Operacija za kompletan prolaps rektuma kod odraslih
Abstract
Background
Complete (full‐thickness) rectal prolapse is a lifestyle‐altering disability that commonly affects older people. The range of surgical methods available to correct the underlying pelvic floor defects in full‐thickness rectal prolapse reflects the lack of consensus regarding the best operation.
Objectives
To assess the effects of different surgical repairs for complete (full‐thickness) rectal prolapse.
Search methods
We searched the Cochrane Incontinence Group Specialised Register up to 3 February 2015; it contains trials from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE in process, ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) as well as trials identified through handsearches of journals and conference proceedings. We also searched EMBASE and EMBASE Classic (1947 to February 2015) and PubMed (January 1950 to December 2014), and we specifically handsearched theBritish Journal of Surgery (January 1995 to June 2014), Diseases of the Colon and Rectum (January 1995 to June 2014) and Colorectal Diseases (January 2000 to June 2014), as well as the proceedings of the Association of Coloproctology meetings (January 2000 to December 2014). Finally, we handsearched reference lists of all relevant articles to identify additional trials.
Selection criteria
All randomised controlled trials (RCTs) of surgery for managing full‐thickness rectal prolapse in adults.
Data collection and analysis
Two reviewers independently selected studies from the literature searches, assessed the methodological quality of eligible trials and extracted data. The four primary outcome measures were the number of patients with recurrent rectal prolapse, number of patients with residual mucosal prolapse, number of patients with faecal incontinence and number of patients with constipation.
Main results
We included 15 RCTs involving 1007 participants in this third review update. One trial compared abdominal with perineal approaches to surgery, three trials compared fixation methods, three trials looked at the effects of lateral ligament division, one trial compared techniques of rectosigmoidectomy, two trials compared laparoscopic with open surgery, and two trials compared resection with no resection rectopexy. One new trial compared rectopexy versus rectal mobilisation only (no rectopexy), performed with either open or laparoscopic surgery. One new trial compared different techniques used in perineal surgery, and another included three comparisons: abdominal versus perineal surgery, resection versus no resection rectopexy in abdominal surgery and different techniques used in perineal surgery.
The heterogeneity of the trial objectives, interventions and outcomes made analysis difficult. Many review objectives were covered by only one or two studies with small numbers of participants. Given these caveats, there is insufficient data to say which of the abdominal and perineal approaches are most effective. There were no detectable differences between the methods used for fixation during rectopexy. Division, rather than preservation, of the lateral ligaments was associated with less recurrent prolapse but more postoperative constipation. Laparoscopic rectopexy was associated with fewer postoperative complications and shorter hospital stay than open rectopexy. Bowel resection during rectopexy was associated with lower rates of constipation. Recurrence of full‐thickness prolapse was greater for mobilisation of the rectum only compared with rectopexy. There were no differences in quality of life for patients who underwent the different kinds of prolapse surgery.
Authors' conclusions
The lack of high quality evidence on different techniques, together with the small sample size of included trials and their methodological weaknesses, severely limit the usefulness of this review for guiding practice. It is impossible to identify or refute clinically important differences between the alternative surgical operations. Longer follow‐up with current studies and larger rigorous trials are needed to improve the evidence base and to define the optimum surgical treatment for full‐thickness rectal prolapse.
PICO
Laički sažetak
Operacija za potpuni rektalni prolaps u odraslih
Važnost pregleda literature
Kompletni rektalni prolaps je stanje kada donji dio crijeva (rektum) postaje labav i ispada (prolabira) pri naprezanju. Ne smije se zamijeniti s hemeroidima, stanja koje nastaje kad vene oko rektuma nabubre. Rektalni prolaps je najčešći kod starijih ljudi, posebno žena, iako je uzrok nejasan. Rektalni prolaps može izazvati komplikacije, poput boli, ulceracije, krvarenje i fekalnu inkontinenciju (nemogućnost kontroliranja rada crijeva). Uobičajen način liječenja prolapsa uključuje kirurški zahvat.
Glavni rezultati pregleda
Kad se usporedi zahvat koji se izvodi putem reza na trbuhu ili rezom kroz anus (poznat kao perinealni pristup), nema razlike u učestalosti ponovne pojave prolapsa ili pojave postoperativnih komplikacija. Kad kirurzi obave operaciju kroz mali otvor na trbuhu (laparoskopske operacije) oporavak može biti brži nego kod otvorene trbušne operacije. Ukoliko je zatvor jedan od glavnih simptoma, crijevna resekcija (uklanjanje dijela crijeva) za vrijeme operacije prolapsa može pomoći. Nije bilo razlike u rezultatima kada su korišteni drugačiji oblici zahvata prilikom analnog pristupa.
Štetni učinci
Nije bilo značajnih problema vezano za štetne učinke različitih vrsta operacije opisanih u ovom pregledu.
Ograničenja sustavnog pregleda
Iako je u ovaj sustavni pregled literature uključeno 15 studija, mnogi od njih imali su različite usporedbe i neki su imali i loše metode, što ograničava upotrebljivost rezultata. Međutim, duža praćenja pacijenata koji su sudjelovali u tim studijama, zajedno s rezultatima studija koje su trenutno u tijeku, mogu dati bolje informacije za budućnost.
Authors' conclusions
Summary of findings
| Perineal compared with abdominal approach for full‐thickness rectal prolapse in adults | ||||||
| Patients: Adults with full‐thickness rectal prolapse Setting: Surgical centres in India, Finland, Serbia, Spain, UK Interventions: perineal versus abdominal surgery | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk (with abdominal approach) | Corresponding risk (with perineal approach) | |||||
| Number of patients with recurrent full‐thickness prolapse | Moderate risk (study population) | OR 0.7 (0.17 to 2.88) | 44 (1 RCT) | ⊕⊕⊕ | A pragmatic trial, participants could be randomised between abdominal or perineal surgery. The abdominal procedure was performed through an open or laparoscopic approach depending on surgeon's preference. For perineal surgery, participants could be randomised to a Delorme's or an Altemeier's procedure. It was the surgeon's choice to participate in either or both of the randomisations. | |
| 263 per 1000 | 200 per 1000 | |||||
| Vaizey incontinence score 3 years post‐op | The mean Vaizey incontinence score 3 years post‐op in the control group was 4.6 | The mean Vaizey incontinence score 3 years post‐op in the intervention group was 5 higher (5.44 lower to 6.24 higher) | — | 16 (1 RCT) | ⊕⊕⊕ | The Vaizey scores ranged from 0 (perfect continence) to 24 (totally incontinent) |
| Number of patients with postoperative complications | Moderate risk (study population) | OR 0.65 (0.19 to 2.23) | 44 (1 RCT) | ⊕⊕⊕ | — | |
| 421 per 1000 | 321 per 1000 (121 to 619) | |||||
| Bowel function (bowel thermometer) 3 years post‐op | The mean bowel function (bowel thermometer) 3 years post‐op in the control group was 52 | The mean bowel function (bowel thermometer) 3 years post‐op in the intervention group was 50 higher (31.69 lower to 27.69 higher) | — | 9 (1 RCT) | ⊕⊕⊕ | Bowel function rated by participants, 0 (worst) to 100 (best) |
| Quality of life score (EQ‐5D) at 3 years | The mean quality of life score (EQ‐5D) at 3 years in the control group was 0.73 | the mean quality of life score (EQ‐5D) at 3 years in the intervention group was 0.86 higher (0.14 lower to 0.4 higher) | — | 14 (1 RCT) | ⊕⊕⊕ | EQ‐5D quality of life scores range from − 0.59 (worst) − 1.0 (perfect health) |
| Straining at 3 years post‐op | Moderate risk (study population) | OR 0.06 (0 to 1.33) | 20 (1 RCT) | ⊕⊕⊕ | — | |
| 455 per 1000 | 48 per 1000 (0 to 526) | |||||
| CI: Confidence interval; OR: Odds Ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for imprecision; single trial with small sample size and wide confidence interval | ||||||
Background
Description of the condition
Complete (full‐thickness) rectal prolapse, also known as procidentia, is the circumferential protrusion through the anus of all layers of the rectal wall. It should not be confused with haemorrhoids, which are enlarged cushions of vascular tissue found within the anal canal in the submucosal space. Full‐thickness rectal prolapse is a distressing and demoralising condition and can result in serious but rare complications such as gangrene and perforation. Although it can occur in any age group, it is most common in older women. This review assesses some surgical techniques in current clinical practice for rectal prolapse in adults.
The underlying cause of rectal prolapse remains unclear, although there are some known risk factors, including an abnormally deep Pouch of Douglas (the cavity between the rectum and the posterior wall of the uterus); lax muscles in the pelvic floor and anal canal; weak internal and external anal sphincters, often with evidence of pudendal nerve neuropathy; and abnormal fixation of the rectum, with a mobile mesorectum and lax lateral ligaments (Madiba 2005). Other predisposing factors include neurological illnesses, connective tissue disorders, and high parity (Schoetz 1985; Marshman 1987; Karasick 1997).
Rectal prolapse may result in acute complications of the prolapse itself (pain, ulceration, bleeding, incarceration and gangrene) or chronic debilitating symptoms such as difficulty maintaining perianal hygiene (faecal incontinence, mucus discharge).
Description of the intervention
The only potentially curative treatment for full‐thickness rectal prolapse is surgery. However, for people who are unfit for surgery, high fibre intake with stool softener may help if constipation is a predominant symptom (Phillips 2005). A range of surgical interventions are available, which are similar in principle although technically different in a number of respects. Differences include the surgical approach to the prolapsed bowel (transabdominal, open versus laparoscopic, perineal), the method of fixation during the rectopexy (suture or mesh), and the performance of a bowel resection (removal of a portion of the intestine). The choice of synthetic material used to perform the rectopexy may also vary and can include nylon, Teflon, Marlex, Ivalon, Gore‐Tex, Vicryl or Dexon. There is little information on the best option for failed primary surgical treatment, but repeat surgery can be successful (Fengler 1997; Pikarsky 2000).
The primary surgical interventions are the following.
-
Anal encirclement operation. This procedure is generally reserved for debilitated or other individuals at high anaesthetic risk. Under local anaesthesia, and after reduction of the prolapse, a subcutaneous suture is encircled around the anal orifice. This is then tightened to prevent further prolapse. A variety of suture materials may be used, including silicone rubber impregnated with Dacron, knitted Dacron or polypropylene mesh.
-
Perineal resection. There are two main methods of perineal resection.
-
Perineal rectosigmoidectomy aims to resect (remove) the redundant bowel via the perineum and anchor the lower rectum to the sacrum through fibrosis in the hope of preventing future prolapse. It may be combined with a procedure to tighten the pelvic muscles (levatorplasty).
-
