Suplementos de ácidos grasos omega‐3 para la fibrosis quística
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | RCT (double‐blind placebo‐controlled). Design: parallel. Duration: 12 months. Location: multicentre (2 centres) in Belgium. | |
| Participants | 15 people with CF, homozygous Del508, PI and over 5 years of age. Age, mean: intervention group 14 years (5.2 ‐ 26.0 years); placebo group 17.5 years (4.6 ‐ 30.4 years). Gender (M:F): intervention group (4:3); placebo group (7:1). Participants all established on azithromycin treatment for at least 3 months. | |
| Interventions | Intervention group (n = 7): oral supplement of omega‐3, 60 mg/kg. Control group (n = 8): identical placebo. | |
| Outcomes | Clinical and nutritional status; lung function, changes in erythrocyte levels of EPA and DHA. | |
| Notes | N = 13 completed, 6 intervention, 7 placebo. Supplement well‐tolerated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block stratification, pre‐defined block list, stratified by pharmacist. 4‐digit sequence generation to individualise participants. |
| Allocation concealment (selection bias) | Unclear risk | No mention of concealment allocation. Stratified by participant weight which could enable recruiter to identify allocation. |
| Blinding (performance bias and detection bias) | Unclear risk | Identical capsules, blinded to person responsible for care, participant and outcome assessor, no description of placebo capsules and 1 dropout was due to fishy taste of capsules. |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rate very simply, clearly explained and justified. 15 participants were recruited, 13 participants completed the study. Study was not analysed as ITT, authors stated they would only analyse ITT if participant attended for first 3‐month follow‐up visit. The 2 participants dropped out prior to this 3‐month follow‐up. |
| Selective reporting (reporting bias) | High risk | Outcomes both primary and secondary were modified from protocol to study. Authors acknowledged change from inflammatory biomarkers as primary outcome due to lack of facilities to test the outcome. We have concern over conclusions made regarding impact of n3 supplementation on antibiotic duration and frequency, (clinical status) as this outcome was not explicitly defined at the study protocol stage. The significant decrease in number of respiratory exacerbations is only reported in the supplement group with data not provided for the placebo group. |
| Other bias | Low risk | No additional bias identified. |
| Methods | RCT. Design: parallel with 4 arms. Duration: 6‐weeks. Location: single centre in the USA (Children's Hospital Medical Center, Seattle). | |
| Participants | 12 children and young adults diagnosed with CF on genotype or sweat test, pancreatic insufficiency and able to complete the spirometric tests. Age, mean (SD): age 12.2 (5.4) years. Gender: 7 males, 5 females. Plasma vitamin A and E levels within the normal range. 13 gender and age‐matched people without CF (mean (SD) age 13.4 (6.3) years), 7 males, 6 females. | |
| Interventions | Intervention group: 8 x 1g capsules fish oil (4 capsules twice daily) containing 3.19 g EPA and 2.21 g DHA. Control: olive oil placebo capsules flavoured to obtain a slight fish taste. | |
| Outcomes | Outcomes included in this review: | |
| Notes | Significantly lower plasma n‐6 fatty acids (linoleic acid and AA) noted at baseline in participants with CF compared with the group who did not have CF. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using a stratified randomised block design. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed in paper. |
| Blinding (performance bias and detection bias) | Low risk | Described as double blind ‐ while the capsules were not described as identical, it was stated that the placebo olive oil capsules were flavoured to obtain a slight fish taste which we agreed would be sufficient to blind participants. |
| Incomplete outcome data (attrition bias) | Low risk | Withdrawals from the study were discussed with explanations (20 out of 25 randomised participants completed the study). Study included all participants in the data analysis, which was performed according to the ITT principle. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available for comparison, so unable to definitely eliminate selective reporting. |
| Other bias | Low risk | No additional bias identified. |
| Methods | RCT. Design: parallel. Duration: 3 months. Location: single centre in Sweden (west Sweden CF Centre). | |
| Participants | 45 children and adults with "severe" CF mutations randomised; 43 commenced study and 35 participants completed the study. 8 participants discontinued the study and the inclusion parameters of these participants did not differ from those who completed the study. Age, range: 7 to 41 years. Gender: 17 males, 18 females. Disease status: 20 participants were chronically infected with Pseudomonas aeruginosa. | |
