Fluoridhaltige Mundspülung zur Vorbeugung von Zahnkaries bei Kindern und Jugendlichen
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Study characteristics | ||
| Methods | Study design: 4‐arm parallel‐group RCT (only 2 relevant arms used), placebo‐controlled Study duration: 2 years | |
| Participants | Participants randomised (numbers for relevant groups NR) 488 children analysed at 2 years (available at final examination) Setting of recruitment and treatment: school | |
| Interventions | Comparison: FR + ptc vs PL + ptc FR group: 0.02 % NaF, 100 ppm F PL group: non‐F rinse School use/supervised, daily (160 rinses/y), 20 mL applied for 1 minute Before application: Both groups had toothbrushing with non‐fluoride toothpaste Postop instruction: NR | |
| Outcomes | 2yNetDFS increment ‐ (E+U)(NCA)cl+(ER)xr PF‐DFS | |
| Declaration of Interest | No information provided | |
| Funding | Financial support for the study provided by the Warner Lambert Research Institute | |
| Notes | Clinical (V) caries assessment by 1 examiner (FOTI used); diagnostic threshold = NCA. Radiographic assessment (postBW) by 1 examiner; diagnostic threshold = ER. State of tooth eruptions included = E/U. Intraexaminer reproducibility checks for incremental caries data (ICC for clinical 0.95, for radiographic 0.8); reversal rate between 12% and 7% of observed DFS increment in study groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a table of random numbers, subjects were allocated within each school to one of four study groups" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: "The study was organized on a double‐blind basis..." “The placebo rinse preparation was identical to the active rinse, except that it did not contain any fluoride” Comment: use of placebo described |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The study was organized on a double‐blind basis..." Comment: blind outcome assessment and use of placebo described |
| Incomplete outcome data (attrition bias) | Unclear risk | Overall dropout for length of follow‐up: 12% in 2 years (all groups) Dropout by group: not reported Reasons for losses: mainly due to moving from the area Comment: numbers lost not high, given length of follow‐up; differential loss between groups not assessable. It is unclear whether reasons for missing outcome data are acceptable and balanced. Caries data used in the analysis pertain to participants present at baseline and at final exams |
| Selective reporting (reporting bias) | Low risk | Outcomes reported PF‐DFS Comment: trial protocol not available. All prespecified outcomes (in Methods) reported in the prespecified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported DFS: 9.10 (6.75) FD, 9.79 (7.28) PL DMFT: 5.71(3.44) FD, 6.06 (3.66) PL DMFS: 10.47 (7.36) FD, 11.05 (7.98) PL Age: 12.33 FD, 12.28 PL Comment: initial caries appears balanced between groups. Age also balanced |
| Free of contamination/co‐intervention? | Low risk | Non‐fluoride toothpaste provided to all for home use (no rinse provided) |
| Study characteristics | ||
| Methods | Study design: 4‐arm parallel‐group quasi‐RCT (only 3 relevant arms used)**, "placebo"‐controlled Study duration: 2.5 years | |
| Participants | Participants randomised: N = 766 420 children analysed at 2.5 years (after exclusions, available at final examination) Setting of recruitment and treatment: school | |
| Interventions | Comparison: FR (2 groups) vs PL FR group 1: 0.2% NaF, 900 ppm F FR group 2: 0.7% SMFP, 900 ppm F PL group: non‐F rinse (aqueous 0.1% NaCl solution) School use/supervised, weekly (32 rinses/y), 10 mL applied for 1 minute Before application: NR Postop instruction: no rinsing, eating or drinking for 1 hour | |
| Outcomes | 2.5yDMFS increment ‐ (CA)(E) DMFT (E/U) Side effects (incomplete data) Dropout | |
| Declaration of Interest | No information provided | |
| Funding | Conselho Nacional de Desenvolvimento Cientifico e Tecnologico, Brazil | |
| Notes | Clinical (VT) caries assessment by 2 examiners, diagnostic threshold = CA. State of tooth eruption included = E/U. Consistency of diagnosis assessed by duplicate examinations annually. Reversals < 5% of DMFS increments in all groups and equally common **Study group of sodium monofluorophosphate solution containing 4% of ethanol not considered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quotes from translation: “The children were 9‐12 year olds and were divided between the two examiners in equal numbers according to gender and age but at random” “For each examiner, and for each gender, the children were ordered firstly in ascending order, according to the number of permanent teeth present, and secondly, according to the number of DMFS. To each group formed in this way, by lot, one of the following rinsing solutions were given...” “Then every set of four records (children) at random were distributed into four groups. In this way, comparability between the experimental groups was achieved. Then at random, each group was assigned to one of the four following rinsing solutions...” Comment: unclear how this method of randomisation could affect selection bias. Method of sequence generation not described ‐ possibly a quasi method |
| Allocation concealment (selection bias) | High risk | Quotes from translation: “The children were 9‐12 year olds and were divided between the two examiners in equal numbers according to gender and age but at random” “For each examiner, and for each gender, the children were ordered firstly in ascending order, according to the number of permanent teeth present, and secondly, according to the number of DMFS. To each group formed in this way, by lot, one of the following rinsing solutions were given...” “Then every set of four records (children) at random were distributed into four groups. In this way, comparability between the experimental groups was achieved. Then at random, each group was assigned to one of the four following rinsing solutions...” Comment: method of sequence generation not described ‐ possibly a quasi‐method |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quotes from translation: “Group D: aqueous solution of sodium chloride 0.1%(control)” "Through the school year, the mouthrinses, prepared weekly at the dental school laboratory, were put in plastic bottles, then accommodated in separate boxes, according to the different rinsing solutions, which were taken to the schools and given to the classroom teachers who had been trained to apply/supervise the procedures during the time of the study. The names of the children, who would use the bottles according to the groups to which they belonged, featured in the lid of the boxes" Comment: use of placebo described. Although blinding of participants indicated, study personnel (teachers carrying out the procedure in the schools) were not blind to group assignment |
| Blinding of outcome assessment (detection bias) | Low risk | Quotes from translation: “Two dentists not involved in treatment conducted the exams” Comment: examiners likely to be unaware of treatment group assignment |
| Incomplete outcome data (attrition bias) | High risk | Overall dropout for length of follow‐up: 45.17% in 2.5 years Comment: numbers lost unduly high for length of follow‐up, and although no differential losses occurred, the reason for exclusion of data is unacceptable. Caries data used in analysis pertain to participants present at final examination (after exclusions were made) |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported Reported at 1, 1.5 and 2.5 years' follow‐up DMFT (E/U) O‐DFS BL‐DFS MD‐DFS DMFS (U) AntDMFS PostDMFS Side effects (incomplete data). Study reported that "no adverse effects were observed" but did not specify what adverse effects were assessed or how they were assessed Comment: trial protocol not available (thesis available). All prespecified outcomes (in Methods) were reported in the prespecified way, but we noted some discrepancy between outcomes actually reported and reporting in Methods |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported DMFS: 10.43 FR1, 10.51 FR2, 10.54 PL DMFT: 5.69 FR1, 5.67 FR2, 5.65 PL Dental age: 19.08 FR1, 19.01 FR2, 19.13 PL Comment: initial caries appears balanced between groups. Dental age also balanced |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
| Study characteristics | ||
| Methods | Study design: 4‐arm parallel‐group RCT (only 2 relevant arms used), placebo‐controlled Study duration: 3 years | |
| Participants | Participants randomised: N = 414 374 children analysed at 3 years (available at final examination) Setting of recruitment and treatment: school | |
| Interventions | Comparison: FR+ptc vs PL+ptc FR group: 0.05% NaF, 230 ppm F PL group: non‐F rinse School use/supervised, daily (160 rinses/y), for half minutes Before application: toothbrushing with non‐fluoride toothpaste in both groups Postop instruction: NR | |
| Outcomes | 3yNetDFS increment ‐ (E+U)(CA)cl+(DR)xr PF‐DFS Dropout | |
| Declaration of Interest | No information provided | |
| Funding | This study was supported by a grant from Colgate‐Palmolive | |
| Notes | Clinical (V) caries assessment by 1 examiner, diagnostic threshold = CA. Radiographic assessment (1 postBW) by 1 examiner; diagnostic threshold = DR. State of tooth eruption included = E/U. Intraexaminer reproducibility checks for incremental clinical and radiographic caries data in 10% sample (ICC score 0.9) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The children were allocated to four groups by stratified random sampling at two levels: school and dental age...” Quote from correspondence: “The allocation to groups was random...” Comment: not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | Quote from correspondence: “The allocation to groups was random with complete concealment of treatment allocation” Comment: not enough information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: “The trial was organised on a double‐blind basis, neither the children nor the examiner being aware of who was receiving test or control products” “Control subjects used the equivalent dentifrice and rinse without fluoride” Comment: use of placebo described |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “The trial was organised on a double‐blind basis, neither the children nor the examiner being aware of who was receiving test or control products” Comment: blind outcome assessment and use of placebo described |
| Incomplete outcome data (attrition bias) | Unclear risk | Overall dropout for length of follow‐up: 9.66% in 3 years Comment: numbers lost not high for length of follow‐up, with no differential losses between groups. It is unclear whether reasons for losses are balanced between groups Caries data used in the analysis pertain to participants present at final examination |
| Selective reporting (reporting bias) | Low risk | Outcomes reported PF‐DFS MD‐BL‐DFS MD‐DFS PostMD‐DFS DMFT (E/U) Anterior DMFT Posterior DMFT DFS (U) Comment: trial protocol not available. All pre‐specified outcomes (in Methods) were reported in the pre‐specified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported DMFS: 8.71(6.42) FR, 8.48(6.29) PL DMFT: 5.30(3.58) FR, 5.26(3.47) PL SAR: 93.00(19.75) FR, 93.61(20.43) PL Comment: initial caries appears balanced between groups (although DFS baseline data NR). SAR also seems balanced |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
| Study characteristics | ||
| Methods | Study design: 2‐arm parallel‐group RCT, placebo‐controlled Study duration: 2 years | |
| Participants | Participants randomised: N = 314 246 children analysed at 2 years (after exclusions based on compliance, present at all examinations) Setting of recruitment and treatment: school | |
| Interventions | Comparison: FR vs PL FR group: 0.2% NaF (900 ppm F) PL group: non‐F rinse School use/supervised, twice a week (60 rinses/y), 10 mL applied for 1 minute Prior to application: NR Postop instruction: NR | |
| Outcomes | 2yDFS scores ‐ (E+U) DMFS* Dropout *Reported match‐pair rather than randomised results ‐ could not be included in meta‐analysis. See ROB section | |
| Declaration of Interest | No information provided | |
| Funding | The study authors thank the pharmacy department of The London Hospital | |
| Notes | Clinical caries assessment, diagnostic threshold NR. Radiographic assessment; diagnostic threshold = NR. State of tooth eruption included = E/U. Diagnostic errors NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “...allocation to either study or control groups was done on a school house basis, allocation to a house being done by school administrative staff randomly” Comment: not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: “The subjects rinsed with...NaF for one minute or similarly with...NaCl if they were in the control group” Comment: use of placebo described |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “The study was conducted as a 2 year CCT on a double‐blind basis" Comment: blind outcome assessment and use of placebo described |
| Incomplete outcome data (attrition bias) | High risk | Overall dropout for length of follow‐up: 21.66% in 2 years Dropout by group: 28/153 (18.3%) FR, 40/161 (24.8%) PL Reasons for losses: exclusions based on compliance Reasons for attrition described with numbers by group: change of residence (18, 12), absent at final examination (5, 7); plus exclusions based on compliance, presence in all examinations and for statistical analysis; no differential group losses Comment: numbers lost not unduly high for length of follow‐up, with no differential losses between groups. Reasons for dropout may not be acceptable or balanced between groups. Caries data used in analysis pertain to participants present at all examinations |
| Selective reporting (reporting bias) | High risk | Outcomes reported DFS scores* ‐ (E+U), reported at 2 years' follow‐up DMFS* DMFT* PostMD‐DMFS CFS CFT Comment: trial protocol not available |
| Baseline characteristics balanced? | High risk | Prognostic factors reported DMFS: 7.10 FR, 8.65 PL Age: 11.5 FR, 11.5 PL Comment: initial caries with some imbalance between groups |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
| Study characteristics | ||
| Methods | Study design: 3‐arm parallel‐group RCT (only 2 relevant arms used), non‐placebo‐controlled Study duration: 2 school years (21 months) | |
| Participants | Participants randomised: N = 109 97 children analysed at 2 years (available at final examination) Setting of recruitment and treatment: school | |
| Interventions | FR+ptc vs NT+ptc NT group: no intervention School use/supervised, fortnightly (17 rinses/y), 10 mL applied for 2 minutes Before application: prior professional prophylaxes with non‐fluoride toothpaste in both groups (+oral hygiene instructions) Postop instruction: NR | |
| Outcomes | 2yDFS increment ‐ (CA) O‐DFS Dropout | |
| Declaration of Interest | No information provided | |
| Funding | The study authors thank the Director General of Health (NZ) for approval to publish the study report | |
| Notes | Clinical (VT) caries assessment by 2 examiners, diagnostic threshold = CA. State of tooth eruption included NR. Reproducibility checks for incremental clinical caries data in 15% sample at each examination (reversal rate < 4% for both examiners) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The children were then stratified according to sex, age and caries experience and allocated randomly to three groups” Quote from correspondence: “We are sure that a random number system was used to allocate the children into groups after stratification...” |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Quotes: "one test group received professional prophylaxes and the other group prophylaxes + fluoride rinses" Comment: no placebo described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: “...one of the examiners, ignorant of the group to which the child belonged” Comment: blind outcome assessment reported but no placebo described |
