Trening na bieżni z odciążeniem masy ciała a zdolność chodzenia po udarze mózgu
Abstract
Background
Treadmill training, with or without body weight support using a harness, is used in rehabilitation and might help to improve walking after stroke. This is an update of the Cochrane review first published in 2003 and updated in 2005 and 2014.
Objectives
To determine if treadmill training and body weight support, individually or in combination, improve walking ability, quality of life, activities of daily living, dependency or death, and institutionalisation or death, compared with other physiotherapy gait‐training interventions after stroke. The secondary objective was to determine the safety and acceptability of this method of gait training.
Search methods
We searched the Cochrane Stroke Group Trials Register (last searched 14 February 2017), the Cochrane Central Register of Controlled Trials (CENTRAL) and the Database of Reviews of Effects (DARE) (the Cochrane Library 2017, Issue 2), MEDLINE (1966 to 14 February 2017), Embase (1980 to 14 February 2017), CINAHL (1982 to 14 February 2017), AMED (1985 to 14 February 2017) and SPORTDiscus (1949 to 14 February 2017). We also handsearched relevant conference proceedings and ongoing trials and research registers, screened reference lists, and contacted trialists to identify further trials.
Selection criteria
Randomised or quasi‐randomised controlled and cross‐over trials of treadmill training and body weight support, individually or in combination, for the treatment of walking after stroke.
Data collection and analysis
Two review authors independently selected trials, extracted data, and assessed risk of bias and methodological quality. The primary outcomes investigated were walking speed, endurance, and dependency.
Main results
We included 56 trials with 3105 participants in this updated review. The average age of the participants was 60 years, and the studies were carried out in both inpatient and outpatient settings. All participants had at least some walking difficulties and many could not walk without assistance. Overall, the use of treadmill training did not increase the chances of walking independently compared with other physiotherapy interventions (risk difference (RD) ‐0.00, 95% confidence interval (CI) ‐0.02 to 0.02; 18 trials, 1210 participants; P = 0.94; I² = 0%; low‐quality evidence). Overall, the use of treadmill training in walking rehabilitation for people after stroke increased the walking velocity and walking endurance significantly. The pooled mean difference (MD) (random‐effects model) for walking velocity was 0.06 m/s (95% CI 0.03 to 0.09; 47 trials, 2323 participants; P < 0.0001; I² = 44%; moderate‐quality evidence) and the pooled MD for walking endurance was 14.19 metres (95% CI 2.92 to 25.46; 28 trials, 1680 participants; P = 0.01; I² = 27%; moderate‐quality evidence). Overall, the use of treadmill training with body weight support in walking rehabilitation for people after stroke did not increase the walking velocity and walking endurance at the end of scheduled follow‐up. The pooled MD (random‐effects model) for walking velocity was 0.03 m/s (95% CI ‐0.05 to 0.10; 12 trials, 954 participants; P = 0.50; I² = 55%; low‐quality evidence) and the pooled MD for walking endurance was 21.64 metres (95% CI ‐4.70 to 47.98; 10 trials, 882 participants; P = 0.11; I² = 47%; low‐quality evidence). In 38 studies with a total of 1571 participants who were independent in walking at study onset, the use of treadmill training increased the walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.08 m/s (95% CI 0.05 to 0.12; P < 0.00001; I2 = 49%). There were insufficient data to comment on any effects on quality of life or activities of daily living. Adverse events and dropouts did not occur more frequently in people receiving treadmill training and these were not judged to be clinically serious events.
Authors' conclusions
Overall, people after stroke who receive treadmill training, with or without body weight support, are not more likely to improve their ability to walk independently compared with people after stroke not receiving treadmill training, but walking speed and walking endurance may improve slightly in the short term. Specifically, people with stroke who are able to walk (but not people who are dependent in walking at start of treatment) appear to benefit most from this type of intervention with regard to walking speed and walking endurance. This review did not find, however, that improvements in walking speed and endurance may have persisting beneficial effects. Further research should specifically investigate the effects of different frequencies, durations, or intensities (in terms of speed increments and inclination) of treadmill training, as well as the use of handrails, in ambulatory participants, but not in dependent walkers.
PICO
Streszczenie prostym językiem
Trening na bieżni z odciążeniem masy ciała a zdolność chodzenia po udarze mózgu
Pytanie badawcze: Chcieliśmy ocenić, czy trening chodzenia na bieżni z odciążeniem masy ciała za pomocą uprzęży – jako jedyna forma treningu lub w połączeniu z innymi jego rodzajami – może poprawić chodzenie w porównaniu z innymi metodami treningu chodzenia lub z niestosowaniem leczenia. Jest to aktualizacja przeglądu opublikowanego po raz pierwszy w 2003 roku, zaktualizowanego później w latach 2005 oraz 2014.
Wprowadzenie: Około 60% osób po przebytym udarze mózgu ma trudności z chodzeniem, a poprawa tej umiejętności jest jednym z głównych celów rehabilitacji. Trening na bieżni z odciążeniem masy ciała (ang. body weight support treadmill training, BWSTT) lub bez takiego odciążenia, wykorzystuje specjalistyczny sprzęt do wspomagania treningu chodzenia.
Charakterystyka badania: Do marca 2017 r. zidentyfikowaliśmy 56 istotnych badań klinicznych z udziałem 3105 uczestników. W 26 badaniach (1410 uczestników) BWSTT porównano z innym leczeniem fizjoterapeutycznym; w 20 badaniach (889 uczestników) trening na bieżni bez odciążenia masy ciała porównano z innym leczeniem fizjoterapeutycznym, niestosowaniem leczenia lub z leczeniem pozorowanym; w 2 badaniach (100 uczestników) BWSTT porównano z treningiem na bieżni bez odciążenia masy ciała; a w 4 badaniach (147 uczestników) nie określono, czy stosowano w nich odciążenie. Średni wiek uczestników wynosił 60 lat, a badania przeprowadzono zarówno w warunkach szpitalnych, jak i ambulatoryjnych.
Kluczowe wnioski: Wyniki tego przeglądu były częściowo niejednoznaczne. U osób po przebytym udarze mózgu trenujących na bieżni z odciążeniem masy ciała lub bez takiego odciążenia, nie stwierdzono większego prawdopodobieństwa poprawy zdolności do samodzielnego chodzenia. Jakość tych danych naukowych była jednak niska. Jednak trening na bieżni z odciążeniem masy ciała lub bez takiego odciążenia może poprawić szybkość chodzenia i wydolność chodu w porównaniu z niewykonywaniem treningu na bieżni. Jakość danych naukowych została oceniona jako umiarkowana. Mówiąc dokładniej, osoby po przebytym udarze, które są w stanie chodzić na początku terapii, wydają się odnosić największe korzyści z tego typu interwencji, ale osoby, które nie są w stanie chodzić samodzielnie na początku terapii, nie odnoszą korzyści. Przegląd ten wykazał, że poprawa szybkości chodzenia i wytrzymałości u osób, które mogą chodzić, nie ma trwałego pozytywnego wpływu. Niepożądane zdarzenia, takie jak upadki i przerwanie treningu, nie występowały częściej u osób korzystających z bieżni.
Dalsza analiza wykazała, że trening na bieżni w pierwszych 3 miesiącach po udarze przynosi jedynie niewielką poprawę szybkości i wytrzymałości chodu. W przypadku osób leczonych na późniejszym etapie (dłużej niż 6 miesięcy po przebytym udarze) efekty były mniejsze. Częstsze treningi na bieżni (na przykład 5 razy w tygodniu) wydają się mieć większy wpływ na szybkość chodzenia i wytrzymałość; nie było to jednak rozstrzygające. Krótkie okresy treningu na bieżni (trwające 4 tygodnie) zapewniły umiarkowaną poprawę prędkości chodzenia, ale nie na tyle, aby mieć znaczenie kliniczne.
W niniejszym przeglądzie nie badano wpływu wieku uczestników, ani rodzaju udaru mózgu.
W praktyce wydaje się, że osoby, które mogą chodzić po udarze, ale nie te, które nie mogą, mogą skorzystać z treningu na bieżni (z odciążeniem masy ciała lub bez niego), aby poprawić swoje zdolności chodzenia. Dalsze badania powinny w szczególności zbadać wpływ różnych częstotliwości, czasu trwania lub intensywności (pod względem przyrostów prędkości i nachylenia) treningu na bieżni, a także korzystania z poręczy. Przyszłe badania kliniczne powinny obejmować osoby, które mogą już chodzić, ale nie osoby niesamodzielne, które nie są w stanie chodzić samodzielnie. Przyszłe badania powinny analizować grupy wiekowe, płeć i rodzaj udaru mózgu, aby sprawdzić, kto może odnieść największe korzyści z tego leczenia.
Jakość danych naukowych
Jakość danych naukowych dotyczących treningu chodzenia na bieżni po udarze mózgu była niska do umiarkowanej. Była ona umiarkowana w odniesieniu do szybkości i wytrzymałości chodu pod koniec leczenia i niska w odniesieniu do poprawy zdolności do samodzielnego chodzenia.
Authors' conclusions
Summary of findings
| Treadmill (with or without body weight support) versus other intervention for walking after stroke | ||||||
| Patient or population: adults who had suffered a stroke and exhibited an abnormal gait pattern | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Treadmill (with or without body weight support) versus other intervention | |||||
| Dropouts ‐ by end of treatment | Study population | See comment | 3105 | ⊕⊕⊝⊝ | Risks were calculated from pooled risk differences | |
| 91 per 1000 | 93 per 1000 | |||||
| Moderate | ||||||
| 31 per 1000 | 32 per 1000 | |||||
| Walking speed (m/s) at end of treatment | The mean walking speed (m/s) at end of treatment in the control groups was | The mean walking speed (m/s) at end of treatment in the intervention groups was | 2323 | ⊕⊕⊕⊝ | ||
| Walking speed (m/s) at end of treatment ‐ dependent in walking at start of treatment | The mean walking speed (m/s) at end of treatment ‐ dependent in walking at start of treatment in the control groups was | The mean walking speed (m/s) at end of treatment ‐ dependent in walking at start of treatment in the intervention groups was | 752 | ⊕⊕⊝⊝ | ||
| Walking speed (m/s) at end of treatment ‐ independent in walking at start of treatment | The mean walking speed (m/s) at end of treatment ‐ independent in walking at start of treatment in the control groups was | The mean walking speed (m/s) at end of treatment ‐ independent in walking at start of treatment in the intervention groups was | 1571 | ⊕⊕⊝⊝ | ||
| Walking endurance (m) at end of treatment | The mean walking endurance (m) at end of treatment in the control groups was | The mean walking endurance (m) at end of treatment in the intervention groups was | 1680 | ⊕⊕⊕⊝ | ||
| Walking endurance (m) at end of treatment ‐ dependent in walking at start of treatment | The mean walking endurance (m) at end of treatment ‐ dependent in walking at start of treatment in the control groups was | The mean walking endurance (m) at end of treatment ‐ dependent in walking at start of treatment in the intervention groups was | 639 | ⊕⊕⊝⊝ | ||
| Walking endurance (m) at end of treatment ‐ independent in walking at start of treatment | The mean walking endurance (m) at end of treatment ‐ independent in walking at start of treatment in the control groups was | The mean walking endurance (m) at end of treatment ‐ independent in walking at start of treatment in the intervention groups was | 1041 | ⊕⊕⊝⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded because 95% CI contains effect size of no difference and the minimal important difference. | ||||||
Background
Description of the condition
Stroke ranks as the sixth highest cause of burden of disease worldwide in terms of disability adjusted life years and is the single most important cause of severe disability in people living in their own homes (Murray 2012). An inability or an impaired ability to walk is a significant contributor to long‐term disability and burden of care after stroke. Approximately one‐third of people surviving acute stroke are unable to walk three months after admission to a general hospital (Langhorne 2009).
High‐quality evidence from systematic reviews indicates that organised (stroke unit) care decreases physical dependence after stroke compared with general medical care (SUTC 2013). This organised care is characterised by early mobilisation and multidisciplinary rehabilitation (including physiotherapy) co‐ordinated by regular team meetings (Langhorne 2002). The effectiveness of specific physiotherapy gait‐training strategies, however, is still not very clear. A review of studies comparing different physiotherapy treatments for participants with stroke concluded that "There is insufficient evidence to conclude that any one physiotherapy approach is more effective in promoting recovery of lower limb function or postural control following stroke than any other approach." (Pollock 2014).
Description of the intervention
Walking on a treadmill, with or without body weight supported via a harness connected to an overhead support system, is a method of treating walking impairments post‐stroke that is becoming increasingly popular. Use of a treadmill permits a greater number of steps to be performed within a training session; that is, it increases the amount of task‐specific practice completed. For example, Hesse 2003 reported that people after stroke can perform up to 1000 steps in a 20‐minute treadmill training session, compared with only 50 to 100 steps during a 20‐minute session of conventional physiotherapy (neurophysiological approach). The speed of the treadmill, the amount of body weight support, and the amount of assistance provided by the physiotherapist can all be adjusted in order to provide a sufficient training intensity. This intervention emerged from research involving spinalised cats (Barbeau 1987) and was first used in clinical settings in the 1980s (Finch 1985). Since then, treadmill training with partial body weight support has been increasingly promoted as a treatment to drive recovery after stroke (Charalambous 2013; Langhorne 2009).
Treadmill training with body weight support is costly in terms of equipment and human resources. In addition, the equipment is not portable, so stroke participants must attend a suitably equipped healthcare facility in order to access this treatment. Several published randomised controlled trials (RCTs) have evaluated treadmill training with or without body weight support (Charalambous 2013; Polese 2013).
How the intervention might work
Improving walking after stroke is one of the main goals of rehabilitation. There is increasing evidence that high‐intensity, repetitive, task‐specific training might result in better gait rehabilitation (French 2016; Langhorne 2009). One example of potentially intensive, repetitive, task‐specific gait‐training is treadmill training. Treadmill training can be used to give patients intensive practice (in terms of high repetitions) of complex gait cycles and is being used as a method for increasing walking speed and walking distance in people who had a stroke. The advantage of treadmill training, compared with walking training overground, may be that higher walking speeds and a higher number of gait cycles can be achieved. Treadmill training, therefore, might be effective at improving walking parameters such as gait speed and walking distance after stroke (Polese 2013).
Why it is important to do this review
Several non‐Cochrane systematic reviews evaluating treadmill training, with and without body weight support, have been published since this Cochrane review first appeared in the Cochrane Library 2003, Issue 3 (e.g. Manning 2003; Teasell 2003; Van Peppen 2004) and more recently updates during 2013 (Charalambous 2013; Polese 2013). However, all of these reviews are now out of date or had some methodological weaknesses (e.g. they did not used a comprehensive search strategy for all relevant databases or were prone to language bias because non‐English studies were not included).
Updating this Cochrane review is required in order to justify the large equipment and human resource cost required to implement treadmill training, as well as to confirm the safety and acceptance of this method of training. The first update of this review was published in 2005 and included 15 trials with 622 participants; the second update was published in 2014 and included already 44 trials with 2658 participants. This is the third update of this Cochrane review. The search for trials was extended from June 2013 to March 2017. The aim of this review is to provide an update of the best available evidence about the above‐mentioned approach.
Objectives
To determine if treadmill training and body weight support, individually or in combination, improve walking ability, quality of life, activities of daily living, dependency or death, and institutionalisation or death, compared with other physiotherapy gait‐training interventions after stroke. The secondary objective was to determine the safety and acceptability of this method of gait‐training.
Methods
Criteria for considering studies for this review
Types of studies
We included truly randomised and quasi‐randomised controlled trials (including cross‐over trials) in the review. We considered procedures such as coin tossing and dice rolling as random. Quasi‐random allocation procedures included allocation by hospital record number or birth date, or alternation. We only included the first arm of the data from cross‐over trials. We assessed concealment, blinding, and the number of withdrawals for all trials, but we did not use these data as inclusion or exclusion criteria.
Treadmill training and body weight support, individually or in combination, must have been implemented in one of the experimental conditions. We were looking for trials that made one of the following comparisons:
-
treadmill training with body weight support versus other physiotherapy, placebo, or no intervention;
-
treadmill training without body weight support versus other physiotherapy, placebo, or no intervention;
-
treadmill training with body weight support versus treadmill training without body weight support; and
-
body weight support (without treadmill training) versus other physiotherapy, placebo, or no intervention.
Treadmill training and body weight support, individually or in combination, may have been implemented with physiotherapy co‐intervention(s). Where co‐intervention(s) were comparable for experimental and control groups, we grouped the trials according to the first four comparisons. In some cases, however, the co‐intervention(s) used were not the same for the treatment and control groups. For example, treadmill training with body weight support may be implemented as one component of a task‐oriented physiotherapy program and compared with non task‐oriented physiotherapy (Richards 1993). Task‐oriented physiotherapy programs involve task and context‐specific training of motor skills based on a movement science or motor relearning framework (Carr 1998). Non‐task‐oriented physiotherapy includes neurophysiological approaches to treatment, such as Bobath (Bobath 1990), Brunnstrom (Brunnstrom 1970), Rood (Goff 1969) and proprioceptive neuromuscular facilitation (Knott 1968). While these trials cannot differentiate the effects of treadmill training and body weight support from other co‐interventions, they do evaluate the intervention as part of a treatment package. We identified such trials and described them separately.
We included trials that evaluated any intensity and duration of treadmill training and body weight support that exceeded a single treatment session. Where necessary, we obtained details of the treatment and control interventions via correspondence with the trialists.
Types of participants
We included trials of adults who had suffered a stroke and exhibited an abnormal gait pattern. We used the World Health Organization's (WHO) definition of stroke: "rapidly developing clinical signs of focal (at times global) disturbance of cerebral function, lasting more than 24 hours or leading to death, with no apparent cause other than that of vascular origin." (Hatano 1976). We defined an abnormal gait pattern as walking with a slow speed, exhibiting kinematic deviations during gait (Moore 1993; Moseley 1993), or an inability to walk.
We envisaged that some trials may have included participants with other types of upper motor neurone lesions (e.g. traumatic brain injury, multiple sclerosis). However, we did not identify any mixed trials. If we identify trials using mixed types of participants in future updates of this review, we will attempt to obtain data for the stroke subgroup only via correspondence with the trialists.
Types of interventions
The primary question was whether treadmill training and body weight support, individually or in combination, could improve walking compared with other gait‐training methods, placebo or no treatment. We therefore included any trial that attempted to evaluate such a comparison. Treadmill training involves walking on a standard treadmill; assistance, feedback or guidance may be provided by a health professional (usually a physiotherapist). Some of the participant's body weight may be supported during this training using a harness attached to an overhead support system. Alternatively, this type of body weight support can be used without a treadmill.
Types of outcome measures
Primary outcomes
The primary analyses focused on the ability to walk, both at the end of the treatment period (that is, immediate or short‐term effects) and at the end of the scheduled follow‐up (that is, long‐term effects). We examined the ability to walk using dichotomous and continuous variables.
The dichotomous variable was 'dependence on personal assistance', where we defined 'dependence' as the inability to walk indoors (with or without a gait aid) without personal assistance or supervision. If reported, we used data from functional scales (or parts of functional scales relating to walking) to define the level of dependence. Suitable scales (with criterion for 'dependence') are:
-
Motor Assessment Scale (Carr 1985), a score of two or less;
-
Functional Independence Measure (Hamilton 1994), a score of five or less for the walking item;
-
Barthel Index (Collin 1988), a score of three (independent, but may use any aid) or less for the ambulation item;
-
Rivermead Mobility Index (Collen 1991), an answer of 'no' to the 'walking inside, with an aid if necessary' item; and
-
Functional Ambulation Category (Holden 1984), a score of two or less.
We used walking dependence at the start of treatment to group trials in each comparison in the analyses.
The continuous variables were:
-
independent walking speed measured over a short distance (e.g. six to 10 metres); and
-
independent walking endurance measured over a long distance (e.g. Six‐Minute Walk Test) expressed as a total distance walked.
These tests could be performed with or without a gait aid, but must have been completed without personal assistance. Wade 1992 reported that independent walking speed over a short distance is a simple, reliable, valid, and sensitive measure of walking performance. Walking over a long distance is a valid (Wade 1992) and reliable (Guyatt 1984) measure of walking endurance with established reference equations (Enright 1998). Where participants could not walk unless assisted, we allocated a speed and distance score of zero.
Secondary outcomes
Secondary outcome measures included participant quality of life, ability to perform activities of daily living, and the combined outcomes of death or dependency, and death or institutional care. Quality of life scales included the Frenchay Activities Index, Medical Outcomes Study Short Form Health Survey Questionnaire, Nottingham Health Profile, Quality of Life Index and Sickness Impact Profile (De Haan 1993).
Activities of daily living scales included the Barthel Index, Modified Rankin Scale and Nottingham Extended Activities of Daily Living Scale (Wade 1992); and the Index of Activities of Daily Living, Instrumental Activities of Daily Living Scale, Functional Activities Questionnaire, and Blessed Functional Activities Scale (Pohjasvaara 1997).
We used the Stroke Unit Trialists' Collaboration definitions for death or dependency and death or institutional care (SUTC 2013). The criterion for dependency is scoring less than 18 on the Barthel Index or greater than two on the Modified Rankin Scale, while institutional care refers to care in a residential home, nursing home, or hospital at the end of the scheduled follow‐up.
We determined the safety and acceptance of treadmill training. We used the prevalence of adverse events during the treatment period as a measure of safety. We categorised adverse events into injurious falls, other injury, major cardiovascular events, and any other adverse outcomes. We examined the reason for participants withdrawing from the studies as a marker for acceptance. We analysed these withdrawal data qualitatively.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. For this update, we extended the search for trials from March 2005 (when the first update of this review was published) to 14 February 2017. We searched for trials in all languages and arranged translation of relevant trial reports published in languages other then English.
Electronic searches
We searched the Cochrane Stroke Group Trials Register (last searched 14 February 2017) and the following electronic bibliographic databases:
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 4) in the Cochrane Library (searched 10 April 2017) (Appendix 1);
-
MEDLINE Ovid (1966 to 14 February 2017) (Appendix 2);
-
Embase Ovid (1980 to 14 February 2017) (Appendix 3);
-
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1982 to 14 February 2017) (Appendix 4);
-
AMED Ovid ( Allied and Complementary Medicine; 1985 to 14 February 2017) (Appendix 5); and
-
SPORTDiscus EBSCO (1949 to 14 February 2017) (Appendix 6).
We developed the search strategies with the help of the Cochrane Information Specialist and adapted the MEDLINE search strategy for the other databases.
We identified and searched the following ongoing trials and research registers:
-
International Standard Randomised Controlled Trial Number Register (www.isrctn.com; searched 9 March 2017);
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 9 March 2017) (Appendix 7);
-
Stroke Trials Register (www.strokecenter.org; searched 9 March 2017); and
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (searched 9 March 2017) (Appendix 8).
Searching other resources
We also:
-
handsearched the following relevant conference proceedings:
-
World Congress of NeuroRehabilitation (2006 to 2016);
-
World Congress of Physical Medicine and Rehabilitation (2005 to 2015);
-
World Congress of Physical Therapy (2007 to 2015);
-
Deutsche Gesellschaft für Neurotraumatologie und Klinische Neurorehabilitation (2005 to 2016);
-
Deutsche Gesellschaft für Neurologie (2005 to 2016);
-
Deutsche Gesellschaft für Neurorehabilitation (2005 to 2016); and
-
Asian Oceania Conference of Physical and Rehabilitation (2008 to 2016);
-
-
screened reference lists of all relevant articles; and
-
contacted trialists, experts, and researchers in our field of study.
Data collection and analysis
Selection of studies
For this update, two review authors (BE and JM) read the titles and abstracts of the records identified from the electronic searches and eliminated obviously irrelevant studies. We retrieved the full texts of the remaining studies and two review authors (BE and JM) ranked the studies as relevant, possibly relevant or irrelevant according to our inclusion criteria (types of studies, participants, aims of interventions). Two review authors (JM, ST) then examined whether the relevant and possibly relevant publications fitted the population, intervention, comparison, outcome (PICO) strategy of our study question. We resolved disagreements by discussion with all authors. If we needed further information, we contacted trial authors.
We excluded studies that did not match our inclusion criteria regarding the type of study, participants or type of interventions and those that were not RCTs.
Data extraction and management
For this update, two review authors (BE, JM) independently extracted trial and outcome data from the selected trials. If one of the review authors was involved in an included trial, another review author extracted the trial and outcome data from that trial. In accordance with the 'Risk of bias' tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we used checklists to independently assess:
-
methods of random sequence generation;
-
methods of allocation concealment;
-
blinding of assessors;
-
blinding of participants;
-
adverse effects and dropouts;
-
important imbalances in prognostic factors at baseline;
-
participants (country, number of participants, age, gender, type of stroke, time from stroke onset to study entry, inclusion and exclusion criteria, cognition, pre‐existing neurological impairment(s), neurological history);
-
comparison (details of interventions in treatment and control groups, duration of treatment, details of co‐interventions in the groups);
-
outcomes and their time point of measurement.
All review authors checked the extracted data for agreement. If these authors could not reach consensus, a researcher not involved in data extraction arbitrated. If necessary, we contacted the researchers to request more information.
Assessment of risk of bias in included studies
For this update of the review, two authors (BE and JM) independently assessed the risk of bias in the included trials in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We described the agreement between authors during the assessment of risk of bias and we resolved disagreement by reaching consensus through discussion. We contacted trialists for clarification and to request missing information.
Measures of treatment effect
For all outcomes representing continuous data, we entered means and standard deviations. We calculated a pooled estimate of the mean difference (MD) with 95% confidence interval (CI). If studies did not use the same outcome measure, we calculated standardised mean differences (SMD) instead of MDs. For all binary outcomes, we calculated risk differences (RD) with 95% CI. For all analyses, we used Cochrane's Review Manager software, RevMan 5.2 (RevMan 2012) and used a random‐effects model.
Unit of analysis issues
In the event that individuals underwent more than one intervention, as in a cross‐over trial, we only used data from the first phase of the study before cross‐over.
If outcomes were repeatedly observed in participants (e.g. follow‐up at four and six weeks), we reported the measures at the longest time point post intervention from each study.
Dealing with missing data
We contacted the relevant principal investigators to retrieve missing data. Where possible, we extracted data to allow an intention‐to‐treat (ITT) analysis in which all randomised participants were analysed
in the groups to which they were originally assigned. We did not make assumptions about loss to follow‐up for continuous data. We analysed results for those who completed the trial.
Assessment of heterogeneity
We used the I² statistic to assess hterogeneity. We used a random‐effects model, regardless of the level of heterogeneity. Thus, in the case of heterogeneity, we did not violate the preconditions of a fixed‐effect model approach.
Assessment of reporting biases
We inspected funnel plots for assessing the risk of publication bias.
Data synthesis
GRADE and 'Summary of findings' table
We created a 'summary of findings Table for the main comparison' using the following outcomes.
-
Walking speed (m/s) at the end of treatment. Scale from: 0 to infinity.
-
Walking speed (m/s) at the end of treatment ‐ dependent in walking at the start of treatment. Scale from: 0 to infinity.
-
Walking speed (m/s) at the end of treatment ‐ independent in walking at the start of treatment. Scale from: 0 to infinity.
-
Walking endurance (m) at the end of the intervention phase. Scale from: 0 to infinity.
-
Walking endurance (m) at the end of treatment ‐ dependent in walking at the start of treatment. Scale from: 0 to infinity.
-
Walking endurance (m) at the end of treatment ‐ independent in walking at the start of treatment. Scale from: 0 to infinity.
-
Dropouts ‐ by the end of treatment. Numbers of dropouts and adverse events.
We used the eight GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, publication bias, large effect, plausible confounding would change the effect, and dose response gradient) to assess the quality of the body of evidence as it related to the studies which contributed data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using GRADEpro GDT software (GRADEpro GDT). We justified all decisions to down‐ or up‐grade the quality of studies using footnotes, and we made comments to aid the reader's understanding of the review, where necessary.
Subgroup analysis and investigation of heterogeneity
We did three subgroup analyses:
-
for time between the stroke and the start of training (first subgroup defined as in the first 3 months after stroke, second subgroup defined by duration of illness of more than 3 months)
-
the intensity of training (subgroups defined by a weekly frequency of 5 times per week, 3 to 4 times a week and 3 times per week or less), and
-
the duration of training (subgroups defined by categories of more than 4 weeks, 4 weeks or less than 4 weeks).
The scientific rationale for defining these categories in subgroups is that these above categories were described in the research (e.g. in study protocols for trials assessing the effects of treadmill training) and they are used in clinical rehabilitation after stroke.
However, for the types of co‐interventions implemented in conjunction with treadmill training, we were not able to conduct a subgroup analysis.
We conducted subgroup analyses according to whether participants in the trials were dependent or independent walkers.
Sensitivity analysis
We performed a sensitivity analysis based on the mehodological quality of trials (involving treadmill training) including true versus quasi‐randomisation, concealed versus unconcealed allocation, and blinded versus non‐blinded outcome assessment.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies
Results of the search
2014 version
For the 2014 version of this review, we identified 12725 potentially relevant trials through electronic searching; we considered 246 full papers and included 44 trials with 2658 participants (Ada 2003; Ada 2010; Ada 2013; Da Cunha Filho 2002; Deniz 2011; Du 2006; Duncan 2011; Eich 2004; Franceschini 2009; Gan 2012; Globas 2011; Hoyer 2012; Jaffe 2004; Kang 2012; Kim 2011; Kosak 2000; Kuys 2011; Langhammer 2010; Laufer 2001; Liston 2000; Luft 2008; MacKay‐Lyons 2013; Macko 2005; Mehrberg 2001; Moore 2010; Nilsson 2001a; Nilsson 2001b; Olawale 2009; Pohl 2002; Richards 1993; Richards 2004; Scheidtmann 1999; Smith 2008; Sullivan 2007; Suputtitada 2004; Takami 2010; Toledano‐Zarhi 2011; Visintin 1998a; Visintin 1998b; Weng 2004; Weng 2006; Werner 2002a; Yang 2010; Yen 2008; Zhang 2008; Zhu 2004)
2017 version
In this update, the searches of the electronic databases and trials registers generated 10700 unique references for screening. After excluding nonrelevant citations, we obtained the full texts of 27 papers; of these, we included 12 trials in the qualitative and quantitative analysis of the review (Bonnyaud 2013; Bonnyaud 2013a; Combs‐Miller 2014; DePaul 2015; Gama 2017; Kim 2016; Mao 2015; Middleton 2014; Park 2013; Park 2015; Ribeiro 2013; Srivastava 2016).
Figure 1 shows the flow diagram for the selection of studies.
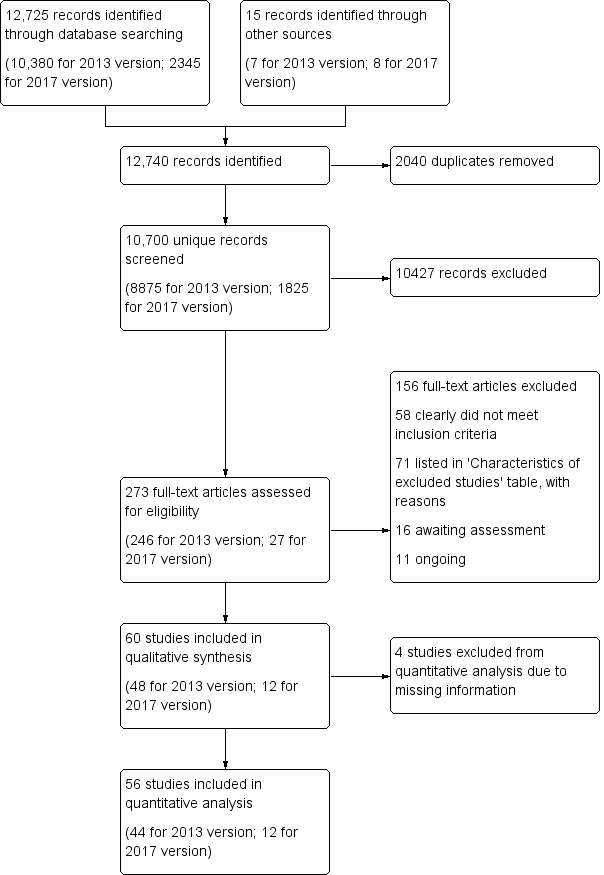
Flow diagram. Please note that the number of full‐texts is not necessarily equal to the number of studies that means that there often are several full‐texts of a single trial (e.g. as is the case for Ada 2003 or DEGAS 2007).
Included studies
We included 56 studies, involving a total of 3105 participants, in the quantitative analysis of this review (see the Characteristics of included studies). Two included studies have been split up into two sub‐studies each (Nilsson 2001; Visintin 1998).
Twenty‐six studies (1410 participants) compared treadmill training with body weight support to another physiotherapy intervention (Analysis 2.2); 20 studies (889 participants) compared treadmill training without body weight support to another physiotherapy intervention, no intervention or sham intervention (Analysis 3.1); two studies (100 participants) compared treadmill training with body weight support to treadmill training without body weight support; and four studies (147 participants) did not state whether they used body weight support or not.
No studies compared body weight support without treadmill training to another physiotherapy intervention.
The data from two studies were subdivided for the analyses and the corresponding participants were not double‐counted. The Nilsson 2001 and Visintin 1998 studies recruited both dependent and independent walkers, so the data were subdivided into two comparisons for each trial. For the Nilsson 2001 trial, we separately analysed data from the 54 participants (26 experimental and 28 control) who were dependent walkers at the start of treatment (Nilsson 2001a) and data from the 19 participants (10 experimental and nine control) who were independent walkers at the start of treatment (Nilsson 2001b). For the Visintin 1998 trial, we performed separate analyses for data from the 59 participants (33 experimental and 26 control) (Visintin 1998a) and 20 participants (10 experimental and 10 control) (Visintin 1998b) who were dependent and independent walkers at the start of treatment, respectively. We obtained these walking dependency data through correspondence with the authors.
The characteristics of participants in the included studies are listed in Table 1. The characteristics of the experimental interventions are listed in Table 2. The outcomes used in the included studies are described in detail in the Characteristics of included studies. The reporting of adverse events and dropouts was incomplete for all trials and described in detail in Table 3 and Table 4. If these data were not explicitly reported, we attempted to obtain the missing information through correspondence with the trialists.
| Study ID | EXP age | CTL age | EXP gender | CTL gender | EXP time post‐stroke | CTL time post‐stroke | EXP paresis side | CTL paresis side |
| Mean 66 (SD 11) years (excluding 1 dropout) | Mean 66 (SD 11) years (excluding 1 dropout) | Men/women 9/4 | Men/women 10/4 | Mean 28 (SD 17) months | Mean 26 (SD 20) months | Left/right 5/8 | Left/right 8/6 | |
| Mean 70 (SD 9) years | Mean 71 (SD 9) years | Men/women 38/26 | Men/women 33/29 | Mean 18 (SD 8) days | Mean 18 (SD 7) days | Left/right 34/30 | Left/right 36/26 | |
| Mean 67 (SD 12) years | Mean 63 (SD 13) years | Men/women 52/16 | Men/women 19/15 | Mean 21 (SD 16) months | Mean 19 (SD 13) months | Left/right 32/34 | Left/right 13/21 | |
| Mean 50 (SD 13) years (including both groups) | Men/women 45/15 (including both groups) | Mean 6 (SD 6) years (including both groups) | Left/right 30/30 (including both groups) | |||||
| Mean 50 (SD 13) years (including both groups) | Men/women 45/15 (including both groups) | Mean 6 (SD 6) years (including both groups) | Left/right 30/30 (including both groups) | |||||
| Mean 45 (SD 21) years | Mean 48 (SD 10) years | Men/women 8/4 | Men/women 10/3 | Mean 6 (SD 6) years | Mean 5 (SD 4) years | Left/right 8/4 | Left/right 8/5 | |
| Mean 56 (SD 8) years | Mean 64 (SD 6) years | Men/women 4/6 | Men/women 7/3 | Mean 62 (SD 49) months | Mean 60 (SD 52) months | Left/right 6/4 | Left/right 6/4 | |
| Mean 57.8 (SD 5.5) years (excluding dropouts) | Mean 58.9 (SD 12.9) years (excluding dropouts) | Men/women 6/0 | Men/women 7/0 | Mean 15.7 (SD 7.7) days | Mean 19.0 (SD 12.7) days | Left/right/bilateral 1/4/1 | Left/right 4/3 | |
| Mean 61.5 (SD 4.7) years | Mean 61.5 (SD 12.5) years | Men/women 8/2 | Men/women 3/7 | Mean 71 (SD 40) days | Mean 81 (SD 47) months | Left/right 6/4 | Left/right 3/7 | |
| Mean 62 (SD 13) years | Mean 61.5 (SD 4.7) years | Men/women 21/14 | Men/women 22/14 | Median 19 (Q1 7, Q2 34) weeks | Median 18 (Q1 10, Q3 30) weeks | Left/right/bilateral 20/12/3 | Left/right/bilateral 17/18/1 | |
| 56 (6) years | 58 (6) years | Men/women 35/32 | Men/women 30/31 | < 3 months | < 3 months | Left/right 31/36 | Left/right 29/32 | |
| Mean 62 (SD 12) years | Mean 63 (SD 13) years | Men/women 159/123 | Men/women 65/61 | Mean 64 (SD 9) days | Mean 63 (SD 8) days | Left/right 121/161 | Left/right 61/65 | |
| Mean 62.4 (SD 4.8) years (all participants) | Mean 64.0 (SD 6.0) years (all participants) | Men/women 17/8 | Men/women 16/9 | Mean 6.1 (SD 2.2) weeks | Mean 6.3 (SD 2.5) weeks | Left/right 14/11 | Left/right 14/11 | |
| Mean 66 (SD 12) years | Mean 71 (SD 12) years | Men/women 28/24 | Men/women 22/23 (only 45 described) | Mean 17 (SD 10) days | Mean 14 (SD 7) days | Left/right 29/23 | Left/right 15/30 (only 45 described) | |
| Mean 59 (SD 8) years | Mean 58 (SD 10) years | Men/women 7/7 (only 14 described) | Men/women 8/6 (only 14 described) | Mean 60 (SD 55) months | Mean 54 (SD 42) months | Left/right 9/5 (only 14 described) | Left/right 6/8 (only 14 described) | |
| Not described | Not described | Not described | Not described | Not described | Not described | Not described | Not described | |
| Mean 69 (SD 7) years | Mean 69 (SD 6) years | Men/women 14/4 (only 18 described) | Men/women 15/3 (only 18 described) | Mean 60 (SD 47) months | Mean 70 (SD 67) months | Left/right 4/14 (only 18 described) | Left/right 9/9 (only 18 described) | |
| Mean 52 (SD 13) years | Mean 52 (SD 6) years | Men/women 20/10 | Men/women 18/12 | Mean 99 (SD 39) days | Mean 96 (SD 42) days | Left/right 17/13 | Left/right 17/13 | |
| Mean 58.2 (SD 11.2) years (excluding dropouts) | Mean 63.2 (SD 8.3) years (excluding dropouts) | Men/women 5/5 (excluding dropouts) | Men/women 7/3 (excluding dropouts) | Mean 3.9 (SD 2.3) years (excluding dropouts) | Mean 3.6 (SD 2.6) years (excluding dropouts) | Left/right 6/4 (excluding dropouts) | Left/right 4/6 (excluding dropouts) | |
| Mean 56 (SD 7) years | Mean 56 (SD 8) years | Men/women 10/10 (excluding dropouts) | Men/women 6/4 (excluding dropouts) | Mean 14 (SD 4) months | Mean 15 (SD 7) months | Left/right 8/12 (excluding dropouts) | Left/right 5/5 (excluding dropouts) | |
| Mean 51 (SD 4) years | Mean 50 (SD 8) years | Men/women 11/9 | Men/women 14/10 | Mean 15 (SD 6) months | Mean 14 (SD 3) months | Left/right 8/12 | Left/right 8/16 | |
| Mean 56.20 (SD 7.56) years | Mean 52.00 (SD 7.27) years | Men/women 4/6 | Men/women 5/5 | Mean 7.5 (SD 4.4) months | Mean 13.3 (SD 16.1) months | Left/right 3/7 | Left/right 4/6 | |
| Mean 74 (SEM 2) years (all participants) | Mean 70 (SEM 2) years | Men/women 13/9 | Men/women 18/16 | Mean 39 (SEM 3) days | Mean 40 (SEM 4) days | Left/right/bilateral 8/12/2 | Left/right/bilateral 12/16/6 | |
| Mean 63 (SD 14) years | Mean 72 (SD 17) years | Men/women 8/7 | Men/women 6/9 | Mean 52 (SD 32) days (excluding dropouts) | Mean 49 (SD 30) days (excluding dropouts) | Left/right 6/9 | Left/right 11/4 | |
| Mean 74 (SD 13) years | Mean 75 (SD 10) years | Men/women 10/11 | Men/women 6/12 | Mean 419 (SD 1034) days | Mean 349 (SD 820) days | Left/right 15/6 | Left/right 13/5 | |
| Mean 66.6 (SD 7.2) years (excluding dropouts) | Mean 69.3 (SD 8.1) years (excluding dropouts) | Men/women 7/6 | Men/women 7/5 | Mean 32.6 (SD 21.2) days | Mean 35.8 (SD 17.3) days | Left/right 5/8 | Left/right 5/7 | |
| Mean 79.1 (SD 6.8) years (all EXP and CTL participants) | Men/women 12/6 | Not reported | Not reported | Not reported | Not reported | |||
| Mean 64 (SD 10) years | Mean 63 (SD 9) years | Men/women 14/20 (excluding dropouts) | Men/women 19/18 (excluding dropouts) | Mean 55 months (excluding dropouts) | Mean 63 months (excluding dropouts) | Left/right 21/12 (excluding dropouts) | Left/right 13/21 (excluding dropouts) | |
