Active chest compression‐decompression for cardiopulmonary resuscitation
Abstract
Background
Active compression‐decompression cardiopulmonary resuscitation (ACDR CPR) uses a hand‐held suction device, applied mid‐sternum, to compress the chest then actively decompress the chest after each compression. Randomised controlled trials testing this device have shown discordant results.
Objectives
To determine the effect of active chest compression‐decompression CPR compared to standard chest compression CPR on mortality and neurological function in adults with cardiac arrest treated either in‐hospital or out‐of‐hospital.
Search methods
We updated the searches of CENTRAL in The Cochrane Library (Issue 12 of 12, 2012), MEDLINE (OVID, 1946 to January week 1 2013), and EMBASE (OVID, 1980 to week 1 2013) on 14 January 2013. We checked the reference list of retrieved articles, contacted experts in the field, and searched ClinicalTrials.gov.
Selection criteria
All randomised or quasi‐randomised studies comparing active compression‐decompression with standard manual chest compression in adults with a cardiac arrest who received cardiopulmonary resuscitation by a trained medical or paramedical team.
Data collection and analysis
We independently extracted data on an intention‐to‐treat basis. When needed, we contacted the authors of the primary studies. If appropriate, we cumulated studies and pooled relative risk (RR) estimates. We predefined subgroup analyses according to setting (out‐of‐hospital or in‐hospital) and attending team composition (with physician or paramedic only).
Main results
In this update, 27 new related publications were found, but they did not all fulfil inclusion criteria or concerned participants already reported in previous publications. In the end, we included 10 trials in this review: Eight were in out‐of‐hospital settings; one was set in‐hospital only; and one had both in‐hospital and out‐of‐hospital components. Allocation concealment was adequate in four studies. The two in‐hospital studies were different in quality and size (773 and 53 participants). Both found no differences between ACDR CPR and STR in any outcome.
Out‐of‐hospital trials cumulated 4162 participants. There were no differences between ACDR CPR and STR for mortality either immediately (RR 0.98, 95% confidence interval (CI) 0.94 to 1.03) or at hospital discharge (RR 0.99, 95% CI 0.98 to 1.01). The pooled RR of neurological impairment of any severity was 1.71 (95% CI 0.90 to 3.25), with a non‐significant trend to more frequent severe neurological damage in survivors of ACDR CPR (RR 3.11, 95% CI 0.98 to 9.83). However, assessment of neurological outcome was limited, and few participants had neurological damage.
There was no difference between ACDR CPR and STR with regard to complications such as rib or sternal fractures, pneumothorax, or haemothorax (RR 1.09, 95% CI 0.86 to 1.38). Skin trauma and ecchymosis were more frequent with ACDR CPR.
Authors' conclusions
Active chest compression‐decompression in people with cardiac arrest is not associated with any clear benefit.
Plain language summary
Active compression‐decompression using a hand‐held device for emergency heart massage
During standard cardiopulmonary resuscitation (heart massage) for cardiac arrest (arrest of the heart), the chest is compressed manually and repeatedly by hand. This is a temporary method that pumps blood and oxygen to the brain via the heart. During standard cardiopulmonary resuscitation, the chest is not manually decompressed. Active chest compression‐decompression is an alternative method of heart massage and uses a hand‐held suction device to compress the chest, then decompress the chest after each compression. Comparison of these techniques showed active chest compression‐decompression to have no advantage and some drawbacks compared to standard cardiopulmonary resuscitation.
Authors' conclusions
Background
Active compression‐decompression resuscitation (ACDR CPR) uses a hand‐held device with a suction cup to perform cardiac massage during cardiopulmonary resuscitation. The operator applies this device mid‐sternum to compress the chest as in standard manual cardiopulmonary resuscitation (STR). The operator then uses the device to actively decompress the chest after each compression (Cohen 1992). This differs from STR, which allows passive relaxation of the chest after each compression. A pressure gauge in the device measures both compression and decompression forces and can be used to adjust the effort.
ACDR CPR was designed to increase the pump action of closed chest massage. Indeed, experimental studies have shown that ACDR CPR, in comparison to STR, increases venous return and left ventricular filling in the decompression phase, leading to improved stroke volume (Tucker 1993), cardiac output (Orliaguet 1995), and arterial pressures (Shultz 1994) in the compression phase. Only one study, involving participants after prolonged resuscitation, found no differences in haemodynamics (Malzer 1996).
Disadvantages of ACDR CPR include the requirement for more training than STR, more physical effort (Shultz 1995), and the fact that it can be more difficult to apply in certain clinical situations. Increased frequency of sternal and rib fractures with ACDR CPR has been reported (Baubin 1999).
The improved haemodynamic associated with ACDR CPR was expected to reduce mortality or neurological damage, or both, when applied to people in cardiac arrest. However, results from clinical trials have been variable and contradictory. The first clinical trials comparing ACDR CPR to STR included small groups of participants, in‐hospital, and showed promising results with the newer technique (Cohen 1993; Tucker 1994). Later randomised controlled trials involving a greater number of participants, mostly in out‐of‐hospital settings, often showed negative effects for the ACDR CPR method (Schwab 1995; Stiell 1996).
