioner. | |
| Intervention used cell phone. Will be evaluated in Cochrane review of mobile phone‐based interventions for smoking cessation (Whittaker 2012).. | |
| Insufficient detail in abstract to include, no full report identified. | |
| Study of 2 different frequencies of telephone counselling for high blood pressure, including smoking cessation counselling. Smoking cessation not reported as an outcome, unclear if smoking cessation measured. | |
| Telephone component cannot be evaluated independently of face‐to‐face counselling. The intervention included in‐hospital counselling by a nurse. | |
| Only 3m follow‐up | |
| Telephone component cannot be evaluated independently of face‐to‐face counselling and offer of pharmacotherapy. | |
| The purpose of the telephone call was to encourage participants to enrol in quitline services | |
| Telephone component cannot be evaluated independently of face‐to‐face counselling. The intervention included in‐hospital counselling by a nurse. Quasi‐random design. | |
| Complex intervention; all participants received telephone counselling. | |
| Main intervention component was face‐to‐face support. Telephone contact in both arms. | |
| Three worksites allocated to different interventions. No way to distinguish variation due to worksite from effect of intervention. | |
| Previously included, recruited only recent quitters so now covered in Cochrane review of relapse prevention (Hajek 2009) | |
| The intervention included 1 session of face‐to‐face counselling with telephone follow‐up. Results, which did not show any intervention effect, are given in Bobo 1998. | |
| No long‐term outcomes yet reported. | |
| Not randomized. Compared participants accepting proactive calls to those choosing only 1 session. | |
| Systems change intervention; trained dental staff in to assess, advise and refer to telephone counselling. | |
| Quitline referral confounded with brief advice, only 3m follow‐up. | |
| The intervention included the opportunity of a motivational telephone call following provider advice and S‐H components. Follow‐up was only 5 ‐ 8 wks. | |
| Smoking status not measured. | |
| Trial of a relapse prevention intervention; participants were abstinent at time of randomization. | |
| All participants had same quitline counselling. | |
| The focus of the intervention was on genetic susceptibility feedback. Effect of telephone support cannot be evaluated independently. | |
| Compares 2 telephone‐based interventions for preventing relapse following group therapy. Now included in Cochrane relapse prevention review (Hajek 2009) | |
| Trial of NRT as opposed to telephone support; same telephone support intervention offered to both groups. | |
| All participants received telephone counselling and NRT, test of additional group counselling. | |
| Telephone support could not be evaluated independently of combined intervention. | |
| Not a controlled trial. Survey of callers to UK quitline. Conservatively assuming that non‐responders at 1 year were continuing smokers and assuming 20% of reported successes would fail biochemical validation gave an adjusted quit rate of 15. 6% (95% CI 12.7% to 18.9%). | |
| Trial in pregnant women. | |
| Telephone intervention purpose was to assess smoking status, interest in making another quit attempt, quit challenges, and treatment preferences, not to assist cessation per se. | |
| Intervention was telephone counselling for nonsmokers wanting to help a smoker. Outcome was calls by smoker to quitline, not cessation. | |
| Short follow‐up. | |
| School as unit of randomization. Telephone counselling confounded by other school‐based initiatives. | |
| Not a controlled trial. A panel sample of callers to the Scottish Smokeline was followed up for 1 year. 607 (71% of original sample) were reached. The quit rate was 23.6%, 8.2% reported not smoking for > 80% of the previous year. It was estimated that 5.9% of the adult smokers in Scotland called during the year. | |
| The amount and timing of telephone contact is unclear. The main component was a S‐H programme, compared to a waiting list control. Total of 40 participants. | |
| Intervention addressed multiple risk factors, number of smokers enrolled not specified. | |
