Pengurusan ubat untuk sawan tonik‐klonik akut termasuk sawan status epileptikus dalam kalangan kanak‐kanak
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised controlled trial carried out over 12 months in Malawi | |
| Participants | 160 children of both sexes and aged 2 months to 12 years presenting to a paediatric emergency department in a generalised seizure. | |
| Interventions | Intranasal lorazepam versus intramuscular paraldehyde | |
| Outcomes | Seizure cessation | |
| Notes | Study conducted in Africa with a high proportion of children with either cerebral malaria or meningitis. Consequently, not readily generalisable to western populations | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Blocked randomisation was done in advance by a computer that randomly generated a table of numbers in batches of ten" Comment: adequate randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: " treatment allocations were sealed in unmarked identical envelopes. Investigators were masked to these allocations before the point of patient treatment. Quote: adequate concealment |
| Blinding (performance bias and detection bias) | Low risk | Study unblinded, but blinding would have been difficult due to the different routes of administration of the 2 study drugs. This is not likely to have affected outcome |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were included in the final analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the Results section |
| Other bias | High risk | A high proportion of the children recruited had either cerebral malaria or meningitis. These comorbidities may have impacted upon the results |
| Methods | Quasi‐randomised controlled trial (odd and even days randomisation of the 2 drugs) over a 12‐month study period | |
| Participants | 102 children of both sexes and aged < 16 years presenting to a single Accident and Emergency department in a tonic‐clonic convulsion including established convulsive status epilepticus. Participants treated included those with an established diagnosis of epilepsy, febrile convulsions and those presenting with a first convulsion. | |
| Interventions | Lorazepam versus diazepam: rectal and intravenous administration. Diazepam dose: 0.3 to 0.4 mg/kg and lorazepam dose: 0.05 to 0.1 mg/kg. These doses were used for both intravenous and rectal routes of administration | |
| Outcomes | Seizure cessation | |
| Notes | Numerous protocol violators in the study who were then excluded from analysis. The study population was small and there were substantial differences in the size of the 2 treatment groups (lorazepam 33 participants and diazepam 53 participants). There was an even larger discrepancy in the children who received the drug rectally; rectal lorazepam (6 children) versus rectal diazepam (19 children) This clearly suggests a higher violation rate for these children who should have received rectal lorazepam. This may have been due to clinician uncertainty about the use of rectal lorazepam, as this drug and route of administration are not used in routine clinical practice | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: " children were assigned..on an odd and even dates basis" Comment: this was done to avoid any delay incurred by another randomisation method. The randomisation method may have contributed to the unequal sizes of the groups |
| Allocation concealment (selection bias) | High risk | As described above, clinicians would be aware of the allocation by whether the day was odd or even |
| Blinding (performance bias and detection bias) | Low risk | The study was unblinded, but this would have been impractical and is not likely to have affected outcome |
| Incomplete outcome data (attrition bias) | High risk | There were a relatively large number of protocol violators (16/102 children, or 16% of the total study population) and these violators were excluded from the analyses. The analysis was therefore not an intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in the Results section |
| Other bias | High risk | Large discrepancy in the 2 routes of administration used in the study, probably due to clinician uncertainty about the use of rectal lorazepam. This discrepancy is likely to have impacted upon results |
| Methods | Randomised controlled trial, not blinded | |
| Participants | 141 children aged 6 ‐ 14 years attending the emergency room of a hospital in New Delhi, India with a seizure, or those having a seizure during attendance 58 out of 141 of the children (41%) had generalised tonic‐clonic seizures but primary outcome results are presented separately for the subgroup of generalised tonic‐clonic seizures | |
| Interventions | Intranasal versus intravenous lorazepam | |
| Outcomes | Cessation of all visible motor activity by 10 minutes | |
| Notes | Results are presented for the subgroup of 58 children with generalised tonic‐clonic seizures Inclusion criteria did not include duration of seizure, unlike most of the studies There was 1 protocol violation when intravenous access could not be obtained in 1 child who was randomised to intravenous lorazepam. This child was treated with intranasal lorazepam. However the results were analysed on an intention‐to‐treat basis and no participants were excluded from the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:" randomisation was done using blocks of variable length" |
| Allocation concealment (selection bias) | Low risk | Quote: "Opaque sealed envelopes containing allocation of randomisation" |
| Blinding (performance bias and detection bias) | Low risk | The study was unblinded; this would have been difficult, due to the different routes of administration and is not likely to have affected outcome |
| Incomplete outcome data (attrition bias) | Low risk | All recruited participants were included in the analysis and analysed on an intention to treat basis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in the Results section |
| Other bias | Low risk | None identified |
| Methods | Randomised controlled trial, not blinded and no placebo | |
| Participants | 98 children of both sexes and aged 3 months to 12 years attending the emergency department of two large paediatric hospitals in Tehran, Iran between April 2007 and April 2008. | |
| Interventions | Buccal midazolam versus rectal diazepam | |
| Outcomes | Cessation of all motor activity within 5 minutes, without respiratory depression and without seizure recurrence | |
| Notes | Buccal midazolam associated with 100% seizure cessation rate, which is higher than expected | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A random number table was used for randomisation" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to assess this |
| Blinding (performance bias and detection bias) | Low risk | The study was unblinded; blinding would have been difficult due to the different routes used, but this is unlikely to have had a significant impact on the results |
| Incomplete outcome data (attrition bias) | Low risk | All recruited participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in the Results section |
| Other bias | Unclear risk | Buccal midazolam associated with 100% seizure cessation rate, which is higher than expected. Unclear whether this high success rate was due to a particular element of the trial design |
| Methods | Prospective quasi‐randomised trial (odd and even days randomisation of the 2 drugs) in 1 centre | |
| Participants | 43 children of both sexes aged 2 months to 12 years who presented with a seizure to the emergency room, regardless of seizure type, aetiology or duration | |
| Interventions | Buccal midazolam versus rectal diazepam | |
| Outcomes | Cessation of convulsive seizure activity within 10 minutes | |
| Notes | Children who were seizing on arrival were included, on the assumption that the seizure was prolonged. This is different from most of the other studies, which require a period of seizure activity lasting 5 ‐ 10 minutes before inclusion and randomisation. However, this should not have introduced bias, as these children should have been equally distributed between the 2 groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Diazepam was given on odd days of the month and midazolam on the even days" Comment: inadequate randomisation |
| Allocation concealment (selection bias) | High risk | See above; no concealment of allocation |
| Blinding (performance bias and detection bias) | Low risk | Study unblinded, but blinding would not have been possible, due to the different routes of administration of the 2 study drugs, so this is not likely to have affected outcome |
| Incomplete outcome data (attrition bias) | Low risk | All recruited participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the Results section |
| Other bias | Low risk | None identified |
| Methods | Prospective randomised study in two centres | |
| Participants | 23 children of both sexes and aged birth to 18 years presenting to an emergency department with a motor seizure lasting at least 10 minutes | |
| Interventions | Intramuscular midazolam versus intravenous diazepam | |
| Outcomes | Seizure cessation within 5 minutes of drug administration | |
| Notes | 1 child was enrolled in the study twice, so is represented in both groups. It was not possible to identify this child in the reported results There was also a protocol violator who was randomised to receive intravenous diazepam but received intramuscular midazolam after 25 minutes, due to unsuccessful intravenous access. This participant was excluded from the analysis and would have skewed the results significantly if he/she had been included. It may have been helpful to know the response time of this child once treatment was administered, as this is an important example of the disadvantages of the intravenous route | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly selected by computer" Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to assess this |
| Blinding (performance bias and detection bias) | Low risk | Blinding would not have been possible, due to the different routes of administration of the 2 study drugs, but this is not likely to have affected outcome |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote "Three children were randomised to receive diazepam but were excluded because their seizures did not persist for 10 minutes." Comment: this is unlikely to have made a significant difference to the analysis Quote "One child was a protocol deviation and was excluded‐ was randomised to diazepam but received midazolam instead due to unsuccessful attempts at IV access" Comment: this child should have been included in the analysis for it to be considered an intention‐to‐treat analysis. However it would have skewed the results significantly, as midazolam was not given until after 25 minutes of attempting intravenous access |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the Results section |
| Other bias | High risk | 1 child was enrolled in the study twice, so is represented in both groups. It was not possible to identify this child in the reported results. Due to the small numbers of children included in the study, this double‐enrolment may have impacted on the results |
| Methods | Double‐blind multicentre randomised trial | |
| Participants | 273 patients aged 3 months up to 18 years presenting with convulsive status epilepticus | |
| Interventions | intravenous diazepam versus intravenous lorazepam | |
| Outcomes | Primary outcomes: Cessation of status epilepticus by 10 minutes without recurrence within 30 minutes | |