| Incomplete outcome data (attrition bias) | Low risk | Overall dropout for length of follow‐up: 11.0% in 2 years Dropout by group: 6/54 FR, 7/55 NT Reasons for losses: leaving school (12 children) Comment: numbers lost not high, given length of follow‐up. No differential losses between groups. Reason for losses acceptable and balanced between groups Caries data used in the analysis pertain to participants available at final examination |
| Selective reporting (reporting bias) | Low risk | Outcomes reported O‐DFS MD‐DFS BL‐DFS Dropout Comment: trial protocol not available. All prespecified outcomes (in Methods) were reported in the prespecified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported DFS: 10.65(6.4) FR, 10.5(6.4) NT Dental age: 21.2(5.7) FR, 21.4(5.0) NT Comment: initial caries appears balanced between groups. Dental age also balanced |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
| Study characteristics | ||
| Methods | Study design: 2‐arm parallel‐group RCT, placebo‐controlled Study duration: 3 years | |
| Participants | Participants randomised: numbers NR 262 children analysed after 3 years (available at final examination) Setting of recruitment and treatment: school | |
| Interventions | Comparison: FR vs PL FR group: 0.2% NaF (900 ppm F) PL group: non‐F rinse School use/supervised, fortnightly (17 rinses/y) Before application: NR Postop instruction: NR | |
| Outcomes | 2yDMFS final scores* ‐ (CA) DMFT *Only results of combined non‐randomised and randomised groups reported (separate results for placebo group not available, data could not be included in meta‐analysis) | |
| Declaration of Interest | No information provided | |
| Funding | The study authors thank the permission of the Director General of Health (NZ) for approval to publish the paper | |
| Notes | Clinical (VT) caries assessment by 1 examiner; diagnostic threshold = CA; state of tooth eruption included NR; diagnostic errors NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The high caries‐risk children were randomly divided into two groups...” Comment: not enough information |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: “...the other used a placebo rinse...” “Mouth rinsing was conducted double‐blind, with the supervisor, the dental nurses and the children being unaware of the composition of the mouth rinsing solution” “...after examination and tentative treatment planning by the dental nurses” Comment: use of placebo described |
| Blinding of outcome assessment (detection bias) | Low risk | Quotes: as above Comment: blind outcome assessment and use of placebo described |
| Incomplete outcome data (attrition bias) | High risk | Overall dropout for length of follow‐up: not reported Reasons for attrition NR: any differential group losses not assessable |
| Selective reporting (reporting bias) | High risk | Outcomes reported Comment: only results of combined non‐randomised and randomised groups reported (separate results for placebo group not available, data could not be included for mea‐analysis) |
| Baseline characteristics balanced? | High risk | Prognostic factors reported No baseline characteristics/values reported |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
| Study characteristics | ||
| Methods | Study design: 3‐arm parallel group RCT, placebo‐controlled Study duration: 2 years | |
| Participants | Participants randomised: N = 614) (numbers randomised to each group NR) 475 children analysed at 2 years (available at final examination, who participated throughout) Setting of recruitment and treatment: schools in a non‐fluoridated community ***History of prior exposure to systemic F was reported by nearly half of panel | |
| Interventions | Comparison: FR (2 groups) vs PL FR group 2 (n = 158): 0.22% NaF group = 1000 ppm F PL group (n = 158): distilled water, coloured and flavoured to simulate active agents School use/supervised, daily (140 rinses/y), 5 mL applied for 1 minute Before application: NR Postop instruction: NR | |
| Outcomes | 2yNetDFS increment ‐ (CA)cl+(ER)xr DFS (U) Side effects (incomplete data) | |
| Declaration of Interest | No information provided | |
| Funding | Supported by NIDR Contract Number NIH 71‐2379 | |
| Notes | Clinical (VT) caries assessment, diagnostic threshold = CA; state of tooth eruption included NR. Radiographic assessment (4 postBW); diagnostic threshold = ER; diagnostic errors NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “After being randomly assigned to one of three treatment groups...” Comment: not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: “A double‐blind clinical trial was conducted...” Comment: described as double‐blinded. No descriptions on how personnel were blinded, but this was probably carried out. Use of placebo described |
| Blinding of outcome assessment (detection bias) | Low risk | Quotes: “A double‐blind clinical trial was conducted...” Comment: described as double‐blinded but method of blinding of outcome assessor not reported. Probably low risk because bitewing radiographs were used Blind outcome assessment and use of placebo described |
| Incomplete outcome data (attrition bias) | High risk | Overall dropout for length of follow‐up: 22.64% in 2 years Comment: numbers lost not unduly high for length of follow‐up. Differential losses not assessable. It is unclear whether reasons for missing outcome data are acceptable and balanced. Caries data used in the analysis pertain to participants present throughout the trial |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported DFS (U) Side effects (incomplete data): Study reported that "no adverse effects were observed" but did not specify what adverse effects were assessed or how these were assessed Comment: trial protocol not available. Prespecified outcomes (in Methods) were reported. However side effects data were incomplete |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported DFS: 6.26(5.09) FR1, 5.46(4.54) FR2, 6.47(5.50) PL No prior exposure to systemic fluoride: 85/159 (53.5%) FR1, 92/158 (58.2%) 81/158 (51.3%) PL Comment: initial caries appears balanced between groups |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
| Study characteristics | ||
| Methods | Study design: 4‐arm parallel‐group RCT (only 2 relevant arms used), placebo‐controlled Study duration: 2 years | |
| Participants | Participants randomised: numbers NR nor obtainable 271 children analysed at 2 years (after exclusions, present for both examinations) Setting of recruitment and treatment: school | |
| Interventions | Comparison: FR vs PL PL group: non‐F rinse (disguised and colour coded) School use/supervised, daily (140 rinses/y), 10 mL applied for 1 minute Before application: no tooth cleaning performed Postop instruction: NR | |
| Outcomes | 2yNetDFS increment ‐ (CA)cl+xr | |
| Declaration of Interest | No information provided | |
| Funding | The study was supported by National Institute of Dental Research, Contract No. NOI‐DE42445 | |
| Notes | Clinical (VT) caries assessment by 2 examiners; diagnostic threshold = CA; state of tooth eruption included NR. Radiographic assessment (2 postBW) by 2 examiners; diagnostic threshold NR; diagnostic errors NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Subjects were randomly assigned to 1 examiner and 1 of 4 treatment groups at the time of the clinical examination” Comment: not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: “A strict double‐blind routine was maintained throughout the course of the investigation” “The placebo and active rinses were disguised and colour coding...” "Supervisors had typed lists indicating the agent code for each subject" Comment: use of placebo described |
| Blinding of outcome assessment (detection bias) | Low risk | Quotes: “A strict double‐blind routine was maintained throughout the course of the investigation” “Subjects always seen by the same examiner and examined without reference to previous findings” Comment: blind outcome assessment and use of placebo described |
| Incomplete outcome data (attrition bias) | High risk | Overall dropout for length of follow‐up: not reported Comment: Reasons for missing outcome data may be unacceptable, and It is unclear whether these are balanced between groups |
| Selective reporting (reporting bias) | Low risk | Outcomes reported Comment: trial protocol not available. All prespecified outcomes (in Methods) were reported in the prespecified way |
| Baseline characteristics balanced? | Unclear risk | Prognostic factors: DFS, dental age and age reported as "balanced" (values not reported) |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
| Study characteristics | ||
| Methods | Study design: 4‐arm parallel‐group RCT (only 3 relevant arms used), placebo‐controlled Study duration: 2.5 years | |
| Participants | Participants randomised: N = 966 524 children analysed at 2.5 years (present for entire trial period) Setting of recruitment and treatment: school | |
| Interventions | Comparison: FR (2 groups) vs PL NaF group 1: 230 ppm F, daily (160 rinses/y) PL group: non‐F rinse (0.1 NaCl) School use/supervised, 10 mL applied for 1 minute Before application: NR Postop instruction: NR | |
| Outcomes | 2.5yNetDMFS increment O‐DMFS Dropout | |
| Declaration of Interest | No information provided | |
| Funding | No information provided | |
| Notes | Clinical (VT) caries assessment by 2 examiners; diagnostic threshold NR. State of tooth eruption included NR; differences between examiner assessments NS (but reproducibility assessment NR). Results presented separately by examiner (combined results considered) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The children were assigned randomly, within each school, to one of three groups” Comment: not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: “A control group of children followed the procedure once a week using a placebo mouthrinse” Comment: use of placebo described |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “The examiners were unaware of any child’s group assignment, and did not have access to records from the baseline examination” Comment: blind outcome assessment and use of placebo described |
| Incomplete outcome data (attrition bias) | High risk | Overall dropout for length of follow‐up: 45.75% in 2.5 years Comment: Numbers lost were high, although no differential loss occurred between groups. It is unclear whether 1 of the reasons for missing outcome data (voluntary withdrawal) is acceptable and balanced. Caries data used in the analysis pertain to participants present throughout the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes reported O‐DMFS MD‐DMFS BL‐DMFS Comment: trial protocol not available. All prespecified outcomes (in Methods) were reported in the prespecified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported DMFS: 4.62 FR1, 4.76 FR2, 4.93 PL Comment: initial caries apparently balanced between groups |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
| Study characteristics | ||
| Methods | Study design: 4‐arm parallel‐group RCT, placebo‐controlled Study duration: 3 years | |
| Participants | Participants randomised: numbers NR nor obtainable 936 children analysed at 3 years Exposure to other fluoride: not obtainable Setting of recruitment and treatment: school | |
| Interventions | FR (3 groups) vs PL FR group 1: 0.02% NaF = 100 ppm F FR group 2: 0.05% NaF = 225 ppm F FR group 3: 0.10% NaF = 450 ppm F PL group: non‐F rinse Before application: NR Postop instruction: NR | |
| Outcomes | 3yDMFS increment | |
| Declaration of Interest | No information provided | |
| Funding | No information provided | |
| Notes | Other data NR nor obtainable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The children were randomly assigned to one of four mouth rinse groups...” Comment: not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “...one of four mouthrinse groups (control, and three concentrations of sodium fluoride) and were followed double‐blinded for three years...” Comment: Study described use of a control mouthrinse, the control is a mouthrinse group that did not rinse with F and it is a DB study |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “...one of four mouthrinse groups (control, and three concentrations of sodium fluoride) and were followed double‐blinded for three years...” Comment: blind outcome assessment reported, although unclear what procedures were used, but use of placebo reported |
| Incomplete outcome data (attrition bias) | High risk | Overall dropout for length of follow‐up: not obtainable |
| Selective reporting (reporting bias) | Low risk | Outcomes reported Comment: trial protocol not available. All prespecified outcomes (in Methods) were reported in the prespecified way |
| Baseline characteristics balanced? | High risk | Prognostic factors reported DMFS: 7.39(8.52) FR1, 6.28(7.77) FR2, 6.79(7.07) FR3, 7.50(8.23) PL Comment: initial caries appears not balanced between groups |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
| Study characteristics | ||
| Methods | Study design: 3‐arm parallel‐group RCT, placebo‐controlled Study duration: 2 years | |
| Participants | Participants randomised: N = 820; numbers by group NR 453 children analysed at 2 years (present in all examinations) Setting of recruitment and treatment: school | |
| Interventions | FR (2 groups) vs PL FR group 2: 0.04% neutral NaF solution (200 ppm F) PL group: non‐F rinse School use/supervised, twice a day (330 rinses/y), 20 mL applied in 2 successive rinses of 30 seconds each Before application: NR Postop instruction: NR | |
| Outcomes | 2yNetDFS increment ‐ cl+xr DMFS Proportion of children with new DFS | |
| Declaration of Interest | No information provided | |
| Funding | The study was supported by a grant from the Warner‐Lambert Company | |
| Notes | Clinical (VT) caries assessment by 1 examiner, diagnostic threshold NR. Radiographic assessment (2‐4 postBW+ 4 anterior) by 1 examiner; diagnostic threshold NR. State of tooth eruption included NR. Diagnostic errors NR. Reversals ranged between 6% and 16% of observed DMFS increment in study groups for combined clinical and x‐ray findings, with rates higher in the test groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “On the basis of age and sex within individual classrooms in each of the three schools, the children were randomly assigned to one of three treatment regimen groups” Comment: not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: “Children in regimen group 3 used the placebo mouthwash which was fluoride free...” “...the children entered the room, announced their name and colour code, picked a colour‐coded cup containing the assigned mouthwash...” Comment: use of placebo described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quotes: “Children in regimen group 3 used the placebo mouthwash which was fluoride free...” “...the children entered the room, announced their name and colour code, picked a colour‐coded cup containing the assigned mouthwash...” “Radiographic findings were added later to the clinical findings” Comment: use of placebo described, but it is unclear whether examiner was blinded |
| Incomplete outcome data (attrition bias) | High risk | Overall dropout for length of follow‐up: 44.76% in 2 years Comment: numbers lost unduly high for length of follow‐up. Differential losses not assessable. It is unclear whether reasons for missing outcome data are acceptable and balanced. Caries data used in the analysis pertain to participants present at all examinations |
| Selective reporting (reporting bias) | Low risk | Outcomes reported DMFS DMFT Proportion of children with new DFS Comment: trial protocol not available. All prespecified outcomes (in Methods) were reported in the prespecified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported DMFT: 3.67(2.81) FR1, 3.87(3.48) FR2, 3.60(2.90) PL DMFS: 5.82(5.18) FR1, 6.17(6.67) FR2, 6.02(6.21) PL Age: 11.8 FR1, 11.4 FR2, 11.8 PL Gender: 75M, 75F (FR1), 70M, 72F (FR2), 71M, 89F (PL) Comment: initial caries appears balanced (although DFS baseline data NR). Other characteristics also balanced |
| Free of contamination/co‐intervention? | Low risk | Non‐fluoride toothpaste and appropriate mouthrinse provided to all for home use |
| Study characteristics | ||
