Buprenorfina para el tratamiento de la abstinencia de opiáceos
Resumen
Antecedentes
La abstinencia controlada es un paso necesario antes del tratamiento sin fármacos o puede representar la variable de evaluación de un tratamiento de sustitución.
Objetivos
Evaluar los efectos de la buprenorfina versus dosis disminuidas gradualmente de metadona, agonistas alfa2adrenérgicos, fármacos sintomáticos o placebo, o diferentes regímenes de buprenorfina para controlar la abstinencia de opiáceos, en cuanto a la intensidad del síndrome de abstinencia experimentado, la duración y la finalización del tratamiento y los efectos adversos.
Métodos de búsqueda
Se hicieron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL, número 11, 2016), MEDLINE (1946 hasta diciembre, semana 1, 2016), Embase (hasta 22 diciembre 2016), PsycINFO (1806 hasta diciembre, semana 3, 2016), y en la Web of Science (hasta 22 diciembre 2016) y se hicieron búsquedas manuales en las listas de referencias de artículos.
Criterios de selección
Ensayos controlados aleatorios de intervenciones que utilizaron buprenorfina para modificar los signos y síntomas de abstinencia en participantes que eran principalmente dependientes de opiáceos. Las intervenciones de comparación incluían la reducción de las dosis de metadona, agonistas alfa2adrenérgicos (clonidina o lofexidina), fármacos sintomáticos o placebo, y diferentes regímenes basados en buprenorfina.
Obtención y análisis de los datos
Se utilizaron los procedimientos metodológicos estándar previstos por la Colaboración Cochrane.
Resultados principales
Se incluyeron 27 estudios con 3048 participantes. Los comparadores principales fueron clonidina o lofexidina (14 estudios). Seis estudios compararon buprenorfina versus metadona, y siete compararon tasas diferentes de reducción de la dosis de buprenorfina. Se evaluaron 12 estudios como en alto riesgo de sesgo en al menos uno de siete dominios de la calidad metodológica. Seis de estos estudios compararon buprenorfina con clonidina o lofexidina y dos con metadona; los otros cuatro estudios compararon diferentes tasas de reducción de la dosis de buprenorfina.
Para la comparación de la buprenorfina y la metadona en dosis disminuidas gradualmente, no fue posible realizar el metanálisis para los resultados de la intensidad de la abstinencia ni los efectos adversos. Sin embargo, la información revelada por los estudios individuales sugirió que la buprenorfina y la metadona tuvieron una capacidad similar para mejorar la abstinencia de opiáceos, sin efectos adversos clínicamente significativos. Los metanálisis que fueron posibles apoyaron la conclusión de ninguna diferencia entre la buprenorfina y la metadona en cuanto a la duración promedio del tratamiento (diferencia de medias [DM] 1,30 días, intervalo de confianza [IC] del 95%: ‐8,11 a 10,72; N = 82; estudios = 2; baja calidad) o las tasas de finalización del tratamiento (cociente de riesgos [CR] 1,04; IC del 95%: 0,91 a 1,20; N = 457; estudios = 5; calidad moderada).
En relación con la clonidina o la lofexidina, la buprenorfina se asoció con una puntuación promedio inferior de la abstinencia (que indicó una abstinencia menos grave) durante el episodio del tratamiento, con un tamaño del efecto que se considera pequeño a moderado (diferencia de medias estandarizada [DME] ‐0,43; IC del 95%: ‐0,58 a ‐0,28; N = 902; estudios = 7; calidad moderada). El tratamiento en los pacientes que recibieron buprenorfina tuvo una duración mayor, con un tamaño del efecto que se considera grande (DME 0,92; IC del 95%: 0,57 a 1,27; N = 558; estudios = 5; calidad moderada) y los mismos tuvieron una mayor probabilidad de completar el tratamiento para la abstinencia (CR 1,59; IC del 95%: 1,23 a 2,06; N = 1264; estudios = 12; calidad moderada). Al mismo tiempo no hubo diferencias significativas en la incidencia de efectos adversos, aunque los abandonos debido a los efectos adversos pueden ser más probables con clonidina (CR 0,20; IC del 95%: 0,04 a 1,15; N = 134; estudios = 3; baja calidad). La diferencia en las tasas de finalización del tratamiento se traduce a un número necesario a tratar para un resultado beneficioso adicional de 4 (IC del 95%: 3 a 6), que indica que por cada cuatro pacientes tratados con buprenorfina, es posible esperar que un paciente adicional finalice el tratamiento que con clonidina o lofexidina.
Para los estudios que comparan diferentes tasas de reducción de la dosis de buprenorfina, el metanálisis fue posible sólo para la finalización del tratamiento, con análisis por separado para los contextos hospitalarios y ambulatorios. Los resultados fueron diversos, y se evaluó la calidad de las pruebas como muy baja. Aún no se conoce qué efecto tiene la tasa de la reducción gradual de la dosis en el resultado del tratamiento.
Conclusiones de los autores
La buprenorfina es más efectiva que la clonidina o la lofexidina para controlar la abstinencia de opiáceos en cuanto a la gravedad de la abstinencia, la duración del tratamiento para la abstinencia y la probabilidad de finalización del tratamiento.
La buprenorfina y la metadona parecen ser igualmente efectivas, aunque los datos son limitados. Sigue siendo posible que el modelo de abstinencia experimentado pueda diferir y que los síntomas de abstinencia puedan resolverse más rápidamente con buprenorfina.
No es posible establecer conclusiones a partir de las pruebas disponibles sobre la efectividad relativa de las diferentes tasas de disminución gradual de la dosis de buprenorfina. Los resultados divergentes de los estudios incluidos en esta revisión indican que puede haber factores múltiples que afectan la respuesta a la tasa de reducción gradual de la dosis. Uno de dichos factores podría ser si el plan de tratamiento inicial incluye una transición al tratamiento de prevención de la recaída posterior con naltrexona. En efecto, la administración de buprenorfina para apoyar la transición al tratamiento con naltrexona es un aspecto digno de investigación adicional.
La mayoría de los participantes en los estudios incluidos en esta revisión fueron hombres. Ninguno de los estudios informó los resultados sobre la base del sexo, lo cual impide la exploración de las diferencias relacionadas con esta variable. La investigación que considere el sexo como un factor que influye en la respuesta al tratamiento para la abstinencia sería relevante para seleccionar el tipo más apropiado de intervención para cada individuo.
PICO
Resumen en términos sencillos
Buprenorfina para el tratamiento de la abstinencia de opiáceos
Pregunta de la revisión
Se examinaron las pruebas acerca del efecto de la buprenorfina para tratar la abstinencia en los pacientes que dependen de fármacos opiáceos (p.ej. heroína u opiáceos farmacéuticos).
Antecedentes
La abstinencia controlada, o desintoxicación, es un primer paso necesario para los tratamientos a largo plazo de la dependencia de opiáceos. La combinación de síntomas incómodos y la necesidad imperiosa e intensa dificulta el cumplimiento de la abstinencia de opiáceos en la mayoría de los pacientes. La buprenorfina es uno de los fármacos usados para tratar la abstinencia de los fármacos opiáceos. Esta revisión consideró si la buprenorfina es más efectiva que la metadona en dosis disminuidas gradualmente, o mejor que la clonidina o la lofexidina, que son otros fármacos que se han usado comúnmente para tratar la abstinencia de opiáceos.
Fecha de la búsqueda
Las pruebas están actualizadas hasta diciembre 2016.
Características de los estudios
Se identificaron 27 ensayos controlados aleatorios (estudios clínicos en que los pacientes se asignan al azar a uno de dos o más grupos de tratamiento), que incluyeron 3048 participantes con dependencia de los opiáceos. Para 21 estudios la edad promedio de los participantes estuvo en el rango de 25 a 40 años ‐ en un estudio la edad promedio fue de 47 años, mientras que en dos estudios que incluían a adolescentes, la edad promedio de los participantes estaba en el rango de 17 a 20 años (3 estudios no informaron la edad promedio de los participantes). En cuatro estudios, todos o casi todos los participantes eran hombres, mientras que en tres estudios menos de la mitad de los participantes eran hombres. En la mayoría de los estudios los hombres comprendían entre la mitad y tres cuartos de los participantes, un equilibrio que es típico de la población de pacientes que dependen de opiáceos. Catorce de los estudios se realizaron en los Estados Unidos, mientras que los estudios restantes se realizaron en otros ocho países. Los estudios compararon buprenorfina con metadona (6 estudios), clonidina o lofexidina (14 estudios), o diferentes tasas de reducción de la dosis de buprenorfina (siete estudios).
Catorce estudios informaron el financiamiento de fuentes diferentes de la industria; en siete estudios, la financiación o los fármacos fueron proporcionados por una compañía farmacéutica. La fuente de financiación no estaba clara para siete estudios.
Resultados clave
En comparación con clonidina o lofexidina, los pacientes que recibieron buprenorfina para la abstinencia de opiáceos tendrán signos y síntomas menos graves, probablemente recibirán tratamiento durante más tiempo, presentarán menos efectos secundarios y tendrán una probabilidad mayor de completar el período programado de tratamiento. La efectividad de la buprenorfina probablemente es similar a las dosis disminuidas gradualmente de metadona, aunque no existe seguridad en cuanto a si los síntomas de abstinencia se resuelven más rápidamente con buprenorfina. Tampoco existe seguridad en cuanto a si la reducción rápida de la dosis de buprenorfina es más efectiva que la reducción lenta y si este hecho depende del contexto de la abstinencia.
Calidad de la evidencia
La calidad de las pruebas se evaluó como muy baja a moderada para la comparación de buprenorfina versus clonidina o lofexidina, baja a moderada para la comparación de buprenorfina versus metadona, y muy baja a baja para la comparación de diferentes tasas de reducción de la dosis. Las pruebas adicionales podrían cambiar los resultados, en particular para la buprenorfina en comparación con metadona y para las diferentes tasas de reducción de la dosis de buprenorfina.
