Agonistas adrenérgicos alfa2 para el tratamiento de la abstinencia de opiáceos
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Double‐blind, controlled study | |
| Participants | Setting: outpatient clinic, Australia. Participants: 31 heroin users seeking withdrawal assistance, with evidence of repeated iv drug use. Group sizes: (1) n = 16, (2) n = 15. Group characteristics not reported. Required to attend clinic with non‐drug‐using family member or partner | |
| Interventions | (1) Clonidine, 15 μg/kg/day, 3 to 4 divided doses, tapered. (2) Placebo. Additional medication used but not reported. Scheduled duration 3 to 7 days | |
| Outcomes | Number with signs or symptoms of withdrawal graded > 2 (on 0 to 4 scale); number successful (completed 3 to 7 days of treatment, no signs of withdrawal, negative supervised urine samples) | |
| Notes | Observers rated 4 signs (pupil diameter, sweating, rhinorrhoea, and lacrimation) and 3 symptoms (abdominal pain, leg cramps, diarrhoea) 0 to 4 each day. Participants rated management of withdrawal good, average, of no use, or terrible. Daily urine testing. Source of funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients ... who requested assistance for heroin withdrawal were advised of the placebo controlled trial being undertaken." Comment: Allocation may have been random, but this was not specifically reported. The participant characteristics were not reported, and there was no discussion of the similarity of the groups |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "The patients were seen on a daily basis by the medical officer and a series of observations were recorded on a standard flow sheet." "Sixteen patients were given clonidine and fifteen received the identical placebo tablets ..." Comment: Use of identical placebo suggests participants were blinded, but the treating medical officer, who was also the observer, may not have been |
| Blinding (performance bias and detection bias) | Low risk | Blinding was uncertain, but these outcomes were unlikely to be influenced by blinding |
| Incomplete outcome data (attrition bias) | Low risk | Outcome of withdrawal episode (success/failure) reported for all participants. Withdrawal severity and adverse effects reported as dichotomous data (e.g. number with withdrawal graded > 2) and missing data unlikely to have clinically relevant impact on these data |
| Selective reporting (reporting bias) | High risk | Participant assessments of withdrawal management not reported. Use of additional medication not reported |
| Other bias | Unclear risk | Quote: "The study was closed after thirty‐one patients had been entered into the trial because of the difficulty in convincing patients that they should be willing to be treated with placebo for the sake of the study." Participants "represented 80% of those presenting during the study period." Comment: Insufficient information to determine the extent of risk of bias from participant's preference for active medication |
| Methods | Randomised, controlled, double‐blind trial. Sample represented 34.7% of total patients admitted to unit during the study | |
| Participants | Setting: inpatient treatment in specialist drug and alcohol unit, London, UK. Participants: 86 opioid dependent by DSM‐IV, using heroin, methadone, or both. Group sizes: (1) n = 42, (2) n = 44. Groups similar on age, gender, body weight, and drug use history. Mean age: 31.7 years. 80% men. Mean duration of opioid use: 10.5 years. 43% also used benzodiazepines | |
| Interventions | Stabilised on methadone (about 60 mg/day) for 3 days prior to detoxification with: (1) Lofexidine, initial dose 0.6 mg/day, increased by 0.4 mg/day until day 4, maintained at 2 mg/day for 3 days, then tapered over 3 days or (2) Methadone, starting dose variable, tapered over 10 days. Both drugs administered twice daily. Diazepam, 3 days of stabilisation then tapered over 21 days for those codependent on benzodiazepines. Scheduled duration of withdrawal treatment 10 days, inpatient stay 21 days | |
| Outcomes | Mean daily withdrawal score (graph); length of stay; mean daily blood pressure (graph); number completing 20 days of treatment; number experiencing dizziness | |
| Notes | Short Opiate Withdrawal Scale (10 items, 0 to 4 severity) completed daily by participants. Study supported by funds from Britannia Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned." Comment: Method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) | Low risk | Quote: "If a patient left before completing the treatment programme, the treatment code was broken and the patient informed which treatment they were taking. Those who completed treatment remained blind to the treatment they had received ..." Comment: Participants at least were blind to treatment allocation, and withdrawal scores were rated by participants |
| Blinding (performance bias and detection bias) | Low risk | The use of a treatment code suggests that personnel may also have been blinded |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "... in the lofexidine group ... six patients dropped out within the first 10 days compared to only one of the methadone treated patients (P = 0.048)." Comment: Withdrawal severity was significantly greater in the lofexidine group in the first 10 days ‐ this difference cannot be attributed to the differential drop‐out. Indeed, data missing due to drop‐out might be expected to increase the difference |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | 37/86 participants simultaneously withdrawing from benzodiazepines; equally distributed between groups |
| Methods | Randomised, placebo‐controlled, double‐blind trial. Polydrug dependence an exclusion criterion (1 excluded postrandomisation) | |
| Participants | Setting: inpatient treatment, Germany. Participants: 50 dependent heroin users. Group sizes: (1) n = 24, (2) n = 25. Groups similar on most characteristics, but at entry clonidine group had longer mean time since last heroin use. Mean age: 26 years. 78% men. 78% unemployed; around 5 previous withdrawal attempts | |
| Interventions | Medication commenced with withdrawal symptoms. (1) Clonidine, 0.1 mg/tablet. (2) Placebo. Day 1, 1 or 2 tablets 3 times a day, increasing to 1 or 2 tablets 3 to 5 times a day depending on symptoms. Dose tapered over 10 days. Both groups given neuroleptics when necessary and counselling. Scheduled duration 10 days | |
| Outcomes | Graph of withdrawal scores; global assessment of efficacy; side effects; number completing treatment | |
| Notes | Withdrawal rated by observers (22 items, 0 to 5 severity) and participants (38 items, "not there" to "hard"). Published in German. English translation obtained. Source of funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The treatment was double‐blind randomised comparison." Comment: The method of sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind stated |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind stated, and these outcomes unlikely to be affected by knowledge of group allocation |
| Incomplete outcome data (attrition bias) | Low risk | Completion of treatment was the only outcome used in analyses for this review |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised, placebo‐controlled, double‐blind trial. Dependence on methadone or other opioids, or polydrug use exclusion criteria | |
| Participants | Setting: inpatient treatment in non‐specialised unit of psychiatric hospital, Lausanne, Switzerland. Participants: 32 heroin users, dependent by DSM‐III‐R. Group sizes: 16 in each group. Groups similar except in frequency of heroin use: participants used heroin (mean ± SD) (1) 3.3 ± 2.1, (2) 4.8 ± 2.4 times a day. Mean age: about 24 years. 72% men. 66% used iv; remainder used by sniffing or smoking; 19% had previously been hospitalised for detoxification | |
| Interventions | (1) Clonidine, max dose 0.6 mg/day, tapered over 7 days. (2) Carbamazepine, max dose 400 mg/day, plus mianserin (atypical antidepressant) to max 90 mg/day for 6 days. Adjunct medications as required, in both groups. Scheduled duration 7 days | |
| Outcomes | Withdrawal scores (graphs); instances of comedication; global satisfaction score; retention to end of treatment; difference in blood pressure; instances medication withheld | |
| Notes | Opiate withdrawal questionnaire (30 items, rated 0 to 3) completed by participants. Intensity of global withdrawal by VAS. Observers rated withdrawal as "very difficult" to "very easy". Study "partially supported by a grant from AKZO‐Organon Switzerland." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomized ... by groups of four." Comment: The method of sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were randomized and on double‐blind conditions allocated to one of the treatment groups." Comment: This information is insufficient to make a judgement on the adequacy of allocation concealment |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind, double‐dummy stated |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind, double‐dummy stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Missing data concerned (VAS) of one patient and exit laboratory tests one patient." Comment: Missing data not sufficient to have significant impact |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised, placebo‐controlled, double‐blind trial | |
