Anesthésiques locaux périduraux par rapport aux analgésiques à base d'opioïdes pour la paralysie gastro‐intestinale postopératoire, les vomissements et les douleurs après une chirurgie abdominale
Résumé scientifique
Contexte
La paralysie gastro‐intestinale, les nausées, les vomissements et les douleurs sont des problèmes cliniques majeurs à la suite d'une chirurgie abdominale. Les techniques anesthésiques et analgésiques qui réduisent la douleur et les nausées et vomissements postopératoires (NVPO), tout en prévenant ou en réduisant l'iléus postopératoire, pourraient réduire la morbidité postopératoire, la durée d'hospitalisation et les coûts hospitaliers. Cette revue a été publiée pour la première fois en 2001 et a été mise à jour par de nouveaux auteurs en 2016.
Objectifs
Comparer les effets de l'analgésie épidurale postopératoire avec des anesthésiques locaux, après une chirurgie abdominale, par rapport aux opioïdes systémiques ou épiduraux postopératoires en termes de rétablissement du transit gastro‐intestinal, de contrôle de la douleur postopératoire, de vomissements postopératoires, d'incidence de fuite anastomotique, de durée de séjour hospitalier.
Stratégie de recherche documentaire
Nous avons identifié les essais cliniques en effectuant des recherches informatisées dans le registre central des essais contrôlés de Cochrane (CENTRAL) (2014, numéro 12), MEDLINE (de 1950 à décembre 2014) et EMBASE (de 1974 à décembre 2014) et en vérifiant les listes de références des essais retenus. Lorsque nous avons relancé la recherche en février 2016, nous avons ajouté 16 nouvelles études potentiellement intéressantes à la liste des « études en attente de classification » et nous intégrerons ces études dans les conclusions formelles de la revue lors de la prochaine mise à jour de la revue.
Critères de sélection
Nous avons inclus les essais contrôlés randomisés en parallèle comparant les effets de l'anesthésie locale péridurale postopératoire par rapport à des traitements basés sur des opioïdes systémiques ou périduraux.
Recueil et analyse des données
Nous avons évalué la qualité des études en utilisant l'outil Risque de biais développé par Cochrane Deux auteurs de la revue ont indépendamment extrait les données et ont jugé la qualité des données probantes selon l'échelle GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group).
Résultats principaux
Nous avons inclus 128 essais avec 8754 participants dans la revue, et 94 essais avec 5846 participants dans l'analyse. Les essais inclus dans la revue ont été financés comme suit: organismes de bienfaisance (n = 19), ressources ministérielles (n = 8), sources gouvernementales (n = 15) et industrie (en partie ou au total) (n = 15). La source de financement n'a pas été précisée pour les autres études.
Les résultats de 22 essais comprenant 1138 participants montrent qu'une péridurale contenant un anesthésique local réduira le temps nécessaire au rétablissement du transit gastro‐intestinal tel que mesuré par le temps nécessaire à la première émission de gaz après une chirurgie abdominale (différence moyenne standardisée (SMD) ‐1,28, intervalle de confiance (IC) à 95% ‐1,71 à ‐0,86 ; données probantes de haute qualité ; équivalent à 17,5 heures). L'effet est proportionnel à la concentration de l'anesthésique local utilisé. Au total, 28 essais, comprenant 1559 participants, ont fait état d'une diminution du délai avant l'apparition des premières selles (SMD ‐0,67, 95 % IC ‐0,86 à ‐0,47 ; données probantes de faible qualité ; équivalent à 22 heures). 35 essais, comprenant 2 731 participants, ont montré que la douleur au mouvement dans les 24 heures suivant l'opération était également réduite (SMD ‐0,89, 95 % IC ‐1,08 à ‐0,70 ; données probantes de qualité moyenne ; équivalent à 2,5 sur une échelle de 0 à 10). D'après les résultats de 22 essais comprenant 1154 participants, nous n'avons pas constaté de différence dans l'incidence des vomissements dans les 24 heures postopératoires (rapport de risque (RR) de 0,84, IC à 95% de 0,57 à 1,23 ; données probantes de faible qualité). Les investigateurs de 17 essais, comprenant 848 participants, n'ont pas constaté de différence dans l'incidence des fuites d'anastomoses gastro‐intestinales (RR 0,74, 95 % IC 0,41 à 1,32 ; données probantes de faible qualité ). Les investigateurs de 30 essais, comprenant 2598 participants, ont noté que l'analgésie péridurale réduisait la durée du séjour hospitalier pour une chirurgie ouverte (CMS ‐0,20, 95 % IC ‐0,35 à ‐0,04 ; données probantes de très faible qualité ; équivalent à une journée). Les données probantes sur les coûts étaient très limitées.
Conclusions des auteurs
Une péridurale contenant un anesthésique local, avec ou sans ajout d'un opioïde, accélère le rétablissement du transit gastro‐intestinal (données probantes de haute qualité). Une péridurale contenant un anesthésique local avec un opioïde diminue la douleur après une chirurgie abdominale (données probantes de qualité moyenne). Nous n'avons pas trouvé de différence dans l'incidence des vomissements ou des fuites anastomotiques (données probantes de faible qualité). Pour la chirurgie ouverte, une péridurale contenant un anesthésique local réduirait la durée du séjour à l'hôpital (données probantes de très faible qualité).
PICOs
Résumé simplifié
Anesthésiques locaux périduraux pour la prévention de la paralysie gastro‐intestinale postopératoire, des vomissements et des douleurs après une chirurgie abdominale
Contexte
La douleur et la paralysie intestinale (absence de mouvement) sont fréquentes après une chirurgie abdominale. Après laparotomie, cholécystectomie laparoscopique et colectomie, environ 10,3 % des patients souffriront d'une paralysie intestinale temporaire. Cela peut prolonger la durée du séjour à l'hôpital et augmenter les coûts de procédure. Parmi les moyens possibles de traiter la douleur après une chirurgie abdominale, on peut citer la péridurale et les injections d'opioïdes (substances apparentées à la morphine ou analgésiques). Une péridurale consiste à insérer un cathéter (un tube étroit) dans l'espace péridural (l'espace virtuel entourant la membrane qui contient le liquide céphalo‐rachidien et la moelle épinière) et à diffuser une solution d'anesthésique local (substance qui coupe la transmission de la douleur au cerveau) (seul ou en combinaison avec des opioïdes) pour anesthésier l'abdomen. Cette revue Cochrane compare les effets d'une péridurale utilisant un anesthésique local avec ceux d'un traitement à base d'opioïdes sur le déroulement postopératoire d’une chirurgie abdominale.
Date des recherches
Les preuves sont à jour jusqu'à décembre 2014. Lorsque nous avons relancé la recherche en février 2016, nous avons ajouté 16 nouvelles études potentiellement intéressantes à la liste des « études en attente de classification » et nous les intégrerons dans les conclusions formelles de la revue de lors de la prochaine mise à jour de la revue.
Caractéristiques des études
Nous avons inclus 128 essais avec 8754 participants des deux sexes, âgés de 33 à 76 ans, dans la revue et 94 essais avec 5846 participants dans l'analyse. Trois essais ont indiqué être officiellement enregistrés.
Sources de financement des études
Les essais inclus dans la revue ont été financés comme suit: organismes de bienfaisance (n = 19), ressources ministérielles (n = 8), sources gouvernementales (n = 15) et industrie (en partie ou au total) (n = 15). La source de financement n'a pas été précisée pour les autres essais.
Résultats principaux
Nous avons constaté qu'une péridurale contenant un anesthésique local réduit le temps nécessaire du rétablissement de la fonction intestinale par rapport à un régime à base d'opioïdes (équivalent à 17 heures). Egalement, une péridurale utilisant un anesthésique local et un opioïde réduit la douleur (équivalent à une diminution de 2,5 sur une échelle de 0 à 10 pour la douleur au mouvement dans les 24 heures suivant l'opération) et le temps passé à l'hôpital (équivalent à une journée) pour une chirurgie ouverte. Nous n'avons trouvé aucune donnée indiquant qu'une péridurale avec un anesthésique local aurait un effet sur l'incidence des vomissements ou la mauvaise cicatrisation intestinale.
Qualité des données
Nous avons estimé que la qualité des données était élevée pour le rétablissement de la fonction gastro‐intestinale, modérée pour le traitement de la douleur, faible pour l'absence d'effet sur les vomissements ou la cicatrisation intestinale et très faible pour la réduction du temps passé à l'hôpital après une chirurgie ouverte.
Authors' conclusions
Summary of findings
| Epidural local anaesthetic compared with opioid‐based regimen for adults | ||||||
| Patient or population: adults | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Opioid‐based regimen | Epidural local anaesthetic | |||||
| Time required to observe first flatus | Mean time required to observe first flatus in the intervention groups was | 1138 | ⊕⊕⊕⊕ | Effect size was proportionate to the | ||
| Time required to observe first faeces | Mean time required to observe first faeces in the intervention groups was | 1559 | ⊕⊕⊝⊝ | Pooled reduction is | ||
| VAS scores on movement at 24 hours | Mean VAS scores on movement at 24 hours in the intervention groups was | 2731 | ⊕⊕⊕⊝ | Pooled reduction | ||
| Vomiting during first 24 hours | Study population | RR 0.84 | 1154 | ⊕⊕⊝⊝ | ||
| 170 per 1000 | 143 per 1000 | |||||
| Low | ||||||
| 50 per 1000 | 42 per 1000 | |||||
| High | ||||||
| 250 per 1000 | 210 per 1000 | |||||
| Anastomotic leak | Study population | RR 0.74 | 848 | ⊕⊕⊝⊝ | ||
| 53 per 1000 | 39 per 1000 | |||||
| Low | ||||||
| 30 per 1000 | 22 per 1000 | |||||
| High | ||||||
| 100 per 1000 | 74 per 1000 | |||||
| Length of hospital stay | Mean length of hospital stay in the intervention groups was | 2598 | ⊕⊝⊝⊝ | Pooled reduction is | ||
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aAllocation concealment and/or blinding of outcome assessors rated as unclear or high risk for 75% or more of included studies for this outcome | ||||||
Background
This is an update of a previously published Cochrane review (Jorgensen 2001).
Description of the condition
In 2011, nearly 29% of hospital stays and 48% of hospital costs in the United States (USA) involved operating room procedures (http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb165.jsp). Among the 15 procedures most commonly performed in the USA were cholecystectomy and common bile duct exploration (129.4 per 100,000 population), abdominal and vaginal hysterectomy (99.4 per 100,000 population), colorectal resection (97.4 per 100,000 population), excision or lysis of peritoneal adhesions (97.4 per 100,000 population), appendicectomy (93.3 per 100,000 population) and oophorectomy (71.3 per 100,000 population). Thus abdominal surgery represents a significant proportion of hospital stays and costs. Gastrointestinal paralysis and postoperative pain are two major issues that need to be taken care of after abdominal surgery.
Gastrointestinal paralysis following abdominal surgery may result in prolonged hospital stay and increased costs. Following laparotomy, laparoscopic cholecystectomy and colectomy, approximately 10.3% of patients will have an ileus (Gan 2015). An ileus occurs more frequently in colectomy than cholecystectomy and more often when performed by laparotomy. Patients with ileus receiving opioids will have an increased length of hospital stay (ranging from 4.8 to 5.7 days), greater total costs (from USD 9,945 to USD 13,055) and a higher 30‐day all‐cause readmission rate (2.3% to 5.3% higher) compared with patients without an ileus (Gan 2015).
In 2000, the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) suggested that pain should be considered as the fifth vital sign, and that under‐treatment of pain would constitute abrogation of a fundamental human right (White 2007). After this statement was issued, an increase in the use of opioids for acute postoperative pain treatment was observed, as was an increase in their side effects (White 2007). It was also noted that postoperative critical respiratory events were encountered more frequently during the first 24 hours after opioid therapy was introduced (Ramachandran 2011).
Description of the intervention
Epidural anaesthesia or analgesia or both consist of injection of a local anaesthetic into the spine outside the dura mater. Epidural local anaesthetic may be used during abdominal surgery, sometimes as a replacement for general anaesthesia but most commonly as a supplement to general anaesthesia for surgery and for postoperative analgesia.
How the intervention might work
Epidural analgesia has been claimed to facilitate many of the steps through which a patient must go before returning to his or her preoperative functional level after a major abdominal surgery, including motility of the gastrointestinal tract (Thörn 1996). As summarized in their review, Holte and Kehlet considered that "the pathogenesis of postoperative ileus is multifactorial, and includes activation of inhibitory reflexes, inflammatory mediators and opioids (endogenous and exogenous)" (Holte 2002). Epidural analgesia may promote a faster return to intestinal transit through various mechanisms including a reduction in opioid administration (Guay 2006), a blockade of sympathetic gut innervation (creating a relative parasympathetic predominance) and a direct effect of systemic local anaesthetics (McCarthy 2010). Thorn et al included 14 participants and demonstrated that the gastrointestinal electromyographic activity of participants who received epidurally administered bupivacaine was different from that of participants who received epidurally administered morphine (Thörn 1996). Oral acetaminophen absorption (as demonstrated by the area under the curve of acetaminophen blood concentrations from zero to 60 minutes) was also greater among participants who received bupivacaine than among those given morphine (Thörn 1996). Thus an epidural containing a local anaesthetic may promote faster gastrointestinal transit return than is attained with systemic opioids.
Among undisturbed participants receiving patient‐controlled morphine analgesia after surgery, abnormal breathing patterns with cyclical airway obstruction are extremely common (Drummond 2013). Provided that pain relief would be at least equivalent to that achieved with opioid therapy, decreasing the quantity of opioids administered (Guay 2006) would make epidural analgesia with a local anaesthetic appear as an interesting alternative in the treatment of acute postoperative pain for the first days after abdominal surgery ‐ the time when pain is most intense. Reducing the quantity of opioids administered after surgery may reduce the rare, but serious, adverse respiratory events associated with administration of opioids for the treatment of postoperative pain.
Why it is important to do this review
This is an update of a previously published Cochrane review (Jorgensen 2001) in which review authors concluded that an epidural with a local anaesthetic reduced the time to return of gastrointestinal transit but with substantial heterogeneity. The effect of additional epidural opioid on gastrointestinal function was unclear. We undertook this review to look for new studies, to update methods and to re‐explore heterogeneity.
Objectives
To compare effects of postoperative epidural analgesia with local anaesthetics versus postoperative systemic or epidural opioids in terms of return of gastrointestinal transit, postoperative pain control, postoperative vomiting, incidence of anastomotic leak, length of hospital stay and costs after abdominal surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel randomized controlled trials (RCTs) in which an epidural containing a local anaesthetic was added to general anaesthesia and was continued or not for postoperative analgesia or was used for postoperative analgesia in one group, and this group was compared with another group given systemic or epidural opioid‐based regimens. We excluded quasi‐randomized trials (e.g. even/odd day of birth, chart number), and we applied no language or publication status restrictions.
Types of participants
We included adult (≥ 16 years old accepted) patients undergoing any abdominal surgery (open or laparoscopic) under general anaesthesia. We excluded trials performed on children, trials performed outside the perioperative period (chronic pain, labour analgesia) and trials in which participants underwent surgery at other surgical sites (i.e. not abdominal surgery).
Types of interventions
Treatment groups received epidural anaesthesia/analgesia containing a local anaesthetic with or without added opioids.
Control groups received an opioid‐based regimen administered by the systemic route or through an epidural.
General anaesthesia was used for all participants during surgery.
We excluded trials comparing various types or various concentrations of local anaesthetics when investigators included no control group without a local anaesthetic (different intervention).
Some substances are not universally accepted as safe for injection in the epidural space. Therefore, we did not retain in the analysis any trial (or subgroup) in which anything other than an opioid or a local anaesthetic or epinephrine was injected into the epidural space (e.g. midazolam, ketamine, tramadol).
Types of outcome measures
Primary outcomes
-
Postoperative paralytic ileus as measured by first passage of flatus.
Secondary outcomes
-
Postoperative paralytic ileus as measured by first passage of faeces (stool).
-
Pain scores (any ascending scale) at rest and on movement at six to eight hours, 24 hours, 48 hours and 72 hours.
-
Incidence of postoperative vomiting: number of participants who had experienced vomiting on day one.
-
Anastomotic leak.
-
Length of hospital stay (LOS).
-
Hospital costs.
Search methods for identification of studies
Figure 1 presents the flow chart for study selection.