| Telephone support could not be evaluated independently of face‐to‐face counselling. | |
| Not a controlled trial. Followed 258 nicotine patch purchasers who enrolled for support program of 4 calls from a trained nurse counsellor. 36% quit rate at 8m. | |
| Not randomized. Smokers chose intensity of support. | |
| Intervention used mobile phone (including text messaging). To be covered by separate Cochrane review (Whittaker 2012). | |
| Systems change intervention; referral to quitline was only 1 component. | |
| Small (n = 39) feasibility study in Emergency Department. Very low rate of follow‐up especially for sustained abstinence outcome (2/39 reached at both follow‐ups). | |
| Evaluated a telephone support system. All smokers recruited had access to the interactive programme. Random subsets were selected for access to messages about nicotine gum, sent a reminder to call, or sent a user's manual. | |
| Study of phone counselling for relapse prevention. | |
| Abstinence data given only for intervention group. | |
| Follow‐up 12 wks. At this point there was no evidence that the addition of a single proactive call 2 days after the TQD increased cessation rates over 6 mailings of tailored materials. | |
| Telephone component cannot be evaluated independently of face‐to‐face counselling. The intervention included brief counselling and NRT. | |
| Telephone component cannot be evaluated independently of face‐to‐face counselling. The intervention included in‐hospital nurse counselling as well as post‐discharge telephone contact, and was compared to a minimal intervention. | |
| Telephone component could not be evaluated independently of combined intervention. | |
| Telephone intervention was a 10‐min reminder call, 2m after face‐to‐face advice to quit prior to surgery. Outcomes combined with an arm given reminder at a face‐to‐face meeting. | |
| Telephone component cannot be evaluated independently of face‐to‐face counselling. The intervention included in‐hospital physician advice and counselling by a nurse as well as post‐discharge telephone contact, and was compared to usual care. | |
| Not a controlled trial. Pre‐test/post‐test study of different referral methods. | |
| All participants had same basic counselling intervention. Test of a mood management component. | |
| All participants had same counselling intervention. Test of tailored written materials, see Cochrane self‐help review (Lancaster 2005b). | |
| Not an evalution of counselling; compared 2 strategies to encourage calls to a Quitline. | |
| Telephone component cannot be evaluated independently of face‐to‐face counselling. The intervention included in‐hospital physician advice and counselling by a nurse as well as post‐discharge telephone contact, and was compared to usual care. | |
| Telephone component could not be evaluated independently of combined intervention. | |
| Not randomized; comparison of work‐based intervention programmes. | |
| Only 3m follow‐up. | |
| Only 12 wks follow‐up. | |
| All participants received telephone counselling. Test of a mood management component. | |
| Intervention used mobile phone (including text messaging). To be covered by separate Cochrane review (Whittaker 2012). | |
| Not randomized. The treated groups were recruited by different means and given different interventions both of which included telephone counselling by nurses or counsellors. | |
| Only 3m follow‐up. | |
| Trial of methods for clinic referral to quitline support. No quitting outcomes. | |
| Recruitment via quitline, but test of providing samples of NRT. | |
| Recruitment via quitline, but test of nicotine‐free cigarettes as an adjunct to NRT. | |
| Comparison of physician‐provided general help to quit smoking with intervention primarily aimed at facilitating quitline use. Both groups had same access to quitline. | |
| Telephone component cannot be evaluated independently of face‐to‐face counselling. | |
| Only 12 wks follow‐up. | |
| Uncontrolled evaluation. Quitline callers followed up at 1 year. | |
| Quitline component was part of a comprehensive intervention including face‐to‐face support. | |
| Only 12 wks follow‐up. | |
| Not randomized; uses a concurrent matched control. | |