| Mean 62 (SD 15) years | Mean 59 (SD 13) years | Men/women 15/9 | Men/women 14/12 | Mean 23 (SD 6) days | Mean 23 (SD 4) days | Left/right 16/8 | Left/right 13/13 | |
| Mean 63 (SD 10) years | Mean 64 (SD 8) years | Men/women 22/10 | Men/women 21/8 | Mean 35 (SD 29) months | Mean 39 (SD 59) months | Left/right 18/14 | Left/right 13/16 | |
| Mean 59.6 (SD 9.2) years | Mean 60.8 (SD 10.7) years | Men/women 10/5 | Men/women 9/4 | Mean 49 (SD 20) months | Mean 48 (SD 17) months | Left/right 6/9 | Left/right 6/7 | |
| Not described | Not described | Not described | Not described | Not described | Not described | Not described | Not described | |
| Mean 61.4 (SD 15.7) years | Mean 60.7 (SD 11.4) years | Men/women 14/9 | Men/women 16/4 | Mean 50.4 (SD 56.8) months | Mean 29 (SD 52) months | Left/right 8/15 | Left/right 8/12 | |
| Mean 50 (SD 15) years (EXP and CTL participants) | Men/women 14/6 (EXP and CTL) | Mean 13 (SD 8) months (EXP and CTL) | Left/right 16/4 (EXP and CTL) | |||||
| Median 54 (range 24 to 67) years (all participants) | Median 56 (range 24 to 66) years | Men/women 20/16 | Men/women 20/17 | Median 22 (range 10 to 56) days | Median 17 (range 8 to 53) days | Left/right/bilateral 21/11/4 | Left/right/bilateral 18/14/5 | |
| Mean 56.8 (SD 6.4) years | Mean 57.0 (SD 7.1) years | Men/women 12/8 | Men/women 22/18 | Mean 10.2 (SD 6.9) months | Mean 10.5 (SD 6.3) months | Left/right 12/8 | Left/right 19/21 | |
| Mean 53 (SD 8) years | Mean 53 (SD 9) years | Men/women 12/8 | Men/women 13/7 | Mean 21 (SD 7) months | Mean 16 (SD 8) months | Left/right 12/9 | Left/right 10/10 | |
| Mean 55 (SD 10) years | Mean 52 (SD 13) years | Men/women 4/5 | Men/women 6/4 | Mean 10 (SD 3) months | Mean 13 (SD 4) months | Left/right 3/6 | Left/right 6/4 | |
| Mean 58.2 (SD 10.5) years for EXP 1 (excluding dropouts) | Mean 61.6 (SD 10.6) years (excluding dropouts) | Men/women 16/4 for EXP 1 | Men/women 13/7 | Mean 16.2 (SD 16.4) weeks for EXP 1 | Mean 16.1 (SD 18.5) weeks | Left/right 15/5 for EXP 1 | Left/right 16/4 | |
| Mean 56 (SD 8) years (without dropouts) | Mean 58 (SD 9) years (without dropouts) | Not described | Not described | Mean 33 (SD 25) months | Mean 20 (SD 10) months | Not described | Not described | |
| Mean 69.6 (SD 7.4) years (all participants) | Mean 67.3 (SD 11.2) years (CTL 1) | Men/women 5/5 | Men/women 2/6 | Mean 8.3 (SD 1.4) days | Mean 8.8 (SD 1.5) days | Left/right 8/2 | Left/right 2/6 | |
| Mean 62.9 (SD 12) years | Mean 60.7 (SD 12) years | Men/women 22/10 | Men/women 21/10 | Mean 52.0 (SD 22) months | Mean 52.6 (SD 18) months | Left/right 15/17 | Left/right 20/11 | |
| Mean 57.7 (SD 11.0) years (all participants) | Men/women 16/14 | Mean 52.2 (SD 29.6) days | Left/right 17/13 | |||||
| Mean 57.8 (SD 7.0) years | Mean 56.0 (SD 8.3) years | Men/women 8/2 | Men/women 4/6 | < 1 year: 8 | < 1 year: 8 | Left/right 4/16 | ||
| Mean group II 47.93 (SD 9.95) years; group III 44.20 (SD 11.70) years | Mean 44.40 (SD 12.31) years | Men/women group II 12/3; group III 12/3 | Men/women 12/3 | Mean group II 442.07 (SD 295.13) days; group III 391.80 (SD 431.10) days | mean 652.20 (SD 579.04) days | left/right group II 6/9; group III 8/7 | Left/right 7/8 | |
| Mean 60.0 (SD 13.3) years | Mean 63.4 (SD 8.4) years | Men/women 34/26 | Men/women 11/9 | Mean 23.8 (SD 15.2) months | Mean 28.4 (SD 19.0) months | Left/right 28/32 | Left/right 10/10 | |
| Mean 61.1 (SD 10.2) years | Mean 64.9 (SD 10.7) years | Men/women 20/4 | Men/women 15/9 | Mean 27.3 (SD 26.6) months | Mean 21.6 (SD 27.7) months | Left/right 9/15 | Left/right 8/16 | |
| Mean 68.6 (SD 8.9) years | Mean 66.9 (SD 10.6) years | Men/women 15/9 | Men/women 7/7 | Mean 14.0 (SD 8.1) days | Mean 13.7 (SD 8.9) days | Left/right 12/12 | Left/right 4/10 | |
| Mean 65 (SD 10) years | Mean 65 (SD 12) years | Men/women 11/3 | Men/women 10/4 | Mean 11 (SD 5) days | Mean 11 (SD 4) days | Not described | Not described | |
| Mean 66.5 (SD 12.8) years (all participants) | Mean 66.7 (SD 10.1) years | Men/women 31/19 | Men/women 28/22 | Mean 68.1 (SD 26.5) days | Mean 78.4 (SD 30.0) days | Left/right 30/20 | Left/right 21/29 | |
| 55.2 (15.4) years | 54.6 (15.2) years | Men/women 17/6 | Men/women 17/5 | Mean 36.1 (SD 11.3) days | Mean 35.6 (SD 14.5) days | Left/right | Left/right | |
| 51 (12) years | 50 (14) years | Men/women 8/5 | Men/women 9/4 | Mean 62 (SD 24) days | Mean 63 (SD 34) days | Left/right | Left/right | |
| Mean 59.7 (SD 10.2) years (all participants) | Mean 60.3 (SD 8.6) years (all participants) | Men/women 8/7 | Men/women 5/10 | Mean 7.4 (SD 2.0) weeks | Mean 6.9 (SD 2.1) weeks | Left/right 7/8 | Left/right 7/8 | |
| Mean 57.2 (SD 9.3) years | Mean 55.0 (SD 10.1) years | Men/women 5/5 | Men/women 5/3 | Mean 1.2 (SD 1.1) years | Mean 1.6 (SD 1.5) years | Left/right 5/5 | Left/right 4/4 | |
| Mean 57.3 (SD 16.4) years | Mean 56.1 (SD 12.7) years | Men/women 3/4 | Men/women 6/1 | Mean 2.0 (SD 0.6) months | Mean 2.0 (SD 2.4) months | Left/right 5/2 | Left/right 3/4 | |
| 63.3 (13.4) years | 62.8 (15.4) years | Men/women | Men/women | 68.7 (25.6) days | 66.3 (23.3) days | Left/right | Left/right | |
| 56.9 (12.9) years | 57.8 (12.16) years | Men/women 6/4 | Men/women 7/3 | Mean 4.1 (SD 4.8) months | Mean 3.1 (SD 4.2) months | Not stated by the authors | Not stated by the authors | |
CTL: control
EXP: experimental
Q1: first quartile (descriptive statistics)
Q2: second quartile
Q3: third quartile
SD: standard deviation
SEM: standard error of the mean
| Study ID | EXP: treadmill | EXP: support | EXP: duration | EXP: frequency | EXP: N weeks | CTL: interventions | CTL: duration | CTL: frequency | CTL: N weeks |
| Gradually increased on an individual basis starting from 0.7 m/s at the start of the first session and finishing at 1.1 m/s at the end of the last session, on average | BWS: no Hand support: yes, use of hand rails if required Assistance from therapist: only if required, 2 participants needed slight help with stepping through for the first 2 weeks | 30 minutes (24, 21, 18, and 15 minutes in treadmill training in the first, second, third and fourth training weeks, respectively) | 3 times per week | 4 weeks | Sham (task‐orientated home program with an intensity insufficient to produce an effect, plus telephone follow‐up once each week) | 30 minutes | 3 times per week (plus encouraged to walk every day) | 4 weeks | |
| Initial speed of the treadmill was set so that the therapist had time to assist the leg to swing through while maintaining a reasonable step length. If a participant was too disabled to walk on a moving treadmill with the assistance of a therapist, then the participant walked on the spot. Once they attained a speed of 0.4 m/s without body weight support, they commenced 10 minutes of overground walking | BWS: yes Hand support: no Assistance from therapist: yes if required | 30 minutes | 5 times per week | Until they achieved independent walking or | Assisted overground walking. Aids such as knee splints, ankle–foot orthoses, parallel bars, forearm support frames and walking sticks could be used as part of | 30 minutes | 5 times per week | Until they achieved independent walking or | |
| Treadmill was run at a comfortable speed and participants were instructed to "walk as slowly as possible" and/or a metronome was used to decrease cadence thereby encouraging larger steps. When necessary, marching‐type steps were included to encourage hip and knee flexion during swing phase to improve toe clearance. When a normal step length was observed, the therapist increased the speed of the treadmill until step length was compromised. Workload was then progressed by increasing the incline of the treadmill. Overground walking was used each session and comprised 20% of intervention time in week 1 and was progressively increased each week so that it comprised 50% of the 30 minutes intervention time in week 8 of training. In week 9, the 4‐month training group returned to 20% overground walking, which was again increased to 50% by week 16 | BWS: no Hand support: no Assistance from therapist: no | 30 minutes | 3 times per week | Group 1: 16 weeks Group 2: eight weeks | Control group received no intervention. | ‐ | ‐ | ‐ | |
| Comfortable walking speed | No BWS | 20 minutes | Single session | ‐ | Overground gait‐training with constant walking speed | 20 minutes | Single session | ‐ | |
| 1 EXP subgroup walking on Participants were instructed to walk without stopping, at their own comfortable speed. The mass fixed to the ankle of the non‐paretic lower limb was 2 kg for women and 4 kg for men | No BWS | 20 minutes | Single session | ‐ | 1 CTL subgroup walking overground without a mass other CTL subgroup walking overground with a mass. Participants were instructed to walk without stopping, at their own comfortable speed. The mass fixed to the ankle of the non‐paretic lower limb was 2 kg for women and 4 kg for men | 20 minutes | Single session | ‐ | |
| Body weight‐supported treadmill training. Rest breaks were allowed as needed, however, breaks were not included in the overall walking time. Walking speed was increased or decreased based on the Borg rating of 11 to 14. Participants were instructed to achieve their fastest possible walking pace on the treadmill at every training session, without exceeding the moderate intensity level on the Borg scale. | BWS: began with | 30 minutes | 5 days per week | 2 weeks | Overground walking training. Rest breaks were allowed as needed, however, breaks were not included in the overall walking time. | 30 minutes | 5 days per week | 2 weeks | |
| Gradually increased in increments of 0.01 m/s, starting at 0.01 m/s | BWS: yes, starting at 30% body weight and progressively decreased to 0% Hand support: not reported Assistance from therapist: not reported | 20 minutes | 5 times per week | 2 to 3 weeks | Task‐orientated gait‐training | 20 minutes | 5 times per week | 2 to 3 weeks | |
| 10‐minute sessions, if necessary separated by 5‐minute resting period, training at comfortable walking speed every 3 to 5 minutes was increased by increments of 0.01 m/s | BWS: yes Hand support: not reported Assistance from therapist: not reported | 60 minutes | 5 times per week | 4 weeks | Range of motion, stretching, strengthening, balance, co‐ordination exercises and conventional ambulation training treatment program with parallel bars | 60 minutes | 5 times per week | 4 weeks | |
| Treadmill training assisted by 1 or more physical therapy staff (physical guidance, at or above 0.89 m/s) | BWS: yes up to 40% of BWS, weaned according to performance Handle use discouraged | Up to 30 minutes | 15 sessions | 5 weeks | Motor learning Walking Programm (practising 7 core walking activities) | Up to 40 minutes | 15 sessions | 5 weeks | |
| Gradually increased starting from 0.1 m/s to 0.5 m/s; interval method, resting period gradually reduced | BWS: yes, initial BWS 30% to 40% weight, gradual reduction Hand support: not reported Assistance from therapist: not reported | 40 minutes | 2 times per day | 4 weeks | Brunnstrom, Bobath, Rood therapy approaches as well as proprioceptive neuromuscular facilitation techniques and motor relearning program, transfer training, trunk stabilisation | 40 minutes | Unclear | 4 weeks | |
| At 0.89 m/s, followed by a progressive program of walking overground for 15 minutes. The treadmill speeds ranged from 0 to 1.6 km per hour, increasing by increments of 0.16 km per hour. | BWS: yes Hand support: not reported Assistance from therapist: yes | 90 minute sessions | 3 times per week | 12 to 16 weeks (30 and 36 exercise sessions | Home exercise as an active control, not as a high‐intensity, task‐specific walking program. Progression through the program was managed by a physical therapist in the home, with the goals of enhancing flexibility, range of motion in joints, strength of arms and legs, co‐ordination, and static and dynamic | 90‐minute sessions | 3 times per week | 12 to 16 weeks (30 and 36 exercise sessions | |
| Speed and inclination increased on an individual basis to achieve a training heart rate. | BWS: yes, the harness was always secured and body weight was minimally supported (0 to 15%) according to participant needs. Hand support: not reported Assistance from therapist: yes, to set the paretic leg, weight shift and hip extension, if required | 30 minutes | 5 times per week | 6 weeks | Not task‐orientated (neurophysiological) | 30 minutes | 5 times per week | 6 weeks | |
| Speed starting from 0.1 m/s and aiming at 1.2 m/s according to the participant's compliance and progress. Conventional treatment was performed for 40 minutes, not immediately | BWS: yes, limited to Hand support: not reported Assistance from therapist: 2 trained | 20 minutes + 40 minutes | 2 times per day | 20 sessions within 5 weeks | 20 sessions of overground gait‐training of 60 minutes each | 60 minutes | 5 times per week | 20 sessions within 5 weeks | |
| Body weight support treadmill training and comfortable treadmill speed was set | BWS: yes, from 30% to 0% of body weight Hand support: allowed Assistance from therapist: allowed | 45 minutes | 3 times per week | 6 weeks | Walking overground at comfortable walking speed | 45 minutes | 3 times per week | 6 weeks | |
| Body weight support treadmill training; treadmill speed was initially started at 0.5 mph | BWS: yes, up to 40% of their body weight supported at the beginning of the Hand support: unclear Assistance from therapist: unclear | Not described | Not described | 8 weeks | Body weight support overground ambulation | Not described | Not described | 8 weeks | |
| Beginning with 10 to 20 minutes) at 60% to 80% of the maximum heart rate reserve (starting with 40% to 50% HRR). Duration was increased as tolerated by 1 to 5 minutes per week Treadmill speed was progressed by 0.1 to 0.3 km/hour every 1 to 2 weeks Training was a group intervention (3 participants trained in parallel) | BWS: no Hand support: allowed Assistance from therapist: unclear Treadmill inclination at 0° | 30 to 50 minutes | 3 times per week | 3 months (39 sessions) | Passive, muscle tone–regulating exercises for the upper and lower extremities with elements of balance training conducted on an outpatient basis in physiotherapy practices or rehabilitation centres. No aerobic fitness training was performed. | 60 minutes | 3 times per week | 3 months (13 weeks) | |
| Treadmill therapy with BWS and on days without TTBWS, conventional gait‐training was conducted | BWS: yes Hand support: not reported Assistance from therapist: not reported | 30 minutes | Daily for the | 30 sessions for a period of a minimum of 10 weeks | Intensive gait‐training (30 minutes) and functional training (30 minutes) daily for a minimum of 10 weeks | 30 minutes | Daily | For a minimum of 10 weeks | |
| Comfortable walking speed (speed not reported), speed was not progressed | BWS: no, harness used to prevent falls only Hand support: yes, use of hand rails, if required Assistance from therapist: no | 60 minutes | 3 times per week | 2 weeks | Task‐orientated (overground obstacle training) | 60 minutes | 3 times per week | 2 weeks | |
| Group 1: treadmill training with optic flow (optic flow was applied and treadmill speed was increased by 0.1 km/hour each time once the participant could walk stably for more than 20 seconds) Group 2: treadmill training without optic flow (treadmill speed was increased by | BWS: no Hand support: allowed but discouraged Assistance from therapist: no | 30 minutes (2 times for 15 minutes with a rest between) | 3 times per week | 4 weeks | General stretching with added range of motion exercises in the less and more affected sides of the trunk, arms and legs for the same time. Exercise therapy was performed using the traditional motor development theory and neurodevelopmental treatment based on motor learning theory. | 30 minutes | 3 times per week | 4 weeks | |
| Gradually increased starting from 0.3 m/s to 0.7 m/s | BWS: no Hand support: no Assistance from therapist: no | 30 minutes | 5 times per week | 6 weeks | Control group received muscle strengthening (seated leg press, knee extension, leg abductor) | 30 minutes | 5 times per week | 6 weeks | |
| Treadmill training with virtual reality in addition to general physical therapy If the participant maintained the speed and felt safe for 20 s, the treadmill speed was then increased by 5% during next training session | BWS: no Hand support: unclear Assistance from therapist: unclear | 30 minutes | 3 times per week | 4 weeks | 2 control groups: 1 control group received community ambulation in addition to general physical therapy, the other control group no additional walking training to general physical therapy | 30 minutes | 3 times per week | 4 weeks | |
| Gradually increased from 0.22 to 0.89 m/s, as tolerated | BWS: yes, starting at 30% body weight and progressively decreased to 0% or eliminated Hand support: yes, use of hand rails, if required Assistance from therapist: yes, assisted with swing phase, foot placement and weight shift, if required | 45 minutes | 5 times per week | 2 to 3 weeks | Not task‐orientated (orthopaedic) | 45 minutes | 5 times per week | 2 to 3 weeks | |
| Walked on the treadmill at an intensity of 40% to 60% heart rate reserve or a Borg Rating of Perceived Exertion of 11 to 14. Participants commenced at an intensity level of 40% heart rate reserve for 30 minutes, progressing each week aiming for a 5% to 10% increase until 60% heart rate reserve was reached. For participants unable to reach 40% heart rate reserve on commencement of treadmill walking, treadmill speeds were set as fast as tolerated and progressed as quickly as possible. Also received task‐oriented physiotherapy, approximately 1 hour per day | BWS: no Hand support: yes, were encouraged to hold the handrail Assistance from therapist: yes, a physiotherapist provided assistance as | 30 minutes | 3 times per week | 6 weeks | Received usual physiotherapy intervention only | Unclear (probably the same as the EXP group) | Unclear (probably the same as the EXP group) | Unclear (probably the same as the EXP group) | |
| Walking speed was started on the lowest level and was increased within the first minutes to the working level. The working load was increased in co‐operation with the participants to a level they felt comfortable with and they felt no insecurity in balance or discomfort otherwise. | BWS: no Hand support: yes Assistance from therapist: no, and no inclination | 30 minutes | (Up to) 5 times per week | Mean of 16 days of inpatient stay (mean 10 walking sessions) | Outdoor walking at a comfortable speed and with the use of ordinary assistive devices, when necessary | 30 minutes | (Up to) 5 times per week | Mean of 17 days of inpatient stay (mean 11 walking sessions) | |
| Comfortable walking speed, speed used and progression not reported | BWS: no Hand support: yes, use of hand rails, if required Assistance from therapist: yes, assisted with swing phase and trunk alignment | 8 to 20 minutes | 5 times per week | 3 weeks | Task‐orientated | 8 to 20 minutes | 5 times per week | 3 weeks | |
| Speed used and progression not reported | BWS: no Hand support: not reported Assistance from therapist: not reported | 60 minutes | 3 times per week | 4 weeks | Task‐orientated | 60 minutes | 3 times per week | 4 weeks | |
| Aerobic intensity of 60% of heart rate reserve. Duration and intensity started low (10 to 20 minutes, 40% to 50% heart rate reserve) and increased approximately for 5 minutes and 5% heart rate reserve every 2 weeks, as tolerated. Treadmill velocity and incline were increased by 0.05 m/s and 1% increments, respectively | BWS: no Hand support: not reported Assistance from therapist: not reported | 40 minutes | 3 times per week | 6 months | 13 supervised traditional stretching movements on a raised mat table with a therapist’s assistance. Each movement was performed actively if possible or passively with a therapist's assistance. Movements included quadriceps, calf, hip and hamstring stretch, low back rotation and stretch, chest stretch, bridging, shoulder shrug, abduction, and flexion, heel slides and short arc of quadriceps | 40 minutes | 3 times per week | 6 month | |
| 5 to 10 minutes of active/passive stretching exercises 10 to 15 minutes of upper extremity training (active exercises and strengthening) 10 to 15 minutes of lower extremity training (active exercises and strengthening) 25 to 30 minutes of BWSTT including warm‐up and cool‐down BWSTT initiated in 5 to 10‐minute bouts at the heart rate achieved at 40% to 50% of baseline VO2 peak. The goal was to achieve a target exercise duration (at least 20 minutes, exclusive of warm‐up and cool‐down) and intensity (heart rates corresponding to 60% to 75% of baseline VO2 peak 27) by the fourth or fifth week. Initially, ambulatory‐independent participants walked at a treadmill speed of 80% to 90% of their self‐paced overground speed Ambulatory‐dependent participants walked at a treadmill speed of 70% to 80% of their overground speed Treadmill speed and grade were gradually increased and percentage of manual and body weight support decreased, as tolerated | BWS: yes 20% to 30% or 40%, if necessary of their body weight Hand support: handrail support was discouraged Assistance from therapist: therapist emphasised trunk and limb alignment, loading of the stance limb, hip extension at terminal stance, and advancement of the swing limb | 40 minutes | 5 times per week (after 6 weeks, 3 times per week) | 6 weeks (plus 6 weeks; total of 48 sessions) | 5 to 10 minutes of active/passive stretching exercises 10 to 15 minutes of upper extremity training (active exercises and strengthening) 10 to 15 minutes of lower extremity training (active exercises and strengthening) 25 to 30 minutes of overground gait‐training | 40 minutes | 5 times per week (after 6 weeks, 3 times per week) | 6 weeks (plus 6 weeks; total of 48 sessions) | |
| Increased from a mean of 0.48 (SE 0.30) m/s at baseline to 0.75 (SE 0.30) m/s at treatment end on an individual basis to achieve a target aerobic intensity of 60% to 70% heart rate reserve (treadmill slope increased from 0% at baseline to 2.2% (SE 2.2) at treatment end) | BWS: no Hand support: yes, use of handrails, if required Assistance from therapist: not reported | 40 minutes (including 5 minutes warm‐up and 5 minutes cool‐down) | 3 times per week | 6 months | Task‐orientated | 40 minutes | 3 times per week | 6 months | |
| Treadmill training, with gradually increased walking speed to 2.5 mph | BWS: yes, gradually decreased Hand support: unclear Assistance from therapist: yes | 30 minutes | 5 times per week | 3 weeks | Individualised overground gait‐training (based on the Bobath Approach) | 30 minutes | 5 times per week | 3 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | |
| Treadmill training, with increasing walking speed | BWS: yes, from 8% to 50%, gradually decreased Hand support: unclear Assistance from therapist: no | 60 minutes | 5 times per week | 10 days | Overground gait‐training | 60 minutes | 5 times per week | 10 days | |
| Intensive locomotor training with walking velocity increased in 0.5 km/h increments until participants’ heart rate reached 80% to 85% of age‐predicted maximum or until the participants' Rating of Perceived Exertion increased to 17 on the Borg scale, and was reduced in 10% increments, as tolerated | BWS: up to 40% partial Hand support: handrail use for balance only Assistance from therapist: therapists did not provide manual assistance | Unclear | 2 to 5 times per week | 4 weeks | Did not receive locomotor training or any other interventions | Unclear | 2 to 5 times per week | 4 weeks | |
| Gradually increased from 0.0 to 2.0 m/s on an individual basis | BWS: yes, starting at 100% body weight and decreased to 0% Hand support: yes, use of a cross bar, if required Assistance from therapist: yes, assisted with swing phase, hip and knee extension during stance phase, and weight shift if required | 30 minutes | 5 times per week | 9 to 10 weeks | Task‐orientated | 30 minutes | 5 times per week | 9 to 10 weeks | |
| Participants walked on a treadmill at a "predetermined natural safe walking speed" | BWS: not reported Hand support: not reported Assistance from therapist: not reported | 60 minutes of therapy, including 25 minutes treadmill training | 3 times per week | 12 weeks | Conventional physiotherapy, CTL 2 received overground gait‐training included in the hourly therapy sessions, whereas CTL 1 received conventional physiotherapy only (active and passive range of motion exercises, strength, and balance training) | 60 minutes | 3 times per week | 12 weeks | |
| Treadmill gait‐training at comfortable walking speed | BWS: not reported Hand support: not reported Assistance from therapist: not reported | 30 minutes twice a day | 5 times per week | 1 week | Overground gait‐training | 30 minutes twice a day | 5 times per week | 1 week | |
| Treadmill training with | BWS: not reported Hand support: not reported Assistance from therapist: not reported | 30 minutes | 5 times per week | 3 weeks | Ground walking with rhythmic auditory stimulation | 30 minutes | 5 times per week | 3 weeks | |
| Speed‐dependent treadmill training (EXP 1) ‐ aggressive increase in speed starting from the highest speed the participant could walk at without stumbling and increasing at 10% increments of this speed several times within a session. The average treadmill speed increased from 0.68 m/s (SD 0.34) at the start of training to 2.05 m/s (SD 0.71) at the end of training; | Speed‐dependent treadmill training BWS: yes, no more than 10% body weight for the first 3 training sessions only (participants always wore an unweighted harness) Hand support: not reported Assistance from therapist: no BWS: yes, no more than 10% body weight for the first 3 training sessions only Hand support: not reported Assistance from therapist: yes, assisted with the walking cycle | 30 minutes | 3 times per week | 4 weeks | Not task‐orientated (neurophysiological) | 45 minutes | 3 times per week | 4 weeks | |
| Treadmill training with partial body weight support at comfortable walking speed | BWS: yes, initially 30%, then decreased Hand support: not reported Assistance from therapist: yes, initially aided | 30 minutes | 3 times per week | 4 weeks | Proprioceptive Neuromuscular Facilitation method (PNF, including waist dissociations, sitting and rising from a chair, anteroposterior and latero‐lateral weight transfer) | 30 minutes | 3 times per week | 4 weeks | |
| Speed used and progression not reported | BWS: no Hand support: not reported Assistance from therapist: not reported | 105 minutes (about 35 minutes in treadmill training) | 5 times per week | 5 weeks | Not task‐orientated (neurophysiological) | 105 minutes | 5 times per week | 5 weeks | |
| Specialised locomotor training including tilt table, reciprocal stepping on a Kinetron device | BWS: no Hand support: not described Assistance from therapist: not described | 60 minutes | 5 times per week | 8 weeks | Conventional physiotherapy (traditional neurodevelopmental approach, task‐oriented motor learning, overground gait‐training, stepping exercises) | 60 minutes | 5 times per week | 8 weeks | |
| Gradually increased from 0.0 to 1.3m/s | BWS: yes, amount of body weight support and progression not reported Hand support: yes, use of hand rails, if required Assistance from therapist: yes, assisted with swing phase, foot placement, hip and knee extension during stance phase, and weight shift, if required | 30 minutes | 5 times per week | 3 weeks | Not task‐orientated (neurophysiological) | 30 minutes | 5 times per week | 3 weeks | |
| Participants walked for 5 minutes with a "slightly hard" rate of perceived exertion (RPE), then the speed was increased by increments of 0.2 m/hour every 10 minutes of walking with a "slightly hard" RPE | BWS: not clearly stated Hand support: not reported Assistance from therapist: only if required, 2 participants needed slight help with stepping through for the first 2 weeks | 20 minutes | 12 times per month | 4 weeks | Sham (weekly phone calls, recording of a daily life log) | Not reported | 1 telephone call per week | 4 weeks | |
| 2 treadmill groups: group 1 with BWS and group 2 without BWS at gradually increased walking speed | BWS: group 1 yes (40%), group 2 no Hand support: yes Assistance from therapist: not described | 30 minutes | 5 times per week | 4 weeks | Overground task‐oriented training | 30 minutes | 5 times per week | 4 weeks | |
| Initially 4 x 5‐minute training bouts at individualised speeds, initially within the range of 0.7 to 1.1 m/s, followed by 15 m overground walking and either (1) sham or (2) progressive resistive leg cycling, or (3) individualised progressive resistive strength training | BWS: yes, initially between 30% and 40% of the participant's weight and being decreased as participants improved Hand support: not described Assistance from therapist: up to 3 therapists assisting in placing of both feet and the pelvis, if necessary | 60 minutes | 4 times per week | 6 weeks | Sham (upper extremity cycle ergometry with minimal physical exertion) | 60 minutes | 4 times per week | 6 weeks | |
| Speed was initiated from 0.044 m/s for 10 minutes, followed by a rest for 5 minutes and then increased by increments of 0.044 m/s for 10 minutes | BWS: yes, 30% during the first week, 20% during the second week, I0% during the third week, and no BWS during the fourth week Hand support: unclear Assistance from therapist: initially 2 therapists assisted in placing the foot and the pelvis | 25 minutes | 7 times per week | 4 weeks | Walking at a self‐adopted speed on a 15 m walkway for 10 minutes, rested 5 minutes, and walked again 10 minutes | 25 minutes | 7 times per week | 4 weeks | |
| For 3 minutes twice (with 4 minute rest); week 1: 0.8 km/hour, week 2: 1.0 km/hour, week 3: 1.3 km/hour | BWS: yes 30% Hand support: yes, use of hand rails, if required Assistance from therapist: not described | 30 minutes control intervention followed by 10 minutes treadmill training either in forward or backward direction | 3 times per week | 4 weeks | Conventional training (stretching, strengthening), including overground walking < 200 m and ADL training | 80 minutes | 5.5 times per week | 4 weeks | |
| Intervention consisted of treadmill training, training on a hand bike machine, and a stationary bicycle | BWS: not stated Hand support: not stated Assistance from therapist: not stated | 90 minutes exercise training, including 35 to 55 minutes treadmill training | 2 times per week | 6 weeks | Home exercise booklet with included instructions for flexibility and muscle strength exercises, participants were encouraged to stick to their normal community routine | NA | NA | 6 weeks | |
| Gradually increased in increments of 0.04 m/s, from 0.23 to 0.42 m/s, on average, on an individual basis | BWS: yes, starting at 40% body weight and progressively decreased to 0% Hand support: yes, use of hand rails, if required Assistance from therapist: yes, assisted with stepping and limb control during stance and swing phases, and weight shift, if required | 20 minutes | 4 times per week | 6 weeks | Task‐orientated (treadmill only) | 20 minutes | 4 times per week | 6 weeks | |
| Initial speed was half of the measured maximal walking speed prior to training session for 5 minutes as a warm‐up, then intervals of higher speed for 10 s were delivered, returning back to warm‐up speed for 2 minutes; in the next phase the speed would be increased or decreased by 10%, respectively | BWS: no Hand support: unclear Assistance from therapist: yes, assisted with foot placing and pelvis rotation | 20 minutes | 5 times per week | 4 weeks | Neuromuscular facilitation techniques | 20 minutes | 5 times per week | 4 weeks | |
| Participants walked backwards on a treadmill with increasing speed | BWS: no Hand support: unclear Assistance from therapist: yes; assisted with foot placing and pelvis rotation | 30 minutes of control intervention and 30 minutes of treadmill training | 5 times per week | 3 weeks | Neuromuscular facilitation techniques including lower limb movements and overground gait exercises | 60 minutes | 5 times per week | 3 weeks | |
| Increased from a mean of 0.32 (SD 0.05) m/s at baseline on an individual basis | BWS: yes, starting at a mean of 8.93% (SD 1.84) body weight and progressively decreased Hand support: yes, use of handrails, if required Assistance from therapist: yes, assisted with foot placement, swing phase, and hip and trunk extension during stance phase, if required | 15 to 20 minutes | 5 times per week | 2 weeks | Task‐orientated | 15 to 20 minutes | 5 times per week | 2 weeks | |
| Additional to the CTL intervention: | BWS: yes Hand support: no, participants were encouraged to refrain from handrails Assistance from therapist: yes, 1 or 2 therapists assisted | 30 minutes + 20 minutes control intervention | 3 times per week | 4 weeks | Stretching, muscle strengthening, balance, and overground walking training | 50 minutes | 3 times per week | 4 weeks | |
| Additional to the CTL intervention: | BWS: yes Hand support: no, participants were encouraged to refrain from handrails Assistance from therapist: yes, 1 or 2 therapists assisted | 30 minutes + 20 minutes of control intervention | 3 times per week | 4 weeks | Stretching, muscle strengthening, balance and overground walking training | 50 minutes | 2 to 3 times per week | 4 weeks | |
| Increased from 0.2 km/hour and 40% weight‐bearing relief according to the participant's capabilities | BWS: yes Hand support: unclear Assistance from therapist: yes, assisted with foot placing, knee extension and pelvis rotation | 30 minutes | 5 times per week | 8 weeks | Not described | Not stated | Not stated | 8 weeks | |
| Walking speed and BWS were individualised to the participants' capabilities (with a mean walking speed of 0.13 m/s at baseline and 0.17 m/s at the end of the intervention phase) | BWS: yes Hand support: unclear Assistance from therapist: unclear | Individualised | 5 times a week | 4 weeks | Individualised conventional motor rehabilitation aiming at improving strength and endurance | Not stated | 5 times a week | 4 weeks |
ADL: activities of daily living
BWS: body weight support
BWSTT: body weight support treadmill training
CTL: control
EXP: experimental
GT: gait trainer
HRR: heart rate reserve
NA: not applicable
PNF: Proprioceptive Neuromuscular Facilitation
RPE: rate of perceived exertion
SE: standard error
SD: standard deviation
TTBWS: treadmill training with body weight support
VO2: volume of oxygen consumption
| Study ID | Injurious falls | Other injuries | Cardiovascular event | Other adverse event |
| EXP = 1 (hip fracture caused by a fall at home after the first week of training) | EXP = 1 (missed post‐treatment measurement session due to low back pain) | EXP = 0 | EXP = 1 (fall during overground component of training but no injuries sustained) | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 47 reports All reports included musculoskeletal problems (back, hip, knee, calf, foot pain, and gout), headaches, dizziness, or chest pain. There were 6 reports of falling, 1 of which resulted in a fracture and none of which occurred during the delivery of the intervention. 2 participants in the experimental group experienced anxiety attributable to being on a treadmill that was severe enough for them to withdraw from the study. | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = 0 | EXP = 16 (fracture) | EXP = 1 (myocardial infarction) | EXP = 139 + 143 (all reported events) | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 1 recurrent stroke, 1 transportation problem | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = 0 | EXP = 0 | EXP = 1 (acute myocardial infarction 2 days after last treatment session) | EXP = 0 | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = 0 | EXP = 1 (knee pain after first 4 treadmill sessions) | EXP = 0 | EXP = 1 (hospitalised after first training session and subsequently died, reason for hospitalisation not reported) | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 11 (5 falls during treadmill training but no injuries sustained; 6 minor medical complications) | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP 1 = 0 | EXP 1 = 0 | EXP 1 = 0 | EXP 1 = 0 | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = 1 (hip fracture) | EXP = 1 (cardiac problems) | EXP = not reported | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = 7 | ||||
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
CTL: control
EXP: experimental
| Study ID | EXP ‐ treatment phase | EXP ‐ follow‐up | CTL ‐ treatment | CTL ‐ follow‐up |
| 1 ‐ hip fracture caused by a fall at home after the first week of training | No dropouts | 1 ‐ moved out of area | 1 ‐ moved out of area | |
| 2 ‐ died 2 ‐ withdrew | No follow‐up period | 2 ‐ died | No follow‐up period | |
| 1 ‐ withdrew | No dropouts | 3 ‐ withdrew | No dropouts | |
| No dropouts | No dropouts | No dropouts | No dropouts | |
| No dropouts | No dropouts | No dropouts | No dropouts | |
| 2 dropouts | No dropouts | No dropouts | No dropouts | |
| 1 ‐ completed fewer than 9 treadmill and body weight support sessions | No follow‐up period | 1 ‐ pulmonary complications (not related to the protocol) | No follow‐up period | |
| Dropouts not stated | Dropouts not stated | Dropouts not stated | Dropouts not stated | |
| 1 dropout | 3 dropouts | 5 dropouts | 4 dropouts | |
| No dropouts | No follow‐up period | No dropouts | No follow‐up period | |
| 35 (12 withdrew, 7 died, 13 moved, 3 other) | Unclear | 11 (2 withdrew, 6 died, 3 moved) | ||
| No dropouts | 1 ‐ refusal | No dropouts | No dropouts | |
| 10 ‐ dropouts | No follow‐up period | 10 ‐ dropouts | No follow‐up period | |
| 2 ‐ dropouts | No dropouts | 2 ‐ dropouts | No dropouts | |
| No dropouts | No follow‐up period | No dropouts | No follow‐up period | |
| 1 ‐ recurrent stroke 1 ‐ transportation problem | 2 dropouts (but unclear which group) | No dropouts | 2 dropouts (but unclear which group) | |
| No dropouts | No follow‐up period | No dropouts | No follow‐up period | |
| 1 ‐ endurance level too low to continue treatment | No dropouts | 2 ‐ medical conditions unrelated to the study (1 participant with arthritis and 1 participant with a heart condition) | No dropouts | |
| 1 ‐ dropout ‐ another treatment 1 ‐ lack of participation | No dropouts | No dropouts | No dropouts | |
| Dropouts not stated | Dropouts not stated | Dropouts not stated | Dropouts not stated | |
| No dropouts | No follow‐up period | 3 dropouts in the control group without additional training | No follow‐up period | |
| 1 ‐ chose to discontinue treatment (did not want to walk on the treadmill) | No follow‐up period | 1 ‐ Stroke progression requiring readmission to acute care | No follow‐up period | |
| 1 ‐ withdrew 1 ‐ fall | 1 ‐ moved 1 ‐ medical condition | No dropouts | No dropouts | |
| 3 ‐ dropouts (unclear reasons) | No follow‐up period | 2 ‐ dropouts (unclear reasons) | No follow‐up period | |
| 2 ‐ discharged prior to completion of data collection | No follow‐up period | 1 ‐ discharged prior to completion of data collection | No follow‐up period | |
| 1 ‐ hospitalised after first treatment and subsequently died (reason for hospitalisation not reported) | No follow‐up period | No dropouts | No follow‐up period | |
| 12 ‐ unrelated medical condition 2 ‐ recurrent stroke 6 ‐ noncompliance | No follow‐up period | 11 ‐ unrelated medical condition 11 ‐ noncompliance | No follow‐up period | |
| 1 ‐ seizure activity 1 ‐ moved | 1 ‐ refused | 2 ‐ medical reasons 1 ‐ disinterest | 1 ‐ refused 1 ‐ lost to follow‐up | |
| 3 ‐ medical conditions (1 participant had sinus surgery, 1 participant had pre‐existing shoulder pain, 1 participant had a gastrointestinal bleed and recurrent stroke) | No follow‐up period | 4 ‐ medical conditions (1 participant had a hernia repair, 1 participant had elective cardiac surgery, 1 participant had a radiculopathy, and 1 participant had a foot infection and poor control of hypertension) | No follow‐up period | |
| 1 ‐ discontinued treatment, cardiovascular instability 2 ‐ discontinued treatment, early discharged | No follow‐up period | 2‐ discontinued treatment, early discharge | No follow‐up period | |
| Missing information | Missing information | Missing information | Missing information | |
| 4 ‐ discontinued treatment, lost to follow‐up, unable to contact | No follow‐up period | 1‐ discontinued treatment, lost to follow‐up, unable to contact | ||
| Authors stated: 10 did not complete the protocol because of noncompliance with study requirements (i.e. not wearing accelerometer, n = 5), early discharge from clinical PT (n = 2), orthopaedic injury which limited walking (n = 1), or previous diagnosis of secondary neurological injuries (n = 2). | ||||
| 2 ‐ chose to discontinue treatment (did not want to walk on the treadmill) | 2 ‐ medical reasons | 1 ‐ chose to discontinue treatment (wanted to walk on the treadmill) | 1 ‐ moved out of area | |
| 2 ‐ did not attend all training sessions | No follow‐up period | 5 ‐ Did not attend all training sessions | No follow‐up period | |
| none | No follow‐up period | None | No follow‐up period | |
| none | No follow‐up period | None | No follow‐up period | |
| 2 ‐ medical conditions (1 participant with bladder infection and fever, and 1 participant with viral infection and fever) from EXP 1 | No follow‐up period | 5 ‐ medical conditions (3 participants with pneumonia and 2 with viral infection and fever) | No follow‐up period | |
| 2 ‐ dropouts | No follow‐up period | 3 ‐ dropouts | No follow‐up period | |
| 1 ‐ reason not reported | No follow‐up data reported | 2 ‐ reason not reported | No follow‐up data reported | |
| 1 ‐ medical conditions (hip fracture) 1 ‐ medical conditions (cardiac problems) | 5 ‐ being unavailable | 1 ‐ reason not stated | 7 ‐ being unavailable | |
| No dropouts | No follow‐up period | No dropouts | No follow‐up period | |
| Dropouts not stated | Dropouts not stated | Dropouts not stated | Dropouts not stated | |
| 4 ‐ dropouts | No follow‐up period | 4 ‐ dropouts | No follow‐up period | |
| 6 ‐ withdrawn by administration 1 ‐ refused to participate | 4 ‐ refused to participate | 2 ‐ withdrawn by administration | 1 ‐ withdrawn by administration 3 ‐ refused to participate | |
| Dropouts not stated | No follow‐up period | Dropouts not stated | No follow‐up period | |
| 3 ‐ for family reasons | No follow‐up period | Dropouts not stated | No follow‐up period | |
| 1 ‐ chose to discontinue treatment | No follow‐up period | No dropouts | No follow‐up period | |
| 2 ‐ chose to discontinue treatment | 14 ‐ medical event, repeated stroke, lack of willingness to participate or moved away from area | 4 ‐ chose to discontinue treatment | 13 ‐ medical event, repeated stroke, lack of willingness to participate or moved away from area | |
| 2 ‐ reasons unknown due to issues of translation | No follow‐up period | 3 ‐ reasons unknown due to issues of translation | No follow‐up period | |
| Dropouts not stated | No follow‐up period | Dropouts not stated | No follow‐up period | |
| No dropouts | No follow‐up period | No dropouts | No follow‐up period | |
| No dropouts | No follow‐up period | No dropouts | No follow‐up period | |
| No dropouts | No follow‐up period | No dropouts | No follow‐up period | |
| Dropouts not stated | No follow‐up period | Dropouts not stated | No follow‐up period | |
| No dropouts | No follow‐up period | No dropouts | No follow‐up period | |
CTL: control
EXP: experimental
PT: physiotherapy
Excluded studies
We excluded 72 studies for various reasons (see Characteristics of excluded studies). Fivteen studies are still awaiting classification, mainly due to being conference abstracts with sparse outcome data reported and we were unable to contact the authors (see the Characteristics of studies awaiting classification). Eleven studies are ongoing (see the Characteristics of ongoing studies).
We excluded all these studies from the main analysis.
Risk of bias in included studies
Two authors (JM and ST) independently assessed the methodological quality of the included trials using the 'Risk of bias' tool (using the categories random sequence generation, allocation concealment and blinding of outcome assessors; Figure 2).

