Vaccines for the common cold
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Study characteristics | ||
| Methods | Design: double‐blind RCT (2 arms) Country: USA (1 site) Clinical setting: Great Lakes Naval Training Center Follow‐up: 9 weeks' basic‐training period Intention‐to‐treat: yes Randomisation unit: participant Analysis unit: participant | |
| Participants | Great Lakes Naval Training Center, new recruits Randomised: 2307 participants Vaccines group: 1139 (49.3%) Vaccines group: 1139 (49.3%) Lost postrandomisation: 0% Analysed participants: Vaccines group: 1139 (49.3%) Gender (number of men): 2307 Inclusion criteria:
Exclusion criteria: not reported | |
| Interventions | Experimental group: the vaccines used were composed of orally administered live adenovirus 4, parenterally administered inactivated adenovirus 4, and parenterally administered inactivated adenovirus 4 and 7 preparations Control group: placebo Co‐interventions:
| |
| Outcomes | This RCT did not specify primary or secondary outcomes. Incidence of admissions of participants with respiratory illness (not only hospitalised participants):
Toxic effects | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Epidemiologic design of this study consisted of the random assignment of one half of the recruits ..." (p 982) Insufficient information to permit a judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit a judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Double‐blind procedure was followed with paramedical personnel administering the appropriate vaccine or placebo to recruits on their third day after arrival at Great Lakes, just prior to initiation of basic training" (p 982) Quote: "Placebo for the parenterally administered vaccines consisted of an injection of physiological saline, and that for the orally administered vaccine consisted of an identical appearing inert gelatin capsule" (p 982) Comment: blinding of participants and key study personnel ensured, and it is unlikely that the blinding could have been broken |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: blinding of outcome assessment was performed with the use of placebo |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol is not available, but it is clear that the published reports include all expected outcomes. However, some outcomes are described in a narrative fashion and not per group. Quote: "... there was no observable toxic reaction to this new live vaccine preparation within the study design." (p 985) |
| Other bias | Unclear risk | The sample size was not reported. There is no table with baseline characteristics of the participants. |
RCT: randomised controlled trial
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Phase I study ‐ did not assess incidence of common cold, did not define common cold | |
| Phase 2a ‐ did not assess incidence of common cold | |
| Phase I study ‐ did not assess incidence of common cold, did not define common cold | |
| Phase I study ‐ did not assess incidence of common cold, did not define common cold | |
| Phase I study ‐ did not assess incidence of common cold, did not define common cold | |
| Phase II study ‐ did not assess incidence of common cold, did not define common cold | |
| Unknown phase ‐ did not assess incidence of common cold, did not define common cold | |
| Unknown phase ‐ did not assess incidence of common cold, did not define common cold | |
| Assessed lower respiratory tract infection | |
| Phase II study ‐ did not assess incidence of common cold, did not define common cold | |
| Phase II study ‐ did not assess incidence of common cold, did not define common cold | |
| Assessed common cold symptoms caused by bacterial infections | |
| Phase I ‐ did not assess incidence of common cold | |
| Wrong design, not RCT | |
| Phase I study ‐ did not assess incidence of common cold, did not define common cold | |
| Wrong design, experimental infection | |
| Duplicate record ‐ conference abstract of Sadoff 2021a | |
| Wrong study design, not RCT | |
| Assessed lower respiratory tract infection | |
| Assessed lower respiratory tract infection | |