| Methods | Study design: 2‐arm parallel group (quasi) RCT, "placebo"‐controlled Study duration: 3 years | |
| Participants | Participants randomised: N = 809 594 children analysed at 2 years (available at final examination) Surfaces affected at start: 7.3 DMFS (from sample randomised) Setting of recruitment and treatment: school | |
| Interventions | FR vs PL PL group: sodium bicarbonate solution* School use/supervised, weekly (30 rinses/y), applied for 1 minute. Rinsing was performed once a week in the morning Before application: NR Postop instruction: Children were instructed not to swallow the solution and not to eat or drink for 30 minutes after rinsing *Test and control solutions look and taste similar | |
| Outcomes | 2yDMFS increment ‐ (E+U) DMFT Dropout | |
| Declaration of Interest | No information provided | |
| Funding | No information provided | |
| Notes | Clinical (VT) caries assessment by 1 examiner, diagnostic threshold NR. State of tooth eruption included = E/U. Diagnostic errors NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotes: “...all children in the same classrooms were divided into two teams. The criteria used for the division were DMFT and DMFS, dental age and score for OHI” Comment: unclear how method of randomisation used affected selection bias. Coin flipping acceptable method of sequence generations but unclear how teams were formed |
| Allocation concealment (selection bias) | Unclear risk | Quote: “A flip of a coin decided which team would be experimental and which team will be controls” Comment: Allocation was done after teams were formed |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The solutions were mixed by the dental staff. The solution used was 0.4% neutral sodium fluoride, with 0.18 % fluoride ion. The placebo consist of a solution of sodium bicarbonate. Both solutions were colourless and almost tasteless. Students act as the monitors who dispense the solution, collected the used cups, kept the time and reminded each other about brushing" Mouth rinsing was conducted in "teams" Comment: blinding likely maintained because both types of solutions look and taste similar |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “In as much as a double‐blind study was being accomplished, neither students nor examiner knew whether a student was a member of the controls or the experimental group” Comment: likely to be at low risk for outcome assessment blinding if blinding was maintained for participants |
| Incomplete outcome data (attrition bias) | Unclear risk | Overall dropout for length of follow‐up: 26.58% in 2 years Comment: numbers lost not unduly high, given length of follow‐up, with no differential losses between groups. It is unclear whether reasons for missing outcome data are acceptable and balanced. Caries data used in the analysis pertain to participants present at final exam |
| Selective reporting (reporting bias) | Low risk | Outcomes reported DMFT DT DF Comment: trial protocol not available. All prespecified outcomes (in Methods) were reported in the prespecified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported DMFS: 7.19 FR, 7.37 PL DMFT: 4.50 FR, 4.59 PL DT: 2.36 FR, 2.49 PL FT: 1.90 FR, 1.85 PL Dental age: 18.53 FR, 18.64 PL OHI: 1.44 FR, 1.47 PL Comment: initial caries appears balanced between groups. Other characteristics also balanced |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
| Study characteristics | ||
| Methods | Study design: 2‐arm parallel group RCT, placebo‐controlled Study duration: 3 years | |
| Participants | Number randomised: 1306 (numbers randomised to each group NR) Number analysed: 1083 children at 3 years (present at final examination) Setting of recruitment and treatment: school **Both groups had been using FR before the study started | |
| Interventions | Comparison: FR vs PL PL group (n = 545): distilled water ‐ peppermint flavoured School use/supervised, fortnightly (17 rinses/y) Before application: NR Postop instruction: NR | |
| Outcomes | 3yCrude postDMFS increment ‐ (CA)(E+U)cl DMFS (U) Proportion of children with new postMDDMFS | |
| Declaration of Interest | No information provided | |
| Funding | Danish Dental Association | |
| Notes | Clinical (VT) caries assessment by dentists at public dental service, diagnostic threshold = CA. Radiographic assessment (2 postBW) by 1 examiner; diagnostic threshold = ER. State of tooth eruption included = E/U. Reproducibility of diagnosis assessed by duplicate radiographic examination of 10% random sample (kappa value 0.72) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “...children from kindergarten through 6th grade were stratified by school and grade and randomly distributed into two groups” Quote from correspondence: “The randomization was done using a table of random numbers” |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: “...the children were allocated to two groups: a fluoride group...and a water (placebo) group” Comment: use of placebo described. Both participants and personnel should be effectively blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quotes: "two bitewings radiographs taken using a standardised method" Comment: objective method used, blinding stated. Blind outcome assessment and use of placebo described |
| Incomplete outcome data (attrition bias) | Unclear risk | Overall dropout for length of follow‐up: 17.08% (223/1306) in 3 years Comment: numbers lost not high for length of follow‐up; differential losses between groups not assessable (study authors were unable to provide the numbers randomised to each group (personal correspondence)), but numbers analysed seem balanced across groups. It is unclear whether reasons for missing outcome data are acceptable and balanced. Caries data used in analysis pertain to participants present at final examination |
| Selective reporting (reporting bias) | Low risk | Outcomes reported: postDMFS (CA)(E+U)cl, reported at 3 years' follow‐up DMFS (U) O‐DMFS MD‐DMFS BL‐DMFS CIR‐xr Comment: trial protocol not available. All prespecified outcomes (in Methods) were reported in the prespecified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported DMFS: 1.43 FR, 1.46 PL SAR: 27.7 FR, 28.6 PL Comment: initial caries appears balanced between groups. SAR also balanced |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
| Study characteristics | ||
| Methods | Study design: 3‐arm parallel‐group RCT; placebo‐controlled Study duration: 2 years | |
| Participants | Participants randomised: N = 947; numbers randomised to each group NR 413 children analysed at 2 years (after exclusions, present in all examinations) Setting of recruitment and treatment: school | |
| Interventions | FR (2 groups) vs PL FR group 1: APF 0.66% = 3000 ppm F FR group 2: NaF 0.66% = 3000 ppm F PL group: non‐F rinse School use/supervised, weekly (25 rinses/y), 8 mL applied twice (16 mL) for 1 minute Before application: NR Postop instruction: NR | |
| Outcomes | 2yNetDMFS increment ‐ (E+U) cl+(ER)xrReported at 1 and 2 years' follow‐up | |
| Declaration of Interest | No information provided | |
| Funding | All mouthwash solutions used in the study were commercially prepared by the Lorvic Corp | |
| Notes | Clinical (VT) caries assessment by 2 examiners, diagnostic threshold NR. Radiographic assessment (5 postBW) by 2 examiners; diagnostic threshold = ER. State of tooth eruption included ‐E/U. Diagnostic errors NR (but examiners calibrated regularly). Reversals ranged between 5% and 10% of observed DMFS increment in study groups for combined clin+xr findings, with rates higher in the test groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The baseline records of the children were stratified according to sex, dental age... Within each stratum, each child was assigned randomly to one of three study groups” Comment: not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: “Group A rinsed their mouths in school once a week with a placebo solution” Comment: use of placebo described |
| Blinding of outcome assessment (detection bias) | Low risk | Quotes: “The examiner did not know the group to which any child was assigned” “Group A rinsed their mouths in school once a week with a placebo solution” Comment: blind outcome assessment and use of placebo described |
| Incomplete outcome data (attrition bias) | High risk | Overall dropout for length of follow‐up: 56.39% in 2 years Comment: numbers lost unduly high, given length of follow‐up. Differential losses not assessable. Reasons for missing outcome data (poor compliance) may be unacceptable, and it is unclear whether they are balanced between groups. Caries data used in the analysis pertain to participants present at baseline and final exams |
| Selective reporting (reporting bias) | Low risk | Outcomes reported Comment: trial protocol not available. All prespecified outcomes (in Methods) were reported in the prespecified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported DMFS: 10.16(9.77) FR1, 11.38(10.60) FR2, 10.81(8.69) PL Comment: initial caries appears balanced between groups |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
| Study characteristics | ||
| Methods | Study design: 3‐arm parallel‐group RCT, placebo‐controlled Study duration: 3 years | |
| Participants | Participants randomised: N = 912; numbers by group NR 598 children analysed at 3 years (present for entire trial period) Setting of recruitment and treatment: school | |
| Interventions | FR (2 groups) vs PL FR group 1: 0.05% NaF 230 ppm F, daily (150 rinses/y) PL group: non‐F rinse School use/supervised, 10 mL applied for 1 minute Before application: NR Postop instruction: NR | |
| Outcomes | 3yNetDMFS increment ‐ (CA)(E)clinReported at 1, 2 and 3 years' follow‐up O‐DMFS | |
| Declaration of Interest | No information provided | |
| Funding | No information provided | |
| Notes | Clinical (VT) caries assessment by 2 examiners; diagnostic threshold = CA (FOTI assessment ‐ loss of translucency on transillumination ‐ for approximal surfaces). State of tooth eruptions included = E; differences between examiner assessments NS (but reproducibility assessment NR). Results presented separately by examiner(combined results considered) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from correspondence: “Using a computer generated table of random numbers, the 912 subjects...were randomly assigned...” |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “Group C (controls) rinsed once a week with a placebo solution” Comment: use of placebo described |
| Blinding of outcome assessment (detection bias) | Low risk | Quotes: “The examiners were unaware of any child’s group assignment, and did not have access to records from the previous examinations” “Group C (controls) rinsed once a week with a placebo solution” Comment: blind outcome assessment and use of placebo described |
| Incomplete outcome data (attrition bias) | Unclear risk | Overall dropout for length of follow‐up: 34.43% in 3 years Comment: numbers lost unduly high, given length of follow‐up. Differential losses between groups not assessable. It is unclear whether reasons for missing outcome data are acceptable and balanced. Caries data used in the analysis pertain to participants present throughout the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes reported DMFS increment (CA)(E)clin, reported at 1, 2 and 3 years' follow‐up O‐DMFS MD‐DMFS BL‐DMFS Comment: trial protocol not available. All prespecified outcomes (in Methods) were reported in the prespecified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported DMFS: 6.06(5.76) FR1, 5.98(5.70) FR2, 6.56(6.00) PL Comment: initial caries appears balanced between groups |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
| Study characteristics | ||
| Methods | Study design: 2‐arm parallel‐group RCT, placebo‐controlled Study duration: 1.6 years | |
| Participants | Participants randomised: N = 493 256 children analysed at 1.6 years (present for entire trial period) Setting of recruitment and treatment: school | |
| Interventions | FR vs PL FR group 1: 0.2% neutral NaF solution (900 ppm F) PL group: non‐F rinse solution School use/supervised, weekly (30 rinses/y), 10 mL applied for 1 minute Before application: NR Postop instruction: NR | |
| Outcomes | 1.6yNetDMFS increment ‐ (E+U)Reported at 1 and 1.6 years' follow‐up DMFT (E/U) Dropout | |
| Declaration of Interest | No information provided | |
| Funding | No information provided | |
| Notes | Clinical (VT) caries assessment by 2 examiners, diagnostic threshold NR. State of tooth eruption included = E/U. Diagnostic errors NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “...according to dental age...sex and previous caries experience of the children, they were randomly assigned to one of the two following study groups...” Comment: not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: “The control group rinsed with a placebo” “A monthly rinsing for the controls seemed to be a reasonable compromise. Because the examiners for this study had no part in administering treatments, a double‐blind method could be maintained strictly” Comment: use of placebo described |
| Blinding of outcome assessment (detection bias) | Low risk | Quotes: “The control group rinsed with a placebo” “A monthly rinsing for the controls seemed to be a reasonable compromise. Because the examiners for this study had no part in administering treatments, a double‐blind method could be maintained strictly” Comment: blind outcome assessment and use of placebo described |
| Incomplete outcome data (attrition bias) | High risk | Overall dropout for length of follow‐up: 48.07% in 1.6 years Comments: numbers lost unduly high, given length of follow‐up, with no differential losses. It is unclear whether reasons for missing outcome data are acceptable and balanced. Caries data used in the analysis pertain to participants present at all exams |
| Selective reporting (reporting bias) | Low risk | Outcomes reported DMFT (E/U) DMFS (U) Comment: trial protocol not available. All prespecified outcomes (in Methods) were reported in the prespecified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported DMFS: 0.90 FR, 0.97 PL DMFT: 0.73 FR, 0.75 PL Comment: initial caries appears balanced between groups |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
| Study characteristics | ||
| Methods | Study design: 2‐arm parallel‐group RCT, placebo‐controlled Study duration: 1.6 years | |
| Participants | Participants randomised: N = 381 208 children analysed at 1.6 years (present for entire trial period) Setting of recruitment and treatment: school | |
| Interventions | FR vs PL FR group 1: 0.2% neutral NaF solution (900 ppm F) PL group: non‐F rinse solution School use/supervised, weekly (30 rinses/y), 10 mL applied for 1 minute Before application: NR Postop instruction: NR | |
| Outcomes | 1.6yNetDMFS increment ‐ (E+U)Reported at 1 and 1.6 years' follow‐up DMFT (E/U) Dropout | |
| Declaration of Interest | No information provided | |
| Funding | No information provided | |
| Notes | Clinical (VT) caries assessment by 2 examiners, diagnostic threshold NR. State of tooth eruption included = E/U. Diagnostic errors NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “...according to dental age...sex and previous caries experience of the children, they were randomly assigned to one of the two following study groups...” Comment: not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: “The control group rinsed with a placebo” “A monthly rinsing for the controls seemed to be a reasonable compromise. Because the examiners for this study had no part in administering treatments, a double‐blind method could be maintained strictly” Comment: use of placebo described |
| Blinding of outcome assessment (detection bias) | Low risk | Quotes: “The control group rinsed with a placebo” “A monthly rinsing for the controls seemed to be a reasonable compromise. Because the examiners for this study had no part in administering treatments, a double‐blind method could be maintained strictly” Comment: blind outcome assessment and use of placebo described |