The Delorme's procedure is a modification of perineal rectosigmoidectomy in which there is no resection of the prolapsed bowel. Instead, the mucosa is stripped and the muscle layer plicated and placed as a buttress above the pelvic floor. It may be combined with levatorplasty.
-
-
Transabdominal rectopexy. The aim of this procedure is to anchor the rectum to the sacrum without resection. There are various modifications to the procedure, including the following.
-
Use of different fixation materials (mesh made from different materials such as nylon, Teflon, Marlex, Ivalon, Gore‐Tex, Vicryl or Dexon, or simple sutures using materials such as prolene).
-
Placement of the mesh (anterior or ventral, posterior, completely or partially encircling the rectum).
-
Full rectal mobilisation with division or preservation of lateral ligaments prior to fixation.
-
Open or laparoscopic access.
-
-
Transabdominal resection. This is similar to perineal resection except that it is performed through the abdomen. However, the extent of the bowel resection (removal) may be more extensive and varied (sigmoid colon, rectosigmoid or subtotal colon). This procedure may be combined with rectopexy (attachment of the rectum to the sacrum), but the redundant colon is resected (removed) first.
-
Transabdominal mobilisation of rectum. This is similar to transabdominal rectopexy, except the rectum is not fixed by suture or mesh, relying on the formation of adhesions between the mobilised rectum and the sacrum without artificial fixation.
Why it is important to do this review
Selection of the surgical technique for rectal prolapse is difficult, and results appear to vary depending on the technique used (Eu 1997). However, studies suggest prolapse recurrence might not relate to the technical complexity of the surgery (Nelson 2001; Raftopoulos 2005). The purpose of this review is to investigate the effectiveness of surgical techniques in current clinical practice for the repair of full‐thickness rectal prolapse in adults. This condition may co‐occur with other pelvic organ prolapse; however, it is beyond the scope of this study to provide a comprehensive review of other conditions. Operative procedures designed to repair specific anal sphincter or pelvic floor defects have been covered in a separate Cochrane Review (Brown 2013), as has the surgical management of rectocoele (Maher 2013). Likewise, we did not include rectal intussusception (internal or occult rectal prolapse) in our review, as it is often associated with other pelvic floor abnormalities, including rectocoele and enterocoele.
Objectives
To assess the effects of different surgical repairs for complete (full‐thickness) rectal prolapse.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and quasi‐RCTs of surgery for managing full‐thickness rectal prolapse.
Types of participants
All adults diagnosed with full‐thickness rectal prolapse.
Types of interventions
Eligible studies whose interventions include one or more of the following, as described in the Background.
-
Anal encirclement operation.
-
Perineal resection.
-
Transabdominal rectopexy.
-
Tranabdominal resection.
-
Transabdominal mobilisation of rectum.
Types of outcome measures
There are a range of different dimensions to the outcome of surgery for rectal prolapse, as reflected in the list of measures below. Nevertheless, because of the dangers of multiple statistical testing and data dependent reporting, we have selected specific measures of poor outcome as primary measures, presenting them in tabular form regardless of whether or not data were available. We also sought data describing the secondary outcomes, but we only tabulated them if data were available.
Primary outcomes
-
Number of people with recurrent full‐thickness rectal prolapse
-
Number of people with residual mucosal prolapse
-
Number of people with faecal incontinence (or incontinence score)
-
Number of people with constipation (or delayed gut transit time)
Secondary outcomes
-
Participant symptoms: failed treatment as judged by the need for additional therapy (drugs, dietary changes, repeat interventions for either full‐thickness or residual mucosal prolapse)
-
Clinical end points
-
Operative time
-
Postoperative morbidity (e.g. bleeding, wound infection, pelvic infection, mesh infection, anastomotic complications)
-
Number of people with adverse effects (e.g. chronic wound pain)
-
Length of hospital stay, recovery time
-
Readmission rates
-
Postoperative mortality
-
Bowel function, including defecatory frequency, defecatory problems, straining
-
-
Physiological measures
-
Anal canal pressure profiles (resting and squeeze pressure of anal sphincters)
-
Rectal compliance (change in rectal pressure in relation to change in volume, a measure of rectal wall stiffness)
-
Rectal capacity
-
Rectal sensation (detection of rectal sensation, i.e. perception of need to defecate when distending a rectal balloon with increasing volume). Three common measurements include threshold volume to distension, desire to defecate and maximum volume.
-
Colonic transit time studies
-
Anorectal angle on videoproctography (this angle may affect continence)
-
-
Health status measures
-
Condition‐specific health outcomes
-
Psychological measures (e.g. the Hospital Anxiety and Depression Scale; Zigmond 1983)
-
General health measures (e.g. the 36‐item Short Form Health Survey (SF‐36); Ware 1993)
-
-
Health economic measures: cost utility based on quality of life assessments
We adopted GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach for assessing the quality of evidence. The primary and secondary outcomes were classified as 'critical', 'important' or 'not important' for decision making from the patients' perspective. The GRADE working group strongly recommends including up to seven outcomes in the summary of findings table (Guyatt 2011; Guyatt 2011a; Guyatt 2013; Guyatt 2013a). We selected the following outcomes:
-
Number of patients with recurrent full‐thickness prolapse
-
Vaizey incontinence score 3 years post‐op
-
Number of patients with postoperative complications
-
Bowel function (bowel thermometer) 3 years post‐op
-
Quality of life score (EQ‐5D) at 3 years
-
Straining at 3 years post‐op
We used GRADEPro in order to generate Summary of Findings Table (GRADEpro GDT 2015).
Search methods for identification of studies
We did not impose any language or date restrictions on the searches. We did not include studies where rectal prolapse surgery was combined with other pelvic organ prolapse surgery in women.
Electronic searches
For this review, we used the search strategy developed for the Incontinence Review Group to identify relevant trials from the Incontinence Group Specialised Register of Controlled Trials. For more details of the search methods used to build the Specialised Register, please see the Group's module in the Cochrane Library (http://onlinelibrary.wiley.com/o/cochrane/clabout/articles/INCONT/frame.html). The Register contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE in process, ClinicalTrials.gov, the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) and handsearches of journals and conference proceedings. Most of the trials in the Cochrane Incontinence Group Specialised Register are also contained in CENTRAL. The date of the most recent search was 3 February 2015. We specify the terms used to search the Incontinence Group Specialised Register in Appendix 1.
We also searched EMBASE and EMBASE Classic (1947 to February 2015) on OvidSP, using the strategy described in Appendix 1.
The review authors also performed an additional specific search of PubMed (January 1950 to December 2014); see Appendix 1 for details.
Searching other resources
The review authors handsearched the reference lists of all relevant articles as well as several journals: the British Journal of Surgery (January 1995 to June 2014), Diseases of the Colon and Rectum (January 1995 to June 2014) and Colorectal Diseases (January 2000 to June 2014). We also handsearched the conference proceedings of the Association of Coloproctology (January 2000 to December 2014).
Data collection and analysis
Selection of studies
Two review authors (ST, SB) examined all the references and abstracts generated from the electronic searches, retrieving the full‐text reports of all potentially relevant trials. The two review authors independently identified trials, resolving any disagreements through discussion with the third reviewer (RLN). Review authors were not blind to the names of authors, institutions or journals. We excluded studies that were not RCTs or if they did not make the prespecified comparisons.
Data extraction and management
Two review authors (ST, SB) independently extracted data from the included trials as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any differences of opinion through group discussion. We had to extract some data directly from figures in the publication, and we tried to obtain other information from the trials' authors (Senapati 2013). All other data were published data only.
Assessment of risk of bias in included studies
Two review authors (ST, SB) independently assessed the methodological quality of identified trials according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed bias according to six domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and 'other' issues. We described the domains for each study as being at 'high', 'low' or 'unclear' risk of bias based on available information.
Measures of treatment effect
We presented dichotomous outcomes as odds ratios (ORs), and continuous outcomes as mean differences (MDs) if scales were comparable or standardised mean differences (SMD) if they were non‐comparable. We used 95% confidence intervals (CIs) to measure precision of treatment effect.
Unit of analysis issues
We considered individual participants to be the unit of allocation.
Dealing with missing data
We contacted trial authors for data that may have been collected but not reported. Where trialists reported the results in terms of the mean and standard error (SE), we calculated the standard deviation (SD) using the relationship defined by the equation:
SD = SE x √s
where s represents the sample size.
When only means and ranges were available, we estimated the standard deviation from the range (range x 0.95/4).
Assessment of heterogeneity
We examined the trial methods and descriptions in the Characteristics of included studies tables to identify clinical heterogeneity. We assessed the statistical heterogeneity using the Chi2 test, where a P value of 0.10 was the cutoff value to determine statistical significance, and the I2 statistic, where 0% to 40% signifies that the statistical heterogeneity might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity and 75% to 100% represents considerable heterogeneity (Higgins 2011).
Assessment of reporting biases
We did not use funnel plots to report biases, as the number of trials for each type of intervention was never more than five.
Data synthesis
We analysed data were using Review Manager (RevMan 2012).
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was not possible due to the limited number of identified trials for each type of intervention.
Sensitivity analysis
Sensitivity analysis was not possible due to the limited number of identified trials for each type of intervention.
Results
Description of studies
Results of the search
We identified a total of 415 records through the literature search and retrieved 31 potentially relevant full‐text articles for further assessment. We finally included 15 eligible studies (20 records) and excluded 3 studies (3 records; Gupta 2006; Nelson 2001; Raftopoulos 2005); see the Characteristics of excluded studies table for details. We also found 10 reports of 5 ongoing studies (Tarquini 2010; DeloRes 2012; Makela‐Kaikkonen 2013; ACTRN12605000748617; NCT00946205). Figure 1 shows the assessment process in a PRISMA flowchart.

