Azithromycine dans le traitement des infections aiguës des voies respiratoires inférieures
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Location: France | |
| Participants | 110 adults with acute lower respiratory tract infection, either acute bacterial bronchitis or pneumonia. Acute bronchitis was defined as bacterial bronchial or bronchopulmonary infection accompanied by the production of purulent sputum. Participants with infectious mononucleosis, chronic or chronic obstructive pulmonary disease without acute infection, or who had received antibiotics within 48 hours prior to the study were excluded | |
| Interventions | 1. Azithromycin 500 mg single dose on day 1 followed by a single dose of 250 mg daily on day 2 to 5 | |
| Outcomes | Cure | |
| Notes | Of the bronchitis cases, 20/48 in the azithromycin group and 19/54 in the amoxycillin/clavulanic acid group were described as acute exacerbation of chronic bronchitis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The study did not report how randomisation was done |
| Allocation concealment (selection bias) | Unclear risk | The concealment process was not reported. Quote: "Of this total, 52 (30 males, 22 females) were randomized to receive oral azithromycin and 58 (39 males, 19 females) to receive oral amoxycillin/CA." |
| Blinding (performance bias and detection bias) | Unclear risk | The study did not provide blinding information |
| Incomplete outcome data (attrition bias) | Low risk | Reasonable, as 6 patients not having clinical assessment were clearly reported. 4 participants were in the azithromycin group (7.7%; 4/52) and 2 participants were in the amoxycillin group (3.5%; 2/58) |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available |
| Other bias | Unclear risk | Baseline characteristics of the 2 treatment groups were not addressed |
| Methods | Multicentre study, participants were randomised to receive either azithromycin or amoxycillin/clavulanic acid. No blinding. Efficacy was evaluated 10 days after the therapy started | |
| Participants | 142 hospitalised or outpatients aged 18 years or more with acute purulent exacerbation of chronic bronchitis. Exclusion criteria: participants treated with other antibiotics 48 hours prior to the study, leucopenia, coagulation disorders, renal dysfunction, HIV/AIDS on immunosuppressive drugs, suspected pneumonia with lung abscess, pleuritis, empyema or active tuberculosis, pregnancy and lactation. Participants: azithromycin group N = 69, amoxycillin/clavulanic acid group N = 73 | |
| Interventions | 1. Azithromycin (Pfizer) 500 mg single dose daily for 3 days | |
| Outcomes | Cure (disappearance of all signs and clinical symptoms of infection by day 10) | |
| Notes | Corticosteroids were allowed, provided this did not exceed 25 mg for prednisolone or its equivalent in both groups. Of the 142 participants, 2 participants dropped out and were not included in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | High risk | Not mentioned in the article |
| Blinding (performance bias and detection bias) | High risk | No blinding |
| Incomplete outcome data (attrition bias) | Low risk | All participants randomised were analysed. 69 in the azithromycin group and 73 in the amoxycillin group |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Baseline characteristics of the 2 treatment groups were not addressed |
| Methods | Participants were randomised in a 2:1 ratio to receive either azithromycin or amoxycillin/clavulanic acid. No blinding. Efficacy was evaluated at 8 to 10 days after the therapy started | |
| Participants | 759 adult participants aged between 18 to 75 years were recruited; 620 had acute tracheobronchitis and 139 had acute exacerbations of chronic bronchitis. A diagnosis of acute tracheobronchitis was based on the presence of at least 2 of the following signs and symptoms: cough, fever 38 ºC or higher, purulent sputum and rhonchi/rales. Participants: azithromycin group N = 501, amoxycillin/clavulanic acid group N = 258 | |
| Interventions | 1. Azithromycin 500 mg once daily for 3 days (2 x 250 mg capsules taken at least 1 hour before or 2 hours after meals) | |
| Outcomes | Cure | |
| Notes | Of 759 participants, 31 participants with various reasons (adverse events, lack of efficacy and lost to follow‐up) discontinued treatment; 9 in the azithromycin group and 22 in the amoxycillin/clavulanate group. 26 out of 31 who dropped out were followed and evaluated. In the analysis, 754 participants were included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. Quote: "Patients were randomized in a 2:1 ratio to receive either azithromycin or amoxicillin/clavulanic acid" |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation was not described |
| Blinding (performance bias and detection bias) | High risk | No blinding |