Mauer et al combined individual participant data of 2866 participants from seven separate out‐of‐hospital randomised controlled trials (Mauer 1999). Pooled data showed in participants treated with ACDR CPR a significant improvement in one‐hour survival (23.8% versus (vs) 20.6% in ACDR CPR and STR groups, respectively) and a trend, which was not significant, towards a better survival to hospital discharge (7% vs 5.8%). Mauer et al found that ACDR CPR neither improved nor impaired neurological outcomes and complication rates. After the publication of this meta‐analysis, several new controlled trials comparing ACDR CPR and STR were done. Again, some showed a benefit using ACDR CPR (Plaisance 1999), and some did not (Skogvoll 1999).
A parallel Cochrane systematic review, which aimed to study the effectiveness of mechanical chest compression devices when compared with manual chest compressions for cardiopulmonary resuscitation, found that evidence from human studies was insufficient to conclude that mechanical chest compression is associated with benefit or harm (Brooks 2011). ACDR CPR is a passive tool, manually driven by the rescuer, who delivers all the energy needed to compress and decompress the chest, so it is as prone ‐ or more ‐ to fatigue as standard manual compression. The 2010 American Heart Association guidelines for cardiopulmonary resuscitation increased the focus on adequate chest compression as a critical component of high‐quality cardiopulmonary resuscitation (American Heart Association 2010). Retrieving the ACDR CPR device might delay immediate application of chest compression, and early fatigue may reduce the performance of compressions performed with this device.
We decided to prepare and maintain a systematic review, analysing the effects of the use of ACDR CPR and STR in both out‐of‐hospital and in‐hospital settings.
Objectives
To determine the effect of active chest compression‐decompression CPR compared to standard chest compression CPR on mortality and neurological function in adults with cardiac arrest treated either in‐hospital or out‐of‐hospital.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised controlled trials comparing ACDR CPR to STR in the defined population and reporting at least one of the defined outcomes. We accepted quasi‐randomised studies because it can be difficult to maintain the allocation process, as the time available for deciding treatment is very limited. Double‐blinding is not possible for this intervention; blinded assessment of outcomes was desired, but we did not consider it a decisive requisite for inclusion of studies.
Types of participants
Adult patients (any age greater than 16 years old) suffering cardiac arrest and undergoing cardiopulmonary resuscitation efforts performed by a medical or paramedical trained team. We considered both out‐of‐hospital and in‐hospital settings, but undertook separate analyses, as patients in these settings are usually different with regard to causes of cardiac arrest and comorbidity. For our purposes, we defined cardiac arrest as sudden presentation of unresponsiveness, absence of spontaneous pulse, and absence of spontaneous respiration in a person not having a known or evident terminal disease.
Types of interventions
Intervention group
-
Active chest compression‐decompression using an appropriate device
Paramedical or medical teams participating in the study must have received specific training in ACDR CPR. Participants could receive STR by a witness (medical or not) until the arrival of the emergency team, but after that they must have received sustained ACDR CPR. We did not consider for inclusion trials alternating ACDR CPR and STR in the same participant.
Control group
-
Standard manual chest compression by a trained paramedical or medical team, using the recommendations for cardiopulmonary resuscitation drawn up by the American Heart Association (American Heart Association 2005), the European Resuscitation Council (European Resuscitation Council 2005), or both
Other interventions
Any intervention other than chest compression procedure, especially those interventions for basic and advanced life support, must have been applied equally in both intervention and control groups and should have been performed in accordance with the aforementioned recommendations. Time response from cardiac arrest to the beginning of resuscitation by the paramedical or medical team was assumed to be equal between treatment groups as a result of the randomisation procedure. If there were imbalances between the groups, this was dealt with by stratification or logistic regression analysis.
Types of outcome measures
Mortality
-
Immediate: participants not recovering spontaneous circulation in spite of resuscitation efforts.
-
To hospital discharge: participants not surviving long enough to be discharged from the hospital where they were first admitted.
-
Long‐term: at three, six, or 12 months, or a combination of the aforementioned, when data were available.
Frequency of resultant neurological impairment in participants surviving until hospital discharge
This was classified as follows:
-
Moderate: impaired functionality or quality of life, but participant self‐sufficient for basic needs of daily life. This corresponds to the Glasgow‐Pittsburgh Cerebral Performance category 2 (moderate disability).
-
Severe: dependency for one or several basic needs of daily life. This corresponds to the Glasgow‐Pittsburgh Cerebral Performance categories 3 and 4 (severe disability, vegetative state).
-
Any resultant neurologic impairment.
If data were available, we also assessed this outcome in the long‐term (three, six, or 12 months, or a combination of the aforementioned).
Complications
-
Relevant complications derived from the applied technique.
-
Sternal and rib fractures.
-
Haemothorax or pneumothorax.
-
Internal organ damage.
We did not consider minor complications such as superficial trauma to the skin.
Search methods for identification of studies
Electronic searches
The following electronic databases were searched last on 14 January 2013:
-
the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, Issue 12 of 12, December 2012;
-
MEDLINE on Ovid (1946 to January week 1 2013); and
-
EMBASE on Ovid (1980 to week 1 2013).