| Notes | Consideration was given to sample size with an estimate of 120 participants per group for 80% power to detect a significant difference between treatments. After an interim analysis halfway through the study, this was increased to 131 participants per group, probably because there was less treatment effect difference than anticipated between the treatment arms. Analysis of data was transparent, with all participants who were randomised analysed on an intention‐to‐treat basis but with further per protocol analysis limited to those with no protocol violation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block randomisation (1:1) with stratification to 3 age groups was performed |
| Allocation concealment (selection bias) | Low risk | Measures taken to ensure allocation concealment |
| Blinding (performance bias and detection bias) | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) | Low risk | All participants who were randomised were analysed on an intention to treat basis. An additional per protocol analysis limited to those with <1 no protocol violation |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the Results section |
| Other bias | Low risk | None identified |
| Methods | Prospective quasi‐randomised study (odd and even days randomisation of the 2 drugs) over 15 months | |
| Participants | 45 children of both sexes and aged 1 month to 13 years presenting to the emergency room with a seizure lasting at least 5 minutes | |
| Interventions | intranasal midazolam versus rectal diazepam | |
| Outcomes | Stopping of seizure within 10 minutes | |
| Notes | Some methodology described unclear, particularly relating to seizure type and aetiology of included children. It is therefore unclear if the population of this study is generalisable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Diazepam was given on odd days of the month and midazolam on the even days" |
| Allocation concealment (selection bias) | High risk | See above; no concealment of allocation |
| Blinding (performance bias and detection bias) | Low risk | Study unblinded, but blinding would not have been possible, due to the different routes of administration of the 2 study drugs, but this is not likely to have affected outcome |
| Incomplete outcome data (attrition bias) | Low risk | All participants were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Quote: "information about previous convulsions and history of antiepileptic medication was obtained.." Comment: this information was not reported in the Results section but as this is not one of the primary outcome measures it is not likely to be significant |
| Other bias | Unclear risk | Unclear description of the seizure type and aetiology of included children, so it is unclear if the population of this study is generalisable |
| Methods | Randomised controlled trial, unblinded | |
| Participants | 120 children aged 6 months to 14 years, attending emergency room with an acute seizure | |
| Interventions | Intravenous diazepam versus midazolam versus lorazepam | |
| Outcomes | Time to seizure cessation | |
| Notes | Unclear exactly when participants were given second dose of drug (range 5 ‐ 20 minutes); the convention would be to wait 10 minutes. Seizure cessation is defined as "Cessation of visible epileptic phenomenon or return of purposeful response to external stimuli within 15 minutes of drug administration". This definition is different from all other included studies and latter part of this definition is not an appropriate criterion for judging seizure cessation, as most individuals following a tonic‐clonic seizure will have a post‐ictal phase in which they do not respond to external stimuli | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by shuffling of envelopes |
| Allocation concealment (selection bias) | Low risk | Allocation by sealed envelopes |
| Blinding (performance bias and detection bias) | Low risk | Study was unblinded, but this is not likely to have affected outcome |
| Incomplete outcome data (attrition bias) | Unclear risk | The 3 excluded participants where IV access was not possible were not included in the analysis. However, as all routes were intravenous this is unlikely to have introduced bias |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | High risk | The definition of the 'Seizure Cessation' outcome used is different from all other included studies and is not an appropriate criterion for judging seizure cessation. This definition is likely to have impacted upon results. |
| Methods | Randomised unblinded study | |
| Participants | 60 children aged 2 months to 15 years old presenting to emergency department with acute seizure episode | |
| Interventions | Intranasal midazolam versus intravenous diazepam | |
| Outcomes | Time needed to control seizure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding (performance bias and detection bias) | Low risk | Study was unblinded, but this is not likely to have affected outcome |
| Incomplete outcome data (attrition bias) | Low risk | All participants were included in the analysis and analysed on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | High risk | Number of children with seizure cessation not reported; we would expect this outcome to be reported |
| Other bias | Low risk | None identified |
| Methods | 12‐month randomised controlled trial | |
| Participants | 44 children of both sexes and aged 6 months to 5 years presenting to a paediatric emergency department with a febrile seizure | |
| Interventions | Intravenous diazepam versus intranasal midazolam | |
| Outcomes | Seizure cessation | |
| Notes | In addition this study evaluated a specific subgroup of children with prolonged convulsive febrile seizures. This is important, as the aetiology of seizures varies across the age ranges during childhood, thereby potentially affecting results | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed in advance with a random number table by a hospital pharmacist not involved in the study" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "and treatment allocations were sealed in opaque envelopes. Investigators were blind to these allocations. Comment: probably done |
| Blinding (performance bias and detection bias) | Low risk | The study was unblinded‐;blinding would have been difficult, due to the different routes of administration. This is not likely to have affected outcome |
| Incomplete outcome data (attrition bias) | Low risk | Outcome data were available for all participants enrolled in the study |
| Selective reporting (reporting bias) | Unclear risk | All prespecified outcomes were reported in the Results section. In the Methods section, seizure cessation was defined as 'successful' if seizures stopped in < 5 minutes, 'successful but delayed' if seizures stopped after 5 ‐ 10 minutes and 'failure' if seizures had not stopped after 10 minutes. However, results seem to be presented only in terms of treatment success and failure. It is unclear if this is selective reporting of results |
| Other bias | Low risk | None identified |
| Methods | Prospective randomised study in 1 centre | |
| Participants | 70 children of both sexes and aged 2 months to 15 years presenting with an acute seizure to the emergency department. | |
| Interventions | intranasal midazolam versus intravenous diazepam | |
| Outcomes | Time from drug treatment to seizure cessation (Treatment successful if seizures stopped within 10 minutes) | |
| Notes | Both treatment arms showed a 100% seizure cessation rate, which is higher than expected | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed in advance with an odd and even number table" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "treatment allocations were sealed in opaque envelopes" Comment: probably done |
| Blinding (performance bias and detection bias) | Low risk | Study unblinded, but blinding would not have been possible, due to the different routes of administration of the 2 study drugs, but this is not likely to have affected outcome |
| Incomplete outcome data (attrition bias) | Low risk | All participants were included in the analysis |
| Selective reporting (reporting bias) | High risk | Time taken to insert intravenous cannula in the intravenous diazepam group should have been included, as this would have a significant effect on the time from arrival to seizure cessation. Other studies comparing intravenous with other routes have included this information |
| Other bias | Unclear risk | Both treatment arms showed a 100% seizure cessation rate, which is higher than expected. Unclear whether this high success rate was due to a particular element of the trial design |
| Methods | Multicentre randomised controlled trial over 3 years 4 months. Randomisation of 2 drugs in weekly blocks | |
| Participants | 177 children of both sexes aged 6 months to 16 years presenting to a children's accident and emergency department with active generalised tonic‐clonic seizures including established convulsive status epilepticus. Children with partial seizures or non‐convulsive status epilepticus were excluded. | |
| Interventions | Buccal midazolam versus rectal diazepam | |
| Outcomes | Seizure cessation without recurrence within 1 hour and without respiratory depression | |
| Notes | 219 convulsive episodes were recorded in the 177 children. Some results are reported only as the number of episodes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " weekly blocks of treatment were randomly selected for each of the four centres. The randomisation sequence was generated ...from a table of random numbers" Comment: probably done |
| Allocation concealment (selection bias) | High risk | Quote: "Allocation was not concealed from attending staff" |
| Blinding (performance bias and detection bias) | Low risk | Study unblinded, but blinding would not have been possible, due to the different routes of administration of the 2 study drugs. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "46 episodes were excluded". 46 episodes were screened for eligibility but did not meet criteria; all participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the Results section |
| Other bias | Unclear risk | None identified |
| Methods | Unblinded randomised trial | |
| Participants | 100 children with convulsive status epilepticus aged 1 month to 16 years | |
| Interventions | Intramuscular midazolam versus rectal diazepam | |
| Outcomes | Seizure cessation after drug administration without recurrence within 60 minutes | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Blinding (performance bias and detection bias) | Low risk | Study was unblinded, but this is not likely to have affected outcome |
| Incomplete outcome data (attrition bias) | Low risk | All participants were included in the analysis and analysed on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the Results section |
| Other bias | Unclear risk | None identified |
| Methods | Placebo‐controlled single‐blinded randomised study in 1 centre | |
| Participants | 330 children of both sexes and aged 3 months to 12 years who presented while convulsing or experienced a seizure lasting > 5 minutes to an emergency department in Uganda. Note 67.3% of children had malaria and 13.7% had cerebral malaria | |
| Interventions | Buccal midazolam versus rectal diazepam | |
| Outcomes | Cessation of visible seizure activity within 10 minutes, without recurrence in the subsequent hour Convulsion lasting > 10 minutes or recurring within 1 hour, defined as treatment failures Time to cessation of convulsions | |
| Notes | Study conducted in Africa with a high proportion of children with either cerebral malaria or meningitis. Consequently, not readily generalisable to western populations | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a computer was used to generate a list of sequential random treatment codes" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "each treatment code ... placed in a opaque envelope, sealed. Investigators were not aware of a patient's treatment allocation" Comment: probably done |
