استفاده از پرتودرمانی پس از جراحی در سرطان سلول غیر‐کوچک ریه
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در حال انجام
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | 1966 to 1977 RCT | |
| Participants | 224 patients Trial data used in subgroup analyses for sex, age and histology | |
| Interventions | Surgery + radiotherapy vs surgery alone | |
| Outcomes | Survival | |
| Notes | 20 small cell participants excluded from meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: stated as randomised in paper; checks run on IPD suggest adequate sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "randomisation carried out via sealed envelope" |
| Blinding (performance bias and detection bias) | Low risk | Comment: trial not blinded owing to the nature of the intervention; outcome not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Comment: individual participant data obtained and checked for all outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: individual participant data obtained and checked for all outcomes |
| Other bias | Low risk | Comment: study apparently free of other sources of bias |
| Methods | 1981 to 1995 RCT | |
| Participants | 317 patients Trial data used in subgroup analyses for sex, age, histology, stage and nodal status | |
| Interventions | Surgery + radiotherapy vs surgery alone | |
| Outcomes | Survival | |
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: stated as randomised in paper; checks run on IPD suggest adequate sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "randomisation carried out via sealed envelope" |
| Blinding (performance bias and detection bias) | Low risk | Comment: trial not blinded owing to the nature of the intervention; outcome not likely influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Comment: individual participant data obtained and checked for all outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: individual participant data obtained and checked for all outcomes |
| Other bias | Low risk | Comment: study apparently free of other sources of bias |
| Methods | 1986 to 1990 RCT | |
| Participants | 106 patients Trial data used in subgroup analyses for sex, age, histology, stage and nodal status | |
| Interventions | Surgery + radiotherapy vs surgery alone | |
| Outcomes | Survival | |
| Notes | Unpublished trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: unpublished trial; checks run on IPD suggest adequate sequence generation |
| Allocation concealment (selection bias) | Low risk | Comment: unpublished, insufficient information provided, but collection of IPD and correspondence with those who supplied the data reassured that data were adequate |
| Blinding (performance bias and detection bias) | Low risk | Comment: trial not blinded owing to the nature of the intervention; outcome not likely influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Comment: individual participant data obtained and checked for all outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: individual participant data obtained and checked for all outcomes |
| Other bias | Low risk | Comment: study apparently free of other sources of bias |
| Methods | 1986 to 1994 RCT | |
| Participants | 189 patients Trial data used in subgroup analyses for sex, age, stage and nodal status | |
| Interventions | Surgery + radiotherapy vs surgery alone | |
| Outcomes | Survival | |
| Notes | Same publication as GETCB 05CB88 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned by centralised telephone procedure" Comment: stated as randomised in paper; checks run on IPD suggest adequate sequence generation |
| Allocation concealment (selection bias) | Low risk | Comment: randomisation by central telephone call |
| Blinding (performance bias and detection bias) | Low risk | Comment: trial not blinded owing to the nature of the intervention; outcome not likely influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Comment: individual participant data obtained and checked for all outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: individual participant data obtained and checked for all outcomes |
| Other bias | Low risk | Comment: study apparently free of other sources of bias |
| Methods | 1988 to 1994 RCT | |
| Participants | 539 patients Trial data used in subgroup analyses for sex, age, stage and nodal status | |
| Interventions | Surgery + radiotherapy vs surgery alone | |
| Outcomes | Survival | |
| Notes | Same publication as GETCB 04CB86 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned by centralised telephone procedure" Comment: stated as randomised in paper; checks run on IPD suggest adequate sequence generation |
| Allocation concealment (selection bias) | Low risk | Comment: randomisation by central telephone call |
| Blinding (performance bias and detection bias) | Low risk | Comment: trial not blinded owing to the nature of the intervention; outcome not likely influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Comment: individual participant data obtained and checked for all outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: individual participant data obtained and checked for all outcomes |
| Other bias | Low risk | Comment: study apparently free of other sources of bias |
| Methods | 1989 to 1997 RCT | |
| Participants | 104 patients Trial data used in subgroup analyses for age, sex, histology and stage | |
| Interventions | Surgery + radiotherapy vs surgery alone | |
| Outcomes | Survival | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "by chance" using computer‐generated model Comment: stated as randomised in paper; checks run on IPD suggest adequate sequence generation |
