작은 무증상 복부 대동맥류 수술
초록
배경
복부 대동맥류(AAA)는 주요 복부동맥의 비정상적인 부풀어오름 이다. 일부 복부 대동맥류는 응급상황으로 나타나 수술을 필요로 하며, 다른 복부 대동맥류는 증상이 없는 상태로 남아 있다. 무증상 복부 대동맥류의 치료는 많은 요인에 따라 다르지만, 동맥류 크기에 따라 파열 위험이 증가하기 때문에 동맥류의 크기가 중요하다. 대규모의 무증상 복부 대동맥류(지름 5.5 cm 이상)는 대개 외과적인 수술로 복원이 되며, 매우 소규모인 복부 대동맥류(지름 4.0 cm 미만)는 초음파로 모니터링된다. 직경 4.0~5.5cm의 무증상 복부 대동맥류의 차후 확대 복원술을 통한 조기 복원과 정김검진의 역할에 대한 논쟁이 계속되고 있다. 이것은 1999년에 처음 출판된 고찰의 네 번째 업데이트다.
목적
직경이 4.0cm에서 5.5cm 사이인 사람의 초기 복원 수술과 정기적인 초음파 검진에 따른 사망률 및 비용, 2차 결과로서 삶의 질 및 동맥류 파열 등을 비교한다.
검색 전략
Cochrane Vascular Information Specialist는 Cochrane Vascular Specialized Register, Central, MEDLINE, 2개의 다른 데이터베이스와 2개의 임상시험 등록 레지스터를 2019년 7월 10일까지 검색했다. 회의 진행과 관련 연구들의 참조 리스트를 조사하였다.
선정 기준
최소 6개월마다 4.0cm에서 5.5cm의 무증상 복부 대동맥류를 가진 환자를 조기 복원 또는 영상 기반 감시로 무작위로 배정하는 무작위 대조 시험을 포함했다. 결과에는 사망률이나 생존이 포함되어야 했다.
자료 수집 및 분석
세 명의 검토 저자가 다른 팀 구성원에 의해 상호 대조하여 추출된 데이터를 독립적으로 추출했다. 결과는 사망률, 비용, 삶의 질, 동맥류 파열이었다. 사망률의 경우 무작위 배정을 따른 1년 및 6년(개방 복원술만)에 멘틀‐헨젤 카이 2 통계에 기초하여 위험비(RR) (혈관내 동맥류 복원만 해당), 위험비(HR)(개방 복원만 해당) 및 95% 신뢰구간(CI)을 추정했다
주요 결과
이 업데이트에 대한 새로운 연구 결과를 찾지 못했다. 참여자 3314명을 대상으로 한 4번의 시험이 포함 기준을 충족시켰다. 초기 개방 복원과 정기검진을 통한 감시를 비교한 시험은 2회, 초기 혈관내 복원(EVAR)을 정기검진을 통한 감시와 비교한 시험은 2회였다. 사망률과 비용에 대한 근거의 확실성을 측정하기 위해 GRADE를 이용했으며 근거의 확실성은 높은 수준부터 낮은 수준까지 다양했다. 비뚤림 위험의 우려와 부정확성의 위험으로 인해 근거의 확실성을 높은 수준에서 중간 및 낮은 수준으로 하향 조정했다(일부 결과는 한 연구에 의해 보고됨).
네 번의 시험 모두 정기검진을 통한 감시집단에서 더 나은 조기 생존률을 보였지만(복원 수술시 30일 수술사망률로 인한) 장기 생존에 차이가 있다는 근거는 보이지 않았다. 한 연구는 초기 개방 복원 수술을 조정 HR이 0.88(95% CI 0.75 ~ 1.02, 평균 추적 10년, HR 1.21, 95% CI 0.95 ~ 1.54, 평균 추적 4.9년)인 초음파를 통한 감시와 비교했다. 초기 개방형 복원 수술과 정기검진을 통한 감시를 비교한 두 시험의 참가자 수치의 통합 분석(최대 후속 7~8년)에서는 생존의 차이가 발견되지 않았다(확대 점수 조정 HR 0.99, 95% CI 0.83~1.18, 2226명의 참가자, 높은 근거 확실성). 부족한 치료 효과는 복부 대동맥류의 직경(P = 0.39), 참여 연령(P = 0.61)또는 여성(HR 0.84, 95% CI 0.62~1.11)에 의해 3년 정도로 달라지지 않았다. 두 연구는 혈관내 복원을 정기검진을 통한 감시와 비교했다. 12개월간 초기 혈관내 복원 수술관련 생존률에 대한 근거는 없었다(RR 1.92, 95% CI 0.73 대 5.06, 846명의 참가자, 낮은 근거 불확실성).
두 번의 임상시험은 비용을 보고했다. 무작위 배정 후 처음 18개월 동안 참가자 1인당 평균 영국 보건 서비스 비용은 감시 집단(복원 수술 집단 GBP 4978과 감시 집단의 GBP 3914 비교; 평균 차이 (MD) GBP 1064, 95% CI 796 ‐ 1332; 1090명의 참가자, 중간 정도의 근거 확실성)보다 높았다. 12년 후에도 비슷한 차이가 있었다. 무작위 배정 후 6개월 동안 참여자의 평균 미국 병원비는 정기검진을 통한 감시 집단보다 초기 혈관내 복원 수술 집단에서 더 높았다(복원 수술 비용 USD 33,471; 정기검진 비용 USD 5520 27,951, 95% CI 25,156 ‐ 30,746; 참여자 614명, 근거 확실성 낮음). 4년 후, 집단 간 총 의료비 차이에 대한 근거는 없었다(수리비 4만8669달러, 감시비 4만6천112달러, 평균 차이 2557달러, 95% CI –8043~13,156; 614명의 참가자; 낮은 근거 확실성)
모든 연구는 삶의 질을 보고했지만 서로 다른 측정 평가를 사용했고 결과는 상충했다.
네 가지 연구 모두 동맥류 파열이 보고되었다. 최대 3년 동안 초기 혈관내 복원 수술 및 정기검진을 통한 감시 실험에서 보고된 파열은 거의 없었다. 개방수술과 정기검진을 통한 감시 실험에서는 최소 6년까지 파열이 있었고 감시 집단에서도 파열이 더 많았지만 대부분 복원 수술의 수치를 넘은 동맥류에서 파열이 발생했다.
연구진 결론
개방형 복원수술 또는 초기 혈관내 복원 수술 시행 여부와 상관없이 소규모 복부 대동맥류(4.0cm ~ 5.5cm) 조기 복원수술의 이점에 대한 근거가 없었고, 적어도 개방 수리에 대해서는 환자 연령과 복부 대동맥류 직경과 무관했다. 따라서, 소규모 복부 대동맥류의 개방 수술 또는 초기 혈관내 복원 수술은 현재 이용 가능한 근거에 의해 뒷받침되지 않는다. 초기 혈관내 복원 수술을 조사 중인 두 임상시험의 장기 데이터는 사용할 수 없으므로, 개방 복원수술에 대해 처음 몇 년 후 결과와 관련하여 확실한 결론을 도출할 수 있을 뿐이다. 이러한 인구들에 관한 자료가 부족할 때 소수민족과 여성의 소규모 복부 대동맥류와 관련된 위험과 관리에 관한 연구가 시급하다.
PICOs
쉬운 말 요약
증상을 일으키지 않는 작은 복부 대동맥류 수술
배경
동맥류는 동맥(혈관의 풍선)이 생기는 것으로 복부 대동맥류(AAA)의 경우 복부의 주요 동맥(아orta)에서 발생한다. AAA 파열은 수술 수리가 빠르지 않는 한 사망을 초래하는데, 이는 달성하기 어렵다. 동맥류 지름이 5.5cm 이상인 사람이나 관련 통증이 있는 사람은 증상을 완화하고 파열과 사망 위험을 줄이는 수술을 권한다. 그러나 수술에는 위험이 따른다. 외과적인 복원 수술은 개방 수술이나 혈관내 복원술(최소한의 외과적 키홀 시술)을 통해 강한 합성 물질로 대동맥을 다시 정렬하는 것으로 구성된다. 소규모 무증상(증상 없음) 복부 대동맥류는 파열 위험이 낮고 정기적인 영상을 통해 모니터링되므로 복부 대동맥류가 자라면 수술로 복원할 수 있다.
