Kortikosteroid sedutan berbanding sistemik untuk rawatan displasia bronkopulmonary pada bayi pramatang yang kurang berat badan yang menerima ventilasi
Appendices
Appendix 1. Previous search methodology
For previous versions of the review, randomised controlled trials comparing inhaled versus systemic corticosteroid therapy in preterm infants were identified from MEDLINE (1966 to 2011) using MeSH headings: infant‐newborn, chronic lung disease, bronchopulmonary dysplasia, anti‐inflammatory agents, steroids; dexamethasone, administration, inhalation; aerosols, budesonide, beclomethasone dipropionate, flunisolide and fluticasone propionate.
Other databases were searched including: Cochrane Central Register of Controlled Trials (The Cochrane Library, Issue 6, 2011), EMBASE (1980 to 2011), CINAHL (1982 to 2011), reference lists of published trials and abstracts published in Pediatric Research or electronically on the Pediatric Academic Societies web site (1990 to 2011). No language restrictions were applied.
For the 2012 update, we searched Clinicaltrials.gov, Controlled‐trials.com and Web of Science, which were not searched for previous reviews.
We used the following search strategies for the 2012 updated searches:
PubMed
((bronchopulmonary dysplasia OR lung diseases OR chronic lung disease) AND (anti‐inflammatory agents OR steroids OR dexamethasone OR inhalation OR aerosols OR budesonide OR beclomethasone dipropionate OR flunisolide OR fluticasone propionate)) AND ((infant, newborn[MeSH] OR newborn OR neon* OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW) AND (randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh])) AND (("2007"[PDat] : "3000"[PDat]))
CINAHL
(bronchopulmonary dysplasia OR lung diseases OR chronic lung disease) AND (anti‐inflammatory agents OR steroids OR dexamethasone OR inhalation OR aerosols OR budesonide OR beclomethasone dipropionate OR flunisolide OR fluticasone propionate) ) and ( ( infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW) AND ( randomised controlled trial OR controlled clinical trial OR randomised OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) 2007 ‐ Present
Cochrane Central Register of Controlled Trials
(bronchopulmonary dysplasia OR lung diseases OR chronic lung disease) AND (anti‐inflammatory agents OR steroids OR dexamethasone OR inhalation OR aerosols OR budesonide OR beclomethasone dipropionate OR flunisolide OR fluticasone propionate) and (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW), from 2007 to 2011
EMBASE
1 ((bronchopulmonary dysplasia or lung diseases or chronic lung disease) and (anti‐inflammatory agents or steroids or dexamethasone or inhalation or aerosols or budesonide or beclomethasone dipropionate or flunisolide or fluticasone propionate)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (1849)
2 (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (603948)
3 (human not animal).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (11849457)
4 (randomised controlled trial or controlled clinical trial or randomised or placebo or clinical trials as topic or randomly or trial or clinical trial).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (1256505)
5 1 and 2 and 3 and 4 (336)
6 limit 5 to yr="2007 ‐Current" (76)
Clinicaltrials.gov
(infant OR newborn) AND (bronchopulmonary dysplasia OR lung disease) AND (anti‐inflammatory agents OR steroids OR dexamethasone OR inhalation OR aerosols OR budesonide OR beclomethasone dipropionate OR flunisolide OR fluticasone propionate)
Controlled‐trials.com
(infant OR newborn) AND (bronchopulmonary dysplasia OR lung disease) AND (anti‐inflammatory agents OR steroids OR dexamethasone OR inhalation OR aerosols OR budesonide OR beclomethasone dipropionate OR flunisolide OR fluticasone propionate)
Appendix 2. Standard search methodology for 2017 update
PubMed
((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]))
EMBASE
(infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomised controlled trial or controlled clinical trial or randomised or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL
(infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomised controlled trial OR controlled clinical trial OR randomised OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library
(infant or newborn or neonate or neonatal or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
Appendix 3. Risk of bias tool
We used the standard methods of Cochrane and Cochrane Neonatal to assess the methodological quality (to meet the validity criteria) of the trials. For each trial, we sought information regarding the method of randomisation, and the blinding and reporting of all outcomes of all the infants enrolled in the trial. We assessed each criterion as low, high, or unclear risk. Two review authors separately assessed each study. We resolved any disagreement by discussion. We added this information to the table Characteristics of included studies. We evaluated the following issues and entered the findings into the risk of bias table:
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorized the method used to generate the allocation sequence as:
a. Low risk (any truly random process e.g. random number table; computer random number generator);
b. High risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number);
c. Unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorized the method used to conceal the allocation sequence as:
a. Low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
b. High risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
c. Unclear risk
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorized the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorized the methods as:
a. Low risk, high risk or unclear risk for participants;
b. Low risk, high risk or unclear risk for personnel;
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorized the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorized the methods as:
a. Low risk for outcome assessors.
b. High risk for outcome assessors.
c. Unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorized the methods as:
a. Low risk (< 20% missing data);
b. High risk (≥ 20% missing data);
c. Unclear risk.
6. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
a. Low risk (where it is clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
b. High risk (where not all the study's pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
c. Unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
a. Low risk;
b. High risk;
c. Unclear risk
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.

