Communication des risques personnalisée pour une prise de décision éclairée concernant les tests de dépistage
Résumé scientifique
Contexte
On observe une tendance à l'implication croissante des patients dans les décisions de santé. Bien que le dépistage soit généralement perçu comme utile pour la santé de la population, des risques sont associés aux tests concernés. Le fait de parvenir à la fois à une bonne implication des consommateurs et à une prise de décision éclairée est désormais considéré comme un objectif important pour les programmes de dépistage. Les estimations personnalisées des risques se sont révélées des méthodes efficaces de communication des risques.
Objectifs
Evaluer les effet de la communication des risques personnalisée sur la prise de décision éclairée par les personnes passant des tests de dépistage. Nous avons également évalué les composantes individuelles qui constituent des décisions éclairées.
Stratégie de recherche documentaire
Deux auteurs ont effectué des recherches dans le registre Cochrane des essais contrôlés (CENTRAL, The Cochrane Library, numéro 3, 2012), MEDLINE (OvidSP), EMBASE (OvidSP), CINAHL (EbscoHOST) et PsycINFO (OvidSP) sans restrictions de langue. Nous avons effectué des recherches de 2006 à mars 2012. La période pour les recherches précédentes s'étendait de 1989 à décembre 2005 pour PsycINFO et de 1985 à décembre 2005 pour les autres bases de données. Pour la version originale de cette revue, nous avons également effectué des recherches dans CancerLit et Science Citation Index (mars 2001). Nous avons par ailleurs examiné les bibliographies et avons procédé à des recherches de références dans les études incluses et d'autres revues systématiques du domaine pour identifier les études manquées lors de la recherche initiale.
Critères de sélection
Les essais contrôlés randomisés intégrant une intervention ayant un « élément de communication des risques personnalisée » pour les personnes subissant des procédures de dépistage et rendant compte de mesures des décisions éclairées, mais aussi de critères de jugement cognitifs, affectifs ou comportementaux concernant la décision de ces personnes de passer ou non un test de dépistage.
Recueil et analyse des données
Deux auteurs ont, de manière indépendante, évalué le risque de biais de chaque essai inclus et extrait les données. Nous avons extrait des données concernant la nature et le contexte des interventions et les données relatives aux critères de jugement pertinents. Nous avons utilisé des méthodes statistiques standard pour combiner les données à l'aide du logiciel RevMan version 5, notamment une analyse selon différents niveaux de détail de la communication des risques personnalisée, différentes conditions de dépistage et des études basées uniquement sur les participants à haut risque plutôt que sur les personnes présentant un risque « moyen ».
Résultats principaux
Nous avons inclus 41 études portant sur 28 700 personnes. Dix‐neuf nouvelles études ont été identifiées dans cette mise à jour, lesquelles s'ajoutent aux 22 études incluses dans les deux précédentes versions de la revue. Trois études mesuraient la décision éclairée concernant le passage d'un test de dépistage après une communication des risques personnalisée dans le cadre de leur intervention. Ces trois études présentaient un faible risque de biais et ont fourni des preuves solides indiquant que les interventions amélioraient la prise de décisions éclairées, bien que les résultats aient été hétérogènes. Globalement, 45,2 % (592/1309) des participants qui recevaient des informations personnalisées sur les risques ont fait des choix éclairés contre 20,2 % (229/1135) des participants qui recevaient des informations générales concernant les risques. Les rapports de cotes (RC) combinés concernant la décision éclairée ont été de 4,48 (intervalle de confiance (IC) à 95 % 3,62 à 5,53 pour les effets fixes) et de 3,65 (IC à 95 % 2,13 à 6,23 pour les effets aléatoires). Neuf études ont mesuré l'accroissement des connaissances au moyen d'échelles différentes. Toutes ces études ont montré un accroissement des connaissances avec une communication des risques personnalisée. Dans trois études, les interventions ont montré une tendance à une plus grande exactitude de la perception des risques, mais les preuves étaient de qualité médiocre. Quatre études sur six ont rapporté des changements non significatifs de l'anxiété après une communication personnalisée des risques aux participants. Globalement, on a observé une faible diminution, non significative, des scores d'anxiété. La plupart des études (32/41) ont mesuré le passage de tests de dépistage après les interventions. Nos résultats (RC 1,15 (IC à 95 % 1,02 à 1,29)) constituent des preuves de qualité médiocre, cohérentes avec un léger effet, indiquant qu'une communication des risques personnalisée, dans laquelle un score de risque était fourni (6 études) ou les participants étaient informés de leur niveau de risque (6 études), augmente le passage de tests de dépistage.
Conclusions des auteurs
Des preuves solides, issues de trois essais, indiquent que les estimations des risques personnalisées incluses dans les interventions de communication pour les programmes de dépistage améliorent les choix éclairés. Cependant, les preuves de l'augmentation des recours à ces tests de dépistage avec des interventions semblables sont faibles et on ignore si cette augmentation est associée à des choix éclairés. Les études ont inclus des programmes de dépistage divers. Par conséquent, les données de cette revue ne nous permettent pas d'établir des conclusions quant aux meilleures interventions pour apporter une communication des risques personnalisée afin d'améliorer les décisions éclairées. Les résultats sont dominés par des découvertes issues du domaine de la mammographie et du cancer colorectal. Il faut donc être prudent en établissant des généralités à partir de ces résultats, et en particulier pour les domaines cliniques autres que la mammographie et le dépistage du cancer colorectal.
Résumé simplifié
Communication des risques personnalisée pour une prise de décision éclairée concernant les tests de dépistage
Le dépistage est généralement considéré comme une méthode efficace et sûre pour prévenir les maladies, mais il existe des risques et des inconvénients liés aux procédures utilisées. Il est important que la personne passant un test de dépistage connaisse les risques de la maladie, mais également les raisons pour lesquelles elle est concernée. Ces informations permettraient aux personnes de prendre une décision éclairée quant au fait de suivre une procédure de dépistage.
Dans cette revue, nous avons examiné des études qui fournissaient des informations personnalisées sur les risques pour chaque participant(e), de façon qu'il (elle) puisse décider ou non de passer un test de dépistage en fonction de son profil de risque personnel. Nous avons trouvé 41 études qui portaient sur un total de 28 700 participants et leur fournissaient de telles informations personnalisées sur les risques. Nous avons intégré les résultats de toutes ces études et avons découvert que lorsqu'un tel profil de risque était inclus dans l'intervention, les participants prenaient des décisions plus éclairées quant au dépistage comparé aux personnes recevant des informations plus générales concernant les risques. Globalement, 45,2 % (592/1309) des participants qui recevaient des informations personnalisées sur les risques ont fait des choix éclairés contre 20,2 % (229/1135) des participants qui recevaient des informations générales concernant les risques.
Nous avons également découvert que ces interventions semblaient accroître les connaissances et pouvaient améliorer l'exactitude de la perception des risques chez les participants aux essais. Cependant, elles n'ont pas affecté de façon significative l'anxiété des participants. Les résultats ont également indiqué que le fait de fournir des informations personnalisées sur les risques de la maladie avait entraîné une légère augmentation du nombre de personnes ayant suivi la procédure de dépistage. Les résultats de cette revue sont dominés par des études dépistant le cancer du sein et le cancer colorectal. Il est nécessaire d'être prudent dans l'application de ces résultats à d'autres types de dépistage.
Authors' conclusions
Summary of findings
| Patient or population: patients with informed decision making about taking screening tests | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk8 | Corresponding risk | |||||
| Control | Personalised risk communication versus general risk information | |||||
| Informed decision ‐ Numerical risk and categorised risk combined | 202 per 1000 | 480 per 1000 | OR 3.65 | 2444 | ⊕⊕⊕⊕ | Random‐effects estimate presented due to significant heterogeneity |
| Uptake of screening test ‐ Numerical and categorised risk combined | 532 per 1000 | 566 per 1000 | OR 1.15 | 6442 | ⊕⊕⊝⊝ | Fixed‐effect estimate |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence (we have not assessed publication bias for any of the groups) | ||||||
| 1 MMIC: Multi‐dimensional Measure of Informed Choice 8Control risk was used as baseline risk, due to lack of studies that measure this in detail to be presented as baseline risk for the population. | ||||||
| Patient or population: patients with informed decision making about taking screening tests | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk10 | Corresponding risk | |||||
| Control | Personalised risk communication versus general risk information | |||||
| Knowledge regarding screening test/condition concerned ‐ calculated risk score (numerical) versus general information | The mean knowledge regarding screening test/condition concerned ‐ calculated risk score (numerical) versus general information in the intervention group was | 588 | ⊕⊕⊕⊝ | SMD 0.4 (0.23 to 0.56) Fixed‐effect estimate | ||
| Knowledge regarding screening test/condition concerned ‐ calculated risk score (categorised) versus general information | The mean knowledge regarding screening test/condition concerned ‐ calculated risk score (categorised) versus general information in the intervention group was | 260 | ⊕⊕⊝⊝ | SMD 0.57 (0.32 to 0.82) Fixed‐effect estimate | ||
| Knowledge regarding screening test/condition concerned ‐ personal risk factor list versus general information | The mean knowledge regarding screening test/condition concerned ‐ personal risk factor list versus general information in the intervention group was | 838 | ⊕⊕⊕⊕ | SMD 0.89 (0.75 to 1.04) Fixed effect estimate | ||
| Knowledge regarding screening test/condition concerned ‐ calculated risk score (numerical) versus general information | 244 per 1000 | 457 per 1000 | OR 2.6 | 1413 | ⊕⊕⊕⊕ high4,6,13,14 | Random‐effects estimate |
| Knowledge regarding screening test / condition concerned ‐ personal risk factor list v general information | 166 per 1000 | 586 per 1000 | OR 7.13 | 2107 | ⊕⊕⊕⊕ | Fixed‐effect estimate |
| Accurately‐perceived risk | 225 per 1000 | 324 per 1000 | OR 1.65 | 1264 | ⊕⊕⊝⊝ | Random‐effects estimate |
| Anxiety ‐ all groups | The mean anxiety in the intervention groups was | 1848 | ⊕⊝⊝⊝ | SMD ‐0.13 (‐0.29 to 0.03) Random‐effects estimate | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence (we have not assessed publication bias for any of the groups) | ||||||
| 1 This study was high risk for reporting bias. Four risk of bias items were low risk and four were unclear risk. Quality down graded by a point. 10Control risk was used as baseline risk due to lack of studies that measure this in detail to be presented as baseline risk for the population. 11Sample size less than the Optimal Information size (OIS). Quality downgraded by one point. 12Both studies were of low risk of bias and hence not downgraded. 13Significant heterogeneity among studies but all studies have same direction of effect and hence quality not down graded. 14Not downgraded for indirectness of evidence. | ||||||
Background
Risk communication is an integral part of modern healthcare practice. It is important in various strategies to help individuals make the right choices. Be it a public screening programme or a matter of starting a new treatment, providing evidence‐based risk and benefit information to patients, and ensuring they understand it, forms the cornerstone of informed decision making (Naik 2012). However, understanding is still limited of how best to present and discuss risks and benefits of health care in general, and screening in particular, for an individual.
Screening: what are the issues around policies and practice?
Screening is defined as "the systematic application of a test or inquiry, to identify individuals at sufficient risk of a specific disorder to warrant further investigation or direct preventive action, amongst persons who have not sought medical attention on account of symptoms of that disorder"(UKNSC 1998).
Many tests and procedures form part of screening programs ‐ programs with the objective of sorting out apparently well persons who probably have a disease, from those who probably do not. They may only highlight a risk of disease, and are not intended to definitely diagnose a disease or condition. Screening is a complex event that can have various outcomes for the participant, some of which can cause unnecessary distress (Collins 2011). Although screening programmes are generally seen as an effective measure to reduce mortality and morbidity in the target populations, there are several issues surrounding them. The evolution of evidence regarding certain screening programmes has led to fierce debate about actual benefits and harms (Bewley 2011; Hackshaw 2012). A recent review of breast cancer screening for example, suggests that it is unclear whether such screening does more harm than good, and that balanced, evidence‐based risk‐benefit information should be provided to help women make an informed decision about undergoing screening (Gøtzsche 2011). Directly harmful effects of screening include: complications arising from the investigation, adverse effects of treatment, unnecessary treatment of persons with true‐positive test results who have inconsequential disease, adverse effects of labelling or early diagnosis, anxiety generated by the investigations and treatment, and the costs or inconvenience incurred during investigations and treatment (Barratt 1999). Other harms, such as failure to detect disease, only apply to those with the condition. In either of these scenarios, Raffle 2001 warns that "harm to uninformed participants leads to anger, bitterness, and potentially to litigation". Furthermore screening can have significant social and psychological costs which are difficult to evaluate and quantify (Stewart‐Brown 1997).
These well recognised potential harms have led to increasing debate about the policy and practice of screening. A review of breast cancer screening policy has been proposed in the United Kingdom (UK), prompted by concerns about the accuracy and transparency of information provided to women about the benefits and harms of screening (Richards 2011). The American Cancer Society has also highlighted the confusion created by screening guidelines, and proposes providing the public with clear guidance about the benefits, limitations, and harms associated with taking a screening test (Brawley 2012).
Informed decision making
An informed decision is defined as one where "a reasoned choice is made by a reasonable individual using relevant information about the advantages and disadvantages of all the possible courses of action, in accord with the individual’s beliefs" (Bekker 1999).
The UK national guidance confirms that the purpose of information about screening is to ensure that participants can make a fully informed choice about whether to take the test (GMC 1998). The UK National Screening Committee some time ago proposed a new definition for screening to incorporate the importance of informed choice in screening:
"a public health service in which members of a defined population, who do not necessarily perceive they are at risk of, or are already affected by, a disease or its complications, are asked a question or offered a test to identify those individuals who are more likely to be helped than harmed by further tests or treatment to reduce the risk of disease or its complications" (UKNSC 2000)
Jepson and colleagues reiterate this policy and recommend evaluating screening programmes to ensure that people invited for screening are genuinely making informed choices, and explore how comprehensive information affects other variables such as uptake, cost effectiveness, and satisfaction (Jepson 2005).
How can informed decisions be measured?
Briss 2004 provides a conceptual framework for informed decision making which includes: knowledge, participation in decision making at a personally desirable level, and consistency between the decision and individual preferences or values. A systematic review by Mullen et al, on measures of informed decision making in cancer screening, observed no single study measuring all of these three aspects (Mullen 2006). They recommended the development and use of validated measures of informed decision making and the use of theoretical frameworks for such screening studies.
Marteau and colleagues have proposed the multi‐dimensional measure of informed choice, which has been validated for Down’s syndrome screening (Michie 2002; Marteau 2001). This is based on assessing the consistency between knowledge, attitudes to tests and choices of uptake of tests. To date, few studies in screening have used this scale as a measure for informed choice (Nagle 2008; Smith 2010; Steckelberg 2011). This scale is promising and could form the basis for development of a universal measure of informed choice for uptake of screening tests. Further research is needed to validate this scale in other areas. Furthermore, increasingly the outcomes described in the literature include 'affective' measures such as satisfaction with the decision made, and 'decisional conflict'. This includes whether an individual feels a decision is consistent with their personal values, and certainty about making the right decision (Edwards 1999a; Holmes‐Rovner 1996; Llewelyn‐Thomas 1995).
Description of the intervention
What is tailoring and why is personalising risk information important?
Tailored health communication refers to providing information to someone based on characteristics that are unique to that person. Kreuter et al define tailoring as: "Any combination of information or change strategies intended to reach one specific person, based on characteristics that are unique to that person, related to the outcome of interest and have been derived from an individual assessment" (Kreuter 1999a; Kreuter 1999b).
Tailoring could address any characteristics such as the person’s educational background, cultural orientation, and general level of comprehension. Many studies have adopted a theoretically‐driven approach and have tailored their interventions on behavioural constructs that are based on behaviour change models (Noar 2007), for example the Transtheoretical Model (Prochaska 1992; Prochaska 1997) and Heath Belief Model (Becker 1974; Rosenstock 1988; Rosenstock 2000).
A high rate of participation is expected in most established screening programmes to ensure that they run effectively. Health authorities may employ a variety of strategies to promote screening and enhance uptake amongst the target populations. Some screening programmes provide information which is personally tailored and more relevant to the person in question. Others provide information about population or 'average' risks of contracting a disease as a basis for discussion or decision making about undergoing screening, or perhaps simply to try to motivate people to attend for tests which are perceived by authorities to be in the person's or population's best interests (Slaytor 1998). Such tactics may be seen as questionable, working against the spirit of informed decision making and consumer autonomy, and amounting to a disregard for the principles of medical ethics and good practice (Beauchamp 2001). Foster and Anderson (Foster 1998) criticise the implementers of the UK's National Cervical Screening Programme, including general practitioners, for their persuasive tactics in getting patients to comply.
According to the Health Belief Model one of the key determinants of health‐related behaviour is perceived threat (Becker 1974). This is made up of two components: the individuals perceiving themselves as susceptible to the disease, and the perceived severity of the disease. Tailoring information using an individual’s specific risk factors is what we describe as 'personalised risk communication', sometimes also called 'individualised risk communication'. This may be based on the individual's own risk factors for a condition (such as age or family history). In some cases it is calculated from an individual's risk factors using formulae derived from epidemiological data, with the information presented as an absolute risk or as a risk score, for example the Gail score for the risk of breast cancer (Gail 1989) and Q‐risk score for cardiovascular risk (Hippisley‐Cox 2008). Risk information may also be categorised into, for example, high, medium or low risk groups. It may be less detailed, involving a listing, for example, of a person's risk factors as a focus for discussion and intervention.
How the intervention might work
Evidence suggests that the format in which risk information is presented affects patients’ understanding and perception of risk (Ahmed 2012). It is assumed that tailored messages are perceived as more relevant to an individual, and are therefore better processed and understood (Barratt 2005). Foster and Anderson (Foster 1998) have emphasised the importance of providing good‐quality information to those undergoing cervical screening procedures. They stress that women should be made aware of the risks (discomfort, over‐treatment of abnormal smears) and limitations (false positives; false negatives), as well as the benefits of the test. Further, they also point out that people have different risks of contracting the cervical cancer, based on factors such as age, sexual activity, social class and smoking. A systematic review of studies with interventions designed to provide tailored information on cancer risk and screening method has shown that personally‐tailored risk information improved subjects' knowledge and realistic perception of cancer risk, compared to the provision of generic risk information only (Albada 2009). Noar et al found in their meta‐analytic review that tailored messages outperformed comparison messages in affecting health behaviour change, lending support to claims made by other authors of narrative reviews that tailoring does in fact 'work' (Noar 2007).