| Incomplete outcome data (attrition bias) | High risk | Overall dropout for length of follow‐up: 45.41% in 1.6 years Comments: numbers lost unduly high, given length of follow‐up, with almost differential losses (51.31% FR, 42.11% PL). It is unclear whether reasons for missing outcome data are acceptable and balanced. Caries data used in the analysis pertain to participants present at all exams |
| Selective reporting (reporting bias) | Low risk | Outcomes reported DMFT (E/U) DMFS (U) Comment: trial protocol not available. All prespecified outcomes (in Methods) were reported in the prespecified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported DMFS: 6.97 FR, 6.48 PL DMFT: 3.59 FR, 3.44 PL Comment: initial caries appears balanced between groups |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
| Study characteristics | ||
| Methods | Study design: 2‐arm parallel‐group RCT, "placebo"‐controlled Study duration: 3 years | |
| Participants | Participants randomised: N = 217 167 children analysed at 3 years (present for entire trial period) Setting of recruitment and treatment: school | |
| Interventions | Comparison: FR vs 'PL' FR group: 0.5% NaF (2250 ppm F) 'PL' group: non‐F rinse (distilled water) School use/supervised, fortnightly (17 rinses/y), 10 mL applied for 2 minutes Before application: NR Postop instruction: NR | |
| Outcomes | 3yDFS increment ‐ (CA)(E)cl DFT Dropout | |
| Declaration of Interest | No information provided | |
| Funding | No information provided | |
| Notes | Clinical (VT) caries assessment by 1 examiner; diagnostic threshold = CA; radiographic assessment (2 postBW) used as an aid but not reported; state of tooth eruption included = E. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quotes: "The children were randomly assigned to test and control groups" “The children selected to be exposed to an experimental measure were divided into 2 groups by assigning every other child in the class register to one group; the remainder to the other group. In these alphabetical register the boys and the girls were entered separately. In this way, both groups comprised an equal number of boys and girls" Comment: not randomised. Alternation used to allocate into groups |
| Allocation concealment (selection bias) | Low risk | Comment: The non‐random method (alternation) used for sequence generation would not allow for allocation concealment. However, because every child in the class was assigned according to the ordering in the class register (alphabetically), lack of allocation concealment could not influence assignment of participants |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quotes: “In the present investigation, which was carried out with control groups,the double‐blind method was used” “The examiner did not know to which group the children belonged” "...fluoride solution in test group and distilled water in control group" “The terms test group and control group were never used, for it was not known until after the investigation which group was a test or a control group. The groups were therefore referred to as the ‘yellow’ one and the ‘green’ one” Comment: Effectiveness of distilled water as a placebo is unclear. Moreover, participants were assigned in alternation, which makes it easier to guess |
| Blinding of outcome assessment (detection bias) | Low risk | Quotes: “In the present investigation, which was carried out with control groups,.the double‐blind method was used” “The examiner did not know to which group the children belonged” “The terms test group and control group were never used, for it was not known until after the investigation which group was a test or a control group. The groups were therefore referred to as the ‘yellow’ one and the ‘green’ one” Comment: radiographic assessment used. Unclear whether examiners were effectively blinded but likely to be low risk |
| Incomplete outcome data (attrition bias) | Unclear risk | Overall dropout for length of follow‐up: 23.04% in 3 years Comment: numbers lost not unduly high, given length of follow‐up, and no differential loss evident between groups. It is unclear whether reasons for missing outcome data are acceptable and balanced. Caries data used in the analysis pertain to participants present throughout the trial |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported DFT O‐DFS MD‐DFS BL‐DFS CAR (annual) Secondary caries Comment: trial protocol available. Prespecified outcomes were reported. However side effects data were incomplete |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported DFS: 14.36(7.47) FR, 14.93(8.47) PL DFT: 9.38(4.15) FR, 9.45(4.26) PL SAR: 67.82(19.82) FR, 64.30(16.85) PL TAR: 9.06(3.60) FR, 8.41(2.99) PL Comment: initial caries appears balanced between groups. Other baseline characteristics (SAR, TAR) also balanced |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
| Study characteristics | ||
| Methods | Study design: 2‐arm parallel‐group RCT, "placebo"‐controlled Study duration: 3 years | |
| Participants | Participants randomised: N = 344 251 children analysed at 3 years (present for entire trial period) Setting of recruitment and treatment: school | |
| Interventions | Comparison: FR vs 'PL' FR group: 0.5% NaF (2250 ppm F) PL group: non‐F rinse (distilled water) School clinic/supervised, 3 times a year (3 rinses/y), 10 mL applied for 2 minutes Before application: NR Postop instruction: NR | |
| Outcomes | 3yDFS increment ‐ (CA)(E)cl DFT Dropout | |
| Declaration of Interest | No information provided | |
| Funding | No information provided | |
| Notes | Clinical (VT) caries assessment by 4 examiners; diagnostic threshold = CA; radiographic assessment (2 postBW) used as an aid but not reported; state of tooth eruption included = E. Diagnostic errors NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quotes: "The children were randomly assigned to test and control groups" “The children selected to be exposed to an experimental measure were divided into 2 groups by assigning every other child in the class register to one group; the remainder to the other group. In these alphabetical register the boys and the girls were entered separately. In this way, both groups comprised an equal number of boys and girls" "The terms test group and control group were never used, for it was not known until after the investigation which group was a test or a control group. The groups were therefore referred to as the ‘yellow’ one and the ‘green’ one” Comment: not randomised. Alternation used to allocate into groups |
| Allocation concealment (selection bias) | Low risk | Comment: The non‐random method (alternation) used for sequence generation would not allow for allocation concealment. However, because each child in the class was assigned according to the order in the class register (alphabetically), lack of allocation concealment could not influence assignment of participants |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quotes: “In the present investigation, which was carried out with control groups, the double‐blind method was used” “The examiner did not know to which group the children belonged” "...fluoride solution in test group and distilled water in control group" “The terms test group and control group were never used, for it was not known until after the investigation which group was a test or a control group. The groups were therefore referred to as the ‘yellow’ one and the ‘green’ one” Comment: use of placebo described |
| Blinding of outcome assessment (detection bias) | Low risk | Quotes: “In the present investigation, which was carried out with control groups,.the double‐blind method was used” “The examiner did not know to which group the children belonged” “The terms test group and control group were never used, for it was not known until after the investigation which group was a test or a control group. The groups were therefore referred to as the ‘yellow’ one and the ‘green’ one” Comment: radiographic assessment used. Unclear whether examiners were effectively blinded but likely to be low risk |
| Incomplete outcome data (attrition bias) | High risk | Overall dropout for length of follow‐up: 27.03% in 3 years Comment: Numbers lost were not high, given length of follow‐up, although differential losses evident between groups. It is unclear whether reasons for missing outcome data are acceptable and balanced. Caries data used in the analysis pertain to participants present throughout the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes reported DFT CAR (annual) Secondary caries Comment: trial protocol available. All prespecified outcomes were reported in the prespecified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported DFS: 5.52(3.14) FR, 5.63(3.12) PL DFT: 3.40(1.62) FR, 3.64(1.85) PL SAR: 32.45(10.39) FR, 33.34(11.23) PL TAR: 5.15(2.27) FR, 5.16(2.66) PL Comment: initial caries appears balanced between groups. Other baseline characteristics (SAR, TAR) also balanced |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
| Study characteristics | ||
| Methods | Study design: 2‐arm parallel‐group RCT, "placebo"‐controlled Study duration: 2 years | |
| Participants | Participants randomised: N = 392 251 children analysed at 2 years (present for entire trial period) Setting of recruitment and treatment: school | |
| Interventions | Comparison: FR vs 'PL' FR group: 0.05% NaF (230 ppm F) PL group: non‐F rinse (tap water) School clinic/supervised, 3 times a year (3 rinses/y), 10 mL applied for 2 minutes Before application: NR Postop instruction: NR | |
| Outcomes | 2yDFS increment ‐ (CA)(E)cl DFT Dropout | |
| Declaration of Interest | No information provided | |
| Funding | No information provided | |
| Notes | Clinical (VT) caries assessment by 2 examiners; diagnostic threshold = CA; radiographic assessment (2 postBW) used as an aid but not reported; state of tooth eruption included = E. Diagnostic errors NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quotes: "The children were randomly assigned to test and control groups" “The children selected to be exposed to an experimental measure were divided into 2 groups by assigning every other child in the class register to one group; the remainder to the other group. In these alphabetical register the boys and the girls were entered separately. In this way, both groups comprised an equal number of boys and girls" "The terms test group and control group were never used, for it was not known until after the investigation which group was a test or a control group. The groups were therefore referred to as the ‘yellow’ one and the ‘green’ one” Comment: not randomised. Alternation used to allocate into groups |
| Allocation concealment (selection bias) | Low risk | Comment: The non‐random method (alternation) used for sequence generation would not allow for allocation concealment. However, because each child in the class was assigned according to ordering in the class register (alphabetically), lack of allocation concealment could not influence assignment of participants |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quotes: “In the present investigation, which was carried out with control groups, the double‐blind method was used” “The examiner did not know to which group the children belonged” "...fluoride solution for test group and tap water for control group" “The terms test group and control group were never used, for it was not known until after the investigation which group was a test or a control group. The groups were therefore referred to as the ‘yellow’ one and the ‘green’ one” Comment: use of placebo described |
| Blinding of outcome assessment (detection bias) | Low risk | Quotes: “In the present investigation, which was carried out with control groups, the double‐blind method was used” “The examiner did not know to which group the children belonged” “The terms test group and control group were never used, for it was not known until after the investigation which group was a test or a control group. The groups were therefore referred to as the ‘yellow’ one and the ‘green’ one” Comment: radiographic assessment used. Unclear whether examiners were effectively blinded but likely to be low risk |
| Incomplete outcome data (attrition bias) | High risk | Overall dropout for length of follow‐up: 35.97% in 2 years Comment: Numbers lost were high, given length of follow‐up, and showed differential losses between groups. It is unclear whether reasons for missing outcome data are acceptable and balanced. Caries data used in the analysis pertain to participants present throughout the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes reported DFT CAR (annual) Secondary caries Comment: trial protocol available. All prespecified outcomes were reported in the prespecified way |
| Baseline characteristics balanced? | Unclear risk | Prognostic factors reported DFS: 6.89(3.10) FR, 7.01(3.63) PL DFT: 4.82(1.71) FR, 4.86(2.11) PL SAR: 51.75(13.88) FR, 53.20(16.04) PL TAR: 8.54(2.88) FR, 8.85(3.29) PL Comment: initial caries appears balanced between groups. Other baseline characteristics (SAR, TAR) also balanced |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
| Study characteristics | ||
| Methods | Study design: 3‐arm parallel‐group RCT, placebo‐controlled Study duration: 2.4 years | |
| Participants | Participants randomised: N = 575 343 children analysed at 2.4 years (after exclusions, present for entire trial period) Setting of recruitment and treatment: school | |
| Interventions | FR (2 groups) vs PL APF group 1: 200 ppm F, daily (160 rinses/y) School use/supervised Before application: NR Postop instruction: NR | |
| Outcomes | 2.4yDFS increment ‐ (E+U) DMFS (U) Dropout | |
| Declaration of Interest | No information provided | |
| Funding | No information provided | |
| Notes | Clinical (VT) caries assessment by 2 examiners, diagnostic threshold = CA. State of tooth eruption included = E/U. Diagnostic errors NR (results from only 1 examiner reported) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The subjects were randomly assigned to three groups...” Comment: not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “One group received a daily placebo mouthwash...” Comment: use of placebo described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quotes: "The examinations were accomplished by 2 examiners working independently" “One group received a daily placebo mouthwash...” Comment: use of placebo described, but It is unclear whether examiners were blinded, although examinations were done independently |
| Incomplete outcome data (attrition bias) | High risk | Overall dropout for length of follow‐up: 40.35% in 2.4 years Comment: numbers lost unduly high for length of follow‐up with no differential loss between groups. It is unclear whether reasons for missing outcome data are balanced, and they may not be acceptable. Caries data used in the analysis pertain to participants present at all exams with more than 75% compliance |
| Selective reporting (reporting bias) | Low risk | Outcomes reported DMFS (U) Comment: trial protocol not available. All prespecified outcomes (in Methods) were reported in the prespecified way |
| Baseline characteristics balanced? | Unclear risk | Prognostic factors reported DMFS: 2.57 FR1, 3.25 FR2, 3.20 PL Age: 8.7 FR1, 8.6 FR2, 8.5 PL Comment: initial caries appears balanced between groups. Age also balanced |
| Free of contamination/co‐intervention? | Low risk | Non‐fluoride toothpaste provided to all for home use (no rinse provided) |
| Study characteristics | ||
| Methods | Study design: 3‐arm parallel‐group RCT, placebo‐controlled Study duration: 2 years (+ 1 year post‐intervention period) | |
| Participants | Participants randomised: N = 1202; numbers randomized to each group NR 743 children analysed at 2 years (available at final examination) Surfaces affected at start: 6.2 DFS Setting of recruitment and treatment: school | |
| Interventions | FR (2 groups) vs PL FR group 2: 0.04% SnF2 = 100 ppm F PL group: non‐F rinse School use/supervised, daily (160 rinses/y), 20 mL applied in 2 successive rinses 30 seconds each Before application: NR Postop instruction: NR | |