| Allocation concealment (selection bias) | Low risk | Comment: computer‐generated randomisation, which was checked by an independent colleague |
| Blinding (performance bias and detection bias) | Low risk | Comment: trial not blinded owing to the nature of the intervention; outcome not likely influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Comment: individual participant data obtained and checked for all outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: individual participant data obtained and checked for all outcomes |
| Other bias | Low risk | Comment: study apparently free of other sources of bias |
| Methods | 1989 to 1998 RCT | |
| Participants | 111 patients Trial data used in subgroup analyses for sex, age, histology, stage and nodal status | |
| Interventions | Surgery + radiotherapy vs surgery alone | |
| Outcomes | Survival | |
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: stated as randomised in paper; checks run on IPD suggest adequate sequence generation |
| Allocation concealment (selection bias) | Low risk | Comment: insufficient information provided in abstract, but collection of IPD and correspondence with those who supplied the data reassured that data were adequate |
| Blinding (performance bias and detection bias) | Low risk | Comment: trial not blinded owing to the nature of the intervention; outcome not likely influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Comment: individual participant data obtained and checked for all outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: individual participant data obtained and checked for all outcomes |
| Other bias | Low risk | Comment: study apparently free of other sources of bias |
| Methods | 1978 to 1985 RCT | |
| Participants | 230 patients Trial data used in subgroup analyses for sex, age, histology, stage and nodal status | |
| Interventions | Surgery + radiotherapy vs surgery alone | |
| Outcomes | Survival | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "permuted block randomisation" Comment: stated as randomised in paper; checks run on IPD suggest adequate sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: treatment assigned by central office |
| Blinding (performance bias and detection bias) | Low risk | Comment: trial not blinded owing to the nature of intervention; outcome not likely influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Comment: individual participant data obtained and checked for all outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: individual participant data obtained and checked for all outcomes |
| Other bias | Low risk | Comment: study apparently free of other sources of bias |
| Methods | 1985 to 1991 RCT | |
| Participants | 163 patients Trial data used in subgroup analyses for sex, age, histology, stage and nodal status | |
| Interventions | Surgery + radiotherapy vs surgery alone | |
| Outcomes | Survival | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised with a table of randomisation according to Snedecor and Cochran" Comment: stated as randomised in paper; checks run on IPD suggest adequate sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "randomised with a table of randomisation according to Snedecor and Cochran"; insufficient information provided |
| Blinding (performance bias and detection bias) | Low risk | Comment: trial not blinded owing to the nature of the intervention; outcome not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Comment: individual participant data obtained and checked for all outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: individual participant data obtained and checked for all outcomes |
| Other bias | Low risk | Comment: study apparently free of other sources of bias |
| Methods | 1986 to 1993 RCT | |
| Participants | 308 patients Trial data used in subgroup analyses for sex, age, histology, stage and nodal status | |
| Interventions | Surgery + radiotherapy vs surgery alone | |
| Outcomes | Survival | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: stated as randomised in paper; checks run on IPD suggest adequate sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "treatment assigned by central office" |
| Blinding (performance bias and detection bias) | Low risk | Comment: trial not blinded owing to the nature of the intervention; outcome not likely influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Comment: individual participant data obtained and checked for all outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: individual participant data obtained and checked for all outcomes |
| Other bias | Low risk | Comment: study apparently free of other sources of bias |
| Methods | 1988 to 1992 RCT | |
| Participants | 74 patients Trial data used in subgroup analyses for sex, age, histology, stage and nodal status | |
| Interventions | Surgery + radiotherapy vs surgery alone | |
| Outcomes | Survival | |
| Notes | Sealed envelope randomisation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: stated as randomised in paper; checks run on IPD suggest adequate sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "randomisation carried out via sealed envelope" |
| Blinding (performance bias and detection bias) | Low risk | Comment: trial not blinded owing to the nature of the intervention; outcome not likely influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Comment: individual participant data obtained and checked for all outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: individual participant data obtained and checked for all outcomes |
| Other bias | Low risk | Comment: study apparently free of other sources of bias |
All trials supplied individual participant data for analysis and therefore are defined as unpublished data, even though most are published.