There is recognition that uptake of tests per se is not necessarily desirable (for example, prostate specific antigen screening and antenatal cystic fibrosis screening are contentious areas). Also some screening tests are only recommended for those who are at high risk for disease susceptibility, for example genetic testing for breast and ovarian cancer (US PSTF 2005). Making an informed choice about taking screening tests, and adherence to the consumer's chosen option, are widely regarded as more desirable goals for risk communication (Edwards 1999a; Liao 1996; Sarfati 1998).
In this light, providing individualised risk information may seem to be more pertinent than providing generic risk information about the disease for which the screening intervention is designed. Personalised risk communication is more likely to be useful in decision making about whether or not to participate in screening, and individuals who perceive their personal risk as high may feel more susceptible to the disease in question. They may weigh this risk information with the harmful effects of screening, leading to an informed choice to take up the test. Similarly, those who consider themselves at low or no risk may opt out of the screening programme.
Why it is important to do this review
Public health impact, the scope and limitations
The UK National Health Service incorporates 11 different screening activities within its national health programme (UK National Screening Portal). In England alone, 2.24 million women were sent invitations to attend breast cancer screening in 2010‐11 (NHS Information Centre 2011a). Invitations to attend cervical cancer screening were sent to 1.79 million women (NHS Information Centre 2011b). Significant volumes of screening procedures can also be expected to be taking place within breast and cervical cancer programmes and other screening programmes globally. People invited for such screening procedures should make informed and balanced choices. However, focusing narrowly on this outcome may ignore conflicting implications for uptake, cost‐effectiveness and other important public health goals. Stakeholders and policy makers must carefully consider these factors. We attempt to organise such evidence, wherever available, in this systematic review. Further research would certainly be necessary to place the findings of this review in context with existing or planned screening programmes.
Other reviews in the field
Risk communication itself may be subdivided by the dyad involved (for example between agencies and the public, or from individual communicators), as well as according to the nature of the risk, which may be familiar or non‐familiar (Vlek 1987). Reviews of 'mass' risk communication, primarily from the environmental health discipline (Covello 1986; Fischhoff 1979; Keeney 1986), and from narrow clinical fields such as familial cancer (Bottorff 1996), are available. Briss 2004 also reviewed interventions that sought to promote informed decisions about cancer screening, but not through 'personalising' risk information. In addressing the effects of personalised methods of risk communication, this review focuses on risk communication which is provided, used and evaluated in healthcare encounters with individuals, couples, or immediate families (for example, parents of young children). As such, the review is broader than previous clinical risk communication reviews (Bottorff 1996) but still aims to be relevant to the community and the clinical needs of healthcare professionals across a range of disciplines. It focuses on the domain of healthcare screening. In contrast, Albada and colleagues (Albada 2009) in their systematic review have reviewed interventions that provided tailored information specifically about cancer risk and screening. Information in the included studies was tailored to the personal characteristics related to behaviour change theories or constructs, and to the participant’s individualised risks. Their main outcome measures were risk perception, cancer knowledge and screening behaviour. A recent systematic review (Stacey 2011) evaluated randomised controlled trials (RCTs) of decision aids that provided information about treatment or screening options and their associated outcomes, compared to standard care. Decision attributes, behavioural, health, and health system effects were studied as outcome measures. Several other reviews have sought to consolidate evidence on the most effective means of increasing screening uptake (Bonfill 2001; Everett 2011; Flight 2004).
This review updates the 2006 version, which identified 22 relevant studies (Edwards 2006).
Objectives
We primarily compare the effects of personalised and general risk communication interventions in promoting an informed decision about participating in health screening. We also compare the effects of these interventions on people's cognitive, affective and behavioural outcomes. These include their knowledge, risk perception, satisfaction with decision making and decisional conflict, emotional well‐being and behaviour (i.e. taking or intending to take screening tests).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Participants were people facing real life decisions (not hypothetical exercises) about whether to undergo screening. They were individuals making decisions alone or on another's behalf (for example, for a young child), or couples making decisions together.
The screening activities involved an investigation performed by a health professional. Examples of these include:
-
mammography;
-
cervical 'Papanicolaou' smears;
-
colorectal cancer screening;
-
prostatic cancer screening (PSA test);
-
antenatal screening (including Down's syndrome, neural tube defects and other fetal anomalies);
-
genetic screening (including breast cancer gene testing);
-
high cholesterol/cardiovascular risk screening;
-
neonatal screening (including cystic fibrosis and Duchenne testing);
-
skin cancer screening;
-
lung cancer screening.
We excluded studies if they described only:
-
mass communication; or
-
military or school or prison‐based interventions (where people are less free to choose than in other healthcare settings).
Types of interventions
The interventions comprise one of the three levels of personalised risk information:
-
individualised risk score or individual actual risk information (i.e. absolute or relative risk information); or
-
categorisations of risk status based on these estimates (for example, high, medium or low risk status); or
-
discussion of personal risk factors relevant to the screening decision (that is, the individual's own characteristics are taken into account in assessing their actual risk or elevated risk status relative to others).
The interventions address decision making about screening tests; that is, testing whose objective is the presumptive identification of unrecognised disease or defect (including genetic markers for disease) by the application of tests or other procedures, which can be applied rapidly. These tests are intended to sort out apparently well people who may have a disease from those who probably do not, and they are not intended to be diagnostic (Rose 1978; Wilson 1968).
The risk communication intervention could come before screening, at the time of the screening intervention, or at the time of counselling or promotion of screening. Personalised risk communication could be delivered via oral, written, video, or electronic (e.g. Internet or CD‐ROM) media.
Personalised risk information is compared to generalised risk communication interventions, including: population risk estimates, general information on risk factors, or general encouragement to acknowledge risks or change risk behaviour.
We excluded studies which simply evaluated health education/promotion to reduce risk factors or increase adherence to screening, without discussion of risks and benefits of undergoing or not undergoing screening; or if general rather than personalised risk communication was the main basis of the intervention.
Types of outcome measures
Primary outcomes
Measures of an informed decision. An informed decision is defined as one where "a reasoned choice is made by a reasonable individual using relevant information about the advantages and disadvantages of all the possible courses of action, in accord with the individual’s beliefs" (Bekker 1999). An example is the Multidimensional Measure of Informed Choice (MMIC), proposed by Marteau and colleagues, based on assessing the consistency between knowledge, attitudes to tests and choices of uptake of tests. This has been validated for Down's syndrome screening (Michie 2002; Marteau 2001).
Secondary outcomes
Other outcome measures focus on identifying changes in any of the following key areas of informed decision making (Llewelyn‐Thomas 1995; Edwards 1999a), if appropriate data were available:
-
'cognitive' outcomes: knowledge of risk, accurate risk perception;
-
'affective' outcomes: anxiety/emotional well‐being, satisfaction with decisions made, decisional conflict, anxiety, intention to take up screening;
-
behavioural outcomes: uptake of tests, adherence to choice regarding screening test, 'appropriate' uptake;
-
health status outcomes: specific status measures or quality of life measures such as SF‐36;
-
economic outcomes: cost of intervention.
Search methods for identification of studies
Electronic searches
Two authors searched:
-
the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 3 2012),
-
MEDLINE (OvidSP) (2005 to March 2012),
-
EMBASE (OvidSP) (2005 to March 2012),
-
CINAHL (EbscoHOST) (2005 to March 2012), and
-
PsycINFO (OvidSP) (2005 to March 2012).
We developed the search strategies from earlier work by Matthews et al (Matthews 1999) and the previous versions of this review (Edwards 2006; Edwards 2003b; Edwards 2003c), as well as that used by Jepson et al (Jepson 2000) in their systematic review. The strategies comprised three layers of search terms ‐ keywords and medical subject headings (MeSH) ‐ aimed at identifying articles about screening involving counselling/education or risk specifically. The goal was for high recall (sensitivity) of literature in the field of risk communication in screening (which may therefore be at the expense of precision (specificity). We searched from 2006 to March 2012 for all databases. We tailored search strategies to the relevant databases, and as appropriate used mainly subject headings (for example, MEDLINE, CINAHL) or the equivalent translated text words (for example, EMBASE). We used 'explode' functions on all subject headings. We have not applied language restrictions to the searches.
The date ranges for the previous searches (Edwards 2006) were from 1989 to December 2005 for PsycINFO and from 1985 to December 2005 for all other databases.
Overall the search date range for the CENTRAL, MEDLINE, EMBASE and CINAHL databases was from 1985 to March 2012. For PsycINFO the overall date range was from 1989 to March 2012.
For the first publication of this review (Edwards 2003b; Edwards 2003c) we also searched CancerLit (1985 to 2001) and Science Citation Index Expanded (searched March 2002). Strategies are available from the lead author upon request.
We present all search strategies in Appendices.
Searching other resources
We also reviewed the reference lists and conducted citation searches of included studies and other systematic reviews in the field (Albada 2009), to identify other potentially‐relevant studies.
Data collection and analysis
Selection of studies
Two authors (GN and HA) independently made an initial selection of potentially‐eligible studies from search outputs (titles and abstracts). Disagreements were resolved by discussion. In cases of doubt about relevance to this review, we retrieved papers in full for final decision making. Papers rejected at this stage were circulated to other review authors to review the decision, and if finally excluded, were listed in the table 'Characteristics of Excluded Studies' with a reason for exclusion given.
Data extraction and management
Two authors (GN and HA) independently extracted and collated on a template key information from all included studies. Data extracted included country of origin, health professional group involved, screening program and patient group involved, setting, sample size, and key outcomes. We also extracted data on differences in baseline risk, for assessment of effect modifiers. We checked consistency of assessment by weighted kappa agreement scores.
We also collected data on the nature/design of the intervention. This included: the type of personalised risk communication in the main intervention: estimate or calculation of numerical risk or risk score; estimated or calculated risk categorised as, for example, high, medium or low level of risk for consumer; or listing of personal risk factors without estimate or calculation of risk level. The nature of the intervention also covered whether specific counselling or behavioural change strategies, or both, were included in the risk communication intervention.
Assessment of risk of bias in included studies
We extracted data to assess the risk of bias of the included studies. Two authors independently assessed all studies against the Risk of Bias tool derived from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved the differences in assessment of the risk of bias included studies by discussion.
The principal elements used for this review are:
-
Random sequence generation (selection bias): Studies that describe the random sequence generation were marked as low risk. If there was no mention of this then the studies were marked as unclear. Quasi‐randomised trials were marked as high risk.
-
Allocation concealment (selection bias): Studies that describe allocation concealment were marked as low risk. If there was no mention of this then the studies were marked as unclear. Studies were marked as high risk if an allocation concealment procedure was clearly not followed.
-
Blinding of participants and personnel (performance bias): Studies that describe blinding in detail were marked as low risk. If there was no mention of this then the studies were marked as unclear. Studies were marked as high risk if a blinding procedure was clearly not followed or the trialists described the participants and personnel in the study as unblinded.
-
Blinding of outcome assessment (detection bias): Studies that describe blinding in detail were marked as low risk. If there was no mention of this then the studies were marked as unclear. Studies were marked as high risk if a blinding procedure was clearly not followed or the trialists described the outcome assessment procedure as unblinded.
-
Incomplete outcome data (attrition bias): Studies were marked as low risk if there was a low attrition rate (less than 20%) or if an intention‐to‐treat principle was adopted. If there was no description of attrition in the study, this element was marked as unclear. If no adjustments were made despite a significant drop out rate studies were marked as high risk for attrition bias.
-
Selective reporting (reporting bias): Studies were marked as low risk if the protocols were available and all outcomes listed in it were addressed. They were marked as unclear if protocols were not available. High risk status was assigned if there was clear evidence of selective reporting based on the outcomes mentioned in the methods section of the included studies.
-
Other biases, e.g. baseline comparability, measure against contamination and funding for the screening test: Studies that describe the components in detail were marked as low risk. If there was no mention of this then the studies were marked as unclear. Studies were marked as high risk if the components were clearly not addressed, or demonstrated biases.
We report the risk of bias assessment in Figures and the table Characteristics of included studies. We conducted the sensitivity analysis by excluding studies that were considered as high risk for each risk of bias item mentioned above and then studying how this affected the results. We have also incorporated these assessments whilst rating the quality of the evidence using the GRADE working group's recommendations (Guyatt 2008).
Measures of treatment effect
Dichotomous data
We present dichotomous data as odds ratios (ORs) with 95% confidence intervals (CIs), calculated from absolute change in numbers between the intervention and control groups.
Continuous data
We present continuous data as mean differences (MDs) with 95% CIs, calculated from the mean change (and the standard deviation) between the intervention and control groups. When the same outcomes were measured on different scales we used standardised mean differences (SMDs) when combining data in the meta‐analysis.
Unit of analysis issues
We included studies randomised at individual and cluster level. For studies that were cluster randomised, we used outcome measures adjusted for clustering in the analysis.
Dealing with missing data
We contacted authors to procure missing data. If data were still unavailable, these studies were still included and reported in the narrative. We did not conduct an intention‐to‐treat (ITT) analysis and data were analysed as reported. We documented loss to follow‐up and assessed it as a source of potential bias. We conducted sensitivity analysis by excluding studies that were considered at high risk for attrition bias.
Assessment of heterogeneity
We used the I2 statistic to assess heterogeneity (Deeks 2011). We expected heterogeneity of the studies in terms of the screening tests addressed, participants and design of intervention. We used a fixed‐effect model to present pooled data where heterogeneity was of low or moderate significance i.e. I2 < 60%. Results from a random‐effects model (which gives a more conservative confidence interval on the estimate of effect size) were examined and presented in cases (in addition to fixed‐effect estimates), where this was of substantial significance i.e. I2 = 60% to 79%. We did not pool the data if there was considerable heterogeneity between the studies i.e. I2 = / > 79%.
Data synthesis
Studies in this review derive from a heterogeneous group of screening programmes. Nevertheless the essential characteristic of the risk communication interventions (personalised elements) rendered data combination appropriate. We first examined studies for narrative synthesis. We then used standard statistical methods described in the Cochrane Handbook (Deeks 2011) to combine data. Data to be studied were entered and meta‐analysed in the statistical software package incorporated within RevMan 5. Outcome data extracted comprised the absolute changes in numbers between groups (for dichotomous variables), and the mean change and standard deviation of the mean change (for continuous variables). From these, we calculated the odd ratios (ORs) for dichotomous variables, and mean differences (MDs) for continuous outcomes. When the same outcomes were measured on different scales we used SMDs when combining data in the meta‐analysis. For cluster randomised studies, we used the generic inverse variance method to incorporate the adjusted ORs and the MDs. We also used the generic inverse variance method to combine studies where the raw data were not available, despite contacting the authors for further information. We recorded the statistical significance of results for all analysed outcomes. Insufficient data were provided in many studies to enable (raw) data entry into RevMan 5.
Subgroup analysis and investigation of heterogeneity
The categorisation of personalised risk communication into three levels of detail given to consumers (numerical risk, categorised risk and listing of personal risk factors) was examined for evidence of a different effect at each level. We also tested for heterogeneity within subgroups.We expected the interventions to be spread across a wide range of screening programmes and populations. Hence we also examined important effect modifiers on the changes in outcomes demonstrated, examining in particular, if the data were available, for the influence of: screening program or condition, setting, professional group involved, type of intervention, differences in baseline risk, high risk status of target population for screening, age, gender, educational level and the funding for the screening procedure. We then analysed different sections of the data, for example according to different conditions for screening, or studies based only on ‘high risk’ participants rather than people at ‘average risk’.
Sensitivity analysis
We included all studies in the meta‐analysis where possible. We performed a series of sensitivity analyses to investigate how the results changed when studies with greater risk of bias were excluded in each risk of bias category.
Results
Description of studies
Results of the search
Twenty‐two studies were already included in the last version of the review (Edwards 2006). From the new searches (2006 onwards), we identified 8371 publications. From these, we excluded 8025 publications after we examined the titles and abstracts. We retrieved 346 potentially‐relevant studies in full‐text for detailed evaluation. Of these, 15 new publications met our inclusion criteria. We also undertook a manual follow‐up of references from key publications and journals, and of key authors. We identified a systematic review by Albada and colleagues (Albada 2009) as being highly relevant to this review. Four studies were added by hand searching reference lists of included studies in the Albada review, bringing the total of newly‐included studies to 19. In total this updated review includes 41 studies. (See Figure 1).

