Les inhibiteurs de la phosphodiestérase 4 dans le traitement de la bronchopneumopathie chronique obstructive
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 24 weeks Intention‐to‐treat analysis: stated | |
| Participants | Setting: 102 centres in Canada, Mexico and the USA Participants: 647 (15 mg cilomilast: 431, placebo: 216) Baseline characteristics: mean age: 65 years, 62% male, mean FEV1% predicted of 49.7%, mean smoking history of 59.9 pack‐years for cilomilast and 56.1 pack‐years for placebo, or current smokers (44% and 47%, respectively) Inclusion criteria: FEV1/FVC ≤ 0.7, FEV1 30% ‐70% with smoking history > 10 pack‐years or current smokers Exclusion criteria: active tuberculosis, lung cancer and bronchiectasis Total number of participant withdrawals: 137 (32%) and 52 (24%) from treatment and control groups, respectively | |
| Interventions | Run‐in: 4 weeks, single‐blind. Placebo tablets to assess suitability Cilomilast 15 mg twice daily Placebo twice daily Concomitant medication
| |
| Outcomes | Primary outcomes: lung function; change in FEV1, SGRQ averaged over 24 weeks Secondary outcomes: incidence rate of COPD exacerbations, adverse events, FVC at the trough, 6‐MWT, post‐exercise dyspnoea | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Described as randomised. No other information available |
| Randomised? | Low risk | "Eligible subjects were randomised in a 2:1 ratio to receive oral cilomilast, 15 mg bid, or placebo for 24 weeks." |
| Method of randomisation described? | Unclear risk | Not stated |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Unclear risk | Not stated |
| Description of withdrawals and drop‐outs? | Low risk | "The primary reasons for the withdrawal of subjects from the study prior to randomisation were the failure to meet inclusion/exclusion criteria (15.4%) and the presence of adverse effects, including COPD exacerbations (8.5%). More subjects receiving cilomilast than placebo withdrew from the double‐blind phase of study (31.8% (n = 137) versus 24.1% (n = 52)." |
| Baseline profile: anticholingeric use | Low risk | 54% in cilomilast; 58% placebo used ipratropium |
| Baseline profile: β2 agonist use | Low risk | 99% in cilomilast; 100% placebo used albuterol. 9% in cilomilast; 12% placebo used salmeterol |
| Baseline profile: corticosteroid use | Low risk | 7% in cilomilast; 8% placebo used triamcinolone. 6% in cilomilast; 7% placebo used beclomethasone |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 24 weeks Intention‐to‐treat analysis: stated | |
| Participants | Setting: 98 centres in Australia and New Zealand, Germany, Spain, South Africa and the UK Participants: 700 (15 mg cilomilast: 474, placebo: 226) Baseline characteristics: mean age: 64.6 years, 80% male, mean FEV1% predicted of 49% with 5.1% reversibility, DLCO was 71% predicted, also with higher rates of chronic bronchitis 80.1%. 45% active smokers Inclusion criteria: aged 40‐80 years, FEV1/FVC ≤ 0.7, FEV1 30%‐70% with smoking history > 10 pack‐years Exclusion criteria: active tuberculosis, lung cancer and bronchiectasis Total number of participant withdrawals: 122 (26%) and 51 (23%) from treatment and control groups, respectively | |
| Interventions | Run‐in: 4 weeks, single‐blind with placebo Cilomilast 15 mg twice daily Placebo twice daily Concomitant medication
| |
| Outcomes | Primary outcomes: lung function; change in FEV1, SGRQ averaged over 24 weeks Secondary outcomes: incidence rate of COPD exacerbations, summary symptom score, FVC at the trough, 6‐MWT, post‐exercise dyspnoea | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Described as randomised. No other information available |
| Randomised? | Low risk | Participants were randomised in a 2:1 ratio to receive oral cilomilast, 15 mg twice daily, or placebo for 24 weeks |
| Method of randomisation described? | Unclear risk | Not stated |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Unclear risk | Not stated |
| Description of withdrawals and drop‐outs? | Low risk | Total number of participants withdrawn 51 (23%) placebo, 122 (26%) cilomilast, primarily due to adverse events, of which most were not from COPD exacerbations |
| Baseline profile: anticholingeric use | Unclear risk | No information available |
| Baseline profile: β2 agonist use | Unclear risk | No information available |
| Baseline profile: corticosteroid use | Unclear risk | No information available |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 12 weeks Analysis was done per‐protocol population | |
| Participants | Setting: not stated Participants: 59 (15 mg cilomilast: 29, placebo: 30) Baseline characteristics: mean age: 61‐62 years, 81% male, 53% active smokers, mean 46 pack‐years, 53%‐58% FEV1 predicted Inclusion criteria: aged 40‐80 years, fixed airflow obstruction, smoking history > 10 pack‐years Exclusion criteria: not stated Total number of participant withdrawals: 4 (14%) and 2 (7%) from treatment and control groups, respectively | |
| Interventions | Run‐in: 4 weeks, single‐blind with placebo Cilomilast 15 mg twice daily Placebo twice daily Concomitant medication
Used alongside short‐acting ß2 agonists (available to all) and anticholingeric drugs (offered to 24%) | |
| Outcomes | Primary outcomes: change in neutrophil percentage in induced sputum Secondary outcomes: FEV1, numbers of subepithelial CD8+ cells, CD 68+ cells, epithelial and subepithelial neutrophils | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Described as randomised. No other information available |
| Randomised? | Low risk | Randomised to a ratio of 1:1 |
| Method of randomisation described? | Unclear risk | Not stated |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Unclear risk | Not stated |
| Description of withdrawals and drop‐outs? | Low risk | "One patient was lost to follow‐up 3 days after randomisation and another withdrawn for non‐compliance 32 days after randomisation. Four patients were withdrawn after adverse events." |
| Baseline profile: anticholingeric use | Unclear risk | No information available |
| Baseline profile: β2 agonist use | Unclear risk | No information available |
| Baseline profile: corticosteroid use | Unclear risk | No information available |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 24 weeks Intention‐to‐treat analysis: stated | |
| Participants | Setting: 110 centres in Belgium, Finland, France, Italy, the Netherlands, Norway, Portugal, Spain and the UK Participants: 711 (15 mg cilomilast: 469, placebo: 242) Baseline characteristics: mean age: 64.6 years, 86% male, mean FEV1 % predicted of 53% with 5.0% reversibility. 38% active smokers Inclusion criteria: FEV1/FVC ≤ 0.7 with smoking history > 10 pack‐years Exclusion criteria: active tuberculosis, lung cancer and bronchiectasis Total number of participant withdrawals: 121 (26%) and 63 (26%) from treatment and control groups, respectively | |
| Interventions | Run‐in: 4 weeks, single‐blind with placebo Cilomilast 15 mg twice daily Placebo twice daily Concomitant medication
| |
| Outcomes | Primary outcomes: lung function; change in FEV1, SGRQ averaged over 24 weeks Secondary outcomes: incidence rate of COPD exacerbations, summary symptom score, FVC at the trough, 6‐MWT, post‐exercise dyspnoea | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Described as randomised. No other information available |
| Randomised? | Low risk | "Eligible patients were randomised to receive either SB 207499 or matching placebo ina ratio of 2:1 for 24 weeks." |
| Method of randomisation described? | Unclear risk | Not stated |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Unclear risk | Not stated |
| Description of withdrawals and drop‐outs? | Low risk | Total number of participants withdrawn 63 (26%) placebo, 121 (26%) cilomilast, primarily due to adverse events, of which most were not from COPD exacerbations |
| Baseline profile: anticholingeric use | Unclear risk | No information available |
| Baseline profile: β2 agonist use | Unclear risk | No information available |
| Baseline profile: corticosteroid use | Unclear risk | No information available |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 24 weeks Intention‐to‐treat analysis: stated | |
| Participants | Setting: 103 centres in the USA Participants: 613 (15 mg cilomilast: 296, placebo: 317) Baseline characteristics: mean age: 63.2 years: placebo and 63.1 years; cilomilast. 47% male: placebo and 46% male: cilomilast. Mean FEV1 % predicted not available Inclusion criteria: aged ≥ 40 years of age. FEV1/FVC ≤ 0.7 with smoking history > 10 pack‐years. ≤ 70%, post‐albuterol reversibility ≤ 15% or ≤ 200 mL (or both), post‐albuterol FEV1 ≤ 70% of predicted normal and at least 1 COPD exacerbation within the 12 months prior to screening Exclusion criteria: not stated Total number of participant withdrawals: 105 (35%) and 76 (24%) from treatment and control groups, respectively | |
| Interventions | Run‐in: not stated Cilomilast 15 mg twice daily Placebo twice daily Concomitant medication
| |
| Outcomes | Primary outcomes: change from baseline to endpoint in trough pre‐bronchodilator FEV1. Change in total score of SGRQ averaged over 24 weeks Secondary outcomes: changes from baseline in clinic trough FVC, time to first level 2 or level 3 COPD exacerbation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Randomised. No other information given |
| Randomised? | Low risk | Participants were randomised to receive either 15 mg of cilomilast or matching placebo twice daily |
| Method of randomisation described? | Unclear risk | Not stated |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Unclear risk | Not stated |
| Description of withdrawals and drop‐outs? | Low risk | Total number of participants withdrawn 76 (24%) placebo, 105 (35%) cilomilast |
| Baseline profile: anticholingeric use | Unclear risk | No information available |
| Baseline profile: β2 agonist use | Unclear risk | No information available |
| Baseline profile: corticosteroid use | Unclear risk | No information available |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 12 weeks Analysis was done per‐protocol population | |
| Participants | Setting: 10 centres in the USA Participants: 65 (15 mg cilomilast: 31, placebo: 34) Baseline characteristics: mean age: 64.4 years: placebo and 66.1 years: cilomilast. 67% male: placebo and 84% male: cilomilast. Mean FEV1 % predicted not available Inclusion criteria: aged 40‐80 years. FEV1/FVC ≤ 0.7 with smoking history > 10 pack‐years. Post‐salbutamol reversibility ≤ 15% or 200 mL and a post‐salbutamol FEV1 at least 1.0 L and between 30% and 70% of predicted Exclusion criteria: not stated Total number of participant withdrawals: 1 (3%) and 1 (3%) from treatment and control groups, respectively | |
| Interventions | Run‐in: not stated Cilomilast 15 mg twice daily Placebo twice daily Concomitant medication
| |
| Outcomes | Primary outcomes: change from baseline at endpoint in neutrophils as a percentage of total cells in induced sputum Secondary outcomes: FVC at the trough, sputum macrophages, eosinophils and lymphocytes as a percentage of total cells in induced sputum. Total cell counts in induced sputum | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Described as randomised. No other information available |
| Randomised? | Low risk | All participants were randomised to receive either cilomilast 15 mg or matching placebo |
| Method of randomisation described? | Unclear risk | Not stated |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Unclear risk | Not stated |
| Description of withdrawals and drop‐outs? | Low risk | Total number of participants withdrawn 1 (3%) placebo, 1 (3%) cilomilast |
| Baseline profile: anticholingeric use | Unclear risk | No information available |
| Baseline profile: β2 agonist use | Unclear risk | No information available |
| Baseline profile: corticosteroid use | Unclear risk | No information available |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 12 weeks Intention‐to‐treat analysis: stated | |
| Participants | Setting: 32 centres in the USA, Canada and Australia Participants: 156 (15 mg cilomilast: 79, placebo: 77) Baseline characteristics: mean age: 64.2 years: placebo and 65 years; cilomilast. 66% male: placebo and 65% male: cilomilast. Mean FEV1 % predicted not available Inclusion criteria: aged 40‐80 years. FEV1/FVC ≤ 0.7 with smoking history > 10 pack‐years. Post‐salbutamol reversibility ≤ 15% or 200 mL and a post‐salbutamol FEV1 at least 1.0 L and between 30% and 70% of predicted. Baseline RV (from plethysmography) ≥ 120% of predicted RV Exclusion criteria: not stated Total number of participant withdrawals: 15 (19%) and 14 (18%) from treatment and control groups, respectively | |
| Interventions | Run‐in: not stated Cilomilast 15 mg twice daily Placebo twice daily Concomitant medication
| |
| Outcomes | Primary outcomes: change from baseline to endpoint in volume of trapped gas (D) Secondary outcomes: lung volume measurements, including SVC and RV, 6‐MWT and exertional dyspnoea | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Described as randomised. No other information available |
| Randomised? | Low risk | Participants were randomised to receive either 15 mg of cilomilast or matching placebo |
| Method of randomisation described? | Unclear risk | Not stated |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Unclear risk | Not stated |
| Description of withdrawals and drop‐outs? | Low risk | Total number of participants withdrawn 14 (18%) placebo, 15 (19%) cilomilast |
| Baseline profile: anticholingeric use | Unclear risk | No information available |
| Baseline profile: β2 agonist use | Unclear risk | No information available |
| Baseline profile: corticosteroid use | Unclear risk | No information available |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 24 weeks Intention‐to‐treat analysis: stated | |
| Participants | Setting: 22 centres in China Participants: 1018 (15 mg cilomilast: 678, placebo: 340) Baseline characteristics: mean age: 63.9 years: placebo and 64.6 years; cilomilast. 91% male: placebo and 93% male: cilomilast. Mean FEV1 % predicted not available Inclusion criteria: aged 40‐75 years. FEV1/FVC ≤ 0.7 with smoking history > 10 pack‐years. Documented history of COPD exacerbations each year for 3 years prior to screening. At least 1 exacerbation in the last year that required oral corticosteroids or antibiotics. Post‐salbutamol reversibility ≤ 15% or 200 mL and a post‐salbutamol FEV1 at least 1.0 L and between 25% and 70% of predicted. % predicted FRC of ≥ 120% from plethysmography Exclusion criteria: not stated Total number of participant withdrawals: 124 (18%) and 35 (10%) from treatment and control groups, respectively | |
| Interventions | Run‐in: not stated Cilomilast 15 mg twice daily Placebo twice daily Concomitant medication
| |
| Outcomes | Primary outcomes: change from baseline to endpoint in trough pre‐bronchodilator FEV1 Secondary outcomes: time to first level 2 or level 3 COPD exacerbation. Level 2 is defined as acute worsening of COPD that requires additional treatment or hospital outpatient visit. Level 3 is hospital admission for treatment. Change from baseline to endpoint RV and FRC. Change from baseline total score of SGRQ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Described as randomised. No other information available |
| Randomised? | Low risk | Participants were randomised to a 2:1 ratio for cilomilast 15 mg to placebo |
| Method of randomisation described? | Unclear risk | Not stated |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Unclear risk | Not stated |
| Description of withdrawals and drop‐outs? | Low risk | Total number of participants withdrawn 35 (10%) placebo, 124 (18%) cilomilast |
| Baseline profile: anticholingeric use | Unclear risk | No information available |
| Baseline profile: β2 agonist use | Unclear risk | No information available |
| Baseline profile: corticosteroid use | Unclear risk | No information available |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 24 weeks Intention‐to‐treat analysis: stated | |
| Participants | Setting: 132 centres in USA and Canada Participants: 825 (15 mg cilomilast: 418, placebo: 407) Baseline characteristics: mean age: 64.4 years: placebo and 64.5 years; cilomilast. 62% male: placebo and 56% male: cilomilast > 50% predicted FEV1 for both groups Inclusion criteria: aged 40‐80 years. FEV1/FVC ≤ 0.7 with smoking history > 10 pack‐years. Post‐salbutamol reversibility ≤ 15% or 200 mL and a post‐salbutamol FEV1 at least 1.0 L and between 30% and 70% of predicted Exclusion criteria: not stated Total number of participant withdrawals: 143 (34%) and 96 (24%) from treatment and control groups, respectively | |
| Interventions | Run‐in: not stated Cilomilast 15 mg twice daily Placebo twice daily Concomitant medication
| |
| Outcomes | Primary outcomes: change from baseline to endpoint in trough pre‐bronchodilator FEV1. Change in total score of SGRQ averaged over 24 weeks Secondary outcomes: post‐exercise breathlessness, clinic trough FVC, time to first level 2 or level 3 COPD exacerbation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Described as randomised. No other information available |
| Randomised? | Low risk | Participants were randomised to receive either 15 mg of cilomilast or matching placebo twice daily |
| Method of randomisation described? | Unclear risk | Not stated |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Unclear risk | Not stated |
| Description of withdrawals and drop‐outs? | Low risk | Total number of participants withdrawn 96 (24%) placebo, 143 (34%) cilomilast |
| Baseline profile: anticholingeric use | Unclear risk | No information available |
| Baseline profile: β2 agonist use | Unclear risk | No information available |
| Baseline profile: corticosteroid use | Unclear risk | No information available |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 52 weeks Intention‐to‐treat analysis: stated | |
| Participants | Setting: 137 centres from 18 countries Participants: 907 (15 mg cilomilast: 455, placebo: 452) Baseline characteristics: mean age: 63.3 years: placebo and 64.6 years; cilomilast. 73% male: placebo and 78% male: cilomilast. 42% current smokers Inclusion criteria: aged 40‐80 years. FEV1/FVC ≤ 0.7 with smoking history > 10 pack‐years. Poor reversibility of airway obstruction, defined by ≤ 10% of predicted normal or ≤ 200 mL (or both) increase in FEV1 after administration of salbutamol 400 µg via MDI at screening; post‐salbutamol FEV1 of between 30%‐70% predicted normal at screening Exclusion criteria: not stated Total number of participant withdrawals: 167 (37%) and 121 (27%) from treatment and control groups, respectively | |
| Interventions | Run‐in: not stated Cilomilast 15 mg twice daily Placebo twice daily Concomitant medication
| |
| Outcomes | Primary outcomes: mean change from baseline in trough pre‐bronchodilator FEV1 averaged over 52 weeks. Incidence rate of level 2 (moderate) and level 3 (severe) COPD exacerbations during the treatment period Secondary outcomes: time to first level 2 or level 3 COPD exacerbation. Quality of life determined by SGRQ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Described as randomised. No other information available |
| Randomised? | Low risk | Participants were randomised to receive either 15 mg of cilomilast or matching placebo twice daily |
| Method of randomisation described? | Unclear risk | A randomisation criteria was used. No other information available |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Unclear risk | Not stated |
| Description of withdrawals and drop‐outs? | Low risk | Total number of participants withdrawn 121 (27%) placebo, 167 (37%) cilomilast |
| Baseline profile: anticholingeric use | Unclear risk | No information available |
| Baseline profile: β2 agonist use | Unclear risk | No information available |
| Baseline profile: corticosteroid use | Unclear risk | No information available |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 12 weeks Intention‐to‐treat analysis: not stated | |
| Participants | Setting: 42 centres in the USA Participants: 306 (15 mg cilomilast: 203, placebo: 103) Baseline characteristics: mean age: 64.3 years: placebo and 65.0 years; cilomilast. 64% male: placebo and 70% male: cilomilast Inclusion criteria: pre‐albuterol FEV1/FVC ≤ 0.7. Post‐albuterol FEV1 between 30% and 70% of predicted Exclusion criteria: not stated Total number of participant withdrawals: 61 (30%) and 14 (14%) from treatment and control groups, respectively | |
| Interventions | Run‐in: not stated Cilomilast 15 mg twice daily Placebo twice daily Concomitant medication
| |
| Outcomes | Primary outcomes: no primary efficacy or safety analyses were defined. Descriptive statistics of change from baseline in minimum and maximum heart rate via 24‐h Holter monitoring reported Secondary outcomes: no secondary efficacy or safety analyses were defined | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Described as randomised. No other information available |
| Randomised? | Low risk | Participants were randomised at 2:1 ratio of cilomilast to placebo |
| Method of randomisation described? | Unclear risk | Not stated |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Unclear risk | Not stated |
| Description of withdrawals and drop‐outs? | Low risk | Total number of participants withdrawn 14 (14%) placebo, 61 (30%) cilomilast |
| Baseline profile: anticholingeric use | Unclear risk | No other information available |
| Baseline profile: β2 agonist use | Unclear risk | No other information available |
| Baseline profile: corticosteroid use | Unclear risk | No other information available |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 18 weeks Intention‐to‐treat analysis: stated | |
| Participants | Setting: 34 centres in the USA, Canada and South America 2) Participants: 199 (15 mg cilomilast: 97, placebo: 102) Baseline characteristics: mean age: 64.7 years: placebo and 63.7 years; cilomilast. 76% male: placebo and 69% male: cilomilast Inclusion criteria: age at least 40 years. FEV1/FVC ≤ 0.7 with smoking history > 10 pack‐years. Baseline FEV1 < 70% predicted normal. Moderate to severe chronic dyspnoea defined by baseline Dyspnoea Index focal score ≤ 7, evidence of hyperinflation defined by RFRC at least 140% of predicted. Exercise limitation, defined as peak symptom limited VO2 < 75% Exclusion criteria: not stated Total number of participant withdrawals: 24 (25%) and 13 (13%) from treatment and control groups, respectively | |
| Interventions | Run‐in: not stated Cilomilast 15 mg twice daily Placebo twice daily Concomitant medication
| |
| Outcomes | Primary outcomes: change from baseline at endpoint in RFRC Secondary outcomes: change from baseline at endpoint in IC during exercise, exertional dyspnoea as measured by the modified Borg scale | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Described as randomised. No other information available |
| Randomised? | Low risk | Participants were randomised to receive either 15 mg of cilomilast or matching placebo twice daily |
| Method of randomisation described? | Unclear risk | Not stated |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Unclear risk | Not stated |
| Description of withdrawals and drop‐outs? | Low risk | Total number of participants withdrawn 13 (13%) placebo, 24 (25%) cilomilast |
| Baseline profile: anticholingeric use | Unclear risk | No information available |
| Baseline profile: β2 agonist use | Unclear risk | No information available |
| Baseline profile: corticosteroid use | Unclear risk | No information available |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 13 weeks Analysis was done per‐protocol population | |
| Participants | Setting: 27 centres in Australia, Canada, Finland, Ireland, Lithuania, Norway, Romania, Slovakia, Slovenia, South Africa, Sweden and the UK Participants: 127 (15 mg cilomilast: 65, placebo: 62) Baseline characteristics: mean age: 63.4 years: placebo and 61.4 years; cilomilast. 76% male: placebo and 72% male: cilomilast Inclusion criteria: aged 40‐80 years. FEV1/FVC ≤ 0.7 with smoking history > 10 pack‐years. Post‐bronchodilator FEV1 between 40% and 80% predicted normal. Poor reversibility of ≤ 10% or 200 mL increase in FEV1 Exclusion criteria: not stated Total number of participant withdrawals: 8 (12%) and 6 (10%) from treatment and control groups, respectively | |
| Interventions | Run‐in: not stated Cilomilast 15 mg twice daily Placebo twice daily Concomitant medication
| |
| Outcomes | Primary outcome: change from baseline at endpoint in CD68+ (macrophages) and CD8+ (cytotoxic T‐lymphocytes) per unit area of tissue Secondary outcome: change from baseline in numbers of sub‐epithelial cells per unit area in biopsy for neutrophil elastase positive (ne+) cells, CD4+, IL‐8 mRNA positive cells, TNF‐alpha mRNA positive cells | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Randomised. No other information available |
| Randomised? | Low risk | Participants were randomised to receive either 15 mg of cilomilast or matching placebo twice daily |
| Method of randomisation described? | Unclear risk | Not stated |
| Blinding? | Low risk | Double‐blinding |
| Method of blinding described? | Unclear risk | Not stated |
| Description of withdrawals and drop‐outs? | Low risk | Total number of participants withdrawn 6 (10%) placebo, 8 (12%) cilomilast |
