استفاده از واکسن آنفلوآنزا برای درمان بیماری مزمن انسدادی ریه (COPD)
چکیده
پیشینه
واکسیناسیون آنفلوآنزا (influenza vaccinations) اخیرا در درمان افراد مبتلا به COPD توصیه میشوند، اما این توصیهها عمدتا بر مبنای شواهد به دست آمده از مطالعات مشاهدهای بوده و تعداد کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) در این خصوص بسیار کم هستند. عفونت آنفلوآنزا منجر به موربیدیتی و مرگومیر بیشتر در افراد مبتلا به COPD میشود، اما احتمال بالقوهای مبنی بر بروز عوارض جانبی در تاثیر واکسیناسیون آنفلوآنزا یا هزینه‐اثربخش نبودن آنها نیز وجود دارد.
اهداف
تعیین اینکه واکسیناسیون آنفلوآنزا در افراد مبتلا به COPD باعث کاهش بیماری ریوی و مرگومیر میشود یا خیر، و اینکه استفاده از این واکسن منجر به بروز حوادث جانبی اضافی میشود یا خیر و اینکه استفاده از این واکسن هزینه‐اثربخش است یا خیر.
روشهای جستوجو
ما پایگاه ثبت کارآزماییهای راههای هوایی در کاکرین، دو پایگاه ثبت کارآزماییهای بالینی، و فهرست منابع مقالات را جستوجو کردیم. تعدادی از شرکتهای دارویی که با آنها تماس گرفتیم نیز منابعی را ارائه کردند. آخرین جستوجو در دسامبر 2017 به اجرا درآمد.
معیارهای انتخاب
RCTهایی که به مقایسه ویروس زنده یا غیر‐فعال شده با دارونما (placebo)، یا به تنهایی یا با یک واکسن دیگر، در افراد مبتلا به COPD پرداخته بودند.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده مرور بهطور مستقل از هم به استخراج دادهها پرداختند. تمامی ورودیها (entries) دو بار چک شدند. برای دستیابی به اطلاعات ازدسترفته با نویسندگان مطالعه و شرکتهای تولید کننده دارو تماس گرفتیم. از روشهای استاندارد مورد انتظار کاکرین استفاده کردیم.
نتایج اصلی
ما 11 RCT را با 6750 شرکتکننده وارد مرور کردیم، اما فقط شش مطالعه از این مطالعات، دربرگیرنده افراد مبتلا به COPD (2469 شرکتکننده) بودند. سایر مطالعات روی افراد مسن و با خطر بالا به اجرا درآمده بودند، به طوری که برخی از آنها دارای بیماری مزمن ریه بودند. مداخلات مقایسه شده با دارونما عبارت بودند از تزریقهای ویروس غیر‐فعال شده و واکسنهای ویروس اینترانازال ضعیف شده (attenuated intranasal virus). برخی مطالعات واکسن غیر‐فعال شده داخل عضلانی و واکسن زنده ضعیف شده اینترانازال را با واکسن عضلانی غیر‐فعال شده و دارونمای اینترانازال مقایسه کرده بودند. مطالعات در انگلستان، آمریکا و تایلند به اجرا درآمده بودند.
واکسن غیر‐فعال شده در مقایسه با دارونما، تعداد کل موارد تشدید شده را به ازای هر شرکتکننده واکسینه شده کاهش داده بود (تفاوت میانگین (MD): 0.37‐؛ 95% فاصله اطمینان (CI): 0.64‐ تا 0.11‐؛ P = 0.006؛ دو RCT؛ 180 شرکتکننده؛ شواهد با کیفیت پائین). این اتفاق به دلیل کاهش در موارد تشدید دیرهنگام بیماری بود که بعد از سه یا چهار هفته اتفاق میافتاد (MD: ‐0.39؛ 95% CI؛ 0.61‐ تا 0.18‐؛ P = 0.0004؛ دو RCT؛ 180 شرکتکننده؛ شواهد با کیفیت پائین). واکنشهای جانبی موضعی در شرکتکنندگان مبتلا به COPD و مسن (تنها اقلیتی از آنها که مبتلا به COPD بودند) که واکسن دریافت کرده بودند، به طور معنیداری بیشتر بود، اما تاثیرات به طور کلی ملایم و گذرا بودند.
هیچ شواهدی درباره تاثیر ویروس زنده ضعیف شده اینترانازال زمانی که به واکسیناسیون غیر‐فعال شده عضلانی افزوده شده بود، وجود نداشت.
دو مطالعه که مرگومیر را برای واکسن آنفلوآنزا در برابر دارونما ارزیابی کرده بودند، برای تشخیص هر گونه تاثیر روی مرگومیر بسیار کوچک بودند. با وجود این، یک مطالعه بزرگ (N = 2215) بیان کرده بود زمانی که ویروس زنده ضعیف شده به واکسیناسیون ویروس غیر‐فعال شده افزوده شده، هیچ تفاوتی در مرگومیر وجود نداشت.
نتیجهگیریهای نویسندگان
بر اساس تعداد محدودی از RCTها با بیش از یک دهه قدمت، که ما توانستیم وارد مرور کنیم، به نظر میرسد واکسن غیر‐فعال شده موارد تشدید بیماری را در افراد مبتلا به COPD کاهش داده بود. اندازه تاثیر با آنچه که در مطالعات مشاهدهای بزرگ دیده شده بود، مشابه و ناشی از کاهش در تشدید بیماری اتفاق افتاده سه هفته بعد از واکسیناسیون یا بیشتر و به دلیل آنفلوآنزا بود. افزایش خفیفی در بروز عوارض جانبی موضعی گذرا به دنبال واکسیناسیون وجود داشت، اما هیچ شواهدی مبنی بر افزایش در تشدید زودهنگام بیماری وجود نداشت. افزودن ویروس زنده ضعیف شده به واکسن غیر‐فعال شده هیچ مزیت اضافیای نشان نداده بود.
PICO
خلاصه به زبان ساده
واکسن آنفلوآنزا برای افراد مبتلا به بیماری مزمن انسدادی ریه (COPD)
سوال مطالعه مروری
آیا واکسنهای آنفلوآنزا، اپیزودهای بیماری تنفسی یا مرگومیر را در افراد مبتلا به بیماری مزمن انسدادی ریه (chronic obstructive pulmonary disease; COPD) کاهش میدهند؟
پیشینه
COPD یک چترواژه (umbrella term) است که برای توصیف بیماریهای ریوی پیشرونده شامل آمفیزم (emphysema)، برونشیت مزمن (chronic bronchitis) و آسم مقاوم (refractory asthma) (غیر‐قابل بازگشت) استفاده میشود. این بیماری به واسطه افزایش تنگی نفس شناخته میشود. تقریبا علیرغم وجود توصیههای جهانی مبنی بر ضرورت دریافت واکسیناسیون سالانه آنفلوآنزا از سوی افراد مبتلا به COPD، کارآزماییهای تصادفیسازی و کنترل شده (مطالعاتی که در آنها تعدادی از افراد مشابه به طور تصادفی برای تست یک داروی خاص، درمان یا مداخله دیگر در دو یا تعداد بیشتری از گروههای درمانی قرار داده میشوند) بسیار کمی تاثیر این درمان را ارزیابی کردهاند. واکسنهای آنفلوآنزا ممکن است با استفاده از ویروس غیر‐فعال شده (ذرههای کوچکی از دیواره ویروس) یا ویروس زنده ضعیف شده (قدرت کاهش یافته اما زنده) ساخته شوند.
برای پاسخ به این سوال، برای شناسایی مطالعات انجام شده در سراسر دنیا که به بررسی استفاده از واکسنهای آنفلوآنزا برای افراد مبتلا به COPD پرداخته بودند، به دقت جستوجو کردیم.
ویژگیهای مطالعه
ما شش مطالعه را با 2469 شرکتکننده مبتلا به COPD، و پنج مطالعه بیشتر را با 4281 شرکتکننده مسن یا با خطر بالا، که نسبتی از آنها مبتلا به بیماری مزمن ریه بودند وارد مرور کردیم.
نتایج کلیدی
ما پارهای از شواهد را با کیفیت متوسط به دست آوردیم مبنی بر اینکه واکسن آنفلوآنزای غیر‐فعال شده موارد تشدید COPD را، به ویژه موارد مربوط به خود ویروس آنفلوآنزا، کاهش داده بود. واکسن ویروس آنفلوآنزای غیر‐فعال شده که به صورت تزریق در داخل عضله تجویز شده بود، با افزایش در بروز عوارض جانبی موضعی گذرا (از جمله درد) در محل تزریق همراه بود. واکسن ویروس غیر‐فعال شده منجر به بروز آنفلوآنزا، یا هر نوع COPD تشدید شده معنیدار نشده بود. افزودن ویروس زنده ضعیف شده به ویروس غیر‐فعال شده، ایمنی بیشتری را برای شرکتکنندگان به همراه نداشت.
کیفیت شواهد
کیفیت شواهد متوسط بود. از سال 2004 تاکنون هیچ کارآزمایی جدیدی انجام نشده است. آخرین جستوجو در منابع علمی را در دسامبر 2017 انجام دادیم.
Authors' conclusions
Summary of findings
| Influenza vaccine compared to placebo for chronic obstructive pulmonary disease (COPD) | ||||||
| Patient or population: chronic obstructive pulmonary disease (COPD) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with Influenza vaccine | |||||
| Total exacerbations per participant | The mean number of total exacerbations per participant ranged across placebo groups from 0.83 to 1.35 | MD 0.37 lower | ‐ | 180 | ⊕⊕⊝⊝ | Despite the effect size, this is based on a very small number of trials and participants. However there was good agreement between these two studies (low I2). One study was not conducted in an epidemic year. We extrapolated some data. Ideally more trials would be done to refine these effect sizes. |
| Early exacerbations per participant | The mean number of early exacerbations per participant ranged across placebo groups from 0.14 to 0.34 | MD 0.01 higher | ‐ | 180 | ⊕⊕⊝⊝ | See above |
| Late exacerbations per participant | The mean number of late exacerbations per participant ranged across placebo groups from 0.48 to 1.21 | MD 0.39 lower | ‐ | 180 | ⊕⊕⊝⊝ | See above |
| Days disability from respiratory illness | not reported | |||||
| Hospital admissions | 76 per 1000 | 26 per 1000 | OR 0.33 | 180 | ⊕⊕⊝⊝ | |
| Mortality | 76 per 1000 | 67 per 1000 | OR 0.87 | 180 | ⊕⊕⊝⊝ | |
| Local effects at injection site | 63 per 1000 | 274 per 1000 | OR 5.57 | 125 | ⊕⊕⊝⊝ | Single study on participants who all had COPD (Wongsurakiat 2004a), but very similar findings in studies with a mixed population (Cate 1977; Govaert 1994) |
| GRADE Working Group grades of evidence | ||||||
| aSmall number of studies which are over ten years old limit our confidence in the generalisability of these findings to currently available vaccines (downgraded once for indirectness). b Studies too small and events too infrequent to detect a consistent effect (downgraded once for imprecision) | ||||||
Background
Description of the condition
Chronic obstructive pulmonary disease (COPD) occurs predominantly in older people who have smoked, and is characterised by progressive airflow obstruction that is largely irreversible. As the disease progresses, exacerbations may occur several times per year, and may require hospital admission. These exacerbations can take several weeks to resolve, during which time considerable morbidity may occur and result in significant health care costs. Infection with influenza is an important cause of excess mortality and morbidity in people with COPD (Rothbart 1995). It may affect the progression of the disease (Centanni 1997), and frequency of exacerbations (Sethi 2002). People with COPD are at an increased risk for respiratory illness‐related hospitalisation during influenza outbreaks, irrespective of age and degree of morbidity (Monto 1987).
Description of the intervention
Annual influenza vaccination is almost universally recommended in COPD guidelines (ATS 1995; ATS 2004; BTS 1997; GOLD 2018; NICE 2010; Siafakas 1995; Yang 2017). The largest body of evidence to support this recommendation comes from observational studies in the older population. In a large, serial cohort study of nearly 150,000 older people, those who had been vaccinated had a reduction of about 32% in the rates of hospitalisation for all respiratory conditions, and a reduction of approximately 50% in all‐cause mortality over their untreated counterparts (Nichol 1998). In people with chronic lung disease, those who were vaccinated had a 52% reduction in hospitalisations and a 70% reduction in death rate during influenza seasons (Nichol 1999). A meta‐analysis of 20 cohort studies of influenza vaccination in older people showed a 56% reduction in respiratory illness, a 53% reduction in pneumonia, a 50% reduction in hospitalisation, and a 68% reduction in deaths from all causes during influenza outbreaks (Gross 1995). The benefit was seen especially in epidemic years, when the vaccine strain was identical or similar to the epidemic strain (Gross 1995). Most studies suggest that vaccination is very cost effective. For example, Nichol and colleagues estimated that vaccination was associated with a reduction in health care costs of about USD 171 per year per high risk person vaccinated (Nichol 1998).
How the intervention might work
The effectiveness of the vaccine depends on the immunocompetence of the vaccine recipient and the degree of similarity between virus strains in the vaccine and those in circulation (ACIP 1999). Most vaccine programmes use an inactivated virus vaccine, which contains three virus strains (usually 2 type A and 1 type B), representing the influenza viruses likely to circulate in the upcoming winter. The vaccine is made from highly purified, egg‐grown viruses that have been inactivated. These vaccines may be whole virus, sub virion, or purified‐surface‐antigen preparations. The mechanism of protection by the vaccine is thought to occur via circulating antibodies to HA (Hemagglutinin) and NA (Neuramidase), acting against severe infection of the lower respiratory tract. Stimulation of cytotoxic T‐cell responses may also be important (Patriarca 1994). In general, older people have lower phagocytic function, and mount less of an immune response to vaccination than younger people (Treanor 1992). To improve vaccine efficacy, live attenuated viruses have been trialled. Levels of secretory anti‐HAs, immunoglobulins (Igs) and anti‐influenza A virus cytotoxic T‐cell responses were better in people with COPD after immunisation with monovalent live attenuated vaccine than with inactivated influenza A virus vaccines (Gorse 1991; Gorse 1995; Gorse 1996). Some investigators have co‐administered more than one type of vaccine, such as cold attenuated virus with inactivated virus vaccine, in an attempt to increase vaccine efficacy in people with COPD (Gorse 1997).