The search strategies employed can be found in Appendix 1 (2008) and Appendix 2 (2010 and 2013).
The RCT filters used for MEDLINE and EMBASE have been updated (Lefebvre 2011).
Searching other resources
We checked the reference list of all identified primary studies and review articles for other potential relevant citations. We contacted the sole company that manufactures ACDR CPR devices (Ambu International A/S) and colleagues working in the field of cardiopulmonary resuscitation for other published and unpublished studies. A search of the clinical trials registry ClinicalTrials.gov was conducted. There was no language restriction.
Data collection and analysis
Selection of studies
Two authors (CL‐L, MM‐B) selected trials for inclusion or exclusion after reading titles and abstracts of studies identified using the search strategy. We compared the selected trials and resolved any discrepancy by discussion and consensus. Using a predefined form, two authors independently read the full text of retained studies and included trials that met the above stated criteria. We checked the articles finally selected for the review to avoid including data published in duplicate.
Data extraction and management
Using a predefined form, two authors (CL‐L, MM‐B) independently extracted data. We checked the forms for agreement and decided differences by discussion and consensus. We extracted all data on the basis of intention‐to‐treat. When needed, we contacted authors of primary studies for additional information or data.
Assessment of risk of bias in included studies
Two authors (CL‐L, MM‐B) independently assessed the methodological quality of the selected studies, addressing adequacy of allocation concealment for randomised trials, which we ranked as yes (low risk of bias), unclear, or no (high risk of bias), following the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008; Higgins 2011). We resolved any differences of opinion by discussion and consensus.
Assessment of heterogeneity
We tested homogeneity between included studies using a fixed‐effect or random‐effects model, as appropriate. If significant heterogeneity was present, we attempted to explain the differences based on the clinical characteristics of the included studies. If a clinically dissimilar study existed, we did not combine it statistically.
Assessment of reporting biases
We carried out a funnel plot to test for the presence of publication bias, based on the data for the primary outcome of immediate mortality.
Data synthesis
We pooled trials using Review Manager 5 (RevMan) software and pooled relative risk calculated for all outcomes. If results for any outcome were significant, we also expressed them as number needed to treat (NNT) to prevent one adverse outcome.
Subgroup analysis and investigation of heterogeneity
We considered predefined subgroups for out‐of‐hospital studies. Personnel within the resuscitation teams were thought to have a possible effect on outcome because ACDR CPR requires more training than standard chest compression, and this could influence its performance. Therefore, we proposed subgroup analyses for studies where the resuscitation team included physicians and paramedics and for studies where resuscitation teams consisted of paramedics without a physician.
Sensitivity analysis
We performed sensitivity analyses by selectively pooling the following:
-
studies having the best methodological quality (allocation concealment adequate (A)); and
-
studies including the greatest number of participants (i.e. > 500 participants).
Results
Description of studies
Results of the search
Our searches identified 231 references in total. We found 27 new publications for this update, of which we assessed two in detail, but none fulfilled our inclusion criteria.
We were aware of two additional controlled trials conducted in Brussels (Belgium) and Thessaloniki (Greece), which were cited as having contributed to the combined analysis of individual participant data performed by Mauer et al (Mauer 1999). However, this analysis offered no separate data from the individual trials. We were unable to obtain any published data and did not receive responses to our letters to the respective main authors.
After reading titles and abstracts, we retained 27 articles for detailed assessment. We excluded 10 articles reporting nine studies: five because they were not randomised (Arai 2001; Gueugniaud 1998; Lefrançois 1998; Panzer 1996; Rivers 1993); one because it included a large proportion of participants with pre‐existing terminal disease (Cohen 1993); two because they combined ACDR CPR with other interventions not performed in controls (an inspiratory impedance threshold device in both cases) (Aufderheide 2011; Wolcke 2003); and one study (two references) because ACDR CPR was applied to all participant groups (Plaisance 2004). We present further details of the excluded studies in the 'Characteristics of excluded studies' section. One further article is a publication in Chinese, which is waiting for translation of the full text.
Finally, 16 articles reporting 10 studies fulfilled the defined inclusion criteria (Characteristics of included studies). Two of these studies included two separate trials respectively. Stiell reported on out‐of‐hospital and in‐hospital participants separately (Stiell‐Inhospital 1996; Stiell‐Prehospital 1996), and Schwab reported on groups of participants in two cities separately (Schwab‐Fresno 1995; Schwab‐S.Francisco 1995). Thus, we included 12 comparisons, cumulating a total of 4988 participants.
Included studies
Setting
Most trials (eight) included only out‐of‐hospital cardiac arrests; one included both out‐of‐ and in‐hospital cardiac arrests (Stiell‐Prehospital 1996, n = 1011; Stiell‐Inhospital 1996, n = 773), and one included only in‐hospital participants (Tucker 1994, n = 53). The trials were conducted between 1993 and 1999 in Europe (France, Germany, Norway, United Kingdom) and North America (Canada, United States).
