袋鼠妈妈护理降低低出生体重婴儿的发病率和死亡率
摘要
研究背景
袋鼠妈妈护理 (Kangaroo Mother Care, KMC) 最初被定义为母亲与其新生儿之间的皮肤接触、频繁且完全母乳喂养或几乎完全母乳喂养,以及早期出院。KMC已被建议作为低出生体重(Low Birthweight, LBW)婴儿传统护理的替代方案。
研究目的
确定是否有证据支持常规护理的最初稳定期之前或之后,在LBW婴儿中将KMC作为常规新生儿护理的替代方案,并评估其有益和不利影响。
检索策略
我们采用了Cochrane新生儿系统综述小组(Cochrane Neonatal Review Group)的标准检索策略。检索的来源包括Cochrane对照试验中心注册库(Cochrane Central Register of Controlled Trials, CENTRAL;2016年第6期)、MEDLINE、Embase、护理和相关健康文献累积索引(Cumulative Index to Nursing and Allied Health Literature, CINAHL)、拉丁美洲和加勒比健康科学信息数据库(Latin American and Caribbean Health Science Information database, LILACS)和人口信息在线(Population Information Online, POPLINE)数据库(均从建库到2016年6月30日),以及世界卫生组织(World Health Organization, WHO)试验注册数据集(截至2016年6月30日)。此外,我们还检索了袋鼠基金会的网页、关于KMC的会议和专题讨论会摘要以及谷歌学术。
纳入排除标准
比较在LBW婴儿中,KMC与常规新生儿护理,或早发KMC与晚发KMC的随机对照试验。
资料收集与分析
我们按照Cochrane新生儿系统综述小组的方法进行数据收集和分析。
主要结果
包括3042名婴儿在内的21项研究符合纳入标准。19项研究评估了LBW婴儿稳定期后的KMC,一项研究评估了LBW婴儿稳定期前的KMC,一项研究比较了相对稳定LBW婴儿的早发KMC和晚发KMC。16项研究评估了间歇的KMC,5项研究评估了连续的KMC。
KMC与常规新生儿护理的对比:在出院或胎龄40到41周时,KMC与死亡(风险比 [risk ratio, RR]=0.60,95%置信区间[confidence interval, CI] [0.39, 0.92];8项试验,1736名婴儿),院内感染/败血症(RR=0.35, 95% CI [0.22, 0.54];5项试验,1239名婴儿)和体温过低(RR=0.28,95% CI [0.16, 0.49];9项试验;989名婴儿,中等质量证据)的风险降低显著相关。在最近的随访中,KMC与死亡率(RR=0.67, 95% CI [0.48, 0.95];12项试验,2293名婴儿;中等质量证据)和严重感染/败血症(RR=0.50, 95% CI [0.36, 0.69];8项试验,1463名婴儿;中等质量证据)风险的降低显著相关。此外,在最近一次随访中发现KMC可以促进体重增加(平均差异 [mean difference, MD]=4.1g/d, 95% CI [2.3, 5.9]; 11项试验, 1198名婴儿;中等质量证据)、身高增长(MD=0.21厘米/周,95% CI [0.03, 0.38];3项试验,377名婴儿)和头围增加(MD=0.14厘米/周,95% CI [0.06, 0.22];4项试验,495名婴儿),还能促进出院时或胎龄40到41周时(RR=1.16,95% CI [1.07, 1.25];6项研究,1453名母亲)以及1‐3个月随访时的完全母乳喂养(RR=1.20,95% CI [1.01, 1.43];5项研究,600名母亲),增加出院时或胎龄40到41周时(RR=1.20,95% CI [1.07, 1.34];10项研究,1696名母亲;中等质量证据)和1‐3个月随访时(RR=1.17,95% CI [1.05, 1.31];9项研究,1394名母亲;低质量证据)的任何母乳喂养(完全母乳喂养或部分母乳喂养),以及一些母婴依恋和家庭环境的测量。12个月校正月龄时,KMC组和对照组婴儿在精神运动发育方面的格里菲斯指数差异没有统计学意义(低质量证据)。敏感性分析表明,纳入具有高偏倚风险的研究不会影响研究结果的总体方向,也不会影响主要结局的治疗效应量。
在相对稳定的婴儿中,早发KMC与晚发KMC:一项试验在73名相对稳定的LBW婴儿中比较了早发连续KMC(出生后24小时内)与晚发连续KMC(出生后24小时后)。研究人员报告两组在死亡率、发病率、严重感染、体温过低、母乳喂养和营养指标方面没有显著差异。早发KMC与住院时间显著缩短有关(MD=0.9天,95% CI [0.6, 1.2])。
作者结论
本更新系统综述的证据支持在LBW婴儿中使用KMC作为传统新生儿护理的替代方案,主要是在资源有限的环境中。还需要更多关于不稳定或相对稳定LBW婴儿早期连续KMC有效性和安全性,以及长期神经发育结局和护理成本的信息。
PICO
简语概要
袋鼠妈妈护理降低低出生体重婴儿的发病率和死亡率
系统综述问题:袋鼠妈妈护理(kangaroo mother care, KMC)是否可以降低低出生体重(low birthweight, LBW)婴儿的发病率和死亡率?
研究背景:LBW婴儿(< 2500g)的常规护理费用昂贵,需要高技能人员和长期后勤支持。KMC已被提议作为LBW婴儿常规新生儿护理的替代方案。KMC的主要组成部分是母亲和新生儿之间的皮肤接触。KMC 的另外两个组成部分是频繁和完全母乳喂养或几乎完全母乳喂养,以及尝试提前出院。
研究特征:我们在2016年6月通过检索医学数据库确定了21项随机对照试验(3042名婴儿)。
主要结果:与常规新生儿护理相比,发现KMC可降低出院时或胎龄40到41周以及最近一次随访时的死亡率、严重感染/败血症、院内感染/败血症、体温过低、严重疾病和下呼吸道疾病。此外,KMC促进了体重、身高和头围的增加、出院时或胎龄40到41周以及1‐3个月随访时的母乳喂养、母亲对婴儿护理方法的满意度、母婴依恋和家庭环境的测量。研究人员指出,在12个月的矫正月龄时,神经发育和神经感觉结局没有差异。
证据质量:大多数关键和重要结局为中等质量证据。
结论:KMC是LBW婴儿常规新生儿护理有效且安全的替代方案,主要是在资源有限的国家。
Authors' conclusions
Summary of findings
| Kangaroo mother care versus conventional neonatal care for reducing morbidity and mortality in low birthweight infants | ||||||
| Patient or population: infants with low birthweight | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional neonatal care | Kangaroo mother care | |||||
| Mortality at latest follow‐up | Study population | RR 0.67 | 2293 | ⊕⊕⊕⊝ | ||
| 60 per 1000 | 40 per 1000 | |||||
| Moderate | ||||||
| 30 per 1000 | 20 per 1000 | |||||
| Severe infection/sepsis at latest follow‐up ‐ stabilized infants | Study population | RR 0.5 | 1463 | ⊕⊕⊕⊝ | ||
| 131 per 1000 | 65 per 1000 | |||||
| Moderate | ||||||
| 162 per 1000 | 81 per 1000 | |||||
| Hypothermia at discharge or at 40 to 41 weeks’ postmenstrual age ‐ stabilized infants | Study population | RR 0.28 | 989 | ⊕⊕⊕⊝ | ||
| 271 per 1000 | 76 per 1000 | |||||
| Moderate | ||||||
| 333 per 1000 | 93 per 1000 | |||||
| Weight gain at latest follow‐up (g/d) ‐ stabilized infants | Mean weight gain at latest follow‐up (g/d) ‐ stabilized infants in the intervention groups ‐ was | 1198 | ⊕⊕⊕⊝ | |||
| Any breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age ‐ stabilized infants | Study population | RR 1.2 | 1696 | ⊕⊕⊕⊝ | ||
| 762 per 1000 | 914 per 1000 | |||||
| Moderate | ||||||
| 743 per 1000 | 892 per 1000 | |||||
| Any breastfeeding at 1 to 3 months' follow‐up ‐ stabilized infants | Study population | RR 1.17 | 1394 | ⊕⊕⊝⊝ | ||
| 711 per 1000 | 832 per 1000 | |||||
| Moderate | ||||||
| 622 per 1000 | 728 per 1000 | |||||
| Griffith quotient for psychomotor development (all subscales) at 12 months' corrected age (copy) | Mean Griffith quotient for psychomotor development (all subscales) at 12 months' corrected age (copy) in the intervention groups was | 579 | ⊕⊕⊝⊝ | |||
| *The basis for the assumed risk (eg, median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aMost of the pooled effect provided by studies with moderate or high risk of bias | ||||||
Background
Description of the condition
Low birthweight (LBW), defined as weight at birth of less than 2500 g, irrespective of gestational age, has an adverse effect on child survival and development, and may even be an important risk factor for adult disease (Barker 1995). Overall, it is estimated that 15% to 20% of all births worldwide are LBW, representing more than 20 million births a year, the great majority of them reported in low‐ and middle‐income countries (WHO 2014). LBW is a major contributor to both neonatal and child mortality (Lawn 2014; UNICEF 2015). A complex process of care named conventional or modern neonatal care includes interventions already proven to lower the burden of neonatal morbidity and mortality. Conventional neonatal care of LBW infants is expensive and requires both trained personnel and permanent logistical support. This complexity is critical, mainly during the stabilization period, until the infant has adapted to autonomous extrauterine life. In low‐ and middle‐income countries, financial and human resources for neonatal care are limited, and hospital wards for LBW infants are often overcrowded. Thus, interventions for LBW infants that reduce neonatal morbidity and mortality and costs would signify an important advance in care.
Description of the intervention
In 1978, Edgar Rey (Rey 1983) proposed and developed kangaroo mother care (KMC) at Instituto Materno Infantil in Santa Fe de Bogotá, Colombia, as an alternative to the conventional contemporary method of care for LBW infants. KMC was initially conceived to address the lack of incubators, the high rate of nosocomial infection, and the occurrence of infant abandonment at the local hospital. The term KMC is derived from similarities to marsupial caregiving. Mothers are used as "incubators" to maintain infants' body temperature, and as the main source of food and stimulation for LBW infants, while they mature enough to face extrauterine life in similar conditions as those born at term. Initially, the method was applied only after the LBW infant had stabilized, because LBW infants need a variable period of conventional care before they are eligible for KMC. Stabilization of respiratory, thermal, and feeding functions has been considered crucial for the success of this intervention. The definition of stabilization is not precise; stabilization has been defined as independent of gestational age and weight, which are the main variables associated with those vital functions. Some recent studies have evaluated the effectiveness of early‐onset KMC (as soon as possible after birth) for LBW infants born in hospitals with little neonatal intensive care capacity (Nagai 2010; Worku 2005). Currently, the definition of KMC is characterized by significant heterogeneity (Chan 2016). The major component of KMC is skin‐to‐skin contact (SSC), by which infants are placed vertically between the mother's breasts firmly attached to the chest and below her clothes. SSC is offered to infants as far as the mother‐infant dyad can tolerate it. Mothers can share the role of provider of SSC with others, especially the babies' fathers. The aim is to empower the mother (parents or caregivers) by gradually transferring the skills and responsibility for becoming the child's primary caregiver and meeting every physical and emotional need (Nyqvist 2010). The other two components of KMC are frequent and exclusive or nearly exclusive breastfeeding and attempts at early discharge from hospital, regardless of weight or gestational age, with strict follow‐up. However, the last two components are less frequently identified as part of KMC (Chan 2016).
Different modalities of KMC have been adopted around the world (Charpak 1996), according to the needs of various settings. This diversity includes exclusive and non‐exclusive breastfeeding, breast or gavage feedings, completely or partially naked, continuous (≥ 20 hours per day) or intermittent (for short periods once or a few times per day and for a variable number of days) SSC with variable duration of exposure, and early‐or‐not hospital discharge.
KMC has been reported to be associated with similar neonatal mortality after stabilization, some reduction of neonatal morbidity, greater quality of mother‐to‐child bonding, and shorter hospital stay and lower costs compared with standard, conventional care of LBW infants. Some researchers have claimed that KMC is the best option if neonatal care units are unavailable; if they are available but are overwhelmed by demand, KMC would allow rationalization of resources by freeing up incubators for sicker infants (Ruiz‐Peláez 2004).
This review covered all randomized controlled trials (RCTs) of KMC with all its components, irrespective of duration of intervention, breastfeeding patterns, and time to discharge from hospital. We performed subgroup analyses for the primary outcome of mortality at discharge or at 40 to 41 weeks' postmenstrual age and at latest follow‐up according to type of KMC (intermittent vs continuous), infant age at initiation of KMC (≤ 10 days vs >10 days), setting in which the trial was conducted (low/middle‐income countries vs high‐income countries), and infant stabilization (before vs after). For all outcomes in stabilized LBW infants, we performed subgroup analyses according to type of KMC (intermittent vs continuous). In addition, we included RCTs that compared early‐onset (starting within 24 hours after birth) versus late‐onset (starting after 24 hours after birth) KMC.
How the intervention might work
The intervention assumes that the mother maintains the infant's body temperature and is the main source of nutrition and stimulation, which are the main components of conventional neonatal care (Rey 1983). SSC would allow that an infant´s demands for care may trigger neuropsychobiological paths that increase maternal behavior and immediate response to infant needs, as well as increased lactogenesis (Diaz‐Rossello 2008). In addition, KMC would empower the mother (parents or caregivers) by gradually transferring the skills and responsibility for becoming the child’s primary caregiver and meeting every physical and emotional need (Nyqvist 2010).
Why it is important to do this review
We undertook this systematic review to determine whether KMC reduces morbidity and mortality in LBW infants. We believe that this review provides a valuable resource for clinicians and policy makers in summarizing current best evidence and highlighting gaps in research.
Objectives
To determine whether evidence is available to support the use of KMC in LBW infants as an alternative to conventional neonatal care before or after the initial period of stabilization with conventional care, and to assess beneficial and adverse effects.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials, including cluster‐randomized trials, in which KMC (continuous or intermittent) was compared with conventional neonatal care in stabilized or non‐stabilized LBW infants. Moreover, we included randomized trials that compared early‐onset KMC versus late‐onset KMC. We excluded trials if they were quasi‐randomized, or if they evaluated effects of KMC in healthy full‐term infants or in those with birthweight ≥ 2500 g, which is the topic of a separate review (Moore 2012), or if they used a cross‐over design, or if they reported only results for physiological parameters, or if they evaluated only the effect of KMC on procedural pain in infants, which is the topic of a separate review (Johnston 2014), or if they assessed the effect of KMC on infant colic or on neonatal transport. In addition, we did not include studies in which KMC was part of a package of interventions for newborn care.
When trials were reported in abstracts, we planned to include them, provided information on study methods was sufficient to allow us to assess eligibility and risk of bias. If insufficient information was reported, we attempted to contact trial authors to request further information before deciding to exclude any study.
Types of participants
LBW Infants (defined as birthweight < 2500 g), regardless of gestational age.
Types of interventions
-
Comparisons of KMC with conventional neonatal care in LBW infants, regardless of infant stabilization status, duration of intervention, and breastfeeding patterns, and irrespective of whether or not discharge from hospital was early.
-
Comparisons of early‐onset KMC with late‐onset KMC in LBW infants, irrespective of infant stabilization status.
Types of outcome measures
We chose primary outcomes to be most representative of the clinically important measures of effectiveness and safety for these infants. Secondary outcomes included other clinical measures of effectiveness, mother‐infant attachment or interaction, satisfaction with care, home environment and father involvement, and costs of care.
Primary outcomes
-
Mortality.
-
-
At discharge or at 40 to 41 weeks' postmenstrual age (from randomization until discharge or 40 to 41 weeks' postmenstrual age).
-
At six months of age or at six months' follow‐up (from randomization until six months of age or six months' follow‐up).
-
At 12 months' corrected age (from randomization until 12 months' corrected age).
-
At latest follow‐up (from randomization until last follow‐up).
-
-
Severe infection/sepsis (as defined in individual studies).
-
Severe illness (as defined in individual studies).
-
Infant growth.
-
-
Weight at discharge or at 40 to 41 weeks' postmenstrual age, and at six and 12 months' corrected age.
-
Weight gain at latest follow‐up.
-
Length at discharge or at 40 to 41 weeks' postmenstrual age, and at six and 12 months' corrected age.
-
Length gain at latest follow‐up.
-
Head circumference at discharge or at 40 to 41 weeks' postmenstrual age, and at six and 12 months' corrected age.
-
Head circumference gain at latest follow‐up.
-
-
Neurodevelopmental and neurosensory impairment.
-
-
Psychomotor development (measured by a validated tool/instrument).
-
Cerebral palsy.
-
Deafness.
-
Visual impairment.
-
Secondary outcomes
-
Nosocomial infection/sepsis (as defined in individual studies).
-
Mild/moderate infection or illness (as defined in individual studies).
-
Lower respiratory tract disease (as defined in individual studies).
-
Diarrhea (as defined in individual studies).
-
Hypothermia (as defined in individual studies).
-
Hyperthermia (as defined in individual studies).
-
Length of hospital stay.
-
Re‐admission to hospital.
-
Breastfeeding.
-
-
Exclusive breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age, and at one to three and at six to 12 months' follow‐up.
-
Any breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age, and at one to two, three, six, and 12 months' follow‐up.
-
Onset of breastfeeding.
-
-
Mother‐infant attachment (measured by a validated tool/instrument).
-
Mother‐infant interaction (measured by a validated tool/instrument).
-
Parental and familial satisfaction (measured by interviews).
-
Home environment and father involvement (measured by a validated tool/instrument).
-
Costs of care.
Search methods for identification of studies
Electronic searches
Appendix 1 shows the search strategy for the 2014 update. For the 2016 update, we used criteria and standard methods of The Cochrane Collaboration and the Cochrane Neonatal Review Group (see the Cochrane Neonatal Group search strategy for specialized register). This included searches of the CENTRAL (Cochrane Central Register of Controlled Trials; 2016, Issue 6) in The Cochrane Library; and MEDLINE via PubMed, Embase, LILACS (Latin American Caribbean Health Sciences Literature), POPLINE (Population Information Online), and CINAHL (Cumulative Index to Nursing and Allied Health Literature) databases (all from inception to June 30, 2016) using the following search terms: (Kangaroo OR skin‐to‐skin OR [skin to skin]), plus database‐specific limiters for RCTs and neonates (see Appendix 2 for the full search strategies for each database). We applied no language restrictions.
We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov), the World Health Organization International Trials Registry and Platform (www.whoint/ictrp/search/en/), and the ISRCTN Registry (International Standard Randomized Controlled Trial Number Registry).
Searching other resources
We also searched the Web page of the Kangaroo Foundation, the International Network of Kangaroo Care, conference and symposia proceedings on KMC, reference lists of identified studies, textbooks, review articles, and Google Scholar. In addition, we performed journal handsearching and contacted investigators involved in the field to locate unpublished studies.
Data collection and analysis
Selection of studies
We used standard methods of The Cochrane Collaboration and its Neonatal Review Group. Two review authors retrieved and reviewed independently all studies deemed suitable to determine inclusion. We resolved disagreements through consensus.
Data extraction and management
Two review authors independently extracted data in duplicate from all reports and recorded them on a piloted form. We performed no blinding of authorship. We extracted the following data for each trial: authors; year of publication; country; level of care; human resources used; inclusion and exclusion criteria; study characteristics; mean or median weight and gestational age at birth, and infant age at enrollment by group; description of interventions; co‐interventions; mean or median duration of KMC; criteria for infant discharge from the hospital; scheme for follow‐up of infants after discharge; numbers randomized and analyzed; numbers of and reasons for withdrawal; and outcomes. Review authors resolved differences in data extracted by discussion and reached consensus. We sought additional information from individual investigators when published information did not include the required details. One review author (AC‐A) entered data into Review Manager software (RevMan 2014), and the other review author (JLD‐R) checked data for accuracy. We processed included trial data as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of risk of bias in included studies
Two review authors who were not associated with any of the trials assessed risk of bias individually in each included trial. Review authors were not blind to author, institution, journal of publication, or results when conducting methodological assessments, as they were familiar with most of the studies. When differences in assessment of risk of bias arose, we reached a consensus. We assessed risk of bias by using the dimensions outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed seven domains related to risk of bias in each included trial because evidence suggests that these are associated with biased estimates of treatment effect: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. We made a judgment about risk of bias for each of the seven domains as "low risk," "high risk," or "unclear risk."
Random sequence generation
"Low risk" of bias: Investigators described a random component in the sequence generation process such as random number table, computer random number generator, shuffling of cards or envelopes, drawing of lots, or computerized minimization.
"High risk" of bias: Investigators described a non‐random component in the sequence generation process, such as odd or even date of birth, based on date or day of admission, based on hospital or clinical record number, or allocated by judgment of the clinician; preference of the participant; availability of the intervention; or results of laboratory tests.
"Unclear risk" of bias: information insufficient to permit judgment of "low risk" or "high risk."
Allocation concealment
"Low risk" of bias: Investigators used an adequate method to conceal allocation, such as central allocation (including telephone or web‐based randomization) or sequentially numbered, opaque, sealed envelopes.
"High risk" of bias: Investigators used a non‐adequate method to conceal allocation, such as open random allocation schedule (eg, a list of random numbers), assignment envelopes without appropriate safeguards, alternation or rotation, date of birth, or case record number.
"Unclear risk" of bias: information insufficient to permit judgment of "low risk" or "high risk."
Blinding of participants and personnel
"Low risk" of bias: As KMC cannot be implemented when masked, we considered adequate blinding of participants and personnel as either of the following: (1) no blinding or incomplete blinding, but review authors judged that the outcome was not likely to be influenced by lack of blinding; or (2) blinding of participants and key study personnel ensured, and unlikely that blinding could have been broken.
"High risk" of bias: either of the following: (1) no blinding or incomplete blinding, and the outcome was likely to be influenced by lack of blinding; or (2) blinding of key study participants and personnel attempted, but likely that blinding could have been broken, and the outcome was likely to be influenced by lack of blinding.
"Unclear risk" of bias: information insufficient to permit judgment of "low risk" or "high risk."
We assessed blinding of participants and personnel separately for each outcome or each class of outcomes (objective and subjective).
Blinding of outcome assessment
"Low risk" of bias: We considered blinding of outcome assessment to be adequate in either of the following: (1) no blinding of outcome assessment, but review authors judged that outcome measurement was not likely to be influenced by lack of blinding; or (2) blinding of outcome assessment ensured, and unlikely that blinding could have been broken.
"High risk" of bias: either of the following: (1) no blinding of outcome assessment, and outcome measurement was likely to be influenced by lack of blinding; or (2) blinding of outcome assessment, but likely that blinding could have been broken, and that outcome measurement was likely to be influenced by lack of blinding.
"Unclear risk" of bias: information insufficient to permit judgment of "low risk" or "high risk."
We assessed blinding of outcome assessment separately for each outcome or each class of outcomes (objective and subjective).
Incomplete outcome data
"Low risk" of bias: any one of the following: (1) no missing outcome data; (2) reasons for missing outcome data unlikely to be related to true outcome; (3) missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; (4) for dichotomous outcome data, proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; (5) for continuous outcome data, plausible effect size among missing outcomes not enough to have a clinically relevant impact on observed effect size; or (6) missing data imputed by appropriate methods.
"High risk" of bias: any one of the following: (1) reasons for missing outcome data likely to be related to true outcome, with imbalance in numbers or reasons for missing data across intervention groups; (2) for dichotomous outcome data, proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; (3) for continuous outcome data, plausible effect size among missing outcomes enough to induce clinically relevant bias impact on observed effect size; (4) "as‐treated" analysis done with substantial departure of the intervention received from that assigned at randomization; or (5) potentially inappropriate application of simple imputation.
"Unclear risk" of bias: reporting of attrition/exclusions insufficient to permit judgment of "low risk" or "high risk."
Selective reporting
"Low risk" of bias: any one of the following: (1) Study protocol was available, and all of the study's prespecified outcomes that were of interest in the review were reported in the prespecified way; or (2) the study protocol was not available, but it was clear that published reports included all expected outcomes, including those that were prespecified.
"High risk" of bias: any one of the following: (1) Not all of the study's prespecified primary outcomes were reported; (2) one or more primary outcomes were reported using measurements, analysis methods, or subsets of data that were not prespecified; (3) one or more reported primary outcomes were not prespecified; (4) one or more outcomes of interest in the review were reported incompletely, so that they could not be entered into a meta‐analysis; or (5) the study report failed to include results for a key outcome that would be expected to have been reported for such a study.
"Unclear risk" of bias: information insufficient to permit judgment of "low risk" or "high risk."
Other bias
"Low risk" of bias: Study appeared to be free of other sources of bias.
"High risk" of bias: At least one important risk of bias was present. For example, the study (1) had a potential source of bias related to the specific study design used; or (2) has been claimed to have been fraudulent; or (3) had extreme baseline imbalance; or (4) used blocked randomization in unblinded trials; or (5) had differential diagnostic activity; or (6) had some other problem.
"Unclear risk" of bias: information insufficient to assess whether an important risk of bias existed, or rationale or evidence insufficient to suggest that an identified problem will introduce bias.
Review authors independently assessed risk of bias in included studies and resolved discrepancies through discussion. We made explicit judgments about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and we explored the impact of the level of bias by undertaking sensitivity analyses (see Sensitivity analysis).
Measures of treatment effect
For dichotomous data, we have presented results as risk ratios (RRs) with 95% confidence intervals (CIs). For continuous data, we have used mean differences (MDs) with 95% CIs. We calculated the number needed to treat for an additional beneficial or harmful outcome (NNTB or NNTH) for outcomes for which investigators reported a statistically significant reduction or increase in risk difference based on control event rates in the included studies.
Unit of analysis issues
The unit of analysis was the participating infant in individually randomized trials. We had planned to include cluster‐randomized trials in the analyses, along with individually randomized trials, but no such trials met our inclusion criteria.
We considered that cross‐over trials would not be feasible for this intervention, and consequently, we did not include such trials.
Dealing with missing data
For included studies, we noted levels of attrition in the Characteristics of included studies tables. We analyzed outcomes on an intention‐to‐treat basis. If this was not clear from the original article, we carried out re‐analysis when possible. We contacted study authors for missing data.
Assessment of heterogeneity
We tested heterogeneity of results among studies by using the quantity I2, which describes the percentage of total variation across studies that is due to heterogeneity rather than to chance (Higgins 2003). A value of 0% indicates no observed heterogeneity, whereas I2 values of 50% or greater indicate a substantial level of heterogeneity. We planned to pool data across studies using the fixed‐effect model if substantial statistical heterogeneity was not present. If we noted substantial heterogeneity (I2 values ≥ 50%), we used a random‐effects model to pool data and made an attempt to identify potential sources of heterogeneity based on subgroup analysis by type of KMC, infant age at initiation of KMC, setting in which the trial was conducted, and risk of bias of trials.
Assessment of reporting biases
We assessed publication and related biases visually by examining the symmetry of funnel plots, and statistically by using Egger's test (Egger 1997). The larger the deviation of the intercept of the regression line from zero, the greater was the asymmetry, and the more likely it was that the meta‐analysis would yield biased estimates of effect. We considered a P value < 0.1 to indicate significant asymmetry, as suggested by Egger.
Data synthesis
We performed statistical analyses using Review Manager software (RevMan 2014) and analyzed outcomes on an intention‐to‐treat basis. If data for similar outcomes from two or more separate studies were available, we combined data in a meta‐analysis and calculated a pooled RR or MD with associated 95% CIs.
Quality of evidence
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: mortality at latest follow‐up, severe infection/sepsis at latest follow‐up, hypothermia at discharge or at 40 to 41 weeks’ postmenstrual age, weight gain at latest follow‐up, any breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age and at one to three months' follow‐up, and psychomotor development at 12 months' corrected age (Griffith quotient for all subscales).
Two authors independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from randomized controlled trials as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We used the GRADEpro 2008 Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades:
-
High: We are very confident that the true effect lies close to that of the estimate of the effect.
-
Moderate: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
-
Low: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
-
Very low: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We performed prespecified subgroup analyses for the primary outcome of mortality at discharge or at 40 to 41 weeks' postmenstrual age and at latest follow‐up according to type of KMC (intermittent vs continuous), daily duration of KMC (< 2 hours vs 6 to 15 hours vs ≥ 20 hours), infant age at initiation of KMC (≤ 10 days vs > 10 days), setting in which the trial was conducted (low/middle‐income countries vs high‐income countries), and infant stabilization status at trial entry (before vs after). For all outcomes in stabilized LBW infants, we performed subgroup analyses according to type of KMC (intermittent vs continuous). We also compared early‐onset KMC (starting within 24 hours post birth) against late‐onset KMC (starting after 24 hours post birth).
It was not possible to perform planned subgroup analyses according to birthweight, gestational age, and type of LBW owing to limited available information.
Sensitivity analysis
We carried out a planned sensitivity analysis to explore the impact of risk of bias on the general direction of findings or on the size of the treatment effect for main outcomes when more than one study contributed data. We did this by excluding trials with high risk of bias in their results as judged by the review authors. For the primary outcomes of "mortality at discharge or at 40 to 41 weeks' postmenstrual age," "mortality at latest follow‐up," ''severe infection/sepsis at latest follow‐up," and "infant growth," we performed sensitivity analyses by excluding trials with unclear allocation concealment and high levels of attrition (> 20%).
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
In the previous version of this review (Conde‐Agudelo 2014), we included 18 trials (Ali 2009; Blaymore Bier 1996; Boo 2007; Cattaneo 1998; Charpak 1997; Eka Pratiwi 2009; Gathwala 2008; Ghavane 2012; Kadam 2005; Nagai 2010; Neu 2010; Ramanathan 2001; Roberts 2000; Rojas 2003; Sloan 1994; Suman 2008; Whitelaw 1988; Worku 2005) and excluded 38 trials (Ahn 2010; Anderson 2003; Arandia 1993; Bera 2014; Bergman 1994; Bergman 2004; Charpak 1994; Chiu 2009; Christensson 1998; Chwo 2002; Dala Sierra 1994; Darmstadt 2006; de Almeida 2010; de Macedo 2007; Feldman 2002; Gregson 2011; Hake Brooks 2008; Huang 2006; Ibe 2004; Kambarami 1998; Kumar 2008; Lai 2006; Lamy Filho 2008; Legault 1993; Legault 1995; Lincetto 2000; Lizarazo‐Medina 2012; Ludington‐Hoe 1991; Ludington‐Hoe 2000; Ludington‐Hoe 2004; Ludington‐Hoe 2006; Miles 2006; Miltersteiner 2005; Mitchell 2013; Ohgi 2002; Sloan 2008; Tallandini 2006; Udani 2008). For this update, the search strategy identified 16 additional studies for possible inclusion, of which we included three (Acharya 2014; Kumbhojkar 2016; Nimbalkar 2014), added one study to awaiting assessment (Holditch‐Davis 2014), and excluded 12 (Badiee 2014; Broughton 2013; Dehghani 2015; Karimi 2014; Kashaninia 2015; Kristoffersen 2016; Lamy Filho 2015; Lyngstad 2014; Mörelius 2015; Samra 2015; Silva 2016; Swarnkar 2016) (Figure 1). One paper by Neu et al (published in 2013) reported additional results of a previously included study (Neu 2010).

