Rocuronio versus sucinilcolina para la inducción de la intubación de secuencia rápida
Información
- DOI:
- https://doi.org/10.1002/14651858.CD002788.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 29 octubre 2015see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Anestesia
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Diem TT Tran (DT), Ethan K Newton (EN), Victoria AH Mount (VM), Jacques S Lee (JL), George A Wells (GW), Jeffrey J Perry (JJP)
Conceiving the review: JJP
Co‐ordinating the review: JJP
Undertaking manual searches: JJP, VM EN
Screening search results: JJP, JL, VM, EN, DT
Organizing retrieval of papers: JJP, VM, EN, DT
Screening retrieved papers against inclusion criteria: JJP, JL, VM, EN, DT
Appraising quality of papers: JJP, JL, VM, EN, DT
Abstracting data from papers: JJP, JL, VM, EN, DT
Data management for the review: JJP, DT
Entering data into Review Manager: JJP, VM, EN, DT
Analysis of Data: JJP, JL, VS, GW, DT
Interpretation of data: JJP, VS, GW, DT
Statistical analysis: JJP, GW, DT
Writing the review: JJP, JL, VM, GW, DT
Securing funding for the review: JJP
Guarantor for the review (one author): JJP
Responsible for reading and checking review before submission: JJP, DT
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
Canadian Association of Emergency Physicians, Canada.
Declarations of interest
Diem TT Tran: none known
Ethan K Newton: none known
Victoria AH Mount: none known
Jacques S Lee: none known
George A Wells: none known
Jeffrey J Perry: none known
Acknowledgements
We would like to thank: Andrew Smith (content editor), Cathal Walsh (statistical editor), and Mary Meyers (consumer referee) for their help and editorial advice during the preparation of this updated systematic review.
We would also like to thank:
Mrs Jessie McGowen who helped generate the initial search strategy.
Dr Gina Neto who assessed foreign language articles.
Mrs Beverly Shea who assisted with methodology of conducting the meta‐analysis.
Mrs Verda Toprak who assessed foreign language articles.
Dr Altan Sahin who assessed foreign language articles.
Version history
| Published | Title | Stage | Authors | Version |
| 2015 Oct 29 | Rocuronium versus succinylcholine for rapid sequence induction intubation | Review | Diem TT Tran, Ethan K Newton, Victoria AH Mount, Jacques S Lee, George A Wells, Jeffrey J Perry | |
| 2008 Apr 23 | Rocuronium versus succinylcholine for rapid sequence induction intubation | Review | Jeffrey J Perry, Jacques S Lee, Victoria AH Sillberg, George A Wells | |
| 2003 Jan 20 | Rocuronium versus succinylcholine for rapid sequence induction intubation | Review | Jeffrey J Perry, Jacques JL Lee, George Wells | |
Differences between protocol and review
We added a subgroup analysis based on detection bias after the meta‐analysis was performed, to try to identify a source for the high statistical heterogeneity.
Notes
August 2015: Methods now include a 'Risk of bias' table, a 'Summary of findings' table and GRADE assessment.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Androstanols [*administration & dosage];
- Intubation, Intratracheal [*methods];
- Neuromuscular Depolarizing Agents [*administration & dosage, adverse effects];
- Neuromuscular Nondepolarizing Agents [*administration & dosage, adverse effects];
- Propofol [administration & dosage];
- Randomized Controlled Trials as Topic;
- Rocuronium;
- Succinylcholine [*administration & dosage, adverse effects];
Medical Subject Headings Check Words
Humans;
PICO

Search flow diagram for this update from July 2007 to February 2015
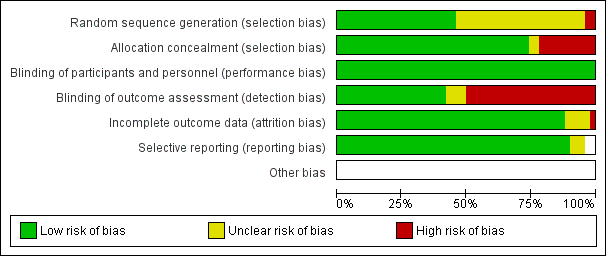
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial
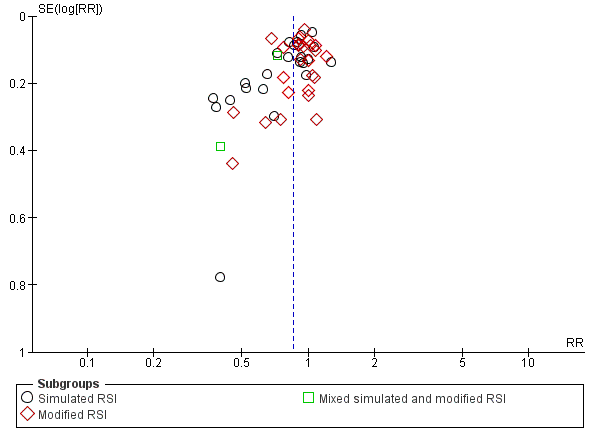
Funnel plot of comparison: Rocuronium any dose versus succinylcholine, outcome: Excellent versus other intubation conditions.