Flow diagram. Study selection from the 2014 search. We reran the search in February 2016, and added 16 potential new studies of interest to the list of ‘Studies awaiting classification'. These studies will be incorporated into the formal review findings during the next review update. We also found one ongoing trial.
Electronic searches
We conducted a comprehensive literature search to identify all published and unpublished RCTs with no language restriction. We searched the following electronic databases to identify potential studies: Cochrane Central Register of Controlled Trials (CENTRAL; 2014, Issue 12) (Appendix 1); MEDLINE (OVID) 1950 to December 2014 (Appendix 2); and EMBASE (OVID) 1974 to December 2014 (Appendix 3). We reran the search in February 2016 and added 16 potential new studies of interest to the list of Studies awaiting classification; we will incorporate these studies into formal review findings during the next review update. We added one study to the Ongoing studies section.
Searching other resources
We also looked at PsycINFO as a source of grey literature in March 2015 (Appendix 4).
Data collection and analysis
Selection of studies
Two review authors (JG and DNN (DNN left the review before its completion) or MN) scanned the titles and abstracts of all reports identified by electronic searching and retrieved full texts of articles for potential inclusion. We excluded duplicate publications by comparing sites and dates of data collection. We stated reasons for excluding retrieved studies under Characteristics of excluded studies and in Figure 1.
Data extraction and management
Two review authors (JG and DNN (DNN left the review before its completion) or MN) independently extracted data. We resolved disagreements by discussion and did not require assistance from a third review author. We extracted events and total number of participants in each group for dichotomous data when available. We extracted mean, standard deviation (SD) and number of participants in each group for continuous data when available. If results were not available in our favoured format or were provided on different scales, we extracted data as P values and number of participants for each group. When we were not able to extract data in any of these formats, we contacted study authors to obtain additional information from their trials. We did not use medians as estimates for means and did not estimate variances from interquartile or range. We extracted sites and dates of data collection (for exclusion of duplicate publications) and factors required for exploration of heterogeneity (see Assessment of heterogeneity). After we had reached agreement, data were entered into RevMan (http://ims.cochrane.org/revman/about‐revman‐5) and into Comprehensive Meta Analysis Version 2.2.044 (www.Meta‐Analysis.com) (for exploration of heterogeneity and assessment of small‐study effects and publication bias) by one review author (JG).
Assessment of risk of bias in included studies
Two review authors (JG, DNN or MN) evaluated the methodological quality of selected studies with no assumption using the risk of bias assessment tool of The Cochrane Collaboration (Higgins 2011). We resolved disagreements by discussion. We rated as unclear elements for which the report provided insufficient information to allow us to make a clear judgement.
-
Generation of the allocation sequence of interventions: We considered randomization adequate if it was generated by a computer or a random number table algorithm. We judged other processes, such as tossing of a coin, adequate if the whole sequence was generated before the start of the trial. We considered the trial as quasi‐randomized if a non‐random system, such as dates, names or identification numbers, was used.
-
Concealment of allocation: We considered concealment adequate if the process that was used prevented patient recruiters, investigators and participants from knowing the intervention allocation of the next participant to be enrolled in the study. We considered concealment inadequate if the allocation method allowed patient recruiters, investigators or participants to know the treatment allocation of the next participant to be enrolled in the study.
-
Blinding of participants and personnel: We considered blinding adequate if the participant and personnel taking care of the participant were blinded to the intervention. We considered blinding inadequate if the participant or personnel were not blinded to the intervention. We rated as unclear trials for which this was only partially adequately addressed (personnel blinded but not participants or vice versa, etc.).
-
Blinding of outcome assessment: We considered blinding adequate if the outcome assessor was blinded to the intervention. We considered blinding inadequate if the outcome assessor was not blinded to the intervention.
-
Incomplete outcome data (attrition bias): We considered the trial adequate if all dropouts or withdrawals were accounted for, and if the number of dropouts was small (< 20%), was similar for both interventions and reasons for dropping out seemed reasonable. We considered the trial inadequate for this specific item if reasons for dropping out of patients were not stated or did not sound reasonable, the number was high (≥ 20%) or the number was highly different between groups.
-
Selective reporting (reporting bias): We considered the trial at low risk of bias if all measurements stated in the Methods section were included in the Results section. We rated the trial as having unclear risk of bias when some of the results were missing or insufficient information was provided (conference abstract). We rated the trial as having high risk of bias when important results (taking study author objectives into account) were mentioned in the Methods section but were not given in the Results section.
-
Any other risk of bias: We considered any other reason that may have influenced study results. Per‐protocol (not intention‐to‐treat) results were considered as introducing potential risk, and we rated these as having unclear risk. Differences between study groups in demographic characteristics were rated as presenting unclear or high risk, depending on their potential influence on review results. We rated study protocols at high risk when other treatment modalities differed markedly (e.g. high steroid dose was given to one group only, epidural local anaesthetic was part of a fast track programme applied to one group only).
Measures of treatment effect
We reported results as risk ratios (RRs) and their 95% confidence intervals (CIs) for dichotomous data (vomiting and gastrointestinal anastomotic leak). Odds ratios (ORs) are not easily understood by clinicians (McColl 1998). All continuous data (time to first flatus, time to first faeces, pain scores, length of hospital stay and costs) included items entered as P values. Therefore it was not possible to provide results as differences in means and their 95% CIs. Instead we provided results as standardized mean differences (SMDs) and their CIs. For SMDs, we considered 0.2 a small difference, 0.5 a medium difference and 0.8 a large difference (Pace 2011). For clinical correspondence, we multiplied the standard deviation (SD) of the control group of a study at low risk of bias, and when a typical SD was available, by the SMD. When we noted an effect, we calculated the number needed to treat for an additional beneficial outcome (NNTB) or an additional harmful outcome (NNTH) on the basis of the odds ratio, as this value is less likely to be influenced by the side (benefit or harm) on which data have been entered (Deeks 2002) (http://www.nntonline.net/visualrx/). When we observed no effect, we calculated optimal size information (number of participants needed for inclusion in a simple large trial) to justify a conclusion based on absence of effect (Pogue 1998) (http://www.stat.ubc.ca/˜rollin/stats/ssize/).
Unit of analysis issues
The unit of analysis was a participant who was individually randomized to the treatment group (intervention or control) in RCTs selected for this review.
Dealing with missing data
We contacted study authors for additional information when published articles did not provide enough information for extraction of data. We made no imputation.
Assessment of heterogeneity
We considered clinical heterogeneity before pooling results and examined statistical heterogeneity before carrying out any meta‐analysis.
We quantified statistical heterogeneity by using the I2 statistic. We qualified the amount as low (< 25%), moderate (50%) or high (≥ 75%) depending on the value obtained for the I2 statistic (Higgins 2003).
Assessment of reporting biases
We assessed publication bias by using a funnel plot, followed by Duval and Tweedie's trim and fill technique (Borenstein 2009; Duval 2000; Duval 2000a). This technique not only assesses whether publication bias is likely, it also yields an estimate of effect size after correction for the possibility of publication bias when such bias is suspected.
Data synthesis
We analysed data with RevMan (http://ims.cochrane.org/revman/about‐revman‐5) and Comprehensive Meta Analysis Version 2.2.044 (www.Meta‐Analysis.com) by using fixed‐effect (I2 < 25%) or random‐effects models (I2 > 25%). For continuous data, all analyses provided data that we could not enter in our favoured format (mean, SD and number of participants). In these situations, we chose not to consider a median as equivalent to a mean and did not estimate SD from quartiles. Instead we entered data into Comprehensive Meta Analysis as P values and numbers of participants. In such cases, mean differences cannot be obtained. We then transferred data in RevMan as generic variance and presented our results as standardized mean differences (SMDs). For SMDs, we considered 0.8 as the cutoff limit for a large effect (Pace 2011). For clinical equivalents, we multiplied the SMD by the SD of a study at low risk of bias, and when a typical SD on a clinical scale was provided (Higgins 2011). For dichotomous data, we provided results as risk ratios (values best understood by clinicians; McColl 1998). For results in which the intervention produced an effect, we calculated the number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH) by using the odds ratio (http://www.nntonline.net/visualrx/). When results were negative, we also calculated optimal information size to ensure that enough participants were included in the retained studies to justify a conclusion based on absence of effect (Pogue 1998) (www.stat.ubc.ca/˜rollin/stats/ssize/b2.html).
Subgroup analysis and investigation of heterogeneity
We explored any amount of heterogeneity > 25% by using Egger's regression intercept (Comprehensive Meta Analysis; to eliminate a small‐study effect), sensitivity analysis, subgrouping or meta‐regression (Comprehensive Meta Analysis) as appropriate. A priori factors for heterogeneity consisted of:
-
level of the epidural (thoracic vs lumbar);
-
type of drug used (local anaesthetic alone (concentration in lidocaine equivalent potency calculated as follows: lidocaine = 1, bupivacaine = 4, chloroprocaine = 1.5, dibucaine = 4, etidocaine = 4, levobupivacaine = 3.9, mepivacaine = 0.8, prilocaine = 0.9, procaine = 0.5, ropivacaine = 3 and tetracaine = 4; Berde 2009)) versus local anaesthetic plus opioid (and type of opioid);
-
timing (pre‐surgical vs post‐surgical incision) and duration of administration (intraoperative only, < 48 hours vs ≥ 48 hours);
-
site of surgery (bowel surgery; gynaecological, urological or vascular surgery);
-
type of surgery (open vs laparoscopic);
-
mean group age;
-
American Society of Anestheiologists (ASA) physical status; and
-
substance used and route of administration of analgesia in the control group (intravenous (with or without use of a patient‐controlled analgesia device) vs epidural (with or without use of a patient‐controlled analgesia device) vs other routes).
Although forest plots for all outcomes were examined while studies were placed in order for all potential heterogeneity factors, to avoid multiple comparisons, we performed analysis (sensitivity, subgrouping or meta‐regression) only when forest plots suggested a statistically significant effect, or when reviewers made the request (open vs laparoscopic surgery). All analysis performed are reported. Analysis included as forest plots in RevMan (Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.9; Analysis 1.10; Analysis 1.11; Analysis 1.12; Analysis 1.13; Analysis 1.14; Analysis 1.15; Analysis 1.17; Analysis 1.18) are those chosen as most interesting for each outcome (i.e. subgrouped to offer maximal possibility of showing subgroup differences).
Sensitivity analysis
We performed sensitivity analysis (defined as excluding a study on its risk of bias or because it appeared as an outlier on a forest plot).
Quality of evidence
We judged the quality of a body of evidence according to the GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) system (Guyatt 2011a) and presented this assessment in summary of findings Table for the main comparison (ims.cochrane.org/revman/gradepro) for all outcomes. For pain, we chose scores on movement at postoperative day one. For risk of bias, we judged the quality of evidence as high when most information was derived from studies at low risk of bias, and downgraded quality by one level when most information was obtained from studies at high or unclear risk of bias (allocation concealment and blinding of outcome assessors), or by two levels when the proportion of information obtained from studies at high risk of bias was sufficient to affect interpretation of results. For inconsistency, we downgraded the quality of evidence by one when the I2 statistic was 50% or higher without satisfactory explanation, and by two levels when the I2 statistic was 75% or higher without explanation. We did not downgrade the quality of evidence for indirectness, as all outcomes were based on direct comparisons were performed on the population of interest and were not surrogate markers (Guyatt 2011b). For imprecision (Guyatt 2011c), we downgraded the quality of evidence by one level when the CI around the effect size was large or overlapped an absence of effect and failed to exclude an important benefit or harm, or when the number of participants was less than the optimal information size; and we downgraded the quality by two levels when the CI was very wide and included both appreciable benefit and harm. For publication bias, we downgraded the quality of evidence by one level when correcting for the possibility of publication bias as assessed by Duval and Tweedie’s fill and trim analysis changed the conclusion. We upgraded the quality of evidence by one when the effect size was large (RR < 0.5 or > 2.0), and by two levels when the effect size was very large (RR < 0.2 or > 5) (Guyatt 2011d). We applied the same rules for OR when basal risk was less than 20%. For SMD, we used 0.8 as the cutoff point for a large effect (Pace 2011). We also upgraded quality by one level when we found evidence of a dose‐related response. We upgraded quality by one level when the possible effect of confounding factors would reduce a demonstrated effect or suggest a spurious effect when results show no effect. When the quality of the body of evidence is high, further research is very unlikely to change our confidence in the estimate of effect. When quality is moderate, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. When quality is low, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. When the quality is very low, any estimate of effect is very uncertain (Guyatt 2008).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; and Characteristics of studies awaiting classification.
Results of the search
We reviewed all included and excluded studies presented in the previous version of the review (Jorgensen 2001). The electronic search yielded 1047 abstracts from The Cochrane Library, 525 from EMBASE and 1257 from MEDLINE (Figure 1). After removal of duplicates, we screened 1606 abstracts. From studies in the previous version of the review, the list of abstracts obtained by the electronic search and the reference lists of relevant studies, we selected 307 studies (of which 216 were new studies) for further evaluation. We excluded 179 studies for various reasons: not a randomized controlled trial (n = 23), cross‐over trial (n = 1), no outcome of interest measured for the actual review (n = 41), different intervention (n = 95), different study population (n = 18) or retracted (n = 1).
Included studies
Sixteen studies are awaiting classification and one is ongoing. We retained 128 trials with 8754 participants (4426 in epidural local anaesthetic groups and 4328 in control groups) in the review and 94 studies with 5846 participants (3010 in epidural local anaesthetic groups and 2836 in opioid‐based regimen groups) in the analysis (Figure 1). We included 34 trials in the review but not in the analysis: 17 because data could not be extracted (Addison 1974; Alpaslan 2010; Beilin 2008; Bellolio 2012; Bisgaard 1990; Carli 1997; Cuschieri 1985; Elkaradawy 2011; Kentner 1996; Lugli 2008; Lugli 2010; Malenkovic 2003; O'Connor 2006; Schulze 1988; Seeling 1991; Tuman 1991; Yeager 1987), 15 because pain scores were the only outcomes of interest measured and local anaesthetics were administered during surgery only or for an unspecified duration (Doruk 2003; El‐Refai 2003; Handley 1997; Katz 2003; Limberi 2003; Liuboshevskii 2012; Ozcan 2004; Ozdilmac 2003; Park 2001; Rockemann 1996; Schricker 2000; Schricker 2002; Schumann 2003; Subramaniam 2000; Watters 1993), one because no outcomes were available at our selected time points (Scheinin 1982) and one because investigators provided insufficient information (Voylenko 2013). The 128 included trials enrolled participants with a mean age between 33 and 76 years of age, with a mean ASA score between 1.41 and 3.45 or from 1 to 2 to 1 to 5, and were published between 1974 and 2014. Researchers in three studies reported that their trial was registered: Fant 2013 (www.ClinicalTrials.gov; identifier: NCT01367418), Levy 2011 (www.ClinicalTrials.gov; identifier: NCT 18926278) and Muehling 2009 (www.ClinicalTrials.gov; identifier: NCT 00615888).
Trials were performed in Australia (n = four: Barratt 2002; Davies 1993; Handley 1997; Peyton 2003); Canada (n = 19; Bois 1997; Boylan 1998; Carli 1997; Carli 2001; Carli 2002; Donatelli 2006; Katz 2003; Lattermann 2007; Lugli 2008; Lugli 2010; Miller 1976; Mondor 2010; O'Connor 2006; Schricker 2000; Schricker 2002; Schricker 2004; St‐Onge 1997; Taqi 2007; Watters 1993); China (n = six; Cai 2007; Hu 2006; Tsui 1997; Wang 2010; Zeng 2003; Zhu 2013); Czech Republic (n = 1; Voylenko 2013); Denmark (n = eight; Bisgaard 1990; Brodner 2001; Hjortsø 1985a; Hjortsø 1985b; Jorgensen 2001; Moiniche 1993; Schulze 1988; Schulze 1992); Egypt (n = three; Elkaradawy 2011; El‐Refai 2003; Fayed 2014); Finland (n = four; Salomaki 1995; Scheinin 1982; Scheinin 1987; Turunen 2009); France (n = five; Jayr 1988; Jayr 1993; Jayr 1998; Mann 2000; Motamed 1998); Germany (n = 10; Heurich 2007; Hubler 2001; Kentner 1996; Muehling 2009; Neudecker 1999; Rockemann 1996; Rockemann 1997; Seeling 1990; Seeling 1990a; Seeling 1991); Greece (n = two; Chalmouki 2010; Limberi 2003); India (n = three; Kumar 2004; Subramaniam 2000; Tyagi 2011); Israel (n = two; Beilin 2003; Beilin 2008); Italy (n = 10; Aceto 2002; Barzoi 2000; Dauri 2003; De Pietri 2006; Giannoni 1999; Lombardo 2009; Luchetti 1996; Marandola 2008; Martella 2012; Siniscalchi 2003); Japan (n = one; Kudoh 2001); Korea (n = one; Hong 2008); Lithuania (n = one; Rimaitis 2003), Romania (n = two; Cindea 2011; Gherghina 2010); Russia (n = one; Liuboshevskii 2012), Serbia (n = 1; Malenkovic 2003); Spain (n = one; Calderon 2004); Sweden (n = six; Ahn 1988; Fant 2013; Rutberg 1984; Thorén 1989; Wallin 1986; Wattwil 1989); Switzerland (n = two; Licker 1994; Riwar 1991); The Netherlands (n = one; Broekema 1998); Turkey (n = eight; Alpaslan 2010; Aygun 2004; Doruk 2003; Erol 2008; Hadimioglu 2012; Ozcan 2004; Ozdilmac 2003; Ozturk 2010); United Kingdom (n = six; Addison 1974; Buggy 2002; Cuschieri 1985; George 1994; Levy 2011; Mallinder 2000); United States of America (n = 17; Benzon 1994; Cronin 2001; Cullen 1985; Ferguson 2009; Gambling 2009; Liu 1995; Norman 1997; Park 2001; Paulsen 2001; Pflug 1974; Schumann 2003; Senagore 2003; Steinberg 2002; Stevens 1998; Welch 1998; Yeager 1987; Zutshi 2005); and Uruguay (n = one; Bellolio 2012).
The funding source of 56/128 (44%) included trials was known. Those trials were funded by charity (n = 19; Beilin 2008; Buggy 2002; Carli 2001; Carli 2002; Cronin 2001; Cullen 1985; Cuschieri 1985; Donatelli 2006; Handley 1997; Jayr 1988; Jorgensen 2001; Mann 2000; Salomaki 1995; Scheinin 1987; Schricker 2000; Schricker 2002; Schricker 2004; Schulze 1992; Taqi 2007), departmental resources (n = eight; Cai 2007; Ferguson 2009; Hu 2006; Levy 2011; Mondor 2010; Tyagi 2011; Wang 2010; Zeng 2003), governmental sources (n = 15; Barratt 2002; Beilin 2003; Boylan 1998; Fant 2013; Heurich 2007; Katz 2003; Lugli 2008; Lugli 2010; Miller 1976; Park 2001; Peyton 2003; Pflug 1974; Schumann 2003; Thorén 1989; Watters 1993) and industry (in part or in total) (n = 15; Benzon 1994; Bois 1997; Gambling 2009; Hjortsø 1985a; Hjortsø 1985b; Jayr 1993; Jayr 1998; Liu 1995; Mallinder 2000; Moiniche 1993; Rutberg 1984; St‐Onge 1997; Schulze 1988; Steinberg 2002; Wallin 1986).
