آمانتادین و ریمانتادین در درمان آنفلوآنزا A در کودکان و سالمندان
چکیده
پیشینه
آنفلوآنزا یک بیماری حاد تنفسی است که توسط ویروسهای آنفلوآنزا A و B ایجاد میشود. عوارض آن ممکن است به خصوص میان کودکان و افراد مسن رخ دهند.
اهداف
ارزیابی اثربخشی و بیخطری (safety) مصرف آمانتادین (amantadine) و ریمانتادین (rimantadine) در پیشگیری، درمان، و کوتاه کردن طول دوره ابتلا به آنفلوآنزا A در کودکان و سالمندان.
روشهای جستوجو
ما CENTRAL (2014، شماره 9)، MEDLINE (1966 تا هفته 4 سپتامبر 2014) و EMBASE (1980 تا اکتبر 2014) را جستوجو کردیم.
معیارهای انتخاب
کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) یا شبه‐RCTهایی که آمانتادین و/یا ریمانتادین را با عدم مداخله، دارونما (placebo)، دیگر داروهای ضد ویروسی یا دوزها یا برنامههای متفاوت تجویز آمانتادین یا ریمانتادین در کودکان و سالمندان مبتلا به آنفلوآنزا A مقایسه کردند.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده مرور بهطور مستقل از هم نتایج جستوجو را ارزیابی کردند. دادهها را با استفاده از روششناسی (methodology) استاندارد کاکرین، استخراج و آنالیز کردیم.
نتایج اصلی
تعداد 12 مطالعه (2494 شرکتکننده: 1586 کودک و 908 سالمند) را شناسایی کردیم که آمانتادین و ریمانتادین را با دارونما، پاراستامول (paracetamol) (یک کارآزمایی: 69 کودک) یا زانامیویر (دو کارآزمایی: 545 سالمند) برای درمان آنفلوآنزا A، مقایسه کردند.
آمانتادین در پیشگیری از ابتلا به آنفلوآنزا A در کودکان موثر بود (773 شرکتکننده، خطر نسبی (RR): 0.11؛ 95% CI؛ 0.04 تا 0.30). خطر احتمالی ابتلا به آنفلوآنزا A در گروه کنترل، 10 مورد در هر 100 فرد در نظر گرفته شد. این خطر در گروه ریمانتادین یک مورد در هر 100 فرد (95% CI؛ 0 تا 3) گزارش شد. با این وجود، کیفیت شواهد پائین بود و بیخطری استفاده از دارو به خوبی ثابت نشد.
برای درمان، ریمانتادین در کاهش تب در روز سوم درمان در کودکان مفید ظاهر شد: یک مطالعه منتخب با خطر پائین سوگیری (bias)، کیفیت متوسط شواهد و 69 شرکتکننده (RR: 0.36؛ 95% CI؛ 0.14 تا 0.91). خطر فرضی 38 مورد در هر 100 فرد بود. خطر مربوطه در گروه ریمانتادین 14 مورد در هر 100 فرد (95% CI؛ 5 تا 34) گزارش شد.
ریمانتادین هیچ تاثیر پروفیلاکتیک در افراد مسن نشان نداد. سطح کیفیت شواهد بسیار پائین بود: 103 شرکتکننده (RR: 0.45؛ 95% CI؛ 0.14 تا 1.41). خطر فرضی 17 مورد در هر 100 فرد بود. خطر مربوطه در گروه ریمانتادین 7 مورد در هر 100 فرد (95% CI؛ 2 تا 23) گزارش شد.
هیچ شواهدی از بروز عوارض جانبی ناشی از درمان با آمانتادین یا ریمانتادین دیده نشد.
هیچ مطالعهای را برای ارزیابی آمانتادین در افراد مسن پیدا نکردیم.
نتیجهگیریهای نویسندگان
کیفیت شواهد همراه با فقدان دانش در مورد بیخطری مصرف آمانتادین و فواید محدود ریمانتادین، نشان نمیدهند که آمانتادین و ریمانتادین در مقایسه با گروه کنترل (دارونما یا پاراستامول) میتوانند در پیشگیری، درمان و کوتاه کردن طول دوره درمان آنفلوآنزا A در کودکان و سالمندان مفید باشند.
PICO
خلاصه به زبان ساده
آمانتادین و ریمانتادین در پیشگیری و درمان آنفلوآنزا A در کودکان و سالمندان
سوال مطالعه مروری
همانطور که توسط سازمان جهانی بهداشت (WHO) توصیه شده است، اوسلتامیویر (oseltamivir) (تامیفلو (Tamiflu)) در حال حاضر برای افراد مبتلا به آنفلوآنزا A استفاده میشود. در همهگیریهای قبلی، این ویروس به آمانتادین و ریمانتادین (amantadine and rimantadine) حساس بود. اگر این داروها بیخطر بوده و سویه در گردش به این داروها حساس باشد، میتوانند جایگزینی باشند برای مدیریت درمانی آنفلوآنزا. بنابراین خواستیم به این سوال پاسخ دهیم که آمانتادین و ریمانتادین میتوانند از ابتلای کودکان و سالمندان به آنفلوآنزا A پیشگیری کرده و آن را درمان کنند یا خیر.
پیشینه
آنفلوآنزای A یک عفونت تنفسی است که باعث سرفه، آبریزش بینی و تب میشود. اکثر نشانهها بدون درمان در عرض سه تا هفت روز برطرف میشوند. با این حال، بستری شدن در بیمارستان، پنومونی و حتی مرگ از عوارض نادر این بیماری، به ویژه در میان کودکان و سالمندان، است. بیماریهای همهگیر (پاندمی) نیز باعث نگرانی هستند.
نتایج کلیدی و کیفیت شواهد
تعداد 12 کارآزمایی (2494 شرکتکننده: 1586 کودک و 908 سالمند) را شناسایی کردیم. ما به دنبال کارآزماییهایی بودیم که آمانتادین یا ریمانتادین را عدم مداخله، دارونما یا داروهای کنترل در کودکان و سالمندان مقایسه کردند. آخرین جستوجوها در اکتبر 2014 تکمیل شدند. چندین پیامد را، از جمله آنفلوآنزا A، طول دوره تب، سرفه، سردرد، تهوع/استفراغ، سرگیجه و تحریک/بیخوابی بررسی کردیم.
اگرچه آمانتادین در پیشگیری از ابتلا به آنفلوآنزا نوع A در کودکان موثر بود، لازم است که در 17 کودک در یک دوره 14 تا 18 هفتهای استفاده شود تا از بروز یک مورد آنفلوآنزا A پیشگیری شود. علاوه بر این، بیخطری دارو به خوبی ثابت نشده است. سطح کیفیت شواهد پائین بود.
اثربخشی هر دو داروی ضد ویروس به سود ریمانتادین در کاهش تب تا روز سوم درمان در کودکان محدود شد. سطح کیفیت شواهد متوسط بود. به نظر نمیرسد که این مزیت توصیه به استفاده از ریمانتادین را برای درمان همه کودکان مبتلا به آنفلوآنزا A توجیه کند.
ریمانتادین تاثیر پروفیلاکتیک (پیشگیرانه) در افراد مسن نشان نداد. سطح کیفیت شواهد بسیار پائین است.
نتیجهگیری
کیفیت شواهد همراه با فقدان دانش در مورد بیخطری مصرف آمانتادین و فواید محدود ریمانتادین، نشان نمیدهند که آمانتادین و ریمانتادین در مقایسه با گروه کنترل (دارونما یا پاراستامول) میتوانند در پیشگیری، درمان و کوتاه کردن طول دوره درمان آنفلوآنزا A در کودکان و سالمندان مفید باشند.
Authors' conclusions
Summary of findings
| Amantadine compared with placebo for prevention and treatment of influenza A in children | ||||||
| Patient or population: children with no influenza A infection (prevention) or with influenza A infection (treatment) Settings: all Intervention: amantadine Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Amantadine | |||||
| Cases of influenza A during prophylaxis (follow‐up:14 to 18 weeks) | Medium risk population | RR 0.11 (0.04 to 0.3) | 773 | ⊕⊕⊝⊝ | ||
| 10 per 100 | 1 per 100 | |||||
| Fever after initiation of treatment (follow‐up: 3 days) | Medium risk population | RR 0.37 (0.08 to 1.75) | 104 | ⊕⊕⊝⊝ | ||
| 23 per 100 | 9 per 100 | |||||
| Cough after initiation of treatment | See comment | See comment | Not estimable | 0 (0) | See comment | No selected trial |
| Dizziness (follow‐up: 7 days) | Medium risk population | RR 6.63 (0.32 to 137.33) | 599 | ⊕⊝⊝⊝ | ||
| 0 per 100 | 0 per 100 | |||||
| Nausea/vomiting (follow‐up: 7 days) | Medium risk population | RR 0.54 (0.15 to 2) | 599 | ⊕⊝⊝⊝ | ||
| 13 per 100 | 7 per 100 | |||||
| Stimulation/insomnia (follow‐up: 7 days) | Medium risk population | RR 0.46 (0.12 to 1.74) | 599 | ⊕⊕⊝⊝ | ||
| 3 per 100 | 7 per 100 | |||||
| CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| *The basis for the assumed risk (e.g. median control group risk across studies) was calculated on the basis of control event rate. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 1Allocation concealment not used or unclear. 2Sparse data. 3Allocation concealment unclear. 4Sparse data, confidence intervals do not rule out potential for null effect or harm. 5High heterogeneity unexplained. | ||||||
| Rimantadine compared with placebo for prevention and treatment of influenza A in children | ||||||
| Patient or population: children with no influenza A infection (prevention) or with influenza A infection (treatment) Settings: any Intervention: rimantadine Comparison: control (placebo or acetaminophen) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Rimantadine | |||||
| Cases of influenza A during prophylaxis (follow‐up: 1 to 35 days) | Medium risk population | RR 0.49 (0.21 to 1.15) | 178 | ⊕⊕⊝⊝ | ||
| 24 per 100 | 12 per 100 | |||||
| Fever after initiation of treatment (follow‐up: 3 days) | Medium risk population | RR 0.36 (0.14 to 0.91) | 69 | ⊕⊕⊕⊝ | ||
| 38 per 100 | 14 per 100 | |||||
| Cough after initiation of treatment (follow‐up: 7 days) | Medium risk population | RR 0.83 (0.63 to 1.1) | 69 | ⊕⊕⊕⊝ | ||
| 81 per 100 | 67 per 100 | |||||
| Dizziness (follow‐up: 35 days) | Medium risk population | RR 3.21 (0.14 to 75.68) | 56 | ⊕⊝⊝⊝ | ||
| 0 per 100 | 0 per 100 | |||||
| Nausea/vomiting (follow‐up: 7 to 35 days) | Medium risk population | RR 0.96 (0.1 to 9.01) | 125 | ⊕⊕⊝⊝ | ||
| 2 per 100 | 2 per 100 | |||||
| Stimulation/insomnia | See comment | See comment | Not estimable | 0 | See comment | No selected trial |
| CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| *The basis for the assumed risk (e.g. median control group risk across studies) was calculated on the basis of control event rate. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 1Allocation concealment unclear. 2Sparse data and confidence intervals do not rule out the potential for no effect or harm | ||||||
| Amantadine compared with placebo for prevention and treatment of influenza A in the elderly | ||||||
| Patient or population: elderly people with no influenza A infection (prevention) or with influenza A infection (treatment) Settings: any Intervention: amantadine Comparison: control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Amantadine | |||||
| Cases of influenza A during prophylaxis | See comment | Not estimable | 0 | See comment | No selected trial | |
| Fever after initiation of treatment | See comment | Not estimable | 0 | See comment | No selected trial | |
| Cough after initiation of treatment | See comment | Not estimable | 0 | See comment | No selected trial | |
| Dizziness | See comment | Not estimable | 0 | See comment | No selected trial | |
| Nausea | See comment | Not estimable | 0 | See comment | No selected trial | |
| Vomiting | See comment | Not estimable | 0 | See comment | No selected trial | |
| Stimulation/insomnia | See comment | Not estimable | 0 | See comment | No selected trial | |
| Patient or population: elderly people with no influenza A infection (prevention) or with influenza A infection (treatment) Settings: any Intervention: rimantadine Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Rimantadine | |||||
| Cases of influenza A during prophylaxis | Medium risk population | RR 0.45 (0.14 to 1.41) | 103 | ⊕⊝⊝⊝ | ||
| 17per 100 | 7 per 100 | |||||
| Fever after initiation of treatment | See comment | 0 | See comment | See comment | No selected trial | |
| Cough after initiation of treatment | See comment | 0 | See comment | See comment | No selected trial | |
| Dizziness (follow‐up: 12 weeks) | Medium risk population | |||||
| 12 per 100 | 11 per 100 (2 to 70) | RR 0.94 | 35 (1) | ⊕⊕⊝⊝ | ||
| Nausea (follow‐up: 8 to 12 weeks) | Medium risk population | RR 1.99 (0.45 to 8.75) | 233 | ⊕⊝⊝⊝ | ||
| 8 per 100 | 15 per 100 | |||||
| Vomiting (follow‐up: 8 to 12 weeks) | Medium risk population | RR 0.99 (0.38 to 2.6) | 233 | ⊕⊕⊝⊝ | ||
| 7 per 100 | 7 per 100 | |||||
| Stimulation/insomnia (follow‐up: 8 to 12 weeks) | Medium risk population | RR 1.61 (0.43 to 6.02) | 233 | ⊕⊕⊝⊝ | ||
| 7 per 100 | 11 per 100 | |||||
| CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| *The basis for the assumed risk (e.g. median control group risk across studies) was calculated on the basis of control event rate. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 1Allocation concealment unclear and 1 study had high withdrawal rate. 2Sparse data and confidence interval do not rule out no effect or harm. 3Allocation concealment unclear 4High heterogeneity unexplained. | ||||||
Background
Description of the condition
Influenza is an acute and usually self limiting respiratory illness caused by influenza A and B viruses, which are members of the Orthomyxoviridae family (Nicholson 1992). Influenza may cause annual epidemics and intermittent pandemics (Sasaki 2011). Typically, seasonal influenza occurs most frequently during autumn and winter in temperate regions, but in some tropical countries it may occur throughout the year with one or two peaks during rainy seasons (Monto 2008; Yang 2010).
Although the natural transmission of the influenza virus predominantly occurs via aerosols dispersed by coughing or sneezing, it is also transmitted by nasal secretions and contact with contaminated surfaces. While all respiratory viruses, including influenza, use the nose as the entry channel, they can also enter through the tear ducts, draining into patients' sinuses and airways (Bitko 2007). The virus particles are deactivated by the ultraviolet rays in sunlight and common disinfectants such as soap (Barik 2012).
The illness is characterised by an abrupt onset of symptoms. These symptoms include headache, fever, general aches, weakness and myalgia, accompanied by respiratory tract signs, particularly cough and sore throat. However, a wide spectrum of clinical presentations may occur, ranging from a mild, febrile upper respiratory illness, to severe prostration and respiratory and systemic signs and symptoms.
The most common complication that occurs during outbreaks of influenza is pneumonia (both viral and bacterial). A number of extra‐pulmonary complications may also occur. These include Reye's syndrome in children (most commonly between two and 16 years of age), myocarditis, pericarditis and central nervous system (CNS) diseases. Again these include encephalitis, transverse myelitis and Guillain‐Barré syndrome (Barik 2012; Wiselka 1994).
An interesting and clinically relevant aspect of pandemic and epidemic influenza that sets it apart from seasonal influenza is the induction of the so‐called cytokine storm, consisting of interleukin‐6, tumour necrosis factor and interferon‐g. Together, these proinflammatory cytokines cause systemic inflammatory response syndrome, leading to multi‐organ failure that includes airway destruction, vascular endothelial damage and plasma leakage (Barik 2012; Cheung 2002)
Description of the intervention
Nowadays there are two main measures for the treatment and prophylaxis of influenza viruses: immunisation using influenza vaccines directly isolated from influenza A and B viruses and antiviral agents (Demicheli 2000; Noah 2013). Vaccination is the primary strategy for the prevention of influenza (Antanova 2012; Hsu 2012). Nevertheless, there are a number of likely scenarios for which effective antiviral agents would be of utmost importance. For example, the available evidence on the safety, efficacy or effectiveness of influenza vaccines for people aged 65 years or older is of poor quality (Jefferson 2010; Thomas 2011). Vaccination among the elderly may not be as effective as their immune systems are less responsive (Sasaki 2011). Influenza vaccines are efficacious in children older than two but little evidence is available for children under two (Demicheli 2012). During any influenza season, antigenic drift in the virus may occur after formulation of the year's vaccine. The vaccine can therefore be less protective and outbreaks can more easily occur in high‐risk populations. In the course of a pandemic, vaccine supplies would be inadequate. Moreover, vaccine production by current methods cannot be carried out with the speed required to halt the progress of a new strain of influenza virus. Therefore, it is likely that vaccines would not be available for those infected by the first wave of the virus (Hayden 2004). Additionally, in a study published in 2013, the author stated that vaccination‐only strategies were not cost‐effective for any pandemic scenario, saving few lives and incurring substantial vaccination costs (Kelso 2013). Vaccination, coupled with long duration social distancing, antiviral treatment and antiviral prophylaxis, was considered to be cost‐effective for moderate and extreme pandemics, as it can save lives while simultaneously reducing the total pandemic cost (Kelso 2013). Antiviral agents therefore form an important part of a rational approach to influenza management (Kelso 2013; Moscona 2005).
