Saringan kanser payudara dengan mamografi
Abstract
Background
A variety of estimates of the benefits and harms of mammographic screening for breast cancer have been published and national policies vary.
Objectives
To assess the effect of screening for breast cancer with mammography on mortality and morbidity.
Search methods
We searched PubMed (22 November 2012) and the World Health Organization's International Clinical Trials Registry Platform (22 November 2012).
Selection criteria
Randomised trials comparing mammographic screening with no mammographic screening.
Data collection and analysis
Two authors independently extracted data. Study authors were contacted for additional information.
Main results
Eight eligible trials were identified. We excluded a trial because the randomisation had failed to produce comparable groups.The eligible trials included 600,000 women in the analyses in the age range 39 to 74 years. Three trials with adequate randomisation did not show a statistically significant reduction in breast cancer mortality at 13 years (relative risk (RR) 0.90, 95% confidence interval (CI) 0.79 to 1.02); four trials with suboptimal randomisation showed a significant reduction in breast cancer mortality with an RR of 0.75 (95% CI 0.67 to 0.83). The RR for all seven trials combined was 0.81 (95% CI 0.74 to 0.87).
We found that breast cancer mortality was an unreliable outcome that was biased in favour of screening, mainly because of differential misclassification of cause of death. The trials with adequate randomisation did not find an effect of screening on total cancer mortality, including breast cancer, after 10 years (RR 1.02, 95% CI 0.95 to 1.10) or on all‐cause mortality after 13 years (RR 0.99, 95% CI 0.95 to 1.03).
Total numbers of lumpectomies and mastectomies were significantly larger in the screened groups (RR 1.31, 95% CI 1.22 to 1.42), as were number of mastectomies (RR 1.20, 95% CI 1.08 to 1.32). The use of radiotherapy was similarly increased whereas there was no difference in the use of chemotherapy (data available in only two trials).
Authors' conclusions
If we assume that screening reduces breast cancer mortality by 15% and that overdiagnosis and overtreatment is at 30%, it means that for every 2000 women invited for screening throughout 10 years, one will avoid dying of breast cancer and 10 healthy women, who would not have been diagnosed if there had not been screening, will be treated unnecessarily. Furthermore, more than 200 women will experience important psychological distress including anxiety and uncertainty for years because of false positive findings. To help ensure that the women are fully informed before they decide whether or not to attend screening, we have written an evidence‐based leaflet for lay people that is available in several languages on www.cochrane.dk. Because of substantial advances in treatment and greater breast cancer awareness since the trials were carried out, it is likely that the absolute effect of screening today is smaller than in the trials. Recent observational studies show more overdiagnosis than in the trials and very little or no reduction in the incidence of advanced cancers with screening.
PICOs
Ringkasan bahasa mudah
Saringan kanser payudara dengan mamografi
Saringan dengan mamografi menggunakan pengimejan X‐ray untuk mencari kanser payudara sebelum ketulan dapat dirasai. Matlamatnya adalah untuk merawat kanser lebih awal, apabila kemungkinan ada penawar. Ulasan ini memasukkan tujuh kajian yang melibatkan 600,000 wanita dalam lingkungan umur 39 hingga 74 tahun yang secara rawak untuk menerima saringan mamogram atau tidak. Kajian yang menyediakan maklumat yang boleh paling dipercayai menunjukkan bahawa pemeriksaan tidak mengurangkan kadar kematian kanser payudara. Kajian yang berkemungkinan lebih berat sebelah ( dilakukan dengan kurang teliti) mendapati bahawa saringan mengurangkan kadar kematian kanser payudara. Walau bagaimanapun, saringanan akan menyebabkan sesetengah wanita mendapat diagnosis kanser walaupun kanser mereka tidak akan membawa kepada kematian atau penyakit. Pada masa ini, adalah tidak mungkin untuk mengenalpasti siapa wanita‐wanita ini, dan mereka mungkin mempunyai payudara atau ketulan yang dibuang dan menerima radioterapi yang tidak perlu. Jika kita menganggap bahawa saringanan mengurangkan kadar kematian kanser payudara sebanyak 15% selepas 13 tahun susulan dan kelebihan diagnosis dan kelebihan rawatan adalah pada 30%, ia bermakna bahawa bagi setiap 2000 wanita dipanggil untuk saringan sepanjang 10 tahun, seorang akan mengelakkan kematian kanser payudara dan 10 wanita yang sihat, yang tidak akan didiagnosis jika tidak ada saringan akan diberi rawatan yang tidak perlu. Tambahan pula, lebih daripada 200 wanita akan mengalami tekanan psikologi penting termasuk kebimbangan dan ketidakpastian bagi bertahun‐tahun kerana penemuan positif yang palsu.
Wanita yang dipanggil untuk saringan perlu diberitahu sepenuhnya kedua‐dua manfaat dan kemudaratannya. Untuk memastikan bahawa keperluan untuk pilihan bermaklumat untuk wanita menimbang sama ada atau tidak untuk menghadiri program saringan dapat dipenuhi, kami telah menulis sebuah risalah yang berasaskan bukti untuk kaum awam yang boleh didapati dalam beberapa bahasa di www.cochrane.dk . Kerana kemajuan besar dalam rawatan dan lebih besar kesedaran kanser payudara daripada kajian yang telah dijalankan, ia adalah berkemungkinan bahawa kesan mutlak saringan hari ini adalah lebih kecil daripada kajian‐ kajian. Kajian pemerhatian terbaru menunjukkan kelebihan diagnosis daripada kajian dan sangat sedikit atau tiada pengurangan dalam insiden kanser berlanjutan dengan saringan.
Authors' conclusions
Background
Breast cancer is an important cause of death among women. Early detection through mass screening with mammography has the potential to reduce mortality, but it also leads to overdiagnosis and overtreatment (IARC 2002). Since screening preferentially identifies slow‐growing tumours (length bias) (Final reports 1977; Fox 1979), the harms of unnecessary treatment of overdiagnosed tumours could reduce or outweigh any potential benefits.
The best way to reliably estimate the effectiveness of screening is with randomised trials. Large trials, involving 650,000 women, have been carried out in North America and Europe (Canada 1980; Edinburgh 1978; Göteborg 1982; Malmö 1976; New York 1963; Stockholm 1981; Two‐County 1977; UK age trial 1991), and several systematic reviews and meta‐analyses have been published (Berry 1998; Blamey 2000; Cox 1997; Demissie 1998; Elwood 1993; Glasziou 1992; Glasziou 1995; Glasziou 1997; Gøtzsche 2000; Gøtzsche 2011; Hendrick 1997; Humphrey 2002; IARC 2002; Kerlikowske 1995; Kerlikowske 1997; Larsson 1996; Larsson 1997; Nelson 2009; Nyström 1993; Nyström 1996; Nyström 1997; Nyström 2000; Nyström 2002; Olsen 2001a; Olsen 2001b; Smart 1995; Swed Cancer Soc 1996; UK review 2012; Wald 1993).
The large number of reviews reflects the controversies surrounding mammography screening and the uncertainties of its effects in women of various ages. There is wide variation in screening policies between different countries, with some countries abstaining from introducing screening partly because of the lack of a documented reduction in all‐cause mortality (Isacsson 1985; Skrabanek 1993; Swift 1993). One area of concern is the potential for radiotherapy treatment of low‐risk women, such as those who have their cancers identified at screening, to increase all‐cause mortality because of adverse cardiovascular effects (EBCTCG 1995; EBCTCG 2000). In addition, there is concern that cause of death has not been ascribed in an unbiased fashion in the trials. Finally, carcinoma in situ is much more likely to be detected with screening mammography and although less than half of the cases will progress to be invasive (Nielsen 1987; Welch 1997) these women will nevertheless be treated with surgery, drugs and radiotherapy.
Meta‐analyses of screening are often deficient (Walter 1999) and few of the meta‐analyses listed above have taken account of the risk of bias in the individual trials or considered harms as well as benefits. We have identified important weaknesses in the trials (Gøtzsche 2000; Gøtzsche 2000a; Gøtzsche 2004; Gøtzsche 2011) and have now updated our Cochrane Review with additional data.
Objectives
To study the effect of screening for breast cancer with mammography on mortality and morbidity.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials. Trials using less reliable randomisation methods were evaluated separately.
We have discussed recent observational studies in this review as these have provided important new knowledge, e.g. in relation to evidence on overdiagnosis and other harms of screening.
Types of participants
Women without previously diagnosed breast cancer.
Types of interventions
Experimental: screening with mammography
Control: no screening with mammography
Types of outcome measures
Mortality from breast cancer
Mortality from any cancer
All‐cause mortality
Use of surgical interventions
Use of adjuvant therapy
Harms of mammography
Search methods for identification of studies
We used a very broad search strategy. We searched PubMed with (breast neoplasms[MeSH] OR "breast cancer" OR mammography[MeSH] OR mammograph*) AND (mass screening[MeSH] OR screen*). This search was supplemented with a search on author names in the author field (Alexander F*, Andersson I*, Baines C*, Bjurstam N*, Duffy S*, Fagerberg G*, Frisell J*, Miller AB, Moss S*, Nystrom L*, Shapiro S, Tabar L*). The latest search was done on 22 November 2012 and 29,222 records were imported into ProCite. Until the 2009 review, these records were searched for author names, cities and eponyms for the trials; thereafter, all new records were browsed. This very broad search strategy, combined with browsing the titles and reading the abstracts when a paper might be relevant for mammography screening, enabled us to assemble also the observational studies of the benefits and harms of screening.
We searched the World Health Organization's International Clinical Trials Registry Platform (22 November 2012) with this strategy, for Recruitment Status ALL: (Condition: breast AND (cancer% OR carcinoma% OR neoplas% OR tumour% OR tumor%) AND Intervention: screen OR mass screen%) OR (Condition: breast AND (cancer% OR carcinoma% OR neoplas% OR tumour% OR tumor%) AND Intervention: mammograph%) OR (Condition: breast neoplasm AND Intervention: mammography).
We scanned reference lists and included letters, abstracts, grey literature and unpublished data to retrieve as much relevant information as possible. There were no language restrictions.
Data collection and analysis
Two authors independently decided which trials to include based on the prestated criteria. Disagreements were resolved by discussion.
We assessed whether the randomisation was adequate and led to comparable groups, following standard criteria as closely as possible (Higgins 2008). We divided the trials into those with adequate randomisation and those with suboptimal randomisation.
Two authors independently extracted methodological and outcome data; disagreements were resolved by discussion. Extracted data included: number of women randomised; randomisation and blinding procedures; exclusions after randomisation; type of mammography; number of screenings and interval between screenings; attendance rate; introduction of screening in the control group; co‐interventions; number of cancers identified; breast cancer mortality; cancer mortality; all‐cause mortality; harms of mammography; and use of surgical interventions, chemotherapy, radiotherapy, tamoxifen and other adjuvant therapy. We contacted the primary investigators to clarify uncertainties.
Statistical methods
We performed intention‐to‐treat analyses, when possible, by including all randomised women. A fixed‐effect model with the Mantel‐Haenszel method was used, and 95% confidence intervals (CI) are presented. In case of heterogeneity in the trial results (P < 0.10), we explored possible causes. We present the analyses in the graphs as risk ratios, for convenience, but also discuss the absolute risk reductions (or increases) and risk differences as these are more important than relative risks for trials in low‐risk populations with few events, such as in the trials we reviewed.
In the trials with suboptimal randomisation, we could not carry out a proper analysis for all‐cause mortality as we did not have access to the necessary data (see 'Risk of bias in included studies') but present the available data in the graphs for the sake of completeness. For breast cancer mortality, our estimates are not formally correct because we were unable to adjust for baseline differences. However, they turned out to be in close agreement with the estimates and CIs published by the trialists. For completeness, we have shown the pooled estimates for the trials with adequate randomisation and those with suboptimal randomisation together, although we believe these summary estimates are likely to be unreliable (see below).
We report outcome data at approximately 7 and 13 years, which were the most common follow‐up periods in the trial reports; and present age groups under 50 years of age and above, which is the age limit that has most often been used by the trialists and in screening programmes.
Results
Description of studies
We identified 11 completed trials. From these we excluded two small trials of several interventions including mammography (Berglund 2000; Dales 1979) and a trial involving 166,600 women where the only intervention was a prevalence screen and where exclusions after randomisation occurred only in the screened group; previous cancer at any site was an exclusion criterion and more than 1500 women were excluded from the screened group, 468 because they had already died (Singapore 1994).
An additional trial in the UK is ongoing (http://www.controlled‐trials.com/ISRCTN33292440). This is an age extension cluster randomised trial, recruiting women aged 47‐49 or 71‐73 years old, and aiming for a sample size of 3 million women. It started in 2010 and is expected to run till the end of 2026.