Study flow diagram: review update
Included studies
Twenty‐one studies, including 3042 infants, fulfilled inclusion criteria, of which 19 evaluated KMC in LBW infants after stabilization (Acharya 2014; Ali 2009; Blaymore Bier 1996; Boo 2007; Cattaneo 1998; Charpak 1997; Eka Pratiwi 2009; Gathwala 2008; Ghavane 2012; Kadam 2005; Kumbhojkar 2016; Neu 2010; Nimbalkar 2014; Ramanathan 2001; Roberts 2000; Rojas 2003; Sloan 1994; Suman 2008; Whitelaw 1988), one evaluated KMC in LBW infants before stabilization (Worku 2005), and one compared early‐onset KMC with late‐onset KMC (Nagai 2010) in relatively stable LBW infants. Sixteen studies were conducted in low‐ or middle‐income countries (India (Ali 2009; Gathwala 2008; Ghavane 2012; Kadam 2005; Kumbhojkar 2016; Nimbalkar 2014; Ramanathan 2001; Suman 2008); Ethiopia (Cattaneo 1998; Worku 2005); Malaysia (Boo 2007); Madagascar (Nagai 2010); Indonesia (Cattaneo 1998; Eka Pratiwi 2009); Nepal (Acharya 2014); Ecuador (Sloan 1994); Colombia (Charpak 1997); and Mexico (Cattaneo 1998)), and five in high‐income countries (United States (Blaymore Bier 1996; Neu 2010; Rojas 2003); United Kingdom (Whitelaw 1988); and Australia (Roberts 2000)). The sample size ranged from 28 (Ramanathan 2001) to 777 (Charpak 1997) (median, 110). Five studies included infants from multiple pregnancies (Ali 2009; Blaymore Bier 1996; Boo 2007; Charpak 1997; Whitelaw 1988), and six included only infants with birthweight ≤ 1500 g (Blaymore Bier 1996; Boo 2007; Ghavane 2012; Ramanathan 2001; Rojas 2003; Whitelaw 1988). Infants with major congenital malformations or severe perinatal complications and parental refusal to participate in the study were reported as meeting exclusion criteria in the great majority of included studies.
Eight studies did not provide data on the percentage of LBW infants meeting eligibility criteria. Among studies conducted in low‐ or middle‐income countries, 37% (Eka Pratiwi 2009) to 87% (Acharya 2014) of LBW infants met eligibility criteria, whereas for studies conducted in high‐income countries, percentages ranged from 19% (Rojas 2003) to 50% (Whitelaw 1988). The mean or median age of LBW infants at enrollment varied from < one hour (Nimbalkar 2014) to 32 days (Roberts 2000) (median, seven days). Median or mean infant age at enrollment was < one day in three studies (Eka Pratiwi 2009; Nimbalkar 2014; Worku 2005), one to 10 days in eight studies (Ali 2009; Cattaneo 1998; Charpak 1997; Gathwala 2008; Kadam 2005; Kumbhojkar 2016; Nagai 2010; Suman 2008), 11 to 20 days in six studies (Ghavane 2012; Neu 2010; Ramanathan 2001; Rojas 2003; Sloan 1994; Whitelaw 1988), and 21 to 32 days in three studies (Blaymore Bier 1996; Boo 2007; Roberts 2000). One study did not report data on infant age at enrollment (Acharya 2014). In the study that compared early‐onset KMC with late‐onset KMC (Nagai 2010), mean age at initiation of KMC was 19.8 hours in the early‐onset KMC group, and 33.0 hours in the late‐onset KMC. Mean or median weight of infants at recruitment ranged from 968 g (Blaymore Bier 1996) to 2076 g (Nagai 2010) (median, 1611 g).
Trials were conducted under a variety of hospital conditions, regulations, and routines. However, descriptions of the KMC intervention shows remarkable consistency across trials. In all instances, the intervention included SSC and encouraged breastfeeding. Early neonatal discharge from hospital was considered only in the Colombian study (Charpak 1997). Among studies evaluating KMC in stabilized LBW infants, 16 used intermittent KMC (Acharya 2014; Ali 2009; Blaymore Bier 1996; Boo 2007; Eka Pratiwi 2009; Gathwala 2008; Ghavane 2012; Kadam 2005; Kumbhojkar 2016; Neu 2010; Nimbalkar 2014; Ramanathan 2001; Roberts 2000; Rojas 2003; Suman 2008; Whitelaw 1988), and three used continuous KMC (Cattaneo 1998; Charpak 1997; Sloan 1994). Only one study provided a detailed definition of stabilization (Nagai 2010). The mean or median duration of KMC per day was < two hours in six studies (Blaymore Bier 1996; Boo 2007; Neu 2010; Roberts 2000; Rojas 2003; Whitelaw 1988), four to seven hours in three studies (Acharya 2014; Ali 2009; Ramanathan 2001), eight to 17 hours in seven studies (Eka Pratiwi 2009; Gathwala 2008; Ghavane 2012; Kadam 2005; Kumbhojkar 2016; Nimbalkar 2014; Suman 2008), and ≥ 20 hours in three studies (Cattaneo 1998; Charpak 1997; Sloan 1994). Studies that evaluated KMC in LBW infants before stabilization (Worku 2005) and compared early‐onset KMC with late‐onset KMC (Nagai 2010) used continuous KMC. In studies evaluating intermittent KMC, the intervention was a combination of SSC and radiant warmer/incubator. Standard neonatal care included infant stay in incubator only (Blaymore Bier 1996; Boo 2007; Charpak 1997; Neu 2010; Roberts 2000; Rojas 2003; Whitelaw 1988) or in radiant warmer only (Acharya 2014; Ali 2009; Kadam 2005; Kumbhojkar 2016; Nimbalkar 2014; Suman 2008; Worku 2005) or in incubator or radiant warmer (Cattaneo 1998; Eka Pratiwi 2009; Gathwala 2008; Ghavane 2012; Ramanathan 2001; Sloan 1994). Information provided to mothers in the conventional neonatal care group on promotion of breastfeeding and on facilitation and promotion of maternal involvement in the care of the neonate, which are critical for the outcomes measured, was not reported in eight trials (Acharya 2014; Blaymore Bier 1996; Charpak 1997; Eka Pratiwi 2009; Kumbhojkar 2016; Nagai 2010; Suman 2008; Worku 2005).
Eleven studies were performed in neonatal intensive care units of tertiary care, public, maternity, or university hospitals (Ali 2009; Boo 2007; Eka Pratiwi 2009; Kadam 2005; Kumbhojkar 2016; Ramanathan 2001; Roberts 2000; Rojas 2003; Sloan 1994; Suman 2008; Whitelaw 1988), four in neonatal units of university hospitals (Cattaneo 1998; Gathwala 2008; Nagai 2010; Worku 2005), two in "kangaroo wards" (KMC infants) and neonatal intensive/intermediate care units of tertiary care hospitals (controls) (Charpak 1997; Ghavane 2012), two in newborn nurseries (Acharya 2014; Blaymore Bier 1996), one in a maternity ward (Nimbalkar 2014), and one in both hospital and home (Neu 2010). Infants were cared for by both doctors and nurses in all but two studies (Ghavane 2012; Neu 2010). In the Ghavane 2012 study, infants in the KMC group were cared for solely by their mothers, who was supervised by a trained nurse. In the Neu 2010 study, the supportive intervention that promoted kangaroo holding of preterm infants by their mothers was performed by an experienced nurse. Nine studies reported clearly on criteria for discharging infants from the hospital (Ali 2009; Boo 2007; Cattaneo 1998; Charpak 1997; Ghavane 2012; Kadam 2005; Kumbhojkar 2016; Ramanathan 2001; Suman 2008). The most commonly reported criteria were (1) good general health of the infant without overt illness; (2) feeding well on exclusive or predominant breastfeeding; (3) weight gain of 10 to 15 g/kg/d for ≥ three consecutive days; (4) stable temperature for ≥ three consecutive days; and (5) mother confident of taking care of the infant at home. In addition, three studies included an infant weight of ≥ 1300 to 1500 g as a discharge criterion. Thirteen studies reported on schemes for follow‐up of infants after discharge from the hospital (Ali 2009; Blaymore Bier 1996; Cattaneo 1998; Charpak 1997; Gathwala 2008; Ghavane 2012; Kumbhojkar 2016; Nagai 2010; Neu 2010; Roberts 2000; Sloan 1994; Suman 2008; Whitelaw 1988). In summary, the most common scheme for follow‐up was the following: weekly until 40 weeks' postmenstrual age, and monthly thereafter until three to six months of age or corrected age. In five studies, the last follow‐up was provided at six months of age or corrected age (Ali 2009; Blaymore Bier 1996; Neu 2010; Roberts 2000; Sloan 1994). Infants were followed up to 12 months of age or corrected age in only two studies (Charpak 1997; Whitelaw 1988).
The main characteristics of the included studies are shown in the table Characteristics of included studies.
Excluded studies
We excluded 50 studies: 21 because they were non‐randomized trials (Ahn 2010; Arandia 1993; Bera 2014; Bergman 1994; Broughton 2013; Charpak 1994; Dala Sierra 1994; de Almeida 2010; de Macedo 2007; Feldman 2002; Gregson 2011; Ibe 2004; Kashaninia 2015; Kristoffersen 2016; Lamy Filho 2008; Legault 1995; Lincetto 2000; Lizarazo‐Medina 2012; Ohgi 2002; Silva 2016; Tallandini 2006), 10 because they included infants with birthweight ≥ 2500 g and did not report results separately for the subgroup of infants with birthweight < 2500 g (Anderson 2003; Chiu 2009; Chwo 2002; Hake Brooks 2008; Huang 2006; Karimi 2014; Lai 2006; Mörelius 2015; Samra 2015; Sloan 2008), seven because they reported only physiological outcomes (Bergman 2004; Dehghani 2015; Ludington‐Hoe 1991; Ludington‐Hoe 2000; Ludington‐Hoe 2004; Ludington‐Hoe 2006; Mitchell 2013), three because the method of generation of allocation to treatment was quasi‐randomized (Kambarami 1998; Miltersteiner 2005; Swarnkar 2016), three because allocation was performed by a cross‐over design (Legault 1993; Lyngstad 2014; Miles 2006), two because KMC was part of a preventive package of interventions for essential newborn care (Darmstadt 2006; Kumar 2008), one because it evaluated only KMC for rewarming hypothermic infants (Christensson 1998), one because it assessed only the effect of KMC on the mental health of mothers (Badiee 2014), one because it evaluated only the effect of KMC on colonization status of newborns’ nostrils (Lamy Filho 2015), and one because it was published as an abstract only, and our attempts to locate full publications or to contact study authors were unsuccessful (Udani 2008).
We have presented the main characteristics of the excluded studies in the table Characteristics of excluded studies.
Risk of bias in included studies
We have depicted the risk of bias in included studies in Figure 2. We judged that no study adequately addressed all seven domains. We judged only two studies to adequately address six domains. The methodological quality of the included trials was mixed, and we carried out a sensitivity analysis to examine the impact of excluding trials at high risk of bias. See Sensitivity analysis. The main threats to validity were performance bias (by lack of blinding of participants, personnel, and outcomes assessors) and selection bias (by lack of information on methods used for concealment of treatment allocation).