| Blinding (performance bias and detection bias) | Low risk | Quote; "Study drugs and placebo were pre‐packaged by a pharmacist not involved with patient care. " Comment: probably done Quote: "Although the study team were not aware which treatment a patient received they were aware of the treatment code, therefore we considered this single‐blinded" Comment: blinding probably adequate as each participant received placebo and study drug |
| Incomplete outcome data (attrition bias) | Low risk | Data analysed on an intention to treat basis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the Results section |
| Other bias | High risk | A high proportion of the children recruited had either cerebral malaria or meningitis. These co‐morbidities may have impacted upon the results. |
| Methods | Partly‐randomised prospective trial in a single centre over 1 year | |
| Participants | 115 children of both sexes aged 1 month to 12 years either presenting to the emergency department with acute convulsions or who developed acute seizures on the ward or PICU | |
| Interventions | intramuscular midazolam versus intravenous diazepam | |
| Outcomes | Mean time from administration of drug to cessation of seizures | |
| Notes | Not all participants were randomised; only those who were randomised are included in the results of this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients who already had an intravenous access present were treated with intravenous diazepam.. patients without an intravenous access were randomised into 2 groups" Comment: randomisation is inadequate, as treatment determined by presence of IV access which may introduce bias (patients not randomised are not included in the review) Method of randomisation of those without an IV access is unclear |
| Allocation concealment (selection bias) | High risk | No information about whether allocation in those without an IV access was concealed. Allocation definitely not concealed in those with an intravenous access |
| Blinding (performance bias and detection bias) | Low risk | Study unblinded, but blinding would not have been possible due to the different routes of administration of the 2 study drugs, but this is not likely to have affected outcome |
| Incomplete outcome data (attrition bias) | Low risk | All participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the Results section |
| Other bias | Low risk | None identified |
| Methods | Randomised prospective trial in a single centre | |
| Participants | 178 children of both sexes aged 1 ‐ 12 years presenting with convulsive status epilepticus (continuous convulsive activity for 5 minutes or more). | |
| Interventions | intravenous lorazepam versus intravenous diazepam‐phenytoin combination | |
| Outcomes | Cessation of seizure activity within 10 minutes and no recurrence over the subsequent 18 hours | |
| Notes | One child received lorazepam despite being randomised to diazepam‐phenytoin. This led to a difference in the number of participants in each group The study protocol states that where access could not be obtained, rectal lorazepam or diazepam would be used instead. The number of participants receiving rectal drugs should have been included in the paper,‐but was clarified through personal communication with the author who informed us that all drugs were given intravenously Both treatment arms showed a 100% seizure cessation rate, which is higher than expected | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Simple randomisation was done using a computer generated random number table" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation was by sealed envelope technique" Comment: probably done |
| Blinding (performance bias and detection bias) | Low risk | The study was unblinded but this is unlikely to have had a significant impact on the results |
| Incomplete outcome data (attrition bias) | Low risk | All participants were included in the analysis. One received lorazepam despite being randomised to diazepam‐phenytoin, i.e. was a protocol violation. Data were analysed on intention‐to‐treat basis. This is unlikely to have had a significant impact on the overall findings of the study |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the Results section |
| Other bias | Unclear risk | Both treatment arms showed a 100% seizure cessation rate, which is higher than expected. Unclear whether this high success rate was due to a particular element of the trial design |
| Methods | Prospective randomised trial in a single centre | |
| Participants | 120 children of both sexes aged 0 ‐ 12 years (mean age 3.2 years) presenting with an episode of convulsion, irrespective of cause and duration. | |
| Interventions | Buccal midazolam versus intravenous diazepam | |
| Outcomes | Cessation of all motor activity within or by 5 minutes of administration of the drug | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation was done using the random number table" Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to assess this |
| Blinding (performance bias and detection bias) | Low risk | Study unblinded, but blinding would not have been possible due to the different routes of administration of the 2 study drugs; this is not likely to have affected outcome |
| Incomplete outcome data (attrition bias) | Low risk | All participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the Results section |
| Other bias | Low risk | None identified |
IV: intravenous
PICU: paediatric intensive care unit
RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Only 38 of 100 participants in this study were under 16 years of age. In addition, this study examined the treatment of benzodiazepine‐refractory status epilepticus, whereas we are concerned with the treatment of children presenting with acute convulsive status epilepticus | |
| This study examined the management of refractory not acute status epilepticus | |
| Most seizures were simple partial seizures as opposed to generalised tonic‐clonic seizures. Study also included children with absence, myoclonic and atonic seizures | |
| The study examined drug management for the long‐term prevention of recurring febrile seizures, rather than management of acute convulsions | |
| This study examined diazepam treatment for clusters of seizures rather than acute convulsions | |
| The study examined drug management for the long‐term prevention of recurring febrile seizures, rather than management of acute convulsions | |
| This study compared intranasal midazolam and rectal diazepam for the treatment of seizures at home, not in a hospital‐based setting, so did not meet our inclusion criteria | |
| This was a study of the use of buccal midazolam for acute seizures in children, but without any comparison or placebo group | |
| This study examined the treatment of children already treated with IV diazepam, phenytoin and phenobarbital and whose seizures had lasted at least 60 minutes. The comparison was between rectal sodium valproate and intravenous midazolam. We excluded this study as it was examining the treatment of refractory status epilepticus | |
| This study was a prospective comparison of intravenous midazolam and lorazepam in 27 paediatric patients. However this was only published in abstract form as conference proceedings, so there was insufficient information on which to base assessment of the trial. Attempts to contact the authors were unsuccessful | |
| This study included children with refractory status epilepticus who were initially treated with intravenous diazepam and 2 doses of intravenous phenytoin, then randomised to either IV SVA or diazepam infusion. We excluded this study as it was examining the management of refractory not acute status epilepticus | |
| This study examined the management of refractory not acute status epilepticus | |
| This was a study of the use of intravenous valproate for acute seizures in children, but without any comparison or placebo group | |
| This was excluded as it was a retrospective audit of practice, comparing two different time periods when different seizure protocols were used. It did not meet our inclusion criteria of being a randomised, quasi‐randomised or controlled study | |
| This study examined the management of refractory not acute status epilepticus | |
| Quasi randomised study of rectal diazepam and buccal midazolam in treating 79 seizure episodes in 18 patients with severe and refractory epilepsy in a residential institution. The study does not make clear how many of the 11 paediatric patients had experienced a tonic‐clonic and not a complex partial or myoclonic seizure when treated with diazepam or midazolam. Only 11 of the 18 patients were aged 16 years or under | |
| This double‐blind, randomised study compared intramuscular midazolam with intravenous lorazepam for the pre‐hospital treatment of status epilepticus in children and adults. As the study did not take place in a hospital setting it did not meet our inclusion criteria. | |
| This study compared continuous midazolam or diazepam infusion in patients with refractory status epilepticus, defined as motor seizures uncontrolled after two doses of diazepam and a phenytoin infusion. We excluded this study as it concerned the management of refractory not acute status epilepticus | |
| The study examined drug management for the long‐term prevention of recurring febrile seizures, rather than management of acute convulsions. | |
| Less than 70% of participants had generalised tonic‐clonic seizures. We contacted the authors to request subgroup data but these were not supplied |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation Show forest plot | 3 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.98, 1.20] |
| Analysis 1.1  Comparison 1 Lorazepam versus diazepam, Outcome 1 Seizure cessation. | ||||
| 1.1 Intravenous | 3 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.94, 1.16] |
| 1.2 Rectal | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.86 [1.47, 5.55] |
| 2 Time from drug administration to stopping of seizures Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2 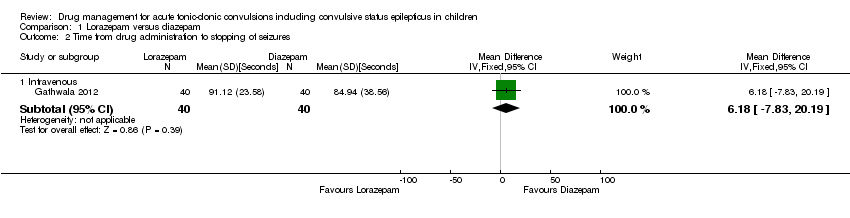 Comparison 1 Lorazepam versus diazepam, Outcome 2 Time from drug administration to stopping of seizures. | ||||
| 2.1 Intravenous | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 6.18 [‐7.83, 20.19] |
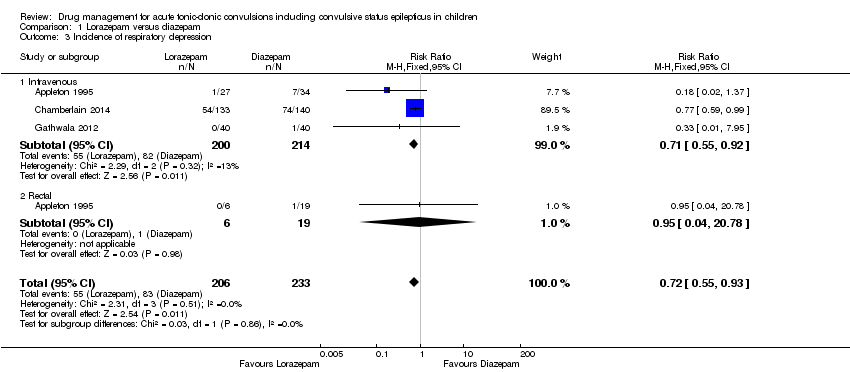
| 3 Incidence of respiratory depression Show forest plot | 3 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.55, 0.93] |
| Analysis 1.3  Comparison 1 Lorazepam versus diazepam, Outcome 3 Incidence of respiratory depression. | ||||
| 3.1 Intravenous | 3 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.55, 0.92] |
| 3.2 Rectal | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.04, 20.78] |
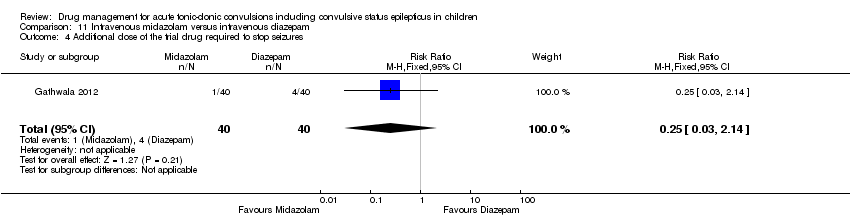
| 4 Additional dose of the trial drug required to stop seizures Show forest plot | 3 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.64, 1.20] |
| Analysis 1.4  Comparison 1 Lorazepam versus diazepam, Outcome 4 Additional dose of the trial drug required to stop seizures. | ||||
| 4.1 Intravenous | 3 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.71, 1.33] |
| 4.2 Rectal | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.56] |
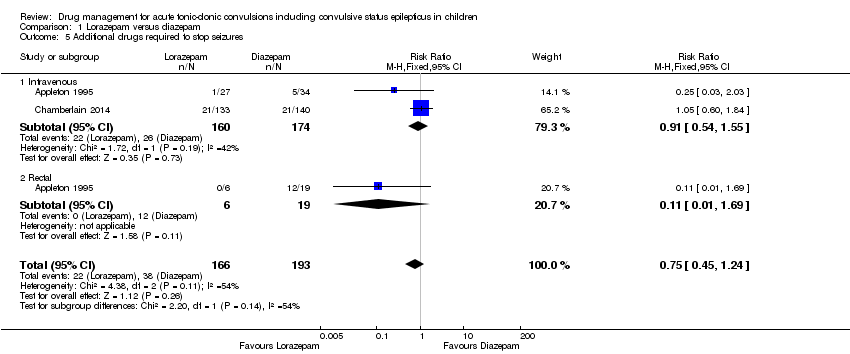
| 5 Additional drugs required to stop seizures Show forest plot | 2 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.45, 1.24] |
