Tratamento medicamentoso para a incontinência fecal em adultos
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Cross‐over randomised controlled trial | |
| Participants | 36 adults (22 women) with passive FI and structurally intact anal sphincters | |
| Interventions | A: 10% phenylephrine gel | |
| Outcomes | Subjective cure: A: 0/36, B: 0/36 | |
| Notes | 15 patients continued with loperamide during the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was carried out by a pharmacist by means of computer generated random numbers" |
| Allocation concealment (selection bias) | Low risk | "The randomization code was kept in the hospital pharmacy and made known to the investigators only after analysis of the completed study." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Repored as "double‐blind" trial but it is not specified who was blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Repored as "double‐blind" trial but it is not specified who was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Was use of a cross over design appropriate? | Low risk | Yes |
| Is it clear that the order of receiving treatment was randomised? | Low risk | Used computer‐generated random numbers. |
| Can it be assumed that the trial was not biased from carry over effects? | Unclear risk | "4 weeks treatment periods separated by a 1 week washout period" |
| Methods | Cross‐over randomised controlled trial | |
| Participants | 12 adults (7 women) with FI after ileoanal pouch surgery for ulcerative colitis | |
| Interventions | A: 10% phenylephrine gel | |
| Outcomes | Cure: A: 4/12, B: 0/12 | |
| Notes | 8 patients continued with loperamide during the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random assignment was performed using random numbers generated by a scientific calculator, and the randomization code was kept in the hospital pharmacy and made known to the investigators only after analysis of the completed study" |
| Allocation concealment (selection bias) | Low risk | "Random assignment was performed using random numbers generated by a scientific calculator, and the randomization code was kept in the hospital pharmacy and made known to the investigators only after analysis of the completed study" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Repored as "double‐blind" trial but it is not specified who was blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Repored as "double‐blind" trial but it is not specified who was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Was use of a cross over design appropriate? | Low risk | Yes |
| Is it clear that the order of receiving treatment was randomised? | Low risk | "Random assignment was performed using random numbers generated by a scientific calculator, and the randomization code was kept in the hospital pharmacy and made known to the investigators only after analysis of the completed study" |
| Can it be assumed that the trial was not biased from carry over effects? | Unclear risk | "...........two four‐week treatment phases separated by a one‐week washout phase." |
| Methods | Randomised controlled trial | |
| Participants | 206 people (145 women) with at least weekly faecal incontinence associated with chronic rectal emptying (40% of patients had daily faecal incontinence for more than 2 years). 178 were available for analysis after the first week of treatment. Patients were aged 65 years or older and residents of long‐term care units. | |
| Interventions | A: 30 g per day of a single osmotic laxative (lactulose) | |
| Outcomes | Mean number of FI episodes per week (n, mean, SD): A: 61, 6 (2.9), B: 62, 6 (2.7) (P = 0.9 after 4 weeks) | |
| Notes | Higher dropout rate in Group I than in Group II | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as "prospective randomized study" but the method of sequence generation not specified |
| Allocation concealment (selection bias) | Unclear risk | Reported as "prospective randomized study" and it is not specified whether or not the allocation was concealed. |
| Blinding of participants and personnel (performance bias) | High risk | "This study could be randomized but not blinded because outcomes were measured daily and because the treatment was provided by the nursing staff." |
| Blinding of outcome assessment (detection bias) | High risk | "This study could be randomized but not blinded because outcomes were measured daily and because the treatment was provided by the nursing staff." |
| Incomplete outcome data (attrition bias) | Unclear risk | No missing data, however 32 participants in Group I and 23 participants in Group II withdrew from the trial. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Was use of a cross over design appropriate? | Low risk | This is a randomised controlled trial and not a cross‐over trial and this domain of risk of bias assessment is not applicable and would be judged as low risk. |
| Is it clear that the order of receiving treatment was randomised? | Low risk | This is a randomised controlled trial and not a cross‐over trial and this domain of risk of bias assessment is not applicable and would be judged as low risk. |
| Can it be assumed that the trial was not biased from carry over effects? | Low risk | This is a randomised controlled trial and not a cross‐over trial and this domain of risk of bias assessment is not applicable and would be judged as low risk. |
| Methods | Randomised controlled trial. | |
| Participants | 10 people (7 women) with passive faecal incontinence and low maximal resting anal pressure but with intact sphincters demonstrated on endoanal ultrasound scan | |
| Interventions | A: 0% gel (placebo containing no active ingredient) | |
| Outcomes | Maximal resting anal pressure: comparable at baseline on all study days | |
| Notes | Washout of 48 hours between daily doses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It is reported that "Gels were applied in a random order..................." however it is not specified how sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | It is reported that "Gels were applied in a random order..................." however it is not specified if the allocation was concealed or no |
| Blinding of participants and personnel (performance bias) | Low risk | It is reported that both the investigator and patients were unaware of the nature of each gel. |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is not specified if the outcome was assessed by the investigator or someone else. |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Was use of a cross over design appropriate? | Low risk | This is a randomised controlled trial and not a cross‐over trial and this domain of risk of bias assessment is not applicable and would be judged as low risk. |
| Is it clear that the order of receiving treatment was randomised? | Low risk | This is a randomised controlled trial and not a cross‐over trial and this domain of risk of bias assessment is not applicable and would be judged as low risk. |
| Can it be assumed that the trial was not biased from carry over effects? | Low risk | This is a randomised controlled trial and not a cross‐over trial and this domain of risk of bias assessment is not applicable and would be judged as low risk. |
| Methods | Cross‐over randomised controlled trial | |
| Participants | 11 adults | |
| Interventions | Initial 2 day drug‐free washout period | |
| Outcomes | Mean stool frequency stated to be significantly lower with oral loperamide (Group A) compared with suppository (Group B, P < 0.02) or placebo washout phase (P < 0.05) | |
| Notes | No useable data (graphical form only) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It is reported that the "Subjects were randomized........", however, method of sequence generation not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | Low risk | "Subjects were blinded to the contents of their medication in all three phases. The investigators were blinded to the contents in the first and third phases." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Was use of a cross over design appropriate? | Low risk | The design seems to be appropriate, however information pertaining to sequence generation and allocation concealment are not provided, and the washout period seems to be short. |
| Is it clear that the order of receiving treatment was randomised? | Unclear risk | Not specified |
| Can it be assumed that the trial was not biased from carry over effects? | High risk | "2‐day drug‐free washout period" |
| Methods | Cross‐over randomised controlled trial. | |
| Participants | 10 adults (7 men and 3 woman) | |
| Interventions | A: loperamide 2 mg + placebo x 2 | |
| Outcomes | No effect on stool frequency | |
| Notes | Study aim was to reduce the adverse effects of orlistat treatment for obesity (oily stools, increased faecal frequency and urgency and 'fecal spotting' (= faecal incontinence) in order to increase compliance with orlistat | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The hospital pharmacy dispensed medication according to a computer generated randomization list." |
