نقش آنتاگونیست اوپیوئیدی با حداقل آرامسازی برای ترک اوپیوئید
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised controlled trial | |
| Participants | Setting: day surgery or mixed inpatient and outpatient, community‐based treatment, Australia N = 80 heroin dependent by DSM‐IV, similar on age, sex, socioeconomic status, and total length of heroin use Average age: 30.6 years 64% men 95% used heroin at least once daily, 6.2% reported using other opioids in addition to heroin, 91% used tobacco, 64% cannabis, 51% alcohol, 45% tranquilizers, 26% amphetamines; 57.8% known to be unemployed | |
| Interventions | Group 1 (n = 41): naloxone (IV) 800 µg in repeated doses plus clonidine, under midazolam sedation; when no withdrawal apparent, oral naltrexone in increasing doses at 30‐minute intervals. Day surgery procedure at private, community‐based clinic Group 2 (n = 39): clonidine, oral 75‐150 µg/day on inpatient (5‐7 days) or outpatient (7‐10 days) basis at a community‐based public facility | |
| Outcomes | Number commencing and number completing detoxification, number commencing naltrexone treatment, number abstinent from heroin in 4 weeks postdetoxification | |
| Notes | Physical withdrawal assessed immediately prior to, and immediately postdetoxification, using part 1 of the Severity of Dependence Questionnaire Source of funding: Department of Health (Western Australia) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...subjects were randomly assigned to one of two detoxification treatment groups." Method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and providers (performance bias) | Low risk | No blinding, but these outcomes considered unlikely to be affected by knowledge of group allocation |
| Blinding of participants and providers (performance bias) | High risk | No blinding, and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessor (detection bias) | Low risk | No blinding, but measurement of these outcomes unlikely to be influenced by lack of blinding |
| Blinding of outcome assessor (detection bias) | High risk | No blinding, and the measurement of these outcomes is likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | High risk | Data on patient satisfaction with detoxification, physical withdrawal scores and craving reported only for participants who completed detoxification, and there was a significant difference in rates of completion for the 2 groups (87.8% vs 28.2%). |
| Selective reporting (reporting bias) | High risk | Most outcomes (withdrawal severity, acceptability of treatment, engagement in further treatment) only reported for those who completed detoxification |
| Other bias: Comparability of cohorts | Low risk | Random allocation and groups similar on baseline characteristics |
| Other bias: Selection of comparison cohort | Low risk | Groups drawn from same population |
| Other bias: Protection against contamination | Low risk | 2 groups were treated by different personnel in different settings: in private and in public, community‐based treatment facility |
| Methods | Prospective cohort study; treatment allocation open and by participant choice | |
| Participants | Setting: inpatient, dedicated unit in psychiatric teaching hospital, UK N = 49 opioid dependent by DSM‐IV; groups similar in clinical characteristics, except higher levels of alcohol consumption in group 1. 4 participants allocated to group 1 decided not to continue with the regimen and switched to group 2; analysis based on n= 26 (group 1) and n = 23 (group 2) Average age 32 71% men | |
| Interventions | Stabilised for 3 days on mean 32 mg (group 1) or 33 mg (group 2) methadone; diazepam prescribed for concurrent alcohol or benzodiazepine dependence Methadone stopped abruptly, then Group 1 (n = 30): lofexidine 2 mg/day for 2 days, plus naloxone 0.8 mg in morning days 3‐6; or Group 2 (n = 19): lofexidine, 1.8 mg day 1, then 1 mg twice a day for 5 days, 0.6 mg twice on day 7. All offered 25 mg naltrexone day 7 Structured care programme targeted at relapse prevention as adjunct before and during detoxification. Up to 28 days inpatient care | |
| Outcomes | Average withdrawal severity (AUC of mean withdrawal score), days in treatment, participants experiencing hypotension, transient delirium, number completing scheduled treatment | |
| Notes | Withdrawal assessed by Short Opiate Withdrawal Scale. Urine screening throughout study. Source of funding: Britannia Pharmaceuticals, UK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Treatment allocation ... was by patient choice." |
| Allocation concealment (selection bias) | High risk | Quote: "Treatment allocation was open and was by patient choice." |
| Blinding of participants and providers (performance bias) | Low risk | No blinding, but these outcomes considered unlikely to be affected by awareness of group allocation |