Some of the eight eligible trials (Canada 1980; Edinburgh 1978; Göteborg 1982; Malmö 1976; New York 1963; Stockholm 1981; Two‐County 1977; UK age trial 1991) comprised slightly different subtrials. The Canadian trial was actually two trials, one covering the age group 40 to 49 years (Canada 1980a) and the other 50 to 59 years (Canada 1980b). The Edinburgh and Malmö trials continued to include women as they passed the lower age limit for entry to the trial, and the Two‐County trial had different randomisation ratios in the two counties (Kopparberg 1977; Östergötland 1978). Most trials covered the age range 45 to 64 years, but the UK age trial invited women aged 39 to 41 years to participate. The Canadian trial was the only one in which the women were individually randomised after invitation and gave informed consent; the others used a variety of procedures based on a prespecified segment of the female population that was randomised to invitation for screening or to a control group.
The number of consecutive screening invitations was in the range of four to nine for all trials except the Stockholm and Two‐County trials, in which a large fraction were invited for only two or three screenings. In the Two‐County trial, the mammographically screened women were encouraged to perform breast self‐examinations once a month on a fixed date (Rapport 1982). This was Swedish policy generally but we do not know for certain whether this was also true for the Göteborg, Malmö and Stockholm trials. Clinical examinations of screened women were performed in New York and Edinburgh. In Canada, in the 40 to 49 year age group, screened women had an annual clinical breast examination whereas control women were examined at the first visit and were taught self‐examination for use thereafter. In the 50 to 59 year age group, all women had their breasts clinically examined annually.
The women in the control group were not invited to screening at any point in time in the New York trial, whereas they were invited for screening after 10 to 13 years of follow up in the Edinburgh, Malmö and UK age trials. In the Canadian trial, most of the women in the control group were invited when the trial ended (Baines 2005). Some women were invited for screening while the trial was still ongoing in the Göteborg, Stockholm and Two‐County trials (see 'Risk of bias in included studies').
In all trials, women in the control groups were offered usual care. This included mammography on indication, that is for suspected malignancy, with the probable exceptions of the New York trial and the first five years of the Two‐County trial.
According to the information we identified, the technical quality of the mammograms and the observer variation was assessed only in the Canadian trial. There are data on diagnostic rates, however, that show that the sensitivity in the trials that followed the New York trial has not consistently improved (Fletcher 1993; IARC 2002). Various combinations of one‐ and two‐view mammography were used (see 'Characteristics of included studies').
Risk of bias in included studies
The trials have been conducted and reported over a long period of time, during which standards for reporting trials have improved. The New York trial, for example, was first reported in 1966 but crucial details on the randomisation method, exclusions and blinding were not published until 20 years later (Aron 1986; Shapiro 1985; Shapiro 1988). Data on use of radiotherapy and chemotherapy in the Kopparberg trial were published 14 years after the main results (Tabar 1999). Below we discuss the trial methodology in detail, which is essential reading to understand the controversies surrounding the effects of screening and the often conflicting information presented. The trials are described consecutively by start date.
The New York trial (New York 1963)
Population studied
The New York trial (also called the Health Insurance Plan (HIP) trial) invited women who were members of an insurance plan and aged 40 to 64 years from December 1963 to June 1966. It reported an individual randomisation within pairs matched by age, family size and employment group (Shapiro 1985). It is not clear whether the randomisation method was adequate; it was described as "alternation" by researchers who contacted one of the trial investigators (Freedman 2004). The entry date for a woman was the date she was scheduled for the examination (Shapiro 1966); the matched control was assigned the same date (Shapiro 1985). The matched pairs method should lead to intervention and control groups of exactly the same size. This is supported by the approximate numbers given in several publications, for example "The women were carefully chosen as 31,000 matched pairs" (Strax 1973). The largest published exact number of women invited is 31,092 (Fink 1972).
Comparability of groups
Postrandomisation exclusions of women with previous breast cancer occurred but this status "was most completely ascertained for screened women," whereas women in the control group "were identified through other sources as having had breast cancer diagnosed before their entry dates" (Shapiro 1988). Using information in the trial reports (Fink 1972; Shapiro 1985; Shapiro 1994), we calculated that 853 (31,092 minus 30,239) women were excluded from the screened group because of previous breast cancer compared with only 336 (31,092 minus 30,756) in the control group. Although it was reported that great care was taken to identify these women, the lead investigator noted that more than 20 years after the trial started some prior breast cancer cases among the controls were unknown to the investigators and those women should have been excluded (Shapiro 1985a). This creates a bias in favour of screening for all‐cause mortality and likely also for breast cancer mortality though the authors have written, without providing data, that ascertainment of cases of previous breast cancer was "nearly perfect" in those women who died from breast cancer (Shapiro 1988).
It is difficult to evaluate whether there were other baseline differences between the groups. In one paper (Shapiro 1972) the text described all randomised women and referred to a table that showed baseline differences as percentages but did not provide the numbers upon which the percentages were based. Footnotes explained that some of the data were based on 10% and 20% samples. The table title referred to women entering the trial in 1964, and not all women as claimed in the text. Assuming that the table title is correct, the data presented in some cases were a 1964 subgroup of 10% and 20% samples. These resulting samples are therefore too small to study other possible baseline differences than those related to differential exclusion of women with previous breast cancer.
Assignment of cause of death
We found no data on the autopsy rate. Assignment of cause of death was unblinded for 72% of the women with breast cancer (Shapiro 1988). The differential exclusions and unblinded assessments make us question the reliability of the reported breast cancer mortality rates.
Likelihood of selection bias
We classified the trial as suboptimally randomised.
The Malmö trial (Malmö 1976)
Population studied
This trial recruited women aged 45 to 69 years. Randomisation was carried out by computer within each birth year cohort (Andersson 1981), dividing a randomly arranged list in the middle (Andersson 1999a). The first publications noted that 21,242 women were randomised to the screening group and 21,240 to the control group (Andersson 1980; Andersson 1981a).
Comparability of groups
A later publication reported four more women in the control group (Andersson 1983) but the main publication (Andersson 1988) reported only 21,088 women in the study group and 21,195 in the control group. It did not account for the 199 or 203 missing women. The number of missing women was largest in the 45 to 50 years age group (137 from the intervention group and 26 or 27 from the control group), mainly because the 1929 birth year cohort was recruited by an independent research project that included mammography (Andersson 2001). The trialists recruited less than the planned 50% of this birth year cohort, but this does not explain why 26 or 27 women were missing from the control group. Exclusion of the 1929 birth year cohort from analysis changes the relative risk for death from breast cancer by only 0.01 (Andersson 2001). For 17 of the 25 birth year cohorts, the size of the study and control groups were identical or differed by only one, as expected. The largest difference in the other eight cohorts, apart from the 1929 one, was 25 fewer women than expected in the study group for the 1921 cohort (Nyström 2002). Thus, the authors of a meta‐analysis of the Swedish trials did not report on all randomised women in Malmö (Nyström 2002).
The date of entry into the trial was defined differently for the two groups. For the mammography group it was the date of invitation (Andersson 1988), and the midpoint of these dates for each birth year cohort defined the date of entry for women in the control group (Andersson 2000). Enrolment began in October 1976 (Andersson 2000) and ended in September 1978 (Andersson 1988). It is not clear whether screening of the control group began in December 1990 (Nyström 2000) or in October 1992 (Nyström 2002). Most women in the control group were never screened (Nyström 2002). We calculated the interval between when screening started in the study group and in the control group (the intervention contrast) to be 19 years (Nyström 2002). In the meta‐analyses of the Swedish trials, breast cancer cases diagnosed before randomisation were explicitly excluded, further reducing the screened group by 393 and the control group by 412 (Nyström 1993); in total 86 more women were excluded from the screened group than the control group. Baseline data on age were not significantly different in the screened group and the control group (Gøtzsche 2000a).
Assignment of cause of death
The autopsy rate for breast cancer cases as presented in the main publication for this trial (Andersson 1988) was high at 76%, but it was halved from 1985 to 1997 (Andersson 2000). Cause‐of‐death assessments were blinded up to 1988 (Andersson 2000).
Likelihood of selection bias
We classified the trial as adequately randomised.
The Malmö II trial (Malmö II 1978)
Population studied
This was an extension of the Malmö trial, called MMST II. Women who reached the age of 45 years were enrolled between September 1978 and November 1990; screening of the control group began in September 1991 (Nyström 2000). The long enrolment period gives an average estimated intervention contrast of eight years. Although the entry criterion for age was stated to be 45 years, the trialists included 6780 women aged 40 to 44 (Nyström 2002).
Comparability of groups
The MMST II trial has been published only in brief (Andersson 1997). We therefore cannot check whether there were differential postrandomisation exclusions. If the same procedure as in the Malmö trial had been followed, the sizes of the study and control group cohorts should not differ by more than one. However, the group size differed more for seven of the 13 birth year cohorts (Nyström 2002). The reported numbers in the individual cohorts do not add up to the reported totals, but to 28 fewer in the study group and 28 more in the control group. Because of an administrative error, the entire 1934 birth year cohort was invited for screening (Andersson 1999b). If this cohort is excluded, there is still a gross imbalance with 5724 women in the study group and only 5289 in the control group, for those aged 45 to 49 years (P = 0.00004, Poisson analysis). In total, there were 9581 and 8212 women in the analyses, respectively (Nyström 2002).
This trial was neither included nor mentioned in the 1993 meta‐analysis of the Swedish trials (Nyström 1993). The lead investigator informed us that it was not conducted according to a formal protocol (Andersson 1999b), whereas the most recent meta‐analysis reported that the trial was conducted with the same protocol as the older part of the trial (Nyström 2002). When the breast cancer mortality rate in the screening group is plotted against the control group rate for eight trials, with data from younger women, the Malmö II trial is a clear outlier (Berry 1998).
Assignment of cause of death
An official registry was used for cause‐of‐death assessments.
Likelihood of selection bias
We classified the trial as suboptimally randomised.
The Two‐County trial (Kopparberg 1977; Two‐County 1977; Östergötland 1978)
Population studied
This trial recruited women 40 years of age and over in Kopparberg and Östergötland; the two subtrials were age‐matched and cluster randomised (21 and 24 clusters, respectively). The selection of clusters was stratified to ensure an even distribution between the two groups with respect to residency (urban or rural), socioeconomic factors and size (Kopparberg 1977; Tabar 1979; Östergötland 1978). The randomisation process and the definition of the date of entry have been inconsistently described; and some women were only 38 years of age, below the inclusion criterion (Nyström 2002). According to the first publications, random allocation of the women in each community block took place three to four weeks before screening started (Fagerberg 1985); all women from a given block entered the trial at the same time and this date was the date of randomisation (Tabar 1985). However, it has also been described that a public notary allocated the clusters in Östergötland by tossing a coin (Nyström 2000) while witnesses were present (Fagerberg, personal communication, 1999). We have been unable to find any detailed description of the randomisation in Kopparberg but found a recent description for the whole trial: "Randomisation was by traditional mechanical methods and took place under the supervision of the trial statistician" (Duffy 2003). Thus it is not clear whether the randomisation was carried out on one occasion or whether it took place over several years.