Surgeries included bariatric surgery (Schumann 2003); cholecystectomy (Addison 1974; Cuschieri 1985; Elkaradawy 2011; Erol 2008; Miller 1976; Moiniche 1993; Rutberg 1984; Schulze 1988; Wallin 1986); surgery of the gastrointestinal tract (Aceto 2002; Ahn 1988; Barratt 2002; Barzoi 2000; Bisgaard 1990; Cai 2007; Calderon 2004; Carli 1997; Carli 2001; Carli 2002; De Pietri 2006; Donatelli 2006; Fayed 2014; Gherghina 2010; Giannoni 1999; Handley 1997; Hjortsø 1985b; Kudoh 2001; Lattermann 2007; Levy 2011; Liu 1995; Liuboshevskii 2012; Luchetti 1996; Lugli 2008; Lugli 2010; Mallinder 2000; Mann 2000; Marandola 2008; Martella 2012; Mondor 2010; Neudecker 1999; Ozdilmac 2003; Paulsen 2001; Rimaitis 2003; Riwar 1991; Rockemann 1997; Scheinin 1987; Schricker 2000; Schricker 2002; Schricker 2004; Senagore 2003; Siniscalchi 2003; Steinberg 2002; Schulze 1992; Taqi 2007; Turunen 2009; Tyagi 2011; Wang 2010; Watters 1993; Welch 1998; Zhu 2013; Zutshi 2005); gynaecological surgery (Cronin 2001; El‐Refai 2003; Ferguson 2009; Hong 2008; Jorgensen 2001; Katz 2003; Licker 1994; Ozcan 2004; Thorén 1989; Tsui 1997; Wattwil 1989); liver surgery (Bellolio 2012); urological surgery (Brodner 2001; Chalmouki 2010; Dauri 2003; Doruk 2003; Fant 2013; Hadimioglu 2012; Hubler 2001; Kentner 1996; O'Connor 2006; Ozturk 2010; Voylenko 2013); vascular surgery (Bois 1997; Boylan 1998; Davies 1993; Lombardo 2009; Muehling 2009; Norman 1997; Tuman 1991); and various abdominal surgeries (Alpaslan 2010; Aygun 2004; Beilin 2003; Beilin 2008; Benzon 1994; Broekema 1998; Buggy 2002; Cindea 2011; Cullen 1985; Gambling 2009; George 1994; Hjortsø 1985a; Hu 2006; Jayr 1993; Jayr 1998; Kumar 2004; Limberi 2003; Malenkovic 2003; Motamed 1998; Park 2001; Peyton 2003; Pflug 1974; Rockemann 1996; Salomaki 1995; Scheinin 1982; Seeling 1990; Seeling 1990a; Seeling 1991; St‐Onge 1997; Subramaniam 2000; Yeager 1987; Zeng 2003). These surgeries were performed by laparoscopy (Hong 2008; Levy 2011; Luchetti 1996; Neudecker 1999; Senagore 2003; Taqi 2007; Turunen 2009); or by open laparotomy (Aceto 2002; Addison 1974; Ahn 1988; Alpaslan 2010; Aygun 2004; Barratt 2002; Barzoi 2000; Beilin 2003; Beilin 2008; Bellolio 2012; Benzon 1994; Bisgaard 1990; Bois 1997; Boylan 1998; Brodner 2001; Broekema 1998; Buggy 2002; Cai 2007; Calderon 2004; Carli 1997; Carli 2001; Carli 2002; Chalmouki 2010; Cindea 2011; Cronin 2001; Cullen 1985; Cuschieri 1985; Dauri 2003; Davies 1993; De Pietri 2006; Donatelli 2006; Doruk 2003; Elkaradawy 2011; El‐Refai 2003; Erol 2008; Fant 2013; Fayed 2014; Ferguson 2009; Gambling 2009; George 1994; Gherghina 2010; Giannoni 1999; Hadimioglu 2012; Handley 1997; Heurich 2007; Hjortsø 1985a; Hjortsø 1985b; Hu 2006; Hubler 2001; Jayr 1988; Jayr 1993; Jayr 1998; Jorgensen 2001; Katz 2003; Kentner 1996; Kudoh 2001; Kumar 2004; Lattermann 2007; Licker 1994; Limberi 2003; Liu 1995; Liuboshevskii 2012; Lombardo 2009; Lugli 2008; Lugli 2010; Malenkovic 2003; Mallinder 2000; Mann 2000; Marandola 2008; Martella 2012; Miller 1976; Moiniche 1993; Mondor 2010; Motamed 1998; Muehling 2009; Norman 1997; O'Connor 2006; Ozcan 2004; Ozdilmac 2003; Ozturk 2010; Park 2001; Paulsen 2001; Peyton 2003; Pflug 1974; Rimaitis 2003; Riwar 1991; Rockemann 1996; Rockemann 1997; Rutberg 1984; Salomaki 1995; Scheinin 1982; Scheinin 1987; Schricker 2000; Schricker 2002; Schricker 2004; Schulze 1988; Schulze 1992; Schumann 2003; Seeling 1990; Seeling 1990a; Seeling 1991; Siniscalchi 2003; Steinberg 2002; Stevens 1998; St‐Onge 1997; Subramaniam 2000; Thorén 1989; Tsui 1997; Tuman 1991; Tyagi 2011; Voylenko 2013; Wallin 1986; Wang 2010; Watters 1993; Wattwil 1989; Welch 1998; Wiedemann 1991; Yeager 1987; Zeng 2003; Zhu 2013; Zutshi 2005).
Epidurals were placed at the thoracic level (Aceto 2002; Addison 1974; Barratt 2002; Barzoi 2000; Bois 1997; Brodner 2001; Broekema 1998; Cai 2007; Carli 1997; Carli 2001; Carli 2002; Chalmouki 2010; Cronin 2001; Cuschieri 1985; Dauri 2003; Davies 1993; De Pietri 2006; Donatelli 2006; Elkaradawy 2011; Erol 2008; Fant 2013; Fayed 2014; Ferguson 2009; George 1994; Hubler 2001; Jayr 1988; Jayr 1993; Jayr 1998; Jorgensen 2001; Kudoh 2001; Kumar 2004; Lattermann 2007; Levy 2011; Liu 1995; Liuboshevskii 2012; Luchetti 1996; Lugli 2008; Lugli 2010; Mann 2000; Marandola 2008; Martella 2012; Moiniche 1993; Mondor 2010; Motamed 1998; Muehling 2009; Neudecker 1999; Norman 1997; Paulsen 2001; Pflug 1974; Rimaitis 2003; Rockemann 1996; Rockemann 1997; Rutberg 1984; Salomaki 1995; Scheinin 1982; Schricker 2000; Schricker 2002; Schricker 2004; Schulze 1988; Schulze 1992; Schumann 2003; Seeling 1990; Seeling 1990a; Senagore 2003; Siniscalchi 2003; Steinberg 2002; Stevens 1998; St‐Onge 1997; Taqi 2007; Thorén 1989; Turunen 2009; Tyagi 2011; Wallin 1986; Wattwil 1989; Zhu 2013; Zutshi 2005), at the lumbar level (Ahn 1988; Alpaslan 2010; Aygun 2004; Beilin 2003; Beilin 2008; Bisgaard 1990; Boylan 1998; Calderon 2004; Doruk 2003; El‐Refai 2003; Giannoni 1999; Hadimioglu 2012; Handley 1997; Hjortsø 1985a; Hong 2008; Hu 2006; Katz 2003; Kentner 1996; Licker 1994; Malenkovic 2003; Mallinder 2000; Miller 1976; Ozcan 2004;Ozdilmac 2003; Riwar 1991; Subramaniam 2000; Tsui 1997; Watters 1993) or at the thoracic or lumbar level (Benzon 1994; Buggy 2002; Gherghina 2010; Heurich 2007; Limberi 2003; O'Connor 2006; Park 2001; Peyton 2003; Tuman 1991; Yeager 1987), or they were placed at an unspecified level (Bellolio 2012; Cindea 2011; Cullen 1985; Gambling 2009; Hjortsø 1985b; Lombardo 2009; Ozturk 2010; Scheinin 1987; Seeling 1991; Voylenko 2013; Wang 2010; Welch 1998; Zeng 2003).
Local anaesthetics were administered only for surgery (Brodner 2001; Gambling 2009; Hadimioglu 2012; Handley 1997; Jayr 1988; Katz 2003; Limberi 2003; Luchetti 1996; Mallinder 2000; Mondor 2010; Norman 1997; Ozcan 2004; Ozdilmac 2003; Park 2001; Rockemann 1996; Subramaniam 2000; Watters 1993), for less than 48 hours (Alpaslan 2010; Aygun 2004; Barzoi 2000; Beilin 2008; Benzon 1994; Buggy 2002; Calderon 2004; Cuschieri 1985; Dauri 2003; Elkaradawy 2011; George 1994; Hjortsø 1985a; Hjortsø 1985b; Jayr 1998; Jorgensen 2001; Kentner 1996; Licker 1994; Lombardo 2009; Marandola 2008; Miller 1976; Moiniche 1993; Neudecker 1999; Rutberg 1984; Salomaki 1995; Scheinin 1982; Seeling 1990a; Senagore 2003; Stevens 1998; Thorén 1989; Wallin 1986; Wang 2010; Wattwil 1989; Zeng 2003), for 48 hours or longer (Aceto 2002; Addison 1974; Ahn 1988; Barratt 2002; Beilin 2003; Bisgaard 1990; Bois 1997; Boylan 1998; Broekema 1998; Cai 2007; Carli 1997; Carli 2001; Carli 2002; Chalmouki 2010; Cindea 2011; Cronin 2001; Cullen 1985; Davies 1993; De Pietri 2006; Donatelli 2006; Erol 2008; Fant 2013; Fayed 2014; Ferguson 2009; Gherghina 2010; Giannoni 1999; Hong 2008; Hu 2006; Hubler 2001; Jayr 1993; Kudoh 2001; Kumar 2004; Lattermann 2007; Levy 2011; Liu 1995; Lugli 2008; Lugli 2010; Malenkovic 2003; Mann 2000; Motamed 1998; Paulsen 2001; Peyton 2003; Pflug 1974; Rimaitis 2003; Riwar 1991; Rockemann 1997; Scheinin 1987; Schricker 2004; Schulze 1988; Schulze 1992; Seeling 1990; Seeling 1991; Steinberg 2002; St‐Onge 1997; Taqi 2007; Tsui 1997; Tuman 1991; Turunen 2009; Tyagi 2011; Zhu 2013; Zutshi 2005) or for an unspecified duration (Bellolio 2012; Doruk 2003; El‐Refai 2003; Liuboshevskii 2012; Martella 2012; Muehling 2009; O'Connor 2006; Ozturk 2010; Schricker 2000; Schricker 2002; Schumann 2003; Siniscalchi 2003; Voylenko 2013; Welch 1998; Yeager 1987). For Heurich 2007, local anaesthetic was administered for 24 hours to participants in the second portion of the trial and for 48 hours to participants in the first portion. The concentration of local anaesthetic varied between 2 and 30 milligrams per millilitre in lidocaine equivalent. Fentanyl (Aygun 2004; Barratt 2002; Beilin 2003; Beilin 2008; Benzon 1994; Bois 1997; Buggy 2002; Calderon 2004; Carli 2001; Carli 2002; Cindea 2011; Dauri 2003; Donatelli 2006; Elkaradawy 2011; Erol 2008; Fayed 2014; George 1994; Hu 2006; Katz 2003; Lattermann 2007; Levy 2011; Licker 1994; Liuboshevskii 2012; Lugli 2008; Lugli 2010; O'Connor 2006; Ozcan 2004; Ozturk 2010; Paulsen 2001; Rimaitis 2003; Salomaki 1995; Schricker 2000; Schricker 2002; Schricker 2004; Seeling 1990; Senagore 2003; Steinberg 2002; Taqi 2007; Tsui 1997; Tuman 1991; Tyagi 2011; Zeng 2003; Zutshi 2005), meperidine (Schumann 2003; St‐Onge 1997), fentanyl or meperidine (Peyton 2003), fentanyl or no opioid (Heurich 2007), morphine (Barzoi 2000; Bisgaard 1990; Boylan 1998; Cai 2007; Ferguson 2009; Giannoni 1999; Hjortsø 1985a; Hjortsø 1985b; Hong 2008; Jayr 1993; Liu 1995; Luchetti 1996; Marandola 2008; Moiniche 1993; Motamed 1998; Ozdilmac 2003; Rockemann 1996; Schulze 1992; Seeling 1991; Stevens 1998; Subramaniam 2000; Wang 2010; Welch 1998; Zhu 2013), extended‐release morphine (Gambling 2009), sufentanil (Aceto 2002; Fant 2013; Gherghina 2010; Lombardo 2009; Mann 2000; Martella 2012; Muehling 2009; Rockemann 1997), morphine or sufentanil (Broekema 1998), morphine or no opioid (Cullen 1985; Doruk 2003) or no opioid (Addison 1974; Ahn 1988; Alpaslan 2010; Brodner 2001; Chalmouki 2010; Cronin 2001; Cuschieri 1985; Davies 1993; De Pietri 2006; El‐Refai 2003; Hadimioglu 2012; Handley 1997; Hubler 2001; Jayr 1988; Jayr 1998; Jorgensen 2001; Kentner 1996; Kudoh 2001; Kumar 2004; Limberi 2003; Malenkovic 2003; Mallinder 2000; Miller 1976; Mondor 2010; Neudecker 1999; Norman 1997; Park 2001; Pflug 1974; Riwar 1991; Rutberg 1984; Scheinin 1982; Scheinin 1987; Schulze 1988; Seeling 1990a; Siniscalchi 2003; Thorén 1989; Turunen 2009; Voylenko 2013; Wallin 1986; Watters 1993; Wattwil 1989; Yeager 1987) was added to the epidural.
Epidural administration of local anaesthetic was started before (Aceto 2002; Addison 1974; Ahn 1988; Alpaslan 2010; Barratt 2002; Barzoi 2000; Beilin 2008; Bellolio 2012; Bisgaard 1990; Boylan 1998; Brodner 2001; Broekema 1998; Buggy 2002; Cai 2007; Carli 1997; Carli 2001; Carli 2002; Chalmouki 2010; Cronin 2001; Cuschieri 1985; Dauri 2003; Davies 1993; De Pietri 2006; Donatelli 2006; Doruk 2003; Elkaradawy 2011; El‐Refai 2003; Erol 2008; Fant 2013; Ferguson 2009; Gambling 2009; George 1994; Gherghina 2010; Giannoni 1999; Hadimioglu 2012; Handley 1997; Heurich 2007; Hjortsø 1985a; Hjortsø 1985b; Hong 2008; Hubler 2001; Jayr 1988; Jayr 1993; Jorgensen 2001; Kentner 1996; Kudoh 2001; Lattermann 2007; Levy 2011; Licker 1994; Limberi 2003; Liu 1995; Liuboshevskii 2012; Lombardo 2009; Luchetti 1996; Lugli 2008; Lugli 2010; Malenkovic 2003; Mallinder 2000; Mann 2000; Marandola 2008; Moiniche 1993; Mondor 2010; Muehling 2009; Neudecker 1999; Norman 1997; O'Connor 2006; Ozcan 2004; Ozdilmac 2003; Ozturk 2010; Park 2001; Peyton 2003; Rimaitis 2003; Riwar 1991; Rockemann 1997; Rutberg 1984; Scheinin 1982; Schricker 2000; Schricker 2002; Schricker 2004; Schulze 1988; Schulze 1992; Schumann 2003; Seeling 1990; Seeling 1991; Senagore 2003; Siniscalchi 2003; Steinberg 2002; Stevens 1998; St‐Onge 1997; Taqi 2007; Thorén 1989; Tsui 1997; Tuman 1991; Turunen 2009; Tyagi 2011; Wallin 1986; Wang 2010; Watters 1993; Wattwil 1989; Yeager 1987; Zeng 2003) or after the surgical incision was made (Aygun 2004; Beilin 2003; Benzon 1994; Bois 1997; Calderon 2004; Cullen 1985; Fayed 2014; Jayr 1998; Hu 2006; Kumar 2004; Martella 2012; Miller 1976; Motamed 1998; Ozcan 2004; Paulsen 2001; Pflug 1974; Salomaki 1995; Scheinin 1987; Seeling 1990a; Welch 1998; Zhu 2013; Zutshi 2005), or before for one group and after for another (Rockemann 1996; Subramaniam 2000). Timing was unclear for two trials (Cindea 2011; Voylenko 2013).
The control group received opioids by the epidural (Barzoi 2000; Benzon 1994; Bisgaard 1990; Cronin 2001; Cullen 1985; Gambling 2009; Hubler 2001; Kumar 2004; Liu 1995; Rutberg 1984; Salomaki 1995; Scheinin 1982; Scheinin 1987; St‐Onge 1997; Subramaniam 2000; Thorén 1989), intrathecal (De Pietri 2006), intramuscular (Addison 1974; Ahn 1988; Broekema 1998; Hjortsø 1985a; Jorgensen 2001; Liuboshevskii 2012; Miller 1976; Moiniche 1993; Ozcan 2004; Ozturk 2010; Rimaitis 2003; Seeling 1990; Wallin 1986; Wattwil 1989; Welch 1998), intravenous (Aceto 2002; Aygun 2004; Barratt 2002; Beilin 2003; Beilin 2008; Bois 1997; Boylan 1998; Brodner 2001; Buggy 2002; Cai 2007; Carli 2001; Carli 2002; Chalmouki 2010; Dauri 2003; Davies 1993; Donatelli 2006; Doruk 2003; Elkaradawy 2011; El‐Refai 2003; Fant 2013; Fayed 2014; Ferguson 2009; George 1994; Gherghina 2010; Giannoni 1999; Hadimioglu 2012; Handley 1997; Hong 2008; Jayr 1998; Kentner 1996; Kudoh 2001; Lattermann 2007; Licker 1994; Limberi 2003; Liu 1995; Lombardo 2009; Luchetti 1996; Lugli 2008; Lugli 2010; Mallinder 2000; Mann 2000; Marandola 2008; Martella 2012; Mondor 2010; Motamed 1998; Muehling 2009; Neudecker 1999; Norman 1997; Ozdilmac 2003; Paulsen 2001; Peyton 2003; Pflug 1974; Riwar 1991; Rockemann 1996; Rockemann 1997; Schricker 2000; Schricker 2002; Schricker 2004; Schumann 2003; Seeling 1990a; Senagore 2003; Siniscalchi 2003; Steinberg 2002; Stevens 1998; Taqi 2007; Tsui 1997; Tyagi 2011; Zeng 2003; Zhu 2013; Zutshi 2005), epidural or intravenous (Heurich 2007; Seeling 1991), intravenous or intramuscular (Cuschieri 1985; Hu 2006; Park 2001; Turunen 2009; Watters 1993; Yeager 1987) or subcutaneous route (Carli 1997; Jayr 1988; Jayr 1993). For Calderon 2004; Hjortsø 1985b; Malenkovic 2003; Schulze 1988; Schulze 1992; and Wang 2010, the exact route of administration of the opioid in the control group was not specified. The opioid administered in the control group was buprenorphine (Kudoh 2001; Malenkovic 2003; Seeling 1990a), fentanyl (Benzon 1994; Cai 2007; Cronin 2001; Erol 2008; Fayed 2014; Hong 2008; Luchetti 1996; Salomaki 1995), fentanyl or piritramide (Heurich 2007), ketobemidone (Wattwil 1989), meperidine (Addison 1974; Liuboshevskii 2012; Miller 1976; Ozcan 2004; Rimaitis 2003; St‐Onge 1997), meperidine or tramadol (Wang 2010), morphine (Barratt 2002; Barzoi 2000; Beilin 2003; Beilin 2008; Bisgaard 1990; Bois 1997; Boylan 1998; Broekema 1998; Buggy 2002; Carli 2001; Carli 2002; Chalmouki 2010; Cindea 2011; Cullen 1985; Cuschieri 1985; Davies 1993; De Pietri 2006; Donatelli 2006; Elkaradawy 2011; El‐Refai 2003; Fant 2013; Ferguson 2009; George 1994; Gherghina 2010; Giannoni 1999; Hadimioglu 2012; Handley 1997; Hjortsø 1985a; Hjortsø 1985b; Jayr 1988; Jayr 1993; Jayr 1998; Jorgensen 2001; Katz 2003; Kumar 2004; Lattermann 2007; Levy 2011; Licker 1994; Limberi 2003; Liu 1995; Lombardo 2009; Lugli 2008; Lugli 2010; Mallinder 2000; Mann 2000; Marandola 2008; Martella 2012; Moiniche 1993; Mondor 2010; Motamed 1998; Neudecker 1999; Norman 1997; Ozdilmac 2003; Ozturk 2010; Park 2001; Pflug 1974; Rockemann 1996; Rockemann 1997; Rutberg 1984; Scheinin 1982; Scheinin 1987; Schricker 2000; Schricker 2002; Schricker 2004; Schulze 1992; Schumann 2003; Seeling 1991; Senagore 2003; Siniscalchi 2003; Steinberg 2002; Stevens 1998; Subramaniam 2000; Taqi 2007; Thorén 1989; Tsui 1997; Zeng 2003; Zhu 2013), morphine or meperidine (Hu 2006; Paulsen 2001; Watters 1993; Welch 1998), morphine or tramadol (Doruk 2003), extended‐release morphine (Gambling 2009), nicomorphine (Schulze 1988), oxycodone (Turunen 2009), papaveratum (Carli 1997), pentazocine (Ahn 1988; Riwar 1991; Wallin 1986), piritramide (Brodner 2001; Kentner 1996; Muehling 2009; Seeling 1990), sufentanil (Hubler 2001) or tramadol (Aceto 2002; Aygun 2004; Dauri 2003; Tyagi 2011). The type of opioid used in the control group was not specified for Peyton 2003; Tuman 1991; Yeager 1987 and Zutshi 2005. The comparator was unclear for Alpaslan 2010; Bellolio 2012; O'Connor 2006 and Voylenko 2013.
Excluded studies
We excluded 179 studies because they were not randomized controlled trials (n = 23), were cross‐over trials (n = 1), had no outcomes of interest measured for the actual review (n = 41), studied a different intervention (n = 95), studied a different population (n = 18) or retracted the report (n = 1). The characteristics of excluded studies and reasons for exclusion are listed under Characteristics of excluded studies and in Figure 1. Some of the studies included in the previous version of this systematic review (Jorgensen 2001) did not include a group without local anaesthetics, as all groups received a neuraxial block with a local anaesthetic during surgery. For this reason (see Characteristics of excluded studies for individual studies), some studies included in the previous version of this review do not appear among our included studies. Likewise, as the result of a different method of data extraction and redefinition of outcomes, some studies excluded in the previous version of this systematic review (Jorgensen 2001) may be included in the current version.
Ongoing studies
We did not specifically look for ongoing studies, but we found one ongoing trial when we reran the search in February 2016. We will search trial registers for the next update.
Studies awaiting classification
We found 16 trials of potential interest when we reran the search in February 2016, five of which contained our selected outcomes. We will evaluate those studies further at the next update.
Risk of bias in included studies
Among the included trials, we identified the following as the most common flaws: insufficient description of the method of randomization, absence of details of allocation concealment and absence of blinding or of information on blinding (Figure 2; Figure 3).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged allocation concealment as appropriate for less than 25% of included trials and marked this as unclear for all other trials (Figure 2).
Blinding
We rated blinding of participants and personnel taking care of the participants or assessing participants' outcomes as adequate for less than 25% of included trials (Figure 2). Despite the difficulty of the exercise, the following trials succeeded in blinding outcome assessors: Barzoi 2000; Benzon 1994; Broekema 1998; Cronin 2001; Cullen 1985; De Pietri 2006; Elkaradawy 2011; El‐Refai 2003; Gambling 2009; Hubler 2001; Jayr 1988; Jayr 1993; Katz 2003; Kumar 2004; Liu 1995; Luchetti 1996; Malenkovic 2003; Mondor 2010; O'Connor 2006; Salomaki 1995; St‐Onge 1997; Subramaniam 2000.
Incomplete outcome data
We determined that complete data were provided for more than 75% of included trials (Figure 2).
Selective reporting
We judged selective reporting as problematic for less than 25% of included trials (Figure 2).
Other potential sources of bias
We judged more than 50% of included trials as having low risk for other potential bias (Figure 2).
Effects of interventions
Primary outcome
Return of gastrointestinal transit
Postoperative paralytic ileus as measured by first passage of flatus
In 22 trials that included 1138 participants (Ahn 1988; Carli 2001; Gherghina 2010; Hjortsø 1985a; Jayr 1988; Jorgensen 2001; Kudoh 2001; Liu 1995; Lombardo 2009; Martella 2012; Paulsen 2001; Riwar 1991; Steinberg 2002; Stevens 1998; Taqi 2007; Thorén 1989; Tyagi 2011; Wallin 1986; Wang 2010; Wattwil 1989; Welch 1998; Zhu 2013), an epidural containing a local anaesthetic reduced the time required for return of gastrointestinal transit after abdominal surgery, as measured by time required before first flatus was observed: standardized mean difference (SMD) ‐1.28, 95% confidence interval (CI) ‐1.71 to ‐0.86; I2 = 90%.(random‐effects model) (Analysis 1.1). Egger's regression intercept showed that part of the heterogeneity might be due to a small‐study effect (P value < 0.0001; two‐tailed). Duval and Tweedie's trim and fill analysis calculated that two trials might be missing to left of mean for an adjusted SMD of ‐1.47 (95% CI ‐1.94 to ‐1.00) (random‐effects model). Keeping only trials in which local anaesthetic was continued after completion of surgery (Ahn 1988; Carli 2001; Gherghina 2010; Hjortsø 1985a; Jorgensen 2001; Kudoh 2001; Liu 1995; Lombardo 2009; Paulsen 2001; Riwar 1991; Steinberg 2002; Stevens 1998; Taqi 2007; Thorén 1989; Tyagi 2011; Wallin 1986; Wang 2010; Wattwil 1989; Zhu 2013) would not affect statistical heterogeneity (I2 = 89%). When this was done, the effect size was similar whether an opioid was (SMD ‐1.14, 95% CI ‐1.73 to ‐0.56; 11 trials including 575 participants) or was not (SMD ‐1.19, 95% CI ‐1.72 to ‐0.66; seven trials including 273 participants) added to the epidural infusion (mixed‐effects analysis): P value for heterogeneity between the two subgroups = 0.92 (Q = 0.011). For the same trials (local anaesthetic continued after surgery), when trials were subgrouped by type of surgery performed, a large effect (SMD ≥ 0.8) was seen for gastrointestinal (SMD ‐1.26, 95% CI ‐1.72 to ‐0.80), abdominal aortic repair (SMD ‐12.86, 95% CI ‐15.98 to ‐9.73), gynaecological (SMD ‐1.24, 95% CI ‐1.86 to ‐0.62) and urological (SMD ‐0.83, 95% CI ‐1.47 to ‐0.18) surgeries only (mixed‐effects analysis). Although a high (gastrointestinal surgery) or moderate (gynaecological surgery) amount of heterogeneity is seen, this heterogeneity comes from the amplitude in effect, because no trial favoured the opioid‐based regimen over the epidural regimen with a local anaesthetic. If the following potency equivalences are assumed ‐ lidocaine = 1, mepivacaine = 0.8, ropivacaine = 3, levobupivacaine = 3.9 and bupivacaine = 4 ‐ for participants undergoing gastrointestinal, abdominal aortic repair, gynaecological or urological surgery, for whom the infusion was used after surgery, higher concentrations (in mg/mL) of local anaesthetic infusion after surgery would increase the amplitude of effect (P value = 0.0008) (Figure 4). For laparoscopic surgery (one study with 50 participants; Taqi 2007), the SMD would be ‐0.81 (95% CI ‐1.39 to ‐0.23). Liu 1995 (mean and SD of the control group 71 ± 13.86 hours, respectively) revealed that 54 participants (27 per group) could eliminate a 15% difference in a simple trial (alpha = 0.05; beta = 0.2; two‐sided test).