Antiviral drugs for influenza are currently divided into two classes: M2 ion channel inhibitors and neuraminidase inhibitors. The first class includes amantadine and rimantadine and the latter zanamivir, oseltamivir, laninamivir (approved in Japan) and peramivir (approved in Japan and Korea) (Barik 2012). M2 ion channel inhibitors affect ion channel activity through the cell membrane and are reported to be effective by interfering with the replication cycle of type A viruses (but not type B). The neuraminidase inhibitors interfere with the release of influenza virus progeny from infected host cells and are effective against influenza A and B (Moscona 2005). Both drug classes have shown partial effectiveness for the prevention and treatment of influenza A viruses, although neuraminidase inhibitors are less likely to promote the development of drug‐resistant influenza (Moscona 2005).
Resistance to M2 inhibitors remained low until 2003 (Bright 2005; Ziegler 1999). An epidemiological study into resistance to amantadine carried out from 1991 to 1995 described a frequency of 1% (16/2017) of resistant variants among H1N1 and H3N2 viruses (Ziegler 1999). However, there was a subsequently a dramatic increase in strains of influenza A (H3N2) with a specific mutation (Ser31Asn). An increase in resistance to amantadine was showed in communities located in Asia and North America (Bright 2005; Bright 2006). This resistance in 70% to 90% of strains occurred despite the absence of sustained selective drug pressure (Bright 2005; Bright 2006).
During the 2005 to 2006 season, 16% of H1N1 and 91% of H3N2 viruses were resistant around the world. Although the estimate for the proportion of resistance in H1N1 viruses was very low, an analysis conducted in China showed that the frequency of resistant H1N1 viruses had greatly increased from 28% (8/29) in the 2004 to 2005 season to 72% (33/46) in the 2005 to 2006 season. Similar studies were conducted in other countries in the 2005 to 2006 season. The following frequencies of resistance were obtained: 45% (13/29) in Europe, 24% (4/17) in Taiwan and 33% (1/3) in Canada (Deyde 2007).
A global pandemic emerged in 2009, caused by a new influenza A strain (H1N1) (WHO 2010a). All influenza A (H1N1) viruses tested in WHO Collaborating Centres to date have been shown to be resistant to amantadine and rimantadine (WHO 2011; WHO 2012).
When an avian influenza A (H7N9) virus was detected as the cause of human infections in China, its susceptibility to antiviral drugs was assessed. The outbreak viruses carried the established adamantine resistance marker. Once again neuraminidase inhibitors remained the only licensed treatment option (Li 2014).
Influenza A resistance to amantadine and rimantadine has been frequently reported over the last few years and, as such, it may seem unnecessary to continue testing sensitivity to these drugs. However, patterns of sensitivity and resistance of influenza viruses to antiviral drugs may change over time and so we consider it necessary to continue monitoring sensitivity and resistance.
How the intervention might work
The use of amantadine and rimantadine for the treatment and prevention of influenza A in adults has already been the topic of a review (Jefferson 2006b). The results of that review confirmed that amantadine and rimantadine had a comparable efficacy and effectiveness in the treatment of influenza A in healthy adults, although their effectiveness in interrupting transmission was probably low. As previous pandemics proved to be susceptible to this class of drugs, it seems reasonable to review the evidence for amantadine and rimantadine for treating and preventing influenza A in children and the elderly (Hayden 2006b).
Why it is important to do this review
Although the disease occurs in all age groups (Pineda Solas 2006), the risks of complications, hospitalisations and deaths from influenza are higher among three groups of people: 1) persons older than 65 years; 2) young children; and 3) persons of any age who have medical conditions that place them at increased risk. Rates of infection are highest amongst children and they are also one of the most important links for transmission (Dolin 2005).
Pandemics occur when influenza spreads globally, infecting 20% to 40% of the world's population in one year. This results in as many as 10 million deaths (WHO 2003). They usually arise in China, where pigs, ducks and humans live in close proximity to each other, and spread westward to the rest of Asia, Europe and the Americas (Bonn 1997). In the past 110 years there have been five pandemics caused by different influenza A viral subtypes. The Spanish influenza pandemic (1918 to 1919) is considered to have caused an estimated 40 million deaths worldwide. Most years, typical influenza epidemics infect 5% to 20% of the population and result in anywhere between 250,000 and 500,000 deaths, according to the World Health Organization (WHO), although other estimates accounting for deaths due to complications of influenza are as high as 1 million to 1.5 million.
In 2009, a new influenza A strain (H1N1) caused a global pandemic. According to the WHO, as of 24 January 2010, more than 214 countries and overseas territories had reported laboratory‐confirmed cases of pandemic influenza H1N1, resulting in at least 18,449 deaths (WHO 2010a).
In an earlier version of a Cochrane review in adults, the review authors stated that neuraminidase inhibitors were effective in reducing symptoms and complications, however there are now doubts about their effectiveness against complications (Jefferson 2014).
In a Cochrane review published in 2007, the review authors concluded that oseltamivir may be considered for the treatment of children aged one to 12 years with influenza infection (Matheson 2007). This antiviral is likely to shorten the duration of symptoms, hasten the return to normal activities and reduce the incidence of secondary complications. Nevertheless, the review authors also concluded that more data were needed to clarify the benefits of neuraminidase inhibitors for the treatment of influenza in asthmatic children (including addressing the potential confounder of prior vaccination).
Nowadays, neuraminidase inhibitors are used as a prescription drug for patients suffering from influenza on the recommendation of the WHO (WHO 2010b). Governments have spent billions of dollars stockpiling neuraminidase inhibitors as a public health measure (WHO 2010b). In previous pandemics, the influenza A virus was susceptible to amantadine and rimantadine. Therefore, these antivirals could be a less expensive alternative in the management of influenza if the circulating strain proves to be susceptible to amantadine and rimantadine (Hayden 2006b). However, we should emphasise the resistance patterns of the pandemic viruses in 2009. All influenza A (H1N1) viruses tested in WHO Collaborating Centres to date were sensitive to zanamivir and all were resistant to amantadine and rimantadine (WHO 2011).
These facts reinforce the importance of conducting and maintaining reviews of a variety of treatments, especially less expensive ones, for the treatment and prevention of influenza.
Objectives
To assess the effectiveness and safety of amantadine and rimantadine in preventing, treating and shortening the duration of influenza A in children and the elderly.
We tested the following hypotheses in comparisons between groups intended for amantadine or rimantadine prophylaxis or treatment compared with control groups:
-
there is no difference in the number of cases of influenza A or in the duration of influenza symptoms; and
-
there is no difference in the number of adverse effects.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs comparing amantadine or rimantadine, or both, with placebo, control drugs, different doses or schedules of amantadine or rimantadine, or both, or no intervention, in children and the elderly.
Types of participants
We included studies where at least 75% of the population was up to 19 years of age, or 65 years of age or older. We also included trials with a wider age range where data by age subgroups were available.
Types of interventions
Comparisons of amantadine or rimantadine, or both, to placebo, control drugs, other antivirals, no interventions or different doses of amantadine or rimantadine, or both, as prophylaxis and/or treatment for influenza A.
Types of outcome measures
Primary outcomes
-
Response to treatment (measured as cases on the specified day of treatment): fever on day three of treatment, cough on day seven of treatment, malaise on day six of treatment and conjunctivitis and eye symptoms on day five of treatment.
-
Cases of influenza, studied in all prophylaxis comparisons, including those in which two antivirals (rimantadine and zanamivir) (Gravenstein 2005; Schilling 1998), and two different doses of rimantadine were compared (Monto 1995).
-
Cases of side effects in children: diarrhoea, exanthema, malaise, muscular limb pain, headache, dyspnoea, dizziness, stimulation/insomnia, nausea, vomiting, arrhythmia, gastrointestinal (GI) symptoms, CNS symptoms, change in behaviour, hyperactivity and tinnitus.
-
Cases of side effects in the elderly: headache, dizziness, stimulation/insomnia, nausea, vomiting, anxiety, confusion, fatigue, depression, impaired concentration, loss of appetite, rash or allergic reaction, seizures or clonic twitching, dry mouth, insomnia or sleeplessness, body weakness and debility.
We used dichotomous outcomes for all the comparisons.
Secondary outcomes
The following outcomes appeared in the protocol but in the end we did not consider them in the analysis, as they were not reported in the included trials: patients' well‐being, admission to hospital, general practitioner (GP) visits and other drugs used. We could not analyse deaths. Although cited by Monto 1995, they were included among other causes of withdrawal.
Search methods for identification of studies
Electronic searches
For this 2014 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL 2014, Issue 9) (accessed 7 October 2014), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (June 2011 to September week 4, 2014) and EMBASE (June 2011 to October 2014).
The search strategy for MEDLINE and CENTRAL is described in Appendix 1. See Appendix 2 for the EMBASE search strategy. We imposed no language or publication restrictions. We used the same search strategy for our previous update in 2011, searching the Cochrane Central Register of Controlled Trials (CENTRAL 2011, Issue 2), MEDLINE (July 2007 to June week 3, 2011) and EMBASE.com (July 2007 to June 2011). Details of the review's initial search are in Appendix 3.
Searching other resources
We searched the trials registries WHO International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov for completed and ongoing trials (latest search 7 October 2014). We screened bibliographies of retrieved articles and reviews in order to identify further trials. We contacted pharmaceutical companies and researchers active in the field for unpublished trials.
Data collection and analysis
Selection of studies
Two review authors (MG and MS) independently applied the selection criteria to all retrieved articles and extracted data using a data extraction form, specifically designed for this review. We resolved disagreements by consensus. We appointed one review author (AC) as arbitrator when necessary.
We entered extracted data into RevMan 2012. Combination of data was dependent on population characteristics and outcomes studied.
Data extraction and management
Two review authors (MG, MS) independently read the retrieved trials and applied the selection criteria. We independently extracted and reviewed data using the data collection form previously developed for this review. Two review authors (MG, MS) resolved disagreements on the quality of the trials by consensus. We appointed a third author (AC) as arbitrator if necessary.
We emailed the authors of primary studies when the complete information sought was not available in study reports. We obtained authors' contact details from the study reports, other recent publications, university directories or by searching the world wide web. We recorded the following data.
-
Setting: hospital, emergency, offices or clinics, primary health care, nursing homes, communities, prisons, military personnel, nursery or day care.
-
Participants: criteria for patients to join the trial, age, gender, diagnostic criteria and co‐morbid conditions.
-
Interventions: placebo, other than amantadine and rimantadine antiviral controls, comparing different doses or schedules of amantadine and/or rimantadine or no intervention.
-
Outcome measures: global symptom improvements, relief, death, cases of influenza, malaise, fever, nausea, arthralgia, rash, headache, systemic and serious side effects, well‐being, admission to hospital, GP visits, other drugs used, cough, coryza, sore throat, hoarseness, vomiting, abdominal pain, insomnia, irritability, behaviour changes and anorexia.
-
Adverse effects: dry mouth, drowsiness/fatigue, constipation, urinary retention, sweating, headache, diarrhoea, palpitations, irritability, blurred vision, dizziness/light headedness and nausea/vomiting and any other systemic and serious side effects.
Assessment of risk of bias in included studies
Two review authors (MG, MS) independently screened trial quality. We resolved disagreements by discussion. We appointed a third author (AC) to act as arbitrator when necessary. We used the criteria recommended by the Cochrane Handbook for Systematic Reviews of Interventions to assess the risk of bias (Higgins 2011). We developed a form and populated it to assess the risk of bias, based on a Cochrane review (Ahovuo‐Saloranta 2014). We indicated if the risk of bias was low, high , or even unclear, indicating either a lack of information or uncertainty over the potential for bias.
1. Sequence generation: was the method used to generate the allocation sequence appropriate to produce comparable groups? We considered that the risk of bias was low if the authors described a random component in the sequence generation process (for example, a random number table, a computerised random number table, coin tossing, shuffling cards or envelopes, throwing dice, drawing of lots). If there was no or insufficient information about the sequence generation process, we marked this domain 'unclear'. We considered that there was a high risk of bias if the sequence was generated by: 1) odds and evens or date of birth; 2) some rule based on date (or day) of admission; 3) some rule based on hospital or clinic record number.
2. Allocation sequence concealment: was the method used to conceal the allocation sequence appropriate to prevent the allocation being known in advance of, or during, enrolment? We marked this domain 'low risk' of bias if the trial authors described adequate concealment (for example, by means of either central allocation, sequentially numbered drug containers of identical appearance, or sequentially numbered, opaque, sealed envelopes) and 'high risk' of bias if: 1) inadequate concealment was documented; 2) allocation concealment was not used (for example, using either an open random allocation schedule, assignment envelopes without appropriate safeguards, alternation or rotation, date of birth or case record number). We marked this domain 'unclear' if: 1) insufficient information about allocation concealment was provided; 2) the information was unclearly reported.