주요 결과
소규모(지름 4.0cm~5.5cm) 무증상 복부 대동맥류를 가지고 있는 3314명의 참가자를 조기 복원치료 집단 또는 정기적인 초음파로 동맥류 성장을 검사(감시)하는 집단으로 무작위 배정하여 수행된 시험을 4건 발견했다. (2019년 7월 10일까지 검색) 감시군에서는 동맥류가 확대되거나 지름 5.5cm에 이르거나 또는 증상이 나타나면 복원수술을 진행하였다. 이 임상시험은 수술 후 30일 이내 사망하는 환자의 수(수술 사망률)에 의해 감시 집단의 더 나은 조기 생존률을 보여주었다. 임상시험 결과 3~8년 동안의 사후 관리 기간 동안 조기 복원술을 진행한 환자(개방 또는 혈관내)와 초음파 정기검진을 통한 감시를 진행한 환자의 장기 생존에는 차이가 없었다. 3년 후, 초음파 정기검진을 통한 감시에 무작위로 배정받았던 사람들의 약 31%가 결국 동맥류를 고쳤으며, 12년 후에는 75%까지 증가했다.
두 번의 임상시험은 비용을 보고했다. 처음 18개월 동안, 초음파 정기검진은 개방형 복원술 또는 혈관내 복원술보다 비용이 낮았다. 4년 후, 한 시험은 혈관내 복원집단과 초음파 정기검진을 통한 감시 집단의 총 의료비가 유사하다는 것을 발견했다. 12년 후, 또 다른 시험에서는 개방형 복원보다 정기검진을 통한 감시의 병원비가 더 저렴하다는 것을 발견했다.
네 가지 시험은 삶의 질을 측정하기 위해 서로 다른 방법을 사용했고 결과는 상반되었다. 정기검진을 통한 감시 집단에서 동맥류 파열 비율은 개방형 복원 수술을 사용한 시험에서 더 높게 나타났지만, 이러한 비율은 참가자를 혈관내 복원술에 적합한 대동맥 해부학을 가진 참가자로 제한하지는 않았다. 대부분의 파열은 이전의 동맥류 직경이 복원 수술의 수치를 넘은 사람들이다.
근거의 신뢰성
개방형 복원술을 사용한 방법은 양호했으며, 개방형 복원술과 정기검진을 통한 감시를 비교한 두 시험은 근거의 신뢰성이 높거나 중간 수준이었다. 혈관내 복원과 정기검진을 통한 감시를 비교한 두 시험의 경우 비뚤림 위험이 불확실 하거나 높았고 근거의 신뢰성은 낮았다. 네 번의 시험은 소규모(지름 4.0cm~5.5cm) 복부 대동맥류의 조기 수술로 전반적인 이점이 없음을 보여준다. 초기 개방 복원술을 정기 검진 치료와 비교한 두 번의 시험에서는 이 결과가 환자의 연령이나 동맥류 크기(4.0cm에서 5.5cm 범위)에 관계없이 유효하다는 것을 발견했다. 게다가, 혈관내 복원에 초점을 맞췄던 두 번의 시험도 정기 검진 치료보다 이득이 없다는 것을 발견했다. 초기 혈관내 수리나 소규모 복부 대동맥류는 현재 근거에 의해 뒷받침되지 않는다.
Authors' conclusions
Summary of findings
| Early open repair compared to ultrasound surveillance for small asymptomatic AAA | ||||||
| Patient or population: small asymptomatic AAA Setting: hospital Intervention: early open repair (open surgery) Comparison: ultrasound surveillance | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with ultrasound surveillance | Risk with early repair | |||||
| Mortality (follow‐up to 6 years) | Study population | HR 0.99 (CI 0.83 to 1.18)a | 2226 (2 RCTs) | ⊕⊕⊕⊕ | No clear evidence to support a difference in survival between early open repair and surveillance. | |
| 0.28 (0.25 to 0.31) | 0.30 (0.27 to 0.33) | |||||
| Costs (per participant) (follow‐up to 18 months) | GBP 3914 | GBP 4978 | MD GBP 1064higher | 1090 | ⊕⊕⊕⊝ | In UKSAT, the mean health service costs per participant were higher in the surgery than the surveillance group, and remained higher at 12‐years of follow‐up. This estimate accounted for semi‐annual surveillance visits, aneurysm repair, and any associated follow‐up. |
| QoLc (follow‐up to 24 months) | See comment | 2226 (2 RCTs) | — | In UKSAT, early‐surgery survivors reported minor improvements in MOS‐20 based current health perceptions and less negative changes in bodily pain (after 1 year). In ADAM, early‐surgery and surveillance groups did not differ for most SF‐36 scales, but the study authors reported that the early‐surgery group had better scores for general health and lower scores for vitality (during the first 2 years); more participants became impotent after early repair compared with surveillance (after 1 year); maximum activity level declined more rapidly over time in the early‐repair group. | ||
| Aneurysm rupture (follow‐up 6 years ) | See comment | 2226 (2 RCTs) | — | In UKSAT, there were 25 ruptures – at least 17 in the surveillance group vs ≥ 6 in the early‐repair group (2 with emergency repairs, group unknown). 15/25 had an aneurysm diameter < 5.5 cm at previous follow‐up. In ADAM, there were 13 ruptures – 11 in the surveillance group and 2 in the early‐repair group (last diameter preceding rupture was not reported). | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AAA: abdominal aortic aneurysm; CI: confidence interval; EVAR: endovascular aneurysm repair; GBP: Great British pounds; HR: hazard ratio; MD: mean difference; MOS‐20: 20‐item Medical Outcomes Study; QoL: quality of life; RCT: randomised controlled trial; SF‐36: 36‐item Short Form. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aFrom pooled individual participant analysis (estimated from Figure 1 in Filardo 2013). | ||||||
| Early endovascular repair compared to ultrasound surveillance for small asymptomatic AAA | ||||||
| Patient or population: small asymptomatic AAA Setting: hospital Intervention: early endovascular repair (EVAR) Comparison: ultrasound surveillance | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with ultrasound surveillance | Risk with early repair | |||||
| Mortality (follow‐up at 1 year) | See comment | RR 1.92 | 846 | ⊕⊕⊝⊝ | Surveillance group: 6 deaths, 408 alive, 126 lost to follow‐up; EVAR: 12 deaths, 420 live, 116 lost to follow‐up. Neither trial reached target sample size. | |
| Costs (follow‐up at 6 months) | USD 5520 | USD 33,471 | MD 27,951 USD higher (25,156 higher to 30,746 higher ) | 614 | ⊕⊕⊝⊝ | Hospital costs were higher in the EVAR group at 6 months. |
| Costs (follow‐up to 48 months) | USD 46,112 | USD 48,669 | MD 2557 USD higher | 614 | ⊕⊕⊝⊝ | No clear evidence to support a difference in total medical costs between groups by 48 months. |
| QoL (follow‐up to 24 months) | See comment | 605 | — | CAESAR reported similar SF‐36 scores between the groups in all domains (after 1 year). PIVOTAL reported no treatment‐related differences in EQ‐5D scores (24 months). | ||
| Aneurysm rupture (overall follow‐up) | See comment | 1088 (2 RCTs) | — | In CAESAR no ruptures observed for aneurysms < 5.5 cm diameter (2 ruptures in the surveillance group that exceeded repair threshold). PIVOTAL reported 1 rupture in the EVAR group and 2 ruptures in the surveillance group. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AAA: abdominal aortic aneurysm; CI: confidence interval; EVAR: endovascular aneurysm repair; MD: mean difference; QoL: quality of life; RCT: randomised controlled trial; RR: risk ratio; USD: United States dollars. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aAs no deaths occurred in the CAESAR surveillance group, summary data from Kaplan‐Meier plots was used to pool data for deaths at one year (CAESAR and PIVOTAL). | ||||||
Background
Description of the condition
An aneurysm is an abnormal dilatation of an artery, the name coming from the Ancient Greek word 'ανεύρυσμα' meaning dilation. The most common arterial aneurysm is found in the infrarenal abdominal aorta of the older population, and known as an abdominal aortic aneurysm (AAA). The most common definition of an AAA is based on the diameter of the abdominal aorta: an abdominal aortic diameter of 3.0 cm or more (usually is more than two standard deviations above the mean diameter for men), is considered to be aneurysmal (Lederle 1988; Lindholt 1999). An alternative definition is that the maximum infrarenal aortic diameter is at least 1.5 times larger than the expected normal infrarenal aortic diameter or suprarenal aortic diameter (Kent 2014). This 1.5‐fold diameter increase also provides a useful basis for the definition of AAA in women in whom the mean aortic diameter is smaller than in men (Rogers 2013).
