Penambahan nutrisi bagi penjagaan pasca‐patah tulang pinggul dalam kalangan orang tua
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Method of randomisation: concealed, computer‐generated programme Intention‐to‐treat analysis: carried out Lost to follow‐up: all participants followed‐up | |
| Participants | Location: ortho‐geriatric unit, Department of Geriatrics, Rabin Medical Center, Petah Tikva, Israel Period of study: May 2010–December 2011 50 participants Inclusion criteria: > 65 years, admitted following hip fracture within 48 h of the injury and orthopaedic surgery was the treatment of choice Exclusion criteria: presented to hospital > 48 h after the injury, receiving steroids and/or immunosuppression therapy; active oncologic disease, multiple fractures, diagnosed dementia, required supplemental nasal oxygen which precluded the measurement of resting energy expenditure (REE) Sex: 33 female, 17 male Age: mean 83 years Fracture type: 40% pertrochanteric, 20% subcapital, 6% subtrochanteric, 6% base of femoral neck, 28% other | |
| Interventions | Timing of intervention: 24 h after surgery for 14 d (a) Calories with an energy goal determined by three REE measurements in first 7 d using indirect calorimetry (IC) (Fitmate, Cosmed, Italy) which was based on hospital‐prepared diets (standard or texture‐adapted). Oral nutritional supplements (ONS) amount adjusted to make up the difference between energy received from hospital food and measured energy expenditure. These ONS were provided in the form of Ensure plus (Abbott Laboratories) containing 355 kcal/237 ml and 13.5 g protein or Glucerna (Abbott Laboratories) containing 237 kcal/237 ml and 9.9 g protein/237 ml. The participant, family and caregivers educated regarding importance of nutritional support and more attention was given to personal food preferences. 24‐h food diaries were filled in by the medical staff, family and caregivers. (b) Usual hospital food (standard or texture‐adapted) and a fixed dose of ONS if already prescribed prior to hospitalisation. Hospital‐prepared diets provided a mean of 1800 kcal and 80 g of protein if meals completely eaten by the participants Allocated: 22/28 Assessed: 22/28 | |
| Outcomes | Length of follow‐up: length of hospital stay Main outcomes: Mortality Length of hospital stay Total complications Infectious complications Pressure ulcers Other outcomes: Protein and energy intakes | |
| Notes | Power calculation indicated needed 66 participants. In view of the slow rate of expected recruitment an interim analysis was planned after 50 participants. In the presence of a positive result, the study was discontinued. No funder reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States "Randomization was performed using a concealed, computer generated program." |
| Allocation concealment (selection bias) | Low risk | States "Randomization was performed using a concealed, computer generated program. RA enrolled participants and assigned them to interventions while YB enrolled patients but was blinded to the intervention." Comment: probably done |
| Blinding of participants and personnel (performance bias) | High risk | No placebo group |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo group. Comment: unlikely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No placebo group. Comment: may have been influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for, with no drop‐outs. |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for, with no drop‐outs. |
| Selective reporting (reporting bias) | Low risk | No protocol available, but expected outcomes reported |
| Other bias | High risk | Power calculation indicated needed 66 participants. In view of the slow rate of expected recruitment an interim analysis was planned after 50 participants. In the presence of a positive result, the study was discontinued. No funder reported |
| Methods | Method of randomisation: quasi‐randomised | |
| Participants | Location: hospital, Nottingham, UK | |
| Interventions | Timing of intervention: nasogastric feeding started within 5 d of surgery, 8 h overnight with tube disconnected during the day, until discharge or death. Feeding stopped if participant did not tolerate tube or removed tube on 3 occasions | |
| Outcomes | Length of follow‐up: until discharge or death | |
| Notes | There was an administrative limit imposed of a maximum of 6 participants being fed at one time. Data presented from 1983 paper for numbers of participants are correct, error in number of participants in 1985 paper. Slight discrepancy with days to reach independent mobility presented in 1984 abstract. Reply from trialists (15 February 2000) gave details of randomisation (on recall: either by date of admission or birth), outcome assessment, inclusion criteria, denominators and baseline comparability | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised. On recall by trialists: "either on the basis of odd and even dates of birth or of admission". |
| Allocation concealment (selection bias) | High risk | Quasi‐randomised. On recall by trialists: "either on the basis of odd and even dates of birth or of admission". |
| Blinding of participants and personnel (performance bias) | High risk | No placebo group. Comment: likely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo group. Comment: unlikely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No placebo group. Comment: may have been influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for, with no drop‐outs. |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for, with no drop‐outs. |
| Selective reporting (reporting bias) | Low risk | Protocol not available, but study report includes all outcomes reported in methods and those that would be expected. Comment: probably done |
| Other bias | High risk | States that Bastow was "supported by a grant from Rousell Laboratories Ltd", manufacturers of Clinifeed nasogastric feed used in trial |
| Methods | Method of randomisation: states double‐blind, but no other details | |
| Participants | Location: hospitals; Nottingham, Leeds and Doncaster, UK | |
| Interventions | Timing of interventions: start time unclear, twice daily for 2 months, | |
| Outcomes | Length of follow‐up: 6 months Food intake (nr) | |
| Notes | Conference abstract only. No denominators for intention‐to‐treat analysis, so cannot use data in analysis. Data on arm muscle circumference, fatigue score and food intake presented for 35 participants completing 2 months of treatment. Request for further details (including denominators) sent 19 May 1999, re‐sent 4 February 2000 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract only. No details provided |
| Allocation concealment (selection bias) | Unclear risk | Abstract only. States "randomized in a double‐blind fashion", no other details provided |
| Blinding of participants and personnel (performance bias) | Low risk | Abstract only. States "double‐blind" and "unlabelled identical sachets" |
| Blinding of outcome assessment (detection bias) | Low risk | Abstract only. Comment: unlikely to have been influenced by unblinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Abstract only. States "double‐blind" and "unlabelled identical sachets". Comment: unclear if done |
| Incomplete outcome data (attrition bias) | Unclear risk | Abstract only. Insufficient details on attrition and exclusions provided |
| Incomplete outcome data (attrition bias) | Unclear risk | Abstract only. Insufficient details on attrition and exclusions provided |
| Selective reporting (reporting bias) | Unclear risk | Abstract only. Insufficient details provided |
| Other bias | Unclear risk | Abstract only. Insufficient details provided. No details on sponsor |
| Methods | Method of randomisation: Factorial design computer‐based randomisation performed by study statistician. Randomisation for the dosage of cholecalciferol was double‐blinded Intention‐to‐treat analysis: carried out Lost to follow‐up: 14% lost to follow‐up | |
| Participants | Location: Triemli City Hospital, Zurich, Switzerland Period of study: screening for recruitment 2005‐2007 173 participants Inclusion criteria: age 65 years or older, surgical repair of acute hip fracture, Folstein Mini‐Mental State Examination score of 15 or more, understand German, able to walk at least 3 m before fracture Exclusion criteria: prior hip fracture at the newly fractured hip, metastatic cancer or chemotherapy in last year, severe visual or hearing impairment, creatinine clearance of 15 mL/min or less, kidney stone in the past 5 years, hypercalcaemia, primary hyperparathyroidism or sarcoidosis Sex: 137 female, 36 male Age: mean 84 years Fracture type: further details not given | |
| Interventions | Timing of intervention: from mean of 4.2 d after hip fracture surgery for 12 months (a) With breakfast, participants took a study capsule containing 1200 IU of cholecalciferol. For breakfast and at bedtime, participants took a tablet containing 400 IU of cholecalciferol and 500 mg of elemental calcium as calcium carbonate (Nycomed, Wädenswil, Switzerland). (b) With breakfast, participants took a placebo capsule (identical in appearance and taste to active tablet). For breakfast and at bedtime, participants took a tablet containing 400 IU of cholecalciferol and 500 mg of elemental calcium as calcium carbonate (Nycomed, Wädenswil, Switzerland). Groups a and b were also randomised to standard or extended physiotherapy Allocated: 86/87 Assessed: 73/75 | |
| Outcomes | Length of follow‐up: 12 months Main outcomes: Mortality Complications Functional status Level of care Putative side effects Other outcomes: Compliance | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States "computer‐based randomization" |
| Allocation concealment (selection bias) | Unclear risk | States "Randomization for the dosage of cholecalciferol was double‐blinded, whereas randomization for PT (physiotherapy) was single‐blinded (all study staff except the treating physiotherapist who instructed the home program were blinded to the PT treatment allocation). Comment: allocation concealment unclear |
| Blinding of participants and personnel (performance bias) | Low risk | States double‐blind and vitamin D placebo identical in appearance and taste |
| Blinding of outcome assessment (detection bias) | Low risk | States double‐blind and vitamin D placebo identical in appearance and taste |
| Blinding of outcome assessment (detection bias) | Low risk | States double‐blind and vitamin D placebo identical in appearance and taste |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for missing data provided and missing data balanced across groups |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for missing data provided and missing data balanced across groups |
| Selective reporting (reporting bias) | High risk | Trial registration on clinicaltrials.gov gives outcomes of numbers of people who fell, disability, health care utilisation and quality of life (EuroQol); not provided in published paper |
| Other bias | Low risk | Funded by Swiss National Foundations, Vontobel Foundation (charitable foundation), |
| Methods | Method of randomisation: sealed opaque envelopes, prepared independently from recruitment | |
| Participants | Location: Hospital Ramon y Cajal, Madrid, Spain | |
| Interventions | Timing of intervention: started 48 h after operation, until hospital discharge | |
| Outcomes | Length of follow‐up: up to hospital discharge | |
| Notes | Emailed 22 January 2009 requesting mortality information. Author replied 23 January confirming no participants had died during the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomized" only. No further details provided |
| Allocation concealment (selection bias) | Low risk | States used of "sealed opaque envelopes". Independent preparation of envelopes: "The investigator recruiting the patients ....had no role in the randomisation process" |
| Blinding of participants and personnel (performance bias) | High risk | No placebo provided |
| Blinding of outcome assessment (detection bias) | Low risk | No details provided on blinding of outcome assessment, but outcome assessment unlikely to have been influenced by unblinding. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided on blinding of outcome assessment, and outcome assessment may have been influenced by unblinding |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for in analysis |
| Incomplete outcome data (attrition bias) | Unclear risk | Denominators unclear for length of hospital stay, length of immobilisation and supplement intake |
| Selective reporting (reporting bias) | Unclear risk | Insufficient details provided |
| Other bias | Unclear risk | Funding source (Fundacion para la Investigacion Biomedica, Hospital Ramon y Cajal, Madrid, Spain) and source of supplemental nutrition (Hospital Ramon y Cajal) do not appear related to manufacturer of the supplements. |
| Methods | Method of randomisation: randomised, open two‐arm trial, using sealed opaque envelopes Intention‐to‐treat analysis: in acute hospital; complications, length of stay, mobilisation not collected after moved to another centre for rehabilitation Lost to follow‐up: 53% lost to complete follow‐up (moved to another centre for rehabilitation) | |
| Participants | Location: Hospital Universitario Ramon y Cajal, Madrid, Spain Period of study: recruitment May 2007–September 2008 60 participants Inclusion criteria: age > 65 years, hip fracture where orthopaedic surgery considered treatment of choice Exclusion criteria: moderate–severe malnutrition (weight loss of > 5% in the previous month or > 10% in the previous 6 months, and/or serum albumin concentrations < 2.7 g/dL), acute and/or chronic renal failure, hepatic insufficiency or cirrhosis (Child B or C), severe heart failure with class III or IV of the New York Heart Association, respiratory failure, gastrointestinal condition precluding adequate oral nutritional intake. Sex: 44 female, 16 male Age: mean 84 years Fracture type: fracture type not given | |
| Interventions | Timing of intervention: from admission (including pre‐operative) until discharge (a) Energy and protein supplements by means of commercial enteral nutrition for oral intake (Fortimel, 200 mL bricks, each provides 20 g protein and 200 kcal, Nutricia Advanced Medical Nutrition – Danone Group) to aim at 40 g of protein and 400 kcal per day (2 bricks a day) and every participant was prescribed a standard or texture‐adapted diet to meet their calculated metabolic rate. The Harris–Benedict equation was employed to calculate the basal metabolic rate and a coefficient of 1.3 was employed to estimate the total metabolic rate. In‐hospital diets provided a mean of 100 g of protein per day (range 80–120 g). (b) Every participant was prescribed a standard or texture‐adapted diet to meet their calculated metabolic rate. The Harris–Benedict equation was employed to calculate the basal metabolic rate and a coefficient of 1.3 was employed to estimate the total metabolic rate. In‐hospital diets provide a mean of 100 g of protein per day (range 80–120 g). Allocated: 30/30 Assessed: 18/14 | |
| Outcomes | Length of follow‐up: until discharge from hospital Main outcomes: Mortality Postoperative hospital stay, Postoperative hospital complications Requiring rehabilitation Other outcomes: Compliance | |
| Notes | Emailed [email protected] 25 November 2014 to enquire about numbers in intervention and control groups going to rehabilitation hospital (text differs from flow chart) and whether data were collected in rehabilitation hospital for complications, mobilisation and length of stay. Replied with further information 26 November 2014 for all these queries | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "patients were randomized using sealed opaque envelopes to yield two groups with 30 patients each." |
| Allocation concealment (selection bias) | Low risk | States "patients were randomized using sealed opaque envelopes to yield two groups with 30 patients each... The investigators who designed the study prepared the envelopes and assigned participants to their groups, but had no contact with the patients throughout the study. The investigator recruiting the patients, administering the interventions and evaluating the outcomes had no role on the randomization process." |
| Blinding of participants and personnel (performance bias) | High risk | No placebo group. Comment: likely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo group. States also "The investigator recruiting the patients, administering the interventions and evaluating the outcomes had no role on the randomization process." Comment: unlikely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No placebo group. States "The investigator recruiting the patients, administering the interventions and evaluating the outcomes had no role on the randomization process." Comment: may have been influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | High risk | Missing outcome data balanced in numbers across intervention (18) and control groups (14), but proportion high enough to likely induce a clinically relevant bias in observed effect size |
| Incomplete outcome data (attrition bias) | High risk | Missing outcome data balanced in numbers across intervention (18) and control groups (14), but proportion high enough to likely induce a clinically relevant bias in observed effect size |
| Selective reporting (reporting bias) | Unclear risk | Insufficient details provided to judge |
| Other bias | Low risk | States "The funding source, Fundacion para la Investigacion Biomedica, Hospital Ramon y Cajal (FIBio‐RyC), Madrid, Spain, had no role in the study design, the collection, analysis, and interpretation of data, the writing of the report, or the decision to submit the paper for publication. The ONS employed in this study were provided by the Hospital Ramo´n y Cajal, Madrid, Spain." |
| Methods | Method of randomisation: alternating numbers | |
| Participants | Location: hospital, Ipswich, UK | |
| Interventions | Timing of intervention: from second day of admission until discharge (including rehabilitation hospital) | |
| Outcomes | Length of follow‐up: no details (21+ days) | |
| Notes | Author provided protocol of trial and information on method of randomisation and outcome assessment. Request for further details (other outcomes, period of follow‐up) sent 19 May 1999, re‐sent 3 February 2000 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternating numbers (information from trial author) |
| Allocation concealment (selection bias) | High risk | Alternating numbers (information from trial author), states randomly assigned with no further details |
| Blinding of participants and personnel (performance bias) | High risk | No placebo group. Comment: likely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo group. Comment: unlikely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No placebo group. Comment: may have been influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient details provided on pressure sores |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient details provided on 2‐stage walking goals |
| Selective reporting (reporting bias) | Low risk | Protocol available and all outcomes provided |
| Other bias | Unclear risk | Source of funding for study unclear |
| Methods | Method of randomisation: quasi‐randomised by year of birth | |
| Participants | Location: hospital, Freemantle, Australia | |
| Interventions | Timing of intervention: started within 2 to 3 d after surgery, for 28 d | |
| Outcomes | Length of follow‐up: 6 months | |
| Notes | Percentages provided in report indicate variation in denominators used. Requests for further details of denominators and mortality during study sent 13 August 2003 and 13 October 2003. Reply received October 2003 giving details of denominators, mortality, withdrawals, and details of vitamin and mineral content of supplement | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Quasi‐randomisation of cases was carried out using their date of birth." |
| Allocation concealment (selection bias) | High risk | "Quasi‐randomisation of cases was carried out using their date of birth" but nurse co‐ordinators and unit dietitian responsible for carrying out the study and collecting the data |
| Blinding of participants and personnel (performance bias) | High risk | No placebo group. Comment: likely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo group. Comment: unlikely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No placebo group. Comment: may have been influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | Missing outcome data balanced in numbers across groups, but reasons for missing outcome data unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Missing outcome data balanced in numbers across groups, but reasons for missing outcome data unclear |
| Selective reporting (reporting bias) | High risk | Hospital mortality, admissions to nursing home, cognitive impairment stated in methods, but not provided |
| Other bias | Unclear risk | Source of funding for study unclear |
| Methods | Method of randomisation: block randomisation of 15. Table of randomisation by statistician not involved in study Intention‐to‐treat analysis: insufficient details provided Lost to follow‐up: insufficient details provided | |
