Azitromicin za akutne infekcije donjih dišnih putova
Abstract
Background
Acute lower respiratory tract infections (LRTI) range from acute bronchitis and acute exacerbations of chronic bronchitis to pneumonia. Approximately five million people die from acute respiratory tract infections annually. Among these, pneumonia represents the most frequent cause of mortality, hospitalisation and medical consultation. Azithromycin is a macrolide antibiotic, structurally modified from erythromycin and noted for its activity against some gram‐negative organisms associated with respiratory tract infections, particularly Haemophilus influenzae (H. influenzae).
Objectives
To compare the effectiveness of azithromycin to amoxycillin or amoxycillin/clavulanic acid (amoxyclav) in the treatment of LRTI, in terms of clinical failure, incidence of adverse events and microbial eradication.
Search methods
We searched CENTRAL (2014, Issue 10), MEDLINE (January 1966 to October week 4, 2014) and EMBASE (January 1974 to November 2014).
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs, comparing azithromycin to amoxycillin or amoxycillin/clavulanic acid in participants with clinical evidence of an acute LRTI, such as acute bronchitis, pneumonia and acute exacerbation of chronic bronchitis.
Data collection and analysis
The review authors independently assessed all potential studies identified from the searches for methodological quality. We extracted and analysed relevant data separately. We resolved discrepancies through discussion. We initially pooled all types of acute LRTI in the meta‐analyses. We investigated the heterogeneity of results using the forest plot and Chi2 test. We also used the index of the I2 statistic to measure inconsistent results among trials. We conducted subgroup and sensitivity analyses.
Main results
We included 16 trials involving 2648 participants. We were able to analyse 15 of the trials with 2496 participants. The pooled analysis of all the trials showed that there was no significant difference in the incidence of clinical failure on about days 10 to 14 between the two groups (risk ratio (RR), random‐effects 1.09; 95% confidence interval (CI) 0.64 to 1.85). A subgroup analysis in trials with acute bronchitis participants showed significantly lower clinical failure in the azithromycin group compared to amoxycillin or amoxyclav (RR random‐effects 0.63; 95% CI 0.45 to 0.88). A sensitivity analysis showed a non‐significant reduction in clinical failure in azithromycin‐treated participants (RR 0.55; 95% CI 0.25 to 1.21) in three adequately concealed studies, compared to RR 1.32; 95% CI 0.70 to 2.49 in 12 studies with inadequate concealment. Twelve trials reported the incidence of microbial eradication and there was no significant difference between the two groups (RR 0.95; 95% CI 0.87 to 1.03). The reduction of adverse events in the azithromycin group was RR 0.76 (95% CI 0.57 to 1.00).
Authors' conclusions
There is unclear evidence that azithromycin is superior to amoxycillin or amoxyclav in treating acute LRTI. In patients with acute bronchitis of a suspected bacterial cause, azithromycin tends to be more effective in terms of lower incidence of treatment failure and adverse events than amoxycillin or amoxyclav. However, most studies were of unclear methodological quality and had small sample sizes; future trials of high methodological quality and adequate sizes are needed.
PICO
Laički sažetak
Azitromicin za akutne infekcije donjih dišnih putova
Istraživačko pitanje
Ovaj Cochrane sustavni pregled napravljen je kako bi se usporedili azitromicin i amoksicilin ili amoksiklav za liječenje akutnih infekcija donjeg dišnog sustava.
Dosadašnje spoznaje
Akutne infekcije donjih dišnih putova jedne su od najčešćih dijagnoza u ordinacijama opće prakse. Dijagnoze se kreću od akutnog bronhitisa i naglog pogoršanja (akutne egzacerbacije) kroničnog bronhitisa do upale pluća. Azitromicin se ubraja u skupinu makrolidnih antibiotika i koristi se za liječenje određenih bakterijskih infekcija.
Razdoblje koje pokriva pretraživanje literature
Analizirane su studije objavljene do studenoga 2014.
Obilježja istraživanja
Analizirane su randomizirane kontrolirane studije i kvazi‐randomizirane kontrolirane studije u kojima su uspoređeni azitromicin i amoksicilin ili kombinacija amoksicilin/klavulanska kiselina kod osoba sa simptomima akutne upale donjih došnih putova kao što su akutni bronhitis, upala pluća ili naglo pogoršanje kroničnog bronhitisa.