| Allocation concealment (selection bias) | Low risk | "The sequence was concealed until the study was completed". |
| Blinding of participants and personnel (performance bias) | Unclear risk | Reported as "double‐blind study", however it is not specified who was blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Reported as "double‐blind study", however it is not specified who was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Was use of a cross over design appropriate? | Low risk | Each participant received placebo and two of the three doses of loperamide for 2 weeks, each separated by a 2‐week washout. The sequence was concealed until the study was completed. |
| Is it clear that the order of receiving treatment was randomised? | Low risk | "The hospital pharmacy dispensed medication according to a computer generated randomization list." |
| Can it be assumed that the trial was not biased from carry over effects? | Low risk | Each participant received placebo and two of the three doses of loperamide for 2 weeks, each separated by a 2‐week washout. |
| Methods | Cross‐over randomised controlled trial. | |
| Participants | 30 adults (8 women) with ileoanal pouches (16 handsewn and 14 stapled), performed for ulcerative colitis. Groups combined for analysis | |
| Interventions | A: loperamide 4 mg three times a day | |
| Outcomes | Number experiencing soiling, day: A: 3/28, B: 7/28; night: A: 1/28, B: 11/28 | |
| Notes | Patients were asked to keep to usual meal times and diet. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | Low risk | Reported as "double blinded" and used "identical capsule" therefore participants must be blinded. Not sure about personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | No complete data |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Was use of a cross over design appropriate? | Low risk | Seems appropriate with seven days washout period in between. |
| Is it clear that the order of receiving treatment was randomised? | Unclear risk | Method of randomisation not specified |
| Can it be assumed that the trial was not biased from carry over effects? | Low risk | There was seven days washout period |
| Methods | Cross‐over randomised controlled trial | |
| Participants | 15 people (14 women) with chronic diarrhoea and FI | |
| Interventions | A: diphenoxylate (2.5 mg) plus atropine sulfate (25 mcg) | |
| Outcomes | Failure rate (number not continent): A: 0/15, B: 3/15 | |
| Notes | Patients were admitted to hospital and given a standardised diet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as "patients were randomized in a double‐blind fashion", however method of sequence generation not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding of participants and personnel (performance bias) | Low risk | "Neither the patients, the laboratory personnel, nor the physicians in charge knew when the patients were lomotil or when they were on placebo until the code was broken after the experiment was completed". |
| Blinding of outcome assessment (detection bias) | Low risk | "Neither the patients, the laboratory personnel, nor the physicians in charge knew when the patients were lomotil or when they were on placebo until the code was broken after the experiment was completed". |
| Incomplete outcome data (attrition bias) | High risk | reported data of only those participants who completed the trial as reported "data on this 4th patient are not included ion this paper...........". The excluded patient had "severe abdominal discomfort" the cause of which is not specified. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Was use of a cross over design appropriate? | Low risk | Seems appropriate |
| Is it clear that the order of receiving treatment was randomised? | Unclear risk | Not specified |
| Can it be assumed that the trial was not biased from carry over effects? | High risk | No washout period |
| Methods | Cross‐over randomised controlled trial | |
| Participants | 17 patients (4 women) with 'J' configuration ileoanal pouches 1 to 4 months after closure of diverting ileostomy (8 patients with ulcerative colitis and 9 with familial adenomatous polyposis) | |
| Interventions | A: sodium valproate 400 mg | |
| Outcomes | Number with FI (soiling): A: 3/17, B: 10/17 | |
| Notes | Sodium valproate has contractile effects on the internal anal sphincter | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified. Reported as "The valproate sodium and placebo series were carried out in random order" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Unclear risk | Protocol not assessed |
| Was use of a cross over design appropriate? | Unclear risk | Seems appropriate |
| Is it clear that the order of receiving treatment was randomised? | Unclear risk | Not specified |
| Can it be assumed that the trial was not biased from carry over effects? | Low risk | There was a 3‐day washout period |
| Methods | Randomised controlled trial | |
| Participants | Adults patients > 21 years old who, having undergone coloproctectomy with ileoanal anastomosis and J‐shaped ileal reservoir, presented with nocturnal faecal incontinence Exclusion criteria: associated anal pathology, active pouchitis, stenotic ileoanal anastomosis, concomitant treatment with loperamide, monoaminoxidase inhibitors and/or tricyclic antidepressants, pregnancy, narrow angle glaucoma, uncontrolled arterial hypertension/coronary disease/cardiac arrhythmias/aortic aneurysm, epilepsy 37 patients initially identified from 98 interviews Reasons for non ‐ inclusion/withdrawal: 9 with associated anal pathology or intercurrent illness, 9 did not complete previous evaluations, 4 refused to participate, 3 did not complete treatment 12 participants included in final analysis Group A: 5 (2 men, 3 women. Mean age: 49.0 [Range 27‐62]) Group B: 7 (6 men,1 woman. Mean age: 38.7 [Range: 24‐63]) | |
| Interventions | A: Cream with active ingredient (10% phenylephrine) B: Placebo cream 0.5 mg cream to be applied digitally around anal margin by patients before going to bed Length of treatment: 1 month (number of days unclear). A faecal incontinence diary was kept for 21 days before treatment and during the treatment month. All patients received 3.5g Plantago ovata thereby guaranteeing minimal fibre intake. Liquid intake restricted to 1.5 L/day | |
| Outcomes | Occurence of faecal incontinence during treatment; median (range) Group A: 5.4 (0‐14) Group B: 9 (0‐19) | |
| Notes | 9 patients with ulcerative colitis (Group A; 5 Group B; 4,). 3 patients previously operated for Familial Adenomatous Polyposis (all Group B). Inconsistent with exclusion criteria of the trial (associated anal pathology). Discrepancy between text and table: texts states 3 men in group A whereas table states 2 men | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Fueron randomizados en dos grupos (A) y (B), utilizando la técnica de muestreo en bloque' 'They were randomised into two groups (A) and (B), using the technique of block sampling' |
| Allocation concealment (selection bias) | Unclear risk | None mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | Technique not mentioned |
| Blinding of outcome assessment (detection bias) | Unclear risk | Technique not mentioned |
| Incomplete outcome data (attrition bias) | High risk | Two participants in Group B and 1 in Group A did not complete treatment, these results were not included in final analysis |
| Selective reporting (reporting bias) | High risk | Results of two participants not completing trial not reported |
| Was use of a cross over design appropriate? | Low risk | This is a randomised controlled trial and not a cross‐over trial and this domain of 'Risk of bias' assessment is not applicable and would be judged as low risk. |
| Is it clear that the order of receiving treatment was randomised? | Low risk | This is a randomised controlled trial and not a cross‐over trial and this domain of 'Risk of bias' assessment is not applicable and would be judged as low risk. |
| Can it be assumed that the trial was not biased from carry over effects? | Low risk | This is a randomised controlled trial and not a cross‐over trial and this domain of 'Risk of bias' assessment is not applicable and would be judged as low risk. |
| Methods | Cross‐over randomised controlled trial (three arms) | |
| Participants | 30 patients with persistent (chronic) diarrhoea for at least 3 months (definition of diarrhoea included urgency and faecal incontinence). Diagnoses included irritable bowel, Crohn's disease, after gastric surgery, ulcerative colitis, diabetes | |
| Interventions | A: loperamide hydrochloride (2 mg) | |
| Outcomes | Number of people with FI: A: 2/25, B: 3/25, C: 6/25 | |