Women were invited to their first screening from October 1977 to January 1980 in Kopparberg (Tabar 1981). The cohorts in Östergötland were defined between May 1978 and March 1981. It is not clear how many women were randomised and reported numbers vary considerably, both for numbers randomised (Table 1) and for numbers of breast cancer deaths, despite similar follow up (Gøtzsche 2004). Documentation of baseline comparability was called for in 1988 (Andersson 1988a) but it appears not to have been published. Since the randomisation was stratified after socioeconomic factors (Tabar 1991), baseline data potentially affecting mortality should exist.
| Study | Age range | Study group | Control group | Reference |
| Malmö | 40‐74 | 21242 | 21240 | |
| 40‐74 | 21242 | 21244 | ||
| 40‐74 | 21088 | 21195 | ||
| Kopparberg | total | 47389 | 22658 | |
| 40‐74 | 39051 | 18846 | ||
| 40‐74 | 38589 | 18582 | ||
| 40‐74 | 38562 | 18478 | ||
| 40‐74 | 38589 | 18582 | ||
| 40‐74 | 38568 | 18479 | ||
| 40‐74 | 38588 | 18582 | ||
| 40‐74 | data not available | data not available | ||
| 40‐49 | 9625 | 5053 | ||
| 40‐49 | data not available | data not available | ||
| 40‐49 | 9582 | 5031 | ||
| 40‐49 | 9650 | 5009 | ||
| Östergötland | total | 47001 | 45933 | |
| 40‐74 | 39034 | 37936 | ||
| 40‐74 | 38491 | 37403 | ||
| 40‐74 | 38405 | 37145 | ||
| 40‐74 | 38491 | 37403 | ||
| 40‐74 | 38942 | 37675 | ||
| 40‐74 | 39105 | 37858 | ||
| 40‐74 | 38942 | 37675 | ||
| 40‐49 | 10312 | 10625 | ||
| 40‐49 | data not available | data not available | ||
| 40‐49 | 10262 | 10573 | ||
| 40‐49 | 10240 | 10411 | ||
| Stockholm | 40‐64 | 40318 | 19943 | |
| 40‐65 (sic) | 38525 | 20651 | ||
| 40‐64 | 40318 | 19943 | ||
| 40‐69 | 39139 | 20978 | ||
| 40‐49 | data not available | data not available | ||
| 40‐49 | 14842 | 7103 | ||
| 40‐49 | 14185 | 7985 | ||
| 40‐49 | 14303 | 8021 | ||
| Göteborg | 40‐59 | 20724 | 28809 | |
| 39‐59 | 21650 | 29961 | ||
| 40‐59 | 21000 | 29200 | ||
| 40‐49 | 10821 | 13101 | ||
| 39‐49 | 11724 | 14217 | ||
| 40‐49 | 10888 | 13203 |
Comparability of groups
The randomisation procedure seems to have led to non‐comparable groups. First, breast cancer mortality in the control group was almost twice as high in Kopparberg compared to Östergötland (0.0021 versus 0.0012, P = 0.02). This was not apparent from the tabulated data (Tabar 1985). The published graphs are also potentially misleading; although adjacent mortality curves look much the same the two y‐axes are differently scaled (Tabar 1995). Second, in Kopparberg more women in the control group were diagnosed with breast cancer before entry to the trial than in the study group. How the diagnostic information was obtained was not described (Tabar 1989) and the number of women excluded for this reason was not stated, but can be calculated by comparing two tables (Tabar 1985; Tabar 1989). More women were excluded from the control group than from the study group (P = 0.03); most of the imbalance occurred in the age group 60 to 69 years (P = 0.007). In Östergötland, numbers of exclusions were very similar, 1.40% versus 1.39%. Third, age‐matching was reported (Tabar 1979; Tabar 1981; Tabar 1985a) but study group women were on average five months older (Nixon 2000), which is a small bias against screening.
We were unable to ascertain when systematic screening of the control group started. The available information is conflicting and the range of the discrepancies amounts to three years for both counties (Arnesson 1995; Duffy 2003; Nyström 1993, ; Nyström 2000; Nyström 2002; Rapport 1982; Tabar 1979; Tabar 1985; Tabar 1992). It seems most likely that screening of the control group in Kopparberg started in 1982, in accordance with the trial protocol (Rapport 1982) and a doctoral thesis (Nyström 2000). In this case, the impression conveyed in the main publication for the trial that screening was offered to the control group after publication of the results in April 1985 is incorrect (Tabar 1985; Tabar 1992). In the protocol, a five‐year intervention period was planned but with a stopping rule based on statistical significance testing every six months (Rapport 1982). The trial publications did not mention the repeated looks at the data (Tabar 1985). We estimated an intervention contrast of five years for Kopparberg and eight years for Östergötland. A valid comparison of benefits and harms of screening should be confined to the period prior to screening of the control group.
No information is available from the primary author of this trial (Atterstam 1999; Prorok 2000; Tabar 2000a). We have not received information from Nyström either on the missing account of the randomisation process in Kopparberg, or from the Swedish National Board of Health (Socialstyrelsen), which funded the trial.
Assignment of cause of death
The autopsy rate was 36% (Projektgruppen 1985). According to an investigator involved with the trial (Crewdson 2002), other Swedish trialists (Nyström 2002), and an IARC report (IARC 2002), cause‐of‐death assessments were not blind. This has been disputed by the lead investigator of the trial (Tabar 2002). In a meta‐analysis of the Swedish trials, a blinded independent endpoint committee reassessed the death classifications (Nyström 1993).
Likelihood of selection bias
We classified the trial as suboptimally randomised and likely to be biased.
The Edinburgh trial (Edinburgh 1978)
Population studied
This trial used cluster randomisation with about 87 clusters (the number varies in different reports); the age group was 45 to 64 years. Coded general practices were stratified by size and allocated by manual application of random numbers. In one district, at least three of the 15 practices initially randomised to the screening group later changed allocation status, and at least four others were added (Alexander 1989). Two of these practices were unintentionally told the wrong group, and three changed allocation group because of "statistical considerations" (Roberts 1984). One practice was included in the follow up even though it was a pilot screening practice that did not participate in the randomisation (Roberts 1990). The trialists have conducted replicate analyses with these women removed (Alexander 2000) but as far as we know the data have not been published.
Comparability of groups
Doubts about the randomisation process were raised by the trialists (Alexander 1989), supported by baseline differences: 26% of the women in the control group and 53% in the study group belonged to the highest socioeconomic level (Alexander 1994), and mammographic screening was associated with an unlikely 26% reduction in cardiovascular mortality (Alexander 1989). Entry dates were defined differently. In most practices the entry date was the date the invitation letter was issued; for women in hospital it was the date their names appeared on a list sent to their general practitioner. The entry date for five practices was not defined. In the control group, the entry date was the date the physician's practice was indexed. Before entry, the general practitioners in the screening practices had to decide whether each woman would be suitable for invitation to screening. Physicians in the control practices decided whether each woman would be eligible to receive a leaflet about breast self‐examination (Roberts 1984). The eligibility criteria were thus broader for the control group and the entry dates seem to be earlier. Practices were enrolled one at a time over a period of 2.5 years, from 1979 to 1981 (Alexander 1989). Women turning 45 years of age and women moving into the city were enrolled on an ongoing basis (Roberts 1984). Recruitment of the control group began in the 10th year of follow up (Alexander 1994). The exclusion procedures were different in the study and control groups (Chamberlain 1981; Roberts 1984) and 338 versus 177 women were excluded because of prior breast cancer (Alexander 1994).
Likelihood of selection bias
This trial was not adequately randomised and was so biased that it cannot provide reliable data. We have therefore shown its results in a separate graph, for completeness only.
The Canadian trial (Canada 1980; Canada 1980a; Canada 1980b)
Population studied
Women aged 40 to 59 years were individually randomised after invitation and giving informed consent. Their names were entered successively on allocation lists, where the intervention was prespecified on each line. An independent review of ways in which the randomisation could have been subverted uncovered no evidence of this (Bailar 1997). Enrolment took place from January 1980 to March 1985 (Canada 1980a).
Comparability of groups
Fifty‐nine women in the age group 40 to 49 years and 54 in the age group 50 to 59 years were excluded after randomisation (Miller 2000; Miller 2002); none were excluded because of previous breast cancer. The comparison groups were nearly identical in size (25,214 versus 25,216 aged 40 to 49 years; and 19,711 versus 19,694 aged 50 to 59 years), and were similar at baseline for age and nine other factors of potential prognostic importance (Baines 1994; Canada 1980; Canada 1980a; Canada 1980b; Miller 2000; Miller 2002). There were more small node‐positive cancers at baseline in the screened group than in the control group among women aged 40 to 49 years, but this is a post‐hoc subgroup finding which is probably a result of the intervention (Baines 1995; Baines 1997; Canada 1980). Several women with positive nodes were probably unrecognised in the control group (Miller 1997a). This is supported by the fact that 47% of women with node‐negative cancer in the usual care group died of breast cancer compared with 28% in the mammography group (Miller 1997). Exclusion of the deaths caused by these cancers did not change the result (Baines 1995; Baines 1997; Canada 1980).
Assignment of cause of death
The autopsy rate was low, 6% (Baines 2001). Cause‐of‐death assessments were blinded for women with diagnosed breast cancer and for other possible breast cancer deaths, for follow up after seven years. For follow up after 13 years, death certificates were used in a minority of cases as some hospitals refused to release clinical records (Miller 2000; Miller 2002).
Likelihood of selection bias
We classified the trial as adequately randomised.
The Stockholm trial (Stockholm 1981)
Population studied
In this trial, women were invited for screening if they were aged 40 to 64 years in 1981 (born 1917 to 1941) and were born on days 1 to10 in a month, or if they were aged 40 to 64 years in 1982 (born 1918 to 1942) and were born on days 21 to 30 in a month (Frisell 1986). Similarly, there were two groups of controls but since they were all born on days 11 to 20 in a month, most women served as controls twice (those born in 1918 to 1941). Invitations were sent successively by ascending order of birth date (Frisell 1989). The date of entry was the date of invitation (Frisell 1991). Enrolment of the first cohort began in March 1981 and ended in April 1982; enrolment of the second cohort began in April 1982 and ended in May 1983 (Frisell 2000a).
Comparability of groups
Since the control women born in 1918 to 1941 served as controls for both subtrials (Frisell 1989a; Frisell 2000b) they should have two entry dates, approximately one year apart, but this was not described. According to the matching there should have been a similar number of women in the screened and control groups in each subtrial, but we found an imbalance in the second subtrial (P = 0.01, Poisson analysis) with 508 more women belonging to the screened group than to the control group (Frisell 1991). Furthermore, in the time period where 19,507 women born from 1918 to 1942 were invited to screening, only 929 women, all born in 1942, were included in the control group (Nyström 2002).
The reported numbers of women in the various subgroups are inconsistent, as are the numbers reported to us in personal communications (Frisell 2000a; Frisell 2000b). Because of the problems related to timing and the overlap of the two control groups, results from the two subtrials were not independent, and the estimates cannot be pooled without correction for dependence. It is not clear how these difficulties were handled in the trialists' analysis (Frisell 1991) or in the Swedish meta‐analyses (Nyström 1993; Nyström 2000; Nyström 2002).
The first trial report did not describe any women excluded after randomisation; only breast cancer cases identified during the intervention period were followed up to ascertain breast cancer deaths (Frisell 1991). Exclusions occurred in later publications but no numbers were given (Frisell 1997; Nyström 1993; Nyström 2000) and the numbers we have received in personal communications have been inconsistent (Frisell 2000a; Frisell 2000b).
Of those attending the first screening, 25% had had a mammogram in the two previous years (Frisell 1989a). Information on screening of the control group varied. A meta‐analysis noted that a few women were screened after three years and most after four years (Nyström 1993), a doctoral thesis stated that the controls were invited for screening from October 1985 (Nyström 2000), and the trialists noted that they were invited during 1986 (Frisell 1989a; Frisell 1991). We estimated an intervention contrast of four years. A valid comparison of benefits and harms of screening should be restricted to this period (Frisell 1991).
Assignment of cause of death
It is not stated whether cause‐of‐death assessments were blinded for this initial period. The autopsy rate was 22% (Nyström 2000).
Likelihood of selection bias
We classified the trial as suboptimally randomised.
The Göteborg trial (Göteborg 1982)
Population studied
This trial included women aged 39 to 59 years. Birth year cohorts were randomised by the city municipality's computer department with the ratio between study group and control group adjusted according to the capacity of the screening unit (Bjurstam 2000; Nyström 2002). The randomisation was by cluster based on date of birth in the 1923 to 1935 cohorts, and by individual birth date for the 1936 to 1944 cohorts (Bjurstam 1997).
Comparability of groups
We found baseline data only on age, and only for those aged 39 to 49 years. Since the allocation ratios were irregular, we could not assess the comparability of groups and adequacy of randomisation. The randomisation ratios were most extreme for the oldest and the youngest birth‐year cohorts randomised in clusters; for 1923, there were 2.0 times as many women in the control group as in the study group, whereas for 1935 there were only 1.1 times as many. Since breast cancer mortality increases with age, this bias favoured screening and can be adjusted for only by comparing the results within each birth‐year cohort before they are pooled (Bjurstam 2003).
Entry dates were not defined but the birth year cohorts were randomised one at a time, beginning with the 1923 cohort in December 1982 and ending in April 1984 with the 1944 cohort. A similar proportion of women were excluded from the study and control groups, 254 (1.2%) and 357 (1.2%), because of previous breast cancer (Bjurstam 2003). Information on screening of the control group varied, ranging from three to seven years after randomisation (Bjurstam 1997; Bjurstam 2003; Nyström 1993, figure; Nyström 2000). We estimated an intervention contrast of five years. A valid comparison of benefits and harms of screening should be confined to this period.
Assignment of cause of death
The autopsy rate was 31% (Nyström 2000). Cause‐of‐death assessments were blinded.
Likelihood of selection bias
We classified the trial as suboptimally randomised.