| Blinding of participants and providers (performance bias) | High risk | No blinding, and these outcomes are likely to be influenced by lack of blinding |
| Blinding of outcome assessor (detection bias) | Low risk | No blinding, but measurement of these outcomes unlikely to be influenced by lack of blinding |
| Blinding of outcome assessor (detection bias) | High risk | No blinding, and measurement of these outcomes likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Level of missing data insufficient to affect outcomes |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias: Comparability of cohorts | Low risk | Groups similar on clinical characteristics at baseline |
| Other bias: Selection of comparison cohort | Low risk | Experimental and control groups drawn from same population |
| Other bias: Protection against contamination | Unclear risk | It is possible that communication between groups could have occurred. |
| Methods | Randomised controlled, double‐blind trial | |
| Participants | Setting: inpatient treatment in drug and alcohol unit, UK N = 89, opioid dependent by DSM‐IV. Severity of dependence marginally less in naloxone group, groups otherwise similar Gender and age not reported. | |
| Interventions | Stabilised for 3 days on mean 34.3 mg/day (group 1, n = 45) and 37.8 mg/day (group 2, n = 44) methadone Methadone stopped abruptly, then lofexidine 1.8‐2.0 mg/day as 3 doses, combined with naloxone 0.8 mg (group 1) or placebo solution by injection days 3‐6 (group 2) | |
| Outcomes | Mean length of stay and number completing treatment, mean withdrawal and craving scores (as graphs and results of statistical tests), number of injections received, use of additional medication | |
| Notes | Withdrawal assessed by Short Opiate Withdrawal Scale. Daily urine screening. Source of funding: Charitable trust and Brittannia Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "subjects ... were randomly assigned..."; method of sequence generation not reported |
| Allocation concealment (selection bias) | Low risk | Double‐blind stated, with medication prepared by pharmacy |
| Blinding of participants and providers (performance bias) | Low risk | Quote: "Antagonist medication was delivered directly from the in‐house pharmacy for each individual patient in order to preserve the double‐blind" |
| Blinding of participants and providers (performance bias) | Low risk | Quote: "Antagonist medication was delivered directly from the in‐house pharmacy for each individual patient in order to preserve the double‐blind" |
| Blinding of outcome assessor (detection bias) | Low risk | Quote: "Antagonist medication was delivered directly from the in‐house pharmacy for each individual patient in order to preserve the double‐blind" |
| Blinding of outcome assessor (detection bias) | Low risk | Quote: "Antagonist medication was delivered directly from the in‐house pharmacy for each individual patient in order to preserve the double‐blind" |
| Incomplete outcome data (attrition bias) | Low risk | No significant difference in retention rates of the 2 groups |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias: Comparability of cohorts | Low risk | Random allocation and groups stated to be similar at baseline |
| Other bias: Selection of comparison cohort | Low risk | Groups drawn from same population |
| Other bias: Protection against contamination | Low risk | Communication between groups could have occurred, but blinding would be expected to nullify the effect |
| Methods | Prospective cohort study. Naltrexone/lofexidine group recruited pre‐admission; lofexidine‐only group recruited from routine admissions selecting this regimen rather than tapered methadone | |
| Participants | Setting: inpatient, UK N = 22, opioid dependent by DSM‐IV, withdrawing from heroin or methadone or both. More women in group 2; groups otherwise similar in demographics and drug use Average age 31 4/11 women in group 1, 0/11 women in group 2 Co‐dependence on benzodiazepines, alcohol or cocaine were exclusion criteria | |
| Interventions | Stabilised for 3 days on mean 40.0 mg (group 1) and 34.72 mg (group 2) methadone Group 1 (n = 11): naloxone 0.8 mg IM day 1 only; naltrexone, 14 mg in 5 doses day 1, increasing to 50 mg day 4, then single dose 50 mg/day; lofexidine, 2.0 mg day 1, tapered to 0.8 mg day 4 then ceased Group 2 (n = 11): lofexidine, 1.8 mg day 1, 2.0 mg days 2‐6, 1.0 mg day 7. Both groups able to request additional 0.4 mg lofexidine | |
| Outcomes | Overall withdrawal severity (AUC analysis of withdrawal score), number requesting extra lofexidine, days in treatment, number with hypotensive side effects, number completing scheduled treatment | |
| Notes | Short Opiate Withdrawal Scale completed by participants 4 times daily for 7 days, then once daily. Urine screening 3 times a week Source of funding: Brittania Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patient choice |
| Allocation concealment (selection bias) | High risk | Open label |
