Использование десмопрессина для минимизации периоперационного переливания крови
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: cardiac surgery Country: Japan Registration: not prospectively registered | |
| Participants | Inclusion criteria: adults undergoing cardiac surgery with cardiopulmonary bypass with a membrane oxygenator Exclusion criteria: not reported Number of participants randomised: 9 Number of participants analysed: 9 Age: desmopressin arm: 52 ± 5 years; placebo arm: 57 ± 5 years Gender: desmopressin arm: male 2, female 3; placebo arm: male 3, female 1 Type of surgery
Duration of surgery: desmopressin arm: 378 ± 54 minutes; placebo arm: 444 ± 59 minutes Duration of cardiopulmonary bypass: desmopressin arm: 173 ± 42 minutes; placebo arm: 167 ± 27 minutes Emergency cases: not reported Antiplatelet agents: not reported Anticoagulants: not reported Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 50 mL 0.9% saline) 15 minutes after heparin reversal over 20 minutes (n = 5) Comparator arm: placebo details not reported (n = 4) | |
| Outcomes | Primary outcome: blood loss 24 hours postoperatively (method for measurement not reported) Secondary outcomes
| |
| Notes | Translated from Japanese to English by Junko Kiriya. A single study was split into 4 groups, with randomisation to DDAVP or placebo, and randomisation to membrane oxygenation or bubble oxygenation. Results have been presented separately for the 2 types of oxygenators (see Aida 1991b below). Blood loss and red cell transfusion requirements were reported as mL/kg, and no weights were reported. Consequently, results from this trial have been reported qualitatively and were not included in meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Unclear risk | Insufficient information for judgement |
| Blinding of outcome assessors | Unclear risk | Insufficient information for judgement |
| Incomplete outcome data | Low risk | All participants accounted for in the final analysis |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | High risk | More pre‐randomisation bleeding in placebo arm than in DDAVP arm |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: cardiac surgery Country: Japan Registration: not prospectively registered | |
| Participants | Inclusion criteria: adults undergoing cardiac surgery with cardiopulmonary bypass with a bubble oxygenator Exclusion criteria: not reported Number of participants randomised: 11 Number of participants analysed: 11 Age: desmopressin arm: 60 ± 8 years; placebo arm: 57 ± 17 years Gender: desmopressin arm: male 4, female 1; placebo arm: male 2, female 4 Type of surgery
Duration of surgery: desmopressin arm: 309 ± 96 minutes; placebo arm: 369 ± 87 minutes Duration of cardiopulmonary bypass: desmopressin arm: 137 ± 80 minutes; placebo arm: 148 ± 25 minutes Emergency cases: not reported Antiplatelet agents: not reported Anticoagulants: not reported Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 50 mL 0.9% saline) 15 minutes after heparin reversal over 20 minutes (n = 5) Comparator arm: placebo details not reported (n = 6) | |
| Outcomes | Primary outcome: blood loss 24 hours postoperatively (method for measurement not reported) Secondary outcomes
| |
| Notes | Translated from Japanese to English by Junko Kiriya. A single study was split into 4 groups with randomisation to DDAVP or placebo, and randomisation to membrane oxygenation or bubble oxygenation. Results have been presented separately for the 2 types of oxygenators (see Aida 1991a above). Blood loss and red cell transfusion requirements were reported as mL/kg, and no weights were reported. Consequently, results from this trial have been reported qualitatively and were not included in meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Unclear risk | Insufficient information for judgement |
| Blinding of outcome assessors | Unclear risk | Insufficient information for judgement |
| Incomplete outcome data | Low risk | All participants accounted for in the final analysis |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | High risk | More pre‐randomisation in placebo arm than in DDAVP arm. Very small number randomised |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: orthopaedic surgery: scoliosis Country: Turkey Registration: not prospectively registered | |
| Participants | Inclusion criteria: idiopathic or congenital scoliosis requiring surgical intervention (anterior, posterior, or sequential surgery) Exclusion criteria: people with malformations, especially of the urinary system Number of participants randomised: 40 Number of participants analysed: 40 Age: desmopressin arm: 14.8 ± 4 years; placebo arm: 14.5 ± 3 years Gender: desmopressin arm: male 5, female 13; placebo arm: male 6, female 16 Type of surgery
Duration of surgery: desmopressin arm: 241.7 ± 82.2 minutes; placebo arm: 202 ± 60.3 minutes Duration of cardiopulmonary bypass: N/A Emergency cases: none Antiplatelet agents: not reported Anticoagulants: not reported Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: all participants Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 100 mL 0.9% saline) at induction of anaesthesia over 20 minutes (n = 18) Comparator arm: placebo (100 mL 0.9% saline) at induction of anaesthesia over 20 minutes (n = 22) | |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | Blood loss and red cell transfusion requirements reported as median and interquartile range, so not included in meta‐analysis. Red cell transfusion reported in mL and converted to units with assumption that 300 mL is equivalent to 1 unit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Unclear risk | Allocation determined by one of the investigators who was not involved in outcome assessment. No information on how allocation was concealed |
| Blinding of participants and personnel | Low risk | Quote: "The solutions were prepared by one of the current authors (AA) who was not involved in the evaluation of the study parameters. The patient, surgeon and anaesthesiologist remained blind to the type of treatment" |
| Blinding of outcome assessors | Low risk | Quote: "The solutions were prepared by one of the current authors (AA) who was not involved in the evaluation of the study parameters. The patient, surgeon and anaesthesiologist remained blind to the type of treatment" |
| Incomplete outcome data | Low risk | Reported all outcomes on all participants |
| Selective outcome reporting | Unclear risk | Presented numerical data only for total blood loss (up to 24 hours) but also collected data on 0, 30, 60, 90, 120, and 150 minutes. These are displayed graphically only with a single P value |
| Other sources of bias | High risk | Five participants were re‐randomised |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: cardiac surgery Country: Sweden Registration: not prospectively registered | |
| Participants | Inclusion criteria: CABG with 3 (or more) veins or internal mammary arterial bypass grafts Exclusion criteria: previous cardiac surgery; previous coagulation disorders; coumarin anticoagulants, heparin, or acetylsalicylic acid within 7 days before surgery Number of participants randomised: 100 Number of participants analysed: 19 Age: desmopressin arm: 57 ± 12 years; placebo arm: 61 ± 5 years Gender: desmopressin arm: male 8, female 2; placebo arm: male 8, female 1 Type of surgery: all undergoing CABG Duration of surgery: not reported Duration of cardiopulmonary bypass: desmopressin arm: 70 ± 23 minutes; placebo arm: 69 ± 12 minutes Emergency cases: none Antiplatelet agents: none Anticoagulants: none Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 50 mL 0.9% saline) 15 minutes after heparin reversal over 15 minutes (n = 10) Comparator arm: placebo (50 mL 0.9% saline) 15 minutes after heparin reversal over 15 minutes (n = 9) | |
| Outcomes | Primary outcome: changes in laboratory measures of haemostasis Secondary outcome: total blood loss (measured by drain output) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Unclear risk | Quote: "The treatment code was not broken until all five blocks were completed". However method of concealment was not reported |
| Blinding of participants and personnel | Unclear risk | Described as a double‐blind study, but method of blinding was not reported. Participants were given DDAVP or placebo; however, facial flushing may have been observed or experienced |
| Blinding of outcome assessors | Unclear risk | Double‐blind, placebo‐controlled trial; method of blinding not reported but placebo available. However, facial flushing may have been observed |
| Incomplete outcome data | High risk | Additional unpublished data for the whole study. Paper reports only 19 of the 100 participants |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | High risk | Funded in part by Ferring AB, Malmo, Sweden (a manufacturer of DDAVP) |
| Methods | Type of study: multi‐centre, 2‐arm, parallel‐group RCT Setting: cardiac surgery Country: USA Registration: not prospectively registered | |
| Participants | Inclusion criteria: age 18 to 75 years; elective cardiac valve operations with, or without, coronary artery bypass Exclusion criteria: recent myocardial infarction (timing not specified); unstable angina; deep vein thrombosis or pulmonary embolism; history of bleeding diathesis or platelet defect; unstable haemodynamic status; pertinent drug allergy; pregnancy Number of participants randomised: 92 Number of participants analysed: 83 Age: desmopressin arm: 61.9 ± 10.7 years; placebo arm: 60.1 ± 9.2 years Gender: desmopressin arm: male 24, female 17; placebo arm: male 21, female 21 Type of surgery: all valve replacements, but not possible to determine how many in each category Duration of surgery: desmopressin arm: 261 ± 70 minutes; placebo arm: 242.5 ± 61.4 minutes Duration of cardiopulmonary bypass: desmopressin arm: 118.6 ± 39 minutes; placebo arm: 111.8 ± 41.5 minutes Emergency cases: none Antiplatelet agents: not reported Anticoagulants: not reported Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: desmopressin arm: epsilon‐aminocaproic acid (dose not specified) 1; placebo arm: none Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 50 mL 0.9% saline) immediately after heparin reversal over 15 minutes (n = 41) Comparator arm: placebo (50 mL 0.9% saline) immediately after heparin reversal over 15 minutes (n = 42) | |
| Outcomes | Primary outcome: total blood loss (measured by drain output) Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Quote: "Randomisation was provided by a series of computer‐generated random numbers" |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Unclear risk | Quote: "All study medication was dispensed in a blinded fashion by the investigator or hospital pharmacy and was administered in a blinded fashion". Medication dispensing was performed by the investigator, so unclear if blinding could be maintained |
| Blinding of outcome assessors | Unclear risk | Quote: "All study medication was dispensed in a blinded fashion by the investigator or hospital pharmacy and was administered in a blinded fashion". Medication dispensing was performed by the investigator, so unclear if blinding could be maintained |
| Incomplete outcome data | High risk | 92 participants enrolled and 9 excluded from analysis: 7 did not receive study drug, 1 inadvertently received drug preoperatively, and 1 had no blood loss data collected (5 in DDAVP arm, 4 in placebo) |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | High risk | One participant in the DDAVP arm received epsilon‐aminocaproic acid. Three participants in the DDAVP arm and 1 participant on the placebo arm received another dose of DDAVP. Funded in part by Rorer Central Research (a manufacturer of DDAVP) |
| Methods | Type of study: multi‐centre, 2‐arm, parallel‐group RCT Setting: cardiac surgery Country: Italy Registration: prospectively registered: NCT00337766 | |
| Participants | Inclusion criteria: age ≥ 18 years; elective cardiac surgery; diffuse intraoperative bleeding without a surgical source or excessive postoperative bleeding from chest tubes defined as 100 mL over 30 minutes, or 2 mL/kg/h for at least 2 hours Exclusion criteria: lack of informed consent; myocardial infarction within previous 7 days Number of participants randomised: 135 Number of participants analysed: 135 Age: desmopressin arm: 64 ± 13.3 years; placebo arm: 62 ± 13.2 years Gender: desmopressin arm: male 50, female 18; placebo arm: male 50, female 17 Type of surgery: all cardiac surgery Duration of surgery: not reported Duration of cardiopulmonary bypass: not reported Emergency cases: none Antiplatelet agents: desmopressin arm: 25; placebo arm: 29 Anticoagulants: not reported Coagulopathy: desmopressin arm: 1; placebo arm: 1 Thrombocytopenia: not reported Antifibrinolytics: a slow intravenous bolus of 1 g tranexamic acid was administered before surgery to all participants. At 1 participating centre, this was followed by a continuous infusion of 400 mg/h during the surgical intervention. At the other 2 participating centres, a further dose of 500 mg was administered following cardiopulmonary bypass Cell salvage: not reported Transfusion protocol: 1 unit of red cells transfused if haemoglobin < 80 g/L | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 50 mL 0.9% saline) administered over 20 minutes in the event of excessive bleeding (n = 68) Comparator arm: placebo (50 mL 0.9% saline) administered over 20 minutes in the event of excessive bleeding (n = 67) | |
| Outcomes | Primary outcome: total number of participants receiving a red cell transfusion Secondary outcomes
| |
| Notes | Trial was stopped after 135/200 participants recruited owing to futility. Total volume of red cells transfused and total blood loss were reported as median and interquartile range, which could not be incorporated into meta‐analysis so this trial has been reported narratively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Quote: "A computer‐generated randomization sequence stratified by center with blocks of 20 was used" |
| Allocation concealment | Low risk | Opaque, sealed envelopes that were sequentially numbered |
| Blinding of participants and personnel | Low risk | Quote: "Patients, managing physicians, and nurses were blinded to treatment assignment for the whole duration of the study. Desmopressin and placebo were prepared in a separate room, as colourless fluids in unlabelled bottles, by personnel who was not involved in patients’ management and data collection" |
| Blinding of outcome assessors | Low risk | Quote: "Patients, managing physicians, and nurses were blinded to treatment assignment for the whole duration of the study. Desmopressin and placebo were prepared in a separate room, as colourless fluids in unlabelled bottles, by personnel who was not involved in patients’ management and data collection" |
| Incomplete outcome data | Low risk | All participants included in final analysis |
| Selective outcome reporting | Low risk | All prespecified outcomes reported |
| Other sources of bias | Low risk | No other clear sources of bias. Supported by departmental funds only |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: cardiac surgery Country: USA Registration: not prospectively registered | |
| Participants | Inclusion criteria: elective CABG surgery Exclusion criteria: none reported Number of participants randomised: 20 Number of participants analysed: 19 Age: desmopressin arm: 61.7 ± 8.1 years; placebo arm: 62.5 ± 6.9 years Gender: desmopressin arm: male 8, female 2; placebo arm: male 6, female 3 Type of surgery: all CABG surgery Duration of surgery: not reported Duration of cardiopulmonary bypass: desmopressin arm: 109 ± 33 minutes; placebo arm: 89 ± 38 minutes Emergency cases: none Antiplatelet agents: desmopressin arm: 6; placebo arm: 5 Anticoagulants: not reported Coagulopathy: none Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: all participants Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 0.9% saline (volume not reported)) immediately after heparin reversal over 10 minutes (n = 10) Comparator arm: placebo (0.9% saline (volume not reported)) immediately after heparin reversal over 10 minutes (n = 9) | |
| Outcomes | Primary outcome: changes in laboratory measures of haemostasis Secondary outcomes
| |
| Notes | Volume of red cells reported as mL rather than in units. Converted into units assuming 1 unit is equivalent to 300 mL | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | High risk | Infusion given over 10 minutes, resulting in notable hypotension and compromising blinding |
| Blinding of outcome assessors | High risk | Infusion given over 10 minutes, resulting in notable hypotension and compromising blinding |
| Incomplete outcome data | Low risk | 20 randomised, 1 excluded owing to 'TEG technical error'; however, this is unlikely to bias results |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | Unclear risk | Insufficient information for judgement |
| Methods | Type of study: single‐centre, 3‐arm, parallel‐group RCT Setting: cardiac surgery Country: Spain Registration: not prospectively registered | |
| Participants | Inclusion criteria: age ≥ 18 years; CABG, valve replacement, annuloplasty, combined valve replacement and CABG, or closure of atrial septal defect Exclusion criteria: emergency operations; history of a bleeding disorder; allergy or previous exposure to aprotinin Number of participants randomised: 149 Number of participants analysed: 140 Age: desmopressin arm: 58 ± 12 years; placebo arm: 54 ± 12 years; aprotinin arm: 57 ± 10 years Gender: desmopressin arm: male 33, female 17; placebo arm: male 31, female 20; aprotinin arm: male 31, female 17 Type of surgery
Duration of surgery: desmopressin arm: 188 ± 41 minutes; placebo arm: 184 ± 55 minutes; aprotinin arm: 178 ± 77 minutes Duration of cardiopulmonary bypass: desmopressin arm: 89 ± 40 minutes; placebo arm: 99 ± 36 minutes; aprotinin arm: 87 ± 25 minutes Emergency cases: none Antiplatelet agents: desmopressin arm: 7; placebo arm: 5; aprotinin arm: 7 Anticoagulants: none Coagulopathy: none Thrombocytopenia: not reported Antifibrinolytics: desmopressin arm: 0; placebo arm: 0; aprotinin arm: all received aprotinin Cell salvage: not reported Transfusion protocol: red cells transfused if haemoglobin < 80 g/L, or if participant was in shock because of haemorrhage | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 50 mL 0.9% saline) immediately after heparin reversal over 20 to 30 minutes; placebo (200 mL 0.9% saline preoperatively, 200 mL in fluid prime, and 50 mL/h from skin incision to skin closure) (n = 50) Comparator arm: placebo (200 mL 0.9% saline preoperatively, 200 mL in fluid prime, 50 mL/h from skin incision to skin closure, and 50 mL immediately after heparin reversal over 20 to 30 minutes) (n = 51) Aprotinin arm: aprotinin (2 million KIU in 200 mL preoperatively, 2 million KIU in 200 mL in fluid prime, 500,000 KIU in 50 mL/h from skin incision to skin closure). placebo (50 mL 0.9% saline) immediately after heparin reversal over 20 to 30 minutes (n = 48) | |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | Blood loss was reported as mL/m2 body surface area. No body surface area data were reported and consequently results from this trial for blood loss have been reported qualitatively rather than included in meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Low risk | Quote: "Sealed envelopes ensured only the pharmacist who prepared the encoded infusions knew whether a patient received desmopressin, aprotinin or placebo" |
| Blinding of participants and personnel | Low risk | Only pharmacist preparing the investigational medicinal product (IMP) was aware of allocation |
| Blinding of outcome assessors | Low risk | Only pharmacist preparing the IMP was aware of allocation |
| Incomplete outcome data | High risk | 140/149 (94%) participants included in the final analysis with clear reasons for exclusions. Participants who returned to theatre with bleeding were excluded from analysis (1 in DDAVP group and 2 in aprotinin group) |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | High risk | Supported in part by QF Bayer, Spain (a manufacturer of aprotinin) |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: cardiac surgery Country: Taiwan Registration: not prospectively registered | |
| Participants | Inclusion criteria: adults undergoing cardiac surgery with cardiopulmonary bypass Exclusion criteria: none reported Number of participants randomised: 48 Number of participants analysed: 48 Age: not reported Gender: not reported Type of surgery: all cardiac surgery with cardiopulmonary bypass (types of surgery not specified) Duration of surgery: not reported Duration of cardiopulmonary bypass: not reported Emergency cases: not reported Antiplatelet agents: not reported Anticoagulants: not reported Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously (volume and type of diluent not reported)) 1 hour after heparin reversal (speed of infusion not specified) (n = 48) Comparator arm: placebo (no details reported) (n = 48) | |
| Outcomes | Primary outcome: changes in laboratory measures of haemostasis Secondary outcomes
| |
| Notes | Despite a worldwide search, we were unable to obtain the full text for this paper. Consequently, we extracted data from the abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Abstract only: insufficient information for judgement |
| Allocation concealment | Unclear risk | Abstract only: insufficient information for judgement |
| Blinding of participants and personnel | Unclear risk | Abstract only: insufficient information for judgement |
| Blinding of outcome assessors | Unclear risk | Abstract only: insufficient information for judgement |
| Incomplete outcome data | Unclear risk | Abstract only: insufficient information for judgement |
| Selective outcome reporting | Unclear risk | Abstract only: no protocol available |
| Other sources of bias | Unclear risk | Abstract only: insufficient information for judgement |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: vascular surgery Country: USA Registration: not prospectively registered | |
| Participants | Inclusion criteria: elective infrarenal aortic aneurysm repair or aortofemoral bypass for occlusive disease Exclusion criteria: aspirin within 7 days of operation; acquired or congenital haemorrhagic diathesis; emergency operation; creatinine ≥ 3 mg/dL; thoracoabdominal reconstruction; aortorenal or visceral bypass Number of participants randomised: 91 Number of participants analysed: 91 Age: desmopressin arm: 62 ± 9 years; placebo arm: 64 ± 8 years Gender: desmopressin arm: male 43, female 0; placebo arm: male 48, female 0 Type of surgery
Duration of surgery: desmopressin arm: 273 ± 73 minutes; placebo arm: 252 ± 76 minutes Duration of cardiopulmonary bypass: N/A Emergency cases: none Antiplatelet agents: none Anticoagulants: not reported Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: all participants Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (20 μg intravenously in 50 mL 0.9% saline) immediately after intravenous heparinisation and just before aortic cross‐clamp application over 15 minutes (n = 43) Comparator arm: placebo (50 mL 0.9% saline) immediately after intravenous heparinisation and just before aortic cross‐clamp application over 15 minutes (n = 48) | |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | Paper reports intraoperative blood loss at 3 points: pre‐clamp (DDAVP given at end of this), during clamp, and after clamp. No combined figure is provided for intraoperative blood loss for period after DDAVP given. Total intraoperative blood loss includes intraoperative blood loss before DDAVP given; consequently, this has not been included in meta‐analysis. Transfusion during the operation could not be calculated, as this was reported as 2 separate groups: clamp and post clamp | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Low risk | Participants were randomised by drawing a sealed envelope |
| Blinding of participants and personnel | Low risk | Quote: "The only person to have knowledge of treatment assignment was the pharmacist who kept records and prepared DDAVP or placebo in identical‐appearing plastic bags of 50 mL normal saline solution" |
| Blinding of outcome assessors | Low risk | The only person to have knowledge of treatment assignment was the pharmacist who kept records |
| Incomplete outcome data | Low risk | All participants included in final analysis |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | Unclear risk | Insufficient information for judgement |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: cardiac surgery Country: France Registration: not prospectively registered | |
| Participants | Inclusion criteria: open heart surgery; significant postoperative blood loss (> 75 mL/m2/h) at any time during the first 6 hours post surgery; prolonged bleeding time (> 10 minutes). Exclusion criteria: < 15 years of age; massive mediastinal haemorrhage requiring reoperation Number of participants randomised: 92 Number of participants analysed: 92 (81 for bleeding outcomes) Age: desmopressin arm: 60 ± 13 years; placebo arm: 55 ± 16 years Gender: desmopressin arm: male 37, female 10; placebo arm: male 32, female 13 Type of surgery
Duration of surgery: not reported Duration of cardiopulmonary bypass: desmopressin arm: 83 ± 31 minutes; placebo arm: 82 ± 30 minutes Emergency cases: not reported Antiplatelet agents: not clear: "participants usually stopped antiplatelet drugs preoperatively" Anticoagulants: none Coagulopathy: prolonged bleeding time for all participants Thrombocytopenia: none Antifibrinolytics: desmopressin arm: aprotinin 2; placebo arm: aprotinin 6 Cell salvage: not reported Transfusion protocol: red cell transfusion if haematocrit < 30% | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 50 mL 0.9% saline) at any time from the end of the operation to 6 hours postoperatively over 30 minutes (n = 47) Comparator arm: placebo (50 mL 0.9% saline) at any time from the end of the operation to 6 hours postoperatively over 30 minutes (n = 45) | |
| Outcomes | Primary outcome: blood loss after 24 hours (measured by volume of suction drainage) Secondary outcomes
| |
| Notes | Blood loss reported in mL/m2, so not included in meta‐analysis and reported narratively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Unclear risk | Insufficient information for judgement: reported as "double‐blind" but no details given |
| Blinding of outcome assessors | Unclear risk | Insufficient information for judgement: reported as "double‐blind" but no details given |
| Incomplete outcome data | High risk | Blood loss reported only for participants who did not undergo reoperation: 3/47 in DDAVP arm and 8/45 in placebo arm required reoperation |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | High risk | Supported by grants from Ferring (a manufacturer of DDAVP) |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: cardiac surgery Country: USA Registration: not prospectively registered | |
| Participants | Inclusion criteria: elective cardiac surgery involving cardiopulmonary bypass; abnormal platelet function after cardiopulmonary bypass (defined as hemoSTATUS < 60% in channel 5) Exclusion criteria: urgent procedures; pre‐existing disorders of haemostasis; treatment with antifibrinolytic or antiplatelet agents within 2 days of surgery; intraoperative microvascular bleeding requiring blood component transfusion Number of participants randomised: 101 Number of participants analysed: 101 Age: desmopressin arm: 64 ± 10 years; placebo arm: 66 ± 10 years Gender: desmopressin arm: male 30, female 20; placebo arm: male 34, female 17 Type of surgery Number of procedures reported rather than procedures undergone by each participant; therefore numbers higher than numbers of participants
Duration of surgery: not reported Duration of cardiopulmonary bypass: desmopressin arm: 147 ± 49 minutes; placebo arm: 146 ± 38 minutes Emergency cases: none Antiplatelet agents: desmopressin arm: 26; placebo arm: 33 Anticoagulants: desmopressin arm: warfarin 3; placebo arm: 0 Coagulopathy: all had hemoSTATUS < 60% in channel 5 Thrombocytopenia: not reported Antifibrinolytics
Cell salvage: not reported Transfusion protocol: no protocol, transfusion given at discretion of treating physicians | |
| Interventions | Intervention arm: DDAVP (0.4 μg/kg intravenously in 50 mL 0.9% saline) over 30 minutes (timing of administration unclear) (n = 50) Comparator arm: placebo (50 mL 0.9% saline) over 30 minutes (timing of administration unclear) (n = 51) | |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Quote: "Randomisation was based on a computer‐generated random‐number table and done according to a sequential allocation schedule" |
| Allocation concealment | Unclear risk | Quote: "Randomisation was based on a computer‐generated random‐number table and done according to a sequential allocation schedule that was generated by an investigator not involved in treatment assignment." Insufficient details for judgement about allocation concealment |
| Blinding of participants and personnel | Low risk | Quote: "Desmopressin and placebo were administered intravenously as colourless fluids from unlabelled syringes, aspirated in a separate room and transported to the operating room by one of the investigators (not masked to treatment status but not involved in the management of the patients). Desmopressin was given as 0.4 g/kg over 30 min, and placebo patients received a corresponding volume of normal saline. Managing physicians, nurses, and patients were masked to treatment status perioperatively, and no protocol violations were noted" |
| Blinding of outcome assessors | Unclear risk | Not clear who assessed outcomes and whether blinded |
| Incomplete outcome data | Low risk | All participants included in final analysis |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | High risk | Material support provided by Rhône‐Poulenc Rone Pharmaceuticals Inc (a manufacturer of DDAVP) |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: cardiac surgery Country: Germany Registration: not prospectively registered | |
| Participants | Inclusion criteria: elective first‐time myocardial revascularisation; aspirin within previous 5 days; male Exclusion criteria: preoperative haemoglobin < 135 g/L; preoperative prolongation of PT or aPTT; any anticoagulant treatment other than aspirin; intraoperative use of aprotinin Number of participants randomised: 40 Number of participants analysed: 39 Age: desmopressin arm: 56 ± 9 years; placebo arm: 58 ± 8 years Gender: desmopressin arm: male 19, female 0; placebo arm: male 20, female 0 Type of surgery: all undergoing elective CABG Duration of surgery: desmopressin arm: 240 ± 37 minutes; placebo arm: 248 ± 48 minutes Duration of cardiopulmonary bypass: desmopressin arm: 88 ± 25 minutes; placebo arm: 81 ± 22 minutes Emergency cases: none Antiplatelet agents: desmopressin arm: aspirin 19; placebo arm: aspirin 20 Anticoagulants: none Coagulopathy: none Thrombocytopenia: not reported Antifibrinolytics: none Cell salvage: all participants Transfusion protocol: red cells transfused if haematocrit < 30% | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 0.9% saline (volume not reported)) 5 minutes after heparin reversal over 15 minutes (n = 19) Comparator arm: placebo (0.9% saline (volume not reported)) 5 minutes after heparin reversal over 15 minutes (n = 20) | |
| Outcomes | Primary outcome: total volume of red cells transfused Secondary outcomes
| |
| Notes | Blood loss and volume of red cells transfused up to 24 hours postoperatively reported as median and range. Consequently, these results have been reported narratively and were not included in meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Low risk | Quote: "the anaesthesiologist and surgeon responsible for postoperative treatment were blinded to the substance given" |
| Blinding of outcome assessors | Unclear risk | Quote: "surgeon responsible for postoperative treatment was blinded", but does not say whether this surgeon was the outcome assessor |
| Incomplete outcome data | High risk | One participant randomised to DDAVP was "excluded from analysis because, in the early postoperative course, he showed excessive bleeding" |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | Low risk | Insufficient information for judgement |
| Methods | Type of study: single‐centre, open‐label, 3‐arm, parallel‐group RCT Setting: orthopaedic surgery Country: Israel Registration: not prospectively registered | |
| Participants | Inclusion criteria: ASA scale 1‐3; undergoing elective total knee replacement Exclusion criteria: New York Heart Association (NYHA) 3 or 4 classified heart failure; chronic renal failure; liver cirrhosis; bleeding disorders; current anticoagulant therapy Number of participants randomised: 30 Number of participants analysed: 30 Age: desmopressin arm: 72 ± 6 years; placebo arm: 72 ± 8 years; tranexamic acid: 71 ± 5 years Gender: desmopressin arm: male 2, female 8; placebo arm: male 3, female 7; tranexamic acid arm: male 4, female 6 Type of surgery: all undergoing elective total knee replacement Duration of surgery: desmopressin arm: 133 ± 16 minutes; placebo arm: 134 ± 14 minutes; tranexamic acid arm: 135 ± 11 minutes Duration of cardiopulmonary bypass: N/A Emergency cases: none Antiplatelet agents: not reported Anticoagulants: none Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: desmopressin arm: 0; placebo arm: 0; tranexamic acid arm: all receiving tranexamic acid Cell salvage: not reported Transfusion protocol: red cells transfused if haematocrit < 27% | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 0.9% saline (volume not reported)) 30 minutes before tourniquet removed over 30 minutes. Followed by infusion of placebo (10 mL/kg) until 12 hours after tourniquet deflated (n = 10) Comparator arm: standard care (n = 10) Tranexamic acid arm: tranexamic acid: 15 mg/kg 30 minutes before tourniquet removed over 30 minutes, then 10 mg/kg/h until 12 hours after tourniquet deflated (n = 10) | |
| Outcomes | Primary outcome: change in laboratory measures of haemostasis Secondary outcomes
| |
| Notes | Volume of red cells transfused reported as mean only. Consequently, results for this outcome are reported narratively and were not included in meta‐analysis. Red cell transfusion reported in mL and converted to units with assumption that 300 mL is equivalent to 1 unit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Quote: "computer generated randomisation table" |
| Allocation concealment | Unclear risk | Insufficient information to make judgement |
| Blinding of participants and personnel | High risk | Control group received standard of care with no intervention or placebo. Participants and personnel were adequately blinded to whether they were in the tranexamic acid or DDAVP arms (but not control). |
| Blinding of outcome assessors | Low risk | Quote: "the decision to transfuse ... was taken by an independent observer who was blinded to treatment modality" |
| Incomplete outcome data | Low risk | Complete outcome data reported for all groups |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | Unclear risk | Insufficient information for judgement |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: orthopaedic surgery Country: Sweden Registration: not prospectively registered | |
| Participants | Inclusion criteria: undergoing total hip replacement Exclusion criteria: prostaglandin synthesis inhibitors Number of participants randomised: 12 Number of participants analysed: 12 Age: not reported Gender: not reported Type of surgery: all undergoing elective total hip replacement Duration of surgery: not reported Duration of cardiopulmonary bypass: N/A Emergency cases: none Antiplatelet agents: not reported Anticoagulants: not reported Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 50 mL 0.9% saline) at start of surgery and again 6 hours postoperatively over 20 to 30 minutes (n = 6) Comparator arm: placebo (50 mL 0.9% saline) at start of surgery and again 6 hours postoperatively over 20 to 30 minutes (n = 6) | |
| Outcomes | Primary outcome: change in laboratory measures of haemostasis Secondary outcomes
| |
| Notes | Blood loss reported as mean only. Consequently, this outcome has been reported narratively and was not included in meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Unclear risk | Reported as double‐blind but with insufficient details of methods |
| Blinding of outcome assessors | Unclear risk | Reported as double‐blind but with insufficient details of methods |
| Incomplete outcome data | Low risk | All participants included in final analysis |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | High risk | Study was supported in part by Ferring AB (a manufacturer of DDAVP). Methods for reporting main outcome (total blood loss) were not clear |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: orthopaedic surgery Country: Sweden Registration: not prospectively registered | |
| Participants | Inclusion criteria: elective total hip replacement Exclusion criteria: > 80 years old; severe vascular, hepatic, or renal disease; prostaglandin synthesis inhibitors Number of participants randomised: 50 Number of participants analysed: 50 Age: desmopressin arm: 64 ± 9 years; placebo arm: 68 ± 9 years Gender: desmopressin arm: male 12, female 13; placebo arm: male 12, female 13 Type of surgery: all undergoing elective total hip replacement Duration of surgery: desmopressin arm: 106 ± 23 minutes; placebo arm: 104 ± 20 minutes Duration of cardiopulmonary bypass: N/A Emergency cases: none Antiplatelet agents: not reported Anticoagulants: not reported Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 50 mL 0.9% saline) at start of surgery and again 6 hours postoperatively over 20 to 30 minutes (n = 25) Comparator arm: placebo (50 mL 0.9% saline) at start of surgery and again 6 hours postoperatively over 20 to 30 minutes (n = 25) | |
| Outcomes | Primary outcome: blood loss: intraoperative and total (measured by estimating blood in surgical swabs, paper drapes, and folds; volume in suction reservoir; and change in haemoglobin preoperatively and postoperatively compared with estimated total blood volume) Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Unclear risk | Reported as performed in a double‐blind fashion but no details of methods |
| Blinding of outcome assessors | Unclear risk | Reported as performed in a double‐blind fashion but no details of methods |
| Incomplete outcome data | Low risk | All participants included in final analysis |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | Unclear risk | DDAVP supplied free of charge by Ferring AB (a manufacturer of DDAVP), but unclear if they had any role in the study design |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: cardiac surgery Country: USA Registration: not prospectively registered | |
| Participants | Inclusion criteria: elective primary CABG Exclusion criteria: warfarin or heparin within 24 hours of surgery; documented coagulopathies or platelet disorders; allergy to DDAVP; renal failure; stroke or venous thromboembolism within 3 months Number of participants randomised: 40 Number of participants analysed: 40 Age: desmopressin arm: 59.9 ± 10.7 years; placebo arm: 59.6 ± 11.2 years Gender: desmopressin arm: male 17, female 3; placebo arm: male 17, female 3 Type of surgery: all undergoing elective CABG Duration of surgery: not reported Duration of cardiopulmonary bypass: desmopressin arm: 50.8 ± 14.4 minutes; placebo arm: 50.7 ± 10.7 minutes Emergency cases: none Antiplatelet agents: none Anticoagulants: none Coagulopathy: none Thrombocytopenia: none Antifibrinolytics: not reported Cell salvage: all participants Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 50 mL 0.9% saline) 5 minutes after heparin reversal over 15 minutes (n = 20) Comparator arm: placebo (50 mL 0.9% saline) 5 minutes after heparin reversal over 15 minutes (n = 20) | |
| Outcomes | Primary outcome: clinically significant hypotension Secondary outcomes
| |
| Notes | Volume of red cells transfused reported as mean only (no standard deviation), so reported narratively and not included in meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information to make judgement |
| Allocation concealment | Low risk | Sealed envelopes used |
| Blinding of participants and personnel | Low risk | Desmopressin and placebo solutions were identical in appearance. Surgeon, anaesthesiologist, and investigator collecting experimental data were unaware of which solution was administered. |
| Blinding of outcome assessors | Low risk | Desmopressin and placebo solutions were identical in appearance. Surgeon, anaesthesiologist, and investigator collecting experimental data were unaware of which solution was administered. |
| Incomplete outcome data | Low risk | All participants included in final analysis |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | Unclear risk | Insufficient information for judgement |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: cardiac surgery Country: USA Registration: not prospectively registered | |
| Participants | Inclusion criteria: elective CABG operations; aspirin within 7 days of surgery Exclusion criteria: valvular heart disease; need for intra‐aortic balloon pump; re‐doing CABG Number of participants randomised: 65 Number of participants analysed: 59 Age: desmopressin arm: 62.2 ± 10.4 years; placebo arm: 62.7 ± 12.3 years Gender: desmopressin arm: male 19, female 10; placebo arm: male 23, female 7 Type of surgery: all undergoing elective CABG Duration of surgery: not reported Duration of cardiopulmonary bypass: desmopressin arm: 80.4 ± 29 minutes; placebo arm: 95.4 ± 25.6 minutes Emergency cases: none Antiplatelet agents: aspirin taken by all participants Anticoagulants: not reported Coagulopathy: not reported Thrombocytopenia: none Antifibrinolytics: not reported Cell salvage: all participants Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 50 mL 0.9% saline) immediately after heparin reversal over 30 minutes (n = 29) Comparator arm: placebo (50 mL 0.9% saline) immediately after heparin reversal over 30 minutes (n = 30) | |
| Outcomes | Primary outcome: total blood loss (measured by weighing surgical sponges, volume in cell saver, and suction drainage) Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient detail for judgement. "Patients were assigned to the DDAVP or placebo groups according to a randomization schedule" |
| Allocation concealment | Unclear risk | Insufficient detail for judgement. "all study medication was dispensed in blinded fashion ...The solutions were prepared by research pharmacists" |
| Blinding of participants and personnel | Low risk | Quote: "The solutions were prepared by a research pharmacist". "Members of all the anaesthesia and surgical teams were blinded to the nature of the DDAVP or placebo infusion" |
| Blinding of outcome assessors | Low risk | Quote: "The solutions were prepared by a research pharmacist". "Members of all the anaesthesia and surgical teams were blinded to the nature of the DDAVP or placebo infusion" |
| Incomplete outcome data | Low risk | Six participants excluded from final analysis (3 in each group): 2 died intraoperatively, 1 inadvertently received DDAVP, 3 required insertion of an intra‐aortic balloon pump. Incomplete outcome data balanced between groups |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | High risk | Supported in part by a grant from Rhone‐Poulenc Rorer (a manufacturer of DDAVP) |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: orthopaedic surgery: spinal Country: Canada Registration: not prospectively registered | |
| Participants | Inclusion criteria: ASA 1‐2; idiopathic scoliosis; undergoing scheduled spinal fusion surgery Exclusion criteria: different surgical technique used; history of bleeding diathesis; ingestion of drugs known to interfere with haemostasis; abnormal bleeding time (> 9 minutes); aPTT > 36 seconds; PT > 25 seconds; TT > 16 seconds; platelet count < 150 × 109/L Number of participants randomised: 31 Number of participants analysed: 30 Age: desmopressin arm: 13.5 ± 1.9 years; placebo arm: 15.1 ± 1.9 years Gender: desmopressin arm: male 1, female 14; placebo arm: male 1, female 14 Type of surgery: all undergoing elective spinal fusion scoliosis surgery Duration of surgery: desmopressin arm: 246 ± 72 minutes; placebo arm: 222 ± 42 minutes Duration of cardiopulmonary bypass: N/A Emergency cases: none Antiplatelet agents: none Anticoagulants: none Coagulopathy: none Thrombocytopenia: none Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: red cell transfusion for bleeding causing 20% to 30% fall in calculated blood volume | |
| Interventions | Intervention arm: DDAVP (10 μg/m2 body surface area intravenously in 100 mL 0.9% saline) at time of first skin incision over 20 minutes (n = 15) Comparator arm: placebo (100 mL 0.9% saline) at time of first skin incision over 20 minutes (n = 15) | |
| Outcomes | Primary outcome: blood loss: intraoperative and total (measurement method not reported) Secondary outcomes
| |
| Notes | Volume of red cells transfused includes both autologous and allogeneic blood transfusion | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Low risk | Quote: "The design was double blind, and the surgeon, the anesthesiologist, and the investigator collecting the experimental data were all unaware of which solution was administered" |
| Blinding of outcome assessors | Low risk | Quote: "the investigators collecting the experimental data were unaware of which solution was administered" |
| Incomplete outcome data | Low risk | All participants included in final analysis |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | Unclear risk | Insufficient information for judgement |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: maxillofacial surgery Country: USA Registration: not prospectively registered | |
| Participants | Inclusion criteria: bimaxillary osteotomy; normal preoperative PT and aPTT; no history of bleeding disorder or easy bruising Exclusion criteria: none reported Number of participants randomised: 20 Number of participants analysed: 20 Age: not reported Gender: desmopressin arm: male 1, female 9; placebo arm: male 4, female 6 Type of surgery: all undergoing bimaxillary osteotomy and osteoplastic genioplasty Duration of surgery: not reported Duration of cardiopulmonary bypass: N/A Emergency cases: none Antiplatelet agents: not reported Anticoagulants: not reported Coagulopathy: none Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (20 μg intravenously in 50 mL 0.9% saline) 30 minutes preoperatively over 30 minutes (n = 10) Comparator arm: placebo (50 mL 0.9% saline) 30 minutes preoperatively over 30 minutes (n = 10) | |
| Outcomes | Primary outcome: blood loss: up to 24 hours postoperatively (measured by estimating blood loss in surgical sponges and suction drainage) Secondary outcomes
| |
| Notes | Total blood loss reported as mean and range, so outcome reported narratively and not included in meta‐analysis. Volume of red cells transfused reported as mean only, so reported narratively and not included in meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Unclear risk | Insufficient information for judgement |
| Blinding of outcome assessors | Low risk | Quote: "the evaluating team was not apprised of the DDAVP recipients" |
| Incomplete outcome data | Low risk | All participants included in final analysis |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | Unclear risk | Insufficient information for judgement |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: cardiac surgery Country: Canada Registration: not prospectively registered | |
| Participants | Inclusion criteria: > 18 years old; elective cardiac surgery involving cardiopulmonary bypass Exclusion criteria: pregnancy; known bleeding disorder such as haemophilia, von Willebrand disease, or immune thrombocytopenic purpura; abnormal coagulation (PT, aPTT or TT); platelet count < 100 × 109/L; clotting parameters that had not returned to normal after cessation of anticoagulant drugs Number of participants randomised: 164 Number of participants analysed: 150 Age: desmopressin arm: < 45 years: 5, 46‐60 years: 27, 61‐75 years: 39, > 75 years: 3; placebo arm: < 45 years: 6, 46‐60 years: 26, 61‐75 years: 39, > 75 years: 5 Gender: not reported Type of surgery
Duration of surgery: desmopressin arm: 306 ± 89 minutes; placebo arm: 318 ± 114 minutes Duration of cardiopulmonary bypass: desmopressin arm: 168 ± 58 minutes; placebo arm: 161 ± 52 minutes Emergency cases: none Antiplatelet agents: desmopressin arm: 16 aspirin, 10 dipyridamole; placebo arm: 11 aspirin, 4 dipyridamole Anticoagulants: not reported Coagulopathy: none Thrombocytopenia: none Antifibrinolytics: not reported Cell salvage: all participants Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 25 mL 0.9% saline) immediately after heparin reversal over 15 minutes (n = 74) Comparator arm: placebo (25 mL 0.9% saline) immediately after heparin reversal over 15 minutes (n = 76) | |
| Outcomes | Primary outcome: blood loss: perioperative and total (measured by weighing surgical sponges, estimating blood on surgical drapes, measuring suction bottles and drain output) Secondary outcomes
| |
| Notes | Blood loss and volume of red cells transfused reported as median and range, so these outcomes were reported narratively and were not included in meta‐analysis. Volume of red cells transfused was reported in mL, and this was converted to units, assuming that 1 unit is equivalent to 300 mL | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Low risk | Quote: "All patients, all treating physicians, and all investigators involved in collecting data, measuring blood loss, or performing and interpreting laboratory tests were blinded to the treatment assigned" |
| Blinding of outcome assessors | Low risk | Quote: "All patients, all treating physicians, and all investigators involved in collecting data, measuring blood loss, or performing and interpreting laboratory tests were blinded to the treatment assigned" |
| Incomplete outcome data | High risk | 14 participants who were randomised were excluded from the outcome results, including 3 participants who died "during surgery or shortly afterward". It is not clear which treatment these participants had been allocated to |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | Low risk | No other clear source of bias. Supported by a grant from the British Columbia Heart Foundation |
| Methods | Type of study: 2‐arm parallel‐group RCT (unclear if single‐centre or multi‐centre trial) Setting: cardiac surgery Country: Brazil Registration: not prospectively registered | |
| Participants | Inclusion criteria: cardiac surgery requiring cardiopulmonary bypass Exclusion criteria: not reported Number of participants randomised: not reported Number of participants analysed: 150 Age: not reported Gender: not reported Type of surgery: all cardiac surgery requiring cardiopulmonary bypass. Information on individual types of surgery not reported Duration of surgery: not reported Duration of cardiopulmonary bypass: not reported Emergency cases: not reported Antiplatelet agents: not reported Anticoagulants: not reported Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 50 mL 0.9% saline) immediately after the end of surgery over 15 minutes (n = 75) Comparator arm: placebo (50 mL 0.9% saline) immediately after the end of surgery over 15 minutes (n = 75) | |
| Outcomes | Primary outcome: blood loss: up to 72 hours (method not reported) Secondary outcomes
| |
| Notes | Abstract only with full text not expected to be published. Blood loss reported as mL/m2, so reported narratively and not included in meta‐analysis. Volume of red cells reported in mL and converted to units by assuming 1 unit to be equivalent to 300 mL. Original author contacted on 23 March 2016, 6 April 2016, and 6 July 2016, but did not respond | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Abstract: insufficient information for judgement |
| Allocation concealment | Unclear risk | Abstract: insufficient information for judgement |
| Blinding of participants and personnel | Unclear risk | Abstract: insufficient information for judgement. Reported as "double‐blinded" but without explanation of methods |
| Blinding of outcome assessors | Unclear risk | Abstract: insufficient information for judgement. Reported as "double‐blinded" but without explanation of methods |
| Incomplete outcome data | Unclear risk | Abstract: insufficient information for judgement |
| Selective outcome reporting | Unclear risk | Abstract: protocol not available |
| Other sources of bias | Unclear risk | Abstract: insufficient information for judgement |
| Methods | Type of study: 2‐arm, parallel‐group RCT (unclear if single‐centre or multi‐centre trial) Setting: cardiac surgery Country: Canada Registration: not prospectively registered | |
| Participants | Inclusion criteria: uncomplicated CABG Exclusion criteria: not reported Number of participants randomised: 62 Number of participants analysed: 59 to 62 Age: desmopressin arm: 61 ± 10 years; placebo arm: 59 ± 9 years Gender: groups reported together: 47 male, 15 female Type of surgery: all CABG requiring cardiopulmonary bypass Duration of surgery: not reported Duration of cardiopulmonary bypass: desmopressin arm: 92 ± 21 minutes; placebo arm: 89 ± 24 minutes Emergency cases: none Antiplatelet agents: desmopressin arm: 12; placebo arm: 13 Anticoagulants: not reported Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 100 mL 0.9% saline) immediately after heparin reversal over 15 minutes (n = 31) Comparator arm: placebo (50 mL 0.9% saline) immediately after heparin reversal over 15 minutes (n = 31) | |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | DDAVP was given towards the end of the procedure; therefore, intraoperative outcome was not used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Computer‐generated random number table |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | High risk | Not blinded; important as outcome includes transfusion |
| Blinding of outcome assessors | High risk | Not blinded; important for measurement of blood loss |
| Incomplete outcome data | High risk | Excluded participants who required re‐exploration for bleeding from further analysis: 2 in DDAVP group and 1 in placebo group |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | Unclear risk | Insufficient information for judgement |
| Methods | Type of study: single‐centre, 4‐arm, parallel‐group, open‐label RCT. Hemșinli 2012a reported DDAVP vs placebo. Hemșinli 2012b reported DDAVP and tranexamic acid vs tranexamic acid. Hemșinli 2012c reported DDAVP vs tranexamic acid Setting: cardiac surgery Country: Turkey Registration: not prospectively registered | |
| Participants | Inclusion criteria: emergency CABG; dual antiplatelet therapy Exclusion criteria: not reported Number of participants randomised: not reported Number of participants analysed: 20 Age: not reported Gender: not reported Type of surgery: all emergency CABG Duration of surgery: not reported Duration of cardiopulmonary bypass: not reported Emergency cases: desmopressin arm: 10; standard care arm: 10 Antiplatelet agents: desmopressin arm: dual antiplatelet therapy 10; standard care arm: dual antiplatelet therapy 10 Anticoagulants: not reported Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously (diluent and diluent volume not reported)) over 20 minutes (timing of infusion not reported) (n = 10) Comparator arm: standard care (n = 10) | |
| Outcomes | Primary outcome: total blood loss (method for measurement not reported) Secondary outcomes: volume of red cells transfused (data not reported in manuscript) | |
| Notes | Abstract only. Blood loss reported as mean (no standard deviation), so data for this outcome are reported narratively and were not included in meta‐analysis. Study investigator, Dr Altun, contacted on 28 June 2016. This study is complete and is planned for publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Abstract: insufficient information for judgement |
| Allocation concealment | Unclear risk | Abstract: insufficient information for judgement |
| Blinding of participants and personnel | High risk | Not blinded. Control arm is standard care, not placebo |
| Blinding of outcome assessors | High risk | Not blinded. Control arm is standard care, not placebo |
| Incomplete outcome data | Unclear risk | Abstract: insufficient information for judgement |
| Selective outcome reporting | High risk | Outcome data make reference to a table that is not provided |
| Other sources of bias | Unclear risk | Abstract: insufficient information for judgement |
| Methods | Type of study: single centre, 4‐arm, parallel‐group, open‐label RCT. Hemșinli 2012a reported DDAVP vs placebo. Hemșinli 2012b reported DDAVP and tranexamic acid vs tranexamic acid. Hemșinli 2012c reported DDAVP vs tranexamic acid Setting: cardiac surgery Country: Turkey Registration: not prospectively registered | |
| Participants | Inclusion criteria: emergency CABG; dual antiplatelet therapy Exclusion criteria: not reported Number of participants randomised: not reported Number of participants analysed: 20 Age: not reported Gender: not reported Type of surgery: all emergency CABG Duration of surgery: not reported Duration of cardiopulmonary bypass: not reported Emergency cases: desmopressin and tranexamic acid arm: 16; tranexamic acid arm: 18 Antiplatelet agents: desmopressin and tranexamic acid arm: 16; tranexamic acid arm: 18 Anticoagulants: not reported Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: desmopressin and tranexamic acid arm: 16; tranexamic acid arm: 18 Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously (diluent and diluent volume not reported)) over 20 minutes (timing of infusion not reported). Tranexamic acid (10 mg/kg intravenously) over 30 minutes, then 1 mg/kg for 10 hours administered at time of first skin incision (n = 16) Comparator arm: tranexamic acid (10 mg/kg intravenously) over 30 minutes, then 1 mg/kg for 10 hours administered at time of first skin incision (n = 18) | |
| Outcomes | Primary outcome: total blood loss (method for measurement not reported) Secondary outcomes: volume of red cells transfused (data not reported in manuscript) | |
| Notes | Abstract only. Blood loss reported as mean (no standard deviation), so data for this outcome are reported narratively and were not included in meta‐analysis. Study investigator, Dr Altun, contacted on 28 June 2016. This study is complete and is planned for publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Abstract: insufficient information for judgement |
| Allocation concealment | Unclear risk | Abstract: insufficient information for judgement |
| Blinding of participants and personnel | Unclear risk | Abstract: insufficient information for judgement |
| Blinding of outcome assessors | Unclear risk | Abstract: insufficient information for judgement |
| Incomplete outcome data | Unclear risk | Abstract: insufficient information for judgement |
| Selective outcome reporting | High risk | Outcome data make reference to a table that is not provided |
| Other sources of bias | Unclear risk | Abstract: insufficient information for judgement |
| Methods | Type of study: single‐centre, 4‐arm, parallel‐group, open‐label RCT. Hemșinli 2012a reported DDAVP vs placebo. Hemșinli 2012b reported DDAVP and tranexamic acid vs tranexamic acid. Hemșinli 2012c reported DDAVP vs tranexamic acid Setting: cardiac surgery Country: Turkey Registration: not prospectively registered | |
| Participants | Inclusion criteria: emergency CABG; dual antiplatelet therapy Exclusion criteria: not reported Number of participants randomised: not reported Number of participants analysed: 28 Age: not reported Gender: not reported Type of surgery: all emergency CABG Duration of surgery: not reported Duration of cardiopulmonary bypass: not reported Emergency cases: desmopressin arm: 10; tranexamic acid arm: 18 Antiplatelet agents: desmopressin arm: 10; tranexamic acid arm: 18 Anticoagulants: not reported Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: desmopressin arm: 10; tranexamic acid arm: 18 Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously (diluent and diluent volume not reported)) over 20 minutes (timing of infusion not reported) (n = 10) Comparator arm: tranexamic acid (10 mg/kg intravenously) over 30 minutes, then 1 mg/kg for 10 hours administered at time of first skin incision (n = 18) | |
| Outcomes | Primary outcome: total blood loss (method for measurement not reported) Secondary outcome: volume of red cells transfused (data not reported in manuscript) | |
| Notes | Abstract only. Blood loss reported as mean (no standard deviation), so data for this outcome are reported narratively and were not included in meta‐analysis. Study investigator, Dr Altun, contacted on 28 June 2016. This study is complete and is planned for publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Abstract: insufficient information for judgement |
| Allocation concealment | Unclear risk | Abstract: insufficient information for judgement |
| Blinding of participants and personnel | Unclear risk | Abstract: insufficient information for judgement |
| Blinding of outcome assessors | Unclear risk | Abstract: insufficient information for judgement |
| Incomplete outcome data | Unclear risk | Abstract: insufficient information for judgement |
| Selective outcome reporting | High risk | Outcome data makes reference to a table that is not provided |
| Other sources of bias | Unclear risk | Abstract: insufficient information for judgement |
| Methods | Type of study: single‐centre, 4‐arm, parallel‐group, RCT. Horrow 1991a reported DDAVP vs placebo. Horrow 1991b reported DDAVP and tranexamic acid vs tranexamic acid and placebo. Horrow 1991c reported DDAVP vs tranexamic acid Setting: cardiac surgery Country: USA Registration: not prospectively registered | |
| Participants | Inclusion criteria: elective cardiac surgery Exclusion criteria: warfarin or oestrogens within 7 days of surgery; active haematuria; serum creatinine ≥ 2 mg/dL; personal or family history of abnormal bleeding; intra‐aortic balloon counterpulsation Number of participants randomised: 84 Number of participants analysed: 82 Age: desmopressin arm: 63 ± 11 years; placebo arm: 64 ± 10 years Gender: not reported Type of surgery
Duration of surgery: not reported Duration of cardiopulmonary bypass: desmopressin arm: 92 ± 34 minutes; placebo arm: 98 ± 33 minutes Emergency cases: none Antiplatelet agents: not reported Anticoagulants: none Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: all participants Transfusion protocol: red cells transfused if haematocrit < 21%, chest tube drainage ≥ 250 mL/h, or haematocrit < 24%, with haemodynamic evidence of hypovolaemia | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously (diluent and diluent volume not reported)) after heparin reversal over 20 minutes (n = 38) Comparator arm: placebo (0.9% saline (diluent volume not reported)) (timing and speed of infusion not reported) (n = 44) | |
| Outcomes | Primary outcome: blood loss (measured by drain output) Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Quote: "A table of random numbers determined patient allocation to one of four groups" |
| Allocation concealment | Low risk | Quote: "Coded infusion bags and sealed envelopes prepared by a pharmacist not involved in the study provided double‐blinded conditions" |
| Blinding of participants and personnel | Low risk | Quote: "Coded infusion bags and sealed envelopes prepared by a pharmacist not involved in the study provided double‐blinded conditions. Actual group assignments became known months after patient participation ended" |
| Blinding of outcome assessors | Low risk | Quote: "Coded infusion bags and sealed envelopes prepared by a pharmacist not involved in the study provided double‐blinded conditions. Actual group assignments became known months after patient participation ended" |
| Incomplete outcome data | Unclear risk | 4 randomised were then excluded postoperatively; 1 developed a rash but 1 in each of the placebo and tranexamic acid groups returned to theatre, and 1 in the combined group "could not be separated from ECC" |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | Low risk | No other clear sources of bias. Supported by a grant from the Mary L Smith Charitable Lead Trust |
| Methods | Type of study: single‐centre, 4‐arm, parallel‐group RCT. Horrow 1991a reported DDAVP vs placebo. Horrow 1991b reported DDAVP and tranexamic acid vs tranexamic acid and placebo. Horrow 1991c reported DDAVP vs tranexamic acid Setting: cardiac surgery Country: USA Registration: not prospectively registered | |
| Participants | Inclusion criteria: elective cardiac surgery Exclusion criteria: warfarin or oestrogens within 7 days of surgery; active haematuria; serum creatinine ≥ 2 mg/dL; personal or family history of abnormal bleeding; intra‐aortic balloon counterpulsation Number of participants randomised: 79 Number of participants analysed: 77 Age: desmopressin and tranexamic acid arm: 63 ± 9 years; tranexamic acid and placebo arm: 65 ± 11 years Gender: not reported Type of surgery
Duration of surgery: not reported Duration of cardiopulmonary bypass: desmopressin and tranexamic acid arm: 92 ± 31 minutes; tranexamic acid and placebo arm: 87 ± 40 minutes Emergency cases: none Antiplatelet agents: not reported Anticoagulants: none Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: all participants treated with tranexamic acid 10 mg/kg loading dose over 30 minutes, then 1 mg/kg/h for 10 hours Cell salvage: all participants Transfusion protocol: red cells transfused if haematocrit < 21%, chest tube drainage ≥ 250 mL/h, or haematocrit < 24% with haemodynamic evidence of hypovolaemia | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously (diluent and diluent volume not reported)) after heparin reversal over 20 minutes. Tranexamic acid 10 mg/kg loading dose after induction of anaesthesia and before first skin incision over 30 minutes, then 1 mg/kg/h for 10 hours (n = 40) Comparator arm: placebo (0.9% saline (diluent volume not reported)) (timing and speed of infusion not reported). Tranexamic acid 10 mg/kg loading dose after induction of anaesthesia and before first skin incision over 30 minutes, then 1 mg/kg/h for 10 hours (n = 37) | |
| Outcomes | Primary outcome: blood loss (measured by drain output) Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Quote: "A table of random numbers determined patient allocation to one of four groups" |
| Allocation concealment | Low risk | Quote: "Coded infusion bags and sealed envelopes prepared by a pharmacist not involved in the study provided double‐blinded conditions" |
| Blinding of participants and personnel | Low risk | Quote: "Coded infusion bags and sealed envelopes prepared by a pharmacist not involved in the study provided double‐blinded conditions. Actual group assignments became known months after patient participation ended" |
| Blinding of outcome assessors | Low risk | Quote: "Coded infusion bags and sealed envelopes prepared by a pharmacist not involved in the study provided double‐blinded conditions. Actual group assignments became known months after patient participation ended" |
| Incomplete outcome data | Unclear risk | 4 randomised participants were excluded postoperatively; 1 developed a rash but 1 in each of placebo and tranexamic acid groups returned to theatre, and 1 in the combined group "could not be separated from ECC" |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | Low risk | No other clear sources of bias. Supported by a grant from the Mary L Smith Charitable Lead Trust |
| Methods | Type of study: single‐centre, 4‐arm, parallel‐group RCT. Horrow 1991a reported DDAVP vs placebo. Horrow 1991b reported DDAVP and tranexamic acid vs tranexamic acid and placebo. Horrow 1991c reported DDAVP vs tranexamic acid Setting: cardiac surgery Country: USA Registration: not prospectively registered | |
| Participants | Inclusion criteria: elective cardiac surgery Exclusion criteria: warfarin or oestrogens within 7 days of surgery; active haematuria; serum creatinine ≥ 2 mg/dL; personal or family history of abnormal bleeding; intra‐aortic balloon counterpulsation Number of participants randomised: 77 Number of participants analysed: 75 Age: desmopressin arm: 63 ± 11 years; tranexamic acid arm: 65 ± 11 years Gender: not reported Type of surgery
Duration of surgery: not reported Duration of cardiopulmonary bypass: desmopressin arm: 92 ± 34 minutes; tranexamic acid arm: 87 ± 40 minutes Emergency cases: none Antiplatelet agents: not reported Anticoagulants: none Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: desmopressin arm: 0; tranexamic acid arm: all participants in tranexamic acid arm treated with tranexamic acid 10 mg/kg loading dose over 30 minutes, then 1 mg/kg/h for 10 hours Cell salvage: all participants Transfusion protocol: red cells transfused if haematocrit < 21%, chest tube drainage ≥ 250 mL/h, or haematocrit < 24% with haemodynamic evidence of hypovolaemia | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously (diluent and diluent volume not reported)) after heparin reversal over 20 minutes (n = 38) Comparator arm: tranexamic acid 10 mg/kg loading dose after induction of anaesthesia and before first skin incision over 30 minutes, then 1 mg/kg/h for 10 hours (n = 37) | |
| Outcomes | Primary outcome: blood loss (measured by drain output) Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Quote: "A table of random numbers determined patient allocation to one of four groups" |
| Allocation concealment | Low risk | Quote: "Coded infusion bags and sealed envelopes prepared by a pharmacist not involved in the study provided double‐blinded conditions" |
| Blinding of participants and personnel | Low risk | Quote: "Coded infusion bags and sealed envelopes prepared by a pharmacist not involved in the study provided double‐blinded conditions. Actual group assignments became known months after patient participation ended" |
| Blinding of outcome assessors | Low risk | Quote: "Coded infusion bags and sealed envelopes prepared by a pharmacist not involved in the study provided double‐blinded conditions" |
| Incomplete outcome data | Unclear risk | 4 randomised participants were then excluded postoperatively; 1 developed a rash but 1 in each of placebo and tranexamic acid groups returned to theatre, and 1 in the combined group "could not be separated from ECC" |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | Low risk | No other clear sources of bias. Supported by a grant from the Mary L Smith Charitable Lead Trust |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: cardiac surgery Country: China Registration: not prospectively registered | |
| Participants | Inclusion criteria: undergoing elective valvular surgery; ASA classification 2‐3; no coronary heart disease or decompensated heart failure; no blood disease (no further information given); normal preoperative coagulation tests and platelet count; no anticoagulant or haemostasis treatment for 1 week before surgery Exclusion criteria: emergency or repeat surgery Number of participants randomised: 102 Number of participants analysed: 102 Age: desmopressin arm: 49 ± 10 years; placebo arm: 53 ± 9 years Gender: desmopressin arm: 21 male, 31 female; placebo arm: 21 male, 29 female Type of surgery: all undergoing elective valve replacement surgery Duration of surgery: desmopressin arm: 215 ± 67 minutes; placebo arm: 205 ± 76 minutes Duration of cardiopulmonary bypass: desmopressin arm: 110 ± 49 minutes; placebo arm: 101 ± 50 minutes Emergency cases: none Antiplatelet agents: not reported Anticoagulants: none Coagulopathy: none Thrombocytopenia: none Antifibrinolytics: all participants received tranexamic acid 30 mg/kg during surgery Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 50 mL 0.9% saline) 30 minutes before cardiac rewarming over 10 minutes (n = 52) Comparator arm: placebo (50 mL 0.9% saline) 30 minutes before cardiac rewarming over 10 minutes (n = 50) | |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information to make judgement |
| Allocation concealment | Unclear risk | Insufficient information to make judgement |
| Blinding of participants and personnel | Unclear risk | Insufficient information for judgement: "a double‐blind method was utilized", but DDAVP was infused over 10 minutes, which may have broken blinding |
| Blinding of outcome assessors | Unclear risk | Insufficient information for judgement: "a double‐blind method was utilized", but DDAVP was infused over 10 minutes, which may have broken blinding |
| Incomplete outcome data | Low risk | All participants included in final analysis |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | Unclear risk | Insufficient information to make judgement |
| Methods | Type of study: 2 separate single‐centre, 2‐arm, parallel‐group RCTs. Karnezis 1994a reported DDAVP vs placebo for participants undergoing total knee replacement. Karnezis 1994b reported DDAVP vs placebo for participants undergoing total hip replacement. Setting: orthopaedic surgery Country: USA Registration: not prospectively registered | |
| Participants | Inclusion criteria: primary total knee replacement Exclusion criteria: history of operative intervention involving hip or knee; coagulation disorder; coronary artery disease; warfarin or hearing within 7 days of procedure; bilateral or revision procedures Number of participants randomised: 36 Number of participants analysed: 36 Age: desmopressin arm: 65 ± 5.3 years; placebo arm: 66 ± 9.3 years Gender: desmopressin arm: 7 male, 10 female; placebo arm: 9 male, 10 female Type of surgery: all undergoing elective total knee replacement surgery Duration of surgery: not reported Duration of cardiopulmonary bypass: N/A Emergency cases: none Antiplatelet agents: not reported Anticoagulants: none Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not used Transfusion protocol: red cells transfused if haematocrit < 22% to 24% | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 50 mL 0.9% saline) 30 minutes before complete closure of the wound over 20 minutes (n = 17) Comparator arm: placebo (50 mL 0.9% saline) 30 minutes before complete closure of the wound over 20 minutes (n = 19) | |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | Blood loss reported graphically but not numerically, so not possible to extract data for meta‐analysis. Volume of red cells reported in mL rather than units. Converted to units with the assumption that 300 mL is equivalent to 1 unit red cells. Three participants returned to theatre for a lateral release, but it was unclear from which groups these participants came | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Quote: "allocated ... with use of a randomization table" |
| Allocation concealment | Unclear risk | Insufficient detail for judgement. Quote: "only pharmacists were aware of the treatment group" |
| Blinding of participants and personnel | Low risk | Quote: "All patients, treating physicians, and investigators collecting the data were blinded to the assigned treatment. Only the pharmacist was aware of the treatment group" |
| Blinding of outcome assessors | Low risk | Quote: "All patients, treating physicians, and investigators collecting the data were blinded to the assigned treatment. Only the pharmacist was aware of the treatment group" |
| Incomplete outcome data | High risk | Unclear which group participants who required reoperation were in. Full numerical data for blood loss not presented |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | Low risk | No other clear source of bias. Quote: "No funds were received in support of study" |
| Methods | Type of study: 2 separate single‐centre, 2‐arm, parallel‐group RCTs. Karnezis 1994a reported DDAVP vs placebo for participants undergoing total knee replacement. Karnezis 1994b reported DDAVP vs placebo for participants undergoing total hip replacement. Setting: orthopaedic surgery Country: USA Registration: not prospectively registered | |
| Participants | Inclusion criteria: primary total hip replacement Exclusion criteria: history of operative intervention involving hip or knee; coagulation disorder; coronary artery disease; warfarin or hearing within 7 days of procedure; bilateral or revision procedures Number of participants randomised: 56 Number of participants analysed: 56 Age: desmopressin arm: 65 ± 7.8 years; placebo arm: 67 ± 6.7 years Gender: desmopressin arm: 12 male, 14 female; placebo arm: 14 male, 16 female Type of surgery: all undergoing elective total hip replacement surgery Duration of surgery: not reported Duration of cardiopulmonary bypass: N/A Emergency cases: none Antiplatelet agents: not reported Anticoagulants: none Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: all participants Transfusion protocol: red cells transfused if haematocrit < 22% to 24% | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 50 mL 0.9% saline) 30 minutes before complete closure of the wound over 20 minutes (n = 26) Comparator arm: placebo (50 mL 0.9% saline) 30 minutes before complete closure of the wound over 20 minutes (n = 30) | |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | Blood loss reported graphically but not numerically, so not possible to extract data for meta‐analysis. Volume of red cells reported in mL rather than units. Converted to units assuming that 300 mL is equivalent to 1 unit red cells. Three participants returned to theatre for a lateral release, but it is unclear which groups these participants were from | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Quote: "allocated ... with use of a randomization table" |
| Allocation concealment | Unclear risk | Insufficient detail for judgement. Quote: "only pharmacists were aware of the treatment group" |
| Blinding of participants and personnel | Low risk | Quote: "All patients, treating physicians, and investigators collecting the data were blinded to the assigned treatment. Only the pharmacist was aware of the treatment group" |
| Blinding of outcome assessors | Low risk | Quote: "All patients, treating physicians, and investigators collecting the data were blinded to the assigned treatment. Only the pharmacist was aware of the treatment group" |
| Incomplete outcome data | High risk | Unclear which group participants who required reoperation were in. Full numerical data for blood loss not presented |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | Low risk | No other clear source of bias. Quote: "No funds were received in support of study" |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: orthopaedic surgery Country: Canada Registration: not prospectively registered | |
| Participants | Inclusion criteria: scheduled spinal fusion with Harrington rod instrumentation Exclusion criteria: bleeding diathesis; aspirin within 14 days; bleeding time > 9 minutes on preoperative screen Number of participants randomised: 35 Number of participants analysed: 35 Age: desmopressin arm: 14.8 ± 3.3 years; placebo arm: 15.3 ± 1.8 years Gender: desmopressin arm: 8 male, 9 female; placebo arm: 7 male, 11 female Type of surgery: all undergoing spinal fusion with Harrington rod instrumentation Duration of surgery: desmopressin arm: 178 minutes (mean); placebo arm: 177 minutes (mean) Duration of cardiopulmonary bypass: N/A Emergency cases: none Antiplatelet agents: none Anticoagulants: not reported Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (10 μg/m2 body surface area intravenously in 0.5 μg/mL (diluent not reported)) immediately after induction of anaesthesia over 20 minutes (n = 17) Comparator arm: placebo (type of placebo and volume not reported) immediately after induction of anaesthesia over 20 minutes (n = 18) | |
| Outcomes | Primary outcome: blood loss intraoperatively and total blood loss (measured by weighing surgical sponges and suction drainage) Secondary outcome: volume of red cells transfused intraoperatively | |
| Notes | All participants given DDAVP 3 days before surgery to assess its effects in addition to DDAVP/placebo immediately before surgery. Blood loss perioperatively and volume of red cells transfused reported as mean and mean difference with 95% confidence intervals. Standard deviations for each outcome have been calculated from these data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Unclear risk | Quote: "The medication was sent to the operating room in a syringe labelled "spinal fusion study medication ...", "Neither the surgeon nor the anaesthetist was aware of the treatment groups to which patients were assigned" Insufficient detail provided about volume and type of placebo for judgement |
| Blinding of outcome assessors | Unclear risk | Quote: "The medication was sent to the operating room in a syringe labelled "spinal fusion study medication...", "Neither the surgeon nor the anaesthetist was aware of the treatment groups to which patients were assigned" |
| Incomplete outcome data | Low risk | All participants included in final analysis (although no data for 5 in each group for duration of surgery). Data missing from 1 participant who received DDAVP and 1 who received placebo for red cell transfusions. No explanation given. However, this represented < 10% total |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | Unclear risk | Desmopressin was provided by Richmond Pharmaceuticals Inc, but unclear if company had a role in study design |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: cardiac surgery Country: Finland Registration: not prospectively registered | |
| Participants | Inclusion criteria: elective primary CABG Exclusion criteria: previous cardiac surgery; coagulation disorder; coumarin anticoagulant, heparin or acetylsalicylic acid within 5 days of surgery Number of participants randomised: 33 Number of participants analysed: 30 Age: desmopressin arm: 57 ± 9 years; placebo arm: 59 ± 5 years Gender: desmopressin arm: 14 male, 1 female; placebo arm: 14 male, 1 female Type of surgery: all undergoing primary CABG Duration of surgery: desmopressin arm: 247 ± 42 minutes; placebo arm: 244 ± 39 minutes Duration of cardiopulmonary bypass: desmopressin arm: 94 ± 19 minutes; placebo arm: 103 ± 23 minutes Emergency cases: none Antiplatelet agents: none Anticoagulants: none Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: red cells transfused if haematocrit < 20% during cardiopulmonary bypass, and if haematocrit < 30% postoperatively | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 100 mL 0.9% saline) immediately after sternal closure over 15 minutes (n = 15) Comparator arm: placebo (100 mL 0.9% saline) immediately after sternal closure over 15 minutes (n = 15) | |
| Outcomes | Primary outcome: laboratory measures of haemostasis Secondary outcomes
| |
| Notes | Volume of red cells transfused reported as mean and range, so reported narratively and not included in meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Unclear risk | Insufficient information for judgement. Study reported it was double‐blind but without giving details |
| Blinding of outcome assessors | Unclear risk | Insufficient information for judgement. Study reported it was double‐blind but without giving details |
| Incomplete outcome data | High risk | Three participants excluded after randomisation without justification in the protocol: 1 in the placebo arm died, and 2 in the DDAVP arm had significant complications |
| Selective outcome reporting | Unclear risk | No protocol available |
| Other sources of bias | Low risk | No other clear sources of bias. The study was supported by grants from the Paulo Foundation and Helsinki University Central Hospital |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: type of surgery not reported Country: USA Registration: not prospectively registered | |
| Participants | Inclusion criteria: undergoing a surgical procedure with expected blood loss < 750 mL Exclusion criteria: none reported Number of participants randomised: not reported Number of participants analysed: 23 Age: not reported Gender: not reported Type of surgery: not reported Duration of surgery: not reported Duration of cardiopulmonary bypass: not reported Emergency cases: not reported Antiplatelet agents: not reported Anticoagulants: not reported Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 10 mL 0.9% saline) after anaesthetic induction (duration of infusion not reported) (n = 12) Comparator arm: placebo (10 mL 0.9% saline) after anaesthetic induction (duration of infusion not reported) (n = 11) | |
| Outcomes | Primary outcome: laboratory measures of haemostasis Secondary outcomes: none reported | |
| Notes | No outcomes of interest to this review were reported in this trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Unclear risk | Insufficient information for judgement |
| Blinding of outcome assessors | Unclear risk | Insufficient information for judgement |
| Incomplete outcome data | Unclear risk | Insufficient information for judgement |
| Selective outcome reporting | Unclear risk | No protocol available |
| Other sources of bias | Unclear risk | Insufficient information for judgement |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: dialysis catheter insertion Country: South Korea Registration: not prospectively registered | |
| Participants | Inclusion criteria: uraemic patients who had not yet started dialysis; undergoing dialysis catheter insertion; prolonged closure time on platelet function analyser‐100 Exclusion criteria: chronic liver disease; infectious diseases (not specified); drugs that interfere with platelet function within 10 days of entering study Number of participants randomised: 48 Number of participants analysed: 48 Age: desmopressin arm: median 60 (range 28‐93) years; placebo arm: median 57 (range 27‐66) years Gender: desmopressin arm: 16 male, 8 female; placebo arm: 15 male, 9 female Type of surgery: all undergoing catheter insertion for dialysis Duration of surgery: not reported Duration of cardiopulmonary bypass: N/A Emergency cases: not reported Antiplatelet agents: none Anticoagulants: not reported Coagulopathy: not reported Thrombocytopenia: none Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 50 mL 0.9% saline) over 30 minutes (timing of infusion not clear) (n = 24) Comparator arm: placebo (50 mL 0.9% saline) over 30 minutes (timing of infusion not clear) (n = 24) | |
| Outcomes | Primary outcome: laboratory measures of haemostasis Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | High risk | No information on blinding; probably an open‐label trial. Outcomes relatively subjective and could be biased by revealing treatment |
| Blinding of outcome assessors | High risk | No information on blinding; probably an open‐label trial. Outcomes relatively subjective and could be biased by revealing treatment |
| Incomplete outcome data | Low risk | All participants included in final analysis |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | Low risk | No other clear source of bias. This study was supported by Baxter Korea (not a manufacturer of DDAVP) |
| Methods | Type of study: single‐centre, 3‐arm, parallel‐group RCT Setting: orthopaedic surgery Country: Finland Registration: not prospectively registered | |
| Participants | Inclusion criteria: seropositive rheumatoid arthritis; scheduled for total hip arthroplasty under spinal anaesthesia Exclusion criteria: revision arthroplasty; contraindications for spinal anaesthesia; hepatic malfunction assessed by "thorough anamnesis"; renal malfunction assessed by serum creatinine; "anamnestic" or diagnosed coagulation disorder; warfarin treatment; any treatment other than acetylsalicylic acid or NSAIDs affecting thrombocyte function or other components of coagulation; later excluded 4 participants who experienced intraoperative surgical problems with ensuing blood loss over 400 mL Number of participants randomised: 75 Number of participants analysed: 71 Age: desmopressin (0.2 μg/kg) arm: 62 ± 13 years; desmopressin (0.4 μg/kg) arm: 59 ± 13 years; placebo arm: 61 ± 13 years Gender: desmopressin (0.2 μg/kg) arm: 14 male, 10 female; desmopressin (0.4 μg/kg) arm: 11 male, 12 female; placebo arm: 17 male, 7 female Type of surgery: all undergoing total hip replacement Duration of surgery: desmopressin (0.2 μg/kg) arm: 102 ± 27 minutes; desmopressin (0.4 μg/kg) arm: 104 ± 24 minutes; placebo arm: 107 ± 33 minutes Duration of cardiopulmonary bypass: N/A Emergency cases: none Antiplatelet agents: none Anticoagulants: none Coagulopathy: none Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: red cells transfused if haemoglobin < 90 g/L | |
| Interventions | Intervention arm 1: DDAVP (0.2 µg/kg intravenously in 50 mL 0.9% saline) at start of surgery over 30 minutes (n = 24) Intervention arm 2: DDAVP (0.4 µg/kg intravenously in 50 mL 0.9% saline) at start of surgery over 30 minutes (n = 23) Comparator arm: placebo (50 mL 0.9% saline) at start of surgery over 30 minutes (n = 24) | |
| Outcomes | Primary outcome: total blood loss (measured by estimating blood loss from surgical swabs and suction drainage) Secondary outcomes
| |
| Notes | Intraoperative data for mean blood loss and volume of red cells transfused estimated from figure. Standard deviation not available, so results reported narratively. DDAVP (0.4 µg/kg) arm used for comparison versus placebo in analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Treatment assignment was determined using a randomisation list |
| Allocation concealment | Low risk | Sealed envelopes |
| Blinding of participants and personnel | Low risk | Study drugs were randomised and prepared by an independent pharmacist, and the study code was stored at the pharmacy department. All participants, personnel, and investigators were blinded to treatment assignment for the duration of the study. Placebo was prepared in identical volume and syringe |
| Blinding of outcome assessors | Low risk | Study drugs were randomised and prepared by an independent pharmacist, and the study code was stored at the pharmacy department. All participants, personnel, and investigators were blinded to treatment assignment for the duration of the study |
| Incomplete outcome data | Unclear risk | Quote: "Four patients had to be excluded during the study due to intraoperative surgical problems with ensuing blood loss over 400 mL, including one from the D 0.2 group [DDAVP 0.2 µg/kg group] due to arterial damage, one from the placebo group due to femoral fracture and two from the D 0.4 group [DDAVP 0.4 µg/kg group] due to other complications with acetabular cup. Thus, all the statistical analyses were based on 71 patients" Judgement comment: an intention‐to‐treat analysis was not used, but only 4 participants were excluded for surgical reasons |
| Selective outcome reporting | Unclear risk | No protocol available to ensure all prespecified outcomes reported |
| Other sources of bias | Unclear risk | Insufficient information for judgement |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: vascular surgery Country: Sweden Registration: not prospectively registered | |
| Participants | Inclusion criteria: elective surgery aortoiliac graft surgery for aortoiliac occlusive disease or aneurysms Exclusion criteria: history of increased bleeding tendency; prolonged preoperative bleeding time; acetylsalicylic acid within 10 days before surgery Number of participants randomised: 50 Number of participants analysed: 50 Age: not reported Gender: desmopressin arm: 19 male, 6 female; placebo arm: 18 male, 7 female Type of surgery
Duration of surgery: desmopressin arm: 197 ± 62 minutes; placebo arm: 215 ± 93 minutes Duration of cardiopulmonary bypass: N/A Emergency cases: none Antiplatelet agents: none Anticoagulants: none Coagulopathy: none Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: red cells transfused if haematocrit < 30% | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 10 mL 0.9% saline) immediately before the start of the operation over 10 minutes (n = 25) Comparator arm: placebo (10 mL 0.9% saline) immediately before the start of the operation over 10 minutes (n = 25) | |
| Outcomes | Primary outcome: blood loss: intraoperatively and in total (measured by estimating blood loss in surgical swabs, suction bottles, and drain output) Secondary outcomes
| |
| Notes | Volume of red cells transfused reported as mL, so converted to units based on the assumption that 1 unit is equivalent to 300 mL. One death occurred, but the study arm in which it occurred was not reported. Death caused by rupture of part of the upper anastomosis of a repair of an abdominal aortic aneurysm, on the sixth postoperative day | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Method of sequence generation not reported |