PRISMA study flow diagram.
Included studies
We have added 3 new trials to this third update of the review (Karas 2011; Senapati 2013; Youssef 2013), bringing the total number of included RCTs to 15. Fourteen trials were published in a full paper format and one trial was published as an abstract. There were a total of 1007 participants included in the 15 trials (Characteristics of included studies table).
-
One trial compared abdominal resectional rectopexy and pelvic floor repair with the perineal approach (Deen 1994).
-
Five trials compared different methods of rectopexy (Speakman 1991; Selvaggi 1993; Winde 1993; Novell 1994; Galili 1997).
-
Three trials examined the difference in abdominal rectopexy with and without division of the lateral ligament (Speakman 1991; Selvaggi 1993; Mollen 2000).
-
Three trials compared polyglycolic acid mesh with polypropylene or polyglactin mesh (Winde 1993; Galili 1997; Mollen 2000).
-
One trial assessed the results of Ivalon sponge rectopexy with open suture rectopexy (Novell 1994).
-
Two trials compared the outcomes of rectopexy with and without bowel resection (Lukkonen 1992; McKee 1992).
-
Two trials compared the outcomes of laparoscopic and open abdominal rectopexy (Boccasanta 1998; Solomon 2002).
-
One trial compared the outcomes between two different techniques used in perineal rectosigmoidectomy (Boccasanta 2006).
-
Two trials examined the cost benefits between laparoscopic and open abdominal rectopexy (Boccasanta 1998; Solomon 2002).
-
One trial compared the outcomes of abdominal rectopexy versus mobilisation of rectum and no rectopexy (Karas 2011).
-
One trial compared the Delorme's procedure with and without levatorplasty (Youssef 2013).
-
One trial made three comparisons: abdominal versus perineal approach, resection versus no resection in abdominal approach and Altemeier's versus Delorme's procedure in perineal approach (Senapati 2013).
All studies apart from Karas 2011 and Senapati 2013 took place in a single surgical centre. Four were conducted at centres in the United Kingdom (Speakman 1991; McKee 1992; Deen 1994; Novell 1994), three in Italy (Selvaggi 1993; Boccasanta 1998; Boccasanta 2006), one in Australia (Solomon 2002) and one each in Finland (Lukkonen 1992), Germany (Winde 1993), Israel (Galili 1997), the Netherlands (Mollen 2000) and Egypt (Youssef 2013). Nineteen countries participated in Karas 2011 (Austria, Brazil, Canada, Czech Republic, Eygpt, Greece, Hungary, India, Iran, Italy, Korea, Lithuania, New Zealand, Poland, Serbia, Spain, Switzerland, Turkey, United States) and five in Senapati 2013 (India, Finland, Serbia, Spain, United Kingdom)
Length of follow‐up varied between and within the trials, lasting:
-
≤ a year (Speakman 1991; Lukkonen 1992; Youssef 2013);
-
> 1 and ≤ 3 years (McKee 1992; Selvaggi 1993; Deen 1994; Boccasanta 1998; Solomon 2002; Boccasanta 2006; Senapati 2013);
-
> 3 years and ≤ 5 years (Winde 1993; Novell 1994; Galili 1997; Mollen 2000; Karas 2011).
We present further details in the Characteristics of included studies table.
Excluded studies
We excluded Gupta 2006 because the participants did not have full‐thickness rectal prolapse. The other two studies were not RCTs (Nelson 2001; Raftopoulos 2005; Characteristics of excluded studies).
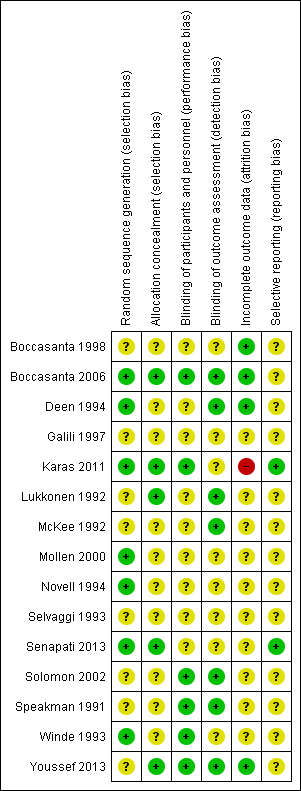
Risk of bias in included studies
Overall, trials included in this review had major methodological limitations, which may have introduced different forms of bias (Figure 2; Figure 3). Despite a potentially heterogenous group of people, only five trials clearly described the inclusion/exclusion criteria (Solomon 2002; Boccasanta 2006; Karas 2011; Senapati 2013; Youssef 2013). Follow‐up periods ranged from 6 months in Lukkonen 1992 to 60 months in Karas 2011.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
With regard to sequence generation, eight trials did not mention methods of randomisation (Speakman 1991; Lukkonen 1992; McKee 1992; Selvaggi 1993; Galili 1997; Boccasanta 1998; Solomon 2002; Youssef 2013).
Only five trials mentioned the allocation concealment methods (Lukkonen 1992; Boccasanta 2006; Karas 2011; Senapati 2013; Youssef 2013). Of these studies, three used sealed envelopes (Lukkonen 1992; Boccasanta 2006; Youssef 2013), and two used central allocation (Karas 2011; Senapati 2013). These methods had a low risk of bias.
Blinding
There were only four trials where the assessors were blind to the interventions given (Speakman 1991; Solomon 2002; Boccasanta 2006; Youssef 2013) and were classified as having a low risk of bias. We assessed three further studies as being at low risk of bias, as there was an outcome assessor independent from the surgical team in Deen 1994, and Lukkonen 1992 and McKee 1992 used objective outcome measurements. We assessed eight studies as having an unclear risk of bias as they did not provide any information on blinding of the assessor (Selvaggi 1993; Winde 1993; Novell 1994; Galili 1997; Boccasanta 1998; Mollen 2000; Karas 2011; Senapati 2013).
Incomplete outcome data
Seven trials mentioned patient withdrawals (McKee 1992; Novell 1994; Solomon 2002; Boccasanta 2006; Karas 2011; Senapati 2013; Youssef 2013). The withdrawal/dropout rate ranged from 0% in Boccasanta 2006 to 10% in Karas 2011, with similar rates between the intervention groups apart from in Karas 2011, in which there were 8/136 (6%) lost to follow‐up in the rectopexy group and 18/116 (16%) in the no rectopexy group. As a result we assessed this trial being at a high risk of attrition bias. We assessed four trials as being at a low risk of attrition bias, as there was no missing data in three (Deen 1994; Boccasanta 1998; Boccasanta 2006), and the dropout rate between the two intervention arms was similar in the other (Youssef 2013).
Selective reporting
Study protocols were available for three studies (Karas 2011; Senapati 2013; Youssef 2013). Both Karas 2011 and Senapati 2013 were assessed to have low risk of reporting bias as the investigators had reported all the outcomes that were pre‐specified the protocols. The protocol for Youssef 2013 was published after the study had finished recruiting participants; although it did not mention a patient satisfaction score, the paper did present one. We considered that this study, together with the rest of the trials, had an unclear reporting bias, as there was insufficient information available to judge whether they were at high or low risk of bias.
Other potential sources of bias
There was considerable variation in the reporting of participant assessment before surgery. Only two trials (Karas 2011; Senapati 2013) carried out a power calculation, and only five reached a sample size greater than 50 participants (Novell 1994; Boccasanta 2006; Karas 2011; Senapati 2013; Youssef 2013). The Characteristics of included studies table contains further details.
Effects of interventions
Altogether, the 15 included trials made the following comparisons:
-
Conventional diathermy and handsewn rectosigmoidectomy versus harmonic scalpel and stapled technique.
-
Comparisons of different perineal approaches.
-
Open abdominal Ivalon sponge rectopexy versus open abdominal suture rectopexy.
-
Open abdominal polyglycolic acid mesh versus open polyglactin or polypropylene mesh rectopexy.
-
Preservation versus division of the lateral ligaments during open mesh rectopexy.
-
Laparoscopic versus open procedure.
-
Abdominal versus perineal approach.
-
Resection versus no resection rectopexy.
-
Rectopexy versus no rectopexy.
There were no trials on anal encirclement operation.
Comparison 1: Conventional diathermy and handsewn rectosigmoidectomy versus harmonic scalpel and stapled technique
Boccasanta 2006 evaluated whether emerging surgical techniques improved patient outcomes, comparing traditional monopolar electrocautery and handsewn anastomosis with harmonic scalpel (Ultracision) and circular stapler during rectosigmoidectomy and pelvic floor repair.
Primary outcomes
The only primary outcome measured was recurrent rectal prolapse, which occurred in 3/20 (15%) people in the handsewn group compared to 2/20 (10%) in the stapled group (Analysis 1.1). There was no significant difference in the incontinence score at 12 months after surgery between both groups (Analysis 1.2).
Secondary outcomes
Investigators reported on five secondary outcomes: postoperative morbidity, length of hospital stay (Analysis 1.3), recovery time (Analysis 1.4), defecating problems (Analysis 1.5) and physiological parameters (Analysis 1.6; Analysis 1.7; Analysis 1.8). Two of 10 people in the handsewn group developed stenosis, which was treated by transanal dilatation. Although defecatory problems (dyschezia, tenesmus, rectal bleeding) and incontinence scores improved from baseline in both groups, the trial was too small to detect statistically significant differences between the groups in any of the physiological outcomes.
Comparison 2: Comparisons of different perineal approaches
Delorme's procedure with levatorplasty versus Delorme's procedure without levatorplasty
One trial examined this comparison (Youssef 2013).
Primary outcomes
There was less prolapse recurrence using the Delorme's with levatorplasty (1/41) compared to Delorme's without levatorplasty (6/41), but the difference was not significant (Analysis 2.1). The number of people with residual faecal incontinence was significantly lower in Delorme's with levatorplasty compared to Delorme's without levatorplasty (OR 0.07, 95% CI 0.01 to 0.56; Analysis 2.2).. There was also no difference in the incidence of constipation between a Delorme's with levatorplasty and a Delorme's without levatorplasty (Analysis 2.3).
Secondary outcomes
Operating time was significantly longer in the Delorme's with levatorplasty group compared to the regular Delorme's group (OR 29.40, 95% CI 22.72 to 36.08; Analysis 2.4). However, there was no difference in postoperative complications or length of hospital stay between these two approaches (Analysis 2.5; Analysis 2.6). Resting and squeeze pressures were significantly higher in the Delorme's with levatorplasty group compared to the Delorme's without levatorplasty group (Analysis 2.7; Analysis 2.8). The rectal sensation improved in both groups after surgery, but the Delorme’s with levatorplasty group required less volume at which rectal sensation was first perceived, suggesting a better functional outcome (Analysis 2.9). The satisfaction score was significantly higher in people who had Delorme's with levatorplasty (Analysis 2.10).
Altemeier's versus Delorme's procedure
One trial examined this comparison (Senapati 2013).
Primary outcomes
There was no significant difference between recurrent rectal prolapse after an Altemeier's procedure (24/102) versus a Delorme's procedure (31/99; Analysis 2.11), and no difference in incontinence score between the Altemeier's and Delorme's procedure groups (Analysis 2.12).
Secondary outcomes
There was no difference in overall bowel function or quality of life score at three years postsurgery (Analysis 2.13; Analysis 2.14).
Comparison 3. Open abdominal Ivalon sponge rectopexy versus open abdominal suture rectopexy
A single trial of 63 participants compared open abdominal Ivalon sponge rectopexy versus open abdominal suture rectopexy; 31 underwent Ivalon sponge rectopexy and 32 underwent suture rectopexy (Novell 1994).
Primary outcomes
The study reported three of the four primary outcomes contemplated: recurrent full‐thickness prolapse, residual faecal incontinence and constipation. There was one recurrent rectal prolapse from each group (Analysis 3.1). At median follow‐up (47 months), 9/31 people (29%) who had had Ivalon sponge rectopexy and 5/32 (16%) of people who had received suture rectopexy complained of faecal incontinence, but the difference was not significant (OR 2.21, 95% CI 0.65 to 7.56; Analysis 3.2). Postoperative constipation occurred in 15/31 (48%) people in the Ivalon sponge rectopexy group, compared with 10/32 (31%) people in the sutured rectopexy group (Analysis 3.3, not significant).
Secondary outcomes
The trial reported three secondary outcomes: postoperative mortality, postoperative morbidity and length of hospital stay. There was no 30‐day mortality in either group. In the Ivalon sponge group, 6/31 (19%) of participants had postoperative complications compared with 3/32 (9%) in the sutured rectopexy group (Analysis 3.4), but this was not significant. Median hospital stay was 14 days in both groups.
Comparison 4. Open abdominal polyglycolic acid mesh versus open polyglactin or polypropylene mesh rectopexy
Two small trials compared different mesh materials for open abdominal rectopexy: Winde 1993 compared polyglycolic acid mesh versus polyglactin mesh, and Galili 1997 compared polyglycolic acid mesh versus polypropylene mesh.
Primary outcomes
There were no recurrent rectal prolapses in either group in Winde 1993, but there was one in Galili 1997 from the group treated with polyglycolic acid mesh (Analysis 4.1). There were no statistically significant differences for residual mucosal prolapse between the trial groups, but the confidence intervals were wide (Analysis 4.2). Three of 15 participants (20%) in Winde 1993 complained of residual faecal incontinence after polyglycolic acid mesh rectopexy, compared with 7/20 (35%) in Galili 1997 after polyglactin mesh rectopexy (Analysis 4.3, not significant). In Galili 1997, there was no statistically significant difference in the faecal incontinence scores (Analysis 4.4) and only two reports of postoperative constipation (Analysis 4.5).
Secondary outcomes
There were few data for participants with postoperative complications (Analysis 4.6). One trial also reported length of stay (Galili 1997), which averaged eight days for both groups. However, the trials were too small to detect differences in of these outcomes.
Comparison 5. Preservation versus division of the lateral ligaments during open mesh rectopexy
Three small trials compared the effects of preservation versus division of lateral ligaments during open mesh rectopexy (Speakman 1991; Selvaggi 1993; Mollen 2000). The Selvaggi trial was only available in abstract format, though, and we could not perform any statistical analyses due to insufficient reporting of data.
Primary outcomes
Rectal prolapse recurred in 4/21 (19%) participants following preservation of the lateral ligaments and in 0/23 participants following division of the ligaments (OR (fixed) 15.35, 95% CI 0.73 to 321.58, Analysis 5.1). In Speakman 1991, 2/12 (17%) participants developed mucosal prolapse following rectopexy with preservation of lateral ligaments compared with 0/14 in the group that had division of lateral ligaments (Analysis 5.2). Mollen 2000 reported the colonic transit times rather than the number of people with constipation due to the operation. If we assume that those with delayed colonic transit were constipated, then 4/21 (19%) people developed constipation after rectopexy with preservation of the lateral ligaments compared with 10/23 (43%) with division (OR (fixed) 0.32 CI 0.08 to 1.23, Analysis 5.3). The numbers were too low to assess differences in the constipation score (Analysis 5.4).
Secondary outcomes
Speakman 1991 reported no major complications in 26 participants (Analysis 5.5), while Mollen 2000 reported several physiological parameters (Analysis 5.6; Analysis 5.7; Analysis 5.8; Analysis 5.9). There was no difference in the parameters between intervention groups except in defecation frequency, which was higher in the group with division of lateral ligaments (MD − 1 episode per day, 95% CI − 1.39 to − 0.61; Analysis 5.6). The authors suggested that the elasticity and capacity of the rectum reduced with division of the lateral ligaments, resulting in more frequent defecation.
Comparison 6. Laparoscopic versus open procedure
Boccasanta 1998 and Solomon 2002 compared laparoscopic mesh rectopexy versus open mesh rectopexy. There was a subsequent cost analysis for participants that were involved in Solomon 2002.
Primary outcomes
Both trials reported on recurrent prolapse: in Solomon 2002, 0/20 participants in the laparoscopic group had a full thickness prolapse recurrence, compared with 1/19 after open surgery (Analysis 6.1). In Boccasanta 1998, 0/8 had a residual mucosal prolapse after laparoscopic repair versus 1/13 in the open group (Analysis 6.2).