| Interventions | Intervention group A: 50 mg/kg per day fatty acid blend capsules containing predominantly EPA and DHA. Intervention group B: placebo capsules containing predominantly saturated fatty acids. Intervention group C: 50 mg/kg per day fatty acid blend capsules containing a high proportion of linoleic acid and AA. Participants increased their pancreatic enzymes by 10% to 20% to maintain normal stools. | |
| Outcomes | Outcomes included in this review: | |
| Notes | Actual dose of EPA and DHA administered not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using random number generator. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed in paper. |
| Blinding (performance bias and detection bias) | Unclear risk | Described as double‐blind; treatment was assumed to be administered as identical capsules; however, 2 participants complained of fish smell in group A. |
| Incomplete outcome data (attrition bias) | Unclear risk | 45 participants were initially randomised, but 2 were excluded due to acute exacerbations and therefore did not enter the study. Withdrawls from study were described: 35 participants completed the study; 8 discontinued because of low compliance (n = 4), stomach pains (n = 2) and weight gain (n = 2). Study did not include all participants in the data analysis, therefore, data were not analysed as ITT. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available for comparison, so unable to definitely eliminate selective reporting. |
| Other bias | High risk | The lack of information on dosage is a potential risk of bias. |
| Methods | RCT. Design: cross‐over study planned. However, significant carry‐over effect of treatment noted at end of study, therefore analysis restricted to the first 6‐week treatment period. Duration: 6 weeks. Location: single centre in Australia (Sydney). | |
| Participants | 19 adolescents and adults diagnosed with CF on genotype, sweat test, or clinically. Age, median (range): 17 years (12 years ‐ 26 years). Gender: 11 males, 5 females. | |
| Interventions | Intervention group: capsules with 2.7 g EPA daily. Control group: identical olive oil placebo capsules. | |
| Outcomes | Outcomes included in this review: | |
| Notes | Initial randomisation gave groups with comparable baseline characteristics except for age. The treatment group also had significantly greater weight, peripheral blood leucocyte and neutrophil counts. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Did not state the randomisation technique. |
| Allocation concealment (selection bias) | Unclear risk | No details were provided in the primary paper. |
| Blinding (performance bias and detection bias) | Low risk | Described as double blind, treatment was administered as 'identical olive oil capsules'. |
| Incomplete outcome data (attrition bias) | Low risk | Of the 19 participants recruited, 3 participants were excluded from the study before analysis due to corticosteroid treatment during the first treatment period. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available for comparison, so unable to definitely eliminate selective reporting. |
| Other bias | Low risk | No additional bias identified. |
| Methods | RCT. Design: cross‐over trial with no washout period. Duration: 1 year (2 x 6‐month periods). Location: single centre in Switzerland (Lausanne CF Center). | |
| Participants | 17 children and young adults with CF and PI; 1 participant discontinued study after 8 months for personal convenience. Age, mean (SD): 18 (9) years). Gender: 10 males, 7 females. | |
| Interventions | Intervention Group: liquid dietary supplement containing PUFA mixture (EPA, DHA, GLA and STA). Control Group: liquid dietary supplement without PUFA mixture. Volume of supplementation was determined according to participant's weight; intake ranged from 100 mg ‐ 300 mg DHA. and 200 mg ‐ 600 mg EPA per day. | |
| Outcomes | Outcomes included in this review: | |
| Notes | Relatively low daily dose of EPA and DHA compared to previous clinical trials. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Did not state the randomisation technique. |
| Allocation concealment (selection bias) | Unclear risk | No details were provided in the primary paper. |
| Blinding (performance bias and detection bias) | Low risk | Described as double blind, placebo treatment was not stated to be identical but it was described as the same liquid dietary supplement as the intervention but without the PUFA mixture. |
| Incomplete outcome data (attrition bias) | Unclear risk | 17 participants were randomised; 16 completed the study and one discontinued after 8 months for personal convenience. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available for comparison, so unable to definitely eliminate selective reporting. |
| Other bias | Low risk | No additional bias identified. |
AA: arachidonic acid
BMI: body mass index
CF: cystic fibrosis
DHA: docosahexaenoic acid
EPA: eicosapentaenoic acid
GLA: gamma‐linolenic acid
ITT: intention‐to‐treat
PI: pancreatic insufficient
PUFA: polyunsaturated fatty acids
RCT: randomised controlled trial
SD: standard deviation
STA: stearidonic acid
