肺癌筛查
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial. | |
| Participants | Men aged 40 to 64 years. Current smokers with a lifetime cigarette consumption of greater than 150,000. Participants were included in the study if their initial prevalence screen was negative. They were excluded if they were not likely to participate for at least five years in periodic screening due to serious disease or other reasons. | |
| Interventions | Intervention group: semi‐annual chest x‐rays and sputum cytology. Control group: one chest x‐ray and sputum cytology at the end of the study. Afterwards, both groups had annual chest x‐rays (no sputum cytology) for a further three years. | |
| Outcomes | Lung cancer survival and mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding of the assessment of cause of death not described. |
| Incomplete outcome data (attrition bias) | High risk | Not reported. |
| Other bias | Unclear risk | Randomisation was stratified by age, smoking status, socioeconomic status, place of residence and occupational exposure, but number of strata used was not specified. Details not provided for all variables at baseline in the published reports. |
| Methods | Controlled (non‐randomised) trial. | |
| Participants | Men aged 40 to 65 years. All men living in the Erfurt county in Germany at the time of the study were included (smokers and non‐smokers); 41,532 men in the intervention group and 102,348 in the control group. | |
| Interventions | Intervention group: chest x‐ray at six‐monthly intervals. Control group: chest x‐ray at 18‐monthly intervals. Screening duration: five years. | |
| Outcomes | Lung cancer survival and mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not used. |
| Allocation concealment (selection bias) | High risk | Not used. |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding of the assessment of cause of death not described. |
| Incomplete outcome data (attrition bias) | Low risk | Withdrawals and drop‐outs adequately described, but losses to follow‐up significantly greater in the control group. |
| Other bias | Low risk | |
| Methods | Randomised controlled trial. | |
| Participants | Men over 45 years of age. 5161 men in the x‐ray‐only group and 5226 in the dual‐screen group. Smokers (at least 1 pack per day). | |
| Interventions | Intervention group: annual chest x‐rays and four‐monthly sputum cytology. Screening duration: five years. | |
| Outcomes | Lung cancer survival and mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | Individuals volunteered for the study by telephoning the Johns Hopkins Lung Project at which time they were randomised into intervention and control groups and given an appointment for screening. A total of 10,828 men were initially randomised, but 441 were automatically disqualified for failing to meet the age or cigarette‐smoking criteria of the study. |
| Blinding (performance bias and detection bias) | Low risk | Blinding of the assessment of cause of death. |
| Incomplete outcome data (attrition bias) | Unclear risk | 1.3% of participants lost to follow‐up but no further details provided. |
| Other bias | Low risk | The investigators reported on 36 baseline variables including multiple age strata, occupational exposures and smoking history. There were significantly more black participants in the control group (621 versus 701, P = 0.009) but this difference was not statistically significant after adjusting for multiple comparisons (P < 0.0014). |
| Methods | Randomised controlled trial. | |
| Participants | Men and women aged 35 to 54 at entry. 5156 people in study group and 5557 in control group. Both smokers and non‐smokers were included (about 17% of participants were smokers in both groups). All were members of Kaiser Permanente Medical Care Progam. | |
| Interventions | Intervention group: encouraged to undergo an annual Multiphasic Health Checkup (MHC) which included a chest x‐ray. Control group: participants not urged to take MHCs but could voluntarily do so as part of the care they received. | |