| Baseline profile: anticholingeric use | Unclear risk | No other information available |
| Baseline profile: β2 agonist use | Unclear risk | No other information available |
| Baseline profile: corticosteroid use | Unclear risk | No other information available |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 6 weeks Intention‐to‐treat analysis: stated | |
| Participants | Setting: 60 centres in Austria, France, Germany, the Netherlands and the UK Participants: 424 (5 mg cilomilast: 109, 10 mg cilomilast: 102, 15 mg cilomilast: 107, placebo: 106) Baseline characteristics: mean age: 62‐63 years, 75%‐78% male, mean FEV1% predicted of 46.8%, mean smoking history of 36‐43 (SD 22.4) pack‐years Inclusion criteria: FEV1/FVC ≤ 0.7 with smoking history > 10 pack‐years Exclusion criteria: asthma, poorly controlled COPD needing hospital visit 6 weeks before study, recent COPD exacerbations or recent corticosteroid use Total number of participant withdrawals: 18 (17%) and 17 (16%) from treatment and control groups, respectively | |
| Interventions | Run‐in: 2 weeks, single‐blind. Placebo tablets to assess compliance Cilomilast 5 mg, 10 mg, 15 mg twice daily Placebo twice daily Concomitant medication
| |
| Outcomes | Primary outcomes: lung function: change in FEV1, SGRQ Secondary outcomes: peak expiratory flow and FVC, the first dose effect of active treatment on FEV1 | |
| Notes | Post‐bronchodilator results not given so pre‐bronchodilator values used in analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Described as randomised. No other information available |
| Randomised? | Low risk | "Eligible patients were randomly assigned to receive a 5, 10, or 15 mg tablet of cilomilast twice daily (morning and evening) or matching placebo for 6 weeks." |
| Method of randomisation described? | Unclear risk | Not stated |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Unclear risk | Not stated |
| Description of withdrawals and drop‐outs? | Low risk | "14 patients (13%) taking cilomilast 15 mg had adverse events leading to patient withdrawal, as did 12 each in the 5 and 10 mg groups (11 and 12%, respectively) and eight (8%) in the placebo group." |
| Baseline profile: anticholingeric use | Unclear risk | Information not available |
| Baseline profile: β2 agonist use | Low risk | 102 (24%) participants had been taking long‐acting ß2‐ agonists; e.g. salmeterol, formoterol |
| Baseline profile: corticosteroid use | Low risk | 331 (78%) individuals had taken other medications for their COPD, the most common being inhaled steroids. 229 (54%) took beclomethasone, budesonide or fluticasone |
| Methods | 14 double‐blind and placebo‐controlled studies (Roflumilast FK1 101; Roflumilast FK1 103; Roflumilast IN‐108; Roflumilast M2‐107; Roflumilast M2‐110; Roflumilast M2‐111; Roflumilast M2‐112; Roflumilast M2‐118; Roflumilast M2‐119; Roflumilast M2‐121; Roflumilast M2‐124; Roflumilast M2‐125; Roflumilast M2‐127; Roflumilast M2‐128) | |
| Participants | See individual studies | |
| Interventions | Roflumilast 500 µg once daily Roflumilast 250 µg once daily Placebo once daily | |
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | See individual studies |
| Randomised? | Low risk | Randomised |
| Method of randomisation described? | Unclear risk | See individual studies |
| Blinding? | Unclear risk | Double‐blind |
| Method of blinding described? | Unclear risk | See individual studies |
| Description of withdrawals and drop‐outs? | Unclear risk | See individuals studies |
| Baseline profile: anticholingeric use | Unclear risk | See individuals studies |
| Baseline profile: β2 agonist use | Unclear risk | See individuals studies |
| Baseline profile: corticosteroid use | Unclear risk | See individuals studies |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 24 weeks Intention‐to‐treat analysis: stated | |
| Participants | Setting: 43 centres in mainland China, Hong Kong and Singapore Participants: 626 (500 µg roflumilast: 313, placebo: 313) Baseline characteristics: mean age: 64 years, 91% male, mean FEV1% predicted of 36%, mean smoking history of 37.2 pack‐years for roflumilast and 37.5 pack‐years for placebo) or current smokers (24% and 29%, respectively) Inclusion criteria: Chinese, Malay or Indian ethnicity, age 40‐80 years with severe‐very severe COPD FEV1/FVC ≤ 0.7, and post‐bronchodilator FEV1 ≤ 50%. Current smokers or ex‐smokers with smoking history > 10 pack‐years or current smokers. 12‐month history of COPD and ≥ 14 puffs of rescue medication during the week prior to randomisation Exclusion criteria: primary bronchiectasis, cystic fibrosis, bronchiolitis, lung resection, lung cancer, interstitial lung disease, or active TB, lower respiratory tract infection, diagnosis of asthma at < 40 years of age, or a1‐antitrypsin deficiency Total number of participant withdrawals: 67 (21.4%) and 50 (16%) from treatment and control groups, respectively | |
| Interventions | Run‐in: 4 weeks, single‐blind. Placebo tablets to assess suitability Roflumilast 500 µg once daily Placebo once daily Concomitant medication Participants were allowed to continue taking fixed combinations of ICS plus LABA or LAMA monotherapy (e.g. tiotropium) if taken at a stable dose for at least 6 months prior to the run‐in period. SAMAs (e.g., ipratropium) were allowed at a constant daily dose as concomitant medication if taken on a regular basis for at least 4 weeks prior to study inclusion. All other COPD treatments were not allowed | |
| Outcomes | Primary outcomes: lung function; change in pre‐bronchodilator FEV1 Secondary outcomes: change in post‐bronchodilator FEV1, FVC, incidence rate of COPD exacerbations, time to first COPD exacerbation, transition dyspnoea index, proportional of participants experiencing a COPD exacerbation, adverse events, changes in body weight, laboratory values, vital signs and physical examination findings | |
| Notes | NCT number: NCT01313494 Funded by Takeda | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | The investigator or anyone at the study site was prevented from knowing the allocation sequence with code labelling. |
| Randomised? | Low risk | The investigators used an automated, interactive voice‐response system to randomly assign participants. |
| Method of randomisation described? | Low risk | The sponsor generated a list of participant numbers using a pseudorandom number generator |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Low risk | Tablets were identical in appearance. |
| Description of withdrawals and drop‐outs? | Unclear risk | Total number of participants that discontinued 50 (16%) placebo, 67 (21.4%) roflumilast |
| Baseline profile: anticholingeric use | Low risk | LAMA: 17.9% for placebo; 20.4% for roflumilast SAMA: 18.2% for placebo; 17.3% for roflumilast |
| Baseline profile: β2 agonist use | Unclear risk | No information available. SABA allowed |
| Baseline profile: corticosteroid use | Low risk | ICS/LABA: 55.9% for placebo; 59.7% for roflumilast |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 52 weeks Intention‐to‐treat analysis: stated | |
| Participants | Setting: 203 centres in 21 countries (see online appendix) Participants: 1945 (500 µg roflumilast: 969; placebo: 966) Baseline characteristics: mean age: 65 years, 75% male, mean FEV1% predicted of 35%, mean smoking history of 48 pack‐years for roflumilast and 48 pack‐years for placebo) or current smokers (42% and 45%, respectively) Inclusion criteria: ≥ 40 years with a smoking history of at least 20 pack‐years and a diagnosis of chronic obstructive pulmonary disease with severe airflow limitation (confirmed by a post‐bronchodilator FEV1/FVC ratio < 0·70 and a post‐bronchodilator FEV1 of ≤ 50% predicted), symptoms of chronic bronchitis, and a history of at least two exacerbations in the previous year Participants must have been taking an ICS–LABA combination for 12 months before the study and a constant dose of an ICS–LABA fixed combination for at least 3 months before enrolment, with placebo tablet compliance of 80%–125% during the 4‐week baseline observation period and with a total cough and sputum score of ≥ 14 (in which the score was a sum of daily scores on 4‐point scales for cough and sputum) recorded in a daily diary during the week preceding the randomisation visit Exclusion criteria: chronic obstructive pulmonary disease exacerbation that was ongoing during the baseline period, or had a diagnosis of asthma or other major lung disease Total number of participant withdrawals: 269 (28%) and 192 (20%) from treatment and control groups, respectively | |
| Interventions | Run‐in: 4 weeks, single‐blind. Placebo tablets to assess suitability Roflumilast 500 µg once daily Placebo once daily Concomitant medication All participants used a fixed‐dose ICS–LABA combination during the baseline and treatment period. If a participant had an exacerbation that needed additional treatment during the study, the investigator could give them up to 40 mg prednisolone, administered systemically, per day for 7–14 days. In the case of purulent sputum or suspected bacterial infection, additional antibiotic therapy was allowed The use of the following treatments was not allowed: oral and parenteral glucocorticosteroids (except to treat acute exacerbations), LABA or ICS mono therapy, SAMA, and any short‐acting β2 agonists (with the exception of salbutamol) or oral β2 agonists Participants already taking inhaled tiotropium bromide (a LAMA) were allowed to continue this treatment | |
| Outcomes | Primary outcomes: rate of moderate‐to‐severe chronic obstructive pulmonary disease exacerbations per patient per year. This was assessed in several ways: rate of exacerbations; % with exacerbation; time to first, second or third exacerbation; NNTB to avoid 1 moderate to severe exacerbation Secondary outcomes: change in post‐bronchodilator FEV1, rate of severe chronic obstructive pulmonary disease exacerbations per patient per year, laboratory values, vital signs, physical examination findings, changes in bodyweight and body‐mass index, and reported adverse events, including mortality | |
| Notes | NCT number: NCT01329029 Sponsored by Takeda | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | All parties involved in the study were masked to treatment assignment |
| Randomised? | Low risk | Randomised |
| Method of randomisation described? | Low risk | Enrolled participants were randomly assigned in a 1:1 ratio, with a block size of 4, by a computerised central randomisation system, the Interactive Voice Response |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Low risk | Roflumilast and placebo were supplied as identical yellow triangular tablets in wallet cards containing 40 tablets |
| Description of withdrawals and drop‐outs? | Unclear risk | 269 participants (28%) discontinued from study in the roflumilast group and 192 (20%) discontinued from the placebo group |
| Baseline profile: anticholingeric use | Unclear risk | LAMA: 69% for placebo; 70% for roflumilast |
| Baseline profile: β2 agonist use | Unclear risk | No group differences stated; however 1900 (98%) of 1935 participants were using a combination of ICS–LABA according to the protocol |
| Baseline profile: corticosteroid use | Unclear risk | As above |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 12 weeks Intention‐to‐treat analysis: stated | |
| Participants | Setting: single centre in USA Participants: 27 (500 µg roflumilast: 11, placebo: 16) Baseline characteristics: mean age: 62 years, 64% male, mean FEV1% predicted of 45%, mean smoking history of 44 pack‐years for roflumilast and 47 pack‐years for placebo) or current smokers (63% and 55%, respectively) Inclusion criteria: > 40 years old with a diagnosis of moderate‐to‐severe COPD as defined by Global Initiative for Chronic Obstructive Lung Disease criteria, current or former cigarette smokers with more than 10 pack‐years of total consumption, chronic bronchitis defined by chronic cough and sputum production lasting at least 3 months for 2 consecutive years Exclusion criteria: asthma as defined by the American Thoracic Society/European Respiratory Society guidelines, clinically significant bronchiectasis, known sensitivity to roflumilast, the use of other methylxanthines (specifically theophylline) within 1 month of screening, changes to maintenance COPD therapy within 1 month of screening Total number of participant withdrawals: 1 (9%) and 1 (6%) from treatment and control groups, respectively | |
| Interventions | Run‐in: no run in Roflumilast 500 µg once daily Placebo once daily Concomitant medication Allowed, except for theophylline. For roflumilast and placebo groups respectively: LAMA was used by 8 (50%) and 6 (55%); ICS or LABA/ICS was used by 10 (63%) 6 (55%) | |
| Outcomes | Primary outcomes: change in induced sputum AcPGP at 12 weeks post‐randomisation in an intention‐to‐treat analysis Secondary outcomes: changes in plasma AcPGP, sputum neutrophil counts, additional sputum biomarkers, 6MWT, the Breathlessness Cough and Sputum Scale, SGRQ scores, and changes in post‐bronchodilator FEV1 at the 12‐week visit | |
| Notes | NCT number: NCT01572948 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Randomised? | Unclear risk | Randomised |
| Method of randomisation described? | Unclear risk | Sealed envelope. Block randomisation schema using a block size of four and an allocation ratio of 1:1. Block randomization was stratified by current smoking status and ICS use |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Low risk | Identical white tablets containing a 30‐day supply of either roflumilast, 500 mg or placebo |
| Description of withdrawals and drop‐outs? | Low risk | No withdrawals described |
| Baseline profile: anticholingeric use | Unclear risk | Not stated |
| Baseline profile: β2 agonist use | Unclear risk | Not stated |
| Baseline profile: corticosteroid use | Unclear risk | Not stated |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 26 weeks Intention‐to‐treat analysis: stated | |
| Participants | Setting: not stated Participants: 516 (roflumilast 250 µg: 175, roflumilast 500 µg: 169, placebo: 172) Baseline characteristics: median age: 61‐62 years. 72% male. Mean 38 to 63 pack‐years. 53% current smokers Inclusion criteria: aged 40‐75 years. FEV1/FVC ≤ 0.7 with smoking history > 10 pack‐years. Reversibility < 12% or 200 mL post‐bronchodilator FEV1 35%‐75% predicted Exclusion criteria: not stated Participant withdrawals: not stated | |
| Interventions | Run‐in: 2 weeks with placebo Roflumilast 500 µg once daily Roflumilast 250 µg once daily Placebo once daily Concomitant medication
| |
| Outcomes | Primary outcomes: post‐bronchodilator FEV1 and FEF between 25%‐75% of vital capacity Secondary outcomes: number of moderate or severe COPD exacerbations which required treatment with oral steroids | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Randomised. No other information available |
| Randomised? | Low risk | Participants randomised in either roflumilast 250 µg, roflumilast 500 µg or placebo groups |
| Method of randomisation described? | Unclear risk | Not stated |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Unclear risk | Not stated |
| Description of withdrawals and drop‐outs? | High risk | Not stated |
| Baseline profile: anticholingeric use | Unclear risk | No information available |
| Baseline profile: β2 agonist use | Unclear risk | No information available |
| Baseline profile: corticosteroid use | Unclear risk | No information available |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 24 weeks Intention‐to‐treat analysis: stated | |
| Participants | Setting: not stated Participants: 518 (roflumilast 500 µg: 200, placebo: 186) Baseline characteristics: mean age: 60 years. 75% male. 62% current smokers. Average of 35 pack‐years Inclusion criteria: aged 40‐75 years. FEV1/FVC ≤ 0.7. Post‐bronchodilator FEV1 35%‐75% of predicted. FEV1 reversibility ≤ 12% and ≤ 200 mL. Pre‐bronchodilator FEV1/FVC ≤ 70% Exclusion criteria: not stated Participant withdrawals not stated | |
| Interventions | Run‐in: 2 weeks with placebo Roflumilast 500 µg once daily for 24 weeks Roflumilast 500 µg once daily for 12 weeks. Placebo once daily for following 12 weeks Concomitant medication
Used alongside short‐acting ß2 agonists (available to all) | |
| Outcomes | Primary outcomes: results for 12/24 week post‐bronchodilator FEV1 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Randomised? | Low risk | "After randomisation, patients received placebo or roflumilast 500 µg once daily for 24 weeks or roflumilast 500 µg for 12 weeks followed by placebo for 12 weeks" |
| Method of randomisation described? | Unclear risk | Not stated |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Unclear risk | Not stated |
| Description of withdrawals and drop‐outs? | High risk | Not stated |
| Baseline profile: anticholingeric use | Unclear risk | No further information available |
| Baseline profile: β2 agonist use | Unclear risk | No further information available |
| Baseline profile: corticosteroid use | Unclear risk | No information available |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 6 months Intention‐to‐treat analysis: stated Responder analysis for the most part | |
| Participants | Setting: 2 centres Participants: 41 (500 µg roflumilast: 30, placebo: 11) Baseline characteristics: not stated Inclusion criteria: not stated Exclusion criteria: not stated Total number of participant withdrawals: not stated | |
| Interventions | Run‐in: not stated Concomitant medication: not stated | |
| Outcomes | Primary outcomes: postbronchodilation: spirometry, body plethysmography, 6MWT, Patient reported outcomes Secondary outcomes: not stated | |
| Notes | NCT number: NCT01480661 Funded by Takeda | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Randomised? | Low risk | Randomised |
| Method of randomisation described? | Unclear risk | Not stated |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Unclear risk | Not stated |
| Description of withdrawals and drop‐outs? | Unclear risk | Not stated |
| Baseline profile: anticholingeric use | Unclear risk | Not stated |
| Baseline profile: β2 agonist use | Unclear risk | Not stated |
| Baseline profile: corticosteroid use | Unclear risk | Not stated |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 12 weeks Intention‐to‐treat analysis: not stated | |
| Participants | Setting: 5 centres in India Participants: 118 recruited (roflumilast 500 µg: 47; roflumilast 200 µg: 46, placebo: 25) Baseline characteristics: mean age: 60 years. 98% male. 41% current smokers. Post‐bronchodilator FEV1 of 57%‐61%. Average of 25 pack‐years Inclusion criteria: not stated Exclusion criteria: not stated Participant withdrawals: roflumilast 500 µg: 13 (28%); roflumilast 200 µg: 7 (15%) and 10 (40%) from control group | |
| Interventions | Roflumilast 250 µg once daily Roflumilast 500 µg once daily Placebo once daily Concomitant medication
| |
| Outcomes | To study the safety and tolerability of roflumilast 250 μg versus roflumilast 500 μg versus placebo | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Randomised? | Low risk | Randomised |
| Method of randomisation described? | Unclear risk | Not stated |
| Blinding? | Low risk | Double‐blind |
| Method of blinding described? | Unclear risk | Not stated |
| Description of withdrawals and drop‐outs? | Low risk | Data as above |
| Baseline profile: anticholingeric use | Unclear risk | No information available |
| Baseline profile: β2 agonist use | Unclear risk | No information available |
| Baseline profile: corticosteroid use | Unclear risk | No information available |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 24 weeks Intention‐to‐treat analysis: not stated | |
| Participants | Setting: Japan Participants: 600 (roflumilast 250 µg: 205, roflumilast 500 µg: 204, placebo: 191) Baseline characteristics: mean age: 70 years. 96% male. Post‐bronchodilator FEV1 not stated. Average of 56 pack‐years. 37% current smokers Inclusion criteria: not stated Exclusion criteria: not stated Total number of participant withdrawals: not stated | |
| Interventions | Run‐in: single‐blind 4 weeks with placebo Roflumilast 500 µg once daily Roflumilast 250 µg once daily Placebo once daily Concomitant medication
| |
| Outcomes | To investigate the efficacy and safety after 24‐week treatment of APTA‐2217 (roflumilast) at doses of 500 µg and 250 µg in people with COPD using placebo as a control To investigate the pharmacokinetics of roflumilast and roflumilast N‐oxide after repeated administration of APTA‐2217 at doses of 500 µg and 250 µg | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Randomised? | Low risk | Randomised |
| Method of randomisation described? | Unclear risk | Not stated |
| Blinding? | Low risk | Double‐blind |
| Method of blinding described? | Unclear risk | Not stated |
| Description of withdrawals and drop‐outs? | High risk | Not described |
| Baseline profile: anticholingeric use | Low risk | Constant dose of short anticholinergics used in 35% (roflumilast 500 µg), 33% (roflumilast 200 µg) and 33% (placebo), respectively |
| Baseline profile: β2 agonist use | Unclear risk | Not described |
| Baseline profile: corticosteroid use | Unclear risk | Not described |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 24 weeks Intention‐to‐treat analysis: stated | |
| Participants | Setting: 159 centres in Australia, Austria, Belgium, Canada, France, Germany, Hungary, Ireland, South Africa, Spain and the UK Participants: 1411 (roflumilast 250 µg: 576, roflumilast 500 µg: 555, placebo: 280) Baseline characteristics: median age: 64 years. 74% male. Post‐bronchodilator FEV1 is 51% for both groups. Average of 42 pack‐years. 45% current smokers Inclusion criteria: aged ≥ 40 with history of COPD > 12 months. FEV1 < 50% predicted, FEV1/FVC ≤ 0.7 with smoking history > 10 pack‐years. Reversibility < 12% or 200 mL. Mean post‐bronchodilator FEV1 30%‐80% predicted Exclusion criteria: asthma, lung cancer or bronchiectasis, long‐term oxygen treatment, recent exacerbation that required a course of systemic corticosteroids, emergency room treatment or hospital admission within 4 weeks before the run‐in period Total number of participant withdrawals: 124 (22%) and 32 (11%) from treatment and control groups, respectively | |
| Interventions | Run‐in: 4 weeks with placebo Roflumilast 500 µg once daily Roflumilast 250 µg once daily Placebo once daily Concomitant medication
| |
| Outcomes | Primary outcomes: post‐bronchodilator FEV1 and SGRQ total score Secondary outcomes: change from baseline in pre‐bronchodilator FEV1, post‐bronchodilator FVC, post‐bronchodilator FEV in 6 seconds and FVC, FEF rate between 25%‐75% of vital capacity and number of moderate or severe COPD exacerbations | |
| Notes | There is inconsistency in the quoting of statistical errors. Within the text and Table 2, data are quoted as "least squares means and SD", however in Figures 2 and 3, SE bars are shown. It is more likely that the results represent SE and not SD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | "No person involved in data analysis had knowledge of the randomisation sequence" |
| Randomised? | Low risk | "Treatment was assigned by the investigators with sequential study numbers according to a block randomisation list in ratios of 2:2:1 (Roflumilast 250 µg, Roflumilast 500 µg, Placebo)." |
| Method of randomisation described? | Low risk | "The randomisation sequence was generated by ALTANA Pharma AG in a blinded manner" |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Low risk | "Medication boxes were labelled with the study protocol number, randomisation number, and visit code; coding prevented the investigator and people at the study centre from knowing which medication was given." |
| Description of withdrawals and drop‐outs? | Low risk | 100 participants discontinued from study from the roflumilast 250 µg group, 124 from the roflumilast 500 µg group and 32 from the placebo group |
| Baseline profile: anticholingeric use | Unclear risk | No information available |
| Baseline profile: β2 agonist use | Unclear risk | No information available |
| Baseline profile: corticosteroid use | Unclear risk | No information available |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 24 weeks Intention‐to‐treat analysis: not stated | |
| Participants | Setting: 36 centres in Argentina, Canada, Columbia, Mexico, Peru and the USA Participants: estimated enrolment of 1000 participants Baseline characteristics: not stated Inclusion criteria:
Exclusion criteria:
Total number of participant withdrawals: not stated | |
| Interventions | Roflumilast 500 µg daily versus placebo | |
| Outcomes | Primary outcomes: pulmonary function | |
| Notes | ClinicalTrials.gov Identifier: nCT00062582 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Randomised? | Low risk | Randomised |
| Method of randomisation described? | Unclear risk | Not stated |
| Blinding? | Low risk | Double‐blind |
| Method of blinding described? | Unclear risk | Not stated |
| Description of withdrawals and drop‐outs? | Unclear risk | Not stated |
| Baseline profile: anticholingeric use | Unclear risk | Not stated |
| Baseline profile: β2 agonist use | Unclear risk | Not stated |
| Baseline profile: corticosteroid use | Unclear risk | Not stated |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 52 weeks Intention‐to‐treat analysis: stated | |
