Konservative Behandlung von durch Prostatektomie bedingte Harninkontinenz
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT | |
| Participants | Time of recruitment: Pre‐operatively Population: 95 men after radical prostatectomy (whole population, with or without UI) Included: men who underwent RP for clinically localized prostate cancer. Patients were not taking anticholinergic drugs or any drug that may affect continence for the duration of the study Excluded: previous urethral, bladder or prostate surgery, prior urinary or faecal incontinence, neurogenic and psychiatric disorders, pre‐operative urinary tract complications, radiotherapy Age (mean, SD): A 57.2 (3.25); B 58.8 (5.4); C 56.3 (6.8) Dropouts: 10 A: 4 (2 received radiotherapy, 2 had post‐operative complications); B: 4 (2 received radiotherapy, 2 refused follow up); C: 2 (2 received radiotherapy) No differential dropout Baseline characteristics: Comparable at baseline | |
| Interventions | Time of intervention: Post‐operative treatment A (26): PFMT alone. At catheter removal men received standard care of verbal and written instructions, active instructions from physiotherapist to perform 3 sets of 15 to 20 contractions daily, for a duration of 3 to 5 seconds with a 6 to 10 second rest period, encouraged to perform exercises before functional activities such as sneezing, coughing, or lifting weight, also in the supine position, sitting, squatting and going up and down stairs. B (26): ES: treatment started one week after catheter removal, patients received 15 minutes of twice weekly electrical stimulation for 12 weeks C (28): PFMT + BFB + ES: treatment started one week after catheter removal, patients received twice weekly treatment with 15 minutes of electrical stimulation and 15 minutes of biofeedback for 12 weeks, instructed to perform 3 series of 10 rapid contractions, 3 sustained contractions of 5, 7 or 10 seconds and then 10 contractions during prolonged expiration in the supine position All patients were given a logbook to complete daily regarding self‐report of exercises Duration of treatment: 12 weeks Follow up: 6, 12 and 24 weeks | |
| Outcomes | Primary outcome (number of men with UI) Number of incontinent men (defined as some pads required and weight gain of the pad > 1 g during the test) Baseline: A 22/26; B 22/26; C 23/28 2 months: A 21/26; B 19/26; C 18/28 3 months: A 17/26; B 12/26; C 20/28 6 months: A 9/26; B 6/26; C 1/28 Other outcomes Leakage weight in grams on 24 hour pad test (mean (SD) N) Baseline: A 791 (380.3) 26; B 790 (399.46) 26; C 785 (311.98) 28 2 months: A 533 (316.53) 26; B 383 (145.87) 26; C 263 (145.87) 28 3 months : A 260 (216.53) 26; B 132 (145.87) 26; C 83 (145.87) 28 6 months : A 123 (116.53) 26; B 98 (105.87) 26; C 36 (95.87) 28 Quality of life (Higher score = worse) Mean scores of IIQ‐7 (mean (SD) N) 2 months: 40 (23) 26; B 36 (25) 26; C 26 (25) 28 3 months: 32 (26) 26; B 29 (28) 26; C 20 (24) 28 6 months: 25 (26) 26; B 23 (24) 26; C 15 (25) 28 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using “a computer‐generated random‐number list” |
| Allocation concealment (selection bias) | Low risk | “sealed envelopes” |
| Blinding of participants (performance bias) | High risk | Blinding to treatment not possible |
| Blinding of personnel (performance bias) | Unclear risk | “One experienced physiotherapist delivered all therapy” |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | Low risk | 4 (2 received radiotherapy, 2 had post‐operative complications); B: 4 (2 received radiotherapy, 2 refused follow‐up); C: 2 (2 received radiotherapy). No differential dropout |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods were reported |
| Financial support | Unclear risk | Not reported. Therefore judged to be unclear risk. |
| Approved by medical ethics committee | Low risk | “At the time of this study, there was no Human Research Ethics Committee established in the faculty, but the study was approved by the postgraduate affairs and departmental committee” |
| Informed consent | Low risk | “All patients signed an informed consent form” |
| ITT analysis | Low risk | Assumed from flow diagram of patients |
| Methods | Randomised: yes | |
| Participants | Recruitment: pre‐operative Included: all men undergoing radical prostatectomy N = 100 consecutive patients with stage T1c to T2c prostate cancer undergoing radical retropubic prostatectomy by a single surgeon randomised into 2 groups | |
| Interventions | Pre‐operative intervention Group A (50) intervention: 2 to 4 weeks prior to surgery, participants underwent a 45 minute session with nurse trained in biofeedback. Patients were instructed to perform graded PFMT. Contractions of 5 to 10 seconds, 10 to 15 repetitions were performed with biofeedback (surface electrodes used to measure muscle strength). Advised to practice the exercises 4 times per day until surgery Group B (50) control: no biofeedback training. Written and brief verbal instructions from a nurse on how to perform PFMT (isolate muscle that stops urine flow, practice 4 times per day, 10 to 15 repetitions) Both groups: Encouraged to perform PME 4 times per day after catheter removal 2 weeks post‐operatively Length of follow‐up: 6 months | |
| Outcomes | Main outcome: time to return of continence measured by number of pads used Continence definition: use of 1 pad or less per day Data collection: at 1, 2, 3, 4, and 6 months post‐operatively There was no significant difference in incontinence between the groups | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | "Randomised" |
| Blinding of participants (performance bias) | High risk | Blinding not possible |
| Blinding of personnel (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessment nurse not involved in intervention |
| Incomplete outcome data (attrition bias) | Unclear risk | Three patients dropped out of the biofeedback arm of the study because they never completed their biofeedback session |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | No description |
| Approved by medical ethics committee | Unclear risk | Not reported |
| Informed consent | Unclear risk | Not reported |
| ITT analysis | Unclear risk | Not specified |
| Methods | Randomised: yes | |
| Participants | Recruitment: pre‐operative Included: all men undergoing radical prostatectomy N = 125 volunteer patients randomised, 13 excluded after randomisation Analysis on N = 112 men aged 53 to 68 years who underwent radical prostatectomy for prostate cancer. To be eligible, the men had to be ambulatory, continent and identified at least 1 week prior to their surgery | |
| Interventions | Pre‐operative intervention Group A (57) intervention: single session of biofeedback (rectal probe to measure intra‐abdominal rectal pressure and external anal sphincter contraction) assisted behavioural training. Feedback and verbal instruction used to teach control of pelvic muscles. Taught to contract sphincter during 2 to 10 seconds periods separated by 2 to 10 seconds of relaxation, dependent on ability. Written instructions for daily at home practice of 45 PFM exercises daily (3 sessions of 15 exercises each time). Additionally instructed to slow or interrupt voiding once daily. Encouraged to exercise daily preoperatively, then resume when catheter removed post‐operatively Group B (55) control: usual care of brief verbal instructions post operatively to interrupt the voiding stream plus any instruction from physician Length of follow‐up: 6 months | |
| Outcomes | Main outcome: Continence definition: 3 consecutive weekly 1 day diaries showing no leakage or a 7 day diary showing no leakage Data collection: 1 day bladder diaries mailed in each week. Questionaire on bladder control, lifestyle and 7 day bladder diary at 6 weeks, 3 months and 6 months post‐surgery Time to continence was significantly reduced in the intervention group. The intervention group had a significantly smaller proportion of those with severe or continual leakage at 6 months, and stress type urine loss. No differences on quality of life, return to work or activities between the groups | |
| Notes | Analysis by "intention to treat". Additional data supplied to KFH by author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified by age and tumour differentiation, then randomised using computer generated random numbers, block size of 4 to ensure equity of number in each group |
| Allocation concealment (selection bias) | Low risk | Computer allocated. "The randomization schedule was implemented by the research nurse, so that interventionists would be blinded to the next group assignment." |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | Low risk | Intervention providers and bladder diary scorers were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Bladder diary scorers were blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | 6 and 4 lost to follow‐up at 6 months; 6 and 7 excluded after randomisation as surgery cancelled |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Low risk | "Supported by Grant RO1 DK50283 from the National Institute for Diabetes and Digestive and Kidney Diseases, National Institutes of Health" "The funding organization did not participate in the design or conduct of the study; collection, management, analysis or interpretation of the data; or the preparation, review or approval of the manuscript." |
| Approved by medical ethics committee | Low risk | "This study was reviewed and approved by the University and VA Medical center Institutional Review Boards for Human use" |
| Informed consent | Unclear risk | "All participants provided informed consent" |
| ITT analysis | Low risk | "intention to treat". Patient flow diagram |
| Methods | Randomised: yes Method of allocation: 100 consecutive patients Blinding: no | |
| Participants | Number of men 100 Recruitment: pre‐operative Included: all men undergoing radical prostatectomy Excluded: impaired mental status, BMI.27, diabetes mellitus, neurological‐rheumatic‐immune disease, neck‐urethral surgery, prior catheterisation, post‐operative catheterisation time longer than 6 days. Aged: 48‐68 years | |
| Interventions | Group A (50) intervention: PFMT both pre and post‐operatively. A structured PFMT program 30 and 15 days before surgery, previous physiotherapist evaluation to provide the patients with feedback about the quality of pelvic floor muscle function, PC test (endurance and contraction quality), breathing co‐ordination, typify muscle contraction as tonic and modify incorrect physical attitudes. This was also repeated after the procedure Group B (50) intervention: PFMT post‐operatively only (no details as to whether this is the same as the treatment pre‐operatively above) Duration of treatment: not stated Length of follow‐up: at one and three months | |
| Outcomes | UI at 1 month: A 33/59; B 47/59 3 month: A 24/59; B 37/59 24 hour pad test, number of subjects with pad test weight of > 150 g 1 month: A 15/59; B 20/59 3 month: A 10/59; B 19/59 Quality of life measured by the ICS male sf questionnaire, mean score 1 month: A 14.6 (6.36) 59; B 18.3 (6.36) 59 3month: A 8.1 (7.04) 59; B 12.2 (6.36) 59 Satisfaction scale (PGI‐I) used only for Group A and 75% reported extreme satisfaction for pre‐operative PFMT | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Individuals were randomised by a computer‐generated list that was centrally maintained". "Permuted block randomisation was used, with a block size of every 10 consecutively enrolled participants" |
| Allocation concealment (selection bias) | Low risk | "Individuals were randomised by a computer‐generated list that was centrally maintained". "Permuted block randomisation was used, with a block size of every 10 consecutively enrolled participants" |
| Blinding of participants (performance bias) | High risk | Blinding not possible |
| Blinding of personnel (performance bias) | Low risk | "The surgeon who performed the procedures was blinded to randomisation allocation throughout the study" |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Only the statistician and the data monitoring committee saw unblinded data" |
| Incomplete outcome data (attrition bias) | Unclear risk | No description. It appears that there were was no loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Therefore judged to be unclear risk |
| Financial support | Low risk | None |
| Approved by medical ethics committee | Low risk | "The study was approved by the university institutional review board" |
| Informed consent | Low risk | Patients were "provided written informed consent" |
| ITT analysis | Low risk | Assumed from patient flow chart |
| Methods | RCT | |
| Participants | Time of recruitment: pre‐operative Population: men having a laparoscopic radical prostatectomy (whole population, with or without UI) Included: patients with prostate cancer, undergoing laparoscopic radical prostatectomy Excluded: neurological disorders, a medical history with invasive perineal and/or rectal surgery, preoperatively existing stress urinary incontinence, radiation, ≥ 75 years Age (mean, SD): A 63.7 (5.3); B 63.7 (5.3) Dropouts: 9 from A (1 unable to understand Dutch, 3 post‐operative radiotherapy, 1 oesophageal cancer, 3 discontinued intervention at own request, 1 excluded due to poor compliance) 8 from B (2 post operative radiotherapy, 1 pelvic lymph node dissection, 1 died of cause unrelated to prostate cancer, 5 discontinued intervention at own request, 1 prolonged catheter) Not differential dropout Baseline characteristics: comparable at baseline | |
| Interventions | Time of intervention: pre‐operative (+ postoperative treatment for all men) A (56): 30 mins of guided PFMT + biofeedback weekly for 4 weeks before surgery, received written instructions to: carry out two sets of 30 contractions during abdominal breathing, one breath between each contraction; restart PFMT after catheter removal (7 to 10 days after surgery) B (46): received written instructions on PFMT after catheter removal (7 to 10 days after surgery) All men were seen before surgery by a physiotherapist, who explained relevant anatomy, anal visual inspection and digital palpation, biofeedback registration with rectal probe All patients received PFMT + biofeedback and/or electrical stimulation if still incontinent after 6 weeks Duration of treatment Follow up: 6 weeks, 3 months, 6 months, 9 months and 12 months after surgery | |
| Outcomes | Primary outcome (number of men with UI) Number of incontinent men (leakage on 24 hour pad test) 12 months: A 20/58; B 9/45 Other outcomes Number of continent men after 1 year (no leakage at all on 24 hour pad test) 12 months: 38/58; B 36/45 Adverse effects: A 0/56; B 0/46 Quality of life King’s Health Questionnaire (KHQ) (mean (SD) N): General health 12 months: A 24.48 (50.7) 56; B 29.64 (50.7) 46 Role limitations 12 months: A 21.36 (22.2) 56; B 17.73 (22.2) 46 Physical limitations 12 months: A 16.49 (15.45) 56; B 13.48 (15.45) 46 Social limitations 12 months: A 7.98 (24.8) 56; B 4.15 (24.8) 46 Personal 12 months: A 18.72 (4.4) 56; B 19.62 (4.4) 46 Emotional 12 months: A 5.08 (7.0) 56; B 4.24 (7.0) 46 Sleep or energy disturbance: A 9.13 (39.0) 56; B 6.13 (39.0) 46 Symptom severity: A 14.62 (86.1) 56; B 10.93 (86.1) 46 | |
| Notes | Trial was stopped early because interim analysis found no benefit for group A Additional information supplied by author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Computer‐generated random numbers with block randomization and variable block size” |
| Allocation concealment (selection bias) | Low risk | "central computer system" |
| Blinding of participants (performance bias) | Unclear risk | “participants were also blinded until their first visit to the pelvic floor physiotherapist” |
| Blinding of personnel (performance bias) | Low risk | “The pelvic floor physiotherapists were blinded to randomization” (to pre‐operative randomisation) |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessor blinded |
| Incomplete outcome data (attrition bias) | Low risk | 9 from A (1 unable to understand Dutch, 3 post‐operative radiotherapy, 1 oesophageal cancer, 3 discontinued intervention at own request, 1 excluded due to poor compliance) 8 from B (2 post‐operative radiotherapy, 1 pelvic lymph node dissection, 1 died of cause unrelated to prostate cancer, 5 discontinued intervention at own request, 1 prolonged catheter á demeure). Not differential dropout |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods were reported |
| Financial support | Low risk | None |
| Approved by medical ethics committee | Low risk | “Medical ethical approval was obtained from the Medical Ethics committee of our university hospital” |
| Informed consent | Low risk | “Informed consent was obtained” |
| ITT analysis | Low risk | Assumed from flow diagram |
| Methods | Randomised: yes | |
| Participants | Recruitment: post‐operative Included: men incontinent post‐radical prostatectomy (≥ 1 g urine loss on 1 hour pad test), one week after catheter removal Excluded: pre‐operative UI N = 66 men completing the trial, 33 in intervention group, 33 in control All participants had a radical retropubic prostatectomy and lived within 75 km of hospital Age range 61 to 67 years | |
| Interventions | Post‐operative intervention A (35) intervention: 9 or less sessions of physiotherapy guided pelvic floor exercises after surgery plus information folder B (44) control: exercise instruction through information folder only Length of follow‐up: 6.5 months Dropouts: A 1, B 2 due to stricture; + A 1, B 3 refused further measurements; + B 5 withdrew consent or 1 did not understand | |
| Outcomes | Continence definition: incontinence defined as loss of at least 1 gram of urine on 1 hour pad test and 4 grams on the 24 hour pad test Main outcome: urinary incontinence on both 1 hour (> 1 g) and 24 hour (> 4 g) pad tests Secondary outcome: urodynamic study (urethral pressure profilometry) Data collection: 1 and 26 weeks after catheter removal Number of wet men at 6 months: A: 17/33, B: 20/33 No significance difference in continence rates between the groups | |
| Notes | Sample size required 96 men in each arm Other data presented as median (IQR) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator to achieve 1:1 ratio |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes, sequentially numbered, opened by trial nurse after result of pad test was known |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | Unclear risk | "The physiotherapist who guided men in the PGPFME group was unaware of the outcome data of both treatment groups" |
| Blinding of outcome assessment (detection bias) | Unclear risk | "The data for outcome assessment (e.g. pad‐tests, voiding diaries) were collected and entered in a data base by a trial nurse who was not involved in the treatment or intervention" |
| Incomplete outcome data (attrition bias) | High risk | 13 dropped out (of which 2 from intervention group) |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods reported |
| Financial support | Unclear risk | No description |
| Approved by medical ethics committee | Low risk | "approved by our institutional review board" |
| Informed consent | Low risk | "informed consent" |
| ITT analysis | Unclear risk | "the concept of an intent to treat analysis was not applied". Authors also state, "Participants were analysed in the group to which they were allocated at randomization" |
| Methods | RCT Cross‐over design | |
| Participants | Time of recruitment: post‐operative Population: 74 men with incontinence after prostate surgery Included: men who were experiencing incontinence more than a year after prostate cancer treatment and using absorbent pads Excluded: no description Age (mean, SD): no description Dropouts: no information Baseline characteristics: no information | |
| Interventions | Time of intervention: post‐operative A: penile compression device (clamp) B: sheath drainage system (sheath) C: body‐worn urinals (BWU) D: pads alone All men tested each device for three weeks and asked to state which device was preferred Duration of treatment: 3 weeks with each device Follow‐up: 3 months | |
| Outcomes | Primary outcome (number of men with UI) Not reported Other outcomes Overall opinion: patient satisfaction questionnaire related to device performance Asked to state which device they preferred: Pads were most highly rated compared with sheaths (P = 0.31), clamps (P < 0.01) and BWUs (P < 0.001) The clamp was rated as more secure, less leaky and less restrictive of clothing choice than the others (P < 0.05) but was more painful than the rest (P<0.002) Three months later asked which products they were actually using and for what activities and circumstances: 30/56 using a combination of devices and pads Quality of life EORTC QLC C30 IIQ‐7 ICIQ‐UI King's Health Questionnaire | |
| Notes | Awaiting further information from author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “random order” cross‐over design |
| Allocation concealment (selection bias) | Unclear risk | “random order” |
| Blinding of participants (performance bias) | High risk | Blinding was not possible for participants |
| Blinding of personnel (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | Unclear risk | No information. Therefore judged to be unclear risk |
| Selective reporting (reporting bias) | High risk | Quality of life outcome not reported |
| Financial support | Low risk | Prostate Cancer UK |
| Approved by medical ethics committee | Low risk | Southampton and South West Hampshire Research Ethics Committee (REC) |
| Informed consent | Low risk | Yes |
| ITT analysis | Unclear risk | Not specified |
| Methods | Randomised: yes Blinding: not described | |
| Participants | Recruitment: post‐operative Included: all men undergoing RRP N = 300 consecutive men post RRP, randomised after catheter removal to 2 groups | |
| Interventions | Post‐operative intervention Group A (150) intervention: formal instruction (3 treatment sessions plus at home exercises) in PFMT using verbal explanation, palpation and visualization of the base of the penis with a mirror, in different positions and prior to sneezing, coughing or lifting Group B (150) control: no formal instruction Length of follow‐up: 12 months | |
| Outcomes | Main outcome: urine loss on 1 hour and 24 hour pad tests plus number of pads used daily Continence definition: 0 to 1 pads per day Data collection: 1, 3, 6, and 12 months Wet (leakage or use of pads) 1 month: A 145/150, B 147/150 3 months: A 115/150, B 129/150 6 months: A 35/150, B 102/150 12 months: A 16/150, B 49/148 Surgical implantation of artificial urinary sphincter: A 2/150, B 3/148 | |
| Notes | 74% of the intervention group achieved continence at 3 months compared to only 30% of the control (a significant difference favouring intervention) Differences between the groups declined between 6 to 12 months, with most participants achieving continence in 1 year | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation, block size of 4 for 2 groups (A and B) with only one permutation code (ABBA) |
| Allocation concealment (selection bias) | Unclear risk | Not stated. Therefore judged to be unclear risk |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description of blinding of pad test or data entry from questionnaires |
| Incomplete outcome data (attrition bias) | Unclear risk | 2 dropped out of control group but none from intervention |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Low risk | "Approved by the Ethics Committee of our Institution" |
| Informed consent | Low risk | "All patients signed an informed consent form" |
| ITT analysis | Unclear risk | Not specified |
| Methods | Randomised: yes Intention to treat: yes | |
| Participants | Recruitment: post‐operative Included: men incontinent post‐radical prostatectomy one week after catheter removal N = 42 consecutive patients | |
| Interventions | Post‐operative intervention Group A (28) intervention: initiated after catheter removal. Intervention group received 15 treatment sessions (3 times per week for 30 minutes) of PFMT with EMG (surface) biofeedback in clinic Group B (14) control: instruction with verbal feedback and an information pamphlet with instructions to perform PME 50 to 100 times daily at home Length of follow‐up: 6 months | |
| Outcomes | Main outcome: incontinence episodes measured by 1 hour pad test and continence questionnaire (pads used, number of incontinence episodes) Continence definition: incontinence defined as a urine loss of > 1 g on the 1 hour pad test; 2 or more pads/day a not deemed a "socially acceptable continence rate" Data collection: baseline, 1, 2, 3 and 6 months Level of incontinence in both groups declined over the 6 months of the study. Control group had less urine loss and appeared to regain continence sooner, but the difference was not significant | |
| Notes | Additional data supplied to KFH by author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Allocation concealment (selection bias) | Unclear risk | Randomised 2:1 to intervention: control groups |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | High risk | 1 dropped out of intervention group but followed control intervention ‐ unclear if analysed as control |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Informed consent | Low risk | "Patients were informed about the aims and perspectives of the study. Eligible patients consented" |
| ITT analysis | Unclear risk | "Analysed using the intention‐to‐treat approach". Authors also state "One of the patients initially randomized to group A could not follow the programme but performed PMEs under verbal instruction" |
| Methods | RCT | |
| Participants | Time of recruitment: pre‐operative Population: 83 men undergoing nerve sparing radical prostatectomy (whole population, with or without UI) Included: sexually active men with an IIEF score of at least 18 without aids, continent pre‐operatively Excluded: condition that may prevent patient being able to have post‐operative treatment with PDE5‐inhibitor Age (mean, range): A 62 (46‐73); B 65 (49 to 76) Dropouts: 12 from group A (3 excluded because underwent non‐nerve sparing surgery, 2 withdrew consent, 1 lost a partner, 6 non‐compliance), 3 from group B (2 excluded because underwent non‐nerve sparing surgery, 1 withdrew consent). Differential dropout Baseline characteristics: comparable except Group A significantly more LUTS pre‐operatively | |
| Interventions | Time of intervention: pre‐operative + post‐operative A (30): pre‐operative session guided PFMT + instruction on how to use penile vibratory stimulation device, instructed to stimulate frenulum once daily, 10 seconds of stimulation then 10 second pause, repeated 10 times for 1 week pre‐operatively, Instructed to restart stimulation after catheter removal for 6 weeks B (38): pre‐operative session guided PFMT All men were offered a PDE5 inhibitor after 1 month post‐operatively and also received telephone contact to ensure compliance with treatment Duration of treatment: 6 weeks Follow up: 3, 6 and 12 months post‐operatively | |
| Outcomes | Primary outcome (number of men with UI) Number of incontinent men (men reporting use of more than one pad daily) 3 months: A 14/42; B 15/41 6 months: A 7/42; B 3/41 12 months: A 3/30; B 2/38 (dropout figures added to 3 and 6 months) Other outcomes Continence rate (patients reporting use of up to one pad daily for security reasons only) 3 months: A 65.5%; B 62.9%, P = 0.83 6 months : A 83.3%; B 91.9%, P = 0.28 12 months : A 90%; B 94.7%, P = 0.46 Median (range) pad use 3 months: A 1 (0 to 6); B 5 (0 to ‐34), P = 0.09 6 months: A 0 (0 to 3); B 1/3 (0 to 6), P = 0.14 12 months: A 0 (0 to 2); B 0 (0 to 3), P = 0.56 Median (range) IIEF‐5 3 months : A 5 (0 to 25); B 5 (0‐25), P = 0.25 6 months : A 10.5 (0 to 25); B 5 (0‐25), P = 0.08 12 months : 18 (0 to 25); B 7.5 (0‐25), P = 0.09 IIEF ≥ 18, n/N (%) 3 months: 5/30 (17); B 4/38 (11), P = 0.46 6 months: 13/30 (43); B 9/38 (24), P = 0.09 12 months: 16/30 (53); B 12/38 (32), P = 0.07 Adverse effects: A: 5/30 reported side effects as a result of penile vibratory stimulation (1 red spots on glans penis, 1 small laceration + some bleeding, 2 complained of soreness, 1 frank pain post‐operatively) B: 0/38 Quality of life Median (range) DAN‐PSS post‐operatively 3 months: A 1 (0 to 34); B 5 (0‐34), P = 0.74 6 months: A 2 (0 to 41); B 1 (0‐48), P = 0.74 12 months: A 3 (0 to 36); B 0.5 (0‐21), P = 0.13 | |
| Notes | Further information provided by authors PDE5 (phosphodiesterase yype 5) inhibitor is used for erectile dysfunction | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized prospective trial" and “randomized by a draw” |
| Allocation concealment (selection bias) | Low risk | Used opaque sealed envelopes |
| Blinding of participants (performance bias) | High risk | “It was not possible to create a believable sham device, which could maintain blinding of the study subjects” |
| Blinding of personnel (performance bias) | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) | High risk | Outcome assessor not blinded |
| Incomplete outcome data (attrition bias) | High risk | 12 from group A (3 excluded because underwent non‐nerve sparing surgery, 2 withdrew consent, 1 lost a partner, 6 non‐compliance), 3 from group B (2 excluded because underwent non‐nerve sparing surgery, 1 withdrew consent). Differential dropout |
| Selective reporting (reporting bias) | Low risk | Outcomes in methods reported |
| Financial support | Low risk | “This study was funded by unrestricted grants from the Velux Foundation and Grosserer L.F. Foghts Foundation” |
| Approved by medical ethics committee | Low risk | “The study was approved by the Danish ethical counsel and the Danish Data protection Agency” |
| Informed consent | Low risk | Assumed as they acquired ethical approval |
| ITT analysis | Low risk | Assumed from patient flow diagram |
| Methods | Randomised: yes | |
| Participants | Recruitment: post‐operative Included: men incontinence post‐radical prostatectomy at 6 weeks post surgery N = 30 men: 6 weeks post‐radical prostatectomy with post‐void residual of < 50 ml; no previous TURP, no urinary tract infection, no neurological conditions | |
| Interventions | Post‐operative intervention. Group A (13): intervention, biofeedback (perineal patch EMG) enhanced PFMT; exercise treatment sessions at 6, 7, 9, 11, and 16 weeks post‐operatively Group B (10): control, completed bladder diary but did not have any other intervention Length of follow‐up: 12 months | |
| Outcomes | Main outcome: urine loss measured by voiding diary, 48 hour pad test (reported as mean grams of urine lost in 24 hours), and incontinence questionnaire Continence definition: not clear. Participants described as "completely dry" or with "significant incontinence" Data collection: 6, 12 and 24 weeks There were no significant differences between treatment or control groups on any of the outcome measures at any of the measurement intervals | |
| Notes | Numbers in the groups unclear as 5 withdrew from the study after initial randomisation. Not clear how many were in each group prior to follow‐up at 6 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Allocation concealment (selection bias) | Unclear risk | "Randomised" |
| Blinding of participants (performance bias) | High risk | Blinding not possible. Therefore judged to be at high risk |
| Blinding of personnel (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | Unclear risk | Five men withdrew after initial randomisation. Dropouts from 25 left at 6 weeks appears to be 10 |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Low risk | None |
| Approved by medical ethics committee | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Informed consent | Low risk | "Informed consent was obtained" |
| ITT analysis | Unclear risk | Not specified |
| Methods | RCT | |
| Participants | Time of recruitment: pre‐operative Population: men having a radical prostatectomy (whole population, with or without UI) Included: men planning to undergo open radical prostatectomy (ORP) or robot‐assisted laparoscopic radical prostatectomy (RARP) Willing to accept ambulatory visits once a week until total continence was achieved; willing to perform measurements pre‐operatively and at 1 month, 3 months, 6 months and 12 months after surgery Excluded: cognitive problems; non‐Dutch speaking; simultaneous other surgery; transport problems; lack of time; psychosocial/other medical problems; refused participation; insisted on preoperative PFMT; not approachable; not enough time between diagnosis and date of planned surgery Age (mean, SD): A 62 (5.90); B 62 (6.33) Dropouts: 6 from A; (1 died, 1 cerebrovascular accident, 3 transport problems, 1 refused further participation) 4 from B: (2 transport problems, 2 refused further participation). Not differential dropout Baseline characteristics: Comparable at baseline | |
| Interventions | Time of intervention: pre‐operative A (85): 30 mins of guided PFMT + biofeedback weekly for 3 weeks before surgery instructed to: carry out 60 contractions a day at home; contract their pelvic floor while coughing, and sitting down or getting up from a chair; restart PFMT on day 4 after surgery while catheter was in situ
B (85): instructed to start PFMT on the day after catheter removal (e.g. 2 to 3 weeks after surgery) All men performed an individual guided exercise programme with digital or EMG biofeedback postoperatively weekly, delivered by a therapist (blinded to group allocation) different from the pre‐operative Group A therapist. This included advice on using PF muscles to prevent leakage during functional activities Duration of treatment: as long as any degree of UI persisted Follow up: 1 month, 3 months, 6 months and 12 months after surgery | |
| Outcomes | Primary outcome (number of men with UI) Number of incontinent men (1 hour pad test defined as ≤ 1 g) 1 month: A 37/85; B 35/86, P = 0.758 3 months: A 15/86; B 15/86, P = 1.000 6 months: A 8/86; B 5/85, P = 0.566 12 months: A 7/81; B 7/83, P = 1.000 Other outcomes Cumulative incidence of number of continent men 1 month: A 44/85; B 44/85 3 months: A 67/85; B 71/85 6 months: A 80/85; B 80/85 12 months: A 83/85; B 81/85 Point prevalence of continence, 1 hour pad test, defined as 0 g 1 month: A 42/85; B 41/86 3 months: A 63/86; B 61/86 6 months: A 76/86; B 73/85 12 months: A 68/81; B 73/83 Point prevalence of continence, VAS scale, defined as ≤ 1/10 1 month: A 35/89; B 38/88 3 months: A 64/88; B 52/87 6 months: A 73/88; B 65/86 12 months: A 72/84; B 62/84 Urine loss on 24 hour pad test in grams (mean (SD) N): 1 month: A 90 (?) 85; B 85 (?) 85 3 months: A 17 (?) 85; B 13 (?) 85 6 months: A 12 (?) 85; B 3 (?) 85 12 months: A 2 (?) 85; B 3 (?) 85 Quality of life International prostate Symptom Score (IPSS), King’s Health Questionnaire (KHQ): data not given Only one aspect of the King’s Health Questionnaire, incontinence impact, favoured A at 3 (P = 0.008) and 6 months (P = 0.024) after surgery | |
| Notes | Some men had pre‐operative incontinence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence of randomisation was carried out using a “computer program” and was “determined by the patients’ presence at the outpatient urology clinic”. It is unclear what influence the patients’ presence had on randomisation |
| Allocation concealment (selection bias) | Low risk | “Allocation to the treatment groups was concealed”. Method not reported |
| Blinding of participants (performance bias) | High risk | Blinding was not possible for participants |
| Blinding of personnel (performance bias) | Low risk | Post‐operative treatment was delivered by a therapist who was blinded to group allocation and treatment delivered by the pre‐operative Group A therapist |
| Blinding of outcome assessment (detection bias) | Low risk | “One blinded and well‐trained assessor performed the measurements” |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts: Group A: 6 (1 died, 1 cerebrovascular accident, 3 transport problems, 1 refused further participation); Group B: 4 (2 transport problems, 2 refused further participation) |
| Selective reporting (reporting bias) | High risk | Results not reported for quality of life outcomes |
| Financial support | Low risk | Unconditional funding from the “Agency for innovation by Science and Technology (Applied Biomedical Research): governmental grant” |
| Approved by medical ethics committee | Low risk | “Ethical approval from the commission on medical ethics of the University Hospitals Leuven” |
| Informed consent | Low risk | Patients “signed written informed consent” |
| ITT analysis | Low risk | “Data were analyzed according to the intention‐to‐treat principle” |
| Methods | RCT | |
| Participants | Time of recruitment: pre‐operative Population: 100 men undergoing a radical prostatectomy (whole population, with or without UI) Included: men undergoing RP for clinically localized prostate cancer. Excluded: patients who had previous pelvic organ surgeries, patients with central or peripheral neurologic diseases Age (mean, SD): not reported Dropouts: not reported Baseline characteristics: not reported | |
| Interventions | Time of intervention: pre‐operative (post‐operative treatment for all men) A (50): pre‐operative PFMT for 2 weeks + post‐operative PFMT programme B (50): post‐operative PFMT programme only Duration of treatment Follow‐up: 3.5, 4.5, 12, 13 and 13.5 months | |
| Outcomes | Primary outcome (number of men with UI) Number of incontinent men (defined as using > 1 pad on pad test) 12 months: A 2/50; B 3/50 13 months: A 2/50; B2/50 Other outcomes Quality of life ICS male SF questionnaire, results not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Patients were divided randomly” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Blinding of participants (performance bias) | High risk | Blinding to treatment not possible |
| Blinding of personnel (performance bias) | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Therefore judged to be unclear risk |
| Financial support | Low risk | None |
| Approved by medical ethics committee | Low risk | “Faculty of Physical Therapy Ethical committee, Cairo University” |
| Informed consent | Low risk | Yes |
| ITT analysis | Unclear risk | Not specified |
| Methods | RCT | |
| Participants | Recruitment: post‐operative Included: men with persistent urinary incontinence at 6 weeks after radical prostatectomy Excluded: radiotherapy planned; unable to comply with study or intervention; previous formal PFMT Age (mean, SD): A 62.4 (5.8); B 62.3 (5.6) | |
| Interventions | A (205): one‐to‐one therapy sessions including PFMT and BT if OAB or urgency symptoms + PFMT and lifestyle leaflet Duration of treatment: 4 sessions in 3 months starting 6 weeks after surgery B (206): control group with standard care + lifestyle leaflet only, no individual PFMT instruction or sessions | |
| Outcomes | UI defined as positive response to ICIQ‐SF questionnaire UI at 3 months: A 172/200, B 176/198 UI at 6 months: A 158/197, B 158/197 UI at 9 months: A 144/191, B 157/194 UI at 12 months: A 148/196, B 151/195 Severe UI at 12 months: A 74/196, B 78/195 UI episodes at 12 months from diaries (mean (SD N): A 3 (3.8) 105, B 2.9 (3) 106 ICI‐Q score at 12 months (mean (SD N): A 4.9 (4.1) 196, B 5.4 (4.5) 195 QoL due to UI at 12 months (mean (SD N): A 1.4 (2) 193, B 1.7 (2.3) 193 Use of pads at 12 months: A 63/159, B 68/161 Men not doing PFMT at 12 months: A 63/191, B 91/189 Erectile dysfunction (no erection): A 105/189, B 105/190 QALYs virtually identical Cost: NHS intervention cost was GBP 181 higher in intervention group (95% CI 107 to 255) Other outcomes: use of other protection, catheters, sheath catheters, urinary frequency, nocturia, faecal incontinence, urgency, constipation, EQ5D, SF‐12 | |
| Notes | Low dropout rates ICI‐Q score: 0 = no UI, no effect on QoL; 21 = maximum amount, frequency and effect on QoL QoL due to UI measured using ICIQ‐SF: 0 = no effect, 10 = maximum effect Compliance with therapy high | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated, minimised on centre, age and pre‐existing urinary incontinence |
| Allocation concealment (selection bias) | Low risk | Remote computer allocation |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible for men |
| Blinding of personnel (performance bias) | High risk | Blinding to intervention not possible for therapists |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcomes from questionnaires completed by men, data entry clerks blinded to group |
| Incomplete outcome data (attrition bias) | Low risk | No differential dropout from the groups |
| Selective reporting (reporting bias) | Low risk | Outcomes in methods were reported |
| Financial support | Low risk | "The trial was funded by the National Institute of Health Research Health Technology Assessment (NIHR HTA) Programme (project number 03‐14‐03) and will be published in full in Health Technology Assessment. HSRU, HERU, and NMAHP RU are funded by the Chief Scientist Office of the Scottish Government Health Directorates" |
| Approved by medical ethics committee | Low risk | "Our trials were approved by the Multicentre Research Ethics Committee, Edinburgh, Scotland and overseen by an independent trial steering committee and a separate independent data monitoring committee" |
| Informed consent | Low risk | "All men gave signed informed consent" |
| ITT analysis | Low risk | "We used intention‐to‐treat analysis" |
| Methods | RCT | |
| Participants | Recruitment: post‐operative Included: men with persistent urinary incontinence at 6 weeks after transurethral resection of the prostate (TURP) Excluded: radiotherapy planned; channel TURP for palliation for prostate cancer; unable to comply with study or intervention; previous formal PFMT Age (mean, SD): A 68.2 (7.7); B 67.9 (8.1) | |
| Interventions | A (220): one‐to‐one therapy sessions including PFMT and BT if OAB or urgency symptoms + PFMT and lifestyle leaflet Duration of treatment: 4 sessions in 3 months starting 6 weeks after surgery B (222): control group with standard care + lifestyle leaflet only, no individual PFMT instruction or sessions | |
| Outcomes | UI defined as positive response to ICIQ‐short form questionnaire UI at 3 months: A 142/205, B 132/208 UI at 6 months: A 140/199, B 129/201 UI at 9 months: A 133/197, B 131/202 UI at 12 months: A 126/194, B 125/203 Severe UI at 12 months: A 48/194, B 49/203 UI episodes at 12 months from diaries (mean (SD N): A 1.4 (2.3) 175, B 1.2 (2.2) 179 ICI‐Q score at 12 months (mean (SD N): A 3.9 (3.7) 194, B 4 (4.3) 203 QoL due to UI at 12 months (mean (SD N): A 1.2 (1.9) 190, B 1.3 (2.2) 199 Use of pads at 12 months: A 24/146, B 24/136 Men not doing PFMT at 12 months: A 66/188, B 154/193 Erectile dysfunction (no erection): A 52/177, B 43/178 QALYs virtually identical Cost: NHS intervention cost was GBP 209 higher in intervention group (95% CI 147 to 271) Other outcomes: use of other protection, catheters, sheath catheters, urinary frequency, nocturia, faecal incontinence, urgency, constipation, EQ5D, SF‐12 | |