| Blinding of participants and providers (performance bias) | Low risk | No blinding, but these outcomes considered unlikely to be affected by knowledge of group allocation |
| Blinding of participants and providers (performance bias) | High risk | No blinding, and these outcomes likely to be influenced by knowledge of group allocation |
| Blinding of outcome assessor (detection bias) | Low risk | No blinding, but assessment of these outcomes considered unlikely to be affected by lack of blinding |
| Blinding of outcome assessor (detection bias) | High risk | No blinding, and assessment of these outcomes likely to be influenced by knowledge of group allocation |
| Incomplete outcome data (attrition bias) | Low risk | AUC analysis adjusts for missing data, and dropout similar in both groups |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias: Comparability of cohorts | Low risk | Groups similar on baseline characteristics |
| Other bias: Selection of comparison cohort | Low risk | Experimental and comparison cohort drawn from same population |
| Other bias: Protection against contamination | Unclear risk | It is possible that communication between the groups could have occurred. |
| Methods | Randomised controlled, double‐blind trial | |
| Participants | Setting: hospital outpatient clinic, Italy N = 152, drug abuse disorder by DSM‐III‐R, withdrawing from heroin. Similarity of groups not reported Age 18‐32 years 82% men | |
| Interventions | Group 1 (n = 33): clonidine (0.15 mg IV 3 times a day) Group 2 (n = 42): clonidine (as group 1) + naltrexone (12.5 mg day 2 then 50 mg/day 3 months) Group 3 (n = 58): clonidine (as group 1) + naloxone (0.2 mg IV day 2, 0.4 mg twice a day on days 3 and 4) then naltrexone (50 mg/day from day 5) Group 4 (n = 19): IV saline + oral placebo Daily clinic attendance with 4 hours IV therapy in morning, 3 hours in afternoon | |
| Outcomes | Mean total withdrawal score at 48 and 72 hours; bar graphs for days 1, 2 and 3 showing ratings for individual items of withdrawal scale; morphine metabolites in urine; Hamilton scale for depression on day 1, day 8 and 6 months | |
| Notes | Withdrawal assessed by observer only using 9‐item scale, mainly of objective signs, each item rated 0 to 5 for severity Source of funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "All the patients were randomly divided into four groups..." Comment: group sizes differed and similarity of the characteristics of the groups was not discussed. The adequacy of sequence generation is doubtful. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and providers (performance bias) | Low risk | Double‐blind stated; these outcomes unlikely to be affected by inadequate blinding |
| Blinding of participants and providers (performance bias) | High risk | Double‐blind stated, but given the differences in group sizes it is doubtful whether the blind was maintained, and these outcomes could be influenced by knowledge of group allocation. |
| Blinding of outcome assessor (detection bias) | Low risk | Double‐blind stated; these outcomes unlikely to be affected by inadequate blinding |
| Blinding of outcome assessor (detection bias) | High risk | Adequacy of blind doubtful, and assessment of these outcomes likely to be influenced by knowledge of group allocation |
| Incomplete outcome data (attrition bias) | Low risk | Dropout in first week similar for 3 groups relevant to this review |
| Selective reporting (reporting bias) | High risk | Analysis on basis of treatment provided |
| Other bias: Comparability of cohorts | Unclear risk | No information about comparability of groups |
| Other bias: Selection of comparison cohort | Low risk | All participants drawn from same population |
| Other bias: Protection against contamination | Unclear risk | It is possible that communication between the groups could have occurred. |
| Methods | Randomised controlled trial | |
| Participants | Setting: outpatient clinic, Italy N = 98 withdrawing from heroin, dependent by DSM‐IV, urine positive for morphine; groups similar in psychiatric and psychometric data Age 18‐36 years 72% men Duration of drug use 2‐6 years | |
| Interventions | Heroin use continued until 12 hours before treatment Group 1 (n = 32): clonidine 0.15 mg/100mL saline IV 6 times/day for 2 days, 0.15 mg 3 times a day for 3 days. Additional 0.15 mg orally each evening. Total 5 days treatment Group 2 (n = 32): clonidine as group 1 for 2 days, plus naloxone as repeated 0.04 mg injections to 0.4 mg 2 days. Naltrexone, orally, 5 mg at 6 pm day 1 (after naloxone injections completed), 50 mg day 2, after same procedure. Clonidine 0.15 mg orally 3 times day 3. Total 3 days treatment Group 3 (n = 34): methadone, oral, 40 mg/day in single dose, tapered over 10 days Treatment in outpatient clinic with those in groups 1 and 2 receiving 4 hours IV therapy morning and afternoon. Unclear whether extent of clinic care same for group treated with methadone. All received counselling. Naltrexone maintenance commenced day 6 (group 1), during detox (group 2) and 5 days after taper (group 3) | |