IPD = individual participant data.
RCT = randomised controlled trial.
RT = radiotherapy.1G
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Data unavailable/eligibility uncertain | |
| Eligible | |
| Eligible |
RCT = randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Essai de phase III comparant une radiothérapie médiastinale conformationnelle post‐opératoire à l’absence de radiothérapie après chirurgie complète chez des patients présentant un carcinome bronchique non à petites cellules (CBNPC) avec envahissement médiastinal N2 |
| Methods | Phase III multi‐centric |
| Participants | |
| Interventions | |
| Outcomes | Evaluation de l’impact de la radiothérapie médiastinale conformationnelle sur la survie sans récidive comparé à l’absence de radiothérapie |
| Starting date | 2006 |
| Contact information | Docteur Cécile Le Péchoux ‐ [email protected] |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Survival Show forest plot | 11 | 2343 | Hazard Ratio (95% CI) | 1.18 [1.07, 1.31] |
| Analysis 1.1  Comparison 1 Surgery + PORT versus surgery alone, Outcome 1 Survival. | ||||
| 2 Local recurrence‐free survival Show forest plot | 11 | 2343 | Hazard Ratio (95% CI) | 1.12 [1.01, 1.23] |
| Analysis 1.2  Comparison 1 Surgery + PORT versus surgery alone, Outcome 2 Local recurrence‐free survival. | ||||
| 3 Distant recurrence‐free survival Show forest plot | 11 | 2343 | Hazard Ratio (95% CI) | 1.13 [1.02, 1.24] |
| Analysis 1.3  Comparison 1 Surgery + PORT versus surgery alone, Outcome 3 Distant recurrence‐free survival. | ||||
| 4 Recurrence‐free survival Show forest plot | 11 | 2343 | Hazard Ratio (95% CI) | 1.10 [0.99, 1.21] |
| Analysis 1.4  Comparison 1 Surgery + PORT versus surgery alone, Outcome 4 Recurrence‐free survival. | ||||
| 5 RT delivery method Show forest plot | 11 | 2343 | Hazard Ratio (95% CI) | 1.18 [1.07, 1.31] |
| Analysis 1.5  Comparison 1 Surgery + PORT versus surgery alone, Outcome 5 RT delivery method. | ||||
| 5.1 Cobalt‐60 only | 1 | 202 | Hazard Ratio (95% CI) | 1.48 [1.09, 2.02] |
| 5.2 Cobalt‐60 and linac | 6 | 1746 | Hazard Ratio (95% CI) | 1.18 [1.05, 1.33] |
| 5.3 Linac only | 4 | 395 | Hazard Ratio (95% CI) | 1.02 [0.80, 1.31] |
| 6 RT dose Show forest plot | 11 | 2343 | Peto Odds Ratio (95% CI) | 1.18 [1.07, 1.31] |
| Analysis 1.6  Comparison 1 Surgery + PORT versus surgery alone, Outcome 6 RT dose. | ||||
| 6.1 < 45 Gy | 2 | 382 | Peto Odds Ratio (95% CI) | 0.93 [0.75, 1.17] |
| 6.2 ≥ 45 Gy | 9 | 1961 | Peto Odds Ratio (95% CI) | 1.25 [1.12, 1.40] |
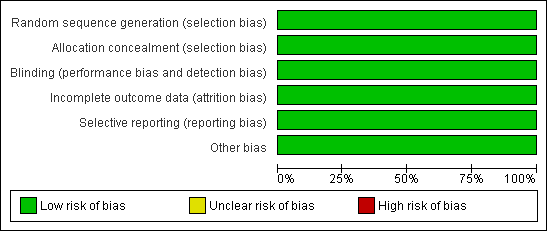
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Overall survival.
