Interrupción quirúrgica de vías nerviosas pelvianas para la dismenorrea primaria y secundaria
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Allocation Concealment: unclear. | |
| Participants | Number of women randomised: 78. | |
| Interventions | Treatment: presacral neurectomy with conservative surgery for endometriosis. | |
| Outcomes | Dysmenorrhoea, measured by a 0 ‐10 analog scale and by a multidimensional scale which included limitation of working ability, systemic symptoms, and need for analgesics. Data reported as mild, moderate or severe pain prior to surgery and 12 months following surgery. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Allocation Concealment: unclear. | |
| Participants | Number of women randomised: 68. | |
| Interventions | Treatment: laparoscopic uterine nerve ablation. | |
| Outcomes | Pain relief measured on a 5 point scale (0 = no pain to 4 = incapacitating pain unresponsive to potent pain relievers and the inability to function). Pain was measured at baseline, 3 months, 12 months and data was dichotomised into success 100 > 50% pain relief or failure 50 > 0% pain relief. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Allocation concealment: stated as maintained securely by storage in sealed, sequentially‐numbered opaque envelopes until the interventions were assigned during the laparoscopic procedure | |
| Participants | Number of women randomised: 123 (108 with dysmenorrhoea) | |
| Interventions | 2 groups: | |
| Outcomes | Changes in non‐menstrual pelvic pain, dysmenorrhoea, deep dyspareunia and dyschezia were assessed primarily by whether there was a decrease in visual analog score for these types of pain of 50% or more from baseline; additionally whether there was a significantly different change in median visual analog score. The numbers requiring further surgery or starting a new medical treatment for pelvic pain and complications were also measured. | |
| Notes | 137/200 agreed to participate; 14 excluded at laparoscopy; follow up at 12 months: 106/123 (86.2%). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Allocation concealment: inadequate. | |
| Participants | Number of women recruited: 39. 18 were excluded due to pathology (endometriosis, PID). | |
| Interventions | Treatment: laparoscopic uterine nerve ablation. | |
| Outcomes | Pain was measured on a five point scale (0 = no pain to 4 = incapacitating pain unresponsive to potent pain relievers and the inability to function). Pain scores for each patient were reported preoperatively and at 3mths and 12mths. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Allocation Concealment: unclear. | |
| Participants | Number of women randomised: 51. | |
| Interventions | Treatment: LUNA with laparoscopic treatment of all visible endometriosis. | |
| Outcomes | Dysmenorrhoea, measured by linear analogue scale (0 ‐ 10) and pain scoring questionnaire. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Allocation concealment: adequate. | |
| Participants | Number of women randomised: 8; also 18 women not randomised but followed up. | |
| Interventions | Treatment: presacral neurectomy and resection of endometriosis. | |
| Outcomes | Relief of pain was reported as the number of women with pain relief in 3 locations. | |
| Notes | Data analysed pooled, and split into protocol (randomised) and non‐protocol (non‐randomised) groups. Study stopped by monitoring committee after 26 participants, as it was considered unethical not to provide those with midline dysmenorrhoea the pain relief that PSN exhibited. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Allocation concealment: adequate. | |
| Participants | Number of women randomised: 180. | |
| Interventions | Treatment: conservative laparoscopic surgery with the addition of uterosacral ligament resection. | |
| Outcomes | Dysmenorrhoea, measured by a 100 mm visual analog scale that ranged from "least possible pain" to "worst possible pain". Frequency was expressed as the number of episodes per each cycle for dysmenorrhoea and chronic pelvic pain. | |
| Notes | Proportion of women satisfied with the treatment were similar. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Allocation concealment: inadequate. | |
| Participants | Number of women randomised: 85. | |
| Interventions | Treatment: laparoscopic uterine nerve ablation with laparoscopic bipolar coagulation of uterine vessels (LBCUV). | |
| Outcomes | Dysmenorrhoea, improvement measured by analgesic use, and scale ‐ completely resolved, significantly improved, slightly improved, unchanged or worsened. Pain data was reported the numbers of women slightly, significantly or completely improved. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Allocation concealment: unclear. | |
| Participants | Number of women randomised: 141. | |
| Interventions | Treatment: laparoscopic presacral neurectomy with conservative surgery for endometriosis. | |
| Outcomes | Dysmenorrhoea, measured by a 100 mm visual analog scale that ranged from "least possible pain" to "worst possible pain". Frequency was expressed as the number of episodes per each cycle for dysmenorrhea and chronic pelvic pain. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
SD = standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Randomised controlled trial comparing LPSN and LUNA (Spanish). Patients had chronic pelvic pain which may or may not have included dysmenorrhoea. Approximately 60 to 80% of women in each treatment group had dysmenorrhoea at baseline. Unable to separate out data for women with dysmenorrhoea at baseline or at end of treatment. | |
| Double blind, randomised trial, interventions were laparoscopic uterine nerve transection combined with vaporisation of endometriosis implants versus no treatment (expectant management only). Therefore data on pain relief as a result of LUNA could not be distinguished from pain relief as a result of the laser vaporisation. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A randomised controlled trial to assess the efficacy of laparoscopic uterosacral nerve ablation (LUNA) in the treatment of chronic pelvic pain. A multi‐centre, prospective randomised‐controlled trial with double blind assessment of outcomes |
| Methods | |
| Participants | New patients presenting to the gynaecology outpatient clinic with pelvic pain (cyclical or non‐cyclical) and/or dyspareunia, and requiring diagnostic laparoscopy for evaluation of these conditions, will be invited to participate. Inclusion criteria: pelvic pain for longer than 6 months duration; pain located within the true pelvis or between and below the anterior iliac crests; associated functional disability; lack of response to medical treatment; diagnostic laparoscopy planned. |
| Interventions | Diagnostic laparoscopy plus uterosacral nerve ablation (experimental group) or laparoscopy without pelvic denervation (control group). |
| Outcomes | Postal questionnaires including visual analogue scale for pain (primary outcome), an index of sexual satisfaction and the EuroQol 5D‐EQ instrument (secondary outcomes) will be administered at 3, 6, 12, 24 and 36 months and 5 and 10 years. The primary assessment of the effectiveness of LUNA will be from comparison of outcomes at the one‐year follow‐up, although the short‐term and longer‐term risks and benefits of LUNA will also be evaluated. |
| Starting date | Date ISRCTN assigned: Oct 2002 |
| Contact information | The LUNA Trial Collaboration, Dr Pallavi Latthe. |
| Notes | ISRCTN41196151 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain relief ‐ up to 6 months Show forest plot | 4 | 258 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.66, 1.99] |
| Analysis 1.1  Comparison 1 LUNA versus control, Outcome 1 Pain relief ‐ up to 6 months. | ||||
| 1.1 Primary dysmenorrhoea | 2 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.56, 3.69] |
| 1.2 Secondary dysmenorrhoea | 3 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.52, 2.02] |
| 2 Pain relief ‐ up to 12 months Show forest plot | 3 | 285 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.72, 1.99] |
| Analysis 1.2  Comparison 1 LUNA versus control, Outcome 2 Pain relief ‐ up to 12 months. | ||||
| 2.1 Primary dysmenorrhoea | 2 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.12 [1.78, 21.03] |
| 2.2 Secondary dysmenorrhoea | 2 | 217 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.43, 1.39] |
| 4 Pain relief up to 36 months Show forest plot | 1 | 116 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.39, 1.80] |
| Analysis 1.4  Comparison 1 LUNA versus control, Outcome 4 Pain relief up to 36 months. | ||||
| 4.2 Secondary dysmenorrhoea | 1 | 116 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.39, 1.80] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain relief ‐ up to 6 months Show forest plot | 1 | 126 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.52 [1.84, 11.09] |
| Analysis 2.1  Comparison 2 PSN versus control, Outcome 1 Pain relief ‐ up to 6 months. | ||||
| 1.2 PSN with surgical treatment of endometriosis vs surgical treatment of endometriosis only | 1 | 126 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.52 [1.84, 11.09] |
| 2 Pain relief ‐ up to 12 months Show forest plot | 2 | 197 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.14 [1.59, 6.21] |
| Analysis 2.2  Comparison 2 PSN versus control, Outcome 2 Pain relief ‐ up to 12 months. | ||||
| 2.2 PSN with surgical treatment of endometriosis vs surgical treatment of endometriosis only | 2 | 197 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.14 [1.59, 6.21] |
| 3 Adverse effects Show forest plot | 1 | 71 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 14.57 [5.04, 42.15] |
| Analysis 2.3  Comparison 2 PSN versus control, Outcome 3 Adverse effects. | ||||
| 3.2 PSN with surgical treatment of endometriosis vs surgical treatment of endometriosis only | 1 | 71 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 14.57 [5.04, 42.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain relief ‐ up to 6 months Show forest plot | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.17, 2.61] |
| Analysis 3.1  Comparison 3 LUNA versus LPSN, Outcome 1 Pain relief ‐ up to 6 months. | ||||
| 1.1 Primary dysmenorrhoea | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.17, 2.61] |
| 2 Pain relief ‐ up to 12 months Show forest plot | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.03, 0.32] |
| Analysis 3.2  Comparison 3 LUNA versus LPSN, Outcome 2 Pain relief ‐ up to 12 months. | ||||
| 2.1 Primary dysmenorrhoea | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.03, 0.32] |
| 3 Adverse effects Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 LUNA versus LPSN, Outcome 3 Adverse effects. | ||||
| 3.3 Primary dysmenorrhoea | 1 | 68 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.02 [0.01, 0.06] |

Sensory afferent nerve supply of the female pelvic organs. C = afferent nerve supply of cervix (illustrated on right side of diagram); O = afferent nerve supply of ovary (illustrated on left side of diagram); U = afferent nerve supply of uterus (illustrated on right side of diagram); P = parasympathetic nerve; S = sympathetic nerve. Copyright approval for reproduction of this figure has been kindly granted by Blackwell Science, publisher of Gynaecological Endoscopy (Johnson 2000).
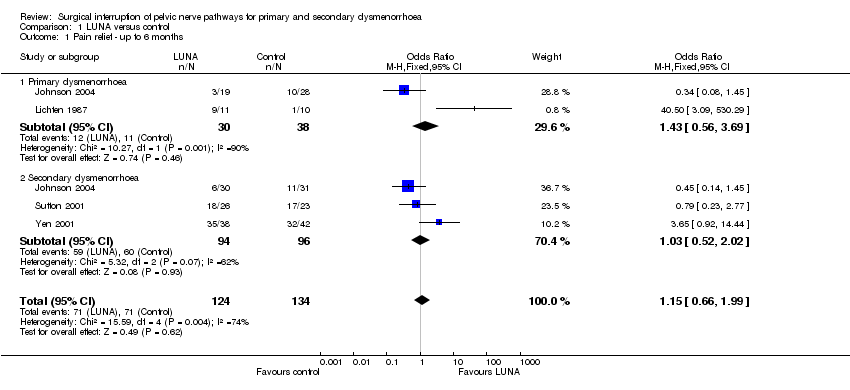
Comparison 1 LUNA versus control, Outcome 1 Pain relief ‐ up to 6 months.

Comparison 1 LUNA versus control, Outcome 2 Pain relief ‐ up to 12 months.

Comparison 1 LUNA versus control, Outcome 4 Pain relief up to 36 months.
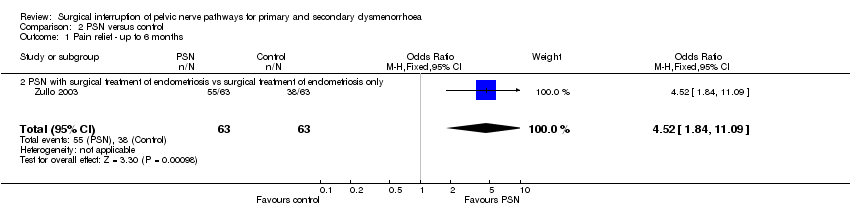
Comparison 2 PSN versus control, Outcome 1 Pain relief ‐ up to 6 months.

Comparison 2 PSN versus control, Outcome 2 Pain relief ‐ up to 12 months.

Comparison 2 PSN versus control, Outcome 3 Adverse effects.

Comparison 3 LUNA versus LPSN, Outcome 1 Pain relief ‐ up to 6 months.

Comparison 3 LUNA versus LPSN, Outcome 2 Pain relief ‐ up to 12 months.
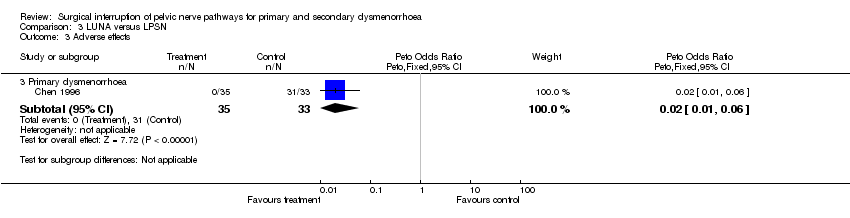
Comparison 3 LUNA versus LPSN, Outcome 3 Adverse effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain relief ‐ up to 6 months Show forest plot | 4 | 258 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.66, 1.99] |
| 1.1 Primary dysmenorrhoea | 2 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.56, 3.69] |
| 1.2 Secondary dysmenorrhoea | 3 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.52, 2.02] |
| 2 Pain relief ‐ up to 12 months Show forest plot | 3 | 285 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.72, 1.99] |
| 2.1 Primary dysmenorrhoea | 2 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.12 [1.78, 21.03] |
| 2.2 Secondary dysmenorrhoea | 2 | 217 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.43, 1.39] |
| 4 Pain relief up to 36 months Show forest plot | 1 | 116 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.39, 1.80] |
| 4.2 Secondary dysmenorrhoea | 1 | 116 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.39, 1.80] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain relief ‐ up to 6 months Show forest plot | 1 | 126 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.52 [1.84, 11.09] |
| 1.2 PSN with surgical treatment of endometriosis vs surgical treatment of endometriosis only | 1 | 126 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.52 [1.84, 11.09] |
| 2 Pain relief ‐ up to 12 months Show forest plot | 2 | 197 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.14 [1.59, 6.21] |
| 2.2 PSN with surgical treatment of endometriosis vs surgical treatment of endometriosis only | 2 | 197 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.14 [1.59, 6.21] |
| 3 Adverse effects Show forest plot | 1 | 71 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 14.57 [5.04, 42.15] |
| 3.2 PSN with surgical treatment of endometriosis vs surgical treatment of endometriosis only | 1 | 71 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 14.57 [5.04, 42.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain relief ‐ up to 6 months Show forest plot | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.17, 2.61] |
| 1.1 Primary dysmenorrhoea | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.17, 2.61] |
| 2 Pain relief ‐ up to 12 months Show forest plot | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.03, 0.32] |
| 2.1 Primary dysmenorrhoea | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.03, 0.32] |
| 3 Adverse effects Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.3 Primary dysmenorrhoea | 1 | 68 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.02 [0.01, 0.06] |