Stiell et al reported and analysed separately data from the two different settings of their trial: out‐of‐hospital and in‐hospital participants. Schwab et al also reported and analysed separately data from the two cities where they conducted their study: Fresno and San Francisco (USA). We maintained the same pattern and pooled each of Stiell and Schwab's trials as two independent substudies.
Participants
All trials excluded people with a terminal disease or evidence of irreversible (i.e. brain) death. Most trials excluded also witnessed arrests, pregnant women, trauma victims, people in hypothermia, and intoxications. Three studies applied additional exclusion criteria: primary respiratory arrest, drowning, airway obstruction, and recent sternotomy.
The mean age of included participants was wide‐ranging: between 65 and 71 years in eight studies, and lower in Goralski 1998 (61.5 years) and Plaisance 1999 (58.5 years). Two trials (Lurie 1994; Nolan 1998) stated that they could include young participants (> eight years old), but the mean age of the participants actually included was 67 years in both studies. Males predominated in all trials (57% to 73%) but one (Tucker 1994), where women represented 57%.
Five studies reported participant medical antecedents, presumed aetiology of the arrest, or both. Antecedents of heart disease (23% to 58%) and suspected cardiac aetiology (40% to 70%) were the most common conditions, followed by respiratory diseases (8% to 16%). Demographic and clinical characteristics were comparable between the ACDR CPR and STR groups in every trial.
Interventions
All trials compared ACDR CPR versus STR. In four of the out‐of‐hospital studies, the rescue teams comprised paramedics only; a physician directed the team in the remaining studies. In all the studies, paramedics and physicians were trained in ACDR CPR, receiving theoretical sessions and practicing with manikins. The frequency of retraining varied: every one month (Plaisance 1999), six months (Mauer 1996), or one year (Luiz 1996; Skogvoll 1999; Stiell‐Inhospital 1996; Stiell‐Prehospital 1996). Five studies did not state if retraining was provided (Goralski 1998; Lurie 1994; Nolan 1998; Schwab‐Fresno 1995; Schwab‐S.Francisco 1995; Tucker 1994).
Mean time from call to arrival of the rescue team ranged from 5.2 to 16 minutes in the out‐of‐hospital studies, depending on geographic and emergency system characteristics. In the two in‐hospital studies, mean response time was only 1.4 and 2 minutes. Mean adrenaline dose used varied from 2.7 mg to 14.3 mg in the different trials. Response times and adrenaline doses were not significantly different between ACDR CPR and STR groups in any individual trial.
Risk of bias in included studies
All included studies were randomised or quasi‐randomised controlled trials. Allocation concealment was adequate (A) in four studies (Goralski 1998; Mauer 1996; Skogvoll 1999; Stiell‐Inhospital 1996; Stiell‐Prehospital 1996); unclear (B) in one (Luiz 1996); and clearly inadequate (C) in five (Lurie 1994; Nolan 1998; Plaisance 1999; Schwab‐Fresno 1995; Schwab‐S.Francisco 1995; Tucker 1994), because they used alternating periods of time (the day, week, or month) to allocate participants and were therefore quasi‐randomised designs.
No study was blinded to the intervention applied, as it is impossible to mask the use of the ACDR device. In four studies, authors conducted explicit intention‐to‐treat analysis. In the remaining six studies, we could extract ‐ from the published data or after contacting authors ‐ data as intention‐to‐treat. No outcome data were missing for the outcomes from each study that were pooled in the review. (Six of the studies did not report useable data on neurological outcomes.)
More details about the risk of bias are given in Figure 1 and Figure 2.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
Effects of interventions
There were few disagreements between the reviewers in selecting primary studies, assessing quality, or extracting data, and they were easily resolved by consensus. The funnel plot graphic was symmetrical, indicating that this test detected no publication bias or other bias.
We gave all results as pooled relative risk (RR) and its 95% confidence interval (CI) (ACDR CPR participants versus STR participants).
In‐hospital studies
We found only two in‐hospital studies fulfilling inclusion criteria (Stiell‐Inhospital 1996; Tucker 1994). The best was the Stiell study, a good‐quality trial (A) that included 733 in‐hospital participants. This trial did not find any significant difference between ACDR CPR and STR groups neither in mortality, immediate (RR 1.01, 95% CI 0.91 to 1.12), or at hospital discharge (RR 1.01, 95% CI 0.96 to 1.06), nor in neurologic impairment in survivors (RR 1.14, 95% CI 0.46 to 2.87) or in complications (RR 0.97, 95% CI 0.49 to 1.93). The Tucker trial was a quality C study, with only 53 participants. No significant difference appeared in this study in any outcome either. Despite the differences in size and quality, the findings of the two in‐hospital studies were similar.
Out‐of‐hospital studies
Mortality
All the out‐of‐hospital studies (cumulating 4162 participants) reported data for immediate mortality. Borderline heterogeneity between studies was found for this outcome (Chi² statistic = 14.81, df = 9, P = 0.10). This heterogeneity was largely due to Plaisance 1999 and disappeared when we removed this study (Chi² statistic = 8.38, df = 8, P = 0.40). We found no effect of ACDR CPR on immediate mortality either using a random‐effects model (RR 0.98, 95% CI 0.94 to 1.03) or a fixed‐effect model (RR 0.98, 95% CI 0.94 to 1.01) or after removing Plaisance 1999 (RR 1.00, 95% CI 0.96 to 1.04).