| Wrong design, not RCT | |
| Assessed common cold symptoms caused by bacterial infections | |
| Phase I study ‐ did not assess incidence of common cold | |
| Phase II study ‐ did not assess incidence of common cold, did not define common cold | |
| Phase II study ‐ did not assess incidence of common cold, did not define common cold | |
| Phase II study ‐ did not assess incidence of common cold, did not define common cold | |
| Phase II study ‐ did not assess incidence of common cold, did not define common cold | |
| Phase I/II study ‐ did not assess incidence of common cold, did not define common cold | |
| Assessed lower respiratory tract infection | |
| Phase II study ‐ did not assess incidence of common cold, did not define common cold | |
| Phase I/II study ‐ did not assess incidence of common cold, did not define common cold | |
| Unknown phase ‐ did not assess incidence of common cold, did not define common cold | |
| Phase I study ‐ did not assess incidence of common cold, did not define common cold | |
| Phase II study ‐ did not assess incidence of common cold, did not define common cold | |
| Unknown phase ‐ did not assess incidence of common cold, did not define common cold | |
| Unknown phase ‐ did not assess incidence of common cold, did not define common cold | |
| Assessed lower respiratory tract infection | |
| Wrong study design, not RCT | |
| Assessed lower respiratory tract infection | |
| Assessed lower respiratory tract infection | |
| Unknown phase ‐ did not assess incidence of common cold, did not define common cold | |
| Phase II study ‐ did not assess incidence of common cold, did not define common cold | |
| Wrong study design, not RCT | |
| Unknown phase ‐ did not assess incidence of common cold, did not define common cold | |
| Assessed respiratory symptoms caused by bacterial infections | |
| Phase I study ‐ did not assess incidence of common cold, did not define common cold | |
| Phase I study ‐ did not assess incidence of common cold, did not define common cold | |
| Wrong study design, not RCT | |
| Wrong study design, not RCT | |
| Unknown phase ‐ did not assess incidence of common cold, did not define common cold | |
| Phase I study ‐ did not assess incidence of common cold, did not define common cold | |
| Wrong study design, not RCT | |
| Phase I study ‐ did not assess incidence of common cold, did not define common cold | |
| Assessed respiratory symptoms caused by bacterial infections | |
| Unknown phase ‐ did not assess incidence of common cold, did not define common cold | |
| Duplicate record ‐ clinical trial register of Langley 2018 | |
| Assessed lower respiratory tract infection | |
| Assessed lower respiratory tract infection | |
| Did not assess incidence of common cold or vaccine safety | |
| Wrong study design, not RCT | |
| Assessed lower respiratory tract infection | |
| Phase I study ‐ did not assess incidence of common cold, did not define common cold | |
| Unknown phase ‐ did not assess incidence of common cold, did not define common cold | |
| Phase III ‐ did not assess incidence of common cold, did not define common cold | |
| Unknown phase ‐ did not assess incidence of common cold, did not define common cold | |
| Unknown phase ‐ did not assess incidence of common cold, did not define common cold | |
| Unknown phase ‐ did not assess incidence of common cold, did not define common cold | |
| Unknown phase ‐ did not assess incidence of common cold, did not define common cold | |
| Phase II ‐ did not assess incidence of common cold, did not define common cold | |
| Wrong study design, not RCT | |
| Wrong intervention, this study assessed human rotavirus associated with gastroenteritis | |
| Assessed lower respiratory tract infection | |
| Assessed lower respiratory tract infection | |
| Wrong intervention, this study assessed human rotavirus associated with gastroenteritis | |
| Wrong intervention, this study assessed human rotavirus associated with gastroenteritis | |
| Phase I ‐ did not assess incidence of common cold, did not define common cold | |