| Participants | Setting: M2‐111 was conducted in 188 centres in 6 countries, and M2‐112 in 159 centres in 14 countries Inclusion criteria: aged ≥ 40 years. Post‐bronchodilator FEV1 < 50% predicted. Reversibility < 15%. Mean post‐bronchodilator FEV1 42%. FEV1/FVC ≤ 0.7 with smoking history > 10 pack‐years. 40% current smokers, 60% ex‐smokers; average 46‐48 pack‐years Exclusion criteria: history of asthma, lung cancer or bronchiectasis, need for long‐term oxygen therapy, known α1antitrypsin deficiency or clinically significant cardiopulmonary co‐morbidity Total number of participant withdrawals: data combined with M2‐112 showing 433 (33%) and 348 (26%) from treatment and control groups, respectively | |
| Interventions | Run‐in: 4 weeks with placebo Roflumilast 500 µg once daily Placebo once daily Concomitant medication
Used alongside corticosteroids, anticholinergics and rescue short‐acting ß2 agonists 54% overall (available to all) | |
| Outcomes | Primary outcomes: change from baseline to endpoint in post‐bronchodilator FEV1 and the number of moderate or severe exacerbations per patient per year Secondary outcomes: change from baseline in SGRQ total score, change from baseline in prebronchial FEV1, post‐bronchodilator FEV in 6 seconds and FVC, FEF rate between 25%‐75% of vital capacity and number of moderate or severe COPD exacerbations requiring systemic corticosteroid treatment per patient per year | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | "Each study participant who qualified was assigned a number in sequential order. Code labelling prevented the investigator and the patient from knowing which drug was administered." |
| Randomised? | Low risk | "randomised (1:1)" |
| Method of randomisation described? | Low risk | "The randomisation list was generated using a multiplicative congruential pseudorandom number generator (program RANDOM, based on Fishman and Moore)." |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Low risk | "There was a stratification of patients according to smoking status (current smokers/ex‐smokers) and treatment with inhaled corticosteroids (yes/no)." |
| Description of withdrawals and drop‐outs? | Low risk | Data combined with M2‐112 |
| Baseline profile: anticholingeric use | Low risk | Data combined with M2‐112 showing 1604 (60%) participants used anticholinergics |
| Baseline profile: β2 agonist use | Low risk | 1463 (55%) participants on short‐acting β2‐agonists |
| Baseline profile: corticosteroid use | Low risk | 1622 (61%) on concomitant ICS |
| Methods | As described in separate studies above and below | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | See individual trials |
| Randomised? | Low risk | See individual trials |
| Method of randomisation described? | Low risk | See individual trials |
| Blinding? | Low risk | See individual trials |
| Method of blinding described? | Low risk | See individual trials |
| Description of withdrawals and drop‐outs? | Low risk | See individual trials |
| Baseline profile: anticholingeric use | Low risk | See individual trials |
| Baseline profile: β2 agonist use | Low risk | See individual trials |
| Baseline profile: corticosteroid use | Low risk | See individual trials |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 52 weeks Intention‐to‐treat analysis: stated | |
| Participants | Setting: 159 centres in 14 countries Participants: 1513 (roflumilast 500 µg: 760, placebo: 753) Baseline characteristics: severe COPD according to GOLD criteria grades III and IV. Mean age: 65 years. 75% male Inclusion criteria: aged ≥ 40 years. Post‐bronchodilator FEV1 < 50% predicted. Reversibility < 15%. Mean post‐bronchodilator FEV1 41%. FEV1/FVC ≤ 0.7 with smoking history > 10 pack‐years. 37% current smokers, 63% ex‐smokers; average 44 pack‐years Exclusion criteria: history of asthma, lung cancer or bronchiectasis, need for long‐term oxygen therapy, known α1antitrypsin deficiency or clinically significant cardiopulmonary co‐morbidity Total number of participant withdrawals: 217 (29%) and 163 (22%) from treatment and control groups, respectively | |
| Interventions | Run‐in: 4 weeks with placebo Roflumilast 500 µg once daily Placebo once daily Concomitant medication
Used alongside corticosteroids, anticholinergics and rescue short‐acting ß2 agonists 54% overall (available to all) | |
| Outcomes | Primary outcomes: change from baseline to endpoint in post‐bronchodilator FEV1 and the number of moderate or severe exacerbations per patient per year Secondary outcomes: change from baseline in SGRQ total score, change from baseline in prebronchial FEV1, post‐bronchodilator FEV in 6 seconds and FVC, FEF rate between 25%‐75% of vital capacity and number of moderate or severe COPD exacerbations requiring systemic corticosteroid treatment per patient per year | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | "Each study participant who qualified was assigned a number in sequential order. Code labelling prevented the investigator and the patient from knowing which drug was administered." |
| Randomised? | Low risk | "randomised (1:1)" |
| Method of randomisation described? | Low risk | "The randomisation list was generated using a multiplicative congruential pseudorandom number generator (program RANDOM, based on Fishman and Moore)." |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Low risk | "There was a stratification of patients according to smoking status (current smokers/ex‐smokers) and treatment with inhaled corticosteroids (yes/no)." |
| Description of withdrawals and drop‐outs? | Low risk | "Over 70% of patients completed the study. The reasons for withdrawal were similar between groups except for adverse events, which occurred more frequently with roflumilast." "Withdrawal due to COPD exacerbations was reported in 3.5 and 3.2% of patients in roflumilast and placebo groups, respectively." |
| Baseline profile: anticholingeric use | Low risk | 739 participants used anticholinergics |
| Baseline profile: β2 agonist use | Low risk | 820 participants on short‐acting β2‐agonists |
| Baseline profile: corticosteroid use | Low risk | 727 participants on beclomethasone dipropionate 2000 µg or less |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 12 weeks Intention‐to‐treat analysis: stated | |
| Participants | Setting: 22 centres in 4 countries Participants: 250 (roflumilast 500 µg: 127, placebo: 123) Baseline characteristics: mean age: 60 years. 73% (roflumilast) versus 84% (placebo) male. Post‐bronchodilator FEV1 55% predicted. Average of 41 pack‐years. 53% current smokers Inclusion criteria: participants were clinically stable patients ≥ 40 years with a smoking history of > 10 pack‐years and a 12‐month history of COPD. Other inclusion criteria included: post‐bronchodilator FEV1 30%‐80% predicted, FEV1/forced vital capacity (FVC) < 0.7 and set plethysmographic FRC and peak oxygen uptake requirements Exclusion criteria: asthma or a lung disease other than COPD; a1‐antitrypsin deficiency; participation in a pulmonary rehabilitation programme within 2 months; supplemental oxygen therapy; a significant medical comorbidity that may influence exercise tolerance Total number of participant withdrawals: 16 (13%) and 12 (10%) from treatment and control groups, respectively | |
| Interventions | Roflumilast 500 µg once daily Placebo once daily Concomitant medication
| |
| Outcomes | Activity‐related dyspnoea (TDI). Spirometry and body plethysmography. Symptom‐limited exercise tests | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Randomised? | Low risk | A 1:1 randomisation ratio was used |
| Method of randomisation described? | Unclear risk | Not stated |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Unclear risk | Not stated |
| Description of withdrawals and drop‐outs? | Low risk | Of the 250 randomised participants, 16 from the roflumilast group and 12 from the placebo group discontinued prematurely |
| Baseline profile: anticholingeric use | Unclear risk | Not stated |
| Baseline profile: β2 agonist use | Unclear risk | Not stated |
| Baseline profile: corticosteroid use | Unclear risk | Not stated |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 12 weeks Intention‐to‐treat analysis: stated | |
| Participants | Setting: 32 centres in 5 countries Participants: 410 (roflumilast 500 µg: 203, placebo: 207) Baseline characteristics: mean age: 68 years. 93% male. Post‐bronchodilator FEV1 50.5% predicted. Average of 44 pack‐years. 69% current smokers Inclusion criteria: former or current smokers with at least a 10 pack‐year history. Aged ≥ 40 years. Post‐bronchodilator FEV1/FVC ≤ 0.7 and FEV1 of 30%‐80%. Clinically stable COPD within 4 weeks prior to baseline Exclusion criteria: history of asthma or other relevant lung disease, COPD exacerbation with the 4 weeks prior to baseline, need for long‐term oxygen therapy, known α1antitrypsin deficiency or clinically significant cardiopulmonary co‐morbidity Total number of participant withdrawals: 40 (20%) and 18 (9%) from treatment and control groups, respectively | |
| Interventions | Run‐in: 4 weeks with placebo Roflumilast 500 µg once daily Placebo once daily Concomitant medication
| |
| Outcomes | Primary outcome: mean change in post‐bronchodilator FEV1 from baseline Secondary outcomes: mean change in pre‐bronchodilator FEV1 from baseline, change in other lung function measures, time to COPD exacerbation, proportion of participants experiencing exacerbations, time to study withdrawal and adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Randomised? | Low risk | Randomised |
| Method of randomisation described? | Low risk | Computer‐generated randomisation list used |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Unclear risk | Not stated |
| Description of withdrawals and drop‐outs? | Low risk | Participant numbers at different stages of the study described in figure |
| Baseline profile: anticholingeric use | Unclear risk | Not stated |
| Baseline profile: β2 agonist use | Unclear risk | Not stated |
| Baseline profile: corticosteroid use | Unclear risk | Not stated |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 12 weeks Intention‐to‐treat analysis: stated | |
| Participants | Setting: 16 centres in 6 countries Participants: estimated enrolment 550 participants Baseline characteristics: not stated Inclusion criteria: people with a history of COPD for at least 12 months as defined by the GOLD criteria, age ≥ 40 years, FEV1/FVC ratio (post‐bronchodilator) ≤ 70%, FEV1 (post‐bronchodilator) ≤ 65% of predicted, FRC (post‐bronchodilator) ≤ 120% of predicted Exclusion criteria: COPD exacerbation indicated by a treatment with systemic glucocorticosteroids not stopped at least 4 weeks prior to the baseline visit, non‐smoker, current smoker or ex‐smoker (smoking cessation at least 1 year ago) with a smoking history of < 10 pack‐years, or suffering from any concomitant disease that might interfere with study procedures or evaluation Total number of participant withdrawals: not stated | |
| Interventions | 500 µg roflumilast tablets once daily versus placebo | |
| Outcomes | Primary outcome: lung function parameters indicative of hyperinflation in people with COPD Secondary outcomes:
| |
| Notes | ClinicalTrials.gov Identifier: NCT00108823 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Randomised? | Low risk | Randomised |
| Method of randomisation described? | Unclear risk | Not stated |
| Blinding? | Low risk | Double‐blind |
| Method of blinding described? | Unclear risk | Not stated |
| Description of withdrawals and drop‐outs? | Unclear risk | Not stated |
| Baseline profile: anticholingeric use | Unclear risk | Not stated |
| Baseline profile: β2 agonist use | Unclear risk | Not stated |
| Baseline profile: corticosteroid use | Unclear risk | Not stated |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 52 weeks Intention‐to‐treat analysis: stated | |
| Participants | Setting: 246 centres in 10 countries Participants: 1513 (roflumilast 500 µg: 760, placebo: 753) Baseline characteristics: mean age: 64 years. 71% male. Post‐bronchodilator FEV1 37.6% predicted. Average of 47 pack‐years. 48% current smokers Inclusion criteria: former or current smokers with at least a 20 pack‐year history. Aged ≥ 40 years. Post‐bronchodilator FEV1/FVC ≤ 0.7. Chronic cough and sputum production. Post‐bronchodilator FEV1 < 50% predicted. At least 1 recorded COPD exacerbation requiring systemic glucocorticosteroids or treatment in hospital in previous year Exclusion criteria: available in the online web appendix (pg 11) Total number of participant withdrawals: 264 (34%) and 234 (31%) from treatment and control groups, respectively | |
| Interventions | Run‐in: 4 weeks with placebo Roflumilast 500 µg once daily Placebo once daily Concomitant medication
| |
| Outcomes | Primary outcomes: mean change in pre‐bronchodilator FEV1 from baseline to each post‐randomisation visit during the treatment period. Mean rate of COPD exacerbations requiring oral or parenteral glucocorticosteroids or requiring hospitalisation or leading to death, per patient per year Secondary outcomes: mean change in post‐bronchodilator FEV1 from baseline to each post‐randomisation visit during the treatment period. Time to mortality due to any reason. Natural log‐transformed C Reactive Protein (mg/L), Mean TDI focal score during the treatment period | |
| Notes | Adverse event data are pooled with numbers from study M2‐125 that followed an identical study design | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | All individuals involved in the studies were unaware of treatment assignment |
| Randomised? | Low risk | "Randomly assigned to oral roflumilast 500 µg once daily or placebo." |
| Method of randomisation described? | Low risk | "The sponsor generated a randomisation list of patient random numbers using a pseudorandom number generator. The investigator used an automated, interactive voice response system to randomly assign patients." |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Low risk | "All individuals involved in the studies were unaware of treatment assignment. Tablets were identical in appearance. The investigator or anyone at the study site was prevented from knowing the allocation sequence with code labelling." |
| Description of withdrawals and drop‐outs? | Low risk | 264 participants discontinued from study in the roflumilast group and 234 discontinued from the placebo group |
| Baseline profile: anticholingeric use | Unclear risk | No other information available |
| Baseline profile: β2 agonist use | Unclear risk | No other information available |
| Baseline profile: corticosteroid use | High risk | Pretreatment of 44% in both roflumilast and placebo groups |
| Methods | As described in separate studies above and below | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | See individual studies |
| Randomised? | Low risk | See individual studies |
| Method of randomisation described? | Low risk | See individual studies |
| Blinding? | Low risk | See individual studies |
| Method of blinding described? | Low risk | See individual studies |
| Description of withdrawals and drop‐outs? | Low risk | See individual studies |
| Baseline profile: anticholingeric use | Low risk | See individual studies |
| Baseline profile: β2 agonist use | Low risk | See individual studies |
| Baseline profile: corticosteroid use | High risk | See individual studies |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 52 weeks Intention‐to‐treat analysis: stated | |
| Participants | Setting: 221 centres in 8 countries Participants: 1571 (roflumilast 500 µg: 773, placebo: 798) Baseline characteristics: mean age: 64 years. 80% male. Average of 48 pack‐years. 35% current smokers Inclusion criteria: former or current smokers with at least a 20 pack‐year history. Aged ≥ 40 years. Post‐bronchodilator FEV1/FVC ≤ 0.7. Chronic cough and sputum production. Post‐bronchodilator FEV1 < 50% predicted. At least 1 recorded COPD exacerbation requiring systemic glucocorticosteroids or treatment in hospital in previous year Exclusion criteria: available in the online web appendix (pg 11) Total number of participant withdrawals: 246 (32%) and 248 (31%) from treatment and control groups, respectively | |
| Interventions | Run‐in: 4 weeks with placebo Roflumilast 500 µg once daily Placebo once daily Concomitant medication
| |
| Outcomes | Primary outcomes: mean change in pre‐bronchodilator FEV1 from baseline to each post‐randomisation visit during the treatment period. Mean rate of COPD exacerbations requiring oral or parenteral glucocorticosteroids or requiring hospitalisation or leading to death, per patient per year Secondary outcomes: mean change in post‐bronchodilator FEV1 from baseline to each post‐randomisation visit during the treatment period. Time to mortality due to any reason. Natural log‐transformed CRP (mg/L), Mean TDI focal score during the treatment period | |
| Notes | Adverse event data are pooled with numbers from study M2‐124 that followed an identical study design | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | All individuals involved in the studies were unaware of treatment assignment |
| Randomised? | Low risk | "Randomly assigned to oral roflumilast 500 µg once daily or placebo." |
| Method of randomisation described? | Low risk | "The sponsor generated a randomisation list of patient random numbers using a pseudorandom number generator. The investigator used an automated, interactive voice response system to randomly assign patients." |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Low risk | "All individuals involved in the studies were unaware of treatment assignment. Tablets were identical in appearance. The investigator or anyone at the study site was prevented from knowing the allocation sequence with code labelling." |
| Description of withdrawals and drop‐outs? | Low risk | 246 patients discontinued from study in the roflumilast group and 248 discontinued from the placebo group |
| Baseline profile: anticholingeric use | Low risk | No other information available |
| Baseline profile: β2 agonist use | Low risk | No other information available |
| Baseline profile: corticosteroid use | High risk | Pretreatment of 40% in both roflumilast and placebo groups |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 24 weeks Intention‐to‐treat analysis: stated | |
| Participants | Setting: 135 centres in 10 countries Participants: 1221 (roflumilast 500 µg: 467, placebo: 468) Baseline characteristics: mean age: 65 years. 71% male. Post‐bronchodilator FEV1 54.7% and 55.3% predicted (roflumilast and placebo). Average of 43 pack‐years. 39% current smokers Inclusion criteria: former or current smokers with (≥ 1 year smoking cessation) and at least a 10 pack‐year history. Aged ≥ 40 years. Post‐bronchodilator FEV1/FVC ≤ 0.7. Post‐bronchodilator FEV1 40% to 70% predicted. Partial reversibility to albuterol with increase from baseline FEV1 of ≤ 12% or 200 mL Exclusion criteria: available in the online web appendix (pg 10) Total number of participant withdrawals: 107 (23%) and 82 (18%) from treatment and control groups, respectively | |
| Interventions | Run‐in: 4 weeks with placebo once a day Roflumilast 500 µg and salmeterol once daily Placebo once daily Concomitant medication
| |
| Outcomes | Primary outcomes: change in mean pre‐bronchodilator FEV1 from baseline to each post‐randomisation visit | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | All individuals involved in the studies were unaware of treatment assignment |
| Randomised? | Low risk | "Randomly assigned to oral roflumilast 500 µg once daily or placebo." |
| Method of randomisation described? | Low risk | "The sponsor generated a randomisation list of patient random numbers using a pseudorandom number generator. The investigator used an automated, interactive voice response system to randomly assign patients." |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Low risk | "All individuals involved in the studies were unaware of treatment assignment. The investigator or anyone at the study site was prevented from knowing the allocation sequence with code labelling. Tablets were identical in appearance." |
| Description of withdrawals and drop‐outs? | Low risk | 107 participants discontinued from study in the roflumilast group and 82 discontinued from the placebo group |
| Baseline profile: anticholingeric use | Unclear risk | No other information available |
| Baseline profile: β2 agonist use | Unclear risk | No other information available |
| Baseline profile: corticosteroid use | Unclear risk | No other information available |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 24 weeks Intention‐to‐treat analysis: stated | |
| Participants | Setting: 85 centres in 7 countries Participants: 910 (roflumilast 500 µg: 372, placebo: 372) Baseline characteristics: mean age: 64 years. 71% male. Post‐bronchodilator FEV1 56.0% and 56.2% predicted (roflumilast and placebo). Average of 44 pack‐years. 40% current smokers Inclusion criteria: former or current smokers with (≥ 1 year smoking cessation) and at least a 10 pack‐year history. Aged ≥ 40 years. Post‐bronchodilator FEV1/FVC ≤ 0.7. Post‐bronchodilator FEV1 40% to 70% predicted. Partial reversibility to albuterol with increase from baseline FEV1 of ≤ 12% or 200 mL Exclusion criteria: available in the online web appendix (pg 10) Total number of participant withdrawals: 62 (17%) and 39 (11%) from treatment and control groups, respectively | |
| Interventions | Run‐in: 4 weeks with placebo once a day Roflumilast 500 µg and tiotropium once daily Placebo once daily Concomitant medication
| |
| Outcomes | Primary outcomes: change in mean pre‐bronchodilator FEV1 from baseline to each post‐randomisation visit | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | All individuals involved in the studies were unaware of treatment assignment |
| Randomised? | Low risk | "Randomly assigned to oral roflumilast 500 µg once daily or placebo." |
| Method of randomisation described? | Low risk | "The sponsor generated a randomisation list of patient random numbers using a pseudorandom number generator. The investigator used an automated, interactive voice response system to randomly assign patients." |
| Blinding? | Low risk | Double‐blinded |
| Method of blinding described? | Low risk | "All individuals involved in the studies were unaware of treatment assignment. The investigator or anyone at the study site was prevented from knowing the allocation sequence with code labelling. Tablets were identical in appearance." |
| Description of withdrawals and drop‐outs? | Low risk | 62 participants discontinued from study in the roflumilast group and 39 discontinued from the placebo group |
| Baseline profile: anticholingeric use | Unclear risk | No information available |
| Baseline profile: β2 agonist use | Unclear risk | No information available |
| Baseline profile: corticosteroid use | Unclear risk | No information available |
| Methods | Parallel‐group study Randomisation: randomised, double‐blind, placebo‐controlled trial Trial duration: 52 weeks Intention‐to‐treat analysis: stated | |
| Participants | Setting: 338 locations across Australia, Argentina, Canada, Chile, Columbia, Italy, Malaysia, Peru, Phillippines, Romania, Russia, Serbia, Spain, Taiwan, Ukraine Participants: 2354 (500 µg roflumilast: 1178; placebo: 1176) Baseline characteristics: mean age: 64 years, 68% male, mean FEV1% predicted of 33%, mean smoking history of 52.2 pack‐years for roflumilast and 53.1 pack‐years for placebo) or current smokers (39% and 40%, respectively) Inclusion criteria: ≥ 40 years with severe‐very severe COPD, chronic bronchitis, ≥ 2 exacerbations and/or hospitalisations in the previous year, and were receiving ICS/LABA with or without LAMA daily for ≥ 3 months Exclusion criteria: participants were excluded if, within the 4 weeks prior to enrolment, they had a moderate or severe COPD exacerbation and/or COPD exacerbation treated with antibiotics or systemic corticosteroids or a lower respiratory tract infection. Other exclusionary criteria included diagnoses of other lung diseases, moderate‐to‐severe liver impairment (Child‐Pugh B or C), HIV or hepatitis infection, current diagnosis of asthma, cancer in the past 5 years, α1‐antitrypsin deficiency, a clinically significant cardiovascular condition, a resting QTc interval > 470 ms, or a body mass index ≥ 45 kg/m Total number of participant withdrawals: 337 (29%) and 254 (21%) from treatment and control groups, respectively | |
| Interventions | Run‐in: 2 weeks, single‐blind. Placebo tablets to assess suitability Roflumilast 500 µg once daily Placebo once daily Concomitant medication ICS/LABA FDC (fluticasone propionate/salmeterol, 250/50 mg (1 inhalation twice a day), or budesonide/formoterol, 160/4.5 mg (2 inhalations twice a day) Participants taking fluticasone propionate/salmeterol, 500/50 mg, at study entry were required to switch to the lower dosage (250/50 mg) before entry. Up to 60% of participants were allowed concomitant LAMA (e.g. tiotropium) if administered for ≥3 months before screening, with no dose change. Those not on LAMA were allowed a SAMA. | |
