儿童急性强直阵挛性惊厥(包括惊厥性癫痫持续状态)的药物管理
摘要
研究背景
强直阵挛性抽搐和惊厥性癫痫持续状态(目前定义为持续至少30分钟的强直阵挛性抽搐)是医疗紧急情况,需要紧急适当的抗惊厥治疗。国际共识认为持续至少5分钟的任何强直阵挛性惊厥应给予抗惊厥药。传统上认为苯二氮卓类药物(地西泮、劳拉西泮、咪达唑仑)为一线药物,苯巴比妥、苯妥英和三聚乙醛为二线药物。这是对2002年首次发表并于2008年更新的Cochrane综述的更新版。
研究目的
评价抗惊厥药物用于治疗任何持续时间的任何急性强直阵挛性惊厥的有效性和安全性,包括在医院或急诊科就诊的已确定惊厥(强直阵挛)癫痫持续状态的儿童。
检索策略
对于最新更新,我们通过Cochrane在线研究注册库(Cochrane Register of Studies Online,CRSO,2017年5月23日)检索了Cochrane癫痫组的专业注册库(Cochrane Epilepsy Group's Specialised Register )(2017年5月23日)、Cochrane对照试验中心注册库(Cochrane Central Register of Controlled Trials,CENTRAL)、MEDLINE(Ovid,1946年至2017年5月23日)、美国临床试验注册平台 ClinicalTrials.gov (2017年5月23日)以及 WHO国际临床试验注册平台 (International Clinical Trials Registry Platform,ICTRP,2017年5月23日)。
纳入排除标准
比较用于治疗包括儿童惊厥性癫痫持续状态在内的急性强直阵挛性惊厥的任何抗惊厥药物的随机和半随机试验。
资料收集与分析
两名评价者独立评估试验以纳入并提取了资料。我们联系了研究作者以获得更多信息。
主要结果
本系统综述纳入18项随机试验,共2199名受试者,涉及一系列药物治疗选择、剂量和给药途径(直肠、口腔、鼻腔、肌肉注射和静脉注射)。这些研究的设计、环境和人群不同,受试者的年龄以及临床表现也不同。在本系统综述中,我们对药物和给药途径进行了许多比较;我们的主要结果如下:
(1)本系统综述仅提供了比较咪达唑仑口腔给药与地西泮直肠给药治疗急性强直阵挛惊厥的低至极低质量证据(癫痫发作停止的风险比(risk ratio,RR)=1.25,95% 置信区间(confidence interval,CI)[1.13, 1.38];4项试验;690名儿童)。然而,效果尚不确定,因此没有足够的证据支持其使用。没有纳入比较咪达唑仑鼻内给药和口腔给药的研究。
(2)研究表明,抗惊厥药口腔给药和鼻内给药与静脉注射抗惊厥药的癫痫停止率相似,例如,劳拉西泮鼻内给药似乎与静脉注射劳拉西泮一样有效(RR=0.96,95% CI [0.82, 1.13];1项试验;141名儿童;高质量证据),而咪达唑仑鼻内给药与静脉注射地西泮一样有效(RR=0.98,95% CI [0.91, 1.06];2项试验;122名儿童;中等质量证据)。
(3) 肌肉注射咪达唑仑与静脉注射地西泮的癫痫发作停止率相似(RR=0.97,95% CI [0.87, 1.09];2项试验;105名儿童;低质量证据)。
(4)对于静脉给药途径,劳拉西泮在停止急性强直性阵挛性惊厥方面似乎与地西泮一样有效:RR=1.04,95% CI [0.94, 1.16];3项试验;414名儿童;低质量证据。此外,我们发现静脉注射咪达唑仑和地西泮(停止癫痫发作的RR=1.08, 95% CI [0.97, 1.21]; 1项试验; 80名儿童;中等质量证据)或静脉注射咪达唑仑和劳拉西泮(停止癫痫发作的RR=0.98, 95% CI [0.91, 1.04]; 1项试验; 80名儿童;中等质量证据)之间无统计学重要差异或临床上重要差异。一般而言,静脉注射抗惊厥药可以更快地停止癫痫发作,但这通常受到建立静脉通路所需时间的影响。
(5)一项试验的有限证据表明,鼻内劳拉西泮在停止急性强直阵挛性惊厥方面可能比肌肉注射三聚乙醛更有效(RR=1.22,95% CI [0.99, 1.52];160名儿童;中等质量证据)。
(6)在纳入的研究中,很少观察和报告不良副作用。呼吸抑制是最常见和最具有临床意义的副作用,据报道,在0%至18%的儿童中观察到这一不良事件的发生。无单个研究表明不同抗惊厥药或其不同给药途径之间的呼吸抑制率存在任何差异;但合并后三项研究(439名儿童)提供的中等质量证据表明劳拉西泮呼吸抑制的发生率显著低于地西泮(RR=0.72,95% CI [0.55, 0.93])。
本系统综述中提供的大部分证据质量大多为中至高。然而,为一些重要结局提供的证据质量从低至极低,特别是在比较非静脉给药途径时。低质量至极低质量的证据中,可用于分析的资料有限且结果不精确;一些研究存在方法学缺陷可能导致结果偏倚;研究环境不适用于更广泛的临床实践,并且一些合并分析存在不一致性。
作者结论
关于抗惊厥药在停止急性强直阵挛性惊厥方面的有效性或安全性,我们尚未发现任何可指导临床实践的新高质量证据。出现不良事件的风险似乎极低,尤其是呼吸抑制。静脉注射劳拉西泮和地西泮的惊厥停止率和呼吸抑制率似乎相似。尽管静脉注射劳拉西泮和静脉注射地西泮可以更快地停止癫痫发作,但建立静脉通路所需的时间可能会削弱这种效果。因此,在没有静脉通路的情况下,对于治疗持续至少5分钟的急性强直阵挛性惊厥,咪达唑仑口腔给药或地西泮直肠给药是可接受的一线抗惊厥药。本系统综述没有提供证据支持将鼻内咪达唑仑或劳拉西泮作为咪达唑仑口腔给药或地西泮直肠给药的替代品。
PICO
简语概要
急性强直阵挛性惊厥(发作)的药物管理(包括儿童的惊厥持续状态)
系统综述问题
本系统综述旨在评价不同抗惊厥药物的使用、不同给药途径,是否会影响急性强直阵挛性惊厥的停止速度。本系统综述还评价了不同的抗惊厥药物是否伴有更少见或不同的严重副作用。
系统综述背景
强直阵挛性惊厥和惊厥性癫痫持续状态是急症。在儿童中,第一种抗惊厥药通常在医院的急症(Accident and Emergency,A&E)室给予。这种药物可以通过多种方式给药,包括静脉(静脉内)、口腔内和两颊间给药(口腔给药)、鼻孔给药(鼻内给药)或直肠给药。首选药物应有效、起效快且无任何严重的不良反应。在这种临床情况下,寻找最有效、最安全的抗惊厥药物研究具有重要意义。
研究特征
我们对所有现有和相关证据进行了评价,这些证据是关于医院急诊科儿童强直阵挛性惊厥的一线抗惊厥治疗药物的有效性和安全性。本系统综述评价了18项随机对照试验(randomised controlled trials, RCTs)的资料;RCTs提供了最可靠的证据。他们研究了不同抗惊厥药物和不同的给药途径。
主要结果
本系统综述共纳入18项RCTs,共2199名儿童,研究了多种不同的抗惊厥药物、药物剂量和药物给药途径。这些研究的设计、环境和纳入的儿童群体存在一些差异,在年龄和临床表现方面(例如他们被纳入试验时惊厥的持续时间)。
对两项试验的分析发现,没有明确证据表明静脉注射劳拉西泮和静脉注射地西泮在停止急诊室内的强直阵挛性惊厥方面有不同的效果。目前尚不明确,在没有静脉通路的情况下,作为强直阵挛性惊厥或惊厥性癫痫持续状态的首次治疗,咪达唑仑口腔给药是否比地西泮直肠给药更有效。没有很强的证据表明鼻内给药途径与静脉给药途径一样有效。因此,没有证据表明它可以用作给药替代途径。
尽管咪达唑仑、劳拉西泮和三聚氰胺等药物可以降低呼吸频率,但这不是常见的并发症,在纳入的研究中也并不常见。这些药物的严重副作用发生率通常极低。
证据质量
许多试验使用了不同的药物、不同的剂量和不同的给药途径。本系统综述的总体结论必须考虑到这一点。大多数试验在大型儿童医院或综合医院的大型儿科进行。这意味着本系统综述中发现的结果可能与世界各地类似的临床情况相关。
本系统综述中提供的证据质量从极低到高不等。由于可用于分析的信息有限,结果不精确,因此为一些结局提供的证据质量低至极低。一些研究的设计也存在差异和问题,这可能影响了研究结果。在一些研究环境中(特定国家/地区)进行的研究证据质量较低,因此结果可能无法反映全球临床实践。
证据检索时间截至2017年5月。
Authors' conclusions
Summary of findings
| Lorazepam compared with diazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Lorazepam Comparison: Diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Diazepam | Lorazepam | |||||
| Seizure cessation Follow‐up: up to 24 hours | 708 per 1000 | 765 per 1000 | RR 1.08 (0.98 to 1.20) | 439 | ⊕⊕⊝⊝ | In two trials, drugs were administered intravenously. In a third trial, drugs were administered intravenously or rectally if intravenous access was not possible Subgroup analysis showed a significant difference by route of intervention (intravenous: RR 1.04 (95% CI 0.94 to 1.16) compared to rectally RR: 2.86 (95% CI 1.47 to 5.55), test of subgroups P = 0.003) |
| Time from drug administration to termination of seizures Follow‐up: up to 24 hours | The mean time to cessation of seizures was 84.94 seconds in the diazepam group | The mean time to cessation of seizures was 6.18 faster (7.83 slower to 20.19 faster) in the lorazepam group | NA | 80 | ⊕⊕⊕⊝ | Drugs were administered intravenously Another trial (where drugs were administered intravenously or rectally) reported similar mean times to seizure cessation. Standard deviations were not available so data could not be entered into analysis |
| Incidence of respiratory depression Follow‐up: up to 24 hours | 356 per 1000 | 256 per 1000 | RR 0.72 (0.55 to 0.93) | 439 | ⊕⊕⊕⊝ | In two trials, drugs were administered intravenously. In a third trial, drugs were administered intravenously or rectally if intravenous access was not possible There was no difference between the routes of intervention (test of subgroups, P = 0.86) |
| Additional drugs required to terminate the seizure: additional dose of study drug Follow‐up: up to 24 hours | 305 per 1000 | 268 per 1000 | RR 0.88 (0.64 to 1.20) | 439 | ⊕⊕⊝⊝ | In two trials, drugs were administered intravenously. In a third trial, drugs were administered intravenously or rectally if intravenous access was not possible Subgroup analysis by route of intervention (intravenous: RR 0.97 (95% CI 0.71 to 1.33) compared to rectally RR: 0.11 (95% CI 0.01 to 1.56), test of subgroups P = 0.11). Two trials also reported whether additional (other) antiepileptic drugs were required to stop the seizure. There were no significant differences overall or by route of intervention |
| Seizure recurrence within 24 hours Follow‐up: up to 24 hours | 266 per 1000 | 229 per 1000 | RR 0.86 (0.61 to 1.20) | 439 | ⊕⊕⊕⊝ | In two trials, drugs were administered intravenously. In a third trial, drugs were administered intravenously or rectally if intravenous access was not possible There was no difference between the routes of intervention (test of subgroups, P = 0.27) |
| Incidence of admissions to the ICU Follow‐up: up to 24 hours | 116 per 1000 | 17 per 1000 | RR 0.15 (0.02 to 0.98) | 86 | ⊕⊕⊝⊝ | In the included trial, drugs were administered intravenously or rectally if intravenous access was not possible There was no difference between the routes of intervention (test of subgroups P = 0.32). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to risk of bias: one included study was quasi‐randomised, which may have led to selection bias and an intention‐to‐treat approach was not used in the study. | ||||||
| Intranasal lorazepam compared with intramuscular paraldehyde for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intranasal lorazepam Comparison: Intramuscular paraldehyde | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intramuscular paraldehyde | Intranasal lorazepam | |||||
| Seizure cessation: within 10 minutes Follow‐up: up to 24 hours | 613 per 1000 | 747 per 1000 | RR 1.22 (0.99 to 1.52) | 160 (1 study) | ⊕⊕⊕⊝ | ‐ |
| Time from drug administration to termination of seizures Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Incidence of respiratory depression Follow‐up: up to 24 hours | No difference was found between either treatment group in terms of clinically important cardiorespiratory events. | NA | 160 (1 study) | ⊕⊕⊝⊝ | ‐ | |
| Additional drugs required to terminate the seizure: 2 or more additional anticonvulsants required Follow‐up: up to 24 hours | 263 per 1000 | 100 per 1000 | RR 0.38 (0.18 to 0.81) | 160 (1 study) | ⊕⊕⊝⊝ | ‐ |
| Seizure recurrence within 24 hours Follow‐up: up to 24 hours | 138 per 1000 | 100 per 1000 | RR 0.73 (0.31 to 1.71) | 160 (1 study) | ⊕⊕⊝⊝ | ‐ |
| Incidence of admissions to the ICU Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to applicability: a high proportion of the children recruited had either cerebral malaria or meningitis. These comorbidities may have impacted upon the results. | ||||||
| Intravenous lorazepam compared with intravenous diazepam/intravenous phenytoin combination for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intravenous lorazepam Comparison: Intravenous diazepam/intravenous phenytoin combination | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous diazepam/intravenous phenytoin combination | Intravenous lorazepam | |||||
| Seizure cessation: within 10 minutes Follow‐up: up to 24 hours | Seizures were stopped for all individuals in the Intravenous diazepam/intravenous phenytoin combination group | Seizures were stopped for all individuals in the Intravenous lorazepam group | RR 1.00 (0.98 to 1.02) | 178 | ⊕⊕⊕⊝ | ‐ |
| Time from drug administration to stopping of seizures Follow‐up: up to 24 hours | There was no significant difference in the median time to seizure cessation (20 seconds in each group). | NA | 178 | ⊕⊕⊕⊝ | ‐ | |
| Incidence of respiratory depression Follow‐up: up to 24 hours | 57 per 1000 | 44 per 1000 (13 to 160) | RR 0.78 (0.22 to 2.82) | 178 | ⊕⊕⊕⊝ | ‐ |
| Additional drugs required to stop the seizure Follow‐up: up to 24 hours | 159 per 1000 | 67 per 1000 (27 to 165) | RR 0.42 (0.17 to 1.04) | 178 | ⊕⊕⊕⊝ | ‐ |
| Seizure recurrence within 24 hours Follow‐up: up to 24 hours | There were no seizure recurrences in either group. | NA | 178 | ⊕⊕⊕⊝ | ‐ | |
| Incidence of admissions to the ICU Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due toapplicability: Both treatment arms showed a 100% seizure cessation rate, which is higher than expected. Unclear whether this high success rate was due to a particular element of the trial design. | ||||||
| Intravenous lorazepam compared with intranasal lorazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intravenous lorazepam Comparison: Intranasal lorazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intranasal lorazepam | Intravenous lorazepam | |||||
| Seizure cessation: within 10 minutes Follow‐up: up to 24 hours | 696 per 1000 | 744 per 1000 | RR 1.07 (0.77 to 1.49) | 58 | ⊕⊕⊕⊝ | There was also no significant difference between treatments for seizure cessation at 1 hour: RR 0.70 (95% CI 0.43 to 1.17) |
| Time from drug administration to stopping of seizures Follow‐up: up to 24 hours | Median time to achieve seizure control from drug administration was 4 minutes in both groups. | NA | 58 | ⊕⊕⊕⊝ | Time taken to achieve intravenous access ranged from 1 to 25 minutes with a median of 4 minutes across all participants in the trial. If this had been included in the response time for the intravenous lorazepam, the results would have been skewed significantly in favour of intranasal lorazepam | |
| Incidence of respiratory depression Follow‐up: up to 24 hours | One child required respiratory support | Two children required respiratory support | NA | 141 | ⊕⊕⊕⊝ | Incidence of respiratory depression was not reported for the subgroup of participants with generalised tonic‐clonic seizures in the trial, therefore these results refer to all participants (including 83 participants without generalised tonic‐clonic seizures). |
| Additional drugs required to stop the seizure Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Seizure recurrence within 24 hours Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Incidence of admissions to the ICU Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded once due to imprecision: imbalance in the number of participants randomised to each intervention with generalised tonic‐clonic seizures and overall direction of effect seems to change when measured at 10 minutes or at 1 hour 2Downgraded once due to imprecision: limited numerical data reported. | ||||||
| Buccal midazolam compared with rectal diazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Buccal midazolam Comparison: Rectal diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Rectal diazepam | Buccal midazolam | |||||
| Seizure cessation: within 5 minutes to 1 hour Follow‐up: up to 24 hours | 584 per 1000 | 730 per 1000 | RR 1.25 (1.13 to 1.38) | 648 (4 trials) 690 seizure episodes | ⊕⊝⊝⊝ | The measurement time of seizure cessation was examined in a subgroup analysis 5 minutes: RR 1.22 (95% CI 1.07 to 1.40, P = 0.004); |
| Time from drug administration to of seizures Follow‐up: up to 24 hours | One trial found no difference between groups in the time from drug administration to seizure cessation One trial reported that both the median treatment initiation time and drug effect time were significantly shorter in the buccal midazolam group than the rectal diazepam group. | NA | 141 (2 trials) | ⊕⊕⊝⊝ | No numerical data presented for either trial | |
| Incidence of respiratory depression Follow‐up: up to 24 hours | 76 per 1000 | 67 per 1000 (46 to 94) | RR 0.88 (0.61 to 1.25) | 648 (4 trials) 690 seizure episodes | ⊕⊕⊝⊝ | ‐ |
| Additional drugs required to stop the seizure: intravenous lorazepam required Follow‐up: up to 24 hours | 573 per 1000 | 332 per 1000 | RR 0.58 (0.42 to 0.79) | 177 (1 trial) 219 seizure episodes | ⊕⊕⊝⊝ | A second trial reported that there was no difference between groups in the need for a second drug |
| Seizure recurrence within 24 hours Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Incidence of admissions to the ICU Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to risk of bias: one included study was quasi‐randomised and one study did not conceal allocation. Both of these studies were at risk of selection bias. | ||||||
| Buccal midazolam compared with intravenous diazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Buccal midazolam Comparison: Intravenous diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous diazepam | Buccal midazolam | |||||
| Seizure cessation Follow‐up: up to 24 hours | 933 per 1000 | 849 per 1000 | RR 0.91 (0.80 to 1.03) | 120 (1 trial) | ⊕⊕⊕⊕ | ‐ |
| Time from drug administration to termination of seizures Follow‐up: up to 24 hours | The mean time to cessation of seizures was 1.13 minutes in the intravenous diazepam group. | The mean time to cessation of seizures was 0.56 minutes higher in the buccal diazepam group (0.29 to 0.83 minutes higher). | NA | 120 (1 trial) | ⊕⊕⊕⊝ | The mean time for initiation of treatment was significantly shorter in the buccal midazolam group (MD ‐1.09 minutes, 95% CI ‐1.31 to ‐0.87) and therefore the mean total time to controlling the seizures was significantly shorter in the buccal midazolam group compared to the intravenous diazepam group (MD ‐0.59, 95% CI ‐0.96 to ‐0.22) |
| Incidence of respiratory depression Follow‐up: up to 24 hours | There were no adverse events in either group | NA | 120 (1 trial) | ⊕⊕⊕⊕ | ‐ | |
| Additional drugs required to stop the seizure Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Seizure recurrence within 24 hours Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Incidence of admissions to the ICU Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to applicability: the route of intervention of the drug has been shown to influence the outcome. | ||||||
| Intranasal midazolam compared with intravenous diazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intranasal midazolam Comparison: Intravenous diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous diazepam | Intranasal midazolam | |||||
| Seizure cessation Follow‐up: up to 24 hours | 967 per 1000 | 948 per 1000 | RR 0.98 (0.91 to 1.06) | 122 (2 trials) | ⊕⊕⊕⊝ | ‐ |
| Time from drug administration to stopping of seizures Follow‐up: up to 24 hours | The mean time to cessation of seizures ranged from 2.5 to 2.94 minutes in the intravenous diazepam group. | The mean time to cessation of seizures was 0.62 minutes higher in the intranasal midazolam group (0.14 lower to 1.38 minutes higher). | NA | 122 (2 trials) | ⊕⊕⊕⊝ | One trial reports that the time for initiation of treatment was significantly shorter in the intranasal midazolam group (MD ‐2.00 minutes, 95% CI ‐3.03 to ‐0.97). The other trial also reports that time for initiation of treatment was significantly shorter in the intranasal midazolam group but does not account for this in analysis |
| Incidence of respiratory depression Follow‐up: up to 24 hours | No adverse events including respiratory depression occurred in either group. | NA | 122 (2 trials) | ⊕⊕⊕⊕ | ‐ | |
| Additional drugs required to stop the seizure Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Seizure recurrence within 24 hours Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Incidence of admissions to the ICU Follow‐up: up to 24 hours | There were no admissions to the ICU in either group | NA | 52 (1 trial) | ⊕⊕⊕⊕ | ‐ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to risk of bias: one of the studies included in this comparison did not report this outcome. As this is an expected outcome, this may be selective reporting. Additionally, in one trial both treatment arms showed a 100% seizure cessation rate, which is higher than expected. Unclear whether this high success rate was due to a particular element of the trial design. | ||||||
| Intranasal midazolam compared with rectal diazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intranasal midazolam Comparison: Rectal diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Rectal diazepam | Intranasal midazolam | |||||
| Seizure cessation: within 10 minutes Follow‐up: up to 24 hours | 591 per 1000 | 869 per 1000 | RR 1.47 (1.00 to 2.16) | 45 | ⊕⊕⊝⊝ | ‐ |
| Time from drug administration to termination of seizures Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Incidence of respiratory depression Follow‐up: | There was no significant difference between the two groups for of cardiorespiratory or adverse effects. | NA | 45 | ⊕⊕⊝⊝ | No numerical data reported | |
| Additional drugs required to stop the seizure Follow‐up: up to 24 hours | 409 per 1000 | 131 per 1000 (41 to 421) | RR 0.32 (0.10 to 1.03) | 45 | ⊕⊕⊝⊝ | ‐ |
| Seizure recurrence within 24 hours Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Incidence of admissions to the ICU Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to risk of bias: one included study was quasi‐randomised, which may have led to selection bias. Additionally, the description of the seizure type and aetiology of the included children was unclear, so it is unclear if the population of this study is generalisable. | ||||||
| Intramuscular midazolam compared with intravenous diazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intramsucular midazolam Comparison: Intravenous diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous diazepam | Intramsucular midazolam | |||||
| Seizure cessation Follow‐up: up to 24 hours | 929 per 1000 | 901 per 1000 | RR 0.97 (0.87 to 1.09) | 105 | ⊕⊕⊝⊝ | ‐ |
| Time from drug administration to stopping of seizures: total time to seizure cessation Follow‐up: up to 24 hours | The mean total time to cessation of seizures was 2.68 minutes lower (3.94 to 1.42 minutes lower) in the intramuscular midazolam group compared to the intravenous diazepam group | NA | 105 | ⊕⊝⊝⊝ | One trial also showed that the initiation of treatment was significantly shorter in the intramuscular midazolam group (MD ‐4.50 minutes (‐6.68 to ‐2.32)) but there was no significant difference between treatments for the time to drug effect (MD 1.10 minutes (95% CI ‐0.91 to 3.11) | |
| Incidence of respiratory depression Follow‐up: up to 24 hours | There were no adverse events or complications in either trial | NA | 105 | ⊕⊕⊝⊝ | ‐ | |
| Additional drugs required to terminate the seizure Follow‐up: up to 24 hours | 71 per 1000 | 96 per 1000 | RR 1.34 (0.35 to 5.13) | 105 | ⊕⊝⊝⊝ | ‐ |
| Seizure recurrence within 24 hours: within one hour Follow‐up: up to 24 hours | 364 per 1000 | 309 per 1000 | RR 0.85 (0.27 to 2.62) | 24 (1 trial) | ⊕⊝⊝⊝ | There was also no significant difference between treatments at within 15 minutes (RR: 0.85 (95% CI 0.06,to12.01) |
| Incidence of admissions to the ICU Follow‐up: up to 24 hours | There were no admissions to the ICU | NA | 81 (1 trial) | ⊕⊕⊕⊝ | ‐ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to risk of bias: in both included trials, methods of randomisation were unclear so the trials may be at risk of selection bias. | ||||||
| Intramuscular midazolam compared with rectal diazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intramuscular midazolam Comparison: Rectal diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Rectal diazepam | Intramuscular midazolam | |||||
| Seizure cessation Follow‐up: up to 24 hours | 940 per 1000 | 959 per 1000 | RR 1.02 (0.93 to 1.12) | 100 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Time from drug administration to stopping of seizures Follow‐up: up to 24 hours | There was a significant difference in time from administration to seizure cessation in favour of midazolam (median 66 seconds, diazepam, median 130 seconds, P < 0.001) | NA | 100 (1 trial) | ⊕⊕⊕⊝ | It is noted that the speed of administration was similarly fast for both medications, so this seems to reflect a medication difference. | |
| Incidence of respiratory depression Follow‐up: up to 24 hours | No patients developed respiratory depression except for one patient who received an accidental double dose of intramuscular midazolam. | NA | 100 (1 trial) | ⊕⊕⊕⊝ | ‐ | |
| Additional drugs required to stop the seizure Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Seizure recurrence within 24 hours Follow‐up: up to 24 hours | Among those with seizures terminated, there were no recurrences at 24 hours | NA | 100 (1 trial) | ⊕⊕⊕⊝ | ||
| Incidence of admissions to the ICU Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to risk of bias: the included study did not conceal allocation so is at risk of selection bias. | ||||||
| Intravenous midazolam compared with intravenous diazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intravenous midazolam Comparison: Intravenous diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous diazepam | Intravenous midazolam | |||||
| Seizure cessation Follow‐up: up to 24 hours | 900 per 1000 | 972 per 1000 | RR 1.08 (0.97 to 1.21) | 80 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Time from drug administration to stopping of seizures Follow‐up: up to 24 hours | The mean time to cessation of seizures was 84.94 seconds in the intravenous diazepam group. | The mean time to cessation of seizures was 7.68 seconds higher in the intravenous midazolam group (6.73 seconds lower to 22.09 seconds higher) . | NA | 80 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Incidence of respiratory depression Follow‐up: up to 24 hours | 25 per 1000 | 8 per 1000 | RR 0.33 (0.01 to 7.95) | 80 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Additional drugs required to stop the seizure: additional dose of the trial drug required Follow‐up: up to 24 hours | 100 per 1000 | 25 per 1000 | RR 0.25 (0.03 to 2.14) | 80 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Seizure recurrence within 24 hours Follow‐up: up to 24 hours | 100 per 1000 | 50 per 1000 | RR 0.50 (0.10 to 2.58) | 80 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Incidence of admissions to the ICU Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to risk of bias: the definition of the 'seizure cessation' outcome is not an appropriate criterion for judging seizure cessation. This definition is likely to have impacted upon results. | ||||||
| Intravenous midazolam compared with intravenous lorazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intravenous midazolam Comparison: Intravenous lorazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous lorazepam | Intravenous midazolam | |||||
| Seizure cessation Follow‐up: up to 24 hours | Seizures were terminated for all children in the Intravenous lorazepam group | Seizures were terminated for 39 out of 40 children in the intravenous midazolam group | RR 0.98 (0.91 to 1.04) | 80 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Time from drug administration to termination of seizures Follow‐up: up to 24 hours | The mean time to cessation of seizures was 91.12 seconds in the intravenous lorazepam group. | The mean time to cessation of seizures was 1.50 seconds higher in the intravenous midazolam group (9.37 seconds lower to 12.37 seconds higher) . | NA | 80 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Incidence of respiratory depression Follow‐up: up to 24 hours | There were no occurrences of respiratory depression in either group | NA | 80 (1 trial) | ⊕⊕⊕⊕ | ‐ | |
| Additional drugs required to terminate the seizure: additional dose of the trial drug required Follow‐up: up to 24 hours | No children in the intravenous lorazepam group required an additional dose of the trial drug. | One child in the intravenous midazolam group required an additional dose of the trial drug. | RR 3.00 (0.13 to 71.51) | 80 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Seizure recurrence within 24 hours Follow‐up: up to 24 hours | 50 per 1000 | 50 per 1000 | RR 1.00 (0.15 to 6.76) | 80 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Incidence of admissions to the ICU Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to risk of bias: the definition of the 'seizure cessation' outcome is not an appropriate criterion for judging seizure cessation. This definition is likely to have impacted upon results. | ||||||
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews (Issue 3, 2008; Appleton 2008).
Description of the condition
Convulsive status epilepticus (CSE) is a medical and neurological emergency and if under‐ or inappropriately treated may result in death or significant morbidity. Convulsive status epilepticus is defined as more than 30 minutes of either continuous seizure activity or two or more sequential seizures without full recovery of consciousness between seizures (Glauser 2016). The 30‐minute definition is based on the duration of convulsive status epilepticus that may lead to irreversible neuronal injury. Since most seizures are brief, and once a seizure lasts more than five minutes it is likely to be prolonged (Shinnar 2001), status treatment protocols are based on a five‐minute definition to minimise both the risk of seizures reaching 30 minutes and potential adverse outcomes associated with treating brief, self‐resolving tonic‐clonic convulsions. It is generally believed that the longer the episode of CSE, the more difficult it is to stop.
When the exact time of onset or duration of the convulsion is not known, any person presenting to the A&E department in an acute tonic‐clonic convulsion tends to be managed according to the definition of status epilepticus, with the primary objective of stopping the convulsion, irrespective of its duration. Most published national and international guidance, including from the National Institute of Health and Care Excellence (NICE) in the UK (NICE 2012), the International League Against Epilepsy (ILAE) (Trinka 2015) and the American Epilepsy Society (AES) (Glauser 2016), recommend treating a tonic‐clonic seizure after five minutes. This is because in over 90% of cases a tonic‐clonic seizure will end spontaneously within four minutes; it is assumed and likely that a seizure that has continued for more than four minutes will not stop spontaneously.
Description of the intervention
Twenty‐five years ago, the first drug used to treat an acute tonic‐clonic convulsion in children was usually administered in the A&E department (Garr 1999). However it is now more common that parents/carers of children with either prolonged or recurrent (serial) convulsions are prescribed ‘rescue’ medications, such as rectal diazepam or buccal/intranasal midazolam to administer at home (or even at school). An epidemiological study published in 2008 demonstrated that 61% of episodes of convulsive status epilepticus in children were treated with pre‐hospital emergency medication and predominantly rectal diazepam (Chin 2008). Over‐treatment may be as potentially damaging as under‐treatment by causing respiratory depression/arrest (with a risk of consequent cerebral hypoxia) or a potentially fatal cardiac arrhythmia.
How the intervention might work
Convulsive status epilepticus is a medical and neurological emergency that may result in death or significant morbidity. The intended aim of the intervention is to stop the acute tonic‐clonic seizure as rapidly as possible, without causing serious and potentially life‐threatening adverse side effects, and avoiding the need for a second‐line treatment.
Why it is important to do this review
This is an update of a Cochrane Review first published in 2002, and updated in 2008. Since the last update (Appleton 2008), there have been a number of newly‐published randomised controlled trials in children. These data contribute to the growing evidence base on the management of acute tonic‐clonic convulsions in children. We therefore consider it appropriate to update the review.
Objectives
To evaluate the effectiveness and safety of the anticonvulsant drugs used to treat any acute tonic‐clonic convulsion of any duration, including established convulsive (tonic‐clonic) status epilepticus in children presenting to a hospital or emergency medical department.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised controlled trials of a parallel design, blinded or unblinded.
Cluster‐randomised and cross‐over trials are not suitable designs for the review, due to the nature of the condition and the treatment.
Types of participants
Children aged between one month and 16 years, presenting to an A&E department or to a hospital ward (direct from the community) in an acute tonic‐clonic convulsion and who received treatment with an anticonvulsant drug, irrespective of the duration of the presenting convulsion.
Children included those presenting with a first convulsion and those with an established diagnosis of epilepsy. Any and all causes of the convulsion (including convulsive status epilepticus) were included in the review. We included studies where 70% or more of the study population had generalised tonic‐clonic seizures (GTC) or secondarily generalised seizures, or where subgroup data for children with GTC were available.
Types of interventions
In children presenting with an acute tonic‐clonic seizure including status epilepticus, we included trials if they compared two or more treatments or two or more treatment protocols of the same anticonvulsant. We included studies comparing first‐line treatments only (i.e. the first treatment a child received at the hospital). Studies of second‐line treatments (e.g. the second treatment given at hospital after a first seizure treatment had failed) were not within the scope of this review. Specific drugs considered within this review included the benzodiazepines (diazepam, lorazepam and midazolam), phenytoin, and paraldehyde. Different routes of drug administration were also analysed where possible, including intravenous (IV), intranasal, buccal, rectal and intramuscular administration. We consider different routes of drug administration separately in analyses.
Types of outcome measures
Primary outcomes
1. Presenting convulsion/episode of convulsive status epilepticus stopped with the drug(s) used.
2. Time taken from administration of any drug in the hospital to stopping of the convulsion.
3. The incidence of specific adverse effects: respiratory depression/arrest; cardiac arrhythmia; hypotension and extravasation of any intravenously‐administered anticonvulsant
Secondary outcomes
1. The need to use additional anti‐epileptic drugs to stop the presenting convulsion.
2. Recurrence of convulsions within 24 hours from stopping of the presenting convulsion.
3. Incidence of admissions to the intensive care unit (ICU).
Search methods for identification of studies
We ran searches for the original review in 2002 and again in 2003, 2005, 2007, 2010, 2011, 2012, 2013, 2014, and 2017. For this update we searched the following databases:
-
Cochrane Epilepsy Group Specialised Register (23 May 2017) using the search strategy outlined in Appendix 1;
-
The Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO, 23 May 2017), using the search strategy outlined in Appendix 2;
-
MEDLINE (Ovid, 1946 to 23 May 2017) using the strategy outlined in Appendix 3;
-
ClinicalTrials.gov (23 May 2017) using the strategy outlined in Appendix 4;
-
WHO International Clinical Trials Registry Platform (ICTRP, 23 May 2017) using the strategy outlined in Appendix 5.
There were no language restrictions.
Data collection and analysis
Selection of studies
Two review authors (Amy McTague and Richard Appleton) independently assessed trials for inclusion. We first screened titles and abstracts, followed by full‐text reports of potentially eligible trials, resolving any disagreements by discussion.
Data extraction and management
All three review authors (Amy McTague, Richard Appleton and Tim Martland) independently extracted the outcome data specified above, as well as the following data. We resolved any disagreements by discussion.
Methodological/trial design
-
Method of randomisation.
-
Method of double‐blinding.
-
Whether any participants had been excluded from the reported analyses.
Participant/demographic information
-
Total number of participants allocated to each treatment group/audited in any protocol.
-
Age/sex.
-
Number and type of background anti‐epileptic drugs.
-
Whether any pre‐hospital emergency anticonvulsant treatment was given.
-
Duration of presenting tonic‐clonic seizure/episode of convulsive status.
-
Cause of acute tonic‐clonic seizure/episode of convulsive status.
Assessment of risk of bias in included studies
Two review authors (Amy McTague and Richard Appleton) independently assessed the risks of bias in the included studies, using the Cochrane 'Risk of bias' tool (Higgins 2011b). We judged whether each study was at high, low or unclear risk of bias in each of the following domains:
-
Random sequence generation;
-
Allocation concealment;
-
Blinding;
-
Incomplete outcome data;
-
Selective outcome reporting.
-
Other potential risks of bias.
We resolved any disagreements by discussion.
Measures of treatment effect
Dichotomous outcomes (e.g. number of children with convulsions stopped, number of children with specific adverse events, etc.) were expressed as risk ratios (RRs) with 95% confidence intervals (CIs). Continuous outcomes (e.g. time to stop the seizure/status episode) were expressed as mean differences (MDs) with 95% CIs.
Unit of analysis issues
We did not anticipate unit‐of‐analysis issues, as the unit of allocation and analysis must be the individual for all included trials, and cross‐over designs would not be suitable for this review, given the acute nature of the convulsions.
The participant was the preferred unit of analysis, but where results were reported in terms of 'episodes' (i.e. the same child being treated for multiple seizures in the same trial), where participant‐specific information could not be extracted we accepted episode‐level information. This is a limitation, as meta‐analysis assumes independence between measurements, and more than one treated seizure per child would not be statistically independent. A consequence of ignoring this unit‐of‐analysis issue could be over‐optimistic confidence intervals.
Where we included studies with multiple treatment arms, multiple treatment doses or different routes of administration, we considered each eligible treatment, dose or route of intervention in separate comparisons.
Dealing with missing data
The analyses conducted in this review aimed to take an 'intention‐to‐treat' approach where possible, i.e. including all randomised participants, analysed in the treatment group to which they were allocated, irrespective of which treatment they actually received.
Where data were missing, we attempted to contact the study authors for this information. If we could not acquire the missing data, we conducted a 'complete‐case' analysis and took account of the limitations of this approach when interpreting results.
Assessment of heterogeneity
We assessed clinical heterogeneity by reviewing the differences across trials in characteristics of recruited participants and treatment protocols. We also estimated heterogeneity statistically using a Chi2 test for heterogeneity and the I2 statistic. We interpreted the I2 statistic as follows (Higgins 2011a):
-
might not be important (I2 values 0% to 40%);
-
may represent moderate heterogeneity (I2 values 30% to 60%);
-
may represent substantial heterogeneity (I2 values 50% to 90%); and
-
considerable heterogeneity (I2 values 75% to 100%).
Assessment of reporting biases
To assess selective reporting bias, we compared the measurements and outcomes planned by the original iInvestigators during the trial with those reported within the published paper, by checking the trial protocols (when available) against the information in the final publication. Where protocols were not available, we compared the 'Methods' and the 'Results' sections of the published papers. We also used our knowledge of the clinical background to identify standard outcome measures usually taken, but not reported by the trial investigators.
If a sufficient number of trials (10 or more) had been included for any comparison, we would have investigated publication bias using a funnel plot.
Data synthesis
We analysed data using the fixed‐effect model in the first instance. Where we found substantial or considerable heterogeneity, we repeated the analysis with a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We assessed clinical and statistical heterogeneity using the methods outlined in Assessment of heterogeneity.
If appropriate, we considered different measurement times of the primary outcome (seizure cessation); i.e. if different trials reported this outcome at different time points or if any trials reported this outcome at multiple time points. In the former case, we also calculated a pooled summary of the measurement time subgroups and performed the Chi2 test for differences between subgroups. In the latter case, where a trial reported multiple time points, we reported subgroup results only and did not pool the results.
Sensitivity analysis
We planned a sensitivity analysis based on the methodological quality of the studies. However, given the small number of studies included in each comparison, we did not deem this sensitivity analysis to be appropriate, but we will consider a sensitivity analysis based on study quality for future updates of the review.
Summary of Findings and Quality of the Evidence (GRADE)
In a post hoc change in line with current Cochrane guidance, for the 2017 update we added a 'Summary of findings' table for each comparison presented in the review, reporting all of the primary and secondary outcomes.
We determined the quality of the evidence using the GRADE approach (GRADEPro 2004), downgrading evidence in the presence of a high risk of bias in at least one study, indirectness of the evidence, unexplained heterogeneity or inconsistency, imprecision of results, or high probability of publication bias. We downgraded evidence by one level if we considered the limitation to be serious, and by two levels if very serious.
Results
Description of studies
Results of the search
The original Cochrane Review (2002) identified a single study (Appleton 1995).
The update in 2008 identified three further studies (Ahmad 2006; Lahat 2000; McIntyre 2005).
For this update, we have identified 14 further studies that meet the main inclusion criteria in addition to the single study in the original review and the three studies identified for the 2008 update. (Arya 2011; Ashrafi 2010; Baysun 2005; Chamberlain 1997; Chamberlain 2014; Fişgin 2002; Gathwala 2012; Javadzadeh 2012; Mahmoudian 2004; Momen 2015; Mpimbaza 2008; Shah 2005; Sreenath 2010; Talukdar 2009).
Full details of searches conducted before 2012 are unavailable. Figure 1 shows the study flow diagram for searches completed between 2012 and 2017, in addition to the studies already listed in the 2008 update of the review.
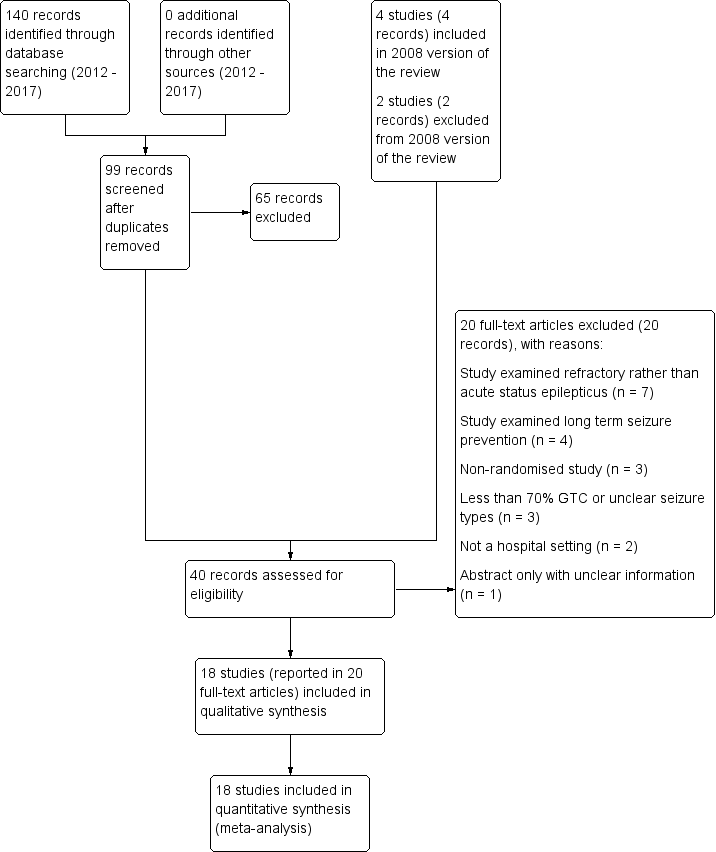
Study flow diagram.
Searches conducted been 2012 and 2017 identified 140 records, including 41 duplicate records. We screened 99 records (title and abstract) for inclusion in the review and excluded 65 clearly irrelevant records. With the four studies included in previous versions of the review and two studies excluded from previous versions of the review, we assessed 40 full‐text articles or clinical trials registry entries. We excluded 20 studies (see Excluded studies) and included 18 studies (reported in 20 full‐text articles or clinical trials registry entries) in the review.
Included studies
We included 18 trials in this review (Ahmad 2006; Appleton 1995; Arya 2011; Ashrafi 2010; Baysun 2005; Chamberlain 1997; Chamberlain 2014; Fişgin 2002; Gathwala 2012; Javadzadeh 2012; Lahat 2000; Mahmoudian 2004; McIntyre 2005; Momen 2015; Mpimbaza 2008; Shah 2005; Sreenath 2010; Talukdar 2009). All were hospital‐based studies.
This section gives a brief description of the characteristics and participants of each included trial; see Characteristics of included studies for further details.
Ahmad 2006 was a 12‐month, open, randomised study comparing intranasal lorazepam (0.1 mg (100 micrograms)/kg) and intramuscular paraldehyde (0.2 mg (200 micrograms)/kg) as the first‐line treatment of children aged two months to 12 years, presenting to a paediatric emergency centre with a generalised convulsion continuing for at least five minutes. The study was carried out in Malawi, Africa. Intramuscular paraldehyde is commonly used as a first‐line treatment for acute tonic‐clonic seizures in sub‐Saharan Africa but is associated with injury around the injection site, sterile abscesses and is incompatible with plastics. Patient demographics were similar in each group. Because of the geographical location of this study most of the children had acute symptomatic seizures, mainly due to acute brain infection (cerebral malaria or bacterial meningitis in two‐thirds of each of the two study groups). Randomisation was allocated in advance by computer in blocks of 10; after identification and treatment of children with hypoglycaemic seizures, investigators opened an unmarked envelope which contained details of treatment allocation. Primary outcome was the clinical cessation of the seizure within 10 minutes of drug administration. Children with features of hepatic or hypertensive encephalopathy or organophosphate poisoning were excluded, as were children who had received an anticonvulsant agent within one hour of presentation. For children in whom clinical seizure activity continued after 10 minutes, investigators followed a locally‐agreed protocol. The study evaluated 160 children of both sexes.
Appleton 1995 was a one‐year open, quasi‐randomised study, comparing lorazepam and diazepam, with the drugs given either intravenously or rectally depending on ease of venous access. This study evaluated 102 children, aged between one month and 16 years, of both sexes, presenting with an acute tonic‐clonic convulsion including established convulsive status epilepticus to an A&E department of a large children's hospital. The study accepted all causes of the convulsion or status, including symptomatic and idiopathic. No child had evidence of acute head trauma, metabolic encephalopathy, bacterial meningitis or herpes simplex encephalitis as a cause of their presenting convulsion. No children were included with known pseudo‐tonic‐clonic convulsions or pseudo‐convulsive, absence or complex partial status. The demography of the two treatment groups was very similar (age; sex; numbers with pre‐existing epilepsy; numbers with a pre‐existing neurological disorder and duration of the presenting convulsion prior to treatment with the two study drugs). Cessation of the seizure was defined as the seizure or episode of status stopping within seven or eight minutes of administration of the first dose of the study anticonvulsant. If the presenting convulsion had not stopped by eight minutes, then a second dose of either lorazepam or diazepam would be given. If this seizure persisted, then an additional anticonvulsant would be given, based on the hospital's protocol for managing convulsive status epilepticus (Garr 1999).
Arya 2011 was a randomised controlled trial comparing intranasal and intravenous (IV) lorazepam for the treatment of convulsive status epilepticus in children. The trial took place in the emergency room of a hospital in New Delhi, India. Inclusion criteria were children aged six to 14 years who presented convulsing or who developed a seizure during the emergency room attendance. Exclusion criteria were receipt of any anti‐epileptic drug (AED) within one hour of enrolment, the presence of severe cardiovascular compromise, and the presence of cerebrospinal fluid (CSF) rhinorrhoea or upper respiratory infection severe enough to prevent intranasal administration. Fifty‐eight children (41%) had GTC, 77 (54%) had partial seizures and six were described as having "others/unclear". The groups were evenly matched for age, gender, seizure type and prior AED administration. The primary outcome measure was cessation of visible motor activity by 10 minutes. Secondary outcome measures were persistent cessation of seizure activity at one hour, time to IV access, time from drug administration to stopping of the seizure and development of hypotension/respiratory depression. Further seizures were treated with intravenous phenytoin.
Ashrafi 2010 was a randomised controlled trial conducted in two large hospitals in Tehran, Iran, comparing buccal midazolam and rectal diazepam for the control of acute convulsive seizures. Ninety‐eight children aged more than three months with an acute prolonged seizure lasting more than five minutes and those convulsing while attending the emergency rooms were enrolled, irrespective of the cause of the seizure. Patients who already had intravenous access or who were younger than three months were excluded. Most (84 patients or 86%) had GTC, with the remainder being myoclonic, focal clonic and focal tonic seizures. There was no significant difference between the two groups for age, sex or seizure type. Randomisation was by a random‐number table to either buccal midazolam (0.3 to 0.5 mg/kg) or rectal diazepam (0.5 mg/kg). The primary outcome measure was cessation of all motor activity in less than five minutes, without respiratory depression and without another seizure. Further seizures were treated with intravenous diazepam. The outcome measures were further defined as treatment initiation time and drug effect time. The authors also examined the convenience of drug use and parental acceptance of the drug/route of administration for each group.
Baysun 2005 was a prospective randomised study of all children attending the emergency room of a children's hospital in Turkey with a seizure, regardless of type, aetiology and whether the seizure was prolonged (this was assumed). No exclusion criteria were stated. Forty‐three children ranging in age from two months to 12 years were recruited and randomised to buccal midazolam (0.25 mg/kg) on even days of the month and rectal diazepam (0.5 mg/kg for under‐fives and 0.3 mg/kg for those aged six or more) on odd days of the month. The two groups did not differ significantly by sex, age, type of seizures or anti‐epileptic drug used. Ten children in the midazolam group and 10 in the diazepam group had GTC. The remaining participants presented with generalised tonic, simple partial and complex partial seizures. Outcome measures were cessation of convulsive seizure activity within 10 minutes, time to response, and need for a second drug. Those who did not respond within 10 minutes were given the alternative drug, i.e. midazolam given to those who had already received diazepam and vice versa.
Chamberlain 1997 was a prospective, open randomised study of the management of children aged 0 to 18 years presenting to the emergency department of two large hospitals in the USA, with motor seizures of at least 10 minutes' duration (all had tonic‐clonic or clonic seizures ‐ clarified in personal communication). The study compared intramuscular midazolam (0.2 mg/kg) with intravenous diazepam (0.3 mg/kg). Children who had established intravenous access or who had already received treatment for this seizure episode were excluded. Primary outcome measures were seizure cessation within five minutes of administration, seizure cessation between five and 10 minutes after administration (defined as delayed seizure control), and treatment failure (lack of cessation by 10 minutes). Those who had treatment failure were subsequently given intravenous diazepam or phenytoin. Other outcome measures included recurrence of seizures, defined as early recurrence if within 15 minutes, or recurrence if within 60 minutes. Twenty‐eight children were identified for enrolment, but three were excluded as their seizures did not persist beyond 10 minutes. One child who was randomised to diazepam was a protocol violation due to failure to establish intravenous access, necessitating treatment with intramuscular midazolam. Twenty‐three children with 24 seizure episodes were studied (one child had two episodes and appears in the study twice, once in each group). The demographics were similar between the two groups.
Chamberlain 2014 was a large multicentre randomised controlled trial conducted in the emergency departments of 11 North American hospitals, comparing intravenous lorazepam with intravenous diazepam for convulsive status epilepticus in children. Inclusion criteria were children aged three months to 18 years with generalised tonic‐clonic status epilepticus. This was defined as three or more seizures in the previous hour, two or more successive seizures with no recovery of consciousness with an ongoing seizure, or an ongoing seizure lasting at least five minutes. Children who had initial focal seizures rapidly evolving to bilaterally convulsive seizures were included. Patients with the following factors were excluded: known pregnancy, significant cardiac arrhythmia, urgent need for surgical intervention and anaesthesia, known contraindication to benzodiazepines or benzodiazepine use in the previous seven days (including pre‐hospital use by ambulance personnel). "Early terminators" were children removed from the study following administration of the study drug due to discovery of an exclusion factor or a refusal to participate by the family. The study assessed 11,630 patients for eligibility, of whom 11,320 were excluded, mainly because they were not having an acute seizure or did not meet the inclusion criteria. The study randomised 310 children to one of the two study drugs. Twenty‐two and 15 children were early terminators from each treatment arm respectively, and were excluded from the efficacy analysis. Further exclusions largely due to protocol deviations resulted in 102 and 107 children being available for per protocol analysis in each treatment arm. The participants in each treatment arm were well‐matched in demographics and seizure aetiology. Primary efficacy outcome measures were cessation of status epilepticus (defined as cessation of generalised convulsive activity with return of consciousness within the four‐hour observation period) within 10 minutes of the initial dose, and seizure freedom for 30 minutes. Secondary outcome measures included latency of drug response (time to cessation of convulsions), need for a dose of study medication, need for further anticonvulsants and sustained seizure freedom for 60 minutes and four hours. Primary safety outcomes were severe respiratory depression (needing assisted ventilation) within four hours of the study drug administration; secondary safety outcomes were aspiration pneumonia, any degree of respiratory depression, time required to return to baseline mental status, and degree of sedation or agitation as measured by the Riker Sedation‐Agitation scale.
Fişgin 2002 was a prospective, randomised, single‐centre study of children aged between one month and 13 years, presenting with an acute seizure to the emergency room of a children's hospital in Turkey. All children who were seizing on arrival were included, as it was presumed that their seizure had been ongoing for at least five minutes.No exclusion criteria are stated and the aetiology of the seizures is not given. Of 45 enrolled in the study, 28 children (14 per treatment group) had generalised tonic‐clonic seizures, the rest presenting with simple focal (10), secondarily generalised (4), tonic (1) and myoclonic (2) seizures. Both febrile and afebrile seizures were included, although only 10% of the participants had a febrile seizure, and were equally distributed between the two groups. Children were randomised on alternate days to rectal diazepam (0.3 mg/kg) or nasal midazolam (0.2 mg/kg). If the seizure continued beyond 10 minutes, the alternative drug was given, i.e. there was cross‐over between the two groups. Persistent convulsions (not clearly defined) were treated with intravenous midazolam by bolus, then infusion. Outcome measures were stopping of the seizure within 10 minutes, response time, and necessity for a second drug. There was no significant difference between the two groups for age or seizure type.
Gathwala 2012 was a randomised controlled trial undertaken in an Indian teaching hospital, comparing intravenous diazepam, midazolam and lorazepam for the treatment of acute convulsive seizures in children. Children aged six months to 14 years presenting with a convulsion to the emergency department were recruited. Children with liver or renal disease, cardiovascular abnormalities, head injury, diabetes mellitus or hypoglycaemia were excluded, as were those whose seizure had already stopped or where intravenous access could not be established. The study assessed 185 children for inclusion, of whom 65 were excluded; 55 did not meet the inclusion criteria, seven declined, and intravenous access could not be established in three. Participants were randomised into three treatment groups, which were evenly matched for demographics, mean duration of seizure, prolonged seizures, those presenting with first episode, and cause of seizure. The primary outcome measure was time to seizure cessation, defined as cessation of visible epileptic phenomena or return of purposeful response to external stimuli within 15 minutes of drug administration. The secondary outcomes were the effects of the drugs, i.e. vomiting, apnoea, somnolence, respiratory depression and requirement for mechanical ventilation. Other secondary outcomes were the number of participants with seizure recurrence, requirement for a second dose of medication, uncontrolled seizures, and the time to seizure recurrence.
Javadzadeh 2012 was a randomised unblinded study of 60 children aged between two months and 15 years, presenting to the emergency department with an acute seizure. Exclusion criteria were patients with prior IV access, previous anticonvulsant treatment, or concurrent respiratory tract infection. Participants were randomised to intranasal midazolam or intravenous diazepam, although all patients were cannulated on arrival. Outcome measures included time needed to control seizure, oxygen saturations, and heart rate pre‐ and post‐treatment.
Lahat 2000 was a 12‐month single‐centre randomised study comparing intranasal midazolam (0.2 mg/kg) and intravenous diazepam (0.3 mg/kg) in the treatment of prolonged febrile seizures (a seizure of at least 10 minutes duration) in children aged six months to five years. The study was carried out in a paediatric emergency department within a general hospital. Patient demographics were similar in both groups. Treatment was successful if the clinical features of the seizure stopped within five minutes. If the seizure stopped at between five and 10 minutes, this was identified as a delayed but successful treatment. Treatment failures (continued seizure activity after 10 minutes) received intravenous diazepam and then phenobarbital in accordance with local guidelines. Randomisation was allocated in advance by a random‐number table, with investigators receiving an opaque envelope with each allocation at the time of administration. Forty‐four children of both sexes were evaluated, with a total of 52 seizure episodes. Children who had received an anticonvulsant or had an intravenous line sited by paramedics prior to hospital attendance were excluded from the study.
Mahmoudian 2004 is a prospective randomised study of children aged two months to 15 years, presenting with an acute seizure to the paediatric emergency department of a general hospital in Iran over a two‐month period. Seventy children who presented with an acute seizure (length not specified) were randomised by an odd‐ and even‐number table to receive 0.2 mg/kg of intravenous diazepam or 0.2 mg/kg of intranasal midazolam. Fifty children presented with GTC, six with simple partial seizures, twelve with complex partial seizures and five with myoclonic seizures (note that multiple seizure types can occur in a single child). Outcome measures were time from treatment to cessation of seizure, with treatment considered successful if the seizure stopped within 10 minutes. Seizures that did not stop within 10 minutes were defined as treatment failures. Treatment failures in the midazolam group were given intravenous diazepam and those in the diazepam group were given intravenous phenobarbitone. Aetiologies of the seizures were reported, and were not evenly distributed between the groups; 14 of the midazolam group versus one of the diazepam group had febrile convulsions, and 10 of the diazepam group versus four in the midazolam group had central nervous system (CNS) infection.
McIntyre 2005 was a 40‐month, multicentre, randomised, controlled trial comparing buccal midazolam (approximately 0.5 mg/kg) with rectal diazepam (0.5 mg/kg) as the first‐line treatment of children aged six months to 15 years, presenting to a paediatric A&E department with active seizures.The primary outcome measure was clinical cessation of the seizure within 10 minutes of drug administration, without seizure recurrence within one hour and without respiratory depression. Children with partial seizures or non‐convulsive status epilepticus were excluded from the trial. Weekly blocks of treatment of either buccal midazolam or rectal diazepam were randomly selected in each of the four participating centres. Participant demographics were similar between groups. Locally‐agreed guidelines were followed in the event of continued seizure activity after the 10‐minute period. The study evaluated 219 seizure episodes in 177 children of both sexes. Separate results were reported both for total episodes and for first presenting episodes, to minimise potential bias of children with multiple entries. In contrast with the other studies included in the previous review, children were not excluded if they had received anticonvulsant agents prior to their attendance at the A&E department.
Momen 2015 was an unblinded randomised trial of 100 children aged one month to 16 years. Inclusion criteria were children older than one month who were convulsing on arrival. The length of the ongoing seizure was not taken into account, so children with relatively short‐lived seizure may have been included. Exclusion criteria were established IV access, prior administration of rectal or nasal benzodiazepines, lack of consent or serial seizures with no recovery of consciousness. In addition, a history of serious adverse reactions to either of the study medications was an exclusion criterion. Participants were randomised to intramuscular midazolam or rectal diazepam. The main outcome measure was seizure cessation without recurrence within 60 minutes. Respiratory rate and blood pressure were also monitored.
Mpimbaza 2008 was a single‐blinded, placebo‐controlled randomised clinical trial in a paediatric emergency unit in Kampala, Uganda. The inclusion criteria were children aged three months to 12 years who presented while convulsing or who experienced a seizure that lasted more than five minutes while in the unit, and who had no documented evidence of having received intravenous diazepam or phenobarbitone in the 24 hours before presentation. Children aged less than three months or more than 12 years, who had evidence of prior treatment or whose convulsion stopped prior to treatment, were excluded. The study recruited 330 participants (note that multiple seizure types can occur in a single participant): 269 (82%) had generalised tonic‐clonic seizures, 18 had tonic seizures, 61 had focal seizures and three had myoclonic seizures. Participants were randomised by a random‐number table to 0.5 mg/kg of rectal diazepam or buccal midazolam. A placebo which was identical in volume and similar in colour was simultaneously given with the study drug. The participants were well‐balanced by age, sex, and type of seizure between the two groups. The primary outcome measure was cessation of visible seizure activity within 10 minutes, without recurrence in the subsequent hour. If the convulsion lasted longer than 10 minutes or recurred within one hour, this was considered a treatment failure and the child was given intravenous diazepam. Secondary outcome measures were the proportion with cessation of convulsions within 10 minutes, the proportion with recurrence in the next hour and within 24 hours of initial control, and time to recurrence within these periods.
Shah 2005 was a prospective controlled quasi‐randomised study of the treatment of acute seizures in children in a tertiary general hospital in Mumbai, India, including children presenting to the emergency department and those who were already admitted to the ward or intensive care unit (ICU). The study enrolled 115 children with an acute seizure (definition unclear) over a one‐year period in a single centre. Those who had already had treatment for the seizure were excluded. Participants who already had intravenous access were treated with 0.2 mg/kg of intravenous diazepam. Those without were randomised to treatment with 0.2 mg/kg of intramuscular midazolam or to the establishment of intravenous access and treatment with intravenous diazepam. Sixty‐three children had generalised tonic‐clonic seizures, and were equally divided between the two treatment groups. Of the remaining participants, 47 had focal, four had tonic and one had clonic seizures. Outcome measures were mean time to cessation of seizures and the presence of adverse effects. Those who did not respond after five minutes were treated with other "anticonvulsants" (not specified).
