Вакцины при простуде
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Design: double‐blind, RCT (2 arms) Country: USA (1 site) Clinical setting: Great Lakes Naval Training Center Follow‐up: 9 weeks' basic‐training period Intention‐to‐treat: yes Randomisation unit: participant Analysis unit: participant | |
| Participants | Great Lakes Naval Training Center, new recruits Randomised: 2307 participants Vaccines group: 1139 (49.3%) Participants receiving intervention: 1139 Vaccines group: 1139 (49.3%)
Lost post‐randomisation: 0% Analysed participants: Vaccines group: 1139 (49.3%) Age median (mean (SD)): did not report Gender (number of men): did not report Inclusion criteria:
Exclusion criteria: not reported | |
| Interventions | Experimental group: the vaccines used were composed of orally administered live adenovirus 4, parenterally administered inactivated adenovirus 4, and parenterally administered inactivated adenovirus 4 and 7 preparations Control group: placebo Co‐interventions
| |
| Outcomes | This RCT did not specify primary or secondary outcomes. Incidence of admissions of participants with respiratory illness (not only hospitalised participants)
Toxic effects | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Epidemiologic design of this study consisted of the random assignment of one half of the recruits ..." (p. 982). Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Double‐blind procedure was followed with paramedical personnel administering the appropriate vaccine or placebo to recruits on their third day after arrival at Great Lakes, just prior to initiation of basic training" (p. 982) Quote: "Placebo for the parenterally administered vaccines consisted of an injection of physiological saline, and that for the orally administered vaccine consisted of an identical appearing inert gelatin capsule" (p. 982) Comment: Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Since recruits with the common cold syndrome rarely require hospitalizations, the effect of the adenovirus vaccines on this clinical entity can not be adequately evaluated." Comment: Although placebo was used, there could be a risk of detection bias because common cold does not require hospitalization |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Comment: The study protocol is not available, but it is clear that the published reports include all expected outcomes. However, some are described in a narrative fashion and not per group. Quote: "... there was no observable toxic reaction to this new live vaccine preparation within the study design." (p. 985) |
| Other bias | High risk | The sample size was not reported. There is no table with basal characteristics of the participants |
RCT: randomised controlled trial
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not RCT | |
| RCT. Did not evaluate common cold | |
| RCT. Did not evaluate common cold | |
| RCT. Did not evaluate common cold | |
| Not RCT | |
| RCT. Did not evaluate common cold | |
| Not RCT | |
| Not RCT | |
| RCT. Did not evaluate common cold | |
| RCT. Did not include healthy people | |
| Not RCT | |
| RCT. Did not evaluate common cold | |
| RCT. Did not evaluate common cold | |
| RCT. Did not evaluate common cold | |
| RCT. Included participants aged < 6 months | |
| Not RCT | |
| RCT. Did not evaluate common cold | |
| RCT. Did not evaluate common cold | |
| Not RCT | |
| RCT. Did not evaluate common cold | |
| RCT. Did not evaluate common cold | |
| RCT. Did not evaluate common cold | |
| RCT. Did not evaluate a vaccine (evaluated a probiotic) | |
| RCT. Did not evaluate common cold | |
| RCT. Included participants aged < 6 months | |
| Non‐vaccine interventions | |
| RCT. Did not evaluate common cold | |
| RCT. Did not evaluate common cold | |
| RCT. Included participants aged < 6 months | |
| RCT. Included pregnant women | |
| Update on vaccines topic | |
| RCT. Did not evaluate common cold | |
| RCT. Did not evaluate common cold | |
| RCT. Did not evaluate common cold | |
| RCT. Did not evaluate common cold | |
| Not RCT | |
| Meta‐analysis | |
| RCT. Did not evaluate common cold | |
| RCT. Did not evaluate common cold | |
| RCT. Did not evaluate common cold | |
| Not RCT | |
| RCT. Did not evaluate common cold | |
| Not RCT | |
| Not RCT |
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of the common cold Show forest plot | 1 | 2307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.45, 2.02] |
| Analysis 1.1 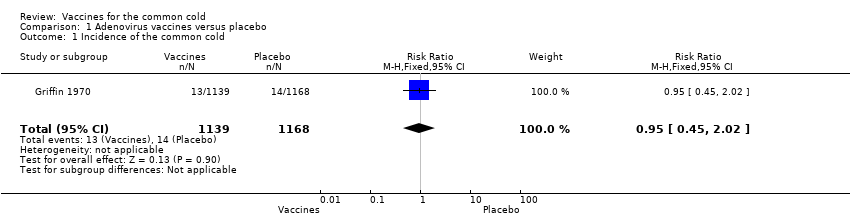 Comparison 1 Adenovirus vaccines versus placebo, Outcome 1 Incidence of the common cold. | ||||
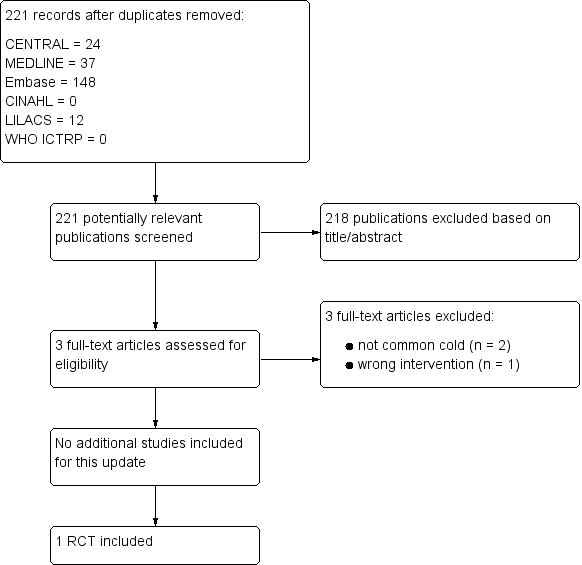
Study flow diagram
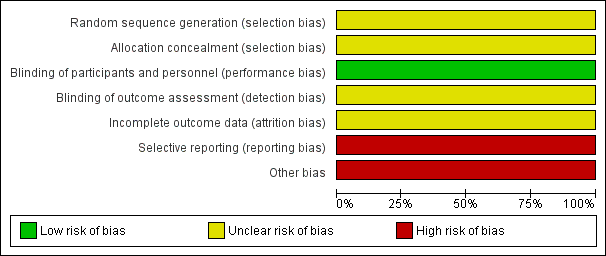
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages

'Risk of bias' summary: review authors' judgements about each risk of bias item for the included study

Comparison 1 Adenovirus vaccines versus placebo, Outcome 1 Incidence of the common cold.
| Virus vaccines compared to placebo for preventing the common cold in healthy people | ||||||
| Patient or population: healthy people | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Virus vaccines for preventing the common cold | |||||
| Incidence of the common cold | Study population | RR 0.95 | 2307 | ⊕⊕⊝⊝ | ||
| 12 per 1000 | 11 per 1000 | |||||
| Vaccine safety | The study stated that there were no adverse events related to the vaccine. | 2307 | ⊕⊕⊝⊝ | |||
| Mortality related to the vaccine ‐ not reported | See comments | See comments | See comments | See comments | See comments | The included study did not report this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| 1Adenovirus vaccine used for preventing the common cold. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of the common cold Show forest plot | 1 | 2307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.45, 2.02] |