| Outcomes | All‐cause mortality and mortality from 'potentially postponable' causes including lung cancer mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patient record numbers (with a concealed code). |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) | Low risk | Blinding of the assessment of cause of death. |
| Incomplete outcome data (attrition bias) | High risk | Follow‐up was poor; in 1980 only 64% of participants were still health plan members and the response rate to follow‐up surveys was only 75%. |
| Other bias | Unclear risk | Statistically significant differences in some baseline variables. |
| Methods | Randomised controlled trial. | |
| Participants | Men over 45 years of age recruited from Mayo Clinic outpatients. Current smokers. 4618 men in the intervention group and 4593 in the control group. Participants were included in the study if their initial prevalence screen x‐ray was normal. | |
| Interventions | Intervention group: four‐monthly chest x‐rays and sputum cytology. Control group: standard Mayo Clinic recommendations to have an annual chest x‐ray and sputum cytology test with their local medical officer. Screening duration: six years. | |
| Outcomes | Lung cancer survival and mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Unclear risk | There were initially 10,933 men interviewed and entered in the prevalence phase of the study. Randomisation for the incidence phase of the study took place on entry to the prevalence study but 16% of men were excluded after randomisation. The exclusions included 91 prevalent lung cancers, six upper respiratory tract cancers, 971 who were ineligible because their life expectancy was less than five years or were thought unable to tolerate lobectomy, and 653 participants who did not complete the prevalence screening. Clinical judgements about eligibility were made by clinicians independent of the study, but the screening group allocation was marked on the participant's record on enrolment and therefore clinicians would have been aware of the allocation at the time of assessing eligibility. Randomisation was undertaken by staff interviewers (not primary investigators) on site, using a random number table, but it is unclear whether or not this was concealed or open (personal communication with Dr Fontana). |
| Blinding (performance bias and detection bias) | Low risk | Blinding of the assessment of cause of death. |
| Incomplete outcome data (attrition bias) | Low risk | Adequate. |
| Other bias | Low risk | The intervention and control groups were well matched for measured known confounders at baseline. Adjusting for these confounders (including smoking history, exposure to non‐tobacco carcinogens and history of other pulmonary diseases) did not significantly alter the results of the study. |
| Methods | Randomised controlled trial. | |
| Participants | Men (current smokers) over 45 years of age. 5072 men in the x‐ray‐only group and 4968 men in the dual‐screen group. | |
| Interventions | Intervention group: annual chest x‐rays and four‐monthly sputum cytology. Control group: annual chest x‐rays (chest x‐rays included postero‐anterior and lateral views). Screening duration: five years. | |
| Outcomes | Lung cancer survival and mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Adequate. Although randomisation took place on site, participants were randomised only after baseline data were entered and they were accepted into the study. Randomisation was co‐ordinated by clerical staff independent of the study investigators. Investigators met with participants only after they were randomised (this information was confirmed by contacting one of the study authors, M. Melamed). |
| Blinding (performance bias and detection bias) | Low risk | Blinding of the assessment of cause of death. |
| Incomplete outcome data (attrition bias) | Low risk | Adequate. |
| Other bias | Low risk | In this study, we examined 13 baseline variables reported in published reports and there was a greater proportion of participants with a history of exposure to asbestos (6% versus 5%, P = 0.03) and nickel (P = 0.03) in the intervention group, but these differences were no longer statistically significant after adjusting for multiple comparisons (P < 0.0039) (Berlin 1984). |
| Methods | Randomised controlled multicentre study. | |
| Participants | Men (59%) and women aged between 55 and 74, with a history of cigarette‐smoking of at least 30 pack‐years and if former smokers had quit within the previous 15 years. Individuals were excluded with a previous diagnosis of lung cancer, or who had undergone CT chest within 18 months before enrolment, or with a history of haemoptysis or unexplained weight loss of more than 6.8 kg in the preceding year. 53,454 persons were enrolled, 26,722 were assigned to screening with low‐dose CT and 26,732 to screening with chest radiography. Participants were recruited by the 33 screening centres. At each screening centre participants were made aware of the trial through direct mailing and use of local radio, newspaper advertisements, outreach including health fairs and presentations to unions an community groups, National Cancer Institute and institutional websites, Internet‐based advertising and public service television and radio announcements. | |
| Interventions | The intervention group were offered a total of three screenings with low‐dose CT at yearly intervals. The control group were offered a total of three screenings with chest radiography (postero‐anterior projection) at yearly intervals. All low‐dose CT scans were acquired using multidetector scanners with a minimum of four channels. The acquisition variables were chosen to reduce exposure to an average effective dose of 1.5 mSv. Low‐dose CT scans that revealed any non‐calcified nodule measuring at least 4 mm in any diameter and radiographic images that revealed any noncalcified nodule or mass were classified as positive "suspicious for" lung cancer. Other abnormalities such as adenopathy or effusion could also be classified as positive. At the third screening round abnormalities suspicious for lung cancer that were stable across the three rounds could be classified as minor abnormalities rather than positive results. No specific nodule‐evaluation approach was mandated by the trial protocol and the recommendations of the interpreting radiologist were reported in writing to the participant and his or her healthcare provider within four weeks of the examination. | |
| Outcomes | The primary outcome was lung cancer mortality; secondary outcomes included all‐cause mortality, incidence of lung cancer, lung cancer case survival (as measured from date of diagnosis), and lung cancer stage distribution. | |
| Notes | The number of lung cancer screening tests conducted outside the NSLT was estimated by self‐administered questionnaires that were mailed to a random sample of approximately 500 participants annually. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised after consent was obtained using a central process (ACRIN website). Randomisation was stratified by age, gender and screening centre, and blocked such that at each centre, each arm had equal numbers of participants within each gender and age category. |
| Allocation concealment (selection bias) | Low risk | As above, participants randomised after consent using a centralised process. |
| Blinding (performance bias and detection bias) | Low risk | Death certificates were obtained for participants who were known to have died. An end point verification team determined whether the cause of death was lung cancer. Deaths selected for review included those with a notation of lung cancer on the death certificate and those occurring among participants ever diagnosed with lung cancer, as well as deaths within six months of a screen suspicious for lung cancer and deaths within 60 days of certain diagnostic evaluation procedures associated with a screen suspicious for lung cancer or a lung cancer diagnosis. Members of the end point verification team were not aware of group assignments. A distinction was made between a death due to lung cancer and a death that resulted from the diagnostic evaluation for or treatment of lung cancer; however the deaths in the latter category were counted as lung cancer deaths in the primary end point analysis. |
| Incomplete outcome data (attrition bias) | Low risk | The vital status was known for 97% of participants in the low‐dose CT group and 96% of the chest radiography group. The median duration of follow‐up was 6.5 years and maximum duration was 7.4 years. According to Consort statement no individuals were excluded from the analysis of the primary outcomes and it is likely that losses to follow‐up were censored. |
| Other bias | Low risk | All prespecified primary outcomes were reported and the intervention and control groups were comparable at baseline. |
| Methods | Cluster‐randomised controlled trial (industrial firms randomised). | |
| Participants | Men aged 40 and over. 29,723 in intervention group and 25,311 in control group. Both smokers and non‐smokers included. | |
| Interventions | Intervention group: six‐monthly chest x‐rays. Control group: chest x‐ray on entry and at the end of the study period. Mobile x‐ray unit used for x‐rays. Screening duration: three years. | |
| Outcomes | Lung cancer survival and mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sampling numbers. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding of the assessment of cause of death not described. |
| Incomplete outcome data (attrition bias) | Low risk | Adequate. |
| Other bias | Unclear risk | Statistically significant differences in some baseline variables. |
| Methods | Randomised controlled multiple cancer (prostate, lung, ovarian and colon cancers) screening study. Multicentre ‐10 screening centres. | |
| Participants | Men and women between the ages of 55 and 74 years. Included smokers and non‐smokers. 77,470 participants in the control group and 77,464 in the intervention group. Exclusions included anyone participating in another cancer screening trial or primary prevention trial. Men who had taken finasteride in the six months before entry or who had had more than one prostate‐specific antigen blood test in the past three years, and individuals who had had colonoscopy, sigmoidoscopy or a barium enema examination in the past three years. Individuals with previous surgical removal of the entire prostate gland, one lung, or the entire colon were also excluded. Women with prior removal of both ovaries were initially excluded, but were allowed to enrol from 1996 onwards. Participants were also excluded if they had a history of any prostate, lung, ovarian or colon cancer or were currently receiving treatment for cancer. Recruitment was targeted to healthy volunteers primarily through direct mail. Enhanced recruitment methods were used to target minority populations. | |
| Interventions | The intervention group were offered a single‐view posterioranterior chest x‐ray at baseline and then annually for three years (a total of four screens including the baseline x‐ray). A chest x‐ray was considered positive when a radiologist identified a mass (> 3 cm), nodule(< 3 cm), infiltrate, or any other abnormality considered suspicious for cancer. Never‐smokers randomised after April 1995 were not offered the final screen. The control group were assigned to usual care (no formalised screening). Participants who received a positive screening result were referred to their primary healthcare provider for further evaluation. The trial protocol did not specify a diagnostic algorithm. Chest radiographic screening in the usual‐care group was assessed by surveying a random sample of just more than 1% of participants using biennial and later annual health status questionnaires. | |
| Outcomes | The primary outcome was lung cancer mortality; secondary outcomes included lung cancer incidence, cancer stage, survival, harms from screening and all‐cause mortality. | |
| Notes | 49.5% of participants in both the intervention and usual‐care groups were men and approximately 52% were former or current smokers in both groups. Adherence to screening was 86.6% for the baseline screen, decreasing to 79% by year three. 91.2% of participants underwent at least one radiographic screening. In the usual‐care group the contamination rate was estimated at 11% during the screening phase of the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation scheme used blocks of random permutations of varying lengths and was stratified by screening centre, gender, and age. |
| Allocation concealment (selection bias) | Low risk | Random assignment was implemented using compiled software and encrypted files loaded on to microcomputers at each of the screening centres. |
| Blinding (performance bias and detection bias) | Low risk | Deaths were ascertained by annual follow‐up questionnaire and where necessary repeat mailings or telephone follow‐up in addition to periodic linkage to the National Death Index. An end point adjudication process was used to assign cause of death. All deaths with causes potentially related to a prostate, ovarian, colorectal or lung cancer were reviewed. Death reviewers were blinded to the trial group of the deceased participant. |
| Incomplete outcome data (attrition bias) | Low risk | Number of losses to follow‐up were not described. For the primary outcome person‐time was measured from randomisation to the earliest death date or date of last follow‐up (censoring date). All individuals randomised were included in the primary analysis. The median follow‐up time in each group was 11.2 years (interquartile range 10.0 to 13 years in each group). |
| Other bias | Low risk | Groups were comparable at baseline and all prespecified outcomes were reported. |
CT: computed tomography
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Screening with low‐dose CT; uncontrolled study. | |
| Pilot randomised controlled trial of low ‐ose CT versus chest x‐ray, small study and no mortality data provided. | |
| Uncontrolled study (screening with low‐dose CT). | |
| Uncontrolled study (screening with low‐dose spiral CT). | |
| Observational, case‐control study. | |
| Small randomised controlled trial of low‐dose spiral CT screening. Feasibility study with no outcome data. | |
| Small randomised controlled trial of low‐dose CT screening. Feasibility study with no mortality data. | |
| Screening with low‐dose CT; uncontrolled study. | |
| Screening with low‐dose CT; uncontrolled study. | |
| Screening with low‐dose CT: uncontrolled study. | |
| Uncontrolled study (screening with low‐dose spiral CT), report on false negative results. | |
| Uncontrolled study (screening with low‐dose CT). | |
| Uncontrolled study. | |
| Uncontrolled study (screening with low‐dose spiral CT). | |
| Observational, case‐control study. | |
| Uncontrolled study (screening with low‐dose spiral CT). | |
| Uncontrolled study (screening with spiral CT). | |
| Uncontrolled study (screening with spiral CT). | |
| Uncontrolled study (screening with low‐dose spiral CT). | |
| Uncontrolled study (screening with CT in asbestos‐exposed workers). | |
| Randomised controlled trial with a total of 523 participants, comparing low‐dose CT screening plus p16 gene methylation detection with chest x‐ray screening. This was a feasibility study and did not include mortality data. |
CT: computed tomography
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | DANTE |
| Methods | Randomised controlled trial. |
| Participants | 2,811 men aged 60 to 75 years, smokers of 20 or more pack‐years. |
| Interventions | Low‐dose CT versus control (no active screening) at baseline and every year for four years. |
| Outcomes | Lung cancer mortality, resectability, stage distribution. |
| Starting date | March 2001. |
| Contact information | |
| Notes | Three‐year preliminary results published in 2009. |
| Trial name or title | DLCST (Danish Lung Cancer Screening Trial). |
| Methods | Randomised controlled trial. |
| Participants | 4104 men and women 50 to 70 years, current or former smokers (at least 20 pack years). |
| Interventions | Five annual low‐dose CT screenings versus no screening. |
| Outcomes | Lung cancer mortality and all‐cause mortality. |
| Starting date | October 2004. |
| Contact information | |
| Notes | Preliminary results published in 2012 but median follow‐up was < 5 years (median 4.81 years), further follow‐up is planned. |
| Trial name or title | ITALUNG |
| Methods | Randomised controlled trial. |
| Participants | 3206 men and women aged 55 to 69 years, smokers and former smokers with at least a 20 pack‐year history of smoking. |
| Interventions | Low‐dose CT screening for four years versus no screening. |
| Outcomes | Lung cancer mortality. |
| Starting date | |
| Contact information | |
| Notes |
| Trial name or title | LUSI (Lung Cancer Screening Intervention trial). |
| Methods | Randomised controlled trial. |
| Participants | 4052 men and women, heavy smokers, aged 50 to 69 years. |
| Interventions | Five annual low‐dose, multislice CT versus no screening. |
| Outcomes | Lung cancer mortality. |
| Starting date | October 2007. |
| Contact information | |
| Notes |
| Trial name or title | MILD (Multicentric Italian Lung Detection project). |
| Methods | Randomised controlled trial. |
| Participants | Men and women aged 49 years and above, current or former smokers (at least 20 pack‐years of smoking) and having quit within 10 years of recruitment. |
| Interventions | Annual low‐dose CT versus biennial low‐dose CT versus control (no active screening). |
| Outcomes | Lung cancer mortality, lung cancer incidence, all‐cause mortality. |
| Starting date | September 2005. |
| Contact information | |
| Notes | The trial is ongoing, preliminary results published in 2012, but median duration of follow‐up was 4.4 years (< 5 years). |
| Trial name or title | Dutch‐Belgian randomised lung cancer screening trial (Nederlands Leuvens Longkanker Screenings Onderzoek). |
| Methods | Multicentre trial, randomised, parallel group, no blinding. |
| Participants | Target number, n = 15,600. Born between 1928 and 1956; current long‐term smokers or quit smoking < 10 years prior. |
| Interventions | 16 detector multislice computed tomography of the chest in year four; pulmonary function test; blood sampling; questionnaires; smoking cessation advice for current smokers, versus smoking cessation advice for current smokers. |
| Outcomes | Primary: reduction in lung cancer mortality. Secondary: cost effectiveness; quality of life. |
| Starting date | August 2003. |
| Contact information | Klaveren RJ van; http://www.nelsonproject.nl. |
| Notes |
CT: computed tomography
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Lung cancer mortality Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1 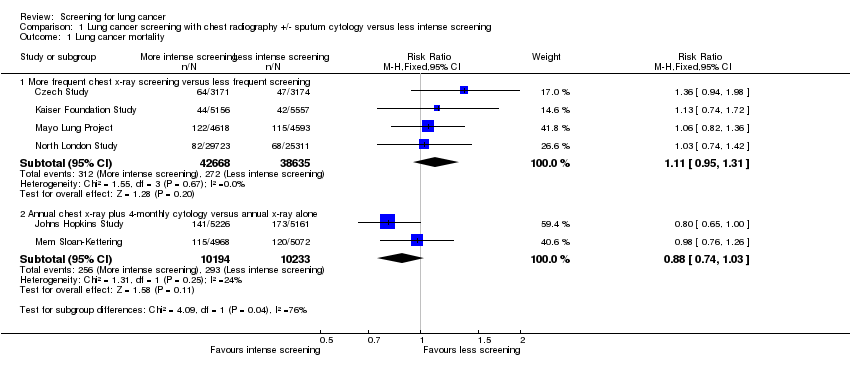 Comparison 1 Lung cancer screening with chest radiography +/‐ sputum cytology versus less intense screening, Outcome 1 Lung cancer mortality. | ||||
| 1.1 More frequent chest x‐ray screening versus less frequent screening | 4 | 81303 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.95, 1.31] |
| 1.2 Annual chest x‐ray plus 4‐monthly cytology versus annual x‐ray alone | 2 | 20427 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.74, 1.03] |
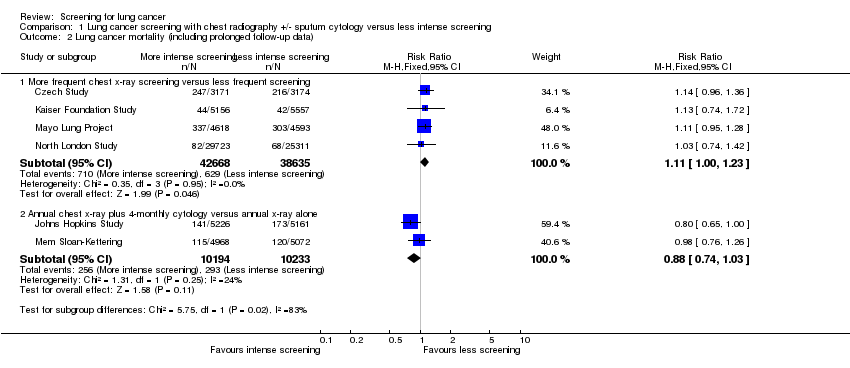
| 2 Lung cancer mortality (including prolonged follow‐up data) Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Lung cancer screening with chest radiography +/‐ sputum cytology versus less intense screening, Outcome 2 Lung cancer mortality (including prolonged follow‐up data). | ||||
| 2.1 More frequent chest x‐ray screening versus less frequent screening | 4 | 81303 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.00, 1.23] |
| 2.2 Annual chest x‐ray plus 4‐monthly cytology versus annual x‐ray alone | 2 | 20427 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.74, 1.03] |
| 3 All‐cause mortality Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Lung cancer screening with chest radiography +/‐ sputum cytology versus less intense screening, Outcome 3 All‐cause mortality. | ||||
| 3.1 More frequent chest x‐ray screening versus less frequent screening | 4 | 170149 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.94, 1.08] |
| 3.2 Annual chest x‐ray plus 4‐monthly cytology versus annual x‐ray alone | 1 | 10040 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.91, 1.15] |
| 4 Lung cancer 5‐year survival Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.4 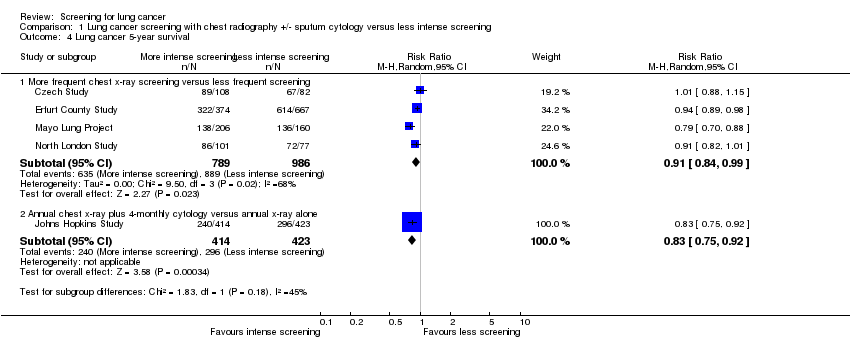 Comparison 1 Lung cancer screening with chest radiography +/‐ sputum cytology versus less intense screening, Outcome 4 Lung cancer 5‐year survival. | ||||
| 4.1 More frequent chest x‐ray screening versus less frequent screening | 4 | 1775 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.84, 0.99] |
| 4.2 Annual chest x‐ray plus 4‐monthly cytology versus annual x‐ray alone | 1 | 837 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.75, 0.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
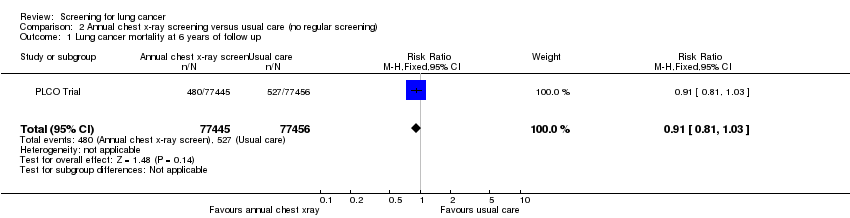
| 1 Lung cancer mortality at 6 years of follow up Show forest plot | 1 | 154901 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.81, 1.03] |
| Analysis 2.1  Comparison 2 Annual chest x‐ray screening versus usual care (no regular screening), Outcome 1 Lung cancer mortality at 6 years of follow up. | ||||
| 2 Lung cancer mortality at 13 years of follow up Show forest plot | 1 | 154901 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.07] |
| Analysis 2.2 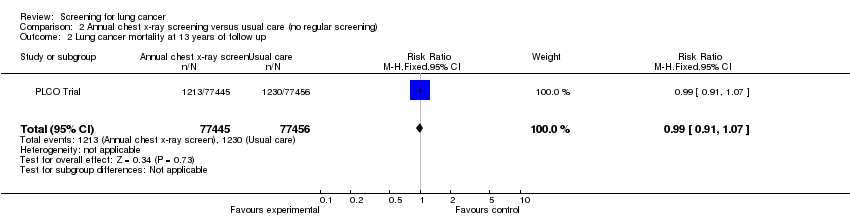 Comparison 2 Annual chest x‐ray screening versus usual care (no regular screening), Outcome 2 Lung cancer mortality at 13 years of follow up. | ||||
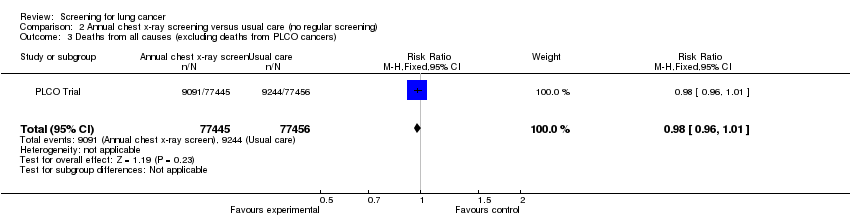
| 3 Deaths from all causes (excluding deaths from PLCO cancers) Show forest plot | 1 | 154901 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.96, 1.01] |
| Analysis 2.3 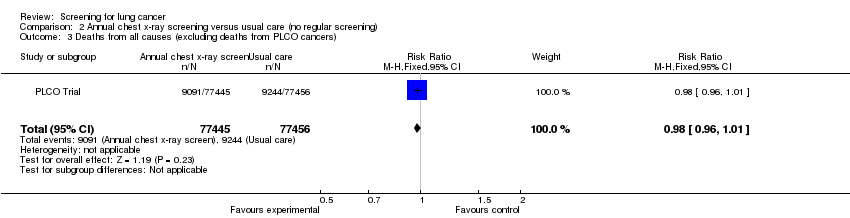 Comparison 2 Annual chest x‐ray screening versus usual care (no regular screening), Outcome 3 Deaths from all causes (excluding deaths from PLCO cancers). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Lung cancer mortality Show forest plot | 1 | 53454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.70, 0.92] |
| Analysis 3.1  Comparison 3 Annual low dose CT screening versus annual chest x‐ray, Outcome 1 Lung cancer mortality. | ||||
| 2 All‐cause mortality Show forest plot | 1 | 53454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.88, 1.00] |
| Analysis 3.2 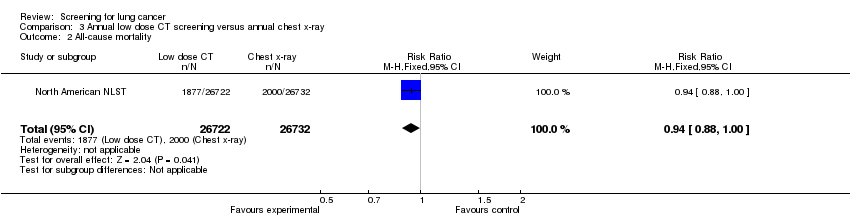 Comparison 3 Annual low dose CT screening versus annual chest x‐ray, Outcome 2 All‐cause mortality. | ||||

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Lung cancer screening with chest radiography +/‐ sputum cytology versus less intense screening, Outcome 1 Lung cancer mortality.
Comparison 1 Lung cancer screening with chest radiography +/‐ sputum cytology versus less intense screening, Outcome 2 Lung cancer mortality (including prolonged follow‐up data).

Comparison 1 Lung cancer screening with chest radiography +/‐ sputum cytology versus less intense screening, Outcome 3 All‐cause mortality.

Comparison 1 Lung cancer screening with chest radiography +/‐ sputum cytology versus less intense screening, Outcome 4 Lung cancer 5‐year survival.
Comparison 2 Annual chest x‐ray screening versus usual care (no regular screening), Outcome 1 Lung cancer mortality at 6 years of follow up.

Comparison 2 Annual chest x‐ray screening versus usual care (no regular screening), Outcome 2 Lung cancer mortality at 13 years of follow up.
Comparison 2 Annual chest x‐ray screening versus usual care (no regular screening), Outcome 3 Deaths from all causes (excluding deaths from PLCO cancers).

Comparison 3 Annual low dose CT screening versus annual chest x‐ray, Outcome 1 Lung cancer mortality.

Comparison 3 Annual low dose CT screening versus annual chest x‐ray, Outcome 2 All‐cause mortality.
| L ung cancer screening with chest radiography +/‐ sputum cytology versus less intense screening for lung cancer | ||||||
| Patient or population: Patients with lung cancer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Lung cancer screening with chest radiography +/‐ sputum cytology versus less intense screening | |||||
| Lung cancer mortality ‐ More frequent chest x‐ray screening versus less frequent screening | 7 per 1000 | 8 per 1000 | RR 1.11 | 81303 | ⊕⊕⊕⊝ | |
| Lung cancer mortality ‐ Annual chest x‐ray plus 4‐monthly cytology versus annual x‐ray alone | 29 per 1000 | 25 per 1000 | RR 0.88 | 20427 | ⊕⊕⊕⊕ | |
| All‐cause mortality ‐ More frequent chest x‐ray screening versus less frequent screening | 83 per 1000 | 84 per 1000 | RR 1.01 | 170149 | ⊕⊕⊝⊝ | |
| All‐cause mortality ‐ Annual chest x‐ray plus 4‐monthly cytology versus annual x‐ray alone | 97 per 1000 | 100 per 1000 | RR 1.03 | 10040 | ⊕⊕⊕⊕ | |
| Lung cancer 5‐year survival ‐ More frequent chest x‐ray screening versus less frequent screening | 902 per 1000 | 820 per 1000 | RR 0.91 | 1775 | ⊕⊕⊝⊝ | |
| Lung cancer 5‐year survival ‐ Annual chest x‐ray plus 4‐monthly cytology versus annual x‐ray alone | 700 per 1000 | 581 per 1000 | RR 0.83 | 837 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 No trials had evidence of adequate allocation concealment and only half had adequate description of drop‐outs. | ||||||
| Annual chest x‐ray screening versus usual care (no regular screening) for lung cancer | ||||||
| Patient or population: Patients with lung cancer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Annual chest x‐ray screening versus usual care (no regular screening) | |||||
| Lung cancer mortality at 6 years of follow‐up | 7 per 1000 | 6 per 1000 | RR 0.91 | 154901 | ⊕⊕⊕⊕ | |
| Lung cancer mortality at 13 years of follow‐up | 16 per 1000 | 16 per 1000 | RR 0.99 | 154901 | ⊕⊕⊕⊕ | |
| Deaths from all causes (excluding deaths from PLCO cancers) | 119 per 1000 | 117 per 1000 | RR 0.98 | 154901 | ⊕⊕⊕⊕ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Annual low dose CT screening versus annual chest x‐ray for lung cancer | ||||||
| Patient or population: Patients with lung cancer | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Annual low‐dose CT screening versus annual chest x‐ray | |||||
| Lung cancer mortality | 17 per 1000 | 13 per 1000 | RR 0.8 | 53454 | ⊕⊕⊕⊕ | |
| All‐cause mortality | 75 per 1000 | 70 per 1000 | RR 0.94 | 53454 | ⊕⊕⊕⊕ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Study | Intervention n (%) | Intervention N | Control n(%) | Control N | Relative risk |
| 108 (3.4%) | 3171 | 82 (2.6%) | 3174 | 1.33 (0.99,1.75) | |
| 374 (0.9%) | 41532 | 667 (0.7%) | 102348 | 1.38 (1.22,1.57) | |
| 585 (12.7%) | 4618 | 500 (10.9%) | 4593 | 1.16 (1.04,1.3) | |
| 132 (0.44%) | 29723 | 97 (0.38%) | 25311 | 1.16 (0.89,1.51) | |
| 238 (4.6%) | 5226 | 246 (4.8%) | 5161 | 0.95 (0.8,1.14) | |
| 176 (3.5%) | 4968 | 178 (3.5%) | 5072 | 1.01 (0.82,1.24) | |
| 1696 (2.2%) | 77445 | 1620 (2.1%) | 77456 | 1.05 (0.98, 1.12) | |
| 1060 (4.0%) | 26722 | 941 (3.5%) | 26732 | 1.13 (1.03, 1.23) | |
| *Data from prolonged period of follow‐up reported post‐study. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Lung cancer mortality Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 More frequent chest x‐ray screening versus less frequent screening | 4 | 81303 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.95, 1.31] |
| 1.2 Annual chest x‐ray plus 4‐monthly cytology versus annual x‐ray alone | 2 | 20427 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.74, 1.03] |
| 2 Lung cancer mortality (including prolonged follow‐up data) Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 More frequent chest x‐ray screening versus less frequent screening | 4 | 81303 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.00, 1.23] |
| 2.2 Annual chest x‐ray plus 4‐monthly cytology versus annual x‐ray alone | 2 | 20427 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.74, 1.03] |
| 3 All‐cause mortality Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 More frequent chest x‐ray screening versus less frequent screening | 4 | 170149 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.94, 1.08] |
| 3.2 Annual chest x‐ray plus 4‐monthly cytology versus annual x‐ray alone | 1 | 10040 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.91, 1.15] |
| 4 Lung cancer 5‐year survival Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 More frequent chest x‐ray screening versus less frequent screening | 4 | 1775 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.84, 0.99] |
| 4.2 Annual chest x‐ray plus 4‐monthly cytology versus annual x‐ray alone | 1 | 837 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.75, 0.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Lung cancer mortality at 6 years of follow up Show forest plot | 1 | 154901 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.81, 1.03] |
| 2 Lung cancer mortality at 13 years of follow up Show forest plot | 1 | 154901 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.07] |
| 3 Deaths from all causes (excluding deaths from PLCO cancers) Show forest plot | 1 | 154901 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.96, 1.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Lung cancer mortality Show forest plot | 1 | 53454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.70, 0.92] |
| 2 All‐cause mortality Show forest plot | 1 | 53454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.88, 1.00] |