| Participants | Setting: inpatient, Italy. Participants: 20 heroin addicts requesting detoxification. Group sizes: 10 in each group. Mean age: 26 years. 75% men | |
| Interventions | (1) Clonidine, 5 μg/kg body weight in 2 doses/day. (2) Placebo. Both groups also received flunitrazepam and Laevosan (lactulose, a synthetic non‐digestible sugar used in the treatment of chronic constipation and hepatic encephalopathy). Scheduled duration of treatment unclear; outcomes reported for first 72 hours | |
| Outcomes | Mean withdrawal score over 72 h, and at 24, 48, and 72 h (reported as mean score only, and with results of analysis of variance); mean scores of individual symptoms (graphs only) | |
| Notes | Details of scale for assessment of withdrawal not reported. Source of funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants "were divided randomly into two groups." Comment: Method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind (participants and observer) stated |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind (participants and observer) stated |
| Incomplete outcome data (attrition bias) | Unclear risk | No drop‐out reported |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Controlled, double‐blind trial. Detoxification preceded admission to drug‐free therapeutic community | |
| Participants | Setting: inpatient treatment, no telephone calls or visitors, Barcelona, Spain. Participants: 45 heroin users, dependent by DSM‐III‐R. Group sizes: (1) n = 26, (2) n = 19. Analysis based on 30/45 participants who completed 12 days of treatment. Of 30 who completed study. Mean age: 23.5 years. 80% men. Mean 4.2 years of heroin use, 1.8 previous supervised withdrawal attempts | |
| Interventions | (1) Clonidine, 0.9 to 1.35 mg/day. (2) Methadone 30 to 45 mg/day. Initial dose based on participant's weight and heroin consumed in previous month. Both drugs given every 8 hours and tapered over 10 days. Flunitrazepam and acetylsalicylic acid (aspirin) as adjunct medications. Psychotherapeutic support given. Naloxone challenges (0.4 mg sc) on day of discharge. Scheduled duration 8 to 10 days | |
| Outcomes | Number of participants with each of 4 withdrawal signs or symptoms (muscular aching, anxiety, weeping, sleep disorders) and each of 4 adverse effects (flatulence, daytime sleeping, asthenia, fatigue during walking); reported as graphs by day of detoxification; mean doses of drugs administered; mean duration for participants who completed treatment; number discharged drug‐free | |
| Notes | Withdrawal rated daily by nurses (19 withdrawal signs, 17 adverse effects rated present/absent). Participants completed State‐Trait Anxiety Inventory Questionnaire on days 1, 2, 3, 4, 7, and 10. Participants monitored by random urine screening. Source of funds research grants, with placebo clonidine provided by Boehringer Ingelheim | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study was conducted in double‐blind fashion ..." Comment: The characteristics of the groups were not compared. Information was insufficient to make a judgement on the adequacy of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind stated; placebos used |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind stated; placebos used |
| Incomplete outcome data (attrition bias) | High risk | 15/26 (58%) participants taking clonidine and 15/19 (79%) participants taking methadone completed treatment. Data on withdrawal symptoms and adverse effects reported only for those who completed treatment. No information on characteristics of participants who dropped out |
| Selective reporting (reporting bias) | Low risk | All outcomes assessed appear to have been reported |
| Other bias | Low risk | None apparent |
| Methods | Randomised, controlled, double‐blind trial | |
| Participants | Setting: home‐based, Manchester, UK. Participants: 50, opioid dependent by DSM‐IV, using methadone or other opiates. Group sizes: (1) n = 26, (2) n = 24. (1) 43%, (2) 71% used iv, otherwise groups similar. Mean age: 28 years. 70% men. Mean 6.9 years opiate use. 66% had previous detoxification experience; 17% employed, 63% supported by relative. | |
| Interventions | All stabilised on methadone (40 mg/day or less) prior to study. (1) n = 26: lofexidine, 0.2 mg/capsule, or (2) n = 24: clonidine 0.1 mg/capsule. Both increased over 3 days to max 8 capsules/day, and tapered over last 3 days. Various adjunct medications available. Total duration of medication unclear. Home‐based treatment with participants visited at least 4 times in week 1, 3 times in week 2, and once in each of weeks 3 and 4. Treatment considered successful if participants opiate‐free by urine test at 4 weeks. Scheduled duration 12 days | |
| Outcomes | Number completing treatment; number with extra home visits; mean withdrawal scores; mean side effects score | |
| Notes | Participants completed Short Opiate Withdrawal Scale (10 items, 0 to 3 severity) during each visit by trial personnel. Financial support provided by Britannia Pharmaceuticals and the "North West Region Medical Innovation Scheme" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned randomly ..." Comment: Although the method of sequence generation was not reported, the similarity of the groups and allocation by the separate pharmacy suggests the method was adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment courses were sent out to patients by Trafford pharmacy, which also conducted the treatment group assignment without knowledge of patient or drug team staff." |
| Blinding (performance bias and detection bias) | Low risk | Participants and treating staff blind to treatment. Drugs prepared in identical capsules |
| Blinding (performance bias and detection bias) | Low risk | Participants and treating staff blind to treatment. Drugs prepared in identical capsules |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Those [participants] stopping early experienced higher maximum SOWS scores." Comment: Differential drop‐out may have reduced mean daily SOWS score in clonidine group to a greater extent than the lofexidine group, but this outcome was not used in this review |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised, placebo‐controlled, double‐blind trial | |
| Participants | Setting: hospital outpatient clinic, Parma, Italy. Participants: 152, drug abuse disorder by DSM‐III‐R, heroin users. Group sizes: (1) n = 33, (2) n = 42, (3) n = 58, (4) n = 19. Similarity of groups not reported. Age: 18 to 32 years. 82% men | |
| Interventions | (1) Clonidine, 0.15 mg iv 3 times a day. (2) Clonidine, 0.15 mg iv 3 times a day + naltrexone, 12.5 mg day 2 then 50 mg/day for 3 months. (3) Clonidine, 0.15 mg iv 3 times a day + naloxone, 0.2 mg iv day 2, 0.4 mg 2 times a day on days 3 and 4, then naltrexone 50 mg/day from day 5. (4) iv saline + oral placebo. Daily clinic attendance with 4 hours iv therapy in morning, 3 hours in afternoon. (Groups 2 and 3 not considered for this review.) Scheduled duration of treatment unclear | |
| Outcomes | Mean total withdrawal score at 48 and 72 hours; bar graphs for days 1, 2, and 3 showing ratings of individual items of withdrawal scale; morphine metabolites in urine; Hamilton Rating Scale for Depression on day 1, day 8, and 6 months | |
| Notes | Withdrawal assessed by observer only using 9‐item scale, mainly of objective signs. Source of funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "All the patients were randomly divided into four groups ..." Comment: Group sizes differed, and similarity of the characteristics of the groups was not discussed. The adequacy of sequence generation is doubtful |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Double‐blind stated, but given the differences in group sizes, it is doubtful whether the blind was maintained for treating personnel, participants, and observers |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind stated. Although it is doubtful whether the blind was maintained, these outcomes are considered unlikely to be affected by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐out in first week higher in placebo compared with other groups. Given the marked difference in withdrawal severity between clonidine and placebo groups, the differential drop‐out is unlikely to have a clinically significant impact on withdrawal scores (the main outcome reported) |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised, controlled trial. Heavy polydrug use, comorbid psychiatric or medical conditions were exclusion criteria | |
| Participants | Setting: outpatient clinic, Parma, Italy. Participants: 98 dependent by DSM‐IV, urine positive for morphine, withdrawing from heroin. Group sizes: (1) n = 32, (2) n = 32, (3) n = 34 (only groups 1 and 3 considered for this review). Groups similar in psychiatric and psychometric data. Age: 18 to 36 years. 72% men. Drug use: 2 to 6 years | |
| Interventions | Heroin use continued until 12 hours before treatment. Withdrawal managed with: (1) Clonidine 0.15 mg/100 mL saline iv 6 times/day for 2 days, 0.15 mg 3 times/day for 3 days, additional 0.15 mg orally each evening. Total 5 days of treatment. (2) Clonidine + naloxone and naltrexone (not considered for this review). (3) Methadone, oral, 40 mg/day in single dose, tapered over 10 days. Treatment in outpatient clinic with those participants in groups (1) and (2) receiving 4 hours iv therapy morning and afternoon. Unclear whether the extent of clinic care was the same for group (3). All received counselling. Drug‐free programme postdetoxification with naltrexone. Naltrexone commenced (1) day 6, (2) during detox, (3) 5 days after taper. Scheduled duration (1) 5, (2) 3, (3) 10 days | |
| Outcomes | Graphs of mean daily withdrawal scores; craving scores before and after detoxification; % of positive urine samples; number accepting naltrexone; % of participants in maintenance naltrexone treatment 3 months after detoxification | |
| Notes | Withdrawal rated by observer (9 items, 0 to 5 severity). Urine testing during detoxification and follow‐up period. Source of funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All the subjects were randomly divided into three groups." Comment: Method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) | High risk | Blinding not discussed; the timing of naltrexone commencement in the treatment protocols differed. This suggests that there was probably no blinding of treatment personnel, and possibly not of participants either |
| Blinding (performance bias and detection bias) | Low risk | These outcomes are considered unlikely to be affected by knowledge of treatment group |
| Incomplete outcome data (attrition bias) | Unclear risk | The 3 groups differed in the proportions who accepted and continued extended naltrexone maintenance treatment, but it is unclear how this difference might translate into missing data; it is also unclear whether differences in drop‐out may have influenced withdrawal scores. (This outcome was not used in this review.) |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Unclear risk | Unclear whether all 3 groups received same amount of clinic care |
| Methods | Randomised, controlled, single‐blind trial | |
| Participants | Setting: inpatient treatment, New Delhi, India. Participants: 120 heroin dependent by ICD‐9. Group sizes: 60 per group. Groups comparable on demographic characteristics. Mean age: 26 years. 100% men. All using heroin by inhalation, mean: (1) 1.8 g/day, (2) 1.3 g/day. Duration of use: about 2.5 years. 51% married, 17% unemployed; male relative to accompany participants in hospital | |
| Interventions | (1) Clonidine, 0.1 mg rising to 0.2 mg 3 times/day. (2) Chlordiazepoxide, 10 mg 3 times/day, plus chlorpromazine, 100 mg day 1, then 200 mg 3 times a day. Drugs tapered when withdrawal symptoms remitted. Additional symptomatic medications as needed. Scheduled duration of treatment not reported | |
| Outcomes | Frequency of 17 withdrawal symptoms | |
| Notes | Participants interviewed for the presence of withdrawal symptoms each morning by person blind to treatment regimen. Source of funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly divided into two equal groups." Comment: Method not reported |
| Allocation concealment (selection bias) | High risk | Method not reported. Study stated as single‐blind (observer only), hence treating doctor and participants probably aware of allocation |
| Blinding (performance bias and detection bias) | Low risk | Observer rating withdrawal symptoms blind to group allocation |
| Blinding (performance bias and detection bias) | Low risk | No objective outcomes reported, other than completion of treatment |
| Incomplete outcome data (attrition bias) | Unclear risk | Drop‐out not reported. Insufficient information to make a judgement |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised, placebo‐controlled, double‐blind trial. Some drop‐out in both groups for logistic reasons associated with prison setting | |
| Participants | Setting: Prison healthcare centre, Winchester, UK. Participants: 68, opioid‐dependent by DSM‐IV, using heroin, methadone, or both. Group sizes: (1) n = 32, (2) n = 36. Groups similar on drug use, demographics, and severity of dependence. Mean age: 30 years. 100% men. Mean 9 years from first use of illicit heroin; some participants also dependent on benzodiazepines | |
| Interventions | Most in custody 24 to 48 h before entering study. Withdrawal managed with: (1) Lofexidine, 0.6 mg/day, increased 0.4 mg/day to max 2 mg/day, tapered to 0 mg/day by day 11. (2) Methadone 30 mg/day, tapered to 0 mg/day over 10 days. Both drugs administered twice a day (supervised). Scheduled duration 10 days | |
| Outcomes | Maximum, minimum withdrawal scores and time of occurrence; overall withdrawal score; use of adjunct medication; retention in treatment; completion of 10 days; reasons for withdrawal from study; incidence of adverse events | |
| Notes | Participants completed withdrawal problem scale (20 items) and Short Opiate Withdrawal Scale (8 items) daily ‐ reported as combined scores. Britannia Pharmaceuticals provided medication and support for independent trial monitor | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The pharmacist who made up the medication used a simple randomisation procedure to allocate each participant to one arm of the trial." |
| Allocation concealment (selection bias) | Low risk | Quote: "The independent pharmacy team at the prison oversaw the randomisation and blinding procedure." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Both the patient and health centre clinicians were blind to the assigned treatment group." |
| Blinding (performance bias and detection bias) | Low risk | Quote: "Both the patient and health centre clinicians were blind to the assigned treatment group." |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Complete sets of withdrawal scale data were created from scores for 63 (92.6%) patients ..." |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised, controlled trial. Not all participants had entered treatment voluntarily. Concurrent medical condition, infectious diseases, mental illnesses exclusion criteria. Endpoint of naloxone challenge used for only 50% of participants | |
| Participants | Setting: inpatient treatment in 5 different rehabilitation centres, China. Participants: 200 opiate dependent by DSM‐III‐R, heroin users. Group sizes: 100 in each group. Methadone group had higher proportion using via oral route (80% compared with 67%). No other differences in demographics or drug use history. Mean age: 24.8 years. 78% men. 74% using orally, rest using iv or both orally and iv; mean duration of addiction: 15.5 months, at admission around 10 hours since last use; 71% had not previously received treatment | |
| Interventions | (1) Clonidine, "sufficient" dose days 1 to 4, tapered days 5 to 8, ceased after day 11. Mean (± SD) max dose day 2: 1.05 ± 0.14 mg. (2) Methadone, max days 1 to 2 then tapered and ceased after day 12. Mean (± SD) max dose day 2: 21.6 ± 5.0 mg. For both drugs, initial dose dependent on body weight, physical condition, heroin intake previous week. Dose titrated against withdrawal and side effects. Scheduled duration 12 days | |
| Outcomes | Mean daily withdrawal score; duration of treatment; side effects score | |
| Notes | Report in Chinese. English translation obtained. Symptoms and vital signs assessed daily using Himmelsbach scale as guide. 21 designated symptoms and vital signs also assessed. Source of funds not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomly divided." Comment: Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Insufficient information reported to determine whether there was any blinding |
| Blinding (performance bias and detection bias) | Low risk | These outcomes were not used in this review, and were unlikely to be affected by a lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | High risk | Rates of completion of withdrawal confounded as not all participants had entered treatment voluntarily, hence there was some compulsion to complete withdrawal |
| Methods | Randomised, controlled, double‐blind trial. Alcohol dependence an exclusion criterion | |
| Participants | Setting: inpatient treatment, Birmingham, UK. Participants: 28 opiate dependent by history and urine screen, admitted for inpatient detoxification. Group sizes: 14 in each group. Characteristics of participants not reported, but groups stated to be well matched with regards to age, sex, and opiate use prior to trial. 68% men | |
| Interventions | All stabilised on methadone for 3 to 4 days prior to study. Methadone stopped on day 3 by substitution with placebo; participants but not observer blind to cessation. Withdrawal managed with: (1) Clonidine, 0.2 mg rising to max 0.9 mg/day. (2) Lofexidine, 0.4 mg rising to max 1.8 mg/day. Methadone placebo stopped day 14. Clonidine or lofexidine tapered over following 4 days. Lorazepam as adjunct medication if needed. Any regular psychoactive medication maintained. Scheduled duration 16 days | |
| Outcomes | Mean daily withdrawal score (graph); mean standing systolic blood pressure (graph); number of participants and number of occasions of use of lorazepam; number of participants reporting side effects and number of patient days of reported side effects | |
| Notes | Withdrawal rated by nurses (scale stated as similar to that used by Gold 1980c). Participants completed VAS. Study supported by Merrell Dow (lofexidine, placebo, technical assistance) and Wellcome (methadone, placebo) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomised double‐blind allocation." Comment: Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind stated; medication prepared in identical capsules |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind stated; medication prepared in identical capsules |