| Not an RCT. All participants called the California Smokers' Helpline and received 1 session of counselling and planned to use NRT. Those who chose to receive further counselling were compared to those who did not. |
CI: confidence interval
m: month(s)
IVR: interactive voice response
NRT: nicotine replacement therapy
S‐H: self help
TC: telephone counselling
TQD: target quit date
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | randomized trial in California Smokers Helpline |
| Participants | 1101 pregnant smokers seeking assistance |
| Interventions | Subjects were randomized to telephone counseling or self help. Those in the self‐help group received a quit kit of written materials, including the American Cancer Society booklet for pregnant smokers. Those in the treatment group received the quit kit plus up to 7 counselling calls (1 pre‐quit, up to 6 follow‐up). |
| Outcomes | Cessation at 3rd trimester (30 day abstinence) |
| Notes | Results presented only as an abstract |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Smoking cessation: data for two years from two different interventions |
| Methods | 9 wk open‐label bupropion phase 300mg daily and NRT for 3 wks combined with behavioural support; smokers randomised in 2 groups, follow‐up for 3, 6, 12 and 24 m |
| Participants | no information |
| Interventions | Group A: 7 wkly one to one counselling sessions; Group B: telephone counselling |
| Outcomes | PPA abstinence and continuous abstinence ? |
| Starting date | |
| Contact information | |
| Notes |
| Trial name or title | Efficacy of Tobacco Quitline for Cancer Survivors |
| Methods | RCT |
| Participants | 950 childhood cancer survivors recruited nationally |
| Interventions | Counsellor‐initiated vs. participant‐initiated tobacco Quit Line with adjunctive nicotine replacement therapy (NRT) in both groups. |
| Outcomes | Cessation at 1 year |
| Starting date | October 2008 |
| Contact information | |
| Notes |
| Trial name or title | Smoking Cessation among Patients with Coronary Heart disease |
| Methods | 2 or more arms, randomised |
| Participants | ≥ 18 yrs and stable cardiac condition after admitted with coronary heart disease at cardiac wards and smoked ≥ 5 cigs/week prior to hospital admission |
| Interventions | Follow‐up measurements 6 months and 12 months after the baseline measurement. |
| Outcomes | Primary: PP abstinence (PPA) from smoking after 6 and 12 m. PPA is considered to be the most sensitive and valid measure of smoking cessation. |
| Starting date | 15 October 2009 |
| Contact information | |
| Notes |
| Trial name or title | Real e Quit (REQ) |
| Methods | randomised pretest‐posttest trial with 12‐ and 26‐week follow‐up |
| Participants | Young adult smokers (< 30; n = 3310) |
| Interventions | Online cessation programme with tailored counselling, e‐cards for social support, quitting testimonials, and a cessation blog compared against a self‐help booklet and telephone quit line |
| Outcomes | Abstinence at 12 and 26 weeks |
| Starting date | |
| Contact information | |
| Notes | Quitline was control condition so may not be eligible for inclusion |
| Trial name or title | Smoking Cessation in Hospitalized Smokers |
| Methods | randomised; open label; NRT at discharge and proactive telephone counselling post hospital discharge, 12‐m cessation rates of hospitalised smokers (2 x 2 factorial design) |
| Participants | hospitalised ≥ 24hrs, ≥ 18 yrs; ≥ 6 CPD |
| Interventions | Genders Eligible for Study: |
| Outcomes | Accepts Healthy Volunteers: |
| Starting date | August 2011 |
| Contact information | |
| Notes |
| Trial name or title | Tobacco Tactics Website for Operating Engineers (BCBSM‐OE) |
| Methods | clustered randomised control, open label |
| Participants | Operating Engineers Local 324 Education Center routine training attendants; ≥ 18 yrs; smoking in the past month, and interested in participating in quit programme |
| Interventions | intervention: Tobacco Tactics website; control: state supported 1‐800 quit‐now telephone hotline |
| Outcomes | Primary: quit rate at 30 d and 6 m follow‐up; 7d PP abstinence; also assess ability to quit at all, no. quit attempts, CPD |
| Starting date | January 2010 |