Ključni rezultati
Analizirani su podatci iz 15 studija koje su uključile 2496 ispitanika. Učinci azitromicina na liječenje u smislu poboljšanja ili neuspješnog liječenja nisu bili bolji u odnosu na amoksicilin ili amoksiklav. Međutim, azitromicin je imao manju učestalost nuspojava nego amoksicilin ili amoksiklav, ali koja nije bila statistički značajna.
Kvaliteta dokaza
Kvaliteta dokaza za glavni rezultat bila je niska jer su samo tri od 15 uključenih studija na odgovarajući način prikrile razvrstavanje ispitanika. Stoga trenutno nema dovoljno dokaza koji bi uvjerljivo pokazali da je azitromicin bolji od amoksicilina ili amoksiklava u liječenju akutnih infekcija donjih dijelova dišnog sustava.
Authors' conclusions
Background
Description of the condition
The spectrum of acute lower respiratory tract infection (LRTI) ranges from acute bronchitis and acute exacerbations of chronic bronchitis to pneumonia. Annually approximately five million people die of acute respiratory tract infections. Among these, pneumonia represents the most frequent cause of mortality, hospitalisation and medical consultation (Bariffi 1995).
Acute bronchitis is one of the most common diagnoses in outpatients. The diagnosis of acute bronchitis is mainly based on the symptom of cough and it is usually mild and self limiting. Acute bronchitis with underlying pulmonary diseases or a prolonged cough of more than two weeks are considered for antibiotic therapy (Knutson 2002). A prospective multicentre study of 359 cases of community‐acquired pneumonia in the United States reported that 58.5% had identifiable pathogens, 32.9% had unknown aetiology and 8.6% had aspiration‐related and post‐obstructive pneumonia. The most frequent aetiologic agent was Streptococcus pneumoniae (S. pneumoniae) (15%), followed by Haemophilus influenzae (H. influenzae) (10.9%), Legionella spp (6.7%) and Chlamydia pneumoniae (C. pneumoniae) (6.1%) (Fang 1990). A study in The Netherlands of 145 adults with LRTI showed that a bacterial cause was found in 43 cases (30%) and a viral cause in 57 cases (39%). Influenza A virus was the most frequently diagnosed micro‐organism. The most frequently identified bacterial agents were H. influenzae (9%) and Mycoplasma pneumoniae (M. pneumoniae) (9%) followed by S. pneumoniae (6%) (Graffelman 2004).
Description of the intervention
Antimicrobial treatment in LRTI has to be effective, partly because of the need to reduce the cost and also due to the problem of increasing resistance to the commonly used antibiotics (Legnani 1997). It has also been suggested that the start of therapy should not be delayed for longer than six hours for diagnostic studies (Brown 1998). The importance of early antimicrobial treatment was supported by a study in elderly patients with pneumonia, which showed that 30‐day mortality was lower after administration of antibiotics within eight hours of arrival at hospital, than after delayed treatment (Meehan 1997). Compliance is also important, particularly in outpatients. A study related to medical compliance for the outpatient management of infectious diseases indicated that there was an inverse relationship between frequency of dose and compliance. A short‐term regimen, requiring administration once a day, was found to have the highest compliance rate ‐ 80% compared to 69% and 38% for administration twice a day and three times a day, respectively (Sclar 1994).
Amoxycillin, an oral antibiotic, constitutes extended spectrum penicillin and is active against many aerobic gram‐negative bacilli encountered in patients with pneumonia. By combining the beta‐lactamase inhibitor clavulanic acid with amoxycillin, the invitro spectrum of penicillin is expanded to include beta‐lactamase producing organisms, which would otherwise be resistant to this drug (Mandell 1994). Amoxycillin has been accepted as one of the first choice antibiotics in patients with community‐acquired LRTI. Amoxycillin‐clavulanic acid is recommended particularly in the high prevalence area of beta‐lactamase producing organisms, and also when an aetiologic agent is not identified (Bartlett 1998; Huchon 1998).
How the intervention might work
Azithromycin is a macrolide antibiotic structurally modified from erythromycin with an expanded spectrum of activity and improved tissue pharmacokinetic characteristics relative to erythromycin. The drug is noted for its activity against some gram‐negative organisms associated with respiratory tract infections, particularly H. influenzae. Azithromycin has similar properties to other macrolides against S. pneumoniae and Moraxella catarrhalis (M. catarrhalis), and is active against atypical pathogens such as Legionella pneumophilae (L. pneumophilae), C. pneumoniae and M. pneumoniae (Dunn 1996).
