Buprenorfina para el tratamiento de la abstinencia de opiáceos
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised, double‐blind, placebo‐controlled study | |
| Participants | Setting: outpatient clinic, USA Participants: N = 8, opioid dependent by DSM‐III‐R, using unspecified opioid drugs Group sizes: group 1: N = 3; group 2: N = 5 Groups similar in withdrawal symptoms and positive urine samples during maintenance phase Average age 39.4 years 87.5% male Average duration opioid use 18.1 years, 87.5% reported i.v. use. One required benzodiazepine detoxification prior to study | |
| Interventions | Buprenorphine solution (or placebo) administered sublingually, plus placebo pills during dose taper Buprenorphine increased to 8 mg/d over first three days, maintained for 28 days, then tapered. Group 1: 36 days (1 mg/4 days then 0.5 mg in last 4 days) Group 2: 12 days (50% every 4 days) Daily clinic attendance Both groups received behavioural counselling (2‐3 sessions of 1 h each). Vouchers for recreational items given for negative urine samples | |
| Outcomes | Change in withdrawal scores per day, % opioid‐negative urine samples, number in treatment for full 9 weeks, % continuously abstinent by week | |
| Notes | Withdrawal rated by participants (20 signs and symptoms in prior 24 h, rated 0 (none) to 9 (severe), max score 180) and observers (8 signs, rated 0 (not at all) to 3 (severe), max score 24) Observed urine and breath alcohol samples 3 times a week. Missed urine samples considered positive Source of funding for trial not reported; report preparation supported by government (NIDA) grants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were assigned randomly . . . by drawing lots" |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not reported |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind stated, with use of placebos. Quote: "Nursing staff who were blind to the treatment conditions completed an observer rating scale" |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind stated, with use of placebos, and these outcomes unlikely to be affected by awareness of treatment allocation |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Missed (urine) samples were considered opioid positive for the purposes of data analyses." "A repeated measures analysis of variance (ANOVA) was performed to examine subject‐ and observer‐rated withdrawal, and mean percentage opioid‐negative urine samples across the treatment groups" Comment: this approach less susceptible to bias due to missing data |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised double‐blind, placebo‐controlled trial. Concurrent dependence on alcohol exclusion criterion | |
| Participants | Setting: inpatient treatment, addiction ward of psychiatric hospital, Tehran, Iran Participants: N = 40, opioid dependent by DSM‐IV, 68% using heroin, 32% opium Group sizes: N = 20 in each group Baseline characteristics of groups similar Average age 31.5 39/40 male Average duration opioid use 9 years; 50% using by injection or multiple routes; 30% employed, 55% married | |
| Interventions | Buprenorphine, i.m. with: Group 1: 4 × 1.5 mg between 12 pm and 6 pm day 1, 4 × 1.5 mg between 6 am and 12 pm day 2 Group 2: 2 × 1.5 mg day 1, tapered to 2 × 0.3 mg day 5 Adjunct medications (indomethacin, trazodone, chlorpromazine, hyoscine) as required. Relapse prevention treatment using naltrexone as aftercare | |
| Outcomes | Withdrawal scores, days in treatment, number completing treatment | |
| Notes | Withdrawal assessed once per day by participants (SOWS) and observer (OOWS) Study conducted without financial support | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned . . . using a computer‐generated list of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not reported |
| Blinding (performance bias and detection bias) | Low risk | Patients and staff with patient contact, and person performing clinical research assessments all blind to group allocation. Placebo used |
| Blinding (performance bias and detection bias) | Low risk | Patients and staff with patient contact, and person performing clinical research assessments all blind to group allocation. Placebo used |
| Incomplete outcome data (attrition bias) | Low risk | 10% dropout in course of study. Missing data replaced by carrying last observation forward |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants | Setting: outpatient clinic, USA Participants: N = 45, opioid dependent, using opiates other than methadone, seeking outpatient detoxification, eligible for methadone maintenance treatment (evidence of recent injecting use part of eligibility criteria) Group sizes: group 1: N = 22; group 2: N = 23, but most analyses based on N = 17 from group 1 and N = 14 from group 2who remained in treatment at least 6 weeks Groups similar on demographics Average age 30 years All male Duration of heroin use, mean ± SD: group 1 6.7 ± 1.9 years; group 2: 9.2 ± 6.1 years | |
| Interventions | Treatment regimens involved 3 weeks stabilisation, 4 weeks dose reduction, 6 weeks placebo dosing with: Group 1: buprenorphine, sublingual solution, 2 mg/d or Group 2: methadone, 30 mg/d, oral Daily clinic attendance All had access to supportive counselling. Scheduled 90‐day treatment | |
| Outcomes | Mean duration in treatment, number in treatment for 6 weeks or more, graphs of retention, mean withdrawal scores and opiate positive or missing urine to week 6 | |
| Notes | Withdrawal rated by subjects 3 times a week (2 scales, each 20 symptoms rated 0‐9) Urine samples collected according to random schedule Study supported by government grants and pharmaceutical company | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised, stratifying for race"; method of sequence generation not specified |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not reported |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Neither the subjects nor the nurses had information about which medication was active nor did they have any information about the dose‐reduction schedule." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Neither the subjects nor the nurses had information about which medication was active nor did they have any information about the dose‐reduction schedule." |
| Incomplete outcome data (attrition bias) | Low risk | Rapid dropout from week 6, towards the end of the dose taper of the two medications, but dropout similar in two groups. Retention main outcome reported after week 6 when dropout > 30% |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants | Setting: inpatient, closed research ward, USA Participants: N = 25 heroin users, with evidence of recent injecting use Group sizes: group 1: N = 12; group 2: N = 13. Analysis primarily on basis of 18 who completed treatment Group similar on demographic characteristics Average age 35 years 44% male Average duration heroin use 11.5 years | |
| Interventions | Morphine used to attenuate withdrawal on admission. Subsequent treatment with: Group 1: buprenorphine, sublingual solution, maximum of 2 mg/dose, total 17 mg over 3 days Group 2: clonidine, maximum 0.3 mg/dose, total 2.7 mg over 5 days Individual counselling twice weekly Total study period 18 days. Placebo administered after completion of active medication phase | |
| Outcomes | Graphs of mean 'urge' and 'need' for an opioid, mean withdrawal score, number completing 10 days of treatment, reasons for cessation | |
| Notes | Visual analogue scales for rating 'urge' and 'need' for an opioid and subjective response to medication Addiction Research Centre scales assessed opioid‐like euphoria, apathetic sedation and dysphoria Daily urine screening Study funded by US government research programme | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned" with stratification based on response to naloxone challenge test. Method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not reported |
| Blinding (performance bias and detection bias) | Low risk | Quote: "double‐blind, double‐dummy . . . design"; extended treatment period to maintain blind |
| Blinding (performance bias and detection bias) | Low risk | Quote: "double‐blind, double‐dummy . . . design" |
| Incomplete outcome data (attrition bias) | Low risk | Analysis of withdrawal based on participants who completed the study. The significant difference in the first 3 days of treatment would be expected to be increased, not decreased, if dropouts had been included. |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised controlled, open‐label study | |
| Participants | Setting: clinical research centre, New York, USA. Inpatient care for 72 h of withdrawal, outpatient for 12 weeks naltrexone maintenance treatment Participants: N = 106, treatment seeking, heroin dependent by DSM‐IV Group sizes: group 1: N = 35; group 2: N = 37; group 3: N = 34 Groups similar on demographic and clinical characteristics Average age 36 72% male 40% using by injection; 36% currently married or cohabiting; 56% currently employed. Major psychiatric illness, active medical illness, dependence on alcohol or drugs other than heroin were exclusion criteria. | |
| Interventions | Group 1 (excluded from review): nalmefene 4 mg i.v. over 30 min, naltrexone 50 mg via nasogastric tube, under propofol anaesthesia (4‐6 hours). Various adjunct medications, including octreotide Group 2: buprenorphine (sublingual, preparation not specified), 8 mg (single dose) day 1, naltrexone 12.5 mg afternoon of day 2, 25 mg 12 hours later, then 50 mg/d Group 3: clonidine, max 1.2 mg/d, discharged day 3, naltrexone 12.5 mg day 7, 25 mg day 8, then 50 mg/d. Clonidine and various adjunct medications available to all groups. Naltrexone maintenance and relapse prevention psychotherapy as aftercare | |
| Outcomes | Graphs of withdrawal severity,.mean weeks in treatment. Number of participants completing inpatient phase, receiving at least one dose of naltrexone, receiving full 50 mg dose of naltrexone. Number retained in treatment over 12 weeks. Number retained 12 weeks who provided 2 or less opiate positive urine samples. Number experiencing serious adverse events | |
