Antibiotik untuk eksaserbasi asma
Abstract
Background
Asthma is a chronic respiratory condition that affects over 300 million adults and children worldwide. It is characterised by wheeze, cough, chest tightness, and shortness of breath. Symptoms typically are intermittent and may worsen over a short time, leading to an exacerbation. Asthma exacerbations can be serious, leading to hospitalisation or even death in rare cases. Exacerbations may be treated by increasing an individual's usual medication and providing additional medication, such as oral steroids. Although antibiotics are sometimes included in the treatment regimen, bacterial infections are thought to be responsible for only a minority of exacerbations, and current guidance states that antibiotics should be reserved for cases in which clear signs, symptoms, or laboratory test results are suggestive of bacterial infection.
Objectives
To determine the efficacy and safety of antibiotics in the treatment of asthma exacerbations.
Search methods
We searched the Cochrane Airways Trials Register, which contains records compiled from multiple electronic and handsearched resources. We also searched trial registries and reference lists of primary studies. We conducted the most recent search in October 2017.
Selection criteria
We included studies comparing antibiotic therapy for asthma exacerbations in adults or children versus placebo or usual care not involving an antibiotic. We allowed studies including any type of antibiotic, any dose, and any duration, providing the aim was to treat the exacerbation. We included parallel studies of any duration conducted in any setting and planned to include cluster trials. We excluded cross‐over trials. We included studies reported as full‐text articles, those published as abstracts only, and unpublished data.
Data collection and analysis
At least two review authors screened the search results for eligible studies. We extracted outcome data, assessed risk of bias in duplicate, and resolved discrepancies by involving another review author. We analysed dichotomous data as odds ratios (ORs) or risk differences (RDs), and continuous data as mean differences (MDs), all with a fixed‐effect model. We described skewed data narratively. We graded the results and presented evidence in 'Summary of findings' tables for each comparison. Primary outcomes were intensive care unit/high dependence unit (ICU/HDU) admission, duration of symptoms/exacerbations, and all adverse events. Seconday outcomes were mortality, length of hospital admission, relapse after index presentation, and peak expiratory flow rate (PEFR).
Main results
Six studies met our inclusion criteria and included a total of 681 adults and children with exacerbations of asthma. Mean age in the three studies in adults ranged from 36.2 to 41.2 years. The three studies in children applied varied inclusion criteria, ranging from one to 18 years of age. Five studies explicitly excluded participants with obvious signs and symptoms of bacterial infection (i.e. those clearly meeting current guidance to receive antibiotics). Four studies investigated macrolide antibiotics, and two studies investigated penicillin (amoxicillin and ampicillin) antibiotics; both studies using penicillin were conducted over 35 years ago. Five studies compared antibiotics versus placebo, and one was open‐label. Study follow‐up ranged from one to twelve weeks. Trials were of varied methodological quality, and we were able to perform only limited meta‐analysis.
None of the included trials reported ICU/HDU admission, although one participant in the placebo group of a study including children with status asthmaticus experienced a respiratory arrest and was ventilated. Four studies reported asthma symptoms, but we were able to combine results for only two macrolide studies of 416 participants; the MD in diary card symptom score was ‐0.34 (95% confidence interval (CI) ‐0.60 to ‐0.08), with lower scores (on a 7 point scale) denoting improved symptoms. Two macrolide studies reported symptom‐free days. One study of 255 adults authors reported the percentage of symptom‐free days at 10 days as 16% in the antibiotic group and 8% in the placebo group. In a further study of 40 children study authors reported significantly more symptom‐free days at all time points in the antibiotic group compared with the usual care group. The same study reported the duration in days of the index asthma exacerbation, again favouring the antibiotic group. One study of a penicillin including 69 participants reported asthma symptoms at hospital discharge; the between‐group difference for both studies was reported as non‐significant.
We combined data for serious adverse events from three studies involving 502 participants, but events were rare; the three trials reported only 10 events: five in the antibiotic group and five in the placebo group. We combined data for all adverse events (AEs) from three studies, but the effect estimate is imprecise (OR 0.99, 95% CI 0.69 to 1.43). No deaths were reported in any of the included studies.
Two studies investigating penicillins reported admission duration; neither study reported a between‐group difference. In one study (263 participants) of macrolides, two participants in each arm were reported as experiencing a relapse, defined as a further exacerbation, by the six‐week time points. We combined PEFR endpoint results at 10 days for two macrolide studies; the result favoured antibiotics over placebo (MD 23.42 L/min, 95% CI 5.23 to 41.60). One study in children reported the maximum peak flow recorded during the follow‐up period, favouring the clarithromycin group, but the confidence interval includes no difference (MD 38.80, 95% CI ‐11.19 to 88.79).
Grading of outcomes ranged from moderate to very low quality, with quality of outcomes downgraded for suspicion of publication bias, indirectness, imprecision, and poor methodological quality of studies.
Authors' conclusions
We found limited evidence that antibiotics given at the time of an asthma exacerbation may improve symptoms and PEFR at follow‐up compared with standard care or placebo. However, findings were inconsistent across the six heterogeneous studies included, two of the studies were conducted over 30 years ago and most of the participants included in this review were recruited from emergency departments, limiting the applicability of findings to this population. Therefore we have limited confidence in the results. We found insufficient evidence about several patient‐important outcomes (e.g. hospital admission) to form conclusions. We were unable to rule out a difference between groups in terms of all adverse events, but serious adverse events were rare.
PICOs
Ringkasan bahasa mudah
Adakah antibiotik selamat dan berkesan sebagai rawatan tambahan untuk eksaserbasi asma?
Latar belakang kepada soalan
Asma adalah keadaan pernafasan jangka panjang lazim yang mempengaruhi orang dewasa dan kanak‐kanak di seluruh dunia. Individu‐individu mungkin mengalami gejala‐gejala yang semakin teruk dalam tempoh yang singkat, yang sering dikenali sebagai eksaserbasi (atau serangan asma). Eksaserbasi biasanya dirawat dengan meningkatkan ubat seseorang (seperti memberi tablet steroid untuk beberapa hari). Kadang kala eksaserbasi boleh dicetus oleh jangkitan seperti virus. Kadang kala, jangkitan bakteria di paru‐paru atau saluran udara mungkin menyebabkan eksaserbasi. Gejala jangkitan bakteria termasuklah berbunyi di dada, demam, dan batuk yang mengeluarkan isipadu besar kahak yang berwarna. Jangkitan bakteria boleh disahkan oleh ujian makmal, sebagai contoh, ujian darah; walau bagaimanapun, ini tidak selalu tersedia di penjagaan primer (di GP). Jangkitan bakteria mungkin memerlukan rawatan dengan antibiotik.
Di dalam ulasan ini, kami ingin mengetahui sama ada antibiotik berguna dan selamat untuk orang dengan eksaserbasi asma. Sebahagian daripada motivasi untuk ulasan ini adalah kebimbangan bahawa antibiotik mungkin terlebih preskripsi untuk orang dengan eksaserbasi asma.
Ciri‐ciri kajian
Kami mencari kajian‐kajian yang membandingkan sekumpulan orang yang diberi sebarang jenis atau dos antibiotik dengan sekumpulan orang yang tidak diberi antibiotik untuk eksaserbasi. Kami hanya memasukkan kajian‐kajian di mana telah ditentukan secara kebetulan siapa yang akan menerima antibiotik. Kami memasukkan kajian orang dewasa dan kanak‐kanak yang dijalankan pada bila‐bila masa dan di mana sahaja di dunia.
Keputusan utama
Kami mendapati enam kajian yang melibatkan 681 orang dewasa dan kanak‐kanak dengan asma. Dua daripada kajian ini dijalankan lebih dari 35 tahun yang lalu.
Secara keseluruhan, kami mendapati sedikit bukti yang menunjukkan antibiotik boleh memperbaiki gejala dan keputusan ujian pernafasan berbanding tanpa antibiotik. Kami tidak pasti tentang keputusan ini kerana hanya sebilangan kecil kajian dan orang yang dimasukkan dalam ulasan kami. Salah satu hasil utama kami ‐ kemasukan ke unit penjagan intensif/unit rawatan separa rapi (ICU/HDU) ‐ tidak dilaporkan.
Kami juga tidak pasti jika orang yang diberi antibiotik mempunyai lebih banyak atau kurang peristiwa buruk (kesan sampingan). Hanya 10 orang (5 diberi antibiotik dan 5 diberi plasebo/tanpa antibiotik) daripada 502 mempunyai peristiwa buruk yang serius.
Kami tidak menemui banyak bukti tentang hasil penting lain, seperti kemasukan ke hospital atau eksaserbasi lain semasa tempoh susulan kajian.
Kajian terbaharu mendapati sukar untuk merekrut orang dengan asma kerana begitu banyak daripada mereka telah diberi antibiotik dan tidak dapat mengambil bahagian.
Kualiti bukti
Secara keseluruhan, kami berkeyakinan rendah terhadap bukti yang dibentangkan dalam ulasan ini. Kami fikir mungkin beberapa kajian antibiotik untuk eksaserbasi asma telah dijalankan tetapi tidak diterbitkan kerana kami menemui sedikit kajian mengenai soalan penting itu. Kami juga bimbang tentang sejauh mana penemuan kajian diaplikasi kepada semua orang dengan serangan asma kerana kebanyakan kajian yang kami dapati hanya merekrut orang‐orang di hospital dan jabatan kecemasan. Juga, dua kajian adalah kajian lama, dan rawatan asma telah banyak berubah dalam tempoh 30 tahun. Oleh kerana hanya beberapa kajian ditemui, dalam beberapa kes kami tidak tahu jika antibiotik lebih baik, lebih buruk atau sama seperti tanpa antibiotik. Akhirnya, kami punyai beberapa kebimbangan tentang perlaksanaan kajian, contohnya, dalam satu kajian kedua‐dua pesakit dan kakitangan kajian mengetahui siapa yang mendapat antibiotik dan yang tidak; ini mungkin mempengaruhi tingkah laku pesakit atau kakitangan.
Kesimpulan
Kami mendapati bukti yang sangat terhad bahawa antibiotik boleh membantu orang yang mengalami serangan asma, dan kami masih tidak pasti. Secara khusus, kami tidak banyak mendapat maklumat mengenai hasil penting seperti kemasukan hospital atau kesan sampingan. Walau bagaimanapun, kesan sampingan yang serius jarang berlaku dalam kajian‐kajian yang kami dapati.
Authors' conclusions
Summary of findings
| Antibiotics compared to placebo/usual care for acute asthma | ||||||
| Patient or population: acute asthma exacerbation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo/usual care | Risk with antibiotics | |||||
| ICU/HDU admission ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | One respiratory arrest in the placebo group in Shapiro 1974. No other studies reported this outcome |
| Symptom score at 10 days. Measured on a 7‐point scale (0 to 6) ; lower score denotes fewer symptoms | Mean symptom score at 10 days ranged from 2 to 2.20 points | MD 0.34 points lower (0.60 lower to 0.08 lower) | ‐ | (2 RCTs) | ⊕⊕⊕⊝a,d MODERATE | |
| All adverse events | 42 per 100 | 41 per 100 | OR 0.99 | 506 | ⊕⊕⊝⊝ | 2 studies in adults and 1 small old study in children with status asthmaticus |
| Serious adverse events Duration 3 days to 3 weeks | 2 per 100 | 2 per 100 | RD 0.00 | 502 | ⊕⊕⊝⊝ | Anticipated absolute effects were calculated using the figures in Figure 1. This is a re‐presentation of the results, but to 4 dp, which allows the calculation to be done |
| Mortality ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No deaths were reported in any of the studies |
| Length of hospital stay, days | Mean length of hospital stay was 2.6 days | MD 0.1 days lower | ‐ | 43 | ⊕⊝⊝⊝ | 1 study reported medians and IQRs and found no significant differences, although data were skewed |
| Relapse after index presentation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| PEFR (GIV) Duration 10 days | Mean PEFR (GIV) ranged from 19.6 to 26.9 L/min (mean difference from baseline) | MD 23.42 L/min (mean difference from baseline) higher | ‐ | 469 | ⊕⊕⊕⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| a‐1 indirectness. Studies mostly recruited from hospital or emergency department. Therefore this review may represent more severe exacerbations and does not apply to people attending the GP and requesting antibiotics. The review does not apply to people who have already received a course of antibiotics. bNo downgrade for risk of bias. One small study excluded 6 participants post hoc, but excluding this study from the meta‐analysis did not affect the results. cNo downgrade. I2 = 0. Different antibiotics were given in each study. dNo downgrade. Only six RCTs have been published on antibiotics for asthma exacerbation. This strongly suggests that unpublished data exist or that clinical trials are seriously lacking for this common intervention. e‐1 imprecision. Confidence intervals include the possibility of important benefit and risk of harm. f‐1 indirectness. Studies mostly recruited from hospital or emergency department. Therefore this review may represent more severe exacerbations and does not apply to people attending the GP and requesting antibiotics. The review does not apply to people who have already received a course of antibiotics. One small study recruited children with status asthmaticus in 1974, when asthma management was different. g‐1 imprecision. Few events. h‐1 risk of bias. Study before good reporting standards introduced. Concerns over study, which excluded six participants, and it is not clear from which arm they were excluded. i‐1 indirectness. Participants were all children with status asthmaticus, and the study was conducted before current asthma management had been introduced (e.g. they all received IV adrenaline). j‐1 imprecision. One small study was included. | ||||||

Forest plot of comparison: 1 Antibiotics versus placebo/usual care, outcome: 1.3 Serious adverse events.
Background
Description of the condition
Asthma is a common chronic, inflammatory condition that affects the airways. It has been estimated that more than 300 million people are affected by asthma worldwide (GAN 2014). The predominant symptom is wheeze, but people with asthma also experience cough, chest tightness, and shortness of breath. There is variation in the severity of symptoms, and typically symptoms are worse at night and in the early morning (GINA 2017). Asthma can be a seriously debilitating condition for adults and children and remains an important cause of mortality. However, the inflammatory airway changes that occur in asthma generally are very responsive to treatment and are reversible. Current first‐line treatment recommendations consist of controller (inhaled corticosteroids (ICSs) with or without long‐acting beta2‐agonists (LABAs)) and reliever medications (BTS/SIGN 2016).
An expert group proposed the following definition for an asthma exacerbation: "a worsening of asthma requiring the use of systemic corticosteroids to prevent a serious outcome" (Fuhlbrigge 2012). This statement equates an exacerbation to both worsening of symptoms and the subsequent need for treatment beyond the patient's routine medication. Further, exacerbations can be classified by their severity using a combination of patient history, examination findings, and vital signs (BTS/SIGN 2016). Exacerbations of asthma are generally acute with multiple possible underlying causes. Most exacerbations are likely to be multi‐factorial, with viral respiratory tract infections implicated in many cases (Jackson 2011). Only a minority of asthma exacerbations are thought to be triggered by bacteria, although evidence is somewhat limited and conflicting (Papadopoulos 2011).
Exacerbations of asthma can be severe and may require urgent treatment. If the exacerbation is severe, guidelines recommend the use of inhaled short‐acting beta2‐agonists (SABAs), systemic corticosteroid treatment (either oral or intravenous), and ipratropium bromide, and, in some cases, magnesium sulphate with supplementary oxygen for patients who are hypoxaemic. Antibiotics are recommended only when clear signs, symptoms, or laboratory test results are suggestive of bacterial infection (BTS/SIGN 2016).
Description of the intervention
"Antibiotics are a type of antimicrobial [that is] used in the treatment or prevention of bacterial infections" (Wikipedia). Macrolides are a class of antibiotic that also displays anti‐inflammatory properties, which may be of additional benefit in people with asthma.
The intervention under review is the administration of antibiotic agents by any means (e.g. intravenously (IV), orally) to patients who present to a healthcare provider with a diagnosis of an asthma exacerbation, in addition to any other treatment they might receive as part of their care. Antibiotics may be administered or prescribed in a primary care setting, if the exacerbation was not severe enough to warrant immediate hospital admission, or in an emergency department (ED) or inpatient setting in the context of a more severe exacerbation. The typical duration of antibiotic treatment for respiratory tract infection varies from five to ten days (Public Health England). Side effects are likely to be antibiotic specific and range from fairly common and mild (e.g. nausea) to rare and serious (e.g. anaphylaxis) (BNF).