Why it is important to do this review
Despite guideline recommendations, vaccination of older people (the age group that includes most people with COPD) is not universal. In the USA in 1997, only 65.5% of older people were vaccinated in the previous year (BRFSS 1998). Yet, the only absolute contraindication to vaccination is chicken egg allergy. Other reasons for not vaccinating include uncertainty about the degree and longevity of protection in the older population and concern about adverse effects (Patriarca 1994). People with COPD, and their doctors, often express concern that vaccination precipitates exacerbations, despite the fact that it is not possible to contract influenza from inactivated virus. Adverse effects usually manifest within 24 hours of vaccination, and can be local or systemic. Several studies have shown that mild local side effects, at the site of injection, are more common in those who were vaccinated, than in those given placebo (Govaert 1994; Nichol 1995). Systemic reactions include myalgia, fatigue, headache, and low‐grade fever. These are more common in females, and after the administration of whole‐virus than sub virion vaccines. Higher doses and levels of pre‐existing antibody also increase the likelihood of these reactions (Cate 1977). The most feared complication of influenza vaccination is Guillain‐Barre Syndrome (GBS). However, this is extremely rare (approximately 1/1,000,000), and the benefits of the vaccine are thought to far out‐weigh the risks of developing vaccine‐associated GBS (ACIP 1999).
This systematic review evaluated the evidence from RCTs that had studied the effect of influenza vaccination in people with COPD. The review was originally published in 2000 (Poole 2000), and updated in 2006 (Poole 2006).
Objectives
To determine whether influenza vaccination in people with COPD:
-
Reduces respiratory illness;
-
Reduces mortality;
-
Is associated with excess adverse events; and
-
Is cost effective.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCT).
Types of participants
We included studies of adults with COPD, as defined by the American Thoracic Society (ATS 1995), or European Respiratory Society (Siafakas 1995). We also included studies with participants who were defined as having chronic bronchitis.
Types of interventions
We included studies randomising people to receive at least one annual influenza vaccination. Influenza vaccination may have been one of the following types: live attenuated whole virus, inactivated, or a split‐virus type vaccine, and may have been administered by either intramuscular injection or intranasal spray.
Types of outcome measures
We classified outcomes as early or late. 'Early' referred to the early post vaccination period, when immunity may not yet have developed, but adverse effects may have occurred
Primary outcomes
-
Exacerbations of COPD, defined as an increase in breathlessness, volume or purulence of sputum, or a combination. This was assessed in terms of both total numbers of exacerbations and participants with one or more exacerbations in the study period.
-
Days of disability from respiratory illness, defined as days in bed, days off work, or days when the person was unable to undertake normal activities
-
Hospital admissions
-
Mortality in the year following vaccination (All cause and respiratory‐related)
Secondary outcomes
-
Change in lung function from baseline, at the end of the study period
-
Adverse effects: acute
-
Acute respiratory illnesses subsequently proven to be influenza‐related
-
Cost effectiveness
Studies would be excluded if they:
-
Did not report outcomes of interest for this review
-
Were not placebo controlled
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Trials Register, which is maintained by the Information Specialist for the Group. The most recent search was conducted in December 2017. The Cochrane Airways Trials Register contains studies identified from several sources:
-
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies Online (crso.cochrane.org);
-
Weekly searches of MEDLINE Ovid SP;
-
Weekly searches of Embase Ovid SP;
-
Monthly searches of PsycINFO Ovid SP;
-
Monthly searches of CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature);
-
Monthly searches of AMED EBSCO (Allied and Complementary Medicine);
-
Handsearches of the proceedings of major respiratory conferences.
Studies contained in the Trials Register are identified through search strategies based on the scope of Cochrane Airways. Details of these strategies, as well as a list of handsearched conference proceedings are in Appendix 1. See Appendix 2 for search terms used to identify studies for this review.
Searching other resources
We searched for additional articles of interest in the bibliographic lists from all full‐text papers we retrieved. In 2006, we searched bibliographies of large reviews of influenza vaccination trials (Galasso 1977; Gross 1995), and recommendations of Advisory Councils (ACIP 1999; BRFSS 1998).
To locate other published or unpublished RCT data in 2006, we contacted pharmaceutical companies that had been involved in the conduct of vaccine trials, the manufacture of vaccines, or both. We contacted the following companies: Smith Kline Beecham, Glaxo‐Wellcome, Merck Sharp and Dohme, Astra, Parke Davis, Wyeth, Pasteur Merieux, and Commonwealth Serum Laboratories. In 2006, we also wrote to authors who had published extensively in the field to ask if they were aware of any further RCTs, published or unpublished.
For the review update (December 2017), we searched clinical trials registries (ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform) for current, recently completed, and ongoing studies.
Data collection and analysis
Selection of studies
For the original review, three review authors (PJP, RWB, EC) independently screened the titles and abstracts for potentially eligible studies and then retrieved the full texts to further screen for inclusion. For the updates this was done by two review authors (PJP and ZK). We resolved disagreement through discussion.
Data extraction and management
Review authors agreed the format of data extraction sheets. Two review authors independently extracted data. One review author entered the data onto the data extraction sheets, and then into Review Manager 4. A second review author double‐checked each entry. For versions of this review published after the 2006 update, we used Review Manager 5 (RevMan 2014).
Assessment of risk of bias in included studies
Two review authors (PJP, ZK) independently assessed the risk of bias for all included studies, using the criteria outlined in the Cochrane Handbook of Systematic Reviews of interventions (Higgins 2011). We resolved disagreements through discussion. We assessed the risk of bias according to the following items:
-
Random sequence generation;
-
Allocation concealment;
-
Blinding;
-
Incomplete outcome data
-
Selective outcome reporting;
-
Other potential bias.
We judged each potential source of bias as high, low or unclear risk of bias. We summarised our judgements of the risk of bias across different studies for each of the items listed.
Measures of treatment effect
We analysed continuous data as mean differences (MD) with 95% confidence intervals (CI) and dichotomous data as odds ratios (OR) with 95% CI or Peto odds ratios (Peto) with 95% CIs where pooling of odds ratios, or approximation to the odds ratio (i.e. for rare events) was required and appropriate i.e. intervention and control groups have similar numbers and number of events were rare (Higgins 2011).
Unit of analysis issues
The participant was the unit of analysis.
Dealing with missing data
Where there were insufficient data in the paper, we requested further data by writing to the author or pharmaceutical company sponsoring the study.
Assessment of heterogeneity
We assessed heterogeneity by visual inspection of the forest plots and calculation of the I² value; which was interpreted broadly as follows:
-
0 to 40%: may represent negligible/low heterogeneity
-
30 to 60%: moderate heterogeneity
-
50 to 90%: substantial heterogeneity
-
75 to 100%: considerable heterogeneity (Higgins 2011).
Assessment of reporting biases
We had planned to assess publication bias using a funnel plot, if there were a sufficient number of studies in a forest plot (more than 10).
Data synthesis
We used a fixed‐effect model and performed a sensitivity analysis with a random‐effects model.
We anticipated that the follow‐up period would be 12 months. Because of the small number of studies involved in this review, we did not annualise the event rate. If further data become available, it may be necessary to annualise the event rate, particularly if follow‐up periods vary.
Subgroup analysis and investigation of heterogeneity
In the case of significant heterogeneity, we had planned to run a sensitivity analysis based on risk of bias. If any heterogeneity could not be explained in terms of risk of bias, we planned the following sub‐group analyses:
-
Type of control group
-
Vaccine type
-
Severity of COPD (by baseline lung function)
-
Setting of study
-
Match between strain of vaccine and infecting strains
-
Age of participants
'Summary of findings' tables
We created a 'Summary of findings' table using the GRADEPro software (GRADEpro GDT 2015). We included all primary outcomes and adverse events. We used the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it related to the studies that contributed data for the prespecified outcomes. We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We justified all decisions to downgrade the quality of studies using footnotes.
Results
Description of studies
Results of the search
For the original review, we screened 105 abstracts of papers from the initial searches. After excluding those that were clearly ineligible, we obtained full texts for 25. An additional 40 articles were identified from bibliographies and references provided by pharmaceutical companies. Commonwealth Serum Laboratories provided another 70 references from an independent search. We obtained full‐text articles when the title and abstract indicated the study was possibly eligible. We reviewed a total of 70 full texts for possible inclusion.
The 2003 search yielded four new abstracts, including two reports of the same eligible study (Gorse 2003; Neuzil 2003). The 2004 search yielded a further five abstracts, including two reports of the same eligible study (Wongsurakiat 2003; Wongsurakiat 2004a). Dr Wongsurakiat identified a further study, published after the 2006 search (Wongsurakiat 2004b).
We did not identify any new eligible studies from searches conducted in December 2017 (see Figure 1 and 'Characteristics of excluded studies' table for further detail).