Authors' conclusions
Summary of findings
| Buprenorphine versus methadone for managing opioid withdrawal | |||||
| Patient or population: Adults with opioid dependence | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | |
| Risk with methadone | Risk with buprenorphine | ||||
| Mean days in treatment | The mean days in treatment in the methadone group was 10.8 or 48.5 days. | The mean days in treatment in the buprenorphine group was 1.3 more (8.1 less to 10.7 more) than in the methadone group. | — | 82 | ⊕⊕⊝⊝ |
| Completion of withdrawal treatment | Study population | RR 1.04 | 457 | ⊕⊕⊕⊝ | |
| 528 per 1000 | 549 per 1000 | ||||
| Moderate | |||||
| 534 per 1000 | 555 per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aStudies were conducted in different settings (inpatient and outpatient). | |||||
| Buprenorphine vs alpha2‐adrenergic agonists for managing opioid withdrawal | |||||
| Patient or population: Adults or adolescents with opioid dependence | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | |
| Risk with alpha2‐adrenergic agonists | Risk with buprenorphine | ||||
| Mean peak withdrawal score, assessed with various scales | Not able to be summarised due to different means of assessment | The mean peak withdrawal score was lower in the buprenorphine group, indicating less severity, with a standardised mean difference of 0.43, which is a moderate effect size | SMD ‐0.43 (‐0.74 to ‐0.13) | 521 | ⊕⊝⊝⊝ |
| Mean overall withdrawal score, with various methods of assessment | Not able to be summarised due to different means of assessment | The mean overall withdrawal score was lower in the buprenorphine group, indicating less severity, with a standardised mean difference of 0.43, which is a moderate effect size | SMD ‐0.43 (‐0.58 to ‐0.28) | 902 | ⊕⊕⊕⊝ |
| Mean days in treatment (with varying scheduled duration) | Mean days in treatment ranged across studies from 21% to 70% of scheduled treatment duration | Mean days in treatment ranged across studies from 25% to 97% of scheduled treatment duration, with a standardised mean difference of 0.92, which is a large effect size. | SMD 0.92 (0.57 to 1.27) | 558 | ⊕⊕⊕⊝ |
| Number experiencing adverse effects | Study population | RR 0.93 | 493 | ⊕⊝⊝⊝ | |
| 289 per 1000 | 269 per 1000 | ||||
| Moderate | |||||
| 127 per 1000 | 118 per 1000 | ||||
| Number with treatment stopped due to adverse effects | Study population | RR 0.20 | 134 | ⊕⊕⊝⊝ | |
| 90 per 1000 | 18 per 1000 | ||||
| Moderate | |||||
| 79 per 1000 | 16 per 1000 | ||||
| Number completing withdrawal treatment | Study population | RR 1.59 | 1264 | ⊕⊕⊕⊝ | |
| 453 per 1000 | 721 per 1000 | ||||
| Moderate | |||||
| 536 per 1000 | 852 per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aRisk of bias in blinding of subjective outcomes. | |||||
| Rapid versus slow taper of buprenorphine dose for managing opioid withdrawal | |||||
| Patient or population: Adults or adolescents with opioid dependence | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | |
| Risk with slow dose taper | Risk with rapid taper | ||||
| Completion of withdrawal treatment − Inpatient | Study population | RR 1.00 | 60 | ⊕⊕⊝⊝ | |
| 900 per 1000 | 900 per 1000 | ||||
| Moderate | |||||
| 900 per 1000 | 900 per 1000 | ||||
| Completion of withdrawal treatment − Outpatient | Study population | RR 0.86 | 647 | ⊕⊝⊝⊝ | |
| 594 per 1000 | 511 per 1000 | ||||
| Moderate | |||||
| 636 per 1000 | 547 per 1000 | ||||
| Number abstinent at completion of dose taper − Inpatient | Study population | RR 1.00 | 40 | ⊕⊕⊝⊝ | |
| 900 per 1000 | 900 per 1000 | ||||
| Moderate | |||||
| 900 per 1000 | 900 per 1000 | ||||
| Number abstinent at completion of dose taper − Outpatient | Study population | RR 0.94 | 619 | ⊕⊝⊝⊝ | |
| 320 per 1000 | 301 per 1000 | ||||
| Moderate | |||||
| 337 per 1000 | 317 per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aSmall number of events (n < 300). | |||||
Background
Description of the condition
Dependence on opioid drugs (heroin, pharmaceutical opiates) is a major health and social issue in most societies. Globally, around 0.2% of adults report unsanctioned use of opioid drugs (Gowing 2015). Despite this low prevalence of opioid use, unsanctioned use of opioid drugs contributes more to the burden of disease than other illicit psychoactive drugs. The burden to the individual user and the community of opioid dependence arises from premature mortality and disability associated with dependent use, with the greatest impact in younger populations of drug users (Gowing 2015), transmission of human immunodeficiency virus (HIV) and hepatitis C, healthcare costs, crime and law enforcement costs, and less tangible costs of family disruption and lost productivity (Mark 2001).
Treatment is central for reducing the harms incurred by individuals and the community from opioid dependence. Managed withdrawal, or detoxification, by itself is not an effective treatment for dependence (Lipton 1983; Mattick 1996). Rates of completion of withdrawal treatment tend to be low, and rates of relapse to opioid use following detoxification are high (Broers 2000; Gossop 1989a; Vaillant 1988). However, withdrawal remains a required first step for many forms of long‐term treatment such as residential rehabilitation and naltrexone maintenance (Kleber 1982). It may also represent the endpoint of an extensive period of substitution treatment with methadone or buprenorphine. As such, the availability of managed withdrawal is essential to an effective and comprehensive treatment system.
The signs and symptoms of the opioid withdrawal syndrome include irritability, anxiety, apprehension, muscular and abdominal pains, chills, nausea, diarrhoea, yawning, lacrimation, sweating, sneezing, rhinorrhoea, general weakness, and insomnia. Symptoms of the opioid withdrawal syndrome usually begin two to three half‐lives after the last opioid dose, that is, 6 to 12 hours for short‐acting opioids such as heroin and morphine, and 36 to 48 hours for long‐acting opioids such as methadone. Following cessation of a short half‐life opioid, symptoms reach peak intensity within two to four days, with most of the obvious physical withdrawal signs no longer observable after 7 to 14 days (Jaffe 1997; Mattick 1996). As with the onset of withdrawal, the duration also varies with the half‐life of the opioid used and the duration of regular use (Tetrault 2009). Opioid withdrawal syndrome is rarely life‐threatening or associated with significant aberrations of mental state (Farrell 1994), but the combination of uncomfortable symptoms and intense craving makes completion of withdrawal treatment difficult for most people (Mattick 1996; Tetrault 2009).
Description of the intervention
For many years routine procedures for managing opioid withdrawal involved suppression of withdrawal with methadone and gradual reduction of the methadone dose (Kleber 1982). This approach derived from observations that the withdrawal syndrome from methadone was milder, though longer, than that from morphine. Methadone's high oral bioavailability, efficacy, and long duration of withdrawal relief (24 to 36 hours) were additional factors that contributed to its widespread use in specialist withdrawal programmes.
Ambivalence to the use of a drug of dependence to treat opioid dependence, government restrictions on prescription of methadone, and consumer dislike of the protracted nature of methadone withdrawal limited, to some extent, the use of methadone for managing opioid withdrawal (Farrell 1994). One widely used non‐opioid alternative, the alpha2‐adrenergic agonist clonidine, can also ameliorate some signs and symptoms of opioid withdrawal (Gossop 1988), but it is associated with adverse effects of sedation and hypotension. Dissatisfaction with methadone and clonidine drove the development of a variety of alternative approaches, in particular buprenorphine.
How the intervention might work
This review assesses the partial agonist, buprenorphine, for managing the signs and symptoms of opioid withdrawal. Research activity has primarily focused on the use of buprenorphine as an alternative medication for opioid substitution treatment, but today physicians often use buprenorphine for managing withdrawal. The rationale behind this treatment approach is that the morphine‐like effects of buprenorphine will suppress the physical signs and symptoms of withdrawal, but people will only experience limited withdrawal signs and symptoms because buprenorphine is a partial agonist (Banys 1994).
There is a complex range of variables that can potentially influence the course and subjective severity of withdrawal, including the type of opioid used, dose taken, concomitant use of other drugs including alcohol, duration of use, general physical health, and psychological factors, such as the reasons for undertaking withdrawal and fear of withdrawal (Farrell 1994; Phillips 1986; Preston 1985). Outcomes of a withdrawal episode may also be influenced by a prior period of substitution treatment, since such treatment is likely to result in a degree of stabilisation in health and social functioning that may facilitate successful withdrawal. Where information is available, we have considered the influence of these variables.
The first, or acute, phase of withdrawal is followed by a period of about six months of a secondary or protracted withdrawal syndrome. This protracted syndrome is characterised by a general feeling of reduced well‐being, which is reflected in measurable abnormal physiological functioning. During this phase, people may periodically experience strong cravings for opioids. Some authors consider that the malaise associated with protracted abstinence is a major factor in relapse (Satel 1993). The protracted nature of withdrawal typically makes the dependence recovery period lengthy and responsive to a range of factors, both social and treatment related. The types of intervention offered following the acute phase of withdrawal to promote recovery and prevent relapse are substantially different from those offered for managing withdrawal and may include psychological and lifestyle counselling, support groups, and pharmacological and medical treatment. We have excluded this long‐term aspect of treatment of opioid dependence from this review because of its substantially different nature.
Why it is important to do this review
This review is one of a series relating to managing opioid withdrawal. Other reviews consider the use of alpha2‐adrenergic agonists (Gowing 2016), the administration of opioid antagonists with minimal sedation (Gowing 2009), their administration under heavy sedation or anaesthesia (Gowing 2010), the use of tapered doses of methadone (Amato 2013), inpatient versus other settings (Day 2005), detoxification treatments for adolescents (Minozzi 2014), and psychosocial and pharmacological treatments for opioid detoxification (Amato 2011).
Objectives
To assess the effects of buprenorphine versus tapered doses of methadone, alpha2‐adrenergic agonists, symptomatic medications or placebo, or different buprenorphine regimens for managing opioid withdrawal, in terms of the intensity of the withdrawal syndrome experienced, duration and completion of treatment, and adverse effects.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled clinical trials that provided detailed information on the type and dose of drugs used and the characteristics of participants treated. Studies also had to provide information on the nature of withdrawal signs and symptoms experienced, the occurrence of adverse effects, or the rates of completion of the treatment episode.
Types of participants
We included studies that involved participants who were primarily opioid dependent and underwent managed withdrawal.
Types of interventions
Experimental interventions involved the administration of buprenorphine for ameliorating the signs and symptoms of opioid withdrawal.
We excluded studies that combined buprenorphine with opioid antagonists administered in order to induce withdrawal. This method of managing withdrawal is covered by a separate Cochrane Review (Gowing 2009).
Comparison interventions involved tapered doses of methadone, alpha2‐adrenergic agonists, symptomatic medications or placebo, or buprenorphine‐based regimens differing in amount, duration, or rate of taper of buprenorphine. For the purpose of this review, we define symptomatic medications as benzodiazepines, antiemetics, antidiarrhoeals, antipsychotics, antispasmodics, muscle relaxants, or non‐opioid analgesics, administered in combination as needed, or according to a defined regimen.
Types of outcome measures
Primary outcomes
We assessed the included studies on the basis of a number of measures.
-
Intensity of withdrawal (with consideration to peak withdrawal scores and average withdrawal score over the duration of withdrawal treatment).
-
Duration of treatment.
-
Nature and incidence of adverse effects.
-
Completion of treatment.
Interventions aimed at the management of acute opioid withdrawal are typically of short duration. As a result, structured psychological therapies are generally not provided as adjuncts to interventions for managing withdrawal, but the episode of withdrawal management does provide the opportunity to inform people who are opioid dependent about the options for further treatment, and to encourage them to engage in treatment appropriate to their needs. The longer the duration of treatment, the more opportunities there are for interaction between treatment services and people who are opioid dependent. The relative time in treatment is also an indicator of the relative acceptability to participants of the interventions being compared. For these reasons, we considered duration of treatment in addition to treatment completion rates.
It is difficult to differentiate adverse effects of treatment from the signs and symptoms of opioid withdrawal. We defined adverse effects as clinically significant signs and symptoms of opioid withdrawal (such as vomiting and diarrhoea) plus any incidents that are not typical components of the opioid withdrawal syndrome (hypotension, dry mouth). Early experience with clonidine, which was developed as a hypotensive agent, was that low blood pressure was a common adverse effect of clonidine treatment. This review therefore considered the occurrence of hypotension or symptoms of hypotension, withholding doses of medication and cessation of treatment as adverse effects.