| Notes | Withdrawal assessed by SOWS, OOWS, and Clinical Institute Narcotic Assessment, 4 times a day during inpatient phase Study funded entirely by grants from US National Institutes of Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ". . .using random, computer‐generated assignments, with stratification by sex. . . . In addition, the Berger‐Exner test was used to confirm that no selection bias in enrolment occurred." |
| Allocation concealment (selection bias) | Low risk | Quote: "All staff remained unaware of the randomisation sequence" |
| Blinding (performance bias and detection bias) | High risk | Patients were not blinded to treatment − sham anaesthesia not ethical. Unclear whether observers were blinded to treatment. |
| Blinding (performance bias and detection bias) | Low risk | These outcomes unlikely to be affected by knowledge of treatment |
| Incomplete outcome data (attrition bias) | Low risk | Losses to follow‐up similar in three groups and main outcomes reported not sensitive to missing data |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Unclear risk | Enrollment stopped at 106 participants (target 150) "because actual differences in withdrawal severity scores and treatment retention were smaller than anticipated, leading to an impractically large recalculated sample size" Comment: it seems unlikely that the stopping of enrolment resulted in bias |
| Methods | Randomised controlled (open‐label) trial | |
| Participants | Setting: residential therapeutic community, USA Participants: N = 20, heroin dependent by DSM‐IV Group sizes: N = 10 in each group Groups similar on demographics and drug use Average age 47 years 65% male 35% using heroin by injection (rest using nasally), 60% also cocaine dependent; 15% married, 20% employed in prior 30 days | |
| Interventions | Buprenorphine/naloxone sublingual tablet, two dose regimens: Group 1: 8 mg plus 24 mg after 30 min on day 1 Group 2: 8 mg day 1, 16 mg day 2, 8 mg day 3 Adjunct medications available as needed Detoxification and monitoring over 7 days; follow‐up evaluations on days 14‐17 | |
| Outcomes | Number completing 7 days of detoxification programme, number completing follow‐up evaluation, mean daily withdrawal score, use of adjunct medication | |
| Notes | Withdrawal assessed daily by Clinical Opiate Withdrawal Scale (COWS) Urine testing 4 times in 7 days of detoxification Source of funding government grants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned in an open‐label fashion"; method of sequence generation not reported. |
| Allocation concealment (selection bias) | High risk | Quote: "patients were randomly assigned in an open‐label fashion". |
| Blinding (performance bias and detection bias) | Unclear risk | Staff who assessed withdrawal symptoms daily were not aware of group assignments, but participants were probably aware of treatment allocation. |
| Blinding (performance bias and detection bias) | Low risk | Treating staff were not aware of group assignments and these outcomes considered unlikely to be affected by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | No significant difference in dropouts between groups |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | High risk | All participants were resident in a therapeutic community. This may have resulted in selection of a highly motivated group who would have done well whatever the treatment. |
| Methods | Randomised controlled, open‐label trial | |
| Participants | Setting: inpatient treatment facility, Srinagar, India Participants: N = 58, opioid dependent by DSM‐IV, treatment seeking. Use of substances of abuse other than opioids was an exclusion criterion Group sizes: N = 29 in each group Groups similar on demographic characteristics Average age 27 years Sex not reported (probably all male) Average 6 years of abuse; 69% employed, 26% married | |
| Interventions | Group 1: buprenorphine/naloxone, sublingual, 4.0/1.0 mg on day 1, 8.0/2.0 mg/d on days 2‐4, 4.0/1.0 mg on days days 5‐7, 2.0/0.5 mg on days 8‐10 Group 2: clonidine, oral, 0.1 mg day 1, increasing to 0.2 mg on days 2‐4, tapered from day 5, ceased on day 10 Both groups received ancillary medications on demand | |
| Outcomes | Average daily withdrawal score, average daily craving score, participants experiencing adverse effects, number completing study | |
| Notes | Withdrawal assessed twice daily with Clinical Opiate Withdrawal Scale (COWS). Craving assessed with visual analogue scale (VAS) Source of funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants were randomised into two groups using computer generated random numbers". |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding (performance bias and detection bias) | High risk | Quote: "open labelled study and blinding was not done" |
| Blinding (performance bias and detection bias) | Low risk | These outcomes are unlikely to be affected by knowledge of the treatment group. |
| Incomplete outcome data (attrition bias) | Low risk | Low rate of attrition, and same in both groups |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised double‐blind, placebo‐controlled trial | |
| Participants | Setting: inpatient, hospital drug dependence unit, Italy Participants: N = 39 from MMT requiring detoxification Group sizes: N = 13 in each group. Analysis based on 32/39 who completed treatment Groups stated to not differ on psychosocial and drug history data Average age 26 years 59% male Average duration opioid dependence 7.5 years, methadone maintenance 3.4 years; 56% using methadone only, 43% using methadone and heroin; polydrug use an exclusion criterion; 41% in stable job | |
| Interventions | All stabilised on methadone, mean 23.7mg/d, tapered to 10 mg/d before study commenced Detoxification managed with: Group 1: buprenorphine intramuscularly, 0.9 mg days 1 and 2, 0.45 mg day 3, 0.15 mg day 4 Group 2: Clonidine, 0.3 to 0.9 mg/d intramuscularly, 6 days or Group 3 (excluded from review): Lefetamine, 60‐240 mg/d intramuscularly, 6 days. This group excluded from this review. Clonidine and lefetamine groups given 5 mg methadone on days 1 and 2; oral and i.m. placebos used to maintain blind, no other drugs allowed. | |
| Outcomes | Graphs of total, objective, subjective and psychological withdrawal scores, adjusted for baseline scores Number completing treatment | |
| Notes | Withdrawal assessed by scale of Bruno and Ferracuti (12 objective, 10 subjective, 5 psychological items, each rated 0‐5). Urine screening used Source of funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were assigned randomly to one of three groups" Comment: Method of sequence generation not reported but groups similar at baseline |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding (performance bias and detection bias) | Low risk | Quote: withdrawal "was rated by a trained psychiatrist, who was blind to study conditions". |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind stated and these outcomes unlikely to be affected by knowledge of intervention |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "The 7 patients who dropped out were equally distributed among the three groups." |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised controlled open‐label trials − separate trials in outpatient and inpatient settings | |
| Participants | Setting: inpatient and outpatient, in various community treatment programmes in USA Participants: N = 113 (inpatient) and N = 231 (outpatient), heroin dependent by DSM‐IV Group sizes: group 1: N = 77; group 2: N = 36 for inpatient study, group 1: N = 157; group 2: N = 74 for outpatient study Groups similar on demographics, drug use history, except cannabis use with group 1 13.9%; group 2 2.6% meeting criteria for cannabis dependence Average age 36 (inpatient) and 39 (outpatient) 68% male Duration of heroin use more than 9 years for outpatient study, less than 7 years for inpatient study. 65% in buprenorphine groups injecting users, 70%‐94% across all groups worked full‐ or part‐time. Those codependent on other drugs not excluded | |
| Interventions | Group 1: buprenorphine/naloxone sublingual tablet, first dose when in mild withdrawal, 4 mg day 1, with further 4 mg 1‐2 hours later, increasing to 16 mg day 3, tapering to 2 mg day 12/13 Group 2: clonidine, 0.05‐0.1 mg every 4‐6 hours day 1, plus transdermal patch from day 2, patch only from day 3, discontinued by day 13 Ancillary medications and counselling available. Medications administered daily. Scheduled duration 13 days | |
| Outcomes | Success, defined as retention for entire study duration and opioid‐free urine on last day. Summary withdrawal, calculated as average of COWS scores across available observations. Average craving score. | |
| Notes | Observers completed COWS (11 signs) daily. Participants completed Adjective Rating Scale for Withdrawal (16 items). Craving assessed by Visual Analogue Scale. Daily urine samples. Source of funding: series of grants from US government (NIDA) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were assigned randomly to bup‐nx or clonidine conditions using a 2:1 ratio in favour of buprenorphine" Comment: based on contacts with the research group, we consider it likely that the method of sequence generation was adequate. |
| Allocation concealment (selection bias) | Low risk | Method of concealment not reported, but based on our knowledge of the research group, we consider it likely that concealment of allocation would have been achieved. |
| Blinding (performance bias and detection bias) | High risk | No blinding |
| Blinding (performance bias and detection bias) | Low risk | No blinding, but these outcomes unlikely to be affected by knowledge of group allocation |