| Outcomes | Graphs of mean daily withdrawal scores, craving scores before and after detox, % of positive urine samples, number accepting naltrexone and % of participants in maintenance naltrexone treatment 3 months after detox | |
| Notes | Withdrawal rated by observer (9 items, 0‐5 severity). Urine testing during detoxification and follow‐up period Source of funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All the subjects were randomly divided into three groups" but the method of sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and providers (performance bias) | Low risk | These outcomes are considered unlikely to be affected by knowledge of group allocation. |
| Blinding of participants and providers (performance bias) | High risk | Blinding not discussed; the timing of naltrexone commencement in the treatment protocols differed. This suggests that there was probably no blinding of treatment personnel, and possibly not participants either |
| Blinding of outcome assessor (detection bias) | Low risk | Assessment of these outcomes are considered unlikely to be affected by knowledge of group allocation. |
| Blinding of outcome assessor (detection bias) | Unclear risk | Insufficient information to permit judgement of low or high risk |
| Incomplete outcome data (attrition bias) | Unclear risk | The 3 groups differed in the proportions who accepted and continued extended naltrexone maintenance treatment, but it is unclear how this difference translates into missing data. It is also unclear whether differences in dropout may have influenced withdrawal scores. (This outcome was not used in this review.) |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias: Comparability of cohorts | Low risk | Groups similar on baseline characteristics |
| Other bias: Selection of comparison cohort | Low risk | All participants drawn from same population |
| Other bias: Protection against contamination | Unclear risk | It is possible that communication between groups could have occurred. |
| Methods | Cohort study design with double‐blind, random allocation to lofexidine + naloxone or lofexidine + placebo. Participants who did not consent to, or were excluded from randomisation received methadone. | |
| Participants | Setting: inpatient, UK N = 137, opiate‐dependent, admitted for inpatient detoxification. No differences between groups 1 and 2, but some differences in group 3. Average age 32 77% men | |
| Interventions | Group 1 (n = 45): lofexidine plus 0.8 mg naloxone on days 3‐6 Group 2 (n = 46): lofexidine plus placebo Group 3 (n = 46): methadone tapered over 10 days | |
| Outcomes | Number completing detoxification, length of stay, abstinence at follow‐up, time to relapse | |
| Notes | No use of rating scales reported Source of funding: charitable trust and Brittannia Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...patients randomly allocated (by computer) to receive lofexidine + naloxone or lofexidine + placebo". This is the only comparison relevant to this review. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and providers (performance bias) | Low risk | Double‐blind stated for groups 1 and 2, and these outcomes considered unlikely to be influenced by knowledge of group allocation |
| Blinding of participants and providers (performance bias) | Low risk | Double‐blind stated for groups 1 and 2 |
| Blinding of outcome assessor (detection bias) | Low risk | Double‐blind stated for groups 1 and 2, and assessment of these outcomes considered unlikely to be influenced by knowledge of group allocation |
| Blinding of outcome assessor (detection bias) | Low risk | Double‐blind stated for groups 1 and 2 |
| Incomplete outcome data (attrition bias) | Low risk | Rates of dropout from groups 1 and 2 similar |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias: Comparability of cohorts | Low risk | Groups 1 and 2 similar on baseline characteristics |
| Other bias: Selection of comparison cohort | Low risk | Groups drawn from same population |
| Other bias: Protection against contamination | Low risk | Communication between groups could have occurred, but blinding would be expected to nullify the effect |
| Methods | Prospective cohort study; participants able to choose treatment group | |
| Participants | Setting: outpatient treatment from primary care medical clinic, USA N = 125 injecting drug users with active opioid addiction, willing to enter long‐term treatment after detoxification. Groups similar in terms of drug use history, craving and withdrawal scores at baseline Average age 33 years 64% men Average age of first heroin use 22 years, average 1.4 prior detoxifications, 95% had history of cocaine use; history of dependence on alcohol, benzodiazepines or sedatives exclusion criterion; 50% employed | |
| Interventions | Group 1 (n = 57): clonidine 0.1‐0.2 mg 4 times a day, tapered over 12 days Group 2 (n = 68): clonidine as group 1, tapered over 5 days + naltrexone 12.5 mg day 1, 25 mg day 2, then 50 mg/day Daily clinic attendance except weekends. Participants in group 2 in clinic to 5 pm day 1 to manage withdrawal from first dose of naltrexone All had access to oxazepam as adjunct medication, with group 2 also receiving ibuprofen, ketorolac and prochlorperazine on day of first naltrexone dose | |
| Outcomes | Number successfully completing detoxification defined by 50 mg dose of naltrexone without acute withdrawal; amount of oxazepam required during detoxification; withdrawal scores reported for baseline only | |
| Notes | Opioid withdrawal scale of 24 items, each rated 0‐3 by participants. No urine screening Source of funding: Research grant, US National Institute on Drug Abuse (NIDA) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Two protocols were offered to patients, who selected their own treatments." |
| Allocation concealment (selection bias) | High risk | Quote: "Two protocols were offered to patients, who selected their own treatments." |
| Blinding of participants and providers (performance bias) | Low risk | No blinding, but these outcomes are considered unlikely to be influenced by knowledge of intervention |
| Blinding of participants and providers (performance bias) | High risk | No blinding, but no subjective data were reported |
| Blinding of outcome assessor (detection bias) | Low risk | No blinding, but assessment of these outcomes considered unlikely to be influenced by knowledge of intervention |
| Blinding of outcome assessor (detection bias) | High risk | No blinding, but no subjective data were reported |
| Incomplete outcome data (attrition bias) | High risk | The 2 groups differed significantly in rates of completion of detoxification. Data on the total amount of oxazepam used during treatment is at risk of bias as a result. |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias: Comparability of cohorts | Low risk | Groups similar on baseline characteristics |
| Other bias: Selection of comparison cohort | Low risk | All participants drawn from same population |
| Other bias: Protection against contamination | Unclear risk | It is possible that communication between groups could have occurred. |
| Methods | Randomised controlled, double‐blind trial | |
| Participants | Setting: outpatient treatment from primary care medical clinic, USA N = 162 heroin dependent with sufficient social support for outpatient detoxification. No substantial differences in sociodemographic or clinical features of groups at baseline Age not reported (between 18 and 50 years) 71% men 62% married, 35% employed | |
| Interventions | Group 1 (n = 55): clonidine 0.1‐0.2 mg every 4 hours as needed days 1‐7, 50 mg naltrexone day 8 Group 2 (n = 54): clonidine as group 1, 12.5 mg naltrexone day 1, increasing to 50 mg day 3 Group 3 (n = 53): buprenorphine 3 mg sublingually days 1‐3 then clonidine as group 1, 25 mg naltrexone day 4, 50 mg day 5 Daily clinic attendance except at weekends | |
| Outcomes | Mean overall and peak withdrawal scores; number retained in treatment for 8 days, number achieving 50 mg maintenance dose naltrexone | |
| Notes | Withdrawal rated by participants (24 items, each rated 0‐3) Source of funding: Research grant, US National Institute on Drug Abuse (NIDA) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned to a treatment group..."; method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and providers (performance bias) | Low risk | Quote: "...staff and patients were blinded to the protocols. Study medications were prepared so that participants received either active or placebo preparations of all medications." However, maintenance of blind difficult due to differential effects of medications. However, these outcomes are considered unlikely to be affected by awareness of intervention. |
| Blinding of participants and providers (performance bias) | Unclear risk | Study undertaken double‐blind, as indicated above, but maintenance of the blind was reported to be difficult |
| Blinding of outcome assessor (detection bias) | Low risk | Assessment of these outcomes considered unlikely to be influenced by knowledge of group allocation |
| Blinding of outcome assessor (detection bias) | Unclear risk | Insufficient information to permit judgement of low or high risk |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "[r]etention did not differ significantly among the groups." |
| Selective reporting (reporting bias) | Low risk | None apparent |
| Other bias: Comparability of cohorts | Low risk | Baseline characteristics of groups similar |
| Other bias: Selection of comparison cohort | Low risk | All participants drawn from same population |