How the intervention might work
Antibiotics act against bacteria and work through a bactericidal or bacteriostatic mechanism of action, either of which assists the body in clearing a bacterial infection (Kohanski 2010). If a bacterial infection is responsible for the exacerbation of asthma, then administration of an appropriate antibiotic may lead to a reduction in symptoms and faster recovery. Bacterial infections with atypical bacterial organisms such as Mycoplasma pneumoniae and Chlamydophila pneumoniae have been associated with acute exacerbations (Blasi 2007). Furthermore, a case has been made for the use of macrolides and potentially ketolides in acute exacerbations because of their concurrent anti‐inflammatory effects (Rollins 2010). However, in a large majority of cases, bacterial infection is not thought to be the underlying cause of the acute exacerbation of asthma; in these cases, the patient should derive little benefit from the administration of antibiotics (Papadopoulos 2011). Moreover, bacterial immunity to antibiotics is an increasing problem and can reduce the efficacy of antibiotic treatment for bacterial infection (Davies 2011).
Why it is important to do this review
Current guidance is clear that for people who present with an acute asthma exacerbation, use of antibiotics should not be routine, and that instead, antibiotics should be prescribed only if the patient's signs and symptoms, such as fever and purulent sputum, for example, suggest that a bacterial infection is present (BTS/SIGN 2016; Longmore 2014). The unnecessary use of antibiotics puts the patient at risk for antibiotic‐related adverse events and increases the probability of increasing antibiotic resistance ‐ a global concern (Davies 2011).
Evidence suggests that a significant number of clinicians are prescribing antibiotics far more widely for patients with an asthma exacerbation than just for those whose presentation suggests that they have a bacterial infection (Kozyrskyj 2006; Paul 2011; Vanderweil 2008). The apparent gulf between guidelines and actual clinical practice, the large numbers of patients treated with antibiotics for acute exacerbations of asthma, and the growing necessity of careful antibiotic stewardship, as well as cost considerations, all highlight the importance of providing a clear overview of the best available evidence on use of antibiotics for acute exacerbations of asthma.
This review aims to clarify the evidence around use of antibiotics in patients who present with an acute exacerbation of asthma. It is an update of a previous Cochrane review that was first published in 2001 and was most recently updated in 2005 (Graham 2001). To the best of our knowledge, no other, more recent reviews have examined this topic.
Objectives
To determine the efficacy and safety of antibiotics in the treatment of asthma exacerbations.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) that were individually randomised in design. Cluster‐randomised trials were eligible for inclusion, but we did not identify any that met our inclusion criteria. We included studies reported in full text, studies published as an abstract only, and unpublished data. We excluded cross‐over trials.
Types of participants
We included studies that recruited children and adults (aged 18 years or over) who presented to the emergency department, primary care, outpatient clinics, or inpatient wards with an asthma exacerbation. We included studies that involved inpatients (who had been admitted for their asthma exacerbation) and outpatients. When studies included participants from more than one setting, we included data from relevant settings if reported separately. We excluded studies that recruited participants with other respiratory diagnoses including pneumonia (confirmed by X‐ray or clinically diagnosed), chronic obstructive pulmonary disease (COPD), and bronchiectasis.
Types of interventions
We included studies comparing antibiotics with placebo or standard care, when standard care did not include an antibiotic. We included studies using intravenous or oral antibiotics, given at any dose and for any duration of treatment. Any co‐interventions were permitted, when they were not part of the randomised treatment (e.g. systemic steroids, inhaled steroids, long‐ or short‐acting beta2‐agonists; ipratropium bromide, magnesium preparations). We excluded studies of prophylactic antibiotics (i.e. not commenced specifically for treatment of an exacerbation).
Types of outcome measures
Primary outcomes
-
Intensive care unit/high dependence unit (ICU/HDU) admission
-
Duration of symptoms/exacerbation (as measured by trialists using, for example, diary cards, symptom scores, and assessments of the time taken to return to normal activities)
-
All adverse events/side effects
Secondary outcomes
-
Mortality
-
Length of hospital admission
-
Relapse after index presentation (as defined by trialists, for example, the need for (further) antibiotics, steroids, admission, or unscheduled healthcare visits)
-
Peak expiratory flow rate (PEFR) (change from baseline preferred)
Reporting in the study one or more of the outcomes listed here is not an inclusion criterion for the review.
Search methods for identification of studies
Electronic searches
We identified studies from the Cochrane Airways Trials Register, which is maintained by the Information Specialist for the Group. The Cochrane Airways Trials Register contains studies identified from several sources.
-
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) through the Cochrane Register of Studies Online (crso.cochrane.org).
-
Weekly searches of MEDLINE Ovid SP 1946 to date.
-
Weekly searches of Embase Ovid SP 1974 to date.
-
Monthly searches of PsycINFO Ovid SP 1967 to date.
-
Monthly searches of Cumulative Index to Nursing and Allied Health Literature (CINAHL) EBSCO 1937 to date.
-
Monthly searches of Allied and Complementary Medicine (AMED) EBSCO.
-
Handsearches of the proceedings of major respiratory conferences.
Studies contained in the Trials Register are identified through search strategies based on the scope of Cochrane Airways. Details of these strategies, as well as a list of handsearched conference proceedings, are provided in Appendix 1. See Appendix 2 for search terms used to identify studies for this review.
We searched the following trials registries.
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov).
-
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch).
We searched the Cochrane Airways Trials Register and additional sources from inception to October 2017, with no restriction on language of publication.
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references. We searched relevant manufacturers' websites for study information.
We searched for errata or retractions from included studies published in full text on PubMed on 10 November 2017.
Data collection and analysis
Selection of studies
Three review authors (of BS, SW, RN, and ED) independently screened titles and abstracts of the search results and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports of all potentially eligible studies, and three review authors (BS, SW, and RN) independently screened them for inclusion, recording reasons for exclusion of ineligible studies. We resolved disagreements through discussion. We identified and excluded duplicates and collated multiple reports of the same study, so that each study, rather than each report, is the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and 'Characteristics of excluded studies' table (Moher 2009).
Data extraction and management
We used a data collection form that had been piloted on at least one study in the review to record study characteristics and outcome data. Two review authors (BS and SW) extracted the following study characteristics from included studies.
-
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and locations, study setting, withdrawals, and date of study.
-
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, and exclusion criteria.
-
Interventions: intervention, comparison, concomitant medications, and excluded medications.
-
Outcomes: primary and secondary outcomes specified and collected and time points reported.
-
Notes: funding for studies and notable conflicts of interest of trial authors.
Three review authors (BS, SW, and RN) then independently extracted outcome data from included studies. We noted in the 'Characteristics of included studies' table if outcome data were not reported in a usable way. We resolved disagreements by reaching consensus or by involving a third person (Chris Cates, Statistical Editor). One review author (RN) transferred data into the Review Manager file (Review Manager (RevMan)). We then double‐checked that data had been entered correctly by comparing data presented in the systematic review with data provided in the study reports. A second review author (RN) spot‐checked study characteristics for accuracy against the study report.
Assessment of risk of bias in included studies
Two review authors (BS and SW) assessed risk of bias independently for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements through discussion and consultation with another review author (RN). We assessed risk of bias according to the following domains.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Other bias.
We judged each potential source of bias as presenting high, low, or unclear risk and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We then summarised risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes when necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a patient‐reported pain scale). When information on risk of bias relates to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for studies that contributed to that outcome
Measures of treatment effect
We analysed dichotomous data as odds ratios (ORs) or risk differences (RDs) when events were rare. We analysed continuous data as mean differences (MDs). If data from rating scales were combined in a meta‐analysis, we ensured that they were entered with a consistent direction of effect (e.g. lower scores always indicate improvement).
We undertook meta‐analyses only when this was meaningful, that is, when treatments, participants, and the underlying clinical question were similar enough for pooling to make sense.
We described skewed data narratively (e.g. as medians and interquartile ranges for each group).
If we had identified single studies that reported multiple trial arms, we planned to include only the relevant arms. If two comparisons (e.g. drug A vs placebo and drug B vs placebo) had been combined in the same meta‐analysis, we planned to either combine the active arms or halve the control group to avoid double‐counting.
When adjusted analyses were available (ANOVA or ANCOVA), we used these as a preference in our meta‐analyses. If both change from baseline and endpoint scores were available for continuous data, we used change from baseline unless we noted a low correlation between measurements for individuals. If a study reported outcomes at multiple time points, we used the latest time point reported.
We used intention‐to‐treat (ITT) or 'full analysis set' analyses when reported (i.e. those for which data had been imputed for participants who were randomly assigned but did not complete the study) instead of completer or per‐protocol analyses.