Why it is important to do this review
Over the past 30 years, strains of S. pneumoniae with diminished susceptibility to penicillin have emerged and spread worldwide (Austrian 1994). Cross‐resistance to other antibiotics has also been reported in many strains of S. pneumoniae that have diminished susceptibility to penicillin and cephalosporin (Goldstein 1996). A number of studies have indicated the importance of M. pneumoniae as the main aetiologic agent in ambulatory patients with pneumonia (Berntsson 1986; Langille 1993; Marrie 1996). Co‐infection by more than one pathogen was also reported, and ranged from less than 10% to 38.9% (Lieberman 1996). The value of routine microbial investigation in all patients with LRTI is uncertain (Woodhead 1991). A survey on the management of 2056 such infections, obtained from general practitioners in France, Germany, Italy, Spain and the UK, reported that microbiological examination was performed in only 7% of cases compared to 22% for chest radiography (Woodhead 1996).
This review compares the effects of azithromycin and amoxycillin or amoxycillin‐clavulanic acid in treating acute LRTI such as acute bronchitis, pneumonia and acute exacerbation of chronic bronchitis in terms of clinical failure, incidence of adverse events and microbial eradication.
Objectives
To compare the effectiveness of azithromycin to amoxycillin or amoxycillin/clavulanic acid (amoxyclav) in the treatment of LRTI, in terms of clinical failure, incidence of adverse events and microbial eradication.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs.
Types of participants
Participants of any age or gender, with clinical evidence of acute LRTI such as acute bronchitis, pneumonia and acute exacerbations of chronic bronchitis.
Types of interventions
Azithromycin of any dose or regimen compared to amoxycillin or amoxycillin/clavulanic acid (amoxyclav).
Types of outcome measures
Primary outcomes
-
Clinical failure (persistence or deterioration of symptoms, death or relapse assessed at about 10 to 14 days after therapy started).
Secondary outcomes
-
Incidence of serious complications.
-
Adverse drug events.
-
Eradication of organism (causative micro‐organism absent from the sputum culture after treatment).
Search methods for identification of studies
Electronic searches
For this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL 2014, Issue 10) (accessed 7 November 2014), which contains the Acute Respiratory Infections Group's Specialised Register, MEDLINE (June 2011 to October week 5, 2014) and EMBASE (June 2011 to November 2014). Previously we searched CENTRAL (2011, Issue 3), MEDLINE (July 2007 to July week 4, 2011) and Embase.com (July 2007 to August 2011). Details of the original search strategy are in Appendix 1.
We used the search strategy described in Appendix 2 to search CENTRAL and MEDLINE. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision), Ovid format (Lefebvre 2011). We adapted the search strategy to search EMBASE (see Appendix 3). There were no language or publication restrictions.
Searching other resources
We searched the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov trials registries (28 January 2015) for completed and ongoing trials. We reviewed the citations in the trials identified by the above searches. We contacted the organisations and individual researchers working in this field for unpublished data and missing data from published trials.
Data collection and analysis
Selection of studies
In the previous versions of this review three review authors (RP, PL and ML) independently screened the results of the searches for potentially relevant studies. In this 2014 update version, three review authors (ML, RP and KM) independently screened the potentially relevant studies. We used an eligibility form to assess these studies for inclusion in the review. We resolved disagreements by discussion.
Data extraction and management
We used a data extraction form to collect information from included trials regarding participants, methods, interventions and outcomes. One review author (RP) extracted data. Another review author (PL) independently cross‐checked the findings. We checked the data sources to avoid multiple publications based on the same data. Extracted data included:
-
the time period and geographical location of the study;
-
baseline characteristics of participants;
-
inclusion/exclusion criteria; and
-
preparation and dosing of treatment regime.
We extracted information on the main outcomes: clinical failure, microbial eradication and adverse events.
Assessment of risk of bias in included studies
In the original review, Panpanich 2004, two review authors (RP, PL) independently assessed trial quality under the following domains:
-
generation of allocation sequence;
-
concealment of treatment allocation;
-
blinding;
-
completeness of the trial.
For the previous update, two review authors (ML, RP) independently assessed the quality of studies included in the review using the 'Risk of bias' tool as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other sources of bias. We classified overall risk of bias as low, unclear or high. We resolved any disagreements between the authors by consensus.
Measures of treatment effect
We carried out statistical analysis using the Review Manager software (RevMan 2014). We reported risk ratio (RR) (95% confidence interval (CI)) of clinical failure, microbial eradication and adverse events for each trial. When trials were sufficiently homogeneous, we pooled the intervention effects.