| Wrong intervention, this study assessed human rotavirus associated with gastroenteritis | |
| Wrong intervention, this study assessed human rotavirus causing gastroenteritis | |
| Duplicate record ‐ clinical trial register of DeVincenzo 2010 | |
| Phase I ‐ did not assess incidence of common cold, did not define common cold | |
| Did not assess incidence of common cold or vaccine safety | |
| Phase I and II ‐ did not assess incidence of common cold, did not define common cold | |
| Phase I ‐ did not assess incidence of common cold, did not define common cold | |
| Phase I ‐ did not assess incidence of common cold, did not define common cold | |
| Phase I ‐ did not assess incidence of common cold, did not define common cold | |
| Phase I ‐ did not assess incidence of common cold, did not define common cold | |
| Terminated, and no results available | |
| Phase I ‐ did not assess incidence of common cold, did not define common cold | |
| Phase I ‐ did not assess incidence of common cold, did not define common cold | |
| Duplicate record ‐ clinical trial register of Langley 2017 | |
| Phase I ‐ did not assess incidence of common cold, did not define common cold | |
| Phase II ‐ did not assess incidence of common cold, did not define common cold | |
| Phase I ‐ did not assess incidence of common cold, did not define common cold | |
| Phase I ‐ did not assess incidence of common cold, did not define common cold | |
| Phase I ‐ did not assess incidence of common cold, did not define common cold | |
| Phase I ‐ did not assess incidence of common cold, did not define common cold | |
| Wrong intervention, not a vaccine | |
| Phase I ‐ did not assess incidence of common cold, did not define common cold | |
| Phase I ‐ did not assess incidence of common cold, did not define common cold | |
| Assessed lower respiratory tract infection | |
| Assessed lower respiratory tract infection | |
| Duplicate record ‐ clinical trial register of Madhi 2020 | |
| Phase I ‐ did not assess incidence of common cold, did not define common cold | |
| Phase I ‐ did not assess incidence of common cold, did not define common cold | |
| Withdrawn, no reasons specified | |
| Phase II ‐ did not assess incidence of common cold, did not define common cold | |
| Duplicate record ‐ clinical trial register of Cunningham 2019 | |
| Phase II ‐ did not assess incidence of common cold, did not define common cold | |
| Assessed lower respiratory tract infection | |
| Phase II ‐ did not assess incidence of common cold, did not define common cold | |
| Phase I ‐ did not assess incidence of common cold, did not define common cold | |
| Withdrawn due to instability of the PreF antigen during manufacturing | |
| Phase I and II ‐ did not assess incidence of common cold, did not define common cold | |
| Duplicate record ‐ clinical trial register of Sadoff 2021a | |
| Phase I ‐ did not assess incidence of common cold, did not define common cold | |
| Phase I ‐ did not assess incidence of common cold, did not define common cold | |
| Duplicate record ‐ clinical trial register of Scaggs 2020 | |
| Terminated | |
| Phase I ‐ did not assess incidence of common cold, did not define common cold | |
| Phase I and II ‐ did not assess incidence of common cold, did not define common cold | |
| Phase II ‐ did not assess incidence of common cold, did not define common cold | |
| Wrong study design, not RCT | |
| Phase IIa ‐ did not assess incidence of common cold | |
| Duplicate record ‐ clinical trial register of Verdijk 2020 | |
| Unknown phase ‐ did not assess incidence of common cold, did not define common cold | |
| Duplicate record ‐ conference abstract of Philpott 2017 | |
| Wrong intervention, study assessed influenza | |
| Unknown phase ‐ did not assess incidence of common cold, did not define common cold | |
| Did not assess incidence of common cold or vaccine safety | |
| Unknown phase ‐ did not assess incidence of common cold, did not define common cold | |
| Wrong design, not RCT | |
| Phase I ‐ did not assess incidence of common cold | |
| Phase II ‐ did not assess incidence of common cold, did not define common cold | |
