Rehabilitasi biopsikososial pelbagai disiplin untuk sakit belakang rendah subakut
Abstract
Background
Low back pain (LBP) is associated with enormous personal and societal burdens, especially when it reaches the chronic stage of the disorder (pain for a duration of more than three months). Indeed, individuals who reach the chronic stage tend to show a more persistent course, and they account for the majority of social and economic costs. As a result, there is increasing emphasis on the importance of intervening at the early stages of LBP.
According to the biopsychosocial model, LBP is a condition best understood with reference to an interaction of physical, psychological, and social influences. This has led to the development of multidisciplinary biopsychosocial rehabilitation (MBR) programs that target factors from the different domains, administered by healthcare professionals from different backgrounds.
This review is an update of a Cochrane Review on MBR for subacute LBP, which was published in 2003. It is part of a series of reviews on MBR for musculoskeletal pain published by the Cochrane Back and Neck Group and the Cochrane Musculoskeletal Group.
Objectives
To examine the effectiveness of MBR for subacute LBP (pain for a duration of six to 12 weeks) among adults, with a focus on pain, back‐specific disability, and work status.
Search methods
We searched for relevant trials in any language by a computer‐aided search of CENTRAL, MEDLINE, Embase, CINAHL, PsycINFO and two trials registers. Our search is current to 13 July 2016.
Selection criteria
We included randomised controlled trials (RCTs) of adults with subacute LBP. We included studies that investigated a MBR program compared to any type of control intervention. We defined MBR as an intervention that included a physical component (e.g. pharmacological, physical therapy) in combination with either a psychological, social, or occupational component (or any combination of these). We also required involvement of healthcare professionals from at least two different clinical backgrounds with appropriate training to deliver the component for which they were responsible.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. In particular, the data extraction and 'risk of bias' assessment were conducted by two people, independently. We used the Cochrane tool to assess risk of bias and the GRADE approach to assess the overall quality of the evidence for each outcome.
Main results
We included a total of nine RCTs (981 participants) in this review. Five studies were conducted in Europe and four in North America. Sample sizes ranged from 33 to 351. The mean age across trials ranged between 32.0 and 43.7 years.
All included studies were judged as having high risk of performance bias and high risk of detection bias due to lack of blinding, and four of the nine studies suffered from at least one additional source of possible bias.
In MBR compared to usual care for subacute LBP, individuals receiving MBR had less pain (four studies with 336 participants; SMD ‐0.46, 95% CI ‐0.70 to ‐0.21, moderate‐quality of evidence due to risk of bias) and less disability (three studies with 240 participants; SMD ‐0.44, 95% CI ‐0.87 to ‐0.01, low‐quality of evidence due to risk of bias and inconsistency), as well as increased likelihood of return‐to‐work (three studies with 170 participants; OR 3.19, 95% CI 1.46 to 6.98, very low‐quality of evidence due to serious risk of bias and imprecision) and fewer sick leave days (two studies with 210 participants; SMD ‐0.38 95% CI ‐0.66 to ‐0.10, low‐quality of evidence due to risk of bias and imprecision) at 12‐month follow‐up. The effect sizes for pain and disability were low in terms of clinical meaningfulness, whereas effects for work‐related outcomes were in the moderate range.
However, when comparing MBR to other treatments (i.e. brief intervention with features from a light mobilization program and a graded activity program, functional restoration, brief clinical intervention including education and advice on exercise, and psychological counselling), we found no differences between the groups in terms of pain (two studies with 336 participants; SMD ‐0.14, 95% CI ‐0.36 to 0.07, low‐quality evidence due to imprecision and risk of bias), functional disability (two studies with 345 participants; SMD ‐0.03, 95% CI ‐0.24 to 0.18, low‐quality evidence due to imprecision and risk of bias), and time away from work (two studies with 158 participants; SMD ‐0.25 95% CI ‐0.98 to 0.47, very low‐quality evidence due to serious imprecision, inconsistency and risk of bias). Return‐to‐work was not reported in any of the studies.
Although we looked for adverse events in both comparisons, none of the included studies reported this outcome.
Authors' conclusions
On average, people with subacute LBP who receive MBR will do better than if they receive usual care, but it is not clear whether they do better than people who receive some other type of treatment. However, the available research provides mainly low to very low‐quality evidence, thus additional high‐quality trials are needed before we can describe the value of MBP for clinical practice.
PICO
Ringkasan bahasa mudah
Rawatan pelbagai disiplin peringkat awal sakit belakang rendah
Soalan ulasan
Kami mengulas bukti mengenai kesan rawatan pelbagai disiplin bagi sakit, kecacatan, dan status kerja dalam kalangan orang‐orang yang telah mengalami sakit belakang rendah selama enam hingga 12 minggu. Kami takrifkan rawatan pelbagai disiplin sebagai rawatan yang mensasarkan fizikal dan juga aspek‐aspek psikologi atau sosial sakit belakang rendah dan melibatkan pasukan penyedia penjagaan kesihatan dengan latar belakang profesional dan latihan yang berbeza. Sebagai contoh, rawatan yang mengintegrasi terapi senaman yang disediakan oleh jurupulih anggota dengan penyesuaian tempat kerja yang disediakan oleh ahli pakar ergonomis, pakar dalam reka bentuk dan persediaan peralatan tempat kerja, akan dianggap sebagai pelbagai disiplin.
Latar belakang
Sakit belakang rendah (LBP) adalah suatu keadaan yang menyebabkan kesakitan dan penderitaan di seluruh dunia dan juga melibatkan kos tinggi kepada masyarakat akibat perbelanjaan penjagaan kesihatan dan tidak hadir bekerja. Kajian terdahulu menunjukkan orang‐orang dengan sakit belakang rendah melebihi tiga bulan kurang berkemungkinan untuk pulih. Hasilnya, terdapat peningkatan penekanan kepada kepentingan intervensi peringkat awal LBP.
Tujuan ulasan ini adalah untuk mengetahui sama ada rawatan pelbagai disiplin adalah lebih baik atau lebih buruk daripada alternatif lain, seperti rawatan biasa (iaitu amalan klinikal semasa) atau rawatan lain (contohnya terapi senaman sahaja) untuk orang‐orang yang mengalami sakit belakang rendah selama enam hingga 12 minggu.
Ciri‐ciri kajian
Carian adalah terkini sehingga Julai 2016.
Lima kajian telah dijalankan di Eropah dan empat di Amerika Utara. Saiz sampel adalah di antara 33 hingga 351. Min umur seluruh kajian adalah di antara 32.0 dan 43.7 tahun. Kebanyakan kajian termasuk sampel campuran peserta lelaki dan perempuan. Para penulis tidak meragui sumber dana kajian‐kajian yang dimasukkan.
Keputusan utama
Secara keseluruhan, kami mendapati rawatan pelbagai disiplin mungkin lebih baik daripada rawatan biasa bagi orang‐orang dengan LBP untuk tempoh enam hingga 12 minggu. Individu yang menerima rawatan pelbagai disiplin mempunyai kurang sakit, kurang kecacatan, peningkatan kemungkinan pulangan untuk kembali bekerja dan kurang hari cuti sakit pada 12 bulan susulan. Walau bagaimanapun, apabila membandingkan rawatan pelbagai disiplin untuk rawatan lain (misalnya intervensi klinikal ringkas termasuk pendidikan dan nasihat mengenai senaman), kami mendapati bahawa rawatan pelbagai disiplin mungkin tidaklah lebih baik daripada rawatan lain. Walaupun kami mengkaji kejadian peristiwa buruk sebagai hasil sekunder, tiada kajian yang dimasukkan melaporkan hasil ini.
Kualiti bukti
Kualiti bukti bagi ulasan ini pada umumnya rendah yang sangat rendah. Ini disebabkan oleh saiz sampel yang kecil dan lain‐lain pembatasan kajian. TLagipun, kami kumpulkan kajian‐kajian dengan pelbagai intervensi dan perbandingan. Misalnya, sesetengah intervensi pelbagai disiplin agak berat (seperti > 30 jam rawatan), sedangkan kajian lain direka bentuk ringkas (seperti < tiga jam). Kebolehubaha di seluruh kajian menjadikannya lebih mencabar untuk mentafsirkan penemuan‐penemuan.
Kesimpulannya, terdapat keperluan untuk kajian rawak terkawal yang lebih besar dan berkualiti tinggi sebelum kita boleh membuat syor muktamad untuk amalan klinikal.
