Vaksin influenza untuk penyakit pulmonari obstruktif kronik (COPD)
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Trials Register
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (the Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| Embase (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the Cochrane Airways Trials Register
COPD search
1. Lung Diseases, Obstructive/
2. exp Pulmonary Disease, Chronic Obstructive/
3. emphysema$.mp.
4. (chronic$ adj3 bronchiti$).mp.
5. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
6. COPD.mp.
7. COAD.mp.
8. COBD.mp.
9. AECB.mp.
10. or/1‐9
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases
Appendix 2. Search strategy to identify relevant trials from the Cochrane Airways Trials Register
Search via Cochrane Register of Studies (CRS Web)
#1 MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive Explode All
#2 MeSH DESCRIPTOR Bronchitis, Chronic
#3 (obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)
#4 COPD:MISC1
#5 (COPD OR COAD OR COBD OR AECOPD):TI,AB,KW
#6 #1 OR #2 OR #3 OR #4 OR #5
#7 MeSH DESCRIPTOR Influenza Vaccines
#8 MeSH DESCRIPTOR Vaccines
#9 (influenza* or flu*) NEAR (vaccin* or immuni* or inoculat*)
#10 flumist
#11 trivalent
#12 CAIV
#13 LAIV
#14 medimmune
#15 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14
#16 #6 AND #15

Study flow diagram for 2018 update

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 1 Total exacerbations per participant.
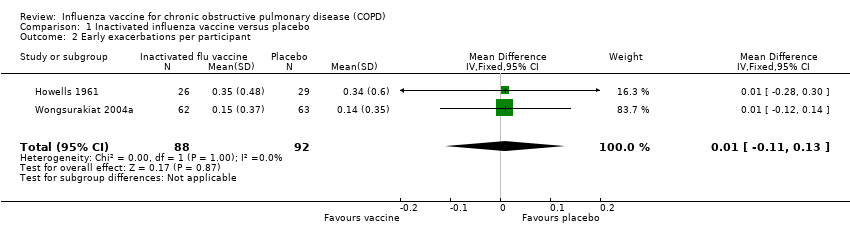
Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 2 Early exacerbations per participant.
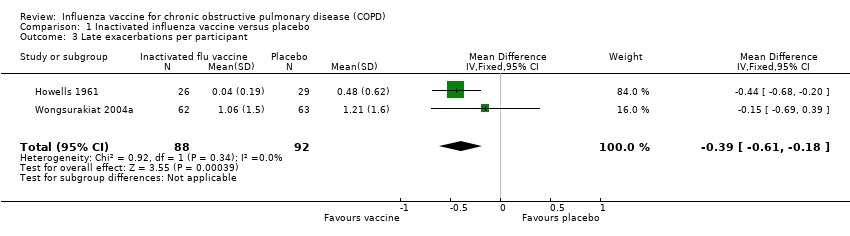
Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 3 Late exacerbations per participant.
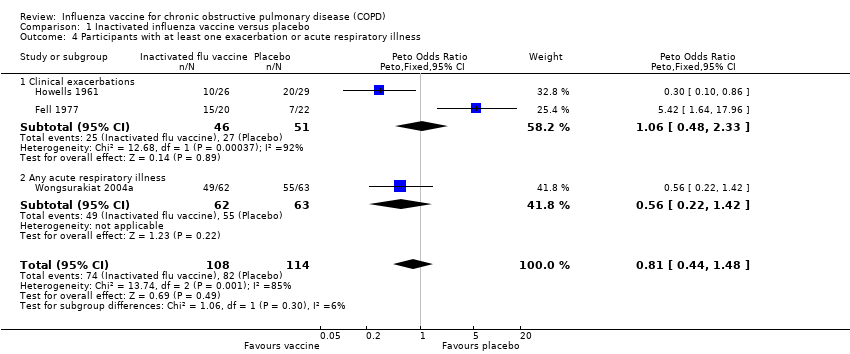
Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 4 Participants with at least one exacerbation or acute respiratory illness.

Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 5 Participants with early exacerbations.

Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 6 Participants with late exacerbations.

Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 7 Hospital admissions.

Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 8 Mortality (all cause).
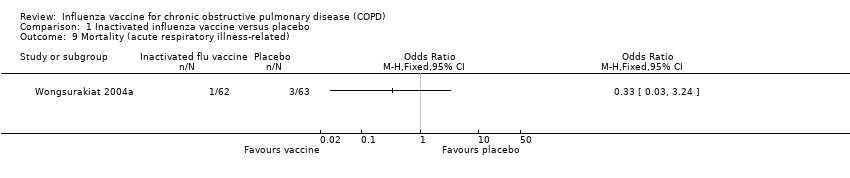
Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 9 Mortality (acute respiratory illness‐related).

Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 10 Overall change in lung function (FEV¹, L).
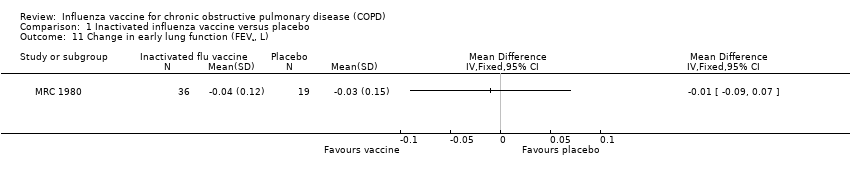
Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 11 Change in early lung function (FEV¹, L).
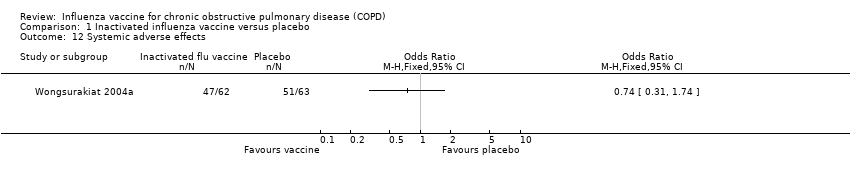
Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 12 Systemic adverse effects.

Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 13 Local effects at injection site.

Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 14 Participants with early breathlessness.
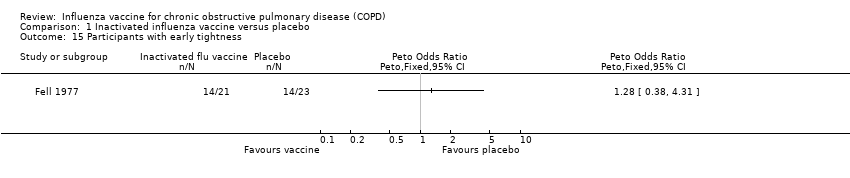
Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 15 Participants with early tightness.

Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 16 Participants with early wheeze.
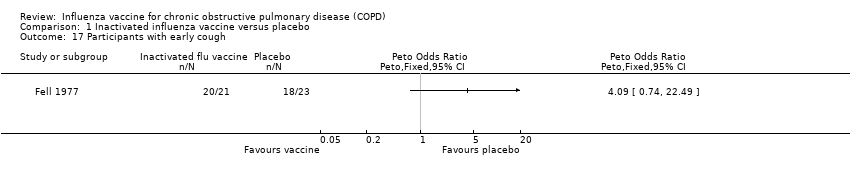
Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 17 Participants with early cough.

Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 18 Acute respiratory illness subsequently documented as influenza‐related.

Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 19 Early acute respiratory illness (ARI).
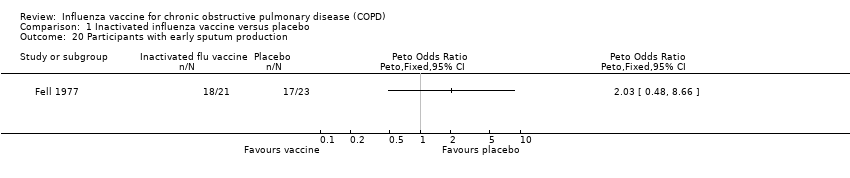
Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 20 Participants with early sputum production.

Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 1 Total exacerbations per participant.
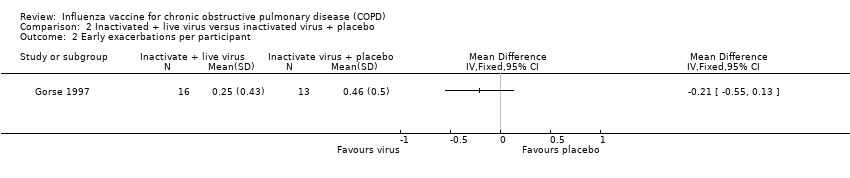
Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 2 Early exacerbations per participant.

Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 3 Late exacerbations per participant.

Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 4 Participants with improvement in exacerbations.
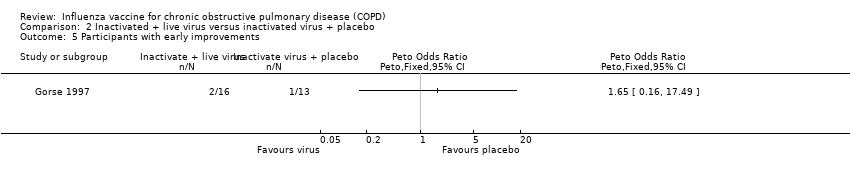
Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 5 Participants with early improvements.

Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 6 Participants with late improvements.

Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 7 Mortality.

Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 8 Early changes in lung function (% predicted FEV¹).

Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 9 Early changes in lung function (FEV¹/FVC %).

Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 10 Post immunisation lung function (FEV¹).

Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 11 Participants with increased lung function (1 category).
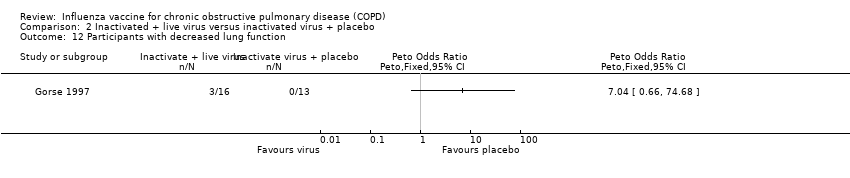
Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 12 Participants with decreased lung function.
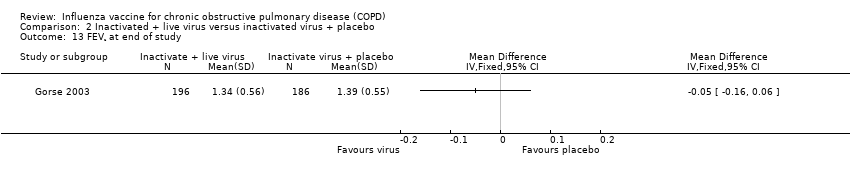
Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 13 FEV¹ at end of study.

Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 14 Participants with adverse effects (new upper respiratory tract symptoms).
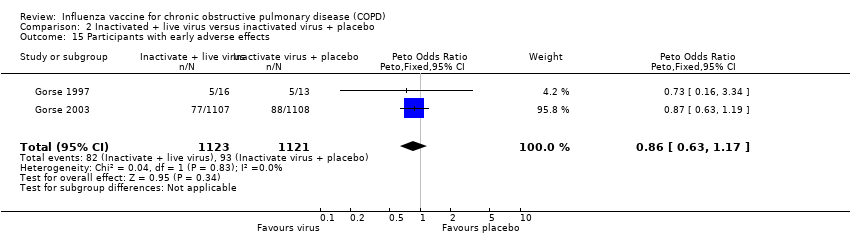
Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 15 Participants with early adverse effects.
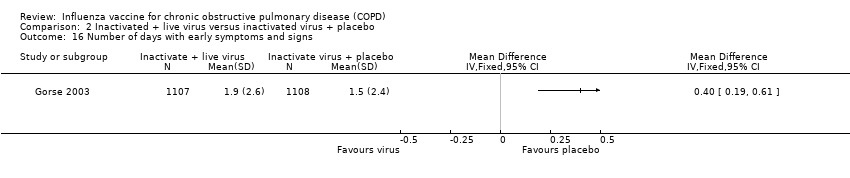
Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 16 Number of days with early symptoms and signs.

Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 17 Number of participants with early adverse effects (by type).

Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 18 Participants with late adverse effects.

Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 19 Acute respiratory illness subsequently documented as influenza‐related.
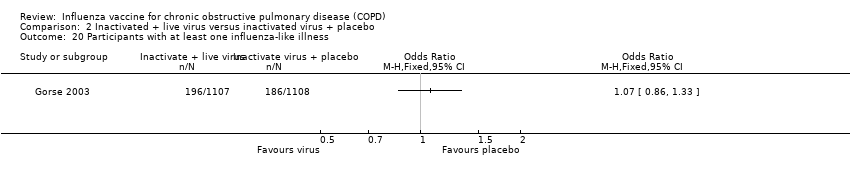
Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 20 Participants with at least one influenza‐like illness.
| Influenza vaccine compared to placebo for chronic obstructive pulmonary disease (COPD) | ||||||
| Patient or population: chronic obstructive pulmonary disease (COPD) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with Influenza vaccine | |||||
| Total exacerbations per participant | The mean number of total exacerbations per participant ranged across placebo groups from 0.83 to 1.35 | MD 0.37 lower | ‐ | 180 | ⊕⊕⊝⊝ | Despite the effect size, this is based on a very small number of trials and participants. However there was good agreement between these two studies (low I2). One study was not conducted in an epidemic year. We extrapolated some data. Ideally more trials would be done to refine these effect sizes. |
| Early exacerbations per participant | The mean number of early exacerbations per participant ranged across placebo groups from 0.14 to 0.34 | MD 0.01 higher | ‐ | 180 | ⊕⊕⊝⊝ | See above |
| Late exacerbations per participant | The mean number of late exacerbations per participant ranged across placebo groups from 0.48 to 1.21 | MD 0.39 lower | ‐ | 180 | ⊕⊕⊝⊝ | See above |
| Days disability from respiratory illness | not reported | |||||
| Hospital admissions | 76 per 1000 | 26 per 1000 | OR 0.33 | 180 | ⊕⊕⊝⊝ | |
| Mortality | 76 per 1000 | 67 per 1000 | OR 0.87 | 180 | ⊕⊕⊝⊝ | |
| Local effects at injection site | 63 per 1000 | 274 per 1000 | OR 5.57 | 125 | ⊕⊕⊝⊝ | Single study on participants who all had COPD (Wongsurakiat 2004a), but very similar findings in studies with a mixed population (Cate 1977; Govaert 1994) |
| GRADE Working Group grades of evidence | ||||||
| aSmall number of studies which are over ten years old limit our confidence in the generalisability of these findings to currently available vaccines (downgraded once for indirectness). b Studies too small and events too infrequent to detect a consistent effect (downgraded once for imprecision) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total exacerbations per participant Show forest plot | 2 | 180 | Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐0.64, ‐0.11] |
| 2 Early exacerbations per participant Show forest plot | 2 | 180 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.11, 0.13] |
| 3 Late exacerbations per participant Show forest plot | 2 | 180 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐0.61, ‐0.18] |
| 4 Participants with at least one exacerbation or acute respiratory illness Show forest plot | 3 | 222 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.81 [0.44, 1.48] |
| 4.1 Clinical exacerbations | 2 | 97 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.48, 2.33] |
| 4.2 Any acute respiratory illness | 1 | 125 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.56 [0.22, 1.42] |
| 5 Participants with early exacerbations Show forest plot | 2 | 180 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.08 [0.52, 2.26] |
| 6 Participants with late exacerbations Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 7 Hospital admissions Show forest plot | 2 | 180 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.33 [0.09, 1.24] |
| 7.1 Clinical exacerbations | 1 | 55 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.01, 2.39] |
| 7.2 Influenza‐related exacerbations | 1 | 125 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.41 [0.09, 1.89] |
| 8 Mortality (all cause) Show forest plot | 2 | 180 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.28, 2.70] |
| 9 Mortality (acute respiratory illness‐related) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Overall change in lung function (FEV¹, L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Change in early lung function (FEV¹, L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12 Systemic adverse effects Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13 Local effects at injection site Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14 Participants with early breathlessness Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 15 Participants with early tightness Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 16 Participants with early wheeze Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 17 Participants with early cough Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 18 Acute respiratory illness subsequently documented as influenza‐related Show forest plot | 2 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.07, 0.48] |
| 18.1 FEV¹ ≥ 70% predicted | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.01, 1.11] |
| 18.2 Participants with chronic bronchitis | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.04, 0.96] |
| 18.3 FEV¹ < 50% predicted | 1 | 47 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 0.99] |
| 18.4 FEV¹ 50% to 69% predicted | 1 | 33 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.07, 2.98] |
| 19 Early acute respiratory illness (ARI) Show forest plot | 1 | 250 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.34, 1.50] |
| 19.1 ARI within 1 week of vaccination | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.24, 4.26] |
| 19.2 ARI between 1 and 4 weeks after vaccination | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.27, 1.50] |
| 20 Participants with early sputum production Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total exacerbations per participant Show forest plot | 2 | 1137 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.35, 0.37] |
| 2 Early exacerbations per participant Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Late exacerbations per participant Show forest plot | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.08, 0.54] |
| 4 Participants with improvement in exacerbations Show forest plot | 1 | 29 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.48 [0.30, 7.42] |
| 5 Participants with early improvements Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 6 Participants with late improvements Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 7 Mortality Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Early changes in lung function (% predicted FEV¹) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Early changes in lung function (FEV¹/FVC %) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Post immunisation lung function (FEV¹) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Participants with increased lung function (1 category) Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 12 Participants with decreased lung function Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 13 FEV¹ at end of study Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14 Participants with adverse effects (new upper respiratory tract symptoms) Show forest plot | 1 | 29 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.89 [0.45, 8.04] |
| 15 Participants with early adverse effects Show forest plot | 2 | 2244 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.86 [0.63, 1.17] |
| 16 Number of days with early symptoms and signs Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 17 Number of participants with early adverse effects (by type) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 COPD | 1 | 2215 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.30, 1.48] |
| 17.2 Dyspnoea | 1 | 2215 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.60, 5.41] |
| 17.3 Pharyngitis | 1 | 2215 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.35, 2.86] |
| 17.4 Flu syndrome | 1 | 2215 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.20, 1.91] |
| 17.5 Rhinitis | 1 | 2215 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.42, 5.34] |
| 17.6 Bronchitis | 1 | 2215 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.50, 8.05] |
| 17.7 Increased cough | 1 | 2215 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.14, 2.51] |
| 17.8 Myalgia | 1 | 2215 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.51 [0.49, 12.96] |
| 17.9 Increased sputum | 1 | 2215 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.17, 3.36] |
| 17.10 Pneumonia | 1 | 2215 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.37, 10.97] |
| 17.11 Asthenia | 1 | 2215 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.37, 10.97] |
| 17.12 Guillain‐Barré syndrome | 1 | 2215 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.19] |
| 17.13 Other | 1 | 2215 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.26, 0.92] |
| 18 Participants with late adverse effects Show forest plot | 2 | 2244 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.33 [1.22, 4.46] |
| 19 Acute respiratory illness subsequently documented as influenza‐related Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 20 Participants with at least one influenza‐like illness Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