| Notes | Not clear how many patients suffered from faecal incontinence at baseline. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | Low risk | Reported as "double blinded" and used "identical capsule" therefore participants must be blinded. Not sure about personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) | High risk | 5 patients failed to attend the clinic regularly and withdrew early in the study. Ten further patients out of remaining 25 patients failed to complete the treatment period for one or more drugs |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Was use of a cross over design appropriate? | Low risk | Seems appropriate |
| Is it clear that the order of receiving treatment was randomised? | Unclear risk | Not stated |
| Can it be assumed that the trial was not biased from carry over effects? | High risk | Patients were instructed to increase the daily dose gradually until control was achieved or side effects became intolerable (but outcomes from last 3 weeks of treatment, after treatment stabilised) |
| Methods | Randomised controlled trial | |
| Participants | 35 patients with low anterior resection with rectal cancer were recruited. 6 participants withdrew and the results were reported of 29 participants; 17 in the treatment group and 12 in the placebo group.All participants had anal incontinence of solid or liquid stools or gas and experienced failure of other treatments with anti‐diarrhoeal agents or biofeedback Exclusion criteria were pregnancy, Ischaemic heart disease, uncontrolled hypertension, aortic aneurysm, treatment with monoamine oxidase inhibitors or tricyclic antidepressants, surgically reparable external sphincter injury, inflammatory bowel disease, or any other | |
| Interventions | A: 30% Phenylephrine (3 g of Phenylephrine HCl in 7 g of white petrolatum) gel B: Identical placebo gel Length of treatment: 0.5 mL of gel was applied topically to the anal margin twice daily for 4 weeks, | |
| Outcomes | Anal incontinence evaluated with Faecal Incontinence Severity Index (FISI) A (n = 17)= Baseline: 32.5(14.5); After: 32.3(14.7) P = 0.940 B (n = 12)= Baseline: 32.1 (11.2); After: 32.4(14.4) P = 0.626 Quality of life assessed with Faecal Incontinence Quality of life (FIQL) scale and included the following domains: lifestyle, coping, depression and embarrassment Lifestyle: A (n = 17)= Baseline: 2.9(0.8); After: 2.9(1.0) P = 0.801 B (n = 12)= Baseline: 2.7(0.5); After: 3.0(0.8) P = 0.269 Coping: A (n = 17)= Baseline: 2.5(0.9); After: 2.8(0.9) P = 0.110 B (n = 12)= Baseline: 2.5(0.5); After: 2.8(0.5) P = 0.119 Depression: A (n = 17)= Baseline: 3.2(0.7); After: 3.2(0.8) P = 0.415 B (n = 12)= Baseline: 3.1(0.5); After: 3.2(0.5) P = 0.554 Embarrassment: A (n = 17)= Baseline: 2.7(0.7); After: 3.0(0.7) P = 0.090 B (n = 12)= Baseline: 2.7(0.6); After: 2.6(0.8) P = 0.855 Manometry: Resting pressure (mmHg): A (n = 17)= Baseline: 30.0(12.3); After: 27.3(12.7) P = 0.362 B (n = 12)= Baseline: 32.6(14.2); After: 27.2(15.0) P = 0.306 Squeezing pressure (mmHg): A (n = 17)= Baseline: 143.3(60.5); After: 160.4(76.9) P = 0.083 B (n = 12)= Baseline: 152.6(86.5); After: 147.1(76.5) P = 0.625 Sustained duration (s): A (n = 17)= Baseline: 41.9(24.5); After: 44.9(48.3) P = 0.848 B (n = 12)= Baseline: 39.6(24.4); After: 32.8(14.4) P = 0.187 Sphincter length (cm): A (n = 17)= Baseline: 3.2(0.9); After: 3.4(0.8) P = 0.368 B (n = 12)= Baseline: 3.5(0.8); After: 3.4(0.8) P = 0.743 High pressure zone (cm): A (n = 17)= Baseline: 2.4(2.1); After: 1.9(0.5) P = 0.378 B (n = 12)= Baseline: 2.1(0.9); After: 2.3(0.9) P = 0.556 Complications: Dermatitis reaction: A = 5/17; B = 1/12 Palpitation: A = 0/17; B = 1/12 Headache: A = 2/17; B = 0/12 | |
| Notes | Not clear how many patients suffered from faecal incontinence at baseline. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A one‐to‐one randomization code was derived by the pharmacy trials coordinator using a computer‐generated random number sequence" |
| Allocation concealment (selection bias) | Low risk | "The randomization code was kept in the hospital pharmacy and made known to the investigators only after the study was completed". |
| Blinding of participants and personnel (performance bias) | Low risk | It is mentioned that the trial was "double blind" and it is also reported that "The placebo and phenylephrine gel were identical in appearance and texture and were supplied in identical containers" therefore it seems that the participants and personnel must be blinded although not specifically stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) | Unclear risk | No incomplete data although 6 participants (2 from the intervention and 4 from the placebo arm) withdrew from the study due to poor compliance |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Was use of a cross over design appropriate? | Low risk | This is a randomised controlled trial and not a cross‐over trial and this domain of risk of bias assessment is not applicable and would be judged as low risk. |
| Is it clear that the order of receiving treatment was randomised? | Low risk | This is a randomised controlled trial and not a cross‐over trial and this domain of risk of bias assessment is not applicable and would be judged as low risk. |
| Can it be assumed that the trial was not biased from carry over effects? | Low risk | This is a randomised controlled trial and not a cross‐over trial and this domain of risk of bias assessment is not applicable and would be judged as low risk. |
| Methods | Randomised controlled trial | |
| Participants | 44 patients with faecal incontinence were included. All participants were women aged 18 years or over. | |
| Interventions | A: Zinc‐aluminium ointment Duration of treatment: Applied on the anal canal mucosa 3 times daily over 4 weeks. | |
| Outcomes | Wexner Faecal Incontinence Score reported before and after the treatment A (n = 24): Before: 16.6 (6‐20); After: 8.5 (0‐11) P < 0.001 B (n = 20): Before: 16.7 (5‐18); After: 13.1(5‐17) P = 0.02 There was a significant difference in the final scores favouring the treatment group (P = 0.001) For Quality of life "Fecal Incontinence Quality of Life (FIQL) score" was used which included the following parameters: lifestyle, conduct, embarrassment and depression. Lifestyle: A (n = 24): Baseline: 2.49(1.06); After: 3.58(1.18) P < 0.001 B (n = 20): Baseline: 2.50(1.01); After: 2.55(1.03) P = 0.151 Conduct: A (n = 24): Baseline: 2.19(1.02); After: 3.12(1.16) P < 0.001 B (n = 20): Baseline: 2.17(0.91); After: 2.37(1.13) P = 0.104 Embarrassment: A (n = 24): Baseline: 1.54(0.82); After: 2.5(1.32) P < 0.001 B (n = 20): Baseline: 1.56(0.74); After: 1.76(0.84) P = 0.043 Depression: A (n = 24): Baseline: 2.51(1.01); After: 3.48(1.17) P = 0.001 B (n = 20): Baseline: 2.46(1.02); After: 2.71(1.13) P = 0.093 The quality of life score increased in both groups but more in the treatment group in all the parameters. Adverse effects/ complications: A = 0/24; B = 0/20 | |
| Notes | It is mentioned that the inclusion criteria were faecal incontinence, minimal sphincter disruption on anal endosonography and it is also stated that "aluminium ointment is effective in the treatment of anal fissure" therefore it seems that all the patients had anal fissure although not specifically stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as "randomized trial" however method of sequence generation not specified |
| Allocation concealment (selection bias) | Unclear risk | Reported as "randomized trial" however method of allocation concealment not specified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Reported as "double‐blind" however it is not specifically mentioned who was blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Reported as "double‐blind" however it is not specifically mentioned who was blinded |
| Incomplete outcome data (attrition bias) | High risk | Earlier it is mentioned that "six were lost to study" but later on it is reported that "one in the treatment group and four in the placebo group withdrew at the beginning of the study" i.e. five patients. They have not specified to which group the sixth patient belonged. Reasons for withdrawal also not reported. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Was use of a cross over design appropriate? | Low risk | This is a randomised controlled trial and not a cross‐over trial and this domain of risk of bias assessment is not applicable and would be judged as low risk. |
| Is it clear that the order of receiving treatment was randomised? | Low risk | This is a randomised controlled trial and not a cross‐over trial and this domain of risk of bias assessment is not applicable and would be judged as low risk. |
| Can it be assumed that the trial was not biased from carry over effects? | Low risk | This is a randomised controlled trial and not a cross‐over trial and this domain of risk of bias assessment is not applicable and would be judged as low risk. |
| Methods | Cross‐over randomised controlled trial | |
| Participants | 26 patients (16 women) with chronic diarrhoea and FI | |
| Interventions | A: loperamide 4 mg three times a day | |
| Outcomes | Episodes of FI per week (n, mean number, range): A: 26, 0.6 (0 to 6), B: 26, 0.9 (0 to 6), P < 0.01 | |
| Notes | Some data only presented graphically, data calculated by approximate measurement | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding of participants and personnel (performance bias) | Low risk | It is stated that "Neither the patient, physician, or technician was aware of the preparation taken during the week. Finally, the technician who performed the objective tests was, in most instances, unaware of the subjective improvement of the patient during the previous week" |
| Blinding of outcome assessment (detection bias) | Low risk | It is stated that "Neither the patient, physician, or technician was aware of the preparation taken during the week. Finally, the technician who performed the objective tests was, in most instances, unaware of the subjective improvement of the patient during the previous week" |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available however, all the outcomes mentioned in the method section were reported |
| Was use of a cross over design appropriate? | Low risk | Seems appropriate |
| Is it clear that the order of receiving treatment was randomised? | Unclear risk | Not stated |
| Can it be assumed that the trial was not biased from carry over effects? | High risk | No washout period |
| Methods | Randomised controlled trial | |
| Participants | 87 patients admitted to a geriatric unit | |
| Interventions | A: Osmotic laxative (lactulose) 15 mL | |
| Outcomes | Number of days when help required from nurses (n/N total trial days): A: 283/801, B: 322/724, P < 0.05 | |
| Notes | Concealment of allocation possibly inadequate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | 30 participants (14 in the treatment group and 16 in the 'no‐treatment' group) were discharged early and did not complete the full trial period |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Was use of a cross over design appropriate? | Low risk | This is a randomised controlled trial and not a cross‐over trial and this domain of risk of bias assessment is not applicable and would be judged as low risk. |
| Is it clear that the order of receiving treatment was randomised? | Low risk | This is a randomised controlled trial and not a cross‐over trial and this domain of risk of bias assessment is not applicable and would be judged as low risk. |
| Can it be assumed that the trial was not biased from carry over effects? | Low risk | This is a randomised controlled trial and not a cross‐over trial and this domain of risk of bias assessment is not applicable and would be judged as low risk. |
| Methods | Cross‐over randomised controlled trial | |
| Participants | 11 patients (8 women) with chronic diarrhoea and FI | |
| Interventions | A: Loperamide oxide 4 mg twice daily | |
| Outcomes | Number of people cured (no diarrhoea or incontinence in 24 hours): A: 7/11, B: 3/11, P < 0.05 | |
| Notes | Heterogeneous disease groups (9 with irritable bowel syndrome, 1 with post‐gastrectomy diarrhoea and one with post‐cholecystectomy diarrhoea) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Reported as "double‐blind study", however it is not specified who was blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Reported as "double‐blind study", however it is not specified who was blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | High risk | it is reported that "the 24‐h faecal output (0800 h to 0800 h) was collected on days 4, 5, and 6 of each treatment week, and the net wet, consistency and dry weight were recorded", however the results are not reported for days 4 and 5 and only reported for day 6. |
| Was use of a cross over design appropriate? | Low risk | seems appropriate |
| Is it clear that the order of receiving treatment was randomised? | Unclear risk | Not reported |
| Can it be assumed that the trial was not biased from carry over effects? | Low risk | There was a washout period of 1 week |
CI = confidence interval
BMI = Body Mass Index
FI = faecal incontinence
IQR = interquartile range
L = litre
mcg = micrograms
mg = milligrams
mL = millilitres
mm Hg = mm mercury
SEM = Standard Error of the Mean
SD = Standard Deviation
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| RCT but intervention does not include a drug. | |
| RCT but excluded as intervention does not include a drug. | |
| Participants had diarrhoea and constipation, and not faecal incontinence. | |
| Not random allocation of patients to intervention groups. | |
| Participants were provided with lactulose to maintain soft stool and did not have faecal incontinence. | |
| Use of psychopharmacological agents (phenothiazine and amphetamine derivatives) for the treatment of incontinent schizophrenic patients. | |
| Participants did not have faecal incontinence. | |
| RCT but excluded as intervention does not include a drug. | |
| Participants had diarrhoea and not faecal incontinence. | |
| Reports results of an RCT comparing biofeedback and Kegel exercise training. | |
| Reports results of an RCT comparing biofeedback and Kegel exercise training. | |
| Participants in both the arms received loperamide and the trial compared low‐residue diet with fibre supplement. | |
| Participants had diarrhoea and not faecal incontinence. | |
| Participants had bowel evacuation problem and not faecal incontinence | |
| Not random allocation of patients to intervention groups. | |
| Participants had post‐operative constipation and not faecal incontinence. | |
| Participants were randomised into 2 groups (valproate and placebo). However, results for faecal incontinence are reported for the entire cohort. | |
| Participants had diarrhoea and not faecal incontinence. | |
| Participants had diarrhoea and not faecal incontinence. | |
| Excluded as intervention does not include a drug ('The Fiber Study'). |
RCT = Randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||||||||||||||||||||
| 1 Number of people failing to achieve full continence Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.1
Comparison 1 DRUG VERSUS PLACEBO, Outcome 1 Number of people failing to achieve full continence. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.1 Loperamide versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.2 Diphenoxylate + atropine versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.3 Phenylephrine gel versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.4 Sodium valproate versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 Number of people failing to improve incontinence Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.2
Comparison 1 DRUG VERSUS PLACEBO, Outcome 2 Number of people failing to improve incontinence. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.1 Loperamide versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.2 Diphenoxylate + atropine versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.3 Phenylephrine gel versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 Number of faecal incontinence episodes Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.3
Comparison 1 DRUG VERSUS PLACEBO, Outcome 3 Number of faecal incontinence episodes. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.1 Loperamide versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.2 Phenylephrine cream versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 Frequency of defecation (per day) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.4
Comparison 1 DRUG VERSUS PLACEBO, Outcome 4 Frequency of defecation (per day). | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.1 Loperamide versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.2 Diphenoxylate + atropine versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.3 Sodium valproate versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 Faecal incontinence score Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.5
Comparison 1 DRUG VERSUS PLACEBO, Outcome 5 Faecal incontinence score. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.1 Loperamide versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.2 Phenylephrine gel versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.3 Zinc aluminium ointment versus placebo ointment | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 Stool weight (grammes in 24 hours) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.6
Comparison 1 DRUG VERSUS PLACEBO, Outcome 6 Stool weight (grammes in 24 hours). | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6.1 Loperamide versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 6.2 Diphenoxylate + atropine versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 Number of people using pads Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.7
Comparison 1 DRUG VERSUS PLACEBO, Outcome 7 Number of people using pads. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7.1 Loperamide versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 8 Number of people with adverse effects Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.8
Comparison 1 DRUG VERSUS PLACEBO, Outcome 8 Number of people with adverse effects. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.1 Loperamide versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.2 Phenylephrine gel versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.3 Sodium valproate versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.4 Zinc aluminium ointment versus placebo ointment | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 Number of people with perianal skin problems Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.9
Comparison 1 DRUG VERSUS PLACEBO, Outcome 9 Number of people with perianal skin problems. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9.1 Sodium valproate versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 10 Maximum resting anal pressure (mmHg) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.10
Comparison 1 DRUG VERSUS PLACEBO, Outcome 10 Maximum resting anal pressure (mmHg). | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10.1 Loperamide versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 10.2 Diphenoxylate + atropine versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 10.3 Phenylephrine gel versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 10.4 Sodium valproate versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 11 Manometry Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.11
Comparison 1 DRUG VERSUS PLACEBO, Outcome 11 Manometry. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11.1 Phenylephrine gel versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 12 Maximum anal squeeze pressure (mmHg) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.12
Comparison 1 DRUG VERSUS PLACEBO, Outcome 12 Maximum anal squeeze pressure (mmHg). | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12.1 Loperamide versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 12.2 Diphenoxylate + atropine versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 13 Duration of squeeze (seconds) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.13
Comparison 1 DRUG VERSUS PLACEBO, Outcome 13 Duration of squeeze (seconds). | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13.1 Diphenoxylate + atropine versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 14 Sensory threshold (cm water) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.14
Comparison 1 DRUG VERSUS PLACEBO, Outcome 14 Sensory threshold (cm water). | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14.1 Loperamide versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 14.2 Diphenoxylate + atropine versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 15 Saline retention test (mL) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.15
Comparison 1 DRUG VERSUS PLACEBO, Outcome 15 Saline retention test (mL). | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15.1 Loperamide versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 15.2 Diphenoxylate + atropine versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 16 Whole‐gut transit time Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.16
Comparison 1 DRUG VERSUS PLACEBO, Outcome 16 Whole‐gut transit time. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 16.1 Loperamide versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 17 Number of soiled items (bedding and or clothing) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.17
Comparison 1 DRUG VERSUS PLACEBO, Outcome 17 Number of soiled items (bedding and or clothing). | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 18 Help required from nurses Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.18
Comparison 1 DRUG VERSUS PLACEBO, Outcome 18 Help required from nurses. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 18.1 Laxative (lactulose) versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 19 Faecal Incontinence Quality of Life (FIQL) score Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.19
Comparison 1 DRUG VERSUS PLACEBO, Outcome 19 Faecal Incontinence Quality of Life (FIQL) score. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 19.1 Zinc aluminium ointment versus placebo ointment | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 19.2 Phenylephrine gel versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 Loperamide versus codeine versus diphenoxylate + atropine sulfate Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.1
Comparison 2 ONE DRUG VERSUS ANOTHER DRUG OR A COMBINATIONOF DRUGS, Outcome 1 Loperamide versus codeine versus diphenoxylate + atropine sulfate. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.1 Solid stool (%) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.2 Number of people with faecal incontinence | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.3 Stool frequency | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.4 Number of people with urgency | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.5 Adverse effects | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.6 Adverse effects causing withdrawal | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 Osmotic laxative (lactulose) versus osmotic laxative + glycerine suppository + enema Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.2
Comparison 2 ONE DRUG VERSUS ANOTHER DRUG OR A COMBINATIONOF DRUGS, Outcome 2 Osmotic laxative (lactulose) versus osmotic laxative + glycerine suppository + enema. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.1 Number of faecal incontinence episodes in 4 weeks | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.2 Number of soiled items (bedding and or clothing) in 4 weeks | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 Oral versus suppository administration of loperamide Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.3
Comparison 2 ONE DRUG VERSUS ANOTHER DRUG OR A COMBINATIONOF DRUGS, Outcome 3 Oral versus suppository administration of loperamide. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 Different doses of oral loperamide Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 2.4
Comparison 2 ONE DRUG VERSUS ANOTHER DRUG OR A COMBINATIONOF DRUGS, Outcome 4 Different doses of oral loperamide. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.1 Stool frequency | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.2 Stool consistency | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.3 Faecal incontinence | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.4 Dose response for faecal incontinence | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.5 Adverse effects | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

PRISMA study flow diagram.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
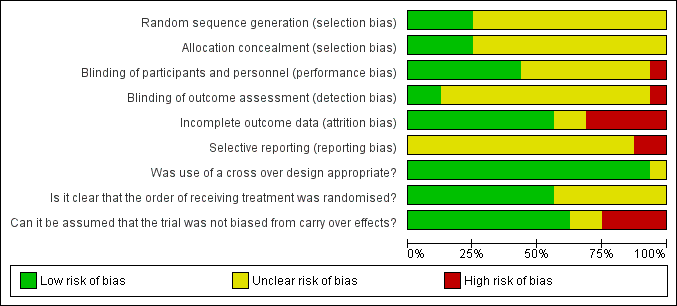
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
| Study | Drug | Placebo | Significance |
| Loperamide versus placebo | |||
| Hallgren 1994 # | 3/28 during the day | 7/28 during the day | |
| Sun 1997 # | 4/11 in 24 hours | 8/11 in 24 hours | P < 0.05 |
| Diphenoxylate + atropine versus placebo | |||
| Harford 1980 # | 0/15 in 24 hours | 3/15 in 24 hours | |
| Phenylephrine gel versus placebo | |||
| Carapeti 2000b # | 8/12 | 12/12 | |
| Sodium valproate versus placebo | |||
| Kusunoki 1990 # | 3/17 | 10/17 | |
Comparison 1 DRUG VERSUS PLACEBO, Outcome 1 Number of people failing to achieve full continence.
| Study | Drug | Placebo |
| Loperamide versus placebo | ||
| Read 1982 # | Episodes of urgency: 7/26 Per cent unformed stool per week (mean, range): 40% (0%‐100%) | Episodes of urgency: 23/26 Per cent unformed stool per week (mean, range): 57% (0%‐100%) (P < 0.001) |
| Sun 1997 # | No improvement in stool consistency: 2/11 Per cent of days with unformed stools: 33% (loperamide oxide) | No improvement in stool consistency: 8/11 Per cent of days with unformed stools: 66% (P < 0.02) |
| Diphenoxylate + atropine versus placebo | ||
| Harford 1980 # | No improvement in stool weight and frequency: 3/15 | No improvement in stool weight and frequency: 3/15 |
| Phenylephrine gel versus placebo | ||
| Carapeti 2000a # | No subjective improvement: 30/36 | 34/36 |
| Carapeti 2000b # | No undefined 'improvement': 6/12 | 11/12 |
Comparison 1 DRUG VERSUS PLACEBO, Outcome 2 Number of people failing to improve incontinence.
| Study | Drug | Placebo |
| Loperamide versus placebo | ||
| Read 1982 # | Mean 0.6 | Mean 0.9 (range 0‐6) |
| Phenylephrine cream versus placebo | ||
| Lumi 2009 | Median 5.4 (range 0‐14) | Median 9 (range 0‐19) |
Comparison 1 DRUG VERSUS PLACEBO, Outcome 3 Number of faecal incontinence episodes.
| Study | Drug | Placebo | Significance |
| Loperamide versus placebo | |||
| Hallgren 1994 # | N 28 mean 4.24 (SD 1.86) | N 28 mean 6.43 (SD 1.99) | |
| Read 1982 # | N 26 mean 1.6 (range 1‐6.3) | N 26 mean 2.4 (range 0‐7.7) | P < 0.001 Wilcoxon's rank sum test for paired data |
| Sun 1997 # | N 11 mean 1.43 (SD 1) | N 11 mean 2 (SD 1) | P < 0.02 |
| Diphenoxylate + atropine versus placebo | |||
| Harford 1980 # | N 15 mean 2.6 (SD 2.71) | N 15 mean 4.9 (SD 3.1) | |
| Sodium valproate versus placebo | |||
| Kusunoki 1990 # | N 17 mean 5.98 (SD 2.97) | N 17 mean 9.65 (SD 3.59) | |
Comparison 1 DRUG VERSUS PLACEBO, Outcome 4 Frequency of defecation (per day).
| Study | Drug | Placebo | Significance |
| Loperamide versus placebo | |||
| Sun 1997 # | visual analogue incontinence | N 11 mean 43 (SD 37) | P = 0.12 |
| Phenylephrine gel versus placebo | |||
| Carapeti 2000a # | N 18 mean 12.5 (SD 3.4) | N 18 mean 12.6 (SD 4.2) | No significant difference |
| Carapeti 2000b # | N 12 mean 12.2 (SD 5.7) | N 12 mean 16.5 (SD 4.4) | |
| Park 2007 | Anal incontinence evaluated with Faecal Incontinence Severity Index (FISI) and reported as mean (SD) n = 17; Baseline: 32.5 (14.5); After: 32.3 (14.7) P = 0.940 | Anal incontinence evaluated with Faecal Incontinence Severity Index (FISI) and reported as mean (SD) n = 12; Baseline: 32.1 (11.2); After: 32.4 (14.4) P = 0.626 | |
| Zinc aluminium ointment versus placebo ointment | |||
| Pinedo 2012 | Wexner Faecal Incontinence Score reported before and after the treatment and reported as mean (SD) n = 24; Before: 16.6 (6‐20); After: 8.5 (0‐11) P = < 0.001 | Wexner Faecal Incontinence Score reported before and after the treatmentand reported as mean (SD) n = 20; Before: 16.7 (5‐18); After: 13.1 (5‐17) P = 0.02 | There was a significant difference in the final scores favouring the treatment group (P = 0.001) |
Comparison 1 DRUG VERSUS PLACEBO, Outcome 5 Faecal incontinence score.
| Study | Drug | Placebo | Significance |
| Loperamide versus placebo | |||
| Read 1982 # | N 26 mean 102 (range 0‐467) | N 26 mean 186 (range 0‐466) | P < 0.001 Wilcoxon's rank sum test for paired data |
| Sun 1997 # | N 11 mean 282 (SD 212) | N 11 mean 423 (SD 163) | P = 0.11 |
| Diphenoxylate + atropine versus placebo | |||
| Harford 1980 # | N 15 mean 256 (SD 333) | N 15 mean 460 (SD 581) | |
Comparison 1 DRUG VERSUS PLACEBO, Outcome 6 Stool weight (grammes in 24 hours).
| Study | Drug | Placebo |
| Loperamide versus placebo | ||
| Hallgren 1994 # | During the day: 1/28 | During the day: |
Comparison 1 DRUG VERSUS PLACEBO, Outcome 7 Number of people using pads.
| Study | Drug | Placebo |
| Loperamide versus placebo | ||
| Read 1982 # | 18/26 (constipation 11, diarrhoea 4, nausea and vomiting 3, abdominal pain 2) | 1/26 (abdominal pain) |
| Sun 1997 # | 6/11 (headache, nausea, dizzyness, abdominal pain, constipation) | 3/11 |
| Phenylephrine gel versus placebo | ||
| Carapeti 2000a # | 3/36 (mild dermatitis after phenylephrine gel application, which settled when drug stopped) | 0/36 |
| Carapeti 2000b # | 0/12 | 0/12 |
| Cheetham 2001 # | 2/10 (burning sensation after phenylephrine gel application, which settled within minutes) | 0/10 |
| Park 2007 | Dermatitis reaction: 5/17; B = 1/12 | Dermatitis reaction: 1/12 |
| Sodium valproate versus placebo | ||
| Kusunoki 1990 # | 8/17 (abdominal pain and nausea) | 0/17 |
| Zinc aluminium ointment versus placebo ointment | ||
| Pinedo 2012 | 0/24 | 0/20 |
Comparison 1 DRUG VERSUS PLACEBO, Outcome 8 Number of people with adverse effects.
| Study | Drug | Placebo |
| Sodium valproate versus placebo | ||
| Kusunoki 1990 # | 3/17 | 9/17 |
Comparison 1 DRUG VERSUS PLACEBO, Outcome 9 Number of people with perianal skin problems.
| Study | Drug | Placebo |
| Loperamide versus placebo | ||
| Hallgren 1994 # | N 28 mean 62 (SD 16) | versus |
| Sun 1997 # | N 11 mean 76 (SD 40) | N 11 mean 69 (SD 35) |
| Diphenoxylate + atropine versus placebo | ||
| Harford 1980 # | N 15 mean 41 (SD 23) | N 15 mean 39 (SD 19) |
| Phenylephrine gel versus placebo | ||
| Carapeti 2000a # | N 18 mean 65 (SD 21) | N 18 mean 54 (SD 21) |
| Carapeti 2000b # | N 12 mean 89 (SD 17) | N 12 mean 75 (SD 14) |
| Cheetham 2001 # | Statistically significant differences between phenylephrine gel (in concentrations of 30% and 40% only) | compared with placebo (P < 0.05) |
| Sodium valproate versus placebo | ||
| Kusunoki 1990 # | N 17 mean 63.6 (SD 12.4) | N 17 mean 42.5 (SD 8.9) |
Comparison 1 DRUG VERSUS PLACEBO, Outcome 10 Maximum resting anal pressure (mmHg).
| Study | Domain | Drug (n = 17); reported as mean (SD) | Placebo (n = 12); reported as mean (SD) |
| Phenylephrine gel versus placebo | |||
| Park 2007 | Resting pressure (mmHg) | Baseline: 30.0 (12.3); After: 27.3 (12.7) P = 0.362 | Baseline: 32.6 (14.2); After: 27.2 (15.0) P = 0.306 |
| Park 2007 | Squeezing pressure (mmHg) | Baseline: 143.3 (60.5); After: 160.4 (76.9) P = 0.083 | Baseline: 152.6 (86.5); After: 147.1 (76.5) P = 0.625 |
| Park 2007 | Sustained duration (s) | Baseline: 41.9 (24.5); After: 44.9 (48.3) P = 0.848 | Baseline: 39.6 (24.4); After: 32.8 (14.4) P = 0.187 |
| Park 2007 | Sphincter length (cm) | Baseline: 3.2 (0.9); After: 3.4 (0.8) P = 0.368 | Baseline: 3.5 (0.8); After: 3.4 (0.8) P = 0.743 |
| Park 2007 | High pressure zone (cm) | Baseline: 2.4 (2.1); After: 1.9 (0.5) P = 0.378 | Baseline: 2.1 (0.9); After: 2.3 (0.9) P = 0.556 |
Comparison 1 DRUG VERSUS PLACEBO, Outcome 11 Manometry.
| Study | Drug | Placebo |
| Loperamide versus placebo | ||
| Hallgren 1994 # | N 28 mean 223 (SD 82) | N 28 mean 219 (SD 93) |
| Sun 1997 # | N 11 mean 163 (SD 86) | N 11 mean 155 (SD 85) |
| Diphenoxylate + atropine versus placebo | ||
| Harford 1980 # | N 15 mean 94 (SD 68) | N 15 mean 96 (SD 68) |
Comparison 1 DRUG VERSUS PLACEBO, Outcome 12 Maximum anal squeeze pressure (mmHg).
| Study | Drug | Placebo |
| Diphenoxylate + atropine versus placebo | ||
| Harford 1980 # | N 15 mean 87 (SD 127) | N 15 mean 86 (SD 127) |
Comparison 1 DRUG VERSUS PLACEBO, Outcome 13 Duration of squeeze (seconds).
| Study | Drug | Placebo |
| Loperamide versus placebo | ||
| Hallgren 1994 # | N 28 mean 29.6 (SD 11.7) | N 28 mean 26.5 (SD 14.9) |
| Diphenoxylate + atropine versus placebo | ||
| Harford 1980 # | N 15 mean 12 (SD 12) | N 15 mean 31 (SD 62) |
Comparison 1 DRUG VERSUS PLACEBO, Outcome 14 Sensory threshold (cm water).
| Study | Drug | Placebo | Significance |
| Loperamide versus placebo | |||
| Sun 1997 # | N 11 mean 223 (SD 274) | versus | P = 0.07 |
| Diphenoxylate + atropine versus placebo | |||
| Harford 1980 # | N 15 mean 492 (SD 461) | N 15 mean 486 (SD 364) | |
Comparison 1 DRUG VERSUS PLACEBO, Outcome 15 Saline retention test (mL).
| Study | Drug | Placebo | Significance |
| Loperamide versus placebo | |||
| Sun 1997 # | N 11 mean 61 hours (SD 13) | N 11 mean 39 hours (SD 15) | Significantly prolonged (P < 0.001) in patients taking loperamide oxide |
Comparison 1 DRUG VERSUS PLACEBO, Outcome 16 Whole‐gut transit time.
| Study | Drug | Placebo | Significance |
| Ryan 1974 | 154 items during trial period | 332 items | P < 0.01 |
Comparison 1 DRUG VERSUS PLACEBO, Outcome 17 Number of soiled items (bedding and or clothing).
| Study | Drug | Placebo | Significance |
| Laxative (lactulose) versus placebo | |||
| Ryan 1974 | 283 days of help | 322 days of help | P < 0.05 during trial period |
Comparison 1 DRUG VERSUS PLACEBO, Outcome 18 Help required from nurses.
| Study | Domains | Drug (n = 17); reported as mean (SD) | Placebo (n = 12); reported as mean (SD) |
| Zinc aluminium ointment versus placebo ointment | |||
| Pinedo 2012 | Lifestyle | Baseline: 2.49 (1.06); After: 3.58 (1.18) P = < 0.001 | Baseline: 2.50 (1.01); After: 2.55 (1.03) P = 0.151 |
| Pinedo 2012 | Conduct | Baseline: 2.19 (1.02); After: 3.12 (1.16) P = < 0.001 | Baseline: 2.17 (0.91); After: 2.37 (1.13) P = 0.104 |
| Pinedo 2012 | Embarrassment | Baseline: 1.54 (0.82); After: 2.5 (1.32) P = < 0.001 | Baseline: 1.56 (0.74); After: 1.76 (0.84) P = 0.043 |
| Pinedo 2012 | Depression | Baseline: 2.51 (1.01); After: 3.48 (1.17) P = 0.001 | Baseline: 2.46 (1.02); After: 2.71 (1.13) P = 0.093 |
| Phenylephrine gel versus placebo | |||
| Park 2007 | Lifestyle | Baseline: 2.9 (0.8); After: 2.9 (1.0) P = 0.801 | Baseline: 2.7 (0.5); After: 3.0 (0.8) P = 0.269 |
| Park 2007 | Coping | Baseline: 2.5 (0.9); After: 2.8 (0.9) P= 0.110 | Baseline: 2.5 (0.5); After: 2.8 (0.5) P = 0.119 |
| Park 2007 | Depression | Baseline: 3.2 (0.7); After: 3.2 (0.8) P = 0.415 | Baseline: 3.1 (0.5); After: 3.2 (0.5) P = 0.554 |
| Park 2007 | Embarrassment | Baseline: 2.7 (0.7); After: 3.0 (0.7) P = 0.090 | Baseline: 2.7 (0.6); After: 2.6 (0.8) P = 0.855 |
Comparison 1 DRUG VERSUS PLACEBO, Outcome 19 Faecal Incontinence Quality of Life (FIQL) score.
| Study | Loperamide | Codeine | Diphenox. + atropine | Significance |
| Solid stool (%) | ||||
| Palmer 1980 # | 67.8% (SD 34) | 58.4% (SD 25.9) | 36.3% (SD 33.3) | Diphenoxylate was associated with a signficantly smaller percentage of solid stools than either loperamide or codeine (P < 0.01) |
| Number of people with faecal incontinence | ||||
| Palmer 1980 # | 2/25 | 3/25 | 6/25 | |
| Stool frequency | ||||
| Palmer 1980 # | N 15 mean 1.8 (SD 0.3) | N 15 mean 1.9 (SD 0.3) | N 15 mean 1.9 (SD 0.3) | |
| Number of people with urgency | ||||
| Palmer 1980 # | 3/16 | 4/17 | 9/17 | Diphenoxylate was significantly worse than loperamide or codenine, P < 0.05 |
| Adverse effects | ||||
| Palmer 1980 # | 22 in 10/25 patients | 29 in 12/25 patients | 39 in 12/25 patients | Significantly more adverse effects with diphenoxylate than loperamide, P < 0.05 |
| Adverse effects causing withdrawal | ||||
| Palmer 1980 # | 4/25 | 4/25 | 5/25 | |
Comparison 2 ONE DRUG VERSUS ANOTHER DRUG OR A COMBINATIONOF DRUGS, Outcome 1 Loperamide versus codeine versus diphenoxylate + atropine sulfate.
| Study | Lactulose | Lactul + supp + enema | Significance |
| Number of faecal incontinence episodes in 4 weeks | |||
| Chassagne 2000 | N 61 mean 24 (SD 11.5 ) | N 62 mean 24 (SD 10.8) | P = 0.9 |
| Number of soiled items (bedding and or clothing) in 4 weeks | |||
| Chassagne 2000 | N 61 mean 80 (SD 16.1) | N 62 mean 78 (SD 20.7) | P = 0.55 |
Comparison 2 ONE DRUG VERSUS ANOTHER DRUG OR A COMBINATIONOF DRUGS, Outcome 2 Osmotic laxative (lactulose) versus osmotic laxative + glycerine suppository + enema.
| Study | Data | Significance |
| Cohen 2001 # | Oral administration resulted in decreased stool frequency compared with suppository administration | P < 0.02 |
Comparison 2 ONE DRUG VERSUS ANOTHER DRUG OR A COMBINATIONOF DRUGS, Outcome 3 Oral versus suppository administration of loperamide.
