Амантадин и римантадин при гриппе А у детей и пожилых людей
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised, parallel, double‐blind comparison of rimantadine with PB. The trial took place during an outbreak of influenza A/H1N1 in Oklahoma | |
| Participants | There was a total of 146 participants, including 76 children, which was our subgroup of interest | |
| Interventions | Rimantadine: 5 mg/kg/d, max: 100 mg/ d (< 10 years) or 200 mg/ d (> 10 years). Oral route. Duration: 5 weeks | |
| Outcomes | Laboratory‐proven infection cases and reported adverse effects | |
| Notes | 1 to 18 years old | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It is reported that "children ... received either rimantadine or PB in a double‐blind, random assignment". Nevertheless, the randomisation method is not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment is not clearly described |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for missing outcome data are unlikely to be related to true outcome |
| Blinding of participants and personnel (performance bias) | Low risk | It is stated that "children ... received either rimantadine or PB in a double‐blind, random assignment" but the specific people who were blinded are not listed |
| Blinding of outcome assessment (detection bias) | Low risk | It is stated that "children ... received either rimantadine or PB in a double‐blind, random assignment" but the specific people who were blinded are not listed |
| Methods | Randomised, parallel, comparison of rimantadine with PB. Multicentre trial that took place during an influenza season for 3 to 4 weeks after the start of treatment | |
| Participants | There was a total of 84 participants, including 46 children, which was our subgroup of interest | |
| Interventions | Rimantadine: 5 mg/kg/d, max: 150 mg/d (= or < 10 years or weighing less than 30 kg) or 200 mg/d (> 9 years who weighed more than 30 kg). Oral route. Duration: 10 days | |
| Outcomes | The outcome of interest was laboratory‐proven infection cases | |
| Notes | 1 to 17 years old | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors stated it was a randomised study and that randomisation is described in another article (Hayden 1989): "all eligible family members ... randomly assigned as a block to receive either rimantadine or PB". The method used is not described |
| Allocation concealment (selection bias) | Low risk | Randomisation was carried out in one of the centres where this multicentric trial was conducted |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for missing outcome data are unlikely to be related to true outcome |
| Blinding of participants and personnel (performance bias) | Low risk | Authors stated it was a double‐blinded trial as described in the other article (Hayden 1989): "the study was double‐blind ... trial". Nevertheless, the specific people who were blinded are not listed |
| Blinding of outcome assessment (detection bias) | Low risk | Authors stated it was a double‐blinded trial as described in the other article (Hayden 1989): "the study was double‐blind ... trial". Nevertheless, the specific people who were blinded are not listed |
| Methods | Randomised, parallel, double‐blind trial in which prophylactic efficacy of rimantadine against influenza A infection in children was evaluated. Rimantadine was compared to PB. The trial took place during a naturally occurring outbreak of influenza A (H3N2) in Oklahoma City, USA, from November, 1984 to March, 1985 | |
| Participants | There was a total of 110 participants from 29 families, including 56 children, which was our subgroup of interest | |
| Interventions | Rimantadine: 5 mg/kg/d, max: 100 mg/d (< 10 years) or 200 mg/d (> 10 years). Oral route | |
| Outcomes | Laboratory‐proven infection cases. Adverse effects | |
| Notes | 1 to 18 years old | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors stated it was a "a randomised ... clinical trial" although randomisation methods are not described |
| Allocation concealment (selection bias) | Unclear risk | The authors state that their "study design has been previously reported" (Clover 1986) but even in that trial, the method of concealment is not clearly described |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for missing outcome data unlikely to be related to outcome |
| Blinding of participants and personnel (performance bias) | Low risk | Authors stated it was "a double‐blind PB controlled clinical trial". Nevertheless, the specific people who are blinded are not listed |
| Blinding of outcome assessment (detection bias) | Low risk | Authors stated it was "a double‐blind PB controlled clinical trial". Nevertheless, the specific people who are blinded are not listed |
| Methods | Randomised, parallel, double‐blind, trial in which amantadine was used as prophylaxis in naturally occurring acute respiratory illness. Amantadine was compared to PB. The trial took place between February 1965 to June 1965 | |
| Participants | There were 293 participants from both sexes (proportion not stated), from 8 to 19 years of age. The participants were volunteers at a school for intellectually handicapped but educable children. Sera pairs tests were obtained in 237 children. Exclusion criteria: children receiving tranquillisers, sympathomimetic amines or anticonvulsives | |
| Interventions | Amantadine: 1 to 2.5 mg/kg (pre‐puberal: 60 mg/dose, 2 x/d, during the first week and 1 x/d during the rest of the period of the study. Older children: 100 mg/dose, 2 x/d, during the first week and 1 x/d during the rest of the period of the study | |
| Outcomes | 4‐fold rises in CF and/or HI tilter against A2/AA/1/65 | |
| Notes | 8 to 19 years old | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors stated "volunteers were assigned to amantadine or the PB group by randomisation", although randomisation method is not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment is not clearly described |
| Incomplete outcome data (attrition bias) | Low risk | It is stated that "The rate of withdrawal ... (the same for the two groups) was small. The reason for withdrawal was discharge from school" |
| Blinding of participants and personnel (performance bias) | Low risk | Although it was "a double‐blind study", the specific people who are blinded are not listed |
| Blinding of outcome assessment (detection bias) | Low risk | Although the trial is described as "a double‐blind study", the specific people who are blinded are not listed |
| Methods | Randomised, parallel, double‐blind comparison of rimantadine with zanamivir. Identical PB (inhaled or tablets) were used. The trial took place in nine long‐term care facilities in the United States over 3 winter seasons. The study was conducted over multiple influenza seasons, therefore some participants were randomised more than once | |
| Participants | There were 231 participants in the rimantadine group and 226 in the zanamivir group (intention‐to‐treat population) of both sexes (29% female in rimantadine group and 30% female in zanamivir group). More than 75% of the participants were 65 years of age or older (90% in rimantadine group and 89% in zanamivir group) | |
| Interventions | Upon an influenza outbreak participants were randomised (1:1) to inhaled zanamivir plus PB or inhaled PB plus zanamivir 100 mg tablets for 14 days | |
| Outcomes | The outcome of interest was laboratory‐proven infection cases | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors describe the trial as "a randomised, parallel comparison of rimantadine with zanamivir" but randomisation methods are not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment is not clearly described |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for missing outcome data are unlikely to be related to the true outcomes |
| Blinding of participants and personnel (performance bias) | Low risk | The trial is described as a "double‐blind comparison of rimantadine with zanamivir. Identical PB (inhaled or tablets) were used". Nevertheless, the specific people who are blinded are not listed |
| Blinding of outcome assessment (detection bias) | Low risk | The trial is described as a "double‐blind comparison of rimantadine with zanamivir. Identical PB (inhaled or tablets) were used". Nevertheless, the specific people who are blinded are not listed |
| Methods | Randomised, parallel, double‐blind comparison of rimantadine with acetaminophen | |
| Participants | 69 children were included, 40 females and 29 males | |
| Interventions | Rimantadine: 6.6 mg/kg/d, max: 150 mg/d (< 9 years) and 200 mg/d (>= 9 years), 2 x/d; by oral route, for 5 days | |
| Outcomes | Mean symptom score of: fever, conjunctivitis, eye symptoms (pain on movement, fever up to 3rd day, conjunctivitis up to 3rd day, eyes symptoms (pain on movement and visual distortion); cough up to 7th day; malaise up to 6th day; CNS symptoms | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It is stated in the published study that "Patients were assigned to the rimantadine or acetaminophen treatment group under a double‐blind, randomised allocation". The investigators also reported in their correspondence to the review authors that a computer random system was used to randomise participants |
| Allocation concealment (selection bias) | Low risk | Participants and investigators enrolling participants could not foresee assignment because a pharmaceutical‐controlled randomisation was used to conceal allocation, as stated in the authors' correspondence to the review authors |
| Incomplete outcome data (attrition bias) | Low risk | 1 "child receiving rimantadine complained of nausea and vomiting and withdrew from the study on the second day". The proportion of missing outcomes compared with observed event risk is not enough to have a clinically relevant impact on the intervention effect estimate |
| Blinding of participants and personnel (performance bias) | Low risk | Although "patients were assigned to the rimantadine or acetaminophen treatment group under a double‐blind, randomised allocation", the specific people who are blinded are not listed |
| Blinding of outcome assessment (detection bias) | Low risk | Although "patients were assigned to the rimantadine or acetaminophen treatment group under a double‐blind, randomised allocation", the specific people who are blinded are not listed |
| Methods | Randomised, parallel, double‐blind comparison of amantadine with PB. This trial took place during an outbreak of influenza in Japan | |
| Participants | There were 355 participants. Although the proportions are not cited, it is stated that the groups are comparable in the following criteria: sex, age, influenza vaccination history, distribution and geometric mean of HI and CF titre in acute sera, interval between onset of symptoms and start of treatment and maximum body temperature before the treatment | |
| Interventions | Amantadine: 50 mg/d (1 to 2 years old); 100 mg/d (3 to 5 years old); 150 mg/d (6 to 10 years old), by oral route, for 7 days | |
| Outcomes | Fever up to 4th day. AE: nausea/vomiting; diarrhoea; exanthema; malaise; muscular, limb pain; headache; dyspnoea; cyanosis; stimulation/insomnia; dizziness; arrhythmia | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that "amantadine or PB was given to the patient at random", although randomisation method is not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment is not described |
| Incomplete outcome data (attrition bias) | Low risk | There were no missing patients, although "four cases were shown to be influenza B and were excluded from statistical analysis" |
| Blinding of participants and personnel (performance bias) | Low risk | Although "amantadine or PB was given to the patient at random by double‐blind method" the specific people who are blinded are not listed |
| Blinding of outcome assessment (detection bias) | Low risk | Although "amantadine or PB was given to the patient at random by double‐blind method" the specific people who are blinded are not listed |
| Methods | Randomised, parallel, double‐blind comparison of amantadine with PB. The trial took place during an outbreak of influenza in the winter of 1968 to 1969 in Japan | |
| Participants | Of the 737 participants, 155 participants of both genders met the inclusion criteria. Although the proportions are not cited, it is stated that the groups are comparable in the following criteria: sex, age, influenza vaccination history, distribution and geometric mean of HI and CF titre in acute sera, interval between onset of symptoms and start of treatment and maximum body temperature before the treatment | |