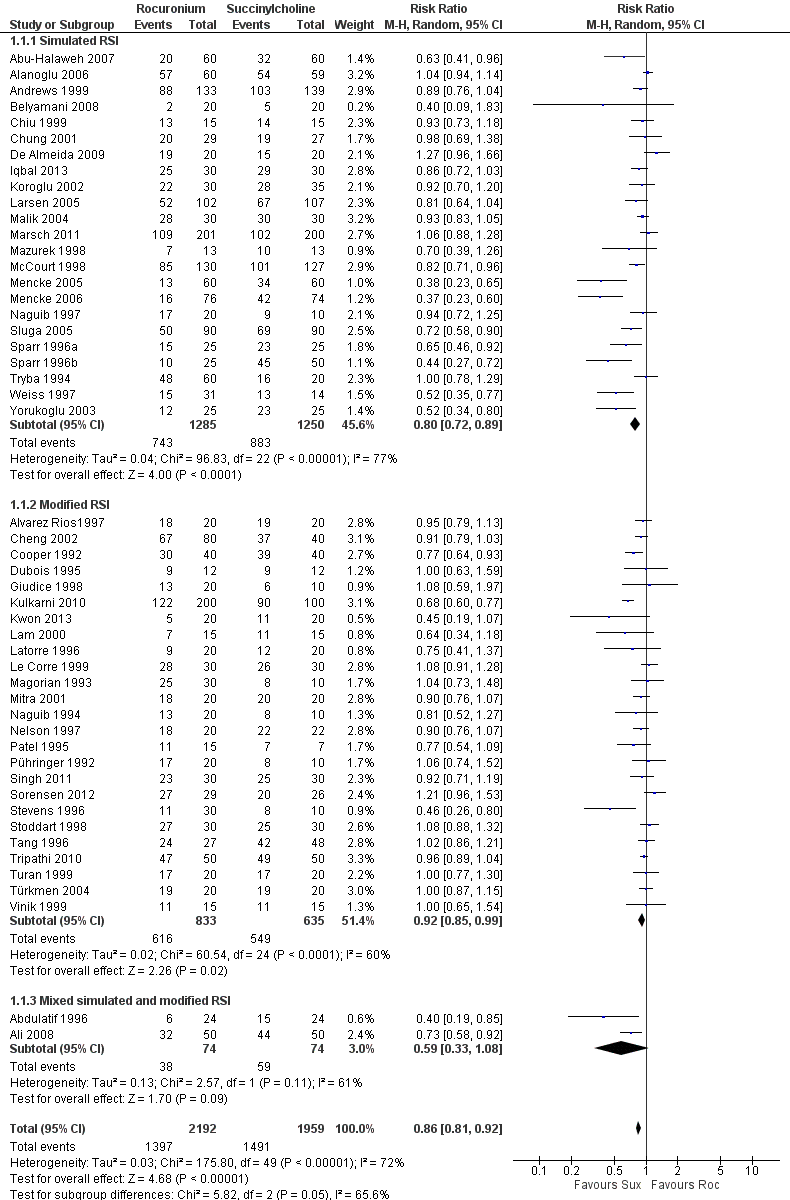
Forest plot of comparison: 1 Rocuronium any dose versus succinylcholine, outcome: 1.1 Excellent versus other intubation conditions

Forest plot of comparison: 3 Rocuronium versus succinylcholine for induction agent, outcome: 3.1 Excellent versus other intubation conditions