Study flow diagram. Search dates from 2006 to March 2012.
Included studies
We describe the main characteristics of the 41 studies, including participants, interventions and outcomes measured, in the table Characteristics of included studies. All were published in English. Thirty‐four were from the USA, four from Australia, one from Canada, one from Germany and one from the UK. There appeared to be increasing interest in the subject over time, with no studies published in the 1980s, 14 studies published in the 1990s and 27 published after 1999. The studies varied in size, with 4 studies involving less than 200 patients, 19 studies involving between 200 and 500 patients, 9 studies involving 500 to 1000 patients and 9 studies involving over 1000 patients.
All included studies were RCTs. Five studies used cluster randomisation (Geller 2006; Glazebrook 2006; Manne 2009; Manne 2010; Nagle 2008), of which one study reported an insignificant cluster effect (Manne 2009). We contacted six authors of included studies, mainly for further clarification of data and to gather information on how the risks were personalised. Three authors responded.
Study setting, recruitment and duration
The patient population and screening programme varied. Most of the studies related to breast cancer screening followed by colorectal cancer screening. They are as illustrated in the table below:
| Screening Programme | Study IDs | Number of studies | |
| Breast cancer group | |||
| i. | Mammography | Bastani 1999; Bloom 2006; Bodurtha 2009; Bowen 2006; Champion 1994; Champion 1995; Champion 2000a; Champion 2002; Champion 2003; Champion 2007; Curry 1993; Jibaja‐Weiss 2003; Kreuter 1996; Lerman 1995; Rimer 2002; Saywell 1999; Schwartz 1999; Skinner 1994 | 18 |
| ii. | Genetic testing for breast cancer | 3
| |
| iii. | Mammography and genetic testing | 2 | |
| Colorectal cancer group | Glanz 2007; Lipkus 2005; Lipkus 2007b; Lee 1991; Manne 2009; Marcus 2005; Rawl 2008; Sequist 2011; Smith 2010; Steckelberg 2011; Trevena 2008 | 11 | |
| Skin cancer group | 3 | ||
| Cervical cancer group | 3 | ||
| Cholesterol testing | 2 | ||
| Prostate cancer group | 1 | ||
| Prenatal test group | 1 | ||
| High risk group
| Bastani 1999; Bloom 2006; Bowen 2002; Bowen 2006; Curry 1993; Geller 2006; Glanz 2007; Glazebrook 2006; Hutchison 1998; Lerman 1995; Lerman 1997; Lipkus 2005; Manne 2010; Myers 1999; Rawl 2008; Schwartz 1999; Skinner 2002 | 17 | |
Most of the studies (35 out of 41) were based in the community, 2 were in an out‐patient department setting and 4 were in primary care settings.
Time of outcome assessment varied from immediately after the intervention to 18 months post intervention. The mean time in months for outcome measurement was 7.26 and the median was 6 months.
Participants
The 41 studies in this review included 28,700 people. The number of people in each study varied from 140 to 3152 (median: 435). Recruitment of participants in most studies was done through clinic lists/GP lists/tumour registries (15 studies). Bowen 2002 had two methods of recruitment. The following table shows the method of recruitment for all studies included in the review.
| Recruitment | Study IDs | Number of studies |
| Clinic/GP lists | Bodurtha 2009; Campbell 1997; Champion 2002; Champion 2003; Champion 2007; Glazebrook 2006; Helmes 2006; Hutchison 1998; Kreuter 1996; Myers 1999; Nagle 2008; Saywell 1999; Sequist 2011; Skinner 1994; Trevena 2008 | 15 |
| Newspaper adverts/community networks | 3 | |
| Community health centre/GP co‐operatives/Lists from agencies/insurance companies | Bowen 2010; Champion 2000a; Curry 1993; Jibaja‐Weiss 2003; Lee 1991; Lipkus 2005; Marcus 2005; Rimer 2002; Smith 2010; Steckelberg 2011 | 10 |
| First degree relatives of index cancer patients/patients with cancer identified from tumour registries | Bastani 1999; Bloom 2006; Bowen 2002; Geller 2006; Glanz 2007; Lerman 1995; Lerman 1997; Manne 2009; Manne 2010; Rawl 2008; Schwartz 1999; Skinner 2002 | 12 |
| Random digit dialling | 2 |
Healthcare group involved
Healthcare groups delivering/developing the interventions were as follows:
| Healthcare Group | Study IDs | Number of studies |
| General practitioners/Family care physicians/Community clinicians | Campbell 1997; Jibaja‐Weiss 2003; Nagle 2008; Sequist 2011; Skinner 2002 | 5 |
| Trained counsellors/genetic counsellors/health educators/health advisors | Bloom 2006; Bowen 2002; Bowen 2006; Bowen 2010; Champion 2007; Geller 2006; Glanz 2007; Helmes 2006; Lerman 1997*; Lipkus 2005; Manne 2009; Manne 2010; Myers 1999; Rimer 2002 | 14 |
| Nursing professionals | Champion 1994; Champion 1995; Champion 2000a; Champion 2002; Champion 2003; Lerman 1995; Lerman 1997*; Saywell 1999 | 8 |
| Information specialists | 1 | |
| Multidisciplinary teams (involving primary care, public health, secondary care professionals, psychologists and/or researchers) | 3 | |
| Not stated | Bastani 1999; Curry 1993; Hutchison 1998; Kreuter 1996; Lee 1991; Lipkus 2007b; Rawl 2008; Schwartz 1999; Skinner 1994; Steckelberg 2011; Trevena 2008 | 11 |
| * Both nursing professionals and counsellors delivered the interventions | ||
Interventions
The personalised risk communication formed components of a wide range of interventions, with some studies using multiple or combined interventions of varying complexity. In general, the interventions used educational counselling (oral), written material such as tailored print material, computer/web‐based interventions, and decision aids. Many studies tailored the interventions using constructs from behaviour change theories. Several studies used more than one theoretical construct to base their interventions. The table Characteristics of included studies provides further information on the interventions. With regard to the interventions targeted at patients, 19 studies reported on single component interventions and 22 reported on multiple component/complex interventions. They are illustrated in the table below:
| Intervention type | Study IDs | Number of studies |
| Studies assessing single component interventions for patients | ||
| Written material/Tailored print | Bastani 1999; Campbell 1997; Curry 1993; Hutchison 1998; Jibaja‐Weiss 2003; Kreuter 1996; Lee 1991; Lipkus 2007b; Marcus 2005; Rawl 2008; Skinner 2002; Skinner 1994 | 12 |
| Face to face education/Counselling | 2 | |
| Telephone counselling | 2 | |
| Computer/web based | 3 | |
| Studies assessing multiple component/complex interventions | ||
| Written material with face to face or telephone education/counselling | Bodurtha 2009; Bowen 2006; Bowen 2010; Champion 1994; Champion 1995; Champion 2000a; Champion 2002; Champion 2003; Champion 2007; Glanz 2007; Helmes 2006; Lerman 1995; Lerman 1997; Lipkus 2005; Manne 2009; Manne 2010; Myers 1999; Rimer 2002; Saywell 1999 | 19 |
| Decision aids | 3 | |
Studies examined interventions based on several behaviour change theoretical constructs. Some studies had a combination approach, incorporating two or more behaviour change theories in their interventions. They are illustrated in the table below:
| Theoretical model | Study IDs | Number of studies |
| Health belief model (Becker 1974) | Bloom 2006; Bodurtha 2009; Champion 1994; Champion 1995; Champion 2000a; Champion 2002; Champion 2003; Champion 2007; Geller 2006; Glazebrook 2006; Lipkus 2005; Lipkus 2007b; Manne 2009; Marcus 2005; Rawl 2008; Saywell 1999; Skinner 1994 | 17 |
| Transtheoretical model (Prochaska 1992; Prochaska 1997) | Bloom 2006; Champion 1995; Champion 2000a; Champion 2002; Champion 2003; Champion 2007; Geller 2006; Manne 2009; Marcus 2005; Rawl 2008; Rimer 2002 | 11 |
| Precaution Adoption Process Model (Weinstein 2002) | 4 | |
| Theory of planned behaviour (Fishbein 1975) | 3 | |
| Elaboration likelihood model (Cacioppo 1984) | 2 | |
| Other | Myers 1999; Skinner 2002; Bloom 2006; Geller 2006; Lipkus 2007b; Manne 2009; Manne 2010 | 7 |
Personalised risk was delivered to the participants in three basic methods. Ten studies had interventions that included a calculated numerical risk estimate, such as from the Gail model for breast cancer risk (Gail 1989). Seven studies had interventions that categorised risk level, such as into 'high’, 'medium’ or 'low’ strata. The other 24 studies examined interventions that included the most basic level of ’personalised risk’, such as the listing of risk factors pertinent to an individual.
| Level of personalised risk communication | Study IDs | Number of studies |
| Calculated numerical risk estimate | Bloom 2006; Bowen 2002; Bowen 2006; Glazebrook 2006; Helmes 2006; Lerman 1995; Nagle 2008; Rimer 2002; Schwartz 1999; Trevena 2008 | 10 |
| Categorised risk estimate | Bastani 1999; Bodurtha 2009; Bowen 2010; Glanz 2007; Lee 1991; Sequist 2011; Skinner 2002 | 7 |
| Listing of personal risk factors | Campbell 1997; Champion 1994; Champion 1995; Champion 2000a; Champion 2002; Champion 2003; Champion 2007; Curry 1993; Geller 2006; Hutchison 1998; Jibaja‐Weiss 2003; Kreuter 1996; Lerman 1997; Lipkus 2005; Lipkus 2007b; Manne 2009; Manne 2010; Marcus 2005; Myers 1999; Rawl 2008; Saywell 1999; Skinner 1994; Smith 2010; Steckelberg 2011 | 24 |
Comparison
In 20 studies, the control participants received general risk information intended to be similar in length to the personal risk intervention being studied, and in 16 studies they received only the usual care. In five studies the interventions for control participants were delayed until the completion of the study.
| Comparison/control group | Study IDs | Number of studies |
| Received general risk information intended to be similar in length to the personal risk intervention being studied | Bastani 1999; Bodurtha 2009; Curry 1993; Glanz 2007; Hutchison 1998; Lee 1991; Lerman 1995; Lipkus 2005; Lipkus 2007b; Manne 2009; Manne 2010; Marcus 2005; Myers 1999; Nagle 2008; Rawl 2008; Schwartz 1999; Skinner 1994; Skinner 2002; Smith 2010; Steckelberg 2011 | 20 |
| Usual care | Campbell 1997; Champion 1994; Champion 1995; Champion 2000a; Champion 2002; Champion 2003; Champion 2007; Geller 2006; Glazebrook 2006; Jibaja‐Weiss 2003; Kreuter 1996; Lerman 1997; Rimer 2002; Saywell 1999; Sequist 2011; Trevena 2008 | 16 |
| Interventions for control participants were delayed until the completion of the study | 5 |
Outcomes
We extracted data on all reported outcomes specified in the review. The primary focus was on measures of an informed decision. We also list below studies reporting other cognitive, affective and behavioural outcomes.
Informed decision
-
Three newly‐identified studies reported informed decision as an outcome measure in their study (Nagle 2008; Smith 2010; Steckelberg 2011). All of these studies used outcome measures based on Marteau’s multi‐dimensional measure of informed choice (Marteau 2001).
Knowledge regarding the screening test
-
Nine studies reported knowledge as one of their outcome measures. Different scales were used, with four studies measuring knowledge as a continuous variable (Glazebrook 2006; Smith 2010; Skinner 2002; Lerman 1997). Five studies dichotomised this outcome, measuring the proportion of participants with adequate knowledge after the intervention (Nagle 2008; Smith 2010; Steckelberg 2011; Trevena 2008; Rimer 2002).
Improvement in risk comprehension
-
Lerman 1995 reported improvement in risk comprehension as an outcome measure.
Perceived risk
-
One study (Bowen 2002) reported perceiving self as an appropriate candidate for the test, and three studies (Lerman 1995; Rimer 2002; Skinner 2002) reported accurately perceived risk as outcome measures. Another study (Helmes 2006) measured perceived risk as the perceived chance of developing the disease, which was not included in the meta‐analysis.
Anxiety
-
Six studies measured anxiety. Different scales were used, with two studies (Nagle 2008; Smith 2010) using the State Trait Anxiety Inventory (STAI), one study (Lerman 1995) using the IES breast cancer distress scale, and three studies (Bloom 2006; Helmes 2006; Skinner 2002) using the breast cancer worry score, on different scales where higher scores correspond to increased anxiety or worry.
Satisfaction with decision
-
Smith 2010 measured satisfaction with the decision made.
Decision conflict
-
This was used by two studies. Smith 2010 measured the proportion of participants with low decision conflict and Nagle 2008 used the decision conflict scale as a continuous measure.
Intention to take the screening test
-
Ten studies (Bodurtha 2009; Bowen 2002; Trevena 2008; Geller 2006; Lee 1991; Skinner 2002; Jibaja‐Weiss 2003; Lerman 1997; Helmes 2006; Bowen 2010) reported this outcome. Three studies (Bowen 2002; Bowen 2010; Helmes 2006) which measured intention to take up breast cancer genetic screening in lower risk participants have been placed in separate meta‐analytic tables (Analysis 1.12; Analysis 1.13). This is because the interventions do not encourage lower risk participants to take up this screening procedure. Currently genetic testing for breast and ovarian cancers are generally not recommended for lower risk individuals (US PSTF 2005).
Uptake of the test
-
Six studies (Bloom 2006; Glazebrook 2006; Nagle 2008; Rimer 2002; Schwartz 1999; Trevena 2008) that provided numerical risk scores, six studies (Bastani 1999; Bodurtha 2009; Bowen 2010; Glanz 2007; Lee 1991; Sequist 2011) that categorised their participants' risk and 20 studies (Campbell 1997; Champion 1994; Champion 1995; Champion 2000a; Champion 2003; Champion 2007; Curry 1993; Geller 2006; Hutchison 1998; Jibaja‐Weiss 2003; Lipkus 2005; Lipkus 2007b; Manne 2009; Manne 2010; Marcus 2005; Myers 1999; Rawl 2008; Saywell 1999; Smith 2010; Steckelberg 2011) that listed their participants' risk factors in their interventions, measured the uptake of the screening procedure.
Appropriate use of cholesterol test
-
Hutchison 1998 measured the appropriate use of a cholesterol test.
Stages of change for uptake of test
-
Three studies (Rawl 2008; Skinner 1994; Champion 2007) reported the movement across the stages of change based on the Transtheoretical model (Prochaska 1992).
Making a recommended behaviour change
-
Kreuter 1996 reported this outcome.
Excluded studies
Several studies were considered closely for inclusion but were eventually excluded. We excluded 29 studies from this review. The primary reason for exclusion (14 studies) was that the intervention did not involve a personalised estimate of the participant's risk. Six studies designed the intervention around participants' health beliefs rather than their risks, and we excluded four studies for not containing a control group. Of the remaining five studies, one was a longitudinal study rather than a randomised trial, one was a study protocol, and in three studies, the outcome could not be regarded as a screening test. See Characteristics of excluded studies. Two studies were still on‐going and could not be included, see Characteristics of ongoing studies.
Risk of bias in included studies
As described in the Characteristics of included studies, six main risk of bias criteria (random sequence generation, allocation concealment, blinding: participants and personnel, blinding: outcome assessors, incomplete outcome data, selective reporting) and three other risk of bias items (baseline comparability, measures against contamination and funding for screening tests) were applied to each study. Only nine studies met four or more of the nine criteria for low risk of bias (Bodurtha 2009; Glazebrook 2006; Manne 2009; Manne 2010; Nagle 2008; Sequist 2011; Smith 2010; Steckelberg 2011; Trevena 2008). The three studies (Nagle 2008; Smith 2010; Steckelberg 2011) reporting informed decision, our primary outcome measures, met six, eight and all of the nine criteria respectively (Figure 2; Figure 3).