| Participants | Location: orthopaedic ward of Geneva University Hospital, Switzerland Period of study: recruited March 1999‐June 2000 45 participants Inclusion criteria: women older than 60 years with a recent hip fracture, i.e. within two weeks, that was attributable to osteoporosis such as occurring on a fall from standing height, and with the ability to give a written informed consent. Exclusion criteria: pathologic fracture; fracture caused by severe trauma; cardiac or pulmonary failure; advanced renal insufficiency with plasma creatinine concentration 200 mmol/L or more; hepatic failure; severe mental impairment; acute illness before the fracture that could interfere with the study protocol; active metabolic bone disease; consumption of protein supplement or of anti‐osteoporotic active drugs or medication known to alter bone metabolism, such as sex hormones or corticosteroids; severe malnutrition (serum albumin level < 15 g/L); life expectancy of less than one year Sex: all female Age: mean 81.3 (SD 7.4) years Fracture type: not given | |
| Interventions | Timing of intervention: from a mean of 10 d post fracture for 28 d a) 20 g milk protein (casein) in 200 ml water, including 550 mg calcium and 500 IU vitamin D3, daily for 28 d b) 20 g whey protein in 200 ml water, including 550 mg calcium and 500 IU vitamin D3, daily for 28 d c) 15 g whey protein and 5 g of essential amino acids in ratio identical to casein in 200 ml water, including 550 mg calcium and 500 IU vitamin D3, daily for 28 d Allocated: 15/15/15 Assessed: unclear | |
| Outcomes | Length of follow‐up: 28 d Main outcomes: Putative adverse events from supplements Other outcomes: Compliance | |
| Notes | Emailed [email protected] 9 October 2014 to ask for further information on outcomes, reply received 14 October 2014 with details of putative side effects and compliance | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States " randomization was performed in blocks of 15 patients...table of randomization was established by a statistician who was not directly involved in the study" |
| Allocation concealment (selection bias) | Low risk | States " randomization was performed in blocks of 15 patients...table of randomization was established by a statistician who was not directly involved in the study" |
| Blinding of participants and personnel (performance bias) | Unclear risk | States "dietician as well as both the medical staff and subjects involved in the study were blinded to the experimental groups" but no further details on how this was achieved |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided and putative adverse events from supplements may have been influenced by unblinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No details provided and compliance may have been influenced by unblinding |
| Incomplete outcome data (attrition bias) | High risk | Numbers in email differ from publication: give 11 dropouts (5 casein, 4 whey, 2 whey and amino acids), with 12 mentioned in publication |
| Incomplete outcome data (attrition bias) | High risk | Numbers in email differ from publication: give 11 dropouts (5 casein, 4 whey, 2 whey and amino acids), with 12 mentioned in publication |
| Selective reporting (reporting bias) | High risk | No details on outcome activities of daily living provided |
| Other bias | High risk | Supported by Novartis Cosumer Health (Berne, Switzerland) |
| Methods | Method of randomisation: computer‐generated random sequence, insufficient indication of adequate safeguards | |
| Participants | Location: hospital, Cardiff, UK | |
| Interventions | Timing of intervention: 2 doses of vitamin preparation given preoperatively, and then 1 dose daily for 5 d postoperatively | |
| Outcomes | Length of follow‐up: 3 months | |
| Notes | Request for further details (method of randomisation, constituents of Parentrovite IVHP, other outcomes) sent. Reply from trialists (27 May 1999) gave details of the intervention, randomisation, and information on fracture type, baseline albumin levels, complications and hospital stay | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Allocation of patients was based on randomly generated numbers (0 or 1)" |
| Allocation concealment (selection bias) | Unclear risk | States "randomly allocated", no further details provided |
| Blinding of participants and personnel (performance bias) | High risk | No placebo |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo group. Comment: unlikely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No placebo group. Comment: may have been influenced by lack of blinding, apart from mental health status which was "assessed by a psychology technician who remained blind as to the treatment group of each patient" |
| Incomplete outcome data (attrition bias) | Unclear risk | Data provided for all participants, apart from putative adverse events (no data provided for control group) |
| Incomplete outcome data (attrition bias) | Unclear risk | Data provided for all participants, apart from putative adverse events (no data provided for control group) |
| Selective reporting (reporting bias) | High risk | Data on outcome final placement not available |
| Other bias | High risk | Bencard provided Parenterovite |
| Methods | Method of randomisation: not stated | |
| Participants | Location: orthopaedic unit in hospital and recovery hospital, Geneva, Switzerland | |
| Interventions | Timing of intervention: from admission to orthopaedic unit to end of stay in second (recovery) hospital, supplement given once daily at 20:00 hours for a mean period of 32 d | |
| Outcomes | Length of follow‐up: 6 months | |
| Notes | Numbers of complications unclear, request for further details sent 24 May 1999, re‐sent 7 February 2000 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomised", no other details provided |
| Allocation concealment (selection bias) | Unclear risk | States "randomised", no other details provided |
| Blinding of participants and personnel (performance bias) | High risk | No placebo group. Comment: likely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo group. Comment: unlikely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No placebo group. Comment: may have been influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | High risk | Data provided for only 25/27 intervention group and 27/32 control group |
| Incomplete outcome data (attrition bias) | High risk | Length of stay data not provided for 6/27 intervention group and 4/32 control group, i.e. length of stay for survivors presented |
| Selective reporting (reporting bias) | Unclear risk | Insufficient details provided |
| Other bias | High risk | Sandoz‐Wander supplied the supplement, but do not appear to have funded the study |
| Methods | Method of randomisation: sequentially numbered opaque sealed envelopes, initially in blocks of 20, later reduced to blocks of 10, prepared by member of staff outside trial, opened sequentially | |
| Participants | Location: single trauma ward, University Hospital of Wales, Cardiff, UK | |
| Interventions | Timing of intervention: unclear when commenced, during stay in acute trauma ward, median 16‐17 d. Dietetic assistant present on ward 6 h/d for 7 d/week | |
| Outcomes | Length of follow‐up: 4 months | |
| Notes | Request for further details on participants with complications sent 15 March 2006. Reply from trialists (15 March 2006) provided number and per cent of live participants having had complications on trauma ward. A letter to the editor in Age and Ageing Advance Access (24 June 2006) by Hewitt and Torgerson pointed out the numerical difference between the two groups was higher than expected given the reported block size of 10. The reply from Duncan indicated that they initially started the study with a block size of 20. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomisation was by sequentially numbered, opaque, sealed envelope method in blocks of 10, prepared by a member of staff not directly involved in the trial." No further details |
| Allocation concealment (selection bias) | Low risk | "Randomisation was by sequentially numbered, opaque, sealed envelope method in blocks of 10, prepared by a member of staff not directly involved in the trial." |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo group. Comment: unlikely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No placebo group. Comment: may have been influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups and unlikely to relate to outcome |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups and unlikely to relate to outcome |
| Selective reporting (reporting bias) | Unclear risk | Appears Waterlow score of pressure sore risk and Abbreviated Mental Test score collected as outcomes, but not provided |
| Other bias | Unclear risk | Funding from Women's Royal Voluntary Service, British Dietetic Assocation, Innovations in Care, Wales Office of Research and Development, Shire Pharmaceuticals (funded nutritional assessments, research assessments) |
| Methods | Method of randomisation: block randomisation conducted by research nurse, using closed, numbered envelopes | |
| Participants | Location: Department of Orthopaedics, Lund University Hospital, Lund, Sweden | |
| Interventions | Timing of intervention: first 10 d in hospital | |
| Outcomes | Length of follow‐up: mean of 120 d | |
| Notes | Emailed on 22nd January 2009 in an attempt to clarify denominators. Author replied 10th February confirming denominators | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomised" with no further details. |
| Allocation concealment (selection bias) | Unclear risk | States "patient were randomised by the research nurse (UBO) to either the control or the treatment group using block randomisation with 40 closed and numbered envelopes in each block". |
| Blinding of participants and personnel (performance bias) | High risk | No placebo intervention |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo group. Comment: unlikely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No placebo group. Comment: may have been influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for, with no dropouts |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for, with no dropouts |
| Selective reporting (reporting bias) | Unclear risk | Insufficient details provided |
| Other bias | Low risk | Funded by Medical Faculty of Lund University |
| Methods | Method of randomisation: computer‐generated assignment, balanced in blocks of 4, with sealed envelopes, opened by pharmacist | |
| Participants | Location: Hospital General de Vic, Barcelona, Spain | |
| Interventions | Timing of intervention: begun within 48 h of study entry, consumed once daily at night for 60 d | |
| Outcomes | Length of follow‐up: 6 months | |
| Notes | Request for further details (including follow‐up data on excluded participants, details of supplement) sent 14 February 2000 and 6 June 2000. Replies from Heidi Guyer (6 March 2000 and 13 June 2000) confirmed assessor blinding, gave other details of methodology and contents of supplement, as well as details of outcome of the excluded participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated assignment, balanced in blocks of 4, with sealed envelopes, prepared by epidemiology unit. "Upon being advised of a patient's inclusion, the pharmacist assigned the patient a study number and opened the envelope ..." |
| Allocation concealment (selection bias) | Low risk | Computer‐generated assignment, balanced in blocks of 4, with sealed envelopes, prepared by epidemiology unit. "Upon being advised of a patient's inclusion, the pharmacist assigned the patient a study number and opened the envelope ..." |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded and reports that supplement and placebo available in 3 flavours that did not differ in taste and appearance |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blinded, although not clear if outcome assessors blinded, but unlikely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blinded, although not clear if outcome assessors blinded, but unlikely to have been influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data balanced in numbers across groups and with similar reasons across groups |
| Incomplete outcome data (attrition bias) | Unclear risk | 5 from intervention group and 3 from control group withdrawn due to protocol violations |
| Selective reporting (reporting bias) | Low risk | No protocol available, but expected outcomes reported |
| Other bias | High risk | Funded by Spanish Ministry of Health and authors thank Clinical Nutrition SA for the preparation of the supplements. 34% of controls and 18% of intervention group on psychotropic medication |
| Methods | Method of randomisation: states "randomly divided" only Intention‐to‐treat: unclear Lost to follow‐up: unclear | |
| Participants | Location: Trauma Center Meidling, Vienna, Austria Period of study: before September 2010 23 participants Inclusion criteria: aged > 65 years with hip fractures (femoral neck, intertrochanteric and subtrochanteric) Exclusion criteria: acute or chronic renal disease, liver failure, severe congestive heart failure, severe pulmonary disease, and any gastrointestinal condition that might preclude the participant from adequate oral nutritional intake Sex: all female Age: mean age 84 years Fracture type: further details not given | |
| Interventions | Timing of intervention: after operation whilst hospitalised a) Oral supplements administered individually when energy and/or protein intake calculated by dietary records did not exceed a level of 20–25 kcal and/or 1–1.5 g protein/kg body weight/ day as recommended by the European Society for Clinical Nutrition and Metabolism per 1000 ml – 4.2 MJ (40% energy as protein), 1.88 mg vitamin A, 13 mcg vitamin D, 23 mg vitamin E, 0.1 mg vitamin K, 190 mg vitamin C, 2.8 mg thiamine, 3.1 mg riboflavin, 34 mg niacin, 3.3 mg pyridoxine, 0.5 mg folate, 10 mg pantothenic acid, 7 mcg vitamin B12, 75 mcg biotin, 500 mg sodium, 2 g potassium, 420 mg magnesium, 2.8 g calcium, 2 g phosphorus, 900 mg chloride, 23 mg zinc, 30 mg iron, 3.4 mg copper, 0.25 mg iodine, 0.13 mg chromate, 1.9 mg fluoride, 6.3 mg manganese, 0.19 mg molybdenum, 0.11 mg selenium b) Usual care Allocated: 14/9 Assessed: 14/9 (numbers not certain) | |
| Outcomes | Length of follow‐up: length of hospitalisation Main outcomes: Length of hospital stay | |
| Notes | Emailed [email protected] 31 December 2014 to request more details of denominators | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomly divided" only |
| Allocation concealment (selection bias) | Unclear risk | States "randomly divided" only |
| Blinding of participants and personnel (performance bias) | High risk | No placebo group. Comment: likely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No placebo group. Comment: may have been influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | Denominators not given for length of stay |
| Selective reporting (reporting bias) | High risk | Length of stay only provided, with no other details of clinical outcomes. Length of stay not included in methods |
| Other bias | Low risk | Funded by Trauma Center, Meidling, Vienna |
| Methods | Method of randomisation: randomised into 3 groups in blocks of 12, using a sealed envelope technique Intention‐to‐treat analysis: appears undertaken Lost to follow‐up: 20% of groups examined here | |
| Participants | Location: 4 university hospitals in Stockholm, Sweden Period of study: before 2014 54 participants Inclusion criteria: age 60 years or older, no severe cognitive impairment (Short Portable Mental Questionnaire score ≥ 3), ambulatory before fracture, body mass index 28 kg/m2 or lower Exclusion criteria: pathological fractures and bisphosphonate treatment within the last year; alcohol/drug abuse or overt psychiatric disorders; abnormal hepatic or renal laboratory parameters such as serum‐alanine aminotransferase or serum‐aspartate‐aminotransferase twice the normal reference range or higher, respectively; serum‐creatinine levels higher than 130 μmol/L or glomerular filtration rate lower than 30 mL/minute; bone metabolic disorders such as primary hyperparathyroidism, osteogenesis imperfecta, Paget’s disease, or myeloma; lactose intolerance, dysphagia, oesophagitis, gastric ulcer, or malignancy; diabetes mellitus associated with nephropathy or retinopathy; active iritis or uveitis Sex: 37 female, 17 male Age: mean 81 years Fracture type: 41% femoral neck fracture, 59% trochanteric fracture | |
| Interventions | Timing of intervention: as soon as participants were stable from a cardiovascular standpoint, able to take food by mouth, and able to sit in an upright position for 1 h after taking their tablets for 6months (a) Fresubin (Fresenius Kabi, Bad Homburg, Germany) protein energy drink, 200 mL twice daily, totaling 600 kcal with 40 g protein and 35 mg risedronate once weekly for 12 months (b) 35 mg risedronate once weekly for 12 months Allocated: 26/28 Assessed: 18/25 | |
| Outcomes | Length of follow‐up: 1 year Main outcomes: Mortality Complications Putative side effects Other outcomes: Compliance | |
| Notes | Emailed [email protected] on 9 December 2014 to enquire if more data on outcomes available. Author provided more details 15 December 2014 A third group ('control') was not included in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomized into three groups in blocks of twelve, using a sealed envelope technique", no details of sequence generation |
| Allocation concealment (selection bias) | Low risk | States "randomized into three groups in blocks of twelve, using a sealed envelope technique" |
| Blinding of participants and personnel (performance bias) | High risk | No placebo intervention |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo group. Comment: unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Unclear risk | No placebo group. Comment: may have been influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | High risk | 8/26 nutrition group lost to follow‐up versus 3/28 in control group |
| Incomplete outcome data (attrition bias) | High risk | 8/26 nutrition group lost to follow‐up versus 3/28 in control group |
| Selective reporting (reporting bias) | Unclear risk | Insufficient details provided |
| Other bias | Unclear risk | About 10% difference in weight between groups, although BMI only differs by 1.3 kg/m2 Fresenius Kabi provided supplement, but states not involved in the planning or implementation of the study, nor in the analyses, conclusions, or manuscript writing |
| Methods | Method of randomisation: not stated | |
| Participants | Location: hospital, Cincinnati, USA | |
| Interventions | Timing of intervention: tube placed in surgery, supplementary feeding began first postoperative night, 11 h per night, continued until participant ate 75% of their calorie needs for 3 consecutive days | |
| Outcomes | Length of follow‐up: no details (21+ days) | |
| Notes | Conference abstract with no denominators, so cannot use data in analysis. Notes taken by Ronald Koretz of an oral conference presentation by Gallagher indicated a quasi‐randomised study with dropouts being placed in control group; thus denominators remain unclear. The notes gave details of total length of stay, numbers pulling out nasogastric tube, mortality, and medical and surgical complications. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract only. States "randomized". No further details provided |
| Allocation concealment (selection bias) | Unclear risk | Abstract only. States "randomized". No further details provided |
| Blinding of participants and personnel (performance bias) | High risk | Abstract only. No placebo group. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | Low risk | Abstract only. No placebo group. Comment: unlikely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Abstract only. No placebo group. Comment: probably not done |
| Incomplete outcome data (attrition bias) | Unclear risk | Abstract only. Insufficient details on attrition and exclusions provided |
| Incomplete outcome data (attrition bias) | Unclear risk | Abstract only. Insufficient details on attrition and exclusions provided |
| Selective reporting (reporting bias) | High risk | Abstract only. Insufficient details provided. Differences found between notes on conference presentation and abstract |
| Other bias | Unclear risk | Abstract only. Insufficient details provided. No details on sponsor |
| Methods | Method of randomisation: block randomised, double‐blind. Randomisation was performed by the Royal Perth Hospital Pharmacy Department, and those involved in this process had no other study involvement. Intention‐to‐treat analysis: not undertaken Lost to follow‐up: 26% did not complete study | |
| Participants | Location: 2 teaching hospitals, Perth, Australia Period of study: before November 2008 95 participants Inclusion criteria: vitamin D‐deficient (serum 25O HD b50 nmol/L) by DiaSorin radioimmunoassay Exclusion criteria: ionised hypercalcaemia, chronic kidney disease (serum creatinine > 150 μmol/L), history of thyrotoxicosis or Cushing's syndrome, concomitant anticonvulsant drug therapy, and use of other medications affecting bone metabolism (including oestrogen, raloxifene, calcitriol, anabolic steroids, bisphosphates, sodium fluoride, oral glucocorticoids > 7.5 mg/day or inhaled glucocorticoids > 1000 μg/day) within the preceding 3 months; poor prognosis or who were unlikely to comply with therapy Sex: not given Age: mean 83 years Fracture type: further details not given | |
| Interventions | Timing of intervention: 3 months from inpatient stay (a) Vitamin D3 1000 IU/d and 1 placebo daily and calcium carbonate equivalent to 600 mg/d (b) Vitamin D2 1000 IU/d and 1 placebo daily and calcium carbonate equivalent to 600 mg/d Allocated: 47/48 Assessed: 36/34 for compliance | |
| Outcomes | Length of follow‐up: 3 months Main outcomes: Mortality, Hypercalcaemia Other outcomes: Compliance | |
| Notes | Boots Health Care provided vitamin D2 and matching placebo. Study funded by Royal Perth Hospital Medical Research Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "Randomization was performed by the Royal Perth Hospital Pharmacy Department, and those involved in this process had no other study involvement", no further details |