| Contact information | |
| Notes |
| Trial name or title | Reaching and Treating Lesbian, Gay, Bisexual, and Transgender (LGBT) Cigarette Smokers |
| Methods | randomised, open label, factorial (4 arm) |
| Participants | ≥ 18 yrs; identify as LGBT |
| Interventions | 1) self help manual, 2) mail‐based self‐help + internet‐based smoking treatment, 3) self‐help manual + telephone counselling, 4) self‐help manual + internet‐based Intervention + telephone counselling |
| Outcomes | Primary: smoking status at 3, 6 and 12 m post enrolment |
| Starting date | February 2008 |
| Contact information | |
| Notes |
| Trial name or title | Evaluating a Telemedicine Smoking Cessation Program in Rural Primary Care Practices |
| Methods | randomised, open label, |
| Participants | ≥ 18 yrs; smoking for ≥ 1 yr |
| Interventions | 1) Telemedicine smoking cessation programme vs 2) Telephone quitline smoking cessation programme |
| Outcomes | Primary: 7d PP abstinence at 3, 6, and 12 m; secondary: prolonged abstinence at 3, 6, and 12 m |
| Starting date | June 2009 |
| Contact information | |
| Notes |
| Trial name or title | Randomised trial of an automated telephone follow‐up system for smoking cessation in smokers with CHD |
| Methods | RCT |
| Participants | hospitalised with CHD at University of Ottawa Heart Institute; ≥ 5 CPD |
| Interventions | 1) Standard care (in hospital nurse counselling and NRT) 2) IVR (interactive voice‐response) group: standard care + IVR |
| Outcomes | Primary: self reported continuous abstinence at 26 and 52 wks post hospital discharge |
| Starting date | |
| Contact information | |
| Notes | Conference abstract report |
| Trial name or title | Evaluating a Telephone‐Based Smoking Cessation Program Among People in the Military (The AFIII Study) |
| Methods | Randomized controlled trial |
| Participants | Adult smokers who are Armed Forces Active Duty personnel, retirees, Reservist, National Guard and family member healthcare beneficiaries |
| Interventions | Proactive versus reactive smoking quit line with adjunctive nicotine replacement therapy (NRT) in both groups. |
| Outcomes | Cessation at 1 year |
| Starting date | April 2008 |
| Contact information | |
| Notes |
| Trial name or title | Telephone Care Coordination for Smokers in VA Mental Health Clinics (TeleQuit MH) |
| Methods | non‐randomised, open label |
| Participants | Smokers who are referred from Mental Health Clinics in VA VISNs 1 and 3 |
| Interventions | Telephone Care Coordination and Telephone Care Coordination with state Quitline |
| Outcomes | Pimary: long‐term smoking abstinence (30‐day PP abstinence) and the rate of program adoption by mental health providers and patients ; 6 months post enrolment; secondary: 30‐day PP abstinence at 2 m (EOT), quit attempt rate (2 and 6 m post enrolment), rate of use of cessation medications (2 and 6 m post enrolment), quarterly VA site‐level performance rates on the VA tobacco performance measures |
| Starting date | November 2009 |
| Contact information | |
| Notes |
| Trial name or title | Evaluation of a Smoking Cessation Intervention for Parents |
| Methods | Randomized controlled trial |
| Participants | Smoking parents, enrolled through children's primary schools |
| Interventions | Proactive telephone counselling (up to seven counsellor‐initiated telephone calls based on cognitive‐behavioural skill building and Motivational Interviewing, distributed over a period of three months) or a control condition |
| Outcomes | Sustained abstinence, 7‐day point prevalence abstinence and 24‐hours point prevalence abstinence at 3 & 12 months |
| Starting date | |
| Contact information | |
| Notes |
| Trial name or title | TeleQuit Smoking Cessation Program |
| Methods | TeleQuit trial, VHA study. randomised by week to either proactive or reactive approach, and to multisession or S‐H |
| Participants | Veterans Administration smoking patients |
| Interventions | Care providers gave brief counselling and provided electronic referral to TeleQuit |
| Outcomes | Cessation at 6 months |
| Starting date | |
| Contact information | |
| Notes | Conference abstract reports preliminary results from the 12 Los Angeles sites, 60 sites in total. |