The UK age trial (UK age trial 1991)
Population studied
This trial included women aged 39 to 41 years who were randomised individually between 1991 and 1997 to an intervention group or a control group, in a ratio of 1:2. Women in the control group received no information about the trial. The trial was undertaken in 23 breast‐screening units in England, Wales, and Scotland. Women were identified from lists of patients from general practitioners held on local Health Authority databases and randomisation was carried out stratified by practice. Prior to this, the general practitioners could remove women with previous breast cancer and others deemed inappropriate to invite for screening. From 1992 onwards the allocations were carried out on the Health Authority computer system with specifically written software. Before this, for women in three early centres, random numbers generated from the coordinating centre computer were applied to the lists.
Comparability of groups
We found baseline data only on age; the mean age was 40.38 and 40.39 years, respectively.
Thirty and 51 women (0.05%) were excluded from analysis for similar reasons in the two groups. The intervention contrast was 10 years. A valid comparison of benefits and harms of screening should be confined to this period.
Assignment of cause of death
There was no information on autopsy rate; information on cause of death was obtained from the central register of the National Health Service.
Likelihood of selection bias
We classified the trial as adequately randomised.
Sources of data used for the meta‐analyses
Deaths ascribed to breast cancer: Alexander 1999; Andersson 1988; Bjurstam 1997; Bjurstam 2003; Frisell 1997; Habbema 1986; Miller 1992a; Miller 1992b; Miller 2000; Miller 2002; Moss 2006; Nyström 1993; Nyström 1993a; Nyström 2002; Roberts 1990; Shapiro 1977; Shapiro 1982; Tabar 1988; Tabar 1995.
Mortality among breast cancer patients: Tabar 1988.
Deaths ascribed to cancer, all patients: Andersson 1988; Aron 1986; Miller 2000; Miller 2002; Shapiro 1988; Tabar 1988.
All‐cause mortality: Andersson 1988; Aron 1986; Bjurstam 1997; Miller 1992a; Miller 1992b; Miller 2000; Miller 2002; Moss 2006; Nyström 2000; Nyström 2002; Projektgruppen 1985; Roberts 1990; Shapiro 1977; Tabar 1989.
Mastectomies and lumpectomies: Andersson 1988; Frisell 1986; Frisell 1989a; Miller 1993; Shapiro 1972; Tabar 1999.
Radiotherapy: Andersson 1988; Benjamin 1996; Shapiro 1972; Tabar 1999.
Chemotherapy and hormone therapy: Andersson 1988; Tabar 1999.
Number of cancers: Andersson 1988; Bjurstam 1997; Frisell 1989a; Miller 1993; Moss 2005; Tabar 1991.
Effects of interventions
Eight trials provided data. We classified three trials as adequately randomised (Canada, Malmö and UK age trial) and four as suboptimally randomised (Göteborg, New York, Stockholm, Two‐County), as was also the extension of the Malmö trial, MMST II. One trial (Edinburgh) was not adequately randomised and cannot provide reliable data; we have therefore only shown its results for completeness, in a separate graph. As the results from the UK age trial were obtained after a mean follow up of 10.7 years, we included them in the results both after 7 and after 13 years. The adequately randomised trials provided 40% of the breast cancer deaths after 13 years (Analysis 1.2).
Deaths ascribed to breast cancer
We judged assignment of breast cancer mortality to be unreliable and biased in favour of screening (see above and 'Discussion'), but included this outcome because it was the main focus in all trials.
The three adequately randomised trials did not find a statistically significant effect of screening on deaths ascribed to breast cancer, relative risk (RR) 0.93 (95% CI 0.79 to 1.09) after 7 years and RR 0.90 (95% CI 0.79 to 1.02) after 13 years. The four suboptimally randomised trials found a beneficial effect: RR 0.71 (95% CI 0.61 to 0.83) after 7 years and RR 0.75 (95% CI 0.67 to 0.83) after 13 years. For all seven trials taken together the RR was 0.81 (95% CI 0.72 to 0.90) after 7 years and RR 0.81 (95% CI 0.74 to 0.87) after 13 years. This result is less reliable, however, than that based on the adequately randomised trials.
The adequately randomised trials did not find a statistically significant effect of screening on deaths ascribed to breast cancer in the youngest age group (under 50 years of age at randomisation except for 7 year data from Malmö for which the limit was 55 years): RR 0.94 (95% CI 0.78 to 1.14) after 7 years and RR 0.87 (95% CI 0.73 to 1.03) after 13 years. The suboptimally randomised trials found an RR of 0.81 (95% CI 0.63 to 1.05) after 7 years and RR of 0.80 (95% CI 0.64 to 0.98) after 13 years. For the oldest age group, the estimates for the adequately randomised trials were RR 0.88 (95% CI 0.64 to 1.20) and RR 0.94 (95% CI 0.77 to 1.15), respectively; for suboptimally randomised trials they were RR 0.67 (95% CI 0.56 to 0.81) and RR 0.70 (95% CI 0.62 to 0.80), respectively.
Deaths ascribed to any cancer
The adequately randomised trials did not find an effect of screening on deaths ascribed to any cancer, including breast cancer (RR 1.02, 95% CI 0.95 to 1.10); the follow up was 10.5 years for Canada and 9 years for Malmö (data were not available for the UK age trial). The suboptimally randomised trials did not provide reliable estimates of cancer mortality (see above); the estimate for the two suboptimally randomised trial that provided data (New York and Two‐County trials) was RR 0.99 (95% CI 0.93 to 1.06).
All‐cause mortality
All‐cause mortality was not significantly reduced (RR 0.98, 95% CI 0.94 to 1.03 after 7 years; and RR 0.99, 95% CI 0.95 to 1.03 after 13 years) for the three adequately randomised trials. The suboptimally randomised trials did not provide reliable estimates of the effects on all‐cause mortality (see 'Risk of bias in included studies' and 'Discussion') and the reported effects were heterogeneous (P = 0.03 after 7 years; P = 0.001 after 13 years). For completeness, the mortality estimates are shown in the graphs.
Surgery
Significantly more breast operations (mastectomies plus lumpectomies) were performed in the study groups than in the control groups: RR 1.31 (95% CI 1.22 to 1.42) for the adequately randomised trials; RR 1.42 (95% CI 1.26 to 1.61) for the suboptimally randomised trials before systematic screening in the control group started (data were available only for Kopparberg and Stockholm). The increased surgery rate could not be explained by the excess of detected tumours at the first screen but seemed to persist, as the mean follow up was seven years for Canada and nine years for Malmö. For Stockholm, the reported data after five years had been transformed according to the smaller size of the control group (Frisell 1989a). We recorrected and found that also for this trial the excess of surgery persisted (RR 1.37 after first round; RR 1.48 after five years).
The number of mastectomies (excluding partial mastectomies, quadrantectomies and lumpectomies) was also significantly increased: RR 1.20 (95% CI 1.08 to 1.32) for the adequately randomised trials; RR 1.21 (95% CI 1.06 to 1.38) for the suboptimally randomised trials.
Radiotherapy
Significantly more women received radiotherapy in the study groups: RR 1.24 (95% CI 1.04 to 1.49) for Malmö after nine years; and RR 1.40 (95% CI 1.17 to 1.69) for Kopparberg before the control group screen.
Other adjuvant therapy
We found little information on other adjuvant therapy. It differed substantially for two of the Swedish trials even though they were carried out at the same time. Chemotherapy was given to only 7% of the breast cancer patients in Malmö but to 31% in Kopparberg before the control group was screened (Analysis 1.17). Conversely, hormone therapy was given to 17% in Malmö, and to 2% in Kopparberg (Analysis 1.18). Information exists from Kopparberg on therapeutic adjuvant therapy given over the years but has not been published (Tabar 1999).
Harms
We found no comparative data on psychological morbidity. Duration of sick leave and mobility of the shoulder were recorded in the Two‐County trial (Rapport 1982) but have not been reported.
Discussion
The decision to embark on the screening programmes was made mainly because of the positive results in the New York and Two‐County trials (Forrest report 1986). Policy makers and many scientists believed that the benefit of screening was well documented. However, information essential to judging the reliability of the trials was often unpublished or published only in Swedish, in theses, letters, conference reports, reviews, or in journals that are not widely read and with titles and abstracts that did not indicate that important data were described. Furthermore, the harms of screening received very little attention.
Breast cancer mortality
The main focus in the screening trials was breast cancer mortality, as very large trials are needed to assess the effect of screening on all‐cause mortality. We cannot assume, however, that a beneficial effect on breast cancer mortality can be translated into improved overall survival. First, screening may increase mortality because of the increased use of radiotherapy. A meta‐analysis predicted that overall, radiotherapy is beneficial for women at high risk of local recurrence. However, it is harmful for women at particularly low risk such as those who have their cancers found by screening. This is primarily because of damage to the coronary arteries and development of heart failure resulting from at least some types of radiotherapy (EBCTCG 2000) and because radiotherapy causes lung cancer. A meta‐analysis of radiotherapy showed that there was a 27% excess mortality from heart disease and a 78% excess mortality from lung cancer (EBCTCG 2005a). This excess mortality becomes important when many healthy women are overdiagnosed.
Second, assessment of cause of death is susceptible to bias. The authors of the Two‐County trial assessed cause of death openly and reported a 24% reduction in breast cancer mortality for Östergötland (Tabar 2000), whereas a meta‐analysis of the Swedish trials based on an official cause of death register reported only a 10% reduction for Östergötland (Nyström 2002). The trial authors reported 10 fewer deaths from breast cancer in the study group despite slightly longer follow up, and 23 more deaths in the control group. They have not provided a plausible explanation of this large discrepancy (Duffy 2002; Tabar 2002). In 2009, "a complete audit of breast cancer cases and deaths" in the Two‐County trial was published, but it is not convincing (Holmberg 2009). There was no blinding; it was not an independent audit; there was no attempt at producing a new data set based on the clinical records (which were only retrieved "where necessary"); and the Two‐County trialists were directly involved with interpretations and resolving disagreements.
The bias seems to favour screening even when cause of death is determined blindly. In the New York trial, differential misclassification might be responsible for about half of the reported breast cancer mortality benefit. A similar number of dubious cases were selected for blinded review from each group, but a much smaller proportion of the screened group were finally classified as having died from breast cancer (Gøtzsche 2004). Furthermore, although the mammographic equipment was standard at the time, its performance was poor. Only 15% of 299 cancers in the study group were detected solely by mammography, and mammography did not identify a single case of minimal breast cancer (< 1 cm) (Thomas 1977). The New York trial reported a 35% reduction in breast cancer mortality after seven years, but we consider it unlikely that it was a true effect.
In conjunction with the first meta‐analysis of the Swedish trials, causes of death were reclassified blindly in some patients (Nyström 1993). Breast cancer was considered the underlying cause of death in 419 of the screened group and 409 of the control group according to Statistics Sweden, and in 418 and 425 cases according to the committee (Nyström 1993). The fact that all 17 reclassifications favoured the screened group suggests differential misclassification. This bias is difficult to avoid (Gøtzsche 2001). Early cancers are treated by lumpectomy and radiotherapy, and radiotherapy reduces the rates of local recurrence by about two‐thirds (EBCTCG 2000). This might increase the likelihood that deaths among screen‐detected breast cancer cases will be misclassified as deaths from other causes (EBCTCG 1995) and that too many deaths in the control group will be misclassified as breast cancer deaths. In fact, for the Swedish trials it was stated that "most patients with locally advanced disease will die due to cancer" and that breast cancer as the underlying cause of death includes women with locally advanced breast cancer, whereas women who have been treated successfully should not be classified as having breast cancer deaths if another specified disease could be the cause of death (Nyström 2000). The use of an official cause of death register as in more recent meta‐analyses (Nyström 2002) cannot solve these problems.
Postrandomisation exclusion of women who already had breast cancer at the time of entry to the trial is another possible source of bias. The exclusions were sometimes made many years after the trial started, or even after it had ended. In the Two‐County trial, only women who were considered to have died from breast cancer were excluded (Nixon 2000), a highly bias‐prone process because those assessing cause of death were not blinded for screening status. Furthermore, the process seemed not to have been adequately monitored as it was not possible to identify prior breast cancers in Östergötland, by cluster (Nixon 2000). It should therefore not be possible to do analyses that respect the clustering with those women excluded, although such analyses have been reported (Tabar 1989; Tabar 1990; Tabar 1991; Tabar 1995). A study that used the same registers as those used by the trialists found that a large number of breast cancer cases and deaths seemed to be missing in reports on the Two‐County trial (Zahl 2006). Another study found that the large reduction in breast cancer mortality agreed poorly with the cancer stages that were reported (Zahl 2001).