Sreenath 2010 was a randomised controlled study of the management of convulsive status epilepticus (defined as continuous convulsive activity lasting for five minutes or more) in children presenting to a single centre in North India. Exclusion criteria were treatment with any anti‐epileptic medication in the preceding four weeks, acute head trauma, history of poisoning and jaundice, suspected renal failure, or diarrhoea presenting with seizures. The study randomised 178 children aged one to 12 years by computer‐generated table to receive lorazepam 0.1 mg/kg or diazepam 0.2 mg/kg. If intravenous access was not present, the drug was given rectally at the same dose. If the seizures recurred within an undefined time frame, a second dose of the same drug was given. The diazepam group were given a loading dose of 18 mg/kg of phenytoin after 15 to 30 minutes, regardless of whether the seizure had recurred. Sixty‐three per cent had generalised tonic‐clonic seizures, while the rest were tonic (10%), clonic (10%), myoclonic (0.5%), simple partial (2%), complex partial (10%), and partial with secondary generalisation (4%). The majority were therefore generalised convulsive or motor seizures. The primary outcome measure was cessation of seizure activity, with treatment considered successful if this occurred within 10 minutes of the first intervention and without recurrence over the next 18 hours. Secondary outcomes were time taken for initial (presenting) convulsion to stop after administration of the first drug, the number of doses of study drug required to treat the initial convulsion, the use of an additional anti‐epileptic drug, the total number of seizures occurring in the first 18 hours following administration of the study drug, the development of respiratory depression, the number of participants requiring transfer to ICU for mechanical ventilation, and the number of participants requiring cross‐over to an alternative regimen (i.e. from diazepam to lorazepam and vice versa).
Talukdar 2009 was a prospective randomised study of 120 children who attended the paediatric emergency department of a Delhi hospital with a seizure, the length of which was not defined. Those with myoclonic, absence and atonic seizures were excluded. The mean age of the participants was 3.2 years, with 73.3% under five years of age and 53.3% under one year. Seventy‐four per cent of the children presented with GTC, while the rest were complex partial or tonic seizures. The groups were not significantly different in age, sex, seizure type or underlying aetiology. Participants were allocated by a random‐number table to 0.2 mg/kg of buccal midazolam or 0.3 mg/kg of intravenous diazepam. The primary outcome measure was cessation of all motor activity within or by five minutes of administration of the drug. The drug response was further analysed as treatment initiation time (time from noting seizure to drug administration), drug effect time (time from drug administration to effect) and total controlling time, a combination of the previous two.
Excluded studies
We excluded 20 studies from the review for the following reasons (see Characteristics of excluded studies for further information):
-
The study evaluated refractory status epilepticus (i.e. children who have failed a first or second treatment for status epilepticus) rather than acute status epilepticus (Agarwal 2007; Arpita 2014; Mahmoudian 2006; Mehta 2007; Mittal 2014; Rosati 2016; Singhi 2002).
-
The study was not randomised or did not have a control group, or both (Kutlu 2003; Morton 2007; Qureshi 2002).
-
Fewer than 70% of included participants had GTCs (or it was unclear how many participants had GTCs) (Bhattacharyya 2006; Scott 1999; Tonekaboni 2012).
-
The study examined drug management for the long‐term prevention of recurring febrile seizures (Camfield 1980; Heckmatt 1976; Strengell 2009) or clusters of seizures (Cereghino 1998), rather than management of acute convulsions.
-
The study was not conducted in a hospital setting (Holsti 2010; Silbergleit 2012).
-
The study was published only as a conference abstract and we could not contact the authors for additional information to assess eligibility (McCormick 1999).
Risk of bias in included studies
The results of our 'Risk of bias' evaluations are summarised in Figure 2 and Figure 3.
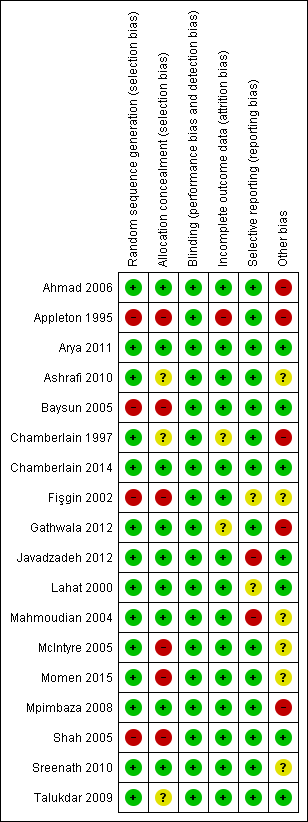
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
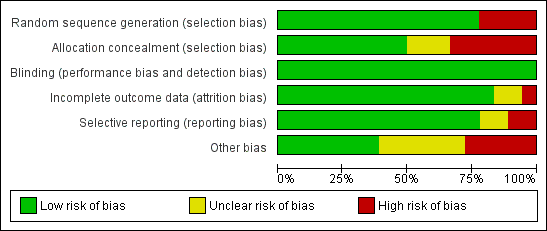
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Fourteen studies reported adequate methods for random sequence generation (e.g. computer‐generated randomisation, block randomisation, etc.) and we judged them to be at low risk of bias. Four studies (Appleton 1995; Baysun 2005; Fişgin 2002; Shah 2005) reported inadequate methods for random sequence generation and we judged these to be at high risk of bias. Three of these studies (Appleton 1995; Baysun 2005; Fişgin 2002) used an alternating ('odd' and 'even') day approach to randomisation and one study (Shah 2005) was partially randomised, including patients attending the emergency department and inpatients in the paediatric ward or intensive care unit. Those who already had intravenous access were selected to receive intravenous diazepam, and those without were further randomised to intramuscular midazolam or intravenous diazepam. For those who were randomised, it was unclear how randomisation was performed.
Regarding allocation concealment, nine studies described adequate methods of concealment such as centralised allocation or sealed opaque envelopes and we judged them to be at low risk of bias. Six studies (Appleton 1995; Baysun 2005; Fişgin 2002; McIntyre 2005; Momen 2015; Shah 2005) did not conceal allocation and we judged them to be at high risk of bias. The remaining three studies did not mention allocation concealment and we judged them to be at unclear risk of bias (Ashrafi 2010; Chamberlain 1997; Talukdar 2009).
Blinding
One study was double‐blinded (Chamberlain 2014) and one trial had single‐blinded participants (Mpimbaza 2008). The remaining sixteen studies were unblinded, and for many of them blinding would have been impractical, due to different routes of intervention. However, given the objective nature of the main outcomes of these studies (e.g. seizure cessation), it is unlikely that the lack of blinding would affect results, so we rated all studies at low risk of bias.
Incomplete outcome data
We judged 15 studies to be at low risk of bias, since all recruited participants were included and analysed on an intention‐to‐treat basis. We judged one study (Appleton 1995) to be at high risk of bias. In this study there were a relatively large number of protocol violators (16 of 102 children, or 16% of the total study population) and these violators were excluded from the analyses. The analysis was therefore not an intention‐to‐treat analysis.
We judged two studies (Chamberlain 1997; Gathwala 2012) as being at unclear risk of bias. In Chamberlain 1997, three children who were randomised to receive diazepam were subsequently excluded, as their seizures did not persist for 10 minutes. There was also a protocol violator who was randomised to receive intravenous diazepam but received intramuscular midazolam after 25 minutes, due to unsuccessful intravenous access. This participant was excluded from the analysis and obviously would have skewed the results significantly if he/she had been included. It may have been helpful to know the response time of this child once treatment was administered, as this is an important example of the disadvantages of the intravenous route. In Gathwala 2012, three children were excluded due to difficulties obtaining intravenous access, and this may have introduced a source of bias. It could be argued that the data cannot be considered to have been analysed on an intention‐to‐treat basis, as these participants were excluded from the analysis. However, given that all routes were intravenous the effect of this is likely to have been small.
Selective reporting
Fourteen studies reported all expected and prespecified outcomes and we judged them to be at low risk of bias. We rated two studies at high risk of bias; Javadzadeh 2012 did not report seizure cessation, which we would expect to be reported, and in Mahmoudian 2004 the authors did not report the time taken to insert intravenous cannulae in the intravenous diazepam group. This would have a significant effect on the time from arrival to seizure cessation. Other studies that compared intravenous with other routes have included this information.
We judged two studies to be at unclear risk of bias. Fişgin 2002 stated that information about previous convulsions and history of anti‐epileptic medication were collected according to the Methods but not reported in the Results section. It is unlikely that this information influenced outcomes, but we are unclear why the information was not reported. Lahat 2000 defined seizure cessation in the Methods section as "successful" if seizures stopped in less than five minutes, "successful but delayed" if seizures stopped after five to 10 minutes, and "failure" if seizures had not stopped after 10 minutes. However, results seem to be presented only in terms of treatment success and failure. It is unclear if this is selective reporting of results.
Other potential sources of bias
We identified additional high risks of bias in five studies. In two studies, a high proportion of the children recruited had either cerebral malaria or meningitis, which may have impacted upon the results (Ahmad 2006; Mpimbaza 2008). In Appleton 1995 there was a large discrepancy in the two routes of administration used in the study, probably due to clinician uncertainty about the use of rectal lorazepam. This discrepancy is likely to have impacted upon results. In Chamberlain 1997, one child was enrolled in the study twice, and is represented in both groups. Due to the small numbers of children included in the study, this double‐enrolment may have impacted on the results. In Gathwala 2012, the definition of the 'seizure cessation' outcome used is different from all the other included studies, and is not an appropriate criterion for judging seizure cessation. This definition is likely to have impacted upon results.
In four studies, it was unclear whether additional bias was present. In three studies (Ashrafi 2010; Mahmoudian 2004; Sreenath 2010), one or both treatment arms showed a 100% seizure cessation rate, which is higher than expected. However, it was unclear whether these unexpected results were due to a particular element of the trial design. In Fişgin 2002, the description of the seizure type and aetiology of participating children was unclear, so we cannot be sure that the population of this study is generalisable.
We found no other biases in the remaining nine studies (Arya 2011; Baysun 2005; Chamberlain 2014; Javadzadeh 2012; Lahat 2000; McIntyre 2005; Momen 2015; Shah 2005; Talukdar 2009).
Effects of interventions
See: Summary of findings for the main comparison Summary of findings ‐ Lorazepam compared with diazepam; Summary of findings 2 Summary of findings ‐ Intranasal lorazepam compared with intramuscular paraldehyde; Summary of findings 3 Summary of findings ‐ Intravenous lorazepam compared with intravenous diazepam/intravenous phenytoin combination; Summary of findings 4 Summary of findings ‐ Intravenous lorazepam compared with intranasal lorazepam; Summary of findings 5 Summary of findings ‐ Buccal midazolam compared with rectal diazepam; Summary of findings 6 Summary of findings ‐ Buccal midazolam compared with intravenous diazepam; Summary of findings 7 Summary of findings ‐ Intranasal midazolam compared with intravenous diazepam; Summary of findings 8 Summary of findings ‐ Intranasal midazolam compared with rectal diazepam; Summary of findings 9 Summary of findings ‐ Intramuscular midazolam compared with intravenous diazepam; Summary of findings 10 Summary of findings ‐ Intramuscular midazolam compared with rectal diazepam; Summary of findings 11 Summary of findings ‐ Intravenous midazolam compared with intravenous diazepam; Summary of findings 12 Summary of findings ‐ Intravenous midazolam compared with intravenous lorazepam
In the 18 included trials, specific drugs (i.e. benzodiazepines (diazepam, lorazepam and midazolam), phenytoin, and paraldehyde) were compared to each other; different routes of drug administration (i.e. intravenous, intranasal, buccal, rectal and intramuscular) of the same drug or different drugs were compared.
Considering the different drugs and different routes of administration, this review makes 12 comparisons. The results of each comparison are also summarised in 'Summary of findings' tables:
summary of findings Table for the main comparison: Lorazepam versus diazepam;
summary of findings Table 2: Intranasal lorazepam versus intramuscular paraldehyde;
summary of findings Table 3: Intravenous lorazepam versus intravenous diazepam/intravenous phenytoin combination;
summary of findings Table 4: Intravenous lorazepam versus intranasal lorazepam;
summary of findings Table 5: Buccal midazolam versus rectal diazepam;
summary of findings Table 6: Buccal midazolam versus intravenous diazepam;
summary of findings Table 7: Intranasal midazolam versus intravenous diazepam;
summary of findings Table 8: Intranasal midazolam versus rectal diazepam;
summary of findings Table 9: Intramuscular midazolam versus intravenous diazepam;
summary of findings Table 10: Intramuscular midazolam versus rectal diazepam;
summary of findings Table 11: Intravenous midazolam versus intravenous diazepam;
summary of findings Table 12: Intravenous midazolam versus intravenous lorazepam.
Table 1 also shows the study‐specific event rates for the outcomes 'Presenting convulsion/episode of convulsive status epilepticus stopped with the drug(s) used,' 'Incidence of respiratory depression' and 'The need to use additional anti‐epileptic drugs to stop the presenting convulsion'.
| Study | Drug | Seizure cessation | Respiratory Depression | Additional drugs required | ||||||
| No. of Events | No. of Children | % | No. of Events | No. of Children | % | No. of Events | No. of Children | % | ||
| IN lorazepam | 60 | 80 | 75 | 0 | 80 | 0 | 8 | 80 | 10 | |
| IM paraldehyde | 49 | 80 | 60 | 0 | 80 | 0 | 21 | 80 | 26 | |
| IV lorazepam | 19 | 27 | 70 | 1 | 27 | 4 | 1 | 27 | 4 | |
| Rectal lorazepam | 6 | 6 | 100 | 0 | 6 | 0 | 0 | 6 | 0 | |
| IV diazepam | 22 | 34 | 65 | 7 | 34 | 21 | 5 | 34 | 15 | |
| Rectal diazepam | 6 | 19 | 32 | 1 | 19 | 5 | 12 | 19 | 63 | |
| IN lorazepam | 16 | 23 | 70 | 1 | 71 | 1 | NR | 23 | NA | |
| IV lorazepam | 26 | 35 | 74 | 2 | 70 | 3 | NR | 35 | NA | |
| Buccal midazolam | 49 | 49 | 100 | 0 | 49 | 0 | 0 | 49 | 0 | |
| Rectal diazepam | 40 | 49 | 82 | 0 | 49 | 0 | 9 | 49 | 18 | |
| Buccal midazolam | 18 | 23 | 78 | 0 | 23 | 0 | 5 | 23 | 22 | |
| Rectal diazepam | 17 | 20 | 85 | 1 | 20 | 5 | 3 | 20 | 15 | |
| IM midazolam | 12 | 13 | 92 | 0 | 13 | 0 | 1 | 13 | 8 | |
| IV diazepam | 10 | 11 | 91 | 0 | 11 | 0 | 1 | 11 | 9 | |
| IV diazepam | 101 | 140 | 72 | 26 | 140 | 16 | 21 | 140 | 15 | |
| IV lorazepam | 97 | 133 | 73 | 26 | 133 | 18 | 21 | 133 | 16 | |
| IN midazolam | 20 | 23 | 87 | 0 | 23 | 0 | 3 | 23 | 13 | |
| Rectal diazepam | 13 | 22 | 60 | 0 | 22 | 0 | 9 | 22 | 40 | |
| IV diazepam | 36 | 40 | 90 | 1 | 40 | 3 | 4 | 40 | 10 | |
| IV midazolam | 39 | 40 | 98 | 0 | 40 | 0 | 1 | 40 | 3 | |
| IV lorazepam | 40 | 40 | 100 | 0 | 40 | 0 | 0 | 40 | 0 | |
| IN midazolam | NR | 30 | NA | NR | 30 | NA | NR | 30 | NA | |
| IV diazepam | NR | 30 | NA | NR | 30 | NA | NR | 30 | NA | |
| IN midazolam | 23 | 26 | 88 | 0 | 26 | 0 | NR | 26 | NA | |
| IV diazepam | 24 | 26 | 92 | 0 | 26 | 0 | NR | 26 | NA | |
| IN midazolam | 35 | 35 | 100 | 0 | 35 | 0 | 0 | 35 | 0 | |
| IV diazepam | 35 | 35 | 100 | 0 | 35 | 0 | 0 | 35 | 0 | |
| Buccal midazolam | 61 | 109 | 56 | 5 | 109 | 5 | 36 | 109 | 33 | |
| Rectal diazepam | 30 | 110 | 27 | 7 | 110 | 6 | 63 | 110 | 57 | |
| IM midazolam | 48 | 50 | 96 | 1 | 50 | 2 | NR | 50 | NA | |
| Rectal diazepam | 47 | 50 | 94 | 0 | 50 | 0 | NR | 50 | NA | |
| Buccal midazolam | 125 | 165 | 76 | 2 | 165 | 1 | NR | 165 | NA | |
| Rectal diazepam | 114 | 165 | 69 | 2 | 165 | 1 | NR | 165 | NA | |
| IM midazolam | 45 | 50 | 90 | 0 | 50 | 0 | 5 | 50 | 10 | |
| IV diazepam | 29 | 31 | 90 | 0 | 31 | 0 | 2 | 31 | 6 | |
| IV lorazepam | 90 | 90 | 100 | 4 | 90 | 4 | 6 | 90 | 7 | |
| IV diazepam with phenytoin | 88 | 88 | 100 | 5 | 88 | 6 | 14 | 88 | 16 | |
| Buccal midazolam | 51 | 60 | 85 | 0 | 60 | 0 | 9 | 60 | 15 | |
| IV diazepam | 56 | 60 | 93 | 0 | 60 | 0 | 4 | 60 | 7 | |
Abbreviations: IM: Intramuscular; IN: Intranasal; IV: Intravenous; NR: Not reported; NA: Not available (percentages could not be calculated where event rate was NR)
*Occurences of respiratory depression were not reported for the subgroup of participants with generalised tonic‐clonic seizures in Arya 2011, therefore these results refer to all participants (including 83 participants without generalised tonic‐clonic seizures).
1. Lorazepam versus diazepam
Three trials recruiting 455 participants compared lorazepam to diazepam (Appleton 1995; Chamberlain 2014; Gathwala 2012). All participants in Chamberlain 2014 and Gathwala 2012 received drugs intravenously; in Appleton 1995, children received the drugs either intravenously or rectally (where intravenous access was not possible). As the route of administration in this trial was not randomised, we test the route of administration for lorazepam and diazepam by subgroup analyses rather than by separate comparisons.
Primary outcomes
1. Presenting convulsion/episode of convulsive status epilepticus stopped with the drug(s) used.
All three studies reported the number of children with their presenting seizure(s) stopped by the trial drug. There was no statistically significant difference between the treatments when administered intravenously; risk ratio (RR) 1.04, 95% confidence interval (CI) 0.94 to 1.16, P = 0.43, Analysis 1.1. There was no heterogeneity present in this analysis (I2 = 0%).
For the 25 participants in Appleton 1995 who received the treatments rectally, lorazepam was statistically significantly more effective for seizure cessation than diazepam; RR 2.86, 95% CI 1.47 to 5.55, P = 0.002, Analysis 1.1. We are cautious when interpreting this result, due to the unbalanced number of children receiving each drug rectally (six received lorazepam and 19 received diazepam).
Overall, for both routes of administration, there was no statistically significant difference between the treatments; RR 1.08, 95% CI 0.98 to 1.20, P = 0.13, low‐quality evidence, Analysis 1.1. We note that this analysis has substantial heterogeneity (I2 = 67%), which may be due to the differences in the results by the different routes of administration (test for subgroup differences: Chi2 = 8.61, df = 1, P = 0.003, I2 = 88.4%).
2. Time taken from administration of any drug in the hospital to stopping the convulsion.
Gathwala 2012 reported that there was no significant difference for time to seizure cessation, with a mean difference 6.18 seconds, 95% CI ‐7.83 to 20.19, P = 0.39, moderate‐quality evidence, Analysis 1.2.
Appleton 1995 reports the mean and range of times for the presenting convulsion to stop is 29 seconds (range 25 to 60) for the intravenous lorazepam, 26 seconds (range 20 to 51) for the intravenous diazepam group, 37 seconds (range 31 to 48) for the rectal lorazepam group, and 38 seconds (range 35 to 49) for the rectal diazepam group. Standard deviations were not available, so we cannot enter data into analysis.
Chamberlain 2014 did not report on this outcome.
3. The incidence of specific adverse effects: respiratory depression/arrest; cardiac arrhythmia; hypotension and extravasation of any intravenously‐administered anticonvulsant
All three studies reported the incidence of respiratory depression. When administered intravenously, there were significantly fewer occurrences of respiratory depression with lorazepam compared to diazepam; RR 0.71, 95% CI 0.55 to 0.92, P = 0.01, 414 children, Analysis 1.3. There was no heterogeneity present in this analysis (I2 = 0%).
For the 25 participants in Appleton 1995 who received the treatments rectally, there was no significant difference between treatments; RR 0.95, 95% CI 0.04 to 20.78, P = 0.98, Analysis 1.3. However, as above we are cautious when interpreting this result, due to the unbalanced number of children receiving each drug rectally.
Overall for both routes of administration, there were significantly fewer occurrences of respiratory depression with lorazepam compared to diazepam; RR 0.72, 95% CI 0.55 to 0.93, P = 0.01, moderate‐quality evidence, Analysis 1.3. There was no heterogeneity present in this analysis (I2 = 0%) and no significant difference between the routes of administration (test for subgroup differences: Chi2 = 0.03, df = 1, P = 0.86, I2 = 0%).
Gathwala 2012 reported that there was a significant increase in somnolence between the diazepam and both the midazolam and lorazepam groups, but other adverse effects were evenly distributed. Chamberlain 2014 also reported that there was an increased incidence of sedation in the lorazepam group (absolute risk difference (ARD) 16.9%, 95% CI 6.1 to 27.7) and increased time taken to return to baseline mental status in the lorazepam group (hazard ratio (HR) 1.96, 95% CI 1.35 to 2.84, P < 0.001).
Secondary outcomes
1. The need to use additional anti‐epileptic drugs to stop the presenting convulsion.
All three studies reported the number of children requiring an extra dose of trial medication to stop the presenting seizure. There was no statistically significant difference between the treatments when administered intravenously (RR 0.97, 95% CI 0.71 to 1.33, P = 0.86, 414 children, Analysis 1.4), rectally (RR 0.11, 95% CI 0.01 to 1.56, P = 0.10, 25 children, Analysis 1.4) or for both routes of administration (RR 0.88, 95% CI 0.64 to 1.20, P = 0.41, low‐quality evidence, Analysis 1.4). Some heterogeneity was present in the combined analysis (I2 = 50%), which is probably due to the differences in routes of administration, although the test for subgroup differences did not reach statistical significance (test for subgroup differences: Chi2 = 2.58, df = 1, P = 0.11, I2 = 61.3%).
Appleton 1995 and Chamberlain 2014 also reported the number of children requiring treatment with additional anti‐epileptic drugs to stop the presenting seizure. There was no statistically significant difference between the treatments when administered intravenously (RR 0.91, 95% CI 0.54 to 1.55, P = 0.73, 334 children, Analysis 1.5), rectally (RR 0.11, 95% CI 0.01 to 1.69, P = 0.11, 25 children, Analysis 1.5) or for both routes of administration (RR 0.75, 95% CI 0.45 to 1.24, P = 0.26, 359 children, Analysis 1.5). Some heterogeneity was present in the analysis combining both routes of administration (I2 = 54%), which is probably due to the differences in routes of administration, although the test for subgroup differences did not reach statistical significance (test for subgroup differences: Chi2 = 2.20, df = 1, P = 0.14, I2 = 54.5%).
2. Recurrence of convulsions within 24 hours from stopping of the presenting convulsion.
All three trials reported the number of children with recurrences of seizures within 24 hours. There was no statistically significant difference between the treatments when administered intravenously (RR 0.91, 95% CI 0.65 to 1.27, P = 0.56, 414 children, Analysis 1.6), rectally (RR 0.19, 95% CI 0.01 to 2.92, P = 0.23, 25 children, Analysis 1.6) or for both routes of administration (RR 0.86, 95% CI 0.61 to 1.20, P = 0.36, moderate‐quality evidence, 439 children, Analysis 1.6). There was no heterogeneity present in the pooled analyses and no differences by route of administration were found (test for subgroup differences: Chi2 = 1.23, df = 1, P = 0.27, I2 = 19.0%).
3. Incidence of admissions to the intensive care unit (ICU)
Appleton 1995 reported the number of admissions to the ICU. There was no statistically significant difference between the intravenously (RR 0.07, 95% CI 0.00 to 1.22, P = 0.07, 61 children, Analysis 1.7), and rectally (RR 0.57, 95% CI 0.03 to 10.51, P = 0.71, 25 children, Analysis 1.7) separately. However, when combining both routes of administration, significantly more children who received diazepam were admitted to the ICU (10 compared to 0 who received lorazepam) (RR 0.15, 95% CI 0.02 to 0.98, P = 0.05, low‐quality evidence, 86 children, Analysis 1.7). There was very little heterogeneity present in the pooled analysis and no difference by route of administration (test for subgroup differences: Chi2 = 0.99, df = 1, P = 0.32, I2 = 0%).
2. Intranasal lorazepam versus intramuscular paraldehyde
One trial (Ahmad 2006), recruiting 160 participants, compared intranasal lorazepam to intramuscular paraldehyde.
Primary outcomes
1. Presenting convulsion/episode of convulsive status epilepticus terminated with the drug(s) used
There was no statistically significant difference between the intranasal lorazepam and intramuscular paraldehyde groups for stopping the presenting seizure, with 60/80 (75%) in the intranasal lorazepam group compared to 49/80 (61%) in the intramuscular paraldehyde group: RR 1.22, 95% CI 0.99 to 1.52, P = 0.07, moderate‐quality evidence, Analysis 2.1.
2. Time taken from administration of any drug in the hospital to stopping the convulsion
This outcome was not reported in the trial.
3. The incidence of specific adverse effects: respiratory depression/arrest; cardiac arrhythmia; hypotension and extravasation of any intravenously‐administered anticonvulsant
There was no difference between the treatment groups for clinically‐important cardiorespiratory events (low‐quality evidence).
Secondary outcomes
1. The need to use additional anti‐epileptic drugs to stop the presenting convulsion
Statistically significantly more children (8/80 (10%)) in the intranasal lorazepam group required two or more additional anticonvulsant doses to stop the seizures, compared to 21/80 children (26%) in the intramuscular paraldehyde group: RR 0.38, 95% CI 0.18 to 0.81, P = 0.01, low‐quality evidence, Analysis 2.2.
2. Recurrence of convulsions within 24 hours from stopping of the presenting convulsion
There was no statistically significant difference between the treatment groups for seizure recurrence within 24 hours: RR 0.73, 95% CI 0.31 to 1.71, P = 0.47, low‐quality evidence, Analysis 2.3.
3. Incidence of admissions to the intensive care unit (ICU)
This outcome was not reported in the trial.
3. Intravenous lorazepam versus intravenous diazepam/intravenous phenytoin combination
One trial (Sreenath 2010), recruiting 178 participants, compared intravenous lorazepam to intravenous diazepam/intravenous phenytoin combination.
Primary outcomes
1. Presenting convulsion/episode of convulsive status epilepticus stopped with the drug(s) used
There was no difference between intravenous lorazepam and intravenous diazepam‐phenytoin combination for seizure cessation within 10 minutes (100% in both groups: RR 1.00, 95% CI 0.98 to 1.02, P = 1.00, moderate‐quality evidence, Analysis 3.1.
2. Time taken from administration of any drug in the hospital to stopping of the convulsion
There was no statistically significant difference in the median time to seizure cessation (20 seconds in each group, moderate‐quality evidence).
3. The incidence of specific adverse effects: respiratory depression/arrest; cardiac arrhythmia; hypotension and extravasation of any intravenously‐administered anticonvulsant
Four participants in the intravenous lorazepam group experienced respiratory depression, compared to five in the intravenous diazepam‐phenytoin combination group. This difference was not statistically significant: RR 0.78, 95% CI 0.22 to 2.82, P = 0.71, moderate‐quality evidence, Analysis 3.2.
Secondary outcomes
1. The need to use additional anti‐epileptic drugs to stop the presenting convulsion
No additional anti‐epileptic drugs were required, as seizures stopped in all children within 10 minutes (Analysis 3.1). However, only six participants in the intravenous lorazepam group required more than one dose of the trial drug to stop the seizures, compared to 14 in the intravenous diazepam‐phenytoin combination group. This difference was not statistically significant: RR 0.42, 95% CI 0.17 to 1.04, P = 0.06, moderate‐quality evidence, Analysis 3.3.
2. Recurrence of convulsions within 24 hours from stopping of the presenting convulsion
There were no seizure recurrences in either group (moderate‐quality evidence). The authors suggest that the lack of recurrences in the diazepam‐phenytoin group may have been due to the addition of phenytoin as a longer‐acting drug.
3. Incidence of admissions to the intensive care unit (ICU)
This outcome was not reported in the trial.
4. Intravenous lorazepam versus intranasal lorazepam
One trial (Arya 2011), recruiting 141 participants, compared intravenous lorazepam to intranasal lorazepam. Results are presented for the subgroup of 58 participants with GTC.
Primary outcomes
1. Presenting convulsion/episode of convulsive status epilepticus stopped with the drug(s) used
There were no statistically significant differences between intravenous and intranasal lorazepam for seizure cessation within 10 minutes: RR 1.07, 95% CI 0.77 to 1.49, P = 0.70, moderate‐quality evidence, or within one hour: RR 0.70, 95% CI 0.43 to 1.17, P = 0.17, Analysis 4.1.
2. Time taken from administration of any drug in the hospital to stopping of the convulsion
Median time to achieve seizure control from drug administration was four minutes in both groups (moderate‐quality evidence).
The authors note that across all participants (including those without GTC), the time taken to achieve intravenous access ranged from one to 25 minutes, with a median of four minutes. If this had been included in the response time for the intravenous lorazepam, the results would have been skewed significantly in favour of intranasal lorazepam.
3. The incidence of specific adverse effects: respiratory depression/arrest; cardiac arrhythmia; hypotension and extravasation of any intravenously‐administered anticonvulsant
Results were not available for the subgroup of participants with GTC. Across all participants (including those without GTC), one child from the intranasal group and two children from the intravenous group required respiratory support ( moderate‐quality evidence) No participant in either group demonstrated significant hypotension.
Secondary outcomes
1. The need to use additional anti‐epileptic drugs to stop the presenting convulsion
This outcome was not reported in the trial.
2. Recurrence of convulsions within 24 hours from stopping of the presenting convulsion
This outcome was not reported in the trial.
3. Incidence of admissions to the intensive care unit (ICU)
This outcome was not reported in the trial.
5. Buccal midazolam versus rectal diazepam
Four trials, recruiting 648 participants, compared buccal midazolam to rectal diazepam (Ashrafi 2010; Baysun 2005; McIntyre 2005; Mpimbaza 2008). One trial (177 participants; McIntyre 2005) reported on 219 seizure episodes; in other words, the same child was randomised and treated for multiple seizures in the same trial. Results are not available at the participant level so results reported for McIntyre 2005 are by episode. This is a limitation, as meta‐analysis assumes independence between measurements, and more than one treated seizure per child would not be statistically independent. A result of ignoring this unit‐of‐analysis issue could be overoptimistic confidence intervals.
Primary outcomes
1. Presenting convulsion/episode of convulsive status epilepticus stopped with the drug(s) used
All four studies reported the number of children with their presenting seizure(s) stopped by the trial drug. Buccal midazolam was statistically significantly more effective than rectal diazepam for seizure cessation: RR 1.25, 95% CI 1.13 to 1.38, P < 0.001, very low‐quality evidence, Analysis 5.1.
However, there was considerable heterogeneity in the analysis (I2 = 81%). When we repeated the analysis with a random‐effects model, there was no statistically significant difference between the treatments: RR 1.23, 95% CI 0.98 to 1.54, P = 0.08. The heterogeneity may be due to the four trials measuring seizure cessation at different time points (Ashrafi 2010 five minutes; RR 1.22, 95% CI 1.07 to 1.40, P = 0.004; Baysun 2005 and Mpimbaza 2008 10 minutes; RR 1.07, 95% CI 0.95 to 1.21, P = 0.26; McIntyre 2005 one hour; RR 2.05, 95% CI 1.45 to 2.91, P < 0.001). We considered these different times in a subgroup analysis and found a significant difference between the subgroups (test for subgroup differences: Chi2 = 12.35, df = 2, P = 0.002, I2 = 83.8%, Analysis 5.1).