| Analysis 1.5  Comparison 1 Lorazepam versus diazepam, Outcome 5 Additional drugs required to stop seizures. | ||||
| 5.1 Intravenous | 2 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.54, 1.55] |
| 5.2 Rectal | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.69] |
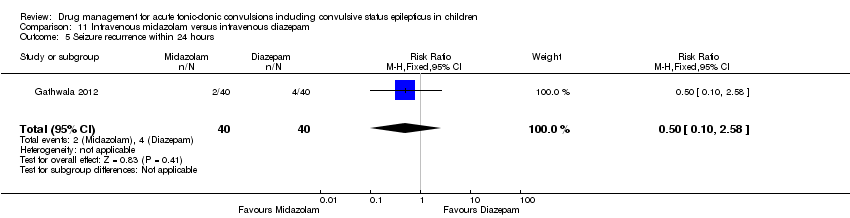
| 6 Seizure recurrence within 24 hours Show forest plot | 3 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.61, 1.20] |
| Analysis 1.6  Comparison 1 Lorazepam versus diazepam, Outcome 6 Seizure recurrence within 24 hours. | ||||
| 6.1 Intravenous | 3 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.65, 1.27] |
| 6.2 Rectal | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 2.92] |
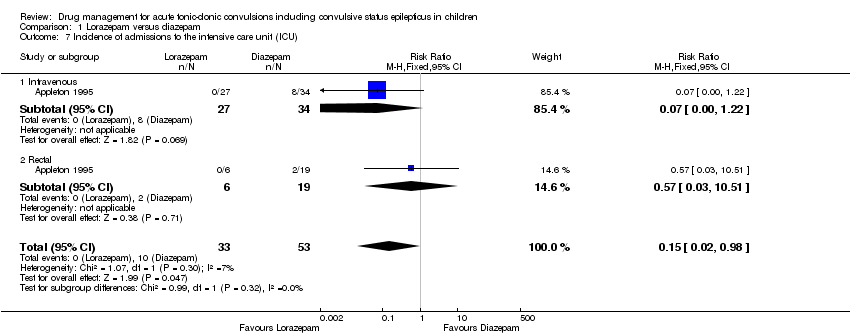
| 7 Incidence of admissions to the intensive care unit (ICU) Show forest plot | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 0.98] |
| Analysis 1.7  Comparison 1 Lorazepam versus diazepam, Outcome 7 Incidence of admissions to the intensive care unit (ICU). | ||||
| 7.1 Intravenous | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.22] |
| 7.2 Rectal | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.03, 10.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
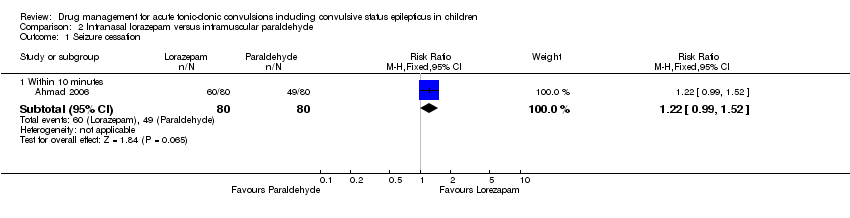
| 1 Seizure cessation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Intranasal lorazepam versus intramuscular paraldehyde, Outcome 1 Seizure cessation. | ||||
| 1.1 Within 10 minutes | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.99, 1.52] |
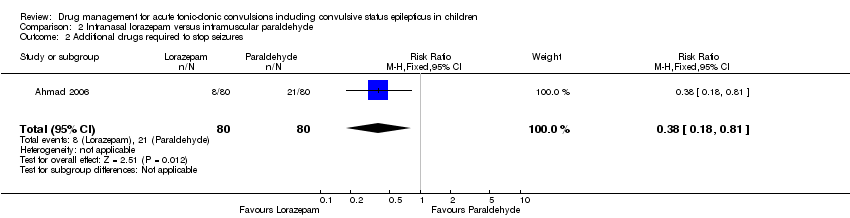
| 2 Additional drugs required to stop seizures Show forest plot | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.18, 0.81] |
| Analysis 2.2  Comparison 2 Intranasal lorazepam versus intramuscular paraldehyde, Outcome 2 Additional drugs required to stop seizures. | ||||
| 3 Seizure recurrence within 24 hours Show forest plot | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.31, 1.71] |
| Analysis 2.3  Comparison 2 Intranasal lorazepam versus intramuscular paraldehyde, Outcome 3 Seizure recurrence within 24 hours. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Intravenous lorazepam versus intravenous diazepam/intravenous phenytoin combination, Outcome 1 Seizure cessation. | ||||
| 1.1 Within 10 minutes | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.98, 1.02] |
| 2 Incidence of respiratory depression Show forest plot | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.22, 2.82] |
| Analysis 3.2  Comparison 3 Intravenous lorazepam versus intravenous diazepam/intravenous phenytoin combination, Outcome 2 Incidence of respiratory depression. | ||||
| 3 Additional drugs required to stop seizures Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.3 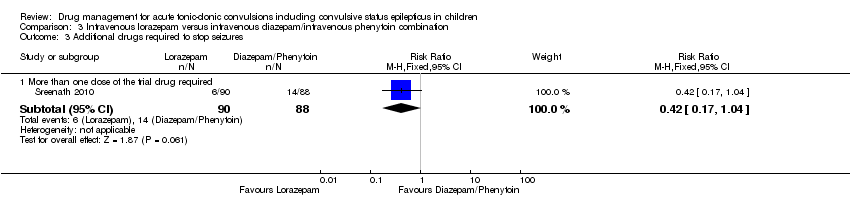 Comparison 3 Intravenous lorazepam versus intravenous diazepam/intravenous phenytoin combination, Outcome 3 Additional drugs required to stop seizures. | ||||
| 3.1 More than one dose of the trial drug required | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.17, 1.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4 Intravenous lorazepam versus intranasal lorazepam, Outcome 1 Seizure cessation. | ||||
| 1.1 Within 10 minutes | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.77, 1.49] |
| 1.2 Within 1 hour | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.43, 1.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation Show forest plot | 4 | 690 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.13, 1.38] |
| Analysis 5.1 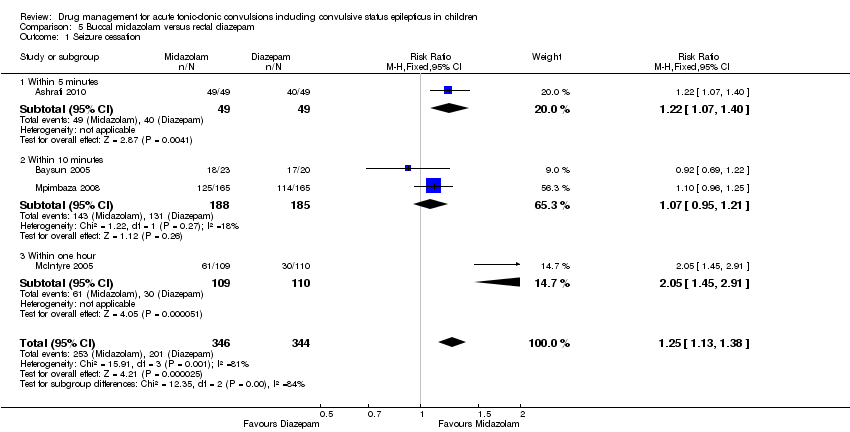 Comparison 5 Buccal midazolam versus rectal diazepam, Outcome 1 Seizure cessation. | ||||
| 1.1 Within 5 minutes | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.07, 1.40] |
| 1.2 Within 10 minutes | 2 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.95, 1.21] |
| 1.3 Within one hour | 1 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [1.45, 2.91] |
| 2 Incidence of respiratory depression Show forest plot | 4 | 690 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.61, 1.25] |
| Analysis 5.2  Comparison 5 Buccal midazolam versus rectal diazepam, Outcome 2 Incidence of respiratory depression. | ||||
| 3 Additional drugs required to stop seizures Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.3  Comparison 5 Buccal midazolam versus rectal diazepam, Outcome 3 Additional drugs required to stop seizures. | ||||
| 3.1 Intravenous lorazepam required | 1 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.42, 0.79] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation within five minutes Show forest plot | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.03] |
| Analysis 6.1  Comparison 6 Buccal midazolam versus intravenous diazepam, Outcome 1 Seizure cessation within five minutes. | ||||
| 2 Time from drug administration to stopping of seizures Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.2 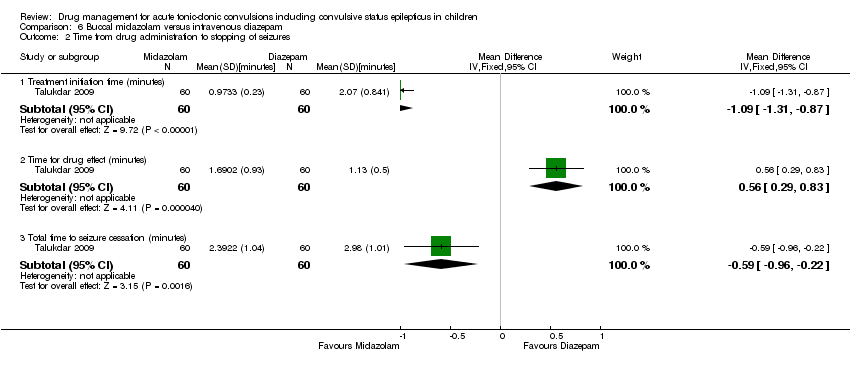 Comparison 6 Buccal midazolam versus intravenous diazepam, Outcome 2 Time from drug administration to stopping of seizures. | ||||
| 2.1 Treatment initiation time (minutes) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐1.09 [‐1.31, ‐0.87] |
| 2.2 Time for drug effect (minutes) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | 0.56 [0.29, 0.83] |
| 2.3 Total time to seizure cessation (minutes) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐0.96, ‐0.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
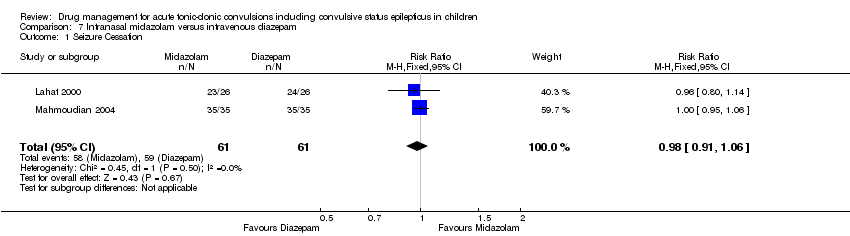
| 1 Seizure Cessation Show forest plot | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.06] |
| Analysis 7.1 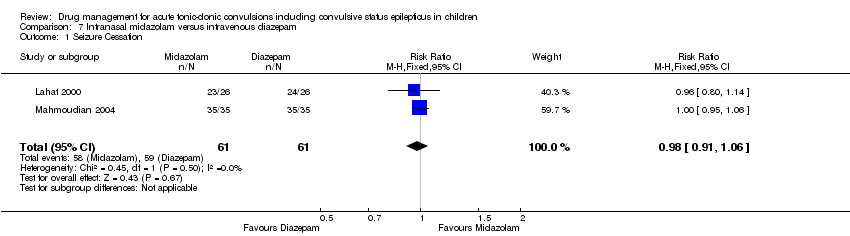 Comparison 7 Intranasal midazolam versus intravenous diazepam, Outcome 1 Seizure Cessation. | ||||
| 2 Time from drug administration to stopping of seizures [minutes] Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 7.2 ![Comparison 7 Intranasal midazolam versus intravenous diazepam, Outcome 2 Time from drug administration to stopping of seizures [minutes].](/cdsr/doi/10.1002/14651858.CD001905.pub3/media/CDSR/CD001905/image_n/nCD001905-CMP-007-02.png) Comparison 7 Intranasal midazolam versus intravenous diazepam, Outcome 2 Time from drug administration to stopping of seizures [minutes]. | ||||
| 2.1 Treatment initiation time (minutes) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐3.03, ‐0.97] |
| 2.2 Time for drug effect (minutes) | 2 | 122 | Mean Difference (IV, Fixed, 95% CI) | 0.62 [‐0.14, 1.38] |
| 2.3 Total time to seizure cessation (minutes) | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [0.24, 1.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 8.1  Comparison 8 Intranasal midazolam versus rectal diazepam, Outcome 1 Seizure cessation. | ||||
| 1.1 Within 10 minutes | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.00, 2.16] |
| 2 Additional drugs required to stop seizures Show forest plot | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.10, 1.03] |
| Analysis 8.2 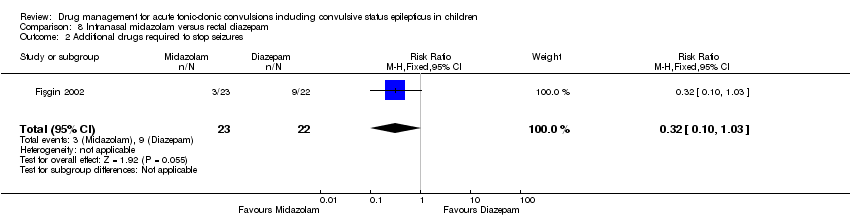 Comparison 8 Intranasal midazolam versus rectal diazepam, Outcome 2 Additional drugs required to stop seizures. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation Show forest plot | 2 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.87, 1.09] |
| Analysis 9.1 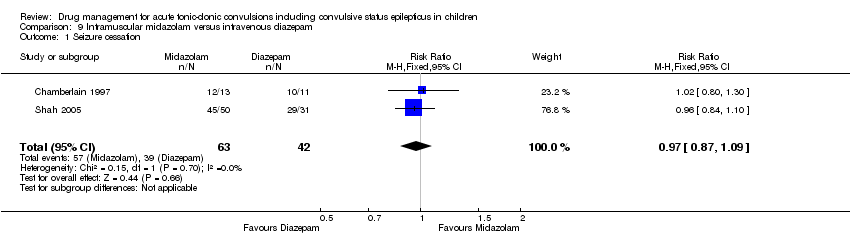 Comparison 9 Intramuscular midazolam versus intravenous diazepam, Outcome 1 Seizure cessation. | ||||
| 2 Time from drug administration to stopping of seizures (minutes) Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| Analysis 9.2  Comparison 9 Intramuscular midazolam versus intravenous diazepam, Outcome 2 Time from drug administration to stopping of seizures (minutes). | ||||
| 2.1 Treatment initiation time (minutes) | 1 | 24 | Mean Difference (Fixed, 95% CI) | ‐4.5 [‐6.68, ‐2.32] |
| 2.2 Time for drug effect (minutes) | 1 | 24 | Mean Difference (Fixed, 95% CI) | 1.1 [‐0.91, 3.11] |
| 2.3 Total time to seizure cessation (minutes) | 2 | 105 | Mean Difference (Fixed, 95% CI) | ‐2.68 [‐3.94, ‐1.42] |
| 3 Additional drugs required to stop seizures Show forest plot | 2 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.35, 5.13] |
| Analysis 9.3 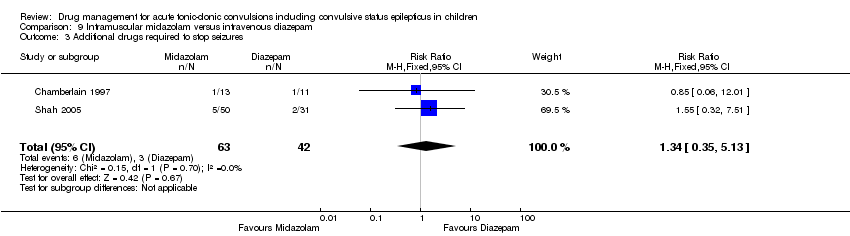 Comparison 9 Intramuscular midazolam versus intravenous diazepam, Outcome 3 Additional drugs required to stop seizures. | ||||
| 4 Seizure recurrence within 24 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 9.4 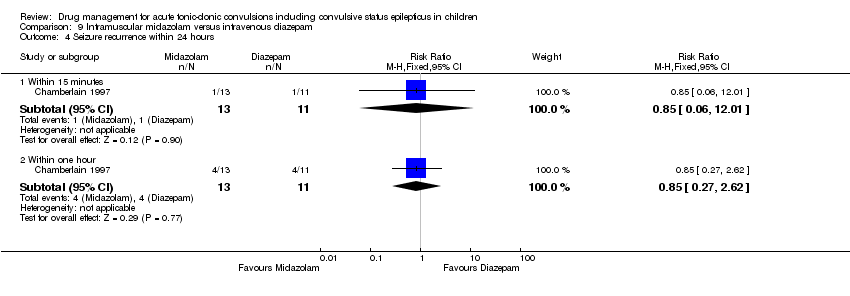 Comparison 9 Intramuscular midazolam versus intravenous diazepam, Outcome 4 Seizure recurrence within 24 hours. | ||||
| 4.1 Within 15 minutes | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.06, 12.01] |
| 4.2 Within one hour | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.27, 2.62] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
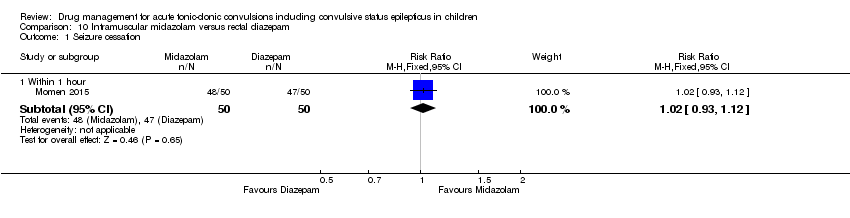
| 1 Seizure cessation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 10.1  Comparison 10 Intramuscular midazolam versus rectal diazepam, Outcome 1 Seizure cessation. | ||||
| 1.1 Within 1 hour | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.93, 1.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
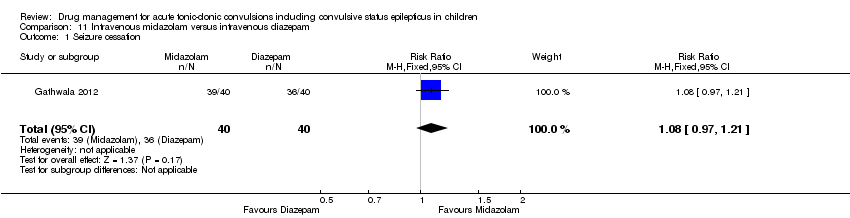
| 1 Seizure cessation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.97, 1.21] |
| Analysis 11.1 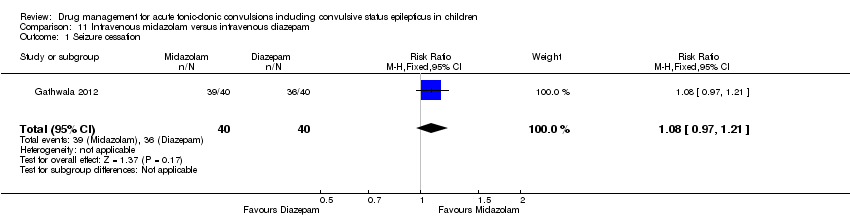 Comparison 11 Intravenous midazolam versus intravenous diazepam, Outcome 1 Seizure cessation. | ||||
| 2 Time from drug administration to stopping of seizures Show forest plot | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 7.68 [‐6.73, 22.09] |
| Analysis 11.2  Comparison 11 Intravenous midazolam versus intravenous diazepam, Outcome 2 Time from drug administration to stopping of seizures. | ||||
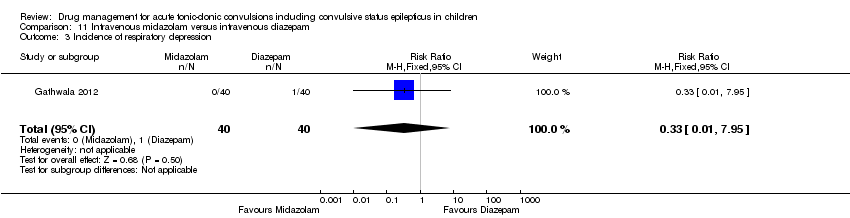
| 3 Incidence of respiratory depression Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| Analysis 11.3 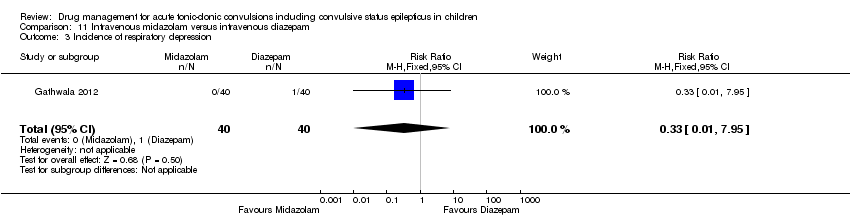 Comparison 11 Intravenous midazolam versus intravenous diazepam, Outcome 3 Incidence of respiratory depression. | ||||
| 4 Additional dose of the trial drug required to stop seizures Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.14] |
| Analysis 11.4  Comparison 11 Intravenous midazolam versus intravenous diazepam, Outcome 4 Additional dose of the trial drug required to stop seizures. | ||||
| 5 Seizure recurrence within 24 hours Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.10, 2.58] |
| Analysis 11.5 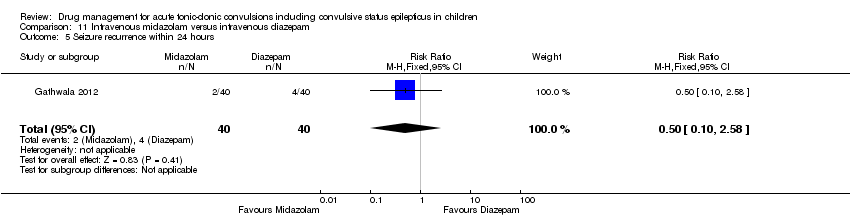 Comparison 11 Intravenous midazolam versus intravenous diazepam, Outcome 5 Seizure recurrence within 24 hours. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.04] |
| Analysis 12.1 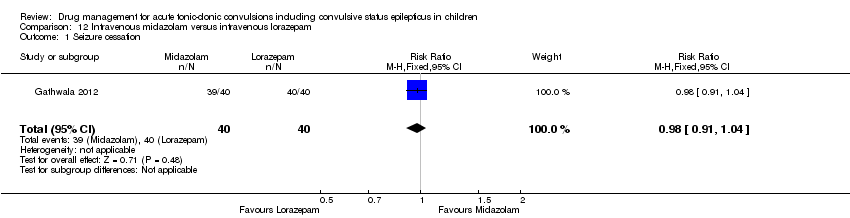 Comparison 12 Intravenous midazolam versus intravenous lorazepam, Outcome 1 Seizure cessation. | ||||
| 2 Time from drug administration to stopping of seizures Show forest plot | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 1.5 [‐9.37, 12.37] |
| Analysis 12.2 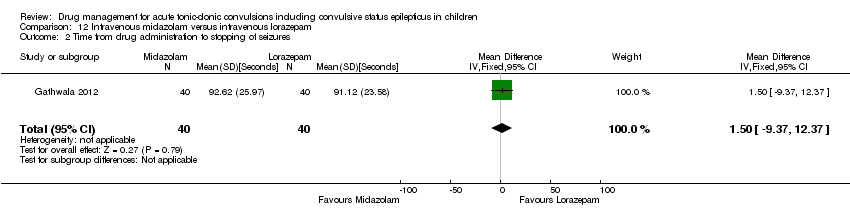 Comparison 12 Intravenous midazolam versus intravenous lorazepam, Outcome 2 Time from drug administration to stopping of seizures. | ||||
| 3 Additional dose of the trial drug required to stop seizures Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.51] |
| Analysis 12.3  Comparison 12 Intravenous midazolam versus intravenous lorazepam, Outcome 3 Additional dose of the trial drug required to stop seizures. | ||||
| 4 Seizure recurrence within 24 hours Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.76] |
| Analysis 12.4 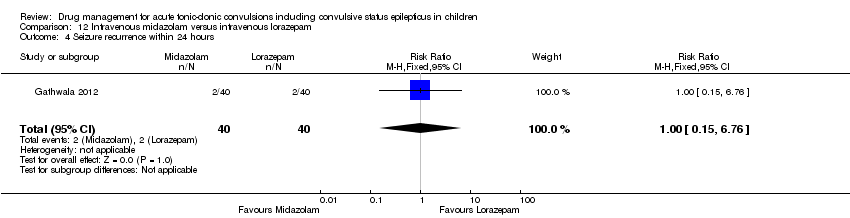 Comparison 12 Intravenous midazolam versus intravenous lorazepam, Outcome 4 Seizure recurrence within 24 hours. | ||||