| Trial name or title | Quit in General Practice: a cluster randomised trial of enhanced in‐practice support for smoking cessation |
| Methods | Cluster randomised trial |
| Participants | Daily smokers presenting to general practitioners |
| Interventions | 1) Quit with Practice Nurse 2) Quitline referral 3) GP usual care |
| Outcomes | Cessation at 3 and 12 months |
| Starting date | |
| Contact information | |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up Show forest plot | 12 | 30182 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.28, 1.49] |
| Analysis 1.1 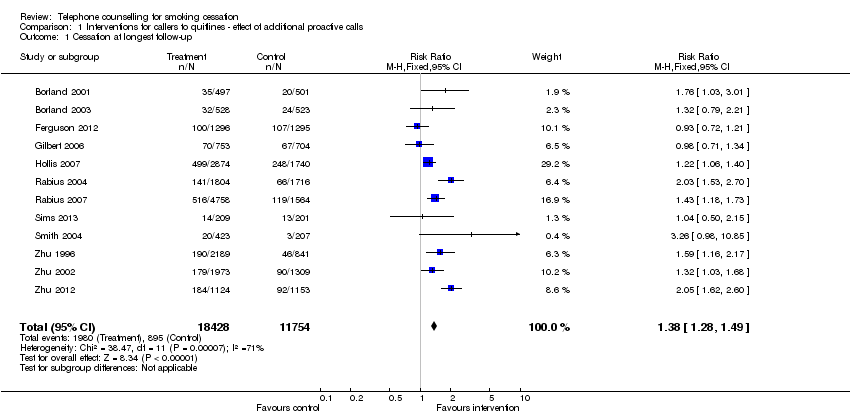 Comparison 1 Interventions for callers to quitlines ‐ effect of additional proactive calls, Outcome 1 Cessation at longest follow‐up. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Interventions for callers to quitlines ‐ comparison of different support during a single call, Outcome 1 Cessation at longest follow‐up. | ||||
| 1.1 Reactive counselling vs self‐help materials | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Stage‐based counselling versus general information | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Tailored counselling versus standard counselling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Long term cessation Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1  Comparison 3 Offer of counselling via quitlines/helplines/hotlines, Outcome 1 Long term cessation. | ||||
| 1.1 Hotline and self‐help materials compared to self help only | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Hotline and self‐help materials for cessation maintenance compared to nothing | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Reactive or proactive counselling vs provider counselling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up ‐ All trials, subgroups by amount of control group support Show forest plot | 51 | 30246 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.20, 1.36] |
| Analysis 4.1 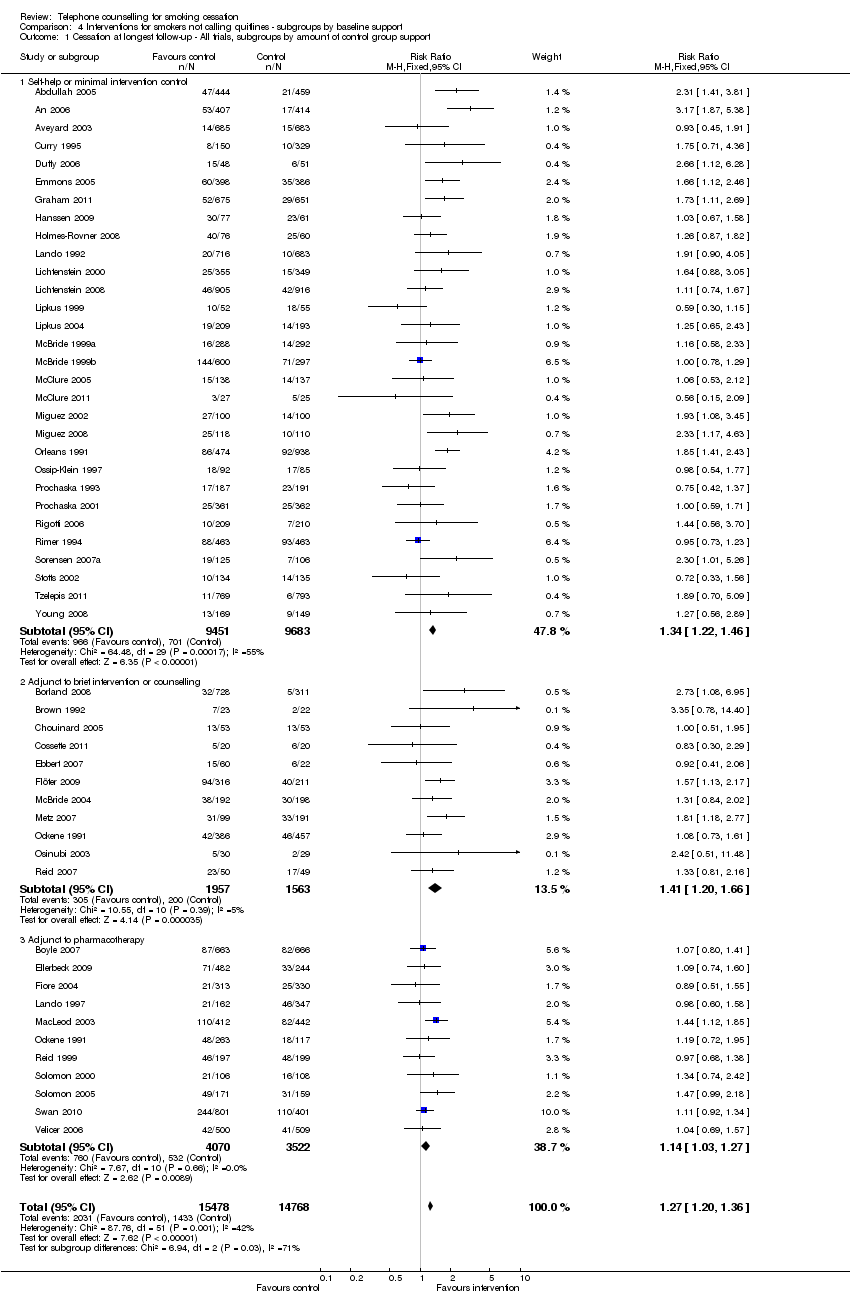 Comparison 4 Interventions for smokers not calling quitlines ‐ subgroups by baseline support, Outcome 1 Cessation at longest follow‐up ‐ All trials, subgroups by amount of control group support. | ||||
| 1.1 Self‐help or minimal intervention control | 30 | 19134 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.22, 1.46] |
| 1.2 Adjunct to brief intervention or counselling | 11 | 3520 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.20, 1.66] |
| 1.3 Adjunct to pharmacotherapy | 11 | 7592 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.03, 1.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up Show forest plot | 51 | 30490 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.20, 1.36] |
| Analysis 5.1  Comparison 5 Interventions for smokers not calling quitlines ‐ subgroups by intensity: 1‐2, 3‐6, >6 calls, Outcome 1 Cessation at longest follow‐up. | ||||
| 1.1 Two sessions or fewer | 9 | 6274 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.91, 1.26] |
| 1.2 3‐6 sessions | 34 | 19736 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.23, 1.42] |
| 1.3 7 sessions or more | 9 | 4480 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.11, 1.50] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Long‐term cessation Show forest plot | 51 | 30246 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.20, 1.36] |
| Analysis 6.1  Comparison 6 Interventions for smokers not calling quitlines ‐ subgroups by motivation, Outcome 1 Long‐term cessation. | ||||
| 1.1 Selected for motivation/ interest in quitting | 16 | 10612 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.19, 1.42] |
| 1.2 Not selected by motivation | 35 | 19634 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.16, 1.38] |

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
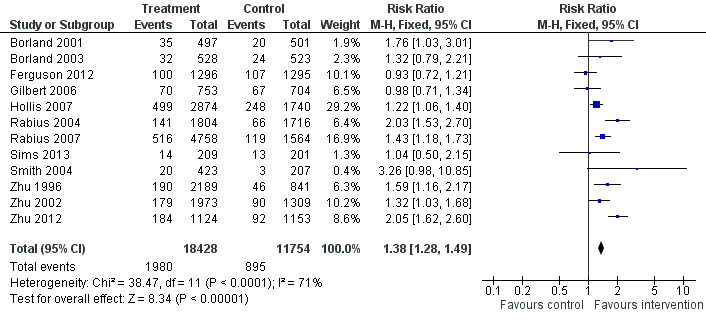
Comparison 3. Interventions for callers to quitlines. Cessation at longest follow‐up
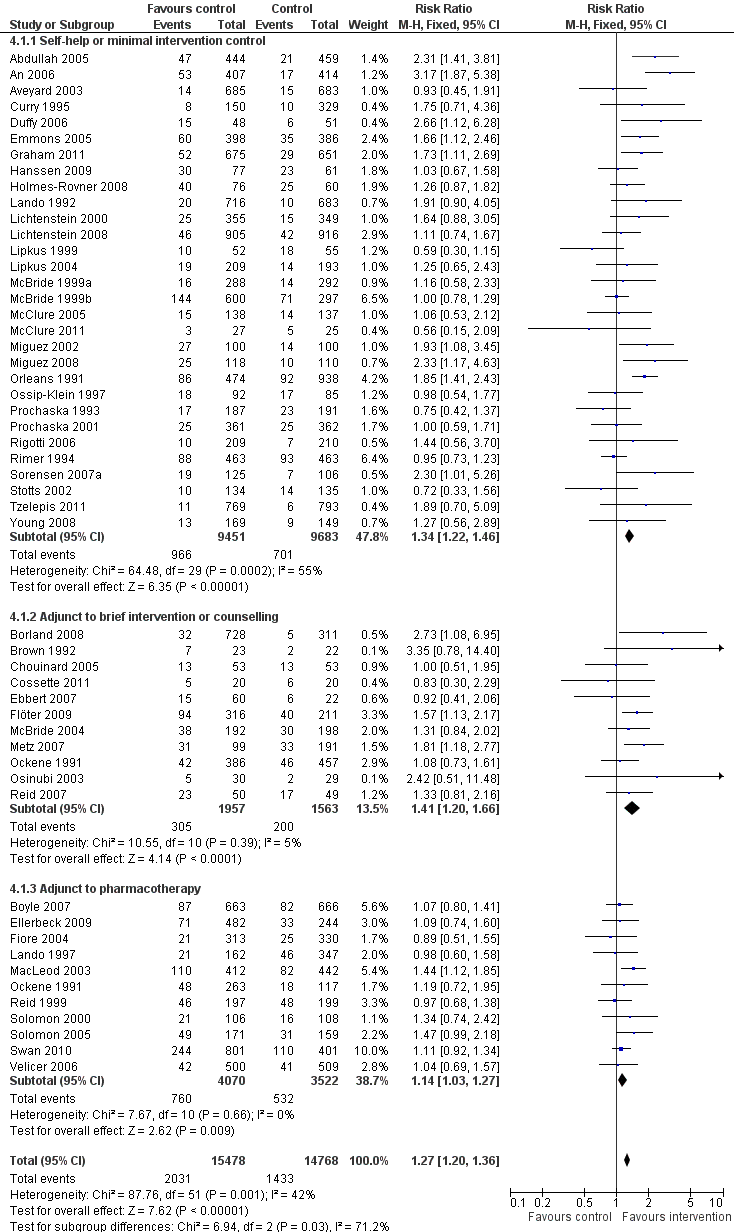
Comparison 4. Interventions for smokers not calling quitlines ‐ subgroups by baseline support. Cessation at longest follow‐up

Comparison 1 Interventions for callers to quitlines ‐ effect of additional proactive calls, Outcome 1 Cessation at longest follow‐up.