Authors' conclusions
Summary of findings
| Patient or population: Subacute low back pain | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence (GRADE) | Comments | ||
| Risk with usual care | Risk with multidisciplinary rehabilitation | ||||||
| Back pain long‐term Higher scores indicated more intense pain Follow‐up: median 12 months | The baseline for the most representative study# (Karjalainen 2003) was 5.7 out of 10 (visual analogue scale). | The risk with MBR was approximately 4.67 (4.60 to 4.73) out of 10. | The mean pain in the intervention group was 0.46 standard deviations lower (0.7 lower to 0.21 lower). | 336 | TOTAL = 532 (5 RCTs) | X X X ◯ MODERATE 1 | This was a small to moderate effect (Cohen 1988) that is probably clinically relevant in this participant group. |
| An additional study that could not be included in meta‐analysis showed no difference between the groups. | ‐ | 196 (1 RCT included in qualitative synthesis). | |||||
| Functional disability at the long term Higher scores indicated more disability Follow‐up: median 12 months. | The baseline for the most representative study# (Karjalainen 2003) was 34 out of 100 (Oswestry Scale). | The risk with MBR was approximately 26.30 (25.2 to 27.4) out of 100. | The mean functional disability in the intervention group was 0.44 standard deviations lower (0.87 lower to 0.01 lower). | 240 | TOTAL = 537 (5 RCTs) | X X ◯◯ | This was a small to moderate effect (Cohen 1988) that is probably clinically relevant in this participant group. |
| Two additional studies could not be included in meta‐analysis. One study showed evidence in favour of MBR and the other showed no difference between the groups. | ‐ | 297 (2 RCTs included in qualitative synthesis). | |||||
| Return‐to‐work at the long term Proportion at work Follow‐up: median 12 months. | Study population | OR 3.19 (1.46 to 6.98) | 170 | X ◯◯◯ | This was a moderate effect that is clinically relevant in this participant group. | ||
| 65 per 100 | 86 per 100 | ||||||
| Sick leave periods at long‐term Cumulative sickness absence periods over the course of the 12‐month follow‐up. | Average sick leave in the usual care group was 997.3 hours (Bultmann 2009). | The risk with MBR was approximately 763.03 (743.3 to 782.3) sick leave hours. | The mean sick leave periods in the intervention group was 0.38 standard deviations lower (0.66 lower to 0.10 lower). | 210 | X X ◯◯ | This was a small to moderate effect (Cohen 1988) that is clinically relevant in this participant group. | |
| Adverse events | N/A | NO EVIDENCE | None of the included studies reported on adverse events. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). #We defined the most representative sample as the study that has the largest weighting in the overall result in RevMan. | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1 Downgraded due to risk of bias, all five trials had high risk of performance bias and detection bias. One trial suffered from unclear risk of selection bias. Another trial suffered from unclear risk of attrition bias (serious bias = 1‐point downgrade). 2 Downgraded due to inconsistency, I2 statistic60% (heterogeneity = 1‐point downgrade). 3 Downgraded due to serious risk of bias, all three trials suffered from risk of performance bias and detection bias. One trial also suffered from unclear risk of selection bias and another trial suffered from unclear risk of attrition bias (very serious bias = 2‐point downgrade). 4 Downgraded due to imprecision, the total number of events was less than 300 (1‐point downgrade). 5 Downgraded due to risk of bias, both trials suffered from risk of performance bias and detection bias. One trial also suffered from unclear risk of attrition bias (serious bias = 1‐point downgrade). 6 Downgraded due to imprecision, total population size < 400 (1‐point downgrade). | |||||||
| Patient or population: Subacute low back pain | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with another treatment | Risk with multidisciplinary rehabilitation | |||||
| Pain at the long term Higher scores indicated more intense pain Follow‐up: median 12 months. | The baseline for the most representative study# (Jensen 2011) was 32.7 out of 60 (LBP Rating Scale). | The risk in the MBR group was approximately 31.02 out of 60. | The mean pain in the intervention group was 0.14 standard deviations lower (0.36 lower to 0.07 higher). | 336 | X X ◯◯ | This difference was not statistically or clinically relevant. |
| Functional disability at the long term Higher scores indicated more severe functional disability. Follow‐up: median 12 months. | The baseline for the most representative study# (Jensen 2011) was 15.6 out of 23 (Roland‐Morris). | The risk in the MBR group was approximately 15.45 out of 23. | The mean functional disability in the intervention group was 0.03 standard deviations lower (0.24 lower to 0.18 higher). | 345 | X X ◯◯ | This difference was not statistically or clinically relevant. |
| Return‐to‐work at long‐term | N/A | N/A | N/A | N/A | NO EVIDENCE | None of the studies that compared MBR to another treatment assessed this outcome. |
| Sick leave periods at long‐term Follow‐up: median 24 months. | Average sick leave in the comparison group was 30 days (Karjalainen 2003). | The risk in the MBR group was approximately 4 (0 to 8) sick leave days. | The mean sick leave days in the intervention group was 0.25 standard deviations lower (0.98 lower to 0.47 higher). | 158 | X ◯◯◯ | There was a difference between the groups but the pooled estimate was imprecise and should not be interpreted. |
| Adverse events | N/A | NO EVIDENCE | None of the included studies reported on adverse events. | |||
| #We defined the most representative sample as the study that has the largest weighting in the overall result in RevMan. *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded due to imprecision, n < 400 (1‐point downgrade). 2Downgraded due to risk of bias, both trials suffered from high risk of performance bias and detection bias. One trial suffered from unclear risk of attrition bias (serious bias = 1‐point downgrade). 3Downgraded due to imprecision 95% confidence interval includes the no effect and the upper or lower confidence limit crosses an effect size of 0.5 (1‐point downgrade). 4Downgraded due to inconsistency, I2 statistic > 60% (1‐point downgrade). 5Downgraded due to risk of bias, the two trials suffered from high risk of performance bias and detection bias (serious bias = 1‐point downgrade). | ||||||
Background
Description of the condition
Low back pain (LBP) is a common condition worldwide, with a one‐month prevalence in the general population of approximately 23% (Hoy 2012). In the latest Global Burden of Disease Study, LBP was identified as the leading cause of disability globally (Vos 2015). It was estimated to be responsible for 72.3 million years lived with disability in 2013, which represents a 57% increase from 1990. In addition to the huge personal toll for individuals and their families, LBP is associated with an enormous societal burden. These costs include healthcare expenditures, as well as the indirect costs related to inability to work or reduced productivity while at work (Dagenais 2008; Luo 2004; Maetzal 2002; Stewart 2003).
Research evidence indicates that the majority of people presenting to healthcare providers with LBP will recover within a few weeks, but a quarter to a third continue to report LBP after 12 months (Hayden 2010). People who reach the chronic stage of LBP (pain for a duration of more than three months) tend to show a more persistent course (Pengel 2003; Hayden 2010); over 50% of these people are not recovered one year later (Menezes Costa 2009). Indeed, it is the small proportion of people with persistent and disabling LBP that account for the majority of social and economic costs (Frymoyer 1991). As a result, there is increasing emphasis on the importance of intervening before symptoms have reached the chronic stage (Chou 2010; Chou 2011). Therefore, in the current review, we focus on subacute LBP, which is defined as back pain with a duration of six to 12 weeks.
Description of the intervention
Multidisciplinary rehabilitation programs acknowledge that although an anatomical or physiological problem can contribute to back pain, psychological factors such as fear, anxiety, mood disturbance, and a tendency to catastrophise may amplify or prolong pain (Main 2012). Similarly, social/environmental factors such as physical job demands, workplace social support, and expectations for resuming work affect long‐term disability (Shaw 2009). These insights have led to the design of interventions to address multiple factors, typically involving a combination of physical, psychological, social and/or work‐related components which are often delivered by a team of clinicians with different skills (Kamper 2014; Guzman 2006). Over time, there has been an increase in research into the multidisciplinary approach due to wider acceptance of the biopsychosocial model (Foster 2011), the ineffectiveness of monotherapies (i.e. the use of single treatments) (Artus 2010), and promising reports from clinical practice. Multidisciplinary biopsychosocial rehabilitation (MBR) may be delivered in multidisciplinary pain clinics, rehabilitation centres, or outpatient settings.
How the intervention might work
The theoretical basis for the intervention comes from the biopsychosocial model (Waddell 1987). According to this theory, chronic LBP involves impairments of physical, psychological and social functioning, and effective treatment requires intervention that specifically addresses these problems. Multidisciplinary biopsychosocial rehabilitation includes elements aimed at improving back‐related physical dysfunction as well as addressing psychological issues or targeting social or work‐related behaviours, or any combination of these.
A large Cochrane Review by Kamper and colleagues (Kamper 2014) found evidence in support of MBR for chronic LBP. They found that participants with chronic LBP receiving multidisciplinary biopsychosocial treatment generally experienced less pain and disability than those receiving usual care or a physical treatment. Although it's unclear whether these effects generalize to the subacute stage of the disorder, accumulating evidence points to the importance of early intervention. Specifically, we know that biopsychosocial risk factors play a role in the transition to chronic LBP (Chou 2011), thus interventions that target these factors in the early stages of LBP may be particularly effective and important to examine.
Why it is important to do this review
Although promising, it is notable that multidisciplinary treatments are labor‐intensive, and their availability, time demands, and costs are important barriers for healthcare providers and consumers (Deyo 2015). The most recent Cochrane review examining the effectiveness of multidisciplinary biopsychosocial treatments among individuals with subacute LBP was published over ten years ago and included only two studies (Karjalainen 2003), thus a careful review of the current state of the literature is long overdue.
Objectives
To examine the effectiveness of MBR for subacute LBP among adults, with a focus on pain, back‐specific disability status, and work status.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs as defined in the Cochrane Handbook (Higgins 2011). We included studies when the full report was peer‐reviewed.
Types of participants
Inclusion criteria
• Adult participants with nonspecific LBP with a mean duration for the current episode greater than six weeks and less than 12 weeks. Given our interest in work status as a primary outcome, participants in the trials were required to be working age (between 18 and 65 years). In samples with mixed durations of pain, more than 75% of the study sample had to have pain that had lasted between six and 12 weeks.
• Participants with or without radiating pain.
Exclusion criteria
• Studies that involved participants with LBP caused by specific pathologies (e.g. infections, neoplasms, metastases, fractures, osteoporosis, rheumatoid arthritis, radiculopathies).
• Studies that involved individuals with LBP during or immediately following pregnancy.
• Studies that recruited participants with postoperative back pain.
Types of interventions
We included studies that investigated a MBR program. This means that the intervention included a physical component (e.g. pharmacological, physical therapy) in combination with either a psychological, social, or occupational component (or any combination of these). We also required involvement of healthcare professionals from at least two different clinical backgrounds.
Physical component
The participant was assessed for physical causes of back pain by a physician, physiotherapist, or other qualified health care professionals, and the participant received pharmacological or exercise/physical therapy (including any of the following: functional restoration, back school, manual therapy, massage, ultrasound, laser therapy, and acupuncture). We excluded surgical interventions.
Psychological component
The participant received group or individual counselling targeting his or her cognitions, emotions, behaviours, beliefs, and/or motives. Cognitive‐behavioral interventions, fear‐avoidance treatment, and motivational interviewing were included here. We expected clinicians to include psychologists, counsellors, and social workers. We excluded any purely educational interventions described in terms of training, advice, skills acquisition, or education (e.g. postural re‐education, advice to stay active).