| Outcomes | 2yNetDFS increment ‐ (E+U)cl+xr DMFS Children with tooth staining/pigmentation, lack of acceptance of the taste, side effects (incomplete data) | |
| Declaration of Interest | No information provided | |
| Funding | The study was supported by a grant from the Warner‐Lambert Company | |
| Notes | Clinical (VT) caries assessment by 2 examiners, diagnostic threshold NR. Radiographic assessment (postBW) by 2 examiners; diagnostic threshold NR. State of tooth eruption included = E/U. Diagnostic errors NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “They were divided by basis of random numbers into three groups selected in such a manner that the sex, age and previous caries experience of each group were closely similar” Comment: not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: “Two of the groups rinsed with the two strengths of the solution and the third rinsed with a placebo” “The three tablets...resembled each other in colour and taste” “The status of each group was not known to anyone actively involved in the study” Comment: use of placebo described |
| Blinding of outcome assessment (detection bias) | Low risk | Quotes: “Two of the groups rinsed with the two strengths of the solution and the third rinsed with a placebo” “The three tablets dissolved in cups...resembled each other in colour and taste” “The status of each group was not known to anyone actively involved in the study” Comment: blind outcome assessment and use of placebo described |
| Incomplete outcome data (attrition bias) | High risk | Overall dropout for length of follow‐up: 38.19% in 2 years Comment: numbers lost unduly high for length of follow‐up. Differential losses not assessable. It is unclear whether reasons for losses are balanced, and they may not be acceptable. Caries data used in the analysis pertain to participants present at final examination |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported DMFS DMFT Increments standardised to 28 teeth and 122 surfaces (E/U) Children with tooth staining/pigmentation, lack of acceptance of the taste, side effects (incomplete data) Comment: trial protocol not available. Prespecified outcomes were reported. However side effects data were incomplete |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported DFS: 6.19 FR1, 6.39 FR2, 6.12 PL DMFT: 3.50 FR1, 3.67 FR2, 3.55 PL SAR: 63.59 FR1, 63.54 FR2, 62.73 PL TAR: 13.53 FR1, 13.45 FR2, 13.32 PL Comment: initial caries appears balanced. Other baseline characteristics (SAR, TAR, age) also balanced |
| Free of contamination/co‐intervention? | Low risk | Non‐fluoride toothpaste provided to all for home use (no rinse provided) |
| Study characteristics | ||
| Methods | Study design: 5‐arm parallel‐group RCT (quasi), non‐placebo‐controlled Study duration: 3 years | |
| Participants | Year study began: 1999 Location: Sweden, 1 city Setting of recruitment and treatment: school Numbers randomised: 788 children ("randomly selected") Numbers analysed: 622 children at 3 years (after exclusions, present for both examinations) Age: all 13 years old Surfaces affected: 1.6 MD‐DFS (SD = 2.8) Background exposure to other fluoride: yes (100% reported F toothpaste used twice a day, 100% reported F varnish applied annually at checkups, but no F in water – “0.1 ppm F”) | |
| Interventions | Comparison: FR (4 groups) vs NT FR group 1: 0.2% NaF, 900 ppm F, 6 rinses/y (initial 3 school days every semester) FR group 2: 0.2% NaF, 900 ppm F, 12 rinses/y (initial 3 and last 3 school days every semester) FR group 3: 0..2% NaF, 900 ppm F, 27 rinses/y (3 consecutive school days every month) FR group 4: 0.2% NaF, 900 ppm F, 20 rinses/y (2 school days (fortnightly) during semesters) NT group: no intervention School use/supervised, 20 mL applied for 1 minute Before application: no toothbrushing before rinsing Postop instruction: Refrain from eating and drinking for 1 hour afterwards | |
| Outcomes | 3‐year postMD‐DFS incidence ‐ (E)(DR/ER)xr Reported at 3 years' follow‐up DS FS Caries progression Dropout | |
| Declaration of Interest | No information provided | |
| Funding | Supported by Swedish Patent Revenue Fund for Research in Preventive Dentistry and the Sigge Perssons & Alice Nybergs Foundation | |
| Notes | Radiographic caries assessment (4 postBW) by 2 examiners; diagnostic threshold = DR and ER; intraexaminer K statistics/kappa values ‐ 0.94 and 0.88 for all scores and for carious surfaces scores only, respectively, interexaminer values NR. State of tooth eruption included = E | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "... Adolescents of five different secondary schools in Mölndal were randomised into five different groups (every school included had five classes within the age group)" Comment: method unclear, quasi‐method likely |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Group 5 (control group) did not rinse" Comment: no placebo described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Two of the authors (E.B. and U.M.S.) read the radiographs simultaneously, using a Comment: blind outcome assessment reported, but no placebo described |
| Incomplete outcome data (attrition bias) | High risk | Overall dropout for length of follow‐up: 166/788 (21%) in 3 years [but 88/788 (11%) in 3 years if no exclusions were performed based on compliance with intervention*] *78 participants were not included in the analysis, on a 'non‐adherence' basis, because they rinsed less than stipulated = 62, or refused to rinse = 16); it is not clear if they had the 3‐year follow‐up examination Comment: numbers lost high for length of follow‐up (FR 4), differential losses between NT and FR groups and among FR groups. Caries data used in analysis pertain to participants present at initial and final examinations |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported Comment: trial protocol not available. All prespecified outcomes (in Methods) were reported; however, although caries prevalence data are fully reported by group (at varying levels of diagnosis) at baseline and at follow‐up, not all caries incidence/increment data are fully reported/tabulated by group and diagnostic threshold |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported Post MD‐DFS (MD‐DFSa+DeS) = 1.68 FR1, 1.44 FR2, 1.79 FR3, 1.75 FR4, 1.45 NT MD‐DS, MD‐FS Comment: initial caries appears balanced between groups |
| Free of contamination/co‐intervention? | Low risk | Quote: "All participants attended dental clinics for regular check‐ups once a year and they were given prophylactic treatment. ...It is custom in Sweden’s dental clinics to treat all children and adolescents with F varnish at their yearly check‐ups and it is standard to brush one’s teeth with F toothpaste twice a day" Comment: no indication of inadvertent application of the intervention to people in the control group (no apparent contamination) or of any additional treatment given to 1 of the groups differentially (no risk of co‐intervention) |
| Study characteristics | ||
| Methods | Study design: 2‐arm parallel‐group RCT, placebo‐controlled Study duration: 2.5 years | |
| Participants | Participants: N= 767 295 children analysed at 2.5 years (available at final examination) Setting of recruitment and treatment: school | |
| Interventions | Comparison: FR vs PL PL group (n = 150): non‐F rinse (no details described) School use/supervised, applied weekly (30 rinses/y) Before application: NR Postop instruction: NR | |
| Outcomes | 2.5yDMFS increment DMFT Dropout | |
| Declaration of Interest | No information provided | |
| Funding | The investigation was financed by the Faculty of Dentistry University of Chile, Laboratorio Chile, Indus Lever and Manufacturas de Cepillos Duralon Ltd. | |
| Notes | Clinical (VT) caries assessment, diagnostic threshold NR. State of tooth eruption included NR. Consistency of diagnosis assessed by duplicate examinations annually. Diagnostic errors NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from translation: “In each school, children were divided at random by the statisticians...” Comment: A random method was likely used |
| Allocation concealment (selection bias) | Unclear risk | Method was not specified |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes from translation: “The study was conducted double‐blind” “..and placebo for the control group” Blind outcome assessment and use of placebo described |
| Blinding of outcome assessment (detection bias) | Low risk | Quotes from translation: “The study was conducted double‐blind” “..and placebo for the control group” Blind outcome assessment and use of placebo described |
| Incomplete outcome data (attrition bias) | High risk | Overall dropout for length of follow‐up: 61.54% in 2.5 years Comment: numbers lost very high, although no differential loss evident between groups (dropout by group: 225/370 FR, 247/397 PL). Caries data used in analysis pertain to participants present at final examinations |
| Selective reporting (reporting bias) | Low risk | Outcomes reported DMFT Comment: trial protocol not available. All prespecified outcomes (in Methods) were reported in the prespecified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported DMFS: 4.38 FR, 4.22 PL DMFT: 2.93 FR, 2.72 PL Comment: initial caries appears balanced between groups |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
| Study characteristics | ||
| Methods | Study design: 5‐arm parallel‐group quasi‐RCT (only 4 relevant arms used, the NT control group not used), "placebo"‐controlled Study duration: 2 years | |
| Participants | Participants randomised (N = 330) 200 children analysed at 2 years (after exclusions, available at final examination) Setting of recruitment and treatment: school | |
| Interventions | Comparison: FR (3 groups) vs 'PL' FR group 1: 0.1% NaF, 450 ppm F, 3 times a week (80 rinses/y) 'PL' group: tap water, 3 times a week (80 rinses/y) School use/supervised, 25 mL applied for 30 seconds Before application: Rinsing with water (tap = drinking water) was carried out first, in all 4 groups, for 30 seconds (followed by another rinse with water in the 'PL' group and rinse with F solution in the treatment groups, as described above) Postop instruction: NR | |
| Outcomes | 2yDMFS increment Dropout | |
| Declaration of Interest | No information provided | |
| Funding | No information provided | |
| Notes | Clinical (VT) caries assessment, diagnostic threshold NR. State of tooth eruption included NR. Diagnostic errors NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote from translation: “For this study, we constituted a control group and four experimental groups numbered 1 to 4, taking into consideration: approximate numbers of children of school age, previous experience of caries and permanent teeth erupted” Comment: not enough information provided Quote from correspondence: “In order to obtain 'homogeneous' groups, children were ordered and pre‐stratified by gender, age, number of permanent teeth present, and by level of DMF, and in this way each one of the groups was formed” |
| Allocation concealment (selection bias) | High risk | Comment: no concealment of allocation indicated/likely |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quotes: “Group V‐ children who rinsed with clean water, three times a week” “...study was conducted double‐blind..." Comment: double‐blinding and use of 'placebo' reported, but methods not described. It was unclear whether the 'placebo' could be distinguished from the active treatment |
| Blinding of outcome assessment (detection bias) | Low risk | Quote from correspondence: "The researcher/examiner did not know to which group the children belonged, and the children were also blind to group assignment" Comment: likely to be low risk because blind outcome assessment and use of 'placebo' described |
| Incomplete outcome data (attrition bias) | High risk | Overall dropout for length of follow‐up: 39.02% (130/330) in 2 years Comment: Numbers lost were high, given length of follow‐up, and it is unclear whether differential losses were noted between groups (because the numbers above were produced after 'statistical' exclusions to keep groups of equal sizes). Reason for missing outcome data is unacceptable. Caries data used in analysis pertain to participants present at final examination (after exclusions) |
| Selective reporting (reporting bias) | Low risk | Outcomes reported Comment: trial protocol not available. All prespecified outcomes (in Methods) were reported in the prespecified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported DMFS: 4.58 FR1, 4.60 FR2, 4.62 FR3, 4.66 ‘PL’ Age: 7 FR1, 7 FR2, 7 FR3, 7 ‘PL’ Dental age: 8.1 FR1, 8.1 FR2, 8.3 FR3, 8.3 ‘PL’ Comment: initial caries appears balanced between groups. Other baseline characteristics (dental age, age) also balanced |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
| Study characteristics | ||
| Methods | Study design: 2‐arm parallel‐group RCT (quasi), non‐placebo‐controlled | |
| Participants | Participants randomised: N = 230 164 children analysed at 2.5 years (available at final examination) Setting of recruitment and treatment: school | |
| Interventions | Comparison: FR vs NT FR group: 0.2% NaF (900 ppm F) NT group: no intervention School use/supervised, weekly (30 rinses/y), 20 mL applied, for 30 seconds Before application: rinsing with drinking water for 30 seconds Postop instruction: no eating or drinking for 30 minutes | |
| Outcomes | 2.5yDMFS increment CAR Dropout | |
| Declaration of Interest | No information provided | |
| Funding | No information provided | |
| Notes | Clinical (VT) caries assessment by 1 examiner, diagnostic threshold NR. State of tooth eruption included NR. Diagnostic errors NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote from translation: “...children were divided at random into 2 groups” Comment: not enough information provided Quote from correspondence: “In order to obtain 'homogeneous' groups, children were ordered and pre‐stratified by gender, age, number of permanent teeth present, and by level of DMF, and then, they were distributed 'at random', to form each one of the groups” |
| Allocation concealment (selection bias) | High risk | Quote from correspondence: “In order to obtain 'homogeneous' groups, children were ordered and pre‐stratified by gender, age, number of permanent teeth present, and by level of DMF, and then, they were distributed 'at random', to form each one of the groups” Comment: no concealment of allocation indicated/likely |
| Blinding of participants and personnel (performance bias) | High risk | Quote from translation: “... received no treatment and served as control” Comment: no placebo used |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quotes from translation: “... received no treatment and served as control” “The clinical examinations were performed by a single examiner without prior knowledge whether the child belonged to the experimental group or control” Comment: blind outcome assessment described, but no placebo used |
| Incomplete outcome data (attrition bias) | High risk | Overall dropout for length of follow‐up: 28.7% (66/230) in 2.5 years Comment: Numbers lost were not high for length of follow‐up but showed differential loss between groups (36.52% FR, 20.87% NT). It is unclear whether reasons for missing data are acceptable. Caries data used in analysis pertain to participants present at final examinations |
| Selective reporting (reporting bias) | Low risk | Outcomes reported CAR Comment: trial protocol not available. All prespecified outcomes (in Methods) were reported in the prespecified way |
| Baseline characteristics balanced? | Low risk | Prognostics factors reported DMFS: 1.4(1.61) FR, 1.4(1.72) NT TAR: 8.3 FR, 8.3 NT Dental age: 9.6 FR, 9.5 NT Comment: initial caries appears balanced between groups. Dental age, TAR also balanced |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
| Study characteristics | ||
| Methods | Study design: 3‐arm parallel‐group RCT, placebo controlled Study duration: 2.4 years | |
| Participants | Participants randomised: N = 464 285 children analysed at 2.4 years (after exclusions, present for entire trial period) Setting of recruitment and treatment: school | |