3. Blinding of participants and personnel: were adequate measures used to blind study participants and personnel from knowing which intervention a participant received? We marked this domain 'low risk' of blinding if the RCT authors stated that: 1) there was no blinding; 2) there was incomplete blinding but the review authors judged that the outcome was not likely to be influenced by said incomplete blinding; 3) blinding of participants and key study personnel was ensured and it is unlikely that the blinding could have been broken. We marked this domain 'high risk' of bias, if the RCT authors described: 1) no blinding; 2) incomplete blinding and the outcome was likely to be influenced by said incomplete blinding; 3) blinding of key study participants and personnel but it was likely that the blinding could have been broken. We marked this domain 'unclear' if there was insufficient information or if the study did not address this outcome.
4. Blinding of outcome assessment: were adequate measures used to blind outcome assessors from knowing which intervention a participant received? We marked this domain 'low risk' of bias if there was: 1) no blinding of outcome assessment but the review authors judged that the outcome measurement was not likely to be influenced by lack of blinding; 2) blinding of outcome assessors was ensured and it is unlikely that the blinding could have been broken. We marked this domain 'high risk' of bias, if: 1) no blinding of outcome assessment was stated and the outcome measurement was likely to be influenced by lack of blinding; 2) there was blinding of outcome assessors but it was likely that the blinding could have been broken. We marked this domain 'unclear' if there was insufficient information or if the study did not address this outcome.
5. Incomplete outcome data describes how complete the data were for the clinical outcomes. Were dropout rates and reasons for withdrawals reported? Were missing data imputed appropriately? We marked this domain 'low risk' of bias if the RCT authors stated that: 1) there were no missing outcome data; 2) the reasons for missing outcome data were unlikely to be related to true outcome: 3) missing outcome data balanced out across intervention groups, with similar reasons for missing data across said groups; 4) the proportion of missing outcomes compared with the observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate; 5) missing data were imputed using appropriate methods. We marked this domain 'high risk' of bias, representing a high risk of attrition bias, if: 1) the reason for missing outcome data was likely to be related to true outcome, with either an imbalance in numbers or reasons for missing data across intervention groups; 2) the proportion of missing outcomes compared with the observed event risk was enough to induce clinically relevant bias in the intervention effect estimate; 3) 'as‐treated' analysis was done with substantial departure of the intervention received from that assigned at randomisation; 4) there was potentially inappropriate application of simple imputation. 'unclear risk of bias' was the expected classification of studies in which there was insufficient reporting of attrition/exclusions to permit the classification of 'low risk' or 'high risk' (e.g. number of randomised patients not stated, no reasons for missing data provided), or if the study did not address this outcome.
We completed a 'Risk of bias' table for each included study (see 'Risk of bias' tables in the Characteristics of included studies table).
Measures of treatment effect
We calculated risk ratios (RRs) and 95% confidence intervals (CI) for each study as all the outcomes studied were dichotomous. We tested for heterogeneity for each outcome.
Unit of analysis issues
In the Gravenstein 2005 trial, the author stated that the study was conducted over three winter seasons and that some participants were randomised more than once. Taking into account that influenza was the outcome of interest and that in each season different influenza viruses emerge, participants that had acquired the infection in one of the seasons could not be considered to be immunologically resistant to influenza in the next season. Consequently, we decided to include all participants described by the trial authors, as this does not seem to produce bias.
In the Crawford 1988 and Clover 1991 studies, eligible family members were randomly assigned as a block to study rimantadine in the prevention of influenza. For the purpose of this review, we selected the children as the subgroup of interest. It could be expected that children from families in the intervention group could be more protected from influenza than children in the control group. Nevertheless, no effect was shown in either of the three trials selected for this comparison (Clover 1986; Clover 1991; Crawford 1988).
Dealing with missing data
We contacted the trial authors to request missing data when data were not clearly provided. We analysed the available data, taking into account the relatively small number and randomness of missing data.
Assessment of heterogeneity
We stored the data extracted from primary studies in the Review Manager software (RevMan 2012). All the outcomes we studied were dichotomous.
We determined whether there were sufficiently homogeneous data to combine when there were two or more selected studies for a given comparison. We grouped the previously selected articles according to the characteristics of interventions, outcomes and populations studied. We had to take into account that pooled studies may still differ from each other even though the initial application of this filter was supposed to reduce the possibility of heterogeneity.
We initially inspected forest plots generated by RevMan 2012 to evaluate the possibility of heterogeneity between studies. We applied the Cochrane test for homogeneity. With this aim we set a P value of 0.1 as the limit for considering the existence of heterogeneity (CCI 2006). We also applied the I2 statistic to quantify heterogeneity among the trials and to verify the impact on the meta‐analysis, considering that some clinical and methodological diversity always occurs in a meta‐analysis. We considered values above 50% to be representative of significant heterogeneity (Higgins 2011), and we explored the causes. We used the subgroup analysis of participants or a subgroup analysis of the studies selected for each comparison when the heterogeneity was relevant to the outcome of the meta‐analysis.
Assessment of reporting biases
We considered assessment of reporting biases to be at risk because of the small number of studies selected for each comparison. Nevertheless, we relied on extensive research and carefully examined the references of the studies found in the search results to avoid reporting biases. We analysed all trials that met the inclusion criteria, independently of the journal's impact factor, the year of publication, the language in which the article was written and the origin of both author and publication. The use of these criteria can be confirmed by checking the lists of included and excluded studies.
Data synthesis
We used the risk ratio (RR) and respective 95% confidence interval (CI) as a summary measure to combine data. We calculated the necessary number of patients to be treated for an individual to benefit from treatment with respect to an outcome (number needed to treat to benefit (NNTB)) and its 95% CI, when a statistical difference was found. We estimated the occurrence of an event in the population, or absolute risk (baseline risk) based on the rate of event occurrence in controls (control group rate (CGR)) for this calculation.
We used the random‐effects model to calculate the summary measure, with the assumption that although the articles could have addressed somewhat different issues, they could be viewed as a family of studies on similar questions. We considered that the articles were a random sample of all studies that addressed the questions we were interested in. Therefore, even considering the possibility of failure of the statistical tests of homogeneity, the combination of similar studies would still be a reasonable procedure. Although it is impossible to state if the articles were really a random sample of all research on an issue, this model is more realistic and less prone to overestimate accuracy (Fletcher 2006).
Subgroup analysis and investigation of heterogeneity
We pre‐specified some subgroup analyses to investigate heterogeneity. We planned to take into account the drugs used for control and treatment, their doses and the previous use of anti‐influenza vaccine(s). However, we stress that the subgroup analysis does not take into account the randomisation processes, so these results must be considered with caution.
Sensitivity analysis
We carried out sensitivity analyses to explore heterogeneity. We conducted subgroup analyses for subsets of participants. We had planned to analyse rimantadine and amantadine separately and together. However, when we identified the use of different antivirals being used as a control, we performed a subgroup analysis. We separated the trials in which the comparison was made using different antiviral medications from those in which the control was made with placebo or other drugs. We also carried out subgroup analyses for subsets of immunised and non‐immunised participants, as well as according to the dosages of antivirals tested in the trials.
Results
Description of studies
Results of the search
We retrieved a total of 33 records in this updated search. Out of a total of 205 abstracts, titles and studies that we retrieved through all the searches, 195 were written in English, three in Russian, two in Czech, three in German, one in French and one in Japanese. We discarded 129 studies. We assessed the remaining 78 articles in detail. It was necessary to contact 46 trial authors to verify that their studies met our selection criteria. We included 12 trials in this review. All of them are published trials and are described in the Characteristics of included studies table. We added another 38 trials in 2011 when we updated this review; we excluded all of them and our conclusions remain unchanged.
We did not identify any new trials for inclusion in this 2014 update. We excluded 20 new trials (Anton 2011; Atiee 2012; Bacosi 2002; Cayley 2012; Cheng 2012; De Vincenzo 2012; Escuret 2012; Gatwood 2012; Hayden 2012; Hsu 2012; Ison 2013; Jiang 2013; Lopez‐Medrano 2012; Louie 2012; Michiels 2013; Sampaio 2011; Santesso 2013; Shah 2012; Singer 2011; Yuen 2012).
Included studies
The 12 included studies were all randomised trials (Clover 1986; Clover 1991; Crawford 1988; Finklea 1967; Gravenstein 2005; Hall 1987; Kitamoto 1968; Kitamoto 1971; Monto 1995; Patriarca 1984; Payler 1984; Schilling 1998); 11 were blinded and one was unblinded (Schilling 1998). The methods of randomisation and the follow‐up period were poorly described in all studies, although we could estimate that follow‐up ranged from eight to 120 days. We classified the included trials into two major groups: those conducted in children and those in the elderly.
Trials in children
Eight selected studies looked at the following.
-
Treatment with amantadine (Kitamoto 1968; Kitamoto 1971) and rimantadine (Hall 1987).
-
Prophylaxis with amantadine (Finklea 1967; Payler 1984) and rimantadine (Clover 1986; Clover 1991; Crawford 1988).
-
Adverse effects due to amantadine (Kitamoto 1968; Kitamoto 1971) and rimantadine (Clover 1986; Crawford 1988; Hall 1987).
For treatment trials and the outcome fever on day three of treatment, the amantadine arm size was 51 and the control arm size was 53 children (Kitamoto 1968; Kitamoto 1971). The rimantadine arm size was 37 and the control arm size was 32 children (Hall 1987). For the other outcomes, cough on day seven, malaise on day six and eye symptoms on day five, we selected just one trial (Hall 1987). The rimantadine arm size was 37 and control arm size was 32 children for each of these outcomes.
In the five prophylaxis trials, we applied wider age ranges for children than the definition stated in the protocol (participants up to 16 years of age). These trials included older participants who were adolescents by the WHO definition (WHO 2007). Data regarding the proportion of the subgroup which strictly fulfilled the age criterion were not available in these studies or by contacting the trial authors. The respective age ranges were one to 17 years (Clover 1991), 13 to 19 years (Payler 1984), one to 18 years (Clover 1986; Crawford 1988), and eight to 19 years of age (Finklea 1967). The amantadine arm size was 368 (Finklea 1967 (104); Payler 1984 (264)) and the control arm size was 373 children (Finklea 1967 (133); Payler 1984 (240)). The rimantadine arm size was 84 (Clover 1986 (35); Clover 1991 (22); Crawford 1988 (27)) and the control arm size was 94 participants (Clover 1986 (41); Clover 1991 (24); Crawford 1988 (29)).
Reported adverse effects of amantadine included exanthema, malaise, muscular limb pain, headache, arrhythmia and stimulation/insomnia. The antiviral arm size was 264 children (Kitamoto 1968 (75); Kitamoto 1971 (189)) and the control arm size was 335 (Kitamoto 1968 (84); Kitamoto 1971 (251)).
A reported adverse effect of amantadine was dyspnoea. The antiviral arm size was 75 and the control arm size was 84 children (Kitamoto 1968). For the adverse effects of hyperreactivity and tinnitus the rimantadine arm size was 27 and the control arm size was 29 children (Crawford 1988).
Nausea/vomiting, diarrhoea and dizziness were described as possible adverse effects for both antivirals. For nausea/vomiting, the amantadine arm size was 264 children (Kitamoto 1968 (75); Kitamoto 1971 (189)) and the control arm size was 335 (Kitamoto 1971 (251); Kitamoto 1968 (84)). The rimantadine arm size was 38 (Crawford 1988 (1); Hall 1987 (37)) and the control arm size was 61 (Crawford 1988 (29); Hall 1987 (32)).
For diarrhoea and dizziness the amantadine arm size was 264 children (Kitamoto 1968 (75); Kitamoto 1971 (189)) and the control arm size was 335 (Kitamoto 1968 (84), Kitamoto 1971 (251)). The rimantadine arm size was 27 and the control arm size was 29 children for these adverse effects (Crawford 1988).
Trials in the elderly
We selected three trials in this age group that reported on prophylaxis with rimantadine; we did not select any treatment trials. We studied the following outcomes.
-
Prophylaxis of laboratory and clinical infection (Monto 1995; Patriarca 1984).
-
Adverse reactions (Monto 1995; Patriarca 1984).
-
Different doses of rimantadine as a prophylactic antiviral (Monto 1995).
-
Comparison to other antivirals in the prophylaxis of influenza (Gravenstein 2005; Schilling 1998).
For prophylaxis of laboratory and clinical infection, the rimantadine (200 mg/day) arm size was 44 (Monto 1995 (26); Patriarca 1984 (18)) and the placebo arm size was 31 participants (Monto 1995 (14); Patriarca 1984 (17)). The trial authors stated they limited this analysis to vaccinated participants in nursing homes with confirmed influenza, as it provided an estimate of the additional protective efficacy of rimantadine. The sample studied by Patriarca 1984 was made up of previously vaccinated participants, so all the participants were analysed (Monto 1995; Patriarca 1984).
In the adverse reaction studies focusing on stimulation/insomnia, confusion, fatigue, nausea, depression, loss of appetite and vomiting, the rimantadine (200 mg/day) arm size was 150 (Monto 1995 (132); Patriarca 1984 (18)) and the placebo arm size was 83 participants (Monto 1995 (66); Patriarca 1984 (17)). All randomly assigned participants were analysed.
In the adverse reaction study focusing on headache, impaired concentration, rash or allergic reaction, seizures or clonic twitching, the rimantadine (200 mg/day) arm size was 132 and the placebo arm size was 66 participants (Monto 1995).
In another adverse reaction study focusing on dizziness and anxiety, the rimantadine (200 mg/day) arm size was 18 and the placebo arm size was 17 participants (Patriarca 1984).
In the unique study evaluating different doses of rimantadine as a prophylactic drug for clinical and confirmed influenza A, the rimantadine (100 mg/day) arm size was 28 and the rimantadine (200 mg/day) arm size was 26 participants (Monto 1995).
Only one selected study focused on adverse effects related to different doses of rimantadine. The studied effects were confusion, depression, impaired concentration, insomnia or sleeplessness, loss of appetite, rash or allergic reaction, seizure or clonic twitching, dry mouth, fatigue or drowsiness, headache, body weakness and debility. The 100 mg/day arm size was 130 and the 200 mg/day arm size was 132 participants (Monto 1995).
We selected two trials for the comparison of rimantadine to another antiviral and the participants were also the elderly (Gravenstein 2005; Schilling 1998). The rimantadine arm size was 254 and the zanamivir arm size was 291 participants. No study used amantadine for this kind of comparison.
Excluded studies
We excluded 212 studies for the following reasons.
-
They were carried out in different age groups.
-
They were not controlled trials.
-
They assessed other drugs.
-
They were non‐human or laboratory studies.
We excluded 20 new trials in this 2014 update (Anton 2011; Atiee 2012; Bacosi 2002; Cayley 2012; Cheng 2012; De Vincenzo 2012; Escuret 2012; Gatwood 2012; Hayden 2012; Hsu 2012; Ison 2013; Jiang 2013; Lopez‐Medrano 2012; Louie 2012; Michiels 2013; Sampaio 2011; Santesso 2013; Shah 2012; Singer 2011; Yuen 2012).
Risk of bias in included studies
The overall risk of bias is presented graphically in Figure 1 and summarised in Figure 2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The trial authors of the 12 included studies stated that participants had been randomly allocated into treatment or control groups. In two of the studies we obtained the following information by contacting the trial authors (Hall 1987; Payler 1984). Hall reported that a computer system was used to randomise participants. The university pharmacy was chosen to allocate and store the study drugs (Hall 1987). In Payler's study, randomisation had been carried out by the statistical department of a pharmaceutical company, which kept the key to the randomisation and only when the study was analysed was the code broken (Payler 1984). There was no mention of any particular randomisation method in the other studies.
Blinding
Ten studies were described as double‐blinded (Clover 1986; Clover 1991; Crawford 1988; Finklea 1967; Gravenstein 2005; Hall 1987; Kitamoto 1968; Kitamoto 1971; Monto 1995; Patriarca 1984). However, only in one trial were blinded people listed (Monto 1995). Although there was no blinding stated in Payler 1984, we judged that the outcome was not likely to be influenced by a lack of blinding. Schilling 1998 was described as an unblinded study; we also judged that the outcomes were unlikely to be influenced by a lack of blinding.
Incomplete outcome data
There were no missing participants in either Kitamoto 1971, Kitamoto 1968 or Payler 1984. The review authors considered that the reasons for missing outcome data were unlikely to be related to true outcome in the following studies: Clover 1986; Clover 1991; Crawford 1988; Finklea 1967; Gravenstein 2005. In the Hall 1987 trial, we considered that the proportion of missing outcomes compared with observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate. On the other hand, we considered the reasons for missing outcome data likely to be related to the true outcome data in two studies (Monto 1995; Patriarca 1984). In Schilling 1998, there was insufficient reporting of exclusion.
Selective reporting
The review authors did not identify any possible sources of reporting biases.
Other potential sources of bias
The review authors did not identify any other possible sources of bias.
Effects of interventions
See: Summary of findings for the main comparison ; Summary of findings 2 ; Summary of findings 3 ; Summary of findings 4
Primary outcomes in children
Amantadine and rimantadine compared to control (placebo and acetaminophen) in the treatment of influenza A in children
In the protocol, we originally planned to study the drug effect on reduction of fever and cough as they are considered the best predictors of influenza diagnosis. After collecting data, we verified that specific timelines for reduction of signs and symptoms were not reported in the included trials. We searched for another way to present an estimation of the response to amantadine and rimantadine in patients with influenza. For this unplanned analysis, we considered the available data and arbitrarily chose a day of antiviral use to evaluate the response to the treatment. This choice was based on the Eccle 2005 study in which clinical manifestations were classified into early and later symptoms. Typically fever may last four to eight days, so we chose day three of treatment as the cut‐off point to which it could be considered that the response to the drug would be useful. Cough is considered a later manifestation that develops slowly and can still be present a week later (Eccle 2005). In the same way, we chose day seven of treatment as the cut‐off point by when the response to the drug could be considered useful.
We also decided to include other treatment outcomes as they were available in Hall's electronic correspondence to us. In the same way, we arbitrarily chose a day of antiviral use to evaluate the response to the treatment to make this unplanned analysis: 'malaise on day six', as it begins early but could still be present for one or two weeks (Eccle 2005; Smith 2006), and 'eye manifestations on day five', as it can occur early on in the course of the illness (Treanor 2005; Wright 2004)
Amantadine was compared to placebo: 104 participants (Kitamoto 1968; Kitamoto 1971), and rimantadine to acetaminophen: 69 participants (Hall 1987).
There was a protective effect of amantadine and rimantadine in the occurrence of fever on day three of antiviral treatment, when trials using both antivirals were combined: 173 participants, risk ratio (RR) 0.39; 95% confidence interval (CI) 0.20 to 0.79 (Analysis 1.1) (Hall 1987; Kitamoto 1968; Kitamoto 1971).
The baseline risk of fever on day three of treatment was 0.28, calculated on the basis of the control group risk (CGR). The number of children needed to treat to benefit (NNTB) to prevent one case of fever on day three of treatment was six (95% CI 4 to 17) (Analysis 1.1).
We also verified a protective effect of rimantadine for this outcome: RR 0.36; 95% CI 0.14 to 0.91 (Analysis 1.1.2). The baseline risk of fever on day three of treatment was 0.38, calculated on the basis of the CGR. The NNTB was five (95% CI 3 to 25) (Analysis 1.1). Just one trial with 69 participants reported this outcome (Hall 1987).
We observed no protective effect of amantadine in the occurrence of fever on day three of treatment: 104 participants, RR 0.37; 95% CI 0.08 to 1.75 (Analysis 1.1.1) (Kitamoto 1968; Kitamoto 1971).
We saw no protective effect of rimantadine regarding the occurrence of any of the following outcomes assessed: malaise on day six (RR 1.04; 95% CI 0.63 to 1.70) (Analysis 1.2), cough on day seven (RR 0.83; 95% CI 0.63 to 1.10) (Analysis 1.3), conjunctivitis on day five (RR 0.17; 95% CI 0.01 to 3.49) (Analysis 1.4), and cases of pain on movement and visual distortion on day five (RR 0.58; 95% CI 0.10 to 3.24) (Analysis 1.5). Just one study with 69 participants reported these outcomes (Hall 1987).
No selected studies reported the use of amantadine for these latter outcomes.
Amantadine and rimantadine compared to control in the treatment of influenza A in the elderly
There was no study selected for this comparison.
Amantadine and rimantadine compared to control (placebo and to specific treatment) in the prophylaxis of influenza A in children
Amantadine was compared to placebo and specific treatment (Finklea 1967; Payler 1984) and rimantadine to placebo (Clover 1986; Clover 1991; Crawford 1988).
The amantadine (Finklea 1967; Payler 1984) and rimantadine trials (Clover 1986; Clover 1991; Crawford 1988) were heterogeneous (Chi2 test 9.27, P value = 0.05, I2 statistic 56.8%) and could not be combined.
A protective effect of amantadine was observed with 773 participants, RR 0.11; 95% CI 0.04 to 0.30 (Analysis 2.1.1). The baseline risk of influenza was 0.10, calculated on the basis of the CGR. The NNTB was 12 (95% CI 9 to 17) for a period ranging from 14 (Payler 1984) to 18 weeks (Finklea 1967).
On the other hand, no protective effect of rimantadine was seen in the prophylaxis of cases of influenza: 178 participants (RR 0.49; 95% CI 0.21 to 1.15) (Analysis 2.1.2) (Clover 1986; Clover 1991; Crawford 1988).
Use of different doses of amantadine and rimantadine for prophylaxis and treatment of influenza in children
There was no selected study conducted in children for this comparison.
Amantadine and rimantadine compared to other antivirals in children
There was no selected study conducted in children for this comparison
Amantadine and rimantadine compared to control (placebo and zanamivir) in the prophylaxis of influenza A in the elderly
Rimantadine was compared to placebo (Monto 1995; Patriarca 1984) and to zanamivir (Schilling 1998). No protective effect of rimantadine was seen regarding the prophylaxis of influenza in the elderly: 191 participants, RR 0.74; 95% CI 0.13 to 4.07 (Analysis 3.1).
Although care must be taken in the interpretation of the Chi2 test due to its low power in detecting heterogeneity in meta‐analyses, we should emphasise the high P value observed in this comparison, considered alongside the I2 statistic value under 50%: Chi2 test 3.28; P value = 0.19, I2 statistic 39%. We decided to explore the reasons for these findings as if the studies were heterogeneous, even though it would result in smaller samples impairing the ability to reach any definitive conclusion (Monto 1995; Patriarca 1984; Schilling 1998).
Monto and Patriarca analysed previously vaccinated participants in blinded trials and used a placebo as control (Monto 1995; Patriarca 1984). Schilling did not state if the participants were vaccinated, although it was stated that the majority of the studied population had been previously immunised (Schilling 1998). This was an unblinded trial in which another antiviral (zanamivir) was used as a control drug.
When we excluded this study (Schilling 1998), the remaining trials, Monto 1995 and Patriarca 1984 were shown to be homogeneous but no protective effect of rimantadine prophylaxis in the occurrence of cases of influenza persisted (103 participants, RR 0.45; 95% CI 0.14 to 1.41) (Analysis 3.2).
Monto 1995 used two different doses of rimantadine in his trial (100 mg/day and 200 mg/day) and Patriarca 1984 used the conventional dose of 200 mg/day. Schilling 1998 used a single dose of 100 mg/day. We also combined Monto's 200 mg/day subgroup with Patriarca's study in which the same dose was administered, but again no protective effect of rimantadine was observed in the prophylaxis of influenza: eight participants, RR 0.44; 95% CI 0.12 to 1.63) (Analysis 3.3) (Monto 1995; Patriarca 1984; Schilling 1998).
Schilling's sample and Monto's 100 mg/day subgroup were heterogeneous and could not be combined (Chi2 test 2.55, P value = 0.11, I2 statistic 60.8%) (Monto 1995; Schilling 1998).
There was no amantadine study selected for comparison.
Use of different doses of amantadine and rimantadine for prophylaxis and treatment of influenza A in the elderly
A reduced rimantadine dose of 100 mg/day was comparable to the full dose of 200 mg daily for prophylaxis of influenza in the elderly, although a wide CI was verified (54 participants, RR 0.93; 95% CI 0.21 to 4.20) (Analysis 4.1). It should be emphasised that there were few data available for these comparisons (Monto 1995).
There was no selected study using different doses of rimantadine in the elderly, nor any selected trial comparing different doses of amantadine for prophylaxis and treatment of influenza in the elderly.
Amantadine and rimantadine compared to other antivirals in the elderly
In Gravenstein's but not in Schilling's study an identical placebo was used (Gravenstein 2005; Schilling 1998). When rimantadine was compared to zanamivir it was shown that zanamivir prevented influenza A more effectively than rimantadine in the elderly (Analysis 5.1).
There was no amantadine trial selected for this comparison in the elderly.
Adverse effects of amantadine and rimantadine compared to control (placebo and acetaminophen) in children
Amantadine was compared to placebo (Kitamoto 1968; Kitamoto 1971). Rimantadine was compared to placebo (Clover 1986; Crawford 1988) and to acetaminophen (Hall 1987).
Amantadine was not related to a higher risk of the following adverse effects in two trials with 599 participants: diarrhoea (RR 0.79; 95% CI 0.42 to 1.47) (Analysis 6.1), exanthema (RR 0.69; 95% CI 0.21 to 2.34) (Analysis 6.2), muscular limb pain (RR 0.85; 95% CI 0.46 to 1.59) (Analysis 6.3), headache (RR 0.73; 95% CI 0.52 to 1.03) (Analysis 6.4) and stimulation and insomnia (RR 0.46; 95% CI 0.12 to 1.74) (Analysis 6.5) (Kitamoto 1968; Kitamoto 1971).
In the same way, amantadine was not related to the outcomes dizziness and dyspnoea. For dizziness there were 655 participants in two studies (Kitamoto 1968; Kitamoto 1971). The RR was 6.63 (95% CI 0.32 to 137.33) (Analysis 6.6.1) and for dyspnoea there were 159 participants in just one trial (Kitamoto 1968). The RR was 0.37 (95% CI 0.02 to 9.02) (Analysis 6.7).