| Allocation concealment (selection bias) | Low risk | States "Randomization was performed by the Royal Perth Hospital Pharmacy Department, and those involved in this process had no other study involvement" |
| Blinding of participants and personnel (performance bias) | Low risk | States double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | States double‐blind and unlikely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | States double‐blind but blinding of outcome assessment not described and may have been influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | High risk | 8/47 on vitamin D3 and 7/48 on vitamin D2 appear not to have been included in follow‐up |
| Incomplete outcome data (attrition bias) | High risk | 8/47 on vitamin D3 and 7/48 on vitamin D2 appear not to have been included in follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient details provided |
| Other bias | Unclear risk | Boots Health Care provided vitamin D2 and matching placebo. Study funded by Royal Perth Hospital Medical Research Foundation |
| Methods | Method of randomisation: sealed, opaque envelopes in blocks of 10, appears stratified by place of residence | |
| Participants | Location: acute care in Hornsby‐Kuringai Hospital and rehabilitation hospitals, Sydney, Australia | |
| Interventions | Timing of intervention: started within 5 d of surgery, given once in the morning and once in the evening for 30 d, served on meal tray in hospital by nurses, given by family or self‐administered out of hospital | |
| Outcomes | Length of follow‐up: 2 months | |
| Notes | Request for further details (blinding of outcome assessors, details of supplement administration, further information on outcomes) sent. Reply from trialists (11 June 1999) gave details of outcome assessor blinding, supplement administration and outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Sealed, numbered opaque envelopes in blocks of 10". Information from Ian Cameron |
| Allocation concealment (selection bias) | Low risk | "Sealed, numbered opaque envelopes in blocks of 10". Information from Ian Cameron |
| Blinding of participants and personnel (performance bias) | High risk | No placebo group. Comment: likely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo group. Comment: unlikely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No placebo group. Comment: may have been influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Only one participant withdrew in control group, data provided by Ian Cameron for all other participants |
| Incomplete outcome data (attrition bias) | Low risk | Only one participant withdrew in control group, data provided by Ian Cameron for all other participants |
| Selective reporting (reporting bias) | Low risk | Thesis provides details that all outcomes reported |
| Other bias | High risk | Mead Johnson pharmaceutical company provided Sustagen supplement |
| Methods | Method of randomisation: computer‐generated randomisation list. Use of numbered envelopes | |
| Participants | Location: teaching hospital, The Hague, the Netherlands | |
| Interventions | Timing of intervention: nasogastric tube placed during surgery or within 12 h afterwards. Feeding started within 24 h of surgery. Intended duration of feeding 2 weeks. Feed administered between 21:00 hours and 05:00 hours to minimise interference with standard hospital diet. | |
| Outcomes | Length of follow‐up: 2 weeks Patient compliance: compliance with tube feeding | |
| Notes | Request for further details (including supplement details and administration, randomisation process, blinding of outcome assessors, details of 11 post‐randomised participants excluded, other outcomes) sent. Reply from trialists (23 June 1999) gave baseline details on all participants randomised, method of randomisation, assessor blinding, supplement details and administration. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomisation list prior to trial was made by computer". "If informed consent a numbered envelope was opened". No information on adequate safeguards |
| Allocation concealment (selection bias) | Unclear risk | "Randomisation list prior to trial was made by computer". "If informed consent a numbered envelope was opened". No information on adequate safeguards |
| Blinding of participants and personnel (performance bias) | High risk | No placebo group. Comment: likely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo group. Comment: unlikely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No placebo group. Comment: may have been influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | 11 participants excluded after randomisation (4 had pressure sores already, 7 pressure sore risk too low), groups not given |
| Incomplete outcome data (attrition bias) | Unclear risk | 11 participants excluded after randomisation (4 had pressure sores already, 7 pressure sore risk too low), groups not given |
| Selective reporting (reporting bias) | Unclear risk | Insufficient details provided |
| Other bias | High risk | Nutricia corp provided support for Nutrison tube feeding and nasogastric tubes |
| Methods | Method of randomisation: quasi‐randomised by date of birth | |
| Participants | Location: hospital, Kuopio, Finland | |
| Interventions | Timing of intervention: start time unclear, 4 months' treatment | |
| Outcomes | Length of follow‐up: 6 months | |
| Notes | Request for further details (timing of intervention, denominators for some outcomes) sent 11 May 1999, returned to sender. Details on method of randomisation received from Jane Robertson on 02 February 1999. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised by date of birth (see Notes) |
| Allocation concealment (selection bias) | Unclear risk | Quasi‐randomised by date of birth, but states "double‐blind" (see Notes) |
| Blinding of participants and personnel (performance bias) | Unclear risk | States "double‐blind". No other details |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient details provided |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient details on attrition and exclusions provided |
| Selective reporting (reporting bias) | Unclear risk | Insufficient details provided |
| Other bias | High risk | Appears sponsored by pharmaceutical company (Laaketehdas Medica, Helsinki, Finland) |
| Methods | Method of randomisation: use of a computer programme, balanced in blocks of four, by independent person | |
| Participants | Location: three centres, Arnhem, Deventer and Nieuwegein, in The Netherlands | |
| Interventions | Timing of intervention: supplemented from immediately postoperative period for four weeks or until discharge, given between regular meals | |
| Outcomes | Length of follow‐up: 28 d or earlier if discharged | |
| Notes | Request for further details (method of randomisation, other complications, adverse events, length of stay, further details of supplement) sent 13/10/03. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a computer programme, balanced in blocks of four, by an independent person. Information from trialists. Comment: probably low risk. |
| Allocation concealment (selection bias) | Low risk | Use of a computer programme, balanced in blocks of four, by an independent person. Information from trialists. |
| Blinding of participants and personnel (performance bias) | Low risk | States "double‐blind" but also states " look and taste of both supplements were not exactly identical, but supplements were given in similar, blinded packages to mask the differences". Comment: probably done. |
| Blinding of outcome assessment (detection bias) | Low risk | States "double‐blind" but also states " look and taste of both supplements were not exactly identical, but supplements were given in similar, blinded packages to mask the differences". Assessed by nurses and unlikely to have been influenced by unblinding. Comment: probably done. |
| Blinding of outcome assessment (detection bias) | Low risk | States "double‐blind" but also states " look and taste of both supplements were not exactly identical, but supplements were given in similar, blinded packages to mask the differences". Assessed by nurses. Comment: probably done. |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for in data. |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for in data. |
| Selective reporting (reporting bias) | Unclear risk | Pressure ulcer reporting agrees with methods, but would expect reporting of other complications |
| Other bias | High risk | Funded by Numico Research BV, nutrition company. |
| Methods | Method of randomisation: states randomised controlled trial, no further details Intention‐to‐treat analysis: no details Lost to follow‐up: no details | |
| Participants | Location: Daejin Medical Center, Bundang Jesaeng General Hospital, Korea Period of study: before September 2012 60 participants Inclusion criteria: aged over 65 years admitted to hospital for hip fracture surgery Exclusion criteria: none provided Sex: not given Age: mean age 81 years Fracture type: further details not given | |
| Interventions | Timing of intervention: 2 weeks postoperatively (a) Oral nutritional supplements, trace element supplements and dietetic counselling (b) Usual care Allocated: 30/30 Assessed: unclear | |
| Outcomes | Length of follow‐up: mean of 120 days Main outcomes: Mortality Complications | |
| Notes | Abstract only. Letter to Dr Kang requesting more details sent 3 October 2014 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract only. States randomized controlled trial, no further details |
| Allocation concealment (selection bias) | Unclear risk | Abstract only. States randomized controlled trial, no further details |
| Blinding of participants and personnel (performance bias) | High risk | Abstract only. No placebo group. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | Low risk | Abstract only. No placebo group. Comment: unlikely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Abstract only. No placebo group. Comment: unclear if done |
| Incomplete outcome data (attrition bias) | Unclear risk | Abstract only. No details provided |
| Incomplete outcome data (attrition bias) | Unclear risk | Abstract only. No details provided |
| Selective reporting (reporting bias) | Unclear risk | Abstract only. Insufficient details provided |
| Other bias | Unclear risk | Abstract only. Insufficient details provided. No details on sponsor |
| Methods | Method of randomisation: computer‐generated randomisation plan in 1:1 ratio. Each study centre had its own randomisation schedule. Randomisation envelopes were opened and used in ascending numerical order. Intention‐to‐treat analysis: not undertaken Lost to follow‐up: 64% | |
| Participants | Location: 6 hospitals, Russia Period of study: 2009‐2010 127 participants Inclusion criteria: age ≥ 45 years, expected to undergo surgical hip fracture repair within 14 d of fracture, admission total protein level ≤ 70 g/L and screening serum albumin ≤ 38 g/L, Subjective Global Assessment score B or C, able to consume foods and beverages orally Exclusion criteria: type 1 diabetes; uncontrolled type 2 diabetes (HbA1c > 8%); active malignancy; chronic, contagious, infectious disease (e.g. active tuberculosis, Hepatitis B or C, or HIV); alcohol or substance abuse; severe dementia; gastrointestinal conditions that may interfere with nutrient intake or digestion, or known allergy or intolerance to any ingredient in supplements Sex: 35 female, 11 male (of 46 evaluated) Age: mean 69 years Fracture type: further details not given | |
| Interventions | Timing of intervention: from before surgery for 28 d a) Ensure TwoCal oral supplements; Abbott Nutrition, Columbus, Ohio, USA; nutritionally complete, energy and protein‐dense drink including 30 vitamins and minerals. A total of two containers (200 mL per container) were given 3 times/d: 100 mL between breakfast and noon meal, 100 mL serving between noon and evening meal, and 200 mL as a snack before going to bed. Provided an additional 798 kcal and 34 g protein/d; and standard hospital food b) Standard hospital food Allocated: ?/? (total 127) Assessed: 22/24 | |
| Outcomes | Length of follow‐up: 28 d Main outcomes: Mortality Functional status Complications Putative side effects Other outcomes: Compliance | |
| Notes | Abstract provides results for only 46 of 127 randomised participants. Emailed Abbott Nutrition 8 October 2014. Dr Menghua Luo replied providing full publication 17 November 2014 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States used "using a computer generated randomization plan on a 1:1 ratio". |
| Allocation concealment (selection bias) | Unclear risk | States "Each study center had its own randomization schedule. As eligible subjects were enrolled, they were assigned a subject number sequentially starting with the first envelope indicating the group assignment. Randomization envelopes were opened and used in ascending numerical order." No indication that envelopes were opaque |
| Blinding of participants and personnel (performance bias) | High risk | No placebo. Comment: probably not done |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo group. Comment: unlikely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No placebo group. Comment: may have been influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | High risk | Only 46 of 127 enrolled assessed. States "72 excluded due to missing records" |
| Incomplete outcome data (attrition bias) | High risk | Only 46 of 127 enrolled assessed. States "72 excluded due to missing records" |
| Selective reporting (reporting bias) | High risk | Insufficient data on adverse events, including denominators. No details of length of stay |
| Other bias | High risk | Supported by Abbott Nutrition, and 3 of the authors were employees |
| Methods | Method of randomisation: not stated | |
| Participants | Location: Illawarra Regional Hospital, Port Kembla Campus, Woolongong, Australia | |
| Interventions | Timing of intervention: started on admission for 10 d, once daily after evening meal | |
| Outcomes | Length of follow‐up: 3 months post‐discharge | |
| Notes | In the trial report, the two supplemented groups were combined for analysis for comparison with control group. 3 subjects eliminated post‐randomisation from analysis because only took protein supplement for 7 d, and 1 eliminated for developing diabetes. Numbers of participants assigned/assessed not always clear. Request for further details sent 4 February 2000 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information: just states "randomised" |
| Allocation concealment (selection bias) | Unclear risk | No information: just states "randomised" |
| Blinding of participants and personnel (performance bias) | High risk | No blinding undertaken |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo group. Comment: unlikely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No placebo group. Comment: may have been influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | High risk | In the trial report, the two supplemented groups were combined for analysis for comparison with control group. Three subjects eliminated post‐randomisation from analysis because only took protein supplement for 7 d, and one eliminated for developing diabetes. Numbers of participants assigned/assessed not always clear |
| Incomplete outcome data (attrition bias) | High risk | In the trial report, the two supplemented groups were combined for analysis for comparison with control group. Three subjects eliminated post‐randomisation from analysis because only took protein supplement for 7 d, and one eliminated for developing diabetes. Numbers of participants assigned/assessed not always clear |
| Selective reporting (reporting bias) | Low risk | Thesis available, all outcomes accounted for |
| Other bias | Unclear risk | No details available on funding source |
| Methods | Method of randomisation: computer‐generated sequence, stratified by admission accommodation. Sealed opaque envelopes, prepared remote from recruitment by pharmacy | |
| Participants | Location: Orthopaedic wards of Flinders Medical Centre, Adelaide, Australia | |
| Interventions | Timing of intervention: from 7 d after fracture, given daily for 6 weeks | |
| Outcomes | Length of follow‐up: 12 months | |
| Notes | Trial population also included 49 other participants (43 with hip fracture), who were allocated to the two other intervention groups: exercise; and nutrition plus exercise. Data from these two groups are not included in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States "The Pharmacy department maintained a computer generated allocation sequence in sealed opaque envelopes." |
| Allocation concealment (selection bias) | Low risk | States "The Pharmacy department maintained a computer generated allocation sequence in sealed opaque envelopes." |
| Blinding of participants and personnel (performance bias) | High risk | No placebo group. Comment: likely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo group but states that research staff were blinded. Comment: unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | Expected outcomes for all trial participants reported (hip fracture patients were a sub group of all participants). |
| Other bias | High risk | Funded bu NHMRC Public Health Research Scholarship, Flinders University‐Industry Collaborative Grant and Nutricia Australia Pty Ltd |
| Methods | Method of randomisation: sealed opaque envelope containing the randomised group from blocks of 12 was drawn for each participant by a member of the ward staff who was not a co‐investigator Intention‐to‐treat analysis: not undertaken, 5 excluded after randomisation Lost to follow‐up: details given | |
| Participants | Location: Department of Rehabilitation of Kowloon Hospital, China Period of study: before June 2012 126 participants Inclusion criteria: 60 years or older, recent low impact osteoporotic fracture of the proximal femur surgically repaired within 4 weeks before recruitment Exclusion criteria: required tube feeding, those in unstable medical condition, BMI ≥ 25, malignancy, conditions with contraindication for high‐protein diet, mentally incapacitated and inability to communicate or understand written consent Sex: 80 female, 41 male (of 121 assessed) Age: mean age 82 years Fracture type: 52 neck of femur, 63 trochanteric, 6 sub‐trochanteric | |
| Interventions | Timing of intervention: started within 3 d of admission to rehabilitation hospital for 4 weeks or until discharged. a) A ready‐to‐use oral liquid nutritional supplement (18–24 g protein and 500 kcal per day). The oral nutritional supplementation was a drink of about 240 ml in volume given twice daily on top of the standard hospital diet. 4 types of nutritional supplements were offered according to participant’s dietary preferences. These were brands Ensure by Abbott, Resource Breeze by Nestle Nutrition (orange or peach flavour), Compleat by Nestle Nutrition and Glucerna by Abbott. Oral 800‐1000 IU vitamin D and tablets containing 1200 mg calcium daily b) Standard hospital diet. Oral 800‐1000 IU vitamin D and tablets containing 1200 mg calcium daily Allocated: 65/61 Assessed: 61/60 | |
| Outcomes | Length of follow‐up: 6 months after discharge Main outcomes: Mortality Complications Rehabilitation hospital stay Functional status, Nursing home and acute hospital care Putative side effects Other outcomes: Compliance | |
| Notes | Emailed [email protected] 5 January 2015 to clarify data for complications. Reply received 6 January 2015 providing numbers of participants with complications in groups Participants recruited if BMI < 25 and mean BMI 21.7, consultant geriatrician advised that participants in this trial be considered under 'malnourished targeted' category of subgroup analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "sealed opaque envelope containing the randomised group from blocks of twelve. |
| Allocation concealment (selection bias) | Low risk | States "sealed opaque envelope containing the randomised group from blocks of twelve was drawn for each patient by a member of the ward staff who was not a co‐investigator" |
| Blinding of participants and personnel (performance bias) | High risk | No placebo group. Comment: likely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Low risk | States that assessment of complications, treatment decisions were made by ward team and not investigators. Although unblinded unlikely to have influenced outcome assessment |
| Blinding of outcome assessment (detection bias) | Low risk | States that assessment of treatment and discharge decisions were made by ward team and not investigators. Functional status assessed by physiotherapist blinded to allocation |
| Incomplete outcome data (attrition bias) | High risk | 4 intervention group and 1 control group excluded by investigators |
| Incomplete outcome data (attrition bias) | High risk | 4 intervention group and 1 control group excluded by investigators |
| Selective reporting (reporting bias) | Low risk | Protocol not available but all expected outcomes accounted provided |
| Other bias | Low risk | Funded by rehabilitation hospital, no commercial sponsorship |
| Methods | Method of randomisation: not stated, stratified by type of hip fracture | |
| Participants | Location: 3 rehabilitation hospitals, USA | |
| Interventions | Timing of intervention: consecutive 28‐d period at least two 8 oz cans/d | |
| Outcomes | Length of follow‐up: 3 months | |
| Notes | Request for further details (mortality, denominators for length of stay, complications) sent 13 October 2004. Details of mortality and denominators received 06 January 2005 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information other than: "randomized, double‐blind, parallel‐group study" |