The largest effects on breast cancer mortality were reported in trials that had long intervals between screenings (Two‐County trial), invited a large fraction of the women to only two or three screenings (Two‐County and Stockholm trials), started systematic screening of the control group after three to five years (Two‐County, Göteborg and Stockholm trials), had only one‐view mammography rather than two views (Two‐County trial), and that had poor equipment for mammography (New York trial); and the cancers found with mammography were considerably smaller in the Canadian trial than in the Two‐County trial (Narod 1997). This suggests that differences in reported effects are related to the risk of bias in the trials rather than to the quality of the mammograms or the screening programmes. The sensitivity of mammographic readings in the trials that followed the New York trial has not consistently improved (Fletcher 1993; IARC 2002) and meta‐analyses have failed to find an association between mammographic quality and breast cancer mortality (Glasziou 1995; Kerlikowske 1995). A meta‐analysis found that the effect of screening was largest in those trials that found fewest node‐positive cancers in the screened group relative to the control group (Gøtzsche 2011). However, the regression line was in the wrong place. A screening effectiveness of zero (same proportion of node‐positive cancers in the screened group as in the control group) predicted a significant 16% reduction in breast cancer mortality after 13 years (95% CI 9% to 23% reduction). This can only occur if there is bias, and there was bias for both variables, assessment of cause of death and of the number of node‐positive cancers.
Several of the trials had clinical examination or regular self‐examination of the breasts as part of their design (see 'Description of studies') but this is not likely to have had a major influence on the effect estimates. The effect of clinical examination is uncertain, and large randomised trials did not find an effect of self‐examination (Kösters 2003).
Cancer mortality
The major difficulty in assessing cause of death might have occurred when the patients were diagnosed with more than one malignant disease (Miller 2001). The importance of autopsy is illustrated by the fact that 21% of the women with breast cancer who died in the Malmö trial had two or three types of different cancers (Andersson 1988a; Janzon 1991). Patients with cachexia and no signs of recurrence of breast cancer would likely be assigned to another type of cancer.
Since cancer mortality is likely to be less subject to bias than breast cancer mortality, we calculated what the expected cancer mortality (including breast cancer mortality) would be if the reported reduction in breast cancer mortality of 29% after seven years for the suboptimally randomised trials (Analysis 1.1) were true. Weighting the four trials that provided data on number of cancer deaths (Analysis 1.7), the expected relative risk was 0.95. However, all‐cancer mortality in these trials was not reduced (RR 1.00, 95% CI 0.96 to 1.05), and this estimate was significantly higher than what was expected (P = 0.02). This provides further evidence that assessment of cause of death was biased in favour of screening. Data from the Two‐County trial (Tabar 1988) illustrates the misclassification directly (Analysis 1.19) (Gøtzsche 2004). Among women with a diagnosis of breast cancer, mortality for other cancers was significantly higher in the screened group and mortality from all other causes also tended to be higher. The increase in mortality for causes other than breast cancer amounts to 38% of the reported decrease in breast cancer mortality in the Kopparberg part of the trial and 56% in the Östergötland part.
It has been shown that belief in the effectiveness of an intervention may influence the decision on which type of cancer caused the patient's death (Newschaffer 2000). Also, lethal complications of cancer treatments are often ascribed to other causes. The size of this misclassification is 37% for cancer generally and 9% for breast cancer (Brown 1993).
All‐cause mortality
The trials were not powered to detect an effect on all‐cause mortality, but it is an important outcome since the findings related to breast cancer mortality may be biased. The complex designs and insufficient reporting precluded us from providing reliable estimates for all‐cause mortality in the trials with suboptimal randomisation. Furthermore, these trials had introduced early screening of the control group or had differentially excluded women after randomisation. Incidentally, however, all‐cause mortality after 13 years was the same in adequately randomised trials and in suboptimally randomised trials (RR 0.99, 95% CI 0.95 to 1.03; and RR 0.99, 95% CI 0.97 to 1.01, respectively).
In 2000, the estimate reported for the four Swedish trials was RR 1.00 (95% CI 0.98 to 1.02) after adjustment for imbalances in age (Nyström 2000). In 2002, the authors reported a 2% (non‐significant) reduction in all‐cause mortality (RR 0.98, 95% CI 0.96 to 1.00) and stated that they would have expected a 2.3% reduction (Nyström 2002). However, the calculation was incorrect and the expected reduction, given their results, was only 0.9% (Gøtzsche 2002a). The error has been acknowledged (The Lancet Erratum 2002; Nyström 2002a) but the published response to our criticism was also incorrect (Nyström 2002b). The reported decrease of 2% in total mortality corresponds to a 10% decrease in all‐cancer mortality, which is not plausible (see 'Cancer mortality' above).
The Östergötland part of the Two‐County trial contributed about half of the deaths in the 2002 report and had a relative risk for all‐cause mortality of 0.98 (Nyström 2002). The women were randomised to only 24 clusters. In the Edinburgh trial there were 87 clusters, but double as many in the invited group belonged to the highest socioeconomic level compared to the control group (Alexander 1994). Socioeconomic factors are strong mortality predictors and could easily explain a 2% reduction in all‐cause mortality, but such data remain unpublished and are also unavailable for the other Swedish trials. It has been reported that pretrial breast cancer incidence and breast cancer mortality were similar in the study group and in the control group in Östergötland (Nyström 2002), but the power of the test was very low (Gøtzsche 2002a). In contrast, another report found that breast cancer mortality was 15% lower in the invited groups in the Two‐Country trial and that correction for this difference changed the estimate of the effect from a 31% reduction to a 27% reduction in breast cancer mortality (Duffy 2003).
It is not clear why the unadjusted and age‐adjusted estimates for all‐cause mortality were the same with an RR of 0.98. The 2002 Swedish meta‐analysis comprised 43,343 deaths whereas in the 2000 meta‐analysis of 27,582 deaths the estimates were RR 1.06 (95% CI 1.04 to 1.08) (Gøtzsche 2000) and RR 1.00 (95% CI 0.98 to 1.02) (Nyström 2000), with non‐overlapping confidence intervals. The Kopparberg part of the Two‐County trial was not available for the 2002 meta‐analysis, but this should not have made any difference since the RR for Kopparberg was 1.00 (95% CI 0.96 to 1.04) (Nyström 2000). The only other difference is that the extended data for the Malmö trial (MSST II) were included, but this trial contributed only 702 deaths (1.6%).
All‐cause mortality has been reported to be lower in the Two‐County trial when the analysis was confined to women with breast cancer (Tabar 2002a). Such subgroup analyses are very unreliable, as are similar analyses in historically controlled studies (Tabar 2001; Tabar 2003a), since many breast cancer cases in the screened groups will have an excellent prognosis because of overdiagnosis and length bias (Berry 2002).
Overdiagnosis and overtreatment
Overdiagnosis is a consequence of cancer screening and an obvious source of harm (IARC 2002). Screening primarily identifies slow‐growing cancers and cell changes that are biologically benign (Doll 1981; Ernster 1996; Fox 1979). This is because slow‐growing tumours have existed for longer than fast‐growing tumours in the detectable range of tumour sizes and are therefore more likely to be detected at a screening session (length bias). Survival of women with screen‐detected cancers is therefore very high, for example 97% in Malmö after 10 years (Janzon 1991). Even within the same stage, it is higher than for cancers detected clinically (Moody‐Ayers 2000).
The level of overdiagnosis and overtreatment was about 30% in the trials that did not introduce early screening in the control group, and somewhat larger in the suboptimally randomised trials before the control group screen. This is apart from the New York trial, which is unreliable since far more breast cancer cases were excluded from the screened group than from the control group (Shapiro 1977; Shapiro 1982; Shapiro 1989). The true increase in surgery is considerably larger than 30%, however. As the excess surgery in the trials is very similar to the increase in diagnoses, reoperations have not been included, although many women are operated upon more than once. In New South Wales, for example, one third of women with carcinoma in situ had either mastectomy alone (19%) or after breast conserving surgery (17%) (Kricker 2000).
Large observational studies support these findings. Incidence increases of 40% to 60% have been reported for Australia, Finland, Norway, Sweden, UK and USA (Barratt 2005; Douek 2003; Fletcher 2003; Gøtzsche 2004; IARC 2002; Jonsson 2005; Morrell 2010; Ries 2002; Zahl 2004. In two additional studies, overdiagnosis was calculated as the percentage of all diagnoses, rather than the percentage of additional diagnoses; correcting for this gives an overdiagnosis of 45% in USA (Bleyer 2012) and 18‐33% in Norway (Kalager 2012). The Norwegian estimate did not include carcinoma in situ and was also an underestimate for other reasons (Jørgensen 2012). A small study from Copenhagen claimed that it is possible to screen without overdiagnosis, but it showed the expected prevalence peak, had very little power and provided no statistical analyses in support of the claim (Olsen 2003). A study that included the whole of Denmark and also non‐screened age groups found 33% overdiagnosis (Jørgensen 2009a). A systematic review that adjusted for decreases in incidence, if any, in older age groups no longer screened, and also for the trend in background incidence, found an overdiagnosis of 35% for invasive cancer and 52% when carcinoma in situ was included, in countries with organised screening programmes (Jørgensen 2009).
Data from the UK show that when screening was extended to the age group 65‐70 years in 2001, a sharp rise in invasive breast cancer incidence occurred in these women although they had been offered screening many times when they were younger and had already contributed to a massive increase in the incidence of DCIS and invasive cancers (Jørgensen 2011). This is difficult to explain unless we assume that many screen‐detected cancers would have regressed spontaneously if left alone, which is supported by a study from Norway with a strong design (Zahl 2008), and by a similarly designed study from Sweden (Zahl 2011). A US study also suggested that breast cancers regress, since the incidence declined much too rapidly after the use of hormone replacement therapy stopped (Chlebowski 2009). Another US study, of the breast cancer incidence and mortality rates during the period 1975 to 2000 when screening was introduced found that, in order to explain the observed trends, it was necessary to postulate that approximately 40% of the observed cancers had limited malignant potential and would have regressed if undetected (Fryback 2006).
Screening increased the number of mastectomies by 20%. Since screening advances the time of diagnosis, a policy change towards more lumpectomies could have led to an overestimate. However, the policy change has occurred slowly (Nattinger 2000) and even in the period 1993 to 1995, 52% of breast surgery in California was mastectomy (Malin 2002). In Stockholm, the increase in mastectomies was larger after five years of screening (25%) than after the first round (16%), and when screening was introduced in Southeast Netherlands, the rate of breast‐conserving surgery increased by 71% while the rate of mastectomy increased by 84% (Gøtzsche 2002) despite the fact that this study did not include carcinoma in situ. The percentage of cases of carcinoma in situ treated by mastectomy declined from 71% in 1983 to 40% in 1993 in USA, but the estimated total numbers of mastectomies for this condition increased almost three‐fold (Ernster 1997). In the UK, mastectomies increased by 36% for invasive cancer and by 422% for carcinoma in situ from 1990 to 2001 (Douek 2003). Carcinoma in situ is more often treated by mastectomy than invasive cancer (Patnick 2012) .
Conversely, use of mammography in the control group would lead to an underestimate of overdiagnosis. In the trials from Malmö and Canada, 24% (Andersson 1988), 17% (Miller 1992b) and 26% (Baines 1994) of the women in the control group reported having received a mammogram during the trial; in the Two‐County trial, it was 13% (Tabar 1985); in the Göteborg trial, 18% of women in the control group received a mammogram in a two‐year period during the trial (Bjurstam 2003). In the Stockholm trial, 25% of those attending the first screening had had a mammogram in the two previous years (Frisell 1989a), and in the Göteborg trial, as many as 51% of the women in the age group 39‐49 had ever received a mammogram (Bjurstam 1997). It is difficult to understand that this trial, with so much contamination reducing the observed benefit, found a 45% reduction in breast cancer mortality.
The documented increase in mastectomies contrasts with assertions by trialists (Tabar 1989), policy makers (Statusrapport 1997; Swed Cancer Soc 1996; Westerholm 1988), websites supported by governmental institutions and advocacy groups (Jørgensen 2004), and invitational letters sent to women invited to screening (Jørgensen 2006; Gøtzsche 2009) that early detection spares patients more aggressive treatments, in particular mastectomy. Publications that base their claims on numbers that include the control group screen (Tabar 2003) are also misleading, as are presentations of relative numbers rather than absolute numbers (Statusrapport 1997). The proportion of breast preserving operations is said to be increasing, but the trend for the number of mastectomies is not revealed. A small study from Florence, without a control group (Paci 2002), was also unreliable (Gøtzsche 2002b). The authors asserted that if screening increased the number of mastectomies, populations in which screening has been introduced should see a subsequent increase. Obviously, since the mastectomy rate has gone down steadily throughout many years, also in countries without screening, it is only to be expected that the authors found a decrease in the mastectomy rate when screening was introduced.