| Allocation concealment | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and personnel | Unclear risk | DDAVP given over 10 minutes and likely to cause facial flushing, resulting in loss of blinding. The abstract states that this was a double‐blind placebo‐controlled study and reported no further details |
| Blinding of outcome assessors | Unclear risk | DDAVP given over 10 minutes and likely to cause facial flushing, resulting in loss of blinding. The abstract states that this was a double‐blind placebo‐controlled study and reported no further details. Assessor for preoperative bleeding was the anaesthetist who was "taking note of suction bottles and counting swabs" |
| Incomplete outcome data | High risk | Quote: "six patients in whom surgical complications significantly contributed to blood loss and transfusion requirements were identified and excluded" |
| Selective outcome reporting | High risk | Quote: "The aim of our study was to investigate if desmopressin given to patients without a history of increased bleeding tendency undergoing aorto‐iliac graft surgery could reduce blood loss and the transfusion requirement. We also aimed to analyse changes in factor VIII/von Willebrand factor (FVIII/vWF) levels and to monitor the safety profile of desmopressin in relation to blood loss" |
| Other sources of bias | Low risk | No other clear sources of bias. Supported by grants from the Swedish Medical Research Council |
| Methods | Type of study: 2‐arm, parallel‐group RCT (unclear whether single‐centre or multi‐centre trial) Setting: orthopaedic surgery Country: Canada Registration: not prospectively registered | |
| Participants | Inclusion criteria: paediatric patients undergoing spine fusion for neuromuscular scoliosis Exclusion criteria: not reported Number of participants randomised: 30 Number of participants analysed: 30 Age: desmopressin arm: 13.4 ± 2.1 years; placebo arm: 13.8 ± 2.8 years Gender: desmopressin arm: 9 male, 7 female; placebo arm: 9 male, 5 female Type of surgery: all undergoing spine fusion Duration of surgery: not reported Duration of cardiopulmonary bypass: N/A Emergency cases: none Antiplatelet agents: not reported Anticoagulants: not reported Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (10 μg/m2 body surface area (route of administration, diluent, and volume of diluent not reported)) immediately after induction of anaesthesia (duration of infusion not reported) (n = 16) Comparator arm: placebo (0.9% saline (volume not reported)) immediately after induction of anaesthesia (duration of infusion not reported) (n = 14) | |
| Outcomes | Primary outcome: blood loss: intraoperatively (measured by estimating blood loss in surgical sponges and suction drainage) Secondary outcomes
| |
| Notes | Perioperative blood loss and perioperative volume of red cells transfused did not distinguish between outcomes before and after administration of DDAVP/placebo. Consequently, these outcomes could not be included in meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Unclear risk | Insufficient information for judgement. Surgical team reported to be blinded to treatment, but no details provided |
| Blinding of outcome assessors | Unclear risk | Insufficient information for judgement |
| Incomplete outcome data | Unclear risk | Insufficient information for judgement |
| Selective outcome reporting | Unclear risk | No protocol available |
| Other sources of bias | Unclear risk | Scoliosis on average 13 degrees more severe in DDAVP group. Desmopressin was supplied by UpJohn Co of Toronto, but unclear if they had a role in study design |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: kidney biopsy Country: Italy Registration: not prospectively registered | |
| Participants | Inclusion criteria: undergoing percutaneous ultrasound‐guided biopsy of the native kidney in the Bari renal unit; aged 16 to 80 years; blood pressure 140/90 mmHg with or without antihypertensive therapy; serum creatinine level < 1.5 mg/dL and/or estimated glomerular filtration rate (GFR) < 60 mL/min/1.73 m2 (calculated by the Modification of Diet in Renal Disease (MDRD) study equation); normal coagulation parameters (bleeding time evaluated by the Simplate method, with values for prothrombin time, partial thromboplastin time, platelets, and fibrinogen in the reference range) Exclusion criteria: solitary kidney; kidney cancer; hydro‐/pyonephrosis; significantly decreased kidney size on ultrasound image; severe obesity (body mass index 30 kg/m2); acute kidney injury Medications that could interfere with haemostasis were withdrawn before the procedure: antiplatelet agents at least 7 days before and heparins 1 to 2 days before the kidney biopsy Number of participants randomised: 162 Number of participants analysed: 162 Age: desmopressin arm: 39.5 ± 14.2 years; placebo arm: 41.7 ± 15 years Gender: desmopressin arm: 45 male, 35 female; placebo arm: 43 male, 39 female Type of surgery: all undergoing percutaneous ultrasound‐guided renal biopsy Duration of surgery: desmopressin arm: median 2 passes (IQR 2 to 3); placebo arm: median 2 passes (IQR 2 to 3) Duration of cardiopulmonary bypass: N/A Emergency cases: none Antiplatelet agents: none Anticoagulants: none Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg subcutaneously (volume and diluent not reported)) 1 hour before the biopsy (duration of infusion not reported) (n = 80) Comparator arm: placebo (1 mL 0.9% saline subcutaneously) 1 hour before the biopsy (duration of infusion not reported) (n = 82) | |
| Outcomes | Primary outcome: number of participants with any bleeding (number of participants with a haematoma ≥ 20 mm diameter or haematuria) Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Quote: "1:1 allocation assignment sequence was generated using random‐number tables; a list divided into blocks of 10 was adequately concealed to prevent attempts to subvert randomisation. Block randomisation was by a computer‐generated random number list prepared by an investigator with no clinical involvement in the trial" |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Unclear risk | A placebo was used and therapy was administered by a nurse not involved in the study, however, it was given subcutaneously leading to risk of facial flushing. It is unclear whether other members of the research team were present when the drug was administered. Facial flushing was not reported as an adverse event, so it is unclear whether it occurred |
| Blinding of outcome assessors | Low risk | Ultrasonographer blinded to randomisation |
| Incomplete outcome data | Low risk | Quote: "All patients were analyzed for the primary and secondary outcomes" |
| Selective outcome reporting | Unclear risk | No protocol available to assess whether all outcomes reported |
| Other sources of bias | Low risk | No other obvious sources of bias. No financial support for the study |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: abdominal, breast, and orthopaedic surgery Country: Netherlands Registration: prospectively registered (ISRCTN10353850) | |
| Participants | Inclusion criteria: > 18 years old; taking a serotonergic antidepressant (fluvoxamine, fluoxetine, paroxetine, sertraline, venlafaxine, lomipramine, citalopram) for at least 2 weeks; undergoing orthopaedic, abdominal, or breast surgery Exclusion criteria: no informed consent; primary haemostasis disorder; hyponatraemia (sodium (serum) < 130 mmol/L); laparoscopic surgery; use of vitamin K antagonists, aspirin, iron supplements, methotrexate, or heparin; acute coronary syndrome (unstable angina or myocardial infarction); spinal anaesthesia during surgery Number of participants randomised: 28 Number of participants analysed: 28 Age: desmopressin arm: 54.2 ± 14.9 years; placebo arm: 49.1 ± 12.1 years Gender: desmopressin arm: 0 male, 14 female; placebo arm: 2 male, 12 female Type of surgery
Duration of surgery: not reported Duration of cardiopulmonary bypass: N/A Emergency cases: none Antiplatelet agents: none Anticoagulants: none Coagulopathy: none Thrombocytopenia: none Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (15 μg if body weight < 50 kg; 30 μg if body weight 50 kg to 100 kg; and 45 μg if body weight > 100 kg (route of administration, diluent, and diluent volume not reported)) (timing and duration of infusion not reported) (n = 14) Comparator arm: placebo (0.9% saline (volume and route of administration not reported) (timing and duration of infusion not reported) (n = 14) | |
| Outcomes | Primary outcome: intraoperative blood loss (measured by estimating blood loss in surgical gauze and drain output) Secondary outcomes: number of participants receiving a red cell transfusion intraoperatively | |
| Notes | Contacted original author, Dr Susanne Marczinski, on 25 February 2016 and 3 March 2016. She provided a published version of the trial in Dutch but was not able to supply any unpublished data. The paper was translated from Dutch into English by Michiel ten Hove. Perioperative blood loss was reported as mean and range. This outcome is reported narratively in this review and was not included in meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Low risk | Both the placebo and the desmopressin ampoule were produced by the production department of the hospital and provided the same label. Because of this, no one at the surgical department knew if participants were randomised to treatment with placebo or with desmopressin |
| Blinding of outcome assessors | Low risk | Both the placebo and the desmopressin ampoule were produced by the production department of the hospital and provided the same label. Because of this, no one at the surgical department knew if participants were randomised to treatment with placebo or with desmopressin |
| Incomplete outcome data | Low risk | All participants included in the final analysis, but this was an interim analysis and it is unclear why the trial was stopped before all participants were recruited |
| Selective outcome reporting | Low risk | All prespecified outcomes from protocol included in final manuscript |
| Other sources of bias | High risk | Plan from protocol was to recruit 45 participants; final publication of results stopped at 28 participants (62% of planned recruitment number). Timing of DDAVP administration unclear. Speed of DDAVP administration (and whether this would have led to facial flushing) also unclear. Sponsored by Hospital Geldersei Vallei |
| Methods | Type of study: single‐centre, 3‐arm, parallel‐group RCT Setting: cardiac surgery Country: USA Registration: not prospectively registered | |
| Participants | Inclusion criteria: CABG without prior cardiac surgery; no aspirin, NSAIDs, coumarin, or heparin administration (10 days before surgery) Exclusion criteria: mediastinal exploration for surgical bleeding or haemodynamic instability pre‐CPB or post‐CPB, but before DDAVP administration Number of participants randomised: 70 Number of participants analysed: 65 Age: desmopressin (2 doses) arm: 63.6 ± 1.8 years; desmopressin (1 dose) arm: 59.8 ± 1.2 years; placebo arm: 61.7 ± 1.5 years Gender: not reported Type of surgery: all undergoing elective CABG Duration of surgery: not reported Duration of cardiopulmonary bypass: desmopressin (2 doses) arm: 85.1 ± 9.2 minutes; desmopressin (1 dose) arm: 88.6 ± 9.4 minutes; placebo arm: 91.3 ± 10.7 minutes Emergency cases: none Antiplatelet agents: none Anticoagulants: none Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: red cells transfused if haemoglobin < 100 g/L | |
| Interventions | Intervention arm 1: DDAVP (0.3 μg/kg intravenously (volume and diluent not reported)) immediately after heparin reversal and again 12 hours postoperatively (duration of infusion not reported) (n = 22) Intervention arm 2: DDAVP (0.3 μg/kg intravenously (volume and diluent not reported)) immediately after heparin reversal (duration of infusion not reported). Placebo (0.9% saline (volume not reported) 12 hours postoperatively (duration of infusion not reported) (n = 21) Comparator arm: placebo (0.9% saline (volume not reported)) immediately after heparin reversal and again 12 hours postoperatively (duration of infusion not reported) (n = 22) | |
| Outcomes | Primary outcome: total blood loss (measured by estimating blood loss in surgical sponges and suction drainage) Secondary outcomes
| |
| Notes | DDAVP (1 dose) group used for comparison vs placebo for main analysis. Blood loss and volume of red cells transfused reported as medians. These outcomes are reported narratively in this review and were not included in meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Unclear risk | Quote: "patients ... randomized into 3 blinded groups" but no further details given |
| Blinding of outcome assessors | Unclear risk | Quote: "patients ... randomized into 3 blinded groups" but no further details given |
| Incomplete outcome data | High risk | Quote: "three patients excluded after randomisation for mediastinal exploration secondary to surgical bleeding" |
| Selective outcome reporting | Unclear risk | No protocol available |
| Other sources of bias | High risk | Study discontinued prematurely without appropriate rationale |
| Methods | Type of study: 2 separate single‐centre, 2‐arm, parallel‐group RCTs. Separated according to post‐cardiopulmonary bypass thromboelastography maximum amplitude (MA). Mongan 1992a reported those with MA > 50 mm and Mongan 1992b reported those with MA ≤ 50 mm Setting: cardiac surgery Country: USA Registration: not prospectively registered | |
| Participants | Inclusion criteria: elective primary CABG Exclusion criteria: preoperative anticoagulation/aspirin within 1 week of surgery; re‐exploration due to surgical bleeding; postoperative evidence of fibrinolysis Number of participants randomised: 3 participants excluded between Mongan 1992a and Mongan 1992b before administration of DDAVP or placebo Number of participants analysed: 86 Age: desmopressin arm: 61.5 ± 9.3 years; placebo arm: 60.9 ± 9.7 years Gender: desmopressin arm: 40 male, 4 female; placebo arm: 36 male, 6 female Type of surgery: all undergoing elective CABG Duration of surgery: not reported Duration of cardiopulmonary bypass: desmopressin arm: 127.6 ± 36.9 minutes; placebo arm: 131.7 ± 31.9 minutes Emergency cases: none Antiplatelet agents: none Anticoagulants: none Coagulopathy: all had thromboelastography MA > 50 mm. No other information on coagulopathies Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: red cells transfused if haematocrit < 24% | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 50 mL 0.9% saline) after heparin reversal and before chest closure over 15 minutes (n = 44) Comparator arm: placebo (50 mL 0.9% saline) after heparin reversal and before chest closure over 15 minutes (n = 42) | |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | Volume of red cells transfused reported as absolute number of red cells. Mean volume of red cells transfused was calculated from this. This outcome is presented narratively and was not included in meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Computer‐generated random number table |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Unclear risk | Insufficient information for judgement |
| Blinding of outcome assessors | Unclear risk | Insufficient information for judgement |
| Incomplete outcome data | Low risk | Three excluded ‐ 1 for fibrinolysis, 2 for surgical bleeding before administration of DDAVP/placebo ‐ prespecified in protocol |
| Selective outcome reporting | Unclear risk | No protocol available |
| Other sources of bias | Unclear risk | Insufficient information for judgement |
| Methods | Type of study: 2 separate single‐centre, 2‐arm, parallel‐group RCTs. Separated according to post‐CPB thromboelastography maximum amplitude (MA). Mongan 1992a reported those with MA > 50 mm and Mongan 1992b reported those with MA ≤ 50 mm Setting: cardiac surgery Country: USA Registration: not prospectively registered | |
| Participants | Inclusion criteria: elective primary CABG Exclusion criteria: preoperative anticoagulation/aspirin within 1 week of surgery; re‐exploration due to surgical bleeding; postoperative evidence of fibrinolysis Number of participants randomised: 3 participants excluded between Mongan 1992a and Mongan 1992b before administration of DDAVP or placebo Number of participants analysed: 29 Age: desmopressin arm: 58.8 ± 10.7 years; placebo arm: 65.8 ± 10 years Gender: desmopressin arm: 9 male, 4 female; placebo arm: 11 male, 5 female Type of surgery: all undergoing elective CABG Duration of surgery: not reported Duration of cardiopulmonary bypass: desmopressin arm: 131.3 ± 41.2 minutes; placebo arm: 136.1 ± 37.3 minutes Emergency cases: none Antiplatelet agents: none Anticoagulants: none Coagulopathy: all had thromboelastography MA ≤ 50 mm. No other information on coagulopathies Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: red cells transfused if haematocrit < 24% | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 50 mL 0.9% saline) after heparin reversal and before chest closure over 15 minutes (n = 13) Comparator arm: placebo (50 mL 0.9% saline) after heparin reversal and before chest closure over 15 minutes (n = 16) | |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | Volume of red cells transfused reported as absolute number of red cells. Mean volume of red cells transfused was calculated from this. This outcome is presented narratively and was not included in meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Computer‐generated random number table |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Unclear risk | Insufficient information for judgement |
| Blinding of outcome assessors | Unclear risk | Insufficient information for judgement |
| Incomplete outcome data | Low risk | Three excluded ‐ 1 for fibrinolysis, 2 for surgical bleeding before administration of DDAVP/placebo ‐ prespecified in protocol |
| Selective outcome reporting | Unclear risk | No protocol available |
| Other sources of bias | Unclear risk | Insufficient information for judgement |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: paediatric cardiac surgery Country: USA Registration: not prospectively registered | |
| Participants | Inclusion criteria: < 40 years old; undergoing complex congenital heart operation requiring cardiopulmonary bypass Exclusion criteria: operation expected to have minimal blood loss; pre‐existing bleeding disorder Number of participants randomised: 60 Number of participants analysed: 60 Age: desmopressin arm: 13.9 ± 10 years; placebo arm: 18.1 ± 9.8 years Gender: desmopressin arm: 15 male, 16 female; placebo arm: 11 male, 18 female Type of surgery
Duration of surgery: desmopressin arm: 450 ± 145 minutes; placebo arm: 447.4 ± 95.6 minutes Duration of cardiopulmonary bypass: desmopressin arm: 141.2 ± 79.8 minutes; placebo arm: 144.2 ± 44.6 minutes Emergency cases: none Antiplatelet agents: desmopressin arm: 3; placebo arm: 1 Anticoagulants: desmopressin arm: 2; placebo arm: 2 Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: no protocol. Decisions about transfusion made postoperatively by staff cardiothoracic surgeon | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 50 mL (10 mL if weight < 10 kg) 0.9% saline) 10 minutes after heparin reversal and after aPTT returned to within 10% of normal over 20 to 30 minutes (n = 31) Comparator arm: placebo (50 mL (10 mL if weight < 10 kg) 0.9% saline) 10 minutes after heparin reversal and after aPTT returned to within 10% of normal over 20 to 30 minutes (n = 29) | |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | Blood loss measured in mL/m2, so this outcome is reported narratively and was not included in meta‐analysis. Transfusion requirements reported in units, so not possible to combine with other paediatric cases; results are reported narratively and were not included in meta‐analysis. Blood loss and transfusion requirements extracted from figures in the original paper (numbers not given in manuscript) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Participants were randomised to 1 of 2 treatment groups (DDAVP or placebo) in blocks of 6 with the use of a random number table with stratification on the basis of previous sternotomy (re‐do) or not (primary) |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Low risk | Only the pharmacist was aware of the solution's identity |
| Blinding of outcome assessors | Low risk | Blinded personnel were also outcome assessors |
| Incomplete outcome data | Low risk | All participants were accounted for in final analysis |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | Unclear risk | Insufficient information for judgement |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: cardiac surgery Country: Turkey Registration: not prospectively registered | |
| Participants | Inclusion criteria: undergoing elective CABG Exclusion criteria: emergency surgery; haemostatic defect; hypertension; diabetes; renal failure Number of participants randomised: 66 Number of participants analysed: 66 Age: desmopressin arm: 59.0 ± 11.5 years; placebo arm: 58.1 ± 12.1 years Gender: desmopressin arm: 24 male, 9 female; placebo arm: 23 male, 10 female Type of surgery: all undergoing elective CABG Duration of surgery: not reported Duration of cardiopulmonary bypass: desmopressin arm: 73.2 ± 32.14 minutes; placebo arm: 65.1 ± 26.72 minutes Emergency cases: none Antiplatelet agents: none Anticoagulants: not reported Coagulopathy: none Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not used Transfusion protocol: red cells transfused if haematocrit < 28% | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 50 mL 0.9% saline) "soon" after heparin reversal over 20 minutes (n = 33) Comparator arm: placebo (50 mL 0.9% saline) "soon" after heparin reversal over 20 minutes (n = 33) | |
| Outcomes | Primary outcome: postoperative blood loss (measured by drain output) Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Quote: "prospectively randomized and allocated equally"; no further details |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Unclear risk | Insufficient information for judgement |
| Blinding of outcome assessors | Unclear risk | Insufficient information for judgement |
| Incomplete outcome data | Low risk | All participants included in final analysis |
| Selective outcome reporting | Unclear risk | Insufficient information for judgement |
| Other sources of bias | Unclear risk | Insufficient information for judgement |
| Methods | Type of study: 2‐arm, parallel‐group RCT (unclear whether single‐centre or multi‐centre study) Setting: cardiac surgery Country: Norway Registration: not prospectively registered | |
| Participants | Inclusion criteria: stable angina pectoris; elective first‐time CABG; taking aspirin Exclusion criteria: treatment with heparin or low molecular weight heparin, oral anticoagulants, NSAIDs or other platelet inhibitors Number of participants randomised: 100 Number of participants analysed: 92 Age: desmopressin arm: 63.1 ± 8.6 years; placebo arm: 64.4 ± 8 years Gender: desmopressin arm: 40 male, 6 female; placebo arm: 36 male, 10 female Type of surgery: all undergoing elective CABG Duration of surgery: desmopressin arm: 133 ± 27 minutes; placebo arm: 135 ± 31 minutes Duration of cardiopulmonary bypass: desmopressin arm: 59 ± 16 minutes; placebo arm: 56 ± 20 minutes Emergency cases: none Antiplatelet agents: all participants were taking aspirin Anticoagulants: none Coagulopathy: none Thrombocytopenia: none Antifibrinolytics: desmopressin arm: 2 g tranexamic acid 3; placebo arm: 2 g tranexamic acid 8 Cell salvage: all participants Transfusion protocol: red cell transfusion if haematocrit < 25% | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 20 mL 0.9% saline) immediately after heparin reversal over 10 minutes (n = 46) Comparator arm: placebo (20 mL 0.9% saline) immediately after heparin reversal over 10 minutes (n = 46) | |
| Outcomes | Primary outcome: blood loss postoperatively (measured by drain output) Secondary outcomes
| |
| Notes | Volume of blood transfused reported as number of blood product donor exposures per participant. This outcome was not included in the analysis for this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Quote: "The Unit for Applied Clinical Research at the Norwegian University of Science and Technology randomized the patients into two groups by means of a computer program, one group receiving desmopressin and the other group receiving placebo" |
| Allocation concealment | Unclear risk | Insufficient information for judgement. Delivered in identical syringes; computer‐generated randomised allocation sequence by independent agent |
| Blinding of participants and personnel | Unclear risk | Quote: "Desmopressin 15 g/mL or placebo was prepared at the hospital pharmacy and delivered in identical 20 mL syringes. The desmopressin group received desmopressin 0.3 g/kg, and the placebo group received a corresponding volume of a 0.9% sodium chloride solution. The injections were given over 10 min at the end of cardiopulmonary bypass (CPB), immediately after the administration of protamine sulfate to neutralize heparin" |
| Blinding of outcome assessors | Low risk | Assessors were blinded |
| Incomplete outcome data | High risk | Did not perform an intention‐to‐treat analysis. Excluded 8 participants in total, including 3 post randomisation for bleeding (deemed surgical) |
| Selective outcome reporting | Unclear risk | No protocol available |
| Other sources of bias | Unclear risk | Imbalance in number of participants treated with tranexamic acid between the 2 groups, although this did not reach statistical significance. Supported by a Norwegian Health Association grant |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: cardiac surgery Country: USA Registration: not prospectively registered | |
| Participants | Inclusion criteria: adults scheduled to undergo elective myocardial revascularisation or single‐valve replacement surgery; left ventricular ejection fraction > 0.40; normal preoperative coagulation profile (prothrombin time, partial thromboplastin time, platelet count, fibrinogen, template bleeding time) Exclusion criteria: haemodynamic instability; preoperative heparin therapy within 48 hours; refusal of blood products (Jehovah’s Witnesses) Participants on aspirin preparations continued to receive aspirin until the evening before surgery Number of participants randomised: 27 Number of participants analysed: 27 Age: desmopressin arm: 60 ± 16 years; placebo arm: 55 ± 15 years Gender: desmopressin arm: 12 male, 2 female; placebo arm: 9 male, 4 female Type of surgery: desmopressin arm: 9 CABG, 5 valve replacements; placebo arm: 8 CABG, 5 valve replacements Duration of surgery: not reported Duration of cardiopulmonary bypass: desmopressin arm: 118 ± 30 minutes; placebo arm: 123 ± 26 minutes Emergency cases: none Antiplatelet agents: desmopressin arm: 4; placebo arm: 5 Anticoagulants: not reported Coagulopathy: none Thrombocytopenia: none Antifibrinolytics: not reported Cell salvage: all participants Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 0.9% saline (volume not reported)) 15 minutes after heparin reversal over 10 minutes (n = 14) Comparator arm: placebo (0.9% saline (volume not reported) 15 minutes after heparin reversal over 10 minutes (n = 13) | |
| Outcomes | Primary outcome: clinically significant hypotension Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Unclear risk | Insufficient information for judgement. "Patients were randomized to receive a blinded infusion", but DDAVP was given over 10 minutes, which may have broken blinding |
| Blinding of outcome assessors | Unclear risk | Insufficient information for judgement. "Patients were randomized to receive a blinded infusion", but DDAVP was given over 10 minutes, which may have broken blinding |
| Incomplete outcome data | Low risk | All participants included in final analysis |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | Unclear risk | Insufficient information for judgement |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: paediatric cardiac surgery Country: USA Registration: not prospectively registered | |
| Participants | Inclusion criteria: paediatric patients ranging in age from 1 day to 16 years of age; scheduled for cardiac operations Exclusion criteria: not reported Number of participants randomised: 112 Number of participants analysed: 95 Age: desmopressin arm: 27 ± 43 months; placebo arm: 24 ± 34 months Gender: desmopressin arm: 29 male, 24 female; placebo arm: 25 male, 17 female Type of surgery
Duration of surgery: not reported Duration of cardiopulmonary bypass: desmopressin arm: 70 ± 30 minutes; placebo arm: 82 ± 36 minutes Emergency cases: not reported Antiplatelet agents: not reported Anticoagulants: not reported Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 0.9% saline (volume not reported)) 5 minutes after heparin reversal over 15 minutes (n = 53) Comparator arm: placebo (0.9% saline (volume not reported)) 5 minutes after heparin reversal over 15 minutes (n = 42) | |
| Outcomes | Primary outcome: blood loss in first 24 hours postoperatively (measured by estimating blood loss in surgical sponges, volume of suction drainage and drain output) Secondary outcome: volume of red cells transfused | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Low risk | Only the pharmacist was not blinded to participants' group assignment. |
| Blinding of outcome assessors | Low risk | Quote: "only pharmacist was not blinded to patient's group assignment" |
| Incomplete outcome data | High risk | No intention‐to‐treat analysis. 17 of 112 participants missing, although they were accounted for ‐ 7 did not receive study drug, 4 had incomplete data, 3 died (non‐hemorrhagic), 2 returned to OR, 1 did not have blood available |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | High risk | Supported in part by a grant from the Rorer Corporation (a manufacturer of DDAVP) |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: cardiac surgery Country: Spain Registration: not prospectively registered | |
| Participants | Inclusion criteria: > 18 years old; valvular heart disease or atrial septal defect Exclusion criteria: emergency surgery; known haemostatic defect; uncontrolled hypertension; renal insufficiency; patients undergoing CABG Number of participants randomised: 100 Number of participants analysed: 100 Age: desmopressin arm: 55 ± 13 years; placebo arm: 53 ± 12 years Gender: desmopressin arm: 19 male, 31 female; placebo arm: 25 male, 25 female Type of surgery
Duration of surgery: not reported Duration of cardiopulmonary bypass: desmopressin arm: 93 ± 43 minutes; placebo arm: 94 ± 40 minutes Emergency cases: none Antiplatelet agents: not reported Anticoagulants: not reported Coagulopathy: none Thrombocytopenia: none Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 50 mL 0.9% saline) immediately after heparin reversal over 15 minutes (n = 50) Comparator arm: placebo (50 mL 0.9% saline) immediately after heparin reversal over 15 minutes (n = 50) | |
| Outcomes | Primary outcome: blood loss intraoperatively, 24 hours postoperatively, and total blood loss (measured by estimating blood loss in surgical sponges and drain output) Secondary outcomes
| |
| Notes | Blood loss reported as mL/m2, so reported narratively and not included in meta‐analysis. Volume of red cells transfused reported in mL, so converted to units, based on the assumption than 300 mL is equivalent to 1 unit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Unclear risk | Insufficient information for judgement |
| Blinding of outcome assessors | Unclear risk | Insufficient information for judgement |
| Incomplete outcome data | Low risk | Reported all outcomes as planned; all participants analysed |
| Selective outcome reporting | Unclear risk | No protocol available |
| Other sources of bias | Unclear risk | DDAVP supplied by Ferring but it is unclear if this was free of charge or if Ferring had a role in study design |
| Methods | Type of study: single‐centre, 4‐arm, parallel‐group RCT Setting: cardiac surgery Country: Spain Registration: not prospectively registered | |
| Participants | Inclusion criteria: > 18 years old; valvular or coronary artery disease Exclusion criteria: emergency surgery; known haemostatic defect; hepatic or renal insufficiency; previous exposure to study drugs; use of other techniques for blood saving Number of participants randomised: 122 Number of participants analysed: 109 Age
Gender
Type of surgery
Duration of surgery: not reported Duration of cardiopulmonary bypass
Emergency cases: none Antiplatelet agents: not reported Anticoagulants: not reported Coagulopathy: none Thrombocytopenia: none Antifibrinolytics: all participants in the aprotinin arm were treated with aprotinin. No other participants were treated with an antifibrinolytic agent Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm 1 (DDAVP 1 dose): DDAVP (0.3 μg/kg intravenously in 50 mL 0.9% saline) immediately after heparin reversal over 20 minutes (n = 25) Intervention arm 2 (DDAVP 2 doses): DDAVP (0.3 μg/kg intravenously in 50 mL 0.9% saline) immediately after heparin reversal and again 6 hours postoperatively over 20 minutes (n = 28) Intervention arm 3 (aprotinin): aprotinin 2 million KIU within 30 minutes after induction of anaesthesia followed by a continuous infusion of 500,000 KIU/h until participant left the operating room, and an additional bolus of 2 million KIU aprotinin in the pump prime by replacement of crystalloid solution (n = 28) Comparator arm (standard care): standard care (n = 28) | |
| Outcomes | Primary outcome: postoperative blood loss (measured by drain output) Secondary outcomes
| |
| Notes | Single dose of DDAVP arm used for comparison with placebo for review. Blood loss and total volume of blood transfused reported as mL/m2, so reported narratively and not included in meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | High risk | Open label |
| Blinding of outcome assessors | High risk | Open label |
| Incomplete outcome data | High risk | Quote: "Of the total number of patients admitted to the study, 13 (4 in the placebo group and 9 in the treatment groups) were excluded from subsequent analysis because they did not complete the study" |
| Selective outcome reporting | Unclear risk | No protocol available |
| Other sources of bias | Unclear risk | Desmopressin supplied by Ferring and aprotinin supplied by Bayer but it is unclear if this was free or if either company had a role in study design |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: cardiac surgery Country: Finland Registration: not prospectively registered | |
| Participants | Inclusion criteria: first‐time CABG procedure; bleeding history unremarkable; normal preoperative blood coagulation tests Exclusion criteria: people who had received acetylsalicylic acid or heparin within 5 days Number of participants randomised: 30 Number of participants analysed: 30 Age: desmopressin arm: 57 ± 9 years; placebo arm: 59 ± 5 years Gender: desmopressin arm: 14 male, 1 female; placebo arm: 14 male, 1 female Type of surgery: all participants undergoing first‐time CABG Duration of surgery: not reported Duration of cardiopulmonary bypass: desmopressin arm: 94 ± 19 minutes; placebo arm: 103 ± 23 minutes Emergency cases: none Antiplatelet agents: none Anticoagulants: none Coagulopathy: none Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: red cells transfused if haematocrit < 30% | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg via side‐port of pulmonary artery catheter introducer in 100 mL 0.9% saline) immediately after sternal closure over 15 minutes (n = 15) Comparator arm: placebo (100 mL 0.9% saline via side‐port of pulmonary artery catheter introducer) immediately after sternal closure over 15 minutes (n = 15) | |
| Outcomes | Primary outcome: clinically significant hypotension Secondary outcomes
| |
| Notes | Blood loss reported as median and range, so this outcome is reported narratively and was not included in meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Unclear risk | Insufficient information for judgement. Reported to be double‐blind, but no details |
| Blinding of outcome assessors | Unclear risk | Insufficient information for judgement. Reported to be double‐blind, but no details |
| Incomplete outcome data | Low risk | All participants included in final analysis |
| Selective outcome reporting | Unclear risk | No protocol available |
| Other sources of bias | High risk | Double‐blind comparison of DDAVP with saline part of a larger trial of DDAVP in patients undergoing CABG: larger trial not published (bleeding outcomes not reported) Supported in part by a grant from the Paulo Foundation, Helsinki, Finland |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: cardiac surgery Country: USA Registration: not prospectively registered | |
| Participants | Inclusion criteria: undergoing CABG with valvular heart disease, atrial septal defects, or undergoing repeat grafting operations for chronically occluded CABGs Exclusion criteria: undergoing primary uncomplicated CABG Number of participants randomised: 72 Number of participants analysed: 70 Age
Gender: desmopressin arm: 21 male, 14 female; placebo arm: 21 male, 14 female Type of surgery
Duration of surgery: desmopressin arm: 373 ± 132 minutes; placebo arm: 392 ± 145 minutes Duration of cardiopulmonary bypass: desmopressin arm: 144 ± 38 minutes; placebo arm: 159 ± 66 minutes Emergency cases: none Antiplatelet agents: not reported Anticoagulants: not reported Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 50 mL 0.9% saline) immediately after heparin reversal over 15 minutes (n = 35) Comparator arm: placebo (50 mL 0.9% saline) immediately after heparin reversal over 15 minutes (n = 35) | |
| Outcomes | Primary outcome: intraoperative blood loss and total blood loss (measured by estimating blood in surgical sponges and suction drainage) Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Low risk | Series of sealed envelopes |
| Blinding of participants and personnel | Unclear risk | "Double‐blind", but no details given |
| Blinding of outcome assessors | Low risk | Surgeons asked to comment whether they thought participant was in intervention or placebo cohort ‐ no correlation with actual allocation |
| Incomplete outcome data | Low risk | Two participants withdrawn from study ‐ 1 died in operating room before receiving the drug and 1 was withdrawn by surgeon (no reason given) before treatment with drug ‐ not included in analysis |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | Low risk | No other clear risk of bias. Supported by grants from the National Institutes of Health |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: orthopaedic surgery Country: Sweden Registration: not prospectively registered | |
| Participants | Inclusion criteria: normal haemostasis; scheduled for elective primary total hip replacement Exclusion criteria: secondary procedure; antiplatelet drug within 10 days of surgery; iron‐deficient anaemia; diabetes mellitus; rheumatoid disease; any disease requiring steroid treatment; abnormal preoperative coagulation status; abnormal bleeding time Number of participants randomised: 80 Number of participants analysed: 79 Age: desmopressin arm: 71 ± 9 years; placebo arm: 68 ± 7 years Gender: desmopressin arm: 20 male, 19 female; placebo arm: 15 male, 25 female Type of surgery: all participants undergoing elective primary total hip replacement surgery Duration of surgery: not reported Duration of cardiopulmonary bypass: N/A Emergency cases: none Antiplatelet agents: none Anticoagulants: none Coagulopathy: none Thrombocytopenia: none Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: red cells transfused if haematocrit < 27% | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 50 mL 0.9% saline) post induction of spinal anaesthesia and again 6 hours after first dose over 15 minutes (n = 39) Comparator arm: placebo (50 mL 0.9% saline) post induction of spinal anaesthesia and again 6 hours after first dose over 15 minutes (n = 40) | |
| Outcomes | Primary outcomes
Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Unclear risk | Insufficient information for judgement. "Double‐blind" but no details reported |
| Blinding of outcome assessors | Unclear risk | Insufficient information for judgement. "Double‐blind" but no details reported |
| Incomplete outcome data | Low risk | One participant excluded as this was an emergency case (fractured neck of femur) and therefore did not meet the inclusion criteria |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | High risk | The study was supported by Ferring AB (the manufacturer of DDAVP) |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: paediatric cardiac surgery Country: Canada Registration: not prospectively registered | |
| Participants | Inclusion criteria: paediatric patients undergoing surgery with cardiac bypass Exclusion criteria: not reported Number of participants randomised: 60 Number of participants analysed: 60 Age: desmopressin arm: 45.9 ± 50.3 months; placebo arm: 64.2 ± 60.1 months Gender: desmopressin arm: 17 male, 13 female; placebo arm: 18 male, 12 female Type of surgery
Duration of surgery: not reported Duration of cardiopulmonary bypass: desmopressin arm: 96.4 ± 37.4 minutes; placebo arm: 93.7 ± 47.1 minutes Emergency cases: not reported Antiplatelet agents: not reported Anticoagulants: not reported Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 0.9% saline (volume not reported)) "on conclusion of cardiopulmonary bypass" over 15 minutes (n = 30) Comparator arm: placebo (0.9% saline (volume not reported but "equal" to DDAVP volume)) "on conclusion of cardiopulmonary bypass" over 15 minutes (n = 30) | |
| Outcomes | Primary outcome: postoperative blood loss (measured by estimating blood loss in surgical sponges and drain output) Secondary outcome: all‐cause mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Low risk | Sealed envelopes |
| Blinding of participants and personnel | Low risk | Only pharmacist aware of treatment allocation |
| Blinding of outcome assessors | Low risk | Only pharmacist aware of treatment allocation |
| Incomplete outcome data | Low risk | All participants included in final analysis |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | Low risk | No other clear sources of bias. Supported in part by a grant from the British Columbia Health Care Research Foundation |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: endoscopic sinus surgery Country: China Registration: registered after study commenced (NCT02125188) | |
| Participants | Inclusion criteria: age 18 to 65 years; first‐time candidates for 2‐side endoscopic sinus surgery; ASA grade 1‐2 Exclusion criteria: history of bleeding disorders; medications that may affect surgical haemostasis; secondary surgery; poorly controlled hypertension; cerebrovascular disease; significant coronary artery disease or arrhythmias; compromised renal or hepatic function; pregnancy Number of participants randomised: 90 Number of participants analysed: 90 Age: desmopressin arm: 45.9 ± 14.9 years; placebo arm: 40.7 ± 17.4 years Gender: desmopressin arm: 26 male, 19 female; placebo arm: 24 male, 21 female Type of surgery: all undergoing endoscopic sinus surgery Duration of surgery: desmopressin arm: 76 ± 20.7 minutes; placebo arm: 82 ± 19.6 minutes Duration of cardiopulmonary bypass: N/A Emergency cases: none Antiplatelet agents: none Anticoagulants: none Coagulopathy: none Thrombocytopenia: none Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg intravenously in 100 mL 0.9% saline) post anaesthetic induction and preoperatively over 20 minutes (n = 45) Comparator arm: placebo (100 mL 0.9% saline) post anaesthetic induction and preoperatively over 20 minutes (n = 45) | |
| Outcomes | Primary outcome: intraoperative blood loss (quality of operative field determined by operating surgeon) Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Quote: "Assignment to the groups was performed by computer‐generated random numbers" |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Unclear risk | Insufficient information on method of blinding. "All of the surgeons were blinded to the patient study groups" |
| Blinding of outcome assessors | Unclear risk | Insufficient information on method of blinding. Surgeons acted as outcome assessors. "All of the surgeons were blinded to the patient study groups" |
| Incomplete outcome data | Low risk | All participants included in final analysis |
| Selective outcome reporting | Low risk | Primary and secondary outcomes same as trial registration |
| Other sources of bias | Low risk | No other clear sources of bias. Supported by Guangdong Provincial Science and Technology Projects of China |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: cardiac surgery Country: Canada Registration: not prospectively registered | |
| Participants | Inclusion criteria: male; < 70 years old; taking aspirin within previous 7 days; undergoing CABG Exclusion criteria: abnormal haematological profile; history of bleeding; repeat coronary bypass surgery; recent heparin intake Number of participants randomised: 44 Number of participants analysed: 44 Age: desmopressin arm: 56.6 (52.9‐60.3) years (mean and range); placebo arm: 61.6 (58.6‐64.6) years (mean and range) Gender: desmopressin arm: 20 male, 0 female; placebo arm: 24 male, 0 female Type of surgery: all undergoing CABG Duration of surgery: not reported Duration of cardiopulmonary bypass: not reported Emergency cases: none Antiplatelet agents: all receiving aspirin Anticoagulants: none Coagulopathy: none Thrombocytopenia: none Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (10 μg/m2 body surface area intravenously in 20 mL 0.9% saline) "after cardiopulmonary bypass" over 20 minutes (n = 20) Comparator arm: placebo (20 mL 0.9% saline) "after cardiopulmonary bypass" over 20 minutes (n = 24) | |
| Outcomes | Primary outcome: postoperative blood loss (measured by volume of suction drainage and estimated cardiopulmonary bypass residual volume) Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Quote: "randomized with restriction in blocks of 10" ‐ exact method unclear |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Unclear risk | Insufficient information for judgement |
| Blinding of outcome assessors | Unclear risk | Insufficient information for judgement |
| Incomplete outcome data | Low risk | All participants included in final analysis |
| Selective outcome reporting | Unclear risk | No protocol available |
| Other sources of bias | High risk | Supported by Ferring Inc (a manufacturer of DDAVP) |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group randomised controlled trial Setting: cardiac surgery Country: UK Registration: not prospectively registered | |
| Participants | Inclusion criteria: elective CABG with cardiopulmonary bypass grafting; men age 30 to 70 years and women who were postmenopausal and < 70 years old Exclusion criteria: reoperation or emergency surgery; heparin or warfarin within 72 hours of surgery; thrombolytic therapy within 7 days before surgery; no informed consent Number of participants randomised: 100 Number of participants analysed: 98 Age: desmopressin arm: 58.8 ± 8.8 years; placebo arm: 55 ± 8.4 years Gender: desmopressin arm: 42 male, 7 female; placebo arm: 38 male, 11 female Type of surgery: all undergoing elective CABG Duration of surgery: not reported Duration of cardiopulmonary bypass: desmopressin arm: 71.4 ± 19 minutes; placebo arm: 74.5 ± 23.3 minutes Emergency cases: none Antiplatelet agents: desmopressin arm: 7; placebo arm: 5 Anticoagulants: none Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: red cells transfused if haematocrit < 30% | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg in 50 mL 0.9% saline) after heparin reversal over 30 minutes (n = 49) Comparator arm: placebo (50 mL 0.9% saline) after heparin reversal over 30 minutes (n = 49) | |
| Outcomes | Primary outcome: postoperative blood loss (measured by estimating blood loss from surgical swabs, suction drainage, and drain output) Secondary outcomes
| |
| Notes | Volume of red cells reported as mean with no standard deviation. This outcome has been reported narratively and was not included in meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Low risk | Quote: "Both desmopressin and placebo were supplied in numbered but otherwise identical 1 mL vials, containing 4μg of active drug (Ferring Pharmaceuticals, Feltham, Middlesex, UK). The ten ampoules supplied for each patient were stored in a refrigerator at 4‐8°C, then diluted in 50 mL isotonic saline prior to administration. A dose of 0.3μg/kg bodyweight was infused intravenously over 30 minutes through a central line, after completion of cardiopulmonary bypass and following reversal of heparin by protamine" |
| Blinding of outcome assessors | Low risk | Quote: "Both desmopressin and placebo were supplied in numbered but otherwise identical 1 mL vials, containing 4μg of active drug (Ferring Pharmaceuticals, Feltham, Middlesex, UK). The ten ampoules supplied for each patient were stored in a refrigerator at 4‐8°C, then diluted in 50 mL isotonic saline prior to administration. A dose of 0.3μg/kg bodyweight was infused intravenously over 30 minutes through a central line, after completion of cardiopulmonary bypass and following reversal of heparin by protamine" |
| Incomplete outcome data | Unclear risk | Insufficient information for judgement |
| Selective outcome reporting | Unclear risk | No protocol available |
| Other sources of bias | High risk | Support and supply of study medication from Ferring Pharmaceuticals (a manufacturer of DDAVP) |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: cardiac surgery Country: Austria Registration: not prospectively registered | |
| Participants | Inclusion criteria: elective bioprosthetic AVR because of severe aortic valve stenosis (defined as a mean gradient of 50 mmHg or an indexed effective orifice area of 0.5 cm2/m2 body surface area); platelet dysfunction (collagen/adenosine diphosphate closure time on platelet function analyser‐100 > 170 seconds) Exclusion criteria: left ventricular ejection fraction < 0.40; body mass index > 40 kg/m2; serum creatinine > 1.5 mg/dL; known hypersensitivity towards DDAVP; active endocarditis; multi‐valvular disease; antiplatelet therapy within 10 days before surgery; any relevant coronary artery disease; inability to give informed consent; mechanical valves Number of participants randomised: 50 Number of participants analysed: 43 Age: desmopressin arm: 72 ± 10 years; placebo arm: 73 ± 8 years Gender: desmopressin arm: 8 male, 12 female; placebo arm: 7 male, 16 female Type of surgery: all undergoing elective bioprosthetic AVR Duration of surgery: desmopressin arm: 295 ± 95 minutes; placebo arm: 273 ± 52 minutes Duration of cardiopulmonary bypass: desmopressin arm: 89 ± 37 minutes; placebo arm: 79 ± 17 minutes Emergency cases: none Antiplatelet agents: none Anticoagulants: none Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: red cells transfused when haemoglobin < 70 g/L in the operating room, or < 80 g/L on the ward | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg in 100 mL 0.9% saline) after induction of anaesthesia over 30 minutes (n = 20) Comparator arm: placebo (100 mL 0.9% saline) after induction of anaesthesia over 30 minutes (n = 23) | |
| Outcomes | Primary outcome: postoperative blood loss (method for measurement not reported) Secondary outcomes
| |
| Notes | Volume of red cells transfused reported in mL, so converted into units based on assumption that 300 mL is equivalent to 1 unit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Quote: "This was a 1:1 block‐randomized (block sizes of 4)" |
| Allocation concealment | Low risk | Quote: "A randomization list was generated with an online program (www. randomization.com) and, for concealment, individually sealed opaque envelops contained the randomization codes for the study nurses who prepared the study drugs. Patients and treating physicians, nurses at the ward, and laboratory technicians were blinded with respect to the randomization code" |
| Blinding of participants and personnel | Low risk | Study nurses prepared and administered study drugs but were not involved in outcome assessment |
| Blinding of outcome assessors | Low risk | Quote: "Patients and treating physicians, nurses at the ward, and laboratory technicians were blinded with respect to the randomization code" |
| Incomplete outcome data | Unclear risk | 7/50 participants excluded: 4 excluded owing to low closure time before administration of DDAVP or placebo; 3 after allocation of study drug (all in the DDAVP arm): 2 for intraoperative situs of porcelain aorta and 1 for requirement of additional CABG |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | Unclear risk | Insufficient information for judgement |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: cardiac surgery Country: USA Registration: not prospectively registered | |
| Participants | Inclusion criteria: undergoing primary cardiac surgery Exclusion criteria: not reported Number of participants randomised: 83 Number of participants analysed: 83 Age: not reported Gender: not reported Type of surgery: all undergoing primary cardiac surgery. No further information reported Duration of surgery: not reported Duration of cardiopulmonary bypass: not reported Emergency cases: none Antiplatelet agents: not reported Anticoagulants: not reported Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: desmopressin arm: epsilon‐aminocaproic acid 8; placebo arm: epsilon‐aminocaproic acid 13 Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg in 50 mL 0.9% saline) after heparin reversal 15 minutes (n = 40) Comparator arm: placebo (50 mL 0.9% saline) after heparin reversal 15 minutes (n = 43) | |
| Outcomes | Primary outcome: postoperative blood loss (measured by drain output) Secondary outcome: number of participants receiving a red cell transfusion | |
| Notes | Blood loss reported separately for mediastinal and pleural blood loss. Mediastinal blood loss figure has been used, as this accounts for approximately 80% of total blood loss at 24 hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Quote: "A card drawn at random determined which vial was to be used" |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Low risk | Identical appearances of bottles (placebo/DDAVP) |
| Blinding of outcome assessors | Low risk | Identical appearances of bottles (placebo/DDAVP) |
| Incomplete outcome data | High risk | Table 1 reports that sample sizes may vary due to missing data but does not indicate how many participants are missing from the analysis |
| Selective outcome reporting | High risk | Total blood loss not reported. Mediastinal and pleural blood loss reported separately |
| Other sources of bias | High risk | Imbalance of participant characteristics; 5 participants in the placebo group receiving DDAVP, along with other differences in postoperative management. Rorer Pharmaceuticals supplied the DDAVP used in the study. Unclear if the company had a role in study design |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: orthopaedic surgery Country: USA Registration: not prospectively registered | |
| Participants | Inclusion criteria: stage IV cerebral palsy and neuromuscular scoliosis; undergoing elective spinal fusion with unit rod instrumentation Exclusion criteria: history of bleeding problems; prolonged PT/aPTT; bleeding time > 7 minutes; thrombocytopenia Number of participants randomised: 21 Number of participants analysed: 21 Age: desmopressin arm: median 13 (range 10‐19) years; placebo arm: median 13 (range 6‐18) years Gender: not reported Type of surgery: all undergoing elective spinal fusion with unit rod instrumentation Duration of surgery: desmopressin arm: median 4.5 hours (range 3.45 to 5.0 hours); placebo arm: median 4.3 hours (range 3.45 to 5.15 hours) Duration of cardiopulmonary bypass: N/A Emergency cases: none Antiplatelet agents: none Anticoagulants: none Coagulopathy: none Thrombocytopenia: none Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: red cells transfused if haemoglobin < 100 g/L | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg in 100 mL 0.9% saline) preoperatively (exact timing unclear) over 15 to 20 minutes (n = 10) Comparator arm: placebo (100 mL 0.9% saline) preoperatively (exact timing unclear) over 15 to 20 minutes (n = 11) | |
| Outcomes | Primary outcome: blood loss intraoperatively and in total (intensive care paediatrician estimated blood loss in surgical bandages) Secondary outcome: total volume of red cells transfused | |
| Notes | Trial stopped early after 21/40 participants recruited, as more blood loss in DDAVP arm than in placebo arm. Blood loss reported as percentage of blood volume, so results reported narratively. Transfusion requirements were reported as median and range, so are reported narratively and were not incorporated into meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Low risk | DDAVP and placebo identical in appearance |
| Blinding of outcome assessors | Low risk | Quote: "surgeon, anaesthesiologist and investigator collecting data were unaware of what solution was being administered" |
| Incomplete outcome data | Low risk | Power calculation was 40 participants. Interim analysis at 21 participants prompted termination. All 21 participants were reported on |
| Selective outcome reporting | Unclear risk | Protocol not available |
| Other sources of bias | Unclear risk | Unclear when DDAVP was administered before surgery. Funding was provided by the Nemours Foundation |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: plastic surgery Country: USA Registration: not prospectively registered 2 trials reported in 1 paper. Wingate 1992a reported extensive procedures; Wingate 1992b reported smaller procedures | |
| Participants | Inclusion criteria: spinal cord injury requiring flap reconstruction of pelvic pressure sores Exclusion criteria: not reported Number of participants randomised: 23 Number of participants analysed: 23 Age: not reported Gender: not reported Type of surgery: all undergoing flap reconstruction Duration of surgery: not reported Duration of cardiopulmonary bypass: N/A Emergency cases: none Antiplatelet agents: not reported Anticoagulants: not reported Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg in 50 mL 0.9% saline) after induction before operation over 20 minutes (n = 14) Comparator arm: placebo (50 mL 0.9% saline) after induction before operation over 20 minutes (n = 9) | |
| Outcomes | Primary outcome: intraoperative blood loss (method for measurement not reported) Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Selected according to a random numbers table |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Low risk | Pharmacist prepared the study drug. "Neither surgical team nor anesthesiologist [was] aware of treatment" |
| Blinding of outcome assessors | Low risk | Pharmacist prepared the study drug. "Neither surgical team nor anesthesiologist [was] aware of treatment" |
| Incomplete outcome data | Low risk | All participants included in final analysis |
| Selective outcome reporting | Unclear risk | No protocol available |
| Other sources of bias | Unclear risk | Insufficient information for judgement |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: plastic surgery Country: USA Registration: not prospectively registered 2 trials reported in 1 paper. Wingate 1992a reported extensive procedures; Wingate 1992b reported smaller procedures | |
| Participants | Inclusion criteria: spinal cord injury requiring flap reconstruction of pelvic pressure sores Exclusion criteria: not reported Number of participants randomised: 21 Number of participants analysed: 21 Age: not reported Gender: not reported Type of surgery: all undergoing flap reconstruction Duration of surgery: not reported Duration of cardiopulmonary bypass: N/A Emergency cases: none Antiplatelet agents: not reported Anticoagulants: not reported Coagulopathy: not reported Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: not reported | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg in 50 mL 0.9% saline) after induction before operation over 20 minutes (n = 8) Comparator arm: placebo (50 mL 0.9% saline) after induction before operation over 20 minutes (n = 13) | |
| Outcomes | Primary outcome: Intraoperative blood loss (method for measurement not reported) Secondary outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Selected according to a random numbers table |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | Low risk | Pharmacist prepared the study drug. "Neither surgical team nor anesthesiologist [was] aware of treatment" |
| Blinding of outcome assessors | Low risk | Pharmacist prepared the study drug. "Neither surgical team nor anesthesiologist [was] aware of treatment" |
| Incomplete outcome data | Low risk | All participants included in final analysis |
| Selective outcome reporting | Unclear risk | No protocol available |
| Other sources of bias | Unclear risk | Insufficient information for judgement |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: hepatic surgery Country: Hong Kong Registration: not prospectively registered | |
| Participants | Inclusion criteria: adults scheduled for hepatectomy Exclusion criteria: coronary artery disease; congenital or acquired coagulation disorders other than liver cirrhosis; blood sodium level < 130 mmol/L; NSAID or aspirin ingestion within 7 days of scheduled surgery; history of thrombovascular disorders or pulmonary thromboembolism Number of participants randomised: 60 Number of participants analysed: 59 Age: desmopressin arm: 47.4 ± 11.3 years; placebo arm: 54.9 ± 11.8 years Gender: desmopressin arm: 20 male, 10 female; placebo arm: 17 male, 13 female Type of surgery: all undergoing hepatectomy Duration of surgery: desmopressin arm: median 405 (range 210‐800) minutes; placebo arm: median 435 (range 180‐780) minutes Duration of cardiopulmonary bypass: N/A Emergency cases: none Antiplatelet agents: none Anticoagulants: none Coagulopathy: none Thrombocytopenia: not reported Antifibrinolytics: not reported Cell salvage: not reported Transfusion protocol: red cell transfusion if haematocrit < 30% | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg in 50 mL 0.9% saline) after induction of anaesthesia over 20 minutes (n = 30) Comparator arm: placebo (50 mL 0.9% saline) after induction of anaesthesia over 20 minutes (n = 29) | |
| Outcomes | Primary outcome: blood loss intraoperatively and in total (measured by estimating blood loss in surgical swabs and drain output) Secondary outcomes
| |
| Notes | Blood loss reported as median and range, so outcome reported narratively and not included in meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Insufficient information for judgement |
| Allocation concealment | Low risk | Quote: "Patient randomization was by drawing a sealed envelope specifying a prescription for either desmopressin or placebo, which was then prepared by an independent investigator and blinded to the patient, attending anesthesiologist and surgeon" |
| Blinding of participants and personnel | Low risk | [DDAVP or placebo] "... was then prepared by an independent investigator and blinded to the patient, attending anesthesiologist and surgeon" |
| Blinding of outcome assessors | Low risk | [DDAVP or placebo] "... was then prepared by an independent investigator and blinded to the patient, attending anesthesiologist and surgeon" |
| Incomplete outcome data | High risk | One participant excluded due to excess bleeding (surgical damage to inferior vena cava). This exclusion was not prespecified and is likely to result in a significant change in the effect estimate |
| Selective outcome reporting | Unclear risk | No protocol available |
| Other sources of bias | Unclear risk | Insufficient information for judgement |
| Methods | Type of study: single‐centre, 2‐arm, parallel‐group RCT Setting: orthopaedic surgery Country: Israel Registration: not prospectively registered | |
| Participants | Inclusion criteria: ASA physical status 1‐3; undergoing elective total knee replacement Exclusion criteria: severe ischaemic heart disease (New York Heart Association grade III or IV); chronic renal failure; liver cirrhosis; bleeding disorders; anticoagulant therapy Number of participants randomised: 40 Number of participants analysed: 40 Age: desmopressin arm: 72 ± 5 years; placebo arm: 71 ± 5 years Gender: desmopressin arm: 3 male, 17 female; tranexamic acid arm: 8 male, 12 female Type of surgery: all undergoing total knee replacement Duration of surgery: desmopressin arm: 128 ± 15 minutes; tranexamic acid arm: 133 ± 13 minutes Duration of cardiopulmonary bypass: N/A Emergency cases: none Antiplatelet agents: not reported Anticoagulants: none Coagulopathy: none Thrombocytopenia: not reported Antifibrinolytics: all participants in tranexamic acid arm treated with tranexamic acid; no participants in DDAVP arm treated with an antifibrinolytic agent Cell salvage: not reported Transfusion protocol: red cells transfused if haematocrit < 27% | |
| Interventions | Intervention arm: DDAVP (0.3 μg/kg (diluent and volume not reported) 30 minutes before deflation of tourniquet (speed of administration not reported). Followed by a constant infusion of intravenous saline until 12 hours after surgery (volume not reported) (n = 20) Comparator arm: tranexamic acid (15 mg/kg) 30 minutes before deflation of tourniquet (speed of administration not reported). Followed by a constant infusion of tranexamic acid 10 mg/kg until 12 hours after surgery (volume not reported) (n = 20) | |
| Outcomes | Primary outcome: total volume of red cells transfused Secondary outcomes
| |
| Notes | Blood loss reported separately for first 12 hours and second 12 hours postoperatively. Data from first 12 hours postoperatively used in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Computer‐generated sequence |
| Allocation concealment | Unclear risk | Insufficient information for judgement |
| Blinding of participants and personnel | High risk | Participants and personnel not blinded to treatment |
| Blinding of outcome assessors | Low risk | Outcome assessor blinded to treatment allocation |
| Incomplete outcome data | Unclear risk | Three participants lost to follow‐up |
| Selective outcome reporting | Unclear risk | No protocol available |
| Other sources of bias | Unclear risk | Insufficient information for judgement |
Abbreviations
ACBG: aortocoronary bypass graft
aPTT: activated partial thromboplastin time
ASA: American Society of Anesthesiologists
ASD: atrial septal defect
AV: atrioventricular
AVR: aortic valve replacement
BCPA: bidirectional cavopulmonary anastomosis
CABG: coronary artery bypass graft
CPB: cardiopulmonary bypass
DDAVP: desmopressin; 1‐deamino‐8‐D‐arginine vasopressin
ECC: extracorporeal circulation
GRF: glomerular filtration rate
HLHS: hypoplastic left heart syndrome
IQR: interquartile range
KIU: Kallikrein Inhibitor Unit
MA: maximum amplitude
MDRD: Modification of Diet in Renal Disease study
MVR: mitral valve replacement
N/A: not applicable
NSAID: non‐steroidal anti‐inflammatory drug
OMC: open mitral commissurotomy
PA: pulmonary artery
PT: prothrombin time
RCA‐RV: right coronary artery‐right ventricle
RCT: randomised controlled trial
TOF: tetralogy of Fallot
TT: thrombin time
TXA: tranexamic acid
VSD: ventricular septal defect
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not a RCT | |
| Not a RCT | |
| Not a RCT | |
| Review article | |
| Wrong participant group: procedures rather than participants randomised | |
| Review article | |
| Not a RCT | |
| Trial did not use the intervention of interest, but used intranasal DDAVP | |
| Trial did not use the intervention of interest, but used intranasal DDAVP | |
| Not a RCT | |
| Not a RCT | |
| Not a RCT | |
| Not a RCT | |
| Not a RCT | |
| Review article | |
| Trial did not use the intervention of interest, but used intranasal DDAVP | |
| Review article | |
| Wrong participant group, used healthy volunteers | |
| Trial did not use the intervention of interest, but was a comparison of thromboelastography with combination thromboelastography and platelet aggregometry | |
| Wrong participant group, used healthy volunteers | |
| Not a RCT | |
| Not a RCT | |
| Wrong participant group, used for participants requiring thromboprophylaxis | |
| Trial did not use the intervention of interest, but used DDAVP versus combination DDAVP and tranexamic acid | |
| Not a RCT | |
| Not a RCT | |
| Trial did not use the intervention of interest, but used intranasal DDAVP | |
| Not a RCT | |
| Wrong participant group, used healthy volunteers | |
| Review article |
Abbreviation
DDAVP: desmopressin; 1‐deamino‐8‐D‐arginine‐vasopressin
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Type of study: single‐centre, parallel‐group, 2‐arm randomised controlled trial (RCT) |
| Participants | Inclusion criteria: age 20 to 70 years; heart transplant surgery Exclusion criteria: history of previous surgery on chest; bleeding disorders or haemophilia; abnormal preoperative coagulation tests or platelet count; contraindications for use of DDAVP (1‐deamino‐8‐D‐arginine‐vasopressin; desmopressin); history of deep vein thrombosis; "Haematologic disease" (not specified); carotid artery stenosis; chronic obstructive pulmonary disease |
| Interventions | Intervention arm: DDAVP 0.3 μg/kg in 2 mL saline before surgery (route of administration not clear) Comparator arm: placebo (2 mL saline) before surgery (route of administration not clear) |
| Outcomes | Primary outcome
Secondary outcomes
|
| Notes |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | DRIVE ‐ Desmopressin for procedures or Radiological InterVEntions |
| Methods | Type of study: single‐centre, parallel‐group, 2‐arm RCT |
| Participants | Inclusion criteria: age 18 years and over; platelet count ≤ 100 × 109/L; inpatient on a critical care ward; due to undergo an invasive procedure Exclusion criteria: active bleeding; history of ischaemic heart disease (myocardial infarction or angina), stroke, or TIA; admission to ICU with traumatic brain injury or seizures; congenital bleeding disorder; pregnant or breastfeeding; history of anaphylaxis to desmopressin |
| Interventions | Intervention arm: DDAVP 0.3 μg/kg in 50 mL 0.9% saline infused intravenously preoperatively over 20 minutes Comparator arm: placebo (50 mL 0.9% saline) infused intravenously preoperatively over 20 minutes |
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | February 2017 |
| Contact information | Trial Manager: Miss Emma Laing ([email protected]) |
| Notes | Expected number of participants: 40 |
| Trial name or title | Desmopressin as treatment for postoperative bleeding after cardiac surgery |
| Methods | Type of study: single‐centre, parallel‐group, 2‐arm RCT |
| Participants | Inclusion criteria: age ≥ 18 years; scheduled for cardiac surgery; excessive postoperative bleeding: > 250 mL for 1 hour, or > 150 mL for 2 hours during first 4 hours Exclusion criteria: a medical condition known to influence the haemostatic system; treated with clopidogrel or systemic steroids during the last week before surgery; INR > 1.5; unable to give written informed consent; unstable people who need transfusion limits other than those used in this study |
| Interventions | Intervention arm: DDAVP 0.3 μg/kg infused intravenously postoperatively in the event of bleeding over 20 minutes Comparator arm: placebo (0.9% saline) infused intravenously postoperatively in the event of bleeding over 20 minutes |
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | March 2009 |
| Contact information | Principal Investigator: Dr Guri Greiff ([email protected]) |
| Notes | Expected number of participants: This trial was stopped because of difficulties with recruitment. 17 participants were recruited. These results are not yet published |
| Trial name or title | DDAVP in the reduction of post‐operative ecchymosis in rhinoplasty |
| Methods | Type of study: single‐centre, parallel‐group, 2‐arm RCT |
| Participants | Inclusion criteria: age 18 to 80 years; undergoing rhinoplasty for which nasal bone osteotomy is necessary Exclusion criteria: heart disease; renal disease with decreased GFR; liver disease |
| Interventions | Intervention arm: DDAVP 0.3 μg/kg infused intravenously preoperatively over 30 minutes Comparator arm: standard care |
| Outcomes | Primary outcome
Secondary outcome
|
| Starting date | December 2013 |
| Contact information | Principal Investigator: Dr Bahman Guyuron ([email protected]) |
| Notes | Expected number of participants: 30 |
| Trial name or title | Study of DDAVP combined with TXA on the blood loss and transfusion need during and after scoliosis correction surgery |
| Methods | Type of study: parallel‐group, 2‐arm RCT |
| Participants | Inclusion criteria: age 8 to 18 years; with idiopathic scoliosis undergoing posterior scoliosis correction surgery; ASA classification: 1‐2 agreed to participate in this study and signed informed consent Exclusion criteria: blood disease, such as anaemia or ITP; history of bleeding or ecchymosis; disorders of platelets, prothrombin time, activated partial thromboplastin time, fibrinogen, D‐dimers; hypertension; cardiac disease, such as unstable angina, myocardial infarction in previous 6 months, cardiac dysfunction, congenital heart disease, pulmonary heart disease; cerebral ischaemia administered with anticoagulants or non‐steroidal anti‐inflammatory drugs; hepatic or kidney dysfunction; blood transfusion in previous month |
| Interventions | Intervention arm: DDAVP 0.3 μg/kg in 100 mL 0.9% saline infused intravenously over 20 minutes before first skin incision Comparator arm: placebo (100 mL 0.9% saline) infused intravenously over 20 minutes before first skin incision |
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | December 2013 |
| Contact information | Principal Investigator: Dr Wen Qi Huang |
| Notes | Expected number of participants: 60 |
Abbreviations
ADP: adenosine diphosphate
ASA: American Society of Anesthesiologists
DDAVP: desmopressin (1‐deamino‐8‐D‐arginine vasopressin)
GFR: glomerular filtration rate
HEME:Haemorrhage Measurement Tool
ICU: intensive care unit
IMP: investigational medicinal product
INR: international normalised ratio
ITP: immune thrombocytopenic purpura
PFA: platelet analyser function
RCT: randomised controlled trial
TIA: transient ischaemic attack
TXA: tranexamic acid
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Red cell volume transfused (intraoperatively) Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Desmopressin vs placebo, Outcome 1 Red cell volume transfused (intraoperatively). | ||||
| 1.1 Adult cardiac surgery | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.22, 1.02] |
| 1.2 Paediatric cardiac surgery | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.87, 1.67] |
| 1.3 Orthopaedic surgery | 3 | 144 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.89, ‐0.11] |
| 1.4 Vascular surgery | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.55, 0.15] |
| 1.5 Plastic surgery | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.23, ‐0.27] |
| 2 Red cell volume transfused (total) Show forest plot | 23 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Desmopressin vs placebo, Outcome 2 Red cell volume transfused (total). | ||||
| 2.1 Cardiac surgery | 14 | 957 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.96, ‐0.08] |
| 2.2 Orthopaedic surgery | 6 | 303 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.67, 0.64] |
| 2.3 Vascular surgery | 2 | 135 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.60, 0.73] |
| 2.4 Hepatic surgery | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐1.27, 0.33] |
| 3 Red cell volume transfused (children only, total) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Desmopressin vs placebo, Outcome 3 Red cell volume transfused (children only, total). | ||||
| 4 Number of participants receiving a red cell transfusion (intraoperatively) Show forest plot | 6 | 349 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.50, 1.09] |
| Analysis 1.4  Comparison 1 Desmopressin vs placebo, Outcome 4 Number of participants receiving a red cell transfusion (intraoperatively). | ||||
| 4.1 Cardiac surgery | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.43, 1.10] |
| 4.2 Plastic surgery | 2 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.45, 1.64] |
| 4.3 Kidney biopsy | 1 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Other | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Number of participants receiving a red cell transfusion (total) Show forest plot | 25 | 1806 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.86, 1.06] |
| Analysis 1.5  Comparison 1 Desmopressin vs placebo, Outcome 5 Number of participants receiving a red cell transfusion (total). | ||||
| 5.1 Cardiac surgery | 17 | 1350 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.82, 1.06] |
| 5.2 Orthopaedic surgery | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.45, 1.64] |
| 5.3 Vascular surgery | 1 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.83, 1.34] |
| 5.4 Paediatric cardiac surgery | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.66, 2.06] |
| 5.5 Plastic surgery | 2 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.45, 1.64] |
| 5.6 Hepatic surgery | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.15, 2.21] |
| 5.7 Kidney biopsy | 1 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.8 Maxillofacial surgery | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.88, 4.54] |
| 6 Blood loss (intraoperative) Show forest plot | 11 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Desmopressin vs placebo, Outcome 6 Blood loss (intraoperative). | ||||
| 6.1 Cardiac surgery | 2 | 87 | Mean Difference (IV, Random, 95% CI) | ‐138.20 [‐623.40, 347.01] |
| 6.2 Orthopaedic surgery | 5 | 224 | Mean Difference (IV, Random, 95% CI) | ‐118.24 [‐278.43, 41.95] |
| 6.3 Vascular surgery | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐525.0 [‐1177.34, 127.34] |
| 6.4 Sinus surgery | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐28.0 [‐31.70, ‐24.30] |
| 6.5 Plastic surgery | 2 | 44 | Mean Difference (IV, Random, 95% CI) | ‐146.02 [‐487.86, 195.83] |
| 7 Blood loss (total) Show forest plot | 28 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Desmopressin vs placebo, Outcome 7 Blood loss (total). | ||||
| 7.1 Adult cardiac surgery | 22 | 1358 | Mean Difference (IV, Random, 95% CI) | ‐135.24 [‐210.80, ‐59.68] |
| 7.2 Orthopaedic surgery | 5 | 241 | Mean Difference (IV, Random, 95% CI) | ‐285.76 [‐514.99, ‐56.53] |
| 7.3 Vascular surgery | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐582.0 [‐1264.07, 100.07] |
| 8 Blood loss (children only, total) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.8 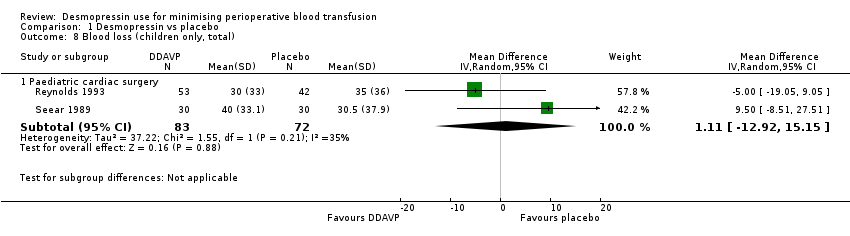 Comparison 1 Desmopressin vs placebo, Outcome 8 Blood loss (children only, total). | ||||
| 8.1 Paediatric cardiac surgery | 2 | 155 | Mean Difference (IV, Random, 95% CI) | 1.11 [‐12.92, 15.15] |
| 9 Number of participants with any bleeding (intraoperatively) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.9 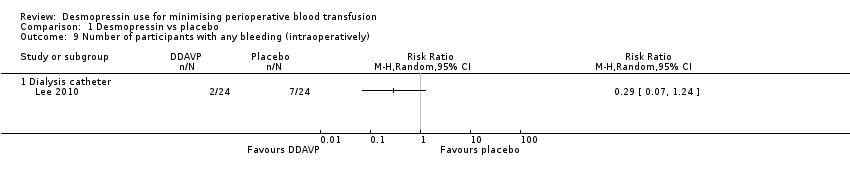 Comparison 1 Desmopressin vs placebo, Outcome 9 Number of participants with any bleeding (intraoperatively). | ||||
| 9.1 Dialysis catheter | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Number of participants with any bleeding (total) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.10  Comparison 1 Desmopressin vs placebo, Outcome 10 Number of participants with any bleeding (total). | ||||
| 10.1 Kidney biopsy | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Reoperation due to bleeding Show forest plot | 23 | 1783 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.66 [0.40, 1.09] |
| Analysis 1.11  Comparison 1 Desmopressin vs placebo, Outcome 11 Reoperation due to bleeding. | ||||
| 11.1 Cardiac surgery | 19 | 1483 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.38, 1.05] |
| 11.2 Orthopaedic surgery | 1 | 30 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Paediatric cardiac surgery | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.93 [0.14, 349.88] |
| 11.4 Dialysis catheter insertion | 1 | 48 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.5 Kidney biopsy | 1 | 162 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 All‐cause mortality Show forest plot | 22 | 1631 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.09 [0.51, 2.34] |
| Analysis 1.12 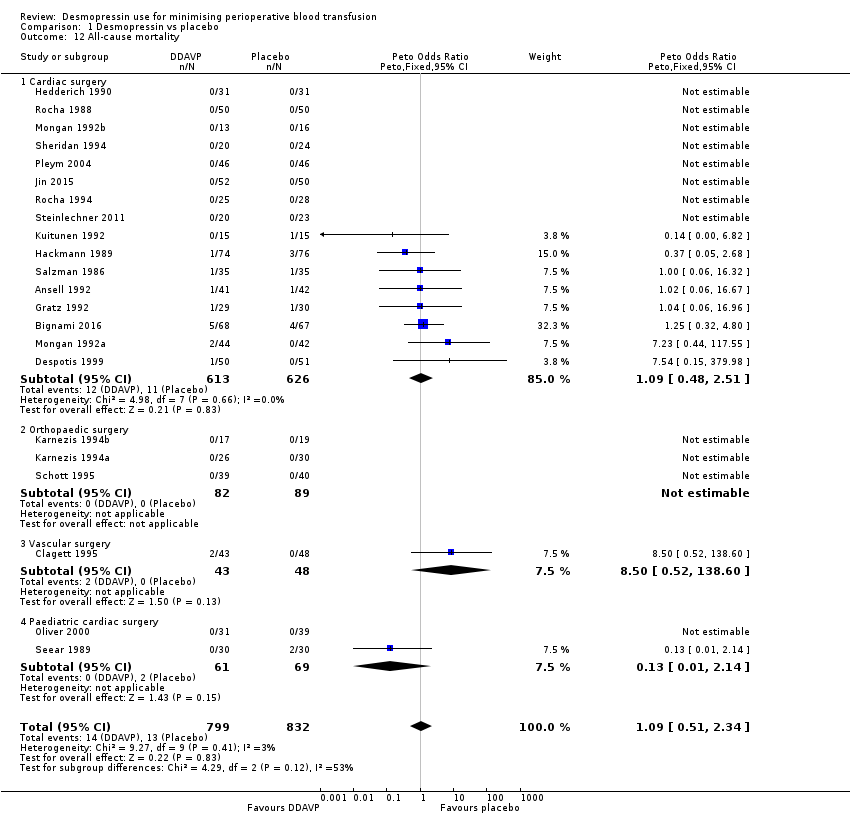 Comparison 1 Desmopressin vs placebo, Outcome 12 All‐cause mortality. | ||||
| 12.1 Cardiac surgery | 16 | 1239 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.09 [0.48, 2.51] |
| 12.2 Orthopaedic surgery | 3 | 171 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Vascular surgery | 1 | 91 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.50 [0.52, 138.60] |
| 12.4 Paediatric cardiac surgery | 2 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.14] |
| 13 All thrombotic events (including myocardial infarction, ischaemic stroke, other arterial thromboembolism, and venous thromboembolism) Show forest plot | 29 | 1984 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.36 [0.85, 2.16] |
| Analysis 1.13 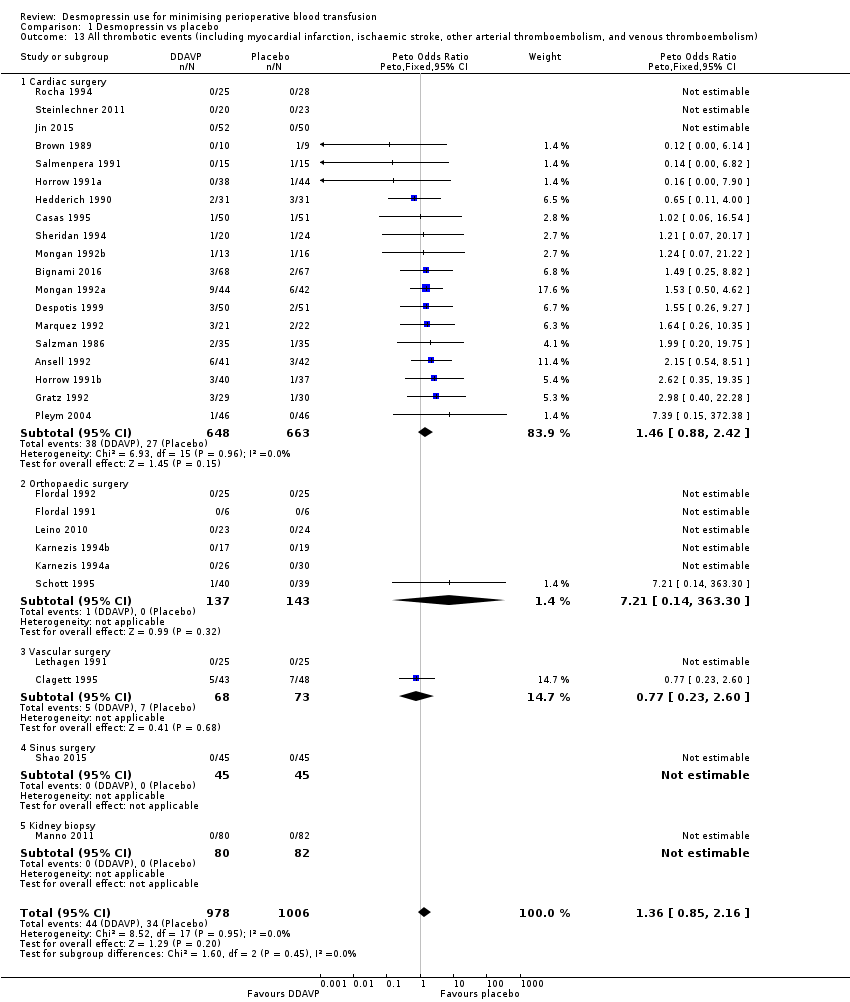 Comparison 1 Desmopressin vs placebo, Outcome 13 All thrombotic events (including myocardial infarction, ischaemic stroke, other arterial thromboembolism, and venous thromboembolism). | ||||
| 13.1 Cardiac surgery | 19 | 1311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.46 [0.88, 2.42] |
| 13.2 Orthopaedic surgery | 6 | 280 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.21 [0.14, 363.30] |
| 13.3 Vascular surgery | 2 | 141 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.23, 2.60] |
| 13.4 Sinus surgery | 1 | 90 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.5 Kidney biopsy | 1 | 162 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Myocardial infarction Show forest plot | 26 | 1704 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.70, 2.46] |
| Analysis 1.14  Comparison 1 Desmopressin vs placebo, Outcome 14 Myocardial infarction. | ||||
| 14.1 Cardiac surgery | 16 | 1031 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.52 [0.77, 3.00] |
| 14.2 Orthopaedic surgery | 6 | 280 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Vascular surgery | 2 | 141 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.11, 2.88] |
| 14.4 Kidney biopsy | 1 | 162 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.5 Sinus surgery | 1 | 90 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Stroke Show forest plot | 19 | 1277 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.95 [0.94, 9.24] |
| Analysis 1.15  Comparison 1 Desmopressin vs placebo, Outcome 15 Stroke. | ||||
| 15.1 Cardiac surgery | 11 | 733 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.95 [0.94, 9.24] |
| 15.2 Orthopaedic surgery | 5 | 201 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Vascular surgery | 1 | 91 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.4 Kidney biopsy | 1 | 162 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.5 Sinus surgery | 1 | 90 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Venous thromboembolism Show forest plot | 20 | 1377 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.17, 3.38] |
| Analysis 1.16 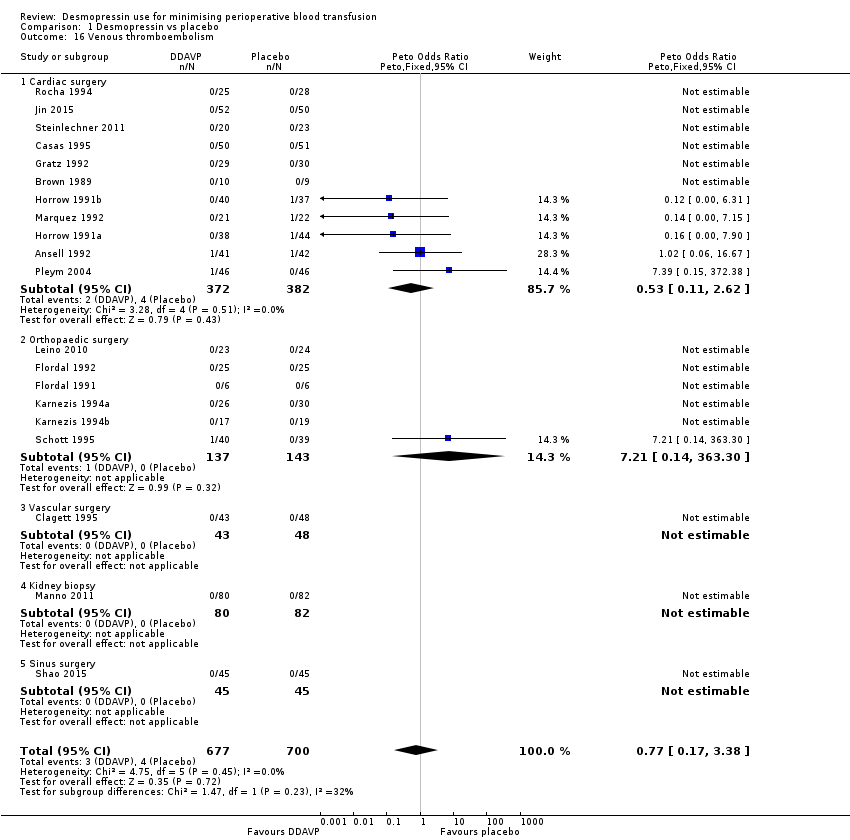 Comparison 1 Desmopressin vs placebo, Outcome 16 Venous thromboembolism. | ||||
| 16.1 Cardiac surgery | 11 | 754 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.53 [0.11, 2.62] |
| 16.2 Orthopaedic surgery | 6 | 280 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.21 [0.14, 363.30] |
| 16.3 Vascular surgery | 1 | 91 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.4 Kidney biopsy | 1 | 162 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.5 Sinus surgery | 1 | 90 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Clinically important hypotension Show forest plot | 18 | 1183 | Risk Ratio (M‐H, Random, 95% CI) | 2.32 [1.37, 3.91] |
| Analysis 1.17  Comparison 1 Desmopressin vs placebo, Outcome 17 Clinically important hypotension. | ||||
| 17.1 Cardiac surgery | 13 | 762 | Risk Ratio (M‐H, Random, 95% CI) | 2.88 [1.32, 6.30] |
| 17.2 Orthopaedic surgery | 2 | 109 | Risk Ratio (M‐H, Random, 95% CI) | 2.05 [0.99, 4.24] |
| 17.3 Paediatric cardiac surgery | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.06, 14.27] |
| 17.4 Sinus surgery | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.5 Kidney biopsy | 1 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Red cell volume transfused (total) Show forest plot | 6 | 388 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.16, ‐0.13] |
| Analysis 2.1  Comparison 2 Desmopressin vs placebo (platelet dysfunction), Outcome 1 Red cell volume transfused (total). | ||||
| 2 Number of participants receiving a red cell transfusion (intraoperatively) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Desmopressin vs placebo (platelet dysfunction), Outcome 2 Number of participants receiving a red cell transfusion (intraoperatively). | ||||
| 3 Number of participants receiving a red cell transfusion (total) Show forest plot | 5 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.66, 1.04] |
| Analysis 2.3  Comparison 2 Desmopressin vs placebo (platelet dysfunction), Outcome 3 Number of participants receiving a red cell transfusion (total). | ||||
| 4 Blood loss (total) Show forest plot | 7 | 422 | Mean Difference (IV, Random, 95% CI) | ‐253.93 [‐408.01, ‐99.85] |
| Analysis 2.4  Comparison 2 Desmopressin vs placebo (platelet dysfunction), Outcome 4 Blood loss (total). | ||||
| 5 Reoperation due to bleeding Show forest plot | 6 | 413 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.39 [0.18, 0.84] |
| Analysis 2.5 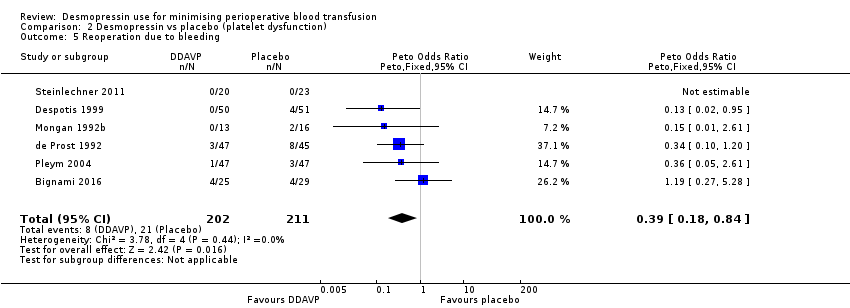 Comparison 2 Desmopressin vs placebo (platelet dysfunction), Outcome 5 Reoperation due to bleeding. | ||||
| 6 All‐cause mortality Show forest plot | 7 | 422 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.72 [0.12, 4.22] |
| Analysis 2.6  Comparison 2 Desmopressin vs placebo (platelet dysfunction), Outcome 6 All‐cause mortality. | ||||
| 7 All thrombotic events (including myocardial infarction, ischaemic stroke, other arterial thromboembolism, and venous thromboembolism) Show forest plot | 7 | 422 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.58 [0.60, 4.17] |
| Analysis 2.7 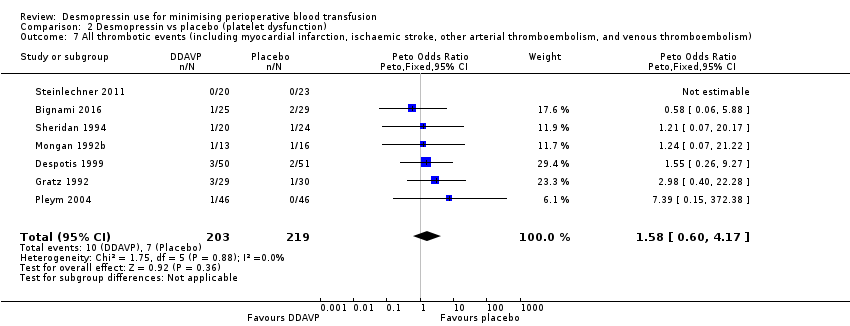 Comparison 2 Desmopressin vs placebo (platelet dysfunction), Outcome 7 All thrombotic events (including myocardial infarction, ischaemic stroke, other arterial thromboembolism, and venous thromboembolism). | ||||
| 8 Myocardial infarction Show forest plot | 5 | 277 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.72 [0.60, 12.37] |
| Analysis 2.8  Comparison 2 Desmopressin vs placebo (platelet dysfunction), Outcome 8 Myocardial infarction. | ||||
| 9 Stroke Show forest plot | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.9  Comparison 2 Desmopressin vs placebo (platelet dysfunction), Outcome 9 Stroke. | ||||
| 10 Venous thromboembolism Show forest plot | 4 | 248 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.56 [0.06, 5.50] |
| Analysis 2.10  Comparison 2 Desmopressin vs placebo (platelet dysfunction), Outcome 10 Venous thromboembolism. | ||||
| 11 Clinically important hypotension Show forest plot | 5 | 315 | Risk Ratio (M‐H, Random, 95% CI) | 6.58 [1.18, 36.76] |
| Analysis 2.11  Comparison 2 Desmopressin vs placebo (platelet dysfunction), Outcome 11 Clinically important hypotension. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Red cell volume transfused (total) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.1 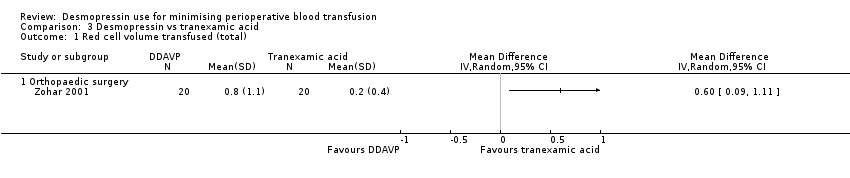 Comparison 3 Desmopressin vs tranexamic acid, Outcome 1 Red cell volume transfused (total). | ||||
| 1.1 Orthopaedic surgery | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of participants receiving a red cell transfusion (total) Show forest plot | 3 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 2.42 [1.04, 5.64] |
| Analysis 3.2  Comparison 3 Desmopressin vs tranexamic acid, Outcome 2 Number of participants receiving a red cell transfusion (total). | ||||
| 2.1 Cardiac surgery | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.82, 2.59] |
| 2.2 Orthopaedic surgery | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 4.15 [1.58, 10.90] |
| 3 Blood loss (total) Show forest plot | 2 | 115 | Mean Difference (IV, Random, 95% CI) | 142.81 [79.78, 205.84] |
| Analysis 3.3  Comparison 3 Desmopressin vs tranexamic acid, Outcome 3 Blood loss (total). | ||||
| 3.1 Cardiac surgery | 1 | 75 | Mean Difference (IV, Random, 95% CI) | 115.0 [35.38, 194.62] |
| 3.2 Orthopaedic surgery | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 180.0 [86.82, 273.18] |
| 4 Reoperation due to bleeding Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.4  Comparison 3 Desmopressin vs tranexamic acid, Outcome 4 Reoperation due to bleeding. | ||||
| 4.1 Cardiac surgery | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 All thrombotic events (including myocardial infarction, ischaemic stroke, other arterial thromboembolism, and venous thromboembolism) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 3.5 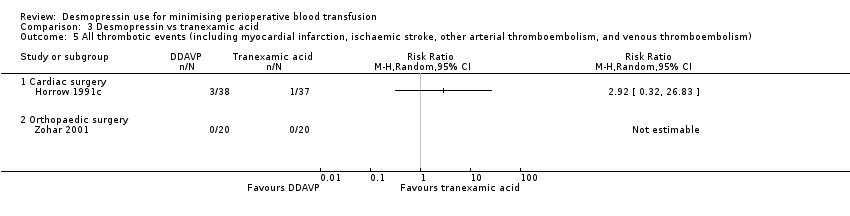 Comparison 3 Desmopressin vs tranexamic acid, Outcome 5 All thrombotic events (including myocardial infarction, ischaemic stroke, other arterial thromboembolism, and venous thromboembolism). | ||||
| 5.1 Cardiac surgery | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Orthopaedic surgery | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Myocardial infarction Show forest plot | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 3.6  Comparison 3 Desmopressin vs tranexamic acid, Outcome 6 Myocardial infarction. | ||||
| 6.1 Cardiac surgery | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Orthopaedic surgery | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Stroke Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 3.7  Comparison 3 Desmopressin vs tranexamic acid, Outcome 7 Stroke. | ||||
| 7.1 Cardiac surgery | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Orthopaedic surgery | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Venous thromboembolism Show forest plot | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 3.8  Comparison 3 Desmopressin vs tranexamic acid, Outcome 8 Venous thromboembolism. | ||||
| 8.1 Cardiac surgery | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Orthopaedic surgery | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants receiving a red cell transfusion (total) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 4.1  Comparison 4 Desmopressin vs aprotinin, Outcome 1 Number of participants receiving a red cell transfusion (total). | ||||
| 1.1 Cardiac surgery | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Reoperation due to bleeding Show forest plot | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.2  Comparison 4 Desmopressin vs aprotinin, Outcome 2 Reoperation due to bleeding. | ||||
| 2.1 Cardiac surgery | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 All‐cause mortality Show forest plot | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 4.3  Comparison 4 Desmopressin vs aprotinin, Outcome 3 All‐cause mortality. | ||||
| 3.1 Cardiac surgery | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 All thrombotic events (including myocardial infarction, ischaemic stroke, other arterial thromboembolism, and venous thromboembolism) Show forest plot | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.4  Comparison 4 Desmopressin vs aprotinin, Outcome 4 All thrombotic events (including myocardial infarction, ischaemic stroke, other arterial thromboembolism, and venous thromboembolism). | ||||
| 4.1 Cardiac surgery | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Myocardial infarction Show forest plot | 2 | 152 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 4.5  Comparison 4 Desmopressin vs aprotinin, Outcome 5 Myocardial infarction. | ||||
| 5.1 Cardiac surgery | 2 | 152 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Stroke Show forest plot | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.6  Comparison 4 Desmopressin vs aprotinin, Outcome 6 Stroke. | ||||
| 6.1 Cardiac surgery | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Venous thromboembolism Show forest plot | 2 | 152 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 4.7  Comparison 4 Desmopressin vs aprotinin, Outcome 7 Venous thromboembolism. | ||||
| 7.1 Cardiac surgery | 2 | 152 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Clinically significant hypotension Show forest plot | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 4.8 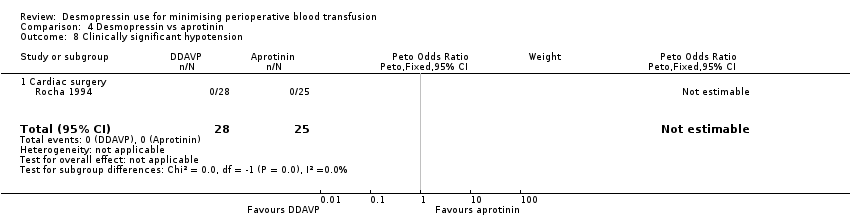 Comparison 4 Desmopressin vs aprotinin, Outcome 8 Clinically significant hypotension. | ||||
| 8.1 Cardiac surgery | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |

Study flow diagram.
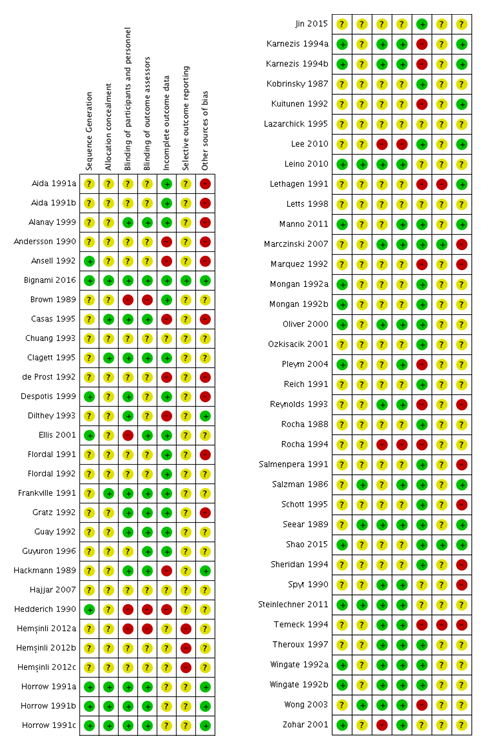
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
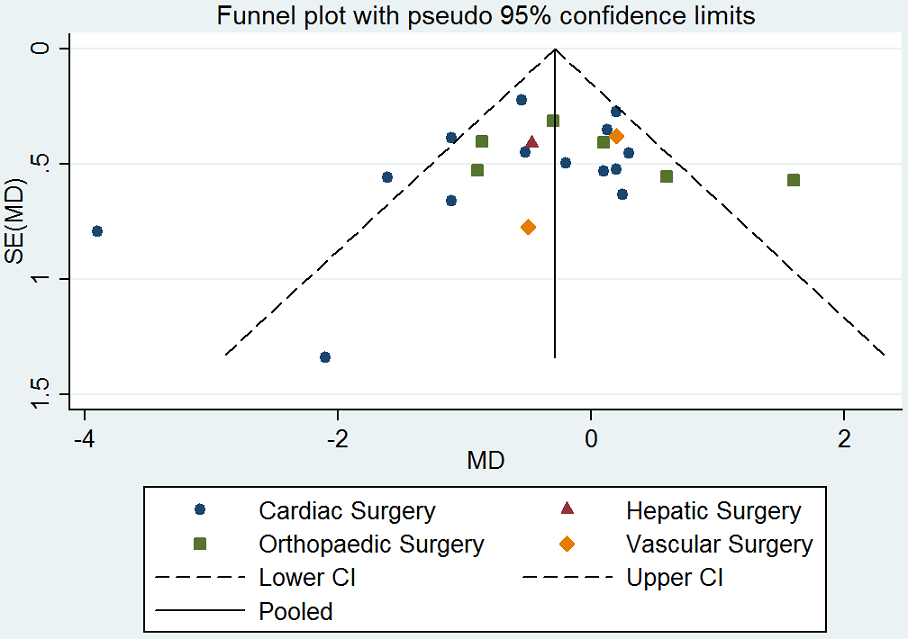
Funnel plot of comparison: desmopressin vs placebo: total red cell volume transfused. CI: confidence interval; MD: mean difference; SE: standard error.
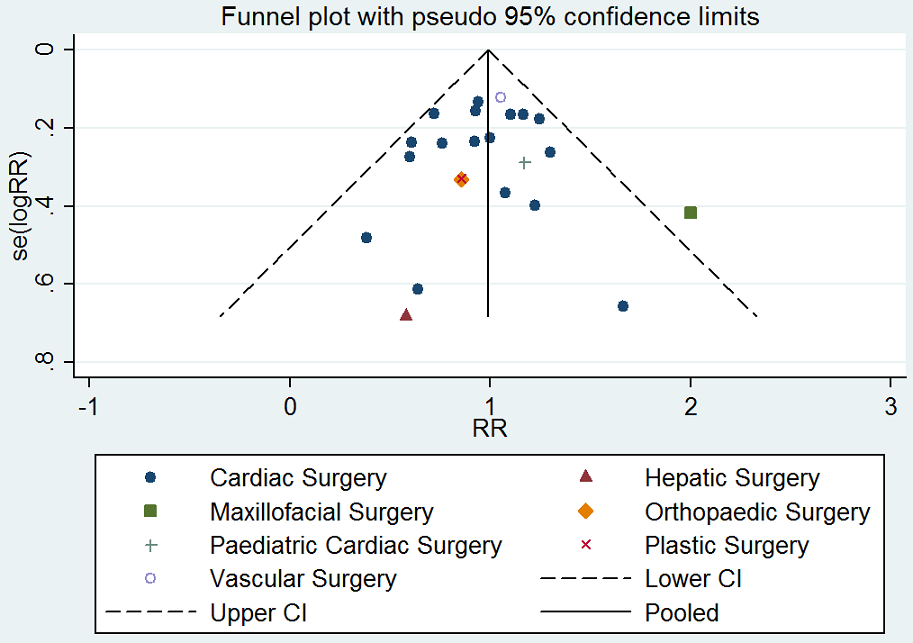
Funnel plot of comparison: desmopressin vs placebo: number of participants receiving a red cell transfusion. CI: confidence interval; RR: relative risk.
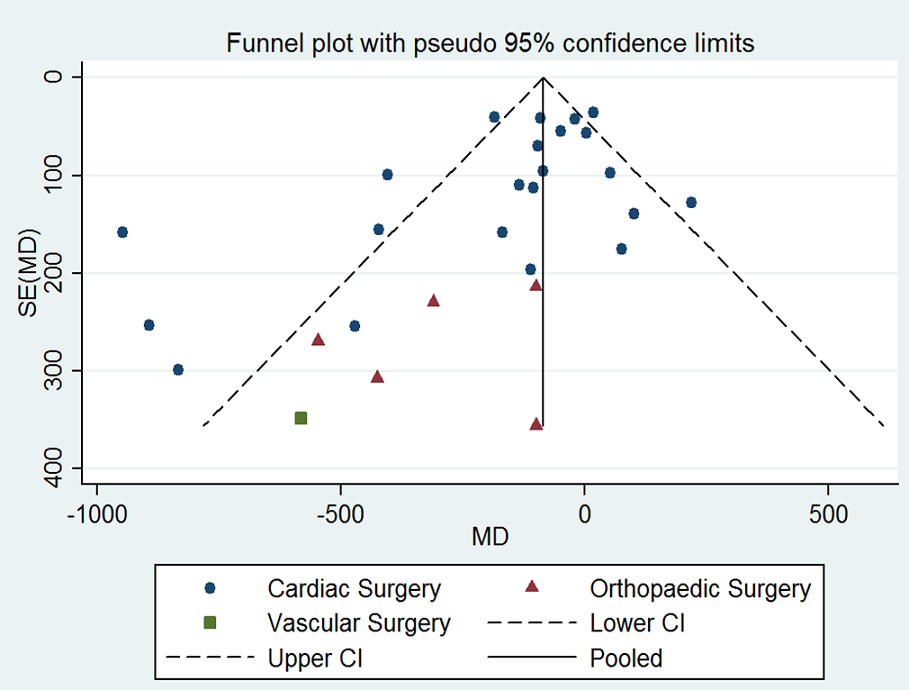
Funnel plot of comparison: desmopressin vs placebo: total blood loss. CI: confidence interval; MD: mean difference; SE: standard error.
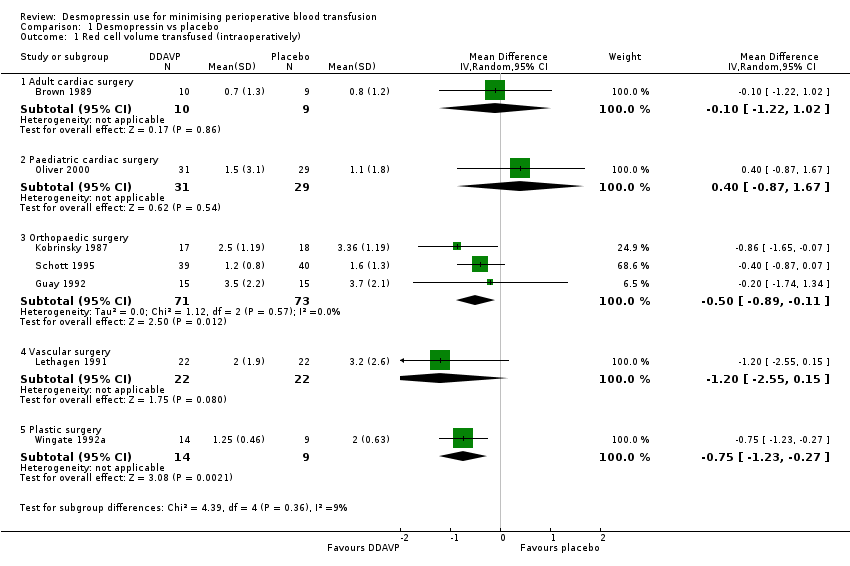
Comparison 1 Desmopressin vs placebo, Outcome 1 Red cell volume transfused (intraoperatively).
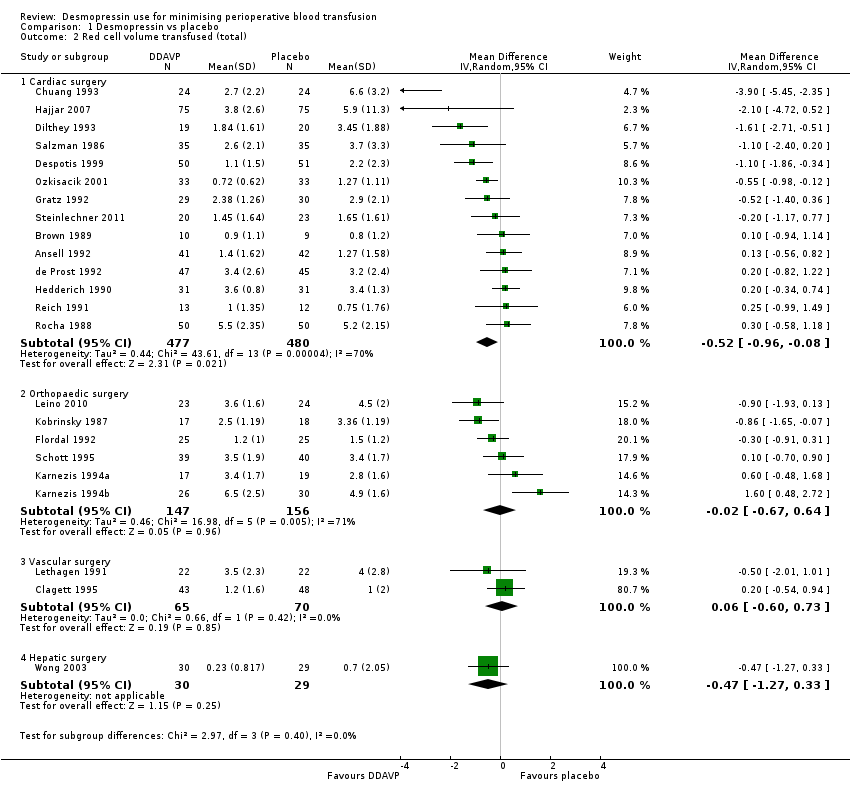
Comparison 1 Desmopressin vs placebo, Outcome 2 Red cell volume transfused (total).