Eight trials (3412 participants) reported mortality at hospital discharge. They were homogeneous. We found no effect of ACDR CPR on mortality at hospital discharge (RR 0.99, 95% CI 0.98 to 1.01).
No study reported long‐term mortality. Plaisance 1999 followed for one year a specific subgroup of participants discharged without neurological damage, but the study did not follow up all the survivors of each treatment group. After contacting the author, further information about participants having neurological injury was not available.
Neurological outcome
Data on neurological outcome in survivors were available from five trials (cumulating 144 survivors) at hospital discharge. There was no heterogeneity between studies. Frequency of severe neurological damage showed a clear trend, without reaching statistical significance, being higher in the ACDR CPR group (RR 3.11, 95% CI 0.98 to 9.83), while moderate neurological damage did not differ between intervention groups (RR 0.98, 95% CI 0.34 to 2.79). The pooled relative risk of neurological impairment of any severity was 1.71 (95% CI 0.90 to 3.25). No study reported long‐term neurological outcomes.
Complications
Six studies (3032 participants) provided data on complications related to treatment. Studies were homogenous. Relevant observed complications (rib or sternal fractures, pneumothorax or haemothorax, internal organ damage) were comparable with both resuscitation techniques (RR 1.09, 95% CI 0.86 to 1.38). Skin trauma or ecchymosis were more frequent with ACDR CPR in all studies.
Subgroup analysis (out‐of‐hospital studies only)
Results for mortality were unchanged when pooling separately trials with and without a physician in the first attending team. The trend toward more frequent neurological damage (of any severity) in the ACDR CPR group was apparent in those studies with only paramedics (RR 2.19, 95% CI 0.93 to 5.13) and disappeared when a physician directed the rescue team (RR 1.14, 95% CI 0.59 to 2.21). However, there were few participants in each subgroup, and it was not possible to analyse neurological outcome by degree of severity given the number of participants.
Sensitivity analysis
All results were unchanged when selectively pooling the best‐quality studies (adequate allocation concealment; four studies) or largest studies (those including > 500 participants: four studies).
Discussion
Summary of main results
This systematic review found no evidence of benefit on mortality with the use of active compression‐decompression cardiopulmonary resuscitation (ACDR CPR) compared with standard manual cardiopulmonary resuscitation (STR) for cardiopulmonary resuscitation. Confidence intervals were small with the trial evidence excluding a benefit greater than 6% relative risk reduction and a harm as big as 3% relative risk increase attributable to ACDR CPR.
With regard to neurologic outcome, a non‐significant difference between treatment groups was observed in out‐of‐hospital studies, with the rate of neurologic impairment of severe degree and any severity tending to be higher in those treated with ACDR CPR. In subgroup analysis, this trend appeared in studies with attending teams comprising paramedics alone, and not in those with paramedics and physicians. However, this difference was not statistically significant, and the assessment of this outcome was limited: The number of participants with neurological impairment was small, assessors were often not blinded to the study intervention applied, and the Glasgow‐Pittsburgh Cerebral Performance Scale is not a precise measure of neurologic function. Also, perceived differences between studies with and without physicians in rescue teams could merely reflect the overall numbers or rescuers attending the participant. These trials did not assess late neurological complications.
Complications were similar with both treatments. Some studies on autopsies of patients after unsuccessful resuscitation found more chest fractures, specifically sternal fractures, with ACDR CPR than with STR, and also a higher risk of iatrogenic cardiac injury (Baubin 1999; Rabl 1996). The clinical trials included in this review were not specifically designed to study complications. Nevertheless, in several of them, autopsies were done in a number of non‐survivors (Stiell‐Inhospital 1996; Schwab‐Fresno 1995; Tucker 1994). In all the studies included in this review and in the pooled results, the observed complications were very similar with both techniques, with the exception of skin trauma or ecchymosis, which were more frequent with ACDR CPR.
Potential biases in the review process
The objective of this systematic review was to assess the overall effectiveness of ACDR CPR in the wide spectrum of clinical practice. A possible limitation of this approach is to pool together trials conducted in different clinical conditions and emergency medical systems. Percentage of participants in ventricular fibrillation, time to receive life support, composition, and training of medical teams are all factors that varied from study to study. Possible benefits of ACDR CPR, or conversely potential dangers, in specific subgroups of people or specific emergency medical systems might have been missed by being diluted in the global pool.
However, results seem to be consistent. Combining the included studies in different ways, by size, quality, team composition, and setting, did not change the main findings. Also, statistically significant heterogeneity between studies was not found, with the only exception of one outcome, immediate mortality, due to one study (Plaisance 1999). Neither the exclusion nor the inclusion (using both fixed‐ and random‐effects models for statistics) of this study modified the results.