Meta‐regression of effects of the concentration of local anaesthetic used (mg/mL) after surgery on the standardized mean difference for return of gastrointestinal transit as measured by the time required to obtain first flatus. P value = 0.0008.
If a trial at low risk of bias (Liu 1995) is used as the standard (SD of the control group 13.86 hours), the difference in gastrointestinal surgery would be equivalent to 17.5 hours.
Quality of evidence
We downgraded evidence by two levels for risk of bias because 75% or more of the studies included for this outcome were rated as having unclear or high risk of bias for allocation concealment and blinding of outcome assessors. We did not downgrade evidence on the basis of inconsistency because a reasonable explanation was found for heterogeneity. We did not downgrade for indirectness because we included direct comparisons for the population of interest, and we did not consider time to first flatus as a surrogate marker for clinical evidence of gastrointestinal transit. We did not downgrade level of evidence for imprecision because the optimal information size was achieved. We did not downgrade evidence for publication bias because applying a correction would not modify the conclusion. We upgraded evidence by one level for a large effect size (SMD > 0.8). We found no evidence of confounding factors to justify upgrading. We upgraded the level of evidence by one for a dose response (increasing the concentration of the local anaesthetic increased the effect size). We rated the level of evidence as high.
Secondary outcomes
Postoperative paralytic ileus as measured by first passage of stool
This outcome was available for 28 trials that included 1559 participants (Ahn 1988; Barzoi 2000; Brodner 2001; Carli 2001; Carli 2002; Chalmouki 2010; Cindea 2011; Giannoni 1999; Hjortsø 1985a; Jorgensen 2001; Kudoh 2001; Levy 2011; Mann 2000; Neudecker 1999; Paulsen 2001; Riwar 1991; Rockemann 1997; Scheinin 1987; Seeling 1990; Steinberg 2002; Stevens 1998; Taqi 2007; Thorén 1989; Turunen 2009; Tyagi 2011; Wallin 1986; Wattwil 1989; Zutshi 2005) in which the duration of local anaesthetic infusion after surgery was known (Analysis 1.2). An epidural with a local anaesthetic infusion after surgery reduces the time required before observation of first faeces (stool): SMD ‐0.67, 95% CI ‐0.86 to ‐0.47; I2 = 69% (random‐effects model). This effect was not seen in one trial in which the local anaesthetic was administered only during surgery (Analysis 1.2). We excluded this trial (Brodner 2001) from the rest of the analysis. When this trial was excluded, effect size (SMD ‐0.71, 95% CI ‐0.90 to ‐0.51) and heterogeneity remained the same (I2 = 69%). Egger's regression intercept showed no significant evidence of a small‐study effect. Duval and Tweedie's trim and fill analysis showed no evidence of publication bias. The addition of an opioid (SMD ‐0.66, 95% CI ‐0.89 to ‐0.44; 16 trials for infusion of a local anaesthetic with an opioid; Barzoi 2000; Carli 2001; Carli 2002; Cindea 2011; Giannoni 1999; Hjortsø 1985a; Levy 2011; Mann 2000; Paulsen 2001; Rockemann 1997; Seeling 1990; Stevens 1998; Steinberg 2002; Taqi 2007; Tyagi 2011; Zutshi 2005) did not significantly modify the amplitude of the effect size (vs SMD ‐0.80, 95% CI ‐1.21 to ‐0.40; 11 trials; Ahn 1988; Chalmouki 2010; Jorgensen 2001; Kudoh 2001; Neudecker 1999; Riwar 1991; Scheinin 1987; Thorén 1989; Turunen 2009; Wallin 1986; Wattwil 1989; P value for heterogeneity between the two subgroups = 0.56, Q = 0.337, mixed=effects analysis). The effect was seen for gastrointestinal (SMD ‐0.78, 95% CI ‐1.03 to ‐0.53; Ahn 1988; Barzoi 2000; Carli 2001; Carli 2002; Kudoh 2001; Levy 2011; Mann 2000; Neudecker 1999; Paulsen 2001; Riwar 1991; Rockemann 1997; Scheinin 1987; Steinberg 2002; Taqi 2007; Turunen 2009; Tyagi 2011; Zutshi 2005), gynaecological (SMD ‐0.62, 95% CI ‐1.23 to ‐0.01; Jorgensen 2001; Thorén 1989; Wattwil 1989) and urological surgery (SMD ‐0.87, 95% CI ‐1.36 to ‐0.38; Chalmouki 2010; Stevens 1998; mixed‐effects analysis) without a statistically significant difference between those subgroups (P value for heterogeneity between subgroups = 0.82, Q = 0.400). This outcome was not available for aortic abdominal surgery. Investigators used thoracic epidural anaesthesia for 21 of these trials and a lumbar epidural for three trials; this information was not available for two trials (Cindea 2011; Scheinin 1987). The concentration of local anaesthetic used after surgery did not influence effect size. For laparoscopic surgery (four studies with 188 participants; Levy 2011; Neudecker 1999; Taqi 2007; Turunen 2009), the SMD would be ‐0.37 (95% CI ‐0.75 to ‐0.00; I2 = 36%; random‐effects model). Kudoh 2001 revealed (mean and SD of control group 114.5 and 28.2 hours, respectively), that 86 participants (43 per group) could eliminate a 15% difference (alpha = 0.05; beta = 0.2; two‐sided test).
If a trial at low risk of bias (Kudoh 2001) is taken as the standard (SD of control group 28.2 hours), the difference in gastrointestinal surgery would be equivalent to 22 hours.
Quality of evidence
We downgraded the quality level by one for risk of bias because 50% or more of included studies were rated as having unclear or high risk for allocation concealment and/or blinding of outcome assessors. We downgraded the level of evidence for inconsistency on the basis of a moderate amount of heterogeneity. We did not downgrade for indirectness, as we included direct comparisons on the population of interest and did not consider time to first faeces as a surrogate marker for clinical evidence of gastrointestinal transit. We did not downgrade for imprecision because the optimal information size was achieved. We found no evidence of publication bias nor of large effect size, confounding factors to justify upgrading or dose‐response effect. We rated the level of evidence as low.
Pain scores at rest and on movement at six to eight hours, 24 hours, 48 hours and 72 hours
Pain scores at rest and on movement at six to eight hours
Findings of 20 trials that included 947 participants (Aygun 2004; Barratt 2002; Beilin 2003; Bois 1997; Boylan 1998; Cai 2007; Calderon 2004; Dauri 2003; De Pietri 2006; Fant 2013; Giannoni 1999; Heurich 2007; Hong 2008; Hubler 2001; Kudoh 2001; Licker 1994; Mann 2000; Motamed 1998; Rutberg 1984; Wiedemann 1991) showed that an epidural containing a local anaesthetic infused after abdominal surgery decreases pain scores at rest at six to eight hours after surgery: SMD ‐0.84, 95% CI ‐1.08 to ‐0.61 (random‐effects model); I2 = 86%. Egger's regression intercept showed no evidence of a small‐study effect. Duval and Tweedie's trim and fill analysis provided no evidence of publication bias. Trials were subgrouped by type of surgery (Analysis 1.3). The effect was seen for gastrointestinal surgery (SMD ‐0.74, 95% CI ‐1.06 to ‐0.42), urological surgery (SMD ‐1.16, 95% CI ‐1.66 to ‐0.67) and aortic abdominal repair (SMD ‐0.63, 95% CI ‐1.00 to ‐0.26) with no statistically significant differences between those three subgroups (Q = 3.604; P value = 0.17) (mixed‐effects analysis). For a trial at low risk of bias with an average SD of 2.75 (Hong 2008), this would be equivalent to a decrease in visual/verbal analogue (VAS) score of 2.3 on a scale from 0 to 10.
Thirteen trials with 617 participants (Barratt 2002; Beilin 2003; Boylan 1998; Cai 2007; Dauri 2003; De Pietri 2006; Fant 2013; Fayed 2014; Heurich 2007; Hubler 2001; Levy 2011; Motamed 1998; Senagore 2003) reported that an epidural containing a local anaesthetic infused after abdominal surgery decreases pain scores on movement (or coughing) at six to eight hours after surgery: SMD ‐1.05, 95% CI ‐1.52 to ‐0.58; I2 = 86%. Egger's regression intercept showed the possibility of a small‐study effect (P value = 0.047; two‐sided test) as part of the heterogeneity. Duval and Tweedie's trim and fill analysis calculated that two trials might be missing to left of mean for an adjusted point of estimate: SMD ‐1.32, 95% CI ‐1.84 to ‐0.79 (random‐effects model). Trials were subgrouped by type of opioid in the control group (Analysis 1.4). An effect was seen for trials in which an epidural with a local anaesthetic was compared with morphine (SMD ‐0.93, 95% CI ‐1.28 to ‐0.59), sufentanil (SMD ‐0.95, 95% CI ‐1.78 to ‐0.12) or tramadol (SMD ‐2.19, 95% CI ‐3.18 to ‐1.20), without a statistically significant difference between morphine and sufentanil (Q = 5.711, P value = 0.06). For a trial at low risk of bias with an average SD of 2.24 (Senagore 2003), the decrease in VAS score would be equivalent to 2.4.
Pain scores at rest and on movement at 24 hours
If the local anaesthetic is continued after surgery, 46 trials that included 3085 participants (Aygun 2004; Barratt 2002; Beilin 2003; Bois 1997; Boylan 1998; Broekema 1998; Buggy 2002; Cai 2007; Carli 2002; Cronin 2001; Cullen 1985; Dauri 2003; De Pietri 2006; Donatelli 2006; Fant 2013; Giannoni 1999; Heurich 2007; Hjortsø 1985b; Hong 2008; Hu 2006; Hubler 2001; Jayr 1993; Kudoh 2001; Kumar 2004; Lattermann 2007; Licker 1994; Mann 2000; Marandola 2008; Moiniche 1993; Motamed 1998; Paulsen 2001; Peyton 2003; Rimaitis 2003; Rockemann 1997; Rutberg 1984; Scheinin 1987; Schricker 2004; Seeling 1990a; St‐Onge 1997; Taqi 2007; Thorén 1989; Wattwil 1989; Wiedemann 1991; Zeng 2003; Zhu 2013; Zutshi 2005) indicated that an epidural with a local anaesthetic decreases pain scores at rest at 24 hours: SMD ‐0.62, 95% CI ‐0.82 to ‐0.43; I2 = 82% (random‐effects model). Egger's regression intercept showed no statistically significant evidence of a small‐study effect. Duval and Tweedie's trim and fill analysis calculated that 13 trials might be missing to left of mean for an adjusted point of estimate: SMD ‐1.01, 95% CI ‐1.22 to ‐0.78 (random‐effects model). Trials were subgrouped by type of opoid included in the epidural (Analysis 1.5). An epidural with a local anaesthetic decreased VAS scores at rest at 24 hours only when morphine (SMD ‐1.32, 95% CI ‐1.87 to ‐0.78), fentanyl (SMD ‐0.55, 95% CI ‐0.77 to ‐0.33) or sufentanil (SMD ‐0.61, 95% CI ‐0.94 to ‐0.29) was added to the epidural infusion. Morphine was more effective than fentanyl (Q = 5.222; P value = 0.02) (mixed‐effects analysis). Trials were subgrouped by type of opioid in the control group (Analysis 1.6). An effect was seen when an epidural with a local anaesthetic was compared with buprenorphine (SMD ‐1.05, 95% CI ‐1.52 to ‐0.57), ketobemidone (SMD ‐1.23, 95% CI ‐1.91 to ‐0.56) or morphine (SMD ‐0.64, 95% CI ‐0.87 to ‐0.41). Investigators reported no statistically significant differences between these three subgroups (Q = 3.86; P value = 0.145) (mixed‐effects analysis). For a trial at low risk of bias (Peyton 2003; SD of the control group 2.5), the decrease in VAS score would be equivalent to 1.6.
Findings of 35 trials including 2731 participants (Barratt 2002; Beilin 2003; Boylan 1998; Broekema 1998; Buggy 2002; Cai 2007; Carli 2001; Carli 2002; Cronin 2001; Dauri 2003; De Pietri 2006; Donatelli 2006; Fant 2013; Fayed 2014; Ferguson 2009; Heurich 2007; Hu 2006; Hubler 2001; Jayr 1993; Lattermann 2007; Levy 2011; Liu 1995; Marandola 2008; Moiniche 1993; Motamed 1998; Peyton 2003; Rimaitis 2003; Rockemann 1997; Schricker 2004; Seeling 1990a; Senagore 2003; St‐Onge 1997; Taqi 2007; Turunen 2009; Zhu 2013) showed that an epidural infusion with a local anaesthetic after abdominal surgery decreases pain scores with movement at 24 hours: SMD ‐0.89, 95% CI ‐1.08 to ‐0.70; I2 = 78% (random‐effects model). Egger's regression intercept showed the possibility of a small‐study effect (P value = 0.004; two‐sided test). Duval and Tweedie's trim and fill analysis showed that six trials might be missing to left of mean for an adjusted point of estimate: SMD ‐1.07, 95% CI ‐1.29 to ‐0.87 (random‐effects model). Trials were subgrouped by type of surgery (Analysis 1.7). An epidural with a local anaesthetic decreases VAS scores on movement at 24 hours after abdominal surgery for a cholecystectomy (SMD ‐0.89, 95% CI ‐1.62 to ‐0.15), gastrointestinal surgery (SMD ‐1.12, 95% CI ‐1.43 to ‐0.80) or abdominal aortic repair (SMD ‐1.70, 95% CI ‐2.43 to ‐0.98). The effect on these three types of surgery was not different (Q = 2.75; P value = 0.25) (mixed‐effects analysis). Trials were subgrouped by type of opioid added to the epidural infusion (Analysis 1.8). An effect was seen when fentanyl (SMD ‐0.95, 95% CI ‐1.20 to ‐0.69), morphine (SMD ‐1.19, 95% CI ‐1.69 to ‐0.69) or sufentanil (SMD ‐0.77, 95% CI ‐1.14 to ‐0.41) was added to the solution (random‐effects model). Researchers reported no statistically significant differences between these three subgroups (Q = 1.22; P value = 0.54) (mixed‐effects analysis). Trials were subgrouped by type of opioid in the control group (Analysis 1.9). An effect was seen when an epidural containing a local anaesthetic was compared with morphine (SMD ‐0.87, 95% CI ‐1.05 to ‐0.69), oxycodone (SMD ‐0.80, 95% CI ‐1.33 to ‐0.26) or tramadol (SMD ‐3.14, 95% CI ‐4.31 to ‐1.97) (random‐effects model). These three subgroups differed (Q = 14.09; P value = 0.001) (mixed‐effects analysis). For laparoscopic surgery (four studies with 206 participants; Levy 2011; Senagore 2003; Taqi 2007; Turunen 2009), the SMD would be ‐0.78 (95% CI ‐1.18 to ‐0.38; I2 = 49%; random‐effects model). Peyton 2003 (mean and SD of the control group: 5.5 and 2.8, respectively on a scale from 0 to 10) required 248 participants (124 per group) to eliminate a difference of 1 in a simple trial (alpha = 0.05; beta = 0.2; two‐sided test).
For a trial at low risk of bias (Peyton 2003; SD of the control group 2.8), the decrease would be equivalent to 2.5.
Quality of evidence for VAS scores on movement at 24 hours
We downgraded level of evidence by two levels for risk of bias because 75% or more of the included studies were rated as having unclear or high risk of bias for allocation concealment and/or blinding of outcome assessors. We did not downgrade for inconsistency because we found reasonable explanations for heterogeneity. We used direct comparisons only with studies performed on the population of interest, and we did not consider pain scores to be surrogate markers for clinical pain. We did not downgrade for imprecision because the optimal information size was achieved. We did not downgrade the level of evidence for the possibility of publication bias because applying a correction for the possibility of one would not modify the conclusion. We upgraded level of evidence by one level for a large effect size (SMD > 0.8). We found no evidence of confounding factors or dose‐response effect to justify upgrading. We rated the level of evidence as moderate.
Pain scores at rest and on movement at 48 hours
If the local anaesthetic was continued for 48 hours or longer, 30 trials with 2466 participants (Barratt 2002; Beilin 2003; Boylan 1998; Broekema 1998; Cai 2007; Carli 2002; Cronin 2001; Cullen 1985; De Pietri 2006; Donatelli 2006; Fant 2013; Giannoni 1999; Heurich 2007; Hu 2006; Hubler 2001; Jayr 1993; Kudoh 2001; Kumar 2004; Lattermann 2007; Mann 2000; Paulsen 2001; Peyton 2003; Rimaitis 2003; Rockemann 1997; Schricker 2004; St‐Onge 1997; Taqi 2007; Wiedemann 1991; Zhu 2013; Zutshi 2005) reported that an epidural containing a local anaesthetic reduces pain scores at rest at 48 hours after surgery: SMD ‐0.47, 95% CI ‐0.71 to ‐0.24; I2 = 80% (random‐effects model). Egger's regression intercept showed no significant evidence of a small‐study effect. Duval and Tweedie's trim and fill analysis calculated that 10 trials might be missing to left of mean for an adjusted point of estimate to SMD ‐0.82 (95% CI ‐1.09 to ‐0.55) (random‐effects model). An epidural infusion with a local anaesthetic alone did not decrease pain scores at rest at 48 hours: SMD 0.38, 95% CI ‐0.49 to 1.25, but an epidural infusion of a local anaesthetic with an opioid did: SMD ‐0.66, 95% CI ‐0.89 to ‐0.43 (random‐effects model) (Analysis 1.10). A meta‐regression of the mean age of participants included in the trial showed that older participants benefit more from an epidural containing a local anaesthetic at 48 hours after abdominal surgery (P value = 0.0002) (Figure 5). The route of administration of the opioid in the control group also influenced the effect size. An epidural containing a local anaesthetic did not improve pain scores at rest at 48 hours when compared with an epidural without a local anaesthetic: SMD 0.24, 95% CI ‐0.22 to 0.70, but it did so when compared with IV (SMD ‐0.74, 95% CI ‐1.04 to ‐0.44; IM (SMD ‐0.40, 95% CI ‐0.70 to ‐0.10) and subcutaneous routes (SMD ‐0.65, 95% CI ‐0.98 to ‐0.33) (Q value for the difference between these four subgroups = 32.46; P value < 0.001) (mixed‐effects analysis). For a trial with low risk of bias (SD 2.1; Peyton 2003), the decrease would be equivalent to 1.0.