Study flow diagram for 2018 update
We included 11 studies (reported in 16 publications) in this review, with 6750 participants. We wrote, requesting more information, on six of them (Cate 1977; Gorse 1995; Govaert 1994/Govaert 1994a; Treanor 1992; Treanor 1994; Wongsurakiat 2004a). Dr Treanor and Dr Cate kindly supplied individual participant data. We received a reply from Dr Gorse but he was unable to supply us with further data. Dr Wongsurakiat kindly provided useful further information.
Included studies
Eleven studies met the entry criteria (see 'Characteristics of included studies' table); all were RCTs, using a parallel group design. All studies were double‐blind and placebo‐controlled, except for Gorse 1995 and Gorse 1997. These two studies were single‐blind and compared intra‐muscular inactivated vaccine and intranasal live attenuated vaccine with intra‐muscular inactivated vaccine and intranasal placebo. Because these studies assessed the additional benefit of a second vaccine, we assessed them as a separate comparison in this review.
Population
Six of the 11 trials in this review studied participants with COPD or chronic bronchitis alone (Fell 1977; Gorse 1997; Gorse 2003; Howells 1961; MRC 1980; Wongsurakiat 2004a). These studies ranged in size from 29 participants in Gorse 1997, to 2215 in Gorse 2003, and included a total of 2469 participants. The other five trials were conducted in older or chronically ill people, or both, a proportion of whom had chronic lung disease. In these studies, the percentage of people with chronic lung diseases varied from 32% in Gorse 1995, to 5% in Cate 1977. From these authors, we sought individual participant data for the subgroup with chronic lung disease, in particular COPD. Where possible, data from the lung disease subgroup are included, although in none of these studies was it possible to ascertain whether this lung disease subgroup had COPD. The studies that included a minority of people with chronic lung disease are described in the discussion section for comparison with the six studies carried out exclusively in people with COPD.
The following descriptions refer only to the six studies specifically investigating influenza vaccination in COPD or chronic bronchitis alone:
Timing: three studies were conducted during winter months (Fell 1977; Gorse 2003; Howells 1961).
Setting: Fell 1977 was in a group practice, and four were in hospital outpatient clinics (Gorse 1997; Gorse 2003; Howells 1961; Wongsurakiat 2004a).
Duration: length of trials varied from as little as three weeks with MRC 1980, to one year (Wongsurakiat 2004a).
Inclusion and exclusion criteria: Howells 1961, MRC 1980, and Fell 1977 studied people with chronic bronchitis. Gorse 1997, Gorse 2003, and Wongsurakiat 2004a specifically studied people with pre‐existing COPD, categorised by FEV1/FVC ratio less than 70% (forced expiratory volume in the first second/Forced vital capacity). Exclusion criteria were varied. They were explicit in Gorse 1997, Gorse 2003, Wongsurakiat 2004a, and MRC 1980, limited in Howells 1961 to Grade 4 bronchitis, and were not reported at all in Fell 1977.
Participant characteristics: the mean age was 67.3 years in the five studies that reported it (Fell 1977; Gorse 1997; Gorse 2003; Howells 1961; Wongsurakiat 2004a). The percentage of males ranged from 64% in Fell 1977 to 100% (Gorse 1997). The latter was a study in US veterans.
Comorbidities: 31% of the treatment group in Gorse 1997 had underlying liver disease. Both treatment and control groups in this study had similar proportions of other underlying diseases. 30% of the treatment group of Fell 1977 was on digoxin, and 8% had coexistent asthma and chronic bronchitis. In Gorse 2003, 95% had comorbidities, and in Wongsurakiat 2004a, this level was 33%.
Smoking history: 97% of the participants of Gorse 1997 had a smoking history, 93% in Fell 1977, 95% in Gorse 2003, and 96% in Wongsurakiat 2004a.
Lung function: the mean peak flow was 280 L/min from the two studies that reported these measurements. Wongsurakiat 2004a stratified the participants in the study by baseline FEV1. Thirty‐six per cent of people had an FEV1 ≥ 70% predicted, 26% an FEV1 of 50% to 69%, and 38% an FEV1 < 50%. The mean baseline FEV1 in Gorse 2003 was 1.38 L (43.5% predicted).
The treatment and control samples were generally well matched, except in Fell 1977, where baseline adverse symptoms were higher in the vaccinated group. This particular study was unusual in that it used the early post vaccination symptoms as the baseline for assessing late post vaccination symptoms. In this study, despite randomisation, there was a significant difference between treatment and control in baseline symptom scores, serum antibody levels, and comorbidities. No details of baseline characteristics were provided by one study (MRC 1980).
Intervention
Vaccination type: two studies used inactivated virus (Howells 1961; Wongsurakiat 2004a). Four studies assessed the effects of live attenuated intranasal virus vaccines (Fell 1977; Gorse 1997; Gorse 2003; MRC 1980), with Gorse 1997 and Gorse 2003 assessing the add‐on benefit of live intranasal virus, while both treatment and control groups received inactivated virus vaccine intramuscularly. We examined these studies separately in the analysis.
Match between vaccine and influenza strains: Fell 1977 reported that their study was carried out in a non‐epidemic year. Wongsurakiat 2004a reported their study was carried out in a non‐epidemic year; however, there was a good match between the influenza that did occur and the serotypes in the vaccine. Gorse 2003 reported a regional outbreak in the study area, with a virus antigenically similar to a vaccine strain. The other studies did not report the match.
Outcome measures
Clinical outcomes that could be evaluated included: exacerbations (Fell 1977; Gorse 1997; Gorse 2003; Howells 1961; Wongsurakiat 2004a), hospitalisations, lung function, adverse effects, and mortality. An assessment of serological outcomes alone was not the purpose of this review. Outcomes were defined as 'early' and 'late' to try and address whether vaccination led to an increase in exacerbations before immunity had developed. We had planned to define 'early' as one to two weeks after vaccination, but Howells 1961 used a period of three weeks, and Wongsurakiat 2004a, four weeks. Wongsurakiat 2004a recorded all acute respiratory infections (ARIs; total of 269 events), which were then subdivided by presentation into common cold (85 events), influenza‐like illnesses (20 events), acute exacerbations (161 events), and pneumonia (three events). Thus, the commonest presentation was 'acute exacerbation' (60% of events). He also conducted an economic evaluation (Wongsurakiat 2003). Gorse 2003a assessed health status by the chronic lung disease index (CLDI), reported by vaccination status.
Dropouts: there was a range of 0% to 19% of participant withdrawals. There were none reported in Gorse 1997. In the MRC 1980 multi‐centre study, 16 participants from one centre had no baseline data and 15 had incomplete records. In Gorse 2003 (the largest study with 2215 participants), 9% dropped out. In Wongsurakiat 2004a, three out of 125 participants dropped out.
Excluded studies
The main reasons for study exclusion were a lack of randomisation, and absence of primary outcome data (Gorse 1988; Gorse 1996; Lama 1998). We excluded another two studies because they lacked a placebo control (Ambrosch 1979; MRC 1959). See 'Characteristics of excluded studies' table for full details.
Risk of bias in included studies
See Figure 2 for a summary of the 'Risk of bias' assessments. We judged many domains as unclear risk of bias, due to lack of detail present in the publications. This is unsurprising, considering all included trials were published in 2004 or earlier, when standards and guidelines for reporting were less stringent.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
We judged three trials (Gorse 2003; Govaert 1994; Wongsurakiat 2004a) at low risk of bias for random sequence generation, but the remaining eight studies reported insufficient information to permit a judgement. We judged three trials (Govaert 1994; Howells 1961; Wongsurakiat 2004a) to be at low risk of bias for concealment of the allocation sequence, while the remaining eight were unclear.
Blinding
We judged four trials to be at low risk for performance bias (Fell 1977; Govaert 1994; Howells 1961; Wongsurakiat 2004a) and three at low risk of detection bias (Fell 1977; Govaert 1994; Howells 1961) as they described suitably identical placebos, however, the remaining studies were unclear; this was due either to not describing the nature of the placebo, the nurse delivering the intervention not being blinded, or a lack of detail about blinding procedures.
One trial did not blind the outcome assessor and consequently, we marked it as high risk of bias (Gorse 1995). We judged three as low risk because they used blinded outcome assessors, but seven studies didn't describe this in sufficient detail to permit a judgement other than unclear.
Incomplete outcome data
We judged one study to be at high risk of attrition bias owing to the fact that 11 of the vaccinated participants were excluded from the study from a single centre (MRC 1980). Seven studies were judged to be at low risk (Fell 1977; Gorse 1997; Govaert 1994; Howells 1961; Treanor 1992; Treanor 1994; Wongsurakiat 2004a), and three were at unclear risk (Cate 1977; Gorse 1995; Gorse 2003).
Selective reporting
All studies were reported in insufficient detail to permit a decision.
Other potential sources of bias
None noted.
Effects of interventions
Influenza vaccination versus placebo
Results that follow relate to the six trials in people with COPD, chronic bronchitis, or both.
Primary outcome
Exacerbations of COPD
Two studies in 180 participants with COPD, chronic bronchitis, or both (both used inactivated virus vaccination) reported continuous data for exacerbation rates (Howells 1961; Wongsurakiat 2004a). Vaccination significantly reduced the number of exacerbations per participant during the follow‐up period (MD –0.37 exacerbations, 95% CI –0.64 to –0.11; P = 0.006; two RCTs, 180 participants; Analysis 1.1).