Secondary outcomes
We also sought to assess data on the number of participants engaged in further treatment following completion of the withdrawal intervention. As indicated in the Background, managed withdrawal by itself is not an effective treatment for dependence. Hence we consider engagement in further treatment to be an outcome of interest.
Search methods for identification of studies
All searches included non‐English language literature. We assessed studies with English abstracts on the basis of the abstract. If we thought the study was likely to meet inclusion criteria, we translated it sufficiently to extract study methods and results.
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 11, 2016), MEDLINE (1946 to December week 1, 2016), Embase (to 22 December 2016), PsycINFO (1806 to December week 3, 2016), and the Web of Science (to 22 December 2016).
We developed a search strategy to retrieve references for all the Cochrane Reviews relating to the management of opioid withdrawal in one operation. We adapted the strategy to each of the major databases and the supporting platform. See Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5.
Searching other resources
We also searched:
-
the reference lists of all relevant papers to identify further studies;
-
some of the main electronic sources of ongoing trials: National Research Register; Current Controlled Trials (www.controlled‐trials.com); ClinicalTrials.gov (clinicaltrials.gov); Osservatorio Nazionale sulla Sperimentazione Clinica dei Medicinali (oss‐sper‐clin.agenziafarmaco.it); and Trialsjournal.com (www.trialsjournal.com);
-
conference proceedings likely to contain trials relevant to the review.
We contacted investigators to seek information about unpublished or incomplete trials.
Data collection and analysis
Selection of studies
One reviewer (LG) assessed each potentially relevant study for inclusion according to the identified inclusion and exclusion criteria. All reviewers confirmed the inclusion and exclusion decisions.
Data extraction and management
We developed a form for recording data on the outcomes of interest, taking into account the different ways that studies might report such data. The outcomes specified in the form were:
-
intensity of withdrawal (peak withdrawal score, overall withdrawal score, number with severe withdrawal, other data such as amount of adjunct medication);
-
duration of withdrawal treatment (days in treatment, other data such as number retained in treatment at a defined point);
-
adverse effects (number experiencing any adverse effects, number with hypotensive effects, number withdrawn from treatment due to adverse effects, other);
-
completion of treatment (number completing the scheduled period of treatment, number abstinent at completion of treatment);
-
postdetoxification (number engaged in further treatment, other such as number abstinent at follow‐up).
One reviewer (LG or DM) extracted key information, in consultation with other review authors where there was any uncertainty. We summarised key findings of studies descriptively in the first instance and considered the capacity for quantitative meta‐analysis. We contacted study authors if we required additional information to include studies in meta‐analyses.
Assessment of risk of bias in included studies
We assessed the methodological quality of included studies according to the approach recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This was based on the evaluation of seven specific methodological domains (namely, sequence generation, allocation concealment, blinding of participants and providers, blinding of outcome assessors, incomplete outcome data, selective outcome reporting, and 'other' issues). For each study, we analysed the seven domains, described them as reported in the study and provided a final judgement on the likelihood of bias in terms of low, high, or unclear risk of bias. We based these judgements on the criteria indicated by Higgins 2011 and their applicability to the addiction field.
We considered blinding separately for subjective and objective outcomes. Lack of blinding is a source of serious risk of bias for subjective outcomes but is less significant with objective outcomes, such as completion of treatment and duration of treatment. We only considered incomplete outcome data for intensity of withdrawal and the nature and incidence of adverse effects. Retention in treatment (duration of treatment) and completion of treatment are frequently primary outcome measures in addiction research. See Appendix 6 for a detailed description of the criteria we considered in the 'Risk of bias' assessment.
Details of the assessments of risk of bias are included in the Characteristics of included studies.
Measures of treatment effect
For dichotomous data (e.g. number completing treatment), we calculated risk ratios (RR), and for continuous data with consistent methods of measurement (e.g. time in treatment), we calculated mean differences (MD). For continuous data with differences between studies in the method of measurement (e.g. withdrawal scores), we calculated standardised mean differences (SMD). According to the approach recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we interpreted SMD of 0.2 to indicate a small effect size, SMD of 0.5 to indicate a moderate effect size, and SMD of 0.8 to indicate a large effect size. We used 95% confidence intervals (CI) to express the uncertainty in each result.
Unit of analysis issues
Where there were trials with multiple arms, we excluded any arms that involved an intervention not defined by the inclusion criteria for this review. Where there were trials with several arms relevant to meta‐analyses, we combined data involving different buprenorphine regimens after checking that the outcomes for the groups were similar.
Assessment of heterogeneity
We assessed statistical heterogeneity using the Chi2 test and its P value, by visual inspection of the forest plots, and through the I2 statistic. A P value of the test lower than 0.10 or an I2 statistic of at least 50% indicated a significant statistical heterogeneity.
Data synthesis
We used Review Manager 5 (RevMan 5) for statistical analyses (RevMan 2014). In all analyses, we used a random‐effects model. We used GRADEpro GDT to prepare the 'Summary of findings' tables (GRADEpro GDT).
Subgroup analysis and investigation of heterogeneity
This review also aimed to consider the following potential sources of heterogeneity through subgroup analyses, as this approach is considered to be associated with less risk of bias.
-
Drug of dependence and severity of dependence (as indicated by duration and level of use).
-
Polydrug use.
-
Concurrent physical and psychiatric illness.
-
Precipitants to the withdrawal episode.
-
Nature of the treatment setting.
-
Nature of adjunct treatment, including other medications to manage symptoms.
The nature of studies that met the inclusion criteria has limited such analyses. Subgroup analysis was possible only for treatment setting in the comparison of buprenorphine with an alpha2 ‐adrenergic agonist (clonidine or lofexidine).
Sensitivity analysis
We did not use risk of bias as a criterion for inclusion in the review. However, we assessed the impact of risk of bias through sensitivity analyses. This involved considering the overall estimate of effect when both including and excluding studies with a high risk of bias in meta‐analysis. We undertook sensitivity analyses where there were at least three studies providing data on the outcome, and where we assessed at least two of these studies as being at low or unclear risk of bias for that outcome. The only domain for which we performed sensitivity analyses was performance and detection bias in subjective outcomes (Analysis 2.1; Analysis 2.2).
Summary of findings table
We assessed the overall quality of the evidence for the primary outcome using the GRADE system. The GRADE Working Group developed a system for grading the quality of evidence that takes into account issues not only related to internal validity but also to external validity, such as directness of results (Atkins 2004; Guyatt 2008a; Guyatt 2008b; Guyatt 2011). The 'Summary of findings' tables present the main findings of a review in a transparent and simple tabular format. In particular, they provide key information concerning the quality of evidence, the magnitude of effect of the interventions examined and the sum of available data on the main outcomes.
The GRADE system assigns grades of evidence as follows.
-
High: we are very confident that the true effect lies close to that of the estimate of the effect.
-
Moderate: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
-
Low: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
-
Very low: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
We downgraded the quality of the studies for the following reasons.
-
Serious (−1) or very serious (−2) study limitation for risk of bias.
-
Serious (−1) or very serious (−2) inconsistency between study results.
-
Some (−1) or major (−2) uncertainty about directness (the correspondence between the population, the intervention, or the outcomes measured in the studies actually found and those under consideration in our systematic review).
-
Serious (−1) or very serious (−2) imprecision of the pooled estimate.
-
Publication bias strongly suspected (−1).
In some situations it is possible to increase grading, but this did not apply to this review.
Results
Description of studies
Results of the search
As indicated in the flow diagram (Figure 1), we identified 3573 records relevant to the management of opioid withdrawal in general. Of these, we excluded 3269 on the basis of title and abstract. We assessed 304 full‐text articles, excluding 192 (without listing reasons). We provide reasons for excluding a further 63 articles (45 studies) that appeared to be relevant, and 49 articles (27 studies) satisfied all the criteria to be included in the review.

Study flow diagram.
Included studies
Twenty‐seven studies (49 reports) involving 3048 participants met the inclusion criteria for this review (see Characteristics of included studies). In total, 2172 participants received buprenorphine.
One study was partially randomised − the first 14 participants were randomly allocated, and the remaining 9 participants had access to the rapid dose reduction regimen only (Wang 1996). We included only the 14 randomly allocated participants in this review. The other studies were randomised controlled trials.
Three studies received some financial support from a pharmaceutical company, and a further three studies reported receiving study medications from a pharmaceutical company. Thirteen studies reported funding from sources other than industry; in seven studies, funding arrangements were unclear.
Major comparisons
The 27 studies made eligible comparisons that fell into three main groups.
-
Buprenorphine versus tapered doses of methadone (6 studies).
-
Buprenorphine versus an alpha2‐adrenergic agonist (14 studies) − Raistrick 2005 used the adrenergic agonist lofexidine, while the other studies used clonidine.
-
Different rates of reduction of buprenorphine (7 studies).
Umbricht 2003 included both clonidine and methadone comparisons, and we consider those results in both relevant comparisons (1 and 2).
In addition, Oreskovich 2005 compared different starting doses of buprenorphine, and Schneider 2000 compared buprenorphine versus oxazepam. As there were no other studies making these comparisons, we do not consider these studies further. This review focuses on the three major comparisons.
Three studies compared regimens that were not defined by the inclusion criteria for this review. Janiri 1994 included a group treated with lefetamine; O'Connor 1997 included a group treated with clonidine plus naltrexone − a separate Cochrane Review assesses this approach (Gowing 2009); and Collins 2005 included a group receiving antagonist‐induced withdrawal under anaesthesia − also covered by a separate Cochrane Review (Gowing 2010). We excluded these groups from the review.
Treatment setting
Buprenorphine versus tapered doses of methadone
Four of the six studies provided treatment on an inpatient basis. One of these studies took place in an AIDS service, participants having been admitted for acute medical illness (Umbricht 2003). Wright 2011 took place in a prison setting, through the prison healthcare centre ‐ in this setting participants would have had less access to opioid drugs than in an outpatient setting, but would have had less supervision than in an inpatient setting. Only Bickel 1988 provided treatment on an outpatient basis.
Buprenorphine versus clonidine or lofexidine
Ten of the 14 studies provided treatment on an inpatient basis. As indicated above, Umbricht 2003 took place in an AIDS service. Ling 2005 included two separate studies: one in an inpatient and one in an outpatient setting.
Different rates of buprenorphine dose reduction
Two of the seven studies provided treatment on an inpatient basis − in Assadi 2004 participants were admitted to an addiction unit, while participants in Hopper 2005 were residents in a therapeutic community. Amass 1994 and Sigmon 2013 provided treatment on an outpatient basis with participants attending a clinic on a daily basis for medication, in Ling 2009 participants attended a clinic once weekly, and Marsch 2016 initially required participants to attend the clinic daily, but after 20 enrolments this was varied to enable participants to attend two to three times weekly. The report for Wang 1996 does not give this detail, but as the scheduled study duration was 12 weeks, we assume that it was undertaken on an outpatient basis.
Participant characteristics
For 21 studies the average age of participants was in the range 25 to 40 years. In one study (Hopper 2005) the average age was 47 years, while in two studies involving adolescents (Marsch 2005, Marsch 2016), the average age of participants was in the range 17 to 20 years. Three studies (O'Connor 1997, Ponizovsky 2006, Wang 1996) did not report the average age of participants.
In 17 of the 27 studies most or all participants were withdrawing from heroin alone or heroin and methadone. In two studies most participants were withdrawing from heroin, but a minority were using opium (Assadi 2004; Nigam 1993). One study was notable in that most participants were withdrawing from prescription opioids (57% oxycodone, 39% buprenorphine) (Sigmon 2013). The remaining seven studies did not report details of the nature of opioid drugs being used, although two studies excluded people dependent on methadone (Bickel 1988; Ling 2009), and two studies compared different rates of buprenorphine taper following a period of substitution treatment with buprenorphine (Amass 1994; Wang 1996). Hence participants in these two studies were effectively withdrawing from buprenorphine.
We had intended to compare outcomes for studies involving participants withdrawing from heroin versus those withdrawing from methadone, but the nature of studies meeting the inclusion criteria did not support this analysis.
There was considerable variability between studies in approach to the use of non‐opioid drugs. Four of the studies excluded participants abusing or dependent on any psychotropic drugs other than opioids (Collins 2005; Janiri 1994; Lintzeris 2002, Nigam 1993). Assadi 2004 and Umbricht 2003 excluded alcohol dependence; Oreskovich 2005 and Wright 2011 excluded people using drugs other than opioids if medically supervised withdrawal was required, and Ponizovsky 2006 excluded people also dependent on benzodiazepines or alcohol. Participants in Amass 1994 and Petitjean 2002 who were dependent on other drugs, in addition to opioids, underwent selective detoxification prior to the study of opioid withdrawal. In Hopper 2005, 60% were also cocaine dependent. All participants in Seifert 2002 and Schneider 2000 were polydrug users.
Opioid use is generally more common amongst men than women. However, in Cheskin 1994 and Marsch 2005, fewer than half the participants were men. In Umbricht 2003, 44% of the group treated with methadone were men, compared to 71% and 69% in the buprenorphine and clonidine groups, respectively. In four studies, authors reported that all participants were men, or they were most likely all men (Bickel 1988; Hussain 2015; Nigam 1993; Ziaaddini 2012). The significance of sex for managing opioid withdrawal is unclear, and hence the potential confounding effect of this variability in gender composition between the studies is also unclear. None of the studies report outcomes on the basis of sex, preventing further exploration of this aspect.
Nigam 1993 (undertaken in India) differed in that all participants used heroin (or opium) by inhalation, a route of administration that is likely to result in lower levels of dependence (Strang 1999). Furthermore, 92% of participants in Nigam 1993 and 70% of participants in Hussain 2015 (also undertaken in India) were employed, a situation that is generally unusual for heroin users from Western countries. Marsch 2005 and Marsch 2016 differed from other studies in that all participants were adolescents.
Fourteen of the studies took place in the USA, while three took place in Germany, two each in the UK, India, and Iran, and one each in Australia, Israel, Italy, and Switzerland.
Treatment regimens
Buprenorphine has low bioavailability when administered orally (Elkader 2005). When used for treating opioid dependence, buprenorphine is most commonly administered sublingually. In early studies of buprenorphine for opioid dependence, participants received buprenorphine sublingually as a solution (Chawarski 2005). Subsequently tablet preparations were developed containing either buprenorphine alone or buprenorphine plus naloxone, a combination that is intended to reduce the risk of recreational and inappropriate use (Larance 2011). More recently a film preparation containing buprenorphine plus naloxone has become available for improved placement under the tongue and reduced risk of recreational use (Lintzeris 2013).
Nine of the 27 studies included in the review reported using sublingual buprenorphine tablets, and a further five studies used the combination buprenorphine‐naloxone tablets. One study (Marsch 2016) commenced participants on sublingual buprenorphine tablets, and transferred them to combination buprenorphine‐naloxone tablets once stable. None of the studies considered for this review used the film preparation.
Three studies reported administering buprenorphine as a sublingual solution (Amass 1994; Bickel 1988; Cheskin 1994), and three studies administered buprenorphine as intramuscular injections (Assadi 2004; Janiri 1994; Umbricht 2003). Participants in Umbricht 2003 had been admitted to hospital for acute medical conditions, and the intramuscular route may have been preferable in this population, but the preparations in the other studies probably reflected a lack of access to sublingual tablets at the time the studies were undertaken.
Six studies did not report details of the buprenorphine preparation administered (Collins 2005; Schneider 2000; Seifert 2002; Steinmann 2008; Wang 1996; Wright 2011).
A number of factors, including route of administration, influence the bioavailability of buprenorphine (Chiang 2003; Strain 2004). Given this complexity, we have not attempted to calculate equivalent doses for buprenorphine used as different formulations and via different routes.
Buprenorphine versus tapered doses of methadone
These six studies varied in both dose of buprenorphine and the scheduled duration of treatment. Umbricht 2003 used the shortest regimen: 3.6 mg buprenorphine (intramuscular) on day 1, tapered to 1.2 mg on day 3. Petitjean 2002 and Seifert 2002 administered buprenorphine in tapered doses over periods of around 10 days; Petitjean 2002 used maximum doses of 16 mg/d (as sublingual tablets), while Seifert 2002 administered maximum doses of 4 mg/d (unspecified route and formulation). Bickel 1988, Steinmann 2008 and Wright 2011 administered buprenorphine over 20 to 21 days, with maximum doses of 2 mg/d (sublingual solution, Bickel 1988), 8 mg/d (unspecified preparation, Wright 2011), or 16 mg/d (unspecified preparation, Steinmann 2008).
Buprenorphine versus alpha2 ‐adrenergic agonists (clonidine or lofexidine)
Two of the 14 studies administered buprenorphine intramuscularly. Janiri 1994 administered 0.9 mg/d for two days and tapered the dose over the next two days. Umbricht 2003 used much higher doses: 3.6 mg on day 1 to 1.2 mg on day 3, tapering the dose by reducing the frequency of injections (from every 4 h on day 1 to every 8 h on day 3). Eight studies administered buprenorphine sublingually, using maximum doses ranging from 1.2 mg/d as buprenorphine tablet in Nigam 1993 to 16 mg/d as buprenorphine/naloxone tablet in Ling 2005. Lintzeris 2002 and Ziaaddini 2012 tailored doses, within limits, in response to individual need; the other studies used fixed‐dose regimens.
Collins 2005 administered a single 8 mg dose of buprenorphine (sublingual, preparation not specified) on the first day of a scheduled three‐day hospitalisation, with naltrexone commenced at 12.5 mg on day 2. O'Connor 1997 also used buprenorphine (3 mg sublingual, preparation not specified) as a transition to naltrexone treatment − participants received combination clonidine plus naltrexone following three days of buprenorphine treatment.
Cheskin 1994, O'Connor 1997 and Umbricht 2003 administered buprenorphine for three days, Janiri 1994, for four days; Lintzeris 2002, Oreskovich 2005, and Ziaaddini 2012, for five days; Marsch 2005 and Raistrick 2005, for seven days; Ponizovsky 2006, Hussain 2015, and Nigam 1993, for 10 days; and Ling 2005, for 13 days.
Different rates of buprenorphine dose reduction
The seven studies that compared different rates of dose‐tapering with buprenorphine varied considerably in the treatment regimens.
The scheduled duration of treatment for the two studies that provided treatment on an inpatient basis (Assadi 2004 and Hopper 2005) was short compared to the studies undertaken on an outpatient basis. For this reason, we separated studies according to treatment setting for analyses.
In Assadi 2004 participants in the experimental group received 4 × 1.5 mg doses of buprenorphine (intramuscular) on the afternoon of day 1, and 4 × 1.5 mg doses (intramuscular) on the morning of day 2, giving a total dose of 12 mg. The comparison group received 3 mg (intramuscular) on day 1, tapered to 0.6 mg on day 5.
Hopper 2005 used a similarly short, high dose regimen, with one group receiving buprenorphine only on day 1 (sublingual tablet, 8 mg followed by 24 mg after 30 min). The comparison group received 32 mg of buprenorphine over three days.
The five studies undertaken in outpatient settings differed in the period of stabilisation on buprenorphine prior to dose‐tapering, the starting dose of buprenorphine and the duration of taper.
In Amass 1994, participants were stabilised on 8 mg buprenorphine for 28 days and the dose was tapered over 12 days or 36 days.
In Ling 2009, the stabilisation period was one month, with a flexible starting dose, and the taper was over seven or 28 days.
In Marsch 2016, the stabilisation period and starting dose was flexible, and the taper was over 28 days or 56 days.
In Sigmon 2013, the stabilisation period was two weeks, with a flexible starting dose, and the taper was over one, two or four weeks.
In Wang 1996, there was no specified stabilisation period, the starting dose was 8 mg and the dose was tapered over 14 days or 8 weeks.
For the purposes of this review, the treatment regimen with buprenorphine tapered over a shorter period of time was defined as "rapid", while the comparison group was defined as "slow". In Sigmon 2013 there was no difference in outcomes for the groups with the buprenorphine dose tapered over one or two weeks; data from these groups were combined as "rapid" dose taper.
Excluded studies
Forty‐five studies (63 reports) did not meet the criteria for inclusion in this review (see Characteristics of excluded studies). The reasons for exclusion were: comparison of interventions other than those defined by the inclusion criteria (17 studies); methodology other than randomised controlled trials (7 studies); no treatment comparison (6 studies); interventions not primarily based on alleviating withdrawal (9 studies), and insufficient data on outcomes defined by the inclusion criteria (14 studies). Some studies fulfilled more than one criteria for exclusion.
Risk of bias in included studies
For summary results of the judged risk of bias across the included studies for each domain, see Figure 2 and Figure 3.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
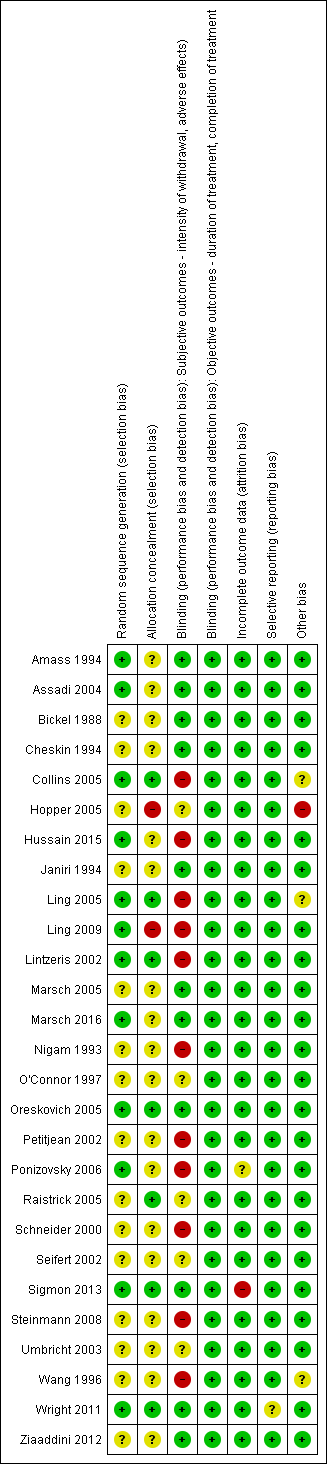
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
We judged five of the six studies comparing buprenorphine versus methadone to be at uncertain risk of bias due to the reporting of insufficient information on the method of random sequence generation and allocation concealment. We judged the other study in this group to be at low risk of allocation bias (Wright 2011).
Similarly we judged 10 of the 14 studies comparing buprenorphine with an alpha2‐adrenergic agonist to be at uncertain risk of bias because of insufficient information on the method of random sequence generation, allocation concealment, or both. We judged the other four studies in this group to be at a low risk of allocation bias.
We judged two of the seven studies comparing different rates of reduction of buprenorphine to be at high risk of allocation bias, as neither of these studies concealed the group allocation (Hopper 2005; Ling 2009). We assessed only Sigmon 2013 to be at low risk of allocation bias; we deemed the risk of bias to be uncertain for the other four studies in this group.
Blinding
We considered the risk of performance and detection bias for objective outcomes (duration and completion of treatment) to be low for all studies, as these outcomes are unlikely to be affected by an awareness of group allocation. This section therefore focuses on the risk of assessment bias in relation to subjective outcomes (intensity of withdrawal, occurrence, and severity of adverse effects).
We judged the following studies to be at high risk of bias due to a lack of blinding.
-
Two of the six studies comparing buprenorphine versus tapered doses of methadone (Petitjean 2002; Steinmann 2008).
-
Six of the 14 studies comparing buprenorphine versus an alpha2‐adrenergic agonist (Collins 2005; Hussain 2015; Ling 2005; Lintzeris 2002; Nigam 1993; Ponizovsky 2006).
-
Two of the seven studies comparing different rates of reduction of buprenorphine (Ling 2009; Wang 1996).
We judged the risk of bias to be uncertain in the following studies.
-
Two studies comparing buprenorphine versus methadone (Seifert 2002; Umbricht 2003).
-
Three studies comparing buprenorphine versus an alpha2‐adrenergic agonist (O'Connor 1997; Raistrick 2005; Umbricht 2003).
We judged the risk of bias to be low in all other studies.
Incomplete outcome data
Retention (duration of treatment) and completion of treatment are primary outcome measures for opioid withdrawal interventions. Hence we only considered the risk of bias due to incomplete data for the outcomes of intensity of withdrawal and adverse effects.
We judged the risk of bias to be high for Sigmon 2013 and uncertain for Ponizovsky 2006. We judged the risk of bias to be low in all other studies.
Selective reporting
We judged the risk of bias to be uncertain for Wright 2011 due to data on adverse effects being collected but not reported. We judged the risk of bias to be low in all other studies.
Other potential sources of bias
We judged the risk of bias to be high for Hopper 2005 as all participants were resident in a therapeutic community. This may have resulted in selection of a highly motivated group who would have done well whatever the treatment.
Collins 2005 and Ling 2005 were both stopped early due to clear benefits from one of the interventions being compared. Potentially this could have introduced bias, but it is difficult to determine the extent of risk.
We also judged Wang 1996 to be at uncertain risk of bias, as there was little information available to assess this study.
Effects of interventions
See: Summary of findings for the main comparison Buprenorphine vs methadone for managing opioid withdrawal; Summary of findings 2 Buprenorphine vs alpha2‐adrenergic agonists for managing opioid withdrawal; Summary of findings 3 Rapid vs slow taper of buprenorphine dose for managing opioid withdrawal
We present results in three sections according to the nature of the comparison: tapered doses of methadone, alpha2‐adrenergic agonist (clonidine or lofexidine), and different rates of buprenorphine dose reduction. We subdivide each of these sections into parts addressing the outcomes of interest to this review: intensity of withdrawal, duration of withdrawal treatment (indicative of both duration of withdrawal and retention in treatment), nature and incidence of adverse effects, and completion of treatment.
Buprenorphine versus tapered doses of methadone
Intensity of withdrawal
Meta‐analysis was not possible for this outcome.
Petitjean 2002 reported no difference in total withdrawal severity, but significantly higher withdrawal scores on day 8 in the buprenorphine group and on days 13 and 16 in the methadone group. Based on the graph presented in the trial report, the average withdrawal score was high in the buprenorphine group to day 12 (the average duration of the buprenorphine taper) before rapidly declining, while in the methadone group the withdrawal score increased steadily to day 16 (the taper was completed on average in 15 days) then declined.
Seifert 2002 presented data as graphs only. These show withdrawal scores to be significantly lower in the buprenorphine group on days 8 and 14. Scores of clinical impression were also significantly lower on days 7 and 14.
Bickel 1988 and Umbricht 2003 found no significant difference between the buprenorphine and methadone groups.
Steinmann 2008 and Wright 2011 did not report data on withdrawal severity.
Duration of withdrawal treatment
Two studies reported data on the average length of stay in treatment (Analysis 1.1). There is no significant difference in the average length of time in treatment in either study (MD 1.30 days, 95% CI −8.11 to 10.72; N = 82; studies = 2). We assessed the quality of evidence for this outcome as low (summary of findings Table for the main comparison).
Seifert 2002 and Steinmann 2008 did not report data on retention in treatment. In Umbricht 2003 the duration of treatment was determined by the acute condition for which the participant was admitted to hospital, rather than by completion of withdrawal treatment. In Wright 2011, the prison setting meant that duration of treatment was potentially affected by a range of factors unrelated to the type of treatment.
Nature and incidence of adverse effects
Three studies stated there were no significant adverse effects in either buprenorphine or methadone groups (Bickel 1988; Seifert 2002; Umbricht 2003). Other studies did not comment on adverse effects.
Completion of treatment
Five studies provided data on completion of treatment (Analysis 1.2). The combined result shows no difference between buprenorphine and methadone in terms of completion of withdrawal treatment (RR 1.04, 95% CI 0.91 to 1.20; N = 457; studies = 5). We assessed the quality of evidence for this outcome as moderate (summary of findings Table for the main comparison).
In Umbricht 2003, the duration of hospital stay depended on the acute condition for which the participant was admitted to hospital, and completion of withdrawal treatment was not relevant.
Buprenorphine versus alpha2‐adrenergic agonists
Intensity of withdrawal
Six studies reported a mean peak withdrawal score (Analysis 2.1). In five of the six studies, the mean peak withdrawal score was lower for those treated with buprenorphine, compared to those treated with an alpha2‐adrenergic agonist. The studies differed in the scale used to assess withdrawal severity, preventing the direct comparison of withdrawal scores across studies, but in all studies a higher score indicates more severe withdrawal. The effect size has instead been estimated as a standardised mean difference. The combined result moderately favours buprenorphine (SMD −0.43, 95% CI −0.74 to −0.13; N = 521; studies = 6). We assessed the quality of evidence for this outcome as very low (summary of findings Table 2), in part due to the high level of unexplained heterogeneity (I2 = 63%).
We assessed three of the studies as being at high risk of bias for this outcome (Hussain 2015; Lintzeris 2002; Nigam 1993). When we excluded these three studies in a sensitivity analysis, the combined result remained moderately in favour of buprenorphine (SMD −0.50, 95% CI −0.92 to −0.09; N = 309; studies = 6).
A mean overall withdrawal score was reported, or able to be calculated, for seven studies (Analysis 2.2). In all seven studies the mean withdrawal score was lower for those treated with buprenorphine, compared to those treated with an alpha2‐adrenergic agonist. We combined data from inpatient and outpatient groups in Ling 2005 in this analysis. The combined result favours buprenorphine with a moderate effect size (SMD −0.43, 95% CI −0.58 to −0.28; N = 902; studies = 7). We assessed the quality of evidence for this outcome as moderate (summary of findings Table 2).
We assessed four of the studies as being at high risk of bias for this outcome (Hussain 2015; Ling 2005; Lintzeris 2002; Nigam 1993). When we excluded these four studies in a sensitivity analysis, the combined result remained significant in favour of buprenorphine with a moderate effect size (SMD −0.37, 95% CI −0.58 to −0.16).
Five other studies also reported less severe withdrawal in those treated with buprenorphine but did not report data in a form that could be included in analyses.
-
Cheskin 1994 reported the mean total (area under the curve) opioid withdrawal symptom score as being 88% higher in the clonidine‐treated group compared to the buprenorphine‐treated group in the first three days.
-
Janiri 1994 stated that buprenorphine was significantly more effective than clonidine in suppressing withdrawal.
-
Oreskovich 2005 reported that 10 of 20 (50%) participants receiving buprenorphine and 1 of 9 (11%) receiving clonidine achieved a suppression of withdrawal in the first 24 hours of treatment. In addition authors stated that Clinical Opiate Withdrawal Scale (COWS) scores were significantly lower in the buprenorphine group over the five days of treatment.
-
Marsch 2005 stated that those in the buprenorphine group reported more positive effects of medication.
-
Ponizovsky 2006 did not rate the severity of withdrawal during the detoxification period; however, after the 10‐day detoxification period both the clonidine and buprenorphine groups had similar ratings on the Clinical Global Impression (CGI) scale, with both groups displaying normal to borderline mental illness scores. The CGI scale is a brief assessment providing the clinician's view of a patient's global functioning, taking into account psychosocial circumstances, symptoms, and behaviour. The assessment is comprised of a one‐item measure evaluating the severity of psychopathology from 1 to 7 and the change in psychopathology using a similar scale, after the initiation of treatment.
In two studies there was no significant difference in withdrawal severity for groups treated with buprenorphine versus clonidine (Collins 2005; Umbricht 2003).
Duration of withdrawal treatment
Four studies reported data on length of treatment (Analysis 2.3). Collins 2005 reported the average weeks in treatment, encompassing the period of inpatient treatment and subsequent naltrexone maintenance treatment. We included these data (converted from weeks to days) in the inpatient subcategory, as this was the setting for detoxification. Ling 2005 studied separate groups in inpatient and outpatient settings − we included both sets of data as two studies in the analysis. Oreskovich 2005 compared two buprenorphine regimens with clonidine. We combined the data and converted results from hours to days prior to analysis. As the studies differed in the scheduled duration of treatment, we calculated a standardised mean difference rather than directly comparing the average days in treatment.
Based on these four studies, participants receiving buprenorphine were likely to be retained in treatment for significantly longer than those receiving clonidine (SMD 0.92, 95% CI 0.57 to 1.27; N = 558; studies = 5). The effect size is large for both inpatient and outpatient settings. We assessed the quality of evidence for this outcome as moderate (summary of findings Table 2).
O'Connor 1997 used eight‐day treatment protocols. The clonidine group received naltrexone on day eight. The buprenorphine group received buprenorphine for three days, then clonidine plus naltrexone, with a dose of 50 mg naltrexone scheduled for day five. Authors did not report the mean length of stay, but there was a comparison of retention in treatment at day 8, the first day on which participants in both treatment protocols had completed detoxification. The authors found no significant difference between the groups, with 65% of those who received clonidine only, and 60% of the buprenorphine group remaining in treatment.
In Umbricht 2003 the duration of treatment was determined by the acute medical condition that led to hospital admission, and not opioid withdrawal. Authors did not report data on treatment duration.
In Ziaaddini 2012, withdrawal managed with buprenorphine or clonidine was a precursor to relapse prevention treatment with naltrexone. The reported duration of treatment included both the withdrawal phase and the subsequent period of naltrexone treatment.
The remaining seven studies did not discuss duration of treatment (Cheskin 1994; Hussain 2015; Janiri 1994; Marsch 2005; Nigam 1993; Ponizovsky 2006; Raistrick 2005).
Nature and incidence of adverse effects
Three studies reported the number of participants who experienced adverse effects (Analysis 2.4). Ling 2005 comprised two separate studies, one in outpatient and one in inpatient settings, and we consider data as two studies in the analysis. The data suggest no significant difference between buprenorphine and an alpha2‐adrenergic agonist in terms of the number of participants experiencing adverse effects (RR 0.93, 95% CI 0.70 to 1.26; N = 493; studies = 4). In addition, Collins 2005 stated there were no serious adverse effects in either the buprenorphine or clonidine group. We assessed the quality of evidence for this outcome as very low (summary of findings Table 2)
A number of studies included discussion of the nature of adverse effects experienced.
Hussain 2015 reported dizziness and dry mouth as common adverse effects in those treated with clonidine, whereas the buprenorphine group experienced headache, constipation, and nausea. Similarly, Nigam 1993 reported giddiness, dry mouth, and constipation in the clonidine group, and nausea, vomiting, and constipation in the buprenorphine group. In Lintzeris 2002, headache was the most commonly reported adverse effect in the buprenorphine group, compared to dizziness and lethargy in those treated with clonidine.
Ling 2005 included continued substance abuse or overdose that resulted in overnight hospitalisation in their definition of adverse events. Fourteen of 157 (8.9%) outpatients receiving buprenorphine experienced serious adverse effects, 10 of which were ongoing substance abuse or overdose. In contrast, 4 of 74 (5.4%) in the clonidine group experienced serious adverse events, 1 of which was ongoing substance abuse. Ling 2005 also reported mean adverse events (± standard deviation (SD)) per participant per day in both inpatients: 1.5 (± 0.8) events for the buprenorphine group versus 2.4 (± 1.6) events for the clonidine group; and in outpatients: 0.7 (± 0.8) events for the buprenorphine group versus 1.2 (± 1.6) events for the clonidine group.
Cheskin 1994 reported a lower respiratory rate, and higher sedation in the buprenorphine group during the three days of treatment. Janiri 1994 reported no significant difference in heart rate or blood pressure. Oreskovich 2005 reported the proportion of occasions when practitioners withheld doses of clonidine (or placebo) due to hypotension: on day 2, physicians withheld 18.4% of the doses in the high dose buprenorphine group, 30.3%in the low dose buprenorphine group, and 38.5% in the clonidine group. On day 3, the proportions were 30.3%, 22.9%, and 38.1%, respectively. As noted by Oreskovich 2005, investigators did not expect to see hypotension in the buprenorphine group; there was no statistically significant difference in the rate of low diastolic blood pressure between the three groups.
These data suggest no significant difference between buprenorphine and clonidine in the incidence of adverse effects, but there are differences in the nature of associated adverse effects and potentially in the severity of adverse effects experienced.
In Ponizovsky 2006 the presence of adverse effects was measured using the Distress Scale for Adverse Symptoms (DSAS) which comprises 22 common adverse effects, each rates 0 (none) to 4 (extreme) for severity. Scores (mean ± SD) were significantly lower for participants receiving buprenorphine (1.81 ± 1.32) compared to those receiving clonidine (6.01 ± 1.42).
Three studies, all taking place in an inpatient setting, reported the number of participants stopping treatment due to adverse effects (Analysis 2.5). This occurred only for the clonidine groups in all three studies (RR 0.20, 95% CI 0.04 to 1.15). However, given the small number of events, we rated the quality of evidence for this outcome as low (summary of findings Table 2).
Collins 2005 reported that no participants in either the buprenorphine or clonidine groups stopped treatment because of adverse effects.
Three studies did not discuss adverse effects (Marsch 2005; O'Connor 1997; Raistrick 2005).
Completion of treatment
We present data on the number of participants who completed the study or the scheduled period of treatment in Analysis 2.6. Two studies reported the number of participants who received a 50 mg dose of naltrexone (Collins 2005; O'Connor 1997). We interpreted these data as indicating the number of participants completing withdrawal treatment, but that may represent a more stringent definition of completion than is the case for other studies. The combined result indicates an advantage for buprenorphine over clonidine in terms of completion of withdrawal treatment in both inpatient (RR 1.74, 95% CI 1.05 to 2.89; N = 539; studies = 6) and outpatient (RR 1.45, 95% CI 1.12 to 1.88; N = 725; studies = 6) settings. The overall result (RR 1.59, 95% CI 1.23 to 2.06; N = 1264; studies = 12) translates to a number needed to treat for an additional beneficial outcome of 4 (95% CI 2.8 to 6.7), indicating that for every four people treated with buprenorphine, we can expect that one additional person will complete treatment than with clonidine. We assessed the quality of evidence for this outcome as moderate (summary of findings Table 2).
In one study, discharge from hospital depended on the acute condition leading to admission, not by completion of withdrawal treatment − 25 of 31 participants in the study were discharged from hospital prior to completion of withdrawal (Umbricht 2003). The remaining study did not report data on completion of treatment (Oreskovich 2005).
Five studies reported outcomes following completion of the study intervention.
-
In Lintzeris 2002, 47 of 58 (81%) in the buprenorphine group engaged in some form of postwithdrawal treatment, compared with 37 of 56 (66%) in the clonidine group. In the 28 days following completion of the study intervention, participants in the buprenorphine group received a mean (± SD) of 19.0 (± 11.2) days of treatment, compared to 11.0 (± 11.0) days for participants in the clonidine group.
-
In Collins 2005, detoxification was followed by naltrexone maintenance treatment. In the buprenorphine group, 9 of 37 (24%) were retained in treatment for 12 weeks, compared to 3 of 34 (9%) in the clonidine group. This difference may be due at least in part to the treatment protocols, with the buprenorphine group receiving the first dose of naltrexone while still in hospital, whereas the clonidine group did not receive it until after hospital discharge.
-
In Raistrick 2005, 37 of 92 (40%) in the buprenorphine group reported abstinence at one‐month follow‐up, compared to 26 of 82 (32%) in the lofexidine group. At this time, 6.5% in the buprenorphine group and 8.5% in the lofexidine group were receiving a substitute prescription, while 14.1% and 8.5%, respectively, were prescribed naltrexone.
-
In Marsch 2005, 61% of the buprenorphine group and 5% of the clonidine group initiated treatment with naltrexone.
-
In Ziaaddini 2012, 52% of participants in the clonidine group commenced naltrexone treatment and remained in treatment for an average of 70.2 days, compared to 53% of participants in the buprenorphine group, who remained in naltrexone treatment for an average of 66.7 days.
Different rates of buprenorphine dose reduction
As noted in Description of studies, the scheduled duration of treatment was shorter for the studies in this group that were undertaken in an inpatient versus outpatient setting. For this reason, we consider results separately according to this variable.
Intensity of withdrawal
No meta‐analysis was possible for this outcome.
Inpatient studies
Assadi 2004 administered buprenorphine over two or five days and found no significant differences in withdrawal severity. However, peak withdrawal occurred earlier with the more rapid treatment protocol, and participants in this group used more adjunct medication.
Hopper 2005 administered buprenorphine over one or three days, and found no significant differences in withdrawal scores or the use of adjunct medications.
Outpatient studies
Amass 1994 tapered buprenorphine over 12 or 36 days in an outpatient setting. While there was no difference in observers' assessment of withdrawal severity, the rate of increase in participant‐rated withdrawal severity was significantly greater in the rapid taper group.
Ling 2009 tapered buprenorphine over 7 or 28 days in an outpatient setting. There were no significant differences between the groups in withdrawal ratings, but participants in the rapid taper group used less concomitant medications.
Sigmon 2013 tapered buprenorphine over one, two, or four weeks in an outpatient setting. Authors reported that those in the four‐week taper group experienced a relatively mild and stable course of withdrawal whereas those in the one‐ and two‐week taper groups experienced a sharp increase in withdrawal during the week following the last dose of buprenorphine. There were no differences between the groups in the use of ancillary medications.
Wang 1996 did not rate withdrawal severity. However, authors reported that all participants in the rapid taper group experienced muscle aches, and most had lengthy insomnia. Participants reported prolonged symptoms even with adjunct medication. In contrast, participants in the gradual reduction group had minimal complaints.
Marsch 2016 did not discuss withdrawal severity.
Duration of withdrawal treatment
No meta‐analysis was possible for this outcome.
Inpatient studies
In Assadi 2004, both groups were scheduled to remain in inpatient treatment for 10 days, with buprenorphine tapered over two or five days during this time. The reported average time in treatment was 9.5 (± 1.8) days for the rapid taper group, compared to 9.8 (± 0.9) days for the five‐day group.
Hopper 2005 did not report data on the average duration of treatment.
Outpatient studies
Marsch 2016 reported an average 37.5 days in treatment for the group with buprenorphine tapered over 56 days, compared to an average 26.4 days for the group with buprenorphine tapered over 28 days. However, the authors also noted a significant effect of the frequency of clinic attendance, with an average 17.3 days in treatment for those required to attend the clinic daily compared to an average 46.7 days for those required to attend on 2 or 3 days each week. No other studies reported data on the average duration of treatment, although Amass 1994 reported that rapid dropout occurred in the rapid taper group once the detoxification phase commenced.
Nature and incidence of adverse effects
No meta‐analysis was possible for this outcome.
Inpatient studies
Assadi 2004 reported no significant difference between the two‐day and five‐day treatment regimens in terms of total adverse effects profile, and no significant difference in any specific adverse effect. They did note that blood levels of one liver enzyme (alanine transaminase, or ALT) had a greater increase from baseline in the group who received the five‐day treatment, but there are multiple factors that can impact liver enzymes, and this difference may not have been related to the buprenorphine regimen.
Hopper 2005 did not comment on adverse effects.
Outpatient studies
Amass 1994, Ling 2009 and Sigmon 2013 did not comment on adverse effects. Wang 1996 stated there were none reported. Marsch 2016 reported that no trial‐specific serious adverse events occurred.
Completion of treatment
We used subgroup analysis to consider data from studies undertaken in inpatient and outpatient settings separately. Due to the substantially different treatment regimens used in the two types of setting, we did not calculate an overall estimate of effect.
Inpatient studies
Two studies reported the number of participants who completed treatment (Analysis 3.1). There was no effect of the rate of dose taper on completion of treatment (RR 1.00, 95% CI 0.84 to 1.18; N = 60; studies = 2). We assessed the quality of evidence for this outcome as low (summary of findings Table 3).
Assadi 2004 confirmed abstinence by a naloxone challenge test at the completion of treatment (Analysis 3.2), finding no effect of the rate of dose taper (RR 1.00, 95% CI 0.81 to 1.23; N = 40; studies = 1). Hopper 2005 did not report the number of participants who were abstinent at the completion of treatment. We assessed the quality of evidence for this outcome as low (summary of findings Table 3).
In Assadi 2004, the total scheduled stay in hospital was 10 days, and naltrexone treatment was available as aftercare. Authors reported no data on this aspect.
Hopper 2005 took place in a residential therapeutic community and presumably study participants subsequently continued in the residential programme. Retention at 14 days was 7 of 10 participants in the 1‐day group and 5 of 10 in the 3‐day group.
Outpatient studies
Four studies that took place in an outpatient setting reported the number of participants who completed treatment (Analysis 3.1). Sigmon 2013 compared treatment regimens with buprenorphine tapered over one, two, or four weeks. We combined data from the one‐ and two‐week regimens prior to entry into the analyses to make the data comparable with that of the other studies.
There was no effect of the rate of dose taper on completion of treatment (RR 0.86, 95% CI 0.44 to 1.70; N = 647; studies = 4; I2 = 80%) However, there is a high degree of heterogeneity in this result, which is likely to reflect the diverse nature of the studies. This, and the small number of participants involved, resulted in our rating the quality of evidence for this outcome as very low (summary of findings Table 3).
Wang 1996 did not clearly report the number of participants who completed treatment.
Four studies reported the number of participants abstinent at the completion of treatment (Analysis 3.2). Marsch 2016 reported that a higher percentage of scheduled urine tests were opioid negative in the group with buprenorphine tapered over 56 days compared with the group with the dose tapered over 28 days (35% versus 17%) but this outcome was also influenced by the frequency of clinic attendance with 20.6% of opioid‐negative urine samples for those required to attend daily, versus 66.2% for those required to attend 2 to 3 days a week. Based on Analysis 3.2, there was no effect of the rate of dose taper on the likelihood of abstinence at completion of treatment (RR 0.94, 95% CI 0.39 to 2.24; N = 610; studies = 4; I2 = 81%). Again there is substantial heterogeneity, and we assessed the quality of evidence for this outcome as being very low (summary of findings Table 3).
Only Sigmon 2013 reported any data on engagement in ongoing treatment. In this study, participants received naltrexone as a second phase of treatment, from the end of the buprenorphine taper to week 12. Eleven of 22 in the four‐week taper group and 10 of 48 in the other groups were retained in treatment to the end of this phase of the study.
Discussion
Summary of main results
Buprenorphine versus tapered doses of methadone
Data comparing buprenorphine versus methadone in tapered doses for managing opioid withdrawal remains limited but are suggestive of buprenorphine and methadone having similar capacity to ameliorate opioid withdrawal, without significant adverse effects. The available data suggest there is no significant difference between buprenorphine and methadone in terms of average treatment duration or rates of completion of withdrawal treatment. We assessed the quality of evidence as low to moderate (summary of findings Table for the main comparison).
There remains a possibility that the pattern of withdrawal symptoms may be different when managing it with buprenorphine versus methadone (Cameron 2001). In Seifert 2002, withdrawal severity was rated only on days 0 (baseline), 2, 8, and 14, while both buprenorphine and methadone were tapered over 11 days. These three assessment points are not sufficient to provide a clear indication of the pattern of withdrawal. Petitjean 2002 reported withdrawal scores on a daily basis up to day 18, while buprenorphine was tapered over an average of 12 days, and methadone an average of 15 days. A feature of managed withdrawal with tapered doses of methadone is that peak withdrawal severity occurs at the end of the methadone taper (Gossop 1989b). The pattern of withdrawal severity for the group treated with methadone in Petitjean 2002 is consistent with this, showing a steady climb in severity to peak around day 15. In contrast, for the buprenorphine group, withdrawal severity is high early in treatment and declines rapidly after day 10. Seifert 2002 also reported that withdrawal scores were significantly lower in the buprenorphine group in the latter stages of treatment. Furthermore, Lintzeris 2002 reported that after ceasing buprenorphine, 5 out of 53 experienced mild and/or transient withdrawal, and 4 of 53 reported moderately severe 'rebound' withdrawal (i.e. the re‐emergence of withdrawal symptoms after ceasing use of the medication for managing withdrawal). These data suggest that most individuals treated with tapered doses of buprenorphine do not experience rebound withdrawal after cessation of buprenorphine. The finding that buprenorphine has similar capacity as methadone for suppressing withdrawal signs and symptoms is consistent with findings in relation to maintenance treatment (Mattick 2014). Overall, however, more information is required on the patterns of withdrawal associated with tapered doses of buprenorphine and methadone in order to draw conclusions on the relative effectiveness of these drugs in terms of amelioration of the signs and symptoms of opioid withdrawal.
Buprenorphine versus alpha2‐adrenergic agonists
The withdrawal scores and descriptive reporting of the withdrawal syndrome experienced by participants support a conclusion that buprenorphine is more effective than alpha2‐adrenergic agonists in ameliorating the signs and symptoms of opioid withdrawal, both in terms of the peak average withdrawal score and the average daily withdrawal score over the withdrawal episode. We assessed the quality of evidence as low to moderate (summary of findings Table 2).
Available data suggest that, compared to alpha2‐adrenergic agonists, buprenorphine is associated with greater retention in treatment, as indicated by a longer mean duration of treatment, in both inpatient and outpatient settings, with moderate quality of evidence (summary of findings Table 2). The clinical significance of a longer period of time in treatment is that it increases the opportunity for interventions to encourage engagement in further treatment following the episode of managed withdrawal.
There was no significant difference between buprenorphine and alpha2‐adrenergic agonists (clonidine or lofexidine) in terms of the number of participants experiencing adverse effects. The nature of adverse effects experienced appears to differ, with dizziness, dry mouth, and lethargy being particularly common with alpha2‐adrenergic agonists and headache being most prominent with buprenorphine, while dropout from treatment because of adverse effects was somewhat more likely with alpha2‐adrenergic agonists. The small number of events and the small number of studies providing data led us to assess the quality of evidence on adverse effects as low to very low (summary of findings Table 2).
Completion of withdrawal treatment is significantly more likely when managing withdrawal with buprenorphine compared to an alpha2‐adrenergic agonist, in either an inpatient or an outpatient setting. The overall result translates to a number needed to treat for an additional beneficial outcome of 4 (95% CI 3 to 6), indicating that for every four people treated with buprenorphine, we can expect that one additional person will complete treatment than with clonidine or lofexidine. We assessed the quality of evidence as moderate (summary of findings Table 2).
Data on engagement in postdetoxification treatment is limited, but four of the five studies reporting information suggest somewhat higher rates of engagement in further treatment following withdrawal managed with buprenorphine versus clonidine or lofexidine. The exception was Ziaaddini 2012, where rates of engagement in naltrexone treatment following withdrawal were similar between buprenorphine and clonidine groups. In contrast, the findings of Collins 2005, Marsch 2005, and Raistrick 2005 suggest higher rates of initiation of naltrexone treatment following withdrawal managed with buprenorphine versus an alpha2‐adrenergic agonist.
Different rates of dose reduction
Based on the two studies undertaken in an inpatient setting with short periods (five days or less) of treatment with buprenorphine, it appears that the rate of taper of the buprenorphine dose may have no significant effect on the intensity of withdrawal or the completion of treatment (Assadi 2004; Hopper 2005). However, the small number of studies and the differing nature of the inpatient settings (a hospital ward in Assadi 2004, and a residential treatment facility in Hopper 2005) make any findings highly uncertain (summary of findings Table 3).
Five studies undertaken in outpatient settings compared buprenorphine regimens of longer duration (up to 56 days). Meta‐analysis of withdrawal scores was not possible, but qualitative comments made by the authors of the studies suggest that there is a more marked increase in withdrawal associated with more rapid rates of tapering of the buprenorphine dose. There were no data on the average duration of treatment, and most studies did not comment on adverse effects. It seems unlikely for there to be differences in the nature or incidence of adverse effects based on the rate of taper of the buprenorphine dose. There was considerable variability between these four studies in rates of completion of withdrawal treatment and abstinence at completion, with low quality evidence (summary of findings Table 3). Two of the four studies were small, exploratory studies, reporting limited data (Amass 1994; Wang 1996). Ling 2009, Marsch 2016 and Sigmon 2013 were larger studies but produced differing results: Ling 2009 found that completion of withdrawal treatment was more likely with a more rapid taper of the dose of buprenorphine, while Sigmon 2013 and Marsch 2016 found that completion of withdrawal treatment was more likely with a slower taper of the dose of buprenorphine. Marsch 2016 involved adolescents, tapered buprenorphine over 28 or 56 days, and found that the frequency of clinic attendance also affected completion rates. In Sigmon 2013, the use of buprenorphine to manage withdrawal was the first phase of the study. In the second phase, study participants received naltrexone maintenance treatment (plus buprenorphine placebo). Participants in Ling 2009 were offered naltrexone treatment as aftercare, but it was not incorporated into the study protocol as a standard component of treatment. The findings in relation to abstinence at completion of the buprenorphine dosing regimen are similarly divergent.
These findings leave the question of whether buprenorphine dose should be tapered rapidly or slowly unanswered and introduce the possibility that it may depend on the context of withdrawal.
Overall completeness and applicability of evidence
Most (14 of 26) of the studies included in this review compared buprenorphine with clonidine (13 studies) or lofexidine (1 study), usually in an inpatient setting (10 studies), for managing withdrawal from heroin alone or heroin and methadone, in relatively short episodes of treatment (3 to 13 days). In this context, the finding that buprenorphine is more effective than clonidine in ameliorating the signs and symptoms of opioid withdrawal, prolonging the duration of treatment, and increasing the likelihood of completion of treatment, is reasonably robust.
Fewer studies (six) compared buprenorphine versus methadone in tapered doses, while the treatment settings were more diverse, and the scheduled duration of treatment tended to be longer (up to 21 days). Historically, methadone was widely used in withdrawal management with a short‐term goal of abstinence, particularly in outpatient settings, but currently the predominant use of methadone is in opioid substitution treatment where the immediate aims are retention in treatment, reduction of illicit opioid use and harms related to opioid use, and improvement of the quality of life, with abstinence a longer‐term goal (WHO 2009). The small number of studies comparing buprenorphine and methadone for managing opioid withdrawal is likely to be a reflection of the predominant use of methadone in opioid substitution treatment.
The small number of studies comparing different dose tapering schedules of buprenorphine, and the diverse findings, indicate that there is still much to learn about how to maximise the effectiveness of buprenorphine when it comes to managing opioid withdrawal in various settings and contexts. There is still very little information about managing withdrawal after a period of substitution treatment with buprenorphine, or indeed methadone, and postwithdrawal arrangements, such as transition to naltrexone, may affect preference for rapid or slow rate of dose taper.
Quality of the evidence
Buprenorphine versus tapered doses of methadone
Six studies involving 436 participants compared buprenorphine versus tapered doses of methadone. Meta‐analysis was possible for only two outcomes, mean days in treatment (2 studies) and number of participants completing withdrawal (5 studies). Study findings were reasonably consistent − we downgraded the quality of the evidence primarily due to the diverse settings in which the studies took place and the small numbers of participants. We assessed the quality of evidence on the duration of treatment as low, and on completion of withdrawal treatment as moderate (summary of findings Table for the main comparison).
Buprenorphine versus alpha2‐adrenergic agonists
Fourteen studies investigated this comparison in 750 people receiving buprenorphine and 615 receiving an alpha2‐adrenergic agonist (103 with lofexidine). Meta‐analysis was possible for the intensity of withdrawal (peak withdrawal score and average withdrawal score across the treatment episode), duration of treatment, adverse effects (number of participants experiencing adverse effects, and number with treatment stopped due to adverse effects), and completion of treatment. All 14 studies contributed to at least one of the meta‐analyses. We assessed the quality of evidence as very low to moderate (summary of findings Table 2) for the six meta‐analyses for the following reasons.
-
Analysis 2.1, mean peak withdrawal score, very low quality due to the risk of bias in the assessment of subjective outcomes, and small number of events.
-
Analysis 2.2, mean overall withdrawal score, moderate quality due to the risk of bias in the assessment of subjective outcomes.
-
Analysis 2.3, mean days in treatment, moderate quality due to different settings and heterogeneity in results.
-
Analysis 2.4, participants experiencing adverse effects, very low quality due to the risk of bias in the assessment of subjective outcomes, different settings, and small number of events.