| Other bias: Protection against contamination | Unclear risk | It is possible that communication between groups could have occurred. The implications of this are unclear given the difficulties in maintaining the blind. |
| Methods | Randomised, placebo‐controlled, double‐blind trial | |
| Participants | Setting: inpatient treatment in research ward, USA. N = 60, heroin dependent by DSM‐III‐R. Groups similar on sociodemographic and drug use characteristics. Average age 32 years 59% men in group 1, 36% men in group 2 30% injecting users; alcohol dependence an exclusion criterion; average days employed in last 30: 8 (SD 2) days (group 1), 4 (SD 1) days (group 2) | |
| Interventions | Buprenorphine, sublingual solution, 12 mg in 2 doses day 1, then single daily dose of 8 mg day 2, 4 mg day 3, 2 mg day 4, plus Group 1 (n = 32): naltrexone, oral, 2 hours after buprenorphine, 12.5 mg days 2 and 3, 25 mg day 4, then 50 mg/day or Group 2 (n = 28): 50 mg oral naltrexone day 8 Symptomatic treatment initiated when withdrawal score above median entry score | |
| Outcomes | Proportion of patients remaining in treatment, day of dropout and reasons for leaving, mean withdrawal score at each time point and daily mean peak scores, proportion of patients with withdrawal scores exceeding median baseline score each day, AUC analysis of withdrawal score, medications used for symptomatic treatment, mean clonidine dose per patient by day of treatment, numbers completing study | |
| Notes | Withdrawal rated by observers 9 times a day using adapted form of Clinical Institute Narcotic Assessment scale (11 items, score 0‐30). Participants completed adjective checklist but results not reported (stated as not robust) Source of funding: research grant, US National Institute on Drug Abuse (NIDA) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[c]omplete patient randomizations was used with a slightly higher probability to be assigned to the NB group to offset a potential for higher dropout rate in this group." Specific method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and providers (performance bias) | Low risk | Double‐blind stated, with placebos used to maintain blind |
| Blinding of participants and providers (performance bias) | Low risk | Double‐blind stated, with placebos used to maintain blind |
| Blinding of outcome assessor (detection bias) | Low risk | Double‐blind stated, with placebos used to maintain blind |
| Blinding of outcome assessor (detection bias) | Low risk | Double‐blind stated, with placebos used to maintain blind |
| Incomplete outcome data (attrition bias) | High risk | 76% in the placebo group and 56% in the naltrexone group completed withdrawal. This difference was not statistically significant, but there was a significant difference in the average length of stay. If participants with more severe withdrawal symptoms were more likely to drop out, data on severity of withdrawal after day 2, is at risk of bias |
| Selective reporting (reporting bias) | Unclear risk | Participants completed adjective checklist to rate withdrawal severity but results not reported (stated as not robust) |
| Other bias: Comparability of cohorts | Low risk | Groups similar on baseline characteristics |
| Other bias: Selection of comparison cohort | Low risk | All participants drawn from same population |
| Other bias: Protection against contamination | Unclear risk | It is possible that communication between groups could have occurred. |
AUC: area under the curve;DSM: Diagnostic and Statistical Manual of Mental Disorders; IM: intramuscularly; IV: intravenously; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Analysis of presentations to hospital emergency department for treatment of adverse effects of antagonist‐induced withdrawal. Retrospective review of records, no treatment comparison | |
| Study of effectiveness of antagonist‐induced withdrawal prior to naltrexone maintenance treatment of heroin dependence; no treatment comparison | |
| Reports outcomes of withdrawal induced by naltrexone with sedation. No treatment comparison. Case series, not controlled study | |
| Reports treatment outcomes for 504 consecutive patients receiving sedation and fluid for 3 days followed by naltrexone for management of opioid withdrawal. No treatment comparison | |
| Cohort study comparing outcomes for heroin‐dependent and methadone‐maintained clients receiving antagonist‐induced withdrawal. No treatment comparison. | |
| Reports 2 case series treated with different regimens of naltrexone combined with clonidine. Not a controlled study. Insufficient data on outcomes defined for this review. | |
| Reports the use of a single dose of naltrexone with adjunct medications on day 2, after cessation of methadone, to accelerate opioid withdrawal. No treatment comparison | |