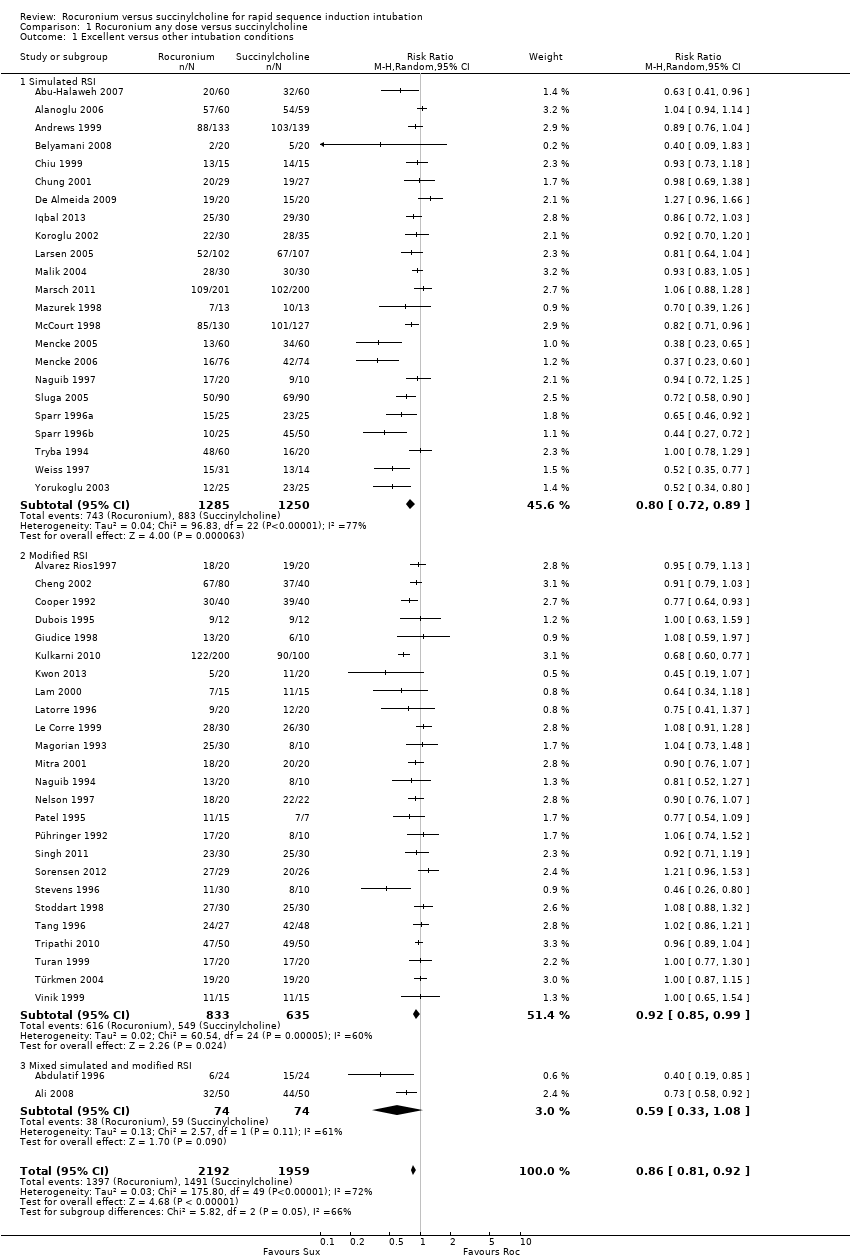
Comparison 1 Rocuronium any dose versus succinylcholine, Outcome 1 Excellent versus other intubation conditions.
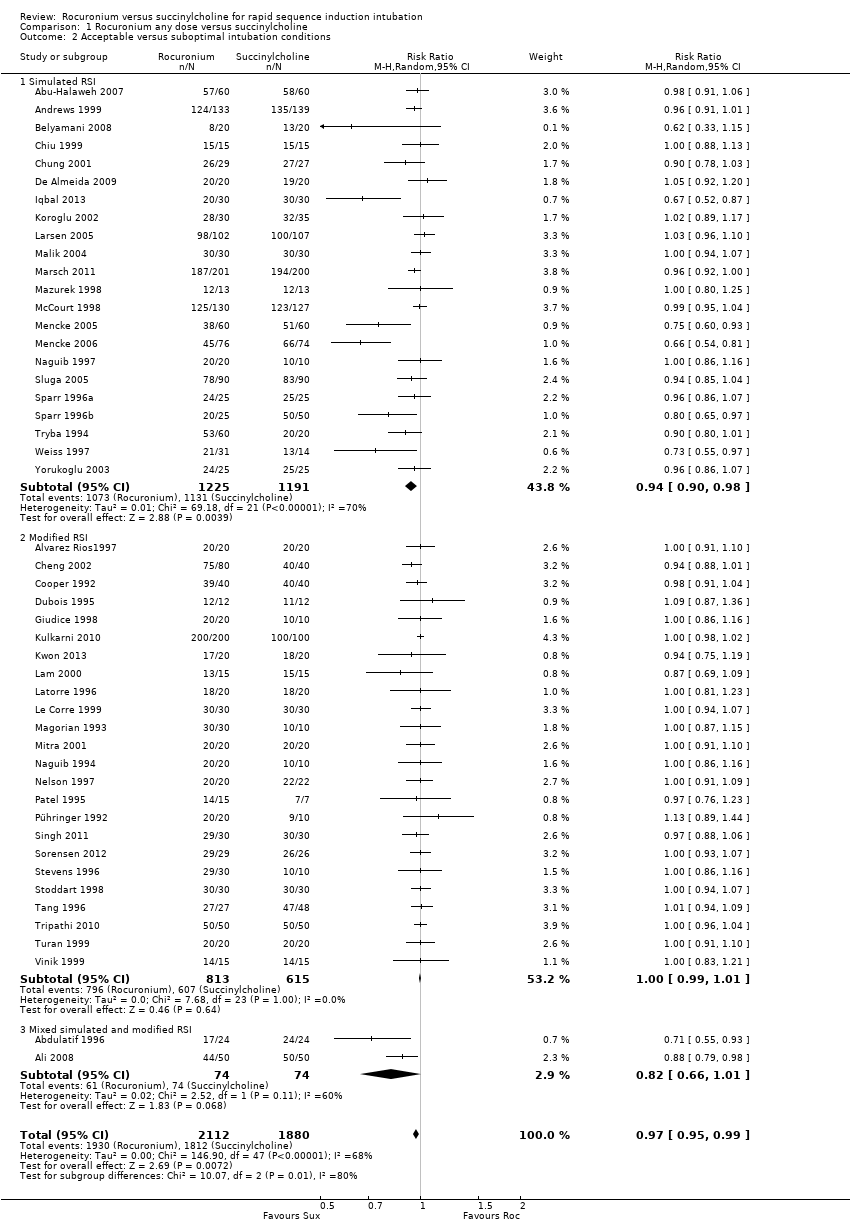
Comparison 1 Rocuronium any dose versus succinylcholine, Outcome 2 Acceptable versus suboptimal intubation conditions.