Comparison 1 Desmopressin vs placebo, Outcome 3 Red cell volume transfused (children only, total).
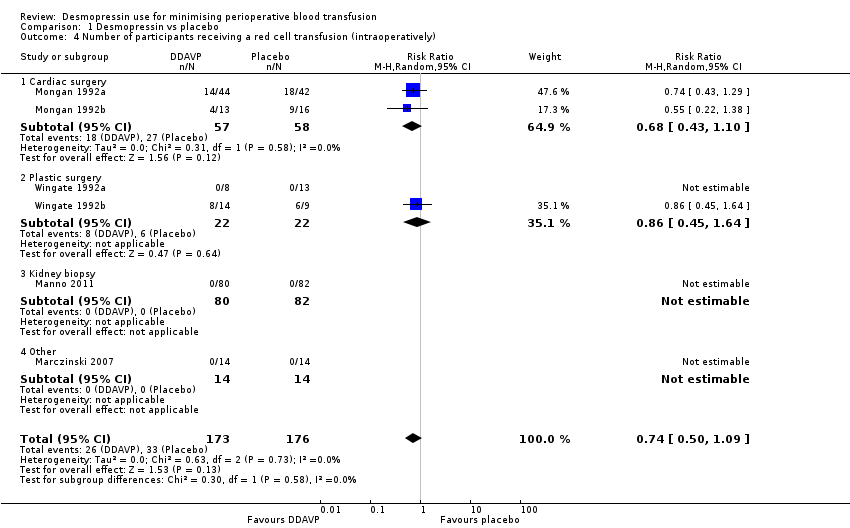
Comparison 1 Desmopressin vs placebo, Outcome 4 Number of participants receiving a red cell transfusion (intraoperatively).
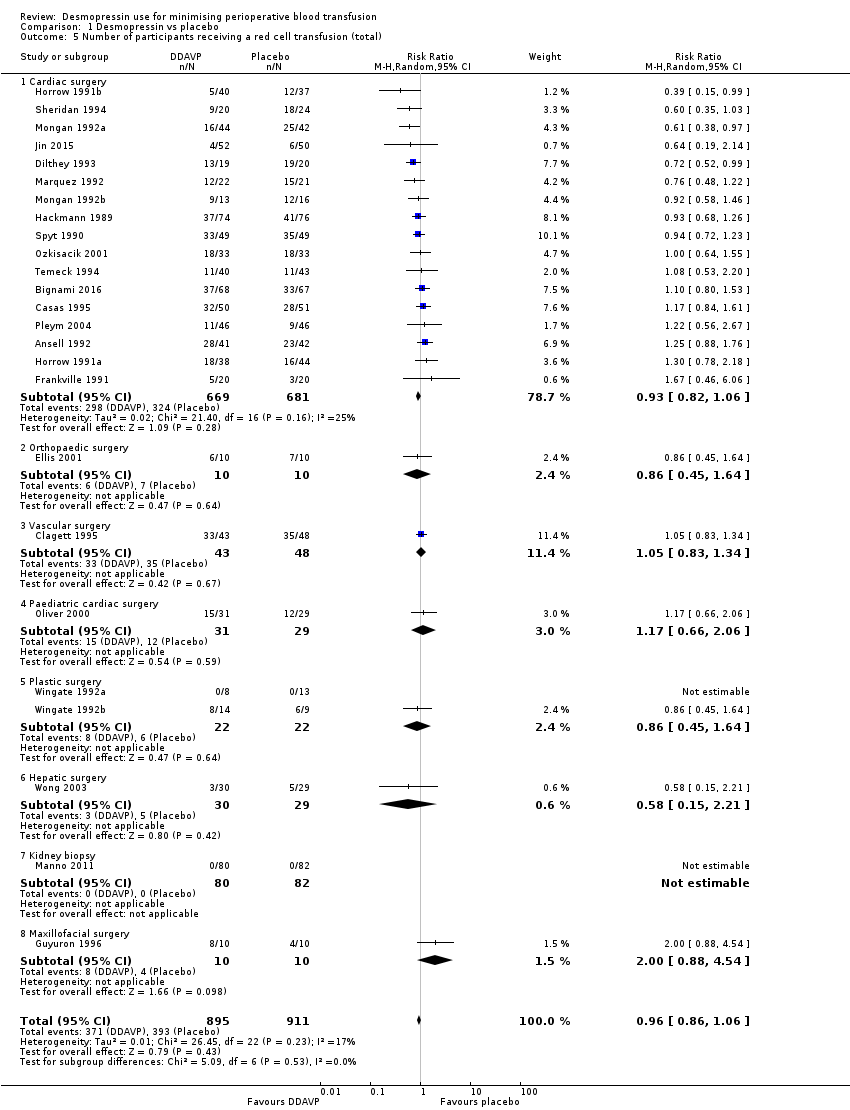
Comparison 1 Desmopressin vs placebo, Outcome 5 Number of participants receiving a red cell transfusion (total).