| Incomplete outcome data (attrition bias) | Low risk | Significantly more dropouts from clonidine groups, but method of analysis (ANCOVA) adjusted for missing data |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Unclear risk | Quote: "the NIDA Data and Safety Monitoring Board (DSMB) recommended that the study be halted prior to collecting the full complement of subjects for the following reasons: the large enrolment status, the consistency of findings overwhelmingly favourable toward the bup‐nx condition and the consideration that additional participant enrolment would not yield meaningful new information. Comment: while early termination of studies is associated with some risk of bias, the extent of recruitment achieved is likely to have minimised this risk |
| Methods | Randomised controlled, open‐label study. Randomisation stratified by buprenorphine dose at end of stabilisation (8, 16 or 24 mg) | |
| Participants | Setting: outpatient, 11 treatment programmes, USA Participants: N = 516, dependent on opioids other than methadone Group sizes: group 1: N = 255; group 2: N = 261 No significant differences between groups in demographics or drug use at baseline Average age 36 years 33% female About 8 years of lifetime heroin use; exclusion criteria included positive urine test for methadone or benzodiazepine, and dependence on alcohol or any drug other than opioids; 35% unemployed in month prior to baseline | |
| Interventions | Buprenorphine/naloxone sublingual tablet (Suboxone), 4‐week stabilisation with flexible dosing for 3 weeks to establish optimal dose, fixed at that dose for week 4, then tapered: Group 1: over 7 days Group 2: over 28 days Psychosocial services as usual for both groups. Once weekly clinic attendance Maximum 5 months covering screening, induction/stabilisation, taper and follow‐up | |
| Outcomes | Mean withdrawal and craving scores at assessment time points, number in treatment at end of taper, number with opioid‐free urine samples at end of taper and at follow‐up | |
| Notes | Participants rated withdrawal with Adjective Rating Scale (16 items each rated 0‐9). Clinicians rated withdrawal with COWS (range 0‐48). Visual analogue scale for overall withdrawal, craving and medication effectiveness. Weekly data collection to week 8. Incentive payments for each 'milestone' visit Source of funding: series of grants from US government (NIDA) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants were assigned randomly. . . stratified by maintenance dose at the end of the stabilization phase". Comment: This sort of randomisation would be expected to be computerised. |
| Allocation concealment (selection bias) | High risk | Quote: "Each treatment site had three sets of randomisation cards (one for each stabilisation dose) prepared in advance. Based on stabilisation dose, the top card in the appropriate stack was selected for the participant." Comment: Concealment of allocation was unlikely meaning the sequence could be easily manipulated |
| Blinding (performance bias and detection bias) | High risk | Study conducted open label |
| Blinding (performance bias and detection bias) | Low risk | Study open label but these outcomes unlikely to be affected by awareness of treatment allocation |
| Incomplete outcome data (attrition bias) | Low risk | No significant differences in baseline characteristics of those who remained in the study to the end of the taper and those who dropped out |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised controlled, open‐label study | |
| Participants | Setting: outpatient, specialist clinic, Australia Participants: N = 114 heroin users, dependent by DSM‐IV Group sizes: group 1: N = 58; group 2: N = 56 Groups similar on demographics and drug use Average age 30 years 65% male About 7 years since first regular heroin use, 90% injecting users; 39% employed full‐ or part‐time, 25% married/de facto. Some polydrug use, but significant other drug dependence an exclusion criterion | |
| Interventions | Group 1: buprenorphine, sublingual tablet, supervised single daily dose around 6 mg/d, adjusted to symptoms, ceased day 5 Group 2: clonidine 0.1‐0.15 mg 4 times a day as required plus symptomatic medications Daily clinic attendance for medication Both groups received supportive counselling during withdrawal. Naltrexone, substitution treatment or counselling available as aftercare. Scheduled duration 8 days | |
| Outcomes | Withdrawal score, treatment retention, time in treatment, days of heroin use, adverse effects, engagement in postwithdrawal treatment | |
| Notes | Withdrawal assessed by SOWS and OOWS (Handelsman) Urine screening used Source of funding: government, buprenorphine provided by manufacturer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a computerized schedule was developed . . . using the technique of dynamic balanced randomisation . . . balancing treatment allocation within each site and across the study as a whole." |
| Allocation concealment (selection bias) | Low risk | Quote: "randomisation was conducted by an independent organization" |
| Blinding (performance bias and detection bias) | High risk | Quote: "patients, treatment providers and outcome assessors (were) aware of group allocation." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "patients, treatment providers and outcome assessors (were) aware of group allocation" but these outcomes unlikely to be affected by knowledge of group allocation. |
| Incomplete outcome data (attrition bias) | Low risk | Difference in dropout cannot account for significant differences between buprenorphine and clonidine treatment |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised double‐blind, placebo‐controlled trial. Participants stratified by sex and past‐month route of opiate use (injection or intranasal) | |
| Participants | Setting: outpatient, university‐based research clinic, USA Participants: N = 36 adolescents (aged 13‐18 years), opiate dependent by DSM‐IV Group sizes: N = 18 in each group Groups similar on demographics and clinical criteria at baseline Average age 17 39% male Average age 15 years at first opiate use; 53% primarily heroin users, 36% injecting users | |
| Interventions | Group 1: buprenorphine, sublingual tablets, commenced at 6 or 8 mg/day according to body weight and opiate use, decreased 2 mg every 7 days Group 2: clonidine, 0.1 mg transdermal patches, 1 day 1, 2 days 2‐6, with optional 3rd days 4‐6, tapered after 7 days. Scheduled treatment duration 28 days Behavioural counselling 3 times a week and incentives contingent on opiate abstinence. All offered naltrexone as aftercare | |
| Outcomes | Number completing scheduled treatment. % urine tests opioid negative. Number initiating treatment with naltrexone | |
| Notes | Withdrawal not assessed. Urine screening used Funding support from government (NIDA) and university grants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned"; method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not reported |
| Blinding (performance bias and detection bias) | Low risk | Quote: "double‐blind, double‐dummy design" |
| Blinding (performance bias and detection bias) | Low risk | Quote: "double‐blind, double‐dummy design" |
| Incomplete outcome data (attrition bias) | Low risk | Retention is major outcome. Analyses of secondary outcomes based on data from first week when retention still high in both groups |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised controlled, double‐blind trial | |
| Participants | Setting: Outpatient, in hospital‐based research clinics in New York City, USA Participants: N=53 adolescents (age 16‐24), opioid‐dependent by DSM‐IV Group sizes: (1) N=28 (2) N=25 Groups similar, except more participants in group (1) were nicotine dependent. Average age 20.5 years 58% male 58% using iv, 81% primary heroin users. Pregnancy, active significant psychiatric disorder, serious medical condition were exclusion criteria. | |
| Interventions | Commenced on sublingual tablets containing buprenorphine only, initial dose 6 or 8 mg based on bodyweight and drug use, additional 2, 4, 6 or 8 mg if withdrawal symptoms after 1 hour. Switched to sublingual buprenorphine/naloxone when stable (after 2 days). Taper adjusted to starting dose to cease after (1) 28 or (2) 56 days. Initially daily clinic attendance required; after 20 enrolments participants able to earn take home doses and clinic attendance required 2‐3 times weekly. All received behavioural counselling and opioid abstinence incentives as adjunct therapies. | |
| Outcomes | Abstinence as percentage of scheduled urine tests documented as negative (missed urine samples assumed to be positive) Retention as number of days with attendance at scheduled visits. | |
| Notes | Urine samples at intake and then randomly at scheduled visits. Source of funding: grant from US government (NIDA); study medication provided by manufacturer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...participants were assigned randomly" "A minimum allocation procedure was used [to balance] groups on participant characteristics likely to influence treatment outcomes. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind stated. Quote: "...each participant received the same number of identical‐appearing tablets ... throughout the trial". No subjective outcomes reported. |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind stated. Quote: "...each participant received the same number of identical‐appearing tablets ... throughout the trial". |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised controlled trial | |
| Participants | Setting: inpatient, hospital, New Delhi, India Participants: N = 72, opioid dependent by DSM‐III‐R. Published analysis based on 44 (of 72 recruited) who completed treatment. Additional information provided by authors Group sizes: group 1: N = 34; group 2: N = 38 Groups similar on demographics, drug use history, withdrawal score at entry Average age 29 All males Duration heroin use 4‐5 years, 90% heroin users (1.5 g/day), rest opium users; no intravenous users; polydrug use an exclusion criterion; 64% married, 92% employed | |