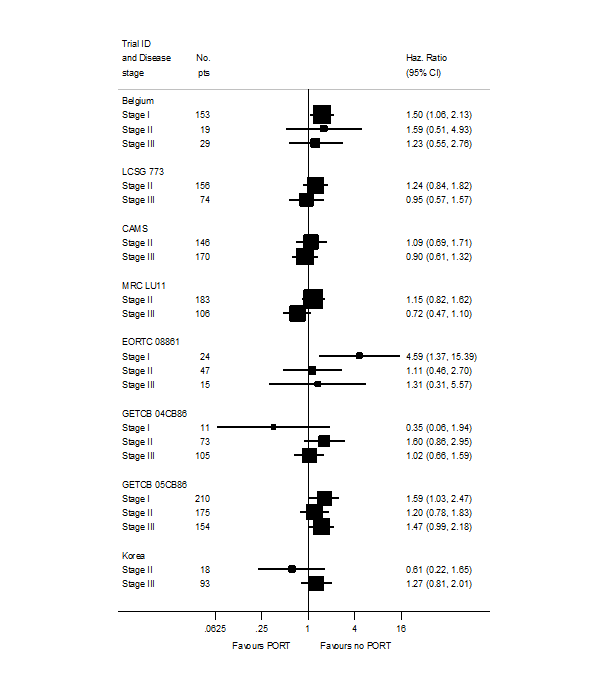
PORT effect on overall survival by trial according to stage.
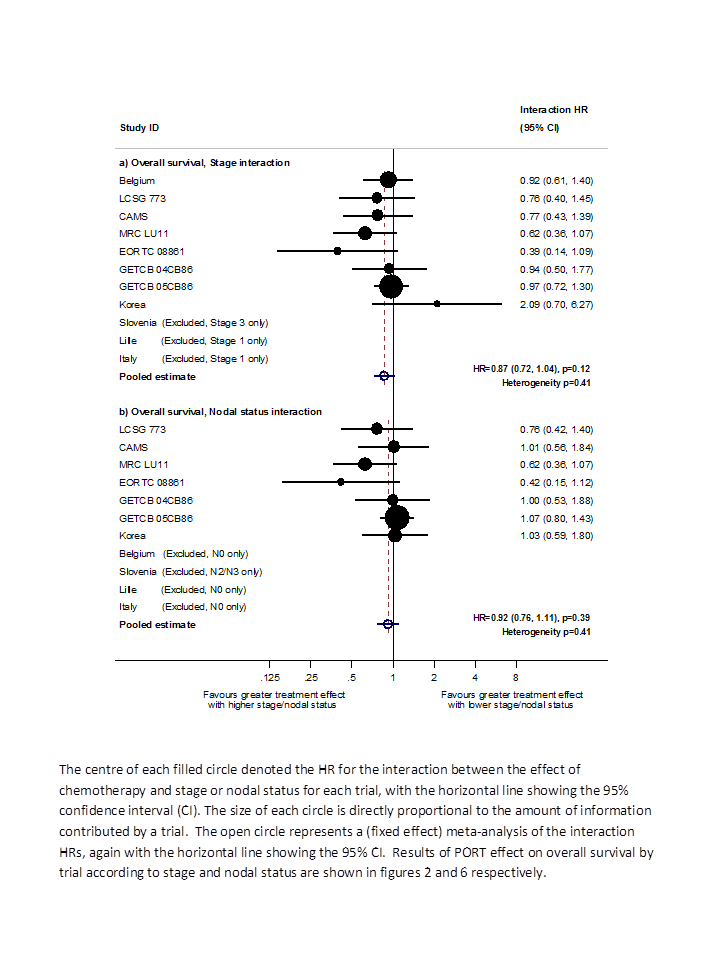
Hazard ratio (HR) for the interaction between the effect of PORT on survival and (a) stage or (b) nodal status.