| Incomplete outcome data (attrition bias) | Low risk | 754 participants (99%; 754/759) were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Baseline characteristics of the 2 treatment groups were not addressed |
| Methods | Multicentre study, 9 study centres in 4 European countries (Belgium, Finland, Germany and UK). Participants were allocated to either treatment group using a randomisation list. No blinding. Efficacy was evaluated at 10 to 15 days after the therapy started | |
| Participants | 251 adult participants aged 18 years or older were recruited, diagnosed by clinical criteria as having acute bronchitis or pneumonia. Participants with life‐threatening conditions, cystic fibrosis or who had received antibiotics in the 48 hours preceding the study were excluded. Participants: azithromycin group N = 125, amoxycillin group N = 126 | |
| Interventions | 1. Azithromycin 500 mg single dose on day 1 followed by 250 mg daily on days 2 to 5 | |
| Outcomes | Cure | |
| Notes | Of 251 randomised participants, 241 were assessed and included in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information for judgement. Quote: "Patients were allocated to either treatment group using a randomizations list" |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation was not described |
| Blinding (performance bias and detection bias) | High risk | No blinding |
| Incomplete outcome data (attrition bias) | Low risk | 96% (241/251) of the 251 randomised participants were included in analysis. 121 out of 125 in the azithromycin group and 120 of 126 in the amoxycillin group were assessed |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Baseline characteristics of the 2 treatment groups were not addressed |
| Methods | Location: The Netherlands | |
| Participants | 118 participants aged 3 months to 12 years with community‐acquired lower respiratory tract infection were recruited. The diagnosis was based on the presence of respiratory signs and symptoms in combination with a positive chest radiograph or clinical evidence of a temperature 38 ºC or higher, cough, leucocytosis > 10,000 cells/mm³. Participants with symptoms for longer than 1 week, weight > 40 kg, or need for parenteral therapy were excluded. Azithromycin group N = 56, co‐amoxyclav group N = 54 | |
| Interventions | 1. Azithromycin suspension 10 mg/kg/day single dose for 3 days | |
| Outcomes | Cure | |
| Notes | Of 118 randomised participants, 110 were clinically evaluated. 8 were excluded; 7 of them did not meet the inclusion criteria, and for 1 participant the informed consent was withdrawn. Compliance was measured by diary card, registered by parents | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done using blocks of 6 at Imro Tarmarko Berghem, The Netherlands |
| Allocation concealment (selection bias) | Low risk | Treatments were provided by the study sponsor in matched placebo suspensions. Randomisation was done using blocks of 6 at Imro Tarmarko Berghem, The Netherlands Quote: "Patients were assigned randomly to treatment with oral azithromycin suspension (10 mg/kg/24 hours) in a single dose for 3 days or co‐amoxiclav suspension (45/11.25 mg/kg/24 h) tds for 10 days" |
| Blinding (performance bias and detection bias) | Low risk | Matched placebo suspensions were used in the 2 treatment groups. Each participant was equally treated for 13 days |
| Incomplete outcome data (attrition bias) | Low risk | At visit 3 (days 10 to 13) 1 patient was lost in each treatment group (azithromycin group N = 55, co‐amoxyclav N = 53) |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available |
| Other bias | Low risk | Baseline characteristics were comparable between the 2 treatment groups |
| Methods | Location: Belgium, multicentre study | |
| Participants | 78 adult participants aged 18 years or older with acute bronchitis, acute exacerbations of chronic bronchitis or pneumonia were recruited. Diagnosis was made based on the clinical signs and symptoms and chest radiology. Participants who received antibiotics in the 48 hours preceding the study were excluded. Participants: azithromycin group N = 41, co‐amoxyclav N = 37 | |
| Interventions | 1. Azithromycin 500 mg (Pfizer) once daily for 3 days | |
| Outcomes | Cure | |
| Notes | 11 out of 78 participants were not clinically evaluated for the following reasons: failure to meet entry criteria, failure to comply with the protocol and adverse events (7 in the azithromycin group and 4 in the co‐amoxyclav group) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study did not report how randomisation was done. Quote: "Patients were randomly assigned to treatment with azithromycin (Pfizer), one 500 mg tablet once daily on 3 consecutive days, or co‐amoxiclav (Augmentin, Beecham Research) 625 mg 3 times daily for 10 days" |
| Allocation concealment (selection bias) | High risk | Information about concealment was not provided. Quote: "Participants were randomly assigned to treatment with azithromycin (Pfizer), one 500 mg tablet once daily on 3 consecutive days, or co‐amoxiclav (Augmentin, Beecham Research) 625 mg 3 times daily for 10 days" |
| Blinding (performance bias and detection bias) | Low risk | Blinding of the study was maintained with matched placebo tablets |
| Incomplete outcome data (attrition bias) | High risk | 17.1% (7/41) of the azithromycin group and 10.8% (4/37) of the amoxycillin/clavulanate group were not clinically evaluable. Quote: "Reasons for exclusion of patients from clinical evaluation were as follows: failure to meet entry criteria (one patient in each treatment group); failure to observe the protocol (3 azithromycin and 2 co‐amoxiclav patients); and treatment incomplete due to an adverse event not necessarily related to treatment (three azithromycin and one co‐amoxiclav patients)" Comment: even though clear explanation was given, the amount of incomplete data was high and not comparable between the 2 groups |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available |
| Other bias | Low risk | Baseline characteristics were comparable between the 2 treatment groups |
| Methods | Location: US, multicentre study | |
| Participants | Participants with community‐acquired pneumonia at 23 centres in the US, aged 6 months to 16 years. Pneumonia was diagnosed by chest X‐ray of acute infiltration and the presence of tachypnoea, with at least 1 of the following: fever, cough, white blood count 12,000/mm³ or more, and respiratory signs of suggestive of pneumonia. Participants with severe or multilobar pneumonia, with evidence of haematologic, renal, hepatic or cardiovascular disease, chronic steroid use or concomitant treatment with other drugs were excluded. Participants aged less than 5 years: azithromycin group N = 129, amoxy‐clavulanic acid group N = 66 | |
| Interventions | 1. Azithromycin oral suspension 10 mg/kg (maximum 500 mg) once on day 1, followed by 5 mg/kg (maximum 250 mg) once daily on days 2 to 5 | |
| Outcomes | Cure | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study did not report how randomisation was done. Quote: "Patients were randomized 2:1 to receive either azithromycin...." |
| Allocation concealment (selection bias) | High risk | No concealment information was available. Quote: "Patients were randomized 2:1 to receive either azithromycin...." |
| Blinding (performance bias and detection bias) | Low risk | Matched placebo was used |
| Incomplete outcome data (attrition bias) | Low risk | 8.1% (25/310) of the azithromycin group and 7.5% (11/146) of the amoxycillin/clavulanate group were excluded from the efficacy analysis |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available |
| Other bias | Low risk | Baseline characteristics were comparable between the 2 treatment groups |
| Methods | Location: The Netherlands, multicentre study | |
| Participants | 99 outpatients from 4 centres in The Netherlands, with clinical evidence of lower respiratory tract infection, either pneumonia or purulent bronchitis or acute exacerbation of chronic bronchitis, were recruited. Participants with a terminal illness, concomitant use of other antibiotics, or with infectious mononucleosis, cystic fibrosis and gastrointestinal absorption abnormality were excluded. Azithromycin group N = 48, co‐amoxyclav group N = 51 | |
| Interventions | 1. Azithromycin 500 mg once daily for 3 days | |
| Outcomes | Cure | |
| Notes | Medication (bronchodilators, adrenergic stimulators or corticosteroids) was given in addition to the study drug to 83% of participants in the azithromycin group and 82% in the co‐amoxyclav group. Compliance was measured by pill count. All 99 randomised participants were evaluated for clinical efficacy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by the Department of Pharmacy at the University Hospital, Utrecht |
| Allocation concealment (selection bias) | High risk | The information about concealment is not provided. Quote: "Patients were randomized to receive either azithromycin as a once‐daily dose of 500 mg (two 250 mg capsules) for three days, or co‐amoxiclav (625 mg capsules) tid for ten days. Randomization was performed by the Department of Pharmacy at the University Hospital, Utrecht." |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "We report the results of a single‐blind comparison of azithromycin with a ten‐day course of coamoxiclav (amoxycillin/clavulanic acid) in patients with acute lower respiratory tract infections". However, it is not clear to whom the single‐blind was applied and no information was available |
| Incomplete outcome data (attrition bias) | Low risk | Analysis of clinical efficacy was performed using data provided from a total of 99 randomised participants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available |
| Other bias | Unclear risk | Baseline characteristics of the 2 treatment groups were not available. However, the authors mentioned that "The two treatment groups were comparable with respect to age, sex ratio, and underlying diseases (not shown)" |
| Methods | Location: The Netherlands, multicentre study | |
| Participants | 144 outpatients were recruited. 123 of them had type I acute exacerbation of chronic bronchitis, 18 had acute purulent bronchitis and 3 had pneumonia. Participants with terminal illness, who were pregnant or lactating, were receiving concomitant antibiotics or had used antibiotics within 48 hours prior to the study treatment, or had infectious mononucleosis, cystic fibrosis or gastrointestinal abnormality that could affect absorption, were excluded. Participants: azithromycin group N = 72, co‐amoxyclav group N = 72 | |
| Interventions | 1. Azithromycin 500 mg once daily for 3 days | |
| Outcomes | Clinical: cure, improvement, failure, relapse | |
| Notes | Medication (bronchodilators, adrenergic stimulators, corticosteroids) was given to 94% of participants in the azithromycin group and 97% in the co‐amoxyclav group. Of 144 randomised participants, only participants diagnosed with type I acute exacerbation of chronic bronchitis (N = 123) were analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by the Department of Pharmacy, University Hospital, Utrecht |
| Allocation concealment (selection bias) | Low risk | Quote: "The enrolled patients were randomized to receive either azithromycin, given as a single dose of 500 mg (two 250‐mg tablets) once daily for 3 days, or co‐amoxiclav 500 mg:125 mg (625‐mg tablets), administered three times daily for 10 days. Randomization was performed by the Department of Pharmacy, University Hospital, Utrecht" |
| Blinding (performance bias and detection bias) | Low risk | Quote: "both antibiotics and matching placebos were supplied in a blister pack, which indicated the time of day when each of the tablets was to be taken." |
| Incomplete outcome data (attrition bias) | High risk | Clinical response was analysed from the majority of enrolled participants: 85.4% (123/144) with diagnosis of type I acute exacerbation of chronic bronchitis (azithromycin group 86.1%; 62/72, co‐amoxyclav group 84.7%; 61/72). However, no reason for the missing data was provided |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available |
| Other bias | Low risk | Baseline characteristics were comparable between the 2 treatment groups |
| Methods | Location: Exequiel Gonzales Cortes Children's Hospital, Santiago, Chile Children who presented with signs of classic bacterial pneumonia were randomly assigned to receive oral amoxycillin or azithromycin | |
| Participants | 48 children aged 1 month to 14 years were enrolled with classic bacterial pneumonia, with high fever and chest findings of crackles or signs of consolidation, and chest X‐rays with segmental, alveolar or lobar consolidation. 1 patient developed serious pneumonia in the first 12 hours of enrolment and was excluded from the study. The remaining 47 completed the study with 23 receiving azithromycin and 24 receiving amoxycillin. The number of children with M. pneumoniae was 8, with 5 in the azithromycin group and 3 in the amoxycillin‐clavulanate group | |
| Interventions | 1. Azithromycin 10 mg/kg once daily for 3 days | |
| Outcomes | 1. Clinical response: fever (> 38 ºC) at 3, 7 and 14 days after intervention | |
| Notes | Outcomes of this study were not relevant to our criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was available |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients from the classic pneumonia group were randomly assigned to receive oral amoxicillin...." Comment: the study did not report the concealment process |
| Blinding (performance bias and detection bias) | Unclear risk | Methods of blinding were not specified. Participants and caregivers may have been aware of their treatment group because the frequency and duration of drug administration was different between the groups. Radiology assessment was blinded Quote: "All chest X‐rays done ... were seen by the same radiologist, who was not familiar with the patients' clinical history and treatment group." |
| Incomplete outcome data (attrition bias) | Low risk | The total randomised participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available |
| Other bias | Low risk | Baseline characteristics were comparable between the 2 treatment groups |
| Methods | Location: The Netherlands. This study was a part of unpublished international multicentre study | |
| Participants | 50 in‐ and outpatients aged 18 years or older with acute exacerbation of chronic bronchitis were recruited. Chronic bronchitis was clinically defined as having 3 levels of severity. Type I exacerbation (most severe grade), type II exacerbation (less severe grade) and type III exacerbation (least severe grade). Participants with a terminal illness or concomitant use of antibiotics within 48 hours prior to treatment were excluded. Participants: azithromycin group N = 25, amoxycillin group N = 25 | |
| Interventions | 1. Azithromycin 500 mg once daily for 3 days | |
| Outcomes | Cure | |
| Notes | All 50 randomised participants were analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was done at Pfizer‐Euroclin, Brussels, Belgium |
| Allocation concealment (selection bias) | Low risk | Quote: "In this double‐blind study, patients were randomized to receive either azithromycin at a dosage of 500 mg (two 250‐mg capsules) once daily for 3 days or amoxicillin at a dosage of 500 mg (two 250‐mg capsules) three times daily for 5 days." Block randomisation was done at Pfizer‐Euroclin, Brussels, Belgium |
| Blinding (performance bias and detection bias) | Low risk | Matched placebo was used. Quote: "Each patient received six capsules per day (six amoxicillin capsules or two azithromycin capsules plus four placebos)" |
| Incomplete outcome data (attrition bias) | Low risk | All 50 randomised participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available |
| Other bias | Unclear risk | Baseline characteristics were comparable between the 2 treatment groups |
| Methods | Location: Italy | |
| Participants | 50 adult participants with acute purulent exacerbation of chronic bronchitis caused by H. influenzae were recruited. Participants: azithromycin group N = 25, amoxycillin/clavulanic acid group N = 25 | |
| Interventions | 1. Azithromycin 500 mg once daily for 3 days | |
| Outcomes | Cure | |
| Notes | All 50 randomised participants were clinically and bacteriological evaluated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to treatment. The actual randomisation is not clear |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation method |
| Blinding (performance bias and detection bias) | Unclear risk | No description of blinding |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were evaluated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available |
| Other bias | Unclear risk | Baseline characteristics of the 2 treatment groups were not addressed |
| Methods | Location: USA, multicentre study | |
| Participants | 70 outpatients aged between 35 and 75 years with a clinical diagnosis of acute bacterial exacerbation of chronic bronchitis were recruited. Participants with pneumonia, bronchitis with concurrent bronchiectasis or active bronchial asthma, or use of antibiotics within 72 hours of enrolment were excluded. Participants: azithromycin group N = 39, amoxycillin/clavulanate group N = 31 | |
| Interventions | 1. Azithromycin 500 mg once on day 1, followed by 250 mg daily on days 2 to 5 | |
| Outcomes | Cure (complete resolution of resolution of acute exacerbation of COPD on day 11) | |
| Notes | 14 participants were excluded from clinical outcome analysis; 8 of 14 had a resistant pathogen (azithromycin 6, amoxycillin/clavulanate 2), 6 had protocol violations (azithromycin 4, amoxycillin/clavulanate 2). Bacteriologic evaluation was performed in 37 participants who had baseline pathogen reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was available |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to receive either azithromycin once daily (two 250‐mg capsules together on day 1, followed by one 250‐mg capsule a day on days 2 through 5) or amoxicillin/clavulanate three times daily (one 500‐mg tablet three times a day for 10 days)" |
| Blinding (performance bias and detection bias) | Low risk | Quote: "To maintain investigator blinded conditions, study medication was assigned and dispensed by an individual other than the investigator responsible for clinical assessments" |
| Incomplete outcome data (attrition bias) | High risk | 25.6% (10/39) of the azithromycin group and 12.9% (4/31) of the amoxycillin/clavulanate group were excluded from evaluation of clinical response at the day 11 end of therapy visit with the reasons explained in the paper: isolation of a resistant pathogen at baseline (6 azithromycin; 2 amoxycillin/clavulanate) or miscellaneous protocol violations, including failure to meet the inclusion criteria, concurrent treatment with another antibiotic, irregular visits or loss to follow‐up (4 azithromycin, 2 amoxycillin/clavulanate) |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available |
| Other bias | Low risk | Baseline characteristics were comparable between the 2 treatment groups |
| Methods | Location: USA, randomised, non‐blinded trial | |
| Participants | 88 participants with community‐acquired pneumonia at the Children's Medical Center of Dallas aged 6 months to 16 years were enrolled. Participants aged 6 months to 5 years: azithromycin group N = 39, amoxy‐clavulanic acid group N = 49 | |
| Interventions | 1. Azithromycin oral suspension 10 mg/kg (maximum 500 mg) once on day 1, followed by 5 mg/kg (maximum 250 mg) once daily for 4 days | |
| Outcomes | Cure | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about how the list of randomised therapy assignments was generated |
| Allocation concealment (selection bias) | Unclear risk | No information was available |
| Blinding (performance bias and detection bias) | High risk | Unblinded treatment |
| Incomplete outcome data (attrition bias) | Low risk | 147 were randomised, 69 to the azithromycin group and 78 to the amoxycillin group. All were analysed |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available |
| Other bias | Low risk | Baseline characteristics were comparable between the 2 treatment groups |
| Methods | Multicentre, double‐blinded trial. Participants were randomly assigned to treatment. Matched placebo tablets were given. Participants were assessed clinically on days 5 and 14 | |
| Participants | 369 participants aged 18 years or more diagnosed with acute bronchitis, or acute infectious exacerbation of chronic bronchitis, or community‐acquired pneumonia were recruited. Acute bronchitis was defined as the presence of purulent sputum together with fever, leucocytosis and/or symptoms suggestive of lower respiratory tract infection. Pregnant and lactating women, participants with a terminal illness, gastrointestinal or hepatic disorders, infectious mononucleosis, or those who had received prior antimicrobial treatment were excluded. Participants: azithromycin group N = 186, co‐amoxyclav group N = 183 | |
| Interventions | 1. Azithromycin (Pfizer) 500 mg once daily for 3 days | |
| Outcomes | Cure | |
| Notes | Of 369 randomised participants, 346 were clinically evaluated; 173 were in the azithromycin group and 173 were in the co‐amoxyclav group. 193 participants who had baseline pathogen were bacteriologically evaluated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about how the randomised assignments were generated |
| Allocation concealment (selection bias) | Unclear risk | No information about concealment was available. Quote: "After enrollment, patients were randomly assigned to one of the treatment groups..." |
| Blinding (performance bias and detection bias) | Low risk | Matched placebo tablets were employed to maintain blinding of the study |
| Incomplete outcome data (attrition bias) | Low risk | 7% (13/186) of the azithromycin group and 5.5 % (10/183) of the amoxycillin/clavulanate group were excluded from the evaluation of clinical outcome. Quote: "Reasons for exclusion were as follows: failure to meet entry criteria (four azithromycin and one co‐amoxiclav‐treated patients); protocol violation (two azithromycin‐treated patients); lost to follow‐up (three patients in each treatment group), adverse events (three azithromycin‐ and six co‐amoxiclav treated patients); and withdrawal from study (one azithromycin‐treated patient)" |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available |
| Other bias | Unclear risk | Baseline characteristics were comparable between the 2 treatment groups |
| Methods | Location: China | |
| Participants | 80 hospitalised participants with acute purulent exacerbation of chronic bronchitis and aged more than 30 years. Participants having antibiotics within 48 hours and with known allergy to beta‐lactam antibiotics, beta‐lactamase inhibitors, serum creatinine > 200 mg/L and immunosuppressant users were excluded | |
| Interventions | 1. Azithromycin iv administration for 5 days, day 1 500 mg and days 2 to 5 250 mg 4 times a day | |
| Outcomes | Cure | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were assigned to treatments. The method of randomisation was not clear |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation method |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were evaluated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available |
| Other bias | Unclear risk | Baseline characteristics of the 2 treatment groups were not addressed |
iv: intravenously
N: number
bid: two times a day
tds: three times a day
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| The in vitro study compared the efficacy of azithromycin and other macrolides with amoxycillin‐clavulanate against Streptococcus pneumoniae and Haemophilus influenzae | |
| The study compared azithromycin with benzyl penicillin or erythromycin in community‐acquired pneumonia | |
| The study compared azithromycin with clarithromycin | |
| A meta‐analysis of various antibiotic comparators including azithromycin versus amoxycillin | |
| The study compared different doses of azithromycin in the treatment of upper and lower respiratory tract infections | |
| The comparators were amoxycillin or erythromycin. The data were analysed in overall results that meant we were not able to get the information specific to amoxycillin | |
| The study compared azithromycin with roxithromycin | |
| The study included participants with upper respiratory infections | |
| A meta‐analysis of various antibiotic comparators not relevant to our compared interventions (azithromycin versus amoxycillin) | |
| The study compared azithromycin with roxithromycin | |
| RCT of single‐dose azithromycin versus 7 days of amoxycillin in treating acute otitis media in Aboriginal children | |
| The study compared azithromycin with other antibiotics | |
| The study compared azithromycin with erythromycin |
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical failure Show forest plot | 15 | 2496 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.85] |
| Analysis 1.1 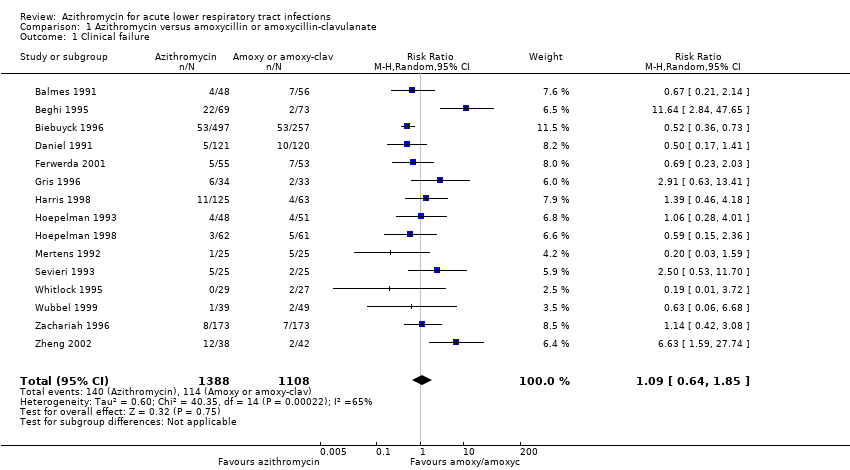 Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 1 Clinical failure. | ||||
| 2 Clinical failure by diagnosis Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 2 Clinical failure by diagnosis. | ||||
| 2.1 Acute bronchitis | 6 | 1296 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.45, 0.88] |
| 2.2 Acute exacerbation of chronic bronchitis | 9 | 808 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.46, 3.32] |
| 2.3 Pneumonia | 5 | 392 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.45, 1.94] |
| 3 Clinical failure by age group Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 3 Clinical failure by age group. | ||||
| 3.1 Adult | 12 | 2112 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.60, 2.20] |
| 3.2 Paediatric | 3 | 384 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.45, 1.94] |
| 4 Clinical failure by dose regimen of azithromycin Show forest plot | 12 | 2112 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.60, 2.20] |
| Analysis 1.4  Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 4 Clinical failure by dose regimen of azithromycin. | ||||
| 4.1 500 mg once daily x 3 | 8 | 1631 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.55, 2.83] |
| 4.2 500 mg single dose followed by 250 mg on day 2 to 5 | 4 | 481 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.25, 3.62] |
| 5 Clinical failure by type of antibiotic in control group Show forest plot | 15 | 2496 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.85] |
| Analysis 1.5 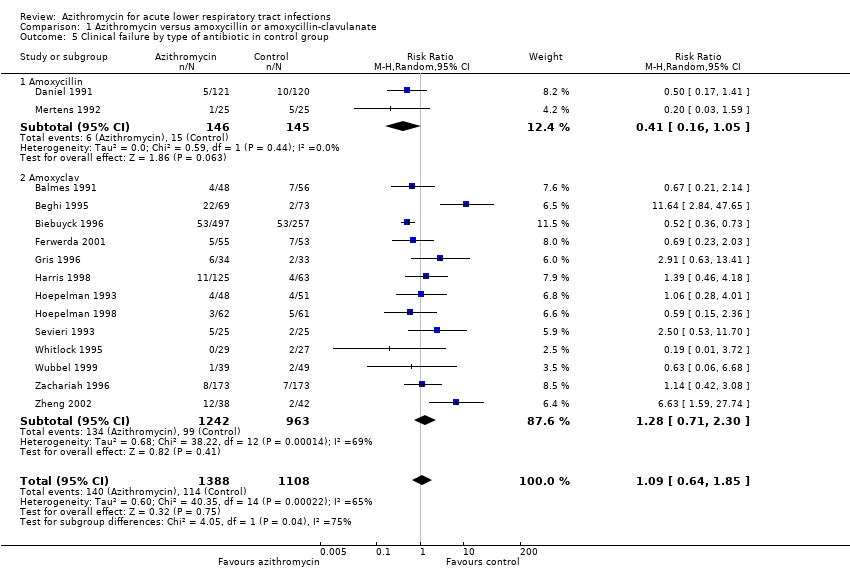 Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 5 Clinical failure by type of antibiotic in control group. | ||||
| 5.1 Amoxycillin | 2 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.16, 1.05] |
| 5.2 Amoxyclav | 13 | 2205 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.71, 2.30] |
| 6 Sensitivity analysis excluding one large trial Show forest plot | 14 | 1742 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.69, 2.09] |
| Analysis 1.6  Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 6 Sensitivity analysis excluding one large trial. | ||||
| 7 Sensitivity analysis excluding three trials with missing data > 10% Show forest plot | 12 | 2250 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.62, 2.03] |
| Analysis 1.7 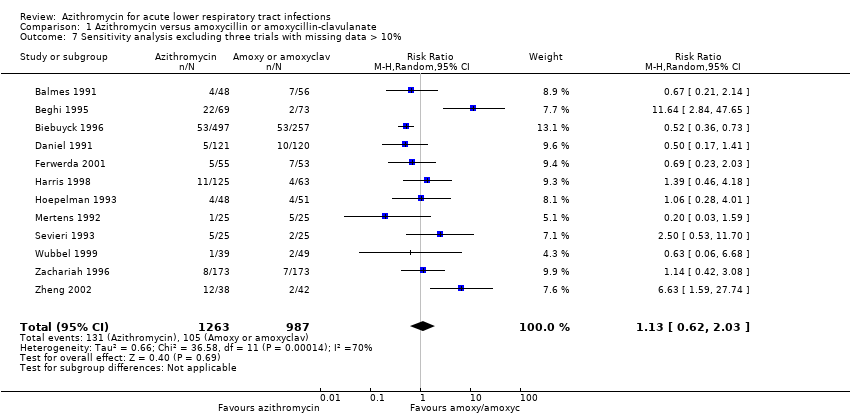 Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 7 Sensitivity analysis excluding three trials with missing data > 10%. | ||||
| 8 Sensitivity analysis with the condition of concealment Show forest plot | 15 | 2496 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.85] |
| Analysis 1.8 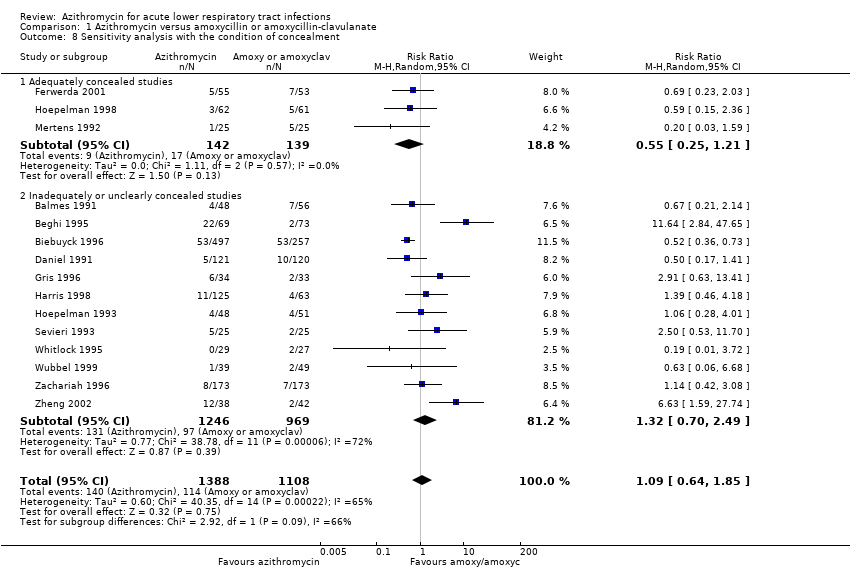 Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 8 Sensitivity analysis with the condition of concealment. | ||||
| 8.1 Adequately concealed studies | 3 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.25, 1.21] |
| 8.2 Inadequately or unclearly concealed studies | 12 | 2215 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.70, 2.49] |
| 9 Microbial eradication Show forest plot | 12 | 961 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.87, 1.03] |
| Analysis 1.9 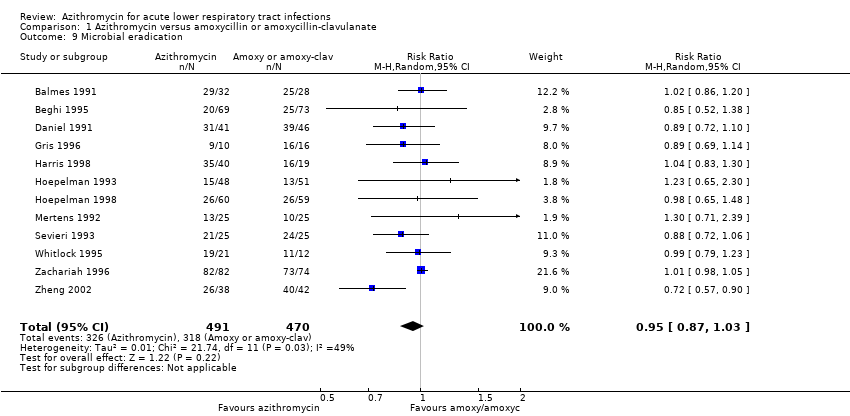 Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 9 Microbial eradication. | ||||
| 10 Adverse events Show forest plot | 12 | 2406 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.57, 1.00] |
| Analysis 1.10  Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 10 Adverse events. | ||||

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, outcome: 1.1 Clinical failure.
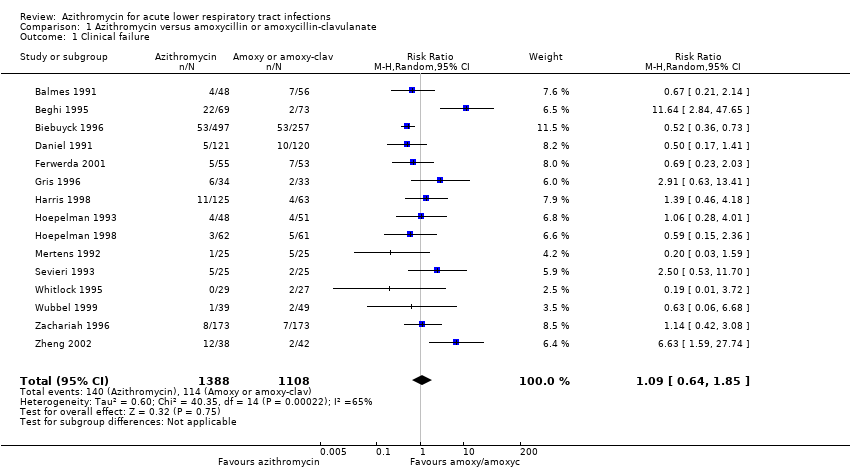
Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 1 Clinical failure.

Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 2 Clinical failure by diagnosis.

Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 3 Clinical failure by age group.

Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 4 Clinical failure by dose regimen of azithromycin.