-
Analysis 2.5, participants with treatment stopped due to adverse effects, low quality due to the small number of events.
-
Analysis 2.6, participants completing treatment, moderate quality due to heterogeneity in results.
Different rates of dose reduction
Seven studies involving 730 participants investigated this comparison. Meta‐analysis was possible only for outcomes of number of participants completing treatment, and number abstinent at completion, and we undertook separate meta‐analyses for inpatient (2 studies) and outpatient (5 studies) settings, as there were substantial differences in treatment regimens between the two. We assessed the quality of evidence as low or very low due to the small number of events, the small size of most of the studies, and heterogeneity in the results. As a result, we have little confidence in the findings for this comparison.
Potential biases in the review process
We did not detect any publication bias and believe that the search strategy was thorough and the study selection and data extraction processes, unbiased. We calculated the mean overall withdrawal score for some studies comparing buprenorphine with an alpha2‐adrenergic agonist using data reported in the studies, and this may have introduced some error.
Agreements and disagreements with other studies or reviews
We are not aware of any other systematic reviews addressing the effectiveness of buprenorphine for managing opioid withdrawal. The finding that buprenorphine has similar efficacy to methadone in terms of the capacity to ameliorate the signs and symptoms of opioid withdrawal is consistent with the findings of another Cochrane Review of the effectiveness of buprenorphine for opioid substitution treatment (Mattick 2014).

Study flow diagram.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Comparison 1 Buprenorphine versus methadone, Outcome 1 Mean days in treatment.
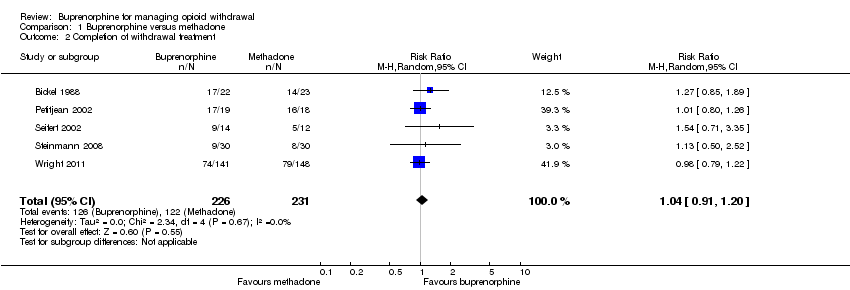
Comparison 1 Buprenorphine versus methadone, Outcome 2 Completion of withdrawal treatment.

Comparison 2 Buprenorphine versus clonidine, Outcome 1 Mean peak withdrawal score.

Comparison 2 Buprenorphine versus clonidine, Outcome 2 Mean overall withdrawal score.

Comparison 2 Buprenorphine versus clonidine, Outcome 3 Mean days in treatment.
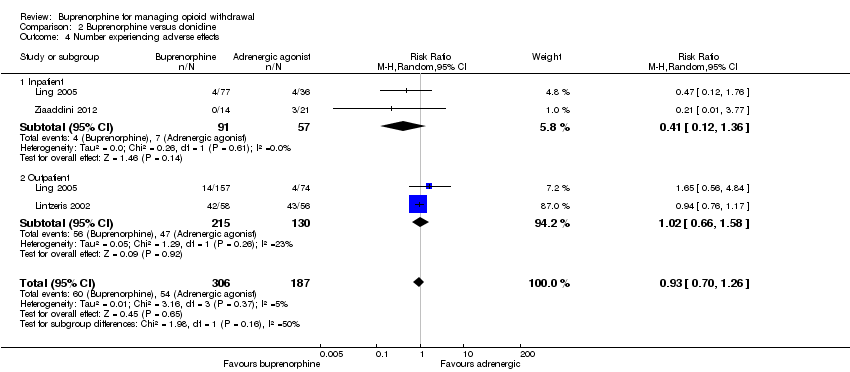
Comparison 2 Buprenorphine versus clonidine, Outcome 4 Number experiencing adverse effects.
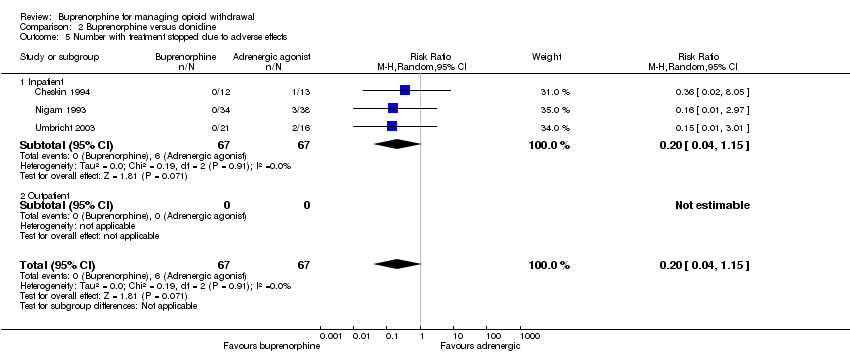
Comparison 2 Buprenorphine versus clonidine, Outcome 5 Number with treatment stopped due to adverse effects.

Comparison 2 Buprenorphine versus clonidine, Outcome 6 Number completing withdrawal treatment.

Comparison 3 Rapid versus slow dose taper, Outcome 1 Completion of withdrawal treatment.

Comparison 3 Rapid versus slow dose taper, Outcome 2 Number abstinent at completion of dose taper.
| Buprenorphine versus methadone for managing opioid withdrawal | |||||
| Patient or population: Adults with opioid dependence | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | |
| Risk with methadone | Risk with buprenorphine | ||||
| Mean days in treatment | The mean days in treatment in the methadone group was 10.8 or 48.5 days. | The mean days in treatment in the buprenorphine group was 1.3 more (8.1 less to 10.7 more) than in the methadone group. | — | 82 | ⊕⊕⊝⊝ |
| Completion of withdrawal treatment | Study population | RR 1.04 | 457 | ⊕⊕⊕⊝ | |
| 528 per 1000 | 549 per 1000 | ||||
| Moderate | |||||
| 534 per 1000 | 555 per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aStudies were conducted in different settings (inpatient and outpatient). | |||||
| Buprenorphine vs alpha2‐adrenergic agonists for managing opioid withdrawal | |||||
| Patient or population: Adults or adolescents with opioid dependence | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | |
| Risk with alpha2‐adrenergic agonists | Risk with buprenorphine | ||||
| Mean peak withdrawal score, assessed with various scales | Not able to be summarised due to different means of assessment | The mean peak withdrawal score was lower in the buprenorphine group, indicating less severity, with a standardised mean difference of 0.43, which is a moderate effect size | SMD ‐0.43 (‐0.74 to ‐0.13) | 521 | ⊕⊝⊝⊝ |
| Mean overall withdrawal score, with various methods of assessment | Not able to be summarised due to different means of assessment | The mean overall withdrawal score was lower in the buprenorphine group, indicating less severity, with a standardised mean difference of 0.43, which is a moderate effect size | SMD ‐0.43 (‐0.58 to ‐0.28) | 902 | ⊕⊕⊕⊝ |
| Mean days in treatment (with varying scheduled duration) | Mean days in treatment ranged across studies from 21% to 70% of scheduled treatment duration | Mean days in treatment ranged across studies from 25% to 97% of scheduled treatment duration, with a standardised mean difference of 0.92, which is a large effect size. | SMD 0.92 (0.57 to 1.27) | 558 | ⊕⊕⊕⊝ |
| Number experiencing adverse effects | Study population | RR 0.93 | 493 | ⊕⊝⊝⊝ | |
| 289 per 1000 | 269 per 1000 | ||||
| Moderate | |||||
| 127 per 1000 | 118 per 1000 | ||||
| Number with treatment stopped due to adverse effects | Study population | RR 0.20 | 134 | ⊕⊕⊝⊝ | |
| 90 per 1000 | 18 per 1000 | ||||
| Moderate | |||||
| 79 per 1000 | 16 per 1000 | ||||
| Number completing withdrawal treatment | Study population | RR 1.59 | 1264 | ⊕⊕⊕⊝ | |
| 453 per 1000 | 721 per 1000 | ||||
| Moderate | |||||
| 536 per 1000 | 852 per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aRisk of bias in blinding of subjective outcomes. | |||||
| Rapid versus slow taper of buprenorphine dose for managing opioid withdrawal | |||||
| Patient or population: Adults or adolescents with opioid dependence | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | |
| Risk with slow dose taper | Risk with rapid taper | ||||
| Completion of withdrawal treatment − Inpatient | Study population | RR 1.00 | 60 | ⊕⊕⊝⊝ | |
| 900 per 1000 | 900 per 1000 | ||||
| Moderate | |||||
| 900 per 1000 | 900 per 1000 | ||||
| Completion of withdrawal treatment − Outpatient | Study population | RR 0.86 | 647 | ⊕⊝⊝⊝ | |
| 594 per 1000 | 511 per 1000 | ||||
| Moderate | |||||
| 636 per 1000 | 547 per 1000 | ||||
| Number abstinent at completion of dose taper − Inpatient | Study population | RR 1.00 | 40 | ⊕⊕⊝⊝ | |
| 900 per 1000 | 900 per 1000 | ||||
| Moderate | |||||
| 900 per 1000 | 900 per 1000 | ||||
| Number abstinent at completion of dose taper − Outpatient | Study population | RR 0.94 | 619 | ⊕⊝⊝⊝ | |
| 320 per 1000 | 301 per 1000 | ||||
| Moderate | |||||
| 337 per 1000 | 317 per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aSmall number of events (n < 300). | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean days in treatment Show forest plot | 2 | 82 | Mean Difference (IV, Random, 95% CI) | 1.30 [‐8.11, 10.72] |
| 2 Completion of withdrawal treatment Show forest plot | 5 | 457 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.91, 1.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean peak withdrawal score Show forest plot | 6 | 521 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.74, ‐0.13] |
| 2 Mean overall withdrawal score Show forest plot | 7 | 902 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.58, ‐0.28] |
| 3 Mean days in treatment Show forest plot | 4 | 558 | Std. Mean Difference (IV, Random, 95% CI) | 0.92 [0.57, 1.27] |
| 3.1 Inpatient setting | 3 | 213 | Std. Mean Difference (IV, Random, 95% CI) | 1.00 [0.33, 1.67] |
| 3.2 Outpatient setting | 2 | 345 | Std. Mean Difference (IV, Random, 95% CI) | 0.82 [0.57, 1.06] |
| 4 Number experiencing adverse effects Show forest plot | 3 | 493 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.70, 1.26] |
| 4.1 Inpatient | 2 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.12, 1.36] |
| 4.2 Outpatient | 2 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.66, 1.58] |
| 5 Number with treatment stopped due to adverse effects Show forest plot | 3 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.04, 1.15] |
| 5.1 Inpatient | 3 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.04, 1.15] |
| 5.2 Outpatient | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Number completing withdrawal treatment Show forest plot | 11 | 1264 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [1.23, 2.06] |
| 6.1 Inpatient | 6 | 539 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [1.05, 2.89] |
| 6.2 Outpatient | 6 | 725 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.12, 1.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Completion of withdrawal treatment Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Inpatient | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.84, 1.18] |
| 1.2 Outpatient | 4 | 647 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.44, 1.70] |
| 2 Number abstinent at completion of dose taper Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Inpatient | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.81, 1.23] |
| 2.2 Outpatient | 4 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.39, 2.24] |