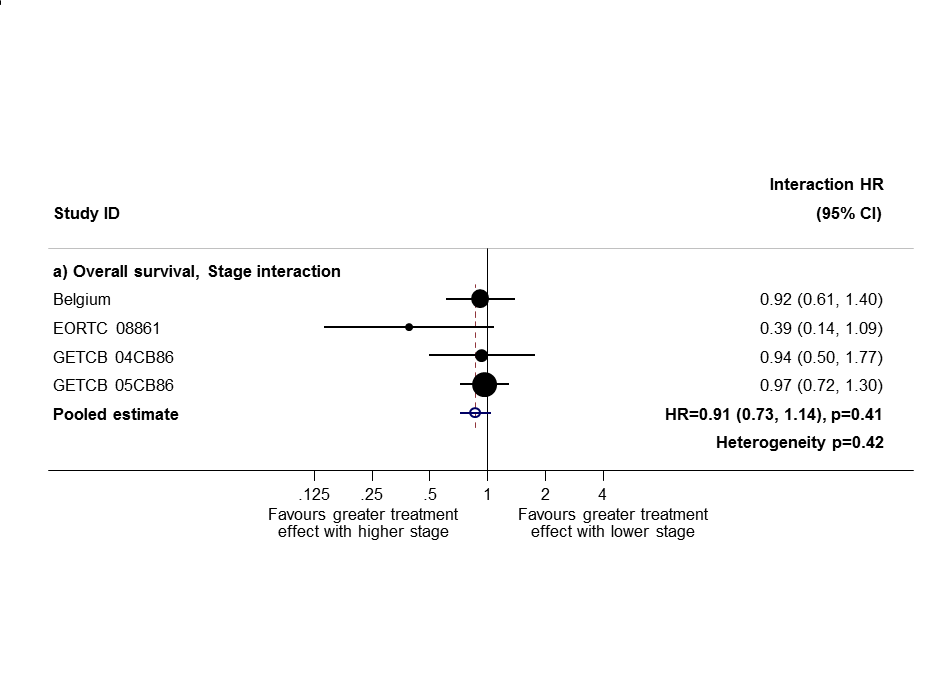
Sensitivity analysis 1: only trials with all stage subgroups included.
