Vitamin K antagonis berbanding dengan berat‐molekul‐rendah heparin untuk rawatan jangka panjang dalam gejala thromboembolism vena
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised parallel‐design single‐institution treatment trial | |
| Participants | Patients (40 allocated to LMWH (20 patients 1.5 mg/kg daily and 20 patients 1.0 mg/kg daily) and 20 to VKA treatment) with PE confirmed on high‐probability ventilation‐perfusion scanning, a positive spiral chest computed tomogram or a conventional pulmonary angiogram, or an intermediate ventilation‐perfusion lung scan in the presence of high clinical suspicion of PE Age, mean ± SD, years: LMWH 55 ± 13/VKA 56 ± 11 Gender, %F: LMWH 75/VKA 70 Location: 1 centre in USA | |
| Interventions | The warfarin arm comprised of a course of continuous infusion intravenous unfractionated heparin for a minimum of 5 days and concomitant warfarin for 90 days. The enoxaparin arm started with a course of 14 days of 1 mg/kg twice‐daily, followed by either a course of 1.5 mg/kg once‐daily, enoxaparin (20 participants), or a course of 1.0 mg/kg once‐daily enoxaparin (20 participants). All participants in the enoxaparin arm received a total of 90 days of enoxaparin. Randomisation into the enoxaparin categories was performed at beginning of trial when participants were initially assigned to enoxaparin or standard/warfarin therapy | |
| Outcomes | Recurrent VTE/DVT: loss of vein compressibility demonstrated on ultrasound Major bleeding: clinically overt and associated with a fall in haemoglobin ≥ 2 g/dL, intracranial or pericardial Mortality data were not provided | |
| Notes | Participants with major bleeding during VKA treatment had an INR of 8.2 and 3.2, respectively Category II trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the method used to generate the randomisation schedule; Brigham and Women’s Hospital (BWH) Investigational Drug Service randomised study participants |
| Allocation concealment (selection bias) | Unclear risk | BWH Investigational Drug Service randomised participants, but how allocation was concealed is not revealed |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial not blinded: Participants receiving standard therapy had drug regimen administered at the principal investigator's office; those receiving LMWH were treated at a different site and underwent echocardiography |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear as to whether those collecting outcomes data were aware of the allocation |
| Incomplete outcome data (attrition bias) | Low risk | Study authors provided a table detailing the reason why 7 participants dropped out |
| Selective reporting (reporting bias) | Low risk | All intended outcomes were reported |
| Other bias | Low risk | None observed |
| Methods | Prospective open single‐centre randomised clinical trial | |
| Participants | 105 patients (50 allocated to LMWH and 55 to VKA treatment) > 40 years of age with DVT, confirmed on venography Age, mean ± SD, years: LMWH 65.3 ± 14.9/VKA 58.6 ± 16.4 Gender, M/F: LMWH 24/26/VKA 23/32 Location: 1 centre in UK | |
| Interventions | Warfarin‐sodium for 3 months (INR of 2.0 to 3.0) compared with a 3‐month course of subcutaneous Fragmin 5000 anti‐Xa units (Kabi 2165 heparin fragment) once daily | |
| Outcomes | Recurrent VTE/DVT: intraluminal filling defect in a deep vein, demonstrated on repeat venography at a site not previously involved, and demonstrated on 2 views Major bleeding: overt bleeding associated with a drop in Hb level ≥ 2 g/dL, transfusion of ≥ 2 blood units if required, or intracranial haemorrhage; other cases were classified as minor Mortality data were provided Blinded outcome assessment was provided by radiologists unaware of treatment allocation | |
| Notes | 3 months of randomised treatment without additional follow‐up Mean INR achieved in the warfarin group was 2.65, with 68.6% between 2.0 and 3.0, 22.8% between 3.1 and 4.0, and 8.6% between 1.7 and 1.9 Category I trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A restricted randomisation list using permuted blocks was prepared using computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Sealed and sequentially numbered envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Open trial, not blind. Compliance of participants randomised to LMWH was monitored |
| Blinding of outcome assessment (detection bias) | Low risk | Independent and blind outcome assessment by radiologists unaware of treatment allocation |
| Incomplete outcome data (attrition bias) | Low risk | Reasons are given for each participant who did not complete the trial |
| Selective reporting (reporting bias) | Unclear risk | All intended outcomes were reported, but timing of outcomes at 2, 4, and 8 weeks was not presented |
| Other bias | Low risk | None observed |
| Methods | Prospective open‐label randomised clinical trial | |
| Participants | 102 patients (50 allocated to LMWH and 52 to VKA treatment) with an episode of DVT confirmed on colour duplex ultrasound Age, mean (range), years: LMWH 59.0 (25 to 91)/VKA 58.2 (23 to 95) Gender, M/F: LMWH 19/31/VKA 22/30 Location: 1 centre in Greece | |
| Interventions | Acenocoumarol arm started with a 5 to 7‐day course of unfractionated heparin followed by acenocoumarol for 6 months (INR 2.0 to 3.0). Tinzaparin group started with a 7 day course of once‐daily subcutaneous tinzaparin 175 anti‐Xa IU continued for 6 months | |
| Outcomes | Recurrent VTE/DVT: presence of new thrombus in a venous segment not found affected on baseline duplex ultrasound scan Major bleeding: overt bleeding associated with a drop in Hb level ≥ 2 g/dL; transfusion of ≥ 2 blood units, if required; intracranial, intraspinal, intraocular, pericardial, or retroperitoneal bleeding, or death; or need for permanent discontinuation of treatment Mortality data were provided Blinded outcome assessment was provided by specialists not involved in the trial, who interpreted all objective diagnostic tests | |
| Notes | 6 months of randomised treatment with 6 months of additional follow‐up INR values in the acenocoumarol arm were 67.2% between 2.0 and 3.0, 13.6% above 3.0, and 19.1% below 2.0 Category I trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐derived treatment schedule |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label, not blind |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded outcome assessment was provided by specialists not involved in the trial, who interpreted all objective diagnostic tests |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs. All data described |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Differences in initial treatment regimens between groups |
| Methods | Prospective open single‐centre randomised clinical trial | |