| Allocation concealment (selection bias) | Unclear risk | No information other than: "randomized, double‐blind, parallel‐group study" |
| Blinding of participants and personnel (performance bias) | Unclear risk | States double‐blind but no further details |
| Blinding of outcome assessment (detection bias) | Low risk | States double‐blind and unlikely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | States double‐blind but no further details, and may have been influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | No details on denominators for complications provided |
| Incomplete outcome data (attrition bias) | High risk | Length of stay data for 4 participants on Boost, and 4 on Ensure not provided. Numbers for purported adverse events, mobility and discharge destination not provided |
| Selective reporting (reporting bias) | Unclear risk | Insufficient details provided |
| Other bias | High risk | Part funded by Mead Johnson, manufacturer of Boost HP |
| Methods | Method of randomisation: randomised in blocks according to computer‐generated randomisation, in‐patient pharmacy co‐ordinated the randomisation and drug distribution Intention‐to‐treat analysis: not carried out Lost to follow‐up: 18/65 lost to follow‐up | |
| Participants | Location: two academic hospital sites, Hamilton, Ontario, Canada Period of study: October 2007‐April 2009 65 participants Inclusion criteria: over age 50 with an acute fragility hip fracture (defined as femoral neck, trochanteric, subtrochanteric or subcapital) which was the result of a minimal trauma accident, defined as a fall from standing height or less Exclusion criteria: pelvic fractures; pathological fractures secondary to malignancy or intrinsic bone disease (e.g. Paget’s disease); pre‐existing bone abnormality; cancer in the past 10 years likely to metastasize to bone; renal insufficiency (creatinine < 30 mls/min); renal stones in past 10 years; hypercalcaemia (primary hyperparathyroidism; granulomatous diseases); hypocalcaemia; stroke within the last 3 months; or had taken hormone replacement therapy, calcitonin, bisphosphates, raloxifene, or parathyroid hormone during the previous 24 months; admitted from long‐term care facilities/nursing homes Sex: 36 female, 25 male Age: mean 69 years Fracture type: further details not given | |
| Interventions | Timing of intervention: day 1 for 90 d (a) Oral placebo bolus day 1, then a daily tablet of 1000 IU vitamin D3 for 90 d (b) 50,000 IU vitamin D2 oral bolus day 1, then a daily tablet of 1000 IU vitamin D3 for 90 d (c) 100,000 IU vitamin D2 oral bolus day 1, then a daily tablet of 1000 IU vitamin D3 for 90 d Allocated: 22/22/21 Assessed: 12/18/17 at 90 d | |
| Outcomes | Length of follow‐up: 90 d Main outcomes: Mortality Adverse events Other outcomes: Compliance | |
| Notes | Emailed [email protected] 6 November 2014 for details of allocation of participants who died or had adverse events. No details received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States "Patients were randomized in blocks according to a computer‐generated randomization list" |
| Allocation concealment (selection bias) | Low risk | States "The central in‐patient pharmacy at McMaster University Medical Centre coordinated the randomization procedure and the distribution of study drugs" |
| Blinding of participants and personnel (performance bias) | Low risk | Blinded, placebo‐controlled trial and states "The medication treatment group was concealed and all participants, study coordinators, physicians, staff, and caregivers were blinded to treatment group allocation" |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded, placebo‐controlled trial and states "The medication treatment group was concealed and all participants, study coordinators, physicians, staff, and caregivers were blinded to treatment group allocation" |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded, placebo‐controlled trial and states "The medication treatment group was concealed and all participants, study coordinators, physicians, staff, and caregivers were blinded to treatment group allocation" |
| Incomplete outcome data (attrition bias) | High risk | 18 participants from 65 lost to follow‐up by 90‐d final follow‐up |
| Incomplete outcome data (attrition bias) | Unclear risk | 18 participants from 65 lost to follow‐up by 90‐day final follow‐up |
| Selective reporting (reporting bias) | Low risk | Principally a study of vitamin D dose responses and adverse events |
| Other bias | High risk | Signficant imbalance in age between two intervention groups (reported P = 0.024). Study supported by Merck Frosst Canada Ltd |
| Methods | Method of randomisation: sealed opaque numbered envelopes Intention‐to‐treat analysis: undertaken Lost to follow‐up: no participants lost to follow‐up | |
| Participants | Location: Peterborough District Hospital, UK Period of study: recruitment January 2003–July 2007 300 participants Inclusion criteria: postoperative haemoglobin level of < 110 g/L within 5 d after hip fracture surgery. Exclusion criteria: participant unwilling to give written informed consent or for whom the relative or next of kin was unavailable or declined to give assent, postoperative haemoglobin level of ‡110 g/L, multiple trauma (defined as either > 2 other fractures or any other fracture requiring surgery other than simple manipulation), participant unable to take oral iron medication because of adverse effects, participant taking iron therapy at time of admission, haemoglobin level of < 110 g/L at time of admission, participant unable to attend routine follow‐up in the hip fracture clinic, age of < 60 years Sex: 245 female, 55 male Age: mean age 82 years Fracture type: 45% intracapsular fracture, 21% intramedullary nail and 34% extramedullary fixation (presumed not intracapsular fractures) | |
| Interventions | Timing of intervention: immediately post‐randomisation for 28 d (a) Oral iron therapy (ferrous sulphate, 200 mg twice daily) (b) No iron supplement Allocated: 150/150 Assessed: 150/150 at 12 months | |
| Outcomes | Length of follow‐up: 12 months Main outcomes: Mortality, Hospital length of stay Putative side effects of treatment | |
| Notes | Emailed Dr Martyn Parker (Martyn.Parker@pbh‐tr.nhs.uk) 16 October 2014 about further details on length of hospital stay data, reply received 16 October 2014 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided on sequence generation |
| Allocation concealment (selection bias) | Low risk | States "randomization was accomplished by opening a sealed opaque numbered envelope for each patient" |
| Blinding of participants and personnel (performance bias) | High risk | No placebo group |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo group. Comment: unlikely to have been influenced by unblinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No placebo group. Comment: may have been influenced by unblinding |
| Incomplete outcome data (attrition bias) | Low risk | Data for all participants randomised provided |
| Incomplete outcome data (attrition bias) | Unclear risk | 13/150 discontinued iron therapy in intervention group and 5 in control group commenced iron therapy. 7/150 in intervention group unable to attend outpatient follow‐up and 16/150 in control group likely to have influenced putative side effects of treatment |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available and unclear if all expected outcomes provided |
| Other bias | Low risk | Non‐pharmaceutical funding (funded by Peterborough Hospital Hip Fracture Fund) |
| Methods | Method of randomisation: randomised into 2 groups independently by a nurse practitioner using computer‐generated random numbers Intention‐to‐treat analysis: 2 participants excluded for moving out of area Lost to follow‐up: 2 participants lost to follow‐up | |
| Participants | Location: Royal Glamorgan Hospital, Llantrisant, Mid Glamorgan, UK Period of study: recruitment February 2005–October 2005 68 participants Inclusion criteria: acute hip fracture confirmed on X‐ray, postoperative anaemia ((Hb between 8–12 g% in men and 8–11 g% in women). Exclusion criteria: pre‐operative serum ferritin less than15 mg/l or more than 200 mg/l, admission CRP > 3, serum iron/total iron binding capacity ratio (TIBC) < 15, TIBC > 60, already on iron tablets, pre‐existing anaemic disorders, underlying medical conditions (malignancy, chronic renal failure, inflammatory bowel disease, chronic peptic ulcer, oesophageal varices, rheumatoid arthritis), medication interfering with iron absorption e.g. antacids, tetracyclines, bisphosphates; no consent Sex: 55 female, 11 male Age: mean age 82 years Fracture type: 53% intertrochanteric fracture, 47% cervical | |
| Interventions | Timing of intervention: from 2nd postoperative day for 4 weeks (a) Oral iron therapy (ferrous sulphate, 200 mg three times daily) (b) No iron supplement Allocated: ?/? Assessed: 32/34 at 4 weeks | |
| Outcomes | Length of follow‐up: 4 weeks Main outcomes: Putative side effects of supplements | |
| Notes | Emailed Mr Prasad ([email protected]) 24 October 2014 about further details on outcomes, replied 24 October 2014 indicating "no deaths or any other complications" in the study or control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States used "using computer generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | States "the patients were then randomised into two groups; independently by a nurse practitioner using computer generated random numbers...The randomisation was implemented by the senior author (JM)." |
| Blinding of participants and personnel (performance bias) | High risk | No placebo group. Comment: likely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | Putative adverse events only primary outcome reported and data only reported for intervention group, clinical staff also not blinded, although states "first author was blinded" |
| Incomplete outcome data (attrition bias) | High risk | Data for putative adverse events only provided for intervention group, also two participants of unknown allocation excluded |
| Selective reporting (reporting bias) | Unclear risk | Insufficient details provided |
| Other bias | Unclear risk | Insufficient details provided |
| Methods | Method of randomisation: states random number table and double‐blind study, but unclear if those who assigned were blinded | |
| Participants | Location: orthopaedic ward in hospital and recovery hospital, Geneva, Switzerland | |
| Interventions | Timing of intervention: mean randomisation time 6.5 (SD 1.9) d after fracture, supplemented 5 d a week for 6 months | |
| Outcomes | Length of follow‐up: 12 months | |
| Notes | Composition of placebo unclear, denominators not clear. Request for further details sent 27 May 1999, re‐sent 7 February 2000 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Using a random number table", no further details provided |
| Allocation concealment (selection bias) | Unclear risk | "Using a random number table, we assigned ..." |
| Blinding of participants and personnel (performance bias) | Low risk | Oral protein supplement and placebo made isocaloric, states "double‐blind". Comment: probably done |
| Blinding of outcome assessment (detection bias) | Low risk | Oral protein supplement and placebo made isocaloric, states "double‐blind" and unlikely to be influenced by unblinding. Comment: probably done |
| Blinding of outcome assessment (detection bias) | Unclear risk | Oral protein supplement and placebo made isocaloric, states "double‐blind" and may have been influenced by unblinding as no details on who assessed outcomes |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for and drop‐outs do not appear to differ between groups |
| Incomplete outcome data (attrition bias) | Unclear risk | No denominators for lengths of stay, activities of daily living unclear |
| Selective reporting (reporting bias) | Unclear risk | Insufficient details provided |
| Other bias | High risk | Study supported by Sandoz Nutrition Ltd |
| Methods | Method of randomisation: multicentre, randomised, open‐label clinical trial Intention‐to‐treat analysis: not carried out, exclusions for poor compliance Lost to follow‐up: 50% lost to follow‐up | |
| Participants | Location: hospitals, Milan, Italy Period of study: up to 2009 107 participants Inclusion criteria: Men and women > 65 y of age with hip fracture who were eligible for surgery Exclusion criteria: dementia; inability to follow instructions; swallowing difficulties; complex ‘pathological’ fractures Sex: 90 female, 17 male Age: mean 80 years Fracture type: 31% intracapsular, 69% extracapsular | |
| Interventions | Timing of intervention: Restorfast for 6 weeks, then Riabylex for further 10 weeks (a) Restorfast sachet once daily (345 mg L‐carnitine, 500 mg calcium, 250 mg magnesium, 5 mcg vitamin D3, 500 mg L‐leucine); followed by one Riabylex daily (1500 mg creatine, 250 mg L‐carnitine, 20 mg coenzyme Q10, nicotinamide 18 mg, pantothenic acid 6 mg, riboflavin 1.6 mg) (b) No intervention Allocated: 54/53 Assessed: 27/26 | |
| Outcomes | Length of follow‐up: 16 weeks Main outcomes: Length of acute hospital stay Time to ambulation Complications: pressure sores Functional status: participants reaching a functional recovery | |
| Notes | Italian speaker (Miriam Brazzelli) extracted data. Funder unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised trial, no further details |
| Allocation concealment (selection bias) | Unclear risk | Randomised trial, no further details |
| Blinding of participants and personnel (performance bias) | High risk | No placebo group. Comment: likely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo group. Comment: unlikely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No placebo group. Comment: may have been influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | High risk | 50% lost to follow‐up, including protocol violations, and because of clinical complications |
| Incomplete outcome data (attrition bias) | High risk | 50% lost to follow‐up, including protocol violations, and because of clinical complications |
| Selective reporting (reporting bias) | Unclear risk | Insufficient details to assess, unusual for no mortality to be reported |
| Other bias | Unclear risk | Italian speaker (Miriam Brazzelli) extracted data. Funder unclear |
| Methods | Method of randomisation: allocation made using sequentially numbered opaque sealed envelopes Intention‐to‐treat analysis: not undertaken (4 participants excluded from analysis as died before surgery although received intervention) Lost to follow‐up: all participants accounted for | |
| Participants | Location: Orthopedic and Trauma Surgery Unit of the Hospital Reina Sofia in Córdoba, Spain Period of study: October 2006–October 2008 200 participants Inclusion criteria: aged over 65 years, surgical management of hip fracture Exclusion criteria: diseases diagnosed before the admission of participant (iron overload disorders, hypersensitivity to oral or parenteral iron preparations, asthma or other severe atopic, active infection or neoplasm), treatment with clopidogrel or with acetylsalicylic acid at dose rates greater than 150 mg/24 h, no surgical indication for the current fracture, disorders impaired coagulation (partial thromboplastin time > 2.5%, international normalised ratio > 1.5), liver disorders with elevated transaminases (aspartase aminotransferase > 70 U/L, alanine aminotransferase > 55 U/L), and chronic kidney failure (creatinine > 2 mg/dL) or patients including in dialysis. Sex: all female Age: mean 83 years Fracture type: 35% intracapsular fracture, 65% extracapsular fracture | |
| Interventions | Timing of intervention: first dose was administered in the first 24 h after admission, always before surgical intervention. The following doses were administered before or after surgery, depending on the time of surgery. (a) 600 mg of iron sucrose IV (Venofer,Vifor France Company, Levallois‐Perret, France) in 3 doses of 200 mg at 48‐h intervals, starting on the day of admission; administration was by slow perfusion of two 100‐mg ampoules diluted in 250 mL of 0.9% saline solution over a 90‐min period (b) no iron supplement Allocated: 100/100 Assessed: 99/97 at 30 d post discharge | |
| Outcomes | Length of follow‐up: 30 d post discharge Main outcomes: Mortality Complications including infections Length of acute hospital stay Purported side effects of treatment | |
| Notes | Emailed [email protected] on 4 November 2014 to clarify length of stay data which differ between text and table. Data from table used for review as no reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "Randomization lists were generated in blocks of 10 to ensure equal group sizes, and allocation was made using sequentially numbered opaque sealed envelopes, so that neither the patient nor the investigator could know which group the subject was assigned to before his or her consent to participation." Comment: sequence generation unclear. |
| Allocation concealment (selection bias) | Low risk | States "Randomization lists were generated in blocks of 10 to ensure equal group sizes, and allocation was made using sequentially numbered opaque sealed envelopes, so that neither the patient nor the investigator could know which group the subject was assigned to before his or her consent to participation." |
| Blinding of participants and personnel (performance bias) | High risk | No placebo group |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo group. Comment: unlikely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No placebo group. Comment: may have been influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | High risk | 3 participants in control group and 1 participant in intervention group excluded as died before surgery although may have had intervention, purported adverse events from iron only provided for intervention group |
| Incomplete outcome data (attrition bias) | High risk | 3 participants in control group and 1 participant in intervention group excluded as died before surgery although may have had intervention, purported adverse events from iron only provided for intervention group |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but all reported and expected outcomes provided |
| Other bias | Low risk | Funded by the Spanish Ministry of Health and Consumer Affairs |
| Methods | Method of randomisation: not stated | |
| Participants | Location: hospital, Bristol, UK | |
| Interventions | Timing of intervention: started after surgery and 24‐36 h of crystalloid intravenous fluids. Intervention provided during waking hours for 10 d | |
| Outcomes | Length of follow‐up: 4 weeks | |
| Notes | Limited functional outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomly selected group of 24 patients were encouraged to drink liquid supplement feeds" |
| Allocation concealment (selection bias) | Unclear risk | States "randomly selected group of 24 patients were encouraged to drink liquid supplement feeds" |
| Blinding of participants and personnel (performance bias) | High risk | No placebo group |
| Blinding of outcome assessment (detection bias) | Low risk | Not likely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No placebo group. Comment: may not have been done |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient details on attrition and exclusions provided |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient details on attrition and exclusions provided |
| Selective reporting (reporting bias) | Unclear risk | Insufficient details provided |
| Other bias | High risk | Imbalance in weights: trochanteric fracture and subcapital fixation supplemented group mean 65 kg, controls 53 kg |
| Methods | Method of randomisation: sealed opaque envelopes opened sequentially | |
| Participants | Location: acute care facility, Little Rock, Arkansas, USA | |
| Interventions | Timing of intervention: small‐bore nasogastric feeding tube placed in theatre or recovery room. Feeding started postoperatively, nightly from 19:00 hours, until volitional intake greater than 90% of predicted requirements for 3 consecutive days or participant discharged home | |
| Outcomes | Length of follow‐up: 6 months | |
| Notes | Pilot study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information from trialists "The actual randomization was prepared by the biostatistician..". No other details provided |
| Allocation concealment (selection bias) | Low risk | "The actual randomization was prepared by the biostatistician.. using sealed envelopes. Security (lined) envelopes were used to assure that the assignment cannot be read without opening the envelope. After consent had been obtained and the baseline assessment was completed, the next envelope was opened to reveal the group assignment ..." Information from trialists |
| Blinding of participants and personnel (performance bias) | High risk | No placebo group. Comment: likely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo group. Comment: unlikely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No placebo group. Comment: may have been influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for, with no dropouts |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for, with no dropouts |
| Selective reporting (reporting bias) | Unclear risk | No protocol available and insufficient details available |
| Other bias | High risk | Funding from Ross Laboratories, who manufactured the nasogastric feed, and Department of Veterans Affairs |
| Methods | Method of randomisation: sealed opaque envelopes opened sequentially | |
| Participants | Location: orthopaedic wards of University Hospital and Department of Veterans Affairs Hospital, Little Rock, Arkansas, USA | |
| Interventions | Timing of intervention: small bore feeding tube placed within 12 h of surgery, confirmed by X‐ray in place until deficit between requirements and oral intake < 480 kcal/day for at least 2 consecutive days or until discharged Given nightly over 11 h | |
| Outcomes | Length of follow‐up: 6 months | |
| Notes | Request for further details on randomisation and tube feeding sent 15 March 2006. Reply, received 14 April 2006, gave further details of randomisation method | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States that "The randomization process was prepared by the biostatistician...Subjects were randomized to either treatment or control within blocks to assure that there were roughly equal numbers of subjects in each group at the end of the study. The block sizes were randomly varied to minimize the ability to deduce the assignment for a particular patient before opening the envelope." Reply from trialists |
| Allocation concealment (selection bias) | Low risk | "The randomization process was prepared by the biostatistician, using a series of sealed envelopes. Security (lined) envelopes were used to assure that the assignment could not be read without opening the envelope. After consent had been obtained and the baseline assessment was completed, the next envelope in order was opened to reveal the group assignment. Each envelope contained a card. The card had the assignment for treatment or control pre‐printed. Space was provided to enter the patient name and ID as well as the date, time and person responsible for randomization. The study nurse completed the card, photocopied it, and returned the original to the biostatistician as a check that the randomization process was progressing appropriately. Subjects were randomized to either treatment or control within blocks to assure that there were roughly equal numbers of subjects in each group at the end of the study. The block sizes were randomly varied to minimize the ability to deduce the assignment for a particular patient before opening the envelope." Reply from trialists |
| Blinding of participants and personnel (performance bias) | High risk | No placebo group. Comment: likely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo group. Comment: unlikely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No placebo group. Comment: may have been influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data for one participant only in intervention group |
| Selective reporting (reporting bias) | Low risk | No protocol available, but expected outcomes reported |
| Other bias | High risk | Control group more than 5 years older. Funded by a National Insititute on Aging Grant. Ross Laboratories supplied nutritional supplements and nasogastric feeding tubes |
| Methods | Method of randomisation: numbered opaque sealed envelopes, unclear if randomisation fully concealed since the envelopes prepared and opened by the same research nurse | |
| Participants | Location: hospital(s) in Stockholm, Sweden | |
| Interventions | Timing of intervention: 6 months, unclear when started | |
| Outcomes | Length of follow‐up: 12 months | |
| Notes | Request for further details (complications) sent. Reply from trialists (14 October 2004) gave further details of infections. Request for further details (randomisation) sent. Reply from trialists (10 November 2004) gave full details of randomisation process | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised, but no further details on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | "Patients were randomised, using opaque sealed envelopes". (Also numbered.) However, the envelopes were prepared and opened by the same research nurse, involved in the trial |
| Blinding of participants and personnel (performance bias) | High risk | No placebo group. Comment: likely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo group. States that " A research nurse not involved in the surgery or clinical decisions assessed all clinical variables." Comment: unlikely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No placebo group. States that " A research nurse not involved in the surgery or clinical decisions assessed all clinical variables." Comment: may have been influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | Two in control group and one in supplement group lost to follow‐up, unlikely to have an impact on outcome assessment |
| Incomplete outcome data (attrition bias) | Unclear risk | Two in control group and one in supplement group lost to follow‐up, unlikely to have an impact on outcome assessment |
| Selective reporting (reporting bias) | Low risk | Protocol not available, but expected outcomes provided |
| Other bias | High risk | Displaced fractures in 75% of controls and 45% of supplement group. Funded by Trygg‐Hansa Insurance Company, the Swedish Orthopaedic Association, the Swedish Research Council, Novo Nordic Fund, Nutricia Nordic AB and Nycomed AB |
| Methods | Method of randomisation: not stated | |
| Participants | Location: orthopaedic ward, hospital and recovery hospital, Geneva, Switzerland | |
| Interventions | Timing of intervention: started on admission to orthopaedic clinic, continued in recovery hospital. Given once daily at 20:00 hours | |
| Outcomes | Length of follow‐up: 7 months | |
| Notes | Post‐randomisation exclusions: 3 in protein intervention group excluded for non‐compliance, 3 controls excluded (2 took protein supplements, one severe diarrhoea), 4 of unspecified group left orthopaedic unit prematurely. Numbers of complications unclear. Request for further details (exclusions, complications) sent 24 May 1999, re‐sent 7 February 2000 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information: just "randomized into two groups" |
| Allocation concealment (selection bias) | Unclear risk | No information: just "randomized into two groups" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Both groups received 250ml supplements daily, but not clear if different in taste or appearance |
| Blinding of outcome assessment (detection bias) | Low risk | Unlikely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Both groups received 250 ml supplements daily, but not clear if different in taste or appearance |
| Incomplete outcome data (attrition bias) | High risk | Post‐randomisation exclusions: 3 in protein intervention group excluded for non‐compliance, 3 controls excluded (2 took protein supplements, one severe diarrhoea), 4 of unspecified group left orthopaedic unit prematurely |
| Incomplete outcome data (attrition bias) | High risk | Post‐randomisation exclusions: 3 in protein intervention group excluded for non‐compliance, 3 controls excluded (2 took protein supplements, one severe diarrhoea), 4 of unspecified group left orthopaedic unit prematurely |
| Selective reporting (reporting bias) | Unclear risk | Insufficient details provided |
| Other bias | High risk | Sandoz‐Wander (Switzerland) supplied the dietary supplements |
| Methods | Method of randomisation: onsite computer randomisation performed Assessor blinding: investigators, participants, medical and nursing staff were blinded to group allocation Intention‐to‐treat analysis: both intention‐to‐treat and per‐protocol analysis approaches adopted Lost to follow‐up: 49 participants | |
| Participants | Location: Medical Centre Alkmaar ‐ The Netherlands and Red Cross Hospital Beverwijk, Netherlands (Acute Hospital) Period of study: recruitment from March 2008‐July 2010 236 randomised with data for 173 participants Inclusion criteria: people with a primary hip fracture scheduled for surgery, aged 75 years or older Exclusion criteria: inability to receive oral intake, major malabsorption, severe renal insufficiency (creatinine clearance < 30 mL/min), participation in another trial Sex: 63/173; Intervention group: 33/80; Control group: 30/93 Age: Mean 84.4 y Fracture type: not stated but fracture fixation methods detailed, 113 hemiarthroplasty (presumed intracapsular fractures), 11 cannulated hip screws, 94 gamma nail (presumed extracapsular fractures) | |
| Interventions | Intervention group: oral taurine capsules (oral) Timing of intervention: commenced pre‐surgery (within 24 h after hospital admission). Continuation of intervention to up to six d postoperatively (a) 3 times/d (scheme 2‐1‐2 capsules of 1.2 g taurine or placebo) to reach 6 g/day daily dose. Intervention continued for those discharged within 6 d post‐op to receive 6 d of intervention. First 2 capsules of the nutritional intervention were provided after receiving informed consent at the same time as baseline data collection. (b) Placebo (microcrystalline cellulose) capsules (oral): commenced pre‐surgery (within 24 h after hospital admission), continuation of intervention: up to 6 d postoperatively. Dose not clearly specified but it was presumed the same scheme as the intervention Allocated: not stated, 236 in total Assessed: 89/98 | |
| Outcomes | Length of follow‐up: 12 months Main outcomes: Mortality Length of hospital stay Morbidity and complications: infection, cardiovascular events, stroke, delirium, requirement for blood transfusion, requirement for reoperation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Computerized randomisation table using block randomisation of 30 patients per block, generated by a local statistician, used by the pharmacological department to label the capsules for the interventions.” (page 12300 section 3.2, Line3‐5) |
| Allocation concealment (selection bias) | Low risk | Allocation “generated by a local statistician, used by the pharmacological department to label the capsules for the interventions.” (page 12300 section 3.2, Line 4‐5) |
| Blinding of participants and personnel (performance bias) | Low risk | “patients…unaware of intervention allocation” (page 12300 section 3.2 line 7) |
| Blinding of outcome assessment (detection bias) | Low risk | “investigators, patients, medical and nursing staff were unaware of interventions allocation” (page 12300 section 3.2, line 7‐9) |
| Blinding of outcome assessment (detection bias) | Low risk | “Two investigators, who were unaware of treatment allocation, independently determines the occurrence of postoperative complications and morbidity” (page 12300 section 3.3, line 4‐6) |
| Incomplete outcome data (attrition bias) | High risk | Reasons for missing data and attrition not clear by group allocation and reason not provided (Figure 1) |
| Incomplete outcome data (attrition bias) | High risk | Reasons for missing data and attrition not clear by group allocation and reason not provided (Figure 1) |
| Selective reporting (reporting bias) | Low risk | Study protocol provided (page 12299, section 3.1, line 8) |
| Other bias | High risk | Underpowered analysis (page 12301 section 3.5, line 1‐2), unrealistic 50% reduction in mortality at 1 year presumed in power calculation |
| Methods | Method of randomisation: computer‐generated random‐number sequence list after pre‐stratification for hospital, gender and age (55‐74 years vs 75 years and above) with allocation ratio 1:1. Independent allocation by phone call to research assistant Intention‐to‐treat analysis: undertaken Lost to follow‐up: 6% lost to follow‐up | |
| Participants | Location: 3 hospitals in South Limburg, Netherlands Period of study: recruitment July 2007‐December 2009 152 participants Inclusion criteria: admitted for surgical treatment of hip fracture, aged ≥ 55 years Exclusion criteria: pathological or periprosthetic fracture; a disease of bone metabolism (Paget’s, hyperparathyroidism); an estimated life expectancy < 1 year due to underlying disease; used an oral nutritional supplement before hospital admission; unable to speak Dutch, lived outside the region or had been bedridden before their hip fracture; dementia or were cognitively impaired, defined as score of < 7 on the Abbreviated Mental Test, as assessed before inclusion Sex: 108 female, 44 male Age: median 79 years Fracture type: 81 neck of femur, 65 pertrochanteric, 6 subtrochanteric | |
| Interventions | Timing of intervention: within 2‐5 d of surgery for 3 months a) 5 dietetic visits to counsel, 5 phone calls, tailored advice stopped when met requirements with diet. Energy‐ and protein‐enriched diet, and recommendations were given with regard to choice, quantity and timing of food products. In addition, participants were advised to consume two bottles of ONS daily in between main meals. The ONS was a milk‐protein based, or a yogurt‐ or juice‐style supplement (Cubitan, Nutridrink Yoghurt style, or Nutridrink Juice style, N.V. Nutricia, Zoetermeer, the Netherlands) providing 2.1 MJ (500 kcal) and 40 g of protein per 500 ml. The dietitian made arrangements to solve any problems, e.g. feeding difficulties, in collaboration with the hospital medical and nursing staff b) Usual care in hospital, rehabilitation clinic or home. Dietetic care or nutritional supplements only provided on request of doctor Allocated: 73/79 Assessed: 73/79 | |
| Outcomes | Length of follow‐up: 1 year Main outcomes: Mortality Complications Length of acute hospital and rehabilitation hospital stay Functional status Readmissions Level of care Quality of life Adverse effects Other outcomes: Compliance Economic outcomes | |
| Notes | Data up to 1 year on mortality and complications taken from thesis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States "the patient was randomised according to a computer‐generated random‐number sequence list after pre‐stratification for hospital, gender and age (55‐74 years vs. 75 years and above)." |
| Allocation concealment (selection bias) | Low risk | States "The researcher made a telephone call to an independent research assistant who took a sequentially numbered and sealed envelope, and informed the researcher to which group the patient had been allocated." |
| Blinding of participants and personnel (performance bias) | High risk | No placebo group. Comment: likely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Low risk | No placebo group. Comment: unlikely to have been influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | No placebo group. Comment: may have been influenced by lack of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | All participants accounted for, with no imbalance in few dropouts |
| Incomplete outcome data (attrition bias) | Unclear risk | All participants accounted for, with no imbalance in few dropouts |
| Selective reporting (reporting bias) | Low risk | Based on PhD thesis, all prespecified and expected outcomes reported |
| Other bias | High risk | Oral nutritional supplements were provided by Nutricia Advanced Medical Nutrition (Danone Research, Centre for Specialized Nutrition, Wageningen, The Netherlands). Unclear extent of involvement in trial |
BMI: body mass index
mosmol/L: milliosmol/L, a measure of osmolality
NHS: UK National Health Service
nr: no results
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Pilot study for RCT of snacks versus oral nutritional supplements. Trial stopped early as only 4 out of 95 patients were eligible for recruitment. No relevant outcomes | |
| RCT of total hip arthroplasty versus osteosynthesis for hip fracture, but not of nutritional supplementation. The second half of each surgical treatment group received nutritional supplementation; thus, the supplementation and control groups were also not concurrent. | |
| Study of protein and energy supplementation after hip fracture. Not a RCT: non concurrent study groups | |
| Prospective, controlled before and after study of new model of nutritional care promoting nutrition as a medicine, multidisciplinary nutritional care, food service enhancements and improved nutrition knowledge and awareness. Not a RCT | |
| RCT. Comparison between 880 mg calcium and 80 mg calcium with 5 mg of anabolic steroid stanozolol. Not both nutrition interventions, and required outcomes not evaluated | |
| Short‐term study on the effect of 250 kcal supplement on the appetite of people with hip or pelvic fracture. Unclear if RCT. No relevant outcomes | |
| Not a RCT: nursing education programme targeting specific problems including nutritional deficits | |
| The 194 ambulatory elderly participants in the trial were unlikely to include people with hip fracture. No response from study author | |
| Randomised trial of oral nutritional supplementation for older women after fracture (hip, pelvis, humerus, femoral shaft). Personal communication from Ian Cameron on 26th November 2014 stated that data for participants with hip fracture are not available | |
| RCT of protein‐rich liquid supplement versus supplement with nandrolone decanoate injections. Not in scope of review | |
| Unable to contact study author. Contacted project supervisor, thesis no longer available | |
| RCT of 250 ml oral supplement providing 20 g protein, 254 kcal, minerals and vitamins. Study reports only effects of supplement on intake of intervention group, compared with control group. No other outcomes provided. French paper ‐ checked by French translator | |
| Not a RCT. Italian paper ‐ checked by Italian translator | |
| Not people with hip fracture | |
| Not people with hip fracture nor a RCT | |
| Quasi‐experimental, pre‐ and post‐test comparison group design without random group assignment of 100 people with hip fractures to nutritional supplements according to nutritional guidelines plus usual care compared with usual care only. Not a RCT | |
| Comparison of 0.25 mcg 1‐alpha‐hydroxyvitamin D3, 100 IU calcitonin and placebo in women after femoral neck fracture. No outcomes of interest reported and probably not a RCT | |
| RCT, involving 150 women after hip fracture, comparing single injection of 300,000 IU vitamin D2, injected vitamin D2 and 1000 mg/d oral calcium, 800 IU/d oral vitamin D3 and 1000 mg/d calcium, or no treatment. Secondary prevention trial | |
| RCT, involving 63 women after hip fracture, comparing nandrolone decanoate (25 mg intramuscularly every 3 weeks), 0.25 mcg 1‐alpha‐hydroxyvitamin D3 daily and 500 mg calcium daily versus 500 mg calcium daily. Thus this evaluated anabolic steroid and vitamin D together. | |
| RCT of daily 1200 mg calcium as calcium carbonate and 1400 IU vitamin D3 versus 200 IU vitamin D3 in people with low‐energy upper and lower limb fractures. No separate data available for the participants with hip fracture | |
| Comparative study of usual nutritional care versus multidisciplinary care for hip fracture; not a RCT | |
| Non‐randomised comparison of standard plan to improve nutritional intake versus usual care for hip fracture | |
| Quasi‐experimental before and after study of best practices for people with hip fracture, with nutritional drink as one component of the intervention (clinical pathway) | |
| Non‐randomised comparison of bran supplements and nursing intervention versus usual nursing care in postoperative orthopaedic patients, mean age 69 years. Unclear if any participant had a hip fracture | |
| Comparative study, not explicitly randomised. Mixed group of hip fracture and femoral shaft fracture participants aged 17‐67 years; thus majority of hip fracture participants were not over 65 years. Russian paper ‐ checked by Russian translator | |
| Randomised trial of older people, of whom 89 had fractures, newly admitted to long‐term medical care. No response from lead author to requests for separate results for participants with hip fracture | |
| RCT of protein and energy supplementation in nursing homes; not specifically directed at people after hip fracture | |
| Not a RCT. Mixed group of orthopaedic patients | |
| RCT of interdisciplinary intervention (geriatric assessment/consultation, discharge planning and rehabilitation in hospital and up to 3 months post discharge, with nutrition only part of the intervention) versus usual care for hip fracture | |
| No usable results published in conference abstract reporting trial of oral supplements for 25 people with hip fracture. No response from authors | |
| No response from author. No information in the two conference abstracts reports of the trial of how many people with hip fracture were included, nor their results | |
| Randomised trial of a multidisciplinary intervention programme for people after hip fracture. The nutritional intervention was only one component of the complex intervention | |
| Intervention and control groups were not concurrent, nor randomised. The trial investigated the effects of active involvement of orthopaedic patients in their own dietary care; thus the intervention was not direct nutritional supplementation but rather a means of enhancing update by patients. Mixed patient population with hip fracture, or undergoing knee or hip arthroplasty | |
| Two hip fracture patients only. Unlikely to be a RCT | |
| Comparative study; not explicitly randomised. Mixed group of hip fracture and femoral shaft fracture participants aged 17‐65 years; thus majority of hip fracture participants were not over 65 years. Russian paper ‐ checked by Russian translator | |
| RCT testing the addition of pear juice or high fibre supplement to normal diet versus normal diet alone in a mixed group of orthopaedic patients admitted for elective surgery or after traumatic fracture. Aimed at the management of constipation and not for improvement of nutritional status; no relevant outcomes | |
| Not RCT. German paper | |
| Quasi‐randomised placebo‐controlled trial of vitamin C: participants recruited with pressure sores, not because of hip fracture, although 9 of the 20 participants had hip fracture | |
| RCT of resistance training and nutrition therapy combined versus attention control for hip fracture. Unable to assess effect of nutrition separately | |