Unit of analysis issues
We did not have any unit of analysis issues in our review. All of the included trials were of parallel design. They had random assignments and data analyses at the patient level.
Dealing with missing data
We excluded the trials with more than 10% of missing data in sensitivity analysis when assessing the influence of missing data on the overall results of clinical failure.
Assessment of heterogeneity
We assessed heterogeneity in the estimates of the treatment effects among the included trials through visual examination of the forest plot and the Chi2 test of heterogeneity, using a 10% level of statistical significance. We also used the I2 statistic to measure inconsistency in results among trials (Higgins 2003). We considered a value greater than 50% to represent substantial heterogeneity.
Assessment of reporting biases
We examined publication bias for clinical failure by visually inspecting a funnel plot.
Data synthesis
We planned to analyse the summary weighted risk ratio and 95% CI using fixed‐effect inverse variance meta‐analysis for combining data where we judged trials sufficiently similar. We used a random‐effects meta‐analysis where there was clinical or methodological heterogeneity between studies sufficient to suggest that treatment effects might differ between trials. We used a random‐effects meta‐analysis to pool the treatment effects where the heterogeneity between studies could not be explained by any potential factor.
Subgroup analysis and investigation of heterogeneity
We conducted a subgroup analysis according to the following prespecified factors:
-
age group;
-
types of respiratory tract infections such as acute bronchitis, acute exacerbation of chronic bronchitis and pneumonia;
-
dose regimen of azithromycin; and
-
type of antibiotic in control group.
Sensitivity analysis
We conducted sensitivity analyses to assess the impact of the potentially important factors on the overall results of clinical failure. The potential factors were:
-
trial quality according to allocation concealment; and
-
trials with more than 10% missing data.
Results
Description of studies
Results of the search
In our original published version of this review, Panpanich 2004, we identified 26 potentially relevant studies. Of these, we included 15 studies and details are presented in the Characteristics of included studies table. In the first update in 2008 we include one more trial (Kogan 2003), which was previously in the Studies awaiting classification section.
In the previous updated search in August 2011 we identified 43 records, of which we assessed three as potentially relevant for inclusion (Dimopoulos 2007; Maimon 2008; Morris 2010). However, we excluded all three studies because one was a trial comparing azithromycin versus amoxycillin in treating acute otitis media and the other two were meta‐analyses.
In the update search in November 2014 we identified 38 records. After considering their titles and abstracts we excluded all of them because they did not satisfy our specified inclusion criteria.
Included studies
We included a total of 16 trials involving 2648 participants. We did not include the Kogan 2003 results in the analyses because the outcomes of clinical response and radiological findings were not relevant to our criteria. Details of the included trials are provided in the Characteristics of included studies table.
All 15 trials in the original review reported numbers of participants cured, improvements, failures and relapses. Microbial eradication was reported in 12 trials (Balmes 1991; Beghi 1995; Daniel 1991; Gris 1996; Harris 1998; Hoepelman 1993; Hoepelman 1998; Mertens 1992; Sevieri 1993; Whitlock 1995; Zachariah 1996; Zheng 2002). No trial reported duration of fever. All trials reported failure at about 10 to 14 days after treatment started.
Study location
Fourteen trials were published in English, one trial was in Italian (Sevieri 1993) and one was in Chinese (Zheng 2002). The studies were conducted between 1991 and 2003 in France, Belgium, The Netherlands, Finland, Germany, UK, USA, Italy, Chile and China.
Participants
Twelve out of 16 trials were conducted in adults. Five trials recruited adult participants either with acute bacterial bronchitis or chronic bronchitis with acute exacerbation or pneumonia (Gris 1996; Hoepelman 1993; Hoepelman 1998; Kogan 2003; Zachariah 1996). Five trials recruited only participants with chronic bronchitis with acute exacerbation (Beghi 1995; Mertens 1992; Sevieri 1993; Whitlock 1995; Zheng 2002). Three trials were conducted in children aged six months to 16 years with community‐acquired pneumonia (Ferwerda 2001; Harris 1998; Wubbel 1999).
Interventions
Azithromycin was compared to amoxycillin‐clavulanic acid in 13 trials (Balmes 1991; Beghi 1995; Biebuyck 1996; Ferwerda 2001; Gris 1996; Harris 1998; Hoepelman 1993; Hoepelman 1998; Sevieri 1993; Whitlock 1995; Wubbel 1999; Zachariah 1996; Zheng 2002). Three trials compared azithromycin to amoxycillin (Daniel 1991; Kogan 2003; Mertens 1992).