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
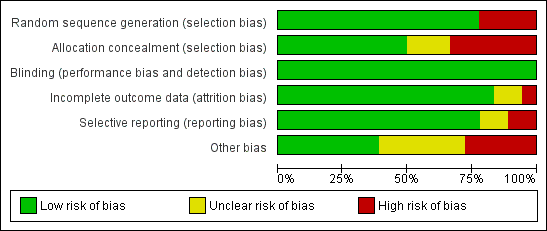
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Lorazepam versus diazepam, Outcome 1 Seizure cessation.

Comparison 1 Lorazepam versus diazepam, Outcome 2 Time from drug administration to stopping of seizures.
Comparison 1 Lorazepam versus diazepam, Outcome 3 Incidence of respiratory depression.

Comparison 1 Lorazepam versus diazepam, Outcome 4 Additional dose of the trial drug required to stop seizures.
Comparison 1 Lorazepam versus diazepam, Outcome 5 Additional drugs required to stop seizures.

Comparison 1 Lorazepam versus diazepam, Outcome 6 Seizure recurrence within 24 hours.
Comparison 1 Lorazepam versus diazepam, Outcome 7 Incidence of admissions to the intensive care unit (ICU).
Comparison 2 Intranasal lorazepam versus intramuscular paraldehyde, Outcome 1 Seizure cessation.
Comparison 2 Intranasal lorazepam versus intramuscular paraldehyde, Outcome 2 Additional drugs required to stop seizures.

Comparison 2 Intranasal lorazepam versus intramuscular paraldehyde, Outcome 3 Seizure recurrence within 24 hours.

Comparison 3 Intravenous lorazepam versus intravenous diazepam/intravenous phenytoin combination, Outcome 1 Seizure cessation.

Comparison 3 Intravenous lorazepam versus intravenous diazepam/intravenous phenytoin combination, Outcome 2 Incidence of respiratory depression.
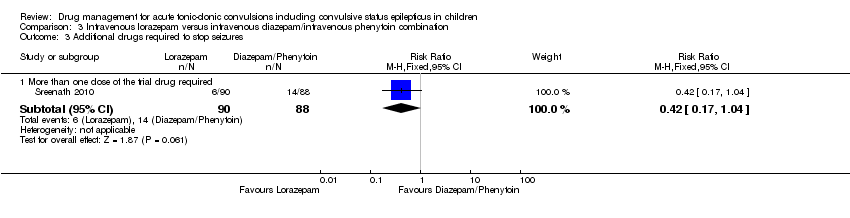
Comparison 3 Intravenous lorazepam versus intravenous diazepam/intravenous phenytoin combination, Outcome 3 Additional drugs required to stop seizures.
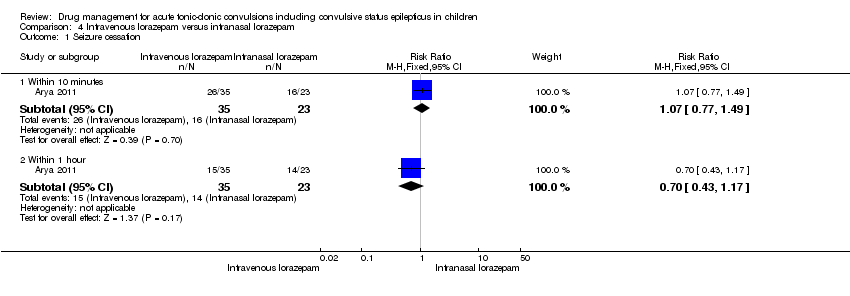
Comparison 4 Intravenous lorazepam versus intranasal lorazepam, Outcome 1 Seizure cessation.

Comparison 5 Buccal midazolam versus rectal diazepam, Outcome 1 Seizure cessation.

Comparison 5 Buccal midazolam versus rectal diazepam, Outcome 2 Incidence of respiratory depression.

Comparison 5 Buccal midazolam versus rectal diazepam, Outcome 3 Additional drugs required to stop seizures.
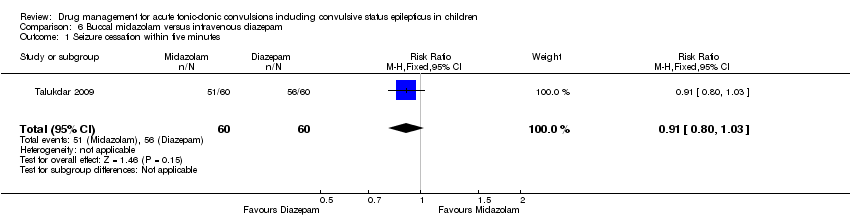
Comparison 6 Buccal midazolam versus intravenous diazepam, Outcome 1 Seizure cessation within five minutes.

Comparison 6 Buccal midazolam versus intravenous diazepam, Outcome 2 Time from drug administration to stopping of seizures.
Comparison 7 Intranasal midazolam versus intravenous diazepam, Outcome 1 Seizure Cessation.
![Comparison 7 Intranasal midazolam versus intravenous diazepam, Outcome 2 Time from drug administration to stopping of seizures [minutes].](/es/cdsr/doi/10.1002/14651858.CD001905.pub3/media/CDSR/CD001905/image_n/nCD001905-CMP-007-02.png)
Comparison 7 Intranasal midazolam versus intravenous diazepam, Outcome 2 Time from drug administration to stopping of seizures [minutes].

Comparison 8 Intranasal midazolam versus rectal diazepam, Outcome 1 Seizure cessation.

Comparison 8 Intranasal midazolam versus rectal diazepam, Outcome 2 Additional drugs required to stop seizures.

Comparison 9 Intramuscular midazolam versus intravenous diazepam, Outcome 1 Seizure cessation.

Comparison 9 Intramuscular midazolam versus intravenous diazepam, Outcome 2 Time from drug administration to stopping of seizures (minutes).

Comparison 9 Intramuscular midazolam versus intravenous diazepam, Outcome 3 Additional drugs required to stop seizures.

Comparison 9 Intramuscular midazolam versus intravenous diazepam, Outcome 4 Seizure recurrence within 24 hours.
Comparison 10 Intramuscular midazolam versus rectal diazepam, Outcome 1 Seizure cessation.
Comparison 11 Intravenous midazolam versus intravenous diazepam, Outcome 1 Seizure cessation.

Comparison 11 Intravenous midazolam versus intravenous diazepam, Outcome 2 Time from drug administration to stopping of seizures.
Comparison 11 Intravenous midazolam versus intravenous diazepam, Outcome 3 Incidence of respiratory depression.
Comparison 11 Intravenous midazolam versus intravenous diazepam, Outcome 4 Additional dose of the trial drug required to stop seizures.
Comparison 11 Intravenous midazolam versus intravenous diazepam, Outcome 5 Seizure recurrence within 24 hours.

Comparison 12 Intravenous midazolam versus intravenous lorazepam, Outcome 1 Seizure cessation.

Comparison 12 Intravenous midazolam versus intravenous lorazepam, Outcome 2 Time from drug administration to stopping of seizures.

Comparison 12 Intravenous midazolam versus intravenous lorazepam, Outcome 3 Additional dose of the trial drug required to stop seizures.