Plaisance 1999 showed better results for immediate mortality than the other trials. This difference could be explained by several reasons. It could be due to a bias in participant selection, as allocation concealment was clearly inadequate. But it could be due also to a better performance of the ACDR CPR technique. In this study, practitioners were experienced in ACDR CPR, and frequent retraining was performed, with rapid rotation of staff to avoid fatigue and conduct of the entire cardiopulmonary resuscitation at the scene of cardiac arrest directed by a physician. It was not possible to compare the outcomes of this study with the other included trials, other than immediate mortality, because not all the participants were assessed until hospital discharge. Participants were followed to discharge only if they had no neurological impairment.
Agreements and disagreements with other studies or reviews
Our results are different from the meta‐analysis by Mauer 1999 with regard to immediate mortality, where they found a significant reduction (odds ratio 0.83, 95% CI 0.695 to 0.99). The main difference seems to be that we pooled more studies than Mauer: the studies by Schwab, Goralski, and Skogvoll, leading to 1296 more participants. All these trials had negative results for ACDR CPR. Mauer et al used individual participant data and analysed one‐hour mortality while we recorded the immediate mortality as defined by authors of each trial, usually in the first minutes after resuscitation. However, the differences are limited to results for early mortality; Mauer and colleagues found no significant difference in mortality at hospital discharge neither.
Other considerations
Difficulty in applying optimal ACDR CPR in clinical settings, reported by several studies, reveal that this procedure, while seemingly relatively simple, is in fact much more complex than STR. This complexity could limit its effectiveness in real practice. In studies on transducer‐equipped manikins, ACDR CPR required a higher physical effort than STR (Shultz 1995). Staff fatigue led to a significant reduction of compression rate and depth, as well as decompression force, after only two minutes in one study (Skogvoll 1997). ACDR CPR mechanical performance was also worse than STR during patient transport (Sunde 1997). In some of the clinical trials included in this review, they had limited available personnel to sustain chest massage, chest massage was performed during transport, or both (Nolan 1998; Schwab‐Fresno 1995; Schwab‐S.Francisco 1995; Stiell‐Prehospital 1996).
The 2010 American Heart Association guidelines for cardiopulmonary resuscitation increased the focus on adequate chest compression as a critical component of high‐quality cardiopulmonary resuscitation, specifically in minimising interruptions in chest compressions, providing compressions of adequate rate and depth, and avoiding leaning between compressions (American Heart Association 2010). The difficulty of applying ACDR CPR during transport and the reduced performance of ACDR CPR observed in some trials because of early fatigue may hinder in real practice the theoretical advantages of ACDR CPR over manual compression.
One additional problem, non‐adherence to chest of the ACDR CPR device, was common. This defect was found in a variable proportion of participants, which ranged from 9% (Lurie 1994; Mauer 1996; Nolan 1998) to 16% (Goralski 1998; Luiz 1996; Stiell‐Inhospital 1996; Stiell‐Prehospital 1996), but was as high as 24% in one study (Skogvoll 1999). In most of those participants, staff were unable to continue ACDR CPR and performed STR in substitution.
There are several important determinants of survival following cardiac arrest that are independent of the technique used. Time from arrest to basic and advanced life support, specially to defibrillation, is a well‐characterised major factor (Eisenberg 1979; Mullie 1989), most important in out‐of‐hospital arrests (Becker 1991; Nichol 1996). Other major factors influencing survival are patient‐intrinsic: primary cause of arrest, initial heart rhythm (Pepe 1993; Zoch 2000), coexisting diseases, and age (de Vos 1999; Ebell 1992).
Most experimental studies concluded that ACDR CPR, compared to STR, improved haemodynamics during cardiopulmonary resuscitation. Despite this physiological advantage, this does not appear to be sufficient to modify the natural evolution of cardiac arrest.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
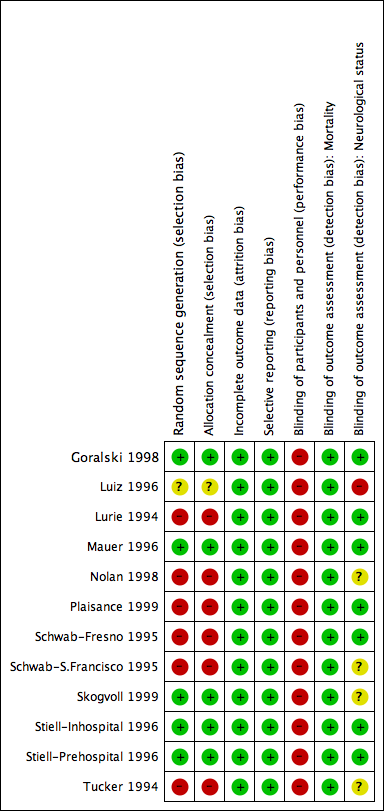
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
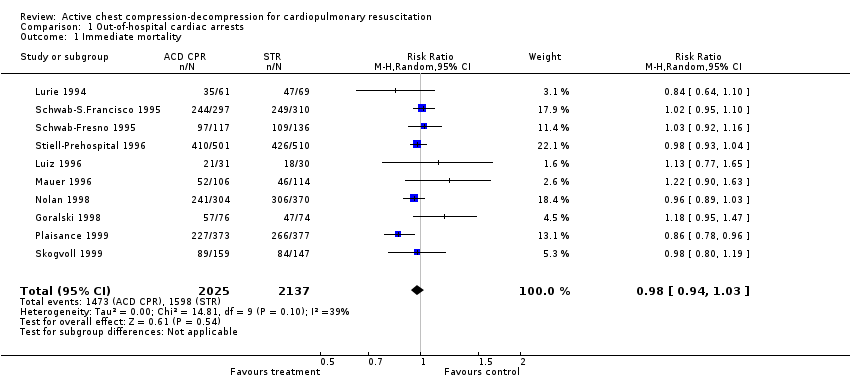
Comparison 1 Out‐of‐hospital cardiac arrests, Outcome 1 Immediate mortality.