| Incomplete outcome data (attrition bias) | Unclear risk | Drop‐out not reported. Unable to assess extent and impact of incomplete data |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised, placebo‐controlled, double‐blind trial. Current alcohol abuse an exclusion criterion. Component of multicentre study ‐ see also Senay 1983 | |
| Participants | Setting: outpatient treatment, Connecticut, USA. Participants: 50 withdrawing from methadone maintenance treatment. Group sizes: 25 in each group. 1 in clonidine group did not commence treatment ‐ did not meet blood pressure criteria. Published report based on remaining 49 participants. Groups similar on age, sex, race, and length of addiction. Mean age: 29.5 years. 76% men. Mean length of addiction: 10 years | |
| Interventions | Comfortable on methadone 20 mg/day for 2 weeks. (1) Clonidine (plus methadone placebo), initial dose 0.3 mg/day, 3 divided doses, tablets; gradual increase to max 1 mg/day by day 6, tapered by 20% to 25% per day from day 11. (2) Methadone (plus clonidine placebo), initial dose 20 mg/day, single daily dose as oral syrup, tapered by 1 mg/day. Chloral hydrate as adjunct medication. Scheduled duration of study 30 days | |
| Outcomes | Retention in treatment; mean withdrawal scores at baseline, weeks 1 to 2 and 3 to 4; max ratings; number using sleep medications; number completing detoxification; incidence of side effects | |
| Notes | Withdrawal rated by nurses (24 items, 0 to 3 severity) and participants (31 items, 1 to 4 severity). Side effects rated by physicians and nurses. Supported by grants from National Institute on Drug Abuse and Boehringer Ingelheim | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomly assigned." Comment: Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) | Low risk | Participants and observers blind |
| Blinding (performance bias and detection bias) | Low risk | Participants and observers blind |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Most clonidine failures typically dropped out of treatment during the first week of the study, whereas the methadone failures tended to stay in the study until the third week" and withdrawal scores were higher for treatment failures compared to successes. Comment: The different timing of drop‐out potentially distorts mean withdrawal scores; this outcome not used in this review. The approach to analysis by study authors reduced the risk of bias |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised controlled trial. Participants allocated at ratio of 1.5:1 for Qigong relative to other 2 groups. Blinding not able to be maintained, but each study group unaware of others. No dropouts; participants in mandatory treatment | |
| Participants | Setting: residential treatment, China. Participants: 86 heroin users, dependent by DSM‐III‐R, urine positive for morphine. Group sizes: (1) n = 26, (2) n = 34, (3) n = 26. No significant difference in baseline data of groups. Mean age: 32 years. 100% men. 79 using by injection, 7 by sniffing; mean 5.5 years of drug use; mean 27 hours between last use and entry to treatment centre | |
| Interventions | (1) Symptomatic medications. (2) Qigong ‐ traditional Chinese health practice. (3) Lofexidine, 0.4 mg twice day 1, 0.6 mg 3 times/day for 3 days, then tapered to cease after day 10. Only groups (1) and (3) considered for this review. Scheduled duration about 10 days | |
| Outcomes | Graph of daily withdrawal scores; Hamilton Anxiety Rating Scale scores days 0, 5, and 10; days to achieve morphine‐negative urine | |
| Notes | Withdrawal rated by observers, 5 levels, 23 symptoms. Source of funds not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned ..." (abstract) and "Qualified subjects were assigned into one of three groups according to the order in which they entered the treatment centre." Comment: Method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Study established with observers blind, but authors noted the blind was difficult to maintain. Blinding of participants was not possible, but participants were not aware of other treatment groups |
| Blinding (performance bias and detection bias) | Low risk | These outcomes unlikely to be affected by lack of blinding, but were confounded by treatment being mandatory. These outcomes not used for this review |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | High risk | Rates of completion of withdrawal confounded as participants were in mandatory treatment |
| Methods | Randomised, controlled, double‐blind trial | |
| Participants | Setting: inpatient treatment in hospital detoxification ward, Taipei, Taiwan. Participants: 80 heroin users, opioid dependent by DSM‐IV. Group sizes: 40 in each group. Groups similar on demographics and drug use history. Mean age: 32 years. 81% men. All Chinese, 61 used iv, 9 im, 10 by smoking; 18% also used methamphetamine; mean duration of heroin use around 4 years; first detoxification attempt for 20% | |
| Interventions | (1) Lofexidine, max dose 1.6 mg/day. (2) Clonidine, max dose 0.6 mg/day, in 4 divided doses. Total dosing period 6 days, with 10 days treatment | |
| Outcomes | Symptom frequency on days 2 and 3; graph of mean scores; median duration of treatment (patient days used as denominator to adjust for different retention rates); number with 1 or more items rated moderate on day of discharge; number of doses omitted due to low blood pressure; retention rates (graph) | |
| Notes | Withdrawal rated by observers 3 times a day (15 items, 0 to 3 severity). Funding support from Britannia Pharmaceuticals and Taiwan Major Chem. & Pharm. Corp. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All study subjects were randomly assigned ..." Comment: Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not reported |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind stated. Medication prepared in identical capsules |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind stated. Medication prepared in identical capsules |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts excluded from analysis of withdrawal scores. Quote: "Significantly fewer subjects had self‐discharged from the lofexidine group than clonidine group at day four ... and at day five." Comment: Withdrawal severity similar for the 2 groups, so differential drop‐out unlikely to have clinically significant impact on outcomes |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised, controlled, double‐blind trial | |
| Participants | Setting: inpatient, Spain. Participants: 88 heroin dependent by DSM‐III‐R, admitted for inpatient detoxification. Group sizes: (1) n = 43, (2) n = 45. Groups stated to be similar. Mean age: 25 years. 80% men. Mean 65 months of addiction. | |
| Interventions | (1) Clonidine 0.9, 1.3 or 1.8 mg/day. (2) Guanfacine, 6, 12, or 18 mg/day. Doses of both medications determined by dose of heroin and body weight. Duration of treatment (9, 12, or 15 days) also dependent on heroin use and body weight. Alprazolam 2 mg/day as adjunct medication | |
| Outcomes | Days of admission; days of treatment; mean scores for individual withdrawal and adverse effects signs and symptoms; changes in blood pressure and heart rate | |
| Notes | 17 withdrawal signs and symptoms and 19 adverse effects assessed. Source of funds not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Assigned to either treatment group ... through a random computer table." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind stated |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind stated, and these outcomes considered unlikely to be affected by knowledge of group allocation |
| Incomplete outcome data (attrition bias) | Unclear risk | Timing of drop‐out not reported. Unclear how withdrawal scores might be affected |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised, placebo‐controlled, double‐blind trial | |
| Participants | Setting: inpatient, treatment services operated by non‐government organisations, Tehran, Iran. Participants: 90 opioid dependent by DSM‐IV, using opium (93%), opium extract (42%), heroin (29%), and crack (74%). Use by injection reported by 20%. Group sizes: 30 in each. Group characteristics similar. Age: 25to 40 years All male. | |
| Interventions | (1) Hab‐o Shefa, preparation of plant extracts used in traditional Iranian medicine, 3 g/day in 4 divided doses, tapered from day 8. (2) Clonidine, 0.2 to 0.4 mg days 1 to 2, 0.6 mg days 3 to 18, 0.4 to 0.2 mg days 20 to 21. (3) Placebo (sugar). Group (1) not considered for this review. All participants received an assisted self help intervention (behavioural therapy and the 12‐step principles). Scheduled duration of treatment 21 days. Naloxone challenge test on day 21 | |
| Outcomes | Overall average scores for withdrawal, craving, depression, side effects and graphs of daily mean withdrawal scores | |
| Notes | Withdrawal assessed with Subjective (13 items, possible scores 0 to 13), Objective (16 items, possible scores 0 to 64), and Clinical (11 items, possible scores 0 to 48) Opiate Withdrawal Scales. Craving assessed by visual analogue scale. Depression assessed with Beck and Hamilton scales. Side effects rates by investigator as present or absent. Source of funds not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computerized random numbers" (Materials and Methods) |
