| 1 Leaving the study early ‐ any reason Show forest plot | 16 | 1305 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.89, 1.38] |
|
| 1.1 short term ‐ up to 6 weeks | 7 | 380 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.84, 1.34] |
| 1.2 medium term ‐ 7 ‐ 26 weeks | 9 | 925 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.76, 1.97] |
| 2 Removed from analysis Show forest plot | 11 | 793 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.54, 1.79] |
|
| 3 Global effect: 1. Not improved (CGI) Show forest plot | 13 | 580 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.68, 1.09] |
|
| 3.1 short term ‐ up to 6 weeks | 6 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.58, 1.21] |
| 3.2 medium term ‐ 7 ‐ 26 weeks | 7 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.65, 1.19] |
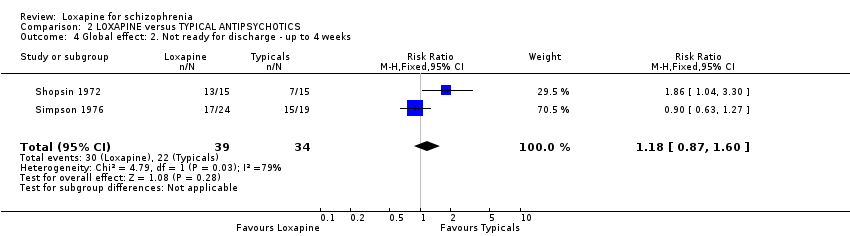
| 4 Global effect: 2. Not ready for discharge ‐ up to 4 weeks Show forest plot | 2 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.87, 1.60] |
|
| 5 Global effect: 3. Needing additional antipsychotic/sedative drugs ‐ up to 6 weeks Show forest plot | 2 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.62, 2.12] |
|
| 6 Global effect: 4. Participant rating of illness Show forest plot | 2 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 6.1 did not feel better ‐ 4 weeks | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.69, 2.21] |
| 6.2 much, or very much better ‐ 4 weeks | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.73, 1.37] |
| 6.3 worse ‐ 4 weeks | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.01] |
| 6.4 would not prefer to stay on medication ‐ 4 weeks | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.78, 1.81] |
| 6.5 prefer another medication ‐ 4 weeks | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.78, 1.81] |
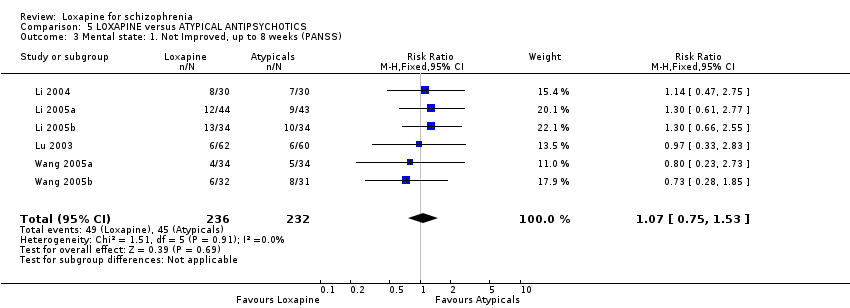
| 7 Mental state: 1a. General ‐ not improved, by 8 weeks (BPRS/PANSS) Show forest plot | 6 | 915 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.76, 1.05] |
|
| 8 Mental state: 1b. General ‐ average endpoint score, by 8 weeks (BPRS, high score=worse) Show forest plot | 3 | 465 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐2.92, ‐0.67] |
|
| 9 Mental state: 1c. General ‐ average endpoint score, by 8 weeks (PANSS, high score=worse) Show forest plot | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐1.75 [‐8.60, 5.10] |
|
| 10 Mental state: 1d. General ‐ average change score (BPRS, high score=worse) Show forest plot | 3 | 465 | Mean Difference (IV, Fixed, 95% CI) | ‐1.38 [‐2.60, ‐0.16] |
|
| 11 Mental state: 2. Specific Show forest plot | 5 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 11.1 anxiety ‐ 6 weeks | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.35, 5.81] |
| 11.2 anxiety ‐ 7 ‐ 26 weeks | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.58] |
| 11.3 behaviour changes (not specified), by 12 weeks | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.59, 1.50] |
| 11.4 depression ‐ 4 weeks | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.19] |
| 11.5 excitement ‐ 4 weeks | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 20.77] |
| 11.6 restlessness ‐ 4 weeks | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.38, 4.16] |
| 11.7 restlessness ‐ 7 ‐ 26 weeks | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.58] |
| 11.8 violence or aggression ‐ 4 weeks | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 20.77] |
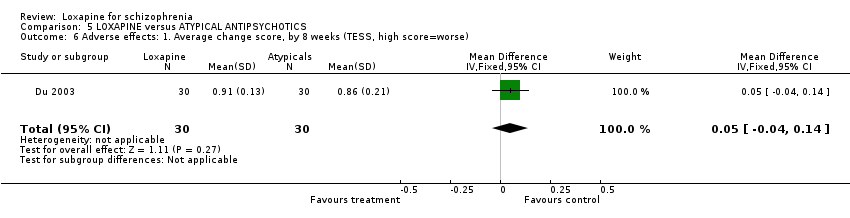
| 12 Adverse effects: 1. Average change score, by 8 weeks (TESS, high score=worse) Show forest plot | 3 | 340 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.11, ‐0.03] |
|
| 13 Adverse effects: 2. Any adverse event Show forest plot | 14 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.88, 1.06] |
|
| 13.1 up to 6 weeks | 7 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.91, 1.13] |
| 13.2 7 ‐ 26 weeks | 7 | 309 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.76, 1.05] |
| 14 Adverse effects: 3. Anticholinergic effects ‐ specific symptoms Show forest plot | 9 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 14.1 blurred vision ‐ up to 6 weeks | 4 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.63, 1.39] |
| 14.2 blurred vision ‐ 12 weeks | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.52, 4.79] |
| 14.3 constipation ‐ 4 weeks | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.92, 1.40] |
| 14.4 constipation ‐ 7 ‐ 26 weeks | 3 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.48, 2.96] |
| 14.5 dry mouth ‐ up to 6 weeks | 3 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.76, 2.39] |
| 14.6 dry mouth ‐ 7 ‐ 26 weeks | 3 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.60, 2.26] |
| 14.7 nasal congestion ‐ 12 weeks | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.11, 3.32] |
| 15 Adverse effects: 4. Cardiovascular problems Show forest plot | 12 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 15.1 hypertension ‐ 12 weeks | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.05, 5.33] |
| 15.2 ECG abnormalites ‐ up to 4 weeks | 2 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.37, 1.90] |
| 15.3 ECG abnormalities ‐ up to 12 weeks | 4 | 456 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.11, 1.47] |
| 15.4 hypotension ‐ 7 ‐ 26 weeks | 5 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.46, 1.52] |
| 15.5 syncope ‐ 8 weeks | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.7 [0.30, 24.43] |
| 15.6 tachycardia ‐ 7 to 26 weeks | 6 | 365 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.78, 1.47] |
| 15.7 unspecified ‐ 12 weeks | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.45, 1.54] |
| 16 Adverse effects: 5. Gastrointestinal problems Show forest plot | 5 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 16.1 abdominal pain ‐ 6 weeks | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.35, 5.81] |
| 16.2 appetite loss ‐ 4 weeks | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.49, 1.70] |
| 16.3 constipation ‐ 8 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 99.95] |
| 16.4 diarrhoea ‐ 4 weeks | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.19] |
| 16.5 diarrhoea ‐ 12 weeks | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.60] |
| 16.6 nausea or vomiting ‐ 4 weeks | 2 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.15, 3.60] |
| 16.7 nausea or vomiting ‐ 12 weeks | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.8 [0.17, 18.75] |
| 16.8 stomach trouble ‐ 4 weeks | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.68] |
| 17 Adverse effects: 6. Movement disorders Show forest plot | 19 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 17.1 agitation ‐ 8 weeks | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.18] |
| 17.2 akathisia ‐ up to 6 weeks | 3 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.52, 2.19] |
| 17.3 akathisia ‐ up to 12 weeks | 3 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.80, 1.88] |
| 17.4 akinesia ‐ 4 weeks | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.51, 158.85] |
| 17.5 dyskinesia ‐ 4 weeks | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.29, 3.03] |
| 17.6 dystonia ‐ up to 6 weeks | 4 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.91, 3.54] |
| 17.7 dystonia ‐ up to 12 weeks | 2 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.59, 2.42] |
| 17.8 extrapyramidial ‐ up to 4 weeks | 4 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.77, 1.23] |
| 17.9 extrapyramidal ‐ up to 12 weeks | 4 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.85, 1.38] |
| 17.10 excess salivation ‐ 4 weeks | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.87, 2.31] |
| 17.11 excess salivation ‐ 7 ‐ 26 weeks | 3 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.39, 2.19] |
| 17.12 fixed stare ‐ 6 weeks | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.20, 3.27] |
| 17.13 heavy muscles ‐ up to 6 weeks | 2 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.21, 2.32] |
| 17.14 muscle cramp ‐ 6 weeks | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.35, 5.81] |
| 17.15 muscle spasm ‐ 6 weeks | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.30, 3.87] |
| 17.16 muscle spasm ‐ 26 weeks | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.20, 19.78] |
| 17.17 needing additional anticholinergic medication ‐ up to 6 weeks | 7 | 302 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.81, 1.33] |
| 17.18 needing additional anticholinergic medication ‐ up to 12 weeks | 3 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.71, 1.72] |
| 17.19 oculogyric crisis ‐ 6 weeks | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.40, 4.48] |
| 17.20 rigidity ‐ up to 6 weeks | 4 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.96, 1.50] |
| 17.21 rigidity ‐ 7 ‐ 26 weeks | 3 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.51, 2.06] |
| 17.22 thick speech ‐ up to 6 weeks | 2 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.44, 3.39] |
| 17.23 tremor ‐ up to 6 weeks | 4 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.81, 1.51] |
| 17.24 tremor ‐ 7 to 26 weeks | 4 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.54, 1.32] |
| 17.25 twisting movement ‐ 8 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 20.90] |