| Study | Outcome information | Significance |
| Stool frequency | ||
| Fox 2005 # | No effect | |
| Stool consistency | ||
| Fox 2005 # | Trend for increased stool consistency from median (IQR) score 0.7 (0.5 to 0.9) with placebo to 0.4 (0.3 to 0.6) with 6 mg dose (0 = all hard, 1 = all liquid) | |
| Faecal incontinence | ||
| Fox 2005 # | Less faecal spotting and incontinence with loperamide vs placebo | P < 0.05 |
| Dose response for faecal incontinence | ||
| Fox 2005 # | Significant positive dose‐response relationship with increasing dose of loperamide (reduced FI with 2 mg dose, and almost no FI with 4 mg and 6 mg doses) | |
| Adverse effects | ||
| Fox 2005 # | Adverse effects: none (specifically no severe constipation with the highest doses) | |
Comparison 2 ONE DRUG VERSUS ANOTHER DRUG OR A COMBINATIONOF DRUGS, Outcome 4 Different doses of oral loperamide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of people failing to achieve full continence Show forest plot | Other data | No numeric data | ||
| 1.1 Loperamide versus placebo | Other data | No numeric data | ||
| 1.2 Diphenoxylate + atropine versus placebo | Other data | No numeric data | ||
| 1.3 Phenylephrine gel versus placebo | Other data | No numeric data | ||
| 1.4 Sodium valproate versus placebo | Other data | No numeric data | ||
| 2 Number of people failing to improve incontinence Show forest plot | Other data | No numeric data | ||
| 2.1 Loperamide versus placebo | Other data | No numeric data | ||
| 2.2 Diphenoxylate + atropine versus placebo | Other data | No numeric data | ||
| 2.3 Phenylephrine gel versus placebo | Other data | No numeric data | ||
| 3 Number of faecal incontinence episodes Show forest plot | Other data | No numeric data | ||
| 3.1 Loperamide versus placebo | Other data | No numeric data | ||
| 3.2 Phenylephrine cream versus placebo | Other data | No numeric data | ||
| 4 Frequency of defecation (per day) Show forest plot | Other data | No numeric data | ||
| 4.1 Loperamide versus placebo | Other data | No numeric data | ||
| 4.2 Diphenoxylate + atropine versus placebo | Other data | No numeric data | ||
| 4.3 Sodium valproate versus placebo | Other data | No numeric data | ||
| 5 Faecal incontinence score Show forest plot | Other data | No numeric data | ||
| 5.1 Loperamide versus placebo | Other data | No numeric data | ||
| 5.2 Phenylephrine gel versus placebo | Other data | No numeric data | ||
| 5.3 Zinc aluminium ointment versus placebo ointment | Other data | No numeric data | ||
| 6 Stool weight (grammes in 24 hours) Show forest plot | Other data | No numeric data | ||
| 6.1 Loperamide versus placebo | Other data | No numeric data | ||
| 6.2 Diphenoxylate + atropine versus placebo | Other data | No numeric data | ||
| 7 Number of people using pads Show forest plot | Other data | No numeric data | ||
| 7.1 Loperamide versus placebo | Other data | No numeric data | ||
| 8 Number of people with adverse effects Show forest plot | Other data | No numeric data | ||
| 8.1 Loperamide versus placebo | Other data | No numeric data | ||
| 8.2 Phenylephrine gel versus placebo | Other data | No numeric data | ||
| 8.3 Sodium valproate versus placebo | Other data | No numeric data | ||
| 8.4 Zinc aluminium ointment versus placebo ointment | Other data | No numeric data | ||
| 9 Number of people with perianal skin problems Show forest plot | Other data | No numeric data | ||
| 9.1 Sodium valproate versus placebo | Other data | No numeric data | ||
| 10 Maximum resting anal pressure (mmHg) Show forest plot | Other data | No numeric data | ||
| 10.1 Loperamide versus placebo | Other data | No numeric data | ||
| 10.2 Diphenoxylate + atropine versus placebo | Other data | No numeric data | ||
| 10.3 Phenylephrine gel versus placebo | Other data | No numeric data | ||
| 10.4 Sodium valproate versus placebo | Other data | No numeric data | ||
| 11 Manometry Show forest plot | Other data | No numeric data | ||
| 11.1 Phenylephrine gel versus placebo | Other data | No numeric data | ||
| 12 Maximum anal squeeze pressure (mmHg) Show forest plot | Other data | No numeric data | ||
| 12.1 Loperamide versus placebo | Other data | No numeric data | ||
| 12.2 Diphenoxylate + atropine versus placebo | Other data | No numeric data | ||
| 13 Duration of squeeze (seconds) Show forest plot | Other data | No numeric data | ||
| 13.1 Diphenoxylate + atropine versus placebo | Other data | No numeric data | ||
| 14 Sensory threshold (cm water) Show forest plot | Other data | No numeric data | ||
| 14.1 Loperamide versus placebo | Other data | No numeric data | ||
| 14.2 Diphenoxylate + atropine versus placebo | Other data | No numeric data | ||
| 15 Saline retention test (mL) Show forest plot | Other data | No numeric data | ||
| 15.1 Loperamide versus placebo | Other data | No numeric data | ||
| 15.2 Diphenoxylate + atropine versus placebo | Other data | No numeric data | ||
| 16 Whole‐gut transit time Show forest plot | Other data | No numeric data | ||
| 16.1 Loperamide versus placebo | Other data | No numeric data | ||
| 17 Number of soiled items (bedding and or clothing) Show forest plot | Other data | No numeric data | ||
| 18 Help required from nurses Show forest plot | Other data | No numeric data | ||
| 18.1 Laxative (lactulose) versus placebo | Other data | No numeric data | ||
| 19 Faecal Incontinence Quality of Life (FIQL) score Show forest plot | Other data | No numeric data | ||
| 19.1 Zinc aluminium ointment versus placebo ointment | Other data | No numeric data | ||
| 19.2 Phenylephrine gel versus placebo | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Loperamide versus codeine versus diphenoxylate + atropine sulfate Show forest plot | Other data | No numeric data | ||
| 1.1 Solid stool (%) | Other data | No numeric data | ||
| 1.2 Number of people with faecal incontinence | Other data | No numeric data | ||
| 1.3 Stool frequency | Other data | No numeric data | ||
| 1.4 Number of people with urgency | Other data | No numeric data | ||
| 1.5 Adverse effects | Other data | No numeric data | ||
| 1.6 Adverse effects causing withdrawal | Other data | No numeric data | ||
| 2 Osmotic laxative (lactulose) versus osmotic laxative + glycerine suppository + enema Show forest plot | Other data | No numeric data | ||
| 2.1 Number of faecal incontinence episodes in 4 weeks | Other data | No numeric data | ||
| 2.2 Number of soiled items (bedding and or clothing) in 4 weeks | Other data | No numeric data | ||
| 3 Oral versus suppository administration of loperamide Show forest plot | Other data | No numeric data | ||
| 4 Different doses of oral loperamide Show forest plot | Other data | No numeric data | ||
| 4.1 Stool frequency | Other data | No numeric data | ||
| 4.2 Stool consistency | Other data | No numeric data | ||
| 4.3 Faecal incontinence | Other data | No numeric data | ||
| 4.4 Dose response for faecal incontinence | Other data | No numeric data | ||
| 4.5 Adverse effects | Other data | No numeric data | ||

