表面活性物质治疗足月和过期产儿的胎粪吸入综合征
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Multicentre (19 centres) study in China. Study period not stated. | |
| Participants | Term and late preterm neonates with MAS, BW > 2500 gm, postnatal age < 36 hrs, a/A pO₂ ratio < 0.22, OI > 15 and needed mechanical ventilation for 1 to 2 hrs without improvement. | |
| Interventions | Sixty‐one term infants with severe MAS were randomly assigned to either a surfactant or a control group within 36 h after birth. The infants in the surfactant group (n=31) received an initial dose of porcine lung‐derived surfactant (Curosurf) at 200 mg/kg, and repeated doses of 200, 100 and 100 mg/kg were given at 6 to 12 h intervals to a maximum of four doses if oxygenation index (OI) deteriorated by > 2 from baseline. | |
| Outcomes | PRIMARY: Reduction of OI to < 10 and an increase of the pretreatment a/A pO₂ ratio of 100% over baseline 24 hrs after surfactant treatment. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information presented |
| Allocation concealment (selection bias) | Low risk | Surfactant or control therapy was randomly assigned by the randomisation centre staff according to sequentially numbered randomisation cards, provided in sealed randomisation envelopes, based on an expected total enrolment of 64 participants. |
| Blinding of participants and personnel (performance bias) | High risk | Surfactant administration was not conducted in a blind manner because that would have required a separate dosing team for each clinic centre. |
| Blinding of outcome assessment (detection bias) | High risk | Staff were aware of group assignment. |
| Incomplete outcome data (attrition bias) | Low risk | 66 infants were enrolled and 5 infants (4 in the surfactant group and 1 in the control group were excluded from the final analysis because of violation of the entry criteria). |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us so we cannot ascertain if there were any deviations from the protocol. |
| Methods | Single‐centre study in the US. Study period not stated. | |
| Participants | Term infants with MAS, requiring assisted ventilation, supplemental oxygen > 50%, MAP > 7cm H₂0, a/A pO₂ ratio < 0.22, age < 6 hrs and no major congenital anomaly. | |
| Interventions | The treatment group (n = 20) received modified bovine surfactant extract (Survanta 150 mg/kg), repeated at 6‐hr intervals for a maximum of 4 doses, infused intratracheally via a side port adaptor over 20 mins. | |
| Outcomes | PRIMARY: Improvement in OI, improvement in a/A pO₂ ratio | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | No specific information provided but the investigators and the physicians and nurses caring for the infants were unaware of the infants' assignment groups. |
| Blinding of participants and personnel (performance bias) | Low risk | The investigators and the physicians and nurses caring for the infants were unaware of the infants' assignment groups. |
| Blinding of outcome assessment (detection bias) | Low risk | The investigators and the physicians and nurses caring for the infants were unaware of the infants' assignment groups. |
| Incomplete outcome data (attrition bias) | Low risk | Outcomes reported for all enrolled infants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us so we cannot ascertain if there were any deviations from the protocol. |
| Methods | Multicentre (44 centres) study in the US. Study period September 1 1992 to October 23 1995. Stratification: primary diagnosis disease severity (oxygenation index) | |
| Participants | Infants > 2000 gm, gestational age > 36 weeks, age < 120 hrs with MAS, PPHN or sepsis and severe respiratory failure but without any major congenital anomalies or IVH > Grade I. | |
| Interventions | The treatment group (n = 87) received modified bovine surfactant extract (Survanta, 100 mg/kg) or air placebo (up to 4 doses prior to ECMO and 4 additional doses if ECMO was required). | |
| Outcomes | PRIMARY: Need for ECMO, severe complications, mortality. | |
| Notes | Only infants with MAS are included in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation. Computer‐generated random numbers Stratification: primary diagnosis disease severity (oxygenation index). |
| Allocation concealment (selection bias) | Low risk | The treatment assignments were made by having the pharmacist or dosing investigator at each site report the primary diagnosis and mean entry oxygen index for each participant to a central randomisation centre, Bio‐Pharm Clinical Services, Inc., which issued a participant number and treatment assignment on the basis of a computer‐generated random number. |
| Blinding of participants and personnel (performance bias) | Low risk | Study treatments were administered by dedicated dosing investigators at each site. The dosing investigators shielded the infant with drapes or a screen during treatment, and all other personnel left the immediate bedside area during the dosing procedure. The dosing investigator took the same amount of time to prepare and administer either treatment, and when treatment was complete, all supplies were stored in a locked area. Dosing investigators were prohibited from participating in any other aspect of the infants care and from revealing the treatment assignment. |