| Interventions | Group 1: buprenorphine, initial dose 0.6 mg/d, maximum 1.2 mg/d, sublingual tablet, in 3 divided doses Group 2: clonidine, initial dose 0.3 mg/d, maximum 0.9 mg/d, oral, in 3 divided doses. Nitrazepam as adjunct medication. Scheduled duration 10 days | |
| Outcomes | Mean daily withdrawal scores. Side effects. Completion rates from personal communication | |
| Notes | Withdrawal assessed daily using Subjective and Objective Opiate Withdrawal Scales (Handelsman). Urine screening used only for some participants Source of funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors advised that subjects were randomly assigned but did not specify the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not reported |
| Blinding (performance bias and detection bias) | High risk | Authors advised study not double‐blind |
| Blinding (performance bias and detection bias) | Low risk | These outcomes unlikely to be affected by knowledge of group allocation |
| Incomplete outcome data (attrition bias) | Low risk | Dropout similar in two groups; withdrawal score comparisons based on those who completed treatment |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised, double‐blind clinical trial | |
| Participants | Setting: outpatient, primary care clinic, USA Participants: N = 162, heroin dependent. Group sizes: group 1: N = 55; group 2: N = 54; group 3: N = 53 Groups similar on sociodemographic and clinical characteristics Age range 18 to 50 years (average not reported) 71% male Average duration of heroin use > 7 years, polydrug users not excluded; 35% employed; 62% married | |
| Interventions | Group 1: clonidine 0.1‐0.2 mg every 4 h as needed days 1‐7. 50 mg naltrexone day 8 Group 2 (excluded from review): clonidine as in group 1, 12.5 mg naltrexone day 1, increasing to 50 mg day 3 Group 3: buprenorphine 3 mg sublingually (preparation not specified) days 1‐3, then clonidine as in group 1, 25 mg naltrexone day 4, 50 mg day 5 Oxazepam, ibuprofen, ketorolac, prochlorperazine as adjunct medication Daily clinic attendance except weekends. Participants referred for further treatment following trial | |
| Outcomes | Mean overall and peak withdrawal scores. Number retained in treatment for 8 days. Number achieving 50 mg maintenance dose naltrexone | |
| Notes | Withdrawal assessment primarily subjective symptoms Funding support: government grant (NIDA) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "participants were randomly assigned"; method of sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "staff and patients were blinded to the protocols". Comment: double‐blind possibly compromised by differential symptoms |
| Blinding (performance bias and detection bias) | Low risk | Quote: "staff and patients were blinded to the protocols" Comment: double‐blind possibly compromised by differential symptoms, but these outcomes unlikely to be affected by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Retention did not differ significantly among the groups" |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | Outcome definition adjusted for differences in medication duration |
| Methods | Double‐blind, double‐dummy, randomised controlled trial | |
| Participants | Setting: inpatient detoxification facility affiliated with University of Washington, USA Participants: N = 30 heroin dependent by DSM‐IV, in moderate withdrawal at time of randomisation. One participant (clonidine group) discontinued due to protocol violation − main analysis on N = 29, complete demographic data on N = 28 Group sizes: group 1: N = 10; group 2: N = 10; group 3: N = 9 Groups similar on main demographic and clinical characteristics Average age 36 63% male Average duration of heroin use 6.5 to 10.3 years; polydrug users excluded if medically supervised withdrawal required for other drug dependence; unemployed group 1 10%, group 2 50%, group 3 25% | |
| Interventions | Buprenorphine, sublingual tablet (Subutex), or placebo in two dose regimens: Group 1: high dose: 8‐8‐8‐4‐2 mg/d or Group 2: low dose: 2‐4‐8‐4‐2 mg/d on days 1‐5 Compared with group 3:clonidine, oral, 0.2‐0.3 mg four times a day for 5 days Ancillary medications available No specific behavioural therapies during withdrawal, but participants assessed for placement in outpatient treatment after completion of the study | |
| Outcomes | Number achieving suppression of withdrawal; mean withdrawal score; chlordiazepoxide use; mean hours to discharge; doses of medication (or placebo) withheld due to hypotension | |
| Notes | Withdrawal assessed by COWS, Adjective Rating Scale for Withdrawal, and Visual Analogue Craving Scale Funding support from University of Washington. Buprenorphine and placebo provided by pharmaceutical company | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned . . . by a computerised random numbers generator" |
| Allocation concealment (selection bias) | Low risk | Quote: "(allocation) by the offsite research pharmacist" |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind, double‐dummy stated |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind, double‐dummy stated |
| Incomplete outcome data (attrition bias) | Low risk | Statistical methods used to adjust for missing data |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised, controlled (open‐label) study | |
| Participants | Setting: inpatient, specialist unit, Switzerland Participants: N = 37, opioid dependent by ICD‐10, using heroin or methadone Group sizes: group 1: N = 19; group 2: N = 18 Groups similar on sociodemographics Average age 32 years 78% male Those with concurrent alcohol or benzodiazepine dependence were detoxified before entering study | |
| Interventions | Medication tailored to declared amount of heroin use and withdrawal signs: Group 1: buprenorphine, sublingual tablets, 8 mg/70 kg in 2 daily doses to max 16 mg/70 kg, reduced in 2 mg steps over average 12 days Group 2: oral methadone, 40 mg/70kg to max 60 mg/70kg, reduced in 10 mg steps to 30 mg/70kg, then 5 mg steps, over total 15 days on average | |
| Outcomes | Withdrawal severity, length of stay, completion rate | |
| Notes | Withdrawal assessed by Short Opiate Withdrawal Scale. Urine testing used Source of funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned". Insufficient information reported on characteristics of groups to form a view on adequacy of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not reported |
| Blinding (performance bias and detection bias) | High risk | No blinding |
| Blinding (performance bias and detection bias) | Low risk | These outcomes unlikely to be affected by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Dropout evenly distributed between groups |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised, open‐label, controlled trial | |
| Participants | Setting: inpatient, 2 separate specialist units, Jerusalem, Israel Participants: N = 200 heroin dependent by ICD‐10 Group sizes: N = 100 in each group Detailed demographics reported only for those who completed detoxification, but authors stated there were no significant differences in sociodemographic characteristics between completers and non‐completers within each group Majority (> 60%) in each group aged between 25 and 44 years 86% male Dependence on benzodiazepines or alcohol an exclusion criterion. 19% married, 88% unemployed | |
| Interventions | Group 1: buprenorphine (Subutex) 10 mg day 1 with extra if needed, tapered to finish day 10 Group 2: clonidine, 0.6 mg/d in divided doses, tapered from day 5 10 days detoxification followed by relapse prevention programme | |
| Outcomes | Number completing treatment. Mean adverse effects score. Adverse effects, well‐being assessed only for those who completed 10‐day detoxification | |
| Notes | Withdrawal severity not reported Study supported in part by pharmaceutical company, and by government | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "we used a random number generator" |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not reported. Groups treated in separate hospitals. |
| Blinding (performance bias and detection bias) | High risk | No blinding |
| Blinding (performance bias and detection bias) | Low risk | These outcomes unlikely to be affected by lack of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | Significantly more dropout from clonidine group. It is unclear how this difference in dropout may have affected the adverse symptoms mean score, which is the only outcome relevant to this review |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Open‐label, randomised controlled trial | |
| Participants | Setting: outpatient, specialist clinic, Leeds, UK Participants: N = 210 heroin dependent by ICD‐10. Data also reported for N = 271 who opted not to be allocated randomly and chose 1 of the 2 treatments Group sizes: group 1: N = 107; group 2: N = 103 Groups similar on baseline variables (but limited data reported) Average age 28 years 75% male | |
| Interventions | Group 1: buprenorphine, sublingual tablet, 4 mg day 1, 6‐8 mg day 2, 6 mg day 3, then tapered and ceased day 7; naltrexone offered 2 days after last buprenorphine Group 2: lofexidine, 0.4 mg 4‐hourly as needed for 4 days; co‐phenotrope, hyoscine, chlordiazepoxide, chlorpromazine as needed day 1, naltrexone 25 mg day 4 Daily clinic attendance | |
| Outcomes | Mean daily withdrawal severity, completion rate, abstinence at 1 month | |
| Notes | Withdrawal assessed by Short Opiate Withdrawal Scale. Urine screening on completion Funding from treatment service with contribution from pharmaceutical company but without input to study design, conduct, or report | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "participants were allocated randomly"; method not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "predetermined random sequence . . . held by an administrator" |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "open‐label randomised controlled trial" and "The researcher was blind to . . . the treatment regimen given" Comment: this suggests that observers, but not participants or treating staff were blind to group allocation. This introduces some risk of bias for measures completed by participants. |
| Blinding (performance bias and detection bias) | Low risk | These outcomes unlikely to be affected by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Peak withdrawal scores recorded on day 2 when missing data similar in both groups (20% and 22%). No other outcome data affected by differences in dropout rates |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | More than half eligible participants chose not to enter the trial, but data reported for these cohorts and outcomes were similar to the groups randomised |
| Methods | Randomised controlled open‐label trial | |
| Participants | Setting: inpatient detoxification ward, Hannover, Germany Participants: N = 27 methadone and/or heroin users, dependent by DSM‐IV Group sizes: group 1: N = 15; group 2: N = 12 Groups similar on sociodemographics and drug use history Average age 31 years 89% male Average duration opioid use 11.9 ± 5.4 years (group 1); 8.7 ± 5.8 years (group 2). All polydrug users. 1st inpatient detoxification for around half of the participants. | |
| Interventions | Group 1: buprenorphine, 3 mg/d for 7 days, then tapered and ceased day 11 Group 2: oxazepam, 90 mg/d for 7 days then tapered and ceased day 15 Both groups received carbamazepine, 900 mg/d for 7 days, then tapered and ceased day 20. Total 21‐day treatment | |
| Outcomes | Graph of withdrawal scores. Number giving 'drug not effective' as reason for dropout. Number NOT completing treatment | |
| Notes | Withdrawal assessed using Short Opiate Withdrawal Scale. Urine screening used Source of funding: Government grant. Buprenorphine provided by pharmaceutical company | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned"; method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not reported |
| Blinding (performance bias and detection bias) | High risk | Open‐label stated (authors suggest that medication effects would have revealed group allocation) |
| Blinding (performance bias and detection bias) | Low risk | These outcomes unlikely to be affected by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "the difference in the dropout rate between the two treatment strategies was not significant." |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Double‐dummy randomised controlled trial | |
| Participants | Setting: inpatient treatment in ward for drug and alcohol abuse, Hannover, Germany Participants: N = 26, opioid dependent (methadone or heroin) by DSM‐IV Group sizes: group 1: 14; group 2: 12 Groups similar on demographics and drug use history, except 6/14 in group 1 and 10/12 in group 2 abused cannabis in addition to opioids Average age 32 years 85% male Average duration of opioid abuse about 10 years First inpatient detoxification for 19%. All polydrug users | |
| Interventions | All given 25 mg methadone, 400 mg carbamazepine on admission then: Group 1: buprenorphine, 4 mg/d for 3 days then tapered to cease day 10, or Group 2: L‐methadone, 20 mg day 1, tapered to cease day 10 All received carbamazepine, 900 mg/d for 6 days, then tapered to cease day 14 | |
| Outcomes | Withdrawal severity, completion rate | |
| Notes | Withdrawal assessed by Short Opiate Withdrawal Scale. Observed urine samples weekly Source of funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned"; method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "double‐dummy, parallel group study design" "Buprenorphine placebo was obtained from the pharmacy" Comment: it is unclear whether participants, treating personnel and observers were all blind to group allocation |
| Blinding (performance bias and detection bias) | Low risk | These outcomes unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "the difference in the dropout rate was not significant" |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised controlled trial, double‐blind, double dummy, with stabilisation dose, abstinence during stabilisation, cocaine use, gender, alcohol dependence, and current pain as stratification variables | |
| Participants | Setting: outpatient, research clinic, Vermont, USA Participants: N = 70, opioid dependent by DSM‐IV Group sizes: group 1: N = 24; group 2: N = 24; group 3: N = 22 Baseline characteristics of groups similar Average age 27 years 69% male Average 4.9 years of opioid abuse; primary drug of abuse oxycodone for 57%, buprenorphine for 39%; 16% used primary by intravenous injection 94% white, 63% employed full‐time, 24% ever married | |
| Interventions | Stabilised on buprenorphine/naloxone for 2 weeks, then dose tapered: Group 1: over 1 week Group 2: over 2 weeks Group 3: over 4 weeks Participants received 5.5 sublingual tablets at each visit containing a combination of either active/placebo buprenorphine so participants and staff were blind to dose, taper and commencement of naltrexone At completion of buprenorphine taper, all groups were commenced on naltrexone, 12.5 mg day 1, 25 mg days 2 and 3, 50 mg day 4, then 50 mg/d All participants received behavioural therapy based on Community Reinforcement Approach Participants visited the clinic daily in weeks 1‐5, then 3 times a week during weeks 6‐12 | |
| Outcomes | Participants abstinent from opioids, retention in treatment, participants receiving naltrexone, withdrawal scores | |
| Notes | Urine samples were collected three times a week. Withdrawal and craving were assessed by visual analogue scales, and the CINA Source of funding government grant (NIDA); buprenorphine and placebo provided by manufacturer through NIDA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants . . . were then randomly assigned . . . Stratification variables included stabilization dose, illicit opioid abstinence during stabilization, past‐month cocaine use, sex, current alcohol dependence,and current chronic pain not reported." Comment: method of sequence generation not reported, but computerisation is likely given the number of stratification variables used |
| Allocation concealment (selection bias) | Low risk | Quote: "Doses were prepared by the hospital’s investigational pharmacy". Comment: although method of allocation concealment not specifically reported, the preparation of identical medications by the hospital pharmacy makes it likely that concealment was adequate |
| Blinding (performance bias and detection bias) | Low risk | Study stated as double‐blind with placebos used so that "neither the participants nor the staff knew the doses received, the duration of taper and the point at which naltrexone therapy began." |
| Blinding (performance bias and detection bias) | Low risk | Study double‐blind and double‐dummy |
| Incomplete outcome data (attrition bias) | High risk | Analysis of withdrawal severity based on a subset of participants (n = 28, 40% of all participants) who were verified to be abstinent from opioids while the buprenorphine dose was being tapered |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised controlled trial | |
| Participants | Setting: inpatient, detoxification unit, Wasserburg, Germany Participants: N = 60 Group sizes: group 1: N = 30; group 2: N = 30 Groups similar on demographics and drug use at baseline Average age 27 years 83% male, duration of heroin use about 7 years | |
| Interventions | Group 1: methadone, 60 mg/d, tapered 2.5‐5 mg/d. Group 2: buprenorphine, 12‐16 mg/d, tapered 0.8‐1.2 mg/d. Study duration 28 days | |
| Outcomes | Craving, withdrawal, use of additional medication, number completing treatment | |
| Notes | Withdrawal assessed with COWS and tailored instrument (24 items) Source of funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned"; method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not reported |
| Blinding (performance bias and detection bias) | High risk | "Due to technical reasons" there was no blinding |
| Blinding (performance bias and detection bias) | Low risk | These outcomes unlikely to be affected by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "The two groups of patients did not differ . . . in the rate at which they successfully completed the trial." |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised, double‐blind clinical trial, with stratification on withdrawal severity, pain, CD4 T‐cell count | |
| Participants | Setting: inpatient, AIDS service, USA Participants: N = 55, HIV positive, opioid‐dependent by self‐report and physical exam, hospitalised for acute medical illness Group sizes: group 1: N = 21; group 2: N = 16; group 3. N = 18 Those in group 1 more likely to have been admitted for fever/cellulitis. Groups otherwise similar Average age 40 years 62% male Duration of heroin use around 18 years. Concurrent alcohol dependence, enrolment in MMT exclusion criteria. | |
| Interventions | All stabilised with morphine 10 mg i.m. every 4 h as needed up to 6 h prior to enrolment in study then 3 day taper with: Group 1: buprenorphine 0.6 mg i.m. every 4 h day 1, every 6 h day 2, every 8 h day 3, Group 2: oral clonidine, 0.2 mg loading dose, 0.1 mg every 4 h day 1, every 6 h day 2, every 8 h day 3, or Group 3: oral methadone, 30 mg day 1, 20 mg day 2, 10 mg day 3. All received clonidine transdermal patch day 4 No adjunct treatment for withdrawal | |
| Outcomes | Withdrawal severity, completion rate, adverse effects, use of supplemental morphine for pain | |
| Notes | Withdrawal assessed by Short Opiate Withdrawal Scale (participants) and Objective Opiate Withdrawal Scale (observers) Source of funding: government grant (NIDA) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients . . . were randomly assigned"; "patients were stratified on four characteristics". Method of sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "To maintain the blind, one active medication and two inactive medications were administered to all participants." Comment: It remains unclear whether participants, treating personnel and observers were all blind to group allocation |