| Notes | Low dropout rates ICI‐Q score: 0= no UI, no effect on QoL; 21 = maximum amount, frequency and effect on QoL QoL due to UI measured using ICIQ‐SF: 0 = no effect, 10 = maximum effect Compliance with therapy high | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated, minimised on centre, age and pre‐existing urinary incontinence |
| Allocation concealment (selection bias) | Low risk | Remote computer allocation |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible for men |
| Blinding of personnel (performance bias) | High risk | Blinding to intervention not possible for therapists |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcomes from questionnaires completed by men, data entry clerks blinded to group |
| Incomplete outcome data (attrition bias) | Low risk | No differential dropout from the groups |
| Selective reporting (reporting bias) | Low risk | Outcomes in methods were reported |
| Financial support | Low risk | "The trial was funded by the National Institute of Health Research Health Technology Assessment (NIHR HTA) Programme (project number 03‐14‐03) and will be published in full in Health Technology Assessment. HSRU, HERU, and NMAHP RU are funded by the Chief Scientist Office of the Scottish Government Health Directorates" |
| Approved by medical ethics committee | Low risk | "Our trials were approved by the Multicentre Research Ethics Committee, Edinburgh, Scotland and overseen by an independent trial steering committee and a separate independent data monitoring committee" |
| Informed consent | Low risk | "All men gave signed informed consent" |
| ITT analysis | Low risk | "We used intention‐to‐treat analysis" |
| Methods | Randomised controlled trial | |
| Participants | Recruitment: post‐operative Included: men incontinent 1 to 16 years after radical prostatectomy (mean years since operation: A 5.1, B 3.9, C 5.1) N = 208 (prior to dropout). Analysis of 172 men at 8 weeks Age between 51 to 84 years % of men with prior PFMT instruction: A 36%, B 56%, C 47% % of men using antimuscarinics: A 16%, B 20%, C 28% % of men with urgency UI: A 1%, B 3%, C 2% % of men with stress UI: A 44%, B 47%, C 44% % of men with mixed UI: A 54%, B 50%, C 54% | |
| Interventions | A (70): behavioural therapy with PFMT alone for 8 weeks B (70): behavioural therapy with biofeedback and electrical stimulation for 8 weeks C (68): control, no treatment for 8 weeks, then offered choice of intervention A or B Behavioural therapy consisted of pelvic floor muscle exercises and bladder control strategies in both groups Dropouts: A 19 at 6 months, 23 at 12 months; B 22 at 6 months, 36 at 12 months; C 3 at 8 weeks Length of follow‐up: 12 months for groups A and B C transferred to treatment at 8 weeks so no further follow up possible | |
| Outcomes | Frequency of UI, mean accidents in a week Number of continent men at 8 weeks: A 11/70, B 12/70, C 4/68 Incontinence episodes per day at 8 weeks (mean, SD, N): A 1.86 (0.56) 58; B 1.71 (0.54) 54; C: 3 (1.17) 64 Change in quality of life at 8 weeks using EPIC UI subscale (bigger change is better, mean, SD, N): A 13.1 (15.5) 58; B 12.3 (14.6) 54; C 2.9 (12.4) 64 Adverse events: A 0/70, B 2/70 (haemorrhoidal irritation), C 0/68 Patient's Global Perceptions of Improvement (much better): A 90%, B 91%, C 10% Completely satisfied with treatment progress: A 47%, B 47%, C not reported Compliance with PFMT and bladder control strategies at 8 weeks: A 100%, B 93% Compliance at 6 months: A 82%, B 84% Compliance at 12 months: A 91%, B 81% | |
| Notes | Some baseline differences between groups, did not quite reach statistical significance High dropout rates No data available for control group after eight weeks as all received treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified by site, type and frequency of UI, generated by computer programme |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes, opened sequentially |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | Unclear risk | Data entry staff blinded to group |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcomes from questionnaires completed by men, data entry staff blinded to group |
| Incomplete outcome data (attrition bias) | High risk | Analysis and reported tables on 172 men |
| Selective reporting (reporting bias) | Low risk | Results of outcomes reported |
| Financial support | Low risk | National Institutes of Health ‐ National Institute of Diabetes and Digestive and Kidney Diseases, grant R01 DK60044‐01A2 |
| Approved by medical ethics committee | Low risk | Approved by "University of Alabama at Birmingham Institutional Review Board" |
| Informed consent | Low risk | Yes |
| ITT analysis | Unclear risk | Not specified |
| Methods | Randomised: yes | |
| Participants | Recruitment: post‐operative Included: men incontinent post‐radical prostatectomy in an inpatient rehabilitation program N= 180 men (prior to dropouts). Randomly assigned to 3 groups (60 in each group) | |
| Interventions | Post‐operative intervention Group B (60) intervention: anal ES plus physiotherapy (PFMT) Group C (60) control: PFMT alone. Length of follow‐up: 3 months | |
| Outcomes | Main outcome: urine loss measure on 1 hour pad test Secondary outcomes: quality of life (QLQ‐C30) Continence definition: self‐reports of incontinence Data collection: admission and discharge from the rehabilitation program and at 3 months after discharge All groups improved on continence and quality of life. Use of ES was only of additional value in a compliant subgroup. Perineal ES was better accepted than anal | |
| Notes | Additional data supplied to KFH by author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Computerised randomisation |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not specified |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of personnel (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | High risk | Dropouts: 22 out of 60 in anal ES group, 4 out of 60 in perineal ES group. No reasons for dropouts given |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Informed consent | Unclear risk | Not reported. Therefore judged to be unclear risk |
| ITT analysis | High risk | No intention‐to‐treat analysis; insufficient information on methods of statistical analysis; interventions unclear and insufficiently specified |
| Methods | RCT | |
| Participants | Time of recruitment: pre‐operative Population: 66 men who underwent TURP (whole population, with or without UI) Included: patients with benign prostatic hyperplasia and underwent TURP, aged 60 to 90 years, remarkable lower urinary tract symptoms (LUTS) with poor response to medication, ambulatory, able to communicate verbally Excluded: indwelling catheter‐dependent postdischarge, neurogenic bladder, dementia or disability affecting verbal communication Age (mean, SD): A 69.67 (6.09); B 71.41 (6.67) Dropouts: 5 (2 catheter still in situ after discharge from hospital, 3 lost to follow‐up). Not differential dropout Baseline characteristics: comparable at baseline | |
| Interventions | Time of intervention: post‐operative treatment A (32): guided PFMT + EMG biofeedback after catheter removal (2 days postoperatively), instructed to: contract pelvic muscles for 5 seconds and relax for 10 seconds. After discharge, patients were instructed to carry out 5 mins of each PFE three times daily. Patients also received motivational telephone interviews once weekly B (29): no description Duration of treatment: 12 weeks Follow up: 1 week, 1 month, 2 months and 3 months | |
| Outcomes | Primary outcome (number of men with UI) Not reported Other outcomes Quality of life SF‐36 scores (mean (SD) N) Physical component 3 months: A 54.86 (8.62) 32; B 49.86 (11.23) 29 Physical functioning 3 months: A 89.69 (17.13) 32; B 85.82 (21.60) 29 Body pain 3 months: A 93.66 (15.16) 32; B 89.48 (22.71) 29 General health 3 months: A 82.03 (14.05) 32; B 64.93 (27.16) 29 Physical role limitation 3 months: A 68.75 (36.48) 32; B 51.72 (38.92) 29 Mental health component 3 months: A 56.21 (6.20) 32; B 48.52 (11.94) 29 Mental role limitation 3 months: A 93.75 (21.48) 32; B 73.81 (37.80) 29 Vitality 3 months: A 80.47 (13.16) 32; B 64.14 (24.02) 29 Mental health 3 months: A 88.00 (10.51) 32; B 77.38 (18.68) 29 Social functioning 3 months: A 90.63 (14.20) 32; B 76.29 (29.57) 29 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly classified" |
| Allocation concealment (selection bias) | Unclear risk | "randomly classified" |
| Blinding of participants (performance bias) | High risk | Blinding to intervention was not possible |
| Blinding of personnel (performance bias) | High risk | Blinding not possible |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | Low risk | 5 (2 catheter still in situ after discharge from hospital, 3 lost to follow‐up). Not differential dropout |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Informed consent | Unclear risk | Not reported. Therefore judged to be unclear risk |
| ITT analysis | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Methods | Randomisation: yes | |
| Participants | Recruitment: post‐operative Included: men incontinent post‐radical prostatectomy or post‐TURP. UI of at least 6 months duration N = 11 patients at least 6 months post‐surgery (4 radical retropubic, 6 radical peritoneal, 1 TURP) | |
| Interventions | Post‐operative intervention Group A (6): intervention: Instruction in PFMT including biofeedback with visual feedback as well as verbal to assist in identifying and discriminating muscles Group B (5): comparator: Instruction in PFMT, squeezing of finger during digital rectal examination Both: weekly visit for a total of 4 clinic visits Length of follow‐up: 12 months | |
| Outcomes | Main outcome: urine loss measure by standardised pad test, bladder diary, subjective estimation of degree of incontinence Secondary outcomes: leak point pressure measured by video‐urodynamics, Joseph Continence Assessment Tool Continence definition: subjective evaluation by participants Data collection: baseline, 3, 6, and 12 months No differences between the groups. Improvement seen in all patients at 12 months | |
| Notes | Data not published in article. Raw data supplied to review author (KFH) who calculated means and standard deviations. These were reviewed by a second review author (KNM) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Allocation concealment (selection bias) | Unclear risk | Reported as "Randomised". No additional information provided |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported. Therefore judged to be unclear risk.\ |
| Incomplete outcome data (attrition bias) | Unclear risk | Three dropouts |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Informed consent | Unclear risk | Not reported. Therefore judged to be unclear risk |
| ITT analysis | High risk | No |
| Methods | Randomised: yes | |
| Participants | Recruitment: post‐operative Included: men with UI after radical prostatectomy Randomised: N = 32 | |
| Interventions | A (16) intervention: extra‐corporeal magnetic innervation (ExMI), treatment sessions were for 20 minutes twice weekly for 8 weeks B (16) control: PFMT alone. Duration of treatment not specified Length of follow‐up: six months | |
| Outcomes | 24 hour pad test, g of urine Baseline: A 655, B 646 1 month: A 147, B 187 2 months: A 33, B 81, P = 0.001 3 months: A 9 (SD 28), B 45 (28), P = 0.001 6 months: Less than 10 g in both groups Number of pads used daily Baseline: A 4.2, B 4.1 I month: A 1.5, B 1.8 2months: A 0.6, B 0.9, P = 0.033 3 months: A 0.1 (0.42), B 0.6 (0.42), P = 0.002 6 months: A 0, B 0.1 Quality of life measured by I‐QoL | |
| Notes | Awaiting further translation ‐ information from abstract only SDs calculated using P values | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description, Chinese language |
| Allocation concealment (selection bias) | Unclear risk | "Randomly assigned" |
| Blinding of participants (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of personnel (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | Unclear risk | No description Therefore judged to be unclear risk |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | Not reported, Chinese language |
| Approved by medical ethics committee | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Informed consent | Unclear risk | Not reported. Therefore judged to be unclear risk |
| ITT analysis | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Methods | RCT | |
| Participants | Time of recruitment: pre‐operative Population: men having a radical prostatectomy (whole population, with or without UI) Included: patients with prostate cancer (stage T2) and candidates for RPP who were referred for treatment Excluded: radiotherapy (previous or after RPP), previous transurethral resection, pre‐existing neurological disease, urinary fistula after RPP, prolonged indwelling urethral catheterization (more than 15 days), clinical situations that rendered the patient unsuitable for surgical procedure, failure to attend all PFMR or electrical stimulation sessions, loss of follow‐up and desistance Age (mean, SD): A 64 (8); B 62 (7); C 60 (8) Dropouts: 9 (2 failed to attend all sessions, 2 desistance, 1 adjuvant radiotherapy, 1 postoperative urethral stenosis, 1 urinary fistula, 1 unsuitable for surgery due to cardiovascular risk, 1 inadequate follow up) Unclear from which group Baseline characteristics: Comparable at baseline | |
| Interventions | Time of intervention: pre‐operative only A (15): standard treatment with verbal instructions for PFMT B (17): pre‐operative guided PFMT, with 10 physiotherapy sessions: contractions of the pelvic floor muscles for 5 seconds in “dorsal decubitus” position for 10 times, in the same position with the waist elevated (10 times), lying down with legs adducted against a plastic ball performed 10 times and standing and flexing the hips to 60̊ (10 times) C (17): pre‐operative PFMT + electrical stimulation during 10 physiotherapy sessions, electrical stimulation was with an anal probe lasting 15 minutes in total, and men also received guided PFMT and followed the same training regime as above Men did not receive PFMT post‐operatively
Duration of treatment: 10 pre‐operative sessions Follow up: 1, 3 and 6 months | |
| Outcomes | Primary outcome (number of men with UI) Not reported Other outcomes 1 hour pad test score (mean (SD) N) 1 month: A 17.6 (38.5) 15; B 29.5 (35.8) 17; C 25.5 (35.4) 17 3 months:14.3 (34.4) 15; B 11.8 (28.4) 17; C 9.6 (18.8) 17 6 months: A 5.5 (14.16) 15; B 25.3 (59) 17; 4.35 (7.3) 17 Quality of life ICIQ‐SF score (mean (SD) N) 1 month: A 7.5 (5) 15; B 14 (3.6) 17; C 9.6 (6.3) 17 3 months: A 5.4 (5.2) 15; B 6.9 (5.8) 17; C 7.2 (6.4) 17 6 months: A 3.7 (5.3) 15; B 4.8 (5.3) 17; C 5.3 (5.5) 17 SF‐36 Results not reported: “There were no differences between groups on the various domains of the SF‐36 (p > 0.05)” | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The patients were randomized (computer generated list using Randomizer, v4)” |
| Allocation concealment (selection bias) | Unclear risk | “The patients were randomized (computer generated list using Randomizer, v4)” |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | Unclear risk | “PFMR was performed in the preoperative period by the same physiotherapist.” |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | Unclear risk | 9 (2 failed to attend all sessions, 2 desistance, 1 adjuvant radiotherapy, 1 post‐operative urethral stenosis, 1 urinary fistula, 1 unsuitable for surgery due to cardiovascular risk, 1 inadequate follow‐up). Unclear from which group |
| Selective reporting (reporting bias) | High risk | Results of SF‐36 not reported |
| Financial support | Low risk | “Sao Paulo State Foundation for Research Support – FAPESP (number 08/54585‐1)” |
| Approved by medical ethics committee | Low risk | “After approval by the ethical committee and internal review board, 58 consecutive males were included in this analysis” |
| Informed consent | Low risk | “All subjects received and signed an informed consent form” |
| ITT analysis | Low risk | Data presented for all men randomised and not excluded. No differential dropout apparent |
| Methods | Randomised controlled clinical trial | |
| Participants | Recruitment: post‐operative Included: men with UI after radical prostatectomy Randomised: N = 24 | |
| Interventions | Group A (12) intervention: extra‐corporeal magnetic innervation (ExMI), the frequency of the pulse field was 10 Hz for 10 minutes, followed by a 3 minute rest and a second treatment of 50 Hz for 20 minutes. This was done twice a week Group B (12) control: PFMT alone, instructions given to carry out 20 mins x 3 a day Duration of treatment: six weeks Length of follow up: 1, 3 and 6 months | |
| Outcomes | Main outcome measures: quality of life scale and the ICI‐Q‐SF 1 month: both scores were decreased with no significant differences between the groups At 3 and 6 months: both scores decreased with group A having a significantly lower (better) score than group B (P < 0.05) | |
| Notes | Information from abstract, awaiting translation of paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "randomly assigned". No additional information provided |
| Allocation concealment (selection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of participants (performance bias) | High risk | Blinding not possible |
| Blinding of personnel (performance bias) | High risk | Blinding not possible |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | Low risk | All 24 patients included in the final analysis |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Low risk | Hospital board, local military university hospital |
| Informed consent | Unclear risk | Not reported. Therefore judged to be unclear risk |
| ITT analysis | Unclear risk | Not specified. Therefore judged to be unclear risk |
| Methods | Randomised: prospective randomised controlled trial Method of allocation: computer generated random numbers Blinding: blinded outcome assessors, not instructors Dropouts: 12 excluded as the couldn't attend regularly for PFMT; 33 continent after surgery and were not randomised; 13 lost to follow‐up in the control group (5 social reasons and 8 non‐responders) Intention to treat: no | |
| Participants | Recruitment: post‐operative Included: men incontinent (UI > 2g/24 hour pad test), post‐radical prostatectomy who were able to attend hospital Excluded: those with a history of preoperative incontinence, significant perioperative complications, rectal lesion, infection, psychiatric neurological disorders, inability to contract PF muscles or weak contraction with increased detrusor activity Mean age: A 66.8 (6.3 years), B 67.9 (5.5 years) | |
| Interventions | Group A (54) intervention: PFMT re‐education program, verbal feedback The training program involved active PFE. Verbal feedback of the contraction was used to instruct the patients to correctly and selectively contract their pelvic muscles while relaxing the abdominal muscles. The strength of the pelvic floor muscles was measured by digital anal control using a score of 0 to 5 ( 0 = no contraction, 5 = good contraction against strong resistance) Initially home practice comprised 45 contractions (3 sessions of 15) per day at home, progressively increasing the number until 90 per day. This was taught by two experienced urologists Group B (53) control: no treatment Duration of treatment: up to a year or until incontinence ceased Length of follow‐up: 1, 3, 6 and 12 months | |
| Outcomes | UI at ‐ 1 month: A 83.3% (45/54), B 97.5% (39/40), P = 0.04 3 months: A 53.7% (29/54), B 77.5% (31/40), P = 0.03 6 months: A 33.3% (18/54), B 60% (24/40), P = 0.01 12 months: A 16.6% (9/54), B 52.5% (21/40), P < 0.01 Subjective assessment of continence using VAS: P = 0.01 at 12 months Quality of lIfe (single question): P = 0.03 at 12 months | |
| Notes | ITT analysis used for data entry, assuming that all 13 men who dropped out of the control group were dry, because of differential dropout of 13 men from B versus none from A with no explanation for difference between groups If unable to contract anal sphincter or strength 2 or less, not randomised. These men were given ES treatment at home with anal probe | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers |
| Allocation concealment (selection bias) | Low risk | Stratified on volume of urine lost on pad test |
| Blinding of participants (performance bias) | High risk | Blinding of intervention not possible |
| Blinding of personnel (performance bias) | High risk | Blinidng of intervention not possible |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinded outcome assessors |
| Incomplete outcome data (attrition bias) | High risk | Differential dropout of 13 from control group, ITT analysis used for data entry by review authors |
| Selective reporting (reporting bias) | Unclear risk | Outcomes in methods reported |
| Financial support | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Low risk | "The study was approved by the Medical Centre Institutional Review Board" |
| Informed consent | Low risk | "All men provided informed consent" |
| ITT analysis | Low risk | Assumed from patient flow chart |
| Methods | RCT | |
| Participants | Time of recruitment: post‐operative Population: men with incontinence after retropubic radical prostatectomy, open or laparoscopic Included: moderate to severe incontinence at 30 days after catheter removal Excluded: lack of cooperation, pre‐operative incontinence, early recovery of continence Age (mean): A 67; B 66.5 Dropouts: “Survey questionnaire were correctly filled in and returned by fewer than 10% of the patients” Baseline characteristics: comparable at baseline | |
| Interventions | Time of intervention: post‐operative treatment A (166): one‐to‐one guided PFMT + biofeedback during first session, second session involved 10 sets of pelvic floor electrical stimulation lasting 15 mins each, instructed to: carry out three sets of 30 contractions a day at home for the first month after catheter removal (16 days after surgery) B (166): received oral and written information on pelvic floor anatomy and on PFME, instructed to: perform three sets of 30 contractions a day at home for the first month after catheter removal (16 days after surgery) and continue for duration of All men received oral and written information on pelvic floor anatomy and on PFME, pelvic floor muscle endurance assessed by digital anal control + PFMT consisting of 3 sets of 30 contractions daily for the first month after catheter removal Duration of treatment Follow up: 3 months, 6 months and 12 months | |
| Outcomes | Primary outcome (number of men with UI) Number of incontinent men (defined as 0 or 2 minipads daily) 3 months: A 36/166; B 81/166 6.5 months: A 1/166; B 28/166 12 months: A 0/166; B 0/166 Other outcomes Median time of continence recovery, days: A 44 ± 2, B 76 ± 4, P ≤ 0.01 Quality of life ICIQ‐male: Results not reported RAND 36‐Item Health Survey questionnaire: results not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Prospectively randomized” Sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | "Prospectively randomized" |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | High risk | Not reported for primary outcome |
| Selective reporting (reporting bias) | High risk | No reporting of primary outcome |
| Financial support | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Informed consent | Unclear risk | Not reported. Therefore judged to be unclear risk |
| ITT analysis | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Methods | Randomised: yes | |
| Participants | Randomised post‐operatively Included: radical prostatectomy, all men after catheter removal Age: Group A mean 61.86 years, Group B, 61.43 years | |
| Interventions | Intervention post‐operative Group A (30) intervention: PFMT plus ES and biofeedback twice a week for 6 weeks ES ‐ a surface electrode was inserted into the anus and pulsed, the intensity was adequate to induce visual lifting of the levator ani and pubococcygeus muscle, considering the level of comfort to the patient Biofeedback ‐ via surface electrodes both perineal and abdominally Group B (30) control: instructions to conduct PFMT ‐ verbal and written instructions at catheter removal and follow‐up visits Duration of treatment: 6 weeks Length of follow up: 3 and 6 months | |
| Outcomes | 24 hour pad test: g/24hrs, mean (SD) 3 months: A 16.67 (30.55), B 136.67 (152.62), P = 0.000 6 months: A 3.47 (14.67), B 27.83 (55.98), P = 0.0004 ICS‐male questionnaire, number of men incontinent, n/N 3 months: A 6/30, B 20/30 6 months: A 1/30, B 10/30 Time to regain continence: A 8 (6.49) weeks, B 13.88 (8.32) weeks, P = 0.003 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Consecutive patients |
| Allocation concealment (selection bias) | Unclear risk | Quote ‐ "Randomized fashion" |
| Blinding of participants (performance bias) | High risk | Blinding not possible |
| Blinding of personnel (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Informed consent | Low risk | "All patients signed an informed consent before randomization" |
| ITT analysis | Unclear risk | Not specified. Therefore judged to be unclear risk |
| Methods | RCT (abstract only) | |
| Participants | Time of recruitment: pre‐operative Population: 70 consecutive men undergoing a laparoscopic radical prostatectomy (whole population, with or without UI) Included: men undergoing RP for clinically localized prostate cancer T1 to T3 Excluded: history of incontinence or overactive bladder, central or peripheral neurologic disease and cognitive impairment Age (mean, SD): not reported Dropouts: 5 lost to follow up, unclear from which group Baseline characteristics: not reported | |
| Interventions | Time of intervention: pre‐operative (post‐operative treatment for all men) A (24): PFMT: 5 sessions of guided PFMT for 2 to 3 weeks pre‐operatively and continued post‐operatively B (25): post‐operative standard care, written instructions for PFMT All men underwent clinical examination of pelvic muscles function using digital perineal testing according to “AIPDA score” and evaluation of voiding symptoms Duration of treatment: Follow up: 1, 3 and 6 months | |
| Outcomes | Primary outcome (number of men with UI) Number of incontinent men (need to wear a pad) No useable data Other outcomes 24 hour pad test Pad use Bladder diary Quality of life Instrument unspecified | |
| Notes | No useable data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomised” |
| Allocation concealment (selection bias) | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Blinding of participants (performance bias) | High risk | Blinding to treatment not possible |
| Blinding of personnel (performance bias) | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | Unclear risk | “Five patients lost at follow up”. Not clear why there were dropouts or from which group |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Informed consent | Unclear risk | Not reported. Therefore judged to be unclear risk |
| ITT analysis | Unclear risk | Not specified. Therefore judged to be unclear risk |
| Methods | Randomised: yes, block procedure | |
| Participants | Recruitment: pre‐operative Included: all men undergoing radical prostatectomy N = 53 men | |
| Interventions | Pre and post‐operative intervention Group A (27) intervention: pre‐operatively received further instruction and practice with PME protocol home exercises and biofeedback (anal probe) (Incare 8900); practiced at home 3 times a week, starting with daily 15 PFMT and increasing by 10 every 4 weeks to a maximum of 35 PFMT Group B (24) control: post‐operatively no further interventions until week 5 when pelvic muscle strength was assessed Both: pre‐operatively, both groups received 30 minutes' prostate education programme and baseline 'perineal muscle evaluation' (not defined); as well all were taught to contract the perineal muscle and hold for a few seconds prior to standing, lifting or coughing and limit the amount of tea, chocolate, alcohol and over‐the‐counter medications Length of follow‐up: 12 weeks | |
| Outcomes | Main outcome: urine loss measured by 24 hour pad test, frequency of micturitions (self‐recorded bladder diary), number of pads used; days to achieve continence from baseline Secondary outcomes: perineal muscle strength (method not described) Continence definition: self‐report of return of continence Data collection: 3 day bladder diaries at weeks 2, 5, 9 and 12. 24 hour pad test at weeks 5 and 12 | |
| Notes | Inclusion of other modalities such as caffeine limitation and using perineal muscles during any event which increased abdominal stress may have masked any treatment benefit Extra information obtained from thesis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block procedure |
| Allocation concealment (selection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | Unclear risk | Two dropouts |
| Selective reporting (reporting bias) | Low risk | Outcomes in methods reported |
| Financial support | Unclear risk | Not reported |
| Approved by medical ethics committee | Low risk | "Permission to conduct this study was obtained from the Univerisity of Florida Health Center Institutional Review Board (IRB)." |
| Informed consent | Low risk | "The informed consent was explained to each subject, and his signature was obtained to confirm consent to participate in the study" |
| ITT analysis | Unclear risk | Not specified. Therefore judged to be unclear risk |
| Methods | Randomised: yes Blinding: physiotherapist blinded to results of control group | |
| Participants | Recruitment: post‐operative Included: men incontinent post‐radical prostatectomy. Median duration of UI 8 weeks post‐surgery, range 4 to 200 weeks N = 63 men (53 completed study) | |
| Interventions | Post‐operative intervention Intervention Group C (21) control: oral and written information about PFMT pre and post‐operatively (standard treatment) Length of follow‐up: 24 weeks | |
| Outcomes | Main outcome: urine loss measured by 24 hour pad test Secondary outcomes: quality of life measures (Incontinence Impact Questionnaire, European Organization for the research and treatment of Cancer‐EORTC QLQ C‐30, version 2), physical symptom inventory (adapted from Herr 1994) Continence definition: ≤ 2 g urine/24 hours Data collection: baseline, 12, 16, 24 weeks after baseline | |
| Notes | Intervention perhaps administered too early ‐ all subjects improved at the same rate; wide range of severity of urinary incontinence at study entry and size of SD of pad test results also may have resulted in Type II error | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were assigned using a computer‐generated random‐number list placed in sealed envelopes at the end of the assessment visit, with patient and researcher opening the sealed envelope" |
| Allocation concealment (selection bias) | Low risk | "Participants were assigned using a computer‐generated random‐number list placed in sealed envelopes at the end of the assessment visit, with patient and researcher opening the sealed envelope" |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | Low risk | Physiotherapist blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | Low risk | 5 (3 from group B, 2 from group A), 3 bladder neck contractures, 1 rectal pain when performing exercises, 1 vacation for 4 months). No differential dropout |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Low risk | "Funding for the research project was received from the Oncology Nurses' Society, Canadian Nurses' Foundation, Caritas Health, Alberta Physiotherapy Association, Edna Minton Foundation, and the University of Alberta" |
| Approved by medical ethics committee | Low risk | "approved by the University of Alberta and Caritas Health Group ethics review boards" |
| Informed consent | Low risk | "All patients signed informed consent" |
| ITT analysis | High risk | Intention‐to‐treat‐analysis not performed |
| Methods | Randomised: yes (order of product testing: in threes to treatment block of 4 periods (1 no device, 3 with devices) Dropouts: none | |
| Participants | Recruitment: post‐operative Included: men incontinent post‐radical prostatectomy who required continuous pad protection for stress incontinence Inclusion criteria: normal perineal and penile sensation, intact penile skin, sufficient manual dexterity N = 12 men | |
| Interventions | Post‐operative intervention Each participant had 4 periods (each lasted 1 day) | |
| Outcomes | Main outcome: 4 hour pad test Secondary outcomes: resistive index, cavernosal flow None of the devices completely eliminated urine loss when applied at a comfortable pressure. Each device showed improvement in terms of urine lost, with Cunningham clamp having the lowest mean loss | |
| Notes | Unable to blind participants and research assistant to intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐generated randomized list of device assignments was prepared by one of the investigators" Block, multiple period crossover design using Latin square configuration |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes, research assistant not involved in study chose envelope |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of outcome assessment (detection bias) | Unclear risk | "A research assistant not directly involved with recruitment or data collection entered the data" |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Financial support | Low risk | "This study was supported by the University of Alberta Internal Allocations Fund and Department of Radiology, University of Alberta Hospital." |
| Approved by medical ethics committee | Low risk | "The Institutional review Board at the University of Alberta approved the study" |
| Informed consent | Low risk | "the study was explained and informed consent obtained" |
| ITT analysis | Unclear risk | Not specified. Therefore judged to be unclear risk |
| Methods | Randomised: yes | |
| Participants | Recruitment: post‐operative (but approached before surgery) Included: men incontinent after radical prostatectomy (> 8 grams urine lost on 24 hour pad test) at 4 weeks post‐surgery N = 217 men from 3 centres with early stage prostate cancer | |
| Interventions | Post‐operative intervention Group A (106) intervention: maximum 24 weekly, 30 minute treatment protocol (30 min biofeedback‐assisted PFMT) and home exercise protocol of 2 to 3 times a day Group B (99) control: verbal and written information on PFME and weekly telephone contact by a urology nurse Both: at 4 weeks post‐surgery, both groups received standardised verbal and written instruction about PFMT and recovery after radical prostatectomy by one dedicated physiotherapist or registered nurse at each site Length of follow‐up: 12 months | |
| Outcomes | Main outcome: grams of urine loss on 24 hour pad test (> 8 g defined as incontinence) Definition of continence: < 8 g of urine loss on 24 hour pad test; subjective continence defined as yes or no Secondary outcome: IPSS, IIQ‐7 (Incontinence Impact Questionnaire), voiding diary, and subjective continence All measures obtained at baseline (pre‐operatively) and at 4, 8, 12, 28 weeks and 1 year post‐operatively 24 hour pad test, mean (SD) N 12 weeks: A 115 (300) 93, B 72 (144) 82 16 weeks: A 76 (259) 94, B 61 (194) 80 28 weeks: A 45 (142) 87, B 35 (101) 74 12 months: A 47 (215) 89, B 8 (10) 78 Dry at 8 weeks: A 20/101 (20%), B 20/88 (23%) Dry at 12 weeks: A 30/93 (32%), B 23/82 (28%) Dry at 16 weeks: A 41/94 (44%), B 32/80 (40%) Dry at 28 weeks: A 41/87 (47%). B 37/74 (50%) Dry at 12 months: A 53/89 60%, B 47/78 60% (< 8 g on pad test) No significant differences between groups on continence or on symptom and quality of life measures or diary at any time point post‐operatively Cost: A: CAD 400; B 240 Adverse events: none in either group The majority of men reported a low impact of incontinence as per the IIQ‐7 and fewer LUTS at 12 months than at baseline on the IPSS. The majority were very satisfied with treatment and support from the continence nurse | |
| Notes | Groups comparable at pre‐operation baseline on PSA, Gleason score, IPPS, IIQ, pad test and voiding diary | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated list of random numbers, random blocked allocation to groups |
| Allocation concealment (selection bias) | Low risk | Group allocation placed in sealed opaque envelopes; opened by participant after initial post‐operation instruction session with therapist |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | Low risk | Therapist blinded to outcome of non‐intervention group; pads weighed by third party |
| Blinding of outcome assessment (detection bias) | Low risk | Data entry by clerk blinded to group |
| Incomplete outcome data (attrition bias) | Unclear risk | Dropouts: control = 7; treatment = 12; no differential dropout |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Therefore judged to be unclear risk |
| Financial support | Low risk | "Funded by the Alberta Heritage Foundation for Medical Research, the Northern Alberta Urology Foundation, and Pfizer Corporation (unrestricted)" |
| Approved by medical ethics committee | Low risk | "Healthcare ethics approval was obtained at all sites" |
| Informed consent | Low risk | "After the consent form was signed, baseline data were collected" |
| ITT analysis | Low risk | Patient flow chart give details of patient dropouts and withdrawals |
| Methods | RCT abstract only | |
| Participants | Time of recruitment: not reported Population: men having laparoscopic radical prostatectomy Included: patients who underwent laparoscopic radical prostatectomy performed by a single surgeon Excluded: not reported Age (mean, SD): not reported Dropouts: not reported Baseline characteristics: comparable at baseline | |
| Interventions | Time of intervention: post‐operative treatment for all men A (20): PFMT + sacral surface therapeutic ES (ssTES), ssTES 2 times a day for 15 minutes each, lasting 1 month after catheter removal (day 5) B (14): PFMT only, carried out alone Duration of treatment: 1 month Follow‐up: 1 month, 3 months, 6 months and 12 months post‐operatively | |
| Outcomes | Primary outcome (number of men with UI) Number of incontinent men (defined as requirement for a pad to keep clothing dry) 6 months: A 3/20; B 6/14 12 months: A 0/20; B 5/14 Other outcomes Recovery rate of urinary continence (defined as no requirement for a pad to keep clothing dry) 6 months: A 17/20, B 8/14 12 months: A 20/20, B 9/14, P = 0.007 Quality of life | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomly assigned” |
| Allocation concealment (selection bias) | Unclear risk | "randomly assigned" |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | Unclear risk | Not enough information. Therefore judged to be unclear risk |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Therefore judged to be unclear risk |
| Financial support | Low risk | None |
| Approved by medical ethics committee | Low risk | “ethics committee of Kitasato university of medicine” |
| Informed consent | Low risk | Yes |
| ITT analysis | Unclear risk | No description. Therefore judged to be unclear risk |
| Methods | Randomised: yes | |
| Participants | Recruitment: pre‐operative Included: men undergoing radical prostatectomy Aged: 59 to 72 years | |
| Interventions | Group A intervention: extra‐corporeal magnetic innervation (ExMI) based pelvic floor device Group B control: PFMT alone Treatment initiated one week after catheter removal Duration of treatment: 10 weeks Length of follow‐up: 12 months | |
| Outcomes | On first day following catheter removal 16.8% of patients were continent Subsequent follow‐up data unclear if N = 105 or 88 subjects. Group numbers not stated UI at ‐ 4 weeks: A 49%, B 56% 3 months: A 36%, B 50% 6 months; A 18%, B 32% Twenty minute pad test at 12 months, significantly better in Group A at 12 months, P =0.004 QoL score and urinary symptom inventory also carried out, numbers not given | |
| Notes | No useable data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized" |
| Allocation concealment (selection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | Unclear risk | One patient withdrew from Group A for non‐medical reasons |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | No description. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Unclear risk | No description. Therefore judged to be unclear risk |
| Informed consent | Unclear risk | No description. Therefore judged to be unclear risk |
| ITT analysis | Unclear risk | Not specified. Therefore judged to be unclear risk |
| Methods | Randomised: yes | |
| Participants | Recruitment: post‐operative Included: men incontinent post‐radical prostatectomy 6 weeks after six week after surgery N = 43 (39 completed study) | |
| Interventions | Post‐operative intervention Group A (21) intervention: PFMT plus biofeedback plus ES directed by physiotherapist Group B (22) control: PFME on their own without medical supervision Length of follow‐up: 12 weeks | |
| Outcomes | Main outcome: urine loss measured by pad test No statistical difference between groups as to recovery of continence | |
| Notes | Abstract only ‐ unable to contact author for further data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Allocation concealment (selection bias) | Unclear risk | "Randomised" |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | Unclear risk | Four dropouts |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | No description. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Unclear risk | No description. Therefore judged to be unclear risk |
| Informed consent | Unclear risk | No description. Therefore judged to be unclear risk |
| ITT analysis | Unclear risk | Not specified. Therefore judged to be unclear risk |
| Methods | Randomised: yes | |
| Participants | Recruitment: Pre‐operative Included: radical prostatectomy, all men Age: Group A 48 to 68 years, Group B 49 to 72 years | |
| Interventions | Intervention: post operative Group A (38) intervention: instructions on PFMT and physiotherapy, 45 minutes weekly. Patients were instructed to perform 3 sets of contractions daily at home, in either a supine, sitting or standing position. Digital anal palpation to teach correct contractions, as well as oral and written instructions DVD of instructions given to those living too far from hospital Group B (42) control: instructions on PFMT alone Duration of treatment: up to 1 year Length of follow‐up: 3, 6 and 12 months | |
| Outcomes | Self‐reported continence (not using pads) 3 months: A 16/35 (46%), B 17/40 (43%), P = 0.73 6 months: A 27/34 (79%), B 22/38 (58%), P = 0.061 12 months: A 33/36 (92%), B 28/39 (72%), P = 0.028 24 hour pad test: g/24hrs, mean (range) 3 months: A 17 (0‐282), B 7 (0‐46), P = 0.53 6 months: A 9 (0‐203), B 2 (0‐12), P = 0.73 12 months: A 2 (0‐55), B 1 (0‐14), P = 0.95 PFM strength (anal squeeze pressure, cm H2O), mean (SD) 3 months: A 50.7 (23.9), B 55.7 (25.6), P = 0.398 6 months: A 56.1 (21.7), B 65.8 (27.0), P = 0.117 12 months: A 64.0 (24.0), B 71.5 (26.2), P = 0.237. | |
| Notes | No SDs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Norwegian University performed the computerised randomisation procedure immediately after pre‐operative test |
| Allocation concealment (selection bias) | Low risk | Norwegian University performed the computerised randomisation procedure immediately after pre‐operative test. Urologist no prior knowledge of randomisation procedure |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | High risk | Drop out rate was 6% Four lost to follow up in physiotherapy group, one lost in instructions only group |
| Selective reporting (reporting bias) | Low risk | Outcomes in methods reported |
| Financial support | Low risk | "The work was funded by The Norwegian Fund for Postgraduate Training in Physiotherapy and The Norwegian Cancer Society" |
| Approved by medical ethics committee | Low risk | "The study was approved by the Regional Committee for Medical and Health Research Ethics" |
| Informed consent | Low risk | "Eighty‐five men provided written informed consent" |
| ITT analysis | Low risk | Assumed from patient flow chart |
| Methods | Randomised: yes | |
| Participants | Recruitment: pre‐operative Included: all men scheduled for radical prostatectomy N = 38 patients with localized carcinoma of the prostate | |
| Interventions | Pre and post‐operative interventions Group A (19) intervention: 2 treatment sessions pre‐operatively. Session 1 consisted of PFMT in a hook lying position Group B (19) control: no formal education on PFMT pre‐operatively, telephone or face to face follow‐up at least monthly Length of follow‐up: 12 months | |
| Outcomes | Main outcome: urine loss measured by number of pads used daily Continence definition: 0 pads or 1 precautionary pad used Data collection: UI questionnaires at 6, 12, 16, 20, 28, and 52 weeks Greater number of the intervention group gained continence earlier than the control group at 3 months (only point of statistical difference). Minimal long‐term effect as continence rates the same at 1 year | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were enrolled in prospective, randomized fashion into a treatment or a control group" |
| Allocation concealment (selection bias) | Unclear risk | "Randomly assigned" |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | Low risk | 1 dropout from each arm. Categorised as incontinent |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Informed consent | Unclear risk | Not reported. Therefore judged to be unclear risk |
| ITT analysis | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Methods | RCT | |
| Participants | Time of recruitment: post‐operative Population: 121 men who underwent radical prostatectomy (whole population, with or without UI) Included: elderly male patients aged ≥ 65 years, clinically localized prostate cancer (cT1 to T2), Eastern Cooperative Oncology Group performance status of 0 or 1, and written informed consent Excluded: adjuvant or neoadjuvant therapy, severe postoperative complications, a history of intrapelvic surgery, diseases that can affect voiding function, and limitations for exercise intervention, such as patients with serious cardiovascular events or spinal or articular disease Age (mean, SD): A 69.1 (5.7); B 69.4 (7.2) Dropouts: A: 7 (1 orthopaedic surgery for a pre‐existing ankle problem, 1 transurethral surgery for urethral stricture, 4 non‐compliance with follow‐up due to a long distance from the centre to the home or personal affairs, 1 new employment after surgery) B: 8 (1 ophthalmologic surgery for a cataract, 2 adjuvant radiotherapy, 4 non‐compliance with follow‐up due to a long distance from the center to the home or personal affairs, 1 new employment after surgery) Not differential dropout Baseline characteristics: comparable at baseline | |
| Interventions | Time of intervention: post‐operative treatment for all men A (26): patients performed Kegel exercises twice weekly, together with other types of exercises which included resistance training and pelvic flexibility. The intervention started 3 weeks after surgery and lasted 12 weeks B (23): ‘In the control group, only kegel exercises were performed’ Duration of treatment: 15 weeks Follow‐up: 1 week before surgery, 3 weeks and 15 weeks after surgery | |
| Outcomes | Primary outcome (number of men with UI) Cumulative number of incontinent men [defined as > 1 g on 24 hour pad test) 15 weeks: A 7/26; B 13/23 Other outcomes Cumulative number of continent men [defined as < 1 g on 24 hour pad test) 15 weeks: A 19/26; B 10/23, P = 0.035 Urine loss in grams using 24 hour pad test (mean (SD) N) 1 week before surgery: A 0 (NR) 26; B 0 (NR) 23 3 weeks post‐operatively: A 60 (NR) 26; B 83 (NR) 23 15 weeks: A 12 (NR) 26; B 46 (NR) 23 Quality of life ICIQ score (mean (SD) N) 1 week before surgery: A 4 (NR) 26; B 3 (NR) 23 3 weeks post‐operatively: A 10 (NR) 26; B 10 (NR) 23 15 weeks: A 6 (NR) 26; B 10 (NR) 23
SF‐36 physical composite score (mean (SD) N) 1 week before surgery: A 57 (NR) 26; B 54 (NR) 23 3 weeks post‐operatively: A 45 (NR) 26; B 44 (NR) 23 15 weeks: A 57 (NR) 26; B 48 (NR) 23
SF‐36 mental composite score (mean (SD) N) 1 week before surgery: A 45 (NR) 26; B 44.6 (NR) 23 3 weeks post‐operatively: A 44 (NR) 26; B 43 (NR) 23 15 weeks: A 49 (NR) 26; B 46 (NR) 23
Beck Depression Inventory (mean (SD) N) 1 week before surgery: A 9 (NR) 26; B 7.4 (NR) 23 3 weeks post‐operatively: A 8 (NR) 26; B 9 (NR) 23 15 weeks: A 6 (NR) 26; B 9 (NR) 23 NR = Not reported | |
| Notes | Details of the combined exercise regime Post‐operative weeks 1 to 4 1) Education about post‐operative symptoms 2) Performing Kegel exercises, recognizing the parapelvic muscles 3) Pelvic floor flexibility fitness: performing pelvic exercises while sitting on a ball Post‐operative weeks 5 to 8 (ball exercises) 1) Performing pelvic exercises while sitting on a ball 2) Performing lower extremity exercises while placing a ball on the wall 3) Lifting a heel on the ball while standing face‐to‐face with the wall 4) Lifting up and down on the ball while spreading and bending legs 5) Performing flank exercises while having a ball in the hand 6) Squeezing the ball with the adductor muscles while lying on a table Post‐operative weeks 9‐12 (elastic band exercises) 1) Lifting the object with an elastic band lateral, anterior, and posterior to the patient’s arms 2) Lifting the legs and then spreading them while attaching an elastic band to the foot Further information provided by author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A random number generator was used to determine the randomization allocation in a 1:1 ratio” |
| Allocation concealment (selection bias) | Low risk | “Sealed envelope, sequentially numbered, and opened by the trial nurse” |
| Blinding of participants (performance bias) | High risk | Blinding of participants was not possible |
| Blinding of personnel (performance bias) | High risk | Blinding not possible |
| Blinding of outcome assessment (detection bias) | Low risk | An independent assessor performed serial measurements |
| Incomplete outcome data (attrition bias) | Unclear risk | A: 7 (1 orthopaedic surgery for a pre‐existing ankle problem, 1 transurethral surgery for urethral stricture, 4 non‐compliance with follow‐up due to a long distance from the centre to the home or personal affairs, 1 new employment after surgery) B: 8 (1 ophthalmologic surgery for a cataract, 2 adjuvant radiotherapy, 4 non‐compliance with follow up due to a long distance from the center to the home or personal affairs, 1 new employment after surgery) No differential dropout |
| Selective reporting (reporting bias) | Low risk | Results of outcomes reported |
| Financial support | Low risk | Unconditional funding from the “Medical Research Institute, Pusan National University Hospital, Busan, Korea.” |
| Approved by medical ethics committee | Low risk | “Our institutional review board approved this prospective, randomized, controlled trial” |
| Informed consent | Low risk | Patients signed “written informed consent” |
| ITT analysis | High risk | No |
| Methods | Randomised: yes. Method of allocation: consecutive patients | |
| Participants | Pre‐operative randomisation Included: all men undergoing radical prostatectomy Pre‐operative intervention Age: not given | |
| Interventions | Group A (N not given) intervention: early pelvic floor rehabilitation program at home twice dally, Kegel exercises Group B (N not given) control: no formal PFMT Duration of treatment: for six months or until continence was achieved Length of follow‐up: at 3 and 6 months | |
| Outcomes | PFM strength: P = 0.002 Quality of life using ICIQ‐SF not significant 24 hour pad test not significant | |
| Notes | No useable data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Consecutive patients. No additional information provided. Therefore judged to be unclear risk |
| Allocation concealment (selection bias) | Unclear risk | Randomised controlled trial. No additional information provided. Therefore judged to be unclear risk |
| Blinding of participants (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of personnel (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Low risk | "FAPESP" (Sao Paulo Research Foundation) |
| Approved by medical ethics committee | Low risk | "COMITE DE ESTICA E PESQUISA ‐ UNICAMP" |
| Informed consent | Low risk | Yes |
| ITT analysis | Unclear risk | Not specified |
| Methods | Randomised: yes | |
| Participants | Recruitment: pre‐operative Included: all men undergoing TURP N = 58 men (55 completed study) with benign prostatic hypertrophy randomised to 2 groups | |
| Interventions | Pre and post‐operative intervention Group A (30) intervention: initial visit before surgery, digital evaluation of pelvic muscle contraction strength. Verbal instruction, feedback and reinforcement on contraction was given to teach selective contraction of anal sphincter and relaxation of abdominal muscles. Verbal and written instruction given for home PFMT. Weekly digital anal reassessment and grading of pelvic muscle contraction by the therapist. Instructed to practice contractions 45 times per day (3 groups of 15 contractions) Group B (28) control: not specified Both A and B: voiding diaries initiated after catheter removal Length of follow‐up: 4 weeks. Data collection at catheter removal and weekly for 4 weeks | |
| Outcomes | Main outcome: urine loss (incontinence episodes) measured by 48 hour bladder diaries completed weekly Secondary outcomes Significant increase in muscle strength in intervention group by week 4 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | B ‐ 'randomised' |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | Low risk | Urologist performing digital evaluation of pelvic floor muscle contraction was blinded to the study group |
| Blinding of outcome assessment (detection bias) | Unclear risk | "One urologist, who was blinded to the study group of the patients, performed only the digital evaluation of the pelvic floor muscle contraction and established and reported the grading during all the visits" |
| Incomplete outcome data (attrition bias) | Unclear risk | Dropouts due to non‐attendance at all clinic appointments (A 2, B 1) |
| Selective reporting (reporting bias) | Low risk | Results reported for outcomes stated in methods |
| Financial support | Unclear risk | No description. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Unclear risk | No description. Therefore judged to be unclear risk |
| Informed consent | Low risk | "Informed consent was given by all patients" |
| ITT analysis | Unclear risk | Not specified |
| Methods | Randomised: yes | |
| Participants | Post‐operative intervention Included: radical prostatectomy, all men after catheter removal Age: 51 to 76 years | |
| Interventions | Group A (36) intervention: PFMT plus BF weekly for 3 months Group B (37) control: PFMT oral instructions only Duration of treatment: weekly until continent or to a maximum of 3 months Length of follow‐up: 3 months after treatment finished | |
| Outcomes | UI severity (24 hour pad test weights) 1 month (N, mean, SD): A 96 g (160) 36, B 355 (423) 37, P = 0.007 3 months: A 51 (119), 36, B 197 (269) 37 6 months: A 40 (77), 36, B 80 (176) 37 ICI‐SF score: 3 months:A 3.4 (3.7), 36, B 6.8 (5.6) 37, P = 0.022 6 months: A 2.7 (3.5), 36, B 4.3 (5.5) 37, P = 0.339 PFM Strength, A versus B: 1 month, P = 0.006; 3 months P < 0.001; 6 months P = 0.799 Quality of life (IIQ): 3 months: A 1.6 (2.7), 36, B 4.3 (6.2) 37 | |
| Notes | Groups comparable at baseline before operation on age, BMI, voiding symptoms and PFMT strength | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Allocation concealment (selection bias) | Unclear risk | "Randomised controlled trial" |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | High risk | Blinding not possible |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | Low risk | 19: A:10 (2 refused further follow‐up, 7 post‐operative complications, 1 radiotherapy); B: 9 (6 refused further follow‐up, 2 post‐operative complications, 1 radiotherapy). No differential dropout |
| Selective reporting (reporting bias) | Low risk | Outcomes in methods reported |
| Financial support | Low risk | Grant FAPESP 2003/07656‐7 (Sao Paulo Research Foundation) |
| Approved by medical ethics committee | Low risk | "institutional review board approval" |
| Informed consent | Low risk | "All patients signed an informed consent before randomization" |
| ITT analysis | Low risk | Assumed from patient flow chart |
| Methods | Randomisation: yes | |
| Participants | Recruitment: pre‐operatively Included: all men undergoing radical prostatectomy Groups comparable at baseline Age range 39 to 74 years Pre‐operative UI 9% | |
| Interventions | Group A (62) intervention: brief verbal instruction in PFMT before operation and offer of one biofeedback session at 2 months after surgery (uptake 33%) plus PFMT for four weeks with biofeedback Group B (64) control: brief verbal instruction in PFMT before operation and offer of one biofeedback session at 2 months after surgery (uptake 46%) | |
| Outcomes | No urinary outcomes provided No between group differences in intensity and distress of lower urinary tract symptoms nor in impact on health‐related quality of life | |
| Notes | No useable data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | The co‐project director who supervised the intervention was responsible for recruitment, but did not have access to the randomisation list The co‐project director who supervised data collection was responsible for concealment of the randomisation list and allocation to the next available assignment on the list to participants sequentially as they enrolled |
| Blinding of participants (performance bias) | High risk | Participants were advised by the research assistant of their group assignment |
| Blinding of personnel (performance bias) | High risk | Questionnaires were filled in by research assistants either in person or by telephone interview |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | Low risk | No significant difference between groups in the number of participants who either withdrew prematurely or were dropped from the study. Questionnaires with > 20% data missing were excluded from analysis. In remainder mean substitution was inputted for missing data |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Low risk | "This study was supported by the American Cancer Society (TPRB‐98‐118‐01‐PBP) and a Rutgers College of Nursing Faculty Research Development Award" |
| Approved by medical ethics committee | Low risk | "Recruitment was initiated in January 1998 after approval of the parent study was obtained from the institutional review boards of both medical centres and the university" |
| Informed consent | Low risk | "Written informed consent was obtained by a research assistant" |
| ITT analysis | Low risk | "Data analysis was by intention‐to‐treat" |
| Methods | Randomisation: randomly assigned via sealed envelopes | |
| Participants | Number of men 54 but no numbers in groups Recuitment: post‐operatively Included: radical prostatectomy, all with UI who were 50 + years, English speaking and were within a 50 mile radius of treatment centre Age: mean 59.5 (6.3) years | |
| Interventions | Group A intervention: routine brief verbal and written PFMT plus one PFMT session and 3 weekly nurse phone calls Group B intervention: routine brief verbal and written PFMT plus four BF enhanced PFMT sessions and 4 weekly nurse phone calls Group C control: routine brief verbal and written PFMT Duration of treatment: 3 months Length of follow‐up: 9 months | |
| Outcomes | Urine stream interruption test (PFM strength) Mishell Uncertainty in Illness Scale Broome Pelvic Muscle self‐Efficacy Scale UI frequency (3 day bladder diary) 24 hour pad test (volume of urine lost) Male Urogenital Distress Inventory (UI distress) Male Urinary Symptom Impact Questionnaire (QoL) | |
| Notes | No useable data in abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Via sealed envelopes |
| Allocation concealment (selection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Low risk | "NIH/NINR" |
| Approved by medical ethics committee | Low risk | "Yes" |
| Informed consent | Low risk | "Following informed consent" |
| ITT analysis | Unclear risk | Not specified. Therefore judged to be unclear risk |
| Methods | Randomisation: yes, single blind Method of allocation: using coloured cards | |
| Participants | Post‐operative intervention Included: men with UI eight weeks after radical prostatectomy Exclusion: previous radiotherapy, anterior transurethral resection, diabetes mellitus and urethral obstruction after surgery Age: median 63.7 years, range 46 to 83 years | |
| Interventions | A (44) intervention: verbal instruction and information on PFMT plus information on life style changes. Additional 15 physiotherapy sessions consisting of intensive PFMT with BF and ES B (32) control: verbal instruction and information on PFMT plus information on life style changes Duration of treatment: no description Length of follow‐up: 6 months | |
| Outcomes | Incontinence Quality of life (I‐QoL, higher score better), mean (SD) Directly after treatment: A 44.23 (14.61), B 37.53 (9.94) At 6 months: A 80.32 (7.01), B 51.69 (16.17), P = 0.001 At 6 months for Group A (44) intervention only: 1 hour pad test: mean urine loss before treatment 54.2 g and after treatment 8.8 g (P > 0.001) VAS severity of UI: before treatment 9.3, after treatment 1.3 (P > 0.001) | |
| Notes | Unexplained disparity between numbers in randomised groups No results for Group B control for pad test or VAS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Coloured cards |
| Allocation concealment (selection bias) | Unclear risk | Method of selection unknown |
| Blinding of participants (performance bias) | High risk | Blinding not possible |
| Blinding of personnel (performance bias) | High risk | Blinding not possible |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | High risk | No information for Group B control for both the one hour pad test and the VAS severity of UI |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Low risk | "None" |
| Approved by medical ethics committee | Low risk | "Medical Ethical Committee of Nossa Senhora das Gracas Hospital in Curitiba, Brazil" |
| Informed consent | Low risk | "after signing informed consent" |
| ITT analysis | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Methods | Randomisation: yes, mathematical table, grouped in blocks of 10 Method of allocation: sealed envelopes by independent third party Blinding: Slingle blind. Independent physiotherapist undertook initial assessment and 4 week outcome assessment Dropouts: 9 before intervention (4, training too time consuming; 1, didn't have TURP; 4, operated elsewhere) Setting: Hospital, Denmark | |
| Participants | Pre‐operative intervention Included: TURP, all men Exclusion: prostate cancer, previous lower urinary tract surgery, neurological disease Age: A 70 (58 to 77) years, B 68 (52 to 79) years | |
| Interventions | Group A (26) intervention: 1 hour individual session with physiotherapist to teach correct contraction for PFMT, three 1 hour group lessons and home training programme Group B (23) control: no pre‐operative physiotherapy. Information about anatomy and physiology and verbal instructions for 2 to 3 days after TURP in the ward Duration of treatment: 4 weeks after surgery Length of follow‐up: 2 and 4 weeks and 3 months after operation | |
| Outcomes | Compliance: A 24/26 attended all 4 training sessions Use of urinary pads per 24 hours, at 4 weeks: A 4/26, B 4/21. At 3 months: A 3/26, B 5/22 UI (pad test weight g/24hrs): 4 weeks (N, Median, range): A 26, 12 (0 to 374), B 23, 4 (0 to 56), P = 0.755 Danish Prostatic Symptom Scale: 3 months (N, median, range): A 26, 3 (0 to 24), B 23, 4.5 (0 to 51), P = 0.754 Also data on muscle function, muscle strength, static endurance and dynamic endurance | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Mathematical table, grouped in blocks of 10 |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes by independent third party |
| Blinding of participants (performance bias) | High risk | Not possible to blind to intervention |
| Blinding of personnel (performance bias) | Unclear risk | Independent physiotherapist undertook initial assessment and 4 week outcome assessment |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | High risk | Nine dropped out before intervention |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Low risk | None |
| Approved by medical ethics committee | Low risk | "This study was approved by the ethical committee in Copenhagen County and followed the Declaration of Helsinki" |
| Informed consent | Low risk | "Informed consent was obtained from the patients" |
| ITT analysis | Unclear risk | Not specified |
| Methods | RCT | |
| Participants | Time of recruitment: pre‐operative Population: men undergoing radical prostatectomy (whole population, with or without UI) Included: men who underwent open retropubic radical prostatectomy for clinically localized prostate cancer (cT1a to cT2b), able to regularly attend an ambulatory schedule Excluded: prior diseases with a possible impact on urinary continence, preoperative radiotherapy and any medical condition that could limit participation in the training programme Age (mean, range): A 67 (60 to 74); B 64 (52 to 74) Dropouts: 1 from A (intolerance to procedure using rectal probe), 1 from B (surgical complication) Not differential dropout Baseline characteristics: comparable at baseline | |
| Interventions | Time of intervention: pre‐operative A (16): on the day before RP + the day after catheter removal, patients received guided PFMT + biofeedback + information about the anatomy of pelvic floor muscles and wrong execution was corrected, also given oral and written instructions on Kegel exercises to be performed at home, instructed to: perform three sets daily for 10 mins, each contraction lasting 5 seconds with 5 seconds of relaxation, contract their pelvic floor while lying, sitting and standing, frequency recorded in training diary, After RP visits at monthly intervals after catheter removal involving assisted biofeedback and motivation for 20 min B (16): after catheter removal, men received standard care, oral and written instructions from urologist on PFMT, Instructed to: start PFMT (e.g. 2 to 3 weeks after surgery), control visits at 3 + 6 months after catheter removal All men were given oral and written instructions post‐operatively to perform PFMT at home, 3 sets daily of 10 min each Duration of treatment: monthly visits as long as patient required pads, including safety pads Follow up: at least 6 months after catheter removal | |
| Outcomes | Primary outcome (number of men with UI) Number of incontinent men (defined as ICIQ‐UI > 0) 1 month: A 10/16; B 16/16, P = 0.02 3 months: A 8/16; B 15/16, P = 0.01 6 months: A 6/16; B 15/16, P = 0.002 Other outcomes Number of continent men (efined as ICIQ‐UI = 0) 1 month: A 6/16; B 0/16, P = 0.02 3 months: A 8/16; B 1/16, P = 0.01 6 months : A 10/16; B 1/16, P = 0.002
Mean number of incontinence episodes per week/24 hours (mean (SD) N) 1 month: A 1.43 (0.82) 16; B 14 (0.82) 16, P = N.S 3 months: A 0.57 (1.47) 16; B 2 (1.47) 16, P = 0.01 6 months: A 0.43 (1.33) 16; B 1.86 (1.33) 16, P = 0.005
Mean number of pads used per week/24 hours (mean (SD) N) 1 month: A 0.46 (0.67) 16; B 0.94 (0.67) 16 P = NS 3 months: A 0.23 (0.63) 16; B 0.91 (0.63) 16 P = 0.005 6 months: A 0.2 (0.57) 16; B 0.66 (0.57) 16 P = 0.03
Quality of life Mean ICIQ‐OAB score (mean (SD) N) 1 month: A 11.5 (3.6) 16; B 14 (3.6) 16, P = NS 3 months: A 11 (0.92) 16; B 11.7 (0.92) 16, P = 0.04 6 months: A 9 (4.1) 16; B 13 (4.1) 16, P = 0.01
Mean UCLA‐PCI score (mean (SD) N) 1 month: A 330 (?) 16; B 260 (?) 16, P = NS 3 months: A 400 (500) 16; B 270 (338) 16, P = 0.006 6 months: A 430 (487) 26; B 275 (311) 16, P = 0.003 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Generated by computer and was stratified with a 1:1 allocation” |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants (performance bias) | High risk | “Participants were unblinded to treatment assignment” |
| Blinding of personnel (performance bias) | Low risk | “Surgeons and person scoring the evaluation questionnaires were blinded throughout the duration of the study” |
| Blinding of outcome assessment (detection bias) | Low risk | “nurse scoring the evaluation questionnaires was blinded” to randomisation |
| Incomplete outcome data (attrition bias) | Low risk | 1 from A (intolerance to procedure) 1 from B (surgical complication). Not differential dropout |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | Not reported |
| Approved by medical ethics committee | Low risk | “Work was carried out in accordance with the ethical standards of the appropriate institutional committee on human experimentation and with the last revision of the Helsinki Declaration |
| Informed consent | Low risk | “All eligible patients gave informed signed consent” |
| ITT analysis | Low risk | Assumed from patient flow diagram |
| Methods | Randomised: yes | |
| Participants | Recruitment: pre‐operative Included: all men, radical prostatectomy Age: 45 to 75 years | |
| Interventions | Group A (19) intervention: PFMT Group B (19) control: no PFMT length of follow‐up: 2, 4 and 8 weeks | |
| Outcomes | Dry at 2 weeks: A 9/19, B 9/19 Dry at 4 weeks: A 9/19, B 9/19 Dry at 8 weeks: A 15/19, B 17/19, P = 0.374 No significant differences for age (P = 0.674), PSA (P = 0.208), Gleason score pre (P = 0.762) and post‐operation (P = 0.824) | |
| Notes | Awaiting translation for more information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Allocation concealment (selection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of participants (performance bias) | High risk | No description, Spanish language |
| Blinding of personnel (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts. Therefore judged to be unclear risk |
| Selective reporting (reporting bias) | Unclear risk | Spanish language |
| Financial support | Unclear risk | Unable to be determined |
| Approved by medical ethics committee | Unclear risk | Unable to be determined |
| Informed consent | Unclear risk | Unable to be determined |
| ITT analysis | Unclear risk | Unable to be determined |
| Methods | Randomised: yes Blinding: yes (outcome assessor not involved with the study) Intention to treat: yes | |
| Participants | Recruitment: post‐operative Included: men incontinent post‐radical prostatectomy 15 days after surgery after catheter removal N = 102 eligible, 98 completed | |
| Interventions | Post‐operative intervention Group A (50) intervention: 1 session of PFMT in hospital before discharge and then saw the physiotherapist for 1 to 2 weeks for as long as UI persisted; 90 daily home exercises sitting, standing and lying; 7 men unable to contract PFM or with weak contraction received electrical stimulation by anal probe Group B (52) control: No formal PFMT instruction but saw the therapist at 1 to 2 weeks and received placebo stimulation and information about aetiology of UI Both A and B: received bladder training to increase bladder capacity Length of follow‐up: 12 months | |
| Outcomes | Main outcome: urine loss measured by 24 and 1 hour pad tests; 24 hour pad test done daily until continence achieved; 1 hour pad test when loss of < 2 g of urine to confirm continence Secondary outcomes: Continence definition: Data collection: subject assessment of continence preoperatively (during screening), and at 1, 6 and 12 months. Daily weighing of pads by participants (24 hour pad test) | |
| Notes | Pragmatic study; policy of management left to clinical judgment as to which protocols to add to PFMT regime. 63 of the eligible subjects were unable to participate because of geographical reasons; demographics and post‐operative variables did not differ from the 102 subjects who were in the treatment groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified by grams of urine loss (< 50 , > 50, < 250, > 250 g) |
| Allocation concealment (selection bias) | Low risk | A ‐ stratified randomisation with sealed envelopes |
| Blinding of participants (performance bias) | Unclear risk | The control group "received placebo electrotherapy that could not affect the pelvic‐floor muscle function." |
| Blinding of personnel (performance bias) | Unclear risk | "The patients in both groups were treated by the same therapist (MVK) until they became continent, within a period of 1 year" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessor not involved with the study |
| Incomplete outcome data (attrition bias) | Unclear risk | Dropouts: 5 |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Low risk | "supported by a grant from the Fund of Scientific Research, Flanders, Belgium" |
| Approved by medical ethics committee | Unclear risk | Not specified |
| Informed consent | Low risk | "All patients included in the study gave written informed consent" |
| ITT analysis | Low risk | "The groups were analysed on an intention‐to‐treat basis" |
| Methods | Randomised: yes | |
| Participants | Recruitment: pre‐operative Included: all men undergoing radical prostatectomy N = 139 randomised (number in each group at various data collection points varied) | |
| Interventions | Post‐operative intervention Group A (47): PFMT alone Group B (46): PFMT + ES; PFMT as above plus instructed by dedicated in ES via surface anal electrode and bio‐impulser (biphasic pulse with 1 second bursts, 5 second pulse width, 2 second pulse trains Group C (46): PFMT + ES + biofeedback. As above plus biofeedback (anal probe) 15 minutes twice daily for 3 months All groups A and B and C: PFMT by physiotherapist, 20‐30 minute sessions for 3 days, instructed to perform exercises twice daily for 3 months plus 3 week rehabilitation program after discharge. Regular interaction with health professional for 6 weeks after surgery, encouraged to performed treatment for 3 months post‐surgery Length of follow‐up: 12 months | |
| Outcomes | Main outcome: urine loss measure by continence questionnaire and 20 minute provocative pad test Continence definition: reported use of 0 to 1 pads on questionnaire (subjective) or loss of less than 1 gram of urine on pad test Data collection: baseline (after catheter removal), 3 months and 12 months post‐operatively Willingness to undergo surgery again: A 73%, B 83%, C 73% Quality of life EORCT QLQ‐C30: scores for physical; role; emotional; social; and global quality of life were not significantly different between the groups at 3 or 12 months (no SDs provided) No significant differences in continence rates between the three groups at baseline, 3 months or 12 months (objective) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Prospective randomized trial" Method of sequence generation not specified |
| Allocation concealment (selection bias) | Unclear risk | "Prospective randomized trial" Method of sequence generation not specified |
| Blinding of participants (performance bias) | High risk | Blinding to intervention not possible |
| Blinding of personnel (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | "Results at baseline after catheter removal, at 3 and 12 months postoperatively were available for 139, 120 and 128 (questionnaires at three different time points) and 116, 79 and 124 (pad test at three different time points) patients, respectively". However, no information about individual groups |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Approved by medical ethics committee | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Informed consent | Low risk | "Informed consent was obtained" |
| ITT analysis | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Methods | Randomised: yes. Blinding: double blind Dropouts: 1 due to pain in the intervention group | |
| Participants | Randomisation: postoperative Included: radical prostatectomy, all with severe post‐operative UI of > 100 g after catheter removal Age: mean 65.7 (7.0) years Pre‐operative intervention | |
| Interventions | All patients instructed pre‐operatively PFMT by nurses and continued after catheter removal A (26) intervention: oral PFMT plus electrical stimulation for 15 minutes twice daily (50 Hz square waves, 300 µsec pulse duration, maximum output 70 mA (5 sec on, 5 sec off duty cycle) B (30) control: oral PFMT plus sham stimulation (output 3 mA, 2 sec on, 13 sec off duty cycle) Duration of treatment: until continent or 12 months Length of follow‐up: 1, 3, 6 and 12 months after treatment Dropouts: A 4/26, B 5/30 (including 2+4 with adverse effects) | |
| Outcomes | Number of incontinent men 1 month: A 18/26, B 29/30 3 months: A 10/24, B 25/29 6 months: A 5/23, B 15/26 12 months: A 3/22, B 8/25 24 hour pad test weights (mean ml, SD, N) 1 month: A 210 (261) 26, B 423 (357) 30 3 months: A 81 (140) 24, B 232 (339) 29 6 months: A 20 (49) 23, B 132 (293) 26 12 months: A 18 (49) 22, B 98 (277) 25 Time until continent in months (mean, SD, N): A 2.71 (2.6) 22, B 6.82 (3.9) 25, P = 0.0006 ICIQ‐SF (mean score SD N; 0 to 21, higher = worse) 1 month: A 10.6 (6) 26, B 14.9 (4.9) 30 3 months: A 5.8 (5.7) 24, B 11.2 (5.7) 29 6 months: A 4.3 (6.2) 23, B 8.2 (5.3) 26 12 months: A 4.2 (6.2) 22, B 5.6 (6.5) 25 ICIQ‐QoL score (mean score SD N; 0 to 21; 0 to 10, higher = worse) 1 month: A 4.2 (3.5) 26, B 6 (3) 30 3 months: A 2.2 (2.3) 24, B 3.7 (2.9) 29 6 months: A 1.6 (3.1) 23, B 2.5 (2.2) 26 12 months: A 1.5 (3.1) 22, B 1.9 (2.5) 25 Adverse effects: A 2/26, B 4/30 (discomfort or anal pain) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By computer |
| Allocation concealment (selection bias) | Low risk | "None of the patients, doctors or medical staff knew which type of stimulation had been assigned until the key code was opened" |
| Blinding of participants (performance bias) | Low risk | Men were blinded to the intervention (sham, low energy stimulation in control group |
| Blinding of personnel (performance bias) | Low risk | Blinding of doctors, nurses and medical staff |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of assessors, medical staff |
| Incomplete outcome data (attrition bias) | Low risk | It is reported that "In the active ES group 2 patients discontinued after 2 and 3 months, respectively, due to urethral stricture at the bladder neck. In the sham group 1 patient discontinued treatment at 7 months because of an increase in prostate specific antigen and he then underwent radiation therapy". However, there is no evidence that dropout was related to trial interventions |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Low risk | "None" |
| Approved by medical ethics committee | Low risk | "Local ethical committee approval" was obtained |
| Informed consent | Low risk | "written informed consent from each subject was obtained" |
| ITT analysis | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Methods | Randomised: yes Method of allocation: not stated Blinding: not mentioned Dropouts: it appears that there are no dropouts but this is not specifically mentioned | |
| Participants | Recruitment: post‐operative Included: 36 men with urinary incontinence, >100g on 24hour pad test, one day after catheter removal Mean age: Group A 67.2 years, Group B 68.2 years, Group C 66.2 years | |
| Interventions | A (12) intervention: anal electrode for 15 minutes twice a day for 1 month B (12) intervention: extra‐corporeal magnetic innervation, neocontrol system, treatment sessions 20 minutes, twice a week for 2 weeks C (12) control: PFMT, digital anal teaching of correct contractions, then verbal and written instructions for home practice Length of follow‐up: 2, 3, 4, 5 and 6 months | |
| Outcomes | 24 hour pad test weight (grams) 3 months: A 34 g, B 7.3 g, C 50 g. 6 months: for all groups less than 10 g Quality of life measured by I‐QOL: improvement in all groups over time, no statistically significant difference between the groups Remaining UI at 6 months: A 2/12, B 1/12, C 2/12 | |
| Notes | Adverse effects: None in any of the groups, no discomfort or irritation from anal probe | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Allocation concealment (selection bias) | Unclear risk | Randomly assigned |
| Blinding of participants (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of personnel (performance bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Blinding of outcome assessment (detection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | Unclear risk | Numbers not given |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Financial support | Unclear risk | Not reported |
| Approved by medical ethics committee | Low risk | "The local ethics committee approved the protocol procedure" |
| Informed consent | Low risk | "Each patient provided written informed consent" |
| ITT analysis | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Methods | Randomised: yes Method of allocation: not stated Blinding: none Dropouts: two did not complete the control follow‐up assessment because they believed the control group was not helpful | |
| Participants | 58 men approached, 33 consented, 3 dropouts Recruitment: post‐operative Included: all incontinent men 6 months after radical prostatectomy | |
| Interventions | Group A (14) intervention: PFMT plus BF using rectal electrical sensor, initial 45 minute session with physical therapist then written instructions to carry out at home three times a day for 10 minutes. Plus support group, 6 meetings in 3 months with a health psychologist Group B (15) control: PFMT plus BF using rectal electrical sensor, initial 45 minute session with physical therapist then written instructions to carry out at home three times a day for 10 minutes | |
| Outcomes | Length of follow‐up: 3 months Frequency of PFMT: 4 to 7 times per week A 12/14, B 6/13, P = 0.077 Use of pad or brief: A 7/14 (50%), B 11/13 (85%), P = 0.057 Not able to control urge to urinate and prevent leakage: A 4/14, B 8/13, P = 0.085 Nocturia per week (mean): A 13, B 15.08, P = 0.484 VAS for severity of UI: A 3.21, B 4.65, P = 0.057 (t = ‐1.902) QoL measured by Illness Intrusiveness Questionnaires (IIRS): A 10.96, B 17.27, P = 0.037 Mann Whitney U = 48.5 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. Therefore judged to be unclear risk |
| Allocation concealment (selection bias) | Unclear risk | "Randomised" |
| Blinding of participants (performance bias) | High risk | Group therapy (unable to blind to intervention) |
| Blinding of personnel (performance bias) | Unclear risk | A research assistant, who was a doctoral candidate in medical anthropology, collected data under supervision |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified. Therefore judged to be unclear risk |
| Incomplete outcome data (attrition bias) | Unclear risk | Two dropouts in the control group |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Financial support | Low risk | "This study was supported by an American Cancer Society pilot research grant and the Frances Payne Bolton School of Nursing at Case Western Reserve University" |
| Approved by medical ethics committee | Unclear risk | Not reported. Therefore judged to be unclear risk |
| Informed consent | Unclear risk | "33 patients consented to participate" |
| ITT analysis | Unclear risk | Not reported. Therefore judged to be unclear risk |
ExMI = extra‐corporeal magnetic innervation; g = gram(s); PFMT = pelvic floor muscle training; TURP = transurethral resection of the prostate; UI = urinary incontinence
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Insufficient information to assess study for inclusion. Abstract only, no data included. Attempts to contact the author for data unsuccessful | |
| Data from study that included male postprostatectomy and female post‐polio patients. Translation obtained as reported in German. Data from the two groups were not separated and therefore not in a usable form | |
| Not measuring incontinence | |
| Insufficient information to assess study for inclusion. Attempts to contact the author for data unsuccessful | |
| Data from study which involved post‐TURP patients. Two groups, treatment and control. Not randomly assigned to groups, first 25 consecutively assigned to control, next 25 to intervention | |
| Descriptive study. No control group | |
| RCT. PFMT + Duloxetine (drug) versus PFMT + placebo | |
| Descriptive pilot study. No control group | |
| Intervention after radiotherapy only | |
| RCT, 58 men after RP. Pharmacological intervention: Duloxetine + PFMT versus PFMT alone | |
| RCT, 112 men after RP. Pharmacological intervention: Duloxetine + PFMT versus PFMT alone | |
| Insufficient information to assess study for inclusion. Data reported in paper presentation and in later published report did not contain sufficient detail of analysis to include in tables of comparison. Attempts to contact authors not successful in providing further data | |
| Pharmacological intervention | |
| Education intervention (refrigerator magnet) not an intervention included in review | |
| A comparative study. Early versus delayed PFMT no randomisation | |
| Measuring erectile dysfunction only | |
| Descriptive study of change in education delivery approach. No control group | |
| Data not separated for men who received radiotherapy and those who underwent prostate surgery | |
| Insufficient information to assess study for inclusion. Abstract only. Attempts to contact authors for further data unsuccessful. Possibly ongoing trial but no further data available | |
| RCT but of written information about diet and general exercise | |
| Comparative study of different types of urinary sheath | |
| Measuring erectile dysfunction, no useable data | |
| Descriptive study. No control group | |
| Not prostatectomy | |
| Measuring "sensory urgency" only, not incontinence | |
| Measuring the validity of a specific test, no useable data | |
| Descriptive study. No control group. Article in Spanish with English abstract | |
| Descriptive study. No control group. Article in Spanish with English abstract | |
| Descriptive study. No control group | |
| Not looking at incontinence. Translation obtained as reported in Chinese | |
| Data for men who received radiotherapy not separated from those who underwent prostate surgery | |
| Not randomised. Translation obtained as reported in Chinese | |
| Not RCT. Physiotherapist‐guided PFMT versus control (retrospective analysis) | |
| Not randomised | |
