Clotiapina para las enfermedades psicóticas agudas
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Allocation: randomised, matched pairs, table of random numbers. | |
| Participants | Diagnosis: schizophrenia. | |
| Interventions | 1. Clotiapine: dose 45 to 90 mg/day orally by day 7, as needed thereafter, range 90‐290 mg/day. N = 2. | |
| Outcomes | General improvement: overall clinical response. Unable to use ‐ | |
| Notes | Trial sponsored by drug company. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Allocation: randomised, pre‐established code. | |
| Participants | Diagnosis: 'psychotic syndromes of a schizophrenic type'. | |
| Interventions | 1. Clotiapine: dose 40‐240 mg/day orally. N = 23. | |
| Outcomes | General improvement: overall clinical response. Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised, table of random numbers. | |
| Participants | Diagnosis: paranoid schizophrenia. | |
| Interventions | 1. Clotiapine: dose 40‐160 mg/day IM from day 1‐5, then 40‐160 mg/day orally until the end of trial. N =1 5. | |
| Outcomes | Global improvement: (degree of improvement on categorical scale). Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised ‐ by toss of a coin. | |
| Participants | Diagnosis: organic (psycho‐active substance) hallucinations, organic delusional disorder, schizophrenia, bipolar disorder (DSM III). | |
| Interventions | 1. Clotiapine: maximum dose 40 mg IM 6 hourly + haloperidol 10 mg/day orally. N = 30. | |
| Outcomes | Mental state: (BPRS). Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Allocation: randomised ‐ by toss of a coin. | |
| Participants | Diagnosis: schizophrenia, acute paranoid reaction, other and unspecific reactive psychosis, unspecified psychosis, bipolar mood disorder‐manic phase (ICD 9). | |
| Interventions | 1. Clotiapine: dose 40 mg IM initially, then 80‐160 mg/day, in divided doses, orally or IM. N = 21. | |
| Outcomes | Adverse effects: (needing anticholinergic medication, frequent side‐effects, pain at site of injection). Unable to use ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Diagnostic Manuals
DSM ‐ Diagnosic and Statistical Manual of Mental Disorders (American Psychiatric Association)
ICD ‐ International Classification of Diseases
General
IM ‐ intramuscular
Ht ‐ Haematocrit
Hb ‐ Haemoglobin
PRSS ‐ Psychiatric Rating Scale for Schizophrenia
RBC ‐ Red Blood Cell
WBC ‐ Wight Blood Cell
ESR ‐ Erithrocyte Sedimentation Rate
IV ‐ Intravenous injection
M ‐ Male
F ‐ Female
SD ‐ Standard Deviation
Scales
BPRS ‐ Brief Psychiatric Rating Scale
CGI ‐ Clinical Global Impression
UKU ‐ Udvalg for Kliniske ndersogelser
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Allocation: not randomised. | |
| Allocation: not randomised. | |
| Allocation: not randomised. | |
| Allocation: randomised. | |
| Allocation: not randomised. | |
| Allocation: not described. | |
| Allocation: not randomised. | |
| Allocation: not randomised. | |
| Allocation: not randomised. | |
| Allocation: not randomised. | |
| Allocation: not randomised. | |
| Allocation: not randomised. | |
| Allocation: randomised. | |
| Allocation: by 'the double‐blind technique', matched pairs according to sex, age and clinical condition ‐ no mention of randomisation. | |
| Allocation: not randomised. | |
| Allocation: not randomised. | |
| Allocation: not randomised. | |
| Allocation: not randomised. | |
| Allocation: not randomised. | |
| Allocation: not randomised. | |
| Allocation: randomised. | |
| Allocation: not randomised. | |
| Allocation: not randomised. | |
| Allocation: not randomised. | |
| Allocation: not randomised. | |
| Allocation: randomised. | |
| Allocation: not randomised. | |
| Allocation: not randomised. |
IM ‐ intramuscular injection
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 General clinical impression: No significant improvement Show forest plot | 3 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.25, 2.66] |
| Analysis 1.1  Comparison 1 CLOTIAPINE versus STANDARD MEDICATION ‐ OTHER ANTIPSYCHOTICS, Outcome 1 General clinical impression: No significant improvement. | ||||
| 2 Hospital and service outcome: Not well enough to be discharged Show forest plot | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.93, 1.16] |
| Analysis 1.2  Comparison 1 CLOTIAPINE versus STANDARD MEDICATION ‐ OTHER ANTIPSYCHOTICS, Outcome 2 Hospital and service outcome: Not well enough to be discharged. | ||||
| 3 Leaving the study early Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 CLOTIAPINE versus STANDARD MEDICATION ‐ OTHER ANTIPSYCHOTICS, Outcome 3 Leaving the study early. | ||||
| 3.1 any reason | 3 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 2.26 [0.40, 12.88] |
| 3.2 adverse effects | 3 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 5.63 [0.28, 111.43] |
| 4 Adverse effects: 1. Movement disorders ‐ use of antiparkinsonian medication Show forest plot | 2 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.03, 4.10] |
| Analysis 1.4  Comparison 1 CLOTIAPINE versus STANDARD MEDICATION ‐ OTHER ANTIPSYCHOTICS, Outcome 4 Adverse effects: 1. Movement disorders ‐ use of antiparkinsonian medication. | ||||
| 5 Adverse effects: 2. Incidence of specific side‐effects Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 CLOTIAPINE versus STANDARD MEDICATION ‐ OTHER ANTIPSYCHOTICS, Outcome 5 Adverse effects: 2. Incidence of specific side‐effects. | ||||
| 5.1 dry mouth | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.4 [0.09, 1.84] |
| 5.2 headache | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [0.92, 3.66] |
| 5.3 insomnia | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.82, 1.70] |
| 5.4 pain at site of injection | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.25, 98.27] |
| 5.5 palpitations | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.10, 2.44] |
| 5.6 rash | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 5.63 [0.28, 111.43] |
| 5.7 seizure | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 69.70] |
| 5.8 sweating ‐ facial | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.78, 2.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 Mental state: No significant improvement ‐ three days after beginning of treatment Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐3.36 [‐8.09, 1.37] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.1  Comparison 2 CLOTIAPINE versus STANDARD MEDICATION ‐ BENZODIAZEPINES, Outcome 1 Mental state: No significant improvement ‐ three days after beginning of treatment. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 Leaving the study early Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.26] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.2  Comparison 2 CLOTIAPINE versus STANDARD MEDICATION ‐ BENZODIAZEPINES, Outcome 2 Leaving the study early. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 Behaviour: Aggression (Overt Aggression Scale, skewed data, high=poor) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.3
Comparison 2 CLOTIAPINE versus STANDARD MEDICATION ‐ BENZODIAZEPINES, Outcome 3 Behaviour: Aggression (Overt Aggression Scale, skewed data, high=poor). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.1 on admission | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.2 by 24 hours | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.3 by 72 hours | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.4 by 1 week | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 Adverse effects: Movement disorders (Simpson‐Angus Scale, skewed data, high=poor) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.4
Comparison 2 CLOTIAPINE versus STANDARD MEDICATION ‐ BENZODIAZEPINES, Outcome 4 Adverse effects: Movement disorders (Simpson‐Angus Scale, skewed data, high=poor). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.1 by 24 hours | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.2 by 72 hours | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.3 by 1 week | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||
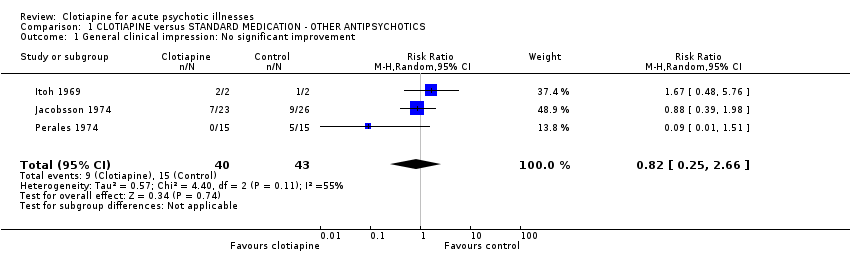
Comparison 1 CLOTIAPINE versus STANDARD MEDICATION ‐ OTHER ANTIPSYCHOTICS, Outcome 1 General clinical impression: No significant improvement.