| Allocation concealment (selection bias) | Low risk | Quote: Medication "in unit size capsules packed in the boxes that were encoded ... for each patient individually and were distributed by a third person who had no contact with ... the investigator [or] the patients". (Materials and Methods) |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind stated. Quote: "A physician ... who ... was blind to capsules content, performed all the clinical assessments". (Materials and Methods, Setting and Ethics) |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind stated |
| Incomplete outcome data (attrition bias) | Low risk | Missing data replaced by last observation carried forward. Analysis of variance applied for two‐way comparisons |
| Selective reporting (reporting bias) | Unclear risk | Average side effects score reported but no details of nature of side effects experienced. Stated that all participants completed the study, but it is not specifically stated whether this meant that all stayed in treatment for 21 days |
| Other bias | Low risk | None apparent |
| Methods | Randomised, placebo‐controlled, double‐blind trial. Participants and observers blind to medication. Polydrug use exclusion criterion. Analysis based on 90 (30 in each group) who completed 12 or more days of treatment | |
| Participants | Setting: inpatient treatment in general hospital detoxification unit, Barcelona, Spain. Participants: 170 heroin dependent by DSM‐III‐R. Group sizes: (1) n = 40, (2) n = 68, (3) n = 62. No differences in characteristics of groups. Mean age: about 24 years. 80% men. Mean duration of opioid use around 5 years. | |
| Interventions | Initial dose of medication dependent on weight and heroin use in previous week. (1) Clonidine, mean (± SD) max dose 1.05 ± 0.1 mg/day. (2) Methadone, mean (± SD) max dose 37.3 ± 4.49 mg/day. (3) Guanfacine, mean (± SD) max dose 3.58 ± 0.41 mg/day. For all drugs, max dose given on days 2 and 3. Drug tapered over 11 days. Benzodiazepines as adjunct medication as needed. Scheduled duration 11 days | |
| Outcomes | Time course (graphs) of withdrawal score, mydriasis, and side effects; mean max withdrawal score; time course of cardiovascular effects; mean duration of treatment for those who completed and those who did not complete treatment; number experiencing side effects; number completing detoxification | |
| Notes | Withdrawal and side effects rated by observers. Participants completed psychometric evaluations. Study supported by Boehringer Ingelheim and Sandoz SAE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Subjects were randomly assigned to ... one of ... three groups" but "In order to achieve 30 patients in each group a total of 170 (40 methadone, 68 clonidine, 62 guanfacine) had to be included." Comment: It is questionable whether a truly random sequence generation could achieve the different group sizes reported |
| Allocation concealment (selection bias) | High risk | Method of allocation not reported, but recruitment continued until 30 participants in each group had completed 12 or more days of treatment, suggesting inadequate concealment of allocation |
| Blinding (performance bias and detection bias) | Low risk | Research nurses (observers) and physician blind to treatment condition |
| Blinding (performance bias and detection bias) | Low risk | Research nurses (observers) and physician blind to treatment condition |
| Incomplete outcome data (attrition bias) | High risk | Analysis based on participants who completed 12 or more days of treatment. No information on participants who dropped out |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised, controlled, double‐blind study undertaken in 3 phases with third group (guanfacine 4 mg/day) introduced in second phase | |
| Participants | Setting: inpatient, Barcelona, Spain. Participants: 144 heroin dependent by DSM‐III‐R. Group sizes: (1) n = 75, (2) n = 43, (3) n = 26. Stated that there were no differences between groups. Mean age: 27.1 years. 71% men. Using heroin mean 656 mg/day, 52% HIV positive | |
| Interventions | Stabilised on methadone, dose dependent on body weight and heroin consumption. Methadone tapered to 10% (methadone group) or 50% (guanfacine groups) of initial dose prior to detoxification. (1) Continued tapered methadone (3 divided doses/day). (2) Guanfacine 3 mg substituted for methadone on day 9. (3) Guanfacine 4 mg substituted for methadone on day 9. Benzodiazepines and hypnotics available as adjunct medication. Scheduled duration 18 days | |
| Outcomes | Mean daily doses medication; retention rate (graph); mean daily withdrawal scores (graphs); mean dose diazepam | |
| Notes | Opiate withdrawal checklist completed by nurses. Opiate withdrawal scale completed by participants. Study supported by grants, but details of grant source unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The pharmaceutical unit of the hospital was responsible for the randomization ..." Comment: Although the method of sequence generation was not reported, the similarity of the groups and allocation by the separate unit suggests the method was adequate |
| Allocation concealment (selection bias) | Low risk | Allocation by pharmacy |
| Blinding (performance bias and detection bias) | Low risk | Nursing staff, treating doctor, and participants blind |
| Blinding (performance bias and detection bias) | Low risk | Nursing staff, treating doctor, and participants blind |
| Incomplete outcome data (attrition bias) | Low risk | No significant difference between groups in length of stay. The similarity in withdrawal scores for the 3 groups indicates that drop‐out is unlikely to have a clinically significant impact on this outcome |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised, controlled, double‐blind trial. Codependence on other drugs or abuse of alcohol exclusion criteria. Component of multicentre study ‐ see also Kleber 1985 | |
| Participants | Setting: outpatient treatment, Chicago, USA. Participants: 61, stabilised on methadone 20 mg/day. Group sizes: (1) n = 30, (2) n = 31. 100% men. Mean duration of addiction: 11.4 years | |
| Interventions | (1) Clonidine, 0.5 mg day 1, additional 0.1 to 0.3 mg/day as needed to max 1.0 mg/day, tapered after day 10. (2) Methadone decreased 1 mg/day. Diuretics and chloral hydrate only adjunct medications, both groups. Scheduled duration of study interventions 30 days | |
| Outcomes | Retention in treatment; number completing detoxification; urine screening results; concomitant medications and illicit drugs; incidence of adverse experiences | |
| Notes | Withdrawal severity not assessed. Study partly supported by funds from Boehringer Ingelheim | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized, double‐blind, parallel group, 30‐day study." Comment: Method of sequence generation not reported, and group similarities not assessed |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not reported |
| Blinding (performance bias and detection bias) | High risk | Observers making symptom ratings blind, but those assessing adverse reactions and vital signs were aware of group allocations |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind stated, and these outcomes considered unlikely to be affected by knowledge of group allocation |
| Incomplete outcome data (attrition bias) | Low risk | Retention is an outcome. Other outcomes reported in such a way that is not influenced by drop‐out |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised controlled trial | |
| Participants | Setting: outpatient, Budapest, Hungary. Participants: 26 intravenous heroin users. Group sizes: (1) n = 16, (2) n = 10. Groups similar on demographics and drug use. Mean age: 24 years. 80.8% men. Mean duration of heroin use: 1.7 years. | |
| Interventions | (1) Tizanidine 3 x 80 mg/day. (2) "Usual" treatment (symptomatic medication). (1) 13/16 and (2) 10/10 received tramadol (narcotic analgesic) with doses reduced by tapering. Various other adjunct medications as required. Scheduled duration 10 days | |
| Outcomes | Comparison of withdrawal scores; graphs of subjective severity of tremor and diarrhoea; number completing withdrawal; number relapsing during follow‐up | |
| Notes | Participants rated withdrawal (7 items, each rated 0 to 5) daily. Published in Hungarian, information extracted with help of interpreter. Source of funds not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were divided into two groups." Comment: Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) | High risk | No blinding reported |
| Blinding (performance bias and detection bias) | Low risk | No blinding, but these outcomes considered unlikely to be affected by knowledge of group allocation |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts during withdrawal |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised controlled trial with stratification on withdrawal severity, pain, cocaine use, CD4 cell count. Groups similar except (1) more likely to have been admitted for fever/cellulitis | |
| Participants | Setting: inpatient treatment, AIDS service, Baltimore, USA. Participants: 55 HIV‐positive, opioid‐dependent by self report and physical examination, hospitalised for acute medical illness. Group sizes: (1) n = 21 (not considered for this review), (2) n = 16, (3) n = 18. (1) 71% (2) 69% (3) 44% men. Mean age (± SD): 39.7 ± 5.6. Duration of heroin use: about 18 years. Concurrent alcohol dependence, enrolment in methadone maintenance treatment both exclusion criteria. 95% to 100% African‐American | |
