Contenido relacionado
Revisiones y protocolos relacionados
Manya Prasad, Pudukode R Krishnan, Reginald Sequeira, Khaldoon Al‐Roomi | 10 septiembre 2014
Rebecca Bresnahan, Mariangela Panebianco, Anthony G Marson | 1 julio 2020
Rebecca Bresnahan, Kirsty J Martin‐McGill, John Williamson, Benedict D Michael, Anthony G Marson | 22 octubre 2019
Jia Liu, Lu-Ning Wang | 11 mayo 2021
Francesco Brigo, Simona Lattanzi, Stanley C Igwe, Masoud Behzadifar, Nicola Luigi Bragazzi | 24 julio 2020
Mariangela Panebianco, Sarah Al-Bachari, Jane L Hutton, Anthony G Marson | 12 enero 2021
JinSong Geng, JianCheng Dong, Youping Li, HengJian Ni, Kui Jiang, Li Li Shi, GuoHua Wang | 2 diciembre 2019
Francesco Brigo, Katherine Jones, Christin Eltze, Sara Matricardi | 7 abril 2021
Corticosteroides, incluida la ACTH, para la epilepsia infantil diferente de los espasmos epilépticos
Vishal Mehta, Colin D Ferrie, J Helen Cross, Gayatri Vadlamani | 18 junio 2015
Lin Song, Fang Liu, Yao Liu, Ruoqi Zhang, Huanhuan Ji, Yuntao Jia | 20 abril 2020
Respuestas clínicas Cochrane
Jane Burch, Agustín Ciapponi | 6 julio 2020














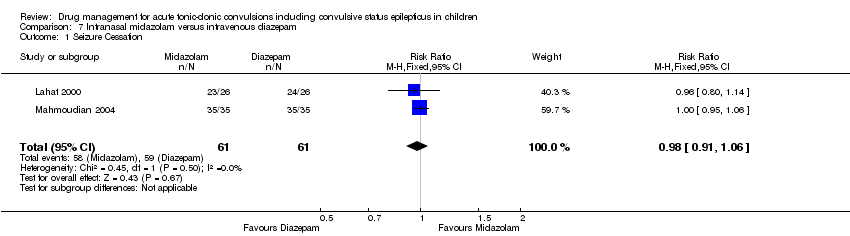
![Comparison 7 Intranasal midazolam versus intravenous diazepam, Outcome 2 Time from drug administration to stopping of seizures [minutes].](/es/cdsr/doi/10.1002/14651858.CD001905.pub3/media/CDSR/CD001905/image_n/nCD001905-CMP-007-02.png)