| Descriptive study. No control group | |
| Data for men who received radiotherapy not separated from those who underwent surgery |
Estim = Electrical stimulation
ExMI = Extra‐corporeal magnetic innervation
TURP = Transurethral resection of the prostate
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Not enough information |
| Participants | 73 men after retropubic radical prostatectomy |
| Interventions | Ultrasound‐guided biofeedback versus biofeedback using verbal instructions and digital biofeedback |
| Outcomes | |
| Notes | Authors to be contacted regarding whether assignment was randomised |
| Methods | Open label RCT |
| Participants | Men scheduled for radical prostatectomy |
| Interventions | Preoperative intensive PFMT with or without proprioceptive training |
| Outcomes | Anal examination to assess pelvic floor muscle function; subjective and objective voiding and incontinence parameters; four tests of pelvic floor muscle function; PGI‐I; ICIQ‐male score |
| Notes | Further information needed from authors |
| Methods | Unclear if randomised, further information from authors required |
| Participants | Time of recruitment: pre‐operative
Population: Men having a radical prostatectomy (whole population, with or without UI)
Included: Men who were candidates for retropubic radical prostatectomy
Excluded: Acquired or congenital disability, cardiovascular diseases requiring the administration of drugs that interfere with voiding, e.g. diuretics and/or alphalytics, problems relating to vesico‐sphincteral innervations, episodes of unstable or transitory continence during their lifetime, any type of neuropathy, other cancers, and psychoaffective disturbances such as depression or insomnia Age (mean, SD): A 68 (?); B 68 (?)
Dropouts: Not reported Baseline characteristics: Comparable at baseline |
| Interventions | Time of intervention: Pre‐operative
A (45): 20 mins of PFMT + biofeedback daily for 15 weeks before surgery, instructed to: carry out exercises during increased abdominal pressure (coughing, extending the abdomen, raising the head and keeping it raised), instructed how to carry out rapid, brief contractions without increasing abdominal activity and how to perform slow contractions
B (45): Instructed to start PFMT at home 15 weeks before surgery
After surgery and removal of catheter, all men were instructed to carry out four series of PF contractions at home on a daily basis for six months
Duration of treatment: 6 months Follow up: 1 month, 3 months and 6 months after surgery |
| Outcomes | Primary outcome (number of men with UI): Number of incontinent men (defined as use of pads): Pre‐operative: A 0/45; B 0/45 1 month: A 42/45; B 42/45 3 months: A 30/45; B 33/45 6 months: A 13/45; B 15/45 Other outcomes: Number of continent men (defined as completely dry without the use of pads): Pre‐operative: A 45/45; B 45/45 1 month: A 3/45; B 3/45 3 months: A 15/45; B 12/45 6 months: A 32/45; B 30/45 |
| Notes | Unclear if randomised |
| Methods | RCT |
| Participants | Men with urinary incontinence after radical prostatectomy |
| Interventions | A: PFMT B: PFMT with additional 'BBS trainer' |
| Outcomes | Quality of life (EORTC questionnaire), impact of incontinence |
| Notes | Awaiting German translation |
| Methods | RCT |
| Participants | Men after radical prostatectomy |
| Interventions | A: PFMT + biofeedback + electrical stimulation B: Whole body vibration C: Guided PFMT |
| Outcomes | International Prostate Symptom Score (IPSS), the enclosed question about quality of life (IPSS‐QL), pad test, pelvic floor strength, maximum uroflow, micturition volume, serum testosterone and blood glucose |
| Notes | Awaiting German translation |
| Methods | RCT |
| Participants | 127 Men with incontinence after “cancer treatment” Radical prostatectomy? |
| Interventions | A: Biofeedback + PFMT + 6 biweekly sessions of problem‐solving therapy delivered through a support group, B: Biofeedback + PFMT + 6 biweekly sessions of Problem‐solving therapy delivered through telephone contact, C (?): Standard care |
| Outcomes | Number of incontinent men [need for wearing a pad] |
| Notes | Further information required about participants + no useable data |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Health Interventions in Men Undergoing Radical Prostatectomy‐ A Randomized Controlled Clinical Trial |
| Methods | RCT |
| Participants | Men undergoing radical prostatectomy |
| Interventions | Intensive fitness intervention |
| Outcomes | Expanded Prostate Cancer Index (EPIC)‐26, RAND‐12 Questionnaire, body weight change, body mass index (BMI) change, blood pressure (BP) change, International Index of Erectile Function (IIEF), Quality of Erection Questionnaire (QEQ) |
| Starting date | December 2012 |
| Contact information | Arthur L Burnett, Johns Hopkins Hospital, Baltimore, United States |
| Notes |
| Trial name or title | Study of Non‐Invasive Viberect Penile Vibratory Stimulation Regimen to Enhance Recovery of Erectile Function/Rigidity and Urinary Control/Continence After Nerve Sparing Radical Prostatectomy (RP) for Clinically Localized Prostate Cancer |
| Methods | RCT |
| Participants | Men with Urinary Incontinence |
| Interventions | Post‐operative use of Viberect device 3 days after catheter removal, daily usage for 7‐10 minutes versus no treatment |
| Outcomes | Recovery of erectile function following radical prostatectomy, IIEF, recovery of continence, EPIC urinary and and sexual domain, AUA, EHS EDITS and TSS questionnaires |
| Starting date | April 2013 |
| Contact information | Arthur L. Burnett, M.D., Johns Hopkins University |
| Notes |
| Trial name or title | Mechanical Nerve Stimulation in the Treatment of Post Prostatectomy Incontinence |
| Methods | RCT |
| Participants | Men with urinary incontinence more than 1 year after radical prostatectomy |
| Interventions | Medical vibrator used daily for 6 weeks |
| Outcomes | 24 hour pad test, Micturition diary, Validated symptom score ICI‐Q, IPSS |
| Starting date | June 2012 |
| Contact information | Copenhagen University Hospital at Herlev |
| Notes |
| Trial name or title | Perioperative Post‐Prostatectomy Incontinence Home Telehealth Program (ProsTel) |
| Methods | RCT |
| Participants | Men undergoing radical prostatectomy |
| Interventions | Guided PFMT using a telehealth device (home messaging unit) |
| Outcomes | Time to continence using ICIQ‐SF, EPIC‐UI, HRQOL, IIQ‐SF, IPSS, patient satisfaction questionnaire, Estimated Percent improvement, Global perception of Improvement |
| Starting date | January 2012 |
| Contact information | Department of Veterans Affairs |
| Notes |
| Trial name or title | A Multicentre, Pilot Randomized Controlled Trial to Examine the Effects of Prehabilitation on Functional Outcomes After Radical Prostatectomy |
| Methods | RCT |
| Participants | Men undergoing radical prostatectomy |
| Interventions | Behavioral: Prehabilitation (PREHAB) |
| Outcomes | Adherence to Prehabilitation Program, Recruitment, Contamination, Study Retention, Physical Fitness, Quality of Life, Psychosocial Wellbeing, Physical Activity, Treatment Complications, Post‐operative length of stay |
| Starting date | November 2013 |
| Contact information | Daniel Santa Mina, University Health Network, Toronto, Ontario, Canada |
| Notes |
| Trial name or title | Trial study of the efficacy of intensive preoperative pelvic floor muscle training to decrease post‐prostatectomy urinary incontinence |
| Methods | RCT |
| Participants | Men with urinary incontinence after radical prostatectomy |
| Interventions | Preoperative guided PFMT 3 weeks before surgery versus PFMT on the day of admission for surgery |
| Outcomes | Pad test, IIQ‐7, SF 12 |
| Starting date | February 2011 |
| Contact information | Sau‐loi NG, Queen Mary Hospital, Hong Kong |
| Notes |
| Trial name or title | Prevention of Urinary Incontinence After Prostatectomy |
| Methods | RCT |
| Participants | Men undergoing radical prostatectomy |
| Interventions | BioFeedback; Functional Electrical Stimulation; Pelvic Floor Muscle training exercises |
| Outcomes | 24‐hour PAD test: Complete continence |
| Starting date | February 2007 |
| Contact information | Carlo Cisari, Azienda Ospedaliero Universitaria Maggiore della Carita |
| Notes |
| Trial name or title | Implementation and scientific evaluation of rehabilitative sports groups for prostate cancer patients: study protocol of the ProRehab Study |
| Methods | "Patient preference RCT"? |
| Participants | Men after radical prostatectomy |
| Interventions | exercise intervention ‐ rehabilitative sports group |
| Outcomes | "quality of life using EORTC‐QLQ‐C30/PR 25, incontinence using pad test and erectile dysfunction using IIEF" |
| Starting date | |
| Contact information | German Sport University Cologne |
| Notes |
Estim = Electrical stimulation
ExMI = Extra‐corporeal magnetic innervation
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinent men Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 1 Number of incontinent men. | ||||
| 1.1 less than 3 months | 7 | 980 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.08] |
| 1.2 within 3‐6 months | 7 | 895 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.83, 1.10] |
| 1.3 within 6‐12 months | 5 | 792 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.73, 1.14] |
| 1.4 after 12 months | 3 | 665 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.60, 1.22] |
| 2 Number of incontinence episodes per day Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 2 Number of incontinence episodes per day. | ||||
| 2.1 less than 3 months | 2 | 400 | Mean Difference (IV, Fixed, 95% CI) | ‐1.09 [‐1.39, ‐0.78] |
| 2.2 within 3‐6 months | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.40, 1.00] |
| 2.3 within 6‐12 months | 1 | 217 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.33, 0.93] |
| 2.4 after first year | 1 | 211 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.82, 1.02] |
| 3 Number of men using pads Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 3 Number of men using pads. | ||||
| 3.1 less than 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 within 3‐6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 within 6‐12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 after 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Pad changes over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4 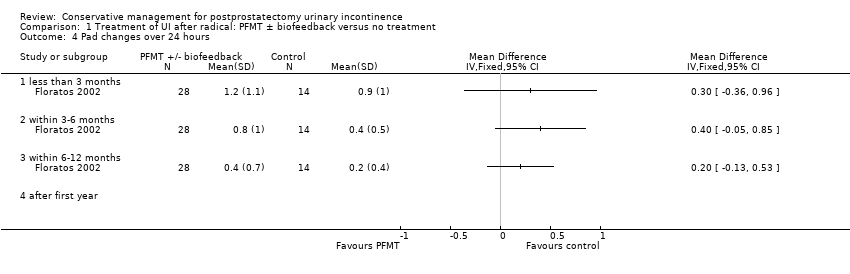 Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 4 Pad changes over 24 hours. | ||||
| 4.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 after first year | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Urinary Incontinence Score (ICIQ‐SF) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5 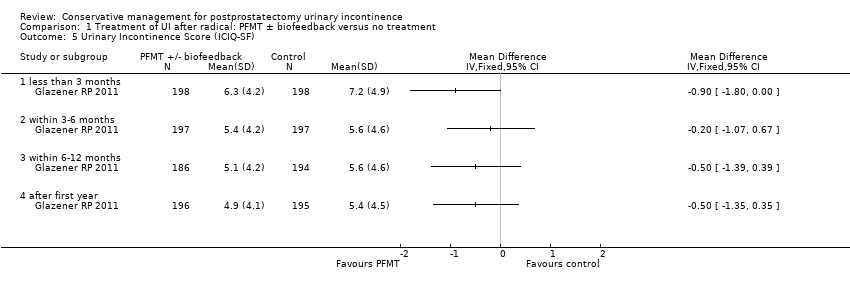 Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 5 Urinary Incontinence Score (ICIQ‐SF). | ||||
| 5.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Quality of life related to urinary incontinence Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.6  Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 6 Quality of life related to urinary incontinence. | ||||
| 6.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.7  Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 7 Adverse events. | ||||
| 8 24 hour pad test (grams of urine lost) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.8 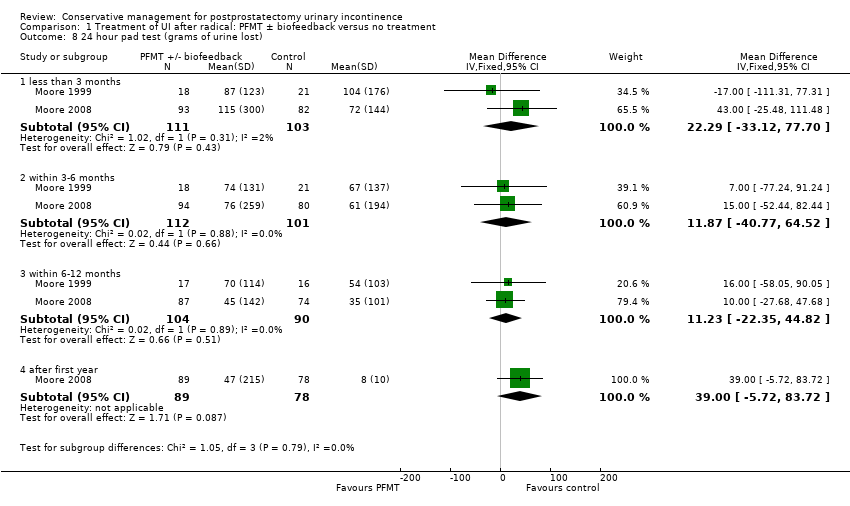 Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 8 24 hour pad test (grams of urine lost). | ||||
| 8.1 less than 3 months | 2 | 214 | Mean Difference (IV, Fixed, 95% CI) | 22.29 [‐33.12, 77.70] |
| 8.2 within 3‐6 months | 2 | 213 | Mean Difference (IV, Fixed, 95% CI) | 11.87 [‐40.77, 64.52] |
| 8.3 within 6‐12 months | 2 | 194 | Mean Difference (IV, Fixed, 95% CI) | 11.23 [‐22.35, 44.82] |
| 8.4 after first year | 1 | 167 | Mean Difference (IV, Fixed, 95% CI) | 39.0 [‐5.72, 83.72] |
| 9 1 hour pad test (grams of urine lost) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.9 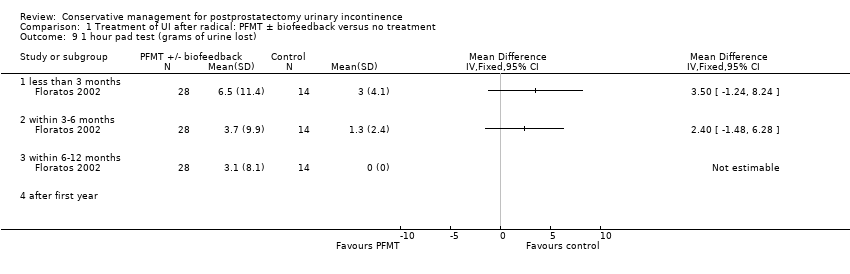 Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 9 1 hour pad test (grams of urine lost). | ||||
| 9.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 after first year | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Number of men not carrying out pelvic floor muscle contractions at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.10  Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 10 Number of men not carrying out pelvic floor muscle contractions at 12 months. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinent men Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1 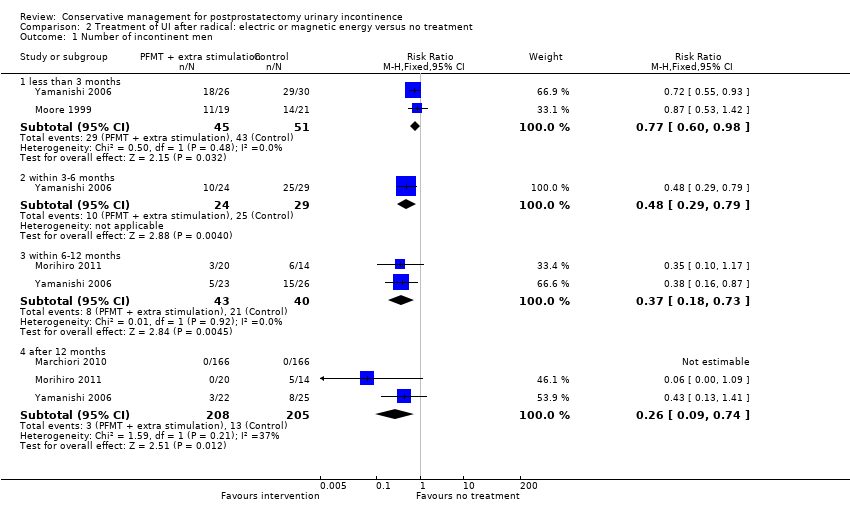 Comparison 2 Treatment of UI after radical: electric or magnetic energy versus no treatment, Outcome 1 Number of incontinent men. | ||||
| 1.1 less than 3 months | 2 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.60, 0.98] |
| 1.2 within 3‐6 months | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.29, 0.79] |
| 1.3 within 6‐12 months | 2 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.18, 0.73] |
| 1.4 after 12 months | 3 | 413 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.09, 0.74] |
| 2 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2 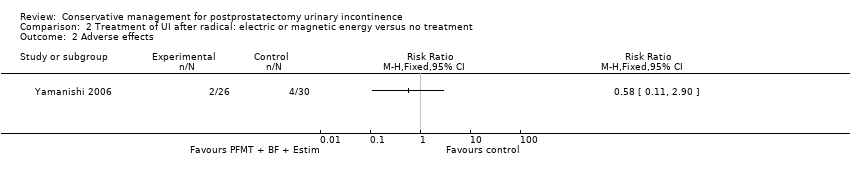 Comparison 2 Treatment of UI after radical: electric or magnetic energy versus no treatment, Outcome 2 Adverse effects. | ||||
| 3 24 hour pad test (grams of urine lost) Show forest plot | 2 | 325 | Mean Difference (IV, Fixed, 95% CI) | ‐16.94 [‐58.21, 24.33] |
| Analysis 2.3 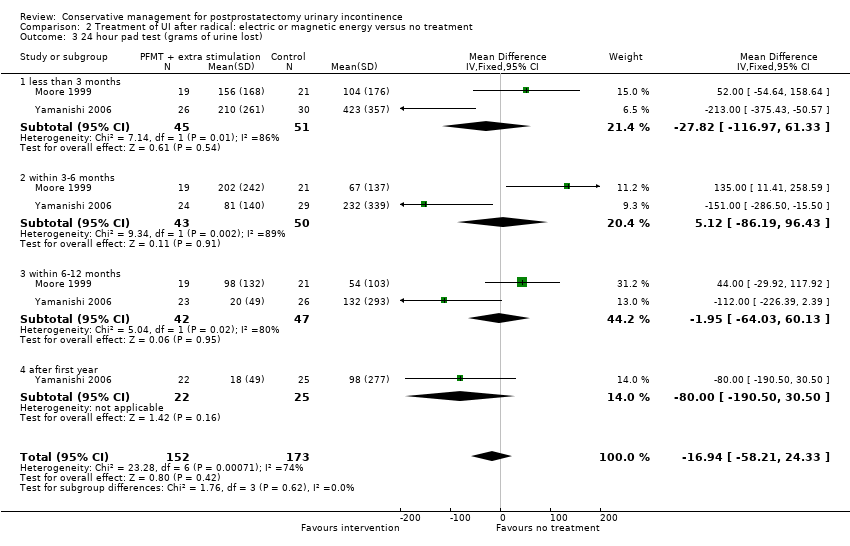 Comparison 2 Treatment of UI after radical: electric or magnetic energy versus no treatment, Outcome 3 24 hour pad test (grams of urine lost). | ||||
| 3.1 less than 3 months | 2 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐27.82 [‐116.97, 61.33] |
| 3.2 within 3‐6 months | 2 | 93 | Mean Difference (IV, Fixed, 95% CI) | 5.12 [‐86.19, 96.43] |
| 3.3 within 6‐12 months | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐1.95 [‐64.03, 60.13] |
| 3.4 after first year | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐80.0 [‐190.50, 30.50] |
| 4 Urinary Incontinence Score (ICIQ‐short form UI score) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2 Treatment of UI after radical: electric or magnetic energy versus no treatment, Outcome 4 Urinary Incontinence Score (ICIQ‐short form UI score). | ||||
| 4.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Urinary Incontinence Quality of Life Score (ICIQ‐short form) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 Treatment of UI after radical: electric or magnetic energy versus no treatment, Outcome 5 Urinary Incontinence Quality of Life Score (ICIQ‐short form). | ||||
| 5.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Time until continent (months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.6  Comparison 2 Treatment of UI after radical: electric or magnetic energy versus no treatment, Outcome 6 Time until continent (months). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinent men at < 3 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.1  Comparison 4 Treatment of UI after radical: combinations of treatments versus no treatment, Outcome 1 Number of incontinent men at < 3 months. | ||||
| 1.1 PFMT + anal Estim + Biofeedback vs no treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of incontinent men within 3‐6 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.2 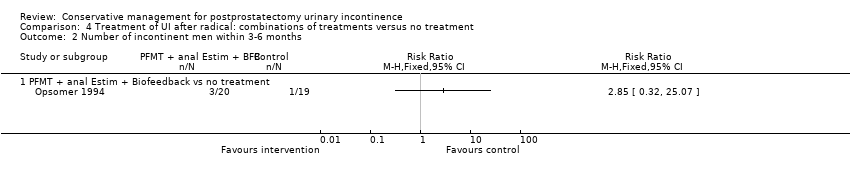 Comparison 4 Treatment of UI after radical: combinations of treatments versus no treatment, Outcome 2 Number of incontinent men within 3‐6 months. | ||||
| 2.1 PFMT + anal Estim + Biofeedback vs no treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of incontinence episodes per day at > 3 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.3 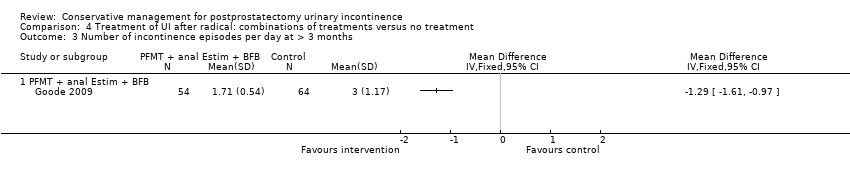 Comparison 4 Treatment of UI after radical: combinations of treatments versus no treatment, Outcome 3 Number of incontinence episodes per day at > 3 months. | ||||
| 3.1 PFMT + anal Estim + BFB | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.4  Comparison 4 Treatment of UI after radical: combinations of treatments versus no treatment, Outcome 4 Adverse effects. | ||||
| 4.1 PFMT + anal Estim + BFB | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinent men at < 3 months Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.1  Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 1 Number of incontinent men at < 3 months. | ||||
| 1.1 PFMT + Anal EStim vs PFMT alone | 2 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.83, 1.12] |
| 2 Number of incontinent men within 3 to 6 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.2 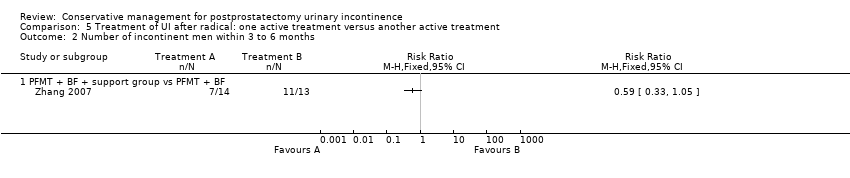 Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 2 Number of incontinent men within 3 to 6 months. | ||||
| 2.1 PFMT + BF + support group vs PFMT + BF | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of incontinent men within 6 to 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.3 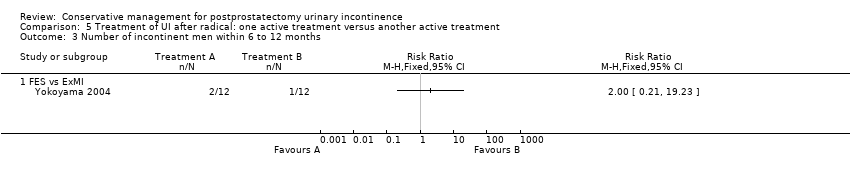 Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 3 Number of incontinent men within 6 to 12 months. | ||||
| 3.1 FES vs ExMI | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of incontinence episodes at < 3 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.4  Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 4 Number of incontinence episodes at < 3 months. | ||||
| 4.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Quality of Life Score (severity of UI) within 3 to 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.5  Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 5 Quality of Life Score (severity of UI) within 3 to 6 months. | ||||
| 5.1 PFMT + BF + support group vs PFMT + BF | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Quality of Life Score (I‐QoL) within 6‐12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.6  Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 6 Quality of Life Score (I‐QoL) within 6‐12 months. | ||||
| 6.1 PFMT + BF + EStim vs PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Quality of Life Score (ICI‐Q‐SF) within 6‐12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.7  Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 7 Quality of Life Score (ICI‐Q‐SF) within 6‐12 months. | ||||
| 7.1 PFMT + ExMI vs PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.8 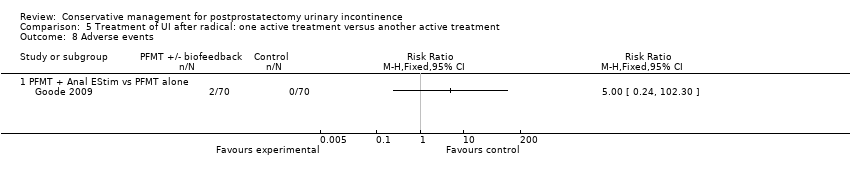 Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 8 Adverse events. | ||||
| 8.1 PFMT + Anal EStim vs PFMT alone | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 1 hour pad test (grams of urine lost): at < 3 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.9 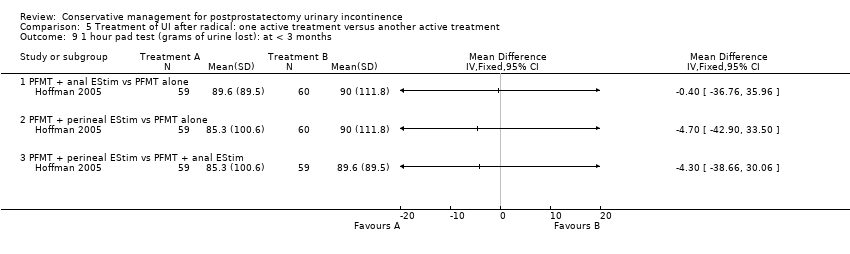 Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 9 1 hour pad test (grams of urine lost): at < 3 months. | ||||
| 9.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 PFMT + perineal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 PFMT + perineal EStim vs PFMT + anal EStim | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 24 hour pad test (grams of urine lost): at < 3 months Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.10  Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 10 24 hour pad test (grams of urine lost): at < 3 months. | ||||
| 10.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 PFMT + visual BF vs PFMT + oral BF | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 24 hour pad test (grams of urine lost): within 3 to 6 months Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.11  Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 11 24 hour pad test (grams of urine lost): within 3 to 6 months. | ||||
| 11.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 PFMT + visual BF vs PFMT + oral BF | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 24 hour pad test (grams of urine lost): within 3 to 6 months Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.12  Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 12 24 hour pad test (grams of urine lost): within 3 to 6 months. | ||||
| 12.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 PFMT + visual BF vs PFMT + oral BF | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 ExMI vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Pad changes over 24 hours within 3 to 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.13  Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 13 Pad changes over 24 hours within 3 to 6 months. | ||||
| 13.1 ExMI vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Number of men not carrying out sufficient PFMT within 3 to 6 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.14 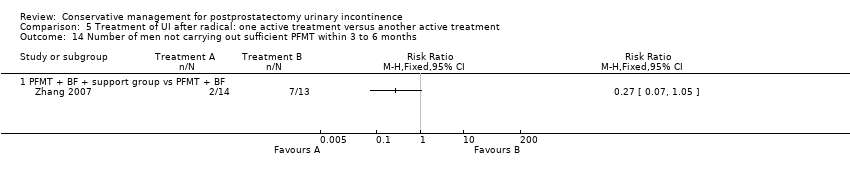 Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 14 Number of men not carrying out sufficient PFMT within 3 to 6 months. | ||||
| 14.1 PFMT + BF + support group vs PFMT + BF | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinent men Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 6.1 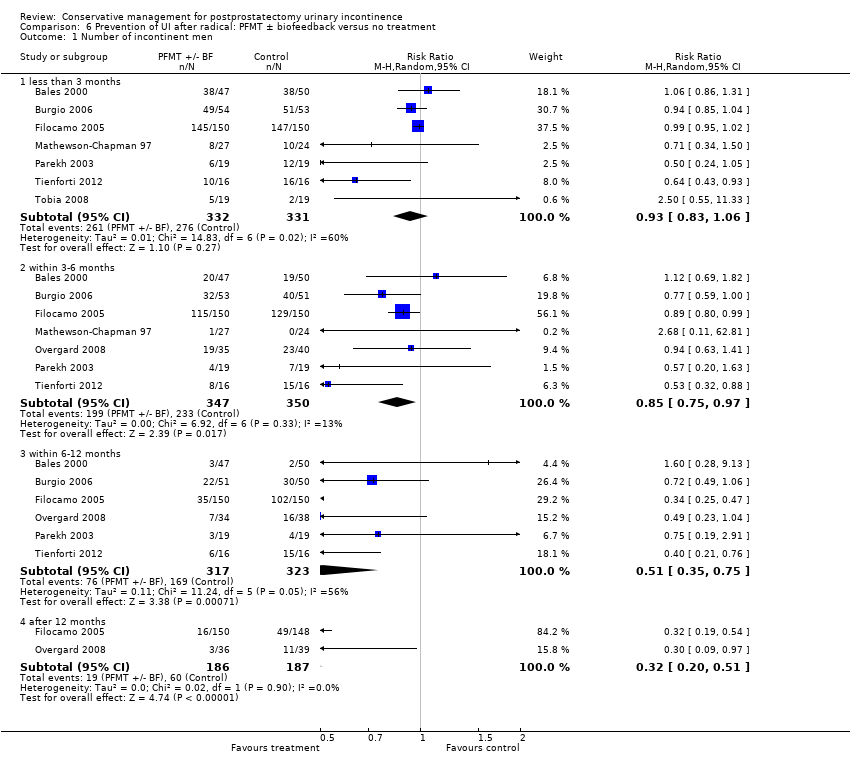 Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 1 Number of incontinent men. | ||||
| 1.1 less than 3 months | 7 | 663 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.83, 1.06] |
| 1.2 within 3‐6 months | 7 | 697 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.75, 0.97] |
| 1.3 within 6‐12 months | 6 | 640 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.35, 0.75] |
| 1.4 after 12 months | 2 | 373 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.20, 0.51] |
| 2 Pad changes over 24 hours Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 6.2 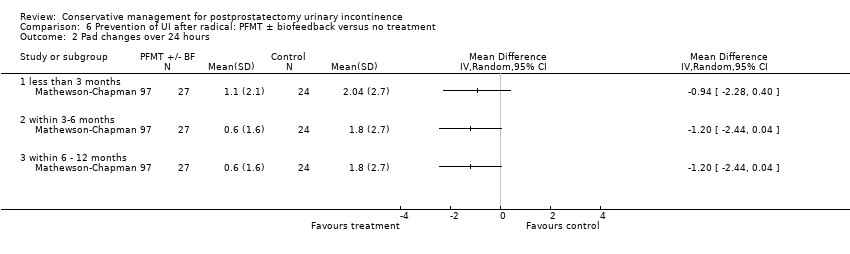 Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 2 Pad changes over 24 hours. | ||||
| 2.1 less than 3 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 within 3‐6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 within 6 ‐ 12 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 1 hour pad test (grams of urine lost) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.3 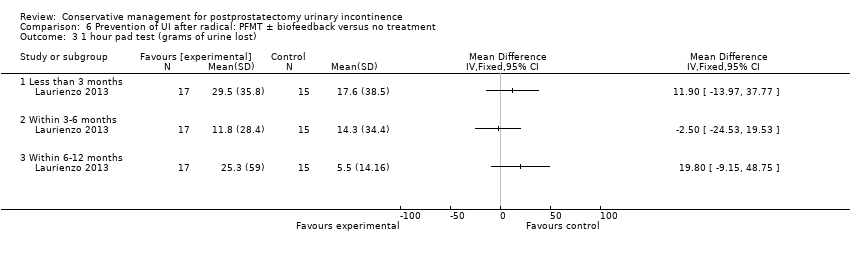 Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 3 1 hour pad test (grams of urine lost). | ||||
| 3.1 Less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 24 hour pad test (gm/24hrs) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 6.4 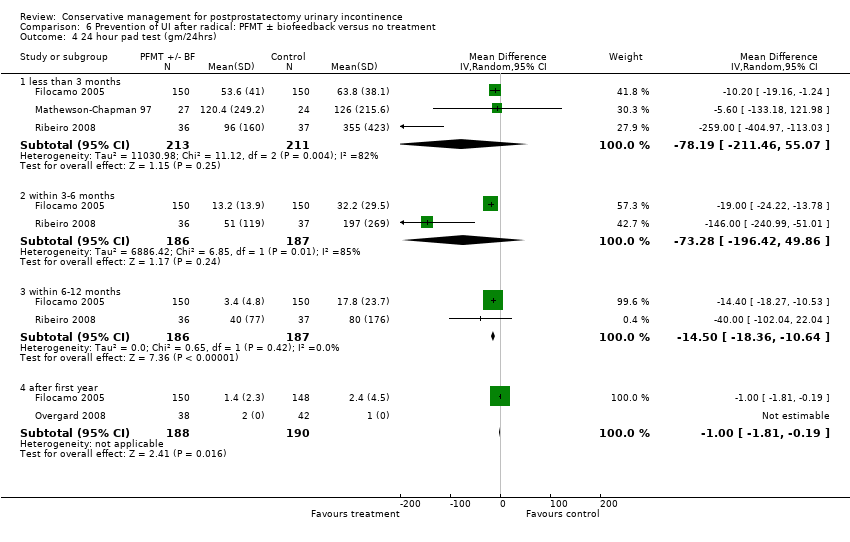 Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 4 24 hour pad test (gm/24hrs). | ||||
| 4.1 less than 3 months | 3 | 424 | Mean Difference (IV, Random, 95% CI) | ‐78.19 [‐211.46, 55.07] |
| 4.2 within 3‐6 months | 2 | 373 | Mean Difference (IV, Random, 95% CI) | ‐73.28 [‐196.42, 49.86] |
| 4.3 within 6‐12 months | 2 | 373 | Mean Difference (IV, Random, 95% CI) | ‐14.50 [‐18.36, ‐10.64] |
| 4.4 after first year | 2 | 378 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐1.81, ‐0.19] |
| 5 Number of incontinence episodes per day Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.5  Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 5 Number of incontinence episodes per day. | ||||
| 5.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 after first year | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Urinary Incontinence Score (ICI‐short form) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 6.6  Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 6 Urinary Incontinence Score (ICI‐short form). | ||||
| 6.1 less than 3 months | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 6.5 [3.45, 9.55] |
| 6.2 within 3‐6 months | 2 | 105 | Mean Difference (IV, Random, 95% CI) | ‐1.21 [‐5.99, 3.56] |
| 6.3 within 6‐12 months | 2 | 105 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐3.19, 1.81] |
| 7 Quality of Life Score (IIQ) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.7  Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 7 Quality of Life Score (IIQ). | ||||
| 7.1 less than 3 months | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 within 6‐12 months | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 after first year | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Pelvic floor muscle strength (anal squeeze pressure, cm H2O) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.8  Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 8 Pelvic floor muscle strength (anal squeeze pressure, cm H2O). | ||||
| 8.1 less than 3 months | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Number of men not carrying out sufficient PFMT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.9  Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 9 Number of men not carrying out sufficient PFMT. | ||||
| 9.1 less than 3 months | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 within 3‐6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 within 6‐12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 after 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Number of men having surgery for incontinence Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.10 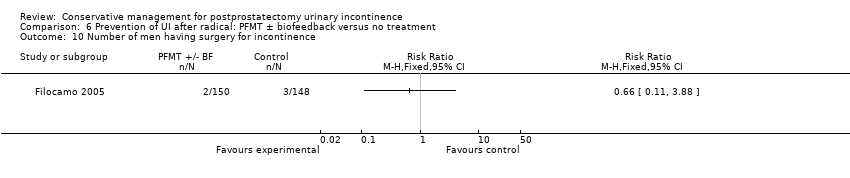 Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 10 Number of men having surgery for incontinence. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 1 hour pad test (grams of urine lost) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.1 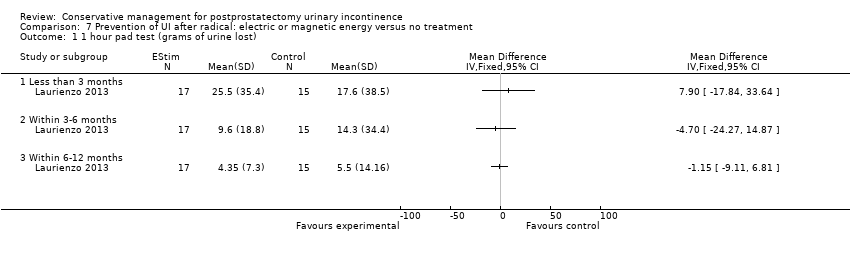 Comparison 7 Prevention of UI after radical: electric or magnetic energy versus no treatment, Outcome 1 1 hour pad test (grams of urine lost). | ||||
| 1.1 Less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 ICIQ‐SF score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.2  Comparison 7 Prevention of UI after radical: electric or magnetic energy versus no treatment, Outcome 2 ICIQ‐SF score. | ||||
| 2.1 Less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinent men within 3 to 6 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.1  Comparison 9 Prevention of UI after radical: combinations of treatments versus no treatment, Outcome 1 Number of incontinent men within 3 to 6 months. | ||||
| 1.1 PFMT + anal Estim + Biofeedback versus no treatment/sham treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of incontinent men within 6 to 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.2 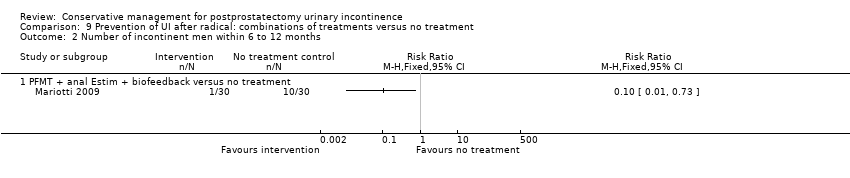 Comparison 9 Prevention of UI after radical: combinations of treatments versus no treatment, Outcome 2 Number of incontinent men within 6 to 12 months. | ||||
| 2.1 PFMT + anal Estim + biofeedback versus no treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 24 hour pad test (grams of urine lost) within 3 to 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.3  Comparison 9 Prevention of UI after radical: combinations of treatments versus no treatment, Outcome 3 24 hour pad test (grams of urine lost) within 3 to 6 months. | ||||
| 3.1 PFMT + anal Estim + Biofeedback versus no treatment/sham treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 24 hour pad test (grams of urine lost) 6 to 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.4  Comparison 9 Prevention of UI after radical: combinations of treatments versus no treatment, Outcome 4 24 hour pad test (grams of urine lost) 6 to 12 months. | ||||
| 4.1 PFMT + anal Estim + Biofeedback versus no treatment/sham treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Time until continent (months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.5  Comparison 9 Prevention of UI after radical: combinations of treatments versus no treatment, Outcome 5 Time until continent (months). | ||||
| 5.1 PFMT + anal Estim + Biofeedback versus no treatment/sham treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinent men at < 3months Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 10.1  Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 1 Number of incontinent men at < 3months. | ||||
| 1.1 PFMT pre and post op vs PFMT post op | 2 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.69, 1.06] |
| 1.2 PFMT + Biofeedback + transcutaneous Estim versus Estim only | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.61, 1.26] |
| 1.3 PFMT + Biofeedback + transcutaneous Estim versus post‐op PFMT | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.57, 1.11] |
| 1.4 Post‐op transcutaneous Estim versus post‐op PFMT | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.67, 1.22] |
| 2 Number of incontinent men within 3 to 6 months Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 10.2  Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 2 Number of incontinent men within 3 to 6 months. | ||||
| 2.1 PFMT pre and post op vs PFMT post op | 2 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.54, 1.04] |
| 2.2 post‐op PFMT + biofeedback + transcutaneous Estim vs post‐op Estim | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.96, 2.49] |
| 2.3 PFMT + general exercise versus PFMT alone | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.23, 0.99] |
| 2.4 Post‐op PFMT + transcutaneous Estim + Biofeedback versus post‐op PFMT | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.76, 1.57] |
| 2.5 Post‐op transcutaneous electrical stimulation versus post‐op PFMT | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.43, 1.16] |
| 3 Number of incontinent men within 6 to 12 months Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.3  Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 3 Number of incontinent men within 6 to 12 months. | ||||
| 3.1 PFMT pre and post op vs PFMT post op | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 post‐op PFMT + Biofeedback + transcutaneous Estim vs post‐op Estim | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Post‐op PFMT + transcutaneous Estim + Biofeedback versus post‐op PFMT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Post‐op transcutaneous Estim versus post‐op PFMT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of incontinent men after 12 months Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 10.4 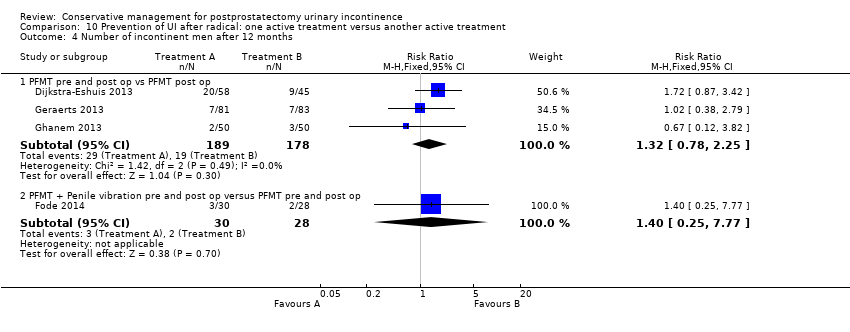 Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 4 Number of incontinent men after 12 months. | ||||
| 4.1 PFMT pre and post op vs PFMT post op | 3 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.78, 2.25] |
| 4.2 PFMT + Penile vibration pre and post op versus PFMT pre and post op | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.25, 7.77] |
| 5 No. with severe incontinence (e.g. pad test weight >150g) at < 3 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.5 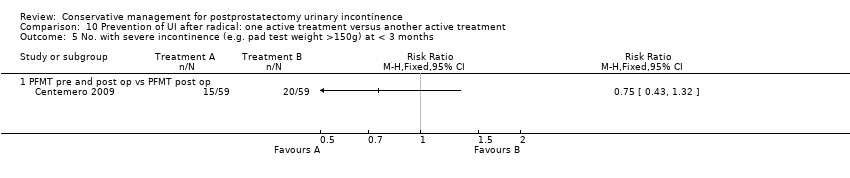 Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 5 No. with severe incontinence (e.g. pad test weight >150g) at < 3 months. | ||||
| 5.1 PFMT pre and post op vs PFMT post op | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 No. with severe incontinence (e.g. pad test weight >150g) at 3 to 6 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.6  Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 6 No. with severe incontinence (e.g. pad test weight >150g) at 3 to 6 months. | ||||
| 6.1 PFMT pre and post op vs PFMT post op | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 20 minute pad test (grams of urine lost): within 3 to 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.7  Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 7 20 minute pad test (grams of urine lost): within 3 to 6 months. | ||||
| 7.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 PFMT + anal EStim + BF vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 PFMT + anal EStim vs PFMT + anal EStim + BF | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 20 minute pad test (grams of urine lost): within 6 to 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.8 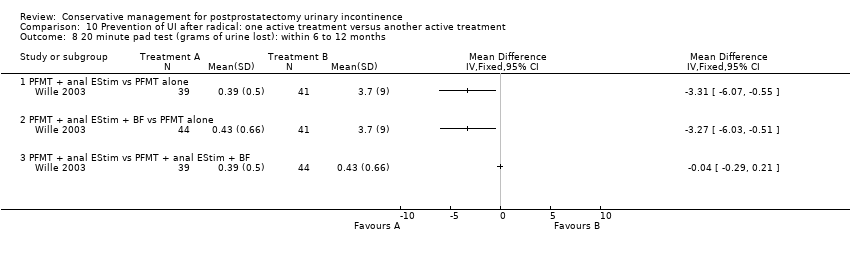 Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 8 20 minute pad test (grams of urine lost): within 6 to 12 months. | ||||
| 8.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 PFMT + anal EStim + BF vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 PFMT + anal EStim vs PFMT + anal EStim + BF | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 1 hour pad test (grams of urine lost) at less than 3 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.9 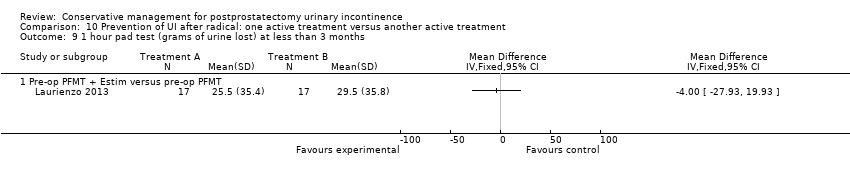 Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 9 1 hour pad test (grams of urine lost) at less than 3 months. | ||||
| 9.1 Pre‐op PFMT + Estim versus pre‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 1 hour pad test (grams of urine lost) within 3‐6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.10  Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 10 1 hour pad test (grams of urine lost) within 3‐6 months. | ||||
| 10.1 Pre‐op PFMT + electrical stimulation versus pre‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 1 hour pad test within 6‐12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.11  Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 11 1 hour pad test within 6‐12 months. | ||||
| 11.1 Pre‐op PFMT + electrical stimulation versus pre‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 24 hour pad test (grams of urine lost) at less than 3 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.12  Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 12 24 hour pad test (grams of urine lost) at less than 3 months. | ||||
| 12.1 PFMT + Biofeedback + transcutaneous Estim versus Estim only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Post‐operative PFMT + transcutaneous Estim + Biofeedback versus post‐operative PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 Post‐operative transcutaneous electrical stimulation versus post‐operative PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 24 hour pad test (grams of urine lost) within 3‐6 months Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.13  Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 13 24 hour pad test (grams of urine lost) within 3‐6 months. | ||||
| 13.1 PFMT + Biofeedback + transcutaneous Estim versus Estim only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Postoperative PFMT + biofeedback + transcutaneous Estim versus postoperative PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.3 Post‐operative transcutaneous Estim only versus post‐operative PFMT only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.4 PFMT + general exercise versus PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 24 hour pad test (grams of urine lost) within 6‐12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.14  Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 14 24 hour pad test (grams of urine lost) within 6‐12 months. | ||||
| 14.1 PFMT + transcutaneous Estim + biofeedback versus Estim only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Post‐op PFMT + transcutaneous Estim + Biofeedback versus post‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.3 Post‐op transcutaneous Estim versus post‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Quality of Life Score (ICS male short form) at < 3 months Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.15  Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 15 Quality of Life Score (ICS male short form) at < 3 months. | ||||
| 15.1 PFMT pre and post op vs PFMT post op | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Urinary Incontinence Quality of Life Score (ICIQ ‐ short form) within 3‐6 months Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.16 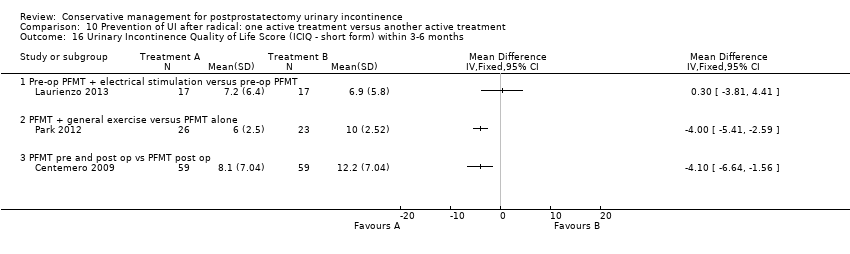 Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 16 Urinary Incontinence Quality of Life Score (ICIQ ‐ short form) within 3‐6 months. | ||||
| 16.1 Pre‐op PFMT + electrical stimulation versus pre‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 PFMT + general exercise versus PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.3 PFMT pre and post op vs PFMT post op | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 Urinary Incontinence Quality of Life Score (ICIQ‐short form) within 6‐12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.17  Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 17 Urinary Incontinence Quality of Life Score (ICIQ‐short form) within 6‐12 months. | ||||
| 17.1 Pre‐op PFMT + electrical stimulation versus pre‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 King's health Questionnaire after 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.18  Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 18 King's health Questionnaire after 12 months. | ||||
| 18.1 General Health | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 Role limitations | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.3 Physical limitations | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.4 Social limitations | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.5 Personal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.6 Emotional | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.7 Sleep/energy disturbance | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.8 Symptom severity | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 Health status measure SF‐36 within 3‐6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.19 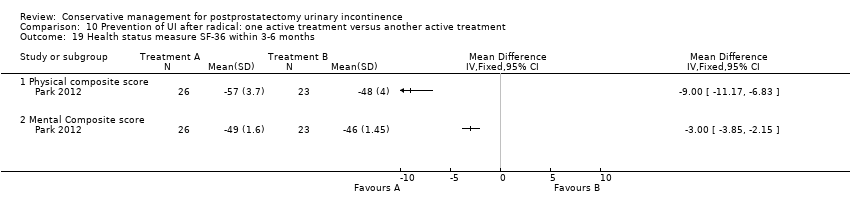 Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 19 Health status measure SF‐36 within 3‐6 months. | ||||
| 19.1 Physical composite score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Mental Composite score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Adverse events Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.20  Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 20 Adverse events. | ||||
| 20.1 PFMT pre and post op vs PFMT post op | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 PFMT + Penile vibration pre and post op versus PFMT pre and post op | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinent men Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 11.1 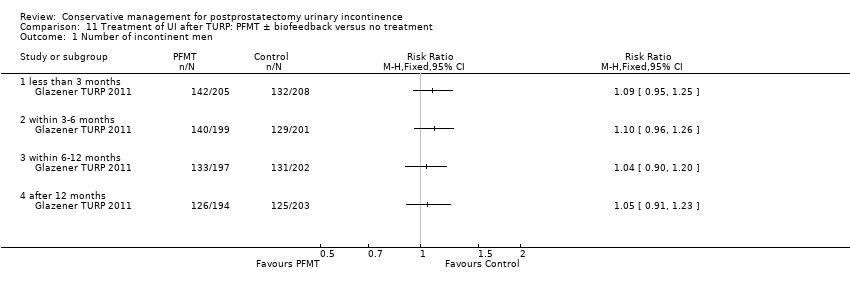 Comparison 11 Treatment of UI after TURP: PFMT ± biofeedback versus no treatment, Outcome 1 Number of incontinent men. | ||||
| 1.1 less than 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 within 3‐6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 within 6‐12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 after 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of incontinence episodes per day Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 11.2  Comparison 11 Treatment of UI after TURP: PFMT ± biofeedback versus no treatment, Outcome 2 Number of incontinence episodes per day. | ||||
| 2.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of men using pads Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 11.3  Comparison 11 Treatment of UI after TURP: PFMT ± biofeedback versus no treatment, Outcome 3 Number of men using pads. | ||||
| 3.1 less than 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 within 3‐6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 within 6‐12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 after 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Urinary Incontinence Score (ICI‐short form) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 11.4  Comparison 11 Treatment of UI after TURP: PFMT ± biofeedback versus no treatment, Outcome 4 Urinary Incontinence Score (ICI‐short form). | ||||
| 4.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Quality of life related to urinary incontinence Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 11.5  Comparison 11 Treatment of UI after TURP: PFMT ± biofeedback versus no treatment, Outcome 5 Quality of life related to urinary incontinence. | ||||
| 5.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of men not carrying out pelvic floor muscle contractions at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 11.6  Comparison 11 Treatment of UI after TURP: PFMT ± biofeedback versus no treatment, Outcome 6 Number of men not carrying out pelvic floor muscle contractions at 12 months. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinent men Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 16.1  Comparison 16 Prevention of UI after TURP: pre or post‐operative PFMT ± biofeedback versus no treatment, Outcome 1 Number of incontinent men. | ||||
| 1.1 less than 3 months | 2 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.21, 1.77] |
| 1.2 within 3‐6 months | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.14, 1.89] |
| 2 Health status measure SF‐36 within 3‐6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 16.2  Comparison 16 Prevention of UI after TURP: pre or post‐operative PFMT ± biofeedback versus no treatment, Outcome 2 Health status measure SF‐36 within 3‐6 months. | ||||
| 2.1 Physical component | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Physical functioning | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Body pain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 General Health | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Physical role limitation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Mental health component | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Mental role limitation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Vitality | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Mental health | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.10 Social functioning | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | |||||||||||||||
| 1 Number of men satisfied with device Show forest plot | Other data | No numeric data | |||||||||||||||||
| Analysis 21.1
Comparison 21 Containment of urinary incontinence from any cause: external penile compression devices (penile clamps) versus no treatment, Outcome 1 Number of men satisfied with device. | |||||||||||||||||||
| 2 Mean urine loss (grams of urine on pad test) Show forest plot | Other data | No numeric data | |||||||||||||||||
| Analysis 21.2
Comparison 21 Containment of urinary incontinence from any cause: external penile compression devices (penile clamps) versus no treatment, Outcome 2 Mean urine loss (grams of urine on pad test). | |||||||||||||||||||
| 3 Penile Doppler blood flow (mean systolic velocity) Show forest plot | Other data | No numeric data | |||||||||||||||||
| Analysis 21.3
Comparison 21 Containment of urinary incontinence from any cause: external penile compression devices (penile clamps) versus no treatment, Outcome 3 Penile Doppler blood flow (mean systolic velocity). | |||||||||||||||||||
| 4 Penile Doppler blood flow (mean resistence to flow index) Show forest plot | Other data | No numeric data | |||||||||||||||||
| Analysis 21.4
Comparison 21 Containment of urinary incontinence from any cause: external penile compression devices (penile clamps) versus no treatment, Outcome 4 Penile Doppler blood flow (mean resistence to flow index). | |||||||||||||||||||
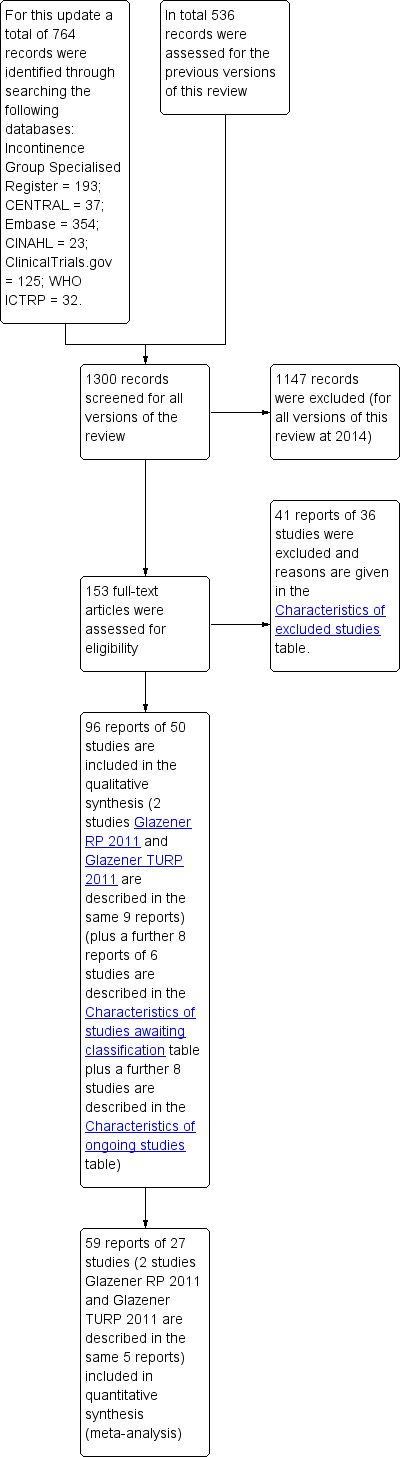
PRISMA study flow diagram.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
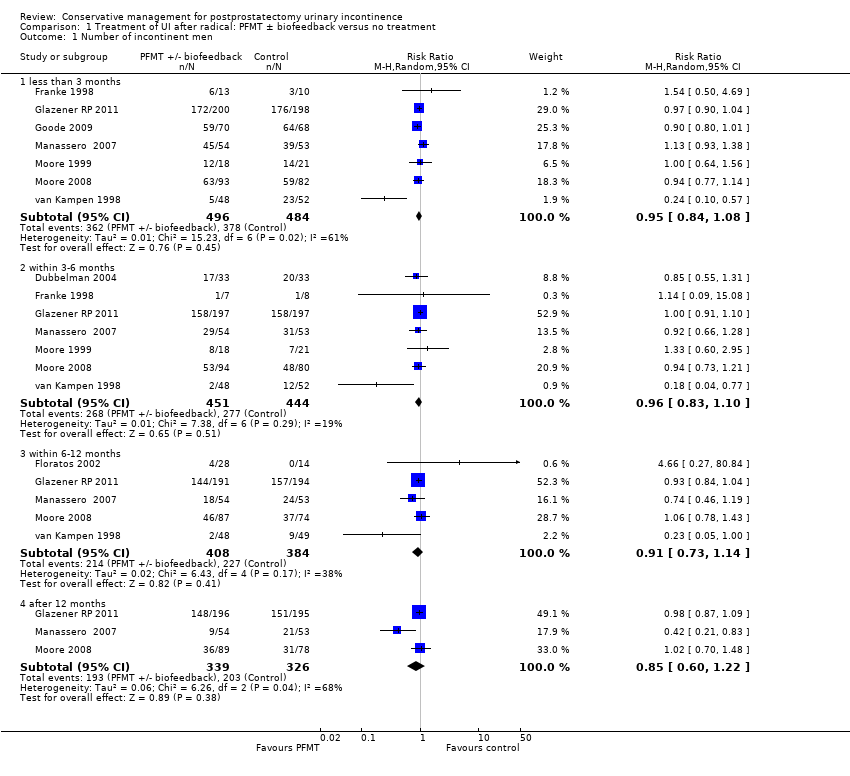
Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 1 Number of incontinent men.

Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 2 Number of incontinence episodes per day.

Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 3 Number of men using pads.
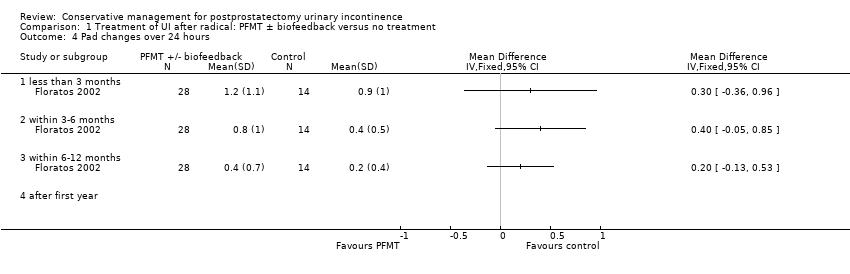
Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 4 Pad changes over 24 hours.
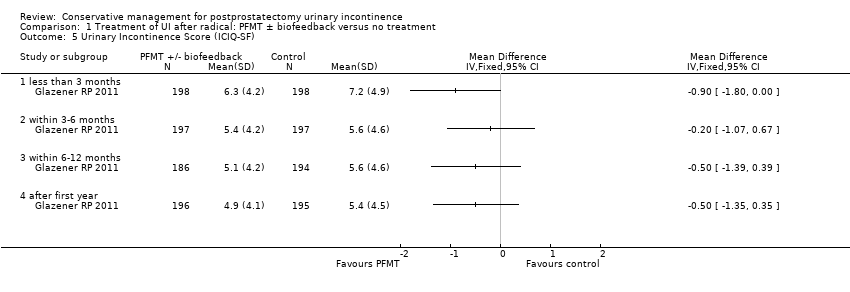
Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 5 Urinary Incontinence Score (ICIQ‐SF).
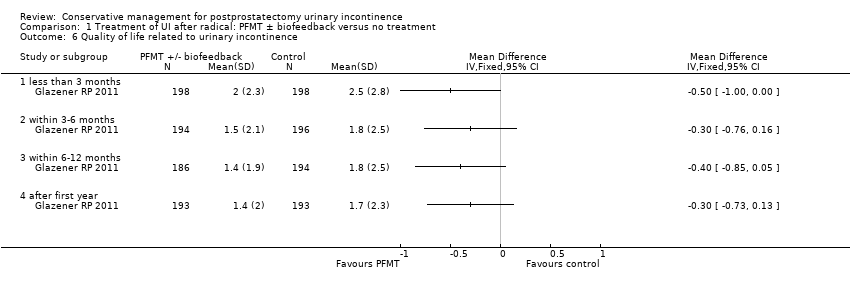
Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 6 Quality of life related to urinary incontinence.
Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 7 Adverse events.
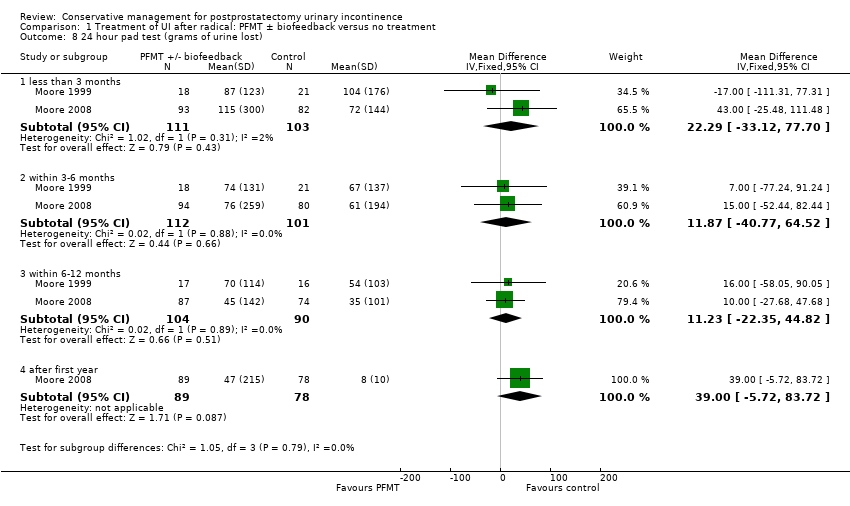
Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 8 24 hour pad test (grams of urine lost).

Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 9 1 hour pad test (grams of urine lost).
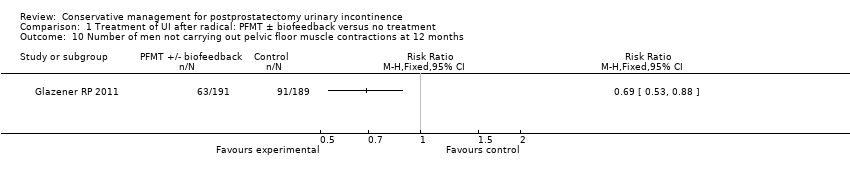
Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 10 Number of men not carrying out pelvic floor muscle contractions at 12 months.

Comparison 2 Treatment of UI after radical: electric or magnetic energy versus no treatment, Outcome 1 Number of incontinent men.

Comparison 2 Treatment of UI after radical: electric or magnetic energy versus no treatment, Outcome 2 Adverse effects.
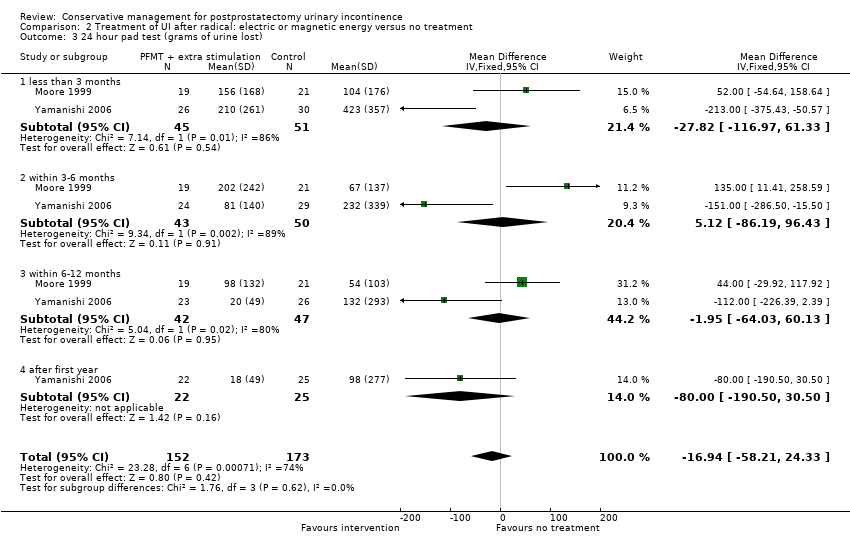
Comparison 2 Treatment of UI after radical: electric or magnetic energy versus no treatment, Outcome 3 24 hour pad test (grams of urine lost).

Comparison 2 Treatment of UI after radical: electric or magnetic energy versus no treatment, Outcome 4 Urinary Incontinence Score (ICIQ‐short form UI score).

Comparison 2 Treatment of UI after radical: electric or magnetic energy versus no treatment, Outcome 5 Urinary Incontinence Quality of Life Score (ICIQ‐short form).

Comparison 2 Treatment of UI after radical: electric or magnetic energy versus no treatment, Outcome 6 Time until continent (months).

Comparison 4 Treatment of UI after radical: combinations of treatments versus no treatment, Outcome 1 Number of incontinent men at < 3 months.

Comparison 4 Treatment of UI after radical: combinations of treatments versus no treatment, Outcome 2 Number of incontinent men within 3‐6 months.
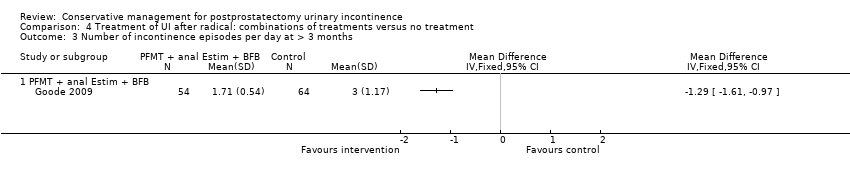
Comparison 4 Treatment of UI after radical: combinations of treatments versus no treatment, Outcome 3 Number of incontinence episodes per day at > 3 months.

Comparison 4 Treatment of UI after radical: combinations of treatments versus no treatment, Outcome 4 Adverse effects.
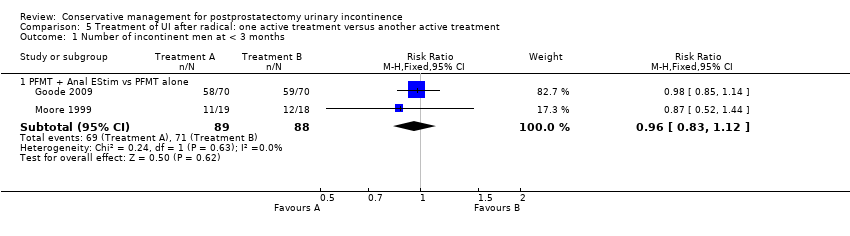
Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 1 Number of incontinent men at < 3 months.

Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 2 Number of incontinent men within 3 to 6 months.

Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 3 Number of incontinent men within 6 to 12 months.
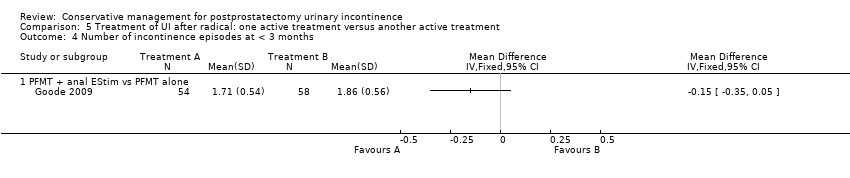
Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 4 Number of incontinence episodes at < 3 months.

Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 5 Quality of Life Score (severity of UI) within 3 to 6 months.

Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 6 Quality of Life Score (I‐QoL) within 6‐12 months.
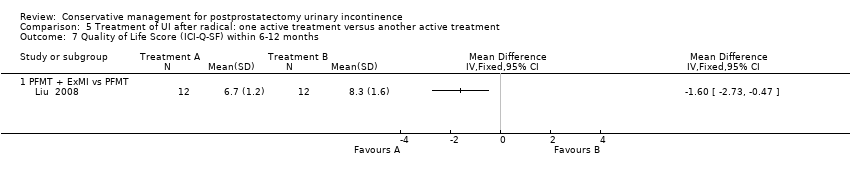
Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 7 Quality of Life Score (ICI‐Q‐SF) within 6‐12 months.
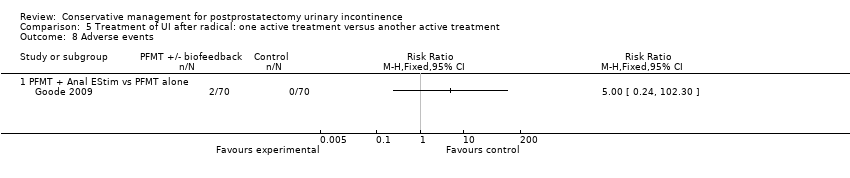
Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 8 Adverse events.
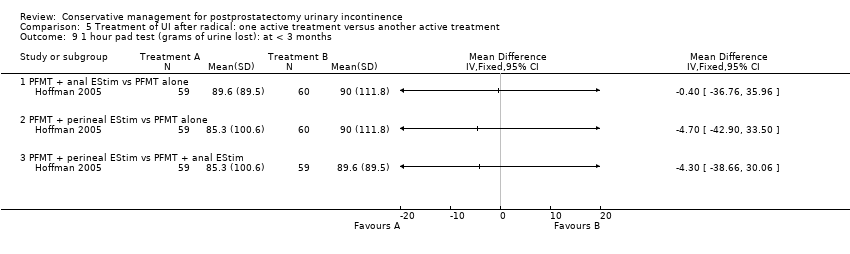
Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 9 1 hour pad test (grams of urine lost): at < 3 months.
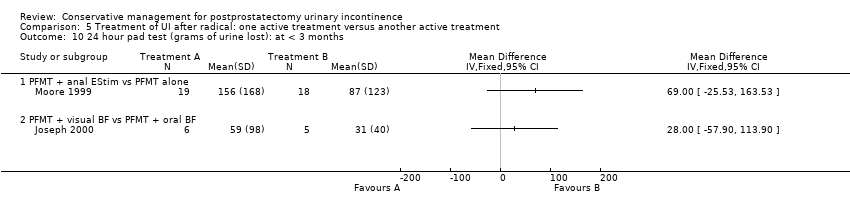
Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 10 24 hour pad test (grams of urine lost): at < 3 months.

Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 11 24 hour pad test (grams of urine lost): within 3 to 6 months.

Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 12 24 hour pad test (grams of urine lost): within 3 to 6 months.
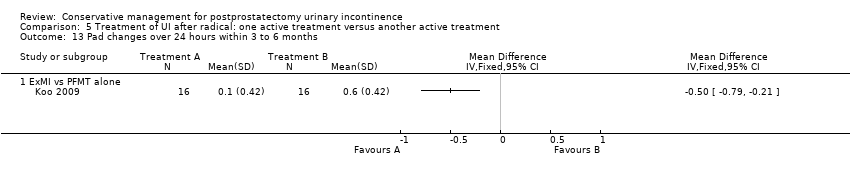
Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 13 Pad changes over 24 hours within 3 to 6 months.

Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 14 Number of men not carrying out sufficient PFMT within 3 to 6 months.

Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 1 Number of incontinent men.

Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 2 Pad changes over 24 hours.
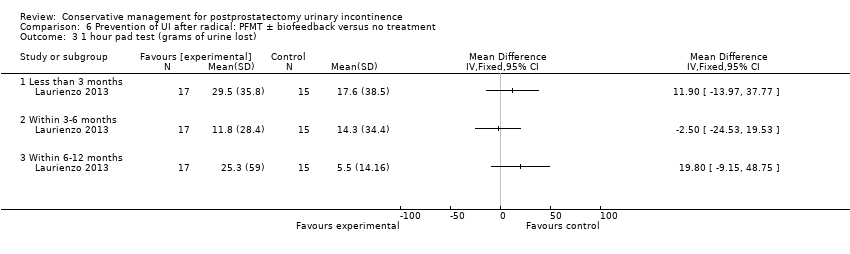
Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 3 1 hour pad test (grams of urine lost).

Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 4 24 hour pad test (gm/24hrs).
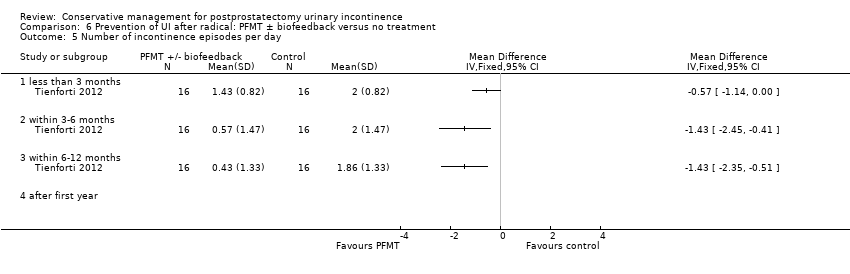
Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 5 Number of incontinence episodes per day.
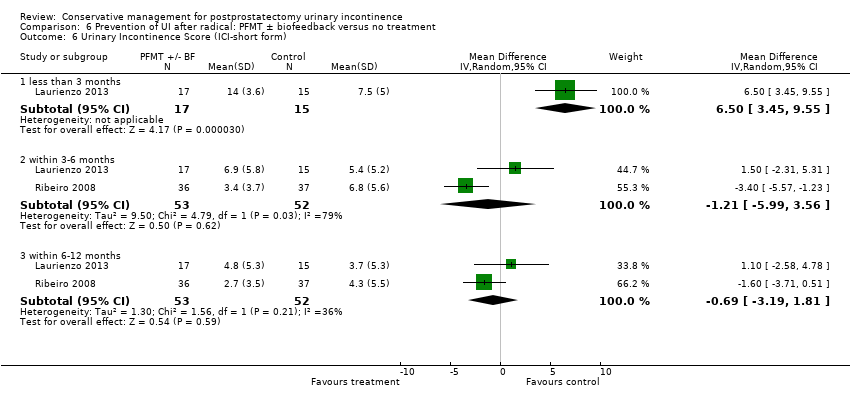
Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 6 Urinary Incontinence Score (ICI‐short form).

Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 7 Quality of Life Score (IIQ).

Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 8 Pelvic floor muscle strength (anal squeeze pressure, cm H2O).

Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 9 Number of men not carrying out sufficient PFMT.

Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 10 Number of men having surgery for incontinence.
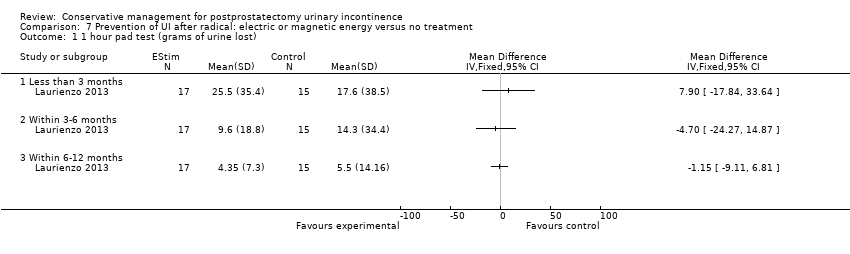
Comparison 7 Prevention of UI after radical: electric or magnetic energy versus no treatment, Outcome 1 1 hour pad test (grams of urine lost).

Comparison 7 Prevention of UI after radical: electric or magnetic energy versus no treatment, Outcome 2 ICIQ‐SF score.
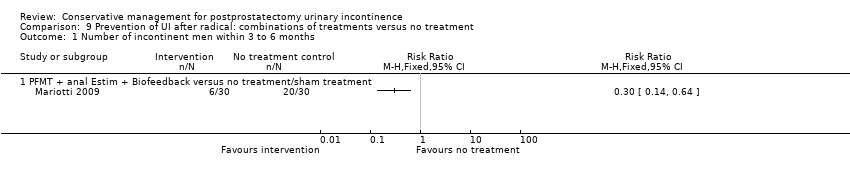
Comparison 9 Prevention of UI after radical: combinations of treatments versus no treatment, Outcome 1 Number of incontinent men within 3 to 6 months.

Comparison 9 Prevention of UI after radical: combinations of treatments versus no treatment, Outcome 2 Number of incontinent men within 6 to 12 months.

Comparison 9 Prevention of UI after radical: combinations of treatments versus no treatment, Outcome 3 24 hour pad test (grams of urine lost) within 3 to 6 months.
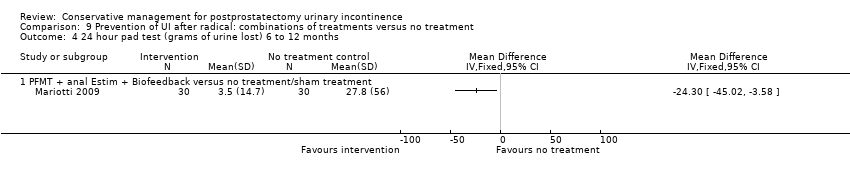
Comparison 9 Prevention of UI after radical: combinations of treatments versus no treatment, Outcome 4 24 hour pad test (grams of urine lost) 6 to 12 months.

Comparison 9 Prevention of UI after radical: combinations of treatments versus no treatment, Outcome 5 Time until continent (months).

Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 1 Number of incontinent men at < 3months.
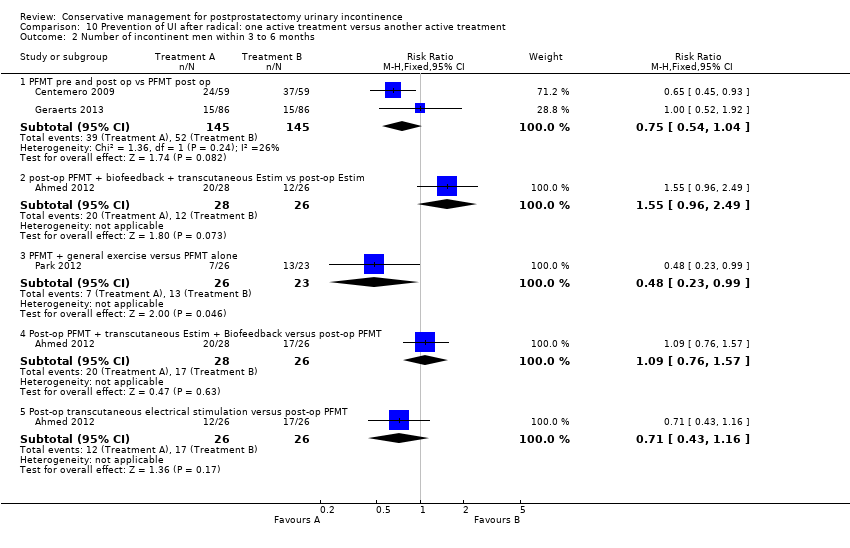
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 2 Number of incontinent men within 3 to 6 months.

Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 3 Number of incontinent men within 6 to 12 months.
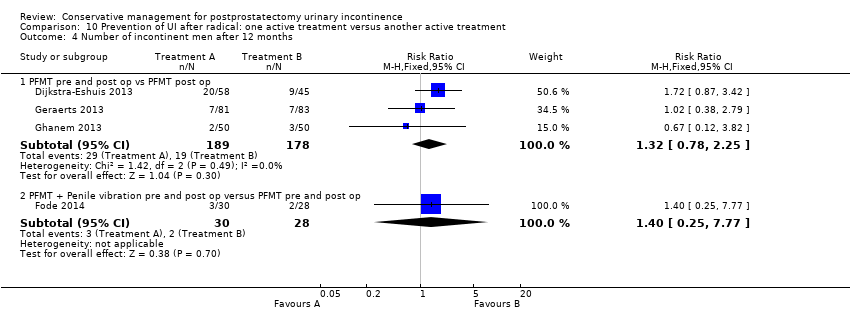
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 4 Number of incontinent men after 12 months.
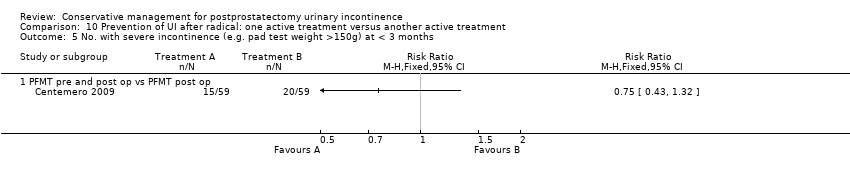
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 5 No. with severe incontinence (e.g. pad test weight >150g) at < 3 months.
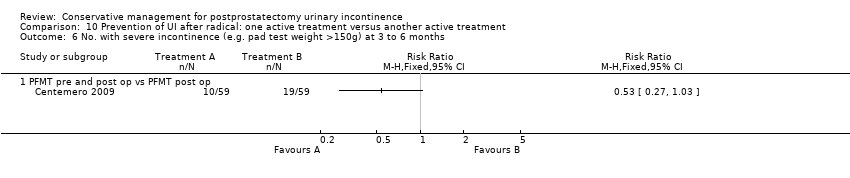
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 6 No. with severe incontinence (e.g. pad test weight >150g) at 3 to 6 months.

Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 7 20 minute pad test (grams of urine lost): within 3 to 6 months.

Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 8 20 minute pad test (grams of urine lost): within 6 to 12 months.
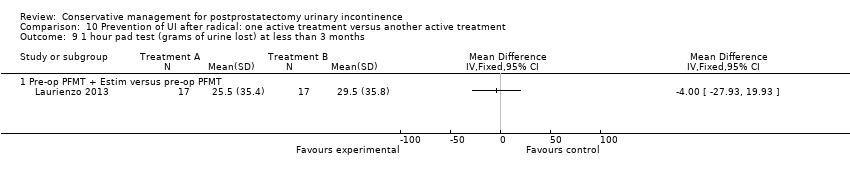
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 9 1 hour pad test (grams of urine lost) at less than 3 months.

Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 10 1 hour pad test (grams of urine lost) within 3‐6 months.
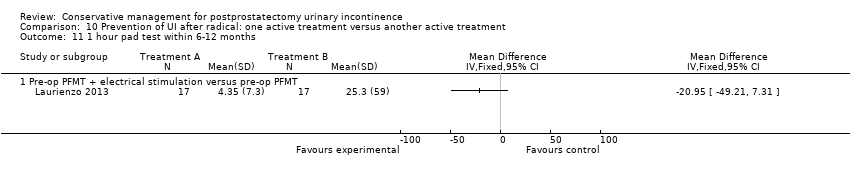
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 11 1 hour pad test within 6‐12 months.
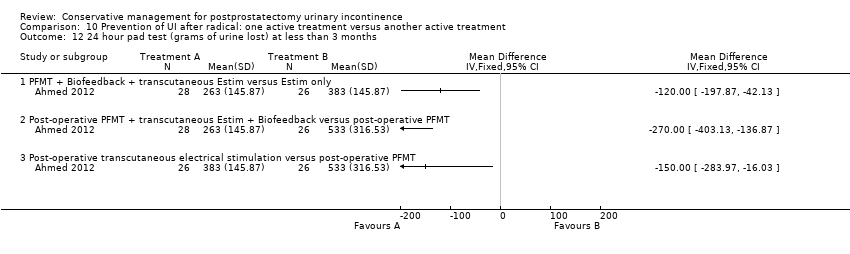
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 12 24 hour pad test (grams of urine lost) at less than 3 months.
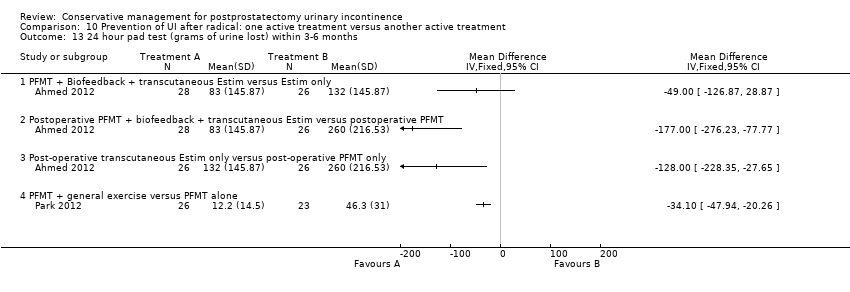
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 13 24 hour pad test (grams of urine lost) within 3‐6 months.

Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 14 24 hour pad test (grams of urine lost) within 6‐12 months.
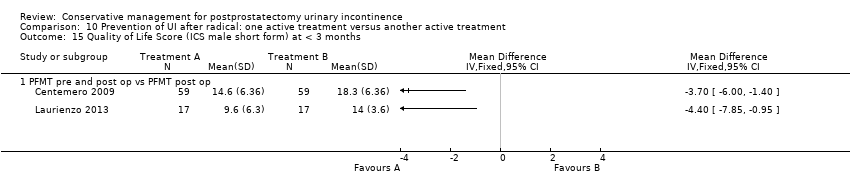
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 15 Quality of Life Score (ICS male short form) at < 3 months.
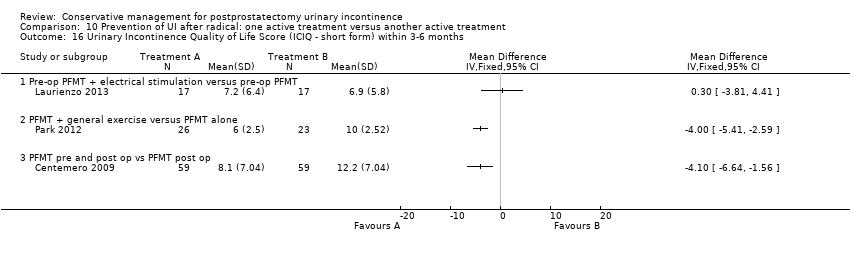
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 16 Urinary Incontinence Quality of Life Score (ICIQ ‐ short form) within 3‐6 months.

Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 17 Urinary Incontinence Quality of Life Score (ICIQ‐short form) within 6‐12 months.
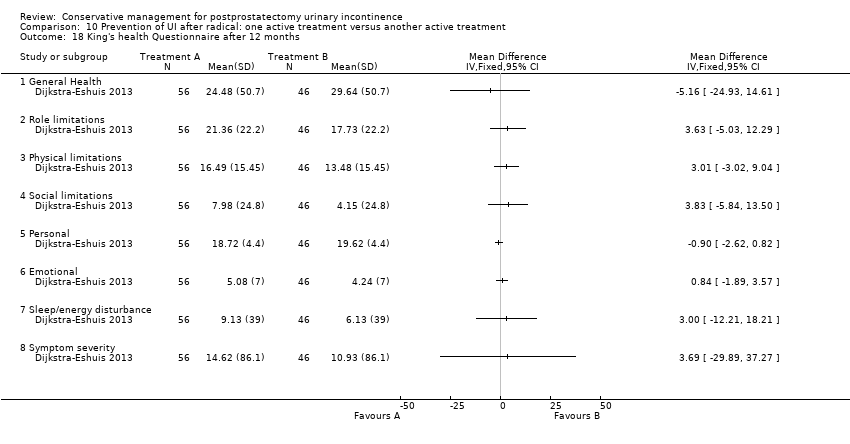
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 18 King's health Questionnaire after 12 months.
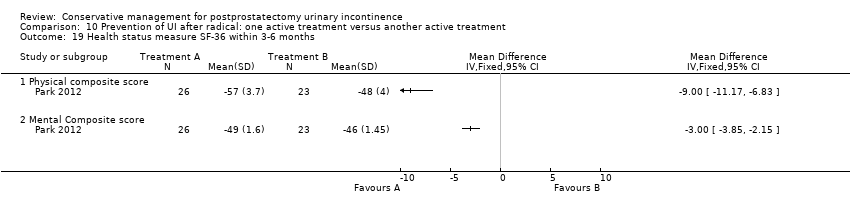
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 19 Health status measure SF‐36 within 3‐6 months.
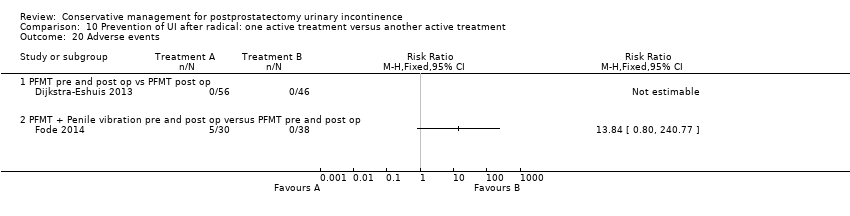
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 20 Adverse events.

Comparison 11 Treatment of UI after TURP: PFMT ± biofeedback versus no treatment, Outcome 1 Number of incontinent men.

Comparison 11 Treatment of UI after TURP: PFMT ± biofeedback versus no treatment, Outcome 2 Number of incontinence episodes per day.

Comparison 11 Treatment of UI after TURP: PFMT ± biofeedback versus no treatment, Outcome 3 Number of men using pads.

Comparison 11 Treatment of UI after TURP: PFMT ± biofeedback versus no treatment, Outcome 4 Urinary Incontinence Score (ICI‐short form).

Comparison 11 Treatment of UI after TURP: PFMT ± biofeedback versus no treatment, Outcome 5 Quality of life related to urinary incontinence.

Comparison 11 Treatment of UI after TURP: PFMT ± biofeedback versus no treatment, Outcome 6 Number of men not carrying out pelvic floor muscle contractions at 12 months.
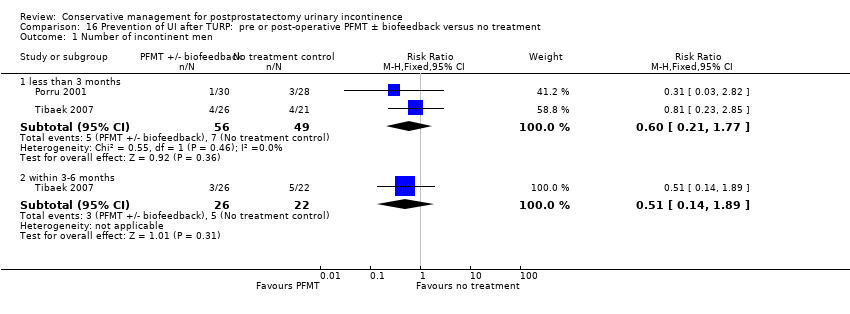
Comparison 16 Prevention of UI after TURP: pre or post‐operative PFMT ± biofeedback versus no treatment, Outcome 1 Number of incontinent men.
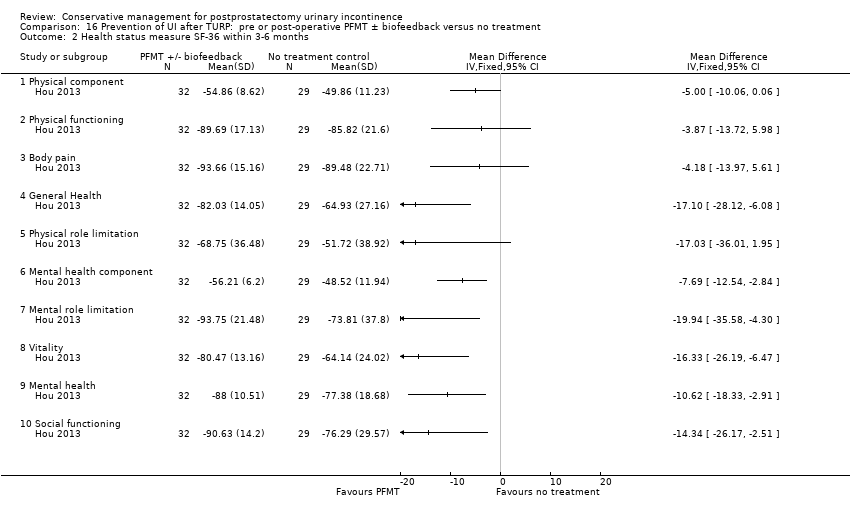
Comparison 16 Prevention of UI after TURP: pre or post‐operative PFMT ± biofeedback versus no treatment, Outcome 2 Health status measure SF‐36 within 3‐6 months.
| Study | Control (no device) | U‐Tex | C3 | Cunningham |
| Moore 2004 | 0/12 | 0/12 | 2/12 | 10/12 |
Comparison 21 Containment of urinary incontinence from any cause: external penile compression devices (penile clamps) versus no treatment, Outcome 1 Number of men satisfied with device.
| Study | Control (no device) | U‐Tex | C3 | Cunningham |
| Moore 2004 | 122.8 gm (SD 130.8) | 53.3 gm (SD 65.7) | 32.3 gm (SD 24.3) | 17.1 gm (SD 21.3) |
Comparison 21 Containment of urinary incontinence from any cause: external penile compression devices (penile clamps) versus no treatment, Outcome 2 Mean urine loss (grams of urine on pad test).
| Study | Control (no device) | U‐Tex | C3 | Cunningham |
| Moore 2004 | N=12 men | N=12 men | N=12 men | N=12 men |
Comparison 21 Containment of urinary incontinence from any cause: external penile compression devices (penile clamps) versus no treatment, Outcome 3 Penile Doppler blood flow (mean systolic velocity).
| Study | Control (no device) | U‐Tex | C3 | Cunningham |
| Moore 2004 | N=12 men | N=12 men | N=12 men | N=12 men |
Comparison 21 Containment of urinary incontinence from any cause: external penile compression devices (penile clamps) versus no treatment, Outcome 4 Penile Doppler blood flow (mean resistence to flow index).
| Treatment of UI after radical: PFMT ±biofeedback versus no treatment; for postprostatectomy urinary incontinence | ||||||
| Patient or population: patients with postprostatectomy urinary incontinence | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Treatment of UI after radical: PFMT ±biofeedback versus no treatment | |||||
| Number of incontinent men ‐ after 12 months | 623 per 1000 | 529 per 1000 | RR 0.85 | 665 | ⊕⊕⊕⊝ | |
| Urinary Incontinence Score (ICI‐SF) ‐ after first year | The mean urinary incontinence score (ici‐short form) ‐ after first year in the intervention groups was | 391 | ⊕⊕⊝⊝ | |||
| Adverse events | See comment | See comment | Not estimable | 138 | ⊕⊕⊕⊕ | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Wide CI (0.60 to 1.22) | ||||||
| Treatment of UI after radical: electric or magnetic energy versus no treatment for postprostatectomy UI | ||||||
| Patient or population: Patients with postprostatectomy UI | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Treatment of UI after radical: electric or magnetic energy versus no treatment | |||||
| Number of incontinent men ‐ after 12 months | 63 per 1000 | 16 per 1000 | RR 0.26 | 413 | ⊕⊕⊕⊝ | |
| Urinary Incontinence Score (ICIQ‐SF UI score) ‐ after 12 months | The mean urinary incontinence score (iciq‐short form ui score) ‐ after 12 months in the intervention groups was | 47 | ⊕⊕⊝⊝ | |||
| Urinary Incontinence Quality of Life Score (ICIQ‐SF) ‐ after 12 months | See comment | See comment | Not estimable | 47 | ⊕⊕⊝⊝ | |
| Adverse events | 133 per 1000 | 77 per 1000 | RR 0.58 | 56 | ⊕⊕⊝⊝ | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Random sequence generation and allocation concealment unclear is 1/2 trials taking part in the meta‐analysis | ||||||
| Treatment of UI after radical: combinations of treatments versus no treatment for postprostatectomy UI | ||||||
| Patient or population: patients with postprostatectomy UI | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Treatment of UI after radical: combinations of treatments versus no treatment | |||||
| Number of incontinent men with 3 to 6 months | 53 per 1000 | 150 per 1000 | RR 2.85 | 39 | ⊕⊝⊝⊝ | |
| Urinary Incontinence Quality of Life Score (ICIQ‐SF) after 12 months | Study population | Not estimable | 0 | See comment | ||
| See comment | See comment | |||||
| Moderate | ||||||
| Adverse events ‐ PFMT + anal EStim + BFB | 0 per 1000 | 0 per 1000 | RR 4.86 | 138 | ⊕⊕⊝⊝ | |
| Economic Analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Random sequence generation and allocation concealment unclear | ||||||
| Treatment of UI after radical: one active treatment versus another active treatment for postprostatectomy UI | ||||||
| Patient or population: Patients with postprostatectomy UI | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Treatment of UI after radical: one active treatment versus another active treatment | |||||
| Number of incontinent men within 6 to 12 months ‐ FES versus ExMI | 83 per 1000 | 167 per 1000 | RR 2 | 24 | ⊕⊝⊝⊝ | |
| Quality of Life Score (ICI‐Q‐SF) within 6 to 12 months ‐ PFMT + ExMI versus PFMT | The mean quality of life score (ICI‐Q‐SF) within 6 to 12 months ‐ PFMT + ExMI versus PFMT in the intervention groups was | 24 | ⊕⊕⊝⊝ | |||
| Adverse events PFMT + Anal EStim versus PFMT alone | 0 per 1000 | 0 per 1000 | RR 5 | 140 | ⊕⊕⊝⊝ | |
| Economic analysis using QALY | Study population | Not estimable | 0 | See comment | ||
| See comment | See comment | |||||
| Moderate | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Random sequence generation and allocation concealment is unclear | ||||||
| Prevention of UI after radical: PFMT ±biofeedback versus no treatment compared to for UI | ||||||
| Patient or population: All men after radical prostatectomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Prevention of UI after radical: PFMT ±biofeedback versus no treatment | ||||||
| Number of incontinent men ‐ after 12 months | 321 per 1000 | 103 per 1000 | RR 0.32 | 373 | ⊕⊕⊕⊝ | |
| Quality of life score assessed using (ICI‐SF UI score) ‐ within 6 to 12 months | The mean quality of life score assessed using (ICI‐SF UI score) ‐ within 6 to 12 months in the intervention groups was | 105 | ⊕⊝⊝⊝ | |||
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Allocation concealment is unclear for Filocamo 2005 which contributes 84.2% weightage | ||||||
| Prevention of UI after radical: electric or magnetic energy versus no treatment for UI | ||||||
| Patient or population: All men after radical prostatectomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prevention of UI after radical: electric or magnetic energy versus no treatment | |||||
| Number of incontinent men after 12 months ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Quality of life score assessed using (ICIQ‐SF score) ‐ within 6 to 12 months | See comment | See comment | Not estimable | 32 | ⊕⊝⊝⊝ | |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Allocation concealment is unclear | ||||||
| Prevention of UI after radical: combinations of treatments versus no treatment compared to for postprostatectomy UI | ||||||
| Patient or population: All men after radical prostatectomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Prevention of UI after radical: combinations of treatments versus no treatment | ||||||
| Number of incontinent men within 6 to 12 months ‐ PFMT + anal EStim + biofeedback versus no treatment | See comment | See comment | Not estimable | 60 | ⊕⊕⊝⊝ | |
| Quality of life Score assessed using (ICIQ‐SF) or (ICIQ‐ SF UI score) ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Sequence generation and allocation concealment are both unclear | ||||||
| Prevention of UI after radical: one active treatment versus another active treatment compared to (PFMT pre and post‐operation versus PFMT post‐operation) for UI | ||||||
| Patient or population: All men after radical prostatectomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| (PFMT pre and post‐operation versus PFMT post‐operation) | Prevention of UI after radical: one active treatment versus another active treatment | |||||
| Number of incontinent men after 12 months | See comment | See comment | Not estimable | 367 | ⊕⊕⊕⊝ | |
| Quality of Life Score assessed using (ICIQ‐SF) or (ICIQ‐SF UI score) after 12 months ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Adverse events | See comment | See comment | Not estimable | 102 | ⊕⊕⊕⊕ | |
| Economic Analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Sequence generation is unclear 2/3 trials and allocation concealment is unclear in 1/3 trials | ||||||
| Prevention of UI after radical: one active treatment versus another active treatment compared to (PFMT + penile vibration pre and post‐operation versus PFMT pre and post‐operation) for | ||||||
| Patient or population: All men after radical prostatectomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| (PFMT + penile vibration pre and post‐operation versus PFMT pre and post‐operation) | Prevention of UI after radical: one active treatment versus another active treatment | |||||
| Number of incontinent men after 12 months | 71 per 1000 | 100 per 1000 | RR 1.4 | 58 | ⊕⊕⊝⊝ | |
| Quality of life Score assessed using (ICIQ‐SF) or (ICIQ‐SF UI score) | Study population | Not estimable | 0 | See comment | ||
| See comment | See comment | |||||
| Moderate | ||||||
| Adverse events | See comment | See comment | Not estimable | 68 | ⊕⊕⊝⊝ | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Not applicable | ||||||
| Prevention of UI after radical: one active treatment versus another active treatment compared to (pre‐operative PFMT + electrical stimulation versus pre‐operative PFMT) for UI | ||||||
| Patient or population: All men after radical prostatectomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| (pre‐operative PFMT + electrical stimulation versus pre‐operative PFMT) | Prevention of UI after radical: one active treatment versus another active treatment | |||||
| Number of incontinent men after 12 months ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Quality of Life Score assessed using (ICIQ‐SF) within 6 to 12 months | See comment | See comment | Not estimable | 34 | ⊕⊝⊝⊝ | |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Allocation concealment is unclear | ||||||
| Treatment of UI after TURP: PFMT ±biofeedback versus no treatment compared to for UI | ||||||
| Patient or population: Men with UI after TURP | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Treatment of UI after TURP: PFMT ±biofeedback versus no treatment | ||||||
| Number of incontinent men‐ after 12 months | See comment | See comment | Not estimable | 1609 | ⊕⊕⊕⊝ | |
| Quality of life Score assessed using Score (ICIQ‐SF UI score) ‐ after 12 months | See comment | See comment | Not estimable | 397 | ⊕⊕⊝⊝ | |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Not applicable | ||||||
| Prevention of UI after TURP: pre or post‐operative PFMT ±biofeedback versus no treatment for UI | ||||||
| Patient or population: All men after TURP | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prevention of UI after TURP: pre or post‐operative PFMT ±biofeedback versus no treatment | |||||
| Number of incontinent men ‐ within 3 to 6 months | 227 per 1000 | 116 per 1000 | RR 0.51 | 48 | ⊕⊝⊝⊝ | |
| Urinary Incontinence Score assessed using (ICIQ‐SF) or (ICIQ‐SF UI score) at 12 months ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Not applicable | ||||||
| Study ID | Intervention | Control |
| A: At catheter removal received standard care of verbal and written instructions, instructed by physiotherapist to perform 3 sets of 15‐20 contractions daily, for a duration of 3‐5 seconds with a 6‐10 second rest period, encouraged to perform exercises before functional activities such as sneezing, coughing, or lifting weight, also in the supine position, sitting, squatting and going up and down stairs
B: ES, treatment started one week after catheter removal, patients received 15 minutes of twice weekly electrical stimulation for 12 weeks
C: PFMT + BFB + ES: Treatment started one week after catheter removal, patients received twice weekly treatment with 15 minutes of electrical stimulation and 15 minutes of biofeedback for 12 weeks, instructed to perform 3 series of 10 rapid contractions, 3 sustained contractions of 5, 7 or 10 seconds and then 10 contractions during prolonged expiration in the supine position
All patients were given a logbook to complete daily regarding self‐report of exercises | ||
| PFMT + biofeedback 45 minute session with nurse trained in biofeedback. Patients were instructed to perform graded PFMT. Contractions of 5‐10 seconds, 10‐15 repetitions were performed with biofeedback (surface electrodes used to measure muscle strength). Advised to practice the exercises 4 times per day until surgery | No biofeedback training Written and brief verbal instructions from a nurse on how to perform PFMT (isolate muscle that stops urine flow, practice 4 times per day, 10‐15 repetitions). | |
| PFMT + biofeedback Single session of biofeedback (rectal probe to measure intra‐abdominal rectal pressure and external anal sphincter contraction) assisted behavioural training. Feedback and verbal instruction used to teach control of pelvic muscles. Taught to contract sphincter during 2‐10 seconds periods separated by 2‐10 seconds of relaxation, dependent on ability. Written instructions for daily at home practice of 45 PFM exercises daily (3 sessions of 15 exercises each time). Additionally instructed to slow or interrupt voiding once daily. Encouraged to exercise daily preoperatively, then resume when catheter removed post‐operatively | Usual care of brief verbal instructions post operatively to interrupt the voiding stream plus any instruction from physician. | |
| Intervention A: PFMT both pre and post‐operatively. A structured PFMT program 30 and 15 days before surgery, previous physiotherapist evaluation to provide the patients with feedback about the quality of pelvic floor muscle function, PC teste (endurance and contraction quality), breathing coordination, typify muscle contraction as tonic and modify incorrect physical attitudes. This was also repeated after the procedure Intervention B: PFMT post‐operatively only | ||
| 30 mins of guided PFMT + biofeedback weekly for 4 weeks before surgery, received written instructions to: carry out two sets of 30 contractions during abdominal breathing, one breath between each contraction; restart PFMT after catheter removal (7‐10 days after surgery) All men were seen before surgery by a physiotherapist, who explained relevant anatomy, anal visual inspection and digital palpation, biofeedback registration with rectal probe, All patients received PFMT + biofeedback or electrical stimulation, or both, if still incontinent after 6 weeks | Received written instructions on PFMT after catheter removal (7‐10 days after surgery) | |
| Nine or less sessions of physiotherapy guided pelvic floor exercises after surgery | Exercise instruction through information folder | |
| Formal instruction (3 treatment sessions plus at home exercises) in PFMT using verbal explanation, palpation and visualization of the base of the penis with a mirror, in different positions and prior to sneezing, coughing or lifting | No formal instruction | |
| Initiated after catheter removal, 15 treatment sessions (3 times per week for 30 minutes) of PFMT with EMG (surface) biofeedback in clinic | Instruction with verbal feedback and an information pamphlet with instructions to perform PFMT 50‐100 times daily at home | |
| Pre‐operative session guided PFMT + instruction on how to use penile vibratory stimulation device. Instructed to stimulate frenulum once daily, 10 seconds of stimulation then 10 second pause, repeated 10 times for 1 week pre‐operatively, instructed to restart stimulation after catheter removal for 6 weeks All men were offered a PDE5 inhibitor after 1 month post‐operatively and also received telephone contact to ensure compliance with treatment | Preoperative session guided PFMT | |
| Biofeedback (perineal patch EMG) enhanced PFMT; exercise treatment sessions at 6, 7, 9, 11, and 16 weeks post‐operatively | No treatment. | |
| Intervention A: PFMT + biofeedback 30 mins of guided PFMT + biofeedback weekly for 3 weeks before surgery. Patients were instructed to carry out 60 contractions a day at home; contract their pelvic floor while coughing, and sitting down or getting up from a chair. Patients were also instructed to restart PFMT on day 4 after surgery while catheter was in situ Intervention B: Instructed to start PFMT on the day after catheter removal (e.g. 2‐3 weeks after surgery) All men: Received weekly individual guided exercise programme with digital or EMG biofeedback after surgery. Advice was given on how to contract pelvic floor muscles to prevent leakage during functional activities. When patients carried out the instructed 60 contractions, they were asked to colour in three squares in their diary to assess compliance | ||
| Pre‐operative PFMT for 2 weeks + postoperative PFMT programme | Postoperative PFMT programme only | |
| Intervention A: Behavioural therapy with PFMT for 8 weeks Intervention B: Behavioural therapy with biofeedback and electrical stimulation for 8 weeks Behavioural therapy consisted of pelvic floor muscle exercises and bladder control strategies in both groups | No treatment | |
| Intervention A: perineal EStim plus physiotherapy (PFMT) Intervention B: anal EStim plus physiotherapy (PFMT) | PFMT alone | |
| Guided PFMT + biofeedback after catheter removal (2 days post‐operatively), instructed to: contract pelvic muscles for 5 seconds and relax for 10 seconds. After discharge, patients were instructed to carry out 5 mins of each PFE three times daily. Patients also received motivational telephone interviews once weekly | No description | |
| Intervention A: Instruction in PFMT including biofeedback with visual feedback as well as verbal to assist in identifying and discriminating muscles Intervention B: Instruction in PFMT, squeezing of finger during digital rectal examination | ||
| ExMI, treatment sessions were for 20 minutes twice weekly for 8 weeks | PFMT alone | |
| A (15): Standard treatment with verbal instructions for PFMT B (17): Pre‐operative guided PFMT, with 10 physiotherapy sessions: contractions of the pelvic floor muscles for 5 seconds in “dorsal decubitus” position for 10 times, in the same position with the waist elevated (10 times), lying down with legs adducted against a plastic ball performed 10 times and standing and flexing the hips to 60̊ (10 times) C (17): Pre‐operative PFMT + ES during 10 physiotherapy sessions, ES was with an anal probe lasting 15 minutes in total, and men also received guided PFMT and followed the same training regime as above Men did not receive treatment post‐operatively | Instructed to start PFMT at home 15 weeks before surgery. | |
| Extra‐corporeal magnetic innervation (ExMI), the frequency of the pulse field was 10Hz for 10 minutes, followed by a 3 minute rest and a second treatment of 50 Hz for 20 minutes. This was done twice a week | PFMT alone, instructions given to carry out 20mins x 3 a day. | |
| PFMT re‐education program, verbal feedback The training program involved active PFE. verbal feedback of the contraction was used to instruct the patients to correctly and selectively contract their pelvic muscles while relaxing the abdominal muscles. the strength of the pelvic floor muscles was measured by digital anal control using a score of 0 to 5 ( 0 = no contraction, 5 = good contraction against strong resistance) Initially home practice comprised 45 contractions (3 sessions of 15) per day at home, progressively increasing the number until 90 per day. This was taught by two experienced urologists | No treatment. | |
| Guided PFMT + biofeedback during first session, second session involved 10 sets of pelvic floor electrical stimulation lasting 15 mins each, instructed to: carry out three sets of 30 contractions a day at home for the first month after catheter removal (16 days after surgery) All men received oral and written information on pelvic floor anatomy and on PFME, pelvic floor muscle endurance assessed by digital anal control | Received oral and written information on pelvic floor anatomy and on PFME, instructed to: perform 30 contractions a day at home for the first month after catheter removal (16 days after surgery) | |
| PFMT plus ES and biofeedback twice a week for 6 weeks ES ‐ a surface electrodes was inserted into the anus and pulsed, the intensity was adequate to induce visual lifting of the levator ani and pubococcygeus muscle, considering the level of comfort to the patient Biofeedback ‐ via surface electrodes both perineal and abdominally | Instructions to conduct PFMT ‐ verbal and written instructions at catheter removal and follow up visits. | |
| PFMT: 5 sessions of guided PFMT for 2‐3 weeks pre‐operatively and continued post‐operatively All men underwent clinical examination of pelvic muscles function using digital perineal testing according to “AIPDA score” and evaluation of voiding symptoms | Postoperative standard care, written instructions for PFMT | |
| Pre‐operatively received further instruction and practice with PME protocol Home exercises and biofeedback (anal probe) (Incare 8900); practiced at home 3 times a week, starting with daily 15 PFMT and increasing by 10 every 4 weeks to a maximum of 35 PFMT. | Post‐operatively no further interventions until week 5 when pelvic muscle strength was assessed. | |
| Intervention A: PFMT alone Intervention B: PFMT plus rectal ES treated by one physiotherapist 30 minutes twice a week for 12 weeks Both included home exercises 3x/day gradually working up to 30 minutes per session lying, standing, sitting; strength, endurance, speed and control with maximum contractions of 5‐10 seconds, 10‐20 second relaxation and 12‐20 repetitions; submaximum contractions at 65‐75% of maximum strength with hold 20‐30 seconds and equal rest time, 8‐10 repetitions; speed was sets of quick repetitive contractions in a 10 second time span; control involved gradual recruitment to maximum contraction in 3 stages with 5 second hold at each stage and a slow release with rest 15‐30 seconds | oral and written information about PFMT pre and post‐ operatively (standard treatment) | |
| Each participant had 4 periods (each lasted 1 day) | ||
| Maximum 24 weekly, 30‐minute treatment protocol (30 min biofeedback‐assisted PFMT) and home exercise protocol of 2‐3 times a day | Verbal and written information on PFME and weekly telephone contact by a urology nurse | |
| PFMT + sacral surface therapeutic electrical stimulation (ssTES), ssTES 2x a day for 15 minutes each, lasting 1 month after catheter removal (day 5) | PFME only, carried out alone | |
| Extra‐corporeal magnetic innervation (EXMI) based pelvic floor device | PFMT alone | |
| PFMT plus biofeedback plus electrical stimulation directed by physiotherapist | PFMT on their own without medical supervision. | |
| Instructions on PFMT and physiotherapy, 45 minutes weekly Patients were instructed to perform 3 sets of contractions daily at home, in either a supine, sitting or standing position. Digital anal palpation to teach correct contractions, as well as oral and written instructions DVD of instructions given to those living too far from hospital | Instructions on PFMT alone. | |
| Two treatment sessions preoperatively. Session 1 consisted of PFMT in a hook lying position | No formal education on PFMT pre‐operatively, telephone or face to face follow‐up at least monthly. | |
| Patients performed Kegel exercises together with other types of exercises which included resistance training and pelvic flexibility. The intervention started 3 weeks after surgery and lasted 12 weeks Details of the combined exercise regime: Post‐operative weeks 1‐4 1) Education about postoperative symptoms 2) Performing Kegel exercises, recognizing the parapelvic muscles 3) Pelvic floor flexibility fitness: performing pelvic exercises while sitting on a ball Post‐operative weeks 5‐8 (ball exercises) 1) Performing pelvic exercises while sitting on a ball 2) Performing lower extremity exercises while placing a ball on the wall 3) Lifting a heel on the ball while standing face‐to‐face with the wall 4) Lifting up and down on the ball while spreading and bending legs 5) Performing flank exercises while having a ball in the hand 6) Squeezing the ball with the adductor muscles while lying on a table Post‐operative weeks 9‐12 (elastic band exercises) 1) Lifting the object with an elastic band lateral, anterior, and posterior to the patient’s arms 2) Lifting the legs and then spreading them while attaching an elastic band to the foot | In the control group, only Kegel exercises were performed | |
| Early pelvic floor rehabilitation program at home twice dally, Kegel exercises | No formal PFMT | |
| Initial visit before surgery, digital evaluation of pelvic muscle contraction strength. Verbal instruction, feedback and reinforcement on contraction was given to teach selective contraction of anal sphincter and relaxation of abdominal muscles. Verbal and written instruction given for home PFMT. Weekly digital anal reassessment and grading of pelvic muscle contraction by the therapist. Instructed to practice contractions 45 times per day (3 groups of 15 contractions) | Not specified | |
| PFMT plus BF weekly for 3 months | PFMT oral instructions only | |
| Intervention A: Brief verbal instruction in PFMT before operation and offer of one biofeedback session at 2 months after surgery (uptake 33%) plus PFMT for four weeks with biofeedback Intervention B: Brief verbal instruction in PFMT before operation and offer of one biofeedback session at 2 months after surgery (uptake 46%) | ||
| Intervention A: routine brief verbal and written PFMT plus one PFMT session and 3 weekly nurse phone calls Intervention B: routine brief verbal and written PFMT plus four BF enhanced PFMT sessions and 4 weekly nurse phone calls | Routine brief verbal and written PFMT. | |
| Verbal instruction and information on PFMT plus information on life style changes. Additional 15 physiotherapy sessions consisting of intensive PFMT with BF and ES | Verbal instruction and information on PFMT plus information on life style changes. | |
| One hour individual session with physiotherapist to teach correct contraction for PFMT, three 1 hour group lessons and home training programme | No pre operative physiotherapy. Information about anatomy and physiology and verbal instructions for 2 to 3 days after TURP in the ward. | |
| PFMT + biofeedback Patients received guided PFMT + biofeedback + information about the anatomy of pelvic floor muscles the day before surgery and after catheter removal. They were also given oral and written instructions on Kegel exercises to be performed at home which involved three sets of contractions daily for 10 mins, contracting their pelvic floor while lying, sitting and standing. The frequency of contractions was recorded in a training diary and visits at monthly intervals after catheter removal involved assisted biofeedback and motivation for 20 min | No biofeedback training Received standard care, oral and written instructions from urologist on PFMT, Instructed to: start PFMT after catheter removal (e.g. 2‐3 weeks after surgery) | |
| PFMT | No PFMT | |
| 1 session of PFMT in hospital before discharge and then saw the physiotherapist for 1‐2 weeks for as long as UI persisted. 90 daily home exercises sitting, standing and lying. 7 men unable to contract PFM or with weak contraction received electrical stimulation by anal probe | No formal PFMT instruction but saw the therapist at 1‐2 weeks and received placebo stimulation and information about aetiology of UI. | |
| Intervention A: PFMT alone Intervention B: PFMT + ES; PFMT as above plus instructed by dedicated in ES via surface anal electrode and bio‐impulser (biphasic pulse with 1 second bursts, 5 second pulse width, 2 second pulse trains Intervention C: PFMT + ES + biofeedback. As above plus biofeedback (anal probe) 15 minutes twice daily for 3 months All groups: PFMT by physiotherapist, 20‐30 minute sessions for 3 days, instructed to perform exercises twice daily for 3 months plus 3 week rehabilitation program after dischargeRegular interaction with health professional for 6 weeks after surgery, encouraged to performed treatment for 3 months post‐surgery | ||
| Oral PFMT plus ES for 15 minutes twice daily Instructed pre‐operatively PFMT by nurses and continued after catheter removal | Oral PFMT plus sham device. Instructed pre‐operatively PFMT by nurses and continued after catheter removal. | |
| Intervention A: anal electrode for 15 minutes twice a day for 1 month Intervention B: extra‐corporeal magnetic innervation, neocontrol system, treatment sessions 20 minutes, twice a week for 2 weeks | PFMT, digital anal teaching of correct contractions, then verbal and written instructions for home practice. | |
| PFMT plus BF using rectal electrical sensor, initial 45 minute session with physical therapist then written instructions to carry out at home three times a day for 10 minutes. Plus support group, 6 meetings in 3 months with a health psychologist | PFMT plus BF using rectal electrical sensor, initial 45 minute session with physical therapist then written instructions to carry out at home three times a day for 10 minutes |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinent men Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 less than 3 months | 7 | 980 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.08] |
| 1.2 within 3‐6 months | 7 | 895 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.83, 1.10] |
| 1.3 within 6‐12 months | 5 | 792 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.73, 1.14] |
| 1.4 after 12 months | 3 | 665 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.60, 1.22] |
| 2 Number of incontinence episodes per day Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 less than 3 months | 2 | 400 | Mean Difference (IV, Fixed, 95% CI) | ‐1.09 [‐1.39, ‐0.78] |
| 2.2 within 3‐6 months | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.40, 1.00] |
| 2.3 within 6‐12 months | 1 | 217 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.33, 0.93] |
| 2.4 after first year | 1 | 211 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.82, 1.02] |
| 3 Number of men using pads Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 less than 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 within 3‐6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 within 6‐12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 after 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Pad changes over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 after first year | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Urinary Incontinence Score (ICIQ‐SF) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Quality of life related to urinary incontinence Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 24 hour pad test (grams of urine lost) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 less than 3 months | 2 | 214 | Mean Difference (IV, Fixed, 95% CI) | 22.29 [‐33.12, 77.70] |
| 8.2 within 3‐6 months | 2 | 213 | Mean Difference (IV, Fixed, 95% CI) | 11.87 [‐40.77, 64.52] |
| 8.3 within 6‐12 months | 2 | 194 | Mean Difference (IV, Fixed, 95% CI) | 11.23 [‐22.35, 44.82] |
| 8.4 after first year | 1 | 167 | Mean Difference (IV, Fixed, 95% CI) | 39.0 [‐5.72, 83.72] |
| 9 1 hour pad test (grams of urine lost) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 after first year | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Number of men not carrying out pelvic floor muscle contractions at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinent men Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 less than 3 months | 2 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.60, 0.98] |
| 1.2 within 3‐6 months | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.29, 0.79] |
| 1.3 within 6‐12 months | 2 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.18, 0.73] |
| 1.4 after 12 months | 3 | 413 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.09, 0.74] |
| 2 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 24 hour pad test (grams of urine lost) Show forest plot | 2 | 325 | Mean Difference (IV, Fixed, 95% CI) | ‐16.94 [‐58.21, 24.33] |
| 3.1 less than 3 months | 2 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐27.82 [‐116.97, 61.33] |
| 3.2 within 3‐6 months | 2 | 93 | Mean Difference (IV, Fixed, 95% CI) | 5.12 [‐86.19, 96.43] |
| 3.3 within 6‐12 months | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐1.95 [‐64.03, 60.13] |
| 3.4 after first year | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐80.0 [‐190.50, 30.50] |
| 4 Urinary Incontinence Score (ICIQ‐short form UI score) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Urinary Incontinence Quality of Life Score (ICIQ‐short form) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Time until continent (months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinent men at < 3 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 PFMT + anal Estim + Biofeedback vs no treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of incontinent men within 3‐6 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 PFMT + anal Estim + Biofeedback vs no treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of incontinence episodes per day at > 3 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 PFMT + anal Estim + BFB | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 PFMT + anal Estim + BFB | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinent men at < 3 months Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 PFMT + Anal EStim vs PFMT alone | 2 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.83, 1.12] |
| 2 Number of incontinent men within 3 to 6 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 PFMT + BF + support group vs PFMT + BF | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of incontinent men within 6 to 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 FES vs ExMI | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of incontinence episodes at < 3 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Quality of Life Score (severity of UI) within 3 to 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 PFMT + BF + support group vs PFMT + BF | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Quality of Life Score (I‐QoL) within 6‐12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 PFMT + BF + EStim vs PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Quality of Life Score (ICI‐Q‐SF) within 6‐12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 PFMT + ExMI vs PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 PFMT + Anal EStim vs PFMT alone | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 1 hour pad test (grams of urine lost): at < 3 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 PFMT + perineal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 PFMT + perineal EStim vs PFMT + anal EStim | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 24 hour pad test (grams of urine lost): at < 3 months Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 PFMT + visual BF vs PFMT + oral BF | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 24 hour pad test (grams of urine lost): within 3 to 6 months Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 PFMT + visual BF vs PFMT + oral BF | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 24 hour pad test (grams of urine lost): within 3 to 6 months Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 PFMT + visual BF vs PFMT + oral BF | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 ExMI vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Pad changes over 24 hours within 3 to 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 ExMI vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Number of men not carrying out sufficient PFMT within 3 to 6 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14.1 PFMT + BF + support group vs PFMT + BF | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinent men Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 less than 3 months | 7 | 663 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.83, 1.06] |
| 1.2 within 3‐6 months | 7 | 697 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.75, 0.97] |
| 1.3 within 6‐12 months | 6 | 640 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.35, 0.75] |
| 1.4 after 12 months | 2 | 373 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.20, 0.51] |
| 2 Pad changes over 24 hours Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 less than 3 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 within 3‐6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 within 6 ‐ 12 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 1 hour pad test (grams of urine lost) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 24 hour pad test (gm/24hrs) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 less than 3 months | 3 | 424 | Mean Difference (IV, Random, 95% CI) | ‐78.19 [‐211.46, 55.07] |
| 4.2 within 3‐6 months | 2 | 373 | Mean Difference (IV, Random, 95% CI) | ‐73.28 [‐196.42, 49.86] |
| 4.3 within 6‐12 months | 2 | 373 | Mean Difference (IV, Random, 95% CI) | ‐14.50 [‐18.36, ‐10.64] |
| 4.4 after first year | 2 | 378 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐1.81, ‐0.19] |
| 5 Number of incontinence episodes per day Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 after first year | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Urinary Incontinence Score (ICI‐short form) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 less than 3 months | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 6.5 [3.45, 9.55] |
| 6.2 within 3‐6 months | 2 | 105 | Mean Difference (IV, Random, 95% CI) | ‐1.21 [‐5.99, 3.56] |
| 6.3 within 6‐12 months | 2 | 105 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐3.19, 1.81] |
| 7 Quality of Life Score (IIQ) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 less than 3 months | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 within 6‐12 months | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 after first year | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Pelvic floor muscle strength (anal squeeze pressure, cm H2O) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 less than 3 months | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Number of men not carrying out sufficient PFMT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 less than 3 months | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 within 3‐6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 within 6‐12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 after 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Number of men having surgery for incontinence Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 1 hour pad test (grams of urine lost) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 ICIQ‐SF score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinent men within 3 to 6 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 PFMT + anal Estim + Biofeedback versus no treatment/sham treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of incontinent men within 6 to 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 PFMT + anal Estim + biofeedback versus no treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 24 hour pad test (grams of urine lost) within 3 to 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 PFMT + anal Estim + Biofeedback versus no treatment/sham treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 24 hour pad test (grams of urine lost) 6 to 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 PFMT + anal Estim + Biofeedback versus no treatment/sham treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Time until continent (months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 PFMT + anal Estim + Biofeedback versus no treatment/sham treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinent men at < 3months Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 PFMT pre and post op vs PFMT post op | 2 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.69, 1.06] |
| 1.2 PFMT + Biofeedback + transcutaneous Estim versus Estim only | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.61, 1.26] |
| 1.3 PFMT + Biofeedback + transcutaneous Estim versus post‐op PFMT | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.57, 1.11] |
| 1.4 Post‐op transcutaneous Estim versus post‐op PFMT | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.67, 1.22] |
| 2 Number of incontinent men within 3 to 6 months Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 PFMT pre and post op vs PFMT post op | 2 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.54, 1.04] |
| 2.2 post‐op PFMT + biofeedback + transcutaneous Estim vs post‐op Estim | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.96, 2.49] |
| 2.3 PFMT + general exercise versus PFMT alone | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.23, 0.99] |
| 2.4 Post‐op PFMT + transcutaneous Estim + Biofeedback versus post‐op PFMT | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.76, 1.57] |
| 2.5 Post‐op transcutaneous electrical stimulation versus post‐op PFMT | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.43, 1.16] |
| 3 Number of incontinent men within 6 to 12 months Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 PFMT pre and post op vs PFMT post op | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 post‐op PFMT + Biofeedback + transcutaneous Estim vs post‐op Estim | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Post‐op PFMT + transcutaneous Estim + Biofeedback versus post‐op PFMT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Post‐op transcutaneous Estim versus post‐op PFMT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of incontinent men after 12 months Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 PFMT pre and post op vs PFMT post op | 3 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.78, 2.25] |
| 4.2 PFMT + Penile vibration pre and post op versus PFMT pre and post op | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.25, 7.77] |
| 5 No. with severe incontinence (e.g. pad test weight >150g) at < 3 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 PFMT pre and post op vs PFMT post op | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 No. with severe incontinence (e.g. pad test weight >150g) at 3 to 6 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 PFMT pre and post op vs PFMT post op | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 20 minute pad test (grams of urine lost): within 3 to 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 PFMT + anal EStim + BF vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 PFMT + anal EStim vs PFMT + anal EStim + BF | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 20 minute pad test (grams of urine lost): within 6 to 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 PFMT + anal EStim + BF vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 PFMT + anal EStim vs PFMT + anal EStim + BF | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 1 hour pad test (grams of urine lost) at less than 3 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Pre‐op PFMT + Estim versus pre‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 1 hour pad test (grams of urine lost) within 3‐6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Pre‐op PFMT + electrical stimulation versus pre‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 1 hour pad test within 6‐12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Pre‐op PFMT + electrical stimulation versus pre‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 24 hour pad test (grams of urine lost) at less than 3 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 PFMT + Biofeedback + transcutaneous Estim versus Estim only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Post‐operative PFMT + transcutaneous Estim + Biofeedback versus post‐operative PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 Post‐operative transcutaneous electrical stimulation versus post‐operative PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 24 hour pad test (grams of urine lost) within 3‐6 months Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 PFMT + Biofeedback + transcutaneous Estim versus Estim only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Postoperative PFMT + biofeedback + transcutaneous Estim versus postoperative PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.3 Post‐operative transcutaneous Estim only versus post‐operative PFMT only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.4 PFMT + general exercise versus PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 24 hour pad test (grams of urine lost) within 6‐12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.1 PFMT + transcutaneous Estim + biofeedback versus Estim only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Post‐op PFMT + transcutaneous Estim + Biofeedback versus post‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.3 Post‐op transcutaneous Estim versus post‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Quality of Life Score (ICS male short form) at < 3 months Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1 PFMT pre and post op vs PFMT post op | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Urinary Incontinence Quality of Life Score (ICIQ ‐ short form) within 3‐6 months Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.1 Pre‐op PFMT + electrical stimulation versus pre‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 PFMT + general exercise versus PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.3 PFMT pre and post op vs PFMT post op | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 Urinary Incontinence Quality of Life Score (ICIQ‐short form) within 6‐12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 17.1 Pre‐op PFMT + electrical stimulation versus pre‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 King's health Questionnaire after 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 18.1 General Health | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 Role limitations | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.3 Physical limitations | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.4 Social limitations | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.5 Personal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.6 Emotional | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.7 Sleep/energy disturbance | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.8 Symptom severity | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 Health status measure SF‐36 within 3‐6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.1 Physical composite score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Mental Composite score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Adverse events Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 20.1 PFMT pre and post op vs PFMT post op | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 PFMT + Penile vibration pre and post op versus PFMT pre and post op | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinent men Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 less than 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 within 3‐6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 within 6‐12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 after 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of incontinence episodes per day Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of men using pads Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 less than 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 within 3‐6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 within 6‐12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 after 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Urinary Incontinence Score (ICI‐short form) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Quality of life related to urinary incontinence Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of men not carrying out pelvic floor muscle contractions at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinent men Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 less than 3 months | 2 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.21, 1.77] |
| 1.2 within 3‐6 months | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.14, 1.89] |
| 2 Health status measure SF‐36 within 3‐6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Physical component | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Physical functioning | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Body pain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 General Health | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Physical role limitation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Mental health component | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Mental role limitation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Vitality | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Mental health | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.10 Social functioning | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of men satisfied with device Show forest plot | Other data | No numeric data | ||
| 2 Mean urine loss (grams of urine on pad test) Show forest plot | Other data | No numeric data | ||
| 3 Penile Doppler blood flow (mean systolic velocity) Show forest plot | Other data | No numeric data | ||
| 4 Penile Doppler blood flow (mean resistence to flow index) Show forest plot | Other data | No numeric data | ||