| Interventions | FR (2 groups) vs PL APF group 1: 200 ppm F, daily (160 rinses/y) School use/supervised Before application: NR Postop instruction: NR | |
| Outcomes | 2.4yNetDMFS increment ‐ (CA) (E+U) DMFS (U) Dropout | |
| Declaration of Interest | No information provided | |
| Funding | No information provided | |
| Notes | Clinical (VT) caries assessment by 2 examiners, diagnostic threshold = CA. State of tooth eruption included = E/U. Diagnostic errors NR (results from only 1 examiner reported) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The subjects were randomly assigned into three groups...” Comment: not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “One group received a daily placebo mouthwash...” Comment: use of placebo described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quotes: "The examinations were accomplished by 2 examiners working independently" “One group received a daily placebo mouthwash...” Comment: use of placebo described, but It is unclear whether examiners were blinded, although examinations were done independently |
| Incomplete outcome data (attrition bias) | High risk | Overall dropout for length of follow‐up: 38.58% in 2.4 years Comment: numbers lost unduly high for length of follow‐up, with some differential loss between groups (43.66% FR1, 34.15% FR2, 38.61% PL). It is unclear whether reasons for missing outcome data are balanced, and they may not be acceptable. Caries data used in the analysis pertain to participants present at all exams with more than 75% compliance |
| Selective reporting (reporting bias) | Low risk | Outcomes reported DMFS (U) Comment: trial protocol not available. All prespecified outcomes (in Methods) were reported in the prespecified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported DMFS: 6.47(4.65) FR1, 6.80(4.60) FR2 6.48(4.98) PL Age: 8.7 FR1, 8.6 FR2, 8.6 PL Comment: initial caries appears balanced between groups. Age also balanced |
| Free of contamination/co‐intervention? | Low risk | Non‐fluoride toothpaste provided to all for home use (no rinse provided) |
| Study characteristics | ||
| Methods | Study design: 2‐arm parallel‐group RCT, "placebo"‐controlled Study duration: 3 years | |
| Participants | Participants randomised: numbers NR nor obtainable 139 children analysed at 3 years Setting of recruitment and treatment: school | |
| Interventions | Comparison: FR vs 'PL' 'PL' group (n = 70): tap water (no F = 0.01 ppm F) School use/supervised, for 3 days every 6 months (6 rinses/y), 10 mL applied Before application: NR Postop instruction: NR | |
| Outcomes | 3ypostMD‐DFS increment ‐ (DR/ER)xr | |
| Declaration of Interest | No information provided | |
| Funding | The study was supported by the County Council of Halland, Sweden | |
| Notes | Radiographic assessment (4 postBW) by 1 examiner; diagnostic threshold = DR and ER. Diagnostic errors NR. State of tooth eruption included NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotes: “A test group was randomly sampled...” “...school children were sampled into two groups...” Comment: not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quotes: “...In the control group, the children rinsed with tap water...” “The study was designed so that the subjects did not know whether their rinsing solution contained fluoride or not” "The same prophylactic information was given to the teenagers during the rinsing procedures in both groups, and the same staff members.. organised the rinsing procedures in the test as well as control groups through the whole study periods" Comment: use of ‘placebo’ described (no description of whether the mouthrinse is identical in appearance or taste to tap water. Staff did not seem to be blinded) |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "the detection and recording of caries and filled surfaces from the bitewing radiographs were performed by one of the authors who was specially trained for the purposed and did not know the origin of the radiographs analysed" Comment: likely to be low risk because blind outcome assessment and use of ‘placebo’ described |
| Incomplete outcome data (attrition bias) | Unclear risk | Numbers randomised not reported. Dropout rate NR nor obtainable. Reasons for attrition NR. Any differential group losses not assessable |
| Selective reporting (reporting bias) | Low risk | Outcomes reported Comment: trial protocol not available. All prespecified outcomes (in Methods) were reported in the prespecified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported PostMDDFS: 1.35 (1.58) FR, 1.16 (1.55) ‘PL’ Comment: initial caries appears balanced between groups |
| Free of contamination/co‐intervention? | Low risk | Quote: “Similar preventive programs were applied to the two groups during the experimental period” Comment: sufficient indication of overall prevention of contamination/co‐intervention |
| Study characteristics | ||
| Methods | Study design: 2‐arm parallel‐group RCT, placebo‐controlled Study duration: 3 years | |
| Participants | Participants randomised: N = 398 Number analysed: 365 children analysed at 3 years (available at final examination) Setting of recruitment and treatment: school | |
| Interventions | Comparison: FR vs PL PL group (n = 191): water, with flavouring solution added School use/supervised, fortnightly (19 rinses/y), 10 mL applied Before application: NR Postop instruction: NR | |
| Outcomes | 3yNetDMFS increment ‐ (CA)(E)cl DMFS (U) Dropout | |
| Declaration of Interest | No information provided | |
| Funding | Supported by a grant from Colgate Palmolive Inc., Copenhagen | |
| Notes | Clinical (VT) caries assessment by dentists at public dental service, diagnostic threshold = CA. Radiographic assessment (2 postBW) by 1 examiner; diagnostic threshold = DR. State of tooth eruption included (E/U). Reproducibility of diagnosis assessed by duplicate radiographic examination of 10% random sample (kappa value 0.72) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The children were stratified according to school and age and subsequently randomly allocated to two groups” Quote from correspondence: “The method of randomisation is not mentioned in the protocol” Comment: not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: "...flavouring solution .... were added" “Both placebo and fluoride solutions were poured into small plastic cups at the dental school and each cup labelled with the child’s name, school and grade” Comment: adequate efforts to ensure that water was an effective placebo, and steps taken to ensure blinding; use of a placebo described |
| Blinding of outcome assessment (detection bias) | Low risk | Quotes: “....the examiners .... did not know to which group the children belonged" Comment: Examiner did not know treatment assignment; definitions and objective outcome measures used (bitewing radiographs) Comment: blind outcome assessment and use of a placebo described |
| Incomplete outcome data (attrition bias) | Low risk | Overall dropout for length of follow‐up: 8.29% in 3 years Comment: numbers lost not unduly high, given length of follow‐up, with no differential losses between groups. It is unclear whether reasons for missing outcome data are acceptable and balanced. Caries data used in the analysis pertain to participants who completed the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes reported DMFS (U) O‐DMFS MD‐DMFS BL‐DMFS PostMDDMFS Comment: trial protocol not available. All prespecified outcomes (in Methods) were reported in the prespecified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported DMFS: 3.56 (2.92) FR, 3.7 (2.49) PL Mean age (months): 106.66(10.52) FR, 108.43(10.70) PL Erupted surfaces: 56.86(17.66) FR, 57.34(15.86) PL Comment: initial caries appears balanced between groups. Other baseline characteristics (erupted surfaces, age) also balanced |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
| Study characteristics | ||
| Methods | Study design: 2‐arm parallel‐group RCT, placebo‐controlled Study duration: 2 school years (1.6 years) | |
| Participants | Participants randomised: N = 890 726 children analysed at 1.6 years (available at final examination) Setting of recruitment and treatment: school | |
| Interventions | Comparison: FR vs PL FR group: 0.1% SnF2, 240 ppm F PL group: non‐F rinse School use/supervised, daily (160 rinses/y), 60 mL applied in 3 successive rinses of 10, 30 and 30 seconds each Before application: NR Postop instruction: NR | |
| Outcomes | 1.6yDMFS increment ‐ cl+xr DMFT Children with tooth staining/pigmentation (incomplete data) Dropout | |
| Declaration of Interest | No information provided | |
| Funding | Sponsors of the study were US Airforce School of Aerospace Medicine under contract no. F41609‐68‐C‐0025, and Procter and Gamble Co. | |
| Notes | Clinical (VT) caries assessment by 2 examiners, diagnostic threshold NR. Radiographic assessment (4 postBW) by 2 examiners; diagnostic threshold NR. State of tooth eruption included NR. Diagnostic errors NR. Results of 1 examiner chosen (findings of both examiners consistent throughout) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “At the time of the first examination, the children were grouped by sex, age...Within these groupings, adjacent subject entries were assigned to test or control groups by random permutations of two” Comment: block randomisation done |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: "Neither the participants nor the examiners were aware of the assignments throughout the test" Comment: use of placebo reported |
| Blinding of outcome assessment (detection bias) | Low risk | Quotes: "Neither the participants nor the examiners were aware of the assignments throughout the test" Comment: blind outcome assessment and use of placebo reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Overall dropout for length of follow‐up: 18.43% in 1.6 years Comment: numbers lost not unduly high, given length of follow‐up, with no differential losses evident between groups. It is unclear whether reasons for missing outcome data are acceptable and balanced. Caries data used in the analysis pertain to participants present at final examination |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported DMFT Children with tooth staining/pigmentation (incomplete data) Comment: trial protocol not available. Prespecified outcomes were reported. However side effects data were incomplete |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported DMFS: 4.90(4.03) FR, 4.80(4.51) PL DMFT: 3.22(2.18) FR, 3.06(2.47) PL Age: 10.38 FR, 10.39 PL Gender: 165 M 183 F (FR), 187 M, 191 F (PL) Comment: initial caries appears balanced between groups. Other baseline characteristics (age, gender) also balanced |
| Free of contamination/co‐intervention? | Low risk | Non‐fluoride toothpaste provided to all for home use (no rinse provided) |
| Study characteristics | ||
| Methods | Study design: 6‐arm parallel‐group RCT (4 relevant arms used), placebo‐controlled Study duration: 2.5 years | |
| Participants | Participants randomised: N = 878 527 children analysed at 2.5 years (available at final examination) Setting of recruitment and treatment: school | |
| Interventions | FR (2 groups) vs PL (2 groups) FR group 1: AmF 250 ppm F FR group 2: NaF 250 ppm F PL group 1: non‐F rinse PL group 2: non‐F rinse School use/supervised, daily (150 rinses/y), 10 mL applied for 1 minute Before application: NR Postop instruction: NR | |
| Outcomes | 2.5yNetDMFS increment ‐ (CA)cl + (DR)xr DMFT Stain score Dropout | |
| Declaration of Interest | No information provided | |
| Funding | Investigation supported by the US National Caries Program under contract no. N01‐DE‐32427 (product formulations by Procter and Gamble Co. and Menley and James Laboratories) | |
| Notes | Clinical (VT) caries assessment by 2 examiners, diagnostic threshold = CA. Radiographic assessment (5 BW) by 2 examiners; diagnostic threshold = DR. State of tooth eruption included NR. Reversal rate between 4% and 9% of observed caries increment in groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The baseline examinations were stratified by race and sex within each school, and ordered by increasing DMFT. Study group assignments were made by random permutations of seven within each stratum” |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “The placebo preparations were all fully formulated like their active fluoride ingredient, but did not have the specific active fluoride ingredient” Comment: use of placebo described |
| Blinding of outcome assessment (detection bias) | Low risk | Quotes: “A double‐blind design was used; neither examiner nor subjects were aware of the type of treatment received” Comment: blinded outcome assessment and use of placebo described |
| Incomplete outcome data (attrition bias) | High risk | Overall dropout for length of follow‐up: 39.98% in 2.5 years Comment: Numbers lost were high, given length of follow‐up, with differential losses evident between groups: 44.71% FR1, 38.06% FR2, 37.42% PL1, 36.91% PL2 Reasons for missing outcome data are not reported. Caries data used in the analysis pertain to participants at final exam |
| Selective reporting (reporting bias) | Low risk | Outcomes reported DMFS increment ‐ (CA) cl + (DR) xr, reported at 2.5 years' follow‐up DMFT Stain score Comment: trial protocol not available. All prespecified outcomes (in Methods) were reported in the prespecified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported DMFS: 3.90(0.34) FR1, 4.30(0.41) PL1,4.36(0.43) FR2, 4.95(0.54) PL2 DMFT: 2.30(0.17) FR1, 2.49(0.20) PL1,2.36(0.20) FR2, 2.72(0.28) PL2 Comment: initial caries appears slightly imbalanced. |
| Free of contamination/co‐intervention? | Low risk | Non‐fluoride toothpaste provided to all for home use (no rinse provided) |
| Study characteristics | ||
| Methods | Study design: 5‐arm parallel‐group RCT, placebo‐controlled Study duration: 2 years | |
| Participants | Participants randomised: N = 2014 1238 children analysed at 2 years (available at final examination) Setting of recruitment and treatment: school | |
| Interventions | FR (4 groups) vs PL NaF group 1: 230 ppm F, daily (160 rinses/y) School use/supervised, 10 mL applied for 1 minute Before application: NR Postop instruction: NR | |
| Outcomes | 2yNetDMFS increment Reported at 1.5 and 2.5 years' follow‐up PostMD‐DFS Dropout | |
| Declaration of Interest | No information provided | |
| Funding | No information provided | |
| Notes | Clinical (VT) caries assessment by 2 examiners, diagnostic threshold NR. Radiographic assessment by 2 examiners; diagnostic threshold NR. State of tooth eruption included NR. Diagnostic errors NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The participants were then allocated to study groups by random permutations of five after stratification by sex and race within each school...” Comment: not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: “Group C rinsed weekly with a placebo solution containing 0.1% NaCl” “The examiners were not aware of group assignments and did not consult baseline findings during the incremental exam” Comment: use of placebo described |
| Blinding of outcome assessment (detection bias) | Low risk | Quotes: “Group C rinsed weekly with a placebo solution containing 0.1% NaCl” “The examiners were not aware of group assignments and did not consult baseline findings during the incremental exam” Comment: blind outcome assessment and use of placebo described |
| Incomplete outcome data (attrition bias) | High risk | Overall dropout for length of follow‐up: 38.53% in 2 years Comment: Numbers lost were unduly high, given length of follow‐up, with no differential loss evident between groups [44.18%(FR1), 38.01%(FR2), 38.53%(FR3), 36.27%(FR4), 35.16%(PL)]. Reasons for missing outcome data are acceptable. Caries data used in analysis pertain to participants present at final examinations |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported PosMD‐DFS Comment: trial protocol not available. All prespecified outcomes (in Methods) were reported in the prespecified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported DMFS: 4.71 FR1, 5.17 FR2, 4.75 FR3, 4.11 FR4, 4.93 PL Comment: Initial caries shows some imbalance, but adjustment made no difference in results ‐ “A covariance analysis utilizing baseline as the covariant, however failed to change the results of the tests” |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