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
We wrote to all trialists requesting clarification of some design features or the provision of missing information in order to complete the quality ratings (correspondence was via email or letter, with a reminder being send after three weeks and then every three months if we did not get a response). If no data were provided or no contact achieved, we used published data only for all analysis.
Three trials used a cross‐over design with random allocation to the order of treatments (Liston 2000; Scheidtmann 1999; Werner 2002a). All other studies used a parallel‐group design with true randomisation or quasi‐randomisation (Laufer 2001) to groups.
We explored publication bias visually by inspecting funnel plots for all comparisons (plots only shown for analyses 1.1 and 1.2 (Figure 3; Figure 4)). Our inspection did not indicate clear evidence for publication bias or our inspection was not suggestive of systematic heterogeneity. The only systematic heterogeneity in the funnel plots was found between categories of people after stroke who were dependent or independent walkers at study onset (as we described in detail above).
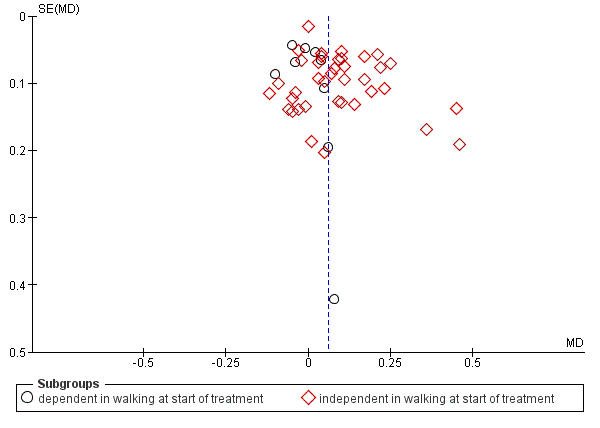
Funnel plot of comparison: 1 Treadmill (with or without body weight support) versus other intervention, outcome: 1.1 Walking speed (m/s) at end of treatment.