Social/occupational component
A social worker, occupational physician, case manager, ergonomist, or vocational therapist assessed the participant’s family, social and/or occupational environment and then provided an appropriate intervention.
Comparisons
We included any type of control intervention, but we evaluated the following comparisons separately:
-
MBR versus usual care
-
MBR versus other intervention
We defined usual care as care reflective of the usual management of these participants within the health care system in which the study was conducted. In contrast, we defined other interventions as interventions that were designed specifically for the RCT.
Types of outcome measures
This review focused on patient‐centred outcomes. They were categorized into three groups according to follow‐up time after randomizations.
-
Short‐term: up to three months
-
Medium‐term: > three months and less than 12 months
-
Long‐term: 12 months or more
We defined long term as our primary follow‐up point. Where a study reported multiple follow‐ups, the time‐points closest to three, six and 12 months were used in the meta‐analyses. Separate meta‐analyses were performed for each follow‐up period.
Primary outcomes
-
Pain
-
Back‐specific disability/functional status
-
Work status (return‐to‐work, sick leave)
Secondary outcomes
-
Generic health or quality of life (QoL)
-
Healthcare service utilization
-
Global improvement
-
Psychological and cognitive function (depression, anxiety, fear avoidance, coping strategies)
-
Adverse events
We reported the findings for the primary outcomes and adverse events in our 'Summary of Findings' tables; summary of findings Table for the main comparison and summary of findings Table 2.
Search methods for identification of studies
Electronic searches
We searched for relevant RCTs and quasi‐RCTs in any language in the following databases to 13 July 2016:
-
Cochrane Central Register of Controlled Trials (CENTRAL, which includes the Cochrane Back and Neck (CBN) group trials register; The Cochrane Library, Issue 6)
-
MEDLINE (Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)) (OvidSP, 1946 to 13 July 2016)
-
Embase (OvidSP, 1980 to 2016 Week 28)
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL, EBSCO, 1981 to 13 July 2016)
-
PsycINFO (OvidSP, 2002 to July Week 1 2016)
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP)
An information specialist from the CBN devised and ran the searches according to CBN guidelines (Furlan 2015). In 2016, MEDLINE (Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)) was searched as it allows multiple subsets of Ovid MEDLINE to be searched at one time. The search strategies can be found in Appendix 1.
Searching other resources
We searched reference lists and contacted authors in the field for additional studies.
Data collection and analysis
The methods for this review are based on current recommendations from Cochrane (Higgins 2011) and the Cochrane Back and Neck Group (Furlan 2015). For each of the steps, review authors worked in a team of four (TJM, DVE, RC, EI) or in pairs to independently screen new studies, assess the risk of bias (RoB), and extract data. There were no language restrictions.
Selection of studies
Four reviewers (TJM, DVE, RC, EI) independently screened titles and abstracts using DistillerSR. We then assigned each selected article to a pair of reviewers who independently screened the titles and abstracts and came to consensus about retrieving the full text article. Finally, reviewers worked in pairs to assess all full‐text articles against the inclusion criteria. Moreover, we worked with translators to review all non‐English studies against the inclusion criteria. At this stage, we also reassessed the included studies from the original review against our inclusion criteria. We resolved any disagreements through discussion.
Data extraction and management
Four authors (TJM, DVE, RC, EI) worked in pairs to independently extract the data for each included study using a standardized form in DistillerSR. We then compared the data and resolved any conflicts through discussion. We extracted data on all patient‐centred outcomes.
Assessment of risk of bias in included studies
See Appendix 2 for a description of the 'risk of bias' assessment.
Measures of treatment effect
We combined the outcome measures from the individual trials through meta‐analysis where possible, taking into account clinical comparability of population, intervention and outcomes between trials. Pooled effect estimates were calculated using random‐effects models.
We analysed dichotomous outcomes by calculating the odds ratio (OR). We analysed continuous outcomes by calculating the mean difference (MD) when the same instrument was used to measure the outcomes or the standardized mean difference (SMD) when different instruments were used to measure the outcomes. We expressed the uncertainty of the effect with 95% confidence intervals (95% CI).
We examined SMD effect sizes using Cohen's 'rules of thumb', where 0.2 represented a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988).
Unit of analysis issues
All included studies randomised participants and analysed results at the individual participant level. However, studies in this review reported repeated observations on participants. To address this unit of analysis issue, we followed the guidance in section 9.3.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In particular, we assessed available data from multiple follow‐up periods of the same treatment groups by conducting separate analyses based on different periods of follow‐up; short, intermediate and long term (see Types of outcome measures section above for more details).
Dealing with missing data
Where medians instead of means were reported, we planned to substitute these into the analysis. Where follow‐up standard deviations were not reported, we planned to use the standard deviation for the same measure at baseline as a substitute. Where neither the baseline nor follow‐up standard deviation was reported, we planned to calculate an estimate of the standard deviation from the same measure reported in other studies within the comparison. We attempted to contact authors of the original studies to supply data where insufficient data were reported in the article.
Data synthesis
We assessed the overall quality of the evidence for each outcome. To accomplish this, we used the GRADE approach (Atkins 2004), as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and in the most recent method guidelines from the Cochrane Back and Neck Group (Furlan 2015). Following the GRADE guidelines, we categorized the quality of evidence as high, moderate, low, or very low. The evidence available to answer each subquestion was graded according to the following domains which are further discussed in Appendix 2 : study design, risk of bias, inconsistency, indirectness (not generalisable), and imprecision. We also planned to assess publication bias, but we were not able to assess this due to the limited number of studies identified.
'Summary of Findings' Tables
We reported the findings from our main comparisons and outcomes in 'Summary of Findings' tables.
Our main comparisons were MBR versus usual care, and MBR versus other treatments, and our main outcomes were pain, back‐specific disability, and work status, at long‐term. See summary of findings Table for the main comparison and summary of findings Table 2.
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analyses based on baseline symptom intensity and intervention intensity. We expected that the treatment effect may vary depending on the severity of the condition in the sample, with more severe samples having the potential for greater improvement over the course of the study. Moreover, we expected that more intense interventions would be associated with greater benefits for participants.
We operationalised symptom intensity and intervention intensity as follows:
• Baseline symptom intensity. We categorized studies according to the mean score at baseline on a pain scale and a back‐specific disability measure. We categorized studies with a mean score of 60% or greater of the scale maximum for both pain and disability as 'higher baseline symptom intensity'. We categorized studies with a mean score of less than 60% of the scale maximum for both pain and disability as 'lower baseline symptom intensity'.
• Intervention intensity. We categorized interventions that involved more than 100 face‐to‐face hours and were delivered on a daily basis as having high‐intensity, and interventions that involved less that 30 hours delivered on a non‐daily basis as low‐intensity. We categorized other interventions as mid‐intensity and excluded them from these subgroup analyses (Guzman 2006; Kamper 2014).
In cases where insufficient information was reported to categorise a study, we planned to exclude the study from the subgroup analysis.
Sensitivity analysis
We planned to perform sensitivity analyses to see if the overall estimates of effectiveness changed when only studies with low risk of selection bias were considered. We categorized studies as having low risk of selection bias if they used both adequate random sequence generation and adequate allocation concealment.
Results
Description of studies
We have listed this information in the Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
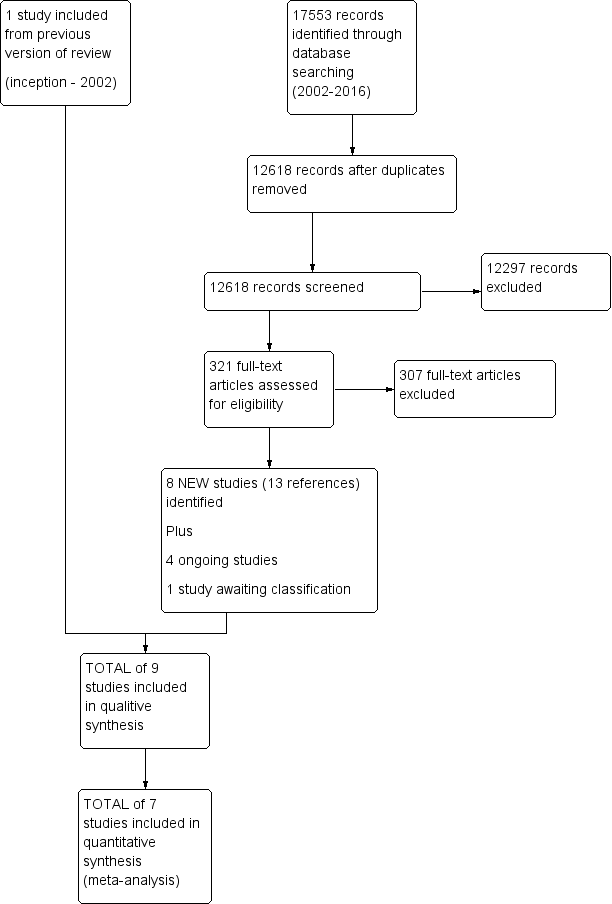
Since the original review, our extensive literature search identified 17553 citations for appraisal against our inclusion and exclusion criteria. We retrieved 321 full‐text articles for further assessment and study selection. Ultimately, eight articles met our inclusion criteria. Additionally, we retained one of the two articles from the original version of this review (i.e. Loisel 1997); we excluded the other (Lindström 1992) because the multidisciplinary intervention was not carried out by clinicians from two or more disciplines. In sum, our review is based on nine studies with a total of 14 references. See Figure 1 for more details.

Study flow diagram.
The search for registered trials identified four ongoing studies (see Characteristics of ongoing studies) and one study awaiting classification (see Characteristics of studies awaiting classification).