Denmark has a unique control group, as only 20% of the population was screened throughout 17 years. The large increase in mastectomies when screening was introduced has not been compensated later or in older age groups (Jørgensen 2011). A study from Norway has confirmed this (Suhrke 2011).
Quality assurance programmes could possibly reduce the surgical activity to some degree, but they could also increase it. In the UK, for example, the surgeons were blamed for not having treated even more women with carcinoma in situ by mastectomy (BASO audit 2000), and the number of women treated by mastectomy almost doubled from 1998 to 2008 (Dixon 2009).
Two to three years after breast cancer treatment, 47% of the women reported pain, usually several times a week (Gärtner 2009). Only half of those with pain reported that it was light (corresponding to 1‐3 on a 10‐point scale). The pain was equally common among those who had had breast‐conserving surgery as among those with a mastectomy, and pain was more common when the women had had radiotherapy. Thus, half of all the overdiagnosed women will suffer from chronic pain, presumably for the rest of their lives.
False‐ positive diagnoses, psychological distress and pain
False‐positive diagnoses can cause considerable and sustained psychological distress (Bülow 2000; Salz 2010), not only until it is known whether or not there is a cancer (Brodersen 2006) but for years after the women are declared free from cancer (Brodersen 2013). Many women experience anxiety, worry, despondency, sleeping problems, negative impact on sexuality and behaviour, and changes in their relationships with family, friends, and acquaintances as well as in existential values (Brodersen 2006; Brodersen 2007; Brodersen 2013; Salz 2010). In a large study that compared women with normal findings, women with false‐positive diagnoses and women with breast cancer, the severity of the psychological distress for women with false‐positive findings was between that for healthy women and those with breast cancer even three years after they had been declared free from cancer (Brodersen 2013). Some women will feel more vulnerable about disease and see a doctor more often (Barton 2001).
In the Stockholm trial, one‐third of women with false‐positive findings were not declared cancer‐free at six months (Lidbrink 1996). In the UK, women who had been declared cancer‐free after additional testing or biopsies were twice as likely to suffer psychological consequences three years later than women who received a clear result after their last mammogram (Brett 2001). In the USA, three months after they had false‐positive results 47% of women who had highly suspicious readings reported that they had substantial anxiety related to the mammogram, 41% had worries about breast cancer, 26% reported that the worry affected their daily mood, and 17% that it affected their daily function (compared to 3% with a normal mammogram) (Lerman 1991). In Norway, 18 months after screening mammography 29% of women with false‐positive results and 13% of women with negative results reported anxiety about breast cancer (Gram 1990).
The cumulative risk of a false‐positive result after 10 mammograms ranges from about 20% to 60% (Barratt 2005; Castells 2006; Christiansen 2000; Elmore 1998; Hofvind 2004; Hubbard 2011; Johns 2010; Njor 2007). It is considerably higher in USA than elsewhere, e.g. the recall rate in women aged 50 to 54 years was 13% to 14% after the first mammogram, compared to 8% in the UK (Smith‐Bindman 2003). The reported percentages are often too low because recalls due to poor technical quality of the mammogram are not included (Hofvind 2004; Johns 2010; Njor 2007), although these women may be just as affected by such recalls as by a real suspicion of cancer (Brodersen 2006). In USA, 19% would have had a biopsy after 10 mammograms (Elmore 1998).
Thus, it seems that screening inflicts important psychological distress for years on more than a tenth of the healthy population of women who attend a screening programme. The women are often not being informed about this risk (Gøtzsche 2009; Jørgensen 2004; Jørgensen 2006; Slaytor 1998; Werkö 1995) or the risk of receiving a diagnosis of carcinoma in situ (Gøtzsche 2009; Jørgensen 2004; Thornton 1997).
About half of the women report that it is painful to have a mammogram taken (Armstrong 2007; Miller 2002a; McNoe 1996), and half of the women who decline an invitation to the second round of screening note that the major reason was that their first mammogram was painful (Elwood 1998).
Other recent reviews of screening
Previous reviews have generally not heeded the methodological quality of the trials, but when the methods were assessed blindly the researchers judged the Canadian trial to be of high quality and the Two‐County trial to be of poor quality (Glasziou 1995).
Prompted by our first Cochrane review in 2001, the US Preventive Services Task Force performed an updated systematic review (Humphrey 2002). It excluded the Edinburgh trial and reported a 16% reduction in breast cancer mortality for all ages. The authors noted that, "the mortality benefit of mammography screening is small enough that biases in the trials could erase or create it" and were concerned whether, across all age groups, the magnitude of benefit is sufficient to outweigh the harms. The Task Force gave mammography screening a grade B recommendation (US Task Force 2002). The Task Force reported a 15% reduction in breast cancer mortality for those aged 39 to 49 years in 2009 and larger effects in older age groups (Nelson 2009). A comprehensive IARC report (IARC 2002) was not a systematic review and paid little attention to the varying quality of the trials; it even included a non‐randomised study in its meta‐analysis. A 2012 UK report was not a systematic review either (UK review 2012). It used data from the Cochrane review for the benefit, but did not adjust the estimation of the effect to account for the varying quality of the trials or the improvements in treatment and breast cancer awareness. The report focussed on breast cancer mortality, and ignored all cause mortality, which may bias its findings in favour of breast screening. It acknowledged that previous estimations of the benefits and harms of mammography screening had been over‐optimistic and acknowledged uncertainties around estimations of the magnitude of effect. It did not use the Cochrane review estimate of overdiagnosis but a smaller one that was diluted because of screening in the control group (Welch 2006).
The meta‐analyses of the Swedish trials are not systematic reviews as they do not include all relevant trials. There is a high risk of bias in cluster randomised trials with few clusters (Puffer 2003) and numbers of randomised women were inconsistently reported (Table 1). In Stockholm, for example, the number of randomised women decreased by 4.5% in the screening group but increased by 3.6% in the control group (Gøtzsche 2000) in the Swedish 1993 review (Nyström 1993) compared to the trial report (Frisell 1997). In the 2000 and 2002 reviews (Nyström 2000; Nyström 2002), numbers have increased by 1.6% in both groups but should have been the same as in the 1993 report since all women were identified through their unique identification number (Nyström 2002), which has been used in Sweden for several decades; exclusions of women with previous breast cancer was completed with the 1993 review; and all three reviews were based on the exact age at randomisation, and the age range was the same. The varying numbers therefore indicate that the randomisation was not respected. The estimates in the Swedish reviews were adjusted for differences in age, but since the distribution of age would be expected to differ over socioeconomic strata such adjustment would be expected to lead to other imbalances (Gøtzsche 2000). Furthermore, simulation studies have shown that adjustments quite often increase bias rather than reduce it (Deeks 2003). The most recent review of the Swedish trials reported a 15% reduction in breast cancer mortality with the follow‐up model (Nyström 2002); another estimate of 21% was based on an 'evaluation model', which is flawed, as it ignores breast cancer deaths among women in the control group whose breast cancer diagnosis was made after the first screening round of the control group (Berry 1998).
What were the absolute effects of screening in the trials?
The largest reported effect in the Swedish trials collectively is a 29% relative reduction in breast cancer mortality for women aged 50 to 69 years, which corresponds to an absolute reduction in breast cancer mortality of 0.1% after 10 years (Nyström 1993). According to the Cochrane Handbook (Higgins 2008), the primary analysis in a systematic review should be based on studies at low risk of bias, and these studies showed only a 7% relative reduction in breast cancer mortality after 7 years and 10% after 13 years. We therefore believe that a realistic estimate is a 10‐15% relative reduction in breast cancer mortality in the trials. This is also what one would expect based on tumour data. The average difference in tumour size between the screened and the control groups was only 5 mm, which predicts a 12% reduction in breast cancer mortality since tumour size is linearly related to the risk of metastasis (Gøtzsche 2012a). The 12% reduction is an overestimate because the small overdiagnosed tumours inflate the difference in size of tumours, which must be less than 5 mm for clinically relevant tumours.
The trials did not find a reduction in all‐cancer mortality and our estimate could therefore be an overestimate. But if we assume the effect is 15%, it means that for every 2000 women invited for screening throughout 10 years, one will avoid dying of breast cancer. This number can be deduced from the first meta‐analysis of the Swedish trials, taking into account that the effect is only half as large as indicated in that paper (Nyström 1993, page 976). It can also be deduced from our review. After seven years (Analysis 1.1), there were 384 deaths from breast cancer in the adequately randomised trials out of 173,061 women in the control group, and a 15% effect corresponds to 326.4 deaths in a study group of the same size, which gives 0.7 women per 2000.
Similarly, if we assume that the level of overdiagnosis is 30%, which might be an underestimate, it means that for every 2000 women invited for screening throughout 10 years, 10 healthy women who would not have had a breast cancer diagnosis if there had not been screening will be diagnosed as cancer patients, and will be treated unnecessarily (see Analysis 1.14; there were 1083 cancers in the control group in the adequately randomised trials out of 66,154 women, which gives 325 overdiagnosed cancers, or 9.8 per 2000). In addition, it is likely that more than 200 women will experience important psychological distress for many months because of false‐positive findings.
What is the effect of screening today?
There have been substantial advances in treatment since the trials were performed. Anti‐hormones and polychemotherapy are effective also when the cancer has metastasized (EBCTCG 2005), and the declines in breast cancer mortality we have seen (Autier 2010) have occurred rather uniformly across prognostic groups (Blamey 2007). An updated meta‐analysis of polychemotherapy showed that some regimens reduce breast cancer mortality by about one third, largely independently of tumour characteristics (EBCTCG 2012). This means that the effect of screening must be smaller today than when the trials were conducted in terms of the number of women who avoid dying of breast cancer.
In order to be effective, screening would of necessity need to lead to a reduction in the number of advanced cancers at diagnosis. In the USA, there has been a very small decrease in advanced cancers (Esserman 2009; Jørgensen 2011). A detailed analysis of a time period spanning 30 years showed that the incidence of early‐stage breast cancer in USA went up from 112 to 234 cases per 100,000 women (a 109% increase) while the incidence of late‐stage cancer decreased by 8%, from 102 to 94 cases per 100,000 women (Bleyer 2012). Moreover, the small decline in advanced cancers was confined to regional disease involving the lymph nodes; there was no reduction in disease with distant metastases. A systematic review of several countries (Australia, Italy, Norway, Switzerland, the Netherlands, UK and the USA) found that, on average, the rate of cancers larger than 20 mm was not affected by screening (Autier 2011). In Norway, screening did not decrease the incidence of cancers in stages III and IV, as the reductions were exactly the same in screened and non‐screened areas (Kalager 2012).
In contrast to screening, increased breast cancer awareness seems to have been important. In Denmark, the average tumour size at diagnosis was 33 mm in 1978‐79, but only 24 mm ten years later, in 1988‐89 (Rostgaard 2010). This change occurred before screening started, and in contrast to screening, breast cancer awareness is unlikely to cause overdiagnosis. The difference of 9 mm is much greater than the average difference between the screened and the control groups in the trials, which was only 5 mm (Gøtzsche 2012a), despite the fact that the small overdiagnosed tumours would tend to spuriously exaggerate the difference. In Canada, the size of clinically detected tumours decreased by 4 mm from 1987 to 1999 (Narod 2011).
There are many poor observational studies claiming large effects of screening, but they often use statistical models with unsupported assumptions or misleading comparisons (Gøtzsche 2010; Gøtzsche 2012). The better studies rely on unmodified data. As noted above, Denmark has a unique control group, as only 20% of the population was screened throughout 17 years. The annual decline in breast cancer mortality in the relevant age group and time‐period was 1% in the screened areas and 2% in the non‐screened areas. In women who were too young to benefit from screening the declines were larger, 5% and 6%, respectively (Jørgensen 2010). Also in the UK, Sweden and Norway, there was no visible effect of screening when age groups were compared (Jørgensen 2010; Kalager 2010; Jørgensen 2011). The Norwegian study (Kalager 2010) was criticized because of short follow‐up, but the follow‐up from start of screening was 6.6 years, which is when an effect was seen in the trials.
A study reported a 15% effect in the USA (Berry 2005), but the authors noted that the decline in breast cancer mortality coincided not only with widespread propagation of screening but also with increasing use of adjuvant therapy. They also noted that slight variations in modelling assumptions could result in marked changes in estimated effects. Further, the statistical models adjusted for an increase in breast cancer incidence, which was inappropriate, as much of this increase was overdiagnosis. Unlike the USA, women below age 50 years are rarely offered screening in Europe. The mean decline in breast cancer mortality between 1989 and 2005 in these women was 37%, whereas it was 21% in women aged 50‐69 years (Autier 2010). The declines began before organised screening in many countries and fitted better with the introduction of tamoxifen, which explains the larger decline in young women who often have oestrogen‐sensitive tumours (Jørgensen 2011). A comparison of three pairs of neighbouring European countries that had introduced screening 10‐15 years apart showed no relation between screening start and the reductions in breast cancer mortality (Autier 2011a); in fact, the reduction in breast cancer mortality was about the same in the six European countries as in USA (Bleyer 2011). An Australian study found that most, if not all, of the reduction in breast cancer mortality could be attributed to adjuvant hormonal and chemotherapy (Burton 2011).
Screening advocates have claimed that screening explains why breast cancer mortality rates are lower in Sweden than in Denmark (Dean 2010), but this difference existed decades before screening. Further, the reductions in breast cancer mortality in the screening period were largest in Denmark, 49% versus 36% in Sweden in women under 50, although half of these women are invited in Sweden versus none in Denmark (Autier 2010). In those aged 50‐69 years, the reduction was 26% in Denmark versus 16% in Sweden, although only 20% of Danish women were invited, versus all in Sweden where more than 80% participated (Autier 2010; IARC 2002). Despite having the longest running programme, the widest invited age range, and the shortest screening interval in Europe (IARC 2002), Sweden has experienced lower reductions in breast cancer mortality than the European median (Autier 2010).
These studies taken in combination cast doubt as to the effectiveness of screening today. Even if screening still reduces breast cancer mortality, the effect on all‐cause mortality remains uncertain. However, both the randomised and non‐randomised studies provide evidence that screening causes substantial overdiagnosis.
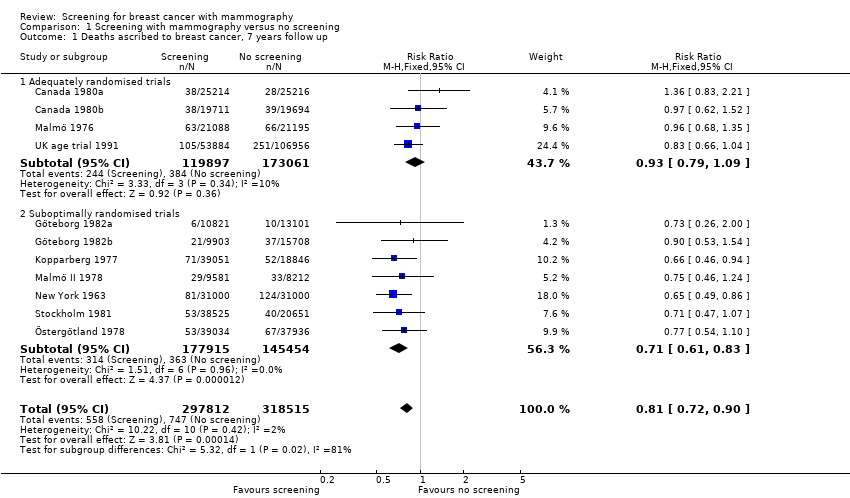
Comparison 1 Screening with mammography versus no screening, Outcome 1 Deaths ascribed to breast cancer, 7 years follow up.
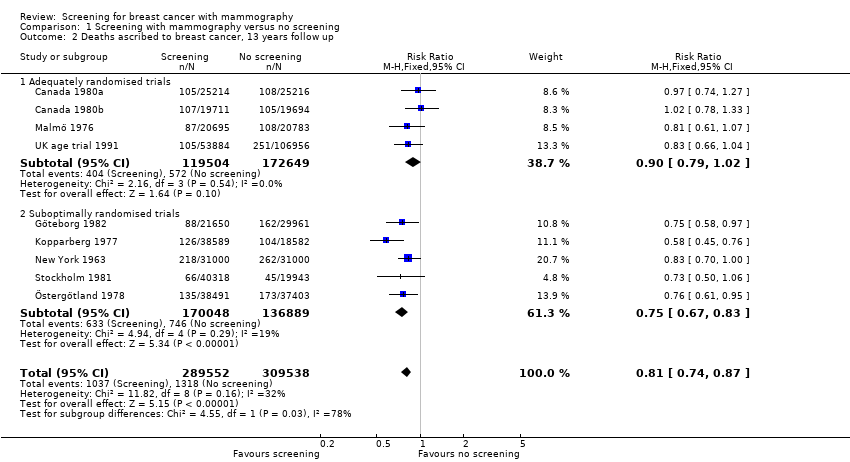
Comparison 1 Screening with mammography versus no screening, Outcome 2 Deaths ascribed to breast cancer, 13 years follow up.

Comparison 1 Screening with mammography versus no screening, Outcome 3 Deaths ascribed to breast cancer, 7 years follow up, women below 50 years of age (Malmö 55).
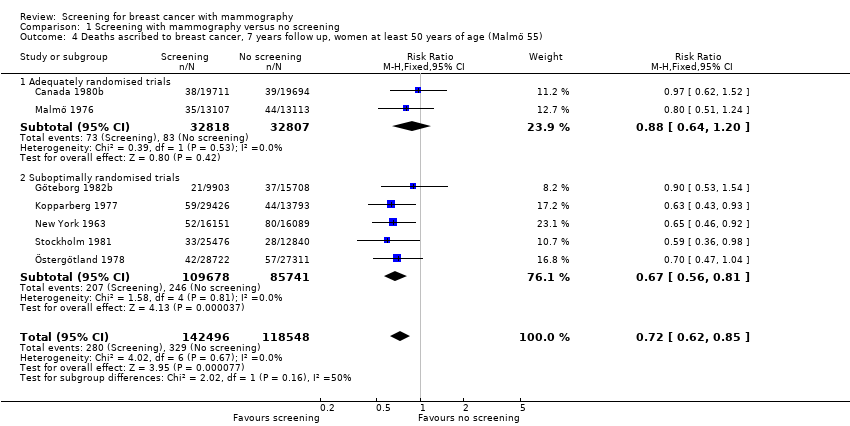
Comparison 1 Screening with mammography versus no screening, Outcome 4 Deaths ascribed to breast cancer, 7 years follow up, women at least 50 years of age (Malmö 55).

Comparison 1 Screening with mammography versus no screening, Outcome 5 Deaths ascribed to breast cancer, 13 years follow up, women below 50 years of age.
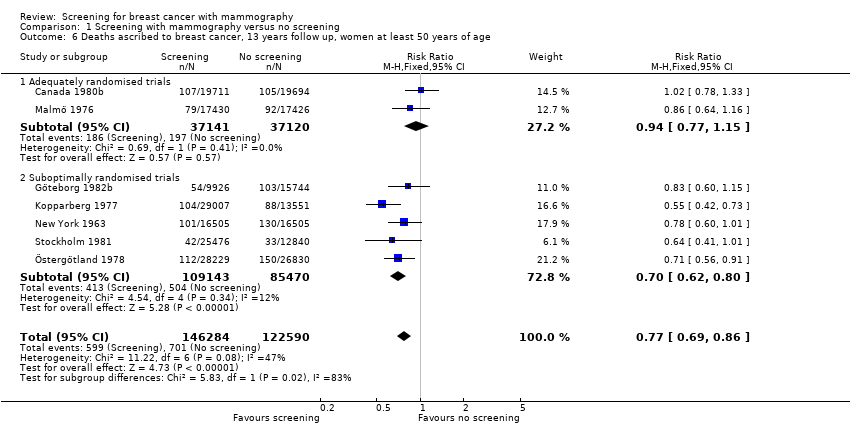
Comparison 1 Screening with mammography versus no screening, Outcome 6 Deaths ascribed to breast cancer, 13 years follow up, women at least 50 years of age.

Comparison 1 Screening with mammography versus no screening, Outcome 7 Deaths ascribed to any cancer, all women.
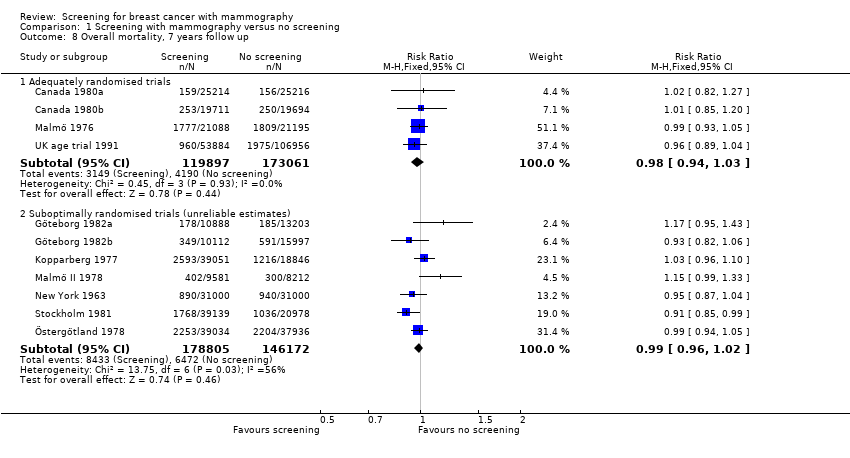
Comparison 1 Screening with mammography versus no screening, Outcome 8 Overall mortality, 7 years follow up.
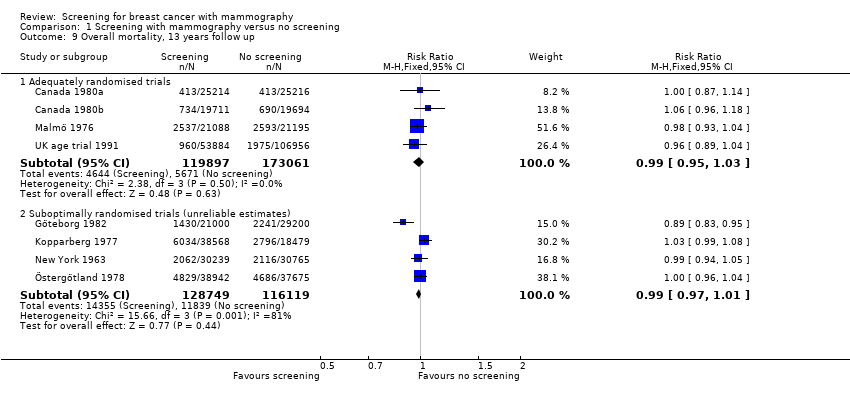
Comparison 1 Screening with mammography versus no screening, Outcome 9 Overall mortality, 13 years follow up.
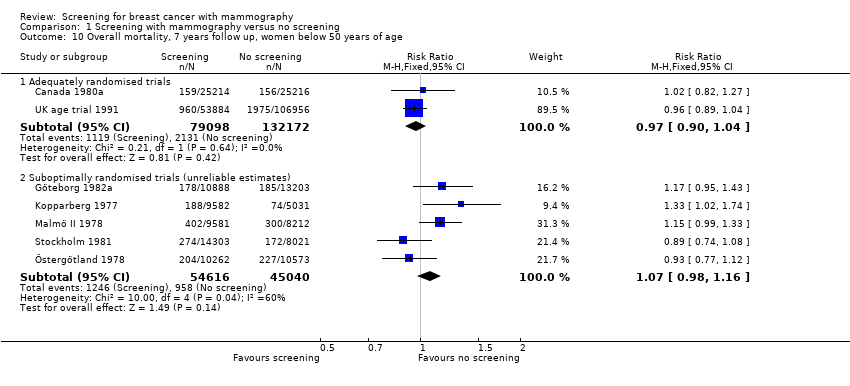
Comparison 1 Screening with mammography versus no screening, Outcome 10 Overall mortality, 7 years follow up, women below 50 years of age.
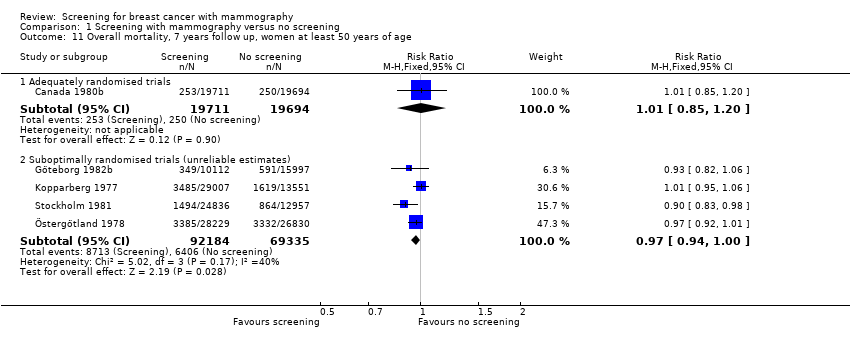
Comparison 1 Screening with mammography versus no screening, Outcome 11 Overall mortality, 7 years follow up, women at least 50 years of age.