| Blinding of outcome assessment (detection bias) | Low risk | With the exception of dosing, the infant's clinical care during the 28 days of the study was provided by clinical investigators who were unaware of the treatment assignment. |
| Incomplete outcome data (attrition bias) | Low risk | All 330 randomised infants accounted for (168 of these infants were enrolled on the basis of MAS, and the remainder on the basis of PPHN or sepsis) . Two infants were later withdrawn from the study when consent was withdrawn. Their limited data were subsequently excluded from analysis. The diagnosis on which their enrolment was based was not stated. |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us so we cannot ascertain if there were any deviations from the protocol |
| Methods | Multicentre (13 centres) study in Chile between March 2001 and June 2003. | |
| Participants | Term infants ≥ 37 weeks of gestation with moderate to severe MAS and respiratory insufficiency within the first 12 hrs after birth. | |
| Interventions | The treatment group (n = 28) received 150 mg /kg/dose (6 ml) of Survanta every 6 hours for a total of 3 doses if they remained intubated. | |
| Outcomes | PRIMARY: Days of mechanical ventilation. | |
| Notes | We obtained unpublished data from Dr A Maturana. In the unpublished manuscript there were 3 more infants enrolled in the surfactant group and 1 more infant enrolled in the control group. We report the outcomes as per the unpublished report, not the referenced abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation scheme using blocks of 4 (as per unpublished manuscript). |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes stratified by centre |
| Blinding of participants and personnel (performance bias) | Low risk | A placebo (air) was used. |
| Blinding of outcome assessment (detection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | Low risk | Outcomes reported for all randomised (as per unpublished manuscript). |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us so we cannot ascertain if there were any deviations from the protocol. |
a/A pO₂ ratio = arterial/alveolar oxygen tension ratio
BW = birth weight
ECMO = extracorporeal membrane oxygenation
IVH = Intraventricular haemorrhage
MAP = mean airway pressure
MAS = meconium aspiration syndrome
OI = oxygen index
PPHN = persistent pulmonary hypertension of the neonate
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Sequential case study; no control group | |
| Not randomised, no control group. As a part of a West German multicentre study (424 participants, 16 hospitals) 10 term neonates ventilated because of severe meconium aspiration syndrome were treated with 1 to 4 doses of 50 mg/kg/BW of a bovine surfactant (Alveofact). Before treatment respiratory distress was severe (median FiO₂: 1.0, median MAD 9.9 mmHg, median OI: 20). Acute improvement ("responders") was shown in 4 participants. All infants survived. Time of mechanical ventilation was 6 to 26 days. High frequency ventilation was applied in 2 non‐responders, ECMO in 1. | |
| Retrospective review; treatment with bronchoalveolar lavage with dilute surfactant preparation Chang 2003 retrospectively reviewed the charts of all term infants with a diagnosis of MAS who had an oxygenation index (OI) > 20 during a 2‐year period. Tracheobronchial lavage was performed with a dilute surfactant suspension (5 mg/mL or 10 mg/mL) to reach a total dose of 60 to 70 mg/kg of phospholipid, administered in aliquots of 2 mL.
The records of 22 patients were reviewed, of whom 12 had undergone lavage. These infants were subdivided into low‐concentration (surfactant concentration, 5 mg/mL; n = 6) and high‐concentration (surfactant concentration, 10 mg/mL; n = 6) subgroups. There were no significant differences in demographic characteristics between the 2 subgroups. The lavaged infants had a significantly higher arterial partial pressure of oxygen (PaO₂) 24 hours after lavage than the infants without lavage (178.3 mm Hg vs 80.6 mm Hg, P < 0.05). The incidence of pneumothorax (1/12 vs 7/10, P < 0.05) and requirement for inhaled nitric oxide (5/12 vs 9/10, P < 0.05) were significantly lower in the lavaged group. All infants tolerated the procedure well except for 2 with transient complications. There were no significant differences in duration of lavage, response and complications between subgroups lavaged at low and high surfactant concentration. | |
| Bronchoalveolar lavage with dilute surfactant preparation Dargaville 2011 evaluated whether lung lavage with surfactant changes the duration of mechanical respiratory support or other outcomes in meconium aspiration syndrome (MAS).