Included studies
We included a total of nine RCTs in this review. Five studies were conducted in Europe and four in North America. Sample sizes ranged from 33 to 351. The mean age across trials ranged between 32.0 and 43.7 years. The majority of studies included mixed samples of male and female participants (% female ranged from approximately 40% to 60%). However, both Campello 2012 and Slater 2009 included mainly male participants (< 20% female across the groups). Eight studies reported lower baseline symptom intensity (< 60% on pain and disability scales), and there were insufficient data to categorize one study (see Characteristics of included studies).
All nine studies looked at MBR interventions with a physical component in combination with a psychological, social, and/or vocational component. Eight of the MBR interventions included an occupational component, which consisted of a worksite visit or a work rehabilitation plan or both, and two studies had a strong psychotherapy focus (Campello 2012; Schiltenwolf 2006). Seven of the MBR interventions were integrated programs, meaning that there was communication between professionals from different disciplines. The interventions ranged in duration from two to 18 weeks, with the exception of Karjalainen 2003, which included only a 1.25 hour session aimed to increase body control and exercising, as well as a 75 minute work‐site visit. None of the studies reported high‐intensity interventions (> 100 hours contact time delivered on a daily basis), three studies reported mid‐intensity interventions (> 30 and < 100 hours contact time), and four studies reported low‐intensity interventions (< 30 hours contact time delivered on a non‐daily basis). There were insufficient data to categorize two studies on intensity. See Table 1 for an overview of the MBR interventions, including the practitioners involved, methods for interdisciplinary collaboration, and the frequency/duration of the intervention.
| Study | Practitioners involved | Methods for interdisciplinary collaboration | Intervention intensity |
| Ergonomist, physiotherapist | "The workplace intervention consisted of a workplace assessment and work adjustments in which all major stakeholders in the return‐to‐work process participated: i.e., the worker, the employer, the occupational physician, and the worker’s general practitioner.” | The entire program consisted of two 1‐hour sessions a week, with 26 sessions maximally (13 weeks) = low intensity | |
| Occupational physician, occupational physiotherapist, chiropractor, psychologist, social worker | “The formulation and implementation of a coordinated, tailored and action‐oriented work rehabilitation plan collaboratively developed by an interdisciplinary team using a feedback guided approach.” | The duration of the intervention was for up to three months; insufficient information to categorize intervention intensity. | |
| Physical therapist, psychologist, physician | “Backs to Work is a coordinated multidisciplinary reconditioning program” | The duration of the intervention was 3 hours per day, 3 days/week for 4 weeks = 36 hours = mid‐intensity | |
| Rehabilitation physician, specialist in clinical social medicine, physiotherapist, social worker, occupational therapist | Coordinated through case manager | The duration of the intervention was 18 weeks, average of 4 meetings with case manager = low intensity | |
| Physician, physiotherapist, company nurse, company physician | Physiotherapist visited patient’s workplace to involve work supervisor and company health care professionals in treatment | The duration of the mini‐intervention was 1.25‐1.5 hours and the worksite visit was approximately 75 minutes = low intensity | |
| Occupational physician, ergonomist, back pain specialist | “All described interventions were provided by a multidisciplinary medical, ergonomic, and rehabilitation staff at the Sherbrooke University Hospital back pain clinic.” | In a previous study using the same protocol (Loisel 1994), the duration of functional rehabilitation therapy ranged from 2 to 13 weeks. Insufficient information to categorize intervention intensity. | |
| Physician, physiotherapist, psychotherapist | This does not appear to be an integrated program. The physiotherapists were blind to treatment condition, indicating no communication between the physiotherapists and psychotherapists. | The duration of the intervention was 6 hours of daily treatment for 15 days in 3 weeks = mid‐intensity | |
| Physician, masters‐level clinician for behavioural medicine intervention | The extent to which health care professionals communicated wasn't clear from the article text. | The duration of the intervention was 6‐10 weeks, 4 hours a week = mid‐intensity | |
| Physical therapy and behavioral medicine sessions “provided by licensed professionals trained in their respective fields;” occupational therapist | Case management sessions and interdisciplinary team conferences held at baseline and discharge. | The duration of the intervention was from 4 to 10 weeks; 6‐9 behavioral medicine sessions; 6‐9 physical therapy sessions; Up to 6 work transitions sessions; one or more case management sessions = low intensity |
Six studies compared MBR to usual care(Anema 2007; Bultmann 2009; Campello 2012; Karjalainen 2003; Loisel 1997; Whitfill 2010). However, it should be noted that Anema 2007 differed from the other studies in that the comparison group included participants receiving usual care, as well as those who received either graded activity alone or the work intervention alone. However, their analyses statistically controlled for the effects of graded activity alone and the work intervention alone, thus the results reflected the difference between the combined intervention (i.e. MBR) and usual care.
Four studies compared MBR to other treatments (Jensen 2011; Karjalainen 2003; Schiltenwolf 2006; Slater 2009). The other treatment comparisons included (1) a 'mini' intervention with features from a light mobilization program and a graded activity program (Karjalainen 2003), (2) a brief clinical intervention including education and advice on exercise (Jensen 2011), (3) a functional restoration program of individual physiotherapy, group therapy in water, workout, and back school (Schiltenwolf 2006), and (4) usual medical care plus nondirective supportive care using a Rogerian counselling approach (Slater 2009).
Excluded studies
We excluded 307 articles (277 English and 30 nonEnglish) after full‐text screening. nonEnglish papers were reviewed by colleagues proficient in the language of the article. The most common reasons for exclusion were: study design other than RCT, inclusion of participants other than those with subacute LBP, and index interventions that did not include two or more elements of the biopsychosocial model or were not delivered by clinicians of different clinical backgrounds. See Characteristics of excluded studies for more information.
Risk of bias in included studies
We identified four studies (Anema 2007; Karjalainen 2003; Loisel 1997; Schiltenwolf 2006) as having lower risk of bias relative to the other included studies. In particular, these studies suffered from risk of bias in only two bias categories (performance bias and detection bias). Moreover, with the exception of Karjalainen 2003, these studies also included outcomes based on administrative data (e.g. return‐to‐work, sick leave data), thereby minimizing the impact of detection bias for these particular outcomes. The results of the 'risk of bias' assessment are summarized in Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We classified seven of the nine included studies as having low risk of selection bias. Methods of ensuring adequate randomizations included computer‐generated random numbers and a lottery system, and treatment allocation was concealed using various methods, including sealed envelopes and conducting the allocation off site. The other two studies (Campello 2012; Whitfill 2010) were classified as having unclear risk of selection bias because they didn't specify the randomizations or allocation concealment methods used.
Blinding
Due to the nature of the MBR intervention, none of the included studies achieved blinding of participants. Indeed, it would have been very difficult to keep participants blind to an intervention that they were actively participating in. However, blinding of personnel was achieved by Slater 2009. In this study assessors and therapists were not told about the alternative treatments and hypotheses, and treatments were conducted in separate areas to prevent cross‐talk.
Measures of pain and disability rely on participant reports of their symptoms, which may be influenced by participants' knowledge of group assignment, as well as their expectations about the effectiveness of different interventions. In fact, in an MBR intervention, where there tends to be a great deal of face‐to‐face time between practitioners and participants, the experience of receiving the intervention may have an important impact on reported outcomes. However, six included studies measured more objective outcomes (e.g. sick leave data from health insurance companies). Thus, for these outcomes, detection bias was low.
Given the challenges of blinding in MBR trials, risk of performance bias and detection bias represented the main source of potential bias in this review. As a result of these study limitations, all of the evidence was downgraded by at least one point in the GRADE assessment (see Quality of the evidence section below).
Incomplete outcome data
We judged six of the nine included studies as having Iow risk of attrition bias. These studies described dropouts in detail and used an intent‐to‐treat analysis, when appropriate. Only one study was rated as having high risk of attrition bias due to over 30% loss to follow‐up in the intervention condition.
Selective reporting
We assessed all but one study in the review as having low risk of reporting bias. The one exception was the study by Slater 2009, which failed to report group differences for some study outcomes.
Other potential sources of bias
There were no additional sources of bias identified.
Effects of interventions
See: Summary of findings for the main comparison Multidiscipinary rehabilitation versus usual care for subacute low back pain at long‐term follow‐up; Summary of findings 2 Multidisciplinary rehabilitation versus other treatment for subacute low back pain at long‐term follow‐up
See the summary of findings from our main comparisons: MBR versus usual care (summary of findings Table for the main comparison) and MBR versus other treatment (summary of findings Table 2). We included all studies in at least one meta‐analysis, with the exception of Anema 2007 and Slater 2009. Anema 2007 reported data in a manner that did not allow for inclusion in the meta‐analysis, and Slater 2009 reported only one outcome (i.e. a dichotomous recovery outcome) that could not be combined with outcomes from other studies.
We estimated standard deviations for all data from Karjalainen 2003 by calculating an estimate of the standard deviation from the same measure reported at baseline in other studies within the comparison. However, in one case this information was not available, so we used ranges to estimate standard deviations by dividing the range by four (sick leave days; Karjalainen 2003). See Characteristics of included studies for (1) findings that could not be incorporated into meta‐analyses and (2) findings for all secondary outcomes.
Multidisciplinary biopsychosocial rehabilitation versus usual care
Pain
Pain intensity short‐term
Very low‐quality evidence from four studies with a total of 272 participants (Bultmann 2009; Campello 2012; Karjalainen 2003; Loisel 1997) showed that MBR was more effective than usual care for short‐term pain improvement (standard mean difference (SMD) ‐0.40, 95% confidence interval (CI) ‐0.74 to ‐0.06) (see Analysis 1.1, Figure 3).

Forest plot of comparison: 1 Multidisciplinary rehabilitation versus usual care, outcome: 1.1 Pain intensity (scales varied from 0 to 10 or 0 to100).
Pain at intermediate‐term
Very low‐quality evidence from two studies with a total of 155 participants (Karjalainen 2003; Loisel 1997) showed that MBR was no better than usual care for intermediate‐term pain improvement (SMD ‐0.34, 95% CI ‐1.0 to 0.31) (see Analysis 1.1, Figure 3).
Pain at long‐term
Moderate quality evidence from five studies with a total of 532 participants reported pain intensity at long‐term. We included four studies with a total of 336 participants in the meta‐analysis (Bultmann 2009; Karjalainen 2003; Loisel 1997; Whitfill 2010) and analysed one study with 196 participants separately (Anema 2007) because results were presented as mean improvements, which could not be combined with means from other studies. Results from the meta‐analysis indicated that MBR was more effective than usual care for long‐term pain improvement (SMD ‐0.46, 95% CI ‐0.70 to ‐0.21) (see Analysis 1.1, Figure 3). Anema 2007 found no differences between the groups. Although it was unclear why the Anema 2007 findings were inconsistent with the pooled effect, it may be due to the comparison group, which included participants who received usual care, as well as those who received graded activity or the work intervention alone. These findings suggested that the combination of graded activity and the work intervention (i.e. MBR) did not have an impact over and above the independent effects of these interventions.
Disability
Disability at short‐term
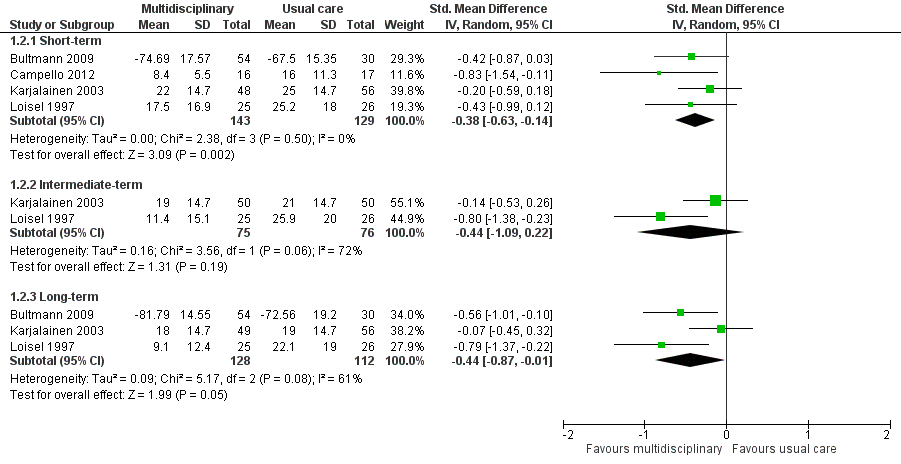
Very low‐quality evidence from four studies with a total of 272 participants (Bultmann 2009; Campello 2012Karjalainen 2003; Loisel 1997) showed that MBR was more effective than usual care for disability in the short term (SMD ‐0.38, 95% CI ‐0.63 to ‐0.14) (see Analysis 1.2, Figure 4). It should be noted that Bultmann 2009 used an inverted Oswestry scale, such that lower scores indicated more severe disability. Therefore, data from this study were reverse‐coded and then entered into the meta‐analysis. This procedure applied to the long‐term disability results presented below. See Characteristics of included studies for more information about the inverted scale.
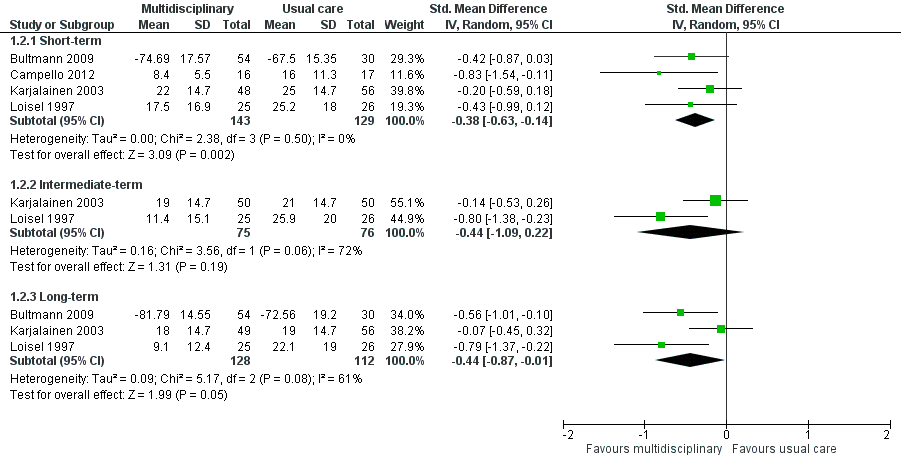
Forest plot of comparison: 1 Multidisciplinary rehabilitation versus usual care, outcome: 1.2 Disability (measured with different continuous scales)
Disability at intermediate‐term
Very low‐quality evidence from two studies with a total of 151 participants (Karjalainen 2003; Loisel 1997) showed that MBR was no better than usual care for disability in the intermediate term (SMD ‐0.44, 95% CI ‐1.09 to 0.22) (see Analysis 1.2, Figure 4).
Diability at long‐term
Low‐quality evidence from five studies with a total of 537 participants reported disability in the long‐term. We included three studies with a total of 240 participants in the meta‐analysis (Bultmann 2009; Karjalainen 2003; Loisel 1997) and reported Whitfill 2010 (101 participants) and Anema 2007 (196 participants) individually. We could not include Anema 2007 in the meta‐analysis because results were presented as mean improvements, which could not be combined with means from other studies, and Whitfill 2010 reported results of a statistical test comparing the two groups but failed to report group means. Results from the meta‐analysis showed that MBR was more effective than usual care for long‐term disability (SMD ‐0.44, 95% CI ‐0.87 to ‐0.01) (see Analysis 1.2, Figure 4). Whitfill 2010 also showed less disability in the MBR group compared to usual care; Anema 2007 found no differences between the groups. Again, it was unclear why the Anema 2007 findings deviated from those of the other studies, but it may be due to the fact that some participants in the comparison group received graded activity or the workplace intervention.
Sick Leave
Sick leave at short‐term
Very low‐quality evidence from one study with 33 participants (Campello 2012) showed that MBR was no better than usual care for short‐term work status; all subjects in both groups were back to duty by the end of the intervention period.
Sick leave at long‐term
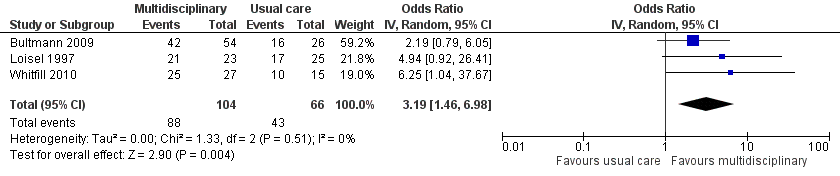
Studies that compared MBR to usual care reported long‐term sick leave using three different outcomes: return‐to‐work, time to return‐to‐work, and sick leave periods.
Very low‐quality evidence from three studies with a total of 170 participants (Bultmann 2009; Loisel 1997; Whitfill 2010) showed that MBR was more effective than usual care for return‐to‐work at the long term (OR 3.19, 95% CI 1.46 to 6.98) (see Analysis 1.3, Figure 5).

Forest plot of comparison: 1 Multidisciplinary rehabilitation versus usual care, outcome: 1.3 Return‐to‐work at long‐term.
Low‐quality evidence from one study of 196 participants (Anema 2007) showed that MBR was no better than usual care for reducing time to return‐to‐work.
Low‐quality evidence from two studies with a total of 210 participants (Bultmann 2009; Karjalainen 2003) showed that MBR was more effective than usual care in reducing sick leave periods at the long term (SMD ‐0.38, 95% CI ‐0.66 to ‐0.10) (see Analysis 1.4).
Secondary outcomes at short‐term
One study with 33 participants (Campello 2012) showed no differences between the groups in pain catastrophising, symptoms of depression and fear‐avoidance beliefs.
Secondary outcomes at intermediate‐term
No secondary outcomes were reported at intermediate‐term.
Secondary outcomes at long‐term
One study with 100 participants (Whitfill 2010) showed less depression in the MBR group compared to the usual care group. In terms of quality of life (measured by the SF‐36); the MBR group showed improved physical functioning compared to the usual care group, but there were no group differences in mental functioning.
One study with 105 participants (Karjalainen 2003) examined quality of life at 12 and 24 months. However, MBR was no more effective than usual care in improving quality of life at both time‐points.
One study of 51 participants (Loisel 1997) reported on generic functional status at 12 months. Results suggested that MBR was more effective in improving functional status compared to usual care.
Multidisciplinary biopsychosocial rehabilitation versus other treatment
Pain
Pain intensity short‐term
Very low‐quality evidence from two studies with a total of 165 participants (Karjalainen 2003; Schiltenwolf 2006) showed that MBR was no more effective than another treatment for short‐term pain improvement (SMD ‐0.09, 95%, CI ‐0.50 to 0.33) (see Analysis 2.1, Figure 6).

Forest plot of comparison: 2 Multidisciplinary rehabilitation versus other treatment, outcome: 2.1 Pain.
Pain at intermediate‐term
Very low‐quality evidence from two studies with a total of 162 participants (Karjalainen 2003; Schiltenwolf 2006) showed that MBR was no better than another treatment for intermediate‐term pain improvement (SMD ‐0.64, 95% CI ‐1.85 to 0.57) (see Analysis 2.1, Figure 6).
Pain at long‐term
Low‐quality evidence from two studies with a total of 336 participants (Jensen 2011; Karjalainen 2003) showed that MBR was no better than another treatment for long‐term pain improvement (SMD ‐0.14, 95% CI ‐0.36 to 0.07) (see Analysis 2.1, Figure 6).
Disability
Disability at short‐term
Low‐quality evidence from two studies with a total of 165 participants (Karjalainen 2003; Schiltenwolf 2006) showed that MBR was no more effective than another treatment for disability at the short term (SMD ‐0.00, 95%, CI ‐0.34 to 0.34) (see Analysis 2.2, Figure 7).