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
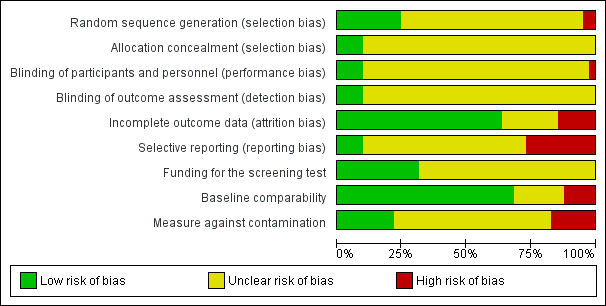
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
The allocation sequence was adequately generated in 10 studies (Bodurtha 2009; Champion 2000a; Champion 2003; Glazebrook 2006; Jibaja‐Weiss 2003; Lee 1991; Nagle 2008; Smith 2010; Steckelberg 2011; Trevena 2008), whereas 2 studies (Campbell 1997; Geller 2006) described a quasi‐random component in the sequence generation process. Other (29) studies did not report sufficient information to determine this risk of bias.
The allocation was adequately concealed only in four studies (Nagle 2008; Smith 2010; Steckelberg 2011; Trevena 2008). The majority of the studies (37) did not describe the allocation concealment in sufficient detail to permit evaluation.
Blinding
The majority of the interventions provided to clients were difficult to blind for clients, providers and outcomes assessors. Therefore, only two studies (Smith 2010; Steckelberg 2011) could blind clients, providers and outcome assessors.
Incomplete outcome data
Twenty‐six studies adequately addressed incomplete outcome data (Bloom 2006; Bodurtha 2009; Bowen 2002; Bowen 2006; Bowen 2010; Campbell 1997; Champion 1994; Champion 1995; Champion 2007; Curry 1993Glanz 2007; Helmes 2006; Lee 1991; Lipkus 2005; Lipkus 2007b; Manne 2009; Manne 2010; Marcus 2005; Myers 1999; Nagle 2008; Schwartz 1999; Sequist 2011; Skinner 2002; Smith 2010; Steckelberg 2011; Trevena 2008) whereas six studies did not (Bastani 1999; Champion 2002; Geller 2006; Hutchison 1998; Rimer 2002; Saywell 1999). The principal reason for the attrition bias was that missing outcomes are enough to induce clinically‐relevant bias in observed effect estimates. Nine studies reported insufficient information to evaluate this criterion.
Selective reporting
Only five studies provided trial registration numbers, for which protocols were available. Of these five studies, one (Trevena 2008) did not contain adequate details for outcome measures in the protocol. The other four studies (Nagle 2008; Sequist 2011; Smith 2010; Steckelberg 2011) reported all planned outcomes and were definitely free of reporting bias. Ten studies (Bloom 2006; Bowen 2006; Bowen 2010; Champion 1995; Champion 2002; Glazebrook 2006; Jibaja‐Weiss 2003; Lee 1991; Manne 2009; Skinner 1994) did not report all the outcomes mentioned in the paper. The other 26 studies reported all outcomes mentioned in the paper, but were marked as unclear due to unavailability of study protocol.
Other potential sources of bias
In 28 studies (Bodurtha 2009; Bowen 2002; Bowen 2006; Bowen 2010; Campbell 1997; Champion 2007; Geller 2006; Glanz 2007; Glazebrook 2006; Helmes 2006; Hutchison 1998; Jibaja‐Weiss 2003; Kreuter 1996; Lerman 1995; Lerman 1997; Lipkus 2005; Lipkus 2007b; Manne 2009; Manne 2010; Marcus 2005; Nagle 2008; Rawl 2008; Rimer 2002; Saywell 1999; Sequist 2011; Skinner 1994; Skinner 2002; Smith 2010; Steckelberg 2011; Trevena 2008) the baseline characteristics of participants in all study arms were comparable to each other. In five studies (Bastani 1999; Champion 1994; Champion 2002; Champion 2003; Hutchison 1998) there were differences in the baseline characteristics of participants between the study arms. The remaining eight studies did not provide adequate information to assess this source of bias.
Nine studies (Bastani 1999; Campbell 1997; Geller 2006; Glazebrook 2006; Hutchison 1998; Manne 2009; Manne 2010; Nagle 2008; Steckelberg 2011) had adequate protection against contamination. We assessed a further seven studies (Bloom 2006; Bowen 2002; Glanz 2007; Lerman 1995; Lerman 1997; Rawl 2008; Schwartz 1999) as being at high risk due to the potential for contamination within recruited members, and without mention of protection against contamination. We assessed as unclear the other 25 studies in which there was no mention of protection against contamination.
We included funding for the screening test as one of the risk of bias elements, as this can influence the intentions and the uptake of screening tests. Only 13 studies (Bodurtha 2009; Glanz 2007; Glazebrook 2006; Helmes 2006; Lipkus 2005; Lipkus 2007b; Manne 2009; Manne 2010; Rawl 2008; Saywell 1999; Sequist 2011; Smith 2010; Steckelberg 2011) reported how participants would have had their screening tests funded; i.e. if tests were provided free of charge by the researchers, if participants themselves had to pay for these tests, or if they were provided by the health organisation or insurance companies.
Effects of interventions
See: Summary of findings for the main comparison Personalised risk communication versus general risk information for informed decision making about taking screening tests; Summary of findings 2 Personalised risk communication versus general risk information for informed decision making about taking screening tests
Informed decision
Our primary outcome of interest was 'informed decision'. This was measured in three newly‐included studies, with the measures based on Marteau’s Multidimensional Measure of Informed choice (Marteau 2001). One study provided personalised risk to the consumers undergoing screening, within interventions that used a calculated risk score (Nagle 2008). The other two studies (Steckelberg 2011; Smith 2010) listed out the personal risk factors to the participants. Overall there is strong evidence from these three trials that personalised risk communication, when presented as a part of complex interventions such as decision aids, promotes informed uptake of screening tests. Overall 45.2% (592/1309) of participants who received personalised risk information made informed choices, compared to 20.2% (229/1135) of participants who received generic risk information. The odds ratio (OR) for the study that provided numerical risk in its intervention (Nagle 2008) was OR 2.08 (95% CI 1.14 to 3.81). The pooled OR for the two studies that used listing of risk factors in their intervention was 4.98 (95% CI 3.97 to 6.24). The overall ORs were 4.48 (95% CI 3.62 to 5.53) for fixed‐effect and 3.65 (95% CI 2.13 to 6.23) for random‐effects. The effect measures showed significant heterogeneity (I2 = 77%), probably due to differences in the type of interventions and the populations within each study. However all studies consistently demonstrated a similar direction of effect, favouring personalised risk communication (see Analysis 1.1 and summary of findings Table for the main comparison).
Knowledge
Nine studies measured knowledge regarding the screening test. Four studies measured this on continuous outcome scales. The scales used varied with studies and we present these as standardised mean differences (SMDs). There were considerable subgroup differences, but all studies showed a significant increase in knowledge with personalised risk communication. We present the SMDs (by fixed‐effects model) in each subgroup below in the table (also see Analysis 1.2 subcategories 1‐3 and summary of findings Table 2):
| Knowledge measured on continuous scales | |
| Subgroup
| Standardised Mean Difference (SMD) |
| Fixed‐effect model | |
| Calculated risk score (numerical) versus general information | 0.40 (95% CI 0.23 to 0.56) |
| Calculated risk score (categorised) versus general information | 0.57 (95% CI 0.32 to 0.82) |
| Personal risk factor list versus general information | 0.89 (95% CI 0.75 to 1.04) |
Five studies measured the proportion of participants with good knowledge in each of their study arms. Again, there were significantly higher proportions of participants with good knowledge within the intervention arms of the studies, when personalised risk communication was delivered:
| Knowledge ‐ measured as proportion with good knowledge | ||
| Subgroup
| Odds Ratios (OR) | |
| Fixed‐effect model | Random‐effects model | |
| Calculated risk score (numerical) versus general information (see Analysis 1.3) | 2.11 (95% CI 1.52 to 2.91) | 2.60 (95% CI 1.27 to 5.34) |
| Personal risk factor list v general information (see Analysis 1.4) | 7.13 (95% CI 5.79 to 8.79) | ‐ |
Risk perception/comprehension
In three studies the interventions showed a trend towards more accurate risk perception (fixed‐effect OR 1.46 (95% CI 1.13 to 1.88); random‐effects OR 1.65 (95% CI 0.96 to 2.81), see Analysis 1.5 ). However the evidence from these studies was of low quality (see summary of findings Table 2). Another study (Bowen 2002) examined whether people perceived themselves as appropriate for the tests after the intervention, with a non‐significant trend towards fewer people perceiving appropriateness of tests after the intervention(see Analysis 1.6). Skinner 2002 studied the effect of tailored print material on the decision to take up genetic screening in women with personal history of breast and ovarian cancer. This study showed that women were more knowledgeable (SMD 0.57 (95% CI 0.32 to 0.82)), and had more accurate perceptions of risk (OR 2.50 (95% CI 1.48 to 4.20)), but fewer intended to take tests after the personalised risk intervention (OR 0.21 (95% CI 0.07 to 0.64)), see Analysis 1.2; Analysis 1.5; Analysis 1.11. Lerman 1995 reported a small (non‐significant) improvement in risk comprehension after personalised risks were delivered (see Analysis 1.17).
Anxiety
Four out of six studies reported non‐significant changes in anxiety following personalised risk communication to the participants. Overall there was a small, non‐significant decrease in anxiety scores in the intervention group (fixed‐effect SMD ‐0.13 (95% CI ‐0.22 to ‐0.03); random‐effects SMD ‐0.13 (95% CI ‐0.29 to 0.03); negative values indicate a decrease in anxiety and favouring personalised risk communication), see Analysis 1.7 and summary of findings Table 2.
Uptake of test
Most studies (32/41) measured the uptake of screening tests following personalised risk communication interventions. There was significant heterogeneity (I2 > 80%) within these studies. Subgroup analysis revealed this was less significant when studies were examined by the levels of risk communication that was provided within their interventions. Heterogeneity was least significant in studies where risk was provided in a more detailed manner, i.e. as a numerical score. There were six studies in this group, with personalised risk communication having little effect on the uptake of screening tests (fixed‐effect OR 0.95 (95% CI 0.78 to 1.15)), see Analysis 1.14 subcategory 01 and summary of findings Table for the main comparison). For risk estimates or calculations which were categorised into high, medium or low strata of risk (six studies), the results were significant ( OR 1.29 (95% CI 1.11 to 1.51) (see Analysis 1.14 subcategory 02 and summary of findings Table for the main comparison). Overall, pooling of these two subgroups yielded a combined OR of 1.15 (95% CI 1.02 to 1.29) – indicative of weak evidence, consistent with a small effect, that personalised risk communication where a risk score was provided or the participants were given their categorised risk, increases uptake of screening tests.
For studies that listed personal risk factors in their interventions (20 studies), there was considerable heterogeneity between studies for uptake of test, and we do not present a pooled estimate for this subgroup. However 10/20 studies (50%) showed a significant increase in the uptake of the screening tests following personalised risk communication interventions (see Analysis 1.15 subcategory 01).
One study (Hutchison 1998) also assessed 'appropriate uptake' of tests (externally‐defined appropriateness: that is, uptake for people at higher risk, and non‐uptake for people at lower risk) and found more appropriate uptake after the personalised risk intervention (OR 1.32 (95% CI 1.14 to 1.55) (Analysis 1.16).
Intention to take up screening test
Seven studies measured the intention to take screening tests, and had considerably heterogeneous results (see Analysis 1.11). Three other studies measured the intention to take up genetic testing in normal or low risk participants. These tests (as discussed earlier in this review) are not necessarily indicated in consumers with such low or average risks. In one study (Bowen 2002) the results were inconclusive (see Analysis 1.12). The other two studies (Helmes 2006; Bowen 2010) demonstrated a significant decrease in participants' intentions to take up the genetic tests following personalised risk communication interventions. The mean difference (MD) for fixed‐effect was ‐0.53 (95% CI ‐0.63 to ‐0.44) and for random‐effects was ‐0.77 (95% CI ‐1.42 to ‐0.13) (negative values indicate a decrease in intention and favour personalised risk communication; see Analysis 1.13).
Decision conflict
Nagle 2008 measured decision conflict regarding prenatal testing for foetal abnormality. The results of this study showed a non‐significant increase in decision conflict following personalised risk communication (see Analysis 1.9).
Satisfaction with decision
Smith 2010 measured satisfaction with decision in interventions to support informed choice about bowel cancer screening and found no change after personalised risk communication (see Analysis 1.10).
Stages of change
Three studies looked at the stages of change based on the Transtheoretical model (Prochaska 1992; Prochaska 1997). Champion 2007 found all interventions were effective (compared to usual care) in moving women forward in mammography stage (versus same or backward). However, the combination of tailored telephone and print interventions (versus usual care) had the largest effect (OR 1.95 (95% CI 1.36 to 2.81)). Rawl 2008 found that the forward movement of stage was slightly higher in the risk tailored group for colorectal cancer screening. Skinner 1994 found that 71% of participants did not change; 14% advanced one stage; 12% 'regressed'; no significant differences between tailored message and control groups were noticed (see Analysis 1.18).
Making a recommended behaviour change
One study (Kreuter 1996) measured the proportion of participants making a recommended behaviour change after personalised risks were communicated. There were slight, non‐significant differences between the groups (OR 0.98 95% CI 0.76 to 1.28).
Quality of life (SF‐36)
One study (Bowen 2010) measured quality of life scores on the SF‐36 scale after interventions to promote breast health behaviours. There was significant improvement on this measure following personalised risk communication(MD 7.10 (95% CI 5.90 to 8.30), see Analysis 1.20).
Subgroup analysis
The results for the studies addressing mammography showed similar effects with uptake of screening and to the overall data set. The numerical calculated risk estimates were associated with lower ORs for uptake of tests (OR 0.84 (95% CI 0.68 to 1.03)) (See Analysis 3.5 and subcategories). The subgroups for categorised risk and listing of risk factors showed considerable heterogeneity with uptake of the tests and it was not appropriate to pool these results (see Analysis 3.6 and Analysis 3.7).