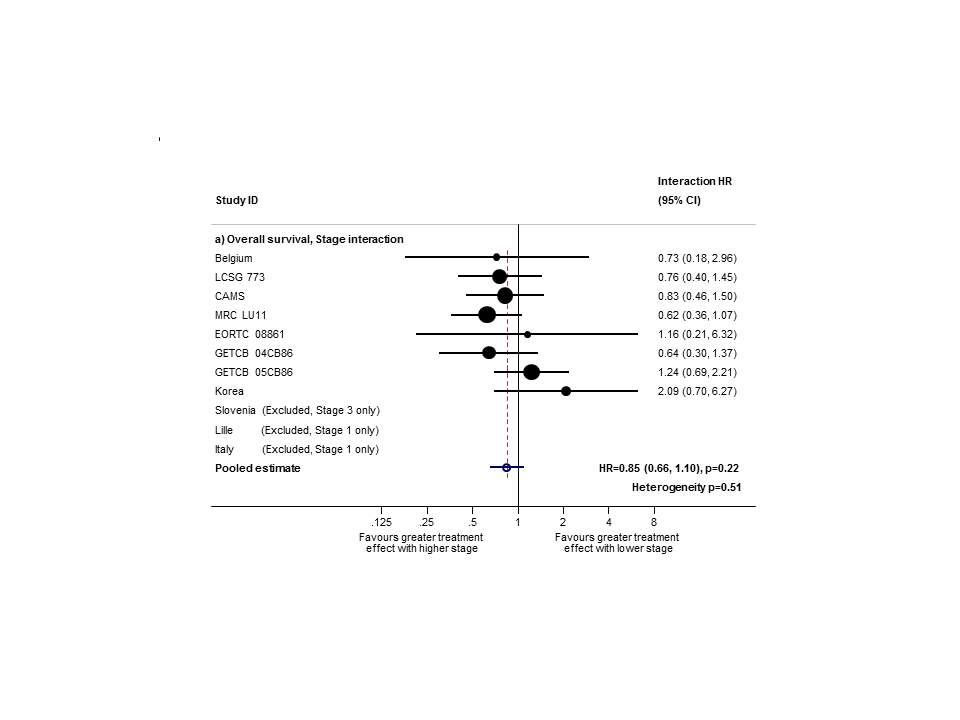
Sensitivity analysis (2): only trials with stage II and III subgroups represented.

PORT effect on overall survival by trial according to nodal status.

Comparison 1 Surgery + PORT versus surgery alone, Outcome 1 Survival.
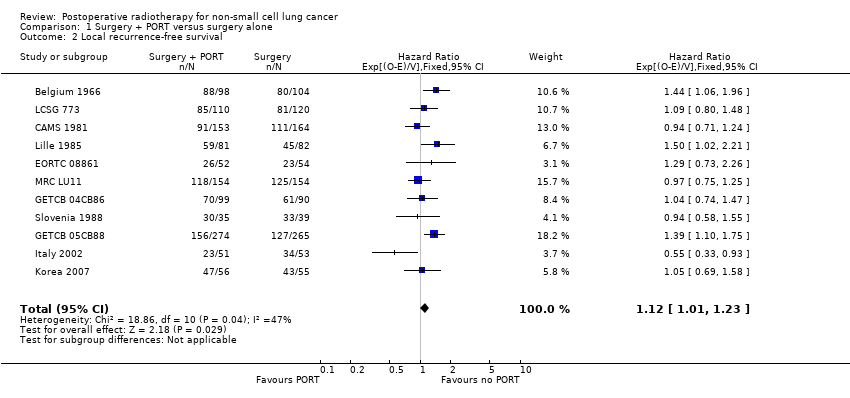
Comparison 1 Surgery + PORT versus surgery alone, Outcome 2 Local recurrence‐free survival.
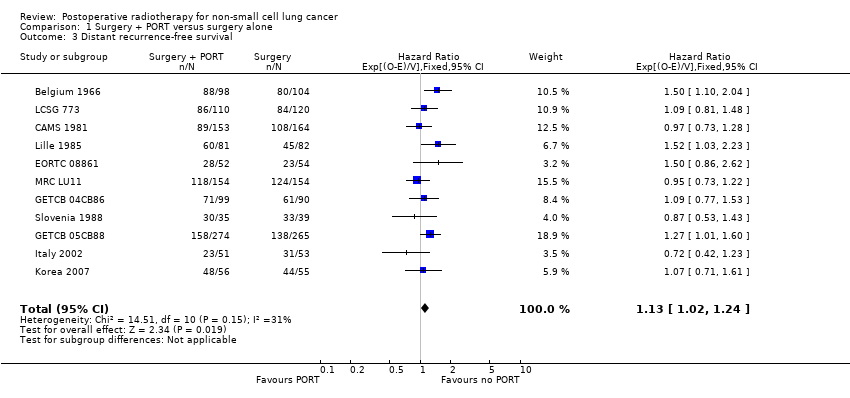
Comparison 1 Surgery + PORT versus surgery alone, Outcome 3 Distant recurrence‐free survival.