Two other primary outcomes measured were incontinence and constipation. Solomon 2002 reported faecal incontinence scores (correlated with severity of faecal incontinence): although both groups improved from baseline, the trials were too small to detect a significant difference (Analysis 6.3). In the other trial, the faecal incontinence scores (the score is correlated with severity of faecal incontinence) were 9 (laparoscopic) versus 10 (open) respectively, without measures of dispersion (Boccasanta 1998).
Because of different assessment criteria for constipation, we could not combine results for this outcome. However, there was no statistically significant difference from baseline or between groups in a Visual Analogue Scale for constipation in Solomon 2002, and in Boccasanta 1998, the numbers were too low to to analyse a difference in constipation (Analysis 6.4).
Secondary outcomes
The two trials reported on three of the same secondary outcomes: operating time, length of hospital stay and cost analysis. In addition, Solomon 2002 reported postoperative morbidity, and Boccasanta 1998 examined some physiological parameters. Operating time was significantly longer in the laparoscopic group (MD 67 min, 95% CI 52 to 83, Analysis 6.5); Solomon 2002's results suggested that postoperative complications were significantly less common in the laparoscopic group (OR 0.15, 95% CI 0.04 to 0.62, Analysis 6.6). Hospital stay was also significantly shorter (MD 2.35 days fewer, 95% CI 1.37 to 3.33, Analysis 6.7). There was no statistically significant difference in measured physiological parameters between the two groups in Boccasanta 1998 (Analysis 6.8; Analysis 6.9; Analysis 6.10; Analysis 6.11). Regarding costs, both studies found that the laparoscopic approach was cheaper (Analysis 6.12), but the trials were carried out in different healthcare systems (USA and Australia; the Australian costs were converted to USD for this analysis).
Comparison 7: Abdominal versus perineal approach
Deen 1994 compared perineal rectosigmoidectomy and pelvic floor repair in 10 people versus abdominal resection rectopexy and pelvic floor repair in another 10. Senapati 2013 compared a perineal procedure in 25 people versus an abdominal procedure in 19. In that study, after initial randomisation, participants could undergo an abdominal or perineal procedure according to surgeon's preference or could be randomised again (Characteristics of included studies table). After full randomisation (2 x 2), 11 participants had resection rectopexy and 12 underwent Altemeier's procedure.
Primary outcomes
Deen 1994 reported data for three primary outcome measures, and Senapati 2013 reported data for two. Both trials reported on recurrence after perineal rectosigmoidectomy (Altemeier's procedure) and resection rectopexy. Overall there was no significant difference between the two approaches (odds ratio (OR) 0.64, 95% confidence interval (CI) 0.12 to 3.55; Analysis 7.1). Assessing outcomes purely from an abdominal versus a perineal approach, Senapati 2013 observed recurrence in 5 of 25 (20%) patients after perineal surgery compared with 5/19 (26%) after abdominal surgery (Analysis 7.2), again showing no significant difference. Two participants from each group in Deen 1994 complained of residual mucosal prolapse after surgery (Analysis 7.3).
There was no significant difference in Vaizey incontinence scores at three years postsurgery between an abdominal or a perineal approach (Analysis 7.4). After perineal surgery, 6/10 people continued to suffer from faecal incontinence, compared with 1/10 after abdominal surgery (Deen 1994). On the other hand, there was no significant difference in residual incontinence between Altemeier's procedure (2/12 people) and resection rectopexy (3/11 people) (OR 2.26, 95% CI 0.61 to 8.40; Analysis 7.5; Senapati 2013)).
Secondary outcomes
Deen 1994 reported four clinical end points: postoperative mortality, postoperative morbidity, need for intervention due to failed therapy and length of hospital stay. There was no mortality in this study. One patient developed anastomotic stricture after rectosigmoidectomy, which was treated with dilatation (Analysis 7.6). Two people had prolonged ileus, and one patient had wound infection after abdominal resection rectopexy (Analysis 7.7). In Senapati 2013, 8/19 people in the abdominal group experienced complications compared with 8/25 in the perineal group (Analysis 7.7). There was no difference in bowel function (from 0 = worst to 100 = best) between perineal and abdominal approaches at three years after surgery (Analysis 7.8). There were more people straining at three years after abdominal surgery than after perineal surgery, although the difference was not significant (Analysis 7.9). Deen 1994 reported three physiological outcomes, finding no significant difference in maximum resting or squeeze pressure between perineal rectosigmoidectomy and resection rectopexy (Analysis 7.10; Analysis 7.11); however, rectal compliance was significantly higher (less stiff) after abdominal surgery (MD − 1.70, 95% CI − 2.37 to − 1.03; Analysis 7.12). There was no difference in EQ‐5D (the EuroQol survey that measures quality of life in five dimensions) score converted to a health utility score between perineal and abdominal approaches at three years after surgery (Analysis 7.13).
Comparison 8: Resection versus no resection rectopexy
Three trials compared resection of redundant bowel in one group versus no resection in the other. In Lukkonen 1992, resection was combined with suture rectopexy and compared with mesh rectopexy alone. In McKee 1992 and Senapati 2013, suture rectopexy was performed in both groups but resection in only one group.
Primary outcomes
Neither Lukkonen 1992 nor McKee 1992 reported any recurrent rectal prolapse. In Senapati 2013, recurrence occurred in 4/32 of the resection group and 9/35 of the no resection group, but the difference was not significant (OR 0.41, CI 0.11 to 1.50; Analysis 8.1). Lukkonen 1992 and McKee 1992 reported residual faecal incontinence, and we were able to obtain data from the authors of Senapati 2013, who confirmed that there was no statistically significant difference between groups (18/56 (32%) incontinent in the resection group versus 20/59 (34%) in the rectopexy group; OR 0.93, 95% CI 0.43 to 2.03; Analysis 8.2). There was again no difference in the Vaizey incontinence score three years after surgery (Senapati 2013; Analysis 8.3). There was significantly less postoperative constipation in the resection group (5/42, 12% versus 20/42, 48%, OR 0.14, 95% CI 0.04 to 0.44; Analysis 8.4).
Secondary outcomes
Data on complications were available in the published report of Lukkonen 1992, and we obtained them from authors for Senapati 2013. In the meta‐analysis, there were complications in 16/47 (34%) in the resection group compared to 11/50 (22%) in the non‐resection group (Analysis 8.5).
There were virtually no differences between groups in bowel function (Analysis 8.6) and in physiological parameters including anal resting pressure (Analysis 8.7), maximum rectal volumes (Analysis 8.8), volume to first sensation (Analysis 8.9) and anorectal angle (Analysis 8.10). There was a difference between groups in rectal compliance, which was significantly higher (more compliant) in the non‐resection group (McKee 1992; Analysis 8.11), possibly because of the kinking of the redundant sigmoid colon onto the rectum, resulting in more constipation in this group of participants. Additionally, gut transit time was faster in the resection group (McKee 1992; Analysis 8.12). There was no difference in the quality of life score between the resection and no resection groups (Senapati 2013; Analysis 8.13).
Comparison 9: Rectopexy versus no rectopexy
One multicentre trial examined this comparison (Karas 2011).
Primary outcome
The only primary outcome measured was recurrence of rectal prolapse. There were 2 cases of this in the rectopexy group and 10 in the no rectopexy group, and the difference was significant (OR (fixed) 6.32, 95% CI 1.36 to 29.47, Analysis 9.1).
Secondary outcomes
There were two deaths in the rectopexy group (both died of pulmonary embolism) and none from the no rectopexy group (Analysis 9.2). There was a non‐significant difference in other complications, which occurred in 23/136 people in the rectopexy group and 11/116 in the no rectopexy group (Analysis 9.3). The median blood loss was 150 ml in the rectopexy group and 100 ml in the no rectopexy group. The median operating time was shorter in the rectopexy group (118 min) than in the no rectopexy group (145 min). There was no difference between groups in median length of hospital stay (six days).
Discussion
Summary of main results
We have identified three new eligible studies since the last review, including two multicentre trials (Karas 2011; Senapati 2013; Youssef 2013). This update included 15 randomised controlled trials with 1007 participants.
There is a tendency to carry out an abdominal procedure in young, fit people because of the perceived reduced recurrence rate compared with perineal procedures, which are reserved for more frail or unfit people. The logic is that these interventions are less invasive and therefore carry less risk of morbidity than abdominal approaches. There were only two trials that compared an abdominal with a perineal approach, but there were insufficient data to confidently comment on the difference in complications (Deen 1994; Senapati 2013). We did not see any obvious difference in recurrence between abdominal or perineal approaches. The participants are still being followed up in Senapati 2013, and it will be interesting to see the long‐term results.
Rectopexy has stood the test of time, and in experienced hands it provides good long‐term results (Byrne 2008; Foppa 2014). Five trials examined different materials used to fix the mobilised rectum, but it appears that essentially there were no differences in primary outcomes (Speakman 1991; Selvaggi 1993; Winde 1993; Novell 1994; Galili 1997).
During rectal mobilisation in rectopexy, the lateral ligaments may be preserved or divided, and evidence from non‐randomised studies suggests division may result in denervation of the rectum due to damage to the parasympathetic component of the inferior hypogastric plexus (Varma 1992; El Muhtaseb 2014). On the other hand, preservation may result in increased recurrence, presumably due to incomplete mobilisation of the rectum. The evidence is limited due to small numbers, but there appears to be more constipation among those with division of the lateral ligaments.
If constipation is one of the main symptoms, sigmoid resection during abdominal rectopexy in conjunction with prolapse repair may be advisable (Madoff 1992). Three trials in this review examined abdominal rectopexy with and without sigmoid resection, and their combined results suggest that resection does avoid constipation (Lukkonen 1992; McKee 1992; Senapati 2013). There were more complications in the resection group, but the difference was not significant.
Two non‐randomised studies suggest fixation is not necessary, and rectal mobilisation alone results in recurrence rates similar to fixation (Nelson 2001; Raftopoulos 2005). If this were true, then the potential complications associated with fixation, such as presacral bleeding or infection associated with foreign bodies used, may be obviated. However, these observations did not come from Level 1 evidence, and a subsequent multicentre trial involving 252 patients compared rectopexy versus no rectopexy (Karas 2011). Although comparatively large, this trial has some limitations, such as the use of non‐standardised surgical techniques (whether open or laparoscopic approach, extent of rectal mobilisation, methods of rectopexy, the addition of sigmoid resection) and the unusually high proportion of males. With these caveats in mind, the results do suggest that recurrence is more likely if the rectum is simply mobilised but not fixed.
Minimally invasive surgery has been gaining popularity. Two trials examined the role of laparoscopy in an abdominal approach. The trial data suggest equivalent low recurrence rates with less morbidity and a more rapid recovery for the laparoscopic group. Although equipment costs are higher, the authors of both studies suggest more rapid hospital discharge results in a cost saving compared with open surgery (Boccasanta 1998; Solomon 2002).
There are two commonly performed perineal approaches: the Delorme’s procedure is widely practised in the UK, while the Altemeier’s procedure is popular in North America. Either procedure can be combined with levatorplasty. The data is limited to two studies, and the evidence favouring one procedure or the other is either conflicting or not statistically significant. This suggests that the evidence is too limited to determine whether one perineal approach is better than another.
Will more information be available in the future to guide our practice in management of full‐thickness rectal prolapse? Results from five ongoing trials will hopefully add to our current understanding and help us to improve the management of rectal prolapse. A multicentre study currently recruiting participants in Germany and Switzerland will compare the Delorme's procedure versus laparoscopic resection rectopexy (DeloRes 2012). A single centre study in Denmark is assessing the difference between laparoscopic posterior rectopexy without mesh and laparoscopic anterior mesh rectopexy (NCT00946205). Another trial is examining robotic‐assisted versus laparoscopic ventral rectopexy in full‐thickness rectal prolapse and intussusception (Makela‐Kaikkonen 2013); this trial has finished recruiting and is awaiting results. One Australian trial compared laparoscopic resection rectopexy with fixation rectopexy (ACTRN12605000748617), and another single centre study compared standard mesh rectopexy with ventral rectopexy (Tarquini 2010). Furthermore, outcomes from longer follow‐up of the trials included in the present review may also provide more information on prolapse recurrence and bowel function.
Overall completeness and applicability of evidence
There are several major weaknesses of this review. The first relates to the plethora of interventions that have been analysed. There is very little consensus about the best treatment for rectal prolapse, apart from the agreement that surgery is the only potential mechanism for cure. Thus, when it comes to surgical intervention, the options are numerous. Should the approach be perineal or abdominal, open or laparoscopic? Should the rectum be mobilised anteriorly/posteriorly/laterally? Should redundant colon be routinely resected in combination with rectal fixation, and how should the rectum be fixed?
For each of these interventions, there are, in turn, numerous outcomes. Although many would agree that the most important universal outcome is recurrence of rectal or mucosal prolapse, some interventions are utilised because they have distinct advantages over other techniques. For instance, constipation can be considered the primary outcome when comparing resection rectopexy with mesh rectopexy, and adverse effects and cost may become primary outcomes when comparing laparoscopic abdominal surgery with open surgery, assuming efficacy is comparable. The different interventions, with the variable reporting of outcomes (including different follow‐up periods for each trial) weaken the power of the meta‐analyses, as often results from only two trials can be combined because of the specificity and heterogeneity of the individual comparisons.
During data synthesis and analysis, we calculated the standard deviation from the range, but this only provides estimated values.
Quality of the evidence
Another significant drawback of this review is the methodological weakness of many of the included trials, which compromises the value of their results. For instance, eight trials did not mention methods of randomisation and only five trials documented the allocation concealment methods. Other potential sources of bias are likely due to lack of blinding in most trials, poor withdrawal and exclusion criteria data, and inadequate follow‐up, which has to be long‐term in order to exclude recurrence. Only five trials had follow‐up more than three years and none more than five years. Having said that, the most recent trials (Karas 2011; Senapati 2013) have larger numbers of participants and improved design.

PRISMA study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
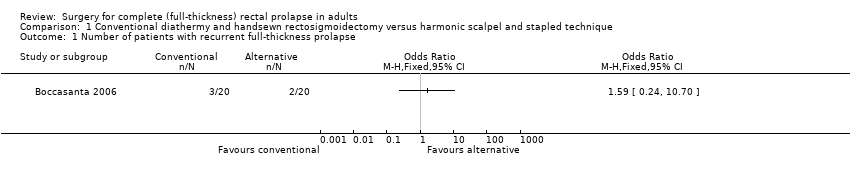
Comparison 1 Conventional diathermy and handsewn rectosigmoidectomy versus harmonic scalpel and stapled technique, Outcome 1 Number of patients with recurrent full‐thickness prolapse.

Comparison 1 Conventional diathermy and handsewn rectosigmoidectomy versus harmonic scalpel and stapled technique, Outcome 2 Incontinence score.

Comparison 1 Conventional diathermy and handsewn rectosigmoidectomy versus harmonic scalpel and stapled technique, Outcome 3 Hospital stay.
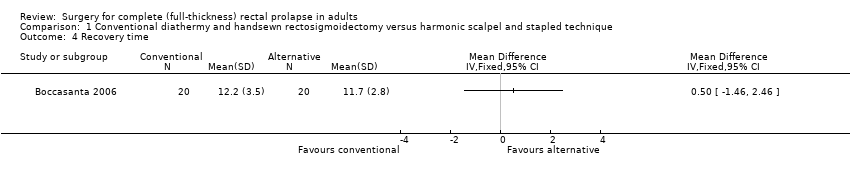
Comparison 1 Conventional diathermy and handsewn rectosigmoidectomy versus harmonic scalpel and stapled technique, Outcome 4 Recovery time.

Comparison 1 Conventional diathermy and handsewn rectosigmoidectomy versus harmonic scalpel and stapled technique, Outcome 5 Number of patients with defecatory problems.

Comparison 1 Conventional diathermy and handsewn rectosigmoidectomy versus harmonic scalpel and stapled technique, Outcome 6 Resting anal pressure (mmHg).

Comparison 1 Conventional diathermy and handsewn rectosigmoidectomy versus harmonic scalpel and stapled technique, Outcome 7 Squeeze pressure (mmHg).

Comparison 1 Conventional diathermy and handsewn rectosigmoidectomy versus harmonic scalpel and stapled technique, Outcome 8 Threshold volume (ml).
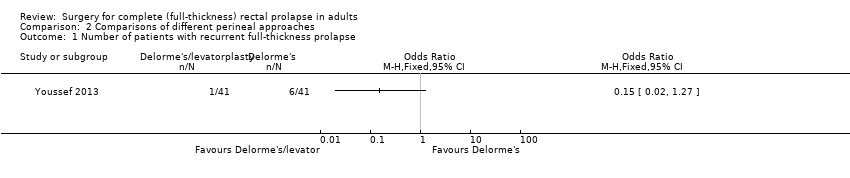
Comparison 2 Comparisons of different perineal approaches, Outcome 1 Number of patients with recurrent full‐thickness prolapse.

Comparison 2 Comparisons of different perineal approaches, Outcome 2 Number of patients with residual faecal incontinence.
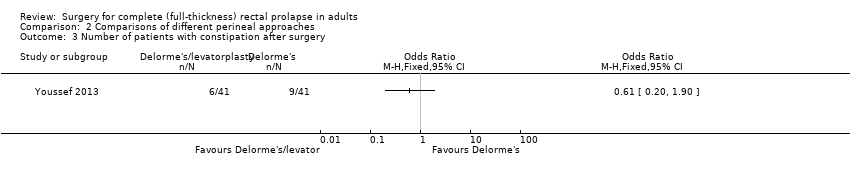
Comparison 2 Comparisons of different perineal approaches, Outcome 3 Number of patients with constipation after surgery.

Comparison 2 Comparisons of different perineal approaches, Outcome 4 Operating time (min).

Comparison 2 Comparisons of different perineal approaches, Outcome 5 Number of patients with postoperative complications.
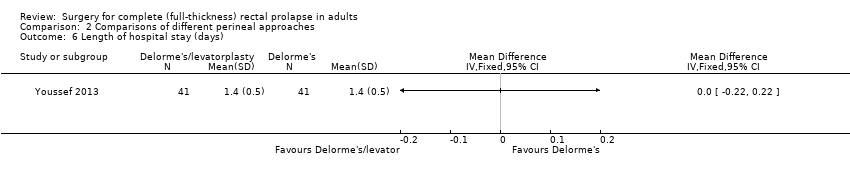
Comparison 2 Comparisons of different perineal approaches, Outcome 6 Length of hospital stay (days).

Comparison 2 Comparisons of different perineal approaches, Outcome 7 Postoperative maximum resting pressure.

Comparison 2 Comparisons of different perineal approaches, Outcome 8 Postoperative maximum squeeze pressure.
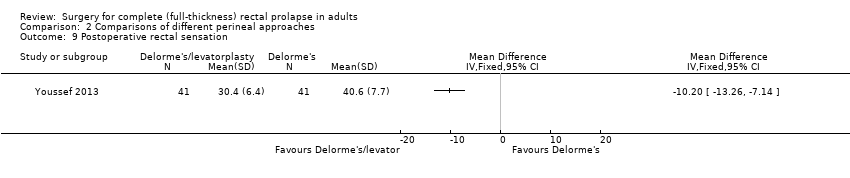
Comparison 2 Comparisons of different perineal approaches, Outcome 9 Postoperative rectal sensation.

Comparison 2 Comparisons of different perineal approaches, Outcome 10 Patient's postoperative satisfaction score.

Comparison 2 Comparisons of different perineal approaches, Outcome 11 Number of patients with recurrent full‐thickness prolapse.
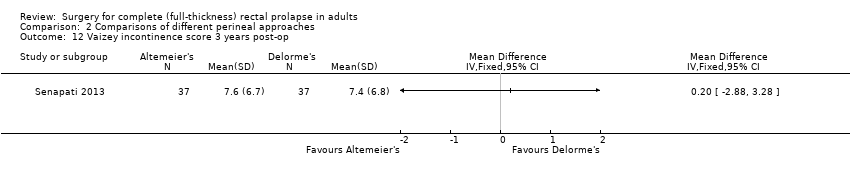
Comparison 2 Comparisons of different perineal approaches, Outcome 12 Vaizey incontinence score 3 years post‐op.

Comparison 2 Comparisons of different perineal approaches, Outcome 13 Bowel function (bowel thermometer) 3 years post‐op.

Comparison 2 Comparisons of different perineal approaches, Outcome 14 Quality of life score (EQ‐5D) at 3 years.

Comparison 3 Open abdominal Ivalon sponge rectopexy versus open abdominal suture rectopexy, Outcome 1 Number of patients with recurrent full‐thickness prolapse.

Comparison 3 Open abdominal Ivalon sponge rectopexy versus open abdominal suture rectopexy, Outcome 2 Number of patients with postoperative faecal incontinence.

Comparison 3 Open abdominal Ivalon sponge rectopexy versus open abdominal suture rectopexy, Outcome 3 Number of patients with constipation after surgery.
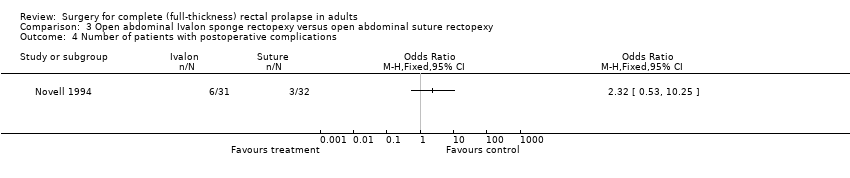
Comparison 3 Open abdominal Ivalon sponge rectopexy versus open abdominal suture rectopexy, Outcome 4 Number of patients with postoperative complications.
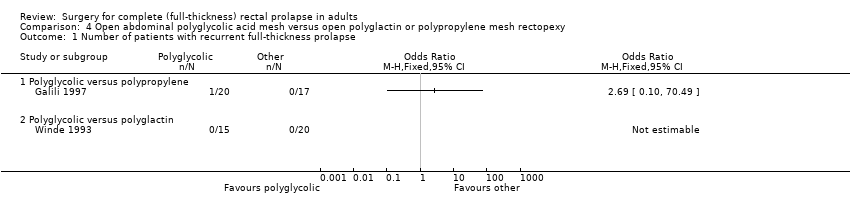
Comparison 4 Open abdominal polyglycolic acid mesh versus open polyglactin or polypropylene mesh rectopexy, Outcome 1 Number of patients with recurrent full‐thickness prolapse.

Comparison 4 Open abdominal polyglycolic acid mesh versus open polyglactin or polypropylene mesh rectopexy, Outcome 2 Number of patients with residual mucosal prolapse.

Comparison 4 Open abdominal polyglycolic acid mesh versus open polyglactin or polypropylene mesh rectopexy, Outcome 3 Number of patients with residual faecal incontinence.
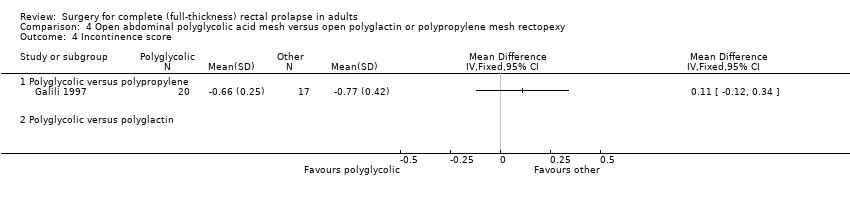
Comparison 4 Open abdominal polyglycolic acid mesh versus open polyglactin or polypropylene mesh rectopexy, Outcome 4 Incontinence score.
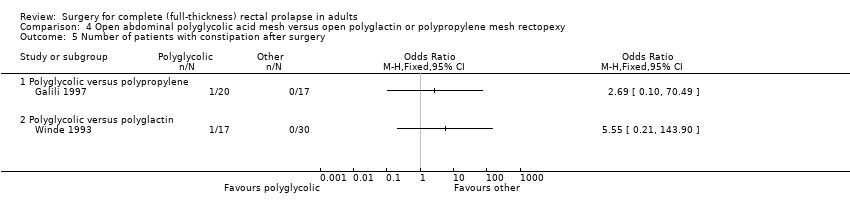
Comparison 4 Open abdominal polyglycolic acid mesh versus open polyglactin or polypropylene mesh rectopexy, Outcome 5 Number of patients with constipation after surgery.

Comparison 4 Open abdominal polyglycolic acid mesh versus open polyglactin or polypropylene mesh rectopexy, Outcome 6 Number of patients with postoperative complications.
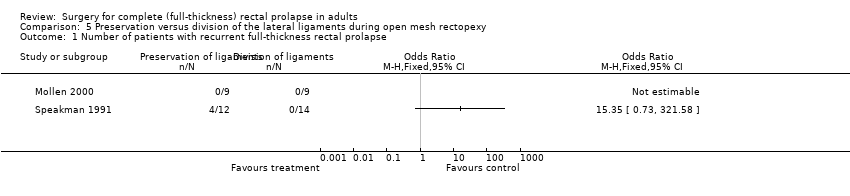
Comparison 5 Preservation versus division of the lateral ligaments during open mesh rectopexy, Outcome 1 Number of patients with recurrent full‐thickness rectal prolapse.

Comparison 5 Preservation versus division of the lateral ligaments during open mesh rectopexy, Outcome 2 Number of patients with residual mucosal prolapse only.

Comparison 5 Preservation versus division of the lateral ligaments during open mesh rectopexy, Outcome 3 Number of patients with constipation.

Comparison 5 Preservation versus division of the lateral ligaments during open mesh rectopexy, Outcome 4 Constipation score.
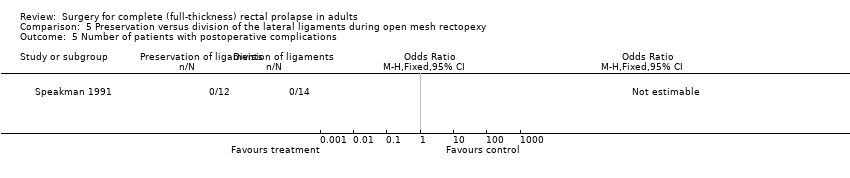
Comparison 5 Preservation versus division of the lateral ligaments during open mesh rectopexy, Outcome 5 Number of patients with postoperative complications.
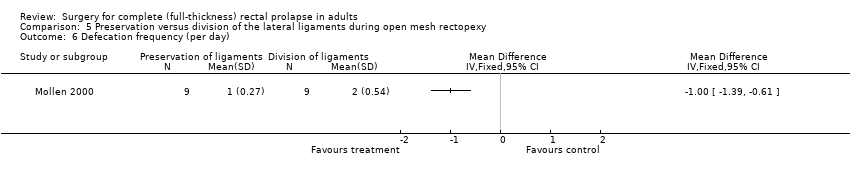
Comparison 5 Preservation versus division of the lateral ligaments during open mesh rectopexy, Outcome 6 Defecation frequency (per day).
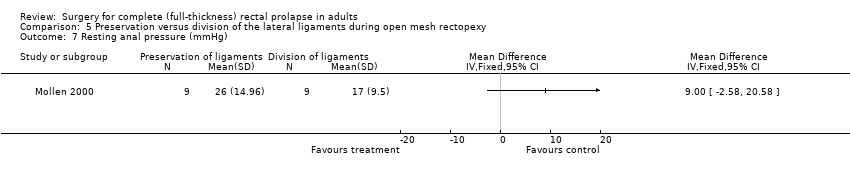
Comparison 5 Preservation versus division of the lateral ligaments during open mesh rectopexy, Outcome 7 Resting anal pressure (mmHg).
Comparison 5 Preservation versus division of the lateral ligaments during open mesh rectopexy, Outcome 8 Anal squeeze pressures (mmHg).

Comparison 5 Preservation versus division of the lateral ligaments during open mesh rectopexy, Outcome 9 Compliance (ml/mmHg).

Comparison 6 Laparoscopic versus open procedure, Outcome 1 Number of patients with recurrent full‐thickness prolapse.

Comparison 6 Laparoscopic versus open procedure, Outcome 2 Number of patients with residual mucosal prolapse only.

Comparison 6 Laparoscopic versus open procedure, Outcome 3 Incontinence score.
Comparison 6 Laparoscopic versus open procedure, Outcome 4 Number of patients with constipation after surgery.
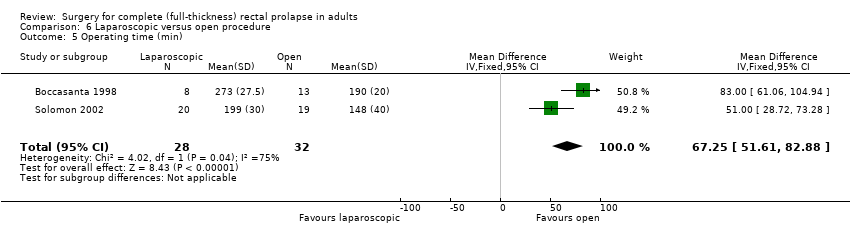
Comparison 6 Laparoscopic versus open procedure, Outcome 5 Operating time (min).

Comparison 6 Laparoscopic versus open procedure, Outcome 6 Number of patients with postoperative complications.

Comparison 6 Laparoscopic versus open procedure, Outcome 7 Length of hospital stay (days).

Comparison 6 Laparoscopic versus open procedure, Outcome 8 Maximum resting anal pressure (cmH2O).

Comparison 6 Laparoscopic versus open procedure, Outcome 9 Maximum squeeze pressure.
Comparison 6 Laparoscopic versus open procedure, Outcome 10 Maximum rectal volume (ml).
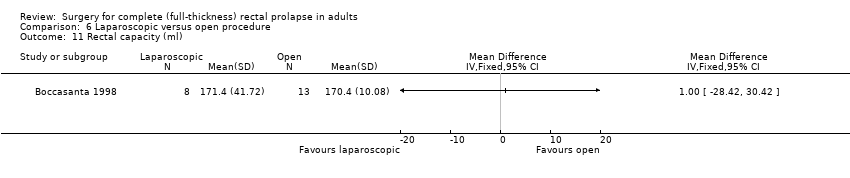
Comparison 6 Laparoscopic versus open procedure, Outcome 11 Rectal capacity (ml).

Comparison 6 Laparoscopic versus open procedure, Outcome 12 Total cost (USD).
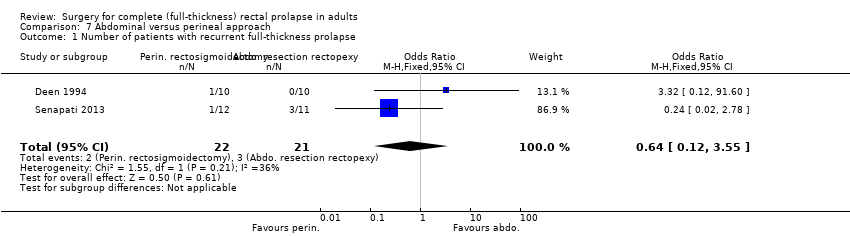
Comparison 7 Abdominal versus perineal approach, Outcome 1 Number of patients with recurrent full‐thickness prolapse.

Comparison 7 Abdominal versus perineal approach, Outcome 2 Number of patients with recurrent full‐thickness prolapse.

Comparison 7 Abdominal versus perineal approach, Outcome 3 Number of patients with residual mucosal prolapse only.
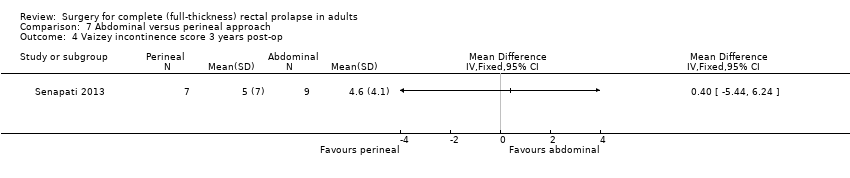
Comparison 7 Abdominal versus perineal approach, Outcome 4 Vaizey incontinence score 3 years post‐op.
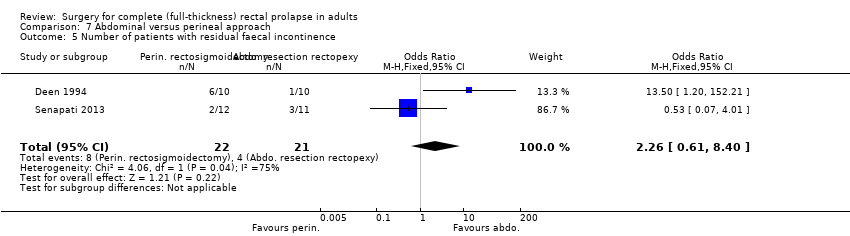
Comparison 7 Abdominal versus perineal approach, Outcome 5 Number of patients with residual faecal incontinence.
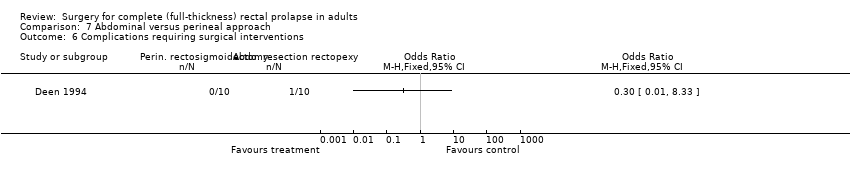
Comparison 7 Abdominal versus perineal approach, Outcome 6 Complications requiring surgical interventions.

Comparison 7 Abdominal versus perineal approach, Outcome 7 Number of patients with postoperative complications.

Comparison 7 Abdominal versus perineal approach, Outcome 8 Bowel function (bowel thermometer) 3 years post‐op.
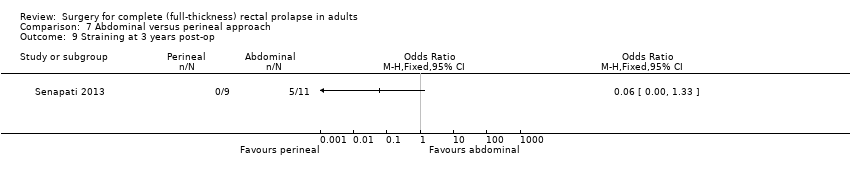
Comparison 7 Abdominal versus perineal approach, Outcome 9 Straining at 3 years post‐op.

Comparison 7 Abdominal versus perineal approach, Outcome 10 Maximum resting pressure (cmH2O).

Comparison 7 Abdominal versus perineal approach, Outcome 11 Maximum squeeze pressure (cmH2O).
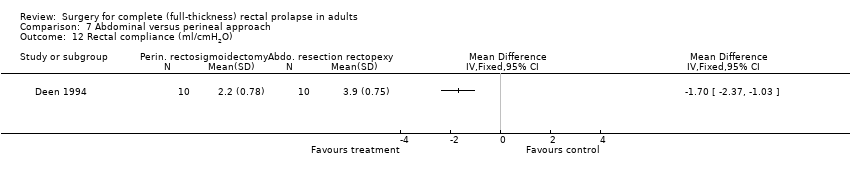
Comparison 7 Abdominal versus perineal approach, Outcome 12 Rectal compliance (ml/cmH2O).

Comparison 7 Abdominal versus perineal approach, Outcome 13 Quality of life score (EQ‐5D) at 3 years.

Comparison 8 Resection versus no resection rectopexy, Outcome 1 Number of patients with recurrent full‐thickness prolapse.

Comparison 8 Resection versus no resection rectopexy, Outcome 2 Number of patients with residual faecal incontinence.

Comparison 8 Resection versus no resection rectopexy, Outcome 3 Vaizey incontinence score 3 years post‐op.

Comparison 8 Resection versus no resection rectopexy, Outcome 4 Number of patients with constipation due to surgery.
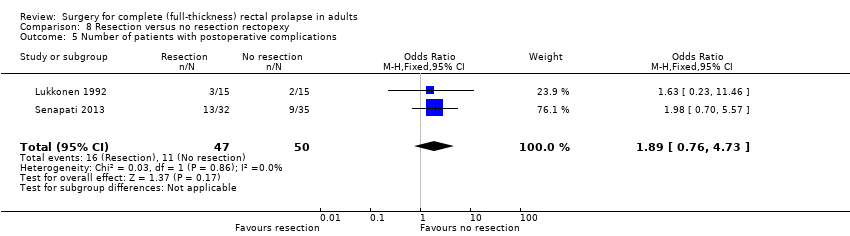
Comparison 8 Resection versus no resection rectopexy, Outcome 5 Number of patients with postoperative complications.

Comparison 8 Resection versus no resection rectopexy, Outcome 6 Bowel function (bowel thermometer) 3 years post‐op.

Comparison 8 Resection versus no resection rectopexy, Outcome 7 Maximum resting anal pressure (mmHg).

Comparison 8 Resection versus no resection rectopexy, Outcome 8 Maximum rectal volumes (ml).

Comparison 8 Resection versus no resection rectopexy, Outcome 9 Volume to first sensation (ml).

Comparison 8 Resection versus no resection rectopexy, Outcome 10 Anorectal angle (postoperative).
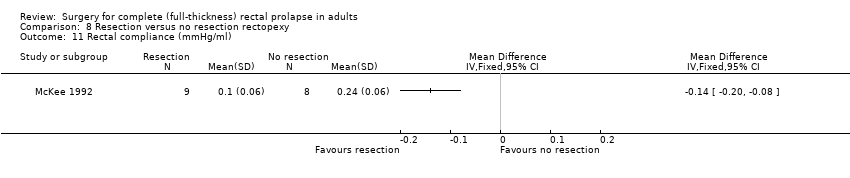
Comparison 8 Resection versus no resection rectopexy, Outcome 11 Rectal compliance (mmHg/ml).
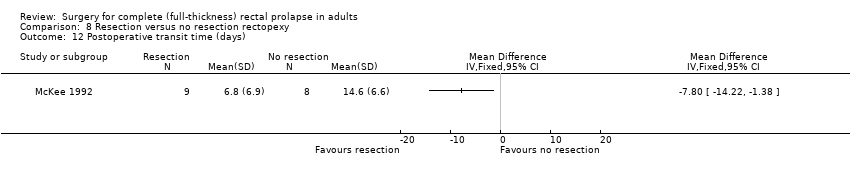
Comparison 8 Resection versus no resection rectopexy, Outcome 12 Postoperative transit time (days).

Comparison 8 Resection versus no resection rectopexy, Outcome 13 Quality of life score (EQ‐5D) at 3 years.

Comparison 9 Rectopexy versus no rectopexy, Outcome 1 Number of patients with recurrent full‐thickness prolapse.
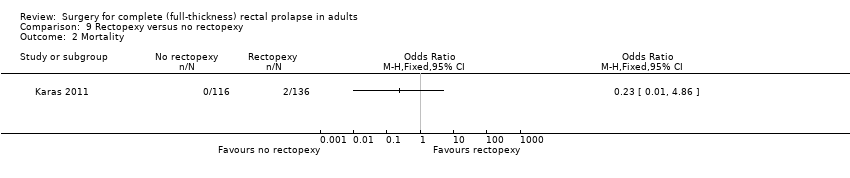
Comparison 9 Rectopexy versus no rectopexy, Outcome 2 Mortality.
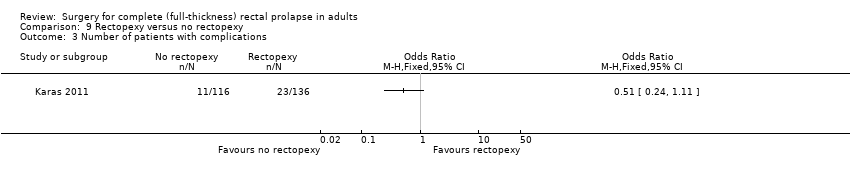
Comparison 9 Rectopexy versus no rectopexy, Outcome 3 Number of patients with complications.
| Perineal compared with abdominal approach for full‐thickness rectal prolapse in adults | ||||||
| Patients: Adults with full‐thickness rectal prolapse Setting: Surgical centres in India, Finland, Serbia, Spain, UK Interventions: perineal versus abdominal surgery | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk (with abdominal approach) | Corresponding risk (with perineal approach) | |||||
| Number of patients with recurrent full‐thickness prolapse | Moderate risk (study population) | OR 0.7 (0.17 to 2.88) | 44 (1 RCT) | ⊕⊕⊕ | A pragmatic trial, participants could be randomised between abdominal or perineal surgery. The abdominal procedure was performed through an open or laparoscopic approach depending on surgeon's preference. For perineal surgery, participants could be randomised to a Delorme's or an Altemeier's procedure. It was the surgeon's choice to participate in either or both of the randomisations. | |
| 263 per 1000 | 200 per 1000 | |||||
| Vaizey incontinence score 3 years post‐op | The mean Vaizey incontinence score 3 years post‐op in the control group was 4.6 | The mean Vaizey incontinence score 3 years post‐op in the intervention group was 5 higher (5.44 lower to 6.24 higher) | — | 16 (1 RCT) | ⊕⊕⊕ | The Vaizey scores ranged from 0 (perfect continence) to 24 (totally incontinent) |
| Number of patients with postoperative complications | Moderate risk (study population) | OR 0.65 (0.19 to 2.23) | 44 (1 RCT) | ⊕⊕⊕ | — | |
| 421 per 1000 | 321 per 1000 (121 to 619) | |||||
| Bowel function (bowel thermometer) 3 years post‐op | The mean bowel function (bowel thermometer) 3 years post‐op in the control group was 52 | The mean bowel function (bowel thermometer) 3 years post‐op in the intervention group was 50 higher (31.69 lower to 27.69 higher) | — | 9 (1 RCT) | ⊕⊕⊕ | Bowel function rated by participants, 0 (worst) to 100 (best) |
| Quality of life score (EQ‐5D) at 3 years | The mean quality of life score (EQ‐5D) at 3 years in the control group was 0.73 | the mean quality of life score (EQ‐5D) at 3 years in the intervention group was 0.86 higher (0.14 lower to 0.4 higher) | — | 14 (1 RCT) | ⊕⊕⊕ | EQ‐5D quality of life scores range from − 0.59 (worst) − 1.0 (perfect health) |
| Straining at 3 years post‐op | Moderate risk (study population) | OR 0.06 (0 to 1.33) | 20 (1 RCT) | ⊕⊕⊕ | — | |
| 455 per 1000 | 48 per 1000 (0 to 526) | |||||
| CI: Confidence interval; OR: Odds Ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for imprecision; single trial with small sample size and wide confidence interval | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients with recurrent full‐thickness prolapse Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Incontinence score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Hospital stay Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Recovery time Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Number of patients with defecatory problems Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Resting anal pressure (mmHg) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Squeeze pressure (mmHg) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Threshold volume (ml) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients with recurrent full‐thickness prolapse Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Number of patients with residual faecal incontinence Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Number of patients with constipation after surgery Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Operating time (min) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Number of patients with postoperative complications Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Length of hospital stay (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Postoperative maximum resting pressure Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Postoperative maximum squeeze pressure Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Postoperative rectal sensation Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Patient's postoperative satisfaction score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Number of patients with recurrent full‐thickness prolapse Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12 Vaizey incontinence score 3 years post‐op Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13 Bowel function (bowel thermometer) 3 years post‐op Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14 Quality of life score (EQ‐5D) at 3 years Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients with recurrent full‐thickness prolapse Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Number of patients with postoperative faecal incontinence Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Number of patients with constipation after surgery Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Number of patients with postoperative complications Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients with recurrent full‐thickness prolapse Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Polyglycolic versus polypropylene | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Polyglycolic versus polyglactin | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of patients with residual mucosal prolapse Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Polyglycolic versus polypropylene | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Polyglycolic versus polyglactin | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of patients with residual faecal incontinence Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Polyglycolic versus polypropylene | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Polyglycolic versus polyglactin | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Incontinence score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Polyglycolic versus polypropylene | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Polyglycolic versus polyglactin | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of patients with constipation after surgery Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Polyglycolic versus polypropylene | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Polyglycolic versus polyglactin | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of patients with postoperative complications Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Polyglycolic versus polypropylene | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Polyglycolic versus polyglactin | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients with recurrent full‐thickness rectal prolapse Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Number of patients with residual mucosal prolapse only Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Number of patients with constipation Show forest plot | 2 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.08, 1.23] |
| 4 Constipation score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Number of patients with postoperative complications Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Defecation frequency (per day) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Resting anal pressure (mmHg) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Anal squeeze pressures (mmHg) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Compliance (ml/mmHg) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients with recurrent full‐thickness prolapse Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Number of patients with residual mucosal prolapse only Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Incontinence score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Number of patients with constipation after surgery Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Operating time (min) Show forest plot | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | 67.25 [51.61, 82.88] |
| 6 Number of patients with postoperative complications Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Length of hospital stay (days) Show forest plot | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐2.35 [‐3.33, ‐1.37] |
| 8 Maximum resting anal pressure (cmH2O) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Maximum squeeze pressure Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Maximum rectal volume (ml) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Rectal capacity (ml) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12 Total cost (USD) Show forest plot | 2 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.84 [‐1.41, ‐0.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients with recurrent full‐thickness prolapse Show forest plot | 2 | 43 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.12, 3.55] |
| 2 Number of patients with recurrent full‐thickness prolapse Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Number of patients with residual mucosal prolapse only Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Vaizey incontinence score 3 years post‐op Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Number of patients with residual faecal incontinence Show forest plot | 2 | 43 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.26 [0.61, 8.40] |
| 6 Complications requiring surgical interventions Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Number of patients with postoperative complications Show forest plot | 2 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.15, 1.37] |
| 8 Bowel function (bowel thermometer) 3 years post‐op Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Straining at 3 years post‐op Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Maximum resting pressure (cmH2O) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Maximum squeeze pressure (cmH2O) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12 Rectal compliance (ml/cmH2O) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13 Quality of life score (EQ‐5D) at 3 years Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients with recurrent full‐thickness prolapse Show forest plot | 3 | 115 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.11, 1.50] |
| 2 Number of patients with residual faecal incontinence Show forest plot | 3 | 115 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.43, 2.03] |
| 3 Vaizey incontinence score 3 years post‐op Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Number of patients with constipation due to surgery Show forest plot | 3 | 84 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.04, 0.44] |
| 5 Number of patients with postoperative complications Show forest plot | 2 | 97 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.76, 4.73] |
| 6 Bowel function (bowel thermometer) 3 years post‐op Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Maximum resting anal pressure (mmHg) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Maximum rectal volumes (ml) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Volume to first sensation (ml) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Anorectal angle (postoperative) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Rectal compliance (mmHg/ml) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12 Postoperative transit time (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13 Quality of life score (EQ‐5D) at 3 years Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of patients with recurrent full‐thickness prolapse Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Mortality Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Number of patients with complications Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