The 11 studies examining risk communication in high‐risk individuals showed trends towards larger ORs for uptake of tests than the other studies. The OR for numerical estimates of risk was 1.05 by fixed‐effect (95% CI 0.79 to 1.40) and 1.08 by random‐effects (95% CI 0.63 to 1.84). The OR for categorised estimates of risk was 1.38 (95%CI 1.06 to1.80). Overall, pooling of these two subgroups yielded a combined OR of 1.22 (95% CI 1.00 to 1.48), indicative of a higher uptake of screening tests when only the high‐risk group studies were analysed (Analysis 5.6).
For studies whose interventions listed personal risk factors, there was considerable heterogeneity between studies for uptake of test, and we do not present a pooled estimate for this subgroup (See Analysis 5.7).
Ten studies measured uptake of tests in colorectal cancer screening programmes. The overall OR for uptake was 1.02 by fixed‐effect (95% CI 0.90 to 1.16) and 1.06 by random‐effects (95% CI 0.82 to 1.37) (see Analysis 6.8).
Sensitivity analysis
We conducted a sensitivity analysis for the uptake of screening tests, by excluding studies that were either at either unclear or high risk of bias for four risk of bias domains that we considered to be most important for this topic. The results were as follows:
Random sequence generation (selection bias)
In this analysis we included only low risk studies. The analysis for this risk of bias domain yielded a lower uptake of tests, with an OR of 1.10 (95% CI 0.88 to 1.39) when the two groups with limited heterogeneity (i.e. those which provided either numerical or categorised risks) were pooled. Five low risk studies accounted for this analysis (Bodurtha 2009; Glazebrook 2006; Lee 1991; Nagle 2008; Trevena 2008). The studies that provided listing of risk factors remained considerably heterogeneous, even after studies with high risk of bias for the above domain were excluded.
Incomplete outcome data (attrition)
In this analysis we included only low risk studies. The analysis for this risk of bias domain yielded similar uptake of tests, with an OR of 1.15 (95% CI 0.99 to 1.35) when the two groups with limited heterogeneity (i.e. those which provided either numerical or categorised risks) were pooled. Nine low risk studies accounted for this analysis (Bloom 2006; Bodurtha 2009; Bowen 2010; Glanz 2007; Lee 1991; Nagle 2008; Schwartz 1999; Sequist 2011Trevena 2008). The studies that provided listing of risk factors remained considerably heterogeneous, even after studies with high risk of bias for the above domain were excluded.
Selective reporting (reporting bias)
In this analysis we only excluded studies that were at high risk for selective reporting. We included low risk studies and the 'unclear' studies which reported all outcomes mentioned in their methods sections, but were marked 'unclear' due to unavailability of study protocol. The analysis for this risk of bias domain reduced the uptake of tests, with an OR of 1.03 (95% CI 0.90 to 1.18) when the two groups with limited heterogeneity (i.e. those which provided either numerical or categorised risks) were pooled. Seven studies accounted for this analysis (Bastani 1999; Bodurtha 2009; Glanz 2007; Nagle 2008; Rimer 2002; Schwartz 1999; Sequist 2011). The studies that provided listing of risk factors remained considerably heterogeneous, even after studies with high risk of bias for the above domain were excluded.
Baseline comparability
The analysis for this risk of bias domain again yielded a similar measure of uptake of tests, with an OR of 1.15 (95% CI 1.00 to 1.33) for fixed‐effect and 1.22 (95% CI 0.94 to 1.57) for random‐effects model (I2 = 63%), when the two groups with limited heterogeneity (i.e. those which provided either numerical or categorised risks) were pooled. Eight studies accounted for this analysis (Bodurtha 2009; Bowen 2010; Glanz 2007; Glazebrook 2006; Nagle 2008; Rimer 2002; Sequist 2011; Trevena 2008). The studies that provided listing of risk factors remained considerably heterogeneous, even after studies with high risk of bias for the above domain were excluded.
The sensitivity analyses for selection bias, attrition bias and baseline comparability did not alter the uptake measure significantly. By excluding the studies that had a high risk for selective reporting bias, the OR for uptake was lower, probably due to the exclusion of studies that may have reported only significant results.
Discussion
Summary of main results
This review updates the second (2006) publication of this review (Edwards 2006). Risk communication, particularly in the field of screening, is evolving with time. We have identified 19 new studies with elements of personalised risk communication in screening, which takes the total number of studies in this review to 41. The earlier two versions of this review (Edwards 2003c; Edwards 2006) included studies that measured outcomes that form elements of an informed decision, but identified no studies measuring informed decision as such. This updated review includes three studies (Nagle 2008; Smith 2010; Steckelberg 2011) that specifically have measured informed decision for the uptake of screening tests after personalised risk communication. From these three studies, there was strong evidence that personalised risk communication enhanced informed decision making, although with heterogeneous results. We expected this heterogeneity due to the varied nature of the interventions and the level of risk communication that was used in each of these studies. However, all studies had significant effect sizes, all favouring personalised risk communication.
Other recent studies have sought to address knowledge, perceived risk, and other elements of informed decision making. Overall these studies showed an increase in knowledge and a trend towards more accurate risk perception when personalised risk was included in their interventions. This field of risk communication in screening mirrors other clinical areas of health care, where there is an increasing trend to involve consumers in their own healthcare choices. In the same way that screening recommendations have developed in recent years from simply seeking to maximise uptake, to promotion of informed choice about participation in screening programmes (Holland 2005), the trial literature also now shows some development towards this.
Further, there was weak evidence to suggest that such interventions promote uptake of screening tests, although effects were small.
Some data also show that when the personalised risk information disclosed is more detailed, the uptake of tests is lower than when the risk communication is less detailed or numerically unspecific (i.e. when categorised into risk groups). Significant heterogeneity was noted among studies that merely listed the personal risk factors in their intervention. Test uptake thus becomes unpredictable when less specific ways of communicating personal risk factors are used. This may reflect the differences in perception of actual risk among participants between studies when such less specific methods of personalised risk communication are employed, but may also reflect the different nature of screening programme where some are more controversial (e.g. mammography and PSA testing) that the others (e.g. colorectal cancer screening).
High risk status an effect modifier
The effects of interventions on screening uptake appeared greater among consumers or patients deemed to be at higher risk of a disease than average. In this group of studies, most favoured the intervention, irrespective of the level of risk communication. This suggests that the 'high risk status' of the consumer may be an important effect modifier for personalised risk communication. There appears to be potential for substantial modification of choices made among this group of consumers. It may be appropriate that the scope for interventions to influence decisions is large in this group of consumers. Equally, however, interventions could be harmful if they are not introduced carefully. Healthcare professionals must take steps to ensure that they are achieving informed decision making by these consumers.
Overall completeness and applicability of evidence
We have used a highly‐sensitive search strategy (Matthews 1999) to identify as many papers as possible in this field, from all relevant databases. The search covers studies published until March 2012, in an effort to outline the most contemporary evidence at the time of publication. To further increase the completeness of this search, we have followed this up by handsearching journals and reference lists, and also have looked at other similar reviews in the field to identify any papers missed in the initial search. This updated review presents a significant increase in the number of included studies, compared to the previous versions. Notably, however, the interventions used in these studies cover a range of methods to deliver the personalised element of risk communication. In addition, the measures of components of informed decision vary widely across studies. The settings in which the studies were conducted include a diverse range of screening programmes. Therefore, data from this review do not allow us to draw a firm conclusion about the best interventions to deliver personalised risk st= in enhancing informed decisions. The results are dominated by findings from the topic area of mammography and colorectal cancer. Caution is therefore required in generalising from these results, particularly for clinical topics other than mammography and colorectal cancer screening.
Further, in all studies the effects are a measure of the ('complex') intervention as a whole rather than that of personalised risk communication per se. However, isolating the effect of personalised risk communication on informed decisions or the uptake of tests, and drawing firm conclusions based on this, poses a great challenge to researchers working in this field.
Quality of the evidence
We included 41 studies in this review. It is difficult to draw general conclusions from this review because these studies varied widely according to interventions provided, outcomes measured, the screening programme, and duration of interventions and follow‐up. A substantial number of people are included in this review (28,700), and the number of participants within each study varied widely, from 140 to 3152 people.
The studies that reported direct measures of informed decision were few (n = 3) in number, but were of very good methodological quality. Most other studies that measured other outcomes (i.e. components of informed decision) were not explicit in reporting elements that could introduce biases into their design, although studies reporting several of the knowledge outcomes were also of high quality. Only five studies (Lerman 1995; Lerman 1997; Manne 2010; Nagle 2008; Smith 2010) provided information of the validity of scales used to measure the outcomes of interest.
We have illustrated the quality of evidence for our main outcomes using GRADE Working Group recommendations (Guyatt 2008) in summary of findings Table for the main comparison and summary of findings Table 2.
Overall, the outcome ‘informed decision’ and several of the knowledge outcomes were subject to less risk of bias and provide better quality of evidence than the other reported outcomes (in particular, screening uptake, accuracy of perceived risk, and anxiety).
Potential biases in the review process
Despite the extensive search strategy, we may have missed some studies of interventions including personalised risk communication for screening tests if they were not indexed in the bibliographic databases appropriately to be identified by our search terms. We made significant efforts to contact trial authors for further data, where these were not available, and for further details of how the risks were personalised. Some data that were not extractable or were unclear were not included, if we did not receive further clarification from the authors.
As expected, the other main limitation of this review lies in the fact that we did not find any studies that examined the effect of personalised risk communication in isolation. All studies studied the effect of their interventions which included personalised risk communication. The interventions as such were diverse in nature, and it is hard to draw firm conclusions on the weight of effect that personalised risk communication has contributed to. There was considerable heterogeneity in some of the groups, in which case we did not pool these data. Many studies that were unclear on the risk of bias items are still included within the overall meta‐analyses, and we present a sensitivity analysis by excluding such studies. Lastly, ways to measure the outcomes were also diverse which may have biased the review. Common validated methods of measuring informed decision are yet to be developed for wider use.
Agreements and disagreements with other studies or reviews
We are not aware of any other systematic reviews that assessed specifically the effect of personalised risk communication on measures of informed decision. Albada 2009 was the most similar review assessing the effects of tailored information based on theoretical (behavioural) constructs and providing cancer risk for individuals undergoing screening. The authors assessed the effects on risk perception, knowledge and screening behaviour. They concluded that interventions based on theoretical constructs increased risk perception and knowledge of cancer as compared to generic interventions, and information tailored to individual risk specifically increased realistic risk perceptions. As with our review, they found limited evidence for enhancing screening behaviour. A recent review (Stacey 2011) also found that decision aids that are tailored to support individual healthcare decisions increased people's involvement, and improved knowledge and realistic perception of outcomes, but had variable effect on choice behaviour.
Noar et al presented the first meta‐analytic review of tailored print interventions for health behaviour change. In contrast with our review, their findings suggest that tailored print information focusing on preventive health behaviours out‐perform generic interventions. However a host of behaviour change studies other than screening are included in their review (Noar 2007).
Briss 2004 studied interventions that were generally consistent in improving individuals’ knowledge about the disease, accuracy of risk perceptions, or knowledge and beliefs about the pros and cons of screening and treatment options. They reported insufficient evidence that such interventions promote informed decisions, due to a paucity of studies that fully measured this outcome. They recommended further research in this area, providing a conceptual framework to evaluate this outcome. We have found only three studies that used informed decision as an outcome measure, reiterating the recommendations proposed by Briss 2004.
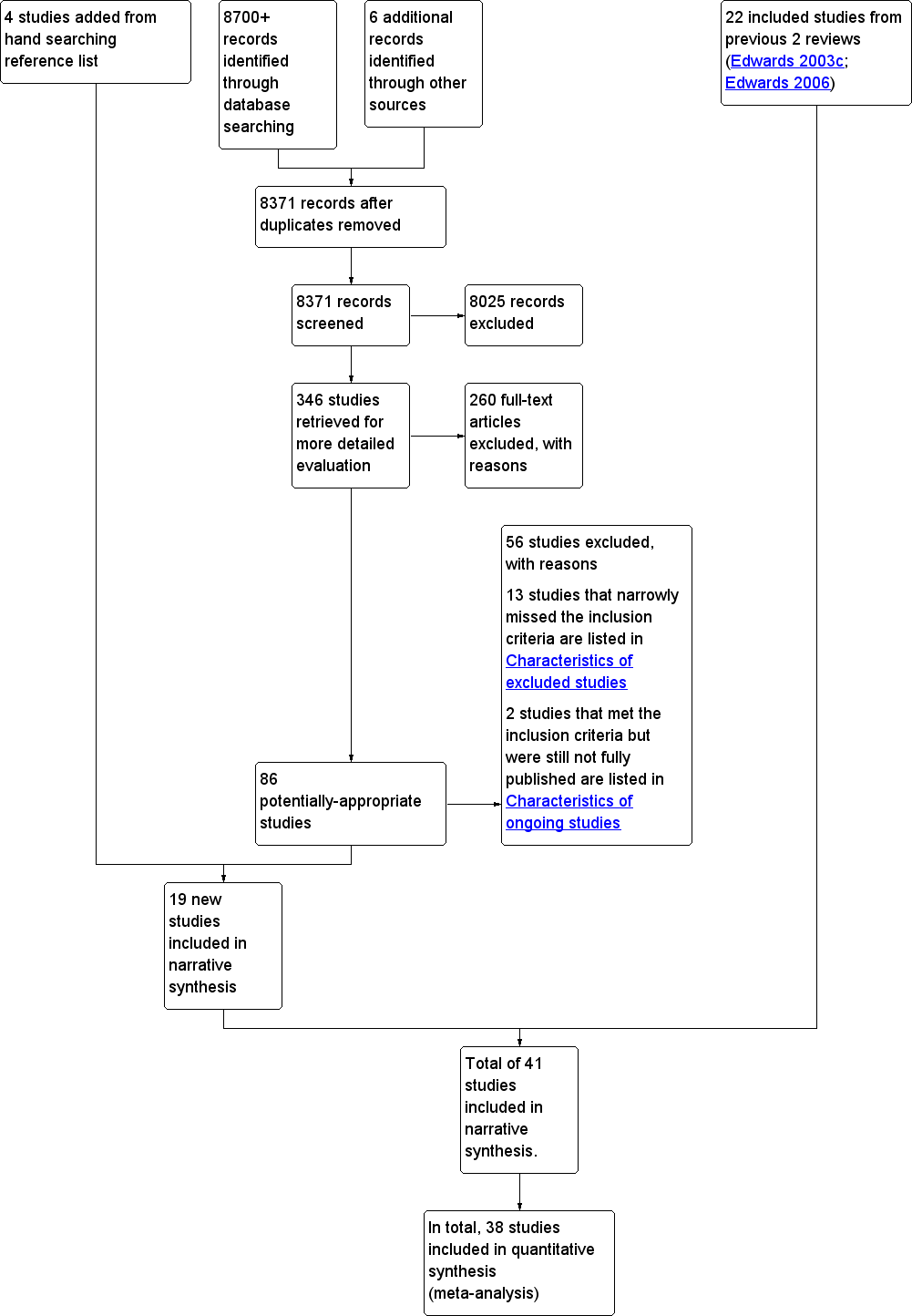
Study flow diagram. Search dates from 2006 to March 2012.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
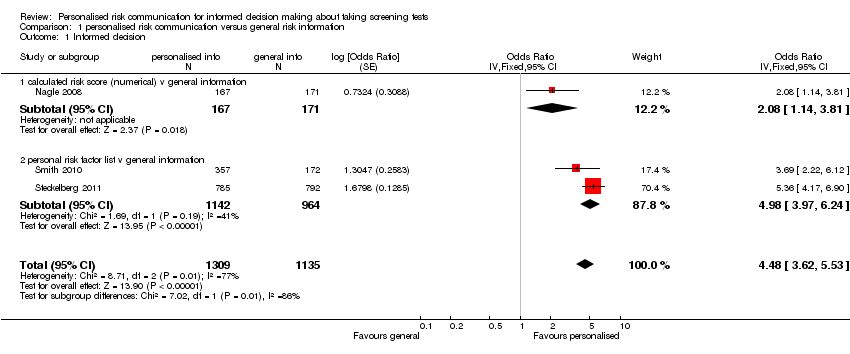
Comparison 1 personalised risk communication versus general risk information, Outcome 1 Informed decision.