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Most of the included studies used adequate methods to generate allocation sequence. Ten studies used random number tables (Acharya 2014; Cattaneo 1998; Charpak 1997; Eka Pratiwi 2009; Gathwala 2008; Nimbalkar 2014; Ramanathan 2001; Rojas 2003; Sloan 1994; Worku 2005), and four studies used shuffling of envelopes (Blaymore Bier 1996; Boo 2007; Roberts 2000; Whitelaw 1988). Other methods of sequence generation used included web‐based random number generator (Ghavane 2012), computer random number generator (Neu 2010), minimization computerized technique (Nagai 2010), block randomization technique (Ali 2009), the sealed envelope method (Kadam 2005), and simple randomization (Kumbhojkar 2016; Suman 2008).
Ten studies used sealed envelopes for concealment of treatment allocation (Boo 2007; Ghavane 2012; Kadam 2005; Kumbhojkar 2016; Neu 2010; Nimbalkar 2014; Roberts 2000; Rojas 2003; Suman 2008; Whitelaw 1988), although only five studies (Ghavane 2012; Neu 2010; Nimbalkar 2014; Rojas 2003; Whitelaw 1988) explicitly stated that the envelopes were opaque, sealed, and numbered. Investigators concealed allocation by using a software that provided automatically random allocation (minimization method) in only one study (Nagai 2010). Ten studies did not report the method of allocation concealment (Acharya 2014; Ali 2009; Blaymore Bier 1996; Cattaneo 1998; Charpak 1997; Eka Pratiwi 2009; Gathwala 2008; Ramanathan 2001; Sloan 1994; Worku 2005).
Blinding
As KMC cannot be implemented when masked, all included studies reported lack of blinding of participants and clinical staff. Only two studies (Ghavane 2012; Nagai 2010) reported that outcome assessors were masked to the intervention group of infants. Neu 2010 reported that four researchers assessed outcome measures, two of whom were blinded to the hypotheses of the study but not to group assignment of mother‐infant dyads. The other two researchers were blinded to group assignment and hypotheses. The remaining trials did not state whether any attempt was made to "blind" outcome assessment.
We consider that performance and observer bias cannot be excluded owing to lack of blinding of participants and clinicians. However, although this could affect assessment of subjective outcomes such as parental and familial satisfaction, mother‐infant attachment, and social and home environment, or objective outcomes such as breastfeeding, length of hospital length, and re‐admission to hospital after discharge, it is much less likely to have affected the primary outcomes (infant mortality, severe infection/sepsis, severe illness, infant growth, and neurodevelopmental disability) and some secondary outcomes of this review (nosocomial infection, mild/moderate infection or illness, hypothermia, and hyperthermia).
Incomplete outcome data
Eight trials had no losses to follow‐up and no exclusions post randomization (Acharya 2014; Kadam 2005; Nagai 2010; Nimbalkar 2014; Ramanathan 2001; Roberts 2000; Rojas 2003; Whitelaw 1988). In seven studies, 1% to 10% of recruited infants were lost to follow‐up (Ali 2009; Blaymore Bier 1996; Charpak 1997; Eka Pratiwi 2009; Gathwala 2008; Ghavane 2012; Sloan 1994). Boo 2007 excluded 12.3% of infants in the KMC group because SSC sessions were carried out on less than 50% of hospital stay days after recruitment. Two trials (Cattaneo 1998; Worku 2005) did not report the number of infants lost to follow‐up or excluded after randomization. Kumbhojkar 2016 did not report the number of infants lost to follow‐up or exclusions, but investigators stated in the Discussion section of the article that "poor follow‐up" was provided in the control group. Suman 2008 had high risk of attrition bias because 22.3% of infants were lost to follow‐up. Moreover, imbalance across intervention groups was evident in numbers for losses to follow‐up (KMC 10.2%; control 33.9%). In addition, 6.4% of infants were omitted from reports of analyses because they did not receive assigned care. Neu 2010 had high risk of attrition bias because 9.2% of infants were lost to follow‐up and 16.1% were excluded post randomization.
Selective reporting
No study protocols were available. We compared outcomes listed in the Methods section of articles against those reported in the Results section. Sixteen studies (Acharya 2014; Blaymore Bier 1996; Boo 2007; Cattaneo 1998; Charpak 1997; Eka Pratiwi 2009; Gathwala 2008; Ghavane 2012; Kadam 2005; Kumbhojkar 2016; Nagai 2010; Neu 2010; Nimbalkar 2014; Roberts 2000; Rojas 2003; Suman 2008) reported all outcomes listed in the Methods section, and we assume that these reports probably included all prespecified variables. Two studies (Ali 2009; Worku 2005) had high risk of bias owing to selective outcome reporting. Worku 2005 did not report the great majority of outcomes listed in the Methods section, such as mild/moderate and severe illness, sepsis, diarrhea, pneumonia, aspiration, weight gain, and mother's feelings. In Ali 2009, non‐significant results such as infant mortality (primary outcome) and weight, length, and head circumference at discharge and follow‐up (secondary outcomes) were mentioned but were not reported adequately. In the remaining three studies, some secondary outcomes listed in the Methods section were not reported (Ramanathan 2001), or they were mentioned but were not reported adequately (Sloan 1994; Whitelaw 1988).
Other potential sources of bias
We did not identify other potential sources of bias in 15 studies (Acharya 2014; Blaymore Bier 1996; Boo 2007; Gathwala 2008; Ghavane 2012; Kadam 2005; Kumbhojkar 2016; Nagai 2010; Neu 2010; Nimbalkar 2014; Ramanathan 2001; Roberts 2000; Rojas 2003; Whitelaw 1988; Worku 2005). Three studies (Ali 2009; Charpak 1997; Eka Pratiwi 2009) used blocked randomization for sequence generation. When blocked randomization is used in an unblinded trial, and when assignments are revealed after individuals are recruited into the trial, it is sometimes possible to predict future assignments. This is particularly the case when blocks are of a fixed size. Cattaneo 1998 carried out randomization in blocks of six with stratification by weight at one of the three participating centers. The trial performed by Sloan 1994 was stopped early because investigators found a highly significant difference in severe morbidity at two months and at six months. Randomized controlled trials that are stopped early are more likely to be associated with greater effect sizes than RCTs not stopped early (Bassler 2010). This difference is independent of the presence of statistical stopping rules and is greatest in smaller studies. In the study by Suman 2008, groups were significantly different at baseline in weight and age at enrollment.
Effects of interventions
Comparison 1. Kangaroo mother care versus conventional neonatal care
The comparison between KMC and conventional neonatal care included 20 studies (2969 infants) and 49 outcomes, of which 24 were reported in more than one study.
Mortality (outcomes 1.1 to 1.4)
Kangaroo mother care was associated with a statistically significant reduction in risk of mortality at discharge or at 40 to 41 weeks’ postmenstrual age (3.2% vs 5.3%; RR 0.60, 95% CI 0.39 to 0.92; I2 = 0%; NNTB = 47, 95% CI 31 to 236; eight trials, 1736 infants) (Analysis 1.1), and at latest follow‐up (4.0% vs 6.0%; RR 0.67, 95% CI 0.48 to 0.95; I2 = 0%; NNTB = 50, 95% CI 32 to 331; 12 trials, 2293 infants; moderate‐quality evidence) (Analysis 1.4) (Figure 3). The significantly decreased risk of death at discharge or at 40 to 41 weeks’ postmenstrual age, and at latest follow‐up, was also demonstrated in the subgroup of studies that used continuous (≥ 20 hours/d) KMC (mortality at discharge or at 40 to 41 weeks’ postmenstrual age: RR 0.60, 95% CI 0.38 to 0.96; I2 = 0%; three trials, 1117 infants; mortality at latest follow‐up: RR 0.67, 95% CI 0.46 to 0.98; I2 = 0%; four trials, 1384 infants), the subgroup of studies in which KMC was initiated within 10 days post birth (mortality at discharge or at 40 to 41 weeks’ postmenstrual age: RR 0.56, 95% CI 0.36 to 0.88; I2 = 0%; five trials, 1412 infants; mortality at latest follow‐up: RR 0.56, 95% CI 0.37 to 0.85; I2 = 0%; six trials, 1489 infants), the subgroup of studies conducted in low/middle‐income countries (mortality at discharge or at 40 to 41 weeks’ postmenstrual age: RR 0.57, 95% CI 0.37 to 0.89; I2 = 0%; seven studies, 1676 infants; mortality at latest follow‐up: RR 0.65, 95% CI 0.45 to 0.93; I2 = 0%; 10 trials, 2162 infants), and the trial in which KMC was used in unstabilized infants (RR 0.57, 95% CI 0.33 to 1.00). The statistically significant beneficial effect of KMC on mortality at discharge or at 40 to 41 weeks’ postmenstrual age and on mortality at latest follow‐up was not demonstrated in the subgroup of trials that used intermittent KMC (< 2 hours/d and between 6 and 15 hours/d), or that initiated KMC after 10 days post birth, or that were conducted in high‐income countries, or that used KMC in stabilized infants.
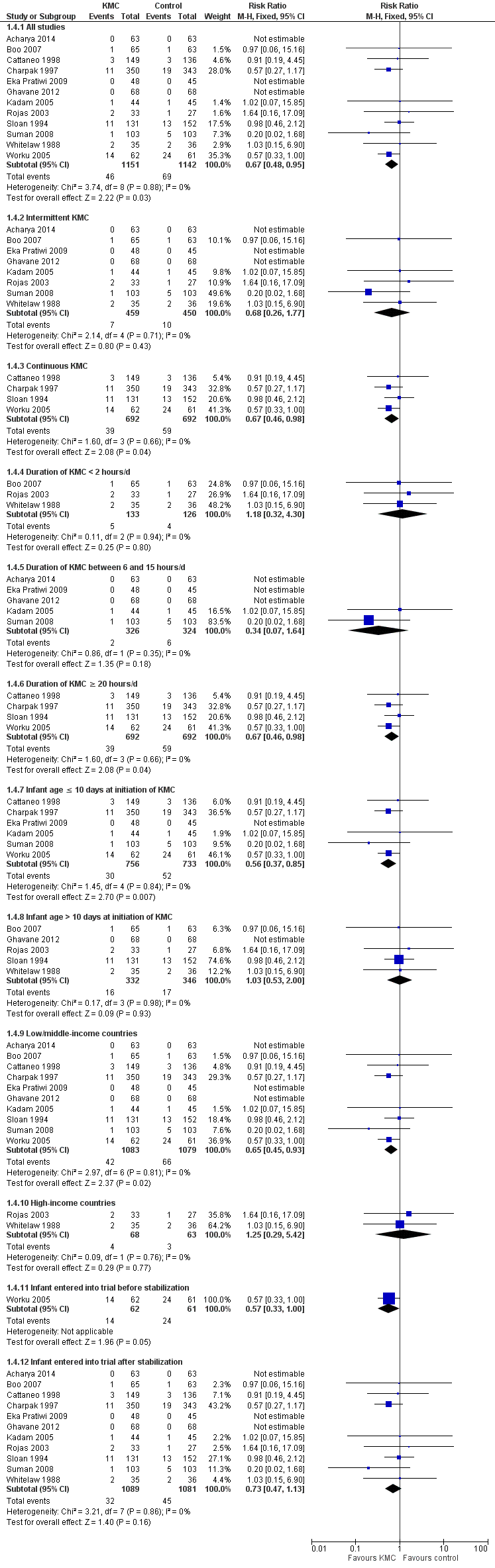
Forest plot of comparison: 1 Kangaroo mother care versus conventional neonatal care, outcome: 1.1 Mortality at latest follow‐up.
The sensitivity analysis limited to studies with adequate concealment of allocation revealed a similar reduction in mortality at discharge or at 40 to 41 weeks’ postmenstrual age, and at latest follow‐up, although this was not statistically significant (mortality at discharge or at 40 to 41 weeks’ postmenstrual age: RR 0.59, 95% CI 0.19 to 1.81; I2 = 0%; five trials; mortality at latest follow‐up: RR 0.68, 95% CI 0.26 to 1.77; I2 = 0%; six trials). Similar results were obtained when we excluded studies with high risk of attrition bias (mortality at discharge or at 40 to 41 weeks’ postmenstrual age: RR 0.64, 95% CI 0.41 to 1.00; I2 = 0%; six studies; mortality at latest follow‐up: RR 0.71, 95% CI 0.49 to 1.01; I2 = 0%; 10 studies).
We found no overall difference in risk of mortality at six months of age or at six months' follow‐up (RR 0.99, 95% CI 0.48 to 2.02; two trials, 354 infants) (Analysis 1.2), and at 12 months’ corrected age (RR 0.57, 95% CI 0.27 to 1.17; one trial, 693 infants) (Analysis 1.3) between KMC infants and controls.
Infection/illness (outcomes 1.5 to 1.14)
In stabilized LBW infants, KMC was associated with a statistically significant reduction in severe infection/sepsis at latest follow‐up (6.6% vs 13.1%; RR 0.50, 95% CI 0.36 to 0.69; I2 = 24%; NNTB = 15, 95% CI 12 to 25; eight trials, 1463 infants; moderate‐quality evidence) (Analysis 1.5) (Figure 4), severe illness at six months' follow‐up (5.3% vs 17.8%; RR 0.30, 95% CI 0.14 to 0.67; NNTB = 8, 95% CI 7 to 17; one trial, 283 infants) (Analysis 1.6), nosocomial infection/sepsis at discharge or at 40 to 41 weeks’ postmenstrual age (4.0% vs 11.4%; RR 0.35, 95% CI 0.22 to 0.54; I2 = 0%; NNTB = 14, 95% CI 11 to 19; five trials, 1239 infants) (Analysis 1.7), lower respiratory tract disease at six months' follow‐up (4.6% vs 12.5%; RR 0.37, 95% CI 0.15 to 0.89; NNTB = 13, 95% CI 9 to 73; one trial, 283 infants) (Analysis 1.9), and hypothermia at discharge or at 40 to 41 weeks' postmenstrual age (7.6% vs 27.1%; RR 0.28, 95% CI 0.16 to 0.49; I2 = 52%; NNTB = 5, 95% CI 4 to 7; nine trials, 989 infants; moderate‐quality evidence) (Analysis 1.11).