| Outcomes | Primary outcomes: rate of moderate or severe COPD exacerbations per patient per year Secondary outcomes: rate of severe exacerbations, rate of moderate or severe antibiotic‐treated COPD exacerbations, and mean change from baseline in predose FEV1 over 52 weeks, frequency of moderate or severe COPD exacerbations, median time to first moderate or severe exacerbation, mean changes from baseline in predose FVC, mean changes from baseline over time in the COPD Assessment Test (CAT), and daily symptoms measured via EXAcerbation of Chronic Pulmonary Disease Tool–Patient Reported Outcomes (EXACT‐PRO), adverse events, Columbia‐Suicide Severity Rating Scale, laboratory parameters and vital signs | |
| Notes | NCT01443845 Sponsored by Activas and Astra Zeneca | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Randomised? | Low risk | Randomised |
| Method of randomisation described? | Unclear risk | Not stated |
| Blinding? | Low risk | Double blind |
| Method of blinding described? | Unclear risk | Not stated |
| Description of withdrawals and drop‐outs? | Unclear risk | 337 participants (29%) discontinued from study in the roflumilast group and 254 (22%) discontinued from the placebo group |
| Baseline profile: anticholingeric use | Unclear risk | LAMA: 47% for placebo; 47% for roflumilast |
| Baseline profile: β2 agonist use | Unclear risk | Combined LABA/ICS Fluticasone propionate/salmeterol FDC 65% for placebo; 65% for roflumilast Budesonide/formoterol FDC 35% for placebo; 35% for roflumilast |
| Baseline profile: corticosteroid use | Unclear risk | As above |
CAT: COPD Assessment Test; COPD: chronic obstructive pulmonary disease; DLCO: diffusing capacity of the lung for carbon monoxide; FDC: fixed dose combination; FEF: forced expiratory flow; FEV1: forced expiratory volume in one second; FRC: functional residual capacity; FVC: forced vital capacity; GOLD: Global Initiative for Chronic Obstructive Lung Disease; IC: inspiratory capacity; ICS: inhaled corticosteroid; L: litre; LABA: Long‐acting beta‐2 agonist; LAMA: Long acting muscarinic antagonist; MDI: metered dose inhaler; mL: millilitre; RFRC: resting functional residue capacity; RV: residual volume; SAMA: Short acting muscarinic antagonist; SD: standard deviation; SE: standard error; SGRQ: St George's Respiratory Questionnaire
SOBQ: Shortness of Breath Questionnaire; SVC: slow vital capacity; TDI: transition dyspnoea index; 6MWT: 6‐minute walk test
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Insufficient data. Only RR of QOL improvement provided | |
| Integrated results from four 24‐week cilomilast trials | |
| Analysis focused on participants with a baseline SGRQ score of ≥ the median SGRQ score only | |
| Endpoint: first dose‐bronchodilator effects only | |
| Treatment Bayer BAY 19‐8004; 11 participants only 1 week in duration | |
| Endpoint: first‐dose bronchodilator effects only | |
| Cross‐over design | |
| Phase II trial. No primary outcome measure investigating lung function. Only 1 trial to date | |
| No study ID or group numbers identified | |
| No SD or SE given | |
| Combining results from 2 pivotal European phase III cilomilast trials | |
| Study 038. Insufficient data available for changes in lung function and exacerbation rates | |
| Treatment QAK423 (Novartis), discontinued. Only 1 trial available | |
| Combined results. Individual studies already included in review | |
| JP108 is an extension study of APTA‐2217‐06 study. After the key‐open of APTA‐2217‐06 study, administration to placebo group would be terminated. Not all participants enrolled in JP106 continued onto the JP108 study | |
| No placebo group | |
| Open label. No placebo group | |
| Open‐label study. Men or women with COPD who successfully completed study 042 or 091 where participants received cilomilast 15 mg twice daily or placebo for 24 weeks in study 042 and 26 weeks in study 091 without tolerability problems. Concomitant COPD medication use allowed, given placebo or placebo/Ariflo during study period | |
| Open‐label study. Men or women with COPD who successfully completed study 039 where participants received cilomilast 15 mg twice daily or placebo for 24 weeks without tolerability problems. Concomitant COPD medication use allowed, given placebo or placebo/Ariflo during study period | |
| Abstract only. Unable to contact study author | |
| No study ID or group numbers identified | |
| Treatment UK‐500,001 (Pfizer). Discontinued | |
| Treatment UK‐500,001 (Pfizer). Discontinued | |
| Although quoted as significant, mean and SD figures not provided | |
| Inhaled therapy |
COPD: chronic obstructive pulmonary disease
RR: risk ratio
QOL: quality of life
SD: standard deviation
SE: standard error
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | An international, 16‐week, randomised, double‐blind, placebo‐controlled, parallel‐group study investigating the effects of roflumilast 500 µg once‐daily versus placebo on inflammatory parameters in bronchial biopsy tissue specimens, sputum and blood serum |
| Participants | 150 participants with COPD and chronic bronchitis for at least 12 months will be recruited into the study and randomized in a 1:1 ratio to receive either roflumilast or placebo |
| Interventions | Roflumilast and placebo |
| Outcomes | The primary endpoint will be the number of CD8+ cells in bronchial biopsy tissue specimens (sub‐mucosa) and the key secondary endpoint will be the number of CD68+ cells assessed by indirect immunohistochemistry |
| Notes | Completed awaiting results |
| Methods | Single‐blind, randomised, placebo‐controlled study was carried out in the Department of Respiratory Medicine at National Institute of Diseases of the Chest and Hospital (NIDCH), Dhaka, Bangladesh |
| Participants | 130 participants were recruited initially and randomly distributed into Group‐A where they got conventional therapy (inhaled salmeterol + fluticasone and tiotropium) and roflumilast (0.5 mg once daily) and Group‐ B where participants got placebo with conventional therapy. Study duration was 3 months |
| Interventions | As above |
| Outcomes | The primary outcome variable was change in mean FEV1 and secondary outcome variable was change in mean CAT score from baseline |
| Notes | No data provided. Study authors contacted |
CAT: COPD Assessment Test
COPD: chronic obstructive pulmonary disease
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Evaluation of the effect of roflumilast in hyperinflated COPD patients using functional respiratory imaging |
| Methods | Parallel RCT |
| Participants | 40 people who are stable on LABA/LAMA therapy and who are prone to dynamics hyperinflation |
| Interventions | Roflumilast and placebo |
| Outcomes | Radiological (CT) changes in airway measures Changes in spirometry and body plethysmography |
| Starting date | September 2015 |
| Contact information | University Hospital of Antwerp |
| Notes | Other Study ID Numbers: FLUI‐2014‐134, EudraCT Estimated study completion date: January 2017 |
| Trial name or title | A multicenter randomized double‐blind clinical study evaluated the safety, pharmacokinetic and pharmacodynamic characteristics of roflumilast in COPD patients |
| Methods | Parallel RCT |
| Participants | People with COPD in China |
| Interventions | Roflumilast and placebo |
| Outcomes | Area under the plasma concentration after versus drug dose Percentage of participants with adverse events of interest Change in pre‐bronchodilator FEV1 during the down titration period |
| Starting date | March 2016 |
| Contact information | Contact: Zheng Jinping |
| Notes | Estimated enrolment: 120 Estimated study completion date: August 2017 |
COPD: chronic obstructive pulmonary disease
FEV1: forced expiratory volume in first second
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 FEV1 (by drug) Show forest plot | 27 | 20585 | Mean Difference (IV, Random, 95% CI) | 51.53 [43.17, 59.90] |
| Analysis 1.1  Comparison 1 PDE4 inhibitor versus placebo, Outcome 1 FEV1 (by drug). | ||||
| 1.1 Roflumilast 500 μg | 17 | 14230 | Mean Difference (IV, Random, 95% CI) | 56.45 [48.01, 64.89] |
| 1.2 Roflumilast 250 μg | 3 | 1033 | Mean Difference (IV, Random, 95% CI) | 56.88 [24.38, 89.38] |
| 1.3 Cilomilast 15 mg | 10 | 5322 | Mean Difference (IV, Random, 95% CI) | 41.03 [23.93, 58.13] |
| 2 FEV1 (by mean COPD severity) Show forest plot | 21 | 16659 | Mean Difference (IV, Fixed, 95% CI) | 52.77 [46.73, 58.82] |
| Analysis 1.2  Comparison 1 PDE4 inhibitor versus placebo, Outcome 2 FEV1 (by mean COPD severity). | ||||
| 2.1 GOLD grade I + II (FEV1 ≥ 50% predicted) | 9 | 4647 | Mean Difference (IV, Fixed, 95% CI) | 51.79 [38.99, 64.59] |
| 2.2 GOLD grade III + IV (FEV1 < 50% predicted) | 12 | 12012 | Mean Difference (IV, Fixed, 95% CI) | 53.06 [46.19, 59.92] |
| 3 FEV1 (Roflumilast 500 μg by mean COPD severity) Show forest plot | 15 | 13742 | Mean Difference (IV, Fixed, 95% CI) | 55.51 [48.88, 62.14] |
| Analysis 1.3  Comparison 1 PDE4 inhibitor versus placebo, Outcome 3 FEV1 (Roflumilast 500 μg by mean COPD severity). | ||||
| 3.1 GOLD grade I + II (FEV1 ≥ 50% predicted) | 6 | 3187 | Mean Difference (IV, Fixed, 95% CI) | 69.86 [53.34, 86.38] |
| 3.2 GOLD grade III + IV (FEV1 < 50% predicted) | 9 | 10555 | Mean Difference (IV, Fixed, 95% CI) | 52.75 [45.52, 59.99] |
| 4 FEV1 (by study duration) Show forest plot | 27 | 19785 | Mean Difference (IV, Fixed, 95% CI) | 49.08 [43.85, 54.32] |
| Analysis 1.4 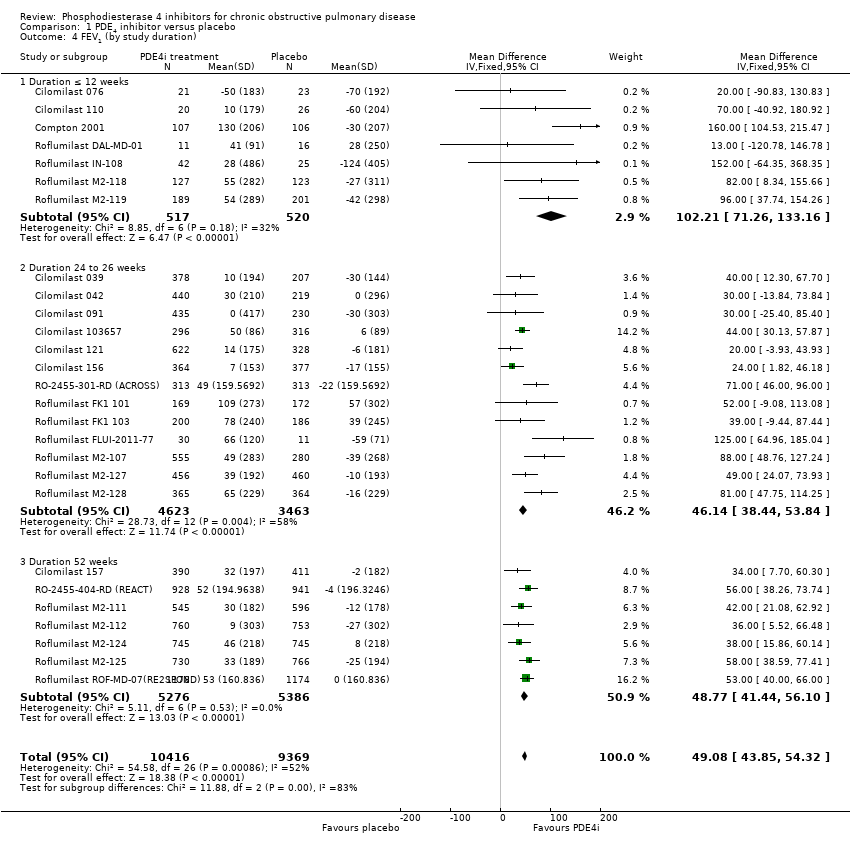 Comparison 1 PDE4 inhibitor versus placebo, Outcome 4 FEV1 (by study duration). | ||||
| 4.1 Duration ≤ 12 weeks | 7 | 1037 | Mean Difference (IV, Fixed, 95% CI) | 102.21 [71.26, 133.16] |
| 4.2 Duration 24 to 26 weeks | 13 | 8086 | Mean Difference (IV, Fixed, 95% CI) | 46.14 [38.44, 53.84] |
| 4.3 Duration 52 weeks | 7 | 10662 | Mean Difference (IV, Fixed, 95% CI) | 48.77 [41.44, 56.10] |
| 5 FEV1 (additional medication) Show forest plot | 27 | 19565 | Mean Difference (IV, Fixed, 95% CI) | 49.08 [43.84, 54.31] |
| Analysis 1.5  Comparison 1 PDE4 inhibitor versus placebo, Outcome 5 FEV1 (additional medication). | ||||
| 5.1 Long‐acting bronchodilator | 2 | 1645 | Mean Difference (IV, Fixed, 95% CI) | 60.52 [40.57, 80.46] |
| 5.2 Corticosteroids | 3 | 2904 | Mean Difference (IV, Fixed, 95% CI) | 42.26 [25.46, 59.05] |
| 5.3 PDE4i treatment only | 19 | 10169 | Mean Difference (IV, Fixed, 95% CI) | 44.78 [37.67, 51.90] |
| 5.4 Various concomitant treatments | 3 | 4847 | Mean Difference (IV, Fixed, 95% CI) | 56.58 [46.91, 66.25] |
| 6 FEV1 (published versus unpublished) Show forest plot | 27 | 19785 | Mean Difference (IV, Fixed, 95% CI) | 49.23 [43.99, 54.46] |
| Analysis 1.6  Comparison 1 PDE4 inhibitor versus placebo, Outcome 6 FEV1 (published versus unpublished). | ||||
| 6.1 Published | 19 | 15244 | Mean Difference (IV, Fixed, 95% CI) | 55.75 [49.44, 62.06] |
| 6.2 Unpublished | 8 | 4541 | Mean Difference (IV, Fixed, 95% CI) | 34.82 [25.44, 44.19] |
| 7 FEV1 (random‐effects model) Show forest plot | 27 | 19785 | Mean Difference (IV, Random, 95% CI) | 51.47 [42.68, 60.26] |
| Analysis 1.7  Comparison 1 PDE4 inhibitor versus placebo, Outcome 7 FEV1 (random‐effects model). | ||||
| 8 FEV1 (roflumilast 500 μg versus 250 μg) Show forest plot | 3 | 1560 | Mean Difference (IV, Fixed, 95% CI) | 22.61 [‐5.95, 51.16] |
| Analysis 1.8  Comparison 1 PDE4 inhibitor versus placebo, Outcome 8 FEV1 (roflumilast 500 μg versus 250 μg). | ||||
| 9 FVC Show forest plot | 16 | 21954 | Mean Difference (IV, Fixed, 95% CI) | 87.28 [74.87, 99.70] |
| Analysis 1.9  Comparison 1 PDE4 inhibitor versus placebo, Outcome 9 FVC. | ||||
| 10 PEF Show forest plot | 5 | 4245 | Mean Difference (IV, Fixed, 95% CI) | 6.54 [3.95, 9.13] |
| Analysis 1.10 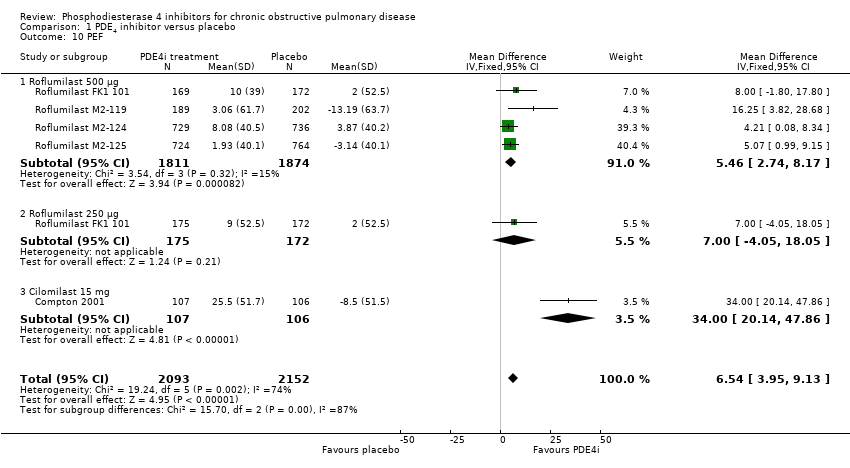 Comparison 1 PDE4 inhibitor versus placebo, Outcome 10 PEF. | ||||
| 10.1 Roflumilast 500 μg | 4 | 3685 | Mean Difference (IV, Fixed, 95% CI) | 5.46 [2.74, 8.17] |
| 10.2 Roflumilast 250 μg | 1 | 347 | Mean Difference (IV, Fixed, 95% CI) | 7.0 [‐4.05, 18.05] |
| 10.3 Cilomilast 15 mg | 1 | 213 | Mean Difference (IV, Fixed, 95% CI) | 34.0 [20.14, 47.86] |
| 11 SGRQ total score Show forest plot | 11 | 7645 | Mean Difference (IV, Fixed, 95% CI) | ‐1.06 [‐1.68, ‐0.43] |
| Analysis 1.11 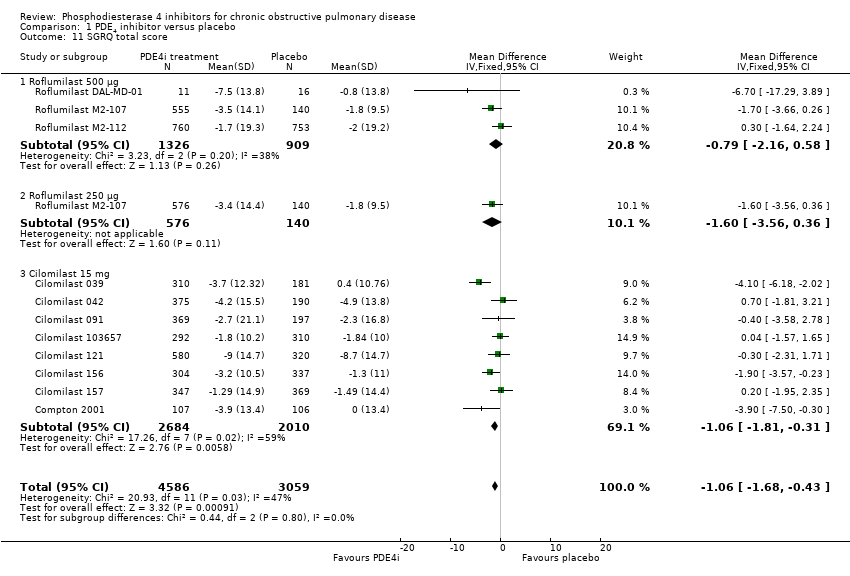 Comparison 1 PDE4 inhibitor versus placebo, Outcome 11 SGRQ total score. | ||||
| 11.1 Roflumilast 500 μg | 3 | 2235 | Mean Difference (IV, Fixed, 95% CI) | ‐0.79 [‐2.16, 0.58] |
| 11.2 Roflumilast 250 μg | 1 | 716 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐3.56, 0.36] |
| 11.3 Cilomilast 15 mg | 8 | 4694 | Mean Difference (IV, Fixed, 95% CI) | ‐1.06 [‐1.81, ‐0.31] |
| 12 SGRQ total score (by published versus unpublished) Show forest plot | 11 | 7069 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐1.65, ‐0.34] |
| Analysis 1.12  Comparison 1 PDE4 inhibitor versus placebo, Outcome 12 SGRQ total score (by published versus unpublished). | ||||
| 12.1 Published | 5 | 3079 | Mean Difference (IV, Fixed, 95% CI) | ‐1.98 [‐3.07, ‐0.89] |
| 12.2 Unpublished | 6 | 3990 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐1.26, 0.40] |
| 13 SGRQ total score (by duration) Show forest plot | 11 | 7069 | Mean Difference (IV, Fixed, 95% CI) | ‐0.99 [‐1.65, ‐0.33] |
| Analysis 1.13  Comparison 1 PDE4 inhibitor versus placebo, Outcome 13 SGRQ total score (by duration). | ||||
| 13.1 Duration < 12 weeks | 2 | 240 | Mean Difference (IV, Fixed, 95% CI) | ‐4.19 [‐7.60, ‐0.78] |
| 13.2 Duration 24 to 26 weeks | 7 | 4600 | Mean Difference (IV, Fixed, 95% CI) | ‐1.18 [‐1.94, ‐0.42] |
| 13.3 Duration 52 weeks | 2 | 2229 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐1.18, 1.69] |
| 14 SGRQ total score (by mean COPD severity) Show forest plot | 8 | 4851 | Mean Difference (IV, Fixed, 95% CI) | ‐1.56 [‐2.39, ‐0.74] |
| Analysis 1.14  Comparison 1 PDE4 inhibitor versus placebo, Outcome 14 SGRQ total score (by mean COPD severity). | ||||
| 14.1 GOLD grade I and II | 3 | 2042 | Mean Difference (IV, Fixed, 95% CI) | ‐1.62 [‐2.80, ‐0.44] |
| 14.2 GOLD grade III and IV | 5 | 2809 | Mean Difference (IV, Fixed, 95% CI) | ‐1.51 [‐2.67, ‐0.34] |
| 15 SGRQ symptom score Show forest plot | 2 | 1048 | Mean Difference (IV, Fixed, 95% CI) | ‐1.53 [‐4.11, 1.06] |
| Analysis 1.15  Comparison 1 PDE4 inhibitor versus placebo, Outcome 15 SGRQ symptom score. | ||||
| 15.1 Roflumilast | 1 | 835 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐3.78, 1.78] |
| 15.2 Cilomilast | 1 | 213 | Mean Difference (IV, Fixed, 95% CI) | ‐4.80 [‐11.73, 2.13] |
| 16 Number of participants with one or more exacerbations (by drug) Show forest plot | 23 | 19948 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.73, 0.83] |
| Analysis 1.16  Comparison 1 PDE4 inhibitor versus placebo, Outcome 16 Number of participants with one or more exacerbations (by drug). | ||||
| 16.1 Roflumilast 500 μg | 13 | 14420 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.73, 0.86] |
| 16.2 Cilomilast | 10 | 5528 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.67, 0.85] |
| 17 Number of participants on roflumilast with one or more exacerbations (additional medication) Show forest plot | 13 | 14420 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.73, 0.86] |
| Analysis 1.17  Comparison 1 PDE4 inhibitor versus placebo, Outcome 17 Number of participants on roflumilast with one or more exacerbations (additional medication). | ||||
| 17.1 Long‐acting bronchodilators | 2 | 1676 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.54, 0.88] |
| 17.2 Corticosteroids | 1 | 2686 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.70, 0.95] |
| 17.3 Treatment only | 7 | 5145 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.67, 0.93] |
| 17.4 Various concomitant treatments | 3 | 4913 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.72, 0.91] |
| 18 Exacerbation rate (inverse variance) Show forest plot | 9 | Rate Ratio (Fixed, 95% CI) | 0.88 [0.83, 0.93] | |
| Analysis 1.18 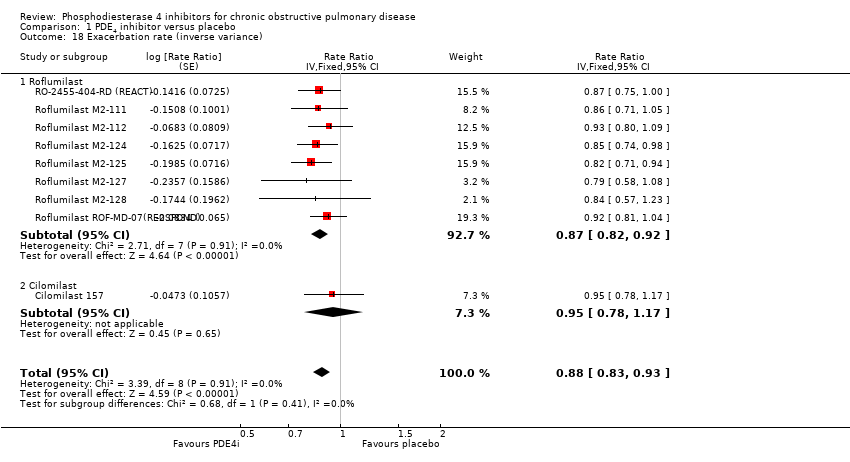 Comparison 1 PDE4 inhibitor versus placebo, Outcome 18 Exacerbation rate (inverse variance). | ||||
| 18.1 Roflumilast | 8 | Rate Ratio (Fixed, 95% CI) | 0.87 [0.82, 0.92] | |
| 18.2 Cilomilast | 1 | Rate Ratio (Fixed, 95% CI) | 0.95 [0.78, 1.17] | |
| 19 Borg Scale Show forest plot | 6 | 2860 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.33, ‐0.05] |
| Analysis 1.19  Comparison 1 PDE4 inhibitor versus placebo, Outcome 19 Borg Scale. | ||||
| 19.1 Cilomilast | 6 | 2860 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.33, ‐0.05] |
| 20 Summary symptom score Show forest plot | 5 | 6186 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.07, 0.03] |
| Analysis 1.20  Comparison 1 PDE4 inhibitor versus placebo, Outcome 20 Summary symptom score. | ||||
| 20.1 Roflumilast | 2 | 4287 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.08, 0.04] |
| 20.2 Cilomilast | 3 | 1899 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.13, 0.06] |
| 21 Shortness of breath questionnaire Show forest plot | 2 | 1633 | Mean Difference (IV, Fixed, 95% CI) | ‐1.09 [‐2.47, 0.28] |
| Analysis 1.21 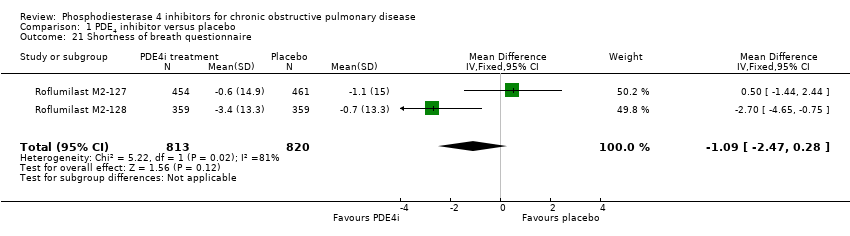 Comparison 1 PDE4 inhibitor versus placebo, Outcome 21 Shortness of breath questionnaire. | ||||
| 22 6‐minute walk test Show forest plot | 5 | 1975 | Mean Difference (IV, Fixed, 95% CI) | 2.09 [‐7.39, 11.57] |
| Analysis 1.22  Comparison 1 PDE4 inhibitor versus placebo, Outcome 22 6‐minute walk test. | ||||
| 22.1 Roflumilast | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 55.0 [‐111.29, 221.29] |
| 22.2 Cilomilast | 4 | 1948 | Mean Difference (IV, Fixed, 95% CI) | 1.92 [‐7.58, 11.41] |
| 23 Number of participants experiencing an adverse effect Show forest plot | 27 | 20988 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.22, 1.37] |
| Analysis 1.23  Comparison 1 PDE4 inhibitor versus placebo, Outcome 23 Number of participants experiencing an adverse effect. | ||||
| 23.1 Roflumilast 500 μg | 13 | 14446 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.24, 1.42] |
| 23.2 Cilomilast 15 mg | 14 | 6542 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.08, 1.36] |
| 24 Number of participants experiencing an adverse event (Roflumilast 500 μg versus 250 μg) Show forest plot | 4 | 1977 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.01, 1.46] |
| Analysis 1.24  Comparison 1 PDE4 inhibitor versus placebo, Outcome 24 Number of participants experiencing an adverse event (Roflumilast 500 μg versus 250 μg). | ||||
| 25 Diarrhoea Show forest plot | 25 | 20181 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.13 [2.76, 3.54] |
| Analysis 1.25  Comparison 1 PDE4 inhibitor versus placebo, Outcome 25 Diarrhoea. | ||||
| 25.1 Roflumilast | 11 | 13639 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.72 [3.15, 4.38] |
| 25.2 Cilomilast | 14 | 6542 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.47 [2.05, 2.98] |
| 26 Nausea Show forest plot | 24 | 20627 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.78 [3.23, 4.43] |
| Analysis 1.26  Comparison 1 PDE4 inhibitor versus placebo, Outcome 26 Nausea. | ||||
| 26.1 Roflumilast 500 μg | 10 | 13229 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.21 [2.57, 4.03] |
| 26.2 Roflumilast 250 μg | 1 | 856 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.97 [0.91, 17.39] |
| 26.3 Cilomilast 15 mg | 14 | 6542 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.37 [3.49, 5.47] |
| 27 Headache Show forest plot | 21 | 18977 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.47, 1.95] |
| Analysis 1.27  Comparison 1 PDE4 inhibitor versus placebo, Outcome 27 Headache. | ||||
| 27.1 Roflumilast 500 μg | 10 | 13327 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.15 [1.76, 2.63] |
| 27.2 Roflumilast 250 μg | 1 | 347 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.24, 3.99] |
| 27.3 Cilomilast 15 mg | 11 | 5303 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.08, 1.62] |
| 28 Vomiting Show forest plot | 11 | 5828 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.01 [2.80, 5.74] |
| Analysis 1.28 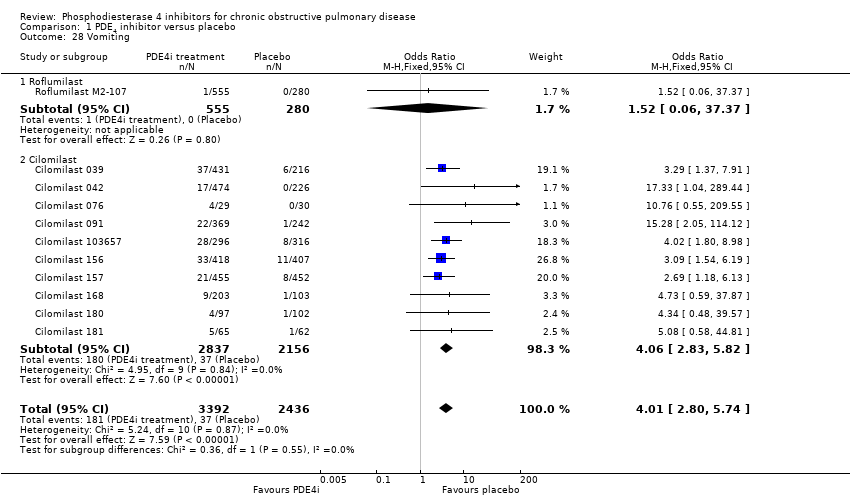 Comparison 1 PDE4 inhibitor versus placebo, Outcome 28 Vomiting. | ||||
| 28.1 Roflumilast | 1 | 835 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.06, 37.37] |
| 28.2 Cilomilast | 10 | 4993 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.06 [2.83, 5.82] |
| 29 Dyspepsia Show forest plot | 13 | 6247 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.17 [2.33, 4.30] |
| Analysis 1.29  Comparison 1 PDE4 inhibitor versus placebo, Outcome 29 Dyspepsia. | ||||
| 29.1 Roflumilast | 1 | 626 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.07 [0.36, 137.40] |
| 29.2 Cilomilast | 12 | 5621 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.13 [2.30, 4.27] |
| 30 Abdominal pain Show forest plot | 13 | 8165 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.04 [1.63, 2.55] |
| Analysis 1.30  Comparison 1 PDE4 inhibitor versus placebo, Outcome 30 Abdominal pain. | ||||
| 30.1 Roflumilast | 2 | 2561 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.76 [1.35, 5.62] |
| 30.2 Cilomilast | 11 | 5604 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.97 [1.55, 2.49] |
| 31 Weight loss Show forest plot | 9 | 12178 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.76 [3.11, 4.54] |
| Analysis 1.31 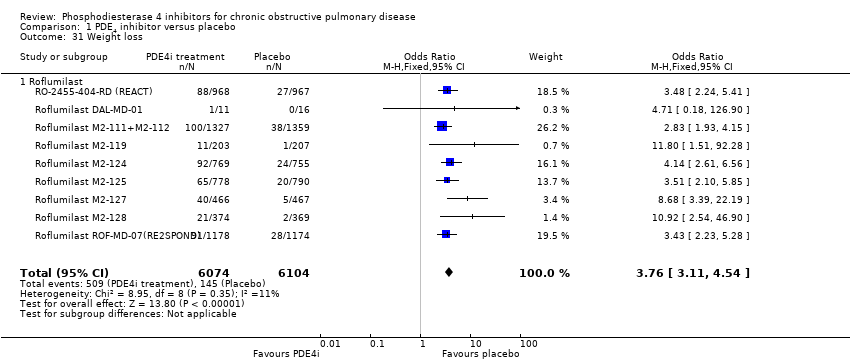 Comparison 1 PDE4 inhibitor versus placebo, Outcome 31 Weight loss. | ||||
| 31.1 Roflumilast | 9 | 12178 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.76 [3.11, 4.54] |
| 32 Influenza‐like symptoms Show forest plot | 9 | 11460 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.87, 1.36] |
| Analysis 1.32  Comparison 1 PDE4 inhibitor versus placebo, Outcome 32 Influenza‐like symptoms. | ||||
| 32.1 Roflumilast 500 μg | 7 | 10147 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.87, 1.41] |
| 32.2 Roflumilast 250 μg | 1 | 347 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.18, 22.00] |
| 32.3 Cilomilast 15 mg | 2 | 966 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.44, 1.75] |
| 33 Upper respiratory tract infection Show forest plot | 20 | 16902 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.79, 1.02] |
| Analysis 1.33 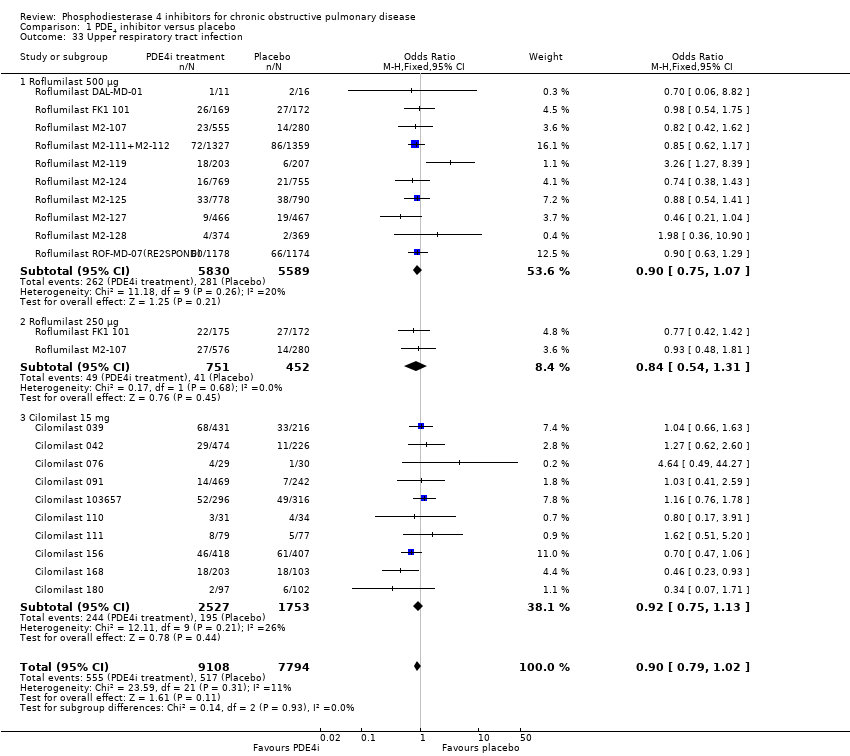 Comparison 1 PDE4 inhibitor versus placebo, Outcome 33 Upper respiratory tract infection. | ||||
| 33.1 Roflumilast 500 μg | 10 | 11419 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.75, 1.07] |
| 33.2 Roflumilast 250 μg | 2 | 1203 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.54, 1.31] |
| 33.3 Cilomilast 15 mg | 10 | 4280 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.75, 1.13] |
| 34 Withdrawals due to adverse events Show forest plot | 28 | 20996 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.74, 2.09] |
| Analysis 1.34 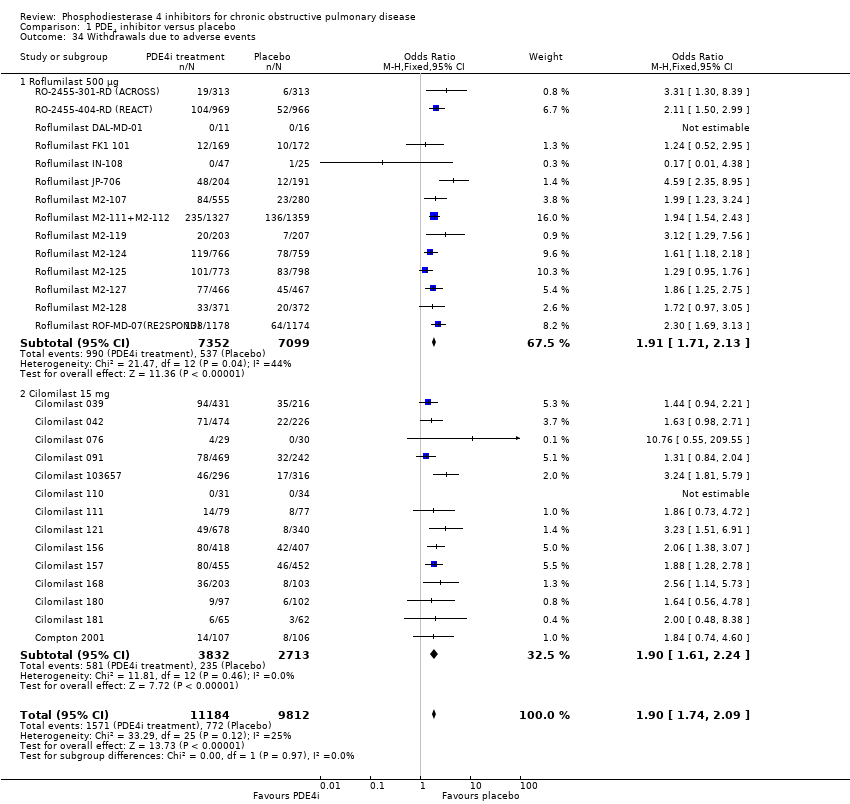 Comparison 1 PDE4 inhibitor versus placebo, Outcome 34 Withdrawals due to adverse events. | ||||
| 34.1 Roflumilast 500 μg | 14 | 14451 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.91 [1.71, 2.13] |
| 34.2 Cilomilast 15 mg | 14 | 6545 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.61, 2.24] |
| 35 Non‐fatal serious adverse events Show forest plot | 24 | 18689 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.07] |
| Analysis 1.35 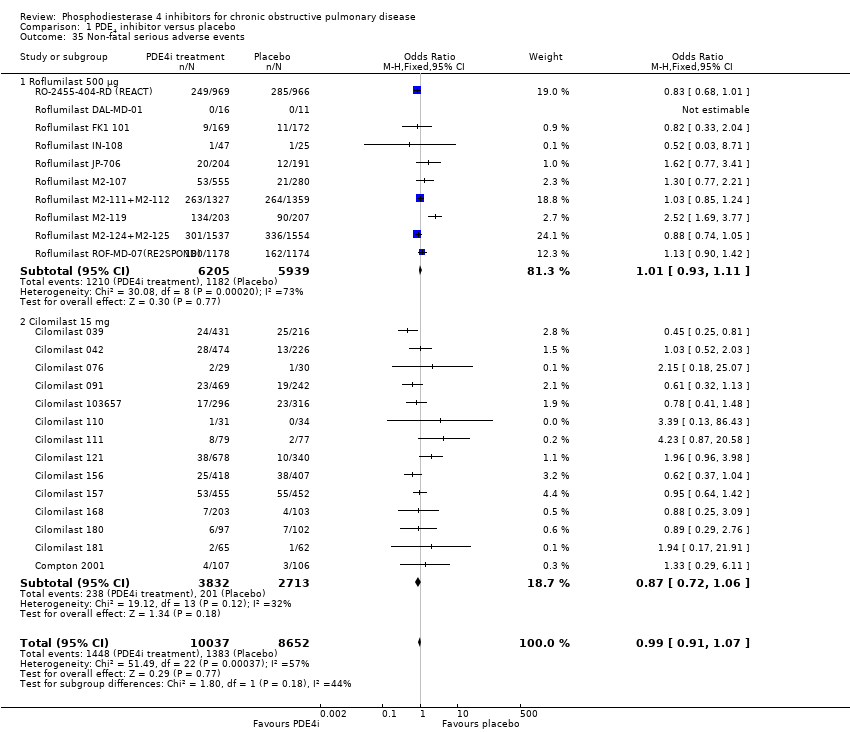 Comparison 1 PDE4 inhibitor versus placebo, Outcome 35 Non‐fatal serious adverse events. | ||||
| 35.1 Roflumilast 500 μg | 10 | 12144 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.93, 1.11] |
| 35.2 Cilomilast 15 mg | 14 | 6545 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.72, 1.06] |
| 36 Mortality Show forest plot | 23 | 19344 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.76, 1.23] |
| Analysis 1.36 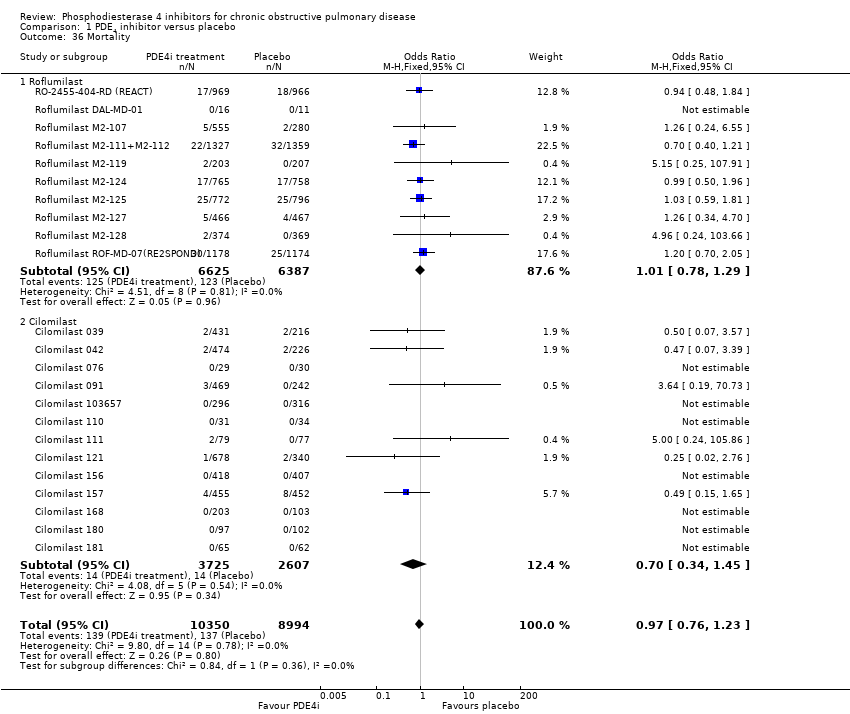 Comparison 1 PDE4 inhibitor versus placebo, Outcome 36 Mortality. | ||||
| 36.1 Roflumilast | 10 | 13012 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.78, 1.29] |
| 36.2 Cilomilast | 13 | 6332 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.34, 1.45] |
| 37 All psychiatric disorders (roflumilast) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.37  Comparison 1 PDE4 inhibitor versus placebo, Outcome 37 All psychiatric disorders (roflumilast). | ||||
| 37.1 Roflumilast 500 μg | 1 | 11168 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.79, 2.54] |
| 37.2 Roflumilast 250 μg | 1 | 6288 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.56, 1.33] |
| 38 Insomnia and sleep disorders (roflumilast) Show forest plot | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.38  Comparison 1 PDE4 inhibitor versus placebo, Outcome 38 Insomnia and sleep disorders (roflumilast). | ||||
| 38.1 Roflumilast 500 μg | 4 | 15482 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.67 [2.11, 3.38] |
| 38.2 Roflumilast 250 μg | 1 | 6288 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.81, 2.70] |
| 39 Anxiety or anxiety disorder (roflumilast) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.39 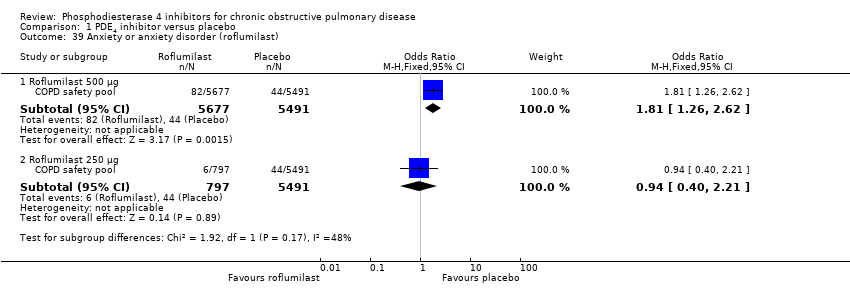 Comparison 1 PDE4 inhibitor versus placebo, Outcome 39 Anxiety or anxiety disorder (roflumilast). | ||||
| 39.1 Roflumilast 500 μg | 1 | 11168 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.26, 2.62] |
| 39.2 Roflumilast 250 μg | 1 | 6288 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.40, 2.21] |
| 40 Depression (roflumilast) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.40  Comparison 1 PDE4 inhibitor versus placebo, Outcome 40 Depression (roflumilast). | ||||
| 40.1 Roflumilast 500 μg | 1 | 11168 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.11, 2.27] |
| 40.2 Roflumilast 250 μg | 1 | 6288 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.20, 1.56] |