Comparison 2 Rocuronium specific dose versus succinylcholine, Outcome 1 Excellent versus other intubation conditions.
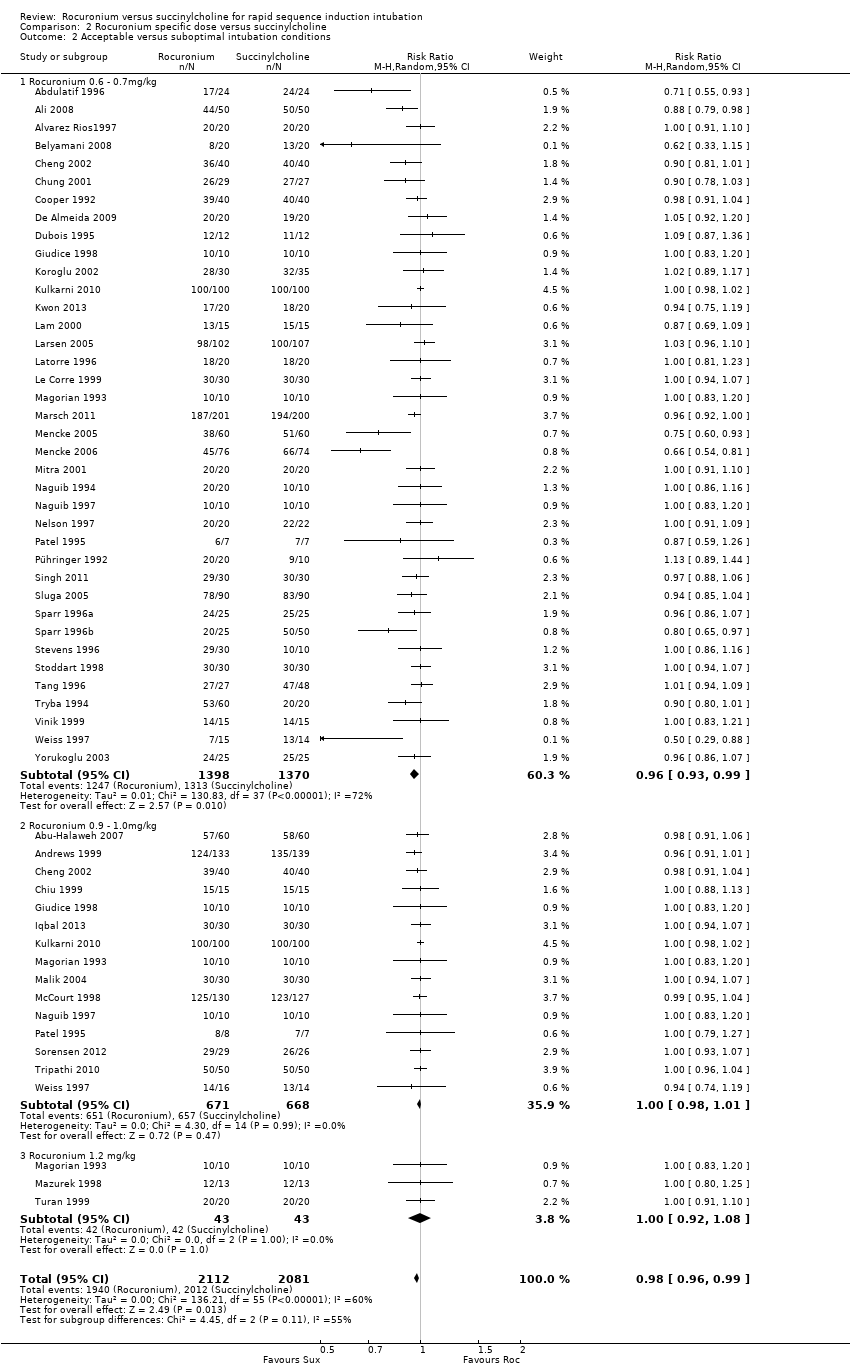
Comparison 2 Rocuronium specific dose versus succinylcholine, Outcome 2 Acceptable versus suboptimal intubation conditions.

Comparison 3 Rocuronium versus succinylcholine for induction agent, Outcome 1 Excellent versus other intubation conditions.
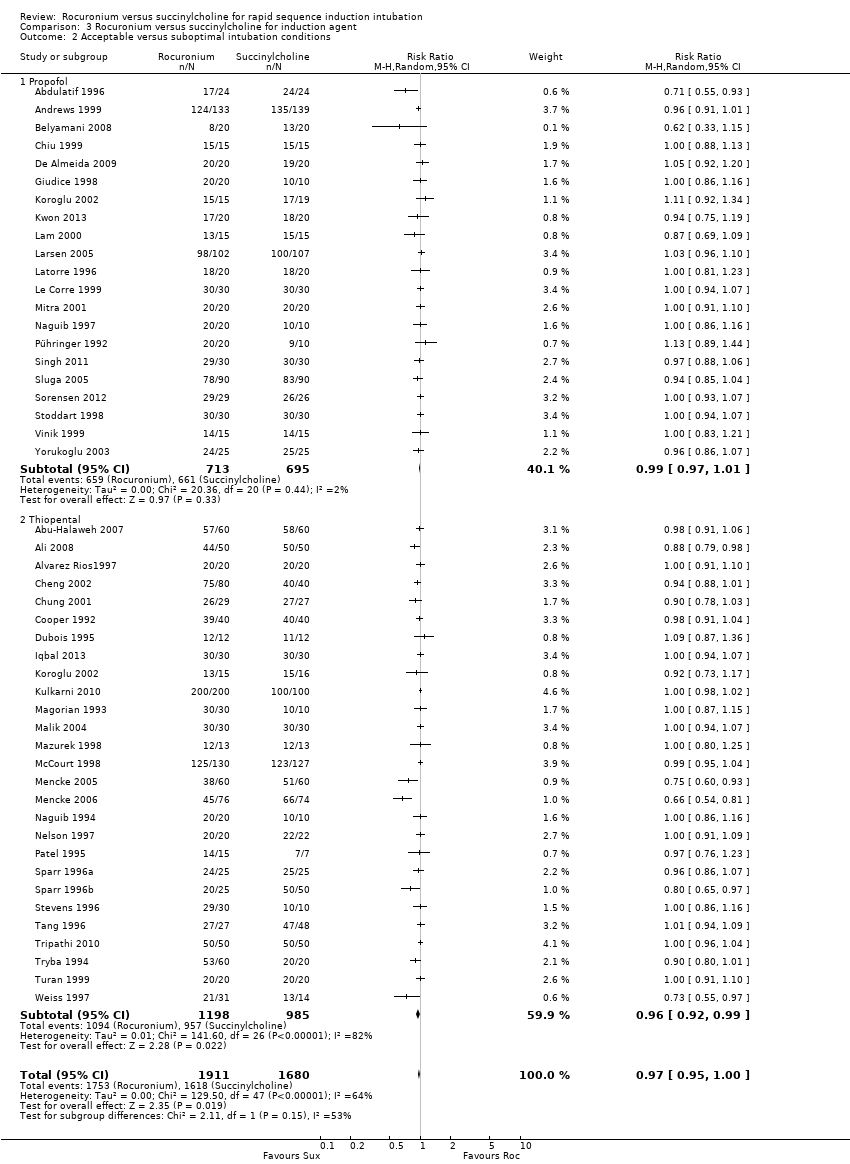
Comparison 3 Rocuronium versus succinylcholine for induction agent, Outcome 2 Acceptable versus suboptimal intubation conditions.

Comparison 4 Rocuronium versus succinylcholine with narcotic, Outcome 1 Excellent versus other intubation outcomes.
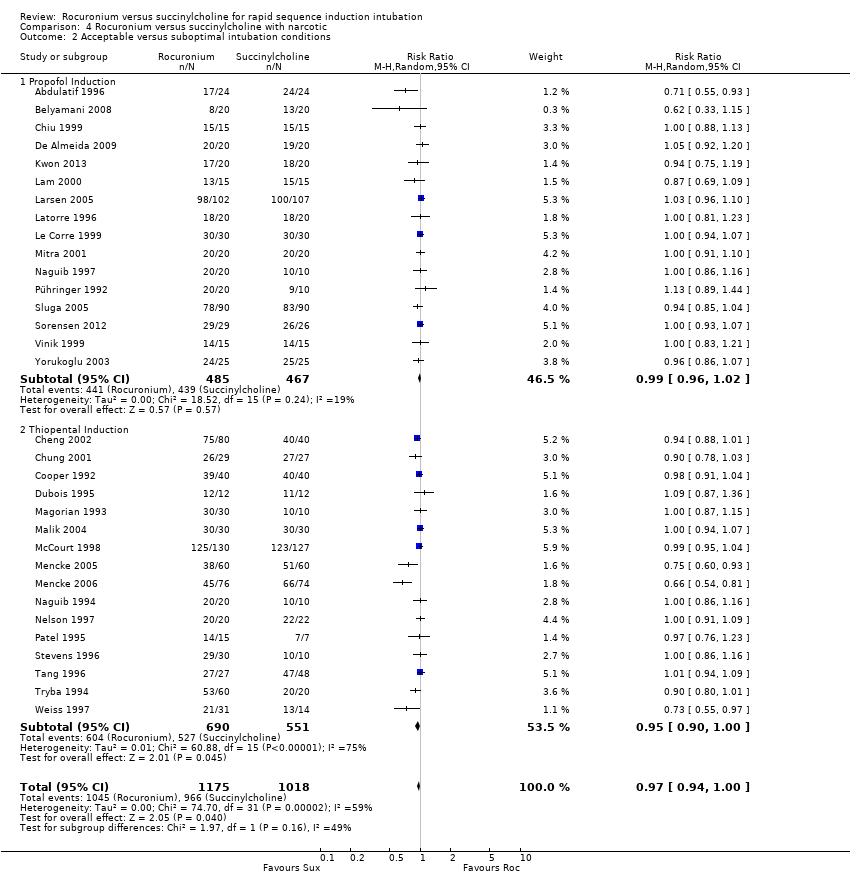
Comparison 4 Rocuronium versus succinylcholine with narcotic, Outcome 2 Acceptable versus suboptimal intubation conditions.
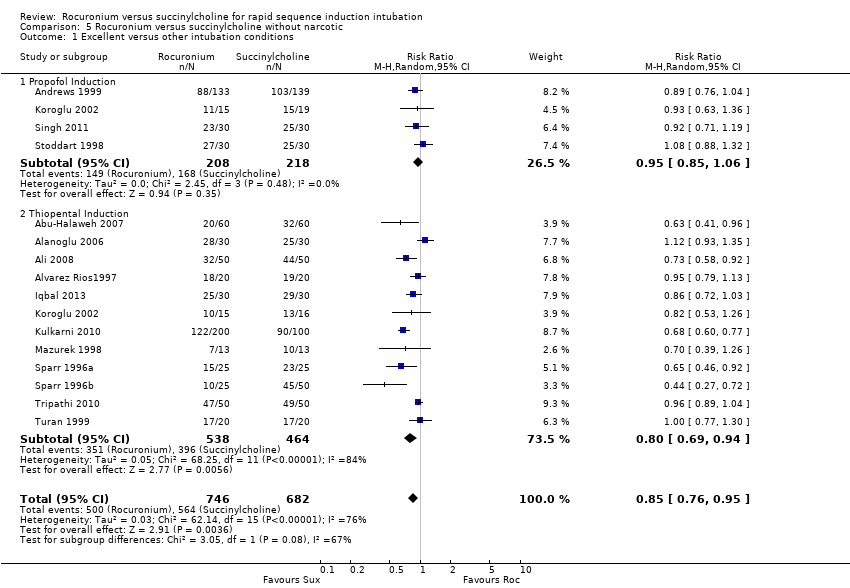
Comparison 5 Rocuronium versus succinylcholine without narcotic, Outcome 1 Excellent versus other intubation conditions.