| Blinding (performance bias and detection bias) | Low risk | These outcomes unlikely to be affected by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) | Low risk | Statistical methods allowed for missing data and variation in time of assessment |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Double‐blind controlled trial, with 14/23 participants randomly allocated | |
| Participants | Setting: not stated − probably outpatient, Milwaukee, USA Participants: N = 23 who had completed maintenance phase of preliminary trial of buprenorphine Group sizes: group 1: N = 14 (N = 7 randomly allocated); group 2: N = 7 Note: this review only used data for the participants who were randomly allocated Participant characteristics not reported (conference abstract only information available) | |
| Interventions | Buprenorphine 8 mg/d, tapered: Group 1: rapidly (14 days) Group 2: slowly (8 weeks) Placebo to total 12 weeks | |
| Outcomes | Descriptive comments on withdrawal; completion rate | |
| Notes | Withdrawal rating scale not used. Urine screening not reported Source of funding: government grant (NIDA) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The first 14 (patients) were randomized into two groups" Comment: method of allocation not reported. Data reported separately for the 14 participants who were allocated randomly, and the remaining 9 participants who were all allocated to group 1. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not reported |
| Blinding (performance bias and detection bias) | High risk | Double‐blind only for those allocated randomly |
| Blinding (performance bias and detection bias) | Low risk | These outcomes unlikely to be affected by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Completion of withdrawal treatment is the only outcome reported |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Unclear risk | Unable to assess (conference abstract only report available) |
| Methods | Randomised, open‐label, controlled trial | |
| Participants | Setting: healthcare services in high, but not maximum, security prisons (2 male, 1 female), England Participants: prisoners using illicit opioids, wanting to detoxify and remain abstinent, in custody at least 28 days Group sizes: group 1: N = 148; group 2: N = 141 Groups similar on baseline characteristics Median age 31 years Median duration of opioid use 10 years; 53% used primarily by injection | |
| Interventions | Group 1: buprenorphine, sublingual (probably tablets) starting dose 8 mg, tapered to 0.4 mg at day 20 Group 2: methadone (oral, 1 mg/1 ml mixture), starting dose 30 mg, tapered to 2 mg (day 20) | |
| Outcomes | Opioid abstinence at 8 days, 1, 3 and 6 months after detoxification. Urinalysis for opioid abstinence at 8 days postdetoxification. Factors predictive of abstinence | |
| Notes | Withdrawal severity not assessed Source of funding: government grant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation sequence (with random block size) was generated using Microsoft |
| Allocation concealment (selection bias) | Low risk | Quote: "Sealed, opaque, consecutively numbered envelopes concealing the name of the allocated intervention were prepared by a researcher who had no contact with participants." |
| Blinding (performance bias and detection bias) | Low risk | Study undertaken open‐label because "blinded study would have necessitated commercial funding to develop dummy preparations . . . [and] there is also potential bias in study findings where there is a commercial funder". Because it was open‐label, subjective outcomes were not used. |
| Blinding (performance bias and detection bias) | Low risk | Study undertaken open‐label, but these outcomes at less risk of bias due to awareness of treatment allocation |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up at primary outcome (abstinence at 8 days postdetoxification) similar for two groups: group 1: 24%; group 2: 29% |
| Selective reporting (reporting bias) | Unclear risk | Data collected on adverse effects but not reported |
| Other bias | Low risk | None apparent |
| Methods | Randomised, double‐blind, controlled trial | |
| Participants | Setting: inpatient psychiatric hospital, Kerman, Iran Participants: opioid dependent by DSM‐IV, randomly selected from individuals referred for detoxification Group sizes: group 1: N = 14; group 2: N = 21 Employed: 42.9% (group 1); 71.4% (group 2); groups otherwise similar Average age 25 years All male 34% married | |
| Interventions | Group 1: buprenorphine, sublingual tablet (plus clonidine placebo), 2 mg day 1, 4 mg day 2, 6 mg day 3, 4 mg day 4, 2 mg day 5 with additional 2‐4 mg if required for severe withdrawal Group 2: clonidine, oral tablets, 0.4 mg day 1, 0.6 mg days 2 and 3, 0.2 mg days 4 and 5, plus additional 0.2‐0.4 mg/d if required for severe withdrawal Actual doses administered not reported | |
| Outcomes | Average change in withdrawal and craving scores on days 1, 2, 3, and 5. Total days in treatment and days receiving naltrexone at follow‐up | |
| Notes | Withdrawal rated by 'psychiatric technician' with COWS (11 items rated 0‐4), and by participants with ARWS (Adjective Rating Withdrawal Scale, 16 items rated 0‐9). Craving assessed with visual analogue scale Funding source intramural | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "participants were randomly allocated to . . . detoxification group." Method of sequence generation not explained |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind stated, and placebos used to maintain blind. Quote: "All drugs and placebos were assigned a code and kept by a person who was not involved in the study" |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All patients took naltrexone at the end of the detoxification period" Comment: this review focuses on the detoxification period, and if all participants completed detoxification there should be no incomplete data |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
COWS: Clinical Opiate Withdrawal Scale; DSM‐IV: Diagnostic and Statistical Manual of Mental Health ‐ 4th edition; DSM‐III‐R: Diagnostic and Statistical Manual of Mental Health ‐ 3rd edition, Revised; i.m.: intramuscular; i.v.: intravenous; MMT: methadone maintenance treatment; OOWS: Objective Opiate Withdrawal Scale; SOWS: Subjective Opiate Withdrawal Scale.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Randomised controlled trial comparing effect of 2 different periods of extended buprenorphine prescription following inpatient detoxification. No data on detoxification outcomes defined for this review. Not primarily about withdrawal management | |
| Comparison of 3 different regimens for transferring participants from methadone to buprenorphine, with subsequent tapering of buprenorphine dose. No comparison during withdrawal phase and insufficient data on outcomes of this phase. | |
| Randomised controlled trial comparing buprenorphine/naloxone for withdrawal management and substitution treatment. Treatment comparison is not one of the modalities included in the review | |
| Clonidine or buprenorphine offered consecutively for management of opioid withdrawal. Not randomised controlled trial | |
| Investigation of buprenorphine as an adjunct to antagonist‐induced withdrawal. The groups differed only in the timing of naltrexone administration. Treatment comparison not one defined by inclusion criteria | |
| Randomised controlled trial comparing 2 different dose regimens of buprenorphine for management of opioid withdrawal. Groups combined for analysis of postdetoxification outcomes. No data on detoxification phase | |
| Comparison of outcomes of opioid withdrawal managed with buprenorphine in specialist clinic or primary health setting. Treatment comparison not defined by inclusion criteria | |
| Randomised controlled trial comparing memantine and placebo as adjuncts to buprenorphine for management of opioid withdrawal. Not one of the comparisons specified for this review | |
| Treatment comparison (WeiniCom, a Chinese herbal preparation with complex pharmacology) is not one of the modalities defined by the inclusion criteria. | |
| Comparison of buprenorphine and lofexidine for management of opioid withdrawal. Not a controlled study − partly retrospective; insufficient outcome data (conference abstract only) | |
| Controlled study comparing buprenorphine plus naloxone from day 5, with tincture of opium. Unclear whether group allocation was random. Treatment comparison (tincture of opium) is not one of the modalities defined by the inclusion criteria. | |
| Comparison of memantine and placebo in conjunction with buprenorphine in terms of capacity to ameliorate withdrawal induced with naloxone. Study more about pharmacology of memantine than management of withdrawal | |
| Randomised controlled trial comparing methadone followed by buprenorphine versus buprenorphine alone for managing opioid withdrawal. Primary treatment approach unclear − it appears that some participants in buprenorphine only group received methadone during first few days of treatment. Comparison is not one specified for this review | |
| Preliminary report of trial comparing methadone and buprenorphine for the treatment of opioid dependence. Insufficient outcome data | |
| Comparison of buprenorphine and clonidine for management of opioid withdrawal. Insufficient data − conference abstract only. Probably preliminary report of Cheskin 1994 | |
| Randomised controlled trial comparing buprenorphine and methadone (60 or 20 mg/d) for 17 weeks of maintenance followed by 8 weeks withdrawal. Comparison not one specified for this review. Focus on maintenance rather than management of withdrawal | |
| Study of withdrawal symptoms following abrupt cessation of methadone or buprenorphine. Focus on pharmacological properties rather than management of withdrawal. Insufficient data − conference abstract only | |
| Comparison of brief (5‐day) and extended (30‐day) buprenorphine treatment for outpatient management of opioid withdrawal. Data drawn from 2 previous studies comparing counselling approaches as adjuncts to buprenorphine. Not randomised controlled trial | |
| Open label study comparing 3 different dose regimens of buprenorphine in terms of illicit opiate use and withdrawal symptoms during 30 days at a fixed dose. Buprenorphine abruptly discontinued after 30 days but no data reported on this withdrawal phase. Study more about substitution treatment than management of withdrawal | |
| Reports use of buprenorphine for 30 days as transition between methadone or heroin and naltrexone, with comparison of reactions to naloxone and naltrexone. No treatment comparison for withdrawal phase | |
| Participants stabilised on methadone or buprenorphine then detoxified by combination of tapered methadone plus lofexidine, or tapered buprenorphine. Insufficient data (conference abstract, preliminary report) | |
| Randomised controlled trial comparing 5‐day buprenorphine detoxification with stabilisation on buprenorphine in hospital and linkage to outpatient opioid substitution treatment. Comparison not one specified for this review. | |
| Investigation of doses of buprenorphine required to suppress opioid withdrawal symptoms. No treatment comparison | |
| Comparison of different dose regimens of buprenorphine, with starting dose determined by duration and dose of heroin use and severity of dependence. Not a randomised controlled trial | |
| Randomised controlled trial comparing DHEA and placebo as adjuncts to buprenorphine for management of opioid withdrawal. Analysis largely in terms of factors affecting response to DHEA treatment. Insufficient outcome data, and not primarily a study of buprenorphine | |
| Randomised controlled trial comparing active and sham transcutaneous electric acupoint stimulation (TEAS) as an adjunct to inpatient detoxification managed with buprenorphine. Focus of study on drug use following detoxification. Insufficient data on withdrawal episode | |
| Comparison of naltrexone‐buprenorphine combination with buprenorphine only for managing opioid withdrawal. Investigation of pharmacology of buprenorphine and naltrexone rather than management of withdrawal | |
| Medication selection based on judgement of treating clinician. Not randomised controlled trial. Focus on sleep parameters − insufficient data on outcomes defined for this review | |
| Comparison of 2 rates of reduction of buprenorphine dose for management of opioid withdrawal. Only information available is conference abstract − contact author advised study was never fully written up. Insufficient information on medications and characteristics of patients, insufficient outcome data | |
| Participants able to choose buprenorphine or methadone for management of opioid withdrawal | |
| Randomised controlled trial comparing buprenorphine detoxification and continuation of buprenorphine substitution treatment for opioid dependence. Comparison not one of the modalities defined by the inclusion criteria | |
| Participants maintained on buprenorphine for 30 days then given challenges with naltrexone or naloxone as process of easing transition to naltrexone maintenance treatment. No treatment comparison | |
| Randomised controlled trial comparing active buprenorphine/naloxone implants with placebo implants and with sublingual buprenorphine/naloxone tablets. Opiate withdrawal and craving were assessed in the context of initiation of substitution (maintenance) treatment rather than managing withdrawal | |
| Randomised controlled trial comparing gabapentin and placebo as adjuncts to buprenorphine for treatment of opioid withdrawal. Intervention is not one defined by the inclusion criteria for this review | |
| Comparison of buprenorphine and dihydrocodeine for management of opioid withdrawal in prison setting. Comparison not one of the modalities defined by inclusion criteria | |
| Randomised placebo‐controlled trial of depot preparation of buprenorphine. Focus is on effectiveness in providing opioid blockade and suppression of withdrawal, but not full withdrawal intervention | |
| Randomised controlled trial comparing 2 different buprenorphine regimens to manage induction onto naltrexone treatment. Intervention not one defined by inclusion criteria for this review. Preliminary report (conference abstract) − insufficient outcome data | |
| Cross‐over study assessing withdrawal syndrome following cessation of morphine or buprenorphine. No intervention to manage withdrawal | |
| Comparison of buprenorphine‐naltrexone combination with buprenorphine followed by naltrexone for management of opioid withdrawal. Treatment comparison not one of the modalities defined by the inclusion criteria | |
| Randomised controlled trial comparing adjunct therapies with phases of buprenorphine maintenance and dose taper. Comparison not one defined by the inclusion criteria for this review | |
| Comparison of buprenorphine and lofexidine for management of opioid withdrawal. Mixed retrospective and prospective data collection − not a controlled study | |
| Reports the use of buprenorphine for management of opioid withdrawal in an outpatient setting. No treatment comparison | |
| Comparison of 12 week buprenorphine maintenance and 14‐day detox in terms of positive urine samples and retention in treatment for opioid dependence. Comparison is not one of the modalities defined by the inclusion criteria | |
| Randomised controlled trial comparing buprenorphine and dihydrocodeine for management of opioid withdrawal in primary care setting. Comparison not one of the modalities defined by the inclusion criteria. | |
| Comparison of buprenorphine and methadone for management of opioid withdrawal. Insufficient information on treatment regimens and outcomes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean days in treatment Show forest plot | 2 | 82 | Mean Difference (IV, Random, 95% CI) | 1.30 [‐8.11, 10.72] |
| Analysis 1.1  Comparison 1 Buprenorphine versus methadone, Outcome 1 Mean days in treatment. | ||||
| 2 Completion of withdrawal treatment Show forest plot | 5 | 457 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.91, 1.20] |
| Analysis 1.2  Comparison 1 Buprenorphine versus methadone, Outcome 2 Completion of withdrawal treatment. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean peak withdrawal score Show forest plot | 6 | 521 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.74, ‐0.13] |
| Analysis 2.1  Comparison 2 Buprenorphine versus clonidine, Outcome 1 Mean peak withdrawal score. | ||||
| 2 Mean overall withdrawal score Show forest plot | 7 | 902 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.58, ‐0.28] |
| Analysis 2.2  Comparison 2 Buprenorphine versus clonidine, Outcome 2 Mean overall withdrawal score. | ||||
| 3 Mean days in treatment Show forest plot | 4 | 558 | Std. Mean Difference (IV, Random, 95% CI) | 0.92 [0.57, 1.27] |
| Analysis 2.3  Comparison 2 Buprenorphine versus clonidine, Outcome 3 Mean days in treatment. | ||||
| 3.1 Inpatient setting | 3 | 213 | Std. Mean Difference (IV, Random, 95% CI) | 1.00 [0.33, 1.67] |
| 3.2 Outpatient setting | 2 | 345 | Std. Mean Difference (IV, Random, 95% CI) | 0.82 [0.57, 1.06] |
| 4 Number experiencing adverse effects Show forest plot | 3 | 493 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.70, 1.26] |
| Analysis 2.4  Comparison 2 Buprenorphine versus clonidine, Outcome 4 Number experiencing adverse effects. | ||||
| 4.1 Inpatient | 2 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.12, 1.36] |
| 4.2 Outpatient | 2 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.66, 1.58] |
| 5 Number with treatment stopped due to adverse effects Show forest plot | 3 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.04, 1.15] |
| Analysis 2.5  Comparison 2 Buprenorphine versus clonidine, Outcome 5 Number with treatment stopped due to adverse effects. | ||||
| 5.1 Inpatient | 3 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.04, 1.15] |
| 5.2 Outpatient | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Number completing withdrawal treatment Show forest plot | 11 | 1264 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [1.23, 2.06] |
| Analysis 2.6  Comparison 2 Buprenorphine versus clonidine, Outcome 6 Number completing withdrawal treatment. | ||||
| 6.1 Inpatient | 6 | 539 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [1.05, 2.89] |
| 6.2 Outpatient | 6 | 725 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.12, 1.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Completion of withdrawal treatment Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Rapid versus slow dose taper, Outcome 1 Completion of withdrawal treatment. | ||||
| 1.1 Inpatient | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.84, 1.18] |
| 1.2 Outpatient | 4 | 647 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.44, 1.70] |
| 2 Number abstinent at completion of dose taper Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 Rapid versus slow dose taper, Outcome 2 Number abstinent at completion of dose taper. | ||||
| 2.1 Inpatient | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.81, 1.23] |
| 2.2 Outpatient | 4 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.39, 2.24] |

Study flow diagram.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Comparison 1 Buprenorphine versus methadone, Outcome 1 Mean days in treatment.
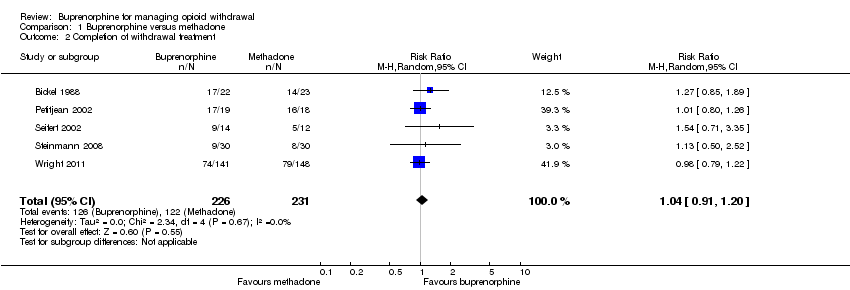
Comparison 1 Buprenorphine versus methadone, Outcome 2 Completion of withdrawal treatment.

Comparison 2 Buprenorphine versus clonidine, Outcome 1 Mean peak withdrawal score.

Comparison 2 Buprenorphine versus clonidine, Outcome 2 Mean overall withdrawal score.

Comparison 2 Buprenorphine versus clonidine, Outcome 3 Mean days in treatment.