Comparison 1 Desmopressin vs placebo, Outcome 6 Blood loss (intraoperative).

Comparison 1 Desmopressin vs placebo, Outcome 7 Blood loss (total).
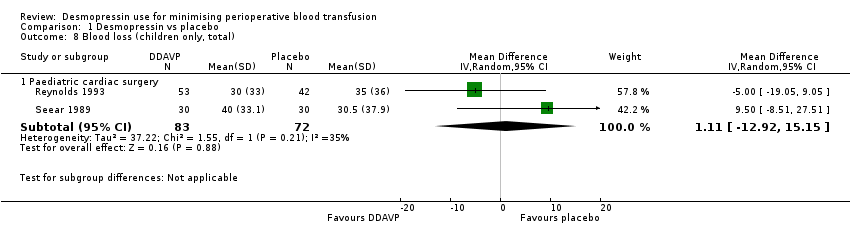
Comparison 1 Desmopressin vs placebo, Outcome 8 Blood loss (children only, total).
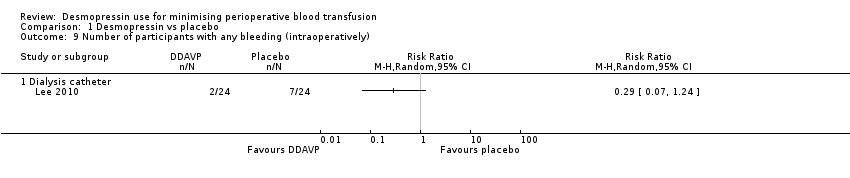
Comparison 1 Desmopressin vs placebo, Outcome 9 Number of participants with any bleeding (intraoperatively).
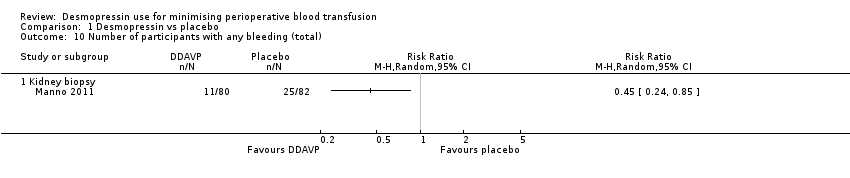
Comparison 1 Desmopressin vs placebo, Outcome 10 Number of participants with any bleeding (total).
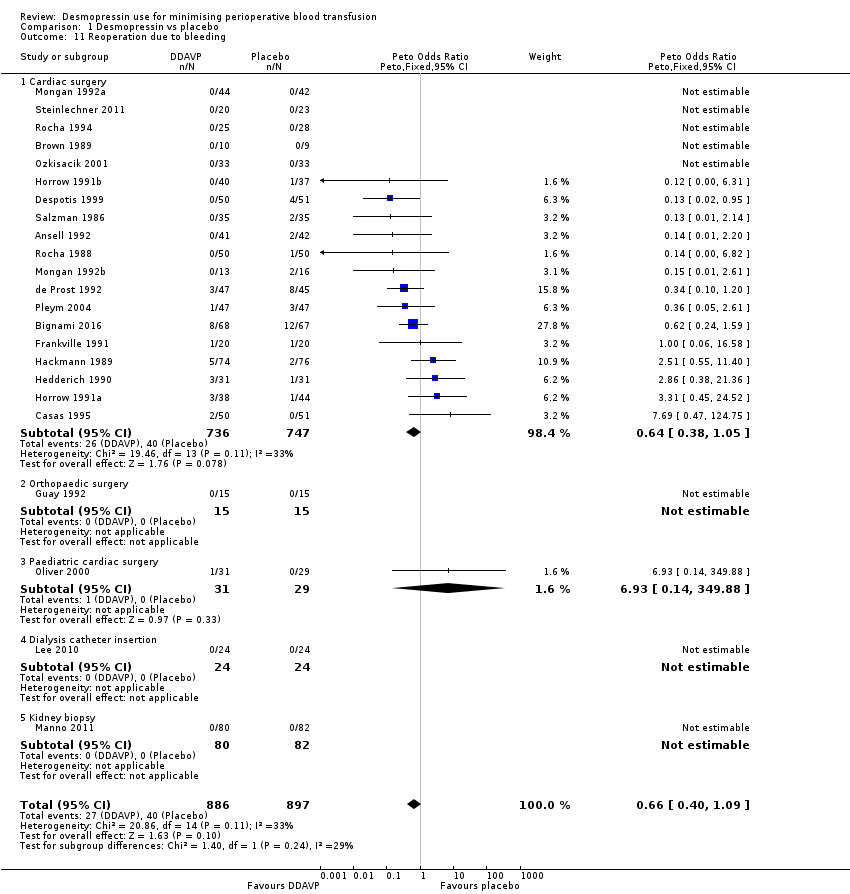
Comparison 1 Desmopressin vs placebo, Outcome 11 Reoperation due to bleeding.

Comparison 1 Desmopressin vs placebo, Outcome 12 All‐cause mortality.
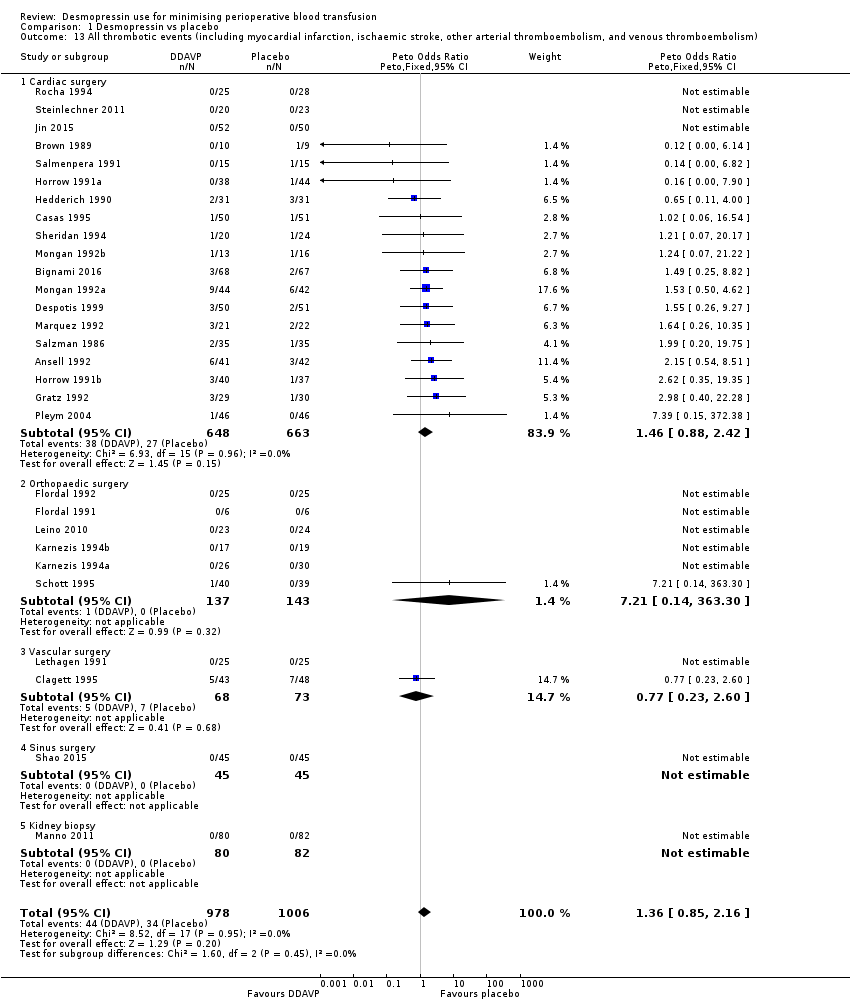
Comparison 1 Desmopressin vs placebo, Outcome 13 All thrombotic events (including myocardial infarction, ischaemic stroke, other arterial thromboembolism, and venous thromboembolism).
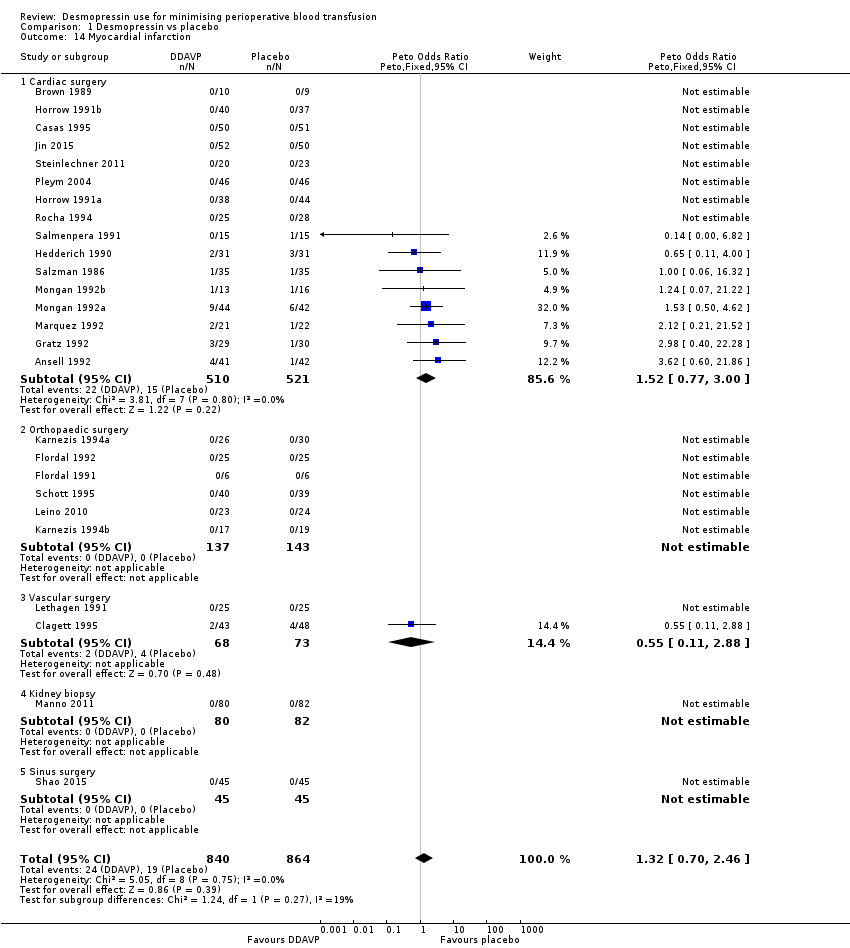
Comparison 1 Desmopressin vs placebo, Outcome 14 Myocardial infarction.