| Interventions | All stabilised with morphine 10 mg im every 4 hours as needed up to 6 hours prior to enrolment in study. 3‐day taper with: (1) Buprenorphine 0.6 mg im every 4 hours day 1, every 6 hours day 2, every 8 hours day 3. (2) Clonidine, oral 0.2 mg loading dose, 0.1 mg every 4 hours day 1, every 6 hours day 2, every 8 hours day 3. (3) Methadone, oral 30 mg day 1, 20 mg day 2, 10 mg day 3. All received clonidine transdermal patch day 4. No adjunct treatment for withdrawal. Scheduled duration 3 days | |
| Outcomes | Withdrawal severity; completion rate; adverse effects; use of supplemental morphine for pain | |
| Notes | Withdrawal assessed by Short Opiate Withdrawal Scale (participants) and Objective Opiate Withdrawal Scale (observers). Supported by National Institute on Drug Abuse Intramural Research Program | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... patients were randomly assigned ..." "... patients were stratified on four characteristics ..." Comment: Method of sequence generation not specifically reported but with stratification on 4 characteristics is likely to be computer based |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not reported |
| Blinding (performance bias and detection bias) | Low risk | Quote: "To maintain the blind, one active medication and two inactive medications were administered to all participants." |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind stated, placebos used |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐out was related to the acute medical condition that was the reason for hospital admission and was unlikely to introduce bias to outcome assessments. Statistical methods allowed for missing data and variation in time of assessment |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised, controlled, double‐blind trial | |
| Participants | Setting: inpatient, hospital, Barcelona, Spain. Participants: 32 heroin users, admitted for treatment of organic disease (mainly infectious disease related to consumption of drugs). Group sizes: (1) n = 14, (2) n = 8, (3) n = 10. Mean age: 23 years. 65% men. | |
| Interventions | (1) Methadone, 30 mg/day. (2) Clonidine, 10 μg/kg/day. (3) Levomepromazine (neuroleptic) 75 mg/day. Doses of all drugs increased until stable, maintained 3 days, then tapered. Treatment scheduled for around 8 days | |
| Outcomes | Mean opioid withdrawal score; mean score of secondary effects; mean score of adjustment to hospital setting; number completing treatment | |
| Notes | Ratings of withdrawal (24 items), side effects (19 items), attitudes and disruptive behaviour during hospitalisation (11 items) daily by single observer. Urine screening used. Source of funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation by random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind stated |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind stated |
| Incomplete outcome data (attrition bias) | Low risk | Difference in drop‐out rates insufficient to distort reported outcomes |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised, placebo‐controlled, double‐blind trial. May include some participants from multicentre study ‐ see Senay 1983 | |
| Participants | Setting: outpatient, New York, USA. Participants: 26 withdrawing from methadone maintenance (n = 19) or heroin or methadone or both (n = 7). Group sizes: 13 in each group. Groups stated as similar. Mean age: 31 years. 85% men. Mean duration of addiction 10 years | |
| Interventions | Stabilised for 3 weeks on methadone 15 to 30 mg/day, then: (1) Clonidine, dose titrated against symptoms and side effects to max 1.2 mg/day. (2) Methadone reduced by 1 mg/day. Clinic visits 3 to 5 times per week. Scheduled duration of study intervention 30 days | |
| Outcomes | Number achieving 10 days opioid free; number initiating naltrexone maintenance treatment | |
| Notes | Ratings of withdrawal not reported. Partial support from National Institute on Drug Abuse | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomly assigned." Comment: Method of sequence generation not reported and insufficient information on group characteristics to make a judgement on adequacy |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) | Low risk | Participants and investigators blind to medication. Investigators not informed of blood pressure measurements to avoid breaking blind |
| Blinding (performance bias and detection bias) | Low risk | Participants and investigators blind to medication. Investigators not informed of blood pressure measurements to avoid breaking blind |
| Incomplete outcome data (attrition bias) | Low risk | Completion of treatment is the only outcome included in analyses for this review. Drop‐out was not clearly reported |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | None apparent |
| Methods | Randomised, placebo‐controlled, double‐blind trial. Use of long‐acting opioids (methadone, l‐alpha‐acetylmethadol, buprenorphine) an exclusion criterion | |
| Participants | Setting: inpatient, multiple sites, USA. Participants: 68 opioid‐dependent by DSM‐IV. Group sizes: (1) n = 35, (2) n = 33. Groups similar on demographics. Mean age: 41 years. 87% men. 67/68 heroin users, 1 using hydromorphone; 67.6% iv users; 17.5% married; 68% worked at least part time | |
| Interventions | Stabilised on morphine sulphate 3 days (up to 100 mg/day in 4 doses sc). (1) Lofexidine 3.2 mg/day. (2) Placebo in 4 divided doses for 4 days. On day 8 (1) lofexidine 1.6 mg/day or (2) placebo. Placebo days 9 to 11. Scheduled duration of treatment 11 days | |
| Outcomes | Mean withdrawal score; number retained in treatment; standing and sitting blood pressure | |
| Notes | Principal measure used was withdrawal assessed with Modified Himmelsbach Opiate Withdrawal Scale (completed by observer), but other scales also used including participant‐completed scales. Study terminated early due to significant findings. Study funded by research grants from National Institute on Drug Abuse. Britannia Pharmaceuticals provided medication and placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Coordinating Center ... generated a randomization sequence for each site separately, in blocks of four, using non‐sequential subject numbers." |
| Allocation concealment (selection bias) | Low risk | Quote: "(Coordinating Center) provided site with a randomization number which corresponded to a specific drug therapy kit that had previously been shipped to the site." |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind stated, medications provided as identical tablets |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind stated, medications provided as identical tablets |
| Incomplete outcome data (attrition bias) | Low risk | Difference in drop‐out insufficient to distort outcomes |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias | Low risk | Trial stopped early due to significant findings |
DSM‐IIIR: Diagnostic and Statistical Manual of Mental Health ‐ Third Edition Revised; DSM‐IV: Diagnostic and Statistical Manual of Mental Health ‐ Fourth Edition; h: hour; HIV: human immunodeficiency virus; ICD: International Classification of Diseases; im: intramuscular; iv: intravenous; max: maximum; sc: subcutaneous; SD: standard deviation; SOWS: Short Opiate Withdrawal Scale; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Comparison of clonidine and baclofen, which is not one of the modalities defined by the inclusion criteria (clonidine is the control intervention for the study) | |
| Compares outcomes for 2 treatment regimens, 1 based on clonidine and 1 on clomethiazole (Heminevrin) plus symptomatic medications. Interventions not offered concurrently, limited information reported on medications and participant characteristics, and data collection probably retrospective | |
| Participants able to choose methadone or lofexidine as treatment approach, with 10‐day and 5‐day lofexidine regimens offered serially. Non‐random allocation introduces risk of bias. This version of review restricted to randomised controlled trials | |
| Randomised controlled trial comparing naloxone and placebo as adjuncts to lofexidine for management of opioid withdrawal. Regimens involving opioid antagonists are not one of the modalities defined by the inclusion criteria for this review | |
| Randomised controlled trial comparing clonidine with reducing doses of methadone. Very little information on participant characteristics. Outcome data limited as no participants completed the study | |
| Study presented as randomised controlled trial, but allocation to groups was alternate, not random. Treatment does not appear to be voluntary ‐ participants arrested and subsequently taken to hospital outpatient department for treatment. Aim to assess effectiveness of tramadol; clonidine used as comparison intervention. Insufficient participant information and insufficient outcome data | |
| Randomised, controlled, double‐blind study comparing clonidine and buprenorphine, which is not one of the modalities defined by the inclusion criteria | |
| Focus appears to be on assessment of withdrawal rather than clonidine as a modality for management of withdrawal. Unclear if participants were undergoing detoxification on a voluntary basis | |
| Comparison of inpatient and outpatient settings for opioid withdrawal managed with lofexidine | |
| Comparison of lofexidine and clonidine when administered in combination with opioid antagonist. Antagonist‐induced withdrawal is not one of the modalities defined by the inclusion criteria | |
| Comparison of clonidine and placebo as adjuncts to tapered methadone for management of opioid withdrawal. Intervention not one defined by inclusion criteria | |
| Effectively single‐group study (placebo controls and double‐blind method used only for first 2 doses of medication). Insufficient outcome data | |
| Compares effect of clonidine on opioid withdrawal for group withdrawing from methadone and group withdrawing from heroin. Insufficient information on treatment and participant characteristics; non‐random allocation | |
| Placebo controls and double‐blind methods used only for first 2 doses of medication and naloxone challenges at the end of withdrawal. 3 groups identified (non‐random allocation) on basis of methadone dose prior to clonidine. No treatment comparison | |
| Reports outcomes of treatment with clonidine for 100 participants withdrawing from methadone. No treatment comparison | |
| Comparison of 3 cohorts of heroin‐dependent people undergoing inpatient detoxification. Not a controlled study ‐ medication regimens variable | |
| Comparison of clonidine and Acetophen (enkephalinase inhibitor), which is not one of the modalities defined by the inclusion criteria. Limited data on treatment outcomes | |
| Compared guanfacine alone with a combination of guanfacine plus propoxyphene, which is not one of the modalities defined by the inclusion criteria | |
| Compared clonidine with lefetamine (an analgesic with partial opioid agonist activity) and buprenorphine, neither of which are modalities defined by the inclusion criteria | |
| Comparison of clonidine, morphine, and placebo in opioid‐dependent participants. Not a complete withdrawal intervention. Morphine was withheld for 24 hours only for tests of the pharmacology of clonidine | |
| Comparison of (1) a calcium channel blocking agent plus dextropropoxyphene, (2) dextropropoxyphene plus a benzodiazepine, and (3) guanfacine with increasing doses of naltrexone from day 4. The comparison modalities were not those defined by the inclusion criteria. Group allocation was sequential, not random | |
| No concurrent treatment comparison ‐ cohort treated with clonidine compared with other cohorts treated with reducing doses of methadone | |
| Randomised controlled trial comparing outcomes of opioid withdrawal managed with clonidine in home or outpatient setting. No treatment comparison as defined by inclusion criteria | |
| Randomised controlled trial comparing dextromethorphan and placebo as adjuncts to clonidine for the management of opioid withdrawal. Comparison is not one defined for this review | |
| Comparison of clonidine with meperidine (pethidine) for management of opioid withdrawal. Group allocation was alternate, not random; meperidine is not one of the modalities defined by the inclusion criteria; and insufficient outcome data were reported | |
| Analysis of outcomes for participants randomly allocated to lofexidine‐naloxone or lofexidine‐placebo, and those ineligible for participation in randomised controlled trial or who refused random allocation and received reducing doses of methadone over 10 days. Significant differences in groups, indicating high risk of bias from allocation method for comparison of lofexidine and methadone. Insufficient outcome data relating to period of acute withdrawal (main outcomes were completion of withdrawal, retention in postdetoxification treatment, and abstinence at follow‐up postdetoxification) | |
| Controlled trial comparing clonidine only with clonidine combined with naltrexone, which is not one of the modalities defined by the inclusion criteria. Group allocation by choice, not random | |
| Randomised controlled trial comparing clonidine only with clonidine plus naltrexone, and buprenorphine followed by clonidine plus naltrexone. Neither of the comparisons are modalities defined by the inclusion criteria | |
| Retrospective study assessing effectiveness of adding stimulant to regimen of symptomatic medication (including clonidine) for management of opioid withdrawal | |
| Comparison of dapiprazole, clonidine, and placebo for management of opioid withdrawal. Methadone administered in decreasing doses for 6 days while doses of study medications increased to maximum day 6. Insufficient outcome data. Unclear if group allocation was random | |
| Randomised controlled trial comparing low‐dose naltrexone and placebo as adjuncts to clonidine for the management of opioid withdrawal. Comparison is not one defined by the inclusion criteria for this review | |
| Randomised controlled trial comparing detoxification in specialist drug dependence unit (managed with reducing doses of methadone) and detoxification in general psychiatric ward of hospital (managed with clonidine). Primary purpose of study was to investigate effect of cue exposure on postwithdrawal outcomes; effect of setting was a secondary study (participants not randomly allocated to setting). Insufficient outcome data. Limited data on modification of signs and symptoms of withdrawal | |
| Comparison of methadone plus clonidine and clonidine alone for management of opioid withdrawal. Non‐concurrent cohort study. Insufficient outcome data | |
| Comparison of lofexidine and symptomatic medications for management of opioid withdrawal. Insufficient outcome data. No details of characteristics of participants. No concurrent treatment comparison |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Peak withdrawal score Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Alpha2‐adrenergic agonist versus placebo, Outcome 1 Peak withdrawal score. | ||||
| 2 Participants with severe withdrawal Show forest plot | 3 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.18, 0.57] |
| Analysis 1.2  Comparison 1 Alpha2‐adrenergic agonist versus placebo, Outcome 2 Participants with severe withdrawal. | ||||
| 3 Completion of treatment Show forest plot | 3 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [1.34, 2.84] |
| Analysis 1.3  Comparison 1 Alpha2‐adrenergic agonist versus placebo, Outcome 3 Completion of treatment. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Peak withdrawal score Show forest plot | 2 | 263 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.02, 0.46] |
| Analysis 2.1  Comparison 2 Alpha2‐adrenergic agonist versus methadone, Outcome 1 Peak withdrawal score. | ||||
| 2 Participants with severe withdrawal Show forest plot | 5 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.81, 1.73] |
| Analysis 2.2  Comparison 2 Alpha2‐adrenergic agonist versus methadone, Outcome 2 Participants with severe withdrawal. | ||||
| 3 Overall withdrawal severity Show forest plot | 3 | 119 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.24, 0.49] |
| Analysis 2.3  Comparison 2 Alpha2‐adrenergic agonist versus methadone, Outcome 3 Overall withdrawal severity. | ||||
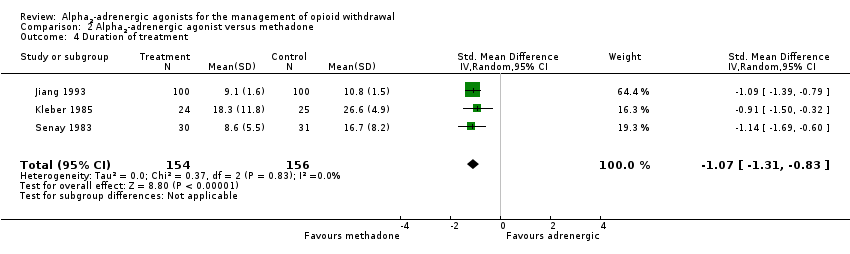
| 4 Duration of treatment Show forest plot | 3 | 310 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.31, ‐0.83] |
| Analysis 2.4  Comparison 2 Alpha2‐adrenergic agonist versus methadone, Outcome 4 Duration of treatment. | ||||
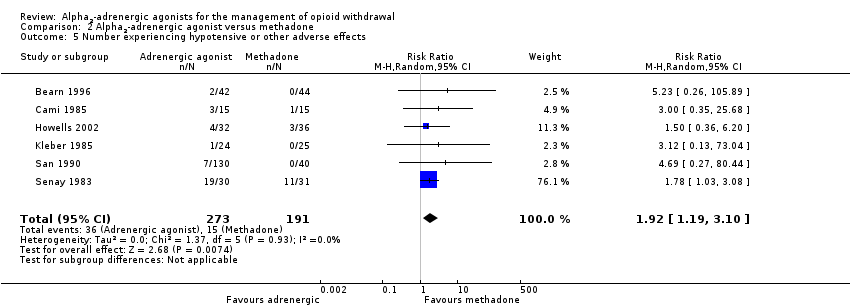
| 5 Number experiencing hypotensive or other adverse effects Show forest plot | 6 | 464 | Risk Ratio (M‐H, Random, 95% CI) | 1.92 [1.19, 3.10] |
| Analysis 2.5  Comparison 2 Alpha2‐adrenergic agonist versus methadone, Outcome 5 Number experiencing hypotensive or other adverse effects. | ||||
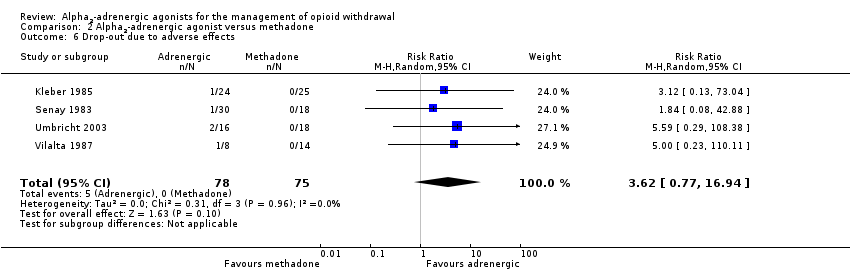
| 6 Drop‐out due to adverse effects Show forest plot | 4 | 153 | Risk Ratio (M‐H, Random, 95% CI) | 3.62 [0.77, 16.94] |
| Analysis 2.6  Comparison 2 Alpha2‐adrenergic agonist versus methadone, Outcome 6 Drop‐out due to adverse effects. | ||||
| 7 Completion of treatment Show forest plot | 9 | 659 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.69, 1.05] |
| Analysis 2.7  Comparison 2 Alpha2‐adrenergic agonist versus methadone, Outcome 7 Completion of treatment. | ||||
| 8 Completion of treatment by opioid Show forest plot | 9 | 657 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.73, 1.11] |
| Analysis 2.8  Comparison 2 Alpha2‐adrenergic agonist versus methadone, Outcome 8 Completion of treatment by opioid. | ||||
| 8.1 Heroin | 4 | 293 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.61, 1.25] |
| 8.2 Methadone | 5 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.80, 1.18] |
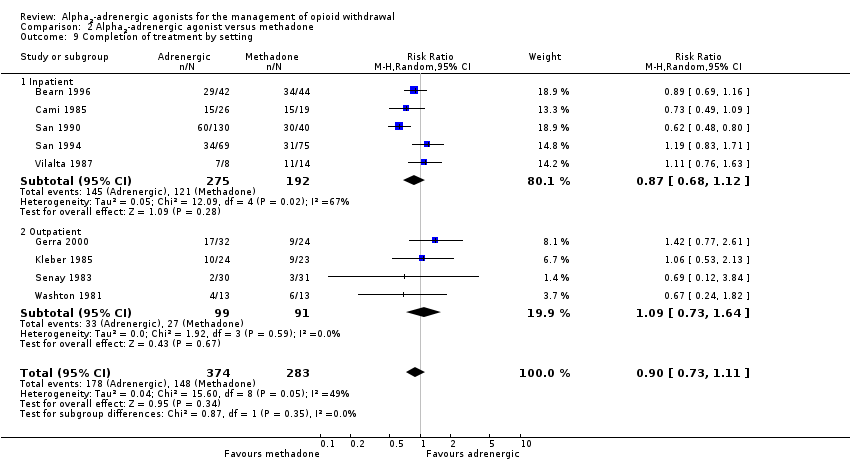
| 9 Completion of treatment by setting Show forest plot | 9 | 657 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.73, 1.11] |
| Analysis 2.9  Comparison 2 Alpha2‐adrenergic agonist versus methadone, Outcome 9 Completion of treatment by setting. | ||||
| 9.1 Inpatient | 5 | 467 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.68, 1.12] |
| 9.2 Outpatient | 4 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.73, 1.64] |

Study flow diagram.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
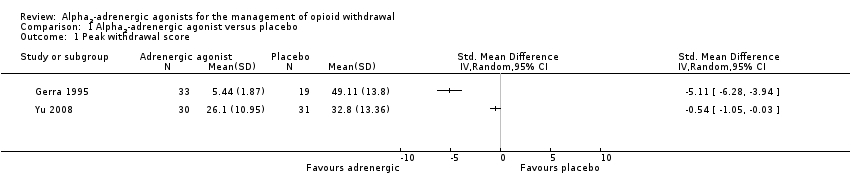
Comparison 1 Alpha2‐adrenergic agonist versus placebo, Outcome 1 Peak withdrawal score.

Comparison 1 Alpha2‐adrenergic agonist versus placebo, Outcome 2 Participants with severe withdrawal.

Comparison 1 Alpha2‐adrenergic agonist versus placebo, Outcome 3 Completion of treatment.