| Participants | 185 patients (93 allocated to LMWH and 92 to VKA treatment) with a first or second episode of DVT confirmed on contrast venography (20 excluded from analysis by trialists (8 LMWH, 12 VKA)) Age, mean (range), years: LMWH 62.7 (19 to 83)/VKA 28.3 (20 to 82) Gender, M/F: LMWH 41/44/VKA 46/34 Location: 1 centre in Spain | |
| Interventions | Coumarin arm started with a 5‐day course of unfractionated heparin followed by coumarin for 3 months (INR 2.0 to 3.0). Enoxaparin group started with a 7‐day course of twice‐daily subcutaneous enoxaparin 40 mg (4000 anti‐Xa IU) and continued with a 3‐month course of once‐daily enoxaparin 40 mg | |
| Outcomes | Recurrent VTE/ DVT: constant intraluminal filling defect in a deep vein not present on the first day Major bleeding: intracranial or retroperitoneal or producing a decrease in Hb level ≥ 2 g/dL, sufficient to necessitate discontinuation of treatment or transfusion of ≥ 2 units of blood Mortality from all causes Blinded outcome assessment was provided by 2 blinded observers, who assessed the outcomes of venograms | |
| Notes | 3 months of randomised treatment and an additional 9 months of follow‐up. All participants stopped after 3 months of treatment Intensity of VKA therapy was 15% INR < 2.0, 64% INR between 2.0 and 3.0, and 21% INR > 3.0 Category I trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐derived treatment schedule |
| Allocation concealment (selection bias) | Unclear risk | Computer‐derived treatment schedule; no other information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial, not blind. Participants in the LMWH group were not hospitalised |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded outcome assessment was provided by 2 blinded observers who assessed outcomes of venograms |
| Incomplete outcome data (attrition bias) | Low risk | Outcomes presented; no loss to follow‐up; 1 participant died of a PE |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Unclear risk | Differences in initial treatment regimens between groups |
| Methods | Prospective open randomised clinical trial | |
| Participants | 200 patients (100 allocated to LMWH and 100 to VKA treatment) with DVT confirmed on venography Age, mean (range), years: 58 (18 to 92) Gender, M/F: 82/118 Location: 1 centre, Germany | |
| Interventions | Phenprocoumon for 3 or 6 months (INR 2.0 to 3.0) compared with 3‐ or 6‐month course of subcutaneous dalteparin‐sodium 5000 IU anti‐Xa once daily | |
| Outcomes | Recurrent VTE | |
| Notes | Different initial therapies were used: 17 participants underwent venous thrombectomy, 18 systemic lysis, 28 regional lysis, and 137 IV unfractionated heparin as initial treatment 3 or 6 months of randomised treatment and an additional 9 months of follow‐up Category II trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
| Methods | Multi‐centre randomised open‐label clinical trial | |
| Participants | 737 patients (369 allocated to LMWH and 368 to VKA treatment) with DVT confirmed on venography or compression ultrasonography Age, < 60 years old/≥ 60 years old: LMWH 187/182, VKA 152/217 Gender, M/F: LMWH 207/162, VKA 188/180 Location: 30 centres across Canada | |
| Interventions | Warfarin arm started with a 6‐day course of unfractionated heparin followed by warfarin for 3 months (INR 2.0 to 3.0). Tinzaparin group received once‐daily subcutaneous tinzaparin 175 anti‐Xa IU/kg of body weight, continued for 3 months | |
| Outcomes | Recurrent VTE/ DVT: previously compressible proximal vein segment not compressible on repeat ultrasonography or venography demonstrating a constant intraluminal filling defect in the deep veins not present on the baseline venogram Recurrent PE: (a) high‐probability lung scan finding; (b) non‐diagnostic lung scan with documented new DVT; (c) spiral computed tomography showing thrombus in the central pulmonary arteries; (d) pulmonary angiography revealing a constant intraluminal filling defect or cut‐off of a vessel > 2.5 mm in diameter; or (e) PE found at autopsy Major bleeding: clinically overt and (a) associated with a fall in Hb ≥ 2 grams/dL, or (b) transfusion of ≥ 2 units of blood, or intracranial or retroperitoneal bleeding occurring in a major joint Mortality data were provided Blinded outcome assessment was provided by a central independent adjudication committee | |
| Notes | 3 months of randomised treatment and an additional 9 months of follow‐up. In the tinzaparin arm, 146 participants continued with warfarin treatment after 3 months of treatment with tinzaparin for a mean of 202 days (median, 258 days). In the warfarin arm, 250 participants continued warfarin treatment after 3 months of allocated treatment for a mean of 156 days (median, 147 days) Participants with major bleeding complications: 1 participant with INR between 3.1 and 3.9, 2 with INR > 4.0 on the day of the bleeding complication Furthermore, a figure in the study publication provides data on INR values throughout the trial Category I trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Derived by computer |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial – not blind |
| Blinding of outcome assessment (detection bias) | Low risk | Central independent adjudication committee interpreted events |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in analysis |
| Selective reporting (reporting bias) | Low risk | All data presented |
| Other bias | Unclear risk | Differences in initial treatment regimens between groups |
| Methods | Multi‐centre open‐label randomised clinical trial | |
| Participants | 480 patients (240 allocated to LMWH and 240 to VKA treatment) with documented, acute, proximal DVT Age, < 60 years old/≥ 60 years old: LMWH 118/122, VKA 122/118 Gender, M/F: LMWH 139/101, VKA 138/102 Location: 22 centres across Canada | |
| Interventions | Participants received tinzaparin 175 IU/kg subcutaneously once daily for 12 weeks, or tinzaparin for 5 days plus oral warfarin, commenced on day 1, INR‐adjusted, and continued for 12 weeks ('usual care'). Participants received 1 in‐clinic injection, then home treatment | |
| Outcomes | Primary efficacy outcome measure was occurrence of objectively documented, symptomatic, recurrent VTE at 12 weeks and at 1 year. Other efficacy outcomes were death rates at 12 weeks and 1 year; participants' self‐reported treatment satisfaction during the treatment period; symptoms of PTS; and incidence of venous leg ulcers as reported by participants. Primary safety outcome measure was occurrence of bleeding (all, major, or minor) during the 12‐week treatment period. Additional safety outcomes included incidence of thrombocytopenia and of bone fracture Blinded outcome assessment was provided by a central independent adjudication committee | |
| Notes | 3 months of randomised treatment and an additional 9 months of follow‐up Category I trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐derived treatment schedule |
| Allocation concealment (selection bias) | Unclear risk | Not clear |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial ‐ not blind |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes judged by a blinded central independent adjudication committee |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes presented. 3 participants lost to follow‐up at 12 months |
| Selective reporting (reporting bias) | Low risk | All outcomes presented |
| Other bias | Unclear risk | Differences in initial treatment regimens between groups |
| Methods | Multi‐centre randomised open‐label parallel‐group trial | |
| Participants | 297 patients (Group A: 98 allocated to 7 ± 2 days of unfractionated heparin followed by a 3‐month course of VKAs; Group B: 105 allocated to 7 ± 2 days of LMWH followed by a 3‐month course of VKAs; Group C: 94 allocated to 3 months of treatment with LMWH, with DVT confirmed on venography Age, years (range): Group A 61.2 (49.9 to 70.5), Group B 61.2 (44.4 to 69.5), Group C 63.2 (45.1 to 70.8) Gender, M, %: Group A 63, 64.3%; Group B 61, 58.1%; Group C 58, 61.7% Location: 27 centres in 3 countries (Poland, Spain, UK) | |
| Interventions | Group A: First coumarin arm started with a 7 ± 2‐day course of unfractionated heparin followed by warfarin for 3 months (INR 2.0 to 3.0) Group B: Second coumarin arm started with a 7 ± 2‐day course of bemiparin 115 anti‐Xa IU/kg once daily, followed by warfarin for 3 months (INR 2.0 to 3.0) Group C: Bemiparin arm received once‐daily subcutaneous tinzaparin 115 anti‐Xa IU/kg of body weight for 10 days, followed by a fixed dose of 3500 anti‐Xa IU for 90 days | |
| Outcomes | Recurrent VTE/ DVT: venography PE: high‐probability lung scan finding Major bleeding: clinically overt and associated with a fall in Hb ≥ 2 g/dL, transfusion of ≥ 2 units of blood, or intracranial or retroperitoneal bleeding Mortality data were provided Blinded outcome assessment was provided | |
| Notes | 3 months of randomised treatment and an additional 28 days of follow‐up Category II trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about generation of the randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | No information about concealment of allocation |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial – not blind |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded assessment of outcomes |
| Incomplete outcome data (attrition bias) | High risk | Drop‐outs were only partially explained |
| Selective reporting (reporting bias) | Low risk | All planned outcomes reported |
| Other bias | Low risk | None observed |
| Methods | Randomised controlled open‐label single‐institution treatment trial | |
| Participants | 40 patients (20 allocated to LMWH and 20 to VKA treatment) with PE confirmed on high‐probability ventilation‐perfusion scanning, a positive contrast chest computed tomogram, or a conventional pulmonary angiogram Age, years, mean ± SD: LMWH 52 ± 17/VKA 51 ± 18 Gender, F: n, %: LMWH 15, 75%/VKA 14, 70% Location: 1 centre in USA | |
| Interventions | Warfarin arm started with a course of enoxaparin (1 mg/kg) twice daily for ≥ 10 doses overlapping 4 days with warfarin continued for 90 days. Enoxaparin arm started with a course of 10 to 18 days at 1 mg/kg twice daily, followed by a 3‐month course of once‐daily subcutaneous enoxaparin 1.5 mg/kg 10 participants were treated with thrombolysis because of right ventricular failure | |
| Outcomes | Recurrent VTE/DVT: filling defect on conventional venography or loss of vein compressibility demonstrated on ultrasound PE: high‐probability lung scan finding, positive contrast chest computed tomogram, or conventional pulmonary angiogram Major bleeding: clinically overt and associated with a fall in Hb ≥ 3 g/dL;or intracranial, intraocular, retroperitoneal, or pericardial bleeding Mortality data were provided Blinded outcome assessment was not provided | |
| Notes | 3 months of randomised treatment; thereafter treatment at the discretion of the treating physician. No follow‐up was provided after 3 months Category II trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked computer randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial – not blind |
| Blinding of outcome assessment (detection bias) | High risk | Independent outcome collection not reported |
| Incomplete outcome data (attrition bias) | Low risk | No trial withdrawals |
| Selective reporting (reporting bias) | Low risk | Prospectively stated outcomes reported |
| Other bias | Low risk | None observed |
| Methods | Prospective open multi‐centre randomised clinical trial | |
| Participants | 202 patients (101 allocated to LMWH and 101 to VKA treatment) with proximal DVT confirmed on contrast phlebography. Evaluable data available for 98 LMWH and 95 VKA participants Age, mean ± SD, years: LMWH 56.6 ± 16.2/VKA 57.8 ± 14.6 Gender, M/F: LMWH 45/53/VKA 49/46 Location: 11 centres in Poland | |
| Interventions | Acenocoumarol for 3 months (INR of 2.0 to 3.0) compared with a 3‐month course of once‐daily subcutaneous nadroparin (85 anti‐Xa units per kilogram) | |
| Outcomes | Recurrent VTE/DVT: new constant intraluminal filling defect compared with baseline venography Major bleeding: overt bleeding associated with a fall in Hb ≥ 2 g/dL with need for transfusion of ≥ 2 units of packed red cells or intracranial or retroperitoneal bleeding Mortality from all causes Blinded outcome assessment was not provided | |
| Notes | 3 months of randomised treatment and an additional 9 months of follow‐up Category II trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial ‐ not blind |
| Blinding of outcome assessment (detection bias) | High risk | Blinded outcome assessment not reported |
| Incomplete outcome data (attrition bias) | Low risk | Withdrawals accounted for |
| Selective reporting (reporting bias) | Low risk | Prospectively stated outcomes reported |
| Other bias | Unclear risk | 3 fatal peripheral or cardiovascular events in the acenocoumarol group are not discussed. Follow‐up treatments after planned 3‐month outcomes differed between groups |
| Methods | Prospective open single‐centre randomised clinical trial | |
| Participants | 158 patients (81 allocated to LMWH and 77 to VKA treatment) with a first DVT episode in this leg confirmed on duplex scan examination Age, mean (95% CI), years: LMWH 65 (62 to 69)/VKA 66 (63 to 70) Gender, M/F: LMWH 31/50/VKA 38/39 Location: 1 centre in Spain | |
| Interventions | Acenocoumarol for 3 or 6 months (INR 2.0 to 3.0) compared with subcutaneous nadroparin adjusted to body weight 2 times daily (1025 anti‐Xa IU/10 kg). Both treatment arms started with a course of ≥ 5 days of treatment with subcutaneous nadroparin twice daily (1025 anti‐Xa IU/10 kg) | |
| Outcomes | Recurrent VTE/DVT: appearance of thrombosis in a previously unaffected venous segment of the ipsilateral or contralateral leg PE: constant intraluminal filling defect on spiral computed tomography or conventional angiography Major bleeding: overt bleeding associated with a decrease ≥ 2 g/dL in Hb level; requirement for blood transfusion of ≥ 2 units; intracranial or retroperitoneal bleeding; or need for permanent discontinuation of treatment. All other episodes of bleeding were defined as minor | |
| Notes | 3 to 6 months of randomised treatment and an additional 6 to 9 months of follow‐up. 44 participants in the acenocoumarol group and 34 in the nadroparin group were treated for 6 months. The remainder were treated for 3 months Control INR values were less than 2.0 in 22.8%, between 2 and 3 in 67.8%, and above 3 in 9.4% of cases Category II trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Generation of allocation sequence not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial ‐ not blind |
| Blinding of outcome assessment (detection bias) | Low risk | Blind outcomes collected by independent panel of physicians |
| Incomplete outcome data (attrition bias) | Low risk | Withdrawals explained |
| Selective reporting (reporting bias) | Low risk | All prospectively started outcomes reported |
| Other bias | Low risk | None observed |
| Methods | Multi‐centre open‐label randomised clinical trial | |
| Participants | 78 patients (all children) (37 allocated to reviparin and 41 to unfractionated heparin plus anticoagulant) with DVT confirmed on venography or compression ultrasound, or PE confirmed on ventilation‐perfusion scan or pulmonary angiogram Age, mean ± SD, years: LMWH 9.4 ± 6.6/VKA 8.7 ± 5.9 Gender, M/F: LMWH 17/20/VKA 19/22 Location: 37 centres in 6 countries (Australia, Canada, Germany, The Netherlands, UK, USA) | |
| Interventions | Interventions started within 48 hours of randomisation 3 months of 100 IU/kg reviparin sodium (Knoll, Germany) compared with 3 months of UFH followed by oral anticoagulants | |
| Outcomes | Recurrent VTE during 3 months of treatment and subsequent 3‐month follow‐up or death due to DVT Other outcomes: ‐ Safety outcomes ‐ Major bleeding defined as clinically significant overt bleeding requiring immediate transfusion of red blood cells, or any retroperitoneal, intracranial, or intra‐articular bleeding ‐ Minor bleeding defined as bruising, oozing around intravenous sites and surgical wounds, small amount of blood from suctioning of endotracheal tubes, small amounts of blood in urine or stool, and minor nosebleeds | |
| Notes | 3 months of randomised treatment and an additional 3 months of follow‐up Category I trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐derived protocol |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial ‐ not blind |
| Blinding of outcome assessment (detection bias) | Low risk | An independent and blinded central adjudication committee assessed all outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Of 78 participants, 66 completed the trial, 17 withdrew, and 5 died |
| Selective reporting (reporting bias) | Low risk | All prospectively stated outcomes were presented |
| Other bias | Unclear risk | Differences in initial treatment regimens between groups |
| Methods | Randomised multi‐centre open‐label trial | |
| Participants | 102 patients (52 allocated to LMWH monotherapy and 50 to LMWH followed by chronic VKA treatment) with objectively confirmed PE (perfusion lung scan or chest computed tomogram) Age, year (range): LMWH 72.4 (25 to 93)/VKA 72.1 (24 to 91) Gender, male, %: LMWH 25, 50%/VKA 28, 53.9% Location: 4 centres in Spain | |
| Interventions | Participants received tinzaparin 175 IU/kg subcutaneously once daily for 6 months, or tinzaparin plus oral acenocoumarol, commenced within 48 hours of the first dose of tinzaparin, INR‐adjusted, and continued for 6 months. In this latter group, tinzaparin was continued until INR was > 2 on 2 consecutive days | |
| Outcomes | Symptomatic, recurrent VTE at 1 month, 3 months, and 6 months (on compression ultrasonography or helical computed tomography) Composite of major and minor clinically relevant bleeding during treatment. Bleeding was defined as major if it was clinically associated with a decrease in Hb levels ≥ 2 g/dL, required a transfusion of ≥ 2 units of red blood cells, or was intracranial or retroperitoneal Other adverse reactions were also reported Blinded outcome assessment was not provided | |
| Notes | Category II trial INR values after discharge were 51.7% of measurements within therapeutic range, 41.5% below, and 6.8% above LEO Pharma provided indemnity and grants to support the study, and 2 study authors reported lecturing or working for LEO Pharma | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We stratified randomization through a central computer‐generated list" |
| Allocation concealment (selection bias) | Unclear risk | Randomisation through a central computer‐generated list ‐ no other information regarding allocation concealment provided |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | "Eight patients did not complete the 6‐month protocol successfully: five (9.7%) randomized to tinzaparin (metastatic cancer, allergy to tinzaparin, vein thrombosis and for two patients the reason was unknown) and three (6%) to VKA (metastatic cancer, inability to reach therapeutic INR and for one patents the reason was unknown)" All withdrawals and reasons for withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Prospectively stated outcomes reported |
| Other bias | Low risk | None observed |
| Methods | Prospective open single‐centre randomised clinical trial | |
| Participants | 187 patients (93 allocated to LMWH and 94 to VKA treatment) with first or second episode of symptomatic DVT confirmed on strain‐gauge plethysmography combined with a positive D‐dimer latex test most often confirmed with contrast venography Age, years, mean: LMWH 65.4/VKA 65.0 Gender, M/F: LMWH 47/46/VKA 54/40 Location: 1 centre in Italy | |
| Interventions | 3 months of conventional treatment with warfarin (INR 2.0 to 3.5), compared with a 3‐month course of enoxaparin 4000 anti‐Xa units once daily. All participants were initially treated with a 10‐day course of subcutaneous unfractionated heparin adjusted to an APTT of about 1.3 to 1.9 times the participant's basal value | |
| Outcomes | Recurrent VTE/DVT: new intraluminal filling defect in the deep veins by repeated venography or, if marked reduction of strain‐gauge plethysmography, coupled with a positive D‐dimer test that followed a negative one PE: defined by single or multiple segmental defects at perfusion scan with no abnormalities on chest radiograph in that area, on positive pulmonary angiogram, or at autopsy Mortality from all causes Blinded outcome assessment was provided by an independent panel of physicians who were unaware of treatment allocation | |
| Notes | 3 months of randomised treatment and an additional 9 months of follow‐up Category I trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐derived generation |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not stated |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial ‐ not blind |
| Blinding of outcome assessment (detection bias) | Low risk | Final adjudication of outcome measures conducted by an independent panel of physicians, 1 of whom was not involved in the trial |
| Incomplete outcome data (attrition bias) | Low risk | Participant exclusions explained and no enrolled participants dropped out |
| Selective reporting (reporting bias) | Low risk | All 3 outcomes reported (recurrent VTE, PE, and bleeding); deaths also reported |
| Other bias | Low risk | None observed |
| Methods | Open‐label prospective randomised clinical trial | |
| Participants | 241 patients (119 allocated to LMWH and 122 to VKA treatment) with an episode of symptomatic DVT confirmed on duplex ultrasonography Age, mean ± SD, years: LMWH 58.9 ± 17.6/VKA: 61.3 ± 16.2 Gender, male, %: LMWH 64, 53.8%/VKA: 70, 57.4% Location: 2 centres in Spain | |
| Interventions | Warfarin arm started with a course of tinzaparin 175 anti‐Xa IU/kg of body weight followed by warfarin for 6 months (INR 2.0 to 3.0). Tinzaparin group received once‐daily subcutaneous tinzaparin 175 anti‐Xa IU/kg of body weight, continued for 6 months | |
| Outcomes | Recurrent VTE/ DVT: previously compressible proximal vein segment no longer compressible on ultrasonography PE: high‐probability lung scan with clinical suspicion, abnormal perfusion scan with documented new DVT or spiral computed tomography showing thrombus in the pulmonary arteries Major bleeding: clinically overt bleeding associated with a fall in Hb ≥ 2 g/dL leading to blood transfusion of ≥ 2 units; or intracranial or retroperitoneal bleeding, or bleeding in a major joint Mortality of all causes Blinded outcome assessment was provided | |
| Notes | 6 months of randomised treatment and an additional 6 months of follow‐up Category II trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label, not blind |
| Blinding of outcome assessment (detection bias) | Low risk | Independently collected outcomes |
| Incomplete outcome data (attrition bias) | Low risk | All outcome data presented |
| Selective reporting (reporting bias) | Low risk | All prospectively stated outcomes reported |
| Other bias | Low risk | None observed |
| Methods | Prospective open single‐centre randomised clinical trial | |
| Participants | 100 patients (50 allocated to LMWH and 50 to VKA treatment) ≥ 75 years of age with a symptomatic proximal DVT confirmed on phlebography Age, mean, years: LMWH 80.9/VKA 79.6 Gender, M/F: LMWH 17/33/VKA 24/26 Location: 1 centre in Spain | |
| Interventions | Acenocoumarol for 3 or 6 months (INR 2.0 to 3.0) compared with once‐daily subcutaneous enoxaparin 40 mg (4000 IU Factor Xa inhibitor). Both treatment arms started with a course of ≥ 10 days of intravenous unfractionated heparin. Starting with a bolus of 5000 IU and followed by 4000 IU administered every 4 hours, with a target APTT of 1.5 to 2.0 times baseline APTT | |
| Outcomes | Recurrent VTE/DVT: new filling defect observed on phlebography PE: pulmonary scintigraphy and/or pulmonary arteriography. Necropsy was performed when necessary Major bleeding: overt bleeding and associated with a decrease in Hb of ≥ 2 g/dLl requiring a blood transfusion; retroperitoneal, intracranial, or intra‐articular, or leading to death. All other episodes of bleeding were defined as minor | |
| Notes | 3 to 6 months of randomised treatment and an additional 6 to 9 months of follow‐up. 7 participants in the acenocoumarol group and 5 in the enoxaparin group were treated for 6 months. The remainder were treated for 3 months Therapeutic compliance was graded as good in 15 (30%) participants (within desired INR range on more than 75% of occasions), acceptable in 28 (56%) participants (within INR target range on 50% to 75% of occasions), and poor in 7 (14%) participants (< 50% of occasions within the target range). In the enoxaparin group, 4 participants reported slight irregularities; in 5 others, the number of vials returned did not correspond exactly with doses needed for that time period Category II trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Closed envelopes used but no further information provided |
| Allocation concealment (selection bias) | Low risk | Closed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label trial ‐ not blind: LMWH administered to hospitalised participants vs acenocoumarol outpatient participants |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes collected by independent specialists |
| Incomplete outcome data (attrition bias) | Low risk | No participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All prospectively stated outcomes were accounted for |
| Other bias | Low risk | None observed |
APTT: activated partial thromboplastin time
CI: confidence interval
DVT: deep venous thrombosis
Hb: haemoglobin
INR: international normalised ratio
IU: international units
LMWH: low‐molecular‐weight heparin
PE: pulmonary embolism
PTS: post‐thrombotic syndrome
SD: standard deviation
UFH: unfractionated heparin
VKA; vitamin K antagonist
VTE: venous thromboembolism
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Composite endpoint trial | |
| Subjective participant‐reported outcomes | |
| Subjective participant‐reported outcomes | |
| Non‐randomised trial |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 16 | 3299 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.60, 1.15] |
| Analysis 1.1  Comparison 1 LMWH versus VKA during allocated treatment (category I and II trials) in participants with VTE, Outcome 1 Incidence of recurrent VTE. | ||||
| 2 Incidence of major bleeding Show forest plot | 16 | 3299 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.32, 0.80] |
| Analysis 1.2  Comparison 1 LMWH versus VKA during allocated treatment (category I and II trials) in participants with VTE, Outcome 2 Incidence of major bleeding. | ||||
| 3 Mortality Show forest plot | 16 | 3299 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.08 [0.75, 1.56] |
| Analysis 1.3  Comparison 1 LMWH versus VKA during allocated treatment (category I and II trials) in participants with VTE, Outcome 3 Mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 12 | 3021 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.79 [0.57, 1.11] |
| Analysis 2.1 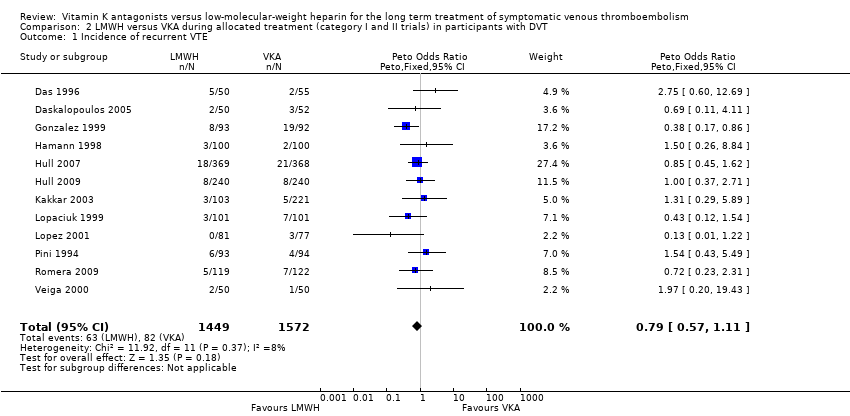 Comparison 2 LMWH versus VKA during allocated treatment (category I and II trials) in participants with DVT, Outcome 1 Incidence of recurrent VTE. | ||||
| 2 Incidence of major bleeding Show forest plot | 12 | 3021 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.33, 0.88] |
| Analysis 2.2 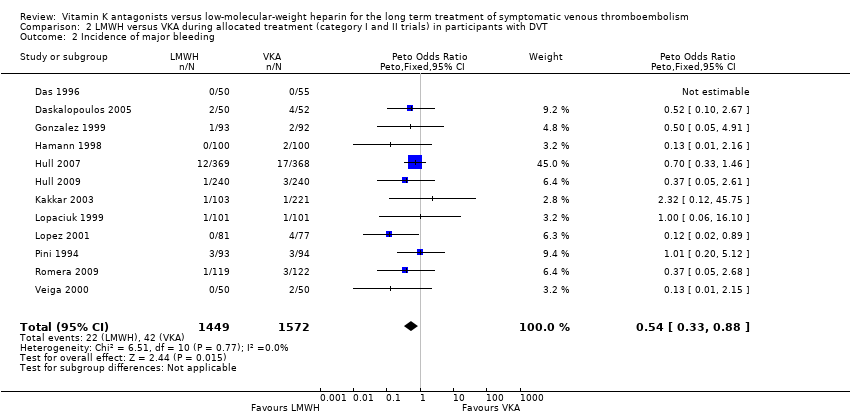 Comparison 2 LMWH versus VKA during allocated treatment (category I and II trials) in participants with DVT, Outcome 2 Incidence of major bleeding. | ||||
| 3 Mortality Show forest plot | 12 | 3021 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.10 [0.75, 1.60] |
| Analysis 2.3  Comparison 2 LMWH versus VKA during allocated treatment (category I and II trials) in participants with DVT, Outcome 3 Mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 3 | 202 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.70 [0.91, 35.60] |
| Analysis 3.1  Comparison 3 LMWH versus VKA during allocated treatment (category I and II trials) in participants with PE, Outcome 1 Incidence of recurrent VTE. | ||||
| 2 Incidence of major bleeding Show forest plot | 3 | 202 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.03, 1.78] |
| Analysis 3.2  Comparison 3 LMWH versus VKA during allocated treatment (category I and II trials) in participants with PE, Outcome 2 Incidence of major bleeding. | ||||
| 3 Mortality Show forest plot | 3 | 202 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.39 [0.51, 57.36] |
| Analysis 3.3  Comparison 3 LMWH versus VKA during allocated treatment (category I and II trials) in participants with PE, Outcome 3 Mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 7 | 1872 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.54, 1.18] |
| Analysis 4.1  Comparison 4 LMWH versus VKA during allocated treatment (category I trials) in participants with VTE, Outcome 1 Incidence of recurrent VTE. | ||||
| 2 Incidence of major bleeding Show forest plot | 7 | 1872 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.36, 1.07] |
| Analysis 4.2  Comparison 4 LMWH versus VKA during allocated treatment (category I trials) in participants with VTE, Outcome 2 Incidence of major bleeding. | ||||
| 3 Mortality Show forest plot | 7 | 1872 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.61, 1.41] |
| Analysis 4.3  Comparison 4 LMWH versus VKA during allocated treatment (category I trials) in participants with VTE, Outcome 3 Mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 2 | 292 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.95 [0.74, 5.19] |
| Analysis 5.1  Comparison 5 Category I trials and the same initial treatment in both groups (unfractionated heparin or LMWH), Outcome 1 Incidence of recurrent VTE. | ||||
| 2 Incidence of major bleeding Show forest plot | 2 | 292 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.20, 5.12] |
| Analysis 5.2  Comparison 5 Category I trials and the same initial treatment in both groups (unfractionated heparin or LMWH), Outcome 2 Incidence of major bleeding. | ||||
| 3 Mortality Show forest plot | 2 | 292 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.89 [0.29, 2.68] |
| Analysis 5.3  Comparison 5 Category I trials and the same initial treatment in both groups (unfractionated heparin or LMWH), Outcome 3 Mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 5 | 1580 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.68 [0.44, 1.03] |
| Analysis 6.1  Comparison 6 Category I trials and initial treatment not the same in both groups (unfractionated heparin compared with LMWH), Outcome 1 Incidence of recurrent VTE. | ||||
| 2 Incidence of major bleeding Show forest plot | 5 | 1580 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.33, 1.04] |
| Analysis 6.2  Comparison 6 Category I trials and initial treatment not the same in both groups (unfractionated heparin compared with LMWH), Outcome 2 Incidence of major bleeding. | ||||
| 3 Mortality Show forest plot | 5 | 1580 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.93 [0.59, 1.46] |
| Analysis 6.3 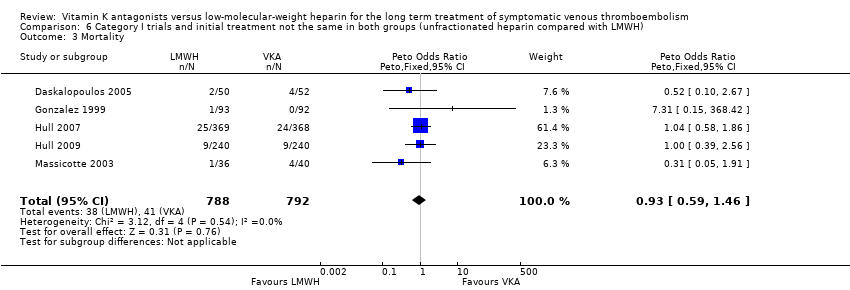 Comparison 6 Category I trials and initial treatment not the same in both groups (unfractionated heparin compared with LMWH), Outcome 3 Mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 10 | 2592 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.12 [0.77, 1.64] |
| Analysis 7.1  Comparison 7 LMWH versus VKA during additional follow‐up (category I and II trials), Outcome 1 Incidence of recurrent VTE. | ||||
| 2 Incidence of major bleeding Show forest plot | 9 | 2112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 7.2  Comparison 7 LMWH versus VKA during additional follow‐up (category I and II trials), Outcome 2 Incidence of major bleeding. | ||||
| 3 Mortality Show forest plot | 10 | 2592 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.71, 1.40] |
| Analysis 7.3  Comparison 7 LMWH versus VKA during additional follow‐up (category I and II trials), Outcome 3 Mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 5 | 1691 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.26 [0.81, 1.98] |
| Analysis 8.1  Comparison 8 LMWH versus VKA during additional nine months of follow‐up (category I trials), Outcome 1 Incidence of recurrent VTE. | ||||
| 2 Incidence of major bleeding Show forest plot | 4 | 1211 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 8.2  Comparison 8 LMWH versus VKA during additional nine months of follow‐up (category I trials), Outcome 2 Incidence of major bleeding. | ||||
| 3 Mortality Show forest plot | 5 | 1691 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.72, 1.55] |
| Analysis 8.3  Comparison 8 LMWH versus VKA during additional nine months of follow‐up (category I trials), Outcome 3 Mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 10 | 2592 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.67, 1.15] |
| Analysis 9.1  Comparison 9 LMWH versus VKA for total period of 12 months of follow‐up (category I and II trials), Outcome 1 Incidence of recurrent VTE. | ||||
| 2 Incidence of major bleeding Show forest plot | 9 | 2112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.56 [0.33, 0.95] |
| Analysis 9.2  Comparison 9 LMWH versus VKA for total period of 12 months of follow‐up (category I and II trials), Outcome 2 Incidence of major bleeding. | ||||
| 3 Mortality Show forest plot | 10 | 2592 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.09 [0.84, 1.43] |
| Analysis 9.3  Comparison 9 LMWH versus VKA for total period of 12 months of follow‐up (category I and II trials), Outcome 3 Mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 5 | 1691 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.70, 1.30] |
| Analysis 10.1  Comparison 10 LMWH versus VKA for total period of 12 months of follow‐up (category I trials), Outcome 1 Incidence of recurrent VTE. | ||||
| 2 Incidence of major bleeding Show forest plot | 4 | 1211 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.72 [0.39, 1.32] |
| Analysis 10.2  Comparison 10 LMWH versus VKA for total period of 12 months of follow‐up (category I trials), Outcome 2 Incidence of major bleeding. | ||||
| 3 Mortality Show forest plot | 5 | 1691 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.05 [0.78, 1.42] |
| Analysis 10.3  Comparison 10 LMWH versus VKA for total period of 12 months of follow‐up (category I trials), Outcome 3 Mortality. | ||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.

Funnel plot of comparison: 2 LMWH versus VKA during three months of allocated treatment (category I and II trials); outcome: 2.1 incidence of recurrent VTE.

Funnel plot of comparison: 2 LMWH versus VKA during three months of allocated treatment (category I and II trials); outcome: 2.2 incidence of major bleeding.

Funnel plot of comparison: 2 LMWH versus VKA during three months of allocated treatment (category I and II trials), outcome; 2.3 mortality.

Comparison 1 LMWH versus VKA during allocated treatment (category I and II trials) in participants with VTE, Outcome 1 Incidence of recurrent VTE.

Comparison 1 LMWH versus VKA during allocated treatment (category I and II trials) in participants with VTE, Outcome 2 Incidence of major bleeding.

Comparison 1 LMWH versus VKA during allocated treatment (category I and II trials) in participants with VTE, Outcome 3 Mortality.
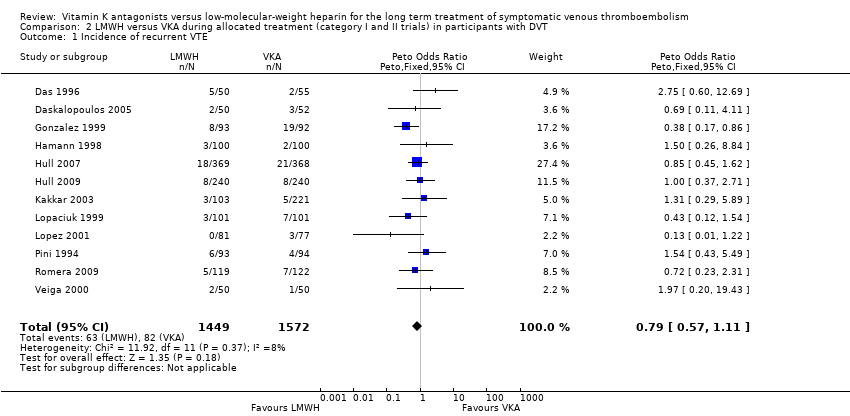
Comparison 2 LMWH versus VKA during allocated treatment (category I and II trials) in participants with DVT, Outcome 1 Incidence of recurrent VTE.

Comparison 2 LMWH versus VKA during allocated treatment (category I and II trials) in participants with DVT, Outcome 2 Incidence of major bleeding.

Comparison 2 LMWH versus VKA during allocated treatment (category I and II trials) in participants with DVT, Outcome 3 Mortality.

Comparison 3 LMWH versus VKA during allocated treatment (category I and II trials) in participants with PE, Outcome 1 Incidence of recurrent VTE.

Comparison 3 LMWH versus VKA during allocated treatment (category I and II trials) in participants with PE, Outcome 2 Incidence of major bleeding.
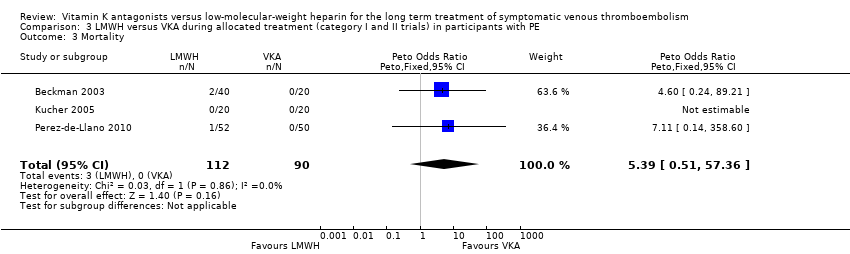
Comparison 3 LMWH versus VKA during allocated treatment (category I and II trials) in participants with PE, Outcome 3 Mortality.

Comparison 4 LMWH versus VKA during allocated treatment (category I trials) in participants with VTE, Outcome 1 Incidence of recurrent VTE.

Comparison 4 LMWH versus VKA during allocated treatment (category I trials) in participants with VTE, Outcome 2 Incidence of major bleeding.

Comparison 4 LMWH versus VKA during allocated treatment (category I trials) in participants with VTE, Outcome 3 Mortality.
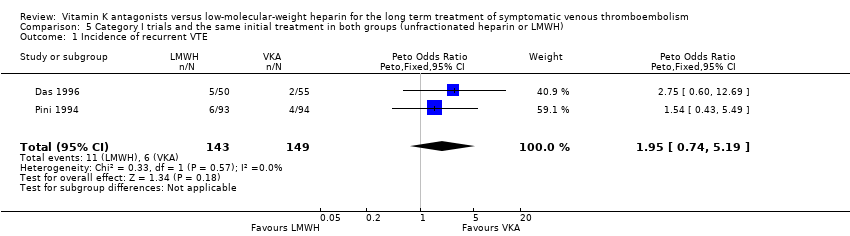
Comparison 5 Category I trials and the same initial treatment in both groups (unfractionated heparin or LMWH), Outcome 1 Incidence of recurrent VTE.

Comparison 5 Category I trials and the same initial treatment in both groups (unfractionated heparin or LMWH), Outcome 2 Incidence of major bleeding.

Comparison 5 Category I trials and the same initial treatment in both groups (unfractionated heparin or LMWH), Outcome 3 Mortality.

Comparison 6 Category I trials and initial treatment not the same in both groups (unfractionated heparin compared with LMWH), Outcome 1 Incidence of recurrent VTE.

Comparison 6 Category I trials and initial treatment not the same in both groups (unfractionated heparin compared with LMWH), Outcome 2 Incidence of major bleeding.

Comparison 6 Category I trials and initial treatment not the same in both groups (unfractionated heparin compared with LMWH), Outcome 3 Mortality.

Comparison 7 LMWH versus VKA during additional follow‐up (category I and II trials), Outcome 1 Incidence of recurrent VTE.

Comparison 7 LMWH versus VKA during additional follow‐up (category I and II trials), Outcome 2 Incidence of major bleeding.

Comparison 7 LMWH versus VKA during additional follow‐up (category I and II trials), Outcome 3 Mortality.

Comparison 8 LMWH versus VKA during additional nine months of follow‐up (category I trials), Outcome 1 Incidence of recurrent VTE.
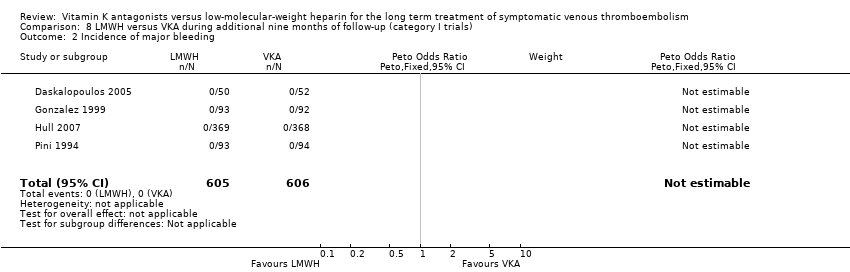
Comparison 8 LMWH versus VKA during additional nine months of follow‐up (category I trials), Outcome 2 Incidence of major bleeding.
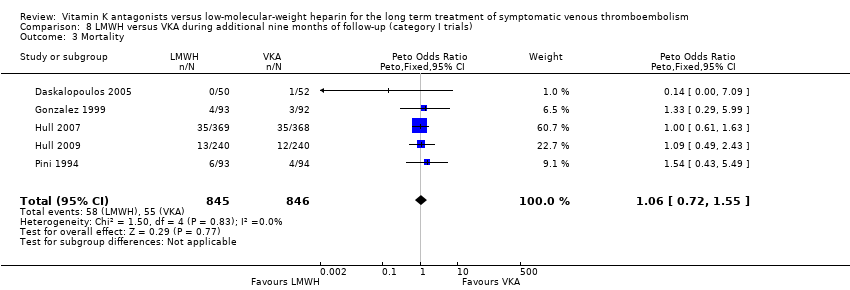
Comparison 8 LMWH versus VKA during additional nine months of follow‐up (category I trials), Outcome 3 Mortality.

Comparison 9 LMWH versus VKA for total period of 12 months of follow‐up (category I and II trials), Outcome 1 Incidence of recurrent VTE.

Comparison 9 LMWH versus VKA for total period of 12 months of follow‐up (category I and II trials), Outcome 2 Incidence of major bleeding.
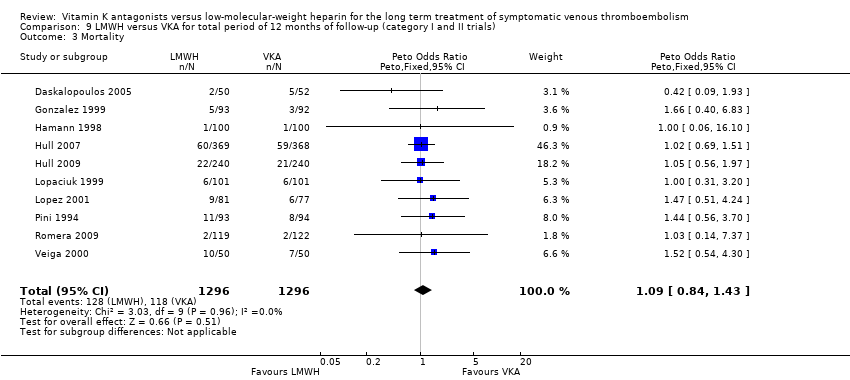
Comparison 9 LMWH versus VKA for total period of 12 months of follow‐up (category I and II trials), Outcome 3 Mortality.

Comparison 10 LMWH versus VKA for total period of 12 months of follow‐up (category I trials), Outcome 1 Incidence of recurrent VTE.

Comparison 10 LMWH versus VKA for total period of 12 months of follow‐up (category I trials), Outcome 2 Incidence of major bleeding.
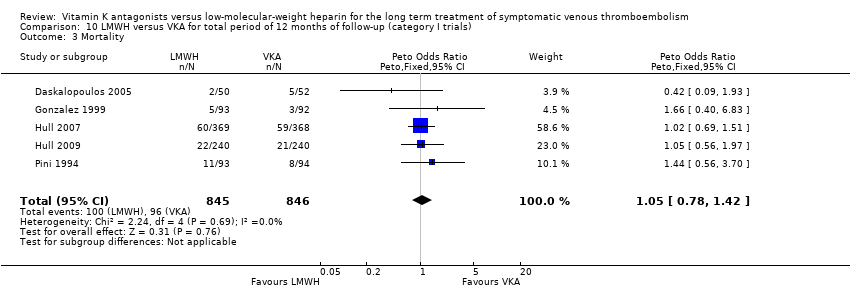
Comparison 10 LMWH versus VKA for total period of 12 months of follow‐up (category I trials), Outcome 3 Mortality.
| LMWH compared with VKA for long term treatment of symptomatic VTE | ||||||
| Patient or population: patients with symptomatic VTE requiring long term treatment (3 months) for symptomatic VTE | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with VKA | Risk with LMWH | |||||
| Incidence of recurrent VTE (treatment duration 3 months) | Study population | Peto OR 0.83 | 3299 | ⊕⊕⊕⊝ | ||
| 51 per 1000 | 42 per 1000 | |||||
| Incidence of major bleeding (treatment duration 3 months) | Study population | Peto OR 0.51 | 3299 | ⊕⊕⊝⊝ | ||
| 29 per 1000 | 15 per 1000 | |||||
| Mortality (treatment duration 3 months) | Study population | Peto OR 1.08 | 3299 | ⊕⊕⊕⊝ | ||
| 35 per 1000 | 37 per 1000 | |||||
| * The basis for the assumed risk with VKA for 'Study population' was the average risk in the VKA group (i.e. total number of participants with events divided by total number of participants in the VKA group included in the meta‐analysis). The risk in the LMWH group (and its 95% confidence interval) is based on assumed risk in the VKA group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aHigh risk of bias due to no blinding but not downgraded, as analysis excluding studies deemed of low methodological quality confirms no clear differences between LMWH and VKA | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 16 | 3299 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.60, 1.15] |
| 2 Incidence of major bleeding Show forest plot | 16 | 3299 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.32, 0.80] |
| 3 Mortality Show forest plot | 16 | 3299 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.08 [0.75, 1.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 12 | 3021 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.79 [0.57, 1.11] |
| 2 Incidence of major bleeding Show forest plot | 12 | 3021 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.33, 0.88] |
| 3 Mortality Show forest plot | 12 | 3021 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.10 [0.75, 1.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 3 | 202 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.70 [0.91, 35.60] |
| 2 Incidence of major bleeding Show forest plot | 3 | 202 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.03, 1.78] |
| 3 Mortality Show forest plot | 3 | 202 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.39 [0.51, 57.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 7 | 1872 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.54, 1.18] |
| 2 Incidence of major bleeding Show forest plot | 7 | 1872 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.36, 1.07] |
| 3 Mortality Show forest plot | 7 | 1872 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.61, 1.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 2 | 292 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.95 [0.74, 5.19] |
| 2 Incidence of major bleeding Show forest plot | 2 | 292 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.20, 5.12] |
| 3 Mortality Show forest plot | 2 | 292 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.89 [0.29, 2.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 5 | 1580 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.68 [0.44, 1.03] |
| 2 Incidence of major bleeding Show forest plot | 5 | 1580 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.33, 1.04] |
| 3 Mortality Show forest plot | 5 | 1580 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.93 [0.59, 1.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 10 | 2592 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.12 [0.77, 1.64] |
| 2 Incidence of major bleeding Show forest plot | 9 | 2112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Mortality Show forest plot | 10 | 2592 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.71, 1.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 5 | 1691 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.26 [0.81, 1.98] |
| 2 Incidence of major bleeding Show forest plot | 4 | 1211 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Mortality Show forest plot | 5 | 1691 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.72, 1.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 10 | 2592 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.67, 1.15] |
| 2 Incidence of major bleeding Show forest plot | 9 | 2112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.56 [0.33, 0.95] |
| 3 Mortality Show forest plot | 10 | 2592 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.09 [0.84, 1.43] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 5 | 1691 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.70, 1.30] |
| 2 Incidence of major bleeding Show forest plot | 4 | 1211 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.72 [0.39, 1.32] |
| 3 Mortality Show forest plot | 5 | 1691 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.05 [0.78, 1.42] |