There were two regimens of azithromycin in the adult trials:
-
azithromycin 500 mg single dose daily for three days (eight trials); and
-
azithromycin 500 mg single dose on day one followed by 250 mg single dose daily on days two to five (four trials).
The regimen of azithromycin in children in two trials was 10 mg/kg single dose on day one and followed by 5 mg/kg once daily on day two to five (Harris 1998; Wubbel 1999). Another trial used 10 mg/kg/day once daily for three days (Ferwerda 2001).
Two trials in children compared azithromycin to amoxycillin/clavulanic acid in participants aged up to five years; and erythromycin in older children (Harris 1998; Wubbel 1999). This review only included the data comparing amoxycillin/clavulanic acid.
Excluded studies
We excluded 13 studies. The main reason for exclusion seen in 10 studies was comparison of azithromycin with other antibiotics not related to amoxycillin. Two studies were meta‐analyses. For more details, see the Characteristics of excluded studies table.
Risk of bias in included studies
Details of the risk of bias of each study are given in the 'Risk of bias' tables in the Characteristics of included studies table. The overall risk of bias is presented graphically in Figure 1 and summarised in Figure 2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
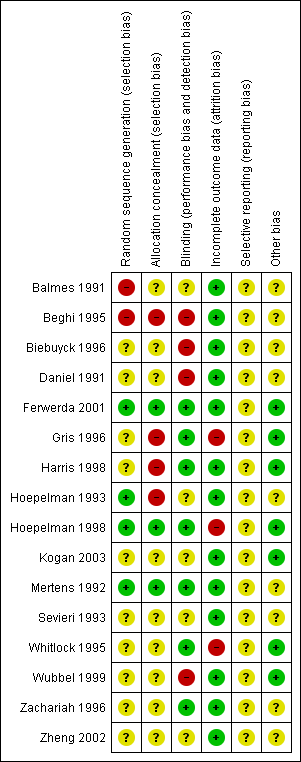
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Four out of 16 included studies had adequate sequence generation for randomisation (Ferwerda 2001; Hoepelman 1993; Hoepelman 1998; Mertens 1992). Three out of 16 included studies had adequate allocation concealment (Ferwerda 2001; Hoepelman 1998; Mertens 1992). We assessed these trials as low risk for selection bias.
Blinding
Seven included studies used double‐blinding by matched placebo in both treatment groups (Ferwerda 2001; Gris 1996; Harris 1998; Hoepelman 1998; Mertens 1992; Whitlock 1995; Zachariah 1996). We assessed them as having low risk of performance and detection biases.
Nine studies either had no description of blinding or unclear information relating to blinding (Balmes 1991; Beghi 1995; Biebuyck 1996; Daniel 1991; Hoepelman 1993; Kogan 2003; Sevieri 1993; Wubbel 1999; Zheng 2002). We assessed these studies to be potentially high risk of bias.
Incomplete outcome data
Three out of 16 included studies had more than 10% missing data for the clinical failure outcome (Gris 1996; Hoepelman 1998; Whitlock 1995). We assessed them to be at high risk of attrition bias and excluded them from the sensitivity analysis. The remaining 13 studies either had analyses of total randomised patients or had missing data of less than 10%.
Selective reporting
Since we did not have access to the protocols for any of the included studies, the risk of bias in selective reporting was unclear for all.
Other potential sources of bias
Six out of 16 included studies had balanced characteristics between the two treatment groups (Ferwerda 2001; Gris 1996; Harris 1998; Hoepelman 1998; Kogan 2003; Whitlock 1995). We assessed them as having a low risk of other potential sources of bias.
We considered that the majority of the included studies, 81.3% (13/16), had a low risk of attrition bias. Among the 16 included studies, only one study had a low risk of bias in five of the six risk of bias domains (Ferwerda 2001).
Effects of interventions
We included 15 trials involving 2496 participants in the analysis. There were 1388 participants who received azithromycin and 1108 who received amoxycillin or amoxyclav. All trials reported the incidence of clinical failure (persistence or deterioration of symptoms or relapse). Eleven trials reported the incidence of microbacterial eradication. There was no evidence of publication bias by visual inspection of the funnel plot (Figure 3).