Seizure cessation data at one hour were also provided by the authors of Mpimbaza 2008, showing that buccal midazolam was significantly more effective than rectal diazepam: RR 1.42, 95% CI 1.06 to 1.90.
The doses of the drugs used in the studies were also different: Baysun 2005 used 0.25 mg/kg for buccal midazolam, whereas Mpimbaza 2008 and McIntyre 2005 used 0.5 mg/kg, and Ashrafi 2010 used 0.3 to 0.5 mg/kg. Furthermore, in Mpimbaza 2008 67.3% of the children had malaria and 13.7% had cerebral malaria; when only children without malaria were analysed, buccal midazolam was statistically significantly more effective than rectal diazepam for seizure cessation (RR 2.11, 95% CI 1.26 to 3.54; data provided by the author). Furthermore, for the subgroup of children with only GTC, 109/135 (80.7%) in the buccal midazolam group compared to 97/134 (72.4%) in the rectal diazepam group had seizure cessation; RR 1.12, 95% CI 0.98 to 1.27; data provided by the author.
2. Time taken from administration of any drug in the hospital to stopping of the convulsion
Baysun 2005 noted that there was no difference between groups in the time from drug administration to seizure cessation, and Ashrafi 2010 reported that both the median treatment initiation time and drug effect time were significantly shorter in the buccal midazolam group than in the rectal diazepam group (low‐quality evidence).
McIntyre 2005 and Mpimbaza 2008 did not report this outcome.
3. The incidence of specific adverse effects: respiratory depression/arrest; cardiac arrhythmia; hypotension and extravasation of any intravenously‐administered anticonvulsant
All four studies reported on the incidence of respiratory depression. Across the four trials, 25/346 in the buccal midazolam groups and 26/344 in the rectal diazepam groups experienced respiratory depression, but this difference was not statistically significant; RR 0.88, 95% 0.61 to 1.25, P = 0.47, low‐quality evidence, Analysis 5.2.
Secondary outcomes
1. The need to use additional anti‐epileptic drugs to stop the presenting convulsion
McIntyre 2005 reported that fewer children in the buccal midazolam group required intravenous lorazepam to stop the seizure compared to the rectal diazepam group; RR 0.58, 95% CI 0.42 to 0.79, P < 0.001, low‐quality evidence, Analysis 5.3. Baysun 2005 also noted no difference in the need for a second drug.
Ashrafi 2010 and Mpimbaza 2008 did not report this outcome.
2. Recurrence of convulsions within 24 hours from stopping of the presenting convulsion
None of the trials reported this outcome.
3. Incidence of admissions to the intensive care unit (ICU)
None of the trials reported this outcome.
6. Buccal midazolam versus intravenous diazepam
One trial (Talukdar 2009), recruiting 120 participants, compared buccal midazolam to intravenous diazepam.
Primary outcomes
1. Presenting convulsion/episode of convulsive status epilepticus stopped with the drug(s) used.
There was no statistically significant difference in seizure cessation rates between the groups treated with buccal midazolam or intravenous diazepam: RR 0.91, 95% CI 0.80 to 1.03, P = 0.15, high‐quality evidence, Analysis 6.1. Both treatments were effective, with 85% seizure cessation rate for buccal midazolam and 93% for intravenous diazepam.
2. Time taken from administration of any drug in the hospital to stopping of the convulsion
The time to control of the seizure from drug administration (time for drug effect) was significantly shorter for intravenous diazepam compared to buccal midazolam (mean difference 0.56 minutes, 95% CI 0.29 to 0.83, P < 0.001, moderate‐quality evidence, Analysis 6.2). However the mean time for initiation of treatment was significantly shorter in the buccal midazolam group (mean difference ‐1.09 minutes, 95% CI ‐1.31 to ‐0.87, P < 0.001, Analysis 6.2), making the mean total time to controlling the seizures significantly shorter in the buccal midazolam group compared to the intravenous diazepam group (mean difference ‐0.59 minutes, 95% CI ‐0.96 to ‐0.22, P = 0.002, Analysis 6.2). The faster drug action of intravenous diazepam is therefore compromised by the need to gain intravenous access.
3. The incidence of specific adverse effects: respiratory depression/arrest; cardiac arrhythmia; hypotension and extravasation of any intravenously‐administered anticonvulsant
There were no significant adverse events in either group (high‐quality evidence).
Secondary outcomes
1. The need to use additional anti‐epileptic drugs to stop the presenting convulsion
This outcome was not reported in the trial.
2. Recurrence of convulsions within 24 hours from stopping of the presenting convulsion
This outcome was not reported in the trial.
3. Incidence of admissions to the intensive care unit (ICU)
This outcome was not reported in the trial.
7. Intranasal midazolam versus intravenous diazepam
Three trials, recruiting 174 participants, compared intranasal midazolam to intravenous diazepam (Javadzadeh 2012; Lahat 2000; Mahmoudian 2004).
Primary outcomes
1. Presenting convulsion/episode of convulsive status epilepticus stopped with the drug(s) used
Two of the trials reported the number of children with their presenting seizure(s) stopped by the trial drug (Lahat 2000; Mahmoudian 2004).
Most of the children in the two trials experienced seizure cessation, with no statistically significant difference between treatments; RR 0.98, 95% CI 0.91 to 1.06, P = 0.67, 122 children, moderate‐quality evidence, Analysis 7.1. There was no heterogeneity present in the analysis (I2 = 0%).
2. Time taken from administration of any drug in the hospital to stopping of the convulsion
Lahat 2000 reported that the mean time for initiation of treatment was significantly shorter in the intranasal midazolam group compared to the intravenous diazepam group (mean difference ‐2.00 minutes, 95% CI ‐3.03 to ‐0.97, P < 0.001, 52 children, Analysis 7.2). Mahmoudian 2004 also stated that the time from seizure onset to treatment was faster in the midazolam group due to cannula insertion in the diazepam group (numerical data not reported).
There was no statistically significant difference between groups in two trials (Lahat 2000; Mahmoudian 2004) in the time to control of the seizure from drug administration (time for drug effect): mean difference 0.62 minutes, 95% CI ‐0.14 to 1.38, P = 0.11, 122 children, moderate‐quality evidence, Analysis 7.2.
Overall, the mean total time to controlling the seizures was significantly shorter in the intravenous diazepam group compared to the intranasal midazolam group in two trials (Javadzadeh 2012; Lahat 2000): mean difference 0.80, 95% CI 0.24 to 1.35, P = 0.005, 112 children, Analysis 7.2. There is considerable heterogeneity in this analysis (I2 = 85%), which probably originated from the calculation of the total time to controlling seizures in the Javadzadeh 2012 trial, which seems to adjust for the time taken to insert an intravenous line.
3. The incidence of specific adverse effects: respiratory depression/arrest; cardiac arrhythmia; hypotension and extravasation of any intravenously‐administered anticonvulsant
Lahat 2000 and Mahmoudian 2004 stated that no adverse events, including respiratory depression, occurred in either group (high‐quality evidence).
Secondary outcomes
1. The need to use additional anti‐epileptic drugs to stop the presenting convulsion
None of the trials reported this outcome.
2. Recurrence of convulsions within 24 hours from stopping of the presenting convulsion
None of the trials reported this outcome.
3. Incidence of admissions to the intensive care unit (ICU)
Mahmoudian 2004 stated that no children required admission to the ICU (high‐quality evidence).
8. Intranasal midazolam and rectal diazepam
One trial (Fişgin 2002), recruiting 45 participants, compared intranasal midazolam and rectal diazepam.
Primary outcomes
1. Presenting convulsion/episode of convulsive status epilepticus stopped with the drug(s) used
Intranasal midazolam was significantly more effective than rectal diazepam in stopping seizures within 10 minutes; 20/23 children with stopped seizures in the intranasal midazolam group, compared to 13/22 in the rectal diazepam group: RR 1.47, 95% CI 1.00 to 2.16, P = 0.05, low‐quality evidence, Analysis 8.1.
2. Time taken from administration of any drug in the hospital to stopping of the convulsion.
This outcome was not reported in the trial.
3. The incidence of specific adverse effects: respiratory depression/arrest; cardiac arrhythmia; hypotension and extravasation of any intravenously‐administered anticonvulsant
There was no significant difference between the two groups for of cardiorespiratory or adverse effects (low‐quality evidence).
Secondary outcomes
1. The need to use additional anti‐epileptic drugs to stop the presenting convulsion
The requirement for a second drug to treat the seizures was higher in the rectal diazepam group (9/22 children compared to 3/23 children in the intranasal midazolam group), but this was not statistically significant; RR 0.32, 95% CI 0.10 to 1.03, P = 0.06, low‐quality evidence, Analysis 8.2.
2. Recurrence of convulsions within 24 hours from stopping of the presenting convulsion
Data were only collected for one hour after seizure onset, so there is no information about seizure recurrence over 24 hours in Fişgin 2002.
3. Incidence of admissions to the intensive care unit (ICU)
This outcome was not reported in the trial.
9. Intramuscular midazolam versus intravenous diazepam
Two trials, recruiting 138 participants, compared intramuscular midazolam to intravenous diazepam (Chamberlain 1997; Shah 2005). Shah 2005 included some non‐randomised participants who received intravenous diazepam as they already had intravenous access; only randomised children are reported in this review. Chamberlain 1997 reported that one child was enrolled in the study twice, so is represented in both groups. It was not possible to identify this child in the reported results so we note that results presented in this section must be interpreted with caution, due to the representation of this child in both treatment groups.
Primary outcomes
1. Presenting convulsion/episode of convulsive status epilepticus stopped with the drug(s) used
Both trials reported the number of children with their presenting seizure(s) stopped by the trial drug.There was no statistically significant difference between the treatments; RR 0.97, 95% CI 0.87 to 1.09, P = 0.66, low‐quality evidence, Analysis 9.1. There was no heterogeneity present in the analysis (I2 = 0%).
2. Time taken from administration of any drug in the hospital to stopping of the convulsion
Chamberlain 1997 reported that the time after arrival to initiating treatment was shorter in the intramuscular midazolam group compared to the intravenous diazepam group (mean difference ‐4.50 minutes, 95% CI ‐6.68 to ‐2.32, P < 0.001, 24 children, Analysis 9.2) and that this offsets the time to drug effect of the two treatments (mean difference 1.10 minutes, 95% CI ‐0.91 to 3.11, P = 0.28, Analysis 9.2), resulting in an overall shorter time to cessation of seizures in the intramuscular midazolam group.
This is demonstrated in both trials, with the mean total cessation time converted from seconds to minutes to allow meta‐analysis; mean difference ‐2.68 minutes, 95% CI ‐3.94 to ‐1.42, P < 0.001, 105 children, very low‐quality evidence, Analysis 9.2
Chamberlain 1997 also reported that delayed seizure control (between five and 10 minutes) occurred in four midazolam participants and one diazepam participant, but this did not reach statistical significance.
3. The incidence of specific adverse effects: respiratory depression/arrest; cardiac arrhythmia; hypotension and extravasation of any intravenously‐ administered anticonvulsant
Chamberlain 1997 reported that there were no significant complications. Shah 2005 reported that there were no occurrences of hypotension or respiratory depression, but identified an important adverse effect in that seven (10.8%) of the children treated with intravenous diazepam developed thrombophlebitis, while none in the intramuscular midazolam group had complications.
Secondary outcomes
1. The need to use additional anti‐epileptic drugs to stop the presenting convulsion
Both trials reported the number of children requiring additional drugs to stop their presenting seizure(s).There was no statistically significant difference between the treatments; RR 1.34, 95% CI 0.35 to 5.13, P = 0.67, 105 children, very low‐quality evidence, Analysis 9.3. There was no heterogeneity in the analysis (I2 = 0%).
2. Recurrence of convulsions within 24 hours from stopping of the presenting convulsion
Chamberlain 1997 reported that after initial seizure cessation four participants in each group had recurrent seizures requiring further medication, with one child from each group having a recurrence within the first 15 minutes. There was no statistically significant difference between groups at 15 minutes (RR 0.85, 95% CI 0.06 to 12.01, P = 0.90) or at one hour (RR 0.85, 95% CI 0.27 to 2.62, P = 0.77, very low‐quality evidence, Analysis 9.4). Shah 2005 did not report this outcome.
3. Incidence of admissions to the intensive care unit (ICU)
Shah 2005 reported that there were no ICU admissions (moderate‐quality evidence). Chamberlain 1997 did not report this outcome.
10. Intramuscular midazolam versus rectal diazepam
One trial (Momen 2015), recruiting 100 participants, compared intramuscular midazolam to rectal diazepam.
Primary outcomes
1. Presenting convulsion/episode of convulsive status epilepticus stopped with the drug(s) used
Presenting convulsions were stopped for most participants (48/50 in the intramuscular midazolam group and 47/50 in the rectal diazepam group) with no significant difference between the treatments: RR 1.02, 95% CI 0.93 to 1.12, P = 0.65, moderate‐quality evidence, Analysis 10.1.
2. Time taken from administration of any drug in the hospital to stopping of the convulsion
Time from administration of drug to seizure cessation was expressed in terms of medians in Momen 2015, so we cannot present data as a forest plot for this review and we report the results narratively.
There was a statistically significant difference in time from administration to seizure cessation in favour of midazolam: median 66 seconds; diazepam: median 130 seconds, P < 0.001 (moderate‐quality evidence). We note that the speed of administration was similar for both medications, so this seems to reflect a medication difference.
3. The incidence of specific adverse effects: respiratory depression/arrest; cardiac arrhythmia; hypotension and extravasation of any intravenously‐administered anticonvulsant
No participants developed respiratory depression, except for one child who received an accidental double‐dose of midazolam (moderate‐quality evidence).
Secondary outcomes
1. The need to use additional anti‐epileptic drugs to stop the presenting convulsion
This outcome was not reported in the trial.
2. Recurrence of convulsions within 24 hours from stopping of the presenting convulsion
Among those who achieved seizure cessation (see Analysis 10.1), there was no recurrence within 60 minutes (moderate‐quality evidence). No data are available for recurrence up to 24 hours.
3. Incidence of admissions to the intensive care unit (ICU)
This outcome was not reported in the trial.
11. Intravenous midazolam versus intravenous diazepam
One trial (Gathwala 2012), recruiting 80 participants, compared intravenous midazolam to intravenous diazepam.
Primary outcomes
1. Presenting convulsion/episode of convulsive status epilepticus stopped with the drug(s) used
The presenting seizure was stopped in most children, with no statistically significant difference between treatment groups: RR 1.08, 95% CI 0.97 to 1.21, P = 0.17, moderate‐quality evidence, Analysis 11.1.
2. Time taken from administration of any drug in the hospital to stopping of the convulsion
There was no statistically significant difference between treatments in the time to cessation of seizures; mean difference 7.68 seconds, 95% CI ‐6.73 to 22.09, P = 0.30, moderate‐quality evidence, Analysis 11.2.
3. The incidence of specific adverse effects: respiratory depression/arrest; cardiac arrhythmia; hypotension and extravasation of any intravenously‐administered anticonvulsant
One child in the intravenous diazepam group and no children in the intravenous midazolam group experienced respiratory depression; this difference was not statistically significant: RR 0.33, 95% CI 0.01 to 7.95, P = 0.50, moderate‐quality evidence, Analysis 11.3. Gathwala 2012 also reported that there was a significant increase in somnolence in the diazepam compared to the midazolam groups, but that other adverse effects were evenly distributed.
Secondary outcomes
1. The need to use additional anti‐epileptic drugs to stop the presenting convulsion
There was no statistically significant difference between treatments in the number of children requiring an additional dose of the trial drug to stop the seizure (one child in the midazolam group and four children in the diazepam group); RR 0.25, 95% CI 0.03 to 2.14, P = 0.21, moderate‐quality evidence, Analysis 11.4.
2. Recurrence of convulsions within 24 hours from stopping of the presenting convulsion
There was no statistically significant difference between treatment groups in the number of children with seizure recurrence within 24 hours (two children in the midazolam group and four children in the diazepam group); RR 0.50, 95% CI 0.10 to 2.58, P = 0.41, moderate‐quality evidence, Analysis 11.5.
3. Incidence of admissions to the intensive care unit (ICU)
This outcome was not reported in the trial.
12. Intravenous midazolam versus intravenous lorazepam
One trial (Gathwala 2012), recruiting 80 participants, compared Intravenous midazolam to intravenous lorazepam.
Primary outcomes
1. Presenting convulsion/episode of convulsive status epilepticus stopped with the drug(s) used
The presenting seizure was stopped in most children in the Gathwala 2012 trial; there was no statistically significant difference between treatment groups; RR 0.98, 95% CI 0.91 to 1.04, P = 0.48, moderate‐quality evidence, Analysis 12.1.
2. Time taken from administration of any drug in the hospital to stopping of the convulsion
There was no significant difference between treatment groups in the time to cessation of seizures; mean difference 1.50 seconds, 95% CI ‐9.37 to 12.37, P = 0.79, moderate‐quality evidence, Analysis 12.2.
3. The incidence of specific adverse effects: respiratory depression/arrest; cardiac arrhythmia; hypotension and extravasation of any intravenously‐administered anticonvulsant
There were no occurrences of respiratory depression in either group in the Gathwala 2012 trial. Gathwala 2012 also reported that other adverse effects were evenly distributed between the groups (high‐quality evidence).
Secondary outcomes
1. The need to use additional anti‐epileptic drugs to stop the presenting convulsion
There was no statistically significant difference between treatment groups in the number of children requiring an additional dose of the trial drug to stop the seizure (one child in the midazolam group and no children in the lorazepam group); RR 3.00, 95% CI 0.13 to 71.51, P = 0.50, moderate‐quality evidence, Analysis 12.3.
2. Recurrence of convulsions within 24 hours from stopping of the presenting convulsion
There was no statistically significant difference between treatment groups in the number of children with seizure recurrence within 24 hours (two children in each group); RR 1.00, 95% CI 0.15 to 6.76, P = 1.00, moderate‐quality evidence, Analysis 12.4.
3. Incidence of admissions to the intensive care unit (ICU)
This outcome was not reported in the trial.
Discussion
Summary of main results
The 14 newly‐identified studies in this updated review include a range of drug treatment options (midazolam, diazepam, lorazepam, paraldehyde and phenytoin), treatment doses (for the same drug) and a range of routes of administration (rectal, buccal, nasal, intramuscular and intravenous). A number of the new studies have evaluated and emphasised the use of non‐intravenous routes. These have included intranasal, intramuscular and buccal routes. The role of the intramuscular route in children is uncertain, particularly in view of its relatively invasive nature as well as potentially serious complications, including trauma to the sciatic nerve.
The 18 studies included in this review vary by design, setting and populations, in their ages and also in their clinical situation. We have conducted many comparisons of drugs and of routes of administration of drugs in this review, with the quality of the evidence for each comparison varying from high to very low, depending on the homogeneity and quality of design of the studies contributing to the comparison.
This update has shown that for intravenous administration, lorazepam and diazepam seem to be associated with similar rates of seizure cessation (Appleton 1995; Chamberlain 2014; Gathwala 2012), but risks of bias in the included studies and heterogeneity of design may have confounded the results.
Two studies in this update have shown that buccal midazolam may be associated with a higher rate of seizure cessation than rectal diazepam (Ashrafi 2010; Mpimbaza 2008). However, we are very uncertain about the estimate of this effect. In part, this reflects the different range of doses of buccal midazolam used in these studies and the different characteristics of their participants. A single study also provides moderate‐ to high‐quality evidence that buccal midazolam may be associated with a higher rate of seizure cessation than intravenous diazepam (Talukdar 2009). However, as for all studies included within this review, with different routes of administration, time to cessation of seizures was influenced by the way it was delivered.
There are currently insufficient data to determine whether there are any significant or clinically important differences in efficacy or safety between the buccal and intranasal routes of administration of midazolam; this issue will only be resolved by at least one robust RCT that compares buccal to intranasal midazolam. The intranasal route of administration was used in five studies and was compared with rectal or intravenous routes (Ahmad 2006; Arya 2011; Fişgin 2002; Javadzadeh 2012; Mahmoudian 2004). Generally, the intranasal/buccal/intramuscular routes appear to show similar rates of the most common (and most clinically important) primary outcome, seizure cessation, compared to intravenous routes of administration. However, the rapid action of the intravenously‐administered drug is compromised by the time taken to achieve intravenous access. This was particularly demonstrated in three studies (Arya 2011; Shah 2005; Talukdar 2009). This is an important issue, particularly in infants but also in older children who are in shock with circulatory collapse, and where intravenous access is likely to be more difficult and therefore delay effective anticonvulsive treatment.
Adverse side effects were observed very infrequently in the included studies. Respiratory depression was the most common and most clinically relevant side effect. Where reported in the study, the frequency ranged from none (Ashrafi 2010; Chamberlain 1997; Fişgin 2002; Mahmoudian 2004; Shah 2005; Talukdar 2009), to 1% to 2% (Arya 2011), almost 6% (Sreenath 2010) and up to almost 18% (Chamberlain 2014). The latter study defined respiratory depression as ‘assisted ventilation’; the incidence of respiratory depression is considerably higher than in the other studies that reported this outcome. None of the studies individually demonstrated any difference in the rates of respiratory depression between the different anticonvulsants or their different routes of administration; but when pooled, three studies provided moderate‐quality evidence that lorazepam was significantly associated with fewer occurrences of respiratory depression than diazepam (RR 0.72, 95% CI 0.55 to 0.93).
Overall completeness and applicability of evidence
The evidence presented in previous versions of the review has supported previously‐published open, anecdotal data. Buccal midazolam has become established as the first‐line non‐intravenous drug, and intravenous lorazepam has become established as the first‐line intravenous drug in treating an acute tonic‐clonic convulsion (and established convulsive status epilepticus) in children. The evidence has contributed to the evidence base for the Status Epilepticus Working Group to revise the convulsive status epilepticus guideline which was first published in 2000 (Working Party 2000) and has been incorporated into the partially revised and updated National Institute for Health and Care Excellence (NICE) Clinical Guideline in Epilepsy (NICE 2012) and the Advanced Paediatric Life Support (APLS) guidelines (APLS 2016).
Most studies were undertaken in unselected populations of children presenting to the Emergency Department (ED) of a single centre, or a group of centres (between three and 11) based either in a general or a children’s hospital. Consequently, these data are likely to be generalisable and applicable to other children with acute tonic‐clonic convulsions in this clinical situation. However, there were two studies undertaken in a very specific population, of African children, in whom cerebral malaria was the cause of the convulsion in 49% to 67% (Ahmad 2006; Mpimbaza 2008 respectively). The treatment arms in Ahmad 2006 were somewhat unusual, comprising intranasal lorazepam and intramuscular paraldehyde; no other study used these treatments. The authors justified the use of paraldehyde on the basis of it's being the “first or second‐line anticonvulsant agent in much of sub‐Saharan Africa because of its favourable safety and efficacy profile”. Paraldehyde has been used as an anticonvulsant for over 50 years. It is currently used when other anticonvulsants, including benzodiazepines or phenytoin and phenobarbital, have failed to stop an acute tonic–clonic convulsion and is often effective (Rowland 2009). The rectal route is preferred, because of the risk of sterile abscesses and damage to the sciatic nerve with the intramuscular route. Rectal paraldehyde is included in the UK’s APLS algorithm (APLS 2016) for the management of status epilepticus.
Two early studies (Chamberlain 1997; Lahat 2000) used a seizure duration of 10 rather than five minutes as the time to institute emergency treatment; all other studies used five or "at least 5" minutes, which is standard international practice.
The age range of the children in the 18 studies varied between birth and under 18 years. Most assessed children aged two months to 12 or 15 years, although two assessed a much narrower age range from two months to approximately five years (Ahmad 2006; Lahat 2000). Most epidemiological studies have demonstrated that more than 80% of children who present with an acute tonic‐clonic convulsion, including convulsive status epilepticus, are under 10 years of age, and of these most will be under five years of age. In addition, most causes of convulsive status epilepticus in children under five will be febrile status or due to an acute symptomatic cause. Consequently, this might introduce some bias in those studies that assessed only young children.
Quality of the evidence
The extent of the evidence provided by this updated review, both in terms of the 14 new studies (making a total of 18 and comprising 2199 participants) and their scientific robustness, has strengthened its quality and its conclusions. We have consequently achieved some of the objectives of the review.
Much of the evidence provided in this review is of moderate to high quality. However, the quality of the evidence provided for some important outcomes is low to very low, particularly for comparisons of non‐intravenous routes of drug administration. We downgraded the quality of the evidence due to imprecise results where limited data were available for analysis or where confidence intervals of effect sizes were wide, making interpretation of results difficult. Quality of the evidence was also downgraded due to the methodological inadequacies of some studies which may have introduced bias into the results, to study settings which were not applicable to wider clinical practice, and to inconsistency in some pooled analyses.
The dose of lorazepam in all preparations (predominantly intravenous, but also rectal and intranasal) was the same in all six studies where it was a treatment arm (0.1 mg/kg). In contrast, the dose of midazolam (in predominantly buccal but also intranasal preparation) ranged from 0.2 to 0.5 mg/kg, and the dose of either rectal or intravenous diazepam varied from 0.2 to 0.5 mg/kg. The reasons for the wide range of doses are not clear. Previous studies had not suggested that respiratory depression was a significant problem with doses of buccal midazolam of 0.5 mg/kg (McIntyre 2005), and the methodology and findings of this large study would have helped to inform subsequent studies on an effective and safe dose.
One potential bias throughout all the studies is how the original trial authors defined cessation of the seizure or convulsion following the intervention. Observer variation and inconsistency is well recognised when deciding when a tonic‐clonic convulsion has stopped. A proposed definition by Appleton (personal opinion) is that a tonic‐clonic convulsion has stopped when there is “no visible sign of ongoing rhythmic clonic activity”. The included studies' definitions of seizure cessation ranged from no definition to “the practitioner’s clinical judgement” to “generalized convulsions have stopped”, “cessation of all visible convulsive activity”, “cessation of all visible motor seizure activity” or “cessation of all motor activity”. The use of ‘motor activity’ is arguably too vague, as the brief, asymmetric and asynchronous myoclonus that commonly follows a tonic‐clonic seizure may be misinterpreted as “ongoing motor activity”; this is likely to impact on the efficacy result and lead to bias between studies.
Potential biases in the review process
It is unlikely that the methods used in this updated review will have introduced any significant bias. We successfully addressed outstanding queries and resolved them in most cases by personal contact with the leading or corresponding authors of the included studies.
We identified all relevant new studies, as far as we could ascertain. The methodology of most of the new studies was more robust than those included in the first review. However, there was some variation in methodology and the reporting of results between these studies, as detailed earlier in the review. Two of the 18 studies reported 100% seizure cessation in both treatment arms (Mahmoudian 2004; Sreenath 2010), and in one treatment arm (Ashrafi 2010), which is unusual as the median seizure‐cessation rate was approximately 75% in all other studies. In addition, the dose of intravenous diazepam in the two studies that reported 100% seizure cessation in both was the lowest used throughout the included studies (0.2 mg/kg).
Agreements and disagreements with other studies or reviews
This review is in broad general agreement with the recently‐published Evidence‐based guideline on the treatment of convulsive status epilepticus in children and adults, published by the Guideline Committee of the American Epilepsy Society (Glauser 2016).
Our findings are also consistent with a recent meta‐analysis (McMullan 2010) of midazolam versus diazepam in children and young adults which included many of the studies in this review. They also concluded that non‐intravenous midazolam was as effective as intravenous diazepam and that buccal midazolam was superior to rectal diazepam.
The above studies would appear to have been subjected to a similar systematic review process.