Comparison 12 Intravenous midazolam versus intravenous lorazepam, Outcome 4 Seizure recurrence within 24 hours.
| Lorazepam compared with diazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Lorazepam Comparison: Diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Diazepam | Lorazepam | |||||
| Seizure cessation Follow‐up: up to 24 hours | 708 per 1000 | 765 per 1000 | RR 1.08 (0.98 to 1.20) | 439 | ⊕⊕⊝⊝ | In two trials, drugs were administered intravenously. In a third trial, drugs were administered intravenously or rectally if intravenous access was not possible Subgroup analysis showed a significant difference by route of intervention (intravenous: RR 1.04 (95% CI 0.94 to 1.16) compared to rectally RR: 2.86 (95% CI 1.47 to 5.55), test of subgroups P = 0.003) |
| Time from drug administration to termination of seizures Follow‐up: up to 24 hours | The mean time to cessation of seizures was 84.94 seconds in the diazepam group | The mean time to cessation of seizures was 6.18 faster (7.83 slower to 20.19 faster) in the lorazepam group | NA | 80 | ⊕⊕⊕⊝ | Drugs were administered intravenously Another trial (where drugs were administered intravenously or rectally) reported similar mean times to seizure cessation. Standard deviations were not available so data could not be entered into analysis |
| Incidence of respiratory depression Follow‐up: up to 24 hours | 356 per 1000 | 256 per 1000 | RR 0.72 (0.55 to 0.93) | 439 | ⊕⊕⊕⊝ | In two trials, drugs were administered intravenously. In a third trial, drugs were administered intravenously or rectally if intravenous access was not possible There was no difference between the routes of intervention (test of subgroups, P = 0.86) |
| Additional drugs required to terminate the seizure: additional dose of study drug Follow‐up: up to 24 hours | 305 per 1000 | 268 per 1000 | RR 0.88 (0.64 to 1.20) | 439 | ⊕⊕⊝⊝ | In two trials, drugs were administered intravenously. In a third trial, drugs were administered intravenously or rectally if intravenous access was not possible Subgroup analysis by route of intervention (intravenous: RR 0.97 (95% CI 0.71 to 1.33) compared to rectally RR: 0.11 (95% CI 0.01 to 1.56), test of subgroups P = 0.11). Two trials also reported whether additional (other) antiepileptic drugs were required to stop the seizure. There were no significant differences overall or by route of intervention |
| Seizure recurrence within 24 hours Follow‐up: up to 24 hours | 266 per 1000 | 229 per 1000 | RR 0.86 (0.61 to 1.20) | 439 | ⊕⊕⊕⊝ | In two trials, drugs were administered intravenously. In a third trial, drugs were administered intravenously or rectally if intravenous access was not possible There was no difference between the routes of intervention (test of subgroups, P = 0.27) |
| Incidence of admissions to the ICU Follow‐up: up to 24 hours | 116 per 1000 | 17 per 1000 | RR 0.15 (0.02 to 0.98) | 86 | ⊕⊕⊝⊝ | In the included trial, drugs were administered intravenously or rectally if intravenous access was not possible There was no difference between the routes of intervention (test of subgroups P = 0.32). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to risk of bias: one included study was quasi‐randomised, which may have led to selection bias and an intention‐to‐treat approach was not used in the study. | ||||||
| Intranasal lorazepam compared with intramuscular paraldehyde for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intranasal lorazepam Comparison: Intramuscular paraldehyde | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intramuscular paraldehyde | Intranasal lorazepam | |||||
| Seizure cessation: within 10 minutes Follow‐up: up to 24 hours | 613 per 1000 | 747 per 1000 | RR 1.22 (0.99 to 1.52) | 160 (1 study) | ⊕⊕⊕⊝ | ‐ |
| Time from drug administration to termination of seizures Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Incidence of respiratory depression Follow‐up: up to 24 hours | No difference was found between either treatment group in terms of clinically important cardiorespiratory events. | NA | 160 (1 study) | ⊕⊕⊝⊝ | ‐ | |
| Additional drugs required to terminate the seizure: 2 or more additional anticonvulsants required Follow‐up: up to 24 hours | 263 per 1000 | 100 per 1000 | RR 0.38 (0.18 to 0.81) | 160 (1 study) | ⊕⊕⊝⊝ | ‐ |
| Seizure recurrence within 24 hours Follow‐up: up to 24 hours | 138 per 1000 | 100 per 1000 | RR 0.73 (0.31 to 1.71) | 160 (1 study) | ⊕⊕⊝⊝ | ‐ |
| Incidence of admissions to the ICU Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to applicability: a high proportion of the children recruited had either cerebral malaria or meningitis. These comorbidities may have impacted upon the results. | ||||||
| Intravenous lorazepam compared with intravenous diazepam/intravenous phenytoin combination for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intravenous lorazepam Comparison: Intravenous diazepam/intravenous phenytoin combination | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous diazepam/intravenous phenytoin combination | Intravenous lorazepam | |||||
| Seizure cessation: within 10 minutes Follow‐up: up to 24 hours | Seizures were stopped for all individuals in the Intravenous diazepam/intravenous phenytoin combination group | Seizures were stopped for all individuals in the Intravenous lorazepam group | RR 1.00 (0.98 to 1.02) | 178 | ⊕⊕⊕⊝ | ‐ |
| Time from drug administration to stopping of seizures Follow‐up: up to 24 hours | There was no significant difference in the median time to seizure cessation (20 seconds in each group). | NA | 178 | ⊕⊕⊕⊝ | ‐ | |
| Incidence of respiratory depression Follow‐up: up to 24 hours | 57 per 1000 | 44 per 1000 (13 to 160) | RR 0.78 (0.22 to 2.82) | 178 | ⊕⊕⊕⊝ | ‐ |
| Additional drugs required to stop the seizure Follow‐up: up to 24 hours | 159 per 1000 | 67 per 1000 (27 to 165) | RR 0.42 (0.17 to 1.04) | 178 | ⊕⊕⊕⊝ | ‐ |
| Seizure recurrence within 24 hours Follow‐up: up to 24 hours | There were no seizure recurrences in either group. | NA | 178 | ⊕⊕⊕⊝ | ‐ | |
| Incidence of admissions to the ICU Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due toapplicability: Both treatment arms showed a 100% seizure cessation rate, which is higher than expected. Unclear whether this high success rate was due to a particular element of the trial design. | ||||||
| Intravenous lorazepam compared with intranasal lorazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intravenous lorazepam Comparison: Intranasal lorazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intranasal lorazepam | Intravenous lorazepam | |||||
| Seizure cessation: within 10 minutes Follow‐up: up to 24 hours | 696 per 1000 | 744 per 1000 | RR 1.07 (0.77 to 1.49) | 58 | ⊕⊕⊕⊝ | There was also no significant difference between treatments for seizure cessation at 1 hour: RR 0.70 (95% CI 0.43 to 1.17) |
| Time from drug administration to stopping of seizures Follow‐up: up to 24 hours | Median time to achieve seizure control from drug administration was 4 minutes in both groups. | NA | 58 | ⊕⊕⊕⊝ | Time taken to achieve intravenous access ranged from 1 to 25 minutes with a median of 4 minutes across all participants in the trial. If this had been included in the response time for the intravenous lorazepam, the results would have been skewed significantly in favour of intranasal lorazepam | |
| Incidence of respiratory depression Follow‐up: up to 24 hours | One child required respiratory support | Two children required respiratory support | NA | 141 | ⊕⊕⊕⊝ | Incidence of respiratory depression was not reported for the subgroup of participants with generalised tonic‐clonic seizures in the trial, therefore these results refer to all participants (including 83 participants without generalised tonic‐clonic seizures). |
| Additional drugs required to stop the seizure Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Seizure recurrence within 24 hours Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Incidence of admissions to the ICU Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded once due to imprecision: imbalance in the number of participants randomised to each intervention with generalised tonic‐clonic seizures and overall direction of effect seems to change when measured at 10 minutes or at 1 hour 2Downgraded once due to imprecision: limited numerical data reported. | ||||||
| Buccal midazolam compared with rectal diazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Buccal midazolam Comparison: Rectal diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Rectal diazepam | Buccal midazolam | |||||
| Seizure cessation: within 5 minutes to 1 hour Follow‐up: up to 24 hours | 584 per 1000 | 730 per 1000 | RR 1.25 (1.13 to 1.38) | 648 (4 trials) 690 seizure episodes | ⊕⊝⊝⊝ | The measurement time of seizure cessation was examined in a subgroup analysis 5 minutes: RR 1.22 (95% CI 1.07 to 1.40, P = 0.004); |
| Time from drug administration to of seizures Follow‐up: up to 24 hours | One trial found no difference between groups in the time from drug administration to seizure cessation One trial reported that both the median treatment initiation time and drug effect time were significantly shorter in the buccal midazolam group than the rectal diazepam group. | NA | 141 (2 trials) | ⊕⊕⊝⊝ | No numerical data presented for either trial | |
| Incidence of respiratory depression Follow‐up: up to 24 hours | 76 per 1000 | 67 per 1000 (46 to 94) | RR 0.88 (0.61 to 1.25) | 648 (4 trials) 690 seizure episodes | ⊕⊕⊝⊝ | ‐ |
| Additional drugs required to stop the seizure: intravenous lorazepam required Follow‐up: up to 24 hours | 573 per 1000 | 332 per 1000 | RR 0.58 (0.42 to 0.79) | 177 (1 trial) 219 seizure episodes | ⊕⊕⊝⊝ | A second trial reported that there was no difference between groups in the need for a second drug |
| Seizure recurrence within 24 hours Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Incidence of admissions to the ICU Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to risk of bias: one included study was quasi‐randomised and one study did not conceal allocation. Both of these studies were at risk of selection bias. | ||||||
| Buccal midazolam compared with intravenous diazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Buccal midazolam Comparison: Intravenous diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous diazepam | Buccal midazolam | |||||
| Seizure cessation Follow‐up: up to 24 hours | 933 per 1000 | 849 per 1000 | RR 0.91 (0.80 to 1.03) | 120 (1 trial) | ⊕⊕⊕⊕ | ‐ |
| Time from drug administration to termination of seizures Follow‐up: up to 24 hours | The mean time to cessation of seizures was 1.13 minutes in the intravenous diazepam group. | The mean time to cessation of seizures was 0.56 minutes higher in the buccal diazepam group (0.29 to 0.83 minutes higher). | NA | 120 (1 trial) | ⊕⊕⊕⊝ | The mean time for initiation of treatment was significantly shorter in the buccal midazolam group (MD ‐1.09 minutes, 95% CI ‐1.31 to ‐0.87) and therefore the mean total time to controlling the seizures was significantly shorter in the buccal midazolam group compared to the intravenous diazepam group (MD ‐0.59, 95% CI ‐0.96 to ‐0.22) |
| Incidence of respiratory depression Follow‐up: up to 24 hours | There were no adverse events in either group | NA | 120 (1 trial) | ⊕⊕⊕⊕ | ‐ | |
| Additional drugs required to stop the seizure Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Seizure recurrence within 24 hours Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Incidence of admissions to the ICU Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to applicability: the route of intervention of the drug has been shown to influence the outcome. | ||||||
| Intranasal midazolam compared with intravenous diazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intranasal midazolam Comparison: Intravenous diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous diazepam | Intranasal midazolam | |||||
| Seizure cessation Follow‐up: up to 24 hours | 967 per 1000 | 948 per 1000 | RR 0.98 (0.91 to 1.06) | 122 (2 trials) | ⊕⊕⊕⊝ | ‐ |
| Time from drug administration to stopping of seizures Follow‐up: up to 24 hours | The mean time to cessation of seizures ranged from 2.5 to 2.94 minutes in the intravenous diazepam group. | The mean time to cessation of seizures was 0.62 minutes higher in the intranasal midazolam group (0.14 lower to 1.38 minutes higher). | NA | 122 (2 trials) | ⊕⊕⊕⊝ | One trial reports that the time for initiation of treatment was significantly shorter in the intranasal midazolam group (MD ‐2.00 minutes, 95% CI ‐3.03 to ‐0.97). The other trial also reports that time for initiation of treatment was significantly shorter in the intranasal midazolam group but does not account for this in analysis |
| Incidence of respiratory depression Follow‐up: up to 24 hours | No adverse events including respiratory depression occurred in either group. | NA | 122 (2 trials) | ⊕⊕⊕⊕ | ‐ | |
| Additional drugs required to stop the seizure Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Seizure recurrence within 24 hours Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Incidence of admissions to the ICU Follow‐up: up to 24 hours | There were no admissions to the ICU in either group | NA | 52 (1 trial) | ⊕⊕⊕⊕ | ‐ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to risk of bias: one of the studies included in this comparison did not report this outcome. As this is an expected outcome, this may be selective reporting. Additionally, in one trial both treatment arms showed a 100% seizure cessation rate, which is higher than expected. Unclear whether this high success rate was due to a particular element of the trial design. | ||||||
| Intranasal midazolam compared with rectal diazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intranasal midazolam Comparison: Rectal diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Rectal diazepam | Intranasal midazolam | |||||
| Seizure cessation: within 10 minutes Follow‐up: up to 24 hours | 591 per 1000 | 869 per 1000 | RR 1.47 (1.00 to 2.16) | 45 | ⊕⊕⊝⊝ | ‐ |
| Time from drug administration to termination of seizures Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Incidence of respiratory depression Follow‐up: | There was no significant difference between the two groups for of cardiorespiratory or adverse effects. | NA | 45 | ⊕⊕⊝⊝ | No numerical data reported | |
| Additional drugs required to stop the seizure Follow‐up: up to 24 hours | 409 per 1000 | 131 per 1000 (41 to 421) | RR 0.32 (0.10 to 1.03) | 45 | ⊕⊕⊝⊝ | ‐ |
| Seizure recurrence within 24 hours Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Incidence of admissions to the ICU Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to risk of bias: one included study was quasi‐randomised, which may have led to selection bias. Additionally, the description of the seizure type and aetiology of the included children was unclear, so it is unclear if the population of this study is generalisable. | ||||||
| Intramuscular midazolam compared with intravenous diazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intramsucular midazolam Comparison: Intravenous diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous diazepam | Intramsucular midazolam | |||||
| Seizure cessation Follow‐up: up to 24 hours | 929 per 1000 | 901 per 1000 | RR 0.97 (0.87 to 1.09) | 105 | ⊕⊕⊝⊝ | ‐ |
| Time from drug administration to stopping of seizures: total time to seizure cessation Follow‐up: up to 24 hours | The mean total time to cessation of seizures was 2.68 minutes lower (3.94 to 1.42 minutes lower) in the intramuscular midazolam group compared to the intravenous diazepam group | NA | 105 | ⊕⊝⊝⊝ | One trial also showed that the initiation of treatment was significantly shorter in the intramuscular midazolam group (MD ‐4.50 minutes (‐6.68 to ‐2.32)) but there was no significant difference between treatments for the time to drug effect (MD 1.10 minutes (95% CI ‐0.91 to 3.11) | |
| Incidence of respiratory depression Follow‐up: up to 24 hours | There were no adverse events or complications in either trial | NA | 105 | ⊕⊕⊝⊝ | ‐ | |
| Additional drugs required to terminate the seizure Follow‐up: up to 24 hours | 71 per 1000 | 96 per 1000 | RR 1.34 (0.35 to 5.13) | 105 | ⊕⊝⊝⊝ | ‐ |
| Seizure recurrence within 24 hours: within one hour Follow‐up: up to 24 hours | 364 per 1000 | 309 per 1000 | RR 0.85 (0.27 to 2.62) | 24 (1 trial) | ⊕⊝⊝⊝ | There was also no significant difference between treatments at within 15 minutes (RR: 0.85 (95% CI 0.06,to12.01) |
| Incidence of admissions to the ICU Follow‐up: up to 24 hours | There were no admissions to the ICU | NA | 81 (1 trial) | ⊕⊕⊕⊝ | ‐ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to risk of bias: in both included trials, methods of randomisation were unclear so the trials may be at risk of selection bias. | ||||||
| Intramuscular midazolam compared with rectal diazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intramuscular midazolam Comparison: Rectal diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Rectal diazepam | Intramuscular midazolam | |||||
| Seizure cessation Follow‐up: up to 24 hours | 940 per 1000 | 959 per 1000 | RR 1.02 (0.93 to 1.12) | 100 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Time from drug administration to stopping of seizures Follow‐up: up to 24 hours | There was a significant difference in time from administration to seizure cessation in favour of midazolam (median 66 seconds, diazepam, median 130 seconds, P < 0.001) | NA | 100 (1 trial) | ⊕⊕⊕⊝ | It is noted that the speed of administration was similarly fast for both medications, so this seems to reflect a medication difference. | |
| Incidence of respiratory depression Follow‐up: up to 24 hours | No patients developed respiratory depression except for one patient who received an accidental double dose of intramuscular midazolam. | NA | 100 (1 trial) | ⊕⊕⊕⊝ | ‐ | |
| Additional drugs required to stop the seizure Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Seizure recurrence within 24 hours Follow‐up: up to 24 hours | Among those with seizures terminated, there were no recurrences at 24 hours | NA | 100 (1 trial) | ⊕⊕⊕⊝ | ||
| Incidence of admissions to the ICU Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to risk of bias: the included study did not conceal allocation so is at risk of selection bias. | ||||||
| Intravenous midazolam compared with intravenous diazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intravenous midazolam Comparison: Intravenous diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous diazepam | Intravenous midazolam | |||||
| Seizure cessation Follow‐up: up to 24 hours | 900 per 1000 | 972 per 1000 | RR 1.08 (0.97 to 1.21) | 80 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Time from drug administration to stopping of seizures Follow‐up: up to 24 hours | The mean time to cessation of seizures was 84.94 seconds in the intravenous diazepam group. | The mean time to cessation of seizures was 7.68 seconds higher in the intravenous midazolam group (6.73 seconds lower to 22.09 seconds higher) . | NA | 80 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Incidence of respiratory depression Follow‐up: up to 24 hours | 25 per 1000 | 8 per 1000 | RR 0.33 (0.01 to 7.95) | 80 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Additional drugs required to stop the seizure: additional dose of the trial drug required Follow‐up: up to 24 hours | 100 per 1000 | 25 per 1000 | RR 0.25 (0.03 to 2.14) | 80 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Seizure recurrence within 24 hours Follow‐up: up to 24 hours | 100 per 1000 | 50 per 1000 | RR 0.50 (0.10 to 2.58) | 80 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Incidence of admissions to the ICU Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to risk of bias: the definition of the 'seizure cessation' outcome is not an appropriate criterion for judging seizure cessation. This definition is likely to have impacted upon results. | ||||||
| Intravenous midazolam compared with intravenous lorazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intravenous midazolam Comparison: Intravenous lorazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous lorazepam | Intravenous midazolam | |||||
| Seizure cessation Follow‐up: up to 24 hours | Seizures were terminated for all children in the Intravenous lorazepam group | Seizures were terminated for 39 out of 40 children in the intravenous midazolam group | RR 0.98 (0.91 to 1.04) | 80 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Time from drug administration to termination of seizures Follow‐up: up to 24 hours | The mean time to cessation of seizures was 91.12 seconds in the intravenous lorazepam group. | The mean time to cessation of seizures was 1.50 seconds higher in the intravenous midazolam group (9.37 seconds lower to 12.37 seconds higher) . | NA | 80 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Incidence of respiratory depression Follow‐up: up to 24 hours | There were no occurrences of respiratory depression in either group | NA | 80 (1 trial) | ⊕⊕⊕⊕ | ‐ | |
| Additional drugs required to terminate the seizure: additional dose of the trial drug required Follow‐up: up to 24 hours | No children in the intravenous lorazepam group required an additional dose of the trial drug. | One child in the intravenous midazolam group required an additional dose of the trial drug. | RR 3.00 (0.13 to 71.51) | 80 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Seizure recurrence within 24 hours Follow‐up: up to 24 hours | 50 per 1000 | 50 per 1000 | RR 1.00 (0.15 to 6.76) | 80 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Incidence of admissions to the ICU Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to risk of bias: the definition of the 'seizure cessation' outcome is not an appropriate criterion for judging seizure cessation. This definition is likely to have impacted upon results. | ||||||
| Study | Drug | Seizure cessation | Respiratory Depression | Additional drugs required | ||||||
| No. of Events | No. of Children | % | No. of Events | No. of Children | % | No. of Events | No. of Children | % | ||
| IN lorazepam | 60 | 80 | 75 | 0 | 80 | 0 | 8 | 80 | 10 | |
| IM paraldehyde | 49 | 80 | 60 | 0 | 80 | 0 | 21 | 80 | 26 | |
| IV lorazepam | 19 | 27 | 70 | 1 | 27 | 4 | 1 | 27 | 4 | |
| Rectal lorazepam | 6 | 6 | 100 | 0 | 6 | 0 | 0 | 6 | 0 | |
| IV diazepam | 22 | 34 | 65 | 7 | 34 | 21 | 5 | 34 | 15 | |
| Rectal diazepam | 6 | 19 | 32 | 1 | 19 | 5 | 12 | 19 | 63 | |
| IN lorazepam | 16 | 23 | 70 | 1 | 71 | 1 | NR | 23 | NA | |
| IV lorazepam | 26 | 35 | 74 | 2 | 70 | 3 | NR | 35 | NA | |
| Buccal midazolam | 49 | 49 | 100 | 0 | 49 | 0 | 0 | 49 | 0 | |
| Rectal diazepam | 40 | 49 | 82 | 0 | 49 | 0 | 9 | 49 | 18 | |
| Buccal midazolam | 18 | 23 | 78 | 0 | 23 | 0 | 5 | 23 | 22 | |
| Rectal diazepam | 17 | 20 | 85 | 1 | 20 | 5 | 3 | 20 | 15 | |
| IM midazolam | 12 | 13 | 92 | 0 | 13 | 0 | 1 | 13 | 8 | |
| IV diazepam | 10 | 11 | 91 | 0 | 11 | 0 | 1 | 11 | 9 | |
| IV diazepam | 101 | 140 | 72 | 26 | 140 | 16 | 21 | 140 | 15 | |
| IV lorazepam | 97 | 133 | 73 | 26 | 133 | 18 | 21 | 133 | 16 | |
| IN midazolam | 20 | 23 | 87 | 0 | 23 | 0 | 3 | 23 | 13 | |
| Rectal diazepam | 13 | 22 | 60 | 0 | 22 | 0 | 9 | 22 | 40 | |
| IV diazepam | 36 | 40 | 90 | 1 | 40 | 3 | 4 | 40 | 10 | |
| IV midazolam | 39 | 40 | 98 | 0 | 40 | 0 | 1 | 40 | 3 | |
| IV lorazepam | 40 | 40 | 100 | 0 | 40 | 0 | 0 | 40 | 0 | |
| IN midazolam | NR | 30 | NA | NR | 30 | NA | NR | 30 | NA | |
| IV diazepam | NR | 30 | NA | NR | 30 | NA | NR | 30 | NA | |
| IN midazolam | 23 | 26 | 88 | 0 | 26 | 0 | NR | 26 | NA | |
| IV diazepam | 24 | 26 | 92 | 0 | 26 | 0 | NR | 26 | NA | |
| IN midazolam | 35 | 35 | 100 | 0 | 35 | 0 | 0 | 35 | 0 | |
| IV diazepam | 35 | 35 | 100 | 0 | 35 | 0 | 0 | 35 | 0 | |
| Buccal midazolam | 61 | 109 | 56 | 5 | 109 | 5 | 36 | 109 | 33 | |
| Rectal diazepam | 30 | 110 | 27 | 7 | 110 | 6 | 63 | 110 | 57 | |
| IM midazolam | 48 | 50 | 96 | 1 | 50 | 2 | NR | 50 | NA | |
| Rectal diazepam | 47 | 50 | 94 | 0 | 50 | 0 | NR | 50 | NA | |
| Buccal midazolam | 125 | 165 | 76 | 2 | 165 | 1 | NR | 165 | NA | |
| Rectal diazepam | 114 | 165 | 69 | 2 | 165 | 1 | NR | 165 | NA | |
| IM midazolam | 45 | 50 | 90 | 0 | 50 | 0 | 5 | 50 | 10 | |
| IV diazepam | 29 | 31 | 90 | 0 | 31 | 0 | 2 | 31 | 6 | |
| IV lorazepam | 90 | 90 | 100 | 4 | 90 | 4 | 6 | 90 | 7 | |
| IV diazepam with phenytoin | 88 | 88 | 100 | 5 | 88 | 6 | 14 | 88 | 16 | |
| Buccal midazolam | 51 | 60 | 85 | 0 | 60 | 0 | 9 | 60 | 15 | |
| IV diazepam | 56 | 60 | 93 | 0 | 60 | 0 | 4 | 60 | 7 | |
| Abbreviations: IM: Intramuscular; IN: Intranasal; IV: Intravenous; NR: Not reported; NA: Not available (percentages could not be calculated where event rate was NR) *Occurences of respiratory depression were not reported for the subgroup of participants with generalised tonic‐clonic seizures in Arya 2011, therefore these results refer to all participants (including 83 participants without generalised tonic‐clonic seizures). | ||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation Show forest plot | 3 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.98, 1.20] |
| 1.1 Intravenous | 3 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.94, 1.16] |
| 1.2 Rectal | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.86 [1.47, 5.55] |
| 2 Time from drug administration to stopping of seizures Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Intravenous | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 6.18 [‐7.83, 20.19] |
| 3 Incidence of respiratory depression Show forest plot | 3 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.55, 0.93] |
| 3.1 Intravenous | 3 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.55, 0.92] |
| 3.2 Rectal | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.04, 20.78] |
| 4 Additional dose of the trial drug required to stop seizures Show forest plot | 3 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.64, 1.20] |
| 4.1 Intravenous | 3 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.71, 1.33] |
| 4.2 Rectal | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.56] |
| 5 Additional drugs required to stop seizures Show forest plot | 2 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.45, 1.24] |
| 5.1 Intravenous | 2 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.54, 1.55] |
| 5.2 Rectal | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.69] |
| 6 Seizure recurrence within 24 hours Show forest plot | 3 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.61, 1.20] |
| 6.1 Intravenous | 3 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.65, 1.27] |
| 6.2 Rectal | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 2.92] |
| 7 Incidence of admissions to the intensive care unit (ICU) Show forest plot | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 0.98] |
| 7.1 Intravenous | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.22] |
| 7.2 Rectal | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.03, 10.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Within 10 minutes | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.99, 1.52] |
| 2 Additional drugs required to stop seizures Show forest plot | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.18, 0.81] |
| 3 Seizure recurrence within 24 hours Show forest plot | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.31, 1.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Within 10 minutes | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.98, 1.02] |
| 2 Incidence of respiratory depression Show forest plot | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.22, 2.82] |
| 3 Additional drugs required to stop seizures Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 More than one dose of the trial drug required | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.17, 1.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Within 10 minutes | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.77, 1.49] |
| 1.2 Within 1 hour | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.43, 1.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation Show forest plot | 4 | 690 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.13, 1.38] |
| 1.1 Within 5 minutes | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.07, 1.40] |
| 1.2 Within 10 minutes | 2 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.95, 1.21] |
| 1.3 Within one hour | 1 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [1.45, 2.91] |
| 2 Incidence of respiratory depression Show forest plot | 4 | 690 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.61, 1.25] |
| 3 Additional drugs required to stop seizures Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Intravenous lorazepam required | 1 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.42, 0.79] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation within five minutes Show forest plot | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.03] |
| 2 Time from drug administration to stopping of seizures Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Treatment initiation time (minutes) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐1.09 [‐1.31, ‐0.87] |
| 2.2 Time for drug effect (minutes) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | 0.56 [0.29, 0.83] |
| 2.3 Total time to seizure cessation (minutes) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐0.96, ‐0.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure Cessation Show forest plot | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.06] |
| 2 Time from drug administration to stopping of seizures [minutes] Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Treatment initiation time (minutes) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐3.03, ‐0.97] |
| 2.2 Time for drug effect (minutes) | 2 | 122 | Mean Difference (IV, Fixed, 95% CI) | 0.62 [‐0.14, 1.38] |
| 2.3 Total time to seizure cessation (minutes) | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [0.24, 1.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Within 10 minutes | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.00, 2.16] |
| 2 Additional drugs required to stop seizures Show forest plot | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.10, 1.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation Show forest plot | 2 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.87, 1.09] |
| 2 Time from drug administration to stopping of seizures (minutes) Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 Treatment initiation time (minutes) | 1 | 24 | Mean Difference (Fixed, 95% CI) | ‐4.5 [‐6.68, ‐2.32] |
| 2.2 Time for drug effect (minutes) | 1 | 24 | Mean Difference (Fixed, 95% CI) | 1.1 [‐0.91, 3.11] |
| 2.3 Total time to seizure cessation (minutes) | 2 | 105 | Mean Difference (Fixed, 95% CI) | ‐2.68 [‐3.94, ‐1.42] |
| 3 Additional drugs required to stop seizures Show forest plot | 2 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.35, 5.13] |
| 4 Seizure recurrence within 24 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Within 15 minutes | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.06, 12.01] |
| 4.2 Within one hour | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.27, 2.62] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Within 1 hour | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.93, 1.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.97, 1.21] |
| 2 Time from drug administration to stopping of seizures Show forest plot | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 7.68 [‐6.73, 22.09] |
| 3 Incidence of respiratory depression Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| 4 Additional dose of the trial drug required to stop seizures Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.14] |
| 5 Seizure recurrence within 24 hours Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.10, 2.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.04] |
| 2 Time from drug administration to stopping of seizures Show forest plot | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 1.5 [‐9.37, 12.37] |
| 3 Additional dose of the trial drug required to stop seizures Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.51] |
| 4 Seizure recurrence within 24 hours Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.76] |