Meta‐regression of the effects of mean age of participants included in the study on VAS scores at rest at 48 hours (P value = 0.0002). Older participants benefit more from an epidural containing a local anaesthetic for an abdominal surgery.
Findings of 27 trials that included 2398 participants (Barratt 2002; Beilin 2003; Boylan 1998; Broekema 1998; Cai 2007; Carli 2001; Carli 2002; Cronin 2001; De Pietri 2006; Donatelli 2006; Fant 2013; Fayed 2014; Ferguson 2009; Heurich 2007; Hu 2006; Hubler 2001; Jayr 1993; Lattermann 2007; Liu 1995; Peyton 2003; Rimaitis 2003; Rockemann 1997; Schricker 2004; St‐Onge 1997; Taqi 2007; Turunen 2009; Zhu 2013) showed that an epidural containing a local anaesthetic decreased pain scores on movement at 48 hours: SMD ‐0.85, 95% CI ‐1.10 to ‐0.60; I2 = 85%. Egger's regression intercept showed that a small‐study effect might be present (P value = 0.002; two‐sided test). Duval and Tweedie's trim and fill analysis calculated that four trials might be missing left of mean for an adjusted point of estimate: SMD ‐1.06, 95% CI ‐1.34 to ‐0.77 (random‐effects model). An effect was not seen (SMD ‐0.56, 95% CI ‐1.71 to 0.58) when a local anaesthetic alone was used, but an epidural containing both a local anaesthetic and an opioid decreased pain scores on movement at 48 hours: SMD ‐0.88, 95% CI ‐1.13 to ‐0.63 (random‐effects model) (Analysis 1.11). A meta‐regression of the mean age of participants included in the trial showed that older participants benefit more from an epidural containing a local anaesthetic at 48 hours after abdominal surgery (P value = 0.002) (Figure 6). An epidural containing a local anaesthetic did not improve pain scores on movement at 48 hours when compared with an epidural without a local anaesthetic: SMD ‐0.17, 95% CI ‐0.88 to 0.54. An epidural containing a local anaesthetic improved pain scores when it was compared with pain treatment administered by intravenous (SMD ‐1.04, 95% CI ‐1.39 to ‐0.68), intramuscular (SMD ‐0.76, 95% CI ‐088 to ‐0.54) and subcutaneous (SMD ‐0.53, 95% CI ‐0.85 to ‐0.21) routes, or by a single intrathecal injection (SMD ‐3.03, 95% CI ‐3.85 to ‐2.22) (Q value for the difference between these four subgroups = 32.92; P value < 0.001) (mixed‐effects analysis). For a trial at low risk of bias with the typical SD of 2.6 (Peyton 2003), the decrease would be equivalent to 2.2.

Meta‐regression of the effects of mean age of participants included in the study on VAS scores on movement at 48 hours (P value = 0.002). Older participants benefit more from an epidural containing a local anaesthetic for an abdominal surgery.
Pain scores at rest and on movement at 72 hours
Fifteen trials that included 1821 participants (Beilin 2003; Broekema 1998; Cai 2007; Carli 2002; Cronin 2001; Cullen 1985; Giannoni 1999; Hubler 2001; Jayr 1993; Kudoh 2001; Mann 2000; Paulsen 2001; Peyton 2003; Rimaitis 2003; Rockemann 1997) showed that an epidural with a local anaesthetic decreases VAS scores at rest at 72 hours: SMD ‐0.56, 95% CI ‐0.88 to ‐0.24; I2 = 90% (random‐effects model) (Analysis 1.12). Egger's regression intercept showed that a small‐study effect might be present (P value = 0.04). Duval and Tweedie's trim and fill analysis calculated that six trials might be missing to left of mean for an adjusted point of estimate of ‐0.99 (95% CI ‐1.45 to ‐0.53) (random‐effects model). We found no effect with a local anaesthetic alone on pain scores at rest at 72 hours (SMD 0.00, 95% CI ‐0.34 to 0.34), but an epidural containing both a local anaesthetic and an opioid decreased pain scores at rest at 72 hours (SMD ‐0.77, 95% CI ‐1.16 to ‐0.39) (random‐effects model). For a trial with low risk of bias (SD 1.7; Peyton 2003), the decrease would be equivalent to 1.0.
Findings of 15 trials with 1873 participants (Beilin 2003; Broekema 1998; Cai 2007; Carli 2001; Carli 2002; Cronin 2001; Fayed 2014; Ferguson 2009; Hubler 2001; Jayr 1993; Liu 1995; Peyton 2003; Rimaitis 2003; Rockemann 1997; Turunen 2009) showed that an epidural with a local anaesthetic decreased pain scores on movement at 72 hours: SMD ‐0.69, 95% CI ‐0.99 to ‐0.39; I2 = 86% (random‐effects model) (Analysis 1.13). Egger's regression intercept showed no significant evidence of a small‐study effect. Duval and Tweedie's trim and fill analysis calculated that six trials might be missing left of mean for an adjusted point of estimate: SMD ‐1.14, 95% CI ‐1.56 to ‐0.72 (random‐effects model). We found no effect when a local anaesthetic alone was infused through the epidural catheter (SMD ‐0.03, 95% CI ‐0.38 to 0.32), but an epidural infusion containing both a local anaesthetic and an opioid decreased pain scores on movement at 72 hours (SMD ‐0.87, 95% CI ‐1.22 to ‐0.51) (random‐effects model). For a trial at low risk of bias (SD 2.5; Peyton 2003), the decrease would be equivalent to 1.7.
Incidence of postoperative vomiting: number of participants who experienced vomiting on day one
From a total of 22 trials with 1154 participants (Aceto 2002; Barzoi 2000; Benzon 1994; Calderon 2004; Carli 2001; Cullen 1985; De Pietri 2006; Erol 2008; Gambling 2009; George 1994; Hong 2008; Jayr 1998; Luchetti 1996; Marandola 2008; Neudecker 1999; Salomaki 1995; Siniscalchi 2003; Steinberg 2002; Taqi 2007; Thorén 1989; Tsui 1997; Wattwil 1989), we did not find a difference in the number of participants who will experience vomiting during the first 24 hours after abdominal surgery performed under general anaesthesia: risk ratio (RR) 0.84, 95% CI 0.57 to 1.23; I2 = 21% (Analysis 1.14). Egger's regression intercept showed no statistically significant small‐study effect. Duval and Tweedie's trim and fill analysis showed that five studies might be missing to right of mean for an adjusted point of estimate: RR 1.05, 95% CI 0.68 to 1.60 (random‐effects model). For laparoscopic surgery (four studies with 160 participants; Hong 2008; Luchetti 1996; Neudecker 1999; Taqi 2007), RR would be 0.50 (95% CI 0.18 to 1.38; I2 = 39% (random‐effects model). For gynaecological surgery, three studies used a high lidocaine equivalent concentration (10 mg/mL; Hong 2008; Thorén 1989; Wattwil 1989) and one study used a low lidocaine equivalent concentration (2.5 mg/mL; Tsui 1997). The incidence was reduced when a high lidocaine equivalent concentration was used (three trials with 112 participants: RR 0.13, 95% CI 0.03 to 0.52; I2 = 0%). For this subgroup, Tweedie's trim and fill analysis showed that two studies might be missing to left of mean for an adjusted point of estimate: RR 0.12, 95% CI 0.04 to 0.37. If an incidence of 32% is assumed, the NNTB for an epidural containing a lidocaine equivalent of 10 mg/mL would be 4 (95% CI 4 to 7).
With a basal rate of 17% in the trial population (summary of findings Table for the main comparison), 1732 participants (866 per group) were required, to eliminate a decrease of 25% in the incidence of vomiting (alpha = 0.05, beta = 0.2; one‐sided test) (http://www.stat.ubc.ca/˜rollin/stats/ssize/b2.html).
Quality of evidence for postoperative vomiting
We downgraded level of evidence by one level for risk of bias because 50% or more of the included studies were rated as having unclear or high risk of bias for allocation concealment and/or blinding of outcome assessors. Heterogeneity was lower than 25%. We included direct comparisons of the population of interest, and we did not consider vomiting as a surrogate marker for food tolerance. We downgraded level of evidence by one level for imprecision because the number of participants included was below the optimal information size. Correcting for publication bias would not modify the conclusion. We found no evidence of a large effect size and identified no confounding factors to justify upgrading. We noted no dose‐response effect when all studies were included. We rated the quality of evidence as low.
Gastrointestinal tract anastomotic leak
From findings of 17 trials with 848 participants (Ahn 1988; Barratt 2002; Brodner 2001; Carli 2002; Giannoni 1999; Levy 2011; Liu 1995; Mallinder 2000; Mann 2000; Paulsen 2001; Rimaitis 2003; Riwar 1991; Scheinin 1987; Schulze 1992; Turunen 2009; Tyagi 2011; Zhu 2013), we did not find a difference in the incidence of anastomotic leak: RR 0.74, 95% CI 0.41 to 1.32; I2 = 0% (Analysis 1.15). Egger's regression intercept showed no significant small‐study effect. Duval and Tweedie's trim and fill analysis calculated that one study might be missing to right of mean for an adjusted point of estimate: RR 0.79, 95% CI 0.40 to 1.55. For laparoscopic surgery (two studies with 118 participants; Levy 2011; Turunen 2009), RR would be 2.47 (95% CI 0.34 to 18.12; I2 = 0%).
With a basal rate of 6% in the study population (summary of findings Table for the main comparison), 5466 participants (2733 per group) were required, to eliminate a decrease of 25% in the incidence of anastomotic leak (alpha = 0.05, beta = 0.2; one‐sided test) in a simple trial (http://www.stat.ubc.ca/˜rollin/stats/ssize/b2.html).
Quality of evidence
We downgraded level of evidence for risk of bias by one level because 50% or more of the included studies were rated as having unclear or high risk of bias for allocation concealment and/or blinding of outcome assessors. We observed no heterogeneity. We included direct comparisons of the population of interest and this is not a surrogate marker. We downgraded level of evidence by one level for imprecision because the number of participants included was below the optimal information size. We found no evidence of publication bias nor of a large effect, confounding factors to justify upgrading or dose‐response effect. We rated the level of evidence as low.
Length of hospital stay
Investigators in 34 trials with 2774 participants (Aceto 2002; Bois 1997; Carli 2001; Carli 2002; Dauri 2003; Davies 1993; Giannoni 1999; Hadimioglu 2012; Jayr 1993; Levy 2011; Liu 1995; Lombardo 2009; Mallinder 2000; Martella 2012; Miller 1976; Mondor 2010; Muehling 2009; Neudecker 1999; Norman 1997; Ozturk 2010; Paulsen 2001; Peyton 2003; Pflug 1974; Rimaitis 2003; Rockemann 1997; Seeling 1990; Senagore 2003; Stevens 1998; Thorén 1989; Turunen 2009; Tyagi 2011; Wattwil 1989; Zhu 2013; Zutshi 2005) reported that an epidural with a local anaesthetic does not reduce length of hospital stay: SMD ‐0.13, 95% CI ‐0.29 to 0.02; I2 = 69% (random‐effects model) (Analysis 1.16). Egger's regression intercept showed no statistically significant evidence of a small‐study effect. Duval and Tweedie's trim and fill analysis calculated that nine trials might be missing to right of mean for an adjusted point of estimate: SMD 0.05, 95% CI ‐0.11 to 0.21. Epidural analgesia reduced length of hospital stay for open surgery (SMD ‐0.20, 95% CI ‐0.35 to ‐0.04; I2 = 68%; 30 studies with 2598 participants (Analysis 1.17) but not for laparoscopic surgery (four studies with 176 participants; Levy 2011; Neudecker 1999; Senagore 2003; Turunen 2009): SMD 0.38, 95% CI ‐0.06 to 0.82; I2 = 49%; random‐effects model). For open surgery, a small‐study effect might occur (P value = 0.03; two‐sided test for Egger's regression intercept). and Duval and Tweedie's trim and fill analysis showed that nine studies might be missing to right of mean for an adjusted point of estimate: SMD 0.00, 95% CI ‐0.16 to 0.16; random‐effects model. Carli 2002 (SD in the control group 5 days) indicated that the reduction for an open surgery would be equivalent to one day. In the same study, 786 participants (393 per group) would be required for a simple trial to eliminate a difference of one day (from eight to seven days) (alpha = 0.05; beta = 0.2; two‐sided test).
Quality of evidence for length of hospital stay for open surgery
We downgraded level of evidence by two levels for risk of bias because 75% or more of the studies included for this outcome were rated as having unclear or high risk of bias for allocation concealment and/or blinding of outcome assessors. We downgraded level of evidence for inconsistency on the basis of a moderate amount of heterogeneity. We included direct comparisons of the population of interest and did not consider length of hospital stay as a surrogate marker. The optimal information size was achieved. We downgraded for publication bias because correcting for the possibility of one would modify the conclusion (absence of effect). We found no evidence of a large effect size nor of a dose‐response effect. We upgraded level of evidence by one level for possible confounding factors, as hospital discharge may not actually reflect readiness for discharge. Participants must be ready to be discharged, but not all participants ready for discharge are discharged at that specific time. Delays may happen for various reasons (e.g. number of days in hospital fixed for each type of surgery at certain centres, no one available to sign for participant discharge, lack of help/assistance with assisted care at home). We found no evidence of a dose‐response effect. We rated the quality of evidence as very low.
Hospital costs
Data for cost were available for two small trials only (Paulsen 2001; Welch 1998) (Analysis 1.18). One trial provided costs related to pain therapy only (Rockemann 1997). These three trials studied participants undergoing open abdominal surgery.
Discussion
An epidural containing a local anaesthetic will decrease the time required for return of gastrointestinal transit, as measured by the time required to observe first flatus after abdominal surgery (Analysis 1.1) (high quality of evidence; equivalent to 17.5 hours; summary of findings Table for the main comparison). The effect is seen for almost every type of abdominal surgery and is proportionate to the concentration of local anaesthetic administered after surgery (Figure 4). The optimal concentration required to obtain the maximal effect without impeding ambulation by producing motor blockade of the lower limbs may need to be determined in future trials/reviews. Adding an opioid to the mixture does not reduce the benefit. The exact duration of administration required may also need to be determined in future trials/reviews. The effect of an epidural with a local anaesthetic on time between surgery and first faeces was not as clear (quality of evidence low; equivalent to 22 hours; summary of findings Table for the main comparison). Administration of the local anaesthetic only during the intraoperative period might not be sufficient to produce an effect. A reduced incidence of vomiting was seen only at relatively high local anaesthetic concentrations and for gynaecological surgeries. Return of gastrointestinal transit is an important goal to achieve after abdominal surgery, as it is generally considered to be required before hospital discharge, and because postoperative ileus will increase hospital costs (Gan 2015). Return of gastrointestinal transit as measured by time to first flatus or bowel movement is often chosen as an outcome measure in clinical trials evaluating recovery after surgery (Neville 2014). However, some study authors consider that passage of flatus or stool may reflect rectal emptying rather than effective gastrointestinal motility. A combination of food tolerance plus defecation has recently been reported as having a good positive predictive value to identify recovery of gastrointestinal transit compared with scintigraphic assessment (van Bree 2014). In the present review, positive effects on gastrointestinal transit translated to an interesting shortening of hospital stay for participants undergoing open surgery (very low quality of evidence). Data on cost were too limited to allow us to make any comment on them.
In 2000, the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) suggested that pain should be considered as the fifth vital sign, and that under‐treatment of pain should constitute abrogation of a fundamental human right (White 2007). After this statement was issued, an increase in the use of opioids for treatment of patients with acute postoperative pain was observed, as was an increase in opioid side effects (White 2007). Regional blockade interrupts pain transmission to the brain and may be used during the surgery itself as a replacement for general anaesthesia (regional anaesthesia), or for treatment of postoperative pain (regional analgesia). In adults, regional analgesic techniques decrease postoperative opioid consumption (Guay 2006), making them a potentially interesting alternative or adjunct to opioid‐based regimens for treatment of postoperative pain, provided of course that the efficacy would be at least equivalent. An epidural with a local anaesthetic has a clinically relevant effect on pain scores (moderate quality of evidence for pain on movement at 24 hours after surgery; summary of findings Table for the main comparison). Not every patient will benefit to the same extent from an epidural with a local anaesthetic. Many factors will increase or decrease the beneficial effects. For patients themselves, the older the patient, the greater the difference in pain scores between an epidural with a local anaesthetic and an opioid‐based regimen (Figure 5; Figure 6). For type of surgery, patients undergoing abdominal aortic repair, gastrointestinal surgery or urological surgery seem to be the ones for which an epidural containing a local anaesthetic would be most beneficial. The type of solution used also matters. Administering only a local anaesthetic through the epidural catheter will not produce a decrease in pain scores, at least from 24 hours and after, and this applies to pain scores both at rest and on movement. Fortunately, as mentioned above, adding an opioid to the mixture does not seem to decrease the benefit of the local anaesthetic for acceleration of gastrointestinal transit return. For the opioid added to the local anaesthetic, morphine, fentanyl and sufentanil seem to be equally effective for pain on movement. When an opioid is administered in the epidural space, blood concentrations achieved will vary according to the type of opioid used. A systemic effect of the opioid is to be expected. The type of opioid used in the control group as well as the route of administration may increase or decrease the difference in pain scores between an epidural containing a local anaesthetic and an opioid‐based regimen. Pain reduction was noted regardless of the type of approach used (open vs laparoscopic). Thus the decision to use epidural analgesia after abdominal surgery versus other modalities has to be considered on a case‐by‐case basis, taking into account patient age, associated co‐morbidities, relative contraindications and type of surgery performed.
When evaluating the benefit of an intervention, one must also consider potential side effects. Because they may increase the risk of hypotension (Davies 2006), some clinicians have hypothesized that secondary extra fluid administration could increase sutures, oedema and anastomotic dehiscence. We did not find a difference in the incidence of anastomotic leak (low quality of evidence). Severe complications such as paraplegia or death related to epidurals when used for perioperative pain treatment in adults are fortunately very rare (1.0 to 6.1 per 100,000 procedures; Cook 2009) and are best evaluated by large prospective trials, as they are rarely reported in randomized controlled trials using neuraxial blocks (Guay 2014).
Summary of main results
An epidural with a local anaesthetic will accelerate the return of gastrointestinal transit by approximately 17 hours. The effect is proportional to the local anaesthetic concentration. To be effective, the solution may need to be administered after surgery ‐ not solely intraoperatively. The effect of an epidural containing a local anaesthetic on gastrointestinal transit will translate to shorter hospital stay for open surgery only. An epidural with a local anaesthetic and an opioid improves pain scores (open or laparoscopic surgeries). Adding an opioid to the solution of local anaesthetic will improve pain scores without affecting gastrointestinal transit. When all studies were included, we did not find an effect on the incidence of vomiting or evidence to support any effect of an epidural with a local anaesthetic on the incidence of anastomotic leak of the gastrointestinal tractus.
Overall completeness and applicability of evidence
Given the high number of participants and studies included in this review, we are confident that our conclusions are valid.
Quality of the evidence
We rated the quality of evidence as high for reduced time to first flatus, and as moderate for pain scores on movement at 24 hours. We rated the quality of evidence as low for absence of effect on vomiting, for reduced time to first faeces and for absence of effect on the incidence of anastomotic leaks. We rated the quality as very low for reduced length of hospital stay for open surgery.
Potential biases in the review process
Our search was quite extensive, and we applied no language restrictions. We are therefore confident that our review reflects actual available information on this topic. We reran the search in February 2016 and found six studies with our selected outcomes. We added five studies to a list of Characteristics of studies awaiting classification (Chen 2015; Khoronenko 2014; Satsuta 2015; Sidiropoulou 2014; Xu 2014) and will incorporate them into formal review findings during the next review update. We added one study under Characteristics of ongoing studies (Li 2015a).
Agreements and disagreements with other studies or reviews
We agree with the previous version of this review (Jorgensen 2001) that an epidural with a local anaesthetic decreases time to return of gastrointestinal tract transit, as measured by time to first flatus after surgery. We found that heterogeneity observed in the previous version can be explained by the variety of local anaesthetic concentrations used in the included studies. We agree that an epidural that would contain only local anaesthetic offers no clear advantage over other modalities of treatment in terms of pain reduction. The epidural solution must contain a mixture of local anaesthetic and opioids to be more effective than other modalities of pain treatment after abdominal surgery.