The prevalence and incidence rates of AAA have decreased since the late 1990s, attributed partially to the decline in smoking (Sampson 2014; Sidloff 2014). Prevalence is negligible before the age of 55 to 60 years, and after this increases steadily with age (Sampson 2014). In 1990, the global prevalence in 75‐ to 79‐year olds was 2423 per 100,000 population versus 2275 per 100,000 population in 2010; there also was a decline in incidence in both high‐ to low–middle income countries (Sampson 2014). At both times, the prevalence was highest in high‐income countries and lowest in Latin America and Central Asia. Population screening studies offer the best evidence regarding the contemporary prevalence of AAA. Data from the Swedish Screening Programme showed the prevalence in 65‐year old men is 1.7% with an additional 0.5% with an already known AAA (Svensjö 2011); 1.3% in the UK National Screening Programme (Jacomelli 2016); and 3.3% in a Danish screening programme (Grøndal 2015). AAAs are approximately four times less common in women versus men, with the pooled prevalence of AAA in women over 60 years of age at 0.7% (Ulug 2016).
The natural history of small AAA is progressive growth for most patients and the majority of cases have no symptoms (asymptomatic). For people with AAA of 3.0 to 5.5 cm in diameter 1. there is no difference in aneurysm growth rates between men and women (both on average 2.2 mm per year but increasing exponentially with AAA diameter); 2. smoking increases aneurysm growth rates by 0.35 mm per year (about 16%); and 3. diabetes is associated with decreased aneurysm growth rates by 0.51 mm per year (approximately 25% reduction) (Sweeting 2012). Randomised trials have not yet identified an effective treatment to limit AAA growth. The risk of AAA rupture is low for people with AAA less than 5.5 cm in diameter (Sweeting 2012); but, following rupture, mortality is very high at 40% to 50% if repaired (Bown 2002). However, about half do not even reach hospital alive giving an overall mortality of about 80%. In recent years, the use of endovascular repair (EVAR) (in suitable patients), rather than open surgical repair of ruptured AAAs, has been associated with lower mortality (Kontopodis 2020).
Smoking is the strongest risk factor for AAA, with an odds ratio greater than 3 for the association (Lederle 2000; Svensjö 2011), and higher in women (Stackelberg 2014). At a pathobiological level, the cause of AAA is multifactorial, with atherosclerosis and inflammation involved (Sakalihasan 2018).
Description of the intervention
For asymptomatic AAAs, management depends on the size of the aneurysm. Intervention should be considered at the point where the risk of AAA rupture outweighs the risk of AAA repair. Clinical guidelines now recommend repair in men after the diameter reaches 5.5 cm, although a lower threshold (5.0 cm) is recommended for women (Wanhainen 2019). For smaller aneurysms, regular surveillance using ultrasonography is recommended (Wanhainen 2019). There are two established forms of AAA repair. The first to be established was open surgical repair, with the AAA being exposed through either a transabdominal or a retroperitoneal incision. The aorta is clamped above and below the aneurysm, the aneurysm sac opened, and a synthetic inlay graft sewn into place proximally and distally, replacing the diseased aorta. The clamps are removed and the aneurysm sac is then wrapped around the graft before closure of the incision layers. The next to be established was endovascular aneurysm repair, where the compressed synthetic graft is delivered into place by catheter, usually from an artery in the groin, and is then placed into position above and below the AAA, expanded in situ and held in place using expandable stents and other methods. Other methods of AAA repair remain experimental.
How the intervention might work
The aim of both forms of intervention is to avoid the risk of AAA rupture and probable death, by relining the dilated segment of the aorta with a synthetic graft. There are several different reasons why this type of intervention might work. First, the thinned and weakened AAA wall is no longer directly subject to arterial pressures, with increased blood pressure being associated with increased risk of AAA rupture (Sweeting 2012). Second, the synthetic graft is stronger than the thinned and aneurysmal aortic wall and can resist the repetitive rise and fall of blood pressure as the pulse is conducted to the lower limbs. Third, the synthetic graft acts as a barrier between the blood and the diseased aorta, so that the biological interactions between blood and tissue which promote aortic degeneration are avoided. Nevertheless, interventions are associated with a risk of operative mortality and morbidity and these risks appear higher for open repair than EVAR (Powell 2017), and are higher in women than men (Ulug 2017). The risk of AAA rupture is not completely eradicated after repair, especially in the case of EVAR (Patel 2016).
Why it is important to do this review
The unclear area of care for small AAAs, resulting from the uncertainty surrounding the risk of rupture versus the risk of intervention and expansion rates identified by the RAND panel (Ballard 1992), highlighted the need for randomised controlled trials (RCT) comparing early surgery and selective surveillance as treatment options. This led to the design of the Aneurysm Detection And Management (ADAM) trial (ADAM), the United Kingdom Small Aneurysm Trial (UKSAT) (UKSAT), and the Canadian trial [pers comm], which used open surgery to perform the repairs. Later, when EVAR became available, the Comparison of surveillance versus Aortic Endografting for Small Aneurysm Repair (CAESAR), and the Positive Impact of endoVascular Options for Treating Aneurysms earLy (PIVOTAL) trials were conducted using EVAR as the surgical option. Most recently, pooling the participant‐level data from the ADAM and UKSAT trials has enabled investigation of the possibility that age or AAA diameter might affect survival differences between early repair and surveillance (Filardo 2013; Filardo 2014). As the current guidelines for management of AAA state, "[d]ebate remains for patients presenting with AAAs between 4.0 cm and 5.4 cm regarding the most appropriate role for either early treatment or surveillance and selective repair for those aneurysms that subsequently enlarge beyond 5.4 cm" (Chaikof 2018). It is now several years since these trials have reported and there have been advances in anaesthesia, intensive care and surgery that might reduce perioperative risks. The epidemiology of AAA is changing, with their onset being delayed and the mean age at AAA repair rising steadily. This update will assess whether there is any new evidence available to help guide management of small AAA.
Objectives
To compare mortality and costs, as well as quality of life and aneurysm rupture as secondary outcomes, following early surgical repair versus routine ultrasound surveillance in people with asymptomatic AAAs between 4.0 cm and 5.5 cm in diameter.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs in which participants were randomly allocated to early surgery versus ultrasound surveillance.
Types of participants
We included men or women of any age with an asymptomatic AAA. The aneurysm was restricted to the abdominal aorta distal to the renal arteries. The maximum antero‐posterior diameter, measured using ultrasound or computed tomography (CT) scanning, must have been at least 4.0 cm and less than 5.5 cm. The aneurysm should have been non‐tender on examination and the participant assessed as generally fit for surgery.
Types of interventions
We included studies involving repair of the aneurysm consisting of insertion of a prosthetic inlay graft either by open surgery (abdominal or retroperitoneal route) or by EVAR. Surveillance of the maximum antero‐posterior diameter was to be performed regularly, with a maximum interval of six months. The timing of early surgery will vary with healthcare system, but should have been within three months of randomisation.
Types of outcome measures
Primary outcomes
The key outcome measures had to include at least one of the following.
-
Mortality: death rate during a specified period of time following randomisation.
-
Direct hospital costs from trial data: all hospital costs using specific survey or standard costing manuals which included inpatient stays, surgery, and outpatient attendances including ultrasound surveillance.
Secondary outcomes
-
Quality of life (QoL): a standard generic measure using a validated instrument encompassing typical domains such as pain, health perceptions, mental health, and physical and social functioning.
-
Rupture: rate of aneurysm rupture diagnosed at postmortem, operation, or certified as the underlying cause of death.
-
Cause of death: mortality by underlying cause of death according to the International Classification of Diseases.
-
Operative mortality: measured as 30‐day or 'in hospital' mortality, or both.