| RCT involving a mixed group of medical, general surgical and orthopaedic patients aged over 75 years. Author indicates that only a few participants had hip fractures | |
| This trial appears to form part of one of three consecutive studies published in the PhD thesis of Driver (Driver LT. Evaluation of supplemental nutrition in elderly orthopaedic patients [PhD thesis]. Surrey (UK): Univ. of Surrey, 1994). All three studies evaluated nutritional supplementation in a combined group of people with hip fracture and elective hip replacement. There were major defects in the randomisation process, as well as numerical discrepancies, which suggest intention‐to‐treat problems. We have been unable to contact Driver to obtain clarification of the status of the three studies, the trial populations and further specific information on the participants with hip fracture. For the purposes of this review, the 3 studies have been represented as 1 trial. | |
| RCT of dietetic counselling versus usual care in a mixed patient group with osteoporotic fractures (forearm, vertebral, hip). Limited outcomes only (energy, protein and calcium intake, weight and BMI) | |
| RCT. Mixed group of people with elective hip replacement and hip fracture. Some participants were excluded from the analysis. Limited outcomes only (haemoglobin and reticulocyte count) |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Unclear if RCT |
| Participants | People with hip fracture |
| Interventions | (a) Oral nutritional supplements enriched with arginine and micronutrients plus standard hospital diet (b) Standard hospital diet |
| Outcomes | Follow‐up: at least 15 d after surgery Outcomes: pressure ulcers, wound infections |
| Notes | Letter to Dr Benati requesting further details sent 7 October 2014 |
| Methods | Multicentre, randomised placebo‐controlled trial |
| Participants | 303 participants aged 65 years or more with osteoporotic hip fracture requiring surgical repair; haemoglobin 90‐120 g/L |
| Interventions | (a) 40,000 IU erythropoietin and ferric carboxymaltose 1000 mg as 20‐min infusion (b) Erythropoietin placebo and ferric carboxymaltose 1000 mg as 20‐min infusion (c) Erythropoietin placebo and ferric carboxymaltose placebo as 20‐min infusion |
| Outcomes | Follow‐up: 60 d after hospital discharge Outcomes: mortality, adverse events, quality of life |
| Notes | Email 22 September 2014 related to status of trial publication. Now published |
| Methods | RCT |
| Participants | 75 participants with lower extremity fracture |
| Interventions | (a) 3 g calcium β‐hydroxy‐β‐methylbutyrate, 1000 IU vitamin D and 36 g protein supplementation and standard postoperative nutrition |
| Outcomes | Follow‐up: 30 d Oucomes: muscle strength, mobilisation time, wound healing, hospitalisations |
| Notes |
| Methods | Controlled trial: "randomly divided" |
| Participants | 46 women with hip fracture, mean age 83 years |
| Interventions | (a) Nutritional therapeutic regime (protocols, protein enriched food, oral and/or parenteral supplementation) (b) Usual care |
| Outcomes | Nutritional biochemistry |
| Notes | Email to Dr Elmadfa on 3 October 2008 asking for further details, and Dr Elmadfa ([email protected]) and Dr Fabian ([email protected]) on 3 November 2016 for further details |
| Methods | Randomised three‐arm trial |
| Participants | 48 women who had surgery for hip fracture |
| Interventions | (a) Vitamin D3 1,500 IU/day (b) Vitamin D3 10,500 IU weekly (c) Vitamin D3 45,000 IU every 28 d |
| Outcomes | Follow‐up: 56 d Outcomes: Hypercalcaemia |
| Notes | Emailed Sophia Ish‐Shalom (s‐ish‐[email protected]) 21 November 2014 requesting details of outcomes relevant to this review |
| Methods | RCT |
| Participants | 50 men and women with fractured neck of femur, at risk of malnutrition |
| Interventions | (a) Liquid multinutrient oral nutritional support |
| Outcomes | Follow‐up: at least 7 d Compliance, patient satisfaction |
| Notes | Emailed [email protected] on 5 September 2014 asking for further details |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Does a high dose fish oil intervention improve outcomes in older adults recovering from hip fracture? |
| Methods | Randomised controlled double‐blind trial |
| Participants | 150 men and women, aged 65 years or over, within 7 d of surgical fixation of femoral fracture, history of recent unexplained weight loss and at risk of further weight loss and current poor appetite, elevated C reactive protein (6 mg/L or more), serum albumin < 35 g/L, raised energy expenditure |
| Interventions | (a) 15 ml/day liquid fish oil orally (4.9 g eicosapentaenoic acid and 3.4 g docosahexaenoic acid) and individualised nutrition therapy |
| Outcomes | Follow‐up: 6, 12 weeks and 12 months |
| Starting date | February 2010 |
| Contact information | Dr Michelle Miller |
| Notes | Emailed [email protected] 5 September 2014 to enquire status of trial. Replied 7 September 2014 that trial completed and results being analysed |
| Trial name or title | REVITAHIP |
| Methods | Multicentre, randomised, controlled, double‐blind trial |
| Participants | 340 men and women aged 65 y or over with hip fracture requiring surgery |
| Interventions | a) 250,000 IU vitamin D3 (5 tablets of 50,000 IU) within 7 d postsurgery b) 5 placebo tablets Followed by daily calcium (500 mg) and vitamin D (800 IU) for 6 months for both groups |
| Outcomes | Follow‐up: 2, 4, 12 and 24 weeks Outcomes: functional status e.g. gait velocity, falls, fractures, quality of life, hospitalisation, morbidity, mortality |
| Starting date | 2010 |
| Contact information | Jenson Mak: [email protected] |
| Notes | Trial completed, results being written up for publication |
| Trial name or title | Does intravenous iron therapy reduce the need for blood transfusion and improve post operative blood count following surgery for broken neck of femur? |
| Methods | Randomised placebo controlled trial |
| Participants | 270 participants with planned surgical fixation of fractured neck of femur |
| Interventions | (a) Single 50 ml infusion of 1000 mg iron polymaltose over 20 min for participants < 70 kg, or 1500 mg for heavier participants (b) Saline placebo |
| Outcomes | Length of stay, mortality |
| Starting date | 1 July 2012 |
| Contact information | Matt Harper Fremantle Hospital PO Box 480 WA 4160 Australia |
| Notes |
| Trial name or title | The effect of taurine on morbidity and mortality in the elderly hip fracture patient |
| Methods | Randomised controlled double‐blind trial |
| Participants | Aged over 75 years, surgery for hip fracture, both genders, number recruited unclear |
| Interventions | (a) 3 g taurine/day or 6 g taurine/day |
| Outcomes | Follow‐up: 1 year |
| Starting date | July 2007, expected completion July 2010 |
| Contact information | Dr Alexander PJ Houdijk |
| Notes | Emailed [email protected] 5 September 2014 to enquire about status of trial. Reply received 18 September 2014 indicating that manuscript in preparation and results not yet available |
| Trial name or title | HIPERPROT‐GER study |
| Methods | Single centre, RCT |
| Participants | 100 participants aged 65 years and over after surgery for hip fracture starting rehabilitation |
| Interventions | (a) 2 bottles Ensure Plus Advance per day for 30 d in hospital (enriched with β‐hydroxy‐β‐methylbutyrate, vitamin D3 and calcium) (b) Usual care |
| Outcomes | Follow‐up: 1 year Outcomes: functional status, mortality |
| Starting date | 2012 |
| Contact information | |
| Notes | Emailed Dr Malafarina 22 September 2014 enquiring about progress with study, replied 25 September 2014 indicating that recruitment continuing |
| Trial name or title | Hip fracture surgery and oral nutritional supplements (HIATUS) |
| Methods | RCT |
| Participants | 24 participants 70 years and over after acute hip fracture and surgical treatment |
| Interventions | (a) Oral nutritional supplement (b) Placebo |
| Outcomes | Short Physical Performance Battery, quality of life |
| Starting date | January 2012 |
| Contact information | Heike Bischoff‐Ferrari University of Zurich Department of Rheumatology and Institute of Physical Medicine Zurich Switzerland 8091 |
| Notes | Sponsored by Nestlé |
| Trial name or title | The effect of intravenous iron on postoperative transfusion requirements in hip fracture patients |
| Methods | Single‐centre RCT |
| Participants | 80 men and women undergoing surgical repair of fractured neck of femur, aged 70 years or more |
| Interventions | (a) 200 mg iron sucrose within 24 h or admission, repeated day 1 after operation and day 2 (b) Usual care |
| Outcomes | Follow‐up: Outcomes: mortality, postoperative infections, cardiovascular complications, length of acute hospital stay, functional status, costs |
| Starting date | June 2012 |
| Contact information | |
| Notes | Emailed Iain Moppett 25 September 2014 to enquire about status of trial, replied 17 November 2014 indicating that trial still in progress |
ADL: activities of daily living
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality by end of study Show forest plot | 20 | 1385 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.79 [0.55, 1.15] |
| Analysis 1.1 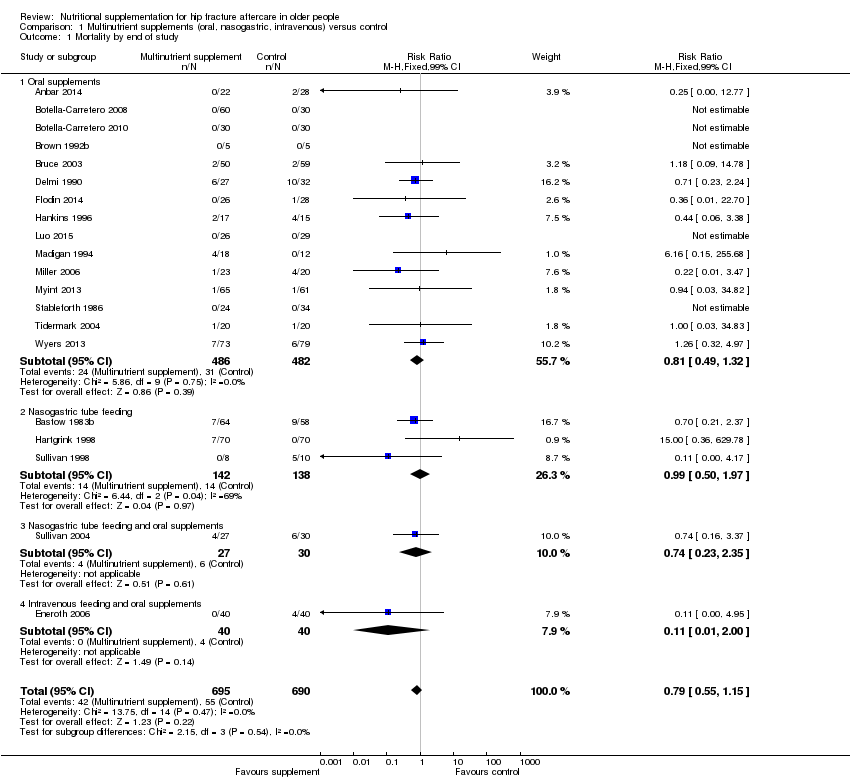 Comparison 1 Multinutrient supplements (oral, nasogastric, intravenous) versus control, Outcome 1 Mortality by end of study. | ||||
| 1.1 Oral supplements | 15 | 968 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.81 [0.49, 1.32] |
| 1.2 Nasogastric tube feeding | 3 | 280 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.99 [0.50, 1.97] |
| 1.3 Nasogastric tube feeding and oral supplements | 1 | 57 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.74 [0.23, 2.35] |
| 1.4 Intravenous feeding and oral supplements | 1 | 80 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.11 [0.01, 2.00] |
| 2 Participants with complications at end of study Show forest plot | 14 | 882 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.69 [0.59, 0.81] |
| Analysis 1.2  Comparison 1 Multinutrient supplements (oral, nasogastric, intravenous) versus control, Outcome 2 Participants with complications at end of study. | ||||
| 2.1 Oral supplements | 11 | 727 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.71 [0.59, 0.86] |
| 2.2 Nasogastric tube feeding | 1 | 18 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.09 [0.73, 1.64] |
| 2.3 Nasogastric tube feeding and oral supplements | 1 | 57 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.11 [0.75, 1.65] |
| 2.4 Intravenous feeding and oral supplements | 1 | 80 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.21 [0.10, 0.46] |
| 3 Participants with complications at end of study: random‐effects model Show forest plot | 14 | 882 | Risk Ratio (M‐H, Random, 99% CI) | 0.70 [0.53, 0.91] |
| Analysis 1.3  Comparison 1 Multinutrient supplements (oral, nasogastric, intravenous) versus control, Outcome 3 Participants with complications at end of study: random‐effects model. | ||||
| 3.1 Oral supplements | 11 | 727 | Risk Ratio (M‐H, Random, 99% CI) | 0.72 [0.58, 0.89] |
| 3.2 Nasogastric tube feeding | 1 | 18 | Risk Ratio (M‐H, Random, 99% CI) | 1.09 [0.73, 1.64] |
| 3.3 Nasogastric tube feeding and oral supplements | 1 | 57 | Risk Ratio (M‐H, Random, 99% CI) | 1.11 [0.75, 1.65] |
| 3.4 Intravenous feeding and oral supplements | 1 | 80 | Risk Ratio (M‐H, Random, 99% CI) | 0.21 [0.10, 0.46] |
| 4 Unfavourable outcome (death or complications) at end of study Show forest plot | 6 | 334 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.67 [0.51, 0.89] |
| Analysis 1.4  Comparison 1 Multinutrient supplements (oral, nasogastric, intravenous) versus control, Outcome 4 Unfavourable outcome (death or complications) at end of study. | ||||
| 4.1 Oral supplements | 6 | 334 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.67 [0.51, 0.89] |
| 4.2 Nasogastric tube feeding | 0 | 0 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] |
| 4.3 Nasogastric tube feeding and oral supplements | 0 | 0 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] |
| 4.4 Intravenous feeding and oral supplements | 0 | 0 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] |
| 5 Unfavourable outcome (death or complications) ‐ oral supplements extra analyses Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Multinutrient supplements (oral, nasogastric, intravenous) versus control, Outcome 5 Unfavourable outcome (death or complications) ‐ oral supplements extra analyses. | ||||
| 5.1 Oral supplements: worst case scenario | 6 | 353 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.81 [0.62, 1.04] |
| 5.2 Oral supplements: Hankins 1996 acute hospital data | 1 | 31 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.96 [0.71, 1.31] |
| 5.3 Oral supplements: Hankins 1996 post discharge | 1 | 31 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.10 [0.50, 2.41] |
| 6 Adverse effects (putatively related to treatment) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Multinutrient supplements (oral, nasogastric, intravenous) versus control, Outcome 6 Adverse effects (putatively related to treatment). | ||||
| 6.1 Oral supplements (mainly diarrhoea or/and vomiting) | 6 | 442 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.99 [0.47, 2.05] |
| 6.2 Nasogatric tube feeding | 1 | 18 | Risk Ratio (M‐H, Fixed, 99% CI) | 8.56 [0.51, 144.86] |
| 6.3 Intravenous feeding and oral supplements | 1 | 57 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.85 [0.49, 7.03] |
| 6.4 Nasogastric tube feeding and oral supplements | 0 | 0 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality by end of study Show forest plot | 20 | 1385 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.79 [0.55, 1.15] |
| Analysis 2.1  Comparison 2 Multinutrient supplements (oral, nasogastric routes, intravenous) versus control (split by nutritional status), Outcome 1 Mortality by end of study. | ||||
| 1.1 Malnourished targeted | 6 | 388 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.55 [0.27, 1.11] |
| 1.2 Malnourished not targeted | 14 | 997 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.92 [0.59, 1.42] |
| 2 Mortality by end of study ‐ oral supplements only Show forest plot | 15 | 968 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.81 [0.49, 1.32] |
| Analysis 2.2 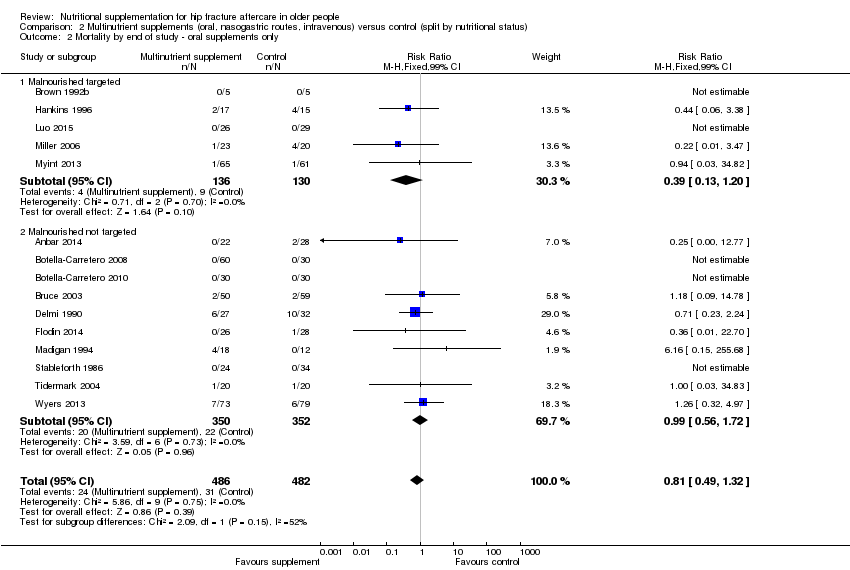 Comparison 2 Multinutrient supplements (oral, nasogastric routes, intravenous) versus control (split by nutritional status), Outcome 2 Mortality by end of study ‐ oral supplements only. | ||||
| 2.1 Malnourished targeted | 5 | 266 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.39 [0.13, 1.20] |
| 2.2 Malnourished not targeted | 10 | 702 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.99 [0.56, 1.72] |
| 3 Participants with complications at end of study Show forest plot | 14 | 882 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.69 [0.59, 0.81] |
| Analysis 2.3 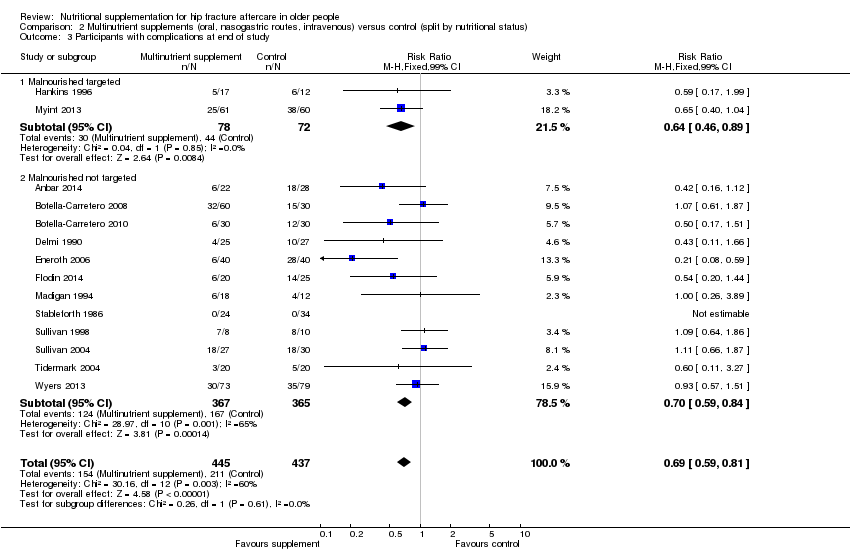 Comparison 2 Multinutrient supplements (oral, nasogastric routes, intravenous) versus control (split by nutritional status), Outcome 3 Participants with complications at end of study. | ||||
| 3.1 Malnourished targeted | 2 | 150 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.64 [0.46, 0.89] |
| 3.2 Malnourished not targeted | 12 | 732 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.70 [0.59, 0.84] |
| 4 Unfavourable outcome (death or complications) at end of study Show forest plot | 6 | 334 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.67 [0.51, 0.89] |
| Analysis 2.4 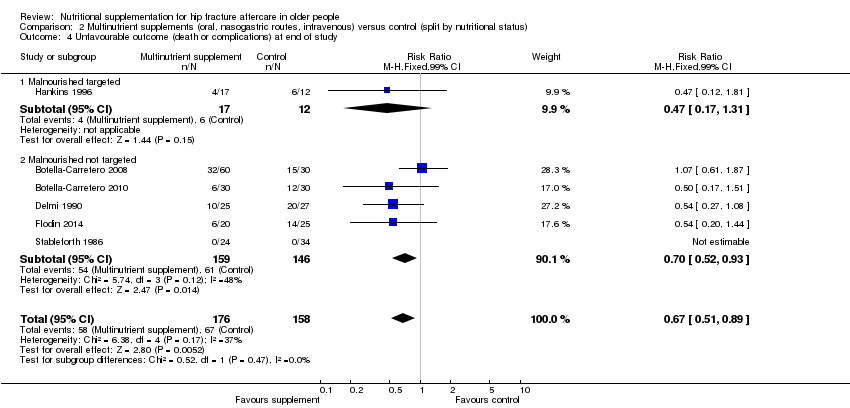 Comparison 2 Multinutrient supplements (oral, nasogastric routes, intravenous) versus control (split by nutritional status), Outcome 4 Unfavourable outcome (death or complications) at end of study. | ||||
| 4.1 Malnourished targeted | 1 | 29 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.47 [0.17, 1.31] |
| 4.2 Malnourished not targeted | 5 | 305 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.70 [0.52, 0.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality by end of study by risk of bias for allocation concealment Show forest plot | 20 | 1385 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.79 [0.55, 1.15] |
| Analysis 3.1 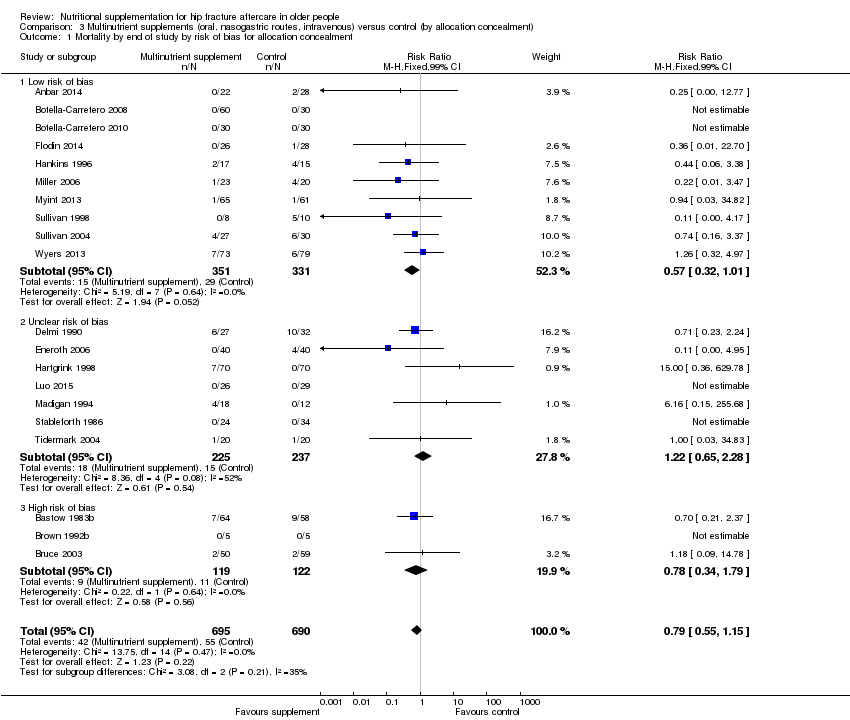 Comparison 3 Multinutrient supplements (oral, nasogastric routes, intravenous) versus control (by allocation concealment), Outcome 1 Mortality by end of study by risk of bias for allocation concealment. | ||||
| 1.1 Low risk of bias | 10 | 682 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.57 [0.32, 1.01] |
| 1.2 Unclear risk of bias | 7 | 462 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.22 [0.65, 2.28] |
| 1.3 High risk of bias | 3 | 241 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.78 [0.34, 1.79] |
| 2 Participants with complications at end of study by risk of bias for allocation concealment Show forest plot | 14 | 882 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.69 [0.59, 0.81] |
| Analysis 3.2  Comparison 3 Multinutrient supplements (oral, nasogastric routes, intravenous) versus control (by allocation concealment), Outcome 2 Participants with complications at end of study by risk of bias for allocation concealment. | ||||
| 2.1 Low risk of bias | 9 | 622 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.78 [0.66, 0.92] |
| 2.2 Unclear risk of bias | 5 | 260 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.38 [0.24, 0.61] |
| 2.3 High risk of bias | 0 | 0 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality by end of study Show forest plot | 4 | 361 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.42 [0.85, 2.37] |
| Analysis 4.1 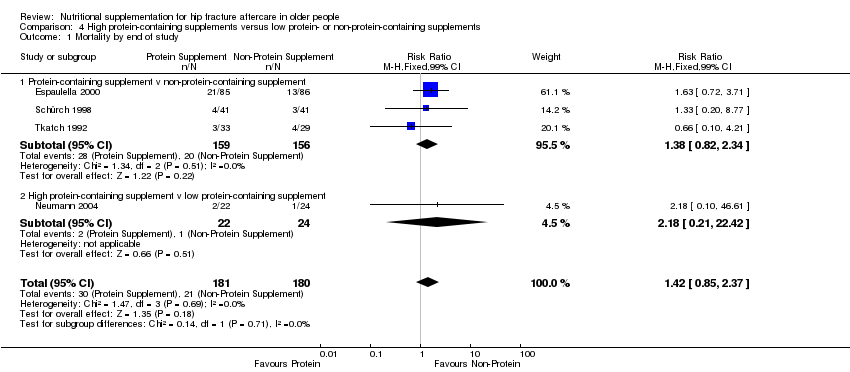 Comparison 4 High protein‐containing supplements versus low protein‐ or non‐protein‐containing supplements, Outcome 1 Mortality by end of study. | ||||
| 1.1 Protein‐containing supplement v non‐protein‐containing supplement | 3 | 315 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.38 [0.82, 2.34] |
| 1.2 High protein‐containing supplement v low protein‐containing supplement | 1 | 46 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.18 [0.21, 22.42] |
| 2 Unfavourable outcome (death or complications) at end of study Show forest plot | 2 | 223 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.78 [0.65, 0.95] |
| Analysis 4.2  Comparison 4 High protein‐containing supplements versus low protein‐ or non‐protein‐containing supplements, Outcome 2 Unfavourable outcome (death or complications) at end of study. | ||||
| 2.1 Protein‐containing supplement v non‐protein‐containing supplement | 2 | 223 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.78 [0.65, 0.95] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality by end of study Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Totals not selected | |
| Analysis 5.1 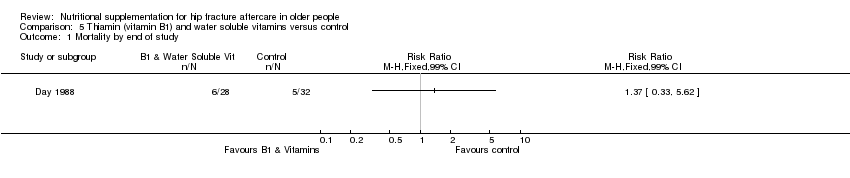 Comparison 5 Thiamin (vitamin B1) and water soluble vitamins versus control, Outcome 1 Mortality by end of study. | ||||
| 2 Participants with complications at end of study Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Totals not selected | |
| Analysis 5.2 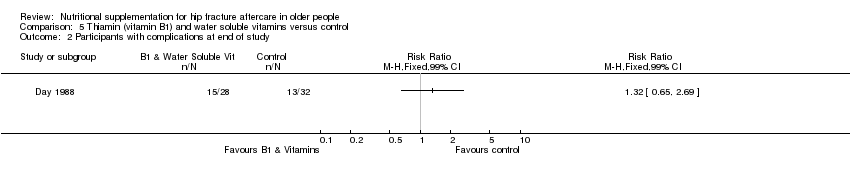 Comparison 5 Thiamin (vitamin B1) and water soluble vitamins versus control, Outcome 2 Participants with complications at end of study. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with complications at end of study Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Totals not selected | |
| Analysis 6.1 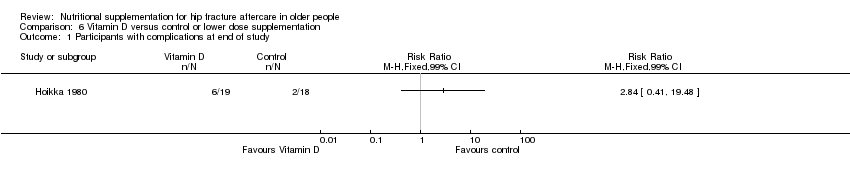 Comparison 6 Vitamin D versus control or lower dose supplementation, Outcome 1 Participants with complications at end of study. | ||||
| 2 Mortality by end of study Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.2 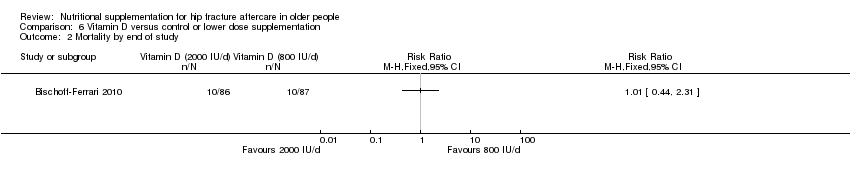 Comparison 6 Vitamin D versus control or lower dose supplementation, Outcome 2 Mortality by end of study. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality by end of study Show forest plot | 3 | 566 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.98 [0.65, 1.46] |
| Analysis 7.1 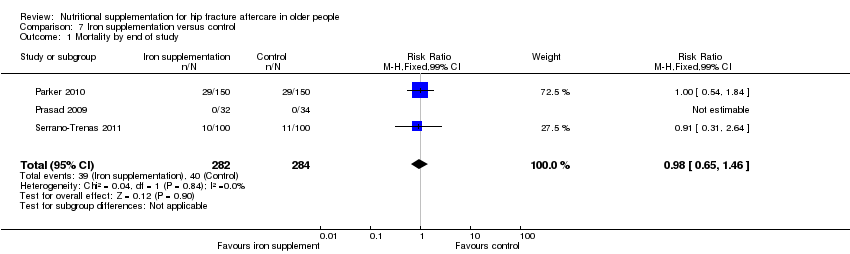 Comparison 7 Iron supplementation versus control, Outcome 1 Mortality by end of study. | ||||
| 2 Participants with complications at end of study Show forest plot | 2 | 266 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.23 [0.63, 2.42] |
| Analysis 7.2  Comparison 7 Iron supplementation versus control, Outcome 2 Participants with complications at end of study. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality by end of study Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Totals not selected | |
| Analysis 8.1  Comparison 8 Taurine versus placebo, Outcome 1 Mortality by end of study. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality by end of study Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Totals not selected | |
| Analysis 9.1  Comparison 9 Dietetic assistants versus usual care, Outcome 1 Mortality by end of study. | ||||
| 2 Participants with complications at end of study Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Totals not selected | |
| Analysis 9.2  Comparison 9 Dietetic assistants versus usual care, Outcome 2 Participants with complications at end of study. | ||||
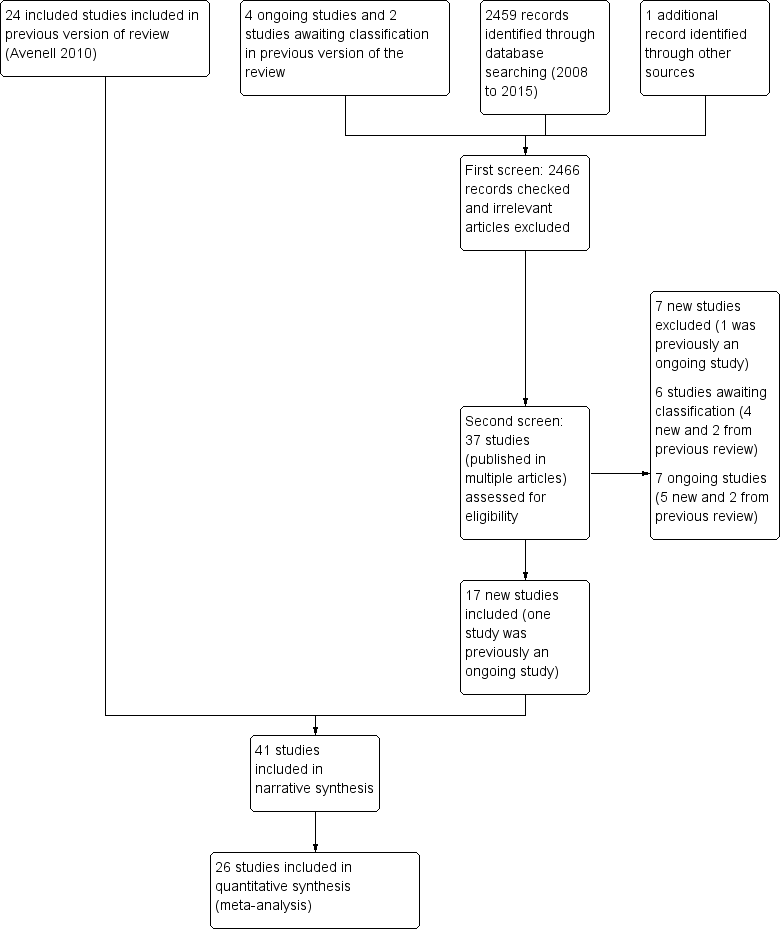
Study flow diagram
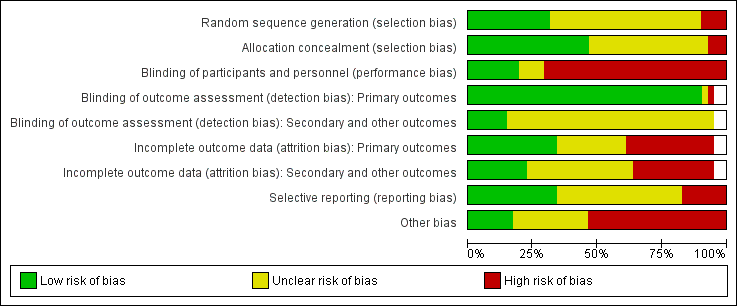
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
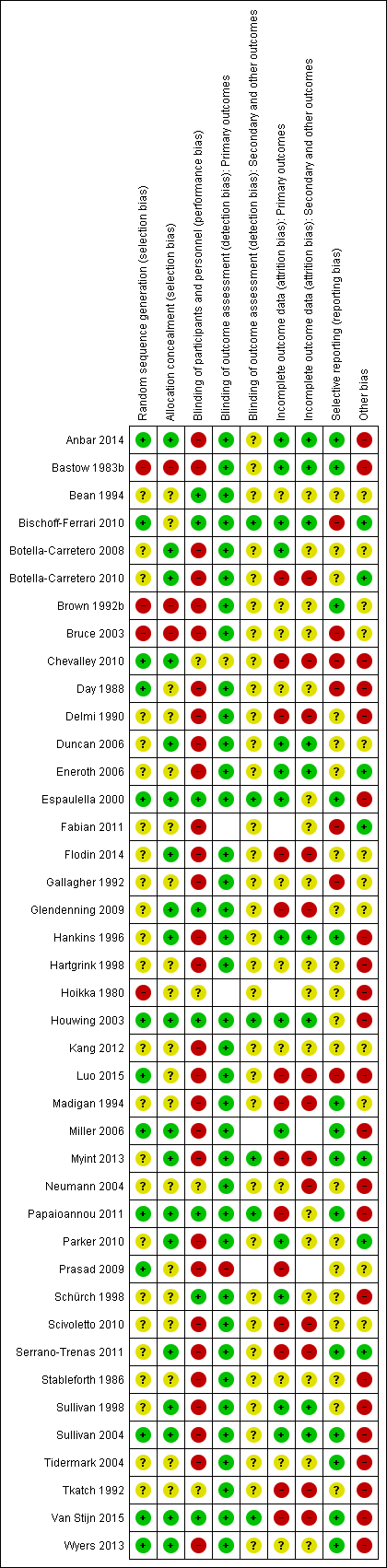
Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Forest plot of comparison: 1 Multinutrient supplements (oral, nasogastric, intravenous) versus control, outcome: 1.1 Mortality by end of study
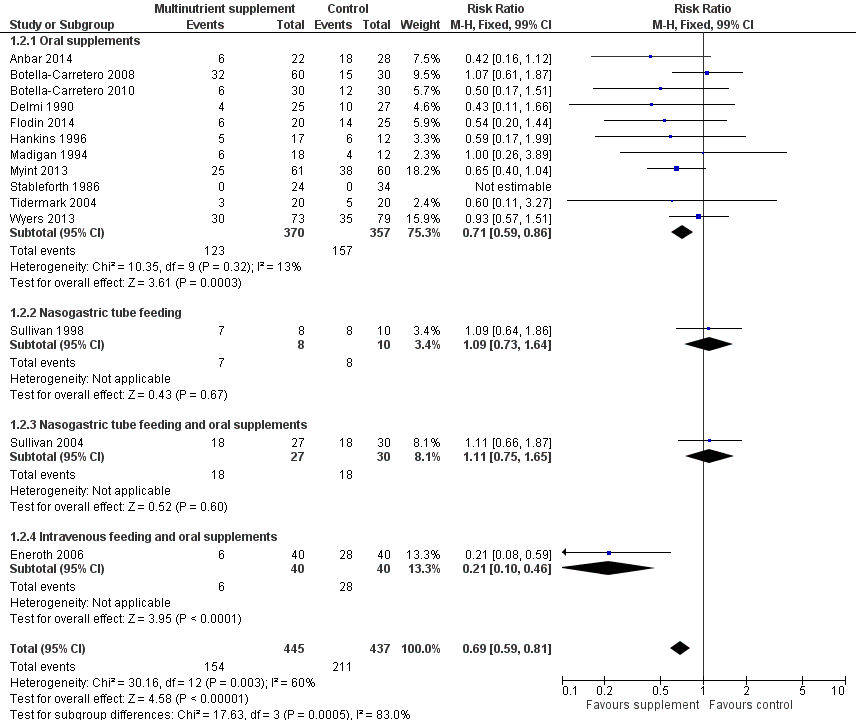
Forest plot of comparison: 1 Multinutrient supplements (oral, nasogastric, intravenous) versus control, outcome: 1.2 Participants with complications at end of study