Randomised controlled trial that enrolled ventilated infants with MAS. Infants randomised to lavage received two 15‐mL/kg aliquots of dilute bovine surfactant instilled into, and recovered from, the lung. Control subjects received standard care, which in both groups included high frequency ventilation, nitric oxide, and, where available, ECMO.
66 infants were randomised, with 1 ineligible infant excluded from analysis. Median duration of respiratory support was similar in infants who underwent lavage and control subjects (5.5 vs. 6.0 days, P = .77). Requirement for high frequency ventilation and nitric oxide did not differ between the groups. Fewer infants who underwent lavage died or required ECMO: 10% (3/30) compared with 31% (11/35) in the control group (odds ratio, 0.24; 95% confidence interval, 0.060 to 0.97). Lavage transiently reduced oxygen saturation without substantial heart rate or blood pressure alterations. Mean airway pressure was more rapidly weaned in the lavage group after randomisation. | |
| Not randomised | |
| Gadzinowski 2008 compared the effectiveness of surfactant treatment either by bolus or surfactant lung lavage followed by inhaled nitric oxide (iNO) therapy in infants with MAS complicated by persistent pulmonary hypertension (PPHN). 13 infants with diagnosis of MAS and PPHN were first treated with conventional respiratory support. Then between 2 and 22 hrs of life they were randomised either to bolus surfactant treatment (n = 6) or surfactant lung lavage (SLL, n = 7) treatment. Then all infants were treated with iNO therapy. The groups were compared with regard to their clinical course: changes in PaO₂, FiO₂, MAP, OI, A‐a oxygen gradient, duration of iNO therapy, length of ventilation and hospitalisation. Complications and mortality were also compared. The results showed that infants treated with SLL had significant improvements in oxygenation, decreases in MAP and A‐a gradients. But there were no significant differences in duration of ventilation, iNO treatment, length of hospitalisation or complications. In conclusion these data show no advantage of SLL therapy over bolus surfactant treatment in infants with MAS complicated by PPHN. | |
| Retrospective, not randomised | |
| Historical controls Hung 2006 assessed the effects of lavage with a small volume of dilute surfactant in neonates with MAS and compared the results with those of historical controls treated with larger volumes. Eleven newborns with MAS were treated using 20 ml of dilute surfactant at a phospholipid concentration of 10 mg/ml (SVL group). Results were compared with those of 9 infants previously treated with large‐volume lavage (LVL group), using 40 ml of dilute surfactant, 5 mg/ml. Measures of oxygenation, including mean PaO₂, oxygenation index, and arterial/alveolar 0₂ ratio, showed no significant difference between the 2 groups. | |
| Saline lavage and surfactant replacement, not randomised | |
| Sequential case study; no control group | |
| Not randomised, historical controls, surfactant used as lavage. Lam 1999 reported a pilot experience on the use of diluted bovine lung surfactant lipid extract solution (Survanta, Ross Laboratories, Ohio, USA) as a tracheobronchial lavage fluid for the treatment of infants with severe MAS.
6 consecutively recruited infants with severe MAS necessitating mechanical ventilation with an oxygen index of ≥ 15 within 6 hours of life during a 1½‐year period were treated with tracheobronchial lavage with 15 mL/kg of diluted surfactant solution (Survanta) at a phospholipid concentration of 5 mg/mL administered in 2‐mL aliquots. The outcome of treatment was assessed by comparison with 6 consecutive historic control infants with equally severe MAS of similar inclusion criteria retrospectively.
The mean oxygen index, mean airway pressure, fraction of inspired oxygen, and arterial/alveolar oxygen tension ratio improved significantly within the first 48 hours after treatment in the lavage group. The duration of ventilation (mean ± SEM, 55.3 ± 4.6 hours vs 131 ± 60 hours) and oxygen therapy (mean ± SEM, 4.1 ± 0.5 days vs 20.8 ± 8.2 days) were also significantly reduced in the lavage‐treated group compared with the control group. All 6 infants in the lavage group survived without sequelae whereas there were 2 deaths in the control group. The process of administering the surfactant lavage was well tolerated with no air leak complications. | |
| Bronchoalveolar lavage with dilute surfactant preparation Lin 2014 evaluated 136 full‐term infants with severe MAS who were admitted to the neonatal intensive care unit. Infants were randomly allocated to pulmonary surfactant (PS) lavage and PS injection groups. In the PS lavage group, infants were treated with endotracheal lavage using 3 to 5 mL of diluted PS (12 mg/mL) each time, and the PS injection group was given PS by intratracheal injection with an initial dose of 200 mg/kg. Blood gas, oxygenation index (OI), and PaO2/FiO2 of the two groups were evaluated before and 2, 12, 24, and 48 hours after the treatment, and the duration of mechanical ventilation, complication rate, and cure rate were compared between the two groups. | |