| 18 Adverse effects: 7. Neurological problems Show forest plot | 9 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 18.1 ataxia ‐ 4 weeks | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.15] |
| 18.2 clumsiness ‐ 26 weeks | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.26] |
| 18.3 confusion/cloudiness ‐ 6 weeks | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.20, 3.27] |
| 18.4 confusion/cloudiness ‐ 7 to 26 weeks | 2 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.61, 13.24] |
| 18.5 dizziness, fainting, weakness ‐ up to 6 weeks | 2 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.37, 2.36] |
| 18.6 dizziness/fainting, weakness ‐ 7 ‐ 26 weeks | 3 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.67, 3.75] |
| 18.7 giddiness ‐ 4 weeks | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.08, 1.89] |
| 18.8 seizures ‐ up to 12 weeks | 3 | 302 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.94 [0.45, 34.72] |
| 18.9 unsteadiness ‐ 26 weeks | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.35, 25.68] |
| 19 Adverse effects: 8. Sleep problems Show forest plot | 12 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 19.1 drowsiness / sedation ‐ up to 6 weeks | 6 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.79, 1.65] |
| 19.2 drowsiness/ sedation ‐ up to 12 weeks | 6 | 408 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.02, 1.86] |
| 19.3 fatigue ‐ 6 weeks | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.17, 2.44] |
| 19.4 insomnia ‐ up to 6 weeks | 3 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.56, 1.81] |
| 19.5 insomnia ‐ up to 12 weeks | 2 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.13, 0.69] |
| 19.6 lethargy ‐ up to 6 weeks | 2 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.77, 3.75] |
| 20 Adverse effects: 9. Weight changes Show forest plot | 4 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 20.1 weight increase ‐ 6 weeks | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.10, 2.16] |
| 20.2 weight increase 12 weeks | 2 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.30, 1.10] |
| 20.3 weight loss ‐ 6 weeks | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.12, 3.19] |
| 20.4 weight loss ‐ 12 weeks | 2 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.58, 3.31] |
| 21 Adverse effects: 10. Others Show forest plot | 16 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 21.1 abnormal blood results ‐ up to 6 weeks | 5 | 235 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.60, 2.22] |
| 21.2 abnormal blood results ‐ up to 12 weeks | 5 | 506 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.76, 1.47] |
| 21.3 anxiety ‐ 8 weeks | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.07, 1.20] |
| 21.4 difficulty swallowing ‐ 6 weeks | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.20, 3.27] |
| 21.5 headache ‐ 6 weeks | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.35, 5.81] |
| 21.6 headache ‐ 12 weeks | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.24, 7.48] |
| 21.7 libido ‐ decrease ‐ 4 weeks | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.11] |
| 21.9 opthalmic changes ‐ 12 weeks | 2 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.15, 6.81] |
| 21.10 ringing in ears ‐ 6 weeks | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.35, 5.81] |
| 21.11 skin problems ‐ rash ‐ 4 weeks | 2 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.09, 1.33] |
| 21.12 skin problems ‐ rash ‐ 7 ‐ 26 weeks | 4 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.05, 0.77] |
| 21.13 swelling of hands/face ‐ 4 weeks | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.28, 1.61] |
| 21.14 swelling of hands/face ‐ 26 weeks | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.26, 96.13] |
| 21.15 tingling sensation ‐ 6 weeks | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.17, 2.44] |
| 21.16 lactation ‐ 12 weeks | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.02, 8.09] |
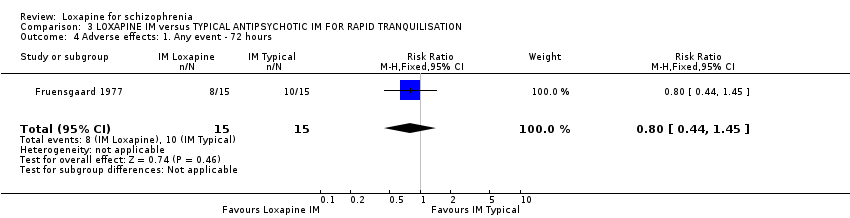
| 21.17 sweating ‐ 72 hours | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.09 [0.20, 21.48] |
| 21.18 sweating ‐ 12 weeks | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 7.09] |
| 21.19 excitement ‐ 12 weeks | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.05 [0.96, 17.12] |
| 21.20 depression ‐ 12 weeks | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.48, 3.02] |
| 21.21 lacrimation ‐12 weeks | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.12, 63.84] |
| 21.22 breathlessness ‐ 12 weeks | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.12, 63.84] |
| 21.23 bulimia ‐ 12 weeks | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.12, 63.84] |
| 21.24 hypersalivation ‐ 8 weeks | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.55, 7.27] |