| Study characteristics | ||
| Methods | Study design: 2‐arm parallel‐group RCT, placebo‐controlled Study duration: 2 school years (1.6 years) | |
| Participants | Participants randomised: N = 491 434 children analysed at 3 years (available at final examination) Surfaces affected at start: 8.8 DMFS Setting of recruitment and treatment: school | |
| Interventions | Comparison: FR vs PL FR group: 0.05% NaF (230 ppm F) PL group: non‐F rinse School use/supervised, daily (160 rinses/y), 7.5 mL applied for 2 minutes Before application: NR Postop instruction: NR | |
| Outcomes | 3yNetDMFS increment ‐ (E+U)(CA)cl+(DR)xr DMFT (E/U) Signs of sensitivity in oral mucosa Dropout | |
| Declaration of Interest | No information provided | |
| Funding | The project was financed by a grant from Colgate‐Palmolive Ltd. | |
| Notes | Clinical (V) caries assessment by 1 examiner, diagnostic threshold = CA/NCA. Radiographic assessment (2postBW) by 1 examiner; diagnostic threshold = ER. State of tooth eruption included = E/U. Intraexaminer reproducibility checks for incremental caries data in 10% sample (ICC score 0.9 for DMFS) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quotes: “248 were allocated to the test and 243 to the control group” “Control and test subjects were arranged randomly within the same school classes” “The distribution of subjects into test and control groups was undertaken using stratified random sampling” Comment: not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: “The trials was organised on a double‐blind basis, neither the subjects not the investigators being aware who was receiving test or control rinses” “...the control rinse was similar in taste and appearance to test rinse except for the omission of sodium fluoride” Comment: use of placebo described |
| Blinding of outcome assessment (detection bias) | Low risk | Quotes: “The trials was organised on a double‐blind basis, neither the subjects not the investigators being aware who was receiving test or control rinses” “...the control rinse was similar except for the omission of sodium fluoride” Comment: blinded outcome assessment and use of placebo described |
| Incomplete outcome data (attrition bias) | Unclear risk | Overall dropout for length of follow‐up: 11.6% in 3 years Comment: numbers lost not high, given length of follow‐up, with no differential loss evident between groups. It is unclear whether reasons for missing outcome data are balanced between groups. Caries data used in the analysis pertain to participants present at the final examination |
| Selective reporting (reporting bias) | Low risk | Outcomes reported DMFT (E/U) PF‐DMFS FS‐DMFS Comment: trial protocol not available. All prespecified outcomes (in Methods) were reported in the prespecified way |
| Baseline characteristics balanced? | Unclear risk | Prognostic factors reported DMFS: 8.74(5.49) FR, 8.88(5.44) PL DMFT: 5.55(3.04) FR, 5.58(3.06) PL Gender: 123 M, 99 F (FR), 121 M, 91 F (PL) Fluoride dentifrice use: 6 FR, 8 PL Comment: initial caries appears balanced between groups. Other baseline characteristics (gender, exposure to fluoride toothpaste) also balanced |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
| Study characteristics | ||
| Methods | Study design: 2‐arm cluster‐randomised trial, non‐placebo‐controlled Study duration: 3 years | |
| Participants | Number randomised: 501 children were "examined at baseline", 29 schools were randomised, number of children per group NR 207 children analysed at 3 years (present at final examination, for which readable x‐rays were available) Surfaces affected at start: 2.7 DFS Setting of recruitment and treatment: elementary schools, The Hague | |
| Interventions | Comparison: FR vs NT NT group: no intervention School use/supervised, weekly (30 rinses/y), 10 mL applied for 1 minute Before application: NR Postop instruction: NR | |
| Outcomes | 3yNetDFS increment (mean converted from median) ‐ (CA/NCA)cl+(DR/ER)xr | |
| Declaration of Interest | No information provided | |
| Funding | Supported by a grant from Het Praeventiefonds | |
| Notes | Clinical (V) caries assessment by 2 examiners; diagnostic threshold = CA/NCA; state of tooth eruption included NR. Radiographic assessment (2 postBW) by 2 examiners; diagnostic threshold = DR/ER; partial recording. Diagnostic errors NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “A sample of 29 schools stratified according to SES and randomly assigned to two groups was selected” Comment: not enough information provided about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information about allocation concealment |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "One group of schools (14) performed rinsing and the other group (15) served as controls" Comment: Control group had no treatment. No placebo described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "The radiographs were interpreted by the same investigators without reference to the clinical examination data" Comment: Clinical and radiographic exams were done independently. Randomisation was by school. It was unclear whether examiners would have known which assignment/school the radiographs were from. Blinded outcome assessment indicated but no placebo described |
| Incomplete outcome data (attrition bias) | High risk | Overall drop‐out for length of follow‐up (reported for individuals within clusters only): 58.7% (207/501) in 3 years Comment: unclear whether recruitment of children was done before clusters (schools) had been randomised. Numbers lost unduly high for length of follow‐up; differential losses between groups not assessable. Reason for missing outcome data unacceptable. Caries data used in analysis pertain to participants with readable radiographs present at final examination (and analysis done at individual level within clusters does not take clustering into account) |
| Selective reporting (reporting bias) | Low risk | Outcomes reported Comment: trial protocol not available. All prespecified outcomes (in Methods) were reported in the prespecified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported DFS: 2.8 FR, 2.6 PL Age: 8 years (both groups combined) Erupted surfaces: 38.3 (both groups combined). Comment: initial caries appears balanced between groups (for individuals within |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
| Study characteristics | ||
| Methods | Study design: 4‐arm parallel‐group RCT (only 2 relevant arms used), placebo‐controlled Study duration: 3 years | |
| Participants | Participants randomised: numbers NR 95 children analysed at 3 years (available at final examination) Surfaces affected at start: 5.8 DMFS (from 1 year sample) Setting of recruitment and treatment: school and school/home | |
| Interventions | FR(Chlor)+ptc vs PL(Chlor)+ptc** FR group: 0.04% NaF (180 ppm F) PL group: non‐F rinse School use/supervised, 5 days every 3 weeks (115 rinses/y), 5 mL applied for 1 minute. Same schedule recommended for evening rinse at home (but no instruction for use of toothpaste given) Before application: prior toothbrushing without toothpaste in both groups (done at school, recommended for home) Postop instruction: not to eat or drink after rinse **Chlorhexidine present in both fluoride and non‐fluoride mouthrinse (thus, other outcomes, such as tooth staining, not relevant for the comparison of interest) | |
| Outcomes | 3yDMFS increment ‐ (CA)cl+(DR)xr | |
| Declaration of Interest | No information provided | |
| Funding | No information provided | |
| Notes | Clinical (VT) caries assessment by 2 examiners; diagnostic threshold = CA (FOTI assessment ‐ loss of translucency on transillumination ‐ for approximal surfaces of anterior teeth); state of tooth eruption included NR. Radiographic assessment; diagnostic threshold = DR ; kappa 0.7 and 0.79 for interexaminer and intraexaminer reliability | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The subjects were randomly divided into 4 groups” Comment: not enough information given |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: “All rinsing solutions were used and other study procedures performed on a double‐blind basis...” “Group CX rinsing with chlorhexidine solution...Group CXF with chlorhexidine‐fluoride solution” “The examiners did not know which group the children belonged to” Comment: use of placebo described |
| Blinding of outcome assessment (detection bias) | Low risk | Quotes: “All rinsing solutions were used and other study procedures performed on a double‐blind basis...” “The examiners did not know which group the children belonged to” Comment: blinded outcome assessment and use of placebo described |
| Incomplete outcome data (attrition bias) | High risk | Overall dropout for length of follow‐up: 17.3% (42/243) in 3 years (all groups) Comment: numbers lost not unduly high for length of follow‐up, but differential losses between groups not assessable. Reason for missing outcome data not reported. Caries data used in analysis pertain to participants available at final examination |
| Selective reporting (reporting bias) | Low risk | Outcomes reported Comment: trial protocol not available. All prespecified outcomes (in Methods) were reported in the prespecified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported DMFS: 5.0(3.7) FR, 6.6(4.4) PL Gender (% Boys): 50 FR, 50 PL Comment: initial caries appears imbalanced, but “adjustment made no difference in the results”. Gender balanced |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
| Study characteristics | ||
| Methods | Study design: 9‐arm parallel‐group RCT (only 3 relevant arms used), non‐placebo‐controlled Study duration: 2 school years | |
| Participants | Participants randomised: N = 597 494 children analysed at 2 years (available at final examination) Surfaces affected at start: 14.7 DMFS (from sample randomised) Setting of recruitment and treatment: school and home/school | |
| Interventions | FR (2 groups) vs NT FR group 1: 0.05% NaF (230 ppm F), 10 mL applied daily (320 rinses/y), unsupervised at home (instructed to be done after toothbrushing every evening) NT group: no intervention Before application: NR Postop instruction: NR | |
| Outcomes | 2yDMFS increment ‐ (CA)cl+(DR)xr MD‐DMFS Proportion of children with new carious lesions ‐ (U)xr Dropout | |
| Declaration of Interest | No information provided | |
| Funding | Financial support from the Swedish Medical Research Council, the City of Goteborg, the County of Stockholm and the National Board of Health, partial support (toothpastes in the trial) by Procter and Gamble Co | |
| Notes | Clinical (VT) caries assessment by 2 examiners, diagnostic threshold = CA; radiographic assessment (BW) by 2 examiners; diagnostic threshold = DR. State of tooth eruption included NR. Interexaminer and intraexaminer reproducibility checks done for clinical caries in 4% and 2% of sample, respectively; duplicate examination of x‐ray records done, and any discrepancies discussed before final diagnosis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The groups were randomly constituted and randomly assigned to the test different test methods, according to a system worked out with the assistance of statisticians...” Comment: It is likely a random method was used |
| Allocation concealment (selection bias) | Unclear risk | Method not specified |
| Blinding of participants and personnel (performance bias) | High risk | Quote: “The study was a blind test as the examination charts did not refer to the treatment or to the code number of the groups” Comment: no placebo described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: “The study was a blind test as the examination charts did not refer to the treatment or to the code number of the groups” Comment: blinded outcome assessment but no placebo described |
| Incomplete outcome data (attrition bias) | Unclear risk | Overall dropout for length of follow‐up: 17.25% in 2 years Comment: Numbers lost were not unduly high for the length of follow‐up, with no differential losses. It is unclear whether reasons for missing outcome data are acceptable and balanced. Caries data used in analysis pertain to participants present at final examinations |
| Selective reporting (reporting bias) | Low risk | Outcomes reported MD‐DMFS FS Proportion of children with new carious lesions (U) xr Comment: trial protocol not available. All prespecified outcomes (in Methods) were reported in the prespecified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported DMFS: 14.4(7.30) FR1, 15.2(8.57) FR2, 14.5(7.42) NT MD‐DMFS: 3.54 FR1, 3.97 FR2, 3.59 NT Comment: initial caries appears balanced between groups |
| Free of contamination/co‐intervention? | Unclear risk | No information provided |
| Study characteristics | ||
| Methods | Study design: 3‐arm parallel‐group RCT, placebo‐controlled Study duration: 3 years | |
| Participants | Participants randomised: N = 925 569 children analysed at 3 years (available at final examination) Surfaces affected at start: 8.4 DFS Setting of recruitment and treatment: school | |
| Interventions | FR (2 groups) vs PL FR group 2: 0.05% neutral NaF solution (230 ppm F) PL group: non‐F rinse solution School use/supervised, weekly (30 rinses/y), 10 mL applied for 1 minute Before application: NR Postop instruction: children instructed not to eat or drink for at least 1/2 hour after rinsing | |
| Outcomes | 3yNetDFS increment ‐ (CA)cl Dropout | |
| Declaration of Interest | No information provided | |
| Funding | No information provided | |
| Notes | Clinical (VT) caries assessment by 1 examiner, diagnostic threshold = CA. State of tooth eruption included NR. Intraexaminer reproducibility checks for incremental caries data in 40% sample (ICC score 0.91) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “...participants were randomly assigned to one of 3 rinsing groups” “Boys and girls were separately, randomly allocated to one of the three colours...” Comment: not enough information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Quotes: "The trial was conducted on a double‐blind basis. Boys and girls...were not informed of the meaning of the colour code. Nor was the examiner allowed to know to which colour code a subject belonged” Comment: use of placebo described |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: as above Comment: blinded outcome assessment and use of placebo described |
| Incomplete outcome data (attrition bias) | High risk | Overall dropout for length of follow‐up: 38.49% in 3 years Comment: numbers lost unduly high for length of follow‐up, with no differential losses between groups. Reasons for missing outcome data are acceptable and balanced. Caries data used in analysis pertain to participants present at final examinations |
| Selective reporting (reporting bias) | Low risk | Outcomes reported Comment: trial protocol not available. All prespecified outcomes (in Methods) were reported in the prespecified way |
| Baseline characteristics balanced? | Low risk | Prognostic factors reported DFS: 8.7(6.6) FR1, 8.2(5.8) FR2, 8.4(6.5) PL Gender: 89 M, 96 F (FR1), 90 M, 102 F (FR2), 93M, 99 F (PL) Comment: initial caries appears balanced between groups. Gender also balanced |
| Free of contamination/co‐intervention? | Low risk | Quote from correspondence: “We ensured that a child did not change the rinse during the study” Comment: overall prevention of contamination/co‐intervention indicated |
Dropout rate based only on groups relevant to the review, on relevant follow‐ups, unless otherwise stated. Baseline caries experience averaged among relevant study arms, and based on the study sample analysed at the end of the study period (final sample), unless otherwise stated. Age range (average age when reported) at the time the study started based on all study participants (or on groups relevant to the review when data were available).
1stm = first permanent molar; AmF = amine fluoride; APF = acidulated phosphate fluoride; CA = lesions showing loss of enamel continuity that can be recorded clinically (undermined enamel, softened floor/walls) or showing frank cavitation; CAR = caries attack rate; CFS = caries‐free surfaces; CFT= caries‐free teeth; Chlor = chlorhexidine diguclonate; CIR = caries incidence rate; cl = clinical examination; d(e)ft/s = decayed (extracted) and filled deciduous teeth or surface; dmft/s = decayed, missing (or extracted) and filled deciduous teeth or surface; D(M)FS/T = decayed (missing) and filled permanent surfaces or teeth; DR = radiolucency into dentin; E = teeth erupted at baseline; ER = any radiolucency in enamel/enamel‐dentin junction; F = fluoride; FR = fluoride mouthrinse; ICC = intraclass correlation co‐efficient (for interrater reliability); M = missing permanent teeth; MD = mesio and distal surfaces; N = numbers; Na = sodium; NaF = sodium fluoride; NCA = non‐cavitated enamel lesions visible as white spots or discoloured fissures; NH4F = ammonium fluoride; NR = not reported; NS = not significant; NT = no treatment control; O = occlusal surfaces; PF = pit and fissure surfaces; PL = placebo mouthrinse; post BW = posterior bite‐wing x‐ray assessment; ppm F = parts per million of fluoride; ptc = prior tooth‐cleaning performed with or without a non‐fluoride paste; RCT = randomised controlled trial; SMFP = sodium monofluorophosphate; SnF2 = stannous fluoride; U = teeth unerupted at baseline; VT = visual‐tactile assessment; xr = radiographic examination.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Fluoride solution swallowed after rinsing (even though no systemic effect should be anticipated for this age group) | |
| Random or quasi‐random allocation not stated. Blind outcome assessment not stated | |
| Additional fluoride‐based intervention associated with fluoride mouthrinse. Blind outcome assessment not stated | |
| Additional non‐fluoride‐based intervention associated with fluoride mouthrinse. Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated or indicated | |
| No relevant outcome reported. Blind outcome assessment not stated. Length of follow‐up of less than 1 year/school year (6 months) | |
| Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely | |
| Additional fluoride‐based intervention associated with fluoride mouthrinse. Clearly not randomised or quasi‐randomised (systematic process of assignment). Length of follow‐up of less than 1 year/school year | |
| Additional interventions associated with fluoride mouthrinse. Not a randomised or quasi‐randomised trial (only 2 clusters (schools) selected, each assigned to 1 of the 2 study groups) | |
| Open outcome assessment | |
| Open outcome assessment reported after contacting study author | |
| Open outcome assessment. Not a randomised or quasi‐randomised trial (selection of 2 clusters only, each assigned to 1 of the 2 groups) | |
| Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely | |
| No random or quasi‐random allocation used (selected group comparisons). Blind outcome assessment not stated and unlikely | |
| Clearly not randomised or quasi‐randomised (concurrent control group taken from another study) | |
| Clearly not randomised or quasi‐randomised (systematic allocation according to participants' characteristics). Blind outcome assessment not stated or indicated | |
| Additional fluoride‐based and non‐fluoride‐based interventions associated with fluoride mouthrinse. Random or quasi‐random allocation not stated | |
| Additional fluoride‐based intervention associated with fluoride mouthrinse. Blind outcome assessment not stated | |
| Additional non‐fluoride‐based intervention associated with fluoride mouthrinse. Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated or indicated | |
| Clearly not randomised or quasi‐randomised (systematic group assignment). Blind outcome assessment not stated and unlikely | |
| Open outcome assessment. Random or quasi‐random allocation not stated or indicated | |
| Fluoride solution swallowed after rinsing (even though no systemic effect should be anticipated for this age group) | |
| Additional fluoride‐based intervention associated with fluoride mouthrinse | |
| Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely | |
| Additional fluoride‐based intervention associated with fluoride mouthrinse | |
| Additional active agent associated with fluoride in mouthrinse. Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely | |
| Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely | |
| Random or quasi‐random allocation not stated. Blind outcome assessment not stated | |
| Random or quasi‐random allocation not stated. Blind outcome assessment not stated and unlikely | |
| Additional intervention associated with fluoride mouthrinse | |
| Not a randomised or quasi‐randomised trial. Only 2 clusters (schools) selected, each assigned to 1 of the 2 study groups. Blind outcome assessment not stated and unlikely | |
| Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely | |
| Additional fluoride‐based intervention associated with fluoride mouthrinse | |
| Random or quasi‐random allocation not stated | |
| Open outcome assessment reported after contacting study author | |
| Additional non‐fluoride‐based intervention associated with fluoride mouthrinse. Blind outcome assessment not stated | |
| Additional intervention associated with fluoride mouthrinse. Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely | |
| Random or quasi‐random allocation not stated or indicated. Outcome assessment not blind | |
| Random or quasi‐random allocation not stated or indicated. Outcome assessment not blind | |
| Open outcome assessment | |
| Clearly not randomised or quasi‐randomised (concurrent control group selected by matching procedure) | |
| Additional active agent associated with fluoride in mouthrinse. Not a randomised or quasi‐randomised trial ‐ only 3 clusters (school classes), each assigned to 1 of the 3 interventions compared | |
| Not a randomised or quasi‐randomised trial ‐ only 3 clusters (schools), each assigned to 1 of the 3 study groups (method of assignment not stated). Outcome assessment not blinded | |
| Random or quasi‐random allocation not stated or indicated. Blind outcome assessment not stated and unlikely | |
| Length of follow‐up of less than 1 year/school year | |
| Random or quasi‐random allocation not stated or indicated | |
| Clearly not randomised or quasi‐randomised (concurrent control group taken from a different population). Open outcome assessment | |
| Clearly not randomised or quasi‐randomised (concurrent control group taken from a different population). Open outcome assessment | |
| Random or quasi‐random allocation not stated | |
| Clearly not randomised or quasi‐randomised (systematic assignment of a few clusters to interventions). Blind outcome assessment not stated and unlikely Note ‐ abstract only, full text not available/obtainable | |
| Additional fluoride‐based intervention associated with fluoride mouthrinse |
Characteristics of studies awaiting classification [ordered by study ID]
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Additional information for this study report still missing |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 D(M)FS increment (PF) ‐ nearest to 3 years (35 trials) Show forest plot | 35 | 15305 | Prevented Fraction (IV, Random, 95% CI) | 0.27 [0.23, 0.30] |
| Analysis 1.1  Comparison 1: Fluoride mouthrinse versus placebo or no treatment, Outcome 1: D(M)FS increment (PF) ‐ nearest to 3 years (35 trials) | ||||
| 1.2 D(M)FT increment (PF) ‐ nearest to 3 years (13 trials) Show forest plot | 13 | 5105 | Prevented Fraction (IV, Random, 95% CI) | 0.23 [0.18, 0.29] |
| Analysis 1.2  Comparison 1: Fluoride mouthrinse versus placebo or no treatment, Outcome 2: D(M)FT increment (PF) ‐ nearest to 3 years (13 trials) | ||||
| 1.3 Developing 1 or more new caries (3 trials) Show forest plot | 3 | 1805 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.46, 1.29] |
| Analysis 1.3  Comparison 1: Fluoride mouthrinse versus placebo or no treatment, Outcome 3: Developing 1 or more new caries (3 trials) | ||||
| 1.4 Lack of acceptability of treatment as measured by leaving study early (4 trials) Show forest plot | 4 | 1700 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.62, 2.83] |
| Analysis 1.4  Comparison 1: Fluoride mouthrinse versus placebo or no treatment, Outcome 4: Lack of acceptability of treatment as measured by leaving study early (4 trials) | ||||
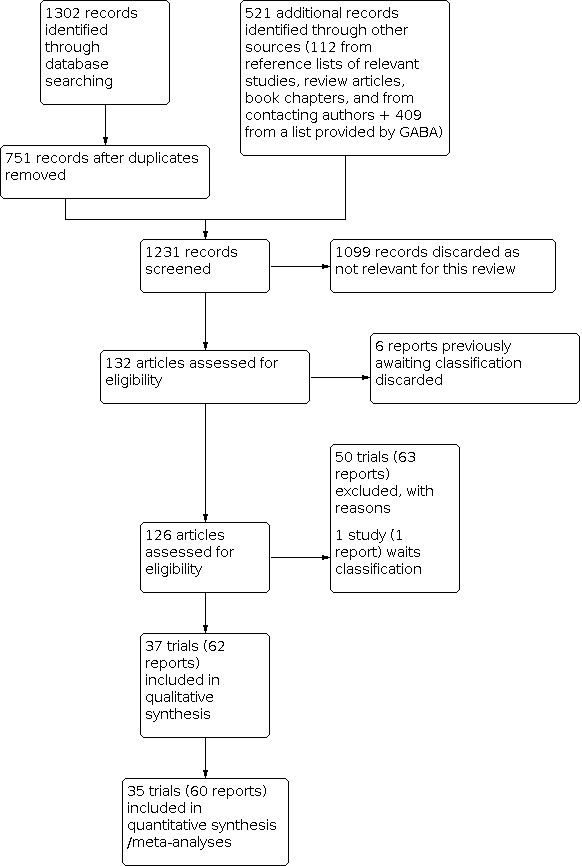
Study flow diagram from 2016 search

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies (a plot of the distribution of judgements (low risk of bias, unclear risk of bias and high risk of bias) across studies for each risk of bias item)

Funnel plot of comparison: 1 Fluoride mouthrinse versus placebo or no treatment, outcome: 1.1 D(M)FS increment (PF) ‐ nearest to 3 years (35 trials)

Comparison 1: Fluoride mouthrinse versus placebo or no treatment, Outcome 1: D(M)FS increment (PF) ‐ nearest to 3 years (35 trials)

Comparison 1: Fluoride mouthrinse versus placebo or no treatment, Outcome 2: D(M)FT increment (PF) ‐ nearest to 3 years (13 trials)

Comparison 1: Fluoride mouthrinse versus placebo or no treatment, Outcome 3: Developing 1 or more new caries (3 trials)

Comparison 1: Fluoride mouthrinse versus placebo or no treatment, Outcome 4: Lack of acceptability of treatment as measured by leaving study early (4 trials)
| Fluoride mouthrinse compared with placebo or no treatment for preventing caries in children and adolescents | ||||||
| Patient or population: children and adolescents | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with placebo or no treatment (assumed risk) | Risk with fluoride mouthrinse (corresponding risk) | |||||
| Changes in caries on the surfaces of permanent teeth measured by D(M)FS increment ‐ nearest to 3 years | Mean increment ranged across control groups from 0.74 to 21.05, median 5.6 | The corresponding mean increment in the intervention group is 3.80 (95% CI 3.64 to 4.00) | PFa 0.27 | 15305 | ⊕⊕⊕⊝ | Large effect: D(M)FS PF 27% (23% to 30%) |
| Changes in caries on the permanent teeth measured by D(M)FT increment ‐ nearest to 3 years | Mean increment ranged across control groups from 0.72 to 8.41, median 3.2 | The corresponding mean increment in the intervention group is 2.46 (95% CI 2.27 to 2.62) | PFa 0.23 | 5105 | ⊕⊕⊕⊝ | Moderate to large effect: D(M)FT PF 23% (18% to 29%) |
| Unacceptability of treatment as measured by leaving study early | 149 per 1000 | 198 per 1000 | RR 1.33 | 1700 | ⊕⊝⊝⊝ | |
| Tooth staining | Study 1: "significant difference" in stain score (from the control) in the group using an amine fluoride mouthrinse: "non‐significant difference" (from the control) in the group using sodium fluoride In 2 trials where stannous fluoride mouthrinsing was tested against placebo rinsing: Study 2: "approximately six children had tenacious staining that required a rubber cup prophylaxis carried out" ‐ no indication as to which groups these children belonged Study 3: "some amount of yellow pigmentation, somewhat more noticeable in the children in the test group" | Study 1: 525 Study 2: 743 Study 3: 726 | ⊕⊝⊝⊝ very lowe | We know little about the risk of tooth staining owing to incomplete reporting | ||
| Signs of acute toxicity during application of treatment (such as nausea/gagging/vomiting) | Not reported in any studies | No data on signs of acute toxicity | ||||
| Mucosal irritation/oral soft tissue allergic reaction | "no cases of mucosal hypersensitivity after periodical examinations of every subject" ‐ reported in 1 study | 434 (1 RCT) | ⊕⊝⊝⊝ very lowe | We know very little about the risk of mucosal irritation/allergic reaction owing to lack of reporting | ||
| *The basis for the assumed risk, the risk in the placebo or no treatment group, was the range and median in the control groups of the studies included in the review. Thecorresponding risk, the risk in the intervention group(and its 95% confidence interval), is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aPF = 1 ‐ (mean increment in control group/mean increment in treatment group) (expressed as percentages). PF values between 1% and 10% are considered to be a small effect; between 10% and 20%, a moderate effect; above 20% a large or substantial effect. cWide confidence interval ‐ small number of participants analysed. dHigh unexplained heterogeneity observed. eIncomplete information from one to three trials with unclear or high risk of bias. Outcome downgraded for concerns of risk of bias and serious imprecision. | ||||||
| Analysis | Number of studies | RE PF estimate | 95% CI | Meta‐analysis P value | Heterogeneity test |
|---|---|---|---|---|---|
| D(M)FS ‐ all studies | 35 | 27% | 23% to 30% | P value < 0.0001 | Chi2 = 58.43 (34 df); P value = 0.006; I² = 42% |
| D(M)FT ‐ all studies | 13 | 23% | 18% to 29% | P value < 0.0001 | Chi2 = 26.04 (12 df); P value = 0.011; I² = 54% |
| D(M)FS = decayed (missing) and filled permanent surfaces | |||||
| Characteristic | Number of studies | Slope estimate | 95% CI | Slope interpretation | P value |
|---|---|---|---|---|---|
| Mean baseline caries | 34 | 0.2% | (‐0.8% to 1.3%) | Increase in PF per unit increase in mean baseline caries | 0.7 |
| Fluoridated water area | 33 | 6.6% | (‐4.8% to 17.9%) | Higher PF in presence of water fluoridation | 0.3 |
| Fluoride dentifrice use | 33 | 4.8% | (‐3.2% to 12%) | Higher PF in presence of fluoride dentifrice use | 0.2 |
| Background fluorides | 33 | 5.8% | (‐1.5% to 13.1%) | Higher PF in presence of background fluoride | 0.12 |
| Rinsing frequency | 34 | 0.4% | (‐4.3% to 5.0%) | Increase in PF per 100 extra applications/y | 0.9 |
| Fluoride concentration in solution | 35 | 1.1% | (‐3.9% to 6.0%) | Increase in PF per 1000 ppm F | 0.7 |
| Intensity (frequency times concentration) | 33 (excludes DePaola 1977) | 8.3% | (‐14% to 31%) | Increase in PF equivalent to doubling from 100 to 200 applications and increasing by 1000 ppm F | 0.5 |
| Control group | 35 | 8.2% | (‐2.0% to 18.4%) | Higher PF for no treatment compared with placebo | 0.11 |
| Dropout | 32 | 0.4% | (‐2.1% to 2.9%) | Increase in PF per 10 dropouts | 0.7 |
| Length of follow‐up | 35 | 1.1% | (‐6.2% to 8.5%) | Increase in PF per extra year of follow‐up | 0.8 |
| D(M)FS = decayed (missing) and filled permanent surfaces | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 D(M)FS increment (PF) ‐ nearest to 3 years (35 trials) Show forest plot | 35 | 15305 | Prevented Fraction (IV, Random, 95% CI) | 0.27 [0.23, 0.30] |
| 1.2 D(M)FT increment (PF) ‐ nearest to 3 years (13 trials) Show forest plot | 13 | 5105 | Prevented Fraction (IV, Random, 95% CI) | 0.23 [0.18, 0.29] |
| 1.3 Developing 1 or more new caries (3 trials) Show forest plot | 3 | 1805 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.46, 1.29] |
| 1.4 Lack of acceptability of treatment as measured by leaving study early (4 trials) Show forest plot | 4 | 1700 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.62, 2.83] |