Comparison 1 CLOTIAPINE versus STANDARD MEDICATION ‐ OTHER ANTIPSYCHOTICS, Outcome 2 Hospital and service outcome: Not well enough to be discharged.

Comparison 1 CLOTIAPINE versus STANDARD MEDICATION ‐ OTHER ANTIPSYCHOTICS, Outcome 3 Leaving the study early.

Comparison 1 CLOTIAPINE versus STANDARD MEDICATION ‐ OTHER ANTIPSYCHOTICS, Outcome 4 Adverse effects: 1. Movement disorders ‐ use of antiparkinsonian medication.

Comparison 1 CLOTIAPINE versus STANDARD MEDICATION ‐ OTHER ANTIPSYCHOTICS, Outcome 5 Adverse effects: 2. Incidence of specific side‐effects.
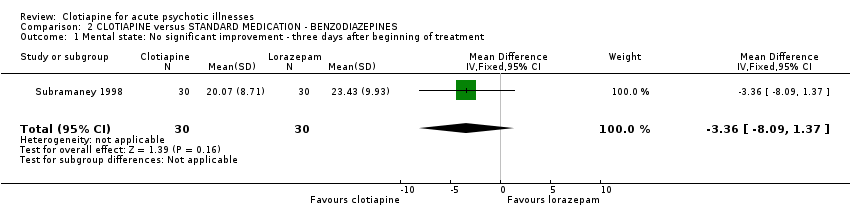
Comparison 2 CLOTIAPINE versus STANDARD MEDICATION ‐ BENZODIAZEPINES, Outcome 1 Mental state: No significant improvement ‐ three days after beginning of treatment.