We determined the number of early or late exacerbations per participant by further interpretation of the data. For the placebo group of Howells 1961, 20 participants experienced 24 exacerbations. Since there were eight participants experiencing early exacerbations, there would have been at least eight early exacerbations. Similarly, since there were 12 participants experiencing late exacerbations, there would have been at least 12 late exacerbations. Thus the assumption was made that in order to make up the total of 24 exacerbations, there were two more early and two more late exacerbations. In support of this conclusion is the statement in the paper that similar numbers of early exacerbations were recorded in both placebo and vaccinated groups. Sensitivity analysis using 12 early and 12 late exacerbations showed no difference in the significance of our results. Wongsurakiat 2004a provided the number of early and late exacerbations without a spread. As the number of early exacerbations was small, it was assumed that these occurred in separate participants, and we calculated the SD accordingly. We allocated the SD of the 'total exacerbations'' per participant provided by the author to the 'late exacerbations'.
While there was no statistically significant effect of vaccination on early exacerbation rates (MD 0.01, 95% CI –0.11 to 0.13; P = 0.87; two RCTs, 180 participants; Analysis 1.2), inactivated influenza vaccination significantly reduced late exacerbation rates (MD –0.39, 95% CI –0.61 to –0.18; P < 0.001; two RCTs, 180 participants; Analysis 1.3).
Wongsurakiat 2004a reported no clear difference in the overall incidence or severity of acute respiratory infections (ARIs) between the vaccination and placebo groups.
Participants with at least one exacerbation or acute respiratory illness in the study period
Three studies, with 444 participants, contributed to this outcome (Fell 1977; Howells 1961; Wongsurakiat 2004a). There was no significant difference between vaccination and placebo‐treated participants with respect to the number of participants having at least one exacerbation or acute respiratory illness (Peto 0.81, 95% CI 0.44 to 1.48; P = 0.49; three RCTs, 444 participants; Analysis 1.4). However, there was significant heterogeneity in this result (P = 0.001), so it must be treated with caution. A sensitivity analysis by vaccine type showed that if only the two studies that used inactivated virus vaccine were included, this heterogeneity was removed, with a reduction in the number of participants with at least one exacerbation or acute respiratory illness in the study period with vaccination (OR 0.42, 95% CI 0.21 to 0.85; P = 0.02; two RCTs, 180 participants). Results from Howells 1961 and Wongsurakiat 2004a showed no significant difference in the number of individual participants with early exacerbations (Peto 1.08, 95% CI 0.52 to 2.26; P = 0.84; two RCTs, 180 participants; Analysis 1.5), but Howells 1961 did show a significant reduction in the number of participants with late exacerbations (Peto 0.13, 95% CI 0.04 to 0.45; P = 0.002; one RCT, 55 participants; Analysis 1.6). In Wongsurakiat 2004a, nine of 76 exacerbations in the vaccination group were early; while 10 of 85 exacerbations in the placebo group were early. We assumed that they each occurred in a different participant. Clearly, over the course of the study, there were some participants who had more than one exacerbation; the number of individual participants in each group who had late exacerbations was not reported.
Days of disability from respiratory illness
Not reported.
Hospital admissions
Two studies, with 180 participants, reported data on this outcome (Howells 1961; Wongsurakiat 2004a). There was no significant effect of vaccination over placebo on hospitalisation (Peto 0.33, 95% CI 0.09 to 1.24; P = 0.52; two RCTs, 180 participants; Analysis 1.7). In Howells 1961 there were no hospitalised participants in the treatment group, and only two in the control group. Wongsurakiat 2004a reported the number of hospitalisations for influenza‐related respiratory infections only. There were two in the vaccine group and five in the placebo group. They reported no clear difference in the severity of acute respiratory infections between groups, including no clear difference in the chance of being hospitalised (P = 0.2 by log rank test). None of the vaccinated participants required mechanical ventilation for acute respiratory infection, whereas five in the placebo group did.
Mortality
Two studies, with 180 participants, reported all‐cause mortality; there was a total of 13 deaths (Howells 1961; Wongsurakiat 2004a. There was no significant difference between vaccine and placebo‐treated groups (Peto 0.87, 95% CI 0.28 to 2.70; P = 0.81; two RCTs, 180 participants; Analysis 1.8). One control participant died during an acute exacerbation (Howells 1961). In Wongsurakiat 2004a, 12 of 125 participants died (eight of which were unrelated to acute respiratory infection); of those that were ARI related there was no demonstrable difference between vaccination and control (OR 0.33, 95% CI 0.03 to 3.24; Analysis 1.9).
Secondary outcomes
Lung function
One small study (55 participants) reported FEV1 changes from baseline (MRC 1980). There was no significant difference between the groups' overall change in FEV1 (MD –0.02, 95% CI –0.12 to 0.08; Analysis 1.10), nor in their change in early FEV1 (MD ‐0.01, 95% CI ‐0.09 to 0.07; Analysis 1.11) but there was insufficient evidence to prove no difference in treatments. Wongsurakiat 2004b reported no clear difference in lung function between groups at one and four weeks after vaccination.
Adverse effects and acute respiratory illness subsequently proven to be influenza‐related
Wongsurakiat 2004b evaluated local and systemic symptoms in the weeks following vaccination. There was no significant difference in the incidence of systemic adverse effects between the treated and placebo groups (OR 0.74, 95% CI 0.31 to 1.74; Analysis 1.12). The only significant difference observed was in the local reaction at the injection site; seen in 27% of vaccinees and 6% of the placebo group (OR 5.57, 95% CI 1.75 to 17.71; Analysis 1.13).
Fell 1977 (44 participants) reported early (within two weeks of vaccination) upper respiratory tract symptoms. One vaccinated participant also developed pleuritic pain. There was no statistically significant difference between vaccinated and control participants in terms of breathlessness or tightness (Peto 1.28, 95% CI 0.38 to 4.31; P = 0.696; Analysis 1.14; Analysis 1.15). The occurrence of a wheeze within the first two weeks was greater in vaccinated participants (Peto 3.57, 95% CI 1.10 to 11.56; P = 0.034; Analysis 1.16).No statistically significant difference between groups was noted for cough (Peto 4.09, 95% CI 0.74 to 22.49; P = 0.106; Analysis 1.17).
Two studies assessed the clinical presentations to see if they were related to influenza virus infection (Howells 1961; Wongsurakiat 2004a). Howells 1961 used the hemagglutination inhibition (HAI) test, and Wongsurakiat 2004a used both serology and virology swabs. Overall, inactivated influenza vaccination resulted in a marked decrease in influenza‐related respiratory infections (OR 0.19, 95% CI 0.07 to 0.48; P < 0.001; two RCTs, 180 participants; Analysis 1.18). The effect was similar whether participants had mild, moderate, or severe COPD, or chronic bronchitis (test for heterogeneity P = 0.73). Influenza accounted for 8% (13/161) of the acute exacerbations in the Wongsurakiat 2004a study. Further, the study demonstrated that there was no significant difference in early ARI (OR 0.72, 95% CI 0.34 to 1.50; Analysis 1.19).
In terms of sputum production no significant difference was noted between the vaccination group and control (Peto 2.03, 95% CI 0.48 to 8.66; P = 0.338; Analysis 1.20). Breathlessness was recorded significantly less often (P < 0.05) in the 5 of 21 participants who had a serological response to vaccination than in the placebo group.
The Treanor 1994 paper reports that 12% of the older people vaccinated with live attenuated virus reported systemic symptoms of malaise and myalgias, as did 10% of inactivated virus vaccinees. The placebo group reported none. Twenty‐six per cent of those who received the live virus reported lower respiratory tract symptoms of hoarseness and non‐productive cough, as did 13% of those who received the inactivated virus, and 9% of those who received placebo. Twenty‐nine per cent of those who received live virus reported upper respiratory tract symptoms of sneezy, runny, or stuffy nose or sore throat, as did 37% of those who received the inactivated virus, and 18% of those who received placebo. Six per cent of those who received the live virus experienced fever, as did 2.5% of those who received the inactivated virus. None of the placebo group reported any febrile illness. In the subgroup of participants with chronic lung disease in this study, of the 20 participants who received some form of influenza vaccination, 11 reported a total of 20 adverse effects, while of the two participants who received the placebo, one participant reported two adverse effects.
The Govaert 1994 paper reports 25% of high risk vaccinees experienced one or more adverse reactions, compared to 16% of those who received placebo, however, effects, if any, appeared to have been mild and transitory. Eleven per cent of all vaccinees experienced systemic effects, as did 9.4% of the placebo recipients. When a multiple regression analysis that looked at the effect of lung disease on systemic adverse reactions was performed, the difference between vaccinees and the placebo group was statistically significant (OR 1.95, 95% CI 1.24 to 3.07). Local effects were experienced by 17.5% of all vaccinees in this study, but in only 7.3% of the placebo group (P < 0.001). This study also showed that differences between the treatment groups for adverse effects reduced with age.
The Cate 1977 paper reports 7.8% of those who received inactivated virus reported mild systemic reactions, and 4.9% reported moderate to severe ones. The control group who received saline placebo reported similar numbers of mild systemic reactions, but no moderate to severe ones. Most systemic reactions resolved within two days of vaccination. In the vaccinated group, 18.5% of participants experienced erythema (local redness) with or without induration (hardening) at the injection site, compared to none in the control group.
Cost effectiveness