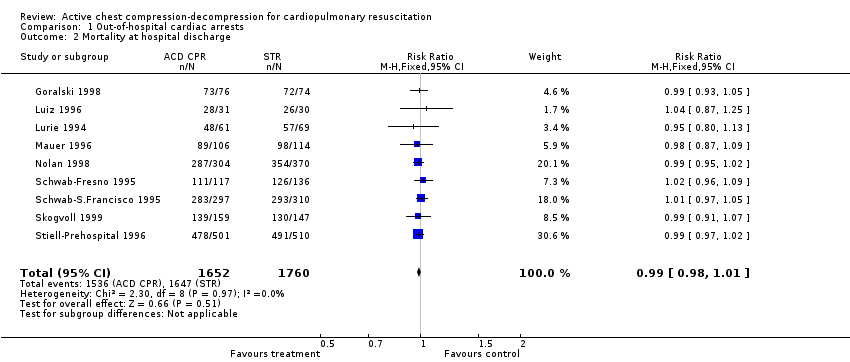
Comparison 1 Out‐of‐hospital cardiac arrests, Outcome 2 Mortality at hospital discharge.

Comparison 1 Out‐of‐hospital cardiac arrests, Outcome 3 Neurological impairment in survivors.

Comparison 1 Out‐of‐hospital cardiac arrests, Outcome 4 Complications.
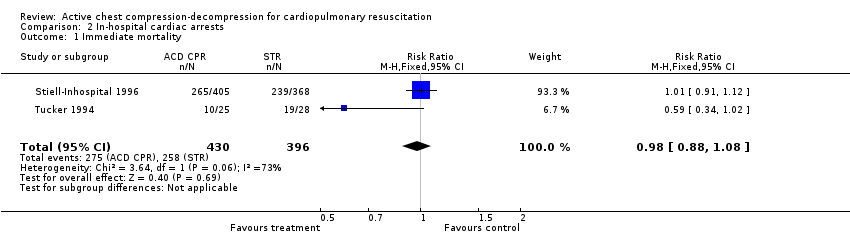
Comparison 2 In‐hospital cardiac arrests, Outcome 1 Immediate mortality.
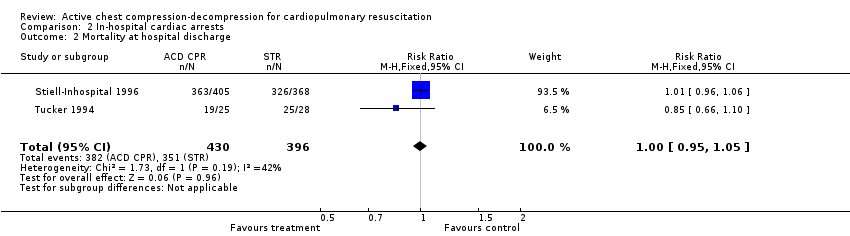
Comparison 2 In‐hospital cardiac arrests, Outcome 2 Mortality at hospital discharge.

Comparison 2 In‐hospital cardiac arrests, Outcome 3 Neurological impairment in survivors.

Comparison 2 In‐hospital cardiac arrests, Outcome 4 Complications.

Comparison 3 Subgroup analysis: physician in first responding team or only paramedics, Outcome 1 Immediate mortality.
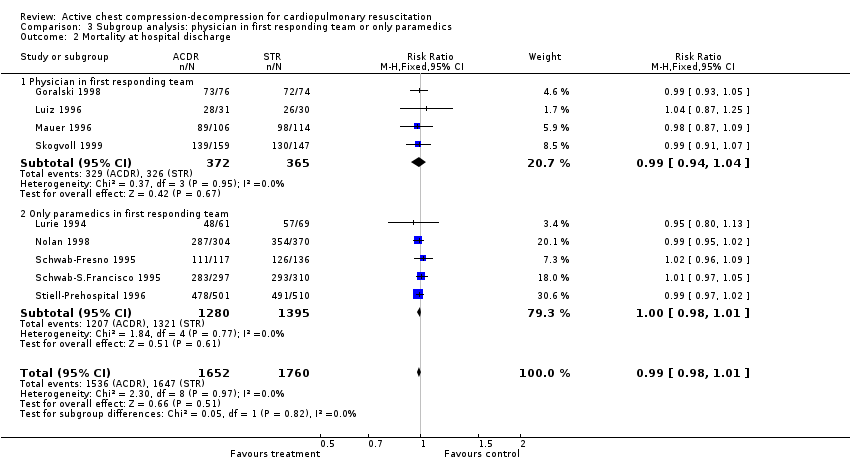
Comparison 3 Subgroup analysis: physician in first responding team or only paramedics, Outcome 2 Mortality at hospital discharge.

Comparison 3 Subgroup analysis: physician in first responding team or only paramedics, Outcome 3 Neurological impairment in survivors (any severity).

Comparison 4 Sensitivity analysis: best‐quality studies, Outcome 1 Immediate mortality.

Comparison 4 Sensitivity analysis: best‐quality studies, Outcome 2 Mortality at hospital discharge.

Comparison 4 Sensitivity analysis: best‐quality studies, Outcome 3 Neurological impairment in survivors (any severity).

Comparison 5 Sensitivity analysis: largest (n > 500) studies, Outcome 1 Immediate mortality.
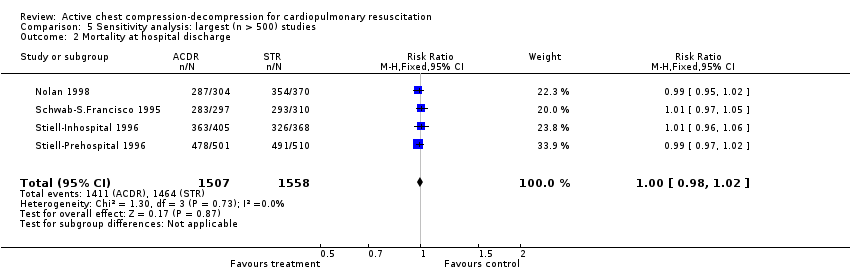
Comparison 5 Sensitivity analysis: largest (n > 500) studies, Outcome 2 Mortality at hospital discharge.

Comparison 5 Sensitivity analysis: largest (n > 500) studies, Outcome 3 Neurological impairment in survivors (any severity).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Immediate mortality Show forest plot | 10 | 4162 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.94, 1.03] |
| 2 Mortality at hospital discharge Show forest plot | 9 | 3412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.98, 1.01] |
| 3 Neurological impairment in survivors Show forest plot | 5 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.06, 2.83] |
| 3.1 Moderate neurological impairment | 4 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.34, 2.79] |
| 3.2 Severe neurological impairment | 4 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.11 [0.98, 9.83] |
| 3.3 Any neurological impairment (any severity) | 5 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.90, 3.25] |
| 4 Complications Show forest plot | 7 | 3032 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.86, 1.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Immediate mortality Show forest plot | 2 | 826 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.88, 1.08] |
| 2 Mortality at hospital discharge Show forest plot | 2 | 826 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.95, 1.05] |
| 3 Neurological impairment in survivors Show forest plot | 2 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.63, 2.25] |
| 3.1 Moderate neurologic impairment | 2 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.10, 2.58] |
| 3.2 Severe neurologic impairment | 2 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.59, 6.50] |
| 3.3 Any neurological impairment (any severity) | 2 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.50, 2.86] |
| 4 Complications Show forest plot | 1 | 773 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.49, 1.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Immediate mortality Show forest plot | 10 | 4162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.94, 1.01] |
| 1.1 Physician in first responding team | 5 | 1487 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.89, 1.04] |
| 1.2 Only paramedics in first responding team | 5 | 2675 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.95, 1.02] |
| 2 Mortality at hospital discharge Show forest plot | 9 | 3412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.98, 1.01] |
| 2.1 Physician in first responding team | 4 | 737 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.94, 1.04] |
| 2.2 Only paramedics in first responding team | 5 | 2675 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.98, 1.01] |
| 3 Neurological impairment in survivors (any severity) Show forest plot | 5 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.90, 3.25] |
| 3.1 Physician in first responding team | 2 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.41, 2.80] |
| 3.2 Only paramedics in first responding team | 3 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.19 [0.93, 5.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Immediate mortality Show forest plot | 12 | 4988 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.95, 1.01] |
| 1.1 Allocation concealment adequate | 5 | 2460 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.96, 1.06] |
| 1.2 Allocation concealment unclear | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.77, 1.65] |
| 1.3 Allocation concealment inadequate | 6 | 2467 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.90, 0.99] |
| 2 Mortality at hospital discharge Show forest plot | 11 | 4238 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.98, 1.01] |
| 2.1 Allocation concealment adequate | 5 | 2460 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.97, 1.02] |
| 2.2 Allocation concealment unclear | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.87, 1.25] |
| 2.3 Allocation concealment inadequate | 5 | 1717 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.97, 1.02] |
| 3 Neurological impairment in survivors (any severity) Show forest plot | 7 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.89, 2.51] |
| 3.1 Allocation concealment adequate | 3 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.75, 2.79] |
| 3.2 Allocation concealment unclear | 1 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.33, 2.37] |
| 3.3 Allocation concealment inadequate | 3 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.09 [0.67, 6.54] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Immediate mortality Show forest plot | 5 | 3815 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.93, 1.00] |
| 2 Mortality at hospital discharge Show forest plot | 4 | 3065 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.98, 1.02] |
| 3 Neurological impairment in survivors (any severity) Show forest plot | 3 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.81, 3.16] |