Comparison 2 CLOTIAPINE versus STANDARD MEDICATION ‐ BENZODIAZEPINES, Outcome 2 Leaving the study early.
| Study | Clotiapine N | Clotiapine mean (SD) | Lorazepam N | Lorazepam Mean (SD) |
| on admission | ||||
| Subramaney 1998 | 30 | 6.43 (4.07) | 30 | 5.87 (3.27) |
| by 24 hours | ||||
| Subramaney 1998 | 30 | 1.33 (2.78) | 30 | 1.83 (3.14) |
| by 72 hours | ||||
| Subramaney 1998 | 30 | 1.30 (2.85) | 30 | 1.17 (2.15) |
| by 1 week | ||||
| Subramaney 1998 | 29 | 1.41(4.14) | 29 | 1.03 (2.26) |
Comparison 2 CLOTIAPINE versus STANDARD MEDICATION ‐ BENZODIAZEPINES, Outcome 3 Behaviour: Aggression (Overt Aggression Scale, skewed data, high=poor).
| Study | Clotiapine N | Clotiapine mean (SD) | Lorazepam N | Lorazepam mean (SD) |
| by 24 hours | ||||
| Subramaney 1998 | 30 | 2.67 (2.43) | 30 | 1.2 (1.81) |
| by 72 hours | ||||
| Subramaney 1998 | 30 | 2.83 (2.15) | 30 | 1.67 (2.47) |
| by 1 week | ||||
| Subramaney 1998 | 30 | 2.07 (1.86) | 30 | 1.37 (2.22) |
Comparison 2 CLOTIAPINE versus STANDARD MEDICATION ‐ BENZODIAZEPINES, Outcome 4 Adverse effects: Movement disorders (Simpson‐Angus Scale, skewed data, high=poor).
| Favoured drug regime | Number |
| haloperidol + lorazepam +/‐ benztropine | 11 |
| droperidol | 4 |
| benzodiazepine (unspecified) alone | 3 |
| droperidol + lorazepam + diphenhydramine | 1 |
| haloperidol + benztropine | 1 |
| Drug of choice | Mean dose (range) |
| diazepam* | 27 (10‐80) |
| haloperidol | 22 (10‐60) |
| chlorpromazine | 162 (50‐400) |
| droperidol | 14 (10‐20) |
| paraldehyde | U/K |
| amytal | U/K |
| lorazepam | U/K |
| nitrazepam** | U/K |
| * most frequent ** least frequent |
| Drug of choice | Mean dose (range) | Frequency of use |
| haloperidol + promethazine | 5 (2.5‐10) + 50 (25‐100) | 61% |
| haloperidol + promethazine + diazepam | 5 (2.5‐10) + 50 (25‐100) + 10 | 15% |
| diazepam | 10 | 9% |
| haloperidol + promethazine + chlorpromazine | 5 + 50 + 25 | 7% |
| chlorpromazine + diazepam + promethazine | 25 + 10 + 50 | 1% |
| chlorpromazine + promethazine | 25 + 50 | 1% |
| chlorpromazine | 25 | 1% |
| diazepam + promethazine | 10 + 50 | 1% |
| haloperidol + diazepam | 5 + 10 | 1% |
| promethazine | 50 | 1% |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 General clinical impression: No significant improvement Show forest plot | 3 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.25, 2.66] |
| 2 Hospital and service outcome: Not well enough to be discharged Show forest plot | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.93, 1.16] |
| 3 Leaving the study early Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 any reason | 3 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 2.26 [0.40, 12.88] |
| 3.2 adverse effects | 3 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 5.63 [0.28, 111.43] |
| 4 Adverse effects: 1. Movement disorders ‐ use of antiparkinsonian medication Show forest plot | 2 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.03, 4.10] |
| 5 Adverse effects: 2. Incidence of specific side‐effects Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 dry mouth | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.4 [0.09, 1.84] |
| 5.2 headache | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [0.92, 3.66] |
| 5.3 insomnia | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.82, 1.70] |
| 5.4 pain at site of injection | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.25, 98.27] |
| 5.5 palpitations | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.10, 2.44] |
| 5.6 rash | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 5.63 [0.28, 111.43] |
| 5.7 seizure | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 69.70] |
| 5.8 sweating ‐ facial | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.78, 2.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mental state: No significant improvement ‐ three days after beginning of treatment Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐3.36 [‐8.09, 1.37] |
| 2 Leaving the study early Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.26] |
| 3 Behaviour: Aggression (Overt Aggression Scale, skewed data, high=poor) Show forest plot | Other data | No numeric data | ||
| 3.1 on admission | Other data | No numeric data | ||
| 3.2 by 24 hours | Other data | No numeric data | ||
| 3.3 by 72 hours | Other data | No numeric data | ||
| 3.4 by 1 week | Other data | No numeric data | ||
| 4 Adverse effects: Movement disorders (Simpson‐Angus Scale, skewed data, high=poor) Show forest plot | Other data | No numeric data | ||
| 4.1 by 24 hours | Other data | No numeric data | ||
| 4.2 by 72 hours | Other data | No numeric data | ||
| 4.3 by 1 week | Other data | No numeric data | ||