Unit of analysis issues
For dichotomous outcomes, we used participants, rather than events, as the unit of analysis. However, when rate ratios were reported in a study, we analysed them on this basis. We meta‐analysed data from cluster‐RCTs only if available data has been adjusted (or could be adjusted), to account for the clustering.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and obtain missing numerical outcome data when possible (e.g. when a study is identified as an abstract only). When this was not possible, and the missing data were thought to introduce serious bias, we took this into consideration when determining the GRADE rating for affected outcomes.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the studies in each analysis. We did not identify substantial heterogeneity in our analyses.
Assessment of reporting biases
If we had been able to pool more than 10 studies, we planned to create and examine a funnel plot to explore possible small‐study and publication biases.
Data synthesis
'Summary of findings' table
We created a 'Summary of findings' table using all pre‐specified outcomes. We used the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to studies that contributed data for the pre‐specified outcomes. We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), along with GRADEpro software (GRADEpro GDT). We justified all decisions to downgrade the quality of studies by using footnotes and made comments to aid the reader's understanding of the review when necessary.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses.
-
Adults (aged 18 years) versus children.
-
Antibiotic type (macrolides versus other).
-
Setting: inpatient versus outpatient.
-
C‐reactive protein (CRP)‐stratified treatment versus non‐CRP‐stratified treatment.
We then planned to use the following outcomes in subgroup analyses.
-
ICU/HDU admission.
-
Duration of symptoms/exacerbation.
-
All adverse events/side effects.
We used the formal test for subgroup interactions provided in Review Manager (Review Manager (RevMan).
Sensitivity analysis
We planned to carry out the following sensitivity analyses while removing the following from the primary outcome analyses.
-
Excluding open‐label trials.
-
Excluding trials at high risk of selection bias.
-
Excluding unpublished data.
-
Comparing results from the fixed‐effect model versus results from the random‐effects model.
Results
Description of studies
Full details of the conduct and characteristics of each included study can be found in the Characteristics of included studies tables, and reasons for exclusion when full texts had to be viewed are given in the Characteristics of excluded studies table.
Results of the search
This review is an update of a previous review (Graham 2001). We fully revised the protocol including background, PICO (population, intervention, comparison, outcomes), and methods and registered the protocol on PROSPERO (Normansell 2017). Therefore we ran a new 'all years' search.
The preliminary searches conducted yielded a total of 429 references ‐ 364 from electronic database searches and 67 from records obtained through searches of clinicaltrials.gov and the World Health Organization (WHO) trials portal (http://apps.who.int/trialsearch/). We excluded most (n = 402) of these references on the basis of the title and abstract. From these references, we identified 27 studies as potentially relevant. Six studies (for which there were 13 records) met the inclusion criteria for this review (Fonseca‐Aten 2006;Graham 1982;Johnston 2006;Johnston 2016; Koutsoubari 2012;Shapiro 1974), and we excluded the other 14 (see Excluded studies section). We have presented a study flow diagram in Figure 2. We conducted the latest search on 17 October 2017.
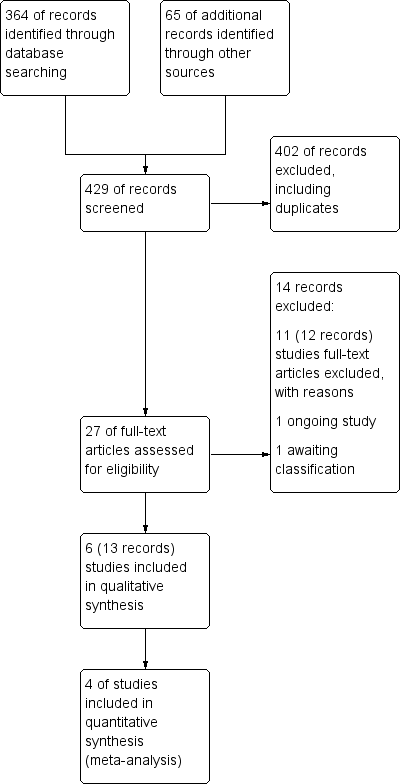
Study flow diagram.
Included studies
Six studies met our inclusion criteria, four of which contributed data to at least one meta‐analysis. These studies included a total of 681 participants who were randomly assigned to comparisons of interest in this review. The largest study included 278 participants (Johnston 2006), and the smallest 40 (Koutsoubari 2012). The mean total number of participants was 114, and the median number was 55. Investigators reported all six trials in full peer‐reviewed articles. We present a summary of the characteristics of included studies in Table 1.
| Study ID | Total n | Country | Age range (years) | Duration of follow‐up | Intervention comparison |
| 43 | USA | 4‐15 | 3‐8 weeks | Clarithromycin (15 mg/kg) vs placebo | |
| 71 | UK | 13‐82 | Unclear | Amoxicillin (300 mg 3 days) vs placebo | |
| 278 | International (multi‐centre) | 17‐68 | 6 weeks | Telithromycin (800 mg/d) vs placebo | |
| 199 | UK | Mean (SD) = 39.9 (14.82) | 6 weeks | Azithromycin (500 mg/d) vs placebo | |
| 40 | Greece | 6‐14 | 12 weeks | Clarithromycin (15 mg/kg/d for 3/52) vs placebo | |
| 50 | USA | 1‐18 | 7 days and 1 to 3 weeks | Hetacillin (ampicillin 100 mg/kg/24 h IV followed by 900 mg PO/d for 6/7) vs placebo |
We attempted to contact authors of Fonseca‐Aten 2006 for more information in July 2017 but were unable to make contact with the named contact person. The lead author of Johnston 2006 and the trial statistician for Johnston 2016 provided additional details on request.
Methods
As per our protocol, all included trials were RCTs, which individually randomised participants to antibiotics versus placebo or usual care. Five studies had post‐treatment follow‐up periods ranging from one to twelve weeks, and only one did not define a follow‐up period (Graham 1982). No study reported a run‐in period, as recruitment was triggered by an unscheduled presentation with an exacerbation. Outcome data were extracted at the end of antibiotic treatment or at the last time point reported. Two studies were conducted in the UK (Graham 1982;Johnston 2016), two in the USA (Fonseca‐Aten 2006; Shapiro 1974), one in Greece (Koutsoubari 2012), and the other (an international study) across multiple centres (Johnston 2006). Most of these studies recruited participants from the emergency department, from an urgent care setting, or when patients were admitted to hospital. One study recruited via parents bringing their children to them if an exacerbation was suspected, before subsequently attending hospital to confirm (Koutsoubari 2012).
Participants
We included studies involving both adults and children. Three studies recruited only children (age range 1 to 18, depending on the specific study (Fonseca‐Aten 2006; Koutsoubari 2012; Shapiro 1974)), two recruited only adults (age range 17 to 68, and 18 to 55 years; Johnston 2006; Johnston 2016), and one included adolescents and adults (age range 14 to 82; Graham 1982). Two studies included information on the ethnicity of participants. Most of those included were of white ethnicity (88.9% for the intervention arm and 94.6% for the control arm) in Johnston 2006, whilst the highest proportions of participants were of black ethnicity (68% and 48%, respectively) in Fonseca‐Aten 2006.
All studies included participants experiencing an asthma exacerbation, but how this was defined varied across the included studies. Of note, in Shapiro 1974, participants were given a diagnosis of "status asthmaticus" (defined as "a lack of response of severe bronchospasm to three subcutaneous injections of 1:1000 aqueous epinephrine given at 15‐minute intervals"). Three studies reported the asthma history of participants in the number of years since diagnosis, and only one reported the severity of asthma of participants and the severity of the exacerbation (Johnston 2016), although another also reported the current exacerbation severity index (Koutsoubari 2012). Both Johnston studies reported the smoking status and pack‐years of participants (Johnston 2006;Johnston 2016), Koutsoubari 2012 reported the percentage of participants exposed to tobacco smoke, and Graham 1982 reported the percentage of current smokers. It is interesting to note that Graham 1982 reported the proportion of participants who had received antibiotic treatment during the week before admission (24.3% in the treatment arm and 17.6% in the control arm); this was an exclusion criterion for most of the included studies.
All but one study explicitly excluded participants with a diagnosed, or strongly suspected, bacterial infection and those who had received recent antibiotic therapy (Koutsoubari 2012). Fonseca‐Aten 2006 excluded children with a diagnosis of bacterial infection needing antibiotics. Graham 1982 excluded participants whose chest X‐rays showed signs of pneumonia. Johnston 2006 excluded participants reporting any antibiotic use within 30 days before enrolment, or with an obvious infection requiring antibiotic treatment. Johnston 2016 excluded participants reporting use of oral or systemic antibiotics within 28 days before enrolment and participants requiring other antibiotic therapy. Finally, Shapiro 1974 excluded participants with evidence of bacterial disease, specifically, any of the following ‐ otitis media, purulent pharyngitis, or fever ‐ and lobular pulmonary infiltrate on admission chest X‐ray who recently received antibiotics.
Interventions
Four studies investigated macrolide antibiotics, with two trialling clarithromycin (Fonseca‐Aten 2006; Koutsoubari 2012), one azithromycin (Johnston 2016), and one telithromycin (part of the subgroup of macrolides known as ketolides) (Johnston 2006). The two remaining studies investigated penicillins, specifically, amoxicillin (Graham 1982) and hetacillin (known now as ampicillin) (Shapiro 1974). All studies compared the antibiotic of choice against a placebo, apart from Koutsoubari 2012, which was an open‐label study with usual care comparison. Both studies investigating clarithromycin were carried out in children and administered the antibiotic at the same dose (15 mg/kg/d). Doses used in each study are detailed in the summary of included studies (Table 1).
Outcomes
Outcomes reported were not consistent across the included studies. Lung function was the most consistently measured outcome, as reported by five of the six included studies. Most studies also reported some measure of participant symptoms at the end of treatment or follow‐up, or time taken for resolution of symptoms. Both Johnston 2006 and Johnston 2016 used an asthma symptom score. Graham 1982 and Shapiro 1974 reported duration of symptoms. Only half of the included studies reported adverse events, and only two explicitly reported serious adverse events (Johnston 2006; Johnston 2016).
Funding
Two trials were sponsored by pharmaceutical companies (Fonseca‐Aten 2006; Johnston 2006), two were funded by governmental agencies (Johnston 2016; Shapiro 1974), and the funding source for two studies was not reported (Graham 1982; Shapiro 1974).
Excluded studies
We excluded 14 records after full‐text assessment. We excluded 11 studies (12 records) with reasons as detailed in Characteristics of excluded studies. One trial is reported to be ongoing (NCT02003911). A further study is awaiting classification; this trial was registered on the EU clinical trials register in 2010, last refreshed on 20 September 2016, with status currently no longer recruiting. We were unable to identify a linked publication, and no contact details were given (EUCTR2010‐018592‐16‐DK). Nine of the excluded studies investigated long‐term use of antibiotics as prophylaxis for asthma rather than as treatment for exacerbations. One study investigated participants with chronic asthma, and another included participants with asthma‐like symptoms, rather than with confirmed asthma.
Risk of bias in included studies
We noted substantial variation in the levels of risk of bias between and within the studies included in this review. Moreover, although we judged few aspects of these studies to have high risk of bias, we found instances in studies when lack of detail on the precise methods used by study authors meant that the level of risk of bias was unclear. Figure 3 provides an overview of our risk of bias judgements.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We found three studies to have low risk of selection bias (Johnston 2006; Johnston 2016; Koutsoubari 2012), and three to have unclear risk (Fonseca‐Aten 2006; Graham 1982; Shapiro 1974). This lack of clarity was due to lack of detail on the exact methods used by study authors.
Blinding
One study used an open‐label design and therefore was at high risk of performance and detection bias (Koutsoubari 2012). All the other studies reported that they were double‐blinded (i.e. participants and personnel), and we considered them to be at low risk of performance bias. Only two studies provided sufficient detail on the method used to blind outcome assessors to indicate low risk of detection bias (Johnston 2006; Johnston 2016). For the other three studies, we judged the risk as unclear.
Incomplete outcome data
Studies varied in the level of risk of attrition bias. We judged Koutsoubari 2012 to be at low risk, as study authors stated that all randomised participants completed the trial. Two participants from the placebo group in Graham 1982 dropped out owing to slow clinical progress, but trialists describe including them in a sensitivity analysis under worst‐case scenario assumptions, which had no impact on the overall results. We therefore judged this study to be at low risk.
We judged Shapiro 1974 to be at high risk, as researchers excluded 6 of the 50 participants initially included: three because they developed signs and symptoms suggesting bacterial disease, and three because of inadvertent failure to administer the study preparation. The distribution between study arms of those excluded for suspected bacterial infection and for protocol violations is not reported. As the study was conducted over 40 years ago, we have not attempted to clarify this with the study authors. We also judged Fonseca‐Aten 2006 to be at high risk, as more than 50% of participants did not complete follow‐up; however, this study did not contribute outcome data to the review.
We considered Johnston 2006 to be at low risk of attrition bias for adverse events; 263 of 270 randomised participants were included in the safety analysis. Re‐inclusion of missing participants under best‐worst case assumptions and worst‐best case assumptions had little impact on the pooled effect estimate for all adverse events or for serious adverse events. More data for symptom scores and PEFR were missing (e.g. 240 out of 270 randomised provided day 10 symptom score data, and 253 out of 270 provided day 10 peak flow readings). We received detailed data tables and the statistical analysis plan from the lead study author. This confirms that missing end‐point PEFR data were imputed from a previous non‐baseline reading, if available. No baseline readings were carried forward. For missing domiciliary PEFR measurements, the average of previous and subsequent readings (if available) was used for imputation, and again, no baseline readings were carried forward. A similar approach was used to deal with missing symptom scores from patient diary cards. Given that we cannot be certain what impact the entirely missing values may have had on the final effect estimate, overall we rated this study as having unclear risk of bias.
We judged Johnston 2016 also to be at low risk for bias for adverse events; correspondence with the trial statistician confirmed that all randomised participants were included in the safety analysis. However, as with Johnston 2006, more data for other outcomes were missing. Twenty per cent of participants missed at least one study visit, 60 did not provide day 10 symptom score data, and 36 did not provide a day 10 peak flow reading. The trial statistician confirmed that mixed (multi‐level) modelling was used; thus all available diary records were included in the model, regardless of availability of the day 10 reading. It is unclear what impact the entirely missing values may have had on the effect estimates; therefore, results should be interpreted with caution. Overall, we judged this trial to be at unclear risk of attrition bias.
Selective reporting
For three studies, the level of risk of reporting bias was unclear. For one, there was low risk, and for two we judged the risk to be high.
We judged Fonseca‐Aten 2006 to be at high risk of reporting bias. We were not able to identify a prospective registration or published protocol, and we found that not all evaluated outcomes were reported numerically, for example, "No clinical differences were demonstrated for clarithromycin therapy vs placebo on visit 3". Attempts to contact study author teams failed. We also judged Shapiro 1974 to be at high risk; we found no prospective registration or protocol and determined that researchers did not evaluate all outcomes numerically (e.g. graphically displayed only), so we could not include these data in the meta‐analysis.
We judged Graham 1982 to be at unclear risk. Again, we were not able to identify a published protocol or a prospective registration, but study authors clearly reported all outcomes described in the methods section. However, study authors used medians and ranges and non‐parametric tests, so we could not combine data in meta‐analyses. Similarly, we were unable to identify a published protocol or registration for Koutsoubari 2012, but we found that study authors clearly reported all outcomes described in the methods. Study authors used medians and interquartile ranges for non‐normal data, so these were not combined in meta‐analyses.
We judged Johnston 2016 to be at low risk of reporting bias; the trial was prospectively registered and outcomes were reported as planned. Of note, the trial team relaxed the inclusion criteria in an attempt to improve recruitment, but this is unlikely to have introduced bias.
We judged Johnston 2006 to be at unclear risk of bias because several outcomes listed in the prospective trial registration were not fully reported, including outcomes of interest for this review (i.e. health status at follow‐up (6 weeks); need for additional medication (e.g. ICS, oral corticosteroid (OCS), bronchodilator); time to next exacerbation of asthma). The lead study author provided the following explanation: "the time‐to‐next‐acute‐exacerbation and need for additional medications data were not included because acquisition of such data in the setting of an acute exacerbation study, not unexpectedly, was so incomplete that a decision was taken not to analyse them".
Other potential sources of bias
In Graham 1982, participants could be included more than once in the trial, as the episode, rather than the individual, was the unit of randomisation: 60 patients experienced 71 exacerbations during the trial. We are unable to determine what effect this had on the effect estimates reported. We detected baseline imbalance between arms in Shapiro 1974, including differences in the mean number of days of wheezing before admission (2.6 in the hetacillin group and 5.8 in the placebo group).
Effects of interventions
Intensive care unit/high dependency unit admission
Children
One child in the placebo group of Shapiro 1974 experienced respiratory arrest shortly after admission and was mechanically ventilated; we assume this child would have received care in an ICU/HDU setting. Of note, this study was conducted in children with status asthmaticus. None of the other included studies reported this outcome.
Duration of symptoms/exacerbation
No studies reported on the duration of symptoms or exacerbations, which was our pre‐specified outcome. However, four studies used different approaches to report asthma symptoms (Graham 1982; Johnston 2006; Johnston 2016; Koutsoubari 2012).
Adults
Graham 1982 reported a physician's assessment of asthma symptoms at hospital discharge (median duration of admission seven days in the amoxicillin group and eight days in the placebo group) on a 4 to 12‐point scale (higher score = worse symptoms) with results presented as medians and ranges. The median (range) score in the amoxicillin group was 5 (4 to 9), and in the placebo group 4 (4 to 8) (n = 69). The same study reported patients' assessment of symptoms at discharge on a visual analogue scale (lower score = worse symptoms), also as medians and ranges. The median (range) score in the amoxicillin group was 33 (range 0 to 250), and in the placebo group 28 (0 to 85) (n = 69). The visual analogue scale was reported to be 10 cm and the results given in millimetres; as the trial was conducted in 1982, we did not attempt to resolve the discrepancy that the range reported exceeds the scale. The between‐group difference for both scores was reported as non‐significant. Graham 1982 also reported the median and range of numbers of days to 50% improvement in symptoms as assessed by both physician and patient. In the amoxicillin group, the median (range) numbers of days was 3 (1 to 6) for the physician assessment and 3 (2 to 10) in the placebo group. For patients' assessment, the scores were 2 (2 to 10) and 2 (2 to 8), respectively.
Participants in Johnston 2006 (a trial of telithromycin) and Johnston 2016 (a trial of azithromycin) were asked to rate their own symptoms using modified diary cards on a 7‐point scale (0 = no symptoms, 6 = severe symptoms). We were able to extract data at 10 days and at six weeks from Johnston 2006, and at 10 days from Johnston 2016. We chose to combine the 10‐day findings using mean differences. Results favour antibiotics over placebo (mean difference (MD) ‐0.34, 95% confidence interval (CI) ‐0.60 to ‐0.08; participants = 416; I2= 0%; studies = 2; Analysis 1.1; moderate‐quality evidence). At six weeks, participants in the intervention group of Johnston 2006 still reported lower symptom scores than those in the control group, but the upper confidence interval includes no between‐group difference (MD ‐0.2, 95% CI ‐0.5 to 0.02; P = 0.066). Johnston 2006 also reported the number of symptom‐free days as a percentage at 10 days (calculated by dividing the number of days when all symptom scores in the diary were zero by the number of days for which the patient provided scores); results favoured telithromycin (16% of days symptom‐free vs 8%; P = 0.006; participants = 255).
Children
Koutsoubari 2012 reported the median and the interquartile range (IQR) for symptom‐free days at 3, 6, and 12 weeks. At 3 weeks, the median (IQR) for the clarithromycin group was 16 (1), and for the control group 12 (2) (n = 40). At 6 weeks, values were reported as 36 (2) and 29 (3), respectively (n = 40), and by 12 weeks, 78 (2) and 69 (6), respectively (n = 40) (P < 0.0001 at all three time points). The same study reported the duration in days of the index asthma exacerbation, also as median and IQR. The median (IQR) in the clarithromycin group was 5 (1), and in the control group 7.5 (1) (n = 40) (P < 0.00001).
All adverse events/side effects
Four studies reported adverse events (Johnston 2006; Johnston 2016; Koutsoubari 2012; Shapiro 1974). We combined data for all adverse events (AEs) from three studies; the confidence interval includes both potential harm or benefit of the intervention (odds ratio (OR) 0.99, 95% CI 0.69 to 1.43; participants = 506; studies = 3; I2 = 0%; low‐quality evidence; Analysis 1.2). Researchers reported no significant difference in the test for subgroup differences between adults and children.
We also combined data for serious adverse events (SAEs). We were able to extract data from three studies, and we analysed the result as a risk difference because events were rare. The pooled result suggests no difference between antibiotic and control, but it should be noted that only 10 events were reported across the three trials (five in the antibiotic group and five in the placebo group) (risk difference (RD) 0.00, 95% CI ‐0.03 to 0.03; participants = 502; studies = 3; I2 = 0%; low‐quality evidence, Analysis 1.3). Study results show no significant difference in the test for subgroup differences between adults and children.
Adults
Pooled data for the adults subgroup were as follows: OR 1.00, 95% CI 0.69 to 1.45.
Children
Shapiro 1974 also reported 'complications' after discharge and noted two in the hetacillin group (one hospitalisation for asthma, and one participant experienced persistent wheezing) and three in the placebo group (fever, diarrhoea, and pulmonary infiltrate in one participants, abdominal discomfort in another, and persistent wheezing in a third).
For serious adverse events, Koutsoubari 2012 reported that no participant required hospital admission during the study; as the definition of a serious adverse event is "death, a life‐threatening adverse event, inpatient hospitalisation or prolongation of existing hospitalisation", we assumed that no participant had experienced an SAE.
Mortality
No deaths were reported in any of the included studies.
Length of hospital admission
Two studies reported admission duration, but we could not combine the results (Graham 1982; Shapiro 1974).
Adults
Graham 1982 reported median (range) duration of admission in days as 7 (3 to 25) in the amoxicillin group and 8 (3 to 6) in the placebo group (n = 69; reported as not significantly different).
Children
Shapiro 1974 reported the mean (SD) duration of admission; the confidence interval includes the possibility of an increase or a decrease in duration of admission in the hetacillin group (MD ‐0.10, 95% CI ‐0.53 to 0.33; participants = 43; studies = 1; very low‐quality evidence, Analysis 1.4).
Relapse after index presentation
One study reported exacerbations in the follow‐up period (Johnston 2006). Two adults in each arm experienced an exacerbation by the six‐week time points (n = 263).
Peak expiratory flow rate
Four studies reported peak expiratory flow rate (PEFR) (Graham 1982; Johnston 2006; Johnston 2016; Koutsoubari 2012).
Adults
We combined endpoint results at 10 days for Johnston 2006 and Johnston 2016 using generic inverse variance (GIV). The result favours antibiotics over placebo with the mean difference exceeding the minimal clinically important difference of 18.79 L/min (Santanello 1999): MD 23.42 L/min, 95% CI 5.23 to 41.60; participants = 416; studies = 2; Analysis 1.5. However, the pre‐specified primary outcome in Johnston 2006 was change in domiciliary morning PEFR. Based on modelled data, the mean difference between groups in the change from baseline was reported as 3.6 L/min (95% CI ‐32.5 to 25.3; P = 0.81).
Children
One study in children reported the maximum peak flow recorded during the follow‐up period (Koutsoubari 2012); results favoured the clarithromycin group, but the confidence interval includes no difference (MD 38.80, 95% CI ‐11.19 to 88.79; participants = 40; studies = 1; Analysis 1.6).
Subgroup and sensitivity analyses
Subgroup analysis
Subgroup analysis was restricted by the small number of trials identified.
Adults (aged 18 years) versus children
Data for adults and children were subgrouped throughout and were reported separately above. Only two meta‐analyses pooled data from adults and children: serious adverse events and all adverse events. No serious events were reported in the one study in children, and the test for subgroup differences was negative (P = 0.99; I2 = 0%). Similarly for all adverse events, no subgroup difference was detected (P = 0.80; I2 = 0%).
Antibiotic type (macrolides vs other)
Only one meta‐analysis pooled data from two different classes of antibiotic: all adverse events. We detected no difference between the two studies investigating a macrolide antibiotic and the one study investigating a penicillin (P = 0.80; I2 = 0%) (analysis not shown); however, the one study investigating penicillin was conducted in 1974, and the two investigating macrolides in 2006 and 2016.
Setting: inpatient versus outpatient
Most of the participants included in this review were recruited in a hospital or emergency department setting. Two trials reported recruiting some participants from urgent care or primary care centres but did not present data disaggregated by setting (Johnston 2006; Johnston 2016).
CRP‐stratified treatment versus non‐CRP‐stratified treatment
None of the included studies reported stratifying treatment by CRP results.
Sensitivity analysis
Excluding open‐label trials
Only one study was reported to be open‐label (Koutsoubari 2012). This study was combined with other studies in one analysis (serious adverse events) but did not contribute events; thus its exclusion has no impact on the effect estimate.
Excluding trials at high risk of selection bias
We did not judge any of the included trials to be at high risk of selection bias. Three trials were at unclear risk for both random sequence generation and allocation concealment, but only one trial contributed to a meta‐analysis (Shapiro 1974): all adverse events. Excluding this trial had minimal impact on the effect estimate (OR 1.00, 95% CI 0.69 to 1.45; participants = 462; studies = 2; I2 = 0%; data not shown).
Excluding unpublished data
We did not include any unpublished data in our meta‐analyses.
Comparing results from the fixed‐effect model versus the random‐effects model
Results show a negligible difference between random‐effects and fixed‐effect models.
Discussion
Summary of main results
This review is an update of a previous review (Graham 2001), and we have run a new 'all years' search. We fully revised the protocol including background, PICO (population, intervention, comparison, outcomes), and methods, and registered it on PROSPERO (Normansell 2017). Six studies met our inclusion criteria, four of which contributed data to at least one meta‐analysis. These studies included a total of 681 adults and children who were randomly assigned to comparisons of interest in this review. Four studies investigated macrolide antibiotics (Fonseca‐Aten 2006; Johnston 2006; Johnston 2016; Koutsoubari 2012), and two studies investigated ampicillin and amoxicillin, respectively (Graham 1982; Shapiro 1974); both studies were conducted over 30 years ago. Five studies compared antibiotics versus placebo, and one was open‐label (Koutsoubari 2012). We were able to perform limited meta‐analysis owing to the small number of trials identified and between‐study heterogeneity.
None of the included trials reported intensive care unit/high dependence unit (ICU/HDU) admission, although one participant in the placebo group of Shapiro 1974 (a study including children with status asthmaticus) experienced respiratory arrest shortly after admission and was mechanically ventilated. None of the included studies reported our outcome of interest ‐ duration of symptoms. Studies provided some data on symptom scores, and four studies reported some measure of symptom‐free days. Overall these favoured antibiotics, but measures show ambiguity.
No deaths were reported in any of the included studies. Four studies reported adverse events, and we were able to combine data for serious adverse events from three studies, but these events were rare; only 10 events were reported across the three trials (five in the antibiotic group and five in the placebo group; 502 participants): risk difference (RD) 0.00, 95% confidence interval (CI) ‐0.03 to 0.03. We combined data for all adverse events (AEs) from three studies; the confidence interval includes potential harm or benefit of the intervention: odds ratio (OR) 0.99, 95% CI 0.69 to 1.43.
One study reported exacerbations in the follow‐up period (Johnston 2006). Two participants in each arm were reported as experiencing an exacerbation within six weeks (n = 263). Four studies reported peak expiratory flow rate (PEFR). We combined endpoint results at 10 days for Johnston 2006 and Johnston 2016, and results favoured antibiotics over placebo, with the mean difference (MD) exceeding the minimal clinically important difference: mean difference (MD) 23.42 L/min, 95% CI 5.23 to 41.60. One study in children reported the maximum peak flow recorded during the follow‐up period (Koutsoubari 2012); results favoured the clarithromycin group, but the confidence interval included no difference: MD 38.80, 95% CI ‐11.19 to 88.79. All three studies that reported PEFR used macrolide antibiotics ‐ therefore the reduction in peak flow could have been due at least in part to the anti‐inflammatory properties.
We were able to perform only very limited subgroup and sensitivity analyses owing to the small number of trials identified, and we found no evidence of important effect modification according to age or class of antibiotic, although this cannot be ruled out.
Overall completeness and applicability of evidence
The evidence presented is considered incomplete because of the small number of relevant trials identified, between‐study heterogeneity limiting meta‐analysis, and the age of two of the six studies ‐ one randomised controlled trial (RCT) was conducted in 1974 (Shapiro 1974), and another in 1982 (Graham 1982). Applicability of evidence from RCTs conducted over 30 years ago is questionable. Shapiro 1974, for example, used a definition of an acute exacerbation of asthma that diverges from that used in modern day practice; furthermore reports show differences between the treatment protocol and current guidelines for the treatment of individuals with acute exacerbations of asthma. Furthermore, most participants were recruited at a hospital, rather than in a primary care setting, and this may limit generalisability to other settings, for example, primary care.
Only four of the six included studies contributed to the meta‐analysis. A large portion of the data was not presented in the study papers in a format compatible with the other studies, or simply was not reported. Fonseca‐Aten 2006 included no clinical data in its study results. Considerable heterogeneity is evident between the outcomes reported in these studies. This discrepancy between outcomes made it difficult to carry out much meaningful meta‐analysis, limiting the completeness of presented evidence. We did not seek to address the benefits or harms of long‐term antibiotic use in asthma; this is the topic of a separate review (Kew 2015).
Five of the six studies included in this review specifically excluded participants if they received the diagnosis of a bacterial infection, or if one was strongly suspected. Consequently, application of review findings is limited to patients with an exacerbation of asthma without signs, symptoms, or investigative findings suggestive of bacterial infection (i.e. those not meeting current guidance to receive antibiotics). However, it would be considered unethical to withhold antibiotics from a patient considered likely to have a bacterial infection; thus current and future studies are unlikely to address this question. Furthermore, although included studies in adults excluded participants with other respiratory comorbidities, such as chronic obstructive pulmonary disease (COPD) or "COPD‐asthma overlap syndrome" (ACOS), this condition may be more difficult to diagnose; thus it is possible that the adult trials included participants with this diagnosis, potentially confounding study results (Soriano 2003). Although none of the included studies presented results stratified by smoking history, the two largest included studies in adults excluded participants with a greater than 10 or 5 pack‐year smoking history, respectively (Johnston 2006; Johnston 2016).
A pertinent observation arising from this review relates to the difficulty of participant recruitment highlighted in Johnston 2016. Trialists were able to recruit a total of only 199 participants out of a total 4582 assessed, with 2044 excluded for receiving prior antibiotic treatment. For every patient randomised, at least 10 patients were excluded for this reason. This suggests that use of antibiotics may be widespread in the United Kingdom, possibly contrary to current guidelines (BTS/SIGN 2016). Guidelines clearly state that antibiotics should not be used for routine treatment of acute asthma exacerbations (BTS/SIGN 2016; GINA 2017). Researchers have found evidence to suggest high levels of antibiotic prescription for acute exacerbations of asthma. In the United States, one study reported this to be as high as 60% (Lindenauer 2016); similarly a study in China reported that almost 75% of patients attended the emergency department for an acute exacerbation and received an antibiotic (Tang 2013); the equivalent figure from a study conducted in the United Kingdom was 57% (Bafadhel 2011). These figures pose a challenge for researchers attempting to carry out studies examining the efficacy and safety of antibiotics for acute exacerbations of asthma.
A further complication for interpretation is that any modest benefits associated with macrolide use may be the result of anti‐inflammatory rather than antibacterial properties of this class of antibiotic (Rollins 2010). Head‐to‐head studies comparing classes of antibiotics would be of limited use in resolving this uncertainty, as effects would be confounded by the different spectrum of bacteria against which antibiotics are effective. Had we identified more studies, we would have had to consider whether pooling older studies using penicillin antibiotics with more recent studies using macrolides made clinical sense. However, the only meta‐analysis in which this occurred was that for all adverse events, in which the two recent Johnston trials using macrolides were pooled with an older study using ampicillin. The test for subgroup differences in this meta‐analysis is negative but underpowered. Issues of appropriate pooling may become more relevant in future updates of this review if additional trials are identified.
Finally, it should be noted that none of the patients were included on the basis of inflammatory markers such as C‐reactive protein (CRP) or procalcitonin, although evidence suggests that these markers were used to beneficial effect to reduce antibiotic prescription for patients with asthma (Long 2014). Access to tests to confirm a bacterial infection is not routinely available to all doctors worldwide, especially in a primary care setting. Moreover, no studies provided information on costs, and none explored potential issues arising from antibiotic resistance.