Comparison 1 personalised risk communication versus general risk information, Outcome 2 knowledge regarding screening test / condition concerned.
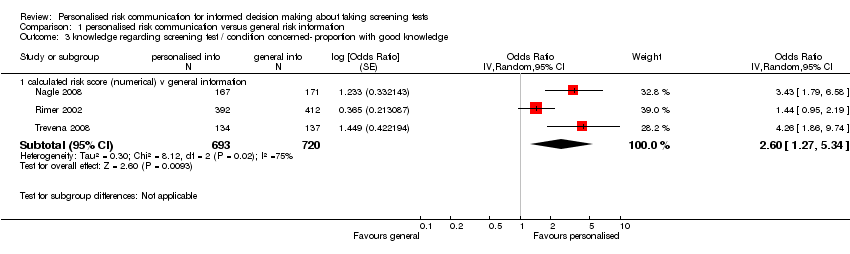
Comparison 1 personalised risk communication versus general risk information, Outcome 3 knowledge regarding screening test / condition concerned‐ proportion with good knowledge.

Comparison 1 personalised risk communication versus general risk information, Outcome 4 knowledge regarding screening test / condition concerned‐ proportion with good knowledge.

Comparison 1 personalised risk communication versus general risk information, Outcome 5 accurately perceived risk.

Comparison 1 personalised risk communication versus general risk information, Outcome 6 perceived risk ‐ perceiving self as appropriate candidate for test.
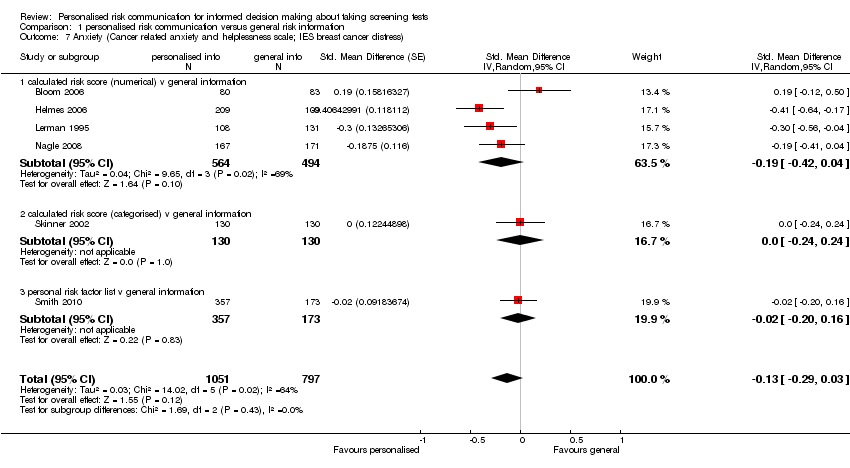
Comparison 1 personalised risk communication versus general risk information, Outcome 7 Anxiety (Cancer related anxiety and helplessness scale; IES breast cancer distress).

Comparison 1 personalised risk communication versus general risk information, Outcome 8 decision conflict (proportion with lower scores).

Comparison 1 personalised risk communication versus general risk information, Outcome 9 decision conflict.
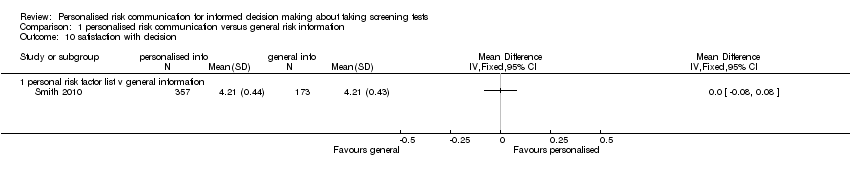
Comparison 1 personalised risk communication versus general risk information, Outcome 10 satisfaction with decision.
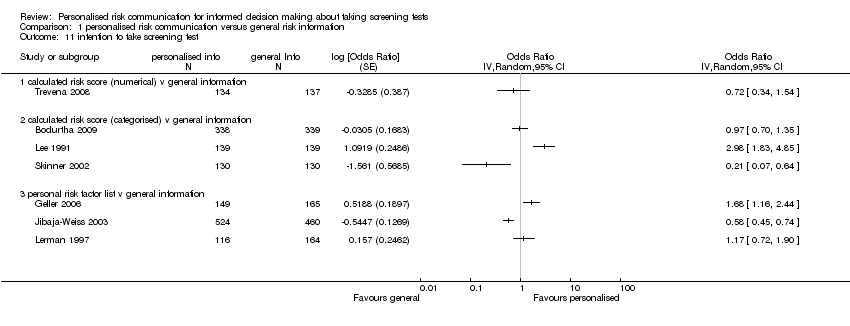
Comparison 1 personalised risk communication versus general risk information, Outcome 11 intention to take screening test.
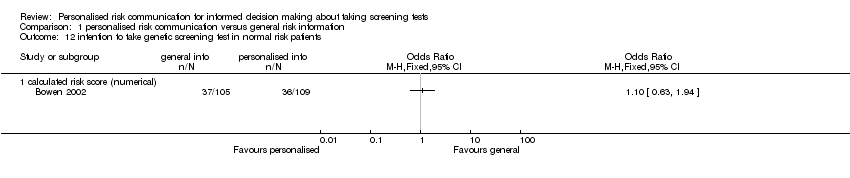
Comparison 1 personalised risk communication versus general risk information, Outcome 12 intention to take genetic screening test in normal risk patients.

Comparison 1 personalised risk communication versus general risk information, Outcome 13 intention to take genetic screening test in normal risk patients.
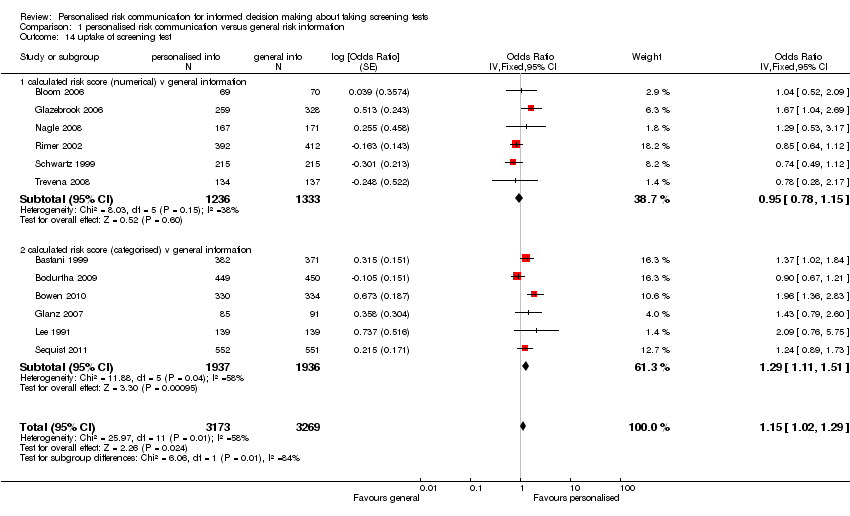
Comparison 1 personalised risk communication versus general risk information, Outcome 14 uptake of screening test.

Comparison 1 personalised risk communication versus general risk information, Outcome 15 uptake of screening test.

Comparison 1 personalised risk communication versus general risk information, Outcome 16 appropriate use of cholesterol test.
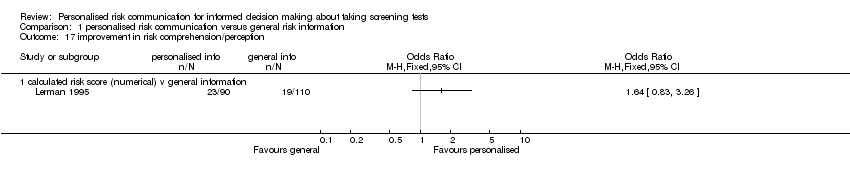
Comparison 1 personalised risk communication versus general risk information, Outcome 17 improvement in risk comprehension/perception.
| Study | |
| personal risk factor list v general information | |
| Champion 2007 | All interventions were effective (compared to usual care) in moving women forward in mammography stage (versus same or backward). However, the combination of tailored telephone and print interventions versus usual care, had the largest effect. (OR 1.949, 95% CI 1.355 to 2.815) |
| Rawl 2008 | Forward movement of stage was:
Forward movement of stage was slightly higher in the risk tailored group. |
| Skinner 1994 | 71% did not change; 14% advanced one stage; 12% 'regressed': no significant differences between tailored message and control. |
Comparison 1 personalised risk communication versus general risk information, Outcome 18 stages of change.

Comparison 1 personalised risk communication versus general risk information, Outcome 19 making a recommended behaviour change.

Comparison 1 personalised risk communication versus general risk information, Outcome 20 Quality of life (SF‐36).

Comparison 2 personalised risk communication versus general risk information for PAP SMEARS, Outcome 1 intention to take screening test.

Comparison 2 personalised risk communication versus general risk information for PAP SMEARS, Outcome 2 uptake of screening test.

Comparison 3 personalised risk communication versus general risk information for MAMMOGRAPHY, Outcome 1 knowledge regarding screening test / condition concerned.

Comparison 3 personalised risk communication versus general risk information for MAMMOGRAPHY, Outcome 2 accuracy of perceived risk.
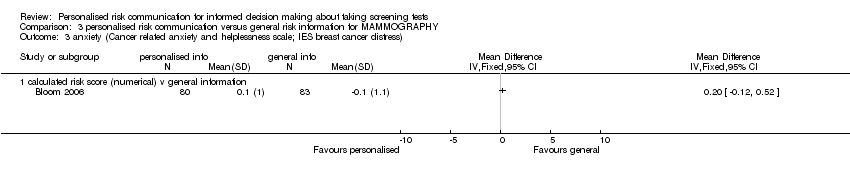
Comparison 3 personalised risk communication versus general risk information for MAMMOGRAPHY, Outcome 3 anxiety (Cancer related anxiety and helplessness scale; IES breast cancer distress).
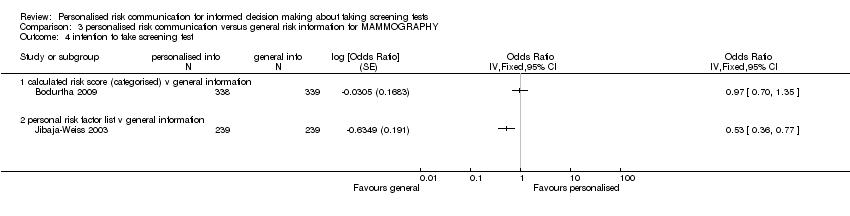
Comparison 3 personalised risk communication versus general risk information for MAMMOGRAPHY, Outcome 4 intention to take screening test.
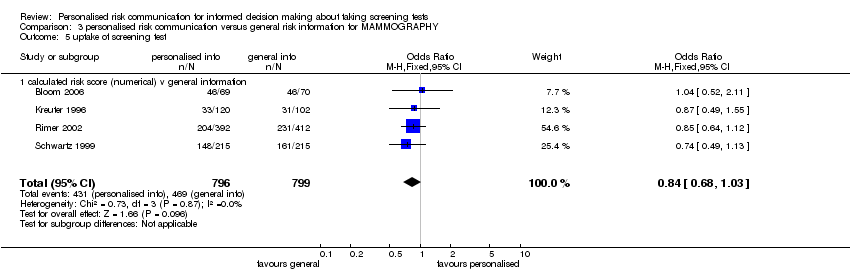
Comparison 3 personalised risk communication versus general risk information for MAMMOGRAPHY, Outcome 5 uptake of screening test.

Comparison 3 personalised risk communication versus general risk information for MAMMOGRAPHY, Outcome 6 uptake of screening test.

Comparison 3 personalised risk communication versus general risk information for MAMMOGRAPHY, Outcome 7 uptake of screening test.
| Study | |
| personal risk factor list v general information | |
| Skinner 1994 | 71% did not change; 14% advanced one stage; 12% 'regressed': no significant differences between tailored message and control. |
Comparison 3 personalised risk communication versus general risk information for MAMMOGRAPHY, Outcome 8 stages of change.

Comparison 4 personalised risk communication versus general risk information for CHOLESTEROL TESTS, Outcome 1 uptake of screening test.

Comparison 4 personalised risk communication versus general risk information for CHOLESTEROL TESTS, Outcome 2 appropriate use of cholesterol test.

Comparison 5 personalised risk communication versus general risk information for 'HIGH RISK' PEOPLE, Outcome 1 knowledge regarding screening test / condition concerned.
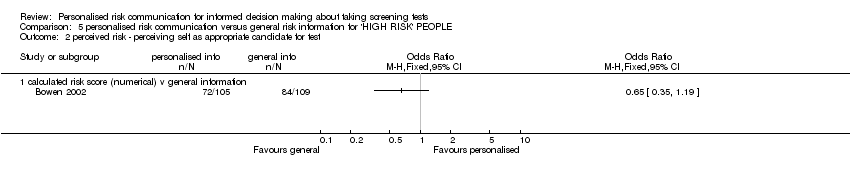
Comparison 5 personalised risk communication versus general risk information for 'HIGH RISK' PEOPLE, Outcome 2 perceived risk ‐ perceiving self as appropriate candidate for test.
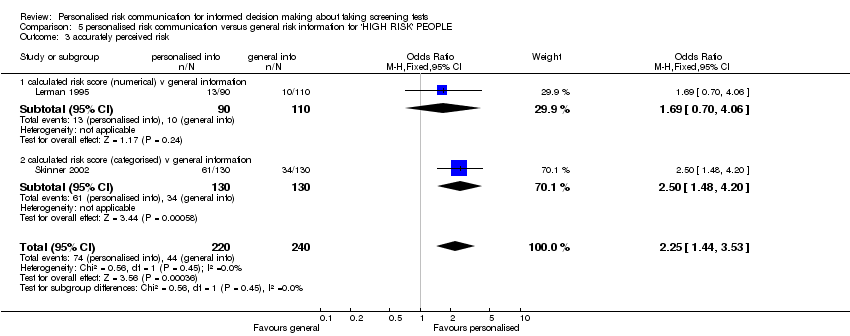
Comparison 5 personalised risk communication versus general risk information for 'HIGH RISK' PEOPLE, Outcome 3 accurately perceived risk.

Comparison 5 personalised risk communication versus general risk information for 'HIGH RISK' PEOPLE, Outcome 4 anxiety (Cancer related anxiety and helplessness scale; IES breast cancer distress).

Comparison 5 personalised risk communication versus general risk information for 'HIGH RISK' PEOPLE, Outcome 5 intention to take screening test.

Comparison 5 personalised risk communication versus general risk information for 'HIGH RISK' PEOPLE, Outcome 6 uptake of screening test.

Comparison 5 personalised risk communication versus general risk information for 'HIGH RISK' PEOPLE, Outcome 7 uptake of screening test.
| Study | |
| personal risk factor list v general information | |
| Rawl 2008 | Forward movement of stage was:
Forward movement of stage was slightly higher in the risk tailored group. |
Comparison 5 personalised risk communication versus general risk information for 'HIGH RISK' PEOPLE, Outcome 8 stages of change.

Comparison 6 personalised risk communication versus general risk information for COLORECTAL SCREENING, Outcome 1 Informed decision.

Comparison 6 personalised risk communication versus general risk information for COLORECTAL SCREENING, Outcome 2 knowledge regarding screening test / condition concerned.
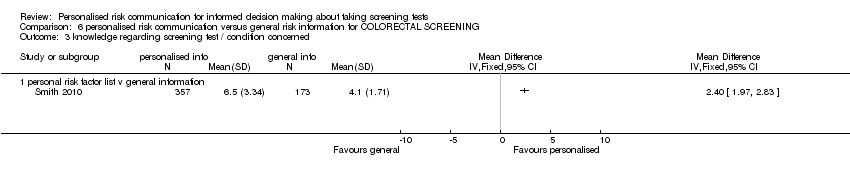
Comparison 6 personalised risk communication versus general risk information for COLORECTAL SCREENING, Outcome 3 knowledge regarding screening test / condition concerned.

Comparison 6 personalised risk communication versus general risk information for COLORECTAL SCREENING, Outcome 4 anxiety (Cancer related anxiety and helplessness scale; IES breast cancer distress).
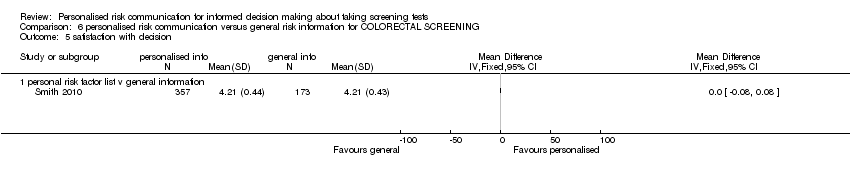
Comparison 6 personalised risk communication versus general risk information for COLORECTAL SCREENING, Outcome 5 satisfaction with decision.

Comparison 6 personalised risk communication versus general risk information for COLORECTAL SCREENING, Outcome 6 Decision conflict (proportion with low scores).
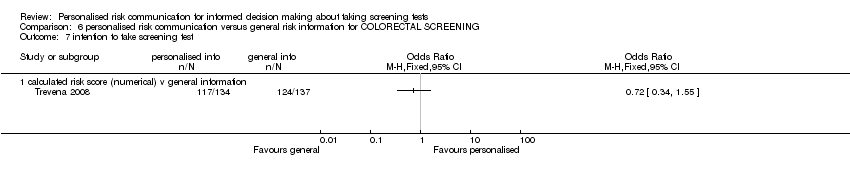
Comparison 6 personalised risk communication versus general risk information for COLORECTAL SCREENING, Outcome 7 intention to take screening test.

Comparison 6 personalised risk communication versus general risk information for COLORECTAL SCREENING, Outcome 8 uptake of screening test.
| Study | |
| personal risk factor list v general information | |
| Rawl 2008 | Forward movement of stage was:
Forward movement of stage was slightly higher in the risk tailored group. |
Comparison 6 personalised risk communication versus general risk information for COLORECTAL SCREENING, Outcome 9 stages of change.

Comparison 7 personalised risk communication versus general risk information for PROSTATE CANCER SCREENING, Outcome 1 uptake of screening test.

Comparison 8 Personalised risk communication versus general risk information for SKIN CANCER SCREENING, Outcome 1 knowledge regarding screening test / condition concerned (Generic Inverse Variance).

Comparison 8 Personalised risk communication versus general risk information for SKIN CANCER SCREENING, Outcome 2 intention to take screening test.
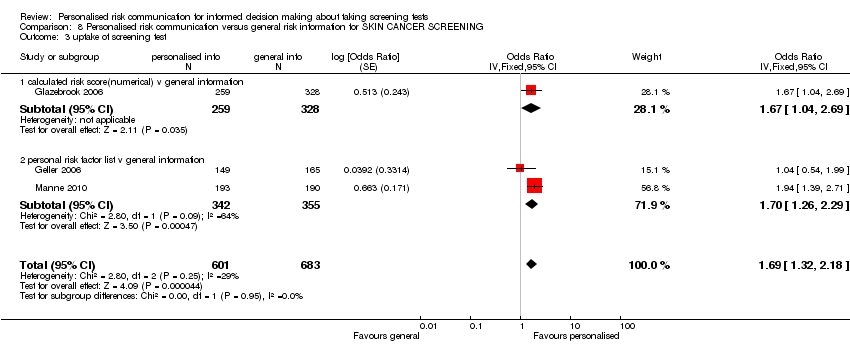
Comparison 8 Personalised risk communication versus general risk information for SKIN CANCER SCREENING, Outcome 3 uptake of screening test.

Comparison 9 Personalised risk communication versus general risk information for PRENATAL TESTING FOR FOETAL ABNORMALITY, Outcome 1 Informed decision.

Comparison 9 Personalised risk communication versus general risk information for PRENATAL TESTING FOR FOETAL ABNORMALITY, Outcome 2 knowledge regarding screening test / condition concerned.

Comparison 9 Personalised risk communication versus general risk information for PRENATAL TESTING FOR FOETAL ABNORMALITY, Outcome 3 uptake of screening test.

Comparison 9 Personalised risk communication versus general risk information for PRENATAL TESTING FOR FOETAL ABNORMALITY, Outcome 4 anxiety.

Comparison 9 Personalised risk communication versus general risk information for PRENATAL TESTING FOR FOETAL ABNORMALITY, Outcome 5 decision conflict.
| Patient or population: patients with informed decision making about taking screening tests | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk8 | Corresponding risk | |||||
| Control | Personalised risk communication versus general risk information | |||||
| Informed decision ‐ Numerical risk and categorised risk combined | 202 per 1000 | 480 per 1000 | OR 3.65 | 2444 | ⊕⊕⊕⊕ | Random‐effects estimate presented due to significant heterogeneity |
| Uptake of screening test ‐ Numerical and categorised risk combined | 532 per 1000 | 566 per 1000 | OR 1.15 | 6442 | ⊕⊕⊝⊝ | Fixed‐effect estimate |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence (we have not assessed publication bias for any of the groups) | ||||||
| 1 MMIC: Multi‐dimensional Measure of Informed Choice 8Control risk was used as baseline risk, due to lack of studies that measure this in detail to be presented as baseline risk for the population. | ||||||
| Patient or population: patients with informed decision making about taking screening tests | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk10 | Corresponding risk | |||||
| Control | Personalised risk communication versus general risk information | |||||
| Knowledge regarding screening test/condition concerned ‐ calculated risk score (numerical) versus general information | The mean knowledge regarding screening test/condition concerned ‐ calculated risk score (numerical) versus general information in the intervention group was | 588 | ⊕⊕⊕⊝ | SMD 0.4 (0.23 to 0.56) Fixed‐effect estimate | ||
| Knowledge regarding screening test/condition concerned ‐ calculated risk score (categorised) versus general information | The mean knowledge regarding screening test/condition concerned ‐ calculated risk score (categorised) versus general information in the intervention group was | 260 | ⊕⊕⊝⊝ | SMD 0.57 (0.32 to 0.82) Fixed‐effect estimate | ||
| Knowledge regarding screening test/condition concerned ‐ personal risk factor list versus general information | The mean knowledge regarding screening test/condition concerned ‐ personal risk factor list versus general information in the intervention group was | 838 | ⊕⊕⊕⊕ | SMD 0.89 (0.75 to 1.04) Fixed effect estimate | ||
| Knowledge regarding screening test/condition concerned ‐ calculated risk score (numerical) versus general information | 244 per 1000 | 457 per 1000 | OR 2.6 | 1413 | ⊕⊕⊕⊕ high4,6,13,14 | Random‐effects estimate |
| Knowledge regarding screening test / condition concerned ‐ personal risk factor list v general information | 166 per 1000 | 586 per 1000 | OR 7.13 | 2107 | ⊕⊕⊕⊕ | Fixed‐effect estimate |
| Accurately‐perceived risk | 225 per 1000 | 324 per 1000 | OR 1.65 | 1264 | ⊕⊕⊝⊝ | Random‐effects estimate |
| Anxiety ‐ all groups | The mean anxiety in the intervention groups was | 1848 | ⊕⊝⊝⊝ | SMD ‐0.13 (‐0.29 to 0.03) Random‐effects estimate | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence (we have not assessed publication bias for any of the groups) | ||||||
| 1 This study was high risk for reporting bias. Four risk of bias items were low risk and four were unclear risk. Quality down graded by a point. 10Control risk was used as baseline risk due to lack of studies that measure this in detail to be presented as baseline risk for the population. 11Sample size less than the Optimal Information size (OIS). Quality downgraded by one point. 12Both studies were of low risk of bias and hence not downgraded. 13Significant heterogeneity among studies but all studies have same direction of effect and hence quality not down graded. 14Not downgraded for indirectness of evidence. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Informed decision Show forest plot | 3 | 2444 | Odds Ratio (Fixed, 95% CI) | 4.48 [3.62, 5.53] |
| 1.1 calculated risk score (numerical) v general information | 1 | 338 | Odds Ratio (Fixed, 95% CI) | 2.08 [1.14, 3.81] |
| 1.2 personal risk factor list v general information | 2 | 2106 | Odds Ratio (Fixed, 95% CI) | 4.98 [3.97, 6.24] |
| 2 knowledge regarding screening test / condition concerned Show forest plot | 4 | Std. Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 calculated risk score (numerical) v general information | 1 | 588 | Std. Mean Difference (Fixed, 95% CI) | 0.40 [0.23, 0.56] |
| 2.2 calculated risk score (categorised) v general information | 1 | 260 | Std. Mean Difference (Fixed, 95% CI) | 0.57 [0.32, 0.82] |
| 2.3 personal risk factor list v general information | 2 | 838 | Std. Mean Difference (Fixed, 95% CI) | 0.89 [0.75, 1.04] |
| 3 knowledge regarding screening test / condition concerned‐ proportion with good knowledge Show forest plot | 3 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 calculated risk score (numerical) v general information | 3 | 1413 | Odds Ratio (Random, 95% CI) | 2.60 [1.27, 5.34] |
| 4 knowledge regarding screening test / condition concerned‐ proportion with good knowledge Show forest plot | 2 | 2107 | Odds Ratio (Fixed, 95% CI) | 7.13 [5.79, 8.79] |
| 4.1 personal risk factor list v general information | 2 | 2107 | Odds Ratio (Fixed, 95% CI) | 7.13 [5.79, 8.79] |
| 5 accurately perceived risk Show forest plot | 3 | 1264 | Odds Ratio (M‐H, Random, 95% CI) | 1.65 [0.96, 2.81] |
| 5.1 calculated risk score (numerical) v general information | 2 | 1004 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.91, 1.64] |
| 5.2 calculated risk score (categorised) v general information | 1 | 260 | Odds Ratio (M‐H, Random, 95% CI) | 2.50 [1.48, 4.20] |
| 6 perceived risk ‐ perceiving self as appropriate candidate for test Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 calculated risk score (numerical) v general information | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Anxiety (Cancer related anxiety and helplessness scale; IES breast cancer distress) Show forest plot | 6 | 1848 | Std. Mean Difference (Random, 95% CI) | ‐0.13 [‐0.29, 0.03] |
| 7.1 calculated risk score (numerical) v general information | 4 | 1058 | Std. Mean Difference (Random, 95% CI) | ‐0.19 [‐0.42, 0.04] |
| 7.2 calculated risk score (categorised) v general information | 1 | 260 | Std. Mean Difference (Random, 95% CI) | 0.0 [‐0.24, 0.24] |
| 7.3 personal risk factor list v general information | 1 | 530 | Std. Mean Difference (Random, 95% CI) | ‐0.02 [‐0.20, 0.16] |
| 8 decision conflict (proportion with lower scores) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 personal risk factor list v general information | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 decision conflict Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 9.1 calculated risk score (numerical) v general information | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 satisfaction with decision Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 personal risk factor list v general information | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 intention to take screening test Show forest plot | 7 | Odds Ratio (Random, 95% CI) | Totals not selected | |
| 11.1 calculated risk score (numerical) v general information | 1 | Odds Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 calculated risk score (categorised) v general information | 3 | Odds Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 personal risk factor list v general information | 3 | Odds Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 intention to take genetic screening test in normal risk patients Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.1 calculated risk score (numerical) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 intention to take genetic screening test in normal risk patients Show forest plot | 2 | Mean Difference (Random, 95% CI) | ‐0.77 [‐1.42, ‐0.13] | |
| 13.1 calculated risk score (numerical) | 1 | Mean Difference (Random, 95% CI) | ‐0.52 [‐0.61, ‐0.43] | |
| 13.2 calculated risk score (categorised) | 1 | Mean Difference (Random, 95% CI) | ‐1.2 [‐1.88, ‐0.52] | |
| 14 uptake of screening test Show forest plot | 12 | 6442 | Odds Ratio (Fixed, 95% CI) | 1.15 [1.02, 1.29] |
| 14.1 calculated risk score (numerical) v general information | 6 | 2569 | Odds Ratio (Fixed, 95% CI) | 0.95 [0.78, 1.15] |
| 14.2 calculated risk score (categorised) v general information | 6 | 3873 | Odds Ratio (Fixed, 95% CI) | 1.29 [1.11, 1.51] |
| 15 uptake of screening test Show forest plot | 20 | Odds Ratio (Random, 95% CI) | Totals not selected | |
| 15.1 personal risk factor list v general information | 20 | Odds Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 appropriate use of cholesterol test Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 16.1 calculated risk score (numerical) v general information | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 improvement in risk comprehension/perception Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 17.1 calculated risk score (numerical) v general information | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 stages of change Show forest plot | Other data | No numeric data | ||
| 18.1 personal risk factor list v general information | Other data | No numeric data | ||
| 19 making a recommended behaviour change Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.1 personal risk factor list v general information | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Quality of life (SF‐36) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 20.1 calculated risk score (categorised) v general information | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 intention to take screening test Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 personal risk factor list v general information | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 uptake of screening test Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 calculated risk score (numerical) v general information | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 personal risk factor list v general information | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 knowledge regarding screening test / condition concerned Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 calculated risk score (numerical) v general information | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 accuracy of perceived risk Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 calculated risk score (numerical) v general information | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 anxiety (Cancer related anxiety and helplessness scale; IES breast cancer distress) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 calculated risk score (numerical) v general information | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 intention to take screening test Show forest plot | 2 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| 4.1 calculated risk score (categorised) v general information | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 personal risk factor list v general information | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 uptake of screening test Show forest plot | 4 | 1595 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.68, 1.03] |
| 5.1 calculated risk score (numerical) v general information | 4 | 1595 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.68, 1.03] |
| 6 uptake of screening test Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 calculated risk score (categorised) v general information | 3 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 uptake of screening test Show forest plot | 8 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 personal risk factor list v general information | 8 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 stages of change Show forest plot | Other data | No numeric data | ||
| 8.1 personal risk factor list v general information | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 uptake of screening test Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 calculated risk score (numerical) v general information | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 calculated risk score (categorised) v general information | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 personal risk factor list v general information | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 appropriate use of cholesterol test Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 personal risk factor list v general information | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 knowledge regarding screening test / condition concerned Show forest plot | 3 | 1156 | Std. Mean Difference (Fixed, 95% CI) | 0.59 [0.47, 0.71] |
| 1.1 calculated risk score (numerical) v general information | 1 | 588 | Std. Mean Difference (Fixed, 95% CI) | 0.40 [0.23, 0.56] |
| 1.2 calculated risk score (categorised) v general information | 1 | 260 | Std. Mean Difference (Fixed, 95% CI) | 0.57 [0.32, 0.82] |
| 1.3 personal risk factor list v general information | 1 | 308 | Std. Mean Difference (Fixed, 95% CI) | 1.02 [0.78, 1.26] |
| 2 perceived risk ‐ perceiving self as appropriate candidate for test Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 calculated risk score (numerical) v general information | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 accurately perceived risk Show forest plot | 2 | 460 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.25 [1.44, 3.53] |
| 3.1 calculated risk score (numerical) v general information | 1 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.70, 4.06] |
| 3.2 calculated risk score (categorised) v general information | 1 | 260 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.50 [1.48, 4.20] |
| 4 anxiety (Cancer related anxiety and helplessness scale; IES breast cancer distress) Show forest plot | 3 | 662 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.14, 0.28] |
| 4.1 calculated risk score (numerical) v general information | 2 | 402 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.16, 0.48] |
| 4.2 calculated risk score (categorised) v general information | 1 | 260 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.28, 0.28] |
| 5 intention to take screening test Show forest plot | 3 | 854 | Odds Ratio (Fixed, 95% CI) | 1.29 [0.97, 1.72] |
| 5.1 calculated risk score (categorised) v general information | 1 | 260 | Odds Ratio (Fixed, 95% CI) | 0.21 [0.07, 0.64] |
| 5.2 personal risk factor list v general information | 2 | 594 | Odds Ratio (Fixed, 95% CI) | 1.47 [1.09, 1.97] |
| 6 uptake of screening test Show forest plot | 5 | 2085 | Odds Ratio (Fixed, 95% CI) | 1.22 [1.00, 1.48] |
| 6.1 calculated risk score (numerical) v general information | 3 | 1156 | Odds Ratio (Fixed, 95% CI) | 1.05 [0.79, 1.40] |
| 6.2 calculated risk score (categorised) v general information | 2 | 929 | Odds Ratio (Fixed, 95% CI) | 1.38 [1.06, 1.80] |
| 7 uptake of screening test Show forest plot | 7 | Odds Ratio (Random, 95% CI) | Totals not selected | |
| 7.1 personal risk factor list v general information | 7 | Odds Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 stages of change Show forest plot | Other data | No numeric data | ||
| 8.1 personal risk factor list v general information | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Informed decision Show forest plot | 2 | 2107 | Odds Ratio (Fixed, 95% CI) | 4.99 [3.98, 6.25] |
| 1.1 personal risk factor list v general information | 2 | 2107 | Odds Ratio (Fixed, 95% CI) | 4.99 [3.98, 6.25] |
| 2 knowledge regarding screening test / condition concerned Show forest plot | 3 | 2378 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.81 [5.57, 8.34] |
| 2.1 calculated risk score (numerical) v general information | 1 | 271 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.26 [1.86, 9.74] |
| 2.2 personal risk factor list v general information | 2 | 2107 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.04 [5.72, 8.67] |
| 3 knowledge regarding screening test / condition concerned Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 personal risk factor list v general information | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 anxiety (Cancer related anxiety and helplessness scale; IES breast cancer distress) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 personal risk factor list v general information | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 satisfaction with decision Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 personal risk factor list v general information | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Decision conflict (proportion with low scores) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 personal risk factor list v general information | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 intention to take screening test Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 calculated risk score (numerical) v general information | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 uptake of screening test Show forest plot | 10 | 5567 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.82, 1.37] |
| 8.1 calculated risk score (numerical) v general information | 1 | 271 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.28, 2.17] |
| 8.2 calculated risk score (categorised) v general information | 3 | 1557 | Odds Ratio (M‐H, Random, 95% CI) | 1.34 [1.01, 1.77] |
| 8.3 personal risk factor list v general information | 6 | 3739 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.68, 1.35] |
| 9 stages of change Show forest plot | Other data | No numeric data | ||
| 9.1 personal risk factor list v general information | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 uptake of screening test Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 personal risk factor list v general information | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 knowledge regarding screening test / condition concerned (Generic Inverse Variance) Show forest plot | 1 | Std. Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 1.1 calculated risk score(numerical) v general information | 1 | Std. Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 intention to take screening test Show forest plot | 1 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| 2.1 Personal risk factor list v general information | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 uptake of screening test Show forest plot | 3 | 1284 | Odds Ratio (Fixed, 95% CI) | 1.69 [1.32, 2.18] |
| 3.1 calculated risk score(numerical) v general information | 1 | 587 | Odds Ratio (Fixed, 95% CI) | 1.67 [1.04, 2.69] |
| 3.2 personal risk factor list v general information | 2 | 697 | Odds Ratio (Fixed, 95% CI) | 1.70 [1.26, 2.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Informed decision Show forest plot | 1 | 338 | Odds Ratio (Fixed, 95% CI) | 2.08 [1.14, 3.81] |
| 1.1 calculated risk score(numerical) v general information | 1 | 338 | Odds Ratio (Fixed, 95% CI) | 2.08 [1.14, 3.81] |
| 2 knowledge regarding screening test / condition concerned Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 calculated risk score(numerical) v general information | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 uptake of screening test Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 calculated risk score(numerical) v general information | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 anxiety Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 calculated risk score(numerical) v general information | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 decision conflict Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 calculated risk score(numerical) v general information | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