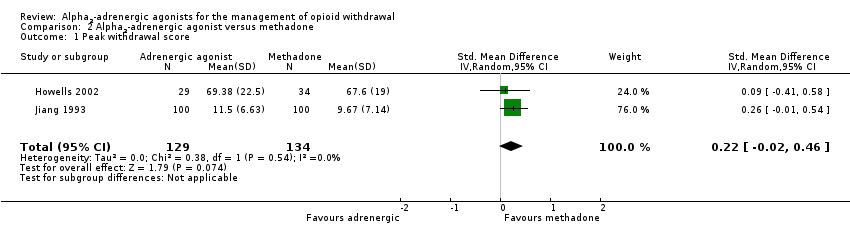
Comparison 2 Alpha2‐adrenergic agonist versus methadone, Outcome 1 Peak withdrawal score.
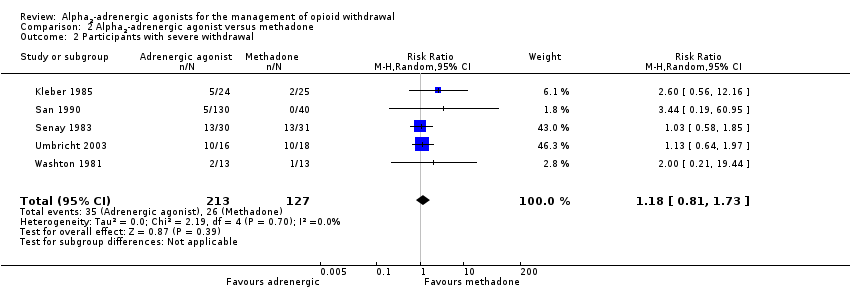
Comparison 2 Alpha2‐adrenergic agonist versus methadone, Outcome 2 Participants with severe withdrawal.
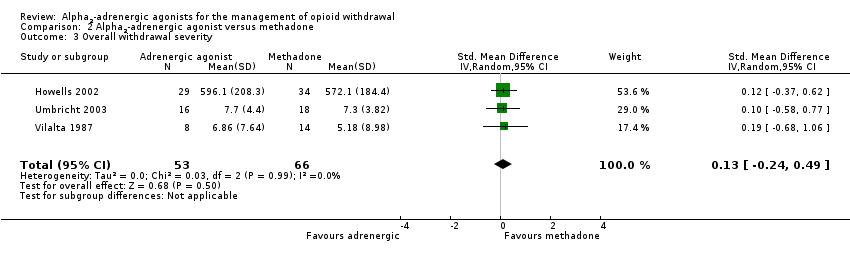
Comparison 2 Alpha2‐adrenergic agonist versus methadone, Outcome 3 Overall withdrawal severity.
Comparison 2 Alpha2‐adrenergic agonist versus methadone, Outcome 4 Duration of treatment.
Comparison 2 Alpha2‐adrenergic agonist versus methadone, Outcome 5 Number experiencing hypotensive or other adverse effects.
Comparison 2 Alpha2‐adrenergic agonist versus methadone, Outcome 6 Drop‐out due to adverse effects.

Comparison 2 Alpha2‐adrenergic agonist versus methadone, Outcome 7 Completion of treatment.
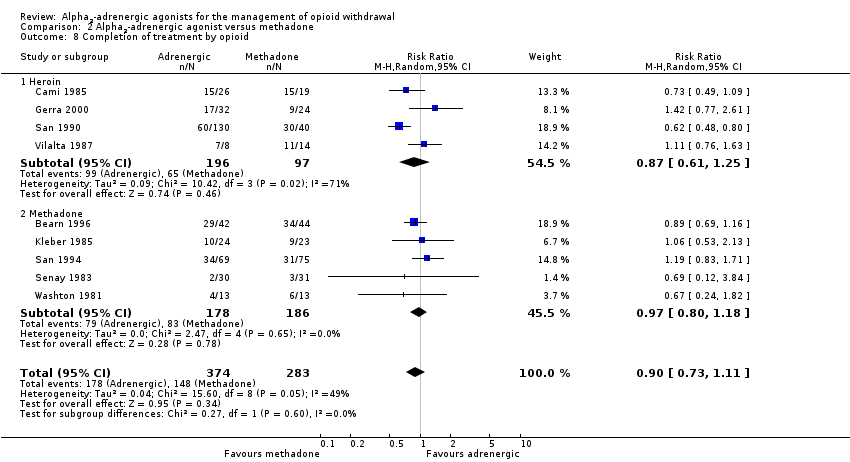
Comparison 2 Alpha2‐adrenergic agonist versus methadone, Outcome 8 Completion of treatment by opioid.
Comparison 2 Alpha2‐adrenergic agonist versus methadone, Outcome 9 Completion of treatment by setting.
| Alpha2‐adrenergic agonist versus methadone for the management of opioid withdrawal | ||||||
| Patient or population: People undergoing managed opioid withdrawal | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Alpha2‐adrenergic agonist versus methadone | |||||
| Participants with severe withdrawal | Study population | RR 1.18 | 340 | ⊕⊕⊝⊝ | ‐ | |
| 205 per 1000 | 242 per 1000 | |||||
| Moderate | ||||||
| 80 per 1000 | 94 per 1000 | |||||
| Peak withdrawal score | ‐ | The mean peak withdrawal score in the intervention groups was | ‐ | 263 | ⊕⊕⊕⊝ | SMD 0.22 (‐0.02 to 0.46) |
| Overall withdrawal severity | ‐ | The mean overall withdrawal severity in the intervention groups was | ‐ | 119 | ⊕⊕⊕⊝ | SMD 0.13 (‐0.24 to 0.49) |
| Duration of treatment | ‐ | The mean duration of treatment in the intervention groups was | ‐ | 310 | ⊕⊕⊝⊝ | SMD ‐1.07 (‐1.31 to ‐0.83) |
| Number experiencing hypotensive or other adverse effects | Study population | RR 1.92 | 464 | ⊕⊕⊝⊝ | ‐ | |
| 79 per 1000 | 151 per 1000 | |||||
| Moderate | ||||||
| 33 per 1000 | 63 per 1000 | |||||
| Drop‐out due to adverse effects | Study population | RR 3.62 | 153 | ⊕⊕⊝⊝ | ‐ | |
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Completion of treatment | Study population | RR 0.85 | 659 | ⊕⊕⊝⊝ | ‐ | |
| 568 per 1000 | 483 per 1000 | |||||
| Moderate | ||||||
| 750 per 1000 | 638 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1One study at risk of selection bias, one at risk of performance and detection bias. | ||||||
| Alpha2‐adrenergic agonist versus placebo for the management of opioid withdrawal | ||||||
| Patient or population: People undergoing managed opioid withdrawal | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Alpha2‐adrenergic agonist versus placebo | |||||
| Participants with severe withdrawal | Study population | RR 0.32 | 148 | ⊕⊕⊕⊝ | ‐ | |
| 589 per 1000 | 188 per 1000 | |||||
| Moderate | ||||||
| 800 per 1000 | 256 per 1000 | |||||
| Completion of treatment | Study population | RR 1.95 | 148 | ⊕⊕⊕⊝ | ‐ | |
| 288 per 1000 | 561 per 1000 | |||||
| Moderate | ||||||
| 333 per 1000 | 649 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Small number of events. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Peak withdrawal score Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Participants with severe withdrawal Show forest plot | 3 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.18, 0.57] |
| 3 Completion of treatment Show forest plot | 3 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [1.34, 2.84] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Peak withdrawal score Show forest plot | 2 | 263 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.02, 0.46] |
| 2 Participants with severe withdrawal Show forest plot | 5 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.81, 1.73] |
| 3 Overall withdrawal severity Show forest plot | 3 | 119 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.24, 0.49] |
| 4 Duration of treatment Show forest plot | 3 | 310 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.31, ‐0.83] |
| 5 Number experiencing hypotensive or other adverse effects Show forest plot | 6 | 464 | Risk Ratio (M‐H, Random, 95% CI) | 1.92 [1.19, 3.10] |
| 6 Drop‐out due to adverse effects Show forest plot | 4 | 153 | Risk Ratio (M‐H, Random, 95% CI) | 3.62 [0.77, 16.94] |
| 7 Completion of treatment Show forest plot | 9 | 659 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.69, 1.05] |
| 8 Completion of treatment by opioid Show forest plot | 9 | 657 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.73, 1.11] |
| 8.1 Heroin | 4 | 293 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.61, 1.25] |
| 8.2 Methadone | 5 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.80, 1.18] |
| 9 Completion of treatment by setting Show forest plot | 9 | 657 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.73, 1.11] |
| 9.1 Inpatient | 5 | 467 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.68, 1.12] |
| 9.2 Outpatient | 4 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.73, 1.64] |