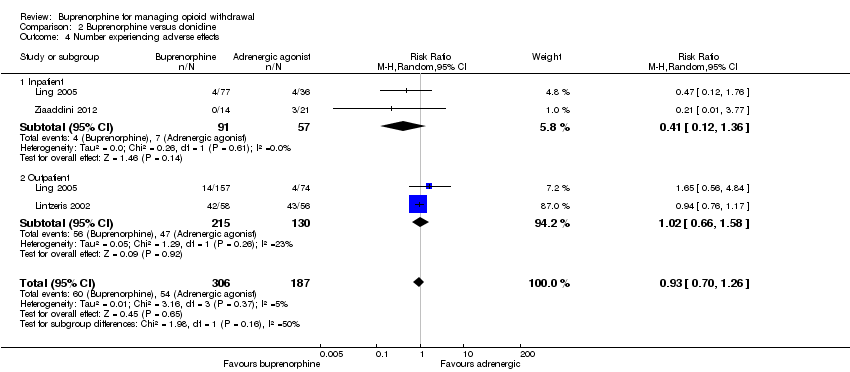
Comparison 2 Buprenorphine versus clonidine, Outcome 4 Number experiencing adverse effects.
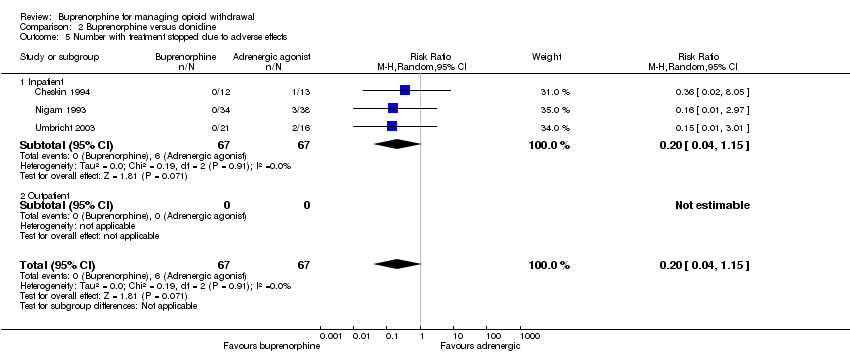
Comparison 2 Buprenorphine versus clonidine, Outcome 5 Number with treatment stopped due to adverse effects.

Comparison 2 Buprenorphine versus clonidine, Outcome 6 Number completing withdrawal treatment.

Comparison 3 Rapid versus slow dose taper, Outcome 1 Completion of withdrawal treatment.

Comparison 3 Rapid versus slow dose taper, Outcome 2 Number abstinent at completion of dose taper.
| Buprenorphine versus methadone for managing opioid withdrawal | |||||
| Patient or population: Adults with opioid dependence | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | |
| Risk with methadone | Risk with buprenorphine | ||||
| Mean days in treatment | The mean days in treatment in the methadone group was 10.8 or 48.5 days. | The mean days in treatment in the buprenorphine group was 1.3 more (8.1 less to 10.7 more) than in the methadone group. | — | 82 | ⊕⊕⊝⊝ |
| Completion of withdrawal treatment | Study population | RR 1.04 | 457 | ⊕⊕⊕⊝ | |
| 528 per 1000 | 549 per 1000 | ||||
| Moderate | |||||
| 534 per 1000 | 555 per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aStudies were conducted in different settings (inpatient and outpatient). | |||||
| Buprenorphine vs alpha2‐adrenergic agonists for managing opioid withdrawal | |||||
| Patient or population: Adults or adolescents with opioid dependence | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | |
| Risk with alpha2‐adrenergic agonists | Risk with buprenorphine | ||||
| Mean peak withdrawal score, assessed with various scales | Not able to be summarised due to different means of assessment | The mean peak withdrawal score was lower in the buprenorphine group, indicating less severity, with a standardised mean difference of 0.43, which is a moderate effect size | SMD ‐0.43 (‐0.74 to ‐0.13) | 521 | ⊕⊝⊝⊝ |
| Mean overall withdrawal score, with various methods of assessment | Not able to be summarised due to different means of assessment | The mean overall withdrawal score was lower in the buprenorphine group, indicating less severity, with a standardised mean difference of 0.43, which is a moderate effect size | SMD ‐0.43 (‐0.58 to ‐0.28) | 902 | ⊕⊕⊕⊝ |
| Mean days in treatment (with varying scheduled duration) | Mean days in treatment ranged across studies from 21% to 70% of scheduled treatment duration | Mean days in treatment ranged across studies from 25% to 97% of scheduled treatment duration, with a standardised mean difference of 0.92, which is a large effect size. | SMD 0.92 (0.57 to 1.27) | 558 | ⊕⊕⊕⊝ |
| Number experiencing adverse effects | Study population | RR 0.93 | 493 | ⊕⊝⊝⊝ | |
| 289 per 1000 | 269 per 1000 | ||||
| Moderate | |||||
| 127 per 1000 | 118 per 1000 | ||||
| Number with treatment stopped due to adverse effects | Study population | RR 0.20 | 134 | ⊕⊕⊝⊝ | |
| 90 per 1000 | 18 per 1000 | ||||
| Moderate | |||||
| 79 per 1000 | 16 per 1000 | ||||
| Number completing withdrawal treatment | Study population | RR 1.59 | 1264 | ⊕⊕⊕⊝ | |
| 453 per 1000 | 721 per 1000 | ||||
| Moderate | |||||
| 536 per 1000 | 852 per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aRisk of bias in blinding of subjective outcomes. | |||||
| Rapid versus slow taper of buprenorphine dose for managing opioid withdrawal | |||||
| Patient or population: Adults or adolescents with opioid dependence | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | |
| Risk with slow dose taper | Risk with rapid taper | ||||
| Completion of withdrawal treatment − Inpatient | Study population | RR 1.00 | 60 | ⊕⊕⊝⊝ | |
| 900 per 1000 | 900 per 1000 | ||||
| Moderate | |||||
| 900 per 1000 | 900 per 1000 | ||||
| Completion of withdrawal treatment − Outpatient | Study population | RR 0.86 | 647 | ⊕⊝⊝⊝ | |
| 594 per 1000 | 511 per 1000 | ||||
| Moderate | |||||
| 636 per 1000 | 547 per 1000 | ||||
| Number abstinent at completion of dose taper − Inpatient | Study population | RR 1.00 | 40 | ⊕⊕⊝⊝ | |
| 900 per 1000 | 900 per 1000 | ||||
| Moderate | |||||
| 900 per 1000 | 900 per 1000 | ||||
| Number abstinent at completion of dose taper − Outpatient | Study population | RR 0.94 | 619 | ⊕⊝⊝⊝ | |
| 320 per 1000 | 301 per 1000 | ||||
| Moderate | |||||
| 337 per 1000 | 317 per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aSmall number of events (n < 300). | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean days in treatment Show forest plot | 2 | 82 | Mean Difference (IV, Random, 95% CI) | 1.30 [‐8.11, 10.72] |
| 2 Completion of withdrawal treatment Show forest plot | 5 | 457 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.91, 1.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean peak withdrawal score Show forest plot | 6 | 521 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.74, ‐0.13] |
| 2 Mean overall withdrawal score Show forest plot | 7 | 902 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.58, ‐0.28] |
| 3 Mean days in treatment Show forest plot | 4 | 558 | Std. Mean Difference (IV, Random, 95% CI) | 0.92 [0.57, 1.27] |
| 3.1 Inpatient setting | 3 | 213 | Std. Mean Difference (IV, Random, 95% CI) | 1.00 [0.33, 1.67] |
| 3.2 Outpatient setting | 2 | 345 | Std. Mean Difference (IV, Random, 95% CI) | 0.82 [0.57, 1.06] |
| 4 Number experiencing adverse effects Show forest plot | 3 | 493 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.70, 1.26] |
| 4.1 Inpatient | 2 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.12, 1.36] |
| 4.2 Outpatient | 2 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.66, 1.58] |
| 5 Number with treatment stopped due to adverse effects Show forest plot | 3 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.04, 1.15] |
| 5.1 Inpatient | 3 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.04, 1.15] |
| 5.2 Outpatient | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Number completing withdrawal treatment Show forest plot | 11 | 1264 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [1.23, 2.06] |
| 6.1 Inpatient | 6 | 539 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [1.05, 2.89] |
| 6.2 Outpatient | 6 | 725 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.12, 1.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Completion of withdrawal treatment Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Inpatient | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.84, 1.18] |
| 1.2 Outpatient | 4 | 647 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.44, 1.70] |
| 2 Number abstinent at completion of dose taper Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Inpatient | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.81, 1.23] |
| 2.2 Outpatient | 4 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.39, 2.24] |

