تاثیر آزیترومایسین در درمان عفونتهای حاد دستگاه تنفسی تحتانی
Información
- DOI:
- https://doi.org/10.1002/14651858.CD001954.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 08 marzo 2015see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Infecciones respiratorias agudas
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
In the original review Ratana Panpanich (RP) designed the protocol, identified studies, extracted data from the included studies and co‐wrote the review.
Peerasak Lerttrakarnnon (PL) co‐wrote the review and extracted data from the included studies. Malinee Laopaiboon (ML) helped analyse data and prepared the final draft of this review.
In the 2011 update ML contributed to all processes. RP contributed to 'Risk of bias' assessment and RP and PL also prepared the final draft of the updated review.
In this 2014 update ML contributed to all processes. RP and KW contributed to screening of studies and the final draft of the updated review.
Sources of support
Internal sources
-
Khon Kaen University, Faculty of Public Health, Thailand.
-
Chiang Mai University, Faculty of Medicine, Thailand.
External sources
-
Effective Health Care Alliance Program, Liverpool School of Tropical Medicine, Liverpool, UK.
-
Thailand Research Fund (Distinguished Research Professor Award), Thailand.
-
Thai Cochrane Network, Thailand.
Declarations of interest
Malinee Laopaiboon: I received an honorarium from the Thailand Research Fund, which is a non‐profit organisation.
Ratana Panpanich: none known.
Kyaw Swa Mya: none known.
Acknowledgements
We thank Professor Paul Garner for his kind advice on the protocol development and plan for data analysis. We are grateful to Dr. Shi Luming for her help in extracting data from the Chinese paper included in the review. We also thank the following people for commenting on the draft review: Gustav Malangu, Chantal Raherison, Mark Jones and Diederik van de Beek. We thank Liz Dooley and Sarah Thorning from the Cochrane Acute Respiratory Infections Group for their assistance with the preparation of this systematic review. The data presented and the views expressed are the responsibility of the review authors.
Version history
| Published | Title | Stage | Authors | Version |
| 2015 Mar 08 | Azithromycin for acute lower respiratory tract infections | Review | Malinee Laopaiboon, Ratana Panpanich, Kyaw Swa Mya | |
| 2008 Jan 23 | Azithromycin for acute lower respiratory tract infections | Review | Malinee Laopaiboon, Ratana Panpanich, Peerasak Lerttrakarnnon | |
| 2004 Oct 18 | Azithromycin for acute lower respiratory tract infections | Review | Ratana Panpanich, Peerasak Lerttrakarnnon, Malinee Laopaiboon | |
| 2000 Jul 24 | Azithromycin for lower respiratory tract infections | Protocol | Ratana Panpanich, P Lerttrakarnnon | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Acute Disease;
- Amoxicillin [*therapeutic use];
- Amoxicillin‐Potassium Clavulanate Combination [*therapeutic use];
- Anti‐Bacterial Agents [*therapeutic use];
- Azithromycin [*therapeutic use];
- Bronchitis [*drug therapy];
- Drug Therapy, Combination;
- Pneumonia [*drug therapy];
- Randomized Controlled Trials as Topic;
- Respiratory Tract Infections [drug therapy];
- Treatment Failure;
Medical Subject Headings Check Words
Humans;
PICO

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, outcome: 1.1 Clinical failure.
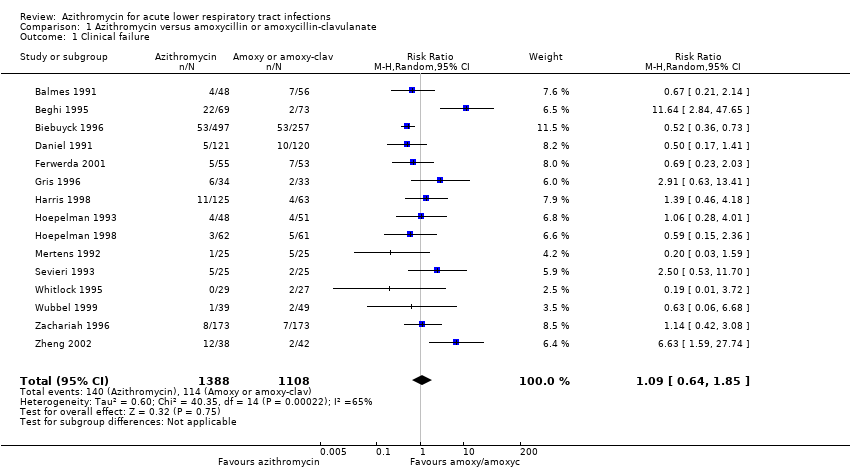
Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 1 Clinical failure.

Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 2 Clinical failure by diagnosis.

Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 3 Clinical failure by age group.

Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 4 Clinical failure by dose regimen of azithromycin.
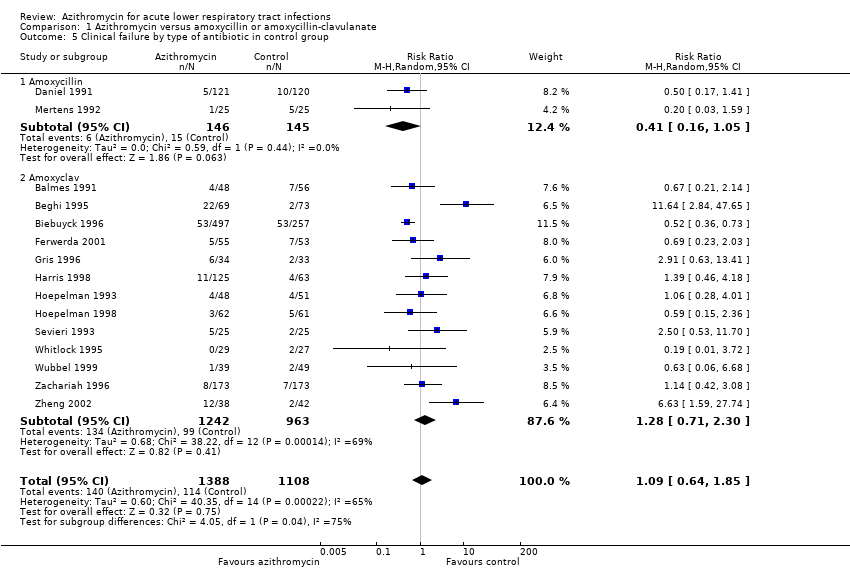
Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 5 Clinical failure by type of antibiotic in control group.

Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 6 Sensitivity analysis excluding one large trial.
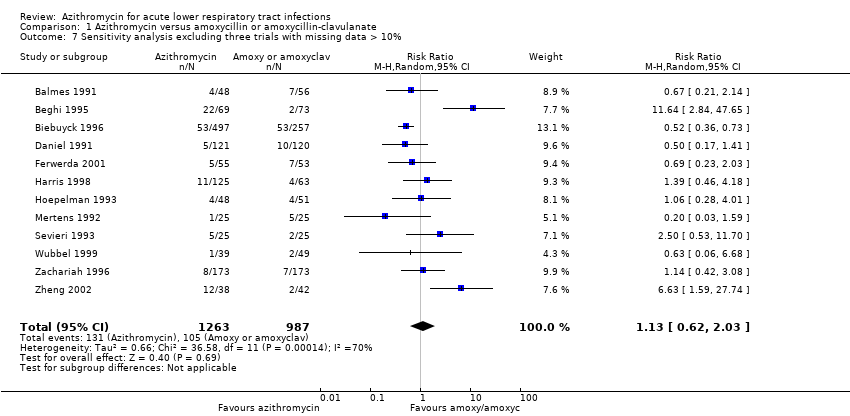
Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 7 Sensitivity analysis excluding three trials with missing data > 10%.

Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 8 Sensitivity analysis with the condition of concealment.
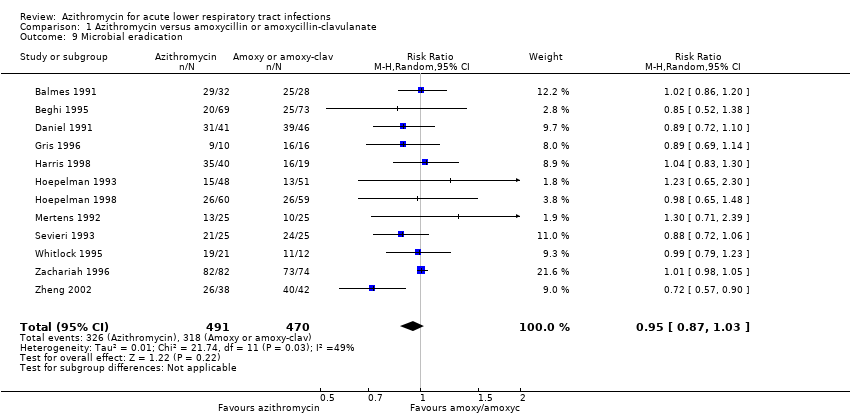
Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 9 Microbial eradication.

Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 10 Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical failure Show forest plot | 15 | 2496 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.85] |
| 2 Clinical failure by diagnosis Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Acute bronchitis | 6 | 1296 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.45, 0.88] |
| 2.2 Acute exacerbation of chronic bronchitis | 9 | 808 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.46, 3.32] |
| 2.3 Pneumonia | 5 | 392 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.45, 1.94] |
| 3 Clinical failure by age group Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Adult | 12 | 2112 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.60, 2.20] |
| 3.2 Paediatric | 3 | 384 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.45, 1.94] |
| 4 Clinical failure by dose regimen of azithromycin Show forest plot | 12 | 2112 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.60, 2.20] |
| 4.1 500 mg once daily x 3 | 8 | 1631 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.55, 2.83] |
| 4.2 500 mg single dose followed by 250 mg on day 2 to 5 | 4 | 481 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.25, 3.62] |
| 5 Clinical failure by type of antibiotic in control group Show forest plot | 15 | 2496 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.85] |
| 5.1 Amoxycillin | 2 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.16, 1.05] |
| 5.2 Amoxyclav | 13 | 2205 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.71, 2.30] |
| 6 Sensitivity analysis excluding one large trial Show forest plot | 14 | 1742 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.69, 2.09] |
| 7 Sensitivity analysis excluding three trials with missing data > 10% Show forest plot | 12 | 2250 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.62, 2.03] |
| 8 Sensitivity analysis with the condition of concealment Show forest plot | 15 | 2496 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.85] |
| 8.1 Adequately concealed studies | 3 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.25, 1.21] |
| 8.2 Inadequately or unclearly concealed studies | 12 | 2215 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.70, 2.49] |
| 9 Microbial eradication Show forest plot | 12 | 961 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.87, 1.03] |
| 10 Adverse events Show forest plot | 12 | 2406 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.57, 1.00] |