Quality of the evidence
Grading of outcomes ranged from moderate to very low quality. Our confidence was reduced for all outcomes by suspicion of publication bias; despite the availability of a common treatment for asthma, we identified only six eligible RCTs, suggesting that unpublished data may exist. However, we were not able to formally explore this by using a funnel plot because we identified an insufficient number of studies. Our confidence was further reduced by indirectness; most of the included trials recruited from emergency care settings, limiting applicability to primary care settings, to which many people with acute exacerbations of asthma initially present. This problem was further compounded by the most recent trial ‐ Johnston 2016 ‐ which struggled to recruit sufficient participants as so many people had already received a course of antibiotics at the time they presented to the emergency department and were therefore excluded. In addition, we downgraded quality for indirectness owing to the age of two of the six studies. Imprecision affected both adverse event outcomes (small numbers of events and few contributing trials) and length of hospital stay. Finally, we had concerns about risk of bias related to lack of blinding in outcomes contributed to by Koutsoubari 2012. We were also concerned about unclear reporting of trial methods for outcomes contributed to by two older trials; these were conducted at a time when methodological practice in conducting trials may have been less rigorous, and when asthma care was different (Graham 1982;Shapiro 1974).
Potential biases in the review process
We conducted this review in accordance with Cochrane standards and by following a pre‐published protocol (Normansell 2017). The updated protocol was reviewed by a Cochrane Airways editor, but it was not formally peer‐reviewed.
Agreements and disagreements with other studies or reviews
Our literature search identified only one systematic review comparing antibiotics with placebo for acute asthma exacerbations, and that was the previous version of this Cochrane Review (Graham 2001). This review found nothing significant to contradict advice given in the BTS Guidelines for Asthma 2016 and the GINA 2017 Guidelines, which recommend not giving antibiotics routinely in acute asthma exacerbations (BTS/SIGN 2016; GINA 2017).

Forest plot of comparison: 1 Antibiotics versus placebo/usual care, outcome: 1.3 Serious adverse events.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Antibiotics versus placebo/usual care, Outcome 1 Symptom score.

Comparison 1 Antibiotics versus placebo/usual care, Outcome 2 All adverse events.
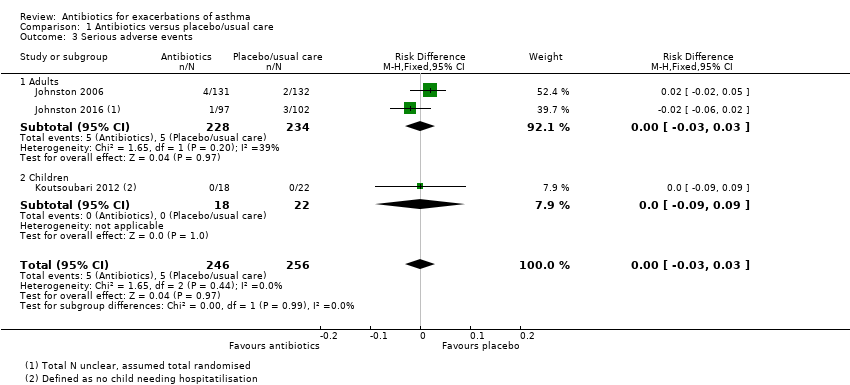
Comparison 1 Antibiotics versus placebo/usual care, Outcome 3 Serious adverse events.

Comparison 1 Antibiotics versus placebo/usual care, Outcome 4 Length of hospital stay (days).

Comparison 1 Antibiotics versus placebo/usual care, Outcome 5 PEF (GIV).

Comparison 1 Antibiotics versus placebo/usual care, Outcome 6 PEF.
| Antibiotics compared to placebo/usual care for acute asthma | ||||||
| Patient or population: acute asthma exacerbation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo/usual care | Risk with antibiotics | |||||
| ICU/HDU admission ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | One respiratory arrest in the placebo group in Shapiro 1974. No other studies reported this outcome |
| Symptom score at 10 days. Measured on a 7‐point scale (0 to 6) ; lower score denotes fewer symptoms | Mean symptom score at 10 days ranged from 2 to 2.20 points | MD 0.34 points lower (0.60 lower to 0.08 lower) | ‐ | (2 RCTs) | ⊕⊕⊕⊝a,d MODERATE | |
| All adverse events | 42 per 100 | 41 per 100 | OR 0.99 | 506 | ⊕⊕⊝⊝ | 2 studies in adults and 1 small old study in children with status asthmaticus |
| Serious adverse events Duration 3 days to 3 weeks | 2 per 100 | 2 per 100 | RD 0.00 | 502 | ⊕⊕⊝⊝ | Anticipated absolute effects were calculated using the figures in Figure 1. This is a re‐presentation of the results, but to 4 dp, which allows the calculation to be done |
| Mortality ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No deaths were reported in any of the studies |
| Length of hospital stay, days | Mean length of hospital stay was 2.6 days | MD 0.1 days lower | ‐ | 43 | ⊕⊝⊝⊝ | 1 study reported medians and IQRs and found no significant differences, although data were skewed |
| Relapse after index presentation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| PEFR (GIV) Duration 10 days | Mean PEFR (GIV) ranged from 19.6 to 26.9 L/min (mean difference from baseline) | MD 23.42 L/min (mean difference from baseline) higher | ‐ | 469 | ⊕⊕⊕⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| a‐1 indirectness. Studies mostly recruited from hospital or emergency department. Therefore this review may represent more severe exacerbations and does not apply to people attending the GP and requesting antibiotics. The review does not apply to people who have already received a course of antibiotics. bNo downgrade for risk of bias. One small study excluded 6 participants post hoc, but excluding this study from the meta‐analysis did not affect the results. cNo downgrade. I2 = 0. Different antibiotics were given in each study. dNo downgrade. Only six RCTs have been published on antibiotics for asthma exacerbation. This strongly suggests that unpublished data exist or that clinical trials are seriously lacking for this common intervention. e‐1 imprecision. Confidence intervals include the possibility of important benefit and risk of harm. f‐1 indirectness. Studies mostly recruited from hospital or emergency department. Therefore this review may represent more severe exacerbations and does not apply to people attending the GP and requesting antibiotics. The review does not apply to people who have already received a course of antibiotics. One small study recruited children with status asthmaticus in 1974, when asthma management was different. g‐1 imprecision. Few events. h‐1 risk of bias. Study before good reporting standards introduced. Concerns over study, which excluded six participants, and it is not clear from which arm they were excluded. i‐1 indirectness. Participants were all children with status asthmaticus, and the study was conducted before current asthma management had been introduced (e.g. they all received IV adrenaline). j‐1 imprecision. One small study was included. | ||||||
| Study ID | Total n | Country | Age range (years) | Duration of follow‐up | Intervention comparison |
| 43 | USA | 4‐15 | 3‐8 weeks | Clarithromycin (15 mg/kg) vs placebo | |
| 71 | UK | 13‐82 | Unclear | Amoxicillin (300 mg 3 days) vs placebo | |
| 278 | International (multi‐centre) | 17‐68 | 6 weeks | Telithromycin (800 mg/d) vs placebo | |
| 199 | UK | Mean (SD) = 39.9 (14.82) | 6 weeks | Azithromycin (500 mg/d) vs placebo | |
| 40 | Greece | 6‐14 | 12 weeks | Clarithromycin (15 mg/kg/d for 3/52) vs placebo | |
| 50 | USA | 1‐18 | 7 days and 1 to 3 weeks | Hetacillin (ampicillin 100 mg/kg/24 h IV followed by 900 mg PO/d for 6/7) vs placebo |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptom score Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | ‐0.34 [‐0.60, ‐0.08] | |
| 1.1 Adults | 2 | Mean Difference (Fixed, 95% CI) | ‐0.34 [‐0.60, ‐0.08] | |
| 1.2 Children | 0 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 All adverse events Show forest plot | 3 | 506 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.69, 1.43] |
| 2.1 Adults | 2 | 462 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.69, 1.45] |
| 2.2 Children | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.12, 5.18] |
| 3 Serious adverse events Show forest plot | 3 | 502 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.03, 0.03] |
| 3.1 Adults | 2 | 462 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.03, 0.03] |
| 3.2 Children | 1 | 40 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.09, 0.09] |
| 4 Length of hospital stay (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 PEF (GIV) Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | 23.42 [5.23, 41.60] | |
| 5.1 Adults | 2 | Mean Difference (Fixed, 95% CI) | 23.42 [5.23, 41.60] | |
| 5.2 Children | 0 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 PEF Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