The studies were heterogeneous for the outcomes malaise (Chi2 test 3.75, P value = 0.05, I2 statistic 73.3%) and nausea/vomiting (Chi2 test 4.26, P value = 0.04, I2 statistic 76.5%), although it seems that the author had used the same protocol. Nevertheless, the heterogeneity for the outcome nausea/vomiting does not seem to be relevant, as amantadine could be related either to an increase or to a reduction in the occurrence of this adverse effect (Kitamoto 1968; Kitamoto 1971).
No cases of arrhythmia were reported in those two trials.
Rimantadine was not related to a higher risk of any of the following adverse effects assessed: central nervous system (CNS) symptoms: one study, 76 participants (RR 0.23; 95% CI 0.01 to 4.70) (Analysis 6.8); change in behaviour: one study, 76 participants (RR 0.23; 95% CI 0.01 to 4.70) (Analysis 6.9); diarrhoea: one study, 56 participants (RR 0.36; 95% CI 0.02 to 8.41) (Analysis 6.1.2); dizziness: one study, 56 participants (RR 3.21; 95% CI 0.14 to 75.68) (Analysis 6.6.2); gastro‐intestinal (GI) manifestations: one study, 76 participants (RR 1.17; 95% CI 0.08 to 18.05) (Analysis 6.10); hyperactivity: one study, 56 participants (RR 0.36; 95% CI 0.02 to 8.41) (Analysis 6.11); tinnitus: one study, 56 participants (RR 3.21; 95% CI 0.14 to 75.68) (Analysis 6.12); cerebellar ataxia: one study, 69 participants (RR 2.61; 95% CI 0.11 to 61.80) (Analysis 6.13) (Clover 1986; Crawford 1988; Hall 1987).
As it was stated, each one of the adverse effects described above was studied in just one included trial, except for nausea and vomiting (Crawford 1988; Hall 1987). In the same way, rimantadine was not related to a higher risk of nausea and vomiting: two studies, 125 participants (RR 0.96; 95% CI 0.10 to 9.01) (Analysis 6.15.2).
Adverse effects related to different doses of amantadine and rimantadine in children
There were no selected studies conducted in children for this comparison.
Adverse effects of amantadine and rimantadine compared to control (placebo) in the elderly
There were two selected studies for these outcomes, both using rimantadine and placebo (Monto 1995; Patriarca 1984).
No effect of rimantadine was seen regarding any of the adverse outcomes assessed in the combined studies: stimulation and insomnia (233 participants, RR 1.61; 95% CI 0.43 to 6.02) (Analysis 7.1), confusion (233 participants, RR 0.79; 95% CI 95% 0.40 to 1.56) (Analysis 7.2), fatigue (233 participants, RR 0.81; 95% CI 0.41 to 1.60) (Analysis 7.3) and vomiting (233 participants, RR 0.99; 95% CI 0.38 to 2.60) (Analysis 7.4) (Monto 1995; Patriarca 1984).
In the same way, rimantadine was not related to the outcomes studied by Monto: headache (198 participants, RR 0.83; 95% CI 0.21 to 3.38) (Analysis 7.5); impaired concentration (198 participants, RR 0.50; 95% CI 0.10 to 2.41) (Analysis 7.6); rash or allergic reaction (198 participants, RR 3.53; 95% CI 0.18 to 67.28) (Analysis 7.7); seizures or clonic twitching (198 participants, RR 2.00; 95% CI 0.23 to 17.54) (Analysis 7.8) and dry mouth (198 participants, RR 0.70; 95% CI 0.23 to 2.12) (Analysis 7.9), as well as in those studied by Patriarca: dizziness (35 participants, RR 0.94; 95% CI 0.15 to 5.97) (Analysis 7.10) and anxiety (35 participants, RR 2.83; 95% CI 0.92 to 8.74) (Analysis 7.11) (Monto 1995; Patriarca 1984).
The articles were heterogeneous just for the occurrence of nausea (test for heterogeneity: Chi2 test 2.02; P value = 0.16; I2 statistic 50.5%). Nevertheless, this heterogeneity does not seem to be relevant as rimantadine could be related either to an increase or to a reduction in the occurrence of nausea in each one of the studies (Patriarca 1984: 35 participants, RR 5.67; 95% CI 0.76 to 42.32 and Monto 1995: 198 participants, RR 1.17; 95% CI 0.47 to 2.90) (Analysis 7.12).
It is important to stress the small samples studied in both trials. There was no amantadine trial selected for comparison.
Adverse effects related to different doses of amantadine and rimantadine in the elderly
There was no protective effect of a reduced dose of rimantadine in the occurrence of the following adverse reactions in the elderly: one study with 262 participants: confusion (RR 0.82; 95% CI 0.41 to 1.65) (Analysis 8.1), depression (RR 0.44; 95% CI 0.12 to 1.65) (Analysis 8.2), impaired concentration (RR 0.68; 95% CI 0.11 to 3.98) (Analysis 8.3), insomnia or sleeplessness (RR 1.02; 95% CI 0.26 to 3.97) (Analysis 8.4), loss of appetite (RR 0.62; 95% CI 0.27 to 1.46) (Analysis 8.5), rash or allergic reaction (RR 0.34; 95% CI 0.04 to 3.21) (Analysis 8.6), seizures or clonic twitching (RR 0.11; 95% CI 0.01 to 2.07) (Analysis 8.7), dry mouth (RR 1.16; 95% CI 0.43 to 3.11) (Analysis 8.8), fatigue or drowsiness (RR 1.14; 95% CI 0.45 to 2.87) (Analysis 8.9), headache (RR 1.02; 95% CI 0.30 to 3.42) (Analysis 8.10) and body weakness or debility (RR 0.91; 95% CI 0.38 to 2.18) (Analysis 8.11) (Monto 1995).
There was no amantadine trial selected for this comparison in the elderly.
Additional outcome (children plus the elderly)
Rimantadine compared to control (placebo) in the prophylaxis of influenza A in children and the elderly
Originally in the protocol we planned only to make the above 12 comparisons. However, whilst analysing the data we considered doing an additional comparison and put the two age groups together. As the small samples studied in rimantadine trials for prophylaxis might have influenced the observed results, we tried to overcome this limitation by combining the trials with rimantadine in children and in the elderly. Rimantadine had no proven effect in preventing influenza in either age group but could be effective when we combined the results from both groups. However, it must be stressed that extraneous characteristics between those groups, other than age or previous immunisations, may have occurred, impairing generalisation of these results. There were five studies selected for this comparison with 156 patients in the treatment group and 125 in the placebo control group (Clover 1986; Clover 1991; Crawford 1988; Monto 1995; Patriarca 1984). The combination of the trials showed a protective effect of rimantadine in preventing influenza A (281 participants, RR 0.49; 95% CI 0.27 to 0.92) (Analysis 9.1).
The baseline risk of influenza A was 0.22, calculated on the basis of the CGR. The NNTB was 9.09 (95% CI 6.25 to 50). We should emphasise that the follow‐up period ranged from 3 to 11 weeks.
The following secondary outcomes appeared in the protocol but in the end we did not consider them in the analysis, as they were not reported in the included trials: patients' well‐being, admission to hospital, general practitioner (GP) visits and other drugs used. We could not analyse deaths. Although cited by Monto 1995, they were included among other causes of withdrawal.
Discussion
Summary of main results
We used a comprehensive search strategy and made every effort to identify relevant studies. In the majority of our comparisons, drawing definitive conclusions was impaired by the small number of studies and participants. The studies demonstrated a decreased incidence of influenza A in children using amantadine during a period ranging from 14 to 18 weeks. The number needed to treat to benefit (NNTB) indicates that for every nine to 17 children receiving amantadine, one case of influenza A can be prevented.
Rimantadine had no proven effect in preventing influenza in either age group but could be effective when we combined the results of both groups. Nevertheless, any inferences from combining these groups must be treated with considerable caution, as they are different clinical groups combined with a small number of studies. Extraneous characteristics between those groups, other than age or previous immunisations, may also have occurred impairing generalisation of these results. Multiple comparisons should also be taken into account in the interpretation of these results.
When amantadine and rimantadine were combined, they appeared to prevent the occurrence of fever on day three in children. However, when analysed separately, this effect was confirmed only for rimantadine. It must be emphasised that there was just one rimantadine trial selected for this outcome (Hall 1987), in which the baseline risk for the occurrence of fever on day three was 38%. For every five children (ranging from three to 25) treated with rimantadine in this unique small sample, it would be possible to prevent one case of fever on day three of treatment.
Overall completeness and applicability of evidence
It could be suggested that amantadine is well tolerated by children, as its use was not related to an increase in the occurrence of the analysed adverse effects. Nevertheless, it may be difficult to distinguish between an adverse effect of the drug and a clinical manifestation of influenza itself. The outcomes muscular pain, headache, malaise, diarrhoea and nausea/vomiting may be adverse effects of amantadine as well as clinical manifestations of influenza in children (MS 2006). In the same way, the outcome dyspnoea (as in Kitamoto 1968) may also occur due to other respiratory diseases, such as asthma, since an asthmatic episode may be triggered by respiratory viruses. So we must emphasise that adverse effects of the drug and clinical manifestations of influenza may had been confounded, since the selected trials were carried out in ill children.
Rimantadine, administered exclusively on a prophylactic basis, was not related to an increase in the occurrence of the analysed adverse effects. In contrast to amantadine studies, just nausea/vomiting could be confounded with influenza manifestations. The other adverse effects could not be confounded, as two of the three selected studies were about prophylaxis and were conducted in children without influenza (Clover 1986; Crawford 1988). The third study was the only one carried out in children with influenza (Hall 1987). Cerebellar ataxia and nausea/vomiting were the studied adverse effects in this trial. Cerebellar ataxia could not be confounded as it had not been described as an influenza manifestation. Cases of nausea/vomiting, which were also cited by Crawford, could have been confounded with influenza manifestations in Hall's article. The side effects nausea/vomiting were described in two studies (Crawford 1988; Hall 1987), while all the other adverse effects were mentioned in just one: diarrhoea, dizziness, hyperreactivity, tinnitus (Crawford 1988), gastrointestinal (GI) symptoms, central nervous system (CNS) symptoms, changes in behaviour (Clover 1986), and cerebellar ataxia (Hall 1987). Rimantadine also was considered to be well tolerated by the elderly, since it was not related to an increase in the incidence of adverse effects in this age group. However, the studied samples were even smaller in the elderly than in the children's age group and this fact may have influenced our results (Monto 1995; Patriarca 1984).
When analysing the adverse reactions to the antivirals, we could not even try to overcome the limitation of the small number of articles and the small samples studied by combining the results of both age groups, as the trial authors had described different outcomes (Clover 1986; Crawford 1988; Hall 1987; Kitamoto 1968; Kitamoto 1971; Monto 1995; Patriarca 1984).
Comparison of different doses of antiviral drugs was available only for rimantadine and was tested in only one study related to the elderly group. There was no selected trial regarding the treatment either in children or in participants using amantadine in both age groups. Both doses were shown to be comparable in the prophylaxis of influenza as well as in the occurrence of adverse effects, with no proven efficacy (Monto 1995).
Data for comparison to other antivirals were available just for rimantadine and zanamivir for prophylaxis of influenza A in the elderly group. This fact allowed a comparison of drugs of the two different classes of antivirals: M2 ion channel inhibitors and neuraminidase inhibitors. Zanamivir more effectively prevented influenza A in the elderly group (Gravenstein 2005; Schilling 1998).
These antivirals proved to be effective prophylactics against influenza in the 1968 Hong Kong pandemic and in the 1977 pandemic‐like event 'Russian influenza'. Although the same resistance marker (Ser31Asn) was present in two isolates of influenza A (H5N1) obtained from patients in China in 2003 and in one lineage of avian and human H5N1 viruses in Thailand, Vietnam and Cambodia, most tested isolates from a second lineage that had been circulating in Indonesia, China, Mongolia, Russia and Turkey appear to be sensitive to amantadine (Hayden 2005; Li 2014). Furthermore, the next pandemic virus may be one that, like H2N2, is susceptible to this class of drug. If the circulating strain were known to be susceptible to M2 inhibitors, these drugs would offer a less costly alternative to other antivirals (neuraminidase inhibitors) for prophylaxis against influenza.
Quality of the evidence
We selected a total of 12 randomised controlled trials (RCTs) (2494 participants: 1586 children and adolescents and 908 elderly participants).
The main factors that affect the strength of evidence are the sparsity of data and the unclear risk of selection bias (Clover 1986; Clover 1991; Crawford 1988; Finklea 1967; Gravenstein 2005; Kitamoto 1968; Kitamoto 1971; Monto 1995; Patriarca 1984; Schilling 1998). We classified two of these studies, both in the elderly, as high risk of bias because of incomplete outcome data (Monto 1995; Patriarca 1984) and a high probability of detection bias (Monto 1995). We considered two trials, both in children and adolescents, to have a low risk of bias (Hall 1987; Payler 1984).
Potential biases in the review process
The use of unpublished data, obtained in electronic correspondence with two of the 12 contact trial authors (Hall 1987; Payler 1984), was the only identified potential bias in this review process.
Agreements and disagreements with other studies or reviews
Although another Cochrane review carried out in adults showed that both amantadine and rimantadine are efficacious and safe in the prophylaxis and treatment of influenza A symptoms (Jefferson 2006b), we could not reach the same conclusion in children and the elderly, except for prophylaxis with amantadine in children. This antiviral was effective in preventing influenza A in children. As in the adults review, rimantadine shortens the duration of fever in children. In 2012, the editorial team considered that the question addressed by this Cochrane review dealing with adults was no longer relevant to decision‐making, as amantadine and rimantadine for influenza A in adults had been replaced by neuraminidase inhibitors and were no longer used.
The M2 ion channel inhibitors are increasingly subject to viral resistance (Goodman 2006; Sleeman 2013). Nevertheless, we consider that in these two especially vulnerable age groups, we should continue to assess the susceptibility of any influenza A outbreak virus to all antiviral drugs, as they may be the first line of defence before an effective vaccine becomes available (Sleeman 2013).
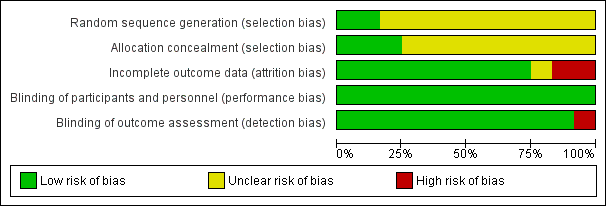
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Amantadine or rimantadine compared to placebo or acetaminophen in the treatment of influenza A in children, Outcome 1 Fever day 3.