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Omega‐3 supplementation compared with large omega‐6 fatty acid source (germ oil) rather than a neutral placebo that contains relatively little omega‐3 or omega‐6 fatty acid (olive oil). | |
| Omega‐3 supplementation compared with large omega‐6 fatty acid source (borache oil) rather than a neutral placebo that contains relatively little omega‐3 or omega‐6 fatty acid (olive oil). | |
| No published outcome data available. | |
| Parenteral (via blood stream), not enteral (oral) supplementation with omega‐3 fatty acids. | |
| Eligibility unclear, attempts made to contact author for further information, but no response received. | |
| Omega‐3 supplementation compared with large omega‐6 fatty acid source (sunflower oil) rather than a neutral placebo that contains relatively little omega‐3 or omega‐6 fatty acid (olive oil). | |
| Omega‐3 supplementation compared with large omega‐6 fatty acid source (corn/soy) rather than a neutral placebo that contains relatively little omega‐3 or omega‐6 fatty acid (olive oil). | |
| Omega‐3 supplementation with large omega‐6 fatty acid source (sunflower oil) rather than a neutral placebo that contains relatively little omega‐3 or omega‐6 fatty acid (olive oil). | |
| Omega‐3 supplementation not compared to placebo. Fish oil compared to actively modified lipid. | |
| Omega‐3 supplementation with large omega‐6 fatty acid source (sunflower oil) rather than a neutral placebo that contains relatively little omega‐3 or omega‐6 fatty acid (olive oil). | |
| Clinical trial currently still in enrolment by invitation. | |
| Omega‐3 supplementation with large omega‐6 fatty acid source (sunflower oil) rather than a neutral placebo that contains relatively little omega‐3 or omega‐6 fatty acid (olive oil). | |
| Eligibility unclear. Attempts made to contact the author for further information , but no response received. | |
| Study to identify a suitable approach for monitoring the incorporation of omega‐3 fatty acids in nutritional studies, not a comparison of omega‐3 fatty acids with control. | |
| Eligibility unclear, attempts made to contact author for further information, but no response received. | |
| Eligibility unclear, attempts made to contact author for further information, but no response received. | |
| Omega‐3 supplementation compared with large omega‐6 fatty acid source (sunflower oil) rather than a neutral placebo that contains relatively little omega‐3 or omega‐6 fatty acid (olive oil). | |
| Omega‐3 supplementation with large omega‐6 fatty acid source (sunflower oil) rather than a neutral placebo that contains relatively little omega‐3 or omega‐6 fatty acid (olive oil). |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Omega‐3 fatty acids versus placebo, Outcome 1 Adverse events. | ||||
| 1.1 Diarrhoea and eructation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 FEV1 % predicted (post‐treatment) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Omega‐3 fatty acids versus placebo, Outcome 2 FEV1 % predicted (post‐treatment). | ||||
| 2.1 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 FVC % predicted (post treatment) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Omega‐3 fatty acids versus placebo, Outcome 3 FVC % predicted (post treatment). | ||||
| 3.1 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 BMI (SD score) (post treatment) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Omega‐3 fatty acids versus placebo, Outcome 4 BMI (SD score) (post treatment). | ||||
| 4.1 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 EPA and DHA % content of neutrophil membrane (post treatment) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 Omega‐3 fatty acids versus placebo, Outcome 5 EPA and DHA % content of neutrophil membrane (post treatment). | ||||
| 5.1 EPA at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 DHA at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Leukotriene B4 to leukotriene B5 ratio (post treatment) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.6  Comparison 1 Omega‐3 fatty acids versus placebo, Outcome 6 Leukotriene B4 to leukotriene B5 ratio (post treatment). | ||||
| 6.1 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 EPA and DHA content of serum phospholipids (change from baseline) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.7  Comparison 1 Omega‐3 fatty acids versus placebo, Outcome 7 EPA and DHA content of serum phospholipids (change from baseline). | ||||
| 7.1 EPA At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 DHA at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 N6/N3 ratio content of serum phospholipids (change from baseline) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.8  Comparison 1 Omega‐3 fatty acids versus placebo, Outcome 8 N6/N3 ratio content of serum phospholipids (change from baseline). | ||||
| 8.1 At 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

Study flow (PRISMA) diagram
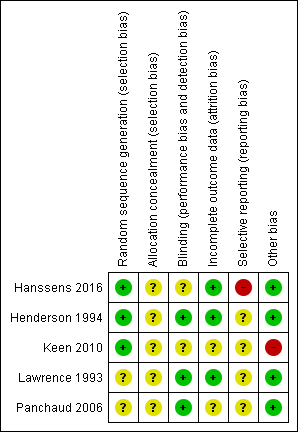
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Omega‐3 fatty acids versus placebo, Outcome 1 Adverse events.

Comparison 1 Omega‐3 fatty acids versus placebo, Outcome 2 FEV1 % predicted (post‐treatment).
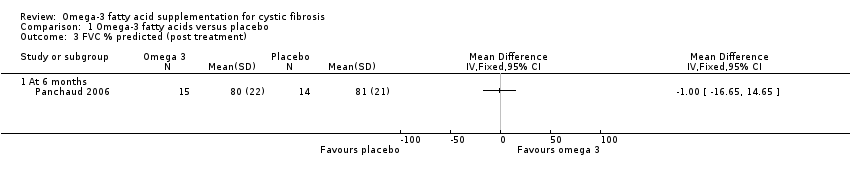
Comparison 1 Omega‐3 fatty acids versus placebo, Outcome 3 FVC % predicted (post treatment).
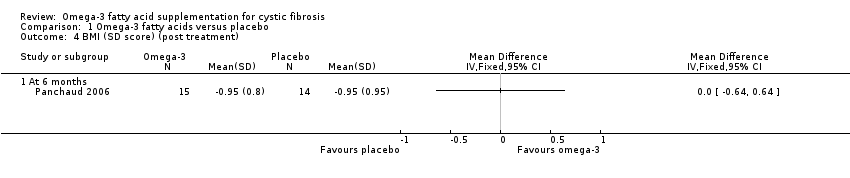
Comparison 1 Omega‐3 fatty acids versus placebo, Outcome 4 BMI (SD score) (post treatment).

Comparison 1 Omega‐3 fatty acids versus placebo, Outcome 5 EPA and DHA % content of neutrophil membrane (post treatment).
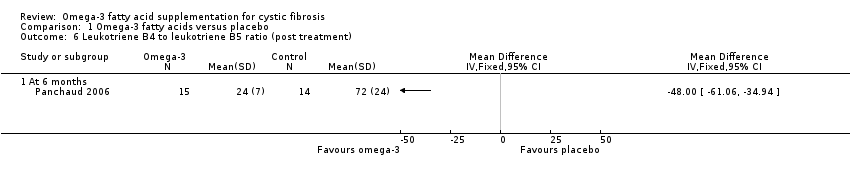
Comparison 1 Omega‐3 fatty acids versus placebo, Outcome 6 Leukotriene B4 to leukotriene B5 ratio (post treatment).

Comparison 1 Omega‐3 fatty acids versus placebo, Outcome 7 EPA and DHA content of serum phospholipids (change from baseline).
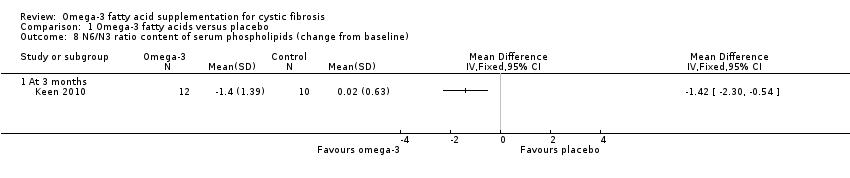
Comparison 1 Omega‐3 fatty acids versus placebo, Outcome 8 N6/N3 ratio content of serum phospholipids (change from baseline).
| Omega‐3 fatty acid supplementation compared with placebo for cystic fibrosis | ||||||
| Patient or population: children and adults with cystic fibrosis Settings: outpatients Intervention: oral omega‐3 supplementation (EPA or DHA, or both) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Omega‐3 supplementation | |||||
| Number of pulmonary exacerbations (median number of exacerbations during the study) Follow‐up: 12 months | The number of exacerbations in the placebo group was greater than in the omega‐3 group (3.5 versus 1.7 (range 1 ‐ 3)). | N/A | 13 | ⊕⊝⊝⊝ | The authors only report the number of exacerbations in the supplemented group compared to the 12 months prior to the trial. Extra data was provided by the study authors to allow a between group comparison. This outcome was not included in the study protocol. | |
| Adverse events: diarrhoea Follow‐up: 6 weeks | 1 study reported drop out due to diarrhoea. 2 out of 7 participants in the fish oil group dropped out and 2 out of 5 participants in the placebo group, OR 0.6 (0.05 to 6.79). | OR 0.6 (0.05 to 6.79) | 12 | ⊕⊝⊝⊝ | Other adverse events included stomach pains (5/35 participants) but the intervention arm wasn't specified (Keen 2010). | |
| FEV1 % predicted Follow‐up: 6 months | The mean FEV1 % predicted was 64% in the control group. | The mean FEV1 % predicted in the intervention group was 2% higher (19.1% lower to 23.11% higher) | MD 2.00 (19.11 to 23.11) | 17 | ⊕⊝⊝⊝ | A further study (n = 16) reported a significant increase from baseline in the EPA group compared to the control group measured in L compared to the placebo group (P = 0.06) (Lawrence 1993). Two studies reported no difference in FEV1 % predicted or lung function (measurement not stated) between groups (Hanssens 2016; Keen 2010) |
| FVC % predicted Follow‐up: 6 months | The mean FVC % predicted in the control group was 81%. | The mean FVC % predicted in the intervention group was 1% lower (16.65 % lower to 14.65 % higher). | MD ‐1.00 (‐16.65 to 14.65) | 17 | ⊕⊝⊝⊝ | 1 study reported a significant rise in FVC (L) in the EPA group (P = 0.01) (Lawrence 1993). 2 studies reported no difference in FVC between groups, but no data were available for analysis (Hanssens 2016; Keen 2010). |
| Growth and nutrition: BMI SD score Follow‐up: 6 months | No significant difference was seen between the PUFA group and the placebo group after 6 months. | MD 0.00 (95 % CI ‐0.64 to 0.64) | 29 | ⊕⊝⊝⊝ | A further study reported on BMI but reported only that BMI z scores remained stable throughout the study (Hanssens 2016). | |
| Biochemical markers of essential fatty acid status: EPA and DHA % content of neutrophil membrane Follow‐up: 6 months | 1 study reported a higher EPA content of the neutrophil membrane in the omega‐3 PUFA‐supplemented group compared to the placebo group, MD 0.90 (95% CI 0.46 to 1.34). In the same study, no difference was observed in DHA membrane concentration between groups, MD 0.10 (95% CI ‐0.45 to 0.65) | 29 | ⊕⊝⊝⊝ | At 6 months, Keen reported a significant increase from baseline in both EPA and DHA content of serum phospholipids in the omega‐3 supplemented group compared to placebo, MD 0.70 (95% CI 0.42 to 0.98) and MD 1.10 (95% CI 0.39 to 1.81), respectively (Keen 2010). | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded twice due to risk of bias within the included study for this outcome. There was uncertainty around allocation concealment and blinding and a high risk of bias due to selective reporting. bDowngraded once due to imprecision from very low participant numbers and low event rates. cDowngraded once due to risk of bias within the included study. It was unclear whether allocation was concealed and whether the outcomes were predefined as there was no protocol available. dDowngraded twice due to very low participant numbers (n = 12) and low event rates. eDowngraded twice due to risk of bias within the trial. The risk of bias was unclear across several domains including; randomisation, allocation concealment, incomplete outcome assessment and selective reporting. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Diarrhoea and eructation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 FEV1 % predicted (post‐treatment) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 FVC % predicted (post treatment) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 BMI (SD score) (post treatment) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 EPA and DHA % content of neutrophil membrane (post treatment) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 EPA at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 DHA at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Leukotriene B4 to leukotriene B5 ratio (post treatment) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 EPA and DHA content of serum phospholipids (change from baseline) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 EPA At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 DHA at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 N6/N3 ratio content of serum phospholipids (change from baseline) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 At 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