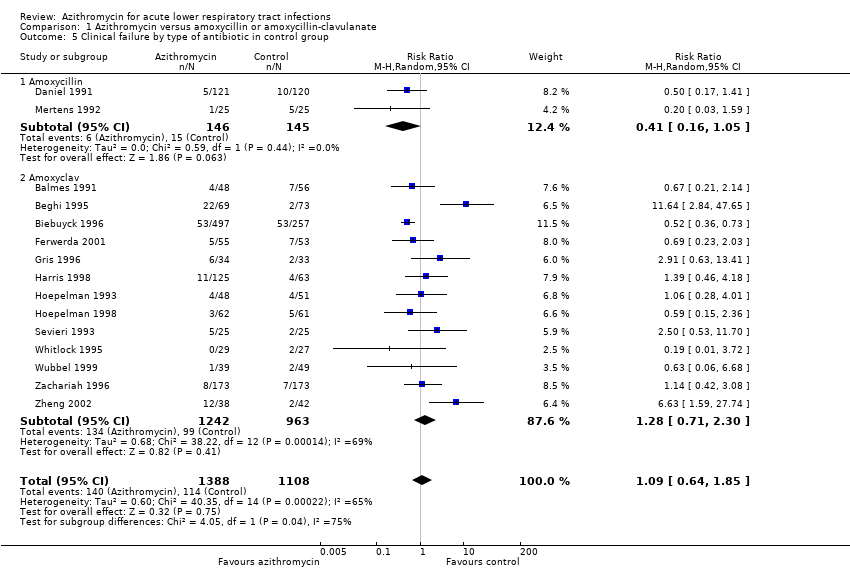
Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 5 Clinical failure by type of antibiotic in control group.

Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 6 Sensitivity analysis excluding one large trial.
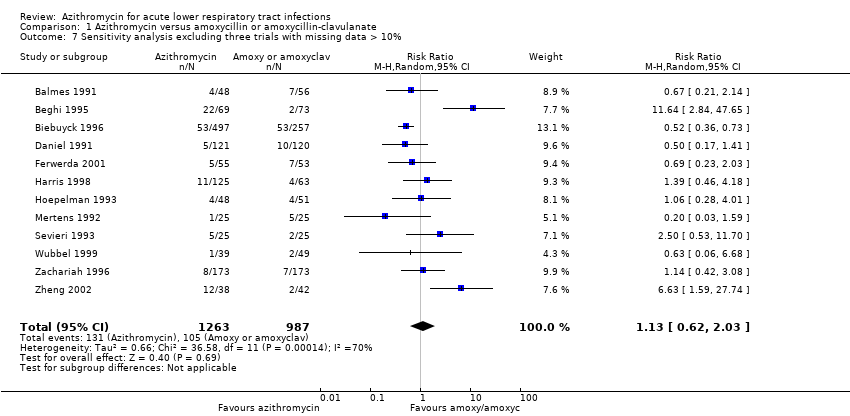
Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 7 Sensitivity analysis excluding three trials with missing data > 10%.

Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 8 Sensitivity analysis with the condition of concealment.
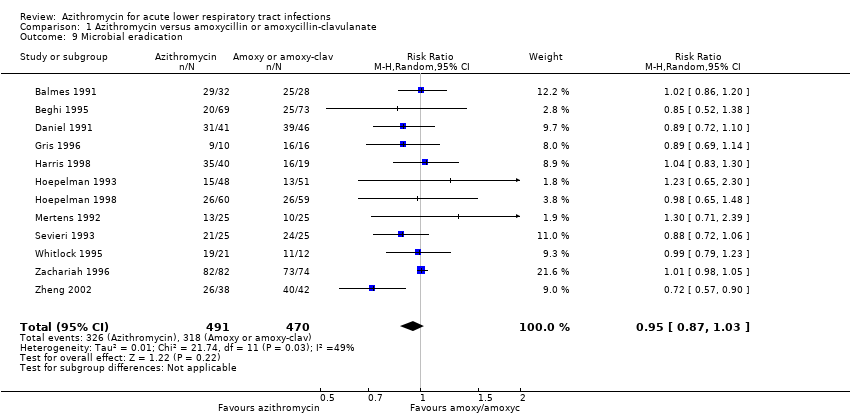
Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 9 Microbial eradication.

Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 10 Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical failure Show forest plot | 15 | 2496 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.85] |
| 2 Clinical failure by diagnosis Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Acute bronchitis | 6 | 1296 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.45, 0.88] |
| 2.2 Acute exacerbation of chronic bronchitis | 9 | 808 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.46, 3.32] |
| 2.3 Pneumonia | 5 | 392 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.45, 1.94] |
| 3 Clinical failure by age group Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Adult | 12 | 2112 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.60, 2.20] |
| 3.2 Paediatric | 3 | 384 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.45, 1.94] |
| 4 Clinical failure by dose regimen of azithromycin Show forest plot | 12 | 2112 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.60, 2.20] |
| 4.1 500 mg once daily x 3 | 8 | 1631 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.55, 2.83] |
| 4.2 500 mg single dose followed by 250 mg on day 2 to 5 | 4 | 481 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.25, 3.62] |
| 5 Clinical failure by type of antibiotic in control group Show forest plot | 15 | 2496 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.85] |
| 5.1 Amoxycillin | 2 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.16, 1.05] |
| 5.2 Amoxyclav | 13 | 2205 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.71, 2.30] |
| 6 Sensitivity analysis excluding one large trial Show forest plot | 14 | 1742 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.69, 2.09] |
| 7 Sensitivity analysis excluding three trials with missing data > 10% Show forest plot | 12 | 2250 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.62, 2.03] |
| 8 Sensitivity analysis with the condition of concealment Show forest plot | 15 | 2496 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.85] |
| 8.1 Adequately concealed studies | 3 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.25, 1.21] |
| 8.2 Inadequately or unclearly concealed studies | 12 | 2215 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.70, 2.49] |
| 9 Microbial eradication Show forest plot | 12 | 961 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.87, 1.03] |
| 10 Adverse events Show forest plot | 12 | 2406 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.57, 1.00] |