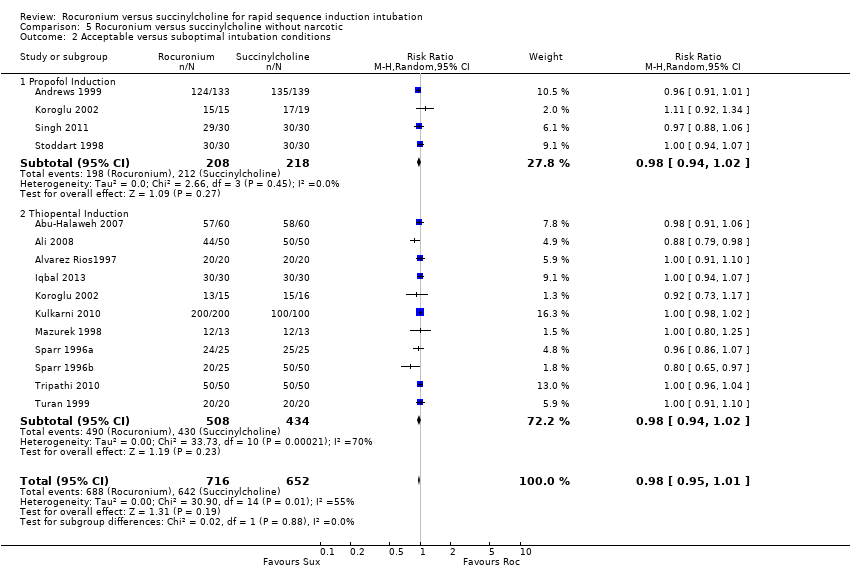
Comparison 5 Rocuronium versus succinylcholine without narcotic, Outcome 2 Acceptable versus suboptimal intubation conditions.

Comparison 6 Comparison of children and adults, Outcome 1 Excellent versus other intubation conditions.

Comparison 6 Comparison of children and adults, Outcome 2 Acceptable versus suboptimal intubation conditions.

Comparison 7 Rocuronium versus succinylcholine in emergency intubation, Outcome 1 Excellent versus other intubation conditions.
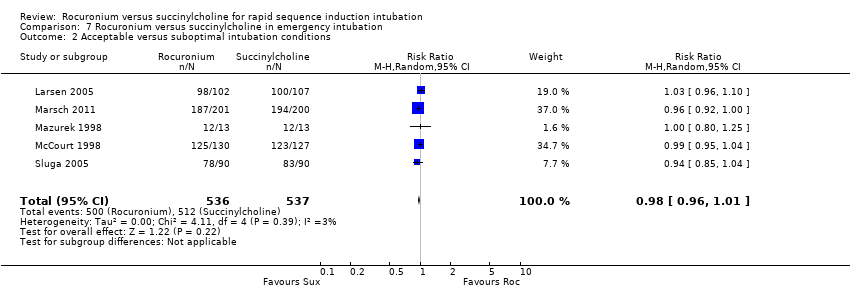
Comparison 7 Rocuronium versus succinylcholine in emergency intubation, Outcome 2 Acceptable versus suboptimal intubation conditions.

Comparison 8 Rocuronium versus succinylcholine by blinding of outcome assessment, Outcome 1 Excellent versus other intubation conditions.