Forest plot of comparison: 1 Kangaroo mother care versus conventional neonatal care, outcome: 1.2 Severe infection/sepsis at latest follow‐up ‐ stabilized infants.
Only the subgroup of trials that used intermittent KMC demonstrated significantly reduced risk of severe infection/sepsis at latest follow‐up and hypothermia at discharge or at 40 to 41 weeks' postmenstrual age. Subgroups of trials that used intermittent or continuous KMC showed a statistically significantly reduced risk of nosocomial infection/sepsis at discharge or at 40 to 41 weeks’ postmenstrual age.
We found no overall difference between KMC infants and controls in risk of mild/moderate infection or illness at latest follow‐up (RR 1.28, 95% CI 0.87 to 1.88) (Analysis 1.8), diarrhea at six months' follow‐up (RR 0.65, 95% CI 0.35 to 1.20) (Analysis 1.10), hyperthermia at discharge or at 40 to 41 weeks' postmenstrual age (RR 0.79, 95% CI 0.59 to 1.05) (Analysis 1.12), and re‐admission to hospital at latest follow‐up (RR 0.60, 95% CI 0.34 to 1.06) (Analysis 1.14).
Intermittent KMC decreased length of hospital stay by 1.6 days, although this difference was not statistically significant (95% CI ‐0.2 to 3.4; P value = 0.08; 11 studies, 1057 infants) (Analysis 1.13). Mean hospital stay from randomization to 41 weeks' postmenstrual age was 4.5 days for KMC infants and 5.6 days for control infants in Charpak 1997. Investigators provided no standard deviations. Cattaneo 1998 reported only median hospital stay, which was 11 days in the KMC group versus 13 days in the control group. Length of hospital stay was two days greater in KMC infants than in control infants in Sloan 1994.
Sensitivity analyses using only studies with adequate allocation concealment demonstrated a similar result for severe infection/sepsis at latest follow‐up (RR 0.40, 95% CI 0.24 to 0.66) and for hypothermia at discharge or at 40 to 41 weeks' postmenstrual age (RR 0.24, 95% CI 0.16 to 0.36). Additional sensitivity analyses did not indicate that removing the study with high risk of attrition bias (Suman 2008) had any important impact on overall effects of KMC on severe infection/sepsis at latest follow‐up (RR 0.54, 95% CI 0.38 to 0.76) and on hypothermia at discharge or at 40 to 41 weeks' postmenstrual age (RR 0.33, 95% CI 0.23 to 0.48).
Infant growth (outcomes 1.15 to 1.26)
Infants given kangaroo mother care gained more weight per day (MD 4.1 g, 95% CI 2.3 to 5.9; 11 trials, 1198 infants; moderate‐quality evidence) (Analysis 1.18) (Figure 5) and had greater increases in length (MD 0.21 cm, 95% CI 0.03 to 0.38; three trials, 377 infants) (Analysis 1.22) and head circumference (MD 0.14 cm, 95% CI 0.06 to 0.22; four trials, 495 infants) (Analysis 1.26) per week than controls. Nevertheless, considerable heterogeneity was evident (I2 > 70%) among trials reporting gain in weight, length, and head circumference. One trial (Charpak 1997) reported that KMC infants had a larger head circumference at 6 months' corrected age than controls (MD 0.34 cm, 95% CI 0.11 to 0.57; 592 infants) (Analysis 1.24). Investigators observed no differences in weight, length, or head circumference at discharge or at 40 to 41 weeks' postmenstrual age (Analysis 1.15; Analysis 1.19; Analysis 1.23) or at 12 months' corrected age (Analysis 1.17; Analysis 1.21; Analysis 1.25), or in weight or length at 6 months’ corrected age (Analysis 1.16; Analysis 1.20). Sloan 1994 reported, "there were no significant differences between the groups in growth indices during the six‐month follow up."

Forest plot of comparison: 1 Kangaroo mother care versus conventional neonatal care, outcome: 1.10 Weight gain at latest follow‐up (g/d) ‐ stabilized infants.
We undertook sensitivity analysis by excluding studies with unclear allocation concealment and high risk of attrition bias to examine the impact on increases in both weight and head circumference. We found no differences in the overall direction of findings.
Neurodevelopmental and neurosensory impairment (outcomes 1.27 to 1.30)
Only one study (Charpak 1997) reported results for neurodevelopmental and neurosensory impairment at one year of corrected age. Researchers found no statistically significant differences between KMC infants and controls in Griffith quotients for psychomotor development (low‐quality evidence) (Analysis 1.27), cerebral palsy (Analysis 1.28), deafness (Analysis 1.29), and visual impairment (Analysis 1.30). A secondary publication of the Charpak 1997 trial reported that the subgroup of KMC infants with birthweight ≤ 1800 g had a higher general developmental quotient than controls at one year of corrected age (P value < 0.01).
Breastfeeding (outcomes 1.31 to 1.40)
Mothers of KMC infants were more likely to be breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age and at one to three months' follow‐up than mothers in the control group. Compared with conventional care, KMC was associated with an increase in the likelihood of exclusive breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age (66.3% vs 56.3%; RR 1.16, 95% CI 1.07 to 1.25; I2 = 39%; NNTB = 11, 95% CI 7 to 25; six studies, 1453 mothers) (Analysis 1.31), and at one to three months' follow‐up (86.9% vs 76.5%; RR 1.20, 95% CI 1.01 to 1.43; I2 = 76%; NNTB = 7, 95% CI 3 to 131; five studies, 600 mothers) (Analysis 1.32), or any (exclusive or partial) breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age (88.9% vs 76.2%; RR 1.20, 95% CI 1.07 to 1.34; I2 = 80%; NNTB = 7, 95% CI 4 to 19; 10 studies, 1696 mothers; moderate‐quality evidence) (Analysis 1.34) (Figure 6), at one to two months' follow‐up (77.9% vs 67.9%; RR 1.33, 95% CI 1.00 to 1.78; I2 = 78%; six studies, 538 mothers) (Analysis 1.35), at 3 months' follow‐up (79.7% vs 69.8%; RR 1.14, 95% CI 1.06 to 1.23; I2 = 41%; five studies, 924 mothers) (Analysis 1.36), and at one to three months' follow‐up (80.4% vs 71.1%; RR 1.17, 95% CI 1.05 to 1.31; I2 = 62%; NNTB = 8, 95% CI 5 to 28; nine studies, 1394 mothers; low‐quality evidence) (Analysis 1.37). It should be noted that heterogeneity was substantial (I2 > 50%) among trials reporting breastfeeding. Overall, investigators found no statistically significant differences between KMC and control for exclusive or any breastfeeding at six to 12 months' follow‐up (Analysis 1.33; Analysis 1.38; Analysis 1.39) and at onset of breastfeeding (Analysis 1.40). However, subgroup analyses showed that intermittent KMC was associated with a significant increase in exclusive breastfeeding at six to 12 months' follow‐up (84.6% vs 55.6%; RR 1.52, 95% CI 1.10 to 2.10; one study, 75 women) and in any breastfeeding at six months' follow‐up (54.7% vs 36.8%; RR 1.50, 95% CI 1.08‐2.08; three studies, 143 women).