Funnel plot of comparison: 1 Multinutrient supplements (oral, nasogastric, intravenous) versus control, outcome: 1.1 Mortality by end of study

Funnel plot of comparison: 1 Multinutrient supplements (oral, nasogastric, intravenous) versus control, outcome: 1.2 Participants with complications at end of study

Comparison 1 Multinutrient supplements (oral, nasogastric, intravenous) versus control, Outcome 1 Mortality by end of study.

Comparison 1 Multinutrient supplements (oral, nasogastric, intravenous) versus control, Outcome 2 Participants with complications at end of study.

Comparison 1 Multinutrient supplements (oral, nasogastric, intravenous) versus control, Outcome 3 Participants with complications at end of study: random‐effects model.

Comparison 1 Multinutrient supplements (oral, nasogastric, intravenous) versus control, Outcome 4 Unfavourable outcome (death or complications) at end of study.

Comparison 1 Multinutrient supplements (oral, nasogastric, intravenous) versus control, Outcome 5 Unfavourable outcome (death or complications) ‐ oral supplements extra analyses.

Comparison 1 Multinutrient supplements (oral, nasogastric, intravenous) versus control, Outcome 6 Adverse effects (putatively related to treatment).

Comparison 2 Multinutrient supplements (oral, nasogastric routes, intravenous) versus control (split by nutritional status), Outcome 1 Mortality by end of study.

Comparison 2 Multinutrient supplements (oral, nasogastric routes, intravenous) versus control (split by nutritional status), Outcome 2 Mortality by end of study ‐ oral supplements only.

Comparison 2 Multinutrient supplements (oral, nasogastric routes, intravenous) versus control (split by nutritional status), Outcome 3 Participants with complications at end of study.

Comparison 2 Multinutrient supplements (oral, nasogastric routes, intravenous) versus control (split by nutritional status), Outcome 4 Unfavourable outcome (death or complications) at end of study.
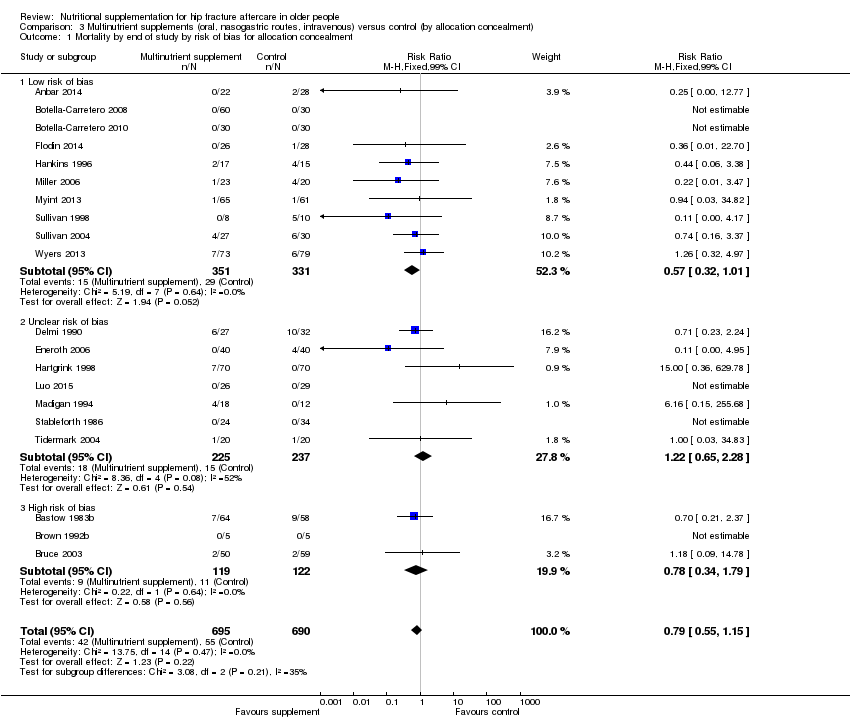
Comparison 3 Multinutrient supplements (oral, nasogastric routes, intravenous) versus control (by allocation concealment), Outcome 1 Mortality by end of study by risk of bias for allocation concealment.

Comparison 3 Multinutrient supplements (oral, nasogastric routes, intravenous) versus control (by allocation concealment), Outcome 2 Participants with complications at end of study by risk of bias for allocation concealment.

Comparison 4 High protein‐containing supplements versus low protein‐ or non‐protein‐containing supplements, Outcome 1 Mortality by end of study.

Comparison 4 High protein‐containing supplements versus low protein‐ or non‐protein‐containing supplements, Outcome 2 Unfavourable outcome (death or complications) at end of study.
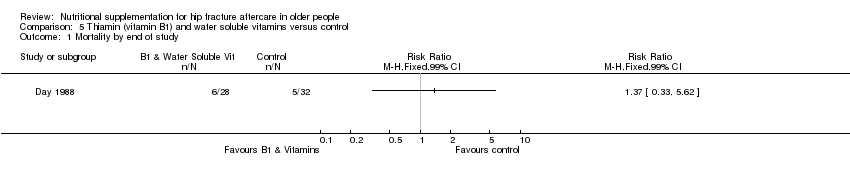
Comparison 5 Thiamin (vitamin B1) and water soluble vitamins versus control, Outcome 1 Mortality by end of study.

Comparison 5 Thiamin (vitamin B1) and water soluble vitamins versus control, Outcome 2 Participants with complications at end of study.

Comparison 6 Vitamin D versus control or lower dose supplementation, Outcome 1 Participants with complications at end of study.
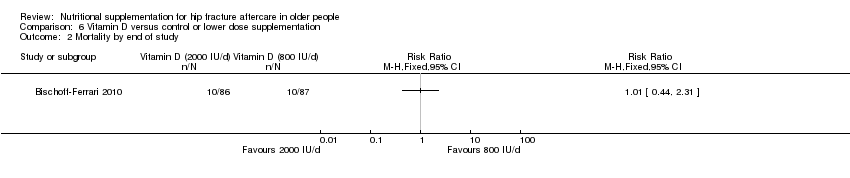
Comparison 6 Vitamin D versus control or lower dose supplementation, Outcome 2 Mortality by end of study.
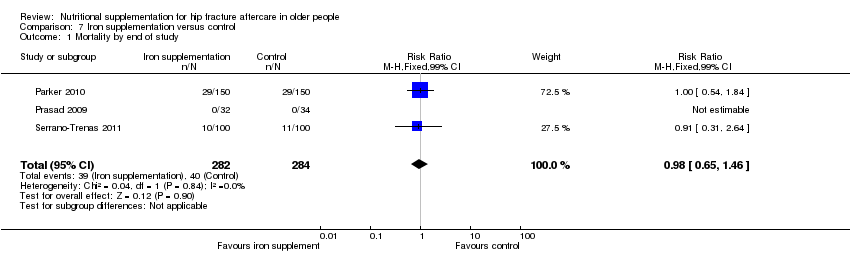
Comparison 7 Iron supplementation versus control, Outcome 1 Mortality by end of study.
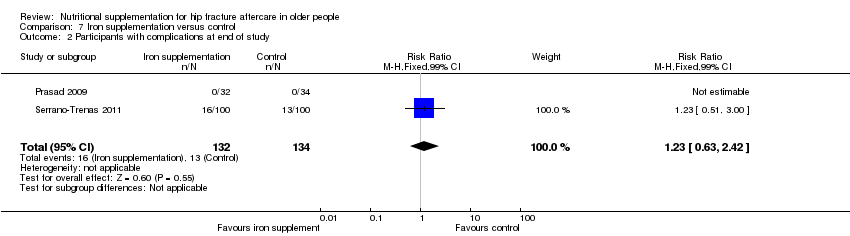
Comparison 7 Iron supplementation versus control, Outcome 2 Participants with complications at end of study.

Comparison 8 Taurine versus placebo, Outcome 1 Mortality by end of study.

Comparison 9 Dietetic assistants versus usual care, Outcome 1 Mortality by end of study.

Comparison 9 Dietetic assistants versus usual care, Outcome 2 Participants with complications at end of study.
| Multinutrient supplements (oral) versus control for hip fracture aftercare in older people | ||||||
| Patient or population: Older people undergoing hip fracture aftercare Comparison: Standard postoperative nutritional support and care in control groups | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Multinutrient supplements (oral) versus control | |||||
| Mortality by end of study Follow‐up: 1‐12 months | Study population | RR 0.81 | 968 | ⊕⊕⊝⊝ | The statistical test for subgroup differences between the results for the 5 trials targeting malnourished participants and those 10 trials not targeting malnourished participants did not confirm a difference between the two subgroups for mortality | |
| 72 per 10001 | 59 per 1000 | |||||
| High risk2 | ||||||
| 250 per 1000 | 203 per 1000 | |||||
| Participants with complications (e.g. pressure sore, chest infection) at end of study | Study population | RR 0.71 | 727 | ⊕⊕⊝⊝ | Only 2 trials targeting malnourished people reported these data | |
| 443 per 10004 | 315 per 1000 | |||||
| Moderate risk5 | ||||||
| 290 per 1000 | 206 per 1000 | |||||
| Unfavourable outcome 7 by end of study Follow‐up: 1‐12 months | Study population | RR 0.67 (0.51 to 0.89) | 334 (6 studies) | ⊕⊝⊝⊝ | Only 1 trial targeting malnourished people reported these data | |
| 500 per 10004 | 335 per 1000 (255 to 445) | |||||
| Putative side effects of treatment (e.g. vomiting and diarrhoea) Follow‐up: during supplementation period | Study population | RR 0.99 (0.47 to 2.05) | 442 (6 studies) | ⊕⊝⊝⊝ | Three of the 6 trials reported no adverse effects | |
| 50 per 10004 | 50 per 1000 (24 to 103) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. The control group risk is the median control group risk across the 9 studies that reported one or more deaths in the control group. | ||||||
| Multinutrient supplements (nasogastric) versus control for hip fracture aftercare in older people7 | ||||||
| Patient or population: Older people undergoing hip fracture aftercare Comparison: Standard postoperative nutritional support and care in control groups | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Multinutrient supplements (nasogastric) versus control | |||||
| Mortality by end of study | Study Population | RR: 0.99 (0.50 to 1.97) | 280 (3 studies) | ⊕⊝⊝⊝ | Only 1 trial targeting malnourished participants reported these data | |
| 156 per 10002 | 155 per 1000 (78 to 308) | |||||
| Participants with complications (e.g. pressure sore, aspiration pneumonia) at end of study | Study Population | RR: 1.09 (0.73 to 1.64) | 18 (1 study) | ⊕⊝⊝⊝ | For consistency we have presented 95% CI here but have used 99% CI for single trial data in the main text: 99% CI 0.64 to 1.86.6 | |
| 800 per 10004 | 872 per 1000 (584 to 1000) | |||||
| Unfavourable outcome Follow‐up: 1‐12 months | See comment | See comment | Outcome not reported | |||
| Putative side effects of treatment (e.g. aspiration pneumonia) Follow‐up: during supplementation period | See comment | See comment | Insufficient data to draw any conclusions. However, poor toleration of tube feeding was noted.1 There was no report of aspiration pneumonia (1 study; 140 participants). One study reported 18 (28% of 64) participants in the intervention group developed diarrhoea ‐ this was ascribed to antibiotics in 16 ‐ but did not report on the control group. One study (18 participants) reported 3 cases of "bloating" in the intervention group; it found no feed‐induced diarrhoea | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence the estimate. | ||||||
| 1. Nasogatric feeding was poorly tolerated but varied between studies. One study reported only 26% of the intervention group tolerated tube feeding for the full two weeks; another reported 78% completed the course (until hospital discharge). | ||||||
| Study ID | Intervention | Control | Mean difference (99% confidence intervaI) | ||||
| Multinutritional oral supplements | |||||||
| 22 | 10.1 | 3.2 | 28 | 12.5 | 5.5 | ‐2.40 days (‐5.60 to 0.80) | |
| 30 | 13.3 | 4.3 | 30 | 12.8 | 4.0 | 0.50 days (‐2.26 to 3.26) | |
| 5 | 27.00 | 10.00 | 5 | 48.00 | 37.00 | ‐21.00 days (‐65.15 to 23.15) | |
| 50 | 17.70 | 9.40 | 58 | 16.60 | 9.20 | 1.10 days (‐3.53 to 5.73) | |
| 18 | 16.00 | 8.00 | 12 | 15.00 | 11.00 | 1.00 day (‐8.51 to 10.51) | |
| 61 | 26.2 | 8.2 | 60 | 29.9 | 11.2 | ‐3.70 days (‐8.30 to 0.90) | |
| Nasogastric tube feeding | |||||||
| 8 | 38.20 | 36.90 | 7 | 23.70 | 20.00 | 14.50 days (‐24.34 to 53.34) | |
| High protein supplements | |||||||
| 85 | 16.40 | 6.60 | 86 | 17.20 | 7.70 | ‐0.80 days (‐3.62 to 2.02) | |
| 18 | 23.20 | 5.52 | 20 | 28.00 | 11.63 | ‐4.80 days (‐12.29 to 2.69) | |
| Iron supplementation versus control | |||||||
| 150 | 18.8 | 17.4 | 150 | 21.3 | 20.6 | ‐2.50 days (‐8.17 to 3.17) | |
| 99 | 13.5 | 7.1 | 97 | 13.1 | 6.9 | 0.40 days (‐2.18 to 2.98) | |
| Vitamin B1 | |||||||
| 28 | 35.00 | 34.00 | 30 | 29.00 | 30.00 | 6.00 days (‐15.75 to 27.75) | |
| Vitamin, mineral and amino acid supplementation versus control | |||||||
| 49 | 15.4 | 6.8 | 47 | 17.9 | 7.3 | ‐2.50 days (‐6.21 to 1.21) | |
| Semi‐essential amino acid | |||||||
| 111 | 13 | 10 | 123 | 13 | 11 | 0.00 days (‐3.54 to 3.54) | |
| SD: standard deviation | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality by end of study Show forest plot | 20 | 1385 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.79 [0.55, 1.15] |
| 1.1 Oral supplements | 15 | 968 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.81 [0.49, 1.32] |
| 1.2 Nasogastric tube feeding | 3 | 280 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.99 [0.50, 1.97] |
| 1.3 Nasogastric tube feeding and oral supplements | 1 | 57 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.74 [0.23, 2.35] |
| 1.4 Intravenous feeding and oral supplements | 1 | 80 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.11 [0.01, 2.00] |
| 2 Participants with complications at end of study Show forest plot | 14 | 882 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.69 [0.59, 0.81] |
| 2.1 Oral supplements | 11 | 727 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.71 [0.59, 0.86] |
| 2.2 Nasogastric tube feeding | 1 | 18 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.09 [0.73, 1.64] |
| 2.3 Nasogastric tube feeding and oral supplements | 1 | 57 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.11 [0.75, 1.65] |
| 2.4 Intravenous feeding and oral supplements | 1 | 80 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.21 [0.10, 0.46] |
| 3 Participants with complications at end of study: random‐effects model Show forest plot | 14 | 882 | Risk Ratio (M‐H, Random, 99% CI) | 0.70 [0.53, 0.91] |
| 3.1 Oral supplements | 11 | 727 | Risk Ratio (M‐H, Random, 99% CI) | 0.72 [0.58, 0.89] |
| 3.2 Nasogastric tube feeding | 1 | 18 | Risk Ratio (M‐H, Random, 99% CI) | 1.09 [0.73, 1.64] |
| 3.3 Nasogastric tube feeding and oral supplements | 1 | 57 | Risk Ratio (M‐H, Random, 99% CI) | 1.11 [0.75, 1.65] |
| 3.4 Intravenous feeding and oral supplements | 1 | 80 | Risk Ratio (M‐H, Random, 99% CI) | 0.21 [0.10, 0.46] |
| 4 Unfavourable outcome (death or complications) at end of study Show forest plot | 6 | 334 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.67 [0.51, 0.89] |
| 4.1 Oral supplements | 6 | 334 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.67 [0.51, 0.89] |
| 4.2 Nasogastric tube feeding | 0 | 0 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] |
| 4.3 Nasogastric tube feeding and oral supplements | 0 | 0 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] |
| 4.4 Intravenous feeding and oral supplements | 0 | 0 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] |
| 5 Unfavourable outcome (death or complications) ‐ oral supplements extra analyses Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 5.1 Oral supplements: worst case scenario | 6 | 353 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.81 [0.62, 1.04] |
| 5.2 Oral supplements: Hankins 1996 acute hospital data | 1 | 31 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.96 [0.71, 1.31] |
| 5.3 Oral supplements: Hankins 1996 post discharge | 1 | 31 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.10 [0.50, 2.41] |
| 6 Adverse effects (putatively related to treatment) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 6.1 Oral supplements (mainly diarrhoea or/and vomiting) | 6 | 442 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.99 [0.47, 2.05] |
| 6.2 Nasogatric tube feeding | 1 | 18 | Risk Ratio (M‐H, Fixed, 99% CI) | 8.56 [0.51, 144.86] |
| 6.3 Intravenous feeding and oral supplements | 1 | 57 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.85 [0.49, 7.03] |
| 6.4 Nasogastric tube feeding and oral supplements | 0 | 0 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality by end of study Show forest plot | 20 | 1385 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.79 [0.55, 1.15] |
| 1.1 Malnourished targeted | 6 | 388 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.55 [0.27, 1.11] |
| 1.2 Malnourished not targeted | 14 | 997 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.92 [0.59, 1.42] |
| 2 Mortality by end of study ‐ oral supplements only Show forest plot | 15 | 968 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.81 [0.49, 1.32] |
| 2.1 Malnourished targeted | 5 | 266 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.39 [0.13, 1.20] |
| 2.2 Malnourished not targeted | 10 | 702 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.99 [0.56, 1.72] |
| 3 Participants with complications at end of study Show forest plot | 14 | 882 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.69 [0.59, 0.81] |
| 3.1 Malnourished targeted | 2 | 150 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.64 [0.46, 0.89] |
| 3.2 Malnourished not targeted | 12 | 732 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.70 [0.59, 0.84] |
| 4 Unfavourable outcome (death or complications) at end of study Show forest plot | 6 | 334 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.67 [0.51, 0.89] |
| 4.1 Malnourished targeted | 1 | 29 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.47 [0.17, 1.31] |
| 4.2 Malnourished not targeted | 5 | 305 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.70 [0.52, 0.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality by end of study by risk of bias for allocation concealment Show forest plot | 20 | 1385 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.79 [0.55, 1.15] |
| 1.1 Low risk of bias | 10 | 682 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.57 [0.32, 1.01] |
| 1.2 Unclear risk of bias | 7 | 462 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.22 [0.65, 2.28] |
| 1.3 High risk of bias | 3 | 241 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.78 [0.34, 1.79] |
| 2 Participants with complications at end of study by risk of bias for allocation concealment Show forest plot | 14 | 882 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.69 [0.59, 0.81] |
| 2.1 Low risk of bias | 9 | 622 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.78 [0.66, 0.92] |
| 2.2 Unclear risk of bias | 5 | 260 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.38 [0.24, 0.61] |
| 2.3 High risk of bias | 0 | 0 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality by end of study Show forest plot | 4 | 361 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.42 [0.85, 2.37] |
| 1.1 Protein‐containing supplement v non‐protein‐containing supplement | 3 | 315 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.38 [0.82, 2.34] |
| 1.2 High protein‐containing supplement v low protein‐containing supplement | 1 | 46 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.18 [0.21, 22.42] |
| 2 Unfavourable outcome (death or complications) at end of study Show forest plot | 2 | 223 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.78 [0.65, 0.95] |
| 2.1 Protein‐containing supplement v non‐protein‐containing supplement | 2 | 223 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.78 [0.65, 0.95] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality by end of study Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Totals not selected | |
| 2 Participants with complications at end of study Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with complications at end of study Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Totals not selected | |
| 2 Mortality by end of study Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality by end of study Show forest plot | 3 | 566 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.98 [0.65, 1.46] |
| 2 Participants with complications at end of study Show forest plot | 2 | 266 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.23 [0.63, 2.42] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality by end of study Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality by end of study Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Totals not selected | |
| 2 Participants with complications at end of study Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 99% CI) | Totals not selected | |