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Information Specialist conducted systematic searches of the following databases for RCTs and controlled clinical trials without language, publication year, or publication status restrictions:
-
Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web; searched 10 July 2019);
-
Cochrane Central Register of Controlled Trials (CENTRAL) Cochrane Register of Studies Online (CRSO; 2019, Issue 6);
-
MEDLINE (Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily, and Ovid MEDLINE) (searched from 1 January 2017 to 10 July 2019);
-
Embase Ovid (searched from 1 January 2017 to 10 July 2019);
-
CINAHL EBSCO (searched from 1 January 2017 to 10 July 2019);
-
AMED Ovid (searched from 1 January 2017 to 10 July 2019).
The Information Specialist modelled search strategies for other databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with adaptations of the highly sensitive search strategy designed by Cochrane for identifying RCTs and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6; Lefebvre 2011). Search strategies for major databases are provided in Appendix 1.
The Information Specialist searched the following trials registries on 10 July 2019:
-
World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch);
-
ClinicalTrials.gov (clinicaltrials.gov).
Searching other resources
We checked the reference lists of relevant studies. We supplemented the searches with information from experts in the field and from handsearches of the following conference proceedings:
-
Society for Vascular Surgery Annual Meeting (through to 15 November 2019);
-
European Society for Vascular Surgery Annual Meeting (through to 15 November 2019).
Data collection and analysis
Selection of studies
We assessed the articles identified by the searches using Covidence software (covidence.org). Initial screening was carried out to remove obviously non‐relevant articles. Remaining articles were then assessed independently in duplicate (JTP and PU) according to the Criteria for considering studies for this review. We listed all studies excluded after full‐text assessment and their reasons for exclusion in the Characteristics of excluded studies table. We constructed a PRISMA diagram to illustrate the study selection process (Figure 1). Any disagreements were resolved by discussion.
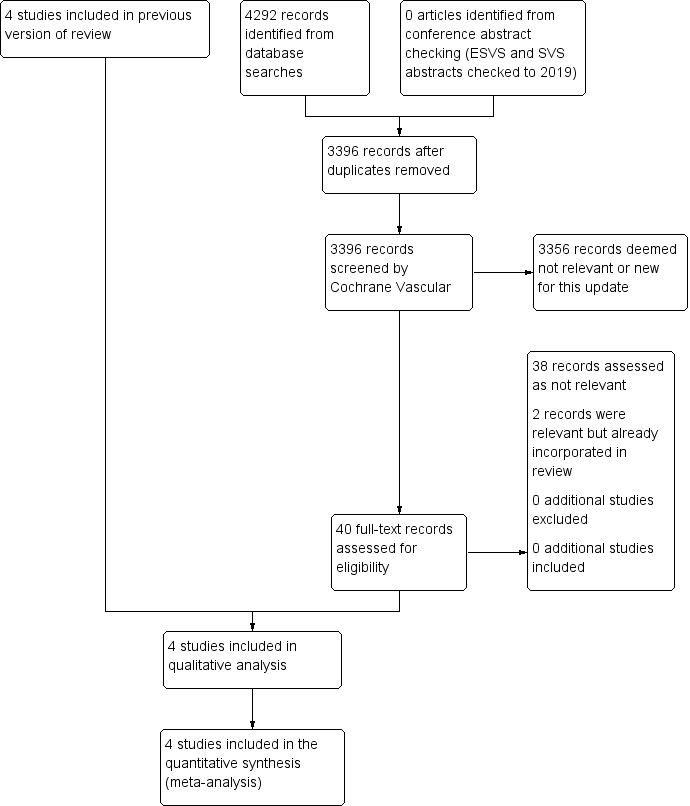
Study flow diagram.
Data extraction and management
For this update, two review authors (JTP and PU) cross‐checked the previous data extractions and re‐extracted data from the two trials comparing EVAR with surveillance. Previously, two review authors (GF and MAMM) abstracted the data, which another team member (DJB) cross‐checked. The data collected on each trial included information on the participants (age and sex distribution, aneurysm size), the interventions (graft type, frequency of ultrasound surveillance), and the outcomes (as specified in Criteria for considering studies for this review).
Assessment of risk of bias in included studies
We discussed each of the trials and agreed on their inclusion or exclusion based on the adequacy of the random allocation, attainment of adequate sample size, and completeness of follow‐up. The nature of the interventions did not permit participants or observers to be blinded, and so this lack did not disqualify trials from inclusion. In addition, we assessed the risk of bias of the included studies using the 'Risk of bias' tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The following domains were assessed and judged to be at low risk of bias, high risk of bias, or unclear risk of bias: selection bias, performance and detection bias, attrition bias, reporting bias, and other sources of bias.
Measures of treatment effect
We estimated risk ratios (RR) (EVAR only), hazard ratios (HR) (open repair only), and 95% confidence intervals (CIs) based on the Mantel‐Haenszel Chi2 statistic to assess the efficacy of the intervention at one year (endovascular and open repair) and six years (open repair only) following randomisation. We estimated the HRs reported for open repair from a participant‐level meta‐analysis that was executed to summarise evidence from the UKSAT and ADAM trials (Filardo 2013; Filardo 2014).
Unit of analysis issues
We used each participant with an AAA of diameter 4.0 cm to 5.5 cm who received early surgical repair versus routine ultrasound surveillance as the unit of analysis.
Dealing with missing data
None of the included studies used single or multiple imputation procedures to deal with missing data. Although the incidence of missing data in the trials comparing open repair with surveillance was very low, the incidence of missing data in the trials comparing EVAR with surveillance was moderate, with 24% of participants lost to follow‐up for mortality and clinical events by 12 months of randomisation.
Assessment of heterogeneity
We assessed heterogeneity using the I2 statistic. We considered I2 values of 50% or greater to indicate substantial heterogeneity. Moreover, we used a Chi2 test to assess heterogeneity in the participant‐level meta‐analysis we executed to summarise evidence from the UKSAT and ADAM trials. If we identified heterogeneity, we explored reasons for it.
Assessment of reporting biases
We intended to use funnel plots for publication bias, However, we did not test for funnel plot asymmetry as the power of the test is low when fewer than 10 trials are included in the analysis (Page 2019). All included studies were assessed for selective reporting bias and published findings on the main study outcome of this review.
Data synthesis
We estimated RRs (EVAR only), HRs (open repair only), and 95% CIs based on Mantel‐Haenszel Chi2 statistic. We calculated the RR summary estimates by employing a fixed‐effect model meta‐analyses approach. We estimated HRs from a participant‐level meta‐analysis that we executed to summarise evidence from the UKSAT and ADAM trials (Filardo 2013; Filardo 2014).
Subgroup analysis and investigation of heterogeneity
We analysed and presented separately studies comparing early EVAR to surveillance and studies comparing early open repair to surveillance. Given the differences in surgical techniques, we did not estimate the overall effect associated with early repair irrespective of the type of surgery compared to surveillance. Accordingly, we executed tests for heterogeneity for each meta‐analysis, one reporting on early EVAR versus surveillance and one reporting on early open repair versus surveillance.
Sensitivity analysis
We included all relevant published studies in this review. We did not carry out a sensitivity analysis due to the small number of included studies in each outcome.
Summary of findings and assessment of the certainty of the evidence
We prepared a 'Summary of findings' table for each of the comparisons 'Early open repair compared to ultrasound surveillance for small asymptomatic AAA' and 'Early endovascular repair compared to ultrasound surveillance for small asymptomatic AAA' to present the main findings from the review. We included the outcomes of mortality, costs, QoL, and aneurysm rupture. See summary of findings Table 1 and summary of findings Table 2. We used GRADEprofiler software to create the tables (GRADEpro GDT). The GRADE criteria were used to rank the certainty of the evidence for each outcome based on risk of bias, inconsistency, indirectness, imprecision, and publication bias (Guyatt 2008). We have provided a description for each step to downgrade the certainty of the evidence.
Results
Description of studies
See Figure 1.
Results of the search
We identified four RCTs from the electronic searches (ADAM; CAESAR; PIVOTAL; UKSAT), and one from personal communication (Canadian trial [pers comm]).