Comparison 1 Desmopressin vs placebo, Outcome 15 Stroke.
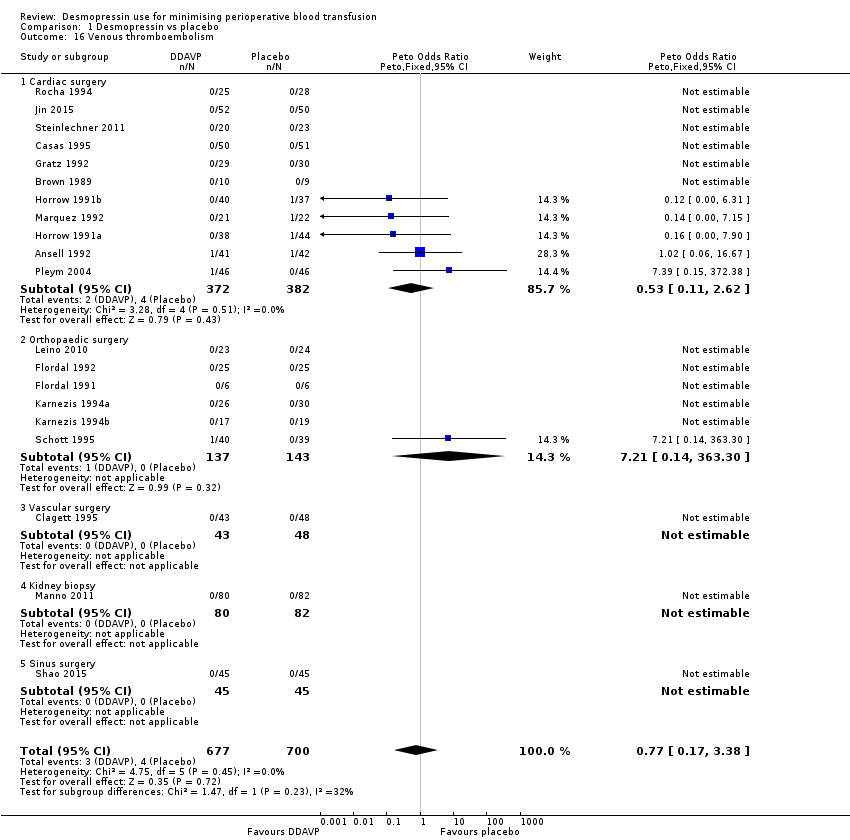
Comparison 1 Desmopressin vs placebo, Outcome 16 Venous thromboembolism.
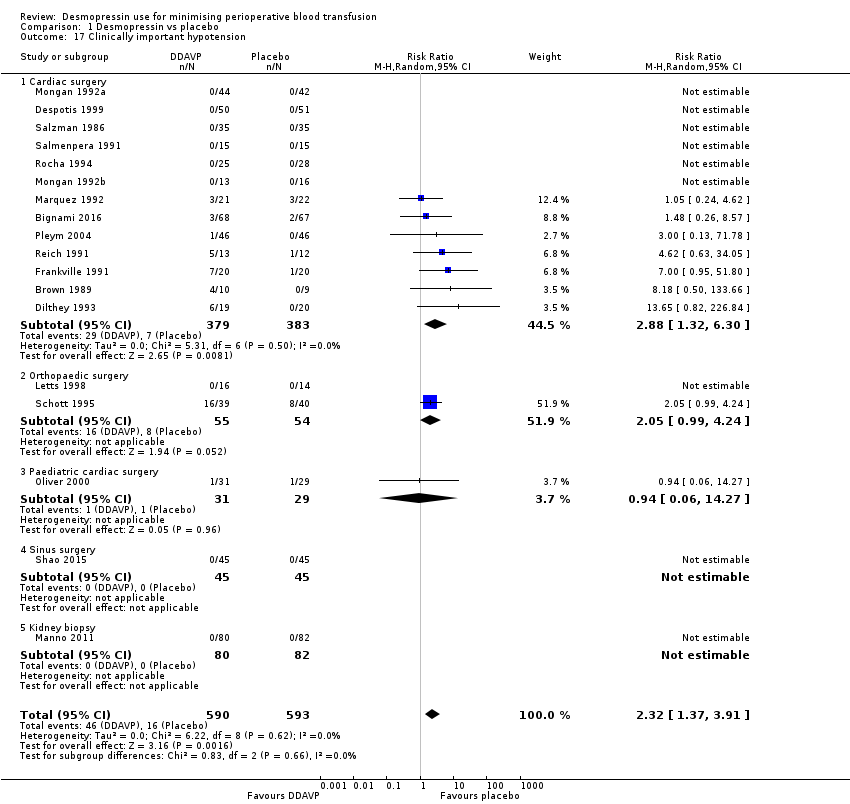
Comparison 1 Desmopressin vs placebo, Outcome 17 Clinically important hypotension.

Comparison 2 Desmopressin vs placebo (platelet dysfunction), Outcome 1 Red cell volume transfused (total).

Comparison 2 Desmopressin vs placebo (platelet dysfunction), Outcome 2 Number of participants receiving a red cell transfusion (intraoperatively).

Comparison 2 Desmopressin vs placebo (platelet dysfunction), Outcome 3 Number of participants receiving a red cell transfusion (total).
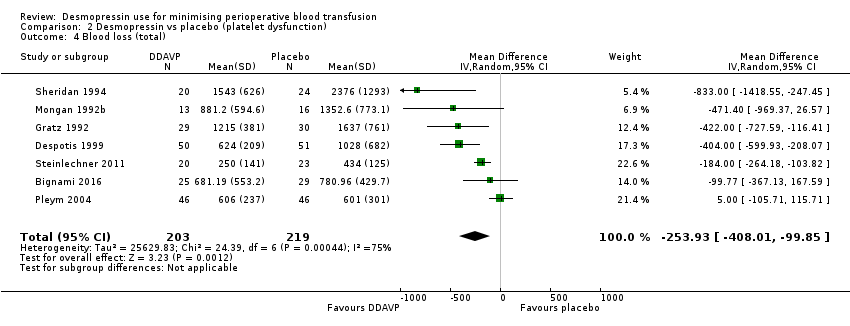
Comparison 2 Desmopressin vs placebo (platelet dysfunction), Outcome 4 Blood loss (total).
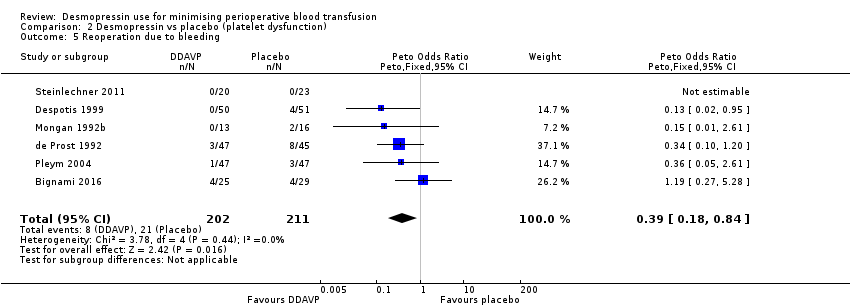
Comparison 2 Desmopressin vs placebo (platelet dysfunction), Outcome 5 Reoperation due to bleeding.

Comparison 2 Desmopressin vs placebo (platelet dysfunction), Outcome 6 All‐cause mortality.
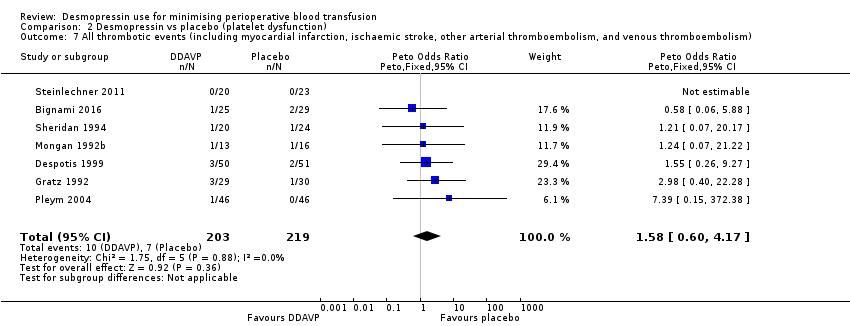
Comparison 2 Desmopressin vs placebo (platelet dysfunction), Outcome 7 All thrombotic events (including myocardial infarction, ischaemic stroke, other arterial thromboembolism, and venous thromboembolism).

Comparison 2 Desmopressin vs placebo (platelet dysfunction), Outcome 8 Myocardial infarction.
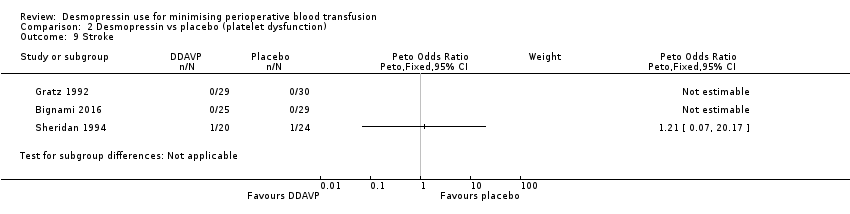
Comparison 2 Desmopressin vs placebo (platelet dysfunction), Outcome 9 Stroke.

Comparison 2 Desmopressin vs placebo (platelet dysfunction), Outcome 10 Venous thromboembolism.
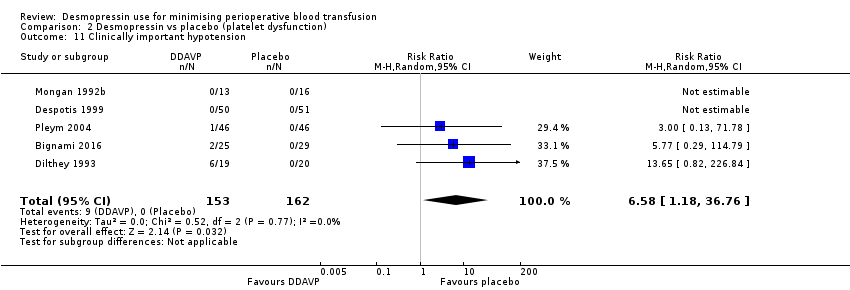
Comparison 2 Desmopressin vs placebo (platelet dysfunction), Outcome 11 Clinically important hypotension.

Comparison 3 Desmopressin vs tranexamic acid, Outcome 1 Red cell volume transfused (total).
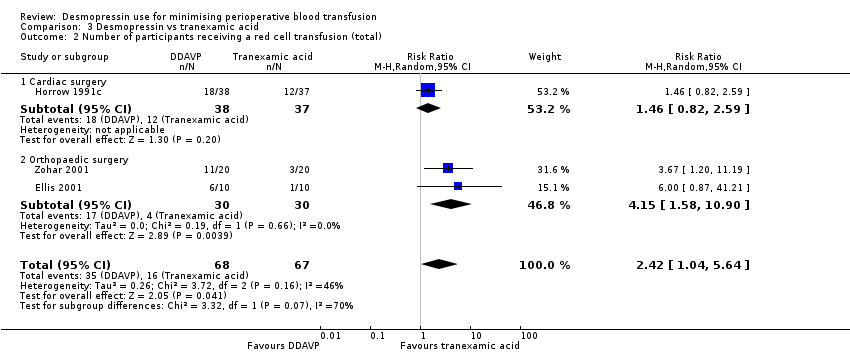
Comparison 3 Desmopressin vs tranexamic acid, Outcome 2 Number of participants receiving a red cell transfusion (total).
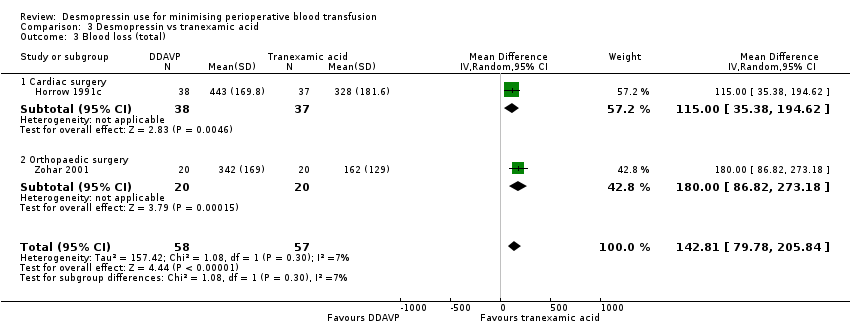
Comparison 3 Desmopressin vs tranexamic acid, Outcome 3 Blood loss (total).
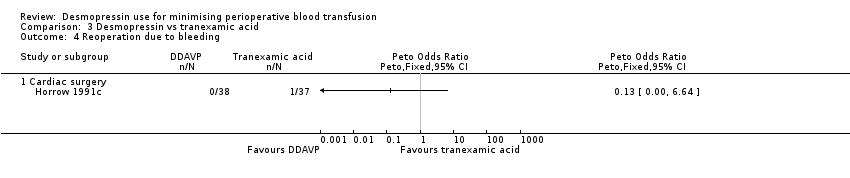
Comparison 3 Desmopressin vs tranexamic acid, Outcome 4 Reoperation due to bleeding.

Comparison 3 Desmopressin vs tranexamic acid, Outcome 5 All thrombotic events (including myocardial infarction, ischaemic stroke, other arterial thromboembolism, and venous thromboembolism).

Comparison 3 Desmopressin vs tranexamic acid, Outcome 6 Myocardial infarction.

Comparison 3 Desmopressin vs tranexamic acid, Outcome 7 Stroke.

Comparison 3 Desmopressin vs tranexamic acid, Outcome 8 Venous thromboembolism.

Comparison 4 Desmopressin vs aprotinin, Outcome 1 Number of participants receiving a red cell transfusion (total).

Comparison 4 Desmopressin vs aprotinin, Outcome 2 Reoperation due to bleeding.
Comparison 4 Desmopressin vs aprotinin, Outcome 3 All‐cause mortality.

Comparison 4 Desmopressin vs aprotinin, Outcome 4 All thrombotic events (including myocardial infarction, ischaemic stroke, other arterial thromboembolism, and venous thromboembolism).

Comparison 4 Desmopressin vs aprotinin, Outcome 5 Myocardial infarction.

Comparison 4 Desmopressin vs aprotinin, Outcome 6 Stroke.
Comparison 4 Desmopressin vs aprotinin, Outcome 7 Venous thromboembolism.

Comparison 4 Desmopressin vs aprotinin, Outcome 8 Clinically significant hypotension.
| Participant or population: participants undergoing surgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with desmopressin | |||||
| Red cell volume transfused (total) | Adult cardiac surgery: red cell volume transfused in the desmopressin group was 0.52 units less (0.96 fewer to 0.08 fewer units, 14 RCTs, 957 participants) | 1454 | ⊕⊕⊝⊝ | Data not pooled due to clinical heterogeneity and reported as subgroups | ||
| Orthopaedic surgery: red cell volume transfused in the desmopressin group was 0.02 units less (0.67 less to 0.64 more units, 6 RCTs, 303 participants) | ||||||
| Vascular surgery: red cell volume transfused in the desmopressin group was 0.06 units more (0.60 less to 0.73 more units, 2 RCTs, 135 participants) | ||||||
| Hepatic surgery: red cell volume transfused in the desmopressin group was 0.47 units less (1.27 less to 0.33 more units, 1 RCT, 59 participants) | ||||||
| Number of participants receiving a red cell transfusion (total) | 450 per 1000 | 436 per 1000 | RR 0.96 | 1806 | ⊕⊕⊕⊝ | |
| Blood loss (total) | Cardiac surgery: total blood loss in the desmopressin group was 135.24 mL less (210.8 mL to 59.68 mL less, 22 RCTs, 1358 participants). | 1643 | ⊕⊝⊝⊝ | Data not pooled owing to clinical heterogeneity and reported as subgroups | ||
| Orthopaedic surgery: total blood loss in the desmopressin group was 285.76 mL less (514.99 mL to 56.53 mL less, 5 RCTs, 241 participants) | ||||||
| Vascular surgery: total blood loss in the desmopressin group was 582 mL less (1264.07 mL less to 100.07 mL more, 1 RCT, 44 participants) | ||||||
| All‐cause mortality | 16 per 1000 | 17 per 1000 | pOR 1.09 (0.51 to 2.34) | 1631 | ⊕⊕⊝⊝ | |
| All thrombotic events (including myocardial infarction, ischaemic stroke, other arterial thromboembolism, and venous thromboembolism) | 34 per 1000 | 44 per 1000 | pOR 1.36 (0.85 to 2.16) | 1984 | ⊕⊕⊝⊝ | |
| Quality of life | Not reported | ‐ | (No studies) | ‐ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level due to risk of bias: inadequate reporting of blinding and incomplete outcome data bDowngraged one level for inconsistency: I2 = 66% cDowngraded one level for inconsistency: I2 = 73% and sensitivity analysis unable to determine cause of heterogeneity dDowngraded one level for suspected publication bias eDowngraded one level due to imprecision, as confidence intervals include both clinically important benefit and clinically important harm | ||||||
| Participant or population: participants with platelet dysfunction undergoing surgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with desmopressin | |||||
| Red cell volume transfused (total) | Red cell volume transfused was 2.6 units | Red cell volume transfused in the desmopressin group was 0.65 units less (1.16 less to 0.13 less) | ‐ | 388 | ⊕⊕⊝⊝ | |
| Number of participants receiving a red cell transfusion (total) | 541 per 1000 | 449 per 1000 | RR 0.83 | 258 | ⊕⊕⊝⊝ | |
| Blood loss (total) | Mean total blood loss was 1098 mL | Total blood loss in the desmopressin group was 253.93 mL less (408.01 mL less to 99.85 mL less) | ‐ | 422 | ⊕⊕⊝⊝ | |
| All‐cause mortality | 14 per 1000 | 10 per 1000 (2 to 59) | pOR 0.72 (0.12 to 4.22) | 422 | ⊕⊝⊝⊝ | |
| All thrombotic events (including myocardial infarction, ischaemic stroke, other arterial thromboembolism, and venous thromboembolism) | 32 per 1000 | 51 per 1000 | pOR 1.58 | 422 | ⊕⊝⊝⊝ | |
| Quality of life | Not reported | ‐ | (No studies) | ‐ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for risk of bias bDowngraded one level for inconsistency due to variation in baseline level of transfusion and blood loss cDowngraded two levels for imprecision, as confidence intervals include clinically important benefit and clinically important harm with low background event rate dDowngraded one level for imprecision | ||||||
| Participant or population: participants undergoing surgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with tranexamic acid | Risk with desmopressin | |||||
| Red cell volume transfused (total) | Mean red cell volume transfused was 0.2 units | Red cell volume transfused in the desmopressin group was 0.6 units more (0.09 more to 1.11 more) | ‐ | 40 | ⊕⊕⊝⊝ | |
| Number of participants receiving a red cell transfusion (total) | 239 per 1000 | 578 per 1000 | RR 2.42 | 135 | ⊕⊝⊝⊝ | |
| Blood loss (total) | Mean blood loss was 270 mL | Total blood loss in the desmopressin group was 142.81 mL more (79.78 mL more to 205.84 mL more) | ‐ | 115 | ⊕⊕⊝⊝ | |
| All‐cause mortality | Not reported | ‐ | (No studies) | ‐ | ||
| All thrombotic events (including myocardial infarction, ischaemic stroke, other arterial thromboembolism, and venous thromboembolism) | 18 per 1000 | 51 per 1000 | RR 2.92 | 115 | ⊕⊝⊝⊝ | |
| Quality of life | Not reported | ‐ | (No studies) | ‐ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for risk of bias bDowngraded one level for indirectness because most types of surgery or procedures were not represented by the included trials cDowngraded one level for imprecision owing to wide confidence intervals dDowngraded two levels for imprecision owing to very wide confidence intervals eOutcome not downgraded for indirectness because already downgraded three levels for other reasons | ||||||
| Participant or population: participants undergoing surgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with aprotinin | Risk with desmopressin | |||||
| Red cell volume transfused (total) | Not reported | ‐ | (No studies) | ‐ | ||
| Number of participants receiving a red cell transfusion (total) | 265 per 1000 | 639 per 1000 | RR 2.41 | 99 | ⊕⊕⊝⊝ | |
| Blood loss (total) | Not reported | ‐ | (No studies) | ‐ | ||
| All‐cause mortality | No deaths in either arm of the trial | Not estimable | 53 | ⊕⊝⊝⊝ | ||
| All thrombotic events (including myocardial infarction, ischaemic stroke, other arterial thromboembolism, and venous thromboembolism) | 14 per 1000 | 13 per 1000 | pOR 0.98 | 152 | ⊕⊝⊝⊝ | |
| Quality of life | Not reported | ‐ | (No studies) | ‐ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for risk of bias bDowngraded one level for indirectness because most types of surgery or procedures were not represented by the included trials cDowngraded two levels for imprecision (no deaths in either arm) dNot downgraded for indirectness because already downgraded three levels for other reasons eDowngraded two levels for imprecision (very wide confidence intervals) | ||||||
| Trial (country) | Number of participants | Surgery type | Cases | Antiplatelet agents | Anticoagulants (%) | Coagulopathy (%) | Thrombocytopenia (%) | Antifibrinolytics (%) | Transfusion protocol | Timing of blood loss or transfusion assessment (hours) |
| (Japan) | 9 | Cardiac | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 24 |
| (Japan) | 11 | Cardiac | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 24 |
| (Turkey) | 40 | Orthopaedic | Elective | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 24 |
| (Sweden) | 19 | Cardiac | Elective | 0 | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| (USA) | 83 | Cardiac | Elective | ‐ | ‐ | ‐ | ‐ | DDAVP: 2‐4a Placebo: 0 | ‐ | 24 |
| (Italy) | 135 | Cardiac | Elective | DDAVP: 38b Placebo: 43b | ‐ | DDAVP: 1‐5 Placebo: 1‐5 | ‐ | 100c | Yes | 24 |
| (USA) | 39 | Cardiac | Elective | DDAVP: 60b Placebo: 50b | ‐ | 0 | ‐ | ‐ | ‐ | 24 |
| (Spain) | 149 | Cardiac | Elective | DDAVP: 14b Placebo: 9‐8b Aprotinin: 14‐6b | 0 | 0 | ‐ | DDAVP: 0 Placebo: 0 Aprotinin: 100d | Yes | 24 |
| (China) | 96 | Cardiac | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 24 |
| (USA) | 91 | Vascular | Elective | 0 | ‐ | ‐ | ‐ | ‐ | ‐ | 72 |
| (France) | 92 | Cardiac | ‐ | 100e | ‐ | ‐ | 0 | DDAVP: 4‐3d Placebo: 13‐3d | Yes | 24 |
| (USA) | 101 | Cardiac | Elective | DDAVP: 52b Placebo: 66b 100f | DDAVP: 6 Placebo: 0 | ‐ | ‐ | DDAVP 50a Placebo 61a | No | 24 |
| (Germany) | 39 | Cardiac | Elective | 100b | 0 | 0 | ‐ | 0 | Yes | 24 |
| (Israel) | 30 | Orthopaedic | Elective | ‐ | ‐ | ‐ | ‐ | DDAVP: 0 TXA: 100c | Yes | 72 |
| (Sweden) | 12 | Orthopaedic | Elective | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 24 |
| (Sweden) | 50 | Orthopaedic | Elective | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| (USA) | 40 | Cardiac | Elective | 0 | 0 | 0 | 0 | ‐ | ‐ | 24 |
| (USA) | 59 | Cardiac | Elective | 100b | ‐ | ‐ | 0 | ‐ | ‐ | 24 |
| (Canada) | 30 | Orthopaedic | Elective | 0 | 0 | 0 | 0 | ‐ | Yes | 24 |
| (USA) | 20 | Maxillofacial | Elective | ‐ | ‐ | 0 | ‐ | ‐ | ‐ | 24 |
| (Canada) | 150 | Cardiac | Elective | DDAVP: 21‐6b Placebo: 14‐5b | ‐ | 0 | 0 | ‐ | ‐ | 24 |
| (Brazil) | 150 | Cardiac | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 72 |
| (Canada) | 62 | Cardiac | Elective | DDAVP: 38‐7b Placebo: 41.9b | ‐ | ‐ | ‐ | ‐ | ‐ | 18 blood loss 48 transfusion |
| (Turkey) | 20 | Cardiac | Emergency | 100b | ‐ | ‐ | ‐ | 0 | ‐ | 30 |
| (Turkey) | 34 | Cardiac | Emergency | 100b | ‐ | ‐ | ‐ | 100c | ‐ | 30 |
| (Turkey) | 28 | Cardiac | Emergency | 100b | ‐ | ‐ | ‐ | DDAVP: 0 TXA: 100c | ‐ | 30 |
| (USA) | 82 | Cardiac | Elective | ‐ | 0 | ‐ | ‐ | 0 | Yes | 12 |
| (USA) | 77 | Cardiac | Elective | ‐ | 0 | ‐ | ‐ | 100c | Yes | 12 |
| (USA) | 75 | Cardiac | Elective | ‐ | 0 | ‐ | ‐ | DDAVP: 0 TXA: 100c | Yes | 12 |
| (China) | 102 | Cardiac | Elective | ‐ | 0 | 0 | 0 | 100c | ‐ | 6 |
| (USA) | 36 | Orthopaedic | Elective | ‐ | 0 | ‐ | ‐ | ‐ | Yes | 24 |
| (USA) | 56 | Orthopaedic | Elective | ‐ | 0 | ‐ | ‐ | ‐ | Yes | 24 |
| (USA) | 35 | Cardiac | Elective | 0 | ‐ | ‐ | ‐ | ‐ | ‐ | 34 |
| (Finland) | 30 | Cardiac | Elective | 0 | 0 | ‐ | ‐ | ‐ | Yes | 16 |
| (USA) | 23 | Not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| (South Korea) | 48 | Dialysis catheter | Elective | 100g | ‐ | ‐ | 0 | ‐ | ‐ | ‐ |
| (Finland) | 71 | Orthopaedic | Elective | 0 | 0 | 0 | ‐ | ‐ | Yes | 96 |
| (Sweden) | 50 | Vascular | Elective | 0 | 0 | 0 | ‐ | ‐ | Yes | ‐ |
| (Canada) | 30 | Orthopaedic | Elective | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Intraoperative only |
| (Italy) | 162 | Kidney biopsy | Elective | 0 | 0 | ‐ | ‐ | ‐ | ‐ | 72 |
| (USA) | 65 | Cardiac | Elective | 0 | 0 | ‐ | ‐ | ‐ | Yes | 24 |
| (Netherlands) | 28 | Orthopaedic/Breast/Abdominal | Elective | 0 | 0 | 0 | 0 | ‐ | ‐ | 48 |
| (USA) | 86 | Cardiac | Elective | 0 | 0 | ‐ | ‐ | ‐ | Yes | 24 |
| (USA) | 29 | Cardiac | Elective | 100h | 0 | ‐ | ‐ | ‐ | Yes | 24 |
| (USA) | 60 | Paediatric cardiac | Elective | DDAVP: 9.7b Placebo: 3.4b | DDAVP: 6.5 Placebo: 6.9 | ‐ | ‐ | ‐ | No | 24 |
| (Turkey) | 66 | Cardiac | Elective | 0 | ‐ | 0 | ‐ | ‐ | Yes | 24 |
| (Norway) | 92 | Cardiac | Elective | 100b | 0 | 0 | 0 | DDAVP: 6.5c Placebo: 17.4c | Yes | 16 |
| (USA) | 27 | Cardiac | Elective | DDAVP: 28.6b Placebo: 38.5b | ‐ | 0 | 0 | ‐ | ‐ | 24 |
| (USA) | 95 | Paediatric cardiac | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 24 |
| (Spain) | 100 | Cardiac | Elective | ‐ | ‐ | 0 | 0 | ‐ | ‐ | 72 |
| (Spain) | 109 | Cardiac | Elective | ‐ | ‐ | 0 | 0 | DDAVP (1): 0 DDAVP (2): 0 Control: 0 Aprotinin: 100d | ‐ | 72 |
| (Finland) | 30 | Cardiac | Elective | 0 | 0 | 0 | ‐ | ‐ | Yes | 16 |
| (USA) | 70 | Cardiac | Elective | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 24 |
| (Sweden) | 79 | Orthopaedic | Elective | 0 | 0 | 0 | 0 | ‐ | Yes | 24 |
| (Canada) | 60 | Paediatric cardiac | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 24 |
| (China) | 90 | Sinus | Elective | 0 | 0 | 0 | 0 | ‐ | ‐ | Intraoperative only |
| (Canada) | 44 | Cardiac | Elective | 100b | 0 | 0 | 0 | ‐ | ‐ | 24 |
| (UK) | 98 | Cardiac | Elective | DDAVP: 14.3b Placebo: 10.2b | 0 | ‐ | ‐ | ‐ | Yes | ˜24 |
| (Austria) | 43 | Cardiac | Elective | 100g | 0 | ‐ | ‐ | ‐ | Yes | 24 |
| (USA) | 83 | Cardiac | Elective | ‐ | ‐ | ‐ | ‐ | DDAVP: 20a Placebo: 30.2a | ‐ | 24 |
| (USA) | 21 | Orthopaedic | Elective | 0 | 0 | 0 | 0 | ‐ | Yes | 24 |
| (USA) | 23 | Plastic | Elective | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 24 |
| (USA) | 21 | Plastic | Elective | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 24 |
| (Hong Kong) | 59 | Hepatic | Elective | 0 | 0 | 0 | ‐ | ‐ | Yes | Intraoperative only |
| (Israel) | 40 | Orthopaedic | Elective | ‐ | 0 | ‐ | ‐ | DDAVP: 0 TXA: 100c | Yes | 12 |
| Blank cells indicate that information was not reported in the original papers | ||||||||||
| Trial | DDAVP dose(s) (μg/kg) | Timing of dose | Timing summary | Comparator(s) |
| Preoperative DDAVP | ||||
| 0.3 | Induction of anaesthesia | Preoperative | Placebo | |
| 0.3 (× 2) | At start of surgery and again after 6 hours | Preoperative | Placebo | |
| 0.3 (× 2) | At start of surgery and again after 6 hours | Preoperative | Placebo | |
| 10 μg/m2 | At time of first skin incision | Preoperative | Placebo | |
| 20 μg | 30 minutes preoperatively | Preoperative | Placebo | |
| 10 μg/m2 | Immediately after induction of anaesthesia | Preoperative | Placebo | |
| 0.3 | After anaesthetic induction | Preoperative | Placebo | |
| 0.3 | Not reported | Preoperative | Placebo | |
| 0.4 | At start of surgery | Preoperative | Placebo | |
| DDAVP 0.2 μg/kg | ||||
| 0.3 | Immediately before start of operation | Preoperative | Placebo | |
| 10 μg/m2 | Immediately after induction of anaesthesia | Preoperative | Placebo | |
| 0.3 | 1 hour before biopsy | Preoperative | Placebo | |
| 15 μg to 45 μg depending on weight | Not reported | Preoperative | Placebo | |
| 0.3 (× 2) | Post induction of anaesthesia and again after 6 hours | Preoperative | Placebo | |
| 0.3 | After induction of anaesthesia | Preoperative | Placebo | |
| 0.3 | After induction of anaesthesia | Preoperative | Placebo | |
| 0.3 | Not reported | Preoperative | Placebo | |
| 0.3 | After induction of anaesthesia | Preoperative | Placebo | |
| 0.3 | After induction of anaesthesia | Preoperative | Placebo | |
| 0.3 | After induction of anaesthesia | Preoperative | Placebo | |
| DDAVP administered at end of operation | ||||
| 0.3 | 15 minutes after reversal of heparin | End of operation | Placebo | |
| 0.3 | 15 minutes after reversal of heparin | End of operation | Placebo | |
| 0.3 | 15 minutes after reversal of heparin | End of operation | Placebo | |
| 0.3 | Immediately after reversal of heparin | End of operation | Placebo | |
| 0.3 | In event of excessive bleeding, after reversal of heparin | End of operation/postoperative | Placebo | |
| 0.3 | Immediately after reversal of heparin | End of operation | Placebo | |
| 0.3 | Immediately after reversal of heparin | End of operation | Placebo | |
| Aprotinina | ||||
| 0.3 | 60 minutes after reversal of heparin | End of operation | Placebo | |
| 20 μg | 15 minutes after heparinisation and before aortic cross‐clamp application | End of operation | Placebo | |
| 0.4 | Unclear | End of operation | Placebo | |
| 0.3 | 5 minutes after reversal of heparin | End of operation | Placebo | |
| 0.3 | Before removal of tourniquet | End of operation | Placebo | |
| Tranexamic acidb | ||||
| 0.3 | 5 minutes after reversal of heparin | End of operation | Placebo | |
| 0.3 | Immediately after reversal of heparin | End of operation | Placebo | |
| 0.3 | Immediately after reversal of heparin | End of operation | Placebo | |
| 0.3 | Immediately after surgery | End of operation | Placebo | |
| 0.3 | Immediately after reversal of heparin | End of operation | Placebo | |
| 0.3 | Immediately after reversal of heparin | End of operation | Placebo | |
| 0.3 | Immediately after reversal of heparin | End of operation | Placebo | |
| 0.3 | Immediately after reversal of heparin | End of operation | Tranexamic acidc | |
| 0.3 | Before cardiac rewarming | End of operation | Placebo | |
| 0.3 | 30 minutes before closure of wound | End of operation | Placebo | |
| 0.3 | 30 minutes before closure of wound | End of operation | Placebo | |
| 0.3 | Immediately after reversal of heparin | End of operation | Placebo | |
| DDAVP 0.3 μg/kg × 2 | ||||
| 0.3 | After reversal of heparin and before chest closure | End of operation | Placebo | |
| 0.3 | After reversal of heparin and before chest closure | End of operation | Placebo | |
| 0.3 | 10 minutes after reversal of heparin | End of operation | Placebo | |
| 0.3 | After reversal of heparin (timing unclear) | End of operation | Placebo | |
| 0.3 | Immediately after reversal of heparin | End of operation | Placebo | |
| 0.3 | 15 minutes after reversal of heparin | End of operation | Placebo | |
| 0.3 | 5 minutes after reversal of heparin | End of operation | Placebo | |
| 0.3 | Immediately after reversal of heparin | End of operation | Placebo | |
| 0.3 | Immediately after reversal of heparin | End of operation | Standard care | |
| Aprotinind | ||||
| DDAVP 0.3 μg/kg × 2 | ||||
| 0.3 | Via pulmonary artery catheter immediately after sternal closure | End of operation | Placebo | |
| 0.3 | Immediately after reversal of heparin | End of operation | Placebo | |
| 0.3 | After reversal of heparin (timing unclear) | End of operation | Placebo | |
| 10 μg/m2 | After reversal of heparin (timing unclear) | End of operation | Placebo | |
| 0.3 | After reversal of heparin (timing unclear) | End of operation | Placebo | |
| 0.3 | After reversal of heparin (timing unclear) | End of operation | Placebo | |
| 0.3 | 30 minutes before deflation of tourniquet | End of operation | Tranexamic acidb | |
| DDAVP administered postoperatively | ||||
| 0.3 | Between end of operation and 6 hours postoperatively | Postoperative | Placebo | |
| 0.3 | Immediately after sternal closure | Postoperative | Placebo | |
| Timing of DDAVP administration unclear | ||||
| 0.3 | Not reported | Not clear | Standard care | |
| 0.3 | Not reported | Not clear | Standard care | |
| 0.3 | Not reported | Not clear | Tranexamic acidc | |
| aAprotinin 2 million KIU in 200 mL preoperatively, 2 million KIU in 200 mL in fluid prime, 500,000 KIU in 50 mL/h from skin incision to skin closure Abbreviation KIU: kilounits | ||||
| Trial | Reason not included in meta‐analysis | DDAVP arm | Placebo arm |
| Orthopaedic surgery | |||
| Reported as mean (no standard deviation) | 0.3 units (n = 23) | 0.5 units (n = 24) | |
| Reported as mean (no standard deviation) | 4.6 units (n = 16) | 5.0 units (n = 14) | |
| Reported as median and range | 51.5 (24 to 98.6) mL/kg (n = 10) | 48.3 (24.5 to 96) mL/kg (n = 11) | |
| Trial | Reason not included in meta‐analysis | DDAVP arm | Placebo arm |
| Adult cardiac surgery | |||
| Reported as mL/kg (mean ± standard deviation) | 8.3 ± 5.6 mL/kg (n = 5) | 10.8 ± 6.3 mL/kg (n = 4) | |
| Reported as mL/kg (mean ± standard deviation) | 10.2 ± 6.4 mL/kg (n = 5) | 13.2 ± 6.6 mL/kg (n = 6) | |
| Reported as median (interquartile range) | 1.7 (2.3) units (n = 18) | 0.6 (1.3) units (n = 22) | |
| Reported as median (interquartile range) | 2 (1 to 4) units (n = 68) | 2 (1 to 3) units (n = 67) | |
| Reported as mean (no standard deviation) | 2.4 units (n = 15) | 2 units (n = 15) | |
| Reported as median (90% confidence interval) | 2 (1 to 8.5) units (n = 74) | 2 (1 to 9.8) units (n = 76) | |
| Reported as mean (range) | 1.3 (0 to 2) units (n = 15) | 1.1 (0 to 3) units (n = 15) | |
| Reported as median only | 2 units (n = 21) | 2 units (n = 22) | |
| Reported as mean only | 0.86 units (n = 44) | 1.79 units n = 42) | |
| Reported as mean only | 2.4 units (n = 13) | 2.2 units (n = 16) | |
| Reported as mL/m2 (mean ± standard deviation) | 740.4 ± 416.3 mL/m2 (n = 25) | 662.8 ± 380.7 mL/m2 (n = 28) | |
| Reported as mean only | 1.38 units (n = 49) | 1.30 units (n = 49) | |
| Orthopaedic surgery | |||
| Reported as mean only | 0.7 units (n = 10) | 1.1 units (n = 10) | |
| Reported as median and range | 64.8 (30.3 to 123.6) mL/kg (n = 10) | 64.9 (33.8 to 110) mL/kg (n = 11) | |
| Maxillofacial surgery | |||
| Reported as mean (no standard deviation) | 0.6 units (n = 10) | 0.9 units (n = 10) | |
| Trial | Reason not included in meta‐analysis | DDAVP arm | Placebo arm |
| Adult cardiac surgery | |||
| Reported as median (90% confidence interval) | 200 (0 to 1150) mL (n = 74) | 200 (0 to 1013) mL (n = 76) | |
| Reported as mL/m2 body surface area | 131 ± 106 mL/m2 (n = 50) | 193 ± 137 mL/m2 (n = 50) | |
| Paediatric cardiac surgery | |||
| Reported as mL/m2 | 49.3 ± 43.7 mL/m2 (n = 31) | 73.6 ± 71.1 mL/m2 (n = 29) | |
| Orthopaedic surgery | |||
| Reported as mean (no standard deviation) | 1200 mL (n = 23) | 1463 mL (n = 24) | |
| Hepatic surgery | |||
| Reported as median (range) | 832.5 (350 to 2955) mL (n = 30) | 800 mL (250 to 7128) mL (n = 29) | |
| Other surgery | |||
| Reported as mean and range | 251 (2 to 1330) mL (n = 14) | 504 (50 to 2100) mL (n = 14) | |
| Trial | Reason not included in meta‐analysis | DDAVP arm | Placebo arm |
| Adult cardiac surgery | |||
| Reported as mL/kg (mean ± standard deviation) | 8.0 ± 1.4 mL/kg (n = 5) | 5.9 ± 1.5 mL/kg (n = 4) | |
| Reported as mL/kg (mean ± standard deviation) | 11.3 ± 10 mL/kg (n = 5) | 7.5 ± 4 mL/kg (n = 6) | |
| Reported as median (interquartile range) | 950 (950) mL (n = 18) | 975 (811) mL (n = 22) | |
| Reported as median (interquartile range) | 575 (422.5 to 770) mL (n = 68) | 590 (476.25 to 1013.75) mL (n = 67) | |
| Reported as mL/m2 body surface area (mean ± standard deviation) | 400 ± 192 mL/m2 (n = 50) | 489 ± 361 mL/m2 (n = 51) | |
| Reported as mL/m2 body surface area (mean ± standard deviation) | 582 ± 410 mL/m2 (n = 44) | 465 ± 303 mL/m2 (n = 37) | |
| Reported as median (range) | 1000 (600 to 1800) mL (n = 19) | 1075 (400 to 1740) mL (n = 20) | |
| Reported as median (90% confidence interval) | 865 (358 to 2495) mL (n = 74) | 783 (300 to 2219) mL (n = 76) | |
| Reported as mL/m2 (mean ± standard deviation) | 258 ± 106 mL/m2 (n = 75) | 526 ± 314 mL/m2 (n = 75) | |
| Reported as mean (no standard deviation) | 1430 mL (n = 10) | 1767 mL (n = 10) | |
| Reported as mean (no standard deviation) | 574 mL (n = 16) | 535 mL (n = 18) | |
| Reported as median only | 1157 mL (n = 21) | 1180 mL (n = 22) | |
| Reported as mL/m2 body surface area (mean ± standard deviation) | 458 ± 206 mL/m2 (n = 50) | 536 ± 304 mL/m2 (n = 50) | |
| Reported as mL/m2 body surface area (mean ± standard deviation) | 551.8 ± 324.1 mL/m2 (n = 28) | 438.7 ± 228.1 mL/m2 (n = 25) | |
| Reported as median (range) | 1020 (530 to 1155) mL (n = 15) | 1100 (425 to 1720) mL (n = 15) | |
| Orthopaedic surgery | |||
| Reported as mean (no standard deviation) | 1320 mL (n = 6) | 1380 mL (n = 6) | |
| Reported as estimated percentage blood loss: median (range) | 147.8% (57% to 428.8%) (n = 10) | 111.2% (65% to 239.5%) (n = 11) | |
| Maxillofacial surgery | |||
| Reported as mean (range) | 675 (380 to 1330) mL (n = 10) | 819 (200 to 1600) mL (n = 10) | |
| Trial | Reason not included in meta‐analysis | DDAVP arm | Tranexamic acid arm |
| Orthopaedic surgery | |||
| Reported as mean only | 0.7 units (n = 10) | 0.1 units (n = 10) | |
| Trial | Reason not included in meta‐analysis | DDAVP arm | Tranexamic acid arm |
| Adult cardiac surgery | |||
| Reported as mean (no standard deviation) | 1430 mL (n = 10) | 535 mL (n = 18) | |
| Trial | Reason not included in meta‐analysis | DDAVP arm | Aprotinin arm |
| Adult cardiac surgery | |||
| Reported as mL/m2 body surface area (mean ± standard deviation) | 740.4 ± 416.3 mL/m2 (n = 25) | 366.1 ± 331.9 mL/m2 (n = 28) | |
| Trial | Reason not included in meta‐analysis | DDAVP arm | Aprotinin arm |
| Adult cardiac surgery | |||
| Reported as mL/m2 body surface area (mean ± standard deviation) | 400 ± 192 mL/m2 (n = 50) | 195 ± 146 mL/m2 (n = 48) | |
| Reported as mL/m2 body surface area (mean ± standard deviation) | 551.8 ± 324.1 mL/m2 (n = 25) | 358.5 ± 156.3 mL/m2 (n = 28) | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Red cell volume transfused (intraoperatively) Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Adult cardiac surgery | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.22, 1.02] |
| 1.2 Paediatric cardiac surgery | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.87, 1.67] |
| 1.3 Orthopaedic surgery | 3 | 144 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.89, ‐0.11] |
| 1.4 Vascular surgery | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.55, 0.15] |
| 1.5 Plastic surgery | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.23, ‐0.27] |
| 2 Red cell volume transfused (total) Show forest plot | 23 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Cardiac surgery | 14 | 957 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.96, ‐0.08] |
| 2.2 Orthopaedic surgery | 6 | 303 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.67, 0.64] |
| 2.3 Vascular surgery | 2 | 135 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.60, 0.73] |
| 2.4 Hepatic surgery | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐1.27, 0.33] |
| 3 Red cell volume transfused (children only, total) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Number of participants receiving a red cell transfusion (intraoperatively) Show forest plot | 6 | 349 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.50, 1.09] |
| 4.1 Cardiac surgery | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.43, 1.10] |
| 4.2 Plastic surgery | 2 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.45, 1.64] |
| 4.3 Kidney biopsy | 1 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Other | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Number of participants receiving a red cell transfusion (total) Show forest plot | 25 | 1806 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.86, 1.06] |
| 5.1 Cardiac surgery | 17 | 1350 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.82, 1.06] |
| 5.2 Orthopaedic surgery | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.45, 1.64] |
| 5.3 Vascular surgery | 1 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.83, 1.34] |
| 5.4 Paediatric cardiac surgery | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.66, 2.06] |
| 5.5 Plastic surgery | 2 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.45, 1.64] |
| 5.6 Hepatic surgery | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.15, 2.21] |
| 5.7 Kidney biopsy | 1 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.8 Maxillofacial surgery | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.88, 4.54] |
| 6 Blood loss (intraoperative) Show forest plot | 11 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Cardiac surgery | 2 | 87 | Mean Difference (IV, Random, 95% CI) | ‐138.20 [‐623.40, 347.01] |
| 6.2 Orthopaedic surgery | 5 | 224 | Mean Difference (IV, Random, 95% CI) | ‐118.24 [‐278.43, 41.95] |
| 6.3 Vascular surgery | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐525.0 [‐1177.34, 127.34] |
| 6.4 Sinus surgery | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐28.0 [‐31.70, ‐24.30] |
| 6.5 Plastic surgery | 2 | 44 | Mean Difference (IV, Random, 95% CI) | ‐146.02 [‐487.86, 195.83] |
| 7 Blood loss (total) Show forest plot | 28 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Adult cardiac surgery | 22 | 1358 | Mean Difference (IV, Random, 95% CI) | ‐135.24 [‐210.80, ‐59.68] |
| 7.2 Orthopaedic surgery | 5 | 241 | Mean Difference (IV, Random, 95% CI) | ‐285.76 [‐514.99, ‐56.53] |
| 7.3 Vascular surgery | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐582.0 [‐1264.07, 100.07] |
| 8 Blood loss (children only, total) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Paediatric cardiac surgery | 2 | 155 | Mean Difference (IV, Random, 95% CI) | 1.11 [‐12.92, 15.15] |
| 9 Number of participants with any bleeding (intraoperatively) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.1 Dialysis catheter | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Number of participants with any bleeding (total) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10.1 Kidney biopsy | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Reoperation due to bleeding Show forest plot | 23 | 1783 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.66 [0.40, 1.09] |
| 11.1 Cardiac surgery | 19 | 1483 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.38, 1.05] |
| 11.2 Orthopaedic surgery | 1 | 30 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Paediatric cardiac surgery | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.93 [0.14, 349.88] |
| 11.4 Dialysis catheter insertion | 1 | 48 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.5 Kidney biopsy | 1 | 162 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 All‐cause mortality Show forest plot | 22 | 1631 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.09 [0.51, 2.34] |
| 12.1 Cardiac surgery | 16 | 1239 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.09 [0.48, 2.51] |
| 12.2 Orthopaedic surgery | 3 | 171 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Vascular surgery | 1 | 91 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.50 [0.52, 138.60] |
| 12.4 Paediatric cardiac surgery | 2 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.14] |
| 13 All thrombotic events (including myocardial infarction, ischaemic stroke, other arterial thromboembolism, and venous thromboembolism) Show forest plot | 29 | 1984 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.36 [0.85, 2.16] |
| 13.1 Cardiac surgery | 19 | 1311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.46 [0.88, 2.42] |
| 13.2 Orthopaedic surgery | 6 | 280 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.21 [0.14, 363.30] |
| 13.3 Vascular surgery | 2 | 141 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.23, 2.60] |
| 13.4 Sinus surgery | 1 | 90 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.5 Kidney biopsy | 1 | 162 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Myocardial infarction Show forest plot | 26 | 1704 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.70, 2.46] |
| 14.1 Cardiac surgery | 16 | 1031 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.52 [0.77, 3.00] |
| 14.2 Orthopaedic surgery | 6 | 280 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Vascular surgery | 2 | 141 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.11, 2.88] |
| 14.4 Kidney biopsy | 1 | 162 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.5 Sinus surgery | 1 | 90 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Stroke Show forest plot | 19 | 1277 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.95 [0.94, 9.24] |
| 15.1 Cardiac surgery | 11 | 733 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.95 [0.94, 9.24] |
| 15.2 Orthopaedic surgery | 5 | 201 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Vascular surgery | 1 | 91 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.4 Kidney biopsy | 1 | 162 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.5 Sinus surgery | 1 | 90 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Venous thromboembolism Show forest plot | 20 | 1377 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.17, 3.38] |
| 16.1 Cardiac surgery | 11 | 754 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.53 [0.11, 2.62] |
| 16.2 Orthopaedic surgery | 6 | 280 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.21 [0.14, 363.30] |
| 16.3 Vascular surgery | 1 | 91 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.4 Kidney biopsy | 1 | 162 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.5 Sinus surgery | 1 | 90 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Clinically important hypotension Show forest plot | 18 | 1183 | Risk Ratio (M‐H, Random, 95% CI) | 2.32 [1.37, 3.91] |
| 17.1 Cardiac surgery | 13 | 762 | Risk Ratio (M‐H, Random, 95% CI) | 2.88 [1.32, 6.30] |
| 17.2 Orthopaedic surgery | 2 | 109 | Risk Ratio (M‐H, Random, 95% CI) | 2.05 [0.99, 4.24] |
| 17.3 Paediatric cardiac surgery | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.06, 14.27] |
| 17.4 Sinus surgery | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.5 Kidney biopsy | 1 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Red cell volume transfused (total) Show forest plot | 6 | 388 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.16, ‐0.13] |
| 2 Number of participants receiving a red cell transfusion (intraoperatively) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Number of participants receiving a red cell transfusion (total) Show forest plot | 5 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.66, 1.04] |
| 4 Blood loss (total) Show forest plot | 7 | 422 | Mean Difference (IV, Random, 95% CI) | ‐253.93 [‐408.01, ‐99.85] |
| 5 Reoperation due to bleeding Show forest plot | 6 | 413 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.39 [0.18, 0.84] |
| 6 All‐cause mortality Show forest plot | 7 | 422 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.72 [0.12, 4.22] |
| 7 All thrombotic events (including myocardial infarction, ischaemic stroke, other arterial thromboembolism, and venous thromboembolism) Show forest plot | 7 | 422 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.58 [0.60, 4.17] |
| 8 Myocardial infarction Show forest plot | 5 | 277 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.72 [0.60, 12.37] |
| 9 Stroke Show forest plot | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 10 Venous thromboembolism Show forest plot | 4 | 248 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.56 [0.06, 5.50] |
| 11 Clinically important hypotension Show forest plot | 5 | 315 | Risk Ratio (M‐H, Random, 95% CI) | 6.58 [1.18, 36.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Red cell volume transfused (total) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Orthopaedic surgery | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of participants receiving a red cell transfusion (total) Show forest plot | 3 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 2.42 [1.04, 5.64] |
| 2.1 Cardiac surgery | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.82, 2.59] |
| 2.2 Orthopaedic surgery | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 4.15 [1.58, 10.90] |
| 3 Blood loss (total) Show forest plot | 2 | 115 | Mean Difference (IV, Random, 95% CI) | 142.81 [79.78, 205.84] |
| 3.1 Cardiac surgery | 1 | 75 | Mean Difference (IV, Random, 95% CI) | 115.0 [35.38, 194.62] |
| 3.2 Orthopaedic surgery | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 180.0 [86.82, 273.18] |
| 4 Reoperation due to bleeding Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 4.1 Cardiac surgery | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 All thrombotic events (including myocardial infarction, ischaemic stroke, other arterial thromboembolism, and venous thromboembolism) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Cardiac surgery | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Orthopaedic surgery | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Myocardial infarction Show forest plot | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Cardiac surgery | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Orthopaedic surgery | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Stroke Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 Cardiac surgery | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Orthopaedic surgery | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Venous thromboembolism Show forest plot | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Cardiac surgery | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Orthopaedic surgery | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants receiving a red cell transfusion (total) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Cardiac surgery | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Reoperation due to bleeding Show forest plot | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.1 Cardiac surgery | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 All‐cause mortality Show forest plot | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Cardiac surgery | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 All thrombotic events (including myocardial infarction, ischaemic stroke, other arterial thromboembolism, and venous thromboembolism) Show forest plot | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 4.1 Cardiac surgery | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Myocardial infarction Show forest plot | 2 | 152 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Cardiac surgery | 2 | 152 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Stroke Show forest plot | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 6.1 Cardiac surgery | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Venous thromboembolism Show forest plot | 2 | 152 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Cardiac surgery | 2 | 152 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Clinically significant hypotension Show forest plot | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.1 Cardiac surgery | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |