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Lorazepam versus diazepam, Outcome 1 Seizure cessation.

Comparison 1 Lorazepam versus diazepam, Outcome 2 Time from drug administration to stopping of seizures.
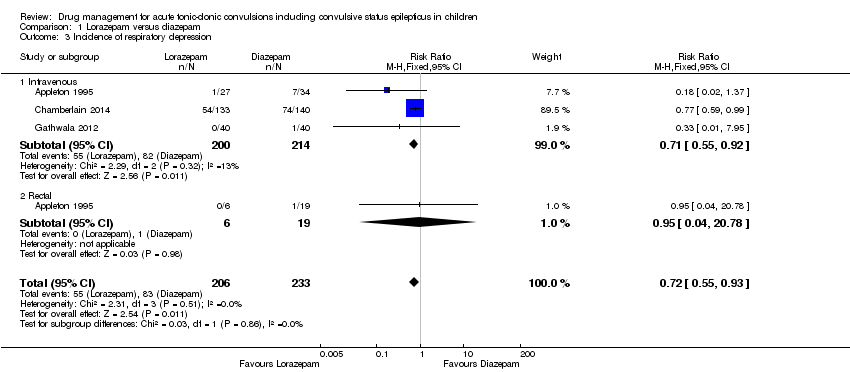
Comparison 1 Lorazepam versus diazepam, Outcome 3 Incidence of respiratory depression.

Comparison 1 Lorazepam versus diazepam, Outcome 4 Additional dose of the trial drug required to stop seizures.
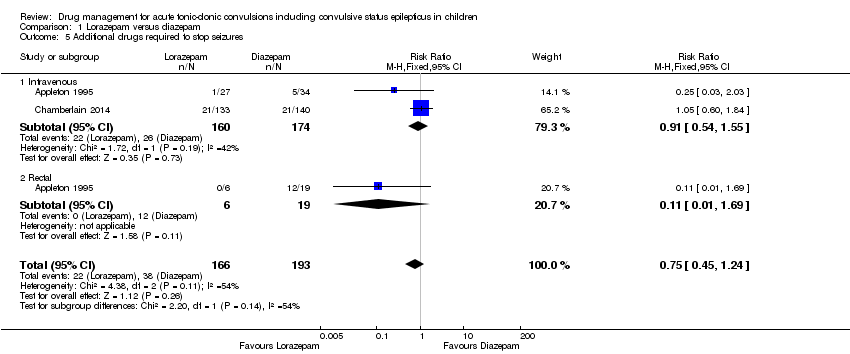
Comparison 1 Lorazepam versus diazepam, Outcome 5 Additional drugs required to stop seizures.

Comparison 1 Lorazepam versus diazepam, Outcome 6 Seizure recurrence within 24 hours.
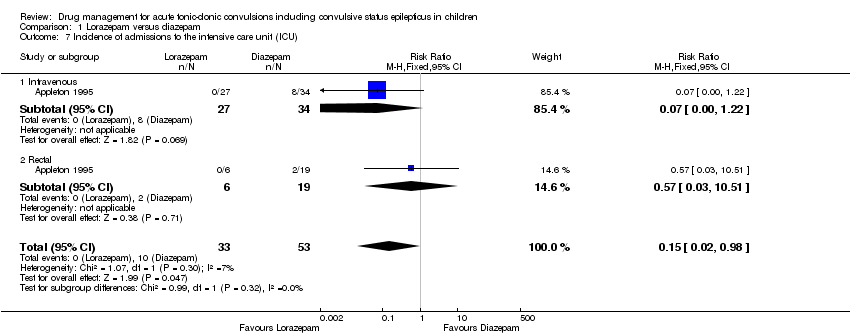
Comparison 1 Lorazepam versus diazepam, Outcome 7 Incidence of admissions to the intensive care unit (ICU).
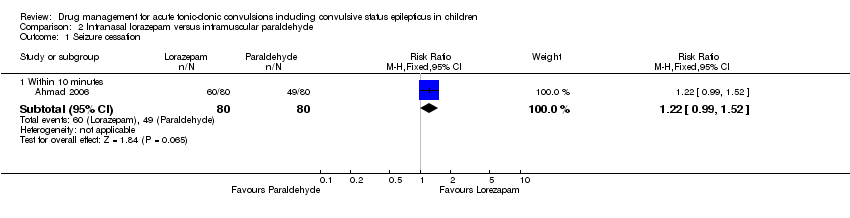
Comparison 2 Intranasal lorazepam versus intramuscular paraldehyde, Outcome 1 Seizure cessation.
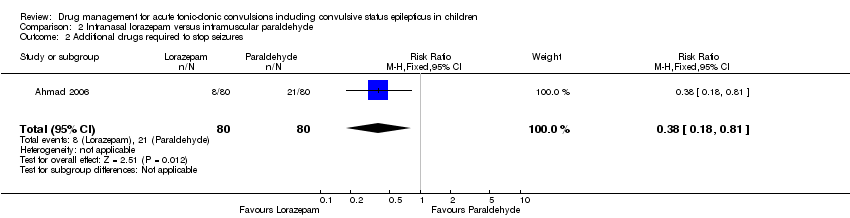
Comparison 2 Intranasal lorazepam versus intramuscular paraldehyde, Outcome 2 Additional drugs required to stop seizures.

Comparison 2 Intranasal lorazepam versus intramuscular paraldehyde, Outcome 3 Seizure recurrence within 24 hours.

Comparison 3 Intravenous lorazepam versus intravenous diazepam/intravenous phenytoin combination, Outcome 1 Seizure cessation.

Comparison 3 Intravenous lorazepam versus intravenous diazepam/intravenous phenytoin combination, Outcome 2 Incidence of respiratory depression.
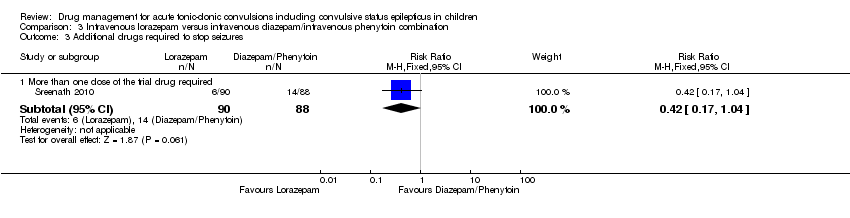
Comparison 3 Intravenous lorazepam versus intravenous diazepam/intravenous phenytoin combination, Outcome 3 Additional drugs required to stop seizures.
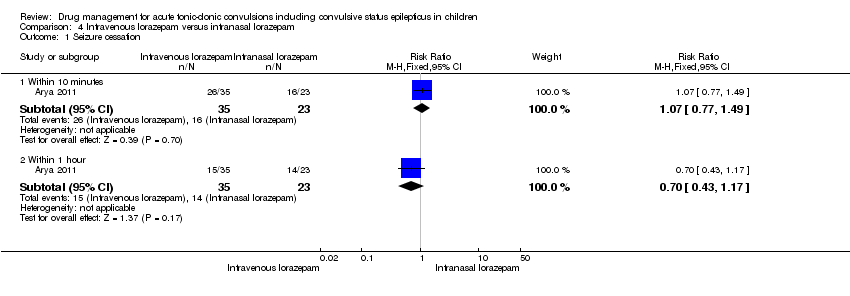
Comparison 4 Intravenous lorazepam versus intranasal lorazepam, Outcome 1 Seizure cessation.

Comparison 5 Buccal midazolam versus rectal diazepam, Outcome 1 Seizure cessation.

Comparison 5 Buccal midazolam versus rectal diazepam, Outcome 2 Incidence of respiratory depression.

Comparison 5 Buccal midazolam versus rectal diazepam, Outcome 3 Additional drugs required to stop seizures.
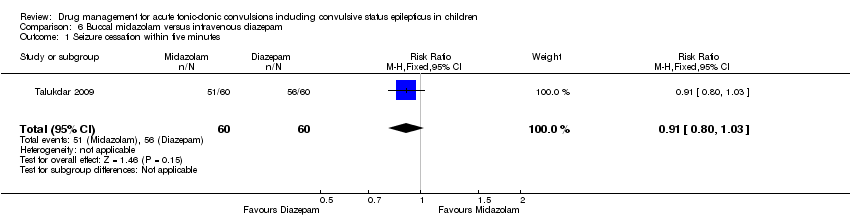
Comparison 6 Buccal midazolam versus intravenous diazepam, Outcome 1 Seizure cessation within five minutes.

Comparison 6 Buccal midazolam versus intravenous diazepam, Outcome 2 Time from drug administration to stopping of seizures.
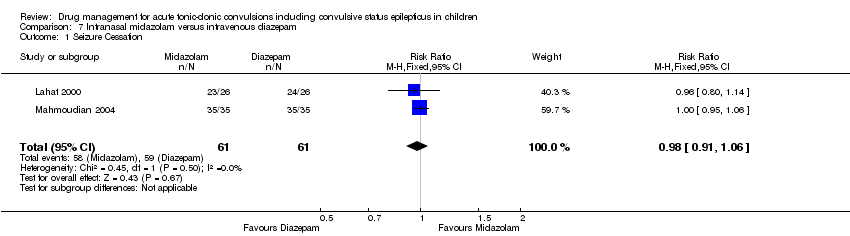
Comparison 7 Intranasal midazolam versus intravenous diazepam, Outcome 1 Seizure Cessation.
![Comparison 7 Intranasal midazolam versus intravenous diazepam, Outcome 2 Time from drug administration to stopping of seizures [minutes].](/es/cdsr/doi/10.1002/14651858.CD001905.pub3/media/CDSR/CD001905/image_n/nCD001905-CMP-007-02.png)
Comparison 7 Intranasal midazolam versus intravenous diazepam, Outcome 2 Time from drug administration to stopping of seizures [minutes].

Comparison 8 Intranasal midazolam versus rectal diazepam, Outcome 1 Seizure cessation.

Comparison 8 Intranasal midazolam versus rectal diazepam, Outcome 2 Additional drugs required to stop seizures.

Comparison 9 Intramuscular midazolam versus intravenous diazepam, Outcome 1 Seizure cessation.

Comparison 9 Intramuscular midazolam versus intravenous diazepam, Outcome 2 Time from drug administration to stopping of seizures (minutes).

Comparison 9 Intramuscular midazolam versus intravenous diazepam, Outcome 3 Additional drugs required to stop seizures.

Comparison 9 Intramuscular midazolam versus intravenous diazepam, Outcome 4 Seizure recurrence within 24 hours.
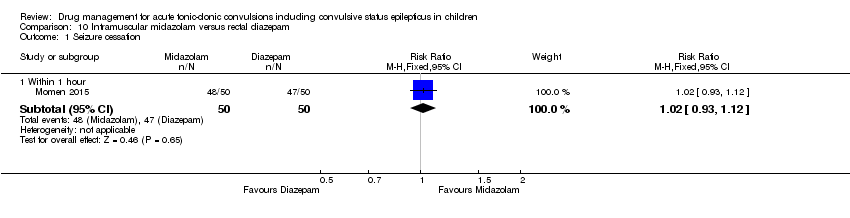
Comparison 10 Intramuscular midazolam versus rectal diazepam, Outcome 1 Seizure cessation.
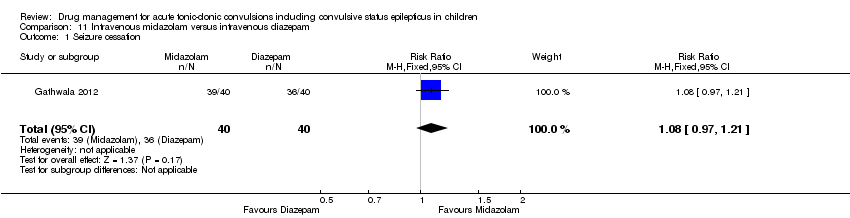
Comparison 11 Intravenous midazolam versus intravenous diazepam, Outcome 1 Seizure cessation.

Comparison 11 Intravenous midazolam versus intravenous diazepam, Outcome 2 Time from drug administration to stopping of seizures.
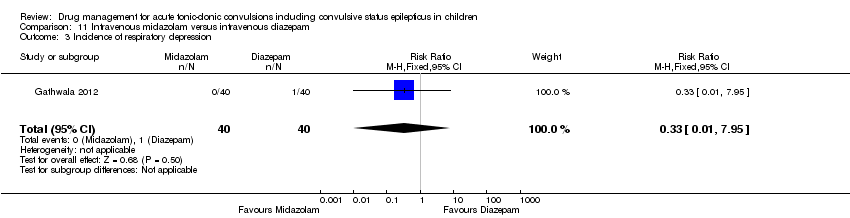
Comparison 11 Intravenous midazolam versus intravenous diazepam, Outcome 3 Incidence of respiratory depression.
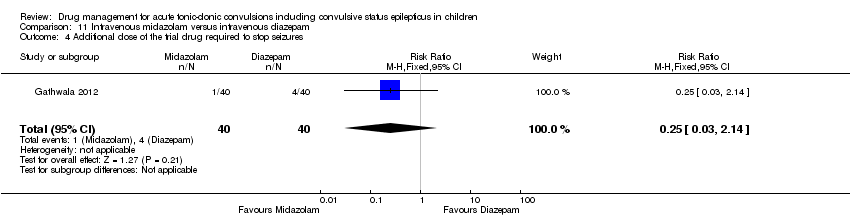
Comparison 11 Intravenous midazolam versus intravenous diazepam, Outcome 4 Additional dose of the trial drug required to stop seizures.
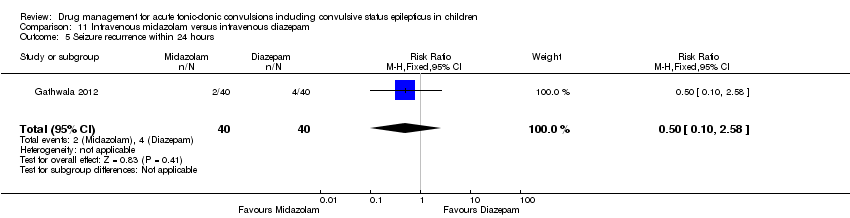
Comparison 11 Intravenous midazolam versus intravenous diazepam, Outcome 5 Seizure recurrence within 24 hours.

Comparison 12 Intravenous midazolam versus intravenous lorazepam, Outcome 1 Seizure cessation.

Comparison 12 Intravenous midazolam versus intravenous lorazepam, Outcome 2 Time from drug administration to stopping of seizures.

Comparison 12 Intravenous midazolam versus intravenous lorazepam, Outcome 3 Additional dose of the trial drug required to stop seizures.

Comparison 12 Intravenous midazolam versus intravenous lorazepam, Outcome 4 Seizure recurrence within 24 hours.
| Lorazepam compared with diazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Lorazepam Comparison: Diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Diazepam | Lorazepam | |||||
| Seizure cessation Follow‐up: up to 24 hours | 708 per 1000 | 765 per 1000 | RR 1.08 (0.98 to 1.20) | 439 | ⊕⊕⊝⊝ | In two trials, drugs were administered intravenously. In a third trial, drugs were administered intravenously or rectally if intravenous access was not possible Subgroup analysis showed a significant difference by route of intervention (intravenous: RR 1.04 (95% CI 0.94 to 1.16) compared to rectally RR: 2.86 (95% CI 1.47 to 5.55), test of subgroups P = 0.003) |
| Time from drug administration to termination of seizures Follow‐up: up to 24 hours | The mean time to cessation of seizures was 84.94 seconds in the diazepam group | The mean time to cessation of seizures was 6.18 faster (7.83 slower to 20.19 faster) in the lorazepam group | NA | 80 | ⊕⊕⊕⊝ | Drugs were administered intravenously Another trial (where drugs were administered intravenously or rectally) reported similar mean times to seizure cessation. Standard deviations were not available so data could not be entered into analysis |
| Incidence of respiratory depression Follow‐up: up to 24 hours | 356 per 1000 | 256 per 1000 | RR 0.72 (0.55 to 0.93) | 439 | ⊕⊕⊕⊝ | In two trials, drugs were administered intravenously. In a third trial, drugs were administered intravenously or rectally if intravenous access was not possible There was no difference between the routes of intervention (test of subgroups, P = 0.86) |
| Additional drugs required to terminate the seizure: additional dose of study drug Follow‐up: up to 24 hours | 305 per 1000 | 268 per 1000 | RR 0.88 (0.64 to 1.20) | 439 | ⊕⊕⊝⊝ | In two trials, drugs were administered intravenously. In a third trial, drugs were administered intravenously or rectally if intravenous access was not possible Subgroup analysis by route of intervention (intravenous: RR 0.97 (95% CI 0.71 to 1.33) compared to rectally RR: 0.11 (95% CI 0.01 to 1.56), test of subgroups P = 0.11). Two trials also reported whether additional (other) antiepileptic drugs were required to stop the seizure. There were no significant differences overall or by route of intervention |
| Seizure recurrence within 24 hours Follow‐up: up to 24 hours | 266 per 1000 | 229 per 1000 | RR 0.86 (0.61 to 1.20) | 439 | ⊕⊕⊕⊝ | In two trials, drugs were administered intravenously. In a third trial, drugs were administered intravenously or rectally if intravenous access was not possible There was no difference between the routes of intervention (test of subgroups, P = 0.27) |
| Incidence of admissions to the ICU Follow‐up: up to 24 hours | 116 per 1000 | 17 per 1000 | RR 0.15 (0.02 to 0.98) | 86 | ⊕⊕⊝⊝ | In the included trial, drugs were administered intravenously or rectally if intravenous access was not possible There was no difference between the routes of intervention (test of subgroups P = 0.32). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to risk of bias: one included study was quasi‐randomised, which may have led to selection bias and an intention‐to‐treat approach was not used in the study. | ||||||
| Intranasal lorazepam compared with intramuscular paraldehyde for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intranasal lorazepam Comparison: Intramuscular paraldehyde | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intramuscular paraldehyde | Intranasal lorazepam | |||||
| Seizure cessation: within 10 minutes Follow‐up: up to 24 hours | 613 per 1000 | 747 per 1000 | RR 1.22 (0.99 to 1.52) | 160 (1 study) | ⊕⊕⊕⊝ | ‐ |
| Time from drug administration to termination of seizures Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Incidence of respiratory depression Follow‐up: up to 24 hours | No difference was found between either treatment group in terms of clinically important cardiorespiratory events. | NA | 160 (1 study) | ⊕⊕⊝⊝ | ‐ | |
| Additional drugs required to terminate the seizure: 2 or more additional anticonvulsants required Follow‐up: up to 24 hours | 263 per 1000 | 100 per 1000 | RR 0.38 (0.18 to 0.81) | 160 (1 study) | ⊕⊕⊝⊝ | ‐ |
| Seizure recurrence within 24 hours Follow‐up: up to 24 hours | 138 per 1000 | 100 per 1000 | RR 0.73 (0.31 to 1.71) | 160 (1 study) | ⊕⊕⊝⊝ | ‐ |
| Incidence of admissions to the ICU Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to applicability: a high proportion of the children recruited had either cerebral malaria or meningitis. These comorbidities may have impacted upon the results. | ||||||
| Intravenous lorazepam compared with intravenous diazepam/intravenous phenytoin combination for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intravenous lorazepam Comparison: Intravenous diazepam/intravenous phenytoin combination | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous diazepam/intravenous phenytoin combination | Intravenous lorazepam | |||||
| Seizure cessation: within 10 minutes Follow‐up: up to 24 hours | Seizures were stopped for all individuals in the Intravenous diazepam/intravenous phenytoin combination group | Seizures were stopped for all individuals in the Intravenous lorazepam group | RR 1.00 (0.98 to 1.02) | 178 | ⊕⊕⊕⊝ | ‐ |
| Time from drug administration to stopping of seizures Follow‐up: up to 24 hours | There was no significant difference in the median time to seizure cessation (20 seconds in each group). | NA | 178 | ⊕⊕⊕⊝ | ‐ | |
| Incidence of respiratory depression Follow‐up: up to 24 hours | 57 per 1000 | 44 per 1000 (13 to 160) | RR 0.78 (0.22 to 2.82) | 178 | ⊕⊕⊕⊝ | ‐ |
| Additional drugs required to stop the seizure Follow‐up: up to 24 hours | 159 per 1000 | 67 per 1000 (27 to 165) | RR 0.42 (0.17 to 1.04) | 178 | ⊕⊕⊕⊝ | ‐ |
| Seizure recurrence within 24 hours Follow‐up: up to 24 hours | There were no seizure recurrences in either group. | NA | 178 | ⊕⊕⊕⊝ | ‐ | |
| Incidence of admissions to the ICU Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due toapplicability: Both treatment arms showed a 100% seizure cessation rate, which is higher than expected. Unclear whether this high success rate was due to a particular element of the trial design. | ||||||
| Intravenous lorazepam compared with intranasal lorazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intravenous lorazepam Comparison: Intranasal lorazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intranasal lorazepam | Intravenous lorazepam | |||||
| Seizure cessation: within 10 minutes Follow‐up: up to 24 hours | 696 per 1000 | 744 per 1000 | RR 1.07 (0.77 to 1.49) | 58 | ⊕⊕⊕⊝ | There was also no significant difference between treatments for seizure cessation at 1 hour: RR 0.70 (95% CI 0.43 to 1.17) |
| Time from drug administration to stopping of seizures Follow‐up: up to 24 hours | Median time to achieve seizure control from drug administration was 4 minutes in both groups. | NA | 58 | ⊕⊕⊕⊝ | Time taken to achieve intravenous access ranged from 1 to 25 minutes with a median of 4 minutes across all participants in the trial. If this had been included in the response time for the intravenous lorazepam, the results would have been skewed significantly in favour of intranasal lorazepam | |
| Incidence of respiratory depression Follow‐up: up to 24 hours | One child required respiratory support | Two children required respiratory support | NA | 141 | ⊕⊕⊕⊝ | Incidence of respiratory depression was not reported for the subgroup of participants with generalised tonic‐clonic seizures in the trial, therefore these results refer to all participants (including 83 participants without generalised tonic‐clonic seizures). |
| Additional drugs required to stop the seizure Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Seizure recurrence within 24 hours Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Incidence of admissions to the ICU Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded once due to imprecision: imbalance in the number of participants randomised to each intervention with generalised tonic‐clonic seizures and overall direction of effect seems to change when measured at 10 minutes or at 1 hour 2Downgraded once due to imprecision: limited numerical data reported. | ||||||
| Buccal midazolam compared with rectal diazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Buccal midazolam Comparison: Rectal diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Rectal diazepam | Buccal midazolam | |||||
| Seizure cessation: within 5 minutes to 1 hour Follow‐up: up to 24 hours | 584 per 1000 | 730 per 1000 | RR 1.25 (1.13 to 1.38) | 648 (4 trials) 690 seizure episodes | ⊕⊝⊝⊝ | The measurement time of seizure cessation was examined in a subgroup analysis 5 minutes: RR 1.22 (95% CI 1.07 to 1.40, P = 0.004); |
| Time from drug administration to of seizures Follow‐up: up to 24 hours | One trial found no difference between groups in the time from drug administration to seizure cessation One trial reported that both the median treatment initiation time and drug effect time were significantly shorter in the buccal midazolam group than the rectal diazepam group. | NA | 141 (2 trials) | ⊕⊕⊝⊝ | No numerical data presented for either trial | |
| Incidence of respiratory depression Follow‐up: up to 24 hours | 76 per 1000 | 67 per 1000 (46 to 94) | RR 0.88 (0.61 to 1.25) | 648 (4 trials) 690 seizure episodes | ⊕⊕⊝⊝ | ‐ |
| Additional drugs required to stop the seizure: intravenous lorazepam required Follow‐up: up to 24 hours | 573 per 1000 | 332 per 1000 | RR 0.58 (0.42 to 0.79) | 177 (1 trial) 219 seizure episodes | ⊕⊕⊝⊝ | A second trial reported that there was no difference between groups in the need for a second drug |
| Seizure recurrence within 24 hours Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Incidence of admissions to the ICU Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to risk of bias: one included study was quasi‐randomised and one study did not conceal allocation. Both of these studies were at risk of selection bias. | ||||||
| Buccal midazolam compared with intravenous diazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Buccal midazolam Comparison: Intravenous diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous diazepam | Buccal midazolam | |||||
| Seizure cessation Follow‐up: up to 24 hours | 933 per 1000 | 849 per 1000 | RR 0.91 (0.80 to 1.03) | 120 (1 trial) | ⊕⊕⊕⊕ | ‐ |
| Time from drug administration to termination of seizures Follow‐up: up to 24 hours | The mean time to cessation of seizures was 1.13 minutes in the intravenous diazepam group. | The mean time to cessation of seizures was 0.56 minutes higher in the buccal diazepam group (0.29 to 0.83 minutes higher). | NA | 120 (1 trial) | ⊕⊕⊕⊝ | The mean time for initiation of treatment was significantly shorter in the buccal midazolam group (MD ‐1.09 minutes, 95% CI ‐1.31 to ‐0.87) and therefore the mean total time to controlling the seizures was significantly shorter in the buccal midazolam group compared to the intravenous diazepam group (MD ‐0.59, 95% CI ‐0.96 to ‐0.22) |
| Incidence of respiratory depression Follow‐up: up to 24 hours | There were no adverse events in either group | NA | 120 (1 trial) | ⊕⊕⊕⊕ | ‐ | |
| Additional drugs required to stop the seizure Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Seizure recurrence within 24 hours Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Incidence of admissions to the ICU Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to applicability: the route of intervention of the drug has been shown to influence the outcome. | ||||||
| Intranasal midazolam compared with intravenous diazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intranasal midazolam Comparison: Intravenous diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous diazepam | Intranasal midazolam | |||||
| Seizure cessation Follow‐up: up to 24 hours | 967 per 1000 | 948 per 1000 | RR 0.98 (0.91 to 1.06) | 122 (2 trials) | ⊕⊕⊕⊝ | ‐ |
| Time from drug administration to stopping of seizures Follow‐up: up to 24 hours | The mean time to cessation of seizures ranged from 2.5 to 2.94 minutes in the intravenous diazepam group. | The mean time to cessation of seizures was 0.62 minutes higher in the intranasal midazolam group (0.14 lower to 1.38 minutes higher). | NA | 122 (2 trials) | ⊕⊕⊕⊝ | One trial reports that the time for initiation of treatment was significantly shorter in the intranasal midazolam group (MD ‐2.00 minutes, 95% CI ‐3.03 to ‐0.97). The other trial also reports that time for initiation of treatment was significantly shorter in the intranasal midazolam group but does not account for this in analysis |
| Incidence of respiratory depression Follow‐up: up to 24 hours | No adverse events including respiratory depression occurred in either group. | NA | 122 (2 trials) | ⊕⊕⊕⊕ | ‐ | |
| Additional drugs required to stop the seizure Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Seizure recurrence within 24 hours Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Incidence of admissions to the ICU Follow‐up: up to 24 hours | There were no admissions to the ICU in either group | NA | 52 (1 trial) | ⊕⊕⊕⊕ | ‐ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to risk of bias: one of the studies included in this comparison did not report this outcome. As this is an expected outcome, this may be selective reporting. Additionally, in one trial both treatment arms showed a 100% seizure cessation rate, which is higher than expected. Unclear whether this high success rate was due to a particular element of the trial design. | ||||||
| Intranasal midazolam compared with rectal diazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intranasal midazolam Comparison: Rectal diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Rectal diazepam | Intranasal midazolam | |||||
| Seizure cessation: within 10 minutes Follow‐up: up to 24 hours | 591 per 1000 | 869 per 1000 | RR 1.47 (1.00 to 2.16) | 45 | ⊕⊕⊝⊝ | ‐ |
| Time from drug administration to termination of seizures Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Incidence of respiratory depression Follow‐up: | There was no significant difference between the two groups for of cardiorespiratory or adverse effects. | NA | 45 | ⊕⊕⊝⊝ | No numerical data reported | |
| Additional drugs required to stop the seizure Follow‐up: up to 24 hours | 409 per 1000 | 131 per 1000 (41 to 421) | RR 0.32 (0.10 to 1.03) | 45 | ⊕⊕⊝⊝ | ‐ |
| Seizure recurrence within 24 hours Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Incidence of admissions to the ICU Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to risk of bias: one included study was quasi‐randomised, which may have led to selection bias. Additionally, the description of the seizure type and aetiology of the included children was unclear, so it is unclear if the population of this study is generalisable. | ||||||
| Intramuscular midazolam compared with intravenous diazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intramsucular midazolam Comparison: Intravenous diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous diazepam | Intramsucular midazolam | |||||
| Seizure cessation Follow‐up: up to 24 hours | 929 per 1000 | 901 per 1000 | RR 0.97 (0.87 to 1.09) | 105 | ⊕⊕⊝⊝ | ‐ |
| Time from drug administration to stopping of seizures: total time to seizure cessation Follow‐up: up to 24 hours | The mean total time to cessation of seizures was 2.68 minutes lower (3.94 to 1.42 minutes lower) in the intramuscular midazolam group compared to the intravenous diazepam group | NA | 105 | ⊕⊝⊝⊝ | One trial also showed that the initiation of treatment was significantly shorter in the intramuscular midazolam group (MD ‐4.50 minutes (‐6.68 to ‐2.32)) but there was no significant difference between treatments for the time to drug effect (MD 1.10 minutes (95% CI ‐0.91 to 3.11) | |
| Incidence of respiratory depression Follow‐up: up to 24 hours | There were no adverse events or complications in either trial | NA | 105 | ⊕⊕⊝⊝ | ‐ | |
| Additional drugs required to terminate the seizure Follow‐up: up to 24 hours | 71 per 1000 | 96 per 1000 | RR 1.34 (0.35 to 5.13) | 105 | ⊕⊝⊝⊝ | ‐ |
| Seizure recurrence within 24 hours: within one hour Follow‐up: up to 24 hours | 364 per 1000 | 309 per 1000 | RR 0.85 (0.27 to 2.62) | 24 (1 trial) | ⊕⊝⊝⊝ | There was also no significant difference between treatments at within 15 minutes (RR: 0.85 (95% CI 0.06,to12.01) |
| Incidence of admissions to the ICU Follow‐up: up to 24 hours | There were no admissions to the ICU | NA | 81 (1 trial) | ⊕⊕⊕⊝ | ‐ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to risk of bias: in both included trials, methods of randomisation were unclear so the trials may be at risk of selection bias. | ||||||
| Intramuscular midazolam compared with rectal diazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intramuscular midazolam Comparison: Rectal diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Rectal diazepam | Intramuscular midazolam | |||||
| Seizure cessation Follow‐up: up to 24 hours | 940 per 1000 | 959 per 1000 | RR 1.02 (0.93 to 1.12) | 100 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Time from drug administration to stopping of seizures Follow‐up: up to 24 hours | There was a significant difference in time from administration to seizure cessation in favour of midazolam (median 66 seconds, diazepam, median 130 seconds, P < 0.001) | NA | 100 (1 trial) | ⊕⊕⊕⊝ | It is noted that the speed of administration was similarly fast for both medications, so this seems to reflect a medication difference. | |
| Incidence of respiratory depression Follow‐up: up to 24 hours | No patients developed respiratory depression except for one patient who received an accidental double dose of intramuscular midazolam. | NA | 100 (1 trial) | ⊕⊕⊕⊝ | ‐ | |
| Additional drugs required to stop the seizure Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| Seizure recurrence within 24 hours Follow‐up: up to 24 hours | Among those with seizures terminated, there were no recurrences at 24 hours | NA | 100 (1 trial) | ⊕⊕⊕⊝ | ||
| Incidence of admissions to the ICU Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to risk of bias: the included study did not conceal allocation so is at risk of selection bias. | ||||||
| Intravenous midazolam compared with intravenous diazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intravenous midazolam Comparison: Intravenous diazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous diazepam | Intravenous midazolam | |||||
| Seizure cessation Follow‐up: up to 24 hours | 900 per 1000 | 972 per 1000 | RR 1.08 (0.97 to 1.21) | 80 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Time from drug administration to stopping of seizures Follow‐up: up to 24 hours | The mean time to cessation of seizures was 84.94 seconds in the intravenous diazepam group. | The mean time to cessation of seizures was 7.68 seconds higher in the intravenous midazolam group (6.73 seconds lower to 22.09 seconds higher) . | NA | 80 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Incidence of respiratory depression Follow‐up: up to 24 hours | 25 per 1000 | 8 per 1000 | RR 0.33 (0.01 to 7.95) | 80 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Additional drugs required to stop the seizure: additional dose of the trial drug required Follow‐up: up to 24 hours | 100 per 1000 | 25 per 1000 | RR 0.25 (0.03 to 2.14) | 80 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Seizure recurrence within 24 hours Follow‐up: up to 24 hours | 100 per 1000 | 50 per 1000 | RR 0.50 (0.10 to 2.58) | 80 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Incidence of admissions to the ICU Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to risk of bias: the definition of the 'seizure cessation' outcome is not an appropriate criterion for judging seizure cessation. This definition is likely to have impacted upon results. | ||||||
| Intravenous midazolam compared with intravenous lorazepam for children with acute tonic‐clonic seizures | ||||||
| Patient or population: Children with acute tonic‐clonic seizures Settings: Hospital inpatients Intervention: Intravenous midazolam Comparison: Intravenous lorazepam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous lorazepam | Intravenous midazolam | |||||
| Seizure cessation Follow‐up: up to 24 hours | Seizures were terminated for all children in the Intravenous lorazepam group | Seizures were terminated for 39 out of 40 children in the intravenous midazolam group | RR 0.98 (0.91 to 1.04) | 80 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Time from drug administration to termination of seizures Follow‐up: up to 24 hours | The mean time to cessation of seizures was 91.12 seconds in the intravenous lorazepam group. | The mean time to cessation of seizures was 1.50 seconds higher in the intravenous midazolam group (9.37 seconds lower to 12.37 seconds higher) . | NA | 80 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Incidence of respiratory depression Follow‐up: up to 24 hours | There were no occurrences of respiratory depression in either group | NA | 80 (1 trial) | ⊕⊕⊕⊕ | ‐ | |
| Additional drugs required to terminate the seizure: additional dose of the trial drug required Follow‐up: up to 24 hours | No children in the intravenous lorazepam group required an additional dose of the trial drug. | One child in the intravenous midazolam group required an additional dose of the trial drug. | RR 3.00 (0.13 to 71.51) | 80 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Seizure recurrence within 24 hours Follow‐up: up to 24 hours | 50 per 1000 | 50 per 1000 | RR 1.00 (0.15 to 6.76) | 80 (1 trial) | ⊕⊕⊕⊝ | ‐ |
| Incidence of admissions to the ICU Follow‐up: NA | Outcome not reported | NA | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded once due to risk of bias: the definition of the 'seizure cessation' outcome is not an appropriate criterion for judging seizure cessation. This definition is likely to have impacted upon results. | ||||||
| Study | Drug | Seizure cessation | Respiratory Depression | Additional drugs required | ||||||
| No. of Events | No. of Children | % | No. of Events | No. of Children | % | No. of Events | No. of Children | % | ||
| IN lorazepam | 60 | 80 | 75 | 0 | 80 | 0 | 8 | 80 | 10 | |
| IM paraldehyde | 49 | 80 | 60 | 0 | 80 | 0 | 21 | 80 | 26 | |
| IV lorazepam | 19 | 27 | 70 | 1 | 27 | 4 | 1 | 27 | 4 | |
| Rectal lorazepam | 6 | 6 | 100 | 0 | 6 | 0 | 0 | 6 | 0 | |
| IV diazepam | 22 | 34 | 65 | 7 | 34 | 21 | 5 | 34 | 15 | |
| Rectal diazepam | 6 | 19 | 32 | 1 | 19 | 5 | 12 | 19 | 63 | |
| IN lorazepam | 16 | 23 | 70 | 1 | 71 | 1 | NR | 23 | NA | |
| IV lorazepam | 26 | 35 | 74 | 2 | 70 | 3 | NR | 35 | NA | |
| Buccal midazolam | 49 | 49 | 100 | 0 | 49 | 0 | 0 | 49 | 0 | |
| Rectal diazepam | 40 | 49 | 82 | 0 | 49 | 0 | 9 | 49 | 18 | |
| Buccal midazolam | 18 | 23 | 78 | 0 | 23 | 0 | 5 | 23 | 22 | |
| Rectal diazepam | 17 | 20 | 85 | 1 | 20 | 5 | 3 | 20 | 15 | |
| IM midazolam | 12 | 13 | 92 | 0 | 13 | 0 | 1 | 13 | 8 | |
| IV diazepam | 10 | 11 | 91 | 0 | 11 | 0 | 1 | 11 | 9 | |
| IV diazepam | 101 | 140 | 72 | 26 | 140 | 16 | 21 | 140 | 15 | |
| IV lorazepam | 97 | 133 | 73 | 26 | 133 | 18 | 21 | 133 | 16 | |
| IN midazolam | 20 | 23 | 87 | 0 | 23 | 0 | 3 | 23 | 13 | |
| Rectal diazepam | 13 | 22 | 60 | 0 | 22 | 0 | 9 | 22 | 40 | |
| IV diazepam | 36 | 40 | 90 | 1 | 40 | 3 | 4 | 40 | 10 | |
| IV midazolam | 39 | 40 | 98 | 0 | 40 | 0 | 1 | 40 | 3 | |
| IV lorazepam | 40 | 40 | 100 | 0 | 40 | 0 | 0 | 40 | 0 | |
| IN midazolam | NR | 30 | NA | NR | 30 | NA | NR | 30 | NA | |
| IV diazepam | NR | 30 | NA | NR | 30 | NA | NR | 30 | NA | |
| IN midazolam | 23 | 26 | 88 | 0 | 26 | 0 | NR | 26 | NA | |
| IV diazepam | 24 | 26 | 92 | 0 | 26 | 0 | NR | 26 | NA | |
| IN midazolam | 35 | 35 | 100 | 0 | 35 | 0 | 0 | 35 | 0 | |
| IV diazepam | 35 | 35 | 100 | 0 | 35 | 0 | 0 | 35 | 0 | |
| Buccal midazolam | 61 | 109 | 56 | 5 | 109 | 5 | 36 | 109 | 33 | |
| Rectal diazepam | 30 | 110 | 27 | 7 | 110 | 6 | 63 | 110 | 57 | |
| IM midazolam | 48 | 50 | 96 | 1 | 50 | 2 | NR | 50 | NA | |
| Rectal diazepam | 47 | 50 | 94 | 0 | 50 | 0 | NR | 50 | NA | |
| Buccal midazolam | 125 | 165 | 76 | 2 | 165 | 1 | NR | 165 | NA | |
| Rectal diazepam | 114 | 165 | 69 | 2 | 165 | 1 | NR | 165 | NA | |
| IM midazolam | 45 | 50 | 90 | 0 | 50 | 0 | 5 | 50 | 10 | |
| IV diazepam | 29 | 31 | 90 | 0 | 31 | 0 | 2 | 31 | 6 | |
| IV lorazepam | 90 | 90 | 100 | 4 | 90 | 4 | 6 | 90 | 7 | |
| IV diazepam with phenytoin | 88 | 88 | 100 | 5 | 88 | 6 | 14 | 88 | 16 | |
| Buccal midazolam | 51 | 60 | 85 | 0 | 60 | 0 | 9 | 60 | 15 | |
| IV diazepam | 56 | 60 | 93 | 0 | 60 | 0 | 4 | 60 | 7 | |
| Abbreviations: IM: Intramuscular; IN: Intranasal; IV: Intravenous; NR: Not reported; NA: Not available (percentages could not be calculated where event rate was NR) *Occurences of respiratory depression were not reported for the subgroup of participants with generalised tonic‐clonic seizures in Arya 2011, therefore these results refer to all participants (including 83 participants without generalised tonic‐clonic seizures). | ||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation Show forest plot | 3 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.98, 1.20] |
| 1.1 Intravenous | 3 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.94, 1.16] |
| 1.2 Rectal | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.86 [1.47, 5.55] |
| 2 Time from drug administration to stopping of seizures Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Intravenous | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 6.18 [‐7.83, 20.19] |
| 3 Incidence of respiratory depression Show forest plot | 3 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.55, 0.93] |
| 3.1 Intravenous | 3 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.55, 0.92] |
| 3.2 Rectal | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.04, 20.78] |
| 4 Additional dose of the trial drug required to stop seizures Show forest plot | 3 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.64, 1.20] |
| 4.1 Intravenous | 3 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.71, 1.33] |
| 4.2 Rectal | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.56] |
| 5 Additional drugs required to stop seizures Show forest plot | 2 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.45, 1.24] |
| 5.1 Intravenous | 2 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.54, 1.55] |
| 5.2 Rectal | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.69] |
| 6 Seizure recurrence within 24 hours Show forest plot | 3 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.61, 1.20] |
| 6.1 Intravenous | 3 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.65, 1.27] |
| 6.2 Rectal | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 2.92] |
| 7 Incidence of admissions to the intensive care unit (ICU) Show forest plot | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 0.98] |
| 7.1 Intravenous | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.22] |
| 7.2 Rectal | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.03, 10.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Within 10 minutes | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.99, 1.52] |
| 2 Additional drugs required to stop seizures Show forest plot | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.18, 0.81] |
| 3 Seizure recurrence within 24 hours Show forest plot | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.31, 1.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Within 10 minutes | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.98, 1.02] |
| 2 Incidence of respiratory depression Show forest plot | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.22, 2.82] |
| 3 Additional drugs required to stop seizures Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 More than one dose of the trial drug required | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.17, 1.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Within 10 minutes | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.77, 1.49] |
| 1.2 Within 1 hour | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.43, 1.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation Show forest plot | 4 | 690 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.13, 1.38] |
| 1.1 Within 5 minutes | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.07, 1.40] |
| 1.2 Within 10 minutes | 2 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.95, 1.21] |
| 1.3 Within one hour | 1 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [1.45, 2.91] |
| 2 Incidence of respiratory depression Show forest plot | 4 | 690 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.61, 1.25] |
| 3 Additional drugs required to stop seizures Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Intravenous lorazepam required | 1 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.42, 0.79] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation within five minutes Show forest plot | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.03] |
| 2 Time from drug administration to stopping of seizures Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Treatment initiation time (minutes) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐1.09 [‐1.31, ‐0.87] |
| 2.2 Time for drug effect (minutes) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | 0.56 [0.29, 0.83] |
| 2.3 Total time to seizure cessation (minutes) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐0.96, ‐0.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure Cessation Show forest plot | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.06] |
| 2 Time from drug administration to stopping of seizures [minutes] Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Treatment initiation time (minutes) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐3.03, ‐0.97] |
| 2.2 Time for drug effect (minutes) | 2 | 122 | Mean Difference (IV, Fixed, 95% CI) | 0.62 [‐0.14, 1.38] |
| 2.3 Total time to seizure cessation (minutes) | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [0.24, 1.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Within 10 minutes | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.00, 2.16] |
| 2 Additional drugs required to stop seizures Show forest plot | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.10, 1.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation Show forest plot | 2 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.87, 1.09] |
| 2 Time from drug administration to stopping of seizures (minutes) Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 Treatment initiation time (minutes) | 1 | 24 | Mean Difference (Fixed, 95% CI) | ‐4.5 [‐6.68, ‐2.32] |
| 2.2 Time for drug effect (minutes) | 1 | 24 | Mean Difference (Fixed, 95% CI) | 1.1 [‐0.91, 3.11] |
| 2.3 Total time to seizure cessation (minutes) | 2 | 105 | Mean Difference (Fixed, 95% CI) | ‐2.68 [‐3.94, ‐1.42] |
| 3 Additional drugs required to stop seizures Show forest plot | 2 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.35, 5.13] |
| 4 Seizure recurrence within 24 hours Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Within 15 minutes | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.06, 12.01] |
| 4.2 Within one hour | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.27, 2.62] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Within 1 hour | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.93, 1.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.97, 1.21] |
| 2 Time from drug administration to stopping of seizures Show forest plot | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 7.68 [‐6.73, 22.09] |
| 3 Incidence of respiratory depression Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| 4 Additional dose of the trial drug required to stop seizures Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.14] |
| 5 Seizure recurrence within 24 hours Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.10, 2.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure cessation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.04] |
| 2 Time from drug administration to stopping of seizures Show forest plot | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 1.5 [‐9.37, 12.37] |
| 3 Additional dose of the trial drug required to stop seizures Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.51] |
| 4 Seizure recurrence within 24 hours Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.76] |