Forest plot of comparison: 2 Multidisciplinary rehabilitation versus other treatment, outcome: 2.2 Disability (Different instruments).
Disability at intermediate‐term
Very low‐quality evidence from two studies with a total of 162 participants (Karjalainen 2003; Schiltenwolf 2006) showed that MBR was no more effective than another treatment for disability at the intermediate term (SMD ‐0.49, 95% CI ‐1.50 to 0.51) (see Analysis 2.2, Figure 7).
Very low‐quality evidence from one study of 65 participants (Slater 2009) showed no difference in the proportion of participants recovered (defined in terms of pain and function) when comparing the MBR group to another treatment.
Disability at long‐term
Low‐quality evidence from two studies with a total of 345 participants (Jensen 2011; Karjalainen 2003) showed that MBR was no better than another treatment for disability at the long term (SMD ‐0.03, 95% CI ‐0.24 to 0.18) (see Analysis 2.2, Figure 7).
Sick Leave
Sick leave at long‐term
Studies that compared MBR to another treatment reported two different sick leave outcomes: time to return‐to‐work and sick leave periods.
Low‐quality evidence from one study of 351 participants (Jensen 2011) showed that MBR was no more effective than another treatment in reducing time to return‐to work.
Very low‐quality evidence from two studies with a total of 158 participants (Karjalainen 2003; Schiltenwolf 2006) showed that MBR was no more effective than another treatment in reducing sick leave periods at the long term (SMD ‐0.25, 95% CI ‐0.98 to 0.47) (see Analysis 2.3).
Secondary outcomes at short‐term
No secondary outcomes were reported at the short‐term.
Secondary outcomes at intermediate‐term
One study of 56 participants examined depression at six months (Schiltenwolf 2006). Results indicated that MBR effectively reduced depressive dysfunction compared to another treatment.
Secondary outcomes at long‐term
One study examined fear‐avoidance and general health (SF‐36) and mental health (SF‐36) at 12 months (numbers of participants ranged from 237 to 244) (Jensen 2011). Results indicated that MBR was no more effective in reducing fear avoidance or improving physical functioning compared to another treatment. However, MBR more effectively improved mental health compared to another treatment.
Subgroup Analyses
We planned to conduct subgroup analyses to examine the treatment effect in studies with higher versus lower baseline symptom intensity and higher versus lower intervention intensity. We were unable to conduct any subgroup analyses because we did not identify any studies that met our criteria for higher baseline symptom intensity, or studies with interventions that met our criteria for higher intervention intensity.
Sensitivity Analyses
We planned to conduct sensitivity analyses to examine the treatment effect for studies with low risk of selection bias. However, we identified too few studies to conduct these analyses, as planned.
Discussion
Summary of main results
This review included nine published RCTs, with data from close to 1000 participants. Overall, we found moderate to very low‐quality evidence in favour of MBR compared to usual care for subacute LBP. In particular, compared to individuals receiving usual care, individuals receiving MBR showed less pain and back‐specific disability, as well as increased likelihood of return‐to‐work and fewer sick leave days at 12‐month follow‐up. When comparing MBR to other treatments, we found low to very low‐quality evidence that MBR was no better than other treatments (i.e. brief intervention with features from a light mobilization program and a graded activity program, functional restoration, brief clinical intervention including education and advice on exercise, and psychological counselling) for reducing pain and disability, and reducing time away from work.
Although more evidence is needed to increase our confidence in these results, the effect estimates for pain and disability were consistent across all follow‐up points. For pain, they translated to about a 1‐point difference on a 10‐point scale, and for disability, they translated to around a 6.5‐ to 9‐point difference on a 100‐point scale. These effect sizes are at the low end of the range of estimates of clinical meaningfulness. Effects on work‐related outcomes were somewhat larger; participants receiving MBR had more than three times the odds of return‐to‐work at one year compared to participants receiving usual care, and fewer sickness absence periods (approximately 215 to 254 hours) at follow‐up.
Our findings suggest that MBR treatments are no better than other treatments for improving outcomes among people with subacute LBP. This was true across all primary outcomes, and at short‐, intermediate‐, and long‐term follow‐up points.
Findings for secondary outcomes
Studies in this review examined secondary outcomes, including symptoms of depression, pain catastrophising, fear‐avoidance beliefs, general health, generic functional status and quality of life. However, we were unable to synthesize results across studies because of a lack of common outcomes across comparisons and follow‐up periods. Results were generally inconsistent across studies ‐ some showed improvements in the MBR group when compared to usual care or another treatment, while others showed no differences between the groups. The most consistent findings related to mental health outcomes at intermediate‐ and long‐term follow‐up. Namely, two studies showed benefits of MBR for reducing symptoms of depression and one showed benefits of MBR for improving mental health more generally. This points to the potential benefits of MBR for psychological well being among participants with subacute LBP and should be considered more fully in future research. However, it should be noted that we did not assess the quality of the evidence for these findings, and it is probably too early to use them as a basis for clinical decision‐making.
Overall completeness and applicability of evidence
This review provides initial answers about the effectiveness of MBR for people with subacute LBP. However, the literature failed to cover all relevant types of participants, interventions, and outcomes. In particular, the evidence was based on a relatively homogenous group of participants. For example, all of the studies were conducted in North America and Europe, so it was unclear whether our findings generalized to people outside of these geographic areas. Moreover, all of the included studies were based on populations with relatively low baseline symptom intensity, thus we were unable to examine whether the treatment effect differed for people with high‐ versus low‐intensity symptoms. However, we categorized symptom intensity based on the cut‐off used by Kamper and colleagues in the Cochrane Review on MBR for chronic LBP (Kamper 2014) (greater than 60% of the maximum possible score on a pain and a disability measure), which may not be valid for people with subacute LBP.
We encountered the same issue when it came to classification of MBR interventions as high versus low‐intensity. In particular, although we noted MBR interventions that ranged in intensity (e.g. 'mini' intervention plus worksite visit (< three hours total)) in Karjalainen 2003 versus 36‐hour intervention in Campello 2012), none fell into the 'high‐intensity' category based on our pre‐established definition (i.e. > 100 face‐to‐face hours delivered on a daily basis) (Kamper 2014). Therefore, we were unable to examine whether intervention intensity influenced the treatment effect. Further consideration of the impact of intervention intensity on treatment outcome will be important for future updates, as it has implications for both the effectiveness and cost‐effectiveness of MBR interventions.
In regard to the outcomes reported, none of the studies in this review assessed adverse events, but given the nature of the intervention, the risk was considered low. Further, work outcomes and healthcare utilisation are key considerations for assessing the effects of MBR in this population, since they are primary determinants of the societal burden of the condition (Maetzal 2002). Many of the included studies did not report these outcomes, and when reported they were measured in different ways. For example, in our analysis of work status at long‐term follow up, we were able to statistically combine only three of the six studies that compared MBR to usual care.
Quality of the evidence
The findings from this review provided mainly low to very low‐quality evidence regarding the effectiveness of MBR in this population. We mainly downgraded studies due to lack of blinding, which increased risk of performance bias, as well as risk of detection bias (i.e. biased self‐reports of pain and disability due to knowledge of treatment group). However, effect sizes were similar for self‐report outcomes (i.e. pain and disability) and behavioral outcomes (i.e. return‐to‐work and sickness absence), which are less susceptible to bias. This consistency across the different types of outcomes suggested that our findings may not be unduly influenced by bias associated with self‐reported outcomes.
We also downgraded the evidence due to inconsistency. There was heterogeneity across participants and interventions, but we were unable to conduct subgroup analyses due to the limited number of studies identified. As a result, we lumped together some studies with a good deal of clinical heterogeneity. For example, we combined a low‐intensity MBR intervention (i.e. 'mini' clinical intervention plus worksite visit (< three hours total) (Karjalainen 2003)) together with more intense interventions, but we were unable to explore the impact of this on our treatment estimates. Despite this, our results were quite consistent across outcomes for the MBR versus usual care comparison, which increases confidence in the treatment estimates.
In regard to the MBR versus 'other treatment' findings, clinical heterogeneity among the 'other treatments' may have contributed to the inconsistent effects across studies. For example, two studies compared MBR to a brief intervention, including a physical examination and advice to stay active (Jensen 2011; Karjalainen 2003), one study compared MBR to a more extensive functional restoration program (Schiltenwolf 2006), and one study included a comparison that was mainly psychological in nature (Slater 2009). With only two studies included in each meta‐analysis, it is likely that further research would change our estimates of effectiveness. Observed inconsistencies may be due to characteristics of the MBR intervention, characteristics of the comparison intervention, or a combination of the two.
We downgraded the evidence due to imprecision for both comparisons. We were dealing with small sample sizes, especially for the analyses looking at work‐related outcomes. Therefore, the quality of the evidence and our confidence in the results will be much improved with the publication of large RCTs that consider common outcomes.
Finally, it should be noted that there is one completed study for which we were unable to find any published articles (see Characteristics of studies awaiting classification).
Potential biases in the review process
First, it is challenging to operationalise the term 'multidisciplinary', and there is no universally accepted definition of MBR (Deyo 2015). Our definition is consistent with both the biopsychosocial model and previous Cochrane Reviews on this topic (Guzman 2006; Karjalainen 2003). However, it is possible that selection of a different definition could result in inclusion of different studies and hence different effect estimates (see Kamper 2014 for a more detailed discussion).
Second, our definition of MBR was quite stringent, as we required involvement of healthcare professionals from at least two different clinical backgrounds. If future work shows that these types of MBR interventions are indeed effective for subacute LBP, a next step will be to examine whether similar effects are observed when less stringent definitions of MBR are applied.
Third, we have included in our meta‐analyses all studies irrespective of their risk of bias. Including studies with high risk of bias may have affected the point estimates, particularly in cases indicating statistical heterogeneity.
Finally, we made the decision a priori to omit non‐published and non‐peer‐reviewed studies from the review, which may have contributed to publication bias given that publication status may be associated with positive study results. Unfortunately the influence of publication bias on our results was difficult to assess due to the limited number of studies contributing to each pooled estimate. However, we have one study awaiting assessment (Rodriguez‐Blanco) with almost the same number of patients as our review (932) which is evidence of publication bias. Publication of this study could substantially change the conclusions of this review.
Agreements and disagreements with other studies or reviews
Our results were fairly consistent with those of the Cochrane Review on MBR for chronic LBP (Kamper 2014). In particular, both reviews found evidence for the effectiveness of MBR when compared to usual care. However, whereas the current review did not find any evidence that MBR was more effective than other treatments for subacute LBP, Kamper and colleagues found that MBR was more effective than other physical interventions for chronic LBP. Although it was unclear why this was the case, it may be due to varying disease trajectories among people with subacute LBP ‐ some will improve regardless of treatment, others will do well with simple monotherapies, and others will only improve with more tailored treatment approaches, such as MBR. In contrast, by the time people reach the chronic stage of LBP with its associated psychosocial stressors (e.g. increased time away from work, greater vulnerability to depression), we can expect a greater proportion of people to benefit from a multidisciplinary approach. If this is the case, it may be more effective to target subgroups of people with subacute LBP who are most likely to benefit from MBR.
We also compared our findings to a Cochrane Review on physical conditioning for workers with LBP (Schaafsma 2013), which shared three included studies with our review (Jensen 2011, Karjalainen 2003, Loisel 1997). Schaafsma and colleagues showed that physical conditioning reduced sickness absence duration compared to usual care among participants with subacute LBP, but only when the intervention took place in the workplace or it included a workplace visit. This finding is consistent with our results, which also suggested that a physical intervention may be effective when combined with a psychological or workplace intervention or both.
An important next step will be to disentangle the impact of the physical, psychological and workplace components and to identify underlying mechanisms. To this end, it would be informative to compare our results with those of other reviews examining monotherapies for subacute LBP, especially those relating to MBR, such as back schools, graded behavioral activity, workplace interventions, and psychological interventions, such as cognitive behavior therapy. However, we were unable to find up‐to‐date reviews that examined these interventions among participants with subacute LBP.
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Multidisciplinary rehabilitation versus usual care, outcome: 1.1 Pain intensity (scales varied from 0 to 10 or 0 to100).
Forest plot of comparison: 1 Multidisciplinary rehabilitation versus usual care, outcome: 1.2 Disability (measured with different continuous scales)
Forest plot of comparison: 1 Multidisciplinary rehabilitation versus usual care, outcome: 1.3 Return‐to‐work at long‐term.
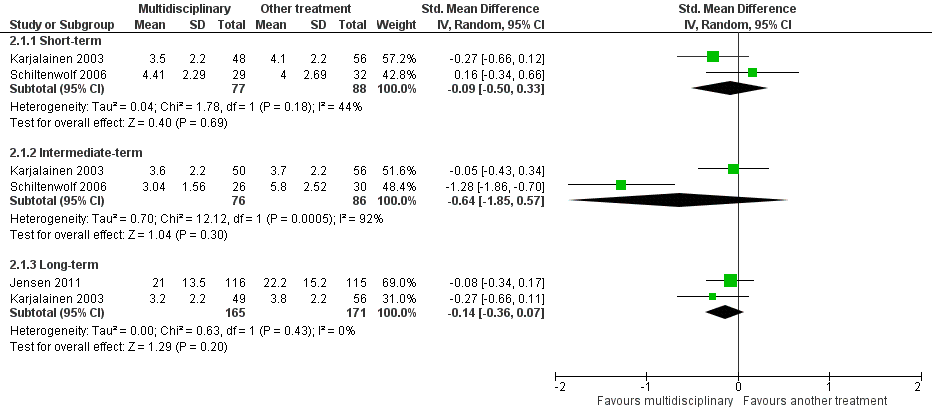
Forest plot of comparison: 2 Multidisciplinary rehabilitation versus other treatment, outcome: 2.1 Pain.

Forest plot of comparison: 2 Multidisciplinary rehabilitation versus other treatment, outcome: 2.2 Disability (Different instruments).

Comparison 1 Multidisciplinary rehabilitation versus usual care, Outcome 1 Pain.
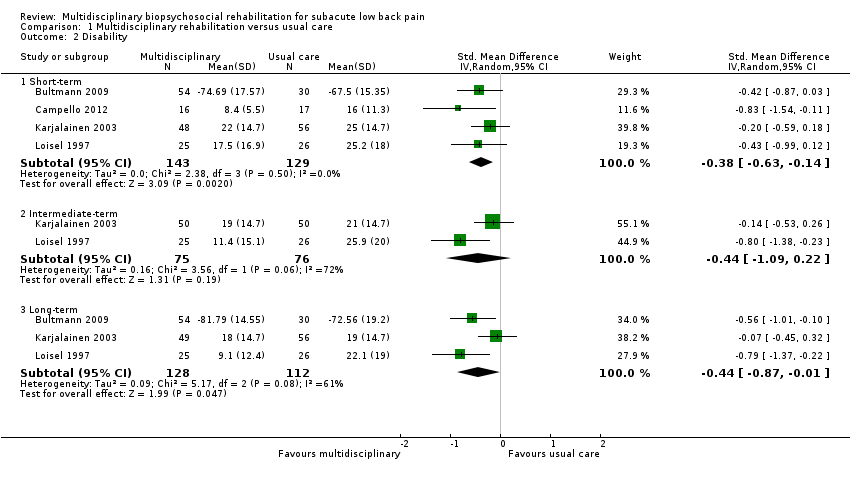
Comparison 1 Multidisciplinary rehabilitation versus usual care, Outcome 2 Disability.
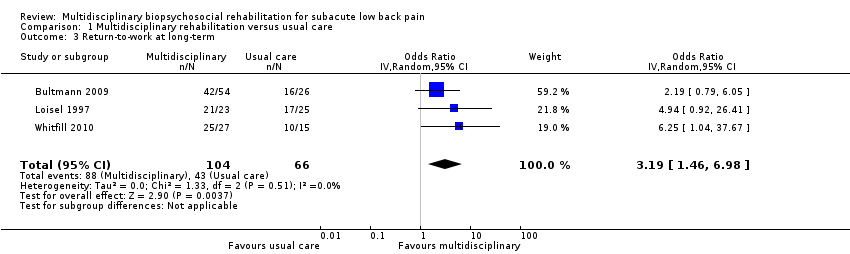
Comparison 1 Multidisciplinary rehabilitation versus usual care, Outcome 3 Return‐to‐work at long‐term.

Comparison 1 Multidisciplinary rehabilitation versus usual care, Outcome 4 Sick leave periods at long‐term.

Comparison 2 Multidisciplinary rehabilitation versus other treatment, Outcome 1 Pain.

Comparison 2 Multidisciplinary rehabilitation versus other treatment, Outcome 2 Disability.
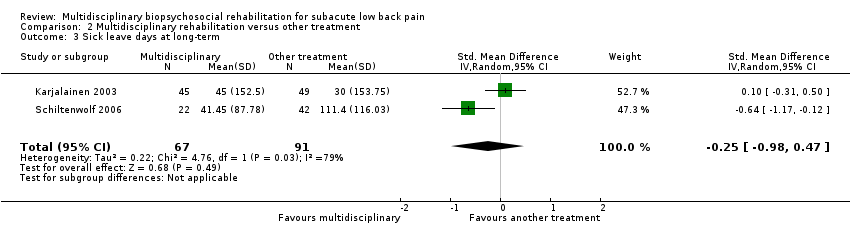
Comparison 2 Multidisciplinary rehabilitation versus other treatment, Outcome 3 Sick leave days at long‐term.
| Patient or population: Subacute low back pain | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence (GRADE) | Comments | ||
| Risk with usual care | Risk with multidisciplinary rehabilitation | ||||||
| Back pain long‐term Higher scores indicated more intense pain Follow‐up: median 12 months | The baseline for the most representative study# (Karjalainen 2003) was 5.7 out of 10 (visual analogue scale). | The risk with MBR was approximately 4.67 (4.60 to 4.73) out of 10. | The mean pain in the intervention group was 0.46 standard deviations lower (0.7 lower to 0.21 lower). | 336 | TOTAL = 532 (5 RCTs) | X X X ◯ MODERATE 1 | This was a small to moderate effect (Cohen 1988) that is probably clinically relevant in this participant group. |
| An additional study that could not be included in meta‐analysis showed no difference between the groups. | ‐ | 196 (1 RCT included in qualitative synthesis). | |||||
| Functional disability at the long term Higher scores indicated more disability Follow‐up: median 12 months. | The baseline for the most representative study# (Karjalainen 2003) was 34 out of 100 (Oswestry Scale). | The risk with MBR was approximately 26.30 (25.2 to 27.4) out of 100. | The mean functional disability in the intervention group was 0.44 standard deviations lower (0.87 lower to 0.01 lower). | 240 | TOTAL = 537 (5 RCTs) | X X ◯◯ | This was a small to moderate effect (Cohen 1988) that is probably clinically relevant in this participant group. |
| Two additional studies could not be included in meta‐analysis. One study showed evidence in favour of MBR and the other showed no difference between the groups. | ‐ | 297 (2 RCTs included in qualitative synthesis). | |||||
| Return‐to‐work at the long term Proportion at work Follow‐up: median 12 months. | Study population | OR 3.19 (1.46 to 6.98) | 170 | X ◯◯◯ | This was a moderate effect that is clinically relevant in this participant group. | ||
| 65 per 100 | 86 per 100 | ||||||
| Sick leave periods at long‐term Cumulative sickness absence periods over the course of the 12‐month follow‐up. | Average sick leave in the usual care group was 997.3 hours (Bultmann 2009). | The risk with MBR was approximately 763.03 (743.3 to 782.3) sick leave hours. | The mean sick leave periods in the intervention group was 0.38 standard deviations lower (0.66 lower to 0.10 lower). | 210 | X X ◯◯ | This was a small to moderate effect (Cohen 1988) that is clinically relevant in this participant group. | |
| Adverse events | N/A | NO EVIDENCE | None of the included studies reported on adverse events. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). #We defined the most representative sample as the study that has the largest weighting in the overall result in RevMan. | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1 Downgraded due to risk of bias, all five trials had high risk of performance bias and detection bias. One trial suffered from unclear risk of selection bias. Another trial suffered from unclear risk of attrition bias (serious bias = 1‐point downgrade). 2 Downgraded due to inconsistency, I2 statistic60% (heterogeneity = 1‐point downgrade). 3 Downgraded due to serious risk of bias, all three trials suffered from risk of performance bias and detection bias. One trial also suffered from unclear risk of selection bias and another trial suffered from unclear risk of attrition bias (very serious bias = 2‐point downgrade). 4 Downgraded due to imprecision, the total number of events was less than 300 (1‐point downgrade). 5 Downgraded due to risk of bias, both trials suffered from risk of performance bias and detection bias. One trial also suffered from unclear risk of attrition bias (serious bias = 1‐point downgrade). 6 Downgraded due to imprecision, total population size < 400 (1‐point downgrade). | |||||||
| Patient or population: Subacute low back pain | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with another treatment | Risk with multidisciplinary rehabilitation | |||||
| Pain at the long term Higher scores indicated more intense pain Follow‐up: median 12 months. | The baseline for the most representative study# (Jensen 2011) was 32.7 out of 60 (LBP Rating Scale). | The risk in the MBR group was approximately 31.02 out of 60. | The mean pain in the intervention group was 0.14 standard deviations lower (0.36 lower to 0.07 higher). | 336 | X X ◯◯ | This difference was not statistically or clinically relevant. |
| Functional disability at the long term Higher scores indicated more severe functional disability. Follow‐up: median 12 months. | The baseline for the most representative study# (Jensen 2011) was 15.6 out of 23 (Roland‐Morris). | The risk in the MBR group was approximately 15.45 out of 23. | The mean functional disability in the intervention group was 0.03 standard deviations lower (0.24 lower to 0.18 higher). | 345 | X X ◯◯ | This difference was not statistically or clinically relevant. |
| Return‐to‐work at long‐term | N/A | N/A | N/A | N/A | NO EVIDENCE | None of the studies that compared MBR to another treatment assessed this outcome. |
| Sick leave periods at long‐term Follow‐up: median 24 months. | Average sick leave in the comparison group was 30 days (Karjalainen 2003). | The risk in the MBR group was approximately 4 (0 to 8) sick leave days. | The mean sick leave days in the intervention group was 0.25 standard deviations lower (0.98 lower to 0.47 higher). | 158 | X ◯◯◯ | There was a difference between the groups but the pooled estimate was imprecise and should not be interpreted. |
| Adverse events | N/A | NO EVIDENCE | None of the included studies reported on adverse events. | |||
| #We defined the most representative sample as the study that has the largest weighting in the overall result in RevMan. *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded due to imprecision, n < 400 (1‐point downgrade). 2Downgraded due to risk of bias, both trials suffered from high risk of performance bias and detection bias. One trial suffered from unclear risk of attrition bias (serious bias = 1‐point downgrade). 3Downgraded due to imprecision 95% confidence interval includes the no effect and the upper or lower confidence limit crosses an effect size of 0.5 (1‐point downgrade). 4Downgraded due to inconsistency, I2 statistic > 60% (1‐point downgrade). 5Downgraded due to risk of bias, the two trials suffered from high risk of performance bias and detection bias (serious bias = 1‐point downgrade). | ||||||
| Study | Practitioners involved | Methods for interdisciplinary collaboration | Intervention intensity |
| Ergonomist, physiotherapist | "The workplace intervention consisted of a workplace assessment and work adjustments in which all major stakeholders in the return‐to‐work process participated: i.e., the worker, the employer, the occupational physician, and the worker’s general practitioner.” | The entire program consisted of two 1‐hour sessions a week, with 26 sessions maximally (13 weeks) = low intensity | |
| Occupational physician, occupational physiotherapist, chiropractor, psychologist, social worker | “The formulation and implementation of a coordinated, tailored and action‐oriented work rehabilitation plan collaboratively developed by an interdisciplinary team using a feedback guided approach.” | The duration of the intervention was for up to three months; insufficient information to categorize intervention intensity. | |
| Physical therapist, psychologist, physician | “Backs to Work is a coordinated multidisciplinary reconditioning program” | The duration of the intervention was 3 hours per day, 3 days/week for 4 weeks = 36 hours = mid‐intensity | |
| Rehabilitation physician, specialist in clinical social medicine, physiotherapist, social worker, occupational therapist | Coordinated through case manager | The duration of the intervention was 18 weeks, average of 4 meetings with case manager = low intensity | |
| Physician, physiotherapist, company nurse, company physician | Physiotherapist visited patient’s workplace to involve work supervisor and company health care professionals in treatment | The duration of the mini‐intervention was 1.25‐1.5 hours and the worksite visit was approximately 75 minutes = low intensity | |
| Occupational physician, ergonomist, back pain specialist | “All described interventions were provided by a multidisciplinary medical, ergonomic, and rehabilitation staff at the Sherbrooke University Hospital back pain clinic.” | In a previous study using the same protocol (Loisel 1994), the duration of functional rehabilitation therapy ranged from 2 to 13 weeks. Insufficient information to categorize intervention intensity. | |
| Physician, physiotherapist, psychotherapist | This does not appear to be an integrated program. The physiotherapists were blind to treatment condition, indicating no communication between the physiotherapists and psychotherapists. | The duration of the intervention was 6 hours of daily treatment for 15 days in 3 weeks = mid‐intensity | |
| Physician, masters‐level clinician for behavioural medicine intervention | The extent to which health care professionals communicated wasn't clear from the article text. | The duration of the intervention was 6‐10 weeks, 4 hours a week = mid‐intensity | |
| Physical therapy and behavioral medicine sessions “provided by licensed professionals trained in their respective fields;” occupational therapist | Case management sessions and interdisciplinary team conferences held at baseline and discharge. | The duration of the intervention was from 4 to 10 weeks; 6‐9 behavioral medicine sessions; 6‐9 physical therapy sessions; Up to 6 work transitions sessions; one or more case management sessions = low intensity |
| Bias Domain | Source of Bias | PossibleAnswers |
| Selection | (1) Was the method of randomizations adequate? | Yes/No/Unsure |
| Selection | (2) Was the treatment allocation concealed? | Yes/No/Unsure |
| Performance | (3) Was the patient blinded to the intervention? | Yes/No/Unsure |
| Performance | (4) Was the care provider blinded to the intervention? | Yes/No/Unsure |
| Detection | (5) Was the outcome assessor blinded to the intervention? | Yes/No/Unsure |
| Attrition | (6) Was the drop‐out rate described and acceptable? | Yes/No/Unsure |
| Attrition | (7) Were all randomised participants analysed in the group to which they were allocated? | Yes/No/Unsure |
| Reporting | (8) Are reports of the study free of suggestion of selective outcome reporting? | Yes/No/Unsure |
| Performance | (9) Were cointerventions avoided or similar? | Yes/No/Unsure |
| Performance | (10) Was the compliance acceptable in all groups? | Yes/No/Unsure |
| Detection | (11) Was the timing of the outcome assessment similar in all groups? | Yes/No/Unsure |
| Other | (12) Are other sources of potential bias unlikely? | Yes/No/Unsure |
| 1 | A random (unpredictable) assignment sequence. Examples of adequate methods are coin toss (for studies with 2 |
| 2 | Assignment generated by an independent person not responsible for determining the eligibility of the patients. |
| 3 | Index and control groups are indistinguishable for the patients or if the success of blinding was tested among |
| 4 | Index and control groups are indistinguishable for the care providers or if the success of blinding was tested |
| 5 | Adequacy of blinding should be assessed for each primary outcome separately. This item should be scored ‐for patient‐reported outcomes in which the patient is the outcome assessor (e.g., pain, disability): the blinding |
| 6 | The number of participants who were included in the study but did not complete the observation period or |
| 7 | All randomised patients are reported/analysed in the group they were allocated to by randomizations for the |
| 8 | All the results from all prespecified outcomes have been adequately reported in the published report of the |
| 9 | If there were no cointerventions or they were similar between the index and control groups. |
| 10 | The reviewer determines if the compliance with the interventions is acceptable, based on the reported |
| 11 | Timing of outcome assessment should be identical for all intervention groups and for all primary outcome |
| 12 | Other types of biases. For example: |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Short‐term | 4 | 272 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.74, ‐0.06] |
| 1.2 Intermediate‐term | 2 | 155 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [1.00, 0.31] |
| 1.3 Long‐term | 4 | 336 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.70, ‐0.21] |
| 2 Disability Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Short‐term | 4 | 272 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.63, ‐0.14] |
| 2.2 Intermediate‐term | 2 | 151 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐1.09, 0.22] |
| 2.3 Long‐term | 3 | 240 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.87, ‐0.01] |
| 3 Return‐to‐work at long‐term Show forest plot | 3 | 170 | Odds Ratio (IV, Random, 95% CI) | 3.19 [1.46, 6.98] |
| 4 Sick leave periods at long‐term Show forest plot | 2 | 210 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.66, ‐0.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Short‐term | 2 | 165 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.50, 0.33] |
| 1.2 Intermediate‐term | 2 | 162 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.85, 0.57] |
| 1.3 Long‐term | 2 | 336 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.36, 0.07] |
| 2 Disability Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Short‐term | 2 | 165 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.34, 0.34] |
| 2.2 Intermediate‐term | 2 | 162 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐1.50, 0.51] |
| 2.3 Long‐term | 2 | 345 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.24, 0.18] |
| 3 Sick leave days at long‐term Show forest plot | 2 | 158 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.98, 0.47] |