| Interventions | Amantadine: 50 mg/d (1 to 2 years old); 100 mg/d (3 to 5 years old); 150 mg/d (6 to 10 years old), by oral route, for 7 days | |
| Outcomes | Fever up to 4th day. AE: nausea/vomiting; diarrhoea; exanthema; malaise; muscular, limb pain; headache; stimulation/insomnia; dizziness; arrhythmia | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The author states "patients were given amantadine or PB according to randomly distributed individual code of the double‐blind method", although the randomisation method is not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment is not described |
| Incomplete outcome data (attrition bias) | Low risk | Although there were no missing outcome data, the author states that "only patients with Hong Kong influenza in whom medication was started within 2 days were included in statistical analysis". "In order to exclude the possible influence of concomitantly administered antipyretics on the defervescent effect of amantadine the same analysis was performed with 134 Hong Kong influenza patients who had received no concomitant antipyretics" |
| Blinding of participants and personnel (performance bias) | Low risk | The author states "patients were given amantadine or PB according to randomly distributed individual code of the double‐blind method". Nevertheless, the specific people who are blinded are not listed |
| Blinding of outcome assessment (detection bias) | Low risk | The author states "patients were given amantadine or PB according to randomly distributed individual code of the double‐blind method". Nevertheless, the specific people who are blinded are not listed |
| Methods | Randomised, parallel, double‐blind comparison of 2 different doses of rimantadine with PB. The trial took place during an outbreak of influenza A/H3N2 during 1993 | |
| Participants | A total of 328 participants, 275 females and 53 males were included | |
| Interventions | Rimantadine: 100 mg/d; rimantadine: 200 mg/d; PB. Ratio: 2:2:1. Duration: up to 8 weeks | |
| Outcomes | Death. AEs: dry mouth, drowsiness/fatigue, headache, irritability, dizziness/light headedness, nausea/vomiting, abdominal pain, body weakness or disability, confusion, depression, impaired concentration, insomnia or sleeplessness, loss of appetite, rash or allergic reaction, seizure or clonic twitching | |
| Notes | 3 groups: rimantadine 100 amantadine 200 and PB | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although the authors state that the participants were randomly assigned to receive active medication (100 or 200 mg of rimantadine per day) or placebo, the randomisation method is not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment is not described |
| Incomplete outcome data (attrition bias) | High risk | Authors stated that an "increased risk of withdrawal from the study only on the basis of perceived side effects was demonstrated among participants in both groups receiving active medication, especially the 200 mg/day group, compared with the placebo group; however, these associations were not statistically significant". The reasons for missing outcome data are likely to be related to true outcome |
| Blinding of participants and personnel (performance bias) | Low risk | It is stated that "staff and residents were blinded to group assignment" |
| Blinding of outcome assessment (detection bias) | High risk | No blinding is stated. The outcome is likely to be influenced by lack of blinding |
| Methods | Randomised, parallel, double‐blind comparison of rimantadine with PB. The trial took place during an outbreak of influenza A (H3N2). Viruses were isolated from patients in the community. The study was conducted from early January to 6 April 1983 | |
| Participants | 35 participants, 68 to 102 years old, of non‐specified gender, all of whom had been vaccinated the previous autumn | |
| Interventions | Rimantadine: 100 mg twice a day; PB. Duration: 80 (+/‐ 4.9) days prophylaxis | |
| Outcomes | Adverse reactions: anxiety, confusion, insomnia, anorexia, fatigue, dizziness, nausea and vomiting | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors stated that "participants ... were randomly assigned to receive either rimantadine or PB". Nevertheless, randomisation method is not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment is not described |
| Incomplete outcome data (attrition bias) | High risk | It was cited that 2 participants from the intervention group withdrew because of side effects. 1 suffered a generalised convulsion of undetermined aetiology (a participant with an underlying idiopathic seizure disorder). 3 later withdrew for no described reasons. 2 participants from the PB group also withdrew. Reasons for missing outcome data are likely to be related to the true outcome, with imbalance in reasons for missing data across intervention and control groups |
| Blinding of participants and personnel (performance bias) | Low risk | It is stated that "a double‐blind, placebo‐control trial" was conducted. Nevertheless, the specific people who were blinded are not listed |
| Blinding of outcome assessment (detection bias) | Low risk | It is stated that "a double‐blind, placebo‐control trial" was conducted. Nevertheless, the specific people who were blinded are not listed |
| Methods | Randomised, parallel trial; blinding is not stated. Amantadine used as prophylaxis in naturally occurring acute respiratory illness. Amantadine was compared to no specific treatment. The trial took place in the autumn of 1982 | |
| Participants | There were 604 randomised students and 536 were analysed. All of them were male, from 13 to 19 years of age. The participants were students of a boarding school. Once the influenza A outbreak had been detected, samples were taken from all boys who were sufficiently unwell to be absent from lessons even if they did not have a fever. Nasopharyngeal aspirates were examined for viruses by rapid immunofluorescent microscopy and tissue culture. Once outbreaks had been identified, only culture methods were used | |
| Interventions | Amantadine: 100 mg/ dose, 1 x/d, during the 14 days | |
| Outcomes | Clinical and laboratory‐proven influenza A | |
| Notes | 13 to 19 years old | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | In correspondence with the review authors, the study authors reported that randomisation had been carried out by the statistical department of a pharmaceutical company |
| Allocation concealment (selection bias) | Low risk | Participants and investigators enrolling participants could not foresee assignment because a pharmaceutical company‐controlled randomisation was used to conceal allocation. They kept the key to the randomisation and only when the study was analysed was the code broken, as stated in the study authors' correspondence with the review authors |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Blinding of participants and personnel (performance bias) | Low risk | Although there was no blinding stated, the review authors judge that the outcome is not likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Although there was no blinding stated, the review authors judge that the outcome is not likely to be influenced by the lack of blinding |
| Methods | Randomised, parallel, unblinded trial. Rimantadine and zanamivir were compared for prophylaxis of influenza A. The trial began in November 1996. The participants were volunteer residents of a nursing home for veterans and their spouses | |
| Participants | 65 volunteers of both sexes received zanamivir and 23 rimantadine | |
| Interventions | Rimantadine: 100 mg/dose, 1 x/day, during 14 days. Zanamivir: 10 mg inhaled bid and 4.4 mg intranasally bid | |
| Outcomes | Clinical and laboratory‐proven influenza A | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors stated that it was a "randomised unblinded study" but the randomisation method is not described |
| Allocation concealment (selection bias) | Unclear risk | Concealment is not described |
| Incomplete outcome data (attrition bias) | Unclear risk | There is insufficient reporting of exclusions. It is stated that "six volunteers receiving zanamivir withdrew. One withdrew due to mild adverse effects". The other reasons for withdrawal are not clear. It is also unclear if there were withdrawals among the rimantadine group |
| Blinding of participants and personnel (performance bias) | Low risk | Although it was a "randomised unblinded study", the review authors judge that the outcome is not likely to be influenced by the lack of blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Although it was a "randomised unblinded study", the review authors judge that the outcome is not likely to be influenced by the lack of blinding |
ACM: acetaminophen
AE: adverse effects
bid: twice a day
CF: complement fixation
CNS: central nervous system
d: day
GI: gastrointestinal
HI: haemagglutination inhibition
NC: not clear
PB: placebo
SCr: serum creatinine
STGO: aspartate aminotransferase
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not a RCT | |
| Not a RCT | |
| Not a RCT | |
| Article about oseltamivir and vaccination | |
| Review article | |
| Pharmacokinetics study of amantadine and rimantadine | |
| Ages of participants were outside protocol age range | |
| Ages of participants were outside protocol age range | |
| Open‐label study of the pharmacokinetic interactions of peramivir with oseltamivir or rimantadine | |
| Ages of participants were outside protocol age range | |
| Article about the treatment of hepatitis C | |
| Ages of participants were outside protocol age range (participants were aged between 17 to 57 years old) | |
| Non‐human trial | |
| Not a RCT | |
| Not a RCT | |
| Non‐human trial | |
| Ages of participants were outside protocol age range (participants were aged between 17 to 57 years old) | |
| Ages of participants were outside protocol age range | |
| Review article about other antiviral drugs | |
| Ages of participants were outside protocol age range | |
| Article focusing influenza surveillance | |
| Analyses by age subgroups of interest were not available | |
| Insufficient data available | |
| Systematic review about the use of other antivirals | |
| Not a RCT | |
| Ages of participants were outside protocol age range (participants were 20 to 60 years old) | |
| Review of the use of the influenza vaccine | |
| Article about neuraminidase inhibitors in healthy adults | |
| Review about neuraminidase inhibitors | |
| Article about strategies for pandemic preparedness | |
| Not a RCT | |
| Article about Chinese medical herbs | |
| The authors studied other antivirals, included other viral infections and the ages of participants were outside protocol age range | |
| Review study with different objectives | |
| Review article | |
| Trial conducted in influenza isolates | |
| Article about chronic hepatitis C | |
| Ages of participants were outside protocol age range (participants were aged between 20 to 39 years old) | |
| Study that compared patient access to pharmaceuticals in the UK and US | |
| Preliminary findings of non‐pharmaceutical intervention trial | |
| Article about an influenza vaccine | |
| Study assessing the prophylactic efficacy of an analogue of amantadine | |
| Review study | |
| Review article | |
| Review study | |
| Ages of human participants were outside protocol age range (participants were aged between 19 to 21 years old). Animals were also studied | |
| Case‐control study | |
| Ages of participants were outside protocol age range (participants were aged between 18 to 45 years old) | |
| Ages of participants were outside protocol age range (participants were aged between 18 to 50 years old) | |
| Randomisation was not stated | |
| Groups characteristics not stated. Analyses by age subgroup of interest not available | |
| Article about oseltamivir | |
| Ages of participants were outside protocol age range | |
| Review study | |
| Article about Alzheimer's | |
| Article about Glycyrrhiza species | |
| Study of the mechanism of action of T‐705 against influenza virus | |
| Non‐human trial | |
| Analyses by age subgroups of interest were not available | |
| Outcomes of interest were not studied | |
| Analyses by age subgroups of interest were not available | |
| Insufficient data available | |
| Trial about drugs that inhibit the virus's neuramidase | |
| Review study | |
| Not a RCT | |
| Pharmacological study | |
| Review article | |
| Study about the molecular basis for resistance of influenza A to amantadine | |
| Ages of participants were outside protocol age range | |
| Ages of participants were outside protocol age range | |
| Ages of participants were outside protocol age range | |
| Ages of participants were outside protocol age range | |
| Pharmacokinetics study in which ages of participants were outside protocol age range | |
| Ages of participants were outside protocol age range | |
| Analysis by age subgroups of interest was not available | |
| Analysis by age subgroups of interest was not available | |
| The drug studied was zanamivir | |
| Not a RCT | |
| Review study | |
| Ages of participants were outside protocol age range | |
| Not a RCT | |
| Study about the human immunodeficiency virus | |
| Study about the human immunodeficiency virus | |
| Systematic review | |
| Not a RCT | |
| Not a RCT | |
| Study of whether combined therapy with 2 classes of anti‐influenza drugs could affect the emergence of resistant virus variants in vitro | |
| Non‐human trial | |
| Non‐human trial | |
| Case series | |
| Review about pharmacokinetics | |
| Ages of participants were outside protocol age range | |
| Study about influenza vaccination | |
| Systematic review about antivirals for influenza in healthy adults | |
| Article about Chinese medicinal herbs | |
| Trial in which a 20‐amino‐acid peptide was used | |
| Article about neurological diseases | |
| Ages of participants were outside protocol age range (participants were aged between 17 to 53 years old) | |
| Not a RCT | |
| Amantadine and/or rimantadine were not tested in this trial | |
| Article about the effect of corticosteroids treatment | |
| Article about complementary and alternative medicine. Not a RCT | |
| Descriptive study to investigate oseltamivir resistance in children treated for influenza | |
| Duplicated results | |
| Ages of participants were outside protocol age range | |
| Ages of participants were outside protocol age range | |
| Ages of participants were outside protocol age range | |
| Ribavirin study in which ages of participants were outside protocol age range (participants were aged between 22 to 42 years old) | |
| Article about multiple sclerosis treatment | |
| Analysis by age subgroups of interest was not available | |
| Ages of participants were outside protocol age range | |
| Article about the use of antivirals for chronic hepatitis C | |
| Non‐human trial | |
| Insufficient data available | |
| Article about the use of amantadine for traumatic brain injury | |
| Outcomes of interest were not studied | |
| Study about an influenza‐like illness | |
| Study about neurologic manifestations in children with influenza B | |
| The authors measured the rates of antiviral and antibiotic prescribing for patients with influenza | |
| The study was conducted in influenza viruses isolated from poultry | |
| Analyses by age subgroups of interest were not available | |
| Article is about hyperreactivity and airway dysfunction in influenza infection and not about treatment or prevention of influenza | |
| Not a RCT | |
| Article about an intravenous neuraminidase inhibitor drug for influenza A | |
| Study of a method for detecting and quantifying influenza A virus replication | |
| Not a RCT | |
| Article was about the use of oseltamivir to control influenza complications after bone marrow transplantation | |
| Not a RCT | |
| Not a RCT | |
| The study was conducted in influenza viruses isolated from poultry | |
| Ages of participants were outside protocol age range | |
| Ages of participants were outside protocol age range | |
| Systematic review of the use of neuraminidase inhibitors | |
| Study of the synthesis and evaluation of dihydrofuran‐fused perhydrophenanthrenes as a new anti‐influenza agent | |
| Review article about treatment of viral hepatitis and oncological conditions | |
| Non‐human trial | |
| Non‐human trial | |
| Article about oseltamivir and zanamivir | |
| Not a clinical trial | |
| Insufficient data available | |
| Article about biophysical aspects of the influenza virus | |
| Ages of participants were outside were outside protocol age (participants were aged between 18 to 24 years old) | |
| Ages of participants were outside protocol age range | |
| Ages of participants were outside protocol age range | |
| A wider age range was considered. Analysis by age subgroups of interest was not available | |
| Randomisation was not stated. Additional information not available | |
| Review study | |
| Analysis by age subgroups of interest was not available | |
| Study about effectiveness of midantan and interferon inducers as means of non‐specific prevention of influenza | |
| Ages of participants were outside protocol age range (participants were aged between 20 to 28 years old) | |
| Not a RCT | |
| Article about a diagnostic test | |
| The aim of the authors was not to study amantadine and rimantadine to prevent or treat influenza | |
| Article about amantadine resistance in clinical influenza A and virus isolates | |
| Insufficient data available | |
| Article about the treatment of juvenile chronic arthritis with antivirals | |
| Ages of participants were outside protocol age range | |
| Not a RCT | |
| Ages of participants were outside protocol age range | |
| Article about neurologic effects of amantadine | |
| Ages of participants were outside protocol age range (participants were aged between 18 to 40 years old) | |
| Ages of participants were outside protocol age range (participants were aged between 18 to 55 years old) | |
| Not a RCT | |
| Not a RCT | |
| Not a RCT | |
| Not a RCT | |
| Article about the efficacy and safety of pardoprunox in patients with early Parkinson's disease | |
| Systematic review | |
| Article about oseltamivir treatment | |
| Not a RCT | |
| Analysis by age subgroups of interest was not available | |
| Review article | |
| Ages of participants were outside protocol age range (participants were aged between 18 to 40 years old) | |
| The purpose of this article was to study the acute effects of amantadine infusions on event‐related potentials | |
| Non‐human trial | |
| Review article | |
| Case‐control study | |
| The trial authors studied different populations. No information was available about clinical outcomes and confirmation of influenza diagnosis | |
| Non‐human article | |
| Review article | |
| Ages of participants were outside protocol age range (participants were aged between 18 to 50 years old) | |
| Ages of participants were outside protocol age range | |
| Ages of participants were outside protocol age range | |
| Ages of participants were outside protocol age range (participants were aged between 18 to 30 years old) | |
| Randomisation was not stated. The groups were not similar at baseline | |
| Study of aetiology and treatment in hospitalised children with pneumonia | |
| Not a study about influenza A | |
| Systematic review | |
| Article about the inhibition of influenza‐virus‐induced cytopathy by sialyglycoconjugates | |
| Article about multiple sclerosis | |
| Insufficient data presented | |
| Ages of participants were outside protocol age range | |
| Ages of participants were outside protocol age range | |
| The drug studied was cyclooctylamine | |
| Not a RCT | |
| Not a RCT | |
| Ages of participants were outside protocol age range | |
| Study about 4 antibody techniques to assess influenza infection | |
| Article about the use of neuraminidase inhibitors in adults | |
| Non‐human trial | |
| Not a RCT | |
| Ages of participants were outside protocol age range (participants were aged between 17 to 54 years old) | |
| Not a RCT | |
| Ages of participants were outside protocol age range | |
| Not a RCT | |
| Analysis by age subgroups of interest was not available | |
| Not a clinical trial | |
| The antiviral studied was oseltamivir | |
| Ages of participants were outside protocol age range (participants were aged between 17 to 20 years old) | |
| Not a RCT | |
| Review article | |
| The purpose of the authors was to study treatment for chronic hepatitis C |
PB: placebo
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fever day 3 Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.1 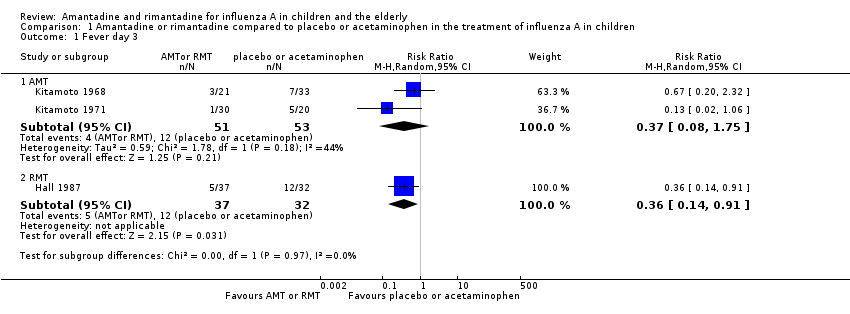 Comparison 1 Amantadine or rimantadine compared to placebo or acetaminophen in the treatment of influenza A in children, Outcome 1 Fever day 3. | ||||
| 1.1 AMT | 2 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.08, 1.75] |
| 1.2 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.14, 0.91] |
| 2 Malaise day 6 Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.2 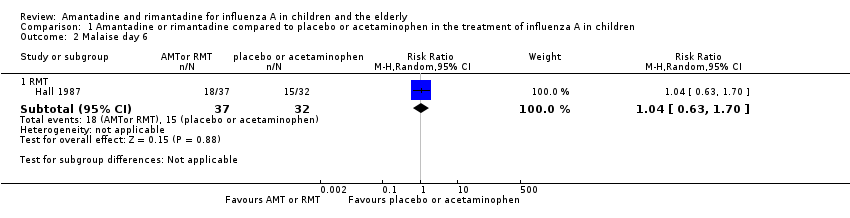 Comparison 1 Amantadine or rimantadine compared to placebo or acetaminophen in the treatment of influenza A in children, Outcome 2 Malaise day 6. | ||||
| 2.1 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.63, 1.70] |
| 3 Cough day 7 Show forest plot | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.63, 1.10] |
| Analysis 1.3  Comparison 1 Amantadine or rimantadine compared to placebo or acetaminophen in the treatment of influenza A in children, Outcome 3 Cough day 7. | ||||
| 3.1 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.63, 1.10] |
| 4 Conjunctivitis day 5 Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.4 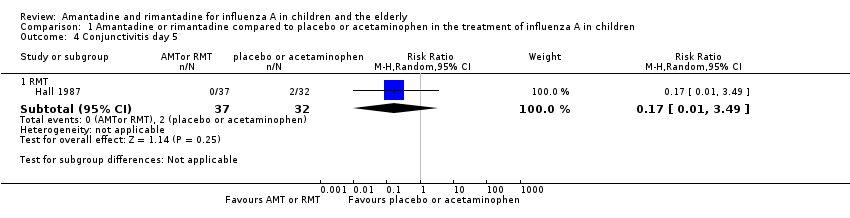 Comparison 1 Amantadine or rimantadine compared to placebo or acetaminophen in the treatment of influenza A in children, Outcome 4 Conjunctivitis day 5. | ||||
| 4.1 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.01, 3.49] |
| 5 Eye symptoms day 5 (pain on movement and visual distortion) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Amantadine or rimantadine compared to placebo or acetaminophen in the treatment of influenza A in children, Outcome 5 Eye symptoms day 5 (pain on movement and visual distortion). | ||||
| 5.1 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.10, 3.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Infection Show forest plot | 5 | 951 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.09, 0.66] |
| Analysis 2.1  Comparison 2 Amantadine and rimantadine compared to placebo and to specific treatment in the prophylaxis of influenza A in children, Outcome 1 Infection. | ||||
| 1.1 AMT | 2 | 773 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.04, 0.30] |
| 1.2 RMT | 3 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.21, 1.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 RMT (proved and clinical infection) Show forest plot | 3 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.13, 4.07] |
| Analysis 3.1  Comparison 3 Amantadine and rimantadine compared to placebo in the prophylaxis of influenza A in the elderly, Outcome 1 RMT (proved and clinical infection). | ||||
| 2 RMT Monto (100 + 200) and Patriarca Show forest plot | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.14, 1.41] |
| Analysis 3.2  Comparison 3 Amantadine and rimantadine compared to placebo in the prophylaxis of influenza A in the elderly, Outcome 2 RMT Monto (100 + 200) and Patriarca. | ||||
| 3 RMT 200 Show forest plot | 2 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.12, 1.63] |
| Analysis 3.3  Comparison 3 Amantadine and rimantadine compared to placebo in the prophylaxis of influenza A in the elderly, Outcome 3 RMT 200. | ||||
| 4 RMT 100 Show forest plot | 2 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.10, 21.10] |
| Analysis 3.4  Comparison 3 Amantadine and rimantadine compared to placebo in the prophylaxis of influenza A in the elderly, Outcome 4 RMT 100. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical and laboratory infection Show forest plot | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.21, 4.20] |
| Analysis 4.1  Comparison 4 Use of different doses of rimantadine for prophylaxis and treatment of influenza A in the elderly, Outcome 1 Clinical and laboratory infection. | ||||
| 1.1 RMT | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.21, 4.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 RMT and zanamivir Show forest plot | 2 | 545 | Risk Ratio (M‐H, Random, 95% CI) | 4.63 [1.46, 14.72] |
| Analysis 5.1 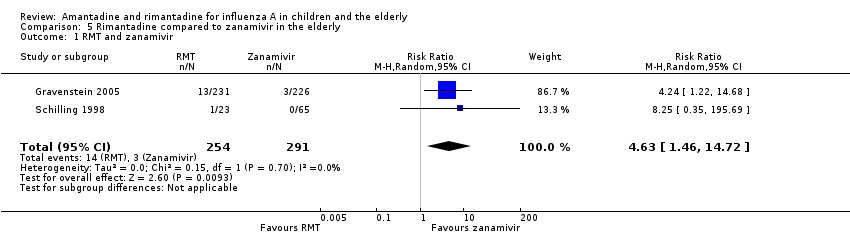 Comparison 5 Rimantadine compared to zanamivir in the elderly, Outcome 1 RMT and zanamivir. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Diarrhoea Show forest plot | 3 | 655 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.42, 1.47] |
| Analysis 6.1  Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 1 Diarrhoea. | ||||
| 1.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.43, 1.53] |
| 1.2 RMT | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.02, 8.41] |
| 2 Exanthema Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.21, 2.34] |
| Analysis 6.2 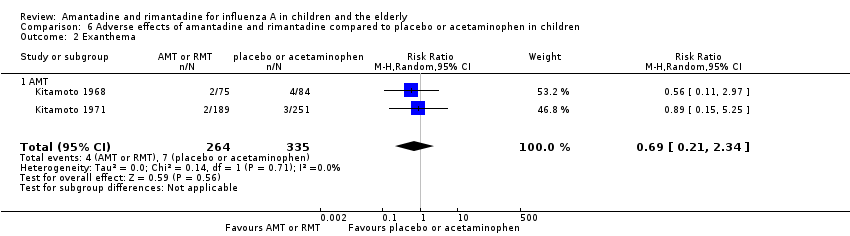 Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 2 Exanthema. | ||||
| 2.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.21, 2.34] |
| 3 Muscular, limb pain Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.46, 1.59] |
| Analysis 6.3 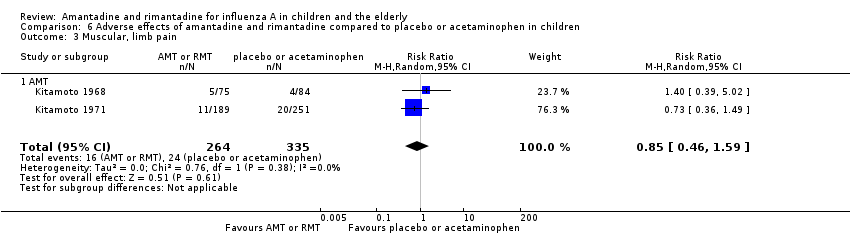 Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 3 Muscular, limb pain. | ||||
| 3.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.46, 1.59] |
| 4 Headache Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.52, 1.03] |
| Analysis 6.4 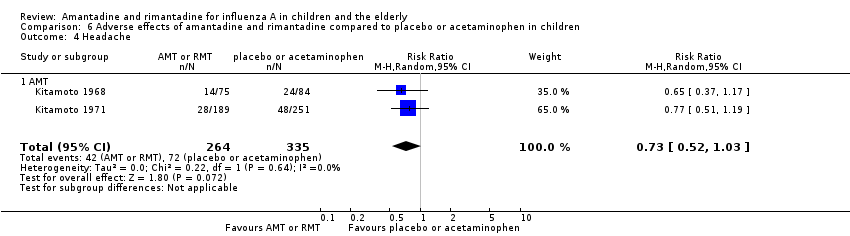 Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 4 Headache. | ||||
| 4.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.52, 1.03] |
| 5 Stimulation/insomnia Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.12, 1.74] |
| Analysis 6.5  Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 5 Stimulation/insomnia. | ||||
| 5.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.12, 1.74] |
| 6 Dizziness Show forest plot | 3 | 655 | Risk Ratio (M‐H, Random, 95% CI) | 4.69 [0.53, 41.75] |
| Analysis 6.6  Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 6 Dizziness. | ||||
| 6.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 6.63 [0.32, 137.33] |
| 6.2 RMT | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 3.21 [0.14, 75.68] |
| 7 Dyspnoea Show forest plot | 1 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 9.02] |
| Analysis 6.7 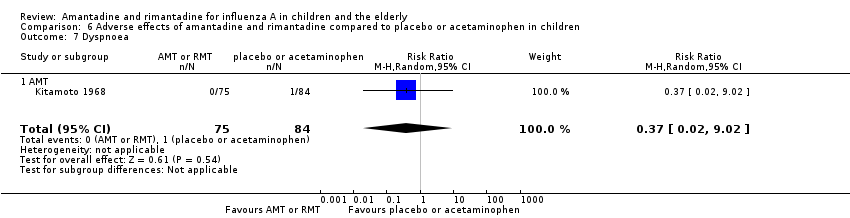 Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 7 Dyspnoea. | ||||
| 7.1 AMT | 1 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 9.02] |
| 8 Central nervous system symptoms Show forest plot | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 4.70] |
| Analysis 6.8 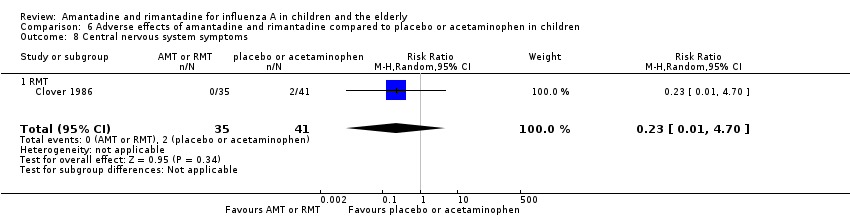 Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 8 Central nervous system symptoms. | ||||
| 8.1 RMT | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 4.70] |
| 9 Change in behaviour Show forest plot | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 4.70] |
| Analysis 6.9  Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 9 Change in behaviour. | ||||
| 9.1 RMT | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 4.70] |
| 10 Gastrointestinal symptoms Show forest plot | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.08, 18.05] |
| Analysis 6.10  Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 10 Gastrointestinal symptoms. | ||||
| 10.1 RMT | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.08, 18.05] |
| 11 Hyperreactivity Show forest plot | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.02, 8.41] |
| Analysis 6.11 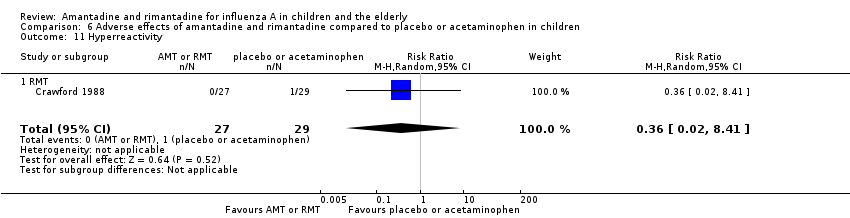 Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 11 Hyperreactivity. | ||||
| 11.1 RMT | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.02, 8.41] |
| 12 Tinnitus Show forest plot | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 3.21 [0.14, 75.68] |
| Analysis 6.12  Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 12 Tinnitus. | ||||
| 12.1 RMT | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 3.21 [0.14, 75.68] |
| 13 Cerebellar ataxia Show forest plot | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 2.61 [0.11, 61.80] |
| Analysis 6.13 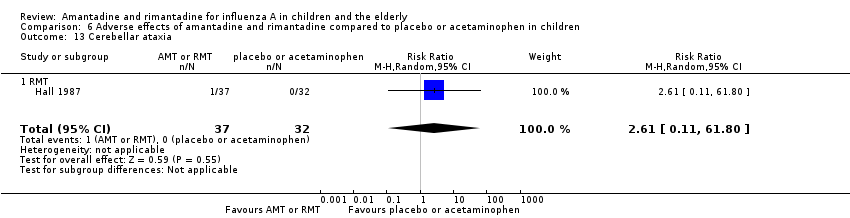 Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 13 Cerebellar ataxia. | ||||
| 13.1 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 2.61 [0.11, 61.80] |
| 14 Malaise Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.41, 1.96] |
| Analysis 6.14  Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 14 Malaise. | ||||
| 14.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.41, 1.96] |
| 15 Nausea/vomiting Show forest plot | 4 | 724 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.24, 1.58] |
| Analysis 6.15 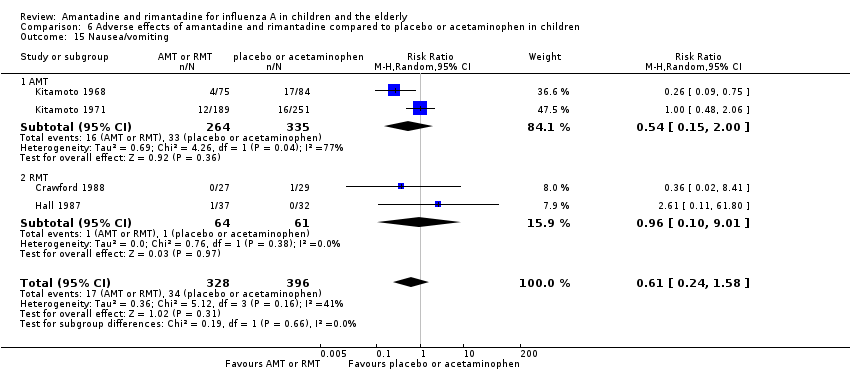 Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 15 Nausea/vomiting. | ||||
| 15.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.15, 2.00] |
| 15.2 RMT | 2 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.10, 9.01] |
| 16 Arrhythmia Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 6.16  Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 16 Arrhythmia. | ||||
| 16.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stimulation/insomnia Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.43, 6.02] |
| Analysis 7.1  Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 1 Stimulation/insomnia. | ||||
| 1.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.43, 6.02] |
| 2 Confusion Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.40, 1.56] |
| Analysis 7.2  Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 2 Confusion. | ||||
| 2.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.40, 1.56] |
| 3 Fatigue Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.41, 1.60] |
| Analysis 7.3  Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 3 Fatigue. | ||||
| 3.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.41, 1.60] |
| 4 Vomiting Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.38, 2.60] |
| Analysis 7.4 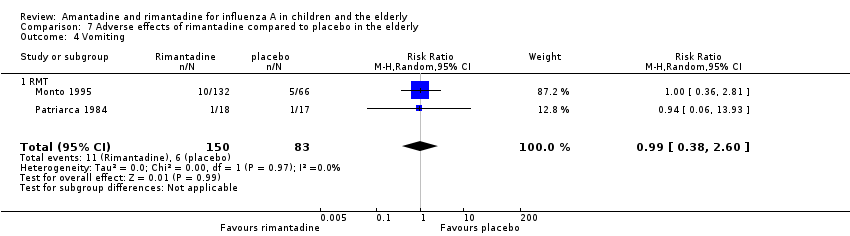 Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 4 Vomiting. | ||||
| 4.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.38, 2.60] |
| 5 Headache Show forest plot | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.21, 3.38] |
| Analysis 7.5  Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 5 Headache. | ||||
| 5.1 RMT | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.21, 3.38] |
| 6 Impaired concentration Show forest plot | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.10, 2.41] |
| Analysis 7.6  Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 6 Impaired concentration. | ||||
| 6.1 RMT | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.10, 2.41] |
| 7 Rash or allergic reaction Show forest plot | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 3.53 [0.18, 67.28] |
| Analysis 7.7 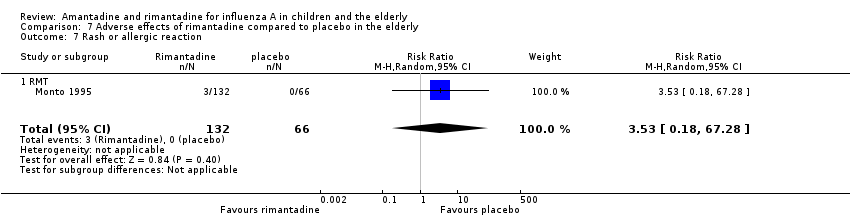 Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 7 Rash or allergic reaction. | ||||
| 7.1 RMT | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 3.53 [0.18, 67.28] |
| 8 Seizures or clonic twitching Show forest plot | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.23, 17.54] |
| Analysis 7.8 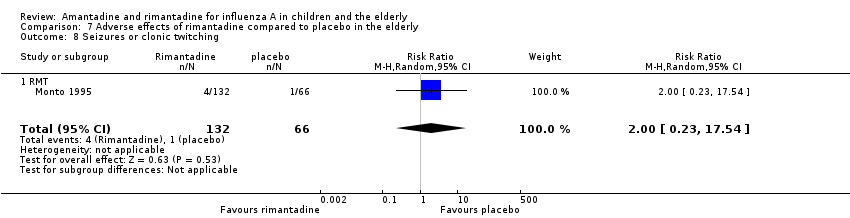 Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 8 Seizures or clonic twitching. | ||||
| 8.1 RMT | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.23, 17.54] |
| 9 Dry mouth Show forest plot | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.7 [0.23, 2.12] |
| Analysis 7.9  Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 9 Dry mouth. | ||||
| 9.1 RMT | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.7 [0.23, 2.12] |
| 10 Dizziness Show forest plot | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.15, 5.97] |
| Analysis 7.10  Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 10 Dizziness. | ||||
| 10.1 RMT | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.15, 5.97] |
| 11 Anxiety Show forest plot | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 2.83 [0.92, 8.74] |
| Analysis 7.11 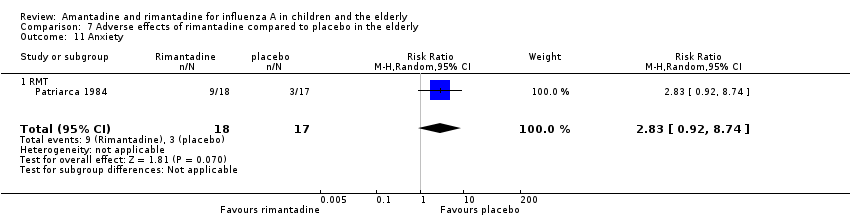 Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 11 Anxiety. | ||||
| 11.1 RMT | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 2.83 [0.92, 8.74] |
| 12 Nausea Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.45, 8.75] |
| Analysis 7.12  Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 12 Nausea. | ||||
| 12.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.45, 8.75] |
| 13 Depression Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.53, 4.98] |
| Analysis 7.13 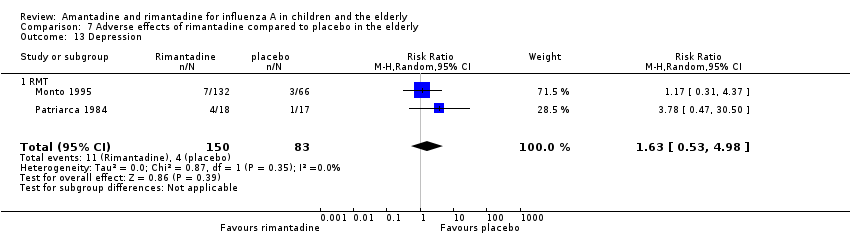 Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 13 Depression. | ||||
| 13.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.53, 4.98] |
| 14 Loss of appetite Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.56, 2.17] |
| Analysis 7.14 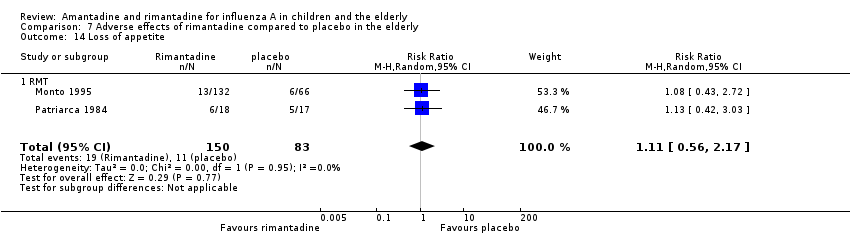 Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 14 Loss of appetite. | ||||
| 14.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.56, 2.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Confusion Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.41, 1.65] |
| Analysis 8.1  Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 1 Confusion. | ||||
| 1.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.41, 1.65] |
| 2 Depression Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.12, 1.65] |
| Analysis 8.2 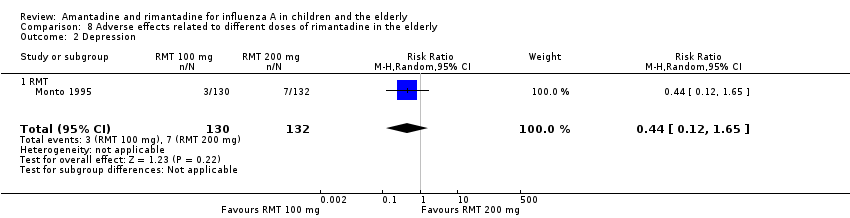 Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 2 Depression. | ||||
| 2.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.12, 1.65] |
| 3 Impaired concentration Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.11, 3.98] |
| Analysis 8.3 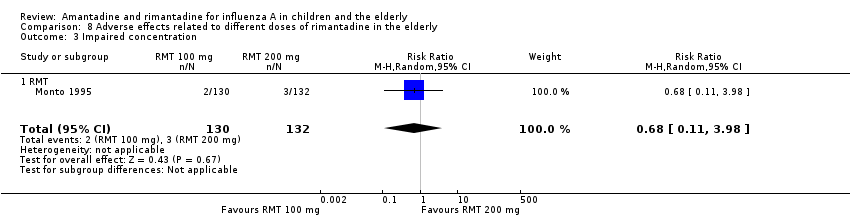 Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 3 Impaired concentration. | ||||
| 3.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.11, 3.98] |
| 4 Insomnia or sleeplessness Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.97] |
| Analysis 8.4 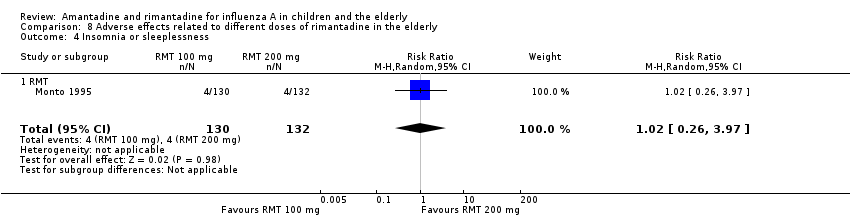 Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 4 Insomnia or sleeplessness. | ||||
| 4.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.97] |
| 5 Loss of appetite Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.27, 1.46] |
| Analysis 8.5  Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 5 Loss of appetite. | ||||
| 5.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.27, 1.46] |
| 6 Rash or allergic reaction Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.04, 3.21] |
| Analysis 8.6 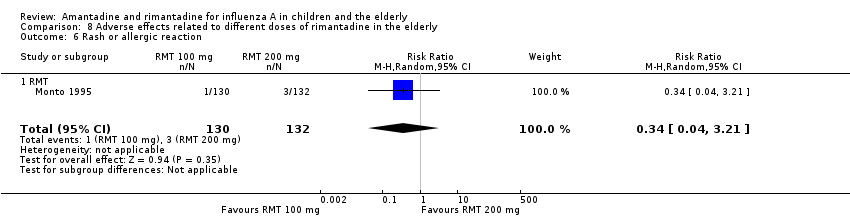 Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 6 Rash or allergic reaction. | ||||
| 6.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.04, 3.21] |
| 7 Seizure or clonic twitching Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.07] |
| Analysis 8.7  Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 7 Seizure or clonic twitching. | ||||
| 7.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.07] |
| 8 Dry mouth Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.43, 3.11] |
| Analysis 8.8 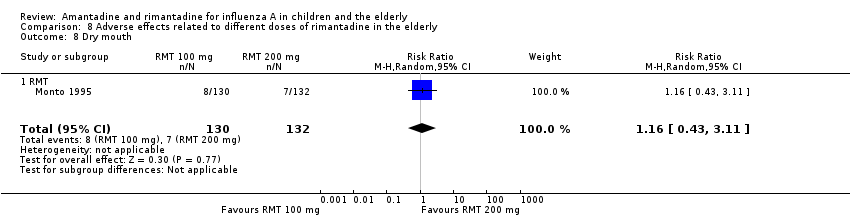 Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 8 Dry mouth. | ||||
| 8.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.43, 3.11] |
| 9 Fatigue and drowsiness Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.45, 2.87] |
| Analysis 8.9 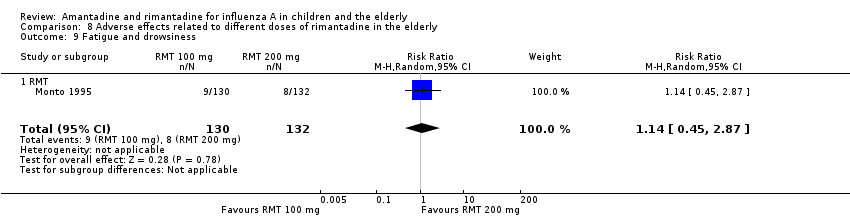 Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 9 Fatigue and drowsiness. | ||||
| 9.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.45, 2.87] |
| 10 Headache Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.30, 3.42] |
| Analysis 8.10 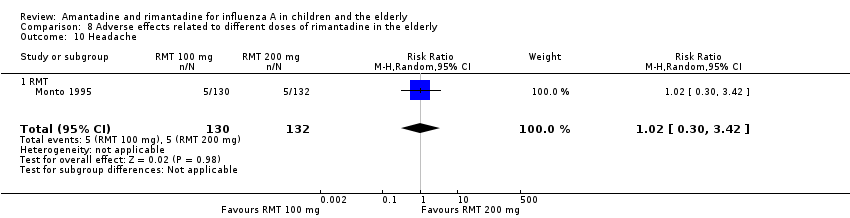 Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 10 Headache. | ||||
| 10.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.30, 3.42] |
| 11 Body weakness or debility Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.38, 2.18] |
| Analysis 8.11 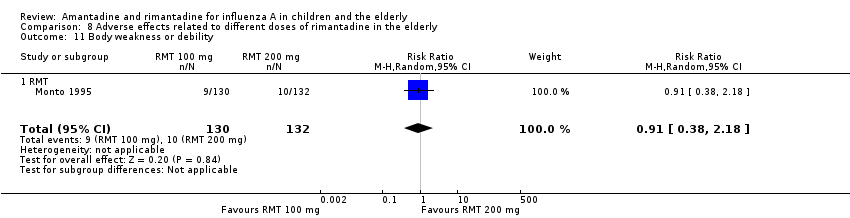 Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 11 Body weakness or debility. | ||||
| 11.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.38, 2.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Infection Show forest plot | 5 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.27, 0.92] |
| Analysis 9.1 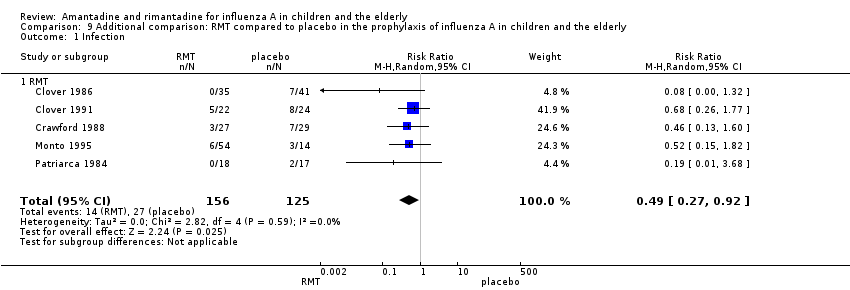 Comparison 9 Additional comparison: RMT compared to placebo in the prophylaxis of influenza A in children and the elderly, Outcome 1 Infection. | ||||
| 1.1 RMT | 5 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.27, 0.92] |
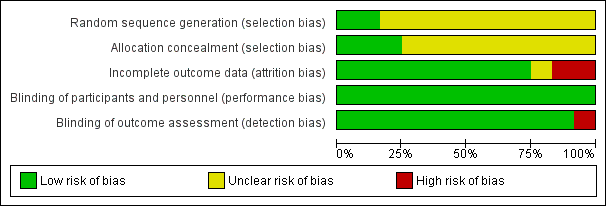
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Amantadine or rimantadine compared to placebo or acetaminophen in the treatment of influenza A in children, Outcome 1 Fever day 3.

Comparison 1 Amantadine or rimantadine compared to placebo or acetaminophen in the treatment of influenza A in children, Outcome 2 Malaise day 6.

Comparison 1 Amantadine or rimantadine compared to placebo or acetaminophen in the treatment of influenza A in children, Outcome 3 Cough day 7.

Comparison 1 Amantadine or rimantadine compared to placebo or acetaminophen in the treatment of influenza A in children, Outcome 4 Conjunctivitis day 5.

Comparison 1 Amantadine or rimantadine compared to placebo or acetaminophen in the treatment of influenza A in children, Outcome 5 Eye symptoms day 5 (pain on movement and visual distortion).
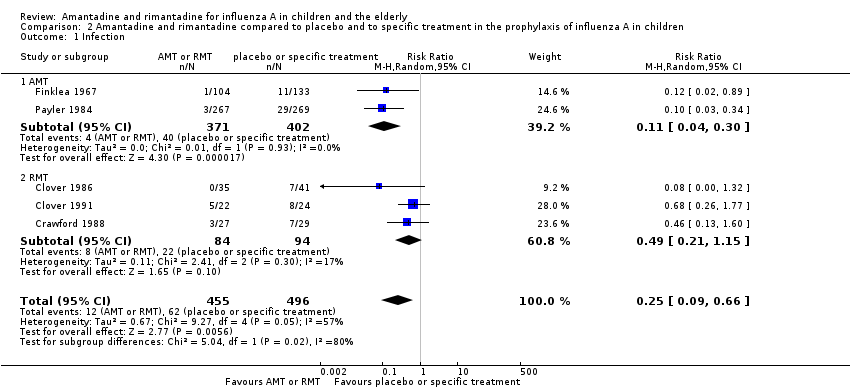
Comparison 2 Amantadine and rimantadine compared to placebo and to specific treatment in the prophylaxis of influenza A in children, Outcome 1 Infection.

Comparison 3 Amantadine and rimantadine compared to placebo in the prophylaxis of influenza A in the elderly, Outcome 1 RMT (proved and clinical infection).

Comparison 3 Amantadine and rimantadine compared to placebo in the prophylaxis of influenza A in the elderly, Outcome 2 RMT Monto (100 + 200) and Patriarca.
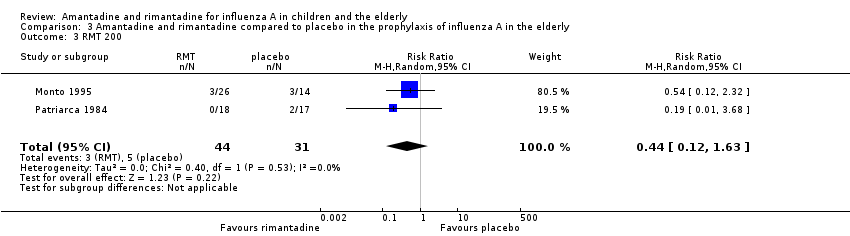
Comparison 3 Amantadine and rimantadine compared to placebo in the prophylaxis of influenza A in the elderly, Outcome 3 RMT 200.

Comparison 3 Amantadine and rimantadine compared to placebo in the prophylaxis of influenza A in the elderly, Outcome 4 RMT 100.

Comparison 4 Use of different doses of rimantadine for prophylaxis and treatment of influenza A in the elderly, Outcome 1 Clinical and laboratory infection.
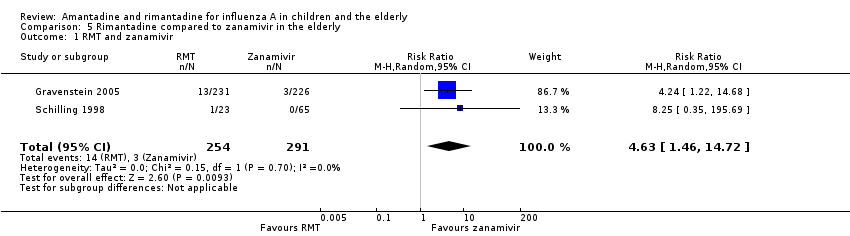
Comparison 5 Rimantadine compared to zanamivir in the elderly, Outcome 1 RMT and zanamivir.

Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 1 Diarrhoea.
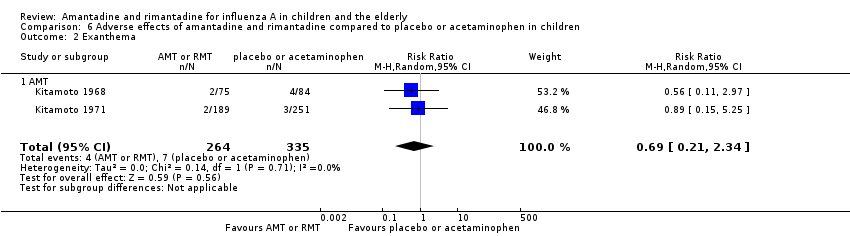
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 2 Exanthema.

Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 3 Muscular, limb pain.

Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 4 Headache.
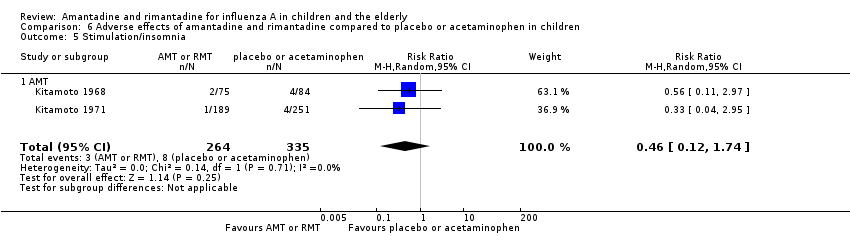
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 5 Stimulation/insomnia.
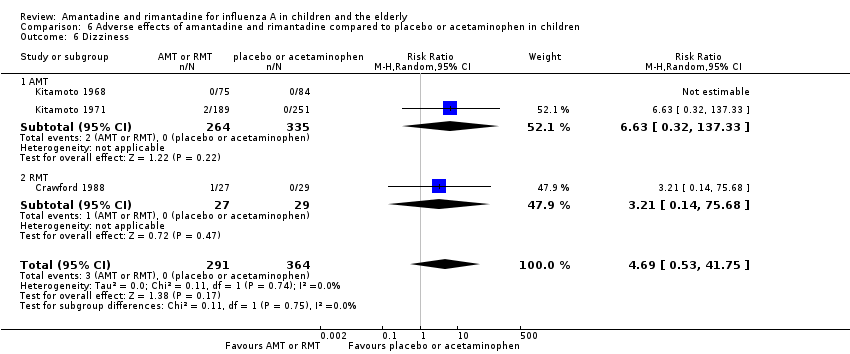
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 6 Dizziness.
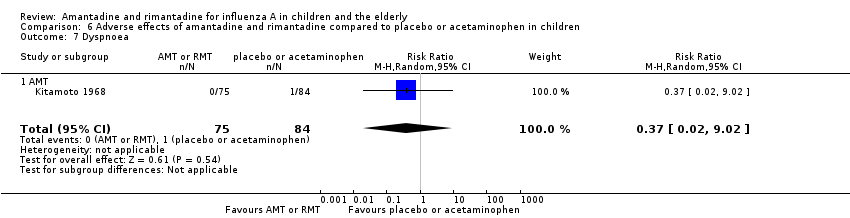
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 7 Dyspnoea.

Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 8 Central nervous system symptoms.
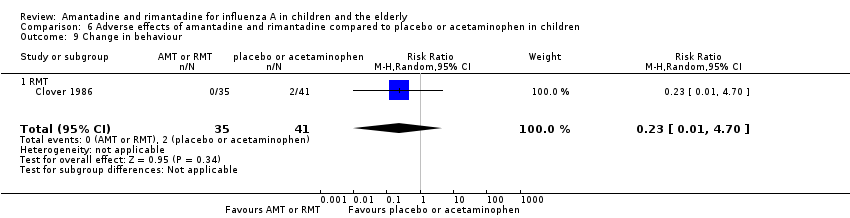
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 9 Change in behaviour.

Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 10 Gastrointestinal symptoms.

Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 11 Hyperreactivity.

Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 12 Tinnitus.
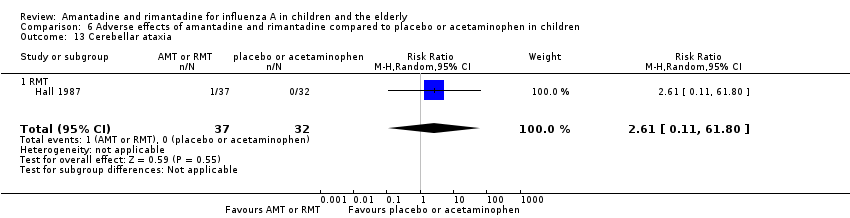
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 13 Cerebellar ataxia.

Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 14 Malaise.
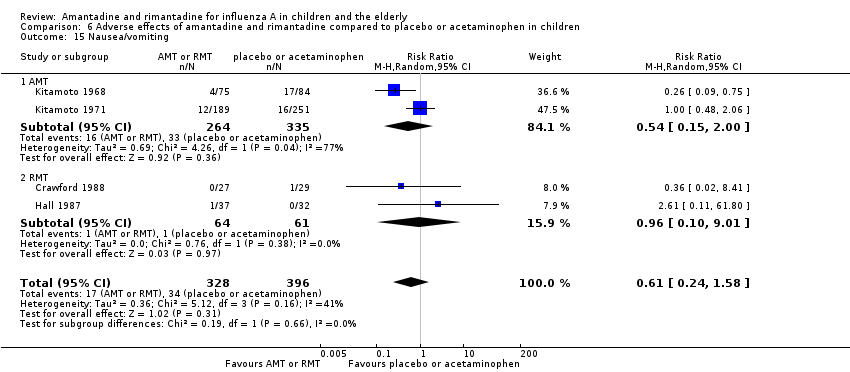
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 15 Nausea/vomiting.

Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 16 Arrhythmia.
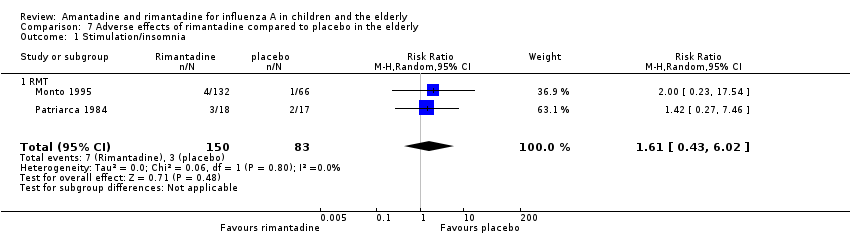
Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 1 Stimulation/insomnia.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 2 Confusion.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 3 Fatigue.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 4 Vomiting.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 5 Headache.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 6 Impaired concentration.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 7 Rash or allergic reaction.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 8 Seizures or clonic twitching.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 9 Dry mouth.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 10 Dizziness.
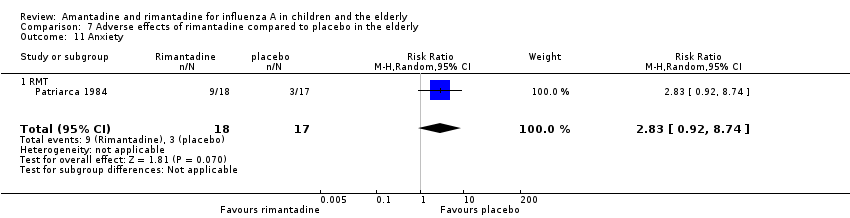
Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 11 Anxiety.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 12 Nausea.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 13 Depression.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 14 Loss of appetite.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 1 Confusion.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 2 Depression.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 3 Impaired concentration.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 4 Insomnia or sleeplessness.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 5 Loss of appetite.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 6 Rash or allergic reaction.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 7 Seizure or clonic twitching.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 8 Dry mouth.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 9 Fatigue and drowsiness.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 10 Headache.
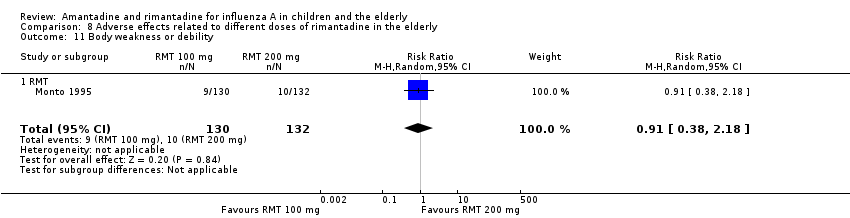
Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 11 Body weakness or debility.
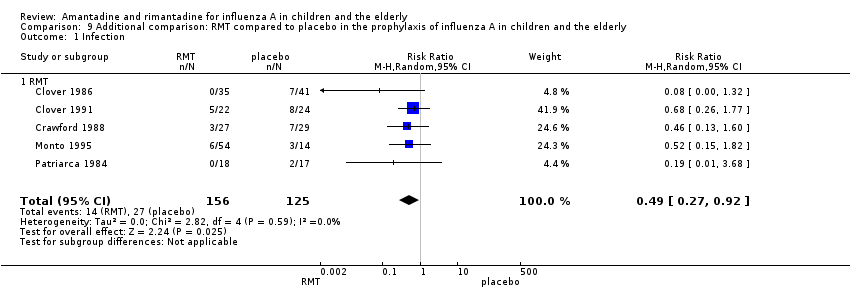
Comparison 9 Additional comparison: RMT compared to placebo in the prophylaxis of influenza A in children and the elderly, Outcome 1 Infection.
| Amantadine compared with placebo for prevention and treatment of influenza A in children | ||||||
| Patient or population: children with no influenza A infection (prevention) or with influenza A infection (treatment) Settings: all Intervention: amantadine Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Amantadine | |||||
| Cases of influenza A during prophylaxis (follow‐up:14 to 18 weeks) | Medium risk population | RR 0.11 (0.04 to 0.3) | 773 | ⊕⊕⊝⊝ | ||
| 10 per 100 | 1 per 100 | |||||
| Fever after initiation of treatment (follow‐up: 3 days) | Medium risk population | RR 0.37 (0.08 to 1.75) | 104 | ⊕⊕⊝⊝ | ||
| 23 per 100 | 9 per 100 | |||||
| Cough after initiation of treatment | See comment | See comment | Not estimable | 0 (0) | See comment | No selected trial |
| Dizziness (follow‐up: 7 days) | Medium risk population | RR 6.63 (0.32 to 137.33) | 599 | ⊕⊝⊝⊝ | ||
| 0 per 100 | 0 per 100 | |||||
| Nausea/vomiting (follow‐up: 7 days) | Medium risk population | RR 0.54 (0.15 to 2) | 599 | ⊕⊝⊝⊝ | ||
| 13 per 100 | 7 per 100 | |||||
| Stimulation/insomnia (follow‐up: 7 days) | Medium risk population | RR 0.46 (0.12 to 1.74) | 599 | ⊕⊕⊝⊝ | ||
| 3 per 100 | 7 per 100 | |||||
| CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| *The basis for the assumed risk (e.g. median control group risk across studies) was calculated on the basis of control event rate. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 1Allocation concealment not used or unclear. 2Sparse data. 3Allocation concealment unclear. 4Sparse data, confidence intervals do not rule out potential for null effect or harm. 5High heterogeneity unexplained. | ||||||
| Rimantadine compared with placebo for prevention and treatment of influenza A in children | ||||||
| Patient or population: children with no influenza A infection (prevention) or with influenza A infection (treatment) Settings: any Intervention: rimantadine Comparison: control (placebo or acetaminophen) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Rimantadine | |||||
| Cases of influenza A during prophylaxis (follow‐up: 1 to 35 days) | Medium risk population | RR 0.49 (0.21 to 1.15) | 178 | ⊕⊕⊝⊝ | ||
| 24 per 100 | 12 per 100 | |||||
| Fever after initiation of treatment (follow‐up: 3 days) | Medium risk population | RR 0.36 (0.14 to 0.91) | 69 | ⊕⊕⊕⊝ | ||
| 38 per 100 | 14 per 100 | |||||
| Cough after initiation of treatment (follow‐up: 7 days) | Medium risk population | RR 0.83 (0.63 to 1.1) | 69 | ⊕⊕⊕⊝ | ||
| 81 per 100 | 67 per 100 | |||||
| Dizziness (follow‐up: 35 days) | Medium risk population | RR 3.21 (0.14 to 75.68) | 56 | ⊕⊝⊝⊝ | ||
| 0 per 100 | 0 per 100 | |||||
| Nausea/vomiting (follow‐up: 7 to 35 days) | Medium risk population | RR 0.96 (0.1 to 9.01) | 125 | ⊕⊕⊝⊝ | ||
| 2 per 100 | 2 per 100 | |||||
| Stimulation/insomnia | See comment | See comment | Not estimable | 0 | See comment | No selected trial |
| CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| *The basis for the assumed risk (e.g. median control group risk across studies) was calculated on the basis of control event rate. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 1Allocation concealment unclear. 2Sparse data and confidence intervals do not rule out the potential for no effect or harm | ||||||
| Amantadine compared with placebo for prevention and treatment of influenza A in the elderly | ||||||
| Patient or population: elderly people with no influenza A infection (prevention) or with influenza A infection (treatment) Settings: any Intervention: amantadine Comparison: control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Amantadine | |||||
| Cases of influenza A during prophylaxis | See comment | Not estimable | 0 | See comment | No selected trial | |
| Fever after initiation of treatment | See comment | Not estimable | 0 | See comment | No selected trial | |
| Cough after initiation of treatment | See comment | Not estimable | 0 | See comment | No selected trial | |
| Dizziness | See comment | Not estimable | 0 | See comment | No selected trial | |
| Nausea | See comment | Not estimable | 0 | See comment | No selected trial | |
| Vomiting | See comment | Not estimable | 0 | See comment | No selected trial | |
| Stimulation/insomnia | See comment | Not estimable | 0 | See comment | No selected trial | |
| Patient or population: elderly people with no influenza A infection (prevention) or with influenza A infection (treatment) Settings: any Intervention: rimantadine Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Rimantadine | |||||
| Cases of influenza A during prophylaxis | Medium risk population | RR 0.45 (0.14 to 1.41) | 103 | ⊕⊝⊝⊝ | ||
| 17per 100 | 7 per 100 | |||||
| Fever after initiation of treatment | See comment | 0 | See comment | See comment | No selected trial | |
| Cough after initiation of treatment | See comment | 0 | See comment | See comment | No selected trial | |
| Dizziness (follow‐up: 12 weeks) | Medium risk population | |||||
| 12 per 100 | 11 per 100 (2 to 70) | RR 0.94 | 35 (1) | ⊕⊕⊝⊝ | ||
| Nausea (follow‐up: 8 to 12 weeks) | Medium risk population | RR 1.99 (0.45 to 8.75) | 233 | ⊕⊝⊝⊝ | ||
| 8 per 100 | 15 per 100 | |||||
| Vomiting (follow‐up: 8 to 12 weeks) | Medium risk population | RR 0.99 (0.38 to 2.6) | 233 | ⊕⊕⊝⊝ | ||
| 7 per 100 | 7 per 100 | |||||
| Stimulation/insomnia (follow‐up: 8 to 12 weeks) | Medium risk population | RR 1.61 (0.43 to 6.02) | 233 | ⊕⊕⊝⊝ | ||
| 7 per 100 | 11 per 100 | |||||
| CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| *The basis for the assumed risk (e.g. median control group risk across studies) was calculated on the basis of control event rate. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 1Allocation concealment unclear and 1 study had high withdrawal rate. 2Sparse data and confidence interval do not rule out no effect or harm. 3Allocation concealment unclear 4High heterogeneity unexplained. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fever day 3 Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 AMT | 2 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.08, 1.75] |
| 1.2 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.14, 0.91] |
| 2 Malaise day 6 Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.63, 1.70] |
| 3 Cough day 7 Show forest plot | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.63, 1.10] |
| 3.1 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.63, 1.10] |
| 4 Conjunctivitis day 5 Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.01, 3.49] |
| 5 Eye symptoms day 5 (pain on movement and visual distortion) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.10, 3.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Infection Show forest plot | 5 | 951 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.09, 0.66] |
| 1.1 AMT | 2 | 773 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.04, 0.30] |
| 1.2 RMT | 3 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.21, 1.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 RMT (proved and clinical infection) Show forest plot | 3 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.13, 4.07] |
| 2 RMT Monto (100 + 200) and Patriarca Show forest plot | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.14, 1.41] |
| 3 RMT 200 Show forest plot | 2 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.12, 1.63] |
| 4 RMT 100 Show forest plot | 2 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.10, 21.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical and laboratory infection Show forest plot | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.21, 4.20] |
| 1.1 RMT | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.21, 4.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 RMT and zanamivir Show forest plot | 2 | 545 | Risk Ratio (M‐H, Random, 95% CI) | 4.63 [1.46, 14.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Diarrhoea Show forest plot | 3 | 655 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.42, 1.47] |
| 1.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.43, 1.53] |
| 1.2 RMT | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.02, 8.41] |
| 2 Exanthema Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.21, 2.34] |
| 2.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.21, 2.34] |
| 3 Muscular, limb pain Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.46, 1.59] |
| 3.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.46, 1.59] |
| 4 Headache Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.52, 1.03] |
| 4.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.52, 1.03] |
| 5 Stimulation/insomnia Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.12, 1.74] |
| 5.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.12, 1.74] |
| 6 Dizziness Show forest plot | 3 | 655 | Risk Ratio (M‐H, Random, 95% CI) | 4.69 [0.53, 41.75] |
| 6.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 6.63 [0.32, 137.33] |
| 6.2 RMT | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 3.21 [0.14, 75.68] |
| 7 Dyspnoea Show forest plot | 1 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 9.02] |
| 7.1 AMT | 1 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 9.02] |
| 8 Central nervous system symptoms Show forest plot | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 4.70] |
| 8.1 RMT | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 4.70] |
| 9 Change in behaviour Show forest plot | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 4.70] |
| 9.1 RMT | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 4.70] |
| 10 Gastrointestinal symptoms Show forest plot | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.08, 18.05] |
| 10.1 RMT | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.08, 18.05] |
| 11 Hyperreactivity Show forest plot | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.02, 8.41] |
| 11.1 RMT | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.02, 8.41] |
| 12 Tinnitus Show forest plot | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 3.21 [0.14, 75.68] |
| 12.1 RMT | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 3.21 [0.14, 75.68] |
| 13 Cerebellar ataxia Show forest plot | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 2.61 [0.11, 61.80] |
| 13.1 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 2.61 [0.11, 61.80] |
| 14 Malaise Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.41, 1.96] |
| 14.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.41, 1.96] |
| 15 Nausea/vomiting Show forest plot | 4 | 724 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.24, 1.58] |
| 15.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.15, 2.00] |
| 15.2 RMT | 2 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.10, 9.01] |
| 16 Arrhythmia Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stimulation/insomnia Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.43, 6.02] |
| 1.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.43, 6.02] |
| 2 Confusion Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.40, 1.56] |
| 2.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.40, 1.56] |
| 3 Fatigue Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.41, 1.60] |
| 3.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.41, 1.60] |
| 4 Vomiting Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.38, 2.60] |
| 4.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.38, 2.60] |
| 5 Headache Show forest plot | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.21, 3.38] |
| 5.1 RMT | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.21, 3.38] |
| 6 Impaired concentration Show forest plot | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.10, 2.41] |
| 6.1 RMT | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.10, 2.41] |
| 7 Rash or allergic reaction Show forest plot | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 3.53 [0.18, 67.28] |
| 7.1 RMT | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 3.53 [0.18, 67.28] |
| 8 Seizures or clonic twitching Show forest plot | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.23, 17.54] |
| 8.1 RMT | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.23, 17.54] |
| 9 Dry mouth Show forest plot | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.7 [0.23, 2.12] |
| 9.1 RMT | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.7 [0.23, 2.12] |
| 10 Dizziness Show forest plot | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.15, 5.97] |
| 10.1 RMT | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.15, 5.97] |
| 11 Anxiety Show forest plot | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 2.83 [0.92, 8.74] |
| 11.1 RMT | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 2.83 [0.92, 8.74] |
| 12 Nausea Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.45, 8.75] |
| 12.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.45, 8.75] |
| 13 Depression Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.53, 4.98] |
| 13.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.53, 4.98] |
| 14 Loss of appetite Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.56, 2.17] |
| 14.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.56, 2.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Confusion Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.41, 1.65] |
| 1.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.41, 1.65] |
| 2 Depression Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.12, 1.65] |
| 2.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.12, 1.65] |
| 3 Impaired concentration Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.11, 3.98] |
| 3.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.11, 3.98] |
| 4 Insomnia or sleeplessness Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.97] |
| 4.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.97] |
| 5 Loss of appetite Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.27, 1.46] |
| 5.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.27, 1.46] |
| 6 Rash or allergic reaction Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.04, 3.21] |
| 6.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.04, 3.21] |
| 7 Seizure or clonic twitching Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.07] |
| 7.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.07] |
| 8 Dry mouth Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.43, 3.11] |
| 8.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.43, 3.11] |
| 9 Fatigue and drowsiness Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.45, 2.87] |
| 9.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.45, 2.87] |
| 10 Headache Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.30, 3.42] |
| 10.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.30, 3.42] |
| 11 Body weakness or debility Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.38, 2.18] |
| 11.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.38, 2.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Infection Show forest plot | 5 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.27, 0.92] |
| 1.1 RMT | 5 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.27, 0.92] |