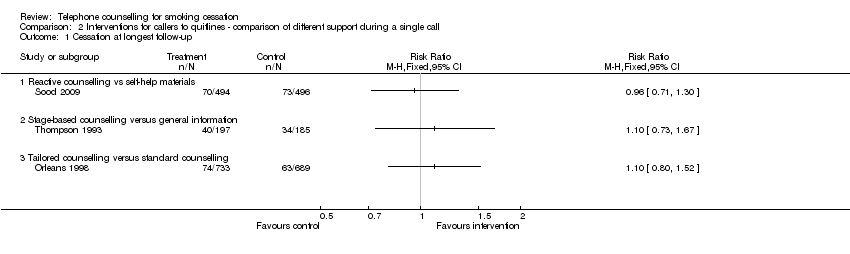
Comparison 2 Interventions for callers to quitlines ‐ comparison of different support during a single call, Outcome 1 Cessation at longest follow‐up.
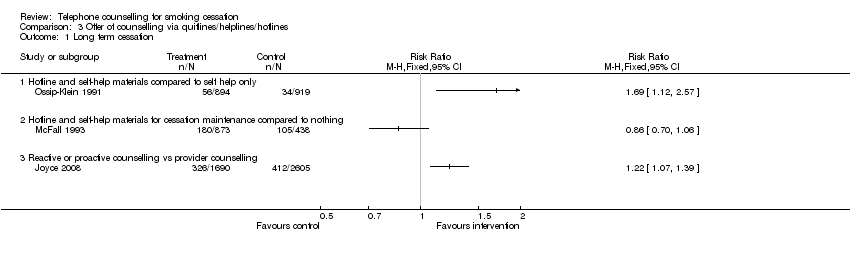
Comparison 3 Offer of counselling via quitlines/helplines/hotlines, Outcome 1 Long term cessation.
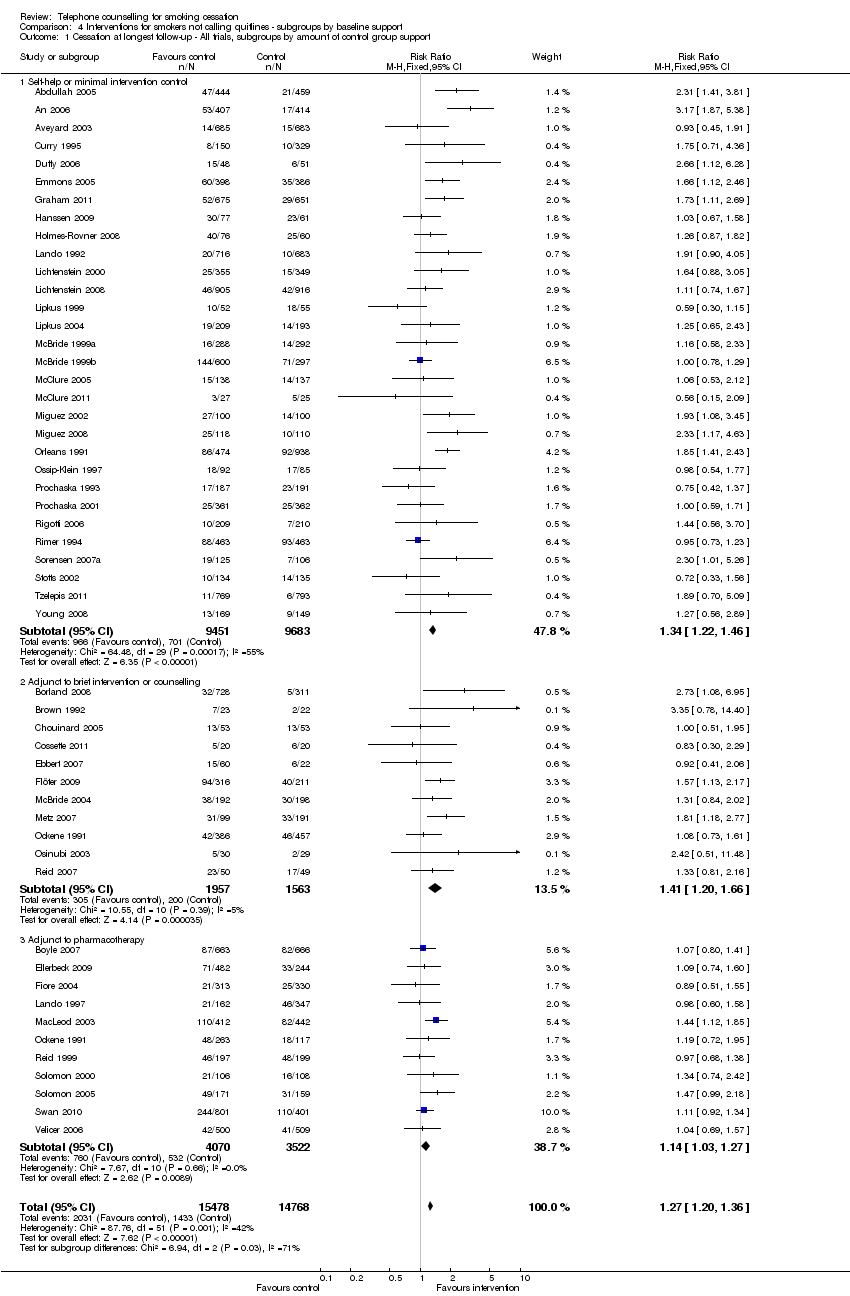
Comparison 4 Interventions for smokers not calling quitlines ‐ subgroups by baseline support, Outcome 1 Cessation at longest follow‐up ‐ All trials, subgroups by amount of control group support.