Comparison 1 Surgery + PORT versus surgery alone, Outcome 4 Recurrence‐free survival.
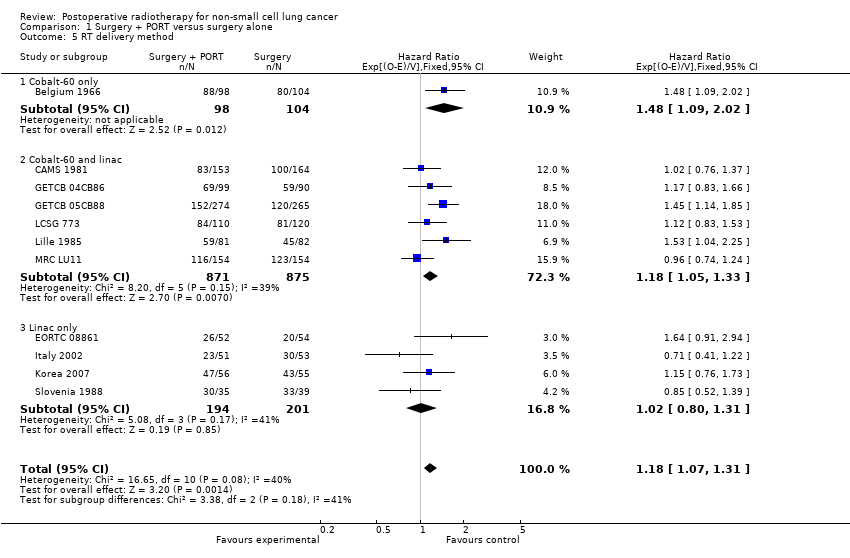
Comparison 1 Surgery + PORT versus surgery alone, Outcome 5 RT delivery method.
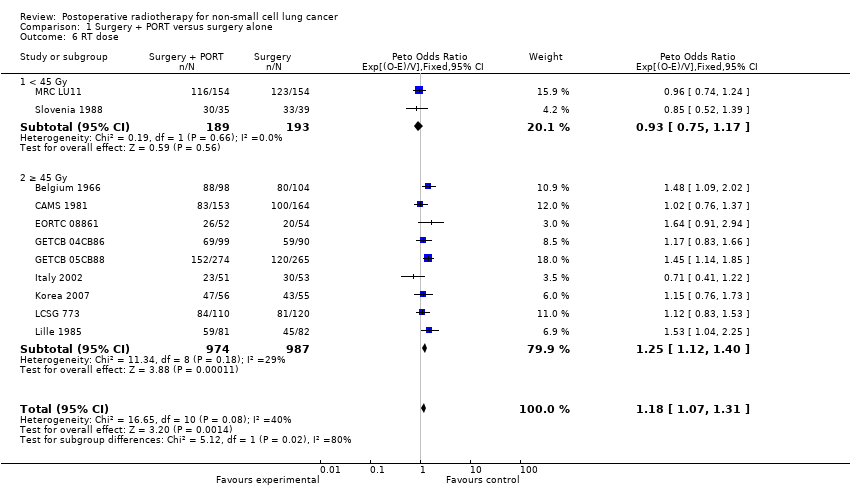
Comparison 1 Surgery + PORT versus surgery alone, Outcome 6 RT dose.