In the control group 33 people out of 100 had an exacerbation of COPD over 6‐52 weeks, compared to 28 (95% CI 27 to 29) out of 100 for the active treatment group.
In the control group 4 people out of 100 had diarrhoea over 6‐52 weeks, compared to 11 (95% CI 10 to 12) out of 100 for the active treatment group.
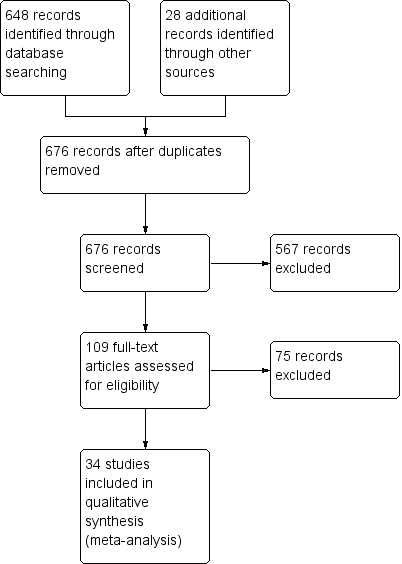
Study flow diagram

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
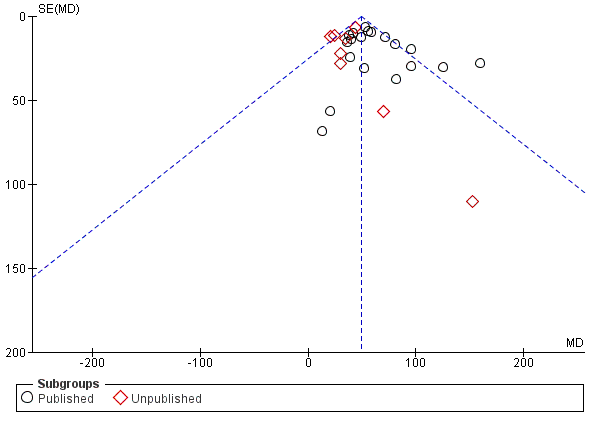
Funnel plot of comparison: 1 PDE4 inhibitor versus placebo, outcome: 1.6 FEV1 (published versus unpublished).