Flow diagram. Study selection from the 2014 search. We reran the search in February 2016, and added 16 potential new studies of interest to the list of ‘Studies awaiting classification'. These studies will be incorporated into the formal review findings during the next review update. We also found one ongoing trial.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Meta‐regression of effects of the concentration of local anaesthetic used (mg/mL) after surgery on the standardized mean difference for return of gastrointestinal transit as measured by the time required to obtain first flatus. P value = 0.0008.

Meta‐regression of the effects of mean age of participants included in the study on VAS scores at rest at 48 hours (P value = 0.0002). Older participants benefit more from an epidural containing a local anaesthetic for an abdominal surgery.

Meta‐regression of the effects of mean age of participants included in the study on VAS scores on movement at 48 hours (P value = 0.002). Older participants benefit more from an epidural containing a local anaesthetic for an abdominal surgery.
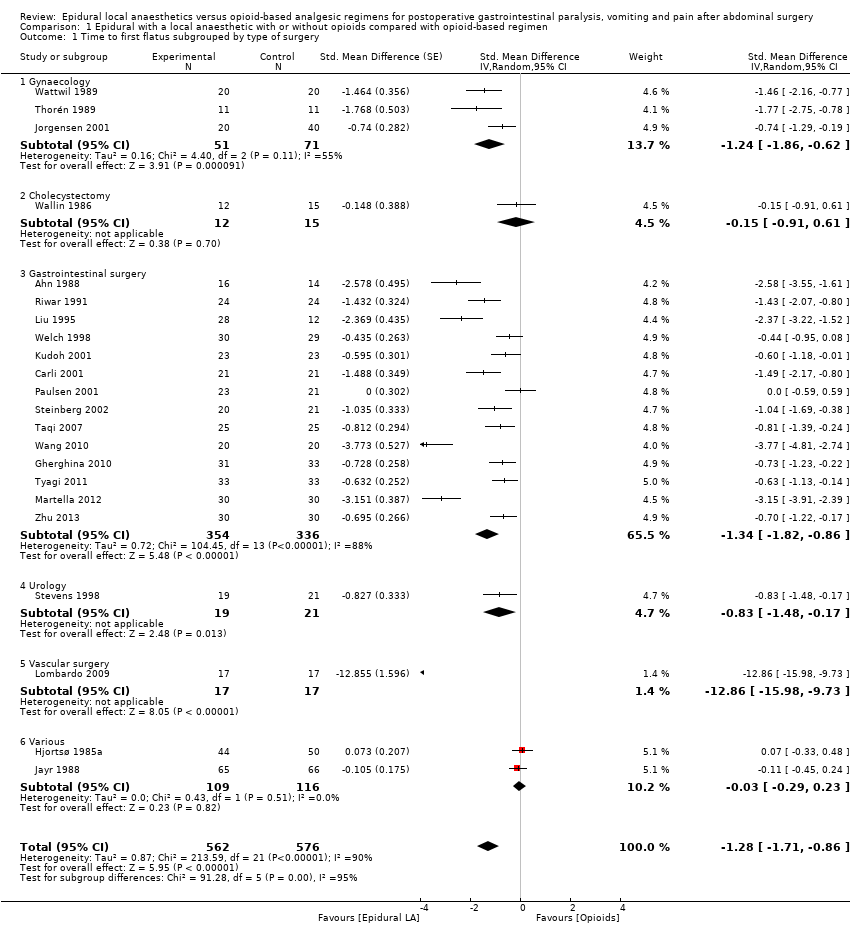
Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 1 Time to first flatus subgrouped by type of surgery.
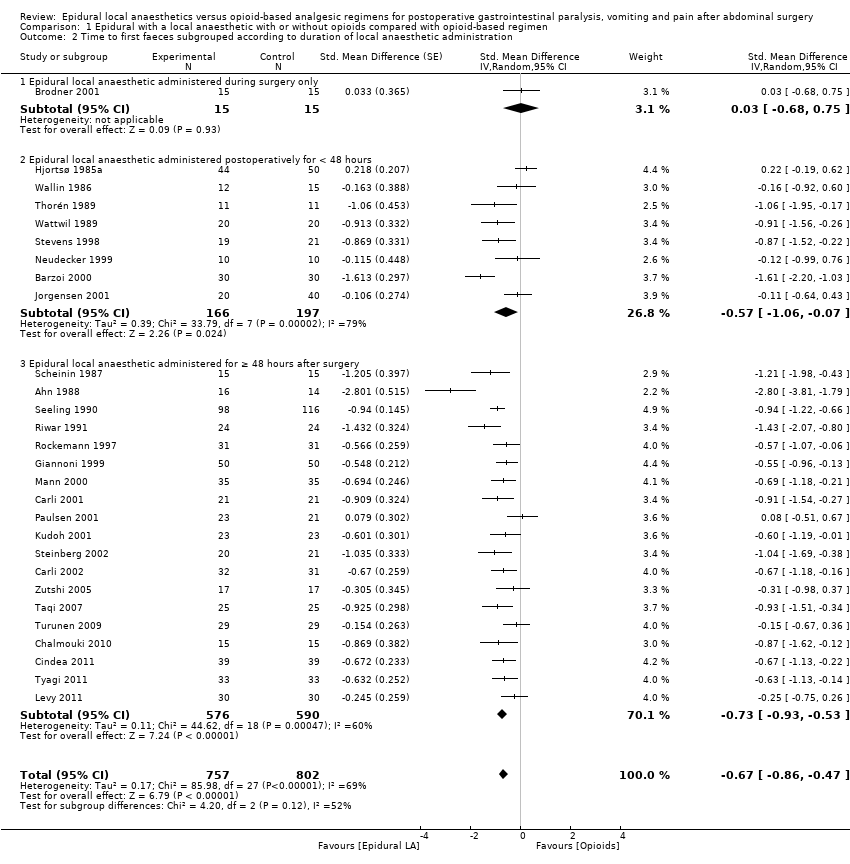
Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 2 Time to first faeces subgrouped according to duration of local anaesthetic administration.

Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 3 Pain scores at rest at 6 to 8 hours after surgery subgrouped by type of surgery.

Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 4 Pain scores on movement at 6 to 8 hours after surgery subgrouped by type of opioid in the control group.
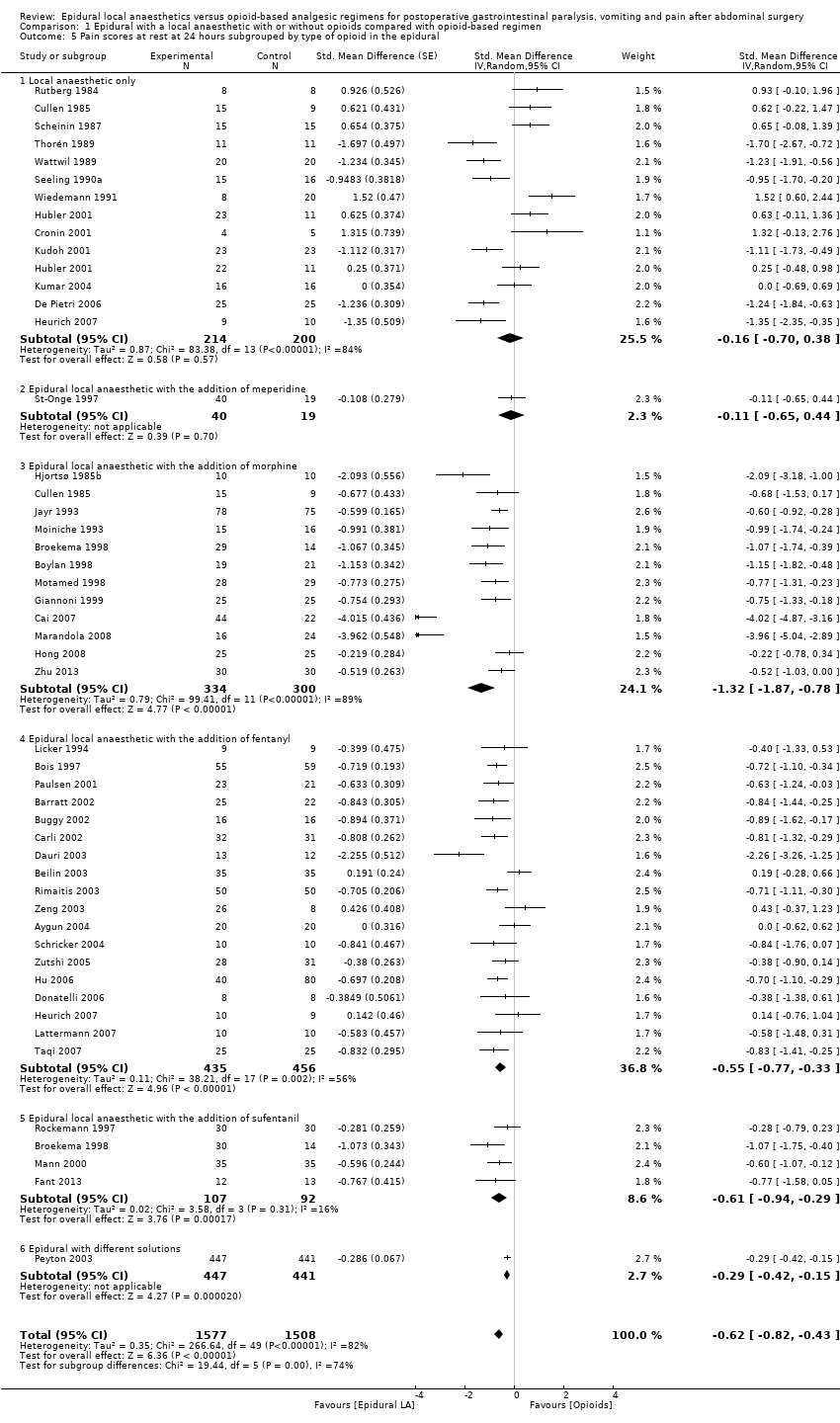
Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 5 Pain scores at rest at 24 hours subgrouped by type of opioid in the epidural.

Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 6 Pain scores at rest at 24 hours subgrouped by opioid in the control group.

Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 7 Pain scores on movement at 24 hours subgrouped by type of surgery.

Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 8 Pain scores on movement at 24 hours subgrouped by type of opioid in the epidural.

Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 9 Pain scores on movement at 24 hours subgrouped by type of opioids in the control group.
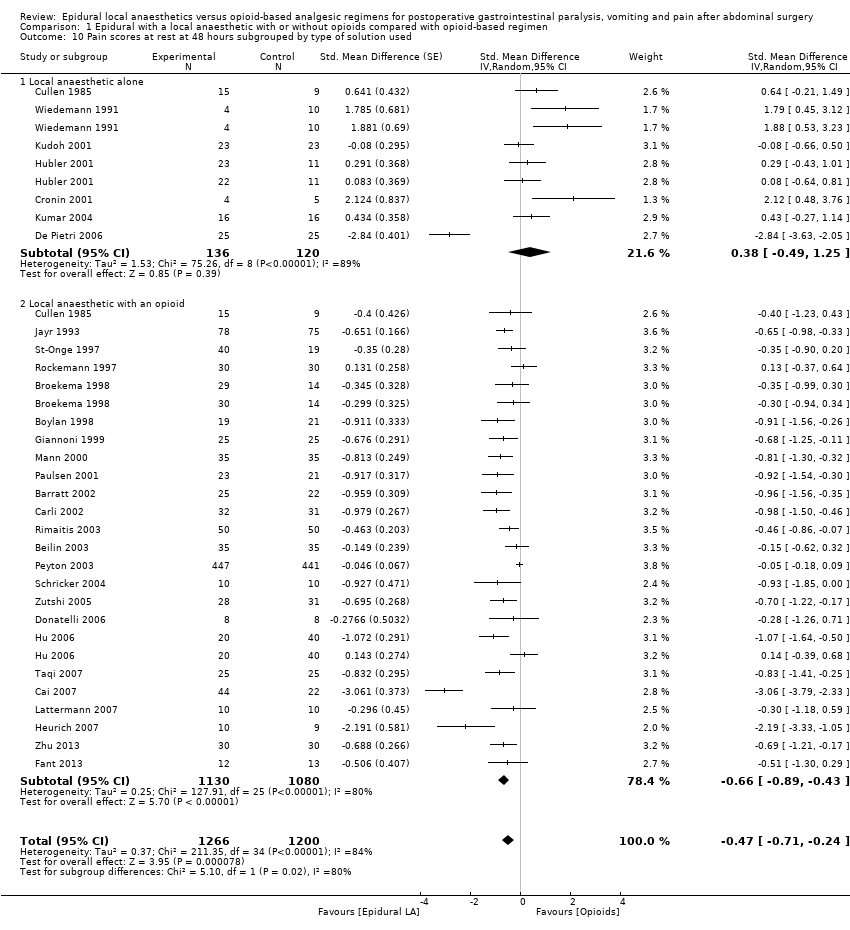
Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 10 Pain scores at rest at 48 hours subgrouped by type of solution used.

Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 11 Pain scores on movement at 48 hours subgrouped by type of solution in the epidural.

Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 12 Pain scores at rest at 72 hours subgrouped by type of solution used.

Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 13 Pain scores on movement at 72 hours subgrouped by type of solution used.
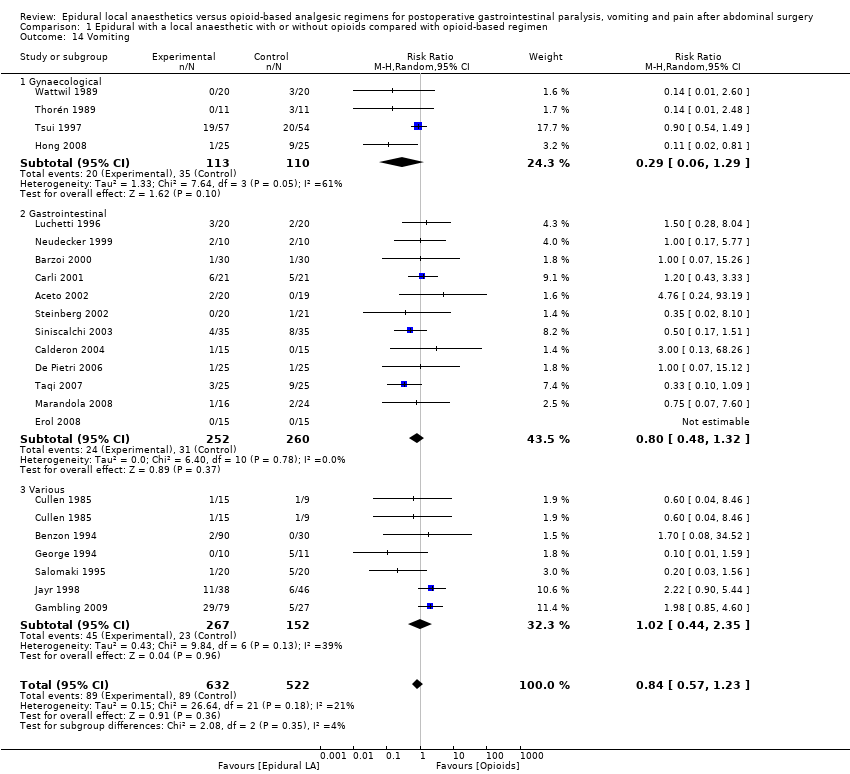
Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 14 Vomiting.

Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 15 Gastrointestinal tract anastomotic leak.
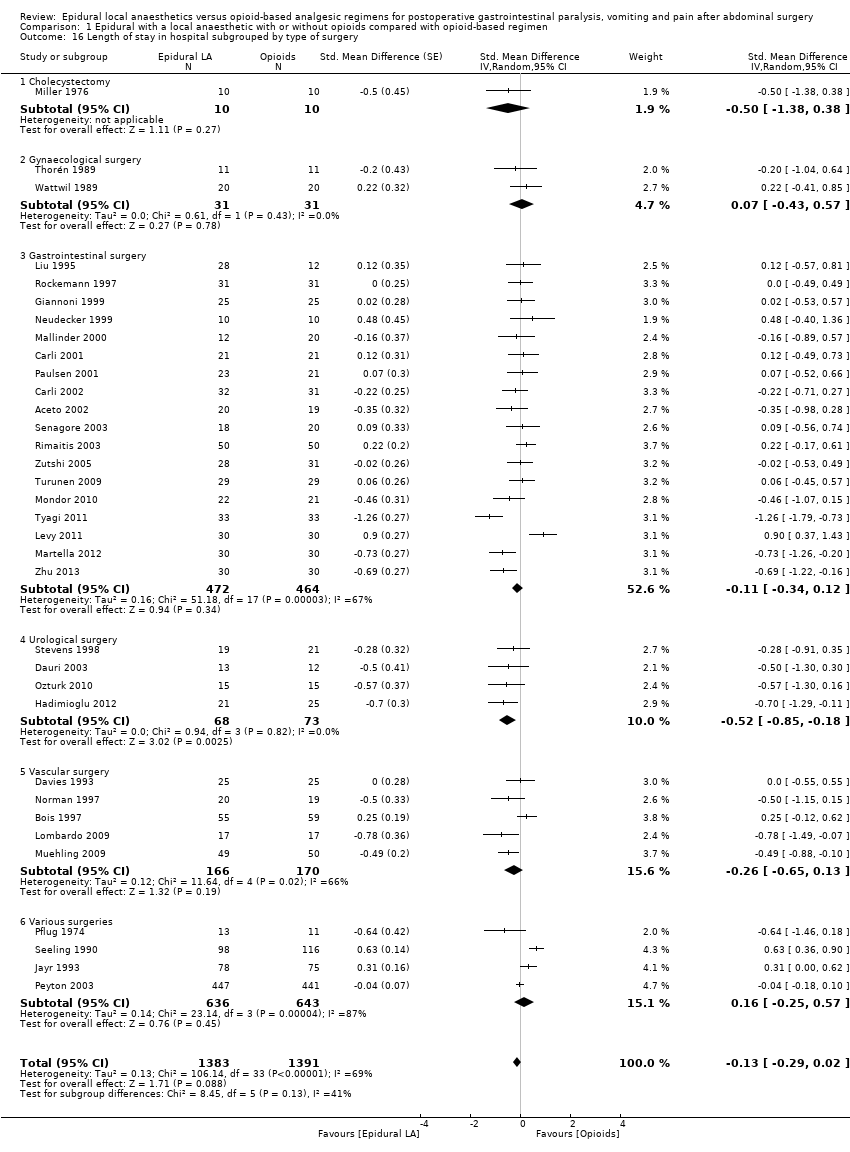
Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 16 Length of stay in hospital subgrouped by type of surgery.

Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 17 Length of stay in hospital subgrouped by surgical site for open surgery only.

Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 18 Costs.
| Epidural local anaesthetic compared with opioid‐based regimen for adults | ||||||
| Patient or population: adults | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Opioid‐based regimen | Epidural local anaesthetic | |||||
| Time required to observe first flatus | Mean time required to observe first flatus in the intervention groups was | 1138 | ⊕⊕⊕⊕ | Effect size was proportionate to the | ||
| Time required to observe first faeces | Mean time required to observe first faeces in the intervention groups was | 1559 | ⊕⊕⊝⊝ | Pooled reduction is | ||
| VAS scores on movement at 24 hours | Mean VAS scores on movement at 24 hours in the intervention groups was | 2731 | ⊕⊕⊕⊝ | Pooled reduction | ||
| Vomiting during first 24 hours | Study population | RR 0.84 | 1154 | ⊕⊕⊝⊝ | ||
| 170 per 1000 | 143 per 1000 | |||||
| Low | ||||||
| 50 per 1000 | 42 per 1000 | |||||
| High | ||||||
| 250 per 1000 | 210 per 1000 | |||||
| Anastomotic leak | Study population | RR 0.74 | 848 | ⊕⊕⊝⊝ | ||
| 53 per 1000 | 39 per 1000 | |||||
| Low | ||||||
| 30 per 1000 | 22 per 1000 | |||||
| High | ||||||
| 100 per 1000 | 74 per 1000 | |||||
| Length of hospital stay | Mean length of hospital stay in the intervention groups was | 2598 | ⊕⊝⊝⊝ | Pooled reduction is | ||
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aAllocation concealment and/or blinding of outcome assessors rated as unclear or high risk for 75% or more of included studies for this outcome | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to first flatus subgrouped by type of surgery Show forest plot | 22 | 1138 | Std. Mean Difference (Random, 95% CI) | ‐1.28 [‐1.71, ‐0.86] |
| 1.1 Gynaecology | 3 | 122 | Std. Mean Difference (Random, 95% CI) | ‐1.24 [‐1.86, ‐0.62] |
| 1.2 Cholecystectomy | 1 | 27 | Std. Mean Difference (Random, 95% CI) | ‐0.15 [‐0.91, 0.61] |
| 1.3 Gastrointestinal surgery | 14 | 690 | Std. Mean Difference (Random, 95% CI) | ‐1.34 [‐1.82, ‐0.86] |
| 1.4 Urology | 1 | 40 | Std. Mean Difference (Random, 95% CI) | ‐0.83 [‐1.48, ‐0.17] |
| 1.5 Vascular surgery | 1 | 34 | Std. Mean Difference (Random, 95% CI) | ‐12.86 [‐15.98, ‐9.73] |
| 1.6 Various | 2 | 225 | Std. Mean Difference (Random, 95% CI) | ‐0.03 [‐0.29, 0.23] |
| 2 Time to first faeces subgrouped according to duration of local anaesthetic administration Show forest plot | 28 | 1559 | Std. Mean Difference (Random, 95% CI) | ‐0.67 [‐0.86, ‐0.47] |
| 2.1 Epidural local anaesthetic administered during surgery only | 1 | 30 | Std. Mean Difference (Random, 95% CI) | 0.03 [‐0.68, 0.75] |
| 2.2 Epidural local anaesthetic administered postoperatively for < 48 hours | 8 | 363 | Std. Mean Difference (Random, 95% CI) | ‐0.57 [‐1.06, ‐0.07] |
| 2.3 Epidural local anaesthetic administered for ≥ 48 hours after surgery | 19 | 1166 | Std. Mean Difference (Random, 95% CI) | ‐0.73 [‐0.93, ‐0.53] |
| 3 Pain scores at rest at 6 to 8 hours after surgery subgrouped by type of surgery Show forest plot | 20 | 947 | Std. Mean Difference (Random, 95% CI) | ‐0.84 [‐1.08, ‐0.61] |
| 3.1 Cholecystectomy | 1 | 16 | Std. Mean Difference (Random, 95% CI) | 0.33 [‐0.65, 1.32] |
| 3.2 Gastrointestinal surgery | 8 | 387 | Std. Mean Difference (Random, 95% CI) | ‐0.74 [‐1.06, ‐0.42] |
| 3.3 Gynaecology | 2 | 68 | Std. Mean Difference (Random, 95% CI) | ‐0.76 [‐1.70, 0.18] |
| 3.4 Urology | 4 | 136 | Std. Mean Difference (Random, 95% CI) | ‐1.16 [‐1.66, ‐0.67] |
| 3.5 Vascular surgery | 2 | 154 | Std. Mean Difference (Random, 95% CI) | ‐0.63 [‐1.00, ‐0.26] |
| 3.6 Various | 4 | 186 | Std. Mean Difference (Random, 95% CI) | ‐1.24 [‐2.18, ‐0.29] |
| 4 Pain scores on movement at 6 to 8 hours after surgery subgrouped by type of opioid in the control group Show forest plot | 13 | 617 | Std. Mean Difference (Random, 95% CI) | ‐1.05 [‐1.52, ‐0.58] |
| 4.1 Epidural LA compared with IV or epidural fentanyl | 3 | 119 | Std. Mean Difference (Random, 95% CI) | ‐1.15 [‐3.91, 1.61] |
| 4.2 Epidural LA compared with IT (De Pietri 2006) or IV (all others) morphine | 8 | 387 | Std. Mean Difference (Random, 95% CI) | ‐0.93 [‐1.28, ‐0.59] |
| 4.3 Epidural LA with opioids compared with IV piritramide | 1 | 19 | Std. Mean Difference (Random, 95% CI) | ‐0.62 [‐1.54, 0.30] |
| 4.4 Epidural LA alone compared with epidural sufentanil | 1 | 67 | Std. Mean Difference (Random, 95% CI) | ‐0.95 [‐1.78, ‐0.12] |
| 4.5 Epidural LA with opioids compared with IV tramadol | 1 | 25 | Std. Mean Difference (Random, 95% CI) | ‐2.19 [‐3.18, ‐1.20] |
| 5 Pain scores at rest at 24 hours subgrouped by type of opioid in the epidural Show forest plot | 46 | 3085 | Std. Mean Difference (Random, 95% CI) | ‐0.62 [‐0.82, ‐0.43] |
| 5.1 Local anaesthetic only | 13 | 414 | Std. Mean Difference (Random, 95% CI) | ‐0.16 [‐0.70, 0.38] |
| 5.2 Epidural local anaesthetic with the addition of meperidine | 1 | 59 | Std. Mean Difference (Random, 95% CI) | ‐0.11 [‐0.65, 0.44] |
| 5.3 Epidural local anaesthetic with the addition of morphine | 12 | 634 | Std. Mean Difference (Random, 95% CI) | ‐1.32 [‐1.87, ‐0.78] |
| 5.4 Epidural local anaesthetic with the addition of fentanyl | 18 | 891 | Std. Mean Difference (Random, 95% CI) | ‐0.55 [‐0.77, ‐0.33] |
| 5.5 Epidural local anaesthetic with the addition of sufentanil | 4 | 199 | Std. Mean Difference (Random, 95% CI) | ‐0.61 [‐0.94, ‐0.29] |
| 5.6 Epidural with different solutions | 1 | 888 | Std. Mean Difference (Random, 95% CI) | ‐0.29 [‐0.42, ‐0.15] |
| 6 Pain scores at rest at 24 hours subgrouped by opioid in the control group Show forest plot | 42 | 2066 | Std. Mean Difference (Random, 95% CI) | ‐0.69 [‐0.91, ‐0.47] |
| 6.1 Epidural local anaesthetic compared with fentanyl | 4 | 144 | Std. Mean Difference (Random, 95% CI) | ‐1.11 [‐3.18, 0.96] |
| 6.2 Epidural local anaesthetic compared with ketobemidone | 1 | 40 | Std. Mean Difference (Random, 95% CI) | ‐1.23 [‐1.91, ‐0.56] |
| 6.3 Epidural local anaesthetic compared with piritramide | 1 | 19 | Std. Mean Difference (Random, 95% CI) | 0.14 [‐0.76, 1.04] |
| 6.4 Epidural local anaesthetic compared with meperidine | 3 | 219 | Std. Mean Difference (Random, 95% CI) | ‐0.86 [‐1.73, 0.00] |
| 6.5 Epidural local anaesthetic compared with sufentanil | 1 | 67 | Std. Mean Difference (Random, 95% CI) | 0.44 [‐0.08, 0.95] |
| 6.6 Epidural local anaesthetic compared with tramadol | 2 | 65 | Std. Mean Difference (Random, 95% CI) | ‐1.09 [‐3.30, 1.12] |
| 6.7 Epidural local anaesthetic compared with morphine | 30 | 1435 | Std. Mean Difference (Random, 95% CI) | ‐0.64 [‐0.87, ‐0.41] |
| 6.8 Epidural local anaesthetic compared with buprenorphine | 2 | 77 | Std. Mean Difference (Random, 95% CI) | ‐1.05 [‐1.52, ‐0.57] |
| 7 Pain scores on movement at 24 hours subgrouped by type of surgery Show forest plot | 35 | 2731 | Std. Mean Difference (Random, 95% CI) | ‐0.85 [‐1.04, ‐0.67] |
| 7.1 Gastrointestinal surgery | 18 | 864 | Std. Mean Difference (Random, 95% CI) | ‐1.12 [‐1.43, ‐0.80] |
| 7.2 Cholecystectomy | 1 | 31 | Std. Mean Difference (Random, 95% CI) | ‐0.89 [‐1.62, ‐0.15] |
| 7.3 Gynaecological surgery | 2 | 144 | Std. Mean Difference (Random, 95% CI) | ‐0.20 [‐0.88, 0.48] |
| 7.4 Urological surgery | 4 | 136 | Std. Mean Difference (Random, 95% CI) | ‐0.66 [‐1.71, 0.40] |
| 7.5 Vascular surgery | 1 | 40 | Std. Mean Difference (Random, 95% CI) | ‐1.70 [‐2.43, ‐0.98] |
| 7.6 Various surgeries | 10 | 1516 | Std. Mean Difference (Random, 95% CI) | ‐0.61 [‐0.81, ‐0.41] |
| 8 Pain scores on movement at 24 hours subgrouped by type of opioid in the epidural Show forest plot | 34 | 1843 | Std. Mean Difference (Random, 95% CI) | ‐0.88 [‐1.09, ‐0.66] |
| 8.1 Local anaesthetic alone | 6 | 234 | Std. Mean Difference (Random, 95% CI) | ‐0.38 [‐0.93, 0.17] |
| 8.2 Epidural meperidine | 1 | 59 | Std. Mean Difference (Random, 95% CI) | 0.31 [‐0.24, 0.86] |
| 8.3 Epidural fentanyl | 16 | 756 | Std. Mean Difference (Random, 95% CI) | ‐0.95 [‐1.20, ‐0.69] |
| 8.4 Epidural sufentanil | 3 | 129 | Std. Mean Difference (Random, 95% CI) | ‐0.77 [‐1.14, ‐0.41] |
| 8.5 Epidural morphine | 10 | 665 | Std. Mean Difference (Random, 95% CI) | ‐1.19 [‐1.69, ‐0.69] |
| 9 Pain scores on movement at 24 hours subgrouped by type of opioids in the control group Show forest plot | 33 | 1796 | Std. Mean Difference (Random, 95% CI) | ‐0.90 [‐1.15, ‐0.66] |
| 9.1 Compared with IV or epidural fentanyl | 4 | 128 | Std. Mean Difference (Random, 95% CI) | ‐0.83 [‐3.13, 1.47] |
| 9.2 Compared with sufentanil | 1 | 67 | Std. Mean Difference (Random, 95% CI) | ‐0.10 [‐0.91, 0.70] |
| 9.3 Compared with meperidine | 3 | 219 | Std. Mean Difference (Random, 95% CI) | ‐0.64 [‐1.57, 0.29] |
| 9.4 Compared with piritramide | 1 | 19 | Std. Mean Difference (Random, 95% CI) | ‐0.78 [‐1.72, 0.15] |
| 9.5 Compared with morphine | 23 | 1249 | Std. Mean Difference (Random, 95% CI) | ‐0.87 [‐1.05, ‐0.69] |
| 9.6 Compared with oxycodone | 1 | 58 | Std. Mean Difference (Random, 95% CI) | ‐0.80 [‐1.33, ‐0.26] |
| 9.7 Compared with tramadol | 1 | 25 | Std. Mean Difference (Random, 95% CI) | ‐3.14 [‐4.31, ‐1.97] |
| 9.8 Compared to buprenorphine | 1 | 31 | Std. Mean Difference (Random, 95% CI) | ‐0.77 [‐1.50, ‐0.04] |
| 10 Pain scores at rest at 48 hours subgrouped by type of solution used Show forest plot | 30 | 2466 | Std. Mean Difference (Random, 95% CI) | ‐0.47 [‐0.71, ‐0.24] |
| 10.1 Local anaesthetic alone | 7 | 256 | Std. Mean Difference (Random, 95% CI) | 0.38 [‐0.49, 1.25] |
| 10.2 Local anaesthetic with an opioid | 24 | 2210 | Std. Mean Difference (Random, 95% CI) | ‐0.66 [‐0.89, ‐0.43] |
| 11 Pain scores on movement at 48 hours subgrouped by type of solution in the epidural Show forest plot | 27 | 2398 | Std. Mean Difference (Random, 95% CI) | ‐0.85 [‐1.10, ‐0.60] |
| 11.1 Local anaesthetic alone | 4 | 184 | Std. Mean Difference (Random, 95% CI) | ‐0.56 [‐1.71, 0.58] |
| 11.2 Local anaesthetic with an opioid | 23 | 2214 | Std. Mean Difference (Random, 95% CI) | ‐0.88 [‐1.13, ‐0.63] |
| 12 Pain scores at rest at 72 hours subgrouped by type of solution used Show forest plot | 15 | 1821 | Std. Mean Difference (Random, 95% CI) | ‐0.56 [‐0.88, ‐0.24] |
| 12.1 Local anaesthetic alone | 4 | 146 | Std. Mean Difference (Random, 95% CI) | ‐0.00 [‐0.34, 0.34] |
| 12.2 Local anaesthetic plus opioids | 12 | 1675 | Std. Mean Difference (Random, 95% CI) | ‐0.77 [‐1.16, ‐0.39] |
| 13 Pain scores on movement at 72 hours subgrouped by type of solution used Show forest plot | 15 | 1873 | Std. Mean Difference (Random, 95% CI) | ‐0.69 [‐0.99, ‐0.39] |
| 13.1 Local anaesthetic alone | 3 | 135 | Std. Mean Difference (Random, 95% CI) | ‐0.03 [‐0.38, 0.32] |
| 13.2 Local anaesthetic with an opioid | 12 | 1738 | Std. Mean Difference (Random, 95% CI) | ‐0.87 [‐1.22, ‐0.51] |
| 14 Vomiting Show forest plot | 22 | 1154 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.57, 1.23] |
| 14.1 Gynaecological | 4 | 223 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.06, 1.29] |
| 14.2 Gastrointestinal | 12 | 512 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.48, 1.32] |
| 14.3 Various | 6 | 419 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.44, 2.35] |
| 15 Gastrointestinal tract anastomotic leak Show forest plot | 17 | 848 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.41, 1.32] |
| 16 Length of stay in hospital subgrouped by type of surgery Show forest plot | 34 | 2774 | Std. Mean Difference (Random, 95% CI) | ‐0.13 [‐0.29, 0.02] |
| 16.1 Cholecystectomy | 1 | 20 | Std. Mean Difference (Random, 95% CI) | ‐0.5 [‐1.38, 0.38] |
| 16.2 Gynaecological surgery | 2 | 62 | Std. Mean Difference (Random, 95% CI) | 0.07 [‐0.43, 0.57] |
| 16.3 Gastrointestinal surgery | 18 | 936 | Std. Mean Difference (Random, 95% CI) | ‐0.11 [‐0.34, 0.12] |
| 16.4 Urological surgery | 4 | 141 | Std. Mean Difference (Random, 95% CI) | ‐0.52 [‐0.85, ‐0.18] |
| 16.5 Vascular surgery | 5 | 336 | Std. Mean Difference (Random, 95% CI) | ‐0.26 [‐0.65, 0.13] |
| 16.6 Various surgeries | 4 | 1279 | Std. Mean Difference (Random, 95% CI) | 0.16 [‐0.25, 0.57] |
| 17 Length of stay in hospital subgrouped by surgical site for open surgery only Show forest plot | 30 | 2598 | Std. Mean Difference (Random, 95% CI) | ‐0.20 [‐0.35, ‐0.04] |
| 17.1 Open vascular surgery | 5 | 336 | Std. Mean Difference (Random, 95% CI) | ‐0.26 [‐0.65, 0.13] |
| 17.2 Open urological surgery | 4 | 141 | Std. Mean Difference (Random, 95% CI) | ‐0.51 [‐0.85, ‐0.18] |
| 17.3 Open cholecystectomy | 1 | 20 | Std. Mean Difference (Random, 95% CI) | ‐0.50 [‐1.39, 0.39] |
| 17.4 Open gynaecological surgery | 2 | 62 | Std. Mean Difference (Random, 95% CI) | 0.07 [‐0.43, 0.57] |
| 17.5 Open gastrointestinal surgery | 14 | 760 | Std. Mean Difference (Random, 95% CI) | ‐0.24 [‐0.47, ‐0.01] |
| 17.6 Open various surgeries | 4 | 1279 | Std. Mean Difference (Random, 95% CI) | 0.15 [‐0.26, 0.57] |
| 18 Costs Show forest plot | 3 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 18.1 Costs related to pain therapy only | 1 | 62 | Std. Mean Difference (Random, 95% CI) | 19.96 [19.57, 20.34] |
| 18.2 Hospital costs | 2 | 103 | Std. Mean Difference (Random, 95% CI) | 0.17 [‐0.22, 0.55] |