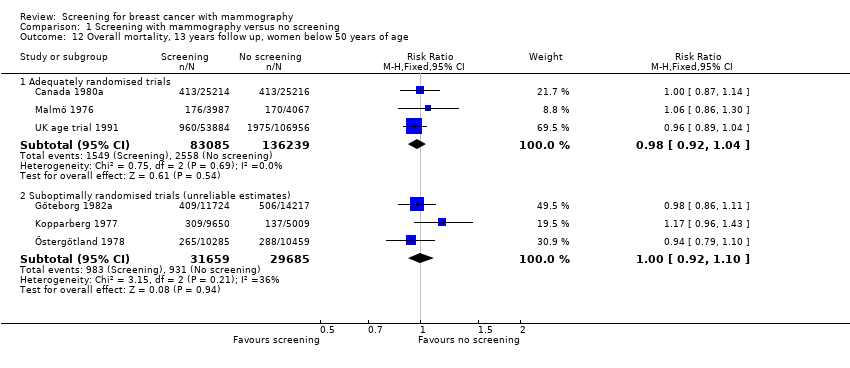
Comparison 1 Screening with mammography versus no screening, Outcome 12 Overall mortality, 13 years follow up, women below 50 years of age.

Comparison 1 Screening with mammography versus no screening, Outcome 13 Overall mortality, 13 years follow up, women at least 50 years of age.
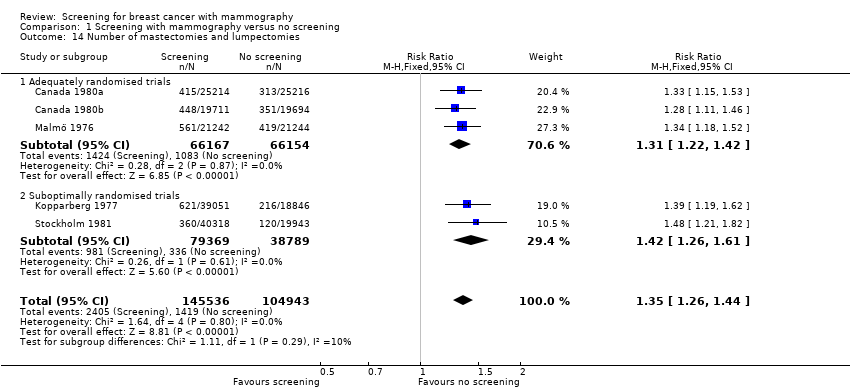
Comparison 1 Screening with mammography versus no screening, Outcome 14 Number of mastectomies and lumpectomies.
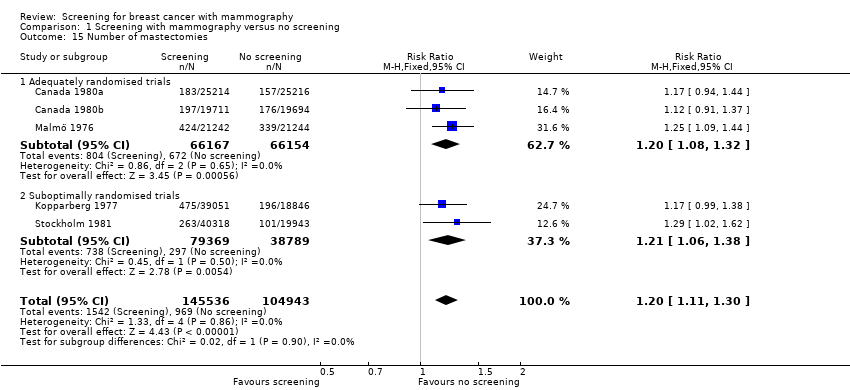
Comparison 1 Screening with mammography versus no screening, Outcome 15 Number of mastectomies.

Comparison 1 Screening with mammography versus no screening, Outcome 16 Number treated with radiotherapy.

Comparison 1 Screening with mammography versus no screening, Outcome 17 Number treated with chemotherapy.

Comparison 1 Screening with mammography versus no screening, Outcome 18 Number treated with hormone therapy.

Comparison 1 Screening with mammography versus no screening, Outcome 19 Mortality among breast cancer patients in the Two‐County study, 7 years follow up.

Comparison 1 Screening with mammography versus no screening, Outcome 20 Results for biased trial.

Comparison 1 Screening with mammography versus no screening, Outcome 21 Number of cancers.
| Study | Age range | Study group | Control group | Reference |
| Malmö | 40‐74 | 21242 | 21240 | |
| 40‐74 | 21242 | 21244 | ||
| 40‐74 | 21088 | 21195 | ||
| Kopparberg | total | 47389 | 22658 | |
| 40‐74 | 39051 | 18846 | ||
| 40‐74 | 38589 | 18582 | ||
| 40‐74 | 38562 | 18478 | ||
| 40‐74 | 38589 | 18582 | ||
| 40‐74 | 38568 | 18479 | ||
| 40‐74 | 38588 | 18582 | ||
| 40‐74 | data not available | data not available | ||
| 40‐49 | 9625 | 5053 | ||
| 40‐49 | data not available | data not available | ||
| 40‐49 | 9582 | 5031 | ||
| 40‐49 | 9650 | 5009 | ||
| Östergötland | total | 47001 | 45933 | |
| 40‐74 | 39034 | 37936 | ||
| 40‐74 | 38491 | 37403 | ||
| 40‐74 | 38405 | 37145 | ||
| 40‐74 | 38491 | 37403 | ||
| 40‐74 | 38942 | 37675 | ||
| 40‐74 | 39105 | 37858 | ||
| 40‐74 | 38942 | 37675 | ||
| 40‐49 | 10312 | 10625 | ||
| 40‐49 | data not available | data not available | ||
| 40‐49 | 10262 | 10573 | ||
| 40‐49 | 10240 | 10411 | ||
| Stockholm | 40‐64 | 40318 | 19943 | |
| 40‐65 (sic) | 38525 | 20651 | ||
| 40‐64 | 40318 | 19943 | ||
| 40‐69 | 39139 | 20978 | ||
| 40‐49 | data not available | data not available | ||
| 40‐49 | 14842 | 7103 | ||
| 40‐49 | 14185 | 7985 | ||
| 40‐49 | 14303 | 8021 | ||
| Göteborg | 40‐59 | 20724 | 28809 | |
| 39‐59 | 21650 | 29961 | ||
| 40‐59 | 21000 | 29200 | ||
| 40‐49 | 10821 | 13101 | ||
| 39‐49 | 11724 | 14217 | ||
| 40‐49 | 10888 | 13203 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Deaths ascribed to breast cancer, 7 years follow up Show forest plot | 11 | 616327 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.72, 0.90] |
| 1.1 Adequately randomised trials | 4 | 292958 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.79, 1.09] |
| 1.2 Suboptimally randomised trials | 7 | 323369 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.61, 0.83] |
| 2 Deaths ascribed to breast cancer, 13 years follow up Show forest plot | 9 | 599090 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.74, 0.87] |
| 2.1 Adequately randomised trials | 4 | 292153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.79, 1.02] |
| 2.2 Suboptimally randomised trials | 5 | 306937 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.67, 0.83] |
| 3 Deaths ascribed to breast cancer, 7 years follow up, women below 50 years of age (Malmö 55) Show forest plot | 9 | 356368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.04] |
| 3.1 Adequately randomised trials | 3 | 227333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.78, 1.14] |
| 3.2 Suboptimally randomised trials | 6 | 129035 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.63, 1.05] |
| 4 Deaths ascribed to breast cancer, 7 years follow up, women at least 50 years of age (Malmö 55) Show forest plot | 7 | 261044 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.62, 0.85] |
| 4.1 Adequately randomised trials | 2 | 65625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.64, 1.20] |
| 4.2 Suboptimally randomised trials | 5 | 195419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.56, 0.81] |
| 5 Deaths ascribed to breast cancer, 13 years follow up, women below 50 years of age Show forest plot | 8 | 329511 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.73, 0.96] |
| 5.1 Adequately randomised trials | 3 | 218697 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.73, 1.03] |
| 5.2 Suboptimally randomised trials | 5 | 110814 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.64, 0.98] |
| 6 Deaths ascribed to breast cancer, 13 years follow up, women at least 50 years of age Show forest plot | 7 | 268874 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.69, 0.86] |
| 6.1 Adequately randomised trials | 2 | 74261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.77, 1.15] |
| 6.2 Suboptimally randomised trials | 5 | 194613 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.62, 0.80] |
| 7 Deaths ascribed to any cancer, all women Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Adequately randomised trials | 3 | 132118 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.95, 1.10] |
| 7.2 Suboptimally randomised trials (unreliable estimates) | 3 | 195871 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.93, 1.06] |
| 8 Overall mortality, 7 years follow up Show forest plot | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Adequately randomised trials | 4 | 292958 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.94, 1.03] |
| 8.2 Suboptimally randomised trials (unreliable estimates) | 7 | 324977 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.96, 1.02] |
| 9 Overall mortality, 13 years follow up Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Adequately randomised trials | 4 | 292958 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.95, 1.03] |
| 9.2 Suboptimally randomised trials (unreliable estimates) | 4 | 244868 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.97, 1.01] |
| 10 Overall mortality, 7 years follow up, women below 50 years of age Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Adequately randomised trials | 2 | 211270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.90, 1.04] |
| 10.2 Suboptimally randomised trials (unreliable estimates) | 5 | 99656 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.98, 1.16] |
| 11 Overall mortality, 7 years follow up, women at least 50 years of age Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Adequately randomised trials | 1 | 39405 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.85, 1.20] |
| 11.2 Suboptimally randomised trials (unreliable estimates) | 4 | 161519 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.94, 1.00] |
| 12 Overall mortality, 13 years follow up, women below 50 years of age Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Adequately randomised trials | 3 | 219324 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.92, 1.04] |
| 12.2 Suboptimally randomised trials (unreliable estimates) | 3 | 61344 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.92, 1.10] |
| 13 Overall mortality, 13 years follow up, women at least 50 years of age Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Adequately randomised trials | 2 | 73634 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.95, 1.04] |
| 13.2 Suboptimally randomised trials (unreliable estimates) | 2 | 98261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.97, 1.02] |
| 14 Number of mastectomies and lumpectomies Show forest plot | 5 | 250479 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.26, 1.44] |
| 14.1 Adequately randomised trials | 3 | 132321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.22, 1.42] |
| 14.2 Suboptimally randomised trials | 2 | 118158 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.26, 1.61] |
| 15 Number of mastectomies Show forest plot | 5 | 250479 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.11, 1.30] |
| 15.1 Adequately randomised trials | 3 | 132321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.08, 1.32] |
| 15.2 Suboptimally randomised trials | 2 | 118158 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.06, 1.38] |
| 16 Number treated with radiotherapy Show forest plot | 2 | 100383 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.16, 1.50] |
| 16.1 Adequately randomised trials | 1 | 42486 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.04, 1.49] |
| 16.2 Suboptimally randomised trials | 1 | 57897 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.17, 1.69] |
| 17 Number treated with chemotherapy Show forest plot | 2 | 100383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.78, 1.19] |
| 17.1 Adequately randomised trials | 1 | 42486 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.39, 1.04] |
| 17.2 Suboptimally randomised trials | 1 | 57897 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.84, 1.34] |
| 18 Number treated with hormone therapy Show forest plot | 2 | 100383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.55, 0.96] |
| 18.1 Adequately randomised trials | 1 | 42486 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.60, 1.08] |
| 18.2 Suboptimally randomised trials | 1 | 57897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.12, 0.72] |
| 19 Mortality among breast cancer patients in the Two‐County study, 7 years follow up Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 Mortality from cancers other than breast cancer | 2 | 2063 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.42 [1.00, 5.85] |
| 19.2 Mortality from causes other than breast cancer | 2 | 2063 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.93, 2.04] |
| 20 Results for biased trial Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 20.1 Deaths ascribed to breast cancer, 7 years follow up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Deaths ascribed to breast cancer, 13 years follow up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.3 Deaths ascribed to breast cancer, 7 years follow up, younger women (below 50 years of age) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.4 Deaths ascribed to breast cancer, 7 years follow up, elderly women (at least 50 years of age) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.5 Deaths ascribed to breast cancer, 13 years follow up, younger women (below 50 years of age) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.6 Deaths ascribed to breast cancer, 13 years follow up, elderly women (at least 50 years of age) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.7 Overall mortality, 7 years follow up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.8 Number treated with radiotherapy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21 Number of cancers Show forest plot | 7 | 512246 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.23, 1.35] |
| 21.1 Adequately randomised trials (after 7‐9 years) | 4 | 292979 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.18, 1.34] |
| 21.2 Suboptimally randomised trials (before control group screen) | 3 | 219267 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.24, 1.44] |