Comparison 1 Amantadine or rimantadine compared to placebo or acetaminophen in the treatment of influenza A in children, Outcome 2 Malaise day 6.

Comparison 1 Amantadine or rimantadine compared to placebo or acetaminophen in the treatment of influenza A in children, Outcome 3 Cough day 7.

Comparison 1 Amantadine or rimantadine compared to placebo or acetaminophen in the treatment of influenza A in children, Outcome 4 Conjunctivitis day 5.

Comparison 1 Amantadine or rimantadine compared to placebo or acetaminophen in the treatment of influenza A in children, Outcome 5 Eye symptoms day 5 (pain on movement and visual distortion).
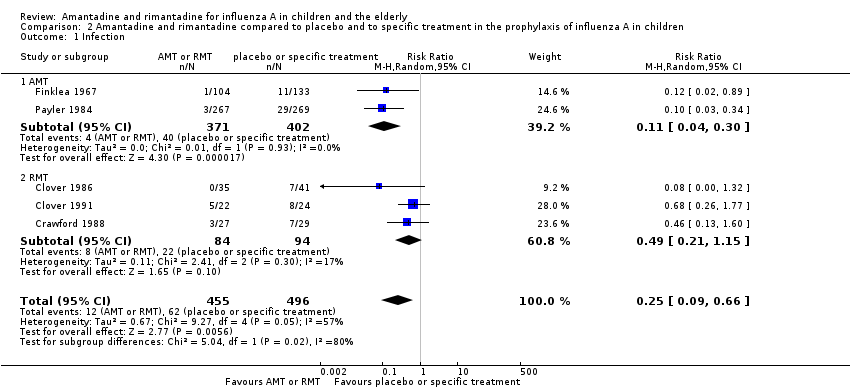
Comparison 2 Amantadine and rimantadine compared to placebo and to specific treatment in the prophylaxis of influenza A in children, Outcome 1 Infection.

Comparison 3 Amantadine and rimantadine compared to placebo in the prophylaxis of influenza A in the elderly, Outcome 1 RMT (proved and clinical infection).

Comparison 3 Amantadine and rimantadine compared to placebo in the prophylaxis of influenza A in the elderly, Outcome 2 RMT Monto (100 + 200) and Patriarca.
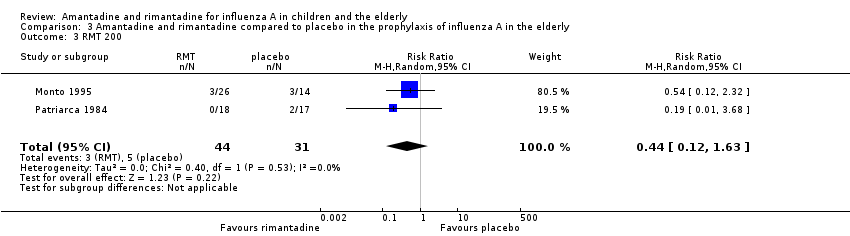
Comparison 3 Amantadine and rimantadine compared to placebo in the prophylaxis of influenza A in the elderly, Outcome 3 RMT 200.

Comparison 3 Amantadine and rimantadine compared to placebo in the prophylaxis of influenza A in the elderly, Outcome 4 RMT 100.

Comparison 4 Use of different doses of rimantadine for prophylaxis and treatment of influenza A in the elderly, Outcome 1 Clinical and laboratory infection.
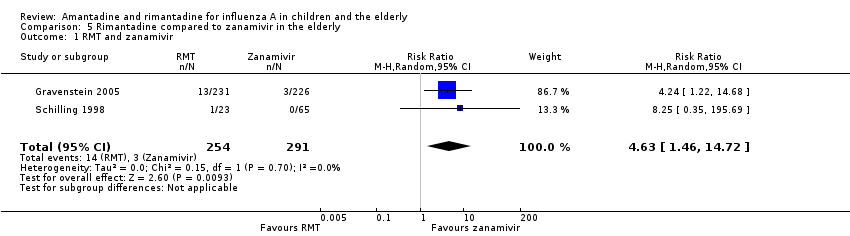
Comparison 5 Rimantadine compared to zanamivir in the elderly, Outcome 1 RMT and zanamivir.

Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 1 Diarrhoea.
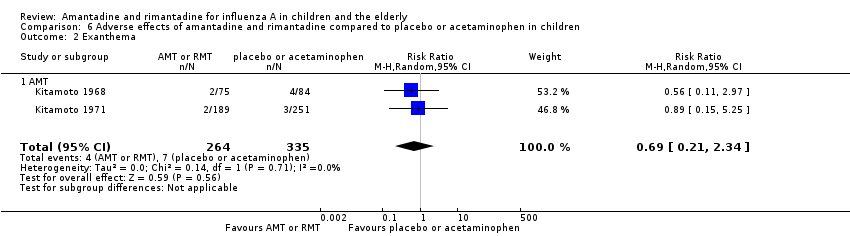
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 2 Exanthema.

Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 3 Muscular, limb pain.

Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 4 Headache.
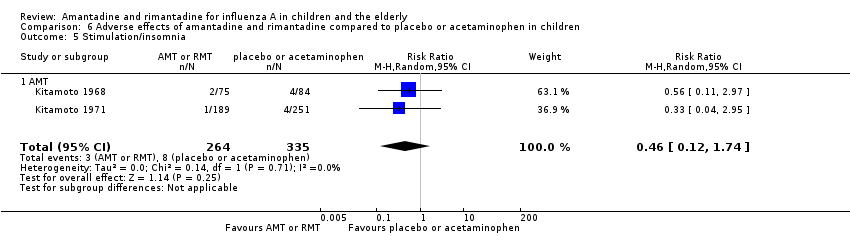
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 5 Stimulation/insomnia.
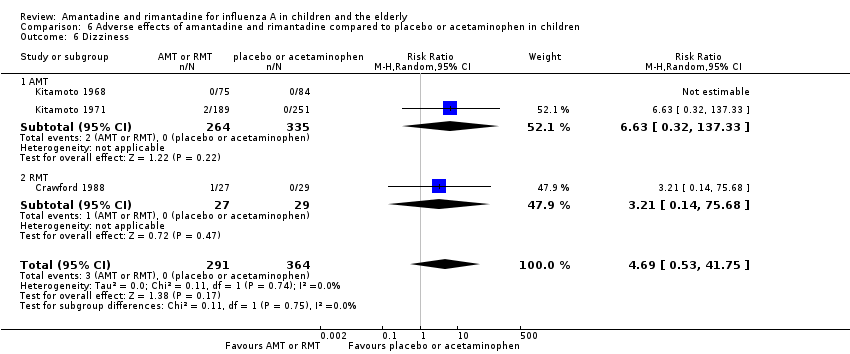
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 6 Dizziness.
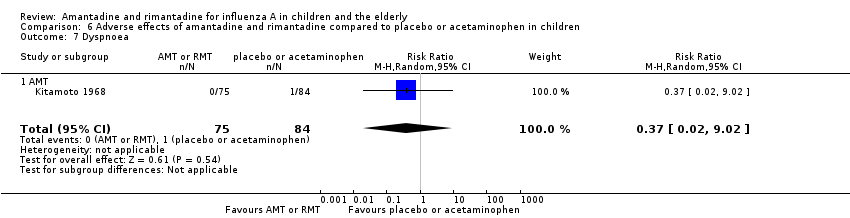
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 7 Dyspnoea.

Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 8 Central nervous system symptoms.
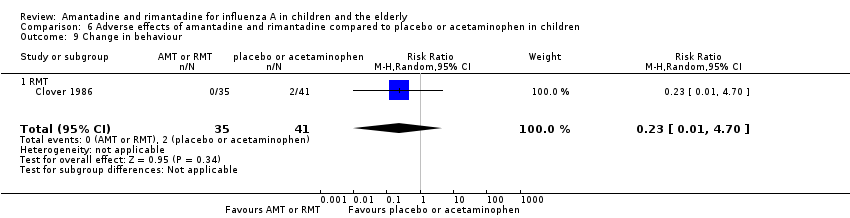
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 9 Change in behaviour.

Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 10 Gastrointestinal symptoms.

Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 11 Hyperreactivity.

Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 12 Tinnitus.
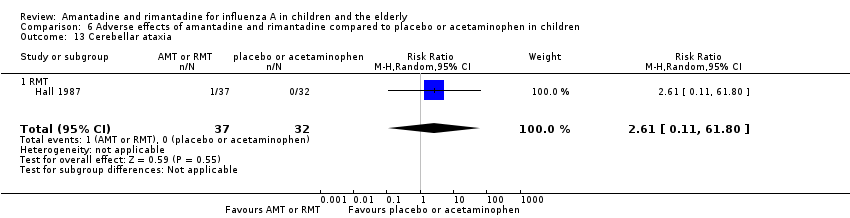
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 13 Cerebellar ataxia.

Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 14 Malaise.
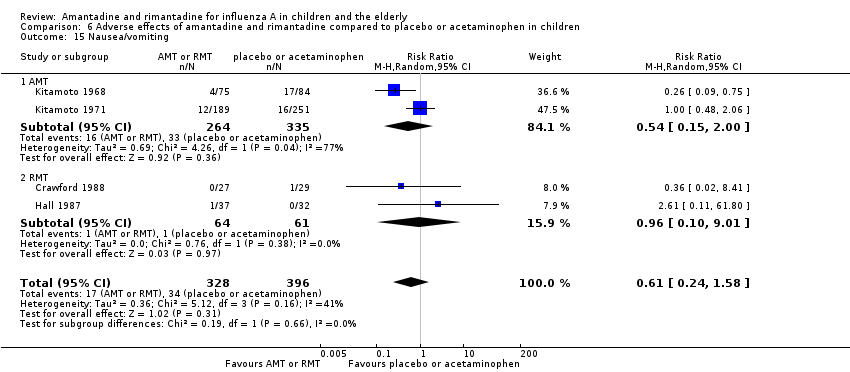
Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 15 Nausea/vomiting.

Comparison 6 Adverse effects of amantadine and rimantadine compared to placebo or acetaminophen in children, Outcome 16 Arrhythmia.
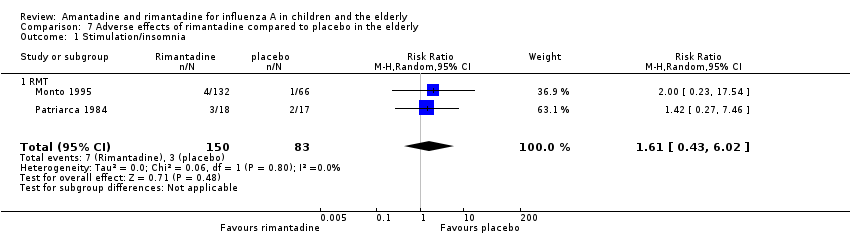
Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 1 Stimulation/insomnia.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 2 Confusion.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 3 Fatigue.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 4 Vomiting.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 5 Headache.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 6 Impaired concentration.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 7 Rash or allergic reaction.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 8 Seizures or clonic twitching.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 9 Dry mouth.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 10 Dizziness.
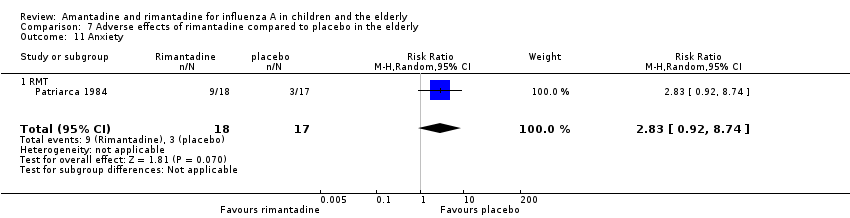
Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 11 Anxiety.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 12 Nausea.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 13 Depression.

Comparison 7 Adverse effects of rimantadine compared to placebo in the elderly, Outcome 14 Loss of appetite.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 1 Confusion.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 2 Depression.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 3 Impaired concentration.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 4 Insomnia or sleeplessness.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 5 Loss of appetite.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 6 Rash or allergic reaction.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 7 Seizure or clonic twitching.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 8 Dry mouth.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 9 Fatigue and drowsiness.

Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 10 Headache.
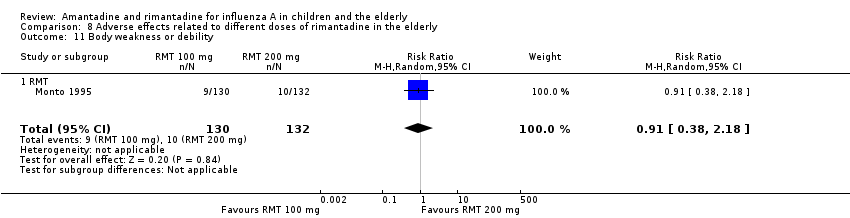
Comparison 8 Adverse effects related to different doses of rimantadine in the elderly, Outcome 11 Body weakness or debility.
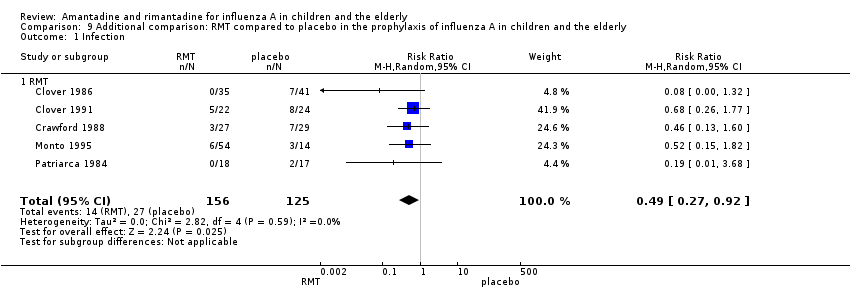
Comparison 9 Additional comparison: RMT compared to placebo in the prophylaxis of influenza A in children and the elderly, Outcome 1 Infection.
| Amantadine compared with placebo for prevention and treatment of influenza A in children | ||||||
| Patient or population: children with no influenza A infection (prevention) or with influenza A infection (treatment) Settings: all Intervention: amantadine Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Amantadine | |||||
| Cases of influenza A during prophylaxis (follow‐up:14 to 18 weeks) | Medium risk population | RR 0.11 (0.04 to 0.3) | 773 | ⊕⊕⊝⊝ | ||
| 10 per 100 | 1 per 100 | |||||
| Fever after initiation of treatment (follow‐up: 3 days) | Medium risk population | RR 0.37 (0.08 to 1.75) | 104 | ⊕⊕⊝⊝ | ||
| 23 per 100 | 9 per 100 | |||||
| Cough after initiation of treatment | See comment | See comment | Not estimable | 0 (0) | See comment | No selected trial |
| Dizziness (follow‐up: 7 days) | Medium risk population | RR 6.63 (0.32 to 137.33) | 599 | ⊕⊝⊝⊝ | ||
| 0 per 100 | 0 per 100 | |||||
| Nausea/vomiting (follow‐up: 7 days) | Medium risk population | RR 0.54 (0.15 to 2) | 599 | ⊕⊝⊝⊝ | ||
| 13 per 100 | 7 per 100 | |||||
| Stimulation/insomnia (follow‐up: 7 days) | Medium risk population | RR 0.46 (0.12 to 1.74) | 599 | ⊕⊕⊝⊝ | ||
| 3 per 100 | 7 per 100 | |||||
| CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| *The basis for the assumed risk (e.g. median control group risk across studies) was calculated on the basis of control event rate. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 1Allocation concealment not used or unclear. 2Sparse data. 3Allocation concealment unclear. 4Sparse data, confidence intervals do not rule out potential for null effect or harm. 5High heterogeneity unexplained. | ||||||
| Rimantadine compared with placebo for prevention and treatment of influenza A in children | ||||||
| Patient or population: children with no influenza A infection (prevention) or with influenza A infection (treatment) Settings: any Intervention: rimantadine Comparison: control (placebo or acetaminophen) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Rimantadine | |||||
| Cases of influenza A during prophylaxis (follow‐up: 1 to 35 days) | Medium risk population | RR 0.49 (0.21 to 1.15) | 178 | ⊕⊕⊝⊝ | ||
| 24 per 100 | 12 per 100 | |||||
| Fever after initiation of treatment (follow‐up: 3 days) | Medium risk population | RR 0.36 (0.14 to 0.91) | 69 | ⊕⊕⊕⊝ | ||
| 38 per 100 | 14 per 100 | |||||
| Cough after initiation of treatment (follow‐up: 7 days) | Medium risk population | RR 0.83 (0.63 to 1.1) | 69 | ⊕⊕⊕⊝ | ||
| 81 per 100 | 67 per 100 | |||||
| Dizziness (follow‐up: 35 days) | Medium risk population | RR 3.21 (0.14 to 75.68) | 56 | ⊕⊝⊝⊝ | ||
| 0 per 100 | 0 per 100 | |||||
| Nausea/vomiting (follow‐up: 7 to 35 days) | Medium risk population | RR 0.96 (0.1 to 9.01) | 125 | ⊕⊕⊝⊝ | ||
| 2 per 100 | 2 per 100 | |||||
| Stimulation/insomnia | See comment | See comment | Not estimable | 0 | See comment | No selected trial |
| CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| *The basis for the assumed risk (e.g. median control group risk across studies) was calculated on the basis of control event rate. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 1Allocation concealment unclear. 2Sparse data and confidence intervals do not rule out the potential for no effect or harm | ||||||
| Amantadine compared with placebo for prevention and treatment of influenza A in the elderly | ||||||
| Patient or population: elderly people with no influenza A infection (prevention) or with influenza A infection (treatment) Settings: any Intervention: amantadine Comparison: control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Amantadine | |||||
| Cases of influenza A during prophylaxis | See comment | Not estimable | 0 | See comment | No selected trial | |
| Fever after initiation of treatment | See comment | Not estimable | 0 | See comment | No selected trial | |
| Cough after initiation of treatment | See comment | Not estimable | 0 | See comment | No selected trial | |
| Dizziness | See comment | Not estimable | 0 | See comment | No selected trial | |
| Nausea | See comment | Not estimable | 0 | See comment | No selected trial | |
| Vomiting | See comment | Not estimable | 0 | See comment | No selected trial | |
| Stimulation/insomnia | See comment | Not estimable | 0 | See comment | No selected trial | |
| Patient or population: elderly people with no influenza A infection (prevention) or with influenza A infection (treatment) Settings: any Intervention: rimantadine Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Rimantadine | |||||
| Cases of influenza A during prophylaxis | Medium risk population | RR 0.45 (0.14 to 1.41) | 103 | ⊕⊝⊝⊝ | ||
| 17per 100 | 7 per 100 | |||||
| Fever after initiation of treatment | See comment | 0 | See comment | See comment | No selected trial | |
| Cough after initiation of treatment | See comment | 0 | See comment | See comment | No selected trial | |
| Dizziness (follow‐up: 12 weeks) | Medium risk population | |||||
| 12 per 100 | 11 per 100 (2 to 70) | RR 0.94 | 35 (1) | ⊕⊕⊝⊝ | ||
| Nausea (follow‐up: 8 to 12 weeks) | Medium risk population | RR 1.99 (0.45 to 8.75) | 233 | ⊕⊝⊝⊝ | ||
| 8 per 100 | 15 per 100 | |||||
| Vomiting (follow‐up: 8 to 12 weeks) | Medium risk population | RR 0.99 (0.38 to 2.6) | 233 | ⊕⊕⊝⊝ | ||
| 7 per 100 | 7 per 100 | |||||
| Stimulation/insomnia (follow‐up: 8 to 12 weeks) | Medium risk population | RR 1.61 (0.43 to 6.02) | 233 | ⊕⊕⊝⊝ | ||
| 7 per 100 | 11 per 100 | |||||
| CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| *The basis for the assumed risk (e.g. median control group risk across studies) was calculated on the basis of control event rate. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 1Allocation concealment unclear and 1 study had high withdrawal rate. 2Sparse data and confidence interval do not rule out no effect or harm. 3Allocation concealment unclear 4High heterogeneity unexplained. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fever day 3 Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 AMT | 2 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.08, 1.75] |
| 1.2 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.14, 0.91] |
| 2 Malaise day 6 Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.63, 1.70] |
| 3 Cough day 7 Show forest plot | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.63, 1.10] |
| 3.1 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.63, 1.10] |
| 4 Conjunctivitis day 5 Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.01, 3.49] |
| 5 Eye symptoms day 5 (pain on movement and visual distortion) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.10, 3.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Infection Show forest plot | 5 | 951 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.09, 0.66] |
| 1.1 AMT | 2 | 773 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.04, 0.30] |
| 1.2 RMT | 3 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.21, 1.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 RMT (proved and clinical infection) Show forest plot | 3 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.13, 4.07] |
| 2 RMT Monto (100 + 200) and Patriarca Show forest plot | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.14, 1.41] |
| 3 RMT 200 Show forest plot | 2 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.12, 1.63] |
| 4 RMT 100 Show forest plot | 2 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.10, 21.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical and laboratory infection Show forest plot | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.21, 4.20] |
| 1.1 RMT | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.21, 4.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 RMT and zanamivir Show forest plot | 2 | 545 | Risk Ratio (M‐H, Random, 95% CI) | 4.63 [1.46, 14.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Diarrhoea Show forest plot | 3 | 655 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.42, 1.47] |
| 1.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.43, 1.53] |
| 1.2 RMT | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.02, 8.41] |
| 2 Exanthema Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.21, 2.34] |
| 2.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.21, 2.34] |
| 3 Muscular, limb pain Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.46, 1.59] |
| 3.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.46, 1.59] |
| 4 Headache Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.52, 1.03] |
| 4.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.52, 1.03] |
| 5 Stimulation/insomnia Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.12, 1.74] |
| 5.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.12, 1.74] |
| 6 Dizziness Show forest plot | 3 | 655 | Risk Ratio (M‐H, Random, 95% CI) | 4.69 [0.53, 41.75] |
| 6.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 6.63 [0.32, 137.33] |
| 6.2 RMT | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 3.21 [0.14, 75.68] |
| 7 Dyspnoea Show forest plot | 1 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 9.02] |
| 7.1 AMT | 1 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 9.02] |
| 8 Central nervous system symptoms Show forest plot | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 4.70] |
| 8.1 RMT | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 4.70] |
| 9 Change in behaviour Show forest plot | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 4.70] |
| 9.1 RMT | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 4.70] |
| 10 Gastrointestinal symptoms Show forest plot | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.08, 18.05] |
| 10.1 RMT | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.08, 18.05] |
| 11 Hyperreactivity Show forest plot | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.02, 8.41] |
| 11.1 RMT | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.02, 8.41] |
| 12 Tinnitus Show forest plot | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 3.21 [0.14, 75.68] |
| 12.1 RMT | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 3.21 [0.14, 75.68] |
| 13 Cerebellar ataxia Show forest plot | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 2.61 [0.11, 61.80] |
| 13.1 RMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 2.61 [0.11, 61.80] |
| 14 Malaise Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.41, 1.96] |
| 14.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.41, 1.96] |
| 15 Nausea/vomiting Show forest plot | 4 | 724 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.24, 1.58] |
| 15.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.15, 2.00] |
| 15.2 RMT | 2 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.10, 9.01] |
| 16 Arrhythmia Show forest plot | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 AMT | 2 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Stimulation/insomnia Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.43, 6.02] |
| 1.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.43, 6.02] |
| 2 Confusion Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.40, 1.56] |
| 2.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.40, 1.56] |
| 3 Fatigue Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.41, 1.60] |
| 3.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.41, 1.60] |
| 4 Vomiting Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.38, 2.60] |
| 4.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.38, 2.60] |
| 5 Headache Show forest plot | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.21, 3.38] |
| 5.1 RMT | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.21, 3.38] |
| 6 Impaired concentration Show forest plot | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.10, 2.41] |
| 6.1 RMT | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.10, 2.41] |
| 7 Rash or allergic reaction Show forest plot | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 3.53 [0.18, 67.28] |
| 7.1 RMT | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 3.53 [0.18, 67.28] |
| 8 Seizures or clonic twitching Show forest plot | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.23, 17.54] |
| 8.1 RMT | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.23, 17.54] |
| 9 Dry mouth Show forest plot | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.7 [0.23, 2.12] |
| 9.1 RMT | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.7 [0.23, 2.12] |
| 10 Dizziness Show forest plot | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.15, 5.97] |
| 10.1 RMT | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.15, 5.97] |
| 11 Anxiety Show forest plot | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 2.83 [0.92, 8.74] |
| 11.1 RMT | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 2.83 [0.92, 8.74] |
| 12 Nausea Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.45, 8.75] |
| 12.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.45, 8.75] |
| 13 Depression Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.53, 4.98] |
| 13.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.53, 4.98] |
| 14 Loss of appetite Show forest plot | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.56, 2.17] |
| 14.1 RMT | 2 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.56, 2.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Confusion Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.41, 1.65] |
| 1.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.41, 1.65] |
| 2 Depression Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.12, 1.65] |
| 2.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.12, 1.65] |
| 3 Impaired concentration Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.11, 3.98] |
| 3.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.11, 3.98] |
| 4 Insomnia or sleeplessness Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.97] |
| 4.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.26, 3.97] |
| 5 Loss of appetite Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.27, 1.46] |
| 5.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.27, 1.46] |
| 6 Rash or allergic reaction Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.04, 3.21] |
| 6.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.04, 3.21] |
| 7 Seizure or clonic twitching Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.07] |
| 7.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.07] |
| 8 Dry mouth Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.43, 3.11] |
| 8.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.43, 3.11] |
| 9 Fatigue and drowsiness Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.45, 2.87] |
| 9.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.45, 2.87] |
| 10 Headache Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.30, 3.42] |
| 10.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.30, 3.42] |
| 11 Body weakness or debility Show forest plot | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.38, 2.18] |
| 11.1 RMT | 1 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.38, 2.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Infection Show forest plot | 5 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.27, 0.92] |
| 1.1 RMT | 5 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.27, 0.92] |