Comparison 8 Rocuronium versus succinylcholine by blinding of outcome assessment, Outcome 2 Acceptable versus suboptimal intubation conditions.
| Rocuronium any dose versus succinylcholine for rapid sequence induction intubation | ||||||
| Patient or population: People requiring rapid sequence induction intubation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk1 | Corresponding risk | |||||
| Succinylcholine | Rocuronium any dose 2 | |||||
| Excellent versus other intubation conditions | Study population | RR 0.86 | 4151 | ⊕⊕⊕⊝ | Risk of bias: 50% of the studies were at high risk for detection bias because the outcome assessor was not blinded to the fasciculations caused by succinylcholine. Inconsistency: High statistical heterogeneity in the studies could not be explained by subgroup analyses. However we did not downgrade because exclusion of trials contributing to heterogeneity did not significantly change the direction or size of effect. | |
| 76 per 100 | 65 per 100 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Assumed risk is the average number of excellent intubations with succinylcholine. 2Rocuronium minimum dose 0.6 mg/kg. Succinylcholine minimal dose is 1mg/kg. | ||||||
| Score | Ease of laryngoscopy | Vocal cords | Intubation response |
| 1. Excellent | Good | Open | None |
| 2. Good | Fair | Open | Diaphragmatic movement |
| 3. Poor | Difficult | Movement | Moderate coughing |
| 4. Impossible | Poor | Closed | Severe coughing or bucking |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 50 | 4151 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.81, 0.92] |
| 1.1 Simulated RSI | 23 | 2535 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.72, 0.89] |
| 1.2 Modified RSI | 25 | 1468 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.85, 0.99] |
| 1.3 Mixed simulated and modified RSI | 2 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.33, 1.08] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 48 | 3992 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.95, 0.99] |
| 2.1 Simulated RSI | 22 | 2416 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.90, 0.98] |
| 2.2 Modified RSI | 24 | 1428 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.99, 1.01] |
| 2.3 Mixed simulated and modified RSI | 2 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.66, 1.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 50 | 4352 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.81, 0.92] |
| 1.1 Rocuronium 0.6 ‐ 0.7mg/kg | 39 | 2808 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.72, 0.88] |
| 1.2 Rocuronium 0.9 ‐ 1.0mg/kg | 16 | 1458 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.89, 1.00] |
| 1.3 Rocuronium 1.2 mg/kg | 3 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.15] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 48 | 4193 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.96, 0.99] |
| 2.1 Rocuronium 0.6 ‐ 0.7mg/kg | 38 | 2768 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.93, 0.99] |
| 2.2 Rocuronium 0.9 ‐ 1.0mg/kg | 15 | 1339 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.98, 1.01] |
| 2.3 Rocuronium 1.2 mg/kg | 3 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.92, 1.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 49 | 3750 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.80, 0.91] |
| 1.1 Propofol | 22 | 1448 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.84, 1.01] |
| 1.2 Thiopental | 28 | 2302 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.73, 0.88] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 47 | 3591 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.95, 1.00] |
| 2.1 Propofol | 21 | 1408 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.97, 1.01] |
| 2.2 Thiopental | 27 | 2183 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.92, 0.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation outcomes Show forest plot | 34 | 2292 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.78, 0.93] |
| 1.1 Propofol Induction | 17 | 992 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.78, 1.01] |
| 1.2 Thiopental Induction | 17 | 1300 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.73, 0.92] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 32 | 2193 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.94, 1.00] |
| 2.1 Propofol Induction | 16 | 952 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.96, 1.02] |
| 2.2 Thiopental Induction | 16 | 1241 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.90, 1.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 15 | 1428 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.76, 0.95] |
| 1.1 Propofol Induction | 4 | 426 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.85, 1.06] |
| 1.2 Thiopental Induction | 12 | 1002 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.69, 0.94] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 14 | 1368 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.95, 1.01] |
| 2.1 Propofol Induction | 4 | 426 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.94, 1.02] |
| 2.2 Thiopental Induction | 11 | 942 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.94, 1.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 50 | 4151 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.80, 0.91] |
| 1.1 Adults | 45 | 3615 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.80, 0.92] |
| 1.2 Children | 5 | 536 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.06] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 48 | 3992 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.95, 0.99] |
| 2.1 Adults | 43 | 3456 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.95, 0.99] |
| 2.2 Children | 5 | 536 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.97, 1.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 5 | 1073 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.73, 0.98] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 5 | 1073 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.96, 1.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Excellent versus other intubation conditions Show forest plot | 50 | 4151 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.81, 0.92] |
| 1.1 Low Risk | 21 | 1880 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.75, 0.92] |
| 1.2 Unclear Risk | 4 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.72, 1.18] |
| 1.3 High Risk | 25 | 2042 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.80, 0.96] |
| 2 Acceptable versus suboptimal intubation conditions Show forest plot | 48 | 3992 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.95, 0.99] |
| 2.1 Low Risk | 23 | 1970 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.94, 1.00] |
| 2.2 Unclear Risk | 3 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.92, 1.07] |
| 2.3 High Risk | 22 | 1912 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.94, 1.00] |