Study flow diagram: review update

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
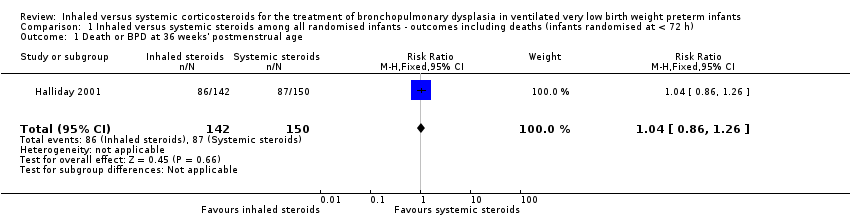
Comparison 1 Inhaled versus systemic steroids among all randomised infants ‐ outcomes including deaths (infants randomised at < 72 h), Outcome 1 Death or BPD at 36 weeks' postmenstrual age.

Comparison 1 Inhaled versus systemic steroids among all randomised infants ‐ outcomes including deaths (infants randomised at < 72 h), Outcome 2 Death or BPD at 28 days of age.

Comparison 1 Inhaled versus systemic steroids among all randomised infants ‐ outcomes including deaths (infants randomised at < 72 h), Outcome 3 Death at 36 weeks' postmenstrual age.

Comparison 1 Inhaled versus systemic steroids among all randomised infants ‐ outcomes including deaths (infants randomised at < 72 h), Outcome 4 Death at 28 days of age.

Comparison 2 Inhaled versus systemic steroids among infants ‐ outcomes including deaths (Infants randomised between 12 and 21 days of age), Outcome 1 Death or BPD at 36 weeks' postmenstrual age.
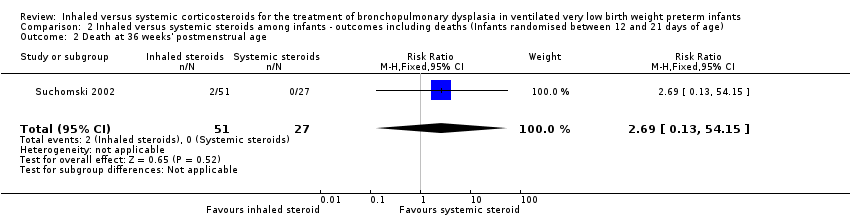
Comparison 2 Inhaled versus systemic steroids among infants ‐ outcomes including deaths (Infants randomised between 12 and 21 days of age), Outcome 2 Death at 36 weeks' postmenstrual age.

Comparison 2 Inhaled versus systemic steroids among infants ‐ outcomes including deaths (Infants randomised between 12 and 21 days of age), Outcome 3 Death at 28 days of age.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 1 BPD at 36 weeks' postmenstrual age.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 2 BPD at 28 days of age.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 3 Need for ventilation among survivors at 36 weeks' postmenstrual age.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 4 Duration of mechanical ventilation among survivors (days).

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 5 Duration of supplemental oxygen among survivors (days).

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 6 Length of hospital stay among survivors (days).