Wongsurakiat calculated the incremental cost effectiveness ratios of inactivated virus vaccination by applying the direct medical costs from a Thai health provider perspective to the results obtained in the RCT (Wongsurakiat 2004a). There were two types of cost: cost of treatment as an outpatient, and cost of hospitalisation. More than 90% of the costs of influenza‐related acute respiratory illness (ARI) were costs of hospitalisation. In people with moderate or severe COPD, more than 90% of the hospital costs were due to costs of treating those who required mechanical ventilation. The costs were based on 1997 prices using Thai Bhat (THB), with vaccination costing 248.40 THB. In the paper the author concluded that cost savings would be 629,538 THB for every 100 people with mild COPD vaccinated, 184 THB for every 100 with moderate COPD, and 680,647 THB for those with severe COPD, i.e. vaccination was very cost effective, but more so in those with more severe COPD.
Live attenuated intranasal vaccine plus inactivated intramuscular vaccine versus placebo intranasal or inactivated intramuscular vaccine
Four studies (2817 participants) evaluated the effect of adding live attenuated virus to inactivated virus vaccination (Gorse 2003; Gorse 1995; Gorse 1997; Treanor 1992). The Gorse 1997 and Gorse 2003 studies were specifically conducted on people with COPD, but the others were carried out in older people, only a minority of whom had lung disease. For simplicity, 'treatment' refers to the live intranasal plus inactivated group, and 'control' to the placebo intranasal plus inactivated vaccine group. Only the Gorse 1997 and Gorse 2003 studies provided data in a form that could be used in analyses.
Primary outcomes
Exacerbations of COPD
There were no significant differences in the total number of exacerbations per participant between the two groups (MD 0.01, 95% CI ‐0.35 to 0.37; P = 0.96; two RCTs; 1137 participants; Analysis 2.1). In the former, exacerbations were defined as the occurrence of increased cough, shortness of breath, sputum production, or a combination. There was no clear difference in either the early exacerbation rate per participant (MD –0.21, 95% CI –0.55 to 0.13; P = 0.23; one RCT, 29 participants; Analysis 2.2), or late exacerbations (MD –0.23, 95% CI –0.08 to 0.54, P = 0.14; one RCT, 29 participants; Analysis 2.3) between groups. In the latter, participants were asked to report any febrile influenza‐like illness (ILI). This was then investigated by serology, swabs, or both to determine if it was influenza‐related.
There was no clear difference in the number of participants who reported improvements in their exacerbations (Peto 1.48, CI 0.30 to 7.42; P = 0.63; one RCT, 29 participants; Analysis 2.4); this finding was consistent when broken down into early improvements (Peto 1.65, 95% CI 0.16 to 17.49; one RCT, 29 participants; Analysis 2.5), and late improvements (Peto 1.26, 95% CI 0.19 to 8.43; one RCT, 29 participants; Analysis 2.6).
In the Gorse 2003a paper, a univariate and stepwise multivariate logistic regression analysis of associations was performed, with at least a 15% improvement or worsening in health status, as measured by the chronic lung disease symptom index. This was a secondary study outcome. Analysis showed that 217 (21%) in the group that received inactive vaccine plus intranasal vaccine had at least a 15% improvement at the end of the study over pre‐vaccine status, compared with 163 (16%) in the control group that had received inactivated virus vaccine alone (OR 1.39, CI 1.10 to1.74).
Days of disability from respiratory illness
Not reported
Hospital admissions
Not reported
Mortality
In the Gorse 2003 paper, the largest study with 2215 participants, there were 64 deaths (3%); there was no difference between intervention and control groups for this outcome (OR 1.14, 95% CI 0.69 to 1.87; one RCT, 215 participants; Analysis 2.7). Of the 64 participants who died, five participants in the treatment group and two in the placebo group had influenza‐like illnesses, four of which were laboratory‐documented.
Secondary outcomes
Lung function
There was no consistent effect on early changes in lung function in Gorse 1997either in terms of percent predicted FEV1 (MD 2.90; 95% CI ‐14.14 to 19.94; one RCT, 20 participants; Analysis 2.8) or FEV1/FVC ratio (MD ‐0.90; 95% CI ‐12.02 to 10.22; one RCT, 29 participants; Analysis 2.9). The results from Gorse 2003 suggested significant effect on lung function in favour of the active virus group (MD ‐0.05; 95% CI ‐0.10 to 0.00, one RCT, 2215 participants; Analysis 2.10), however, there was a significant difference between the two study groups at baseline reported in the paper, with the active group being lower, and improving more. The investigators reported that they did not believe this to be clinically important. The lung functions of the two groups at the end of the study were similar. Gorse 1997 reported that more participants in the active virus group experienced a one category improvement in lung function compared to control, however, this was found to be non‐significant (Peto 4.00; 95% CI 0.68 to 23.60; one RCT, 29 participants; Analysis 2.11). The investigators also reported that a small number of participants in the active virus group experienced a one‐category decrease in lung function, however, once again this was found to be non‐significant (Peto 7.04; 95% CI 0.66 to 74.68; one RCT, 29 participants; Analysis 2.12). Gorse 2003 measured FEV1at the end of the study period and found that there was no evidence of an effect on lung function for those receiving the active virus compared to control (MD ‐0.05; 95% CI ‐0.16 to 0.06; one RCT, 382 participants; Analysis 2.13).
The Treanor 1994 paper reported no significant differences in lung function between groups. From data supplied by the author, for the subgroup with underlying chronic lung disease, those who were vaccinated had a mean decrease in FEV1 from 1.8 litres to 1.6 litres, whereas for the one placebo recipient for whom lung function was recorded, there was a small increase in FEV1.
Adverse effects
There were no significant differences in the reports of new upper respiratory tract symptoms between the groups in the Gorse 1997 study (Peto 1.89, 95% CI 0.45 to 8.04; P = 0.39; one RCT, 29 participants; Analysis 2.14).
There were no statistically significant differences between treatment and control for any adverse effects in the early period (Peto 0.86, 95% CI 0.63 to 1.17; P = 0.34; two RCTs, 2244 participants; Analysis 2.15). When evaluating the results of Gorse 2003 alone, the number of days participants displayed early signs and symptoms favoured control (MD 0.40; 95% CI 0.19 to 0.61; one RCT, 2215 participants; Analysis 2.16). Furthermore, analysis of the number of participants with early adverse events, divided in to sub‐groups by type, showed no evidence of an effect between intervention or control for any single adverse effect; however, the pooled effect was significantly in favour of the active virus group (OR 0.49; 95% CI 0.26 to 0.92; P = 0.027; one RCT, 2215 participants; Analysis 2.17).
There was some evidence of an effect for adverse effects in the late period following vaccination, favouring the control group (Peto 2.33, 95% CI 1.22 to 4.46; P = 0.011; two RCTs, 2244 participants; Analysis 2.18). Gorse 2003 stated in their paper that the proportion of participants with adverse effects at least possibly related to immunisation, did not differ between groups. There were, however, significantly fewer participants with early signs and symptoms in the group receiving inactivated virus vaccine only, as well as a smaller number of participants with late adverse effects. In this group, there was a total number of 99 events in 88 individuals (7.9% of total).
From one RCT (Gorse 2003) there was no evidence of an effect between active virus and control for the outcome of influenza‐related ARI (OR 0.84; 95% CI 0.57 to 1.24; one RCT, 2215 participants; Analysis 2.19). Further, data from the same study demonstrated no statistical difference in the number of participants with at least one influenza‐like illness between groups (OR 1.07; 95% CI 0.86 to 1.33; one RCT, 2215 participants; Analysis 2.20).
In Treanor 1992 (523 participants), which was a study with a lung disease subgroup, 24% of the treatment group experienced respiratory illnesses compared to 28% of the control group. In the treatment group, 12% of participants experienced an influenza‐like illness, compared to 16% of the control group. In the control group, there were two cases of laboratory‐documented influenza A infection, which resulted in hospitalisation. One death due to influenza virus A infection occurred in the control group, compared to none in the treatment group. This study also showed that 10.1% of the treatment group experienced early adverse reactions, compared to 8.3% of the control group. In the treatment group, 3.9% of participants reported early systemic effects consisting of headache, myalgias, malaise, or fatigue, compared to 5.0% of the control group. Of the control group, 1.1% reported fever, and 2.7% of them reported respiratory symptoms consisting of rhinitis or pharyngitis. However, 2.2% of the treatment group reported fever, and 6.7% reported respiratory symptoms. Five per cent of the live virus vaccinees experienced sore arms, compared to 18% of the inactivated virus vaccinees. None of the placebo group reported sore arms. The tendency for inactivated virus to cause local side effects to a greater extent than live virus was statistically significant in this study (P = 0.02).
In the Gorse 1995 paper it was reported that 12% of all participants experienced transient, mild pain at the site of local intramuscular injection.
Acute respiratory illness subsequently proven to be influenza‐related
Not reported
Cost effectiveness
Not reported
Discussion
Summary of main results
This systematic review evaluated the few RCTs that have reported on the effects of influenza vaccination in people with COPD. Despite iterative and exhaustive searches, we only identified 11 studies that met our inclusion criteria, with only six of these having been performed solely on 2562 people with COPD or chronic bronchitis. The entry criteria for these studies were variously reported, but where reported, they showed that the majority of participants had a smoking history and airway obstruction. The earlier studies enrolled younger participants than the more recent studies. The other studies in the review included older participants, those with chronic illnesses, or both, of whom a subset had chronic lung disease.