Funnel plot of comparison: 1 Treadmill (with or without body weight support) versus other intervention, outcome: 1.2 Walking endurance (m) at end of treatment.
Allocation
Twenty‐nine of the 56 included studies described appropriately the method of random sequence generation (see Figure 2).
Twenty‐three of the 56 included studies described appropriately the method of concealing allocation of participants to groups (see Figure 2).
Blinding
Twenty‐five of the 56 included studies described the outcome assessors as being blinded to group allocation (see Figure 2).
Incomplete outcome data
Twenty‐three of the 56 included studies described incomplete outcome data; however, the dropouts appeared not to be substantial. The dropouts were balanced between the groups and therefore do not appear to indicate potential bias.
Selective reporting
For the majority of studies, particularly the older trials, we could not find study protocols. In these cases we assessed whether all the outcomes listed in the methods section of the publication were then reported in the results section.
In most cases, where these study protocols were available, there was no evidence of selective reporting of outcomes relevant to this review.
Other potential sources of bias
We were not aware of other potential sources of bias.
Effects of interventions
Comparison 1: Treadmill (with or without body weight support) versus other intervention
Outcome 1.1: Walking speed (m/s) at the end of the treatment
Forty‐seven studies, with a total of 2323 participants, provided data for walking velocity (metres per second, m/s) at study end (Analysis 1.1).
Overall, the use of treadmill training in walking rehabilitation for people after stroke increased walking velocity significantly. The pooled mean difference (MD, random‐effects model) for walking velocity was 0.06 m/s (95% CI 0.03 to 0.09; P < 0.0001; level of heterogeneity I2 = 44%; moderate‐quality evidence) (Analysis 1.1).
In nine studies, with a total of 752 participants who were dependent in walking at study onset, the use of treadmill training in walking rehabilitation for people after stroke did not increase walking velocity significantly. The pooled mean difference (MD, random‐effects model) for walking velocity was ‐0.01 m/s (95% CI ‐0.06 to 0.03; P = 0.52; level of heterogeneity I2 = 0%; low‐quality evidence) (Analysis 1.1).
In 38 studies, with a total of 1571 participants who were independent in walking at study onset, the use of treadmill training in walking rehabilitation for people after stroke increased walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.08 m/s (95% CI 0.05 to 0.12; P < 0.00001; level of heterogeneity I2 = 49%; low‐quality evidence) (Analysis 1.1).
We found statistically significant subgroup differences in walking velocity between dependent and independent walkers (Chi2 = 11.94, df = 1, P = 0.0005).
Outcome 1.2: Walking endurance (m) at the end of treatment
Twenty‐eight trials, with a total of 1680 participants, provided data for walking endurance (walking capacity; metres (m) walked in six minutes) at study end (Analysis 1.2).
Overall, the use of treadmill training in walking rehabilitation for people after stroke did not increase walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was 14.19 m (95% CI 2.92 to 25.46; P = 0.09; level of heterogeneity I2 = 27%; moderate‐quality evidence) (Analysis 1.2).
In five studies, with a total of 639 participants who were dependent in walking at study onset, the use of treadmill training in walking rehabilitation for people after stroke did not increase walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was ‐5.09 m (95% CI ‐23.41 to 13.22; P = 0.59; level of heterogeneity I2 = 0%; low‐quality evidence) (Analysis 1.2).
In 23 studies, with a total of 1041 participants who were independent in walking at study onset, the use of treadmill training in walking rehabilitation for people after stroke increased walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was 19.72 m (95% CI 6.61 to 32.83; P = 0.003; level of heterogeneity I2 = 27%; low‐quality evidence) (Analysis 1.2).
We found statistically significant subgroup differences in walking endurance between dependent and independent walkers (Chi2 = 4.66, df = 1, P = 0.03).
Comparison 2: Treadmill and body weight support versus other interventions
Outcome 2.1: Dependence on personal assistance to walk at end of the treatment
Nineteen studies, with a total of 1210 participants, measured dependence on personal assistance to walk at the end of the treatment (Analysis 2.1).
Overall, the use of treadmill training with body weight support in walking rehabilitation for people after stroke did not increase the chance of walking independently compared with other physiotherapy interventions (RD 0.00, 95% CI ‐0.02 to 0.02; P = 0.92; level of heterogeneity I2 = 0%) (Analysis 2.1).
In eight studies, with a total of 814 participants who were dependent in walking at study onset, the use of treadmill training with body weight support in walking rehabilitation for people after stroke did not increase the chance of walking independently compared with other physiotherapy interventions (RD ‐0.00, 95% CI ‐0.03 to 0.03; P = 0.92; level of heterogeneity I2 = 0%) (Analysis 2.1).
In 11 studies, with a total of 396 participants who were independent in walking at study onset, the use of treadmill training with body weight support in walking rehabilitation for people after stroke did not increase the chance of walking independently compared with other physiotherapy interventions (RD ‐0.00, 95% CI ‐0.03 to 0.03; P = 1.00; level of heterogeneity I2 = 0%) (Analysis 2.1).
We did not find statistically significant differences between dependent and independent walkers (Chi2 = 0.01, df = 1, P = 0.94).
Outcome 2.2: Walking speed (m/s) at end of the treatment
Twenty‐six studies, with a total of 1410 participants, provided data for walking velocity (metres per second, m/s) at study end (Analysis 2.2).
Overall, the use of treadmill training with body weight support in walking rehabilitation for people after stroke increased walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.07 m/s (95% CI 0.02 to 0.11; P = 0.005; level of heterogeneity I2 = 52%) (Analysis 2.2).
In eight studies, with a total of 738 participants who were dependent in walking at study onset, the use of treadmill training with body weight support in walking rehabilitation for people after stroke did not increase walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was ‐0.01 m/s (95% CI ‐0.06 to 0.03; P = 0.51; level of heterogeneity I2 = 0%) (Analysis 2.2).
In 18 studies, with a total of 672 participants who were independent in walking at study onset, the use of treadmill training with body weight support in walking rehabilitation for people after stroke did increase walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.11 m/s (95% CI 0.06 to 0.17; P < 0.0001; level of heterogeneity I2 = 42%) (Analysis 2.2).
We found statistically significant subgroup differences in walking velocity between dependent and independent walkers (Chi2 = 14.88, df = 1, P = 0.0001).
Outcome 2.3: Walking endurance (m) at end of the treatment
Fifteen trials, with a total of 1062 participants, provided data for walking endurance (walking capacity; metres (m) walked in six minutes) at study end (Analysis 2.3).
Overall, the use of treadmill training with body weight support in walking rehabilitation for people after stroke did not increase walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was 20.79 m (95% CI 0.43 to 41.14; P = 0.05; level of heterogeneity I2 = 51%) (Analysis 2.3).
In five studies, with a total of 639 participants who were dependent in walking at study onset, the use of treadmill training with body weight support in walking rehabilitation for people after stroke did not increase walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was ‐5.09 m (95% CI ‐23.41 to 13.22; P = 0.59; level of heterogeneity I2 = 0%) (Analysis 2.3).
In 10 studies, with a total of 423 participants who were independent in walking at study onset, the use of treadmill training with body weight support in walking rehabilitation for people after stroke increased walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was 36.91 m (95% CI 11.14 to 62.68; P = 0.005; level of heterogeneity I2 = 39%) (Analysis 2.3).
We found statistically significant subgroup differences in walking endurance between dependent and independent walkers (Chi2 = 6.78, df = 1, P = 0.009).
Outcome 2.4: Dependence on personal assistance to walk at end of scheduled follow‐up
Five studies, with a total of 285 participants, measured dependence on personal assistance to walk at the end of scheduled follow‐up (Analysis 2.4).
In two studies, with a total of 170 participants who were dependent in walking at study onset, the use of treadmill training with body weight support in walking rehabilitation for people after stroke did not increase the chance of walking independently compared with other physiotherapy interventions (RD ‐0.02, 95% CI ‐0.18 to 0.15; P = 0.83; level of heterogeneity I2 = 40%) (Analysis 2.4).
In three studies, with a total of 115 participants who were independent in walking at study onset, the use of treadmill training with body weight support in walking rehabilitation for people after stroke did not increase the chance of walking independently compared with other physiotherapy interventions (RD 0.00, 95% CI ‐0.05 to 0.05; P = 1.00; level of heterogeneity I2 = 0%) (Analysis 2.4).
Outcome 2.5: Walking speed (m/s) at end of scheduled follow‐up
Twelve trials, with a total of 944 participants, provided data for walking velocity (metres per second, m/s) at the end of scheduled follow‐up (Analysis 2.5).
Overall, the use of treadmill training with body weight support in walking rehabilitation for people after stroke did not increase walking velocity at the end of scheduled follow‐up significantly. The pooled MD (random‐effects model) for walking velocity was 0.03 m/s (95% CI ‐0.05 to 0.10; P = 0.50; level of heterogeneity I2 = 55%) (Analysis 2.5).
In three studies, with a total of 556 participants who were dependent in walking at the end of scheduled follow‐up, the use of treadmill training with body weight support in walking rehabilitation for people after stroke did not increase walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was ‐0.05 m/s (95% CI ‐0.13 to 0.03; P = 0.20; level of heterogeneity I2 = 0%) (Analysis 2.5).
In nine studies, with a total of 388 participants who were independent in walking at the end of scheduled follow‐up, the use of treadmill training with body weight support in walking rehabilitation for people after stroke did not increase walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.06 m/s (95% CI ‐0.03 to 0.15; P = 0.19; level of heterogeneity I2 = 55%) (Analysis 2.5).
Outcome 2.6: Walking endurance (m) at end of scheduled follow‐up
Ten trials, with a total of 882 participants, provided data for walking endurance (walking capacity; metres (m) walked in six minutes) at the end of scheduled follow‐up (Analysis 2.6).
Overall, the use of treadmill training with body weight support in walking rehabilitation for people after stroke did not increase walking endurance at the end of scheduled follow‐up significantly. The pooled MD (random‐effects model) for walking endurance was 21.64 m (95% CI ‐‐4.70 to 47.98; P = 0.11; level of heterogeneity I2 = 47%) (Analysis 2.6).
In two studies, with a total of 510 participants who were dependent in walking at study onset, the use of treadmill training with body weight support in walking rehabilitation for people after stroke did not increase walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was ‐6.78 m (95% CI ‐34.57 to 21.02; P = 0.63; level of heterogeneity I2 = 0%) (Analysis 2.6).
In eight studies, with a total of 372 participants who were independent in walking at study onset, the use of treadmill training with body weight support in walking rehabilitation for people after stroke did not increase walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was 31.55 m (95% CI 0.57 to 62.53; P = 0.05; level of heterogeneity I2 = 41%) (Analysis 2.6).
Comparison 3: Treadmill training without body weight support versus other interventions
Outcome 3.1: Walking speed (m/s) at the end of the treatment
Twenty trials, with a total of 889 participants who were ambulatory at study onset, provided data for walking velocity (metres per second, m/s) at the end of the treatment (Analysis 3.1).
Overall, the use of treadmill training without body weight support in gait rehabilitation for ambulatory people after stroke increased walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.05 m/s (95% CI 0.01 to 0.09; P = 0.01; level of heterogeneity I2 = 26%) (Analysis 3.1).
Outcome 3.2: Walking endurance (m) at end of treatment
Thirteen trials, with a total of 608 participants, provided data for walking endurance (walking capacity; metres (m) walked in six minutes) at the end of the treatment (Analysis 3.2).
Overall, the use of treadmill training without body weight support in gait rehabilitation for people after stroke did not increase walking endurance significantly. The pooled MD (random‐effects model) for walking velocity was 9.25 m (95% CI ‐1.99 to 20.50; P = 0.11; level of heterogeneity I2 = 0%) (Analysis 3.2).
Comparison 4: Treadmill and body weight support versus treadmill only
In this update of the review, we found only one additional study for this outcome (Srivastava 2016). Only two trials with 99 participants were included in this comparison (Srivastava 2016; Visintin 1998) (more details may be found in Analysis 4.1, Analysis 4.1; Analysis 4.2; Analysis 4.3; Analysis 4.4; Analysis 4.5; Analysis 4.6).
Because there are only sparse data for this comparison, we decided not to pool these studies and to describe the study results without presenting 'totals' and without applying inference tests (Analysis 4.1, Analysis 4.1; Analysis 4.2; Analysis 4.3; Analysis 4.4; Analysis 4.5; Analysis 4.6).
Comparison 5: Adverse events for all included trials
Outcome 5.1: Adverse events during the treatment
Twenty‐four trials, with a total of 1504 participants, provided data for adverse events during the treatment (Analysis 5.1).
Overall, the use of treadmill training with or without body weight support in gait rehabilitation for people after stroke did not increase the risk of adverse events during the treatment (RD (random‐effects model) 0.02, 95% CI ‐0.01 to 0.05; P = 0.14; level of heterogeneity I2 = 51%). The adverse events during the treatment are described in detail for each trial in Table 3.
Comparison 6: Dropouts for all included trials
Outcome 6.1: Dropouts
6.1.1: Dropouts by the end of the treatment
Fifty‐six trials, with a total of 3105 participants, provided data for dropouts at study end (Analysis 6.1).
Overall, the use of treadmill training with or without body weight support in gait rehabilitation for people after stroke did not increase the risk of participants dropping out by the end of the treatment (RD (random‐effects model) 0.00, 95% CI ‐0.01 to 0.01; P = 0.74; level of heterogeneity I² = 0%). The reasons for dropouts and all adverse events during the treatment are described in detail for each trial in Table 3 and Table 4.
6.1.2: Dropouts by the end of scheduled follow‐up (cumulative)
Fourteen trials, with a total of 780 participants, provided data for dropouts by the end of scheduled follow‐up (cumulative) (Analysis 6.1).
Overall, the use of treadmill training with or without body weight support in gait rehabilitation for people after stroke did not increase the risk of participants dropping out by the end of scheduled follow‐up (cumulative) (RD (random‐effects model) ‐0.02, 95% CI ‐0.06 to 0.03; P = 0.47; level of heterogeneity I2 = 0%). The reasons for dropouts are described in detail for each trial in Table 3 and Table 4.
Comparison 7: Sensitivity analysis: by trial methodology
Outcome 7.1: Walking speed (m/s) at the end of the treatment (all trials involving treadmill training)
To examine the robustness of the results, we specified variables (adequate sequence generation process, adequate concealed allocation and blinded assessors for primary outcome) in a sensitivity analysis that we believed could influence the size of the effect observed for walking speed (m/s) at the end of the treatment (Analysis 7.1). We included both participants who were dependent and independent in walking at study onset.
7.1.1: trials with adequate sequence generation process
We included 27 trials, with a total of 1242 participants, that had an adequate sequence generation process (Analysis 7.1). The use of treadmill training in walking rehabilitation for people after stroke increased walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.03 m/s (95% CI 0.00 to 0.06; P = 0.02; level of heterogeneity I2 = 5%).
7.1.2: trials with adequate concealed allocation
We included 21 trials, with a total of 1266 participants, that had adequate concealed allocation (Analysis 7.1). The use of treadmill training in walking rehabilitation for people after stroke increased walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.06 m/s (95% CI 0.01 to 0.10; P = 0.008; level of heterogeneity I2 = 26%).
7.1.3: trials with blinded assessors for the primary outcome
We included 24 trials, with a total of 1554 participants, that had blinded assessors for the primary outcome (Analysis 7.1). The use of treadmill training in walking rehabilitation for people after stroke increased walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.06 m/s (95% CI 0.02 to 0.11; P = 0.008; level of heterogeneity I2 = 38%).
Comparison 8: Subgroup analysis: treadmill (with or without body weight support) versus other, by duration of illness (independent in walking only)
Outcome 8.1: Walking speed (m/s) at the end of the treatment
In our planned subgroup analysis comparing walking speed at the end of the intervention phase in people in the acute and chronic phases of stroke, we arranged all included studies in one of two subgroups (acute and chronic phase).
8.1.1 Acute phase: less than or equal to three months after stroke, independent in walking
Eleven trials, with a total of 347 participants, investigated people in the acute or subacute phase, defined as less than or equal to three months after stroke (Analysis 8.1). The use of treadmill training in walking rehabilitation for people after stroke increased walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.15 m/s (95% CI 0.07 to 0.23; P = 0.0002; level of heterogeneity I2 = 44%).
8.1.2 Chronic phase: more than three months after stroke, independent in walking
Twenty‐six trials, with a total of 1209 participants, investigated people in the chronic phase, defined as more than three months after stroke (Analysis 8.1). The use of treadmill training in walking rehabilitation for people after stroke increased walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.06 m/s (95% CI 0.02 to 0.10; P = 0.001; level of heterogeneity I2 = 39%).
We did not find statistically significant differences in walking velocity between participants treated in the acute/subacute phase compared with participants treated in the chronic phase after stroke (Chi2 = 3.95, df = 1, P = 0.05).
Outcome 8.2: Walking endurance (m) at the end of the treatment
8.2.1 Acute phase: less than or equal to three months after stroke, independent in walking
Five trials, with a total of 178 participants, investigated people in the acute or subacute phase, defined as less than or equal to three months after stroke (Analysis 8.2). The use of treadmill training in walking rehabilitation for people after stroke increased walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was 48.6 m (95% CI 23.97 to 73.32; P = 0.0001; level of heterogeneity I² = 6%).
8.2.2 Chronic phase: more than three months after stroke, independent in walking
Eighteen trials, with a total of 863 participants, investigated people in the chronic phase, defined as more than three months after stroke (Analysis 8.2). The use of treadmill training in walking rehabilitation for people after stroke did not increase walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was 10.69 m (95% CI ‐0.28 to 21.66; P = 0.06; level of heterogeneity I² = 2%).
We found statistically significant differences in walking endurance between participants treated in the acute/subacute phase compared with participants treated in the chronic phase after stroke (Chi2 = 7.59, df = 1, P = 0.006).
Comparison 9: Subgroup analysis: treadmill (with or without body weight support) versus other interventions, by intensity (frequency) of training (independent in walking only)
In our planned subgroup analysis comparing walking speed at the end of the intervention phase at different intensities (frequencies) of training, we arranged all included studies in one of three subgroups (treadmill training five times per week or more, three to four times per week, less than three times per week or unclear frequency).
Outcome 9.1: Walking speed (m/s) at the end of the treatment
9.1.1 Treadmill training five times per week or more
Nineteen trials, with a total of 671 participants, investigated people with an intensity (frequency) of training of five times per week or more (Analysis 9.1). The use of treadmill training in walking rehabilitation for people after stroke increased walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.04 m/s (95% CI 0.02 to 0.07; P = 0.0004; level of heterogeneity I² = 64%).
9.1.2 Treadmill training three to four times per week
Sixteen trials, with a total of 784 participants, investigated people with an intensity (frequency) of training three to four times per week (Analysis 9.1). The use of treadmill training in walking rehabilitation for people after stroke increased walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.08 m/s (95% CI 0.03 to 0.12; P = 0.0008; level of heterogeneity I² = 22%).
9.1.3 Treadmill training less than three times per week or unclear frequency
Three trials, with a total of 116 participants, investigated people with an intensity (frequency) of training less than three times a week (Analysis 9.1). The use of treadmill training in walking rehabilitation for people after stroke did not increase walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.02 m/s (95% CI ‐0.06 to 0.10; P = 0.61; level of heterogeneity I² = 0%).
We did not find statistically significant differences in walking velocity between participants treated at different intensities of training (Chi2 = 2.09, df = 2, P = 0.35).
Outcome 9.2: walking endurance (m) at the end of the treatment
9.2.1 Treadmill training five times per week
Nine trials, with a total of 392 participants, investigated people with an intensity (frequency) of training of five times a week or more (Analysis 9.2). The use of treadmill training in walking rehabilitation for people after stroke increased walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was 27.25 m (95% CI 5.37 to 49.13; P = 0.01; level of heterogeneity I² = 45%).
9.2.2 Treadmill training three to four times per week
Thirteen trials, with a total of 621 participants, investigated people with an intensity (frequency) of training of three to four times per week (Analysis 9.2). The use of treadmill training in walking rehabilitation for people after stroke did not increase walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was 12.41 m (95% CI ‐3.15 to 27.97; P = 0.12; level of heterogeneity I² = 10%).
9.2.3 Treadmill training less than three times per week or unclear
One trial, with a total of 28 participants, investigated people with an intensity (frequency) of training of less than three times a week (Analysis 9.2). The use of treadmill training in walking rehabilitation for people after stroke did not increase walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was ‐15.00 m (95% CI ‐133.26 to 103.26; P = 0.80; level of heterogeneity not applicable).
We did not find statistically significant differences in walking endurance between participants treated at different intensities of training (Chi2 = 1.46, df = 2, P = 0.48).
Comparison 10: Subgroup analysis: treadmill (with or without body weight support) versus other interventions, by duration of training period (independent in walking only)
In our planned subgroup analysis comparing walking speed at the end of the intervention phase after different durations of treatment, we arranged all included studies into one of three subgroups (treadmill training duration of more than four weeks, equal to four weeks or less than four weeks).
Outcome 10.1 Walking speed (m/s) at the end of the treatment
10.1.1 Treadmill training duration of more than four weeks
Fourteen trials, with a total of 802 participants, investigated people with a duration of training of more than four weeks (Analysis 10.1). The use of treadmill training in walking rehabilitation for people after stroke increased walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.05 m/s (95% CI 0.01 to 0.09; P = 0.02; level of heterogeneity I2 = 0%).
10.1.2 Treadmill training duration of four weeks
Thirteen trials, with a total of 404 participants, investigated people with a duration of training of four weeks (Analysis 10.1). The use of treadmill training in walking rehabilitation for people after stroke increased walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.13 m/s (95% CI 0.07 to 0.19; P < 0.0001; level of heterogeneity I2 = 30%).
10.1.3 Treadmill training duration of less than four weeks
Eleven trials, with a total of 365 participants, investigated people with a duration of training of less than four weeks (Analysis 10.1). The use of treadmill training in walking rehabilitation for people after stroke increased walking velocity significantly. The pooled MD (random‐effects model) for walking velocity was 0.08 m/s (95% CI 0.01 to 0.14; P = 0.03; level of heterogeneity I2 = 63%).
We found statistically significant differences in walking velocity between participants treated with training for different durations (Chi2 = 8.68, df = 2, P = 0.01).
Outcome 10.2: Walking endurance (m) at the end of the treatment
In our planned subgroup analysis comparing walking endurance at the end of the intervention phase after different durations of treatment, we arranged all included studies into one of three subgroups (treadmill training duration of more than four weeks, equal to four weeks, or less than four weeks).
10.2.1 Treadmill training duration of more than four weeks
Twelve trials, with a total of 706 participants, investigated people with a duration of training of more than four weeks (Analysis 10.2). The use of treadmill training in walking rehabilitation for people after stroke increased walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was 19.09 m (95% CI 2.29 to 35.88; P = 0.03; level of heterogeneity I2 = 0%).
10.2.2 Treadmill training duration of four weeks
Five trials, with a total of 146 participants, investigated people with a duration of training of four weeks (Analysis 10.2). The use of treadmill training in walking rehabilitation for people after stroke did not increase walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was 29.40 m (95% CI ‐4.75 to 63.54; P = 0.09; level of heterogeneity I2 = 65%).
10.2.3 Treadmill training duration of less than four weeks
Four trials, with a total of 129 participants, investigated people with a duration of training of less than four weeks (Analysis 10.2). The use of treadmill training in walking rehabilitation for people after stroke did not increase walking endurance significantly. The pooled MD (random‐effects model) for walking endurance was 9.82 m (95% CI ‐15.48 to 35.13; P = 0.45; level of heterogeneity I2 = 13%).
We did not find statistically significant differences in walking endurance between participants treated with training for different durations (Chi2 = 0.85, df = 2, P = 0.66).
Other outcomes
We did not analyse the secondary outcomes of participant quality of life, ability to perform activities of daily living, and the combined outcomes of death or dependency, and death or institutional care either because these variables were not reported or due to insufficient data in many of the included studies.
We did not perform the planned subgroup analyses for the types of co‐interventions implemented in conjunction with treadmill training due to insufficient data.
Discussion
Summary of main results
The aim of this review was to evaluate the effect of treadmill training and body weight support, individually or in combination, for walking after stroke. We included 56 trials with 3105 participants in this update. Overall, the use of treadmill training with body weight support did not increase the chance of walking independently compared with people after stroke receiving other physiotherapy interventions, but not treadmill training. The use of treadmill training with body weight support in walking rehabilitation for people after stroke did increase the walking velocity and walking endurance significantly compared with other physiotherapy interventions.
Overall, treadmill training with or without body weight support produced statistically significant higher walking speed and endurance, 0.06 m/s and 14 m respectively, compared with people not receiving treadmill training. For people who could walk independently at the start of treatment, treadmill training with or without body weight support produced statistically significant higher walking speed and endurance, 0.09 m/s and 20 m respectively, compared with people not receiving treadmill training. These results raise the question: how clinically relevant are these statistically significant effects?
For people after stroke, Flansbjer 2005 described the smallest possible change (the standard error of measurement (SEM) and the smallest real clinical differences (95% SRD). The SEMs and the 95% SRDs for walking speed were 0.07 m/s and 0.15 to 0.25 m/s and the SEMs and the 95% SRDs for walking endurance were 18.6 m and 37 to 66 m. Our results might, according to Flansbjer 2005, be interpreted as follows: the overall effects of treadmill training, with or without body weight support, can not be measured in practice and should not be interpreted as a clinically relevant improvement.
We did not find any benefit for people after stroke who could not walk independently at the start of treatment. We did not find enough studies of the effects of treadmill training, with or body weight support, on activities and quality of life to draw any appropriate conclusions, nor did we find enough studies of the effects of body weight support without treadmill training to draw any appropriate conclusions.
Adverse events and dropouts did not occur more frequently in people receiving treadmill training and these were not judged to be clinically serious events.
Our subgroup analysis showed that, for people after stroke who walked independently, treadmill training in the first three months after stroke produced walking speeds that were statistically but not clinically relevant (Flansbjer 2005). For people treated in the chronic phase, the effects on walking speed were lower (and not clinically relevant). However, the subgroup differences did not differ significantly.
Our subgroup analysis showed that, for people after stroke who walked independently, treadmill training in the first three months after stroke produced a walking endurance that was statistically and clinically relevant (Flansbjer 2005). For people treated in the chronic phase, the effects on walking endurance were lower (not clinically relevant). The subgroup differences did differ significantly, indicating that people treated in the first three months after stroke had higher gains in walking endurance compared with training in the chronic phase after stroke.
Our subgroup analysis showed that, for people after stroke who walked independently, treadmill training with higher intensities (frequency of training: five times versus three to four times versus less than three times per week) may produce greater effects on walking speed and endurance. However, this trend toward subgroup differences was not significant.
Possible conclusions based on our findings are that treadmill training can be used when people after stroke can walk independently and when improvement of walking speed and endurance is the aim of therapy. The greatest effect of treadmill training is to be expected in the first three months after stroke. It was, however, not absolutely clear from this review if therapists should apply particular periods or particular frequencies of treatments, for example, training for five times a week or for four weeks.
Overall completeness and applicability of evidence
The results of this review seem to be quite generalisable to inpatient settings in industrialised countries. However, there are factors producing uncertainty for generalisations.
-
The investigated study population was quite heterogeneous (e.g. age, time post‐stroke, severity of stroke and especially walking ability).
-
The investigated experimental and control conditions were heterogeneous (e.g. type of training, frequency, and duration of training; some studies had no active control group or were compared with no intervention).
Hence, the results may be of limited applicability for all people after stroke.
Quality of the evidence
We found heterogeneity regarding trial design (parallel‐group or cross‐over design, two or more intervention groups), but it is not clear if this could have limited the quality of the evidence. Furthermore, in our sensitivity analysis examining the effects of methodological quality on the effectiveness of the intervention, we found that the benefits (improving walking speed) were relatively robust when we removed trials with an inadequate sequence generation process, inadequate concealed allocation, and no blinded assessors for the primary outcome (Analysis 7.1).
Although the methodological quality of the included trials generally seemed moderate (Figure 2), trials investigating treadmill training with or without body weight support are subject to potential methodological limitations. These limitations included inability to blind the therapist and participants, so‐called contamination (provision of the intervention to the control group), and co‐intervention (when the same therapist unintentionally provided additional care to either treatment or comparison group). All these potential methodological limitations introduced the possibility of performance bias. However, as discussed previously, this was not supported in our sensitivity analyses by methodological quality.
Potential biases in the review process
The methodological rigour of Cochrane reviews minimises bias in the process of conducting systematic reviews. We are confident that our detailed search strategy, combined with detailed handsearching efforts, identified all relevant trials. It is possible that we did not identify studies published in the grey literature, but it would be unlikely that this would have a significant impact on our results. Because the grey literature tends to include trials with relatively small numbers of participants and inconclusive results, inclusion of this literature may have actually decreased the size of the effect detected in our review (McAuley 2000).
Another potential source for the introduction of bias could have been that one of the review authors (JM) was involved in conducting and analysing one of the included trials (Pohl 2002). However, the third review author (BE) extracted the outcome data from raw data and described the risk of bias of this trial. Excluding Pohl 2002 from the pooled analyses did not change the results significantly, so we believe that this one trial has not biased our overall evidence.
Agreements and disagreements with other studies or reviews
There are several recent reviews about treadmill training, with or without body weight support; for example, two reviews were published in 2013 (Charalambous 2013; Polese 2013).
The review of Polese 2013 included nine studies of treadmill training with 977 participants and concluded that treadmill training resulted in faster walking than no intervention or a non‐walking intervention immediately after the intervention period (MD 0.14 m/s, 95% CI 0.09 to 0.19). The review of Charalambous 2013 included 15 studies of treadmill training and concluded that treadmill‐based interventions post‐stroke may increase and retain walking speed, but a pooled analysis with forest plots was not provided. In comparison, we found more studies (44 studies included in this update) than in the reviews of Charalambous 2013 and Polese 2013 and we found smaller effects on walking speed, MD 0.07 m/s, 95% CI 0.03 to 0.11 (based on 35 included studies of treadmill training with 1891 participants). These differences could be due to the comprehensive search in our review update and to our inclusion of studies not published in English. This update is the most comprehensive review about the topic to date.
In this update of the review, we have found significant effects for walking velocity and endurance but not for dependence, and we also found that people who have had a stroke and who can walk independently profit more from treadmill training than those who cannot walk. Initially, this might be difficult to interpret. However, we believe that the overall results of this review were somewhat 'confounded' by the results of people who could not walk. We found evidence that this participant group may not profit from treadmill training. Treadmill training appears, therefore, to be an appropriate adjunct intervention that might improve certain important walking parameters, such as speed and endurance for people who are already able walk alone. This might appear a little ironic to researchers because treadmill training with body weight support was designed to get non‐ambulatory walkers walking. Another Cochrane review found evidence that the chance of regaining independent walking ability after stroke increases when electromechanical and robotic‐assisted gait‐training devices are used in combination with physiotherapy (Mehrholz 2017). Interestingly, whereas independent walking improved, neither walking velocity nor walking capacity improved. Perhaps one conclusion could be that different interventions are suitable for different participants. For example, for severely affected people who cannot walk independently, electromechanical and robotic‐assisted gait‐training devices in combination with physiotherapy are recommended (Mehrholz 2017). However, when people who have had a stroke recover and start walking, then treadmill training may improve important walking parameters such as speed and endurance, as our update showed. Therefore, the combination of approaches should be considered.
Finally, it should be mentioned that treadmill training in and of itself is perhaps not the 'main issue'. We believe that treadmill training just offers a very easy approach for high‐intensity, repetitive, task‐specific walking training, which is recommended for gait rehabilitation (Langhorne 2009).

Flow diagram. Please note that the number of full‐texts is not necessarily equal to the number of studies that means that there often are several full‐texts of a single trial (e.g. as is the case for Ada 2003 or DEGAS 2007).
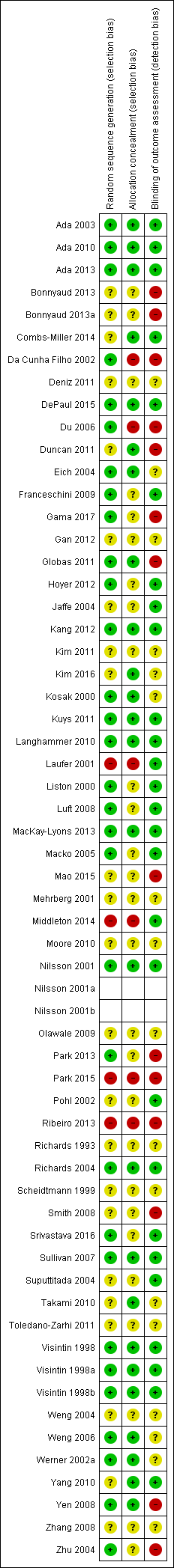
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
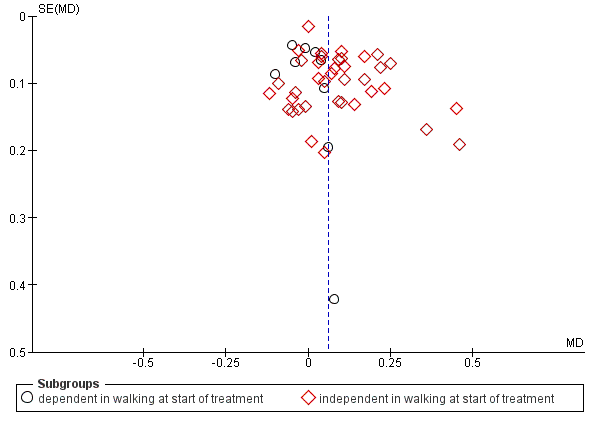
Funnel plot of comparison: 1 Treadmill (with or without body weight support) versus other intervention, outcome: 1.1 Walking speed (m/s) at end of treatment.

