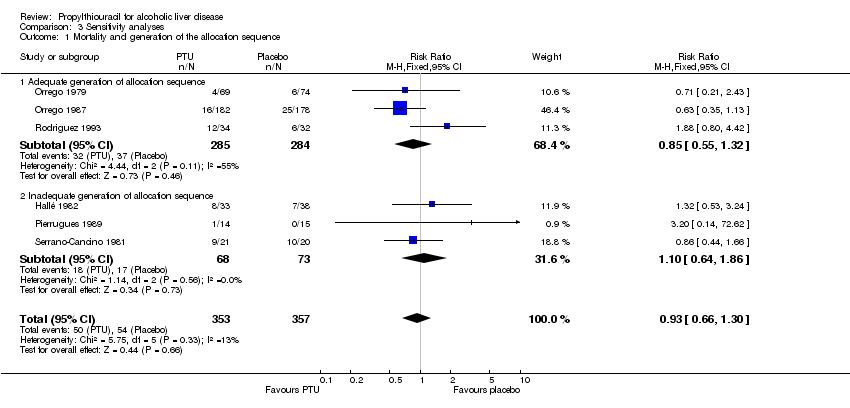
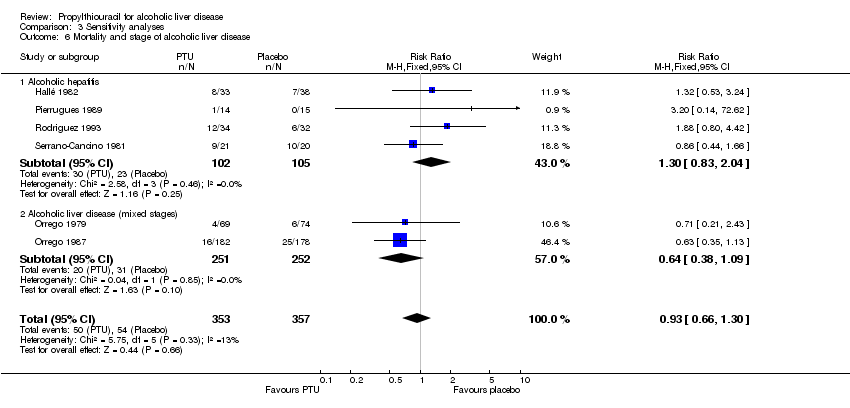
| 1 Mortality Show forest plot | 6 | 710 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.66, 1.30] |
|
| 2 Liver‐related mortality Show forest plot | 4 | 640 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.50, 1.29] |
|
| 3 Variceal bleeding Show forest plot | 4 | 640 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.63, 3.46] |
|
| 4 Hepatic encephalopathy Show forest plot | 3 | 574 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.38, 1.83] |
|
| 5 Total number of complications Show forest plot | 4 | 640 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.78, 1.44] |
|
| 6 Improvement of composite clinical and laboratory index Show forest plot | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐2.65, 4.65] |
|
| 7 Composite clinical and laboratory index Show forest plot | 1 | 133 | Mean Difference (IV, Fixed, 95% CI) | 1.82 [1.23, 2.42] |
|
| 7.1 Change in score of patients with initial index 0‐13 | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.21, 2.01] |
| 7.2 Change in score of patients with initial index 14‐26 | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 2.2 [1.49, 2.91] |
| 8 Composite clinical and laboratory index Show forest plot | 1 | 359 | Mean Difference (IV, Fixed, 95% CI) | 0.73 [0.04, 1.42] |
|
| 8.1 Patients who completed the trial (change) | 1 | 116 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [0.37, 3.43] |
| 8.2 Patients who dropped out the trial (change) | 1 | 194 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.33, 1.33] |
| 8.3 Patients who were not compliant to the trial (change) | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.08, 2.08] |
| 9 S‐bilirubin (mg/dl) Show forest plot | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.57, 1.57] |
|
| 10 S‐aspartate aminotransferase (U/I) Show forest plot | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | 5.0 [‐1.94, 11.94] |
|
| 11 S‐alanine aminotransferase (U/I) Show forest plot | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐13.85, 13.85] |
|
| 12 Prothrombin time (%) Show forest plot | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐23.40, 15.40] |
|
| 13 S‐albumin (g/dl) Show forest plot | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐1.28, 1.50] |
|
| 14 Liver histology (disappearance of Mallory bodies) Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.26, 4.45] |
|
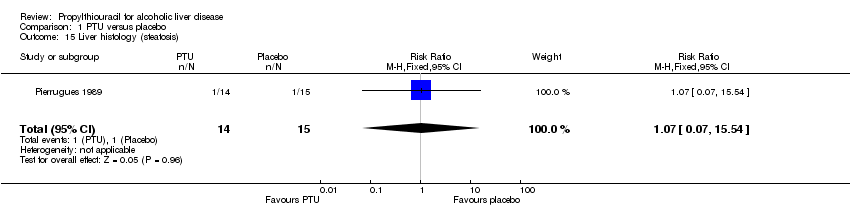
| 15 Liver histology (steatosis) Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.07, 15.54] |
|
| 16 Liver histology (cholestasis) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.87, 4.09] |
|
| 17 Liver histology (hepatitis) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.96, 1.59] |
|
| 18 Liver histology (hyaline necrosis) Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.79, 1.48] |
|