Influenza vaccination versus placebo
There was no evidence of any significant effect on hospitalisation, mortality rates, lung function decline, or exercise tolerance between the vaccine and placebo groups. For the infrequent outcomes of hospitalisation and mortality, the studies were probably too small to detect any difference. One participant in the control group in Gorse 2003 developed Guillain–Barré syndrome, although further details of severity and outcome were not provided. Thus, this review showed no evidence of an increase in Guillain–Barré syndrome with vaccination.
We did not intend to evaluate serological outcomes, such as a significant rise in antibody titre in this review. However, some authors looked at outcomes in the subgroup with a serological response to vaccination. The limited data from these comparisons was consistent with that of the clinical outcomes alone. It also suggested that if people seroconverted, they had fewer adverse effects.
Neuzil 2003 and Wongsurakiat 2004a studied the clinical presentation of symptomatic laboratory‐documented influenza (LDI). Using stepwise logistic regression, Neuzil 2003 found that during an influenza outbreak period, only fever and myalgia were associated with LDI. Together, they had a positive predictive value of 41%. In Wongsurakiat 2004a, the most specific presentation of LDI was 'influenza‐like illness' (namely generalised aches, fever and headache, with or without respiratory tract symptoms). However, LDI occurred in only 10% of participants, indicating a low positive predictive value of this symptom complex for LDI. The conclusion was that it was difficult to diagnose influenza infection clinically, with certainty, in people with COPD.
The one cost‐effectiveness analysis that was conducted, based on an RCT, suggested that inactivated virus vaccination was highly cost‐effective in people with COPD, particularly those with severe airways obstruction. This analysis took into account direct health care costs only, and not indirect costs, or any future health care costs that might be incurred by people with COPD living longer. It was conducted in a non‐epidemic year, and therefore, underestimated the benefits that would be gained in an epidemic year.
Influenza vaccinations were generally well tolerated. There was a significant increase in local effects ranging from pain at the site of injection, to erythema, with or without induration, but all effects appeared to be mild and transitory. These findings were consistent with results from Nichol 1994, a large, well conducted randomised placebo‐controlled trial of influenza vaccination in healthy adults, in which they observed no significant side effects of vaccination, except for arm soreness (63.8% of vaccinees compared to 24.1% placebo; P = 0.001).
One of the main barriers to increasing vaccination rates in people with COPD is the concern of patients and their health professionals that vaccination may increase early exacerbations, before immunity has developed. The evidence in this review showed that inactivated virus vaccination did not have a significant impact, on either the total number of early exacerbations, or on the number of people with COPD who had early exacerbations or early acute respiratory illnesses.
Live attenuated intranasal plus inactivated intramuscular versus placebo intranasal plus inactivated intramuscular vaccination
Because of the risk of influenza and the lesser immunogenicity of vaccines in the elderly (including people with COPD), there is interest in the extra protection afforded by the addition of live attenuated virus to inactivated virus vaccination. This approach to clinical trial design has the advantage that high‐risk groups are not denied vaccination, but larger numbers of participants are needed if the study is to have sufficient power to detect an effect. The studies in this review showed that there was no greater protective effect of live plus inactivated vaccine over inactivated vaccine alone in any of the clinical outcomes of interest. On the other hand, there may be a slight increase in adverse effects with the combination, although this was seen only in Gorse 2003, and the investigators did not regard it as significant.
Overall completeness and applicability of evidence
Recent literature searches indicated that new RCTs for influenza vaccine in COPD have stagnated, with no studies meeting our inclusion criteria since 2006. This is perhaps a reflection of consistent recommendations in major clinical guidelines for COPD management over the last decade, based on existing observational studies and limited placebo controlled RCTs. The most recent updated search for this review returned a number of studies investigating immunostimulants for COPD management, and while outside the scope of this review, may present an adjunct to disease management, by preventing respiratory tract infection and subsequent COPD exacerbations (Collet 1997).
Quality of the evidence
The main issue was that there were few RCTs of influenza vaccinations in COPD, and data were generally not reported in the same way. Where data were combined, confidence intervals were wide, making it difficult to determine whether 'no evidence of effect' actually meant 'evidence of no effect', or an effect was missed. However, the studies that were found were of satisfactory methodological quality, with almost all low or unclear risk of bias assessments in all domains. The effects observed in RCTs were internally consistent, biologically plausible, and supported by observational studies. Furthermore, result from the two main studies had little heterogeneity between them.
Potential biases in the review process
We performed the review in accordance with a pre‐published protocol to reduce biases. However, we did make some post hoc changes, See Differences between protocol and review.
Agreements and disagreements with other studies or reviews
In the original Cochrane Review in 2000, we wrote "the strong recommendations in current guidelines make it ethically difficult now to conduct large, randomised, placebo‐controlled trials of influenza vaccination, even though it would appear desirable to do so". Interestingly, and without our knowledge, such a trial had been conducted but not reported at the time (Wongsurakiat 2004a). The authors believed the study to be justified on the grounds that prior to 1997, influenza vaccine had been unavailable in Thailand. This study of inactivated influenza vaccination tracked 125 participants over one year following vaccination, with only three dropouts. An analysis with the results from Howells 1961, another carefully conducted RCT, showed that inactivated influenza vaccine significantly reduced COPD exacerbations with an effectiveness ((1‐RR) x 100%) of over 60%. Moreover, inactivated influenza vaccination had an effectiveness of over 80% in reducing influenza‐related acute respiratory illness (ARI).
The effectiveness of vaccination was confined to late exacerbations, i.e. those occurring more than three to four weeks after inactivated virus vaccination. The investigators chose to study this time period specifically, in order to allow time for immunity to develop. The authors of Wongsurakiat 2004a and others made the point that the effectiveness of the vaccine in reducing exacerbations depended on how much influenza‐related ARI was present during the study period, i.e. whether there was an epidemic or not. Fell 1977 and Wongsurakiat 2004a were conducted in non‐epidemic years, whereas Howells 1961 was undertaken in an epidemic year. Influenza virus caused 8% of the 'acute exacerbation' and 10% of the 'influenza‐like illness' presentations in Wongsurakiat 2004a, but was responsible for 37% of the acute exacerbations in Howells 1961.
To further emphasise this point, the findings of Howells 1961 and Wongsurakiat 2004a were both consistent with Govaert 1994a, a large (N = 1906), high quality RCT conducted in older people, 9% of whom had chronic lung disease. It assessed the effect of inactivated influenza virus vaccination on the development of influenza or influenza‐like illnesses. When such illnesses were diagnosed by clinical assessment, the relative risk for influenza‐related illness was 0.53 (95% CI 0.39 to 0.73). When the diagnosis was made using the International Classification of Health Problems in Primary Care (ICHPPC‐2‐Defined), the relative risk was 0.83 (95% CI 0.65 to 1.05). However, post hoc analysis showed that during an epidemic, the relative risk for influenza‐related illness diagnosed by clinical assessment was 0.41 (95% CI 0.28 to 0.61), and by ICHPPC‐2‐Defined criteria was 0.74 (95% CI 0.24 to 1.00). This study also demonstrated an overall halving of influenza risk by vaccination. Results for subgroups of those participants at high risk (including those with lung conditions) and those over the age of 70 years were not statistically significant, but the numbers in each group were small. In Treanor 1994, a study of older people who were in institutions where laboratory‐documented outbreaks of influenza A occurred, only 8% of the treatment group, compared to 20% of the control group, had respiratory illnesses. Similarly, only 4% of the treatment group, compared to 11% of the control group, experienced an influenza‐like illness. Inactivated influenza vaccination is likely to have an even greater effect in epidemic years than seen in this review.
Even though the number of RCTs (and participants) was relatively small, the effectiveness of influenza vaccination seen in this review was consistent with that seen in large observational studies. In one of these, involving 1900 older people with chronic lung disease, those who were vaccinated had a halving of the risk of hospitalisation for pneumonia, and a 70% reduction in the risk of death during influenza seasons (Nichol 1999). A meta‐analysis of 20 cohort studies of influenza vaccination in the older population showed a 56% reduction in respiratory illnesses and a 50% reduction in hospitalisation (Gross 1995). Most of these studies had been conducted in epidemic years.