Forest plot of comparison: 1 Kangaroo mother care versus conventional neonatal care, outcome: 1.34 Any breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age ‐ stabilized infants.
Statistically significant positive effects of KMC on breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age and at one to three and six months' follow‐up were demonstrated in the subgroup of trials that used intermittent KMC but not in the subgroup of trials that used continuous KMC. In addition, an increase in the likelihood of any breastfeeding at one to two months' follow‐up was demonstrated in the subgroup of three trials (131 infants) conducted in high‐income countries (RR 2.02, 95% CI 1.28 to 3.21; I2 = 23%).
Parental and familial satisfaction (outcome 1.41)
Only one study (Cattaneo 1998) evaluated parental and familial satisfaction with method of infant care. Mothers in the KMC group were more satisfied with the method of care than were mothers in the control group (91% vs 78%; RR 1.17, 95% CI 1.05 to1.30; 269 mothers) (Analysis 1.41). Investigators found no significant differences in satisfaction with method of care between fathers and families of KMC and control groups.
Mother‐infant attachment or interaction (outcomes 1.42 to 1.49)
Three studies (Charpak 1997; Gathwala 2008; Roberts 2000) reported results on mother‐infant attachment, and one (Neu 2010) on mother‐infant interaction.
A secondary publication of the Charpak 1997 trial reported two series of outcomes that were assessed as manifestations of mother‐infant attachment. The first was the mother's feelings and perceptions of her premature birth experience, measured through a "mother's perception of premature birth questionnaire" using a Likert scale (1 to 5), 24 hours after birth and when the infant reached 41 weeks' postmenstrual age. The second outcome was derived from observations of the mother's and child's responsiveness to each other during breastfeeding, using a "nursing child assessment feeding scale." Researchers compared a total of nine items between KMC and control groups according to the interval between birth and start of the intervention (one to two days, three to 14 days, and longer than 14 days), as well as admission of the infant to the neonatal intensive care unit (NICU) (yes or not), for a total of 45 comparisons. Overall, scores on six comparisons (mother's sense of competence [interval between birth and start of intervention of one to two days], mother's sense of competence [infant admitted to NICU], mother's sense of competence [infant not admitted to NICU], mother’s feelings of worry and stress [interval between birth and start of intervention of one to two days], mother’s sensitivity [interval between birth and start of intervention > 14 days], and infant responsiveness [interval between birth and start of intervention > 14 days]) were significantly higher in the KMC group than in the control group. Scores on two comparisons (mother’s perceptions of social support [interval between birth and start of intervention > 14 days, and infant not admitted to NICU]) were significantly lower in the KMC group than in the control group. Results showed no significant differences in scores for the remaining 37 comparisons (Analysis 1.42; Analysis 1.43; Analysis 1.44).
Gathwala 2008 evaluated mother‐infant attachment at three months' follow‐up through a structured maternal interview that used attachment questions scored in such a manner that a higher score indicated greater attachment. The total attachment score for the KMC group (24.46 ± 1.64) was significantly higher than that obtained for the control group (18.22 ± 1.79) (Analysis 1.45).
Roberts 2000 measured maternal stress levels in the NICU and mothers' perceptions of their maternal competence. Only the score on the scale for "relationship with the infant" was significantly higher in the KMC group (4.4 ± 0.46) than in the control group (3.4 ± 1.16). Researchers found no significant differences between KMC and control group scores on nursery environment, infant appearance, staff behavior and communication, and parental confidence in their parenting abilities (Analysis 1.46; Analysis 1.47).
Neu 2010 evaluated the mother‐infant interaction at six months of age by using the Stiil‐Face Paradigm tool. Mother‐infant dyads in the KMC group showed more symmetrical, and less asymmetrical, co‐regulation than mother‐infant dyads in the control group (Analysis 1.48). Multivariate analysis showed no differences between groups in infant vitality during the neutral face portion of the Stiil‐Face procedure. A secondary publication of the Neu 2010 study reported that KMC infants had similar scores for behavioral regulation and development to those of infants who experienced nurse‐supported blanket holding at 40 to 44 weeks' postmenstrual age (Analysis 1.49).
Home environment and father involvement (outcome 1.50)
One trial (Charpak 1997) evaluated home environment and father involvement at 12 months' corrected age through a structured interview administered to parents during a home visit. The total Home Observation for Measurement of the Environment (HOME) score was significantly higher among kangaroo families (0.28 ± 0.24) than in conventional care families (‐0.51 ± 0.26) (Analysis 1.50). Scores on father involvement were not reported, but study authors claimed that KMC increased father involvement (the father's sense of responsibility and competence).
Costs of care
No study reported data on mean (SD) total medical and non‐medical costs for KMC and control groups. The overall cost was "about 50% less for KMC" in the Cattaneo 1998 study. Specifically, the cost was US $19,289 for KMC and US $39,764 for conventional care. In the Sloan 1994 study, "costs of neonatal care were greater in the control than in the KMC group." Overall, the cost of hospital stay and postneonatal care at five months was US $741 greater for the control than the KMC group. However, data were available for only 49 infants (24 KMC, 25 control) at six months' follow‐up.
All funnel plots showed no asymmetry, either visually or in terms of statistical significance (P value > 0.10 for all, by Egger's test).
Comparison 2. Early‐onset kangaroo mother care versus late‐onset kangaroo mother care in relatively stable infants
Only one trial (Nagai 2010), which was considered at low risk of bias, compared early‐onset KMC versus late‐onset KMC in relatively stable LBW infants. Early continuous KMC was begun as soon as possible, within 24 hours post birth, and late continuous KMC was begun after complete stabilization, generally after 24 hours post birth. This study included a total of 73 LBW infants (early 37, late 36). Investigators reported no statistically significant differences between early‐onset KMC and late‐onset KMC for mortality at four weeks of age (RR 1.95, 95% CI 0.18 to 20.53) (Analysis 2.1) and at six months of age (RR 1.00, 95% CI 0.15 to 6.72) (Analysis 2.10), morbidity (RR 0.49, 95% CI 0.18 to 1.28) (Analysis 2.2) and severe infection (RR 0.42, 95% CI 0.12 to 1.49) (Analysis 2.3) at four weeks of age, re‐admission to hospital at four weeks of age (RR 1.95, 95% CI 0.18 to 20.53) (Analysis 2.4) and at six to 12 months of age (RR 1.00, 95% CI 0.32 to 3.16) (Analysis 2.11), hypothermia (RR 0.58, 95% CI 0.15 to 2.27) (Analysis 2.5), hyperthermia (RR 1.05, 95% CI 0.56 to 1.99) (Analysis 2.6), weight gain at four weeks of age (MD 58.9 g, 95% CI ‐116.9 to 234.6) (Analysis 2.7), exclusive breastfeeding at four weeks of age (RR 0.94, 95% CI 0.85 to 1.04) (Analysis 2.8), and stunting (RR 0.83, 95% CI 0.46 to 1.48) (Analysis 2.12), severe stunting (RR 0.67, 95% CI 0.17 to 2.73) (Analysis 2.13), wasting (RR 0.10, 95% CI 0.01 to 1.77) (Analysis 2.14), severe wasting (RR 0.00, 95% CI 0.00 to 0.00) (Analysis 2.15), underweight (RR 0.49, 95% CI 0.21 to 1.14) (Analysis 2.16), and severe underweight (RR 0.22, 95% CI 0.03 to 1.88) (Analysis 2.17) at six to 12 months of age. However, compared with late‐onset KMC, early‐onset KMC was associated with a statistically significant reduction in body weight loss from birth to 48 hours post birth (MD 43.3 g, 95% CI 5.5 to 81.1) (Analysis 2.7) and in length of hospital stay (MD 0.9 days, 95% CI 0.6 to 1.2) (Analysis 2.9). In addition, early‐onset KMC was associated with a non‐significant increase in the likelihood of exclusive breastfeeding at six months of age (41.4% vs 15.4%; RR 2.69, 95% CI 0.99 to 7.31) (Analysis 2.8).
Discussion
Summary of main results
This updated systematic review of 20 randomized controlled trials (RCTs) comparing kangaroo mother care (KMC) and conventional neonatal care found compelling evidence that KMC is associated with a reduction in mortality at discharge or at 40 to 41 weeks' postmenstrual age and at latest follow‐up, severe infection/sepsis, and hypothermia, and an increase in weight gain and in exclusive or any breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age and at one to three months' follow‐up. Moreover, growing evidence indicates that KMC reduces the risk of nosocomial infection/sepsis at discharge or at 40 to 41 weeks’ postmenstrual age, and increases the gain in length and head circumference, maternal satisfaction with the method, maternal‐infant attachment, and home environment. One trial (Charpak 1997) reported no significant differences between KMC infants and controls in a variety of neurodevelopmental and neurosensory outcomes at one year of corrected age.
Overall, continuous KMC led to a reduction in mortality at discharge or at 40 to 41 weeks' postmenstrual age and at latest follow‐up, and in nosocomial infection/sepsis, severe illness, and lower respiratory tract disease, and an increase in weight gain, maternal satisfaction with the method, and some measures of mother‐infant attachment and home environment. On the other hand, intermittent KMC was associated with a decrease in the risk of severe infection/sepsis, nosocomial infection/sepsis, and hypothermia, and an increase in weight, length, and head circumference gain, exclusive or any breastfeeding at discharge or 40 to 41 weeks' postmenstrual age and at one to three months' follow‐up, and mother‐infant attachment at three months' follow‐up.
Subgroup analyses showed that decreased risk of death at discharge or at 40 to 41 weeks' postmenstrual age and at latest follow‐up was demonstrated in the subgroup of trials that used continuous KMC (≥ 20 hours/d), the subgroup of trials in which KMC was initiated within 10 days post birth, the subgroup of trials conducted in low/middle‐income countries, and the trial in which KMC was used in unstabilized infants. Sensitivity analysis suggested that inclusion of studies with high risk of bias did not affect the general direction of findings nor the size of the treatment effect, although the beneficial effect of KMC on mortality turned non‐significant or marginally significant.
One small high‐quality trial (Nagai 2010) suggested that early‐onset KMC, compared with late‐onset KMC, is associated with a significant reduction in body weight loss from birth to 48 hours post birth and in length of hospital stay, and a marginally significant increase in the likelihood of exclusive breastfeeding at six months of age, with no significant difference in mortality, morbidity, severe infection, re‐admission to hospital, hypothermia, hyperthermia, exclusive breastfeeding at four weeks of age, or infant nutritional indicators at six to 12 months of age.
Overall completeness and applicability of evidence
Participants in the included trials reflect the population for which this intervention is currently considered, that is, low birthweight (LBW)/preterm infants. Sixteen trials, including all five trials that evaluated continuous KMC, were conducted in hospitals in low/middle‐income countries. Mortality at discharge was the only outcome reported in the sole trial (Worku 2005) that compared KMC with conventional neonatal care in LBW infants before stabilization. The remaining 48 outcomes were reported in 19 trials that evaluated KMC in stabilized LBW infants. We were unable to draw conclusions about the effectiveness of KMC in unstabilized LBW infants. Given these factors, the great majority of results of our meta‐analysis can be applied only to stabilized LBW infants in low/middle‐income countries. However, the beneficial effect of KMC on any breastfeeding at one to two months' follow‐up was also found among stabilized LBW infants in high‐income countries.
As only a small trial compared early‐onset KMC with late‐onset KMC, review authors could draw no firm conclusions regarding apparent differences between these two types of management.
One randomized controlled cluster trial (Sloan 2008) assessed the effect of community‐based KMC on overall neonatal mortality, infant mortality, and LBW neonatal mortality; investigators assigned 4165 infants in rural Bangladesh to community‐based KMC or control without KMC. Unfortunately, we did not include this study in the review because 40% overall and 65% of newborns who died were not weighed at birth, and missing birthweight was differential for study group. Results show no difference in overall neonatal mortality rate or infant mortality rate. However, for infants whose modeled birthweight was ≤ 2000 g, the neonatal mortality rate was 9.5% in the community‐based KMC group and 22.5% in the control group (adjusted odds ratio 0.37, 95% confidence interval [CI] 0.16 to 0.86).
Quality of the evidence
Overall, we assessed the quality of the evidence as moderate for most critical and important outcomes (summary of findings Table for the main comparison). We evaluated the risk of bias in included studies by addressing seven specific domains (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias) discussed in section Risk of bias in included studies. Review authors judged that 12 studies adequately addressed at least four domains (Acharya 2014; Blaymore Bier 1996; Boo 2007; Ghavane 2012; Kadam 2005; Kumbhojkar 2016; Nagai 2010; Neu 2010; Nimbalkar 2014; Roberts 2000; Rojas 2003; Whitelaw 1988). Five studies adequately addressed three domains (Charpak 1997; Eka Pratiwi 2009; Gathwala 2008; Ramanathan 2001; Suman 2008), and four adequately addressed two or fewer domains (Ali 2009; Cattaneo 1998; Sloan 1994; Worku 2005).
Overall, the quality of the studies was mixed, although sensitivity analysis suggests that inclusion of studies with high risk of bias did not affect the general direction of findings nor the size of the treatment effect. Nevertheless, lack of blinding of outcome assessors in most studies and the unclear method of allocation concealment might present problems in terms of the overall quality of evidence. Investigators must make every effort to improve research quality.
For some of the results described in the review (hypothermia, weight gain, breastfeeding, and length of hospital stay), evidence shows high levels of statistical heterogeneity. Some of this heterogeneity may have occurred as a result of clinical heterogeneity, for example, different definitions of hypothermia were used, or women may not have been asked about breastfeeding in the same way in different trials. Results of meta‐analysis with substantial heterogeneity should be interpreted cautiously.
Potential biases in the review process
We attempted to reduce bias in the review process wherever possible. Two review authors independently assessed the risk of bias and findings of included studies. We tried to contact authors of studies with missing data but obtained limited response. Despite differences in the timing of outcome measurements among studies, we proceeded with meta‐analyses for several outcomes, as intervention effects were consistent among studies, although to varying degrees. Only one study reported about 50% of outcomes evaluated in the review, precluding convincing conclusions on the effect of KMC on such outcomes.
The beneficial effects of KMC on mortality at discharge or at 40 to 41 weeks' postmenstrual age and at latest follow‐up, severe infection/sepsis, and nosocomial infection found in our meta‐analyses are enhanced by the impressive statistical homogeneity observed among trials (I2 = 0% to 7%).
To date, only one study (Charpak 1997) reported neurodevelopmental results at one year of corrected age. Longer‐term assessments of neurodevelopmental outcomes have not been published yet, and some caution should perhaps be exercised in applying these findings at 12 months' corrected age, because it has been suggested that assessments done at a relatively young age may be insufficiently predictive of longer‐term neurodevelopmental outcomes, particularly with regard to cognitive functioning (Roberts 2010).
Agreements and disagreements with other studies or reviews
Previous versions of this review
Our assessment of the evidence was similar to that provided in the previous version of this review (Conde‐Agudelo 2014), which concluded that "the evidence from this updated review supports the use of KMC in LBW infants as an alternative to conventional neonatal care mainly in resource‐limited settings." In the current version of this review, we included three additional trials and results of infant neurobehavior at 40 to 44 weeks' postmenstrual age of a previously included study. Notwithstanding, the conclusions of this updated review have not changed in relation to those of the previous version of the review. It should be noted that the first two versions of this review (Conde‐Agudelo 2000; Conde‐Agudelo 2003), including only three trials (n = 1362 infants), had concluded that "although KMC appears to reduce severe infant morbidity without any serious deleterious effect reported, there is still insufficient evidence to recommend its routine use in LBW infants." In these earlier versions, biases related to blinding of participants, personnel and outcome assessors were assessed within a single domain. In the current version , we assessed blinding of participants and personnel in a domain that was separate from bias related to blinding of outcome assessment, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The findings of the current updated version of this review allow us to reassert that evidence is sufficient for review authors to recommend the use of KMC in stabilized LBW infants.
Other systematic reviews on KMC
Lawn 2010 performed a systematic review and meta‐analysis to estimate the effect of KMC on neonatal mortality due to direct complications of preterm birth. This review included observational studies and excluded RCTs that initiated KMC after the first week of life. In the meta‐analysis of RCTs, which included three studies (Charpak 1997; Suman 2008; Worku 2005) that provided data on neonatal specific mortality, KMC was associated with a reduction in neonatal death among infants < 2000 g (risk ratio [RR] 0.49, 95% CI 0.29 to 0.82; I2 = 0%; 988 infants). In the meta‐analysis of three observational studies, KMC was associated with decreased risk of neonatal death in infants < 2000 g (RR 0.68, 95% CI 0.58 to 0.79; I2 = 54%; 8151 infants). Another meta‐analysis, which included five RCTs, showed that KMC reduced significantly the risk of severe morbidity (RR 0.34, 95% CI 0.17 to 0.65; I2 = 70%; 1520 infants).
Boundy 2016 conducted a systematic review and meta‐analysis of RCTs and observational studies to assess the effect of KMC on neonatal outcomes among infants of any birthweight or gestational age. Studies with fewer than 10 participants, lack of a comparison group without KMC, and not reporting a quantitative association were excluded. Among LBW infants < 2000 g, KMC was associated with a significant decrease in the risk of mortality at latest follow‐up (RR 0.64, 95% CI 0.46 to 0.89; I2 = 72%; 15 studies [9 RCTs and 6 observational studies]).
The results of our review suggest that KMC reduces the risk of mortality at discharge or at 40 to 41 weeks' postmenstrual age and at latest follow‐up. Our estimated effects were similar to those of Boundy 2016 and were smaller than those of Lawn 2010. Differences between the findings of this updated review and those of Lawn 2010 and Boundy 2016 are explained by inclusion of only RCTs and of a greater number of studies in our review.

Study flow diagram: review update

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Kangaroo mother care versus conventional neonatal care, outcome: 1.1 Mortality at latest follow‐up.
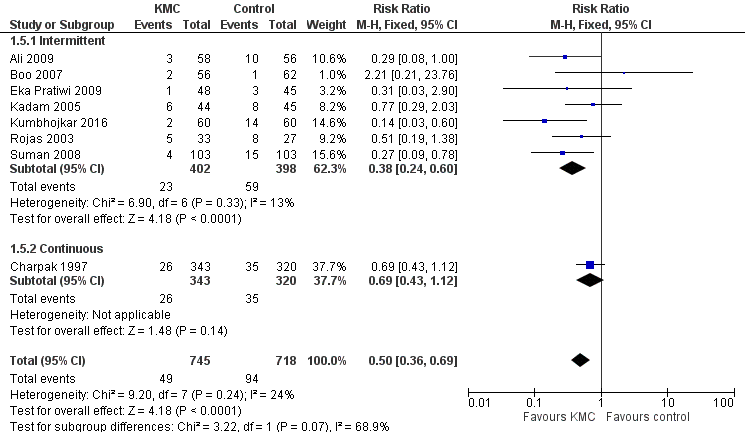
Forest plot of comparison: 1 Kangaroo mother care versus conventional neonatal care, outcome: 1.2 Severe infection/sepsis at latest follow‐up ‐ stabilized infants.

Forest plot of comparison: 1 Kangaroo mother care versus conventional neonatal care, outcome: 1.10 Weight gain at latest follow‐up (g/d) ‐ stabilized infants.