Comparison 1 PDE4 inhibitor versus placebo, Outcome 1 FEV1 (by drug).

Comparison 1 PDE4 inhibitor versus placebo, Outcome 2 FEV1 (by mean COPD severity).

Comparison 1 PDE4 inhibitor versus placebo, Outcome 3 FEV1 (Roflumilast 500 μg by mean COPD severity).
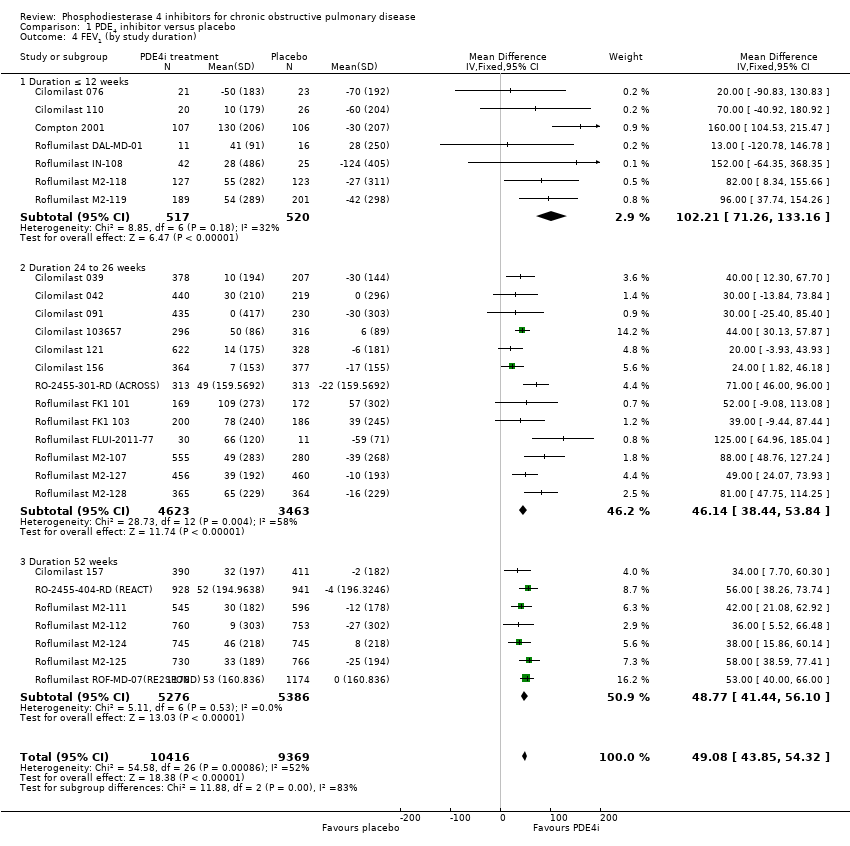
Comparison 1 PDE4 inhibitor versus placebo, Outcome 4 FEV1 (by study duration).

Comparison 1 PDE4 inhibitor versus placebo, Outcome 5 FEV1 (additional medication).

Comparison 1 PDE4 inhibitor versus placebo, Outcome 6 FEV1 (published versus unpublished).
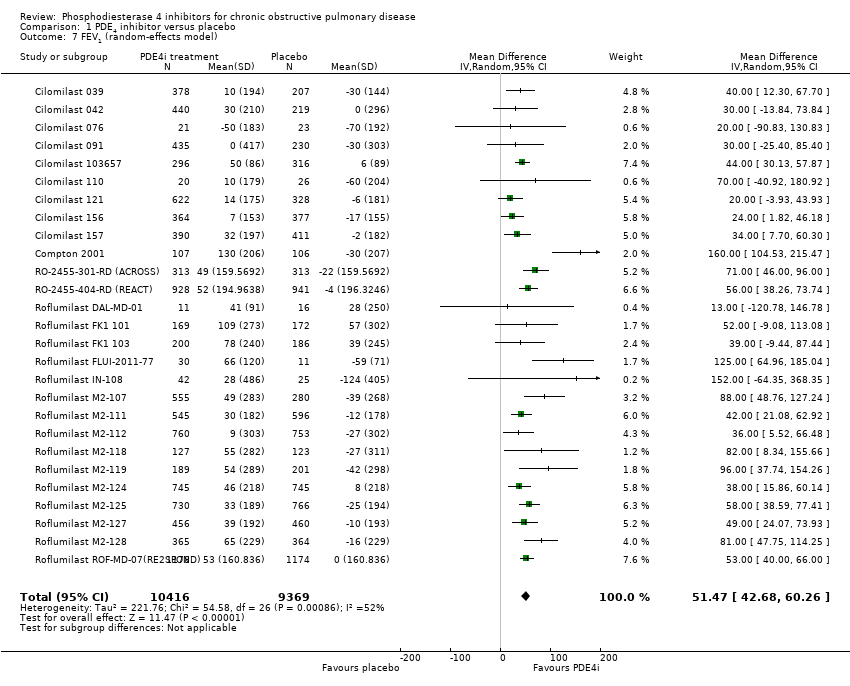
Comparison 1 PDE4 inhibitor versus placebo, Outcome 7 FEV1 (random‐effects model).
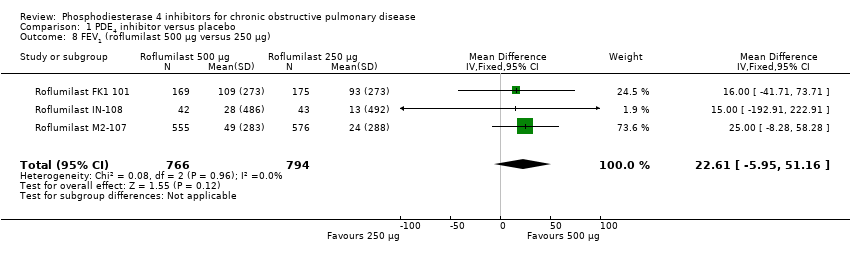
Comparison 1 PDE4 inhibitor versus placebo, Outcome 8 FEV1 (roflumilast 500 μg versus 250 μg).
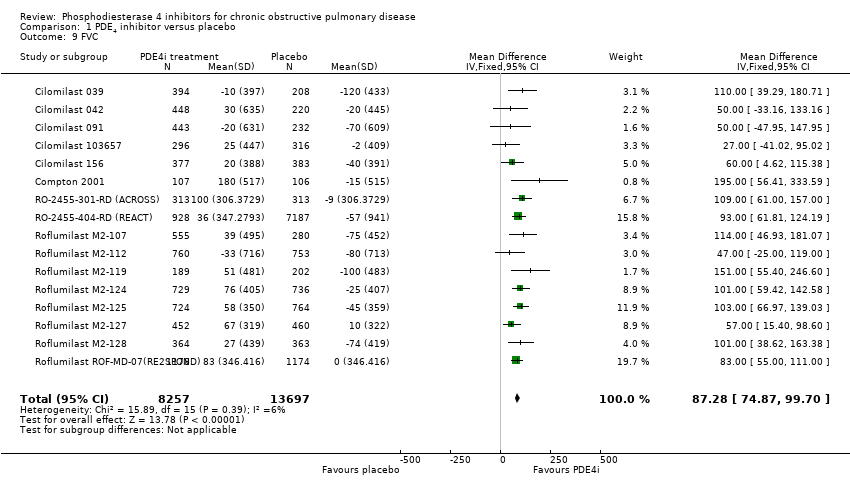
Comparison 1 PDE4 inhibitor versus placebo, Outcome 9 FVC.
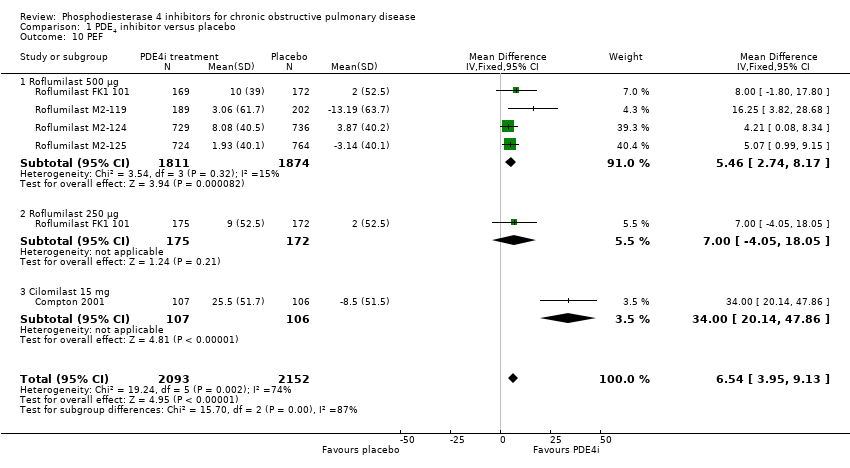
Comparison 1 PDE4 inhibitor versus placebo, Outcome 10 PEF.

Comparison 1 PDE4 inhibitor versus placebo, Outcome 11 SGRQ total score.
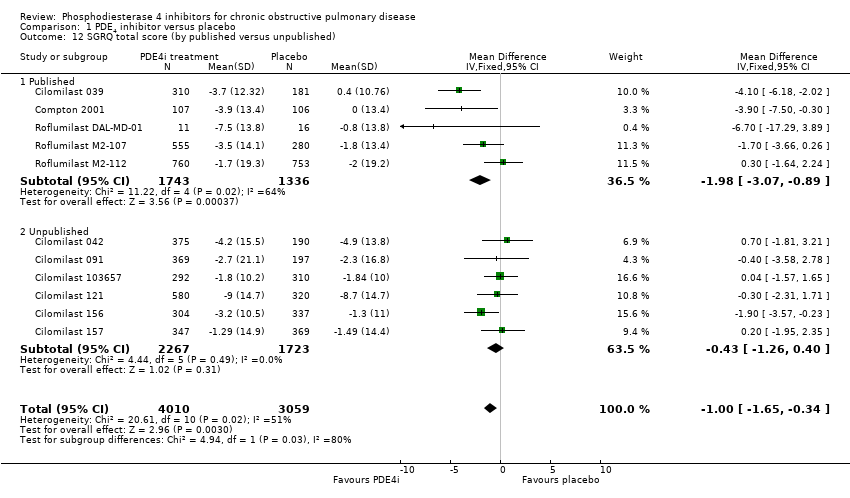
Comparison 1 PDE4 inhibitor versus placebo, Outcome 12 SGRQ total score (by published versus unpublished).

Comparison 1 PDE4 inhibitor versus placebo, Outcome 13 SGRQ total score (by duration).

Comparison 1 PDE4 inhibitor versus placebo, Outcome 14 SGRQ total score (by mean COPD severity).
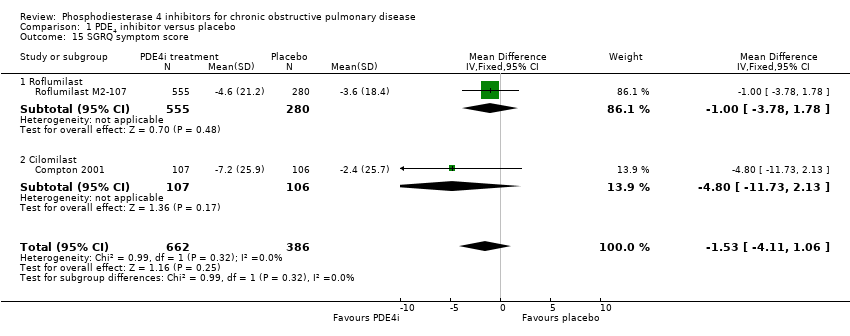
Comparison 1 PDE4 inhibitor versus placebo, Outcome 15 SGRQ symptom score.

Comparison 1 PDE4 inhibitor versus placebo, Outcome 16 Number of participants with one or more exacerbations (by drug).

Comparison 1 PDE4 inhibitor versus placebo, Outcome 17 Number of participants on roflumilast with one or more exacerbations (additional medication).
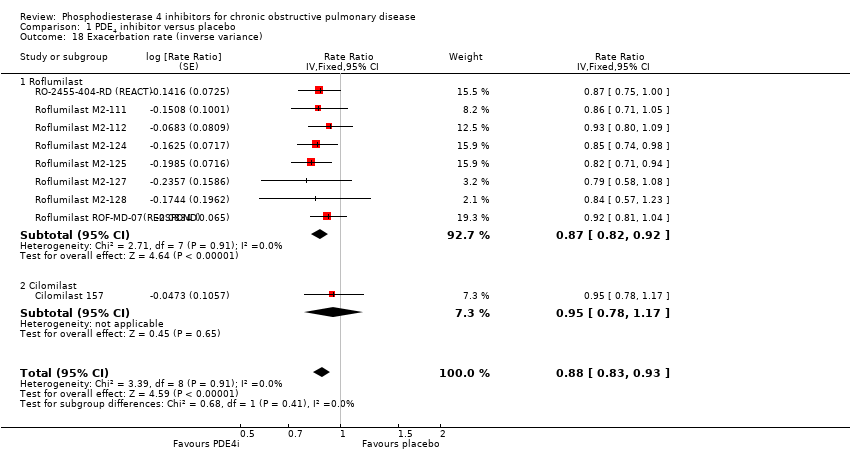
Comparison 1 PDE4 inhibitor versus placebo, Outcome 18 Exacerbation rate (inverse variance).

Comparison 1 PDE4 inhibitor versus placebo, Outcome 19 Borg Scale.

Comparison 1 PDE4 inhibitor versus placebo, Outcome 20 Summary symptom score.

Comparison 1 PDE4 inhibitor versus placebo, Outcome 21 Shortness of breath questionnaire.
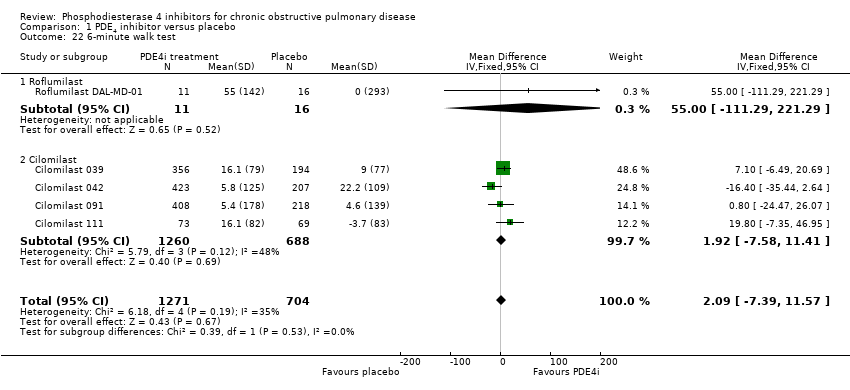
Comparison 1 PDE4 inhibitor versus placebo, Outcome 22 6‐minute walk test.

Comparison 1 PDE4 inhibitor versus placebo, Outcome 23 Number of participants experiencing an adverse effect.
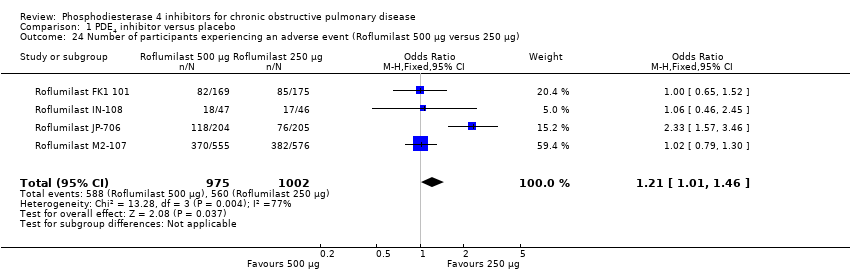
Comparison 1 PDE4 inhibitor versus placebo, Outcome 24 Number of participants experiencing an adverse event (Roflumilast 500 μg versus 250 μg).
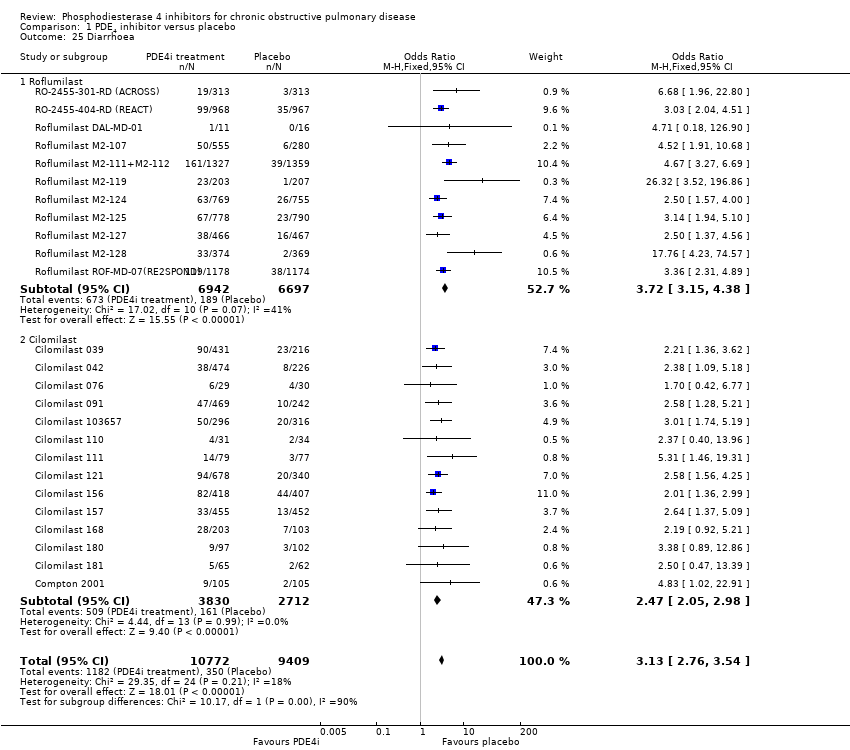
Comparison 1 PDE4 inhibitor versus placebo, Outcome 25 Diarrhoea.

Comparison 1 PDE4 inhibitor versus placebo, Outcome 26 Nausea.
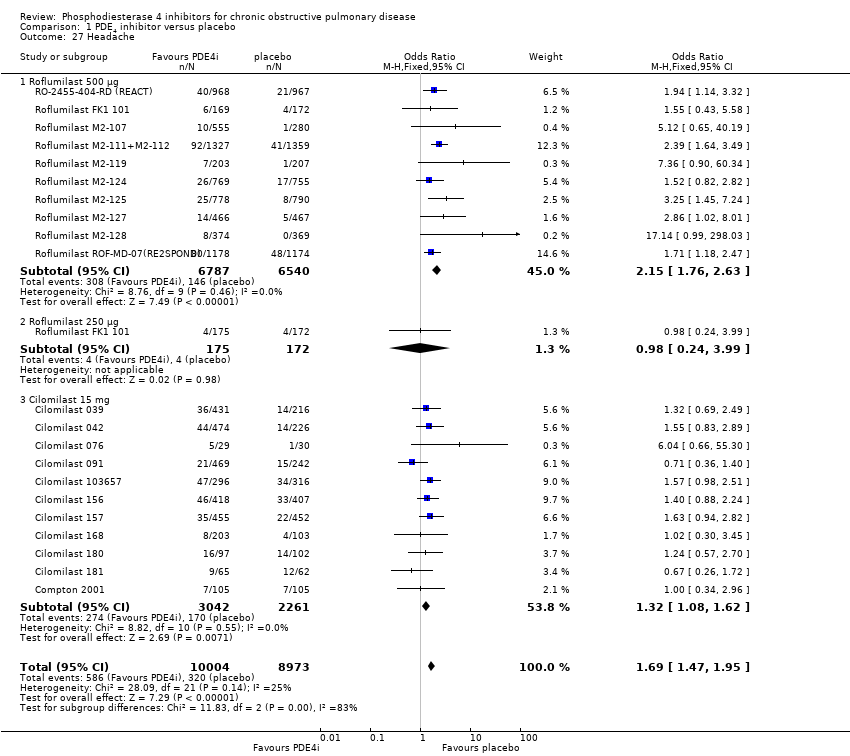
Comparison 1 PDE4 inhibitor versus placebo, Outcome 27 Headache.

Comparison 1 PDE4 inhibitor versus placebo, Outcome 28 Vomiting.
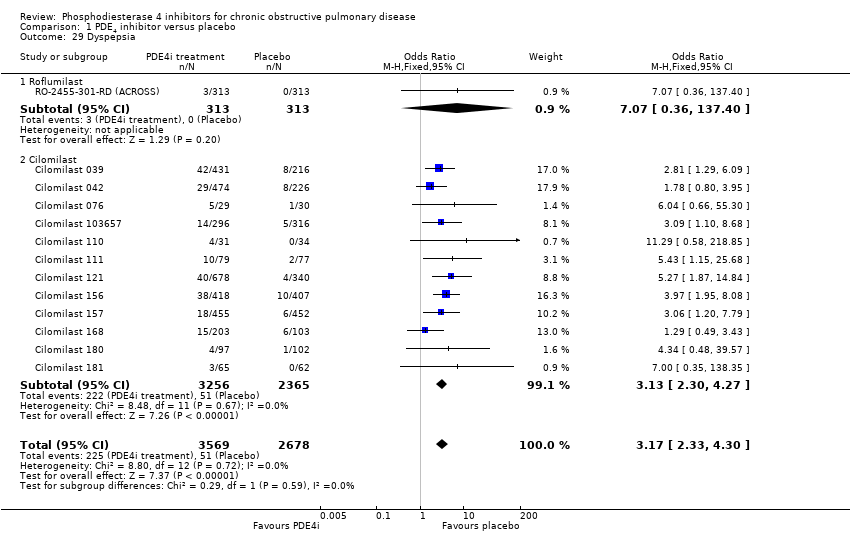
Comparison 1 PDE4 inhibitor versus placebo, Outcome 29 Dyspepsia.
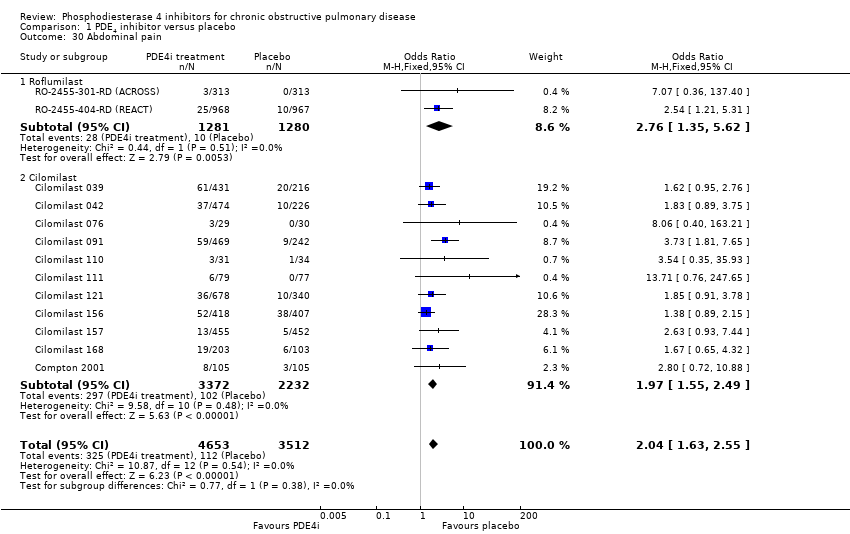
Comparison 1 PDE4 inhibitor versus placebo, Outcome 30 Abdominal pain.

Comparison 1 PDE4 inhibitor versus placebo, Outcome 31 Weight loss.

Comparison 1 PDE4 inhibitor versus placebo, Outcome 32 Influenza‐like symptoms.

Comparison 1 PDE4 inhibitor versus placebo, Outcome 33 Upper respiratory tract infection.
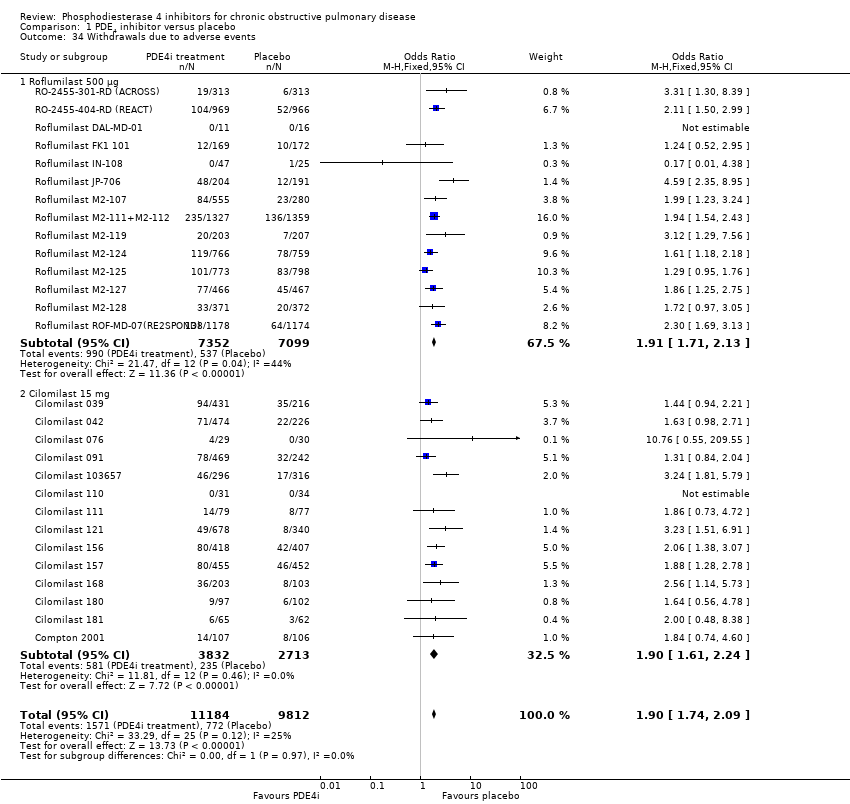
Comparison 1 PDE4 inhibitor versus placebo, Outcome 34 Withdrawals due to adverse events.

Comparison 1 PDE4 inhibitor versus placebo, Outcome 35 Non‐fatal serious adverse events.

Comparison 1 PDE4 inhibitor versus placebo, Outcome 36 Mortality.

Comparison 1 PDE4 inhibitor versus placebo, Outcome 37 All psychiatric disorders (roflumilast).
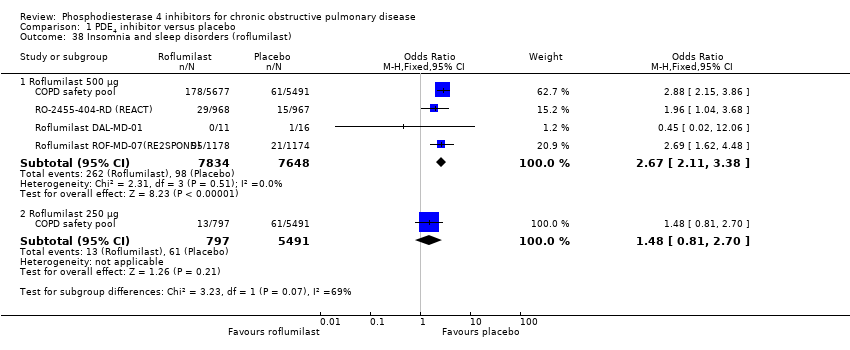
Comparison 1 PDE4 inhibitor versus placebo, Outcome 38 Insomnia and sleep disorders (roflumilast).

Comparison 1 PDE4 inhibitor versus placebo, Outcome 39 Anxiety or anxiety disorder (roflumilast).

Comparison 1 PDE4 inhibitor versus placebo, Outcome 40 Depression (roflumilast).
| Phosphodiesterase 4 inhibitors compared to placebo for chronic obstructive pulmonary disease | ||||||
| Patient or population: people with stable chronic obstructive pulmonary disease | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Phosphodiesterase IV inhibitors | |||||
| Change in FEV1 lung function | The mean change in FEV1 lung function in the control groups was | The mean change in FEV1 lung function in the intervention groups was | 20,585 | ⊕⊕⊕⊝ | ||
| Change in quality of life | The mean change in quality of life in the control groups was an improvement of | The mean change in quality of life in the intervention groups was | 7645 | ⊕⊕⊕⊝ | Lower scores on SGRQ represent improved quality of life. This result does not reach the minimum clinically important difference for this scale. | |
| COPD exacerbations | 33 per 100 | 28 per 100 | OR 0.78 | 19,948 | ⊕⊕⊕⊕ | See Figure 1 |
| Adverse events | 64 per 100 | 69 per 100 | OR 1.29 | 20,988 | ⊕⊕⊕⊝ | This outcome includes participants who reported COPD exacerbations as an adverse event |
| Gastrointestinal side effects | 4 per 100 | 11 per 100 | OR 3.13 | 20,181 | ⊕⊕⊕⊕ | Diarrhoea was the most commonly reported gastrointestinal side effect. See Figure 2 Weight loss was more common, and may be a result of diarrhoea |
| Psychiatric adverse events (roflumilast 500 µg) | 35 per 1000 | 71 per 1000 | OR 2.13 | 11,168 | ⊕⊕⊕⊝ | Pooled data from FDA website, not individual trial reports |
| Mortality (all‐cause) | 1 per 100 | 1 per 100 | OR 0.97 | 19,344 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1There was a greater proportion of participant withdrawals in the treatment (24%) compared with the control group (19%), but not sufficient to warrant downgrading the quality of evidence. 6There were very few events, leading to wide confidence intervals. | ||||||
| Search date: | No. of references for which we sought full text |
| December 2008 | 53 |
| January 2010 | 5 |
| August 2010 | 12 |
| June 2013 | 20 |
| October 2016 | 28 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 FEV1 (by drug) Show forest plot | 27 | 20585 | Mean Difference (IV, Random, 95% CI) | 51.53 [43.17, 59.90] |
| 1.1 Roflumilast 500 μg | 17 | 14230 | Mean Difference (IV, Random, 95% CI) | 56.45 [48.01, 64.89] |
| 1.2 Roflumilast 250 μg | 3 | 1033 | Mean Difference (IV, Random, 95% CI) | 56.88 [24.38, 89.38] |
| 1.3 Cilomilast 15 mg | 10 | 5322 | Mean Difference (IV, Random, 95% CI) | 41.03 [23.93, 58.13] |
| 2 FEV1 (by mean COPD severity) Show forest plot | 21 | 16659 | Mean Difference (IV, Fixed, 95% CI) | 52.77 [46.73, 58.82] |
| 2.1 GOLD grade I + II (FEV1 ≥ 50% predicted) | 9 | 4647 | Mean Difference (IV, Fixed, 95% CI) | 51.79 [38.99, 64.59] |
| 2.2 GOLD grade III + IV (FEV1 < 50% predicted) | 12 | 12012 | Mean Difference (IV, Fixed, 95% CI) | 53.06 [46.19, 59.92] |
| 3 FEV1 (Roflumilast 500 μg by mean COPD severity) Show forest plot | 15 | 13742 | Mean Difference (IV, Fixed, 95% CI) | 55.51 [48.88, 62.14] |
| 3.1 GOLD grade I + II (FEV1 ≥ 50% predicted) | 6 | 3187 | Mean Difference (IV, Fixed, 95% CI) | 69.86 [53.34, 86.38] |
| 3.2 GOLD grade III + IV (FEV1 < 50% predicted) | 9 | 10555 | Mean Difference (IV, Fixed, 95% CI) | 52.75 [45.52, 59.99] |
| 4 FEV1 (by study duration) Show forest plot | 27 | 19785 | Mean Difference (IV, Fixed, 95% CI) | 49.08 [43.85, 54.32] |
| 4.1 Duration ≤ 12 weeks | 7 | 1037 | Mean Difference (IV, Fixed, 95% CI) | 102.21 [71.26, 133.16] |
| 4.2 Duration 24 to 26 weeks | 13 | 8086 | Mean Difference (IV, Fixed, 95% CI) | 46.14 [38.44, 53.84] |
| 4.3 Duration 52 weeks | 7 | 10662 | Mean Difference (IV, Fixed, 95% CI) | 48.77 [41.44, 56.10] |
| 5 FEV1 (additional medication) Show forest plot | 27 | 19565 | Mean Difference (IV, Fixed, 95% CI) | 49.08 [43.84, 54.31] |
| 5.1 Long‐acting bronchodilator | 2 | 1645 | Mean Difference (IV, Fixed, 95% CI) | 60.52 [40.57, 80.46] |
| 5.2 Corticosteroids | 3 | 2904 | Mean Difference (IV, Fixed, 95% CI) | 42.26 [25.46, 59.05] |
| 5.3 PDE4i treatment only | 19 | 10169 | Mean Difference (IV, Fixed, 95% CI) | 44.78 [37.67, 51.90] |
| 5.4 Various concomitant treatments | 3 | 4847 | Mean Difference (IV, Fixed, 95% CI) | 56.58 [46.91, 66.25] |
| 6 FEV1 (published versus unpublished) Show forest plot | 27 | 19785 | Mean Difference (IV, Fixed, 95% CI) | 49.23 [43.99, 54.46] |
| 6.1 Published | 19 | 15244 | Mean Difference (IV, Fixed, 95% CI) | 55.75 [49.44, 62.06] |
| 6.2 Unpublished | 8 | 4541 | Mean Difference (IV, Fixed, 95% CI) | 34.82 [25.44, 44.19] |
| 7 FEV1 (random‐effects model) Show forest plot | 27 | 19785 | Mean Difference (IV, Random, 95% CI) | 51.47 [42.68, 60.26] |
| 8 FEV1 (roflumilast 500 μg versus 250 μg) Show forest plot | 3 | 1560 | Mean Difference (IV, Fixed, 95% CI) | 22.61 [‐5.95, 51.16] |
| 9 FVC Show forest plot | 16 | 21954 | Mean Difference (IV, Fixed, 95% CI) | 87.28 [74.87, 99.70] |
| 10 PEF Show forest plot | 5 | 4245 | Mean Difference (IV, Fixed, 95% CI) | 6.54 [3.95, 9.13] |
| 10.1 Roflumilast 500 μg | 4 | 3685 | Mean Difference (IV, Fixed, 95% CI) | 5.46 [2.74, 8.17] |
| 10.2 Roflumilast 250 μg | 1 | 347 | Mean Difference (IV, Fixed, 95% CI) | 7.0 [‐4.05, 18.05] |
| 10.3 Cilomilast 15 mg | 1 | 213 | Mean Difference (IV, Fixed, 95% CI) | 34.0 [20.14, 47.86] |
| 11 SGRQ total score Show forest plot | 11 | 7645 | Mean Difference (IV, Fixed, 95% CI) | ‐1.06 [‐1.68, ‐0.43] |
| 11.1 Roflumilast 500 μg | 3 | 2235 | Mean Difference (IV, Fixed, 95% CI) | ‐0.79 [‐2.16, 0.58] |
| 11.2 Roflumilast 250 μg | 1 | 716 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐3.56, 0.36] |
| 11.3 Cilomilast 15 mg | 8 | 4694 | Mean Difference (IV, Fixed, 95% CI) | ‐1.06 [‐1.81, ‐0.31] |
| 12 SGRQ total score (by published versus unpublished) Show forest plot | 11 | 7069 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐1.65, ‐0.34] |
| 12.1 Published | 5 | 3079 | Mean Difference (IV, Fixed, 95% CI) | ‐1.98 [‐3.07, ‐0.89] |
| 12.2 Unpublished | 6 | 3990 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐1.26, 0.40] |
| 13 SGRQ total score (by duration) Show forest plot | 11 | 7069 | Mean Difference (IV, Fixed, 95% CI) | ‐0.99 [‐1.65, ‐0.33] |
| 13.1 Duration < 12 weeks | 2 | 240 | Mean Difference (IV, Fixed, 95% CI) | ‐4.19 [‐7.60, ‐0.78] |
| 13.2 Duration 24 to 26 weeks | 7 | 4600 | Mean Difference (IV, Fixed, 95% CI) | ‐1.18 [‐1.94, ‐0.42] |
| 13.3 Duration 52 weeks | 2 | 2229 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐1.18, 1.69] |
| 14 SGRQ total score (by mean COPD severity) Show forest plot | 8 | 4851 | Mean Difference (IV, Fixed, 95% CI) | ‐1.56 [‐2.39, ‐0.74] |
| 14.1 GOLD grade I and II | 3 | 2042 | Mean Difference (IV, Fixed, 95% CI) | ‐1.62 [‐2.80, ‐0.44] |
| 14.2 GOLD grade III and IV | 5 | 2809 | Mean Difference (IV, Fixed, 95% CI) | ‐1.51 [‐2.67, ‐0.34] |
| 15 SGRQ symptom score Show forest plot | 2 | 1048 | Mean Difference (IV, Fixed, 95% CI) | ‐1.53 [‐4.11, 1.06] |
| 15.1 Roflumilast | 1 | 835 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐3.78, 1.78] |
| 15.2 Cilomilast | 1 | 213 | Mean Difference (IV, Fixed, 95% CI) | ‐4.80 [‐11.73, 2.13] |
| 16 Number of participants with one or more exacerbations (by drug) Show forest plot | 23 | 19948 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.73, 0.83] |
| 16.1 Roflumilast 500 μg | 13 | 14420 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.73, 0.86] |
| 16.2 Cilomilast | 10 | 5528 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.67, 0.85] |
| 17 Number of participants on roflumilast with one or more exacerbations (additional medication) Show forest plot | 13 | 14420 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.73, 0.86] |
| 17.1 Long‐acting bronchodilators | 2 | 1676 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.54, 0.88] |
| 17.2 Corticosteroids | 1 | 2686 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.70, 0.95] |
| 17.3 Treatment only | 7 | 5145 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.67, 0.93] |
| 17.4 Various concomitant treatments | 3 | 4913 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.72, 0.91] |
| 18 Exacerbation rate (inverse variance) Show forest plot | 9 | Rate Ratio (Fixed, 95% CI) | 0.88 [0.83, 0.93] | |
| 18.1 Roflumilast | 8 | Rate Ratio (Fixed, 95% CI) | 0.87 [0.82, 0.92] | |
| 18.2 Cilomilast | 1 | Rate Ratio (Fixed, 95% CI) | 0.95 [0.78, 1.17] | |
| 19 Borg Scale Show forest plot | 6 | 2860 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.33, ‐0.05] |
| 19.1 Cilomilast | 6 | 2860 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.33, ‐0.05] |
| 20 Summary symptom score Show forest plot | 5 | 6186 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.07, 0.03] |
| 20.1 Roflumilast | 2 | 4287 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.08, 0.04] |
| 20.2 Cilomilast | 3 | 1899 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.13, 0.06] |
| 21 Shortness of breath questionnaire Show forest plot | 2 | 1633 | Mean Difference (IV, Fixed, 95% CI) | ‐1.09 [‐2.47, 0.28] |
| 22 6‐minute walk test Show forest plot | 5 | 1975 | Mean Difference (IV, Fixed, 95% CI) | 2.09 [‐7.39, 11.57] |
| 22.1 Roflumilast | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 55.0 [‐111.29, 221.29] |
| 22.2 Cilomilast | 4 | 1948 | Mean Difference (IV, Fixed, 95% CI) | 1.92 [‐7.58, 11.41] |
| 23 Number of participants experiencing an adverse effect Show forest plot | 27 | 20988 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.22, 1.37] |
| 23.1 Roflumilast 500 μg | 13 | 14446 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.24, 1.42] |
| 23.2 Cilomilast 15 mg | 14 | 6542 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.08, 1.36] |
| 24 Number of participants experiencing an adverse event (Roflumilast 500 μg versus 250 μg) Show forest plot | 4 | 1977 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.01, 1.46] |
| 25 Diarrhoea Show forest plot | 25 | 20181 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.13 [2.76, 3.54] |
| 25.1 Roflumilast | 11 | 13639 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.72 [3.15, 4.38] |
| 25.2 Cilomilast | 14 | 6542 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.47 [2.05, 2.98] |
| 26 Nausea Show forest plot | 24 | 20627 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.78 [3.23, 4.43] |
| 26.1 Roflumilast 500 μg | 10 | 13229 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.21 [2.57, 4.03] |
| 26.2 Roflumilast 250 μg | 1 | 856 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.97 [0.91, 17.39] |
| 26.3 Cilomilast 15 mg | 14 | 6542 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.37 [3.49, 5.47] |
| 27 Headache Show forest plot | 21 | 18977 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.47, 1.95] |
| 27.1 Roflumilast 500 μg | 10 | 13327 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.15 [1.76, 2.63] |
| 27.2 Roflumilast 250 μg | 1 | 347 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.24, 3.99] |
| 27.3 Cilomilast 15 mg | 11 | 5303 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.08, 1.62] |
| 28 Vomiting Show forest plot | 11 | 5828 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.01 [2.80, 5.74] |
| 28.1 Roflumilast | 1 | 835 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.06, 37.37] |
| 28.2 Cilomilast | 10 | 4993 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.06 [2.83, 5.82] |
| 29 Dyspepsia Show forest plot | 13 | 6247 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.17 [2.33, 4.30] |
| 29.1 Roflumilast | 1 | 626 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.07 [0.36, 137.40] |
| 29.2 Cilomilast | 12 | 5621 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.13 [2.30, 4.27] |
| 30 Abdominal pain Show forest plot | 13 | 8165 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.04 [1.63, 2.55] |
| 30.1 Roflumilast | 2 | 2561 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.76 [1.35, 5.62] |
| 30.2 Cilomilast | 11 | 5604 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.97 [1.55, 2.49] |
| 31 Weight loss Show forest plot | 9 | 12178 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.76 [3.11, 4.54] |
| 31.1 Roflumilast | 9 | 12178 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.76 [3.11, 4.54] |
| 32 Influenza‐like symptoms Show forest plot | 9 | 11460 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.87, 1.36] |
| 32.1 Roflumilast 500 μg | 7 | 10147 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.87, 1.41] |
| 32.2 Roflumilast 250 μg | 1 | 347 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.18, 22.00] |
| 32.3 Cilomilast 15 mg | 2 | 966 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.44, 1.75] |
| 33 Upper respiratory tract infection Show forest plot | 20 | 16902 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.79, 1.02] |
| 33.1 Roflumilast 500 μg | 10 | 11419 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.75, 1.07] |
| 33.2 Roflumilast 250 μg | 2 | 1203 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.54, 1.31] |
| 33.3 Cilomilast 15 mg | 10 | 4280 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.75, 1.13] |
| 34 Withdrawals due to adverse events Show forest plot | 28 | 20996 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.74, 2.09] |
| 34.1 Roflumilast 500 μg | 14 | 14451 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.91 [1.71, 2.13] |
| 34.2 Cilomilast 15 mg | 14 | 6545 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.61, 2.24] |
| 35 Non‐fatal serious adverse events Show forest plot | 24 | 18689 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.07] |
| 35.1 Roflumilast 500 μg | 10 | 12144 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.93, 1.11] |
| 35.2 Cilomilast 15 mg | 14 | 6545 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.72, 1.06] |
| 36 Mortality Show forest plot | 23 | 19344 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.76, 1.23] |
| 36.1 Roflumilast | 10 | 13012 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.78, 1.29] |
| 36.2 Cilomilast | 13 | 6332 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.34, 1.45] |
| 37 All psychiatric disorders (roflumilast) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 37.1 Roflumilast 500 μg | 1 | 11168 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.79, 2.54] |
| 37.2 Roflumilast 250 μg | 1 | 6288 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.56, 1.33] |
| 38 Insomnia and sleep disorders (roflumilast) Show forest plot | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 38.1 Roflumilast 500 μg | 4 | 15482 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.67 [2.11, 3.38] |
| 38.2 Roflumilast 250 μg | 1 | 6288 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.81, 2.70] |
| 39 Anxiety or anxiety disorder (roflumilast) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 39.1 Roflumilast 500 μg | 1 | 11168 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.26, 2.62] |
| 39.2 Roflumilast 250 μg | 1 | 6288 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.40, 2.21] |
| 40 Depression (roflumilast) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 40.1 Roflumilast 500 μg | 1 | 11168 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.11, 2.27] |
| 40.2 Roflumilast 250 μg | 1 | 6288 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.20, 1.56] |