| Describes procedure, demographics and symptoms of opioid‐dependent patients undergoing antagonist‐induced withdrawal at home. No treatment comparison | |
| Reports outcomes of 2 treatment protocols combining naltrexone and clonidine, but differing in timing of naltrexone commencement. Results combined by reporting outcomes against days of clonidine/naltrexone treatment. No treatment comparison. | |
| Reports outcomes of naltrexone‐induced withdrawal with or without an amino acid supplement. Method of selection of the group receiving the amino acid supplement not reported and insufficient detail of participant characteristics to determine comparability of 2 groups. Insufficient outcome data, unclear whether data collection retrospective | |
| Reports use of naltrexone to facilitate withdrawal from buprenorphine in cohort who were unable to reduce below 2 mg buprenorphine due to withdrawal distress. No treatment comparison. | |
| Comparison of antagonist‐induced withdrawal with or without anaesthesia for management of opioid withdrawal. Comparison is not one of the modalities defined by the inclusion criteria | |
| Compares outcomes of antagonist‐induced withdrawal with minimal sedation for group of patients treated in everyday clinical practice compared with those treated as part of a clinical trial (De Jong 2005). No treatment comparison | |
| Randomised controlled trial comparing 2 treatment regimens that differ only in the timing of commencement of naltrexone. Both regimens involve 3 days of buprenorphine (12 mg, 8 mg, 4 mg) with naltrexone commenced either day 2 or day 4. Trial incomplete at the time of this report, but no further report located. Insufficient outcome data | |
| Randomised controlled trial comparing clonidine and lofexidine for managing symptoms of antagonist‐induced withdrawal. Antagonist‐induced withdrawal regimens identical except for adjunct medications | |
| Observational study of 2 groups receiving naltrexone or naltrexone plus buprenorphine. Medication commenced in context of detoxification but focus of study on relapse prevention (12 weeks treatment) | |
| Open‐label study investigating the acceptability of antagonist‐induced withdrawal followed by naltrexone maintenance treatment. No treatment comparison | |
| Chart review of 20 consecutive patients to assess the incidence of delirium during antagonist‐induced withdrawal. Retrospective data collection, no treatment comparison | |
| Reports the use of clonidine and naltrexone to manage opioid withdrawal in an outpatient setting. No treatment comparison | |
| Investigation of effects of repeated doses of naloxone in parolees participating in an aftercare abstinence programme. No treatment comparison, not a full withdrawal intervention | |
| Reports outcomes for 20 patients treated with antagonist‐induced withdrawal with "high level" of sedation and various adjunct medications. No treatment comparison | |
| Reports the administration of naltrexone (0.125 mg initially then increasing) during 6‐day tapered methadone detoxification. No treatment comparison. One of series of studies leading to Mannelli 2009. | |
| Randomised controlled trial comparing tapered methadone plus: placebo versus naltrexone 0.125 mg versus naltrexone 0.25 mg. Intervention not one defined by selection criteria of review | |
| Reports the use of low dose naltrexone and buprenorphine to facilitate transition from opioid dependence to sustained release naltrexone (Vivitrol). No treatment comparison | |
| Comparison of 2 dose regimens of antagonist‐induced withdrawal. Consecutive case series, not a controlled study. Insufficient data on outcomes and participants | |
| Assessment of effectiveness of buprenorphine‐clonidine to manage transition from opioid dependence to sustained‐release naltrexone (Depotrex or Vivitrol). No treatment comparison | |
| Randomised controlled trial of antagonist‐induced withdrawal with or without acupuncture. Investigation of effectiveness of acupuncture, not antagonist‐induced withdrawal | |
| Study of effects of naltrexone administered to patients receiving buprenorphine/naltrexone combination or buprenorphine alone. Focus on pharmacology of buprenorphine rather than management of withdrawal | |
| Comparison of trazodone and clonidine in management of symptoms associated with antagonist‐induced withdrawal. Focus on capacity of trazodone to ameliorate withdrawal, not effectiveness of antagonist‐induced withdrawal. | |
| Comparison of 2 regimens of antagonist‐induced withdrawal. 2 case series, not a controlled study. Insufficient outcome data | |
| Randomised controlled trial comparing very low dose naltrexone (0.125 mg) and placebo as adjuncts to standard clonidine‐based regimen for managing withdrawal. Naltrexone dose insufficient to induce withdrawal. Insufficient data on outcomes defined for review. Conference abstract currently only information available | |
| Reports use of antagonist‐induced withdrawal for opioid dependence. No treatment comparison | |
| Comparison of antagonist‐induced withdrawal with minimal sedation or anaesthesia and continued methadone maintenance treatment. Comparisons not those defined by the criteria for this review | |
| Reports use of antagonist‐induced withdrawal. No treatment comparison | |
| Comparison of nifedipine and clonidine as adjuncts to antagonist‐induced withdrawal. Study abandoned after 2/2 treated with nifedipine experienced delirium following the introduction of naltrexone. Insufficient outcome data | |
| Preliminary (conference abstract) report of randomised controlled trial comparing graduated doses of oral naltrexone with a 7‐day regimen of tapered buprenorphine, following a single day of buprenorphine treatment, to manage transition from opioid dependence to sustained‐release (injectable) naltrexone. Trial ongoing. No data on withdrawal syndrome | |
| Comparison of 2 regimens of antagonist‐induced wtihdrawal, differing in day on which naltrexone was administered (day 2 or day 3). Treatments offered consecutively making control of allocation and performance bias difficult. Limited data reported |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Peak withdrawal severity Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Antagonist‐adrenergic combination versus adrenergic agonist, Outcome 1 Peak withdrawal severity. | ||||
| 1.1 Naltrexone | 2 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Naloxone | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Overall withdrawal severity Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Antagonist‐adrenergic combination versus adrenergic agonist, Outcome 2 Overall withdrawal severity. | ||||
| 2.1 Naltrexone | 3 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Naloxone | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Completion rate Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Antagonist‐adrenergic combination versus adrenergic agonist, Outcome 3 Completion rate. | ||||
| 3.1 Naltrexone | 4 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Naloxone | 6 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |

Flow diagram of literature search

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
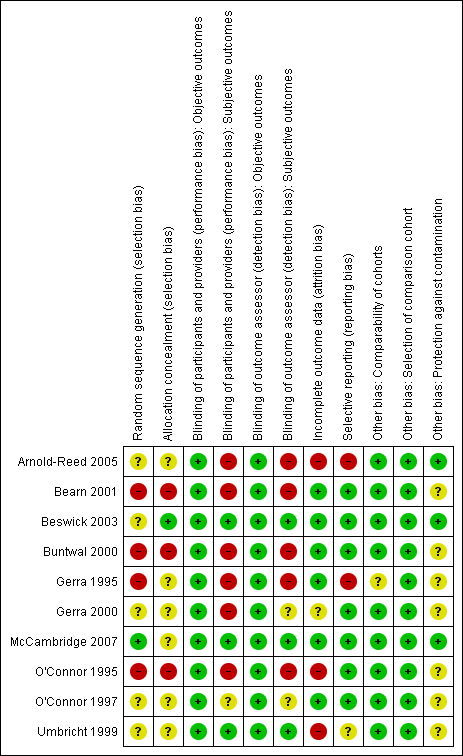
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Comparison 1 Antagonist‐adrenergic combination versus adrenergic agonist, Outcome 1 Peak withdrawal severity.
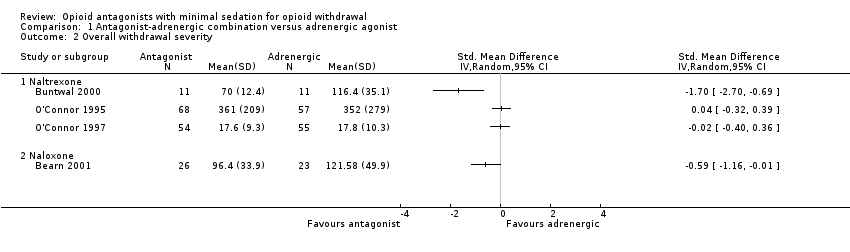
Comparison 1 Antagonist‐adrenergic combination versus adrenergic agonist, Outcome 2 Overall withdrawal severity.

Comparison 1 Antagonist‐adrenergic combination versus adrenergic agonist, Outcome 3 Completion rate.
| Antagonist‐adrenergic combination compared to adrenergic agonist for opioid withdrawal | |||
| Patient or population: opioid dependent adults | |||
| Outcomes | Impact | № of participants | Quality of the evidence |
| Peak withdrawal severity ‐ Naltrexone | In 1 study peak withdrawal severity was similar for the 2 types of intervention. In the other study peak withdrawal was more severe in the group receiving antagonist‐adrenergic combination. | 184 | ⊕⊝⊝⊝ |
| Peak withdrawal severity ‐ Naloxone | This comparison reported by only 1 study, which found more severe withdrawal with naloxone‐clonidine combination. | 91 | ⊕⊝⊝⊝ |
| Overall withdrawal severity ‐ Naltrexone | No difference in overall withdrawal severity for 2 studies; in 1 study overall severity significantly less for antagonist‐adrenergic combination. | 256 | ⊕⊝⊝⊝ |
| Overall withdrawal severity ‐ Naloxone | This comparison reported by only 1 study, which found less severe overall withdrawal with naloxone‐lofexidine combination. | 49 | ⊕⊝⊝⊝ |
| Completion rate ‐ Naltrexone | Completion rate with adrenergic agonist only ranged from 42% to 94%. Completion rate with antagonist‐adrenergic agonist combination ranged from 73% to 95% | 330 | ⊕⊝⊝⊝ |
| Completion rate ‐ Naloxone | Completion rate with adrenergic agonist only ranged from 28% to 94%. Completion rate with antagonist‐adrenergic agonist combination ranged from 73% to 98% | 463 | ⊕⊝⊝⊝ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||
| GRADE Working Group grades of evidence | |||
| aOne study at high risk of bias. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Peak withdrawal severity Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Naltrexone | 2 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Naloxone | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Overall withdrawal severity Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Naltrexone | 3 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Naloxone | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Completion rate Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Naltrexone | 4 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Naloxone | 6 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |