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 7 Intraventricular haemorrhage grade III‐IV.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 8 Periventricular leukomalacia.
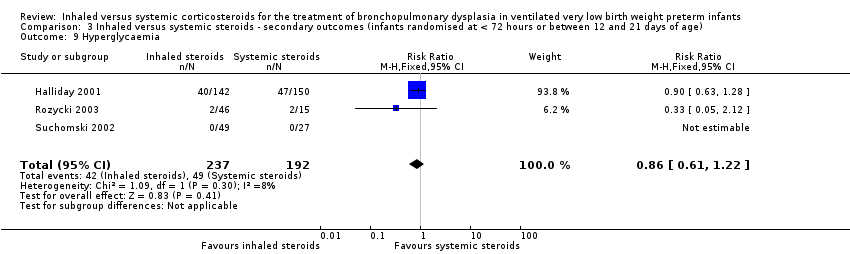
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 9 Hyperglycaemia.
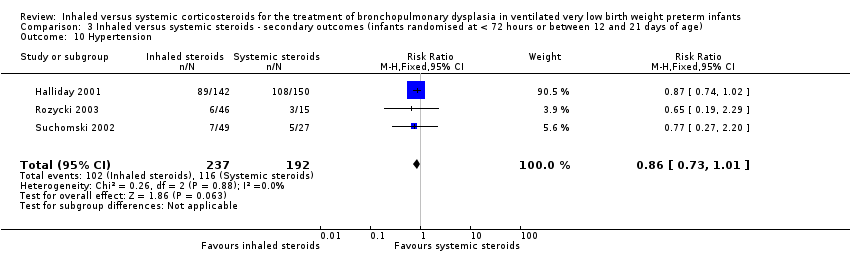
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 10 Hypertension.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 11 Necrotising enterocolitis.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 12 Gastrointestional bleed.
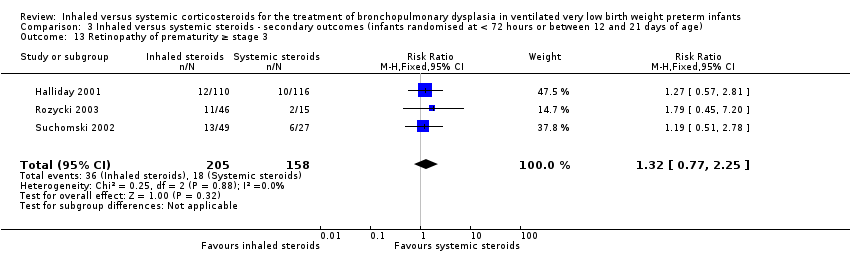
Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 13 Retinopathy of prematurity ≥ stage 3.

Comparison 3 Inhaled versus systemic steroids ‐ secondary outcomes (infants randomised at < 72 hours or between 12 and 21 days of age), Outcome 14 Culture‐proven sepsis.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 1 General conceptual ability (GCA) score at 7 years.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 2 Child behaviour check list (CBLC) at 7 years.
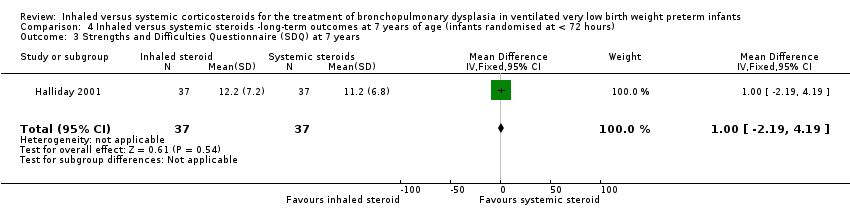
Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 3 Strengths and Difficulties Questionnaire (SDQ) at 7 years.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 4 Cerebral palsy at 7 years.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 5 Moderate/severe disability at 7 years.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 6 Death or moderate/severe disability at 7 years.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 7 Systolic blood pressure of > 95th percentile at 7 years.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 8 Diastolic blood pressure of > 95th percentile at 7 years.

Comparison 4 Inhaled versus systemic steroids ‐long‐term outcomes at 7 years of age (infants randomised at < 72 hours), Outcome 9 Ever diagnosed as asthmatic by 7 years.
| Inhaled steroids compared with systemic steroids for BPD (infants randomised at < 72 hours of age) | ||||||
| Patient or population: Neonates with developing BPD Settings: Neonatal intensive care unit Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| Death or BPD at 36 weeks' postmenstrual age | High risk population | RR 1.04 (95% CI 0.86 to 1.26) | 292 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this single study was high. The study was not blinded at all sites. Only 35/150 infants randomised to systemic steroids received full course while 33/142 infants randomised to inhaled steroids received full course. Results were presented in intention to treat analyses including deaths occurring after 72 hours of age. We downgraded the quality of the evidence by one step. Precision: Precison for the point estimate was acceptable Presence of publication bias: N/A. | |
| 580 per 1000 | 606 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Inhaled steroids compared with systemic steroids for BPD (infants randomised between 12 and 21 days of age) | ||||||
| Patient or population: Neonates with developing BPD Settings: Neonatal intensive care unit Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| Death or BPD at 36 weeks' postmenstrual age | High risk population | RR 0.94 (95% CI 0.83 to 1.05) | 78 (1) | ⊕⊕⊝⊝ | Bias: The risk of bias for this single study was high. There was no blinding of the intervention or outcome measurements. We downgraded the quality of the evidence by one level. Precision: The precision for the point estimate was low as the sample size was small Presence of publication bias: N/A. | |
| 963 per 1000 | 902 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Inhaled steroids compared with systemic steroids for BPD (infants randomised at < 72 hours or between 12 and 21 days of age) | ||||||
| Patient or population: Neonates with developing BPD Settings: Neonatal intensive care unit Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| BPD at 36 weeks' postmenstrual age | High risk population | RR 1.08 (95% CI 0.88 to 1.32) | 429 (3) | ⊕⊕⊝⊝ | Bias: The risk of bias for these three studies was high. There was blinding of randomisation in all three studies. There was no blinding of the intervention or outcome measurements at all sites in the largest study (Halliday 2001). In Rozycki 2003 there was blinding of the intervention but blinding of outcome assessment was unclear. In Suchomski 2002 there was no blinding of the intervention or outcomes measurements. We downgraded the quality of the evidence by two levels. Precision: The precision for the point estimate was high as the sample size was quite large. Presence of publication bias: N/A. We did not create a funnel plot as there were only three trials included in the analysis. | |
| 422 per 1000 | 485 per 1000 | |||||
| Hyperglycaemia | High risk population | RR 0.86 (95% CI 0.61 to 1.22) | 429 (3) | ⊕⊕⊝⊝ | Bias: The risk of bias for these three studies was high. There was blinding of randomisation in all three studies. There was no blinding of the intervention or outcome measurements at all sites in the largest study (Halliday 2001). In Rozycki 2003 there was blinding of the intervention but blinding of outcome assessments was unclear. In Suchomski 2002 there was no blinding of the intervention or outcome measurements. We downgraded the quality of the evidence by two levels. Heterogeneity/consistency: There was no heterogeneity (I² = 8%). Precision: The precision for the point estimate was high as the sample size was quite large. Presence of publication bias: N/A. We did not create a funnel plot as there were only three trials included in the analysis. | |
| 255 per 1000 | 177 per 1000 | |||||
| Hypertension | High risk population | RR (RR 0.86, 95% CI 0.73 to 1.01) | 429 (3) | ⊕⊕⊝⊝ | Bias: The risk of bias for these three studies was high. There was blinding of randomisation in all three studies. There was no blinding of the intervention or outcome measurements at all sites in the largest study (Halliday 2001). In Rozycki 2003 there was blinding of the intervention but blinding of outcome assessments was unclear. In Suchomski 2002 there was no blinding of the intervention or outcome measurements. We downgraded the quality of the evidence by two steps. Heterogeneity/consistency: There was no heterogeneity (I² = 0%). Precision: The precision for the point estimate was high as the sample size was quite large. Presence of publication bias: N/A. We did not create a funnel plot as there were only three trials included in the analysis. | |
| 604 per 1000 | 430 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Inhaled steroids compared with systemic steroids for BPD ‐ long‐term outcomes at 7 years of age (infants randomised at < 72 hours of age) | ||||||
| Patient or population: Neonates with developing BPD Settings: NICU Intervention: Inhaled steroids Comparison: Systemic steroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic steroids | Inhaled steroids | |||||
| General conceptual ability (GCA) score at 7 years The test has a standardisation mean of 100 and SD of 15 | The mean GCA score in the control group was 90.2 | The mean GCA score in the intervention groups was 3.4 units lower | MD ‐3.40 (95% CI ‐12.38 to 5.58) | 74 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the Quality of the evidence by one step. Presence of publication bias: N/A. |
| Moderate/severe disability at 7 years | 135 per 1000 | 189 per 1000 | RR 1.40 (95% CI 0.49 to 4.01) | 74 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the Quality of the evidence by one step. Presence of publication bias: N/A. |
| Death or moderate/severe disability at 7 years | 418 per 1000 | 423 per 1000 | RR 1.01 (95% CI 0.65 to 1.58) | 107 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the Quality of the evidence by one step. Presence of publication bias: N/A. |
| Systolic blood pressure > 95th percentile at 7 years | 353 per 1000 | 194 per 1000 | RR 0.55 (95% CI 0.25 to 1.23) | 70 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the quality of the evidence by one step. Presence of publication bias: N/A. |
| Diastolic blood pressure > 95th percentile at 7 years | 121 per 1000 | 167 per 1000 | RR (1.38, 95% CI 0.43 to 4.45) | 69 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the quality of the evidence by one step. Presence of publication bias: N/A. |
| Ever diagnosed as asthmatic by 7 years | 528 per 1000 | 459 per 1000 | RR 0.87 (95% CI 0.55 to 1.39) | 73 (1) | ⊕⊕⊕⊝ | Bias: The risk of bias for this outcome was low. This outcome was reported in a subset of infants, who had been enrolled in the trial in Ireland and the UK. The assessors of all the long‐term outcomes were blinded to the original treatment group allocation. Heterogeneity/consistency: Heterogeneity was N/A as there was only one study included in the analysis. Precision: Precison for the point estimate was low because of the small sample size. We downgraded the quality of the evidence by one step. Presence of publication bias: N/A. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or BPD at 36 weeks' postmenstrual age Show forest plot | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.86, 1.26] |
| 2 Death or BPD at 28 days of age Show forest plot | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.90, 1.12] |
| 3 Death at 36 weeks' postmenstrual age Show forest plot | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.62, 1.49] |
| 4 Death at 28 days of age Show forest plot | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.52, 1.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or BPD at 36 weeks' postmenstrual age Show forest plot | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.83, 1.05] |
| 2 Death at 36 weeks' postmenstrual age Show forest plot | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.13, 54.15] |
| 3 Death at 28 days of age Show forest plot | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.13, 54.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 BPD at 36 weeks' postmenstrual age Show forest plot | 3 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.88, 1.32] |
| 2 BPD at 28 days of age Show forest plot | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.18] |
| 3 Need for ventilation among survivors at 36 weeks' postmenstrual age Show forest plot | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.30, 4.06] |
| 4 Duration of mechanical ventilation among survivors (days) Show forest plot | 2 | 368 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐5.22, 4.63] |
| 5 Duration of supplemental oxygen among survivors (days) Show forest plot | 2 | 368 | Mean Difference (IV, Fixed, 95% CI) | ‐4.91 [‐20.87, 11.06] |
| 6 Length of hospital stay among survivors (days) Show forest plot | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐13.0 [‐33.22, 7.22] |
| 7 Intraventricular haemorrhage grade III‐IV Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.33, 2.40] |
| 8 Periventricular leukomalacia Show forest plot | 2 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.34, 2.13] |
| 9 Hyperglycaemia Show forest plot | 3 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.61, 1.22] |
| 10 Hypertension Show forest plot | 3 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.73, 1.01] |
| 11 Necrotising enterocolitis Show forest plot | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.50, 1.85] |
| 12 Gastrointestional bleed Show forest plot | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.41, 1.93] |
| 13 Retinopathy of prematurity ≥ stage 3 Show forest plot | 3 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.77, 2.25] |
| 14 Culture‐proven sepsis Show forest plot | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.79, 1.45] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 General conceptual ability (GCA) score at 7 years Show forest plot | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐12.38, 5.58] |
| 2 Child behaviour check list (CBLC) at 7 years Show forest plot | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐4.75, 5.15] |
| 3 Strengths and Difficulties Questionnaire (SDQ) at 7 years Show forest plot | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐2.19, 4.19] |
| 4 Cerebral palsy at 7 years Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.35, 2.72] |
| 5 Moderate/severe disability at 7 years Show forest plot | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.49, 4.01] |
| 6 Death or moderate/severe disability at 7 years Show forest plot | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.65, 1.58] |
| 7 Systolic blood pressure of > 95th percentile at 7 years Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.25, 1.23] |
| 8 Diastolic blood pressure of > 95th percentile at 7 years Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.43, 4.45] |
| 9 Ever diagnosed as asthmatic by 7 years Show forest plot | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.55, 1.39] |