Funnel plot of comparison: 1 Treadmill (with or without body weight support) versus other intervention, outcome: 1.2 Walking endurance (m) at end of treatment.
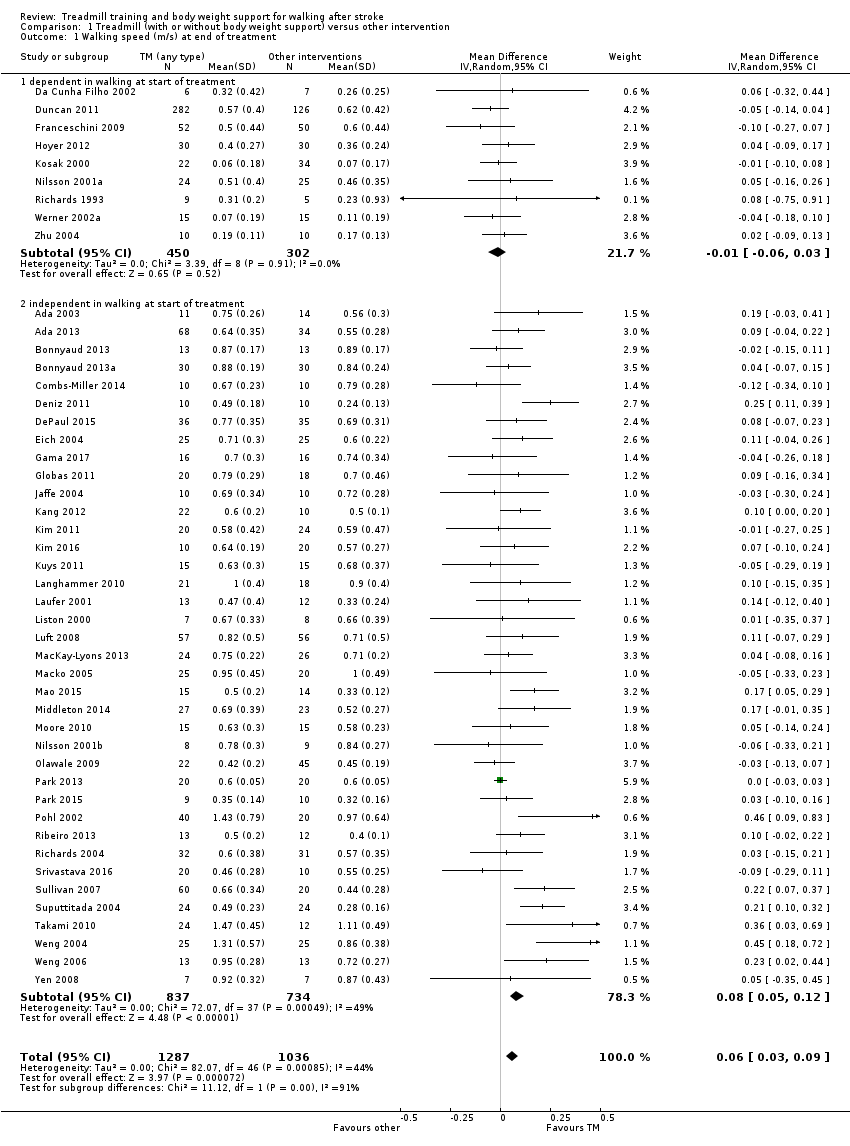
Comparison 1 Treadmill (with or without body weight support) versus other intervention, Outcome 1 Walking speed (m/s) at end of treatment.

Comparison 1 Treadmill (with or without body weight support) versus other intervention, Outcome 2 Walking endurance (m) at end of treatment.

Comparison 2 Treadmill and body weight support versus other interventions, Outcome 1 Dependence on personal assistance to walk at end of treatment.

Comparison 2 Treadmill and body weight support versus other interventions, Outcome 2 Walking speed (m/s) at end of treatment.
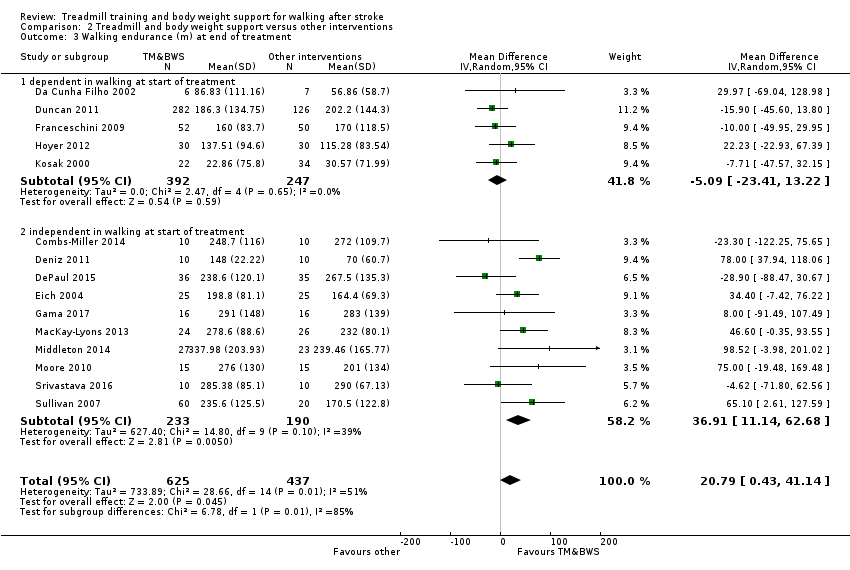
Comparison 2 Treadmill and body weight support versus other interventions, Outcome 3 Walking endurance (m) at end of treatment.
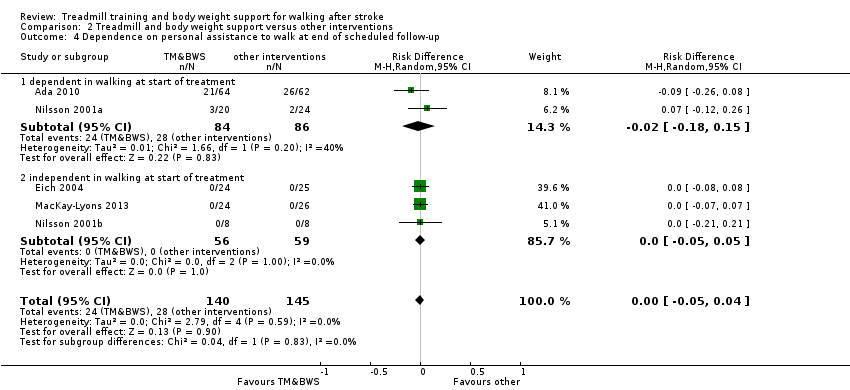
Comparison 2 Treadmill and body weight support versus other interventions, Outcome 4 Dependence on personal assistance to walk at end of scheduled follow‐up.
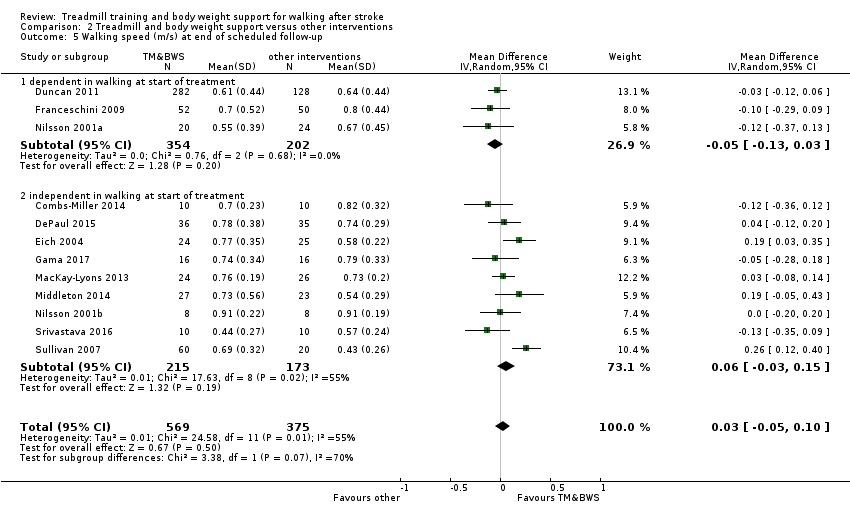
Comparison 2 Treadmill and body weight support versus other interventions, Outcome 5 Walking speed (m/s) at end of scheduled follow‐up.

Comparison 2 Treadmill and body weight support versus other interventions, Outcome 6 Walking endurance (m) at end of scheduled follow‐up.
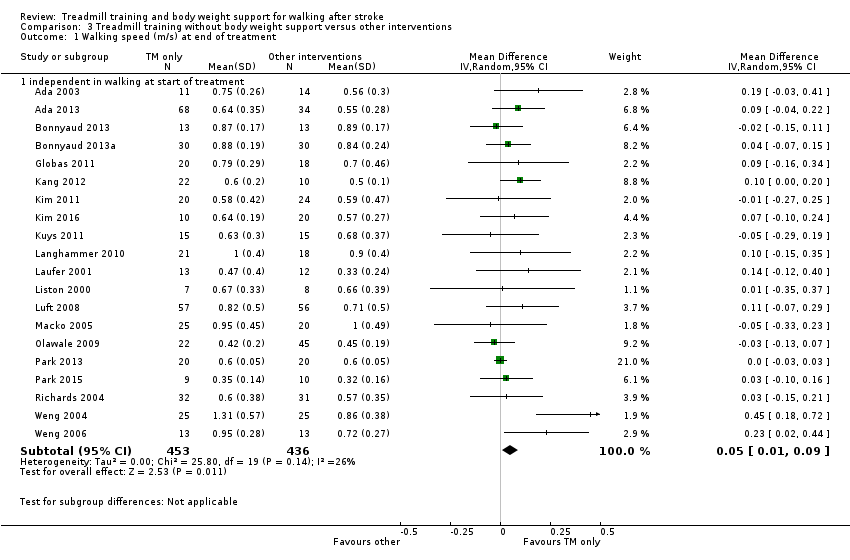
Comparison 3 Treadmill training without body weight support versus other interventions, Outcome 1 Walking speed (m/s) at end of treatment.

Comparison 3 Treadmill training without body weight support versus other interventions, Outcome 2 Walking endurance (m) at end of treatment.

Comparison 4 Treadmill and body weight support versus treadmill only, Outcome 1 Dependence on personal assistance to walk at end of treatment.

Comparison 4 Treadmill and body weight support versus treadmill only, Outcome 2 Walking speed (m/s) at end of treatment.
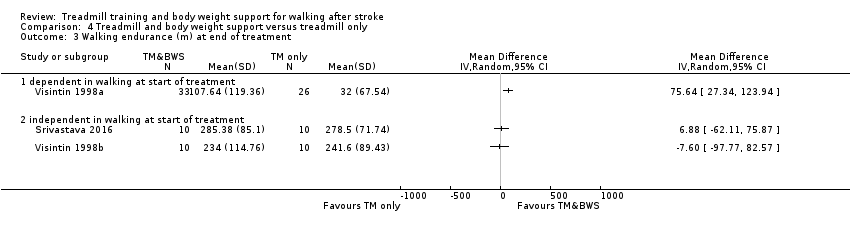
Comparison 4 Treadmill and body weight support versus treadmill only, Outcome 3 Walking endurance (m) at end of treatment.

Comparison 4 Treadmill and body weight support versus treadmill only, Outcome 4 Dependence on personal assistance to walk at end of scheduled follow‐up.
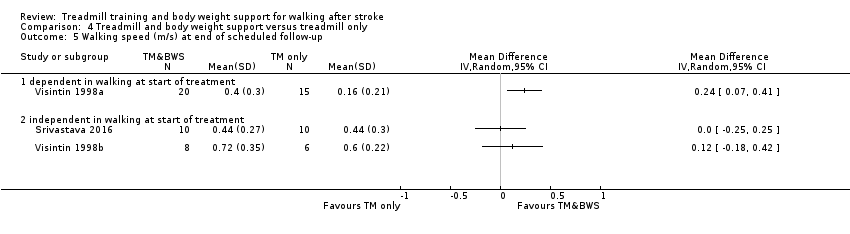
Comparison 4 Treadmill and body weight support versus treadmill only, Outcome 5 Walking speed (m/s) at end of scheduled follow‐up.

Comparison 4 Treadmill and body weight support versus treadmill only, Outcome 6 Walking endurance (m) at end of scheduled follow‐up.

Comparison 5 Adverse events for all included trials, Outcome 1 Adverse events during the treatment.

Comparison 6 Dropouts for all included trials, Outcome 1 Dropouts.
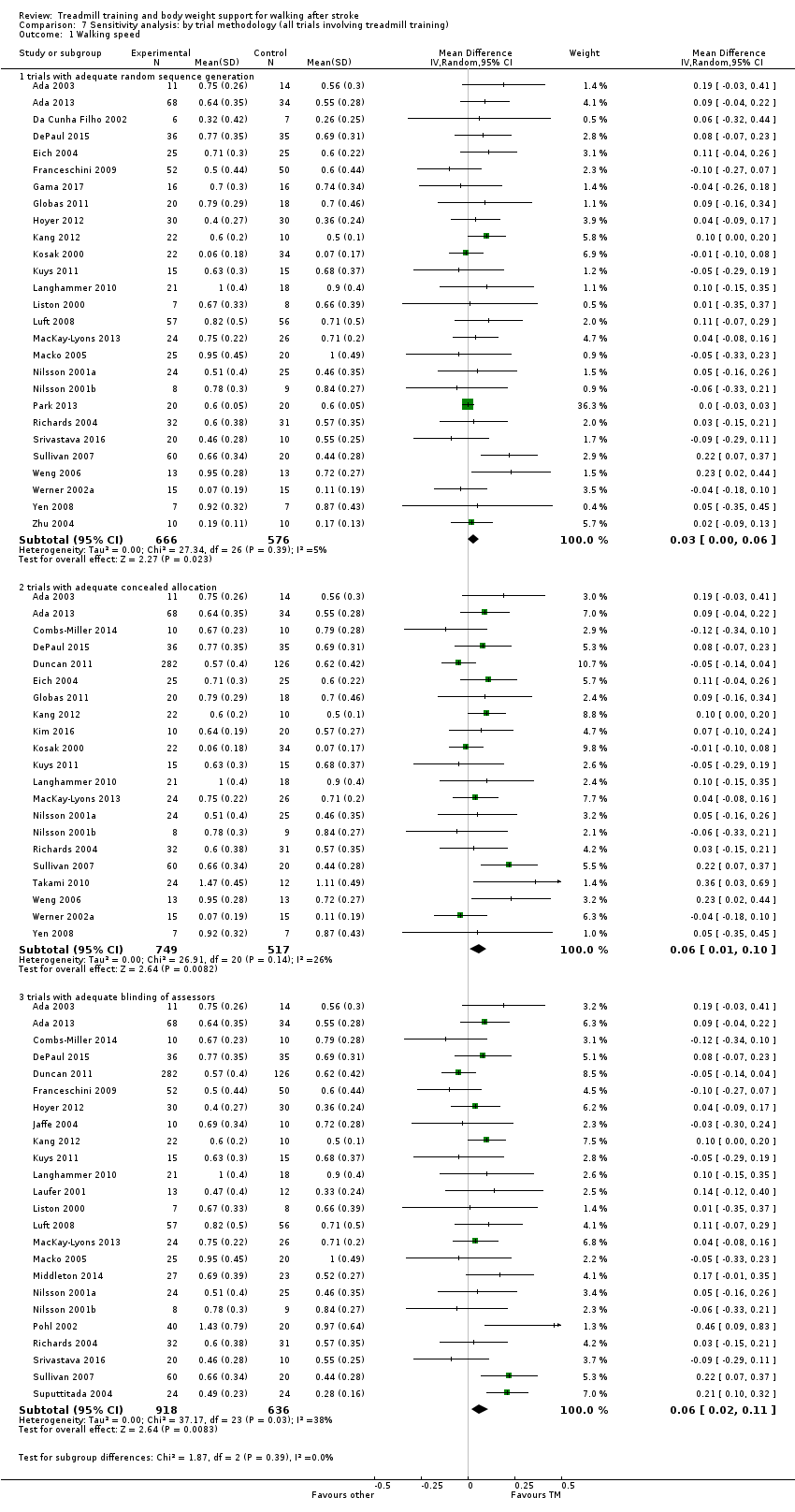
Comparison 7 Sensitivity analysis: by trial methodology (all trials involving treadmill training), Outcome 1 Walking speed.
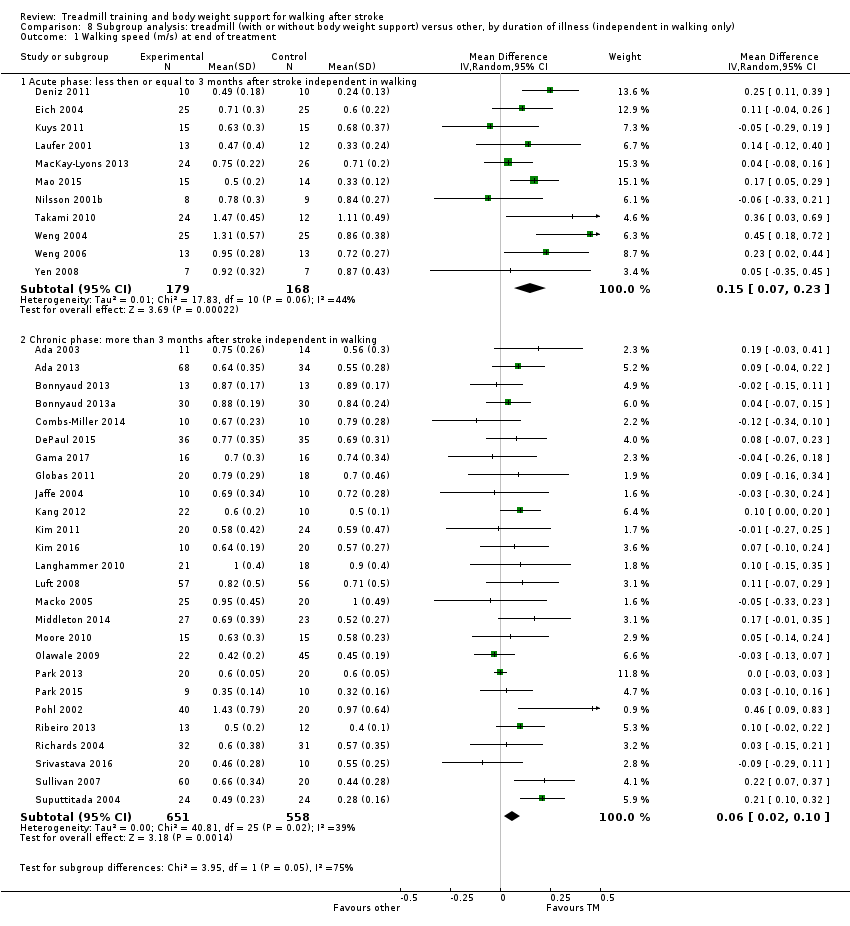
Comparison 8 Subgroup analysis: treadmill (with or without body weight support) versus other, by duration of illness (independent in walking only), Outcome 1 Walking speed (m/s) at end of treatment.

Comparison 8 Subgroup analysis: treadmill (with or without body weight support) versus other, by duration of illness (independent in walking only), Outcome 2 Walking endurance (m) at end of treatment.
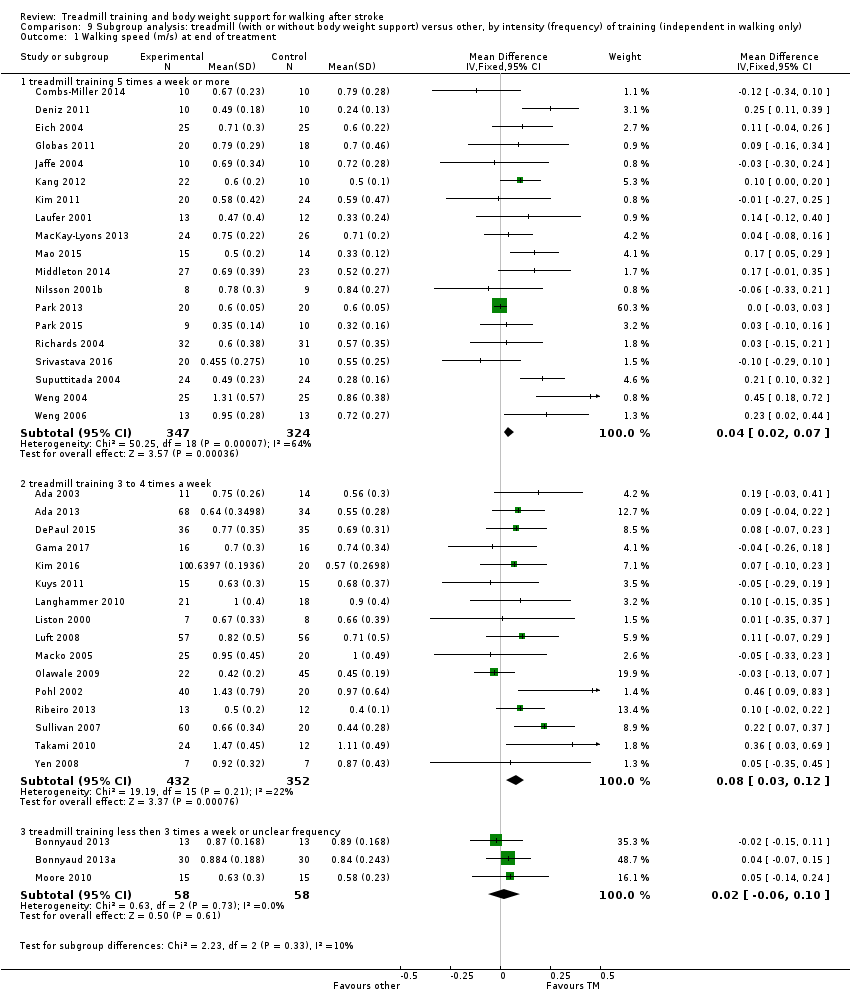
Comparison 9 Subgroup analysis: treadmill (with or without body weight support) versus other, by intensity (frequency) of training (independent in walking only), Outcome 1 Walking speed (m/s) at end of treatment.
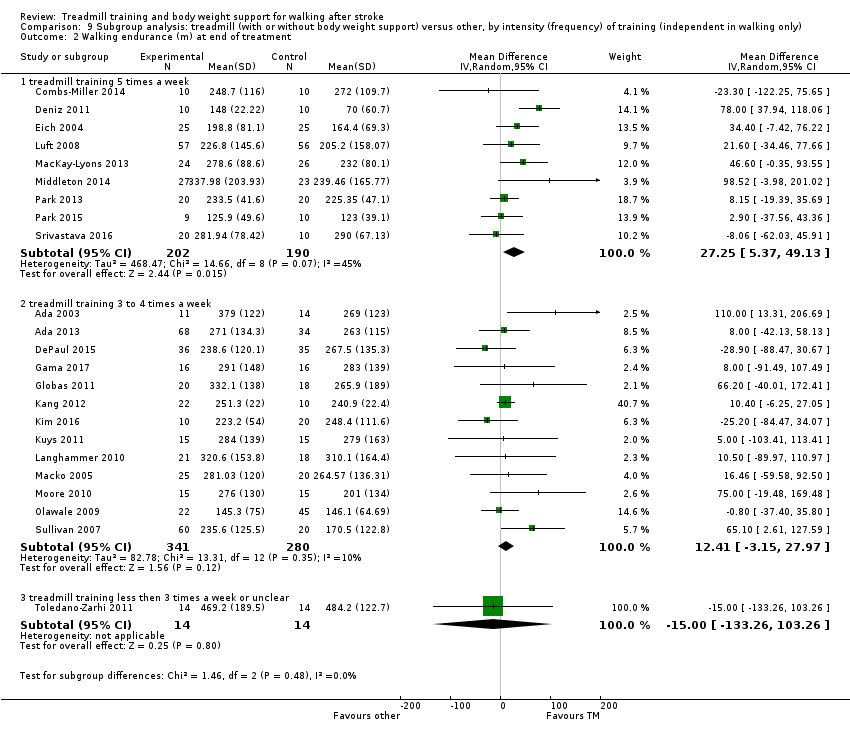
Comparison 9 Subgroup analysis: treadmill (with or without body weight support) versus other, by intensity (frequency) of training (independent in walking only), Outcome 2 Walking endurance (m) at end of treatment.

Comparison 10 Subgroup analysis: treadmill (with or without body weight support) versus other, by duration of training period (independent in walking only), Outcome 1 Walking speed (m/s) at end of treatment.
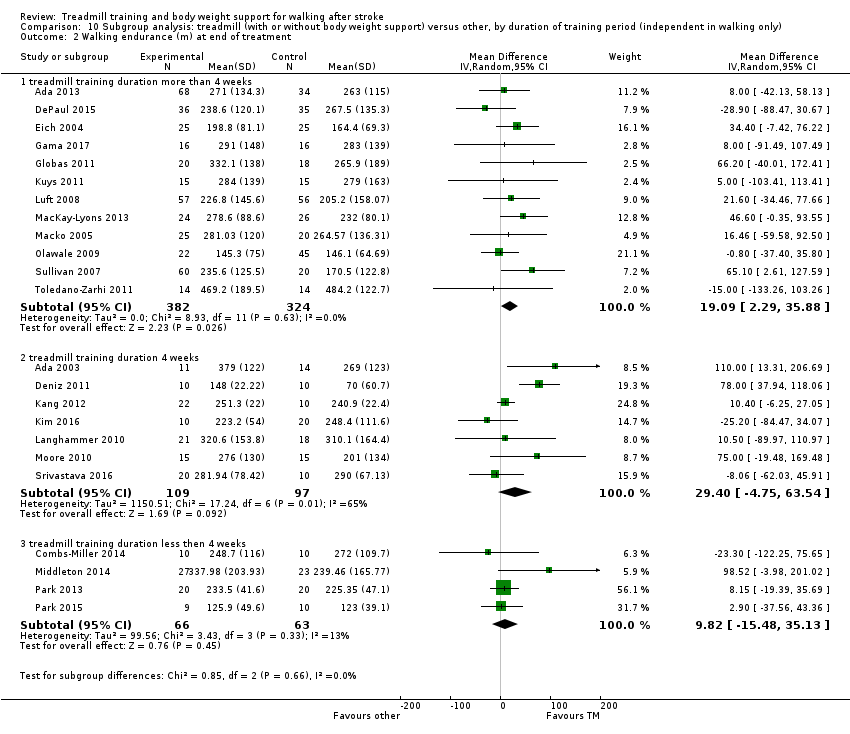
Comparison 10 Subgroup analysis: treadmill (with or without body weight support) versus other, by duration of training period (independent in walking only), Outcome 2 Walking endurance (m) at end of treatment.
| Treadmill (with or without body weight support) versus other intervention for walking after stroke | ||||||
| Patient or population: adults who had suffered a stroke and exhibited an abnormal gait pattern | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Treadmill (with or without body weight support) versus other intervention | |||||
| Dropouts ‐ by end of treatment | Study population | See comment | 3105 | ⊕⊕⊝⊝ | Risks were calculated from pooled risk differences | |
| 91 per 1000 | 93 per 1000 | |||||
| Moderate | ||||||
| 31 per 1000 | 32 per 1000 | |||||
| Walking speed (m/s) at end of treatment | The mean walking speed (m/s) at end of treatment in the control groups was | The mean walking speed (m/s) at end of treatment in the intervention groups was | 2323 | ⊕⊕⊕⊝ | ||
| Walking speed (m/s) at end of treatment ‐ dependent in walking at start of treatment | The mean walking speed (m/s) at end of treatment ‐ dependent in walking at start of treatment in the control groups was | The mean walking speed (m/s) at end of treatment ‐ dependent in walking at start of treatment in the intervention groups was | 752 | ⊕⊕⊝⊝ | ||
| Walking speed (m/s) at end of treatment ‐ independent in walking at start of treatment | The mean walking speed (m/s) at end of treatment ‐ independent in walking at start of treatment in the control groups was | The mean walking speed (m/s) at end of treatment ‐ independent in walking at start of treatment in the intervention groups was | 1571 | ⊕⊕⊝⊝ | ||
| Walking endurance (m) at end of treatment | The mean walking endurance (m) at end of treatment in the control groups was | The mean walking endurance (m) at end of treatment in the intervention groups was | 1680 | ⊕⊕⊕⊝ | ||
| Walking endurance (m) at end of treatment ‐ dependent in walking at start of treatment | The mean walking endurance (m) at end of treatment ‐ dependent in walking at start of treatment in the control groups was | The mean walking endurance (m) at end of treatment ‐ dependent in walking at start of treatment in the intervention groups was | 639 | ⊕⊕⊝⊝ | ||
| Walking endurance (m) at end of treatment ‐ independent in walking at start of treatment | The mean walking endurance (m) at end of treatment ‐ independent in walking at start of treatment in the control groups was | The mean walking endurance (m) at end of treatment ‐ independent in walking at start of treatment in the intervention groups was | 1041 | ⊕⊕⊝⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded because 95% CI contains effect size of no difference and the minimal important difference. | ||||||
| Study ID | EXP age | CTL age | EXP gender | CTL gender | EXP time post‐stroke | CTL time post‐stroke | EXP paresis side | CTL paresis side |
| Mean 66 (SD 11) years (excluding 1 dropout) | Mean 66 (SD 11) years (excluding 1 dropout) | Men/women 9/4 | Men/women 10/4 | Mean 28 (SD 17) months | Mean 26 (SD 20) months | Left/right 5/8 | Left/right 8/6 | |
| Mean 70 (SD 9) years | Mean 71 (SD 9) years | Men/women 38/26 | Men/women 33/29 | Mean 18 (SD 8) days | Mean 18 (SD 7) days | Left/right 34/30 | Left/right 36/26 | |
| Mean 67 (SD 12) years | Mean 63 (SD 13) years | Men/women 52/16 | Men/women 19/15 | Mean 21 (SD 16) months | Mean 19 (SD 13) months | Left/right 32/34 | Left/right 13/21 | |
| Mean 50 (SD 13) years (including both groups) | Men/women 45/15 (including both groups) | Mean 6 (SD 6) years (including both groups) | Left/right 30/30 (including both groups) | |||||
| Mean 50 (SD 13) years (including both groups) | Men/women 45/15 (including both groups) | Mean 6 (SD 6) years (including both groups) | Left/right 30/30 (including both groups) | |||||
| Mean 45 (SD 21) years | Mean 48 (SD 10) years | Men/women 8/4 | Men/women 10/3 | Mean 6 (SD 6) years | Mean 5 (SD 4) years | Left/right 8/4 | Left/right 8/5 | |
| Mean 56 (SD 8) years | Mean 64 (SD 6) years | Men/women 4/6 | Men/women 7/3 | Mean 62 (SD 49) months | Mean 60 (SD 52) months | Left/right 6/4 | Left/right 6/4 | |
| Mean 57.8 (SD 5.5) years (excluding dropouts) | Mean 58.9 (SD 12.9) years (excluding dropouts) | Men/women 6/0 | Men/women 7/0 | Mean 15.7 (SD 7.7) days | Mean 19.0 (SD 12.7) days | Left/right/bilateral 1/4/1 | Left/right 4/3 | |
| Mean 61.5 (SD 4.7) years | Mean 61.5 (SD 12.5) years | Men/women 8/2 | Men/women 3/7 | Mean 71 (SD 40) days | Mean 81 (SD 47) months | Left/right 6/4 | Left/right 3/7 | |
| Mean 62 (SD 13) years | Mean 61.5 (SD 4.7) years | Men/women 21/14 | Men/women 22/14 | Median 19 (Q1 7, Q2 34) weeks | Median 18 (Q1 10, Q3 30) weeks | Left/right/bilateral 20/12/3 | Left/right/bilateral 17/18/1 | |
| 56 (6) years | 58 (6) years | Men/women 35/32 | Men/women 30/31 | < 3 months | < 3 months | Left/right 31/36 | Left/right 29/32 | |
| Mean 62 (SD 12) years | Mean 63 (SD 13) years | Men/women 159/123 | Men/women 65/61 | Mean 64 (SD 9) days | Mean 63 (SD 8) days | Left/right 121/161 | Left/right 61/65 | |
| Mean 62.4 (SD 4.8) years (all participants) | Mean 64.0 (SD 6.0) years (all participants) | Men/women 17/8 | Men/women 16/9 | Mean 6.1 (SD 2.2) weeks | Mean 6.3 (SD 2.5) weeks | Left/right 14/11 | Left/right 14/11 | |
| Mean 66 (SD 12) years | Mean 71 (SD 12) years | Men/women 28/24 | Men/women 22/23 (only 45 described) | Mean 17 (SD 10) days | Mean 14 (SD 7) days | Left/right 29/23 | Left/right 15/30 (only 45 described) | |
| Mean 59 (SD 8) years | Mean 58 (SD 10) years | Men/women 7/7 (only 14 described) | Men/women 8/6 (only 14 described) | Mean 60 (SD 55) months | Mean 54 (SD 42) months | Left/right 9/5 (only 14 described) | Left/right 6/8 (only 14 described) | |
| Not described | Not described | Not described | Not described | Not described | Not described | Not described | Not described | |
| Mean 69 (SD 7) years | Mean 69 (SD 6) years | Men/women 14/4 (only 18 described) | Men/women 15/3 (only 18 described) | Mean 60 (SD 47) months | Mean 70 (SD 67) months | Left/right 4/14 (only 18 described) | Left/right 9/9 (only 18 described) | |
| Mean 52 (SD 13) years | Mean 52 (SD 6) years | Men/women 20/10 | Men/women 18/12 | Mean 99 (SD 39) days | Mean 96 (SD 42) days | Left/right 17/13 | Left/right 17/13 | |
| Mean 58.2 (SD 11.2) years (excluding dropouts) | Mean 63.2 (SD 8.3) years (excluding dropouts) | Men/women 5/5 (excluding dropouts) | Men/women 7/3 (excluding dropouts) | Mean 3.9 (SD 2.3) years (excluding dropouts) | Mean 3.6 (SD 2.6) years (excluding dropouts) | Left/right 6/4 (excluding dropouts) | Left/right 4/6 (excluding dropouts) | |
| Mean 56 (SD 7) years | Mean 56 (SD 8) years | Men/women 10/10 (excluding dropouts) | Men/women 6/4 (excluding dropouts) | Mean 14 (SD 4) months | Mean 15 (SD 7) months | Left/right 8/12 (excluding dropouts) | Left/right 5/5 (excluding dropouts) | |
| Mean 51 (SD 4) years | Mean 50 (SD 8) years | Men/women 11/9 | Men/women 14/10 | Mean 15 (SD 6) months | Mean 14 (SD 3) months | Left/right 8/12 | Left/right 8/16 | |
| Mean 56.20 (SD 7.56) years | Mean 52.00 (SD 7.27) years | Men/women 4/6 | Men/women 5/5 | Mean 7.5 (SD 4.4) months | Mean 13.3 (SD 16.1) months | Left/right 3/7 | Left/right 4/6 | |
| Mean 74 (SEM 2) years (all participants) | Mean 70 (SEM 2) years | Men/women 13/9 | Men/women 18/16 | Mean 39 (SEM 3) days | Mean 40 (SEM 4) days | Left/right/bilateral 8/12/2 | Left/right/bilateral 12/16/6 | |
| Mean 63 (SD 14) years | Mean 72 (SD 17) years | Men/women 8/7 | Men/women 6/9 | Mean 52 (SD 32) days (excluding dropouts) | Mean 49 (SD 30) days (excluding dropouts) | Left/right 6/9 | Left/right 11/4 | |
| Mean 74 (SD 13) years | Mean 75 (SD 10) years | Men/women 10/11 | Men/women 6/12 | Mean 419 (SD 1034) days | Mean 349 (SD 820) days | Left/right 15/6 | Left/right 13/5 | |
| Mean 66.6 (SD 7.2) years (excluding dropouts) | Mean 69.3 (SD 8.1) years (excluding dropouts) | Men/women 7/6 | Men/women 7/5 | Mean 32.6 (SD 21.2) days | Mean 35.8 (SD 17.3) days | Left/right 5/8 | Left/right 5/7 | |
| Mean 79.1 (SD 6.8) years (all EXP and CTL participants) | Men/women 12/6 | Not reported | Not reported | Not reported | Not reported | |||
| Mean 64 (SD 10) years | Mean 63 (SD 9) years | Men/women 14/20 (excluding dropouts) | Men/women 19/18 (excluding dropouts) | Mean 55 months (excluding dropouts) | Mean 63 months (excluding dropouts) | Left/right 21/12 (excluding dropouts) | Left/right 13/21 (excluding dropouts) | |
| Mean 62 (SD 15) years | Mean 59 (SD 13) years | Men/women 15/9 | Men/women 14/12 | Mean 23 (SD 6) days | Mean 23 (SD 4) days | Left/right 16/8 | Left/right 13/13 | |
| Mean 63 (SD 10) years | Mean 64 (SD 8) years | Men/women 22/10 | Men/women 21/8 | Mean 35 (SD 29) months | Mean 39 (SD 59) months | Left/right 18/14 | Left/right 13/16 | |
| Mean 59.6 (SD 9.2) years | Mean 60.8 (SD 10.7) years | Men/women 10/5 | Men/women 9/4 | Mean 49 (SD 20) months | Mean 48 (SD 17) months | Left/right 6/9 | Left/right 6/7 | |
| Not described | Not described | Not described | Not described | Not described | Not described | Not described | Not described | |
| Mean 61.4 (SD 15.7) years | Mean 60.7 (SD 11.4) years | Men/women 14/9 | Men/women 16/4 | Mean 50.4 (SD 56.8) months | Mean 29 (SD 52) months | Left/right 8/15 | Left/right 8/12 | |
| Mean 50 (SD 15) years (EXP and CTL participants) | Men/women 14/6 (EXP and CTL) | Mean 13 (SD 8) months (EXP and CTL) | Left/right 16/4 (EXP and CTL) | |||||
| Median 54 (range 24 to 67) years (all participants) | Median 56 (range 24 to 66) years | Men/women 20/16 | Men/women 20/17 | Median 22 (range 10 to 56) days | Median 17 (range 8 to 53) days | Left/right/bilateral 21/11/4 | Left/right/bilateral 18/14/5 | |
| Mean 56.8 (SD 6.4) years | Mean 57.0 (SD 7.1) years | Men/women 12/8 | Men/women 22/18 | Mean 10.2 (SD 6.9) months | Mean 10.5 (SD 6.3) months | Left/right 12/8 | Left/right 19/21 | |
| Mean 53 (SD 8) years | Mean 53 (SD 9) years | Men/women 12/8 | Men/women 13/7 | Mean 21 (SD 7) months | Mean 16 (SD 8) months | Left/right 12/9 | Left/right 10/10 | |
| Mean 55 (SD 10) years | Mean 52 (SD 13) years | Men/women 4/5 | Men/women 6/4 | Mean 10 (SD 3) months | Mean 13 (SD 4) months | Left/right 3/6 | Left/right 6/4 | |
| Mean 58.2 (SD 10.5) years for EXP 1 (excluding dropouts) | Mean 61.6 (SD 10.6) years (excluding dropouts) | Men/women 16/4 for EXP 1 | Men/women 13/7 | Mean 16.2 (SD 16.4) weeks for EXP 1 | Mean 16.1 (SD 18.5) weeks | Left/right 15/5 for EXP 1 | Left/right 16/4 | |
| Mean 56 (SD 8) years (without dropouts) | Mean 58 (SD 9) years (without dropouts) | Not described | Not described | Mean 33 (SD 25) months | Mean 20 (SD 10) months | Not described | Not described | |
| Mean 69.6 (SD 7.4) years (all participants) | Mean 67.3 (SD 11.2) years (CTL 1) | Men/women 5/5 | Men/women 2/6 | Mean 8.3 (SD 1.4) days | Mean 8.8 (SD 1.5) days | Left/right 8/2 | Left/right 2/6 | |
| Mean 62.9 (SD 12) years | Mean 60.7 (SD 12) years | Men/women 22/10 | Men/women 21/10 | Mean 52.0 (SD 22) months | Mean 52.6 (SD 18) months | Left/right 15/17 | Left/right 20/11 | |
| Mean 57.7 (SD 11.0) years (all participants) | Men/women 16/14 | Mean 52.2 (SD 29.6) days | Left/right 17/13 | |||||
| Mean 57.8 (SD 7.0) years | Mean 56.0 (SD 8.3) years | Men/women 8/2 | Men/women 4/6 | < 1 year: 8 | < 1 year: 8 | Left/right 4/16 | ||
| Mean group II 47.93 (SD 9.95) years; group III 44.20 (SD 11.70) years | Mean 44.40 (SD 12.31) years | Men/women group II 12/3; group III 12/3 | Men/women 12/3 | Mean group II 442.07 (SD 295.13) days; group III 391.80 (SD 431.10) days | mean 652.20 (SD 579.04) days | left/right group II 6/9; group III 8/7 | Left/right 7/8 | |
| Mean 60.0 (SD 13.3) years | Mean 63.4 (SD 8.4) years | Men/women 34/26 | Men/women 11/9 | Mean 23.8 (SD 15.2) months | Mean 28.4 (SD 19.0) months | Left/right 28/32 | Left/right 10/10 | |
| Mean 61.1 (SD 10.2) years | Mean 64.9 (SD 10.7) years | Men/women 20/4 | Men/women 15/9 | Mean 27.3 (SD 26.6) months | Mean 21.6 (SD 27.7) months | Left/right 9/15 | Left/right 8/16 | |
| Mean 68.6 (SD 8.9) years | Mean 66.9 (SD 10.6) years | Men/women 15/9 | Men/women 7/7 | Mean 14.0 (SD 8.1) days | Mean 13.7 (SD 8.9) days | Left/right 12/12 | Left/right 4/10 | |
| Mean 65 (SD 10) years | Mean 65 (SD 12) years | Men/women 11/3 | Men/women 10/4 | Mean 11 (SD 5) days | Mean 11 (SD 4) days | Not described | Not described | |
| Mean 66.5 (SD 12.8) years (all participants) | Mean 66.7 (SD 10.1) years | Men/women 31/19 | Men/women 28/22 | Mean 68.1 (SD 26.5) days | Mean 78.4 (SD 30.0) days | Left/right 30/20 | Left/right 21/29 | |
| 55.2 (15.4) years | 54.6 (15.2) years | Men/women 17/6 | Men/women 17/5 | Mean 36.1 (SD 11.3) days | Mean 35.6 (SD 14.5) days | Left/right | Left/right | |
| 51 (12) years | 50 (14) years | Men/women 8/5 | Men/women 9/4 | Mean 62 (SD 24) days | Mean 63 (SD 34) days | Left/right | Left/right | |
| Mean 59.7 (SD 10.2) years (all participants) | Mean 60.3 (SD 8.6) years (all participants) | Men/women 8/7 | Men/women 5/10 | Mean 7.4 (SD 2.0) weeks | Mean 6.9 (SD 2.1) weeks | Left/right 7/8 | Left/right 7/8 | |
| Mean 57.2 (SD 9.3) years | Mean 55.0 (SD 10.1) years | Men/women 5/5 | Men/women 5/3 | Mean 1.2 (SD 1.1) years | Mean 1.6 (SD 1.5) years | Left/right 5/5 | Left/right 4/4 | |
| Mean 57.3 (SD 16.4) years | Mean 56.1 (SD 12.7) years | Men/women 3/4 | Men/women 6/1 | Mean 2.0 (SD 0.6) months | Mean 2.0 (SD 2.4) months | Left/right 5/2 | Left/right 3/4 | |
| 63.3 (13.4) years | 62.8 (15.4) years | Men/women | Men/women | 68.7 (25.6) days | 66.3 (23.3) days | Left/right | Left/right | |
| 56.9 (12.9) years | 57.8 (12.16) years | Men/women 6/4 | Men/women 7/3 | Mean 4.1 (SD 4.8) months | Mean 3.1 (SD 4.2) months | Not stated by the authors | Not stated by the authors | |
| CTL: control | ||||||||
| Study ID | EXP: treadmill | EXP: support | EXP: duration | EXP: frequency | EXP: N weeks | CTL: interventions | CTL: duration | CTL: frequency | CTL: N weeks |
| Gradually increased on an individual basis starting from 0.7 m/s at the start of the first session and finishing at 1.1 m/s at the end of the last session, on average | BWS: no Hand support: yes, use of hand rails if required Assistance from therapist: only if required, 2 participants needed slight help with stepping through for the first 2 weeks | 30 minutes (24, 21, 18, and 15 minutes in treadmill training in the first, second, third and fourth training weeks, respectively) | 3 times per week | 4 weeks | Sham (task‐orientated home program with an intensity insufficient to produce an effect, plus telephone follow‐up once each week) | 30 minutes | 3 times per week (plus encouraged to walk every day) | 4 weeks | |
| Initial speed of the treadmill was set so that the therapist had time to assist the leg to swing through while maintaining a reasonable step length. If a participant was too disabled to walk on a moving treadmill with the assistance of a therapist, then the participant walked on the spot. Once they attained a speed of 0.4 m/s without body weight support, they commenced 10 minutes of overground walking | BWS: yes Hand support: no Assistance from therapist: yes if required | 30 minutes | 5 times per week | Until they achieved independent walking or | Assisted overground walking. Aids such as knee splints, ankle–foot orthoses, parallel bars, forearm support frames and walking sticks could be used as part of | 30 minutes | 5 times per week | Until they achieved independent walking or | |
| Treadmill was run at a comfortable speed and participants were instructed to "walk as slowly as possible" and/or a metronome was used to decrease cadence thereby encouraging larger steps. When necessary, marching‐type steps were included to encourage hip and knee flexion during swing phase to improve toe clearance. When a normal step length was observed, the therapist increased the speed of the treadmill until step length was compromised. Workload was then progressed by increasing the incline of the treadmill. Overground walking was used each session and comprised 20% of intervention time in week 1 and was progressively increased each week so that it comprised 50% of the 30 minutes intervention time in week 8 of training. In week 9, the 4‐month training group returned to 20% overground walking, which was again increased to 50% by week 16 | BWS: no Hand support: no Assistance from therapist: no | 30 minutes | 3 times per week | Group 1: 16 weeks Group 2: eight weeks | Control group received no intervention. | ‐ | ‐ | ‐ | |
| Comfortable walking speed | No BWS | 20 minutes | Single session | ‐ | Overground gait‐training with constant walking speed | 20 minutes | Single session | ‐ | |
| 1 EXP subgroup walking on Participants were instructed to walk without stopping, at their own comfortable speed. The mass fixed to the ankle of the non‐paretic lower limb was 2 kg for women and 4 kg for men | No BWS | 20 minutes | Single session | ‐ | 1 CTL subgroup walking overground without a mass other CTL subgroup walking overground with a mass. Participants were instructed to walk without stopping, at their own comfortable speed. The mass fixed to the ankle of the non‐paretic lower limb was 2 kg for women and 4 kg for men | 20 minutes | Single session | ‐ | |
| Body weight‐supported treadmill training. Rest breaks were allowed as needed, however, breaks were not included in the overall walking time. Walking speed was increased or decreased based on the Borg rating of 11 to 14. Participants were instructed to achieve their fastest possible walking pace on the treadmill at every training session, without exceeding the moderate intensity level on the Borg scale. | BWS: began with | 30 minutes | 5 days per week | 2 weeks | Overground walking training. Rest breaks were allowed as needed, however, breaks were not included in the overall walking time. | 30 minutes | 5 days per week | 2 weeks | |
| Gradually increased in increments of 0.01 m/s, starting at 0.01 m/s | BWS: yes, starting at 30% body weight and progressively decreased to 0% Hand support: not reported Assistance from therapist: not reported | 20 minutes | 5 times per week | 2 to 3 weeks | Task‐orientated gait‐training | 20 minutes | 5 times per week | 2 to 3 weeks | |
| 10‐minute sessions, if necessary separated by 5‐minute resting period, training at comfortable walking speed every 3 to 5 minutes was increased by increments of 0.01 m/s | BWS: yes Hand support: not reported Assistance from therapist: not reported | 60 minutes | 5 times per week | 4 weeks | Range of motion, stretching, strengthening, balance, co‐ordination exercises and conventional ambulation training treatment program with parallel bars | 60 minutes | 5 times per week | 4 weeks | |
| Treadmill training assisted by 1 or more physical therapy staff (physical guidance, at or above 0.89 m/s) | BWS: yes up to 40% of BWS, weaned according to performance Handle use discouraged | Up to 30 minutes | 15 sessions | 5 weeks | Motor learning Walking Programm (practising 7 core walking activities) | Up to 40 minutes | 15 sessions | 5 weeks | |
| Gradually increased starting from 0.1 m/s to 0.5 m/s; interval method, resting period gradually reduced | BWS: yes, initial BWS 30% to 40% weight, gradual reduction Hand support: not reported Assistance from therapist: not reported | 40 minutes | 2 times per day | 4 weeks | Brunnstrom, Bobath, Rood therapy approaches as well as proprioceptive neuromuscular facilitation techniques and motor relearning program, transfer training, trunk stabilisation | 40 minutes | Unclear | 4 weeks | |
| At 0.89 m/s, followed by a progressive program of walking overground for 15 minutes. The treadmill speeds ranged from 0 to 1.6 km per hour, increasing by increments of 0.16 km per hour. | BWS: yes Hand support: not reported Assistance from therapist: yes | 90 minute sessions | 3 times per week | 12 to 16 weeks (30 and 36 exercise sessions | Home exercise as an active control, not as a high‐intensity, task‐specific walking program. Progression through the program was managed by a physical therapist in the home, with the goals of enhancing flexibility, range of motion in joints, strength of arms and legs, co‐ordination, and static and dynamic | 90‐minute sessions | 3 times per week | 12 to 16 weeks (30 and 36 exercise sessions | |
| Speed and inclination increased on an individual basis to achieve a training heart rate. | BWS: yes, the harness was always secured and body weight was minimally supported (0 to 15%) according to participant needs. Hand support: not reported Assistance from therapist: yes, to set the paretic leg, weight shift and hip extension, if required | 30 minutes | 5 times per week | 6 weeks | Not task‐orientated (neurophysiological) | 30 minutes | 5 times per week | 6 weeks | |
| Speed starting from 0.1 m/s and aiming at 1.2 m/s according to the participant's compliance and progress. Conventional treatment was performed for 40 minutes, not immediately | BWS: yes, limited to Hand support: not reported Assistance from therapist: 2 trained | 20 minutes + 40 minutes | 2 times per day | 20 sessions within 5 weeks | 20 sessions of overground gait‐training of 60 minutes each | 60 minutes | 5 times per week | 20 sessions within 5 weeks | |
| Body weight support treadmill training and comfortable treadmill speed was set | BWS: yes, from 30% to 0% of body weight Hand support: allowed Assistance from therapist: allowed | 45 minutes | 3 times per week | 6 weeks | Walking overground at comfortable walking speed | 45 minutes | 3 times per week | 6 weeks | |
| Body weight support treadmill training; treadmill speed was initially started at 0.5 mph | BWS: yes, up to 40% of their body weight supported at the beginning of the Hand support: unclear Assistance from therapist: unclear | Not described | Not described | 8 weeks | Body weight support overground ambulation | Not described | Not described | 8 weeks | |
| Beginning with 10 to 20 minutes) at 60% to 80% of the maximum heart rate reserve (starting with 40% to 50% HRR). Duration was increased as tolerated by 1 to 5 minutes per week Treadmill speed was progressed by 0.1 to 0.3 km/hour every 1 to 2 weeks Training was a group intervention (3 participants trained in parallel) | BWS: no Hand support: allowed Assistance from therapist: unclear Treadmill inclination at 0° | 30 to 50 minutes | 3 times per week | 3 months (39 sessions) | Passive, muscle tone–regulating exercises for the upper and lower extremities with elements of balance training conducted on an outpatient basis in physiotherapy practices or rehabilitation centres. No aerobic fitness training was performed. | 60 minutes | 3 times per week | 3 months (13 weeks) | |
| Treadmill therapy with BWS and on days without TTBWS, conventional gait‐training was conducted | BWS: yes Hand support: not reported Assistance from therapist: not reported | 30 minutes | Daily for the | 30 sessions for a period of a minimum of 10 weeks | Intensive gait‐training (30 minutes) and functional training (30 minutes) daily for a minimum of 10 weeks | 30 minutes | Daily | For a minimum of 10 weeks | |
| Comfortable walking speed (speed not reported), speed was not progressed | BWS: no, harness used to prevent falls only Hand support: yes, use of hand rails, if required Assistance from therapist: no | 60 minutes | 3 times per week | 2 weeks | Task‐orientated (overground obstacle training) | 60 minutes | 3 times per week | 2 weeks | |
| Group 1: treadmill training with optic flow (optic flow was applied and treadmill speed was increased by 0.1 km/hour each time once the participant could walk stably for more than 20 seconds) Group 2: treadmill training without optic flow (treadmill speed was increased by | BWS: no Hand support: allowed but discouraged Assistance from therapist: no | 30 minutes (2 times for 15 minutes with a rest between) | 3 times per week | 4 weeks | General stretching with added range of motion exercises in the less and more affected sides of the trunk, arms and legs for the same time. Exercise therapy was performed using the traditional motor development theory and neurodevelopmental treatment based on motor learning theory. | 30 minutes | 3 times per week | 4 weeks | |
| Gradually increased starting from 0.3 m/s to 0.7 m/s | BWS: no Hand support: no Assistance from therapist: no | 30 minutes | 5 times per week | 6 weeks | Control group received muscle strengthening (seated leg press, knee extension, leg abductor) | 30 minutes | 5 times per week | 6 weeks | |
| Treadmill training with virtual reality in addition to general physical therapy If the participant maintained the speed and felt safe for 20 s, the treadmill speed was then increased by 5% during next training session | BWS: no Hand support: unclear Assistance from therapist: unclear | 30 minutes | 3 times per week | 4 weeks | 2 control groups: 1 control group received community ambulation in addition to general physical therapy, the other control group no additional walking training to general physical therapy | 30 minutes | 3 times per week | 4 weeks | |
| Gradually increased from 0.22 to 0.89 m/s, as tolerated | BWS: yes, starting at 30% body weight and progressively decreased to 0% or eliminated Hand support: yes, use of hand rails, if required Assistance from therapist: yes, assisted with swing phase, foot placement and weight shift, if required | 45 minutes | 5 times per week | 2 to 3 weeks | Not task‐orientated (orthopaedic) | 45 minutes | 5 times per week | 2 to 3 weeks | |
| Walked on the treadmill at an intensity of 40% to 60% heart rate reserve or a Borg Rating of Perceived Exertion of 11 to 14. Participants commenced at an intensity level of 40% heart rate reserve for 30 minutes, progressing each week aiming for a 5% to 10% increase until 60% heart rate reserve was reached. For participants unable to reach 40% heart rate reserve on commencement of treadmill walking, treadmill speeds were set as fast as tolerated and progressed as quickly as possible. Also received task‐oriented physiotherapy, approximately 1 hour per day | BWS: no Hand support: yes, were encouraged to hold the handrail Assistance from therapist: yes, a physiotherapist provided assistance as | 30 minutes | 3 times per week | 6 weeks | Received usual physiotherapy intervention only | Unclear (probably the same as the EXP group) | Unclear (probably the same as the EXP group) | Unclear (probably the same as the EXP group) | |
| Walking speed was started on the lowest level and was increased within the first minutes to the working level. The working load was increased in co‐operation with the participants to a level they felt comfortable with and they felt no insecurity in balance or discomfort otherwise. | BWS: no Hand support: yes Assistance from therapist: no, and no inclination | 30 minutes | (Up to) 5 times per week | Mean of 16 days of inpatient stay (mean 10 walking sessions) | Outdoor walking at a comfortable speed and with the use of ordinary assistive devices, when necessary | 30 minutes | (Up to) 5 times per week | Mean of 17 days of inpatient stay (mean 11 walking sessions) | |
| Comfortable walking speed, speed used and progression not reported | BWS: no Hand support: yes, use of hand rails, if required Assistance from therapist: yes, assisted with swing phase and trunk alignment | 8 to 20 minutes | 5 times per week | 3 weeks | Task‐orientated | 8 to 20 minutes | 5 times per week | 3 weeks | |
| Speed used and progression not reported | BWS: no Hand support: not reported Assistance from therapist: not reported | 60 minutes | 3 times per week | 4 weeks | Task‐orientated | 60 minutes | 3 times per week | 4 weeks | |
| Aerobic intensity of 60% of heart rate reserve. Duration and intensity started low (10 to 20 minutes, 40% to 50% heart rate reserve) and increased approximately for 5 minutes and 5% heart rate reserve every 2 weeks, as tolerated. Treadmill velocity and incline were increased by 0.05 m/s and 1% increments, respectively | BWS: no Hand support: not reported Assistance from therapist: not reported | 40 minutes | 3 times per week | 6 months | 13 supervised traditional stretching movements on a raised mat table with a therapist’s assistance. Each movement was performed actively if possible or passively with a therapist's assistance. Movements included quadriceps, calf, hip and hamstring stretch, low back rotation and stretch, chest stretch, bridging, shoulder shrug, abduction, and flexion, heel slides and short arc of quadriceps | 40 minutes | 3 times per week | 6 month | |
| 5 to 10 minutes of active/passive stretching exercises 10 to 15 minutes of upper extremity training (active exercises and strengthening) 10 to 15 minutes of lower extremity training (active exercises and strengthening) 25 to 30 minutes of BWSTT including warm‐up and cool‐down BWSTT initiated in 5 to 10‐minute bouts at the heart rate achieved at 40% to 50% of baseline VO2 peak. The goal was to achieve a target exercise duration (at least 20 minutes, exclusive of warm‐up and cool‐down) and intensity (heart rates corresponding to 60% to 75% of baseline VO2 peak 27) by the fourth or fifth week. Initially, ambulatory‐independent participants walked at a treadmill speed of 80% to 90% of their self‐paced overground speed Ambulatory‐dependent participants walked at a treadmill speed of 70% to 80% of their overground speed Treadmill speed and grade were gradually increased and percentage of manual and body weight support decreased, as tolerated | BWS: yes 20% to 30% or 40%, if necessary of their body weight Hand support: handrail support was discouraged Assistance from therapist: therapist emphasised trunk and limb alignment, loading of the stance limb, hip extension at terminal stance, and advancement of the swing limb | 40 minutes | 5 times per week (after 6 weeks, 3 times per week) | 6 weeks (plus 6 weeks; total of 48 sessions) | 5 to 10 minutes of active/passive stretching exercises 10 to 15 minutes of upper extremity training (active exercises and strengthening) 10 to 15 minutes of lower extremity training (active exercises and strengthening) 25 to 30 minutes of overground gait‐training | 40 minutes | 5 times per week (after 6 weeks, 3 times per week) | 6 weeks (plus 6 weeks; total of 48 sessions) | |
| Increased from a mean of 0.48 (SE 0.30) m/s at baseline to 0.75 (SE 0.30) m/s at treatment end on an individual basis to achieve a target aerobic intensity of 60% to 70% heart rate reserve (treadmill slope increased from 0% at baseline to 2.2% (SE 2.2) at treatment end) | BWS: no Hand support: yes, use of handrails, if required Assistance from therapist: not reported | 40 minutes (including 5 minutes warm‐up and 5 minutes cool‐down) | 3 times per week | 6 months | Task‐orientated | 40 minutes | 3 times per week | 6 months | |
| Treadmill training, with gradually increased walking speed to 2.5 mph | BWS: yes, gradually decreased Hand support: unclear Assistance from therapist: yes | 30 minutes | 5 times per week | 3 weeks | Individualised overground gait‐training (based on the Bobath Approach) | 30 minutes | 5 times per week | 3 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | |
| Treadmill training, with increasing walking speed | BWS: yes, from 8% to 50%, gradually decreased Hand support: unclear Assistance from therapist: no | 60 minutes | 5 times per week | 10 days | Overground gait‐training | 60 minutes | 5 times per week | 10 days | |
| Intensive locomotor training with walking velocity increased in 0.5 km/h increments until participants’ heart rate reached 80% to 85% of age‐predicted maximum or until the participants' Rating of Perceived Exertion increased to 17 on the Borg scale, and was reduced in 10% increments, as tolerated | BWS: up to 40% partial Hand support: handrail use for balance only Assistance from therapist: therapists did not provide manual assistance | Unclear | 2 to 5 times per week | 4 weeks | Did not receive locomotor training or any other interventions | Unclear | 2 to 5 times per week | 4 weeks | |
| Gradually increased from 0.0 to 2.0 m/s on an individual basis | BWS: yes, starting at 100% body weight and decreased to 0% Hand support: yes, use of a cross bar, if required Assistance from therapist: yes, assisted with swing phase, hip and knee extension during stance phase, and weight shift if required | 30 minutes | 5 times per week | 9 to 10 weeks | Task‐orientated | 30 minutes | 5 times per week | 9 to 10 weeks | |
| Participants walked on a treadmill at a "predetermined natural safe walking speed" | BWS: not reported Hand support: not reported Assistance from therapist: not reported | 60 minutes of therapy, including 25 minutes treadmill training | 3 times per week | 12 weeks | Conventional physiotherapy, CTL 2 received overground gait‐training included in the hourly therapy sessions, whereas CTL 1 received conventional physiotherapy only (active and passive range of motion exercises, strength, and balance training) | 60 minutes | 3 times per week | 12 weeks | |
| Treadmill gait‐training at comfortable walking speed | BWS: not reported Hand support: not reported Assistance from therapist: not reported | 30 minutes twice a day | 5 times per week | 1 week | Overground gait‐training | 30 minutes twice a day | 5 times per week | 1 week | |
| Treadmill training with | BWS: not reported Hand support: not reported Assistance from therapist: not reported | 30 minutes | 5 times per week | 3 weeks | Ground walking with rhythmic auditory stimulation | 30 minutes | 5 times per week | 3 weeks | |
| Speed‐dependent treadmill training (EXP 1) ‐ aggressive increase in speed starting from the highest speed the participant could walk at without stumbling and increasing at 10% increments of this speed several times within a session. The average treadmill speed increased from 0.68 m/s (SD 0.34) at the start of training to 2.05 m/s (SD 0.71) at the end of training; | Speed‐dependent treadmill training BWS: yes, no more than 10% body weight for the first 3 training sessions only (participants always wore an unweighted harness) Hand support: not reported Assistance from therapist: no BWS: yes, no more than 10% body weight for the first 3 training sessions only Hand support: not reported Assistance from therapist: yes, assisted with the walking cycle | 30 minutes | 3 times per week | 4 weeks | Not task‐orientated (neurophysiological) | 45 minutes | 3 times per week | 4 weeks | |
| Treadmill training with partial body weight support at comfortable walking speed | BWS: yes, initially 30%, then decreased Hand support: not reported Assistance from therapist: yes, initially aided | 30 minutes | 3 times per week | 4 weeks | Proprioceptive Neuromuscular Facilitation method (PNF, including waist dissociations, sitting and rising from a chair, anteroposterior and latero‐lateral weight transfer) | 30 minutes | 3 times per week | 4 weeks | |
| Speed used and progression not reported | BWS: no Hand support: not reported Assistance from therapist: not reported | 105 minutes (about 35 minutes in treadmill training) | 5 times per week | 5 weeks | Not task‐orientated (neurophysiological) | 105 minutes | 5 times per week | 5 weeks | |
| Specialised locomotor training including tilt table, reciprocal stepping on a Kinetron device | BWS: no Hand support: not described Assistance from therapist: not described | 60 minutes | 5 times per week | 8 weeks | Conventional physiotherapy (traditional neurodevelopmental approach, task‐oriented motor learning, overground gait‐training, stepping exercises) | 60 minutes | 5 times per week | 8 weeks | |
| Gradually increased from 0.0 to 1.3m/s | BWS: yes, amount of body weight support and progression not reported Hand support: yes, use of hand rails, if required Assistance from therapist: yes, assisted with swing phase, foot placement, hip and knee extension during stance phase, and weight shift, if required | 30 minutes | 5 times per week | 3 weeks | Not task‐orientated (neurophysiological) | 30 minutes | 5 times per week | 3 weeks | |
| Participants walked for 5 minutes with a "slightly hard" rate of perceived exertion (RPE), then the speed was increased by increments of 0.2 m/hour every 10 minutes of walking with a "slightly hard" RPE | BWS: not clearly stated Hand support: not reported Assistance from therapist: only if required, 2 participants needed slight help with stepping through for the first 2 weeks | 20 minutes | 12 times per month | 4 weeks | Sham (weekly phone calls, recording of a daily life log) | Not reported | 1 telephone call per week | 4 weeks | |
| 2 treadmill groups: group 1 with BWS and group 2 without BWS at gradually increased walking speed | BWS: group 1 yes (40%), group 2 no Hand support: yes Assistance from therapist: not described | 30 minutes | 5 times per week | 4 weeks | Overground task‐oriented training | 30 minutes | 5 times per week | 4 weeks | |
| Initially 4 x 5‐minute training bouts at individualised speeds, initially within the range of 0.7 to 1.1 m/s, followed by 15 m overground walking and either (1) sham or (2) progressive resistive leg cycling, or (3) individualised progressive resistive strength training | BWS: yes, initially between 30% and 40% of the participant's weight and being decreased as participants improved Hand support: not described Assistance from therapist: up to 3 therapists assisting in placing of both feet and the pelvis, if necessary | 60 minutes | 4 times per week | 6 weeks | Sham (upper extremity cycle ergometry with minimal physical exertion) | 60 minutes | 4 times per week | 6 weeks | |
| Speed was initiated from 0.044 m/s for 10 minutes, followed by a rest for 5 minutes and then increased by increments of 0.044 m/s for 10 minutes | BWS: yes, 30% during the first week, 20% during the second week, I0% during the third week, and no BWS during the fourth week Hand support: unclear Assistance from therapist: initially 2 therapists assisted in placing the foot and the pelvis | 25 minutes | 7 times per week | 4 weeks | Walking at a self‐adopted speed on a 15 m walkway for 10 minutes, rested 5 minutes, and walked again 10 minutes | 25 minutes | 7 times per week | 4 weeks | |
| For 3 minutes twice (with 4 minute rest); week 1: 0.8 km/hour, week 2: 1.0 km/hour, week 3: 1.3 km/hour | BWS: yes 30% Hand support: yes, use of hand rails, if required Assistance from therapist: not described | 30 minutes control intervention followed by 10 minutes treadmill training either in forward or backward direction | 3 times per week | 4 weeks | Conventional training (stretching, strengthening), including overground walking < 200 m and ADL training | 80 minutes | 5.5 times per week | 4 weeks | |
| Intervention consisted of treadmill training, training on a hand bike machine, and a stationary bicycle | BWS: not stated Hand support: not stated Assistance from therapist: not stated | 90 minutes exercise training, including 35 to 55 minutes treadmill training | 2 times per week | 6 weeks | Home exercise booklet with included instructions for flexibility and muscle strength exercises, participants were encouraged to stick to their normal community routine | NA | NA | 6 weeks | |
| Gradually increased in increments of 0.04 m/s, from 0.23 to 0.42 m/s, on average, on an individual basis | BWS: yes, starting at 40% body weight and progressively decreased to 0% Hand support: yes, use of hand rails, if required Assistance from therapist: yes, assisted with stepping and limb control during stance and swing phases, and weight shift, if required | 20 minutes | 4 times per week | 6 weeks | Task‐orientated (treadmill only) | 20 minutes | 4 times per week | 6 weeks | |
| Initial speed was half of the measured maximal walking speed prior to training session for 5 minutes as a warm‐up, then intervals of higher speed for 10 s were delivered, returning back to warm‐up speed for 2 minutes; in the next phase the speed would be increased or decreased by 10%, respectively | BWS: no Hand support: unclear Assistance from therapist: yes, assisted with foot placing and pelvis rotation | 20 minutes | 5 times per week | 4 weeks | Neuromuscular facilitation techniques | 20 minutes | 5 times per week | 4 weeks | |
| Participants walked backwards on a treadmill with increasing speed | BWS: no Hand support: unclear Assistance from therapist: yes; assisted with foot placing and pelvis rotation | 30 minutes of control intervention and 30 minutes of treadmill training | 5 times per week | 3 weeks | Neuromuscular facilitation techniques including lower limb movements and overground gait exercises | 60 minutes | 5 times per week | 3 weeks | |
| Increased from a mean of 0.32 (SD 0.05) m/s at baseline on an individual basis | BWS: yes, starting at a mean of 8.93% (SD 1.84) body weight and progressively decreased Hand support: yes, use of handrails, if required Assistance from therapist: yes, assisted with foot placement, swing phase, and hip and trunk extension during stance phase, if required | 15 to 20 minutes | 5 times per week | 2 weeks | Task‐orientated | 15 to 20 minutes | 5 times per week | 2 weeks | |
| Additional to the CTL intervention: | BWS: yes Hand support: no, participants were encouraged to refrain from handrails Assistance from therapist: yes, 1 or 2 therapists assisted | 30 minutes + 20 minutes control intervention | 3 times per week | 4 weeks | Stretching, muscle strengthening, balance, and overground walking training | 50 minutes | 3 times per week | 4 weeks | |
| Additional to the CTL intervention: | BWS: yes Hand support: no, participants were encouraged to refrain from handrails Assistance from therapist: yes, 1 or 2 therapists assisted | 30 minutes + 20 minutes of control intervention | 3 times per week | 4 weeks | Stretching, muscle strengthening, balance and overground walking training | 50 minutes | 2 to 3 times per week | 4 weeks | |
| Increased from 0.2 km/hour and 40% weight‐bearing relief according to the participant's capabilities | BWS: yes Hand support: unclear Assistance from therapist: yes, assisted with foot placing, knee extension and pelvis rotation | 30 minutes | 5 times per week | 8 weeks | Not described | Not stated | Not stated | 8 weeks | |
| Walking speed and BWS were individualised to the participants' capabilities (with a mean walking speed of 0.13 m/s at baseline and 0.17 m/s at the end of the intervention phase) | BWS: yes Hand support: unclear Assistance from therapist: unclear | Individualised | 5 times a week | 4 weeks | Individualised conventional motor rehabilitation aiming at improving strength and endurance | Not stated | 5 times a week | 4 weeks | |
| ADL: activities of daily living | |||||||||
| Study ID | Injurious falls | Other injuries | Cardiovascular event | Other adverse event |
| EXP = 1 (hip fracture caused by a fall at home after the first week of training) | EXP = 1 (missed post‐treatment measurement session due to low back pain) | EXP = 0 | EXP = 1 (fall during overground component of training but no injuries sustained) | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 47 reports All reports included musculoskeletal problems (back, hip, knee, calf, foot pain, and gout), headaches, dizziness, or chest pain. There were 6 reports of falling, 1 of which resulted in a fracture and none of which occurred during the delivery of the intervention. 2 participants in the experimental group experienced anxiety attributable to being on a treadmill that was severe enough for them to withdraw from the study. | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = 0 | EXP = 16 (fracture) | EXP = 1 (myocardial infarction) | EXP = 139 + 143 (all reported events) | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 1 recurrent stroke, 1 transportation problem | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = 0 | EXP = 0 | EXP = 1 (acute myocardial infarction 2 days after last treatment session) | EXP = 0 | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = 0 | EXP = 1 (knee pain after first 4 treadmill sessions) | EXP = 0 | EXP = 1 (hospitalised after first training session and subsequently died, reason for hospitalisation not reported) | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 11 (5 falls during treadmill training but no injuries sustained; 6 minor medical complications) | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP 1 = 0 | EXP 1 = 0 | EXP 1 = 0 | EXP 1 = 0 | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = 1 (hip fracture) | EXP = 1 (cardiac problems) | EXP = not reported | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = 7 | ||||
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = 0 | EXP = 0 | EXP = 0 | EXP = 0 | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| EXP = not reported | EXP = not reported | EXP = not reported | EXP = not reported | |
| CTL: control | ||||
| Study ID | EXP ‐ treatment phase | EXP ‐ follow‐up | CTL ‐ treatment | CTL ‐ follow‐up |
| 1 ‐ hip fracture caused by a fall at home after the first week of training | No dropouts | 1 ‐ moved out of area | 1 ‐ moved out of area | |
| 2 ‐ died 2 ‐ withdrew | No follow‐up period | 2 ‐ died | No follow‐up period | |
| 1 ‐ withdrew | No dropouts | 3 ‐ withdrew | No dropouts | |
| No dropouts | No dropouts | No dropouts | No dropouts | |
| No dropouts | No dropouts | No dropouts | No dropouts | |
| 2 dropouts | No dropouts | No dropouts | No dropouts | |
| 1 ‐ completed fewer than 9 treadmill and body weight support sessions | No follow‐up period | 1 ‐ pulmonary complications (not related to the protocol) | No follow‐up period | |
| Dropouts not stated | Dropouts not stated | Dropouts not stated | Dropouts not stated | |
| 1 dropout | 3 dropouts | 5 dropouts | 4 dropouts | |
| No dropouts | No follow‐up period | No dropouts | No follow‐up period | |
| 35 (12 withdrew, 7 died, 13 moved, 3 other) | Unclear | 11 (2 withdrew, 6 died, 3 moved) | ||
| No dropouts | 1 ‐ refusal | No dropouts | No dropouts | |
| 10 ‐ dropouts | No follow‐up period | 10 ‐ dropouts | No follow‐up period | |
| 2 ‐ dropouts | No dropouts | 2 ‐ dropouts | No dropouts | |
| No dropouts | No follow‐up period | No dropouts | No follow‐up period | |
| 1 ‐ recurrent stroke 1 ‐ transportation problem | 2 dropouts (but unclear which group) | No dropouts | 2 dropouts (but unclear which group) | |
| No dropouts | No follow‐up period | No dropouts | No follow‐up period | |
| 1 ‐ endurance level too low to continue treatment | No dropouts | 2 ‐ medical conditions unrelated to the study (1 participant with arthritis and 1 participant with a heart condition) | No dropouts | |
| 1 ‐ dropout ‐ another treatment 1 ‐ lack of participation | No dropouts | No dropouts | No dropouts | |
| Dropouts not stated | Dropouts not stated | Dropouts not stated | Dropouts not stated | |
| No dropouts | No follow‐up period | 3 dropouts in the control group without additional training | No follow‐up period | |
| 1 ‐ chose to discontinue treatment (did not want to walk on the treadmill) | No follow‐up period | 1 ‐ Stroke progression requiring readmission to acute care | No follow‐up period | |
| 1 ‐ withdrew 1 ‐ fall | 1 ‐ moved 1 ‐ medical condition | No dropouts | No dropouts | |
| 3 ‐ dropouts (unclear reasons) | No follow‐up period | 2 ‐ dropouts (unclear reasons) | No follow‐up period | |
| 2 ‐ discharged prior to completion of data collection | No follow‐up period | 1 ‐ discharged prior to completion of data collection | No follow‐up period | |
| 1 ‐ hospitalised after first treatment and subsequently died (reason for hospitalisation not reported) | No follow‐up period | No dropouts | No follow‐up period | |
| 12 ‐ unrelated medical condition 2 ‐ recurrent stroke 6 ‐ noncompliance | No follow‐up period | 11 ‐ unrelated medical condition 11 ‐ noncompliance | No follow‐up period | |
| 1 ‐ seizure activity 1 ‐ moved | 1 ‐ refused | 2 ‐ medical reasons 1 ‐ disinterest | 1 ‐ refused 1 ‐ lost to follow‐up | |
| 3 ‐ medical conditions (1 participant had sinus surgery, 1 participant had pre‐existing shoulder pain, 1 participant had a gastrointestinal bleed and recurrent stroke) | No follow‐up period | 4 ‐ medical conditions (1 participant had a hernia repair, 1 participant had elective cardiac surgery, 1 participant had a radiculopathy, and 1 participant had a foot infection and poor control of hypertension) | No follow‐up period | |
| 1 ‐ discontinued treatment, cardiovascular instability 2 ‐ discontinued treatment, early discharged | No follow‐up period | 2‐ discontinued treatment, early discharge | No follow‐up period | |
| Missing information | Missing information | Missing information | Missing information | |
| 4 ‐ discontinued treatment, lost to follow‐up, unable to contact | No follow‐up period | 1‐ discontinued treatment, lost to follow‐up, unable to contact | ||
| Authors stated: 10 did not complete the protocol because of noncompliance with study requirements (i.e. not wearing accelerometer, n = 5), early discharge from clinical PT (n = 2), orthopaedic injury which limited walking (n = 1), or previous diagnosis of secondary neurological injuries (n = 2). | ||||
| 2 ‐ chose to discontinue treatment (did not want to walk on the treadmill) | 2 ‐ medical reasons | 1 ‐ chose to discontinue treatment (wanted to walk on the treadmill) | 1 ‐ moved out of area | |
| 2 ‐ did not attend all training sessions | No follow‐up period | 5 ‐ Did not attend all training sessions | No follow‐up period | |
| none | No follow‐up period | None | No follow‐up period | |
| none | No follow‐up period | None | No follow‐up period | |
| 2 ‐ medical conditions (1 participant with bladder infection and fever, and 1 participant with viral infection and fever) from EXP 1 | No follow‐up period | 5 ‐ medical conditions (3 participants with pneumonia and 2 with viral infection and fever) | No follow‐up period | |
| 2 ‐ dropouts | No follow‐up period | 3 ‐ dropouts | No follow‐up period | |
| 1 ‐ reason not reported | No follow‐up data reported | 2 ‐ reason not reported | No follow‐up data reported | |
| 1 ‐ medical conditions (hip fracture) 1 ‐ medical conditions (cardiac problems) | 5 ‐ being unavailable | 1 ‐ reason not stated | 7 ‐ being unavailable | |
| No dropouts | No follow‐up period | No dropouts | No follow‐up period | |
| Dropouts not stated | Dropouts not stated | Dropouts not stated | Dropouts not stated | |
| 4 ‐ dropouts | No follow‐up period | 4 ‐ dropouts | No follow‐up period | |
| 6 ‐ withdrawn by administration 1 ‐ refused to participate | 4 ‐ refused to participate | 2 ‐ withdrawn by administration | 1 ‐ withdrawn by administration 3 ‐ refused to participate | |
| Dropouts not stated | No follow‐up period | Dropouts not stated | No follow‐up period | |
| 3 ‐ for family reasons | No follow‐up period | Dropouts not stated | No follow‐up period | |
| 1 ‐ chose to discontinue treatment | No follow‐up period | No dropouts | No follow‐up period | |
| 2 ‐ chose to discontinue treatment | 14 ‐ medical event, repeated stroke, lack of willingness to participate or moved away from area | 4 ‐ chose to discontinue treatment | 13 ‐ medical event, repeated stroke, lack of willingness to participate or moved away from area | |
| 2 ‐ reasons unknown due to issues of translation | No follow‐up period | 3 ‐ reasons unknown due to issues of translation | No follow‐up period | |
| Dropouts not stated | No follow‐up period | Dropouts not stated | No follow‐up period | |
| No dropouts | No follow‐up period | No dropouts | No follow‐up period | |
| No dropouts | No follow‐up period | No dropouts | No follow‐up period | |
| No dropouts | No follow‐up period | No dropouts | No follow‐up period | |
| Dropouts not stated | No follow‐up period | Dropouts not stated | No follow‐up period | |
| No dropouts | No follow‐up period | No dropouts | No follow‐up period | |
| CTL: control | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Walking speed (m/s) at end of treatment Show forest plot | 47 | 2323 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.03, 0.09] |
| 1.1 dependent in walking at start of treatment | 9 | 752 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.06, 0.03] |
| 1.2 independent in walking at start of treatment | 38 | 1571 | Mean Difference (IV, Random, 95% CI) | 0.08 [0.05, 0.12] |
| 2 Walking endurance (m) at end of treatment Show forest plot | 28 | 1680 | Mean Difference (IV, Random, 95% CI) | 14.19 [2.92, 25.46] |
| 2.1 dependent in walking at start of treatment | 5 | 639 | Mean Difference (IV, Random, 95% CI) | ‐5.09 [‐23.41, 13.22] |
| 2.2 independent in walking at start of treatment | 23 | 1041 | Mean Difference (IV, Random, 95% CI) | 19.72 [6.61, 32.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dependence on personal assistance to walk at end of treatment Show forest plot | 19 | 1210 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.02] |
| 1.1 dependent in walking at start of treatment | 8 | 814 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.03, 0.03] |
| 1.2 independent in walking at start of treatment | 11 | 396 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 2 Walking speed (m/s) at end of treatment Show forest plot | 26 | 1410 | Mean Difference (IV, Random, 95% CI) | 0.07 [0.02, 0.11] |
| 2.1 dependent in walking at start of treatment | 8 | 738 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.06, 0.03] |
| 2.2 independent in walking at start of treatment | 18 | 672 | Mean Difference (IV, Random, 95% CI) | 0.11 [0.06, 0.17] |
| 3 Walking endurance (m) at end of treatment Show forest plot | 15 | 1062 | Mean Difference (IV, Random, 95% CI) | 20.79 [0.43, 41.14] |
| 3.1 dependent in walking at start of treatment | 5 | 639 | Mean Difference (IV, Random, 95% CI) | ‐5.09 [‐23.41, 13.22] |
| 3.2 independent in walking at start of treatment | 10 | 423 | Mean Difference (IV, Random, 95% CI) | 36.91 [11.14, 62.68] |
| 4 Dependence on personal assistance to walk at end of scheduled follow‐up Show forest plot | 5 | 285 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.05, 0.04] |
| 4.1 dependent in walking at start of treatment | 2 | 170 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.18, 0.15] |
| 4.2 independent in walking at start of treatment | 3 | 115 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 5 Walking speed (m/s) at end of scheduled follow‐up Show forest plot | 12 | 944 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.05, 0.10] |
| 5.1 dependent in walking at start of treatment | 3 | 556 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.13, 0.03] |
| 5.2 independent in walking at start of treatment | 9 | 388 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.03, 0.15] |
| 6 Walking endurance (m) at end of scheduled follow‐up Show forest plot | 10 | 882 | Mean Difference (IV, Random, 95% CI) | 21.64 [‐4.70, 47.98] |
| 6.1 dependent in walking at start of treatment | 2 | 510 | Mean Difference (IV, Random, 95% CI) | ‐6.78 [‐34.57, 21.02] |
| 6.2 independent in walking at start of treatment | 8 | 372 | Mean Difference (IV, Random, 95% CI) | 31.55 [0.57, 62.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Walking speed (m/s) at end of treatment Show forest plot | 20 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 independent in walking at start of treatment | 20 | 889 | Mean Difference (IV, Random, 95% CI) | 0.05 [0.01, 0.09] |
| 2 Walking endurance (m) at end of treatment Show forest plot | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 independent in walking at start of treatment | 13 | 608 | Mean Difference (IV, Random, 95% CI) | 9.25 [‐1.99, 20.50] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dependence on personal assistance to walk at end of treatment Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 dependent in walking at start of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 independent in walking at start of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Walking speed (m/s) at end of treatment Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 dependent in walking at start of treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 independent in walking at start of treatment | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Walking endurance (m) at end of treatment Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 dependent in walking at start of treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 independent in walking at start of treatment | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Dependence on personal assistance to walk at end of scheduled follow‐up Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 dependent in walking at start of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 independent in walking at start of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Walking speed (m/s) at end of scheduled follow‐up Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 dependent in walking at start of treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 independent in walking at start of treatment | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Walking endurance (m) at end of scheduled follow‐up Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 dependent in walking at start of treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 independent in walking at start of treatment | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse events during the treatment Show forest plot | 24 | 1504 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.01, 0.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dropouts Show forest plot | 56 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 by end of treatment phase | 56 | 3105 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.01] |
| 1.2 by end of scheduled follow‐up (cumulative) | 14 | 780 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.06, 0.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Walking speed Show forest plot | 36 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 trials with adequate random sequence generation | 27 | 1242 | Mean Difference (IV, Random, 95% CI) | 0.03 [0.00, 0.06] |
| 1.2 trials with adequate concealed allocation | 21 | 1266 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.01, 0.10] |
| 1.3 trials with adequate blinding of assessors | 24 | 1554 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.02, 0.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Walking speed (m/s) at end of treatment Show forest plot | 37 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Acute phase: less then or equal to 3 months after stroke independent in walking | 11 | 347 | Mean Difference (IV, Random, 95% CI) | 0.15 [0.07, 0.23] |
| 1.2 Chronic phase: more than 3 months after stroke independent in walking | 26 | 1209 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.02, 0.10] |
| 2 Walking endurance (m) at end of treatment Show forest plot | 23 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Acute phase: less then or equal to 3 months after stroke independent in walking | 5 | 178 | Mean Difference (IV, Random, 95% CI) | 48.64 [23.97, 73.32] |
| 2.2 Chronic phase: more than 3 months after stroke independent in walking | 18 | 863 | Mean Difference (IV, Random, 95% CI) | 10.69 [‐0.28, 21.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Walking speed (m/s) at end of treatment Show forest plot | 38 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 treadmill training 5 times a week or more | 19 | 671 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [0.02, 0.07] |
| 1.2 treadmill training 3 to 4 times a week | 16 | 784 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [0.03, 0.12] |
| 1.3 treadmill training less then 3 times a week or unclear frequency | 3 | 116 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.06, 0.10] |
| 2 Walking endurance (m) at end of treatment Show forest plot | 23 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 treadmill training 5 times a week | 9 | 392 | Mean Difference (IV, Random, 95% CI) | 27.25 [5.37, 49.13] |
| 2.2 treadmill training 3 to 4 times a week | 13 | 621 | Mean Difference (IV, Random, 95% CI) | 12.41 [‐3.15, 27.97] |
| 2.3 treadmill training less then 3 times a week or unclear | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐15.0 [‐133.26, 103.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Walking speed (m/s) at end of treatment Show forest plot | 38 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 treadmill training duration more than 4 weeks | 14 | 802 | Mean Difference (IV, Random, 95% CI) | 0.05 [0.01, 0.09] |
| 1.2 treadmill training duration 4 weeks | 13 | 404 | Mean Difference (IV, Random, 95% CI) | 0.13 [0.07, 0.19] |
| 1.3 treadmill training duration less then 4 weeks | 11 | 365 | Mean Difference (IV, Random, 95% CI) | 0.08 [0.01, 0.14] |
| 2 Walking endurance (m) at end of treatment Show forest plot | 23 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 treadmill training duration more than 4 weeks | 12 | 706 | Mean Difference (IV, Random, 95% CI) | 19.09 [2.29, 35.88] |
| 2.2 treadmill training duration 4 weeks | 7 | 206 | Mean Difference (IV, Random, 95% CI) | 29.40 [‐4.75, 63.54] |
| 2.3 treadmill training duration less then 4 weeks | 4 | 129 | Mean Difference (IV, Random, 95% CI) | 9.82 [‐15.48, 35.13] |