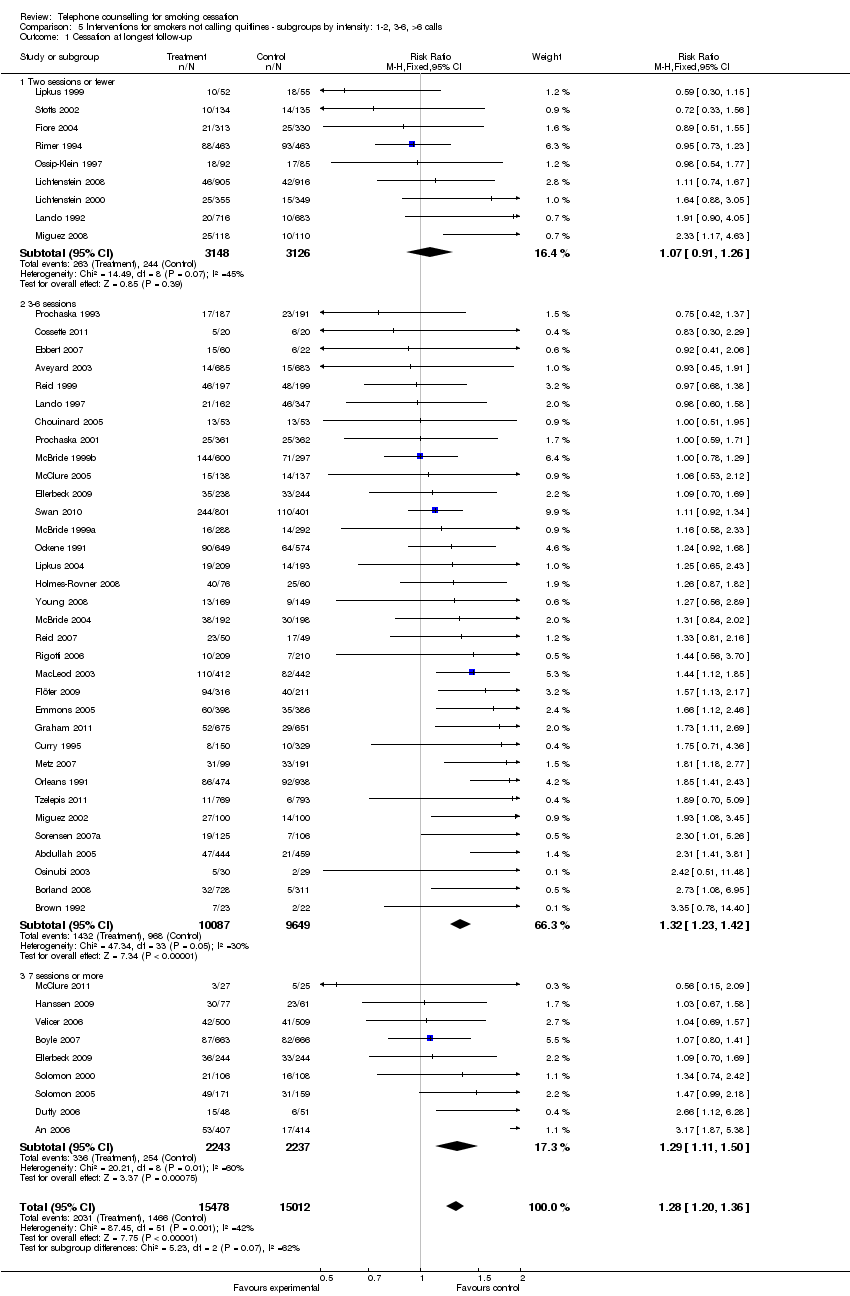
Comparison 5 Interventions for smokers not calling quitlines ‐ subgroups by intensity: 1‐2, 3‐6, >6 calls, Outcome 1 Cessation at longest follow‐up.