Forest plot of comparison: 1 Kangaroo mother care versus conventional neonatal care, outcome: 1.34 Any breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age ‐ stabilized infants.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 1 Mortality at discharge or at 40 to 41 weeks' postmenstrual age.
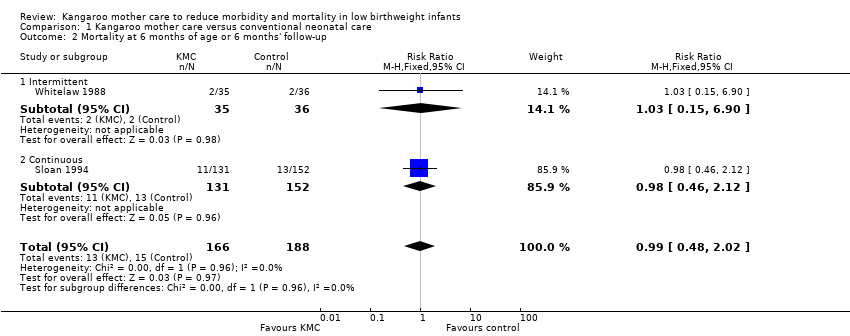
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 2 Mortality at 6 months of age or 6 months' follow‐up.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 3 Mortality at 12 months' corrected age.
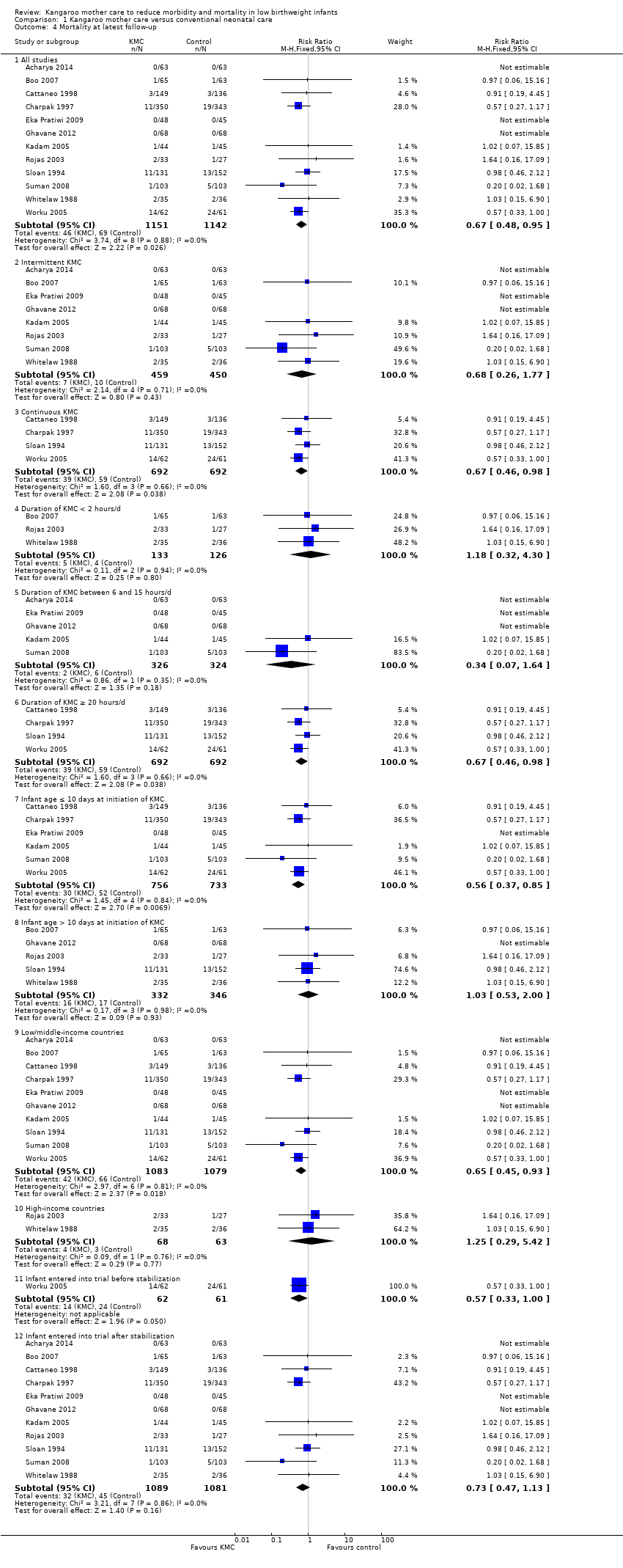
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 4 Mortality at latest follow‐up.
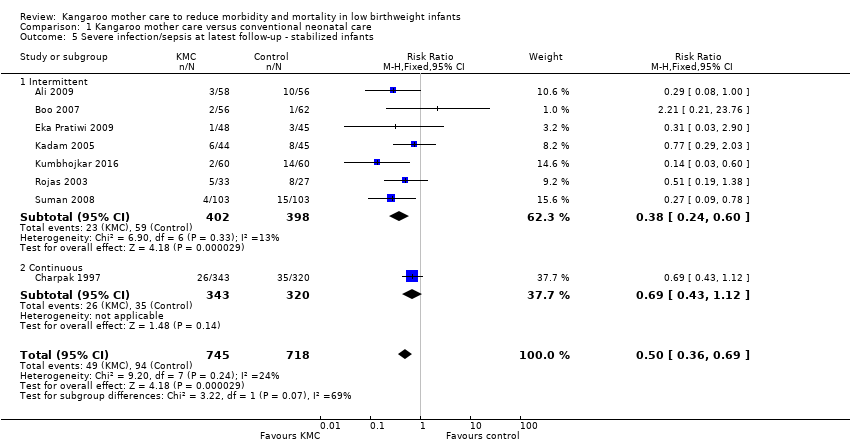
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 5 Severe infection/sepsis at latest follow‐up ‐ stabilized infants.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 6 Severe illness at 6 months' follow‐up ‐ stabilized infants.
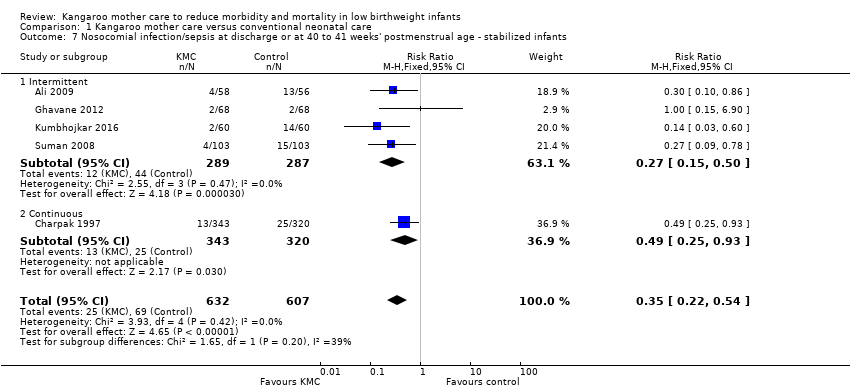
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 7 Nosocomial infection/sepsis at discharge or at 40 to 41 weeks' postmenstrual age ‐ stabilized infants.
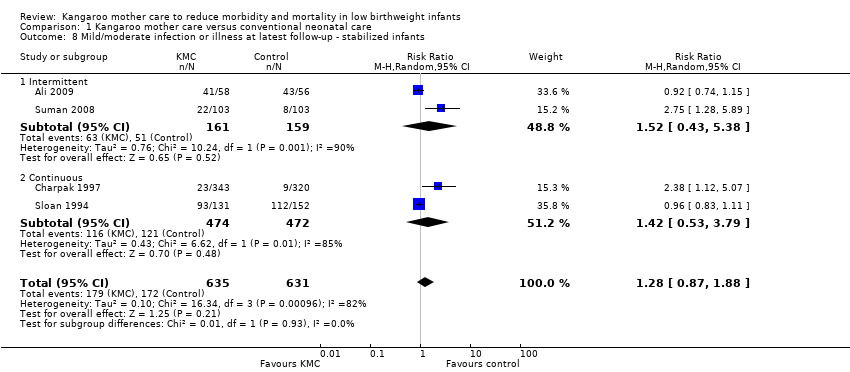
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 8 Mild/moderate infection or illness at latest follow‐up ‐ stabilized infants.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 9 Lower respiratory tract disease at 6 months' follow‐up ‐ stabilized infants.
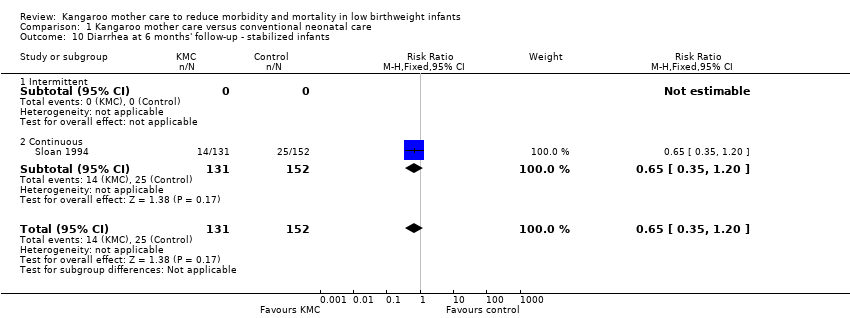
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 10 Diarrhea at 6 months' follow‐up ‐ stabilized infants.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 11 Hypothermia at discharge or at 40 to 41 weeks’ postmenstrual age ‐ stabilized infants.
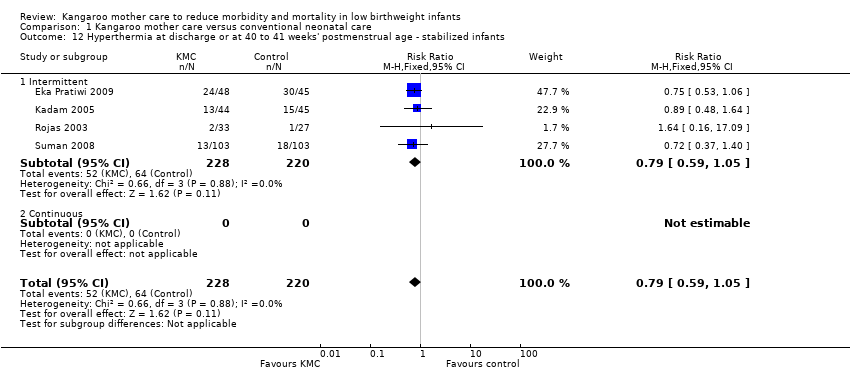
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 12 Hyperthermia at discharge or at 40 to 41 weeks' postmenstrual age ‐ stabilized infants.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 13 Length of hospital stay (days) ‐ stabilized infants.
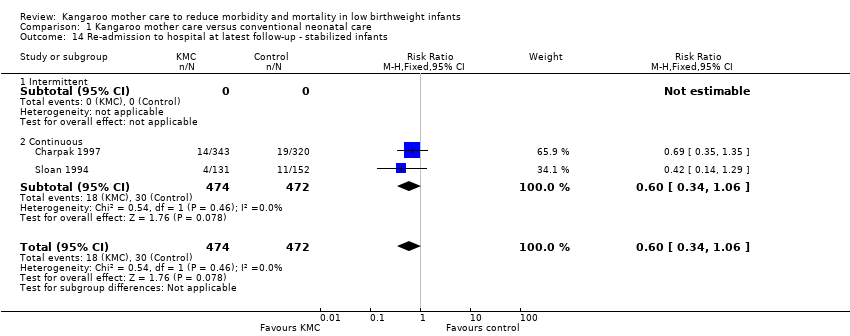
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 14 Re‐admission to hospital at latest follow‐up ‐ stabilized infants.
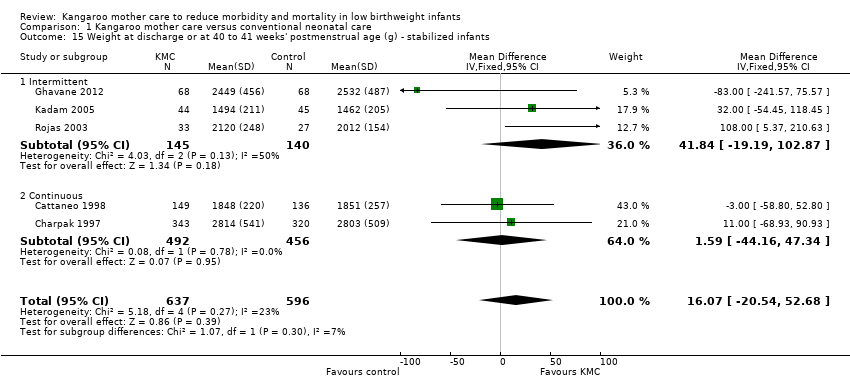
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 15 Weight at discharge or at 40 to 41 weeks' postmenstrual age (g) ‐ stabilized infants.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 16 Weight at 6 months' corrected age (g) ‐ stabilized infants.
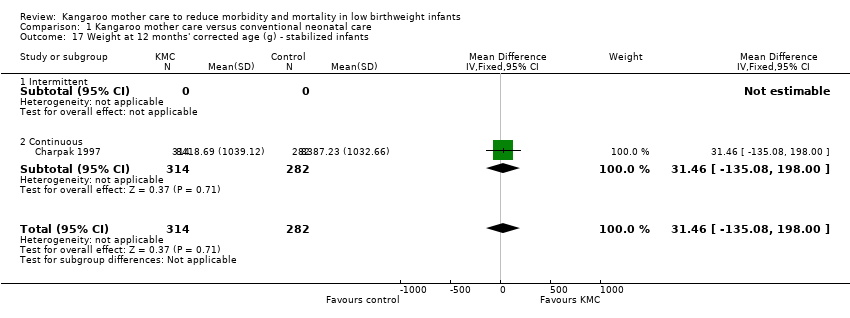
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 17 Weight at 12 months' corrected age (g) ‐ stabilized infants.
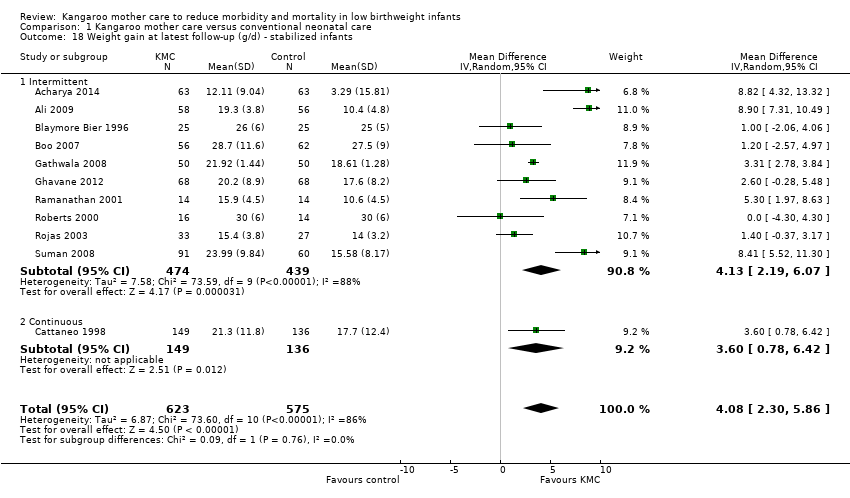
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 18 Weight gain at latest follow‐up (g/d) ‐ stabilized infants.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 19 Length at discharge or at 40 to 41 weeks' postmenstrual age (cm) ‐ stabilized infants.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 20 Length at 6 months' corrected age (cm) ‐ stabilized infants.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 21 Length at 12 months' corrected age (cm) ‐ stabilized infants.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 22 Length gain at latest follow‐up (cm/wk) ‐ stabilized infants.
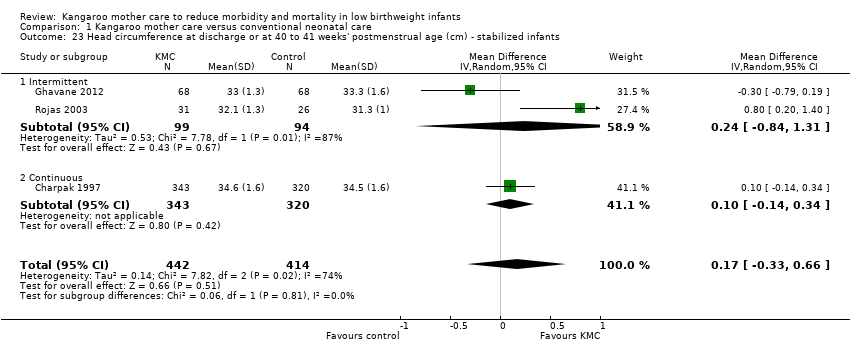
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 23 Head circumference at discharge or at 40 to 41 weeks' postmenstrual age (cm) ‐ stabilized infants.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 24 Head circumference at 6 months' corrected age (cm) ‐ stabilized infants.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 25 Head circumference at 12 months' corrected age (cm) ‐ stabilized infants.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 26 Head circumference gain at latest follow‐up (cm/wk) ‐ stabilized infants.
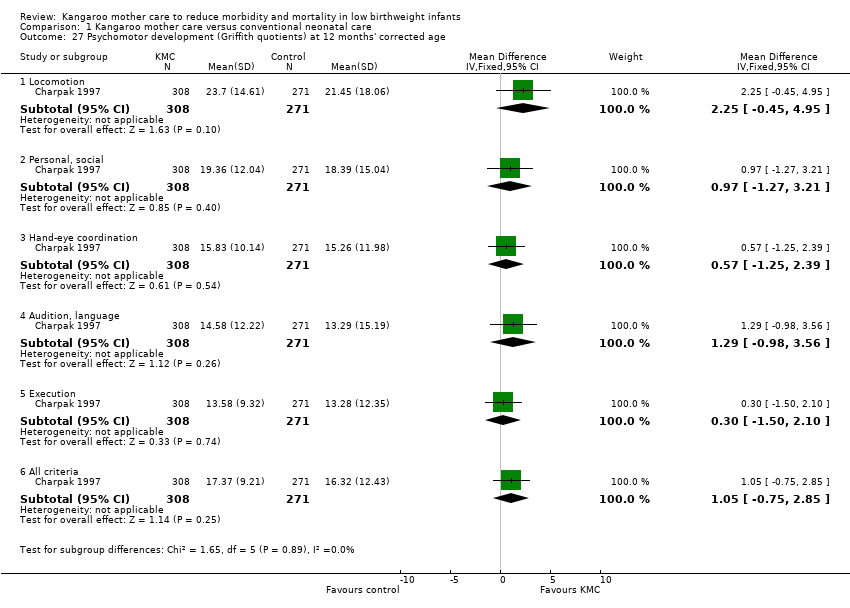
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 27 Psychomotor development (Griffith quotients) at 12 months' corrected age.
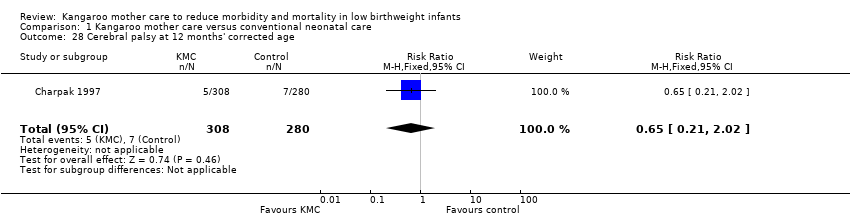
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 28 Cerebral palsy at 12 months' corrected age.
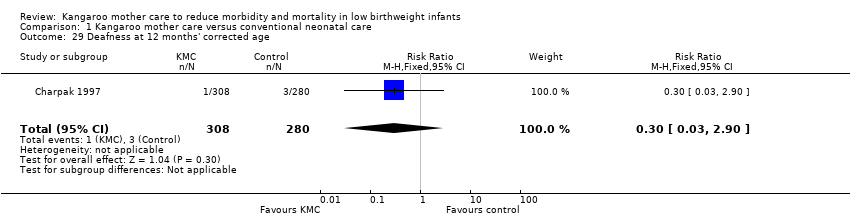
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 29 Deafness at 12 months' corrected age.
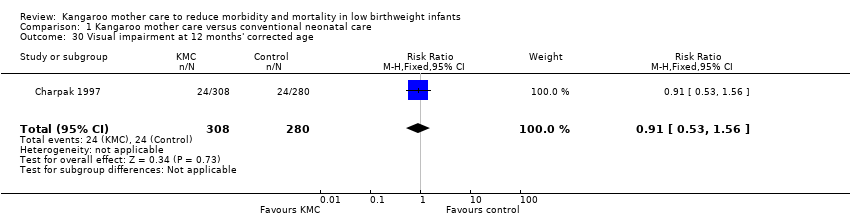
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 30 Visual impairment at 12 months' corrected age.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 31 Exclusive breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age ‐ stabilized infants.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 32 Exclusive breastfeeding at 1 to 3 months' follow‐up ‐ stabilized infants.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 33 Exclusive breastfeeding at 6 to 12 months' follow‐up ‐ stabilized infants.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 34 Any breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age ‐ stabilized infants.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 35 Any breastfeeding at 1 to 2 months' follow‐up ‐ stabilized infants.
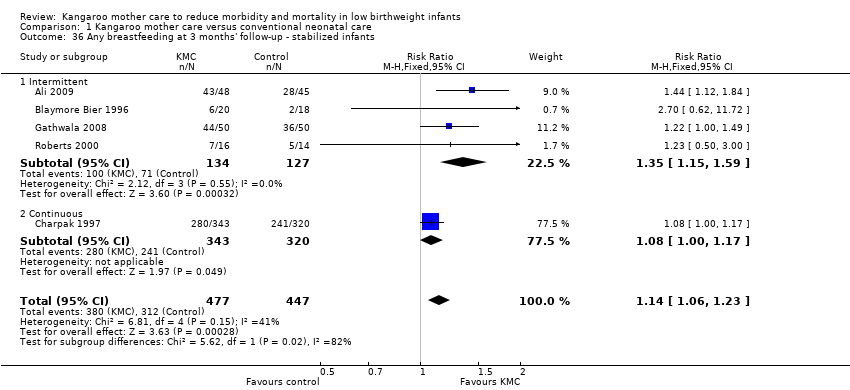
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 36 Any breastfeeding at 3 months' follow‐up ‐ stabilized infants.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 37 Any breastfeeding at 1 to 3 months' follow‐up ‐ stabilized infants.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 38 Any breastfeeding at 6 months' follow‐up ‐ stabilized infants.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 39 Any breastfeeding at 12 months' follow‐up ‐ stabilized infants.
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 40 Onset of breastfeeding (days) ‐ stabilized infants.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 41 Parental and familial satisfaction (continuous KMC).