Study flow diagram for 2018 update

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 1 Total exacerbations per participant.
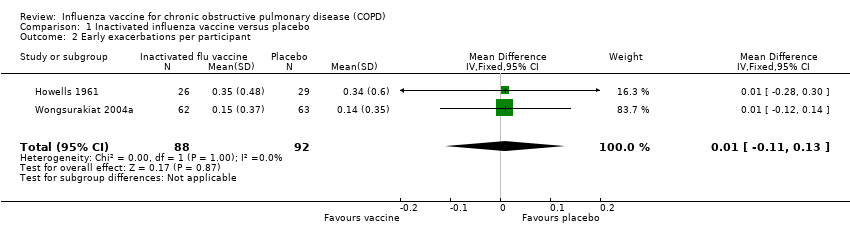
Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 2 Early exacerbations per participant.
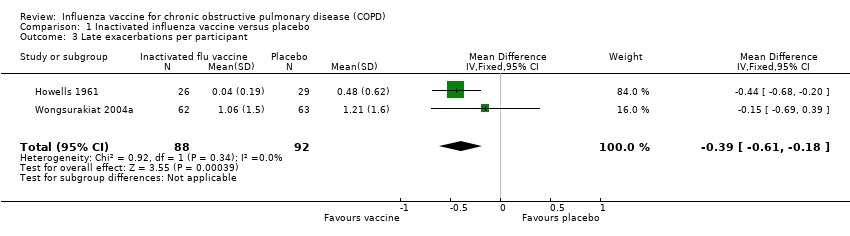
Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 3 Late exacerbations per participant.
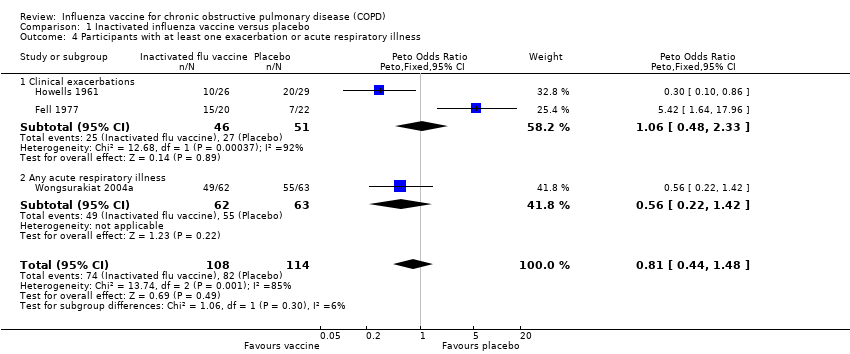
Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 4 Participants with at least one exacerbation or acute respiratory illness.

Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 5 Participants with early exacerbations.

Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 6 Participants with late exacerbations.

Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 7 Hospital admissions.

Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 8 Mortality (all cause).
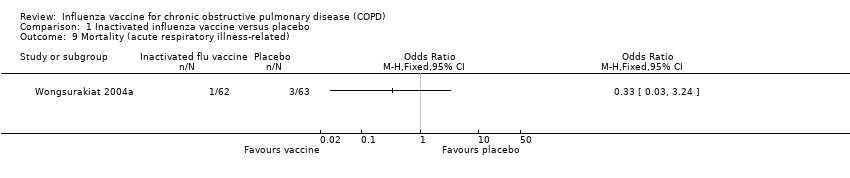
Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 9 Mortality (acute respiratory illness‐related).

Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 10 Overall change in lung function (FEV¹, L).
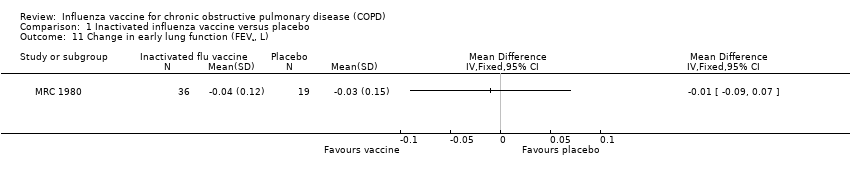
Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 11 Change in early lung function (FEV¹, L).
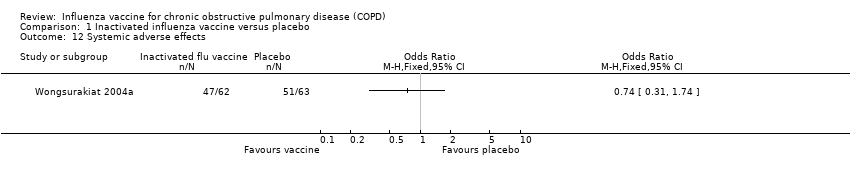
Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 12 Systemic adverse effects.

Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 13 Local effects at injection site.

Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 14 Participants with early breathlessness.
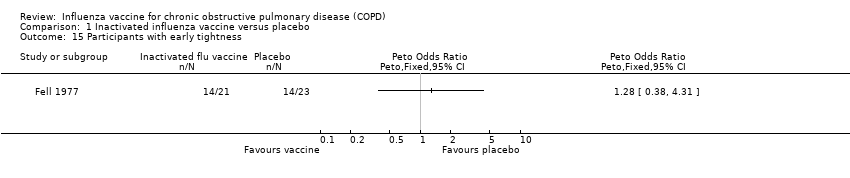
Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 15 Participants with early tightness.

Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 16 Participants with early wheeze.
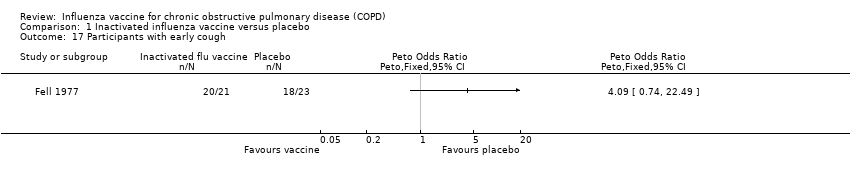
Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 17 Participants with early cough.

Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 18 Acute respiratory illness subsequently documented as influenza‐related.

Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 19 Early acute respiratory illness (ARI).
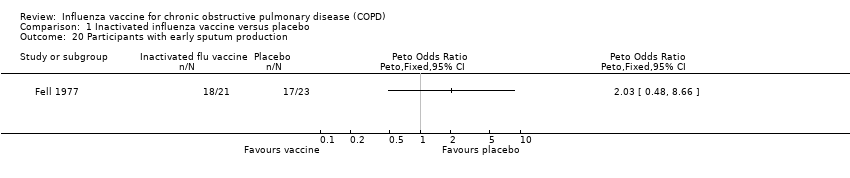
Comparison 1 Inactivated influenza vaccine versus placebo, Outcome 20 Participants with early sputum production.

Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 1 Total exacerbations per participant.
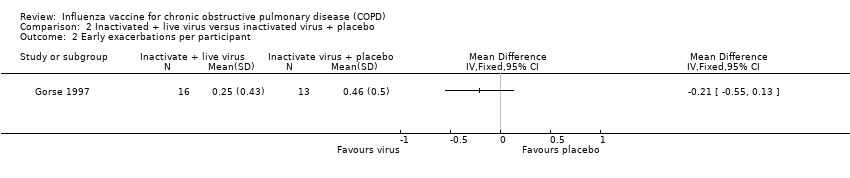
Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 2 Early exacerbations per participant.

Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 3 Late exacerbations per participant.

Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 4 Participants with improvement in exacerbations.
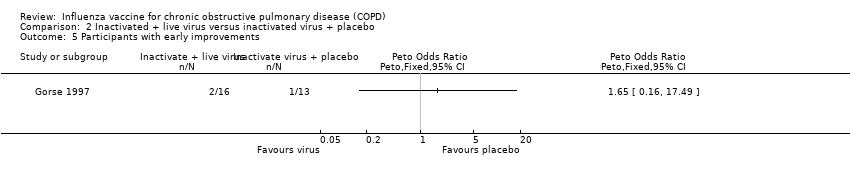
Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 5 Participants with early improvements.

Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 6 Participants with late improvements.

Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 7 Mortality.

Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 8 Early changes in lung function (% predicted FEV¹).

Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 9 Early changes in lung function (FEV¹/FVC %).

Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 10 Post immunisation lung function (FEV¹).

Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 11 Participants with increased lung function (1 category).
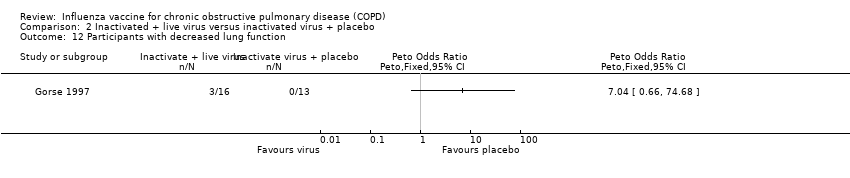
Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 12 Participants with decreased lung function.
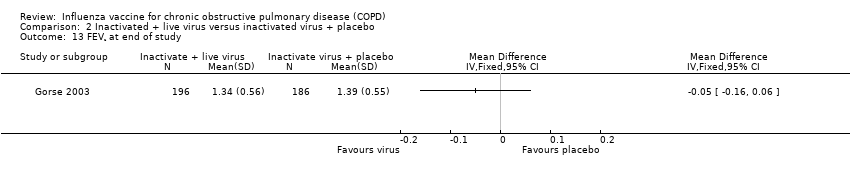
Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 13 FEV¹ at end of study.

Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 14 Participants with adverse effects (new upper respiratory tract symptoms).
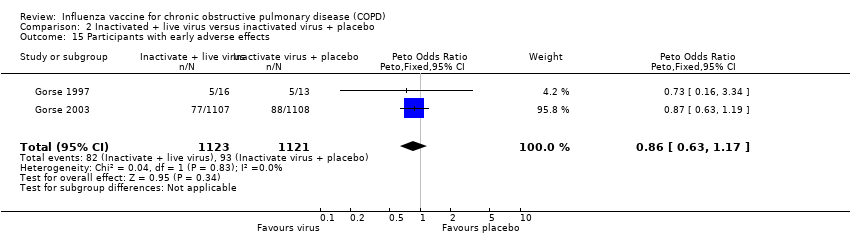
Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 15 Participants with early adverse effects.
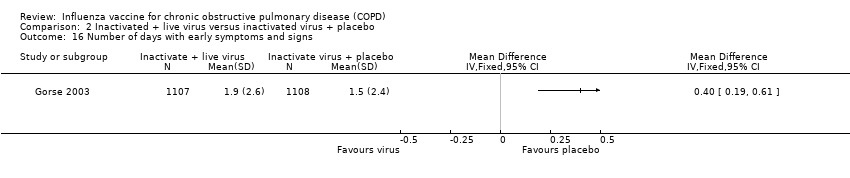
Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 16 Number of days with early symptoms and signs.

Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 17 Number of participants with early adverse effects (by type).

Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 18 Participants with late adverse effects.

Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 19 Acute respiratory illness subsequently documented as influenza‐related.
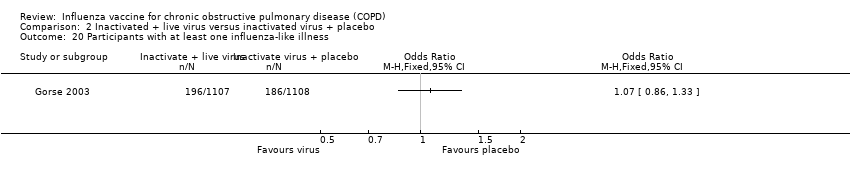
Comparison 2 Inactivated + live virus versus inactivated virus + placebo, Outcome 20 Participants with at least one influenza‐like illness.
| Influenza vaccine compared to placebo for chronic obstructive pulmonary disease (COPD) | ||||||
| Patient or population: chronic obstructive pulmonary disease (COPD) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with Influenza vaccine | |||||
| Total exacerbations per participant | The mean number of total exacerbations per participant ranged across placebo groups from 0.83 to 1.35 | MD 0.37 lower | ‐ | 180 | ⊕⊕⊝⊝ | Despite the effect size, this is based on a very small number of trials and participants. However there was good agreement between these two studies (low I2). One study was not conducted in an epidemic year. We extrapolated some data. Ideally more trials would be done to refine these effect sizes. |
| Early exacerbations per participant | The mean number of early exacerbations per participant ranged across placebo groups from 0.14 to 0.34 | MD 0.01 higher | ‐ | 180 | ⊕⊕⊝⊝ | See above |
| Late exacerbations per participant | The mean number of late exacerbations per participant ranged across placebo groups from 0.48 to 1.21 | MD 0.39 lower | ‐ | 180 | ⊕⊕⊝⊝ | See above |
| Days disability from respiratory illness | not reported | |||||
| Hospital admissions | 76 per 1000 | 26 per 1000 | OR 0.33 | 180 | ⊕⊕⊝⊝ | |
| Mortality | 76 per 1000 | 67 per 1000 | OR 0.87 | 180 | ⊕⊕⊝⊝ | |
| Local effects at injection site | 63 per 1000 | 274 per 1000 | OR 5.57 | 125 | ⊕⊕⊝⊝ | Single study on participants who all had COPD (Wongsurakiat 2004a), but very similar findings in studies with a mixed population (Cate 1977; Govaert 1994) |
| GRADE Working Group grades of evidence | ||||||
| aSmall number of studies which are over ten years old limit our confidence in the generalisability of these findings to currently available vaccines (downgraded once for indirectness). b Studies too small and events too infrequent to detect a consistent effect (downgraded once for imprecision) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total exacerbations per participant Show forest plot | 2 | 180 | Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐0.64, ‐0.11] |
| 2 Early exacerbations per participant Show forest plot | 2 | 180 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.11, 0.13] |
| 3 Late exacerbations per participant Show forest plot | 2 | 180 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐0.61, ‐0.18] |
| 4 Participants with at least one exacerbation or acute respiratory illness Show forest plot | 3 | 222 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.81 [0.44, 1.48] |
| 4.1 Clinical exacerbations | 2 | 97 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.48, 2.33] |
| 4.2 Any acute respiratory illness | 1 | 125 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.56 [0.22, 1.42] |
| 5 Participants with early exacerbations Show forest plot | 2 | 180 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.08 [0.52, 2.26] |
| 6 Participants with late exacerbations Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 7 Hospital admissions Show forest plot | 2 | 180 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.33 [0.09, 1.24] |
| 7.1 Clinical exacerbations | 1 | 55 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.01, 2.39] |
| 7.2 Influenza‐related exacerbations | 1 | 125 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.41 [0.09, 1.89] |
| 8 Mortality (all cause) Show forest plot | 2 | 180 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.28, 2.70] |
| 9 Mortality (acute respiratory illness‐related) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Overall change in lung function (FEV¹, L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Change in early lung function (FEV¹, L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12 Systemic adverse effects Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13 Local effects at injection site Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14 Participants with early breathlessness Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 15 Participants with early tightness Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 16 Participants with early wheeze Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 17 Participants with early cough Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 18 Acute respiratory illness subsequently documented as influenza‐related Show forest plot | 2 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.07, 0.48] |
| 18.1 FEV¹ ≥ 70% predicted | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.01, 1.11] |
| 18.2 Participants with chronic bronchitis | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.04, 0.96] |
| 18.3 FEV¹ < 50% predicted | 1 | 47 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 0.99] |
| 18.4 FEV¹ 50% to 69% predicted | 1 | 33 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.07, 2.98] |
| 19 Early acute respiratory illness (ARI) Show forest plot | 1 | 250 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.34, 1.50] |
| 19.1 ARI within 1 week of vaccination | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.24, 4.26] |
| 19.2 ARI between 1 and 4 weeks after vaccination | 1 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.27, 1.50] |
| 20 Participants with early sputum production Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total exacerbations per participant Show forest plot | 2 | 1137 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.35, 0.37] |
| 2 Early exacerbations per participant Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Late exacerbations per participant Show forest plot | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.08, 0.54] |
| 4 Participants with improvement in exacerbations Show forest plot | 1 | 29 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.48 [0.30, 7.42] |
| 5 Participants with early improvements Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 6 Participants with late improvements Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 7 Mortality Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Early changes in lung function (% predicted FEV¹) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Early changes in lung function (FEV¹/FVC %) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Post immunisation lung function (FEV¹) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Participants with increased lung function (1 category) Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 12 Participants with decreased lung function Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 13 FEV¹ at end of study Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14 Participants with adverse effects (new upper respiratory tract symptoms) Show forest plot | 1 | 29 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.89 [0.45, 8.04] |
| 15 Participants with early adverse effects Show forest plot | 2 | 2244 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.86 [0.63, 1.17] |
| 16 Number of days with early symptoms and signs Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 17 Number of participants with early adverse effects (by type) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 COPD | 1 | 2215 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.30, 1.48] |
| 17.2 Dyspnoea | 1 | 2215 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.60, 5.41] |
| 17.3 Pharyngitis | 1 | 2215 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.35, 2.86] |
| 17.4 Flu syndrome | 1 | 2215 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.20, 1.91] |
| 17.5 Rhinitis | 1 | 2215 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.42, 5.34] |
| 17.6 Bronchitis | 1 | 2215 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.50, 8.05] |
| 17.7 Increased cough | 1 | 2215 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.14, 2.51] |
| 17.8 Myalgia | 1 | 2215 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.51 [0.49, 12.96] |
| 17.9 Increased sputum | 1 | 2215 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.17, 3.36] |
| 17.10 Pneumonia | 1 | 2215 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.37, 10.97] |
| 17.11 Asthenia | 1 | 2215 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.37, 10.97] |
| 17.12 Guillain‐Barré syndrome | 1 | 2215 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.19] |
| 17.13 Other | 1 | 2215 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.26, 0.92] |
| 18 Participants with late adverse effects Show forest plot | 2 | 2244 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.33 [1.22, 4.46] |
| 19 Acute respiratory illness subsequently documented as influenza‐related Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 20 Participants with at least one influenza‐like illness Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