| Phase II ‐ did not assess incidence of common cold, did not define common cold | |
| Phase I ‐ did not assess incidence of common cold, did not define common cold | |
| Phase I ‐ did not assess incidence of common cold, did not define common cold | |
| Phase II ‐ did not assess incidence of common cold, did not define common cold | |
| Did not assess incidence of common cold or vaccine safety | |
| Phase I ‐ did not assess incidence of common cold, did not define common cold | |
| Wrong study design, not RCT | |
| Duplicate record ‐ conference abstract of Madhi 2020 | |
| Unknown phase ‐ did not assess incidence of common cold, did not define common cold | |
| Did not assess incidence of common cold or vaccine safety | |
| Assessed lower respiratory tract infection | |
| Duplicate record ‐ conference abstract of Verdijk 2020 | |
| Phase I ‐ did not assess incidence of common cold, did not define common cold | |
| Wrong study design, not RCT | |
| Did not assess incidence of common cold or vaccine safety | |
| Phase I ‐ did not assess incidence of common cold, did not define common cold | |
| Wrong study design, not RCT | |
| Wrong study design, not RCT | |
| Did not assess incidence of common cold or vaccine safety |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Study name | Evaluating the safety and immune response to a single dose of a respiratory syncytial virus (RSV) vaccine in infants and children |
| Methods | Randomised clinical trial; parallel assignment |
| Participants | 105 |
| Interventions | Biological: RSV ΔNS2 Δ1313 I1314L vaccine; other: placebo |
| Outcomes | Frequency and severity of vaccine‐related solicited AEs; proportion of participants that develop 4‐fold or greater rises in RSV neutralising antibody titre following vaccination |
| Starting date | June 2013 |
| Contact information | Jocelyn San Mateo; 410‐614‐4306; [email protected] |
| Notes |
| Study name | Evaluating the infectivity, safety, and immunogenicity of a respiratory syncytial virus vaccine (RSV 6120/∆NS2/1030s) in RSV‐seropositive children and RSV‐seronegative infants and children |
| Methods | Randomised clinical trial; parallel assignment |
| Participants | 45 |
| Interventions | Biological: RSV 6120/∆NS2/1030s; other: placebo |
| Outcomes | Grades of: study product‐related solicited AEs (RSV‐seropositive participants); study product‐related solicited AEs (RSV‐seronegative participants); study product‐related unsolicited AEs (RSV‐seropositive participants); study product‐related unsolicited AEs (RSV‐seronegative participants); study product‐related SAEs (RSV‐seropositive participants). Frequency of infection with: RSV (RSV‐seropositive participants); with RSV (RSV‐seronegative participants). Peak titre of vaccine virus shed (RSV‐seropositive participants). Duration of virus shedding in nasal washes (RSV‐seropositive participants). RSV‐neutralising serum antibody titre (RSV‐seropositive participants). IgG serum antibody titres to RSV F glycoprotein ELISA (RSV‐seropositive participants) |
| Starting date | 13 October 2017 |
| Contact information | Ruth A Karron |
| Notes |
| Study name | Evaluating the infectivity, safety, and immunogenicity of the recombinant live‐attenuated RSV vaccines RSV ΔNS2/Δ1313/I1314L or RSV 276 in RSV‐seronegative infants and children 6 to 24 months of age |
| Methods | Randomised clinical trial; parallel assignment |
| Participants | 80 |
| Interventions | Biological: RSV ΔNS2/Δ1313/I1314L; biological: RSV 276; other: placebo |
| Outcomes | Grades of: study product‐related solicited AEs; product‐related unsolicited AEs; product‐related SAEs; number of participants infected with RSV; peak titre of vaccine virus shed; duration of virus shedding in nasal washes. Frequency of: ≥ 4‐fold rise in RSV serum neutralising antibody titre; RSV neutralising antibody responses; ≥ 4‐fold rise in serum antibody titres to RSV F glycoprotein; antibody responses to RSV F glycoprotein |
| Starting date | 4 October 2017 |
| Contact information | Ruth A Karron |
| Notes |