| T stage | N stage | M stage | Meta‐analysis stage | AJCC stage |
| 0, 1, 2, X, iS | 0 | 0 | I | I |
| 0, 1, 2, X, iS | 1 | 0 | II | II |
| Any | 2, 3 | 0 | III | III non‐metastatic |
| 3, 4 | Any | 0 | III | III non‐metastatic |
| Any | Any | 1 | IV | Any metastatic |
| AJCC = American Joint Committee on Cancer. | ||||
| T stage | N stage | M stage | Meta‐analysis stage |
| 1, 2 | 0 | 0 | I |
| 1, 2 | 1 | 0 | II |
| 3 | 0 | 0 | II |
| 1, 2 | 2 | 0 | III |
| 3 | 1, 2 | 0 | III |
| Any | Any | 1 | IV |
| Characteristic | Postoperative RT | Surgery only | Total |
| AGE (data from 11 trials) | |||
| < 54 years | 294 | 327 | 621 |
| 55 to 59 years | 267 | 261 | 528 |
| 60 to 64 years | 290 | 276 | 566 |
| > 65 years | 312 | 315 | 627 |
| Unknown | 0 | 1 | 1 |
| SEX (data from 11 trials) | |||
| Male | 988 | 992 | 1980 |
| Female | 175 | 187 | 362 |
| Not recorded | 0 | 1 | 1 |
| HISTOLOGY (data from 9 trials) | |||
| Adenocarcinoma | 195 | 218 | 413 |
| Squamous | 522 | 545 | 1067 |
| Other | 66 | 54 | 120 |
| Unknown | 380 | 363 | 743 |
| META‐ANALYSIS STAGE (data from 11 trials) | |||
| I | 328 | 338 | 666 |
| II | 353 | 366 | 719 |
| III | 463 | 455 | 918 |
| IV | 1 | 0 | 1 |
| Unknown | 18 | 21 | 39 |
| WHO PERFORMANCE STATUS (data from 4 trials; not used) | |||
| Good (0, 1) | 195 | 196 | 391 |
| Poor (2, 3, 4) | 77 | 83 | 160 |
| Unknown | 22 | 21 | 43 |
| Trend or interaction 1998 | Trend or interaction 2005 | Trend or interaction 2010 'old' methods | Trend or interaction 2010 'new' methods and TNM changes | |
| Age | P = 0.34 | P = 0.44 | P = 0.32 | P = 0.20 |
| Sex | P = 0.94 | P = 0.92 | P = 0.84 | P = 0.49 |
| Histology | P = 0.75 | P = 0.61 | P = 0.42 | P = 0.38 |
| Stage | P = 0.0003 | P = 0.003 | P = 0.003 | P = 0.12 |
| Nodal status | P = 0.016 | P = 0.02 | P = 0.03 | P = 0.39 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Survival Show forest plot | 11 | 2343 | Hazard Ratio (95% CI) | 1.18 [1.07, 1.31] |
| 2 Local recurrence‐free survival Show forest plot | 11 | 2343 | Hazard Ratio (95% CI) | 1.12 [1.01, 1.23] |
| 3 Distant recurrence‐free survival Show forest plot | 11 | 2343 | Hazard Ratio (95% CI) | 1.13 [1.02, 1.24] |
| 4 Recurrence‐free survival Show forest plot | 11 | 2343 | Hazard Ratio (95% CI) | 1.10 [0.99, 1.21] |
| 5 RT delivery method Show forest plot | 11 | 2343 | Hazard Ratio (95% CI) | 1.18 [1.07, 1.31] |
| 5.1 Cobalt‐60 only | 1 | 202 | Hazard Ratio (95% CI) | 1.48 [1.09, 2.02] |
| 5.2 Cobalt‐60 and linac | 6 | 1746 | Hazard Ratio (95% CI) | 1.18 [1.05, 1.33] |
| 5.3 Linac only | 4 | 395 | Hazard Ratio (95% CI) | 1.02 [0.80, 1.31] |
| 6 RT dose Show forest plot | 11 | 2343 | Peto Odds Ratio (95% CI) | 1.18 [1.07, 1.31] |
| 6.1 < 45 Gy | 2 | 382 | Peto Odds Ratio (95% CI) | 0.93 [0.75, 1.17] |
| 6.2 ≥ 45 Gy | 9 | 1961 | Peto Odds Ratio (95% CI) | 1.25 [1.12, 1.40] |