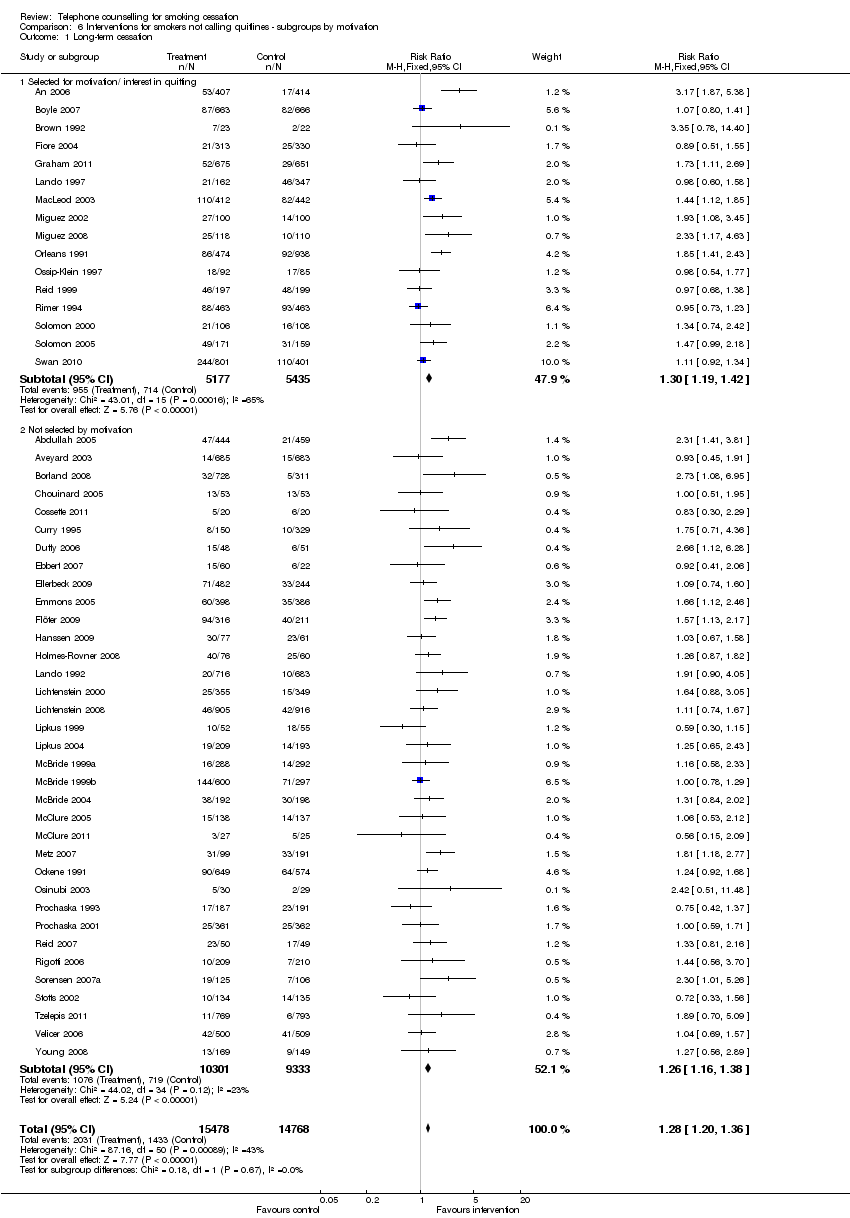
Comparison 6 Interventions for smokers not calling quitlines ‐ subgroups by motivation, Outcome 1 Long‐term cessation.
| Interventions for callers to quitlines ‐ effect of additional proactive calls for smoking cessation | ||||||
| Patient or population: callers to quitlines | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Additional proactive calls | |||||
| Smoking cessation | Study population | RR 1.38 | 30182 | ⊕⊕⊕⊝ | ||
| 76 per 10001 | 105 per 1000 | |||||
| Low | ||||||
| 50 per 10001 | 69 per 1000 | |||||
| High | ||||||
| 150 per 10001 | 207 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Low control rate reflects lower end of range evident in trials; 4/12 had control rates < 5%. High control rate likely to be applicable for people also using pharmacotherapy | ||||||
| Proactive telephone counselling for smokers not calling quitlines | ||||||
| Patient or population: smokers not calling quitlines | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Proactive telephone counselling | |||||
| Cessation at longest follow‐up ‐ All trials, subgroups by amount of control group support | 97 per 10001 | 123 per 1000 | RR 1.27 | 30246 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Based on crude average of events/total, with participants lost to follow‐up assumed to be smoking. Interquartile range in trials 6‐20%. Higher baseline cessation rates typical amongst motivated populations receiving pharmacotherapy and some support Relative addtional benefit of telephone intervention may be smaller in this setting. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up Show forest plot | 12 | 30182 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.28, 1.49] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Reactive counselling vs self‐help materials | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Stage‐based counselling versus general information | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Tailored counselling versus standard counselling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Long term cessation Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Hotline and self‐help materials compared to self help only | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Hotline and self‐help materials for cessation maintenance compared to nothing | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Reactive or proactive counselling vs provider counselling | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up ‐ All trials, subgroups by amount of control group support Show forest plot | 51 | 30246 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.20, 1.36] |
| 1.1 Self‐help or minimal intervention control | 30 | 19134 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.22, 1.46] |
| 1.2 Adjunct to brief intervention or counselling | 11 | 3520 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.20, 1.66] |
| 1.3 Adjunct to pharmacotherapy | 11 | 7592 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.03, 1.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation at longest follow‐up Show forest plot | 51 | 30490 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.20, 1.36] |
| 1.1 Two sessions or fewer | 9 | 6274 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.91, 1.26] |
| 1.2 3‐6 sessions | 34 | 19736 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.23, 1.42] |
| 1.3 7 sessions or more | 9 | 4480 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.11, 1.50] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Long‐term cessation Show forest plot | 51 | 30246 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.20, 1.36] |
| 1.1 Selected for motivation/ interest in quitting | 16 | 10612 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.19, 1.42] |
| 1.2 Not selected by motivation | 35 | 19634 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.16, 1.38] |