Included studies
See Characteristics of included studies table. We included four RCTs involving 3314 participants, which fulfilled the criteria for consideration in the present review (ADAM; CAESAR; PIVOTAL; UKSAT). We used results from analyses of pooled participant‐level data from the UKSAT and ADAM trials in the comparison of early open repair to selective surveillance (Filardo 2013; Filardo 2014).
All four trials enrolled participants with small (4.0 cm to 5.5 cm) non‐tender, asymptomatic AAAs who were considered fit for surgery. The trials excluded participants who were considered unfit for surgery, had symptoms associated with the aneurysm, were unable to attend the follow‐up visit, or were unable to give informed consent. The ADAM study further excluded people who had received a revascularisation procedure within three months of enrolment, had a myocardial infarction within six months of enrolment, or were expected to survive less than five years because of invasive cancer or another life‐threatening disease. The CAESAR trial, besides excluding people not anatomically suitable for EVAR, further excluded people who had severe comorbidities or a suprarenal or thoracic aorta of 4.0 cm or greater in diameter, or that needed urgent repair. The PIVOTAL study further excluded people who had had an abdominal or thoracic repair, an aneurysm originating 1.0 cm or less from the most distal main renal artery, life expectancy of less than three years, Society for Vascular Surgery score greater than two with the exception of age and controlled hypertension, baseline serum creatinine level greater than 2.5 mg/dL, or when the participant did not meet the indications for use of the endograft device. Costs were investigated for PIVOTAL in a study within a study (PIVOTAL Economic Study), involving the same participants, in parallel with the PIVOTAL trial.
Age inclusion criteria were 50 to 79 years (ADAM), 50 to 79 years (CAESAR), 40 to 90 years (PIVOTAL), and 60 to 76 years (UKSAT). Despite the relatively wider age range eligible for inclusion in the ADAM, CAESAR, and PIVOTAL trials, the majority of the participants fell within the same age range as the UKSAT trial: 88% (ADAM), approximately 70% (CAESAR), and approximately 70% (PIVOTAL). This is perhaps unsurprising given that AAA prevalence is much higher in older age groups.
Study designs were similar, with participants randomly allocated to either early surgery or selective surveillance. All trials except UKSAT recommended surgery within one month of randomisation; UKSAT recommended surgery within three months of randomisation. In the four trials, most participants assigned to the early‐surgery group received endovascular or standard open repair within six weeks of randomisation. Likewise, in all four trials, participants assigned to selective surveillance were followed, without repair, at regular intervals (at minimum once every six months), and surgery was performed within six weeks if 1. the aneurysm reached 5.5 cm in diameter; or 2. the aneurysm enlarged by a minimum of 0.7 cm in six months (ADAM), 1.0 cm in one year (UKSAT), greater than 1.0 cm in one year (CAESAR), or a minimum of 0.5 cm between two six‐month assessments (PIVOTAL); or 3. the aneurysm became symptomatic. The primary outcomes of all trials was mortality. Only PIVOTAL and UKSAT measure cost, and all trials assess QoL using a variety of validated questionnaires.
Adherence to assigned treatment was very high across the four trials (UKSAT had the lowest adherence rate at 92.6%), and at the end of the trials, mortality status was ascertained in 100% (ADAM; UKSAT) and in 50% of participants (CAESAR; PIVOTAL). Approximately 62% (ADAM), 48% (CAESAR), 31% (PIVOTAL), and 75% (UKSAT) of the participants in the selective‐surveillance group eventually underwent aneurysm repair.
In total, 3314 participants with asymptomatic AAAs of antero‐posterior diameter 4.0 cm to 5.5 cm were randomised to early repair (1680 participants: 569 in ADAM, 182 in CAESAR, 366 in PIVOTAL, and 563 in UKSAT; 50.7%) or routine ultrasound or CT surveillance every six months (three months if diameter 5.0 cm to 5.5 cm in ADAM and UKSAT) (1634 participants: 567 in ADAM, 178 in CAESAR, 362 in PIVOTAL, and 527 in UKSAT; 49.3%). The primary outcome of the included studies was all‐cause mortality and secondary outcomes were AAA‐related death, morbidity, and QoL. Follow‐up for vital status ranged from 3.5 to 8.0 years (mean 4.9 years) in the ADAM trial; median 32.4 months (interquartile range (IQR) 21.0 to 44.1) in the early EVAR group and 30.9 (IQR 18.3 to 45.3) in the surveillance group in the CAESAR trial; 20 (standard deviation (SD) 12 months; range 0 to 41 months) in the PIVOTAL trial; and up to 12 years (range 8 to 12 years; mean 10 years) in the UKSAT trial.
Excluded studies
We excluded one trial that did not fulfil the criteria for consideration (Canadian trial [pers comm]). This study ended early because of inadequate recruitment and was not sufficiently complete for inclusion in this review. See Characteristics of excluded studies table.
Risk of bias in included studies
See Figure 2 and Figure 3 for the 'Risk of bias' summary.

Risk of bias graph: review authors' judgements about each risk of bias item for the outcomes of mortality and cost presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The methods of randomisation of the included studies ensured good balance across study groups as they all used independent automated computer randomisation, either by telephone or the Internet (ADAM; CAESAR; PIVOTAL; UKSAT). Adherence to assigned treatment was high, with the lowest adherence rate across the four trials at 92.6% (ADAM; CAESAR; PIVOTAL; UKSAT). Therefore, risk of selection bias was low.
Blinding
The nature of the interventions did not permit the blinding of participants or observers to which treatment group they were in, so we judged all studies at unclear risk of performance bias. For the outcome of mortality, vital status was assessed using the same methodology for both participants in the early‐repair group and participants in the routine ultrasound surveillance group in each trial and near complete results were available as a result of low lost‐to‐follow‐up rate. Therefore, any misclassification which might have occurred would have been non‐differential and its impact on the trial results would be limited. So, for the main outcome of mortality, the risk of detection bias was low in all studies (ADAM; CAESAR; PIVOTAL; UKSAT). The risk of bias was low for costs (PIVOTAL; UKSAT). However, as QoL was patient reported; not complete in any of the studies; and reported using a variety of validated questionnaires, the risk for detection bias was unclear for this outcome (ADAM; CAESAR; PIVOTAL; UKSAT).
Incomplete outcome data
We ascertained mortality status to six years in 100% of participants in ADAM and UKSAT. The loss to clinical follow‐up was low; so we judged the risk of attrition bias in these trials as low. The attrition bias for CAESAR and PIVOTAL was notable for both mortality and clinical follow‐up. In CAESAR, within 12 months of randomisation, 13% of participants overall were lost to follow‐up for both mortality and clinical follow‐up (21/182 participants in the endovascular group and 24/178 participants in the surveillance group), so we judged the risk of attrition bias as unclear. In PIVOTAL, within 12 months of randomisation 27% of participants overall were lost to follow‐up (similar in both randomised groups, 95/366 participants in the endovascular group and 102/362 participants in the surveillance group), so we judged the risk of attrition bias as high for mortality.
The health economic analysis of the PIVOTAL trial that was undertaken separately by the Duke Clinical Research Institute only included 614/728 participants randomised (Characteristics of included studies table). The reason for this was due to not all patients being treated at hospitals that generated detailed patient bills. Missing information was balanced between groups, so this was judged to represent an unclear risk of bias for the outcome of cost.
Selective reporting
All included studies published findings on the primary outcome of mortality and reported on the outcomes preplanned in their protocols (ADAM; CAESAR; PIVOTAL; UKSAT). Risk of selective reporting bias was low (ADAM; CAESAR; PIVOTAL; UKSAT).
Other potential sources of bias
The CAESAR trial was originally funded by Cook Medical. In December 2006, during the enrolment phase of the trial, Cook Medical withdrew sponsorship, and the trial continued as full spontaneous research. According to the CAESAR study team, the design, data collection, data analysis, data interpretation, and writing of reports regarding the trial were at all times conducted independently from the sponsor. However, we could not exclude a possible conflict of interest in the CAESAR trial given that the sponsor of the study, Cook Medical, withdrew and so this was judged at high risk of bias. The PIVOTAL trial was sponsored by Medtronic Vascular, who hold the PIVOTAL trial study database. Two members of the PIVOTAL research team who received funding from, and were consultants for, Medtronic declared conflicts of interest; a third member of the PIVOTAL research team had previously been a consultant for Medtronic and so this was judged at high risk of bias. The Vascular Surgery Academic Co‐ordinating Center of the Cleveland Clinic was independently responsible for the conduct of the PIVOTAL trial and its analysis. Also, neither CAESAR or PIVOTAL reached their recruitment target. Other potential sources of bias for the outcomes of mortality and cost for remaining trials included in this review were low (ADAM; UKSAT). In all of the trials potential other sources of bias for QoL outcomes were unclear (ADAM; CAESAR; PIVOTAL; UKSAT).
Effects of interventions
See: Summary of findings 1 Early open repair compared to ultrasound surveillance for small asymptomatic abdominal aortic aneurysms; Summary of findings 2 Early endovascular repair compared to ultrasound surveillance for small asymptomatic abdominal aortic aneurysms
Open repair compared to surveillance
Mortality
Two studies compared early open repair with surveillance (ADAM; UKSAT). In both studies, the 30‐day elective operative mortality in the early‐surgery group (UKSAT: 5.5%; ADAM: 2.1%) led to an early mortality disadvantage in this study group. However, in both studies after a mean follow‐up of three years, there was no difference in mortality between groups.
In the UKSAT study, the long‐term mortality (follow‐up range: 8 to 12 years, mean 10 years) was 63.9% in the early‐surgery group and 67.3% in the surveillance group. The UKSAT investigators found no clear evidence to support a difference in long‐term survival between the early‐surgery and surveillance groups (adjusted HR 0.88, 95% CI 0.75 to 1.02). However, the HRs were non‐proportional among study groups, as revealed by the survival curves crossing approximately at the three‐year mark; the risk associated with operative mortality in the early‐repair group showed an initial survival disadvantage for this group compared to the selective‐surveillance group. The estimated adjusted HRs were in the direction of greater benefit of early surgery for younger participants and those with larger aneurysms, but none of the tests for interaction showed a clear effect.
At the end of the ADAM trial follow‐up (range 3.5 to 8.0 years, mean 4.9 years), the observed mortality in the early‐repair group was 25.1% and in the selective‐surveillance group was 21.5%. However, as in the UKSAT study, there was no clear evidence of a difference in long‐term survival between study groups (adjusted HR 1.21, 95% CI 0.95 to 1.54). The study authors did not report violation of the proportional hazard assumption. Study results showed a possible modification of effect with age and AAA size but none of the tests for interaction were reported as significant. Moreover, the analysis of the pooled participant‐level data from the ADAM and UKSAT trials demonstrated no clear difference in survival between early open repair and surveillance, regardless of participant age or aneurysm diameter (Filardo 2013; Filardo 2014). In women only, combined results for survival to three years showed no clear difference between study groups (HR 0.84, 95% CI 0.62 to 1.11). There were too few women enrolled for any longer‐term analyses (Filardo 2014 and unpublished data).
We performed meta‐analyses of mortality up to six years to assess the effect of open repair versus surveillance (ADAM; UKSAT). The analysis of pooled participant‐level data from the UKSAT and ADAM trials conducted to assess mortality up to six years found no clear evidence to support a difference in survival between early open repair and surveillance (HR 0.99, 95% CI 0.83 to 1.18; 2 trials, 2226 participants; high‐certainty evidence; summary of findings Table 1). This analysis is reported in Filardo 2013 and Filardo 2014. Additional analyses conducted using this pooled data set showed this lack of a clear difference in survival persisted regardless of participant age or AAA diameter within the range of 4.0 cm to 5.5 cm (Filardo 2013; Filardo 2014).
Costs
For open repair compared to surveillance, only UKSAT provided information on costs. There were no cost data for ADAM.
In UKSAT, the mean health service costs per participant over the four to six years' follow‐up period postrandomisation were higher in the surgery than the surveillance group (GBP 4978 with surgery versus GBP 3914 with surveillance; mean difference (MD) GBP 1064, 95% CI 796 to 1332; 1090 participants; moderate‐certainty evidence; Analysis 1.1). This estimate accounted for semi‐annual surveillance visits, aneurysm repair, and any associated follow‐up. For example, if surveillance was conducted only once per annum, the mean cost difference in favour of surveillance widened to GBP 1256 (95% CI 990 to 1522). A 25% increase in cost of aneurysm repair further increased the difference to GBP 1636 (95% CI 1340 to 1932). After 12 years, the resource consequences of early surgery had increased costs by GBP 1326 (95% CI 960 to 1692).
Quality of life
Both studies comparing open repair with surveillance reported QoL using different validated questionnaires (ADAM; UKSAT).
UKSAT assessed QoL using the 20‐item Medical Outcomes Study short‐form. At the time of randomisation, QoL was similar in the two groups, but early‐surgery participants reported minor improvements in current health perceptions and less negative changes in bodily pain at one year after randomisation.
ADAM assessed QoL using the 36‐item Short Form (SF‐36) form. The early‐surgery and surveillance groups did not differ for most SF‐36 scales at most of the time points measured, but the study authors reported that the early‐surgery group had better scores for general health, particularly during the first two years following randomisation, but had lower scores for vitality. ADAM reported that more participants became impotent after randomisation to early repair compared with surveillance, a difference that only became apparent more than one year after randomisation. In ADAM, maximum activity level declined more rapidly over time in the early‐repair group.
Aneurysm rupture
In UKSAT, during six years of follow‐up there were 25 ruptures, of which two participants survived. It is not clear how the ruptures were distributed between the randomised groups, though at least 17 ruptures occurred in the surveillance group and at least six ruptures occurred in the early‐surgery group. Of these 25 aneurysm ruptures, only 15 ruptures had occurred in participants with aneurysms of less than 5.5 cm in diameter. In UKSAT, as in other studies, the rupture rate was almost four times higher in women compared with men (Sweeting 2012).
In ADAM, over a period of similar follow‐up, there were 13 ruptures, of whom 11 were in the surveillance group and two in the early‐surgery group. Of the 11 participants in the surveillance group, there were seven deaths, and in the early‐surgery group there was one death. The last diameter before rupture was not recorded, therefore, we were unable to assimilate the data from two studies.
Other outcomes
30‐day operative mortality is reported under 'Mortality'.
Endovascular repair compared to surveillance
Mortality
Two studies compared early EVAR with surveillance (CAESAR; PIVOTAL). In both trials, the 30‐day operative mortality in the early‐repair group (CAESAR: 0.6%; PIVOTAL: 0.3%) led to an early disadvantage in terms of survival in this study group. The lower 30‐day mortality rate observed in the CAESAR and PIVOTAL studies, compared to the UKSAT and ADAM trials, was expected due to the use of EVAR.
In CAESAR, one year after randomisation, there were no deaths in the surveillance group, and four deaths in the endovascular group. At the end of the CAESAR trial follow‐up (maximum: 54 months, median: 32.4 months), the estimated all‐cause mortality for the early‐repair group was 14.5% and for the selective‐surveillance group was 10.1%. However, there was no clear evidence of a difference between the two groups for long‐term survival (HR 0.76, 95% CI 0.30 to 1.93). The authors did not report a violation of the proportional hazard assumption.
In PIVOTAL, one year after randomisation, there were six deaths in the surveillance group, and eight deaths in the endovascular group. At the end of the PIVOTAL trial follow‐up (range 0 to 41 months, mean 20 (SD 12 months)), the estimated all‐cause mortality for both groups was 4.1%, and long‐term survival showed no clear difference between groups (HR 1.01, 95% CI 0.49 to 2.07). The authors reported no evidence of non‐proportional hazards between groups over time.
In both CAESAR and PIVOTAL trials, there was increasing loss to follow‐up over time. Since at 12 months after randomisation there were no deaths in the surveillance group of CAESAR, the number of deaths at later time points were unclear and no formal meta‐analysis was possible. As no participant‐level data were available, we have used summary data from Kaplan‐Meier plots to pool data for deaths at 12 months (early EVAR: 12 deaths among 432 participants; surveillance group: 6 deaths among 414 participants), which showed no clear evidence of a difference in survival (RR 1.92, 95% CI 0.73 to 5.06; 2 trials, 846 participants; low‐certainty evidence).
Costs
For the EVAR versus surveillance comparison, the PIVOTAL Economic Study investigated costs (Eisenstein 2013), which is a study within PIVOTAL using the same participants. No cost data were available for CAESAR.
The PIVOTAL Economic Study reported higher total medical costs (including AAA‐related clinic visits and imaging studies, AAA repair (endovascular device or open surgery), and other inpatient care (including secondary procedures, emergency department visits, other hospitalisations, and rehabilitation and skilled nursing facility care) in the early EVAR group at six months (USD 33,471 in the EVAR group versus USD 5520 in the surveillance group; MD USD 27,951, 95% CI 25,156 to 30,746; 1 trial, 614 participants; low‐certainty evidence). However, there were greater total medical costs in the surveillance group in months 7 to 48 (USD 40,592 versus USD 15,197 in the EVAR group; MD USD 25,395, 95% CI 15,184 to 35,605). These differences balanced out across the full 48 months studied such that there was no clear evidence to support a difference in total medical costs between the two interventions (USD 48,669 in the EVAR group versus USD 46,112 in the surveillance group; MD USD 2557, 95% CI –8043 to 13,156; 1 trial, 614 participants; low‐certainty evidence).
Quality of life
Both studies that compared EVAR with surveillance reported QoL using different measurements (CAESAR; PIVOTAL).
CAESAR assessed QoL using the SF‐36 form and reported comparable scores in the early EVAR and surveillance groups at randomisation. At six months, the total SF‐36 and the physical and mental domain scores had all increased with respect to baseline in the early‐repair group, while participants in the surveillance group scored lower. However, by one year after randomisation, the two groups had similar SF‐36 scores in all domains.
The PIVOTAL Economic Study assessed QoL between the early EVAR and surveillance groups using the EQ‐5D instrument, and reported results from 710 participants who completed the EQ‐5D instrument at baseline, 12, and 24 months. There were no clear differences between the intervention groups at baseline on any of the EQ‐5D domains, and no treatment‐related differences in either the QoL domains or the utility score at 12 or 24 months' follow‐up. Participants in the EVAR group reported lower visual analogue scale scores at 12 months, but this difference did not persist at 24 months.
Aneurysm rupture
Both CAESAR and PIVOTAL trials selected participants according to aneurysm morphology criteria, which may have influenced the rupture rates (Powell 2008). In addition, the selection criteria in the PIVOTAL trial included only people with 4 cm to 4.9 cm diameter aneurysms. Both trials provided three years of participant follow‐up data.
Aneurysm ruptures were not different between the groups in either trial. There were two aneurysm ruptures in the surveillance arm of the CAESAR trial, both in participants in whom the aneurysm exceeded the repair threshold. In the PIVOTAL trial, there was one rupture in the EVAR group and two in the surveillance group.
Other outcomes
Aneurysm‐related mortality (elective mortality and rupture‐related mortality) was low and not different between the groups in both the CAESAR and PIVOTAL trials. The CAESAR trial also reported that 4/182 participants had conversion to open repair, 16.4% participants in the surveillance group had lost suitability for EVAR by three years, re‐interventions occurred in 10 participants in the EVAR group but no participant in the surveillance group, and that the mean aneurysm growth rate was less than 2 mm/year.
Discussion
The results from the four included trials suggest no overall advantage to early repair for small AAA (ADAM; CAESAR; PIVOTAL; UKSAT). Furthermore, the more recent trials focused on the efficacy of EVAR and found no benefit (CAESAR; PIVOTAL), and analysis of the pooled participant‐level data from the earlier open‐repair trials showed that the lack of any treatment‐related survival benefit was consistent across all participant ages and aneurysm diameters within the small AAA range (Filardo 2013; Filardo 2014). Thus, the currently available evidence supports neither early open nor early EVAR of small AAAs. Our results affirm the Society for Vascular Surgery and the European Society for Vascular Surgery's strong recommendation in favour of surveillance for people with a fusiform AAA of 4.0 cm to 5.4 cm (Chaikof 2018). While the development of endovascular technology offers an improvement in operative mortality compared to open surgery and better short‐term survival in general (Lederle 2009; Prinssen 2004; United Kingdom EVAR Trial Investigators 2010), its efficacy is limited by high rates of re‐operation for complications unique to EVAR over longer follow‐up, including stent migration, stent wire fracture, metal fatigue, graft insertion site problems, and endoleak (Becquemin 2011; De Bruin 2010; EVAR Trial Participants 2005; Wilt 2006). For small AAA in particular, early EVAR does not appear to offer advantages compared to surveillance (see Analysis 1.1), and its use could expose patients to unnecessary risk and ultimately higher healthcare costs (Ballard 2012). Likewise, Analysis 1.1 shows that early open repair offers no better outcomes compared to surveillance for people with small AAAs.
However, it should be kept in mind that the results presented are derived from RCT settings which, particularly for the surveillance group, may not reflect current practice in terms of either the resources available for care or the patient compliance with follow‐up schedules that can be expected. Thus, while we can conclude that there is no clear evidence to support a difference in efficacy between early repair and surveillance in small AAAs, the question regarding effectiveness requires further investigation, particularly for small AAAs approaching the 5.5 cm cut‐off, where one meta‐analysis suggested an eight‐month surveillance interval is needed to adequately manage the risk of expansion past 5.5 cm (Bown 2013), and poor compliance with surveillance could move patients' risks towards greater benefit with early repair. As there is currently no registry containing surveillance data for small AAAs, a large, prospective, population‐based comparative effectiveness study is needed (Ballard 2012).
Future research should include investigating the possible differences in QoL between the various management strategies available for small AAA, taking into account that these might differ by age, and the evidence that moderate exercise (rather than the strict limitation on physical activity previously advised for people with unrepaired small AAA) benefits patients under surveillance (Myers 2010; Tew 2012).
The apparently lower risks of rupture associated with aneurysms morphologically suitable for EVAR and the apparently higher rates of rupture in women need to be elucidated. There is some evidence that these risks differ in men and women with AAA (Abedi 2009; Lo 2013; McPhee 2007; Mehta 2012; UKSAT), but studies to date have generally included very few women, and, in the absence of sufficient data to rigorously examine the competing risks and the timing of intervention in women (Rudarakanchana 2013), recommendations for management in women remain a 'best guess' guided largely by the evidence available for men.
Establishing optimal treatment guidelines for people with small AAAs becomes even more relevant to improving public health and patient outcomes when the likelihood of increased AAA screening in the future is taken into account. The evidence from three randomised population screening trials, summarised in one Cochrane Review, shows the benefits of screening older men for AAA (Cosford 2007). A national screening programme for all men aged 65 years and older runs in the UK (Jacomelli 2016; Oliver‐Williams 2019), and the U.S. Preventive Services Task Force (USPSTF) recommends AAA screening for men aged 65 to 75 years who have ever smoked (US Preventive Task Force). The Society for Vascular Surgery has also recommended screening of all men aged 60 to 85 years for AAA; women aged 60 to 85 years with cardiovascular risk factors; and men and women aged 50 years and older with a family history of AAA (Kent 2004). These recommendations are based on evidence that screening for AAA and repair of large AAAs (5.5 cm or more in diameter) leads to decreased AAA‐specific mortality. However, the USPSTF also indicates that there is possible evidence of harms of screening and early treatment, including an increased number of surgeries with associated clinically significant morbidity and mortality, and short‐term psychological harms (US Preventive Task Force). These harms are of most concern for people with aneurysms in the 4.0 cm to 5.5 cm range, for whom current treatment guidelines are ambiguous.
Summary of main results
See summary of findings Table 1 and summary of findings Table 2. Findings from this review indicate that there was no survival advantage with early repair compared to selective surveillance in people with asymptomatic aneurysms sized 4.0 cm to 5.5 cm in diameter. Results from the UKSAT, ADAM, CAESAR, and PIVOTAL trials showed no clear evidence to support a difference in survival between the study treatment groups; the analysis of the pooled participant‐level data from the ADAM and UKSAT studies showed that this held true regardless of participant age or AAA diameter for open repair (Filardo 2013; Filardo 2014). In the absence of long‐term, participant‐level data for the PIVOTAL and CAESAR trials, we cannot draw firm conclusions about the long‐term effects of early EVAR. However, findings to date suggest no advantage to early surgery for small AAA, and the currently available evidence supports neither early open nor early EVAR of small AAAs. The Society for Vascular Surgery and the European Society for Vascular Surgery guidelines strongly recommend surveillance for men with a fusiform AAA of less than 5.5 cm diameter but suggest a reduced diameter threshold for elective repair in women (Chaikof 2018; Wanhainen 2019). The UK National Institute for Health and Care Excellence (NICE) recommends a diameter threshold for elective repair of 5.5 cm in both men and women (NICE 2020).
Overall completeness and applicability of evidence
This review was based on all trials to date that were suitable for inclusion. However, one limitation of the present review is the low proportion of women and non‐white races in the trials. The gender imbalance is exacerbated by the late onset of the disease in women and by the approximately three times higher prevalence of AAA in men than in women, and that black race has a strong negative association with AAA (Lederle 1997; Lederle 2000). Thus, while it is indisputable that the study results might be difficult to generalise to women and non‐white men, this review provides critical data that can benefit the population with the highest prevalence of AAA and, therefore, the vast majority of people with AAA. Future research regarding the management of small AAA should focus on ethnic minorities and women, as data regarding these populations are lacking. In particular, future research should assess whether the AAA‐management recommendations, which are based on studies in which women are under‐represented, are applicable to women given their smaller body frames and, therefore, smaller abdominal aortas. This is critical given the evidence that risk of rupture, risks associated with repair, and progression of disease may differ between men and women (Abedi 2009; Brown 2003; Lo 2013; McPhee 2007; Mehta 2012; RESCAN Collaborators 2013; UKSAT). Another limitation of the evidence is that, although cost data can be summarised, there are no summary data for cost‐effectiveness.
Quality of the evidence
The UKSAT, ADAM, CAESAR, and PIVOTAL trials were very similar in design and, more importantly, were all well‐conducted studies. All relevant studies were identified and included in this review. Moreover, all relevant data were obtained. In summary, bias was a concern for the CAESAR and PIVOTAL trials due to considerable loss to follow‐up of participants within 12 months, the inability of either trial to reach the planned recruitment numbers and potential bias concerning conflicts of interest. There were no such concerns for the ADAM and UKSAT trials. Therefore the certainty of evidence for mortality summarised in this review is high for early open repair compared to surveillance (ADAM; UKSAT), but low for early EVAR compared to surveillance (CAESAR; PIVOTAL). For costs, we downgraded the certainty of the evidence from high to moderate for early open repair compared to surveillance for imprecision as only UKSAT provided data for this outcome. Our certainty in the evidence for costs in early EVAR compared to surveillance was downgraded from high to low due to risk of bias concerns and imprecision as only PIVOTAL provided cost data.
Potential biases in the review process
Two members of the review authors (GF, MAMM) independently abstracted the data, which were cross‐checked by other team members (DJB). To further reduce bias, the role of JTP and DB (trialists in the UKSAT (JTP) and ADAM (DB) studies and authors in the present review) in abstracting the data was limited to cross‐checking the information abstracted. Strengths of the present review regarding potential biases are: 1. all relevant studies were identified and included in the review; 2. all the studies included in the review had similar designs and methods; 3. relevant data for all studies were obtained; and 4. all the studies included in the review shared the same main primary outcome, and this outcome is the outcome of interest for this review.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the only systematic review published to date on this topic. Our findings are consistent with contemporary data regarding the safety of surveillance in more recent evidence from nationwide screening programmes for AAA in men in England and Sweden (Oliver‐Williams 2019; Wanhainen 2016).

Risk of bias graph: review authors' judgements about each risk of bias item for the outcomes of mortality and cost presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1: Early repair compared to ultrasound surveillance for small asymptomatic AAA, Outcome 1: Health service costs
| Early open repair compared to ultrasound surveillance for small asymptomatic AAA | ||||||
| Patient or population: small asymptomatic AAA Setting: hospital Intervention: early open repair (open surgery) Comparison: ultrasound surveillance | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with ultrasound surveillance | Risk with early repair | |||||
| Mortality (follow‐up to 6 years) | Study population | HR 0.99 (CI 0.83 to 1.18)a | 2226 (2 RCTs) | ⊕⊕⊕⊕ | No clear evidence to support a difference in survival between early open repair and surveillance. | |
| 0.28 (0.25 to 0.31) | 0.30 (0.27 to 0.33) | |||||
| Costs (per participant) (follow‐up to 18 months) | GBP 3914 | GBP 4978 | MD GBP 1064higher | 1090 | ⊕⊕⊕⊝ | In UKSAT, the mean health service costs per participant were higher in the surgery than the surveillance group, and remained higher at 12‐years of follow‐up. This estimate accounted for semi‐annual surveillance visits, aneurysm repair, and any associated follow‐up. |
| QoLc (follow‐up to 24 months) | See comment | 2226 (2 RCTs) | — | In UKSAT, early‐surgery survivors reported minor improvements in MOS‐20 based current health perceptions and less negative changes in bodily pain (after 1 year). In ADAM, early‐surgery and surveillance groups did not differ for most SF‐36 scales, but the study authors reported that the early‐surgery group had better scores for general health and lower scores for vitality (during the first 2 years); more participants became impotent after early repair compared with surveillance (after 1 year); maximum activity level declined more rapidly over time in the early‐repair group. | ||
| Aneurysm rupture (follow‐up 6 years ) | See comment | 2226 (2 RCTs) | — | In UKSAT, there were 25 ruptures – at least 17 in the surveillance group vs ≥ 6 in the early‐repair group (2 with emergency repairs, group unknown). 15/25 had an aneurysm diameter < 5.5 cm at previous follow‐up. In ADAM, there were 13 ruptures – 11 in the surveillance group and 2 in the early‐repair group (last diameter preceding rupture was not reported). | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AAA: abdominal aortic aneurysm; CI: confidence interval; EVAR: endovascular aneurysm repair; GBP: Great British pounds; HR: hazard ratio; MD: mean difference; MOS‐20: 20‐item Medical Outcomes Study; QoL: quality of life; RCT: randomised controlled trial; SF‐36: 36‐item Short Form. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aFrom pooled individual participant analysis (estimated from Figure 1 in Filardo 2013). | ||||||
| Early endovascular repair compared to ultrasound surveillance for small asymptomatic AAA | ||||||
| Patient or population: small asymptomatic AAA Setting: hospital Intervention: early endovascular repair (EVAR) Comparison: ultrasound surveillance | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with ultrasound surveillance | Risk with early repair | |||||
| Mortality (follow‐up at 1 year) | See comment | RR 1.92 | 846 | ⊕⊕⊝⊝ | Surveillance group: 6 deaths, 408 alive, 126 lost to follow‐up; EVAR: 12 deaths, 420 live, 116 lost to follow‐up. Neither trial reached target sample size. | |
| Costs (follow‐up at 6 months) | USD 5520 | USD 33,471 | MD 27,951 USD higher (25,156 higher to 30,746 higher ) | 614 | ⊕⊕⊝⊝ | Hospital costs were higher in the EVAR group at 6 months. |
| Costs (follow‐up to 48 months) | USD 46,112 | USD 48,669 | MD 2557 USD higher | 614 | ⊕⊕⊝⊝ | No clear evidence to support a difference in total medical costs between groups by 48 months. |
| QoL (follow‐up to 24 months) | See comment | 605 | — | CAESAR reported similar SF‐36 scores between the groups in all domains (after 1 year). PIVOTAL reported no treatment‐related differences in EQ‐5D scores (24 months). | ||
| Aneurysm rupture (overall follow‐up) | See comment | 1088 (2 RCTs) | — | In CAESAR no ruptures observed for aneurysms < 5.5 cm diameter (2 ruptures in the surveillance group that exceeded repair threshold). PIVOTAL reported 1 rupture in the EVAR group and 2 ruptures in the surveillance group. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AAA: abdominal aortic aneurysm; CI: confidence interval; EVAR: endovascular aneurysm repair; MD: mean difference; QoL: quality of life; RCT: randomised controlled trial; RR: risk ratio; USD: United States dollars. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aAs no deaths occurred in the CAESAR surveillance group, summary data from Kaplan‐Meier plots was used to pool data for deaths at one year (CAESAR and PIVOTAL). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Health service costs Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1.1 Open repair (GBP) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1.2 EVAR (USD) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |