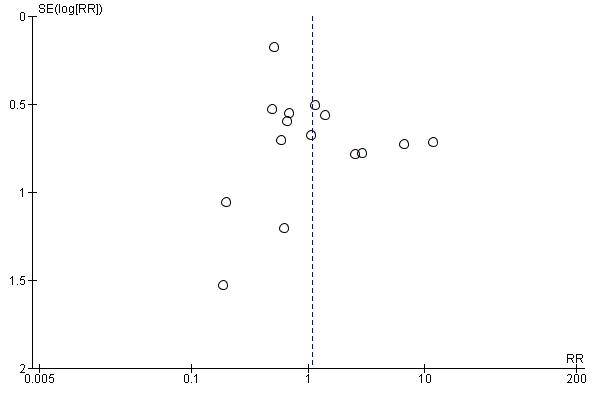
Funnel plot of comparison: 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, outcome: 1.1 Clinical failure.
Primary outcome
1. Clinical failure
The pooled analysis of all trials showed that the incidence of clinical failure on day 10 to 14 in the azithromycin group was 10.1% (140/1388) compared to 10.3% (114/1108) in the amoxycillin or amoxyclav group. There was no statistical significance in the difference in incidence of clinical failure between the two groups (risk ratio (RR) 1.09; 95% confidence interval (CI) 0.64 to 1.85, random‐effects model) (Analysis 1.1). However, the heterogeneity between trials was significant with an I2 statistic of 65.3% (P value = 0.0002).
Heterogeneity would be anticipated with the variation in age groups and types of diagnoses between trials. Subgroup analysis stratified by age groups showed no significant difference in treatment effects between the azithromycin group and the amoxycillin or amoxyclav group in either adults (RR 1.15; 95% CI 0.60 to 2.20, random‐effects model) or children (RR 0.93; 95% CI 0.45 to 1.94) (Analysis 1.3).
In a subgroup analysis of trials with acute bronchitis participants, the incidence of clinical failure was significantly lower in the azithromycin group compared to amoxycillin or amoxyclav (RR 0.63; 95% CI 0.45 to 0.88, random‐effects model) (Analysis 1.2). In analysis of trials with acute exacerbation of chronic bronchitis participants, there was significant heterogeneity between trials with an I2statistic of 75.5% (P value = 0.0001) and clinical failure was not significantly different between the groups.
We considered the impact of study quality (according to adequate concealment) on the pooled results for clinical failure in a sensitivity analysis. The reduction of clinical failure in azithromycin‐treated participants was RR 0.55 (95% CI 0.25 to 1.21) in three adequately concealed studies, compared to RR 1.32 (95% CI 0.70 to 2.49), restricted to 12 studies with inadequate concealment.
We also performed a sensitivity analysis by excluding the largest trial (Biebuyck 1996). The result showed that the overall effect of azithromycin compared to amoxycillin or amoxyclav for reducing clinical failure was a RR of 1.20 (95% CI 0.69 to 2.09). This figure was quite similar to the result for the total of 15 trials (RR 1.09; 95% CI 0.64 to 1.85) (Analysis 1.5).
When excluding the three trials with more than 10% missing data (Gris 1996; Hoepelman 1998; Whitlock 1995), a sensitivity analysis of the remaining 12 trials showed that the overall effect of azithromycin compared to amoxycillin or amoxyclav on reducing clinical failure was a RR of 1.13 (95% CI 0.62 to 2.03). This figure was quite similar to the result for the total of 15 trials (RR 1.09; 95% CI 0.64 to 1.85).
Secondary outcomes
1. Incidence of serious complications
No trials reported death.
2. Adverse drug events
Twelve trials reported adverse events. The most frequent adverse events were mild to moderate gastrointestinal symptoms, nausea, vomiting and diarrhoea. The others reported were headache, insomnia, rash and transient laboratory liver function changes. One large trial reported a higher number of participants discontinuing amoxyclav treatment because of adverse events compared to the azithromycin group: 7% compared to 1.2% respectively (Biebuyck 1996). The overall incidence of adverse events in the azithromycin group was 17.9% (244/1363) compared to 23.6% (246/1043) in the amoxycillin or amoxyclav group. The reduction of adverse events in the azithromycin group was a RR of 0.76 (95% CI 0.57 to 1.00) (Analysis 1.10).
3. Eradication of organism
Twelve trials reported the incidence of microbial eradication. The pooled analysis showed that the incidence of microbial eradication in the azithromycin group was 66.4% (326/491) compared to 67.6% (318/470) in the amoxicillin or amoxyclav group. There was no significant difference between the two groups (RR 0.95; 95% CI 0.87 to 1.03, fixed‐effect model) (Analysis 1.9).
Discussion
Summary of main results
The results of this review showed that the effect of azithromycin compared to amoxycillin or amoxyclav on clinical failure, microbial eradication and adverse events measured at about day 10 to 14 was not statistically significant. However, in a group of participants with acute bronchitis of a suspected bacterial cause, the incidence of clinical failure was significantly lower in the azithromycin group.
Overall completeness and applicability of evidence
The 15 analysed studies were published over a period of 11 years (1991 to 2002). Fourteen out of the 15 included studies were from a wide range of high‐income countries. Eighty per cent (12/15) of the included studies were conducted in adults. There were few differences in the doses of azithromycin and the context of each study. The pooled results may be applied to similar settings.
Quality of the evidence
There were some limitations that related to the quality of the included trials (Figure 1; Figure 2). The most important was that adequately concealed treatment allocation was performed in only three trials and 53% of the studies (8/15) either did not report this information or reported insufficient information about blinding. The results of these studies should be interpreted with caution.
Potential biases in the review process
We followed the Cochrane Acute Respiratory Infections Group's guidelines for conducting the review. However, publication bias may remain a possible (but unknown) source of important bias.
Agreements and disagreements with other studies or reviews
There are no other systematic reviews on this topic.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, outcome: 1.1 Clinical failure.
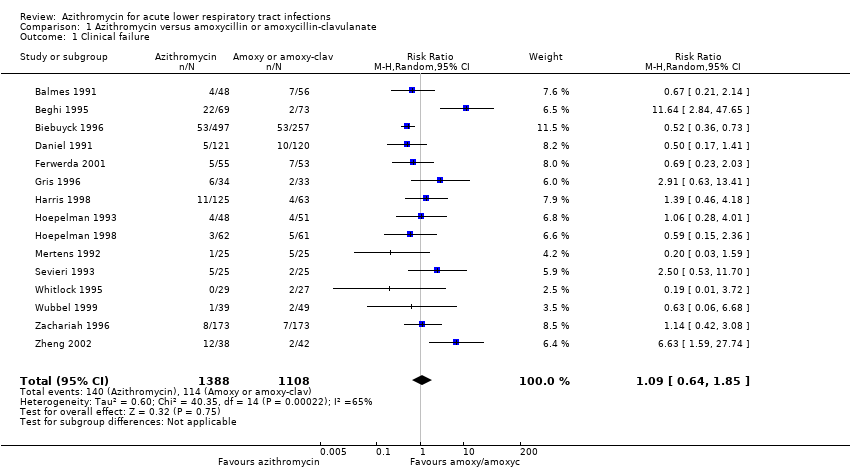
Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 1 Clinical failure.

Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 2 Clinical failure by diagnosis.

Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 3 Clinical failure by age group.

Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 4 Clinical failure by dose regimen of azithromycin.
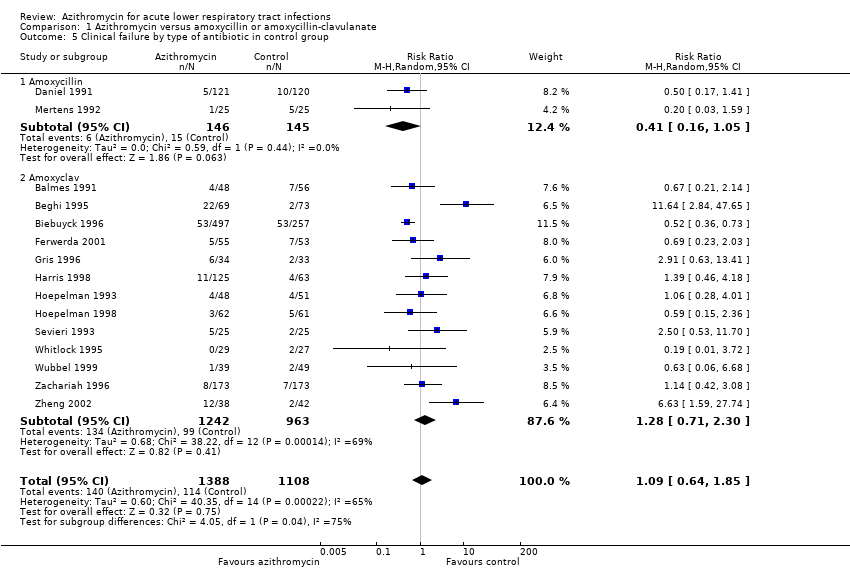
Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 5 Clinical failure by type of antibiotic in control group.

Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 6 Sensitivity analysis excluding one large trial.