| Study name | Evaluating the infectivity, safety and immunogenicity of respiratory syncytial virus vaccines, RSV 6120/∆NS1 and RSV 6120/F1/G2/∆NS1, in RSV‐seropositive children and RSV‐seronegative infants and children |
| Methods | Randomised clinical trial; parallel assignment |
| Participants | 75 |
| Interventions | Biological: RSV 6120/∆NS1; biological: RSV 6120/F1/G2/∆NS1; other: placebo |
| Outcomes | Grades of study product‐related: solicited AEs; unsolicited AEs; SAEs. Frequency of infection with RSV. Peak titre of vaccine virus shed. Duration of virus shedding in nasal washes. Frequency of ≥ 4‐fold rise in RSV‐neutralising antibody titre. Frequency of ≥ 4‐fold rise in IgG antibody responses to RSV F glycoprotein. Frequency of symptomatic, medically attended respiratory and febrile illness in the RSV‐seronegative (group 2) vaccine and placebo recipients who experience natural infection with wt RSV during the RSV season. Severity of symptomatic, medically attended respiratory and febrile illness in the RSV‐seronegative (group 2) vaccine and placebo recipients who experience natural infection with wt RSV during the RSV season. Frequency of antibody responses in the RSV‐seronegative vaccine and placebo recipients who experience natural infection with wt RSV during the RSV season. Measurement of mucosal antibody titres to vaccine |
| Starting date | 25 June 2018 |
| Contact information | Kristi Herbert; 410‐502‐3333; [email protected] |
| Notes |
| Study name | Safety and immunogenicity of a single dose of the recombinant live‐attenuated RSV vaccines RSV ΔNS2/Δ1313/I1314L, RSV 6120/ΔNS2/1030s, RSV 276 or placebo, delivered as nose drops to RSV‐seronegative children 6 to 24 months of age |
| Methods | Randomised clinical trial; parallel assignment |
| Participants | 160 |
| Interventions | Biological: RSV ΔNS2/Δ1313/I1314L vaccine; RSV 6120/ΔNS2/1030s vaccine; RSV 276 vaccine; other: placebo |
| Outcomes | Primary: frequency of Grade 1 or higher solicited AEs [Time Frame: measured through Day 28]; frequency of Grade 2 or higher lower respiratory illnesses [Time Frame: measured through Day 28]; frequency of serious AEs [Time Frame: measured through Day 56]; frequency of ≥ 4‐fold rise in serum RSV‐neutralising antibody titre [Time Frame: measured through Day 56] Secondary: frequency of ≥ 4‐fold rise in serum RSV F IgG [Time Frame: measured through Day 56]; titre of serum RSV F IgG [Time Frame: measured at the Day 56 visit]; titre of serum RSV‐neutralising antibodies [Time Frame: measured at the Day 56 visit]; frequency of RSV‐MAARI [Time Frame: measured through the last day of the RSV season, which will occur between 5 and 12 months after study entry, depending on when the participant enrolls in the study]; maximum grade (if more than 1 illness within a participant) of RSV‐MAARI [Time Frame: measured through the last day of the RSV season, which will occur between 5 and 12 months after study entry, depending on when the participant enrolls in the study]; frequency of RSV‐MAALRI [Time Frame: measured through the last day of the RSV season, which will occur between 5 and 12 months after study entry, depending on when the participant enrolls in the study]; maximum grade (if more than 1 illness within a participant) of RSV‐MAALRI [Time Frame: measured through the last day of the RSV season, which will occur between 5 and 12 months after study entry, depending on when the participant enrolls in the study] |
| Starting date | 16 May 2019 |
| Contact information | Coleen Cunningham and Ruth Karron |
| Notes |
| Study name | A phase 2B placebo‐controlled, randomised study of a RSV vaccine in pregnant women |
| Methods | Randomised clinical trial; parallel assignment |
| Participants | 650 |
| Interventions | Biological: RSV vaccine; other: placebo |
| Outcomes | Percentage of participants reporting: local reactions and systemic events from day of vaccination (Day 1) until Day 7; AEs within 1 month after vaccination; obstetric complications, MAEs and SAEs throughout the study. Percentage of infant participants: with specific birth outcomes; with AE from birth to 1 month of age; with SAE, AE of special interest (congenital anomalies, developmental delay), and MAE through 12 months of age. Immune responses measured by RSV neutralising antibody titres in maternal participants. Geometric mean ratio for RSV neutralising antibody titres in maternal participants |