| Case series Lista 2006 evaluated the efficacy and safety of bronchoalveolar lavage (BAL) with diluted porcine surfactant in mechanically ventilated term infants with severe acute respiratory distress syndrome (ARDS) due to MAS.
Eight consecutive mechanically ventilated term infants with severe ARDS due to MAS underwent BAL with 15 mL/kg of diluted (5.3 mg phospholipid/mL) surfactant saline suspension (porcine surfactant, Curosurf). Treatment was administered slowly in aliquots of 2.5 mL. The mean age of neonates at treatment was 3.5 (range 1 ‐ 8) hours. Heart rate, systemic blood pressure and oxygen saturation were monitored continuously. Arterial blood gases were measured immediately before treatment, and again at 3 and 6 hours post‐treatment. Chest x‐rays were taken 6 and 24 hours after treatment.
Radiological improvement was evident in all 8 infants 6 hours post‐treatment. Compared with pre‐BAL values, significant improvements (P < 0.05) in mean values for partial pressure of oxygen in arterial blood, partial pressure of carbon dioxide in arterial blood, pH, arterial/alveolar O₂ ratio and oxygenation index were documented at 3 and 6 hours after BAL. In all participants, tracheal fluids that had been meconium‐stained prior to BAL were clear of meconium after BAL. Only one infant required nitric oxide therapy for transient pulmonary hypertension. No adverse sequelae of treatment occurred during the study. | |
| Bronchoalveolar lavage with dilute surfactant preparation for the treatment of meconium aspiration syndrome | |
| Bronchoalveolar lavage with dilute surfactant preparation Wiswell 2002 compared treatment with bronchoalveolar lavage using dilute Surfaxin with standard therapy in a population of newborn infants with MAS.
Inclusion criteria were 1) gestational age ≥ 35 weeks, 2) enrolment within 72 hours of birth, 3) diagnosis of MAS, 4) need for mechanical ventilation, and 5) an oxygenation index ≥ 8 and ≤ 25. Infants were randomised to either lavage with Surfaxin or standard care (2:1 proportion). In lavaged infants, a volume of 8 mL/kg dilute Surfaxin (2.5 mg/mL) was instilled into each lung over approximately 20 seconds followed by suctioning after 5 ventilator breaths. The procedure was repeated twice. The third and final lavage was with a more concentrated solution (10 mg/mL) of Surfaxin. |
ECMO = extracorporeal membrane oxygenation
MAP = mean airway pressure
MAS = meconium aspiration syndrome
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 4 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.41, 2.39] |
| Analysis 1.1  Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 1 Mortality. | ||||
| 2 Treatment with ECMO Show forest plot | 2 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.46, 0.91] |
| Analysis 1.2  Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 2 Treatment with ECMO. | ||||
| 3 Pneumothorax Show forest plot | 3 | 269 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.08, 0.05] |
| Analysis 1.3  Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 3 Pneumothorax. | ||||
| 4 Pulmonary interstitial emphysema Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 4 Pulmonary interstitial emphysema. | ||||
| 5 Air leaks (pneumothorax, pneumomediastinum, interstitial emphysema) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 5 Air leaks (pneumothorax, pneumomediastinum, interstitial emphysema). | ||||
| 6 Duration of assisted mechanical ventilation (days) Show forest plot | 3 | 158 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.41, 1.62] |
| Analysis 1.6  Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 6 Duration of assisted mechanical ventilation (days). | ||||
| 7 Duration of supplemental oxygen (days) Show forest plot | 2 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐2.83, 3.64] |
| Analysis 1.7  Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 7 Duration of supplemental oxygen (days). | ||||
| 8 Need for supplemental oxygen at discharge Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 8 Need for supplemental oxygen at discharge. | ||||
| 9 Chronic lung disease (age at diagnosis not stated) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 9 Chronic lung disease (age at diagnosis not stated). | ||||
| 10 Intraventricular haemorrhage (any grade) Show forest plot | 2 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.31, 1.46] |
| Analysis 1.10  Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 10 Intraventricular haemorrhage (any grade). | ||||
| 11 Severe intraventricular haemorrhage Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.11  Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 11 Severe intraventricular haemorrhage. | ||||
| 12 Duration of hospital stay (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.12  Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 12 Duration of hospital stay (days). | ||||

Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 1 Mortality.

Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 2 Treatment with ECMO.

Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 3 Pneumothorax.

Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 4 Pulmonary interstitial emphysema.

Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 5 Air leaks (pneumothorax, pneumomediastinum, interstitial emphysema).
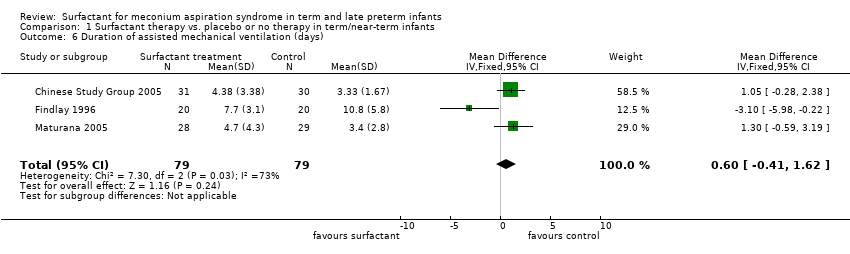
Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 6 Duration of assisted mechanical ventilation (days).

Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 7 Duration of supplemental oxygen (days).

Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 8 Need for supplemental oxygen at discharge.
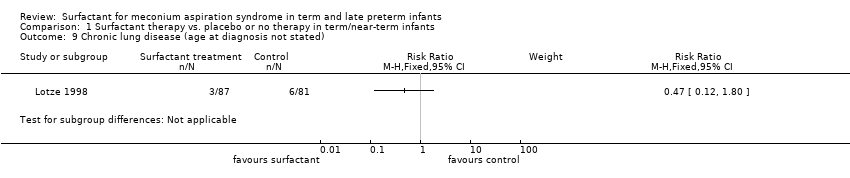
Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 9 Chronic lung disease (age at diagnosis not stated).

Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 10 Intraventricular haemorrhage (any grade).

Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 11 Severe intraventricular haemorrhage.

Comparison 1 Surfactant therapy vs. placebo or no therapy in term/near‐term infants, Outcome 12 Duration of hospital stay (days).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 4 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.41, 2.39] |
| 2 Treatment with ECMO Show forest plot | 2 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.46, 0.91] |
| 3 Pneumothorax Show forest plot | 3 | 269 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.08, 0.05] |
| 4 Pulmonary interstitial emphysema Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5 Air leaks (pneumothorax, pneumomediastinum, interstitial emphysema) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6 Duration of assisted mechanical ventilation (days) Show forest plot | 3 | 158 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.41, 1.62] |
| 7 Duration of supplemental oxygen (days) Show forest plot | 2 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐2.83, 3.64] |
| 8 Need for supplemental oxygen at discharge Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9 Chronic lung disease (age at diagnosis not stated) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10 Intraventricular haemorrhage (any grade) Show forest plot | 2 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.31, 1.46] |
| 11 Severe intraventricular haemorrhage Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12 Duration of hospital stay (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |