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 42 Mother‐infant attachment: mother's feelings and perceptions according to interval between birth and start of intervention, and infant admission to NICU.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 43 Mother‐infant attachment: mother's responses to the infant according to interval between birth and start of intervention, and infant admission to NICU.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 44 Mother‐infant attachment: infant's responses to the mother according to interval between birth and start of intervention, and infant admission to NICU.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 45 Mother‐infant attachment at 3 months' follow‐up.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 46 Mother‐infant attachment: stress in NICU.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 47 Mother‐infant attachment: parenting skills.
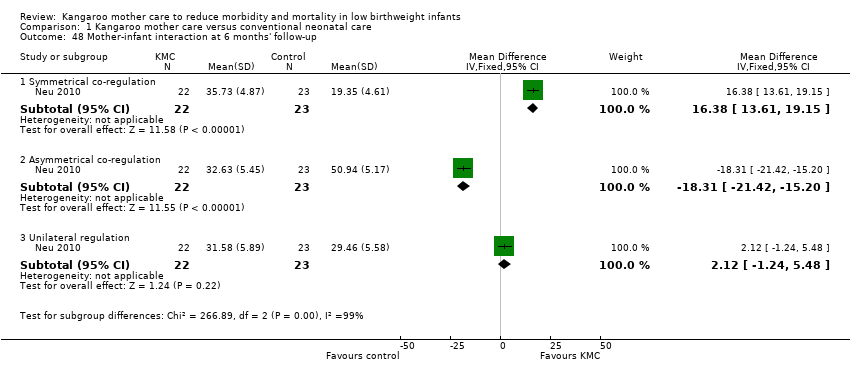
Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 48 Mother‐infant interaction at 6 months' follow‐up.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 49 Infant behavior at 40 to 44 weeks’ postmenstrual age.

Comparison 1 Kangaroo mother care versus conventional neonatal care, Outcome 50 Social and home environment.
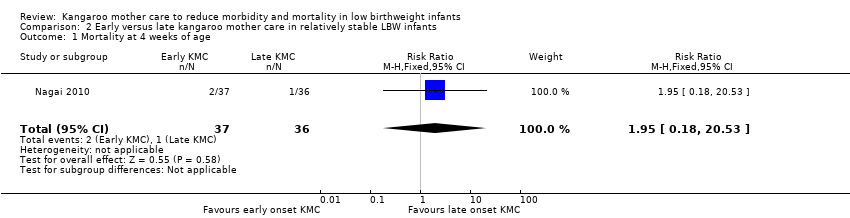
Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 1 Mortality at 4 weeks of age.

Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 2 Morbidity at 4 weeks of age.
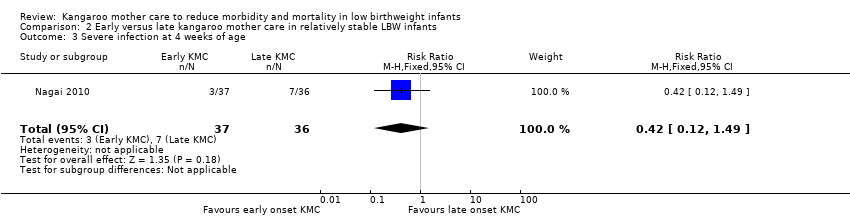
Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 3 Severe infection at 4 weeks of age.
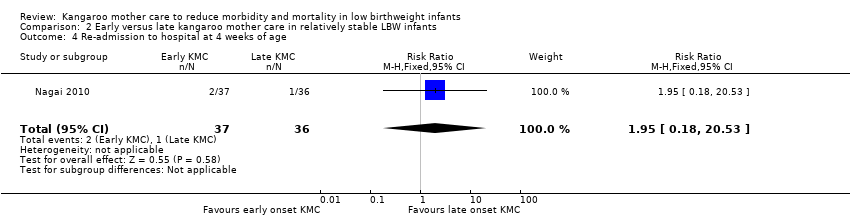
Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 4 Re‐admission to hospital at 4 weeks of age.
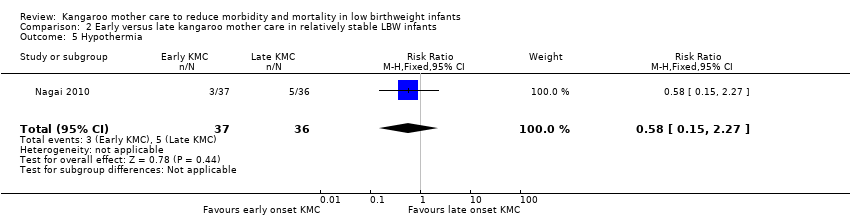
Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 5 Hypothermia.

Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 6 Hyperthermia.
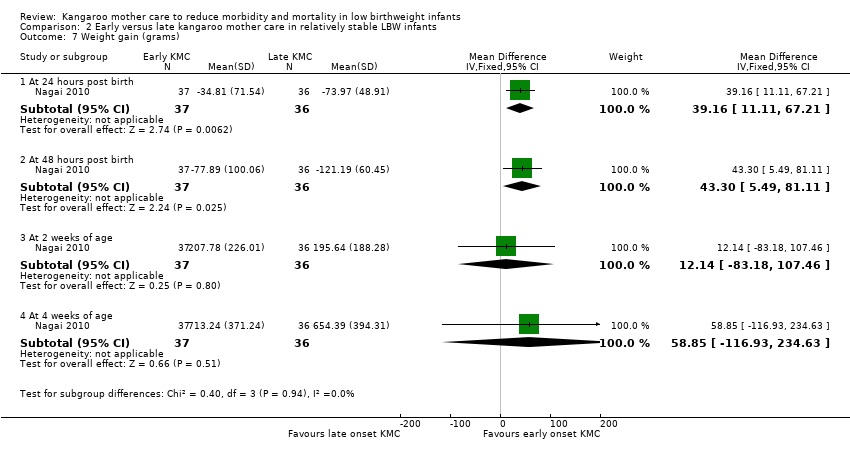
Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 7 Weight gain (grams).

Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 8 Exclusive breastfeeding.

Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 9 Length of hospital stay (days).
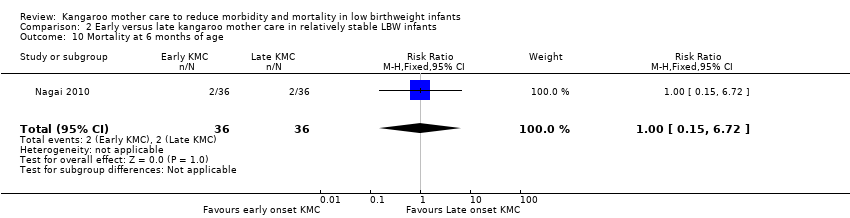
Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 10 Mortality at 6 months of age.
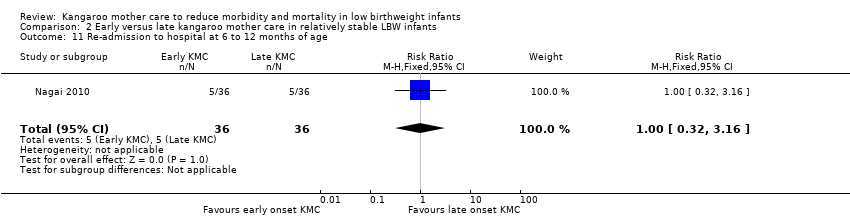
Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 11 Re‐admission to hospital at 6 to 12 months of age.

Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 12 Stunting at 6 to 12 months of age.

Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 13 Severe stunting at 6 to 12 months of age.
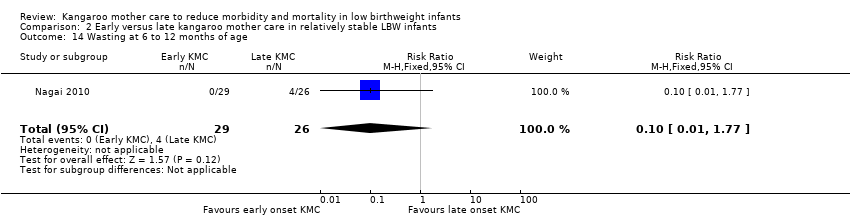
Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 14 Wasting at 6 to 12 months of age.

Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 15 Severe wasting at 6 to 12 months of age.

Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 16 Underweight at 6 to 12 months of age.
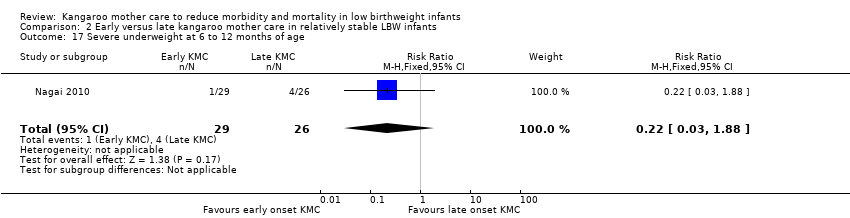
Comparison 2 Early versus late kangaroo mother care in relatively stable LBW infants, Outcome 17 Severe underweight at 6 to 12 months of age.
| Kangaroo mother care versus conventional neonatal care for reducing morbidity and mortality in low birthweight infants | ||||||
| Patient or population: infants with low birthweight | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional neonatal care | Kangaroo mother care | |||||
| Mortality at latest follow‐up | Study population | RR 0.67 | 2293 | ⊕⊕⊕⊝ | ||
| 60 per 1000 | 40 per 1000 | |||||
| Moderate | ||||||
| 30 per 1000 | 20 per 1000 | |||||
| Severe infection/sepsis at latest follow‐up ‐ stabilized infants | Study population | RR 0.5 | 1463 | ⊕⊕⊕⊝ | ||
| 131 per 1000 | 65 per 1000 | |||||
| Moderate | ||||||
| 162 per 1000 | 81 per 1000 | |||||
| Hypothermia at discharge or at 40 to 41 weeks’ postmenstrual age ‐ stabilized infants | Study population | RR 0.28 | 989 | ⊕⊕⊕⊝ | ||
| 271 per 1000 | 76 per 1000 | |||||
| Moderate | ||||||
| 333 per 1000 | 93 per 1000 | |||||
| Weight gain at latest follow‐up (g/d) ‐ stabilized infants | Mean weight gain at latest follow‐up (g/d) ‐ stabilized infants in the intervention groups ‐ was | 1198 | ⊕⊕⊕⊝ | |||
| Any breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age ‐ stabilized infants | Study population | RR 1.2 | 1696 | ⊕⊕⊕⊝ | ||
| 762 per 1000 | 914 per 1000 | |||||
| Moderate | ||||||
| 743 per 1000 | 892 per 1000 | |||||
| Any breastfeeding at 1 to 3 months' follow‐up ‐ stabilized infants | Study population | RR 1.17 | 1394 | ⊕⊕⊝⊝ | ||
| 711 per 1000 | 832 per 1000 | |||||
| Moderate | ||||||
| 622 per 1000 | 728 per 1000 | |||||
| Griffith quotient for psychomotor development (all subscales) at 12 months' corrected age (copy) | Mean Griffith quotient for psychomotor development (all subscales) at 12 months' corrected age (copy) in the intervention groups was | 579 | ⊕⊕⊝⊝ | |||
| *The basis for the assumed risk (eg, median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aMost of the pooled effect provided by studies with moderate or high risk of bias | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at discharge or at 40 to 41 weeks' postmenstrual age Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 All studies | 8 | 1736 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.39, 0.92] |
| 1.2 Intermittent KMC | 5 | 619 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.19, 1.81] |
| 1.3 Continuous KMC | 3 | 1117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.38, 0.96] |
| 1.4 Duration of KMC < 2 hours/d | 2 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.22, 7.73] |
| 1.5 Duration of KMC between 6 and 15 hours/d | 3 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.07, 1.64] |
| 1.6 Duration of KMC ≥ 20 hours/d | 3 | 1117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.38, 0.96] |
| 1.7 Infant age ≤ 10 days at initiation of KMC | 5 | 1412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.36, 0.88] |
| 1.8 Infant age > 10 days at initiation of KMC | 3 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.22, 7.73] |
| 1.9 Low/middle‐income countries | 7 | 1676 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.37, 0.89] |
| 1.10 High‐income countries | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.16, 17.09] |
| 1.11 infant entered into trial before stabilization | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.33, 1.00] |
| 1.12 infant entered into trial after stabilization | 7 | 1613 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.32, 1.23] |
| 2 Mortality at 6 months of age or 6 months' follow‐up Show forest plot | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.48, 2.02] |
| 2.1 Intermittent | 1 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.15, 6.90] |
| 2.2 Continuous | 1 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.46, 2.12] |
| 3 Mortality at 12 months' corrected age Show forest plot | 1 | 693 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.27, 1.17] |
| 3.1 Intermittent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Continuous | 1 | 693 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.27, 1.17] |
| 4 Mortality at latest follow‐up Show forest plot | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 All studies | 12 | 2293 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.48, 0.95] |
| 4.2 Intermittent KMC | 8 | 909 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.26, 1.77] |
| 4.3 Continuous KMC | 4 | 1384 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.46, 0.98] |
| 4.4 Duration of KMC < 2 hours/d | 3 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.32, 4.30] |
| 4.5 Duration of KMC between 6 and 15 hours/d | 5 | 650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.07, 1.64] |
| 4.6 Duration of KMC ≥ 20 hours/d | 4 | 1384 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.46, 0.98] |
| 4.7 Infant age ≤ 10 days at initiation of KMC | 6 | 1489 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.37, 0.85] |
| 4.8 Infant age > 10 days at initiation of KMC | 5 | 678 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.53, 2.00] |
| 4.9 Low/middle‐income countries | 10 | 2162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.45, 0.93] |
| 4.10 High‐income countries | 2 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.29, 5.42] |
| 4.11 Infant entered into trial before stabilization | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.33, 1.00] |
| 4.12 Infant entered into trial after stabilization | 11 | 2170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.47, 1.13] |
| 5 Severe infection/sepsis at latest follow‐up ‐ stabilized infants Show forest plot | 8 | 1463 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.36, 0.69] |
| 5.1 Intermittent | 7 | 800 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.24, 0.60] |
| 5.2 Continuous | 1 | 663 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.43, 1.12] |
| 6 Severe illness at 6 months' follow‐up ‐ stabilized infants Show forest plot | 1 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.14, 0.67] |
| 6.1 intermittent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Continuous | 1 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.14, 0.67] |
| 7 Nosocomial infection/sepsis at discharge or at 40 to 41 weeks' postmenstrual age ‐ stabilized infants Show forest plot | 5 | 1239 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.22, 0.54] |
| 7.1 Intermittent | 4 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.15, 0.50] |
| 7.2 Continuous | 1 | 663 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.25, 0.93] |
| 8 Mild/moderate infection or illness at latest follow‐up ‐ stabilized infants Show forest plot | 4 | 1266 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.87, 1.88] |
| 8.1 Intermittent | 2 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.43, 5.38] |
| 8.2 Continuous | 2 | 946 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.53, 3.79] |
| 9 Lower respiratory tract disease at 6 months' follow‐up ‐ stabilized infants Show forest plot | 1 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.15, 0.89] |
| 9.1 Intermittent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Continuous | 1 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.15, 0.89] |
| 10 Diarrhea at 6 months' follow‐up ‐ stabilized infants Show forest plot | 1 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.35, 1.20] |
| 10.1 Intermittent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Continuous | 1 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.35, 1.20] |
| 11 Hypothermia at discharge or at 40 to 41 weeks’ postmenstrual age ‐ stabilized infants Show forest plot | 9 | 989 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.16, 0.49] |
| 11.1 Intermittent | 9 | 989 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.16, 0.49] |
| 11.2 Continuous | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Hyperthermia at discharge or at 40 to 41 weeks' postmenstrual age ‐ stabilized infants Show forest plot | 4 | 448 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.59, 1.05] |
| 12.1 Intermittent | 4 | 448 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.59, 1.05] |
| 12.2 Continuous | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Length of hospital stay (days) ‐ stabilized infants Show forest plot | 11 | 1057 | Mean Difference (IV, Random, 95% CI) | ‐1.61 [‐3.41, 0.18] |
| 13.1 Intermittent | 11 | 1057 | Mean Difference (IV, Random, 95% CI) | ‐1.61 [‐3.41, 0.18] |
| 13.2 Continuous | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Re‐admission to hospital at latest follow‐up ‐ stabilized infants Show forest plot | 2 | 946 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.34, 1.06] |
| 14.1 Intermittent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Continuous | 2 | 946 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.34, 1.06] |
| 15 Weight at discharge or at 40 to 41 weeks' postmenstrual age (g) ‐ stabilized infants Show forest plot | 5 | 1233 | Mean Difference (IV, Fixed, 95% CI) | 16.07 [‐20.54, 52.68] |
| 15.1 Intermittent | 3 | 285 | Mean Difference (IV, Fixed, 95% CI) | 41.84 [‐19.19, 102.87] |
| 15.2 Continuous | 2 | 948 | Mean Difference (IV, Fixed, 95% CI) | 1.59 [‐44.16, 47.34] |
| 16 Weight at 6 months' corrected age (g) ‐ stabilized infants Show forest plot | 1 | 591 | Mean Difference (IV, Fixed, 95% CI) | 78.19 [‐52.26, 208.64] |
| 16.1 Intermittent | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Continuous | 1 | 591 | Mean Difference (IV, Fixed, 95% CI) | 78.19 [‐52.26, 208.64] |
| 17 Weight at 12 months' corrected age (g) ‐ stabilized infants Show forest plot | 1 | 596 | Mean Difference (IV, Fixed, 95% CI) | 31.46 [‐135.08, 198.00] |
| 17.1 Intermittent | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Continuous | 1 | 596 | Mean Difference (IV, Fixed, 95% CI) | 31.46 [‐135.08, 198.00] |
| 18 Weight gain at latest follow‐up (g/d) ‐ stabilized infants Show forest plot | 11 | 1198 | Mean Difference (IV, Random, 95% CI) | 4.08 [2.30, 5.86] |
| 18.1 Intermittent | 10 | 913 | Mean Difference (IV, Random, 95% CI) | 4.13 [2.19, 6.07] |
| 18.2 Continuous | 1 | 285 | Mean Difference (IV, Random, 95% CI) | 3.60 [0.78, 6.42] |
| 19 Length at discharge or at 40 to 41 weeks' postmenstrual age (cm) ‐ stabilized infants Show forest plot | 3 | 856 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.69, 0.48] |
| 19.1 Intermittent | 2 | 193 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐1.51, 1.04] |
| 19.2 Continuous | 1 | 663 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.36, 0.36] |
| 20 Length at 6 months' corrected age (cm) ‐ stabilized infants Show forest plot | 1 | 590 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.18, 0.64] |
| 20.1 Intermittent | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.2 Continuous | 1 | 590 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.18, 0.64] |
| 21 Length at 12 months' corrected age (cm) ‐ stabilized infants Show forest plot | 1 | 586 | Mean Difference (IV, Fixed, 95% CI) | 0.31 [‐0.17, 0.79] |
| 21.1 Intermittent | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.2 Continuous | 1 | 586 | Mean Difference (IV, Fixed, 95% CI) | 0.31 [‐0.17, 0.79] |
| 22 Length gain at latest follow‐up (cm/wk) ‐ stabilized infants Show forest plot | 3 | 377 | Mean Difference (IV, Random, 95% CI) | 0.21 [0.03, 0.38] |
| 22.1 Intermittent | 3 | 377 | Mean Difference (IV, Random, 95% CI) | 0.21 [0.03, 0.38] |
| 22.2 Continuous | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Head circumference at discharge or at 40 to 41 weeks' postmenstrual age (cm) ‐ stabilized infants Show forest plot | 3 | 856 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.33, 0.66] |
| 23.1 Intermittent | 2 | 193 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.84, 1.31] |
| 23.2 Continuous | 1 | 663 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.14, 0.34] |
| 24 Head circumference at 6 months' corrected age (cm) ‐ stabilized infants Show forest plot | 1 | 592 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [0.11, 0.57] |
| 24.1 Intermittent | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Continuous | 1 | 592 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [0.11, 0.57] |
| 25 Head circumference at 12 months' corrected age (cm) ‐ stabilized infants Show forest plot | 1 | 597 | Mean Difference (IV, Fixed, 95% CI) | 0.39 [‐0.00, 0.78] |
| 25.1 Intermittent | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 Continuous | 1 | 597 | Mean Difference (IV, Fixed, 95% CI) | 0.39 [‐0.00, 0.78] |
| 26 Head circumference gain at latest follow‐up (cm/wk) ‐ stabilized infants Show forest plot | 4 | 495 | Mean Difference (IV, Random, 95% CI) | 0.14 [0.06, 0.22] |
| 26.1 Intermittent | 4 | 495 | Mean Difference (IV, Random, 95% CI) | 0.14 [0.06, 0.22] |
| 26.2 Continuous | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Psychomotor development (Griffith quotients) at 12 months' corrected age Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 27.1 Locomotion | 1 | 579 | Mean Difference (IV, Fixed, 95% CI) | 2.25 [‐0.45, 4.95] |
| 27.2 Personal, social | 1 | 579 | Mean Difference (IV, Fixed, 95% CI) | 0.97 [‐1.27, 3.21] |
| 27.3 Hand‐eye coordination | 1 | 579 | Mean Difference (IV, Fixed, 95% CI) | 0.57 [‐1.25, 2.39] |
| 27.4 Audition, language | 1 | 579 | Mean Difference (IV, Fixed, 95% CI) | 1.29 [‐0.98, 3.56] |
| 27.5 Execution | 1 | 579 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐1.50, 2.10] |
| 27.6 All criteria | 1 | 579 | Mean Difference (IV, Fixed, 95% CI) | 1.05 [‐0.75, 2.85] |
| 28 Cerebral palsy at 12 months' corrected age Show forest plot | 1 | 588 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.21, 2.02] |
| 29 Deafness at 12 months' corrected age Show forest plot | 1 | 588 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.03, 2.90] |
| 30 Visual impairment at 12 months' corrected age Show forest plot | 1 | 588 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.53, 1.56] |
| 31 Exclusive breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age ‐ stabilized infants Show forest plot | 6 | 1453 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.07, 1.25] |
| 31.1 Intermittent | 4 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.11, 1.35] |
| 31.2 Continuous | 2 | 942 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.00, 1.24] |
| 32 Exclusive breastfeeding at 1 to 3 months' follow‐up ‐ stabilized infants Show forest plot | 5 | 600 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [1.01, 1.43] |
| 32.1 Intermittent | 3 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.12, 1.65] |
| 32.2 Continuous | 2 | 379 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.96, 1.10] |
| 33 Exclusive breastfeeding at 6 to 12 months' follow‐up ‐ stabilized infants Show forest plot | 3 | 810 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.95, 1.76] |
| 33.1 Intermittent | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.10, 2.10] |
| 33.2 Continuous | 2 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.66, 1.86] |
| 34 Any breastfeeding at discharge or at 40 to 41 weeks' postmenstrual age ‐ stabilized infants Show forest plot | 10 | 1696 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [1.07, 1.34] |
| 34.1 Intermittent | 8 | 754 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.07, 1.41] |
| 34.2 Continuous | 2 | 942 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.93, 1.40] |
| 35 Any breastfeeding at 1 to 2 months' follow‐up ‐ stabilized infants Show forest plot | 6 | 538 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.00, 1.78] |
| 35.1 Intermittent | 4 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [1.30, 2.75] |
| 35.2 Continuous | 2 | 379 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.96, 1.10] |
| 36 Any breastfeeding at 3 months' follow‐up ‐ stabilized infants Show forest plot | 5 | 924 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.06, 1.23] |
| 36.1 Intermittent | 4 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.15, 1.59] |
| 36.2 Continuous | 1 | 663 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [1.00, 1.17] |
| 37 Any breastfeeding at 1 to 3 months' follow‐up ‐ stabilized infants Show forest plot | 9 | 1394 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.05, 1.31] |
| 37.1 Intermittent | 6 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.18, 1.64] |
| 37.2 Continuous | 3 | 1042 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [1.00, 1.11] |
| 38 Any breastfeeding at 6 months' follow‐up ‐ stabilized infants Show forest plot | 5 | 952 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.98, 1.29] |
| 38.1 Intermittent | 3 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.08, 2.08] |
| 38.2 Continuous | 2 | 809 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.92, 1.24] |
| 39 Any breastfeeding at 12 months' follow‐up ‐ stabilized infants Show forest plot | 1 | 589 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.65, 1.21] |
| 39.1 Intermittent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 39.2 Continuous | 1 | 589 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.65, 1.21] |
| 40 Onset of breastfeeding (days) ‐ stabilized infants Show forest plot | 2 | 295 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐1.64, 1.70] |
| 40.1 Intermittent | 2 | 295 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐1.64, 1.70] |
| 40.2 Continuous | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 41 Parental and familial satisfaction (continuous KMC) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 41.1 Mother satisfied with method | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.05, 1.30] |
| 41.2 Father satisfied with method | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.91, 1.14] |
| 41.3 Family satisfied with method | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.13] |
| 42 Mother‐infant attachment: mother's feelings and perceptions according to interval between birth and start of intervention, and infant admission to NICU Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 42.1 Sense of competence ‐ interval of 1 to 2 days | 1 | 170 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [0.14, 0.68] |
| 42.2 Sense of competence ‐ interval of 3 to 14 days | 1 | 177 | Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.08, 0.58] |
| 42.3 Sense of competence ‐ interval > 14 days | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.17, 0.59] |
| 42.4 Sense of competence ‐ infant admitted to NICU | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | 0.54 [0.07, 1.01] |
| 42.5 Sense of competence ‐ infant not admitted to NICU | 1 | 406 | Mean Difference (IV, Fixed, 95% CI) | 0.24 [0.05, 0.43] |
| 42.6 Worry and stress ‐ interval of 1 to 2 days | 1 | 170 | Mean Difference (IV, Fixed, 95% CI) | 0.31 [0.04, 0.58] |
| 42.7 Worry and stress ‐ interval of 3 to 14 days | 1 | 177 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.20, 0.38] |
| 42.8 Worry and stress ‐ interval > 14 days | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐0.70, 0.12] |
| 42.9 Worry and stress ‐ infant admitted to NICU | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐0.1 [‐0.60, 0.40] |
| 42.10 Worry and stress ‐ infant not admitted to NICU | 1 | 406 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.06, 0.30] |
| 42.11 Social support ‐ interval of 1 to 2 days | 1 | 170 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.35, 0.23] |
| 42.12 Social support ‐ interval of 3 to 14 days | 1 | 177 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.34, 0.22] |
| 42.13 Social support ‐ interval > 14 days | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐0.84, ‐0.10] |
| 42.14 Social support ‐ infant admitted to NICU | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.52, 0.42] |
| 42.15 Social support ‐ infant not admitted to NICU | 1 | 406 | Mean Difference (IV, Fixed, 95% CI) | ‐0.2 [‐0.39, ‐0.01] |
| 43 Mother‐infant attachment: mother's responses to the infant according to interval between birth and start of intervention, and infant admission to NICU Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 43.1 Mother's sensitivity ‐ interval of 1 to 2 days | 1 | 170 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.02, 0.06] |
| 43.2 Mother's sensitivity ‐ interval of 3 to 14 days | 1 | 177 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.05, 0.03] |
| 43.3 Mother's sensitivity ‐ interval > 14 days | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [0.01, 0.11] |
| 43.4 Mother's sensitivity ‐ infant admitted to NICU | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.04, 0.08] |
| 43.5 Mother's sensitivity ‐ infant not admitted to NICU | 1 | 406 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.00, 0.04] |
| 43.6 Mother's response to child's distress ‐ interval of 1 to 2 days | 1 | 170 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.08, 0.02] |
| 43.7 Mother's response to child's distress ‐ interval of 3 to 14 days | 1 | 177 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |
| 43.8 Mother's response to child's distress ‐ interval > 14 days | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.04, 0.06] |
| 43.9 Mother's response to child's distress ‐ infant admitted to NICU | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.01, 0.11] |
| 43.10 Mother's response to child's distress ‐ infant not admitted to NICU | 1 | 406 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.05, 0.01] |
| 43.11 Mother's response to child's socioemotional growth fostering ‐ interval of 1 to 2 days | 1 | 170 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.04, 0.06] |
| 43.12 Mother's response to child's socioemotional growth fostering ‐ interval of 3 to 14 days | 1 | 177 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.06, 0.02] |
| 43.13 Mother's response to child's socioemotional growth fostering ‐ interval > 14 days | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.00, 0.10] |
| 43.14 Mother's response to child's socioemotional growth fostering ‐ infant admitted to NICU | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.12, 0.02] |
| 43.15 Mother's response to child's socioemotional growth fostering ‐ infant not admitted to NICU | 1 | 406 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.01, 0.05] |
| 43.16 Mother's response to child's cognitive growth fostering ‐ interval of 1 to 2 days | 1 | 170 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.04, 0.08] |
| 43.17 Mother's response to child's cognitive growth fostering ‐ interval of 3 to 14 days | 1 | 177 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.10, 0.02] |
| 43.18 Mother's response to child's cognitive growth fostering ‐ interval > 14 days | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [0.00, 0.14] |
| 43.19 Mother's response to child's cognitive growth fostering ‐ infant admitted to NICU | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.17, 0.03] |
| 43.20 Mother's response to child's cognitive growth fostering ‐ infant not admitted to NICU | 1 | 406 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.01, 0.07] |
| 44 Mother‐infant attachment: infant's responses to the mother according to interval between birth and start of intervention, and infant admission to NICU Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 44.1 Clarity of cues ‐ interval of 1 to 2 days | 1 | 170 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.04, 0.06] |
| 44.2 Clarity of cues ‐ interval of 3 to 14 days | 1 | 177 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.03, 0.07] |
| 44.3 Clarity of cues ‐ interval > 14 days | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.05, 0.05] |
| 44.4 Clarity of cues ‐ infant admitted to NICU | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.07, 0.05] |
| 44.5 Clarity of cues ‐ infant not admitted to NICU | 1 | 406 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.01, 0.05] |
| 44.6 Responsiveness ‐ interval of 1 to 2 days | 1 | 170 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.06, 0.02] |
| 44.7 Responsiveness ‐ interval of 3 to 14 days | 1 | 177 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.02, 0.06] |
| 44.8 Responsiveness ‐ interval > 14 days | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [0.01, 0.09] |
| 44.9 Responsiveness ‐ infant admitted to NICU | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.07, 0.05] |
| 44.10 Responsiveness ‐ infant not admitted to NICU | 1 | 406 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.01, 0.05] |
| 45 Mother‐infant attachment at 3 months' follow‐up Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 6.24 [5.57, 6.91] |
| 45.1 Total attachment score at 3 months' follow‐up | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 6.24 [5.57, 6.91] |
| 46 Mother‐infant attachment: stress in NICU Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 46.1 Nursery environment score | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.51, 0.71] |
| 46.2 Infant appearance score | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.62, 0.62] |
| 46.3 Relationship with the infant score | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [0.35, 1.65] |
| 46.4 Staff behavior and communication score | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.95, 1.15] |
| 47 Mother‐infant attachment: parenting skills Show forest plot | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.89, 0.09] |
| 47.1 Total score at discharge | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.89, 0.09] |
| 48 Mother‐infant interaction at 6 months' follow‐up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 48.1 Symmetrical co‐regulation | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 16.38 [13.61, 19.15] |
| 48.2 Asymmetrical co‐regulation | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐18.31 [‐21.42, ‐15.20] |
| 48.3 Unilateral regulation | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 2.12 [‐1.24, 5.48] |
| 49 Infant behavior at 40 to 44 weeks’ postmenstrual age Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 49.1 Attention | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.40, 0.98] |
| 49.2 Autonomic organization | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.41, 0.79] |
| 49.3 Motor | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.22, 0.82] |
| 49.4 Orientation | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.72, 0.34] |
| 49.5 Autonomic | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.89, 1.11] |
| 49.6 State regulation | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.95, 0.33] |
| 49.7 Robust crying | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.90, 0.58] |
| 49.8 State stability | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.93, 1.57] |
| 50 Social and home environment Show forest plot | 1 | 338 | Mean Difference (IV, Fixed, 95% CI) | 0.79 [0.74, 0.84] |
| 50.1 HOME environment total score at 12 months' corrected age | 1 | 338 | Mean Difference (IV, Fixed, 95% CI) | 0.79 [0.74, 0.84] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at 4 weeks of age Show forest plot | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.18, 20.53] |
| 2 Morbidity at 4 weeks of age Show forest plot | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.18, 1.28] |
| 3 Severe infection at 4 weeks of age Show forest plot | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.12, 1.49] |
| 4 Re‐admission to hospital at 4 weeks of age Show forest plot | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.18, 20.53] |
| 5 Hypothermia Show forest plot | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.15, 2.27] |
| 6 Hyperthermia Show forest plot | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.56, 1.99] |
| 7 Weight gain (grams) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 At 24 hours post birth | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | 39.16 [11.11, 67.21] |
| 7.2 At 48 hours post birth | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | 43.3 [5.49, 81.11] |
| 7.3 At 2 weeks of age | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | 12.14 [‐83.18, 107.46] |
| 7.4 At 4 weeks of age | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | 58.85 [‐116.93, 234.63] |
| 8 Exclusive breastfeeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 At 24 hours of age | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.67, 1.57] |
| 8.2 At 2 weeks of age | 1 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.89, 1.12] |
| 8.3 At 4 weeks of age | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.85, 1.04] |
| 8.4 At 6 months of age | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.99, 7.31] |
| 9 Length of hospital stay (days) Show forest plot | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.24, ‐0.56] |
| 10 Mortality at 6 months of age Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.72] |
| 11 Re‐admission to hospital at 6 to 12 months of age Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.32, 3.16] |
| 12 Stunting at 6 to 12 months of age Show forest plot | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.46, 1.48] |
| 13 Severe stunting at 6 to 12 months of age Show forest plot | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.17, 2.73] |
| 14 Wasting at 6 to 12 months of age Show forest plot | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.1 [0.01, 1.77] |
| 15 Severe wasting at 6 to 12 months of age Show forest plot | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Underweight at 6 to 12 months of age Show forest plot | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.21, 1.14] |
| 17 Severe underweight at 6 to 12 months of age Show forest plot | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.03, 1.88] |