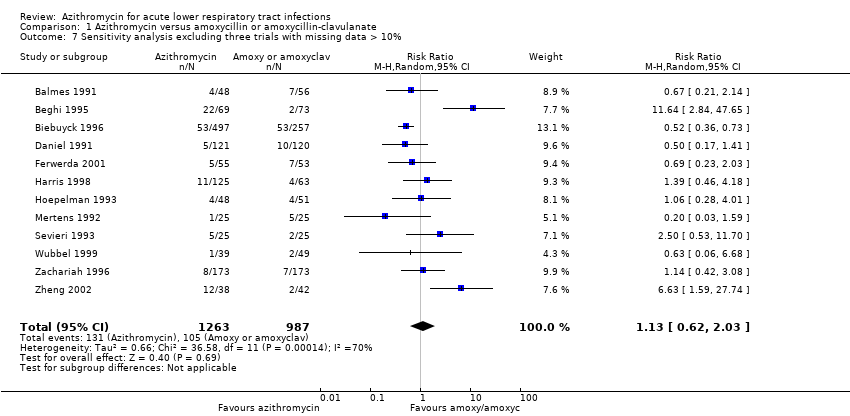
Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 7 Sensitivity analysis excluding three trials with missing data > 10%.

Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 8 Sensitivity analysis with the condition of concealment.
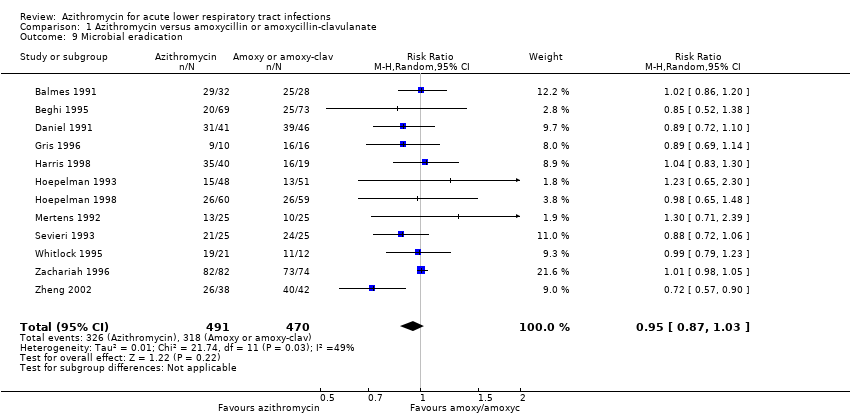
Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 9 Microbial eradication.

Comparison 1 Azithromycin versus amoxycillin or amoxycillin‐clavulanate, Outcome 10 Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical failure Show forest plot | 15 | 2496 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.85] |
| 2 Clinical failure by diagnosis Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Acute bronchitis | 6 | 1296 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.45, 0.88] |
| 2.2 Acute exacerbation of chronic bronchitis | 9 | 808 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.46, 3.32] |
| 2.3 Pneumonia | 5 | 392 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.45, 1.94] |
| 3 Clinical failure by age group Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Adult | 12 | 2112 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.60, 2.20] |
| 3.2 Paediatric | 3 | 384 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.45, 1.94] |
| 4 Clinical failure by dose regimen of azithromycin Show forest plot | 12 | 2112 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.60, 2.20] |
| 4.1 500 mg once daily x 3 | 8 | 1631 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.55, 2.83] |
| 4.2 500 mg single dose followed by 250 mg on day 2 to 5 | 4 | 481 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.25, 3.62] |
| 5 Clinical failure by type of antibiotic in control group Show forest plot | 15 | 2496 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.85] |
| 5.1 Amoxycillin | 2 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.16, 1.05] |
| 5.2 Amoxyclav | 13 | 2205 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.71, 2.30] |
| 6 Sensitivity analysis excluding one large trial Show forest plot | 14 | 1742 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.69, 2.09] |
| 7 Sensitivity analysis excluding three trials with missing data > 10% Show forest plot | 12 | 2250 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.62, 2.03] |
| 8 Sensitivity analysis with the condition of concealment Show forest plot | 15 | 2496 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.85] |
| 8.1 Adequately concealed studies | 3 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.25, 1.21] |
| 8.2 Inadequately or unclearly concealed studies | 12 | 2215 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.70, 2.49] |
| 9 Microbial eradication Show forest plot | 12 | 961 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.87, 1.03] |
| 10 Adverse events Show forest plot | 12 | 2406 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.57, 1.00] |