| Starting date | 7 August 2019 |
| Contact information | Pfizer |
| Notes |
| Study name | Study of safety, reactogenicity and immunogenicity of GlaxoSmithKline's (GSK) respiratory syncytial virus (RSV) maternal unadjuvanted vaccine in healthy pregnant women (aged 18 to 40 years) and their infants |
| Methods | Randomised clinical trial; parallel assignment |
| Participants | 420 |
| Interventions | Biological: RSVPreF3 formulation 2; biological: RSVPreF3 formulation 3; other: placebo |
| Outcomes | Percentage of maternal participants reporting: solicited administration site events; solicited systemic events; with haematological and biochemical laboratory abnormality at baseline; with haematological and biochemical laboratory abnormality at Day 8; unsolicited AEs; at least 1 SAE; with AEs leading to study withdrawal; with at least 1 MAE; pregnancy outcomes; pregnancy‐related AESIs; neonatal AESIs; at least 1 SAE; AEs leading to study withdrawal; at least 1 MAE. RSVPreF3 IgG‐specific antibody concentration in terms of GMCs at Day 1, before vaccination for each group, and by age category. RSVPreF3 IgG antibody GMCs at Day 31. RSVPreF3 IgG antibody GMCs at delivery. RSV‐A neutralising antibody GMTs at Day 1, before vaccination. RSV‐A neutralising antibody GMTs at Day 31. RSV‐A neutralising antibody GMTs at delivery. RSVPreF3 IgG antibody GMCs in infants born to maternal participants. RSV‐A neutralising antibody GMTs in infants born to maternal participants. Geometric mean ratio between cord blood and maternal RSVPreF3 IgG‐specific antibody concentrations |
| Starting date | 5 November 2019 |
| Contact information | GlaxoSmithKline |
| Notes |
| Study name | A study of a vaccine against respiratory syncytial virus (RSV) when given alone and together with a vaccine against diphtheria, pertussis and tetanus (Tdap) viruses followed by a 2nd dose of the RSV vaccine to healthy non‐pregnant women |
| Methods | Randomised clinical trial; parallel assignment |
| Participants | 509 |
| Interventions | Biological: RSVPreF3 formulation 3; biological: RSVPreF3 formulation 2; biological: Boostrix‐ex‐US; other: placebo |
| Outcomes | Percentage of participants with: at least 1 solicited local AE for each study group, after the 1st vaccination; at least 1 solicited general AE for each study group, after the 1st vaccination. Percentage of participants with: any unsolicited AEs for each study group, after the 1st vaccination; at least 1 SAE for each study group, after the 1st vaccination; at least 1 solicited local AE for each study group, after the 2nd vaccination; at least 1 solicited general AE for each study group, after the 2nd vaccination; any unsolicited AEs for each study group, after the 2nd vaccination; at least 1 SAE for each study group, after the 2nd vaccination. Humoral immune response in terms of RSV A neutralising antibody GMTs for each group, at screening. RSV A neutralising antibody GMTs for each group at Day 8, after the 1st vaccination. RSV A neutralising antibody GMTs for each group at Day 31, after the 1st vaccination. Humoral immune response in terms of RSV PreF3 IgG antibody GMCs for each group, at screening. RSV PreF3 IgG GMCs for each group, at Day 8, after the 1st vaccination. RSV PreF3 IgG GMCs for each group, at Day 31, after the 1st vaccination |
| Starting date | 5 November 2019 |
| Contact information | GlaxoSmithKline |
| Notes |
| Study name | Safety and efficacy of BARS13 in the elderly |
| Methods | Randomised clinical trial; parallel assignment |
| Participants | 120 |
| Interventions | Drug: recombinant respiratory syncytial virus vaccine (BARS13)/placebo; drug: recombinant respiratory syncytial virus vaccine (BARS13); drug: placebo |
| Outcomes | Incidence and severity of vaccine‐related AEs including the following solicited AEs; incidence and severity of vaccine‐related AEs including the following solicited AEs; incidence and severity of vaccine‐related AEs including the following solicited AEs; occurrence of any SAE; occurrence of any clinically significant clinical laboratory abnormalities |
| Starting date | 24 May 2021 |
| Contact information | Xuefen Huai: +8618351991682; [email protected] |
| Notes |
| Study name | A phase 3, randomised, open‐label, multi‐country study to evaluate the immunogenicity, safety, reactogenicity and persistence of a single dose of the RSVPreF3 OA investigational vaccine and different revaccination schedules in adults aged 60 years and above |
| Methods | Randomised clinical trial; parallel assignment |
| Participants | 1720 |
| Interventions | Biological: RSVPreF3 OA investigational vaccine |
| Outcomes | Humoral immune response in terms of RSV‐A neutralising antibody GMTs; RSV‐A neutralising antibody GMTs; humoral immune response in terms of RSV‐B neutralising antibody titres; humoral immune response in terms of RSV‐B neutralising antibody titres; humoral immune response in terms of RSVPreF3 IgG antibody GMCs; humoral immune response in terms of RSV‐A neutralising antibody GMTs; cell‐mediated immune response in terms of frequency of RSVPreF3‐specific cluster of differentiation (CD)4+ and/or CD8+ T cells expressing at least 2 activation markers; number of participants with at least 1 solicited administration‐site event and solicited systemic event; number of participants with SAEs; number of participants with a fatal SAE, related SAE, and related pIMDs |
| Starting date | 15 February 2021 |
| Contact information | GlaxoSmithKline |
| Notes |
| Study name | A phase III, double‐blind, randomised, placebo‐controlled study to evaluate the safety, reactogenicity and immune response of a single intramuscular dose of unadjuvanted RSV maternal vaccine, in high‐risk pregnant women aged 15 to 49 years and infants born to the vaccinated mother |
| Methods | Randomised clinical trial; parallel assignment |
| Participants | 353 |
| Interventions | Biological: RSV MAT; drug: placebo |
| Outcomes | Percentage of maternal participants reporting solicited administration site events; percentage of maternal participants reporting solicited systemic events; percentage of maternal participants reporting unsolicited AEs; percentage of maternal participants reporting SAEs, (S)AEs leading to study withdrawal, and medically attended adverse events; percentage of maternal participants reporting pregnancy outcomes; percentage of maternal participants reporting pregnancy‐related AESIs; humoral immune response in terms of RSV MAT IgG‐specific antibody concentrations at pre‐dosing (Day 1) for maternal participants; geometric mean ratio between cord blood and maternal RSV MAT IgG‐specific antibody concentrations; humoral immune response in terms of RSV‐A neutralising antibody titres at delivery for infant participants |
| Starting date | 3 August 2021 |
| Contact information | GlaxoSmithKline |
| Notes |
| Study name | A phase 2/3, randomised, observer‐blind, placebo‐controlled study to evaluate the safety and efficacy of mRNA‐1345, an mRNA vaccine targeting respiratory syncytial virus (RSV), in adults ≥ 60 years of age |
| Methods | Randomised clinical trial; parallel assignment |
| Participants | 34,000 |
| Interventions | mRNA‐1345; placebo |
| Outcomes | Number of participants with solicited local and systemic adverse reactions up to 7 days postinjection; number of participants with unsolicited AEs up to 28 days postinjection; number of participants with medically attended AEs, AESIs, SAEs, and AEs leading to withdrawal up to 24 months postinjection; VE of mRNA‐1345 to prevent a first episode of RSV‐LRTD within the period of 14 days postinjection up to 12 months postinjection; VE of mRNA‐1345 to prevent RT‐PCR confirmed protocol‐defined RSV‐LRTD, defined as 100*(1 − RR), where RR is the ratio of attack rates in the mRNA‐1345 group and the placebo group; VE of mRNA‐1345 to prevent a first episode of RSV‐ARD within the period of 14 days postinjection up to 12 months postinjection; VE of mRNA‐1345 to prevent RT‐PCR confirmed protocol‐defined RSV‐ARD, defined as 100*(1 − RR), where RR is the ratio of attack rates in the mRNA‐1345 group and the placebo group; VE of mRNA‐1345 to prevent hospitalisations associated with RSV‐ARD or RSV‐LRTD within the period of 14 days postinjection up to 12 months postinjection; VE of mRNA‐1345 to prevent RT‐PCR confirmed protocol‐defined RSV‐ARD or RSV‐LRTD, defined as 100*(1 − RR), where RR is the ratio of attack rates in the mRNA‐1345 group and the placebo group; GMT of serum RSV neutralising and binding antibodies (Abs); geometric mean fold‐rise of postbaseline/baseline Ab titres; proportion of participants with ≥ 4‐fold increases in Ab titres from baseline |
| Starting date | 17 November 2021 |
| Contact information | Moderna Clinical Trials Support Center: 1‐877‐777‐7187; [email protected] |
| Notes |
| Study name | A randomised, double‐blind, phase 3 trial to assess clinical efficacy, safety and reactogenicity of the recombinant MVA‐BN® ‐RSV vaccine in adults ≥ 60 years of age |
| Methods | Randomised clinical trial; parallel assignment |
| Participants | 20,000 |
| Interventions | Biological: MVA‐BN‐RSV vaccine; biological: Tris buffered saline |
| Outcomes | Occurrence of LRTD; occurrence of ARD; occurrence of any SAEs; occurrence of complications and hospitalisations; occurrence of any grade 3 or higher adverse events; RSV‐specific T‐cell responses; RSV‐specific serum neutralising antibody titres; RSV‐specific serum IgG antibody titres; occurrence of solicited systemic adverse events |
| Starting date | April 2022 |
| Contact information | Heinz Weidenthaler: 004989255446 ext 300; hwe@bavarian‐nordic.com |
| Notes |
AEs: adverse events
AESIs: adverse events of special interest
ARD: acute respiratory disease
ARs: adverse reactions
CS: clinically significant
ELISA: enzyme‐linked immunosorbent assay
GMCs: geometric mean concentrations
GMTs: geometric mean titres
IgG: immunoglobulin G
LRTD: lower respiratory tract disease
MAE: medically attended adverse event
pIMDs: potential immune‐mediated disorders
RSV: respiratory syncytial virus
RSV‐ARD: respiratory syncytial virus‐associated acute respiratory disease
RSV‐LRTD: respiratory syncytial virus‐associated lower respiratory tract disease
RSV‐MAARI: respiratory syncytial virus‐associated medically attended acute respiratory illness
RSV‐MAALRI: respiratory syncytial virus‐associated medically attended acute lower respiratory illness
RT‐PCR: reverse transcription polymerase chain reaction
SAEs: serious adverse events
Tdap: diphtheria, pertussis, and tetanus
VE: vaccine efficacy
wt RSV: wild‐type respiratory syncytial virus
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Incidence of the common cold Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1: Adenovirus vaccines versus placebo, Outcome 1: Incidence of the common cold | ||||

PRISMA flowchart
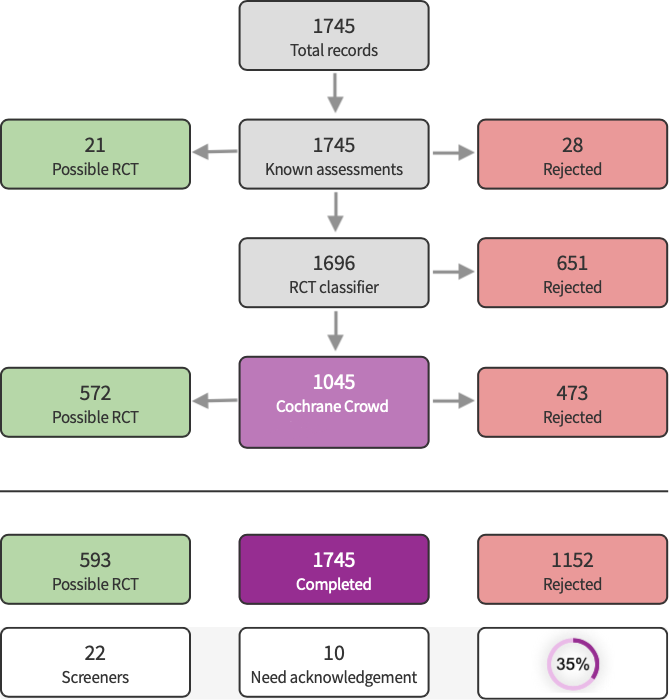
Screen4Me summary diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages.

Risk of bias summary: review authors' judgements about each risk of bias item for the included study.

Comparison 1: Adenovirus vaccines versus placebo, Outcome 1: Incidence of the common cold
| Virus vaccines compared to placebo for preventing the common cold in healthy people | ||||||
| Patient or population: young, healthy men in a military facility | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Placebo | Virus vaccines for preventing the common cold | |||||
| Incidence of the common cold Number of participants with common cold by group | Study population | RR 0.95 | 2307 | ⊕⊝⊝⊝ |
| |
| 12 per 1000 | 11 per 1000 | |||||
| Vaccine safety Follow‐up: mean 9 weeks | The study reported that there were no differences between groups in vaccine‐related adverse events. | 2307 | ⊕⊝⊝⊝ |
| ||
| Mortality: vaccine related and all cause ‐ not reported Follow‐up: mean 9 weeks | See comments | The included study did not report this outcome. | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level due to unclear risk of bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Incidence of the common cold Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |

